Abstract
Sex-differences exist in tobacco smoking behaviors. Nicotine, the primary addictive ingredient in tobacco smoke indirectly affects γ-amino butyric acid (GABA) function. Previous studies reported sex by smoking interactions in brain GABA levels. The goal of the present study was to evaluate if there is a sex by smoking interaction at the GABAA-benzodiazepine receptors (GABAA-BZRs), as well as relationships between GABAA-BZR availability and behavioral variables before and after 1wk of smoking cessation. Twenty six women (8 nonsmokers, age 36.0 ± 13.4y; 19 smokers, age 34.6 ± 8.9y) and twenty five men (8 nonsmokers, age 37.9 ± 13.8y; 17 smokers, 34.1 ± 12.4y) were imaged using [123I]iomazenil and single photon emission computed tomography (SPECT). Smokers were imaged at baseline 7h after the last cigarette. A significantly great number of men were able to abstain from smoking for 1 week (p=0.003). There were no significant differences in nicotine dependence and cigarette craving, mood or pain sensitivity between male and female smokers. There was a significant effect of sex across all brain regions (frontal, parietal, anterior cingulate, temporal and occipital cortices, and cerebellum; p<0.05), with all women (smokers and nonsmokers combined) having a higher GABAA-BZR availability than all men. There was a negative correlation between GABAA-BZR availability and craving (p≤;0.02) and pain sensitivity (p=0.04) in female but not male smokers. This study provides further evidence of a sex-specific regulation of GABAA-BZR availability in humans and demonstrates the potential for GABAA-BZRs to mediate tobacco smoking craving and pain symptoms differentially in female and male smokers.
Keywords: craving, GABAA-BZR, imaging, pain sensitivity, sex, smoking
Introduction
The experience of tobacco smoking differs between women and men. Women find the smell and taste of cigarette smoke to be more reinforcing (Perkins et al., 2001), receive greater relief from cigarettes after acute abstinence, and develop tolerance to nicotine's effects more quickly than men (Fant et al., 1996). However, women tend to smoke fewer cigarettes, inhale less, and smoke cigarettes with less nicotine, resulting in less nicotine intake than men [reviewed in (Grunberg et al., 1991)]. Despite this, women have greater difficulty abstaining from cigarettes than men and have higher rates of relapse (Bohadana et al., 2003, Pomerleau et al., 2005).
Nicotine, the main addictive ingredient in tobacco smoke, acts at the nicotinic acetylcholine receptors (nAChRs) that in turn stimulate γ-aminobutyric acid (GABA) function in the thalamus, hippocampus, and throughout the cerebral cortex (Domino et al., 2000; Erhardt et al., 2002; Fedele et al., 1998; Ghatan et al., 1998; Mansvelder et al., 2002; Meshul et al., 2002; Reid et al., 2000). While acute nicotine administration enhances brain GABA activity (Lena et al., 1993, McMahon et al., 1994b, a), there is limited evidence on nicotine-induced alterations in GABAA receptor levels. One rodent ex vivo study detected a 25% increase in cortical GABAA-benzodiazepine receptors (GABAA-BZRs) in response to chronic nicotine treatment (Magata et al., 2000). However, a preliminary study conducted in our laboratory did not reveal a significant effect of tobacco smoking on the GABAA-BZR availability. Specifically, we did not detect significant differences in GABAA-BZR availability in living human tobacco smokers compared to nonsmokers, nor did we observe a difference in GABAA-BZR availability from baseline to abstinence in a small subset of smokers who agreed to abstain from smoking for 5 weeks and repeated the scanning protocol (Esterlis et al., 2009).The difference observed between the above mentioned rodent study and our human study may be due to a sex-specific regulation of GABA levels by sex-steroid hormones, which in nonsmoking women are highest during the follicular phase and lowest in the luteal phase (Epperson et al., 2002). However, in the smoking women, the interaction of GABA by menstrual cycle phase is disrupted and GABA levels do not fluctuate across the menstrual cycle (Epperson et al., 2005). In addition to losing the GABA cyclicity, Epperson and colleagues found that female smokers have overall lower GABA levels than female nonsmokers, but there was no difference between male smokers compared to male nonsmokers. Thus, although tobacco smoking does not appear to regulate GABAA-BZR availability in a combined sample of male and female smokers, there is a smoking by sex-specific regulation of GABA neurotransmitter levels shown in previous studies.
Tobacco smoking also appears to modify the relationship between GABAA-BZR availability and mood symptoms. Specifically, GABAA-BZR availability was negatively correlated with subsyndromal anxiety and depressive symptoms in nonsmokers but not smokers (Esterlis et al., 2009). It was hypothesized that smoking disrupts the relationship between receptor availability and mood symptoms, or that the GABAA-BZRs function differently in smokers. Sex differences were not examined in that preliminary study. However, sex differences in the GABA system have been identified among individuals with anxiety disorders (Pande et al., 2000) with preliminary reports of lower GABAA-BZR availability in women as compared to men with panic disorder (Malizia et al., 1998), and a trend toward lower GABAA-BZR availability was observed in alcohol-dependent women as compared to controls (Lingford-Hughes et al., 2000). Evidence from animal models of anxiety also suggests that this receptor is mediated differentially in female versus male rodents (Nett et al., 1999, Chadda and Devaud, 2004, Kyrozis et al., 2006).
In addition to GABA's known ability to modulate anxiety and mood, this neurotransmitter has been shown to be involved in pain processes. For example, direct and indirect GABA receptor agonists have antinociceptive qualities in animal models of pain (Levy and Proudfit, 1977; Vaught et al., 1985; Zoen and Enna, 1985). Nicotine also has well-demonstrated antinociceptive qualities (Fertig et al., 1986, Pomerleau, 1986, Craft and Milholland, 1998, Girdlera et al., 2005), which differ by sex. Specifically, female rodents appear to be more sensitive to nicotine's antinociceptive effects than males (Craft and Milholland, 1998, Chen et al., 2009). These sex-specific differences in nicotine sensitivity are likely mediated by the sex steroids (e.g., estrogen) that inhibit nicotine-induced antinociception (Damaj, 2001, Chen et al., 2009).
In the present study, we used [123I]iomazenil and SPECT brain imaging to investigate sex-specific differences in GABAA-BZR availability and its relationship to tobacco craving, mood and pain sensitivity in male and female tobacco smokers. [123I]iomazenil has been validated as safe radioligand to use in humans in vivo, with good test-retest reliability (Abi-Dargham et al., 1995) and low nondisplaceable binding (Abi-Dargham et al., 1994) to measure GABAA-BZR availability in vivo. It is sensitive to the BZ binding site on the GABAA receptor. Based on the evidence that female smokers have lower brain GABA levels compared to female nonsmokers (Epperson et al., 2005), have greater difficulty quitting smoking than men (Bohadana et al., 2003, Pomerleau et al., 2005), and are more likely to smoke in response to negative affect (Husky et al., 2007), we hypothesized a sex by smoking interaction such that altered GABAA-BZR availability would be observed in female, but not male, smokers as compared to age- and sex-matched controls. Further, our exploratory hypotheses were that female smokers would exhibit lower sensitivity to pain and greater negative mood symptoms during acute smoking cessation compared to male smokers, and that sensitivity to pain and greater number of negative mood symptoms would be positively associated with GABAA-BZR availability in female smokers.
METHODS AND MATERIALS
Subjects
Twenty six women (8 nonsmokers, age 36.0 ± 13.4y; 19 smokers, age 34.6 ± 8.9y) and twenty five men (8 nonsmokers, age 37.9 ± 13.8y; 17 smokers, 34.1 ± 12.4y) participated. [There is an overlap between subjects participating in the current study and subjects described in our previous study (Esterlis et al., 2009). Specifically, the nonsmoking sample between the two studies is the same. Since that time, we recruited additional smokers (n=20) which now allows for comparison of the sex-specific differences in GABAA-BZR availability and relationship to clinical variables.] All subjects provided written informed consent to participate in this study, which was approved by the Human Investigation and Radiation Safety Committees at the Yale University School of Medicine (New Haven, CT), and the screening appointment was also approved by the Human Subjects Subcommittee at the West Haven VACHS (CT). The experiments were carried out in accordance with the Declaration of Helsinki as adopted and promulgated by the National Institutes of Health and the EU. Eligibility was evaluated via structured interview, physical examination, laboratory blood tests, urine drug screen, and an electrocardiogram. None of the subjects had a history or evidence of a serious medical or neurological illness, psychiatric disorder, or substance abuse or dependence (except for nicotine dependence in smokers). Subjects had not used psychotropic substances other than alcohol and tobacco for at least one year and reported no marijuana use for at least one month preceding the study. None of the subjects tested positive for drugs on a urine drug screen. All subjects were advised to abstain from alcohol for one week preceding the SPECT scan and smokers were asked to maintain their normal smoking routine prior to the scan day. Following the SPECT scan, smokers were asked to abstain from smoking for 1 week. Those who were able to abstain repeated the craving, mood, and pain assessments described below. Contingency management techniques with monitory reinforcements were used to help smokers abstain from smoking as described below. All women had a regular menstrual cycle, did not use hormonal contraceptives, and were not pregnant or lactating. Women were scheduled to be imaged during the early follicular phase of the menstrual cycle; however, due to technical and scheduling issues, some smoking and nonsmoking subjects were scanned during the late follicular or early luteal phase. Female hormone levels were obtained on the day of the SPECT scans for all female subjects. Nonsmokers were defined as individuals who smoked less than 40 cigarettes in their lifetime and none for two years before the study, as well as carbon monoxide levels ≤ 8ppm and plasma cotinine levels <15 ng/mL. Smokers were defined as have been smoking at least ≥ 10cig/day for at least 1 year; carbon monoxide levels > 11 ppm; plasma nicotine levels > 10 ng/mL; plasma cotinine levels of >150 ng/mL.
Assessments
Study timeline and procedures are outlined in Figures 1a and 1b. Specifically, at the baseline screening appointment (about 2 weeks prior to SPECT scan), subjects were administered the Structural Clinical Interview (SCID-I) for the Diagnostic and Statistical Manual – TR (DSM-IV-TR), and the Fagerström Test of Nicotine Dependence – FTND (Heatherton et al., 1991) to assess nicotine dependence. Nicotine withdrawal symptoms were assessed using the Minnesota Withdrawal Questionnaire (MWQ) (Hatsukami et al., 1984), and craving was assessed using the Tiffany Smoking Urges Questionnaire (Tiffany and Drobes, 1991). Two factors of Tiffany Smoking Urges Questionnaire were employed here: desire (positive symptoms associated with wanting a cigarette; e.g., “I have an urge to smoke”) and relief (withdrawal relief expected if cigarette is smoked; e.g., “Nothing would be better than smoking a cigarette right now”). Subsyndromal depressive symptoms including the Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977). Nicotine craving and withdrawal, and assessment of depression were repeated on the day of the SPECT scan (questionnaires were administered approximately 2 hours after the last cigarette in active smokers), in addition to measurement of state and trait anxiety symptoms (Spielberger's State-Trait Anxiety Inventory – STAI) (Spielberger and Corsuch, 1983).
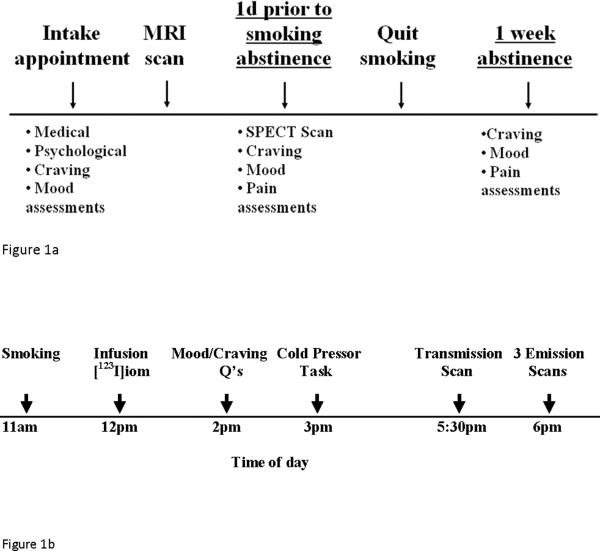
Figure 1.
Figure 1a outlines the overall study design. Subject participation initiated with a screening appointment that included a physical, ECG, blood and urine tests, as well as psychological and craving assessments. Once subjects were eligible to participate, they completed an MRI scan. One day prior to smoking cessation, they underwent a SPECT scan and completed additional assessments as well as cold pressor task. Then subjects abstained from tobacco smoking for ~1week via behavioral reinforcement and contingency management techniques. At the end of the abstinence period they again completed assessments and cold pressor task. Figure 1b outlines study design on the day of the SPECT scan for a representative subject. Subjects smoked their last cigarette (at 11am) about 1h prior to injection of the radiotracer (12pm). Following ensuring no adverse reactions to [123I]iomazenil, subject filled out tobacco craving/withdrawal related and mood questionnaires (2pm). At 3pm, subject participated in cold pressor task. Then, 5.5h after injection of radiotracer (5:30pm), subjects underwent a transmission scan. Following, three emission scans were obtained at 6pm.
The cold pressor task was administered to measure sensitivity to and tolerance of pain, as in our previous report (Esterlis et al., 2009) on the SPECT scan day as well as at 1 week of smoking abstinence. In brief, subjects were required to keep their dominant hand in warm water for 60 seconds. The subjects were then instructed to put the same hand in circulating cold water bath for 90 seconds, but were allowed to remove it if they started to experience intolerable pain (Walsh et al., 1989). Time of first report of pain was recorded as pain sensitivity, and time of hand withdrawal from the cold water was recorded as pain tolerance.
Cotinine (the primary metabolite of nicotine) levels were measured in plasma and urine at the screening appointment and on the day of the SPECT scan in smokers and nonsmokers and daily during the 1 week of abstinence in smokers. Cotinine levels were measured in plasma as described previously (Staley et al., 2006, Esterlis et al., 2009). Urinary cotinine levels were monitored daily during the nicotine withdrawal period using NicAlert cotinine test strips (Nymox Pharmaceutical Corporation, Hasbrouck Heights, NJ).
Contingency Management for Smoking Cessation
Contingency management and brief behavioral reinforcement techniques were used to achieve 1 week of abstinence in smokers as described previously (Staley et al., 2006; (Esterlis et al., 2009). In brief, carbon monoxide (CO) levels ≤ 8 ppm and urine cotinine levels < 100 ng/ml were used to confirm abstinence from any form of nicotine. Smokers were compensated twice daily (morning and afternoon) during the week of abstinence contingent on a breath CO reading and urine cotinine level indicating abstinence.
[123I]Iomazenil SPECT Imaging and Magnetic Resonance Imaging
All subjects participated in one [123I]iomazenil SPECT scan. [123I]iomazenil was prepared as described previously (Zoghbi et al., 1992) with the radiochemical purity > 98.0%. The specific activity of [123I]iomazenil was too high to be measured with the ultraviolet (UV) detector on the high pressure liquid chromotography (HPLC) system. Given the sensitivity of the UV/HPLC system, the specific activity was estimated as greater than 5,000 Ci.mmol−1. All participants were pretreated with saturated potassium iodide to reduce thyroid uptake of 123I. The duration of infusion was 6.5 hours with a mean ± SD total injected dose of 223.5 ± 1.2 MBq for male nonsmokers, 210.4 ± 13.6 MBq for male smokers, 218.5 ± 27.4 MBq for female nonsmokers and 219.2 ± 12.4 MBq for female smokers with a bolus to infusion ratio for all subjects of 3.83 ± 0.02 hours. Prior to imaging, five external fiducial markers containing 0.037 MBq of 123I were placed on the scalp to provide a common reference for co-registration with emission images. Smokers smoked their last cigarette before coming to the SPECT imaging laboratory and thus were imaged about 7h after the last cigarette. Simultaneous transmission and emission scans (STEP) were acquired with a line source containing 740 MBq of cobalt 57 on a PRISM 3000XP 3-headed camera (Phillips, Cleveland, Ohio). Three consecutive 12-minute emission scans were acquired in continuous mode after ~6h of [123I]iomazenil infusion. Three venous blood samples were collected at the midpoint of the second emission scan for assessment of plasma and free parent of [123I]iomazenil (Zoghbi et al., 1992). [123I]iomazenil SPECT has high, 83% to 90%, reproducibility (Abi-Dargham et al., 1995).
Magnetic resonance imaging was performed on a Sonata 1.5T Siemens (Siemens AG, München Germany). Axial images were acquired parallel to the anteroposterior commissural line with an echo time of 5 milliseconds; repetition time of 24 milliseconds; matrix 256×192; number of excitations of 1; field of view of 24 cm; and 128 contiguous slices with a thickness of 1.3 mm.
Image Analysis
SPECT images were processed and volume of interest analyses were performed as described previously (Staley et al., 2005, Esterlis et al., 2009). Emission data from SPECT scans were reconstructed using filtered backprojection from counts acquired in the 123I photopeak (159 keV) with a 20% symmetric window using a Butterworth filter (power factor = 10, cutoff = 0.24 cm). An attenuation map was reconstructed from the transmission and flood data and was used for non-uniform attenuation correction (Rajeevan et al., 1998). The magnetic resonance image was co-registered to the emission image and reoriented to the inter-commissural plane and standardized 2-dimensional region of interest templates were placed on the parietal, frontal, anterior cingulate, occipital, temporal cortices, thalamus, striatum and cerebellum. Three-dimensional volumes of interest were transferred to co-registered SPECT images. Regional activities from right and left hemispheres were averaged, decay corrected, and expressed as kilobecquerels per cubic centimeter using a calibration factor of 0.000912 μCi/cpm derived from a 123I distributed source phantom. Regional activities (kilobecquerels per cubic centimeter) were normalized to free [123I]iomazenil (kilobecquerels per milliliter) plasma levels (Abi-Dargham et al., 1994). Equilibrium distribution volume (VT/fp) was the main outcome measure, which is proportional to brain region activity divided by free parent (Innis et al., 2007). Two raters analyzed the data and the mean outcome measure is used for all analyses. Interrater was <10% variability between raters across regions.
Statistical Analyses
All analysis were performed using SPSS version 16.0 (SPSS Inc. Headquarters, Chicago, IL). Univariate analyses of variance (ANOVA) were conducted to compare subject groups on demographic variables. The sex by smoking interaction in [123I]iomazenil VT/fp was examined with sex and smoking as independent variables with multivariate analysis of variance (MANOVA). Spearman's correlation coefficient was used for the exploratory analyses to assess associations between clinical variables (smoking, pain) and regional [123I]iomazenil VT/fp for all smokers. Different slopes for women and men were estimated to illustrate the relationship between significant variables and brain receptor availability. Statistical significance was set at p<.05, two-tailed.
RESULTS
Subject Characteristics
There were no differences in number of years smoked, the number of cigarettes smoked per day, or nicotine dependence score between men and women smokers (Table 1). Overall, at baseline, smokers endorsed significantly greater number of symptoms on the CES-D (M = 8.2 ± 6.0) as compared to nonsmoking counterparts (M = 4.3 ± 3.7; F=5.71, p=0.02); however, none of the scores met criteria for depression. We thus used the CES-D score as covariate in the later analyses. There were no significant sex-differences in number of depressive symptoms endorsed (F=0.02, p=0.90). There were no significant differences in female hormone levels between smoking (estradiol 82.5 ± 40.1 pg/mL, progesterone 3.5 ± 6.6 ng/mL, follicular stimulating hormone 10.5 ± 20.1 mIU/mL) and nonsmoking (estradiol 78.8 ± 56.7 pg/mL, progesterone 2.2 ± 4.1 ng/mL, follicular stimulating hormone 20.5 ± 33.8 mIU/mL) women at time of study participation (F=0.32, p=0.81). Specifically, 2 of 8 female nonsmokers were imaged in the late follicular/early luteal phase, and 8 of 18 female smokers were imaged in the in the late follicular/early luteal phase. Phase of menstrual cycle was defined as follows: early follicular - progesterone 0–1 ng/mL, estradiol 11–55 pg/mL; late follicular phase – progesterone <2.5 ng/mL, estradiol 56–212 ng/mL; luteal phase – progesterone 2.6–21.5 ng/mL, estradiol <247 ng/mL.
Table I.
Demographics of Nonsmokers and Smokers*
| MNS | MS | FNS | FS | |
|---|---|---|---|---|
| N=8 | N=17 | N=8 | N=19 | |
| Age (SD) | 37.9±13.8 | 34.0±12.4 | 36.0±13.4 | 34.6±8.9 |
| Race | 2AA | 6AA | 3AA | 6AA |
| 6C | 1AS | 1AS | 1HS | |
| 9C | 4C | 12C | ||
| Years smoked | N/A | 13.8±8.3 | N/A | 16.0±9.2 |
| Cigarettes/day | N/A | 18.0±6.4 | N/A | 18.0±9.7 |
| FTND | N/A | 6.0±2.7 | N/A | 7.0±1.9 |
| CES-D1 | 4.6±3.3 | 8.0±5.9 | 4.0±4.2 | 8.0±6.2 |
Abbreviations: MNS — male nonsmokers, MS — male smokers, FNS — female nonsmokers, FS — female smokers; AA — African American, AS — Asian, HS — Hispanic, C — Caucasian; FTND — Fagerstrom Test for Nicotine Dependence
Data are presented as mean ± SD.
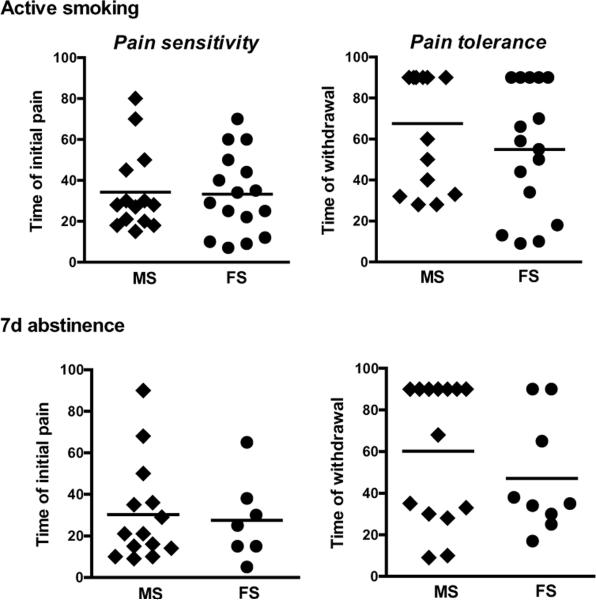
Men and women smokers did not differ significantly in cigarette craving or withdrawal on the day of the SPECT scan or at 1 week of abstinence (Table 2). Furthermore, pain tolerance and pain sensitivity did not differ between men and women smokers (Table 2, Figure 3). Of the smoking subjects, significantly more men than women were able to abstain from smoking for 1 week to repeat assessments (7 of 19 women and 14 of 17 men were able to abstain from smoking; F=10.6, p=0.003). There were no significant differences in demographic, psychological, hormonal levels or smoking variables between subjects who were able to abstain from smoking for 1 week and those who were not.
Table 2.
Mood and craving assessments on scan day and at 1 week tobacco abstinence*
| MNS (n=8) | MS (n=17) | FNS (n=8) | FS (n=19) | |
|---|---|---|---|---|
| Scan day Assessments | ||||
| Tiffany Desire | N/A | 32.0 (±8.6) | N/A | 28.0 (±10.0) |
| Tiffany Withdrawal | N/A | 17.0 (±9.4) | N/A | 19.0 (±11.3) |
| MNWS | N/A | 3.0 (±3.0) | N/A | 3.0 (±4.7) |
| CES-D2 | 6.0 (±2.3) | 7.0 (±6.0) | 2.0 (±0.8) | 9.0 (±6.3) |
| STAI State | 26.8 (±6.2) | 31.0 (±9.4) | 21.0 (±0.8) | 30.0 (±9.4) |
| STAI Trait | 30.8 (±4.8) | 31.0 (±9.9) | 25.0 (±5.13) | 33.0 (±9.4) |
| Pain Sensitivity | N/A | 32.0 (±21.2) | N/A | 33.2 (±19.6) |
| Pain Tolerance | N/A | 67.6 (±27.4) | N/A | 54.9 (±30.9) |
| 1 -week Abst Assessments | ||||
| Tiffany Desire | N/A | 22.0 (±11.6) | N/A | 16.0 (±9.3) |
| Tiffany Withdrawal | N/A | 13.6 (±10.0) | N/A | 12.7 (±9.4) |
| MNWS | N/A | 1.6 (±2.2) | N/A | 6.5 (±5.1) |
| CES-D | N/A | 2.8 (±3.3) | N/A | 12.0 (±10.4) |
| STAI State | N/A | 27.5 (±6.2) | N/A | 33.1 (±13.1) |
| STAI Trait | N/A | 27.8 (±7.6) | N/A | 34.0 (±11.6) |
| Pain Sensitivity | N/A | 34.3 (±20.0) | N/A | 33.3 (±19.6) |
| Pain Tolerance | N/A | 67.6 (±27.4) | N/A | 54.9 (±30.9) |
MNS — male nonsmokers, MS — male smokers, FNS —female nonsmokers, FS —female smokers; MNWS — Minnesota Withdrawal Scale; CES-D — Center for Epidemiological Studies Depression Scale; STAI — Speilberger's State/Trait Anxiety Inventory
Data are presented as mean ± SD.
Figure 3.
All smokers completed cold pressor task on SPECT scan day, and smokers who were able to abstain from cigarettes for 1 week also completed the cold pressor task then. We did not detected significant differences in pain sensitivity or tolerance between male and female smokers at either appointment.
GABAA-BZR availability
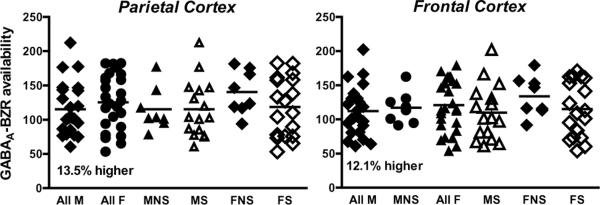
We did not observe a significant sex by smoking interaction in GABAA-BZR availability (MANCOVA; F=1.39, p=0.24) (Table 3). However, we observed an overall main effect of sex such that women had significantly greater numbers of available receptors compared to men regardless of smoking status (Hotelling's Trace F=2.48, p=0.04) (Figure 2). This effect was significant across all regions examined. Power analysis revealed power at 76.9%. We also performed secondary analyses to investigate if the GABAA-BZRs may be involved in ability to abstain from tobacco smoking. There were no significant differences in GABAA-BZR availability between individuals who were able to abstain from smoking and those who were not (all p values > 0.10). Furthermore, there were no significant differences in receptor availability in female nonsmokers and smokers by phase of the menstrual cycle.
Table 3.
GABAA-BZR availability by experimental group.
| Brain Region | Male nonsmokers (N=8) | Male smokers (N=17) | Female nonsmokers (N=8) | Female smokers (N=19) |
|---|---|---|---|---|
| Parietal Cortex | 115.14 ± 31.40 | 115.19 ± 41.63 | 140.44 ± 31.90 | 118.55 ± 43.24 |
| Frontal Cortex | 117.06 ± 23.21 | 109.65 ± 40.65 | 133.96 ± 29.13 | 115.04 ± 40.63 |
| Anterior Cingulate | 141.51 ± 26 22 | 128.71 ± 46.21 | 153.28 ± 37.05 | 131.34 ± 42.66 |
| Temporal Cortex | 130.62 ± 26.61 | 122.70 ± 41.58 | 142.91 ± 31.40 | 124.63 ± 40.53 |
| Occipital Cortex | 189.85 ± 36.72 | 173.99 ± 62.95 | 197.24 ± 36.76 | 171.94 ± 56.59 |
| Cerebellum | 110.52 ± 21.12 | 90.37 ± 34.02 | 111.81 ± 30.06 | 95.19 ± 34.69 |
Mean ± standard deviation reported.
Figure 2.
Illustrated are scatter plots with GABAA-BZR availability data for all men, all women, male nonsmokers (MNS), male smokers (MS), female nonsmokers (FNS), female smokers (FS). . Although no sex by smoking interaction was detected, women overall had significantly greater receptor availability as compared to men (shown here 13.5% difference in the parietal and 12.1% difference in the frontal cortices).
Sex differences in the relationship between receptor availability and smoking variables
We observed a significant negative association between GABAA-BZR availability and craving for cigarettes on the SPECT scan day in the full smoking sample (e.g., men and women) in all cortical regions and cerebellum (rhofrontal =−0.41, p=.02; rhoparietal =−0.41, p=.02; rhoanterior cingulate =−0.46, p=.007; rhotemporal =−0.41, p=.02; rhooccipital =−0.40, p=.02; rhocerebellum =−0.40, p=.02). However, when stratified by sex, this was significant in women (rhofrontal =−0.54, p=.02; rhoparietal =−0.57, p=.01; rhoanterior cingulate =−0.69, p=.002; rhotemporal =−0.52, p=.03; rhooccipital =−0.57, p=.01; rhocerebellum =−0.55, p=.02) but not men smokers.
GABAA-BZR availability and pain and mood measures
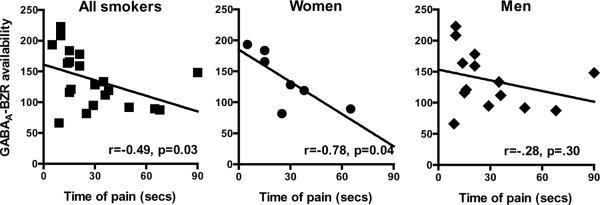
There were no significant correlations between pain tolerance or sensitivity and GABAA-BZR availability in women or men smokers on the day of SPECT scan, 2hrs after last cigarette. Furthermore, there were no significant differences in pain variables on the scan day between subjects who were able to abstain from smoking for one week and those who were not. Interestingly, there was a negative association between pain sensitivity at 1 week of abstinence and pre-quit GABAA-BZR availability in the full smoking sample in all cortical regions (rhofrontal =−0.49, p=.03, rhoparietal =−0.48, p=.03, rhoanterior cingulate =−0.49, p=.03, rhotemporal =−0.50, p=.02, rhooccipital =−0.51, p=.02) and cerebellum (rho=−0.52, p=.02) (Figure 3). When stratified by sex, this association was significant in the women smokers in most cortical regions (rhofrontal =−0.78, p=.04, rhoparietal =−0.78, p=.04, rhotemporal =−0.78, p=.04, rhooccipital =−0.78, p=.04); no significant correlation was observed in men smokers. Furthermore, there were no significant associations between receptor availability and mood measures in smokers (as a group, or by sex).
DISCUSSION
In the present study we evaluated sex and tobacco smoking effects on GABAA-BZR availability and associated behavioral correlates in psychiatrically healthy smokers and nonsmokers during active smoking and at 1 week of smoking abstinence. We found that women have significantly higher GABAA-BZR availability as compared to men regardless of smoking status, and that sex may in part mediate the relationship between GABAA-BZR availability and tobacco craving and pain sensitivity. Consistent with our previous findings (Esterlis et al., 2009), we did not detect a significant correlation between receptor availability and mood variables in this larger smoking sample.
The finding of sex differences in GABAA-BZR availability and a role for sex in GABAA-BZR modulation of craving and pain experiences is consistent with our hypothesis. It is well established that endogenous sex-specific neurosteroids regulate brain development, including the size of the nucleus in neurons and the size of synaptic vesicles, which in turn lead to differences in structural volume, connectivity, and neurotransmitter distribution (Krishnan et al., 2009, Veliskova, 2009). Sex differences have been demonstrated in a number of drug-taking behaviors and reactivity to various drugs, tobacco smoking being most relevant to the present experiment (Grunberg et al., 1991). In an attempt to account for the confounding effects of the menstrual cycle on hormonal variability in female subjects, we attempted to image all women in the early follicular phase of the menstrual cycle, when both estrogen and progesterone levels are low; however, some smoking and nonsmoking women were imaged during the late follicular or early luteal phase. Overall, it appears that GABAA-BZR availability is differentially regulated in men and women and this may stem from neurosteroid effects and sexual differentiation of the brain during critical times of human development.
The finding of higher GABAA-BZR availability in women compared to men was surprising and contrary to the proposed hypothesis based on the study by Epperson and colleagues (Epperson et al., 2005). They previously found that GABA cycling regulated by the menstrual cycle was significantly disrupted in smoking women and chronic tobacco smoking suppressed GABA levels in female smokers compared to nonsmokers. Thus, since the GABA system is known to be influenced by many variables, including menstrual cycle, mental status, and smoking, we took precautions to image psychiatrically healthy male and female smokers and nonsmokers, and to obtain female hormone levels on the day of the scan day in order to examine potential differences in menstrual cycle between female smokers and nonsmokers. Contrary to the proposed hypothesis we did not detect a sex by smoking interaction. Interestingly, we observed that, regardless of smoking status, women had higher GABAA-BZR availability than men throughout the cortical regions and the cerebellum. This suggests that although GABA levels differ in a sex by smoking interaction, GABAA-BZR availability may not be sensitive to the effects of smoking. The potential insensitivity of the GABAA-BZRs to tobacco smoking in healthy human subjects is consistent with our previous study (Esterlis et al., 2009) demonstrating no differences in GABAA-BZR availability between smoking and nonsmoking healthy individuals, or between active smoking and prolonged (5wk) abstinence, although the previous report did not examine a sex-by-smoking interaction. In addition, the binding site for the neurotransmitter GABA and the BZ binding site on the GABAA receptor are distinct. Specifically, [123I]iomazenil binds to the BZ binding site on the GABAA receptor and therefore does not compete for binding with endogenous GABA. In this study, we are not directly measuring altered GABA levels.
We also observed that sex may mediate the relationship between GABAA-BZR availability and tobacco craving and pain sensitivity, but not the relationship between GABAA-BZR and mood. Specifically, craving for cigarettes on the day of the scan was negatively correlated with GABAA-BZR availability in female smokers but not male smokers, i.e., higher receptor availability in all cortical regions and cerebellum was associated with less craving for cigarettes in women. Additionally, pain sensitivity measured at 1 week of tobacco abstinence, but not on scan day, was negatively correlated with receptor availability measured prior to abstinence in female but not male smokers. That is, women with higher GABAA-BZR availability during active smoking were more sensitive to pain at 1 week of smoking abstinence. Although our sample of women who were able to abstain from smoking for 1 week to complete the pain sensitivity task was small, there were no differences in the demographic, mood, or pain variables at baseline between these women and those who dropped out of the study. In our previous study, we did not detect any significant associations between receptor availability and pain measures; however, the sample size of smokers was much smaller (n=16) and we were not able to evaluate this relationship in a sex-specific manner. Furthermore, consistent with our previous report, there were no significant associations between mood and GABAA-BZR availability in smokers, examined as a whole group and by sex. This later finding was contrary to our exploratory hypothesis, as we had expected female smokers to exhibit more negative mood symptoms as compared to male smokers and this to be in part regulated by the GABAA-BZRs.
One limitation of the current study is the lack of pain measures in nonsmokers. This data would have allowed for a smoking by sex comparison of receptor availability in order to assess if a relationship between pain measures and receptor availability exists in female nonsmokers, or if it is specific to female smokers. We thus cautiously proceed with the conclusion that even in the absence of psychological and behavioral differences between female and male smokers in the current study, the GABAergic system regulation of these variables differs significantly between the sexes. Our findings suggest that sexual dimorphism in GABAA-BZR availability in smokers may lead to sex differences in the experience of tobacco smoking and craving, and it is likely that regulation of pain and craving in female smokers may be different as compared to male smokers and may be one of the caveats to their poorer success at quitting smoking. Specifically, the higher GABAA-BZR availability in combination with lower circulating GABA levels in female smokers suggests that there may be more available GABAA receptors during acute abstinence leading to increased pain.
Another limitation of the study was that the cold pressor task was terminated at 90 s. About one third of the subjects were able to keep their hand in the water for the duration of the task, and thus their pain tolerance score may have been affected by this. It is possible that if there was no experimenter-induced ceiling to the task, we may have found a relationship between pain tolerance and GABAA-BZR availability. Furthermore, we only assessed receptor availability pre-quit and we are unable to examine the relationship between pain measures and post-quit GABAA-BZR availability. However, considering our previous findings that during the course of abstinence changes in GABAA-BZRs are not observed and there is no differences in receptor availability between smokers and nonsmokers, we believe the current association between GABAA-BZRs and pain sensitivity at 1 week of abstinence may be clinically meaningful.
The last limitation is that we did not collect hormone levels in male subjects. This limits interpretation of the causation of the higher GABAA-BZR availability finding in women as compared to men. Furthermore, although it is likely that hormones may also mediate the relationship between GABAA-BZR and craving or pain sensitivity, we cannot conclude this with certainty.
Future imaging studies should evaluate the interaction between genetic variables, imaging, pain and craving data in a sex-specific manner. The GABAA-BZR has been under investigation as a target for new wave of antihyperalgesic drugs (Lio et al., 2011). Since a reduction in GABAergic transmission is associated with inflammatory and neuropathic pain, novel treatment drugs that have antinociceptive qualities without the side-effects of benzodiazepines have potential, and our study suggests this may be an important target for pain modulation in smoking women.
In conclusion, these findings suggest the GABAA-BZR may play an important role in mediating sex differences associated with smoking. We found that in female, but not male, smokers there is a relationship between GABAA-BZR availability and craving and pain variables. Although these findings are exploratory in nature and need to be replicated, they are critically important in light of the evidence that women have a greater difficulty achieving smoking abstinence and relapse sooner than men (Bohadana et al., 2003, Pomerleau et al., 2005). Thus, this receptor site may be a potential target of novel pharmaceuticals for smoking cessation in women.
Figure 4.
There is a negative association between receptor availability and pain sensitivity (ie, time to report pain) in the full sample of smokers at 1 week of abstinence. However, when the data were stratified by sex, the relationship was significant in women but not men. Specifically, female smokers with higher receptor availability prior to smoking cessation were more sensitive to pain at 1 week of abstinence.
Acknowledgements
We thank the nuclear technologists, at the Institute for Neurodegenerative Disorders for conducting all SPECT scans in the current study, especially Jane Bartosik. We also thank Louis Amici for metabolite analyses and C. Neill Epperson for intellectual contribution. This study was completed under a grant provided to Julie K. Staley and is dedicated in her memory and her advancement of understanding sex-differences in nicotine addiction.
Funding: Support contributed by TTURC P5015632 and VA Career Development Award −1, K02 DA21863, K01 DA20651, and T32 DA07238-16. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, or the National Institutes of Health.
Footnotes
Author's contribution JKS and SSO were responsible for study concept and design. FB was responsible for radiosynthesis of the radiotracer and metabolite analyses. SMS was responsible for subject recruitment and evaluation, as well as contingency management appointments and clinical issues. SM aided with statistical data analysis and interpretation, as well as results conceptualizations. KK and DL aided with image analysis and manuscript review. JPS aided with all aspects associated with imaging human subjects, as well as imaging data interpretation and manuscript preparation. SSO and SKS aided with manuscript preparation and critical review. KPC aided with all aspects of the study, as well as data interpretation and manuscript preparation. IE was responsible for all aspects of the study, including subject recruitment and evaluation, imaging and imaging data analysis, statistical analyses and interpretation, literature review and manuscript preparation. All authors critically reviewed content and approved final version for publication.
Dr. Seibyl has equity interest in Molecular Neuroimaging, LLC. Dr. O'Malley: member, American College of Neuropsychopharmacogy workgroup, the Alcohol Clinical Trial Initiative, sponsored by Alkermes, Abbott Laboratories, Eli Lilly & Company, GlaxoSmithKline, Johnson & Johnson Pharmaceuticals, Lundbeck, and Schering Plough; partner, Applied Behavioral Research; medication supplies, Pfizer; contract, Nabi Biopharmaceuticals; Advisory Board, Gilead Pharmaceuticals; consultant, Alkermes, GlaxoSmithKline, Brown University, University of Chicago; Scientific Panel of Advisors, Hazelden Foundation. The other authors (Esterlis, McKee, Kirk, Stiklus, Bois, Cosgrove, Lee, Krishnan-Sarin) declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting potential conflict of interest.
References
- Abi-Dargham A, Gandelman M, Zoghbi S, Laruelle M, Baldwin R, Randall P, Zea-Ponce Y, Charney D, Hoffer P, Innis R. Reproducibility of SPECT measurement of benzodiazepine receptors in human brain with iodine-123-iomazenil. J Nucl Med. 1995;36:167–175. [PubMed] [Google Scholar]
- Abi-Dargham A, Laruelle M, Seibyl J, Rattner Z, Baldwin R, Zoghbi S, Zea-Ponce Y, Bremner J, Hyde T, Charney D, Hoffer P, Innis R. SPECT measurement of benzodiazepine receptors in human brain with [123I]Iomazenil: kinetic and equilibrium paradigms. J Nucl Med. 1994;35:228–238. [PubMed] [Google Scholar]
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: Influence of baseline smoking behavior. Nicotine & Tobacco Research. 2003;5:111–116. doi: 10.1080/1462220021000060482. [DOI] [PubMed] [Google Scholar]
- Chadda R, Devaud L. Sex differences in effects of mild chronic stress on seizure risk and GABAA receptors in rats. Pharmacol Biochem Behav. 2004;78:495–504. doi: 10.1016/j.pbb.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cui Y, Lin J, Xiang Q, Liu W, Wang T. Modulatory role of estradiol in nicotinic antinociception in adult female rats. Life Science. 2009;85:91–96. doi: 10.1016/j.lfs.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Craft R, Milholland R. Sex differences in cocaine- and nicotine-induced antinociception in the rat. Brain Res. 1998;809:137–140. doi: 10.1016/s0006-8993(98)00841-5. [DOI] [PubMed] [Google Scholar]
- Damaj M. Influence of gender and sex hormones on nicotine acute pharmacological effects in mice. J Pharmacol Exp Therap. 2001;296:132–140. [PubMed] [Google Scholar]
- Epperson C, Czarkowski K, Gueorguieva R, Jatlow P, Sanacora G, Rothman D, Krystal J, Mason G. Sex, GABA, and nicotine: The impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance. Biological Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson C, Haga K, Mason G, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- Esterlis I, Cosgrove K, Batis J, Bois F, Kloczynski T, Stiklus S, Perry E, Tamagnan G, Seibyl J, Makuch R, Krishnan-Sarin S, O'Malley S, Staley J. GABAA-benzodiazepine receptor availability in smokers and nonsmokers: Relationship to subsyndromal anxiety and depression. Synapse. 2009;63:1089–1099. doi: 10.1002/syn.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fant R, Everson D, DAyton G, Pickworth W, Henningfield J. Nicotine Dependence in Women. J Am Womens Med Assoc. 1996;51:19–28. [PubMed] [Google Scholar]
- Fertig J, Pomerleau O, Sanders B. Nicotine-produced antinociception in minimally deprived smokers and ex-smokers. Addict Behav. 1986;11:239–248. doi: 10.1016/0306-4603(86)90052-3. [DOI] [PubMed] [Google Scholar]
- Girdlera S, Maixnerb W, Naftela H, Stewartc P, Moretza R, Lighta K. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Grunberg N, Winders S, Wewers M. Gender differences in tobacco use. Health Psychology. 1991;10:143–153. [PubMed] [Google Scholar]
- Hatsukami D, Hughes J, Pickens R, Svikis D. Tobacco withdrawal symptoms: An experimental analysis. Psychopharmacology. 1984;84:231–236. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerstrom K. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Brit J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Husky M, Mazure C, Paliwal P, McKee S. Gender differences in the comorbidity of smoking behavior and major depression. Drugand Alcohol Dependence. 2007;93:176–179. doi: 10.1016/j.drugalcdep.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R, Cunningham V, Delforge J, Fujita M, Gjedde A, Gunn R, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe R, Knudsen G, Knuuti J, Lammertsma A, Laruelle M, Logan J, Maguire R, Mintun M, Morris E, Parsey R, Price J, Slifstein M, Sossi V, Suhara T, Votaw J, Wong D, Carson R. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow & Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. PNAS. 2009;106:16692–16697. doi: 10.1073/pnas.0906293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A, Chudomel O, Moshé S, Galanopoulou A. Sex-dependent maturation of GABAA receptor-mediated synaptic events in rat substantia nigra reticulata. Neurosci Lett. 2006;398:1–5. doi: 10.1016/j.neulet.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Lena C, Changeux JP, Mulle C. Evidence for “preterminal” nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J Neurosci. 1993;13:2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A, Acton P, Gacinovic S, Boddington S, Costa D, Pilowsky L, Ell P, Marshall E, Kerwin R. Levels of γ-aminobutyric acid-benzodiazepine receptors in abstinent, alcohol dependent women: Preliminary findings from an 123I-iomazenil single photon emission tomography study. Alc Clin Exp Res. 2000;24:1449–1455. [PubMed] [Google Scholar]
- Lio AD, Benke D, Besson M, Desmeules J, Daali Y, Wang Z, Edwankar R, Cook J, Zeilhofer H. HZ166, a novel GABA(A) receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology. 2011;60:626–632. doi: 10.1016/j.neuropharm.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magata Y, Kitano H, Shiozaki T, Iida Y, Nishizawa S, Saji H, Konishi J. Effect of chronic (−) nicotine treatment on rat cerebral benzodiazepine receptors. Nuclear Med & Biol. 2000;27:57–60. doi: 10.1016/s0969-8051(99)00078-5. [DOI] [PubMed] [Google Scholar]
- Malizia A, Cunningham V, al CBe Decreased benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry. 1998;55:715–720. doi: 10.1001/archpsyc.55.8.715. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Yoon KW, Chiappinelli VA. Electrophysiological evidence for presynaptic nicotinic receptors in the avian ventral lateral geniculate nucleus. J Neurophysiol. 1994a;71:826–829. doi: 10.1152/jn.1994.71.2.826. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Yoon KW, Chiappinelli VA. Nicotinic receptor activation facilitates GABAergic neurotransmission in the avian lateral spiriform nucleus. Neuroscience. 1994b;59:689–698. doi: 10.1016/0306-4522(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Nett S, Jorge-Rivera R, Myers M, Clark A, Henderson L. Properties and sex-specific differences of GABAA receptors in neurons expressing gamma1 subunit mRNA in the preoptic area of the rat. J Neurophysiol. 1999;81:192–203. doi: 10.1152/jn.1999.81.1.192. [DOI] [PubMed] [Google Scholar]
- Pande A, Pollack M, Crockatt J, Greiner M, Chouinard G, Lydiard B, Taylor C, Dager S, Shiovitz T. Placebo-Controlled Study of Gabapentin Treatment of Panic Disorder. Journal of Clinical Psychopharmacology. 2000;20:467–471. doi: 10.1097/00004714-200008000-00011. [DOI] [PubMed] [Google Scholar]
- Perkins K, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effect of visual and olfactory cigarette smoke stimuli. Nicotine & Tobacco Research. 2001;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Pomerleau O. Nicotine as a psychoactive drug: Anxiety and Pain Reduction. Psychopharmacology Bulletin. 1986;22:865–869. [PubMed] [Google Scholar]
- Pomerleau O, Pomerleau C, Mehringer A, Snedecor S, Ninowski R, Sen A. Nicotine dependence, depression, and gender: characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine & Tobacco Research. 2005;7:91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rajeevan N, Zubal I, Rambsy S, Zoghbi S, Seibyl J, Innis R. Significance of nonuniform attenuation correction in quantitative brain SPECT imaging. J Nucl Med. 1998;39:1719–1726. [PubMed] [Google Scholar]
- Spielberger C, Corsuch R, editors. Manual for State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto CA: 1983. [Google Scholar]
- Staley J, Dyck Cv, Weinzimmer D, Brenner E, Baldwin R, Tamagnan G, Riccardi P, Mitsis E, Seibyl J. Iodine-123-5-IA-85380 SPECT Measurement of Nicotinic Acetylcholine Receptors in Human Brain by the Constant Infusion Paradigm:Feasibility and Reproducibility. J Nucl Med. 2005;46:1466–1472. [PubMed] [Google Scholar]
- Staley J, Krishnan-Sarin S, Cosgrove K, Krantzler E, Frohlich E, Perry E, Dubin J, Estok K, Brenner E, Baldwin R, Tamagnan G, Seibyl J, Jatlow P, Picciotto M, London E, O'Malley S, Dyck Cv. Human tobacco smokers in early abstinence have higher levels of beta2-nicotinic acetylcholine receptors than nonsmokers. Journal of Neuroscience. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany S, Drobes D. The development and initial validation of a questionnaire on smoking urges. British Journal of Addictions. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Veliskova J. Sex differences in seizure susceptibility. In: Schwarzkroin P, editor. Encyclopedia of basic epilepsy research. Academic Press; Oxford UK: 2009. pp. 1–4. [Google Scholar]
- Walsh NE, Shoenfeld L, Ramamurthy S, Hoffman J. Normative model for cold pressor test. American Journal of Phys and Med Rehabilitation. 1989;68:6–11. doi: 10.1097/00002060-198902000-00003. [DOI] [PubMed] [Google Scholar]
- Zoghbi S, Baldwin R, Seibyl J, Al-Tikriti M, Zea-Ponce Y, Laurelle M, Sybirska E, Woods S, Goddard A, Charney D, Smith E, Hoffer PB, Innis R. Pharmacokinetics of the SPECT benzodiazepine receptor radioligand [123I]iomazenil in human and nonhuman primates. Nucl Med Biol-Int J Rad App B. 1992;19:881–888. doi: 10.1016/0883-2897(92)90174-w. [DOI] [PubMed] [Google Scholar]