Abstract
Of the parameters that determine glucose disposal and progression to diabetes in humans: first-phase insulin secretion, glucose effectiveness, insulin sensitivity, and the disposition index, only insulin sensitivity can be reliably measured in conscious mice. To determine the importance of the other parameters in murine glucose homeostasis in lean and obese states, we developed the frequently sampled intravenous glucose tolerance test (FSIVGTT) for use in unhandled mice. We validated the conscious FSIVGTT against the euglycemic clamp for measuring insulin sensitivity in lean and obese mice. Insulin resistant mice had increased first-phase insulin secretion, decreased glucose effectiveness and a reduced disposition index, qualitatively similar to humans. Intriguingly, while insulin secretion explained most of the variation in glucose disposal in lean mice, glucose effectiveness and the disposition index more strongly predicted glucose disposal in obese mice. Disposition index curves identified individual diet-induced obese mice as having compensated or decompensated insulin secretion. Conscious FSIVGTT opens the door to apply mouse genetics to the determinants of in vivo insulin secretion, glucose effectiveness and disposition index, and further validates the mouse as a model of metabolic disease.
INTRODUCTION
It is widely assumed by mouse researchers that regulation of rodent glucose disposal is similar to human. We now know from hyperinsulinemic euglycemic clamps that factors regulating insulin sensitivity in conscious mice are relevant to human health and disease (1–3). However, glucose homeostasis is dependent not only on insulin sensitivity, but also on insulin secretion and non-insulin mediated glucose uptake, termed glucose effectiveness (4–8). The disposition index (DI), one of the best predictors of progression to diabetes in humans, is currently not quantifiable in conscious mice. According to recently released guidelines from the Consortium of Mouse Metabolic Phenotyping Centers, metabolic testing under anesthesia yields results that are not physiological (9). Thus, new methods are required to measure these metabolic parameters in conscious unhandled mice in order to fully exploit the potential of mouse genetics in dissecting the pathogenesis of metabolic disease.
Based on work begun in the 1980’s (10–12) it is possible in humans to achieve an integrated view of glucose homeostasis across a disease spectrum from normal glucose tolerance (NGT) to impaired glucose tolerance (IGT) and diabetes, using the frequently sampled intravenous glucose tolerance test (FSIVGTT) with mathematical modeling. The FSIVGTT has become a widely utilized tool in human research, and has helped delineate key principles of glucose homeostasis such as the role of declining first phase insulin secretion in glucose intolerance, and the predictive value of the disposition index in progression to diabetes (13–15). In contrast, the techniques routinely used in conscious mice, euglycemic clamps and intraperitoneal challenge with glucose or insulin, cannot independently assess these metabolic parameters to provide a coordinated view of glucose disposal. Moreover, some data in rats and anesthetized mice actually suggest that the role of first phase insulin release in glucose disposal may be minor (16) to nonexistent (17) in rodents, raising the question that rodent metabolic physiology may differ significantly from human.
In order to examine the roles of first phase insulin secretion, glucose effectiveness and insulin sensitivity on murine glucose disposal, we developed techniques to perform the FSIVGTT in lean and obese conscious mice. We find that in conscious mice, as in humans, first phase insulin secretion, glucose effectiveness and the disposition index impact glucose disposal to varying degrees in health and obesity.
METHODS AND PROCEDURES
Mouse husbandry
All mouse studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. C57BL/6J and B6.V-Lepob/J (the Jackson Laboratories), or C57BL/6NTac (Taconic) male mice were housed in controlled temperature, humidity, and 12-hour light-dark cycle with ad libitum access to mouse chow and water. DIO mice were fed chow containing 60% kCal% fat (Research Diets D12492) for 16 weeks, starting at 8 weeks of age. 6J, 6NTac and Ob/Ob mice were 9–11 weeks old. At FSIVGTT, mice weighed 24.8 ± 0.5 g (6J), 24.7 ± 0.6 g (6NTac), 40.0 ± 1.0 g (DIO) and 50.7 ± 1.3 g (Ob/Ob); at clamp, mice weighed 23.2 ± 0.8 g (6J), 39.2 ± 0.4 g (DIO) and 54.8 ± 01.4 g (Ob/Ob). The pre-procedure fasted weights were, on average, 9.0 ± 0.6% below fed-state preoperative weights, with obese mice losing slightly more (10.9 ± 0.8%) than lean mice (7.5 ± 0.8%).
Catheter implantation and maintenance
Detailed protocols for surgical catheterization, catheter maintenance, blood pressure and heart rate recording, arterial blood sampling and red blood cell return can be found in the online supplement to (18). Briefly, mice were anesthetized with inhaled 2% isoflurane, and micro-renathane catheters (MRE-025; Braintree Scientific, Braintree, MA) were inserted into the left femoral artery and vein, sutured in place, stabilized with superglue, tunneled subcutaneously to exit the skin at the upper back, taped to a wire attached to posterior cervical muscles for stiffness (792500; A-M Systems, Sequim, WA), and connected to a 360 degree dual channel swivel designed for mice (375/D/22QM; Instech). Catheter patency was maintained by continuous sterile infusion of 7ul/hr saline containing 20 units/ml heparin (APP Pharmaceuticals) using a syringe pump with multisyringe adaptor (R99-EM; Razel Scientific Instruments).
FSIVGTT
FSIVGTT was performed after three days recovery from catheter implantation. All blood samples were taken from the arterial catheter in unrestrained, unhandled, conscious mice. After five hours of fasting, two baseline blood samples (20 μl, −10, 0 minutes) were obtained. At time zero, a 1 g/kg bolus of 50% dextrose (Hospira) was injected through the venous catheter over 15 seconds. 20 μl of blood was sampled at 1, 2, 4, 8, 12, 16, 20, 30, and 60 minutes for glucose and insulin. Additional samples for glucose alone, ~2 μl, were obtained at 3, 5, 6, 10, 14, 18, 25, 40 and 50 minutes. All samples were obtained through the arterial catheter. After centrifugation (8000 rpm for 4 minutes) and plasma removal, pooled red cells were resuspended in 20 μl sterile heparinized saline (100U/ml) and re-infused post-sampling at −10, 0, 12, 14, 18, 20, 30, 40 minutes to maintain blood volume. Blood pressure was monitored continuously throughout the protocol; averaging all mice, there were minimal changes in blood pressure from the beginning (100 ± 1 mmHg) to the end (98 ± 2 mmHg) of the FSIVGTT. The hematocrit at the end of the procedure averaged 36 ± 1%. Mouse stress levels during our chronic catheterization and blood sampling protocols are low, as measured by plasma corticosterone levels (18, 19), and as evidenced by the low basal blood glucose measured in the Ob/Ob mice, which exhibit increased sensitivity to stress (Figure 2a) (20). To determine the effect of dosing the intravenous glucose bolus based on lean body mass rather than total body weight, three Ob/Ob mice were subjected to FSIVGTT using 1g/kg lean body mass. As shown in Supplemental Figure 1, this dosing strategy resulted in insufficient insulin secretory response to model FSIVGTT parameters in these mice.
Figure 2. In conscious mice, FSIVGTT-derived insulin sensitivity is comparable to gold-standard hyperinsulinemic euglycemic clamp.
FSIVGTT performed in Ob/Ob mice revealed higher peak blood glucose than lean mice, with slower decay (A), as well as elevated basal and peak plasma insulin (B). Insulin sensitivity measurements obtained from hyperglycemic euglycemic clamp (C) or FSIVGTT (D) were similar. To confirm this finding in a second model of insulin resistance, FSIVGTT was performed in mice with diet induced obesity (E–F). DIO mice showed increased peak blood glucose with a rapid return to baseline; first phase insulin secretion was markedly increased in DIO mice relative to lean mice. Insulin sensitivity measurements obtained by hyperinsulinemic euglycemic clamp (G) and FSIVGTT (H) in DIO mice were similar. Lean controls in panels A, B, D, E, F and H include the seven lean mice from Figure 1 with sufficient insulin secretion to model Si-FSIVGTT (6J-High and 6J-Med from Figure 3 below). Data are represented as mean ± s.e.m.
Euglycemic hyperinsulinemic clamp
Detailed protocols can be found in the online supplement to (21). Mice were catheterized as above, and allowed to recover for three days. After a five hour fast, human insulin was infused at a constant rate (a prime-continuous infusion of 20 mU/kg/min; Novolin R, Novo Nordisk), a variable rate of 50% dextrose was co-infused through the venous catheter to maintain plasma glucose at 100–120 mg/dl. Blood glucose levels were sampled through the arterial catheter as described above, at 10 minute intervals.
Biochemical assays
Blood glucose was measured using a Prodigy Autocode glucometer (~1 μl whole blood; Diagnostic Devices Inc). Plasma insulin was measured by radioimmunoassay (5 μl for lean mice, 2 μl for obese mice; Linco sensitive rat insulin RIA kit, Millipore).
Mathematical modeling
Based on glucose and insulin curves, glucose disposal was divided into three phases: mixing phase (0–2 minutes), glucose effectiveness-dominated phase (2–5 minutes) and insulin-dominated phase (5–60 minutes). The mixing phase was not used for Si or Sg modeling. AIRg was based on insulin values between 0–4 minutes. All Si calculations are multiplied by 104. Data was modeled using MinMod software modified for phase durations as stated above and Sg starting estimate. Alternative mathematical methodology for estimating insulin sensitivity from FSIVGTT data on anesthetized mice have been published (22).
Statistical analyses
Data were expressed as mean ± standard error. All statistical analyses were performed using GraphPad Prism (GraphPad Software). P values were calculated by Student’s t-test when only two groups were compared, or by one-way ANOVA when more than two groups were compared, using log-transformed data when Bartlett’s test for unequal variance showed P<0.05. P<0.05 was considered significant.
RESULTS
FSIVGTT glucose and insulin curves demonstrate rapid glucose disposal and vigorous insulin secretion in conscious lean mice
The FSIVGTT protocol (Figure 1A) was designed to obtain a sufficient number of samples to accurately model individual mouse blood glucose and plasma insulin curves, yet minimize impact on mouse hemodynamics. After an intravenous bolus of glucose into healthy lean C57BL/6NTac (6NTac) mice, blood glucose levels rose rapidly to peak one minute post-injection (Figure 1B). Subsequently, blood glucose declined quickly and dipped slightly below baseline, normalizing before fifteen minutes. Reproducibility among mice was high, resulting in tight curves with minimal variability.
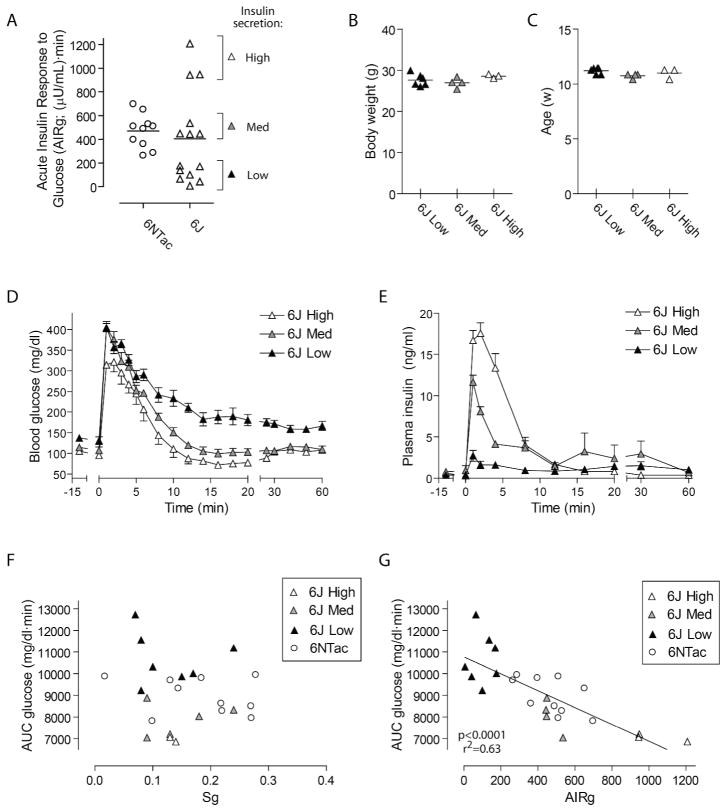
Figure 1. FSIVGTT glucose and insulin curves are rapid and reproducible in conscious lean mice.
Based on pilot data (not shown), the blood sampling protocol (A) was designed to accurately represent glucose and insulin curves while minimizing sampling frequency. (B–C) FSIVGTT was performed on C57BL/6NTac mice (6NTac; n=10). Shown are blood glucose (B) and plasma insulin (C) as a function of time. Note the extremely rapid return to baseline by 15 minutes after IV glucose bolus. (D–E) The FSIVGTT protocol was applied to a second inbred strain, C57BL/6J (6J; n=14). Note the reproducibility of data in similar lean strains. 6NTac curves are re-plotted in (D–E) to facilitate comparison. Data are represented as mean ± s.e.m.
Plasma insulin levels rose rapidly, peaking one minute after glucose injection (Figure 1C). These measurements represent the endogenous mouse pancreatic insulin secretory response; exogenous insulin was not administered. On average, peak insulin concentration was 12.9-fold higher than basal levels. Plasma insulin levels declined rapidly, stabilizing well before fifteen minutes post-injection. Plasma insulin curves also showed a high degree of reproducibility among 6NTac mice.
The FSIVGTT protocol was applied to a second, related strain of healthy lean inbred mice, C57BL/6J (6J), to determine whether similar strains would generate similar blood glucose and plasma insulin curves. Blood glucose in 6J mice also peaked at one minute and returned to baseline before fifteen minutes, although this group as a whole did not dip below baseline (Figure 1D). 6J mice had lower basal plasma insulin levels (Figure 1E) than 6NTac mice (0.6 ± 0.1 ng/ml vs. 1.0 ± 0.2 ng/ml, p=0.04) but peak insulin levels were not statistically different (8.3 ± 1.7 ng/ml vs. 11.0 ± 1.1 ng/ml). The average fold increase in peak plasma insulin level attained for 6J mice was 19.0-fold over basal. Overall curve shapes were comparable in 6NTac and 6J mice, demonstrating the reproducibility of FSIVGTT among similar strains.
Insulin sensitivity measurements by FSIVGTT in conscious mice are comparable to euglycemic hyperinsulinemic clamp
FSIVGTT was performed on conscious obese leptin-deficient Ob/Ob (C57BL/6J background) and control 6J mice (Figure 2A–B). Controls were the subset of 6J mice from Figure 1 with sufficient insulin secretion to model insulin sensitivity (see below for details on insulin secretion in 6J mice). Despite their obese state, unhandled conscious Ob/Ob mice had similar basal blood glucose levels to control mice (Figure 2A). Basal plasma insulin levels were elevated in Ob/Ob mice (7.7 ± 1.3 vs. 0.7 ± 0.2 ng/ml, p=0.0001; Figure 2B). After an intravenous bolus of glucose, Ob/Ob mice attained higher blood glucose levels than controls (527 ± 23 vs. 381 ± 12 mg/dl, p<0.0001). Blood glucose declined more slowly in Ob/Ob mice, not reaching basal levels even by one hour post-injection. AUC glucose was significantly higher in Ob/Ob mice: 14265 ± 726 vs. 9087 ± 306 mg/dl*min; p<0.0001. Plasma insulin levels attained a higher peak in Ob/Ob mice than controls (31.2 ± 3.4 vs. 8.3 ± 1.7 ng/ml, p<0.0001).
Hyperinsulinemic euglycemic clamps were performed on a separate set of Ob/Ob and lean mice. Blood glucose was clamped similarly (104 ± 4 mg/dl (Ob/Ob) vs. 104 ± 5 mg/dl (6J)). Plasma insulin levels for obese mice were 7.6 ± 4.6 ng/ml (baseline) and 26.7 ± 4.7 ng/ml (clamp) and for lean controls were 0.8 ± 0.4 ng/ml (baseline) and 6.7 ± 4.4 ng/ml (clamp). Glucose infusion rates were reduced in Ob/Ob mice relative to controls, resulting in a significantly lower Si-clamp (Figure 2C). When insulin sensitivity was mathematically modeled from FSIVGTT blood glucose and plasma insulin curves, the measured degree of insulin resistance (Si-FSIVGTT) was similar to the clamp result (Figure 2D).
To test whether FSIVGTT-derived insulin sensitivity measurements would parallel hyperinsulinemic euglycemic clamp measurements in a second model of insulin resistance, FSIVGTT was performed on 6J mice with diet induced obesity (DIO; Figure 2E–F). Basal blood glucose and plasma insulin were similar in DIO and control 6J mice. After intravenous glucose bolus, peak blood glucose (545 ± 11 vs. 381 ± 12 mg/dl, p<0.0001) and plasma insulin (36.0 ± 5.9 vs. 8.3 ± 1.7 ng/ml, p<0.0001) were significantly elevated in DIO mice. Compared with Ob/Ob mice, DIO mice showed faster glucose elimination, returning near baseline by 30 minutes. Euglycemic clamp was performed in a separate set of DIO mice (clamped BG 105 ± 1 mg/dl; plasma insulin 1.1 ± 0.3 ng/ml (baseline) and 15.2 ± 1.2 ng/ml (clamp)). As with Ob/Ob mice, DIO clamp- and FSIVGTT-derived insulin sensitivity measurements were similar (Figure 2G–H).
First-phase insulin secretion determines the kinetics of glucose disposal in lean mice
Prior work has suggested that glucose disposal after intravenous glucose challenge in rodents, in contrast to humans, may be largely insulin-independent (16, 17). We asked whether first phase insulin secretion was relevant to glucose disposal in conscious mice. While the population of 6NTac mice showed a fairly uniform acute insulin response to glucose (AIRg, defined as AUC insulin between 0–4 minutes), 6J mice had a surprisingly variable AIRg (Figure 3A). This finding was not related to technical artifact, as low-secretors were distributed among high-secretors with respect to both FSIVGTT procedure date and insulin radioimmunoassay batch, and there was a high degree of consistency among multiple insulin measurements from individual mice. 6J mice are all homozygous for the Nicotinamide nucleotide transhydrogenase (Nnt) mutation, which can reduce insulin secretion (23–25). Differences in AIRg in 6J mice were not due to mouse body weight (Figure 3B), age (Figure 3C), or peak blood glucose (Figure 3D). Although the cause of the variable AIRg in 6J mice remains unexplained, we did observe that, compared to the highest secretors, low secretors demonstrated higher basal blood glucose (131 ± 7 vs. 101 ± 4 mg/dl, p<0.05) and consumed more chow in the postoperative period (12.6 ± 0.5 vs. 8.6 ± 0.3 g, p<0.01).
Figure 3. Glucose disposal in conscious mice is dependent on first phase insulin secretion.
(A) 6J mice showed widely variable AIRg relative to 6NTac mice. 6J mice were separated into three groups based on AIRg <200 (Low), 200–600 (Med) or >600 (High). (B–C) The variability in insulin secretion was not due to differences in mouse body weight (B) or age (C). FSIVGTT blood glucose curves (D) showed that mice with High AIRg had the fastest glucose disposal, while mice with Low AIRg had delayed glucose disposal. (E) FSIVGTT insulin curves illustrate the marked variability in insulin secretion among 6J mice, and demonstrate the consistency among measurements over time for each group. (F–G) For lean mice, AUC glucose was unrelated to Sg (F) but was tightly correlated with AIRg (G). Data for (D–E) are represented as mean ± s.e.m.
We made use of the variable insulin secretion in 6J mice to determine whether the endogenous insulin secretory response impacted glucose disposal rates. Comparing the plasma insulin curves from Low, Med and High secretors (Figure 3E) demonstrated an average increase over basal of 6-fold (Low), 23-fold (Med) and 42-fold (High). Basal insulin levels were not different between the three groups.
The blood glucose curves of Low, Med and High insulin secretors (Figure 3D) demonstrated the impact of endogenous insulin secretion on glucose disposal. High secretors displayed an extremely rapid glucose disposal, dipping below baseline before fifteen minutes post-injection, similar to 6NTac mice (compare with Figure 1B). Mice with intermediate insulin secretion showed intermediate glucose disposal, returning to baseline by fifteen minutes but without dipping below baseline. Low secretors showed markedly delayed glucose disposition, and did not achieve baseline blood glucose levels by one hour post-injection. When all lean mice were examined together, variation in AUC for glucose between 0–60 minutes was unrelated to glucose effectiveness (Sg, Figure 3F), but showed a strong inverse correlation with AIRg (Figure 3G), confirming the importance of the first phase insulin secretory response for determining variation in glucose disposal in conscious lean mice.
Obese, insulin resistant mice show increased AIRg and reduced Sg relative to lean mice
To determine whether conscious mice show the metabolic changes seen in humans with obesity, including increased AIRg and reduced Sg, we compared these parameters between lean, DIO and Ob/Ob mice. The AIRg and Sg data from 6NTac and 6J mice were combined for these analyses (“Lean”). DIO and Ob/Ob mice had approximately 3–4 fold higher AIRg than lean mice, confirming that the first phase insulin secretory response to hyperglycemia was accentuated (Figure 4A). In addition, Sg was significantly lower in DIO and Ob/Ob mice than in lean mice (Figure 4B), suggesting that reduced glucose effectiveness may contribute to glucose intolerance in obese mice, as it does in humans.
Figure 4. Obese mice with IGT have increased first-phase insulin secretion but reduced glucose effectiveness relative to lean mice.
Modeling FSIVGTT glucose and insulin curves to quantify first-phase insulin secretion (AIRg) and glucose effectiveness (Sg) revealed that DIO and Ob/Ob mice had increased AIRg (A) and reduced Sg (B) compared with lean mice. Data are represented as mean ± s.e.m.
Different parameters determine variation in glucose disposal in lean mice than obese mice
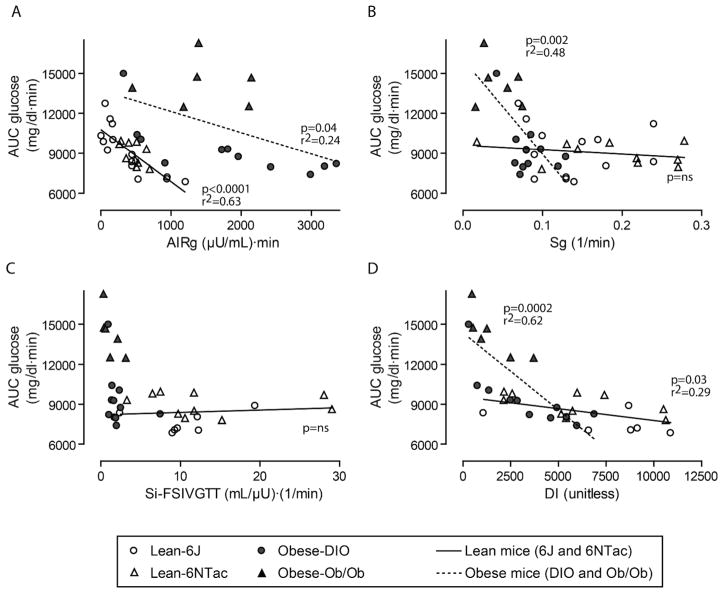
To learn which metabolic parameters correlate best with glucose disposal in lean versus obese mice, each of the four principal FSIVGTT modeled outcomes (AIRg, Sg, Si and DI) were plotted separately against AUC for glucose (Figure 5A–D). By linear regression, AIRg was the most important factor in lean mice, explaining 63% of the variation in glucose disposal (Figures 3G and 5A), but was only loosely correlated with AUC glucose in obese mice (Figure 5A). Intriguingly, variation in Sg was irrelevant to AUC glucose in lean mice, but explained nearly half of the variation in glucose disposal in obese mice (Figures 3F and 5B). The degree of insulin resistance did not independently predict variation in AUC glucose for either lean or obese mice (Figure 5C). Although the DI only nominally predicted AUC glucose in lean mice, DI was the strongest predictor of glucose disposal in obese mice, accounting for 62% of the variation (Figure 5D). These findings suggest that in the setting of obesity, the mouse insulin secretory response was most relevant when taken in context of the degree of insulin sensitivity, as is true for humans (e.g. (13)).
Figure 5. Variation in glucose disposal is predominantly determined by insulin secretion in lean mice, but by Sg and DI in obese mice.
(A) In lean mice, AIRg was significantly correlated with AUC glucose; in obese mice, variation in AIRg accounted for less of the variation in glucose disposal. (B) Sg variation over a wide range was unrelated to AUC glucose in lean mice, but strongly predicted glucose disposal in obese mice. (C–D) On its own, Si did not account for variation in AUC glucose for either lean or obese mice, but DI, the product of Si and AIRg, predicted AUC glucose across the entire cohort of mice (p<0.0001, r2=0.46, curve not shown), attributable to a strong effect in obese mice. Data shown in (C–D) exclude 6J mice with insufficient insulin secretion to model insulin sensitivity. When DIO and Ob/Ob groups were analyzed separately for these parameters, significant correlations were observed for AIRg (A): DIO (p=0.01, r2=0.50), and DI (D): Ob/Ob (p=0.05, r2=0.65) and DIO (p=0.005, r2=0.60); all other correlations were non-significant.
Disposition index identifies metabolic decompensation in individual DIO mice
The disposition index, defined as the product of AIRg and Si, is a strong predictor of progression to glucose intolerance in humans. Conceptually, DI defines the hyperbolic relationship between insulin resistance and insulin secretion; metabolic compensation is maintained in the face of decreasing insulin sensitivity only if insulin secretion increases. In conscious mice, when AIRg was evaluated in the context of Si, the data approximated a hyperbolic relationship, as expected (Figure 6A). Comparing the lean versus obese groups, the average DI was reduced in both DIO and Ob/Ob mice (Figure 6B), indicating that despite increased AIRg, the insulin secretory response was insufficient to maintain metabolic compensation. When the mean DI curves for lean (compensated) versus Ob/Ob (most decompensated) mice were plotted (Figure 6C), the obese DI curve (dashed line) was downward-shifted relative to the lean curve (solid line). Of note, when the DIO mice, which on average had an intermediate DI, were individually plotted against these curves (Figure 6D) some clustered near the compensated curve, whereas the others approximated the decompensated curve, suggesting that the conscious FSIVGTT may have sufficient sensitivity to identify metabolic decompensation in individual mice, while simultaneously measuring insulin sensitivity, insulin secretion, and glucose effectiveness.
Figure 6. Disposition index curves identify individual DIO mice as metabolically compensated or decompensated.
(A) AIRg expressed as a function of Si for all mice showed a roughly hyperbolic relationship between these parameters. Comparing average DI for the lean versus obese groups (B) showed that DI was markedly lower in Ob/Ob mice, with an intermediate phenotype in DIO mice. (C) Hyperbolic DI curves were defined using data from lean mice (compensated; solid curve) and Ob/Ob mice (decompensated; dashed curve). The mean DI for each group is plotted. When DIO mice were individually plotted against these curves (D), the DIO cohort was split, with some mice falling near the compensated curve, and others near the decompensated curve.
DISCUSSION
With this work we demonstrate the feasibility of performing FSIVGTT in conscious, unhandled mice. This technology represents a critical step forward for mouse metabolic research, as it allows simultaneous accurate measurement of insulin sensitivity, glucose effectiveness, first phase insulin secretion, and the disposition index. Using this technique we have made several novel observations. Importantly, these data confirm the widely assumed concept that glucose disposal in the mouse is governed by similar parameters to human metabolism, which include first phase insulin secretion and glucose effectiveness in addition to insulin sensitivity. In lean mice, endogenous first phase insulin secretion determined glucose disposal kinetics. Insulin resistant obese mice had increased AIRg and reduced glucose. Notably, the parameters responsible for glucose disposal kinetics varied between lean and obese mice. Finally, mice with metabolic decompensation showed the same downward shift in the DI curve seen in humans with metabolic decompensation (7).
The value of accurately measuring first phase insulin secretion and Sg in vivo should not be understated. Advances in diabetes research using mouse models have been weighted towards understanding insulin sensitivity, due to availability of the euglycemic clamp, while simultaneous measurement of first phase insulin secretion has not been possible. Currently available techniques to measure mouse first phase insulin secretion either require anesthesia(16, 26, 27), or remove the islet from the natural environment, which alters insulin secretion (1, 28). Note that the FSIVGTT does not measure second phase insulin secretion. The relative contributions of secretion and clearance of insulin cannot be inferred from these data, although the protocol could be adapted to include c-peptide measurements. The variability in insulin secretion observed in genetically identical 6J mice remains unexplained. Interestingly, variability has been demonstrated in these mice for other outcomes, including oncogenesis, stress response, weight gain on high fat diet, and tissue-dependent mRNA expression (29–32). Even more surprising, 6J mice are genetically non-identical at the locus encoding the insulin degrading enzyme (IDE), which regulates circulating insulin concentration (33). Although we present insulin secretion that varied between 6-fold and 42-fold as three distinct groups, it is likely that insulin secretion in 6J mice represents a continuum, just as in humans (11). Peak circulating insulin during the FSIVGTT in lean conscious mice was higher than steady-state levels achieved during euglycemic clamps, despite the high insulin infusion rate of 20 mU/kg/min.
FSIVGTT in conscious mice will allow new types of analyses of in vivo first phase insulin secretory capacity, non-insulin mediated glucose disposal, and the disposition index. This work opens the door to applying genetic and environmental studies, as well as correlation with tissue morphological, histochemical and molecular analyses, to better understand the determinants of these previously inaccessible parameters. For example, by combining FSIVGTT and histology we can separate the roles of beta cell mass and beta cell function in models of diabetes, by combining FSIVGTT with genetics we can identify determinants of high- versus low-secretors of insulin, and Sg, in obesity-related glucose intolerance (1).
Glucose kinetics after an intravenous bolus of glucose into healthy, un-anesthetized mice were extremely rapid, approximately three times faster than published human glucose curves (11). This difference may be due to the smaller volume of distribution, increased heart rate, shorter circulation time, and accelerated glucose turnover. Other than the faster kinetics, the shapes of mouse curves resembled human curves (11); in lean healthy mice blood glucose dipped below basal levels and in obese mice with IGT glucose disposal was prolonged.
Our data contradict prior evidence that glucose disposal in mice (16) and rats (17) is independent of first phase insulin secretion. Differences in outcomes between our study and prior mouse studies may be due to the effects of anesthesia on glucose metabolism (34–36). In anesthetized mice, glucose disposal after intravenous glucose challenge is markedly slower than in conscious mice, only approaching baseline by 50 minutes post-injection in lean mice (16, 37–40). Differences from the prior rat study (17) may be related to variability among species, the glucose dose, or fast duration (overnight in the rats versus five hours in our study) (41).
In lean mice, the strongest predictor of glucose disposal was AIRg. This observation is consistent with human studies, in which the etiology of impaired glucose tolerance was noted to be different between lean and obese people, with impaired disposal principally attributable to AIRg in lean individuals (11). Our finding that the AIRg was increased in obese conscious mice is consistent with a large body of data on the effect of insulin resistance on insulin secretion across species. Interestingly, in obese mice the AIRg did not predict glucose disposal as effectively as it did in lean mice, but considering Si and AIRg together (DI) strongly predicted glucose disposal in obese mice.
Obese mice also had reduced Sg, as is seen in humans with IGT and type 2 diabetes (42). In people, Sg and DI independently predict progression to type 2 diabetes in individuals at high risk for diabetes (13, 43–45). We have found that Sg correlates with glucose disposal in obese mice but not in lean mice. As the gold standard technique for measurement of Sg is the FSIVGTT, use of this technique in conscious mice will allow further investigation into the regulation of this much-debated metabolic parameter.
This technique has some limitations. Modeling Si is dependent on adequate endogenous insulin secretion; modification of the technique to include an exogenous insulin bolus several minutes after the glucose injection would surmount this obstacle. Also, FSIVGTT cannot differentiate insulin resistance arising from liver, fat or muscle.
In summary, we have used the FSIVGTT to demonstrate that the metabolic parameters determining glucose disposal in conscious mice are similar to published human findings. This technique allows quantification of critical elements of glucose metabolism that were not previously quantifiable in unhandled mice: first phase insulin secretion, glucose effectiveness, and the disposition index extending the reach of mouse genetics to interrogate new arenas in metabolism.
Supplementary Material
Acknowledgments
We gratefully acknowledge the thoughtful input of Drs. Andrew Stewart, Rupangi Vasavada, Don Scott and Robert O’Doherty, University of Pittsburgh. This work was supported by National Institutes of Health: HL063767 (CPOD), DK076562 (LCA), DK29867 and DK27619 (RNB) and by the American Diabetes Association: 7-11-BS-04 (LCA).
Footnotes
Conflict of interest statement:
The authors declare no conflicts of interest with this work.
Author contributions: C.P.O. conceived and directed the experiments. B.Z. performed the surgeries. L.C.A., Y.W., E.J.L., S.S., and L.C.R. contributed to data collection. D.S. and R.N.B. performed the mathematical modeling. A.G-O. assisted with insulin measurements. L.C.A. and C.P.O. analyzed the data. L.C.A., A.G-O., R.N.B., and C.P.O. wrote and revised the manuscript.
DISCLOSURE STATEMENT
The authors have no conflicts of interest.
References
- 1.Berglund ED, Li CY, Poffenberger G, Ayala JE, Fueger PT, Willis SE, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008 Jul;57(7):1790–9. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JK, Zisman A, Fillmore JJ, Peroni OD, Kotani K, Perret P, et al. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J Clin Invest. 2001 Jul;108(1):153–60. doi: 10.1172/JCI10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulkarni RN, Almind K, Goren HJ, Winnay JN, Ueki K, Okada T, et al. Impact of genetic background on development of hyperinsulinemia and diabetes in insulin receptor/insulin receptor substrate-1 double heterozygous mice. Diabetes. 2003 Jun;52(6):1528–34. doi: 10.2337/diabetes.52.6.1528. [DOI] [PubMed] [Google Scholar]
- 4.Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993 Dec 30;329(27):1988–92. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 5.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999 Sep;104(6):787–94. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care. 2001 Jan;24(1):89–94. doi: 10.2337/diacare.24.1.89. [DOI] [PubMed] [Google Scholar]
- 7.Bergman RN, Finegood DT, Kahn SE. The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest. 2002 Jun;32(Suppl 3):35–45. doi: 10.1046/j.1365-2362.32.s3.5.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Bergman RN, Pacini G, Porte D., Jr Pathogenesis of age-related glucose intolerance in man: insulin resistance and decreased beta-cell function. J Clin Endocrinol Metab. 1985 Jan;60(1):13–20. doi: 10.1210/jcem-60-1-13. [DOI] [PubMed] [Google Scholar]
- 9.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. Sep-Oct;3(9–10):525–34. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985 Winter;6(1):45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 11.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981 Dec;68(6):1456–67. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987 Mar;79(3):790–800. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenzo C, Wagenknecht LE, Rewers MJ, Karter AJ, Bergman RN, Hanley AJ, et al. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Care. 2010 Sep;33(9):2098–103. doi: 10.2337/dc10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008 Nov 20;359(21):2220–32. doi: 10.1056/NEJMoa0801869. [DOI] [PubMed] [Google Scholar]
- 15.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993 Nov;42(11):1663–72. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 16.Pacini G, Thomaseth K, Ahren B. Contribution to glucose tolerance of insulin-independent vs. insulin-dependent mechanisms in mice. Am J Physiol Endocrinol Metab. 2001 Oct;281(4):E693–703. doi: 10.1152/ajpendo.2001.281.4.E693. [DOI] [PubMed] [Google Scholar]
- 17.McArthur MD, You D, Klapstein K, Finegood DT. Glucose effectiveness is the major determinant of intravenous glucose tolerance in the rat. Am J Physiol. 1999 Apr;276(4 Pt 1):E739–46. doi: 10.1152/ajpendo.1999.276.4.E739. [DOI] [PubMed] [Google Scholar]
- 18.Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O’Donnell CP, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes. 2007 Jul;56(7):1792–801. doi: 10.2337/db06-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoe T, Alonso LC, Romano LC, Rosa TC, O’Doherty RM, Garcia-Ocana A, et al. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol. 2008 Feb 1;586(3):899–911. doi: 10.1113/jphysiol.2007.143586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowland NE, Dunn AJ. Effect of dexfenfluramine on metabolic and neurochemical measures in restraint-stressed ob/ob mice. Physiol Behav. 1995 Oct;58(4):749–54. doi: 10.1016/0031-9384(95)00105-r. [DOI] [PubMed] [Google Scholar]
- 21.Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O’Doherty RM, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med. 2007 Apr 15;175(8):851–7. doi: 10.1164/rccm.200610-1527OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacini G, Ahren M, Ahren B. Reappraisal of the intravenous glucose tolerance index for a simple assessment of insulin sensitivity in mice. Am J Physiol Regul Integr Comp Physiol. 2009 May;296(5):R1316–24. doi: 10.1152/ajpregu.90575.2008. [DOI] [PubMed] [Google Scholar]
- 23.Toye AA, Lippiat JD, Proks P, Shimomura K, Bentley L, Hugill A, et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005 Apr;48(4):675–86. doi: 10.1007/s00125-005-1680-z. [DOI] [PubMed] [Google Scholar]
- 24.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes. 2006 Jul;55(7):2153–6. doi: 10.2337/db06-0358. [DOI] [PubMed] [Google Scholar]
- 25.Wong N, Blair AR, Morahan G, Andrikopoulos S. The deletion variant of nicotinamide nucleotide transhydrogenase (Nnt) does not affect insulin secretion or glucose tolerance. Endocrinology. 2010 Jan;151(1):96–102. doi: 10.1210/en.2009-0887. [DOI] [PubMed] [Google Scholar]
- 26.Curry DL, Bennett LL, Grodsky GM. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968 Sep;83(3):572–84. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- 27.Maechler P, Gjinovci A, Wollheim CB. Implication of glutamate in the kinetics of insulin secretion in rat and mouse perfused pancreas. Diabetes. 2002 Feb;51(Suppl 1):S99–102. doi: 10.2337/diabetes.51.2007.s99. [DOI] [PubMed] [Google Scholar]
- 28.Nunemaker CS, Wasserman DH, McGuinness OP, Sweet IR, Teague JC, Satin LS. Insulin secretion in the conscious mouse is biphasic and pulsatile. Am J Physiol Endocrinol Metab. 2006 Mar;290(3):E523–9. doi: 10.1152/ajpendo.00392.2005. [DOI] [PubMed] [Google Scholar]
- 29.Prehn RT. Nongenetic variability in susceptibility to oncogenesis. Science. 1975 Dec 12;190(4219):1095–6. doi: 10.1126/science.1188386. [DOI] [PubMed] [Google Scholar]
- 30.Jakovcevski M, Schachner M, Morellini F. Individual variability in the stress response of C57BL/6J male mice correlates with trait anxiety. Genes Brain Behav. 2008 Mar;7(2):235–43. doi: 10.1111/j.1601-183X.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang LN, Morgan DG, Clapham JC, Speakman JR. Factors Predicting Nongenetic Variability in Body Weight Gain Induced by a High-Fat Diet in Inbred C57BL/6J Mice. Obesity (Silver Spring) 2011 Jun 30; doi: 10.1038/oby.2011.151. [DOI] [PubMed] [Google Scholar]
- 32.Vedell PT, Svenson KL, Churchill GA. Stochastic variation of transcript abundance in C57BL/6J mice. BMC Genomics. 2011;12:167. doi: 10.1186/1471-2164-12-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watkins-Chow DE, Pavan WJ. Genomic copy number and expression variation within the C57BL/6J inbred mouse strain. Genome Res. 2008 Jan;18(1):60–6. doi: 10.1101/gr.6927808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka T, Nabatame H, Tanifuji Y. Insulin secretion and glucose utilization are impaired under general anesthesia with sevoflurane as well as isoflurane in a concentration-independent manner. J Anesth. 2005;19(4):277–81. doi: 10.1007/s00540-005-0341-1. [DOI] [PubMed] [Google Scholar]
- 35.Schlosser S, Spanholtz T, Merz K, Dennler C, Banic A, Erni D, et al. The choice of anesthesia influences oxidative energy metabolism and tissue survival in critically ischemic murine skin. J Surg Res. 2010 Aug;162(2):308–13. doi: 10.1016/j.jss.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Johansen O, Vaaler S, Jorde R, Reikeras O. Increased plasma glucose levels after Hypnorm anaesthesia, but not after Pentobarbital anaesthesia in rats. Lab Anim. 1994 Jul;28(3):244–8. doi: 10.1258/002367794780681723. [DOI] [PubMed] [Google Scholar]
- 37.Filipsson K, Pacini G, Scheurink AJ, Ahren B. PACAP stimulates insulin secretion but inhibits insulin sensitivity in mice. Am J Physiol. 1998 May;274(5 Pt 1):E834–42. doi: 10.1152/ajpendo.1998.274.5.E834. [DOI] [PubMed] [Google Scholar]
- 38.Ahren B, Pacini G. Dose-related effects of GLP-1 on insulin secretion, insulin sensitivity, and glucose effectiveness in mice. Am J Physiol. 1999 Dec;277(6 Pt 1):E996–E1004. doi: 10.1152/ajpendo.1999.277.6.E996. [DOI] [PubMed] [Google Scholar]
- 39.Aston-Mourney K, Wong N, Kebede M, Zraika S, Balmer L, McMahon JM, et al. Increased nicotinamide nucleotide transhydrogenase levels predispose to insulin hypersecretion in a mouse strain susceptible to diabetes. Diabetologia. 2007 Dec;50(12):2476–85. doi: 10.1007/s00125-007-0814-x. [DOI] [PubMed] [Google Scholar]
- 40.Kooptiwut S, Zraika S, Thorburn AW, Dunlop ME, Darwiche R, Kay TW, et al. Comparison of insulin secretory function in two mouse models with different susceptibility to beta-cell failure. Endocrinology. 2002 Jun;143(6):2085–92. doi: 10.1210/endo.143.6.8859. [DOI] [PubMed] [Google Scholar]
- 41.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006 Feb;55(2):390–7. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 42.Taniguchi A, Fukushima M, Sakai M, Nagata I, Doi K, Nagasaka S, et al. Insulin secretion, insulin sensitivity, and glucose effectiveness in nonobese individuals with varying degrees of glucose tolerance. Diabetes Care. 2000 Jan;23(1):127–8. doi: 10.2337/diacare.23.1.127. [DOI] [PubMed] [Google Scholar]
- 43.Goldfine AB, Bouche C, Parker RA, Kim C, Kerivan A, Soeldner JS, et al. Insulin resistance is a poor predictor of type 2 diabetes in individuals with no family history of disease. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2724–9. doi: 10.1073/pnas.0438009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osei K, Rhinesmith S, Gaillard T, Schuster D. Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans: implications for primary diabetes prevention. Diabetes Care. 2004 Jun;27(6):1439–46. doi: 10.2337/diacare.27.6.1439. [DOI] [PubMed] [Google Scholar]
- 45.Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992 Oct 17;340(8825):925–9. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.