Abstract
RNase P, which catalyzes tRNA 5′-maturation, typically comprises a catalytic RNase P RNA (RPR) and a varying number of RNase P proteins (RPPs): 1 in bacteria, at least 4 in archaea and 9 in eukarya. The four archaeal RPPs have eukaryotic homologs and function as heterodimers (POP5•RPP30 and RPP21•RPP29). By studying the archaeal Methanocaldococcus jannaschii RPR's cis cleavage of precursor tRNAGln (pre-tRNAGln), which lacks certain consensus structures/sequences needed for substrate recognition, we demonstrate that RPP21•RPP29 and POP5•RPP30 can rescue the RPR's mis-cleavage tendency independently by 4-fold and together by 25-fold, suggesting that they operate by distinct mechanisms. This synergistic and preferential shift toward correct cleavage results from the ability of archaeal RPPs to selectively increase the RPR's apparent rate of correct cleavage by 11 140-fold, compared to only 480-fold for mis-cleavage. Moreover, POP5•RPP30, like the bacterial RPP, helps normalize the RPR's rates of cleavage of non-consensus and consensus pre-tRNAs. We also show that archaeal and eukaryal RNase P, compared to their bacterial relatives, exhibit higher fidelity of 5′-maturation of pre-tRNAGln and some of its mutant derivatives. Our results suggest that protein-rich RNase P variants might have evolved to support flexibility in substrate recognition while catalyzing efficient, high-fidelity 5′-processing.
INTRODUCTION
The biogenesis of transfer RNAs (tRNAs) involves 5′- and 3′-maturation, intron splicing (where applicable) and nucleotide modification before their use in translation (1). RNase P, the endonuclease that catalyzes the 5′-maturation, is typically a ribonucleoprotein (RNP) complex except for some organellar variants (2,3). It contains one catalytic RNase P RNA (RPR) and a varying number of RNase P proteins (RPPs): 1 in bacteria, at least 4 in archaea and 9 in eukarya (4–9). Although pre-tRNA cleavage is associated with the RPR (10–12), both RPR and RPP(s) are essential for cellular viability (13–15). While the bacterial RPP is unrelated to archaeal/eukaryal RPPs, four archaeal RPPs, which share homology with eukaryal counterparts, function as binary complexes (POP5•RPP30 and RPP21•RPP29) (16–20). The increasing protein content in the RNase P holoenzyme (10% in bacterial, 50% in archaeal and 70% in eukaryal RNase P) is accompanied by a decrease in the cleavage activity of the respective RPRs (bacterial, 10/min > archaeal, 10−1/min > eukaryal, 10−5/min; pH 6) (11,16,21). However, since all the holoenzymes display similar kcat/Km values (7), it is evident that archaeal and eukaryal RNase P, in contrast to their bacterial counterparts, display an acute functional dependence on multiple RPPs. We have focused on this aspect in the current investigation, particularly on how the multiple RPPs contribute to the RPR's fidelity of processing.
Given the wide variation in pre-tRNA sequences, several studies have focused on mapping the common determinants that permit the collective recognition and efficient cleavage of pre-tRNAs by RNase P. How does RNase P specifically hydrolyze the phosphodiester bond between the first nucleotide in the mature tRNA (N+1) and its preceding nucleotide in the 5′-leader (N−1) (Figure 1)? Chemical modification interference mapping, crosslinking, nucleotide analog interference mapping and mutagenesis studies have identified a suite of interactions between pre-tRNAs and bacterial RNase P (22,23). Notably, these interactions include (i) recognition of the 5′-leader of pre-tRNAs by amino acid residues in a cleft in bacterial RPP (24–28); (ii) the base at N−1 (typically a U) in the pre-tRNA and the adenosine at position 248 in the RPR (A248—Escherichia coli RPR numbering) (29–31); (iii) the T stem–loop (TSL) in the pre-tRNA and the P7–P11 region in the RPR, referred to as the TSL-binding site (TBS) (32–34); and (iv) Watson–Crick base pairing of the 3′-terminal RCC sequence of the pre-tRNA and a conserved GGU sequence in the L15 loop of RPR (23,35). A recent crystal structure of a bacterial RNase P holoenzyme-tRNA (ES) complex (36) confirms these intermolecular interactions. However, not all these contacts are required for accurate or efficient cleavage; hierarchy (if any) among them remains to be determined. In a related vein, differences between recognition of consensus (possessing the sequences/structures enumerated above) and non-consensus pre-tRNAs are also unclear.
Figure 1.
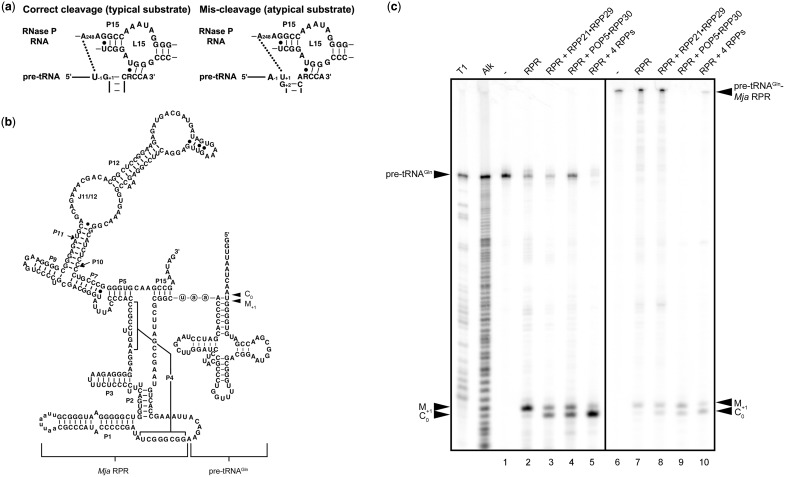
Archaeal RPPs affect the RPR's fidelity of processing of a non-consensus substrate. (a) Possible interactions around the cleavage site between nucleotides in typical (consensus) or atypical (non-consensus) pre-tRNAs and the bacterial RPR. (b) Secondary structure of the self-cleaving pre-tRNAGln-Mja RPR used in this study. The site of tethering of pre-tRNAGln to Mja RPR is depicted. The 5′-aau-3′ spacer is shown in circled lowercase letters. The secondary structure follows conventional representations for all RPRs (100). (c) Rescue of RPR's mis-cleavage by the RPPs. T1, the G ladder generated by RNase T1 digestion of pre-tRNAGln. Alk, the ladder obtained from alkaline hydrolysis of pre-tRNAGln. Uncleaved pre-tRNAGln is shown in lane 1. Cleavage of pre-tRNAGln by Pfu RPR, RPR + RPP21•RPP29, RPR + POP5•RPP30 and RPR + both RPP complexes is shown in lanes 2–5, respectively. Lanes 6 and 7 represent uncleaved and self-cleaved pre-tRNAGln-Mja RPR, respectively. The cleaved 5′-leader products generated by addition of Mja RPP21•RPP29, POP5•RPP30 and four RPPs to pre-tRNAGln-Mja RPR are shown in lanes 8–10. M+1, the 5′-leader generated from mis-cleavage between positions +1 and +2 in pre-tRNAGln; C0, the 5′-leader from cleavage at the correct site between positions −1 and +1 in pre-tRNAGln.
Results from previous investigations suggest that bacterial and eukaryal RNase P employ different recognition determinants during pre-tRNA processing. First, a comparative study examining the ability of partially purified Escherichia coli (Eco) and HeLa RNase P holoenzymes to cleave deletion derivatives of Thermus thermophilus (Tth) pre-tRNAGly, a non-consensus substrate, concluded that while both enzymes are indifferent to the presence of an anticodon stem, deletion of the D stem–loop caused a 30-fold decrease in human RNase P activity but only 3-fold in Eco RNase P (37). That such an inference might partly be substrate identity dependent is borne out by another investigation with HeLa RNase P which reported that removal of the D stem–loop in Eco pre-tRNATyr caused Vmax/Km to decrease by only 2-fold (38). Second, a model substrate comprising a 12-bp stem (similar to the acceptor-T-stem helical stack) capped with a terminal loop and flanked by a 5′-leader and 3′-RCCA trailer was shown to be cleaved by bacterial but not by partially purified human and frog RNase P (38–40). However, if this model substrate contains even a 1-nt bulge as a linker between the T and acceptor stems, it is recognized and cleaved by the native human and frog RNase P holoenzymes (38,39). A subsequent study showed that the human RPR (without RPPs) surprisingly could cleave a similar model substrate lacking a bulge, hinting that the multiple RPPs in the holoenzyme RNP complex must alter the RPR's substrate recognition (11). Third, cleavage of pre-tRNAHis by bacterial RNase P generates an 8-bp acceptor stem while maturation by eukaryotic RNase P yields a 7-bp acceptor stem (29,41,42). Finally, in contrast to the bacterial holoenzyme, partially purified Schizosaccharomyces pombe RNase P catalyzed correct cleavage of various derivatives of a non-consensus pre-tRNA (S. pombe pre-tRNASer with G−1) that differed in 5′- and 3′-flanking sequences (43).
The abovementioned observations motivate studies to uncover the molecular basis for parallels and differences in substrate recognition by RNase P from the three domains of life. In this study, we have focused particularly on the roles of the multiple archaeal/eukaryal RPPs in the fidelity of processing of non-consensus pre-tRNAs. Bacterial RNase P processes precursors to 4.5S RNA and tmRNA, select viral RNAs, C4 antisense RNA from bacteriophage P1 and P7, and some mRNAs, not all of which have a tRNA-like motif (44–49). Recently, human and yeast RNase P have been implicated in processing certain short-lived non-coding (nc) RNAs and mRNAs, and shown to even cleave single-stranded (ss) RNAs (50–56); intriguingly, common recognition determinants (tRNA-like or otherwise) in these substrates are not apparent. While the biological significance of processing these non-tRNA substrates by human/yeast RNase P remains to be uncovered, these findings reveal an unexpected expansion in the repertoire of substrates of eukaryotic RNase P and provide a possible basis for the association of the eukaryotic (and archaeal) RPR with multiple RPPs.
Here, we provide the first evidence that archaeal RPPs engender progressive and synergistic changes in shifting the cognate RPR's preference toward correct cleavage in vitro of pre-tRNAGln, a non-consensus substrate. By comparing the processing of pre-tRNAGln and several of its mutant derivatives, we also found that in vitro reconstituted archaeal and native archaeal/eukaryal RNase P exhibit both a higher fidelity in cleavage-site selection and a greater tolerance of structural deviations from the pre-tRNA consensus. We discuss the implications of these findings for the evolution of RNase P, especially the versatility and plasticity gains that might have resulted from association with multiple protein cofactors.
MATERIALS AND METHODS
Construction of pre-tRNAGln-AAU-Mja RPR
The cloning of pBT7-pre-tRNAGln-AAU-Mja RPR was carried out in two steps. First, two fragments, one encoding the Synechocystis pre-tRNAGln and the other encoding Methanocaldococcus jannaschii (Mja) RPR, were obtained separately by PCR using primer pairs pGln-S3-M GF + pGln-S3-M GR and pGln-S3-M MF + pGln-S3-M MR, respectively (Supplementary Table S1); the plasmids pT7-Gln (57) and pBT7-pre-tRNATyr-UAU-Mja RPR (19) served as the corresponding templates. These two PCR products, each with a 19-nt overlap, were annealed and extended, and the extended product digested with BamHI and ligated to StuI- and BamHI-digested pBT7 (58) to obtain pBT7-pre-tRNAGln-UAU-Mja RPR. Second, to change the linker sequence from 5′-UAU-3′ to 5′-AAU-3′, we employed PCR-based mutagenesis. We designed primers ptGln-AAU-M F and ptGln-linker-M R (Supplementary Table S1) to flank the linker nucleotides and having 5′ extensions to replace the linker sequence. The primers are oriented outward to ensure amplification of the entire pBT7-pre-tRNAGln-UAU-Mja RPR plasmid with the new linker sequence. The resulting PCR product was circularized by ligation with T4 DNA ligase and transformed into Eco DH5α. Transformants were then screened to identify those harboring the desired mutation, which were subsequently confirmed by DNA sequencing.
Construction of mutant derivatives of Synechocystis sp. PCC6803 pre-tRNAGln
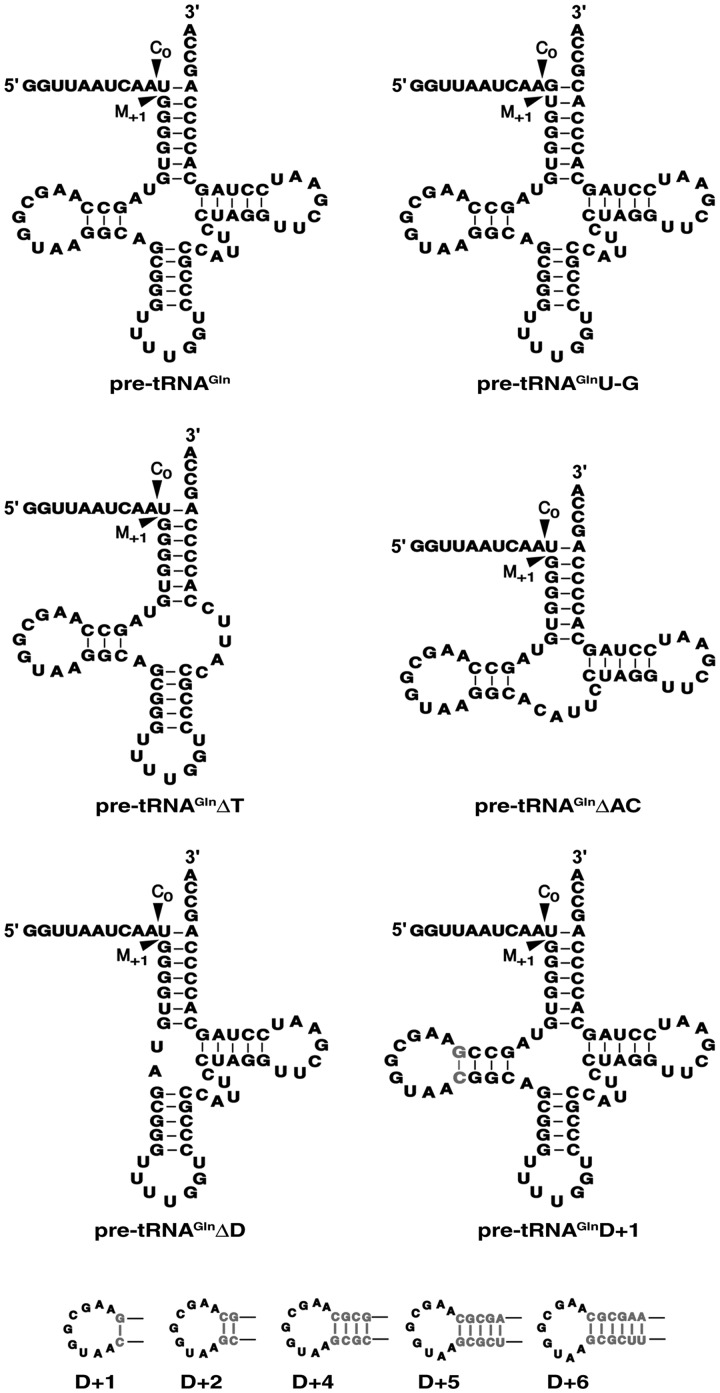
The genes encoding various mutant derivatives of Synechocystis pre-tRNAGln (Figure 3) were cloned using a PCR-based strategy. The entire coding sequence was generated either from fill-in of an annealed pair of primers (Supplementary Table S2) designed to have an overlapping complement or through PCR-based mutagenesis with pT7-Gln (57) serving as the template. In some cases (where cloning into pUC19 was involved), the forward primer included the T7 RNA polymerase promoter sequence and a BamHI site, while in others the forward primer was designed for cloning as a blunt-ended fragment into StuI-digested pBT7 (58). In all cases, the reverse primer included either a HindIII or an EcoRI site for subcloning and a BstNI site for linearizing the template for subsequent run-off transcription. All cloned sequences were confirmed by DNA sequencing before further use.
Figure 3.
Schematic depicting pre-tRNAGln and its mutant derivatives used in this study.
In vitro transcription of RNAs used in this study
All RNAs used in this study were generated by T7 RNA polymerase-mediated run-off transcription. In the case of pre-tRNAGln and its derivatives, appropriate plasmid DNAs digested with BstNI served as the template. Eco and Pyrococcus furiosus (Pfu) RPRs were generated from FokI-digested pJA2′ and EcoRI-digested pBT7-PfuRPR, respectively (20,59). For the self-cleaving pre-tRNAGln-AAU-Mja RPR, the template for transcription was generated by PCR using the high-fidelity Phusion DNA polymerase (New England Biolabs) with pBT7-pre-tRNAGln-AAU-Mja RPR as the template, and 5′-TAATACGACTCACTATAGGTTAATCAATGGGGTGTAG-3′ (forward) and 5′-CTATTTCGGCTTGCACCCC-3′ (reverse) as primers. Note that the forward primer includes the T7 RNA polymerase promoter (underlined sequence). These RNAs were subjected to extensive dialysis to remove unincorporated nucleotide triphosphates and their concentrations determined based on the absorbance at 260 nm and respective extinction coefficient.
To obtain radiolabeled RNAs, the appropriate transcripts were either 5′-labeled with [γ-32P]-ATP and T4 polynucleotide kinase or internally labeled by including [α-32P]-ATP in the in vitro transcription reaction. All labeled transcripts were then gel-purified using denaturing gel electrophoresis.
Purification of bacterial and archaeal RPPs
Recombinant versions of Eco, Mja and Pfu RPPs were obtained after overexpression in Eco and subsequent purification as described previously (16,20,59,60).
Partial purification of Arabidopsis thaliana (Ath) nuclear RNase P
Arabidopsis cultured suspension cells were grown at room temperature under continuous fluorescent white light (60 μmol/m2/s) in Gamborg B5 medium (Caisson Laboratories, Inc.) supplemented with 1.1 mg/l 2,4-dichlorophenoxy acetic acid and 0.5 g/l 2-(N-morpholino)ethanesulfonic acid. Seven-day-old cells were harvested by filtering through two layers of Miracloth and immediately stored at −80°C until subsequent use to prepare a whole cell extract. Ion-exchange chromatography of a clarified crude lysate using sequential SP-, DEAE- and Q-Sepharose (GE Healthcare) yielded a 400-fold purified native, nuclear RNase P. Active fractions at each step were identified with pre-tRNA processing assays using tobacco chloroplast pre-tRNAGly as the substrate. A detailed protocol will be published elsewhere (Lai, L. B. and Gopalan, V., manuscript in preparation).
RNase P assays
General
Pre-tRNA cleavage assays were performed in a thermal cycler and the reactions terminated using one assay-volume of stop dye [10 M urea, 1 mM EDTA, 0.05% (w/v) xylene cyanol, 0.05% (w/v) bromophenol blue, 20% (v/v) phenol]. The reaction products were then subjected to denaturing PAGE using either 10% or 20% (w/v) polyacrylamide/7 M urea gel electrophoresis, respectively, depending on whether the resolving length was 40 cm (e.g. Figures 1 and 4) or 18 cm (for the kinetic data shown in Figure 2).
Figure 4.
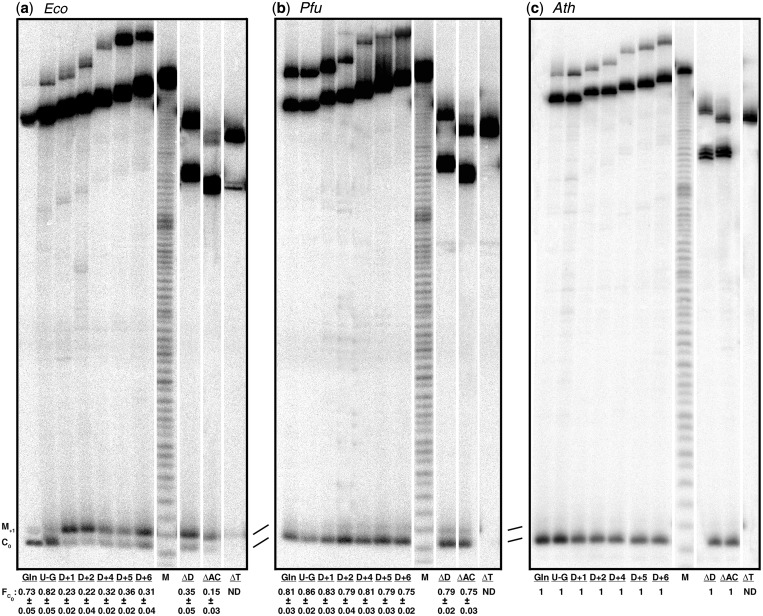
Comparison of correct and aberrant cleavage of pre-tRNAGln and its mutant derivatives by different RNase P holoenzymes. Representative assay gels depicting the cleavages by (a) Eco, (b) Pfu and (c) Ath RNase P. All lanes are from the same gel but they have been reordered for better illustration; a thin white line between lanes indicates such reshuffling. FC0 values, indicating the fraction of cleavage at the correct site relative to the total cleavage [PC0/(PC0 + PM+1)] are listed at the bottom of each panel and were obtained by quantitation of the correct and mis-cleaved products. Mean and standard deviation values were calculated from three independent experiments. ND, not detectable. The lane labels are as specified in Figure 3 with Gln indicating the wild-type pre-tRNAGln. M, the ladder obtained from alkaline hydrolysis of pre-tRNAGln.
Figure 2.
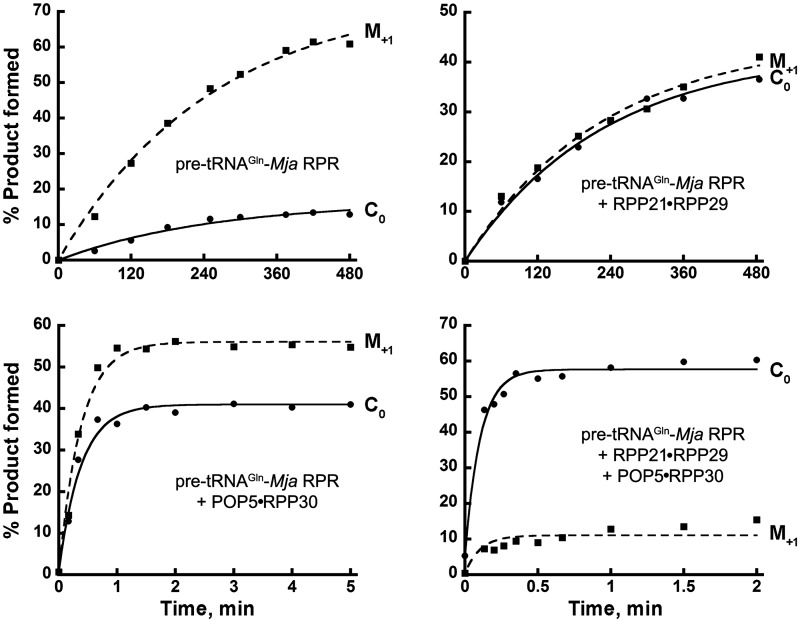
Effect of Mja RPPs on cleavage-site selection and the rate of self-cleavage of pre-tRNAGln-Mja RPR. Time courses depicting self-processing of pre-tRNAGln-Mja RPR with and without RPPs at the correct (C0; circles) and the mis-cleaved (M+1; squares) site. The mean and standard deviation values reported in Table 2 were calculated from three independent experiments, a representative of which is shown here.
Reconstitution of bacterial and archaeal RNase P
While Eco RNase P was reconstituted using 15 nM RPR and 150 nM RPP, Pfu and Mja RNase P were assembled from 20 to 40 nM RPR and a 5- or 10-fold excess amount of recombinant RPP21•RPP29 + POP5•RPP30. The buffers and methods employed for assembly are described in detail elsewhere (16,20,59,60). For the self-cleavage reactions with pre-tRNAGln-Mja RPR, we followed the procedure described previously for pre-tRNATyr-Mja RPR (19,61); typically, it involved assembling 50 nM of the self-cleaving construct and 500 nM of the RPPs.
Cleavage assays
To compare the trans cleavage of pre-tRNAGln by Pfu RNase P with the cis cleavage that occurs during processing of pre-tRNAGln-Mja RPR (Figure 1c), we employed single-turnover assays with Pfu RNase P. Two 2 nM of 5′-labeled pre-tRNAGln was incubated at 55°C with either 15 µM Pfu RPR alone or 200 nM Pfu RPR reconstituted with 2 µM RPPs (Figure 1c, lanes 1–5). The times of incubation were 9 h for the reactions with Pfu RPR and Pfu RPR + RPP21•RPP29, 30 min for Pfu RPR + POP5•RPP30 and 10 min for Pfu RPR + 4 RPPs.
For calculating the rate of self-cleavage of pre-tRNAGln-Mja RPR with and without RPPs, we largely followed the experimental approach outlined in Chen et al. (61) for pre-tRNATyr-Mja RPR. Some modifications were necessary to ensure robust cleavage and to reduce aggregation-related problems. Through empirical testing, we arrived at the optimal conditions suitable for a biphasic set-up: a pre-incubation that permits RPR assembly with RPPs while minimizing self-processing, and a cleavage reaction that is initiated by switching to conditions that engender maximal activity. The conditions for self-cleavage of pre-tRNATyr-Mja RPR with and without POP5•RPP30 were the same as in Chen et al. (60). While the first assembly phase of pre-tRNAGln-Mja RPR + RPP21•RPP29 was unchanged from Chen et al. (61) the cleavage reaction was performed in 200 mM instead of 100 mM Mg(OAc)2. For the reaction with all four RPPs, both phases were performed in 50 mM MES, pH 6 instead of pH 5.4, and the assembly was carried out in 25 mM Ca(OAc)2 instead of 1 mM Mg(OAc)2.
To assess the extent of mis-cleavage of pre-tRNAGln and its mutant derivatives, we performed assays under multiple-turnover conditions (Figure 4). Either a reconstituted (Eco, Pfu and Mja) or a partially purified native (Ath) RNase P variant was added to 500 nM pre-tRNAGln (wild-type or a mutant derivative), a trace amount of which was internally labeled. Incubations were performed at 37°C for Eco/Ath RNase P and 55°C for Mja/Pfu RNase P. We chose incubation periods that allowed nearly two-thirds cleavage of the substrate since our goal was to ascertain the amount of correctly and aberrantly cleaved products (and not their initial velocities of formation). The assay reactions contained 10 mM HEPES (pH 7.5 at 23°C), 10 mM Mg(OAc)2, 400 mM NH4(OAc) and 5%(v/v) glycerol for Eco RNase P; 50 mM Tris–HCl (pH 8 at 23°C), 30 mM MgCl2, 800 mM NH4(OAc) for Pfu and Mja RNase P; and 20 mM Tris–HCl (pH 8 at 23°C), 5 mM MgCl2 for Ath RNase P.
Data analysis
The RNase P reaction contents separated by denaturing PAGE were visualized by phosphorimaging (Typhoon, GE Healthcare) and quantitated with ImageQuant (GE Healthcare) to determine the amount of correctly (PC0) and aberrantly (PM+1) cleaved products. These data were then used to calculate FC0 and kobs for pre-tRNAGln processing; FC0 is the fraction of cleavage at the correct site relative to the total cleavage [PC0/(PC0 + PM+1)].
According to the parallel pathways mechanism in Scheme 1, formation of both PC0 and PM+1 should exhibit the same time dependence; therefore, FC0 [PC0/(PC0 + PM+1)] should remain constant throughout the time course. Also, while kobs = kC0 + kM+1, the ratio of the two products reflects the overall kinetics of each path (i.e. PC0/PM+1 = kC0/kM+1). To obtain the kobs values for self-cleavage of pre-tRNAGln-Mja RPR in the absence and presence of RPPs, we fit either PC0 or PM+1 depending on which afforded the higher signal-to-noise ratio (i.e. PC0 for the reaction with four RPPs and PM+1 for the remainder, Figure 2). For example, in the RPR alone reaction, we first obtained kobs by non-linear least squares fitting [PM+1]t = AmpM+1×(1 − e−kobs•t) using Kaleidagraph software (Synergy). This kobs was in turn used to obtain AmpC0 via [PC0]t = AmpC0×(1 − e−kobs•t). For each time course being studied, the PC0/(PC0 + PM+1) values were individually computed at each time point and then averaged to determine the FC0. The apparent rate constants for correct (kC0) and aberrant cleavage (kM+1) were then obtained from kC0=kobs×FC0 and kM+1=kobs×(1 − FC0).
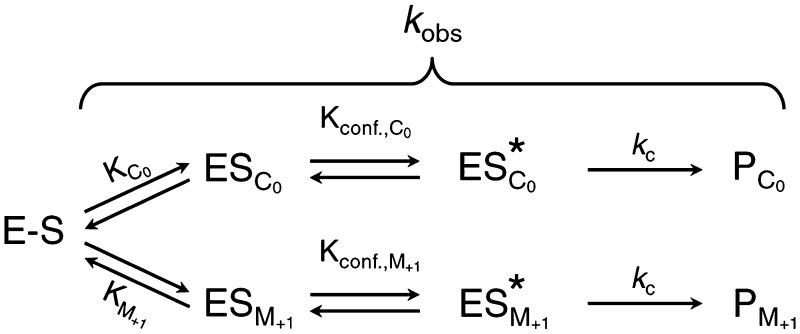
Scheme 1.
E–S refers to pre-tRNAGln-Mja RPR with or without RPPs, and ES is the substrate-docked state.
Three independent time courses were analyzed to obtain a measure of standard deviation for kobs, kC0, kM+1 and FC0 (Table 2); only a representative time course is shown in Figure 2. The standard errors for the best-fit values of kobs did not exceed 13% (RPR alone), 14% (RPR + RPP21•RPP29), 10% (RPR + POP5•RPP30) and 17% (RPR + 4 RPPs); the correlation coefficient was always ≥0.98. AmpM+1 and AmpC0 were used to obtain the Amptotal for each of the self-cleavage reactions. Note that the Amptotal values are ∼90% for all reactions except those with RPR + 4 RPPs, where a fraction of the RPR appears to have assembled into unproductive/partial RNP complexes resulting in a one-third lower amplitude (RPR, 93 ± 7; RPR + RPP21•RPP29, 86 ± 3; RPR + POP5•RPP30, 96 ± 1; RPR + 4 RPPs, 61 ± 2).
Table 2.
Effect of Mja RPPs on the rate of cleavage and cleavage-site selection of pre-tRNAGln-Mja RPR at 55°C at pH 6a
| pre-tRNAGln-Mja RPR | kobs/min | kC0/min | kM+1/min | kC0/kM+1 | FC0 |
|---|---|---|---|---|---|
| Alone | 0.004 ± 0.0004 | 0.0007 ± 0.0001 | 0.0031 ± 0.0003 | 0.2 | 0.18 ± 0.01 |
| + RPP21•RPP29 | 0.004 ± 0.0001 | 0.0021 ± 0.0001 | 0.0023 ± 0.0001 | 0.9 | 0.47 ± 0.02 |
| + POP5•RPP30 | 2.5 ± 0.1 | 1.1 ± 0.03 | 1.4 ± 0.07 | 0.8 | 0.43 ± 0.01 |
| + Both complexes | 10.5 ± 0.8 | 7.8 ± 1.3 | 1.5 ± 0.5 | 5.2 | 0.84 ± 0.03 |
| pre-tRNATyr-Mja RPR | kobs/minb | ||||
|---|---|---|---|---|---|
| Alone | 0.2 ± 0.04 | ||||
| + RPP21•RPP29 | 0.24 ± 0.04 | ||||
| + POP5•RPP30 | 20.5 ± 0.32 | ||||
| + Both complexes | 21.7 ± 0.16 |
| pre-tRNATyr-Mja RPR versus pre-tRNAGln-Mja RPR | kobs,pre-tRNATyr/ kC0,pre-tRNAGln c | ||||
|---|---|---|---|---|---|
| Alone | 286 | ||||
| + RPP21•RPP29 | 114 | ||||
| + POP5•RPP30 | 19 | ||||
| + Both complexes | 2.8 |
aSee ‘Materials and Methods' section for a description of how kobs, kC0, kM+1 and FC0 were calculated. All assays were performed under optimal conditions for each catalytic entity.
bThese data for pre-tRNATyr−Mja RPR experiments are recalculated from Table 1 of reference (19). In this earlier publication, the rates reported for a self-cleaving pre-tRNATyr−Mja RPR were at pH 5.4 and not pH 5.1 as was documented. To facilitate comparison of the pre-tRNAGln−Mja RPR and pre-tRNATyr−Mja RPR cleavage experiments, the rates observed at pH 5.4 with pre-tRNATyr−Mja RPR were multiplied by 4 to obtain rates that would have been observed at pH 6 should they have been manually measurable. We demonstrated previously a linear relationship between log kobs and pH (19).
cThe relative activity was obtained by dividing the kobs of pre-tRNATyr-Mja RPR by the apparent rate of correct cleavage (kC0) of pre-tRNAGln-Mja RPR.
RESULTS
Overall approach and rationale
Most bacterial pre-tRNAs possess a U−1 and G+1C+72 pair (62,63), and changing the N−1 or N+1N+72 nucleotide identity affects substrate ground-state binding, rate of pre-tRNA processing, and cleavage-site selection by bacterial RNase P (30,31,64–69). However, there are natural deviations from such a consensus and these pre-tRNAs are often mis-cleaved by bacterial RNase P, presumably due to mis-docking in the active site (Figure 1a). For example, bacterial pre-tRNAGln, which has a U−1 and U+1A+72, is cleaved by Eco RNase P correctly at C0 (between U−1 and U+1) and incorrectly at M+1 (between U+1 and G+2) (65). Considering the interaction between U−1 in a typical pre-tRNA and A248 of the Eco (bacterial) RPR that is important for substrate positioning and cleavage (Figure 1a, left panel) (30), the presence of U+1 and G+2 in pre-tRNAGln probably engenders an interaction between U+1 in pre-tRNAGln and A248 in the RPR, thereby shifting the cleavage site from C0 to M+1 (Figure 1a, right panel) (65). Because cleavage at M+1 by Eco RNase P occurs at only 15% to 25% frequency, the U+1–A248 interaction is only one of several determinants that dictate the choice of cleavage site (30,32). The single bacterial RPP, which enhances the RPR's affinity for pre-tRNA/catalytically relevant Mg2+ and the rate of pre-tRNA cleavage (25,69–71), was reported to not influence the extent of mis-cleavage of pre-tRNAGln (65). Similarly, both Eco and Tth RPRs mis-cleave a human pre-tRNAGly (C−1, a non-consensus substrate) with ∼20% frequency regardless of the presence of their cognate RPP (37). We postulated that if the multiple archaeal (and eukaryal) RPPs provide additional substrate-recognition determinants (72) that guide the RPR's cleavage-site selection, they might engender progressive and even possibly synergistic changes in the cognate RPR's ability to cleave at the correct site of a non-consensus substrate. Prior to embarking on such an investigation, we first sought to examine the N−1 and N+1 identity variations in all tRNAs.
Although undertaken before (30), for an up-to-date analysis of the phylogenetic variation in the N−1 and N+1 identity, we examined the genomic tRNA database (62). We found that U−1 and G+1 is the predominantly favored identity in all three domains of life (Table 1), although the preference for N−1 is not as pronounced in Archaea and Eukarya. There are several instances when both the U−1 and G+1 substrate-recognition determinants are absent in all life forms, motivating an examination of how departure from the consensus with regard to recognition determinants is dealt with by bacterial/archaeal/eukaryal RNase P.
Table 1.
Phylogenetic variation of the N−1 and N+1 identity in pre-tRNAs
| Domain | Total tRNAs | Percent of tRNAs with indicated nucleotide identity |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A−1 | C−1 | G−1 | U−1 | A+1 | C+1 | G+1 | U+1 | ||
| Bacteria | 34 782 | 19 | 19 | 8 | 54 | 4 | 9 | 81 | 7 |
| Archaea | 2497 | 26 | 18 | 13 | 43 | 8 | 2 | 90 | ∼0.25 |
| Eukarya | 37 988 | 31 | 17 | 11 | 41 | 5 | 2 | 76 | 16 |
We chose to use a cyanobacterial pre-tRNAGln (A−1 and U+1A+72, Figure 1b) (57) as a reporter for our studies comparing the influence of N−1 and N+1 identity on cleavage fidelity of RNase P from all three domains of life. While there are many non-consensus variants, our choice of this pre-tRNAGln as a model was based in part on the following distribution of pre-tRNAs with A−1 and U+1: Bacteria, 430 cases from a total of 34 782; Archaea, 1 from a total of 2497; Eukarya, 808 from a total of 37 988. Two points of note: first, the total number of archaeal tRNAs in the database is only a fraction of those reported for the bacterial/eukaryal counterparts; second, this compilation does not make assumptions on expression/function.
By exploiting our ability to assemble the archaeal RNase P holoenzyme step-wise from recombinant subunits (16–20), we quantitated the contribution of individual subsets of archaeal RPPs on cleavage-site selection and rate of processing of pre-tRNAGln by the cognate RPR. Moreover, by introducing structural alterations in pre-tRNAGln, we sought to further accentuate its mis-cleavage by bacterial RNase P and examined if such deviations from the native fold might somehow be tolerated by the archaeal and eukaryal variants.
The two archaeal RPP binary complexes differentially affect the RPR's processing fidelity and rate
We first investigated cleavage of pre-tRNAGln by Pfu RNase P and determined the fraction of cleavage at the correct site relative to the total cleavage: FC0 = PC0/(PC0+PM+1); PC0 is the product generated from cleavage at C0 (between N−1 and N+1) and PM+1 is that from miscleavage at M+1 (between N+1 and N+2). Pfu RPR alone predominantly miscleaves pre-tRNAGln and exhibits an FC0 = 0.11 ± 0.03; with addition of either Pfu RPP21•RPP29 or POP5•RPP30, FC0 increases to ∼0.57 ± 0.03 or 0.59 ± 0.01, respectively, and further to 0.92 ± 0.01 when both complexes are present (Figure 1c, lanes 1–5). This cumulative effect of RPP21•RPP29 and POP5•RPP30 has not been reported before in any multi-protein RNase P complex. It is notable that the trend persists with some pre-tRNAGln mutant derivatives that we tested (data not shown). These results contrast with FC0 being invariant during cleavage of select non-consensus pre-tRNAs by the Eco RPR both in the presence and absence of the RPP (37,65).
While the trans cleavage assays (under single-turnover conditions) reported above for Pfu RNase P provided qualitative insights on the role of RPPs, we sought to determine the apparent rates of correct and aberrant cleavage of pre-tRNAGln by an archaeal RPR with and without RPPs. By performing a titration of the enzyme over trace amounts of a consensus pre-tRNA substrate, we previously determined the maximal rate (kobs) under saturating conditions of an archaeal RPR with and without RPPs (16). A similar approach with the non-consensus substrate pre-tRNAGln and Pfu RNase P was not possible due to the poor RPR cleavage rates at low E:S ratios and the attendant inability to accurately determine kobs by fitting rate versus [E]. To circumvent this problem, we employed a cis construct that exhibits rate-limiting cleavage (19), and permits a comparison of RPR rates with and without RPPs in the context of uniform substrate binding. We appreciate that substrate interactions may have different thermodynamic or kinetic contributions in the cis context compared to trans cleavage. However, our previous studies on archaeal RNase P variants have revealed that inferences on roles of RPPs drawn from cis and trans cleavage studies mirror each other (16,19) and thereby lend merit to employment of the cis construct where technical advantages favor its use.
We exploited a design similar to the self-cleaving pre-tRNATyr-S3-Mja RPR, which we had optimized earlier with regard to spacer length (3 nt, S3) and site of tethering, and whose rate-determining cleavage step we had extensively characterized both in the absence and presence of RPPs (19). For the study here, we swapped pre-tRNATyr with pre-tRNAGln and replaced the 5′-UAU-3′ spacer in pre-tRNATyr-S3-Mja RPR with a 5′-AAU-3′ spacer to obtain pre-tRNAGln-S3-Mja RPR. This change was introduced to prevent possible base pairing between A−1 and U+73 in the case of the original UAU spacer. For simplicity, we will refer to the new construct as pre-tRNAGln-Mja RPR (Figure 1b). Since the success of this strategy depends on pre-tRNA anchoring in the cis construct mimicking the trans scenario, the site of pre-tRNA tethering is critical. Our choice in pre-tRNAGln-Mja RPR was based on several findings on bacterial RNase P including a recent crystal structure of the RNase P holoenzyme-tRNA complex (36), which reveals base pairing of the 3′-RCC in the pre-tRNA and the L15 loop in the bacterial RPR. Therefore, we conjugated the 3′-end of pre-tRNAGln to the L15 loop-equivalent in the Mja RPR (Figure 1b). Control experiments described below validate this choice.
To verify that the trend observed during cis cleavage with Mja RNase P indeed parallels trans cleavage reactions catalyzed by Pfu RNase P, we examined cleavage of pre-tRNAGln-Mja RPR in the absence and presence of RPPs using a single time-point measurement (Figure 1c, lanes 6–10). While pre-tRNAGln-Mja RPR displayed FC0 = 0.14, POP5•RPP30 and RPP21•RPP29 independently increased FC0 to ∼0.4 and together to ∼0.8. This trend was further confirmed using time-course experiments which yielded FC0 values of 0.18 ± 0.01 (−RPPs), 0.47 ± 0.02 (+ RPP21•RPP29), 0.43 ± 0.01 (+POP5•RPP30), and 0.84 ± 0.03 (+ both complexes) (Table 2 and Figure 2). We also found that Mja RPR assembled with Mja RPP21•RPP29 + POP5•RPP30 displays FC0 =0.8–0.9 during trans cleavage of pre-tRNAGln (data not shown). Collectively, these data allay concerns about an artificial bias in cleavage-site selection arising from use of the cis-cleaving pre-tRNAGln-Mja RPR in lieu of a trans cleavage reaction. These data also reveal that the two broad classes of euryarchaeal RNase P [type A (e.g. Pfu) and M (e.g. Mja)] (12,73) behave in a similar fashion despite the fact that only the type A RPRs support pre-tRNA cleavage in the absence of RPPs.
The time-course analyses of the self-cleavage of pre-tRNAGln-Mja RPR in the absence and presence of RPPs also allowed us to calculate the apparent rates of formation of correct and mis-cleaved products (kC0 and kM+1). For data analysis, we employed the approach typically used to analyze first-order parallel reactions (29,31,33,69). Here, kobs for self-processing of pre-tRNAGln-Mja RPR is the sum of the rates of formation of PC0 and PM+1. Our proposed kinetic framework (Scheme 1) for this self-cleavage parallels that for trans cleavage by bacterial RNase P, which was formulated based on the growing experimental evidence supporting an initial encounter complex (E + S ⇆ ES) that undergoes a conformational change to ES* to optimally position the pre-tRNA and catalytic Mg2+ for efficient cleavage (74–77). During self-cleavage, the initial binding step of the trans reaction is replaced with a substrate-docking step (E − S ⇆ ESC0 and E − S ⇆ ESM+1, where ESC0 and ESM+1 are two distinct docked states that result in different products) (Scheme 1). Thus, kobs cumulatively depends on the equilibrium constants for the docking steps and the rates of chemical cleavage of ESC0 and ESM+1. With kobs and FC0 in hand, we could calculate both kC0 and kM+1 (see ‘Materials and Methods’ section for details).
The disappearance of pre-tRNAGln-Mja RPR indeed followed first-order kinetics both in the absence and presence of RPPs (Figure 2). These assays were performed at pH 6 and 55°C; the lower pH helped to slow down the reaction to enable manual determination of the rates of cleavage. We summarize the key observations regarding how addition of RPPs to the RPR increased the rate and favored correct cleavage. First, there was a ∼2625-fold increase in kobs upon addition of the both RPP complexes to the RPR, with ∼625-fold due to addition of POP5•RPP30 alone (Table 2). Second, while kC0 is only one-fifth of kM+1 during self-cleavage of pre-tRNAGln-Mja RPR, addition of RPPs to this cis construct progressively and substantively increased the bias toward C0. Although both binary RPPs independently normalize the rates of correct and mis-cleavage, their effect on kC0 and kM+1 differs significantly (Table 2). RPP21•RPP29 increased kC0 by 3-fold and decreased kM+1 by one-fourth, while POP5•RPP30 increased kC0 and kM+1 by 1570 - and 450-fold, respectively. In the presence of both complexes, kC0 and kM+1 increased by 11 140 - and 480-fold, respectively, reflecting a synergistic effect of the four RPPs selectively on the rate and fidelity of correct cleavage. The RPP-mediated preferential shift toward C0 is illustrated by kC0/kM+1 = 0.2 for the cis construct-alone reaction, 0.8–0.9 upon addition of either binary RPP, and 5.2 in the presence of four RPPs (Table 2).
Based on an in-depth study of mis-cleavage that occurred upon disrupting interactions between bacterial RNase P and the consensus nucleobase and 2′-hydroxyl at N−1 of the pre-tRNA, Zahler et al. (31) demonstrated lower affinity and cooperativity of Mg2+ binding for the mis-cleavage path relative to the correct cleavage. We sought to examine this possibility during cleavage of pre-tRNAGln-Mja RPR. Decreasing the Mg2+ concentration from 100 to 25 mM in the assay did not affect the FC0 of pre-tRNAGln-Mja RPR + POP5•RPP30, but it did predictably lower the rates for both the correct and aberrant cleavage (data not shown). While it is conceivable that the FC0 value might change at even lower Mg2+ concentrations, the weak activity at <25 mM Mg2+ for the RNP assembled with POP5•RPP30 precluded such experiments.
The significance of N+1N+72 for pre-tRNA cleavage-site selection decreases from bacterial > archaeal > eukaryal RNase P
Given the effect of archaeal RPPs on the RPR's processing fidelity of pre-tRNAGln, we sought to compare an RNase P holoenzyme from each domain of life with regard to cleavage-site selection of non-consensus substrates. We used Eco, Pfu and Ath RNase P as bacterial, archaeal and eukaryal representatives, respectively, to compare the fidelity of processing of pre-tRNAGln and its mutant derivatives (Figure 3). Eco and Pfu RNase P holoenzymes were assembled in vitro from recombinant constituents purified using established methods (16,20,59,60). Ath RNase P was isolated from 7-day-old cultured suspension cells using sequential ion-exchange chromatography to yield a 400-fold purified preparation (data not shown; Lai, L. B. and Gopalan, V., unpublished results). Although studies on yeast and human nuclear RNase P show that the holoenzyme is made up of an RPR and 9 to 10 RPPs, there is still some uncertainty about the make-up of plant nuclear RNase P. Computational searches have uncovered plant homologs for a few of the known eukaryotic RPPs (78), but the identity of the plant nuclear RPR has proven elusive. Based on micrococcal nuclease and proteinase K sensitivity, however, plant nuclear RNase P (from monocots and dicots) appears to function as an RNP complex (79–81). This finding contrasts with the plant organellar version (2) whose catalytic activity is attributable to a single polypeptide [see also (82)]. For the purpose of this study, it suffices to state that the partially purified nuclear Ath RNase P used herein is an RNP complex likely resembling human/yeast nuclear RNase P, albeit of unknown composition.
To understand the influence of the pre-tRNA N−1 and N+1 identity on cleavage-site selection by Eco, Pfu and Ath RNase P, we studied the processing of pre-tRNAGln under multiple-turnover conditions. Eco, Pfu and Ath RNase P cleaved wild-type pre-tRNAGln with FC0 of 0.73 ± 0.05, 0.81 ± 0.03 and 1, respectively (Figure 4). Since most pre-tRNAs contain G+1, we substituted U+1A+72 in pre-tRNAGln with G+1C+72 to generate pre-tRNAGlnU-G (Figure 3). While this substrate resulted in modestly higher cleavage at C0 and a ∼3-fold increase in overall cleavage rate for Eco RNase P (Figure 4; data not shown), some mis-cleavage remains (FC0 = 0.82 ± 0.05), consistent with the idea that multiple determinants dictate cleavage-site selection (30,32).
Consensus tertiary structure of pre-tRNAGln is more important for fidelity of cleavage by bacterial RNase P but not the archaeal or eukaryal variants
Upon deletion of the D-stem, increased mis-cleavage of Tth pre-tRNAGly by Eco RPR both in the presence and absence of RPP (wild-type, 3%; mutant, ∼20%) was observed (37). To determine if there are differences in the relative importance of structural elements in the pre-tRNA for cleavage-site selection by the RNA- and protein-rich RNase P variants, we examined the fidelity of cleavage of three stem–loop deletion derivatives of pre-tRNAGln: ΔD, ΔAC and ΔT (Figure 3). ΔD and ΔAC were cleaved by Eco RNase P with FC0 of 0.35 ± 0.05 and 0.15 ± 0.03, respectively, compared to 0.73 ± 0.05 observed with the wild-type (Figure 4). In contrast, Pfu and Ath RNase P cleaved ΔD and ΔAC with FC0 of ∼0.8 and 1, respectively, nearly identical to their cleavage of wild-type pre-tRNAGln. Interestingly, RNase P from all three domains of life failed to cleave ΔT, an observation reminiscent of previous reports on the importance of the TSL for bacterial and eukaryal RNase P recognition (37,38).
The L-shaped tertiary structure of the tRNA is dependent on the interaction between the D and TΨC loops. In fact, the importance of this interaction for tertiary structure is borne out both by the high sequence conservation of the loops and the structural compensation observed in some tRNAs to ensure preservation of the overall fold (63,83). It is therefore not unexpected that functional groups made available by the architecture of the D-TΨC loop interaction are exploited for a productive interaction with RNase P (32,33,84). As interactions between the TSL in the pre-tRNA and the TBS in the bacterial RPR do influence cleavage-site selection (32), we speculated that substrate recognition might be affected if the local TSL structure is altered, for example, by disrupting the canonical D-TΨC loop interaction. Therefore, we extended the D stem in pre-tRNAGln by 1–6 bp (D+ variants; Figure 3), and tested these mutant derivatives for correct and aberrant cleavage. Akin to the results with pre-tRNAGlnΔD, all the D+ variants were cleaved by Eco RNase P with FC0 of 0.2–0.3 (Figure 4), while Pfu and Ath RNase P cleaved with FC0 of ∼0.8 and 1, respectively.
We performed various control experiments to verify that the dissimilarity in cleavage-site selection between bacterial and archaeal/eukaryal RNase P was mainly due to their structural/functional variability. First, to eliminate differences in assay conditions as a possible reason for disparities in fidelity of processing (Figure 4), we tested Eco RNase P at 55°C and in 30 mM Mg2+, conditions employed for assaying in vitro assembled Pfu RNase P. At these conditions, the mis-cleavage trend of Eco RNase P is largely unchanged compared to assays performed at 37°C and 10 mM Mg2+, which are optimal for Eco RNase P (not shown). The reciprocal experiment wherein Pfu RNase P is tested under conditions optimal for Eco RNase P could not be performed since the in vitro reconstituted Pfu RNase P shows poor activity at 37°C and 10 mM Mg2+. Second, to ensure that the observations with Pfu RNase P are not restricted to type A archaeal RNase P, we examined cleavage of the different pre-tRNAGln derivatives by in vitro reconstituted RNase P from Mja (19), a type M RNase P. The cleavage-site selection exhibited by Mja RNase P mirrored its Pfu counterpart (not shown). Finally, despite the robust biochemical reconstitution of archaeal RNase P, the possibility remained that our observed cleavage-site bias toward C0 with in vitro reconstituted archaeal RNase P (Figure 4) could differ from that of native archaeal RNase P. The availability of partially purified RNase P from Methanococcus maripaludis (17) allowed us to dispel this concern—this native holoenzyme preparation processed pre-tRNAGln and its derivatives with FC0 = 0.8 to 0.9, consistent with our findings on Pfu and Mja RNase P assembled in vitro using the respective RPR + 4 RPPs (Figure 4; data not shown). Thus, the contribution to fidelity of archaeal RPPs other than RPP21•RPP29 and POP5•RPP30 in the native enzyme (17,85) has to be small given that FC0 = 0.8 to 0.9 even in the presence of only these four RPPs.
DISCUSSION
Archaeal RPPs synergistically influence the RPR's cleavage-site selection and rate of processing of pre-tRNAGln
The synergistic effect of the archaeal RPPs on the cognate RPR's rate and fidelity of processing of pre-tRNAGln (Figure 2 and Table 2) could be rationalized by the interaction of RPP binary complexes with different parts of the pre-tRNA either directly or through the RPR. Archaeal RPRs, like their bacterial counterparts, can be demarcated into a specificity (S) domain that binds the pre-tRNA's TSL, and a catalytic (C) domain that cleaves the pre-tRNA; moreover, RPP21•RPP29 and POP5•RPP30 footprint on the S and C domains, respectively (19,86). In the context of these specific interactions with the RPR, we contend that cleavage-site selection is influenced by (i) RPP21•RPP29 interacting with the TSL region, and (ii) POP5•RPP30 with sequence/structures near the cleavage site (e.g. the 5′-leader) in the pre-tRNA. Several observations support this idea. First, a recent study examining Pfu RPR-mediated cleavages of model substrates, with either an intact T loop or a GAAA tetraloop, revealed that the Pfu RPR's S domain could recognize the TSL in these model substrates only in the presence of RPP21•RPP29 (87). Second, given the striking similarity in the tertiary structures of archaeal (and possibly eukaryal) POP5 and bacterial RPP (88,89), and the ability of the bacterial RPP to recognize the 5′-leader of the pre-tRNA (25–27), it is likely that POP5 plays a similar role. Lastly, a gel-shift analysis demonstrated that of the seven recombinant human RPPs tested, only RPP21 and RPP14 (a paralog of POP5) bound pre-tRNATyr in a specific manner (90), although the sites of interaction in the pre-tRNA were not mapped.
We stress that inter-domain cooperation in RNase P catalysis is critical for the synergy observed with the two RPP complexes. The ability of RPP21•RPP29 to position an atypical pre-tRNA optimally in the S domain must somehow enable efficient and correct cleavage by the C domain complexed with POP5•RPP30. The distinct functions of the two RPR domains, with or without their associated RPPs, make inter-domain crosstalk obligatory. The recent finding of an S-domain mutation in the TBS of Eco RPR that changes the nature and rate of mis-cleavage of a model substrate (by the C domain) reinforces this idea of inter-domain cooperation in cleavage-site selection (91).
The above structural perspective helps inform a kinetic model to understand how archaeal RPPs increase FC0 by their ability to selectively favor the rate of correct cleavage (kC0) relative to mis-cleavage (kM+1). Such preferential increases in kC0 might result either from favorably affecting substrate docking for correct cleavage [by changing KC0, the equilibrium constant for E – S ⇆ ESC0, or through enhancing the rate of cleavage at C0 (by changing either Kconf, the equilibrium constant for ES ⇆ ES*, or kc that dictates ES* → E + P; the latter is not considered for reasons discussed elsewhere (16,19,70)]. In fact, since binding/docking and cleavage are coupled (Scheme 1), a synergistic increase in the rate of correct cleavage of a non-consensus pre-tRNA would be expected in the presence of both RPP complexes if each RPP pair selectively contributed to KC0 or Kconf, a premise supported by previous studies (16,19).
Our earlier single-turnover kinetic studies involving cis and trans cleavage of a consensus pre-tRNA revealed that while RPP21•RPP29 promotes substrate binding by 16-fold through a decrease in KS (the dissociation constant for ES formation), POP5•RPP30 increases the archaeal RPR's cleavage rate by ∼100-fold probably by enhancing Kconf (16,19). In a related vein, we believe that RPP21•RPP29 favorably influences KC0 at the expense of KM+1 and thereby increases kC0/kM+1 during self-cleavage of pre-tRNAGln-Mja RPR. The ability of POP5•RPP30 to increase the rate of pre-tRNAGln-Mja RPR cleavage at C0 (1570-fold) and M+1 (450-fold) is presumably due to its promoting ES* formation for both correct cleavage and mis-cleavage (ESC0 ⇆ ES*C0 and ESM+1 ⇆ ES*M+1; Table 2), albeit preferentially for ES*C0 thus accounting for the increase in kC0/kM+1. Hence, the synergistic increase in the correct cleavage that we observed in the presence of both RPP complexes must reflect their collective ability to favor formation of both ESC0 and ES*C0, and the cumulative gains from affecting both concomitantly.
POP5•RPP30 normalizes the rate of processing of consensus and non-consensus pre-tRNAs
Our studies uncovered the ability of POP5•RPP30 to increase the archaeal RPR’s cleavage rate of the non-consensus pre-tRNAGln to that observed with pre-tRNATyr, a consensus representative. The rate of self-processing of pre-tRNAGln-Mja RPR alone (at C0) is 286-fold slower than that for pre-tRNATyr-Mja RPR (Table 2). However, when both RPP complexes are present, the rates of self-processing by these two conjugates differ only by 2.8-fold. This remarkable narrowing of the difference in rates is largely due to the 1570-fold increase in kC0 facilitated by POP5•RPP30 (Table 2). These findings mirror the observation that bacterial RPP normalizes the cognate RPR's rate of cleavage of different pre-tRNAs by altering its energetic contributions to substrate binding and enhancing the rate of RPR-mediated cleavage (25,69,70). To better understand this functional convergent evolution, it would be instructive to compare the mechanisms used by archaeal POP5 and bacterial RPP to equalize the processing rates of different pre-tRNAs by their cognate RPRs.
Similarities and differences in substrate recognition by RNase P from the three domains of life
All three RNase P variants recognize and cleave pre-tRNAGln with deleted D or AC stem but not a T stem. Such recognition features noted before for bacterial and eukaryal RNase P (with other pre-tRNAs) can now be extended to archaeal RNase P. The indispensable nature of the TSL for pre-tRNA recognition by RNase P in all three domains of life may be attributable to the coevolution of RNase P and pre-tRNAs. RNase P-mediated 5′-maturation of pre-tRNAs has been suggested to precede 3′-maturation and intron splicing (1,92). Since RNase P processes all pre-tRNAs, some recognition motifs must be universally conserved in all of them. If an intron is present, it is typically located in the anticodon loop of pre-tRNAs (1), occasionally in the D and variable loops, and seldom in the T loop (93). Thus, if RNase P evolved to recognize a largely invariant module in all pre-tRNAs, then the rarely disrupted TSL seems a good choice.
Our results suggest that despite thematic parallels, bacterial RNase P is more reliant on the native tertiary structure of atypical pre-tRNAs such as pre-tRNAGln for correct cleavage compared to archaeal and eukaryal RNase P. This assertion is borne out by results from previous studies (31,32,43,65) and also by our observation that when the native tertiary structure of pre-tRNAGln is perturbed (e.g. pre-tRNAGlnD+ variants, Figure 3), the mis-cleavage at M+1 is enhanced from ∼30% to 70% (with FC0 decreasing from 0.7 to 0.3; Figure 4). The ability of protein-rich archaeal and eukaryal RNase P, in contrast to their bacterial cousins, to process pre-tRNAGln and its mutant derivatives with FC0 = 0.8 to 1 reflects their high fidelity even when dealing with non-native pre-tRNA structures and indicates an unexpected tolerance to structural deviations from the pre-tRNA consensus.
Protein-rich RNase P confers more flexibility in substrate-recognition?
In a primordial RNA world setting, if the RPR processed only a few ncRNAs and pre-tRNAs (extant or earlier versions), maintaining a common suite of recognition determinants would not have imposed significant evolutionary constraints. However, sequence drift would have been inevitable given the recombinogenic potential of tRNA-encoding genes (92,94) and the subsequent functional specialization of tRNAs. Thus, strict adherence to the RPR's recognition determinants would have been difficult. It has been suggested that association of the bacterial RPR with an RPP likely provided a countermeasure to alleviate possible recognition/catalytic defects in the RPR caused by sequence variation in pre-tRNAs (25,69,70). Since nucleotide identities at −1 and +1 positions in tRNAs (Table 1) are not as conserved in eukarya as in bacteria, eukaryal RPPs, with unique RNA-binding motifs and a combinatorial capability to fulfill a minimum threshold of contacts, might permit equally efficient binding/cleavage of multiple pre-tRNAs or related RNAs that share few common determinants (not only at N−1 and N+1/N+72 but elsewhere in the substrate). For example, two human ncRNAs, the 7-kb MALAT1 that is up-regulated in many cancers and the 20-kb Men β involved in the formation of paraspeckles, have a tRNA-like structure, albeit lacking consensus structural elements, and are processed by human RNase P (50,51). Our finding that archaeal and eukaryal RNase P cleave with high fidelity even those substrates that deviate significantly from the pre-tRNA consensus sequence/fold suggests that the multiple archaeal and eukaryal RPPs could promote functional versatility while retaining both speed and accuracy (6,72,95).
With the endonucleolytic activity residing in the RPR, association with multiple RPPs might have facilitated remodeling the archaeal/eukaryal RPR's active site to enable a broader role for protein-rich RNase P in processing and turnover of RNAs that lack tRNA motifs. The ability of yeast RNase P to cleave certain short-lived ncRNAs, mRNAs, and artificial ssRNAs suggests accommodation of a broad range of substrates with little resemblance to pre-tRNAs (52–56). Functional diversification through partial alterations in the subunit composition of an RNP is also exemplified by yeast/human RNase MRP, an RNase P-related endonuclease involved in maturation of rRNAs and turnover of select mRNAs (96–98). RNase MRP contains an RNA subunit that is structurally related to the RPR and up to 10 protein subunits, of which eight are shared with RNase P. A SELEX-based approach revealed that yeast RNase MRP displays broad substrate specificity in vitro including cleavage of ssRNAs (99); although this trait is shared with RNase P, the two enzymes use different recognition determinants during cleavage of ssRNAs. These observations collectively support the premise that increased protein content in these related catalytic RNPs might underlie their functional plasticity, echoing the key inference from this study.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Tables 1 and 2.
FUNDING
National Science Foundation grants [MCB 0238233 (CAREER), DBI 0509744 (SGER) and MCB 0843543 to V.G.]; and a National Institutes of Health Grant (GM067807 to M.P.F. and V.G.). Funding for open access charge: National Science Foundation.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to Drs. Patricia Chan and Todd Lowe (University of California, Santa Cruz) for making changes to the genomic tRNA database that enables users to determine the N−1 and N+1 variations. The authors thank Professor E. J. Behrman (OSU) for comments on the manuscript.
REFERENCES
- 1.Phizicky EM, Hopper AK. tRNA biology charges to the front. Genes Dev. 2010;24:1832–1860. doi: 10.1101/gad.1956510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gobert A, Gutmann B, Taschner A, Gossringer M, Holzmann J, Hartmann RK, Rossmanith W, Giege P. A single Arabidopsis organellar protein has RNase P activity. Nat. Struct. Mol. Biol. 2010;17:740–744. doi: 10.1038/nsmb.1812. [DOI] [PubMed] [Google Scholar]
- 3.Holzmann J, Frank P, Loffler E, Bennett KL, Gerner C, Rossmanith W. RNase P without RNA: identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell. 2008;135:462–474. doi: 10.1016/j.cell.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Esakova O, Krasilnikov AS. Of proteins and RNA: the RNase P/MRP family. RNA. 2010;16:1725–1747. doi: 10.1261/rna.2214510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans D, Marquez SM, Pace NR. RNase P: interface of the RNA and protein worlds. Trends Biochem. Sci. 2006;31:333–341. doi: 10.1016/j.tibs.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Jarrous N, Gopalan V. Archaeal/eukaryal RNase P: subunits, functions and RNA diversification. Nucleic Acids Res. 2010;38:7885–7894. doi: 10.1093/nar/gkq701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai LB, Vioque A, Kirsebom LA, Gopalan V. Unexpected diversity of RNase P, an ancient tRNA processing enzyme: challenges and prospects. FEBS Lett. 2010;584:287–296. doi: 10.1016/j.febslet.2009.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker SC, Engelke DR. Ribonuclease P: the evolution of an ancient RNA enzyme. Crit. Rev. Biochem. Mol. Biol. 2006;41:77–102. doi: 10.1080/10409230600602634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClain WH, Lai LB, Gopalan V. Trials, travails and triumphs: An account of RNA catalysis in RNase P. J. Mol. Biol. 2010;397:627–646. doi: 10.1016/j.jmb.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 11.Kikovska E, Svard SG, Kirsebom LA. Eukaryotic RNase P RNA mediates cleavage in the absence of protein. Proc. Natl Acad. Sci. USA. 2007;104:2062–2067. doi: 10.1073/pnas.0607326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pannucci JA, Haas ES, Hall TA, Harris JK, Brown JW. RNase P RNAs from some Archaea are catalytically active. Proc. Natl Acad. Sci. USA. 1999;96:7803–7808. doi: 10.1073/pnas.96.14.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamberlain JR, Lee Y, Lane WS, Engelke DR. Purification and characterization of the nuclear RNase P holoenzyme complex reveals extensive subunit overlap with RNase MRP. Genes Dev. 1998;12:1678–1690. doi: 10.1101/gad.12.11.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozeki H, Sakano H, Yamada S, Ikemura T, Shimura Y. Temperature-sensitive mutants of Escherichia coli defective in tRNA biosynthesis. Brookhaven Symp. Biol. 1975:89–105. [PubMed] [Google Scholar]
- 15.Schedl P, Primakoff P. Mutants of Escherichia coli thermosensitive for the synthesis of transfer RNA. Proc. Natl Acad. Sci. USA. 1973;70:2091–2095. doi: 10.1073/pnas.70.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen WY, Pulukkunat DK, Cho IM, Tsai HY, Gopalan V. Dissecting functional cooperation among protein subunits in archaeal RNase P, a catalytic ribonucleoprotein complex. Nucleic Acids Res. 2010;38:8316–8327. doi: 10.1093/nar/gkq668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho IM, Lai LB, Susanti D, Mukhopadhyay B, Gopalan V. Ribosomal protein L7Ae is a subunit of archaeal RNase P. Proc. Natl Acad. Sci. USA. 2010;107:14573–14578. doi: 10.1073/pnas.1005556107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall TA, Brown JW. Archaeal RNase P has multiple protein subunits homologous to eukaryotic nuclear RNase P proteins. RNA. 2002;8:296–306. doi: 10.1017/s1355838202028492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulukkunat DK, Gopalan V. Studies on Methanocaldococcus jannaschii RNase P reveal insights into the roles of RNA and protein cofactors in RNase P catalysis. Nucleic Acids Res. 2008;36:4172–4180. doi: 10.1093/nar/gkn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai HY, Pulukkunat DK, Woznick WK, Gopalan V. Functional reconstitution and characterization of Pyrococcus furiosus RNase P. Proc. Natl Acad. Sci. USA. 2006;103:16147–16152. doi: 10.1073/pnas.0608000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Willkomm DK, Hartmann RK. Minor changes largely restore catalytic activity of archaeal RNase P RNA from Methanothermobacter thermoautotrophicus. Nucleic Acids Res. 2009;37:231–242. doi: 10.1093/nar/gkn915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christian EL, Zahler NH, Kaye NM, Harris ME. Analysis of substrate recognition by the ribonucleoprotein endonuclease RNase P. Methods. 2002;28:307–322. doi: 10.1016/s1046-2023(02)00238-4. [DOI] [PubMed] [Google Scholar]
- 23.Kirsebom LA, Trobro S. RNase P RNA-mediated cleavage. IUBMB Life. 2009;61:189–200. doi: 10.1002/iub.160. [DOI] [PubMed] [Google Scholar]
- 24.Crary SM, Niranjanakumari S, Fierke CA. The protein component of Bacillus subtilis ribonuclease P increases catalytic efficiency by enhancing interactions with the 5′ leader sequence of pre-tRNAAsp. Biochemistry. 1998;37:9409–9416. doi: 10.1021/bi980613c. [DOI] [PubMed] [Google Scholar]
- 25.Koutmou KS, Zahler NH, Kurz JC, Campbell FE, Harris ME, Fierke CA. Protein-precursor tRNA contact leads to sequence-specific recognition of 5′ leaders by bacterial ribonuclease P. J. Mol. Biol. 2010;396:195–208. doi: 10.1016/j.jmb.2009.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niranjanakumari S, Stams T, Crary SM, Christianson DW, Fierke CA. Protein component of the ribozyme ribonuclease P alters substrate recognition by directly contacting precursor tRNA. Proc. Natl Acad. Sci. USA. 1998;95:15212–15217. doi: 10.1073/pnas.95.26.15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rueda D, Hsieh J, Day-Storms JJ, Fierke CA, Walter NG. The 5′ leader of precursor tRNAAsp bound to the Bacillus subtilis RNase P holoenzyme has an extended conformation. Biochemistry. 2005;44:16130–16139. doi: 10.1021/bi0519093. [DOI] [PubMed] [Google Scholar]
- 28.Tsai HY, Masquida B, Biswas R, Westhof E, Gopalan V. Molecular modeling of the three-dimensional structure of the bacterial RNase P holoenzyme. J. Mol. Biol. 2003;325:661–675. doi: 10.1016/s0022-2836(02)01267-6. [DOI] [PubMed] [Google Scholar]
- 29.Brannvall M, Fredrik Pettersson BM, Kirsebom LA. The residue immediately upstream of the RNase P cleavage site is a positive determinant. Biochimie. 2002;84:693–703. doi: 10.1016/s0300-9084(02)01462-1. [DOI] [PubMed] [Google Scholar]
- 30.Zahler NH, Christian EL, Harris ME. Recognition of the 5′ leader of pre-tRNA substrates by the active site of ribonuclease P. RNA. 2003;9:734–745. doi: 10.1261/rna.5220703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zahler NH, Sun L, Christian EL, Harris ME. The pre-tRNA nucleotide base and 2′-hydroxyl at N(-1) contribute to fidelity in tRNA processing by RNase P. J. Mol. Biol. 2005;345:969–985. doi: 10.1016/j.jmb.2004.10.080. [DOI] [PubMed] [Google Scholar]
- 32.Brannvall M, Kikovska E, Wu S, Kirsebom LA. Evidence for induced fit in bacterial RNase P RNA-mediated cleavage. J. Mol. Biol. 2007;372:1149–1164. doi: 10.1016/j.jmb.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Loria A, Pan T. Recognition of the T stem-loop of a pre-tRNA substrate by the ribozyme from Bacillus subtilis ribonuclease P. Biochemistry. 1997;36:6317–6325. doi: 10.1021/bi970115o. [DOI] [PubMed] [Google Scholar]
- 34.Pan T, Loria A, Zhong K. Probing of tertiary interactions in RNA: 2′-hydroxyl-base contacts between the RNase P RNA and pre-tRNA. Proc. Natl Acad. Sci. USA. 1995;92:12510–12514. doi: 10.1073/pnas.92.26.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirsebom LA, Svard SG. Base pairing between Escherichia coli RNase P RNA and its substrate. EMBO J. 1994;13:4870–4876. doi: 10.1002/j.1460-2075.1994.tb06814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiter NJ, Osterman A, Torres-Larios A, Swinger KK, Pan T, Mondragon A. Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA. Nature. 2010;468:784–789. doi: 10.1038/nature09516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardt WD, Schlegl J, Erdmann VA, Hartmann RK. Role of the D arm and the anticodon arm in tRNA recognition by eubacterial and eukaryotic RNase P enzymes. Biochemistry. 1993;32:13046–13053. doi: 10.1021/bi00211a014. [DOI] [PubMed] [Google Scholar]
- 38.Yuan Y, Altman S. Substrate recognition by human RNase P: identification of small, model substrates for the enzyme. EMBO J. 1995;14:159–168. doi: 10.1002/j.1460-2075.1995.tb06986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrara G, Calandra P, Fruscoloni P, Tocchini-Valentini GP. Two helices plus a linker: a small model substrate for eukaryotic RNase P. Proc. Natl Acad. Sci. USA. 1995;92:2627–2631. doi: 10.1073/pnas.92.7.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClain WH, Guerrier-Takada C, Altman S. Model substrates for an RNA enzyme. Science. 1987;238:527–530. doi: 10.1126/science.2443980. [DOI] [PubMed] [Google Scholar]
- 41.Burkard U, Willis I, Soll D. Processing of histidine transfer RNA precursors. Abnormal cleavage site for RNase P. J. Biol. Chem. 1988;263:2447–2451. [PubMed] [Google Scholar]
- 42.Holm PS, Krupp G. The acceptor stem in pre-tRNAs determines the cleavage specificity of RNase P. Nucleic Acids Res. 1992;20:421–423. doi: 10.1093/nar/20.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krupp G, Kahle D, Vogt T, Char S. Sequence changes in both flanking sequences of a pre-tRNA influence the cleavage specificity of RNase P. J. Mol. Biol. 1991;217:637–648. doi: 10.1016/0022-2836(91)90522-8. [DOI] [PubMed] [Google Scholar]
- 44.Alifano P, Rivellini F, Piscitelli C, Arraiano CM, Bruni CB, Carlomagno MS. Ribonuclease E provides substrates for ribonuclease P-dependent processing of a polycistronic mRNA. Genes Dev. 1994;8:3021–3031. doi: 10.1101/gad.8.24.3021. [DOI] [PubMed] [Google Scholar]
- 45.Bothwell AL, Garber RL, Altman S. Nucleotide sequence and in vitro processing of a precursor molecule to Escherichia coli 4.5 S RNA. J. Biol. Chem. 1976;251:7709–7716. [PubMed] [Google Scholar]
- 46.Hartmann RK, Heinrich J, Schlegl J, Schuster H. Precursor of C4 antisense RNA of bacteriophages P1 and P7 is a substrate for RNase P of Escherichia coli. Proc. Natl Acad. Sci. USA. 1995;92:5822–5826. doi: 10.1073/pnas.92.13.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inokuchi H. A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc. Natl Acad. Sci. USA. 1994;91:9223–9227. doi: 10.1073/pnas.91.20.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Altman S. Polarity effects in the lactose operon of Escherichia coli. J. Mol. Biol. 2004;339:31–39. doi: 10.1016/j.jmb.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 49.Mans RM, Guerrier-Takada C, Altman S, Pleij CW. Interaction of RNase P from Escherichia coli with pseudoknotted structures in viral RNAs. Nucleic Acids Res. 1990;18:3479–3487. doi: 10.1093/nar/18.12.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sunwoo H, Dinger ME, Wilusz JE, Amaral PP, Mattick JS, Spector DL. MEN epsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 2009;19:347–359. doi: 10.1101/gr.087775.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilusz JE, Freier SM, Spector DL. 3′ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell. 2008;135:919–932. doi: 10.1016/j.cell.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coughlin DJ, Pleiss JA, Walker SC, Whitworth GB, Engelke DR. Genome-wide search for yeast RNase P substrates reveals role in maturation of intron-encoded box C/D small nucleolar RNAs. Proc. Natl Acad. Sci. USA. 2008;105:12218–12223. doi: 10.1073/pnas.0801906105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marvin MC, Clauder-Munster S, Walker SC, Sarkeshik A, Yates JR, 3rd, Steinmetz LM, Engelke DR. Accumulation of noncoding RNA due to an RNase P defect in Saccharomyces cerevisiae. RNA. 2011;17:1441–1450. doi: 10.1261/rna.2737511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marvin MC, Walker SC, Fierke CA, Engelke DR. Binding and cleavage of unstructured RNA by nuclear RNase P. RNA. 2011;17:1429–1440. doi: 10.1261/rna.2633611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samanta MP, Tongprasit W, Sethi H, Chin CS, Stolc V. Global identification of noncoding RNAs in Saccharomyces cerevisiae by modulating an essential RNA processing pathway. Proc. Natl Acad. Sci. USA. 2006;103:4192–4197. doi: 10.1073/pnas.0507669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang L, Altman S. A noncoding RNA in Saccharomyces cerevisiae is an RNase P substrate. RNA. 2007;13:682–690. doi: 10.1261/rna.460607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pascual A, Vioque A. Substrate binding and catalysis by ribonuclease P from cyanobacteria and Escherichia coli are affected differently by the 3′ terminal CCA in tRNA precursors. Proc. Natl Acad. Sci. USA. 1999;96:6672–6677. doi: 10.1073/pnas.96.12.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai HY, Lai LB, Gopalan V. A modified pBluescript-based vector for facile cloning and transcription of RNAs. Anal. Biochem. 2002;303:214–217. doi: 10.1006/abio.2001.5567. [DOI] [PubMed] [Google Scholar]
- 59.Vioque A, Arnez J, Altman S. Protein-RNA interactions in the RNase P holoenzyme from Escherichia coli. J. Mol. Biol. 1988;202:835–848. doi: 10.1016/0022-2836(88)90562-1. [DOI] [PubMed] [Google Scholar]
- 60.Gopalan V, Baxevanis AD, Landsman D, Altman S. Analysis of the functional role of conserved residues in the protein subunit of ribonuclease P from Escherichia coli. J. Mol. Biol. 1997;267:818–829. doi: 10.1006/jmbi.1997.0906. [DOI] [PubMed] [Google Scholar]
- 61.Chen WY, Xu Y, Cho IM, Oruganti SV, Foster MP, Gopalan V. Cooperative RNP assembly: complementary rescue of structural defects by protein and RNA subunits of archaeal RNase P. J. Mol. Biol. 2011;411:368–383. doi: 10.1016/j.jmb.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan PP, Lowe TM. GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 2009;37:D93–D97. doi: 10.1093/nar/gkn787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sprinzl M, Horn C, Brown M, Ioudovitch A, Steinberg S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1998;26:148–153. doi: 10.1093/nar/26.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kikovska E, Brannvall M, Kirsebom LA. The exocyclic amine at the RNase P cleavage site contributes to substrate binding and catalysis. J. Mol. Biol. 2006;359:572–584. doi: 10.1016/j.jmb.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 65.Kikovska E, Brannvall M, Kufel J, Kirsebom LA. Substrate discrimination in RNase P RNA-mediated cleavage: importance of the structural environment of the RNase P cleavage site. Nucleic Acids Res. 2005;33:2012–2021. doi: 10.1093/nar/gki344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirsebom LA, Svard SG. The kinetics and specificity of cleavage by RNase P is mainly dependent on the structure of the amino acid acceptor stem. Nucleic Acids Res. 1992;20:425–432. doi: 10.1093/nar/20.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Svard SG, Kirsebom LA. Several regions of a tRNA precursor determine the Escherichia coli RNase P cleavage site. J. Mol. Biol. 1992;227:1019–1031. doi: 10.1016/0022-2836(92)90518-o. [DOI] [PubMed] [Google Scholar]
- 68.Loria A, Pan T. Recognition of the 5′ leader and the acceptor stem of a pre-tRNA substrate by the ribozyme from Bacillus subtilis RNase P. Biochemistry. 1998;37:10126–10133. doi: 10.1021/bi980220d. [DOI] [PubMed] [Google Scholar]
- 69.Sun L, Campbell FE, Yandek LE, Harris ME. Binding of C5 protein to P RNA enhances the rate constant for catalysis for P RNA processing of pre-tRNAs lacking a consensus (+ 1)/C(+ 72) pair. J. Mol. Biol. 2010;395:1019–1037. doi: 10.1016/j.jmb.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun L, Campbell FE, Zahler NH, Harris ME. Evidence that substrate-specific effects of C5 protein lead to uniformity in binding and catalysis by RNase P. EMBO J. 2006;25:3998–4007. doi: 10.1038/sj.emboj.7601290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun L, Harris ME. Evidence that binding of C5 protein to P RNA enhances ribozyme catalysis by influencing active site metal ion affinity. RNA. 2007;13:1505–1515. doi: 10.1261/rna.571007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gopalan V. Uniformity amid diversity in RNase P. Proc. Natl Acad. Sci. USA. 2007;104:2031–2032. doi: 10.1073/pnas.0611193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris JK, Haas ES, Williams D, Frank DN, Brown JW. New insight into RNase P RNA structure from comparative analysis of the archaeal RNA. RNA. 2001;7:220–232. doi: 10.1017/s1355838201001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsieh J, Fierke CA. Conformational change in the Bacillus subtilis RNase P holoenzyme–pre-tRNA complex enhances substrate affinity and limits cleavage rate. RNA. 2009;15:1565–1577. doi: 10.1261/rna.1639409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsieh J, Koutmou KS, Rueda D, Koutmos M, Walter NG, Fierke CA. A divalent cation stabilizes the active conformation of the B. subtilis RNase P x pre-tRNA complex: a role for an inner-sphere metal ion in RNase P. J. Mol. Biol. 2010;400:38–51. doi: 10.1016/j.jmb.2010.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koutmou KS, Hsieh J, Fierke CA. In: Ribonuclease P. Liu FAS, editor. New York: Springer Verlag; 2009. pp. 93–112. [Google Scholar]
- 77.Pomeranz Krummel DA, Altman S. Multiple binding modes of substrate to the catalytic RNA subunit of RNase P from Escherichia coli. RNA. 1999;5:1021–1033. doi: 10.1017/s1355838299990416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosenblad MA, Lopez MD, Piccinelli P, Samuelsson T. Inventory and analysis of the protein subunits of the ribonucleases P and MRP provides further evidence of homology between the yeast and human enzymes. Nucleic Acids Res. 2006;34:5145–5156. doi: 10.1093/nar/gkl626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franklin SE, Zwick MG, Johnson JD. Characterization and partial purification of two pre-tRNA 5′-processing activities from Daucus carrota (carrot) suspension cells. Plant J. 1995;7:553–563. doi: 10.1046/j.1365-313x.1995.7040553.x. [DOI] [PubMed] [Google Scholar]
- 80.Pulukkunat DK. Columbus, OH: The Ohio State University; 2002. M.S. Thesis. [Google Scholar]
- 81.Reckard JF., 3rd . Columbus, OH: The Ohio State University; 2000. M.S. Thesis. [Google Scholar]
- 82.Lai LB, Bernal-Bayard P, Mohannath G, Lai SM, Gopalan V, Vioque A. A functional RNase P protein subunit of bacterial origin in some eukaryotes. Mol. Genet. Genomics. 2011;286:359–369. doi: 10.1007/s00438-011-0651-y. [DOI] [PubMed] [Google Scholar]
- 83.Ioudovitch A, Steinberg SV. Structural compensation in an archaeal selenocysteine transfer RNA. J. Mol. Biol. 1999;290:365–371. doi: 10.1006/jmbi.1999.2901. [DOI] [PubMed] [Google Scholar]
- 84.Kahle D, Wehmeyer U, Krupp G. Substrate recognition by RNase P and by the catalytic M1 RNA: identification of possible contact points in pre-tRNAs. EMBO J. 1990;9:1929–1937. doi: 10.1002/j.1460-2075.1990.tb08320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fukuhara H, Kifusa M, Watanabe M, Terada A, Honda T, Numata T, Kakuta Y, Kimura M. A fifth protein subunit Ph1496p elevates the optimum temperature for the ribonuclease P activity from Pyrococcus horikoshii OT3. Biochem. Biophys. Res. Commun. 2006;343:956–964. doi: 10.1016/j.bbrc.2006.02.192. [DOI] [PubMed] [Google Scholar]
- 86.Xu Y, Amero CD, Pulukkunat DK, Gopalan V, Foster MP. Solution structure of an archaeal RNase P binary protein complex: formation of the 30-kDa complex between Pyrococcus furiosus RPP21 and RPP29 is accompanied by coupled protein folding and highlights critical features for protein-protein and protein-RNA interactions. J. Mol. Biol. 2009;393:1043–1055. doi: 10.1016/j.jmb.2009.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sinapah S, Wu S, Chen Y, Pettersson BM, Gopalan V, Kirsebom LA. Cleavage of model substrates by archaeal RNase P: role of protein cofactors in cleavage-site selection. Nucleic Acids Res. 2011;39:1105–1116. doi: 10.1093/nar/gkq732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawano S, Nakashima T, Kakuta Y, Tanaka I, Kimura M. Crystal structure of protein Ph1481p in complex with protein Ph1877p of archaeal RNase P from Pyrococcus horikoshii OT3: implication of dimer formation of the holoenzyme. J. Mol. Biol. 2006;357:583–591. doi: 10.1016/j.jmb.2005.12.086. [DOI] [PubMed] [Google Scholar]
- 89.Wilson RC, Bohlen CJ, Foster MP, Bell CE. Structure of Pfu Pop5, an archaeal RNase P protein. Proc. Natl Acad. Sci. USA. 2006;103:873–878. doi: 10.1073/pnas.0508004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jarrous N, Reiner R, Wesolowski D, Mann H, Guerrier-Takada C, Altman S. Function and subnuclear distribution of Rpp21, a protein subunit of the human ribonucleoprotein ribonuclease P. RNA. 2001;7:1153–1164. doi: 10.1017/s1355838201010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu S, Chen Y, Lindell M, Mao G, Kirsebom LA. Functional coupling between a distal interaction and the cleavage site in bacterial RNase P RNA-mediated cleavage. J. Mol. Biol. 2011;411:384–396. doi: 10.1016/j.jmb.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 92.Hartmann RK, Gossringer M, Spath B, Fischer S, Marchfelder A. The making of tRNAs and more - RNase P and tRNase Z. Prog. Mol. Biol. Transl. Sci. 2009;85:319–368. doi: 10.1016/S0079-6603(08)00808-8. [DOI] [PubMed] [Google Scholar]
- 93.Marck C, Grosjean H. Identification of BHB splicing motifs in intron-containing tRNAs from 18 archaea: evolutionary implications. RNA. 2003;9:1516–1531. doi: 10.1261/rna.5132503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.She Q, Shen B, Chen L. Archaeal integrases and mechanisms of gene capture. Biochem. Soc. Trans. 2004;32:222–226. doi: 10.1042/bst0320222. [DOI] [PubMed] [Google Scholar]
- 95.Marvin MC, Engelke DR. RNase P: increased versatility through protein complexity? RNA Biol. 2009;6:40–42. doi: 10.4161/rna.6.1.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chu S, Archer RH, Zengel JM, Lindahl L. The RNA of RNase MRP is required for normal processing of ribosomal RNA. Proc. Natl Acad. Sci. USA. 1994;91:659–663. doi: 10.1073/pnas.91.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gill T, Cai T, Aulds J, Wierzbicki S, Schmitt ME. RNase MRP cleaves the CLB2 mRNA to promote cell cycle progression: novel method of mRNA degradation. Mol. Cell. Biol. 2004;24:945–953. doi: 10.1128/MCB.24.3.945-953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmitt ME, Clayton DA. Nuclear RNase MRP is required for correct processing of pre-5.8S rRNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 1993;13:7935–7941. doi: 10.1128/mcb.13.12.7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Esakova O, Perederina A, Quan C, Berezin I, Krasilnikov AS. Substrate recognition by ribonucleoprotein ribonuclease MRP. RNA. 2011;17:356–364. doi: 10.1261/rna.2393711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brown JW. The ribonuclease P database. Nucleic Acids Res. 1999;27:314. doi: 10.1093/nar/27.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.