Abstract
OBJECTIVE
To estimate the prevalence of chronic conditions among children admitted to U.S. pediatric intensive care units (PICU) and to assess whether patients with complex chronic conditions (CCC) experience PICU mortality and prolonged LOS risk beyond that predicted by commonly-used severity-of-illness risk-adjustment models.
DESIGN, SETTING, & PATIENTS
Retrospective cohort analysis of 52,791 pediatric admissions to 54 U.S. PICUs that participated in the Virtual Pediatric Intensive Care Unit Performance System (VPS) database in 2008.
MEASUREMENTS
Hierarchical logistic regression models, clustered by PICU site, for PICU mortality and length of stay (LOS) > 15 days. Standardized mortality ratios (SMR) adjusted for severity-of-illness score alone and with CCC.
MAIN RESULTS
Fifty-three percent of PICU admissions had a CCC, 18.5% had a non-complex chronic conditions (NCCC). The prevalence of these conditions and their organ system subcategories varied considerably across sites. The majority of CCC subcategories were associated with significantly greater odds of PICU mortality (odds ratios [OR] 1.25–2.9, all P values <0.02) compared to having a non-complex chronic condition (NCCC) or no chronic condition, after controlling for age, gender, trauma, and severity-of-illness. Only respiratory, gastrointestinal, and rheumatologic/orthopedic/psychiatric CCC were not associated with increased odds of PICU mortality. All subcategories were significantly associated with prolonged LOS. All NCCC subcategories were either not associated or negatively associated with PICU mortality, and most were not associated with prolonged LOS, compared to having no chronic conditions. Among this group of PICUs, adding CCCs to risk-adjustment models led to greater model accuracy but did not substantially change unit-level SMRs.
CONCLUSIONS
Children with CCC were at greater risk for PICU mortality and prolonged LOS than those with no chronic conditions, but the magnitude of risk varied across subcategories. Inclusion of CCCs into models of PICU mortality improved model accuracy but had little impact on SMRs.
Keywords: Child; Intensive Care Units, Pediatric; Mortality; Length of Stay; Chronic Disease; Risk Adjustment
Introduction
Children with complex chronic conditions (CCC) have considerable, on-going care needs (1–3), more so than children with non-complex chronic conditions (NCCC) (4). Children with CCC accounted for 10–17% of hospitalized children in 2006 (5–6), and this prevalence has increased since the 1990s (6–7). These children may be more highly represented among patients admitted to pediatric intensive care units (PICU), as indicated by single-site studies (8–11). However, the prevalence of CCC among a broader population of PICU patients is unknown.
Although the prevalence of CCC among hospitalized children is increasing, the two most commonly-used risk-adjustment models for severity-of-illness—Pediatric Index of Mortality (PIM2) (12) and Pediatric Risk of Mortality (PRISM III) (13)— include few chronic conditions in their models. These risk-adjustment models are utilized for predicting mortality and comparing performance across PICUs (14). Given the disproportionate hospital mortality seen among children with CCC compared to children without chronic conditions (7, 15–16), it is necessary to assess the impact of CCC on mortality beyond that predicted by these risk models. In addition, knowing whether CCC confer additional risk may be necessary for improving future risk-adjustment models.
Therefore, we sought to estimate the prevalence and variation of prevalence of CCCs among children admitted to U.S. PICUs, using a multi-institutional dataset. We subsequently sought to determine if having any CCC and which CCC subcategories were associated with increased risk of PICU mortality and prolonged length of stay (LOS) above that predicted by commonly-used risk-adjustment models. Finally, we sought to determine if inclusion of CCC into risk-adjustment models improved prediction of PICU mortality at the unit level, as measured by standardized mortality ratios (SMRs).
Material and Methods
Data Source and Cohort Selection
We performed a retrospective cohort study of consecutive patients <21 years of age admitted in 2008 to 70 U.S. PICUs in 32 states that participated in the Virtual Pediatric Intensive Care Unit Performance System (VPS, LLC, Alexandria, VA). VPS is a collaboration between the National Association of Children's Hospitals and Related Institutions, National Outcomes Center of Children's Hospital and Health System in Wisconsin, and Children's Hospital Los Angeles. It contains encounter-level information entered by VPS-trained persons at the individual sites. Annual certification of data definitions, routine inter-rater reliability testing, and automated and manual data cleaning queries ensure data quality. Upon approval of our research proposal, data was provided by VPS. No endorsement or editorial restriction of the interpretation of these data or opinions of the authors have been implied or stated. University of California, San Francisco Committee on Human Subjects approved the study.
Patients were excluded if their sites did not submit data for variables selected for the final regression models. To look for clinically relevant selection bias in our sample, included and excluded patients were compared across characteristics, and substantial differences were described.
Chronic Conditions
The original definition for CCC was developed by Feudtner et al (1): “Any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or 1 organ system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.” This definition was accompanied by a now widely-used list of International Classification of Diseases, 9th revision (ICD-9) codes of diagnoses that they determined met this definition (17). However, in reviewing this diagnoses list, we found that there were diagnoses that were missing (eg, diabetes mellitus and primary pulmonary hypertension), some that were not complex (eg, benign adenomas and fibromas), or not chronic (eg, premature atrial contractions and ventricular tachycardia). For these reasons, all VPS diagnoses were reviewed by a group of blinded experts, which included researchers of children with CCC, pediatric intensivists, a pediatric cardiac intensivist, a pediatric oncologist/hematologist, and a rheumatologist to determine which met Feudtner et al's original definition. The resulting modified list was used in our analyses, and a comparison of this list to Feudtner's is provided in the Appendix. In order to ensure capture of chronic conditions, when a patient had an acute condition or status code that necessarily implied an underlying chronic condition (eg, tracheostomy complication), the patient was counted as having a chronic condition. The remaining diagnoses that could be expected to last more than twelve months but not meet the additional CCC criteria were designated as NCCC. To be consistent with Feudtner's CCC subcategories, chronic conditions were further grouped into nine organ systems (cardiovascular, respiratory, neuromuscular, congenital/genetic abnormalities, oncologic, metabolic/endrocrinologic, renal, gastrointestinal, and hematologic/immunologic). A tenth miscellaneous subcategory was created to incorporate rheumatologic, orthopedic, and psychiatric conditions.
Study Design and Variables
Hierarchical mixed-effects logistic regression models for PICU mortality and prolonged PICU stay with random effects at the unit-level were fitted using the VPS cohort to estimate the association between predictor and outcome variables.
The primary predictor was the presence of a chronic condition, stratified into three levels of complexity: CCC, NCCC, and no chronic condition. Other potential independent variables included PIM2 score for severity-of-illness on admission, age, gender, race, elective versus non-elective admission, peri-operative status, trauma status, and having a previous PICU admission. Operative and elective statuses were excluded from the models to avoid collinearity, as they are included in PIM2. Age was converted into an ordinal variable (0–3 months, 3–24 months, >2 years of age), as there are age specific effects for different CCCs.
The primary outcome was death during PICU admission. A dichotomous variable was created for the secondary outcome, prolonged PICU LOS, in order to provide odds ratios (OR). Prolonged LOS was defined as continuous care in a PICU for > 95th percentile of the entire cohort's LOS (18). LOS in days was calculated by subtracting the PICU admission date and time from the PICU discharge date and time.
Statistical Analysis
Patients were stratified by chronic condition status. Patients who had both a CCC and a NCCC were analyzed in the CCC group. When patients had multiple PICU admissions, these admissions were analyzed as unique encounters.
Variables were excluded if they were collinear (spearman rho >0.75) with chronic condition status or severity-of-illness scores. Demographic and clinical characteristics were presented as proportions or medians and interquartile ranges (IQR). Comparisons of these characteristics between groups were performed using Pearson's chi-squared test or Kruskal-Wallis rank test, as appropriate.
Mixed-effects logistic regression models, clustered by PICU site, were used to estimate the association between predictors and outcomes, presented as adjusted odds ratios with 95% confidence intervals (CI). Predictors for the final regression models were included if their P-value was <0.2 and were not collinear with other variables, although age and gender were retained as important clinical covariates. To assess model accuracy, C-statistics with 95% CI were calculated. The impact of adding CCC on accuracy was assessed using a chi-squared test of equality. Hosmer-Lemeshow statistics were calculated to evaluate calibration. Clinically relevant interactions between chronic condition status and other variables were explored and described if significant.
In subanalyses, to evaluate the relevance of number of chronic conditions, regressions were performed using a variable for the number of stratified chronic conditions (ie, no chronic condition, one NCCC, >2 NCCC, one CCC, and >2 CCC). In addition, we used PRISM III scores in lieu of PIM2 in order to explore significant differences when this scoring system is used to risk-adjust severity-of-illness in a mortality model. Finally, a subanalysis using Feudtner's original list of diagnoses was performed to determine the sensitivity of results to different CCC diagnoses lists.
Finally, to explore whether greater inclusion of CCC improves comparison of observed versus expected PICU mortality across units, we estimated SMR and 95% CI for each unit using PIM2 alone and plus the CCC subcategories that statistically significant contributed to risk predictions for individual patients. SMRs were calculated by dividing the mean observed unit mortality by the mean predicted mortality rate, as determined by the risk-adjustment model. The difference between the SMRs determined by the two models was assessed using a Bland-Altman method of comparison (19).
Stata 11 (StataCorp LP, College Station, TX) was used for statistical analyses.
Results
Chronic Conditions Stratification and Prevalence
In 2008, 70 PICUs submitted data to the VPS database on 67,629 admissions. Sixteen (23%) units were excluded because they did not supply the requisite data for analysis (Figure 1). Ultimately, 52,791 (78%) admissions from 54 (77%) units composed our study cohort.
Figure 1.
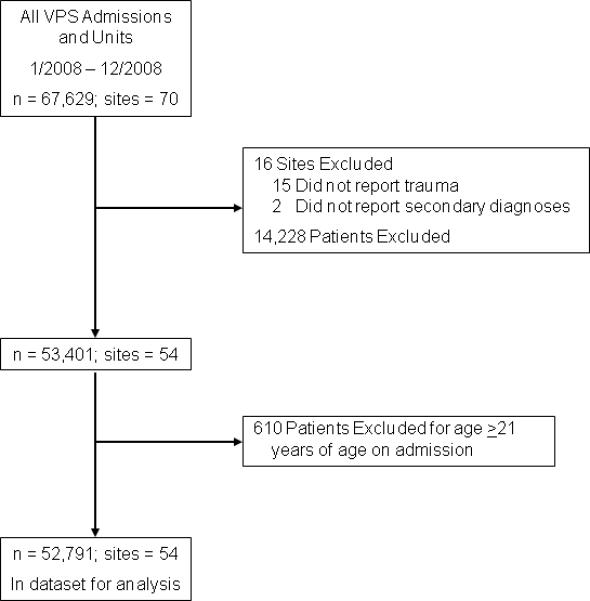
Flow chart of included and excluded PICU sites and patients
There were no statistically significant differences between included and excluded patients in terms of gender, age, elective/operative status, PIM2 score, LOS, or mortality. Compared to excluded patients, included patients were more likely to have been admitted electively (30% v. 24%).
The demographic and clinical characteristics of the study patients, stratified by chronic condition status, are presented in Table 1. Fifty-three percent of the cohort had a CCC; 18.5% had a NCCC. The prevalence of chronic conditions in patients admitted to PICUs varied considerably across the 54 units and subcategories (Table 2).
Table 1.
Patient characteristics by chronic condition status
| Characteristic, % (95% CI) | No Chronic Condition n=15,146 | Non-Complex Chronic Condition n=9,777 | Complex Chronic Condition n=27,868 |
|---|---|---|---|
| Total admissions | 28.7 (28.3–29.1) | 18.5 (18.2–18.9) | 52.8 (52.4–53.2) |
| Age, months, median (IQR)* | 33 (6–135) | 63 (15–156) | 59 (12–152) |
| Male sex* | 58.6 (57.8–59.4) | 55.6 (54.6–56.6) | 54.5 (54–55.1) |
| Race1 | |||
| Caucasian* | 50.7 (49.8–51.6) | 50.6 (49.5–51.7) | 57.3 (56.7–57.9) |
| African American* | 16.1 (15.5–16.7) | 23.5 (22.6–24.4) | 16.2 (15.7–16.7) |
| Hispanic* | 20 (19.3–20.7) | 15.3 (14.5–16.1) | 16.3 (15.8–16.7) |
| Asian/Indian/Pacific Islander | 2.9 (2.6–3.2) | 2.8 (2.4–3.1) | 2.8 (2.6–3) |
| American Indian | 0.7 (0.5–0.8) | 0.5 (0.4–0.7) | 0.5 (0.4–0.6) |
| Other/Mixed* | 5 (4.6–5.4) | 3.9 (3.5–4.3) | 4 (3.8–4.3) |
| Unspecified† | 4.7 (4.3–5.1) | 3.4 (3–3.8) | 2.9 (2.7–3.3) |
| No. chronic diagnoses | |||
| 1 diagnosis | – | 88.1 (87.5–88.8) | 71.4 (70.8–71.9) |
| 2 diagnoses | – | 11.9 (11.2–12.5) | 28.6 (28.1–29.2) |
| Organ categories of chronic conditions2 | |||
| Cardiovascular | – | 7.4 (7.2–7.6) | 11.5 (11.3–11.8) |
| Respiratory | – | 11.6 (11.3–11.8) | 7.7 (7.5–7.9) |
| Neuromuscular | – | 2.7 (2.6–2.8) | 19.3 (18.9–19.6) |
| Congenital/Genetic | – | 4.1 (3.9–4.3) | 5.1 (4.9–5.3) |
| Oncologic | – | 1 (0.9–1.1) | 7.8 (7.6–8) |
| Metabolic/Endocrinologic | – | 0.7 (0.6–0.8) | 8.9 (8.7–9.2) |
| Renal | – | 0.8 (0.7–0.8) | 1.9 (1.8–2) |
| Gastrointestinal | – | 0.5 (0.5–0.6) | 6.2 (6–6.4) |
| Hematologic/Immunologic | – | – | 4 (3.8–4.2) |
| Other | – | 7.9 (7.7–8.1) | 1 (0.9–1.1) |
| Nonelective admission* | 90.1 (90.4–91.4) | 59.5 (58.5–60.5) | 62.1 (61.5–62.7) |
| Pre-/Post-operative* | 21 (20.4–21.7) | 46.2 (5.2–47.2) | 47.5 (46.9–8.1) |
| Origin† | |||
| OR/PACU/Procedure suite | 15.4 (14.8–15.9) | 44.1 (43.2–45.1) | 41.1 (40.5–41.7) |
| Emergency Department | 67.5 (66.8–68.3) | 42.1 (41.1–43) | 34.3 (33.8–34.9) |
| General ward | 12.3 (11.7–12.8) | 9.4 (8.8–10) | 14.4 (14–14.8) |
| Another ICU | 1.6 (1.4–1,8) | 1.6 (1.4–1.9) | 3.8 (3.6–4) |
| Intermediate unit | 1.5 (1.3–1.7) | 1.1 (0.9–1.3) | 2.7 (2.5–2.9) |
| Chronic/Rehab facility | 0.1 (0.1–0.2) | 0.1 (0–0.2) | 0.4 (0.3–0.4) |
| Outpatient/Home | 1.3 (1.1–1.5) | 1.5 (1.2–1.7) | 2.9 (2.7–3.1) |
| Other | 0.3 (0.2–0.4) | 0.1 (0.1–0.2) | 0.4 (0.3–0.4) |
| Reason for admission3 | |||
| Respiratory* | 35.4 (34.6–36.2) | 45.6 (44.6–46.6) | 27.3 (26.8–27.9) |
| Neurologic* | 31.9 (31.1–32.7) | 14.9 (14.1–15.7) | 21.7 (21.2–22.2) |
| Cardiovascular/Shock* | 9.6 (9.1–10.1) | 17.2 (16.4–18) | 21 (20.6–21.5) |
| Metabolic* | 3.8 (3.5–4.1) | 1.2 (1–1.4) | 10.9 (10.6–11.3) |
| Hemorrhage/Coagulopathy* | 1 (2.7–3.3) | 1 (0.8–1.2) | 2.3 (2.1–2.4) |
| Procedure | 0.9 (0.7–1.1) | 0.9 (0.7–1.1) | 1.1 (1–1.2) |
| Other* | 15.4 (14.8–16) | 19.2 (18.4–20) | 15.6 (15.2–16.1) |
| Trauma* | 25.7 (25–26.4) | 3.4 (3–3.7) | 2.3 (2.1–2.5) |
| Disposition* | |||
| General ward | 67.3 (66.5–68) | 67.8 (66.7–68.6) | 60.9 (60.3–61.5) |
| Another ICU | 1.4 (1.2–1.6) | 1.5 (1.3–1.7) | 2.6 (2.5–2.8) |
| Intermediate unit | 9.9 (9.4–10.4) | 11.4 (10.7–12) | 15.8 (15.3–16.2) |
| OR | 0.5 (0.4–0.6) | 0.1 (0.1–0.2) | 0.5 (0.4–0.6) |
| Chronic/Rehab facility | 0.6 (0.4–0.7) | 0.4 (0.3–0.5) | 1.3 (1.2–1.5) |
| Home | 17.2 (16.6–17.8) | 16.7 (16–17.5) | 14.5 (14.1–14.9) |
| Morgue/Medical examiner | 2.2 (2–2.5) | 0.3 (0.2–0.4) | 3.9 (3.6–4.1) |
| Other | 1 (0.8–1.1) | 1.9 (1.6–2.2) | 0.5 (0.4–0.6) |
| PIM2 risk of mortality, %, median (IQR)* | 0.9 (0.8 – 1.6) | 0.3 (0.2 – 1) | 1 (0.3 – 3.2) |
| PICU mortality* | 2.2 (2–2.5) | 0.3 (0.2–0.4) | 3.9 (3.6–4.1) |
| LOS, days, median (IQR)* | 1.4 (0.8 – 3) | 1.2 (0.9 – 2.3) | 1.9 (1 – 4.8) |
| LOS > 15 days* | 3.1 (2.8–3.3) | 1.6 (1.3–1.8) | 7.4 (7–7.7) |
P < 0.01;
P < 0.001 by Pearson's chi-squared test or Kruskal-Wallis rank test
CI, confidence interval; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; OR, operating room; PACU, post-anesthesia care unit; PIM, Pediatric Index of Mortality
Based on 45,412 (80%) admissions of patients from 43 sites that supplied race
Percentages sum to over 100% because some patients had multiple conditions in different organ categories
Based on 48,641 (92%) admissions of patients from 49 sites that supplied reason for admission
Table 2.
Prevalences of chronic conditions in patients admitted to 54 U.S. PICUs
| Condition | Median % | IQR | Range |
|---|---|---|---|
| Any NCCC | 17.9 | 15.5 – 20.9 | 8.9 – 33.2 |
| Any CCC | 49.7 | 40.4 – 58.7 | 22.4 – 70.6 |
| Cardiovascular | |||
| NCCC | 7.3 | 3.5 – 10 | 1 – 14 |
| CCC | 8.8 | 4.7 – 16.9 | 0.9 – 26.9 |
| Respiratory | |||
| NCCC | 10.1 | 6.5 – 13.3 | 2.3 – 26.9 |
| CCC | 7 | 4.9 – 9.8 | 1.5 – 24.2 |
| Neuromuscular | |||
| NCCC | 2.4 | 1.6 – 3.2 | 0 – 7.2 |
| CCC | 17.3 | 14 – 22.6 | 4.8 – 31.9 |
| Congenital/Genetic | |||
| NCCC | 3.1 | 1.6 – 5 | 0.4 – 10 |
| CCC | 4.2 | 2.3 – 6.1 | 0 – 12.1 |
| Oncologic | |||
| NCCC | 0.7 | 0.5 – 1 | 0 – 3.5 |
| CCC | 6.6 | 5.1 – 8.6 | 0.3 – 17.4 |
| Metabolic/Endocrinologic | |||
| NCCC | 0.4 | 0.2 – 0.9 | 0 – 4.9 |
| CCC | 7.9 | 6.5 – 11.8 | 2.1 – 20 |
| Renal | |||
| NCCC | 0.4 | 0.1 – 0.7 | 0 – 4.1 |
| CCC | 1.5 | 0.7 – 2.5 | 0 – 9.9 |
| Gastrointestinal | |||
| NCCC | 0.4 | 0 – 0.6 | 0 – 2.3 |
| CCC | 4 | 1.8 – 9.4 | 0 – 33.6 |
| Hematologic/Immunologic | |||
| NCCC | – | – | – |
| CCC | 3 | 1.8 – 4.1 | 0.2 – 14.2 |
| Other | |||
| NCCC | 6.5 | 4.7 – 11.3 | 0 – 19.4 |
| CCC | 0.4 | 0 – 0.6 | 0 – 1.1 |
CCC, complex chronic condition; IQR, interquartile range; NCCC, non-complex chronic condition
PICU Mortality
The incidence of PICU mortality was 2.7% (1,448 deaths). The majority of these deaths, 1,078 (74%), were children with CCC. Admission type (elective or not; peri-operative status) and having a previous PICU admission were excluded due to collinearity with chronic condition status or PIM2. In multivariable analyses, race and reason for admission were not significant covariates. The adjusted odds ratios for the included predictor variables derived from the model for PICU mortality using chronic conditions stratified by complexity and organ subcategories are presented in Table 3. Except for respiratory, gastrointestinal, and other conditions, CCC in every category had significant and greater odds of PICU mortality than their NCCC counterparts. The risk of PICU mortality varied considerably between different organ system categories. The C-statistic for our mortality model was 0.905 (95% CI 0.897–0.913), indicating excellent accuracy. C-statistics for models adjusting for PIM2 alone and plus gender, age, and trauma were 0.888 (95% CI 0.879–0.897). The test of equality between these models was P<0.0001, indicating adding CCC resulted in a significant change in accuracy. The P-value of the Hosmer-Lemeshow statistic was 0.07, indicating acceptable calibration.
Table 3.
Adjusted odds ratios for predictor variables in hierarchical logistic models of PICU mortality and prolonged length of stay (> 15 days)
| Variable | Mortality |
Prolonged LOS |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Chronic conditions (reference is no chronic condition) | ||||
| Cardiovascular | ||||
| NCCC | 0.71 (0.55–0.93) | 0.013 | 1.55 (1.34–1,79) | <0.001 |
| CCC | 1.25 (1.05–1.48) | 0.013 | 2.4 (2.2–2.7) | <0.001 |
| Respiratory | ||||
| NCCC | 1.05 (0.77–1.42) | 0.76 | 0.89 (0.73–1.08) | 0.24 |
| CCC | 0.86 (0.69–1.08) | 0.19 | 2 (1.77–2.3) | <0.001 |
| Neuromuscular | ||||
| NCCC | 0.97 (0.65–1.45) | 0.89 | 1.28 (1–1.62) | 0.047 |
| CCC | 1.49 (1.28–1.74) | <0.001 | 1.72 (1.55–1.91) | <0.001 |
| Congenital/genetic abnormality | ||||
| NCCC | 1.09 (0.73–1.63) | 0.67 | 0.83 (0.65–1.06) | 0.14 |
| CCC | 1.44 (1.13–1.84) | 0.003 | 1.31 (1.13–1.52) | <0.001 |
| Oncologic | ||||
| NCCC | 1.89 (0.94–3.8) | 0.07 | 1.52 (0.99–2.3) | 0.053 |
| CCC | 2.6 (2.2–3.2) | <0.001 | 2.1 (1.79–2.4) | <0.001 |
| Metabolic/endocrinologic | ||||
| NCCC | 1.08 (0.43–2.7) | 0.87 | 1.59 (0.99–2.7) | 0.075 |
| CCC | 2.1 (1.74–2.6) | <0.001 | 1.66 (1.45–1.91) | <0.001 |
| Renal | ||||
| NCCC | 0.95 (0.42–2.2) | 0.91 | 0.91 (0.57–1.45) | 0.69 |
| CCC | 1.88 (1.32–2.7) | <0.001 | 1.66 (1.31–2.1) | <0.001 |
| Gastrointestinal | ||||
| NCCC | 0.83 (0.28–2.5) | 0.75 | 1.79 (1.16–2.8) | 0.008 |
| CCC | 1.2 (0.96–1.5) | 0.11 | 1.76 (1.54–2) | <0.001 |
| Hematologic/immunologic | ||||
| NCCC | – | – | – | – |
| CCC | 2.9 (2.4–3.6) | <0.001 | 1.71 (1.46–2) | <0.001 |
| Other | ||||
| NCCC | 0.85 (0.7–1.03) | 0.29 | 1.12 (0.94–1.35) | 0.19 |
| CCC | 1.42 (0.79–2.6) | 0.25 | 1.62 (1.09–2.4) | 0.018 |
| Age | ||||
| 0–3 months (reference) | ||||
| 3–24 months | 0.85 (0.7–1.04) | 0.11 | 0.61 (0.54–0.69) | <0.001 |
| > 2 years | 0.75 (0.62–0.9) | 0.003 | 0.37 (0.33–0.42) | <0.001 |
| Male gender | 0.84 (0.74–0.95) | 0.005 | 1.02 (0.94–1.11) | 0.59 |
| Trauma | 1.5 (1.22–1.85) | <0.001 | 0.97 (0.82–1.15) | 0.74 |
| PIM2 score | 2.6 (2.5–2.7) | <0.001 | 1.5 (1.47–1.54) | <0.001 |
CCC, complex chronic condition; LOS, length of stay; NCCC, non-complex chronic condition; OR, odds ratios; PIM, Pediatric Index of Mortality
When number of chronic conditions was analyzed, having one NCCC was associated with a 66% lower odds of PICU mortality (OR 0.34; 95% CI 0.22–0.53, P<0.001) compared to children with no chronic condition, adjusting for age, gender, trauma, and severity-of-illness. Having >2 NCCC was not associated with PICU mortality (OR 0.9; 95% CI 0.39–2.1, P=0.81) compared to children with no chronic condition. Increasing number of CCCs was associated with increasing odds of PICU mortality compared to having no chronic condition—the adjusted odds ratio for one CCC was 1.7 (95% CI 1.43–2) and 2.9 (95% CI 2.4–3.5) for >2 CCC.
In our subanalysis using PRISM III, 41,583 (61%) patients from 39 (56%) units were analyzed. The associations were not significantly different than the PIM model (Supplemental Table 4), except for the magnitude for some CCC subcategories' odd ratios. Only gastrointestinal CCC were newly associated with PICU mortality.
In the subanalysis using Feudtner's CCC list, 29,280 (55.5%) of admissions were identified as having a CCC. When the association of CCC subcategories to PICU mortality were compared using Feudtner's and our modified CCC lists, some significant differences were found (Supplemental Table 5). Specifically, using our list, cardiovascular and neuromuscular CCC were associated with PICU mortality, and gastrointestinal CCC were not.
SMRs for the 54 units are presented in Figure 2. There was little difference between SMRs adjusting for PIM2 score and those adjusting for PIM2 and statistically significant CCC (cardiovascular, neuromuscular, oncologic, metabolic/endocrinologic, renal, and hematologic/immunologic). On Bland-Altman analysis, the mean difference between SMRs was <0.001 (95% limit of agreement −0.06 to 0.06).
Figure 2.
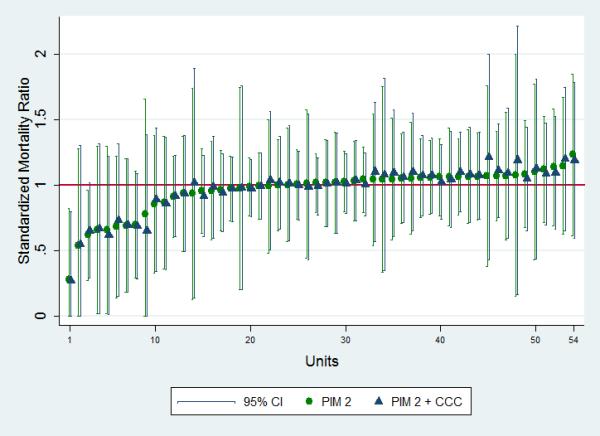
Standardized Mortality Ratios and 95% confidence intervals of 54 PICUs adjusting for PIM2 scores and PIM2 scores plus four high-risk CCC subcategories
Length of Stay
For our secondary outcome of prolonged PICU LOS, the 95th percentile of physical LOS for the entire cohort was 15 days. Children with CCC had 77% of admissions with LOS >15 days.
The adjusted OR for prolonged LOS derived from the regression model for prolonged LOS are presented in Table 3. CCC in every organ category had greater odds of prolonged LOS than their NCCC counterparts, except those in the miscellaneous category. Among NCCC, only cardiovascular, neuromuscular, and gastrointestinal subcategories had increased odds of prolonged LOS, compared to having no chronic condition. The C-statistic for our LOS model was 0.81 (95% CI 0.8–0.82); the Hosmer-Lemeshow statistic P value was 0.26.
Having one NCCC was weakly associated with decreased odds of prolonged LOS (OR 0.81, 95% CI 0.66–0.99; P=0.043), compared to having no chronic condition. Having >2 NCCC was not associated with prolonged LOS (P=0.51). As with mortality, having more CCCs was associated with increasing odds of prolonged LOS. The adjusted odds ratios were 2.1 (95% CI 1.83–2.3) when a patient had one CCC compared to having no chronic condition and 4.6 (95% CI 4–5.2) for >2 CCC.
No statistically significant interactions were found between chronic condition status and other covariates in the models for either outcome.
Discussion
Children with CCC are recognized as having on-going, substantial healthcare needs (3). While they are a relatively small proportion of children in the U.S., they are highly represented among hospitalized children (5–11, 15). Using a multi-institutional database, we demonstrated that children with chronic conditions comprised more than 70% of PICU admissions, and those with CCC comprised just over one-half. We demonstrated that patients with CCC have significant risk for PICU mortality and prolonged LOS beyond that predicted by commonly-used severity-of-illness models. Finally, we showed that the accuracy of predicting PICU mortality was significantly improved with the inclusion of CCC and other covariates, compared to adjusting for severity-of-illness alone, as indicated by a higher C-statistic and significant test of equality.
Earlier studies had not estimated the prevalence of children with CCC admitted to U.S. PICUs. Using 2004–2005 VPS data, Typpo and colleagues found that 52% of children admitted to 28 PICUs had a chronic condition, but the distinction between CCC and NCCC was not examined (20). Using the 2006 Kids' Inpatient Database, Odetola et al. showed that 41% of children with respiratory failure or need for mechanical ventilation possessed a CCC, but they were not able to identify which children were in a PICU or assess critical illnesses other than respiratory failure (15). Our prevalence estimates are the first to be based on an U.S. multi-institutional cohort, to stratify chronic conditions into two levels of complexity, and to show that the prevalence of these conditions and their subcategories varied across PICUs.
Previous studies have addressed the association between chronic conditions and mortality. Feudtner et al. examined all pediatric hospitalizations in Pennsylvania over multiple years and estimated the association between hospital mortality and different CCC subcategories (21). However, they could not distinguish between PICU and non-PICU patients, and they used a proprietary, infrequently-used severity-of-illness risk-adjustment. Odetola et al. did not risk-adjust but only compared mortality of children with CCC to those without chronic conditions (15). Typpo et al. found an association between PICU mortality and having >3 chronic conditions. While they examined organ subcategories and controlled for acute organ dysfunction, they did not examine CCC nor incorporate a commonly-used mortality risk-adjustment (20). In order to address these limitations we: a) focused exclusively on PICU patients in a large database; b) used common and valid severity-of-illness risk-adjustment systems; and c) distinguished between chronic conditions by level of complexity and by organ system. We found that children with any CCC, except respiratory, had greater odds of PICU mortality compared to children with no chronic condition, after controlling for severity-of-illness. Importantly, the magnitude of risk varied across organ system subcategories (ranging from an OR of 1.2 for gastrointestinal [P=NS] to 2.9 [P<0.001] for hematologic/immunologic). All patients with CCC had significantly greater odds of prolonged LOS. Finally, no NCCC subcategories were associated with greater risk of PICU mortality, but some were with prolonged LOS, compared to children with no chronic conditions. Cardiovascular NCCC were associated with statistically lower odds of PICU mortality, compared to no chronic conditions.
The independent association of CCC with PICU mortality demonstrates that many CCC confer a significant risk to patients above that predicted by PIM and PRISM. Developed in 2003 and 1996, respectively, PIM2 and PRISM III incorporate few chronic conditions. PIM2 adjusts for seven high-risk diagnoses, including neuro-degenerative disorders, certain immunodeficiencies, certain cardiovascular conditions, leukemia, and lymphoma (12). PRISM III adjusts for chromosomal abnormalities and oncologic conditions (13). However, when we estimated SMRs using PIM2 with and without statistically significant CCC subcategories, there was no substantive difference. The lack of difference may reflect our sample containing mostly tertiary PICUs, which care for similarly ill and complex patients. Therefore, institutions were similarly affected by the increased risk of mortality. Further research should address whether CCC rates are lower at community PICUs and whether inclusion of CCCs into risk-adjustment models has more impact when a more diverse sample of PICUs is evaluated.
Our study has a number of limitations. First, chronic conditions often have a range of severity, sequelae, and chronicity that is often not captured in diagnosis coding. Admittedly, this results in some subjectivity inherent in labeling CCC. Nonetheless, identifying children with CCC through diagnoses codes has become an accepted approach. Originally, Feudtner and colleagues constructed their list of CCC using clinical knowledge and a review of Washington State death certificates (1), and this list has become widely used (5–7, 15, 22). However, we felt there were some weaknesses in this list that warranted modification. More specifically, Feudtner's list contains diagnoses that our expert panel assessed to be not complex (eg, benign tumors and tics) or not chronic (eg, ventricular fibrillation), assuming patients receive the standard of care. In addition, there were several CCC that were missing, including several hematologic and rheumatologic conditions, such as hemophilia and systemic lupus erythematosus. Thus, using their original definition of a CCC, we developed a modified list from the available VPS diagnoses (Appendix) and used it in our analyses. Overall, there is considerable agreement between the two lists, with a few notable exceptions and important additions in our list.
Some of the differences in the measures of association in the PICU mortality model when Feudtner's CCC list and our modified list were used are readily explainable. Using the modified list, cardiovascular and neuromuscular CCCs were associated with increased PICU mortality. These differences are likely attributed to a more discriminatory cardiac diagnosis list that excluded several non-chronic and non-complex conditions and a more expansive list of neuromuscular diagnoses that met Feudtner's CCC definition. Also, using the modified list, gastrointestinal CCCs were not associated with PICU mortality. The only difference from Feudtner's in the modified list was the inclusion of status codes for ostomies and liver transplant, suggesting these conditions may not increase risk for PICU mortality beyond current severity-of-illness adjustments.
Other limitations include a lack of institutional characteristics in our models. Besides annual PICU volume, we were not provided with institutional characteristics. However, hierarchical regression models take into account that subjects receiving care at the same hospitals should not be treated as independent observations, rather they are “clustered within” institutions. In addition, our study is limited to PICU outcomes only, as VPS does not contain information on pre- or post-PICU care. Thus, we could not analyze other important outcomes, such as hospital mortality or LOS. Finally, besides the primary diagnosis, which by VPS definition is present at time of PICU admission, there was no designation of the timing of onset of chronic conditions in relation to admission. VPS diagnoses did not have a “present on admission” flag. So while all diagnoses pertinent to the patient's ICU course were required to be documented, it is conceivable that a small number of conditions first manifested during the admission. However, we were examining chronic conditions, which manifest over time and are unlikely to first appear during a PICU admission.
Conclusions
PICUs are a locus of care for children with CCC and are the likely place of many of their deaths. Knowing the prevalence of CCC in children admitted to PICUs and their contribution to PICU mortality and LOS aids in understanding the impact of CCC on these children and on acute care resources. Our findings add credence to assertions that greater efforts should be directed towards identifying and addressing the needs of children with CCC in order to minimize this impact. For PICU mortality benchmarking, additional inclusion of high-risk CCC subcategories into future risk-adjustment models would improve model accuracy compared to currently-used models, but may lead to similar comparisons of observed versus expected PICU mortality across units that care for children with CCC.
Supplementary Material
Acknowledgments
We thank Jay Berry, MD, MPH, Steven Dubois, MD, David Moromisato, MD, and Jinoos Yazdany, MD, MPH, for their assistance in identifying complex chronic conditions. We thank John Boscardin, PhD, for reviewing our statistical methods and analysis. We also thank the Virtual Pediatric Intensive Care Unit Performance System for providing the data for this study and, especially, Christine Gall, MS, RN, of the National Outcomes Center for all her assistance.
This study was performed at the University of California, San Francisco.
Funding/Support: Dr. Edwards is supported by a National Institutes of Health K12 HD 047349. Dr. Houtrow is supported by a National Institutes of Health K12 H001097-12. Dr. Vasilevskis is supported by a National Institutes of Health K23 AG040157. Dr. Vasilevskis also receives support from the Geriatric Research, Education and Clinical Center (GRECC) and the Clinical Research Training Center of Excellence, Department of Veterans Affairs Medical Center, Tennessee Valley Healthcare System, Nashville, TN.
Abbreviations
- CCC
complex chronic condition
- CI
confidence interval
- ICD
International Classification of Diseases
- IQR
interquartile range
- LOS
length of stay
- OR
odds ratio
- NCCC
non-complex chronic condition
- PIM
Pediatric Index of Mortality
- PICU
pediatric intensive care unit
- PRISM
Pediatric Risk of Mortality score
- PPV
positive pressure ventilation
- SMR
standardized mortality ratio
- VPS
Virtual Pediatric Intensive Care Unit Performance System
Footnotes
Conflict of Interest Disclosures: The authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106(1 Pt 2):205–209. [PubMed] [Google Scholar]
- 2.Srivastava R, Stone BL, Murphy NA. Hospitalist care of the medically complex child. Pediatr Clin North Am. 2005;52:1165–1187. x. doi: 10.1016/j.pcl.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SK, Simon TD, Srivastava R. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127:529–538. doi: 10.1542/peds.2010-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feudtner C, Feinstein JA, Satchell M, Zhao H, Kang TI. Shifting place of death among children with complex chronic conditions in the United States, 1989–2003. JAMA. 2007;297:2725–2732. doi: 10.1001/jama.297.24.2725. [DOI] [PubMed] [Google Scholar]
- 5.Benneyworth BD, Gebremariam A, Clark SJ, Shanley TP, Davis MM. Inpatient health care utilization for children dependent on long-term mechanical ventilation. Pediatrics. 2011;127:e1533–41. doi: 10.1542/peds.2010-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns KH, Casey PH, Lyle RE, Bird TM, Fussell JJ, Robbins JM. Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126:638–646. doi: 10.1542/peds.2009-1658. [DOI] [PubMed] [Google Scholar]
- 7.Simon TD, Berry J, Feudtner C, Stone BL, Sheng X, Bratton SL, Dean JM, Srivastava R. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126:647–655. doi: 10.1542/peds.2009-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremer R, Leclerc F, Lacroix J, Ploin D, GFRUP/RMEF Chronic Diseases in PICU Study Group Children with chronic conditions in pediatric intensive care units located in predominantly French-speaking regions: Prevalence and implications on rehabilitation care need and utilization. Crit Care Med. 2009;37:1456–1462. doi: 10.1097/CCM.0b013e31819cef0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham RJ, Dumas HM, O'Brien JE, Burns JP. Congenital neurodevelopmental diagnoses and an intensive care unit: defining a population. Pediatr Crit Care Med. 2004;5:321–328. doi: 10.1097/01.pcc.0000128892.38431.2b. [DOI] [PubMed] [Google Scholar]
- 10.Briassoulis G, Filippou O, Natsi L, Mavrikiou M, Hatzis T. Acute and chronic paediatric intensive care patients: current trends and perspectives on resource utilization. QJM. 2004;97:507–518. doi: 10.1093/qjmed/hch087. [DOI] [PubMed] [Google Scholar]
- 11.Namachivayam P, Shann F, Shekerdemian L, Taylor A, van Sloten I, Delzoppo C, Daffey C, Butt W. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med. 2010;11:549–555. doi: 10.1097/PCC.0b013e3181ce7427. [DOI] [PubMed] [Google Scholar]
- 12.Slater A, Shann F, Pearson G. Paediatric Index of Mortality (PIM) Study Group. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 13.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Marcin JP, Pollack MM. Review of the acuity scoring systems for the pediatric intensive care unit and their use in quality improvement. J Intensive Care Med. 2007;22:131–140. doi: 10.1177/0885066607299492. [DOI] [PubMed] [Google Scholar]
- 15.Odetola FO, Gebremariam A, Davis MM. Comorbid illnesses among critically ill hospitalized children: Impact on hospital resource use and mortality, 1997–2006. Pediatr Crit Care Med. 2010;11:457–463. doi: 10.1097/PCC.0b013e3181c514fa. [DOI] [PubMed] [Google Scholar]
- 16.Feudtner C, Christakis DA, Zimmerman FJ, Muldoon JH, Neff JM, Koepsell TD. Characteristics of deaths occurring in children's hospitals: implications for supportive care services. Pediatrics. 2002;109:887–893. doi: 10.1542/peds.109.5.887. [DOI] [PubMed] [Google Scholar]
- 17.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:E99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 18.Marcin JP, Slonim AD, Pollack MM, Ruttimann UE. Long-stay patients in the pediatric intensive care unit. Crit Care Med. 2001;29:652–657. doi: 10.1097/00003246-200103000-00035. [DOI] [PubMed] [Google Scholar]
- 19.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 20.Typpo KV, Petersen NJ, Petersen LA, Mariscalco MM. Children with chronic illness return to their baseline functional status after organ dysfunction on the first day of admission in the pediatric intensive care unit. J Pediatr. 2010;157:108–113. e1. doi: 10.1016/j.jpeds.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feudtner C, Hexem KR, Shabbout M, Feinstein JA, Sochalski J, Silber JH. Prediction of pediatric death in the year after hospitalization: a population-level retrospective cohort study. J Palliat Med. 2009;12:160–169. doi: 10.1089/jpm.2008.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seferian EG, Lackore KA, Rahman AS, Naessens JM, Williams AR. Comparison of chronic illness among children receiving mechanical ventilation in a cohort of children's hospitals in 1991 and 2001. J Pediatr. 2006;149:788–792. doi: 10.1016/j.jpeds.2006.08.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


