Abstract
Background & Aims
Gallbladder carcinoma (GBCa), a type of biliary tract cancer (BTC), has proven challenging to treat demonstrating the need for more effective therapeutic strategies. In our current study, we examined the therapeutic effects of the histone deacetylase (HDAC) inhibitor PCI-24781 against GBCa that developed in BK5.erbB2 mice.
Methods
PCI-24781 [50 mg/kg/day] and control solution were delivered to BK5.erbB2 mice for four weeks. The therapeutic effect of PCI-24781 was evaluated via ultrasound biomicroscopy (USBM) throughout the experiment and histological analyses at the end of the experiment. To investigate potential mechanisms for the therapeutic effects of PCI-24781 on GBCa in BK5.erbB2 mice, PCI-24781-treated gallbladders were subjected to Western blot and RT-PCR analysis. The inhibitory effect of PCI-24781 on the growth of BTC cells was compared to the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) and gemcitabine. To study the role of miRNAs in GBCa tumorigenesis, the expression profile of 368 miRNAs in GBCas from BK5.erbB2 (both treated and untreated) and wild-type mice was analyzed.
Results
Treatment of BK5.erbB2 mice with PCI-24781 for one month prevented 79% of GBCa cases from progression and showed a clinical effect in 47% of cases. We also confirmed a potent inhibitory effect on tumor cell growth in human BTC cell lines treated with PCI-24781. This effect was associated with down-regulation of erbB2 mRNA and erbB2 protein/activity and up-regulation of acetylated histone and acetylated tubulin. Treatment with PCI-24781 resulted in decreased expression of Muc4, an intramembrane ligand for erbB2, in BTC cells. PCI-24781 had more effect on inhibiting growth of BTC cells than SAHA. In addition, PCI-24781 effectively inhibited the growth of gemcitabine-resistant cells. MiRNA profiling revealed that the expression of several miRNAs were significantly altered in GBCa in the BK5.erbB2 mouse compared to normal gallbladder, including up-regulated miR21, which was down-regulated by PCI-24781.
Conclusion
These results indicate that PCI-24781 potently inhibits the growth of BTC cells by decreasing erbB2 expression and activity as well as regulating altered miRNA expression. PCI-24781 may have potential value as a novel chemotherapeutic agent against human BTC in which erbB2 is overexpressed.
Introduction
GBCa has proven challenging to treat due to both it’s poor sensitivity to conventional therapies and its inability to be detected at an early stage. These difficulties lead to a poor overall prognosis [1] and demonstrate the need for better therapeutic modalities. Overexpression of erbB2 has been reported in a significant percentage of human BTC and believed to be one of the major mechanisms underlying BTC carcinogenesis [1–5]. The established frequency of erbB2 overexpression in all BTCs ranges from 6.2 to 65.0% [1, 4, 5], which may be due to differences in experimental method, the use of different antibodies for immunostaining, or the different criteria used for evaluation. Other evidence for the involvement of erbB2 in the development of BTC malignancies comes from chemically-induced animal models using furan [6] and transfected cell model [2].
We previously reported on the development of GBCa in transgenic mice where expression of a rat erbB2 cDNA is targeted to the basal layer of epithelial tissues by the bovine keratin 5 (BK5) promoter (BK5.erbB2 mice) [7]. GBCa develops in approximately 80% of these transgenic mice by 2 months of age. Similarities between GBCa in BK5.erbB2 mice and humans include histopathological and molecular characteristics [7, 8].
HDACs contribute to the regulation of a limited number of genes involved in cell growth, differentiation, and survival [9]. It has been hypothesized that aberrant patterns of histone acetylation maintain the transformed state of human tumors, a state that can be reversed by inhibiting these HDACs. Changes in expression of HDACs have been reported in various cancers [9].
More than ten different HDAC inhibitors, including a broad-spectrum phenylhydroxamic acid HDAC, PCI-24781 (Pharmacyclics, Sunnyvale, CA), are currently undergoing clinical trials [10].
In this study, we examined the therapeutic effects of PCI-24781 against GBCa that develops in BK5.erbB2 mice. To elucidate the mechanism of these inhibitory effects, the altered expressions and activities of erbB2, its downstream signaling molecules, Muc4, and the expression profile of miRNAs were also determined after treatment.
Materials and Methods
Human BTC Cells and Chemicals
Human BTC cell lines TGBC and Sk-ChA-1 [11] were provided by Dr. Takeshi_Todoroki (Tsukuba University, Ibaraki, Japan). PCI-24781 was provided by Pharmacyclics, Inc. (Sunnyvale, CA).
Animals and Treatment Protocols
BK5.erbB2 mice are maintained in the University of Texas M. D. Anderson Cancer Center, Science Park – Research Division. Twenty-eight candidate BK5.erbB2 mice were screened for the presence of GBCas via USBM (Visual Sonics, Toronto, Canada) at 2 months of age in order to ensure that each group had an initial GBCa incidence of 100%. PCI-24781 [50 mg/kg/0.2 ml/day, i.p. injection twice a day], dissolved in 50 mM sodium lactate buffer (pH 4.2), was delivered to 19 BK5.erbB2 mice five consecutive days per week for four weeks. The same solution without PCI-24781 was delivered to 9 BK5.erbB2 mice as control. The animals were monitored twice weekly to evaluate systemic toxicity and body weight. The sizes of tumors in the mice were monitored via USBM every two-weeks. Feeding was stopped 24 hours prior to USBM analysis for gallbladder volume maximization. The determination of the tumor size via USBM was performed as a blinded experiment. All experiments were carried out with strict adherence to institutional guidelines for minimizing distress in animals.
Histological Analysis
After being fixed in formalin and embedded in paraffin, 5 µm serial sections (28 µm between each section) of biliary tract tissue were cut and stained with H&E. Utilization of BrdU as a proliferation marker was performed as previously described [8].
Immunofluorescence Staining
The expression and localization of erbB2, phosphorylated erbB2 (p-erbB2), EGFR, and p-EGFR were determined using immunofluorescence on sections of gallbladders as described previously [7]. Sections were analyzed using a laser confocal microscope (Zeiss 510 Meta).
Cell Growth
Growth of human BTC cells treated with PCI-24781, SAHA, or gemcitabine was determined via the Cell Proliferation Assay Kit (Promega, Madison, WI) according to the manufacturer’s protocol. Each experiment was performed at least three to four times.
Western Blot Analysis
Epithelial cell lysates were prepared from three pooled mouse gallbladders, as described previously [12]. Anti-Muc4 antibody was kindly provided by Dr. Surinder Batra (University of Nebraska). All experiments were done in triplicate and experiments were repeated at least twice.
Evaluation of Therapeutic Efficacy of PCI-24781
The final diagnosis of GBCa tumors was based on images generated through USBM and histological analyses. The criteria for evaluation of therapeutic effects are as follows: Complete Response (CR): complete disappearance of a previous tumor; Partial Response (PR): >30% reduction of all the measurable lesions in the tumor; Minimum Change (MC): <30% change in the measurable size of the tumor compared with the original tumor; Progressive Disease (PG): >30% increase in the size of all measurable lesions in the original tumor or the appearance of new lesion(s).
Detection of Levels of mRNA by Real Time PCR
erbB2 mRNA transcript levels were assessed as previously described [13].
Primary Culture of Gallbladder Epithelial Cells from BK5.erbB2 and Wild-type Mice
The long-term culture of gallbladder epithelial cells from both BK5.erbB2 and wild-type mice were prepared as previously described [14].
Micro RNA Microarray
To compare the expression of miRNA in GBCa to that in control gallbladders, RNA from 8 GBCas from BK5.erbB2 mice and 30 gallbladders from wild-type mice were isolated. In addition, to study the effect of PCI-24781 on the expression of miRNAs in GBCa, the expression of miRNAs in GBCas treated with PCI-24781 was compared to that in untreated GBCas. A microarray was performed and the expression profiles of 368 miRNAs were analyzed at the University of Texas MD Anderson Cancer Center, Science Park Molecular Biology Core. The ratios of miRNA were the average value of three independent experiments.
Detection of Plasma Concentration of PCI-24781 in BK5.erbB2 Mice
The plasma concentration of PCI-24781 in BK5.erbB2 mice was measured by administering PCI-24781 (50 mg/kg/day in 50 mM sodium lactate buffer, pH 4.2) i.p. to these mice twice daily for 2 weeks. Blood samples were collected 4 hours after the last injection and plasma was assayed for PCI-24781 by liquid chromatography with tandem mass spectrometry as previously described [10].
Statistical Analysis
All of the data are expressed as the mean ± SD. Statistical significance was determined by the Mann-Whitney U-test and the Fisher’s exact test. p < 0.05 was considered to be significant.
Results
Therapeutic Efficacy of PCI-24781 on GBCa in BK5.erbB2 Mice
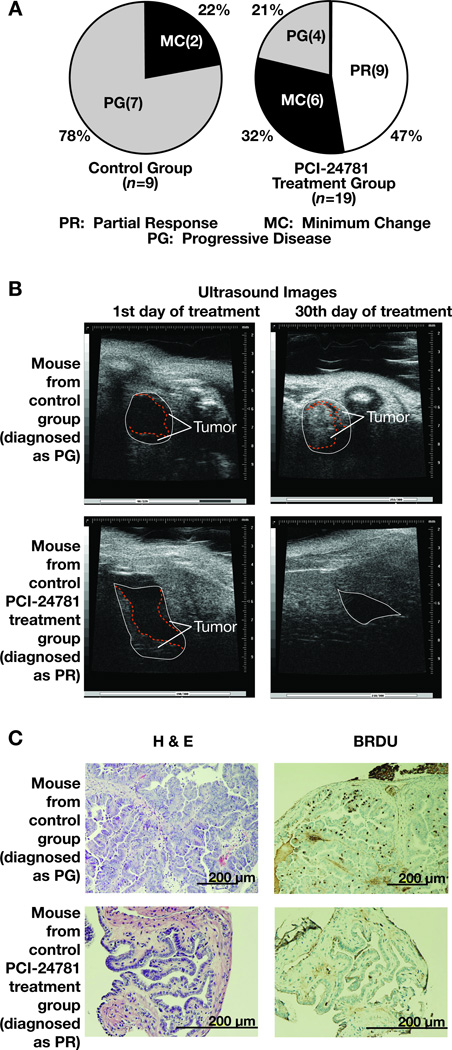
All mice were screened for GBCa via USBM at the start of treatment. Serial sections stained with H&E were analyzed to verify the therapeutic evaluation determined by USBM. Of the control group (n=9), seven mice (78%) were diagnosed as Progressive Disease (PG) and only two cases (22%) were diagnosed as Minimum Change (MC) (Fig. 1A left). While no diagnosis of Complete Response (CR) was made in the treated group, nearly half (47%) of the treated mice were diagnosed as Partial Response (PR). Six cases (32%) from this group were diagnosed as MC and only four cases (21%) were diagnosed as PG (Fig. 1A right). Thus, nearly 80% of treated mice showed either therapeutic efficacy (PR) or prevention from progression (MC). These effects are statistically significant compared with the control group. None of the treated BK5.erbB2 mice showed any signs of toxicity, neurogenic abnormalities or significant difference in body weight gain.
Figure 1. Therapeutic effects of PCI-24781 on GBCa in BK5.erbB2 mice.
A, Therapeutic evaluation of PCI-24781 on GBCa in BK5.erbB2 mice. PR: Partial Response, MC: Minimum Change, and PG: Progressive Disease;
B, Therapeutic effects determined by ultrasound analysis.
Representative ultrasound images of GBCa diagnosed as PG from a non-treated BK5.erbB2 mouse (upper panels) and GBCa diagnosed as PR receiving PCI-24781 (lower panels).
C, Therapeutic effect determined by histological analysis.
Upper panels, GBCa diagnosed as PG from a non-treated BK5.erbB2 mouse; Lower panels, typical GBCa diagnosed as PG from a BK5.erbB2 mouse receiving PCI-24781. Left, H&E staining; Right, BrdU staining.
To examine the level of PCI-24781 in the serum, blood samples were collected 4 hours after the last treatment (50 mg/kg/day, i.p. injection twice a day for 2 weeks). The concentration of PCI-24781 in the plasma of BK5.erbB2 mice was 3.2 ± 0.6 nM (Suppl 1). In a previous study, this plasma concentration was sufficient to inhibit growth of xenografted tumors [10]. Therefore, the in vivo effects noted in BK5.erbB2 mice are likely attributable to the therapeutic effects of PCI.
Typical USBM images shown in Figure 1B (upper panel) revealed that the gallbladder epithelial tumors in the untreated BK5.erbB2 mice dramatically progressed by the end of the experiment, leading to complete luminal obstruction with tumor cells.
Typical images of a gallbladder diagnosed as PR from a mouse treated with PCI-24781 in Figure 1B (lower panel) reveal that the tumors significantly diminished after four weeks of treatment.
Histological Examination of Gallbladders from BK5.erbB2 Mice Treated with PCI-24781
H&E (Fig. 1C, left) and BrdU (Fig. 1C, right) staining demonstrated that the gallbladder lumen in BK5.erbB2 mice was completely covered by well-differentiated adenocarcinoma with a high number of BrdU-positive (proliferating) cells. The lower panel of Figure 1C shows H&E and BrdU staining of a gallbladder from a treated mouse diagnosed as PR, shown in Figure 1B. A majority of the epithelial cells developed minor nuclear atypia and formed a monolayer. The ratio of BrdU-positive cells in treated gallbladder was significantly lower than that in the untreated gallbladder (0.7 ± 0.2% and 4.2 ± 0.5, respectively). Interestingly, thickened stromal tissue was also observed in cases diagnosed as PR (Fig. 1C, left lower). This may indicate the reactivation of regenerative processes after PCI-24781-mediated regression of tumor cells.
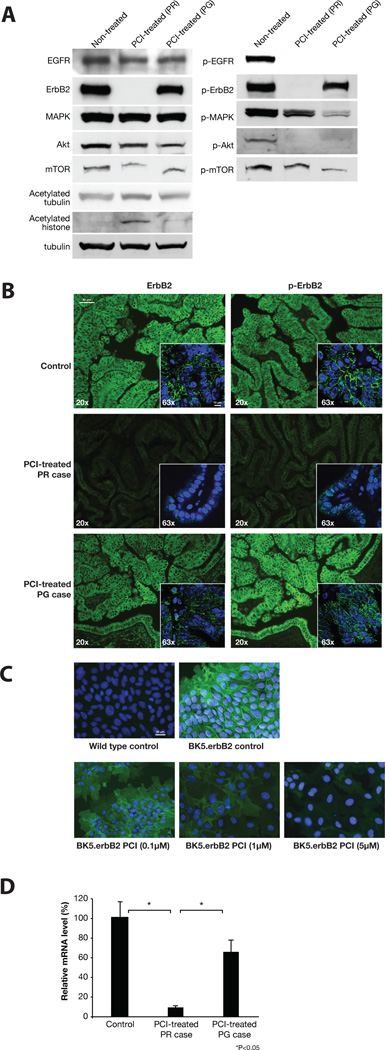
Significant Reduction of Total and Phosphorylated Levels of ErbB2 in Gallbladders of PCI-24781-Treated BK5.erbB2 Mice
Four gallbladders diagnosed as PR and PG in treated BK5.erbB2 mice, and gallbladders diagnosed as PG in non-treated BK5.erbB2 mice were pooled into groups. In gallbladders diagnosed PR, the levels of total erbB2, p-erbB2, p-EGFR (not total EGFR), and the phosphorylated forms of their downstream signaling molecules such as p-MAPK, p-Akt, and p-mTOR were significantly decreased compared to those of gallbladders from non-treated BK5.erbB2 mice (Fig. 2A). In gallbladders diagnosed as PG, levels of downstream signaling molecules were decreased to a similar level as the PR cases, however, the degree of reduction in levels of total erbB2 and p-erbB2 were not remarkable compared to those found in PR cases (Fig. 2A). Relatively high levels of acetylated tubulin and acetylated histone, markers of HDAC inhibitor activity, were observed only in PR cases (Fig. 2A).
Figure 2. Effect of PCI-24781 on the level of erbB2.
A, Western blot analysis of the levels of erbB2/EGFR and their downstream molecules in gallbladder from non-treated BK5.erbB2 mice (left); treated gallbladders diagnosed as PR (middle); treated gallbladders diagnosed as PG (right). B, ErbB2 and p-erbB2 expression in gallbladder from non-treated BK5.erbB2 mice (upper); treated gallbladders diagnosed as PR (middle); treated gallbladders diagnosed as PG (lower). (scale bar, 40µm); C, Effect of PCI-24781 on the level of erbB2 in primary cultured BK5.erbB2 gallbladder cells treated with PCI-24781.(scale bar, 20µm); D, Relative erbB2 mRNA levels in gallbladders treated with PCI-24781. Gallbladders from non-treated BK5.erbB2 mice (left); treated gallbladders diagnosed as PR (middle); treated gallbladders diagnosed as PG (right).
Down-regulation of both erbB2 and p-erbB2 in treated PR cases was confirmed by immunofluorescence analysis (Fig. 2B, middle panels). In PR cases, the levels of membrane-bound total and p-erbB2 were significantly reduced. In both non-treated (Fig. 2B, upper panels) and treated PG cases (Fig. 2B, lower panels), high amounts of membrane-bound total and p-erbB2 were observed. In addition, to confirm the ability of PCI-24781 to decrease the erbB2 level in primary cultured gallbladder epithelial cells, cells from both BK5.erbB2 and wild-type mice were exposed to 0.1, 1, and 5 µM of PCI-24781 for 18 hours. ErbB2 expression was significantly higher in cells from BK5.erbB2 mice than that found in wild-type mice (Fig. 2C), and that a majority of erbB2 protein was localized in the cytoplasmic membrane. Treatment with PCI-24781 resulted in dose-dependent decreased expression of erbB2 and reduction in cell number in cells from BK5.erbB2 mice (Fig. 2C).
PCI-24781 Strongly Decreased ErbB2 mRNA Levels in Gallbladders of BK5.erbB2 Mice
Three gallbladders from each group (non-treated BK5.erbB2 diagnosed as PG, treated BK5.erbB2 diagnosed as PR, and treated BK5.erbB2 diagnosed as PG) were pooled and erbB2 mRNA levels were determined by quantitative RT-PCR. Compared to untreated mice, the level of rat-transgene erbB2 mRNA was markedly decreased in PR cases of treated mice and moderately decreased in PG cases of treated mice (Fig. 2D). Relative percentages of mRNA levels in PR and PG cases were 9.5% and 66%, respectively, compared to those of untreated mice (100%). We confirmed that this effect was not due to inhibition of the BK5 promoter by PCI-24781 by using an additional BK5 promoter-driven mouse model (data not shown). These results indicate that erbB2 mRNA levels were associated with the therapeutic outcome of PCI-24781 treatment. Levels of endogenous mouse erbB2 were significantly low (less than 1 × 10–5 % of rat mRNA levels) in the gallbladder of BK5.erbB2 mice.
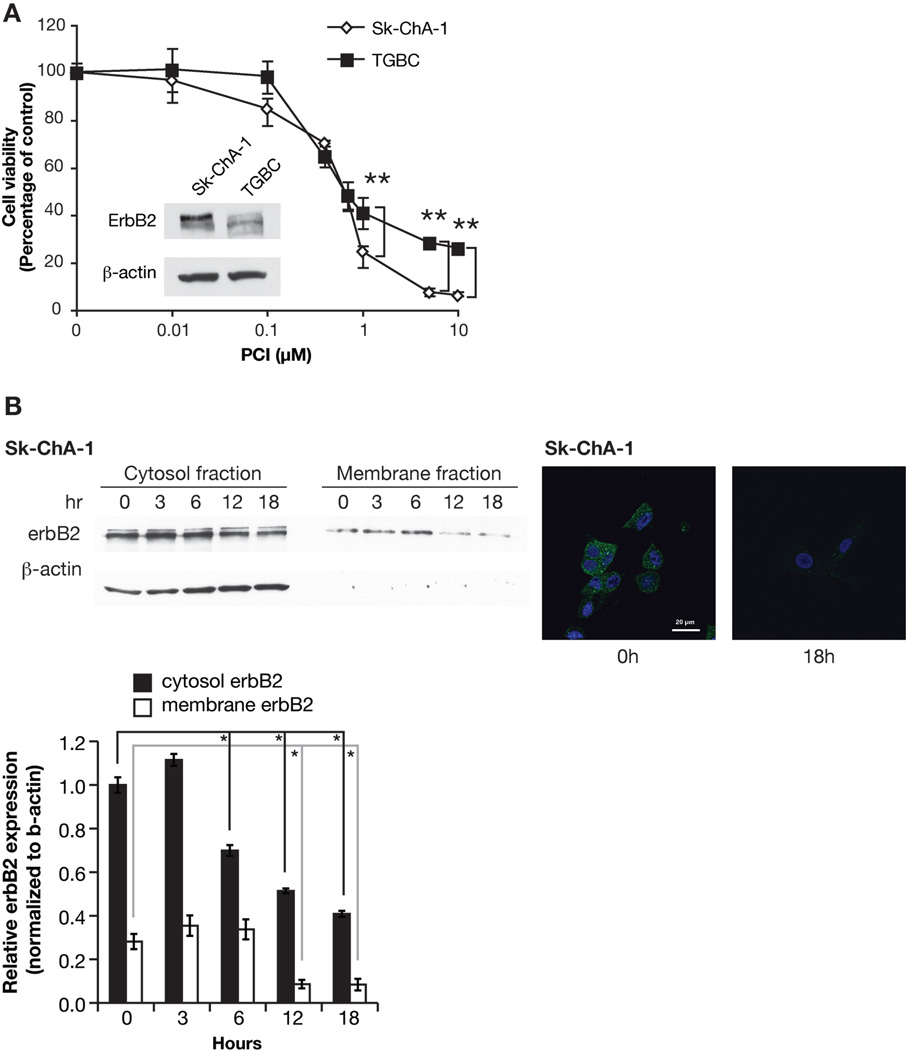
Inhibitory Effect of PCI-24781 on the Growth of Human BTC Cells
We also examined the effect of PCI-24781 on growth of two human BTC cell lines, Sk-ChA-1 and TGBC. PCI-24781 treatment at concentrations from 0.1 – 10 µM resulted in growth inhibition in both cell lines (Fig. 3A). Sk-ChA-1 cells, which have significantly higher erbB2 levels than TGBC cells (Fig. 3A), were more susceptible to PCI-24781 at a concentration of 1 µM or higher. The IC50 values of Sk-ChA-1 and TGBC cells were 0.85 and 1.25 µM, respectively. This inhibition was shown to be associated with dose-dependent induction of apoptosis via TUNEL assay (data not shown).
Figure 3. Inhibitory effect of PCI-24781 on the growth of cells from the human BTC cell lines Sk-ChA1 and TGBC.
A, Cell viability (measured as percentage of control) determined by MTT assay. The Western blot insert shows the level of erbB2 in Sk-ChA-1 and TGBC cells. **, p<0.01 ; B, Time course changes of erbB2 level in cytosol and membrane fraction of Sk-ChA-1 cells treated with PCI-24781 (left upper); Immunostaining of erbB2 in Sk-ChA-1 cells 18 hours after the treatment (right); Changes in ratios of total erbB2 in the cytosolic fraction (black columns) versus membrane fraction (white columns) normalized to the levels of b-actin at 0 hours in Sk-ChA-1 cells (left lower). *, p<0.05
Localization of erbB2 After Treatment with PCI-24781 in Human BTC Cells
Figure 3B (left panel) shows the change of erbB2 level in cytoplasmic and membrane fractions in Sk-ChA-1 cells treated with 0.5 µM PCI-24781 for 0 – 18 hours as determined by Western blot analysis (Fig. 3B, upper left panel) and densitimetric analysis (Fig. 3B, lower panel). After 12 hours of treatment, the level of total erbB2 protein in both fractions started to decrease and continued decreasing after 18 hours of treatment. This reduction of erbB2 was confirmed by immunohistochemical analysis in the cells treated for 18 hours (Fig. 3B, upper right panel).
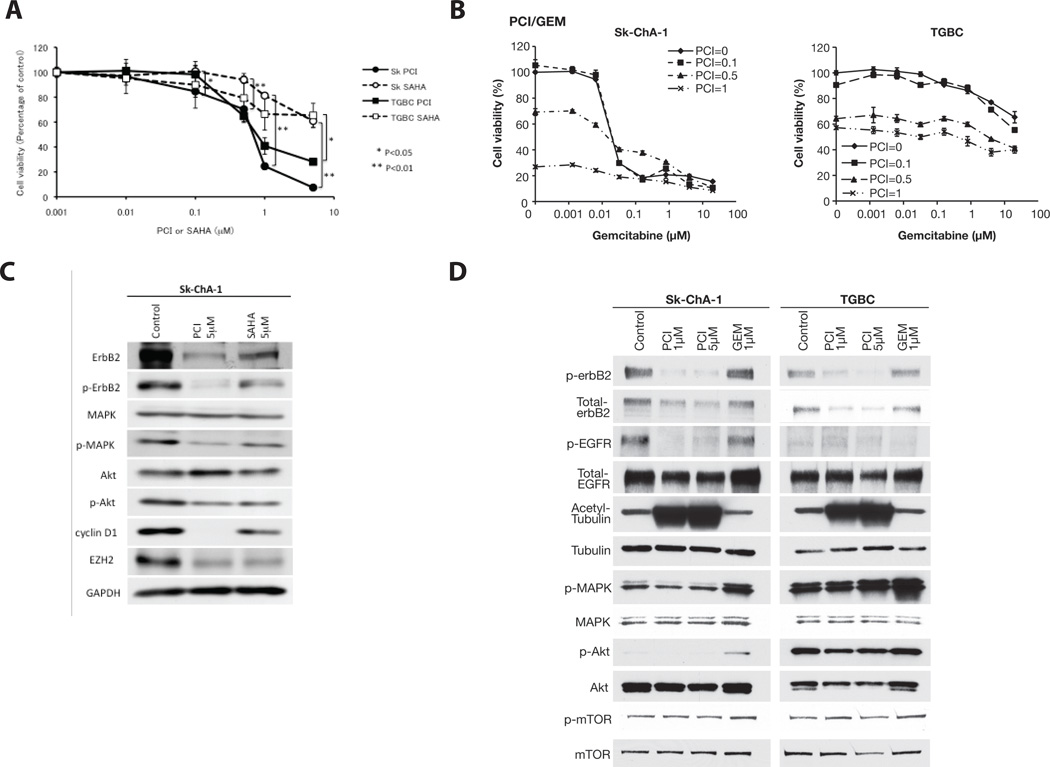
Comparison of the Inhibitory Effect of PCI-24781 to SAHA on the Growth of Human BTC Cells
To compare the inhibitory effect of PCI-24781 on human BTC cells to that of the HDAC inhibitor SAHA, Sk-ChA-1 and TGBC cells were exposed to various concentrations of SAHA for 72 hours. At a concentration of 0.5 µM and higher, PCI-24781 showed a more potent inhibitory effect on the viability of both Sk-ChA-1 and TGBC cells than that of SAHA (p<0.05) (Fig. 4A).
Figure 4. Comparison of inhibitory effect of SAHA and gemcitabine to PCI-24781 on the cell viability of human BTC cells (A and B) and Western blot analysis (C and D).
Cells were treated with PCI-24781, SAHA, and gemcitabine for 72, and 18 hours for the cell viability and Western blot analysis, respectively. Line graphs show cell viability (cell number of non-treated cells =100%) at various doses of PCI-24781, SAHA, and gemcitabine 72 hours after the treatment.
Comparison of the Inhibitory Effect of PCI-24781 to Gemcitabine on the Growth of Human BTC Cells
We compared the inhibitory effects of PCI-24781 to gemcitabine, a first line drug for the treatment of human BTC [15]. Sk-ChA-1 and TGBC cells were exposed to various concentrations of gemcitabine. Growth of Sk-ChA-1 cells was significantly inhibited with 0.05 µM gemcitabine alone, although gemcitabine treatment at 0.1 µM and above resulted in the same degree of inhibition (Fig. 4B). On the contrary, TGBC cells were resistant to treatment with gemcitabine alone, with 67% of cells viable after treatment at a concentration of 20 µM (Fig. 4B). No statistical significance was found in the additive effects by two compounds (data not shown).
Status of erbB2, EGFR, and their Downstream Signaling Molecules in Human BTC Cells Treated with PCI-24781, SAHA, and Gemcitabine
Although SAHA induced significant down-regulation of total erbB2 and p-erbB2, the PCI-24781 showed a more potent inhibitory effect (Fig. 4C). Treatment of PCI-24781 and SAHA in Sk-ChA-1 cells resulted in a reduction of the phosphorylated levels of MAPK and Akt, but not total protein levels (Fig. 4C). These results indicate that both PCI-24781 and SAHA have potent inhibitory effects on erbB2 and its downstream signaling molecules. In addition, both PCI-24781-and SAHA-treated Sk-ChA-1 cells showed down-regulation of the polycomb group protein EZH2, which is known to interact with HDACs [16] (Fig. 4C). PCI-24781 showed a more potent inhibitory effect on the level of cyclin D1 than SAHA (Fig. 4C).
Figure 4D also shows that treatment with PCI-24781 (at both 1 µM and 5 µM, for 18 hours) significantly reduced the levels of p-erbB2, total erbB2, p-EGFR, p-MAPK, p-Akt (confirmed by a long exposure, data not shown), and total Akt in both SK-ChA-1 and TGBC cell lines. These reductions were associated with a dose-dependent upregulation of the acetylated tubulin level.
The effect of gemcitabine on the signaling molecules was also determined. Treatment of cells from the two cell lines with 1 µM gemcitabine for 18 hours did not induce any changes in levels of p-erbB2, total erbB2, p-EGFR or total EGFR (Fig. 4D). However, treatment led to an up-regulation of p-MAPK in both cell lines. As expected, there was no accumulation of acetylated tubulin in either cell line treated with gemcitabine.
PCI-24781 Down-regulates the Expression of Muc4 in Human BTC Cells
Furthermore, we investigated whether PCI-24781 alters the expression of Muc4 mucin in human BTC cells. The high expression level of Muc4 in both cell lines was significantly decreased after 72 hours of treatment with 0.5 µM PCI-24781 (Suppl. 2).
MiRNAs profiling in GBCa
Nine miRNAs were significantly up-regulated and thirteen miRNAs were down-regulated in GBCas in BK5.erbB2 mice (>2.2 fold, p<0.05) compared to gallbladders in untreated mice (Table 1A). Several miRNAs were significantly deregulated in GBCa compared to normal gallbladder. Treatment with PCI-24781 significantly decreased the expression of some of these miRNAs, including miR-21, miR-142-3p, miR-142-5p, and miR-223, which were up-regulated in GBCas (Table 1A and B). PCI-24781 also induced a significant up-regulation in the expression of miR-122, which was down-regulated in GBCas (Table 1A and B).
Table 1.
Hold change in the A) GBCas from BK5.erbB2 mice vs. GBs from wild-type mice (> 2.2 hold), and B) BK5.erbB2 GBCas treated with PCI-24781 vs. untreated GBCas (> 2.2 hold).
| A. Hold change in the GBCas from BK5. erbB2 mice vs. GBs from wild-type mice (>2.2 hold) | |||
|---|---|---|---|
| Down-regulated | Up-regulated | ||
| miR-665 | −8.233 | miR-106a | 2.414 |
| miR-714 | −3.882 | miR-96 | 2.499 |
| miR-763 | −2.880 | miR-223 | 2.612 |
| miR-466f-3p | −2.750 | miR-27a | 2.627 |
| miR-145 | −2.612 | miR-17 | 2.700 |
| miR-193 | −2.588 | miR-15b | 2.793 |
| miR-467e | −2.511 | miR-142-5p | 2.914 |
| miR-143 | −2.337 | miR-142-3p | 3.582 |
| miR-881 | −2.323 | miR-21 | 5.361 |
| miR-720 | −2.311 | ||
| miR-706 | −2.303 | ||
| miR-122 | −2.255 | ||
| miR-378 | −2.241 | ||
| B. Hold change in the GBCas treated with PCI-24781 vs. GBCas untreated from BK5. erbB2 mice (>2.2 hold) | |||
|---|---|---|---|
| Down-regulated | Up-regulated | ||
| miR-223 | −8.642 ± 2.539 | mmu-miR-193 | 2.273 ± 0.214 |
| mmu-miR-21 | −4.509 ± 0.554 | mmu-miR-101b | 2.348 ± 0.221 |
| mmu-miR-142-3p | −4.360 ± 0.920 | hsa-miR-576-3p | 2.600 ± 0.200 |
| mmu-miR-142-5p | −4.417 ± 0.975 | mmu-miR-451 | 2.652 ± 0.251 |
| mmu-miR-205 | −3.702 ± 1.195 | hsa-miR-620 | 3.335 ± 0.315 |
| mmu-miR-141 | −2.189 ± 0.208 | hsa-miR-122 | 11.141 ± 1.031 |
| hsa-miRPlus-B1114 | −2.219 ± 0.121 | mmu-miR-122 | 29.204 ± 14.189 |
Discussion
In the current study, we show that the novel HDAC inhibitor PCI-24781 displayed potent therapeutic efficacy against GBCa that developed in BK5.erbB2 mice. The treatment of these mice for one month prevented 79% of GBCa cases from progression (Fig. 1). We confirmed this potent inhibitory effect on tumor cell growth in vitro using human BTC cell lines treated with PCI-24781.
Western blot analyses suggested that down-regulation of total erbB2 and perbB2 is the critical molecular element mediating the inhibitory effect of PCI-24781 (Fig. 2). An in vivo therapeutic study in BK5.erbB2 mice showed that GBCas sensitive to PCI-24781 treatment (diagnosed as PR) had greatly reduced levels of p-erbB2 and total erbB2 compared to GBCas refractory to treatment (diagnosed as PG). These results indicate that the inhibitory effect of PCI-24781 was not potent enough to reduce the levels of p-erbB2 and total erbB2 in the PG cases. This hypothesis is supported by results showing that the level of erbB2 mRNA was significantly decreased in gallbladders diagnosed as PR, but not in those diagnosed as PG (Fig. 2).
We also observed molecular alterations associated with the inhibition of cell growth, such as decreased levels of p-erbB2, erbB2, and their downstream signaling molecules, in BTC cell lines treated with PCI-24781 (Fig. 4). Other studies support this finding, showing that there is a strong correlation between the growth inhibition of HDAC inhibitors and reduction in the levels of erbB2 transcripts and proteins [17, 18].
Furthermore, we have shown that PCI-24781 induces the down-regulation of Muc4 expression in human BTC cells (Suppl. 2). Muc4 is known to act as an intramembrane ligand for erbB2, inducing its limited phosphorylation via one of the EGF domains [19, 20]. It has been reported that the inhibition of Muc4 leads to a decrease in the expression of erbB2 and p-erbB2 in pancreatic cancer cells and that inhibition of histone deacetylase was associated with Muc4 repression [21]. Since our results showed that PCI-24781 has the ability to down-regulate both erbB2 and Muc4 expression in human BTC cells, PCI-24781 treatment seems to be a valuable modality for the treatment of BTC.
Protein levels of cyclinD1 and EZH2 were down-regulated in Sk-ChA-1 cells following PCI-24781 treatment (Fig. 4). EZH2 is a transcriptional repressor over-expressed in GBCa but not in normal epithelium [22] that is involved in controlling cell growth and tumor progression [23]. Our results indicate that PCI-24781 may regulate not only erbB2 levels and activity, but also the cell cycle and histone methyltransferases that contribute to the epigenetic silencing of target genes in cancer cells.
There are conflicting reports regarding the survival benefit of additional chemotherapy for BTCs [24]. 45.4% of chemotherapy regimens over the past ten years have included gemcitabine [25]. In this study, PCI-24781 was found to have a potent inhibitory effect on cells that showed resistance to gemcitabine (Fig. 4). This result indicates that PCI-24781 has a potential therapeutic value in patients with gemcitabine-resistant BTC.
In the current study, PCI-24781 was found to possess a more potent inhibitory effect on the growth of both Sk-ChA-1 and TGBC cells than SAHA. Although SAHA is one of the most clinically applied HDAC inhibitors for the treatment of multiple myeloma [26], treatment with PCI-24781 might be a promising approach against GBCa.
MiRNAs are a class of small non-coding RNA genes that regulate gene expression during development and differentiation, although their targets are still elusive. MiRNAs were recently found to be abnormally expressed in several types of cancer cells [27]. Our results indicate that aberrant histone deacetylation may induce altered expression of several miRNAs, including up-regulation of miR-21, miR-142-3p, miR-142-5p, and miR-223, as well as down-regulation of miR-122 in GBCas in BK5.erbB2 mice (Table 1). Since miR-21 has been characterized as an antiapoptotic miRNA and is up-regulated in many cancer types including cholangiocarcinoma cells [28], miR-21 may also play an important role in development of GBCa in BK5.erbB2 mice. Down-regulation of miR-122 is found in human and mouse hepatocellular carcinoma (HCC) [29]. Our results indicate that down-regulation of miR-122 may also be a marker of GBCa. Interestingly, we found miR-101 was significantly up-regulated in GBCa treated with PCI-24781. It has been reported that both the expression and function of EZH2 in cancer cell lines is inhibited by miR-101 [30] and that EZH2 activates the transcription of cyclin D1 in breast cancer cells [23]. These studies suggest PCI-24781-induced miR-101 may regulate the expression of both EZH2 and cyclin D1 in GBCa as well. We postulate that the post-transcriptional effect of HDAC inhibition on these miRNAs may play an important role in the anticancer activity of PCI-24781
In summary, our results indicate PCI-24781 has potential potent therapeutic value as a novel chemotherapeutic agent against human BTC with elevated erbB2 expression.
Supplementary Material
PCI-24781 (50 mg/kg/day, i.p. injection, 5 consecutive days per week, 0.2 mL for each injection) was delivered for 2 weeks to BK5.erbB2 mice. Mouse serum was collected 4 hours after the last injection. Control serum samples were collected from BK5.erbB2 mice injected with sodium lactate buffer.
Cells were treated with 0.5 µM PCI-24781 for 96 hours.
Acknowledgments
We thank Shawna Johnson for the excellent technical assistance.
Financial support
This work was supported by the Grants, CA102575 (NCI), CA016672 (NCI), and ES007784 (NIEHS).
Abbreviations
- GBCa
gallbladder carcinoma
- HDAC
histone deacetylase
- EGFR
epidermal growth factor receptor
- BTC
biliary tract cancer
- BK5
bovine keratin 5
- BrdU
bromodeoxyuridine
- USBM
ultrasound biomicroscopy
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- SAHA
suberoylanilide hydroxamic acid
- miRNA
micro RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
This study was partially supported by Pharmacyclics, Inc. and the authors declare no conflict of interest.
References
- 1.Kiguchi K, DiGiovanni J. CR Thomas CF., Jr . Biliary Tract and Gallbladder Cancer. New York: Demos Medical Publishing, LLC; 2009. Role of Growth Factor Signalling Pathways; pp. 19–34. [Google Scholar]
- 2.Sirica AE. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2008;14:7033–7058. doi: 10.3748/wjg.14.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiedmann M, Feisthammel J, Bluthner T, Tannapfel A, Kamenz T, Kluge A, et al. Novel targeted approaches to treating biliary tract cancer: the dual epidermal growth factor receptor and ErbB-2 tyrosine kinase inhibitor NVP-AEE788 is more efficient than the epidermal growth factor receptor inhibitors gefitinib and erlotinib. Anticancer Drugs. 2006;17:783–795. doi: 10.1097/01.cad.0000217433.48870.37. [DOI] [PubMed] [Google Scholar]
- 4.Kim YW, Huh SH, Park YK, Yoon TY, Lee SM, Hong SH. Expression of the c-erb-B2 and p53 protein in gallbladder carcinomas. Oncol Rep. 2001;8:1127–1132. doi: 10.3892/or.8.5.1127. [DOI] [PubMed] [Google Scholar]
- 5.Ogo Y, Nio Y, Yano S, Toga T, Koike M, Hashimoto K, et al. Immunohistochemical expression of HER-1 and HER-2 in extrahepatic biliary carcinoma. Anticancer Res. 2006;26:763–770. [PubMed] [Google Scholar]
- 6.Sirica AE, Radaeva S, Caran N. NEU overexpression in the furan rat model of cholangiocarcinogenesis compared with biliary ductal cell hyperplasia. Am J Pathol. 1997;151:1685–1694. [PMC free article] [PubMed] [Google Scholar]
- 7.Kiguchi K, Carbajal S, Chan K, Beltran L, Ruffino L, Shen J, et al. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61:6971–6976. [PubMed] [Google Scholar]
- 8.Kiguchi K, Ruffino L, Kawamoto T, Ajiki T, Digiovanni J. Chemopreventive and therapeutic efficacy of orally active tyrosine kinase inhibitors in a transgenic mouse model of gallbladder carcinoma. Clin Cancer Res. 2005;11:5572–5580. doi: 10.1158/1078-0432.CCR-04-2603. [DOI] [PubMed] [Google Scholar]
- 9.Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: What are the cancer relevant targets? Cancer Lett. 2009;277:8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Buggy JJ, Cao ZA, Bass KE, Verner E, Balasubramanian S, Liu L, et al. CRA-024781: a novel synthetic inhibitor of histone deacetylase enzymes with antitumor activity in vitro and in vivo. Mol Cancer Ther. 2006;5:1309–1317. doi: 10.1158/1535-7163.MCT-05-0442. [DOI] [PubMed] [Google Scholar]
- 11.Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, et al. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579–596. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- 12.Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24:1695–1702. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]
- 13.Riggs PK, Angel JM, Abel EL, DiGiovanni J. Differential gene expression in epidermis of mice sensitive and resistant to phorbol ester skin tumor promotion. Mol Carcinog. 2005;44:122–136. doi: 10.1002/mc.20127. [DOI] [PubMed] [Google Scholar]
- 14.Kuver R, Savard C, Nguyen TD, Osborne WR, Lee SP. Isolation and long-term culture of gallbladder epithelial cells from wild-type and CF mice. In Vitro Cell Dev Biol Anim. 1997;33:104–109. doi: 10.1007/s11626-997-0030-5. [DOI] [PubMed] [Google Scholar]
- 15.Okusaka T, Ishii H, Funakoshi A, Yamao K, Ohkawa S, Saito S, et al. Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2006;57:647–653. doi: 10.1007/s00280-005-0095-3. [DOI] [PubMed] [Google Scholar]
- 16.Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol. 2005;23:3971–3993. doi: 10.1200/JCO.2005.16.600. [DOI] [PubMed] [Google Scholar]
- 17.Scott GK, Marden C, Xu F, Kirk L, Benz CC. Transcriptional repression of ErbB2 by histone deacetylase inhibitors detected by a genomically integrated ErbB2 promoter-reporting cell screen. Mol Cancer Ther. 2002;1:385–392. [PubMed] [Google Scholar]
- 18.Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- 19.Price-Schiavi SA, Andrechek E, Idris N, Li P, Rong M, Zhang J, et al. Expression, location, and interactions of ErbB2 and its intramembrane ligand Muc4 (sialomucin complex) in rat mammary gland during pregnancy. J Cell Physiol. 2005;203:44–53. doi: 10.1002/jcp.20200. [DOI] [PubMed] [Google Scholar]
- 20.Carraway KL, Ramsauer VP, Haq B, Carothers Carraway CA. Cell signaling through membrane mucins. Bioessays. 2003;25:66–71. doi: 10.1002/bies.10201. [DOI] [PubMed] [Google Scholar]
- 21.Vincent A, Ducourouble MP, Van Seuningen I. Epigenetic regulation of the human mucin gene MUC4 in epithelial cancer cell lines involves both DNA methylation and histone modifications mediated by DNA methyltransferases and histone deacetylases. FASEB J. 2008;22:3035–3045. doi: 10.1096/fj.07-103390. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi J, Sasaki M, Sato Y, Itatsu K, Harada K, Zen Y, et al. Histone deacetylase inhibitor (SAHA) and repression of EZH2 synergistically inhibit proliferation of gallbladder carcinoma. Cancer Sci. 2010;101:355–362. doi: 10.1111/j.1349-7006.2009.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi B, Liang J, Yang X, Wang Y, Zhao Y, Wu H, et al. Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells. Mol Cell Biol. 2007;27:5105–5119. doi: 10.1128/MCB.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida T, Matsumoto T, Sasaki A, Morii Y, Aramaki M, Kitano S. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg. 2002;137:69–73. doi: 10.1001/archsurg.137.1.69. [DOI] [PubMed] [Google Scholar]
- 25.Konstantinidis IT, Deshpande V, Genevay M, Berger D, Fernandez-del Castillo C, Tanabe KK, et al. Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: a single-institution experience. Arch Surg. 2009;144:441–447. doi: 10.1001/archsurg.2009.46. discussion 447. [DOI] [PubMed] [Google Scholar]
- 26.Richardson P, Mitsiades C, Colson K, Reilly E, McBride L, Chiao J, et al. Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leuk Lymphoma. 2008;49:502–507. doi: 10.1080/10428190701817258. [DOI] [PubMed] [Google Scholar]
- 27.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 28.Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 29.Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia MA, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 30.Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCI-24781 (50 mg/kg/day, i.p. injection, 5 consecutive days per week, 0.2 mL for each injection) was delivered for 2 weeks to BK5.erbB2 mice. Mouse serum was collected 4 hours after the last injection. Control serum samples were collected from BK5.erbB2 mice injected with sodium lactate buffer.
Cells were treated with 0.5 µM PCI-24781 for 96 hours.