Abstract
In sub-Saharan Africa, sepsis is an important cause of mortality but optimal sepsis management including fluid resuscitation, early antibiotic administration and patient monitoring is limited by a lack of supplies and skilled health workers.
OBJECTIVE
To evaluate whether early, monitored sepsis management provided by a study medical officer can improve survival among patients with severe sepsis admitted to two public hospitals in Uganda.
DESIGN, SETTING and PATIENTS
A prospective before and after study of an intervention cohort (n=426) with severe sepsis receiving early, monitored sepsis management compared to an observation cohort (n=245) of similarly ill patients with severe sepsis receiving standard management after admission to the medical wards of two Ugandan hospitals.
INTERVENTION
Early sepsis management provided by a dedicated study medical officer comprised of fluid resuscitation, early antibiotics and regular monitoring in the first 6 hours of hospitalization.
MEASUREMENTS
Kaplan-Meier survival and unadjusted and adjusted Cox proportional hazards analysis were used to compare the effect of early, monitored sepsis management on 30-day mortality between the intervention cohort (enrolled May 2008 to May 2009) and observation cohort (enrolled July 2006 to November 2006).
RESULTS
The majority (86%) of patients in both cohorts were HIV-infected. Median fluid volume provided in the first 6 hours of hospitalization was higher in intervention than observation cohort patients (3000 vs. 500 mL, p<0.001) and a greater proportion of intervention cohort patients received antibacterial therapy in less than one hour (67% vs 30.4%, p<0.001). Mortality at 30 days was significantly lower in the intervention cohort compared to the observation cohort (33.0% vs 45.7%, log-rank p=0.005). After adjustment for potential confounders, the hazard of 30-day mortality was 26% less in the intervention cohort compared to the observation cohort (adjusted HR=0.74, 95% CI=0.55–0.98). Mortality among the 13% of intervention patients who developed signs of respiratory distress was associated with baseline illness severity rather than fluid volume administered.
CONCLUSION
Early, monitored management of severely septic patients in Uganda improves survival and is feasible and safe even in a busy public referral hospital.
Keywords: Sepsis, Management Bundle, Fluid Therapy, Uganda, Africa, Mortality, Pulmonary Edema
INTRODUCTION
In low-income countries, bloodstream infections are common and likely contribute to a high incidence of severe sepsis (1, 2). The management of patients with severe sepsis in resource-constrained settings, however, has been minimally evaluated (3, 4). In comparison, sepsis management guidelines in high-income settings have resulted in improved survival (5–7). These guidelines focus on utilizing sepsis bundles which include early fluid resuscitation, initiation of broad-spectrum antimicrobial treatment, and other interventions in response to the patient’s condition. Similar evidence-based guidelines which take into account resource constraints and etiologic agents associated with local ecology could potentially provide an invaluable tool for practitioners to improve survival in critically ill septic patients from low-resource settings.
In 2006, we conducted the first prospective observational study in sub-Saharan Africa of the management and outcomes of hospitalized adult patients with severe sepsis syndromes (8). These Ugandan patients had high mortality (43%) and were predominantly HIV-infected (85%). We found that the components of sepsis management bundles were not adequately provided. For instance, patients in our cohort received <10% of the minimum recommended 20 mL/kg/hr of fluid volume in the first 6 hours after presentation (5). In addition, 52 different antibacterial regimens were administered during the study period and less than 1/3 of patients received their antimicrobial therapy within 1 hour of hospital presentation.
In response, we conducted an intervention study of severely septic adults admitted to two public hospitals in Uganda to prospectively evaluate the impact on mortality of early monitored sepsis management, focusing on fluid resuscitation administered by a dedicated study medical officer.
MATERIALS and METHODS
Study design and site description
Using a before and after study design, we enrolled 426 severely septic patients admitted to the medical wards of Mulago National Referral Hospital and Masaka Regional Referral Hospital between May 2008 and May 2009 (intervention cohort). We compared mortality in this intervention population to that in our earlier prospectively enrolled observational study (observation cohort), which was conducted at the same hospitals. In both hospitals, mechanical ventilation and invasive hemodynamic monitoring were not available for ward patients. An intensive care unit with more advanced care exists in Mulago Hospital but no study patients from the medicine wards were transferred to this unit during the period of either study. Further site descriptions and site resource capacity are described in detail elsewhere (8). There were no major changes in resources on the medical wards at Mulago Hospital or Masaka Regional Referral Hospital during the time that lapsed between the observation and intervention cohorts.
Intervention cohort
Adult patients (age ≥ 18 years) triaged to the hospitals’ Medical Casualty Units during daytime hours (from 9 am to 4 pm, excluding weekends and holidays) were approached for enrollment. Inclusion criteria were: 1) suspected infection as determined by the admitting medical officer; 2) two of the following: a) axillary temperature >37.5°C or <35.5°C, b) heart rate >90 beats/min, c) respiratory rate >20 breaths/min; 3) systolic blood pressure (SBP) ≤ 100 mmHg; and 4) whole blood lactate concentration >2.5 mmol/L or Karnofsky Performance Scale (KPS) score ≤40. Criterion 4 was used to select highest-risk patients as these parameters independently predicted mortality in our previous observational study (8, 9). In addition, eligible patients required accompaniment from an attendant, usually the patient’s next of kin. Patients with suspected surgical or obstetric emergencies or shock without suspected infection were excluded.
The fluid resuscitation protocol consisted of a one liter intravenous fluid challenge of normal saline in the first 60 minutes and 500 mL boluses every 30 minutes in the ensuing five hours (for a total maximum of six liters during the first six hours). A study medical officer administered fluid to study patients and evaluated vital signs every hour during the fluid administration period. If SBP increased by ten mmHg from baseline for two consecutive measurements over 90 mmHg, the medical officer stopped fluids but continued to monitor vital signs every hour during the fluid administration period. If the patient developed signs of iatrogenic pulmonary edema – defined as either a 3% decrease in oxygen saturation, an increase in respiratory rate by five breaths per minute, or new crackles on lung examination – the medical officer determined whether it was necessary to stop fluids, provide supplemental oxygen, order a chest radiograph, and/or administer furosemide. As the primary medical officer for the first 6 hours of a patient’s hospitalization, study medical officers also ensured early administration of initial empiric antibacterial treatment. A standard antibiotic regimen, however, was not supplied as part of the protocol so type of empiric antibacterial regimen was limited to available medications in the casualty ward. After the initial 6 hours of monitored care provided by the study medical officer, patient management was handed over to the non-study primary medical team.
Observation cohort
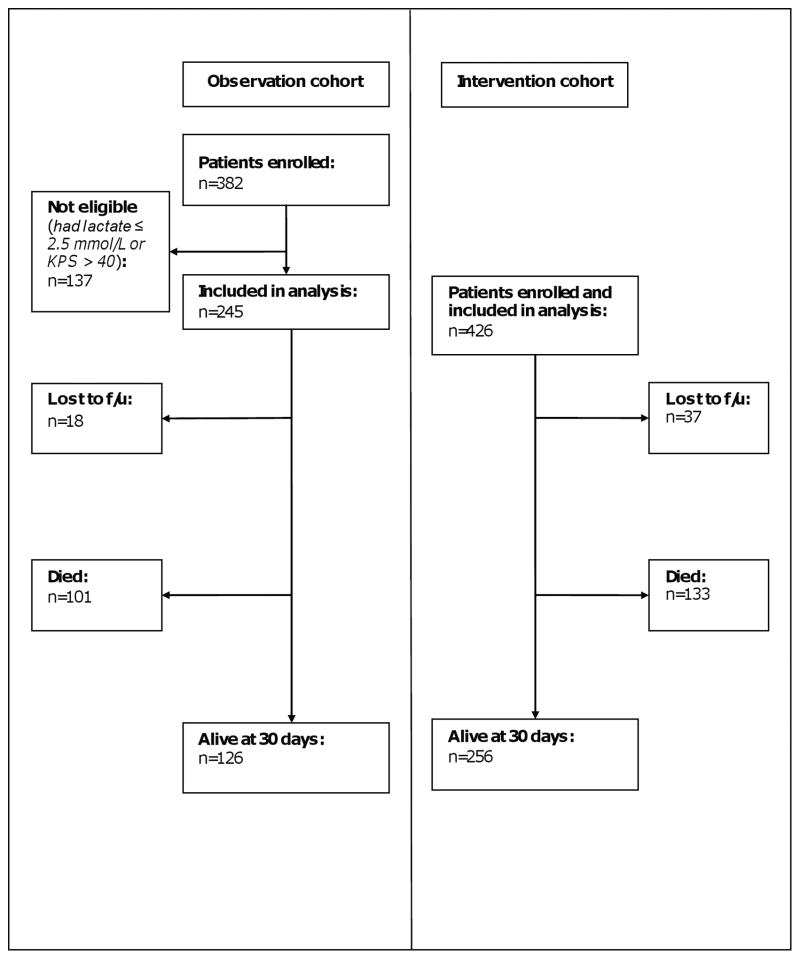
Between July 2006 and November 2006, we enrolled 382 adult patients in an observational study of the management and outcomes of severely septic patients (8). Enrollment times were similar for both the intervention and observation cohorts. The subset of 245 patients from this cohort who fulfilled the inclusion criteria used for the intervention cohort was analyzed as the comparator population for the intervention cohort (Figure 1). All patient management was provided by the primary medical team, including fluid resuscitation, antibacterial administration and patient monitoring.
Figure 1.
Study composition and 30-day survival
Assessment of mortality
The primary study endpoint was mortality, 30 days after hospital admission. Identical approaches for measuring mortality were used for both cohorts. For patients who died in the hospital, the date of death was recorded; for those still hospitalized at 30 days, vital status at that time was recorded. For patients who survived and were discharged before 30 days, they or their family were contacted by mobile phone at least 30 days after admission to obtain vital status information. For those who died between discharge and the follow-up phone call, the date of death was recorded; if the exact date of death was not available, the date of death was recorded as the date of the follow-up phone call. If patients did not have access to a telephone, they were offered nominal transport reimbursement at the time of discharge to return for a post-discharge assessment of vital status.
Evaluation of attendants
In the intervention cohort, we separately evaluated whether patient attendants could serve as an alert mechanism for the study medical officer providing fluid resuscitation. Attendants were informed about the importance of fluid resuscitation and instructed to alert the medical officer when fluid bottles were nearly empty. The study medical officers provided fluid resuscitation according to protocol whether or not they were alerted by the patient attendant.
Ethical review
Ethical approval was obtained from the research and/or ethics committees of the University of Virginia, Makerere University, Mulago Hospital, Infectious Disease Institute, and Uganda National Council of Science and Technology. Written informed consent was obtained from each patient or a surrogate if the patient was too obtunded to provide consent.
Laboratory studies
Blood was collected for complete blood counts, electrolytes, CD4+ T-cell (CD4) counts, malaria thick smears, aerobic blood cultures, HIV serology, and lactate concentration (8). For positive bacterial blood cultures, antibiotic susceptibility testing was performed. Mycobacterial cultures were performed at both sites for the intervention cohort and at Mulago Hospital only for the observation cohort.
Definitions
Bacteremia was defined as bacterial isolation from ≥1 blood culture bottle; coagulase-negative staphylococci were considered contaminants. Using criteria adapted from previous studies, we defined whether patients received appropriate anti-bacterial therapy, based on whether or not the organism was susceptible to the anti-bacterial agent used (10). If susceptibility to the agent was not tested for that particular organism or if blood cultures were negative, appropriate broad-spectrum therapy was based on normative guidelines for empiric management of the typical pathogens causing severe sepsis at the study sites (11). If blood cultures were positive for Mycobacterium tuberculosis, empiric antibacterial therapy was deemed appropriate if it included rifampin, isoniazid, pyrazinamide, and ethambutol. Two physicians trained in infectious diseases (STJ, WMS) independently applied these rules to each patient to evaluate whether empiric anti-bacterial therapy was appropriate; a third infectious diseases-trained physician (AW) served as a tie-breaker in cases of disagreement.
Sample size and safety assessment
Based on the mortality rate in the observation subset (8), a sample size of 426 for the intervention cohort was calculated to detect at least a 13% difference in the primary outcome of 30-day mortality between study groups with a power of 80% and a two-sided significance level of 0.05. An independent data safety monitoring board met three times during the study to review whether the number of deaths in the intervention cohort exceeded the number of deaths in the observation cohort. Since the study was not a randomized controlled trial, no parameters were set for early halting of the study if the number of deaths in the intervention cohort was less than in the observation cohort.
Statistical analysis
Statistical analyses were performed using SPSS version 17.0 (SPSS Inc., USA). Baseline characteristics of the two study groups were compared using a chi-square test for comparisons of proportions and the Mann-Whitney U test for comparisons of continuous variables. Kaplan–Meier estimates of mortality for the two study groups were compared with the log-rank test. Patients lost to follow-up before 30 days contributed follow-up time until their last known assessment of vital status and were censored thereafter.
Cox proportional hazards regression analysis was performed to evaluate the difference in the unadjusted and adjusted hazard for 30-day mortality between the intervention cohort and observation cohort. To assess for confounding of the effect of the intervention on 30-day mortality, the following covariates were included in the model: sex, age, temperature, heart rate, respiratory rate, blood pressure, white blood cell (WBC) count, hemoglobin concentration, platelet count, admission KPS score, Glasgow Coma Score, HIV serostatus, CD4 count, highly active anti-retroviral therapy (HAART), prophylactic trimethoprim-sulfamethoxazole, malaria blood smear result and aerobic blood culture result. In order to determine the best fit of continuous variables in the model, -2 likelihood ratios (LR) for different variable formulations (e.g., continuous, dichotomous based on clinical break-points, and quadratic) were compared. The formulation with the lowest −2LR was included in the model along with the remaining covariates and backwards eliminated if the significance level was greater than 0.05. The final parsimonious model was comprised of statistically significant, independent predictors of 30-day mortality.
To demonstrate their relationship to 30-day mortality, sepsis management variables describing volume of fluid resuscitation and timing to antibacterial administration were evaluated in a separate multivariate Cox proportional hazards regression model. This model did not contain the primary cohort exposure variable but contained the same potential confounders as above in addition to a covariate for empiric administration of antimalarial therapy. Only covariates with a significance level less than or equal to 0.2 at the univariate level were included in the multivariate analysis. Again, covariates were backwards eliminated from the multivariate model if the significance level was greater than 0.05. Indicator variables for strata of volume resuscitation (with ≤ 1000mL as the referent group) and time to antibacterial administration (with no antibiotics as the referent group) were included in the model.
RESULTS
Participant characteristics
Patients enrolled in both cohorts were similar in age, SBP, admission KPS scores, lactate levels, and proportion infected with HIV (Table 1). Compared to observation cohort patients, a greater proportion of intervention cohort patients were tachycardic and male; also, hypothermia and lower hemoglobin concentrations occurred more frequently in intervention cohort patients than observation cohort patients. Among those who were HIV-infected, more patients in the intervention cohort were taking HAART and trimethoprim-sulfamethoxazole prophylaxis at the time of hospitalization and had higher CD4 counts compared to the observation cohort.
Table 1.
Enrollment characteristics of intervention and observation cohorts
| Characteristic | Intervention cohort (n=426)a | Observation cohort (n=245)a | p-value |
|---|---|---|---|
| Demographics: | |||
|
| |||
| Age in years; median (IQR) | 34 (27–40) | 34 (28–41) | 0.4 |
|
| |||
| Female [n (%)] | 219 (51.4) | 150 (61.2) | 0.01 |
|
| |||
| Admission vital signs: | |||
|
| |||
| Temperature<35.5°C [n (%)] | 33 (7.8) | 31 (12.9) | 0.04 |
|
| |||
| Temperature>37.5°C [n (%)] | 243 (59.0) | 136 (56.4) | 0.5 |
|
| |||
| Heart rate, beat/min; median (IQR) | 127.5 (113–143) | 120 (106–134.8) | 0.001 |
|
| |||
| Respiratory rate, breaths/min; median (IQR) | 36 (28–42) | 38 (30–44) | 0.2 |
|
| |||
| Systolic blood pressure, mmHg; median (IQR) | 85 (78–90) | 81 (70–90) | 0.5 |
|
| |||
| HIV descriptors: | |||
|
| |||
| Number HIV-infected [n(%)] | 362 (86.2) | 207 (85.2) | 0.7 |
|
| |||
| CD4 count in lymphocytes/mm3; median (IQR)b | 63 (15–178) | 43 (11–109) | 0.003 |
|
| |||
| Number unaware of HIV status [n(%)]b | 71 (19.7) | 55 (26.6) | 0.1 |
|
| |||
| Number on trimethoprim-sulfamethoxazole prophylaxis [n (%)]b | 238 (64.7) | 105 (50.5) | 0.001 |
|
| |||
| Number on HAART [n(%)]b | 81 (23.5) | 27 (13.0) | 0.003 |
|
| |||
| Other clinical variables: | |||
|
| |||
| Admit KPS; median (IQR) | 40 (30–50) | 40 (30–50) | 0.1 |
|
| |||
| Admit Glasgow Coma Score, median (IQR) | 15 (15–15) | 15 (15–15) | 0.1 |
|
| |||
| Lactate concentration, mmol/L; median (IQR)c | 3.9 (3.1–4.9) | 3.7 (2.9–4.9) | 0.2 |
|
| |||
| White blood cell count, cells/mL median (IQR) | 4800 (2800–8800) | 5500 (3200–9400) | 0.2 |
|
| |||
| Platelet count, cells/mL; median (IQR) | 197,000 (109,750–303,750) | 212,000 (123,250–363,000) | 0.04 |
|
| |||
| Hemoglobin in g/dL; median (IQR) | 9.2 (7.5–11.3) | 8.4 (6.3–10.5) | 0.001 |
|
| |||
| Malaria-positive smear [n(%)] | 63 (14.8) | 38 (15.5) | 0.8 |
|
| |||
| Aerobic blood culture positive [n(%)] | 49 (12.5) | 34 (13.9) | 0.6 |
|
| |||
| • non-typhoidal Salmonella | • 10 (2.3) | • 16 (6.5) | — |
| • Streptococcus pneumoniae | • 22 (5.2) | • 4 (1.6) | |
| • Staphylococcus aureus | • 7 (1.6) | • 7 (2.9) | |
| • Escherichia coli | • 3 (0.7) | • 3 (1.2) | |
| • Cryptococcus neoformans | • 6 (1.4) | • 3 (1.2) | |
| • Proteus species | • 0 (0) | • 1 (0.4) | |
| • Pseudomonas species | • 1 (0.2) | • 0 (0) | |
|
| |||
| Mycobacterium tuberculosis bacteremia [n(%)]d | 86 (20.1) | 18 (12.0) | 0.04 |
Due to the occurrence of missing data found in <5% of the variables, numbers may not add up to total n;
Denominator is number HIV-infected;
Portable whole blood lactate levels were not obtained on all patients [observation cohort (n=154); intervention cohort (n=417)];
Mycobacterial blood cultures were only obtained on patients at the Mulago National Referral Hospital site (n = 150) in the observation cohort; they were obtained at both study sites (n = 426) for the intervention cohort
Fluid management
The median volume of fluid provided to intervention patients in the first six hours of hospitalization was 3000 mL (IQR 2500–4000) (Table 2). This volume was significantly greater than the median 500 mL (IQR 300–1000) administered to observation patients (p<0.001); less than 1% of patients in the observation cohort received more than 3000 mL. A significantly higher percentage of intervention patients received fluids in the initial hour of admission compared to observation patients (97.1% vs 55.1%, p<0.001). Patients from the intervention cohort experienced a significantly larger median increase in SBP at six hours than patients from the observation cohort (12 vs 5 mmHg, p<0.001).
Table 2.
Comparison of fluid and antimicrobial management between intervention and observation cohorts
| Fluid management variables | Intervention cohort (n=426)a | Observation cohort (n=245)a | p-value |
|---|---|---|---|
| Volume of fluid administered in first 6 hours in mL; median (IQR) | 3000.0 (2500–4000) | 500.0 (300–1000) | <0.001 |
| Volume of fluid administered in first 24 hours in mL; median (IQR) | 3500.0 (2510–4500) | 1000.0 (500–1500) | <0.001 |
| < 1 hour to administration of initial fluids [n (%)] | 404 (97.1) | 135 (55.1) | <0.001 |
| Change in SBP in first 6 hours in mmHg; median (IQR) | 12.0 (2.2–25.0) | 5.0 (0–19.5) | <0.001 |
| Strata of volume resuscitation administered in first 6 hours [n (%)] | <0.001c | ||
| • 0–1000 mLb | 5 (1.2) | 200 (81.6) | |
| • 1001–2500 mL | 144 (34.2) | 43 (17.6) | |
| • 2501–3500 mL | 132 (31.4) | 0 (0.0) | |
| • >3500 mL | 140 (33.3) | 2 (0.8) | |
| Antimicrobial management variables | |||
| Time to administration of antimicrobials [n (%)] | <0.001c | ||
| • Never givenb | 0 (0) | 21 (8.8) | |
| • <1 hour | 284 (67) | 73 (30.4) | |
| • Between 1 and 6 hours | 128 (30.2) | 50 (20.8) | |
| • >6 hours | 12 (2.8) | 96 (40) | |
| Inappropriate antibacterial regimen administered [n (%)] | 344 (81.1) | 223 (95.3) | <0.001 |
| Administration of antimalarial as empiric therapy [n (%)] | 66 (15.5) | 53 (21.6) | 0.04 |
Due to the occurrence of missing data found in <5% of the variables, numbers may not add up to total n;
Referent category with respect to indicator variables;
Global p-value for indicator variables compared to referent category
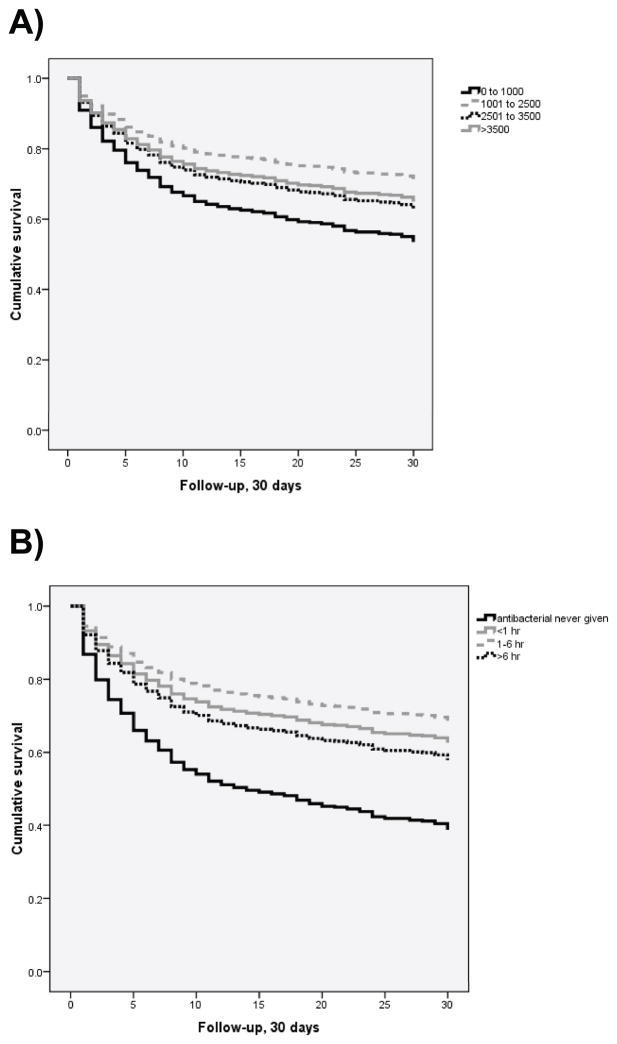
At the univariate level, a decreased hazard for 30-day mortality existed in patients receiving fluid volumes greater than 1000 mL of normal saline (Figure 2a). Compared to patients receiving less than 1000 mL in the first 6 hours of hospitalization, patients receiving between 1000 and 2500 mL of fluid in the first 6 hours had a significantly decreased hazard of 30-day mortality (HR 0.54, 95%CI 0.38–0.77, p=0.001). In addition, patients receiving more than 3500 mL had a significantly decreased hazard of 30-day mortality and patients receiving between 2500 and 3500 mL had a trend towards increased survival (Table 4).
Figure 2.
Kaplan-Meier survival curves comparing 30-day mortality for categories of fluid resuscitation volume (panel A) and timing of antibacterial administration (panel B)
Table 4.
Unadjusted and adjusted Cox proportional hazards analysis of independent predictors of 30-day mortality including factors associated with sepsis management
| Factor | Unadjusted Hazard Ratio (95% CI) | p | Adjusted Hazard Ratio (95% CI) | p |
|---|---|---|---|---|
| Female sex | 0.84 (0.65–1.09) | 0.19 | 0.68 (0.51–0.91) | 0.008 |
| Age, 18 to 29 years olda | 0.98 (0.65–1.48) | 0.94 | -- | -- |
| Age, 30 to 44 years olda | 1.26 (0.87–1.85) | 0.22 | -- | -- |
| HIV-infected | 2.16 (1.23–3.59) | 0.003 | -- | NS |
| Taking trimethoprim-sulfamethoxazole prophylaxis | 1.20 (0.92–1.55) | 0.18 | -- | NS |
| Taking HAART | 1.18 (0.85–1.64) | 0.33 | -- | -- |
| Admission Karnofsky Performance Scale scoreb | 0.69 (0.62–0.76) | <0.001 | 0.71 (0.62–0.81) | <0.001 |
| (Admission Glasgow Coma Scale score)2 b | 0.89 (0.86–0.92) | <0.001 | 0.92 (0.89–0.96) | <0.001 |
| Temperature>37.5°Cc | 1.17 (0.86–1.57) | 0.32 | -- | NS |
| Temperature<35.5°Cc | 1.96 (1.28–2.99) | 0.002 | 1.80 (1.12–2.91) | 0.02 |
| Admission (heart rate)2 per 104 beats/min | 1.44 (1.14–1.82) | 0.002 | 1.52 (1.15–2.02) | 0.004 |
| Admission respiratory rate, breaths/min | 1.24 (1.12–1.38) | <0.001 | 1.21 (1.05–1.38) | 0.007 |
| Admission systolic blood pressure, mmHg | 0.87 (0.80–0.94) | <0.001 | 0.86 (0.79–0.94) | 0.001 |
| CD4 count per 20 cells/mm3 | 0.93 (0.91–0.96) | <0.001 | 0.96 (0.93–0.98) | 0.002 |
| (Admission white blood cell count)2 per 107 cells/mL | 1.03 (1.00–1.06) | 0.06 | 1.04 (1.01–1.07) | 0.005 |
| (Admission hemoglobin)2 per 10 g/dL | 0.92 (0.90–0.95) | <0.001 | -- | NS |
| Admission platelets per 105 cells/mL | 0.82 (0.74–0.90) | <0.001 | 0.86 (0.78–0.96) | 0.006 |
| Positive malaria blood smear | 1.14 (0.80–1.62) | 0.48 | -- | -- |
| Positive aerobic blood culture | 1.12 (0.77–1.63) | 0.55 | -- | -- |
| Antimicrobial taken prior to hospitalization | 0.98 (0.75–1.27) | 0.85 | -- | -- |
| Antimalarial given during hospitalization | 0.69 (0.48–0.99) | 0.05 | -- | NS |
| Time to initial antibiotics, <1 hourd | 0.49 (0.28–0.87) | 0.02 | 0.44 (0.21–0.89) | 0.02 |
| Time to initial antibiotics, 1 to 6 hoursd | 0.40 (0.22–0.73) | 0.003 | 0.29 (0.14–0.62) | 0.001 |
| Time to initial antibiotics, >6 hoursd | 0.58 (0.31–1.07) | 0.08 | 0.39 (0.19–0.81) | 0.01 |
| Volume of fluids in the first 6 hours, 1001 to 2500 mLe | 0.54 (0.38–0.77) | 0.001 | 0.54 (0.35–0.82) | 0.004 |
| Volume of fluids in the first 6 hours, 2501 to 3500 mLe | 0.74 (0.52–1.06) | 0.10 | 0.72 (0.47–1.11) | 0.14 |
| Volume of fluids in the first 6 hours, >3500 mLe | 0.69 (0.48–0.98) | 0.04 | 0.61 (0.39–0.94) | 0.02 |
Compared to age ≥ 45 years old;
Per 10-unit increase;
Compared to 35.5 to 37.5°C;
Compared to patients receiving no initial antibiotics;
Compared to patients receiving ≤ 1000 mL in the first 6 hours of hospitalization
Antimicrobial management
A significantly greater proportion of intervention patients received antibacterial therapy in less than one hour compared to observation patients (67% vs. 30.4%, p<0.001) (Table 2). In addition, a significantly smaller proportion of intervention patients were provided an antibacterial regimen categorized as inappropriate compared to observation patients (81% vs 95.3%, p<0.001). Regarding other antimicrobial therapy, more observation patients received anti-malarial therapy as part of their empiric antimicrobial regimen than intervention patients (21.6% vs. 15.5%, p=0.04).
At the univariate level, early receipt of antibiotics was significantly associated with a decreased hazard of 30-day mortality compared to receiving no antibiotics (Figure 2b). Both antibiotic administration in less than 1 hour (HR 0.49, 95%CI 0.28–0.87, p=0.02) and between 1 and 6 hours (HR 0.40, 95%CI 0.22–0.73, p=0.003) were significantly associated with a decreased hazard for 30-day mortality (Table 4).
Patient follow-up
Vital status at 30 days after hospital admission was available for 390 (91.3%) intervention cohort patients and 227 (92.7%) observation cohort patients (p=0.6). Eighteen (7.3%) of 245 patients from the observation cohort were lost to follow-up by 30 days, after a median 9.5 days (IQR 5.7–13.0). In the intervention cohort, 37 (8.7%) of 426 patients were lost to follow-up after a median five days (IQR 3.0–10.5).
Impact of early sepsis management on mortality
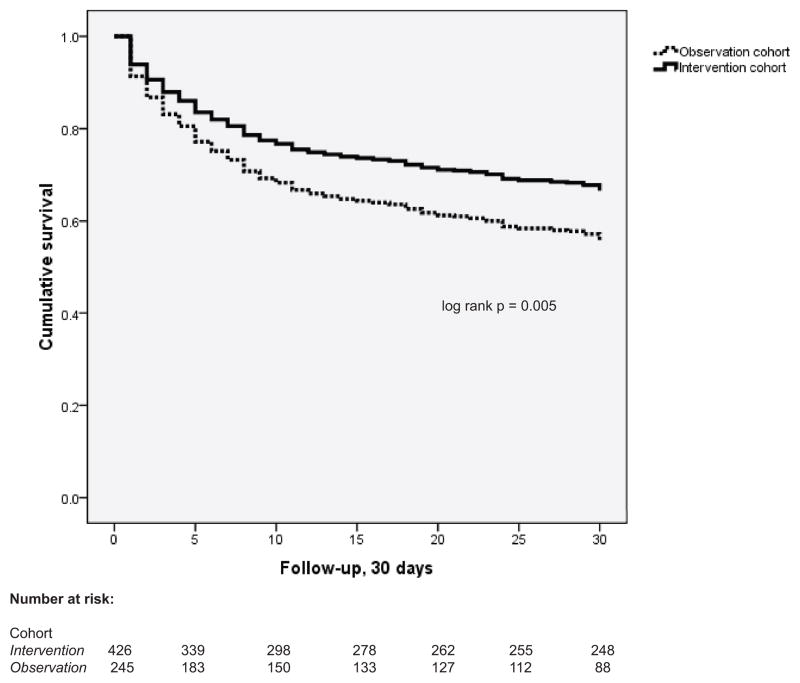
Mortality at 30 days in patients from the intervention cohort was 33.0%, compared to 45.7% in the observation cohort (p=0.005, Figure 3). In unadjusted Cox proportional hazards analysis, the hazard of 30-day mortality was significantly reduced in intervention patients compared to observation patients (HR 0.70, 95% CI 0.54–0.90, p=0.006). Adjustment for potential confounders did not significantly alter the relationship between the intervention cohort and 30 day mortality (adjusted HR 0.74, 95%CI 0.55–0.98, p=0.038) (Table 3). In a separate Cox proportional hazards analysis evaluating the independent effect of the fluid and antibacterial management variables on 30-day mortality, antibiotic administration and increased volume of fluids were independently associated with a decreased hazard of 30-day mortality (Table 4).
Figure 3.
Kaplan-Meier survival curves comparing 30-day mortality between study groups
Table 3.
Multivariate Cox proportional hazards analysis of the relationship between cohort and 30-day mortality adjusted for potential confounders
| Factor | Adjusted Hazard Ratio (95% CI) | p |
|---|---|---|
| Intervention cohorta | 0.74 (0.55–0.98) | 0.04 |
| Female sex | 0.64 (0.48–0.85) | 0.002 |
| Admission Karnofsky Performance Scale scorec | 0.78 (0.68–0.89) | <0.001 |
| (Admission Glasgow Coma Scale score)2 c | 0.92 (0.89–0.96) | <0.001 |
| (Admission heart rate)2 per 105 beats/min | 1.37 (1.05–1.79) | 0.02 |
| Admission respiratory rate per 10 breaths/min | 1.22 (1.07–1.39) | 0.004 |
| Admission systolic blood pressure per 10 mmHg | 0.86 (0.79–0.94) | 0.001 |
| CD4 count per 20 cells/mm3 | 0.95 (0.92–0.98) | 0.001 |
| (Admission white blood cell count)2 per 107 cells/mL | 1.04 (1.01–1.07) | 0.003 |
| (Admission hemoglobin)2 per 10 g/dL | 0.96 (0.94–100) | 0.03 |
| Admission platelets per 105 cells/mL | 0.86 (0.78–0.96) | 0.006 |
Compared to observation cohort;
Compared to normal range of temperature (35.5°C–37.5°C);
Per 10-unit increase
Adverse events from fluid resuscitation in the intervention cohort
Changes in the respiratory examination were noted in 56 (13.1%) of 426 intervention cohort patients during the period of fluid resuscitation. Of those 56 patients, 44 (78.6%) experienced a 3% decrease in oxygen saturation, 18 (32.1%) developed new crackles on lung examination, and 26 (46.4%) developed an increase in respiratory rate by five breaths per minute. Patients with respiratory examination changes did not receive a significantly greater median volume of fluid resuscitation than patients without examination changes [3500 (IQR 2365–4500) vs. 3000 (IQR 2500–4000) mL, p=0.1]. At the univariate level, 30-day mortality was significantly greater in patients with respiratory deterioration compared to patients without respiratory deterioration [48.1% (25/52) vs 31.1% (103/331), HR 1.78, 95%CI 1.15=2.75, p=0.010]. After adjusting for baseline indicators of illness severity (admission KPS score, lactate concentration, and respiratory rate) and for fluid volume provided in the first six hours, baseline indicators of illness severity were significantly associated with an increased hazard for 30-day mortality. In contrast, the development of respiratory examination changes was no longer significantly related to 30-day mortality (HR 1.03, 95%CI 0.32–3.34, p=1.0), nor was the interaction term between fluid volume and the development of changes in respiratory examination (p=0.8).
Role of patient attendants in the intervention cohort
The majority (76.8%, 328/412) of patient attendants could alert the study team medical officer when fluid bottles were nearly empty. The main reasons for attendants being unable to alert the medical officer (n=84) were that the attendant was no longer present (72.3%), the attendant failed to understand the instructions (25.3%) or the attendant on the ward was different from the one who received instructions from the study team (6.1%).
DISCUSSION
In a prospective intervention study conducted at two Ugandan public hospitals, we demonstrated that patients with severe sepsis receiving early monitored management (including fluid and antibiotic administration) had a 30% decreased hazard for 30-day mortality compared with patients receiving standard management. Compared to observation cohort patients, intervention cohort patients received significantly greater and earlier volumes of fluid as well as earlier antibiotic administration over the first six hours of hospitalization. We separately showed that fluid and antibiotic administration were independently associated with a decreased hazard of mortality. This study is the first to show that improved management of adult hospitalized patients with severe sepsis can lead to increased survival in a resource-constrained setting such as Uganda.
While fluid resuscitation remains a cornerstone in the management of septic patients with its benefits reported in animal studies, no definitive studies have evaluated the benefit attributable to fluids alone on survival in adults with severe sepsis(12). To date, the benefits of fluid resuscitation in adult sepsis patients from highly-resourced settings are reported in the context of goal-directed therapy with other treatment modalities including inotrope usage and blood transfusion (16). Our study demonstrates an independent and significant association with increased survival in patients who received greater than 1L of normal saline within the first 6 hours of hospitalization. We, however, were unable to show improvement in survival with increasing increments of fluid resuscitation even after adjusting for covariates associated with illness severity. To be sure, the intervention was comprised of fluids provided by a study attendant who additionally provided regular monitoring and influenced antimicrobial management within the first 6 hours of hospitalization. Thus, we cannot attribute the improvement in mortality to fluids alone; rather, we attribute the intervention effect to a management bundle comprised of fluids, antibiotics and early monitoring by a dedicated health officer. Further studies in high and low-resourced settings are required to more definitively evaluate for a “dose response” to increasing volumes of fluid resuscitation.
Importantly, our results demonstrate that fluid resuscitation can be safely administered with few additional resources and with clear clinical benefit. Importantly, we showed that a median of three liters of fluid can be administered under close monitoring without increasing mortality from respiratory compromise. A small proportion (13%) of intervention patients experienced a change in respiratory examination after initiation of the intervention; those who experienced this change, however, did not receive a significantly greater amount of fluid than those who did not experience a change in respiratory examination. While there was no significant association between respiratory examination changes and mortality after adjustment for measures of illness severity (including admission KPS score and lactate concentration) and volume of fluid resuscitation, baseline illness severity remained an independent predictor of mortality suggesting that baseline illness severity and not fluid administration explained any potential relationship between respiratory status and mortality hazard.
In addition, we report that administration of antibiotics occurred significantly more frequently in intervention cohort patients than observation cohort patients and was independently associated with a decreased hazard for 30-day mortality. We were, however, unable to show an incremental decrease in survival with increasing time to antibiotics as has been shown in previous studies (10, 13). Misclassification bias introduced by the antibacterial administration categories available from both cohorts (i.e.,< 1 hour, 1–6 hours and >6 hours) may have contributed to our inability to report improved outcome with earlier time to administration of antibacterials. Accordingly, it is possible that the majority of patients in the 1–6 hour category received antibacterials closer to 1 hour which would have skewed the effect of that category relative to the less than 1 hour category. In addition, this limitation could be a result of the high proportion of inappropriate antibacterials administered in both cohorts. If our categorization of appropriateness of antibacterial regimens is accurate, then the likelihood of seeing a benefit of early antibacterial administration is low. Notably, we ultimately did not include in the model the covariate describing appropriateness of the antibacterial regimen because of the risk for introducing further misclassification. Given the high frequency of culture negative sepsis and lack of culture and sensitivity data for these patients (68.3% in the intervention cohort and 78.8% in the observation cohort), the majority of antibacterial regimens required inference about appropriateness. Future studies should be conducted to evaluate the additional benefit of early, appropriate antimicrobial treatment for severely septic patients in settings like Uganda.
A lack of material supplies and skilled health workers contributes to the inability to achieve optimal sepsis management in settings like Uganda (14, 15). In busy hospitals which admit many patients, addition of a health worker dedicated to the provision of fluid resuscitation, early antibiotics and monitoring of critically ill patients may improve survival. Alternatively, hospitals with more health workers or fewer patients may be able to decrease mortality in severely septic patients with a sepsis management bundle without requiring an extra health worker dedicated to the management of critically ill patients. We also show an approach to augment management of severely ill patients with the use of a patient attendant. With more than 75% of the attendants successful in alerting the designated medical officer when fluid bottles were nearly empty, using patient attendants as an alert mechanism during resuscitation is a feasible option for busy health providers managing severely septic patients in resource-constrained settings and further studies evaluating this topic are needed.
The before and after observational study design is a major limitation of this study as there are both known and unknown potentially confounding variables that may not be balanced in a non-randomized study. While a randomized clinical trial would have unequivocally demonstrated the effect of the intervention on mortality, this study design was considered unethical at the time the study was being reviewed given the clinical benefits of fluid resuscitation supported by physiologic understanding and data from other settings (12, 16). While standard management of critically ill patients did not change at study sites during this period, a stepped-wedge community trial design for future studies of severely ill hospitalized patients might enable better assessment of the intervention effect (17). Also, recent findings on the potential detrimental effects of fluid resuscitation in severely infected East African children may introduce sufficient equipoise to justify to ethical review committees that a randomized clinical trial is indeed warranted to evaluate the benefits of fluid resuscitation and other sepsis management components in adults from settings like Uganda (18).
Another major limitation for the study is the two-year time gap which occurred between the two study cohorts. A potential source of bias introduced by this gap includes a Hawthorne effect by which overall management of patients with severe sepsis could have improved in the intervention cohort as an indirect consequence of conducting the observational study. Early management of patients with severe sepsis in the intervention cohort, however, was provided by the study medical officer and, therefore, care of intervention cohort patients for the first 6 hours of their hospitalization was directly influenced by this medical officer. After that period, it did not appear that patient care differed between cohorts as illustrated in Table 2 in which the measured volume of fluid administered in the period between 6 and 24 hours was the same (500 mL) for both cohorts. Also, the majority of patients in both cohorts were HIV-infected, reflecting high morbidity among persons with HIV in this setting (2). We observed greater HAART use in the intervention cohort, but adjustment for HAART and CD4 count in the multivariate analysis did not alter the effect of early sepsis management on 30-day mortality, likely since patients were severely immunosuppressed with CD4 counts less than 70 lymphocytes/mm3 in both cohorts. Finally, selection bias may have been introduced from the limitation that patients were only enrolled during daytime hours excluding weekends. The period of enrollment was primarily limited by the business hours during which collaborating laboratory institutions were open to process blood samples for blood cultures and investigations. Importantly, the period of study enrollment was the same for both cohorts so it is unlikely that this limitation disproportionately affected the results of either the observation or intervention cohorts.
CONCLUSIONS
This study demonstrates to health providers working in resource-constrained settings that a simple and inexpensive intervention can save lives and is feasible even in a busy public hospital. Provision of fluid resuscitation, antimicrobials and regular monitoring can occur with minimal or perhaps no additional staffing and can be augmented by patient attendants. Similar studies which assess the feasibility and cost effectiveness of other interventions for averting the high mortality of severely septic patients are needed to develop an evidence-based sepsis bundle which is applicable to low-income settings.
Acknowledgments
Mulago National Referral Hospital site – Medical officers: Nelson Kalema, Cassim Kalisa, Kasozi Kimuli, Gyaviira Makanga, Penelope Miremba, Charles Musoke, Elizabeth Nalintya, Angelo Nganizi, Victor Tumukunde, Salim Wakabi; Nursing officers: Ketty Akullo, Rose Banura, Helen Bileti, Frances Bugembe, Dorothy Ezataru, Lornah Hamalwa, Florence Kalawa, Florence Kateregga, Frederick Mutyabule, Doreen Nabawanuka, Josephine Nabulime, Sheila Nakato, Justine Nakiyimbwa, Sarah Nansikombi, James Oweikanga, Harriet Tibakabikoba; Laboratory support: Moses Joloba, Henry Kajumbula, Samson Omongot, Hannington Twinomugwezi; Data entrants: Jessica Nanyombi, Zalika Ngati; Administrative support: Jackie Mabweijano; Masaka Regional Referral Hospital site – Medical officers: Julius Amumpe, Emmanuel Lule, Henry Kyobe, Francis Ssali, Arthur Lubogo Mutaawe; Clinical officer: Patrick Dhikusooka, Simon Muwanga; Nursing officers: Fredrick Batte, Rose Emerin Nabadda, Judith Owokuhaisa, Scholastica Sekayiba; Laboratory support: James Ahimbisibwe, Simon Aluma, Pius Opendi, Joseph Ouma, Patricia Wagana; Administrative supervision: Nathan Kenya-Mugisha University of Washington – Statistical support: Amalia Magaret
Financial Support:
Primary funding for the study was from an Investigator-Initiated Award provided by Pfizer, Inc. Pfizer, Inc. had no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; or the preparation, review or approval of the manuscript. Additional non-grant support was provided by the Rakai Health Sciences Program through the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. In addition, Dr. Wald is supported by a National Institutes of Health K24 (AI 071113).
Footnotes
Institutions where the work was performed:
- Mulago National Referral Hospital, Kampala, Uganda
- Masaka Regional Referral Hospital, Masaka, Uganda
The authors have not disclosed any potential conflicts of interest
References
- 1.World Health Organization. The Global Burden of Disease: 2004 update. 2008 [cited 2010 28 July]; Available from: http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html.
- 2.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(6):417–32. doi: 10.1016/S1473-3099(10)70072-4. Epub 2010/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker JU, Theodosis C, Jacob ST, Wira CR, Groce NE. Surviving sepsis in low-income and middle-income countries: new directions for care and research. Lancet Infect Dis. 2009;9(9):577–82. doi: 10.1016/S1473-3099(09)70135-5. Epub 2009/08/22. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AC, West TE, Limmathurotsakul D, Peacock SJ. Strategies to reduce mortality from bacterial sepsis in adults in developing countries. PLoS Med. 2008;5(8):e175. doi: 10.1371/journal.pmed.0050175. Epub 2008/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34(1):17–60. doi: 10.1007/s00134-007-0934-2. Epub 2007/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrer R, Artigas A, Levy MM, Blanco J, Gonzalez-Diaz G, Garnacho-Montero J, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299(19):2294–303. doi: 10.1001/jama.299.19.2294. Epub 2008/05/22. [DOI] [PubMed] [Google Scholar]
- 7.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38(2):367–74. doi: 10.1097/CCM.0b013e3181cb0cdc. Epub 2009/12/26. [DOI] [PubMed] [Google Scholar]
- 8.Jacob ST, Moore CC, Banura P, Pinkerton R, Meya D, Opendi P, et al. Severe sepsis in two Ugandan hospitals: a prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS One. 2009;4(11):e7782. doi: 10.1371/journal.pone.0007782. Epub 2009/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore CC, Jacob ST, Pinkerton R, Meya DB, Mayanja-Kizza H, Reynolds SJ, et al. Point-of-care lactate testing predicts mortality of severe sepsis in a predominantly HIV type 1-infected patient population in Uganda. Clin Infect Dis. 2008;46(2):215–22. doi: 10.1086/524665. Epub 2008/01/04. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–48. doi: 10.1378/chest.09-0087. Epub 2009/08/22. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert DNMR, Ellopoulos GM, Chambers HF, Saag MS. The Sanford Guide to Antimicrobial Therapy. 40. Sperryville, VA: Antimicrobial Therapy; 2010. [Google Scholar]
- 12.Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002;30(8):1686–92. doi: 10.1097/00003246-200208000-00002. Epub 2002/08/07. [DOI] [PubMed] [Google Scholar]
- 13.Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–53. doi: 10.1097/CCM.0b013e3181cc4824. Epub 2010/01/06. [DOI] [PubMed] [Google Scholar]
- 14.Dunser MW, Baelani I, Ganbold L. A review and analysis of intensive care medicine in the least developed countries. Crit Care Med. 2006;34(4):1234–42. doi: 10.1097/01.CCM.0000208360.70835.87. Epub 2006/02/18. [DOI] [PubMed] [Google Scholar]
- 15.Baker T. Critical care in low-income countries. Trop Med Int Health. 2009;14(2):143–8. doi: 10.1111/j.1365-3156.2008.02202.x. Epub 2009/02/12. [DOI] [PubMed] [Google Scholar]
- 16.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. Epub 2002/01/17. [DOI] [PubMed] [Google Scholar]
- 17.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28(2):182–91. doi: 10.1016/j.cct.2006.05.007. Epub 2006/07/11. [DOI] [PubMed] [Google Scholar]
- 18.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. NEJM. 2011;362 (26):2483–95. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]