Abstract
Although stress can suppress growth and proliferation, cells can induce adaptive responses that allow them to maintain these functions under stress. While numerous studies have focused on the inhibitory effects of stress on cell growth, less is known on how growth-promoting pathways influence stress responses. We have approached this question by analyzing the effect of mammalian target of rapamycin (mTOR), a central growth controller, on the osmotic stress response. Our results showed that mammalian cells exposed to moderate hypertonicity maintained active mTOR, which was required to sustain their cell size and proliferative capacity. Moreover, mTOR regulated the induction of diverse osmostress response genes, including targets of the tonicity-responsive transcription factor NFAT5 as well as NFAT5-independent genes. Genes sensitive to mTOR-included regulators of stress responses, growth and proliferation. Among them, we identified REDD1 and REDD2, which had been previously characterized as mTOR inhibitors in other stress contexts. We observed that mTOR facilitated transcription-permissive conditions for several osmoresponsive genes by enhancing histone H4 acetylation and the recruitment of RNA polymerase II. Altogether, these results reveal a previously unappreciated role of mTOR in regulating transcriptional mechanisms that control gene expression during cellular stress responses.
INTRODUCTION
The mammalian target of rapamycin (mTOR) Ser/Thr kinase belongs to the family of PI3-kinase-related kinases (PIKK) and is a central regulator of cell growth and proliferation due to its ability to activate the biosynthesis of proteins, nucleic acids and lipids in response to growth-promoting signals (1). The pool of mTOR molecules is distributed into two main protein complexes with distinct functions, mTORC1 and mTORC2, which are defined by specific accessory proteins, such as raptor in mTORC1 (2,3), and rictor and Sin1 in mTORC2 (4,5). mTORC1 is activated by growth factors, nutrients and energy. The mTORC1 complex has substantial influence in growth and proliferation due to its ability to regulate the translation of diverse proteins involved in ribosome biogenesis and cell cycle control (1), by activating the ribosomal S6 subunit kinases (S6K) 1 and 2 (6), and inactivating the translation repressor 4E-BP1 (7). mTORC1 also influences, by direct and indirect mechanisms, the expression of genes involved in the control of metabolism, ribosomal biogenesis, growth and proliferation (8–12). mTORC2 was first described as a regulator of the actin cytoskeleton (4,13), but also regulates cell growth, differentiation and proliferation (14), at least in part by enhancing the activity of mTORC1 via activation and stabilization of Akt (15,16). mTORC1 and mTORC2 differ in their sensitivity to rapamycin, a bacterial product that binds to the intracellular chaperone FKBP12 to form a complex capable of binding with high affinity and specificity to the FRB domain of mTOR. This domain is accessible to the rapamycin-FKBP12 complex when mTOR is alone or forming part of mTORC1. Rapamycin rapidly suppresses the activity of mTORC1 towards many, although not all, of its substrates (17,18), but does not generally inhibit mTORC2 (19). However, it prevents the formation of new mTORC2 complexes, which in the long term can lead to the inhibition of mTORC2-dependent functions (19).
Besides its responsiveness to growth regulatory conditions, mTOR is also sensitive to diverse stressors, such as hypoxia, DNA damage, oxidative stress and osmotic stress (20). DNA damage induces the p53-dependent expression of Sestrins 1 and 2 (21), and hypoxia induces the HIF-1α-mediated expression of REDD1 and REDD2 (22), all of which can activate TSC2 (20,21). TSC2 has GTPase activity and inhibits mTORC1 by converting its activator Rheb-GTP to Rheb-GDP (23). Energy stress in general causes the increase in the intracellular [AMP]/[ATP] ratio and engages the AMP-activated kinase AMPK, which phosphorylates and activates TSC2 (24). AMPK can also inhibit mTORC1 directly by phosphorylating raptor (25). The decrease in mTOR activity in cells exposed to stress serves to slow down or arrest growth and proliferation-promoting processes when cells face conditions that can damage critical components such as DNA. The importance of inhibiting mTOR during stress is illustrated by the finding that TSC2−/− cells cannot shut down mTOR when exposed to glucose deprivation or DNA damage and exhibit mTOR-dependent accumulation of p53 and cell death (26,27). The capacity of mTOR to influence stress responses is also evidenced by its ability to increase the translation and activity of the hypoxia-inducible factors HIF-1α and HIF-2α (28,29). These observations raise the question of whether growth signaling pathways are merely less active under stress or may also regulate the quality and magnitude of stress responses. We have addressed this question by analyzing the effect of mTOR in the response of mammalian cells to hypertonic stress.
The effects of hypertonic stress on mammalian cells depend on its intensity and duration. In mammals, normal tonicity in most tissues is ∼300 mOsm/kg, corresponding to a concentration of extracellular sodium plus potassium ions of ∼150 mM. An exception is the renal medulla, where local tonicity can reach 600–1700 mOsm/kg. However, this elevated tonicity is not entirely caused by sodium ions, but is in part contributed by a high concentration of urea, which actually helps renal medullary cells to tolerate hypertonicity (30). Hypertonicity levels of ∼400 mOsm/kg and higher can also occur in other sites, such as the skin and respiratory and gastrointestinal mucosa, as result of hypernatremia caused by dietary and ambient conditions (31–33). In addition, systemic hypernatremia with plasma tonicities of 360–430 mOsm/kg, which would affect multiple tissues, can result from severe dehydration and osmoregulatory disorders (34–40). Studies have shown that elevated hypertonicity (>600 mOsm/kg) can cause double-strand DNA breaks (41) and induce a genotoxic stress response (30), with activation of ATM and the checkpoint effectors p53 and Chk2 (42,43). However, cells exposed to moderate osmostress conditions of 400–500 mOsm/kg, which are more likely to reflect physiopathological settings, exhibit only a transient genotoxic stress-like response and reversible cell cycle arrest without evident DNA damage (44), and can maintain their proliferative capacity under prolonged hypertonic conditions (40,44–48). Mammalian cells react to osmostress by activating the transcription factor NFAT5/TonEBP, which contains a Rel-like DNA-binding domain homologous to those of NF-κB and the calcineurin-dependent NFATc proteins (49–51). NFAT5 controls the expression of numerous osmoprotective gene products, including chaperones of the Hsp family (52,53), enzymes involved in osmolyte synthesis, and osmolyte transporters (49,54–56), and regulates the adaptation to hypertonicity in cell types as diverse as renal medullary cells (46), embryonic fibroblasts (46,47), neurons (39) or lymphocytes (40,44,47). NFAT5 deficiency in the mouse causes a severe renal dysfunction (46) and T-cell immunodeficiency associated with the inability of NFAT5-deficient cells to adapt to hypertonic stress conditions in vivo (40,47).
With regard to mTOR and osmostress, it had been described that elevated hypertonicity (600–900 mOsm/kg) inactivated the mTOR pathway (57–59), although some had reported a transient stimulation of S6K1 activity by hypertonic shock (60). A possible mechanism for mTOR inhibition during osmostress might involve AMPK, since this kinase can be activated by elevated hypertonicity (61,62). However, intense hypertonic stress might not accurately reflect conditions in which cells would mount an adaptive response, since acute elevation of tonicity >600 mOsm/kg can induce cell death in hours (45), and impair the osmoprotective function of NFAT5 (47). On the other hand, we and others had observed that the activity of NFAT5 in response to more moderate osmostress (500 mOsm/kg) was enhanced in cells with active PI3-kinase (63) or stimulated with mitogens (64), which suggests that growth-promoting signals might have a positive effect on survival responses to osmostress. In this regard, earlier work had shown that at least in some organisms, such as yeast, TOR promoted cell survival to salt stress (65,66).
In view of these observations, we asked whether mTOR was active in mammalian cells exposed to moderate osmostress conditions, and if so, whether it contributed to the induction of an adaptive gene expression program. We report that hypertonicity conditions of 500 mOsm/kg do not inactivate mTORC1 nor mTORC2 in mouse embryo fibroblasts (MEFs). Moreover, mTOR activity was required for cell growth and proliferation under osmostress. By using pharmacological mTOR inhibitors and mRNA microarray analysis of MEFs, we found that mTOR regulated the expression of osmostress-sensitive genes, which comprised regulators of stress responses, cell survival and proliferation, and included NFAT5-dependent and -independent targets, revealing an influence of mTOR on genes with different transcriptional requirements. Among them, we identified REDD1, previously characterized as an mTOR inhibitor in other stress contexts. Suppression of REDD1 affected the expression of various genes induced by osmotic stress, suggesting a regulatory role in the osmostress response. Our results also showed that mTOR enhanced the acetylation of histone H4 at the promoters and transcribed regions of osmostress-induced genes, and the recruitment of RNA polymerase II, which suggests that it can regulate the transcription of stress response genes by influencing their chromatin configuration and RNA polymerase function. These results reveal a previously unappreciated role of mTOR in regulating transcriptional mechanisms that control gene expression during cellular stress responses.
MATERIALS AND METHODS
Cells
The human embryonic kidney cell line HEK293 was obtained from the American Type Culture Collection (ATCC). HEK293T cells were kindly provided by Josep Lluís Parra (Vall d'Hebron Institute of Oncology). AMPKα1/α2 double knockout MEFs were kindly provided by Benoit Viollet (INSERM, Institut Cochin, CNRS and Université Paris Descartes) and Tomi Makela (Institute of Biotechnology, University of Helsinki). Briefly, MEFs were obtained from 12.5-day mouse embryos from Prkaa1(AMPKα1)−/−, Prkaa2(AMPKα2)-floxed mice (AMPKα1−/−, AMPKα2fl/fl) of mixed background (Bl6/CD1) (67). Deletion of AMPKα2 was done by infecting the cells in vitro with Adeno-Cre, and cells were immortalized at passage 2 with a carboxy-terminal fragment of p53 (68,69). Wild-type-AMPK MEFs were immortalized by the same procedure. Wild-type and NFAT5-deficient MEFs were prepared from 13.5-day littermate embryos (129sv background) using the NIH3T3 protocol to obtain spontaneously immortalized cells (46). HEK293 cells and MEFs were maintained in Dulbecco's modified Eagle's Medium (DMEM) (Gibco) supplemented with 10% heat-inactivated fetal bovine serum, 4 mM l-glutamine (Gibco), 1 mM sodium pyruvate (Gibco) and 50 μM β-mercaptoethanol (Gibco). Splenocytes from 8- to 12-weeks-old wild-type and Nfat5−/− mice (46) were isolated by density gradient sedimentation with Lymphoprep (Axis-Shield PoC AS) and stimulated with 2.5 µg/ml concanavalin A (Sigma-Aldrich) plus 25 ng/ml of IL-2 (Chiron) during 24 h in culture medium containing DMEM supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 50 µM β-mercaptoethanol, penicillin–streptomycin and non-essential amino acids (Gibco) and then cultured under isotonic or hypertonic conditions as indicated in the figure legend.
Hypertonic stress
The osmolarity of the culture medium was measured in a VAPRO 5520 vapor pressure osmometer (Wescor). Culture medium with supplements had an osmolality of 330 mOsm/kg, and was adjusted to 300 mOsm/kg by adding 10% sterile H2O (Milli-Q Biocel A10, Millipore). Media were made hypertonic by adding NaCl from a sterile 4 M stock solution in water. Over an isotonic baseline of 300 mOsm/kg, addition of 50 mM NaCl raised the osmolarity to 400 mOsm/kg and 100 mM NaCl to 500 mOsm/kg. In some experiments, sorbitol (200 mM final concentration) was used to induce osmotic stress.
Real-time quantitative PCR (RT–qPCR)
Total RNA was isolated using the High Pure RNA isolation kit (Cat. 11 828 665 001, Roche) following manufacturer’s instructions. 1–2 μg of total RNA were retrotranscribed to cDNA using SuperScript III reverse transcriptase and random primers (Invitrogen). For real-time quantitative PCR, LightCycler 480 SYBR Green I Master Mix (Cat. 11 608 521, Roche) and a LightCycler480 system apparatus (Roche) were used following the instructions provided by the manufacturers. Samples were normalized to L32 mRNA levels using the LightCycler 480 SW 1.5 software (Roche).
Microarray experiments and analysis
NIH3T3-immortalized wild-type and NFAT5-deficient MEFs (46) from passages 30–35 were plated (175 000 cells in 60-mm dishes) in isotonic medium and 2 days later were either left untreated or treated for 8 h with 500 mOsm/kg. When indicated, the mTOR catalytic inhibitor Torin1 was added 1 h before hypertonicity (500 mOsm/kg) treatment. Cells were lysed in RLT buffer (300 µl, RNeasy system, QIAGEN Cat. 74 104) and total RNA was isolated using the manufacturer protocol. The RNA integrity was assessed using Agilent 2100 Bioanalyzer (Agilent), and only samples with high integrity [RNA integrity number (RIN) > 7.5] were subsequently used in microarray experiments. Amplification, labeling and hybridizations were performed according to protocols from Ambion and Affymetrix. Briefly, 250 ng of total RNA were amplified using the Ambion® WT Expression Kit (Ambion/Applied Biosystems), labeled using the WT Terminal Labeling Kit (Affymetrix Inc), and then hybridized to Mouse Gene 1.0 ST Array (Affymetrix) in a GeneChip® Hybridization Oven 640. Washing and scanning were performed using the Hybridization Wash and Stain Kit and the GeneChip® System of Affymetrix (GeneChip® Fluidics Station 450 and GeneChip® Scanner 3000 7G). Three independent microarray hybridizations were performed for each experimental condition: cells maintained at 300 mOsm/kg or exposed to 500 mOsm/kg (8 h) without or with 100 nM Torin1 in the case of wild-type MEFs, or 300 mOsm/kg and 500 mOsm/kg for Nfat5−/− MEFs. Microarray data analysis was performed as follows: after quality control of raw data, it was background-corrected, quantile-normalized and summarized to a gene level using the robust multi-chip average (RMA) (70) obtaining a total of 28 853 transcript clusters, excluding controls, which roughly correspond to genes. Core annotations (version NetAffx 30, human genome 18) were used to summarize data into transcript clusters. Linear Models for Microarray (LIMMA) (71), a moderated t-statistics model, was used for detecting differentially expressed genes between the conditions in study. Correction for multiple comparisons was performed using false discovery rate. Genes with an adjusted P-value < 0.05 or with a P < 0.01 for those comparisons with few results after adjusting P-values were selected as significant. Hierarchical cluster analysis was also performed to analyze how data aggregated and linear model for regression purposes. All data analysis was performed in R (version 2.11.1) with packages aroma.affymetrix, Biobase, Affymetrix, LIMMA and genefilter. The microarray data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE27485.
Cell viability, cell-size determination and proliferation assays
Flow cytometry was done with a BD LSR flow cytometer (BD Biosciences). For viability and cell-cycle analysis, cells were labeled with 5 μg/ml of the DNA dye Hoechst 33342 (SIGMA) in suspension in cytometry tubes for 1 h at 37°C in a water bath. Viability was determined by forward and side scatter parameters (FSC/SSC) in the total population of cells. Non-viable cells were readily identified by their distinct position in the FSC/SSC plots. Cell size (FSC parameter) was analyzed in the population of cells in G1 phase, after staining with the DNA dye Hoechst 33342. Cell proliferation was analyzed in CFDA-SE-labeling experiments. Briefly, MEFs were labeled with 5 µM carboxyfluorescein diacetate succinimidyl ester (CFDA-SE, Molecular Probes, Invitrogen) at Day 0 and then analyzed at 48 and 72 h after labeling. The decrease in CFDA-SE fluorescence intensity in cells, which was proportional to the number of cell divisions, was analyzed by two-color flow cytometry in the population of live cells, identified by staining with Hoechst 33342 and excluding cells with a sub-G0/G1 DNA content.
RNA interference assays
ON-TARGETplus SMARTpool small interfering RNA (siRNA) pools were purchased from Dharmacon: nontargeting scramble (D-001810-10), mouse Ddit4/REDD1 (L-056656-01) and mouse Ddit4l/REDD2 (L-056952-00). siRNA (40 nM) was transfected using Lipofectamine 2000 (Invitrogen) in a six-well plate format following the manufacturer’s protocol.
Chromatin immunoprecipitation
Two days before stimulation 0.4 × 106 MEFs (wild-type-AMPK) were plated in 10-cm diameter dishes and 1 day before applying the stimulus the medium was replaced by fresh isotonic one. Cells were treated during the indicated times with 500 mOsm/kg with or without Torin1 (100 nM) pretreatment. Cells were fixed with 1% formaldehyde for 10 min at room temperature and with continuous agitation. Formaldehyde was then quenched with glycine (final concentration of 125 mM) for 5 min. After washing the plates twice with cold PBS and once with cold PBS + PMSF, cells were lysed in SDS lysis buffer [50 mM Tris–HCl pH 8.1, 1% SDS, 10 mM EDTA and protease inhibitor cocktail set III, EDTA-free (Cat. 539 134, Calbiochem)] for 5–10 min and then stored at −80°C for at least 24 h. Lysates were sonicated in 1.5 ml polypropylene tubes with a bath sonicator (Diagenode Bioruptor) for eight cycles of 30 s on and 30 s off on high power for cells stimulated for ≤4 h and six cycles for cells stimulated 8 h, to obtain DNA fragments between 500 and 1000 bp, and centrifuged 10 min at 18 000 × g to remove insoluble debris. Supernatants were collected and 10% of each sample was separated to use as a measure of chromatin input for normalization. The rest of the sample was diluted 10 times in ChIP dilution buffer (16.7 mM Tris–HCl pH 8.1, 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl and protease inhibitor cocktail set III, EDTA free) for immunoprecipitation. Samples were precleared with protein A-sepharose beads (Amersham, cat. 17-0780-01) that had been pre-adsorbed with 57 µg/ml salmon sperm DNA (Roche, cat. 11 467 140 001) and 0.1 µg/µl of bovine serum albumin (BSA) (New England Biolabs) by rocking for 1 h at 4°C. After removing the preclearing beads, the specific antibodies were added to the lysates and incubated overnight at 4°C in rotation. Protein A-Sepharose beads pre-adsorbed with 60 µg/ml salmon sperm DNA and 0.1 µg/µl of BSA were then added, incubated for 3–4 h at 4°C, and then washed once with low salt wash buffer (20 mM Tris–HCl pH 8.1, 0.1% SDS, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl), once with high salt wash buffer (20 mM Tris–HCl pH 8.1, 0.1% SDS, 1% Triton X-100, 2 mM EDTA, 500 mM NaCl), once with LiCl immune complex wash buffer (10 mM Tris–HCl pH 8.1, 250 mM LiCl, 1% NP40, 1% sodium deoxycholate, 1 mM EDTA) and twice with 1× TE (10 mM Tris–HCl pH 8.1 and 1 mM EDTA). To elute the DNA, beads were incubated with 200 µl elution buffer (1% SDS and 100 mM NaHCO3) for 20 min at room temperature with shaking. To reverse the crosslinking, samples were incubated overnight at 65°C with 1 µg RNase per sample (Roche, cat. 11 119 915 001) and 200 mM NaCl final concentration. DNA was purified using the QIAGEN PCR purification system (Cat. 28 104). DNA was then subjected to real time quantitative PCR (RT–qPCR) with the primers described in Supplementary Methods. Immunoprecipitated DNA from each sample was normalized to its respective chromatin input.
Statistical analysis
The statistical significance of the experimental data was determined with paired Student's t-test.
Reagents, antibodies and primers, western blots, luciferase reporter assays and fluorescence microscopy
The detailed list of reagents (AICAR, rapamycin, Torin1 and general chemicals), antibodies (western blot, chromatin immunoprecipitation and immunofluorescence) and primers used for mRNA analysis and chromatin immunoprecipitation is provided in Supplementary ‘Materials and Methods’ section. Likewise, the description of western blotting, luciferase reporter assays and fluorescence microscopy experiments are also included in Supplementary ‘Materials and Methods’ section.
RESULTS
mTOR remains active under sustained osmotic stress and regulates the induction of osmostress response genes
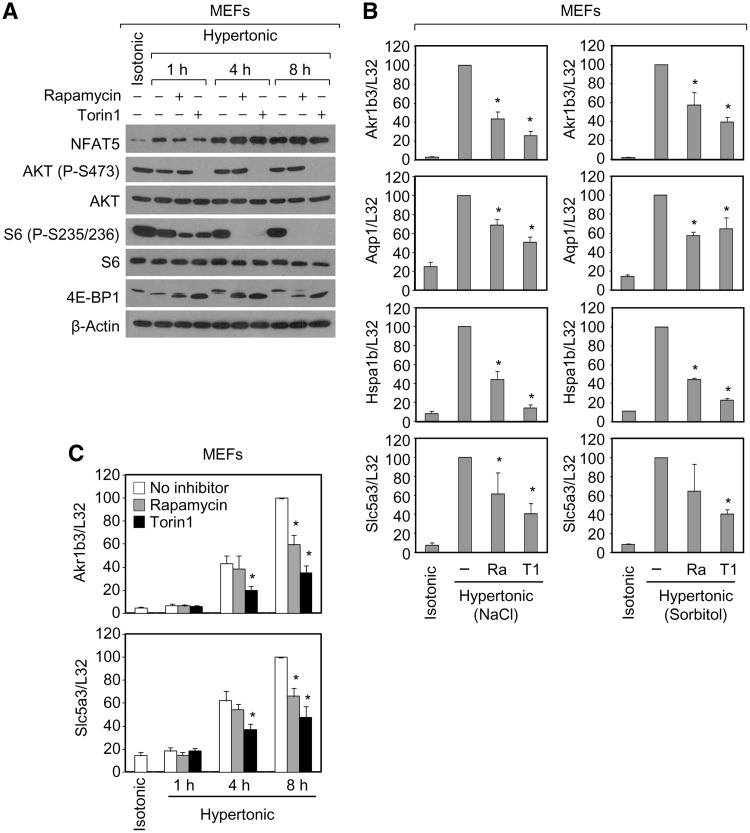
As a first approximation to analyze whether mTOR could influence the osmotic stress response in mammalian cells, we first tested whether this pathway was active during hypertonic stress conditions of 500 mOsm/kg, that were strong enough to induce a robust osmoadaptive gene expression response but not so high that they would suppress cell growth and proliferative capacity. Analysis of diagnostic substrates downstream mTORC1 (phosphorylated-S235/236 in the ribosomal subunit S6, and phosphorylation-dependent electrophoretic mobility shift of 4E-BP1) and mTORC2 (phosphorylated S473 of Akt) showed that although osmostress caused a partial inhibition of both mTOR complexes in MEFs, they retained substantial activity under hypertonic stress (Figure 1A and Supplementary Figure S1A). The induction of NFAT5 observed in the same experiment served as an indicator of the ongoing osmostress response. This experiment also showed that rapamycin specifically inhibited mTORC1, but not mTORC2, in MEFs exposed to osmotic stress, whereas both mTOR complexes were efficiently inhibited by Torin1, an ATP-competitive mTOR inhibitor whose mechanism of action is unrelated to that of rapamycin (18) (Figure 1A). We then tested the effect of rapamycin and Torin1 on the expression of the endogenous mRNAs of several osmoresponsive genes in response to hypertonic stress (500 mOsm/kg) induced by either hypernatremia or sorbitol (Figure 1A). Comparison of both inhibitors on the induction of Akr1b3 (aldose reductase), Aqp1 (aquaporin 1), Hspa1b (Hsp70.1) and Slc5a3 (sodium/myoinositol cotransporter, SMIT) showed that Torin1 was moderately stronger than rapamycin (Figure 1B). Relative mRNA levels for the osmostress-induced genes were obtained after normalization to L32 mRNA, which we had previously compared with another usual normalization control, Gapdh, and confirmed as insensitive to mTOR inhibitors (Supplementary Figure S1B). Experiments also showed that rapamycin and Torin1 caused a stronger inhibition of gene expression at later time points (Figure 1C), in parallel with the progressive inactivation of mTOR (Figure 1A). Analysis of independent cell types yielded similar results, as we observed that induction of Hspa1b, Slc5a3 and Slc6a6 (sodium and chloride-dependent taurine transporter, TauT) by osmotic stress (400 mOsm/kg) in mitogen-activated splenocytes was also sensitive to rapamycin (Supplementary Figure S2A), and that rapamycin caused a significant inhibition of the osmostress-induced, NFAT5-activated ORE-Luc reporter activity in the human embryonic kidney cell line HEK293 and MEFs (Supplementary Figure S2B). The finding that mTORC2 was active in rapamycin-treated MEFs exposed to osmostress indicated that the downregulation of gene expression caused by rapamycin was due to the inhibition of mTORC1, and suggested that the greater potency of Torin1 likely reflected its ability to inhibit mTORC1-dependent functions better than rapamycin (18). This interpretation was also supported by the observation that Torin1 caused a complete dephosphorylation of the mTORC1 substrate 4E-BP1 (Figure 1A), whereas rapamycin had a partial effect, in agreement with previous publications (17,18).
Figure 1.
Effect of rapamycin and Torin1 on the induction of osmostress response genes. (A) MEFs immortalized with a p53-carboxy-terminal fragment (immortalized MEFs) were cultured in isotonic (300 mOsm/kg) or hypertonic medium (500 mOsm/kg, upon addition of 100 mM NaCl) without or with rapamycin (50 nM) or Torin1 (100 nM). NFAT5, phospho-S6 (Ser 235/236), S6, phospho-AKT (Ser 473), AKT, 4E-BP1 and β-actin (loading control) were detected by Western blotting. One representative experiment is shown (other experiments are shown in Supplementary Figure S1). (B) RNA was isolated from immortalized MEFs cultured in isotonic (300 mOsm/kg) medium or medium made hypertonic (500 mOsm/kg) by addition of 100 mM NaCl or 200 mM sorbitol, during 8 h without or with 50 nM rapamycin (Ra) or 100 nM Torin1 (T1). mRNA abundance for Akr1b3, Aqp1, Hspa1b and Slc5a3 normalized to L32 mRNA is represented relative to hypertonic conditions (100%). Bars represent the mean ± SEM of five independent experiments (*P < 0.05). (C) RNA was isolated from immortalized MEFs cultured in isotonic (300 mOsm/kg) or hypertonic medium (500 mOsm/kg, upon addition of 100 mM NaCl) without or with rapamycin (50 nM) or Torin1 (100 nM). mRNA abundance for Akr1b3 and Slc5a3 normalized to L32 mRNA is represented relative to the 8-h time point (100%). Bars represent the mean ± SEM of four independent experiments (*P < 0.05).
Since it had been reported that AMPK can be transiently activated by intense osmotic stress conditions (>600 mOsm/kg) (61,62), we tested whether the lower osmostress levels used in our assays activated AMPK. Our results showed that hypertonic conditions of 500 mOsm/kg had minimal or no effect on the LKB1-mediated phosphorylation of AMPKα in T172, in contrast with the strong phosphorylation induced by the AMPK activator AICAR (Supplementary Figure S3A). Experiments using AMPKα1/α2-double knockout MEFs (Supplementary Figure S3B) showed that wild-type and AMPKα-null cells exhibited similar inducibility and rapamycin sensitivity of osmostress response genes (Supplementary Figure S3C), indicating that AMPK does not have a strong effect on mTOR activity and expression of osmoregulatory genes under moderate osmostress conditions.
Identification of mTOR-regulated genes in the osmotic stress response
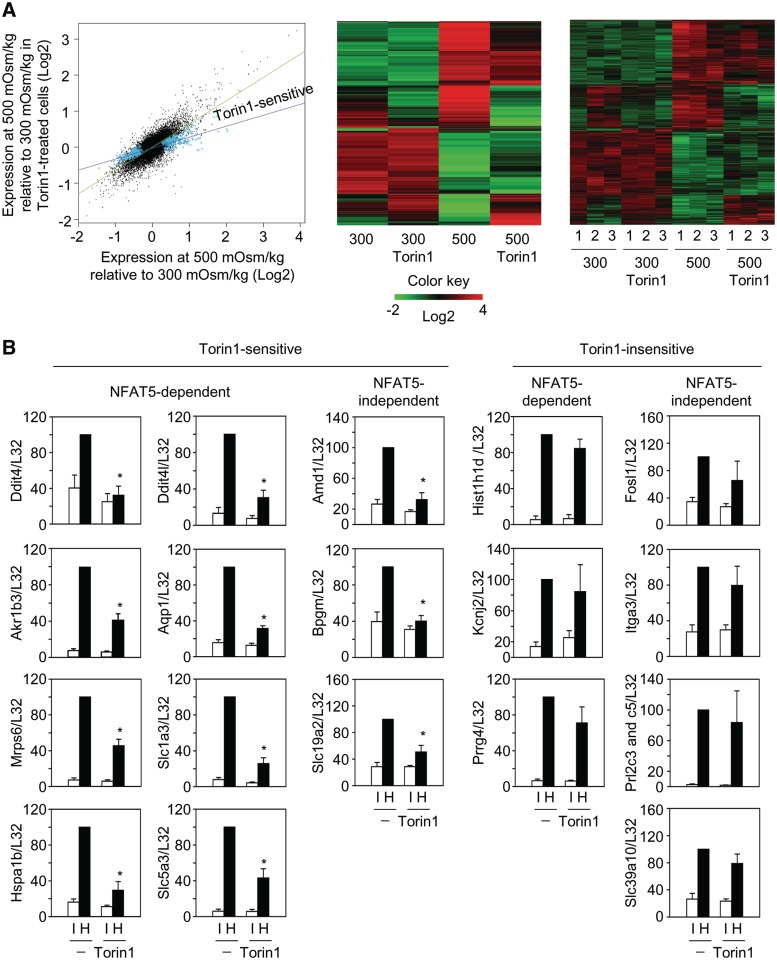
The results above showed that mTOR was active in proliferating cells exposed to physiopathologic osmostress conditions and contributed to enhance the expression of several osmoprotective genes. In order to get a broader picture of genes whose expression was regulated by osmostress and sensitive to mTOR, we used RNA microarray analysis in MEFs. RNA from three independently performed experiments was analyzed with the Affymetrix Mouse Gene 1.0 ST array. The experiments included a parallel analysis of osmoresponsive genes in NFAT5-deficient MEFs to identify NFAT5-dependent genes. Cells were exposed to hypertonic stress for 8 h, since our previous experiments (Figure 1C) had shown that this time point was sufficient for robust induction of osmotic stress response genes, but not so long that cells had fully adjusted to a hypertonic environment (48) nor to the prolonged inhibition of mTOR (12). For these assays, we used Torin1 instead of rapamycin because it had a stronger effect in downregulating osmostress response genes and we considered that it would allow a clearer identification of mTOR-regulated genes when comparing independent samples in the microarray analysis. The results revealed that Torin1 significantly affected the expression of numerous genes in cells exposed to osmotic stress (Figure 2A). We identified 107 genes that were induced by osmotic stress ≥2 times with a P < 0.01, of which 24 (22%) were repressed by Torin1 by at least 35% (Supplementary Table S1). This analysis also showed that another 74 genes were repressed by ≥50% by osmotic stress, and the inhibition of eight of them (11%) was attenuated by Torin1 (Supplementary Table S2). We detected few mRNAs that were consistently inhibited by Torin1 at 8 h, and none upregulated, in non-stressed cells (Supplementary Table S3). This modest effect likely reflected that inhibition of mTOR for the last 9 h in cells that until then had been growing actively had a limited impact on the representation of preexisting mRNAs. However, the combination of osmotic stress and Torin1 downregulated the expression of 12 genes, and increased the expression of 2, that were not significantly affected by either osmotic stress or Torin1 alone (Supplementary Table S4). These results indicated that mTOR regulated the induction and repression of a selective pattern of osmostress responsive genes. Comparison of wild-type and NFAT5-deficient MEFs showed that 74% (79 of 107) of the genes induced by osmostress were regulated by NFAT5, of which 17 (22%) were Torin1-sensitive (Supplementary Table S1). Of 28 NFAT5-independent genes, Torin1 inhibited the induction of 7 (25%) of them. Therefore, although a majority of the genes strongly induced by osmostress were NFAT5-regulated, there was no association between the dependence on NFAT5 and sensitivity to mTOR inhibition.
Figure 2.
Identification of osmostress-regulated genes and their sensitivity to mTOR inhibition. (A) Scatter plot in which the gray dots represent the expression pattern of the entire dataset and the blue crosses the genes whose induction by osmostress was repressed by Torin1 or whose repression by osmostress was attenuated by Torin1. The panels in the right show the heat maps of the gene probes found to be regulated by osmostress and their sensitivity to Torin1. Expression levels correspond to the log2 of the mean of three independent samples per condition (center panel) and for each individual sample (right panel). The brightness of red (induced) and green (downregulated) represents the magnitude of the change in the expression of each gene. Gene names and values are provided in Supplementary Tables S1–S4. (B) RT–qPCR validation of Torin1 sensitivity of osmoresponsive genes identified in the microarray analysis. RNA from the three individual samples used for the microarray analysis plus one additional experiment were analyzed by RT–qPCR for the expression of the indicated genes and normalized to L32. Samples correspond to wild-type MEFs cultured in isotonic (I, white bars) (300 mOsm/kg) or hypertonic (H, black bars) medium (500 mOsm/kg) during 8 h without or with Torin1 (100 nM). RNA levels are represented relative to the amount of mRNA in hypertonic conditions, which was given an arbitrary value of 100. Bars represent the mean ± SEM of four independent experiments (*P < 0.05).
We validated by RT–qPCR a sample of 14 genes chosen by their robust induction by osmostress in the microarray experiments, and also illustrative of Torin1-sensitive and -insensitive, as well as NFAT5-dependent and -independent genes (Figure 2B). This analysis confirmed the sensitivity to Torin1 of the NFAT5-dependent genes Ddit4, Ddit4l, Slc1a3 and the NFAT5-independent Amd1, Bpgm and Slc19a2 (Figure 2B). Also consistent with the microarray data, Torin1 had minimal or no effect on the induction of the NFAT5-dependent Hist1h1d and Kcnj2, and the NFAT5-independent genes Fosl1, Itga3, Prl2c3/c5 and Slc39a10 (Figure 2B). We observed that Akr1b3, Aqp1 and Mrps6 had not scored as Torin1-sensitive with sufficient statistical significance in the microarray, but showed to be inhibited by Torin1 when analyzed by RT–qPCR. This result likely reflects differences in sensitivity between both techniques, and suggests that the number of osmoresponsive genes that could be affected by mTOR inhibition might be greater than detected in the microarray experiments. Analysis of several genes representative of different degrees of sensitivity to Torin1, Amd1, Bpgm, Ddit4, Ddit4l and Mrps6, in an independent MEF line confirmed that they were also inhibited by rapamycin (Supplementary Figure S4), similarly to what we had previously observed with Akr1b3, Aqp1, Hspa1b and Slc5a3 (Figures 1B and 2B).
Besides genes with already known osmoprotective functions, such as Akr1b3, Aqp1, Hspa1b and Slc5a3 (38,49,72,73), mTOR regulated the expression of others that had not been previously shown to respond to osmostress, although they had been described in the context of other stress responses: Ddit4 and Ddit4l in hypoxia, DNA damage and oxidative stress (74), Figf/VEGF-D (75) in hypoxia, and Slc19a2 in DNA damage (76) (Supplementary Tables S1–S5). Some of the Torin1-inhibited genes encoded for positive regulators of proliferation: Amd1 (77), Figf/VEGF-D (78) and Tacstd2/Trop2 (79) (Supplementary Table S1). The set of genes repressed by osmostress and whose repression was attenuated by Torin1 included Atg10, a regulator of autophagosome formation (80), and Gstz1, which participates in cellular responses to oxidative stress (81). (Supplementary Table S2). Another gene found in this analysis was Sesn2, whose basal expression was not affected by osmostress, but was reduced by Torin1 in isotonic and hypertonic conditions (Supplementary Table S3). Sesn2 can be induced by DNA damage, and its product Sestrin 2 has been shown to inhibit mTOR via TSC2 (21). Torin1 also enhanced the expression of Pdk4 and Pim1 in cells exposed to osmostress (Supplementary Table S4). These genes have been described in the context of cellular responses to starvation or inhibition of growth signaling (82,83). The stress-related function of the different genes is summarized in Supplementary Table S5. It is important to note that the expression of several of these genes was affected to a greater or lesser degree, but not eliminated, upon inhibiting mTOR (Supplementary Tables S1–S4), suggesting that this pathway regulated their magnitude of induction but was not an absolute requirement.
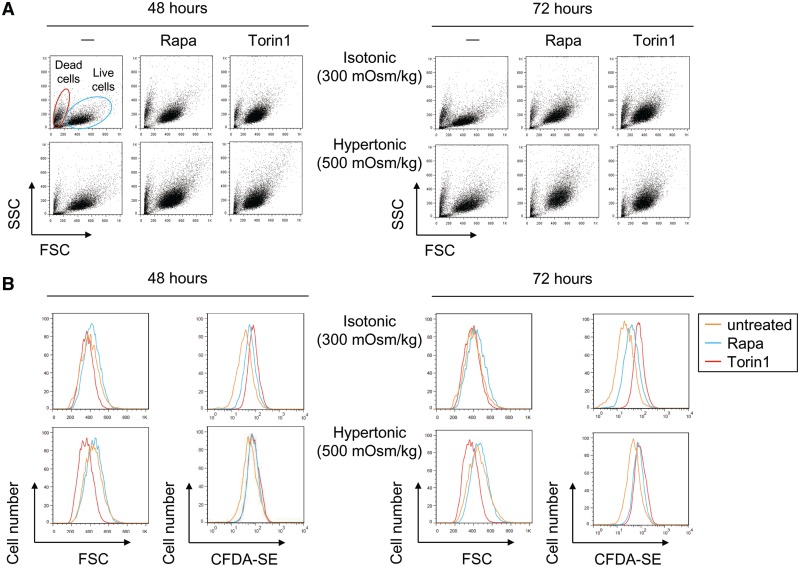
The finding that mTOR regulated the expression of a complex pattern of genes raised the question of how they might contribute to cell functions under stress. However, since the contribution of mTOR to their magnitude of expression varied depending on the particular gene, we considered that trying to reproduce the effect of mTOR inhibition by suppressing or overexpressing combinations of genes would be complex. Nonetheless, we tested whether mTOR activity was important to sustain growth and proliferation capacity in cells exposed to osmostress. For this approach, MEFs were labeled with the fluorescent dye CFDA-SE and then cultured for 48 or 72 h in isotonic or hypertonic medium with or without rapamycin or Torin1. As cells proliferate, the concentration of CFDA-SE per cell decreases as the initial label is distributed to daughter cells in successive rounds of division. Besides CFDA-SE fluorescence, we monitored the overall viability and cell size by flow cytometry. MEFs exposed to osmotic stress proliferated more slowly than those maintained in isotonic conditions, but showed comparable viability and cell size (Figure 3A and B). Inhibition of mTOR impaired cell proliferation and caused a decrease in their size, clearly evident in Torin1-treated cells, in both isotonic and hypertonic conditions (Figure 3B). Visual examination of the cells indicated no obvious signs of toxicity under the conditions tested (Supplementary Figure S5). Altogether, these results indicate that mTOR was required to sustain growth and proliferative capacity during osmostress.
Figure 3.
Effect of osmotic stress and mTOR inhibitors on cell growth and proliferative capacity. Immortalized MEFs were labeled with CFDA-SE and cultured during 48 or 72 h in isotonic (300 mOsm/kg) or hypertonic medium (500 mOsm/kg, upon addition of 100 mM NaCl) without or with rapamycin (50 nM) or Torin1 (100 nM). (A) Cell viability (FSC/SSC dot plots), (B) size (FSC parameter) and relative proliferation (proportional to dilution of CFDA-SE signal) were analyzed by flow cytometry. The FSC parameter in (B) was analyzed in cells in G1, gated by staining the culture with the DNA dye Hoechst 33342. Flow cytometry graphics are representative of three independent experiments with similar results.
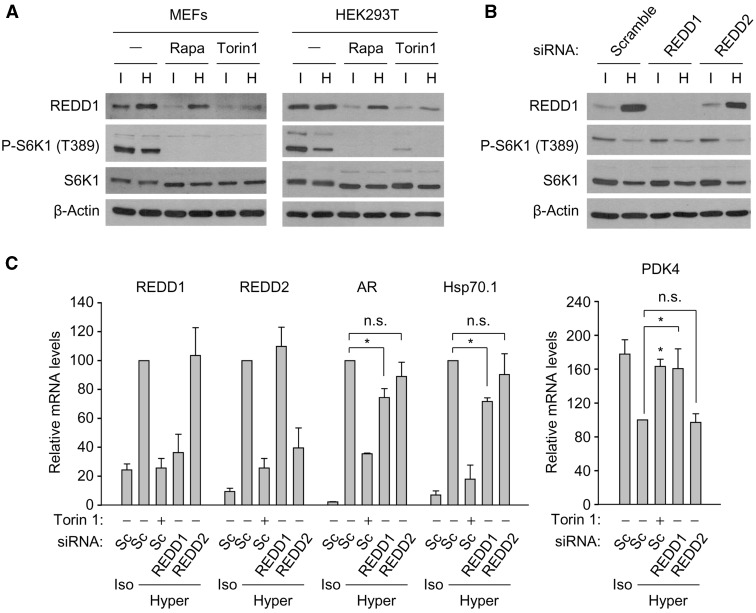
We were intrigued by the finding that hypertonic stress upregulated the mTOR-dependent expression of REDD1 and REDD2 mRNAs, two homologous genes whose products had been described to inhibit mTOR in certain contexts, such as hypoxia (74,84). REDD1 protein, like its mRNA, was induced by osmotic stress in a rapamycin and Torin1-sensitive manner (Figure 4A). We could not asses the induction of REDD2 protein due to the lack of antibodies validated in the literature. We focused on REDD1 and tested its effect on the activity of mTOR and induction of osmoresponsive genes. Suppression of REDD1 with siRNA did not affect the phosphorylation of the mTORC1 substrate S6K1 (Figure 4B), which suggested that induction of endogenous REDD1 by osmotic stress did not inhibit mTOR, in contrast to its inhibitory effect described in other stress responses. However, loss of REDD1 in MEFs caused a decrease in the induction of aldose reductase and Hsp70.1 by osmotic stress, without affecting the induction of REDD2 (Figure 4C). In the same set of experiments, downregulation of REDD2 with siRNA did not cause significant changes in the induction of osmostress response genes nor in the activity of mTORC1 (Figure 4B and C). However, since we have not confirmed the induction of REDD2 protein, we are cautious about the interpretation of this result regarding the potential lack of effect of endogenous REDD2. Our microarray analysis showed that inhibition of mTOR in cells exposed to osmostress enhanced the expression of Pdk4 (pyruvate dehydrogenase kinase 4) (Supplementary Table S4). Since Pdk4 had been recently shown to be repressed by REDD1 in MEFs (85), we tested whether mTOR-mediated induction of REDD1 during osmostress was involved in the inhibition of Pdk4, and found that suppression of REDD1 prevented the downregulation of PDK4 by osmotic stress to a similar extent as Torin1 (Figure 4C). Altogether, these results indicated that although the effects of REDD1 were moderate, it could modulate, both positively (aldose reductase, Hsp70.1) and negatively (PDK4), the expression of several osmoresponsive genes.
Figure 4.
mTOR-sensitive induction of REDD1 by osmotic stress. (A) Immortalized MEFs or HEK293T cells were cultured during 8 h in isotonic (300 mOsm/kg) or hypertonic medium (500 mOsm/kg, upon addition of 100 mM NaCl) without or with rapamycin (50 nM) or Torin1 (100 nM). REDD1, phospho-S6K1 (Thr 389), S6K1 and β-actin (loading control) were detected by Western blotting. (B) Immortalized MEFs were transfected with control non-targeting siRNA (scramble) or siRNA specific for REDD1 or REDD2. After 24 h, they were cultured during 8 h in isotonic (300 mOsm/kg) or hypertonic medium (500 mOsm/kg, upon adding 100 mM NaCl). (C) Effect of suppressing REDD1 and REDD2 on the osmostress-mediated induction of REDD1, REDD2, aldose reductase (AR), Hsp70.1, and PDK4. RNA levels for each gene and condition were normalized to L32 mRNA and are represented relative to the amount of mRNA in hypertonic conditions in cells transfected with control scramble siRNA, which was given an arbitrary value of 100. Bars represent the mean ± SEM of three independent experiments (*P < 0.05; n.s. not statistically significant).
mTOR regulates osmostress-induced changes in chromatin configuration and RNA pol II recruitment to osmoresponsive genes
Next, we analyzed the effect of mTOR inhibition on transcriptional mechanisms potentially involved in the induction of osmostress responsive genes. We observed that Torin1 did not inhibit the initial recruitment of NFAT5 to the Akr1b3 (aldose reductase) enhancer but caused its partial dissociation from chromatin at later time points (≥4 h) (Figure 5A). We also observed that Torin1, and rapamycin to a lesser extent, reduced the basal constitutive nuclear accumulation of NFAT5 at 8 h, but did not impair its translocation induced by osmotic stress (Supplementary Figure S6). These results indicated that the activity of NFAT5 was reduced upon prolonged mTOR inhibition. However, seeing that Torin1 inhibited the induction of only a set (22%) of NFAT5-dependent genes, as well as a similar proportion (25%) of NFAT5-independent ones, we considered that NFAT5 might not be a primary target of mTOR, and wondered whether Torin1 might affect other transcription regulatory mechanisms. Prompted by a recent work showing that hypertonic stress-induced rapid changes in the chromatin configuration of the aldose reductase gene (86), we asked whether mTOR could regulate these processes in NFAT5-dependent and -independent osmostress-responsive genes. In agreement with Tong et al. (86), our analysis of the Akr1b3 (aldose reductase) gene showed that hypertonic stress-induced extensive acetylation of histone H4 at different regions since the first hour of stimulation, followed by eviction of nucleosomes from the transcription start site (TSS) and upstream regions (Figure 5B). Torin1 inhibited the acetylation of H4 throughout an extended region of ∼7 kb by 4 h, but did not affect nucleosome eviction (Figure 5B). We then tested the effect of osmostress and Torin1 on H4 acetylation and eviction in two other genes: the NFAT5-regulated Ddit4l (REDD2), and the NFAT5-independent Bpgm (2,3-bisphosphoglycerate mutase). The increase in H4 acetylation induced by osmotic stress in these genes was rather modest in comparison with Akr1b3, but was still Torin1-sensitive (Supplementary Figure S7). Similarly to what we had observed in the Akr1b3 gene, osmotic stress caused a progressive nucleosome eviction in Ddit4l and Bpgm that was not affected by Torin1.
Figure 5.
Effect of Torin1 on the recruitment of NFAT5 to the Akr1b3 (aldose reductase) gene and its chromatin configuration in response to osmostress. (A) Chromatin from immortalized MEFs cultured in isotonic (300 mOsm/kg) or hypertonic medium (500 mOsm/kg, upon addition of 100 mM NaCl) without or with Torin1 (100 nM) was immunoprecipitated with preimmune rabbit serum (Pre) or a mixture of two rabbit polyclonal antibodies specific for NFAT5 (NFAT5). Immunoprecipitated chromatin was amplified with primers for a genomic region located at −1.13-kb upstream of the TSS that contains three osmotic responsive elements (ORE), and normalized to its respective total chromatin input. Results are represented relative to the sample of 4 h of hypertonicity treatment (arbitrary value of 1). Bars represent the mean ± SEM of four independent experiments (*P < 0.05). (B) Schematic representation of the Akr1b3 gene showing the location of the primers used for different regions. Chromatin from immortalized MEFs cultured in isotonic (300 mOsm/kg) or hypertonic medium (500 mOsm/kg) without or with Torin1 (100 nM) was immunoprecipitated with a control rabbit IgG, an acetylated histone H4-specific rabbit antibody or a histone H4-specific rabbit antibody. Immunoprecipitated chromatin was normalized to its respective total chromatin input. Upper graphics show the ratio of acetylated H4 to total H4 at different time points and conditions relative to a sample of untreated cells (300 mOsm/kg, time 0, arbitrary value of 1). Lower panels correspond to the immunoprecipitation with the H4-specific antibody in the same samples. Results represent the mean ± SEM of four independent experiments (*P < 0.05).
We also analyzed whether inhibition of mTOR affected the recruitment of RNA polymerase II, a more direct indicator of transcriptional activity. Osmotic stress induced a rapid and substantial recruitment of RNA pol II to the TSS and transcribed region (exon 2) of Akr1b3 (Supplementary Figure S8). Inhibition of the osmostress-induced RNA pol II recruitment by Torin1 was detected in 1 h, and preceded the partial dissociation of NFAT5 from the Akr1b3 enhancer, indicating that inhibition of RNA pol II was not due to the dissociation of NFAT5 from upstream regions. Further analysis showed that both rapamycin and Torin1 were significantly effective at inhibiting the binding of RNA pol II to the TSS of Akr1b3 (Figure 6), as well as the recruitment to the TSS and exon2 of active forms of RNA pol II involved in transcription initiation and elongation, identified respectively by phosphorylated Ser5 and Ser2 in the heptad repeat of the carboxy-terminal domain (CTD) of its largest subunit Rbp1 (87). Regarding Bpgm, we observed a moderate effect of osmostress in enhancing the recruitment of RNA pol II and its phosphorylated Ser5 and Ser2 forms than for the Akr1b3 gene, but nonetheless this effect was significant and inhibited by either rapamycin or Torin1 (Figure 6). For Ddit4l, binding of RNA pol II to its TSS and transcribed regions was minimally increased by osmostress compared to Akr1b3 and Bpgm (Figure 6). The modest effect of osmostress in enhancing H4 acetylation of Ddit4l and its occupancy by RNA pol II suggested that the osmostress-induced mTOR-sensitive mechanism for upregulating its mRNA could either involve chromatin-regulatory events different from those regulating Akr1b3 and Bpgm, or additional posttranscriptional mechanisms acting on RNA processing or stability.
Figure 6.
Effect of rapamycin and Torin1 on the recruitment of RNA pol II to osmostress responsive genes. Formaldehyde-crosslinked chromatin from immortalized MEFs cultured during 4 h in isotonic (300 mOsm/kg) or hypertonic medium (500 mOsm/kg, upon addition of 100 mM NaCl) without or with 50 nM rapamycin (Ra) or 100 nM Torin1 (T1) was immunoprecipitated with a control rabbit IgG, antibodies specific for RNA pol II or for phosphorylated Ser5 or Ser2 in its CTD heptad repeat. Immunoprecipitated chromatin was analyzed with primers corresponding to the proximal promoter/TSS and transcribed exonic regions of Akr1b3 (intron 1–exon 2), Bpgm (exon 2) and Ddit4l (exon 3) genes, and normalized to its respective total chromatin input for each sample. Results are shown relative to the sample of 4 h of hypertonicity treatment (arbitrary value of 1), and represent the mean ± SEM of three independent experiments (*P < 0.05).
DISCUSSION
Our study reveals a previously unappreciated capacity of mTOR to regulate the osmostress response by influencing the transcription of stress-responsive genes. We used hypertonic stress conditions (500 mOsm/kg) sufficient to induce a robust cellular response, but moderate enough so that cells could adapt to it. These conditions allowed cells to maintain mTORC1 and mTORC2 functioning and did not activate AMPK. The finding that rapamycin suppressed mTORC1, but not mTORC2, in cells exposed to osmostress, together with the comparable inhibitory effect of rapamycin and Torin1 on several osmoresponsive genes indicate that at least mTORC1 is capable of osmoregulatory function. Nonetheless, our results do not rule out that mTORC2 might also regulate the expression of some of the osmostress-sensitive genes identified in the microarray analysis.
Inhibition of mTOR affected gene expression in different ways: some genes required mTOR to be optimally induced, others were not particularly responsive to hypertonicity but needed mTOR to maintain their expression under stress conditions, and even a few were downregulated during osmostress in an mTOR-dependent manner. The set of genes whose expression was sensitive to mTOR in cells subjected to osmotic stress was enriched in genes associated with stress responses, and included genes whose expression had not been previously associated with osmotic stress but were known to respond to other stressor such as DNA damage, hypoxia or oxidative stress (Supplementary Table S5). The finding that mTOR inhibition affected the magnitude of induction of most of them but did not suppress their expression suggests that the mTOR pathway may play a modulatory role rather than being absolutely required. The particular sensitivity of a set of osmoresponsive genes to mTOR activity might represent one facet of the larger regulatory effect of metabolic and cell growth status on gene expression patterns, and indicate that the profile of stress responses elicited in actively growing cells differs from those of cells with lower biosynthetic and metabolic activity.
Cells kept under sustained osmotic stress maintained their size, indicative of active biosynthesis, and could proliferate, though at a slower rate than unstressed cells. Both functions required active mTOR, which indicated that its net effect in cells exposed to osmostress is to sustain growth and cycling capacity. Inhibition of mTOR in cells exposed to moderate osmostress up to 72 h arrested proliferation and growth, but did not decrease cell viability. This could suggest that cells can survive despite expressing lower levels of those genes that are more dependent on mTOR, or that the restriction in growth-promoting processes enforced by mTOR inhibition could have a protective effect under stress. Both possibilities are not mutually exclusive. Inhibiting mTOR reduced the expression of 22% of osmostress-induced genes, to a greater or lesser extent depending on the gene, but did not suppress the entire osmostress response. By contrast, lack of NFAT5 affected a much greater proportion of osmostress-inducible genes (74%). The interpretation that cells exposed to osmostress could tolerate better the inhibition of mTOR than the lack of NFAT5 is in agreement with the low renal toxicity of rapamycin in patients (88), in contrast with the severe renal dysfunction of NFAT5-deficient mice (46). Since cells in the renal medulla are naturally exposed to elevated hypertonicity, a substantial inhibition of their adaptive response would be highly deleterious, as evidenced by the atrophy of the renal medulla observed in NFAT5-deficient mice (46), consistent with the dependence of a majority of osmoresponsive genes on this factor. It is also possible that inhibiting mTOR could have an overall protective effect under stress despite that cells expressed lower levels of osmoregulatory gene products. At least in other stress contexts, such as DNA damage or glucose deprivation, inhibition of mTOR helps to maintain cell viability and prevent replicative senescence, whereas enforced mTOR activation in those conditions leads to cell death (26,27,89,90). The biological meaning of the reciprocal regulation between mTOR and stress responses is still largely unexplored. Since cells will likely be exposed to a variety of stressors, intrinsic and extrinsic, as well as growth signals throughout their lifespan, an adequate balance of mTOR function could be relevant to cope with stress and maintain growth capacity.
It was noticeable that mTOR enhanced the induction of Ddit4 and Ddit4l in response to osmostress. The products of these genes can inhibit the activity of mTORC1 in other stress contexts, such as hypoxia (74,84), but our results indicated that REDD1 did not seem to inhibit mTORC1 during the osmostress response. In this regard, independent studies have shown that REDD1 can be induced by other stimuli, such as insulin, without causing mTOR inhibition (91), and that REDD1−/− MEFs do not exhibit higher mTORC1 activity than wild-type ones under normal growth conditions (85). Nonetheless, it is possible that induction of REDD1 by osmostress could cooperate with other types of stress to facilitate the inhibition of mTOR. We observed that suppression of REDD1 altered the expression of some mTOR-sensitive, osmostress-regulated genes, which suggested a novel regulatory role of this protein. A recent work by Horak et al. (85) described that REDD1 decreases the production of mitochondrial reactive oxygen species, and this leads to the destabilization of HIF-1α. Suppression of REDD1 increased HIF-1α levels and enhanced the expression of several HIF-1α-regulated genes in normoxic conditions, as well as other genes not known to be HIF-1α targets, such as Pdk4 (85). Since hypertonicity can cause oxidative stress, which contributes to the activation of osmoresponsive genes (92), and can also activate HIF-1α (93), it can be speculated that osmostress-induced REDD1 could serve to modulate the expression of genes sensitive to oxidative stress and HIF-1α. Future work should elucidate this question.
mTOR could modulate the expression of a set of osmoresponsive genes by regulating epigenetic modifications locally and facilitating the recruitment of RNA pol II, as shown here for Akr1b3 and Bpgm. On the other hand, another mTOR-regulated gene, Ddit4l, exhibited a lesser responsiveness to osmostress for histone H4 acetylation and RNA pol II recruitment than Akr1b3 and Bpgm. This could suggest that Ddit4l has an active-like chromatin configuration, as usually found in primary response genes that are poised for rapid induction (94). Alternatively, the mTOR-sensitive upregulation of its mRNA by osmostress might involve chromatin-regulatory events different from those regulating Akr1b3 and Bpgm, or post-transcriptional mechanisms affecting its RNA processing or stability. How is this achieved, and which are the regulators involved are open questions. The more pronounced sensitivity of a group of osmoresponsive genes to mTOR suggests that it might modulate their expression through a limited set of regulators particularly relevant for those genes, rather than affecting the general transcription machinery of the cell. With regard to NFAT5, we observed that its sustained binding to the Akr1b3 enhancer was reduced upon prolonged mTOR inhibition. This effect could contribute to decrease the expression of aldose reductase mRNA and is likely associated with the loss of acetylation in histone H4 at this gene (86). However, other evidences indicated that NFAT5 was not a primary determinant in the sensitivity of osmoresponsive genes to mTOR: dissociation of RNA pol II from Akr1b3 transcribed regions upon mTOR inhibition occurred before NFAT5 dissociated from its enhancer; and inactivation of mTOR also inhibited the osmostress-induced acetylation of H4 and RNA pol II recruitment at the NFAT5-independent Bpgm gene, and impaired the expression of similar proportions of NFAT5-dependent and -independent genes. In addition, >75% of the NFAT5-regulated genes were induced in an mTOR-independent manner, and inactivation of mTOR did not impair the synthesis and nuclear translocation of NFAT5 induced by osmotic stress. In view of these results, it is conceivable that mTOR might act upon different transcriptional regulatory mechanisms, possibly involving diverse transcription factors and chromatin modifiers, to achieve the induction or repression of stress responsive genes. In other contexts, mTOR has been shown to enhance the induction of stress–response transcription factors such as p53 and HIF-1α (27,28), and influence histone acetylation at specific genes by diverse mechanisms, such as inducing MyoD in mammalian muscle cells, which suppresses the expression of the histone deacetylase HDAC4 (95), or by inhibiting the association of the HDACs Rpd3/Sin3 and Sir2 with nutrient responsive genes in yeast (96,97) and promoting the recruitment to them of histone acetyl transferase Esa1 (98).
The notion that mTOR can be an active regulator of stress responses has been often overlooked under the consideration that stress in general inhibits mTOR and this serves to restrict cell growth and proliferation during adverse conditions. However, our findings, together with those from other laboratories, suggest that cells can use this pathway to adjust the type and magnitude of stress–responses to their growth status. The observation that mTOR inhibition affected the expression of a group of hypertonicity-responsive genes in proliferating cells also suggests that actively growing cells exhibit, in an mTOR-regulated manner, specific stress responses that differ from those of less metabolically active cells in their intensity and patterns of genes induced or suppressed. Since growing cells have different needs than less active ones, it is not unexpected that they may turn on particular gene expression programs when facing stress. Elucidating the functional relevance of this selectivity in different cell types deserves further investigation. The activity of mTOR can be modulated by multiple variables including growth factors, cytokines, nutrients, energy availability, the cell cycle phase and stress. Our results support a role of mTOR in tuning the transcriptional regulation of specific gene expression patterns to inputs from stress and growth-regulatory signals.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Tables 1–5, Supplementary Figures 1–8, Supplementary Methods and Supplementary References [99,100].
FUNDING
Ministry of Science and Innovation of Spain (Grant numbers BFU2008-01070, SAF2011-24268) and Distinció de la Generalitat de Catalunya per a la Promoció de la Recerca Universitària (to J.A.); the Ramón y Cajal and I3 Researcher Programmes and Ministry of Science and Innovation of Spain (Grant numbers SAF2006-04913, SAF2009-08066) and Marie Curie International Reintegration Programme (Grant number MCIRG 516308 to C.L-R.); Fundació la Marató TV3 (Grant numbers 030230/31, 080730), Spanish Ministry of Health (Fondo de Investigación Sanitaria, Red HERACLES) (Grant number RD06/0009/1005. FEDER) and Generalitat de Catalunya (Grant numbers SGR-00478, 2009 SGR 601) for research in the laboratories of C. L-R. and J.A.; FPI predoctoral fellowship of the Ministry of Science and Innovation of Spain (to M.C.O.); FI predoctoral fellowship from the Generalitat de Catalunya (to B.M.); FI-IQUC predoctoral fellowship from the Generalitat de Catalunya (to K.D.-E.). Funding for open access charge: Ministry of Science and Innovation of Spain (Grant numbers BFU2008-01070, SAF2011-24268 to J.A.).
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to acknowledge Lara Nonell and Eulàlia Puigdecanet at the Microarray Service (SAM) of IMIM-Hospital del Mar for microarray processing and analysis. David M. Sabatini (Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology and Howard Hughes Medical Institute) and Nathanael S. Gray (Dana Farber Cancer Institute and Harvard Medical School) are thanked for generously providing Torin1. The authors thank Benoit Viollet (INSERM, Institut Cochin, CNRS, and Université Paris Descartes) and Tomi Makela (Institute of Biotechnology, University of Helsinki) for the wild-type-AMPK and AMPKα-deficient immortalized MEF lines. The authors thank Xavier Sanjuán (Advanced Light Microscopy Unit at the Centre for Genomic Regulation) for expert assistance with confocal microscopy. The authors thank Adam Espí for technical assistance, and Jordi Minguillón and Patricia Arreba for preliminary experiments on the sensitivity of NFAT5 to mTOR inhibitors. Members of the JA and CL-R laboratories are acknowledged for helpful discussions.
REFERENCES
- 1.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 2.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 3.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 4.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee-Fruman KK, Kuo CJ, Lippincott J, Terada N, Blenis J. Characterization of S6K2, a novel kinase homologous to S6K1. Oncogene. 1999;18:5108–5114. doi: 10.1038/sj.onc.1202894. [DOI] [PubMed] [Google Scholar]
- 7.Hara K, Yonezawa K, Kozlowski MT, Sugimoto T, Andrabi K, Weng QP, Kasuga M, Nishimoto I, Avruch J. Regulation of eIF-4E BP1 phosphorylation by mTOR. J. Biol. Chem. 1997;272:26457–26463. doi: 10.1074/jbc.272.42.26457. [DOI] [PubMed] [Google Scholar]
- 8.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol. Cell. Biol. 2002;22:5575–5584. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grolleau A, Bowman J, Pradet-Balade B, Puravs E, Hanash S, Garcia-Sanz JA, Beretta L. Global and specific translational control by rapamycin in T cells uncovered by microarrays and proteomics. J. Biol. Chem. 2002;277:22175–22184. doi: 10.1074/jbc.M202014200. [DOI] [PubMed] [Google Scholar]
- 10.James MJ, Zomerdijk JC. Phosphatidylinositol 3-kinase and mTOR signaling pathways regulate RNA polymerase I transcription in response to IGF-1 and nutrients. J. Biol. Chem. 2004;279:8911–8918. doi: 10.1074/jbc.M307735200. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez RH, Lee JS, Francesconi M, Castellani G, Neretti N, Sanders JA, Sedivy J, Gruppuso PA. Regulation of gene expression in hepatic cells by the mammalian target of rapamycin (mTOR) PLoS One. 2010;5:e9084. doi: 10.1371/journal.pone.0009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 14.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Oh WJ, Wu CC, Kim SJ, Facchinetti V, Julien LA, Finlan M, Roux PP, Su B, Jacinto E. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent akt polypeptide. EMBO J. 2010;29:3939–3951. doi: 10.1038/emboj.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc. Natl Acad. Sci. USA. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 21.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 25.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choo AY, Kim SG, Vander Heiden MG, Mahoney SJ, Vu H, Yoon SO, Cantley LC, Blenis J. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol. Cell. 2010;38:487–499. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CH, Inoki K, Karbowniczek M, Petroulakis E, Sonenberg N, Henske EP, Guan KL. Constitutive mTOR activation in TSC mutants sensitizes cells to energy starvation and genomic damage via p53. EMBO J. 2007;26:4812–4823. doi: 10.1038/sj.emboj.7601900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J. Biol. Chem. 2007;282:20534–20543. doi: 10.1074/jbc.M611782200. [DOI] [PubMed] [Google Scholar]
- 29.Toschi A, Lee E, Gadir N, Ohh M, Foster DA. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. J. Biol. Chem. 2008;283:34495–34499. doi: 10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol. Rev. 2007;87:1441–1474. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 31.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 32.Boucher RC, Stutts MJ, Bromberg PA, Gatzy JT. Regional differences in airway surface liquid composition. J. Appl. Physiol. 1981;50:613–620. doi: 10.1152/jappl.1981.50.3.613. [DOI] [PubMed] [Google Scholar]
- 33.Chuang AI, Ito S. Ambient tonicity and intestinal cytochrome CYP3A. Expert Opin. Drug Metab. Toxicol. 2010;6:883–893. doi: 10.1517/17425251003781912. [DOI] [PubMed] [Google Scholar]
- 34.Dmitrieva NI, Burg MB. Hypertonic stress response. Mutat. Res. 2005;569:65–74. doi: 10.1016/j.mrfmmm.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 35.Cooke CR, Wall BM, Jones GV, Presley DN, Share L. Reversible vasopressin deficiency in severe hypernatremia. Am. J. Kidney Dis. 1993;22:44–52. doi: 10.1016/s0272-6386(12)70165-8. [DOI] [PubMed] [Google Scholar]
- 36.Papadimitriou A, Kipourou K, Manta C, Tapaki G, Philippidis P. Adipsic hypernatremia syndrome in infancy. J. Pediatr. Endocrinol. Metab. 1997;10:547–550. doi: 10.1515/jpem.1997.10.5.547. [DOI] [PubMed] [Google Scholar]
- 37.McDill BW, Li SZ, Kovach PA, Ding L, Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc. Natl Acad. Sci. USA. 2006;103:6952–6957. doi: 10.1073/pnas.0602087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J. Biol. Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 39.Loyher ML, Mutin M, Woo SK, Kwon HM, Tappaz ML. Transcription factor tonicity-responsive enhancer-binding protein (TonEBP) which transactivates osmoprotective genes is expressed and upregulated following acute systemic hypertonicity in neurons in brain. Neuroscience. 2004;124:89–104. doi: 10.1016/j.neuroscience.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 40.Berga-Bolanos R, Drews-Elger K, Aramburu J, Lopez-Rodriguez C. NFAT5 regulates T lymphocyte homeostasis and CD24-dependent T cell expansion under pathologic hypernatremia. J. Immunol. 2010;185:6624–6635. doi: 10.4049/jimmunol.1001232. [DOI] [PubMed] [Google Scholar]
- 41.Kultz D, Chakravarty D. Maintenance of genomic integrity in mammalian kidney cells exposed to hyperosmotic stress. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2001;130:421–428. doi: 10.1016/s1095-6433(01)00440-8. [DOI] [PubMed] [Google Scholar]
- 42.Dmitrieva N, Kultz D, Michea L, Ferraris J, Burg M. Protection of renal inner medullary epithelial cells from apoptosis by hypertonic stress-induced p53 activation. J. Biol. Chem. 2000;275:18243–18247. doi: 10.1074/jbc.M000522200. [DOI] [PubMed] [Google Scholar]
- 43.Sheen MR, Kim SW, Jung JY, Ahn JY, Rhee JG, Kwon HM, Woo SK. Mre11-Rad50-Nbs1 complex is activated by hypertonicity. Am. J. Physiol. Renal Physiol. 2006;291:F1014–F1020. doi: 10.1152/ajprenal.00153.2006. [DOI] [PubMed] [Google Scholar]
- 44.Drews-Elger K, Ortells MC, Rao A, Lopez-Rodriguez C, Aramburu J. The transcription factor NFAT5 is required for cyclin expression and cell cycle progression in cells exposed to hypertonic stress. PLoS One. 2009;4:e5245. doi: 10.1371/journal.pone.0005245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dmitrieva NI, Michea LF, Rocha GM, Burg MB. Cell cycle delay and apoptosis in response to osmotic stress. Comp Biochem. Physiol A Mol. Integr. Physiol. 2001;130:411–420. doi: 10.1016/s1095-6433(01)00439-1. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Rodriguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI, Bronson RT, Igarashi P, Rao A, Olson EN. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc. Natl Acad. Sci. USA. 2004;101:2392–2397. doi: 10.1073/pnas.0308703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc. Natl Acad. Sci. USA. 2004;101:10673–10678. doi: 10.1073/pnas.0403139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SD, Choi SY, Lim SW, Lamitina ST, Ho SN, Go WY, Kwon HM. TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress: organic osmolyte-dependent and -independent pathways. Am. J. Physiol. Renal Physiol. 2011;300:F707–F715. doi: 10.1152/ajprenal.00227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl Acad. Sci. USA. 1999;96:2538–2542. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Rodriguez C, Aramburu J, Rakeman AS, Rao A. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with fos and jun. Proc. Natl Acad. Sci. USA. 1999;96:7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez-Rodriguez C, Aramburu J, Jin L, Rakeman AS, Michino M, Rao A. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001;15:47–58. doi: 10.1016/s1074-7613(01)00165-0. [DOI] [PubMed] [Google Scholar]
- 52.Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol. Cell. Biol. 2002;22:5753–5760. doi: 10.1128/MCB.22.16.5753-5760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kojima R, Randall JD, Ito E, Manshio H, Suzuki Y, Gullans SR. Regulation of expression of the stress response gene, Osp94: identification of the tonicity response element and intracellular signalling pathways. Biochem. J. 2004;380:783–794. doi: 10.1042/BJ20040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ito T, Fujio Y, Hirata M, Takatani T, Matsuda T, Muraoka S, Takahashi K, Azuma J. Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (TonE-binding protein) pathway and contributes to cytoprotection in HepG2 cells. Biochem. J. 2004;382:177–182. doi: 10.1042/BJ20031838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakayama Y, Peng T, Sands JM, Bagnasco SM. The TonE/TonEBP pathway mediates tonicity-responsive regulation of UT-A urea transporter expression. J. Biol. Chem. 2000;275:38275–38280. doi: 10.1074/jbc.M004678200. [DOI] [PubMed] [Google Scholar]
- 56.Ito T, Fujio Y, Takahashi K, Azuma J. Degradation of NFAT5, a transcriptional regulator of osmotic stress-related genes, is a critical event for doxorubicin-induced cytotoxicity in cardiac myocytes. J. Biol. Chem. 2007;282:1152–1160. doi: 10.1074/jbc.M609547200. [DOI] [PubMed] [Google Scholar]
- 57.Parrott LA, Templeton DJ. Osmotic stress inhibits p70/85 S6 kinase through activation of a protein phosphatase. J. Biol. Chem. 1999;274:24731–24736. doi: 10.1074/jbc.274.35.24731. [DOI] [PubMed] [Google Scholar]
- 58.Chen D, Fucini RV, Olson AL, Hemmings BA, Pessin JE. Osmotic shock inhibits insulin signaling by maintaining Akt/protein kinase B in an inactive dephosphorylated state. Mol. Cell. Biol. 1999;19:4684–4694. doi: 10.1128/mcb.19.7.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naegele S, Morley SJ. Molecular cross-talk between MEK1/2 and mTOR signaling during recovery of 293 cells from hypertonic stress. J. Biol. Chem. 2004;279:46023–46034. doi: 10.1074/jbc.M404945200. [DOI] [PubMed] [Google Scholar]
- 60.Van der Kaay J, Beck M, Gray A, Downes CP. Distinct phosphatidylinositol 3-kinase lipid products accumulate upon oxidative and osmotic stress and lead to different cellular responses. J. Biol. Chem. 1999;274:35963–35968. doi: 10.1074/jbc.274.50.35963. [DOI] [PubMed] [Google Scholar]
- 61.Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J. Cell. Sci. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- 62.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell. Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Irarrazabal CE, Burg MB, Ward SG, Ferraris JD. Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: role in osmoprotective transcriptional regulation. Proc. Natl Acad. Sci. USA. 2006;103:8882–8887. doi: 10.1073/pnas.0602911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morancho B, Minguillon J, Molkentin JD, Lopez-Rodriguez C, Aramburu J. Analysis of the transcriptional activity of endogenous NFAT5 in primary cells using transgenic NFAT-luciferase reporter mice. BMC Mol. Biol. 2008;9:13. doi: 10.1186/1471-2199-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crespo JL, Daicho K, Ushimaru T, Hall MN. The GATA transcription factors GLN3 and GAT1 link TOR to salt stress in saccharomyces cerevisiae. J. Biol. Chem. 2001;276:34441–34444. doi: 10.1074/jbc.M103601200. [DOI] [PubMed] [Google Scholar]
- 66.Weisman R, Choder M. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J. Biol. Chem. 2001;276:7027–7032. doi: 10.1074/jbc.M010446200. [DOI] [PubMed] [Google Scholar]
- 67.Vaahtomeri K, Ventela E, Laajanen K, Katajisto P, Wipff PJ, Hinz B, Vallenius T, Tiainen M, Makela TP. Lkb1 is required for TGFbeta-mediated myofibroblast differentiation. J. Cell. Sci. 2008;121:3531–3540. doi: 10.1242/jcs.032706. [DOI] [PubMed] [Google Scholar]
- 68.Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, et al. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bjorklund MA, Vaahtomeri K, Peltonen K, Viollet B, Makela TP, Band AM, Laiho M. Non-CDK-bound p27 (p27(NCDK)) is a marker for cell stress and is regulated through the Akt/PKB and AMPK-kinase pathways. Exp. Cell Res. 2010;316:762–774. doi: 10.1016/j.yexcr.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 70.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 71.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3:Article 3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 72.Shim EH, Kim JI, Bang ES, Heo JS, Lee JS, Kim EY, Lee JE, Park WY, Kim SH, Kim HS, et al. Targeted disruption of hsp70.1 sensitizes to osmotic stress. EMBO Rep. 2002;3:857–861. doi: 10.1093/embo-reports/kvf175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haussinger D. The role of cellular hydration in the regulation of cell function. Biochem. J. 1996;313:697–710. doi: 10.1042/bj3130697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J. Biol. Chem. 2005;280:9769–9772. doi: 10.1074/jbc.C400557200. [DOI] [PubMed] [Google Scholar]
- 75.Nilsson I, Rolny C, Wu Y, Pytowski B, Hicklin D, Alitalo K, Claesson-Welsh L, Wennstrom S. Vascular endothelial growth factor receptor-3 in hypoxia-induced vascular development. FASEB J. 2004;18:1507–1515. doi: 10.1096/fj.03-1276com. [DOI] [PubMed] [Google Scholar]
- 76.Lo PK, Chen JY, Tang PP, Lin J, Lin CH, Su LT, Wu CH, Chen TL, Yang Y, Wang FF. Identification of a mouse thiamine transporter gene as a direct transcriptional target for p53. J. Biol. Chem. 2001;276:37186–37193. doi: 10.1074/jbc.M104701200. [DOI] [PubMed] [Google Scholar]
- 77.Nishimura K, Nakatsu F, Kashiwagi K, Ohno H, Saito T, Igarashi K. Essential role of S-adenosylmethionine decarboxylase in mouse embryonic development. Genes Cells. 2002;7:41–47. doi: 10.1046/j.1356-9597.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- 78.Liu YH, Lin CY, Lin WC, Tang SW, Lai MK, Lin JY. Up-regulation of vascular endothelial growth factor-D expression in clear cell renal cell carcinoma by CD74: a critical role in cancer cell tumorigenesis. J. Immunol. 2008;181:6584–6594. doi: 10.4049/jimmunol.181.9.6584. [DOI] [PubMed] [Google Scholar]
- 79.Cubas R, Zhang S, Li M, Chen C, Yao Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol. Cancer. 2010;9:253. doi: 10.1186/1476-4598-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell. Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blackburn AC, Matthaei KI, Lim C, Taylor MC, Cappello JY, Hayes JD, Anders MW, Board PG. Deficiency of glutathione transferase zeta causes oxidative stress and activation of antioxidant response pathways. Mol. Pharmacol. 2006;69:650–657. doi: 10.1124/mol.105.018911. [DOI] [PubMed] [Google Scholar]
- 82.Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch. Biochem. Biophys. 2000;381:1–7. doi: 10.1006/abbi.2000.1946. [DOI] [PubMed] [Google Scholar]
- 83.Fox CJ, Hammerman PS, Thompson CB. The pim kinases control rapamycin-resistant T cell survival and activation. J. Exp. Med. 2005;201:259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horak P, Crawford AR, Vadysirisack DD, Nash ZM, DeYoung MP, Sgroi D, Ellisen LW. Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc. Natl Acad. Sci. USA. 2010;107:4675–4680. doi: 10.1073/pnas.0907705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tong EH, Guo JJ, Xu SX, Mak K, Chung SK, Chung SS, Huang AL, Ko BC. Inducible nucleosome depletion at OREBP-binding-sites by hypertonic stress. PLoS One. 2009;4:e8435. doi: 10.1371/journal.pone.0008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sims RJ, 3rd, Belotserkovskaya R, Reinberg D. Elongation by RNA polymerase II: the short and long of it. Genes Dev. 2004;18:2437–2468. doi: 10.1101/gad.1235904. [DOI] [PubMed] [Google Scholar]
- 88.Kahan BD. Fifteen years of clinical studies and clinical practice in renal transplantation: reviewing outcomes with de novo use of sirolimus in combination with cyclosporine. Transplant. Proc. 2008;40:S17–S20. doi: 10.1016/j.transproceed.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 89.Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging. 2010;2:344–352. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging. 2010;2:924–935. doi: 10.18632/aging.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Regazzetti C, Bost F, Le Marchand-Brustel Y, Tanti JF, Giorgetti-Peraldi S. Insulin induces REDD1 expression through hypoxia-inducible factor 1 activation in adipocytes. J. Biol. Chem. 2010;285:5157–5164. doi: 10.1074/jbc.M109.047688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou X, Ferraris JD, Cai Q, Agarwal A, Burg MB. Increased reactive oxygen species contribute to high NaCl-induced activation of the osmoregulatory transcription factor TonEBP/OREBP. Am. J. Physiol. Renal Physiol. 2005;289:F377–F385. doi: 10.1152/ajprenal.00463.2004. [DOI] [PubMed] [Google Scholar]
- 93.Zhou B, Ann DK, Li X, Kim KJ, Lin H, Minoo P, Crandall ED, Borok Z. Hypertonic induction of aquaporin-5: novel role of hypoxia-inducible factor-1alpha. Am. J. Physiol. Cell. Physiol. 2007;292:C1280–C1290. doi: 10.1152/ajpcell.00070.2006. [DOI] [PubMed] [Google Scholar]
- 94.Hargreaves DC, Horng T, Medzhitov R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell. 2009;138:129–145. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun Y, Ge Y, Drnevich J, Zhao Y, Band M, Chen J. Mammalian target of rapamycin regulates miRNA-1 and follistatin in skeletal myogenesis. J. Cell Biol. 2010;189:1157–1169. doi: 10.1083/jcb.200912093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsang CK, Bertram PG, Ai W, Drenan R, Zheng XF. Chromatin-mediated regulation of nucleolar structure and RNA pol I localization by TOR. EMBO J. 2003;22:6045–6056. doi: 10.1093/emboj/cdg578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ha CW, Huh WK. Rapamycin increases rDNA stability by enhancing association of Sir2 with rDNA in saccharomyces cerevisiae. Nucleic Acids Res. 2010;39:1336–1350. doi: 10.1093/nar/gkq895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rohde JR, Cardenas ME. The tor pathway regulates gene expression by linking nutrient sensing to histone acetylation. Mol. Cell. Biol. 2003;23:629–635. doi: 10.1128/MCB.23.2.629-635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rodriguez A, Flemington EK. Transfection-mediated cell-cycle signaling: considerations for transient transfection-based cell-cycle studies. Anal. Biochem. 1999;272:171–181. doi: 10.1006/abio.1999.4156. [DOI] [PubMed] [Google Scholar]
- 100.Estrada-Gelonch A, Aramburu J, Lopez-Rodriguez C. Exclusion of NFAT5 from mitotic chromatin resets its nucleo-cytoplasmic distribution in interphase. PLoS One. 2009;4:e7036. doi: 10.1371/journal.pone.0007036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.