1. Introduction
Nanoparticulate carriers (NP) offer a suitable means for delivering diagnostic, imaging or therapeutic agents, including small and large molecules, gene vectors, biosensor, and nanotubes [1-3]. NP pose several advantages: (a) improve the solubility of hydrophobic compounds, (b) protect a molecule from undesirable interactions with biological milieu components and improve its stability, (c) provide controlled release of the contents, and (d) favorably alter the pharmacokinetics and biodistribution. Several nanomedicines are used clinically in the treatment of cancer, e.g., Doxil® [4], Abraxane® [5].
The utility of NP depends on their ability to reach their sites of action. Potential target sites include tumor vasculature (e.g., anti-angiogenics), tumor interstitium (e.g., diagnostics or therapeutics targeting extracellular proteins), cell membrane (e.g., antibodies), and intracellular compartments such as the cytosol (e.g., RNAi, drugs targeting cytosolic proteins) [6,7], nucleus (e.g., DNA gene vectors, DNA-active drugs) [8,9], and other intracellular compartments including mitochondria, Golgi apparatus and endoplasmic reticulum (ER) [10-12]. Delivery of NP from the injection site to various target sites within a solid tumor involves multiple kinetic steps, i.e., transport via blood to tumors, extravasation from tumor vasculature, interstitial transport, binding to cell membrane, internalization and intracellular trafficking (Fig. 1). This report provides an overview of the processes with respect to whole tumor (organ level), tumor interstitium (sub-organ level), and cellular/intracellular compartments (subcellular level). The discussion is focused on the physiological and biological barriers to NP delivery, followed by the experimental approaches and NP designs to break down such barriers.
Fig.1.
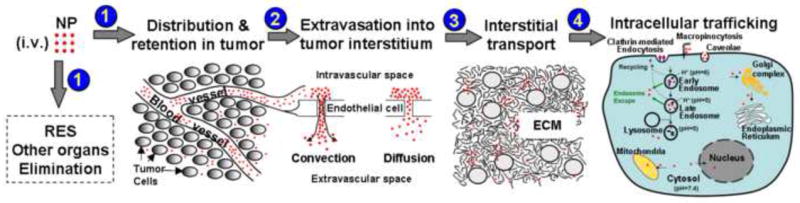
Processes for nanoparticulate carriers (NP) transport from injection site to target sites. (1) Transport and distribution to tumors and other organs via systemic circulation, including elimination by cells of reticuloendothelial system (RES). (2) Extravasation from tumor vasculature. (3) Interstitial transport to reach individual tumor cells. (4) Endocytosis and intracellular trafficking to sub-cellular organelles (early and late endosomes, lysosomes, Golgi complex, endoplasmic reticulum, cytosol, mitochondria, nucleus).
2. Delivery of NP to solid tumors (organ level)
After systemic administration (e.g., intravenous injection), NP are distributed to different organs via blood circulation, and at the same time undergo elimination (e.g., metabolism by the liver, excretion by the kidney). NP are also removed by cells (e.g., macrophages, Kupffer cells) in the reticuloendothelial system (RES) [13,14]. As described below, the delivery of NP to a solid tumor is determined by physiological factors including tumor blood vasculature, lymphatic drainage and tumor interstitial fluid pressure (IFP) and the physicochemical properties of NP such as surface characteristics (charge and hydrophilicity) and particle size. Please refer to our earlier reviews on similar subjects for additional references [15-17].
2.1. Distribution and retention of NP in tumors after systemic delivery
Perfusion and drainage of tissues involve blood and lymphatic vessels. In a solid tumor, NP leave the intravascular space within a vessel to enter the interstitium (i.e., extravasation). Transport across vessel walls is by diffusion, convection through capillary pores, and, to a minor extent, transcytosis. Diffusion is driven by concentration gradients. Transvascular fluid transport is driven by pressure gradients across intravascular and interstitial space. Leakiness in tumor vessels promotes NP extravasation, but also elevates IFP and reduces transvascular fluid transport. Capillary pore size poses the upper size limit for the extravasated NP.
2.1.1. Tumor blood flow and blood vasculature
Blood flow within a tissue is determined by the arteriole-venule (A-V) pressure difference and flow resistance. Blood vessels in the tumor interior are mainly veins/venules, with a few arteries/arterioles in tumor periphery. Hence, the A-V pressure difference is negligible in the central region but is greater in the periphery. This partly explains the lower blood flow in the center and the higher blood flow in the periphery of a tumor.
Blood vessel distribution within a tumor depends on the tumor size and the locations within a tumor. Small tumors (<2 mm) are perfused by vasculature originating from surrounding host tissues, whereas further growth are usually accompanied by newly formed microvessels [16]. Vascularization is inversely related to tumor size. Larger tumors show a higher ratio of avascular/seminecrotic-to-perfused regions and greater distances between capillaries, e.g., the intercapillary distance is ~50 μm in vascularized regions of a rat mammary adenocarcinoma tumor and ~300 μm in the larger tumors in human patients [18]. Within a tumor, vascularization status and distribution of blood vessels vary depending on the location and tumor size. A solid tumor comprises three regions: (a) avascular necrotic region with no vasculature, (b) seminecrotic region containing capillaries, pre-and post-capillaries, and (c) stably perfused region containing many venous vessels and few (2 to 5) arteriolar vessels. Larger tumors usually show higher avascular-to-well-perfused area ratio and greater distance between capillaries, with blood vessel density decreasing from tumor periphery to the center. These intra-tumoral vasculature heterogeneities contribute to uneven drug distribution within solid tumors and partly explain the lower average weight-adjusted drug concentration in larger tumors.
Tumor blood vessels are generally leaky due to the discontinuity of the endothelium (Fig. 2). In transplanted rodent tumors, the pore size of tumor microvessels varies from 100 to 780 nm in diameter depending on the anatomic location (e.g., smaller in cranial tumors compared to subcutaneous tumors) and the tumor growth (e.g., smaller in regressing tumors) [19]. Elevated levels of vasoactive and growth factors (bradykinin, nitric oxide, vascular endothelial growth factor, basic fibroblast growth factor) in tumors result in enhanced vascular permeability and dilatation [20]. Due to vessel leakiness, the major pathway of NP transport across tumor microvascular wall is by extravasation via diffusion and/or convection through the discontinuous endothelial junctions, whereas transcytosis plays a relatively minor role. Extravasation of molecules is associated with fluid movement across vasculature wall, which in turn depends on the hydrostatic pressure gradient between intravascular space (i.e., microvascular pressure or MVP) and interstitial space (i.e., interstitial fluid pressure or IFP) and the osmotic pressure gradient (due to differences in protein levels).
Fig.2.
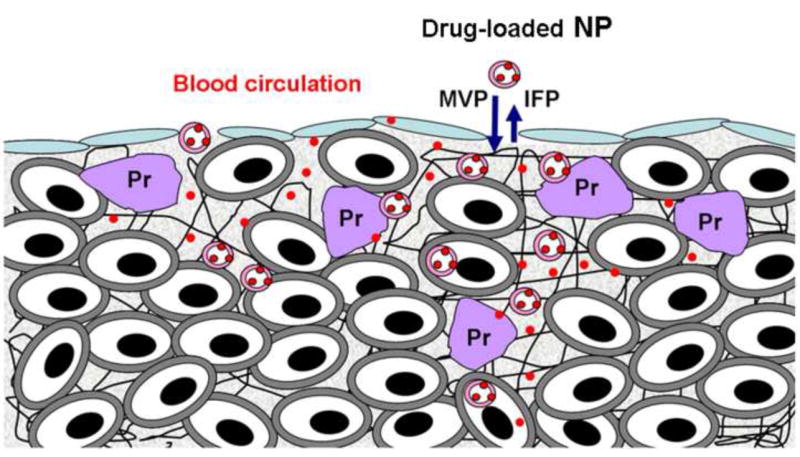
Determinants of interstitial transport of nanoparticulate carriers (NP) in tumors. (1) Absence of lymphatics reduces the clearance of interstitial fluid and soluble proteins, resulting in high interstitial fluid pressure (IFP), thus reducing the pressure gradient between microvascular pressure (MVP) and IFP and the associated convective transport. (2) Physical barriers due to presence of extracellular matrix proteins (Pr) or high cell density reduce diffusive and convective transport. (3) NP binding to proteins and cell membrane reduces the free concentration available for transport.
2.1.2. Lack of lymphatics
Lymphatic vessels are widely distributed throughout the body, and are more permeable to fluid and solutes compared to blood capillaries. The major function of the lymphatic system is to return the interstitial fluid to the blood circulation. In most normal and inflammatory tissues, macromolecules are cleared via the lymphatic system and large particles such as tumor cells detached from a primary tumor can enter the lymph by passing between the endothelial cells of the lymphatic capillaries. Solid tumors lack functional lymphatic drainage which reduces NP clearance from tumor interstitium.
2.1.3. Barriers to convective transvascular transport
Presence of blood and solutes in tumor interstitium, together with the absence of lymphatic drainage results in high IFP, which reduces the hydrostatic pressure gradient and thereby the convective transvascular transport. Usually IFP is uniformly high in the center with a sharp decline in the periphery of a tumor [21]. Changes in IFP parallel changes in MVP [22]. Jain, Baxter and collaborators have demonstrated elevated IFP as an important barrier to extravasation [23,24] (see also 3.1).
2.2. Tumor vs. normal tissues
There are several major differences between the blood and lymph vasculatures in tumor and normal tissues. First, tumor blood vessels are generally more heterogeneous in distribution, density, length and diameter, and are larger in size and more permeable. For example, relative to normal subcutaneous tissues, blood vasculature in the periphery of subcutaneous rat hepatoma tumors shows larger volume fraction (50 vs. 20%), a larger surface area and a longer length per unit volume (70 vs. 20 mm2/mm3 and 160 vs. 36 cm/mm3), whereas the tumor center shows lower values. Similarly, the vascularized regions of rat colon tumors showed larger capillaries (5–20 vs. 5–8 μm diameter) and larger venules (15–70 vs. 12–50 μm) relative to normal colonic tissues [16].
Second, resistance to blood flow in a vessel is determined by viscosity of blood and vessel geometry (length and diameter). Compared to normal tissues, tumors show greater blood viscosity due to the presence of tumor cells and large molecules (e.g., proteins and collagen), larger and longer vessels, similar arterial pressure but a lower venous pressure, with the net result of a greater flow resistance and lower average blood flow in tumors [25]. On the whole, the average blood flow in tumors is lower than in normal tissues.
Third, in most normal tissues, the typical distance in the tight junctions between endothelial cells in microvessels is less than 2 nm and the typical pore size in post-capillary venules is less than 6 nm, with the exception of kidney and the two RES organs (liver and spleen). The latter two groups have larger pore sizes, 50-150 nm. In contrast, tumor vessels are more leaky with pore sizes exceeding 100 nm (see above).
Fourth, the lack of functional lymphatic drainage in tumors poses two opposing effects on NP delivery and transport; it reduces NP clearance from tumor interstitium but also increases IFP and thereby limits the fluid flow and retards convection-mediated transvascular and interstitial transport.
The net effect of the unique features of tumor vasculature is the preferential extravasation and retention of NP relative to normal tissues [19]; these properties are being used for passive tumor targeting (see 2.3.1).
2.3. Experimental approaches and NP formulation designs
The following sections dicuss the experimental approaches for improving the delivery and targeting of NP to solid tumors. Readers are referred to an excellent review on liposome modifications for these purposes [26].
2.3.1. Passive targeting via EPR effect; Effect of particle size
The defective lymphatic drainage in solid tumors decreases the clearance of high molecular weight compounds from tumor interstitium. This, together with leaky tumor blood vessels that allow large molecules to extravasate, result in accumulation and retention of these compounds in solid tumors, a phenomenon recognized as the enhanced permeability and retention (EPR) effect [27,28]. In general, retention plays a larger role in EPR relative to extravasation. EPR is predominant for compounds with molecular weights larger than 40 KD but negligible for smaller molecules that readily redistribute to blood circulation via diffusion and/or convection. EPR is affected by the tumor size with a greater EPR in smaller tumors, probably because of the greater vessel density allowing for more extravasation as compared to larger tumors containing larger avascular regions. The vessel pore size in a majority of experimental tumors ranges from 380 nm to 780 nm. After extravasation, the smaller (100-200 nm) particles are transported to a greater distance and are more dispersed in the interstitium relative to larger (380-780 nm) particles that are more localized in peri-vascular space [29]. Therefore, limiting the size of NP to less than 200 nm can promote extravasation as well as interstitial transport.
2.3.2. Surface modifications of NP to confer stealth property
NP are recognized as foreign particles by cells in the RES, which causes NP entrapment in RES and consequently the rapid clearance of NP from blood circulation. Surface modification has been used to minimize RES entrapment and to increase the blood circulation time. NP surface modification agents include ganglioside GM1 [30], Tween 80 [31], peptide library-PE [32], poly-N-vinylpyrrolidones [33], L-amino-acid-based biodegradable polymer–lipid conjugates [34], polyvinyl alcohol [35], and polyethylene glycol (PEG). The latter is the agent of choice and the most widely used. Pegylation of NP, by increasing the hydrophilicity, forming an 3-D repulsing structure and creating an impermeable layer over NP surface, hinders NP recognition and absorption by opsonin [36,37]. These effects subsequently decrease the overall immune response and inhibit RES uptake. The degree of pegylation affects the stealth properties of NP. For example, at least 0.5 mol% of PEG2000 is required to confer stealth properties to liposomes, whereas higher than 15 mol% of PEG2000 destroys the phospholipid bilayer [38,39]. On the other hand, pegylation decreases the transport of NP in tumor interstitium (due to increased particle size) and decreases the cellular internalization (due to increased surface hydrophilicity and decreased binding to cell surface) [40,41]. These limitations can be overcome by using detachable PEG or by conjugating the hydroxyl end of PEG with cell surface targeting ligands [42,43] (see 4.3).
2.3.3. Targeting the acidic microenvironment in tumor matrix
Solid tumors, in part due to the high glycolysis, usually shows acidic pH [44]. The pH value in tumor matrix is affected by tumor histology, tumor volume and the location within a tumor, and can be as low as 5.7 [45,46]. pH gradient affects the ionization and intratumoral distribution and cellular uptake of ionizable drugs in a tumor [47]. NP comprising pH-sensitive polymers (e.g., poly(L-histidine) and poly-sulfonamide), which are negatively charged and stable at pH 7.4 but become neutralized and leaky at acidic pH, are used to target the tumor extracellular matrix or to promote the release of their contents in intracellular organelles such as the acidic endosomes [48] (see also 4.3).
2.3.4. Targeting tumor vasculature by electrostatic interaction
The luminal endothelial membrane in vessels is negatively charged and therefore can be targeted by cationic NP through electrostatic interactions [49-51]. This approach leads to more rapid and more extensive NP extravasation and retention in tumors relative to passive targeting via the EPR effect [52]. For example, doxorubicin-loaded cationic liposomes and cationic lipid-DNA complexes show greater tumor targeting compared to neutral liposomes, sterically stabilized neutral liposomes, and anionic pegylated liposomes of similar sizes [52-54]. The density of positive charge on NP affects mainly the partitioning between the interstitial and vascular compartments in a solid tumor and not the total uptake [55]. Cationic liposomes are also preferentially taken up by angiogenic tumor endothelium, due to binding through electrostatic interactions and subsequent internalization via clathrin-coated vesicles; this results in 15- to 33-fold greater uptake relative to endothelial cells in normal tissues [54]. However, because the negative charge on luminal endothelial membrane is also found in normal tissues, this mode of targeting is quantitative rather than qualitative. Greater tumor selectivity is achieved by conjugating NP with tumor endothelium specific ligands; e.g., pegylated liposomes conjugated with cyclic RGD peptides that recognize αvβ3 integrin receptors that are over-expressed in proliferating tumor vasculature endothelium relative to normal endothelium [56,57].
3. Interstitial transport (sub-organ level)
In general, after entering a tumor via blood circulation, a molecule is transported across the interstitial space to reach individual tumor cells. The transport processes for small molecules released from NP is by diffusion and convection, whereas the transport for NP, due to its large size and small diffusivity, generally relies more on convection (except when the size of NP is smaller than the pore size in the interstitium). Barriers to NP transport in tumor interstitium and the experimental approaches to overcome such barriers are as follows.
3.1. Barriers to convective transvascular and interstitial transport
The high IFP in a solid tumor together with the lack of a functional lymphatic system reduces the fluid flow and convection-mediated transport. In addition, the higher IFP relative to the surrounding normal tissues results in a net convective flow outward from the tumor core and thereby reduces the interstitial transport to cells in the interior portions [58,59] (Fig. 2). Agents that have been used to lower tumor IFP include imatinib mesylate [60], bevacizumab [61], tumor necrosis factor-alpha (TNF-α) [62,63], Fc:TβRII [64], hyaluronidase [65], dexamethasone [66], cereport [67], prostaglandin E1-methyl ester (PGE1) [68], taxanes (paclitaxel and docetaxel) [69], thalidomide [70], nicotinamide [71] and pentoxifylline [72]. Table 1 summarizes their potential IFP-lowering mechanisms and their effects on mean arterial blood pressure, tumor blood flow and MVP. Their effects on IFP are often tumor type- and dose-dependent. The limitations of these agents include toxicity to normal tissue or lack of tumor selectivity.
Table 1.
Agents affecting IFP and tumor blood flow and pressures.
| Agents [Ref] | Possible mechanisms of lowering IFP | MABP | Blood flow | MVP |
|---|---|---|---|---|
| Imatinib mesylate[60] | Platelet-derived growtd factor antagonist (anti-PDGF), decreases contraction and interaction of stromal fibroblasts witd extracellular matrix | Unknown | Unknown | Decrease |
| Bevacizumab [61] | Vascular endothelial growth factor antagonist (anti-VEGF) | Unknown | Increase | Decrease |
| TNF-α [62,63] | Tumor necrosis factor-alpha, destruct tumor vessel and improve vascular permeability and thus increase the pressure gradient across the vessel | Decrease | Unknown | Decrease |
| Fc:TβRII [64] | TGFβ antagonist, inhibits intra-tumoral macrophage activation to lower IFP, normalizes tumor blood vessels | Unknown | No change | No change |
| Hyaluronidase [65] | Degrades hyaluronan to reduce the physical resistance of extracellular matrix | Unknown | Unknown | Decrease |
| Dexamethasone [66] | Reduces vascular hydraulic conductivity | Unknown | Unknown | Decrease |
| Cereport [67] | Bradykinin agonist, increases vascular surface area and pore size | Decrease | Increase | Decrease |
| Prostaglandin E1-methyl ester [68] | Inhibits stromal fibroblast contraction | Unknown | Unknown | Decrease |
| Taxanes (paclitaxel and docetaxel) [69] | Induces apoptosis and reduces tumor cell density, decompress blood vessels and increases the vascular surface area | Unknown | Increase | Decrease |
| Thalidomide [70] | Angiogenesis inhibitor, produces transient vascular normalization initially | Unknown | Increase | Decrease |
| Nicotinamide [71] | Reduces the heterogeneity of micro-regional perfusion | Decrease | Increase in some but not all tumors | Decrease |
| Pentoxifylline [72] | Alter erythrocyte deformability and thus decrease viscous blood resistance and/or geometric resistance | No change | Increase | Decrease |
IFP, interstitial fluid pressure. MABP, mean arterial blood pressure. MVP, microvascular pressure.
Fick’s First Law indicates negligible diffusive transport in a gel for solutes with sizes exceeding or near the pore size. Accordingly, the diffusive transport of NP is negligible in a tumor with high cell density or, conversely, a low fraction of interstitial space. Our laboratory used an in vitro system devoid of blood flow (and therefore no IFP and no convective transport) to study the barriers to diffusional transport of albumin-bound drugs (~7 nm in diameter); this system comprises 3-dimensional histocultures of 1 mm3 tumors fragments. By comparing the kinetics of uptake and efflux and the spatial distribution in histocultures with different compositions and architectures, we identified tumor cell density as the key barrier to diffusive transport in tumor interstitium [15,73]. We further showed that treatments with commonly used drugs (paclitaxel, doxorubicin), by inducing apoptosis and thereby reducing tumor cell density and increasing the fraction of interstitial space (referred to as tumor-priming, Fig. 3), enhance the diffusive transport in vitro. Similar tumor-priming effects were observed in tumor-bearing animals; apoptosis-inducing treatments produce a transient reduction of tumor cell density and a transient increase in interstitial space. These changes in turn increase the diameter of patent vessels, leading to greater transvascular flow and interstitial transport (diffusive and convective) [74,75]. The optimal time window for these effects is 24 to 96 hr after an intravenous paclitaxel injection. As discussed below, these changes are sufficient to improve the efficacy of nanomedicines in animals [76]. Our findings have since been verified by Jain et al.; their latest publication shows that the void space produced by cancer cell apoptosis enhances the dispersion and efficacy of oncolytic herpes simplex virus [77].
Fig.3.
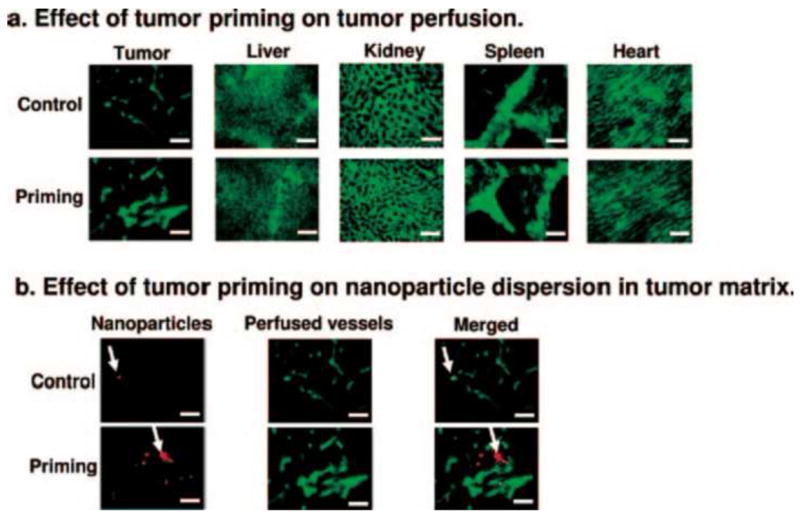
Effects of tumor priming on tumor perfusion and dispersion of nanoparticles (NP) in tumor matrix. (A) Effect of tumor priming on tumor perfusion. (B) Effect of tumor priming on NP dispersion in tumor matrix. NP (red fluorescence), perfused vessels (green fluorescence, perfusion marker 3,3-diheptyloxacarbocyanine iodide), NP merged with perfused vessels (yellow). Arrows indicate NP locations. Note the co-localization of NP with perfused vessels in the control group and the greater dispersion of NP away from vessels in the tumor priming group. Bar, 100 μm. (Reproduced from Ref [76])
It is noteworthy that the effects of paclitaxel tumor-priming on vasculature, blood flow, and delivery and interstitial transport of NP, due to the higher susceptibility of tumor cells to apoptosis, are tumor-specific and not observed in noncancerous tissues including liver, kidney, heart and the organs enriched in reticuloendothelial system (spleen, liver) (Fig. 3). For example, paclitaxel tumor priming enhances the delivery of Doxil® (doxorubicin in pegylated liposomes, 85 nm diameter) selectively to tumors without affecting the normal tissues, resulting in greater tumor regression and prolonged survival without enhancing the host toxicity [76]. The tumor selectivity of tumor-priming and the fact that paclitaxel or other apoptosis-inducing chemotherapy are standards of care for cancer patients offer a unique opportunity to improve NP transport in tumor interstitium without the risk of additional toxicity.
3.2. Extracellular matrix
Extracellular matrix (ECM) comprises fibrous proteins (e.g., collagen, elastin) and polysaccharides (e.g., hyaluronan, glycosaminoglycan (GAG)) [78,79]. These proteins are a source of physical resistance to diffusional transport and are associated with lower hydraulic conductivity and lower convective flow in the interstitium. Collagen appears to contribute more to transport resistance compared to GAG or hyaluronan, e.g., diffusion coefficient of IgG is inversely related to the collagen content in a tumor. Enzymes that degrade tumor ECM materials, such as collagenase and hyaluronidase, promote intra-tumoral dispersion of small molecules, macromolecules (e.g., monoclonal antibody) and NP (e.g., liposomes) [80,81]; with a greater effect for collagenase [82]. Comparison between collagenase and hyaluronidase on the delivery of molecules with different sizes shows that collagenase is more effective in improving macromolecules delivery, whereas hyaluronidase was more efficient to enhance the delivery of smaller molecules such as doxorubicin [83].
3.3. Binding barrier
Binding of NP to extracellular matrix proteins and cell membrane (e.g., receptors) reduces the free drug/NP concentration available for interstitial transport. The binding barrier is more significant for active-targeting NP due to the high specificity and affinity between the targeting ligands and receptors [84]. In 3-dimensional cell spheroids, drugs that do not bind to cellular macromolecules or cannot cross cell membranes (e.g., 5-fluorouracil, cisplatin, inulin, thymidine-5’-triphosphate, sucrose, and nonspecific antibody) are evenly dispersed in 3-D thyroid tumor cell spheroids within 15 min. In contrast, drugs that bind to cellular macromolecules (e.g., doxorubicin, paclitaxel, daunomycin, actinomycin D, methotrexate, specific antibody binding to cell surface proteins) remain localized in the periphery of spheroids and 3-D tumor histocultures (e.g., penetration of ~10 cell layer after 24 hr) [16].
4. NP cellular and subcellular compartments targeting
The majority of research in this area is focused on targeting the membrane proteins enriched in tumor cells. More recent efforts have focused on targeting NP delivery to the cytosol and nucleus. Transport of NP in a cell, e.g., from the cell membrane to the nucleus, involves several processes: (a) attachment of NP to cell membrane through binding to non-specific or specific binding sites, (b) internalization of NP through endocytosis, (c) entrapment of NP in endosomes (eventually fuse with lysosomes), caveosomes or macropinosomes, (d) release of NP from these structures, (e) NP transport in cytosol and uptake by other intracellular cytoplasmic organelles, and (f) transport of NP into the nucleus.
4.1. Targeting the cell membrane
NP binding to cell membrane is a popular approach for designing active-targeting NP. NP bind to cell membrane through non-specific or specific binding (Fig. 4). While such binding in general facilitates the subsequent internalization of NP, there are situations where binding do not lead to internalization. For example, insulin-coated magnetic nanoparticles bind to specific surface receptors without the ensuing endocytosis [85].
Fig.4.
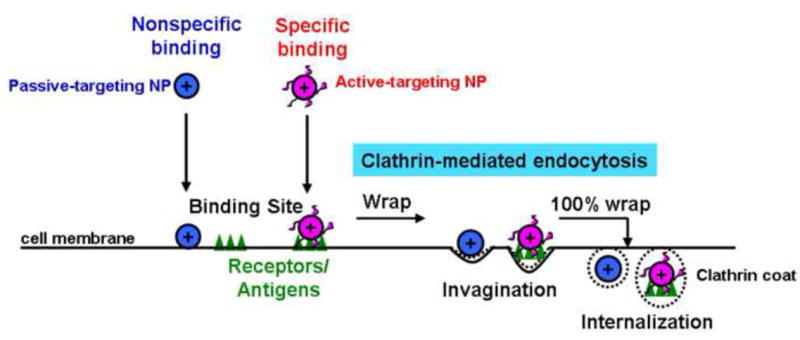
Clathrin-mediated uptake of nanoparticles (NP) into cells. Passive-targeting NP are absorbed to cell membrane components via nonspecific binding, and active-targeting NP via specific binding to membrane receptors or antigens. The primary internalization of bound NP is the clathrin-mediated endocytosis. The recruited NP-binding cell surface components/receptors form clathrin-coated pits to wrap NP and internalization occurs upon complete wrapping.
For nonspecific binding, the components on cell surface are protease-sensitive and the process requires calcium ions for optimal binding [86]. Interactions between a particle and a cell, e.g., due to long-range van der Waals forces arising from fluctuations in the electric dipole moments of molecules, or the depletion and bridging forces due to the density gradients of macromolecules surrounding a cell that give rise to osmotic pressure to induce cell aggregation, can either enhance or reduce the binding [87,88]. Interactions that reduce NP binding include short-range Born repulsive forces due to molecular interactions resulting from overlapping electron clouds, steric repulsive forces induced by the cell membrane surface glycocalyx, electrostatic double layer forces resulting from counterions attracted by cell membrane surface potential, and entropic protrusion and undulation forces arising from molecular fluctuations of hydrocarbon chains [87,89]. Interactions that favor binding include hydrophobic forces, loss of hydrogen bonding, aggregation of non-polar molecules, or hydration [90]. Positive NP surface charge also promotes binding. For example, cationic liposomes usually shows higher extent of cellular uptake compared to neutral liposome and anionic liposome [91]. On the contrary, pegylation, in part by decreasing the positive surface charge, inhibits NP binding to cell surface [92].
Specific binding of NP to cell membrane is achieved by attaching ligands (e.g., antibodies, peptides, proteins, small molecules and carbohydrates) that bind to membrane receptors or antigens [93]. Considerable efforts have been expended on this approach; the most popular ligands are monoclonal antibodies or Fab fragments specific to tumor cell antigens [94-96], peptide [97], folate [98], and transferrin [99,100]. Most of these ligands are internalized by the clathrin-mediated endocytosis and are therefore subjected to the same considerations and limitations (see below). The main considerations for designing active-targeting NP are the density of ligands on particle surface, cell selectivity, stability, and ability to bypass the P-glycoprotein mediated multi-drug resistance mechanism [101-103]. The latter includes liposomes that use folate receptor- and transferrin receptor-mediated endocytosis [104,105]. Conjugations of ligands to liposomal surface typically involve chemical reactions to yield amide, disulfide and thioether bonds [106,107].
4.2. Internalization/endocytosis
Readers are referred to an excellent recent review on the mechanisms of endocytosis [108]. For NP that cannot directly cross the plasma membrane, their uptake into cells is mediated by phagocytic and pinocytic endocytosis [109]. Phagocytosis occurs in specialized cells (e.g., cells in RES) whereas pinocytosis is used by all cells. The three types of pinocytosis can be characterized as fluid-phase, absorptive, and receptor-mediated endocytosis [110]. Fluid phase endocytosis is a low-efficiency and nonspecific process, is primarily driven by the extracellular concentration, and is less prominent compared to absorptive and receptor-mediated endocytosis. For the latter two, NP are concentrated on the cell surface through non-specific or specific binding followed by internalization.
A major mechanism of NP internalization is the clathrin-mediated endocytosis. This process involves recruiting NP-binding cell surface receptors and forming clathrin-coated pits that engulf NP (Fig. 4). The coated vesicle buds into cell and after shedding the coat, the vesicle fuses with endosomes that move from the plasma membrane to lysosomes (Fig. 1). The membrane proton pump V-ATPase in endosomes causes influx of protons, resulting in a continuous drop in pH as endosomes mature from early endosomes (pH 6.2-6.3) to late endosomes (pH 5.0-5.5). Late endosomes fuse with lysosomes (pH 4.8-5.4) that contain degradative enzymes [111,112]. The content of early endosomes can be recycled via tubules to the cell surface or released into the cytosol. NP unable to escape from endosomes are subjected to degradation in the enzyme-rich lysosomes.
Other less prominent endocytosis mechanisms include (a) caveolae-mediated endocytosis that involves caveolin1-positive structures (which include large neutral pH intracellular structures, small vesicles and tubules) with the endocytosed NP located in caveosomes, and (b) clathrin- and caveolae-independent endocytosis that is dependent on cholesterol [108]. Macropinocytosis, also cholesterol-dependent, is involved in the fluid-phase endocytosis of macromolecules in tumor cells with active membrane or cytoskeletal (e.g., actin) activity; the endocytosed NP are located in macropinosomes [113] (Fig. 5).
Fig.5.
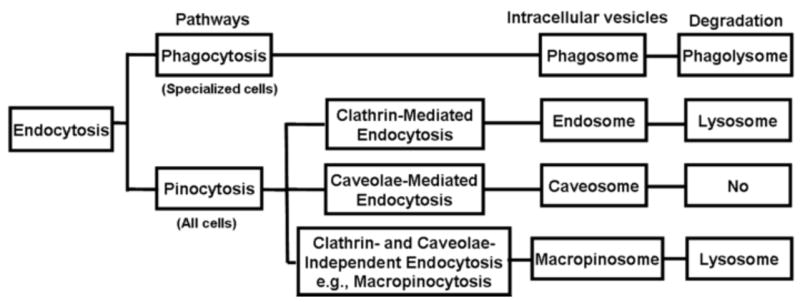
Processes for endocytosis, intracellular vesicular formation and degradation. Caveolae-mediated endocytosis may avoid lysosomal degradation. Macropinocytosis is used as an example of clathrin- and caveolae-independent endocytosis.
Cell penetrating peptides (CPP) are used to promote NP endocytosis. CPP can cross the cell membrane and is able to induce fusion between lipids membranes and leakage of liposome-entrapped compounds when exposed to low pH. CPP are mainly oligocationic in nature and are derived from viral, insect or mammalian proteins with membrane translocation properties, e.g., transactivator of transcription (TAT) family, penetratin, and chimeric peptide transportan, with TAT being the most popular [114-117]. CPP-mediated delivery involves multiple mechanisms. For TAT-modified liposomes, TAT binds to negatively-charged GAG on the cell surface through ionic interactions. TAT can also interact with cell membrane lipid rafts in a receptor-independent manner, stimulating a rapid internalization by macropinocytosis, followed by a pH drop and destabilization of the macropinosome vesicle lipid bilayer [118-120]. The strong adsorption of the CPP derived from the flock house virus coat protein on the cell membrane via GAG induces macropinocytosis [121]. CPP-modified liposomes can also be internalized via clathrin-coated pits or caveolin-dependent endocytosis [122-124]. The extent and mechanism of CPP-mediated internalization is determined by multiple factors, including CPP concentration, types of cells, and cell-specific membrane components [125]. For example, endocytosis of octaarginine (R8)-modified liposomes is density-dependent. Liposomes carrying R8 at a high density are internalized by macropinocytosis and then efficiently escape from macropinosomes to cytosol. In contrast, liposomes carrying R8 at a low density are taken up via clathrin-mediated endocytosis and subsequently degraded in endosomes/lysosomes [126,127]. It is noteworthy that enhanced internalization of liposomes by CPP does not necessary lead to greater efficacy. It has been reported that TAT peptide (TATp)–liposomes, after internalization, remained intact inside the cytoplasm for 1 hr, migrated to the perinuclear zone at 2 hr, and disintegrated after 9 hr [128]. However, it is unclear whether the internalized liposomes were present in macropinosomes, lysosomes or cytosol [129]. In addition, although it has been shown that adding TAT to doxorubicin-loaded liposome promoted drug internalization, TAT did not improve its antitumor activity [130]. Taken together, the available experimental evidence suggests that the internalized drug/TAT-linked NP remains entrapped in endo/lysosomal compartments and dose not reach the intended targets.
Non-endocytic pathways for liposomal NP internalization are mainly through fusing the lipid bilayer with the plasma membrane or through transfer of lipids between liposomes and membrane, resulting in concomitant release of liposome contents into the cytosol [131]. These non-endocytic processes are less prominent compared to endocytosis.
4.3. Escape/release from endosomes to cytosol
Successful endosomal escape enables NP to target the cytosol, which is the site of actions for multiple chemotherapeutic drugs and RNAi. Because NP generally cannot directly cross the endosomal membrane, strategies are to use agents to induce fusion between NP and endosomal membrane, in order to disrupt the membrane and promote the NP release from endosomes to cytosol. These processes also promote a microtubule-driven pathway that enables NP to escape from the early endosomes to enter the trans-Golgi network, Golgi apparatus, endoplasmic reticulum (ER), and cytosol, and thereby bypass the lysosomes [132] (Fig. 6).
Fig.6.
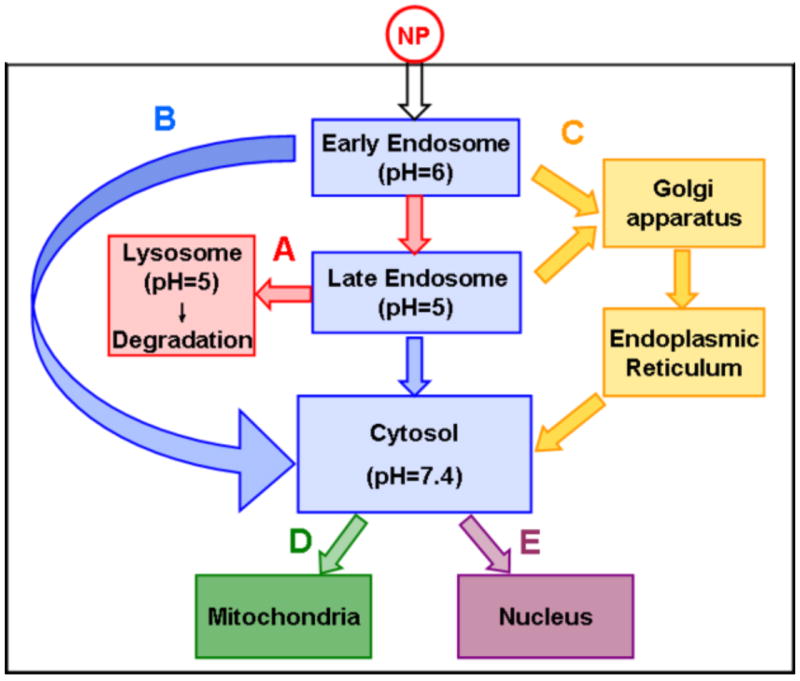
Intracellular trafficking of nanoparticles (NP). NP can undergo several processes: (A) Transport from early endosome to late endosome and then to lysosomes, and undergoes degradation in lysosomes. (B) Released from early/late endosomes into the cytosol. (C) Transport from early/late endosome to Golgi complex and endoplasmic reticulum, followed by release to the cytosol. After reaching cytosol, NP may enter mitochondria (D) or nucleus (E).
Agents used to promote NP escape/release from endosomes include pH-sensitive peptides, pH-buffering polymers, and fusogenic lipids. An example of pH-sensitive peptides is the peptide GALA, a 30 amino acid synthetic peptide with a glutamic acid-alanine-leucine-alanine repeat, which has an amphiphilic structure [133]. A decrease in endosomal pH from 6 to 5 leads to a decrease in negative charges of glutamic acids side-chain of GALA and causes a change of the structure from a random coil to an amphipathic α-helix, and thereby promotes GALA binding to endosomal membranes and results in membrane disruption [134,135]. The topology of GALA is critical to its function; successful endosomal escape is accomplished only when GALA is exposed on the surface of liposomes [136,137]. Virosomes, comprising liposomes modified with fusogenic viral envelope protein that serves as a CPP, induce pH-dependent destabilization of endosomal membranes [138-140]. diINF-7 is another pH-dependent fusogenic peptide that has been used to promote the release of diphtheria toxin A chain encapsulated in immunoliposomes [141]. Polymers with buffering capability at pH 5-6, such as poly(L-histidine) and polyethylenimine, induce the proton sponge effect. The unsaturated amino groups in the polymers absorb and sequester protons in the acidic endosomes, which cause the influx of chloride ions and water molecules, promote osmotic swelling of the endosomes and disrupt the endosome membrane [142]. An example of fusogenic lipids is dioleoylphosphatidylethanolamine (DOPE) [143,144].
Pegylation may inhibit lipid mixing/fusion between liposomes and endosomal membrane and thereby hinder the drug release from endosome to the cytosol [145]. A strategy to overcome this is to use a cleavable PEG-lipid. Most cleavable PEG-lipids are designed to take advantage on unique intracellular microenvironment, such as low pH, targeting a specific enzyme in endosomes, or targeting the reductive conditions in the cytosol [146-148]. An example is the combined use of the CPP octaarginine, cleavable PEG-lipids, and the pH-sensitive fusogenic GALA peptide to make liposomes for delivering siRNA to the cytosol [149,150].
4.4. Delivery to nucleus
Delivery of therapeutics targeting nuclear materials, e.g., DNA-directed therapeutics, requires transport into the nucleus. The nuclear envelope has a double membrane structure and is punctuated by nuclear pore complexes (NPC). Drugs or NP can enter the nucleus via passive, active and endosome-mediated transport. Passive cytosol-nucleus transport uses the aqueous channel of NPC, e.g., small molecules or small NP of up to 9 nm diameter (<50 kDa) [151,152]. Larger molecules, e.g., DNA, enter the nucleus during mitosis when the nuclear membrane breaks down. However, the viscous nature of the cytoplasm makes it very unlikely to attain nuclear localization by diffusion alone [153].
Active, energy-dependent nuclear transport requires the presence of specific targeting sequences, e.g., nuclear localization signal (NLS), that mediate the interaction of candidate molecules or NP with transport proteins such as importins [154,155]. Nuclear delivery of plasmid DNA is achieved by conjugation with NLS, e.g., derived from SV40 antigen. In cultured cells, binding of NLS-liposomes to the nucleus increases in a NLS density-dependent manner. Interestingly, this process appears to be enhanced by pegylation, such that the efficiency of NLS-PEG-liposomes binding with the nucleus is greater than the binding of unpegylated liposomes even at low NLS density [156]. Combination of NLS and pH sensitive fusogenic lipid enables the transfer of plasmid DNA into the nucleus in an energy-dependent manner [157].
A third type of nuclear transport mechanisms involves the endosome trafficking in cells, and can occur in several ways. Endosomes carry the NP away from the plasma membrane to perinuclear locations. This action shields NP from early release into the cytosol and the subsequent degradation by cytoplasmic nucleases [158]. Trafficking of endosomes to perinuclear locations bring the drug/NP in close proximity to NPC and thereby enhances the nuclear entry of the released cargo. Fusing of drug/NP-loaded endosomes directly with the nuclear membrane enables direct entry into the nucleus. For this mechanism, the cytoskeleton, usually microtubules, is involved in the transport and the perinuclear localization of NP.
4.5. Other subcellular organelles
Several recent studies explore targeted delivery to the mitochondrion due to its importance in apoptosis, e.g., release of cytochrome C from mitochondrion to cytosol activates caspase-dependent apoptosis [159]. Liposomes comprising egg phosphatidyl choline, cholesterol and a fusogenic lipid, and modified with a CPP (i.e., octaarginine) show mitochondria-targeting, apparently via a membrane fusion mechanism [160].
Golgi apparatus and ER are the key organelles of the secretory pathway. Proteins are synthesized in the ER and transported to the Golgi apparatus, utilizing specific transporters associated with antigen processing [12]. Golgi and ER are also involved in the lysosomal bypass of NP, i.e., NP may travel from endosomes to the trans-Golgi network, Golgi apparatus, ER, and finally cytosol (Fig. 6). Liposomes comprising fusogenic lipids use a lysosome-bypass route to reach the ER [132].
5. Conclusions and Perspectives
Nanotechnology has become an important tool in translational cancer research. As NP is versatile, can be made of different types of materials, and can have different sizes, surface charges and surface modifications, there is the potential to tailor the design of NP for its intended function. On the other hand, studies in the last 20+ years have identified the multiple barriers to NP delivery and transport in solid tumors and have shown that many of the processes and determinants of NP transfer from the administration site to the target sites are nonlinear, interdependent, and changes with time.
With respect to NP properties, some are beneficial for one transport process but detrimental to others (e.g., a larger NP size promotes the EPR effect but reduces its interstitial transport). For example, NP are frequently surface-modified with targeting ligands, but binding of ligands to cell surface receptors limits NP transport. Similar complexities exist with respect to intended targets in a solid tumor. Tumor properties, biological in nature, are dynamic and altered by a variety of variables, and can produce diverse and at times unexpected effects on NP disposition. Such diverse and dynamic tumor properties create uncertainties on the fate of NP at target sites and hence questions on the NP design. For example, tumor size and structure affect the vascularization status and alter the NP delivery to tumor cells, low interstitial pressure in necrotic regions (e.g., caused by radiation) alters the convective flow, and high levels of intercellular junction proteins reduce transport.
Important considerations for a pharmaceutical or translational scientist in developing NP cancer therapeutics and diagnostics include the following. What are the NP-protein/cell binding characteristics that would yield an optimal balance between tumor selectivity and tumor penetration? Pegylation increases circulation times but also decreases the endocytosis of NP, and some newer NP are designed to shed the pegylation over time. What are the range of % pegylation and the rate of “de-pegylation” to enable optimal tumor targeting? When is the increase in EPR of a larger NP offset by its reduced transport rate? Is a pH-sensitive NP, designed to promote the endosomal escape, likely to do better or worse in human tumors that are typically larger and have a more acidic environment relative to mouse tumor models used for activity evaluation? Do chemotherapy-induced changes in vasculature and vessel pore size favor extravasation of larger NP? What will happen if NP is co-administered with chemotherapy, radiation, or anti-angiogenic? How will intra-tumoral heterogeneity (e.g., vascularization) affect the delivery of NP? Is one type of NP more effective than another, in tumors with specific properties (e.g., more vs. less vascularized, small vs. large size, expression of MDR1 efflux protein)? Should one design NP in anticipation of intratumoral heterogeneity in the transport mechanisms (diffusion vs. convection) in different parts of a tumor? What are the short- and long-term effects of antiangiogenic therapy? What are the magnitudes of errors if the NP design/selection do not take into account the diverse/dynamic tumor properties?
Due to a lack of predictive models to indicate how changes in NP and tumor properties will affect the NP disposition at target sites, the development and evaluation of NP is mostly experience-based (e.g., trial-and-error). While this approach is feasible and has yielded useful NP products such as Abraxane® (albumin-coated paclitaxel nano crystals) and Doxil® (pegylated liposomal doxorubicin), we propose that predictive models that enable an investigator to forecast the fate of NP at target sites and NP-cell-protein interactions, under diverse conditions, may accelerate the design and development of NP cancer therapeutics and diagnostics.
Acknowledgments
This work is supported in part by a research grant R43CA134047 from the National Cancer Institute, DHHS. Yinghuan Li is supported by the China Scholarship Council Fellowship. The authors thank Dr. Jiabi Zhu for his critical reading of the review.
Abbreviations
- CPP
cell penetrating peptides
- ECM
extracellular matrix
- EPR
enhanced permeability and retention
- ER
endoplasmic reticulum
- GAG
glycosaminoglycan
- GALA
a 30 amino acid synthetic peptide with a glutamic acid-alanine-leucine-alanine repeat
- IFP
interstitial fluid pressure
- MVP
microvascular pressure
- NLS
nuclear localization signal
- NP
nanoparticulate carriers
- NPC
nuclear pore complexes
- PEG
polyethylene glycol
- Pgp
P-glycoprotein
- RES
reticuloendothelial system
- RNAi
RNA interference
- siRNA
small interfering RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ting G, Chang CH, Wang HE. Cancer nanotargeted radiopharmaceuticals for tumor imaging and therapy. Anticancer Res. 2009;29:4107–4118. [PubMed] [Google Scholar]
- 2.Jiang S, Gnanasammandhan MK, Zhang Y. Optical imaging-guided cancer therapy with fluorescent nanoparticles. Journal of the Royal Society Interface. 2010;7:3–18. doi: 10.1098/rsif.2009.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JH, von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, Bhatia SN, Sailor MJ. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:981–986. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon R, Gabizon AA. Clinical pharmacology of liposomal anthracyclines: Focus on pegylated liposomal doxorubicin. Clinical Lymphoma & Myeloma. 2008;8:21–32. doi: 10.3816/clm.2008.n.001. [DOI] [PubMed] [Google Scholar]
- 5.Fader AN, Rose PG. Abraxane for the Treatment of Gynecologic Cancer Patients With Severe Hypersensitivity Reactions to Paclitaxel. International Journal of Gynecological Cancer. 2009;19:1281–1283. doi: 10.1111/IGC.0b013e3181a38e2f. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Nakase I, Kawabata N, Yu HH, Pujals S, Imanishi M, Giralt E, Futaki S. Cytosolic Targeting of Macromolecules Using a pH-Dependent Fusogenic Peptide in Combination with Cationic Liposomes. Bioconjugate Chemistry. 2009;20:953–959. doi: 10.1021/bc800530v. [DOI] [PubMed] [Google Scholar]
- 7.Obata Y, Tajima S, Takeoka S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J Control Release. 2010;142:267–276. doi: 10.1016/j.jconrel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Wagstaff KM, Jans DA. Nuclear drug delivery to target tumour cells. European Journal of Pharmacology. 2009;625:174–180. doi: 10.1016/j.ejphar.2009.06.069. [DOI] [PubMed] [Google Scholar]
- 9.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Advanced Drug Delivery Reviews. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y, Harashima H. Mitochondrial drug delivery systems for macromolecule and their therapeutic application to mitochondrial diseases. Advanced Drug Delivery Reviews. 2008;60:1439–1462. doi: 10.1016/j.addr.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Tarrago-Trani MT, Storrie B. Alternate routes for drug delivery to the cell interior: Pathways to the Golgi apparatus and endoplasmic reticulum. Advanced Drug Delivery Reviews. 2007;59:782–797. doi: 10.1016/j.addr.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao M, Alving CR. Delivery of lipids and liposomal proteins to the cytoplasm and Golgi of antigen-presenting cells. Advanced Drug Delivery Reviews. 2000;41:171–188. doi: 10.1016/s0169-409x(99)00064-2. [DOI] [PubMed] [Google Scholar]
- 13.Moghimi SM, Patel HM. Serum factors that regulate phagocytosis of liposomes by Kupffer cells. Biochem Soc Trans. 1993;21:128S. doi: 10.1042/bst021128s. [DOI] [PubMed] [Google Scholar]
- 14.Moghimi SM, Davis SS. Innovations in avoiding particle clearance from blood by kupffer cells - cause for reflection. Critical Reviews in Therapeutic Drug Carrier Systems. 1994;11:31–59. [PubMed] [Google Scholar]
- 15.Au JLS, Jang SH, Wientjes MG. Clinical aspects of drug delivery to tumors. Journal of Controlled Release. 2002;78:81–95. doi: 10.1016/s0168-3659(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 16.Jang SH, Wientjes MG, Lu D, Au JLS. Drug delivery and transport to solid tumors. Pharmaceutical Research. 2003;20:1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA Therapeutics: Barriers and Carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Less JR, Skalak TC, Sevick EM, Jain RK. Microvascular architecture in a mammary carcinoma: branching patterns and vessel dimensions. Cancer Res. 1991;51:265–273. [PubMed] [Google Scholar]
- 19.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular-Permeability in A Human Tumor Xenograft - Molecular-Size Dependence and Cutoff Size. Cancer Research. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 20.Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Research. 1997;57:765–772. [PubMed] [Google Scholar]
- 21.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - An obstacle in cancer therapy. Nature Reviews Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 22.Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 1992;52:5110–5114. [PubMed] [Google Scholar]
- 23.Jain RK. Determinants of Tumor Blood-Flow - A Review. Cancer Research. 1988;48:2641–2658. [PubMed] [Google Scholar]
- 24.Jain RK. Transport of Molecules in the Tumor Interstitium - A Review. Cancer Research. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 25.Peters W, Teixeira M, Intaglietta M, Gross JF. Microcirculatory studies in rat mammary carcinoma. I. Transparent chamber method, development of microvasculature, and pressures in tumor vessels. J Natl Cancer Inst. 1980;65:631–642. [PubMed] [Google Scholar]
- 26.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 27.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discovery Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 29.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unezaki S, Maruyama K, Ishida O, Takahashi N, Iwatsuru M. Enhanced tumor targeting of doxorubicin by ganglioside GM1-bearing long-circulating liposomes. J Drug Target. 1993;1:287–292. doi: 10.3109/10611869308996086. [DOI] [PubMed] [Google Scholar]
- 31.Yang T, Cui FD, Choi MK, Cho JW, Chung SJ, Shim CK, Kim DD. Enhanced solubility and stability of PEGylated liposomal paclitaxel: In vitro and in vivo evaluation. International Journal of Pharmaceutics. 2007;338:317–326. doi: 10.1016/j.ijpharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Riche EL, Erickson BW, Cho MJ. Novel long-circulating liposomes containing peptide library-lipid conjugates: Synthesis and in vivo behavior. Journal of Drug Targeting. 2004;12:355–361. doi: 10.1080/10611860412331285279. [DOI] [PubMed] [Google Scholar]
- 33.Torchilin VP, Levchenko TS, Whiteman KR, Yaroslavov AA, Tsatsakis AM, Rizos AK, Michailova EV, Shtilman MI. Amphiphilic poly-N-vinylpyrrolidones: synthesis, properties and liposome surface modification. Biomaterials. 2001;22:3035–3044. doi: 10.1016/s0142-9612(01)00050-3. [DOI] [PubMed] [Google Scholar]
- 34.Metselaar JM, Bruin P, de Boer LWT, de Vringer T, Snel C, Oussoren C, Wauben MHM, Crommelin DJA, Storm G, Hennink WE. A novel family of L-amino acid-based biodegradable polymer-lipid conjugates for the development of long-circulating liposomes with effective drug-targeting capacity. Bioconjugate Chemistry. 2003;14:1156–1164. doi: 10.1021/bc0340363. [DOI] [PubMed] [Google Scholar]
- 35.Shehata T, Ogawara K, Higaki K, Kimura T. Prolongation of residence time of liposome by surface-modification with mixture of hydrophilic polymers. International Journal of Pharmaceutics. 2008;359:272–279. doi: 10.1016/j.ijpharm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Torchilin VP, Omelyanenko VG, Papisov MI, Bogdanov AA, Trubetskoy VS, Herron JN, Gentry CA. Poly(Ethylene Glycol) on the Liposome Surface - on the Mechanism of Polymer-Coated Liposome Longevity. Biochimica et Biophysica Acta-Biomembranes. 1994;1195:11–20. doi: 10.1016/0005-2736(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 37.Torchilin VP. Polymer-coated long-circulating microparticulate pharmaceuticals. Journal of Microencapsulation. 1998;15:1–19. doi: 10.3109/02652049809006831. [DOI] [PubMed] [Google Scholar]
- 38.Dos Santos N, Allen C, Doppen AM, Anantha M, Cox KAK, Gallagher RC, Karlsson G, Edwards K, Kenner G, Samuels L, Webb MS, Bally MB. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: Relating plasma circulation lifetimes to protein binding. Biochimica et Biophysica Acta-Biomembranes. 2007;1768:1367–1377. doi: 10.1016/j.bbamem.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Kenworthy AK, Hristova K, Needham D, McIntosh TJ. Range and magnitude of the steric pressure between bilayers containing phospholipids with covalently attached poly(ethylene glycol) Biophys J. 1995;68:1921–1936. doi: 10.1016/S0006-3495(95)80369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu NZ, Da D, Rudoll TL, Needham D, Whorton AR, Dewhirst MW. Increased Microvascular Permeability Contributes to Preferential Accumulation of Stealth Liposomes in Tumor-Tissue. Cancer Research. 1993;53:3765–3770. [PubMed] [Google Scholar]
- 41.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular Permeability and Interstitial Penetration of Sterically Stabilized (Stealth) Liposomes in A Human Tumor Xenograft. Cancer Research. 1994;54:3352–3356. [PubMed] [Google Scholar]
- 42.Hatakeyama H, Akita H, Kogure K, Oishi M, Nagasaki Y, Kihira Y, Ueno M, Kobayashi H, Kikuchi H, Harashima H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Therapy. 2007;14:68–77. doi: 10.1038/sj.gt.3302843. [DOI] [PubMed] [Google Scholar]
- 43.Adlakha-Hutcheon G, Bally MB, Shew CR, Madden TD. Controlled destabilization of a liposomal drug delivery system enhances mitoxantrone antitumor activity. Nature Biotechnology. 1999;17:775–779. doi: 10.1038/11710. [DOI] [PubMed] [Google Scholar]
- 44.Tannock IF, Rotin D. Acid Ph in Tumors and Its Potential for Therapeutic Exploitation. Cancer Research. 1989;49:4373–4384. [PubMed] [Google Scholar]
- 45.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, Mcfarlane JD. Extracellular Ph Distribution in Human Tumors. International Journal of Hyperthermia. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 46.van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdan S, Galons JP, Gillies RJ. In vivo imaging of extracellular pH using 1H MRSI. Magn Reson Med. 1999;41:743–750. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 47.Gao ZG, Lee DH, Kim DI, Bae YH. Doxorubicin loaded pH-sensitive micelle targeting acidic extracellular pH of human ovarian A2780 tumor in mice. Journal of Drug Targeting. 2005;13:391–397. doi: 10.1080/10611860500376741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Control Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalra AV, Campbell RB. Development of 5-FU and doxorubicin-loaded cationic liposomes against human pancreatic cancer: Implications for tumor vascular targeting. Pharmaceutical Research. 2006;23:2809–2817. doi: 10.1007/s11095-006-9113-3. [DOI] [PubMed] [Google Scholar]
- 50.Bode C, Trojan L, Weiss C, Kraenzlin B, Michaelis U, Teifel M, Alken P, Michel MS. Paclitaxel encapsulated in cationic liposomes: A new option for neovascular targeting for the treatment of prostate cancer. Oncology Reports. 2009;22:321–326. [PubMed] [Google Scholar]
- 51.Ko YT, Falcao C, Torchilin VP. Cationic Liposomes Loaded with Proapoptotic Peptide D-(KLAKLAK)(2) and Bcl-2 Antisense Oligodeoxynucleotide G3139 for Enhanced Anticancer Therapy. Molecular Pharmaceutics. 2009;6:971–977. doi: 10.1021/mp900006h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Lee A, Lu YH, Lee RJ. Vascular targeting of doxorubicin using cationic liposomes. International Journal of Pharmaceutics. 2007;337:329–335. doi: 10.1016/j.ijpharm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Dellian M, Yuan F, Trubetskoy VS, Torchilin VP, Jain RK. Vascular permeability in a human tumour xenograft: molecular charge dependence. Br J Cancer. 2000;82:1513–1518. doi: 10.1054/bjoc.1999.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thurston G, McLean JW, Rizen M, Baluk P, Haskell A, Murphy TJ, Hanahan D, McDonald DM. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J Clin Invest. 1998;101:1401–1413. doi: 10.1172/JCI965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, Torchilin VP, Munn LL. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Research. 2002;62:6831–6836. [PubMed] [Google Scholar]
- 56.Hynes RO. A reevaluation of integrins as regulators of angiogenesis. Nature Medicine. 2002;8:918–921. doi: 10.1038/nm0902-918. [DOI] [PubMed] [Google Scholar]
- 57.Schottelius M, Laufer B, Kessler H, Wester HJ. Ligands for mapping alphavbeta3-integrin expression in vivo. Acc Chem Res. 2009;42:969–980. doi: 10.1021/ar800243b. [DOI] [PubMed] [Google Scholar]
- 58.Aukland K, Reed RK. Interstitial-Lymphatic Mechanisms in the Control of Extracellular Fluid Volume. Physiological Reviews. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 59.Hassid Y, Eyal E, Margalit R, Furman-Haran E, Degani H. Non-invasive imaging of barriers to drug delivery in tumors. Microvascular Research. 2008;76:94–103. doi: 10.1016/j.mvr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogawa Y, Kawamura T, Furuhashi M, Tsukamoto K, Shimada S. Improving chemotherapeutic drug penetration in melanoma by imatinib mesylate. Journal of Dermatological Science. 2008;51:190–199. doi: 10.1016/j.jdermsci.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 61.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nature Medicine. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kristensen CA, Nozue M, Boucher Y, Jain RK. Reduction of interstitial fluid pressure after TNF-alpha treatment of three human melanoma xenografts. British Journal of Cancer. 1996;74:533–536. doi: 10.1038/bjc.1996.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lejeune FJ. Clinical use of TNF revisited: improving penetration of anti-cancer agents by increasing vascular permeability. J Clin Invest. 2002;110:433–435. doi: 10.1172/JCI16493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salnikov AV, Roswall P, Sundberg C, Gardner H, Heldin NE, Rubin K. Inhibition of TGF-beta modulates macrophages and vessel maturation in parallel to a lowering of interstitial fluid pressure in experimental carcinoma. Lab Invest. 2005;85:512–521. doi: 10.1038/labinvest.3700252. [DOI] [PubMed] [Google Scholar]
- 65.Brekken C, Hjelstuen MH, Bruland OS, Davies CD. Hyaluronidase-induced periodic modulation of the interstitial fluid pressure increases selective antibody uptake in human osteosarcoma xenografts. Anticancer Research. 2000;20:3513–3519. [PubMed] [Google Scholar]
- 66.Kristjansen PEG, Boucher Y, Jain RK. Dexamethasone Reduces the Interstitial Fluid Pressure in A Human Colon Adenocarcinoma Xenograft. Cancer Research. 1993;53:4764–4766. [PubMed] [Google Scholar]
- 67.Emerich DF, Dean RL, Snodgrass P, Lafreniere D, Agostino M, Wiens T, Xiong H, Hasler B, Marsh J, Pink M, Kim BS, Perdomo B, Bartus RT. Bradykinin modulation of tumor vasculature: II. activation of nitric oxide and phospholipase A2/prostaglandin signaling pathways synergistically modifies vascular physiology and morphology to enhance delivery of chemotherapeutic agents to tumors. J Pharmacol Exp Ther. 2001;296:632–641. [PubMed] [Google Scholar]
- 68.Salnikov AV, Iversen VV, Koisti M, Sundberg C, Johansson L, Stuhr LB, Sjoquist M, Ahlstrom H, Reed RK, Rubin K. Lowering of tumor interstitial fluid pressure specifically augments efficacy of chemotherapy. Faseb Journal. 2003;17:1756–+. doi: 10.1096/fj.02-1201fje. [DOI] [PubMed] [Google Scholar]
- 69.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: Clinical implications. Cancer Research. 1999;59:3776–3782. [PubMed] [Google Scholar]
- 70.Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, De Wever J, Martinive P, Gregoire V, Feron O, Gallez B. Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clinical Cancer Research. 2005;11:743–750. [PubMed] [Google Scholar]
- 71.Peters CE, Chaplin DJ, Hirst DG. Nicotinamide reduces tumour interstitial fluid pressure in a dose- and time-dependent manner. British Journal of Radiology. 1997;70:160–167. doi: 10.1259/bjr.70.830.9135442. [DOI] [PubMed] [Google Scholar]
- 72.Lee I, Boucher Y, Demhartner TJ, Jain RK. Changes in Tumor Blood-Flow, Oxygenation and Interstitial Fluid Pressure-Induced by Pentoxifylline. British Journal of Cancer. 1994;69:492–496. doi: 10.1038/bjc.1994.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuh HJ, Jang SH, Wientjes MG, Weaver JR, Au JLS. Determinants of paclitaxel penetration and accumulation in human solid tumor. Journal of Pharmacology and Experimental Therapeutics. 1999;290:871–880. [PubMed] [Google Scholar]
- 74.Lu Z, Tsai M, Lu D, Wang J, Wientjes MG, Au JLS. Tumor-Penetrating Microparticles for Intraperitoneal Therapy of Ovarian Cancer. Journal of Pharmacology and Experimental Therapeutics. 2008;327:673–682. doi: 10.1124/jpet.108.140095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jang SH, Wientjes MG, Au JLS. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: Effect of treatment schedule. Journal of Pharmacology and Experimental Therapeutics. 2001;296:1035–1042. [PubMed] [Google Scholar]
- 76.Lu D, Wientjes MG, Lu Z, Au JLS. Tumor priming enhances delivery and efficacy of nanomedicines. Journal of Pharmacology and Experimental Therapeutics. 2007;322:80–88. doi: 10.1124/jpet.107.121632. [DOI] [PubMed] [Google Scholar]
- 77.Nagano S, Perentes JY, Jain RK, Boucher Y. Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors. Cancer Res. 2008;68:3795–3802. doi: 10.1158/0008-5472.CAN-07-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chauhan VP, Lanning RM, Diop-Frimpong B, Mok W, Brown EB, Padera TP, Boucher Y, Jain RK. Multiscale Measurements Distinguish Cellular and Interstitial Hindrances to Diffusion In Vivo. Biophysical Journal. 2009;97:330–336. doi: 10.1016/j.bpj.2009.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koike C, Mckee TD, Pluen A, Ramanujan S, Burton K, Munn LL, Boucher Y, Jain RK. Solid stress facilitates spheroid formation: potential involvement of hyaluronan. British Journal of Cancer. 2002;86:947–953. doi: 10.1038/sj.bjc.6600158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eikenes L, Bruland OS, Brekken C, Davies CDL. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Research. 2004;64:4768–4773. doi: 10.1158/0008-5472.CAN-03-1472. [DOI] [PubMed] [Google Scholar]
- 81.Eikenes L, Tari M, Tufto I, Bruland OS, Davies CD. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx (TM)) in human osteosarcoma xenografts. British Journal of Cancer. 2005;93:81–88. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eikenes L, Tufto I, Schnell EA, Bjorkoy A, Davies CD. Effect of Collagenase and Hyaluronidase on Free and Anomalous Diffusion in Multicellular Spheroids and Xenografts. Anticancer Research. 2010;30:359–368. [PubMed] [Google Scholar]
- 83.Erikson A, Tufto I, Bjonnum AB, Bruland OS, Davies CD. The Impact of Enzymatic Degradation on the Uptake of Differently Sized Therapeutic Molecules. Anticancer Research. 2008;28:3557–3566. [PubMed] [Google Scholar]
- 84.Juweid M, Neumann R, Paik C, Perezbacete MJ, Sato J, Vanosdol W, Weinstein JN. Micropharmacology of Monoclonal-Antibodies in Solid Tumors - Direct Experimental-Evidence for A Binding-Site Barrier. Cancer Research. 1992;52:5144–5153. [PubMed] [Google Scholar]
- 85.Gupta AK, Berry C, Gupta M, Curtis A. Receptor-mediated targeting of magnetic nanoparticles using insulin as a surface ligand to prevent endocytosis. Ieee Transactions on Nanobioscience. 2003;2:255–261. doi: 10.1109/tnb.2003.820279. [DOI] [PubMed] [Google Scholar]
- 86.Dijkstra J, van Galen M, Scherphof G. Influence of liposome charge on the association of liposomes with Kupffer cells in vitro. Effects of divalent cations and competition with latex particles. Biochim Biophys Acta. 1985;813:287–297. doi: 10.1016/0005-2736(85)90244-5. [DOI] [PubMed] [Google Scholar]
- 87.Shi WD, Wang JZ, Fan XJ, Gao HJ. Size and shape effects on diffusion and absorption of colloidal particles near a partially absorbing sphere: Implications for uptake of nanoparticles in animal cells. Physical Review e. 2008;78 doi: 10.1103/PhysRevE.78.061914. [DOI] [PubMed] [Google Scholar]
- 88.Bechinger C, Rudhardt D, Leiderer P, Roth R, Dietrich S. Understanding depletion forces beyond entropy. Physical Review Letters. 1999;83:3960–3963. [Google Scholar]
- 89.Israelachvili JN, Wennerstrom H. Entropic Forces Between Amphiphilic Surfaces in Liquids. Journal of Physical Chemistry. 1992;96:520–531. [Google Scholar]
- 90.Chandler D. Interfaces and the driving force of hydrophobic assembly. Nature. 2005;437:640–647. doi: 10.1038/nature04162. [DOI] [PubMed] [Google Scholar]
- 91.Miller CR, Bondurant B, Mclean SD, McGovern KA, O’Brien DF. Liposome-cell interactions in vitro: Effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry. 1998;37:12875–12883. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- 92.Du H, Chandaroy P, Hui SW. Grafted poly-(ethylene glycol) on lipid surfaces inhibits protein adsorption and cell adhesion. Biochim Biophys Acta. 1997;1326:236–248. doi: 10.1016/s0005-2736(97)00027-8. [DOI] [PubMed] [Google Scholar]
- 93.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 94.Matsudaira H, Asakura T, Aoki K, Searashi Y, Matsuura T, Nakajima H, Tajiri H, Ohkawa K. Target chemotherapy of anti-CD147 antibody-labeled liposome encapsulated GSH-DXR conjugate on CD147 highly expressed carcinoma cells. Int J Oncol. 2010;36:77–83. [PubMed] [Google Scholar]
- 95.Yang T, Choi MK, Cui FD, Kim JS, Chung SJ, Shim CK, Kim DD. Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. Journal of Controlled Release. 2007;120:169–177. doi: 10.1016/j.jconrel.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 96.Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. Journal of Controlled Release. 2004;100:135–144. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 97.Mamot C, Drummond DC, Greiser U, Hong K, Kirpotin DB, Marks JD, Park JW. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to. Cancer Res. 2003;63:3154–3161. [PubMed] [Google Scholar]
- 98.Xiang GY, Wu J, Lu YH, Liu ZL, Lee RJ. Synthesis and evaluation of a novel ligand for folate-mediated targeting liposomes. International Journal of Pharmaceutics. 2008;356:29–36. doi: 10.1016/j.ijpharm.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hatakeyama H, Akita H, Maruyama K, Suhara T, Harashima H. Factors governing the in vivo tissue uptake of transferrin-coupled polyethylene glycol liposomes in vivo. International Journal of Pharmaceutics. 2004;281:25–33. doi: 10.1016/j.ijpharm.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 100.Eavarone DA, Yu XJ, Bellamkonda RV. Targeted drug delivery to C6 glioma by transferrin-coupled liposomes. Journal of Biomedical Materials Research. 2000;51:10–14. doi: 10.1002/(sici)1097-4636(200007)51:1<10::aid-jbm2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 101.Zhou Y, Daryl C, Zou H, Hayes ME, Adams GP, Kirpotin DB, Marks JD. Impact of single-chain fv antibody fragment affinity on nanoparticle targeting of epidermal growth factor receptor-expressing tumor cells. Journal of Molecular Biology. 2007;371:934–947. doi: 10.1016/j.jmb.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park JW, Benz CC, Martin FJ. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol. 2004;31:196–205. doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 103.Kitamura N, Shigematsu N, Nakahara T, Kanoh M, Hashimoto J, Kunieda E, Kubo A. Biodistribution of immunoliposome labeled with Tc-99m in tumor xenografted mice. Annals of Nuclear Medicine. 2009;23:149–153. doi: 10.1007/s12149-008-0222-4. [DOI] [PubMed] [Google Scholar]
- 104.Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, Gabizon A. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clinical Cancer Research. 2000;6:1949–1957. [PubMed] [Google Scholar]
- 105.Kobayashi T, Ishida T, Okada Y, Ise S, Harashima H, Kiwada H. Effect of transferrin receptor-targeted liposomal doxorubicin in P-glycoprotein-mediated drug resistant tumor cells. International Journal of Pharmaceutics. 2007;329:94–102. doi: 10.1016/j.ijpharm.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 106.Hansen CB, Kao GY, Moase EH, Zalipsky S, Allen TM. Attachment of Antibodies to Sterically Stabilized Liposomes - Evaluation, Comparison and Optimization of Coupling Procedures. Biochimica et Biophysica Acta-Biomembranes. 1995;1239:133–144. doi: 10.1016/0005-2736(95)00138-s. [DOI] [PubMed] [Google Scholar]
- 107.Zalipsky S, Hansen CB, deMenezes DEL, Allen TM. Long-circulating, polyethylene glycol-grafted immunoliposomes. Journal of Controlled Release. 1996;39:153–161. [Google Scholar]
- 108.Doherty GJ, McMahon HT. Mechanisms of Endocytosis. Annual Review of Biochemistry. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 109.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- 110.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 111.Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. Journal of Cell Science. 2010;123:1183–1189. doi: 10.1242/jcs.066399. [DOI] [PubMed] [Google Scholar]
- 112.Aubry L, Klein G, Martiel JL, Satre M. Kinetics of Endosomal Ph Evolution in Dictyostelium-Discoideum Amebas - Study by Fluorescence Spectroscopy. Journal of Cell Science. 1993;105:861–866. doi: 10.1242/jcs.105.3.861. [DOI] [PubMed] [Google Scholar]
- 113.Mercer J, Helenius A. Virus entry by macropinocytosis. Nature Cell Biology. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 114.Futaki S. Arginine-rich peptides: potential for intracellular delivery of macromolecules and the mystery of the translocation mechanisms. International Journal of Pharmaceutics. 2002;245:1–7. doi: 10.1016/s0378-5173(02)00337-x. [DOI] [PubMed] [Google Scholar]
- 115.Gupta B, Torchilin VP. Transactivating transcriptional activator-mediated drug delivery. Expert Opin Drug Deliv. 2006;3:177–190. doi: 10.1517/17425247.3.2.177. [DOI] [PubMed] [Google Scholar]
- 116.Yuan JP, Kramer A, Eckerdt F, Kaufmann M, Strebhardt K. Efficient internalization of the polo-box of polo-like kinase 1 fused to an antennapedia peptide results in inhibition of cancer cell proliferation. Cancer Research. 2002;62:4186–4190. [PubMed] [Google Scholar]
- 117.Padari K, Saalik P, Hansen M, Koppel K, Raid R, Langel I, Pooga M. Cell transduction pathways of transportans. Bioconjugate Chemistry. 2005;16:1399–1410. doi: 10.1021/bc050125z. [DOI] [PubMed] [Google Scholar]
- 118.Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. Journal of Biological Chemistry. 2003;278:35109–35114. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- 119.Vives E, Richard JP, Rispal C, Lebleu B. TAT peptide internalization: Seeking the mechanism of entry. Current Protein & Peptide Science. 2003;4:125–132. doi: 10.2174/1389203033487306. [DOI] [PubMed] [Google Scholar]
- 120.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nature Medicine. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 121.Nakase I, Hirose H, Tanaka G, Tadokoro A, Kobayashi S, Takeuchi T, Futaki S. Cell-surface Accumulation of Flock House Virus-derived Peptide Leads to Efficient Internalization via Macropinocytosis. Molecular Therapy. 2009;17:1868–1876. doi: 10.1038/mt.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vendeville A, Rayne F, Bonhoure A, Bettache N, Montcourrier P, Beaumelle B. HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Molecular Biology of the Cell. 2004;15:2347–2360. doi: 10.1091/mbc.E03-12-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fittipaldi A, Ferrari A, Zoppe M, Arcangeli C, Pellegrini V, Beltram F, Giacca M. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. Journal of Biological Chemistry. 2003;278:34141–34149. doi: 10.1074/jbc.M303045200. [DOI] [PubMed] [Google Scholar]
- 124.Raagel H, Saalik P, Hansen M, Langel U, Pooga M. CPP-protein constructs induce a population of non-acidic vesicles during trafficking through endo-lysosomal pathway. Journal of Controlled Release. 2009;139:108–117. doi: 10.1016/j.jconrel.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 125.Koppelhus U, Awasthi SK, Zachar V, Holst HU, Ebbesen P, Nielsen PE. Cell-dependent differential cellular uptake of PNA, peptides, and PNA-peptide conjugates. Antisense & Nucleic Acid Drug Development. 2002;12:51–63. doi: 10.1089/108729002760070795. [DOI] [PubMed] [Google Scholar]
- 126.Khalil IA, Kogure K, Futaki S, Harashima H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. Journal of Biological Chemistry. 2006;281:3544–3551. doi: 10.1074/jbc.M503202200. [DOI] [PubMed] [Google Scholar]
- 127.Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, Jones AT, Sugiura Y, Futaki S. Cellular uptake of arginine-rich peptides: Roles for macropinocytosis and actin rearrangement. Molecular Therapy. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 128.Torchilin VP. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv Drug Deliv Rev. 2008;60:548–558. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 129.Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D’Souza GG. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc Natl Acad Sci U S A. 2003;100:1972–1977. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tseng YL, Liu JJ, Hong RL. Translocation of liposomes into cancer cells by cell-penetrating peptides penetratin and TAT: A kinetic and efficacy study. Molecular Pharmacology. 2002;62:864–872. doi: 10.1124/mol.62.4.864. [DOI] [PubMed] [Google Scholar]
- 131.Pagano RE, Weinstein JN. Interactions of Liposomes with Mammalian-Cells. Annual Review of Biophysics and Bioengineering. 1978;7:435–468. doi: 10.1146/annurev.bb.07.060178.002251. [DOI] [PubMed] [Google Scholar]
- 132.Mustata RC, Grigorescu A, Petrescu SM. Encapsulated cargo internalized by fusogenic liposomes partially overlaps the endoplasmic reticulum. Journal of Cellular and Molecular Medicine. 2009;13:3110–3121. doi: 10.1111/j.1582-4934.2009.00724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li WJ, Nicol F, Szoka FC. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Advanced Drug Delivery Reviews. 2004;56:967–985. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 134.Nicol F, Nir S, Szoka FC. Effect of phospholipid composition on an amphipathic peptide-mediated pore formation in bilayer vesicles. Biophysical Journal. 2000;78:818–829. doi: 10.1016/S0006-3495(00)76639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nir S, Nieva JL. Interactions of peptides with liposomes: pore formation and fusion. Progress in Lipid Research. 2000;39:181–206. doi: 10.1016/s0163-7827(00)00004-7. [DOI] [PubMed] [Google Scholar]
- 136.Kakudo T, Chaki S, Futaki S, Nakase I, Akaji K, Kawakami T, Maruyama K, Kamiya H, Harashima H. Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: An artificial viral-like delivery system. Biochemistry. 2004;43:5618–5628. doi: 10.1021/bi035802w. [DOI] [PubMed] [Google Scholar]
- 137.Sasaki K, Kogure K, Chaki S, Nakamura Y, Moriguchi R, Hamada H, Danev R, Nagayama K, Futaki S, Harashima H. An artificial virus-like nano carrier system: enhanced endosomal escape of nanoparticles via synergistic action of pH-sensitive fusogenic peptide derivatives. Analytical and Bioanalytical Chemistry. 2008;391:2717–2727. doi: 10.1007/s00216-008-2012-1. [DOI] [PubMed] [Google Scholar]
- 138.Kaneda Y. Virosomes: evolution of the liposome as a targeted drug delivery system. Advanced Drug Delivery Reviews. 2000;43:197–205. doi: 10.1016/s0169-409x(00)00069-7. [DOI] [PubMed] [Google Scholar]
- 139.Sarkar DP, Ramani K, Tyagi SK. Targeted gene delivery by virosomes. Methods Mol Biol. 2002;199:163–173. doi: 10.1385/1-59259-175-2:163. [DOI] [PubMed] [Google Scholar]
- 140.Moser C, Metcalfe IC, Viret JF. Virosomal adjuvanted antigen delivery systems. Expert Rev Vaccines. 2003;2:189–196. doi: 10.1586/14760584.2.2.189. [DOI] [PubMed] [Google Scholar]
- 141.Mastrobattista E, Koning GA, van Bloois L, Filipe ACS, Jiskoot W, Storm G. Functional characterization of an endosome-disruptive peptide and its application in cytosolic delivery of immunoliposome-entrapped proteins. Journal of Biological Chemistry. 2002;277:27135–27143. doi: 10.1074/jbc.M200429200. [DOI] [PubMed] [Google Scholar]
- 142.Jiang HL, Kim TH, Kim YK, Park IY, Cho MH, Cho CS. Efficient gene delivery using chitosan-polyethylenimine hybrid systems. Biomedical Materials. 2008;3 doi: 10.1088/1748-6041/3/2/025013. [DOI] [PubMed] [Google Scholar]
- 143.Simoes S, Moreira JN, Fonseca C, Duzgunes N, de Lima MCP. On the formulation of pH-sensitive long circulation times. Advanced Drug Delivery Reviews. 2004;56:947–965. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 144.Foerg C, Ziegler U, Fernandez-Carneado J, Giralt E, Rennert R, Beck-Sickinger AG, Merkle HP. Decoding the entry of two novel cell-penetrating peptides in HeLa cells: Lipid raft-mediated endocytosis and endosomal escape. Biochemistry. 2005;44:72–81. doi: 10.1021/bi048330+. [DOI] [PubMed] [Google Scholar]
- 145.Song LY, Ahkong QF, Rong Q, Wang Z, Ansell S, Hope MJ, Mui B. Characterization of the inhibitory effect of PEG-lipid conjugates on the intracellular delivery of plasmid and antisense DNA mediated by cationic lipid liposomes. Biochimica et Biophysica Acta-Biomembranes. 2002;1558:1–13. doi: 10.1016/s0005-2736(01)00399-6. [DOI] [PubMed] [Google Scholar]
- 146.Boomer JA, Qualls MM, Inerowicz HD, Haynes RH, Patri VS, Kim JM, Thompson DH. Cytoplasmic Delivery of Liposomal Contents Mediated by an Acid-Labile Cholesterol-Vinyl Ether-PEG Conjugate. Bioconjugate Chemistry. 2009;20:47–59. doi: 10.1021/bc800239b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu H, Deng YH, Chen DW, Hong WW, Lu Y, Dong XH. Esterase-catalyzed dePEGylation of pH-sensitive vesicles modified with cleavable PEG-lipid derivatives. Journal of Controlled Release. 2008;130:238–245. doi: 10.1016/j.jconrel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 148.Maeda T, Fujimoto K. A reduction-triggered delivery by a liposomal carrier possessing membrane-permeable ligands and a detachable coating. Colloids and Surfaces B-Biointerfaces. 2006;49:15–21. doi: 10.1016/j.colsurfb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 149.Hatakeyama H, Ito E, Akita H, Oishi M, Nagasaki Y, Futaki S, Harashima H. A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. Journal of Controlled Release. 2009;139:127–132. doi: 10.1016/j.jconrel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 150.Sakurai Y, Hatakeyama H, Akita H, Oishi M, Nagasaki Y, Futaki S, Harashima H. Efficient Short Interference RNA Delivery to Tumor Cells Using a Combination of Octaarginine, GALA and Tumor-Specific, Cleavable Polyethylene Glycol System. Biological & Pharmaceutical Bulletin. 2009;32:928–932. doi: 10.1248/bpb.32.928. [DOI] [PubMed] [Google Scholar]
- 151.Kau TR, Way JC, Silver PA. Nuclear transport and cancer: From mechanism to intervention. Nature Reviews Cancer. 2004;4:106–117. doi: 10.1038/nrc1274. [DOI] [PubMed] [Google Scholar]
- 152.Wente SR. Gatekeepers of the nucleus. Science. 2000;288:1374–1377. doi: 10.1126/science.288.5470.1374. [DOI] [PubMed] [Google Scholar]
- 153.Campbell EA, Hope TJ. Role of the cytoskeleton in nuclear import. Advanced Drug Delivery Reviews. 2003;55:761–771. doi: 10.1016/s0169-409x(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 154.Alvisi G, Poon IK, Jans DA. Tumor-specific nuclear targeting: promises for anti-cancer therapy? Drug Resist Updat. 2006;9:40–50. doi: 10.1016/j.drup.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 155.Harel A, Forbes DJ. Importin beta: Conducting a much larger cellular symphony. Molecular Cell. 2004;16:319–330. doi: 10.1016/j.molcel.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 156.Kurihara D, Akita H, Kudo A, Masuda T, Futaki S, Harashima H. Effect of Polyethyleneglycol Spacer on the Binding Properties of Nuclear Localization Signal-Modified Liposomes to Isolated Nucleus. Biological & Pharmaceutical Bulletin. 2009;32:1303–1306. doi: 10.1248/bpb.32.1303. [DOI] [PubMed] [Google Scholar]
- 157.Nakamura T, Moriguchi R, Kogure K, Minoura A, Masuda T, Akita H, Kato K, Hamada H, Futaki S, Harashima H. Delivery of condensed DNA by liposomal non-viral gene delivery system into nucleus of dendritic cells. Biological & Pharmaceutical Bulletin. 2006;29:1290–1293. doi: 10.1248/bpb.29.1290. [DOI] [PubMed] [Google Scholar]
- 158.Elouahabi A, Ruysschaert JM. Formation and intracellular trafficking of lipoplexes and polyplexes. Molecular Therapy. 2005;11:336–347. doi: 10.1016/j.ymthe.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 159.Martinou JC, Desagher S, Antonsson B. Cytochrome c release from mitochondria: all or nothing. Nature Cell Biology. 2000;2:E41–E43. doi: 10.1038/35004069. [DOI] [PubMed] [Google Scholar]
- 160.Yamada Y, Akita H, Kamiya H, Kogure K, Yamamoto T, Shinohara Y, Yamashita K, Kobayashi H, Kikuchi H, Harashima H. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochimica et Biophysica Acta-Biomembranes. 2008;1778:423–432. doi: 10.1016/j.bbamem.2007.11.002. [DOI] [PubMed] [Google Scholar]


