Abstract
Previous studies have suggested that serotonergic neurons in the midbrain raphe complex have a functional topographic organization. Recent studies suggest that stimulation of a bed nucleus of the stria terminalis-dorsal raphe nucleus pathway by stress-, anxiety- and fear-related stimuli modulates a subpopulation of serotonergic neurons in the dorsal part of the dorsal raphe nucleus (DRD) and caudal part of the dorsal raphe nucleus (DRC) that participates in facilitation of anxiety responses. In contrast, recent studies suggest that activation of a spinoparabrachial pathway by peripheral thermal or immune stimuli excites subpopulations of serotonergic neurons in the ventrolateral part of the dorsal raphe nucleus/ventrolateral periaqueducal gray (DRVL/VLPAG) region and interfascicular part of the dorsal raphe nucleus (DRI). Studies support a role for serotonergic neurons in the DRVL/VLPAG in inhibition of panic-like responses, and serotonergic neurons in the DRI in antidepressant-like effects. Thus, data suggest that while some subpopulations of serotonergic neurons in the dorsal raphe nucleus play a role in facilitation of anxiety-like responses, others play a role in inhibition of anxiety- or panic-like responses, while others play a role in antidepressant-like effects. Understanding the anatomical and functional properties of these distinct serotonergic systems may lead to novel therapeutic strategies for the prevention and/or treatment of affective and anxiety disorders. In this review, we describe the anatomical and functional properties of subpopulations of serotonergic neurons in the dorsal raphe nucleus, with a focus on those implicated in symptoms of anxiety and affective disorders, the DRD/DRC, DRVL/VLPAG, and DRI.
Keywords: dorsal raphe nucleus, serotonin, 5-hydroxytryptamine, 5-HT, topography, anatomy, hodology
Brain serotonergic systems control diverse physiologic and behavioral functions including motor control, appetite, sleep-wake cycles, as well as emotional behavior and emotional states. Recent studies suggest that subregions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR), nuclei that contain the majority of forebrain-projecting serotonergic neurons, are differentially responsive to stress-related stimuli (for review, see Hale and Lowry, 2010). Most of these studies have focused on the DR (B6 and B7 according to the nomenclature of Dahlström and Fuxe, 1964), a densely packed region of serotonergic neurons located in the caudal midbrain and rostral pons. The DR can be divided into several subregions, based on its anatomy, hodology, and functional topography, encompassing the rostral, dorsal, ventral, ventrolateral, interfascicular, and caudal parts. In previous reviews of the anatomical and functional topography of serotonergic systems, we have comprehensively reviewed midbrain and pontine serotonergic systems (Hale and Lowry, 2010; Lowry et al, 2008a), or focused on anxiety-related serotonergic systems (Hale et al, 2011b; Lowry et al, 2005; Lowry and Hale, 2010; Lowry et al, 2008b). In this review, we describe the anatomical and functional properties of subpopulations of serotonergic neurons in the DR, focusing on comparisons/contrasts of anxiety-related serotonergic circuits (conflict anxiety and anti-panic-related circuits) with depression-related serotonergic circuits.
Recent studies in humans suggest that brain serotonergic systems are dysfunctional in patients with anxiety and affective disorders. Patients with panic disorder show a 4-fold increase in brain serotonin turnover compared with healthy controls (Esler et al, 2007) and this increase is positively correlated with disease severity. Likewise, patients with major depressive disorder (MDD) show elevated brain serotonin turnover compared with healthy controls (Barton et al, 2008), an effect that is associated with carriage of the s allele of the serotonin transporter (slc6a4) gene. Following effective treatment with selective serotonin reuptake inhibitor antidepressant drugs, brain serotonin turnover is substantially reduced in both panic disorder (Esler et al, 2007) and MDD patients (Barton et al, 2008). Moreover, patients with panic disorder with and without comorbid major depression show reduced serotonin 1A (5-HT1A) receptor binding in the DR as well the anterior and posterior cingulate cortices (Neumeister et al, 2004) and patients with MDD show reduced 5-HT1A receptor binding in the DR and in the anterior cingulate, ventrolateral prefrontal and orbital cortices (Drevets et al, 1999). Consistent with an important role for serotonergic signaling in the pathophysiology of MDD, a functional polymorphism in the htr1a gene, leading to impaired repression, is associated with MDD (for review, see Le Francois et al, 2008; Lemonde et al, 2003).
Consistent with increased brain serotonin turnover and alterations in 5-HT1A receptor binding in patients with anxiety and affective disorders, depressed suicide patients have elevated expression of tph2 mRNA, which encodes tryptophan hydroxylase 2, the rate-limiting enzyme in the biosynthesis of serotonin (5-hydroxytryptamine, 5-HT; Bach-Mizrachi et al, 2006; Bach-Mizrachi et al, 2008). Conversely, chronic antidepressant treatment decreases tryptophan hydroxylase (TPH)-immunoreactive cell numbers in rats (MacGillivray et al, 2010). Allelic variation in tryptophan hydroxylase 2 has been identified as a predictor of depression (Zill et al, 2004; Zhang et al, 2005; Haghighi et al, 2008), suicide risk among depressed patients (Lopez de Lara et al, 2007) and responses to antidepressant treatment (Peters et al, 2004). Importantly, however, the elevation of tph2 mRNA expression in depressed suicides, at least in some studies, is restricted to specific subregions of the DR. For example, compared with matched non-psychiatric controls, drug-free depressed suicide victims show trends for increased tph2 mRNA expression in the dorsal raphe nucleus dorsal part (DRD; 136% of control) and the dorsal raphe nucleus ventrolateral part (DRVL; 130% of control), but not in the dorsal raphe nucleus ventral part (DRV; 86% of control; Bach-Mizrachi et al, 2006). Similarly, TPH protein expression is increased in the DRD, but not in the DRVL, DRV or dorsal raphe nucleus interfascicular part (DRI), of depressed suicide victims with alcohol dependence compared with matched non-psychiatric controls (Bonkale et al, 2006).
Consequently, it is important to understand mechanisms through which subregions of the DR could be independently regulated. One mechanism through which subregions of the DR could be independently regulated is through altered synaptic input to specific subpopulations of serotonergic neurons (Hale and Lowry, 2010). Indeed, subregions of the DR receive differential afferent input from forebrain and brainstem sites. In addition, functional neuroanatomical data support the hypothesis that stress-related stimuli activate serotonergic neurons in the DR in a stimulus-specific manner. In other words, different stress-related stimuli that are potentially relevant to anxiety and affective disorders activate serotonergic neurons in different subregions of the DR. These subregions in turn give rise to unique patterns of neural output to forebrain limbic structures, which could be relevant to specific symptoms of anxiety and affective disorders. Stress-induced activation of specific subpopulations of serotonergic neurons in the DR, and increases in serotonergic neurotransmission in specific forebrain structures innervated by those neurons, could be dependent on a number of factors, including 1) increases in excitatory synaptic input to specific populations of serotonergic neurons, 2) disinhibition of specific populations of serotonergic neurons, and 3) differential autoinhibitory influences of 5-HT1A receptors. Differential autoinhibitory influences may exist under baseline conditions (Beck et al, 2004; Commons, 2008; Crawford et al, 2010), or may emerge as a consequence of prior stress and functional desensitization of 5-HT1A receptor inhibitory mechanisms (Rozeske et al, 2011).
The importance of 5-HT1A receptor autoregulation in determining basal and stimulus-induced increases in serotonergic neurotransmission has been demonstrated using both immediate-early gene and microdialysis approaches. For example, Kathryn Commons has shown that administration of the 5-HT1A receptor antagonist, WAY-100635, increases c-Fos expression in the lateral wings (DRVL/VLPAG region), and caudal ventral DR, but not in other subregions of the DR, suggesting that the lateral wings and caudal ventral DR are under significant tonic inhibition by 5-HT1A autoreceptors. In the same study, forced swimming in rats increased c-Fos expression only in serotonergic neurons located within the caudal dorsal and caudal ventral parts of the DR (Commons, 2008); however, following administration of the 5-HT1A receptor antagonist, WAY-100635, forced swimming also increased c-Fos expression in the rostral dorsal and rostral ventral DR. Together, these studies suggest that 5-HT1A autoreceptors are an important determinant of basal and stress-induced activity of different subpopulations of serotonergic neurons. Consistent with the idea that 5-HT1A receptor autoinhibition is an important determinant of regional differences in serotonergic neurotransmission, acute administration of fluoxetine (30 mg/kg, s.c.) increases extracellular serotonin concentrations in the medial prefrontal cortex, but not the dorsal lateral prefrontal cortex in rats (Beyer and Cremers, 2008). However, following pretreatment with WAY-100635, fluoxetine administration induces a significant, two-fold, increase in extracellular serotonin concentrations within the dorsal lateral prefrontal cortex, and potentiates fluoxetine-induced increases in extracellular serotonin concentrations in the medial prefrontal cortex. Although synaptic excitatory and inhibitory inputs to subpopulations of serotonergic neurons, together with 5-HT1A autoreceptor activity, are clearly important determinants of regional differences in basal and stimulus-induced serotonergic neurotransmission, other factors are also likely to be important, including 1) regional differences in the density of serotonergic nerve terminals, 2) regional differences in tryptophan hydroxylase expression or its activity, 3) regional differences in the density of the presynaptic serotonin transporter at nerve terminals, 4) regional differences in postsynaptic serotonin transporters, such as the corticosterone-sensitive organic cation transporter 3 (OCT3) (Gasser et al, 2006; Gasser et al, 2009), and 5) regional differences in the rate of serotonin metabolism (Dhingra et al, 1997).
In this review, we highlight the unique patterns of neural input to specific subregions of the DR, the unique patterns of neural output from specific subregions of the DR, and the functional anatomy of different subregions of the DR. Specifically we will discuss an anxiety-related serotonergic system located in the DRD/DRC region and an anti-panic and antidepressant-like serotonergic system located in the DRVL/VLPAG and DRI regions (Fig. 1). The functional neuroanatomical evidence presented below is in broad support of a model of the dual role of serotonin in the control of anxiety and panic proposed by Deakin, Graeff and colleagues (Graeff et al, 1996). In this model serotonin released in the amygdala serves to increase anxiety-like behavior and increase highly integrated defensive behaviors while serotonin neurons terminating in the dorsal periaqueductal gray region serve to inhibit panic-like responses (Deakin and Graeff, 1991; Graeff, 1990; Graeff et al, 1996). We begin, however, by discussing the evidence for the emergence of functionally distinct populations of serotonin neurons during development.
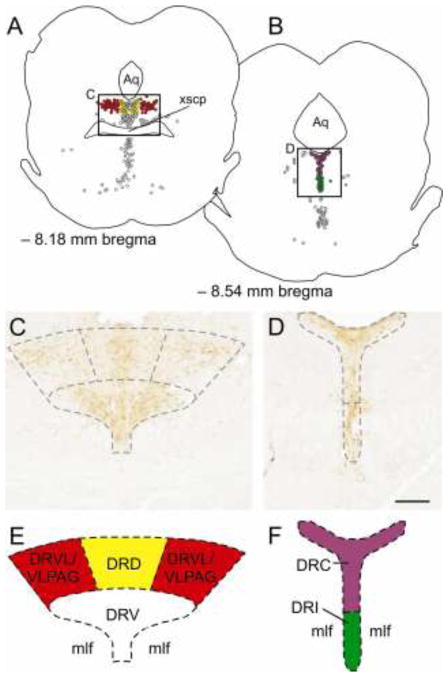
Figure 1.
The dorsal raphe nucleus can be divided into several subregions based on anatomical, functional and hodological evidence. A) Camera lucida drawing showing a coronal section of the rat midbrain/pons at −8.18 mm bregma. Each dot represents one serotonergic neuron. An anxiety-related serotonergic system in the dorsal raphe nucleus, dorsal part (DRD) is represented by yellow dots, while an anti-panic serotonergic system in the dorsal raphe ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG) is represented by red dots. The black box in A is shown in the photomicrograph in C. C) Photomicrograph showing tryptophan hydroxylase-immunoreactive neurons (orange/brown cytosolic staining) in the dorsal raphe nucleus at −8.18 mm bregma. Tryptophan hydroxylase (TPH) is the rate-limiting enzyme in the biosynthesis of serotonin and is commonly used as a marker of serotonergic neurons. Dashed lines show the boundaries for the subregions of the dorsal raphe nucleus which are depicted in E. E) Schematic illustration showing the location of an anxiety-related serotonergic system in the DRD (yellow) and an anti-panic serotonergic system in the DRVL/VLPAG (red). B) Camera lucida drawing showing a coronal section of the rat midbrain/pons at −8.54 mm bregma. Each dot represents one serotonergic neuron. An anxiety-related serotonergic system in the dorsal raphe nucleus, caudal part (DRC) is represented by purple dots, while an antidepressant-like serotonergic system in the dorsal raphe nucleus, interfascicular part (DRI) is represented by green dots. The black box in B is shown in the photomicrograph in D. D) Photomicrograph showing TPH immunoreactive neurons in the dorsal raphe nucleus at −8.54 mm bregma. Dashed lines show the boundaries for the subregions of the dorsal raphe nucleus that are depicted in F. F) Schematic illustration showing the location of an anxiety-related serotonergic system in the DRC (purple) and an antidepressant-like serotonergic system in the DRI (green).
Functional subpopulations of serotonergic neurons are derived from different genetic lineages
Anatomically and functionally different subpopulations of serotonergic neurons follow different neurodevelopmental pathways. For example serotonergic neurons in the DR derive exclusively from rhombomere 1 in the developing brain while serotonergic neurons in the MnR are derived from rhombomere 2 (Jensen et al, 2008). As well as determining the ultimate location of the serotonergic cell bodies, the rhombomeric origin of serotonergic cell groups also contributes to the organization of their projections (Bang et al, 2012). Recent evidence with transgenic mice lacking Pet1, a transcription factor that is necessary for the differentiation of the majority of serotonin neurons, suggests that functionally related subpopulations of serotonergic neurons may have distinct genetic lineages. In Pet1 knockout mice, a Pet1 independent population of serotonergic neurons in the DR differentiates and sends projections to functionally related forebrain targets that are implicated in stress- and anxiety-related behavior, including the central and basolateral nuclei of the amygdala, paraventricular hypothalamus, bed nucleus of the stria terminalis (BNST), and the infralimbic and agranular insular cortices (Kiyasova and Gaspar, 2011; Kiyasova et al, 2011).
Dorsal raphe nucleus, dorsal part (DRD)/dorsal raphe nucleus, caudal part (DRC) system
Functional neuroanatomic studies suggest that there is a subpopulation of serotonergic neurons, located predominantly in the DRD and DRC subdivisions, that plays a role in facilitation of anxiety-like behavior and anxiety states. The DRD is considered, based on hodological and functional neuroanatomical criteria, to be an anxiety-related subregion of the DR. It is closely related, anatomically and functionally, with the DRC, which lies directly caudal to the DRD, adjacent to the cerebral aqueduct. The afferent projections to the DRD have been described in detail by Peyron and colleagues (Peyron et al, 1998), and by Lee and colleagues (2003), while the afferent projections to the DRC have been described by Lee and colleagues (2003); it should be noted that in this review we describe differential inputs to different subregions of the DR relative to other subregions of the DR and not in terms of absolute or exclusive afferent input. The DRD and DRC receive common afferent input from a number of brain structures implicated in the control of anxiety-related behavior and anxiety states (Fig. 2). These include forebrain structures providing top-down regulation of serotonergic function, including the infralimbic and prelimbic cortices, the lateral habenula, the BNST and the central nucleus of the amygdala (although the BNST and the central nucleus of the amygdala appear to more strongly innervate the DRD). These regions giving rise to projections to the DRD/DRC have been shown to be part of a distributed system controlling anxiety-related behavior and anxiety states (Singewald et al, 2003). Consistent with these anatomical relationships, overexpression of corticotropin-releasing factor in the BNST increases CRF type 2 (CRF2) receptor expression selectively in the DRD subregion (Sink et al, 2012). In turn, the DRD and DRC have reciprocal projections to anxiety-related structures, through the dorsal raphe forebrain bundle tract (Azmitia and Segal, 1978; also see Fig. 3). These include the medial prefrontal cortex (Van Bockstaele et al, 1993) and related limbic structures, including the basolateral (Hale et al, 2008) and central amygdaloid nuclei (Commons et al, 2003), BNST (Petit et al, 1995), nucleus accumbens (Van Bockstaele et al, 1993), dorsal hypothalamic area (Commons et al, 2003) and dorsolateral periaqueductal gray (Stezhka and Lovick, 1997). In addition, the DRD and DRC contain a subpopulation of serotonergic neurons that project to the cerebral ventricles via the dorsal raphe periventricular tract (Hale et al, 2010; Simpson et al, 1998; Lowry et al, 2008a; Mikkelsen et al, 1997).
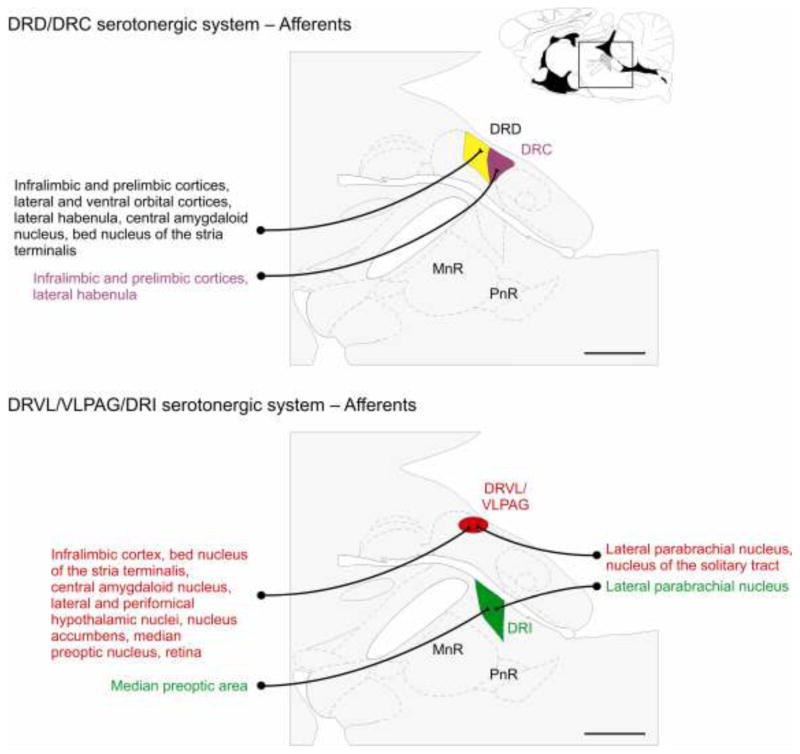
Figure 2.
The anxiety-related serotonergic system comprises the dorsal raphe nucleus, dorsal part (DRD) and dorsal raphe nucleus, caudal part (DRC) subregions of the dorsal raphe nucleus (DR), while the anti-panic/antidepressant serotonergic system comprises the dorsal raphe nucleus, ventrolateral part/ventrolateral part of the periaqueductal gray (DRVL/VLPAG) and dorsal raphe nucleus, interfascicular part (DRI) subregions of the DR. These subregions receive topographically organized afferents from forebrain and brainstem regions. Schematic illustrations of midline sagittal sections of the rat brainstem show subdivisions of the DR adapted from a standard rat brain atlas (Paxinos and Watson, 1998). The full sagittal section is shown in the top right corner for reference; the box shows the area of the brainstem illustrated in the main figure. The subdivisions of the DR and the forebrain and brainstem structures giving rise to selected prominent afferent projections to each subdivision are color-coordinated; DRC, purple; DRD, yellow/black text; DRI, green; DRVL/VLPAG, red. Abbreviations: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral part of the periaqueductal gray; MnR, median raphe nucleus; PnR, pontine raphe nucleus. Scale bar, 1 mm.
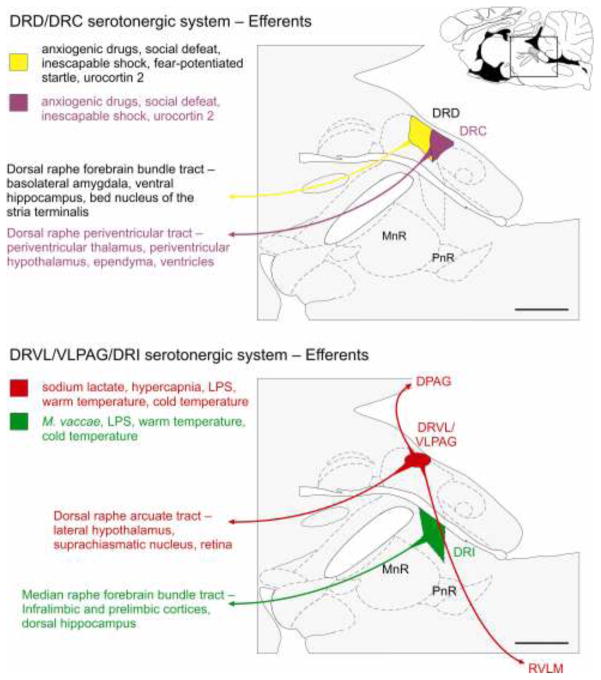
Figure 3.
The anxiety-related serotonergic system comprises the dorsal raphe nucleus, dorsal part (DRD) and dorsal raphe nucleus, caudal part (DRC) subregions of the dorsal raphe nucleus (DR), while the anti-panic/antidepressant serotonergic system comprises the dorsal raphe nucleus, ventrolateral part/ventrolateral part of the periaqueductal gray (DRVL/VLPAG) and dorsal raphe nucleus, interfascicular part (DRI) subregions of the DR. These subregions send topographically organized projections to forebrain and brainstem regions via distinct serotonergic tracts. Schematic illustrations of midline sagittal sections of the rat brainstem show subdivisions of the DR adapted from a standard rat brain atlas (Paxinos and Watson, 1998). The full sagittal section is shown in the top right corner for reference; the box shows the area of the brainstem illustrated in the main figure. There is an anatomic and functional topography among the different subregions of the DR. The lists on the left side of the figure indicate physiologic, pharmacologic and behavioral stimuli known to activate serotonergic neurons in different subdivisions of the DR. The subdivisions of the DR, the ascending (Azmitia and Segal, 1978) and descending serotonergic tracts, as well as the efferent projection regions are color-coordinated; DRC, purple; DRD, yellow/black text; DRI, green; DRVL/VLPAG, red. Abbreviations: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral part of the periaqueductal gray; LPS, lipopolysaccharide; MnR, median raphe nucleus; PnR, pontine raphe nucleus. Scale bar, 1 mm.
Functional neuroanatomical studies indicate that serotonergic and non-serotonergic neurons in the DRD/DRC are responsive to anxiety-related stimuli (Table 1 and 2). A number of anxiogenic stimuli have been found to increase c-Fos expression in DRD serotonergic neurons, including anxiogenic drugs with diverse pharmacological properties, such as caffeine and N-methyl-β-carboline-3-carboxamide (FG-7142), an inverse partial agonist at the benzodiazepine allosteric site on the GABAA receptor (Abrams et al, 2005), the anxiety-related neuropeptide, urocortin 2 (Hale et al, 2010; Staub et al, 2005; Staub et al, 2006), and exposure to social defeat (Gardner et al, 2005). In contrast, some stress or anxiety-related stimuli seem to selectively affect the DRD, while others seem to selectively affect the DRC, suggesting that there may be some functional heterogeneity. For example, fear-potentiated startle, relative to exposure to the conditioned stimulus alone, increases c-Fos expression in the mid-rostrocaudal DRD, but not the DRC (Spannuth et al, 2011). In addition, the DRD, but not the DRC, responds with attenuated c-Fos expression following repeated social defeat, relative to acute defeat (Paul et al, 2011). The DRD contains a dense plexus of neurokinin 1 receptor-immunoreactive fibers (Commons, 2008) and the DRD, but not the DRC, responds to microinjections of substance P with predominantly excitation (Valentino et al, 2003). Based on the anatomical considerations above, it may be stimuli that predominantly involve activation of afferents arising from the central amygdaloid nucleus, or the BNST that preferentially activate DRD serotonergic neurons. In contrast, exposure of rats to warm ambient temperature increases c-Fos expression in the DRC, but not the mid-rostrocaudal DRD (Hale et al, 2011a). Similarly, random unpredictable noise stress, or treatment of brain slices with corticotropin-releasing factor, increase in vivo and in vitro, respectively, tryptophan hydroxylase activity in the DRC, but not the mid-rostrocaudal DRD (Evans et al, 2009). Further studies with a high level of anatomical precision should help distinguish the anatomical and functional properties of subpopulations of serotonergic neurons within the DRD and DRC regions.
Table 1.
Stress-related or other physiologic stimuli increase c-Fos expression in serotonergic neurons in subregions of the dorsal raphe nucleus
| Stimuli | rostral DR | DRD | DRV | DRVL/VLPAG | DRC | DRI | Reference | |
|---|---|---|---|---|---|---|---|---|
| Social Defeat | Rat (Male, Long Evans) | + | + | + | Gardner et al, 2005 | |||
| Social Defeat | Rat (Male, Long Evans) | + | + | + | + | + | + | Paul et al, 2011 |
| Repeated Social Defeat | Rat (Male, Long Evans) | + | + | + | + | + | + | Paul et al, 2011 |
| Aggressive interaction (A) | Syrian hamster (Male, Mesocricetus auratus) | + | Cooper et al, 2009 | |||||
| Open-field exposure | Rat (Male, Wistar) | + | + | + | Bouwknecht et al, 2007 | |||
| Fear-conditioning | Rat (Male, Sprague Dawley) | + | + | + | + | + | + | Spannuth et al, 2011 |
| Fear-potentiated startle | Rat (Male, Sprague Dawley) | + | Spannuth et al, 2011 | |||||
| Anxiogenic drugs | Rat (Male, Wistar) | Abrams et al, 2005 | ||||||
| – mCPP | + | |||||||
| – Caffeine | + | + | + | |||||
| – Yohimbine | + | |||||||
| – FG-7142 | + | + | ||||||
| Urocortin 2 (i.c.v.) | Rat (Male, Wistar) | + | + | + | + | + | Staub et al 2005 | |
| Urocortin 2 (i.c.v.) | Rat (Male, Wistar) | + | + | Staub et al, 2006 | ||||
| Urocortin 2 (i.c.v.) | Rat (Male, Wistar) | + | + | Hale et al, 2010 | ||||
| Warm ambient temperature | Rat (Male, Wistar) | + | + | + | + | Hale et al, 2011 | ||
| Swim stress (15 min) | Rat (Male, Wistar) | Kelly et al, 2011 | ||||||
| – 19 °C water temperature |
+ | + | + | + | + | + | ||
| – 25 °C water temperature |
+ | + | + | + | ||||
| – 35 °C water temperature |
+ | + | + | + | ||||
| Swim stress/WAY-100635 | Rat (Male, Sprague Dawley) | Commons, 2008 | ||||||
| – swim stress (15 min) | + | + | ||||||
| – WAY-100635 | + | + | ||||||
| – Swim stress + WAY-100635 | + | + | + | + | ||||
| Sodium lactate | Rat (Male, Sprague Dawley) | Johnson et al, 2008 | ||||||
| – L-allylglycine (B) | – (C) | – (D) | – (D) | |||||
| – D-allylglycine | + | + | + | + | ||||
| Hypercapnia | Rat (Male, Sprague Dawley) | + | + | + | + | + | Johnson et al, 2005 | |
| Lipopolysaccharide | Mouse (Male, BALB/c) | + | + | Hollis et al, 2006 | ||||
| Mycobacterium vaccae | Mouse (Male, BALB/c) | + | Lowry et al, 2007 |
In hamsters classified as having lost the social interaction compared with those that won the social interaction and novel cage controls
In this model of anxiety with a vulnerability to panic, L-allylglycine, or its inactive enantiomer, D-allylglycine (control) was infused into the dorsomedial hypothalamus (DMH). This results in disinhibition of the DMH and rats that are prone to panic following intravenous infusion of sodium lactate
decreased c-Fos expression in serotonergic neurons compared with L-allylglycine-treated rats infused with vehicle
decreased c-Fos expression in serotonergic neurons compared with D-allylglycine-treated rats infused with sodium lactate
Table 2.
Stress-related or other physiologic stimuli increase c-Fos expression in non-serotonergic cells in subregions of the dorsal raphe nucleus
| Stimuli | rostral DR | DRD | DRV | DRVL/VLPAG | DRC | DRI | Reference | |
|---|---|---|---|---|---|---|---|---|
| Social Defeat | Rat (Male, Long Evans) | + | + | Gardner et al, 2005 | ||||
| Social Defeat | Rat (Male, Long Evans) | + | + | + | + | + | + | Paul et al, 2011 |
| Repeated Social Defeat | Rat (Male, Long Evans) | + | + | + | + | + | + | Paul et al, 2011 |
| Open-field exposure | Rat (Male, Wistar) | + | + | + | + | + | + | Bouwknecht et al, 2007 |
| Fear-conditioning | Rat (Male, Sprague Dawley) | + | + | + | + | + | + | Spannuth et al, 2011 |
| Fear-potentiated startle | Rat (Male, Sprague Dawley) | Spannuth et al, 2011 | ||||||
| Anxiogenic drugs | Rat (Male, Wistar) | Abrams et al, 2005 | ||||||
| – mCPP | + | + | ||||||
| – Caffeine | + | + | + | + | + | + | ||
| – Yohimbine | + | |||||||
| – FG-7142 | + | + | + | |||||
| Urocortin 2 (i.c.v.) | Rat (Male, Wistar) | + | + | Staub et al, 2005 | ||||
| Urocortin 2 (i.c.v.) | Rat (Male, Wistar) | Staub et al, 2006 | ||||||
| Urocortin 2 (i.c.v.) | Rat (Male, Wistar) | + | + | Hale et al, 2010 | ||||
| Warm ambient temperature | Rat (Male, Wistar) | + | + | Hale et al, 2011 | ||||
| Swim stress (15 min) | Rat (Male, Wistar) | Kelly et al, 2011 | ||||||
| – 19 °C water temperature |
+ | + | + | + | + | + | ||
| – 25 °C water temperature |
+ | + | + | + | + | + | ||
| – 35 °C water temperature |
+ | + | + | + | + | + | ||
| Sodium lactate (A) | Rat (Male, Sprague Dawley) | Johnson et al, 2008 | ||||||
| – L-allylglycine (B) | ||||||||
| – D-allylglycine | ||||||||
| Lipopolysaccharide | Mouse (Male, BALB/c) | Hollis et al, 2006 |
c-Fos expression in non-serotonergic neurons was assessed in the DRVL/VLAG region only. There were no differences between sodium lactate and vehicle treated rats
In this model of anxiety with a vulnerability to panic, L-allylglycine, or its inactive enantiomer, D-allylglycine (control) was infused into the dorsal medial hypothalamus (DMH). This results in disinhibition of the DMH and rats that are prone to panic following intravenous infusion of sodium lactate
Although effects have not been localized specifically to the DRD or the DRC, inescapable stress (IS), relative to escapable stress (ES), increases c-Fos expression in the mid-rostrocaudal and caudal DR, a region that includes the DRD and DRC subregions (Grahn et al, 1999). The differential activation of DRD/DRC serotonergic neurons by IS, relative to ES, is due to activation of glutamatergic afferents from the medial prefrontal cortex during ES, which are thought to act on local γ-aminobutyric acid- (GABA-) synthesizing neurons to inhibit serotonergic neurons in the DRD/DRC (Amat et al, 2005). Retrograde tracing studies combined with c-Fos immunohistochemistry have demonstrated that the DR-projecting prefrontal cortical neurons that are preferentially activated during ES are predominantly localized within the prelimbic cortex (Baratta et al, 2009). A functional role of serotonergic neurons in a BNST DRD/DRC circuit in the behavioral consequences of IS has been well established and has been described in several excellent review articles (Maier and Watkins, 1998; Maier and Watkins, 2005). Lesion of the BNST prevents the behavioral consequences of IS (Hammack et al, 2004). Injection of CRF into the mid-rostrocaudal DR, but not the rostral DR, mimics the behavioral consequences of inescapable stress (IS), including escape deficits and increased fear conditioning, in a model of learned helplessness (Hammack et al, 2002). Likewise, injection of Ucn 2, a member of the CRF family of neuropeptides with high affinity for the CRF2 receptor, into the mid-rostrocaudal DR induces learned helplessness (Hammack et al, 2003). Furthermore, microinjection of the anxiogenic drug methyl–6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM) into the mid-rostrocaudal DR enhances fear conditioning and interferes with shuttlebox escape learning, behaviors characteristic of learned helplessness (Maier et al, 1995a). Conversely, inhibition of the mid-rostrocaudal DR using microinjection of the 5-HT1A receptor agonist, 8-hydroxy-N,N-dipropyl-2-aminotetralin (8-OH-DPAT; Maier et al, 1995b) or blockade of the CRF2 receptor using microinjection of the CRF2 receptor antagonist, antisauvagine-30 (ASV-30; Hammack et al, 2003) block the behavioral consequences of IS and electrolytic lesion of the mid-rostrocaudal DR blocks the behavioral effects of peripheral injection of DMCM (Maier et al, 1995a).
Microinjection of Ucn 2 into the caudal DR increases c-Fos expression in serotonergic neurons in the DRD and increases extracellular concentrations of serotonin in the basolateral amygdala (Amat et al, 2004), a major forebrain target of the DRD (Hale et al, 2008) supporting a role of anxiety-related serotonergic neurons arising from the DRD/DRC region. Consistent with this hypothesis, the anxiogenic effects of IS can be blocked, 24 h later, by antagonism of 5-HT2C receptors in the basolateral amygdala (Christianson et al, 2010). Together, these data suggest that IS sensitizes a subpopulation of anxiety-related serotonergic neurons that project to, among other areas, the basolateral amygdala, and sensitization of this subpopulation of serotonergic neurons is necessary and sufficient for the behavioral consequences of IS in a model of learned helplessness.
The caudal part of the DR (DRC) shares some properties with the mid-rostrocaudal DRD, including a subpopulation of serotonergic neurons that projects to the ventricular system (Mikkelsen et al, 1997). Our recent studies suggest that these ventricle-projecting serotonergic neurons area activated by intracerebroventricular injection of Ucn 2 (Hale et al, 2010), suggesting that this population of neurons forms part of a stress- or anxiety-related neuronal circuit. The role for a Ucn 2-sensitive ventricle-projecting serotonergic system is unclear, however activation of these neurons may play a role diverse physiological functions including the clearance of bioactive molecules and metabolites following stress-related stimuli (Nguyen et al, 2001) or neurogenesis in the subventricular zone (Banasr et al, 2001; Brezun and Daszuta, 1999).
Dorsal raphe nucleus, ventrolateral part (DRVL)/ventrolateral periaqueductal gray (VLPAG)/dorsal raphe nucleus, interfascicular part (DRI) system
Functional neuroanatomic studies suggest that there is a subpopulation of serotonergic neurons located predominantly in the DRVL/VLPAG and DRI subdivisions of the DR that plays a role in anti-panic and antidepressant-like effects. The DRVL/VLPAG region, referred to as the lateral wings of the DR, is located on either side of the midline in the mid-rostrocaudal part of the DR and contains large multipolar serotonergic neurons (Fig. 1). The DRVL/VLPAG region contains a population of interneurons expressing the inhibitory neurotransmitter, GABA (Day et al, 2004) that appears to play a role in local inhibition of serotonergic neurons (Jolas and Aghajanian, 1997). The DRVL/VLPAG region receives afferent projections from brainstem regions associated with autonomic control (Fig. 2), including the lateral parabrachial nucleus (Lee et al, 2003), nucleus of the solitary tract (Herbert, 1992), as well as afferent projections from viscerosensory glossopharyngeal and vagal nerves (Herbert and Saper, 1992). The DRVL/VLPAG region also receives afferents from limbic forebrain regions controlling autonomic and emotional states including the BNST (Gray and Magnuson, 1992; Holstege et al, 1985), central nucleus of the amygdala (Rizvi et al, 1991), lateral and perifornical hypothalamic nuclei (Lee et al, 2003; Luiten et al, 1985) i.e., the region containing orexin-synthesizing neurons (Nambu et al, 1999), median preoptic area (Holstege, 1995) and the infralimbic cortex (Hurley et al, 1991). Amygdala priming following repeated infusion of the urocortin 1, a CRF1/CRF2 receptor ligand, into the basolateral amygdala, which results in a chronic anxiety-like state and vulnerability to panic-like responses to sodium lactate (Lee et al, 2008), selectively alters tph2 mRNA expression in the DRVL/VLPAG region (Donner et al, 2012). Consistent with the hypothesis that the DRVL/VLPAG is an important component of the neuronal circuitry controlling the autonomic and behavioral components of emotional states, the DRVL/VLPAG sends efferent projections to brain structures controlling the fight or flight response (Fig. 3) including the rostral ventrolateral medulla (RVLM; Bago et al, 2002; Underwood et al, 1999), dorsolateral periaqueductal gray (DLPAG: Beitz, 1982; Stezhka and Lovick, 1997) and lateral hypothalamus (Ljubic-Thibal et al, 1999).
Like the DRVL/VLPAG, the pattern of afferent and efferent projections to and from the DRI suggests that this region also plays an important role in the control of emotional behavior (Fig. 2 and 3). The DRI contains a population of serotonergic neurons organized in bilateral columns, oriented in the vertical plane and located between the medial longitudinal fasciculi (Azmitia and Gannon, 1986). The DRI receives afferent input from the median preoptic nucleus (Holstege, 1995) and lateral parabrachial nucleus (Lee et al, 2003; Saper and Loewy, 1980). The DRI sends efferent projections to cortical regions controlling behavioral responses to stress-related stimuli including the dorsolateral prefrontal, medial orbital and anterior cingulate cortices (Porrino and Goldman-Rakic, 1982) as well as the mediodorsal thalamus (Krout et al, 2002; Groenewegen, 1988), dorsal and ventral hippocampus (Amaral and Cowan, 1980; Azmitia and Segal, 1978; Bobillier et al, 1979; Kohler and Steinbusch, 1982; Kohler et al, 1982; Pasquier and Reinoso-Suarez, 1978; Vertes and Fass, 1988; Vertes et al, 1999; Wyss et al, 1979), and entorhinal cortex (Kohler and Steinbusch, 1982).
The DRVL/VLPAG and the DRI tend to be co-activated under conditions associated with inhibition of panic-like physiologic and behavioral responses, and under conditions associated with antidepressant-like effects on behavior (Table 1). For example, DRVL/VLPAG and DRI serotonergic neurons are activated by panicogenic agents, such as sodium lactate, or hypercapnia (Johnson et al, 2007; Johnson et al, 2008; Johnson et al, 2010). In a rodent model of anxiety with increased vulnerability to panic, rats that receive chronic microinfusion of the GABA synthesis inhibitor L-allylglycine into the dorsomedial/perifornical hypothalamus respond to intravenous (i.v.) sodium lactate infusion with robust panic-likeo responses whereas control rats that receive the inactive enantiomer, D-allylglycine, do not (Johnson and Shekhar, 2006). However, rats treated with D-allylglycine show increased c-Fos expression in DRVL/VLPAG and DRI serotonergic neurons in response to i.v. sodium lactate infusion whereas panic-prone rats treated with L-allylglycine do not (Johnson et al, 2008), consistent with the hypothesis that activation of DRVL/VLPAG/DRI serotonergic neurons suppress panic-like physiologic and behavioral responses.
Co-activation of DRVL/VLPAG and DRI serotonergic neurons has also been reported following nicotine administration, exposure to warm or cold ambient temperature and peripheral immune system activation. Recent evidence suggests that acute peripheral administration of nicotine, which is known to be anxiolytic (Brioni et al, 1993; Cheeta et al, 2001b; Cheeta et al, 2001a), activates serotonergic neurons in the DRVL/VLPAG and rostral DR (Sperling and Commons, 2011). Conversely, chronic exposure to nicotine results in an inhibition of DRVL/VLPAG and DRI serotonergic neurons (Sperling and Commons, 2011). We have recently shown that serotonergic neurons in the DRVL/VLPAG and DRI, but not the DRD, are activated following exposure to warm ambient temperature (Hale et al, 2011a), or following exposure to cold swim (Kelly et al, 2011). The number of serotonergic neurons expressing c-Fos in the DRVL/VLPAG following exposure to warm ambient temperature is positively correlated with body temperature leadiong to the suggestion the DRVL/VLPAG and DRI may play a role in thermoregulation. Consistent with this hypothesis, peripheral immune system activation, using the pyrogen, lipopolysaccharide, has convergent effects on c-Fos expression in serotonergic neurons in the DRVL/VLPAG and DRI (Hollis et al, 2006).
Serotonergic neurons in the DRI are activated by administration of the saprophytic, non-pathogenic bacterium, Mycobacterium vaccae (Lowry et al, 2007). Consistent with the pattern of efferent projections arising from the DRI, activation of DRI serotonergic neurons is associated with increased concentrations of serotonin and the serotonin metabolite, 5-hydroxyoindoleacetic acid (5-HIAA) in the medial prefrontal cortex and with antidepressant-like behavioral effects in the forced swim test (Lowry et al, 2007), suggesting that serotonergic neurons in the DRI are an important component of an antidepressant-related neuronal system. Consistent with this hypothesis, overexpression of the serotonin 1B (5-HT1B) autoreceptor in the caudal DR (which includes the DRI) is associated with antidepressant-like effects in the forced swim test (McDevitt et al, 2011).
Interactions between subregions of the dorsal raphe nucleus
Functional neuroanatomic evidence suggests that serotonergic systems in the DR form part of a distinct anxiety-facilitating system including the DRD and DRC and an anti-panic or antidepressant-like system in the DRVL/VLPAG and DRI, however the mechanism through which these systems are controlled are not fully understood. One potential mechanism is that activation of distinct subpopulation of serotonergic neurons is driven by synaptic input from afferent projection regions unique to each subregion of the DR. This “synaptically-driven specificity” model of serotonergic neuronal function has been reviewed extensively elsewhere (Hale and Lowry, 2010). A complementary mechanism may involve direct interactions between the subregions in the DR itself. For example, neuroanatomic studies indicate that neurons within the DRVL/VLPAG region project to the DRD and DRV (see Fig. 1B and 1C in Peyron et al, 1998), and selective lesion of the DRVL/VLPAG serotonergic neurons results in increases in tryptophan hydroxylase gene expression in the DRV (Ljubic-Thibal et al, 1999), suggesting that the DRVL/VLPAG serotonergic neurons provide tonic inhibitory input to DRV serotonergic neurons. Consistent with this hypothesis, activation of DRVL/VLPAG serotonergic neurons following acute nicotine exposure is associated with inhibition of DRV serotonergic neurons (Sperling and Commons, 2011).
Future directions
An important objective for future studies is to determine the functional consequences of selective lesion or microinjection of pharmacological compounds into specific subregions of the DR on serotonergic neurotransmission and physiological and behavioral responses. A few studies have begun to address this important question. For example, Neumaier and colleagues have demonstrated that overexpression of 5-HT1B receptors in the caudal DR increases swimming in the forced swim test and reduces conditioned freezing (McDevitt et al, 2011). Meanwhile, small volume microinjections (250 nl) of corticotropin-releasing factor into the caudal, but not rostral, DR, induces escape deficits and increases fear conditioning 24 h later in a model of learned helplessness (Hammack et al, 2002). Further studies, using even smaller volume injections (e.g. 50–100 nl) to isolate functional properties of different subregions of the DR, are an important objective for future studies.
We are not yet at a point where we can use knowledge about anatomical and functional subdivisions of the DR to develop novel therapeutic strategies for treatment of anxiety or affective disorders. There are a number of steps that should be taken to bring us closer to this aim. First, new studies are required involving selective lesions or microinjections of pharmacological compounds into specific subregions of the DR to further elucidate functional properties of subdivisions of the DR. Second, studies designed to understand the afferent pathways and mechanisms controlling specific subpopulations of serotonergic neurons are required in order to provide opportunities to modulate serotonergic function through modulation of afferent signalling mechanisms. Third, further understanding of the genetic lineages and molecular properties of subpopulations of serotonergic neurons are required to identify unique molecular and cellular properties of subpopulations of serotonergic neurons that can be used to selectively target them.
Although we have focused in this review on serotonergic systems, a comprehensive approach to understanding the role of monoaminergic systems in anxiety and affective disorders will take into account reciprocal interactions among serotonergic, dopaminergic, and noradrenergic systems. For example, both dopaminergic (Guiard et al, 2008) and noradrenergic systems (Vandermaelen and Aghajanian, 1983) stimulate serotonergic neuronal firing, while serotonergic systems play important roles in modulating both dopaminergic and noradrenergic neuronal activity (Di Giovanni et al, 2008; Singewald et al, 1999; Szabo et al, 1999).
Conclusions
Recent functional neuroanatomic evidence supports the hypothesis of an anxiety-facilitating serotonergic system in the DRD/DRC regions of the DR and an anti-panic and/or antidepressant-like serotonergic system located in the DRVL/VLPAG and DRI regions of the DR. Understanding the anatomic and functional properties of these distinct serotonergic systems may lead to novel therapeutic strategies for the prevention and/or treatment of affective and anxiety disorders.
Acknowledgments
The project described was supported by R01MH086539 (CAL) and R01MH065702 (AS/CAL) from the National Institute of Mental Health and Grant No. 0845550 (CAL) from the National Science Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, or the National Science Foundation.
Reference List
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. 1980;189:573–591. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ. The primate serotonergic system: A review of human and animal studies and a report on Macaca fascicularis. In: Fahn S, et al., editors. Myoclonus. Raven Press; New York: 1986. pp. 407–468. [PubMed] [Google Scholar]
- Azmitia EC, Jr, Segal M. An autoradiographic analysis of the differential ascending projection of the dorsal and median raphe nuclei in the rat. Journal Of Comparative Neurology. 1978;179:651–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008;13:507–513. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago M, Marson L, Dean C. Serotonergic projections to the rostroventrolateral medulla from midbrain and raphe nuclei. Brain Research. 2002;945:249–258. doi: 10.1016/s0006-8993(02)02811-1. [DOI] [PubMed] [Google Scholar]
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. European Journal of Neuroscience. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci. 2009;30:1111–1116. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton DA, Esler MD, Dawood T, Lambert EA, Haikerwal D, Brenchley C, Socratous F, Hastings J, Guo L, Wiesner G, Kaye DM, Bayles R, Schlaich MP, Lambert GW. Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Archives of General Psychiatry. 2008;65:38–46. doi: 10.1001/archgenpsychiatry.2007.11. [DOI] [PubMed] [Google Scholar]
- Beck SG, Pan YZ, Akanwa AC, Kirby LG. Median and dorsal raphe neurons are not electrophysiologically identical. Journal of Neurophysiology. 2004;91:994–1005. doi: 10.1152/jn.00744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Cremers TI. Do selective serotonin reuptake inhibitors acutely increase frontal cortex levels of serotonin? European Journal of Pharmacology. 2008;580:350–354. doi: 10.1016/j.ejphar.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Bobillier P, Seguin S, Dugueurce A, Lewis BD, Pujol JF. The efferent connections of the nucleus raphe centralis superior in the rat as revealed by autoradiography. Brain Research. 1979;166:1–8. doi: 10.1016/0006-8993(79)90644-9. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Turecki G, Austin MC. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse. 2006;60:81–85. doi: 10.1002/syn.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Brioni JD, O’Neill AB, Kim DJ, Decker MW. Nicotinic receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. Eur J Pharmacol. 1993;238:1–8. doi: 10.1016/0014-2999(93)90498-7. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Irvine EE, Kenny PJ, File SE. The dorsal raphe nucleus is a crucial structure mediating nicotine’s anxiolytic effects and the development of tolerance and withdrawal responses. Psychopharmacology (Berl) 2001a;155:78–85. doi: 10.1007/s002130100681. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Tucci S, File SE. Antagonism of the anxiolytic effect of nicotine in the dorsal raphe nucleus by dihydro-beta-erythroidine. Pharmacol Biochem Behav. 2001b;70:491–496. doi: 10.1016/s0091-3057(01)00641-4. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biological Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG. Evidence for topographically organized endogenous 5-HT-1A receptor-dependent feedback inhibition of the ascending serotonin system. Eur J Neurosci. 2008;27:2611–2618. doi: 10.1111/j.1460-9568.2008.06235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience. 2009;161:680–690. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LK, Craige CP, Beck SG. Increased intrinsic excitability of lateral wing serotonin neurons of the dorsal raphe: a mechanism for selective activation in stress circuits. Journal of Neurophysiology. 2010 doi: 10.1152/jn.01132.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in cell bodies of brain stem neurons. Acta Physiologica Scandinavica. 1964;62:5–55. [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha(1b) adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. Journal Of Comparative Neurology. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JFW, Graeff FG. 5-HT and mechanisms of defence. Journal of Psychopharmacology. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Dhingra NK, Raju TR, Meti BL. Selective reduction of monoamine oxidase A and B in the frontal cortex of subordinate rats. Brain Res. 1997;758:237–240. doi: 10.1016/s0006-8993(96)01477-1. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di MV, Pierucci M, Esposito E. Serotonin-dopamine interaction: electrophysiological evidence. Progress in Brain Research. 2008;172:45–71. doi: 10.1016/S0079-6123(08)00903-5. [DOI] [PubMed] [Google Scholar]
- Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depression and Anxiety. 2012 doi: 10.1002/da.21925. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biological Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert E, Alvarenga M, Socratous F, Richards J, Barton D, Pier C, Brenchley C, Dawood T, Hastings J, Guo L, Haikerwal D, Kaye D, Jennings G, Kalff V, Kelly M, Wiesner G, Lambert G. Increased brain serotonin turnover in panic disorder patients in the absence of a panic attack: reduction by a selective serotonin reuptake inhibitor. Stress. 2007;10:295–304. doi: 10.1080/10253890701300904. [DOI] [PubMed] [Google Scholar]
- Evans AK, Heerkens JL, Lowry CA. Acoustic stimulation in vivo and corticotropin-releasing factor in vitro increase tryptophan hydroxylase activity in the rat caudal dorsal raphe nucleus. Neurosci Lett. 2009;455:36–41. doi: 10.1016/j.neulet.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26:8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA. Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol. 2009;512:529–555. doi: 10.1002/cne.21921. [DOI] [PubMed] [Google Scholar]
- Graeff FG. Brain defense systems and anxiety. In: Roth M, Burrows GD, Noyes R, editors. Handbook of Anxiety. Vol. 3. Elsevier; Amsterdam: 1990. pp. 307–354. [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacology, Biochemistry and Behavior. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Research. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol. 2008;11:625–639. doi: 10.1017/S1461145707008383. [DOI] [PubMed] [Google Scholar]
- Haghighi F, Bach-Mizrachi H, Huang YY, Arango V, Shi S, Dwork AJ, Rosoklija G, Sheng HT, Morozova I, Ju J, Russo JJ, Mann JJ. Genetic architecture of the human tryptophan hydroxylase 2 Gene: existence of neural isoforms and relevance for major depression. Mol Psychiatry. 2008;13:813–820. doi: 10.1038/sj.mp.4002127. [DOI] [PubMed] [Google Scholar]
- Hale MW, Dady KF, Evans AK, Lowry CA. Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling. Experimental Neurology. 2011a;277:271–281. doi: 10.1016/j.expneurol.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Bouwknecht JA, Evans AK, Stamper CE, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience. 2008;157:733–748. doi: 10.1016/j.neuroscience.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2010;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA. Development by environment interactions controlling tryptophan hydroxylase expression. Journal of Chemical Neuroanatomy. 2011b;41:219–226. doi: 10.1016/j.jchemneu.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Stamper CE, Staub DR, Lowry CA. Urocortin 2 increases c-Fos expression in serotonergic neurons projecting to the ventricular/periventricular system. Experimental Neurology. 2010;224:271–281. doi: 10.1016/j.expneurol.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. Journal of Neuroscience. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H. Evidence for projections from medullary nuclei onto serotonergic and dopaminergic neurons in the midbrain dorsal raphe nucleus of the rat. Cell Tissue Res. 1992;270:149–156. doi: 10.1007/BF00381889. [DOI] [PubMed] [Google Scholar]
- Herbert J, Saper CB. Organization of medullary adrenergic and noradrenergic projections to the periaqueductal gray matter in the rat. Journal Of Comparative Neurology. 1992;315:34–52. doi: 10.1002/cne.903150104. [DOI] [PubMed] [Google Scholar]
- Hollis JH, Evans AK, Bruce KP, Lightman SL, Lowry CA. Lipopolysaccharide has indomethacin-sensitive actions on Fos expression in topographically organized subpopulations of serotonergic neurons. Brain, Behavior, and Immunity. 2006;20:569–577. doi: 10.1016/j.bbi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Holstege G. The basic, somatic, and emotional components of the motor system in mammals. In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego: 1995. pp. 137–154. [Google Scholar]
- Holstege G, Meiners L, Tan K. Projections of the bed nucleus of the stria terminalis to the mesencephalon, pons, and medulla oblongata in the cat. Exp Brain Res. 1985;58:379–391. doi: 10.1007/BF00235319. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. Journal Of Comparative Neurology. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nature Neuroscience. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Fitz SD, Hollis JH, Moratalla R, Lightman SL, Shekhar A, Lowry CA. Induction of c-Fos in ‘panic/defence’-related brain circuits following brief hypercarbic gas exposure. J Psychopharmacol. 2010 Jan 15;2010 doi: 10.1177/0269881109353464. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Johnson PL, Lowry CA, Truitt W, Shekhar A. Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J Psychopharmacol. 2008;22:642–645. doi: 10.1177/0269881107082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Shekhar A. Panic-prone state induced in rats with GABA dysfunction in the dorsomedial hypothalamus is mediated by NMDA receptors. J Neurosci. 2006;26:7093–7104. doi: 10.1523/JNEUROSCI.0408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural Pathways Underlying Lactate-Induced Panic. Neuropsychopharmacology. 2007;33:2093–2107. doi: 10.1038/sj.npp.1301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolas T, Aghajanian GK. Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Research. 1997;755:229–245. doi: 10.1016/s0006-8993(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Donner NC, Hale MW, Lowry CA. Swim stress activates serotonergic and nonserotonergic neurons in specific subdivisions of the rat dorsal raphe nucleus in a temperature-dependent manner. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, Gaspar P. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J Neurosci. 2011;31:2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyasova V, Gaspar P. Development of raphe serotonin neurons from specification to guidance. Eur J Neurosci. 2011;34:1553–1562. doi: 10.1111/j.1460-9568.2011.07910.x. [DOI] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V, Steinbusch H. The distribution and origin of serotonin-containing fibers in the septal area: a combined immunohistochemical and fluorescent retrograde tracing study in the rat. Journal Of Comparative Neurology. 1982;209:91–111. doi: 10.1002/cne.902090109. [DOI] [PubMed] [Google Scholar]
- Kohler C, Steinbusch H. Identification of serotonin and non-serotonin-containing neurons of the mid-brain raphe projecting to the entorhinal area and the hippocampal formation. A combined immunohistochemical and fluorescent retrograde tracing study in the rat brain. Neuroscience. 1982;7:951–975. doi: 10.1016/0306-4522(82)90054-9. [DOI] [PubMed] [Google Scholar]
- Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. Journal Of Comparative Neurology. 2002;448:53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- Le Francois B, Czesak M, Steubl D, Albert PR. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology. 2008;55:977–985. doi: 10.1016/j.neuropharm.2008.06.046. [DOI] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Research. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. Journal of Neuroscience. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubic-Thibal V, Morin A, Diksic M, Hamel E. Origin of the serotonergic innervation to the rat dorsolateral hypothalamus: retrograde transport of cholera toxin and upregulation of tryptophan hydroxylase mRNA expression following selective nerve terminals lesion. Synapse. 1999;32:177–186. doi: 10.1002/(SICI)1098-2396(19990601)32:3<177::AID-SYN4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Lopez de Lara C, Brezo J, Rouleau G, Lesage A, Dumont M, Alda M, Benkelfat C, Turecki G. Effect of tryptophan hydroxylase-2 gene variants on suicide risk in major depression. Biological Psychiatry. 2007;62:72–80. doi: 10.1016/j.biopsych.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A. Topographical organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti JM, Pandi-Perumal BL, Jacobs BL, Nutt DL, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Birkhauser; Basel: 2008a. pp. 25–68. [Google Scholar]
- Lowry CA, Hale MW. Serotonin and the neurobiology of anxious states. In: Mûller CP, Jacobs BL, editors. Handbook of the Behavioral Neurobiology of Serotonin. Elsevier; Amsterdam: 2010. pp. 379–398. [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Annals Of The New York Academy Of Sciences. 2008b;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hollis JH, de VA, Pan B, Brunet LR, Hunt JR, Paton JF, van KE, Knight DM, Evans AK, Rook GA, Lightman SL. Identification of an immune-responsive mesolimbocortical serotonergic system: Potential role in regulation of emotional behavior. Neuroscience. 2007;146:756–772. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Luiten PG, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Research. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- MacGillivray L, Lagrou LM, Reynolds KB, Rosebush PI, Mazurek MF. Role of serotonin transporter inhibition in the regulation of tryptophan hydroxylase in brainstem raphe nuclei: time course and regional specificity. Neuroscience. 2010;171:407–420. doi: 10.1016/j.neuroscience.2010.08.055. [DOI] [PubMed] [Google Scholar]
- Maier SF, Busch CR, Maswood S, Grahn RE, Watkins LR. The dorsal raphe nucleus is a site of action mediating the behavioral effects of the benzodiazepine receptor inverse agonist DMCM. Behav Neurosci. 1995a;109:759–766. doi: 10.1037//0735-7044.109.4.759. [DOI] [PubMed] [Google Scholar]
- Maier SF, Grahn RE, Watkins LR. 8-OH-DPAT microinjected in the region of the dorsal raphe nucleus blocks and reverses the enhancement of fear conditioning and interference with escape produced by exposure to inescapable shock. Behav Neurosci. 1995b;109:404–412. doi: 10.1037//0735-7044.109.3.404. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LF. Stressor controllability, anxiety, and serotonin. Cognitive Therapy Res. 1998;22:595–613. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Hiroi R, Mackenzie SM, Robin NC, Cohn A, Kim JJ, Neumaier JF. Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior. Biol Psychiatry. 2011;69:780–787. doi: 10.1016/j.biopsych.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JD, Hay-Schmidt A, Larsen PJ. Central innervation of the rat ependyma and subcommissural organ with special reference to ascending serotoninergic projections from the raphe nuclei. Journal Of Comparative Neurology. 1997;384:556–568. [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Chin WC, O’Brien JA, Verdugo P, Berger AJ. Intracellular pathways regulating ciliary beating of rat brain ependymal cells. J Physiol. 2001;531:131–140. doi: 10.1111/j.1469-7793.2001.0131j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier DA, Reinoso-Suarez F. The topographic organization of hypothalamic and brain stem projections to the hippocampus. Brain Research Bulletin. 1978;3:373–389. doi: 10.1016/0361-9230(78)90106-5. [DOI] [PubMed] [Google Scholar]
- Paul ED, Hale MW, Lukkes JL, Valentine MJ, Sarchet DM, Lowry CA. Repeated social defeat increases reactive emotional coping behavior and alters functional responses in serotonergic neurons in the rat dorsal raphe nucleus. Physiol Behav. 2011;104:272–282. doi: 10.1016/j.physbeh.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Peters EJ, Slager SL, McGrath PJ, Knowles JA, Hamilton SP. Investigation of serotonin-related genes in antidepressant response. Mol Psychiatry. 2004;9:879–889. doi: 10.1038/sj.mp.4001502. [DOI] [PubMed] [Google Scholar]
- Petit J-M, Luppi P-H, Peyron C, Rampon C, Jouvet M. VIP-like immunoreactive projections from the dorsal raphe and caudal linear nuclei to the bed nucleus of the stria terminalis demonstrated by a double immunohistochemical method in the rat. Neurosci Lett. 1995;193:77–80. doi: 10.1016/0304-3940(95)11669-n. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit J-M, Rampon C, Jouvet M, Luppi P-H. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Goldman-Rakic PS. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. Journal Of Comparative Neurology. 1982;205:63–76. doi: 10.1002/cne.902050107. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. Journal Of Comparative Neurology. 1991;303:121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF. Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. Journal of Neuroscience. 2011;31:14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Simpson KL, Fisher TM, Waterhouse BD, Lin RC. Projection patterns from the raphe nuclear complex to the ependymal wall of the ventricular system in the rat. Journal Of Comparative Neurology. 1998;399:61–72. doi: 10.1002/(sici)1096-9861(19980914)399:1<61::aid-cne5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Singewald N, Kaehler ST, Philippu A. Noradrenaline release in the locus coeruleus of conscious rats is triggered by drugs, stress and blood pressure changes. Neuroreport. 1999;10:1583–1587. doi: 10.1097/00001756-199905140-00035. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biological Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry. 2012 doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spannuth BM, Hale MW, Evans AK, Lukkes JL, Campeau S, Lowry CA. Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fear-potentiated startle. Neuroscience. 2011;179:104–119. doi: 10.1016/j.neuroscience.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Commons KG. Shifting topographic activation and 5-HT1A receptor-mediated inhibition of dorsal raphe serotonin neurons produced by nicotine exposure and withdrawal. Eur J Neurosci. 2011;33:1866–1875. doi: 10.1111/j.1460-9568.2011.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub DR, Evans AK, Lowry CA. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF(2)) receptors in the regulation of subpopulations of serotonergic neurons. Brain Research. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Research. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- Stezhka VV, Lovick TA. Projections from dorsal raphe nucleus to the periaqueductal grey matter: studies in slices of rat midbrain maintained in vitro. Neurosci Lett. 1997;230:57–60. doi: 10.1016/s0304-3940(97)00464-3. [DOI] [PubMed] [Google Scholar]
- Szabo ST, de Montigny C, Blier P. Modulation of noradrenergic neuronal firing by selective serotonin reuptake blockers. British Journal of Pharmacology. 1999;126:568–571. doi: 10.1038/sj.bjp.0702343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MD, Arango V, Bakalian MJ, Ruggiero DA, Mann JJ. Dorsal raphe nucleus serotonergic neurons innervate the rostral ventrolateral medulla in rat. Brain Research. 1999;824:45–55. doi: 10.1016/s0006-8993(99)01181-6. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Bey V, Pernar L, Commons KG. Substance P Acts through local circuits within the rat dorsal raphe nucleus to alter serotonergic neuronal activity. J Neurosci. 2003;23:7155–7159. doi: 10.1523/JNEUROSCI.23-18-07155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Research. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Research. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fass B. Projections between the interpeduncular nucleus and basal forebrain in the rat as demonstrated by the anterograde and retrograde transport of WGA-HRP. Exp Brain Res. 1988;73:23–31. doi: 10.1007/BF00279657. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. Journal Of Comparative Neurology. 1999;407:555–582. [PubMed] [Google Scholar]
- Wyss JM, Swanson LW, Cowan WM. A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience. 1979;4:463–476. doi: 10.1016/0306-4522(79)90124-6. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R, Moller HJ, Bondy B, Ackenheil M. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]