ABSTRACT
BACKGROUND
We know little about how much time low-income patients and physicians spend discussing pain during primary care visits.
OBJECTIVE
To measure the frequency and duration of pain-related discussions at a primary care clinic serving mostly low-income black patients; to investigate variables associated with these discussions.
DESIGN
We measured the frequency and duration of pain-related discussions using video-recorded primary care visits; we used multiple regression to evaluate associations between discussions and patient self-report variables.
PARTICIPANTS
A total of 133 patients presenting to a primary care clinic for any reason; 17 family medicine residents.
MAIN MEASURES
Independent variables were pain severity, health status, physical function, chief complaint, and whether the patient and physician had met previously. Dependent variables were presence of pain-related discussions and percent of total visit time spent discussing pain.
KEY RESULTS
Sixty-nine percent of visits included pain-related discussions with a mean duration of 5.9 min (34% of total visit time). Increasing pain severity [OR 1.69, 95% CI (1.18, 2.41)] and pain-related chief complaints [OR 4.10, 95% CI (1.39, 12.12)] were positively associated with the probability of discussing pain. When patients discussed pain, they spent 4.5% more [95% CI (0.60, 8.37)] total visit time discussing pain for every one-point increase in pain severity. Better physical function was negatively associated with the probability of discussing pain [OR 0.65, 95% CI (0.48, 0.86)], but positively associated with the percent of total visit time spent discussing pain [3% increase; 95% CI (0.32, 5.75)] for every one-point increase in physical function). Patients and physicians who had met previously spent 11% less [95% CI (-21.65, -0.55)] total visit time discussing pain. Pain severity was positively associated with time spent discussing pain only when patients and physicians had not met previously.
CONCLUSIONS
Pain-related discussions comprise a substantial proportion of time during primary care visits. Future research should evaluate the relationship between time spent discussing pain and the quality of primary care pain management.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-011-1960-x) contains supplementary material, which is available to authorized users.
KEY WORDS: pain, patient-physician communication, direct observation, time, primary care, patient-physician relationship, black patients
INTRODUCTION
Pain is one of the most common reasons that patients seek primary care1 and is a significant source of lost productivity among Americans.2 Over one-quarter of Americans experience pain on a regular basis.3,4 The prevalence and severity of pain is even greater for patients from vulnerable populations such as veterans,5 racial and ethnic minorities,6,7 and patients with multiple chronic illnesses.8,9 Patients and physicians report that discussions about pain in primary care are often time-consuming, difficult, and frustrating.10–12
However, little information exists about how much time patients and physicians actually spend discussing pain in primary care or about patient characteristics associated with time spent discussing pain. This information could help primary care physicians better prepare for pain-related discussions with their patients. Existing research has not demonstrated a relationship between patients’ reports of pain severity and whether pain is discussed in primary care visits.13,14 Patients are more likely than physicians to prioritize pain management,15 but how patients’ chief complaints influence time spent discussing pain remains unclear.16 Finally, the influence of patient-physician relationships on time spent discussing pain during primary care is unknown,12,16 despite the importance of these relationships in pain management.17,18
One important reason for this knowledge gap is that most studies about pain-related communication use indirect measures such as participant recall and medical records. Such measures provide useful information, but medical records often do not accurately reflect the content of pain-related discussions in primary care,19,20 and participant recall measures are prone to many types of cognitive biases.21,22 Direct observation is not subject to these limitations and so can provide additional information about patient-physician communication.23,24
We directly observed video-recorded primary care visits to measure the frequency and percent of total visit time spent discussing pain. We evaluated visits at a clinic serving predominately low-income, black patients because this population experiences more frequent and more severe pain than the general population.6,7 We investigated whether pain severity, health status, chief complaint, and whether patient and physician had met previously were associated with the presence and/or percent of total visit time spent discussing pain.
METHODS
Study Design and Data Sources
We used data from a previously conducted, IRB-approved study of primary care visits at a Detroit clinic. Participants in this primary study were 17 family medicine residents and 156 patients recruited without regard to medical condition or the presence of pain. Eighty-three percent of physicians and 73% of patients approached agreed to participate. Further details of recruitment are available elsewhere.25,26 All patient participants were established clinic patients but had not necessarily been seen by the physician they saw during the study. Patients completed questionnaires immediately before their visit that included demographic information, an open-ended question about chief complaint (“What was the main reason you saw a doctor today?”), and the Medical Outcomes Study 20-item health survey (SF-20). The SF-20 asks patients to rate their average bodily pain during the past 4 weeks on a 6-point scale (none, very mild, mild, moderate, severe, very severe). It also contains subscales measuring general health, physical function, and mental health.27 Visits were video-recorded using an established method.28 Due to technical problems, only 133 visits were recorded; these visits constitute the sample for our study.
The primary study also measured total visit time (defined as the total time the patient and physician spent in the room together) using The Observer Video-Pro (v.5),29 and collected information about the presence or absence of common chronic illnesses from patients’ medical charts.
Coding of Video Recordings
We watched several video-recorded visits from the primary study and developed a coding system to identify and describe pain-related discussions. We then iteratively applied the coding system and refined it until they could train research assistants to code visits reliably. The final coding system defined pain-related discussions as any mention of physical pain using at least one of the following terms: pain, ache, hurt, sore, burn, or tender. It included discussions of the description, history, diagnosis, and treatment of physical pain. The coding system excluded discussions of other sensations such as itching, numbness, or tingling, and discussions in which patients denied having pain.
The coding system documented the duration of pain-related discussions to the nearest second. Pain-related discussions began with the first mention of pain and ended with the first mention of either a non-pain topic or a different pain. The coding system included discussions of a medical condition causing pain only when pain was the patient’s primary concern related to that condition. For visits that included pain-related discussions, the coding system documented whether the physician and patient had met at least once previously and descriptive information about pain-related discussions (e.g., pain location, who initiated the discussion of pain, and whether the patient and physician had discussed the pain previously). The complete coding manual is available online (Appendix).
We applied our coding system in two steps. First, one author (SGH) watched all visits to identify those that included pain-related discussions. When the presence of a pain-related discussion was uncertain, we discussed and/or watched the relevant visit segments together until we reached consensus about whether a pain-related discussion was present.
Second, we trained three research assistants to apply our coding system to all visits that included pain-related discussions. After training was complete, two of the three research assistants independently coded each visit that included pain-related discussions. Research assistants resolved disagreements about beginning and ending times of less than 4 s in favor of longer discussions. They resolved other disagreements by reviewing the visits together and discussing discrepancies in order to reach consensus.30 One author (SGH) resolved any disagreements that persisted after discussion between coders by reviewing the visit segments in question. That author independently coded 15% of all visits to monitor coder accuracy. For each visit, we summed all pain-related discussions to calculate the total time spent discussing pain and the percent of total visit time spent discussing pain.
Coding of Chief Complaint and Chronic Illness Score
We classified chief complaints collected during the primary study as pain-related when they contained one of the following terms: pain, ache, hurt, sore, burn, or tender. Each author coded the chief complaints independently (agreement = 97%) and resolved disagreements through discussion. We also used data from the primary study to calculate a chronic illness score (range 0–5) for each patient indicating the presence or absence of the five most common chronic illnesses unrelated to pain (diabetes, hypertension, hyperlipidemia, asthma, and heart disease).31–33
Data Analysis
Our primary analyses involved two regressions. First, we conducted a logistic regression with presence of pain-related discussion as the dependent variable. We included chief complaint (pain-related versus not pain-related), pain severity, and the SF-20 subscales for physical function and general health as independent variables. We included total visit time as a covariate, because patients may discuss more topics, including pain, during longer visits. Our patient sample was relatively homogeneous and we had limited sample size, so we included patient demographic variables and chronic illness score as covariates only when they had significant bivariate associations with the dependent variable.
Second, for visits that included pain-related discussions, we conducted a linear regression with the percent of total visit time spent discussing pain as the dependent variable. We included the same independent variables and covariates as in the logistic regression, in addition to whether the patient and physician had met previously. As in the logistic regression, we included patient demographics and chronic illness score as covariates only when they had significant bivariate associations with the dependent variable.
We used the generalized estimating equation (GEE) with robust standard error estimates to account for patients’ being clustered within physicians.34,35 Variables from the primary study contained a small number of missing values (3% for SF-20 measures, 7% for chief complaint), which we imputed using the chained equation method.36,37 Imputation did not meaningfully change our results, so for simplicity we have reported results without imputation. As a sensitivity analysis, we repeated our analyses using a fixed-effects two-level hierarchical model. We checked regression assumptions by inspecting observed-expected tables (logistic regression) and residual plots (linear regression). We performed analyses using Stata 11.1 (College Station, TX).
RESULTS
Table 1 reports baseline participant and visit characteristics. Most patients (98.5%) self-identified as black. Median annual income was less than $30,000; median education was 1 to 2 years of college. Over 80% of physicians reported their ethnicity as Asian or Indian/Pakistani and were international medical graduates.
Table 1.
Participant Characteristics
| All visits | Pain discussed | Pain not discussed | ||
|---|---|---|---|---|
| Patient characteristics | (n = 133) | (n = 92) | (n = 41) | p value* |
| Demographics | ||||
| Male (%) | 24.1 | 21.7 | 29.3 | p = 0.35 |
| Mean age [years] (SD) | 44.0 (14.1) | 44.2 (14.0) | 43.5 (14.5) | p = 0.81 |
| Black race (%) | 98.5 | 97.8 | 100 | p = 0.82 |
| Highest education (%) | ||||
| < High school diploma | 29.0 | 24.4 | 39.0 | p = 0.19 |
| High school diploma | 54.2 | 58.9 | 43.9 | |
| College graduate | 16.8 | 16.7 | 17.1 | |
| Annual income (%) | ||||
| <$10,000 | 28.7 | 32.6 | 20.0 | p = 0.65 |
| $10,000–$49,999 | 55.1 | 52.8 | 60.0 | |
| >$50,000 | 15.5 | 13.5 | 20.0 | |
| SF-20 subscale scores† | ||||
| Pain severity, mean (SD) | 3.8 (1.4) | 4.1 (1.3) | 3.0 (1.5) | p < 0.001 |
| General health, mean (SD) | 14.9 (4.5) | 14.2 (4.4) | 16.4 (4.5) | p = 0.01 |
| Mental health, mean (SD) | 19.2 (4.8) | 19.0 (4.8) | 19.4 (4.7) | p = 0.67 |
| Physical function, mean (SD) | 3.9 (2.1) | 3.4 (2.1) | 5.0 (1.5) | p < 0.001 |
| Visit characteristics | ||||
| Total visit time [min], mean (SD) | 17.7 (7.7) | 18.5 (7.6) | 16.0 (7.5) | p = 0.09 |
| Pain-related chief complaint (%) | 19.5 | 21.6 | 14.3 | p = 0.36 |
| Chronic illness score, mean (SD)‡ | 0.84 (0.89) | 0.78 (0.78) | 0.98 (1.1) | p = 0.66 |
| Physician characteristics | (n = 17) | |||
| Demographics | ||||
| Male (%) | 47.0 | |||
| Mean Age [years] (SD) | 31 (3.4) | |||
| Ethnicity (%) | ||||
| Indian/Pakistani | 47.1 | |||
| Asian | 35.3 | |||
| White | 11.8 | |||
| Black | 5.9 | |||
| International medical graduate(%) | 88.2 | |||
*P values were calculated using t-tests for continuous variables and chi-squared tests for categorical variables
†SF-20 subscales are coded so that higher numbers correspond to increased health and/or increased pain severity
‡Chronic illness score indicates the number of documented chronic illnesses (rage 0–5)
Sixty-three percent of patients reported at least moderate pain; 69% of visits contained pain-related discussions. Table 2 shows that pain-related discussions were common even for patients who reported no pain on the SF-20. Compared to patients who did not discuss pain, patients who discussed pain reported significantly greater pain severity, decreased physical function, and decreased general health on the SF-20 (Table 1). No patient demographics were associated with the presence of pain-related discussions in bivariate analyses.
Table 2.
Distribution of Pain Severity and Pain-Related Discussions
| Pain severity (SF-20) | Number of patients | Pain discussed (%) |
|---|---|---|
| No pain | 13 | 38.5 |
| Very mild | 14 | 42.9 |
| Mild | 22 | 59.1 |
| Moderate | 39 | 74.3 |
| Severe | 29 | 89.7 |
| Very severe | 14 | 78.6 |
Table 3 shows results of the logistic regression. After controlling for other independent variables, pain severity [OR 1.69, 95% CI (1.18, 2.41)] and a pain-related chief complaint [OR 4.10, 95% CI (1.39, 12.12)] remained significantly positively associated with the probability that pain was discussed. Better physical function remained significantly negatively associated with the probability that pain was discussed [OR 0.65, 95% CI (0.48, 0.86)].
Table 3.
Likelihood of Discussing Pain Based on Patient-Level Variables
| Variable | Presence of pain discussion | ||
|---|---|---|---|
| Unadjusted OR | Adjusted OR* | 95% CI | |
| Pain severity† | 1.75 | 1.69 | 1.18, 2.41 |
| Physical function† | 0.63 | 0.65 | 0.48, 0.86 |
| General health† | 0.89 | 1.03 | 0.95. 1.11 |
| Pain-related chief complaint | 1.68 | 4.10 | 1.39, 12.12 |
*Adjusted odds ratios are adjusted for the listed independent variables as well as for total visit time
†Higher values correspond to increased health and/or increased pain severity
Among visits that included pain-related discussions, patients and physicians discussed pain for a mean of 5.9 min (SD 4.6; median = 5.4; interquartile range 2.1 to 8.4) or 34% of the total visit time (SD 24%; median = 30%, IQR 14% to 52%). Patients who discussed pain mentioned a median of two different pains (mean = 1.8). The most common pain categories were musculoskeletal (31%) and headache (16%). Across all visits (including visits without pain-related discussions) patients and physicians spent 23% of total visit time discussing pain.
In bivariate analyses of visits that included pain-related discussions, pain severity and better physical function were both significantly positively associated with percent of total visit time spent discussing pain. Patients and physicians who had met previously spent a significantly lower percent of total visit time discussing pain. Neither chief complaint nor chronic illness score was significantly associated with percent of total visit time spent discussing pain in bivariate analyses. Patient age was the only demographic variable significantly associated with the percent of total visit time spent discussing pain, so we included age as a covariate in the linear regression.
In the linear regression, both pain severity and better physical function remained significantly positively associated with percent of total visit time spent discussing pain. For every one-point increase in pain severity on the six-point SF-20 item, patients and physicians spent on average 4.5% more of total visit time discussing pain [95% CI (0.60, 8.37)]. For every one-point improvement in physical function on the seven-point SF-20 subscale, patients and physicians spent on average 3% more total visit time discussing pain [95% CI (0.32, 5.75)]. Better physical function was negatively associated with the probability of discussing pain, but when pain was discussed, better physical function was positively associated with percent of total visit time spent discussing pain.
Whether patients and physicians had met previously had the largest effect on the percent of total visit time spent discussing pain. On average, patients and physicians who had met previously spent 11% less [95% CI (-21.65, -0.55)] total visit time discussing pain compared to patients and physicians who had not. Older age was also significantly negatively associated with the percent of total visit time spent discussing pain. There was no significant association between chief complaint and percent of total visit time spent discussing pain. Table 4 shows predicted means for independent variables that were statistically significant in the linear regression. Using a hierarchical model instead of GEE to control for clustering within physician did not substantively change the results of either regression.
Table 4.
Association Between Time Spent Discussing Pain and Patient-Level Variables
| Visit characteristic | Percent of total visit time spent discussing pain* | 95% CI |
|---|---|---|
| Pain severity (SF-20) | ||
| Severe | 45.5 | 37.8, 53.3 |
| Moderate | 32.1 | 24.4, 42.7 |
| No pain | 23.1 | 5.8, 40.4 |
| Physical function (SF-20) | ||
| Poor | 26.4 | 13.4, 39.4 |
| Moderate | 35.5 | 27.3, 43.7 |
| Excellent | 44.6 | 34.7, 54.6 |
| Patient and physician had met previously | ||
| No | 42.3 | 30.2, 54.3 |
| Yes | 31.2 | 25.0, 37.3 |
*Values are predicted means adjusted for the listed independent variables as well as for total visit time, chief-complaint, patient age, and patient general health status (SF-20)
We performed exploratory analyses to investigate the large effect of patient and physician having met previously on the percent of total visit time spent discussing pain. Patients who had met the physician previously were significantly older than patients who had not. We found no other significant differences in demographics or SF-20 subscales; mean pain severity was the same for both groups. We found no significant difference in the proportion of patients with pain-related chief complaints or in who initiated pain discussions. The effect of having met previously persisted when we controlled for the number of new pains discussed during visits and for chronic illness score.
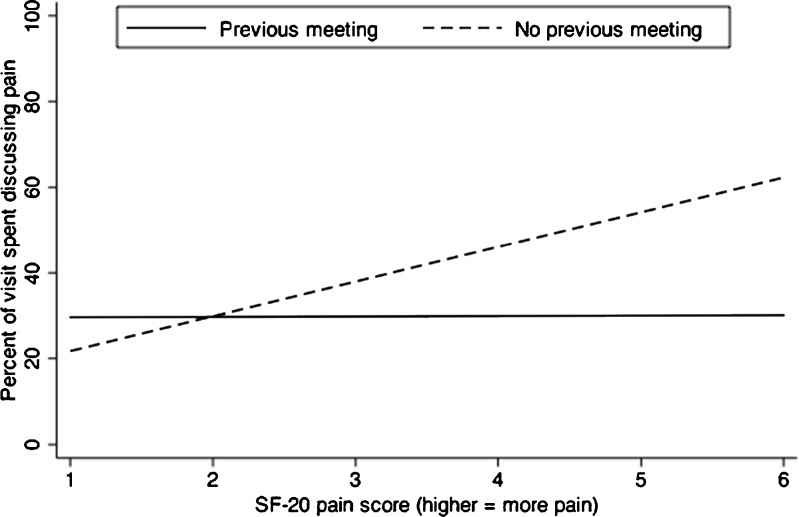
We found a statistically significant interaction between pain severity and having met previously (Fig. 1). When patients and physicians had met previously, pain severity was not associated with the percent of total visit time spent discussing pain. When they had not, pain severity was significantly positively associated with a greater percent of total visit time spent discussing pain.
Figure 1.
Relationship between SF-20 pain score and percent of total visit time spent discussing pain. Lines represent predicted values from multiple linear regression. For visits in which the patient and physician had not met previously (dashed line), pain severity was positively associated with the percent of total visit time spent discussing pain. For visits in which the patient and physician had met previously (solid line), the percent of total visit time spent discussing pain was independent of pain severity.
DISCUSSION
In our sample, most visits include discussions about pain, and these discussions took up on average one-third of the total visit time. In addition, across all visits in our sample, patients and physicians spent 23% of their time together talking about pain. In a recent study of time spent discussing pain among elderly, mostly white patients, fewer than half (48%) of primary care visits included pain-related discussions, lasting a median of 2.3 min.38 In comparison, pain-related discussions in our study were more frequent and longer, even though patients in our sample were younger and had similar visit lengths. Several studies have shown that black race7 and lower socioeconomic status5,39 are associated with greater pain severity. Our findings suggest that this greater burden of pain among low-income black patients translates into frequent and lengthy pain-related discussions during primary care visits.
In our sample, patients who reported better baseline physical function on the SF-20 were less likely to discuss pain with their physicians, but when these patients did discuss pain, they spent a higher percent of total visit time discussing pain compared to patients who reported poor physical function. Pain is a very common cause of functional limitation.40 Therefore, one possible explanation is that among patients who reported poor physical function, discussions of pain-related functional limitations crowded out explicit discussions of pain. Our coding system only counted discussions of functional limitation as pain-related discussions if pain was mentioned explicitly. This explanation is consistent with our finding that older age was associated with a smaller percent of time spent discussing pain; if discussions of pain-related functional limitations did crowd out discussions of pain, this phenomenon would be more common among older patients.
Whether patients had met the physician previously substantially moderated the association between pain severity and percent of total visit time spent discussing pain. One possible explanation is that patients and physicians who had met previously were more likely to have discussed pain in detail during previous visits. However, the effect of having met previously persisted after we controlled for the number of new pains discussed during each visit. Another possible explanation is that visits in which patients and physicians had not met previously were more likely to be acute care visits and so were more likely to involve discussions of acute (rather than chronic) pain.
Our study has several limitations. As mentioned, differences in discussions of acute versus chronic pain may confound our findings relating to patient and physician having met previously. We were able to control for chief complaint and chronic illness, but we were unable to distinguish between acute and chronic pain. Coders often could not distinguish between acute and chronic pain, especially when discussions were brief. In addition, many patients discussed multiple different pains, and may have discussed both acute and chronic pains during a single visit. Future studies that distinguish between acute versus chronic pain could investigate whether the effect of having met previously on time spent discussing pain persists for both acute and chronic pain. Second, our pain severity measure, the single SF-20 item, was designed for assessing chronic pain and so may be less accurate than other measures for assessing acute pain.41 Third, physicians in our study were family medicine residents and were mostly Asian international medical graduates, so our findings may not generalize to attending physicians or other physician populations. However, black patients are more likely than white patients to receive primary care from international medical graduates, to see non-black physicians, and to receive care in clinics similar to the one in our study.42–44 Therefore, the visits in our sample have characteristics typical of many low-income black patients’ experiences in primary care. Finally, whether patients and physicians have met previously is a potentially imperfect measure of continuity of care.45,46 However, patients and physicians can often establish meaningful relationships after a single visit, so our measure is a reasonable one that could be reliably coded from video-recorded visits.
Few studies have focused on pain-related communication within low-income black patient populations, even though this population suffers from a substantial pain burden and racial disparities in pain management are well documented.6,7 The substantial amount of time spent discussing pain in our sample provides another rationale for better understanding the content of pain-related communication among low-income black patients. Information about factors associated with pain-related discussions in this patient population can inform strategies for improving communication about pain that may help reduce or eliminate these disparities.47,48
Our findings highlight that pain-related discussions are common and comprise a substantial proportion of time during routine primary care visits involving low-income black patients. Future studies could evaluate whether lengthy pain-related discussions crowd out discussion of other topics during primary care visits, which has been suggested by previous studies.16 Future studies should also evaluate whether the large amount of time spent discussing pain is one reason that physicians and patients commonly identify discussions about pain as difficult and frustrating.10,11,49 Finally, the components of appropriate pain management in primary care have been difficult to measure or even define using indirect methods,12 so approaches that combine direct observation and self-report measures may provide important information for untangling the relationships between the substantial amount of time devoted to pain during primary care visits and the quality and effectiveness of pain management.50
Electronic supplementary material
(DOC 77 kb)
Contributors
The authors wish to thank Rodney A. Hayward, MD, and Hwa-Jung Choi, PhD, for assistance with data analysis and imputation. They also thank colleagues at the University of Michigan and Wayne State University/Karmanos Cancer Institute for helpful comments on earlier versions of this manuscript.
Funders
Dr Henry is supported by the US Department of Veterans Affairs and the Robert Wood Johnson Foundation Clinical Scholars program, which also funded this project. The primary study was funded by NICHD grant R21 HD050450 (L. Penner) and NCI Center grant P30CA22453 (Karmanos Cancer Institute/Wayne State University).
Prior Presentations
Preliminary findings were presented at the Robert Wood Johnson Foundation Clinical Scholars program annual meeting; Atlanta, GA; 2-5 November 2010; and the Society for General Internal Medicine annual meeting; Phoenix, AZ, 4-7 May 2011.
Conflicts of Interest
The authors declare that they do not have a conflict of interest.
References
- 1.Gureje O, Korff M, Simon GE, Gater R. Persistent pain and well-being—a World Health Organization study in primary care. JAMA. 1998;280:147–51. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–54. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 3.Krueger AB, Stone AA. Assessment of pain: a community-based diary survey in the USA. Lancet. 2008;371:1519–25. doi: 10.1016/S0140-6736(08)60656-X. [DOI] [PubMed] [Google Scholar]
- 4.Relieving Pain in America: a Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 5.Kerns R, Otis J, Rosenberg R, Reid MC. Veterans’ reports of pain and associations with ratings of health, health-risk behaviors, affective distress, and use of the healthcare system. J Rehabil Res Dev. 2003;40:371–9. doi: 10.1682/JRRD.2003.09.0371. [DOI] [PubMed] [Google Scholar]
- 6.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009;10:1187–204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Shavers VL, Bakos A, Sheppard VB. Race, ethnicity, and pain among the US adult population. J Health Care Poor Underserved. 2010;21:177–220. doi: 10.1353/hpu.0.0255. [DOI] [PubMed] [Google Scholar]
- 8.Mantyselka PT, Turunen JHO, Ahonen RS, Kumpusalo EA. Chronic pain and poor self-rated health. JAMA. 2003;290:2435–42. doi: 10.1001/jama.290.18.2435. [DOI] [PubMed] [Google Scholar]
- 9.Butchart A, Kerr EA, Heisler M, Piette JD, Krein SL. Experience and management of chronic pain among patients with other complex chronic conditions. Clin J Pain. 2009;25:293–8. doi: 10.1097/AJP.0b013e31818bf574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthias MS, Parpart AL, Nyland KA, et al. The patient-provider relationship in chronic pain care: Providers’ perspectives. Pain Med. 2010;11:1688–97. doi: 10.1111/j.1526-4637.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 11.Upshur CC, Bacigalupe G, Luckmann R. "They don’t want anything to do with you": patient views of primary care management of chronic pain. Pain Med. 2010;11:1791–8. doi: 10.1111/j.1526-4637.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- 12.Dosa D, Teno J. Haven’t got time for the pain. J Gen Intern Med. 2010;25:889–90. doi: 10.1007/s11606-010-1414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zubkoff L, Lorenz KA, Lanto AB, et al. Does screening for pain correspond to high quality care for veterans? J Gen Intern Med. 2010;25:900–5. doi: 10.1007/s11606-010-1301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mularski RA, White-Chu F, Overbay D, Miller L, Asch SM, Ganzini L. Measuring pain as the 5th vital sign does not improve quality of pain management. J Gen Intern Med. 2006;21:607–12. doi: 10.1111/j.1525-1497.2006.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zulman DM, Kerr EA, Hofer TP, Heisler M, Zikmund-Fisher BJ. Patient-provider concordance in the prioritization of health conditions among hypertensive diabetes patients. J Gen Intern Med. 2010;25:408–14. doi: 10.1007/s11606-009-1232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krein SL, Hofer TP, Holleman R, Piette JD, Klamerus ML, Kerr EA. More than a pain in the neck: how discussing chronic pain affects hypertension medication intensification. J Gen Intern Med. 2009;24:911–6. doi: 10.1007/s11606-009-1020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan M, Ferrell B. Ethical challenges in the management of chronic nonmalignant pain: Negotiating through the cloud of doubt. J Pain. 2005;6:2–9. doi: 10.1016/j.jpain.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Matthias MS, Bair MJ. The patient-provider relationship in chronic pain management: where do we go from here? Pain Med. 2010;11:1747–9. doi: 10.1111/j.1526-4637.2010.00998.x. [DOI] [PubMed] [Google Scholar]
- 19.Stange KC, Zyzanski SJ, Smith TF, et al. How valid are medical records and patient questionnaires for physician profiling and health services research? A comparison with direct observation of patient visits. Med Care. 1998;36:851–67. doi: 10.1097/00005650-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Krebs EE, Bair MJ, Carey TS, Weinberger M. Documentation of pain care processes does not accurately reflect pain management delivered in primary care. J Gen Intern Med. 2010;25:194–9. doi: 10.1007/s11606-009-1194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith DM, Brown SL, Ubel PA. Mispredictions and misrecollections: challenges for subjective outcome measurement. Disabil Rehabil. 2008;30:418–24. doi: 10.1080/09638280701625237. [DOI] [PubMed] [Google Scholar]
- 22.Hertwig R, Fanselow C, Hoffrage U. Hindsight bias: how knowledge and heuristics affect our reconstruction of the past. Memory. 2003;11:357–77. doi: 10.1080/09658210244000595. [DOI] [PubMed] [Google Scholar]
- 23.Ory MG, Yuma PJ, Hurwicz ML, et al. Prevalence and correlates of doctor-geriatric patient lifestyle discussions: analysis of ADEPT videotapes. Prev Med. 2006;43:494–7. doi: 10.1016/j.ypmed.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Bensing JM, Roter DL, Hulsman RL. Communication patterns of primary care physicians in the United States and The Netherlands. J Gen Intern Med. 2003;18:335–42. doi: 10.1046/j.1525-1497.2003.10735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penner LA, Dovidio JF, Edmondson D, et al. The experience of discrimination and black-white health disparities in medical care. J Black Psychol. 2009;35:180–203. doi: 10.1177/0095798409333585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penner LA, Dovidio JF, West TV, et al. Aversive racism and medical interactions with black patients: a field study. J Exp Soc Psychol. 2010;46:436–40. doi: 10.1016/j.jesp.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart AL, Hays RD, Ware JE. The MOS short-form general health survey: reliability and validity in a patient population. Med Care. 1988;26:724–32. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Albrecht TL, Ruckdeschel JC, Ray FL, et al. A portable, unobtrusive device for videorecording clinical interactions. Behave Res Methods. 2005;37:165–9. doi: 10.3758/BF03206411. [DOI] [PubMed] [Google Scholar]
- 29.Noldus LPJJ, Trienes RJH, Hendriksen AHM, Jansen H, Jansen RG. The observer video-pro: new software for the collection, management, and presentation of time-structured data from videotapes and digital media files. Behav Res Methods Instrum Comput. 2000;32:197–206. doi: 10.3758/BF03200802. [DOI] [PubMed] [Google Scholar]
- 30.Harris J, Pryor J, Adams S. The challenge of intercoder agreement in qualitative inquiry. Electronic Emissary; 1997; Available from: http://emissary.wm.edu/templates/content/publications/intercoder-agreement.pdf. Accessed 1 December 2011.
- 31.Hsaio C, Cherry DK, Beatty PC, Rechtsteiner EA. National ambulatory medical care survey: 2007 summary. Hyattsville, Maryland: National Center for Health Statistics. Available from: http://www.cdc.gov/nchs/data/nhsr/nhsr027.pdf. Accessed 1 December 2011. [PubMed]
- 32.Kerr EA, Heisler M, Krein SL, et al. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients’ treatment priorities and self-management? J Gen Intern Med. 2007;22:1635–40. doi: 10.1007/s11606-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rigler SK, Studenski S, Wallace D, Reker DM, Duncan PW. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16:420–8. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 34.Hanley JA, Negassa A, Edwardes MDd, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–75. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 35.Diggle P, Heagerty P, Liang K-L, Zeger SL. Analysis of Longitudinal Data. 2. Oxford, New York: Oxford University Press; 2002. [Google Scholar]
- 36.Royston P. Multiple imputation of missing values: update. Stata J. 2005;5:188–201. [Google Scholar]
- 37.Royston P. Multiple imputation of missing values: further update of ice, with an emphasis on categorical variables. Stata J. 2009;9:466–77. [Google Scholar]
- 38.Tai-Seale M, Bolin J, Bao X, Street R. Management of chronic pain among older patients: inside primary care in the US. Eur J Pain. 2011;[in press]. [DOI] [PMC free article] [PubMed]
- 39.Rios RR, Zautra AJ. Socioeconomic disparities in pain: the role of economic hardship and daily financial worry. Health Psychol. 2011;30:58–66. doi: 10.1037/a0022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruckenthal P, Reid MC, Reisner L. Special issues in the management of chronic pain in older adults. Pain Med. 2009;10:S67–78. doi: 10.1111/j.1526-4637.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 41.Krebs EE, Bair MJ, Damush TM, Tu W, Wu J, Kroenke K. Comparative responsiveness of pain outcome measures among primary care patients with musculoskeletal pain. Med Care. 2010;48:1007–14. doi: 10.1097/MLR.0b013e3181eaf835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howard DL, Bunch CD, Mundia WO, et al. Comparing United States versus international medical school graduate physicians who serve African-American and white elderly. Health Serv Res. 2006;41:2155–81. doi: 10.1111/j.1475-6773.2006.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varkey AB, Manwell LB, Williams ES, et al. Separate and unequal: clinics where minority and nonminority patients receive primary care. Arch Intern Med. 2009;169:243–50. doi: 10.1001/archinternmed.2008.559. [DOI] [PubMed] [Google Scholar]
- 44.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907–15. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 45.Saultz JW. Defining and measuring interpersonal continuity of care. Ann Fam Med. 2003;1:134–43. doi: 10.1370/afm.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers J, Curtis P. The concept and measurement of continuity in primary care. Am J Public Health. 1980;70:122–7. doi: 10.2105/AJPH.70.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burgess DJ, Ryn M, Crowley-Matoka M, Malat J. Understanding the provider contribution to race/ethnicity disparities in pain treatment: insights from dual process models of stereotyping. Pain Med. 2006;7:119–34. doi: 10.1111/j.1526-4637.2006.00105.x. [DOI] [PubMed] [Google Scholar]
- 48.Klonoff EA. Disparities in the provision of medical care: an outcome in search of an explanation. J Behav Med. 2009;32:48–63. doi: 10.1007/s10865-008-9192-1. [DOI] [PubMed] [Google Scholar]
- 49.Eggly S, Tzelepis A. Relational control in difficult physician-patient encounters: negotiating treatment for pain. J Heal Commun. 2001;6:323–33. doi: 10.1080/108107301317140814. [DOI] [PubMed] [Google Scholar]
- 50.Donabedian A. The quality of care: how can it be assessed? JAMA. 1988;260:1743–8. doi: 10.1001/jama.1988.03410120089033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 77 kb)