Abstract
Levamisole and pyrantel are old (1965) but useful anthelmintics that selectively activate nematode acetylcholine ion-channel receptors; they are used to treat roundworm infections in humans and animals. Interest in their actions has surged, giving rise to new knowledge and technical advances, including an ability to reconstitute receptors that reveal more details of modes of action/resistance. We now know that the receptors are plastic and may form diverse species-dependent subtypes of receptor with different sensitivities to individual cholinergic anthelmintics. Understanding the biology of the levamisole receptors is expected to inform other studies on anthelmintics (ivermectin and emodepside) that act on ion-channels.
Keywords: Levamisole, nematode, acetylcholine, ion channel receptor, subtypes, oocyte expression, anthelmintic, resistance
Levamisole and pyrantel: interest and new knowledge
One-third of the human population of the world is at risk of helminth infection, and infection is also very common in animals. Regrettably, there are still no effective vaccines for controlling these infections, so that both treatment and prophylaxis rely on anthelmintic drugs. The continued use of these drugs has given rise to concerns over levels of resistance and promoted the search for new knowledge and understanding that might slow its progress. The cholinergic anthelmintics, which include levamisole and pyrantel, are an important group of anthelmintics and like other major anthelmintics (ivermectin and emodepside) target parasite ion channels. Levamisole selectively opens a restricted subgroup of nematode acetylcholine receptor (AChR) ion channels in nematode nerve and muscle. Opening of AChR channels produces depolarization [1], entry of calcium through the opened channels, and an increase in sarcoplasmic calcium, producing spastic muscle contraction [2]; the parasite is then unable to maintain its location (often in the intestine) and is then swept away, effecting the cure. Interest in this class of anthelmintic has increased recently because application of new methods has demonstrated the presence of diverse receptor subtypes and different cholinergic anthelmintic subtype selectivities. It has also allowed better mechanistic explanations of resistance and the development of exciting novel compounds such as monepantel and derquantel. This review describes new knowledge and insights that have increased our understanding of the biology of these receptors.
Levamisole-sensitive AChRs show pharmacological- and species-dependent diversity
The very small currents (1 pA = 1×10-12 A) that flow through single receptor channels activated by levamisole or pyrantel have been recorded from muscle of the parasitic nematodes Ascaris suum [3], Oesophagostomum dentatum [4] and Brugia malayi [5] using the patch-clamp technique (Figure 1). In A. suum there is the L-type (G35 pS) that is preferentially activated by levamisole, the N-type (G25 pS) that is preferentially activated by nicotine and the B-type (G45 pS) that is preferentially activated by bephenium [6]. At the single-channel level, high concentrations of levamisole activated all three AChR subtypes (Box 1). In the strongyle species, O. dentatum, that belongs to the same phylogenetic clade as Haemonchus contortus and Caenorhabditis elegans, a fourth levamisole-activated AChR subtype with a conductance of 40 pS has also been observed (Figure 1d) [4]. We point out that in the free-living nematode, C. elegans, that levamisole activates only one conductance subtype of 30 pS, which is not activated by nicotine and is pharmacologically different from some of the parasite levamisole receptors that can be activated by nicotine [7]. Together, these observations show that levamisole activates a diverse range of receptor subtypes which are separated by their detailed pharmacology, channel conductances and species. This diversity emphasizes a need for molecular and functional characterization of these receptors in different parasitic nematode species as well as in C. elegans.
Figure 1.
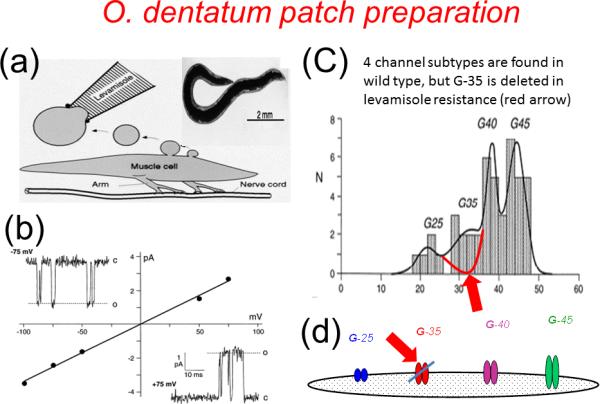
AChR single-channel currents from Oesophagostomum dentatum using the patch-clamp technique [4,43]. (a) Diagram of the production of the membrane vesicles and patch-pipette used to record the levamisole activated AChR channel currents; (inset) adult female O. dentatum. (b) Examples of levamisole activated channel currents recorded at -75mV and +75mV patch potentials and the current voltage plot showing a linear relationship, zero reversal potential, and slope giving a channel conductance of 42 pS. (c) Channel conductance distribution for the anthelmintic sensitive isolate (wild type) showing the four peaks (fitted using the sum of 4 normal distributions). The peaks illustrate the presence of four subtypes present: G25 pS, G35 pS, G40 pS and G45 pS. In the levamisole-resistant (LEVR) isolate there were only three subtypes present because the G35 pS subtype was missing. In the pyrantel resistant isolate, the four G subtypes were present and included the G 35 pS subtype, but there were fewer channels present in the muscle compared with the anthelmintic sensitive (wild) isolate. (d) Diagram of the different channel subtypes detected and presented by size on a basis of the conductances in O. dentatum; G 35 pS is not present in in levamisole resistant isolates, but present in pyrantel isolates.
The C. elegans model of the levamisole receptor
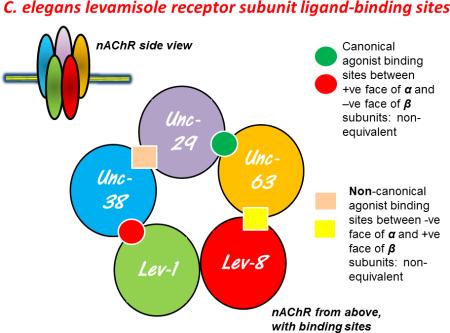
Although levamisole was developed for treatment of parasitic nematodes, the intensive study of the effects of levamisole in the C. elegans model nematode has given us tremendous insight into what may be happening in parasitic nematodes. Starting from the early days of Sydney Brenner [8], levamisole (tetramisole) has been used in genetic studies of C. elegans, so that we now have a better understanding of the molecular mechanisms associated with levamisole AChR signaling. Each AChR is composed of five subunits formed in a ring in the membrane with the channel pore in the center (Figure I, Box 2); the subunits are either α subunits with two vicinal cysteines present or β (non- α) subunits that lack these cysteines [9, 10]. There are at least 27 genes for AChR subunits in C. elegans. The levamisole receptor channel was found to be composed of five different subunits [11]. Each subunit is about 500 amino acids in length and harbors 4 transmembrane regions (M1, M2, M3, and M4). The M2 region forms the lining of the ion-channel pore. In C. elegans (Box 2; Figure 2), three separate genes code for the levamisole α subunits: unc-63, unc-38, lev-8, and two genes code for the β subunits, unc-29 and lev-1 [12-15]. The canonical agonist binding sites are assumed to be between the positive face of the α subunit and the negative face of the adjacent β subunit; the adjacent pairs of subunits are not identical, so the agonist binding sites are not equivalent (Box 2, Figure I). Evidence from other preparations leads us to believe that at least two agonist molecules need to bind to open the receptor channel effectively. In addition to the canonical sites, anthelmintics may also bind to non-canonical sites formed between the positive face of the β subunit and the negative face of an α subunit and increase opening of the ion-channel [16-18]. Thus the site of action and the potency of a cholinergic anthelmintic will depend upon the channel subunit composition.
Figure 2.
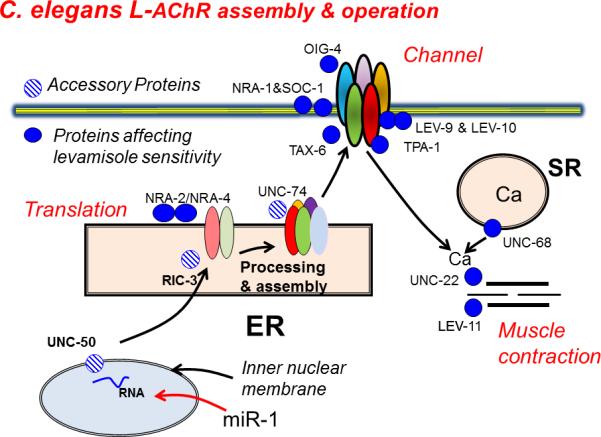
Assembly and function of levamisole receptors. C. elegans levamisole sensitive AChR channels in the muscle membrane are formed by five subunits (UNC-38, blue; UNC-29, purple; UNC-63, yellow; LEV-1, green; and LEV-8, red) with normal function being supported by OIG-1, NRA-1, NRA-2, NRA-4, SOC-1, TAX-6, TPA-1, LEV-9 and LEV-10 proteins. Once the levamisole AChR channel opens, calcium enters and its signal is amplified by the ryanodine receptor (UNC-68); the increased calcium then initiates contraction, requiring the proteins UNC-22 and LEV-11. The expression of the levamisole receptor subunits requires RNAs encoding the five subunits together with three C. elegans ancillary proteins involved in AChR assembly (RIC-3), folding (UNC-74) and trafficking (UNC-50) [24, 27, 45]. MicroRNA (miR-1) is also known to regulate and modulate the expression of subunit RNA. Abbreviations: ER, endoplasmic reticulum; SR sarcoplasmic reticulum.
In addition to the requirement for the subunits, full function of the levamisole channel is associated with proteins derived from at least seven other genes: lev-9, lev-10, tpa-1, tax-6, soc-1, nra-1, and oig-4 (Figure 2) [19-21]; it is anticipated that other associated proteins will be recognized in the future. Null mutants of these genes can produce a degree of levamisole resistance. The gene unc-68, which encodes a ryanodine receptor [22], is associated with amplifying the calcium signal following opening of the levamisole receptor, and two other genes, unc-22 and lev-11 [14], are associated with contractile elements of muscle and produce levamisole resistance by reducing the muscle response to the drug. In addition to these genes, other null mutants of genes that can produce levamisole resistance are involved in processing and assembly of the subunits; these genes are nra-2, nra-4, ric-3, unc-74 and unc-50 [23-25]. RIC-3 is a small transmembrane protein, which is a chaperone promoting nAChR folding in the endoplasmic reticulum [26]. The gene unc-74 encodes a thioredoxin protein required for the expression of levamisole AChR subunits [27]. The gene unc-50 encodes a transmembrane protein in the Golgi apparatus [24]. In unc-50 mutants, levamisole AChR subunits are directed to lysosomes for degradation. These three ancillary proteins have been widely conserved through evolution from nematodes to humans and have been used for the expression of levamisole receptors in Xenopus oocytes. In addition to these proteins, microRNAs (miRNA) are also involved in regulation of receptor subunit expression; miR-1 is one specific miRNA that binds to the mRNA of UNC-63 and UNC-29 but not LEV-1 or LEV-8 [28]. A null mutant of miR-1 leads to reduced sensitivity to levamisole; although the mechanism for this is unclear, it is suggested to involve a change in receptor expression [28].
Molecular diversity: levamisole AChR subunits in parasitic nematodes
Molecular cloning and bioinformatic searches in parasitic nematode genomes and transcriptome databanks have identified homologs of unc-29, unc-38 and unc-63 all across the phylum Nematoda [29, 30]. The conservation of these genes in distantly related nematode species may reflect the common and important role they play in the function of the levamisole AChR. It is noteworthy that in the trichostrongylid species, H. contortus and Teladorsagia circumcincta, that there are four distinct unc-29 paralogs which have been identified [30]. Unlike the unc-29, unc-38 and unc-63 genes, orthologs of the lev-1 gene have only been identified in Clade V nematode species (C. elegans and trichostrongylids). There is currently no evidence for lev-1 orthologs in nematodes of Clade III species (A. suum or B. malayi) nor in Clades I, II or IV. Intriguingly, lev-1 homologs from H. contortus, T. circumcincta and Trichostrongylus colubriformis lack a signal peptide raising questions about the contribution of LEV-1 to the function of levamisole AChRs [30]. Because AChR subunit oligomerization takes place within the endoplasmic reticulum, the absence of a signal peptide for Hco-LEV-1, Tci-LEV-1 and Tco-LEV-1 suggests that the subunit is processed differently; perhaps it is not involved in levamisole AChR construction, or it could associate with other levamisole AChR subunits using a molecular pathway that remains to be identified. Finally, there is also no evidence to date for a gene orthologous to lev-8 in the trichostrongylids, despite intensive laboratory and bioinformatic searches [29, 30]. However, homologs of the acr-8 gene that are closely related to lev-8 appear to be well conserved in parasitic nematode species [31]. The similarity between H. contortus, T. circumcincta and T. colubriformis ACR-8 homologs and the C. elegans sequence is spread over the length of the primary amino acid sequence. However, the motif, YxxCC, which is part of the ligand binding site is conserved for trichostrongylid ACR-8 and C. elegans LEV-8 (YPGCC versus YAGCC for Cel-ACR-8), suggesting that conservation of these residues is associated with specific agonist-binding properties. It has been hypothesized that the lev-8 and acr-8 genes arose from a duplication that occurred after the divergence between strongyloidea and rhabditoidea [31]. Alternatively, a common ancestor that had both lev-8 and acr-8 may have occurred with the lev-8 being lost in the trichostrongylids. Another persuasive observation for ACR-8 being a subunit of levamisole AChR subtypes in parasitic nematodes is that levamisole resistance in H. contortus has been associated with the presence of truncated ACR-8 subunits [32] (Box 3).
Functional diversity: Xenopus expression of different levamisole AChR subtypes
After cloning C. elegans levamisole receptor subunits, it became possible to express the receptor in Xenopus oocytes. Robust expression of the levamisole receptor from C. elegans required injection of cRNA of five subunits (UNC-38, UNC-63, LEV-8, UNC-29 and LEV-1) (Figure 3a) and three ancillary proteins (UNC-74, RIC-3 and UNC-50) [31]. The maximum response to acetylcholine is much greater than the response to levamisole with no nicotine response with this expressed C. elegans levamisole AChR; the lack of response to nicotine demonstrates that the C. elegans receptor is pharmacologically different from the levamisole receptors of some parasitic nematodes. Expression of an acetylcholine and nicotine-sensitive C. elegans muscle homomeric receptor was achieved by injecting ACR-16 cRNA (Figure 3b) [12, 33].
Figure 3.
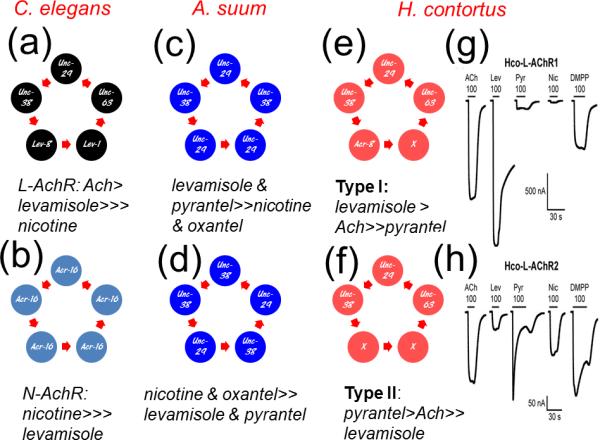
Diagram of possible stoichiometric arrangements of some different AChRs from C. elegans, A. suum and H. contortus and the relative potencies of anthelmintic agonists. (a) The C. elegans levamisole AChR. (b) The C. elegans muscle nicotine AChR. (c) The A. suum AChR where levamisole is more potent than nicotine when more cRNA for UNC-29 than UNC-38 is injected. (d) The A. suum AChR where nicotine is more potent than levamisole when more cRNA for UNC-38 than UNC-29 is injected into the Xenopus oocytes for expression. (e) The H. contortus L-AChR 1 receptor, which is most sensitive to levamisole. (f) The H. contortus L- AChR-2, which is most sensitive to pyrantel [31]. (g) Currents recorded from the Xenopus oocyte expressing H. contortus Hco-L-AChR1 receptor when 100 μM acetylcholine (ACh), 100 μM levamisole (Lev), 100 μM pyrantel (Pyr), 100 μM nicotine (Nic) or 100 μM dimethyphenylpiperazinium (DMPP) are applied. Levamisole is the most potent agonist. (h) Currents recorded from the Xenopus oocyte expressing H. contortus Hco-L-AChR2 receptor when 100 μM acetylcholine (ACh), 100 μM levamisole (Lev), 100 μM pyrantel (Pyr), 100 μM nicotine (Nic) or 100 μM dimethyphenylpiperazinium (DMPP) are applied. Pyrantel produces the biggest peak response.
Two levamisole-sensitive AChR subtypes from A. suum required only two subunits (UNC-38 and UNC-29) and did not require the accessory proteins [34]. Two types of receptor were produced: (i) one type, more sensitive to levamisole and pyrantel than nicotine, was observed when more UNC-29 than UNC-38 cRNA was injected (Figure 3c; Box 1); (ii) the other type that was more sensitive to oxantel and nicotine than levamisole, was observed when more UNC-38 than UNC-29 cRNA was injected (Figure 3d). It is suggested that two types of levamisole receptor are produced by different stoichiometric combinations of UNC-38 and UNC-29: the levamisole/pyrantel receptor by (UNC-38)2:(UNC-29)3 and the nicotine, oxantel receptor by (UNC-38)3:(UNC-29)2. These observations illustrate that the levamisole AChR subunit structure and anthelmintic sensitivity can vary and is plastic.
Expression of the receptor subunits derived from H. contortus, which is more phylogenetically related to C. elegans, was also different. Expression of a functional receptor highly responsive to levamisole required the presence of 4 subunits (UNC-63, UNC-29, UNC-38, and ACR-8) and the three ancillary proteins (Figure 3e) [31]. This receptor, referred to as Hco-L-AChR-1, is more sensitive to levamisole than acetylcholine and is poorly responsive to pyrantel and nicotine (Figure 3f). When the cRNA for ACR-8 is omitted, the UNC-63, UNC-29, UNC-38 subunit combination gave rise to a second, pharmacologically different, levamisole AChR referred to as Hco-L-AChR-2 (Figure 3f). The Hco-L-AChR-2 was more sensitive to pyrantel and acetylcholine than levamisole. Therefore, two distinct subtypes of recombinant H. contortus levamisole AChR could be distinguished, possibly mirroring some of the levamisole AChR diversity revealed with single-channel recording experiments performed on the closely related species O. dentatum (Figure 1). The critical role for the ACR-8 subunit in levamisole sensitivity is highlighted by the fact that the Hco-L-AChR-1 and Hco-L-AChR-2 only differ by the presence or absence of Hco-ACR-8. In C. elegans, the functional expression of the C. elegans levamisole AChR did not require ACR-8 subunits [12]. Of interest in parasitic nematodes is the potential role that ACR-8 plays in levamisole resistance, since absence of ACR-8 in the expressed receptors (Figure 3f) gives rise to the Hco-L-AChR-2 receptor, which is less sensitive to levamisole but highly responsive to pyrantel. Thus, an acr-8 null mutant parasitic nematode isolate may be sensitive to pyrantel but not levamisole.
Modeling the levamisole receptor
The family of ligand gated ion-channels is present in nearly all animal groups and has also been found in bacteria, predating eukaryotes [35]. The subunit genes of this family may be extremely divergent in sequence but retain a highly conserved structure and specific functional motifs (Box 2). This allows the use of known crystal structures to serve as templates for the prediction of related ion-channel structures and identification of acetylcholine and nicotine within the ligand binding pocket of the AChR [36,37]. The models identify potentially important hydrogen bonds formed upon ligand binding and are able to discriminate between compounds with high and low experimentally determined LD50 values. In the future we could use this approach to examine levamisole and pyrantel binding to the various levamisole-sensitive AChRs of identified parasitic nematodes. The next challenge is for in silico prediction of ligand receptor interaction for novel compounds. The example of cytisinoid derivatives and their affinity for acetylcholine binding protein demonstrates that it is possible to achieve a good correlation over a wide range of predicted binding energy and IC50 values [38].
The neurotransmitter binding pocket lies between two adjacent subunits of the channel pentamer (Box 2), formed from three loops of the positive subunit (known as the A, B and C loops) and three beta-strands of the negative subunit (loops D, E and F). The closed, unbound form of the Torpedo fish AChR is an asymmetric pentomer that becomes symmetrical in the presence of acetylcholine [39]. Comparison of a refined version of this structure and that of the channel produced in the open channel configuration shows that the c-loop closes around the bound neurotransmitter, a movement of perhaps 8 Å, accompanied by significant changes in the β-sheet regions of the ligand binding domain and rotation of the transmembrane domain, opening a path for the flow of cations [36, 39].
Modeling interactions of such flexible structures is still being developed and simulation of the ligand-receptor interaction will require either an ensemble of receptors in different physical states, or use of molecular dynamics to produce time simulations of the ligand bound structure. Snapshots can be evaluated to determine the stability of ligand binding and changes in flexibility of residues that interact with the ligand [40]. Although still at the limits of protein modeling, these techniques will help us to understand details of nAChR function and their interaction with levamisole and other anthelmintic compounds.
Resistance: mechanisms of resistance for parasites in vivo
In sensitive O. dentatum parasites, levamisole readily activates and opens the receptor channels (Figure 1b) frequently. In the levamisole and pyrantel resistant isolates the channels open infrequently and there are fewer receptor channels present in the muscle membrane [4, 43]. A histogram of single-channel conductances from the anthelmintic sensitive isolate of O. dentatum [4, 43] shows the presence of four separate channel conductance subtypes: G24 pS, G35 pS, G40 pS, G45 pS (Figure 1c,d). Interestingly, in the levamisole resistant isolate the G35 pS peak is missing, but in the pyrantel resistant isolate this peak is still present [4,43]. These observations suggest that resistance is associated with an overall reduction in the number of receptors, that levamisole resistance may be associated specifically with loss of the G 35 pS subtype but that pyrantel resistance may be different and is not associated with a loss of a specific subtype. We might expect that the changes associated with resistance may lead to subtle changes in motility without compromising the ability of the parasite to complete its life cycle. The changes in motility have been detected by detailed phenotyping [41,42].
Phenotyping sensitive and resistant isolates: microfluidics
We have seen above that there are a range of different levamisole receptor subtypes suggesting some redundancy and that there may be differences between sensitive and resistant isolates. It is now possible to screen and observe effects of drugs such as levamisole affecting motion of L3 larvae of parasitic nematodes with greater resolution using computer tracking and microfluidic technology [41, 42]. These multi-parameter microfluidic bioassays were developed to observe the innate locomotory properties of larval movement along with transient and real time responses to the application of anthelmintics within a single experiment. An electrical field is used in the platform to guide the movement of the larvae (electrotaxis) into and out of the drug wells. Video recording of the experiment along with automatic worm tracking software reveals important information about changes in the locomotion of the worm during entry exposure and exit periods from the drug. The tracking software used can measure subtle locomotion changes in the microfluidic device in the presence or in the absence of drugs. The approach allows quantification of transient drug effects and measurement of locomotory parameters like the sinusoidal properties of the larval movement (velocity, wavelength, frequency and amplitude). The more precise measurements of locomotion can detect levamisole resistance in the absence of drugs from changes in the parameters of locomotion, as well as changes associated with application of anthelmintics [41, 42]. This new method of quantitative phenotyping offers a new way to detect and investigate anthelmintic resistance and anthelmintic interactions that affect the phenotype of locomotion associated with subtle changes in receptor subtypes.
Investigating levamisole resistance at the molecular level in parasitic nematodes
Four sets of proteins have been found to contribute to levamisole resistance in C. elegans. The four sets are: (i) Levamisole sensitive AChR subunits, (ii) proteins involved in processing and assembly of levamisole AChR channel subunits; (iii) proteins involved in regulating the levamisole AChR channel; and (iv) proteins involved in the calcium-contraction signaling cascade (Figure 2; Box 3). In parasitic nematodes, searches for expression or sequence polymorphisms associated with levamisole resistance mainly focused on levamisole AChR subunits. In the strongylid parasite, Ancylostoma caninum (dog hookworm), genes orthologous to unc-38, unc-63 and unc-29 have reduced expression in a highly pyrantel-resistant isolate when compared with a low pyrantel-resistant isolate [44]. By contrast, in the trichostrongylid species H. contortus, T. circumcincta and T. colubriformis, expression of complete coding mRNAs corresponding to unc-38, unc-63, unc-29 and lev-1 homologs were found to be similar in both levamisole susceptible and resistant isolates. However, in addition to full-length coding mRNAs corresponding to unc-63 orthologs, abbreviated isoforms (unc-63b) were found to be specifically expressed in some levamisole resistant isolates from the three nematode species, suggesting a possible role of the truncated UNC-63 subunit in levamisole resistance [30]. Transcript levels may also change with resistance [49]. Recent progress with the functional expression of nematode levamisole AChRs in Xenopus oocytes has provided a unique opportunity to test the role of truncated subunits in levamisole resistance [31].
The truncated Hco-unc-63b transcript in an H. contortus isolate encoded a protein of 343 amino acids that has two transmembrane domains (TM1 and TM2). In Xenopus oocytes, expression of the truncated Hco-UNC-63b along with the full length Hco-unc-63a, Hco-unc-29.1, Hco-unc-38 and Hco-acr-8 cRNA produced a dose-dependent inhibition of the Hco-L-AChR-1 expression [31]. The results show that the truncated Hco-unc-63b is dominant negative, inhibiting the wild type Hco-UNC-63 subunits and therefore could induce a levamisole-resistant phenotype in parasites expressing this mutant form. Expression of the truncated UNC–63b is predicted to reduce expression of both the Hco-L-AChR-1 and Hco-L-AChR-2 receptors. Hence, if such a dominant negative effect also occurs in vivo, a cross resistance to levamisole and pyrantel could be expected in the H. contortus isolates expressing Hco-UNC-63b. The role of truncated ACR-8, which is associated with levamisole resistance [32] remains to be tested in expression experiments.
Concluding remarks
Better molecular and functional platforms have allowed a tremendous increase in our understanding of: (i) the biology of the levamisole AChR channel; (ii) how there are a number of diverse pharmacological subtypes; (iii) how the subunit structure is plastic and affects drug sensitivity; and (iv) how subunit truncation (UNC-63 or ACR-8) or reduced subunit expression may play a role in levamisole resistance. There is optimism that the understanding of the molecular and pharmacological mechanism (or mechanisms) of resistance will allow molecular diagnostic tests for resistance and better pharmacological therapies to be used, including combination drug therapy. The current advances in levamisole receptor molecular biology have bridged the gap between genes and the physiological expression of anthelmintic sensitivity. Further examination of the various receptor subtypes in important parasites such as filaria, hookworm and whipworm, the range of nematode parasite AChR channels in muscle receptors and other tissues, and the changes brought about by resistance and altered subunit composition will provide a deeper understanding of AChR functions and the mechanisms that control the evolution of their diversity. In the future, new approaches that include novel methods to measure small changes in phenotype such as microfluidics, the development of molecular techniques to manipulate parasites in vivo and computer simulation of the ligand receptor interaction will usher in a new awakening for receptor research. Over the next ten years we should expect to see a more detailed map of AChR function as well as new anthelmintics that target these receptors.
Box 1. Levamisole AChRs of nematodes in vivo are composed of diverse species-dependent subtypes.
They have different channel conductances, and the subtypes may be separated pharmacologically.
Table I.
Main muscle nAChR channel conductances opened by different anthelmintic in nematodesa
| Species | Clades | Drugs | Conductances | (pS)e | Refs. | ||
|---|---|---|---|---|---|---|---|
| A. suum | III | ACh | 25-35 | - | 40-50 | [46] | |
| Lev | 23 | 33 | 43 | [6] | |||
| Nic | 26 | 36 | - | [47] | |||
| Pyr | 22 | - | 41 | [52] | |||
| Beph | - | 36 | 43 | [6] | |||
| C. elegans | V | ||||||
| lev-10 mutantb | ACh | 30 | [7] | ||||
| Lev | 29.3 | [7] | |||||
| lev-10;lev-1 mutantc | Lev | 26.9 | [7] | ||||
| lev-10;lev-8 mutantd | ACh | 27.4 | [7] | ||||
| Lev | 30 | [7] | |||||
| O. dentatum | V | Lev | 22 | 35 | 40 | 45 | [4] |
| [43] | |||||||
In C. elegans only one was separated (approx. 30 pS), in A. suum up to three subtypes (approx. G25 pS, G35 pS and G45 pS) were separated, and in O. dentatum four were separated (approx. G25 pS, G35 pS, G40 pS and G45 pS).
C. elegans lev-10 mutant
lev-1 mutant
lev-8 mutant
Conductances
Abbreviations: ACh, acetylcholine; Lev, levamisole; Nic, nicotine; Pyr, pyrantel Beph, bephenium
Box 2.
Figure I. Possible canonical and non-canonical ligand binding sites. The sites are based on the C. elegans levamisole AChR consensus structure. These AChRs are composed of five heterologous subunits; each may be an α or β subunit, about 500 amino acids in length. In C. elegans there are five genes coding for the levamisole AChR channel: unc-38, unc-29, unc-63, lev-1, and lev-8. The canonical agonist binding sites are formed at the interface of two subunits, between the positive surface of the subunit that contributes the principle agonist binding surface and the negative surface of the adjacent β subunit that provides the complimentary agonist binding surface. Thus the receptor subunit composition and arrangements affect the binding sites and pharmacology of the receptor. The canonical binding sites for acetylcholine or other agonists are not identical (non-synonymous) if either of the adjacent subunits forming the binding sites is different. There may also be non-canonical binding sites on the negative face of the subunits [47].
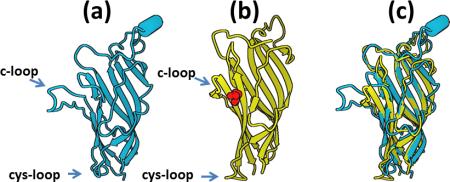
Figure II. Open and closed channel configurations. (a) The torpedo fish acetylcholine receptor (2BG9) and (b): the bacterial proton gated channel (3EAM) is separated by more than 2 billion years of sequence change and yet the structures remain remarkably similar. Only the extracellular ligand binding domain is shown in the illustration, viewed parallel to the plasma membrane; the transmembrane domains extend from the bottom of each structure but are not shown. The characteristic cys-loop is labeled and the ligand binding pocket, indicated in red in the center, is enclosed by the c-loop. A majority of the ligand binding domain is present as two curved beta-sheets that can be seen in almost identical positions when the two structures are superimposed (c). The looped regions of both structures also occupy very similar configurations between the two.
Box 2. Levamisole AChR channels and models of subunit structure.
Levamisole AChR channels
Levamisole and pyrantel are nematode selective cholinergic (acetylcholine) agonists that open non-selective cation ion-channel receptors (AChRs) present on nematode body muscle. The drugs bind to canonical and/or non-canonical binding sites on the receptors (Figures I and II). Opening of these channels produces depolarization, entry of calcium, and muscle contraction; the nematode parasite becomes paralyzed and is ejected from its location, effecting a cure. The ligand binding sites are between adjacent subunits of the receptor and include canonical and non-canonical binding sites.
Modeling ligand binding and the levamisole AChR channel.
The structure of the nematode levamisole AChR channel and subunits is predictable because the structure of ligand-gated ion-channel subunits is conserved (Figure II).
These structures can predict that of other ion-channels by providing an initial approximate configuration for energy minimization techniques that yield optimal predicted structures [50, 51]. The predictions are used in docking studies where configurations of a ligand are evaluated to find the most likely pose identified as that with the lowest energy. The intrinsic flexibility of ion-channels is essential to their function but creates problems for docking studies in silico. This can be seen by the dramatic change is position of the c-loop between the closed channel configuration (Figure IIa): and the open form (Figure IIb). Presence of the ligand stabilizes the c-loop around the ligand. This change is followed by a series of other structural changes that ultimately results in rotation of the M2 transmembrane that opens the channel.
Box 3. Levamisole resistance.
C. elegans levamisole resistance
The degree of our understanding of the mechanisms of levamisole resistance depends on species of nematode studied. Levamisole resistance in C. elegans has been associated with null mutants of 21 genes [12-15,21-28]. Five of the genes are: unc-38, unc-29, unc-63, lev-1, and lev-8, which code for subunits of the levamisole AChR channel; seven of the genes: oig-4, nra-1, soc-1, tax-6, tpa-1, lev-9 and lev-10, are associated with the proper distribution and maintaining the normal opening of the receptor channel. Three genes associated with calcium release or calcium-sensitive contraction are: unc-68, unc-22 and lev-11. Three genes, known as accessory proteins are: unc-50, ric-3 and unc-74. These accessory proteins along with nra-2 and nra-4 are associated with the processing, assembly and delivery of the ion-channel receptor to the membrane.
Resistance in nematode parasites
In O. dentatum levamisole resistance has been associated with a reduced number of receptor channels at the muscle surface and a loss of the G35 pS conductance subtypes of levamisole AChR channel [4], whereas pyrantel resistance was associated with a reduced number of all conductance subtypes [48]. In A. caninum, pyrantel resistance has been associated with reduced expression levels of UNC-38, UNC-63, UNC-29 but not ACR-8. No functional change was detected in the amino acid sequence of either of these proteins nor in the bases coding for the genes (single nucleotide polymorphism: SNPs). In H. contortus, levamisole resistance has been associated with truncated forms of UNC-63. With loss of or truncation of ACR-8, the isolate is expected to be less sensitive to levamisole but still sensitive to pyrantel. Thus it is suggested that loss of ACR-8 subunits may lead to a selective loss of sensitivity to levamisole but not pyrantel. Loss of UNC-63, however, is predicted to lead to a loss of sensitivity to both levamisole and pyrantel. The role of the other C. elegans genes in resistance to levamisole in parasitic nematodes remains to be investigated.
Transcriptomic approach for identifying resistance genes
In addition to the candidate gene strategy, based on C. elegans, a global comparative transcriptomic approach is another useful tool for identification of novel parasitic genes involved in levamisole resistance. This non-hypothesis driven approach using an amplified restriction fragment technique (cDNA-AFLP) has been used to identify genes differentially expressed in levamisole-resistant and susceptible isolates of H. contortus [30]. More than 17 000 transcript-derived fragments (TDFs) were amplified and 26 TDFs displayed differential expression including 11 TDFs specifically present in resistant isolates. Among those candidates, HAX was specifically expressed in the two resistant isolates and presented strong homology with the acr-8 gene. HAX corresponded to a truncated H. contortus acr-8 mRNA isoform (Hco-acr-8b) containing the two first exons and a part of intron 2. The specific expression of Hco-acr-8b was confirmed in other H. contortus levamisole resistant isolates highlighting its interest as a potent marker for levamisole resistance [32,49].
Acknowledgments
The authors acknowledge support of all of our colleagues in parasitology and specifically for this work from the Institut National de la Recherche Agronomique (INRA) to C.L.C. and C.N, the Chateaubriand fellowship from the Embassy of France in the US, grants R56 AI047194 – 11 to R.J.M and R21AI092185-01 to A.P.R from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Glossary
- Cholinomimetics
drugs which mimic the actions of acetylcholine and activate acetylcholine receptors.
- Cholinergic anthelmintic
antinematodal drugs which interact with acetylcholine receptors.
- Conductance subtypes
a classification of receptor ion channels based on conductance groupings; G25 (nAChRs with conductances near 25 pS); G35 (nAChRs with conductances near 35 pS); G40 (nAChRs with conductances near 40 pS); G45 (nAChRs with conductances near 45 pS).
- IC50
The dose of a drug that inhibits 50% of the response.
- LD50
The dose of a drug that kills 50% of the population under test.
- Null mutants
defective gene mutants where the genes are non-functional.
- Patch-clamp technique
an electrophysiological technique for recording the very small currents that flow through single ion-channels.
- Phylogenetic clade
a subgroup within a phylogenetic tree that all trace back to a common point.
- Hco-lev-1, Tci-lev -1,Tco-lev-1, Cel-acr-8
C. elegans genes are labeled and referred to by a three letter code (e.g. lev and acr, followed by an number); to refer homologues of these genes in parasitic nematodes, three letters from the species are added (a gene from Haemonchus contortus which is a homologue of C. elegans lev-1 is referred to as Hco-lev-1; Tci is Teladorsagia circumcincta; Tco is Trichostrongylus colubriformis).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest
References
- 1.Puttachary S, et al. Levamisole and ryanodine receptors. II: An electrophysiological study in Ascaris suum. Mol Biochem Parasitol. 2010;171:8–16. doi: 10.1016/j.molbiopara.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson AP, et al. Levamisole and ryanodine receptors. I: A contraction study in Ascaris suum. Mol Biochem Parasitol. 171:1–7. doi: 10.1016/j.molbiopara.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson SJ, Martin RJ. Levamisole-activated single-channel currents from muscle of the nematode parasite Ascaris suum. Br.J.Pharmacol. 1993;108:170–178. doi: 10.1111/j.1476-5381.1993.tb13458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson AP, et al. Resistance to levamisole resolved at the single-channel level. FASEB J. 1999;13:749–760. doi: 10.1096/fasebj.13.6.749. [DOI] [PubMed] [Google Scholar]
- 5.Robertson AP, et al. Single-channel recording from adult Brugia malayi. Invert Neurosci. 2010;11:53–57. doi: 10.1007/s10158-011-0118-1. [DOI] [PubMed] [Google Scholar]
- 6.Qian H, et al. Pharmacology of N-, L- and B-subtypes of nematode nAChR resolved at the single-channel level in Ascaris suum. FASEB J. 2006 doi: 10.1096/fj.06-6264fje. [DOI] [PubMed] [Google Scholar]
- 7.Qian H, et al. Levamisole resistance resolved at the single-channel level in Caenorhabditis elegans. FASEB J. 2008 doi: 10.1096/fj.08-110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Changeux JP, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. 2005;308:1424–1428. doi: 10.1126/science.1108595. [DOI] [PubMed] [Google Scholar]
- 10.Corringer PJ, et al. Critical elements determining diversity in agonist binding and desensitization of neuronal nicotinic acetylcholine receptors. J.Neurosci. 1998;18:648–657. doi: 10.1523/JNEUROSCI.18-02-00648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones AK, Sattelle DB. Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. Bioessays. 2004;26:39–49. doi: 10.1002/bies.10377. [DOI] [PubMed] [Google Scholar]
- 12.Boulin T, et al. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci U S A. 2008;105:18590–18595. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Culetto E, et al. The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor alpha subunit. J.Biol.Chem. 2004;279:42476–42483. doi: 10.1074/jbc.M404370200. [DOI] [PubMed] [Google Scholar]
- 14.Fleming JT, et al. Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J.Neurosci. 1997;17:5843–5857. doi: 10.1523/JNEUROSCI.17-15-05843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towers PR, et al. The Caenorhabditis elegans lev-8 gene encodes a novel type of nicotinic acetylcholine receptor alpha subunit. J.Neurochem. 2005;93:1–9. doi: 10.1111/j.1471-4159.2004.02951.x. [DOI] [PubMed] [Google Scholar]
- 16.Seo S, et al. The positive allosteric modulator morantel binds at noncanonical subunit interfaces of neuronal nicotinic acetylcholine receptors. J. Neurosci. 2009;29:8734–8742. doi: 10.1523/JNEUROSCI.1859-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu TY, et al. Morantel allosterically enhances channel gating of neuronal nicotinic acetylcholine alpha 3 beta 2 receptors. Mol Pharmacol. 2008;74:466–475. doi: 10.1124/mol.107.044388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans AM, Martin RJ. Activation and cooperative multi-ion block of single nicotinic-acetylcholine channel currents of Ascaris muscle by the tetrahydropyrimidine anthelmintic, morantel. Br.J.Pharmacol. 1996;118:1127–1140. doi: 10.1111/j.1476-5381.1996.tb15515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin RJ, Robertson AP. Mode of action of levamisole and pyrantel, anthelmintic resistance, E153 and Q57. Parasitology. 2007;134:1093–1104. doi: 10.1017/S0031182007000029. [DOI] [PubMed] [Google Scholar]
- 20.Fleming JT, et al. Molecular cloning and in vitro expression of C. elegans and parasitic nematode ionotropic receptors. Parasitology. 1996;113(Suppl):S175–S190. doi: 10.1017/s0031182000077969. [DOI] [PubMed] [Google Scholar]
- 21.Gally C, et al. A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans. Nature. 2004;431:578–582. doi: 10.1038/nature02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maryon EB, et al. unc-68 encodes a ryanodine receptor involved in regulating C. elegans body-wall muscle contraction. J Cell Biol. 1996;134:885–893. doi: 10.1083/jcb.134.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almedom RB, et al. An ER-resident membrane protein complex regulates nicotinic acetylcholine receptor subunit composition at the synapse. EMBO J. 2009;28:2636–2649. doi: 10.1038/emboj.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eimer S, et al. Regulation of nicotinic receptor trafficking by the transmembrane Golgi protein UNC-50. EMBO J. 2007;26:4313–4323. doi: 10.1038/sj.emboj.7601858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottschalk A, et al. Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. EMBO J. 2005;24:2566–2578. doi: 10.1038/sj.emboj.7600741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halevi S, et al. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002;21:1012–1020. doi: 10.1093/emboj/21.5.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haugstetter J, et al. Identification and characterization of a novel thioredoxin-related transmembrane protein of the endoplasmic reticulum. J. Biol. Chem. 2005;280:8371–8380. doi: 10.1074/jbc.M413924200. [DOI] [PubMed] [Google Scholar]
- 28.Simon DJ, et al. The microRNA miR-1 regulates a MEF-2-dependent retrograde signal at neuromuscular junctions. Cell. 2008;133:903–915. doi: 10.1016/j.cell.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williamson SM, et al. The cys-loop ligand-gated ion channel gene family of Brugia malayi and Trichinella spiralis: a comparison with Caenorhabditis elegans. Invert.Neurosci. 2007;7:219–226. doi: 10.1007/s10158-007-0056-0. [DOI] [PubMed] [Google Scholar]
- 30.Neveu C, et al. Genetic diversity of levamisole receptor subunits in parasitic nematode species and abbreviated transcripts associated with resistance. Pharmacogenet Genomics. 20:414–425. doi: 10.1097/FPC.0b013e328338ac8c. [DOI] [PubMed] [Google Scholar]
- 31.Boulin T, Fauvin A, Charvet C, Cortet J, Cabaret J, Bessereau J-L, Neveu C. Functional Reconstitution of Haemonchus contortus Acetylcholine Receptors in Xenopus Oocytes Provides Mechanistic Insights into Levamisole Resistance. Br J Pharmac. 2011;164:1421–1432. doi: 10.1111/j.1476-5381.2011.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fauvin A, et al. cDNA-AFLP analysis in levamisole-resistant Haemonchus contortus reveals alternative splicing in a nicotinic acetylcholine receptor subunit. Mol Biochem Parasitol. 170:105–107. doi: 10.1016/j.molbiopara.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Raymond V, et al. Anthelmintic actions on homomer-forming nicotinic acetylcholine receptor subunits: chicken alpha7 and ACR-16 from the nematode Caenorhabditis elegans. Neuroscience. 2000;101:785–791. doi: 10.1016/s0306-4522(00)00279-7. [DOI] [PubMed] [Google Scholar]
- 34.Williamson SM, et al. The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: formation of two distinct drug targets by varying the relative expression levels of two subunits. PLoS Pathog. 2009;5:e1000517. doi: 10.1371/journal.ppat.1000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen GQ, et al. Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature. 1999;402:817–821. doi: 10.1038/45568. [DOI] [PubMed] [Google Scholar]
- 36.Brejc K, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 37.Le Novere N, et al. Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proc Natl Acad Sci U S A. 2002;99:3210–3215. doi: 10.1073/pnas.042699699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abin-Carriquiry JA, et al. In silico characterization of cytisinoids docked into an acetylcholine binding protein. Bioorg Med Chem Lett. 20:3683–3687. doi: 10.1016/j.bmcl.2010.04.092. [DOI] [PubMed] [Google Scholar]
- 39.Unwin N. Acetylcholine receptor channel imaged in the open state. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- 40.Brandsdal BO, et al. Free energy calculations and ligand binding. Adv Protein Chem. 2003;66:123–158. doi: 10.1016/s0065-3233(03)66004-3. [DOI] [PubMed] [Google Scholar]
- 41.Chen B, et al. Microfluidic bioassay to characterize parasitic nematode phenotype and anthelmintic resistance. Parasitology. 138:80–88. doi: 10.1017/S0031182010001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carr JA, et al. A microfluidic platform for high-sensitivity, real-time drug screening on C. elegans and parasitic nematodes. Lab Chip. 11:2385–2396. doi: 10.1039/c1lc20170k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson AP, et al. Pyrantel resistance alters nematode nicotinic acetylcholine receptor single-channel properties. Eur.J.Pharmacol. 2000;394:1–8. doi: 10.1016/s0014-2999(00)00135-7. [DOI] [PubMed] [Google Scholar]
- 44.Kopp SR, et al. High-level pyrantel resistance in the hookworm Ancylsotoma caninum. Vet.Parasitol. 2006:299–204. doi: 10.1016/j.vetpar.2006.08.036. Vet Parasitol. [DOI] [PubMed] [Google Scholar]
- 45.Halevi S, et al. Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J. Biol. Chem. 2003;278:34411–34417. doi: 10.1074/jbc.M300170200. [DOI] [PubMed] [Google Scholar]
- 46.Pennington AJ, Martin RJ. A patch-clamp study of acetylcholine-activated ion channels in Ascaris suum muscle. J.Exp.Biol. 1990;154:201–221. doi: 10.1242/jeb.154.1.201. [DOI] [PubMed] [Google Scholar]
- 47.Levandoski MM, et al. Single-channel properties of N- and L-subtypes of acetylcholine receptor in Ascaris suum. Int.J.Parasitol. 2005;35:925–934. doi: 10.1016/j.ijpara.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Robertson AP, et al. Pyrantel resistance alters nematode nicotinic acetylcholine receptor single-channel properties. Eur. J. Pharmacol. 2000;394:1–8. doi: 10.1016/s0014-2999(00)00135-7. [DOI] [PubMed] [Google Scholar]
- 49.Williamson SM, et al. Candidate anthelmintic resistance-associated gene expression and sequence polymorphisms in a triple-resistant field isolate of Haemonchus contortus. Mol Biochem Parasitol. 2011;180:99–105. doi: 10.1016/j.molbiopara.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Abin-Carriquiry JA, et al. In silico characterization of cytisinoids docked into an acetylcholine binding protein. Bio. Med. Chem. Let. 2010;20:3683–3687. doi: 10.1016/j.bmcl.2010.04.092. [DOI] [PubMed] [Google Scholar]
- 51.Brandsdal BO, et al. Free Energy Calculations and Ligand Binding. In: Valerie D, editor. Advances in Protein Chemistry. Academic Press; 2003. pp. 123–158. [DOI] [PubMed] [Google Scholar]
- 52.Robertson SJ, et al. The action of pyrantel as an agonist and an open channel blocker at acetylcholine receptors in isolated Ascaris suum muscle vesicles. Eur. J .Pharmacol. 1994;271:273–282. doi: 10.1016/0014-2999(94)90784-6. [DOI] [PubMed] [Google Scholar]