Abstract
Although the overall prognosis in childhood acute lymphoblastic leukaemia (ALL) is good, outcome after relapse is poor. Recurrence is frequently characterised by the occurrence of disease at extramedullary sites such as the central nervous system and testes. Subpopulations of blasts able to migrate to such areas may have a survival advantage and give rise to disease recurrence. Gene expression profiling of 85 diagnostic pre-B-ALL bone marrow samples revealed higher 5T4 oncofoetal antigen transcript levels in cytogenetic high-risk subgroups of patients (p < 0.001). Flow cytometric analysis determined that bone marrow from relapse patients have a significantly higher percentage of 5T4 positive leukemic blasts than healthy donors (p = 0.005). The high-risk Sup-B15 pre-B-ALL line showed heterogeneity in 5T4 expression, and the derived, 5T4+ (Sup5T4) and 5T4− (Sup) subline cells, displayed differential spread to the omentum and ovaries following intraperitoneal inoculation of immunocompromised mice. Consistent with this, Sup5T4 compared to Sup cells show increased invasion in vitro concordant with increased LFA-1 and VLA-4 integrin expression, adhesion to extracellular matrix and secretion of matrix metalloproteases (MMP-2/-9). We also show that 5T4 positive Sup-B15 cells are susceptible to 5T4 specific superantigen antibody-dependent cellular toxicity providing support for targeted immunotherapy in high risk pre-B-ALL.
Keywords: Pre-B cell acute lymphoblastic leukemia, childhood cancer, 5T4 oncofoetal antigen, immunotherapy, CXCR4, CXCL12, chemotaxis, invasion
Introduction
Although modern chemotherapeutic regimens cure over 80% of children with Pre-B-ALL, the outcome for those who relapse remains poor (1). Cytogenetic subtypes are associated with a higher or lower risk of relapse have been identified but these are not predictive for individual patient outcome (2). For example, hyperdiploidy and ETV6-RUNX1 are the most common cytogenetic abnormalities in B-ALL accounting for 50% of cases and are associated with a favourable prognosis but 20% will relapse. In contrast to de novo disease, relapse is associated with an increased recurrence of disease at extramedullary sites such as the central nervous system (CNS) and testes (2-5). We have recently shown that a subpopulation of pre-B lymphoblasts with organised cytoskeletal structures and invasive characteristics give rise to CNS disease in a murine model of childhood ALL (6). Pre-B lymphoblasts, like most haematopoietic cells, express the chemokine receptor CXCR4 and are chemotactic toward its ligand CXCL12. CXCL12 is expressed by bone marrow stroma but also by stromal cells of the central nervous system and gonads among other tissues (7) and thus CXCR4 expressing tumour cells may be expected to migrate to such areas. In fact, CXCR4 expression in lymphoblasts has been reported as predictive of extramedullary organ infiltration in childhood ALL (8). We have recently demonstrated a role for the 5T4 glycoprotein in the regulation of CXCL12/CXCR4 chemokine mediated chemotaxis (9). 5T4 upregulation is also a marker of loss of embryonic stem cell pluripotency and in this context may function to facilitate chemotaxis, together with concomitant epithelial mesenchymal transition, a process also characteristic of epithelial cancer (10).
The expression of 5T4 on normal tissue is restricted, but it is upregulated in many carcinomas and overexpression in tumours has been associated with a poorer clinical outcome (11). Recently, it has been shown that 5T4 is expressed on tumour initiating cells and is associated with the worse clinical outcome in non-small cell lung cancer (12). While mature hematopoietic cells lack the expression of 5T4, open access microarray data suggest that 5T4 is present during the early stages of normal B cell development in particular at the pro- and pre-B cell phase (13) (Supplementary Fig. S1). Here we have investigated 5T4 expression in pre-B-ALL disease and shown an association with high risk of relapse which is correlated to invasive and chemotactic behaviour.
Materials and Methods (with further details in Supplementary Information#)
Primary human leukemia samples
Excess bone marrow material was obtained at diagnosis from children with ALL or from healthy sibling bone marrow donors after consent. The studies were approved by Tameside and Glossop Research Ethics Committees (Manchester, UK; Reference 07/Q1402/56).
Gene expression analysis#
Gene expression on diagnostic cells from 85 pre-B-ALL patients was analyzed as described previously (14-16). qPCR standard methods were used.
Leukaemia cell lines
SD1 and REH cell lines were obtained from Cancer Research UK Central Cell Service and Sup-B15, TOM-1 and NALM-6 lines from DSMZ (Braunschweig, Germany). Cell line authentication was performed routinely as previously described (17). Cell lines were cultured at 37°C, 5% CO2 in RPMI-1640 GlutaMAX (Invitrogen, Paisley, UK) with 10% or 20% fetal bovine serum (FBS) (Sera Laboratories International, Sussex, UK). MTT Vybrant analysis (Invitrogen) was used to assess growth and metabolic rate.
Flow cytometry#
Multi-parameter analysis of cell suspensions from patient / healthy bone marrow/ leukemic cell lines was performed using a LSRII flow cytometer (Beckton Dickinson, UK). Dead cells were excluded using 7-aminoactinomycin (Cambridge Bioscience); and labelled with Alexa-700 conjugated anti-human CD19 and Qdot 605 conjugated anti-human CD10 (Invitrogen) to identify human B-lineage ALL cells. The percentage values plotted for the flow cytometric analysis on CD19+ CD10+ B cells from patients’ samples were calculated by subtracting the background from the values obtained when test samples were labelled with for 5T4 expression. A 9-colour multiplex FACS analysis was performed on these samples as we investigated the expression of a larger set of surface markers. Thus, we were unable to use isotype control antibodies as negative controls and the background was determined on unlabelled cells. Cells were labelled with mouse anti-human 5T4 mAb (mAb-h5T4) (in house) followed by PE conjugated rabbit polyclonal anti-mouse IgG F(ab)2 antibody (DAKO). 5T4 positive and negative Sup-B15 sub-lines were established following fluorescence activated cell sorting (FACS) for 5T4 surface expression and the phenotypes regularly assessed and maintained as > 95 % purity. Cells were also analysed by flow cytometry with antibodies against CD34 (ab18228, Abcam), CD20 (Dako) CXCR4 (clone 12G5, Abcam), alpha L (559875, BD Pharmingen) and alpha 4 integrin (ab25247, Abcam).
CXCR4 modulation was induced in Sup-B15 subline cells by incubation with either 30 or 100ng/ml CXCL12 chemokine for up to 2 hours. The cells were then washed with ice cold acidic buffer (50 mM glycine/HCl, pH3, 100 mM NaCl) for 2 min, followed by two washes with ice cold FACS buffer (0.1% NaN3, 0.2% BSA). CXCR4 expression was assessed by flow cytometry using two layer immunofluorescence with anti CXCR4 antibody (12G5, Abcam) or an isotype matched control at 4°C for 1 hour followed by rabbit anti-mouse immunoglobulins/RPE F(ab’)2 (Dako) at 4°C for 30 min.
SDS-PAGE, western blotting and immunoprecipitation#
Migration/ Adhesion assays#
An automated assay using light-impermeable Boyden chambers (FluoroBlock) and a FLUOstar Omega micro plate-reader (BMG Labtech) was able to continually measure cellular migration of DiIC (Cambridge Bioscience; 1:2000) labelled cells in a gradient (1-10% serum or 0-100ng/ml CXCL12) or invasion through matrigel (BD BioScience). To measure adhesion, cells were labelled with 5mM 5-chloromethylfluorescein diacetate (CMFDA; Cell Tracker Green Dye Molecular ProbesT, Invitrogen) and binding to various adhesion proteins (ICAM-1 & VCAM-1, R&D; Fibronectin, laminin and collagen, BD) coating NUNC Maxisorp flat-bottomed clear plates was determined by FLUOstar Omega.
Gelatin Zymography#
Sup-B15 subline cells were incubated in serum-free RPMI (2×106 cells/ml) at 37°C for 40 hours and conditioned media (CM) collected for gelatin zymography.
Generation of mCherry/Luciferase positive leukemic cell lines
Sup-B15 cells were transduced with retroviral vector rKat coexpressing the firefly luciferase (Luc2) and mCherry (6) by centrifugation in viral supernatant at 1200xg for 3-4 hours in the presence of 4μg/ml polybrene. After 14 days of culture, mCherry positive cells were sorted using an Influx FACS sorter.
In vivo bioluminescence imaging of engrafted leukemic cells#
Six to eight week old severe combined immunodeficient NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mice (Harlan, UK) (6-9 female mice/group) received intraperitoneal (i.p.) injections of mCherry-Luciferase (Luc) positive leukemia cells 1-5 x106 cells. Engraftment and growth of Sup5T4 and Sup mCherry-Luc cells were assessed at varying time points by bioluminescent imaging monitored using the IVIS system 100 (Caliper Life Sciences Ltd, Runcorn, UK) (6). All animals were sacrificed by 4-6 weeks and selected tissues were further analysed by human CD45 immunohistochemistry to identify the leukemic cells as previously described (6). In addition Sup5T4 or Sup cells (5×106) were injected intravenously (i.v.) into female mice and their bone marrow (n = 4) and ovaries (n = 4) harvested at day 30. The surface expression of 5T4 on the population of infiltrating mCherry-Luc cells positive leukemic cells was examined by flow cytometry. Leukemic dissemination in female mice after intraperitoneal injection in animals given 50μg of mAb 5T4 at day one and then either 2 or 3 times /week for three weeks (n = 6 /group) was analysed.
Superantigen antibody dependent cellular cytoxicity (SADCC)#
SADCC targeting 5T4 in vitro was performed as previously (18) with naptumomab estafenatox /ABR-217620 provided by Active Biotech, Lund, Sweden (19).
Statistical analysis
Data were analysed by unpaired Student t-test or One-Way Anova (GraphPad Prism, CA or Microsoft Excel softwares).
Results
Leukemic cells from patients in the high-risk cytogenetic subset of childhood pre-B-ALL and relapse disease express high levels of 5T4
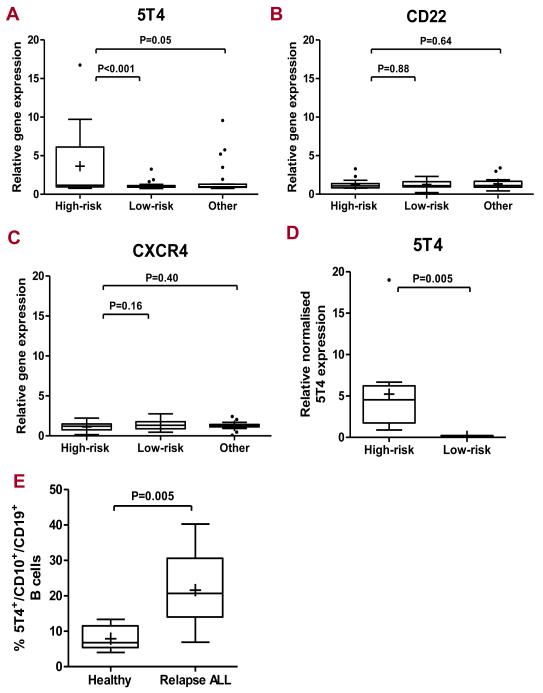
To investigate the expression of the oncofetal antigen 5T4 by pre-B cell blasts from leukemic patients, we examined the data from our previously published microarray data from 85 children with pre-B-ALL stratified by cytogenetics for risk of relapse High risk cytogenetics included mixed-lineage leukaemia (MLL) (n = 4), BCR-ABL (n = 4), iAMP21 (n = 9) and hypodiploid abnormalities (n = 3) and the low risk subset, TCF3-PBX1 (n = 2), hyperdiploidy (n = 24) and ETV6-RUNX1 (n = 17). Seventeen percent (15/85) of the cohort of pre-B-ALL patients had high 5T4 transcript levels (> 2-fold). The high risk cytogenetic category patients showed significantly higher 5T4 transcript levels than the low risk (p < 0.0001) or ‘other’ groups (p=0.05) (Fig 1A). By contrast, there were no significant differences in gene expression between the patient subsets for CD22, a surface glycoprotein associated with > 90% of childhood B-ALL and targeted by antibodies in clinical trials (20), (Fig 1B) or CXCR4 where expression has been associated with migration of B cells in ALL (Fig 1C). Quantitative PCR analysis for 5T4 confirmed higher 5T4 gene expression in lymphoblasts obtained from the high risk cytogenetic patients as compared to patients with a low risk cytogenetic genotype as controls (ETV6-RUNX-1 genotype) (p <0.005) (Fig 1D). Flow cytometric analysis of bone marrow aspirates from pre-B-ALL patients with relapse disease showed significantly higher percentage of 5T4 positive CD10+CD19+ B blasts compared with healthy donors and immunofluorescence staining of cytospins identified strongly 5T4 positive blasts (Fig 1E, S2).
Figure 1. Leukemic cells from patients in high-risk cytogenetic subset of childhood pre-B-ALL and refractory disease express high levels of 5T4 mRNA and protein.
Box-plot representation of relative 5T4 (A), CD22 (B) and CXCR4 (C) microarray expression in leukemic blasts in high risk (n = 20), low risk (n = 43) and other (n = 22) patient groups. The y-axis represents the relative gene expression levels of either 5T4, CD22 or CXCR4. Box plots represent the interquartile range of values, the whiskers represent the smallest and largest values for each category, and the horizontal lines and cross symbols denote the median and mean respectively; outlier values are represented by asterisks; One-way ANOVA statistical analysis. (D) Box-plot representation of 5T4 quantification in high and low risk subsets in TaqMan Q-PCR assay; representation as in (C); Student-t test analysis. (E) Flow cytometry on CD19+ CD10+ B cell blasts from patients with multiple relapse disease (n = 8: 2 normal karyotype, 1 haploid, 1 hyperdiploid,1 hypodiploid, 1 ETV6-RUNX1 and 2 unknown) in comparison to healthy donors (n = 6); the y-axis represents the percentage of 5T4 positive CD19 CD10 B cells (% of 5T4 labelled samples – background). The Pre-B-ALL patients with relapse disease showed significantly higher proportions of 5T4 positive CD10+ CD19+ B blasts compared with healthy donors (means = 21.6 vs 7.9 respectively; p < 0.01, student-t test analysis.
5T4 expression by pre-B-ALL cell lines
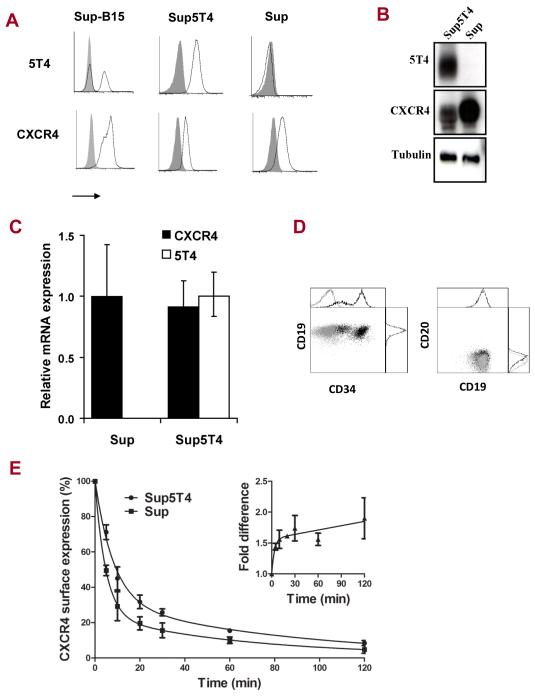
Five pre-B-ALL cell lines derived from high risk (SD1, Sup-B15, TOM-1) or low risk (REH and NALM-6) patients were assessed for surface expression of 5T4 and CXCR4. All lines except SD1 cells expressed cell surface CXCR4 while only Sup-B15 (strong positive variable subpopulation) and TOM-1 cells (weakly positive) showed typical punctate 5T4 membrane expression (Fig S3A, B). The proportion of 5T4 positive Sup-B15 cells and the expression levels on TOM-1 cells varied depending on culturing conditions and passage number but the mechanisms underwriting this dynamic process remain unknown. From the parental Sup-B15 line, we routinely established 5T4 positive (Sup5T4) and negative (Sup) sublines by FACS sorting (Fig 2A); Sup5T4 cells continued to generate negative cells whereas the Sup phenotype was stable. The sublines were authenticated and matched the parental Sup-B15 cell genotype (Table S1). The 5T4 status of these cells was routinely assessed by flow cytometry to ensure > 95% homogeneity. Western blotting confirmed that the Sup cells lack intracellular and surface 5T4 (Fig 2B), while qPCR analyses determined that 5T4 expression was regulated at the mRNA level (Fig 2C). Both sublines were positive for CXCR4 transcripts and protein, with Sup 5T4 negative cells expressing slightly higher CXCR4 levels than the Sup5T4 cells. Interestingly, Sup5T4 cells seem to have a more immature phenotype, expressing medium to high levels of the stem cell marker CD34, in contrast to Sup cells which are CD34 negative. Both sublines express high levels of CD19 and are negative for the mature B cell marker CD20 (Fig 2D). There is evidence that some 5T4 and CXCR4 molecules colocalize at the cell surface in Sup5T4 cells (Fig S3C). Evidence for direct interaction of CXCR4 and 5T4 was investigated by western blot analysis with anti-CXCR4 of SDS-PAGE of mAb-5T4 immunoprecipitates from Sup5T4 cells but it was not possible to identify 46kD CXCR4 molecules (not shown). However, the rate and extent of CXCR4 modulation by CXCL12 was different in the subline cells, consistent with a differential stabilisation of CXCR4 on 5T4 positive cells (Fig 2E). The insert of Fig 2E shows Sup5T4 cells retain 1.6 fold higher surface expression of CXCR4 compared to the Sup cells even after 2 hours of chemokine exposure.
Figure 2. Characterization of Sup-B15 5T4+ (Sup5T4) and 5T4− (Sup) sublines.
Analysis of CXCR4 and 5T4 expression by (A) Flow cytometry, (B) Western blot (C) qPCR. (D) Flow cytometric phenotyping for CD19, CD20 and CD34 (Sup=light grey; Sup5T4=black). (E) Average results from three independent experiments showing differential CXCL12 modulation (30ng) of cell surface CXCR4 expression (fluorescence index determined by flow cytometry) on Sup5T4 compared to Sup cells; * is p <0.05. After treatment with 30ng of CXCL12, the half-life of CXCR4 detected by flow cytometry is 10.6 and 5.8min for Sup5T4 and Sup respectively; a 1.8 fold difference in rate of CXCR4 modulation. (Inset) Fold difference in CXCR4 expression by Sup5T4/Sup with time of modulation by CXCL12. Similar results were seen at 100ng CXCL12 treatment.
5T4 positive Sup-B15 leukemic cells display enhanced CXCL12/CXCR4 mediated chemotaxis which is inhibited by AMD3100 and mAb-h5T4
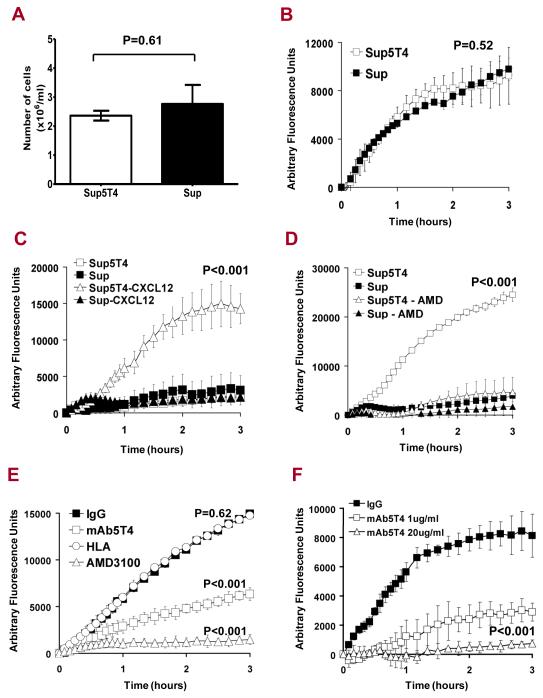
While Sup5T4 and Sup cells show similar growth characteristics (Fig 3A) and inherent motility in a serum gradient (Fig 3B), Sup5T4 cells displayed enhanced migration toward CXCL12 compared to their 5T4 negative counterparts (p<0.001) (Fig 3C). This chemotactic behaviour was blocked by the specific CXCR4 inhibitor, AMD3100 (p < 0.001) (Fig 3D). We then investigated whether this CXCL12 driven chemotaxis could be influenced by an antibody recognizing 5T4, as demonstrated in murine embryonic cells (8). Treatment of Sup5T4 cells with mAb-h5T4 significantly inhibited the CXCL12 dependent chemotaxis as compared to isotype control IgG or an irrelevant antibody to monomorphic HLA (W6/32) (p < 0.001) (Fig 3E, F).
Figure 3. Growth, migration and CXCL12/CXCR4 mediated chemotaxis in 5T4 positive and negative Sup-B15 leukemic cells.
(A) Growth at 3 days after seeding of 5×105 Sup5T4 (white) and Sup (black) cells. Migration of Sup5T4 (□) and Sup (■) cells over 3 hours assessed using a modified fluorescent Boyden chamber assay: (B) with equal motility in a gradient FCS (1-10%); (C) Sup5T4 and Sup cells show no migration with no gradient (□,■) but only Sup5T4 (Δ) but not Sup (▲) show chemotaxis in a CXCL12 gradient (0-100ng/ml). (D) Sup5T4 chemotaxis (□) is blocked by AMD3100 whereas Sup show no chemotaxis (■/▲). (E) CXCL12 mediated chemotaxis by Sup5T4 cells is specifically and significantly diminished by AMD3100 (Δ) or mAb-h5T4 treatment (□) but not by isotype control IgG (■) or an irrelevant antibody (○)(anti-monomorphic HLA; clone W6/32). (F). Dose dependent blocking of CXCL12 chemotaxis by mAb-h5T4.
Sup5T4 cells are more invasive, have a higher metabolic rate and greater MMP activity than their 5T4 negative counterparts
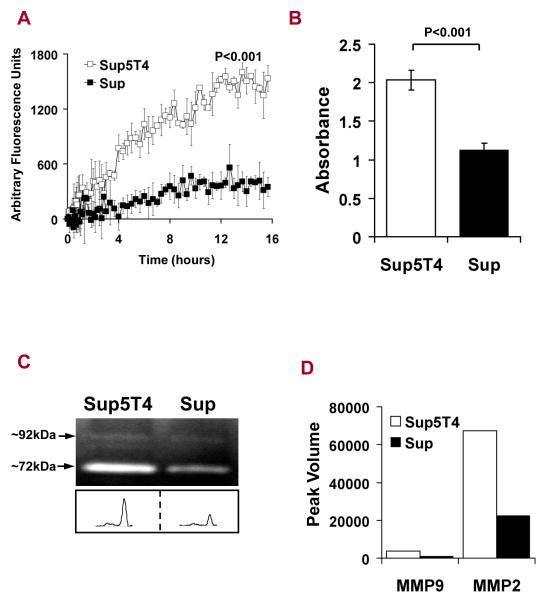
To further investigate factors that may influence leukemia dissemination, we assessed the invasiveness of the Sup-B15 sublines over a 16 hour period using Boyden chambers coated with a layer of Matrigel. Sup5T4 are inherently more invasive than Sup cells (p < 0.001) (Fig 4A) and have a higher metabolic rate than Sup cells (p<0.001) (Fig 4B). In order to invade, cells must remodel their environment by breaking down physical barriers; this process involves the secretion of active matrix metalloproteases (MMP). CM from both Sup-B15 sublines degraded gelatin at an apparent molecular weight of ~ 92 and ~ 72kDa, corresponding to the sizes of human MMP-9 and MMP-2, respectively (Fig 4C). The activity of MMP-2 was greater (~ 20-fold) than MMP-9 activity in CM from both sublines (Fig 4C). More importantly, the gelatinolytic activity of both enzymes in CM from the Sup5T4 was approximately ~3 fold-higher than that from Sup cells (Fig 4D), consistent with the enhanced invasive and metabolic properties of Sup5T4 cells.
Figure 4. Sup5T4 cells are more invasive, have enhanced MMP-2/-9 activity and a higher metabolic rate than Sup cells.
(A) Sup5T4 cells (□) are markedly more invasive through matrigel than Sup cells (■). (B) Metabolism of MTT into formazan is 1.80-fold higher in Sup5T4 than Sup cells. (C) Gelatin zymogram of conditioned media from both sublines demonstrate the proteolytic activity by MMP-9 and MMP-2 (molecular weights ~ 92 and 72 kDa). (D) Densitometry plot shows that MMP-9 and MMP-2 activity from Sup5T4 (white) is 3.86 and 3.01-fold greater than from Sup cells (black), respectively.
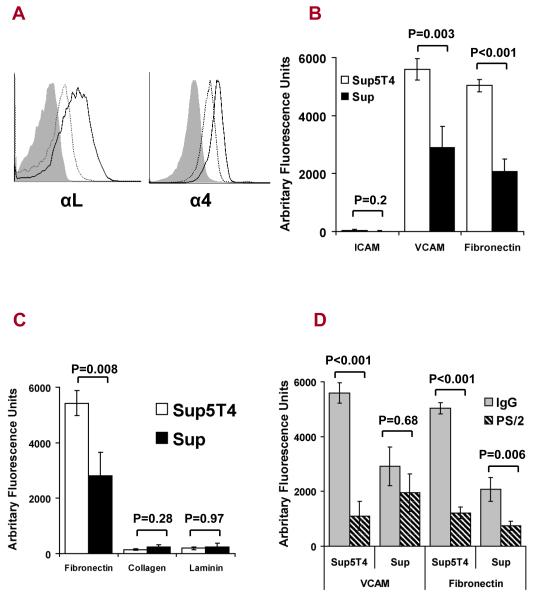
Sup5T4 cells have enhanced integrin expression with increased attachment to extracellular matrix (ECM) and cell adhesion molecules
Upregulation and activation of integrins is associated with increased invasion through enhanced recognition and response to many different ECM proteins including fibronectin, laminin and collagen (21). In addition, LFA-1 (αLβ2) and VLA-4 (α4β1) have been implicated in the dissemination of leukemic cells (22). Sup5T4 cells express higher surface levels of both αL (LFA-1) and α4 (VLA-4) than the Sup cells (Fig 5A). We next examined whether the increased integrin expression on Sup5T4 cells correlated with increased integrin function. VLA-4 has two major ligands (VCAM-1, fibronectin), while LFA-1 is known to bind only ICAM-1. Sup5T4 cells show significantly higher binding to VCAM-1 (p = 0.003) and fibronectin (<0.001) compared to Sup cells, however no binding to ICAM-1 was detected by cells from either subline despite the increased expression of LFA-1 on the Sup5T4 cells (Fig 5B). Neither subline bound to collagen or laminin excluding a functional role for many other integrin family members (Fig 5C). The adhesion of Sup-B15 cells to both fibronectin and VCAM-1 mediated specifically by VLA-4 was confirmed by inhibition using a function-perturbing anti-α4 integrin antibody (PS/2) (Fig 5D). Pre-treatment with the anti-α4 integrin antibody reduced the adhesion of Sup5T4 cells to VCAM-1 and fibronectin by 80% and 75% respectively, whilst adhesion of Sup cells was reduced to a lesser extent (33% VCAM, 64% fibronectin). Collectively, these data confirm that VLA-4 is important in the adhesion of the Sup-B15 cells, and that expression and function of this integrin are upregulated in the 5T4 positive cells.
Figure 5. Sup5T4 compared to Sup cells have upregulated integrin expression and function, with increased attachment to extra cellular matrix (ECM) and cell adhesion molecules.
(A) FACS analysis of integrin αL and α4 subunits on the Sup5T4 (solid black line) and Sup (dotted black line) sublines; isotype control (solid grey). (B) Sup5T4 show significantly higher binding to VCAM-1 and Fibronectin but do not bind to ICAM-1. (C). Sup5T4 and Sup cells fail to bind collagen or laminin. (D) Inhibition of attachment of the Sup-B15 subline cells to VCAM-1 and fibronectin by pre-treatment with anti-VLA-4 antibody (PS/2) and not isotype control .
Differential dissemination and infiltration by Sup-B15 sublines in a mouse xenograft model
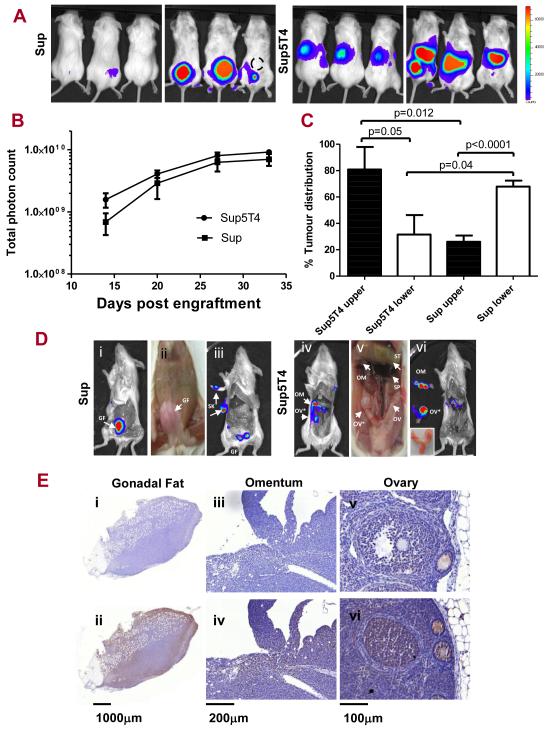
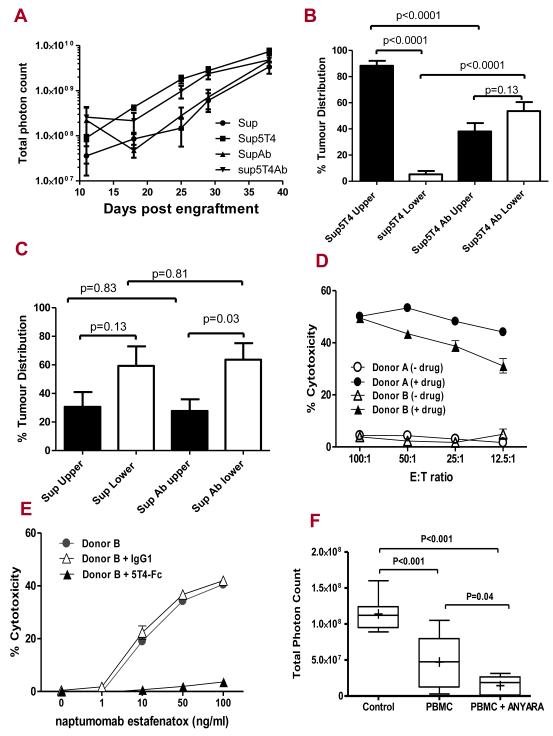
To study their ability to disseminate and infiltrate tissues, we established an animal model where Sup and Sup5T4 cells, stably transduced with the firefly luciferase gene, were transplanted intraperitoneally into NSG immune-deficient female mice and tumour growth and spread was monitored over time by bioluminescent imaging. In the several independent experiments, there was always a 100% take rate of Sup5T4 cells (27/27) whereas Sup cells showed slightly reduced tumourgenicity and sometimes a delay in time to engraftment (15/18). In the experiment illustrated in Figure 6, there was 100% engraftment of both sublines and their growth rates in vivo appeared similar by day 20 (Fig 6B). Most importantly early after engraftment, it was evident that Sup5T4 cells had a distinct dissemination pattern from Sup cells, which was maintained for over a month. Thus the proportional distribution of tumour between the upper and lower parts of the abdominal cavity was significantly different between the sublines (Fig 6C, S4). To further investigate the anatomical sites of leukemia dissemination, the animals were dissected and particular organs bioimaged. Both Sup and Sup5T4 cells most often migrated to and expanded within the gonadal fat tissue while Sup5T4 compared to Sup cells had a much greater propensity to spread to the omentum (19/20 versus 1/12) and ovaries (9/20 versus 2/12). Figures 6D and E show the bioluminescence imaging of dissected mice plus the CD45 immunohistochemistry of the isolated tissues for the typical infiltration of the gonadal fat by Sup cells and the additional targeting of the ovary /omentum by Sup5T4 cells respectively. We next tested whether treatment with mAb-h5T4 prior to, and subsequently 2 or 3 times weekly influenced tumour engraftment or growth of the subline cells in the peritoneal cavity. In the experiment illustrated, the Sup cells show a greater lag time to tumour expansion compared to half the dose of Sup5T4 cells; the growth of neither subline is significantly different with antibody treatment (Fig 7A). Interestingly, in vivo mAb-h5T4 treatment can have a direct affect on the spread of Sup5T4 which shows a distribution similar to that of the Sup cells (Fig 7B). By contrast, with or without antibody treatment, mice given Sup cells show the same preferential lower abdomen distribution (Fig 7C).
Figure 6. Sup5T4 cells are more invasive in vivo and show a high percentage of of ovary and omentum infiltration in a pre-B-ALL murine model.
(A) NSG mice (n=6) were injected with mcherry/luciferase positive Sup5T4 or Sup cells i.p and dissemination measured by bioluminescent imaging over time. Representative images (dorsal and ventral) of 3 mice at day 27 following engraftment are shown. Bioluminescence values are indicated as photons cm−2 s−1. Dashed circle denote where the i.p. injection was administered. (B) The proportionate distribution for the upper and lower abdomen (normalized to total photon count) shows the distinctive spread of the two sublines. (C) Similar in vivo growth of the sublines measured by total photon count over time. (D) Examples of the typical dissemination pattern after dissection to expose the organs in the peritoneal cavity. D (i, ii, iii) are from the second Sup mouse of (A) and show the Sup bioluminescent signal associated with the gonadal fat (GF), after the removal of the skin (SK) and reduction following GF removal and exposure of the internal organs of the abdomen. D (iv, v, vi) are from the second Sup5T4 mouse of (A) and show Sup5T4 dissemination where the skin and the gonodal fat has been removed and organs of abdomen exposed showing bioluminescence associated with an enlarged right ovary (OV*) (inset D v), (non infiltrated ovary; OV) and the omentum (OM), which is a lymphoid organ located between the stomach (ST) and spleen (SP). Removal and imaging of these organs confirmed their infiltration (D vi). (E) CD45 and control labelling shows Sup cells infiltration of the gonadal fat (E i, ii) and of Sup5T4 infiltration of omentum (E iii, iv); CD45 labelling of Sup5T4 infiltrated right ovary and uninvolved left ovary (E v, vi).
Figure 7. In vivo mAb-h5T4 treatment alters Sup5T4 but not Sup ip tumour targeting and 5T4 specific superantigen therapy of pre-B-ALL.
(A) Growth of Sup (4×106) or Sup5T4 (2×106) ip with or without 2/week treatment with mAb-h5T4. mAb-h5T4 treatment does not significantly alter the growth of either tumour; at day 25, Sup v Sup + mAb (p=0.46) and Sup5T4 v Sup5T4 + mAb (p=0.11). The mAb-h5T4 treatment, (B) significantly changes the pattern of dissemination of Sup5T4 cells to that of the Sup cells; (C) does not significantly change the pattern of dissemination of Sup cells. (D) Chromium release cytotoxicity of Sup-B15 cells at different effector to target (E:T) ratios of human donor PMBC (activated with SEA 100ng/ml for 5 days) requires 5T4-superantigen fusion protein (100ng/ml). Drug alone gave spontaneous release similar to the medium. (E) Killing of Sup-B15 cells at (25:1) E:T ratio with increasing concentration of naptumomab estafenatox is blocked by 5T4hIgFc (500 ng/ml) and not by IgG1(500 ng/ml). (F) In vivo therapy of Sup5T4 cells at day 27: naptumomab estafenatox with activated PBMC treatment gives significant reduction in tumour burden compared to activated PBMC treated animals or untreated mice. Box plots represent the interquartile range of values, the whiskers represent the smallest and largest values for each category, and the horizontal lines and cross symbols denote the median and mean respectively.
To determine if the 5T4 status of these cells was maintained in vivo, Sup5T4 or Sup cells were injected intravenously to lead to widespread dissemination (data not shown) and the expression of 5T4 on the mCherry/Luc leukemic cells recovered from the bone marrow and ovaries of these mice at day 30 was determined by flow cytometry (Fig S5). Both sublines engrafted into the bone marrow and maintained their original 5T4 status, however, only Sup5T4 cells were recoverable from the ovaries in the mice.
5T4 specific superantigen therapy of pre-B-ALL
We next tested the susceptibility of Sup5T4 cells to a 5T4 antibody-superantigen fusion protein, naptumomab estafenatox/ABR-217620, which with human peripheral blood mononuclear cells (PBMC) gives superantigen dependent cellular toxicity (SADCC). Figure 7D shows that significant and 5T4-specific T cell mediated cytotoxicity was only observed when activated PBMC and 5T4 antibody-superantigen fusion protein were present and this was specifically blocked by soluble 5T4-hIgFc protein (Fig 7E). The efficacy of this strategy was then tested in our in vivo model and mice treated with naptumomab estafenatox and activated PBMC markedly reduced the total leukemia burden compared to the untreated group (p<0.001). Although activated PBMC alone also delivered non-specific tumour control (p<0.001), this was significantly less effective than treatment with the drug and PBMC (p=0.04) (Fig 7F; S6).
Discussion
Precursor-B cell acute lymphoblastic leukaemia (pre B-ALL) (4, 14, 15) is characterized by the accumulation of malignant counterparts of normal B cell precursors which are influenced by the CXCL12/CXCR4 axis (23, 24). Common sites of childhood B-ALL relapse involve the bone marrow and extramedullary sites such as the gonads and CNS. All these niches express high levels of CXCL12 and therefore modulation of the CXCL12/CXCR4 axis is being explored for the possible therapeutic benefits in ALL and other hematological malignancies (25-27). We have recently demonstrated a role for the 5T4 glycoprotein in facilitating functional expression of CXCR4 and the chemotactic response to its ligand CXCL12 (9). The possibility that a functional influence of leukaemia spread might involve 5T4 expression is supported by the demonstration that a) the high risk of relapse group of patients had a significantly higher 5T4 transcript level and b) patients with relapse disease had a higher proportion of 5T4 positive B cell blasts. Some non-high risk cytogenetic biopsies also showed elevated 5T4 expression and since there is also differential relapse within the lower risk groups, a prospective and systematic analysis of the 5T4 marker with respect clinical outcome is warranted.
In parallel with these observations, two of three B-ALL cell lines derived from patients with a high risk cytogenetic subtype (BCR-ABL positive), were 5T4 positive. Sup-B15 showed a variable proportion of strongly 5T4 positive cells, which allowed us to routinely generate antigen positive (Sup5T4) and negative (Sup) sublines for further study. The existence of a dynamic population of 5T4 positive cells in the Sup-B15 line may reflect the existence of a pre-B-ALL stem cell population or at least residual capacity for differentiation to/from a more invasive state. Leukemia initiating phenotypes defined by in vivo engraftment of blasts from ALL patients in immune-deficient mice appear to be heterogeneous. In some studies, only a more immature stem cell-like phenotype (either CD34+ CD19− or CD34+ CD38−) was able to engraft and initiate the leukaemia in the mice (28, 29), whereas others have reported better engraftment with a more mature CD19+ phenotype (30, 31). Interestingly, a recent study has documented ALL immunophenotypic differences (e.g CD34 expression) that are unrelated to gene-rearrangement profiles reflecting differentiation associated epigenetic changes (32). Sup5T4 cells seem to have a more immature stem cell-like phenotype, expressing medium to high levels of the stem cell marker CD34 in contrast to Sup cells which are CD34 negative. Both sublines express high levels of CD19 (earliest B-lymphoid restricted marker) and are negative for CD20 (upregulated in cells undergoing VDJ rearrangement) (33). It is thought that B precursor ALL cell behaviour mirrors their “normal” counterparts during development, however, whilst normal B cell development is tightly regulated and follows a sequential order, ALL blasts seems to be able to move back and forth within these development stages enabling the different subpopulations to initiate leukemia (34). This plasticity and dynamic process may explain the fact that in vitro conditions without the required signals from the bone marrow microenvironment, SupB-15 cells lose the expression of 5T4 possibly reflecting change from a more immature pre-BI cell (CD34+ CD19+ CD20) to a large pre-BII cell (CD34− CD19+ CD20−) phenotype.
Importantly, Sup5T4 compared to Sup cells showed a more invasive phenotype in vitro and in vivo. Thus, Sup5T4 cells have significantly increased chemotaxis to CXCL12, integrin expression, adhesion to ECM/ adhesion molecules and secretion of functional MMPs in vitro. How 5T4 facilitates CXCL12 chemotaxis is unknown. In mouse embryonic cells 5T4 appears to stabilize CXCR4 expression at the plasma membrane providing for activation of the ERK signaling pathway leading to chemotaxis (9). In Sup-B15 cells membrane expression of CXCR4 is clearly not dependent on 5T4 but functional chemotaxis may require expression as suggested by blockade with a 5T4 specific mAb and reduced CXCR4 modulation by CXCL12. 5T4 may increase stability of CXCR4 expression at the plasma membrane and/or act as a receptor associated molecule providing allosteric contributions to effective ligand signaling (35-37). Interestingly, CXCR4 receptor dysfunctions in WHIM syndrome are associated with impaired desensitization and receptor internalization leading to enhanced chemokine responses (38).
Following intraperitoneal injection of female NSG mice, the subline cells initially showed similar engraftment and growth but Sup5T4 cells exhibit differential spread to the omentum and ovaries in the assessment period. Overall the Sup cells appear to show slightly reduced tumourgenicity and an increased lag time compared to Sup5T4 cells. Interestingly, the infiltration of the ovary is clearly apparent in 35 −50 % of ALL patients at autopsy (5, 39, 40), however less frequently reported than the testes (3-5). No gross Sup5T4 infiltration of the testes was detected in males after one month of engraftment (unpublished). Early reports on the dissemination of tumour cells in peritoneal tissues after intraperitoneal inoculation document the infiltration of sites rich in “milky spots”, such as the omentum, mesentery and gonadal fat (41, 42). Importantly, the omentum is thought to produce early haematopoetic progenitors throughout life and its stroma shows the constitutive expression of IL-7, FLt3 ligand and CXCL12, which are potent cofactors for the growth of B cell precursors (43). In addition, it has been demonstrated that tumour cells injected into the peritoneal cavity bind and grow on to these immune aggregates due to the presence of VCAM-1 and ICAM-1 positive mesothelial cells that overlay these sites (44).
It is reasonable to speculate that tissues like the omentum and ovary attract the Sup5T4 B-ALL cells through the 5T4/CXCR4 dependent chemotaxis axis and/or with enhanced adhesion of the elevated levels of VLA-4 on these leukemic cells to VCAM-1 expressed by the microenvironment (45, 46). A further critical component for tumour cell invasion is provided by the enhanced secretion of MMPs by the Sup5T4 cells allowing breakdown of the tissue basement membranes (47, 48). Invasiveness of many haematological malignancies involves the overexpression of MMP-2 and MMP-9 and these can be induced following exposure to chemokines or after cell adhesion to ECM (47, 49, 50). Indeed, secretion of MMP-9 has been reported to be a marker of poorer prognosis in childhood ALL (47), and higher gelatinase levels were found in leukemic bone marrow compared to normal aspirates with low level MMP-9 correlated with increased survival (51). Interestingly, leukaemic blasts have greater MMP-2 activity than normal lymphocytes (22) as is seen with Sup5T4 versus Sup cells.
The differentiation status of the Sup5T4 may reflect a more stem-like state but also possibly the hijacking of functional components of normal B cell development which enables cells to migrate from the bone marrow to secondary lymphoid organs. A study by Wang et al (52) identified a subpopulation of CD34+CD38+CD19+ Philadelphia positive Sup-B15 ALL cells expressing the endothelial marker VE-cadherin with leukemic stem cell-like properties, such as the formation of hematopoietic colonies after long term culturing with bone marrow stromal cells, induction of sprouting and reactivation of early stem cell genes. In the current study, no difference in the expression of VE-cadherin and E-cadherin was observed when comparing Sup and Sup5T4 cells (unpublished), but as discussed by Wang et al (52), the BCR-ABL Philadelphia translocation alone may promote VE-cadherin expression and therefore that would identical in both Sup and Sup5T4 sublines.
Even with the current successful B-ALL treatments, approximately 1 in 4 children with ALL will relapse (53). Once relapse in the extramedullary sites occurs, the leukemic cells can eventually spread to other extramedullary sites or repopulate the bone marrow, negating all therapeutic gains. Interestingly, in animal models, the inhibitor AMD3100 which blocks CXCL12/CXCR4 activation can sensitize ALL cells to chemotherapy possibly by mobilizing leukemic cells into the circulation where they can be more effectively treated with drugs (54). Targeting the tumour associated 5T4 antigen with antibody to deliver superantigen dependent cellular cytotoxicity (SADCC) (11, 19) or a toxin (12) offers a novel approach relevant to the high risk of relapse patients. Our data show that Sup5T4 cells are sensitive to SADCC in vitro and confirm therapeutic activity against Sup5T4 xenografts in vivo. Our data also show that 5T4 antibodies may have a direct effect on the seeding pattern in vivo, and although the mechanisms are unknown it is interesting that 5T4 antibody responses to 5T4 vaccination (TroVax) in patients correlates with clinical outcome (55). A possible next step would be to conduct phase II efficacy studies in eligible patients with 5T4-expressing leukemic blasts, followed on by studies enrolling patients with relapsed-refractory ALL 5T4-expressing blasts, using targeted immunotherapy as pre and/or post-transplant consolidation therapy.
Supplementary Material
Acknowledgments
We would like to thank all people involved in the PICR core facilities and in particular the BRU. This work was supported by programme grants from Cancer Research UK to PLS (C480/A12328) and VS. AG was supported by Seville Health Authority Spain.
Footnotes
Conflict of interest.
Rights to use 5T4 as a target for superantigen therapy is licensed by Cancer Research Technology Ltd to Active Biotech and PLS has received payments from CRT relating to clinical trial milestones under this agreement. PLS is a member of the SAB of Oxford BioMedica. All other authors declare no conflict of interests.
Supplementary information is available at Leukemia’s website
References
- 1.Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010 Dec 11;376(9757):2009–2017. doi: 10.1016/S0140-6736(10)62002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moorman AV, Ensor HM, Richards SM, Chilton L, Schwab C, Kinsey SE, et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 2010 May;11(5):429–438. doi: 10.1016/S1470-2045(10)70066-8. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs JE, Hastings C. Isolated extramedullary relapse in childhood acute lymphocytic leukemia. Curr Hematol Malig Rep. 2010 Oct;5(4):185–191. doi: 10.1007/s11899-010-0063-9. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan S, Wade R, Moorman AV, Mitchell C, Kinsey SE, Eden TO, et al. Temporal changes in the incidence and pattern of central nervous system relapses in children with acute lymphoblastic leukaemia treated on four consecutive Medical Research Council trials, 1985-2001. Leukemia. 2010 Feb;24(2):450–459. doi: 10.1038/leu.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid H, Marsden HB. Gonadal infiltration in children with leukaemia and lymphoma. J Clin Pathol. 1980 Aug;33(8):722–729. doi: 10.1136/jcp.33.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland M, Castro FV, Alexander S, Smith D, Liu J, Walker M, et al. RAC2, AEP, and ICAM1 expression are associated with CNS disease in a mouse model of pre-B childhood acute lymphoblastic leukemia. Blood. 2011 Jul 21;118(3):638–649. doi: 10.1182/blood-2010-09-307330. [DOI] [PubMed] [Google Scholar]
- 7.Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev. 2000 Oct;177:175–184. doi: 10.1034/j.1600-065x.2000.17715.x. [DOI] [PubMed] [Google Scholar]
- 8.Crazzolara R, Kreczy A, Mann G, Heitger A, Eibl G, Fink FM, et al. High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. Br J Haematol. 2001 Dec;115(3):545–553. doi: 10.1046/j.1365-2141.2001.03164.x. [DOI] [PubMed] [Google Scholar]
- 9.Southgate TD, McGinn OJ, Castro FV, Rutkowski AJ, Al-Muftah M, Marinov G, et al. CXCR4 mediated chemotaxis is regulated by 5T4 oncofetal glycoprotein in mouse embryonic cells. PLoS One. 2010;5(4):e9982. doi: 10.1371/journal.pone.0009982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer HL, Eastham AM, Merry CL, Southgate TD, Perez-Campo F, Soncin F, et al. E-cadherin inhibits cell surface localization of the pro-migratory 5T4 oncofetal antigen in mouse embryonic stem cells. Mol Biol Cell. 2007 Aug;18(8):2838–2851. doi: 10.1091/mbc.E06-09-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkord E, Shablak A, Stern PL, Hawkins RE. 5T4 as a target for immunotherapy in renal cell carcinoma. Expert Rev Anticancer Ther. 2009 Dec;9(12):1705–1709. doi: 10.1586/era.09.152. [DOI] [PubMed] [Google Scholar]
- 12.Damelin M, Geles KG, Follettie MT, Yuan P, Baxter M, Golas J, et al. Delineation of a cellular hierarchy in lung cancer reveals an oncofetal antigen expressed on tumor-initiating cells. Cancer Res. 2011 Jun 15;71(12):4236–4246. doi: 10.1158/0008-5472.CAN-10-3919. [DOI] [PubMed] [Google Scholar]
- 13.van Zelm MC, van der Burg M, de Ridder D, Barendregt BH, de Haas EF, Reinders MJ, et al. Ig gene rearrangement steps are initiated in early human precursor B cell subsets and correlate with specific transcription factor expression. J Immunol. 2005 Nov 1;175(9):5912–5922. doi: 10.4049/jimmunol.175.9.5912. [DOI] [PubMed] [Google Scholar]
- 14.Dirks WG, MacLeod RA, Nakamura Y, Kohara A, Reid Y, Milch H, et al. Cell line cross-contamination initiative: an interactive reference database of STR profiles covering common cancer cell lines. Int J Cancer. 2010 Jan 1;126(1):303–304. doi: 10.1002/ijc.24999. [DOI] [PubMed] [Google Scholar]
- 15.van Delft FW, Horsley S, Colman S, Anderson K, Bateman C, Kempski H, et al. Clonal origins of relapse in ETV6-RUNX1 acute lymphoblastic leukemia. Blood. 2011 Jun 9;117(23):6247–6254. doi: 10.1182/blood-2010-10-314674. [DOI] [PubMed] [Google Scholar]
- 16.Strefford JC, van Delft FW, Robinson HM, Worley H, Yiannikouris O, Selzer R, et al. Complex genomic alterations and gene expression in acute lymphoblastic leukemia with intrachromosomal amplification of chromosome 21. Proc Natl Acad Sci U S A. 2006 May 23;103(21):8167–8172. doi: 10.1073/pnas.0602360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Forsberg G, Ohlsson L, Brodin T, Bjork P, Lando PA, Shaw D, et al. Therapy of human non-small-cell lung carcinoma using antibody targeting of a modified superantigen. Br J Cancer. 2001 Jul 6;85(1):129–136. doi: 10.1054/bjoc.2001.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borghaei H, Alpaugh K, Hedlund G, Forsberg G, Langer C, Rogatko A, et al. Phase I dose escalation, pharmacokinetic and pharmacodynamic study of naptumomab estafenatox alone in patients with advanced cancer and with docetaxel in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2009 Sep 1;27(25):4116–4123. doi: 10.1200/JCO.2008.20.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindler J, Gajavelli S, Ravandi F, Shen Y, Parekh S, Braunchweig I, et al. A phase I study of a combination of anti-CD19 and anti-CD22 immunotoxins (Combotox) in adult patients with refractory B-lineage acute lymphoblastic leukaemia. Br J Haematol. 2011 Aug;154(4):471–476. doi: 10.1111/j.1365-2141.2011.08762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002 Feb;2(2):91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel A, Kollet O, Peled A, Abel L, Nagler A, Bielorai B, et al. Unique SDF-1-induced activation of human precursor-B ALL cells as a result of altered CXCR4 expression and signaling. Blood. 2004 Apr 15;103(8):2900–2907. doi: 10.1182/blood-2003-06-1891. [DOI] [PubMed] [Google Scholar]
- 23.Juarez J, Dela Pena A, Baraz R, Hewson J, Khoo M, Cisterne A, et al. CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia. 2007 Jun;21(6):1249–1257. doi: 10.1038/sj.leu.2404684. [DOI] [PubMed] [Google Scholar]
- 24.Juarez JG, Thien M, Dela Pena A, Baraz R, Bradstock KF, Bendall LJ. CXCR4 mediates the homing of B cell progenitor acute lymphoblastic leukaemia cells to the bone marrow via activation of p38MAPK. Br J Haematol. 2009 May;145(4):491–499. doi: 10.1111/j.1365-2141.2009.07648.x. [DOI] [PubMed] [Google Scholar]
- 25.Parameswaran R, Yu M, Lim M, Groffen J, Heisterkamp N. Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia. 2011 Aug;25(8):1314–1323. doi: 10.1038/leu.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juarez J, Bradstock KF, Gottlieb DJ, Bendall LJ. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003 Jul;17(7):1294–1300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]
- 27.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009 Jun 11;113(24):6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cobaleda C, Gutierrez-Cianca N, Perez-Losada J, Flores T, Garcia-Sanz R, Gonzalez M, et al. A primitive hematopoietic cell is the target for the leukemic transformation in human philadelphia-positive acute lymphoblastic leukemia. Blood. 2000 Feb 1;95(3):1007–1013. [PubMed] [Google Scholar]
- 29.Cox CV, Evely RS, Oakhill A, Pamphilon DH, Goulden NJ, Blair A. Characterization of acute lymphoblastic leukemia progenitor cells. Blood. 2004 Nov 1;104(9):2919–2925. doi: 10.1182/blood-2004-03-0901. [DOI] [PubMed] [Google Scholar]
- 30.Castor A, Nilsson L, Astrand-Grundstrom I, Buitenhuis M, Ramirez C, Anderson K, et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat Med. 2005 Jun;11(6):630–637. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- 31.Hong D, Gupta R, Ancliff P, Atzberger A, Brown J, Soneji S, et al. Initiating and cancer-propagating cells in TEL-AML1-associated childhood leukemia. Science. 2008 Jan 18;319(5861):336–339. doi: 10.1126/science.1150648. [DOI] [PubMed] [Google Scholar]
- 32.Obro NF, Marquart HV, Madsen HO, Ryder LP, Andersen MK, Lausen B, et al. Immunophenotype-defined sub-populations are common at diagnosis in childhood B-cell precursor acute lymphoblastic leukemia. Leukemia. 2011 Oct;25(10):1652–1657. doi: 10.1038/leu.2011.136. [DOI] [PubMed] [Google Scholar]
- 33.Hystad ME, Myklebust JH, Bo TH, Sivertsen EA, Rian E, Forfang L, et al. Characterization of early stages of human B cell development by gene expression profiling. J Immunol. 2007 Sep 15;179(6):3662–3671. doi: 10.4049/jimmunol.179.6.3662. [DOI] [PubMed] [Google Scholar]
- 34.le Viseur C, Hotfilder M, Bomken S, Wilson K, Rottgers S, Schrauder A, et al. In childhood acute lymphoblastic leukemia, blasts at different stages of immunophenotypic maturation have stem cell properties. Cancer Cell. 2008 Jul 8;14(1):47–58. doi: 10.1016/j.ccr.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006 Jan;109(1-2):173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Kofuku Y, Yoshiura C, Ueda T, Terasawa H, Hirai T, Tominaga S, et al. Structural basis of the interaction between chemokine stromal cell-derived factor-1/CXCL12 and its G-protein-coupled receptor CXCR4. J Biol Chem. 2009 Dec 11;284(50):35240–35250. doi: 10.1074/jbc.M109.024851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin JB. Chemokine signaling in cancer: one hump or two? Semin Cancer Biol. 2009 Apr;19(2):116–122. doi: 10.1016/j.semcancer.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bachelerie F. CXCL12/CXCR4-axis dysfunctions: Markers of the rare immunodeficiency disorder WHIM syndrome. Dis Markers. 2010;29(3-4):189–198. doi: 10.3233/DMA-2010-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slasky BS, Straub WH, Deutsch M. Acute lymphocytic leukemia of the ovary: the value of sonography. J Comput Tomogr. 1982 Sep;6(2):161–165. doi: 10.1016/0149-936x(82)90030-3. [DOI] [PubMed] [Google Scholar]
- 40.Pais RC, Kim TH, Zwiren GT, Ragab AH. Ovarian tumors in relapsing acute lymphoblastic leukemia: a review of 23 cases. J Pediatr Surg. 1991 Jan;26(1):70–74. doi: 10.1016/0022-3468(91)90430-2. [DOI] [PubMed] [Google Scholar]
- 41.Hagiwara A, Takahashi T, Sawai K, Taniguchi H, Shimotsuma M, Okano S, et al. Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res. 1993 Feb 1;53(3):687–692. [PubMed] [Google Scholar]
- 42.Tsujimoto H, Takhashi T, Hagiwara A, Shimotsuma M, Sakakura C, Osaki K, et al. Site-specific implantation in the milky spots of malignant cells in peritoneal dissemination: immunohistochemical observation in mice inoculated intraperitoneally with bromodeoxyuridine-labelled cells. Br J Cancer. 1995 Mar;71(3):468–472. doi: 10.1038/bjc.1995.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinho Mde F, Hurtado SP, El-Cheikh MC, Borojevic R. Haemopoietic progenitors in the adult mouse omentum: permanent production of B lymphocytes and monocytes. Cell Tissue Res. 2005 Jan;319(1):91–102. doi: 10.1007/s00441-004-0998-z. [DOI] [PubMed] [Google Scholar]
- 44.Sorensen EW, Gerber SA, Sedlacek AL, Rybalko VY, Chan WM, Lord EM. Omental immune aggregates and tumor metastasis within the peritoneal cavity. Immunol Res. 2009 Feb 28; doi: 10.1007/s12026-009-8100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imai Y, Shimaoka M, Kurokawa M. Essential roles of VLA-4 in the hematopoietic system. Int J Hematol. May;91(4):569–575. doi: 10.1007/s12185-010-0555-3. [DOI] [PubMed] [Google Scholar]
- 46.Rettig MP, Ansstas G, Dipersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. Jan;26(1):34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider P, Costa O, Legrand E, Bigot D, Lecleire S, Grassi V, et al. In vitro secretion of matrix metalloprotease 9 is a prognostic marker in childhood acute lymphoblastic leukemia. Leuk Res. 2010 Jan;34(1):24–31. doi: 10.1016/j.leukres.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 48.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 49.Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g. acute leukemia. Crit Rev Oncol Hematol. 2004 May;50(2):87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Stefanidakis M, Koivunen E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood. 2006 Sep 1;108(5):1441–1450. doi: 10.1182/blood-2006-02-005363. [DOI] [PubMed] [Google Scholar]
- 51.Lin LI, Lin DT, Chang CJ, Lee CY, Tang JL, Tien HF. Marrow matrix metalloproteinases (MMPs) and tissue inhibitors of MMP in acute leukaemia: potential role of MMP-9 as a surrogate marker to monitor leukaemic status in patients with acute myelogenous leukaemia. Br J Haematol. 2002 Jun;117(4):835–841. doi: 10.1046/j.1365-2141.2002.03510.x. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, O’Leary H, Fortney J, Gibson LF. Ph+/VE-cadherin+ identifies a stem cell like population of acute lymphoblastic leukemia sustained by bone marrow niche cells. Blood. 2007 Nov 1;110(9):3334–3344. doi: 10.1182/blood-2007-01-068122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Berg H, de Groot-Kruseman HA, Damen-Korbijn CM, de Bont ES, Schouten-van Meeteren AY, Hoogerbrugge PM. Outcome after first relapse in children with acute lymphoblastic leukemia: a report based on the Dutch Childhood Oncology Group (DCOG) relapse all 98 protocol. Pediatr Blood Cancer. 2011 Aug;57(2):210–216. doi: 10.1002/pbc.22946. [DOI] [PubMed] [Google Scholar]
- 54.Yu M, Gang EJ, Parameswaran R, Stoddart S, Fei F, Schmidhuber S, et al. AMD3100 sensitizes acute lymphoblastic leukemia cells to chemotherapy in vivo. Blood Cancer Journal. 2011;1:e14. doi: 10.1038/bcj.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrop R, Shingler WH, McDonald M, Treasure P, Amato RJ, Hawkins RE, et al. MVA-5T4-induced immune responses are an early marker of efficacy in renal cancer patients. Cancer Immunol Immunother. 2011 Jun;60(6):829–837. doi: 10.1007/s00262-011-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.