Summary
Since its discovery in 1956, rhinovirus (RV) has been recognized as the most important virus producing the common cold syndrome. Despite its ubiquity, little is known concerning the pathogenesis of RV infections, and some of the research in this area has led to contradictions regarding the molecular and cellular mechanisms of RV-induced illness. In this article, we discuss the pathogenesis of this virus as it relates to RV-induced illness in the upper and lower airway, an issue of considerable interest in view of the minimal cytopathology associated with RV infection. We endeavor to explain why many infected individuals exhibit minimal symptoms or remain asymptomatic, while others, especially those with asthma, may have severe, even life-threatening, complications (sequelae). Finally, we discuss the immune responses to RV in the normal and asthmatic host focusing on RV infection and epithelial barrier integrity and maintenance as well as the impact of the innate and adaptive immune responses to RV on epithelial function.
Keywords: Rhinovirus, asthma, pathogenesis, viral-induced asthma exacerbations
Introduction
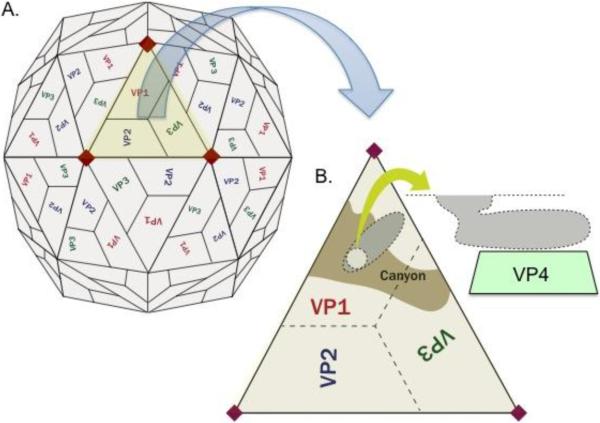
Rhinovirus (RV) was first isolated in 1956 by Dr. Winston Price at Johns Hopkins University and was quickly determined to be the most common cause of cold symptoms in adults [1, 2]. It is a positive sense, single-stranded non-enveloped RNA virus of the picornavirus family with well over 100 serotypes discovered to date [3]. The RNA genome serves as an mRNA, which encodes both structural (capsid) proteins and non-structural proteins that are involved in viral genome replication and virion assembly. Upon entry into a cell the viral genome is translated into a polyprotein, which in turn undergoes proteolytic cleavage to produce the structural and non-structural gene products. The RNA genome is packaged within a protein coat consisting of 4 viral capsid proteins 1, 2, 3, and 4 (VP1, VP2, VP3, and VP4) [3, 4](figure). Amino acid differences in one or more of these capsid proteins confer the antigenic differences among individual RV strains or serotypes. The serotypes can be classified as HRV-A, -B, or -C viruses based upon genetic homology [1, 3, 5].
Figure 1.
(A) Rhinovirus is a non-enveloped, spherical virus composed of a protein shell surrounding the naked RNA genome. The protein capsid consists of 4 polypeptides, viral capsid protein 1 (VP1), VP2, VP3, and VP4, in an icosahedral formation. (B) A hydrophobic pocket or “canyon” exists within VP1, which is the likely point of contact for ICAM-1 [4, 64, 65]. VP4 is located on the internal surface of the virus and is important in assembly of the virus during replication and infection of new cells [66].
Over 90% of the known RV serotypes of the HRV-A and -B families utilize ICAM-1 as their cell entry receptor, while the minor group receptor, low-density lipoprotein (LDL), is used by 10 serotypes [4, 6]. HRV binds ICAM-1 near the site of LFA-1 attachment and, as a consequence of binding, the virus loses its protein capsid. Though somewhat controversial, this uncoating process is thought to occur via intermediate particles characterized by the loss of VP4 and the externalization of the hydrophobic N-termini of VP1, and ultimately this leads to transmigration of viral RNA through the host cell membrane [4].
HRV-C has more recently emerged as a virus of interest, particularly in RV-induced exacerbations of asthma[7]. The genomes of several strains of HRV-C have been recently sequenced, but, to date, the structural information has not as yet shed light on a potential cellular receptor and the receptor it employs to infect epithelial cells remains unclear. Based upon structural modeling studies, this is unlikely to be either ICAM-1 or the LDL receptor. Gern, J. et al. were the first to grow HRV-C in vitro, utilizing sinus mucosal tissue as the cellular substrate for in vitro HRV-C replication [3]. At present, HRV-C infection has been studied to only a limited extent and little is known regarding pathogenic mechanisms unique to this RV subtype. Consequently, the remainder of this review will focus on findings involving infection with HRV-A and -B.
Upper and Lower Respiratory Tract Disease Pathogenesis
In non-asthmatic individuals, symptoms of RV infection are generally limited to the upper respiratory tract. Rhinorrhea and nasal obstruction, the most prominent symptoms, are associated with a neutrophilic inflammatory response that is associated with increased vascular permeability and stimulation of mucus hypersecretion. Cough is a less common but bothersome manifestation of rhinovirus URI. The pathogenesis of cough may involve irritation from posterior pharyngeal drainage or direct infection of the large airways. Gwaltney, J. et al. [8] demonstrated sinus involvement in many individuals with typical common cold symptoms. The sinus disease resolved without intervention suggesting that these upper respiratory illnesses should be more accurately characterized as a viral rhinosinusitis. However, the inflammation associated with obstruction of sinus openings and secondary Eustachian tube dysfunction can predispose to acute bacterial sinusitis and otitis media, respectively.
In contrast, lower respiratory symptoms associated with RV infection are most prominent in patients who have underlying asthma or other chronic lung disease. These symptoms include cough, shortness of breath, chest tightness, and wheezing [9–13]. The basis for these lower respiratory symptoms has been a source of controversy concerning mechanisms of RV pathogenesis. Specifically, the underlying debate centers on the extent to which RV can infect cells of the lower respiratory tract and, as such, whether bronchial infection forms the basis for respiratory symptoms, as opposed to reflecting indirect influences related to the immune response to the upper airway infection. There are a variety of potential barriers to infection of lungs by RV including the temperature sensitivity of replication of the virus. RV replicates optimally at 33° C, a temperature significantly lower than that of bronchial airway epithelium [14]. It is noteworthy that RV has been concomitantly isolated with bacterial pathogens in 24–54% of children and 10–18% of adults with pneumonia [15–17]. From these studies it is unclear if RV is ever the cause of the agent for the development of pneumonia. Thus, more important to respiratory tract infectious disease pathogenesis during RV infection, may be the capacity of RV to predispose to concomitant or subsequent infection with other respiratory pathogens. For instance, human tracheal epithelial cells simultaneously infected with RV14 and Strept. pneumoniae show increased adherence of the Strept. [18]. Similarly, macrophages exposed to RV showed impaired responsiveness of pattern recognition receptors (PRRs) following exposure to bacterial toll-like receptors (TLR) agonists e.g. lipopolysaccharide and lipoteichoic acid [19]. Furthermore, studies implicating RV involvement in lower airway pathology based on the detection of RV antigens (or genomes) in lower respiratory tract airways are confounded by the inability to exclude upper airway-derived RV contamination of bronchial samples. This is certainly problematic with sputum analysis, but even bronchoscopically-obtained samples can be contaminated during the bronchoscope's passage through the upper airway. However, several studies support the presence of RV in the lower airway [20, 21] including work showing RV by in situ hybridization after experimental RV16 infection [13]. Work by this group also demonstrates that while RV serotypes replicate optimally at 33° i.e., the temperature of the upper respiratory tract, the higher temperature of the lower airways is not an absolute barrier to RV replication [22]. The preponderance of current opinion therefore supports the concept that RV likely can productively infect cells of the lower airways.
Clinical and Subclinical Infections
Early studies utilizing tissue culture isolation to detect RV in the nasal secretions of patients with cold symptoms undoubtedly under-reported the frequency of RV infections. Since the advent of nucleic acid-based detection, it is possible to more reliably discern the actual prevalence of RV infection. However, the application of sensitive PCR based detection techniques immediately led to a quandary regarding the issue of the prevalence of asymptomatic infection with RV. This confirmed earlier suspicions regarding the likely prevalence of asymptomatic infection, recognized from the challenge model. RV is detected by RT-PCR in ~12–22% of asymptomatic individuals. Many of these may be false positives. Alternatively, if these do reflect non-infectious colonization, a mechanism for long-term survival of the RV in the nares in the absence of infection is not obvious. Plausibly, less virulent strains of RV could produce an asymptomatic infection. However, a study of asymptomatic individuals with positive PCR tests for RV found that asymptomatic infection was usually associated with simultaneous symptomatic infection in family members [5]. Similarly, in our own experience from experimental infections with RV39, quantitative RV titers from asymptomatic or minimally symptomatic subjects were equivalent to those with the worst symptoms. Along with the striking observations regarding the absence of direct cytopathology produced by RV (discussed next), these observations suggest that it is the nature and extent of the immune response to the virus that determines the symptom profile and not the severity or direct pathology caused by the infection itself.
Pathogenic Influences of RV on the Epithelium
While other respiratory viruses such as influenza and respiratory syncytial virus destroy the airway epithelial barrier, studies demonstrate that RV by itself does not cause cytopathology. For these studies, monolayers of adenoid tissue were infected with RV and, at the time of peak secreted viral titers, no detectable damage or other cytopathic effect was observed [23]. This is consistent with the failure to observe cytopathology in RV-infected nasal or bronchial biopsy tissue. Infection does, however, disrupt epithelial barrier function. The effects of RV to increase vascular leakage and mucus secretion reflect in part this ability of the RV to disrupt the epithelial barrier, specifically the disruption of tight junctions. Studies utilizing cultured human nasal epithelial cells showed decreased zona occluden-1, claudin-1, and E-cadherin mRNA and protein levels after infection with RV [24]. This is consistent with observations regarding the disruption of airway epithelial apical junctions by poly dI:dC [25]. In addition to increasing permeability, this disruption of the epithelial barrier will facilitate translocation of pathogens (including non-RV pathogens) and their soluble products, and expose basolateral epithelial receptors, where TLR and other PRRs are prominently located.
Immune Response to Rhinovirus
In the absence of an ability to ascribe the presence and extent of symptoms to either virus titer or cytopathology, we propose that it is the characteristics of the host response to RV that are the primary determinant of symptoms. The host response to the virus includes those mediated by the innate, humoral, and cellular immune systems. To some extent these distinct responses represent a continuum with the progressive evolution of more severe (and more symptomatic) responses, although the specific sequence of this continuum may vary from patient to patient.
Innate Immunity
In the absence of pre-existing humoral immunity (discussed below) or presumably other mucosal surface-associated factors, RV will infect the epithelium and this will initially lead to the induction of an innate immune response. This occurs very rapidly as evinced by our studies showing appearance of type I interferon along with a drop in airway pH less than 24 hrs after experimental infection [26]. Early innate detection of RV depends on the host's ability to recognize RV-associated pattern recognition receptors including via TLR and other PRRs (e.g. retinoic acid inducible gene- I (RIG-I) and melanoma differentiation associated gene-5 (MDA-5)). RV capsid is recognized by TLR2 on the epithelial surface, whereas, after internalization and initiation of RV-directed RNA translation, RV-associated ssRNA and dsRNA are recognized by endosomal TLR3, TLR7, and TLR8. In addition, dsRNA is also recognized by MDA-5 and RIG-I [27, 28]. Engagement of these receptors induces cytokine expression including type I (IFN-α/-ß) and type III interferons (IL-28A, IL-28B, and IL-29), but also IL-6, IL-12, and IL-15. IFNs directly restrict virus replication but these and other cytokines including IL-12 and IL-15 play important roles in cytotoxic and natural killer cell differentiation, survival, and recruitment [29]. Elicited NK cells are an important early source of IFN-γ. IL-6 is involved in numerous facets of innate immunity that influence RV elimination [30] and an IL-6 single nucleotide polymorphism predicts worse illness [31]. Other important cytokines released by RV-infected epithelium include IL-1ß and IL-11.
Arguably the most important determinants of the clinical outcome of RV infection comprise growth factors, such as G-CSF and GM-CSF, and chemokines, such as CXCL8 (IL- 8), CXCL5 (ENA-78), CXCL10 (IP-10), and CCL5 (RANTES), that together drive granulocyte recruitment, survival and activation. These granulocytes are primarily neutrophils, reflecting especially the activities of CXCL8 and CXCL10. These mediators appear rapidly in nasal lavage fluid and serum of RV-infected patients and their concentrations parallel increases in peripheral blood neutrophils. The role – if any – of PMN in RV eradication is unclear but the ensuing neutrophil-laden nasal exudate is one of the more characteristic features of “colds” and the early expression of CXCL8 and CXCL10 links to the presence of symptomatic RV infections [32]. A neutrophilic exudate has also been associated with increases in kallikrein, which drives the production of kallidin and bradykinin [33]. These kinins are elevated in the nasal washes of subjects with symptomatic RV infections, particularly in those with allergies and asthma [34–37]. Eosinophils can also be robustly expressed [38]. The induction of eosinophilia may influence the ability of RV to produce nasal symptoms by enhancing bystander allergic reactions (discussed below). In contrast, eosinophils, in part through their ability to secrete numerous potent RNAses, appear to promote virus eradication [39].
Humoral Immune Responses
The therapeutic importance of humoral immune responses to RV is increasingly recognized. In experimental RV inoculation, B cell responses in the form of mucosal RV serotype-specific IgA were detected by day 3 and IgG at days 7–8 [40]. A role for this humoral response is suggested by observations that the presence of serotype-specific neutralizing IgG antibodies precludes subsequent challenge infection following experimental inoculation with an RV of that serotype [41]. It should be emphasized that given the need for neutralizing antibody to be present at the nasal mucosa boundary, it is likely that secretory IgA would be the actual determinant of protective humoral immunity. Antibodies could contribute to viral clearance by acting as neutralizing antibodies, e.g., blocking cellular attachment ligands, opsonizing the virus for presentation to phagocytic cells, or by initiating NK cell-mediated antibody-dependent cellular cytotoxicity. In addition to direct virus neutralization, pre-existing antibodies may also serve to mediate antibody-facilitated antigen uptake and promote more rapid and effective cellular immune responses.
The concept that humoral immune responses have primary importance in preventing and eradicating infection is further derived from observations regarding the increased frequency and severity of infection in patients with humoral immune failure (e.g., common variable immune deficiency). In these conditions, RV was the most common virus producing respiratory infections [42]. This was not corrected with replacement immunoglobulin, further implicating the need for serotype-specific antibodies, which could be lacking in any given commercial immunoglobulin preparation.
Cellular Immune Responses
In the absence of neutralizing antibodies or an effective innate immune response, RV-specific T-cells become central in virus eradication. The rapidity with which viral titers begin to decline after an RV infection, usually at ~72 hours, precludes the possibility that this reflects the de novo activation of naïve RV serotype-specific T cells. This observed timeframe is only consistent with activation of pre-existing effector/memory T cells, which must therefore responding to shared epitope(s) displayed by the infecting RV. In unpublished work (Woodfolk, J. and Kwok, W., personal communication), an HLA-DR4-restricted CD4-specific epitope of RV39 VP1 was found to map to a region of the molecule that is conserved across RV groups A, B and C. These observations imply that CD4+ T cells induced by one RV strain are capable of responding to other strains and could drive the observed rapid and potent T cell recall response.
Both CD4- [43] and CD8-specific T cell responses develop as consequences of RV infection. CD4 cells are largely Th1-like and their production of IFN-γ contributes to the anti-viral immune response, but these CD4 cells will also facilitate development of the humoral immune response. CD8 T cells are likely central to the adaptive immune response driving RV eradication, although their presence and role has not been extensively evaluated. In our unpublished studies, these cells can be identified after infection and are also characterized by robust IFN-γ production. An additional mechanism that may contribute to the degree of symptoms developing with RV infection could reflect – similar to other respiratory virus-targeting immune responses – the propensity of these cells to concomitantly express IL-10 and thereby mitigate bystander immune-mediated damage [44, 45].
Mechanism of Asthma Exacerbations in Association with RV Infections
Any discussion of RV-associated disease pathogenesis must appreciate the striking capacity of this virus to drive asthma exacerbations. Among children, 80 to 85% of asthma exacerbations are associated with upper respiratory viral infections [46, 47] and RV consistently accounts for ~60–70% of these virus-associated exacerbations [48–55]. For example, in our studies, viral infections were identified in 61% of children aged 3–18 years hospitalized with an asthma exacerbation. RV accounted for 77% of all positive tests and was the only virus significantly associated with asthma [56]. It should be noted, however, that RV infections are common and most do not produce exacerbations ([57] and unpublished data) and, similarly, asthma exacerbations are not a frequent response to experimental RV challenges, including in our published studies [58, 59].
Determining the underlying mechanisms for asthma exacerbations caused by RV has remained elusive. One theory entertains the notion that asthmatics have a deficient innate immune response to the virus. A study of bronchial epithelial cells observed decreased IFN-ß expression in asthmatics [60] and more recently, the same group has reported that asthmatics have deficient IL-15 [61]. These authors posit that this deficiency led to increased virus load and prolonged symptoms during experimental RV16 infections.
However, other studies including our unpublished work do not confirm that asthmatics exhibit more robust RV replication during an infection when compared to non-asthmatics [62]. Our studies show a strong correlation of the ability of RV to induce an asthma exacerbation to the presence of relevant aeroallergen sensitization [59]. The concept that RV may act to synergize with a bystander IgE-mediated allergic reaction to drive asthma exacerbations is supported by a multi-center trial showing the ability of anti-IgE therapy (omalizumab) to block the seasonally-observed pattern of asthma exacerbations, which included subjects infected with RV [63]. Although the mechanism by which RV could enhance an ongoing allergic reaction is not known, one possible contribution pertains to the observation previously discussed regarding the ability of RV to alter intracellular connections between epithelium, a process that would allow free access of allergens to mucosal tissue.
Summary
A debate remains as to whether it is the inherent pathogenicity of RV that leads to the associated symptoms, or whether it is the environment in which the virus replicates that determines the induction of symptoms. We argue that it is that various facets of the immune response to the virus that are important in restricting the infection but simultaneously drive the symptoms of RV infection, as the virus itself is not cytopathic. Whether features of physical barrier function, the innate immune system, or the adaptive immune response determine the pathogenesis of this virus, or different combinations acting in different subjects remains to be determined. Enhancing the understanding of these mechanisms will dictate future directives for treatment in patients, especially those whose asthma undergoes severe exacerbations by this virus.
Highlights
Rhinovirus is a ubiquitous virus and the usual cause of the common cold, yet little is known regarding its pathogenic mechanisms.
The upper and lower airways are the primary targets of RV, but, surprisingly, this virus causes little cytopathology.
Many patients will have positive tests to RV, yet remain subclinical.
The host epithelial barriers and both innate and adaptive immune responses influence the reaction of the host.
The various immune responses lead to the distinct outcomes from subclinical to severe and even life-threatening infections.
Acknowledgements
The authors would like to acknowledge Dr. Judith Woodfolk for her gracious review and edits of this paper. Also, we appreciate the work of Zach Kennedy for the illustrations included within this review.
Dr. Larry Borish is funded by NIH RO1 AI057483 and R21 AI1090413. Dr. Thomas Braciale has funding from NIH R01 AI15608, R01 HL33391, U19 AI83024 and R21 AI1090413. Dr. Ron Turner has received funding from Henkel, Inc, Johnson and Johnson Pharmaceutical Research and Development, NIH 5R43 AI085683, and NIH 5RO1 AI066367. Dr. Peter Heymann has funding provided by Novartis and the Cove Creek foundation. Dr. Josh Kennedy received funding from NIH 5T32 AI007496-17 and the University of Virginia Children's Hospital Grant-in-Aid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
*Articles of special interest
**Articles of outstanding interest
- 1.Peltola V, Waris M, Osterback R, Susi P, Hyypia T, Ruuskanen O. Clinical effects of rhinovirus infections. J Clin Virol. 2008 Dec;43(4):411–4. doi: 10.1016/j.jcv.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Price WH. The Isolation of a New Virus Associated with Respiratory Clinical Disease in Humans. Proc Natl Acad Sci U S A. 1956 Dec;42(12):892–6. doi: 10.1073/pnas.42.12.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **3.Bochkov YA, Palmenberg AC, Lee WM. Molecular modeling, organ culture and reverse genetics for a newly identified human rhinovirus C. Nat Med. 2011 May;17(5):627–32. doi: 10.1038/nm.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]; This novel paper describes techniques for the previously uncultured rhinovirus C utilizing sinus epithelium. The genotype of this virus is also described.
- 4.Bella J, Rossmann MG. ICAM-1 receptors and cold viruses. Pharm Acta Helv. 2000 Mar;74(2–3):291–7. doi: 10.1016/S0031-6865(99)00056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jartti T, Jartti L, Peltola V, Waris M, Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J. 2008 Dec;27(12):1103–7. doi: 10.1097/INF.0b013e31817e695d. [DOI] [PubMed] [Google Scholar]
- 6.Vlasak M, Roivainen M, Reithmayer M. The minor receptor group of human rhinovirus (HRV) includes HRV23 and HRV25, but the presence of a lysine in the VP1 HI loop is not sufficient for receptor binding. J Virol. 2005 Jun;79(12):7389–95. doi: 10.1128/JVI.79.12.7389-7395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Bizzintino J, Lee WM, Laing IA. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. 2011 May;37(5):1037–42. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this recent study, patients with asthma exacerbations had rhinovirus C (RVC) more often, and infection this virus was associated with more severe disease.
- 8.Gwaltney JM, Jr., Phillips CD, Miller RD, Riker DK. Computed tomographic study of the common cold. N Engl J Med. 1994 Jan 6;330(1):25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 9.Collinson J, Nicholson KG, Cancio E. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax. 1996 Nov;51(11):1115–22. doi: 10.1136/thx.51.11.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkerts G, Busse WW, Nijkamp FP, Sorkness R, Gern JE. Virus-induced airway hyperresponsiveness and asthma. Am J Respir Crit Care Med. 1998 Jun;157(6 Pt 1):1708–20. doi: 10.1164/ajrccm.157.6.9707163. [DOI] [PubMed] [Google Scholar]
- 11.Las Heras J, Swanson VL. Sudden death of an infant with rhinovirus infection complicating bronchial asthma: case report. Pediatr Pathol. 1983 Jul-Sep;1(3):319–23. doi: 10.3109/15513818309040669. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson KG, Kent J, Hammersley V, Cancio E. Risk factors for lower respiratory complications of rhinovirus infections in elderly people living in the community: prospective cohort study. Bmj. 1996 Nov 2;313(7065):1119–23. doi: 10.1136/bmj.313.7065.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadopoulos NG, Bates PJ, Bardin PG. Rhinoviruses infect the lower airways. J Infect Dis. 2000 Jun;181(6):1875–84. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 14.Killington RA, Stott EJ, Lee D. The effect of temperature on the synthesis of rhinovirus type 2 RNA. J Gen Virol. 1977 Sep;36(3):403–11. doi: 10.1099/0022-1317-36-3-403. [DOI] [PubMed] [Google Scholar]
- 15.Jennings LC, Anderson TP, Beynon KA. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008 Jan;63(1):42–8. doi: 10.1136/thx.2006.075077. [DOI] [PubMed] [Google Scholar]
- 16.Juven T, Mertsola J, Waris M, Leinonen M, Ruuskanen O. Clinical response to antibiotic therapy for community-acquired pneumonia. Eur J Pediatr. 2004 Mar;163(3):140–4. doi: 10.1007/s00431-003-1397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas EC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005 Aug 1;41(3):345–51. doi: 10.1086/431588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizuka S, Yamaya M, Suzuki T. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis. 2003 Dec 15;188(12):1928–39. doi: 10.1086/379833. [DOI] [PubMed] [Google Scholar]
- 19.Oliver BG, Lim S, Wark P. Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax. 2008 Jun;63(6):519–25. doi: 10.1136/thx.2007.081752. [DOI] [PubMed] [Google Scholar]
- 20.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997 Mar;155(3):1159–61. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 21.Halperin SA, Eggleston PA, Hendley JO, Suratt PM, Groschel DH, Gwaltney JM., Jr. Pathogenesis of lower respiratory tract symptoms in experimental rhinovirus infection. Am Rev Respir Dis. 1983 Nov;128(5):806–10. doi: 10.1164/arrd.1983.128.5.806. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulos NG, Sanderson G, Hunter J, Johnston SL. Rhinoviruses replicate effectively at lower airway temperatures. J Med Virol. 1999 May;58(1):100–4. doi: 10.1002/(sici)1096-9071(199905)58:1<100::aid-jmv16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Winther B, Gwaltney JM, Hendley JO. Respiratory virus infection of monolayer cultures of human nasal epithelial cells. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):839–45. doi: 10.1164/ajrccm/141.4_Pt_1.839. [DOI] [PubMed] [Google Scholar]
- **24.Yeo NK, Jang YJ. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope. 2010 Feb;120(2):346–52. doi: 10.1002/lary.20764. [DOI] [PubMed] [Google Scholar]; RV infection led to decreased mRNA expression of zona occludens proteins and microscopic evidence of altered tight junctions in primary cultured human nasal epithelium.
- **25.Rezaee F, Meednu N, Emo JA. Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. J Allergy Clin Immunol. 2011 Dec;128(6):1216–24. e11. doi: 10.1016/j.jaci.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper suggests that viral counterparts can lead to disassembly of the tight junctions of airway epithelial cells, leaving the airways vulnerable to infection and allergen infiltration.
- 26.Ngamtruakulpanit LVJ, Nguyen A, Urban P, Hom S, Smith A, Gaston B, Turner R, Hunt J. Exhaled breath condensate acidification during rhinovirus colds. American Thoracic Society 99th International Conference; Seattle. 2003. [Google Scholar]
- **27.Triantafilou K, Vakakis E, Richer EA, Evans GL, Villiers JP, Triantafilou M. Human rhinovirus recognition in non-immune cells is mediated by Toll-like receptors and MDA-5, which trigger a synergetic pro-inflammatory immune response. Virulence. 2011 Jan-Feb;2(1):22–9. doi: 10.4161/viru.2.1.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]; These experiments demonstrated that TLRs play a crucial role in HRV recognition. They also suggest that after viral replication and generation of dsRNA the type I IFN inflammatory response is mediated by MDA-5.
- **28.Slater L, Bartlett NW, Haas JJ. Co-ordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 2010;6(11):e1001178. doi: 10.1371/journal.ppat.1001178. [DOI] [PMC free article] [PubMed] [Google Scholar]; The innate immune response to RV is the first-line of defense against RV. This study shows that in a murine model, TLR3, RIG-1, and MDA5 and inducible RNA helicases are important in coordinated recognition of rhinovirus infection.
- 29.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001 Jan 1;97(1):14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 30.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- *31.Doyle WJ, Casselbrant ML, Li-Korotky HS. The interleukin 6 –174 C/C genotype predicts greater rhinovirus illness. J Infect Dis. 2010 Jan 15;201(2):199–206. doi: 10.1086/649559. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study of adults with experimental rhinovirus infection, polymorphisms in the interleukin-6 gene were associated with greater symptoms. The investigators also evaluated polymorphisms in the IFN-γ gene and found that patients with certain polymorphism were more susceptible to sero-conversion to the virus that was given experimentally.
- 32.Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000 Dec;162(6):2226–31. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- 33.Lauredo IT, Forteza RM, Botvinnikova Y, Abraham WM. Leukocytic cell sources of airway tissue kallikrein. Am J Physiol Lung Cell Mol Physiol. 2004 Apr;286(4):L734–40. doi: 10.1152/ajplung.00129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Igarashi Y, Skoner DP, Doyle WJ, White MV, Fireman P, Kaliner MA. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol. 1993 Nov;92(5):722–31. doi: 10.1016/0091-6749(93)90016-9. [DOI] [PubMed] [Google Scholar]
- 35.Naclerio RM, Proud D, Lichtenstein LM. Kinins are generated during experimental rhinovirus colds. J Infect Dis. 1988 Jan;157(1):133–42. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- 36.Proud D, Naclerio RM, Gwaltney JM, Hendley JO. Kinins are generated in nasal secretions during natural rhinovirus colds. J Infect Dis. 1990 Jan;161(1):120–3. doi: 10.1093/infdis/161.1.120. [DOI] [PubMed] [Google Scholar]
- 37.Christiansen SC, Eddleston J, Bengtson SH. Experimental rhinovirus infection increases human tissue kallikrein activation in allergic subjects. Int Arch Allergy Immunol. 2008;147(4):299–304. doi: 10.1159/000144037. [DOI] [PubMed] [Google Scholar]
- 38.Proud D, Leigh R. Epithelial cells and airway diseases. Immunol Rev. 2011 Jul;242(1):186–204. doi: 10.1111/j.1600-065X.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 39.Avila PC, Abisheganaden JA, Wong H. Effects of allergic inflammation of the nasal mucosa on the severity of rhinovirus 16 cold. J Allergy Clin Immunol. 2000 May;105(5):923–32. doi: 10.1067/mai.2000.106214. [DOI] [PubMed] [Google Scholar]
- 40.Message SD, Johnston SL. The immunology of virus infection in asthma. Eur Respir J. 2001 Dec;18(6):1013–25. doi: 10.1183/09031936.01.00228701. [DOI] [PubMed] [Google Scholar]
- 41.Alper CM, Doyle WJ, Skoner DP, Buchman CA, Cohen S, Gwaltney JM. Prechallenge antibodies moderate disease expression in adults experimentally exposed to rhinovirus strain hanks. Clin Infect Dis. 1998 Jul;27(1):119–28. doi: 10.1086/514634. [DOI] [PubMed] [Google Scholar]
- 42.Kainulainen L, Vuorinen T, Rantakokko-Jalava K, Osterback R, Ruuskanen O. Recurrent and persistent respiratory tract viral infections in patients with primary hypogammaglobulinemia. J Allergy Clin Immunol. 2010 Jul;126(1):120–6. doi: 10.1016/j.jaci.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gern JE, Dick EC, Kelly EA, Vrtis R, Klein B. Rhinovirus-specific T cells recognize both shared and serotype-restricted viral epitopes. J Infect Dis. 1997 May;175(5):1108–14. doi: 10.1086/516449. [DOI] [PubMed] [Google Scholar]
- *44.Sun J, Cardani A, Sharma AK. Autocrine regulation of pulmonary inflammation by effector T-cell derived IL-10 during infection with respiratory syncytial virus. PLoS Pathog. 2011 Aug;7(8):e1002173. doi: 10.1371/journal.ppat.1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]; In a murine model of RSV, this group demonstrated that effector T cell-derived IL-10 limits the excess pulmonary inflammation and thereby acts to maintain critical lung function. This research suggests a critical role of effector T cells in limiting disease severity that may apply to RV infections.
- *45.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009 Mar;15(3):277–84. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]; These studies show that IL-10 produced by effector T cells, especially CD8+ effector cells, also dampens the immune responses to influenza virus infection, and blocking this cytokine was lethal in a mouse model. The studies again support a similar role for cytotoxic T cell-derived IL-10 in ameliorating RV-mediated symptoms and asthma exacerbations..
- 46.Johnston SL, Pattemore PK, Sanderson G. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. Bmj. 1995 May 13;310(6989):1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chauhan AJ, Inskip HM, Linaker CH. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet. 2003 Jun 7;361(9373):1939–44. doi: 10.1016/S0140-6736(03)13582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. Bmj. 1993 Oct 16;307(6910):982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston SL, Pattemore PK, Sanderson G. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996 Sep;154(3 Pt 1):654–60. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 50.Johnston NW, Johnston SL, Duncan JM. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005 Jan;115(1):132–8. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kling S, Donninger H, Williams Z. Persistence of rhinovirus RNA after asthma exacerbation in children. Clin Exp Allergy. 2005 May;35(5):672–8. doi: 10.1111/j.1365-2222.2005.02244.x. [DOI] [PubMed] [Google Scholar]
- 52.Khetsuriani N, Kazerouni NN, Erdman DD. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007 Feb;119(2):314–21. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray CS, Poletti G, Kebadze T. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006 May;61(5):376–82. doi: 10.1136/thx.2005.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sears MR. Epidemiology of asthma exacerbations. J Allergy Clin Immunol. 2008 Oct;122(4):662–8. doi: 10.1016/j.jaci.2008.08.003. quiz 9–70. [DOI] [PubMed] [Google Scholar]
- 55.Wos M, Sanak M, Soja J, Olechnowicz H, Busse WW, Szczeklik A. The presence of rhinovirus in lower airways of patients with bronchial asthma. Am J Respir Crit Care Med. 2008 May 15;177(10):1082–9. doi: 10.1164/rccm.200607-973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heymann PW, Carper HT, Murphy DD. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004 Aug;114(2):239–47. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walter MJ, Castro M, Kunselman SJ. Predicting worsening asthma control following the common cold. Eur Respir J. 2008 Dec;32(6):1548–54. doi: 10.1183/09031936.00026808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halperin SA, Eggleston PA, Beasley P. Exacerbations of asthma in adults during experimental rhinovirus infection. Am Rev Respir Dis. 1985 Nov;132(5):976–80. doi: 10.1164/arrd.1985.132.5.976. [DOI] [PubMed] [Google Scholar]
- 59.Zambrano JC, Carper HT, Rakes GP. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol. 2003 May;111(5):1008–16. doi: 10.1067/mai.2003.1396. [DOI] [PubMed] [Google Scholar]
- 60.Wark PA, Johnston SL, Bucchieri F. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005 Mar 21;201(6):937–47. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laza-Stanca V, Message SD, Edwards MR. The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations. PLoS Pathog. 2011 Jul;7(7):e1002114. doi: 10.1371/journal.ppat.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Denlinger LC, Sorkness RL, Lee WM. Lower airway rhinovirus burden and the seasonal risk of asthma exacerbation. Am J Respir Crit Care Med. 2011 Nov 1;184(9):1007–14. doi: 10.1164/rccm.201103-0585OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Busse WW, Morgan WJ, Gergen PJ. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011 Mar 17;364(11):1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clinical research performed in inner city children shows that treatment with omalizumab (anti-IgE humanized antibodies) effectively blocks the seasonal peaks seen with asthma exacerbations during the fall and spring. This suggests that exacerbations of bystander allergic reactions plays an important and perhaps necessary role in the pathogenesis of RV-induced asthma exacerbations.
- 64.Olson NH, Kolatkar PR, Oliveira MA. Structure of a human rhinovirus complexed with its receptor molecule. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):507–11. doi: 10.1073/pnas.90.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colonno RJ, Condra JH, Mizutani S, Callahan PL, Davies ME, Murcko MA. Evidence for the direct involvement of the rhinovirus canyon in receptor binding. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5449–53. doi: 10.1073/pnas.85.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuchs R, Blaas D. Uncoating of human rhinoviruses. Rev Med Virol. 2010 Sep;20(5):281–97. doi: 10.1002/rmv.654. [DOI] [PubMed] [Google Scholar]