Abstract
This study identifies the IL-25 receptor, IL-17RB, is an important mediator of both innate and adaptive pulmonary type 2 immune responses. Allergen exposure upregulated IL-25 and induced type 2 cytokine production in a novel granulocytic population, termed Type 2 Myeloid (T2M) cells. Il17rb−/− mice exhibited reduced lung pathology following chronic allergen exposure and decreased cytokine production in T2M cells and CD4+ T-lymphocytes. Airway instillation of IL-25 induced IL-4 and IL-13 production exclusively in T2M cells demonstrating their importance in generating T cell-independent inflammation. The adoptive transfer of T2M cells reconstituted IL-25-mediated responses in Il17rb−/− mice. High dose dexamethasone treatment did not reduce the IL-25-induced T2M pulmonary response. Finally, a similar IL-4/IL-13 producing granulocytic population was identified in peripheral blood of asthmatics. These data establish IL-25/IL-17RB as targets for innate and adaptive immune responses in chronic allergic airways disease, and identify T2M cells as a novel steroid-resistant cell population.
Current therapies for asthma focus on reducing the frequency and severity of exacerbations by attenuating bronchiole inflammation and airway hyperreactivity. The limited efficacy of treatments, such as inhaled corticosteroids, to reduce the inflammatory response in some chronic asthmatics illustrates the need for further research into mechanisms underlying asthma’s pathophysiology1–3. Three epithelial-derived type 2 inflammation associated cytokines, IL-25, TSLP, and IL-33, may represent targets for therapeutic treatment in severe asthma. IL-25, in particular, has been reported to enhance responses and further exacerbate allergic disease. In an original study4, the systemic injection of IL-25 into Rag−/− mice induced type 2 cytokine expression and eosinophilia, demonstrating T cell-independent mechanisms could drive type 2 inflammation.
The IL-17 family member IL-25 (IL-17E) regulates multiple aspects of mucosal immunity by promoting type 2 inflammation via production of IL-4, IL-5, and IL-134–6. Pulmonary IL-25 is produced by eosinophils7,8, mast cells, and airway epithelial cells and stimulates asthma-like inflammation characterized by airway hyperreactivity, mucus production, airway eosinophilia, and increased serum IgE9. The induction of these responses requires binding of a noncovalently bound IL-25 homodimer to the IL-17RA/IL-17RB heterodimer10, of which IL-17RB represents an IL-25 specific moiety11. IL-25 is known to enhance Th2 effector functions via Act1 and TRAF6 dependent NFκB activation12–15. Multiple cell types in the lung, including memory16 and effector14,16 T cells, invariant NKT cells17, APCs, and airway smooth muscle, have been characterized as expressing IL-17RB, whereas eosinophils do not7. Several studies have also identified additional IL-25 responsive type 2 cytokine producing non-B, non-T cell (NBNT) populations5,18–20, and a recent investigation reported increased expression of IL-25 and its receptor in the airways of asthmatics after allergen provocation21.
The present study reports that repeated allergen exposure upregulated both pulmonary IL-25 and IL-17RB in a murine model of persistent allergic airway disease, and induced the accumulation of a novel IL-4 and IL-13 producing IL-17RB+ Type 2 Myeloid (T2M) population in the lung. T2M cells are granulocytes driven by IL-25, and are both pathogenic and steroid resistant. Moreover, IL-4 and IL-13 producing T2M-like cells were identified in the peripheral blood of asthmatics.
Results
Chronic allergen drives type 2 cytokine production in myeloid cells
Several reports have linked IL-25 expression to the severity of allergic asthma6,7,9,22,23. The induction of type 2 cytokine expression following the pulmonary instillation of allergen (Fig. 1) was accompanied by increased mRNA expression of Il25 and Il17rb (Fig. 1c) and tracked with the severity of the developing disease as depicted by histology (Fig. 1a). Neither Ifnγ, other IL-17 family members, Il33, nor its receptor Il1rl1 were upregulated with our model of cockroach allergen challenge (Fig. 1b,c), indicating that type 2 inflammation represents the dominant response induced by this model.
Figure 1. Allergen exposure increases pulmonary IL-25 and IL17RB, and recruits bone marrow-derived IL17RB+ IL-4 and IL-13 producing myeloid cells to the lung.
Allergen-induced inflammation was localized to the lungs of C57BL/6J mice (n = 3 per group) via a series of 6 allergen challenges. (a) Time course of representative PAS staining, taken 6 hrs post indicated allergen challenge. Upper row scale bar, 400 µm; lower row, 100 µm. (b) Time course of pulmonary IL-4, IL-5, IL-13, IFN-γ and IL-17a mRNA expression 6 hours post indicated allergen challenge. (*P < 8.44E−05, #P = 0.0008, +P = 0.001). (c) Time course of IL-25, IL17RB, IL-33, IL-17b, and IL17d mRNA expression 6 hours post indicated allergen challenge. ST2 and IL-22 transcripts were not detectable. (*P < 0.007). (d and e) IL-17RB+ lung subsets from naïve and allergen sensitized C57BL/6J mice (n = 5 per group) were assessed by flow cytometry for IL-4 and IL-13 production. (f) Representative flow plots of intracellular cytokine staining in IL-17RB+ CD11b+ Gr-1mid cells from naïve and allergen sensitized C57BL/6J mice. Gray: n-1 staining, black: naïve IL-17RB+ CD11b+ Gr-1mid, red: allergen challenged IL-17RB+ CD11b+Gr-1mid (g) Pulmonary IL-17RB−/− CD11b+ Gr-1mid populations are significantly increased following allergen sensitization. (*P = 0.0026). Results are representative of two independent experiments. (h) Morphology of myeloid cells isolated from the lungs of allergen-sensitized mice. Cells were sorted as CD11b+ Gr-1mid FcγR+ IL-17RB+ CD4− CD8− B220− IL-7Rα− Sca1− c-kit− and stained with H+E. Scale bar 50 µm. All data are presented as mean ± s.e.m.
We have previously identified a pathologically relevant population of IL-17RB+ myeloid cells with the capacity to produce IL-4 during chronic allergic airway disease7. While CD4+ T cells are present following antigen sensitization, the most numerous IL-17RB+ IL-4/IL-13 producing population in the lung were CD11b+ myeloid cells (Fig. 1d,e). We also examined the capacity of innate Lin− ckit+ Sca1+ IL-17RB+ NBNT cells to produce type 2 cytokines. Lin− ckit+ Sca1+ IL-17RB+ cells comprised a relatively rare population in the lungs of both naïve and allergen-challenged mice (range 250–1000), and were not increased following allergen sensitization (Fig. 1d,e). Few myeloid IL-4 and IL-13 producing cells were present in the lungs of naïve mice, and the IL-17RB+ cells preferentially produced type 2 cytokine. Myeloid IL-17RB+ cells represented the major NBNT IL-4/13 cytokine producing population in the lung, outnumbering cytokine producing CD4+ T cells 68:1 (see Supplemental Fig. 1).
Despite significant increases in IL-4 and IL-13 producing myeloid cells in environments with elevated levels of IL-25, not all pulmonary IL-17RB+ myeloid populations appeared to produce these cytokines. Analysis of IL-4 and IL-13 production in IL-17RB+ myeloid subsets, based on levels of Gr-1 expression, allowed for the identification of two distinct IL-17RB+ myeloid populations (Fig. 1f). A comparison of total cell numbers between naïve and allergic animals identified significant increases in both Gr-1mid (Fig. 1g) and Gr-1hi subsets in the lungs of allergic mice, however the IL-17RB+ CD11b+ Gr-1mid population produced IL-4 and IL-13 while the Gr-1hi population did not (data not shown). Isolation of the Gr-1mid subset by FACS identified a granulocytic population with a circular partially segmented nucleus and relatively high nucleus to cytoplasm ratio (Fig. 1h, see Supplemental Fig. 2). These data inspired our next set of experiments, an investigation of IL-17RB’s involvement in the production of type 2 cytokines in this granulocytic IL-17RB+ population.
Il17rb−/− mice exhibit decreased type 2 inflammation
To further explore the overall role of IL-17RB in allergic asthma, Il17rb−/− mice were sensitized to allergen. The loss of the IL-25-specific receptor protected Il17rb−/− mice from allergen-induced inflammation (Fig. 2). Il17rb−/− allergic mice exhibited a dramatic reduction in peribronchial and perivascular inflammation, eosinophilic infiltrates, and mucus production (Fig. 2a). Pulmonary expression of type 2 cytokines as well as the eosinophil-associated chemokine CCL11 were significantly decreased in lungs of Il17rb−/− mice (Fig. 2b). Il17rb−/− draining lymph node cells re-stimulated with antigen produced significantly less type 2 cytokines than wild type lymph node cells (Fig. 2c). Interestingly, a short-term model of allergen sensitization that utilized fewer allergen challenges revealed no phenotypic differences in Il17rb−/− mice (see Supplemental Fig. 3) suggesting that IL-17RB is most relevant during more chronic allergic responses when IL-25 production is significantly increased.
Figure 2. Il17rb−/− mice are protected from allergen induced type 2 inflammation, and type 2 cytokine production in CD11b+ Gr-1+ myeloid cells is IL-17RB dependent.
(a) PAS staining of lungs from WT and Il17rb−/− mice following chronic allergen sensitization. Scale bar 200 µm. (b) Lungs from allergic mice (n = 5 animals per group) were harvested 24 h post final allergen challenge and whole lung homogenates were analyzed for cytokine production by bioplex. (*P = 0.001, #P = 0.048, +P < 0.05 versus WT allergen). (c) Cytokine production from draining lymph nodes cells of allergic mice (n = 5 animals per group). Bars represent the mean ± s.e.m. from triplicate wells. (N.D. = not detected, *P = 2.46E−05, #P = 8.79E−08, +P = 4.00E−06 versus WT allergen). (d) Flow cytometric analysis of lungs from allergic WT and Il17rb−/− mice (n = 5 animals per group), (*P < 0.03). (e) Intracellular cytokine staining for IL-4 and IL-13 producing cells in allergic WT and Il17rb−/− mice (n = 4 animals per group). Results are gated on CD11b+ Gr-1mid cells. Bars represent the mean ± s.e.m. for each group (*P < 0.05, #P < 0.0009). Data are representative of two independent experiments.
There was no detectable difference in total numbers of CD4+ or CD8+ T lymphocyte subsets between the lungs of allergen sensitized Il17rb−/− or WT mice. However, in vitro re-stimulation experiments, as well as in vivo adoptive transfer studies with Rag2−/− mice, demonstrated that CD4+ T cell responses were altered in Il17rb−/− animals and could not efficiently transfer a type 2 immune response to the Rag2−/− recipients (see Supplemental Fig. 4). These data were similar to findings published by several investigators establishing a role of IL-25 in type 2 responses9,10,16. Il17rb−/− mice also exhibited significant reductions in the CD11b+ Gr-1mid myeloid cells previously identified as a source of type 2 cytokines in allergic animals (Fig. 2d). Intracellular cytokine staining of the CD11b+ Gr-1mid population verified that Il17rb−/− mice had a significant reduction in type 2 cytokine producing cells relative to WT mice (Fig. 3e). Reduced lymph node cytokine production, altered CD4+ T cell function, the reduction in type 2 cytokines, and a dramatic decrease in pulmonary myeloid cell infiltrates indicated that multiple cell types were affected by the absence of IL-17RB.
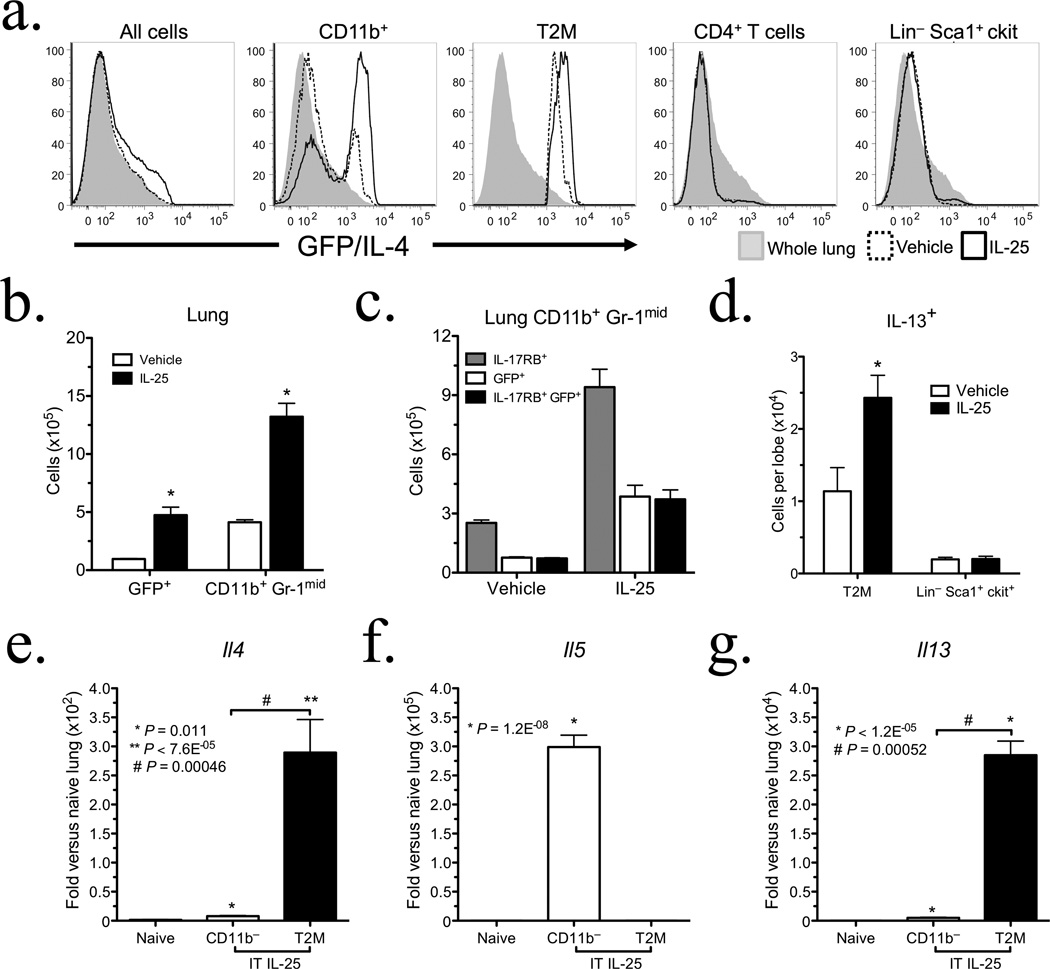
Figure 3. T2M cells represent the primary source of type 2 cytokines following pulmonary IL-25 administration.
4 get mice (n = 4 animals per group) were IT dosed with vehicle or 0.5 µg IL-25 for 4 days, and the inflammatory response was investigated 24 h post final IT. (a) Histograms of lung tissue from 4get mice treated with vehicle or IL-25, gated on total lung, CD11b+, T2M, CD4+ Lymphocytes, and Lin− Sca+ c-kit+ cells respectively. (b) GFP+ and CD11b+ Gr-1mid populations in the lung were assessed by flow cytometry, (*P < 0.026). (c) Pulmonary IL-17RB+ CD11b+ GFP/IL-4+ cell numbers following IL-25 administration. Data are representative of two independent experiments. (d) Pulmonary IL-13+ populations following IL-25 treatment (n = 5 animals per group, *P = 0.038). (e, f, and g) QPCR analysis of IL-4, IL-5, and IL-13 transcripts in T2M cells. Cells were isolated from C57BL/6J mice dosed with 0.5 µg IL-25 for 4 days (n = 5 animals per group), and plated in triplicate. T2M cells were isolated using MACS magnetic bead enrichment followed by FACS. mRNA was isolated from naïve C57BL/6J mice, CD11b depleted lung from IL-25 treated mice, and T2M cells isolated from IL-25 treated mice. All data are presented as mean ± s.e.m.
IL-25-induced inflammation is T2M cell dependent
We adapted a model of antigen-independent IL-25-induced pulmonary inflammation4 to directly assess the effects of IL-25 on type 2 cytokine producing cells in vivo, thereby avoiding the confounding pro-inflammatory effects of antigen-specific activation. Recombinant murine IL-25 was instilled into the airways of IL-4-IRES-eGFP (4get) mice. 4get mice express GFP in cells in which the IL-4 promoter is transcriptionally active, and were used to identify cells poised to produce type 2 cytokines. As has been reported previously4, the intratracheal administration of IL-25 induced a type 2 type inflammatory response, characterized by airway hyperreactivity, eosinophil infiltrates, mucus production, and the upregulation of inflammatory genes including Il25 and Il17rb (see Supplemental Fig. 5). IL-25 instillation induced the expression of GFP/IL-4 in myeloid but not other cell subsets (Fig. 3a), with approximately 80% of GFP/IL-4+ cells being CD11b+. The CD11b+ Gr-1mid IL-17RB+ subset, termed T2M cells to describe their propensity for type 2 cytokine production, demonstrated particularly dramatic enrichment for GFP/IL-4 expression. While IL-25 treated mice showed no increase in other GFP/IL-4+ populations, IL-25 administration significantly increased CD11b+ Gr-1mid infiltrates (Fig. 3b). Figure 3c illustrates that among this population, all GFP+ cells were also IL-17RB+, indicating that IL-25 acts on IL-25 responsive myeloid cells in part by activating transcription at the IL-4 promoter. Flow cytometric analysis further confirmed that the predominant pulmonary source of IL-13 following IL-25 administration were T2M cells (Fig. 3d). Lin− ckit+ Sca-1+ IL-17RB+ cells, a population identified as a source of IL-25-induced IL-13 in the gut19, were not altered by pulmonary IL-25 administration (Fig. 3f).
To verify that myeloid cells were producing type 2 cytokines in response to IL-25 administration, we isolated T2M cells from the lungs of IL-25 treated mice and assessed type 2 transcripts in this population. T2M cells exposed to IL-25 in vivo exhibited dramatic increases in Il4 and Il13 transcripts, whereas Il5 was derived from a CD11b− cell population that remains ill-defined in our studies (Fig. 3e–g). In addition to the pulmonary effects of intratracheal IL-25 administration, T2M cells were also identified in the bone marrow of 4get mice following IL-25 treatments (see Supplemental Fig. 6). Thus, IL-25 had both local and systemic effects linked to development of T2M cells.
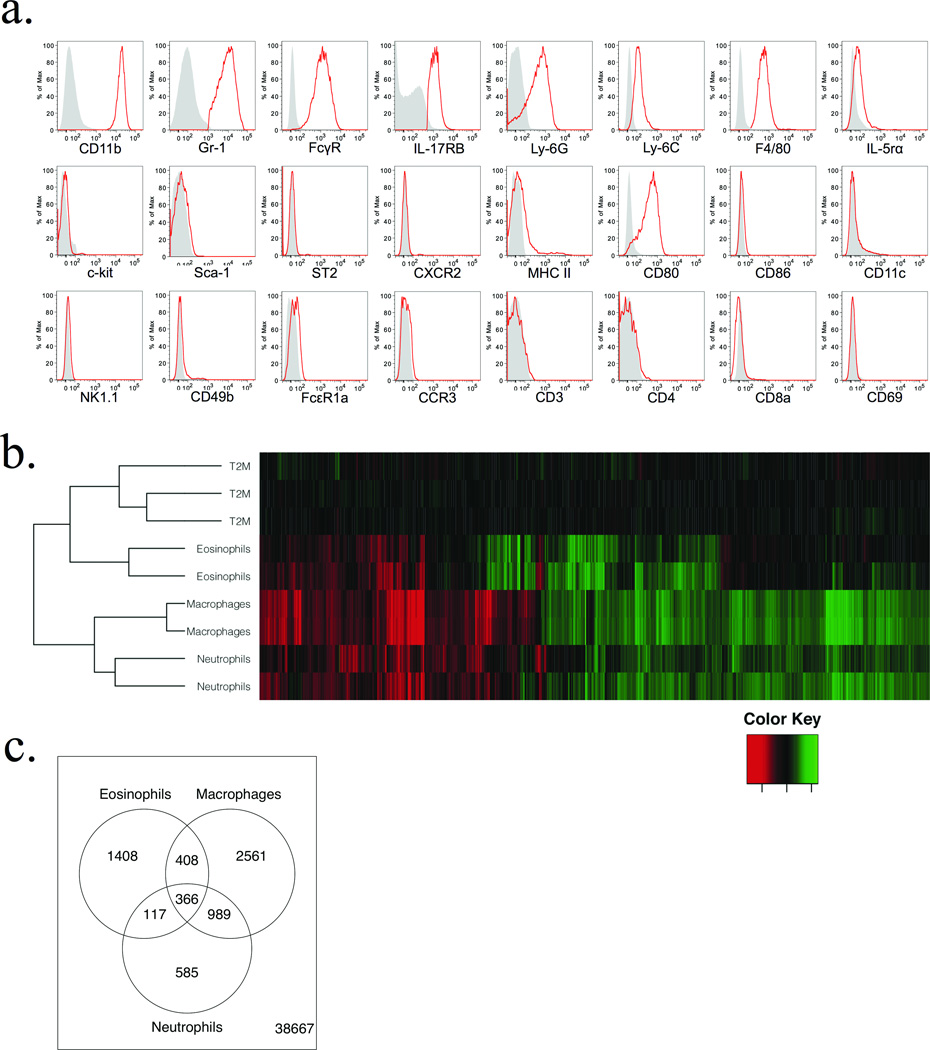
Pulmonary T2M cells are defined by a distinct combination of cell surface antigens (Fig. 4a). Similar expression patterns were observed in T2M cells derived from other tissues, including spleen, bone marrow, and peripheral blood (data not shown). T2M cells did not express the neutrophil-specific receptor CXCR2, nor did they express eosinophil protein markers such as IL-5rα or CCR3. FACS isolated T2Ms did not produce detectable transcripts of myeloperoxidase, major basic protein, or eosinophil peroxidase (data not shown), further supporting them as a separate granulocytic population. Microarray analysis of T2M cells compared to other isolated myeloid cell populations (Fig. 4b,c, see Supplemental Fig. 2) provides further evidence that this population represents a distinct granulocytic subset most closely related to eosinophils.
Figure 4. Patterns of surface receptor expression and a comparison of microarray profiles define T2M cells as a distinct granulocytic subset.
(a) Characterization of pulmonary T2M cells by cell surface receptor expression. 4get mice were challenged (as previously described) with intratracheal IL-25 to induce recruitment of T2M cells to the lung. T2M cells were identified by gating on CD11b+ Gr1mid IL-17RB+ GFP/IL-4+ cells, and expression of various surface markers was then assessed. Gray shaded area: isotype, red line; T2M cells. Data are representative of 2 independent experiments. (b) Hierarchical clustering and heat map generated from microarray analysis of pulmonary T2M cells compared to Eosinophils, Neutrophils, and Macrophages. Colors illustrate fold changes among 1,880 probes for which T2M cells exhibited a minimum 3 fold difference in expression from 2 or more cell types, and an average expression value of at least 26, normalized to average T2M expression levels. (c) Venn diagram illustrating differences in locus expression between T2M cells and other myeloid populations. 366 probes were differentially expression between T2M cells and all cell types analyzed, 1,880 probes differed between T2M cells and at least 2 of the comparison populations.
T2M cells are steroid resistant and pathologically relevant
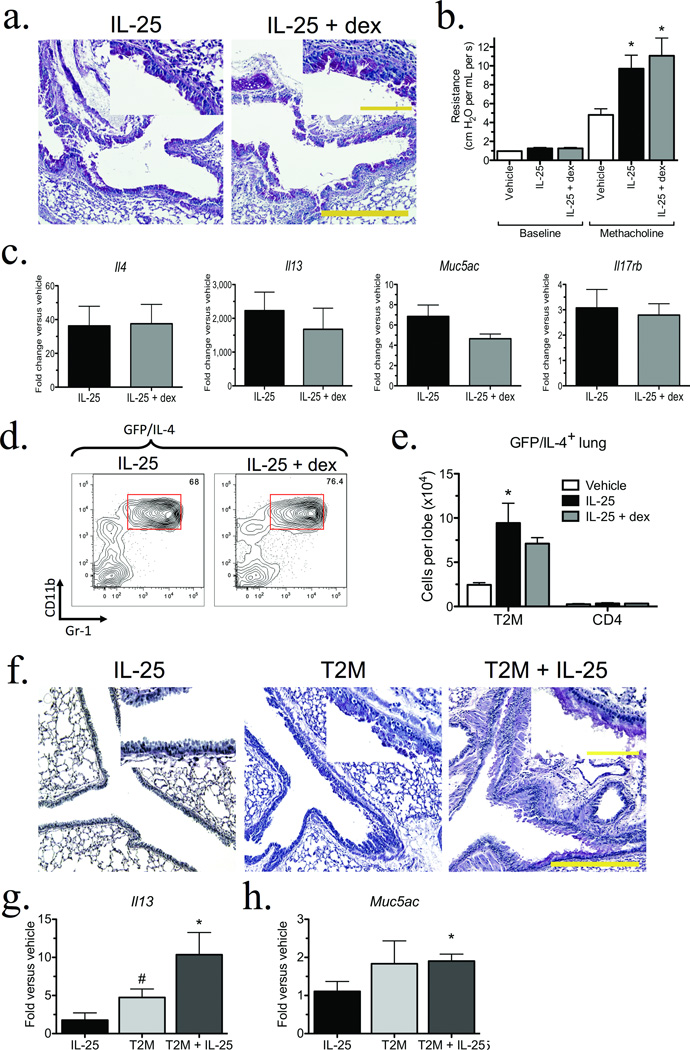
To examine potential clinical implications of type 2 cytokine production in the T2M population, we next examined how steroid treatment affected IL-25-induced pulmonary inflammation. To focus on the T2M response, 4get animals were treated with IL-25 as in previous experiments, with or without dexamethasone. Histologic examination (Fig. 5a) and measurements of airway hyperreactivity (Fig. 5b) indicated that IL-25-induced responses were not significantly altered by dexamethasone. QPCR analyses demonstrated significant increases in type 2 cytokines, mucus genes, and Il17rb that were unaffected by dexamethasone administration (Fig. 5c). Flow cytometric analysis measured equivalent numbers of IL-25-induced GFP/IL-4+ myeloid cells in animals treated with or without dexamethasone (Fig. 5d,e). To verify that dexamethasone treatment was effective, we examined splenic cell subsets (Supplementary Table 1). Dexamethasone significantly reduced total splenocytes, with a specific reduction in CD4+ and CD8+ T cells as well as eosinophils, but had no effect on splenic T2M cells. Overall these data present a striking finding that the IL-25-induced T2M cells are resistant to high dose glucocorticoid treatment.
Figure 5. T2M cells are steroid resistant, and are sufficient to induce airway pathology in IL-17RB−/− mice.
(a) Representative PAS staining indicates IL-25-induced mucus production in 4get mice (n = 5 per group) is not altered by dexamethasone administration. Scale bar 400 µm; inset 100 µm. (b) Airway hyperreactivity (*P < 0.01 versus methacholine treated vehicle). (c) QPCR analysis of whole lung following dexamethasone treatment. (d) Representative flow plots of GFP/IL-4+ CD11b+ Gr-1mid pulmonary populations. (e) Total numbers of pulmonary GFP/IL-4+ cells. Bars represent the mean ± s.e.m. of 4 mice per group. *P < 0.01 versus vehicle alone. Data are representative of two independent experiments. (f) Representative histology from recipients of T2M transfer stained with PAS, 24 hours post final IL-25 administration. Scale bar 400 µm; inset 100 µm. T2M cells were isolated by MACS enrichment and FACS, and 2.0×105 cells were instilled into the airways of Il17rb−/− mice. Mice received 4 total treatments, consisting of 0.5 µg IL-25, T2M cells alone, or IL-25 + T2M cells. (g) QPCR expression of IL-13 following T2M transfer (*P = 0.017, #P = 0.042). (h) QPCR expression for the mucus specific gene muc5ac (*P < 0.026). Bars represent the mean ± s.e.m. of 4 mice per group. Results are representative of 3 independent experiments.
In order to determine if IL-17RB+ T2M cells were sufficient to induce pulmonary inflammation, T2M cells from IL-25 treated mice were isolated and instilled into the airways of Il17rb−/− mice with recombinant IL-25. The transfer of T2M cells from IL-25 treated WT mice into Il17rb−/− recipients, coupled with instillations of IL-25, induced mucus production and inflammation in otherwise IL-25 insensitive Il17rb−/− animals (Fig. 5f). Recipients of T2M cells significantly increased Il13 transcripts, and the transfer of T2M cells with IL-25 further upregulated Il13 expression (Fig. 5g) as well as the mucus-specific gene Muc5ac (Fig. 5h). In separate experiments, T2M transfer exacerbated the inflammatory response in WT recipients and increased Muc5ac transcripts to levels comparable to those observed in IL-25 treated WT animals (see Supplemental Fig. 7). A similar pattern of increased mucus specific gene expression was observed following the adoptive transfer of T2M cells into allergen-sensitized recipients (see Supplemental Fig. 7). Therefore, in the context of increased pulmonary IL-25 levels, T2M cells are sufficient to induce pulmonary inflammation, IL-13, and mucus production; all hallmarks of allergic asthma.
T2M–like cells are increased in asthmatics
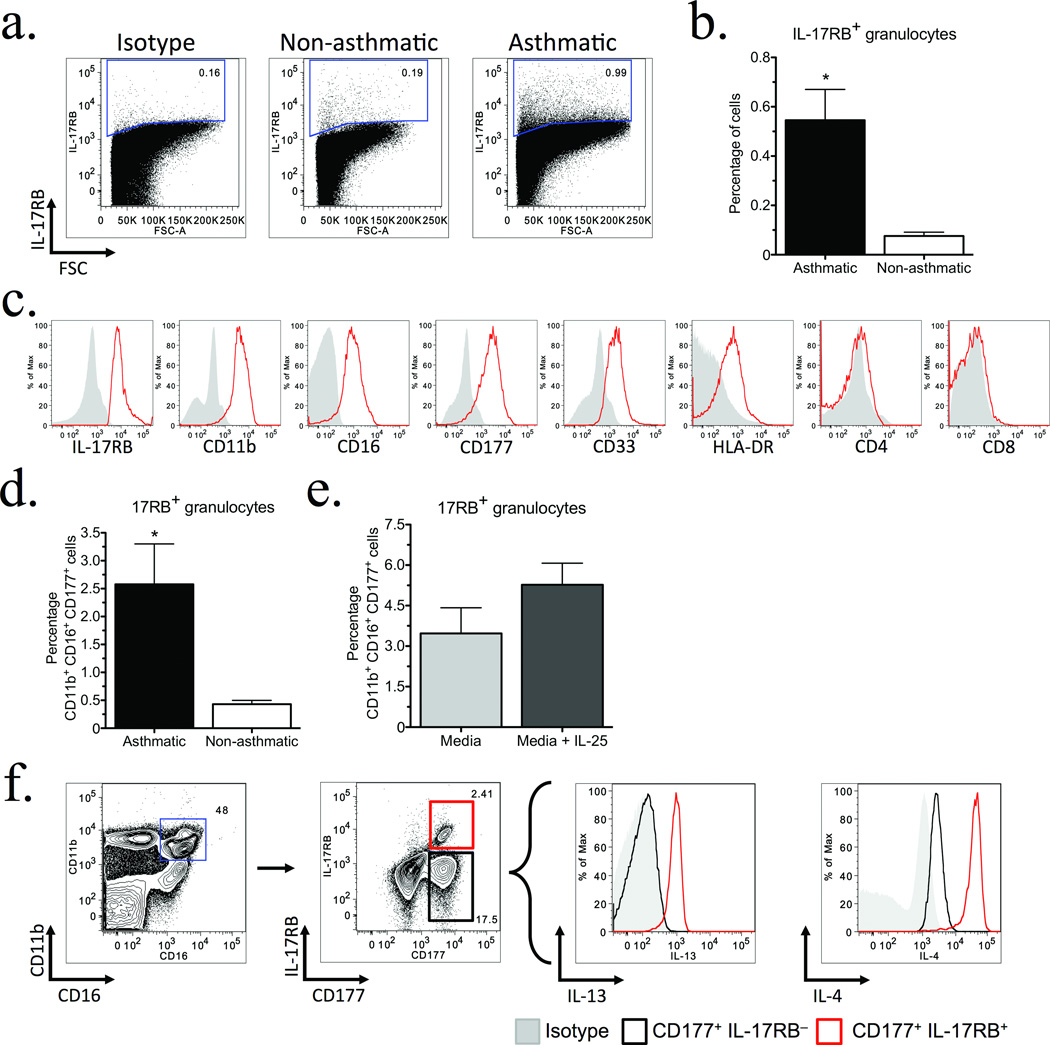
In order to assess whether a population analogous to T2M cells is present in humans and may be clinically relevant, asthmatic volunteers were recruited from the University of Michigan Asthma Clinic, and the expression of IL-17RB in peripheral blood was compared to non-asthmatic volunteers. Flow cytometric analysis identified significantly increased numbers of granulocytic IL-17RB+ cells in asthmatic patients (Fig. 6a,b). The granulocytic IL-17RB+ population co-expressed CD11b, CD16, and the Ly6 family member/myeloid progenitor/neutrophil marker CD177 (Fig. 6c). In addition, most IL-17RB+ cells were CD33+, weakly expressed HLA-DR, and were not CD4+ or CD8+.
Figure 6. An IL-4 and IL-13 producing population analogous to T2M cells is identifiable in peripheral blood and significantly increased in Asthmatics.
Granulocytes were isolated from peripheral blood samples donated by asthmatic (n = 9) or non-asthmatic (n = 8) volunteers and analyzed by flow cytometry. (a) Representative dot plots of granulocytes stained for IL-17RB, normalized to 400k events. (b) Percent total IL-17RB+ granulocytes isolated from peripheral blood of volunteer donors. Bars represent the mean ± s.e.m. for each group, (*P = 0.0031). (c) Representative histograms of IL-17RB+ granulocytes from an asthmatic donor indicate the majority of IL-17RB+ granulocytes are CD11b+ CD16+ CD177+. Cells were gated on total IL-17RB+ cells and assessed for surface marker expression. (d) Percent total CD11b+ CD16+ CD177+ IL-17RB+ granulocytes from volunteer donors. Bars represent the mean ± s.e.m. for each group, (*P = 0.023). (e) Percent total CD11b+ CD16+ CD177+ IL-17RB+ cells from whole blood of volunteer asthmatic donors, cultured in vitro for 2 hours ± 50 ng mL−1 IL-25. (f) Representative intracellular cytokine staining for IL-4 and IL-13 from whole blood from an asthmatic volunteer, cultured for 2 hours with RPMI 1640.
Based on the above findings, we focused our analysis upon IL-17RB+ CD11b+ CD16+ CD177+ cells and identified a significantly increased percentage of these cells in asthmatics (Fig. 6d) that was further elevated following in vitro stimulation with IL-25 (Fig. 6e). This IL-17RB+ subset produced both IL-4 and IL-13, whereas IL-17RB− cells did not. Thus, a population with similar cell surface receptor expression and phenotype as murine T2M cells can be identified in peripheral blood, is significantly elevated in asthmatics, and represents a source of both IL-4 and IL-13.
Discussion
IL-25 has been established as a regulator of type 2 inflammation and multiple reports have described its ability to exacerbate inflammatory responses at mucosal epithelia, including those in allergic asthma. The present study used a mouse model of chronic allergic asthma to identify both T and non-T IL-25 responsive cells involved in pulmonary inflammation. While previous studies have established that targeting IL-25 leads to the reduction of type 2 responses22,23, this study is the first to characterize how deficiency in IL-17RB reduces the pathology of allergic asthma induced by a common environmental allergen. Other reports, including one from our laboratory, have demonstrated that eosinophils produce IL-257,8, thus linking IL-25 production to eosinophilia induced by the allergic response. These data are consistent with clinical studies, as peripheral blood mononuclear cells from patients with severe allergic rhinitis exhibit increased IL-17RB24, and polymorphisms in IL-17RB have been associated with increased risk for severe asthma25. Furthermore, a recent study has identified that allergen-induced expression of IL-25 and its receptor in atopic asthmatics correlates with disease severity21.
Il17rb−/− mice had reduced allergen-induced pathology, including a significant reduction in type 2 cytokines primarily associated with T2M cells that were most prominent during persistent allergen-induced disease. The transfer of IL-25-induced T2M cells recapitulated lung pathology in IL-25 treated Il17rb−/− mice, demonstrating the sufficiency of T2M cells to mediate pathogenic responses. T2M cells appear to be steroid resistant in vivo and represent a distinct granulocytic population, which may have been identified in a model of pulmonary inflammation during N. brasiliensis infection26. T cell function was also altered in Il17rb−/− mice, demonstrating that in chronic allergic disease both T cell and non-T cell populations are contributors to type 2 cytokine-mediated pathophysiology.
While T lymphocytes are responsible for driving allergen-specific type 2 responses, multiple reports have identified critical roles for innate immune populations. Models of N. brasiliensis infection have identified novel IL-25 responsive cells in the gut, characterized as Lin− ckit+ Sca-1+ IL-13+, that play an important role in the clearance of enteric helminthes18–20. Recent studies have identified a similar innate lymphocytic cell population in the gut, lung, and nasal polyps of humans, further supporting their potential role in human disease27. In our studies, this population was present at low numbers in the lung, and did not increase following allergen or IL-25 administration. Previous reports have identified other non-lymphoid populations as sources of type 2 cytokines that can contribute to the allergic environment, including basophils, mast cells, eosinophils, and macrophages28–38. We did not detect significant IL-17RB expression in any of these populations in the lungs of allergen challenged animals. Thus, it appears that there are both Lin+ and Lin− IL-25 responsive cells whose recruitment may depend upon the type of mucosal surface (gut versus lung), both with the capacity to produce type 2 cytokines in an antigen-independent manner.
Analysis of surface markers and presence in the bone marrow during allergen and IL-25-induced responses indicate that T2M cells are derived from the bone marrow. Their presence in peripheral blood of humans with asthma may represent an induced cell population that may be recruited and accumulate in the lung during persistent or exacerbated disease. Because IL-17RB+ subsets could be distinguished based upon the intensity of Gr-1 expression, and in light of the differing capacity for IL-4 and IL-13 production between Gr-1mid and Gr-1hi populations, IL-17RB+ Gr-1hi cells may have functions that overlap with other CD11b+ Gr-1+ populations, such as myeloid suppressor cells39,40. A recent study reported that both IL-17RA and IL-17RB can be expressed on the surface of human neutrophils41. Clinical studies of patients with steroid resistant asthma demonstrated that a neutrophilic inflammatory response is predominant, while animal studies have suggested that steroid resistant Th17 cells may explain neutrophil-mediated responses42–50. Since IL-17RA is required for functional IL-17A and IL-25 signaling, these cytokines may share downstream signals induced following ligand binding that provide a common link for steroid resistance. The development of T2M cells likely depends upon an overall type 2 immune environment and perhaps IL-25 itself.
Our data also indicate that, based on the rapid accumulation of myeloid cells in lungs following IL-25 administration, there is a pool of cytokine producing IL-25 responsive cells capable of amplifying a type 2 response. In allergic individuals, T2M cells may play an important role in the immediate response to an environmental allergen, priming the system for a type 2 response by producing cytokines prior to T lymphocyte activation. T2M cells could also contribute to chronic disease, as airway epithelial damage stimulates IL-25 secretion. This concept may be especially relevant in asthmatics, as our studies identified increased numbers of T2M-like cells in circulation of asthmatics that may be recruited upon an exacerbation and accumulate in the lungs. The induction of IL-25 in airways by pathogens51, allergens, or other noxious stimuli may amplify the severity of the response by activating steroid resistant T2M cells, especially in patients with underlying pulmonary disease. A complete understanding of the development and function of T2M cells will require further investigation, however our findings suggest that they represent an intriguing biomarker and possible therapeutic target for the treatment of severe asthma.
Materials and Methods
Animals and allergen model
6–8 week old female C57BL/6J, BALBc, 4get, and Rag2−/− mice were purchased from The Jackson Laboratory. Il17rb−/− mice were provided by A.L. Budelsky (Amgen). Clinical skin test grade cockroach allergen (HollisterStier) was used for allergen sensitization as previously described52. Animals were immunized systemically (day 0) via intraperitoneal (IP) and subcutaneous injections of allergen emulsified with Incomplete Freund’s Adjuvant. Mice were given 4 intranasal allergen challenges (1.5 µg in 15 µl PBS on days 14, 18, 22, and 26), followed by two intra-tracheal (IT) administrations (5 µg in 50 µl PBS on days 30 and 32). Tissues were analyzed 24 hours post final allergen challenge. All animal experiments were reviewed and approved by the University of Michigan University Committee on Care and Use of Animals.
Airway hyperreactivity (AHR)
AHR was measured using direct ventilation mouse plethysmography, specifically designed for low tidal volumes (Buxco Research Systems), as previously described53,54.
Lung histology
Serial 6 µM sections were obtained from paraffin embedded, formalin-fixed left lungs stained with H+E or periodic acid-Schiff (PAS).
Primary cell isolation
Lung tissue was processed via enzymatic digestion as previously described7. Draining LN and spleen cells were dispersed by mechanical disruption through a 40 µM filter. Bone marrow was isolated by flushing femurs and tibias with 1× PBS and 1% FCS through a 40 µM filter. Cell numbers were quantified following RBC lysis.
LN restimulation
LN cells (4×105 per well, plated in triplicate) were restimulated with 10 µL mL−1 allergen, 10 ng mL−1 IL-25, or both. RNA was isolated after 2 hours in culture; supernatants were analyzed for protein production after 48 hours in culture.
mRNA and Protein Quantification
Whole lung, LN, and bone marrow RNA was isolated using TRIzol (Invitrogen). RNA from cells sorted by FACS was isolated with a microprep kit (Qiagen), DNase treated (Invitrogen), and reverse transcribed with Superscript III (Invitrogen). mRNA was assessed using quantitative PCR analysis (TaqMan) with primers and probe sets from Applied Biosystems. Expression of genes of interest was normalized to Gapdh. Protein was quantified by Bioplex (Bio-Rad) according to manufacturer’s instructions.
Flow cytometry
Populations were assessed using standard techniques. Samples for intracellular staining were treated with 0.5 µL mL−1 brefeldin A, 0.5 µL mL−1 monensin, 0.5 ng mL−1 PMA, and 500 ng/mL ionomycin and incubated for 6 hours at 37°C, 5% CO2. Cells were stained according to manufacturer’s instructions (BD Biosciences fix/perm kit). Data were collected on a BD Biosciences LSR II flow cytometer and on a BD Biosciences FACSAria, and analyzed using FlowJo software (Tree Star). Cellular surface markers of spleen cell controls were not altered by collagenase treatment as compared to untreated cells, consistent with previous studies55–57. All antibodies used in these studies are listed in supplemental material.
Intra-tracheal IL-25 administration and dexamethasone treatments
6–8 week old female C57BL/6J, Balbc, 4get, or Il17rb−/− mice received daily intra-tracheal injections (0.5 µg recombinant IL-25 (R&D) in 50 µL PBS) for 4 days10. Dexamethasone (Sigma) was administered in 2 doses (3 mg per kg per day) via IP injection, 1 hour prior the first and third IL-25 injections. Tissues were analyzed 24 hours after final IL-25 administration.
T2M Isolation and Adoptive Transfers
T2M cells were isolated from the lungs of C57BL/6J mice treated with intra-tracheal IL-25, as described above, by enriching for CD11b+ cells with MACS (Miltenyi), followed by FACS. Isolated cells (2.0×105 per recipient) were instilled into the airways of Il17rb−/− mice via intratracheal injection in 50 µL PBS. Recipients received four transfers total at 24 hour intervals. Lung tissue was harvested 24 hours post final adoptive transfer.
Microarrays
Pooled cells from IL-25 treated 4get mice (n = 4 mice per sample) were sorted by FACS and RNA isolated for microarray analysis. Affymetrix Mouse 430 2.0 microarrays were processed in the University of Michigan DNA Sequencing Core using the WT-Pico V.2 kit.
Clinical studies
All human studies were performed in accordance with an approved University of Michigan Institutional Review Board protocol after legal consent from adult volunteers. Subjects were recruited from the University of Michigan Asthma Clinic and had been diagnosed with asthma based on clinical assessment and pulmonary testing consistent with 2007 NIH/NAEPP guidelines. Patients (n = 9) were persistent asthmatics and on daily controller medication. Healthy control subjects (n = 8) had not previously been diagnosed with asthma. The protocol used to assess inflammatory subsets is described in supplemental material.
Statistical analysis
All data are presented as mean ± s.e.m. Data were evaluated by one-way ANOVA and, where appropriate, further evaluated with the parametric Student-Newman-Keuls test for multiple comparisons or the nonparametric Mann-Whitney rank-sum test. For microarray analysis, expression values for each gene were assessed using a robust multi-array average (RMA). The Affymetrix package of bio-conductor implemented in the R statistical language was used to analyze probesets.
Supplementary Material
Acknowledgments
We thank J. Connett for comments on the manuscript, K. Augusztiny for valuable insight and perspective, members of the Lukacs, Kunkel, and Hogaboam labs for many helpful discussions, and the University of Michigan Flow Cytometry and DNA Sequencing Cores for technical assistance. This work was supported by US National Institutes of Health grants R01 HL059173 and R01 HL036302 (to N.W.L.), and National Institute of General Medical Studies 3T32GM007863-31S1 (to the University of Michigan Medical Scientist Training Program and B.C.P.)
Footnotes
GEO Accession Number
Author Contributions
B.C.P. conceived the study, performed experiments and data analyses, and wrote the manuscript. A.L.B. and M.A.S. provided intellectual contributions through technical advice and experimental design. A.P.B. coordinated recruitment of asthmatic patients. N.W.L conceived and supervised the study and wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Wang W, Li JJ, Foster PS, Hansbro PM, Yang M. Potential therapeutic targets for steroid-resistant asthma. Curr Drug Targets. 2010;11:957–970. doi: 10.2174/138945010791591412. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa Y, Calhoun WJ. Phenotypic characterization of severe asthma. Curr Opin Pulm Med. 2010;16:48–54. doi: 10.1097/MCP.0b013e3283357d15. [DOI] [PubMed] [Google Scholar]
- 3.Adcock IM, Ford PA, Bhavsar P, Ahmad T, Chung KF. Steroid resistance in asthma: mechanisms and treatment options. Curr Allergy Asthma Rep. 2008;8:171–178. doi: 10.1007/s11882-008-0028-4. [DOI] [PubMed] [Google Scholar]
- 4.Fort MM, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 5.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamachi T, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 7.Dolgachev V, Petersen BC, Budelsky AL, Berlin AA, Lukacs NW. Pulmonary IL-17E (IL-25) production and IL-17RB+ myeloid cell-derived Th2 cytokine production are dependent upon stem cell factor-induced responses during chronic allergic pulmonary disease. J Immunol. 2009;183:5705–5715. doi: 10.4049/jimmunol.0901666. [DOI] [PubMed] [Google Scholar]
- 8.Terrier B, et al. IL-25: a cytokine linking eosinophils and adaptative immunity in Churg-Strauss syndrome. Blood. doi: 10.1182/blood-2010-02-267542. [DOI] [PubMed] [Google Scholar]
- 9.Sharkhuu T, et al. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin Exp Allergy. 2006;36:1575–1583. doi: 10.1111/j.1365-2222.2006.02595.x. [DOI] [PubMed] [Google Scholar]
- 10.Rickel EA, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 12.Claudio E, et al. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol. 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swaidani S, et al. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong CK, Li PW, Lam CW. Intracellular JNK, p38 MAPK and NF-kappaB regulate IL-25 induced release of cytokines and chemokines from costimulated T helper lymphocytes. Immunol Lett. 2007;112:82–91. doi: 10.1016/j.imlet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Maezawa Y, et al. Involvement of TNF receptor-associated factor 6 in IL-25 receptor signaling. J Immunol. 2006;176:1013–1018. doi: 10.4049/jimmunol.176.2.1013. [DOI] [PubMed] [Google Scholar]
- 16.Wang YH, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stock P, Lombardi V, Kohlrautz V, Akbari O. Induction of airway hyperreactivity by IL-25 is dependent on a subset of invariant NKT cells expressing IL-17RB. J Immunol. 2009;182:5116–5122. doi: 10.4049/jimmunol.0804213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 19.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saenz SA, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corrigan CJ, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol. 2011;128:116–124. doi: 10.1016/j.jaci.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 22.Angkasekwinai P, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballantyne SJ, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, et al. Allergen challenge of peripheral blood mononuclear cells from patients with seasonal allergic rhinitis increases IL-17RB, which regulates basophil apoptosis and degranulation. Clin Exp Allergy. 40:1194–1202. doi: 10.1111/j.1365-2222.2010.03542.x. [DOI] [PubMed] [Google Scholar]
- 25.Jung JS, et al. Association of IL-17RB gene polymorphism with asthma. Chest. 2009;135:1173–1180. doi: 10.1378/chest.08-1595. [DOI] [PubMed] [Google Scholar]
- 26.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mjosberg JM, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 28.Paul WE. Interleukin-4 production by Fc epsilon R+ cells. Skin Pharmacol. 1991;4(Suppl 1):8–14. [PubMed] [Google Scholar]
- 29.Seder RA, et al. Production of interleukin-4 and other cytokines following stimulation of mast cell lines and in vivo mast cells/basophils. Int Arch Allergy Appl Immunol. 1991;94:137–140. doi: 10.1159/000235345. [DOI] [PubMed] [Google Scholar]
- 30.van Panhuys N, et al. Basophils Are the Major Producers of IL-4 during Primary Helminth Infection. J Immunol. 2011 doi: 10.4049/jimmunol.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torrero MN, Hubner MP, Larson D, Karasuyama H, Mitre E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J Immunol. 2010;185:7426–7434. doi: 10.4049/jimmunol.0903864. [DOI] [PubMed] [Google Scholar]
- 32.Steinfelder S, et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshimoto T, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 34.Perrigoue JG, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang HB, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bandeira-Melo C, et al. IL-16 promotes leukotriene C(4) and IL-4 release from human eosinophils via CD4- and autocrine CCR3-chemokine-mediated signaling. J Immunol. 2002;168:4756–4763. doi: 10.4049/jimmunol.168.9.4756. [DOI] [PubMed] [Google Scholar]
- 37.Holtzman MJ, et al. Immune pathways for translating viral infection into chronic airway disease. Adv Immunol. 2009;102:245–276. doi: 10.1016/S0065-2776(09)01205-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim EY, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peranzoni E, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 40.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garley M, Jablonska E, Grabowska SZ, Piotrowski L. IL-17 family cytokines in neutrophils of patients with oral epithelial squamous cell carcinoma. Neoplasma. 2009;56:96–100. doi: 10.4149/neo_2009_02_96. [DOI] [PubMed] [Google Scholar]
- 42.Hansbro PM, Kaiko GE, Foster PS. Cytokine/anti-cytokine therapy - novel treatments for asthma? Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bullens DM, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drews AC, et al. Neutrophilic airway inflammation is a main feature of induced sputum in nonatopic asthmatic children. Allergy. 2009;64:1597–1601. doi: 10.1111/j.1398-9995.2009.02057.x. [DOI] [PubMed] [Google Scholar]
- 45.Kikuchi S, Nagata M, Kikuchi I, Hagiwara K, Kanazawa M. Association between neutrophilic and eosinophilic inflammation in patients with severe persistent asthma. Int Arch Allergy Immunol. 2005;137(Suppl 1):7–11. doi: 10.1159/000085425. [DOI] [PubMed] [Google Scholar]
- 46.Fukakusa M, et al. Oral corticosteroids decrease eosinophil and CC chemokine expression but increase neutrophil, IL-8, and IFN-gamma-inducible protein 10 expression in asthmatic airway mucosa. J Allergy Clin Immunol. 2005;115:280–286. doi: 10.1016/j.jaci.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 47.Linden A, Hoshino H, Laan M. Airway neutrophils and interleukin-17. Eur Respir J. 2000;15:973–977. doi: 10.1034/j.1399-3003.2000.15e28.x. [DOI] [PubMed] [Google Scholar]
- 48.Jatakanon A, et al. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160:1532–1539. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 49.Vazquez-Tello A, et al. Induction of glucocorticoid receptor-beta expression in epithelial cells of asthmatic airways by T-helper type 17 cytokines. Clin Exp Allergy. 2010;40:1312–1322. doi: 10.1111/j.1365-2222.2010.03544.x. [DOI] [PubMed] [Google Scholar]
- 50.McKinley L, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaiko GE, Phipps S, Angkasekwinai P, Dong C, Foster PS. NK Cell Deficiency Predisposes to Viral-Induced Th2-Type Allergic Inflammation via Epithelial-Derived IL-25. J Immunol. 185:4681–4690. doi: 10.4049/jimmunol.1001758. [DOI] [PubMed] [Google Scholar]
- 52.Berlin AA, Hogaboam CM, Lukacs NW. Inhibition of SCF attenuates peribronchial remodeling in chronic cockroach allergen-induced asthma. Lab Invest. 2006;86:557–565. doi: 10.1038/labinvest.3700419. [DOI] [PubMed] [Google Scholar]
- 53.Campbell EM, Kunkel SL, Strieter RM, Lukacs NW. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- 54.Campbell E, Hogaboam C, Lincoln P, Lukacs NW. Stem cell factor-induced airway hyperreactivity in allergic and normal mice. Am J Pathol. 1999;154:1259–1265. doi: 10.1016/S0002-9440(10)65377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukacs NW, et al. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol. 2010;185:2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kallal LE, Hartigan AJ, Hogaboam CM, Schaller MA, Lukacs NW. Inefficient lymph node sensitization during respiratory viral infection promotes IL-17-mediated lung pathology. J Immunol. 2010;185:4137–4147. doi: 10.4049/jimmunol.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smit JJ, et al. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One. 2008;3:e1720. doi: 10.1371/journal.pone.0001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.