Abstract
A hypothalamic neuropeptide, gonadotropin-releasing hormone (GnRH), is the primary factor regulating gonadotropin secretion. An inhibitory hypothalamic neuropeptide for gonadotropin secretion was, until recently, unknown, although gonadal sex steroids and inhibin can modulate gonadotropin secretion. Findings from the last decade, however, indicate that GnRH is not the sole hypothalamic regulatory neuropeptide of vertebrate reproduction, with gonadotropin-inhibitory hormone (GnIH) playing a key role in the inhibition of reproduction. GnIH was originally identified in birds and subsequently in mammals and other vertebrates. GnIH acts on the pituitary and on GnRH neurons in the hypothalamus via a novel G protein-coupled receptor (GPR147). GnIH decreases gonadotropin synthesis and release, inhibiting gonadal development and maintenance. Such a down-regulation of the hypothalamo-pituitary-gonadal (HPG) axis may be conserved across vertebrates. Recent evidence further indicates that GnIH operates at the level of the gonads as an autocrine/paracrine regulator of steroidogenesis and gametogenesis. More recent evidence suggests that GnIH also acts both upstream of the GnRH system and at the level of the gonads to appropriately regulate reproductive activity across the seasons and during times of stress. The discovery of GnIH has fundamentally changed our understanding of hypothalamic control of reproduction. This review summarizes the discovery, progress and prospect of GnIH, a key regulator of vertebrate reproduction.
Keywords: gonadotropins, gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), melatonin, stress, hypothalamus, pituitary, reproduction, reproductive behavior
1. Introduction
In vertebrates, the reproductive axis integrates information from a wide range of systems via direct and indirect neurochemical inputs. Many of the neuropeptidergic pathways involved in the transduction of environmental stimuli into neuroendocrine signals have been well studied. A classic example of such regulation is the gonadotropin-releasing hormone (GnRH) system. GnRH, a hypothalamic decapeptide, regulates secretion of both of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and acts as a key neurohormone for vertebrate reproduction. Since the discovery of GnRH in the brain of mammals at the beginning of 1970s [7,41], several other GnRHs have been identified in the brain of a variety of vertebrates [29,35,43,44,52,53,70,71,104]. It has also been generally accepted that GnRH is the only hypothalamic regulator of the release of pituitary gonadotropins, and that no other neuropeptide has a direct influence on the reproductive axis. Some neurochemicals and peripheral hormones [e.g., γ-aminobutyric acid (GABA), opiates, gonadal sex steroids, inhibin] can modulate gonadotropin release to a degree, but GnRH was considered to be unusual among hypothalamic neuropeptides in that it appeared to have no hypothalamic antagonist. However, this dogma was challenged by the discovery in 2000 of a vertebrate hypothalamic neuropeptide that inhibits pituitary gonadotropin release [85].
In a search for novel neuropeptides regulating the release of pituitary hormones, Tsutsui and colleagues identified a novel hypothalamic dodecapeptide [Ser-Ile-Lys-Pro-Ser-Ala-Tyr-Leu-Pro-Leu-Arg-Phe-NH2 (SIKPSAYLPLRFamide)] that directly acts on the pituitary to inhibit gonadotropin release in quail and termed it gonadotropin-inhibitory hormone (GnIH; [85]). This was the first demonstration of a hypothalamic neuropeptide inhibiting gonadotropin release in any vertebrate. From the past 10 years of research, we now know that GnIH exists in all avian species studied, including quail, chickens, starlings, sparrows and zebra finches, and acts as a key neurohormone for the regulation of avian reproduction by decreasing gonadotropin release and synthesis [3–5,14,54,66,79–89,91,93,95–97,101,103].
Tsutsui and colleagues have further identified GnIH orthologs in other vertebrates from fish to humans (for reviews, see [38,75,80,81,83,84,87,100]). As in birds, mammalian GnIH orthologs act to inhibit gonadotropin release in several mammalian species, such as rats, hamsters, sheep and cows [16,24,30,31,39,51,65,90]. Furthermore, a fish GnIH ortholog also inhibits gonadotropin release in fish [106], indicating a conserved role for GnIH and its orthologs in the control of the HPG axis across species. Thus, GnIH, a newly discovered hypothalamic neuropeptide, and its orthologs appear to act as key neurohormones controlling vertebrate reproduction, generally (for reviews, see [80,81,83]).
The discovery of GnIH has changed our understanding about regulation of the reproductive axis markedly in the past decade, but we are only at the beginning of an exciting new era of research on reproductive neurobiology. Herein we provide a broad overview of the structure, biosynthesis, mode of action and functional significance of GnIH in the regulation of vertebrate reproduction.
2. Discovery of GnIH, a new key regulator of vertebrate reproduction
2.1. Brief history of the discovery of GnIH
GnIH was discovered in the brain of the Japanese quail Coturnix japonica in search of a novel hypothalamic neuropeptide having a C-terminal Arg-Phe-NH2 motif (RFamide peptide) [85]. RFamide peptides were first isolated in invertebrate species in the late 1970’s. The initial RFamide peptide, Phe-Met-Arg-Phe-NH2 (FMRFamide), is a cardioexcitatory molecule isolated from the ganglia of the venus clam Macrocallista nimbosa [60]. After this discovery, numerous RFamide peptides that act as neurotransmitters, neuromodulators and peripheral hormones have been identified in various invertebrate phyla, including cnidarians, nematodes, annelids, molluscs, and arthropods. Subsequently, immunohistochemical studies suggested the presence of RFamide peptides in the nervous system of vertebrates. Importantly, FMRFamide-immunoreactive (-ir) neurons terminated in the vicinity of the pituitary gland, suggesting a role of some unknown RFamide peptide(s) in the regulation of pituitary function. In 2000, Tsutsui and colleagues discovered that this novel RFamide peptide localized in the hypothalamo-hypophysial system, in contrast to GnRH, actively inhibits gonadotropin release in quail and termed it GnIH [85].
It was believed for a long time that GnRH is the only hypothalamic regulator of pituitary gonadotropin synthesis and release. From the past 10 years of research, however we now know that GnIH exists in all avian species studied (Table 1), and regulates avian reproduction by decreasing gonadotropin release and synthesis via action on the GnRH system and the anterior pituitary gland, mediated via GPR147 [3–5,14,54,66,79–89,91,93,95–97,101,103) (Table 2). After the discovery of GnIH in birds, GnIH orthologs have been further identified in a number of other vertebrates from fish to humans [for reviews, see 80,81,83,84] (Table 1). Importantly, as in birds, mammalian GnIH orthologs [known as RFamide-related peptides (RFRPs)] act to inhibit gonadotropin release across mammalian species [16,24,30,31,38,39,51,90] (Table 2). In addition, RFRP-3, a mammalian GnIH ortholog, has been shown to inhibit GnRH-stimulated gonadotropin synthesis in mammalian pituitary gonadotropes [65] (Table 2). Recently, an inhibitory action of a fish GnIH ortholog was also reported in goldfish [106] (Table 2). In general, GnIH and its orthologs seem to act similarly across vertebrate species to regulate reproduction, although some exceptions exist (further described below)
Table 1.
Amino acid sequences of GnIH and its orthologs in vertebrates.
| Animal | Name | Sequence | Reference |
|---|---|---|---|
|
Mammal
| |||
| Human | RFRP-1 | MPHSFANLPLRFa | Ubuka et al., 2009b |
| RFRP-3 | VPNLPQRFa | Ubuka et al., 2009b | |
|
| |||
| Macaque | RFRP-1* | MPHSVTNLPLRFa | Ubuka et al., 2009a |
| RFRP-3 | SGRNMEVSLVRQVLNLPQRFa | Ubuka et al., 2009a | |
|
| |||
| Bovine | RFRP-1 | SLTFEEVKDWAPKIKMNKPVVNKMPPSAANLPLRFa | Fukusumi et al., 2001 |
| RFRP-3 | AMAHLPLRLGKNREDSLSRWVPNLPQRFa | Yoshida et al., 2003 | |
|
| |||
| Ovine | RFRP-1* | SLTFEEVKDWGPKIKMNTPAVNKMPPSAANLPLRFa | Clarke et al., 2008 |
| RFRP-3* | VPNLPQRFa | Clarke et al., 2008 | |
|
| |||
| Rat | RFRP-1* | SVTFQELKDWGAKKDIKMSPAPANKVPHSAANLPLRFa | Ukena et al., 2002 |
| RFRP-3 | ANMEAGTMSHFPSLPQRFa | Ukena et al., 2002 | |
|
| |||
| Hamster | RFRP-1 | SPAPANKVPHSAANLPLRFa | Ubuka et al., 2011 |
| RFRP-3 | TLSRVPSLPQRFa | Ubuka et al., 2011 | |
|
| |||
|
Bird
| |||
| Quail | GnIH | SIKPSAYLPLRFa | Tsutsui et al., 2000 |
| GnIH-RP-1* | SLNFEEMKDWGSKNFMKVNTPTVNKVPNSVANLPLRFa | Satake et al., 2001 | |
| GnIH-RP-2 | SSIQSLLNLPQRFa | Satake et al., 2001 | |
|
| |||
| Chicken | GnIH* | SIRPSAYLPLRFa | Ikemoto et al., 2005 |
| GnIH-RP-1* | SLNFEEMKDWGSKNFLKVNTPTVNKVPNSVANLPLRFa | Ikemoto et al., 2005 | |
| GnIH-RP-2* | SSIQSLLNLPQRFa | Ikemoto et al., 2005 | |
|
| |||
| Sparrow | GnIH* | SIKPFSNLPLRFa | Osugi et al., 2004 |
| GnIH-RP-1* | SLNFEEMEDWGSKDIIKMNPFTASKMPNSVANLPLRFa | Osugi et al., 2004 | |
| GnIH-RP-2* | SPLVKGSSQSLLNLPQRFa | Osugi et al., 2004 | |
|
| |||
| Starling | GnIH | SIKPFANLPLRFa | Ubuka et al., 2008 |
| GnIH-RP-1* | SLNFDEMEDWGSKDIIKMNPFTVSKMPNSVANLPLRFa | Ubuka et al., 2008 | |
| GnIH-RP-2* | GSSQSLLNLPQRFa | Ubuka et al., 2008 | |
|
| |||
| Zebra finch | GnIH | SIKPFSNLPLRFa | Tobari et al., 2010 |
| GnIH-RP-1* | SLNFEEMEDWRSKDIIKMNPFAASKMPNSVANLPLRFa | Tobari et al., 2010 | |
| GnIH-RP-2* | SPLVKGSSQSLLNLPQRFa | Tobari et al., 2010 | |
|
| |||
|
Amphibian
| |||
| Frog | fGRP/R-RFa | SLKPAANLPLRFa | Koda et al., 2002; Chartrel et al., 2002 |
| fGRP-RP-1 | SIPNLPQRFa | Ukena et al., 2003b | |
| fGRP-RP-2 | YLSGKTKVQSMANLPQRFa | Ukena et al., 2003b | |
| fGRP-RP-3 | AQYTNHFVHSLDTLPLRFa | Ukena et al., 2003b | |
|
| |||
| Newt | nLPXRFa-1 | SVPNLPQRFa | Chowdhury et al., 2011 |
| nLPXRFa-2 | MPHASANLPLRFa | Chowdhury et al., 2011 | |
| nLPXRFa-3 | SIQPLANLPQRFa | Chowdhury et al., 2011 | |
| nLPXRFa-4 | APSAGQFIQTLANLPQRFa | Chowdhury et al., 2011 | |
|
| |||
|
Fish
| |||
| Goldfish | gfLPXRFa-1* | PTHLHANLPLRFa | Sawada et al., 2002b |
| gfLPXRFa-2* | AKSNINLPQRFa | Sawada et al., 2002b | |
| gfLPXRFa-3 | SGTGLSATLPQRFa | Sawada et al., 2002b | |
Putative peptides. LPXRFamide (X = L or Q) carboxyl peptide consensus sequences are shown in bold.
Table 2.
Physiological functions of GnIH and its orthologs in vertebrates.
| Animal | Name | Physiological Function | Reference |
|---|---|---|---|
|
Mammal
| |||
| Human | RFRP-1 | Stimulation of prolactin release (i.c.v.) | Hinuma et al., 2000 |
|
| |||
| Bovine | RFRP-3* | Inhibition of GnRH-elicited gonadotropin release (in vitro) | Kadokawa et al., 2009 |
| Inhibition of gonadotropin release (i.v.) | Kadokawa et al., 2009 | ||
|
| |||
| Ovine | RFRP-3* | Inhibition of GnRH-elicited gonadotropin release (in vitro) | Clerke et al., 2008 |
| Inhibition of gonadotropin release (i.v.) | Clerke et al., 2008 | ||
| Inhibition of GnRH-elicited gonadotropin synthesis and release (in vitro) | Sari et al., 2009 | ||
|
| |||
| Rat | RFRP-1* | Stimulation of adrenocorticotropic hormone, oxytocin release (i.c.v.) | Kaewwongse at al., 2011 |
|
| |||
| RFRP-3 | Stimulation of adrenocorticotropic hormone, oxytocin release (i.c.v.) | Kaewwongse at al., 2011 | |
| Inhibition of gonadotropin release (i.c.v.) | Johnson et al., 2007 | ||
| Inhibition of gonadotropin release (i.v.) | Murakami et al., 2008 | ||
| Inhibition of GnRH-elicited gonadotropin release (in vitro) | Murakami et al., 2008 | ||
| Inhibition of reproductive behavior (i.c.v.) | Johnson et al., 2007 | ||
| Stimulation of feeding behavior (i.c.v.) | Johnson et al., 2007 | ||
| Stimulation of feeding behavior (i.c.v.) | Murakami et al., 2008 | ||
|
| |||
| Hamster | RFRP-1 | Inhibition of gonadotropin release (i.c.v.) | Ubuka et al., 2011 |
|
| |||
| RFRP-3 | Inhibition of gonadotropin release (i.c.v.) | Ubuka et al., 2011 | |
| Inhibition of gonadotropin release (i.p.) | Kriegsfeld et al., 2006 | ||
|
| |||
|
Bird
| |||
| Quail | GnIH | Inhibition of gonadotropin release (in vitro) | Tsutsui et al., 2000 |
| Inhibition of gonadotropin synthesis and release (i.p.) | Ubuka et al., 2006 | ||
|
| |||
| Chicken | GnIH* | Inhibition of gonadotropin synthesis and release (in vitro) | Ciccone et al., 2004 |
| Stimulation of feeding behavior (i.c.v.) | Tachibana et al., 2005 | ||
|
| |||
| GnIH-RP-1* | Stimulation of feeding behavior (i.c.v.) | Tachibana et al., 2005 | |
|
| |||
| GnIH-RP-2* | Stimulation of feeding behavior (i.c.v.) | Tachibana et al., 2005 | |
|
| |||
| Sparrow | GnIH* | Inhibition of GnRH-elicited gonadotropin release (i.v.) | Osugi et al., 2004 |
| Inhibition of gonadotropin release (i.v.) | Osugi et al., 2004 | ||
| Inhibition of gonadotropin release (i.c.v.) | Bentley et al., 2006 | ||
| Inhibition of reproductive behavior (i.c.v.) | Bentley et al., 2006 | ||
|
| |||
|
Amphibian
| |||
| Frog | fGRP | Stimulation of GH release (in vitro, i.p.) | Koda et al., 2002 |
|
| |||
| fGRP-RP-2 | Stimulation of GH/PRL release (in vitro, i.p.) | Ukena et al., 2003b | |
|
| |||
|
Fish
| |||
| Goldfish | gfLPXRFa-1* | Stimulation of gonadotropin and GH release (in vitro) | Amano et al., 2006 |
|
| |||
| gfLPXRFa-2* | Stimulation of gonadotropin and GH release (in vitro) | Amano et al., 2006 | |
|
| |||
| gfLPXRFa-3 | Stimulation of gonadotropin and GH release (in vitro) | Amano et al., 2006 | |
|
| |||
| Zebrafish | zfLPXRFa-3* | Inhibition of gonadotropin release (i.p.) | Zhang et al., 2010 |
Putative peptides. i.c.v.: intracerebroventricular administration; i.v.: intravenous administration; i.p.: intraperitoneal administration.
2.2. Structure, functions and biosynthesis of GnIH
GnIH has a previously-unreported dodecapeptide structure, SIKPSAYLPLRFamide [85] (Table 1). Its C-terminus is identical to chicken LPLRFamide that was reported to be the first RFamide peptide isolated in vertebrates [20], which is likely to be a degraded fragment of GnIH [for reviews, see 80,81,83,87, 100]. After the isolation of GnIH in the quail brain, the precursor polypeptide for GnIH was examined [66]. A cDNA that encodes GnIH precursor polypeptide was identified by a combination of 3′ and 5′ rapid amplification of cDNA ends (3′/5′ RACE) in quail [66] and other avian species, such as chickens, sparrows, zebra finches and starlings (for reviews, see [80,81,83]). The GnIH precursor encodes one GnIH and two GnIH-related peptides (GnIH-RP-1 and GnIH-RP-2) possessing an LPXRFamide (X = L or Q) sequence at their C-termini in all birds (Table 1). These peptide sequences are flanked by a glycine C-terminal amidation signal and a single basic amino acid on each end as an endoproteolytic site. GnIH was further isolated as a mature peptide in starlings [91] and zebra finches [79] and GnIH-RP-2 was also identified in quail [66] (Table 1). In addition, cDNAs that encode LPXRFamide peptides similar to GnIH have been investigated in mammals by a gene database search [27]. The cDNAs identified from mammalian brain encode three GnIH orthologs, RFRP-1, -2, and -3, in bovines and humans and two GnIH orthologs, RFRP-1 and -3, in rodents (for reviews, see [80,81,83]). RFRP-1 and -3 are both LPXRFamide peptides, but RFRP-2 is not an LPXRFamide peptide. The mammalian GnIH orthologs, RFRP-1 and/or RFRP-3 were also identified as mature peptides in bovines [23,105], rats [98], hamsters [90], monkeys [92] and humans [94] (Table 1). Subsequently, it was shown that RFRP-3 inhibits gonadotropin synthesis and/or release in mammals [16,30,31,51,65,90] (Table 2). Accordingly, at least RFRP-3 acts as a functional ortholog of GnIH in terms of reducing gonadotropin secretion (for reviews, see [80,81,83]). More recently, it was found that RFRP-1 also inhibits gonadotropin release in hamsters [90] (Table 2).
3. Progression of GnIH research
3.1. Mode of action and functional significance of GnIH on the HPG axis in birds
3.1.1. Pituitary action of GnIH on gonadotropin secretion
GnIH neurons are located in the paraventricular nucleus (PVN) in the hypothalamus and their major projections reach the median eminence of the hypothalamus [85,96,101]. Based on the observation that GnIH-ir neurons project to the external layer of the median eminence in quail, the first study on GnIH [85] focused on the effect of GnIH on pituitary gonadotropin release (see Table 2). In songbirds, intravenous (i.v.) injection of GnIH can rapidly reduce circulating LH in breeding white-crowned sparrows (Zonotrichia leucophrys gambelii) in addition to inhibiting GnRH-induced LH release in non-breeding song sparrows (Melospiza melodia) in laboratory conditions [54]. GnIH administration in vivo and in vitro inhibits the synthesis of LHβ- and FSHβ-subunits within the pituitary gland of quail and chickens [14,97], indicating a dual role for GnIH within the pituitary-acting over different time-frames to reduce first the release of gonadotropins into the circulation followed by inhibition of LH and FSH synthesis. Thus, it has become clear that GnIH in birds is an important regulator of pituitary gonadotropin synthesis in addition to gonadotropin release [3,14,54,97]. Despite our published data on the distribution of GnIH in the median eminence and GnIH receptor (GnIH-R) in the pituitary, there are some inconsistencies in the literature. For example, rufous-winged sparrows (Aimophila carpalis) do not seem to exhibit immunoreactive GnIH in the median eminence [74] – despite expressing GnIH-R mRNA in the pituitary (McGuire, Deviche and Bentley, unpublished data). Nor do peripheral injections of GnIH rapidly inhibit LH secretion in this species [19]. It is possible that there is another source of GnIH that can influence pituitary gonadotropin release. For example, in some avian species there appear to be GnIH projections to the pars nervosa (Bentley and Wingfield, unpublished data), so GnIH could potentially be released directly into the peripheral circulation. The gonads are also a source of GnIH synthesis, but it is not known if gonadal GnIH can pass into the blood stream (see later section). It is possible that there is dynamic temporal regulation of GnIH in the median eminence, as there is certainly dynamic regulation of GnIH:GnRH contact in the hypothalamus of rufous-winged sparrows, as in mammals (see later section). Obviously, one cannot assume that the findings from a few species are applicable to all– especially given the huge variety of reproductive strategies amongst avian species. It is clear that further comparative work is needed in this area.
3.1.2. Central action of GnIH on physiology and behavior
In addition to the effects of GnIH on the pituitary, the contact of GnRH neurons by GnIH axons is perhaps the most conserved property of GnIH:GnRH interactions across vertebrate classes. Initially discovered in house sparrows, Passer domesticus [4], contact of GnRH neurons by GnIH has been observed in all other vertebrates studied to date, including humans [94]. In birds, GnIH neurons project to GnRH-I and -II neurons and presumably inhibit the action of these two types of GnRH via the GnIH-R GPR147 in European starlings, Sturnus vulgaris [91]. Experimental support of this notion comes from Bentley et al. [3], in which centrally-infused GnIH inhibit circulating LH and reduced copulation solicitation in female white-crowned sparrows (copulation solicitation is thought to be regulated largely by GnRH-II in this species) (Table 2). This finding agrees with the social regulation of GnIH described later. Further, rhodaminated GnIH was shown to bind to putative GnRH-II neurons and, in a later study on European starlings, Sturnus vulgaris, GnRH-I and-II neurons were shown to express GnIH-R mRNA [91]. A separate study investigated GnIH in another songbird species, rufous-winged sparrow, Aimophila carpalis. This bird is a resident of the Sonoran desert and breeds after the onset of summer rain (monsoon). Birds caught during the monsoon (breeding) had fewer, less densely labeled GnIH-ir cell bodies than birds caught before the monsoon (large gonads but not breeding). There were also fewer GnIH-ir fibers in the preoptic area of birds caught during the monsoon season –indicating fewer contacts with GnRH neurons [74]. Thus, physiological, behavioral and histological evidence in several species combine to indicate a direct role for GnIH in regulation of GnRH in the brain of songbirds (Table 2). In contrast to the highly-clustered cell bodies in the PVN, GnIH neuronal fibers are widely distributed in diencephalic and mesencephalic regions in birds, suggesting the regulation of other, non-reproductive behaviors [4,91,101]. For example, central administration of GnIH stimulates feeding behavior in chickens [78] (Table 2). Because of its widespread distribution, it would not be surprising to find that GnIH regulates, or is regulated by, other behaviors.
There is evidence in quail that GnIH might be involved in reproductive termination in response to the nocturnal melatonin signal [13,89] – see section 3.3. Also, melatonin implants delayed the onset of clutch initiation in female great tits (Parus major) but did not affect clutch size, body mass, or timing of onset of activity [26]. In contrast, GnIH and GnRH expression appear to be positively correlated in other species [4,10]. Such a relationship seems confusing and perhaps counter-intuitive to begin with; after all, why should an inhibitory hormone increase during the breeding season [18]? We suspect that in some species, GnIH most likely acts to induce a temporary pause in reproductive effort without shutting down the whole HPG axis, which would cause the individual to miss the opportunity to breed in that year. Such a missed opportunity in a single year could dramatically reduce reproductive success in short-lived songbirds. This temporary cessation of reproduction occurs in response to unpredictable environmental cues, such as stress, or mate/nest availability. In an experiment on European starlings in which nesting opportunities were limited, birds which outcompeted others for nest boxes (‘winners’) had significantly fewer numbers of GnIH-producing cells than those without nest boxes (‘losers’) at the beginning of the breeding season [10]. This relationship changed with breeding stage; once “winners” had begun to incubate eggs, their GnIH content increased significantly above that of “losers”. Thus, whereas birds appeared reproductively capable across treatments, our data indicate that hypothalamic GnIH may serve as a modulator of reproductive behaviors in response to social environment. Consequently, hypothalamic GnIH may serve as a modulator of reproductive behaviors in response to social environment [10]. How this pattern of GnIH expression during long-days in a naturally-housed songbird relates to the increased expression and release of GnIH by short-day induced melatonin action in laboratory quail [13,89] (see section 3.3.) remains to be seen. Studies on wild quail might provide some valuable answers to this question (see [9]).
3.1.3. Gonadal GnIH action
Vertebrate gonads are the sites of synthesis and action of many peptides that were initially classified as neuropeptides. The gonadal GnIH system was first discovered in European starlings and Japanese quail [6]. The presence of transcripts for GnIH and GnIH-R in the gonads and associated tissues, and the expression of GnIH and GnIH-R by specific cell types involved in steroid biosynthesis and gamete maturation indicates the likely importance of GnIH as a paracrine/autocrine regulator within reproductive tissues (for a review, see [45]). To gauge functional significance of gonadal GnIH in males, the effect of GnIH on the secretion of testosterone was investigated using gonadotropin-stimulated cultured house sparrow testes. In this study, testosterone secretion from gonadotropin-stimulated cultured house sparrow testes was significantly decreased by the application of GnIH in vitro [46]. In a separate study, seasonal and possibly melatonin-dependent regulation of gonadal GnIH in European starlings was identified: GnIH and melatonin administration significantly decreased testosterone secretion from LH/FSH-stimulated testes before, but not during, breeding [47]. Thus, local inhibition of sex steroid secretion appears to be regulated seasonally at the level of the gonad, perhaps most strongly prior to the onset of breeding. New evidence in support of this phenomenon comes from an experiment on free-living female great tits, in which birds implanted with melatonin at the beginning of the breeding season delayed their onset of egg-laying relative to controls [26]. It is not yet known if this effect of melatonin occurs via the hypothalamus or if it is a direct effect on the ovary, or both. Thus, although the GnRH system in the hypothalamus has been considered the final integration point of vertebrate reproductive responses to environmental cues, the gonads seem capable of responding directly to endocrine proxies of environmental stimuli such as changes in day length.
In addition to melatonin receptors, avian gonads express glucocorticoid receptor. Environmental cues such as stress and food availability are detectable in the plasma (through glucocorticoids and absence/presence of metabolic fuels). We recently showed that, in addition to regulation by the brain, the gonads of European starlings (Sturnus vulgaris) directly respond to stressful stimuli by modulating sex steroid secretion (McGuire, Kangas and Bentley, under review). Under tissue culture conditions, we showed that physiologically-relevant levels of corticosterone and metabolic stress (via use of the glucose utilization inhibitor 2-deoxy-D-glucose and the fatty acid oxidation inhibitor ethyl 2-mercaptoacetate (2DG/MA)) can directly decrease testosterone and estradiol secretion from LH/FSH-stimulated testes and ovaries. This effect is regulated seasonally. Prior to the breeding season, testes and ovaries respond to corticosterone and 2DG/MA by significantly decreasing gonadal steroid release. Within the breeding season, the testes do not respond to these cues of stress, while the ovaries respond only to corticosterone. This seasonal difference in response may be due in part to the influence of these cues of stress on gonadal neuropeptide expression: corticosterone up-regulates GnIH expression in the testes while metabolic stress up-regulates GnIH in the ovaries. There is no effect of treatment on gonadal GnRH. Thus, the gonads can directly respond to stressful stimuli during a time of critical importance to the onset of breeding.
3.2. Mode of action and functional significance of GnIH on the HPG axis in mammals
As in birds, GnIH has been found in the brains of all mammalian species studied to date, including humans [16,23,38,90,92,94,98,105] (Table 1). As described previously, the cDNA for GnIH encodes two LPXRFamide peptides, RFRP-1 and RFRP-3, in mammals (Table 1). Across mammals, administration of GnIH suppresses gonadotropin secretion [16,31,39,58,59, 65,90] (Table 2). Most studies to date have applied RFRP-3 as the mammalian ortholog of avian GnIH [16,31,58]. However, our recent study showed a strong suppressive effect of RFRP-1 on LH in Siberian hamsters [90]. Consistent with results for GnIH across mammalian species, injections of the GnIH-R antagonist, RF9, result in rapid and sustained, dose-dependent increases in LH in male and female mice and rats [59]. Together, these findings suggest that RFRP-1 and/or -3 operate as GnIH in mammalian species. Whether RFRP-1 and -3 operate synergistically in mammals to inhibit HPG axis activity or whether each peptide dominates differentially across species remains an interesting avenue for further investigation.
3.2.1. Pituitary action of GnIH on gonadotropin secretion
In rodents, GnIH cell bodies are clustered in the dorsomedial nucleus of the hypothalamus (DMH) with extensive projections to hypothothamic and limbic structures [30,38,90]. In other species, such as sheep, GnIH neurons are located in both the PVN and more widely distributed throughout the mediobasal hypothalamus [76]. With regard to reproduction, GnIH neurons have been shown to project to the external layer of the median eminence of Syrian hamsters, with GPR147 expression observed in pituitary in the same investigation [24]. These findings suggested that, as in birds, GnIH might act at the pituitary level to suppress gonadotropin secretion. In sheep, GnIH administration down-regulates LHβ- and FSHβ-subunit expression [65] and results in marked reductions in LH pulse amplitude without affecting pulse frequency [16] (Tabel 2). In cows, repeated administration of GnIH leads to reduced LH pulse frequency [31] (Table 2), indicating the possibility of different modes of action across species. Taken together, these findings provide convincing evidence that the function of GnIH is conserved in mammals as well as in birds.
Some disparity across studies and species has drawn into question the generality of the actions of GnIH directly on the pituitary. In one study in rats, for example, GnIH fibers were not detected in the external layer of the median eminence [63]. In the same study, peripheral injections of the retrograde tracer, Fluorogold, did not label GnIH cell bodies whereas a majority of cells bodies were labeled following central injections of GnIH. Finally, in this study, i.v. injections of GnIH did not affect basal LH concentrations, but GnRH-induced LH secretion was modestly suppressed. This latter finding is consistent with work by another group indicating that GnIH had minimal impact on LH secretion in cultured rat pituitary, but significantly suppressed GnRH-induced LH secretion in vitro [51] (Table 2). Curiously, in this study, peripheral injections of GnIH led to gross reductions in LH at 15 min post injection whereas central injections did not impact LH concentrations. In another study of rats, peripheral injections of the GRP147/NPFF receptor antagonist, RF9, led to modest increases in LH in vivo, whereas application of RF9 to pituitary cultures was without effect [59]. In contrast, central injections of this receptor antagonist markedly stimulated LH release. Another study using ovariectomized female rats treated with a low dose of estrogen, found no effect of central GnIH injections on LH [2]. Together, these findings make it difficult to reconcile the specific impact of GnIH on the pituitary, at least in rats. The disparity across studies likely results from differences in the time course of sample collection, antibody, peptide and tissue preparation, and other differences in experimental procedures and the reproductive state of the animals investigated. Investigators working together to explore both the central and peripheral impact of currently available GnIH peptides and antagonists within the same studies/groups of animals will be necessary to begin to clarify the inconsistencies observed.
3.2.2. Central action of GnIH on physiology and behavior
As in birds, GnIH neurons project monosynaptically to GnRH cells [30,38,76] and GnRH neurons express the GnIH-R GPR147 [90]. Direct application of GnIH inhibits neuronal firing in GnRH cells and this inhibitory action of GnIH persists when amino acid transmission is blocked, providing strong evidence for a direct inhibitory role of GnIH on the GnRH neuronal system [21,102]. Similarly, GnIH administration decreases the activational state of GnRH cells (as measured by FOS co-expression) [2]. Together, these findings, combined with those indicating a suppressive action of central GnIH and RF9 on LH concentrations [2,39,58,59,63], point to a potent role for GnIH in GnRH inhibition.
Several lines of evidence indicate an important role for GnIH in mammalian reproductive function and behavior. In females, GnIH appears to play a significant role in mediating estradiol negative feedback to restrain the reproductive axis throughout most of the ovulatory cycle [2,24,39], with acute injections of estradiol increasing GnIH/FOS expression in ovariectomized hamsters. At the time of the preovulatory LH surge and estradiol positive feedback, GnIH activity is reduced, presumably to allow for stimulation of ovulation [24]. Treatment of female rats with GnIH results in marked inhibition of GnRH neuronal activity at the time of the LH surge, providing support for this possibility [2]. Likewise, in one recent finding, treating mice for 4 days with estrogen led to a decrease in GnIH mRNA expression [50], suggesting a mechanism for removal of GnIH suppression during times of high estrogen stimulation. The discrepancy in the impact of estrogen in mice and hamster may result from the timing at which the brains were collected for analysis – hamster brains were collected prior to the LH surge whereas it was not reported when in the day mouse brains were sampled. Analogous to findings in female white-crowned sparrows [3], central GnIH injections lead to reductions in male sexual motivation and performance in male rats [30], suggesting that a coordinated removal of GnIH inhibition might be important for coordinating female reproductive motivation with the timing of optimal fertility.
In mammals, GnIH appears to play an important role in monitoring internal and external status and integrating this information to control reproductive functioning precisely and maximize reproductive success [8]. For example, GnIH neurons project to neuropeptide-Y, pro-opiomelanocortin, orexin and melanin concentrating cells in the ovine brain [15,61], suggesting an important role for GnIH in communicating current energetic status to the reproductive system. Likewise, administration of GnIH increases feeding in rats [30]. Recent findings suggest that GnIH system may communicate inhibitory information to the reproductive axis during times of stress [32,36] (see section 3.3). In male rats, GnIH cells express glucocorticoid receptors and GnIH expression is up-regulated by acute and chronic stress. The role of the GnIH system in suppressing reproduction during times of stress may not be common across species; stress does not affect GnIH peptide or mRNA expression in sheep [55]. Finally, several studies suggest that GnIH may play a role in seasonal breeding. At odds with an inhibitory role for GnIH in mediating seasonal breeding, GnIH is reduced during non-breeding season in hamster and Soay sheep [17,42,56,62,90] (see section 3.3). However, our recent findings in Siberian hamsters indicate that GnIH can have stimulatory actions in short-day animals and suppression of GnIH in winter may be important for reproductive suppression [90] (Table 2). In contrast to findings in rodents and Soay sheep, GnIH is increased during the non-breeding season in Blackface sheep [76], a seasonal pattern consistent with an inhibitory role for this peptide during the non-breeding season in this species. Together, these findings suggest a potentially non-conserved role for GnIH across seasonal breeding species. Direct manipulations of GnIH in long- and short-day breeding rodents and ungulates under different reproductive states will help to begin to clarify the role of this neuropeptide in mammalian seasonal breeding.
3.2.3. Gonadal GnIH action
As is the case in birds, GnIH and its receptor are robustly expressed in Syrian hamster testis [107], indicating that a role for GnIH in gonadal function may be common to all vertebrates. In hamsters, GnIH expression is confined to seminiferous tubules, with spermatocytes and spermatids expression the GnIH-R GPR147, suggesting an important role in spermatogenesis. In macaque, GnIH is expressed in Leydig cells, Sertoli cells, spermatogonia and spermatocytes [46], suggesting roles in spermatogenesis and sperm production. Analagous to findings in males, GnIH is also found in the granulosa cells of mouse ovarian follicles during proestrus and estrus and in the luteal cells during diestrus 1 and 2 [73], and macaque granulosa cells and oocytes [46]. In mice, GnIH was closely associated with GnRH expression in mouse ovarian tissue [72], suggesting that a coordinated balance between GnRH and GnIH activity plays an important role in follicular development and atresia throughout the ovulatory cycle.
3.3. Regulatory mechanisms of GnIH expression in birds and mammals
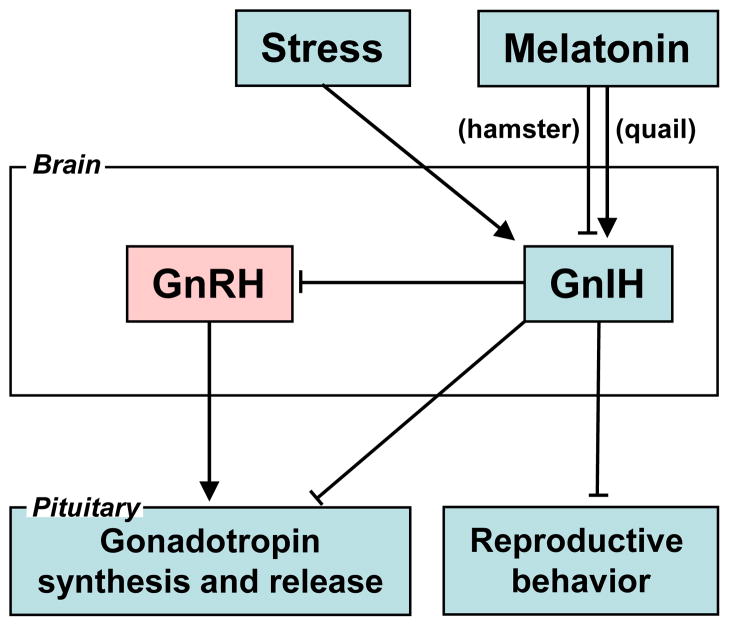
To understand the physiological role of GnIH, Ubuka et al. [89] investigated the mechanisms that regulate GnIH expression in quail. Pinealectomy (Px) combined with orbital enucleation (Ex) (Px plus Ex) decreased the expression of GnIH precursor mRNA and content of mature GnIH peptide in the diencephalon. Melatonin administration to Px plus Ex birds caused a dose-dependent increase in expression of GnIH precursor mRNA and production of mature peptide. The expression of GnIH was photoperiodically controlled and increased under short-day photoperiods, when the duration of melatonin secretion increases. In situ hybridization of Mel1c mRNA combined with immunocytochemistry for GnIH revealed that Mel1c mRNA was expressed in GnIH- ir neurons in the PVN. Melatonin receptor autoradiography further revealed specific binding of melatonin in the PVN. It was further shown that melatonin stimulates the release of GnIH in quail in vitro [13]. Accordingly, melatonin appears to act directly on GnIH neurons through its receptor to induce GnIH expression and release in birds (Fig. 1).
Fig. 1. GnIH actions within the brain and pituitary.
GnIH inhibits gonadotropin synthesis and release by directly acting on the pituitary or by inhibiting the activity of GnRH neurons. GnIH can also inhibit reproductive behavior by possibly acting within the brain. Stress induces the expression of GnIH in birds and mammals. Melatonin induces the expression of GnIH in quail, whereas melatonin inhibits the expression of GnIH in hamsters.
In hamsters, reproductive status is primarily driven by day length (photoperiod). Photoperiodic mammals rely on the annual cycle of changes in nocturnal secretion of a pineal hormone, melatonin, to drive their reproductive responses [8]. If GnIH is involved in the control of reproductive functions in hamsters, the expressions of GnIH precursor mRNA and GnIH peptides should be regulated by photoperiod and melatonin. Ubuka et al. [90] found that expression of GnIH precursor mRNA, GnIH-immunoreactivity in GnIH-ir perikarya and GnIH-ir fiber density decreased under short-day photoperiod compared to long-day in Siberian hamsters. This inhibitory effect of short-day was not seen if the hamsters were pinealectomized, and melatonin administration inhibited the expressions of GnIH precursor mRNA, GnIH-immunoreactivity in GnIH-ir perikarya and GnIH-ir fiber density. It was further shown that the percentage of GnRH-ir neurons receiving GnIH-ir fiber terminals decreased in short-day photoperiod. These results are in line with the results in Syrian hamsters [42,62] and sheep [17]. These findings are consistent with the possibility that the activity of GnIH neurons decreases in short-day by the inhibitory action of pineal melatonin in hamsters (Fig. 1). On the other hand, it was shown that melatonin directly induces GnIH (RFRP) mRNA expression in the rat GnIH cell line (rHypoE-7) [25]. The mechanism of different actions of melatonin on GnIH (RFRP) expression across species is an open question (Fig. 1).
Stress can also inhibit reproduction in birds and mammals. Calisi et al. [11] examined the effects of capture-handling stress on the numbers of GnIH neurons in the hypothalamus of adult male and female house sparrows. There were more GnIH-positive neurons in birds in fall versus those sampled in the spring, and a significant increase in GnIH positive neurons was seen in stressed birds in spring. These data imply an influence of stress upon the GnIH system that changes over the annual cycle of reproduction. It is likely that this effect of immobilization stress is mediated directly via glucocorticoid action on GnIH neurons via glucocorticoid receptor, as in rats [36]. It is not known if house sparrows GnIH neurons express glucocorticoid receptor, but we have recently generated data indicating this to be the case in European starlings. We found glucocorticoid receptor co-localized with GnIH-ir cells in the European starling hypothalamus (Calisi and Bentley, unpublished data). Kirby et al. [36] investigated how acute immobilization stress alters GnIH mRNA and protein levels in adult male rats. GnIH mRNA and protein levels increased immediately following stress but returned to a lower level 24 h after stress. Thus, glucocorticoid receptor expression by GnIH neurons is evident in birds and mammals, and is likely to be an evolutionarily conserved phenomenon. GnIH may therefore be a mediator of stress-induced reproductive disruption in birds and mammals (Fig. 1).
3.4. Phylogenetic Aspects of GnIH structure and functions
From evolutionary aspects of GnIH structure and function, several studies have been conducted to identify GnIH orthologs in lower vertebrates as in birds and mammals. As summarized in Table 1, GnIH orthologs have now been documented in a variety of vertebrates from fish to mammals, including humans: RFRPs in mammals [23,90,92,94,98,105], frog growth hormone (GH)-releasing peptide (fGRP) and fGRP-related peptides (fGRP-RPs) in amphibians [37,67,99] and goldfish LPXRFamide peptide (gfLPXRFa) in teleosts [68]. In amphibians, a GnIH ortholog with a C-terminal LPLRFamide motif was identified in the hypothalamus of bullfrogs Rana catesbeiana [37] (Table 1). The fGRP precursor also encodes one fGRP and three fGRP-RPs [67], later identified as mature peptides [99] (Table 1). At the same time, fGRP was independently purified from the European green frog Rana esculenta as R-RFa [12]. Subsequently, from the brain of goldfish Carassius auratus, a cDNA that encodes three fish GnIH orthologs (gfLPXRFa-1, -2, and -3) was characterized and gfLPXRFa-3 was identified as a mature peptide [68] (Table 1). Thus, the presence of GnIH and its orthologs is a conserved property in vertebrates.
All the identified GnIH and its orthologs possess a common C-terminal LPXRFamide motif (Table 1). GnIH and its orthologs form a new group in the RFamide peptide family (for reviews, see [80,81,83]). Extensive studies over the past decade have shown that vertebrate brains produce a variety of RFamide peptides.
In birds and mammals, GnIH and the mammalian GnIH ortholog RFRP-3 act to inhibit gonadotropin release and synthesis (for reviews, see [80,81,83]) (Table 2). The mammalian GnIH ortholog RFRP-1 also inhibits gonadotropin release in hamsters [90] (Table 2). In contrast to the impact of GnIH in birds and mammals, fGRP and fGRP-RP-2 stimulate the release of GH and/or prolactin (PRL) in amphibians [37,99] (Table 2). On the other hand, gfLPXRFa-1, -2 and -3 stimulate the release of gonadotropins and GH but not PRL in sockeye salmon Oncorhynchus nerka [1], whereas zebrafish (zf)LPXRFa-3 inhibits gonadotropin release in goldfish [106] (Table 2). Thus, all of the identified GnIH and its orthologs regulate pituitary function in vertebrates, but hypophysiotropic activities are diverse in lower vertebrates (Table 2).
Vertebrates have their own reproductive strategies to adapt to their external environments. GnIH and its orthologs regulate the secretion of gonadotropins in most animals and PRL in some animals; both of these hormones are involved in the regulation of vertebrate reproduction. Therefore, GnIH and its orthologs are considered to be evolutionarily-conserved components of the regulatory mechanisms of reproduction in vertebrates. Further studies are necessary to clarify the generality and diversity of functions of GnIH and its orthologs on the secretion of pituitary hormones, in particular gonadotropins, in various vertebrates in relation to their adaptive significance and evolution.
4. Future prospects for GnIH research
4.1. Evolutionary origin of GnIH structure and function
As described above, all the identified GnIH and its orthologs possessed a C-terminal LPXRFamide motif and thus they were designated as LPXRFamide peptides (for reviews, see [80,81,83,87,100]). Although GnIH and its orthologs exist in gnathostomes (jawed vertebrates) from teleosts to humans, the evolutionary history of this gonadotropin-inhibitory system is still unknown because the existence of GnIH was not investigated in agnathans (jawless vertebrates), the most ancient lineage of vertebrates whose lineage dates back over 500 million years [28]. Therefore, it is necessary to identify GnIH orthologs in agnathans to reveal when during the course of evolution animals acquired this hypothalamic gonadotropin-inhibitory system. Lampreys and hagfish are the only two extant members of agnathans. Recently, a candidate of the evolutionary origin of GnIH was found in the brain of sea lampreys Petromyzon marinus, one of the most ancient vertebrate species [83; Osugi et al., unpublished]. To clarify evolutionary origin of GnIH structure and function, further studies are in progress in the sea lamprey as an excellent model for understanding the evolution of the HPG axis in vertebrates [77].
4.2. Interaction of GnIH with kisspeptin for balancing reproduction
In contrast to GnIH, kisspeptin, a neuropeptide encoded by the Kiss1 gene, acts as a pronounced stimulatory regulator of the GnRH system. KISS1was originally identified as a human metastasis suppressor gene, suppressing metastases of human melanomas and breast carcinomas without affecting tumorigenicity (for a review, see [57]). In 2003, marked interest in kisspeptin as a stimulatory modulator of the reproductive axis occurred following the observation that individuals with mutations in the gene GPR54, the receptor for kisspeptin, exhibit hypogonadotropic hypogonadism and fail to enter puberty [69], with analogous results seen in knock-out mice lacking the Gpr54 gene [69]. Since these initial discoveries, numerous contributions by researchers working at varying levels of analysis indicate that kisspeptin is a critical regulator of sexual differentiation and maturation as well as normal, adult reproductive functioning across mammalian species, including humans [33,34,40,57,64].
In addition to GnIH, kisspeptin forms another new group in the RFamide peptide family (for reviews, see [80,81,83]. Despite the fact that all experiments on GnIH and kisspeptin indicate that both have pronounced actions on the HPG axis in mammals, the specific neural loci at which the combined contribution of both of these neurochemicals are integrated and summate to impact downstream reproductive function has not been explored. One question of interest is whether GnIH and kisspeptin act on the same or distinct populations of GnRH cells to regulate the combined output of this ensemble. One study to date demonstrated that GnIH inhibits kisspeptin-activated GnRH firing rate in vitro [102], suggesting interactions at the cellular level at the same GnRH targets. However, to gain insight into the general pattern of GnIH and kisspeptin innervation of the GnRH system, extensive tract tracing and combined electrophysiological studies are required.
Further evidence for the importance of interactions between GnIH and kisspeptin in reproductive function comes from recent work using GnIH and kisspeptin receptor antagonists. For instance, infusions of the kisspeptin receptor antagonist, peptide-234, grossly suppress the LH surge in female rats [64]. Likewise, administration of the GnIH-R antagonist, RF9, augments the gonadotropin-releasing impact of kisspeptin [59]. Together, these findings underscore the importance of maintaining the proper balance in the activity of these opposing neuropeptidergic systems to ensure that both are not regulated simultaneously in the matching directions, resulting in a loss of any downstream impact on target effector systems.
Evidence presented throughout this review indicates that an individual’s internal milieu and the external environment affect both kisspeptin and GnIH. By having both systems sensitive to reproductively-relevant stimuli, and responding in opposing directions, precision can be gained beyond that which can be achieved by either system acting alone. Because these two neuropeptides play key roles in reproduction, it is attractive to speculate that, kisspeptin and GnIH serve to communicate an array of excitatory and inhibitory signals that regulate the HPG axis, summating the contribution of each and, together, producing the appropriate downstream response. By each system responding to both positive and negative stimuli, in addition to cross-talk between the systems, a more accurate depiction of current conditions can be relayed to the reproductive axis for final integration and analysis to maximize reproductive success.
4.3. Application of GnIH research to human reproductive dysfunction
The identification of GnIH in the human hypothalamus [94] and the demonstration that human GnIH has potent inhibitory actions on gonadotropin secretion [16] opened up new vistas for understanding the regulation of human reproduction (for reviews, see [80,81,83]) (Fig. 1). Many factors such as stress, anorexia, diabetes, obesity and photoperiod inhibit gonadotropin secretion. The possible role of GnIH in mediating these effects has been implicated in animals [11,13,17,36,42,62,89,90] (Fig. 1). Currently GnRH analogs are extensively employed to manipulate the hypothalamic-pituitary-gonadal axis in various clinical conditions [22,48,49]. In view of GnIH’s potent inhibitory action on gonadotropin expression and release in animals (Fig. 1), GnIH has the potential of an alternative medicine to inhibit gonadotropins and steroid hormones. Accordingly, this endogenous inhibitor of gonadotropin secretion clearly has therapeutic potential in the treatment of hormone-dependent diseases, such as precocious puberty, endometriosis, uterine fibroids, benign prostatic hyperplasia and prostatic and breast cancers. Human GnIH may also have potential as a novel contraceptive.
5. Conclusion
In sum, the discovery of GnIH has fundamentally changed our understanding of hypothalamic control of reproduction. In addition, the variety of actions and modes of regulation of GnIH raise interesting questions as to the evolution of this neuropeptide and its adaptive significance. For example, why do long days down-regulate GnIH in some species and up-regulate it in others? How do these opposing regulatory roles evolve? Why are the physiological effects of GnIH more varied in lower vertebrates, and what were the selection pressures that caused GnIH to become an inhibitor of reproduction in birds and mammals? Why is more than one mature peptide cleaved from the same precursor polypeptide when they often have similar functions (but not always)? Likewise, anomalies in data both within and across species and laboratories deserve further exploration to fully understand the origins, functions and regulation of this neuropeptide and the complexity of its impact.
Research Highlights.
GnIH is a key neurohormone controlling vertebrate reproduction.
The mode of action and functional significance of GnIH are described.
Future prospects for GnIH studies are discussed.
Acknowledgments
Grant support: Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (22132004 and 22227002 to K.T.), National Institutes of Health Grant (HD-050470 to L.J.K.), National Science Foundation Integrative Organismal Systems Grants (0641188, 0956338 and 0920753 to G.E.B.).
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (22132004 and 22227002 to K. T.), National Institutes of Health Grant (HD-050470 to L. J. K.) and National Science Foundation Integrative Organismal Systems Grants (0641188, 0956338 and 0920753 to G. E. B.). We are grateful to the following collaborators, J. C. Wingfield, P. J. Sharp, I. J. Clarke, H. Vaudry, R. P. Millar, A. Sower, G. Bedecarrats, T. Osugi, V. S. Chowdhury, E. Saigoh, H. Yin, Y. Tobari, Y. L. Son, K. Inoue, Y. Muneoka, K. Ukena, H. Teranishi, Y. Fujisawa, M. Kikuchi, S. Ishii, O. Koizumi, M. Ueno, H. Minakata, H. Satake, M. Hisada, T. Kawada, N. L. McGuire, R. Calisi, N. Perfito, S. O’Brien, I. T. Moore, J. P. Jensen, G. J. Kaur, D. W. Wacker, N. A. Ciccone, I. C. Dunn, T. Boswell, S. Kim, Y. C. Huang, J. Reid, J. Jiang, P. Deviche, T. W. Small, M. A. Ottinger, T. Tachibana, M. Furuse, D. F. Mei, A. Mason, E. M. Gibson, S. A. Humber, S. Jain, W. P. Williams III, S. Zhao, I. P. Sari, Y. Qi, J. T. Smith, H. C. Parkington, J. Iqbal, Q. Li, A. Tilbrook, K. Morgan, A. J. Pawson, M. Murakami, T. Matsuzaki, T. Iwasa, T. Yasui, M. Irahara, M. A. Johnson, G. S. Fraley, M. Binns, P. A. Cadigan, H. Lai, M. Kitani, A. Suzuuchi, V. Pham, P. A. Cadigan, S. Kikuyama, N. Chartrel, K. Yamamoto, A. Koda, S. Ohta, K. Sawada, E. Iwakoshi-Ukena, I. Hasunuma, P. Singh, A. Krishna, R. Sridaran, M. Amano, S. Moriyama, M. Iigo, S. Kitamura, N. Amiya, K. Yamamori and H. Kawauchi.
Footnotes
Disclosure Statement: The authors have nothing tof disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amano M, Moriyama S, Iigo M, Kitamura S, Amiya N, Yamamori K, Ukena K, Tsutsui K. Novel fish hypothalamic neuropeptides stimulate the release of gonadotrophins and growth hormone from the pituitary of sockeye salmon. J Endocrinol. 2006;188:417–423. doi: 10.1677/joe.1.06494. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology. 2009;150:1834–1840. doi: 10.1210/en.2008-1359. [DOI] [PubMed] [Google Scholar]
- 3.Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006;49:550–555. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J Neuroendocrinol. 2003;15:794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- 5.Bentley GE, Tsutsui K, Kriegsfeld LJ. Recent studies of gonadotropin-inhibitory hormone (GnIH) in the mammalian hypothalamus, pituitary and gonads. Brain Res. 2010;1364:62–71. doi: 10.1016/j.brainres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Bentley GE, Ubuka T, McGuire NL, Chowdhury VS, Morita Y, Yano T, Hasunuma I, Binns M, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen Comp Endocrinol. 2008;156:34–43. doi: 10.1016/j.ygcen.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Burgus R, Butcher M, Amoss M, Ling N, Monahan M, Rivier J, Fellows R, Blackwell R, Vale W, Guillemin R. Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF) (LH- hypothalamus-LRF-gas chromatography-mass spectrometry-decapeptide-Edman degradation) Proc Natl Acad Sci U S A. 1972;69:278–282. doi: 10.1073/pnas.69.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronson FH. Mammalian Reproductive Biology. University of Chicago Press; Chicago: 1989. [Google Scholar]
- 9.Calisi RM, Bentley GE. Lab and field experiments: are they the same animal? Horm Behav. 2009;56:1–10. doi: 10.1016/j.yhbeh.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Calisi RM, Díaz-Muñoz SL, Wingfield JC, Bentley GE. Social and breeding status are associated with the expression of GnIH. Genes Brain Behav. 2011;10:557–564. doi: 10.1111/j.1601-183X.2011.00693.x. [DOI] [PubMed] [Google Scholar]
- 11.Calisi RM, Rizzo NO, Bentley GE. Seasonal differences in hypothalamic EGR-1 and GnIH expression following capture-handling stress in house sparrows (Passer domesticus) Gen Comp Endocrinol. 2008;157:283–287. doi: 10.1016/j.ygcen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Chartrel N, Dujardin C, Leprince J, Desrues L, Tonon MC, Cellier E, Cosette P, Jouenne T, Simonnet G, Vaudry H. Isolation, characterization, and distribution of a novel neuropeptide, Rana RFamide (R-RFa), in the brain of the European green frog Rana esculenta. J Comp Neurol. 2002;448:111–127. doi: 10.1002/cne.10253. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury VS, Yamamoto K, Ubuka T, Bentley GE, Hattori A, Tsutsui K. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology. 2010;151:271–280. doi: 10.1210/en.2009-0908. [DOI] [PubMed] [Google Scholar]
- 14.Ciccone NA, Dunn IC, Boswell T, Tsutsui K, Ubuka T, Ukena K, Sharp PJ. Gonadotrophin inhibitory hormone depresses gonadotrophin alpha and follicle-stimulating hormone beta subunit expression in the pituitary of the domestic chicken. J Neuroendocrinol. 2004;16:999–1006. doi: 10.1111/j.1365-2826.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 15.Clarke IJ, Qi Y, Puspita Sari I, Smith JT. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Front Neuroendocrinol. 2009;30:371–378. doi: 10.1016/j.yfrne.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149:5811–5821. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- 17.Dardente H, Birnie M, Lincoln GA, Hazlerigg DG. RFamide-related peptide and its cognate receptor in the sheep: cDNA cloning, mRNA distribution in the hypothalamus and the effect of photoperiod. J Neuroendocrinol. 2008;20:1252–1259. doi: 10.1111/j.1365-2826.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- 18.Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- 19.Deviche P, Small T, Sharp P, Tsutsui K. Control of luteinizing hormone and testosterone secretion in a flexibly breeding male passerine, the Rufous-winged Sparrow,Aimophila carpalis. Gen Comp Endocrinol. 2006;149:226–235. doi: 10.1016/j.ygcen.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Dockray GJ, Reeve JR, Jr, Shively J, Gayton RJ, Barnard CS. A novel active pentapeptide from chicken brain identified by antibodies to FMRFamide. Nature. 1983;305:328–330. doi: 10.1038/305328a0. [DOI] [PubMed] [Google Scholar]
- 21.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150:2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 22.Emons G, Schally AV. The use of luteinizing hormone releasing hormone agonists and antagonists in gynaecological cancers. Hum Reprod. 1994;9:1364–1379. doi: 10.1093/oxfordjournals.humrep.a138714. [DOI] [PubMed] [Google Scholar]
- 23.Fukusumi S, Habata Y, Yoshida H, Iijima N, Kawamata Y, Hosoya M, Fujii R, Hinuma S, Kitada C, Shintani Y, Suenaga M, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. Characteristics and distribution of endogenous RFamide-related peptide-1. Biochim Biophys Acta. 2001;1540:221–232. doi: 10.1016/s0167-4889(01)00135-5. [DOI] [PubMed] [Google Scholar]
- 24.Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gingerich S, Wang X, Lee PK, Dhillon SS, Chalmers JA, Koletar MM, Belsham DD. The generation of an array of clonal, immortalized cell models from the rat hypothalamus: analysis of melatonin effects on kisspeptin and gonadotropin-inhibitory hormone neurons. Neuroscience. 2009;162:1134–1140. doi: 10.1016/j.neuroscience.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Greives TJ, Kingma SA, Beltrami G, Hau M. Melatonin delays clutch initiation in a wild songbird. Biol Lett. doi: 10.1098/rsbl.2011.1100. published online 14 December 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2:703–708. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 28.Janvier P. Palaeontology: modern look for ancient lamprey. Nature. 2006;443:921–924. doi: 10.1038/443921a. [DOI] [PubMed] [Google Scholar]
- 29.Jimenez-Liñan M, Rubin BS, King JC. Examination of guinea pig luteinizing hormone-releasing hormone gene reveals a unique decapeptide and existence of two transcripts in the brain. Endocrinology. 1997;138:4123–4130. doi: 10.1210/endo.138.10.5454. [DOI] [PubMed] [Google Scholar]
- 30.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51:171–180. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadokawa H, Shibata M, Tanaka Y, Kojima T, Matsumoto K, Oshima K, Yamamoto N. Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domest Anim Endocrinol. 2009;36:219–224. doi: 10.1016/j.domaniend.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Kaewwongse M, Takayanagi Y, Onaka T. Effects of RFamide-related peptide (RFRP)-1 and RFRP-3 on oxytocin release and anxiety-related behaviour in rats. J Neuroendocrinol. 2011;23:20–27. doi: 10.1111/j.1365-2826.2010.02077.x. [DOI] [PubMed] [Google Scholar]
- 33.Kauffman AS. Sexual differentiation and the Kiss1 system: hormonal and developmental considerations. Peptides. 2009;30:83–93. doi: 10.1016/j.peptides.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King JA, Millar RP. Structure of chicken hypothalamic luteinizing hormone-releasing hormone. I. Structural determination on partially purified material. J Biol Chem. 1982;257:10722–10728. [PubMed] [Google Scholar]
- 36.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A. 2009;106:11324–11329. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koda A, Ukena K, Teranishi H, Ohta S, Yamamoto K, Kikuyama S, Tsutsui K. A novel amphibian hypothalamic neuropeptide: isolation, localization, and biological activity. Endocrinology. 2002;143:411–419. doi: 10.1210/endo.143.2.8630. [DOI] [PubMed] [Google Scholar]
- 38.Kriegsfeld LJ, Gibson EM, Williams WP, 3rd, Zhao S, Mason AO, Bentley GE, Tsutsui K. The roles of RFamide-related peptide-3 in mammalian reproductive function and behaviour. J Neuroendocrinol. 2010;22:692–700. doi: 10.1111/j.1365-2826.2010.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luque RM, Córdoba-Chacón J, Gahete MD, Navarro VM, Tena-Sempere M, Kineman RD, Castaño JP. Kisspeptin regulates gonadotroph and somatotroph function in nonhuman primate pituitary via common and distinct signaling mechanisms. Endocrinology. 2011;152:957–966. doi: 10.1210/en.2010-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43:1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- 42.Mason AO, Duffy S, Zhao S, Ubuka T, Bentley GE, Tsutsui K, Silver R, Kriegsfeld LJ. Photoperiod and reproductive condition are associated with changes in RFamide-related peptide (RFRP) expression in Syrian hamsters (Mesocricetus auratus) J Biol Rhythms. 2010;25:176–185. doi: 10.1177/0748730410368821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyamoto K, Hasegawa Y, Minegishi T, Nomura M, Takahashi Y, Igarashi M, Kangawa K, Matsuo H. Isolation and characterization of chicken hypothalamic luteinizing hormone-releasing hormone. Biochem Biophys Res Commun. 1982;107:820–827. doi: 10.1016/0006-291x(82)90596-4. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto K, Hasegawa Y, Nomura M, Igarashi M, Kangawa K, Matsuo H. Identification of the second gonadotropin-releasing hormone in chicken hypothalamus: evidence that gonadotropin secretion is probably controlled by two distinct gonadotropin-releasing hormones in avian species. Proc Natl Acad Sci U S A. 1984;81:3874–3878. doi: 10.1073/pnas.81.12.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGuire NL, Bentley GE. A functional neuropeptide system in vertebrate gonads: Gonadotropin-inhibitory hormone and its receptor in testes of field-caught house sparrow (Passer domesticus) Gen Comp Endocrinol. 2010a;166:565–572. doi: 10.1016/j.ygcen.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 46.McGuire NL, Bentley GE. Neuropeptides in the gonads: from evolution to pharmacology. Front Pharmacol. 2010b;1:114. doi: 10.3389/fphar.2010.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGuire NL, Kangas K, Bentley GE. Effects of melatonin on peripheral reproductive function: regulation of testicular GnIH and testosterone. Endocrinology. 2011;152:3461–3470. doi: 10.1210/en.2011-1053. [DOI] [PubMed] [Google Scholar]
- 48.Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- 49.Millar RP, Zhu YF, Chen C, Struthers RS. Progress towards the development of non-peptide orally-active gonadotropin-releasing hormone (GnRH) antagonists: therapeutic implications. Br Med Bull. 2000;56:761–772. doi: 10.1258/0007142001903346. [DOI] [PubMed] [Google Scholar]
- 50.Molnár CS, Kalló I, Liposits Z, Hrabovszky E. Estradiol down-regulates RF-amide-related peptide (RFRP) expression in the mouse hypothalamus. Endocrinology. 2011;152:1684–1690. doi: 10.1210/en.2010-1418. [DOI] [PubMed] [Google Scholar]
- 51.Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199:105–112. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- 52.Okubo K, Amano M, Yoshiura Y, Suetake H, Aida K. A novel form of gonadotropin-releasing hormone in the medaka,Oryzias latipes. Biochem Biophys Res Commun. 2000a;16:298–303. doi: 10.1006/bbrc.2000.3476. [DOI] [PubMed] [Google Scholar]
- 53.Okubo K, Suetake H, Usami T, Aida K. Molecular cloning and tissue-specific expression of a gonadotropin-releasing hormone receptor in the Japanese eel. Gen Comp Endocrinol. 2000b;119:181–192. doi: 10.1006/gcen.2000.7511. [DOI] [PubMed] [Google Scholar]
- 54.Osugi T, Ukena K, Bentley GE, O’Brien S, Moore IT, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone in Gambel’s white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol. 2004;182:33–42. doi: 10.1677/joe.0.1820033. [DOI] [PubMed] [Google Scholar]
- 55.Papargiris MM, Rivalland ET, Clarke IJ, Smith JT, Pereira A, Tilbrook AJ. Evidence that RF-amide related peptide-3 is not a mediator of the inhibitory effects of psychosocial stress on gonadotrophin secretion in ovariectomised ewes. J Neuroendocrinol. 2011;23:208–215. doi: 10.1111/j.1365-2826.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 56.Paul MJ, Pyter LM, Freeman DA, Galang J, Prendergast BJ. Photic and nonphotic seasonal cues differentially engage hypothalamic kisspeptin and RFamide-related peptide mRNA expression in Siberian hamsters. J Neuroendocrinol. 2009;21:1007–1014. doi: 10.1111/j.1365-2826.2009.01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. Physiological roles of the kisspeptin/GPR54 system in the neuroendocrine control of reproduction. Prog Brain Res. 2010a;181:55–77. doi: 10.1016/S0079-6123(08)81005-9. [DOI] [PubMed] [Google Scholar]
- 58.Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, Dijcks FA, Blomenröhr M, Pinilla L, van Noort PI, Tena-Sempere M. Characterization of the inhibitory roles of RFRP3, the mammalian ortholog of GnIH, in the control of gonadotropin secretion in the rat: in vivo and in vitro studies. Am J Physiol Endocrinol Metab. 2010b;299:E39–46. doi: 10.1152/ajpendo.00108.2010. [DOI] [PubMed] [Google Scholar]
- 59.Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, Dijcks FA, Blomenröhr M, Pinilla L, van Noort PI, Tena-Sempere M. Characterization of the potent gonadotropin-releasing activity of RF9, a selective antagonist of RF-amide-related peptides and neuropeptide FF receptors: physiological and pharmacological implications. Endocrinology. 2010c;151:1902–1913. doi: 10.1210/en.2009-1259. [DOI] [PubMed] [Google Scholar]
- 60.Price DA, Greenberg MJ. Structure of a molluscan cardioexcitatory neuropeptide. Science. 1977;197:670–671. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- 61.Qi Y, Oldfield BJ, Clarke IJ. Projections of RFamide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J Neuroendocrinol. 2009;21:690–697. doi: 10.1111/j.1365-2826.2009.01886.x. [DOI] [PubMed] [Google Scholar]
- 62.Revel FG, Saboureau M, Pévet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149:902–912. doi: 10.1210/en.2007-0848. [DOI] [PubMed] [Google Scholar]
- 63.Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150:1413–1420. doi: 10.1210/en.2008-1287. [DOI] [PubMed] [Google Scholar]
- 64.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology. 2009;150:5549–5556. doi: 10.1210/en.2009-0775. [DOI] [PubMed] [Google Scholar]
- 66.Satake H, Hisada M, Kawada T, Minakata H, Ukena K, Tsutsui K. Characterization of a cDNA encoding a novel avian hypothalamic neuropeptide exerting an inhibitory effect on gonadotropin release. Biochem J. 2001;354:379–385. doi: 10.1042/0264-6021:3540379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sawada K, Ukena K, Kikuyama S, Tsutsui K. Identification of a cDNA encoding a novel amphibian growth hormone-releasing peptide and localization of its transcript. J Endocrinol. 2002a;174:395–402. doi: 10.1677/joe.0.1740395. [DOI] [PubMed] [Google Scholar]
- 68.Sawada K, Ukena K, Satake H, Iwakoshi E, Minakata H, Tsutsui K. Novel fish hypothalamic neuropeptide. Eur J Biochem. 2002b;269:6000–6008. doi: 10.1046/j.1432-1033.2002.03351.x. [DOI] [PubMed] [Google Scholar]
- 69.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 70.Sherwood N, Eiden L, Brownstein M, Spiess J, Rivier J, Vale W. Characterization of a teleost gonadotropin-releasing hormone. Proc Natl Acad Sci U S A. 1983;80:2794–2798. doi: 10.1073/pnas.80.9.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sherwood NM, Sower SA, Marshak DR, Fraser BA, Brownstein MJ. Primary structure of gonadotropin-releasing hormone in lamprey brain. J Biol Chem. 1986;15:4812–4819. [PubMed] [Google Scholar]
- 72.Singh P, Krishna A, Sridaran R, Tsutsui K. Immunohistochemical localization of GnRH and RFamide-related peptide-3 in the ovaries of mice during the estrous cycle. J Mol Histol. 2011b;42:371–381. doi: 10.1007/s10735-011-9340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh P, Krishna A, Tsutsui K. Effects of gonadotropin-inhibitory hormone on folliculogenesis and steroidogenesis of cyclic mice. Fertil Steril. 2011a;95:1397–1404. doi: 10.1016/j.fertnstert.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 74.Small TW, Sharp PJ, Bentley GE, Millar RP, Tsutsui K, Mura E, Deviche P. Photoperiod-independent hypothalamic regulation of luteinizing hormone secretion in a free-living Sonoran desert bird, the Rufous-winged Sparrow (Aimophila carpalis) Brain Behav Evol. 2008;71:127–142. doi: 10.1159/000111459. [DOI] [PubMed] [Google Scholar]
- 75.Smith JT, Clarke IJ. Gonadotropin inhibitory hormone function in mammals. Trends Endocrinol Metab. 2010;21:255–260. doi: 10.1016/j.tem.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sower SA, Freamat M, Kavanaugh SI. The origins of the vertebrate hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) endocrine systems: new insights from lampreys. Gen Comp Endocrinol. 2009;161:20–29. doi: 10.1016/j.ygcen.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 78.Tachibana T, Sato M, Takahashi H, Ukena K, Tsutsui K, Furuse M. Gonadotropin-inhibiting hormone stimulates feeding behavior in chicks. Brain Res. 2005;1050:94–100. doi: 10.1016/j.brainres.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 79.Tobari Y, Iijima N, Tsunekawa K, Osugi T, Okanoya K, Tsutsui K, Ozawa H. Identification of gonadotropin-inhibitory hormone in the zebra finch (Taeniopygia guttata): Peptide isolation, cDNA cloning and brain distribution. Peptides. 2010;31:816–826. doi: 10.1016/j.peptides.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 80.Tsutsui K. A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): Biosynthesis, mode of action and functional significance. Prog Neurobiol. 2009;88:76–88. doi: 10.1016/j.pneurobio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol. 2010a;31:284–295. doi: 10.1016/j.yfrne.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Tsutsui K, Bentley GE, Ciccone N. Structure, action and functional significance of GnIH. In: Dawson A, Sharp PJ, editors. Functional Avian Endocrinology. Narosa Publishing House; New Delhi: 2005. pp. 73–82. [Google Scholar]
- 83.Tsutsui K, Bentley GE, Kriegsfeld LJ, Osugi T, Seong JY, Vaudry H. Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinol. 2010b;22:716–727. doi: 10.1111/j.1365-2826.2010.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsutsui K, Bentley GE, Ubuka T, Saigoh E, Yin H, Osugi T, Inoue K, Chowdhury VS, Ukena K, Ciccone N, Sharp PJ, Wingfield JC. The general and comparative biology of gonadotropin-inhibitory hormone (GnIH) Gen Comp Endocrinol. 2007a;153:365–370. doi: 10.1016/j.ygcen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 86.Tsutsui K, Ubuka T, Yin H, Osugi T, Ukena K, Bentley GE, Ciccone N, Inoue K, Chowdhury VS, Sharp PJ, Wingfield JC. Mode of action and functional significance of avian gonadotropin-inhibitory hormone (GnIH): a review. J Exp Zool. 2006;305A:801–806. doi: 10.1002/jez.a.305. [DOI] [PubMed] [Google Scholar]
- 87.Tsutsui K, Ukena K. Hypothalamic LPXRF-amide peptides in vertebrates: identification, localization and hypophysiotropic activity. Peptides. 2006;27:1121–1129. doi: 10.1016/j.peptides.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 88.Tsutsui K, Ubuka T, Yin H, Osugi T, Ukena K, Bentley GE, Sharp PJ, Wingfield JC. Review: Discovery of gonadotropin-inhibitory hormone in a domesticated bird, its mode of action and functional significance. J Ornithol. 2007b;148(Supplement 2):515–520. [Google Scholar]
- 89.Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc Natl Acad Sci U S A. 2005;102:3052–3057. doi: 10.1073/pnas.0403840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, Tsutsui K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology. 2011a doi: 10.1210/en.2011-1110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ubuka T, Kim S, Huang YC, Reid J, Jiang J, Osugi T, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology. 2008;149:268–278. doi: 10.1210/en.2007-0983. [DOI] [PubMed] [Google Scholar]
- 92.Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol. 2009a;517:841–855. doi: 10.1002/cne.22191. [DOI] [PubMed] [Google Scholar]
- 93.Ubuka T, McGuire NL, Calisi RM, Perfito N, Bentley GE. The control of reproductive physiology and behavior by gonadotropin-inhibitory hormone. Integr Comp Biol. 2008;48:560–569. doi: 10.1093/icb/icn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS ONE. 2009b;4:e8400. doi: 10.1371/journal.pone.0008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ubuka T, Mukai M, Wolfe J, Beverly R, Clegg S, Wang A, Hsia S, Li M, Krause JS, Mizuno T, Fukuda Y, Tsutsui K, Bentley GE, Wingfield JC. RNA interference of gonadotropin-inhibitory hormone gene induces arousal in songbirds. PLoS ONE. 2011b doi: 10.1371/journal.pone.0030202. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ubuka T, Ueno M, Ukena K, Tsutsui K. Developmental changes in gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica) hypothalamo-hypophysial system. J Endocrinol. 2003;178:311–318. doi: 10.1677/joe.0.1780311. [DOI] [PubMed] [Google Scholar]
- 97.Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006;147:1187–1194. doi: 10.1210/en.2005-1178. [DOI] [PubMed] [Google Scholar]
- 98.Ukena K, Iwakoshi E, Minakata H, Tsutsui K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett. 2002;512:255–258. doi: 10.1016/s0014-5793(02)02275-5. [DOI] [PubMed] [Google Scholar]
- 99.Ukena K, Koda A, Yamamoto K, Kobayashi T, Iwakoshi-Ukena E, Minakata H, Kikuyama S, Tsutsui K. Novel neuropeptides related to frog growth hormone-releasing peptide: isolation, sequence, and functional analysis. Endocrinology. 2003b;144:3879–3884. doi: 10.1210/en.2003-0359. [DOI] [PubMed] [Google Scholar]
- 100.Ukena K, Tsutsui K. A new member of the hypothalamic RF-amide peptide family, LPXRF-amide peptides: structure, localization, and function. Mass Spectrom Rev. 2005;24:469–486. doi: 10.1002/mas.20031. [DOI] [PubMed] [Google Scholar]
- 101.Ukena K, Ubuka T, Tsutsui K. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res. 2003a;312:73–79. doi: 10.1007/s00441-003-0700-x. [DOI] [PubMed] [Google Scholar]
- 102.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587:1401–1411. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yin H, Ukena K, Ubuka T, Tsutsui K. A novel G protein-coupled receptor for gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica): identification, expression and binding activity. J Endocrinol. 2005;184:257–266. doi: 10.1677/joe.1.05926. [DOI] [PubMed] [Google Scholar]
- 104.Yoo MS, Kang HM, Choi HS, Kim JW, Troskie BE, Millar RP, Kwon HB. Molecular cloning, distribution and pharmacological characterization of a novel gonadotropin-releasing hormone ([Trp8] GnRH) in frog brain. Mol Cell Endocrinol. 2000;164:197–204. doi: 10.1016/s0303-7207(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 105.Yoshida H, Habata Y, Hosoya M, Kawamata Y, Kitada C, Hinuma S. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim Biophys Acta. 2003;1593:151–157. doi: 10.1016/s0167-4889(02)00389-0. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Li S, Liu Y, Lu D, Chen H, Huang X, Liu X, Meng Z, Lin H, Cheng CH. Structural diversity of the GnIH/GnIH receptor system in teleost: its involvement in early development and the negative control of LH release. Peptides. 2010;31:1034–1043. doi: 10.1016/j.peptides.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 107.Zhao S, Zhu E, Yang C, Bentley GE, Tsutsui K, Kriegsfeld LJ. RFamide-related peptide and messenger ribonucleic acid expression in mammalian testis: association with the spermatogenic cycle. Endocrinology. 2010;151:617–627. doi: 10.1210/en.2009-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]