Abstract
Objectives
The renal capacity for sodium excretion is impaired by a reduction in the glomerular ultrafiltration coefficient and by enhancement of the fractional tubular sodium reabsorption (FRNa), leading to a nondipper circadian blood pressure (BP) rhythm. Angiotensin II in the systemic circulation can be easily filtered across the glomerular capillary walls and stimulates renal proximal tubular angiotensinogen (PT-AGT) production, leading to the activation of intrarenal angiotensin II, which is known to augment the FRNa in animal models.
Methods
We performed an immunohistochemical investigation to determine the contribution of PT-AGT to enhancement of FRNa and the nondipper circadian BP rhythm in 40 consecutive patients with primary IgA nephropathy (IgAN).
Results
Immunostaining for PT-AGT was increased in the IgAN patients compared with control individuals (P = 0.04), and correlated directly with the FRNa (r = 0.39, P = 0.01) and the night/day ratio of BP (r = 0.38, P = 0.02), but not creatinine clearance (r = −0.008, P = 0.9). The night/day ratio of BP was determined by both creatinine clearance (r = −0.36, P = 0.03) and FRNa (r = 0.47, P = 0.006).
Conclusion
Tubular sodium reabsorption is stimulated by intrarenal angiotensin II, as indicated by PT-AGT, and contributes to the genesis of the nondipper BP rhythm. Further studies are needed to evaluate whether or not treatment to prevent sodium retention is useful for patients who exhibit increased PT-AGT in renal biopsies.
Keywords: angiotensinogen, blood pressure, circadian rhythm, immunohistochemistry, sodium
INTRODUCTION
In healthy individuals, night-time blood pressure (BP) essentially declines by 10–20% compared with the daytime BP (i.e. dippers). Patients who do not exhibit a BP dip at night are classified as nondippers [1]. We have advocated that in patients whose kidneys exhibit impaired sodium excretion, sodium retention occurs during the day, which prevents the nocturnal BP dip to promote pressure natriuresis [2,3]. Subsequently, our group has further postulated that the renal capacity for sodium excretion is impaired by two mechanisms: reduction of the glomerular ultrafiltration coefficient (KF), and enhancement of the fractional tubular sodium reabsorption (FRNa; tubular reabsorption to filtered load ratio) [4]. A good example of the former mechanism was well illustrated in our previous study that demonstrated inverse relationships between the glomerular filtration rate (GFR) and the night/day ratios of BP and urinary sodium excretion rate (UNaV) in patients with biopsy-proven glomerular disease [5]. Examples of the latter mechanism were shown in the patients whose renal function was preserved, and in those studies, the enhancement in FRNa also impaired renal sodium excretion [6]. In fact, our group has reported that treatment with diuretics, which inhibited FRNa, restored the nondipper circadian BP rhythm [7,8].
Meanwhile, we have developed concise and accurate methods to measure human angiotensinogen (AGT) [9,10], and have proposed that angiotensin II (Ang II) in the systemic circulation can be easily filtered across the glomerular capillary walls, to stimulate proximal tubular expression of AGT and further augment production of Ang II [11]. In this manner, the proximal tubule is the primary site for AGT synthesis, leading to increases in intrarenal Ang II production [12,13]. We have also found that in Ang II-infused hypertensive rats, concomitant treatment with an Ang II type 1 receptor (AT1R) blocker (ARB), olmesartan, substantially prevented the increase in kidney levels of Ang II and AGT [14]. In this model, immunoreactivity for proximal tubular AGT (PT-AGT) changed in parallel with the kidney content of Ang II and AGT [14], suggesting that PT-AGT could represent the intrarenal pathophysiological state of Ang II. A variety of animal models have shown that inappropriate activation of intrarenal Ang II impaired renal sodium excretion through various direct and indirect mechanisms. Among the direct actions, Ang II stimulates tubular sodium reabsorption at various segments along the pathway from the proximal [15] to distal tubules [16] and even in the collecting ducts [17], and also enhances tubuloglomerular feedback sensitivity to produce sustained decreases in the distal nephron delivery volume [18]. These antinatriuretic effects are known to be inhibited by ARBs via the AT1R in animal models [14,19]. In humans, we also have reported that treatment with olmesartan increased daytime UNaV leading to the restoration of a nondipper BP rhythm [20]. In another study, we found that suppression of FRNa was the mechanism underlying the effect of ARB, to normalize the circadian BP rhythm, similar to diuretics [21]. We speculate that in these patients with chronic kidney disease (CKD), intrarenally-activated Ang II had stimulated FRNa before the treatment and that the ARB acted against the intrarenal Ang II activation to increase UNaV, because olmesartan prevented proximal tubular AGT production in the aforementioned animal models [14]. However, it has not yet been shown whether intrarenally-activated Ang II augments FRNa in humans. These findings encouraged us to test the hypothesis that patients with increased PT-AGT, indicating activated intrarenal Ang II, exhibit enhanced FRNa, contributing to a nondipper-type of circadian BP rhythm.
METHODS
The study was an immunohistochemical study approved by the Ethics Review Committee of the Nagoya City University Graduate School of Medical Sciences, and was conducted in accordance with the Declaration of Helsinki. All of the IgA nephropathy (IgAN) and control individuals provided written informed consent.
Patients and study design
To be eligible for the study, patients had to have a biopsy-proven primary IgAN, and undergone both 24-h ambulatory BP measurement and separate day and night-time urine collection at the time of their initial renal biopsy, as described below. The diagnosis was confirmed by the presence of mesangial proliferative glomerulonephritis with predominant deposition of IgA in the glomerular mesangial regions. Patients with secondary IgAN, such as Henoch Schönlein purpura, systemic lupus erythematosus or liver disease, were excluded. We also excluded patients with concomitant diabetes mellitus, nephrotic syndrome, and those receiving antihypertensive agents, diuretics, glucocorticoids, or immunosuppressive agents, because these could influence the circadian BP rhythm or AGT expression. Accordingly, 40 consecutive patients were eligible for this study.
Histological study
Renal specimens were obtained by percutaneous needle biopsy, and were processed by standard techniques for light, immunofluorescence, and electron microscopy. The samples for light microscopic examination and immunohistochemistry were fixed in 10% buffered formalin and embedded in paraffin. Serial 3-μm-thick sections were stained with hematoxylin and eosin, Masson’s trichrome, periodic acid-Schiff, and methenamine silver methods for light microscopic examination. The samples were coded and examined by two independent observers who were blinded to any clinical data. Modifying the previous studies [22,23], histological changes were scored as follows: percentage of glomeruli showing global obsolescence; glomerular sclerosis (0 = none, 1 = segmental, or global in <25%, 2 = global in 25–50%, and 3 = global in >50% of glomeruli); mesangial proliferation (0 = none, 1 = mild: 3–4 mesangial cells per peripheral lobule, 2 = severe segmental: >4 mesangial cells per peripheral lobule, 3 = severe global proliferation); increase in mesangial matrix (0 = none, 1 = mild, 2 = marked segmental: the width of the mesangial interspaces between capillaries exceeding three mesangial cells, 3 = marked global increase); interstitial fibrosis (0 = none, 1 ≤ 25%, 2 = 26–50%, and 3 > 50% of the cortical area); tubular atrophy (0 = none, 1 ≤ 25%, 2 = 26–50%, and 3 > 50% of the cortical tubules); arteriolar hyalinosis (0 = none, 1 = mild hyalinosis in at least 1 arteriole, 2 = severe hyalinosis in at least 1 arteriole, 3 = severe hyalinosis in many arterioles); arteriosclerosis (0 = none, 1 ≤ 25%, 2 = 26–50%, 3 > 50% luminal narrowing by fibroelastic intimal thickening in the most severely affected vessel).
Immunohistochemical analysis for PT-AGT was performed at Tulane University with a mouse monoclonal antihuman AGT antibody (IBL Co. Ltd., Takasaki, Japan) using a robotic system (Autostainer; Dako Cytomation, Glostrup, Denmark) to apply the exact same conditions to all slides to obtain the results expressed in arbitrary units, without knowledge of the patients’ clinical course, as described previously [9,10,14]. The control specimens for the immunohistochemistry were obtained from patients who had undergone renal biopsy for the evaluation of microscopic hematuria or chance albuminuria but were proven not to have any kidney diseases (n = 4). These specimens showed normal glomerular morphology and negative immunofluorescence; although one of the four control patients revealed nonspecific tubular atrophy and interstitial fibrosis in less than 10% of the cortical area.
Blood pressure measurement and urine collection
Twenty-four-hour BP monitoring and daytime (0600–2100 h) and night-time (2100–0600 h) urine collection were conducted on the same day under hospitalization, 2 days before renal biopsy. Prior to these examinations, the patients consumed a hospital-served diet containing 7 g/day of salt for at least 1 week, and were asked to get up at 0600 h and start bed-rest at 2100 h. The urine collections were combined to calculate 24-h creatinine clearance (ml/min), which was used as an index for GFR. Filtered sodium load was calculated as the product of the GFR and plasma sodium concentration (SNa), and tubular sodium reabsorption was calculated as the difference between filtered sodium load and absolute urinary sodium excretion [21]. FRNa was then calculated as the tubular sodium reabsorption to filtered sodium load ratio. Blood samples were collected only once at 0600 h, which was the marginal point between day and night-time. Blood samples for evaluating plasma renin activity (PRA), plasma aldosterone concentration (PAC) and Ang II were centrifuged at 3000 r.p.m. for 10 min at 4°C, and were frozen immediately and stored at −35°C until assay. PRA, PAC, and Ang II were then assayed by radioimmunoassay at an external analysis center (SRL, Inc., Hachioji, Japan). During the 24-h BP monitoring, BP was monitored noninvasively every 30 min with a validated automatic device (ES-H531; Terumo, Tokyo, Japan). The BP values were not considered valid for analysis if data were missing continuously for 2 h or if the patients awoke during the night and had difficulty falling asleep again. Mean arterial pressure (MAP) was calculated as the diastolic BP plus one-third of the pulse BP. Daytime BP was calculated as the average of the 30 readings between 0600 and 2100 h, whereas night-time BP was the average of the remaining 18 readings. Patients, whose nocturnal fall in MAP was more than 10% from daytime to night-time, were classified as dippers, whereas the others were classified as nondippers.
Statistical analysis
Results are expressed as the mean ± SD. Data distribution was tested by Kolmogorov–Smirnov test. Significance of differences was examined using unpaired t-test or by the nonparametric Mann–Whitney U-test, when appropriate. Categorical variables were compared using the chi-squared test. Correlations among quantitative variables were evaluated by the least-squares method, and correlations with categorical variables were tested by Spearman rank correlation test. Forward stepwise multiple regression analysis was conducted to compare the contribution of each independent variable to the night/day ratio of MAP. The independent variables were clinical variables, and histological variables. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS software, version 15.0 J (SPSS Inc, Chicago, Illinois, USA).
RESULTS
At the time of biopsy, the patients (n = 40, 18 women and 22 men) were aged 31 ± 11 (range 17–58) years and with a BMI 22.7 ± 4.6 kg/m2. Their renal biopsies had been performed for the evaluation of microscopic hematuria or brown color gross hematuria, both of which should be accompanied by red cell casts, indicating glomerulonephritis.
Unlike our previous studies [3,5,20,21], the significance of the inverse relationship between creatinine clearance and the night/day ratio of MAP was weakened (r = −0.21, P = 0.2). The FRNa correlated positively with the night/day ratio of MAP (r = 0.36, P = 0.02). The night/day ratio of MAP showed no significant relationship with PRA (r = 0.07, P = 0.7), PAC (r = 0.12, P = 0.5), or serum Ang II concentration (r = 0.19, P = 0.2). When analyzed by stepwise multiple regression analysis (R2 = 0.22, P = 0.01), the main determinants of night/day ratio of MAP were creatinine clearance (r = −0.36, F = 4.9, P = 0.03) and FRNa (r = 0.47, F = 8.4, P = 0.006), rather than age, sex, BMI, PRA, PAC or Ang II.
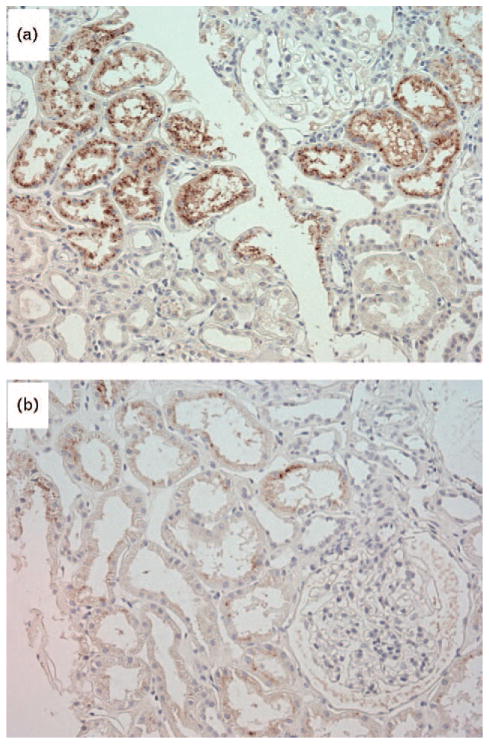
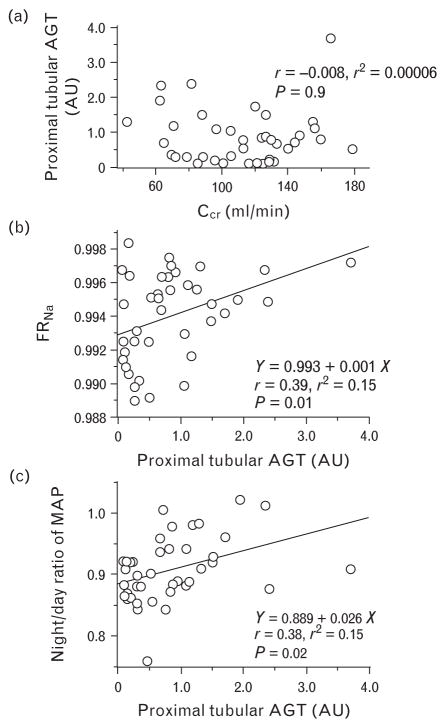
We did not find any differences in demographics between the patients with IgAN and the control individuals (Table 1). Compared with control individuals, the IgAN patients exhibited significantly higher immunoreactivity for AGT expression in their proximal tubules (P = 0.04) (Fig. 1, Table 2). Although PT-AGT did not correlate with creatinine clearance (r = −0.008, P = 0.9; Fig. 2a), it did exhibit direct correlations with the FRNa (r = 0.39, P = 0.01; Fig. 2b), the night/day ratio of MAP (r = 0.38, P = 0.02, Fig. 2c), and that of UNaV (r = 0.33, P = 0.04). None of the PRA (r = −0.12, P = 0.5), PAC (r = −0.03, P = 0.8), or serum Ang II concentrations (r = 0.27, P = 0.1) showed any significant correlations with PT-AGT. Compared with dipper patients (n = 19), nondipper patients (n = 21) exhibited higher values of FRNa (P = 0.03) and PT-AGT (P = 0.02).
TABLE 1.
Demographics and clinical variables
| IgAN (n = 40) | Control (n = 4) | P value | |
|---|---|---|---|
| Male/female | 18/22 | 2/2 | 0.9 |
|
| |||
| Age (years) | 31 ± 11 | 45 ± 16 | 0.1 |
|
| |||
| BMI (kg/m2) | 23 ± 5 | 22 ± 4 | 0.9 |
|
| |||
| Creatinine clearance (ml/min) | 111 ± 33 | 113 ± 23 | 0.9 |
|
| |||
| SBP (mmHg) | |||
| Day | 117 ± 13 | 115 ± 19 | 0.8 |
| Night | 107 ± 13 | 105 ± 15 | 0.8 |
| 24 h | 113 ± 13 | 112 ± 18 | 0.8 |
|
| |||
| DBP (mmHg) | |||
| Day | 74 ± 11 | 79 ± 16 | 0.4 |
| Night | 67 ± 10 | 70 ± 15 | 0.6 |
| 24 h | 72 ± 10 | 76 ± 16 | 0.4 |
|
| |||
| Night/day ratio of MAP | 0.91 ± 0.05 | 0.89 ± 0.05 | 0.7 |
|
| |||
| Night/day ratio of UNaV | 0.81 ± 0.47 | 0.80 ± 0.46 | 0.9 |
|
| |||
| Fractional tubular sodium reabsorption | 0.994 ± 0.003 | 0.994 ± 0.002 | 0.9 |
|
| |||
| Plasma renin activity (ng/ml per h) | 1.7 ± 1.6 | 0.7 ± 0.6 | 0.3 |
|
| |||
| Plasma aldosterone concentration (pg/ml) | 135 ± 93 | 37 ± 15 | 0.08 |
|
| |||
| Plasma angiotensin II (pg/ml) | 9.7 ± 5.2 | 7.5 ± 0.7 | 0.6 |
Values are means ± SD. IgAN, IgA nephropathy; MAP, mean arterial pressure (mmHg); UNaV, urinary sodium excretion rate (mmol/h).
FIGURE 1.
Immunoreactivity for angiotensinogen in the proximal tubules. Proximal tubular angiotensinogen immunostaining was more intense in the patients with IgA nephropathy (a) compared with that in the control individuals (b). Counter-stained with hematoxylin.
TABLE 2.
Histological changes in IgAN vs. controls
| IgAN (n = 40) | Control (n = 4) | P value | ||
|---|---|---|---|---|
| Proximal tubular AGT | 0.40 ± 4.24 | 0.08 ± 5.02 | 0.04 | |
|
| ||||
| Glomerular lesionsa | ||||
| Glomerular obsolescence | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.6 | |
| Glomerular sclerosis | 0.6 ± 0.6 | 0.0 ± 0.0 | 0.02 | |
| Mesangial proliferation | 0.4 ± 0.5 | 0.0 ± 0.0 | 0.01 | |
| Mesangial matrix increase | 0.4 ± 0.5 | 0.0 ± 0.0 | 0.005 | |
|
| ||||
| Tubulo-interstitial and vascular lesions | ||||
| Interstitial fibrosis | No. of patients (%) | |||
| 0 (absent) | 24 (60.0) | 3 (75.0) | 0.7 | |
| 1 (≤25%) | 10 (25) | 1 (25.0)b | ||
| 2 (26–50%) | 6 (15) | 0 | ||
| 3 (>50%) | 0 | 0 | ||
| Tubular atrophy | No. of patients (%) | |||
| 0 (absent) | 31 (77.5) | 3 (75.0) | 0.8 | |
| 1 (≤25%) | 6 (15.0) | 1 (25.0)b | ||
| 2 (26–50%) | 3 (7.5) | 0 | ||
| 3 (>50%) | 0 | 0 | ||
| Arteriolar hyalinosis | No. of patients (%) | |||
| 0 (absent) | 36 (90.0) | 4 (100) | 0.5 | |
| 1 (mild) | 4 (10.0) | 0 | ||
| 2 (severe, any) | 0 | 0 | ||
| 3 (severe, many) | 0 | 0 | ||
| Arterial intimal thickening | No. of patients (%) | |||
| 0 (absent) | 33 (82.5) | 4 (100) | 0.4 | |
| 1 (≤25%) | 7 (17.5) | 0 | ||
| 2 (26–50%) | 0 | 0 | ||
| 3 (>50%) | 0 | 0 | ||
Values are means ± SD. AGT, angiotensinogen (arbitrary unit); IgAN, IgA nephropathy.
Glomeruli were scored individually and the mean scores calculated for all nonsclerosed glomeruli semiquantitatively.
Less than 10% was involved. An adequate specimen for the light microscopy containing sufficient cortical area was considered as a biopsy with at least 10 glomeruli. Consequently, a total of 1290 and 70 glomeruli in the specimens from IgAN and control individuals, respectively, were observed.
FIGURE 2.
Relationships of the proximal tubular angiotensinogen with clinical variables. (a) Proximal tubular angiotensinogen (AGT; AU, arbitrary unit) showed no significant correlation with creatinine clearance (Ccr, ml/min), but showed direct correlations with both tubular sodium reabsorption to filtered sodium load ratio (FRNa) (b) and night/day ratio of the mean arterial pressure (MAP) (c).
Scores for the glomerular sclerosis, mesangial proliferation, and mesangial matrix increase were higher in the patients with IgAN than in the control individuals, whereas the scores for glomerular obsolescence, tubulointerstitial and vascular features were not different between the two groups (Table 2). In the patients with IgAN, we could not find any significant correlations of the night/day ratio of MAP with glomerular obsolescence (ρ = −0.05, P = 0.8), glomerular sclerosis (ρ = −0.23, P = 0.1), mesangial proliferation (ρ = −0.10, P = 0.5), mesangial matrix increase (ρ = −0.16, P = 0.3), interstitial fibrosis (ρ = −0.006, P = 0.9), tubular atrophy (ρ = −0.02, P = 0.9), or arterial intimal thickening (ρ = −0.03, P = 0.9), whereas it correlated with arteriolar hyalinosis (ρ = −0.42, P = 0.009). When analyzed by stepwise multiple regression analysis (R2 = 0.28, P = 0.002), the main determinants of night/day ratio of MAP among the biopsy findings were PT-AGT (r = 0.29, F = 4.2, P = 0.04) and arteriolar hyalinosis (r = −0.38, F = 7.2, P = 0.01), rather than glomerular obsolescence, glomerular sclerosis, mesangial proliferation, mesangial matrix increase, interstitial fibrosis, tubular atrophy or intimal thickening.
DISCUSSION
The present study is the first to demonstrate that IgAN patients with increased AGT immunopositivity in their renal proximal tubules exhibited the nondipper circadian BP rhythm in association with enhanced tubular sodium reabsorption. Although most of the circulating AGT is produced by the liver, AGT in the systemic circulation cannot be filtered across the glomerular capillary walls because of its molecular size [24]. On the contrary, circulating Ang II can be filtered by the glomeruli to be internalized into proximal tubular cells by AT1R-dependent mechanisms, and stimulates AGT production in the proximal tubules [11]. The proximally formed AGT is secreted into the tubular lumen, leading to further intraluminal Ang II formation throughout the nephron, because all of the components necessary to generate intrarenal Ang II [i.e. renin, prorenin, Ang I, and angiotensin-converting enzyme (ACE)] are present along the nephron [25,26]. Furthermore, AGT is the only known substrate for renin, and the level of AGT in humans and rats is close to the Michaelis-Menten constant (Km) value for renin [27,28], supporting the concept that PT-AGT can suggest the means by which systemic Ang II stimulates proximal tubular AGT production and thereby how proximal tubular AGT production could augment the intrarenal production of Ang II. In fact, as mentioned above, immunostaining of PT-AGT changes in parallel with the kidney content of Ang II and AGT [14]. In this manner, PT-AGT can represent the pathophysiological state of intrarenal Ang II. In animal studies, intrarenal Ang II is known to impair renal sodium excretion via various mechanisms. For instance, Ang II stimulates tubular sodium reabsorption at various segments along the pathway from the proximal to the collecting ducts [15–17], and also enhances tubuloglomerular feedback sensitivity to decrease urinary sodium excretion [18]. Combined with these observations, our present findings indicate that even in humans, activated intrarenal Ang II enhances tubular sodium reabsorption to elicit the nondipper circadian BP rhythm.
Guyton [29] proposed that the pressure–natriuresis relationship must be affected in the genesis of hypertension [29,30]. Assuming the pressure–natriuresis curve as a linear relationship and plotting the MAP (X-axis) and UNaV (Y-axis) during the low and high-salt diet, our group [31] proposed that UNaV could be characterized as a first-order function of MAP, where A and B represent the extrapolated X-intercept and the slope, respectively:
| (1) |
The slope (B) originally represents the renal sodium excretion capacity [31], and the (MAP – A) is equal to the net effective filtration pressure across the glomerular capillary walls (ΔPF):
| 31 | (2) |
Glomerular filtration rate is the product of the whole kidney ultrafiltration coefficient (KF) and ΔPF [32]:
| (3) |
UNaV is the difference between the filtered sodium load and tubular sodium reabsorption (tNa):
| (4) |
Eqs. (1), (2), (3) and (4) can be solved for B as follows:
| (5) |
Thus, this relationship suggests that renal sodium excretion capacity is diminished by two mechanisms: reduced KF, or increased FRNa [4]. As the KF is reduced in glomerulonephritis, the reserved filtration surface area, which has not been utilized for ultrafiltration, is lost until filtration pressure disequilibrium appears, and therefore, the GFR is not attenuated. Once filtration pressure disequilibrium appears, glomerular hypertension occurs to compensate for GFR. In this manner, there is a functional reserve in KF before the decrease in GFR. In other words, normal GFR does not necessarily mean normal glomerular KF. In contrast, KF is surely diminished when the decrease in GFR is noted. Therefore, we can consider the decrease in GFR as a representative of the diminished KF especially in patients with glomerular disease. In fact, we have previously reported the inverse relationships between the creatinine clearance, as a measure of GFR, and the night/day ratios of BP and UNaV in patients with glomerular disease [5]. The example of the latter mechanism has been shown in the patients, whose renal function was preserved [6]. Unlike our previous studies [3,5,20,21], the inverse relationship between creatinine clearance and the night/day ratio of MAP never reached statistical significance in the present study. Therefore, we have to seriously consider the consistency of our present finding with the previous results regarding the relationship between GFR and night/day ratio of MAP. We speculate that the lack of significance in the relationship between the GFR and the night/day ratio of MAP is probably due in part to the homogeneity of the patients’ renal function: all of our study patients had relatively preserved renal function and thus the distribution of renal function was not wide enough to show a statistically valid relationship between renal function and other variables. In fact, we were only able to illustrate the significance of the latter mechanism in the population whose renal function was relatively normal [6]. Therefore, we selected the present patient population, not only because we had shown increased PT-AGT in patients with IgAN [33], but also because the latter mechanism was expected to be detectable in patients with preserved renal function. In this manner, we believe that our study results were not inconsistent with our previous findings. This interpretation is supported by the persistence of the inverse relationship between GFR and the night/day ratio of MAP in simple regression analysis, although it never reached statistical significance, and by the fact that our present study also showed that the GFR and FRNa were significant independent determinants of the night/day ratio of MAP in multivariate analysis.
We have postulated that impaired renal capacity for sodium excretion causes insidious sodium retention during the day, which prevents the nocturnal BP from dipping [2,3,5], and that sufficient natriuresis will restore the circadian BP rhythm. Indeed, our colleagues have reported that treatment with diuretics can ameliorate the circadian BP rhythm [7,8]. We have also recently reported that treatment with the ARB, olmesartan, increased the daytime UNaV by suppressing FRNa to restore a nondipper BP rhythm in patients with CKD [20,21]. Therefore, we speculated that olmesartan could inhibit the FRNa, which had been inappropriately stimulated by Ang II prior to the treatment. The present study supports this concept, because it demonstrates a significant correlation between the increased PT-AGT and the nondipper BP rhythm, in association with the enhancement of FRNa.
Previously, we reported that in CKD patients, nocturnal hypertension would elevate glomerular capillary pressure to augment the nocturnal albuminuria [5]. In addition, Lurbe et al. [34] reported that in patients with type 1 diabetes, the progression from normoalbuminuria to microalbuminuria was less likely in dippers than in nondippers. Furthermore, the Randomized Olmesartan and Diabetes Microalbuminuria Prevention study [35] showed that in type 2 diabetes patients, the time to the onset of microalbuminuria was extended by preemptive treatment with olmesartan, which was effective in restoring the nondipper circadian BP rhythm in our previous studies [20,21]. These findings suggest that the nondipper circadian BP rhythm augments the glomerular preload to advance renal dysfunction most notably at night, and that restoration of the nondipper BP rhythm appears to be a positive indicator for the prevention of CKD progression.
One limitation of our study was the small number of control individuals that had undergone renal biopsy and were proven to not have any kidney diseases; consequently, statistical assessment of the differences between the groups lacked sufficient power for the comparison in Table 1. However, we presented the data of the control individuals as a negative control to validate the accuracy of the immunohistochemical staining. Furthermore, a total of 1290 and 70 glomeruli in the specimens from IgAN and control individuals, respectively, as well as accompanying numerous tubules, were observed. Therefore, there remained statistical meaningfulness in Table 2. Further studies will be needed to determine whether the increased PT-AGT in renal biopsy specimens could be a marker of the patient’s predisposition towards future progression of nephropathy, and whether treatment to prevent sodium retention is useful for those patients who exhibit increased PT-AGT in renal biopsy.
In conclusion, this study demonstrates that increased PT-AGT, a marker for intrarenal Ang activity, is associated with an increased night/day MAP ratio and FRNa in patients with IgAN, suggesting a potential role of ARB in these patients.
Abbreviations
- AGT
angiotensinogen
- Ang
angiotensin
- ARB
angiotensin II type 1 receptor blocker
- AT1R
angiotensin II type 1 receptor
- BP
blood pressure
- Ccr
creatinine clearance
- CKD
chronic kidney disease
- FRNa
fractional tubular sodium reabsorption
- GFR
glomerular filtration rate
- IgAN
IgA nephropathy
- KF
glomerular ultrafiltration coefficient
- Km
Michaelis-Menten constant
- MAP
mean arterial pressure
- PAC
plasma aldosterone concentration
- PRA
plasma renin activity
- PT-AGT
proximal tubular angiotensinogen
- SNa
plasma sodium concentration
- tNa
tubular sodium reabsorption
- UNaV
urinary sodium excretion
Footnotes
Conflicts of interest
For all the authors, there are no conflicts of interest.
References
- 1.O’Brien E, Sheridan J, O’Malley K. Dippers and nondippers. Lancet. 1988;2:397. doi: 10.1016/s0140-6736(88)92867-x. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda M, Goto N, Kimura G. Hypothesis on renal mechanism of nondipper pattern of circadian blood pressure rhythm. Med Hypotheses. 2006;67:802–806. doi: 10.1016/j.mehy.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda M, Mizuno M, Yamanaka T, Motokawa M, Shirasawa Y, Nishio T, et al. Patients with renal dysfunction require a longer duration until blood pressure dips during the night. Hypertension. 2008;52:1155–1160. doi: 10.1161/HYPERTENSIONAHA.108.115329. [DOI] [PubMed] [Google Scholar]
- 4.Kimura G, Brenner BM. The renal basis for salt sensitivity in hypertension. In: Laragh JH, Brenner BM, editors. Hypertension pathophysiology, diagnosis, and management. 2. New York: Raven Press; 1995. pp. 1569–1588. [Google Scholar]
- 5.Fukuda M, Munemura M, Usami T, Nakao N, Takeuchi O, Kamiya Y, et al. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65:621–625. doi: 10.1111/j.1523-1755.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuroda S, Uzu T, Fujii T, Nishimura M, Nakamura S, Inenaga T, Kimura G. Role of insulin resistance in the genesis of sodium sensitivity in essential hypertension. J Hum Hypertens. 1999;13:257–262. doi: 10.1038/sj.jhh.1000800. [DOI] [PubMed] [Google Scholar]
- 7.Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–1638. doi: 10.1161/01.cir.100.15.1635. [DOI] [PubMed] [Google Scholar]
- 8.Uzu T, Harada T, Namba T, Yamamoto R, Takahara K, Yamauchi A, Kimura G. Thiazide diuretics enhance nocturnal blood pressure fall and reduce proteinuria in immunoglobulin A nephropathy treated with angiotensin II modulators. J Hypertens. 2005;23:861–865. doi: 10.1097/01.hjh.0000163156.37363.47. [DOI] [PubMed] [Google Scholar]
- 9.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 12.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol. 1992;263 (5 Pt 1):E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 13.Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, Tang SS. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol. 1999;276 (2 Pt 2):F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 14.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster VL, Kokko JP, Jacobson HR. Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J Clin Invest. 1984;73:507–515. doi: 10.1172/JCI111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen ME, Hall JE, Montani JP, Guyton AC, Langford HG, Cornell JE. Mechanisms of angiotensin II natriuresis and antinatriuresis. Am J Physiol. 1985;249 (2 Pt 2):F299–F307. doi: 10.1152/ajprenal.1985.249.2.F299. [DOI] [PubMed] [Google Scholar]
- 17.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 18.Braam B, Navar LG, Mitchell KD. Modulation of tubuloglomerular feedback by angiotensin II type 1 receptors during the development of Goldblatt hypertension. Hypertension. 1995;25:1232–1237. doi: 10.1161/01.hyp.25.6.1232. [DOI] [PubMed] [Google Scholar]
- 19.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT1 receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda M, Yamanaka T, Mizuno M, Motokawa M, Shirasawa Y, Miyagi S, et al. Angiotensin II type 1 receptor blocker, olmesartan, restores nocturnal blood pressure decline by enhancing daytime natriuresis. J Hypertens. 2008;26:583–588. doi: 10.1097/HJH.0b013e3282f2fded. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda M, Wakamatsu-Yamanaka T, Mizuno M, Miura T, Tomonari T, Kato Y, et al. Angiotensin receptor blockers shift the circadian rhythm of blood pressure by suppressing tubular sodium reabsorption. Am J Physiol Renal Physiol. 2011;301:F953–F957. doi: 10.1152/ajprenal.00167.2011. [DOI] [PubMed] [Google Scholar]
- 22.Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13:142–148. doi: 10.1681/ASN.V131142. [DOI] [PubMed] [Google Scholar]
- 23.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, et al. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 24.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 26.Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 27.Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res. 1971;5:86–89. doi: 10.1093/cvr/5.1.86. [DOI] [PubMed] [Google Scholar]
- 28.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 29.Guyton AC. Circulatory physiology III. Philadelphia: WB Saunders; 1980. Arterial pressure and hypertension. [Google Scholar]
- 30.Hall JE, Mizelle HL, Hildebrandt DA, Brands MW. Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension. 1990;15 (6 Pt 1):547–559. doi: 10.1161/01.hyp.15.6.547. [DOI] [PubMed] [Google Scholar]
- 31.Kimura G, Imanishi M, Sanai T, Kawano Y, Kojima S, Yoshida K, et al. Intrarenal hemodynamics in patients with essential hypertension. Circ Res. 1991;69:421–428. doi: 10.1161/01.res.69.2.421. [DOI] [PubMed] [Google Scholar]
- 32.Gomez DM. Evaluation of renal resistances, with special reference to changes in essential hypertension. J Clin Invest. 1951;30:1143–1155. doi: 10.1172/JCI102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, et al. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;358:156–163. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 35.Haller H, Ito S, Izzo JL, Jr, Januszewicz A, Katayama S, Menne J, et al. ROADMAP Trial Investigators. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]