Abstract
Objective
The quality of one’s personal relationships has been linked to morbidity and mortality across different diseases. As a result, it is important to examine more integrative mechanisms that might link relationships across diverse physical health outcomes. In this study, we examine associations between relationships and telomeres which predict general disease risk. These questions are pursued in the context of a more comprehensive model of relationships that highlights the importance of jointly considering positive and negative aspects of social ties.
Method
136 individuals from a community sample (ages 48 to 77) completed the social relationships index which allows a determination of relationships that differ in their positive and negative substrates (i.e., ambivalent, supportive, aversive, indifferent). Telomere length was determined from peripheral blood mononuclear cells via quantitative PCR.
Results
Participants who had a higher number of ambivalent ties in their social networks evidenced shorter telomeres. These results were independent of other relationships types (e.g., supportive), as well as standard control variables (e.g., age, health behaviors, medication use). Gender moderated the links between ambivalent ties and telomere length with these associations seen primarily in women. Follow-up analyses revealed that the links between ambivalent ties and telomeres were primarily due to friendships, parents, and social acquaintances.
Conclusions
Consistent with epidemiological findings, these data highlight a novel and integrative biological mechanism by which social ties may impact health across diseases, and further suggests the importance of incorporating both positivity and negativity in the study of specific relationships and physical health.
Keywords: Relationships, telomeres, gender, physical health
The quality and quantity of one’s social relationships are reliably related to physical health outcomes (Berkman, Glass, Brissette, & Seeman, 2000; DeVogli, Chandola, & Marmot, 2007; House, Landis, & Umberson, 1988; Uchino, 2004). For instance, Holt-Lunstad, Smith, & Layton (2010) conducted a meta-analysis of 148 studies comprised of over 308,000 participants. They found evidence that social support was related to about a 50% lower risk for future mortality (Holt-Lunstad et al., 2010). These links were not moderated by standard control variables and appeared consistent across different causes of death. These data suggest that the quality of one's social relationships may play a general role across major diseases.
Research examining links between relationships and health-relevant physiological pathways is consistent with the above epidemiological links. Low social support and high social negativity are related to greater cardiovascular reactivity during acute stress and elevated ambulatory blood pressure (ABP) during daily life (Gerin, Pieper, Levy, & Pickering, 1992; Ewart, Taylor, Kraemer, & Agras, 1991; Steptoe, Lundwall, & Cropley, 2000). Importantly, both cardiovascular reactivity and ABP are linked to the development of future cardiovascular problems (Chida & Steptoe, 2010; Pickering, Shimbo, & Haas, 2006). Social support and social negativity have also been linked to immune-mediated processes that have implications for infectious and malignant diseases (Graham et al., 2009). However, very little work has examined general biological mechanisms that can link these disease-specific biological pathways with epidemiological studies that tend to focus on all-cause mortality.
Recent research on cellular processes related to biological aging provides a framework for examining more general associations (Blackburn, 2000). Telomeres are repetitive structures at the end of chromosomes that help to promote its stability (Dahse, Fiedler, & Ernst, 1997; Saretzki & von Zglinicki, 2002). However, with each successive replication of the cell, telomeres shorten and when a critical threshold is met the result is cellular senescence. This mechanism serves several critical genomic purposes, including the prevention of chromosomal fusions and unregulated cellular activity (Chan & Blackburn, 2003). Importantly, Cawthon and colleagues (2003) found that shorter telomere length was associated with a three times greater mortality risk for heart disease and an eight times greater mortality risk from infectious diseases up to 20 years later. Thus, one aim of this study was to provide a test of links between relationships and a general biological index of cellular aging (i.e., telomeres).
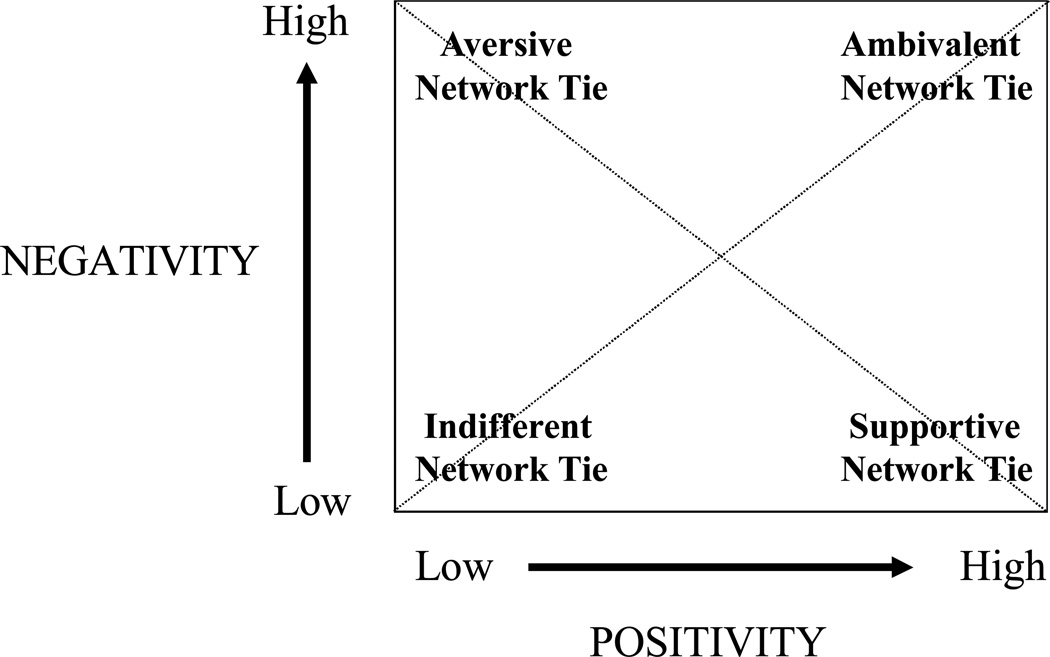
A second and major aim of our study was to examine this question in the context of a comprehensive model on the health effects of relationships (Uchino, Holt-Lunstad, Uno, & Flinders, 2001, see Figure 1). Although a relatively small body of research has linked telomeres to psychosocial processes (e.g., Epel et al., 2004; Kiecolt-Glaser et al., 2011), no study that we are aware of has examined it in the context of both positivity and negativity in relationship functioning. Relationship positivity refers broadly to interpersonal processes that facilitate successful relationship maintenance and growth such as helpful support and guidance (Uchino, 2004). In comparison, relationship negativity refers to interactions that involve conflict, insensitivity, and interference that are generally detrimental to relationships (Brooks & Dunkel Schetter, 2011). Importantly, most of the prior literature on social relationships and health has examined these dimensions of relationships separately. However, positive and negative aspects of relationships are separable dimensions and hence can be uncoupled (e.g., just positive) or co-occur (both positive and negative, Uchino et al., 2001). We have argued that social network members can be classified as either being socially supportive (i.e., high positivity/low negativity), aversive (i.e., low positivity/high negativity), or indifferent (i.e., low in both positivity/negativity). A unique aspect of Figure 1 made salient by our framework is represented in the high positivity / high negativity cell and represents a network member who is a source of both positivity and negativity (ambivalence). For instance, conflict with close family members in which socialization processes or past supportive interactions dictate a positive relationship might result in strong perceptions of ambivalence in these relationships.
Figure 1.
A general framework for examining positive and negative aspects of relationships on health.
Our prior work is consistent with the possibility that despite the positivity in such ambivalent ties, they appear to be related to worse health outcomes. We have argued that such ambivalent ties are harmful because they tend to be unpredictable and hence associated with increased interpersonal stress (Uchino, Holt-Lunstad, Uno, Campo, & Reblin, 2007). Consistent with this reasoning, such ambivalent ties are related to increased cardiovascular reactivity (Holt-Lunstad et al., 2007; Reblin, Uchino, & Smith, 2010) and higher ABP during daily life (Holt-Lunstad, Uchino, & Smith, Cerny, & Nealey-Moore, 2003). Moreover, individuals who are sources of ambivalence tend to make-up close to half of important social network members so there is ample opportunity for such ties to broadly influence health-relevant outcomes (Campo et al., 2009). In the present study, we thus predicted that the number of ambivalent network ties would be related to shorter telomeres. Consistent with the health benefits of support, we also predicted that the number of supportive ties would be related to longer telomeres. Aversive and indifferent ties were not predicted to be linked to telomeres given their low prevalence and / or limited influence (Campo et al., 2009). We also examined if any of these links were independent of other psychosocial factors previously associated with telomere length (e.g., perceived stress; Epel et al., 2004).
A related aim of this study was to examine the moderation of these predicted associations via the demographic factors of age and gender. Age is of interest because it has implications for the processes underlying any associations. Consistent with an age-related difference in disease risk, age is related to shorter telomeres (Frenck, Blackburn, & Shannon, 1998; Iwama et al., 1997). Thus, if age moderates these links, it suggests a developmental process by which over time these ties cumulatively impact on cellular aging. Alternatively, it could indicate that older adults are more sensitive or influenced by these relationship processes (Kiecolt-Glaser & Glaser, 2001). However, the lack of moderation via age suggests that these processes have an impact on cellular aging regardless of the stage of the lifespan.
A second potentially important moderator is gender. In an early review of relationships and mortality, Shumaker and Hill (1989) argued that women are more likely to be negatively influenced by being socially integrated because it can result in greater role strain or conflict. The self-esteem of women also appears to be more contingent on satisfactory relationships due to self-representations that overlap with close others (Cambron, Acitelli, & Pettit, 2009). As a result, women may be more reactive to conflict in close relationships compared to men (Beals & Rook, 2006; Kiecolt-Glaser & Newton, 2001). It would thus follow that links between relationship processes and cellular aging might be stronger in women compared to men.
Method
Participants
One hundred thirty-six relatively healthy participants (83 men and 53 women) were included in this study.1 We recruited a middle-age to older adult sample between 48 and 77 (Mage=60.2, SD=6.71) to increase variability in telomere length. Many older adults are on some form of medication so we only excluded individuals who (a) were on immunosuppressive treatment (e.g., corticosteroid therapy) and/or (b) had cancer or HIV due to concerns about potential effects on peripheral blood mononuclear cells (PBMCs) which we used for the determination of telomere length. These factors (e.g., corticosteroid therapy, treatment regimen)are known to influence the functioning of immune cells (Abbas & Lichtman, 2003) but there was little evidence on the extent of such influences on telomere length determined from these cells at the time of the study so we conservatively used them as exclusion criteria.
Procedure
Eligible participants were scheduled for an appointment at the Social Neuroscience Laboratory. Following informed consent, participants were first rechecked against the exclusion criteria upon their arrival for their session. Participants then completed demographic and health questionnaires, the perceived stress scale, the interpersonal support evaluation list, and the social relationships index (see below). Following completion of the questionnaires, approximately 20 cc of blood was drawn and treated with EDTA to prevent clotting. Telomere length was determined using PBMCs which were separated using density gradient centrifugation with Ficoll-Hypaque. Following the blood draw, participants were debriefed and received $75.00 for their participation.
Measures
Social Relationships Index (SRI)
The SRI instructs individuals to list the initials of individuals in the following domains: (a) spouse / significant other, (b) father, (c) mother, (d) other family, (e) friends, (f) co-workers, and (g) social acquaintances. The categories of other family, friends, co-workers, and social acquaintances are limited to 5 people in order to keep completion of the SRI to a manageable time frame. These network members are then rated in terms of how helpful and upsetting they are (1 = not at all, 6 = extremely) when the participant needs emotional, tangible, and informational support (see Campo et al., 2009 for psychometric information). Based on our prior work (see Figure 1, Uchino et al., 2007), we operationalized different categories of social relationships as the total number of individuals in one’s network who were sources of indifference (i.e., "1" on both positivity and negativity), support (i.e., "2" or greater on positivity and only a “1” on negativity), aversion (i.e., only a “1” on positivity and "2" or greater on negativity), or ambivalence (i.e., "2" or greater on both positivity and negativity).
Perceived Stress Scale (PSS)
The PSS contains 10 items and measures general perceptions of stress. The Cronbach’s alpha for the PSS has been reported at .75 (Cohen & Williamson, 1987). As evidence for the validity of the PSS, Cohen and Williamson (1987) found that individuals relatively high in perceived stress evidence poorer physical health and higher scores on health service utilization than individuals relatively low in perceived stress. The internal consistency of the scale for our study was similarly high (.91 ).
Interpersonal Support Evaluation List (ISEL)
The ISEL contains 40 questions (Cohen et al., 1985) and assesses the specific dimensions of appraisal, self-esteem, belonging, and tangible support. Cohen et al. (1985) report that the internal consistencies of the scales range from .60 to .92; with a four week test-retest reliability of .87 for the total scale, .87 for appraisal support, .82 for belonging support, .71 for self-esteem support, and .80 for tangible support. The reliability of the ISEL has also been established over a 6 month period (Cohen et al., 1985). For the present study the internal consistency of the total scale was high (.95 ).
Health Assessment
A standardized health questionnaire provided information on the following potential health-related variables: medications / medical condition, weekly exercise habits, use of tobacco products (no, yes), weekly alcohol consumption, and body mass index. The health behavior questionnaire has been used in a large longitudinal study on the chronic stress of caregiving for a relative with Alzheimer’s Disease and its effects on physiological function (see Kiecolt-Glaser et al., 1991).
Telomere length
DNA was extracted from isolated PBMCs using the reagents and protocols contained in Qiagen’s Gentra Puregene Blood Kit, Catalog # 158389, with the exception that the red blood cell (RBC) lysis step was skipped, as it was unnecessary due to prior removal of RBCs by centrifugation. Relative telomere lengths were then determined for the PBMCs using quantitative PCR. For each DNA sample, we measured the factor by which the sample differed from a reference DNA sample in its ratio of telomere repeat copy number to single copy gene copy number. This ratio is proportional to the average telomere length (T/S ratio, see Cawthon, 2002). These assays were run in several batches as each batch consisted of 96 participants DNA samples per quantitative PCR run. The inter-assay geometric mean of the coefficient of variation for this assay was 3.13% based on examining the variation in the mean T/S ratio across days (Cawthon, 2009). The intra-assay geometric mean of the coefficient of variation was 5.22%. This was determined by examining variations in the T/S of samples across triplicate repeat measurements of the samples, collected in the same run (Cawthon, 2009). Outliers were determined within triplicate measurements of T/S for a given sample when PCR amplification of either the telomere target and/or the single copy gene target failed for one of the three replicates, that replicate was removed from the analysis. When amplification of two or more of the three replicates of a sample failed, the assay was repeated in triplicate for that DNA sample. The Shapiro-Wilk test of normality which is appropriate for this sample size was .99 indicating a high degree of normality in the resulting T/S ratio data.
Results
Sample Characteristics and Overview of Statistical Analyses
As shown in Table 1, our sample was primarily white middle-age to older adults with a higher percentage of men compared to women. Average body mass was in the overweight range with moderate exercise and relatively low tobacco and alcohol use. Table 1 also lists the major medical conditions for which participants were taking medications. As might be expected, medication use in this sample was primarily related to cardiovascular conditions, especially statins (21%) and antihypertensive medication (8.8%). However, medication use for gastroesophageal reflux (13.2%), thyroid disease (11%), menopause (8.1%), allergies (7.4%), osteoporosis (5.9%), and diabetes (2.2%) was also present.
Table 1.
Main sample characteristics.
| Variable | Sample |
|---|---|
| Mean age (SD, range) | 60.2 (6.71, 48–77) |
| Ethnicity (Percent white) | 93% |
| Gender (Percent women) | 39% |
| Health-related variables (SD, range) | |
| Mean body mass | 26.3 (4.28, 18–47) |
| Mean average hours of weekly exercise | 4.3 (5.15, 0–30) |
| Mean average weekly alcohol consumption | 3.4 (5.20, 0–28) |
| Tobacco use (Percent users) | 10% |
| Percent of main sample medication use / chronic condition | |
| Statin -Cardiovascular | 20.6% |
| Gastroesophageal reflux | 13.2% |
| Thyroid Disease | 11.0% |
| Hypertension | 8.8% |
| Hormone replacement - menopause | 8.1% |
| Allergies | 7.4% |
| Osteoporosis | 5.9% |
| Diabetes | 2.2% |
We utilized simultaneous regression analyses to examine the independent links between these relationship categories and telomere length. Based on prior work, we controlled for age, gender, body mass, health behaviors (e.g., exercise), and medication / conditions (e.g., statin) that have been linked to telomere length (Brouilette et al., 2007; Cherkas et al., 2008; Lee, Im, Kim, Lee, & Shim, 2007; Pavanello et al., 2011; Sampson, Winterbone, Hughes, Dozio, & Hughes, 2006; Valdes et al., 2005; Valdes et al., 2007). We then included all relationship categories in the regression analysis to examine their independent links to telomeres. Moderated regression analyses were utilized to examine potential statistical interactions between each relationship category and age / gender controlling for the main covariates and other relationship categories. These analyses were conducted by entering the centered main effects, followed by the cross-product term based on these centered variables (e.g., age X ambivalent ties, Aiken & West, 1990). Secondary analyses were then performed that statistically controlled for other factors that have been previously linked to telomeres (e.g., perceived stress). Finally, follow-up analyses examined if any hypothesized links were due to more specific types of relationships (e.g., parents, friends).
Social Relationships and Telomere Length
As shown in Table 2, we replicated the well-known link between age and shorter telomeres (β=−.30, t(120)=3.46, p<.001). The only other covariate that was significantly related to telomere length was alcohol consumption which was also related to shorter telomeres (β=−.17, t(120)=2.0, p<.05). Importantly, consistent with our predictions, the number of ambivalent network ties was an independent predictor of shorter telomeres (β=−.20, t(120)=2.15, p<.05). Inconsistent with our predictions, the number of supportive ties did not significantly predict telomere length.2
Table 2.
Results from simultaneous regression analyses linking social ties to telomere length.
| Predictor | β | t-value | p-level |
|---|---|---|---|
| Age | −.30 | 3.46 | .001 |
| Gender | −.02 | 0.20 | .84 |
| BMI | −.16 | 1.77 | .08 |
| Statin use | −.02 | 0.22 | .83 |
| Diabetes | −.06 | 0.71 | .48 |
| Hypertension | .15 | 1.79 | .08 |
| Osteoporosis | −.01 | 0.09 | .93 |
| HRT | .06 | 0.68 | .50 |
| Tobacco use | .10 | 1.20 | .23 |
| Weekly exercise | −.15 | 1.73 | .09 |
| Weekly alcohol | −.17 | 2.00 | .048 |
| Indifferent Ties | −.16 | 1.80 | .07 |
| Supportive Ties | −.06 | 0.62 | .54 |
| Aversive Ties | −.02 | 0.21 | .83 |
| Ambivalent Ties | −.20 | 2.15 | .03 |
Moderational Analyses by Age and Gender
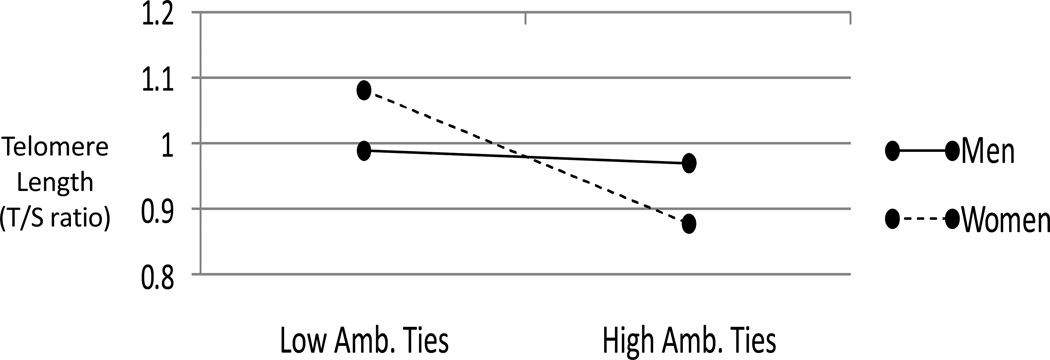
We next conducted analyses examining if the links between relationship categories and telomeres were moderated by age or gender. For instance, the effects of relationships on outcomes might be stronger in older adults who are already showing age-related declines in physiological function (Uchino, Birmingham, & Berg, 2010) or women who may be more sensitive to relationship-based processes (Beals & Rook, 2006; Kiecolt-Glaser & Newton, 2001). Age did not moderate any links between relationships and telomeres. However, a significant interaction emerged between the number of ambivalent ties and gender in predicting telomere length (β=−.17, t(119)=2.09, p<.05). Follow-up analyses investigated these links separately for men and women. As shown in Figure 2, consistent with prior work on gender and relationship processes, the number of ambivalent ties predicted shorter telomere length in women (p<.05) but not men (n.s.).
Figure 2.
Predicted telomere length for men and women one standard deviation above and below the mean for the number of ambivalent network ties.
Secondary Analyses
We next examined the unique links between ambivalent network ties and telomere length by considering other factors that have been associated with either telomeres or general health outcomes (Epel et al., 2004). First, we controlled for perceived stress levels as some studies have found links between greater perceived stress and shorter telomeres (Epel et al., 2004). In our sample, perceived stress was not a significant independent predictors of telomeres (p=.68), perhaps due to the lower levels of perceived stress as most prior studies have used chronically stressed individuals. Nevertheless, statistically controlling for perceived stress did not alter the link between the number of ambivalent ties and shorter telomeres (β=−.20, t(119)=2.16, p<.05), or the ambivalent ties X gender interaction (β=−.17, t(118)=2.08, p<.05). Finally, we tested whether the links between ambivalent ties and telomeres were independent of general measures of perceived support. In these analyses, perceived support did not independently predict telomeres and the significant links above were unchanged when considering general perceptions of support (p's<.05). In sum, these secondary analyses suggest that ambivalent ties were a relatively unique predictors of shorter telomere length.
Follow-up Analyses of Specific Relationship Domains
Finally, we examined if our main results were driven by overall network ambivalence or to specific relationship domains such as a significant other, parents, other family, friends, co-workers, or social acquaintances. Repeating our main analyses, we found a significant link between parental ambivalence and shorter telomeres (β=−.22, t(120)=2.66, p<.01). In addition, the number of ambivalent friends was significantly related to shorter telomeres (β=−.19, t(120)=2.20, p<.05). Similar analyses were conducted breaking down the ambivalent ties X gender interaction and two significant relationship domains emerged. These domains included interactions between gender and the number of ambivalent (a) social acquaintance (β=−.24, t(119)=2.86, p<.01) and (b) friends (β=−.22, t(119)=2.75, p<.01). In both cases, these interactions reflected a significant link between ambivalence and shorter telomeres in women. The results overall suggest that ambivalence in these specific domains were primary contributors to the links between ambivalent ties and cellular aging.
Discussion
In this study, we examined more general biological pathways that might link relationships that differ in their underlying positivity and negativity to broad-based physical health outcomes. Consistent with our predictions, we found the number of ambivalent ties to predict greater cellular aging even after considering a stringent set of control variables (e.g., age, health behaviors, medication use). Moreover, these links were moderated by gender as they were particularly evident in women. Follow-up analyses revealed that these associations were driven primarily by parents, friendships, and social acquaintances. Other studies have examined links between telomere length and psychosocial factors such as stress, marriage, and childhood adversities (Epel et al., 2004; Kiecolt-Glaser et al., 2011; Mainous et al., 2011). However, this is the first study to provide a more comprehensive analysis of links between positive and negative relationship functioning and cellular aging.
A first aim of this study was to examine more general biological processes that might influence links between relationships and mortality across diverse diseases. Telomeres provide a framework for examining such associations as shorter telomeres are strong predictors of mortality across different diseases including cardiovascular disease, cancer, and infectious diseases (Cawthon et al., 2003; Epel et al., 2008). For instance, in the population-based Bruneck Study of Italy, shorter telomeres predicted cancer incidence and mortality (Willeit et al., 2010a), as well as advanced cardiovascular disease risk (Willeit et al., 2010b). Importantly, our primary results were not moderated by age suggesting that the detrimental links with ambivalent ties was evident across both middle age and older adults. This could be significant because midlife appears to be a time of particular vulnerability for co-morbid chronic diseases (Booth, Kapral, Fung, Tu, 2006) and hence ambivalent ties may place individuals at-risk for the earlier development and exacerbation of health problems.
We also found evidence that the link between ambivalent ties and telomere length was moderated by gender. That is, the association between ambivalent ties and shorter telomeres was primarily evident in women. This is consistent with prior work suggesting that conflict in close relationships is linked to deleterious alterations in cardiovascular, endocrine, and immune function in women (Kiecolt-Glaser & Newton, 2001). The mechanisms responsible for such associations may be related to women's more complex relational schemas. For instance, women tend to be more affected by adverse interpersonal processes because their self-esteem maybe more contingent on interpersonal functioning (Cambron et al., 2009). Women may thus be more sensitive to or expend greater effort in response to relationship concerns (Smith et al., 2011). However, this is one of the first studies to show such a link when both relationship negativity and positivity are present (i.e., ambivalence) so future research will be needed to examine associated psychological pathways.
Follow-up analyses of specific relationship domains suggested that the ambivalent ties main effect was driven primarily by parents and friends, whereas the ambivalent ties X gender interaction was due primarily to social acquaintances and friends. Although more work on the specificity of these findings is needed, it is important for at least two reasons. First, it suggest that these associations cannot simply be explained by a general bias to view relationships in an ambivalent manner as one would expect such a mechanism to influence perceptions across relationship domains. Second, the one consistent finding was related to friendships, which is generally understudied in health psychology as most prior work in the area has focused on adolescent friendships (Wills, Gibbons, Gerrard, & Brody, 2000). A greater focus on middle-age and older adult friendships is needed because such relationships are voluntary (in contrast to obligatory familial ties) and hence have important properties in their own right (Adams & Ueno, 2006; Ueno & Adams, 2006). It is interesting to note that most of this work has focused on the benefits of voluntary ties with the assumption that such ties can be severed if they become non-rewarding (Berbier & Schulte, 2000; Thoits, 1992). Ambivalent ties represent a more complex friendship and thus have features that make them difficult to sever. For instance, ambivalent friendships are typically maintained due to a sense of commitment and other positive aspects that accompany them (Bushman & Holt-Lunstad, 2009). Although there may be some psychological and behavioral benefits to ambivalent friendships, the present study suggests these friendships may come with "hidden" physiological costs, especially for women. 3 Ambivalent social acquaintances also appear to have biological costs but might be maintained more by situational constraints (e.g., neighbor).
We also found more specific links between ambivalence towards parents and shorter telomeres. This association was not moderated by gender suggesting more general links across men and women. Very little work has been done examining the links between offsprings' ambivalence towards their parents and its association with health. Fingerman and colleagues (2008) examined mental health outcomes and found that parental ambivalence was related to lower psychological well-being (e.g., greater depression). Prior work is also suggestive of the interpersonal basis for parental ambivalence as it appear to be strongly rooted in relationship tensions such as unsolicited advice, personality differences, child rearing, and past relationship problems (Birditt, Miller, Fingerman, & Lefkowitz, 2009). Future integrative research will be needed to test more complex models that track the interpersonal processes that contribute to parental ambivalence and subsequent health outcomes.
Consistent with our predictions, we did not find the number of aversive or indifferent ties to be associated with telomeres, perhaps due to their lower frequency or depth of interaction. However, inconsistent with our predictions, we did not find the number of supportive ties to predict telomere length. In ancillary analyses, we tested the possibility that supportive ties might be particularly important in the context of relatively high stress (i.e., buffering model of support). However, the number of supportive ties X perceived stress interaction was not significant (p=.77). Cohen and Wills (1985) argued that one requirement of a strong test of the buffering hypothesis is a main effect of stress which we did not find in this study so one would need to be appropriately cautious about this null finding. It is also important to note that our assessment of supportive ties consists of the number of such relationship-specific network members. These relationship-specific measures appear related to other processes, that unless accounted for, might obscure associations with relevant health outcomes. For instance, receiving support in specific relationships is related to providing support; and providing support may have more consistent beneficial associations with health outcomes (Brown et al., 2003; Canevello & Crocker, in press). Future research aimed at separating out these different processes will help clarify aspects of supportive ties that are most strongly tied to physical health outcomes.
There are several limitations of this study. First, although these data suggest that ambivalent ties was detrimental primarily to women (particularly for friendships and social acquaintances), it is important to note that we assessed relationship positivity and negativity in a support-seeking context given its importance in prior work (Cohen, 2004). This may have limited our ability to show some associations in men because their relationships tend to be more activity-based while women's relationships tend to focus more on self-disclosure and support (Adams & Ueno, 2006). Future work will be needed to assess relationship positivity and negativity in other relational domains (e.g., activities) which might show stronger links for men or other relationships domains (e.g., co-workers). Second, although there is evidence linking telomeres to general disease morbidity and mortality, more research is needed on such links. Finally, longitudinal studies that follow changes in relationship processes and telomeres over time will be necessary to draw stronger inferences. These limitations notwithstanding, these data are the first to link relationship positivity and negativity to cellular aging. These data thus provide important mechanistic information on a more general biological mechanism by which aspects of one's social life may be linked to overall disease morbidity and mortality.
Acknowledgments
Support for this research was generously provided by grant number R21 AG029239 from the National Institute on Aging and Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea
An additional 23 participants were run but for various reasons did not have telomere data (e.g., blood draw or blood separation problems). Importantly, comparing these individuals with participants who had complete data on age (the only demographic factor related to telomere length) revealed no significant group differences (mean age 60.1 vs. 60.6, p>.70).
We also repeated all analyses by examining a less restrictive model which only controlled for age and gender as the health assessment contained missing data for 18 individuals. Importantly, all study results were the same using the less restrictive model with the larger sample.
We also conducted ancillary analyses on measures of perceived stress and depression (Radloff, 1977) controlling for age and gender and considering all relationship types (i.e., ambivalent, supportive, aversive, indifferent) in simultaneous regression analyses. Consistent with the possibility of "hidden' physiological costs, the main effect for the number of ambivalent ties and the ambivalent ties X gender interaction were not significant in any of these analyses involving psychological outcomes (p's>.25).
Contributor Information
Bert N. Uchino, Department of Psychology and Health Psychology Program, University of Utah
Richard M. Cawthon, Department of Human Genetics, University of Utah
Timothy W. Smith, Department of Psychology and Health Psychology Program, University of Utah
Kathleen C. Light, Department of Anesthesiology, University of Utah
Justin McKenzie, Department of Psychology, University of Utah.
McKenzie Carlisle, Department of Psychology, University of Utah.
Heather Gunn, Department of Psychology, University of Utah.
Wendy Birmingham, Department of Psychology, University of Utah.
Kimberly Bowen, Department of Psychology, University of Utah.
References
- Abbas AK, Lichtman AH. Cellular and molecular immunology. Philadelphia, PA: Saunders; 2003. [Google Scholar]
- Adams RG, Ueno K. Middle-Aged and Older Adult Men’s Friendships. In: Bedford VH, Formaniak B, editors. Men in relationships: A new look from a life course perspective. New York: Springer; 2006. pp. 103–124. [Google Scholar]
- Beals K, Rook KS. Gender differences in negative social exchanges: Frequency, reactions, and impact. In: Bedford VH, Turner BF, editors. Men in relationships: A new look from a life course perspective. New York: Springer; 2006. pp. 197–217. [Google Scholar]
- Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Social Science and Medicine. 2000;51:843–857. doi: 10.1016/s0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Berbrier M, Schulte A. Binding and nonbinding integration: The relational costs and rewards of social ties on mental health. Research in Community and Mental Health. 2000;11:3–27. [Google Scholar]
- Birditt KS, Miller LM, Fingerman KL, Lefkowitz ES. Tensions in the parents and adult child relationship: Links to solidarity and ambivalence. Psychology and Aging. 2009;24:287–295. doi: 10.1037/a0015196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000 Nov;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: A population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- Brooks K, Dunkel Schetter C. Social negativity and health. Social and Personality Psychology Compass. (in press) [Google Scholar]
- Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Nilesh JS. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- Brown SL, Nesse RM, Vinokur AD, Smith DM. Providing social support may be more beneficial than receiving it: Results from a prospective study of mortality. Psychological Science. 2003;14:320–327. doi: 10.1111/1467-9280.14461. [DOI] [PubMed] [Google Scholar]
- Bushman BB, Holt-Lunstad J. Understanding social relationship maintenance among friends: Why we don’t end those frustrating friendships. Journal of Social and Clinical Psychology. 2009;28:749–778. [Google Scholar]
- Cambron JM, Acitelli LK, Pettit JW. Explaining gender differences in depression: An interpersonal contingent self-esteem perspective. Sex Roles. 2009;61:751–761. [Google Scholar]
- Campo RA, Uchino BN, Holt-Lunstad J, Vaughn AA, Reblin M, Smith TW. The assessment of positivity and negativity in social networks: The reliability and validity of the Social Relationships Index. Journal of Community Psychology. 2009;37:471–486. [Google Scholar]
- Canevello A, Crocker J. Social goals and its implications for health. Social and Personality Compass. (in press) [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Research. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003 Feb;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chan SRWL, Blackburn EH. Telomeres and telomerase. Philosophical Transactions of the Royal Society Lond. B. 2003;359:109–121. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity at leisure time and leukocyte telomere length. Archives of Internal Medicine. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. American Psychologist. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cohen S, Mermelstein RJ, Kamarck T, Hoberman HM. Sarason IG, Sarason B. Social support: Theory, research, and application. The Hague, Holland: Martinus-Niijhoff; 1985. Measuring the functional components of social support; pp. 73–94. [Google Scholar]
- Cohen S, Williamson GM. Perceived stress in a probability sample in the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. London: Sage; 1987. pp. 31–67. [Google Scholar]
- Cosley BJ, McCoy SK, Saslow LR, Epel ES. Is compassion for others stress buffering? Consequences of compassion and social support for physiological reactivity to stress. Journal of Experimental Social Psychology. 2010;46:816–823. [Google Scholar]
- Dahse R, Fiedler W, Ernst G. Telomeres and telomerase: Biological and clinical importance. Clinical Chemistry. 1997;43:708–714. [PubMed] [Google Scholar]
- De Vogli R, Chandola T, Marmot MG. Negative aspects of close relationships and heart disease. Archives of Internal Medicine. 2007;167:1951–1957. doi: 10.1001/archinte.167.18.1951. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. PNAS. 2004 Dec;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging. 2008;4:81–88. doi: 10.18632/aging.100007. PMCID: PMC2830080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart CK, Taylor CB, Kraemer HC, Agras WS. High blood pressure and marital discord: Not being nasty matters more than being nice. Health Psychology. 1991;10:155–163. doi: 10.1037//0278-6133.10.3.155. [DOI] [PubMed] [Google Scholar]
- Fingerman KL, Hay EL, Birditt KS. The best of ties, the worst of ties: Close, problematic, and ambivalent social relationships. Journal of Marriage and Family. 2004 Aug;66:792–808. [Google Scholar]
- Fingerman K, Pitzer L, Lefkowitz E, Birditt K, Mroczek D. Ambivalent relationship qualities between adults and their parents: Implications for the well-being of both parties. Journals of Gerontology: Psychological Sciences. 2008;63:P362–PP371. doi: 10.1093/geronb/63.6.p362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. PNAS. 1998 May;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin W, Pieper C, Levy R, Pickering TG. Social support in social interaction: A moderator of cardiovascular reactivity. Psychosomatic Medicine. 1992;54:324–336. doi: 10.1097/00006842-199205000-00008. [DOI] [PubMed] [Google Scholar]
- Graham JE, Glaser R, Loving TJ, Malarkey WB, Stowell JR, Kiecolt-Glaser JK. Cognitive word use during marital conflict attenuates increases in inflammatory cytokines. Health Psychology. 2009;28:621–630. doi: 10.1037/a0015208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Layton B. Social relationships and mortality: A meta-analysis. PLoS Medicine. 2010;7:1–20. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Uchino BN, Smith TW, Cerny CB, Nealey-Moore JB. Social relationships and ambulatory blood pressure: Structural and qualitative predictors of cardiovascular function during everyday social interactions. Health Psychology. 2003;22:388–397. doi: 10.1037/0278-6133.22.4.388. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Uchino BN, Smith TW, Hicks A. On the importance of relationship quality: The impact of ambivalence in friendships on cardiovascular functioning. Annals of Behavioral Medicine. 2007;33:1–12. doi: 10.1007/BF02879910. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Iwama H, Ohyashiki K, Ohyashiki JH, Hayashi S, Yahata N, Ando K, Shay JW. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Human Genetics. 1997;102:397–402. doi: 10.1007/s004390050711. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic Medicine. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Stress and immunity: Age enhances the risk. Current Directions in Psychological Science. 2001;10:18–21. [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Lee D, Im J, Kim J, Lee H, Shim J. Effect of Long-Term Hormone Therapy on Telomere Length in Postmenopausal Women. Yonsei Medical Journal. 2005;46:471–479. doi: 10.3349/ymj.2005.46.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainous AG, Everett CJ, Diaz VA, Baker R, Mangino M, Codd V, Samani NJ. Leukocyte telomere length and marital status among middle-aged adults. Age and Ageing. 2011;40:73–78. doi: 10.1093/ageing/afq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A, Ferrara SD, Montisci M, Baccarelli A. Shortened telomeres in individuals with abuse in alcohol consumption. International Journal of Cancer. 2011;129:983–992. doi: 10.1002/ijc.25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering TG, Shimbo D, Haas D. Ambulatory blood pressure monitoring. New England Journal of Medicine. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- Pierce GR, Sarason IG, Sarason BR. General and relationships-specific perceptions of social support: Are two constructs better than one? Journal of Personality and Social Psychology. 1991;61:1028–1039. doi: 10.1037//0022-3514.61.6.1028. [DOI] [PubMed] [Google Scholar]
- Reblin M, Uchino BN, Smith TW. Provider and recipient factors that may moderate the effectiveness of received support: Examining the effects of relationship quality and expectations for support on behavioral and cardiovascular reactions. Journal of Behavioral Medicine. 2010;33:423–431. doi: 10.1007/s10865-010-9270-z. [DOI] [PubMed] [Google Scholar]
- Salpea KD, Humpries SE. Telomere length in atherosclerosis and diabetes. Atherosclerosis. 2010;209:35–38. doi: 10.1016/j.atherosclerosis.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29:283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- Sarason IG, Sarason BR, Shearin EN. Social support as an individual difference variable: Its stability, origins, and relational aspects. Journal of Personality and Social Psychology. 1986;50:845–855. [Google Scholar]
- Saretzki G, Von Zglinicki T. Replicative aging, telomeres, and oxidative stress. Annals New York Academy of Sciences. 2002;959:24–29. doi: 10.1111/j.1749-6632.2002.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Hill DR. Gender differences in social support and health. Health Psychology. 1991;10:102–111. doi: 10.1037//0278-6133.10.2.102. [DOI] [PubMed] [Google Scholar]
- Smith TW, Cribbet MR, Nealey-Moore JB, Uchino BN, Williams PG, MacKenzie J, Thayer JF. Matters of the variable heart: Respiratory sinus arrhythmia response to marital interaction and associations with marital quality. Journal of Personality and Social Psychology. 2011;100:103–119. doi: 10.1037/a0021136. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Lundwall K, Cropley M. Gender, family structure and cardiovascular activity during the working day and evening. Social Science & Medicine. 2000;50:531–539. doi: 10.1016/s0277-9536(99)00324-x. [DOI] [PubMed] [Google Scholar]
- Thoits PA. Identity structures and psychological well-being: Gender and marital status comparisons. Social Psychology Quarterly. 1992;55:236–256. [Google Scholar]
- Uchino BN. Social support and physical health: Understanding the health consequences of our relationships. New Haven, CT: Yale University Press; 2004. [Google Scholar]
- Uchino BN. What a lifespan perspective might tell us about why distinct measures of support have differential links to physical health. Journal of Social and Personal Relationships. 2009;26:53–62. doi: 10.1177/0265407509105521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Birmingham W, Berg CA. Are older adults less or more physiologically reactive?: A meta-Analysis of age-related differences in cardiovascular reactivity to laboratory tasks. Journal of Gerontology: Psychological Sciences. 2010;65B:154–162. doi: 10.1093/geronb/gbp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Holt-Lunstad J, Uno D, Flinders JB. Heterogeneity in the social networks of young and older adults: Prediction of mental health and cardiovascular reactivity during acute stress. Journal of Behavioral Medicine. 2001;24:361–382. doi: 10.1023/a:1010634902498. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Holt-Lunstad J, Uno D, Campo R, Reblin M. The social neuroscience of relationships: An examination of health relevant pathways. In: Harmon-Jones E, Winkielman P, editors. Social neuroscience: Integrating Biological and Psychological Explanations of Social Behavior. New York: Guilford; 2007. pp. 474–492. [Google Scholar]
- Ueno K, Adams RG. Adult friendship: A decade review. In: Noller P, Feeney J, editors. Close relationships: Functions, forms, and processes. New York: Psychology Press; 2006. pp. 151–169. [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Richards JB, Gardner JP, Swaminathan R, Kimura M, Xiaobin L, Aviv A, Spector TD. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporosis International. 2007;18:1203–1210. doi: 10.1007/s00198-007-0357-5. [DOI] [PubMed] [Google Scholar]
- Willeit P, J Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, Gasperi A, Weger S, Oberhollenzer F, Reindl M, Kronenberg F, Kiechl S. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arteriosclerosis, Thrombosis, & Vascular Biology. 2010;30:1649–1656. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- Willeit PJ, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. Journal of the American Medical Association. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- Wills TA, Gibbons FX, Gerrard M, Brody GH. Protection and vulnerability processes relevant for early onset of substance use: A test among African American children. Health Psychology. 2000;19:253–263. doi: 10.1037//0278-6133.19.3.253. [DOI] [PubMed] [Google Scholar]