Abstract
Thiazolidinediones (TZDs) are agonists at peroxisome proliferator-activated gamma-type (PPAR-γ) receptors and are used clinically for the treatment of type 2 diabetes where they have been shown to reestablish insulin sensitivity, improve lipid profiles, and reduce inflammation. Recent work also suggests that TZDs may be beneficial in Alzheimer’s disease (AD), ameliorating cognitive decline early in the disease process. However, there have been only a few studies identifying mechanisms through which cognitive benefits may be exerted. Starting at 10 months of age, the triple transgenic mouse model of AD (3×Tg-AD) with accelerated amyloid-β (Aβ) deposition and tau pathology was treated with the TZD pioglitazone (PIO-Actos®) at 18 mg/Kg body weight/day. After four months, PIO-treated animals showed multiple beneficial effects, including improved learning on the active avoidance task, reduced serum cholesterol, decreased hippocampal amyloid-β and tau deposits, and enhanced short- and long-term plasticity. Electrophysiological membrane properties and post-treatment blood glucose levels were unchanged by PIO. Gene microarray analyses of hippocampal tissue identified predicted transcriptional responses following TZD treatment as well as potentially novel targets of TZDs, including facilitation of estrogenic processes and decreases in glutamatergic and lipid metabolic/cholesterol dependent processes. Taken together, these results confirm prior animal studies showing that TZDs can ameliorate cognitive deficits associated with AD-related pathology, but also extend these findings by pointing to novel molecular targets in the brain.
Keywords: 3×Tg-AD, aging, hippocampus, long-term potentiation, microarray analysis, pioglitazone, PPAR, T2DM
INTRODUCTION
Alzheimer’s disease (AD) is the most widespread cause of dementia and its incidence will continue to increase rapidly as the population ages [1]. Although intensive research efforts have provided considerable insights into the biology of AD, the underlying processes mediating the progressive decline in cognitive function are still poorly understood. However, a potentially important observation on the pathogenesis of AD has emerged from studies showing that the risk of AD and mild cognitive impairment (MCI) is increased in subjects with metabolic syndrome (characterized by insulin resistance) and Type 2 diabetes mellitus (T2DM) [2–6]. This link has suggested that drugs used to treat diabetes may also be efficacious in AD. In particular, the thiazolidinediones (TZDs), drugs that restore insulin sensitivity and reestablish glucose homeostasis, have been shown to exert multiple beneficial effects on age-related cognitive decline [7–11]. For example, the TZD rosiglitazone improved cognition in patients with AD or MCI [10], and the TZD pioglitazone (PIO) exerted positive effects on cognitive and functional outcomes in patients with MCI and diabetes [11]. Further, adjunct treatment with rosiglitazone appears to provide some relief from cognitive decline in MCI patients with T2DM [7]. In addition, factors related to lipid metabolism appear to play a role in determining sensitivity to the possible cognition enhancing effects of TZDs. Thus, rosiglitazone improves cognition in non-diabetic elderly patients with probable or mild-to-moderate AD [8] lacking the E4 allele of the apolipoprotein E (APOE-4), but not in APOE-4 carriers.
TZDs are synthetic peroxisome proliferator-activated receptor γ (PPAR-γ) agonists that regulate expression of genes that play a role in glucose handling and adipogenesis. Peripheral targets include insulin receptors, glucose transporters, fatty acid synthase, and several key regulators of adipocyte differentiation [12–15]. Importantly, however, clinical studies with AD patients have also raised the possibility that TZDs might impact cognitive function through mechanisms other than glucose regulation. That is, TZDs have been shown not only to reestablish glucose regulation and normal lipid levels, but also to reduce inflammation and amyloid-β (Aβ) peptide as well as increase cerebral blood flow in humans [11, 16, 17].
Studies in animal models have also identified multiple potential mechanisms of neuroprotection conferred by TZDs. In addition to improvements in glucoregulation, the beneficial effects of TZDs in several murine models of AD have been linked to reductions in inflammatory cytokines [18–21], oxidative stress [22], Aβ deposits [18, 23, 24], glial activation [18, 22], tau phosphorylation [23, 24], or glucocorticoid signaling [25–28]. In the Tg2576 model of AD which develops diabetes, TZDs reestablish insulin sensitivity, but also reduce soluble Aβ40 fragments [29] suggesting that the improvement in cognition in this model may occur independently of an effect on glucose regulation [30].
Given that the pathways through which TZDs potentially modulate cognitive decline and AD-related pathology are poorly understood, we conducted an extensive multidisciplinary analysis of the CNS effects of chronic TZD exposure in a mouse model of AD pathology. The 3×Tg-AD model was chosen because it exhibits a wide range of biomarkers of AD, including cognitive decline, synaptic impairment, elevated Aβ peptides and neuritic plaques, as well as hyperphosphorylated tau proteins and neurofibrillary tangles [31]. We used behavioral, electrophysiological, biochemical, and gene microarray approaches to examine the impact of long-term TZD treatment in vivo on multiple markers of AD-related pathology. Of the two clinically relevant TZDs, PIO was chosen because of its greater lipophilicity and potential higher access to the brain [32].
This is one of the first multidisciplinary studies of chronic exposure to a TZD looking at both central and peripheral markers in a mouse model of AD pathology. The results showed that long-term PIO significantly attenuated several pathological markers of AD. Peripheral glucose concentrations were not altered although several major pathways in the brain were associated with the improvement of outcome measures, suggesting that PIO acted, at least in part, through mechanisms other than glucose regulation.
MATERIALS AND METHODS
Animals
Experiments were conducted in compliance with the institutional guidelines of the Animal Care and Use Committee at the University of Kentucky. Twenty-nine, 10 month-old female triple transgenic mice overexpressing AβPPSWE and TauP301L, and carrying a PS1M146V knock-in mutation (3×Tg-AD)were maintained in a 10-h dark and 14-h light cycle in groups of three or four per cage. These animals were randomly separated into two groups, one receiving a control diet (n = 15, CTRL), the other a PIO-enriched diet (n = 14, PIO). Animals were maintained on these diets for 14 weeks and were, therefore, 14–15 months of age when all experiments began. CTRL and PIO diet were sustained until all data were acquired. Animals weights and food consumption were recorded three times per week throughout the duration of the study. For analyses on animal weights, data were averaged into a weekly mean for each animal. Approximate daily food consumption per animal was determined from weekly measurements of food weight, divided by the number of days between feedings and the number of animals in the cage.
Diets
Actos® was purchased through the division of laboratory animal research facility at the University of Kentucky and was crushed to a fine powder. Two different diets were formulated by Harlan Laboratories, Inc. (Madison, WI) including a control diet (AIN-93G, 18.8% Kcal from protein, 63.9% Kcal from carbohydrates, and 17.2% Kcal from fat; TD.94045), and a PIO diet formulated at 84 ppm and also combined into AIN-93G. Calculated PIO dosages based on average food consumption were approximately 18 mg/kg body weight and were determined using the daily average food intake, the concentration of drug in the diet, and the average weight of the mice. Takeda Pharmaceuticals (Osaka, Japan), the manufacturer of Actos®, recommends doses of PIO should not exceed 20 mg/Kg in the mouse.
Behavior
After 14 weeks of PIO treatment, the one-way active avoidance paradigm, a hippocampal-dependent task [33], was used to assess learning. On the week prior to behavioral characterization, two animals housed in the same cage and on the PIO supplemented diet began to show signs of paresis in caudal extremities (hind limbs and tail) and were excluded from the behavioral analyses even though no hypermetria or proprioceptive deficits were noted. Once the condition started, no overt sign of worsening was seen (well groomed, no change in body weight). Mice (12 PIO and 15 CTRL) were thus trained in an active avoidance task composed of two adjacent chambers (a dark and a light one) separated by a guillotine door (Med Associates Inc., St. Albans, VT). The computer-controlled foot shock delivery (0.8 mA, 25 s foot shock) was always delivered in the dark chamber following a 4 s grace period. Mice were acclimated to the dimly lit training room for 20 min before each session. On day one, the mice were placed into the light compartment for 120 s during which they could move freely to either chamber, no shock was delivered. In order to determine chamber preference (light or dark) baseline activity was recorded as time spent in each chamber. Starting on day two, mice were trained on the task with three trials per session for a total of four sessions over three days. Day two of training consisted of one morning session, day three consisted of a morning and an afternoon session and day four consisted of one morning session. During a single trial, the mice were introduced into the dark chamber and given 4 s to escape to the light chamber. The guillotine door was closed immediately following the escape to the light chamber. Time to exit the dark chamber was recorded. The mouse was then removed from the light chamber and placed in a holding cage for 1 min until subsequent trials. Avoidance learning data are presented as percent avoidance across sessions (sum of the total number of avoidances across trials divided by the total number of trials *100) or mean escape latency (raw and ranked). While some degree of sensory dysregulation has been noted in several other models of AD [34, 35], in the 3×Tg-AD, no overt sign of visuo-motor dysfunction has been noted [36] and these animals show the least amount of retinal Aβ deposition and pathology compared to other models [37].
Blood collection and tissue isolation
At the end of the study, eight mice from each group were anesthetized with phenobarbital (IP – 100 mg/kg) and transcardially perfused with ice-cold 0.9% saline. Blood from these non-fasted animals was collected via cardiac puncture from the right ventricle immediately prior to perfusion. Brains were removed and bisected, one hemisphere was reserved for immunohistochemical staining, while the other hemisphere was dissected in order to isolate the hippocampus (see microarrays section) and the cortex (soluble/insoluble Aβ measurements). The hearts and adrenal glands were harvested, weighed, and flash frozen in liquid nitrogen. Heart weights and adrenal weights were normalized to body weight (mg/g) for each mouse. Livers were harvested and flash frozen for histology.
Serum analysis
Collected blood (as above and from some of the animals used for electrophysiology) was centrifuged at 3,000 rpm for 10 min in BD Microtainer tubes. Plasma was aliquoted into centrifuge tubes and stored at −80°C until analysis. Serum from each animal was shipped on ice to the Comparative Pathology Laboratory at UC Davis, and analyzed on a Roche COBAS Mira Plus Chemistry Analyzer. Quantitative data are reported on several blood markers obtained from 10–15 samples/group.
Liver histology
To determine the extent of lipid accumulation in the liver of PIO and CTRL mice, oil red O staining was performed. Frozen livers were sectioned (10 µm) onto glass slides and stored at −80°C until further use. The tissue was thawed and allowed to air dry for 30 min, fixed in ice-cold 10% formalin for 5 min, and then washed three times in distilled water. Sections were placed in 100% propylene glycol, stained in 5% oil red O stain (Sigma Aldrich) for 8 min at 60°C, and subsequently placed in 85% propylene glycol. Slides were rinsed in distilled water and counterstained with hematoxylin. Liver sections from four randomly selected animals from each group were imaged on a Nikon microscope (TE200) through a 20 X lens using a spectral analysis camera (Nuance, CRi, Woburn, MA). Images were analyzed with ImageJ software (NIH) using the color threshold function to identify hues of reds in the section associated with the oil red O stain pattern. The percent area stained was used for statistical analysis.
Microarrays
Hippocampi were dissected from the left hemispheres of mouse brain (n = 8 per group) following intracardial saline perfusion. Tissue was flash-frozen on dry ice and stored in −80°C freezer until RNA extraction. RNA was prepared using TRIzol reagent precipitation with ethanol and reconstituted in RNAase-free water. Quality control results: [RNA] = 401 ± 26 ng/ µl (Nanodrop); RNA Integrity Number 8.5 ± 0.04 (Bioanalyzer). RNA labeling was performed as described previously [38–41], and according to standard Affymetrix procedures. Labeled cRNA for each animal was individually hybridized to an Affymetrix Gene Chip Mouse Genome microarray (430v2). All arrays passed standard Affymetrix quality control: GAPDH 3′–5′ ratio 0.78 ± 0.004; RawQ 2.48 ± 0.04; Background noise 72.9 ± 1.5; Scaling factor (based on target intensity of 500) 1.31 ± 0.33;% Present 62.0 ± 0.3 and were not significantly different across the two groups (T-test p > 0.1 for all measures). Visual inspection of residual sign images (Affy PLM [42]) revealed no major image defects. The gcRMA probe level algorithm (‘justgcRMA’ command run in Bioconductor in the R operating environment) calculated signal intensities [43, 44]. Only unique probe sets/genes with ‘A’ grade annotations and at least 3 chips with signal intensities >4.3 were retained for further analysis. Values were transferred to Excel (2007, Microsoft), Bioconductor [43], MultiExperiment Viewer [MEV, [45] and the DAVID suite of bioinformatic tools [46] for subsequent analysis. Specific statistical procedures are outlined in Results. The signal intensity (gcRMA) and images (.cel files) have been deposited to the MIAME compliant Gene Expression Omnibus (GEO) database [47] - accession # GSE32536].
CA1 Aβ and tau immunostaining
Right brain hemispheres from each mouse (n = 8 per group) were removed after saline perfusion and placed in 4% paraformaldehyde by weight in 0.4 M phosphate buffer for ~4 h. The brains were then switched to a 30% sucrose solution (by weight) in 0.04% phosphate buffer for ~24 h in the fridge. Prior to placing the brains in a −20°C freezer for storing, they were immersed in an antifreeze solution composed of sucrose (15% by weight) and ethylene glycol (33% by volume) in 0.04 M phosphate buffer. Brain sections (35 µm) were immunohistochemically stained for human Aβ (W0–2 antibody, The Genetics Co., Switzerland; specific for human Aβ4–10) [48], or PHF-1 (generously provided by Dr. Peter Davies, Albert Einstein college of medicine, NY), which recognizes tau phosphorylated at Ser396 and Ser40. Secondary antibody was goat anti-mouse biotin, followed by ExtraAvidin (Sigma, USA). Sections were visualized using Ni-enhanced diaminobenzidine (DAB). Dorsal hippocampus sections were digitized on a Leica microscope equipped with a 40× objective and an Olympus DP70 camera. Images were converted to gray scale using Paint Shop Pro V7. Aβ densities were measured using ImageJ and following an automatic thresholding process for each image using the “make binary” function. A region of interest was defined around stratum pyramidal in area CA1 (excluding large extracellular deposits/plaques) to obtain measures of integrated density (mean gray value *area), and of percent area covered by immunostained neurons (Aβ-positive cells). For the PHF-1 positive cell counts, immnunostained cells were quantified in area CA1 in two sections containing the dorsal hippocampus per brain, from sections adjacent to the sections stained for W0–2.
Soluble and insoluble Aβ levels
RIPA extraction
Previously published techniques were used to quantify soluble and insoluble Aβ levels [49, 50]. Cortical tissue (50–100 mg) taken at the time of hippocampal dissection for microarrays from 7 CTRL and 8 PIO mice was frozen on dry ice for later analysis (one sample was lost). The tissue was thawed on ice and placed in enough radio-immunoprecipitation assay (RIPA) buffer to create equal concentrations of 0.1 mg/µL of tissue. The solution was then placed under a polytron tissue homogenizer set on medium speed. Brain homogenates (500 µL) were aliquoted into microcentrifuge tubes and then centrifuged at 100,000 g for 1 h. The supernatant was collected and the remaining pellet was reserved for formic acid (FA) extraction.
Formic acid extraction
The pellet was sonicated in 70% FA for 30 s (500 ms pulse duration). The homogenate was then centrifuged at 100,000 g for 1 h. The aqueous layer was collected and stored at −80°C until further use.
ELISA
RIPA and FA samples were thawed on ice and vortexed. FA samples were neutralized with 1 : 20 dilution in tris phosphate buffer. Sandwich ELISA was conducted using Ab9 and 4G8 antibodies and detected with neutravidin-HRP and read with a Victor plate reader. Data are presented as Aβ measured in fmol/mg tissue.
Slice preparation, extracellular and intracellular recordings
The remaining mice in each group (n = 6 PIO and n = 7 CTRL) were used for electrophysiological recordings. Hippocampal slices were split across two electrophysiological setups, one monitoring field potentials before and after long-term potentiation (LTP) induction, the other measuring intracellular potentials (e.g., afterhyperpolarization (AHP), synaptic hyperpolarization). All data were acquired using pClamp 8.0 (Molecular Devices MDS, Toronto, Canada) software through a Digidata 1320A A/D converter (Molecular Devices), and quantification of potentials (e.g., amplitude and duration of AHPs, field EPSPs) was obtained with Clampfit software (Molecular Devices). Each setup used Axoclamp 2A amplifiers (Molecular Devices).
LTP measures
One mouse from each group provided unviable tissue and data could not be recorded, therefore, LTP data was obtained from 5 PIO and 6 CTRL mice. 7 slices from 4 CTRL animals and 6 slices from 3 PIO animals provided stable data for inclusion into statistical analyses of LTP induction and maintenance (see criterion for inclusion below). Mice were anesthetized in a CO2 filled chamber prior to decapitation. Brains were quickly removed and placed in oxygenated artificial cerebrospinal fluid (ACSF) of the following composition (in mM): 114 NaCl, 3 KCl, 10 Glucose, 1.25 KH2PO4, 26 NaHCO3, 8 MgCl2, 0.1 CaCl2. Hippocampi were transversely sectioned with a Vibratome 3000 (Bannockburn, IL) yielding 350 µm slices. Slices were transferred to an interface-type recording chamber containing oxygenated recording ACSF (rACSF) with the following composition (in mM): 114 NaCl, 3 KCl, 10 Glucose, 1.25 KH2PO4, 26 NaHCO3, 2.5 CaCl2 and 1.3 MgCl2. The recording chamber was kept at 32°C and warm moist 95% O2/5% CO2 was continually pumped into the chamber. Individual slices were recorded after at least a 2 h incubation period in the chamber. A twisted, bipolar Teflon-coated stainless steel stimulating electrode was placed on the Schaeffer collaterals, while the glass recording electrode (3–15 MΩ) was positioned in the stratum radiatum. Stimulation (baseline and LTP) was delivered through a pair of SD9K stimulators (Astro Med Inc., Grass Instr., Warwick, RI). Prior to initiation of data acquisition on dendritic field potentials (fEPSPs), the slice was stimulated once every 20 s for 10 min at a minimal stimulation setting (~10–20% of the I/O). Current outputs (I/O) were recorded during stepwise increases in voltage at the stimulating electrode to determine maximum output responses. Prior to LTP induction, baseline fEPSPs were recorded for 20 min at a stimulation setting set to 50% of the maximum EPSP (determined by I/O). LTP was induced using a 1 s train of pulses delivered at 100 Hz using the same settings for stimulation amplitude and duration as those used during baseline. EPSPs were recorded for 30 min post-LTP induction. For each slice, baseline EPSP slopes averaged across the 20 min prior to LTP induction were used to normalize EPSP slopes after LTP induction. Data are reported on the slope of the descending phase of the EPSP and on the amplitude of the EPSP measured as the maximum deflection from baseline. Slices with responses drifting up or down greater than 20% across the 20 min baseline were excluded from the analysis. All EPSPs obtained after LTP induction (30 min) were used for statistical analysis using a 2-way RM-ANOVA with time and drug as factors.
Intracellular recordings
Ca2+-dependent AHP were recorded from 11 cells from 7 CTRL animals and 13 cells from 6 PIO treated animals. Passive membrane properties were not different between the two animal groups (Table 2), suggesting that cells with comparable baseline characteristics were analyzed. The tissue preparation was shared with the LTP setup and a similar holding chamber was used to maintain the tissue. The rACSF consisted of the same solution except for final concentrations of CaCl2 (2.0 mM) and MgCl2 (2.0 mM). Slices were placed in a perfusion chamber (RC22C, Warner Instruments, Co., Hamden, CT) and maintained in a continuous flow of oxygenated ACSF pre-heated at 32°C using a TC2Bip/HPRE2 in line heating system (Cell Micro Controls, Northfolk, VA). This setup was mounted on the stage of a Nikon E600FN inverted microscope. As previously described [51], cells were impaled with sharp microelectrodes filled with 2 M KMeSO4 and 10 mM HEPES, pH 7.4 (tip resistance 90–150 MΩ), pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) using a P80 pipette puller (Sutter Instruments, Novato, CA). All experiments were performed in current clamp mode with bridge balance compensation and capacitance neutralization. Signal was digitized at 2 kHz and low-pass filtered at 1 kHz. Recordings of membrane input resistance (IR) were obtained in response to 800 ms, 200 pA hyperpolarizing current injections using an Axoclamp 2B amplifier (Molecular Devices, MDS, Toronto, Canada) while holding the cell at −70 mV. To generate an afterhyperpolarization (AHP) cells were held at −65 mV (baseline) and depolarized with a 100 ms current injection in order to generate three Na+ action potentials. AHPs were elicited every 30 s and at least 6 AHPs were averaged for each cell. The medium AHP (mAHP) was measured as the peak hyperpolarization immediately after the offset of the depolarizing current injection, the slow AHP (sAHP) was measured 800 ms after the end of the current injection. The AHP duration was measured from the end of the depolarizing step until return to baseline. Maximal membrane hyperpolarization during repetitive synaptic activation (3, 7 and 15 Hz) at action potential threshold was also measured during a 10 s stimulation [52]. Only neurons with input resistance >40 MΩ, holding current <500 pA and overshooting action potentials were included in the analysis.
Table 2.
Electrophysiological parameters
| CTRL | PIO | |
|---|---|---|
| Electrode resistance (MΩ) | 117.2 ± 6.50 | 121.1 ± 7.55 |
| Input resistance (MΩ) | 56.4 ± 6.37 | 66.8 ± 5.19 |
| Holding current (pA) | −361.7 ± 47.04 | −276.2 ± 28.27 |
| Action potential height (mV) | 68.9 ± 3.40 | 71.1 ± 3.00 |
| Current injection (pA) | 230.3 ± 18.43 | 214.1 ± 18.73 |
Data represent Mean ± SEM on measure of electrode resistance, input resistance, holding current necessary to hold the cell at −70 mV, action potential height (measured from −65 mV), and intracellular current injection necessary to elicit 3 action potentials (duration 100 ms). No significant difference was found in any of these parameters across treatment groups (T-test, p > 0.05).
Statistics
Unless otherwise noted in Results, T-tests or repeated measure ANOVAs (RM-ANOVAs) were used for statistical analyses (α = 0.05).
RESULTS
Body weights and food consumption
Analysis of animal weights in the week prior to introduction of the purified diets revealed a significant effect across groups with heavier animals seen in the CTRL group (p = 0.04, T-test). Weight data in the next 13 weeks of the study were therefore normalized to the weight of each animal during the first week. This analysis revealed no main effect of drug on weight gained across 13 weeks (F(1,26) = 1.37; p = 0.25; RM-ANOVA) and while animal weights fluctuated minimally during this period, CTRL animals always remained slightly heavier than PIO animals (p < 0.0001, T-test; Table 1). A similar analysis on food intake across the same period revealed no significant difference in grams of food consumed per week per cage in the two groups (F(1,7) = 1.30, p = 0.29, RM-ANOVA). Average body weights and food consumption across the 14 weeks are presented in Table 1.
Table 1.
Blood chemistry panel, animal weights, and food consumption. Blood serum markers in animals were measured following a 4 month treatment with either a control (CTRL) or a PIO-enriched diet (PIO)
| CTRL | PIO | |
|---|---|---|
| Calcium (mg/dL) | 9.3 ± 0.39 | 8.5 ± 0.42 |
| Glucose (mg/dL) | 167.4 ± 19.23 | 169.5 ± 18.16 |
| BUN (mg/dL) | 26.4 ± 4.29 | 24.1 ± 5.78 |
| ALT (U/L) | 93.1 ± 27.95 | 43.4 ± 4.17 |
| CK (U/L) | 799.4 ± 192.73 | 1207.1 ± 252.65 |
| Uric acid (mg/dL) | 1.7 ± 0.24 | 2.2 ± 0.23 |
| Cholesterol (mg/dL) | 113.9 ± 13.40 | 57.4 ± 5.67# |
| HDL (mg/dL) | 63.6 ± 7.91 | 33.6 ± 3.29** |
| LDL (mg/dL) | 11.3 ± 1.46 | 7.9 ± 0.96 |
| Triglyceride (mg/dL) | 101.8 ± 19.42 | 55.4 ± 3.51* |
| Body weight (g) | 34.6 ± 2.42 | 29.3 ± 0.79*** |
| Food (g/mouse/day) | 2.9 ± 0.42 | 2.6 ± 0.14 |
Abbreviations: BUN, blood urea nitrogen; ALT, alanine aminotransferase; CK, creatine kinase; HDL, high density lipoprotein; LDL, low density lipoprotein. Body weights and food intake (Food) are also presented and were averaged across the duration of the study.
p < 0.05,
p < 0.01,
p < 0.001
p < 0.0001 indicate a significant effect of PIO (T-test). Data represent Mean ± SEM.
Serum analysis
Table 1 also presents post-treatment results of the serum analysis on a selected panel of peripheral markers. No sign of diabetes, renal or liver dysfunction, calcium dysregulation, or heart damage were observed. Compared to CTRL mice, PIO mice showed significant reductions in cholesterol, HDL, and triglyceride levels. A strong trend for reductions in LDL levels was also seen (p = 0.056; T-test).
Organ weights and liver histology
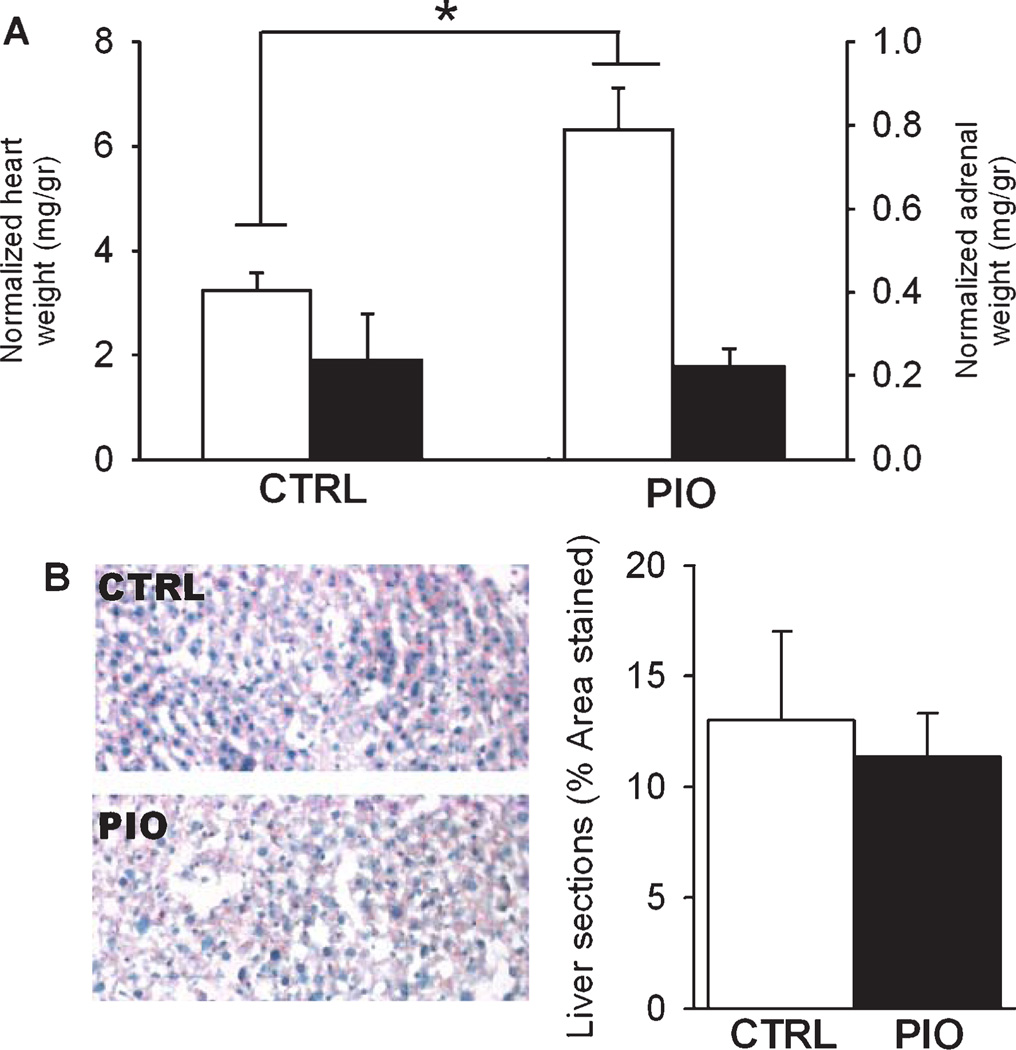
TZDs have been associated with adverse cardiovascular events in several clinical trials [53] which prompted us to harvest and weigh animal hearts. A significant increase in heart weight was seen in the PIO group (p < 0.0001, T-test; Fig. 1A).While the basis for this effect is unclear, it is unlikely that the doubling in heart weight can be attributed to weight gain over the course of the study as the PIO-treated group only showed a 2% increase in body weight. Furthermore, an effect on fluid retention is unlikely because it appears higher PIO doses are necessary for this effect in rodents [54]. Clearly, further studies are necessary to address the basis for potential adverse cardiovascular effects of PIO.
Fig. 1.
Assessment of pioglitazone effects on peripheral tissues. A) Heart (left axis, white bars) and adrenal weights (right axis, black bars) were normalized to body weight (mg/gr) in animals fed either control diet (CTRL) or PIO-enriched diet (PIO). B) Left, representative examples of liver sections stained with oil red O and hematoxylin from a CTRL and PIO-treated animal. Right, data showing percent of sections stained for lipids (% area stained) in both groups. Data represent Mean ± SEM, *p < 0.05, T-test.
Because prior studies have shown a possible link between TZD actions and stress hormones [25], we measured adrenal weights in all animals. There were no significant group differences on this measure (p = 0.72; Fig. 1A). Given the significant decrease in plasma cholesterol, we also tested for evidence of lipid accumulation in the liver of PIO and CTRL mice using oil red O staining. Staining of liver sections with oil red O revealed no difference in the PIO compared to the CTRL animals (p = 0.72; Fig. 1B).
CA1 Aβ staining/levels
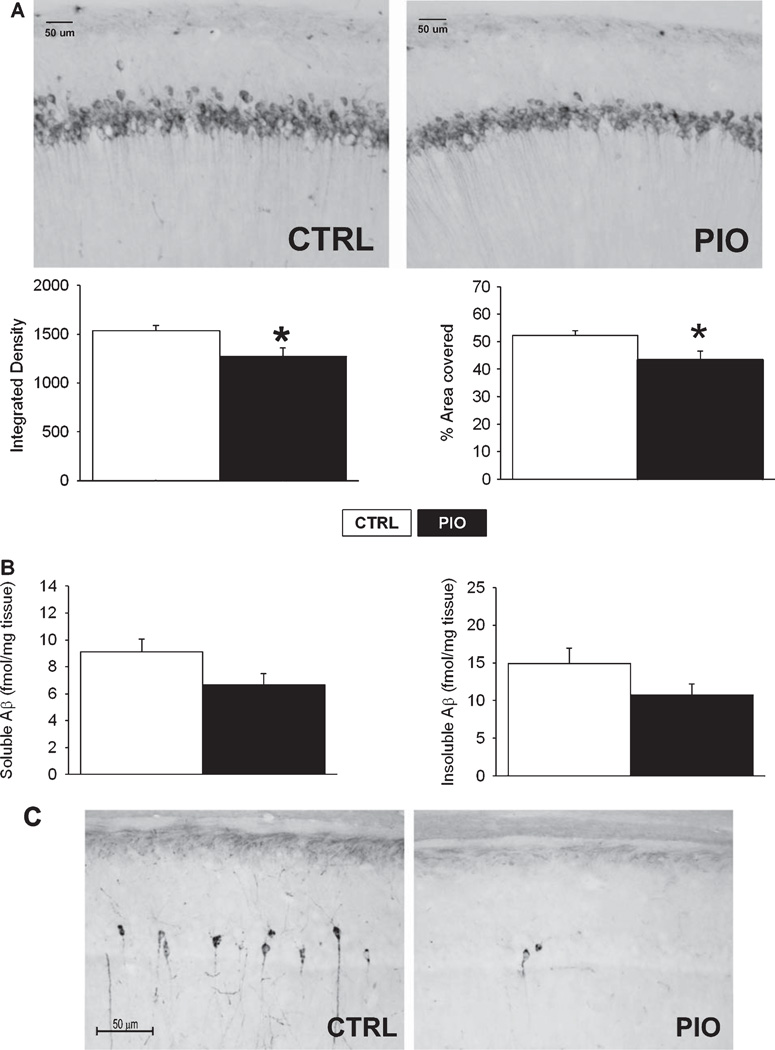
In prior animal and culture studies, TZDs have been shown to reduce Aβ deposits [18, 22, 28, 29, 55]. We therefore monitored Aβ staining in brain sections of dorsal hippocampus. Sections were immunostained with the W02 antibody (Fig. 2) to quantify intracellular Aβ accumulation within area CA1 neurons (stratum pyramidale), and we also quantified soluble and insoluble Aβ fractions from leftover cortices obtained during tissue preparation for microarray experiments. We chose to quantify intracellular Aβ because it is thought to contribute importantly to disease progression [56]. PIO significantly reduced Aβ staining in the CA1 region of the hippocampus. The surface area analyzed (region of interest, ROI) was not different between the 2 groups (p = 0.21; T-test; data not shown), however, the mean gray value in this ROI (p = 0.025; T-test; data not shown), the integrated density (mean grey value *area; p = 0.022; T-test; Fig. 2A left), and the percent area covered by immunostained CA1 neurons (p = 0.025; Fig. 2A right) were all significantly reduced by PIO treatment. Furthermore, a trend for a decrease in the soluble Aβ fraction was seen in these animals compared to those fed the CTRL diet (p = 0.07; Fig. 2C left). While the insoluble Aβ fraction was also decreased, this did not approach significance in animals on the PIO diet (p = 0.13; Fig. 2C right).Correlation between intracellular Aβ density and cholesterol in 16 animals for which both variables were measured yielded a positive correlation which yielded a trend (p = 0.076; r2 = 0.21; data not shown).
Fig. 2.
Hippocampal Aβ levels and tau immunoreactivity. A) Top, representative examples of CA1 area photomicrographs taken from animals fed either control diet (CTRL) or PIO-enriched diet (PIO) and stained using WO2 antibody against the Aβ peptide. Bottom, quantification of WO2 staining shows decreases in integrated Aβ density, and in the area covered by Aβ positive cells (percent). B) Results from Sandwich ELISA method to measure soluble (left) and insoluble (right) Aβ fractions. While not significant, a trend was seen in the soluble fraction decreasing with PIO treatment (p = 0.07). C) Photomicrographs of hippocampal sections stained with PHF-1 antibody to monitor the presence of hyperphosphorylated tau. A significant reduction in tau immnunoreactivity was seen (see text). Data represent Mean ± SEM, T-test, *p < 0.05.
CA1 phosphorylated-tau positive neurons
Prior work has demonstrated TZDs can reduce phosphorylated tau pathology in culture and animal models of AD [23, 24, 57]. Here, we quantified phosphorylated tau-labeled cells and monitored the impact of PIO on this unique pathology in the transgenic animal. A significant and striking decrease in phosphorylated tau positive cells was seen (Fig. 2C). As such, the mean number of PHF-1-positive neurons per section was 7.81 ± 2.01 for the CTRL animals, and 1.18 ± 0.23 for the PIO-treated animals (p = 0.005, T-test).
Active avoidance learning
Light/dark preference
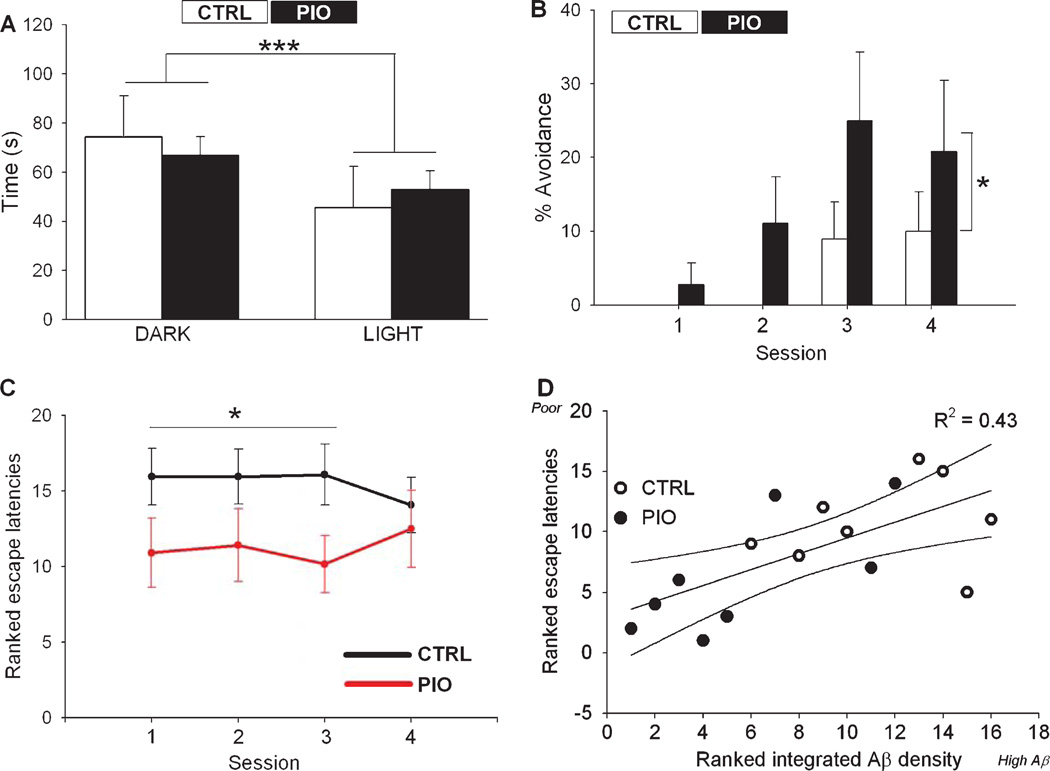
On day one of training, each mouse was allowed to explore the dark and light chambers of the active-avoidance shuttle box for 120 s. Both groups of animals showed a significant preference for the dark side of the chamber (F(1,48) = 31.1; p = 0.0001; Fig. 3A). There was no difference between treatment groups on measures of time spent in the dark chamber (in seconds, CTRL: 74.4 ± 4.48, PIO: 66.9 ± 2.34), the mean number of crossings (CTRL: 7.60 ± 0.92, PIO 9.83 ± 0.99), or the time to first crossing (in seconds, CTRL: 20.9 ± 5.6, PIO: 18.0 ± 3.6), indicating no overt drug-related difference in ambulatory behavior, fear or anxiety levels.
Fig. 3.
Active avoidance learning. A) Pretraining measures on time spent in each compartment indicate all animals spend more time in the dark rather than the light chamber (***p < 0.001, Two-way ANOVA). B) Percentage avoidance in PIO fed mice was significantly greater than in the CTRL group across all training sessions (*p < 0.05 Two-way ANOVA). For graphical comparison only, the percentage avoidance from non-transgenic control mice (non-Tg) is also shown (inset). C) Ranked escape latencies across the first 3 sessions show better performance in the PIO fed mice compared to the CTRL animals (*p < 0.05 Two way ANOVA). D) Ranked escape latencies (higher numbers indicate poorer performance) correlated well with ranked integrated Aβ densities (higher numbers indicate greater Aβ density), as increased Aβ deposits were seen in animals with poor learning (r = 0.66, p < 0.01). Data represent Mean ± SEM.
Avoidance learning
PIO significantly improved learning in the early phases of training (sessions 1–3), showing greater percent avoidance compared to CTRL animals (F(1,25) = 5.08, p = 0.03; RM-ANOVA; Fig. 3B), as well as a reduced mean escape latency across all sessions (F(1,25) = 4.85; p = 0.04, RM-ANOVA, data not shown). The ranked escape latency measured across all sessions (each session = 3 trials, see methods) also was significantly reduced in the PIO group compared to the CTRL group (F(1,25) = 6.42; p = 0.017, RM-ANOVA; Fig. 3C). In sixteen behaviorally characterized animals for which Aβ immunostaining data were available, a correlation analysis revealed that active avoidance learning was worse in animals displaying larger Aβ deposits (p = 0.006; r2 = 0.43; Fig.3D).
Electrophysiology
Membrane hyperpolarization
While measures of cell health (Table 2) and passive membrane properties were not different than those recorded in cells from F344 rats [51, 58–60], the AHPs recorded from 3×Tg-AD animals were surprisingly small. Perhaps due to this floor effect, PIO-treated animals did not show significant reductions in the AHP. Neither the sAHP (p = 0.84, T-test) or mAHP amplitude (p = 0.94, T-test), nor the AHP duration (p = 0.97, T-test) were significantly modified by PIO (Fig. 4A). A subset of cells (9 cells from 6 CTRL animals and 7 cells from 7 PIO animals) was subjected to short-term plasticity stimulation protocols using repetitive synaptic activation. The degree of hyperpolarization was very similar to that seen in young adult F344 rats [59] indicating that synaptic hyperpolarization processes were intact in the 3×Tg-ADanimals. Interestingly, PIO caused a significant increase in synaptic hyperpolarization amplitude (Fig. 4B) at the three frequencies tested (F(1, 39) = 8.57; p = 0.006; ANOVA; Fig. 4B), a phenotype typically seen in younger animals [59].
Fig. 4.
Membrane hyperpolarization measures. A) Example of afterhyperpolarization (top) elicited intracellularly with three action potentials and recorded in a 3×Tg-AD mouse fed the control diet (action potentials are truncated). Bar graphs show the amplitude of the medium afterhyperpolarization (mAHP) and slow AHP (sAHP) as well as the duration of the duration recorded in mice fed either control diet (CTRL) or PIO-enriched diet (PIO) (T-test, p > 0.05). Note that the AHPs are particularly small in this animal model. B) Top, train of action potentials elicited synaptically with repetitive stimulation for 10 s at 7 Hz. Bottom, PIO animals showed a significant increase in maximal membrane hyperpolarization (measured from baseline – dashed line, Δ mV) at the three frequencies tested. *p < 0.05, Two-way ANOVA. Data represent Mean ± SEM.
Long-term potentiation
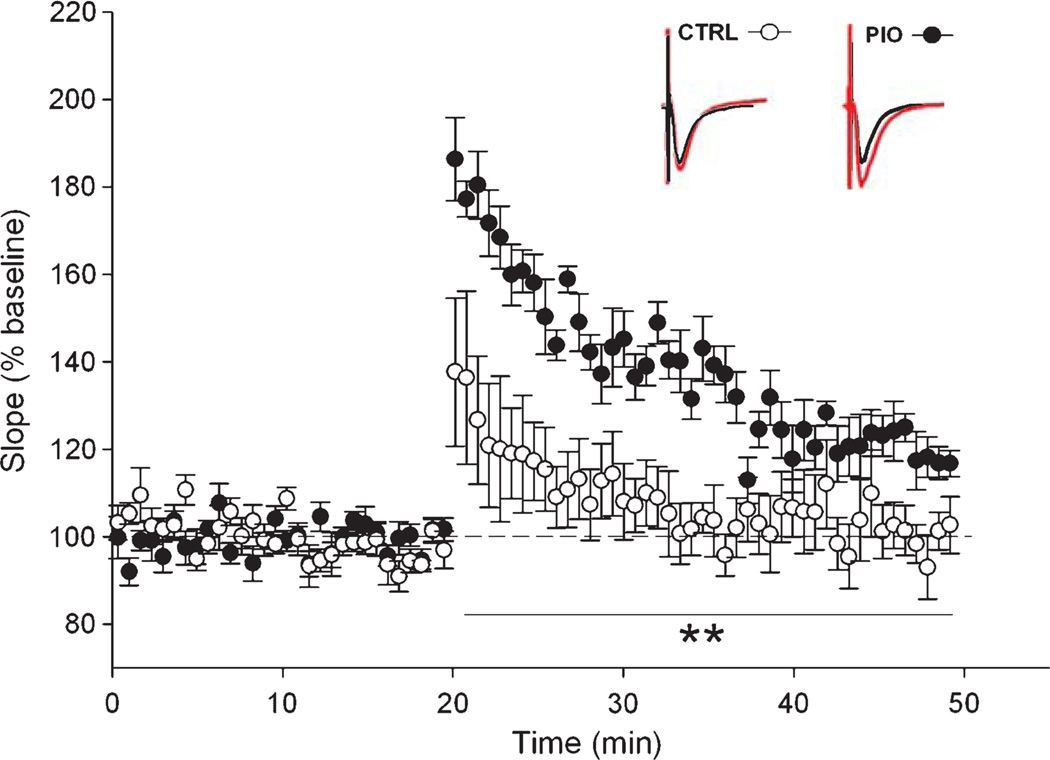
The mean baseline EPSP prior to tetanization of the pathway was not significantly different between groups (in mV, CTRL: −1.65 ± 0.13, PIO: −1.64 ± 0.08; data not shown). Immediately after tetanization and for the next 30 min, slices from PIO-treated animals showed robust potentiation of the EPSP compared to CTRL animals on measures of both EPSP slopes (F(1,11) = 14.5; p < 0.005; RM-ANOVA; Fig. 5) and amplitudes (F(1,11) = 8.55; p < 0.05; RM-ANOVA, data not shown). These results are consistent with prior evidence for a deficit in LTP maintenance in 3×Tg-AD animals [31].
Fig. 5.
High frequency induced synaptic potentiation. Normalized EPSP slopes measured in CTRL (white circles) and PIO (black circles) fed animals during baseline and following a 1 s 100 Hz stimulation burst. All measures taken after LTP induction were significantly greater in the PIO group. Inserts represent EPSP traces taken before LTP induction (black), and in the last 5 minutes of recording (red). Data represent Mean ± SEM, **p < 0.01, RM-ANOVA.
Microarray analyses
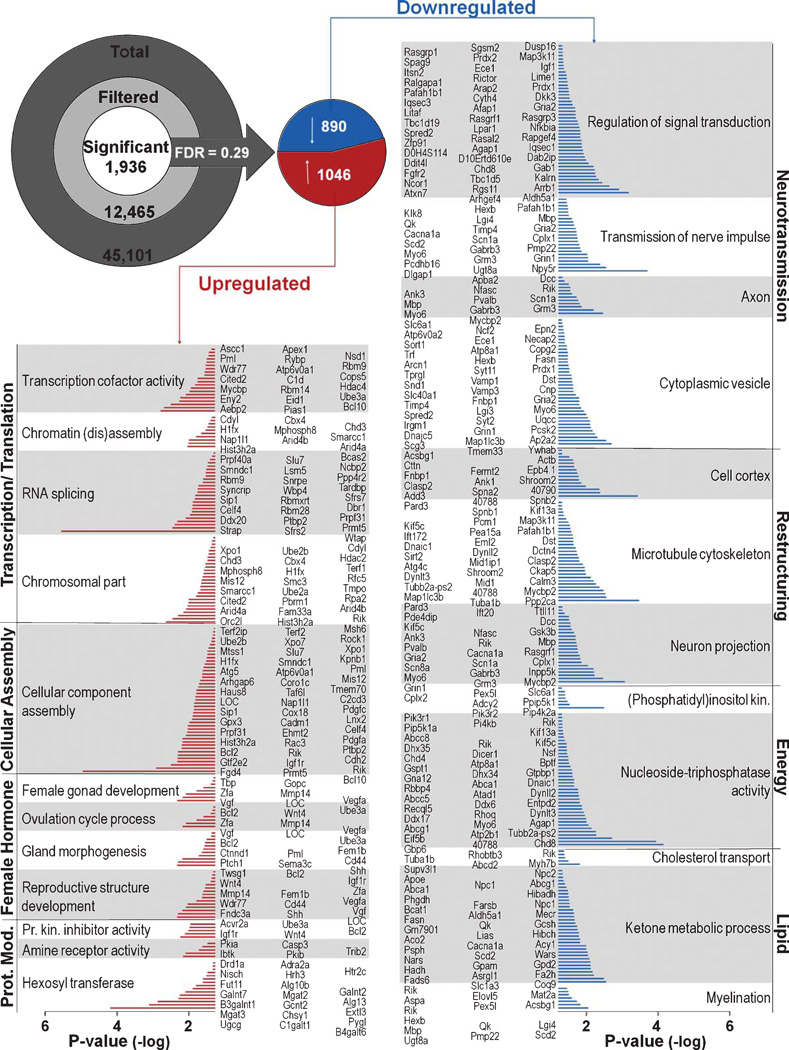
Figure 6 (upper left) displays the flowchart for the microarray analysis, yielding a total of 1936 significant genes split into up and down elements that all showed sensitivity to the PIO treatment. Approximately 7% of those genes (136/1936) contained consensus PPARβ-response elements (PPREs - Motif Finder, Partek Inc., MO) and represent potential direct transcriptional targets of PIO (supplementary Table 1; available online: http://www.j-alz.com/issues/30/vol30-4.html#supplementarydata07). Characterization of upregulated and downregulated genes using DAVID analysis returned a series of functional categories. These were heuristically combined into over-arching groups based on biological processes. Individual genes are shown graphically where the length of each bar represents the −log p value for that message (Fig. 6).With the exception of few extremely significant genes (p < 0.00001), the majority of genes sensitive to PIO falls between p values of 0.05 and 0.005. The overall list of significant genes is shown in supplementary Table 1.
Fig. 6.
Statistical approach and gene clustering. Inset shows the significant genes (up and down) that were identified from a total list of 1936 (FDR = 0.29). An unbiased DAVID analysis shows overrepresentation categorization into clusters sensitive to PIO in the up (left) and down (right) direction. The length of each bar represents the p value associated with this gene. Representing the most populated categories, were genes associated with DNA binding (UP - red) and neurotransmission (DOWN - blue).
From the DAVID analysis, a striking number of RNA targets associated with female hormone processes were shown to be upregulated as were DNA binding processes (Fig. 6, left). Of note, in the downregulated categories (right), decreases in lipid metabolism, myelination, cholesterol transport, energy dependent processes, and glutamatergic neurotransmission were also seen. While the DAVID analysis does not support an overall PIO-mediated suppression of inflammatory processes, several individual candidates known to play a role in inflammatory processes were identified and found to be significantly down regulated by PIO (supplementary Table 1). These included tumor necrosis factor alpha-induced protein 2 (Tnfaip2), tumor necrosis factor receptor type 1-associated DEATH domain protein (Tnfrsf1a), interleukin-1 receptor accessory protein (Il1rap), complement C1q-like proteins 3 (C1ql3) and 2 (C1ql2), and three NFκB-related messages (Nfkbia, Tank, and Tnik). In addition, cytokine signaling may have also been suppressed by upregulation of 2 genes known to be suppressors in this pathway (Socs2 and Socs5), as well as by upregulation in lipin-1 (Lpin1) which has been shown to reduce secretion of inflammatory markers [61].
Several other processes associated with aging or AD included two mRNA species linked to lipid shuttling and Aβ processing (apolipoprotein E (APOE) and amyloid β A4 precursor protein (apba2)) which were found to be decreased by PIO. We also found PIO-mediated increases in lipin-2 (Lpin2) message, another gene associated with lipid homeostasis. Message for the cardiac ryanodine receptor type 2 (Ryr2), an integral protein mediating Ca2+-induced Ca2+-release (CICR) in excitable cells was significantly reduced by PIO as was 11-β dehydrogenase type 1 (Hsd11b1) message. This later enzyme generates the active form of glucocorticoids and is a sought after pharmacological target since it directly impacts glucocorticoid levels in the brain and downregulation improves learning [62, 63]. PIO-mediated downregulation of genes associated with glutamatergic neurotransmission included the genes for glutamate transporters (Slc1a1 and Slc1a2), metabotropic glutamate receptor 3 (Grm3), AMPA receptor 2 (Gria2), glutamate receptor delta-2 subunit (Grid2), and the zeta-1 subunit of the NMDA receptor (Grin1). Trends for down regulation (p < 0.1) were also seen for the glutamate receptor subunits epsilon-3 (Grin2c) and epsilon 1 (Grin2A), as well as for the metabotropic glutamate receptor 1 (Grm1). While several RNA species associated with glutamatergic neurotransmission were also found to be upregulated by PIO treatment, including glutamate receptor delta-1 subunit (Grid1) and glutamate receptor subunit 3A (Grin3A), this does not necessarily detract from a relative loss of glutamatergic function given that Grin3A confers reduced Ca2+ permeability through the NMDA receptor [64], and Grid1’s function outside of the auditory system is not yet clear [65].
DISCUSSION
Because of potential links between AD and diabetes, we investigated whether the anti-diabetic drug PIO could attenuate cognitive impairment and markers of pathology in an accelerated mouse model of AD. Treatment was initiated at 10 months, a time point when hippocampal Aβ deposits are well-established and pathology is still ongoing in this model [31]. Following 4 months of treatment, when animals were 14 months of age, we found PIO improved performance in the active avoidance task, increased short- and long-term hippocampal plasticity (synaptic hyperpolarization and LTP, respectively), reduced Aβ and tau staining, and altered expression of several known pharmacologically-relevant gene targets (e.g., TNF-α, NFκB- and interleukin-related genes). In addition, microarray analyses also revealed several novel targets of PIO known to be associated with cognition, including estrogenic and glutamatergic pathways. Interestingly, it appears that PIO also selectively targeted some of the same processes that are dysregulated by the transgenes (e.g., lipid/fatty acid metabolism, mitochondrial energy processes, and synaptic neurotransmission [66, 67]), providing further support for the potential efficacy of PIO in the treatment of AD.
Pioglitazone facilitates learning and improves short-and long-term plasticity
Consistent with prior reports of PPAR-γ agonists in the brain [68, 69], improved LTP maintenance was seen in animals treated with PIO (Fig. 5). PIO also counteracted learning deficits on the active avoidance task (Fig. 3B) suggesting that PIO may have improved learning through actions on LTP expression. Although optimal NMDA- and AMPA-mediated functions are essential for LTP [70], aberrantly elevated glutamatergic neurotransmission has been seen in AD models [71–74]. Consistent with this aberrant glutamatergic activity, memantine, a weak NMDA antagonist used in the treatment of AD [73–75] enhances LTP in models of aging and AD [76–78], restores neuronal encoding in aging (i.e., hippocampal place field plasticity) [79], and slows cognitive decline in the 3×Tg-AD model [78]. Together with evidence that PIO is able to reduce NMDA-mediated Ca2+ currents and levels [80], the permissive effect of PIO on learning and LTP reported here appears to resemble some aspects of memantine action. Along these lines, it is interesting to note that drugs that target the glutamatergic system can be used to treat mood disorders [81]. Thus, the effects observed here on glutamate signaling may also underlie the purported ability of PIO to reduce symptoms of depression [82] and may hold relevance for the AD population which suffers from a high degree of depression.
Additional electrophysiological analyses identified a PIO-mediated increase in membrane hyperpolarization during repetitive synaptic stimulation (Fig. 4B). The synaptically-driven membrane hyperpolarization is typically larger in younger compared to older neurons [59] and thus, the effect of PIO seen here appears to confer a younger phenotype. This greater hyperpolarization may also have contributed to improved learning in the PIO-treated animals by enhancing signal to noise and facilitating throughput during synaptic communication. These actions also are very similar to those of memantine in AD models [73, 83]. Moreover, the effect of PIO on synaptic hyperpolarization may also provide an underlying mechanism for the reported ability of PIO to reduce seizures in mouse models of epilepsy [84, 85].Another mechanism possibly underlying the effects of PIO on learning and plasticity may depend in part on changes in hyperpolarization-activated cation channels (HCN). Our microarray analysis showed that PIO significantly downregulated Pex5l, a gene that promotes HCN incorporation into dendritic membranes [86]. Since knockdown of HCN is associated with an improvement in learning and LTP [87], inhibition of Pex5l (and hence HCN) may also underlie the beneficial actions of PIO on learning and LTP seen here.
Another measure of intracellular hyperpolarization, the Ca2+-dependent afterhyperpolarization (AHP) (Fig. 4A) was unaffected by PIO. The Ca2+-dependent AHP is larger with aging and is considered a major electrophysiological biomarker of brain aging [60, 88]. We also noted that when a similar number of action potentials was used to evoke the AHP, it was markedly smaller in 3×Tg-AD mice compared to our prior measures in aged F344 rats [58, 59]. While we have previously shown that PIO reduces the AHP in aged rats [54], perhaps the small amplitude AHPs in the 3×Tg model precludes additional reductions by PIO. Smaller AHPs in these animals have previously been noted [89]. Future studies will be needed to resolve differences between animal models of aging and those of AD. Interestingly, however, the reduced AHPs are also consistent with increased excitability and seizures in 3×Tg and other models of AD [90–92]. Overall, the results from the electrophysiology experiments show that PIO induces effects consistent with mechanisms associated with improved learning.
Role of PIO in the periphery
Given the evidence for potentially causal associations between dyslipidemia and cognitive decline and AD [2–4, 93–96], we tested whether the effects of PIO on serum lipid levels (decreased cholesterol, HDL and triglycerides, Table 1) were correlated with improved learning. No significant correlations were detected between any of the lipids measured and learning. We also investigated whether decreased serum lipid levels were associated with brain Aβ levels [93, 97] and observed a trend toward a positive correlation between serum cholesterol and brain Aβ (p = 0.076, please see Results). It is noteworthy that while serum lipid levels were not correlated with learning, we did observe that Aβ levels and behavioral performance were inversely correlated (Fig. 3D) as seen in other AD experimental models and in humans [98–101].
Transcriptional effects in hippocampus and associations with cognition
Lipid-related genes/pathways
Microarray results indicate that lipid metabolism was significantly downregulated by PIO in the hippocampus. Contrary to what is typically seen in the periphery, where PIO mediates lipid storage and adipose tissue expansion through upregulation of genes such as lipoprotein lipase, acyl-CoA synthetase, fatty-acid binding protein 4, and adipocyte protein 2 [12–15], in the brain, an alternative process of reduced fatty acid metabolism seems to emerge (Fig. 6, bottom right). Of interest, we identified many mitochondrial/peroxisomal enzymes linked to the processing/synthesis of various fatty acids (Fasn, Mecr, Scd2, Hadh, Fads6, Fa2h, Elovl5, Acsbg1), glycerolipids (Gpam, Gpd2), or myelin (Npc1, Qk, Mbp, Lgi4, Acsbg1), which were all shown to be downregulated by PIO. We also observed a significant downregulation in genes mediating cholesterol transport (Fig. 6). Taken together, it seems that in the brain PIO may target different lipid-related genes and elicit changes opposite to those seen in the periphery.
Glutamatergic neurotransmission genes/pathways
In addition to the effects of PIO on the glutamate-dependent electrophysiological measures highlighted above (i.e., LTP and synaptic hyperpolarization), several categories of genes associated with glutamatergic neurotransmission also were affected by PIO. These include genes for glutamate transporters (Slc1a1 and Slc1a2), metabotropic glutamate receptor 3 (Grm3), AMPA receptor 2 (Gria2), glutamate receptor delta-2 subunit (Grid2), and the zeta-1 subunit of the NMDA receptor (Grin1), which were all downregulated. Thus, consistent with evidence that the 3×Tg model has aberrant glutamatergic neurotransmission [71–74] the PIO-mediated downregulation of molecules involved in glutamate signaling may have rescued some aspects of the phenotype resulting in improvements in cognition, perhaps similar to the effect of memantine in AD and related models [78, 80, 102].
Estrogen-related genes/pathways
An important series of genes upregulated by PIO in the hippocampus and associated with cognition, are those linked to the steroid hormone estrogen (Fig. 6). Numerous prior studies have reported on the beneficial impact of estrogen in brain aging [103–110] and in female 3×Tg-AD mice as well [109, 111, 112]. Thus, increased estrogen-dependent signaling may also contribute to the improved learning seen with PIO in female 3×-Tg-AD animals. While estrogen levels in 3×Tg-AD mice are unaltered until approximately 12 months of age [111, 112], it remains to be determined whether some effects of PIO on estrogen-related gene targets occur through changes in circulating estrogen levels. It is also unclear whether the estrogen-dependent changes observed here also extend to males. However, it is important to note that TZDs have been shown to have beneficial effects in the brains of male and female mice.
Amyloid processing genes/pathways
Work from several groups has provided evidence for the role of PPAR-γ agonists in reducing Aβ levels through inhibition of β-secretase 1 (BACE-1) [18, 113], by increasing insulin-degrading enzyme (IDE) [28], Abca1-mediated clearance [24], or microglia mediated phagocytosis [24]. Our microarray analyses on hippocampal tissue did not provide supporting evidence for changes in BACE-1 or IDE messages, but did show a significant decrease in Abca1 message levels with PIO. Further studies are needed to determine whether the duration of treatment, type of TZD used, potential adaptation to the TZD, differences in TZD dose [18, 29], or animal models can account for these discrepancies.
CONCLUSIONS
To our knowledge, this is the first comprehensive microarray analysis on the long-term effects of PIO in the brain of a transgenic mouse model of AD. PIO also exerted beneficial effects on pathological biomarkers of AD, counteracted impaired learning, and enhanced synaptic plasticity in these same animals. Insights from the genome-wide analyses regarding the mechanisms of PIO reveal multiple novel targets related to estrogen, glutamate, and lipid signaling in the brain. Interestingly several processes related to lipid signaling, such as myelinogenesis and cholesterol trafficking, are increased with aging and cognitive decline [38, 39] and here we show that PIO can oppose these actions. Finally, our results also demonstrate the efficacy of PIO treatment, even if initiated when pathological processes are prominent and ongoing, suggesting that PIO could represent a clinically-relevant approach in the management of AD, particularly given its pleiotropic effects.
Supplementary Table 1. This table lists all genes significantly affected by PIO treatment. One of the column headers indicates the presence or absence of the consensus PPAR-γ DNA binding sequence. Other information about direction of change, p value, gene symbol and function are also listed. Please see the Microsoft Excel spreadsheet at http://www.j-alz.com/issues/30/vol30-4.html#supplementarydata07.
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank Professor F.M. LaFerla (Director, Institute for memory impairment and neurological disorders, University of California, Irvine) for initially providing the animals to Professor L. Hersh (Biochemistry Chairperson, University of Kentucky).
This work was supported by National Institute on Aging Grants: AG029268, AG010836, AG020251, AG033649, AG034605, and a National Center for Research Resources Grant: NCRR-P20-RR15592.
Footnotes
Supplementary data available online: http://www.j-alz.com/issues/30/vol30-4.html#supplementarydata07
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1227).
REFERENCES
- 1.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H-C. Deaths: Preliminary data for 2009. Natl Vital Stat Rep. 2011;59-4:1–68. [PubMed] [Google Scholar]
- 2.Hartmann T. Cholesterol, A beta and Alzheimer’s disease. Trends Neurosci. 2001;24:S45–S48. doi: 10.1016/s0166-2236(00)01990-1. [DOI] [PubMed] [Google Scholar]
- 3.Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer’s disease: An epidemiological perspective. Eur J Pharmacol. 2008;585:119–129. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 5.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: A lifespan perspective. Lancet Neurol. 2008;7:184–190. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 6.Messier C, Gagnon M. Cognitive decline associated with dementia and type 2 diabetes: The interplay of risk factors. Diabetologia. 2009;52:2471–2474. doi: 10.1007/s00125-009-1533-2. [DOI] [PubMed] [Google Scholar]
- 7.Abbatecola AM, Lattanzio F, Molinari AM, Cioffi M, Mansi L, Rambaldi P, DiCioccio L, Cacciapuoti F, Canonico R, Paolisso G. Rosiglitazone and cognitive stability in older individuals with type 2 diabetes and mild cognitive impairment. Diabetes Care. 2010;33:1706–1711. doi: 10.2337/dc09-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 9.Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care. 2006;29:345–351. doi: 10.2337/diacare.29.02.06.dc05-1626. [DOI] [PubMed] [Google Scholar]
- 10.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: A preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32:1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Berger JP, Akiyama TE, Meinke PT. PPARs: Therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Francis GA, Fayard E, Picard F, Auwerx J. Nuclear receptors and the control of metabolism. Annu Rev Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- 14.de Souza CJ, Eckhardt M, Gagen K, Dong M, Chen W, Laurent D, Burkey BF. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes. 2001;50:1863–1871. doi: 10.2337/diabetes.50.8.1863. [DOI] [PubMed] [Google Scholar]
- 15.Bogacka I, Xie H, Bray GA, Smith SR. The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care. 2004;27:1660–1667. doi: 10.2337/diacare.27.7.1660. [DOI] [PubMed] [Google Scholar]
- 16.Esposito K, Ciotola M, Merante D, Giugliano D. Rosiglitazone cools down inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:1413–1414. doi: 10.1161/01.ATV.0000223874.94624.11. [DOI] [PubMed] [Google Scholar]
- 17.Wang TD, Chen WJ, Cheng WC, Lin JW, Chen MF, Lee YT. Relation of improvement in endothelium-dependent flow-mediated vasodilation after rosiglitazone to changes in asymmetric dimethylarginine, endothelin-1, and C-reactive protein in nondiabetic patients with the metabolic syndrome. Am J Cardiol. 2006;98:1057–1062. doi: 10.1016/j.amjcard.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, Van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta 1–42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 19.Feinstein DL. Therapeutic potential of peroxisome proliferator-activated receptor agonists for neurological disease. Diabetes Technol Ther. 2003;5:67–73. doi: 10.1089/152091503763816481. [DOI] [PubMed] [Google Scholar]
- 20.Lacombe P, Mathews PM, Schmidt SD, Breidert T, Heneka MT, Landreth GE, Feinstein DL, Galea E. Effect of anti-inflammatory agents on transforming growth factor beta over-expressing mouse brains: A model revised. J Neuroinflammation. 2004;1:11. doi: 10.1186/1742-2094-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landreth GE, Heneka MT. Anti-inflammatory actions of peroxisome proliferator-activated receptor gamma agonists in Alzheimer’s disease. Neurobiol Aging. 2001;22:937–944. doi: 10.1016/s0197-4580(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 22.Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer’s disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.To AW, Ribe EM, Chuang TT, Schroeder JE, Lovestone S. The epsilon3 and epsilon4 alleles of human APOE differentially affect tau phosphorylation in hyperinsulinemic and pioglitazone treated mice. PLoS ONE. 2011;6:e16991. doi: 10.1371/journal.pone.0016991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escribano L, Simon AM, Gimeno E, Cuadrado-Tejedor M, Lopez de Maturana R, Garcia-Osta A, Ricobaraza A, Perez-Mediavilla A, Del Rio J, Frechilla D. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: Mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology. 2010;35:1593–1604. doi: 10.1038/npp.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escribano L, Simon AM, Perez-Mediavilla A, Salazar-Colocho P, Del Rio J, Frechilla D. Rosiglitazone reverses memory decline and hippocampal glucocorticoid receptor down-regulation in an Alzheimer’s disease mouse model. Biochem Biophys Res Commun. 2009;379:406–410. doi: 10.1016/j.bbrc.2008.12.071. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Bueno B, Madrigal JL, Lizasoain I, Moro MA, Lorenzo P, Leza JC. Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. Biol Psychiatry. 2005;57:885–894. doi: 10.1016/j.biopsych.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen WA, Flynn ER. Insulin resistance contributes to aberrant stress responses in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis. 2004;17:500–506. doi: 10.1016/j.nbd.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Rivera J, Denner L, Dineley KT. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav Brain Res. 2011;216:255–261. doi: 10.1016/j.bbr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 32.Brunmair B, Staniek K, Lehner Z, Dey D, Bolten CW, Stadlbauer K, Luger A, Furnsinn C. Lipophilicity as a determinant of thiazolidinedione action in vitro: Findings from BLX-1002, a novel compound without affinity to PPARs. Am J Physiol Cell Physiol. 2011;300:C1386–C1392. doi: 10.1152/ajpcell.00401.2010. [DOI] [PubMed] [Google Scholar]
- 33.Munoz C, Grossman SP. Spatial discrimination, reversal and active or passive avoidance learning in rats with KA-induced neuronal depletions in dorsal hippocampus. Brain Res Bull. 1981;6:399–406. doi: 10.1016/s0361-9230(81)80010-x. [DOI] [PubMed] [Google Scholar]
- 34.Phillips M, Boman E, Osterman H, Willhite D, Laska M. Olfactory and visuospatial learning and memory performance in two strains of Alzheimer’s disease model mice–a longitudinal study. PLoS ONE. 2011;6:e19567. doi: 10.1371/journal.pone.0019567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J Neurosci. 2010;30:505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterniczuk R, Antle MC, Laferla FM, Dyck RH. Characterization of the 3×Tg-AD mouse model of Alzheimer’s disease: Part 2. Behavioral and cognitive changes. Brain Res. 2010;1348:149–155. doi: 10.1016/j.brainres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Alexandrov PN, Pogue A, Bhattacharjee S, Lukiw WJ. Retinal amyloid peptides and complement factor H in transgenic models of Alzheimer’s disease. Neuroreport. 2011;22:623–627. doi: 10.1097/WNR.0b013e3283497334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. Hippocampal and cognitive aging across the lifespan: A bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci. 2009;29:1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: Microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A. 2004;101:2173–2178. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: Statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolstad BM, Collin F, Brettschneider J, Simpson K, Cope L, Irizarry R, Speed TP. Quality assessment of affymetrix GeneChip data. In: Gentleman R, Carey V, Huber W, Irizarry R, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer; 2005. pp. 33–47. [Google Scholar]
- 43.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Z, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F. A model-based background adjustment for oligonucleotide expression arrays. JASA. 2004;99:909–917. [Google Scholar]
- 45.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: A free, open-source system for microarray data management and analysis. BioTechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 46.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 47.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Muertter RN, Edgar R. NCBI GEO: Archive for high-throughput functional genomic data. Nucleic Acids Res. 2009;37:D885–D890. doi: 10.1093/nar/gkn764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Groen T, Miettinen P, Kadish I. Transgenic AD model mice, effects of potential anti-AD treatments on inflammation, and pathology. J Alzheimers Dis. 2011;24:301–313. doi: 10.3233/JAD-2011-101479. [DOI] [PubMed] [Google Scholar]
- 49.Pop V, Head E, Berchtold NC, Glabe CG, Studzinski CM, Weidner AM, Murphy MP, Cotman CW. Abeta aggregation profiles and shifts in APP processing favor amyloidogenesis in canines. Neurobiol Aging. 2012;33:108–120. doi: 10.1016/j.neurobiolaging.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beckett TL, Niedowicz DM, Studzinski CM, Weidner AM, Webb RL, Holler CJ, Ahmed RR, LeVine H, 3rd, Murphy MP. Effects of nonsteroidal anti-inflammatory drugs on amyloid-beta pathology in mouse skeletal muscle. Neurobiol Dis. 2010;39:449–456. doi: 10.1016/j.nbd.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+release. J Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gant JC, Thibault O. Action potential throughput in aged rat hippocampal neurons: Regulation by selective forms of hyperpolarization. Neurobiol Aging. 2009;30:2053–2064. doi: 10.1016/j.neurobiolaging.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 54.Henriksen K, Byrjalsen I, Nielsen RH, Madsen AN, Larsen LK, Christiansen C, Beck-Nielsen H, Karsdal MA. A comparison of glycemic control, water retention, and musculoskeletal effects of balaglitazone and pioglitazone in diet-induced obese rats. Eur J Pharmacol. 2009;616:340–345. doi: 10.1016/j.ejphar.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 55.Camacho IE, Serneels L, Spittaels K, Merchiers P, Dominguez D, De Strooper B. Peroxisome-proliferator-activated receptor gamma induces a clearance mechanism for the amyloid-beta peptide. J Neurosci. 2004;24:10908–10917. doi: 10.1523/JNEUROSCI.3987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 57.d’Abramo C, Ricciarelli R, Pronzato MA, Davies P. Troglitazone, a peroxisome proliferator-activated receptor-gamma agonist, decreases tau phosphorylation in CHOtau4R cells. J Neurochem. 2006;98:1068–1077. doi: 10.1111/j.1471-4159.2006.03931.x. [DOI] [PubMed] [Google Scholar]
- 58.Blalock EM, Phelps JT, Pancani T, Searcy JL, Anderson KL, Gant JC, Popovic J, Avdiushko MG, Cohen DA, Chen KC, Porter NM, Thibault O. Effects of long-term pioglitazone treatment on peripheral and central markers of aging. PLoS ONE. 2010;5:e10405. doi: 10.1371/journal.pone.0010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gant JC, Thibault O. Action potential throughput in aged rat hippocampal neurons: Regulation by selective forms of hyperpolarization. Neurobiol Aging. 2008;30:2053–2064. doi: 10.1016/j.neurobiolaging.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thibault O, Hadley R, Landfield PW. Elevated post-synaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: Relationship to impaired synaptic plasticity. J Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HB, Kumar A, Wang L, Liu GH, Keller SR, Lawrence JC, Jr, Finck BN, Harris TE. Lipin 1 represses NFATc4 transcriptional activity in adipocytes to inhibit secretion of inflammatory factors. Mol Cell Biol. 2010;30:3126–3139. doi: 10.1128/MCB.01671-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yau JL, Noble J, Seckl JR. 11beta-hydroxysteroid dehydrogenase type 1 deficiency prevents memory deficits with aging by switching from glucocorticoid receptor to mineralocorticoid receptor-mediated cognitive control. J Neurosci. 2011;31:4188–4193. doi: 10.1523/JNEUROSCI.6145-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seckl JR, Walker BR. 11beta-hydroxysteroid dehydrogenase type 1 as a modulator of glucocorticoid action: From metabolism to memory. Trends Endocrinol Metab. 2004;15:418–424. doi: 10.1016/j.tem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Tong G, Takahashi H, Tu S, Shin Y, Talantova M, Zago W, Xia P, Nie Z, Goetz T, Zhang D, Lipton SA, Nakanishi N. Modulation of NMDA receptor properties and synaptic transmission by the NR3A subunit in mouse hippocampal and cerebrocortical neurons. J Neurophysiol. 2008;99:122–132. doi: 10.1152/jn.01044.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmid SM, Hollmann M. To gate or not to gate: Are the delta subunits in the glutamate receptor family functional ion channels? Mol Neurobiol. 2008;37:126–141. doi: 10.1007/s12035-008-8025-0. [DOI] [PubMed] [Google Scholar]
- 66.Chou JL, Shenoy DV, Thomas N, Choudhary PK, Laferla FM, Goodman SR, Breen GA. Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer’s disease. J Proteomics. 2011;74:466–479. doi: 10.1016/j.jprot.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 67.Martin B, Brenneman R, Becker KG, Gucek M, Cole RN, Maudsley S. iTRAQ analysis of complex proteome alterations in 3×TgAD Alzheimer’s mice: Understanding the interface between physiology and disease. PLoS ONE. 2008;3:e2750. doi: 10.1371/journal.pone.0002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Costello DA, O’Leary DM, Herron CE. Agonists of peroxisome proliferator-activated receptor-gamma attenuate the Abeta-mediated impairment of LTP in the hippocampus in vitro. Neuropharmacology. 2005;49:359–366. doi: 10.1016/j.neuropharm.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Loane DJ, Deighan BF, Clarke RM, Griffin RJ, Lynch AM, Lynch MA. Interleukin-4 mediates the neuroprotective effects of rosiglitazone in the aged brain. Neurobiol Aging. 2009;30:920–931. doi: 10.1016/j.neurobiolaging.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Baudry M, Lynch G. Remembrance of arguments past: How well is the glutamate receptor hypothesis of LTP holding up after 20 years? Neurobiol Learn Mem. 2001;76:284–297. doi: 10.1006/nlme.2001.4023. [DOI] [PubMed] [Google Scholar]
- 71.Woltjer RL, Duerson K, Fullmer JM, Mookherjee P, Ryan AM, Montine TJ, Kaye JA, Quinn JF, Silbert L, Erten-Lyons D, Leverenz JB, Bird TD, Pow DV, Tanaka K, Watson GS, Cook DG. Aberrant detergent-insoluble excitatory amino acid transporter 2 accumulates in Alzheimer disease. J Neuropathol Exp Neurol. 2010;69:667–676. doi: 10.1097/NEN.0b013e3181e24adb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 73.Lipton SA. Failures and successes of NMDA receptor antagonists: Molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1:101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McShane R, Areosa Sastre A, Minakaran N. Meman tine for dementia. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003154.pub5. CD003154. [DOI] [PubMed] [Google Scholar]
- 75.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63:49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 76.Frankiewicz T, Pilc A, Parsons CG. Differential effects of NMDA-receptor antagonists on long-term potentiation and hypoxic/hypoglycaemic excitotoxicity in hippocampal slices. Neuropharmacology. 2000;39:631–642. doi: 10.1016/s0028-3908(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 77.Pieta Dias C, Martins de Lima MN, Presti-Torres J, Dornelles A, Garcia VA, Siciliani Scalco F, Rewsaat Guimaraes M, Constantino L, Budni P, Dal-Pizzol F, Schroder N. Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience. 2007;146:1719–1725. doi: 10.1016/j.neuroscience.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 78.Martinez-Coria H, Green KN, Billings LM, Kitazawa M, Albrecht M, Rammes G, Parsons CG, Gupta S, Banerjee P, LaFerla FM. Memantine improves cognition and reduces Alzheimer’s-like neuropathology in transgenic mice. Am J Pathol. 2010;176:870–880. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burke SN, Maurer AP, Yang Z, Navratilova Z, Barnes CA. Glutamate receptor-mediated restoration of experience-dependent place field expansion plasticity in aged rats. Behav Neurosci. 2008;122:535–548. doi: 10.1037/0735-7044.122.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pancani T, Phelps JT, Searcy JL, Kilgore MW, Chen KC, Porter NM, Thibault O. Distinct modulation of voltage-gated and ligand-gated Ca2+ currents by PPAR-gamma agonists in cultured hippocampal neurons. J Neurochem. 2009;109:1800–1811. doi: 10.1111/j.1471-4159.2009.06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Machado-Vieira R, Ibrahim L, Henter ID, Zarate CA., Jr Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav. 2012;100:678–687. doi: 10.1016/j.pbb.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kemp DE, Ismail-Beigi F, Ganocy SJ, Conroy C, Gao K, Obral S, Fein E, Findling RL, Calabrese JR. Use of insulin sensitizers for the treatment of major depressive disorder: A pilot study of pioglitazone for major depression accompanied by abdominal obesity. J Affect Disord. 2012;136:1164–1173. doi: 10.1016/j.jad.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: Preclinical evidence. Int J Geriatr Psychiatry. 2003;18:S23–S32. doi: 10.1002/gps.938. [DOI] [PubMed] [Google Scholar]
- 84.Abdallah DM. Anticonvulsant potential of the peroxisome proliferator-activated receptor gamma agonist pioglitazone in pentylenetetrazole-induced acute seizures and kindling in mice. Brain Res. 2010;1351:246–253. doi: 10.1016/j.brainres.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 85.Okada K, Yamashita U, Tsuji S. Ameliorative effect of pioglitazone on seizure responses in genetically epilepsy-susceptible EL mice. Brain Res. 2006;1102:175–178. doi: 10.1016/j.brainres.2006.04.108. [DOI] [PubMed] [Google Scholar]
- 86.Lewis AS, Vaidya SP, Blaiss CA, Liu Z, Stoub TR, Brager DH, Chen X, Bender RA, Estep CM, Popov AB, Kang CE, Van Veldhoven PP, Bayliss DA, Nicholson DA, Powell CM, Johnston D, Chetkovich DM. Deletion of the hyperpolarization-activated cyclic nucleotide-gated channel auxiliary subunit TRIP8b impairs hippocampal Ih localization and function and promotes antidepressant behavior in mice. J Neurosci. 2011;31:7424–7440. doi: 10.1523/JNEUROSCI.0936-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 88.Disterhoft JF, Oh MM. Alterations in intrinsic neuronal excitability during normal aging. Aging Cell. 2007;6:327–336. doi: 10.1111/j.1474-9726.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 89.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arsenault D, Julien C, Tremblay C, Calon F. DHA improves cognition and prevents dysfunction of entorhinal cortex neurons in 3×Tg-AD mice. PLoS ONE. 2011;6:e17397. doi: 10.1371/journal.pone.0017397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sparks DL. Coronary artery disease, hypertension, ApoE, and cholesterol: A link to Alzheimer’s disease? Ann N Y Acad Sci. 1997;826:128–146. doi: 10.1111/j.1749-6632.1997.tb48466.x. [DOI] [PubMed] [Google Scholar]
- 94.Sparks DL. The early and ongoing experience with the cholesterol-fed rabbit as a model of Alzheimer’s disease: The old, the new and the pilot. J Alzheimers Dis. 2008;15:641–656. doi: 10.3233/jad-2008-15410. [DOI] [PubMed] [Google Scholar]
- 95.Schreurs BG. The effects of cholesterol on learning and memory. Neurosci Biobehav Rev. 2010;34:1366–1379. doi: 10.1016/j.neubiorev.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mielke JG, Nicolitch K, Avellaneda V, Earlam K, Ahuja T, Mealing G, Messier C. Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice. Behav Brain Res. 2006;175:374–382. doi: 10.1016/j.bbr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Petanceska SS, DeRosa S, Sharma A, Diaz N, Duff K, Tint SG, Refolo LM, Pappolla M. Changes in apolipoprotein E expression in response to dietary and pharmacological modulation of cholesterol. J Mol Neurosci. 2003;20:395–406. doi: 10.1385/JMN:20:3:395. [DOI] [PubMed] [Google Scholar]
- 98.Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H, 3rd, Kraner SD, Norris CM. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parvathy S, Davies P, Haroutunian V, Purohit DP, Davis KL, Mohs RC, Park H, Moran TM, Chan JY, Buxbaum JD. Correlation between Abetax-40-, Abetax-42-, and Abetax-43-containing amyloid plaques and cognitive decline. Arch Neurol. 2001;58:2025–2032. doi: 10.1001/archneur.58.12.2025. [DOI] [PubMed] [Google Scholar]
- 100.Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Howlett DR, Richardson JC, Austin A, Parsons AA, Bate ST, Davies DC, Gonzalez MI. Cognitive correlates of Abeta deposition in male and female mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. Brain Res. 2004;1017:130–136. doi: 10.1016/j.brainres.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 102.Allahtavakoli M, Shabanzadeh A, Roohbakhsh A, Pourshanazari A. Combination therapy of rosiglitazone, a peroxisome proliferator-activated receptor-gamma ligand, and NMDA receptor antagonist (MK-801) on experimental embolic stroke in rats. Basic Clin Pharmacol Toxicol. 2007;101:309–314. doi: 10.1111/j.1742-7843.2007.00127.x. [DOI] [PubMed] [Google Scholar]
- 103.Yi KD, Chung J, Pang P, Simpkins JW. Role of protein phosphatases in estrogen-mediated neuroprotection. J Neurosci. 2005;25:7191–7198. doi: 10.1523/JNEUROSCI.1328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brewer LD, Dowling AL, Curran-Rauhut MA, Landfield PW, Porter NM, Blalock EM. Estradiol reverses a calcium-related biomarker of brain aging in female rats. J Neurosci. 2009;29:6058–6067. doi: 10.1523/JNEUROSCI.5253-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2011 doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar A, Foster TC. 17beta-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol. 2002;88:621–626. doi: 10.1152/jn.2002.88.2.621. [DOI] [PubMed] [Google Scholar]
- 107.Wu TW, Wang JM, Chen S, Brinton RD. 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: A potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 108.Brinton RD. The healthy cell bias of estrogen action: Mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Foy MR, Baudry M, Diaz Brinton R, Thompson RF. Estrogen and hippocampal plasticity in rodent models. J Alzheimers Dis. 2008;15:589–603. doi: 10.3233/jad-2008-15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Simpkins JW, Singh M. More than a decade of estrogen neuroprotection. Alzheimers Dement. 2008;4:S131–S136. doi: 10.1016/j.jalz.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 111.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3×Tg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD. 17beta-Estradiol regulates insulin-degrading enzyme expression via an ERbeta/PI3-K pathway in hippocampus: Relevance to Alzheimer’s prevention. Neurobiol Aging. 2011;32:1949–1963. doi: 10.1016/j.neurobiolaging.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, Walter J, Klockgether T, van Leuven F, Heneka MT. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci U S A. 2006;103:443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.