Abstract
A major goal of translation research in autism is to characterize the physiological and psychological processes underlying behavioral abnormalities. Since autism reflects impairments in social motivation, we modified the mouse three-chamber social approach apparatus for use as a social conditioned place preference arena. We paired one of two unique contexts with social interactions in juvenile mice for five or ten conditioning sessions in BTBR T+tf/J mice and a control strain with normal approach behaviors (C57BL/6J) since the BTBR T+tf/J inbred mouse strain displays a variety of behavioral alterations analogous to symptoms of autism spectrum disorders. While C57BL/6J mice formed a conditioned place preference to the context associated with social interactions, particularly those receiving ten days of conditioning, BTBR T+tf/J mice did not. Neither absence of social proximity nor avoidance due to high rates of autogrooming appeared to underlie the impaired positive incentive value of the unconditioned social stimulus in the BTBR T+tf/J strain. These data contribute to a growing body of evidence suggesting that the BTBR T+tf/J strain shows impairments in all diagnostic domains of autism including social motivation. Additionally, social conditioning testing might provide an important social motivation measure in other rodent models of neuropsychiatric disorders characterized by social abnormalities.
Keywords: Mouse Model, Autism, Social Conditioned Place Preference, BTBR, Three Chamber
1.1 Introduction
Autism spectrum disorder (ASD) is a highly heritable, but varied set of neurodevelopmental abnormalities characterized by deficits in reciprocal social interactions, communication, and restricted or repetitive behaviors [1]. Rodent models of ASD have proven informative in the recapitulation of the syndrome, in revealing physiological abnormalities and in pre-clinical drug screening [26].
Since ASD is conceptualized as a social disorder, a major research emphasis is on elucidating brain systems that regulate social behavior, and in particular, those that are disturbed by environmental or genetic processes [6,13]. Social behavior encompasses a broad accumulation of constructs (emotion, and sexual and aggressive motivation) and is modulated by a variety of stimuli and sensory modalities (olfactory, visual and tactile); therefore, some specificity is required in the interpretation of behavioral variables in social behavior tests [13]. One way to approximate the positive incentive (pro-social or affiliative) motivation of social interaction in rodents is through tests which measure value of social interactions via conditioning. Relative motivation for social interaction in rodents has been tested with operant lever-pressing for social contact [11], through conditioned place preferences (CPP) to interactions with littermates [22, 23] and through exposure to familiar and unfamiliar conspecifics [5, 28, 29]. Notably, the degree of social-conditioned place preference in rodents corresponds directly to variability in social motivation secondary to degree of previous social isolation [29]. In rats, social interactions are sufficiently reinforcing in their ability to reverse cocaine CPP [12]; however, restricting tactile contact with an unconditioned social stimulus markedly attenuates or blocks social CPP [15]. The degree of conditioned place preference can therefore be considered an index of the positive incentive value [3] of social contact.
Social motivation measures may provide complementary measures in animal models of ASD [7]. In fact, attenuated conditioned place preference to conspecifics has been demonstrated in fmr1 mutant mice, a model for Fragile X mental retardation disorder [21]. In terms of translation, social motivation testing may be critical in revealing brain substrates of impaired affiliative social behavior in ASD [8, 30] and perhaps the development of such impairments through altered assignment of reward to social stimuli [9].
In the current study, a social reinforcement paradigm was utilized to analyze social conditioned place preference in BTBR T+tf/J (BTBR) mice. We present a modification of the three-chamber social approach apparatus for the analysis of place preference stemming from context-specific access to a juvenile conspecific. By applying this social conditioning task to compare juvenile C57BL/6J (B6) mice to the socially-impaired BTBR strain, we were able to demonstrate differential place preferences to a context associated with social interactions.
2.1 Materials and Methods
2.1.1 Subjects
C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice were bred from stock obtained from The Jackson Laboratory (Bar Harbor, ME). Subject mice were weaned on postnatal day 21–25 and housed with one to seven same-sex littermates, depending on litter size, in standard individually ventilated cages under a 12:12h light-dark schedule (lights on at 06:00). All mice were individually identified with numbered ear tags. An equal number of male and female mice from each strain were tested beginning at 25 - 30 days of age, at least 24 hours after being weaned. Both sexes were used since previous reports have noted no sex differences in sociability within B6 and BTBR mice [27, 32]. Mean age, standard error and range of ages of mice at the onset of conditioning in each of the four groups are as follows: B6 5 Day: 28.7 ± 0.75, range 26–31; 10 Day: 27.5 ± 0.56, range 26–30; BTBR 5 Day: 26.0 ± 0.00, range 26–26; 10 Day: 25.6 ± 0.16, range 25–26. This range of ages represents experimental constraints that permitted conditioning of cohorts of multiple groups simultaneously.
2.1.2 Social Conditioned Place Preference
Three-chamber arenas constructed according to Nadler et al. [20] were modified so that the two outer compartments included alternating 3.8 cm black and white stripes on the walls; one chamber contained vertical stripes while the other contained horizontal stripes (Figure 1). Arenas measured 41 (L) × 70 (W) × 28 (H) cm in total with each compartment measuring 70 (L) × 20 (W). The center compartment was comprised of solid black Plexiglas walls. Two separate arenas were used simultaneously during testing- each of which had the opposite pattern of stripes to eliminate confounding influences of baseline stripe orientation or side preference. On the first day of testing (Day 0), all subjects were placed in the middle compartment and the doors were lifted to permit full access to all three compartments. Mice could freely explore the entire arena for twenty minutes during which time an observer manually recorded the duration of time in each of the two striped outer compartments, with hand-held stopwatches. This session served as habituation and permitted determination of baseline side preferences for each subject. Mice were then randomly assigned to either 5 or 10 days of social conditioning, creating four groups: B6 5 day, B6 10 day, BTBR 5 day, and BTBR 10 day. In total, twenty mice from each strain were used, representing an equal sex ratio, with ten each per condition. On the day following baseline testing, and for 5 or 10 consecutive days, social conditioning was performed. Mice were randomly assigned to receive contact with a conspecific (unconditioned stimulus) in either the horizontally- or vertically- striped compartment (conditioned stimulus). During conditioning, doors were left closed and the mice were placed into the non-social chamber and permitted to explore alone for 10 minutes, then the mouse was immediately removed and placed into the opposite compartment which contained an unfamiliar age-matched male or female B6 mouse which had been placed in the “social” chamber immediately prior to introducing the subject mouse. The subject mouse and stimulus mouse were then permitted 10 additional minutes to explore or interact. The side in which the subject mouse was initially placed was alternated daily; therefore, the order in which they received the social and non-social sessions was counterbalanced daily. Stimulus mice were never present in any of the arenas during the non-social conditioning session. An unfamiliar group of six naïve male and female B6 mice (n=3/sex) served as stimulus animals. B6 mice were chosen because they display normal sociability. Furthermore, a recent study [31] demonstrated that sociability phenotypes of B6 and BTBR mice are independent of stimulus strain. Since a total of six mice served as stimuli, subject mice conditioned for 10 days were exposed to mice they were in contact with previously for the last four out of the ten days, while 5 day conditioned mice were exposed to unfamiliar mice daily. Sex of the stimulus mouse was alternated daily for each subject. On the three consecutive days following the last conditioning session, mice were tested daily for place preference during a free exploration session for 20 minutes, exactly as they were tested during the baseline period. Repeated testing provided insight into the strength of the reinforcer as indexed by the rate of place-preference extinction.
Figure 1.
Social conditioned place preference arena. Two such arenas were used simultaneously for social conditioning. Each contained opposing orientations of stripes to reduce the influence of side biases. Sliding guillotine doors separated compartments.
2.1.3 Social Interactions
The degree of social conditioned place preference may be directly influenced by the quality or quantity of interactions during conditioning. Mouse strains avoiding social contact might show impaired social conditioning secondary to inadequate social contact during conditioning. To account for this possibility, videotapes of conditioning sessions were sub-sampled for the presence of social proximity. Proximity was defined as maintaining a body distance (excluding tail) of less than or equal to 2 cm at any point in a ten second scan collected every 60 seconds for each session per day. Therefore, the presence/absence of proximity was sub-sampled for ten seconds at each of the nine minutes (1–10, since the first minute was excluded) for five or ten days, yielding at total of 45, and 90 scans for the two conditions, respectively.
2.1.4 Self-Grooming
BTBR mice show consistent enhancements of self-grooming rates compared to B6 control mice [18]. BTBR mice may avoid social contact in order to perform high rates of such body care activities. This hypothesis is predicated on the prediction that vigilance in a social context and self-grooming might be state behaviors at odds with one another. Therefore, during baseline and the first testing session, the time spent self-grooming was scored and calculated as percentage time [grooming duration (seconds)/chamber duration (seconds) × 100]; the calculated value on the first test day was then subtracted from the baseline value to obtain a change in percent time spent grooming in each of the three compartments during testing. We predicted that BTBR mice, but not B6 mice would spend more proportional time grooming on the side they associate with social isolation.
All sessions were videotaped from above with a digital camcorder connected to a DVD recorder. Side preference testing was performed in real time by two observers; social proximity and self-grooming were scored offline from DVDs by an assistant blind to the strain and condition of the mouse using Noldus Observer Software. Testing arenas were sanitized with a 70% ethanol solution and dried between subjects. Conditioning and testing were performed between 08:00 and 13:00 hours under standard fluorescent lighting. Experiments and husbandry were performed according to protocols approved by the University of Hawaii Laboratory Animal Service Animal Care and Use Committee.
2.2 Statistical Analysis
For three consecutive test days after conditioning, percent preference for the side to which the subject mouse was socially conditioned was calculated [time in social compartment/ (time in nonsocial compartment + time in social compartment) x 100] and for graphical and statistical purposes, the baseline preference (calculated in the same manner) for the social side was subtracted. Therefore, values represent the change in percent from baseline, in the preference for the social conditioned chamber. A two-way repeated measures analysis of variance (ANOVA) was performed with Strain and Conditioning Days as the two factors and Test Day as the repeated measures variable. On account of a-priori hypotheses, mean pairwise differences in chamber preference scores after social conditioning were compared to the baseline measures using paired t-tests to determine any significant change from baseline. To determine strain differences independent of testing day, a two-way ANOVA was performed utilizing normalized (baseline subtracted) social compartment preference scores collapsed across all three days of testing. Similarly, two-way repeated measures ANOVAs were utilized to compare mean percentages of scan observations during conditioning in which the mouse was in proximity to the stimulus between the two strains within the five- and ten-day conditioning groups. A series of one-way ANOVAs was performed to compare mean durations of grooming during the habituation and first testing day as well as the difference of the two, to compare proportion of time spent grooming in each of the three chambers.
3.1 Results
3.1.1 Social Conditioned Place Preference
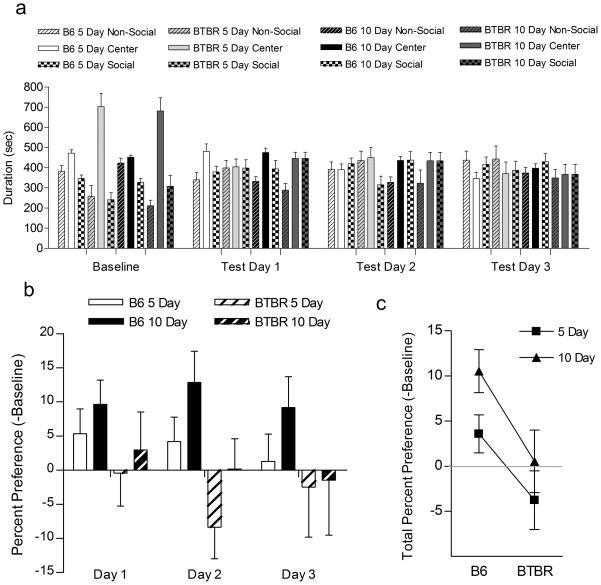
Figure 2a presents mean duration of time in each of the three chambers at baseline and at each day of testing. Change in social compartment preferences (baseline subtracted) are displayed in figure 2b. Positive values indicate preference for the compartment associated with the social interactions while negative values indicate an aversion or decrease in preference for the social compartment, relative to baseline. Two- way repeated measures ANOVA revealed a significant main effect of Strain (F(1,36)=4.196, p=0.048), indicating that BTBR mice showed decreased percent preference for the socially-conditioned side compared to B6 mice. The main effect for the number of Conditioning Days (F(1,36)=1.765, p=0.192) failed to reach statistical significance. Strain x Conditioning Days (F(1,36)=0.099, p=0.755), and Strain x Conditioning Days x Test Day (F(2,72)=0.295, p=0.746) interactions were not statistically significant. Paired t-tests revealed that on each day of testing, B6 mice conditioned for 10 days showed a significant increase in the mean preference for the social chamber relative to their baseline preference (Day 1: t(9)=−3.129, p=0.012; Day 2: t(9)=−2.894, p=0.018; Day 3: t(9)=−2.356, p=0.043). The reinforcement potential of the social conditioning appears strong since there was no evident extinction of social compartment place-preference in B6 mice conditioned for ten days. When mean chamber preferences were collapsed across the three days (Figure 2c), two-way ANOVA revealed a significant effect of strain (F(1,116)=9.914, p=0.003). Interestingly, with the mean values collapsed across all three days of testing, the Conditioning Days main effect bordered statistical significant (F(1,116)=3.867, p=0.052) suggesting that the amount of conditioning might influence the strength of place preference formation. However, the interaction between Strain and number of Conditioning Days failed to reach statistical significance (F(1,116)=0.216, p=0.643). Figure 2b presents percent preference (- baseline) for the socially-conditioned side across three days of testing in B6 and BTBR 5- and 10-day conditioned mice.
Figure 2.
Social conditioned place preference testing for B6 and BTBR mice. Mean time spent in each of the three compartments of the conditioned place preference arena during baseline and testing (a). Percent change in compartment preference after social conditioning in B6 and BTBR mice (b). B6 mice, particularly those conditioned for ten days, showed increased preference (controlling for baseline) for the side associated with social interactions indicated by positive numbers. However, BTBR mice failed to develop a place preference to repeated social interactions over three consecutive days of testing. When preference was collapsed across three days of testing, B6 mice showed a clear preference for the social conditioned side (c), relative to baseline (subtracted) and to BTBR mice. Values represent Mean ± 1 S.E.M. Simple comparisons (test day vs. baseline): * p<0.05. N=10/group
3.1.2 Social Scans
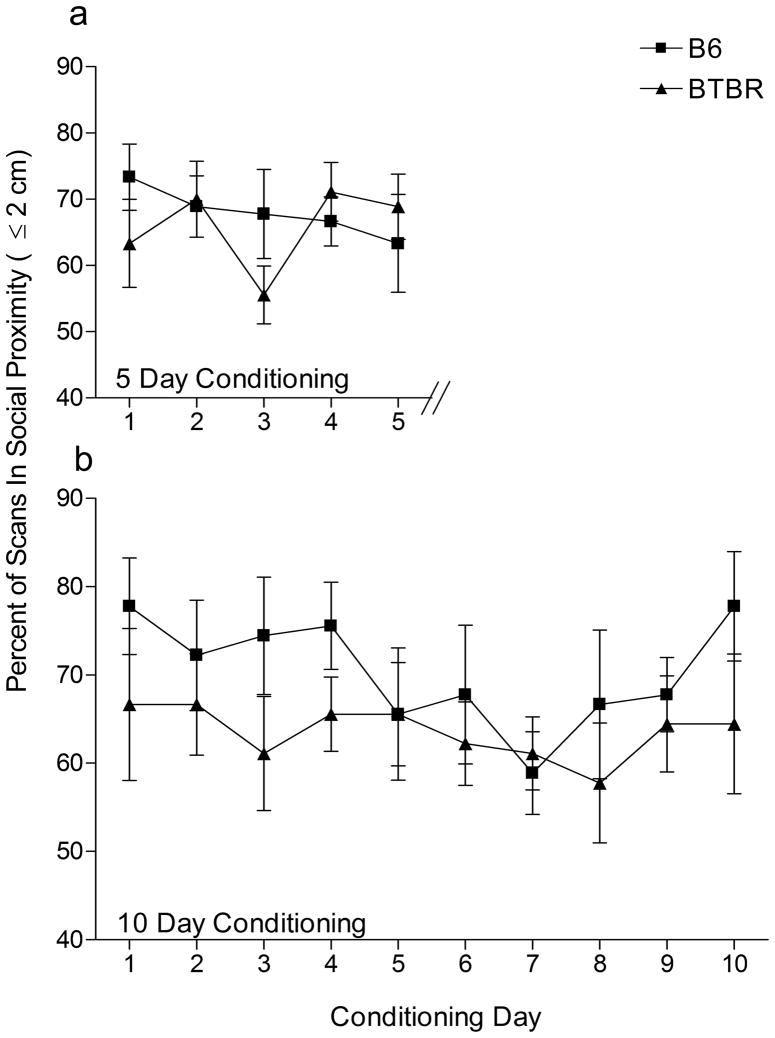
For mice conditioned for five days, there were no significant main effects of Strain or Day, or a Strain x Day interaction (F(1,72)= 0.212, p=0.651; F(4,72)= 0.880, p=0.481; F(4,72)=1.502, p=0.211, respectively). Similarly, there were no significant main effects of Strain or Day, or Strain x Day interaction for mice conditioned for ten days to social interactions (F(1,162)= 4.148, p=0.057; F(9,162)= 0.850, p=0.572; F(9,162)=, 0.391, p=0.938 respectively). These analyses indicate that although there was a slight decrease in the percent time BTBR mice were in proximity during conditioning, this effect was not statistically significant. BTBR mice showed similar proportions of time in proximity during conditioning and this precludes an explanation of a lack of opportunity for social learning due to social distance. Figure 3 presents mean percentages of scan observations of social proximity for five (a) and ten (b) day conditioned mice.
Figure 3.
Scan sampling for social proximity during social conditioning. For mice conditioned for five days (a), no significant strain difference was found in the mean proportion of scan samples in which mice were in proximity to stimuli mice across the five days. Similarly, no significant strain difference in social proximity was noted for mice conditioned for ten days (b). Values represent Mean ± 1 S.E.M. N=10/group.
3.1.3 Self-Grooming
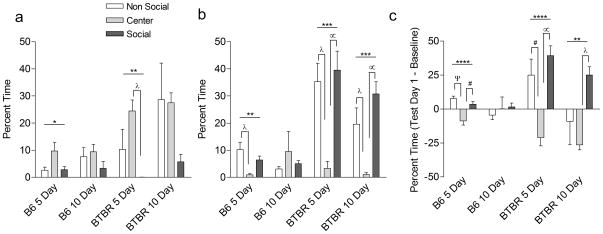
Table I lists the results of one-way ANOVA for mean percent time self-grooming in each of the three chambers at baseline, test day one as well as the difference (subtracted) analyses. Figure 4 presents the mean percent time self-grooming in each of the three chambers of the conditioning apparatus during the baseline session (a) as well as at the first day of testing (b) and the difference between the two (c), which represents the mean change in percent time grooming after social conditioning. Although significant differences in time spent grooming in each of the three chambers were noted, most of the variance contributing to the difference was between the outer two conditioning chambers and the center. If BTBR mice were avoidant of social interactions because of their pre-occupation with self-grooming, we would expect decreased percent time grooming in the social compartment after conditioning and this was not noted. In fact, BTBR mice spent as much time self-grooming in the social chamber as they did the non-social chamber (Figure 4b,c). This suggests that BTBR mice are not avoiding social contact or failing to be reinforced to the presence of a conspecific in order to groom themselves in a context associated with isolation.
Table I.
ANOVA comparisons of mean rates of grooming in each of the three chambers at baseline and testing, as well as the difference between baseline and testing.
| Session | Strain | Conditioning Days | One-Way ANOVA |
|---|---|---|---|
| Baseline | B6 | 5 Day | F(2,27)=3.974, p=0.031 |
| B6 | 10 Day | F(2,27)=1.183, p=0.322 | |
| BTBR | 5 Day | F(2,27)=6.292, p=0.005 | |
| BTBR | 10 Day | F(2,27)=2.470, p=0.103 | |
|
| |||
| Test One | B6 | 5 Day | F(2,27)=7.093, p=0.003 |
| B6 | 10 Day | F(2,27)=0.594, p=0.559 | |
| BTBR | 5 Day | F(2,27)=11.579, p=0.0002 | |
| BTBR | 10 Day | F(2,27)=11.768, p=0.0002 | |
|
| |||
| Subtracted | B6 | 5 Day | F(2,27)=13.958, p=0.00007 |
| B6 | 10 Day | F(2,27)=0.326, p=0.724 | |
| BTBR | 5 Day | F(2,27)=13.283, p=0.0001 | |
| BTBR | 10 Day | F(2,27)=5.895, p=0.008 | |
Figure 4.
Analyses of self-grooming before and after social conditioning. Mean percentages of time spent self-grooming during baseline (a), test day one (b), and the difference between baseline and test day as a measure in change in self-grooming preferences for mice before and after social conditioning. Values represent Mean ± 1 S.E.M. Horizontal lines denote significant main effects per condition: * p≤0.05, ** p≤0.01, *** p≤0.001, **** p≤0.0001. Bonferroni pairwise post-hoc comparisons: # p≤0.05, λ p≤0.01, ∝p≤0.001, ψ p≤0.0001.
4.1 Discussion
Utilizing the commonly-utilized three-chamber apparatus for sociability testing, we have validated a novel approach to analyze direct social motivation deficits by demonstrating an absence of place-preference elicited by an animate social incentive in BTBR mice- a strain known to exhibit face and construct validity for autism. Though our results revealed that the number of conditioning days did not contribute a statistically significant influence on the variance, the means (Figure 1b) strongly indicate that more days of conditioning elicits a stronger place preference, an effect noted previously [28].
Subject mouse behavior was assessed to determine if the amount of voluntary social contact during conditioning was related to differences in social conditioned place preference. We noted that both strains, independent of social phenotype, showed similar rates of social proximity during conditioning. Therefore, the absence of conditioned place preference to social interactions in BTBR mice cannot be solely explained by avoidance of social interactions during conditioning sessions.
Since BTBR mice groom themselves at abnormally high levels and at highly stereotyped patterns [19,24], we predicted that BTBR mice might avoid social stimuli in order to perform bouts of self-grooming, which is a time when mice cannot engage in high rates of vigilance. Our results demonstrate that BTBR mice spent a similar proportion of time grooming in the area associated with social interactions after conditioning compared to the non-social conditioned chamber. Even when eliminating the baseline distinctions in grooming preferences, BTBR mice were not preferentially grooming in the non-social compartment and avoiding self-grooming while inside of the social compartment. These results suggest that social deficits in BTBR mice are not secondary to avoidance of social stimuli in order to engage in self-grooming.
An inherent issue in learning-based assays of motivation is potential cognitive disturbances in the candidate model. BTBR mice have been shown to possess a deficit in footshock-based fear conditioning [16]. However, in the same study MacPherson et al. [16] demonstrated intact object recognition abilities in BTBR mice. Furthermore, Silverman et al. [27] noted that BTBR mice have a higher nociception threshold, suggesting that the aforementioned fear conditioning impairment may have something to do with sensory, rather than cognitive disparities. Newer work by Amodeo and colleagues [2] indicates that BTBR mice have impairments in reversal learning, an impairment that is highly relevant to restricted interests and insistence on sameness deficits in ASD. Acquisition and maintenance of a rewarded contingency are required for CPP, so the perseveration deficits noted in the BTBR mice [2] would presumably have little or no effect since the BTBR mice in the aforementioned study readily acquired the contingencies, but had impairments in switching them. Performance in social learning assays as well as the responses to non-social positive reinforcers (psychostimulants or sweetened solutions) should be examined to determine whether learning and/or memory deficits influence social conditioning performance in the BTBR strain.
An additional limitation is that one cannot rule out aggression and sex as unconditioned stimulus in these tasks since rodents will operantly respond to contexts associated with agonistic and sexual interactions [4,17,18]. However, despite the lack of formal assessment, observers noted no instances of sexual or aggressive interactions during the manually-scored conditioning sessions. Another consideration is whether subjects should be individually housed subjects prior to conditioning; this manipulation has been shown to increase social play in juvenile rats [29] and may increase the positive incentive value of the unconditioned stimulus [10]. Single housing of male subjects might cause increased drive for agonistic interactions. The study presented herein utilized juvenile, socially-housed mice; a design which may have effectively diminished aggressive or sexual motivating factors in the conditioned response. Revealing strain or genotype distinctions in social conditioned place preference without social isolation may be a preferable approach simply because the social CPP paradigm might represent learning resultant to the removal of a negative reinforcer rather than the introduction of a positive one. Other important parameters are whether the stimulus animals are siblings or unfamiliar, as well as their age and strain. Indeed, the current study included slight (~1–2 day), but consistent differences in the age of juvenile B6 and BTBR mice. Kennedy et al, [14] compared social interactions of 25- vs. 44-day old mice in three studies, finding an inconsistent picture, with differences between the two ages found in one study; no difference in another; and differences in females only, in a third. These data suggest that the much smaller age differences, from the same range, in the present study (38 vs. 43 days on average, at the end of the test period) should not be a factor in the finding of reduced social place conditioning for BTBR mice. Finally, conditioning to the social and non-social sides of the apparatus occurred successively, in counterbalanced order, within the same daily session. This procedure is uncommon in CPP studies as the reinforcing events, typically drug states, often have carry-over effects to the non-reinforced side. We opted to conduct these sessions in rapid succession to allow opportunities for immediate contrast between the two, visually different, conditioning chambers. Nevertheless, future research is needed to establish optimal parameters for social conditioned place preference testing in rodent models of ASD.
By utilizing place-preference conditioning testing, we have modified the standard three-chamber sociability test for the use as a social reinforcement assay. Our results strongly suggest that BTBR mice lack social motivation. We propose social conditioning might provide an alternative and/or complementary test, to social approach paradigms for social impairments in rodent models of ASD. This technique may aide in determining neurobiological system impairments and novel therapeutics in animal models of ASD and other forms of psychiatric illness in which social motivation is impaired.
Highlights.
The three-chamber arena was modified to use for conditioned place preference assessment
Juvenile conspecifics were utilized as unconditioned stimuli
C57BL/6J and BTBR T+tf/J mice were compared after five or ten days of conditioning
BTBR T+tf/J mice showed impaired social conditioned place preference
Acknowledgments
Funded by NIH MH081845 to R.J.B. Samantha O’Hanlon and Amy Vasconcellos assisted in data collection and analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association [APA] Diagnostic and Statistical Manual of Mental Disorders. 4. 2000. Diagnostic criteria for 299.00 Autistic Disorder. text revision ed. [Google Scholar]
- 2.Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;277(1):64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 4.Bell MR, Meerts SH, Sisk CL. Male Syrian hamsters demonstrate a conditioned place preference for sexual behavior and female chemosensory stimuli. Horm Behav. 2010;58(3):410–4. doi: 10.1016/j.yhbeh.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51(4):667–72. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 6.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends Cogn Sci. 2012;16(4):231–9. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17(4):448–59. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Devel Psychopathology. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- 9.Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. 2002;73:700–17. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45(3):153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 11.Evans MJ, Duvel A, Funk ML, Lehman B, Sparrow J, Watson NT, Neuringer A. Social reinforcement of operant behavior in rats: a methodological note. J Exp Anal Behav. 1994;62:149–56. doi: 10.1901/jeab.1994.62-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritz M, El Rawas R, Salti A, Klement S, Mardo MT, Kemmler G, et al. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol. 2011;16(2):273–84. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- 13.Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy BC, Panksepp JB, Wong JC, Krause EJ, Lahvis GP. Age-dependent and strain-dependent influences of morphine on mouse social investigation behavior. Behav Pharmacol. 2011;22(2):147–59. doi: 10.1097/FBP.0b013e328343d7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kummer K, Klement S, Eggart V, Mayr MJ, Saria A, Zernig G. Conditioned place preference for social interaction in rats: contribution of sensory components. Front Behav Neurosci. 2011;5:1–5. doi: 10.3389/fnbeh.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacPherson P, McGaffigan R, Wahlsten D, Nguyen PV. Impaired fear memory, altered object memory and modified hippocampal synaptic plasticity in split-brain mice. Brain Res. 2008;1210:179–88. doi: 10.1016/j.brainres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Martínez M, Guillén-Salazar F, Salvador A, Simón VM. Successful intermale aggression and conditioned place preference in mice. Physiol Behav. 1995;58(2):323–8. doi: 10.1016/0031-9384(95)00061-m. [DOI] [PubMed] [Google Scholar]
- 18.Mathews JT, Abdelbaky P, Pfaff D. Social and sexual motivation in the mouse. Behav Neurosci. 2005;119(6):1628–39. doi: 10.1037/0735-7044.119.6.1628. [DOI] [PubMed] [Google Scholar]
- 19.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7(2):152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 20.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for rapid quantitation of autism-like social deficits in mice. Genes Brain Behav. 2004;3:303–14. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 21.Pacey LKK, Doss L, Cifelli C, van der Kooy D, Heximer SP, Hampson DR. Genetic deletion of regulator of G-protein signaling 4 (RGS4) rescues a subset of fragile X phenotypes in the FMR1 knockout mouse. Mol Cell Neurosci. 2011;46:563–72. doi: 10.1016/j.mcn.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in juvenile mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6(7):661–71. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pearson BL, Pobbe RLH, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. 2011 Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–35. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverman JL, Yang M, Lord C, Crawley JN. Behavioral phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11(7):490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity ad neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171(4):1197–208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thiel KJ, Okun AC, Niesewander JL. Social reward –conditioned place preference: a model revealing an interaction between cocaine and social context reward in rats. Drug Alcohol Depend. 2008;96(3):202–12. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trezza V, Damsteegt R, Vanderschuren LJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol. 2009;19(9):659–69. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waterhouse L, Fein D, Modahl C. Neurofunctional mechanisms in autism. Psychol Rev. 1996;103:457–489. doi: 10.1037/0033-295x.103.3.457. [DOI] [PubMed] [Google Scholar]
- 31.Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, Silverman JL, Crawley JN. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. doi: 10.1016/j.physbeh.2011.12.025. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29(8):1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]