Abstract
The objective of this study was to describe: the time of onset and offset of bone mineral density (BMD) loss relative to the date of the final menstrual period (FMP); the rate and amount of BMD decline during the 5 years before and the 5 years after the FMP; and the independent associations between age at final menstrual period (FMP), body mass index (BMI) and race/ethnicity with rates of BMD loss during this time interval. The sample included 242 African-American, 384 Caucasian, 117 Chinese and 119 Japanese women, pre- or early perimenopausal at baseline, who had experienced their FMP and for whom an FMP date could be determined. Loess-smoothed curves showed that BMD loss began 1 year before the FMP and decelerated (but did not cease) 2 years after the FMP, at both the lumbar spine (LS) and femoral neck (FN) sites. Piece-wise, linear, mixed effects regression models demonstrated that during the 10-year observation period, at each bone site, the rates and cumulative amounts of bone loss were greatest from 1 year before through 2 years after the FMP, termed the transmenopause. Postmenopausal loss rates, those occurring between 2 and 5 years after the FMP, were less than those observed during transmenopause. Cumulative, 10-year LS BMD loss was 10.6%; 7.38% was lost during the transmenopause. Cumulative FN loss was 9.1%; 5.8% was lost during the transmenopause. Greater BMI and African American heritage were related to slower loss rates, while the opposite was true of Japanese and Chinese ancestry.
Keywords: Menopause, perimenopause, bone mineral density, ethnic differences, longitudinal cohort
INTRODUCTION
Bone loss begins prior to the cessation of menses. Dual energy X-ray absorptiometry detects unequivocal decline in bone mineral density during late perimenopause, when women have experienced between 3 and 11 months of amenorrhea, whereas little, if any, loss is seen during early perimenopause, when menstrual cycles are irregular but there has not yet been a gap of at least 3 months between periods (1–3). Menstrually-defined menopause transition (MT) categories, which classify stages of the menopause according to menstrual irregularity or number of months of amenorrhea, are imprecise predictors of when the final menstrual period (FMP) will occur. Women who are in early or late perimenopause may be more or less proximal to their FMP and rates of bone mineral density (BMD) loss may therefore differ within menstrually-defined stages. Similary, the time at which bone loss decelerates after the FMP cannot be discriminated using menstrually-classified MT stages.
A more precise description of onset and offset of bone loss can be obtained my modeling BMD change in relation to the FMP date. Using this approach, two longitudinal studies of Caucasian women found BMD loss accellerated about 2 year before to the FMP and slowed, but did not cease, about 2 years after it (4, 5). However, sample sizes in these investigations were modest and neither included minority women, one important consideration, because ethinic-specific patterns of bone loss during the MT could contribute to the known ethnic variation in fracture rates (6–9).
This analysis examines rates of BMD change in relation to the observed date of the FMP, in contrast to menopause transition stages, in a multiethnic cohort of African-American, Caucasian, Chinese and Japanese mid-life women. The objectives of this study were to: 1) describe the timing of the onset and offset of accelerated BMD loss in relation to FMP date; 2) quantify the rate and amount of BMD decline at the lumbar spine (LS) and femoral neck (FN) during the 5 years before and after the FMP; and 3) assess whether body mass index, ethnic/racial origin or age at FMP influenced the rate of BMD loss.
METHODS
Study sample
SWAN is a multi-site, community-based, longitudinal cohort study of the MT (10). Eligibility criteria were: age between 42 and 52 yr, intact uterus and at least one intact ovary, not currently using hormone therapy, at least one menstrual period in the 3 months before screening, and self-identification as a member of one of 5 eligible ethnic groups. Participants were enrolled at 7 sites: Boston, Chicago, Detroit, Pittsburgh, Los Angeles, Newark, and Oakland (N=3302). All sites enrolled Caucasians. Boston, Chicago, Detroit, and Pittsburgh enrolled African-Americans and the remaining 3 sites enrolled Japanese, Hispanic and Chinese women, respectively. The Chicago and Newark sites did not measure BMD, leaving a potential of 2413 participants for the SWAN bone density cohort. Of these, 2335 were enrolled in the bone cohort at baseline. The current analysis includes data from baseline to follow-up visit 10; only bone cohort participants who had a determinable natural (not surgical) FMP date were eligible. Hormone therapy use and other pharmacological agents that affect bone (i.e., tamoxifen, raloxifene, GnRH agonists, corticosteroids or osteoporosis treatments) were exclusions, applied at baseline. The inception cohort size was 862. Data from women who initiated bone-active medicine were censored at the time of first use. Please see the supplemental Figure (Web-only) for a flow diagram of the sample derivation. Participants gave written informed consent and sites obtained IRB approval.
Outcomes
LS and FN BMD (g/cm2) were measured annually using Hologic instruments (Hologic, Inc., Waltham, Massachusetts). Three sites used Hologic 4500A models throughout. Two sites upgraded from 2000 to 4500A models at follow-up visit 8. These sites scanned 40 women on both their old and new machines to develop cross-calibration regression equations. A standard quality control program, conducted in collaboration with Synarc, Inc., included daily phantom measurements, 6 month-cross-calibration with a circulating anthropomorphic spine standard, local site review of all scans, central review of scans that met problem-flagging criteria and central review of a 5% random sample of scans. Short-term in vivo measurement variability was 0.014 g/cm2 (1.4%) for the LS and 0.016 g/cm2 (2.2%) for the FN.
Primary predictor
The primary exposure, the number of months before or after the FMP that the BMD was taken, was computed using the month and year of the FMP and the month and year of each annual BMD assessment. FMP date was determined by annual, standardized interview. FMP date was defined as the last menstrual bleeding date reported during the visit immediately prior to the first visit when the participant was classified as post menopausal (had 12 months of amenorrhea).
Other predictors
Age [years], self-defined race/ethnicity [African-American, Caucasian, Chinese, Japanese], menstrual bleeding patterns, hormone therapy use [yes/no, time-varying], use of any medication that affects bone density [yes/no, time-varying]) were obtained using annual, standardized interviews. Menopause transition stages [time-varying, based on reported annual bleeding patterns] were defined as: premenopausal (regular menses, no change from individual’s pattern), early perimenopausal (menses within the prior 3 months but less predictable than individual’s pattern), late perimenopausal (at least 3 months but less than 12 consecutive months of amenorrhea) and postmenopausal (12 or more months without menses). Weight (kilograms, time-varying) and height (meters) were assessed annually, using calibrated scales and stadiometers. Body mass index (BMI, [weight in kilograms/(height in meters) 2]) was calculated annually.
Data Analysis
Characteristics of bone cohort participants included and excluded from analysis were compared using t-tests (continuous variables) and chi-squared tests (categorical variables). To analyze change in BMD in relation to FMP date, we used a staged approach, consisting of 1) non-parametric, loess-based selection of the functional form of the BMD trajectory in relation to FMP date, 2) piece-wise linear regression to determine knot placement for the parametric BMD trajectory and 3) piece-wise linear regression with fixed knots to estimate BMD decline rates during each phase of the trajectory. First, the loess method was used on repeated annual LS or FN measurements; each participant’s BMD was normalized to her baseline (11).
In steps 2 and 3, we used mixed effects regression to fit piece-wise linear models to repeated measurements of baseline-normalized LS or FN BMD (in separate models) as functions of time before or after FMP, using linear splines with fixed knots at FMP minus 1 year and FMP plus 2 years. To account for within-woman correlation between repeated observations, we included random effects for the intercept and 3 slopes (allowing the intercept and slopes to vary from woman to woman). In step 2, we tested model adequacy and appropriateness of knot locations by running null models with only random effects and no fixed effects. The fraction of within-woman variance in BMD explained by the 3-segment, piecewise-linear, null model was 84.2% for LS BMD and 71.7% for FN BMD. We evaluated knot selection by examining the change in the explained proportion of within-woman variance (pseudo R-square) when the knots were varied around FMP minus one year and FMP plus 2 years. The amount of explained variance was unambiguously lessened by knot movement (in 6 month intervals) away from FMP minus 1 year. However, the explained fraction was unaltered by subtracting or adding 6 months to the knot at FMP plus 2 years. We therefore chose FMP plus 2 years for the knot placement, because it represented the mid-point (indicating gradual deceleration, in contrast to the fairly rapid acceleration at FMP minus 1 year). The explained fraction of within woman variance also did not change when we used raw (un-normalized) or log transformed BMD instead of baseline-normalized BMD. We present baseline-normalized BMD for ease of interpretation: the regression slopes are equivalent to percentage changes in BMD from baseline. Because we adjusted the models for baseline BMD, individual differences in starting BMD do not influence estimated percentages.
In the third step, we added age at FMP, race/ethnicity and baseline BMI to the mixed effects piece-wise models, as fixed effects on the intercept and 3 slopes, to assess how each influenced the rate of BMD decline during each segment of the longitudinal, piece-wise model. The BMD trajectories were divided into 3 linear segments in relation to FMP date (time 0): years -5 to -1 relative to the FMP, termed pre-transmenopause; years -1 to +2 relative to the FMP, termed transmenopause; and years +2 to +5 after the FMP, termed postmenopause. We also modeled the effect of change in BMI since baseline on BMD values at follow-up. Models were adjusted for baseline BMD and clinical site. The effects of each predictor on the slopes for each segment were combined to obtain total effects on BMD decline during the 10 year period. Results are expressed as means and 95% confidence intervals; 95% confidence intervals that exclude 1 are considered statistically significant. Analyses were conducted using SAS version 9.2.
RESULTS
The analytic sample consisted of 242 African-American, 384 Caucasian, 117 Chinese and 119 Japanese women. At baseline, mean value of age was 46.7 years (standard deviation, [SD] 2.6 years), mean age at FMP was 51.6 (SD, 2.4 years) and average body mass index was 27.4 kg/m2 (SD, 7 kg/m2). The baseline percentages of premenopausal and early perimenopausal women were 58% and 41%, respectively; 16% were current smokers. These characteristics were similar to those of the SWAN bone cohort participants who were not included (data not shown). In the analysis sample, the mean number of BMD’s per woman was 9 and the median was 10 (of a maximum possible 10).
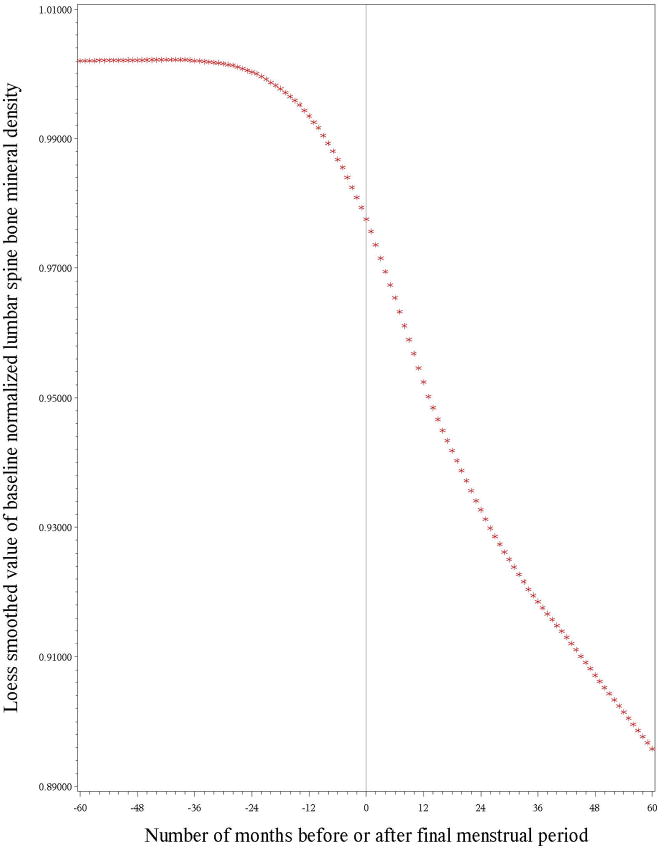
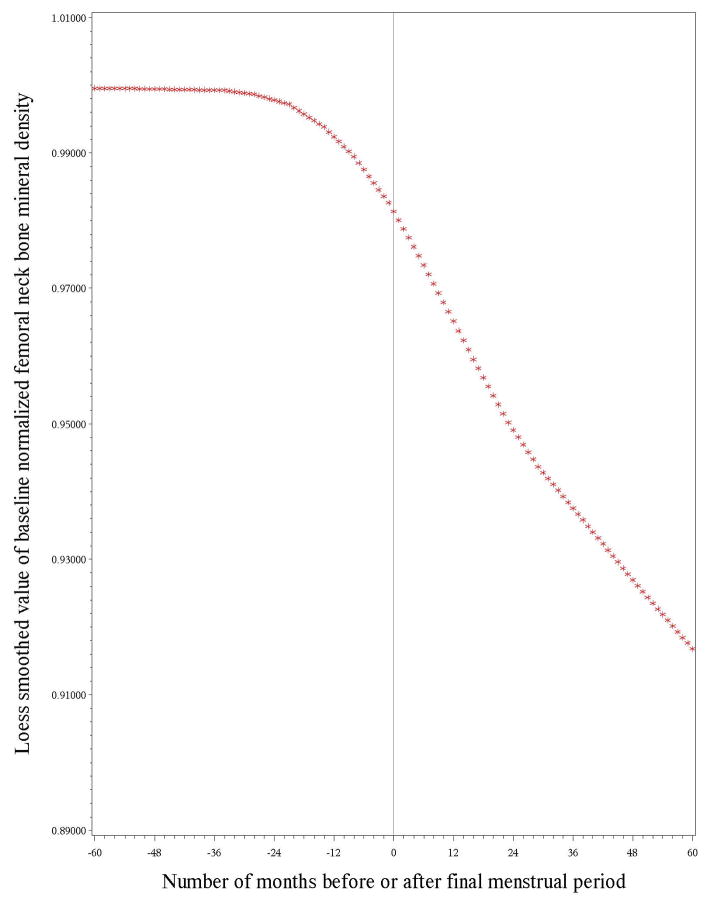
Figures 1 and 2 illustrate the longitudinal loess plots of mean LS and FN BMD as a function of number of months before or after the FMP (based on the date each BMD was obtained and the FMP date). At both bone sites, there appeared to be no decline in BMD prior to one year before the FMP, bone loss began one year before the FMP and decelerated, but did not cease, 2 years after the FMP. The loess plots also showed that trajectories were essentially linear within each of these 3 time intervals. To construct piece-wise regressions, we therefore divided the BMD trajectories into 3 linear segments in relation to FMP date. The first segment consisted of the period from 5 years prior to the FMP to 1 year prior to the FMP, termed pre-transmenopause. The second segment spanned the interval from 1 year prior to the FMP through 2 years after the FMP, termed transmenopause. The final segment started 2 years after the FMP and ended 5 years after the FMP, termed postmenopause. (See methods for tests of adequacy of break-point [knot] selections).
Figure 1.
illustrates the longitudinal trajectory of baseline-normalized lumbar spine bone mineral density (BMD) values in relation to the amount of time before (negative numbers) or after (positive numbers) the final menstrual period (FMP [time zero]). This loess plot illustrates no measurable decline in lumbar spine BMD during the interval between 5 years and 1 year before the FMP, BMD loss starting 1 year prior to the FMP that continued for 2 years after the FMP, and a deceleration, but not cessation, of BMD loss 2 years after the FMP.
Figure 2.
presents the longitudinal trajectory of baseline-normalized femoral neck bone mineral density (BMD) values relative to time before or after the FMP (FMP [time zero]). The span between 5 years and 1 year before the FMP was characterized by no measurable drop in BMD. This loess plot illustrates no measurable decline in femoral neck BMD during the interval between 5 years and 1 year before the FMP, BMD loss starting 1 year prior to the FMP that continued for 2 years after the FMP, and a deceleration, but not cessation, of BMD loss 2 years after the FMP.
Table 1 summarizes the results of the piece-wise linear models that quantified LS BMD loss in each of the 3 segments, i.e., pre-transmenopause, transmenopause and postmenopause. Caucasian women with average baseline LS BMD of 1.066 gm/cm2, average baseline BMI of 27.1 kg/m2 and average age at FMP of 51.6 years are the reference sample. The slopes shown for the Caucasian referent (row 1) are absolute slopes, reflecting the average rate of change in BMD during each segment. Caucasian transmenopausal change in LS BMD was −2.46% per year and postmenopausal change was −1.04% annually; summed 10-year change was −10.6%.
Table 1.
Annual rates of change of lumbar spine (LS) bone mineral density (BMD) in relation to the date of the final menstrual period (FMP) and the influence of body mass index (BMI), race and age at the FMP on LS BMD before, during and after the FMP a
| Annual BMD slopes during each time interval before and after the FMP (95% Confidence Interval) | Cumulative BMD change b(95% Confidence Interval) | |||
|---|---|---|---|---|
| Pre-Transmenopause 5 to 1 yr before FMP | Transmenopause 1 yr before to 2 yr after FMP | Postmenopause 2 yr to 5 yr after FMP | ||
| Caucasian referent c | −0.02% (−0.08%, +0.05%) | −2.46% (−2.61%, −2.31%) | −1.06% (−1.21%, −0.91%) | −10.6% (−11.2%, −10.0%) |
| Baseline BMI (per kg/m2) d | +0.008% (+0.001%, +0.015%) | +0.063% (+0.046, +0.080%) | +0.018% (+0.001%, +0.035%) | +0.28% (+0.21%, +0.34%) |
| Race: Japanese |
+0.01% (−0.12%, +0.14%) |
+0.20% (−0.12%, +0.52%) |
−0.04% (−0.35%, +0.28%) |
+0.5% (−0.7%, +1.8%) |
| Chinese | −0.14% (−0.27%, −0.01%) | −0.23% (−0.54%, +0.08%) | −0.22% (−0.51%, +0.06%) | −2.0% (−3.1%, −0.7%) |
| African American | −0.07% (−0.18%, +0.04%) | +0.27% (+0.02%. +0.52%) | +0.13% (−0.11%, +0.37%) | +0.9% (−0.1%, +1.9%) |
| Increasing age at FMP (yr) | +0.002% (−0.020%, +0.016%) | −0.050% (−0.091%, −0.009%) | +0.040% (+0.002%, +0.079%) | −0.04% (−0.19%, +0.12%) |
In addition to variables listed in table, model is also adjusted for baseline BMD and clinical site. Slope referent values are for Caucasian women of average age at FMP (51.7 years), average baseline BMD (1.066 gms/cm2 at the lumbar spine), and average BMI at baseline (27.1 kg/m2).
Cumulative change during 10-year period, spanning 5 years before and 5 years after the final menstrual period.
Statistically significant associations are shown in bold italic typeface; significance test of non-zero slopes for Caucasian referent; significance test of difference between slopes in Caucasian referent and slopes in the other specified groups.
Slopes for BMI, race and age at FMP, when added to the Caucasian slope referent values, give the slope in women who have each of these characteristics.
Also shown in Table 1 are the associations of BMI (per kg/m2), race/ethnicity and age at FMP with slopes in each of the segments. The figures shown in rows 2 to 6 are relative slopes: when added to the slope values of the Caucasian referent, the figures in rows 2 to 6 of the table yield the average slopes in women who have that alternate characteristic. For example, higher BMI was associated with less bone loss in all segments of the curve, indicated by positive coefficients in pre-transmenopause, transmenopause and postmenopause (+0.008%, +0.063%, +0.018%, respectively, per BMI unit). These positive coefficients do not indicate that women with greater BMI gained bone. Rather, they show that average rates of bone loss (given in row 1 of Table 1) were lessened by these amounts in women with a BMI 1 unit higher than the sample average. Caucasians with BMI values one standard deviation (7.5 kg/m2) above average would still lose bone —a ten year total of 8.50% —but a statistically smaller amount than the sample average 10-year loss of 10.6%. Being African-American was associated with less transmenopausal spinal BMD loss (2.19% per year) and a 10-year BMD change of −9.6%, borderline statistically significantly lower that the Caucasian 10-year rate. During the pre-transmenopausal segment, Chinese women lost LS BMD at a faster rate than Caucasians and Chinese 10-year LS BMD loss was 12.6%.
Results for FN BMD are presented in Table 2. Caucasian women with sample-average BMI, age at FMP and baseline FN BMD (0.832 gm/cm2) lost 1.76% annually during the transmenopausal interval. They lost 1.15% of FN BMD annually during the postmenopausal segment. Total 10-year FN loss was 9.1%. Higher BMI was related to less BMD loss, but only during the transmenopausal interval. The transmenopausal annual FN FMD loss rate was 1.42% in African Americans, 2.13% in Japanese and 2.17% in Chinese women. Compared to Caucasians, 10-year FN BMD loss was greater in Asians and less in African Americans.
Table 2.
Annual rates of change of femoral neck (FN) bone mineral density (BMD) in relation to the date of the final menstrual period (FMP) and the influence of body mass index (BMI), race and age at the FMP on FN BMD before, during and after the FMP a
| Annual BMD slopes during each time interval before and after the FMP (95% Confidence Interval) | Cumulative BMD change b(95% Confidence Interval) | |||
|---|---|---|---|---|
| Pre-Transmenopause 5 to 1 yr before FMP | Transmenopause 1 yr before to 2 yr after FMP | Postmenopause 2 yr to 5 yr after FMP | ||
| Caucasian referent c | −0.06% (−0.13%, +0.01%) | −1.76% (−1.92%, −1.61%) | −1.12% (−1.32%, −1.04%) | −9.1% (−9.7%, −8.5%) |
| Baseline BMI (per kg/m2) d | +0.001% (−.008%, +0.010%) | +0.025% (+0.006%, +0.044%) | +0.001% (−0.015%, +0.017%) | +0.08% (+0.01%, +0.16%) |
| Race: Japanese |
−0.05% (−0.20%, +0.11%) |
−0.37% (−0.70%, −0.04%) |
−0.12% (−0.41%, +0.17%) |
−1.7% (−2.9%, −0.38%) |
| Chinese | −0.06% (−0.22%, +0.09%) | −0.41% (−0.73%, −0.09%) | +0.11% (−0.15%, +0.37%) | −1.2% (−2.4%, +0.0%) |
| African American | −0.02% (−0.14%, +0.11%) | +0.34% (+0.08%, +0.61%) | +0.03% (−0.19%, +0.26%) | +1.1% (+0.1%, +2.1%) |
| Increasing age at FMP (yr) | +0.013% (−0.008%, +0.035%) | −0.055% (−0.098%, −0.013%) | +0.000% (−0.035%, +0.035%) | −0.11% (−0.27%, +0.05%) |
In addition to variables listed in table, model is also adjusted for baseline femoral neck BMD and clinical site. Slope referent values are for Caucasian women of average age at FMP (51.7 years), average baseline BMD (0.832 gms/cm2 at the femoral neck), and average BMI at baseline (27.1 kg/m2).
Cumulative change during 10-year period, spanning 5 years before and 5 years after the final menstrual period.
Statistically significant associations are shown in bold italic typeface; significance test of non-zero slopes for Caucasian referent; significance test of difference between slopes in Caucasian referent and slopes in the other specified groups.
Slopes for BMI, race and age at FMP, when added to the Caucasian slope referent values, give the slope in women who have each of these characteristics.
Later age at FMP was related to greater loss of both bone sites during the transmenopause, but had no effect on the 10-year cumulative loss (Tables 1 and 2). Not shown in the tables, women whose BMI changed during the 10-year period had an ending LS BMD that was higher by 0.10% per increasing BMI unit [95% CI = 0.06, 0.14%] and FN BMD that was higher by 0.35% per unit increase in BMI [95% CI = 0.30, 0.40%].
It is not possible to know the FMP date prospectively. Menstrually-defined menopause transition categories, based on bleeding patterns, are therefore used to in an attempt to stage the transition. The inference is that the later the transition stage, the closer to the FMP. To assess the usefulness of stages based on bleeding patterns (i.e., premenopause, early perimenopause, late perimenopause and postmenopause) in gauging where women are in the bone loss trajectory, we mapped the menstrually based stages onto each yearly interval prior to and after the FMP (Table 3). During the year before the FMP, when bone loss accelerated, 68% of participants who were observed were still classified as early perimenopausal based on bleeding patterns. Even in the year following the FMP, 30% of observations were in women still classified as early perimenopausal according to bleeding patterns. In the year immediately preceding the FMP, only 30% of BMD observations were in women classified as late perimenopausal. In the year immediately after the FMP, 62% of observations were in women classified as late perimenopausal. Table 3 also provides the crude mean BMD values during each yearly time interval prior to and after the FMP. The patterns of crude mean bone loss correspond closely to the loess plots (Figures 1 and 2) and to the piece-wise regression models (Tables 1 and 2).
Table 3.
Number of observations made in each 12-month period prior to and after the final menstrual period (FMP) and the relation between time to or from FMP and menstrually-defined menopause transition stages
| Menstrually-defined menopause transition stages a | |||||||
|---|---|---|---|---|---|---|---|
| Number of months before (negative sign) or after (positive sign) FMP b | Number of Observations | Crude Lumbar Spine BMD c | Crude Femoral Neck BMD c | Premenopausal Number, % | Early Perimenopausal Number, % | Late Perimenopausal Number, % | Postmenopausal Number, % |
| −60 to −49 | 485 | 1.07 | 0.83 | 178 (36.8%) | 304 (62.8%) | 2 (0.4%) | 0 (0.0%) |
| −48 to −37 | 563 | 1.07 | 0.83 | 155 (27.6%) | 396 (70.5%) | 11 (1.9%) | 0 (0.0%) |
| −36 to −25 | 618 | 1.07 | 0.83 | 116 (18.8%) | 481 (77.8%) | 21 (3.4%) | 0 (0.0%) |
| −24 to −13 | 697 | 1.07 | 0.83 | 64 (9.2%) | 552 (79.7%) | 77 (11.1%) | 0 (0.0%) |
| −12 to FMP | 742 | 1.05 | 0.82 | 32 (4.3%) | 503 (68.0%) | 205 (27.7%) | 0 (0.0%) |
| FMP to +12 | 871 | 1.03 | 0.82 | 8 (0.9%) | 266 (30.5%) | 535 (61.4%) | 62 (7.1%) |
| 13 to 24 | 703 | 1.00 | 0.79 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 703 (100%) |
| 25 to 36 | 602 | 0.99 | 0.78 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 602 (100%) |
| 37 to 48 | 456 | 0.97 | 0.77 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 456 (100%) |
| 49 to 60 | 372 | 0.97 | 0.77 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 372 (100%) |
Menstrually-defined menopause stages are based on self-reported bleeding patterns obtained by annual interview. Menopause transition stage categories are: premenopausal, characterized by regular menses; early perimenopausal, defined as menses within the prior 3 months but less predictable compared to participant’s prior pattern; late perimenopausal defined as having had at least 3 months, but less than 12 consecutive months, of amenorrhea; and postmenopausal characterized by having experienced 12 or more months without menses.
The number of months either before or after the FMP that each on-study BMD was obtained. In this manuscript, based on patterns of BMD loss in relation to the FMP, the following terminology is used. The time interval between 5 years and 1 year prior to the FMP is called “pre-transmenopause”. The interval spanning 1 year before to 2 years after the FMP is termed the “transmenopause”. The interval between 2 and 5 years after the FMP is called the “postmenopause”. These FMP-based categories are distinct from the menstrually-based menopause transition category definitions given in footnote “a”.
Crude mean values of lumbar spine or femoral neck BMD in each one-year interval before or after the FMP
DISCUSSION
In the time span consisting of 5 years before and 5 years after the FMP, change in BMD was divisible into 3 linear phases. Bone loss was not evident during the pre-transmenopause, except in Chinese women, who had a small annual decline. At both the LS and FN, BMD loss began 1 year before the FMP and slowed 2 years after it and this transmenopausal loss was greater at the LS than the FN. Postmenopausal loss rates, defined here as starting 2 years after the FMP, were of similar magnitude at each bone site and were less than transmenopausal rates of loss. Cumulative, 10-year LS BMD loss was 10.6%; 7.38% was lost during the transmenopause. Cumulative, 10-year FN loss was 9.1%; 5.8% was lost during the transmenopause. Base-case estimates of bone loss rates were based on Caucasians with sample-average characteristics. Greater BMI and African American heritage were related to slower loss rates, while the opposite was true of Japanese and Chinese ancestry.
The trajectory of menopause-related bone change is best captured by anchoring it to the FMP, as was done a decade ago in an 8-year longitudinal study of 75 initially-premenopausal, Caucasian women, in which an exponential curve was used to characterize BMD loss relative to FMP date (4). In that study, LS and FN bone loss accelerated 2 years before the FMP. Loss continued for 3 to 4 years after the FMP at the LS and for about 1.5 years after the FMP at the FN. These estimated times of onset and offset of transmenopausal loss ostensibly differ from SWAN’s, but the former study did not report parametric testing of the acceleration and deceleration points. Due to differences in statistical modeling used in the former and the current study, it is not feasible to compare their estimates of transmenopausal bone loss. However, in the former study, cumulative BMD losses in the period spanning 4 years before and 4 years after the FMP were 10% at the spine and 9.5% at the hip, similar to SWAN’s 10-year cumulative losses. Using a combination of splines and piece-wise linear models in a longitudinal sample of 183 Caucasian women, the Michigan Bone Health and Metabolism Study (MBHMS) found that spine BMD loss accelerated 2 years before and continued for the 2 years after the FMP, while the FN BMD acceleration began about 2–3 years before the FMP and lasted for 2 years after it (5). Differences in estimated acceleration and deceleration times between MBHMS and SWAN may be due in part to the smaller sample size in the former study and to the challenge of estimating velocity changes when these are gradual. In the MBHMS, spine and hip losses during the interval spanning 1 year prior to through 2 years after the FMP were 8.3% and 4.7%, respectively, concordant with SWAN’s estimates of 7.3% and 5.3% during the same interval at the same bone sites. Dissimilarities in estimated timing of transmenopausal acceleration and deceleration are less important than similarities among these 3 analyses, each of which demonstrate a period of rapid bone loss in the few years before and after the FMP, more pronounced at the LS than at the FN.
Transmenopausal BMD loss was greater at the LS than at the FN, concordant with the higher proportion of trabecular bone at the former compared to the latter site (12, 13). Riggs, Khosla and Melton originally proposed that accelerated, early postmenopausal bone loss affected trabecular bone to a greater degree than it affected cortical bone and that the subsequent, slower rate of BMD loss was similar in both bone compartments (14). The initial, accelerated phase was ascribed to the loss of a tonic estrogen effect on bone turnover; the slower phase to estrogen-deficiency-caused secondary hyperparathyroidism. Newer, CT-based studies still find a menopausal acceleration of trabecular bone loss (more pronounced at the lumbar spine than at the distal tibia or radius) but newly report that trabecular loss begins in women during their 20’s while tibial and radial cortical bone losses do not differ from no loss until the MT (15). We did not observe bone loss before the transmenopause, likely due to the lesser sensitivity of DXA compared to CT. The MT (and concomitant change in estradiol and other factors) appears to play a major role in onset of cortical bone loss and the amplification of trabecular bone loss in midlife women (16, 17).
Body mass, racial/ethic origin and age at FMP were each associated with to bone loss rates, but their effects were manifest during different segments of the bone loss curves and differed at the LS and FN sites. Higher baseline BMI was related to slower rates of LS bone loss during all phases, while at the FN it was associated with slower loss during the transmenopause. Nonetheless, when cumulated over 10-years, higher BMI predicted slower bone loss rates at both bone sites, in accord with most (1, 3, 5, 18), but not all (1), longitudinal studies of the MT. Unlike SWAN’s initial longitudinal findings, we found BMI-independent, racial/ethnic variation in cumulative 10-year bone loss, mainly due to differences in the rates of transmenopausal bone loss. The discordance between SWAN’s first longitudinal report and the current one is likely due to a doubling of the follow-up time and also to our use of the FMP-date-based primary predictor. Racial/ethnic differences in cumulative, 10-year bone loss were small, on the order of 1–2%. This absolute difference in amount of bone loss is unlikely to explain racial/ethnic variations in fracture rates (6–9). However, it is intriguing that the racial/ethnic variations in bone loss rates were almost entirely confined to the transmenopausal segment, which may have long-term impact on structural integrity (discussed below). Finally, the effect of increasing age at FMP on bone loss rate, also isolated to the transmenopause, was quite small and is not likely to be of clinical or biological significance.
Mapping the menstrually-defined MT stages onto the number of years before or after the FMP (Table 3) pointed out that menopause transition stages were not useful clinical signals of the onset of transmenopausal BMD loss. In the year before to the FMP, 70% of women were classified as early perimenopausal and only 30% of women were in late perimenopause. This result appears counter to prior reports that found minimal BMD loss during early perimenopause and a dramatic increase in BMD in late perimenopause—but careful scrutiny will demonstrate that the findings are indeed compatible and provide complementary information (1–3). In the current study, 60% to 80% of the BMD measures that were made in the years spanning 5 years to 1 year before the FMP (when no BMD loss occurred) were in early perimenipausal women; therefore, when one computes average BMD loss among all women classifed as early perimenopausal, it is predominantly influenced by this 4-year period of with no loss. The time span during which women were in late perimenopause was shorter, mainly one year before and one year after the FMP, consistent with the higher rates of BMD loss computed for this stage when menstrually-based classifications are used. But only 28% of women had reached late perimenopause when rapid BMD loss began, demonstrating that late perimenopause is not a clinically sensitive indicator that substantive BMD loss is starting. Finally, it may seem counterintuitive that 30% of participants were classified as early perimenopausal the year after their FMP occurred. However, the FMP date can only be known in retrospect; these are women who have “more abrupt” natural menopause—i.e., who transition directly from irregular menses to no menses without having had a menstrual gap of at least 3 months.
Does accelerated BMD loss during the transmenopause have clinical implications? On average, the absolute quantity of BMD lost during the 3-year transmenopausal phase, 7.4% at the LS and 5.3% at the FN, is unlikely to result in a BMD value sufficiently low to meet even the most conservative treatment recommendations. For example, a Caucasian woman with a baseline FN BMD at the 5th percentile for SWAN Caucasians, 0.69 g/cm2 (a T score −1.4), would have a femoral neck BMD of ~0.64 g/cm2 two years after the FMP (a T score of −1.8). But absolute decline in BMD may be less critical than the rapid bone turnover that it signals. Rapid turnover may damage skeletal structural integrity, though loss of trabecular elements, diminished trabecular connectivity, weakened trabeculae and erosion of the endosteal cortex (19). During the MT, histomorphometry and 3-D micro CT demonstrate declines in trabecular number, enlargement of trabecular spacing and conversion of trabecular plates to rods, in direct correspondence with increases in activation frequency (19, 20). Concern about irreparable architectural damage to bone has led some to advocate for short-term anti-resorptive therapy during the MT in an attempt to prevent such damage (21). While we concur that the major import of transmenopausal accelerated bone loss may be its threat to microarchitecture, we do not believe that the currently available data are sufficient to recommend treatment. Rather, further characterization of this phenomenon is essential.
Strengths of this analysis include its large sample size, number of FMP’s observed, ability to compare patterns of bone loss directly among women from four ethic/racial groups and multiple longitudinal measurements. The analysis method, linking patterns of bone loss to the FMP, newly points out the incapacity of menstrually-defined MT stages to signal the onset of transmenopausal BMD loss. Study limitations include some uncertainty the in the timing of the acceleration and deceleration of BMD loss, especially the latter, which was much less distinct. Ten year loss rates were computed to militate against this uncertainty in knot placement. Because SWAN enrolled women who were in their mid-forties, we cannot capture the period of time earlier than 5 years before the FMP; additional follow-up will permit us to extend observations beyond 5 years post-FMP. Non-Caucasian sample sizes were large enough to detect racial/ethnic differences in BMD trajectories, but not large enough for us to test for interactions within race. Two sites changed Hologic bone densitometer models; however, in-vivo cross-calibration protocols were done.
In conclusion, this analysis confirms that there is a period of rapid BMD loss that brackets the FMP and commences about one year before it and newly reports that transmenopausal BMD loss is independently influenced by ethnicity and body mass. Future work should determine whether rapid transmenopausal bone loss permanently damages bone microarchitecture or bone strength. Clinically useful signals that presage the onset of transmenopausal BMD loss also require elucidation.
Supplementary Material
Acknowledgments
Study of Women’s Health Across the Nation (SWAN) Acknowledgement
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011, Rachel Wildman, PI 2010 –2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Sherry Sherman 1994 – present; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Financial Disclosures
All authors state that they have no conflicts of interest.
Author roles
Gail A. Greendale: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
MaryFran Sowers: Study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
Weijuan Han: Statistical analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Mei-Hua Huang: Study concept and design; acquisition of data; statistical analysis; critical revision of the manuscript for important intellectual content; study supervision
Joel S. Finkelstein: Study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
Carolyn J. Crandall: Critical revision of the manuscript for important intellectual content
Jennifer S. Lee: Critical revision of the manuscript for important intellectual content
Arun S. Karlamangla: Study concept and design; statistical analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Contributor Information
Gail A. Greendale, Email: ggreenda@mednet.ucla.edu, Division of Geriatrics, David Geffen School of Medicine at UCLA, 10945 Le Conte Avenue, Suite 2339, Los Angeles, CA 90095-1687
MaryFran Sowers, Department of Epidemiology, School of Public Health, University of Michigan.
Weijuan Han, Division of Geriatrics, David Geffen School of Medicine at UCLA.
Mei-Hua Huang, Division of Geriatrics, David Geffen School of Medicine at UCLA.
Joel S. Finkelstein, Endocrine Unit, Department of Medicine, Massachusetts General Hospital
Carolyn J. Crandall, Division of General Internal Medicine, David Geffen School of Medicine at UCLA
Jennifer S. Lee, Division of Endocrinology, Department of Internal Medicine, University of California-Davis
Arun S. Karlamangla, Division of Geriatrics, David Geffen School of Medicine at UCLA
References
- 1.Guthrie JR, Ebeling PR, Hopper JL, Barrett-Connor E, Dennerstein L, Dudley EC, Burger HG, Wark JD. A prospective study of bone loss in menopausal Australian-born women. Osteoporos Int. 1998;8(3):282–290. doi: 10.1007/s001980050066. [DOI] [PubMed] [Google Scholar]
- 2.Sowers M, Crutchfield M, Bandekar R, Randolph JF, Shapiro B, Schork MA, Jannausch M. Bone mineral density and its change in pre-and perimenopausal white women: the Michigan Bone Health Study. J Bone Miner Res. 1998 Jul;13(7):1134–1140. doi: 10.1359/jbmr.1998.13.7.1134. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008 Mar;93(3):861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recker R, Lappe J, Davies K, Heaney R. Characterization of the perimenopausal bone loss: a prospective study. J Bone Miner Res. 2000 Oct;15(10):1965–73. doi: 10.1359/jbmr.2000.15.10.1965. [DOI] [PubMed] [Google Scholar]
- 5.Sowers MR, Zheng H, Jannausch ML, McConnell D, Nan B, Harlow S, Randolph JF., Jr Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J Clin Endocrinol Metab. 2010 May;95(5):2155–62. doi: 10.1210/jc.2009-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman SL, Madison RE. Decreased incidence of hip fracture in Hispanics, Asians, and blacks: California Hospital Discharge Data. Am J Public Health. 1998 Nov;78(11):1482–1483. doi: 10.2105/ajph.78.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross PD, Norimatsu H, Davis JW, Yano K, Wasnich RD, Fujiwara S, Hosoda Y, Melton LJ., 3rd A comparison of hip fracture incidence among native Japanese, Japanese Americans, and American Caucasians. Am J Epidemiol. 1991 Apr;133(8):801–809. doi: 10.1093/oxfordjournals.aje.a115959. [DOI] [PubMed] [Google Scholar]
- 8.Farmer ME, White LR, Brody JA, Bailey KR. Race and sex differences in hip fracture incidence. Am J Public Health. 1984 Dec;74(12):1374–1380. doi: 10.2105/ajph.74.12.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005 Feb;20(2):185–94. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 10.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause Biology and Pathobiology. San Diego, CA: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 11.Cleveland WS, Devlin SJ. Locally weighted regression: An approach to regression analysis by local fitting. J Amer Statist Assoc. 1988;83(403):596–610. [Google Scholar]
- 12.Riggs BL, Melton LJ, III, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Roueleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004 Dec;19(12):1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 13.Kuiper JW, Van Kuijk C, Grashuis JL. Distribution of trabecular and cortical bone related to geometry: A quantitative computed tomography study of the femoral neck. Invest Radiol. 1997 Feb;32(2):83–9. doi: 10.1097/00004424-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Riggs BL, Khosla S, Melton LJ., 3rd A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998 May;13(5):763–73. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 15.Riggs BL, Melton LJ, III, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008 Feb;23:205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khosla S, Melton LJ, 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res. 2011 Mar;26(3):441–51. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010 Jun;31(3):266–300. doi: 10.1210/er.2009-0024. [Epub 2010 Jan 5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor BC, Schreiner PJ, Stone KL, Fink HA, Cummings SR, Nevitt MC, Bowman PJ, Ensrud KE. Long-term prediction of incident hip fracture risk in elderly white women: study of osteoporotic fractures. J Am Geriatr Soc. 2004 Sep;52(9):1479–86. doi: 10.1111/j.1532-5415.2004.52410.x. [DOI] [PubMed] [Google Scholar]
- 19.Recker R, Lappe J, Davies KM, Heaney R. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res. 2004 Oct;19(10):1628–33. doi: 10.1359/JBMR.040710. [DOI] [PubMed] [Google Scholar]
- 20.Akhter MP, Lappe JM, Davies KM, Recker RR. Transmenopausal changes in the trabecular bone structure. Bone. 2007 Jul;41(1):111–6. doi: 10.1016/j.bone.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Zaidi M, Turner CH, Canalis E, Pacific R, Sun L, Iqbal J, Guo XE, Silverman S, Epstein S, Rosen CJ. Bone loss or lost bone: rationale and recommendations for the diagnosis and treatment of early postmenopausal bone loss. Curr Osteoporos Rep. 2009 Dec;7(4):118–26. doi: 10.1007/s11914-009-0021-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.