Abstract
Women are twice as likely as men to suffer from stress-related psychiatric disorders, like unipolar depression and post-traumatic stress disorder. Although the underlying neural mechanisms are not well characterized, the pivotal role of stress in the onset and severity of these diseases has led to the idea that sex differences in stress responses account for this sex bias. Corticotropin-releasing factor (CRF) orchestrates stress responses by acting both as a neurohormone to initiate the hypothalamic-pituitary-adrenal (HPA) axis and as a neuromodulator in the brain. One target of CRF modulation is the locus coeruelus (LC)-norepinephrine system, which coordinates arousal components of the stress response. Hypersecretion of CRF and dysregulation of targets downstream from CRF, such as the HPA axis and LC-norepinephrine system, are characteristic features of many stress-related psychiatric diseases, suggesting a causal role for CRF and its targets in the development of these disorders. This review will describe sex differences in CRF and the LC-norepinephrine system that can increase stress-sensitivity in females, making them vulnerable to stress-related disorders. Evidence for gonadal hormone regulation of hypothalamic CRF is discussed as an effect that can lead to increased HPA axis activity in females. Sex differences in the structure of LC neurons that create the potential for hyperarousal in response to emotional stimuli are described. Finally, sex differences at the molecular level of the CRF1 receptor that make the LC-norepinephrine system more reactive in females are reviewed. The implications of these sex differences for the treatment of stress-related psychiatric disorders also will be discussed.
Mood and anxiety disorders are debilitating mental illnesses that affect 20% population and are the leading cause of disability in the United States for younger adults (Mark et al. 2007; Bromet et al. 2011). They particularly present a challenge to women’s health, because women are twice as likely to suffer from these disorders as men. Stress is a common factor in these disorders. Stressor exposure is associated with the onset and severity of the mood disorder, unipolar depression, and several anxiety disorders. In the case of the post-traumatic stress disorder (PTSD), the disease is precipitated by a traumatic event. There is some evidence to suggest that the sex bias in these diseases is due different types of stressors experienced by men vs. women. For example, men experience more physical attacks and serious accidents, while women are more likely to experience interpersonal assaults and sexual abuse (Breslau et al. 1998; Breslau 2002; Perkonigg et al. 2000; Kessler et al. 1995; Olff et al. 2007; Tolin and Foa 2006). Although the type of stressor exposure likely plays a role the sex bias, there is also mounting evidence to suggest that women respond differently to stressful events than men. For example, when women and men are confronted with similar stressful events (e.g., car accidents) women are more likely to develop psychopathology (Iteke et al. 2011). Stressful life events also are more highly correlated with symptoms of anxiety and depression in women than men (Sandanger et al. 2004). Importantly, the sex bias in PTSD remains after adjusting for the type of trauma, preexisting psychiatric disorders, and sex differences in reporting (Breslau 2009; Tolin and Foa 2006; Breslau et al. 1999). Together these data suggest that the increased incidence of stress-related psychiatric diseases in women is biologically determined.
Corticotropin releasing factor (CRF) is a 41amino acid-containing neuropeptide that orchestrates the stress response by acting at the level of the pituitary to initiate the hypothalamic-pituitary-adrenal (HPA) axis response to stress, as well as centrally to modulate limbic and brain monoamine systems that are important in autonomic and behavioral components of the stress response (Owens and Nemeroff 1991; Valentino and Van Bockstaele 2008; Valentino and Commons 2005). Dysregulation of CRF has been linked to the pathophysiology of mood and anxiety disorders (Bremner et al. 1997; Nemeroff 1996). This review will focus on mechanisms by which sex differences in CRF function can lead to increased stress sensitivity in females. First, we will discuss how sex differences in CRF expression can lead to dysregulation of the HPA axis response to stress, an effect linked to certain types of depression and anxiety disorders. Second, sex differences in the morphology of locus coeruleus (LC) cells will be discussed as a potential determinant of emotional arousal. Finally, we describe sex differences in the function of the CRF1 receptors in the LC-arousal system that can lead to hyperarousal, a core symptom of many stress-related diseases in females.
CRF dysregulation in stress-related psychiatric disorders
CRF is a key regulator of the HPA axis response to stress. Stressors initiate CRF release from neurons in the paraventricular nucleus of the hypothalamus (PVN), which is transported via the portal blood vessel system to the pituitary where it stimulates pituitary corticotropes to secrete adrenocorticotropic hormone (ACTH) into the circulatory system. Circulating ACTH then initiates glucocorticoid release from the cortex of the adrenal glands. Glucocorticoids in turn act at the level of the pituitary and hypothalamus to terminate the stress response acting as negative feedback system. In addition to its role in the initiation of the HPA axis, CRF also acts centrally to regulate behavioral and autonomic responses to stress. This is thought to involve CRF-producing neurons in limbic regions such as the bed nucleus of the stria terminalis and central nucleus of the amygdala, regions which are known for mediating anxiety and emotional responses (Swanson et al. 1983; Walker et al. 2003). During stress, release of limbic CRF can modulate monoamine systems that have been implicated in mood and cognition (Rodaros et al. 2007; Wanat et al. 2008; Lodge and Grace 2005; Valentino and Van Bockstaele 2008; Valentino and Commons 2005). Although activation of both the HPA axis and central monoaminergic systems by CRF during acute stress is adaptive, the inappropriate or persistent activation of these systems can have adverse consequences leading to psychopathology.
There is evidence for hypersecretion or overactivity of CRF in patients suffering from stress-related disorders. Increased concentrations of CRF in the cerebrospinal fluid have been reported in depression and PTSD (Nemeroff et al. 1984; Baker et al. 1999; Bremner et al. 1997; Sautter et al. 2003; Banki et al. 1992). Effective treatments for depression, such as antidepressant medication and electroconvulsive shock therapy, decrease cerebrospinal fluid CRF levels, supporting the idea that increased CRF levels are responsible for depressive symptoms (Heuser et al. 1998; De Bellis et al. 1993; Nemeroff et al. 1991). Centrally, changes in CRF and CRF1 receptor expression have been observed in the brains of patients with stress-related psychiatric disorders. Specifically, depressed subjects have a greater number CRF expressing neurons in the PVN, and higher PVN levels of CRF and CRF1 receptor mRNA (Raadsheer et al. 1994; Wang et al. 2008). CRF expression is increased in the LC and dorsal raphe monoaminergic nuclei of depressed subjects (Austin et al. 2003; Bissette et al. 2003). High levels of CRF also are found in the cortex of suicide victims, and this increase is accompanied by decreased CRF1 receptor expression, which may be a compensatory response to cortical CRF hypersecretion (Merali et al. 2004). Multiple genomic studies have identified single nucleotide polymorphisms on the genes for CRF and the CRF1 receptor that are linked to development and/or severity of depression, PTSD, and panic disorder (Smoller et al. 2003; Amstadter et al. 2011; Liu et al. 2006). Treatments for stress-related mental illnesses also support a central role for CRF in the etiology of these diseases. Anxiolytic treatments alter CRF expression in several brain regions, suggesting that this may be an important mechanism of action for these drugs (Owens et al. 1989; Owens et al. 1991). Moreover in a clinical trial, depressed patients treated with a CRF1 receptor antagonist showed a reduction in symptoms of depression and anxiety (Zobel et al. 2000). Together these findings imply a causal role for CRF in the development of depression and certain anxiety disorders.
Sex differences in hypothalamic CRF function
In order to understand the biological basis for sex differences in stress-related psychiatric disorders, researchers have investigated sex differences in stress response systems. One of the most widely reported findings is that female rodents have higher levels of HPA axis hormones than males. Specifically, basal (i.e., unstressed) levels of the glucocorticoid, corticosterone, are elevated in female compared to male rats (Kitay 1961; Viau et al. 2005; Weinstock et al. 1998). There is a circadian rhythm to glucocorticoid release, and although both male and female rats display this rhythm, females have higher peak corticosterone levels and greater average corticosterone concentrations over a 24 h period than males (Critchlow et al. 1963). In response to stress, female rodents release more ACTH and corticosterone than males and their stress hormone levels remain elevated for a longer period of time (Livezey et al. 1985; Handa et al. 1994; Kitay 1961; Rivier 1999; Viau et al. 2005; Weinstock et al. 1998; Iwasaki-Sekino et al. 2009; Heinsbroek et al. 1991; Seale et al. 2004). In contrast to the reliable findings in rodents, reports of sex differences in ACTH and cortisol levels in humans are equivocal. Some studies find elevated stress-induced levels of these hormones in women, while others find the opposite (i.e., men are higher) or no difference (Uhart et al. 2006; Kirschbaum et al. 1999; Kirschbaum et al. 1995; Seeman et al. 2001; Kirschbaum et al. 1992; Kudielka and Kirschbaum 2005; Gallucci et al. 1993; Heuser et al. 1994; Friedmann and Kindermann 1989; Collins and Frankenhaeuser 1978). These discrepant findings are attributed to differences in the type and duration of stressor exposure, and other demographic factors such as the age of the subjects and their disease status (Kudielka and Kirschbaum 2005; Seeman et al. 2001; Paris et al. 2010).
Despite the discrepancies, the higher levels of HPA axis hormones found in female rodents and in some human studies could indicate greater activation of the HPA axis by CRF in females. In support of this, some studies report that female rodents have increased CRF expression in the PVN under basal conditions, particularly when estrogen levels are high (Viau et al. 2005; Iwasaki-Sekino et al. 2009; Duncko et al. 2001; but see Sterrenburg et al. 2012; Sterrenburg et al. 2011; Desbonnet et al. 2008). Certain stressors, such as early life stress, footshock, and psychological stress, increase CRF expression in the PVN selectively in female rats (Desbonnet et al. 2008; Iwasaki-Sekino et al. 2009). In contrast, restraint stress and chronic mild stress increase CRF mRNA in the PVN to a larger degree in males, however, this increase may not have been observed in females in some cases because of their high basal CRF expression (Sterrenburg et al. 2012; Sterrenburg et al. 2011; Duncko et al. 2001; Viau et al. 2005). Recently, sex-specific epigenetic modifications of the CRF gene have been identified following stress, underscoring the important contribution of epigenetic factors to differences in the CRF expression (Sterrenburg et al. 2012; Sterrenburg et al. 2011). Taken together these studies implicate greater CRF release from the PVN of females in their enhanced endocrine responses to stress.
Demographic data demonstrate that the higher rate of depression in women emerges after puberty and then decreases after menopause, suggesting a role for ovarian hormones (Kessler 2003). Because of this link, many studies have investigated gonadal hormone regulation of CRF expression. Gonadal steroid hormone receptors co-localize with CRF in neurons in PVN (Bao et al. 2005; Bao et al. 2006). The promoter region of the CRF gene contains both estrogen and androgen response elements, providing a means by which gonadal hormones can regulate CRF gene expression (Vamvakopoulos and Chrousos 1993; Bao et al. 2006). In rodents, estrogen stimulates CRF expression in the PVN, while androgens suppress this expression (Bohler et al. 1990; Lund et al. 2004; Haas and George 1988; Bingaman et al. 1994; Figueiredo et al. 2007). Although similar studies in humans are limited, Bao and Swaab (2007) found more hypothalamic CRF neurons in men with conditions that increased their estrogen levels above those of healthy male controls (i.e., estrogen therapy or estrogen producing tumors) (Bao and Swaab 2007). However, this same study failed to find the predicted decrease in CRF expressing neurons in women following menopause. This underscores the complexity of the interaction between sex hormones and CRF in humans.
Taken together, these studies suggest that increased hypothalamic levels of CRF in females, particularly during times of high estrogen secretion, can lead to sex differences in HPA axis hormone secretion and contribute to the sex bias in stress-related psychiatric disease. Sex differences in the HPA axis can also be attributed to other non-CRF related factors, such as increased glucocorticoid metabolism in males or impaired glucocorticoid negative feedback in females (Weiser and Handa 2009; Redei et al. 1994; Herbst et al. 1960; Troop 1959). Nonetheless, evidence for increased hypothalamic CRF expression in females certainly warrants further investigation, particularly in human studies, as a mechanism underlying female vulnerability to mood and anxiety disorders.
The LC-norepinephrine system and arousal
A core symptom of several stress-related psychiatric disorders is hyperarousal. Patients suffering from PTSD experience symptoms linked to hyperarousal, including hypervigilance (e.g., difficulty concentrating, sleeping, controlling anger, etc.) and re-experiencing (i.e., the replaying of the traumatic event) (O’Donnell et al. 2004; Southwick et al. 1999). Hyperarousal also is associated with ruminations that characterize melancholic depression (Gold et al. 1996; Gold and Chrousos 2002, 1999). The brain circuitry thought to mediate these arousal features is the LC-norepinephrine system (O’Donnell et al. 2004; Southwick et al. 1999; Gold et al. 1996; Gold and Chrousos 2002; Koob 1999).
The LC is a compact nucleus of norepinephrine-containing neurons located in the pons. A divergent efferent projection system arises from these neurons allowing for norepinephrine release throughout the neuraxis (Swanson and Hartman 1975). The LC serves as the major source of norepinephrine for the forebrain and the sole source of norepinephrine for the cortex and hippocampus (Abercrombie et al. 1988; Aston-Jones et al. 1995).
The physiological characteristics of LC neurons have led to predictions about the function of this system in behavior and in the response to stress. LC neurons discharge spontaneously (tonic) and their firing rate is positively correlated with electroencephalic (EEG) and behavioral indices of arousal (Aston-Jones and Bloom 1981a; Berridge and Foote 1991; Berridge et al. 1993). Relatively low firing rates are associated with slow wave sleep or quiet waking, and higher discharge rates are associated with more active waking (Aston-Jones and Bloom 1981a; Foote et al. 1980). A characteristic feature of LC neurons that has suggested a role in vigilance is their activation by multimodal sensory stimuli. This results in a burst of synchronous discharge (phasic) that precedes orientation to the stimulus (Aston-Jones and Bloom 1981b; Foote et al. 1980). These observations have suggested a role of LC neurons in mediating arousal and facilitating attention towards discrete stimuli. More recent studies are refining our ideas about the role of the LC-norepinephrine system in behavior. In an elegant series of studies in which LC neurons were recorded in monkeys performing an oddball discrimination task, Aston-Jones and colleagues found that different modes of LC discharge are associated with distinct behaviors (Clayton et al. 2004; Aston-Jones and Cohen 2005). In the phasic discharge mode, LC neurons fire synchronously and are responsive to sensory stimuli. This mode of firing is associated with focused attention and staying on-task. In contrast, when LC neurons fire at a high spontaneous or tonic rate, they are less synchronous and less responsive to sensory stimuli. This mode of firing is associated with labile attention, behavioral flexibility and going off task, but also with poor performance on tasks requiring focused attention. These investigators suggested the compelling hypothesis that the LC is involved in decision processes and whether to stay on task or scan the environment in order to shift ongoing behavior to one that might be more adaptive.
The LC, CRF and stress
The LC-norepinephrine system is often activated in parallel with the HPA axis in response to stressors, and this activation is mediated at least in part by CRF (Aston-Jones et al. 1996; Page et al. 1993; Valentino and Van Bockstaele 2005). CRF axon terminals synaptically target norepinephrine neurons of the LC (Valentino and Van Bockstaele 2008; Van Bockstaele et al. 1996, 1998; Van Bockstaele et al. 1999). CRF microinfusion into the LC increases neuronal discharge rate and this is temporally correlated to an activation of cortical electroencephalographic activity (EEG), indicative of arousal (Curtis et al. 1997). That this is a direct effect of CRF on LC neurons is supported by the findings that CRF has similar effects in LC slice preparations even in the presence of tetrodotoxin (Jedema and Grace 2004). At the same time that CRF increases tonic LC discharge, it attenuates phasic sensory-evoked discharge, thereby biasing LC activity towards a high tonic–low phasic state that would favor scanning the environment and promote behavioral flexibility (Valentino and Foote 1988; Valentino and Foote 1987). Consistent with this, moderate doses of CRF administered directly into the LC increased cognitive flexibility in rodents performing the attentional set shifting task (Snyder et al. 2011). These effects of CRF to increase arousal and behavioral flexibility would be adaptive in a dynamic environment, as in acute stress.
Stressors mimic the effects of CRF on LC discharge and these can be prevented by local administration of CRF. For example, hypotensive stress increases LC tonic discharge rate, while inhibiting sensory-evoked phasic discharge, similar to the effects of CRF (Valentino and Wehby 1988; Curtis et al. 1997). Moreover, blocking CRF1 receptors within the LC prevents these electrophysiological changes (Valentino and Wehby 1988; Valentino et al. 1991; Curtis et al. 1994). Similarly, non-noxious visceral stimuli, such as colon distention, increase tonic LC discharge via activation of CRF receptors, supporting the idea that stress-induced electrophysiological changes in LC activity are due to CRF (Lechner et al. 1997; Kosoyan et al. 2005).
Stress also alters downstream endpoints of LC activation through CRF-dependent mechanisms. For example, LC activation by hypotensive stress is temporally correlated to cortical EEG activation and this is prevented by intra-LC administration of a CRF antagonist (Page et al. 1993). This particular finding provided evidence that the arousal component of the stress response is mediated by local CRF actions within the LC. In addition to EEG endpoints, norepinephrine release and c-fos expression in forebrain targets are similarly increased by both stress and intra-LC infusions of CRF (Page and Abercrombie 1999; Rassnick et al. 1998; Hajos-Korcsok et al. 2003). Finally, blocking CRF receptors in the LC prevents the effects of stress on several endpoints of LC activation, including expression of tyrosine hydroxylase (TH; the norepeniphrine synthetic enzyme) and release of cortical norepinephrine (Melia and Duman 1991; Kawahara et al. 2000; Smagin et al. 1997).
The findings reviewed above support the concept that CRF released during stress acts as a neuromodulator to bias LC activity towards a high tonic mode of discharge. This in turn will increase arousal, decrease focused attention and promote behavioral flexibility, effects that would be adaptive in responding to an acute challenge. In this way, the LC plays an important role in mediating a cognitive limb of the stress response. However, if this system is engaged inappropriately or if the response continues long after the stress is terminated, these effects would be maladaptive.
CRF communication with the LC
The nuclear core of the LC where norepinephrine-containing cell bodies are clustered is only moderately innervated by CRF-containing fibers, which derive from Barrington’s nucleus, the PVN, and the nucleus paragigantocellularis (Reyes et al. 2005; Valentino et al. 1992; Valentino et al. 1996). In contrast, a dense CRF terminal field lies within the dorsolateral peri-coerulear region (peri-LC) into which LC dendrites extend for several hundred microns (Shipley et al. 1996; Valentino et al. 1992). CRF projections from the PVN, as well as limbic regions, including the central nucleus of the amygdala and the bed nucleus of the stria terminalis, preferentially innervate the dendrites that extend into this peri-LC zone (Van Bockstaele et al. 1998:Van Bockstaele, 1999 #442). Because the limbic system is critical for coordinating emotional responses to stress, this limbic input into the peri-LC may be an anatomical substrate for an arousal response to emotional events. This contrasts with CRF regulation of the LC core which derives more from autonomically-related nuclei, including Barrington’s nucleus and the nucleus paragigantocellularis (Valentino et al., 1992; 1996).
Ultrastructural studies revealed that CRF-immunoreactive axon terminals form synaptic specializations with LC dendrites in both the core and peri-LC regions (Van Bockstaele et al. 1996). The majority of these synapses are asymmetric, the type associated with excitatory neurotransmission (Van Bockstaele et al. 1996). Thus, it is not surprising that many CRF-containing axon terminals co-localize glutamate, although co-localization with enkephalin and GABA also is occasionally observed (Tjoumakaris et al. 2003; Valentino and Van Bockstaele 2001). Given the evidence for co-localization of CRF and GABA in the extended amygdala, it is possible that CRF-GABA terminals arise from there, whereas the CRF-glutamate axon terminals arise from other CRF-containing neurons (Day et al. 1999). Some CRF axon terminals are apposed to unlabeled terminals that form synaptic specializations with LC dendrites. This may be the structural basis for indirect presynaptic modulation of LC activity (Van Bockstaele et al. 1996).
Sexual dimorphism in LC structure
Given the role of the LC in mediating the arousal component of the stress response and that hyperarousal is a core symptom of stress-related disorders, sexual dimorphism of LC neurons could contribute to sex differences in these diseases. Some studies have found that LC neurons are more numerous in female compared to male rats (Guillamon et al. 1988; Garcia-Falgueras et al. 2006; Garcia-Falgueras et al. 2005). However, this did not generalize across rat strains (Garcia-Falgueras et al. 2006; Garcia-Falgueras et al. 2005). Convergent findings from our laboratory suggest that LC dendrites are more extensive, more complex and may process more information in female rats compared to males (Bangasser et al. 2011). An examination of TH immunoreactivity revealed that LC dendrites of female rats were denser and extended further into the peri-LC region than those of male rats (Bangasser et al. 2011). Morphological analysis of individual juxtacellularly labeled LC neurons confirmed that LC dendritic trees of females vs. males were longer and had more branch points and ends (Bangasser et al. 2011). Furthermore, Sholl analysis revealed that LC dendrites of females had a more complex pattern of branching than dendrites of males. Immunoreactive labeling for synaptophysin, a synaptic vesicle protein, was also greater in females, particularly in the dorsolateral peri-LC where limbic afferents terminate, indicating that LC dendrites of females receive more input (Bangasser et al. 2011).
Sex differences in LC dendritic structure are particularly relevant to stress because of the topographical organization of LC afferents within the LC and peri-LC region described above. The enhanced extension of LC dendritic trees into the peri-LC and their greater complexity increases their probability of contacting limbic afferents that relay emotion-related information. Importantly, contacts between CRF terminals and LC dendrites occur predominantly in the peri-LC, so the receipt of CRF inputs would be greater within this zone. Enhanced synaptophysin expression also suggests that the LC of females is receives more inputs from afferent structures. Thus, the LC of female rodents appears to be structured to process more information, particularly emotion-related information that may be carried in part by CRF. This structure would support a greater arousal response to emotional stimuli. Consistent with this idea, imaging has shown that noxious stimuli produce a greater activation of an emotional-arousal circuit that is comprised of the amygdala and LC in women compared to men (Labus et al. 2008).
Sex differences in CRF1 receptor signaling
In addition to sex differences in LC structure, there are also functional sex differences in LC neuronal responses to CRF. A comparison of LC spontaneous and sensory-evoked discharge rates in male and female rats revealed no difference (Curtis et al., 2006). However, LC activation by hypotensive challenge, which activates LC neurons through CRF, was greater in female rats. Notably, this effect was unrelated to adult hormonal status of either males or females. Sex differences in LC responses to hypotension were attributed to differences in postsynaptic sensitivity to CRF because the CRF dose-response curve for LC activation was shifted to the left in females compared to males (Curtis et al. 2006).
A history of prior stress is an important determinant of LC responses to CRF. For example, prior CRF administration desensitizes LC neurons to subsequent CRF administration, and prior exposure to auditory or hypotensive stress produce cross-desensitization to a high dose of CRF (Conti and Foote 1995, 1996; Conti et al. 1997). In male rats, prior footshock or swim stress produce a complex change in the CRF dose-response curve for LC activation such that the linear portion of the curve is shifted to the left (indicative of sensitization) and the maximum is decreased (Curtis et al. 1995; Curtis et al. 1999; Curtis et al. 2006). Interestingly, this effect of prior stress is seen only in males. Thus, prior swim stress had little effect on the CRF dose response curve in females, perhaps because they were already at ceiling levels for CRF activation (Curtis et al. 2006).
The increased sensitivity of female LC neurons to CRF is a postsynaptic effect seen as a parallel shift to the left in the CRF dose-response curve in females compared to males and therefore is likely due to increased CRF receptor signaling. The complex shift in the CRF dose response curve seen only in males following stress, also suggested sex differences at the level of CRF receptor signaling. CRF activation of LC neurons is mediated by CRF1 receptors (Jedema and Grace 2004; Schulz and Lehnert 1996). These are G-protein coupled receptor (GPCR) that preferentially signal through the cyclic adenosine monophosphate (cAMP) pathway by coupling to the GTP-binding protein Gs, although they can couple to other G-proteins and activate other signaling cascades (Chen et al. 1986; Battaglia et al. 1987; De Souza 1995; Grammatopoulos et al. 2001). The CRF-induced increase in LC neuronal firing requires CRF1 receptor activation of the cAMP signaling cascade, which is then thought to increase the firing rate by phosphorylating potassium channels (Jedema and Grace 2004). Therefore, the increased CRF sensitivity of LC neurons in females could be due to sex differences in cAMP signaling. In support of this, pretreatment with a cAMP antagonist (Rp-cAMP-S) revealed that the majority of the LC response to CRF in unstressed female rats was cAMP mediated, whereas in unstressed males only 50% of the LC response to CRF was cAMP-mediated (Bangasser et al. 2010). In stressed males, their sensitized response to CRF was completely dependent on cAMP (Bangasser et al. 2010). CRF1 receptor immunoprecipitation studies from cortical tissue confirmed sex differences in CRF1- Gs coupling that were suggested by the LC electrophysiology studies. Furthermore, they suggested that sex differences in LC responses to CRF were the result of differential coupling of the Gs protein to the CRF1 receptor (Bangasser et al. 2010). Thus, in the unstressed condition CRF1-Gs coupling, as determined by the amount of Gs pulled down with CRF1 receptor, was greater in unstressed females compared to males and this was seen in both intact and ovariectomized females. Following swim stress, CRF1-Gs coupling increased in males only and this increased to a magnitude that matched that of unstressed females. These sex differences in CRF1-Gs coupling mirrored sex differences in LC electrophysiology and also were not dependent on adult hormonal status as they were seen with both ovariectomized and intact rats. It is noteworthy that these were the first data to link sex differences in physiology to sex differences in GPCR coupling and signaling.
Sex differences in CRF1 receptor signaling could potentially play a role in the sexually dimorphic LC dendritic morphology described above. In addition to its effects on LC physiology, CRF can regulate the morphology of LC dendrites. For example, CRF increases neurite outgrowth in LC-like CATHa cells (Cibelli et al. 2001). Additionally, CRF accelerated the growth and branching of LC dendrites in organotypic slice cultures (Swinny and Valentino 2006). This effect was CRF1 receptor mediated and required the activation protein kinase A (PKA), a downstream target of cAMP activation (Swinny and Valentino 2006). The greater CRF1-Gs association in females is consistent with the more extensive and complex LC dendritic tree. However, direct evidence for causality between CRF and sex differences in LC dendritic morphology has yet to be established.
Sex differences in CRF1 receptor trafficking
Excessive CRF is thought to occur in stress-related psychiatric diseases. However, cellular mechanisms can protect against excessive stimulation of the CRF1 receptor. Homogolous desensitization and internalization, a process by which receptors are trafficked from the cell membrane to the cytosol, enable CRF receptive cells to regulate their responses to excessive ligand or agonist binding (Krupnick and Benovic 1998; Premont 2005; Lefkowitz 1998). Desensitization of the CRF1 receptor is initiated by GRK3 phosphorylation of a serine or threonine residue on the carboxy terminus of the receptor (Oakley et al. 2007; Teli et al. 2005). This promotes recruitment and binding of β-arrestin2, a protein that uncouples the receptor from its G-protein and targets it to clathrin-coated pits for endocytosis (Hauger et al. 2009; Holmes et al. 2006; Oakley et al. 2007). Once internalized the receptor can be recycled back to the membrane or degraded. Both swim stress and intra-LC CRF administration induce CRF1 receptor internalization in LC dendrites of male rats (Bangasser et al. 2010; Reyes et al. 2006; Reyes et al. 2008). Receptor internalization is apparent at 1 and 24 hours after swim stress, and by 24 hours many receptors are associated with mulitvesicular bodies that target receptors for degradation (Reyes et al. 2008). This cellular event correlates with a decrease in the maximum magnitude of LC activation produced by CRF. CRF1 receptor desensitization and internalization in LC neurons of male rats could protect against excessive LC activation by CRF during conditions of repeated stress or CRF hypersecretion.
Notably, prior exposure to swim stress did not decrease the maximum magnitude of LC activation by CRF in females, suggesting that CRF1 receptors fail to desensitize and/or internalize in females following stress (Curtis et al. 2006). An immunoelectron microscopy study confirmed differences in internalization (Bangasser et al. 2010). Unlike stressed male rats that had a high proportion of internalized receptors in their LC dendrites, in stress female rats the majority of CRF1 receptors were associated with the plasma membrane. Interestingly, in unstressed females, a substantial proportion of CRF1 receptors were cytoplasmic, suggesting that stress may actually recruit CRF1 receptors to the plasma membrane in females. This sex difference in CRF1 receptor trafficking could be attributed to sex differences in CRF1 receptor association with β-arrestin2 (Bangasser et al. 2010). In the unstressed condition, CRF1 receptor association with β-arrestin2 was comparable in both sexes. Swim stress increased CRF1-β-arrestin2 association in male rats, consistent with the role of β-arrestin2 in the initiation of internalization. In contrast, swim stress did not affect CRF1 association with β-arrestin2 in females, which remained comparable to the unstressed state. This explains the inability of CRF1 receptors to internalize in stressed females. As for CRF1-Gs coupling, the differences in association with β-arrestin2 were unrelated to adult hormonal status. Together the results suggested that the decreased ability of CRF1 receptors in females to associate with β-arrestin2 compromises the process of CRF1 receptor internalization and this is expressed physiologically as an inability to decrease the maximal CRF response after prior stress. The loss of this important cellular adaptation is another potential mechanism that confers vulnerability to excessive CRF in females and particularly one that could contribute to the core symptom of hyperarousal. It is noteworthy that the decreased ability of CRF1 receptors in females to associate with β-arrestin2 also explains the enhanced association of CRF1 receptors with Gs because β-arrestin2 sterically hinders binding of Gs to GPCRs.
In addition to sex differences in β-arrestin2 binding, sex differences in other downstream regulators of receptor trafficking and recycling may also critically establish sex-specific CRF1 receptor localization. In cell culture, internalized human CRF1 receptors are transited via the GTPase Rab5 to early endosomes and then rapidly recycled via a Rab4-mediated endosomal pathway (Holmes et al. 2006). Future studies investigating sex differences in these GTPases may demonstrate, for example, that decreased Rab4 activity in males accounts for the CRF1 receptor internalization that persists 24 h after stressor exposure.
Differences in association of CRF1 receptors with G proteins and β-arrestin2 may suggest structural differences in the receptor. These could include sex differences in agonist-induced receptor conformation or other post-translational modifications, such as phosphorylation of the carboxy tail. Although no sex differences were found in threonine phosphorylation, this does not preclude differences in serine or tyrosine phosphorylation or in other post-translational modifications (e.g., sulfation, glycosylation, etc.) (Bangasser et al. 2010). Alternatively, sex differences in G proteins and β-arrestin2 themselves or other intercellular regulators may establish these effects.
Sex Biased Signaling
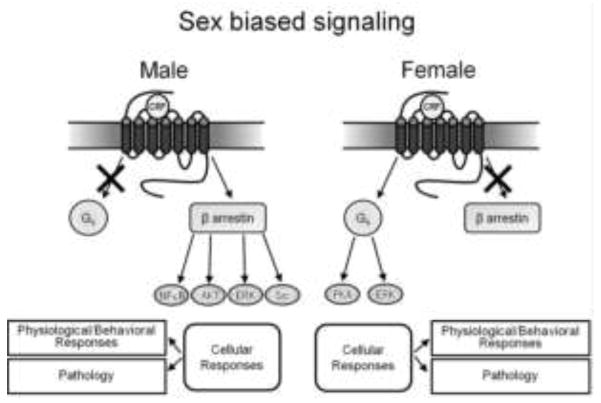
The molecular and cellular sex differences described above would render females more sensitive to acute stress and less able to adapt to chronic stress or excess CRF. New knowledge about the function of β-arrestins in cells suggests even broader implications of the findings. In addition to its role in receptor internalization, β-arrestin2 can also activate several G-protein-independent signaling cascades by scaffolding signaling molecules, including mitogen-activated protein kinase (e.g., ERK2, JNK3, and p38), tyrosine kinases (e.g., c-SRC, Hck, Fgr, Yes), as well as AKT, PI3 kinase and RhoA (for review see, Lefkowitz and Shenoy 2005; Violin and Lefkowitz 2007; DeWire et al. 2007; Shukla et al. 2011). The decreased ability of female CRF1 receptors to associate with -arrestin2 and increased ability to associate with Gs suggests that CRF1 receptor signaling is sex biased, such that in female neurons there is a bias away from β-arrestin2-related signaling and towards Gs-related signaling. In male neurons the pattern is opposite. In this scenario, CRF released during stress would engage different signaling pathways in males and females, resulting in unique cellular functions that translate to distinct behavioral and/or pathological responses (Fig. 1). Consequences of these sex differences would be magnified when CRF is in excess as has been proposed in depression and post-traumatic stress disorder. In these conditions, sex differences in the signaling cascades engaged by CRF could underlie sex differences in the neurobehavioral expression of the disease.
Figure 1.
This schematic depicts the predicted model of sex biased signaling. In males the CRF1 receptor associates with β-arrestin2, which sterically hinders Gs association and biases signaling towards β-arrestin2-related pathways such as NFκB, AKT, ERK, and Src. In females, the CRF1 receptor does not associate with β-arrestin2 as well and is more highly coupled to Gs, which signals through cAMP and sometimes ERK. Note that in both males and females CRF activates the receptor, but the signaling events are unique because of sex specific interactions of CRF1 receptors with binding partners. The differential cellular responses can translate to different physiological and behavioral responses to the same stressor and different pathology.
Sex biased CRF1 receptor signaling has important therapeutic implications for novel compounds that can shift the bias of CRF1 receptor signaling from Gs- to β-arrestin2-related pathways. By shifting CRF signaling towards a β-arrestin2 pathway, such compounds could potentially make females more resilient to the pathophysiological consequences of stress, particularly hyperarousal. “Biased agonists” are currently being designed to direct receptor-mediated cellular events towards specific pathways in an effort to promote efficacy for desired effects while diminishing adverse effects (Whalen et al. 2011). The angiotensin II receptor is being targeted in this way to take advantage of the anti-apototic effects that are mediated through -arrestin, while at the same time hindering the G-protein mediated effects that result in hypertension (Ahn et al. 2004; Violin et al. 2006; Violin and Lefkowitz 2007; Revankar et al. 2004 ). Biased agonists such as these are currently available for the angiotensin II and β-adrenergic receptors (Wei et al. 2003; Ahn et al. 2004; Whalen et al. 2011; Drake et al. 2008). Because CRF1 receptor mediated Gs-cAMP signaling increases LC firing rate and arousal, pharmaceuticals aimed at shifting signaling from G-protein mediated to β-arrestin-mediated may reduce the hyperarousal symptoms associated with stress-related psychiatric diseases.
A potential role for hormones in sex differences in the LC
Most sex differences are established by gonadal hormones. Although fluctuating gonadal hormones directly regulate CRF expression, they do not appear to modulate sex difference in CRF1 receptor function or sex differences in LC dendritic morphology. Electrophysiological responses of LC neurons to endogenous CRF elicited by hypotensive stress were no different in intact vs. castrated males, suggesting no contribution of circulating testosterone to this effect (Curtis et al. 2006). In female rats, heightened electrophysiological responses to endogenous CRF were similar under several hormonal conditions tested: ovariectomy (removal of the ovaries), ovariectomy with estrogen plus progesterone replacement, and intact cycling females (Curtis et al. 2006). Consistent with this, there were no sex differences in CRF1 receptor binding of Gs or β-arrestin2 in cycling vs. ovariectomized females, further indicating that circulating ovarian hormones do not contribute to these effects (Bangasser et al.). With respect to dendritic morphology, there was no change in LC dendritic density or the size of the LC dendritic field at different stages of the estrous cycle of females, suggesting that these effects are not linked to circulating estrogen and/or progesterone (Bangasser et al. 2011).
Although sex differences in CRF1 receptor function and dendritic morphology appear unrelated to adult hormonal status, it remains possible that gonadal hormone surges that occur during the perinatal or pubertal period establish these effects. During the perinatal period, testosterone transiently rises masculinizing the male brain (Swerdloff et al. 1992). This perinatal testosterone surge can establish sex difference in the HPA axis (Seale et al. 2005b; Seale et al. 2005a; McCormick et al. 1998). This surge also masculinizes the volume and neurochemistry of the bed nucleus of the stria terminalis and density of dendritic spines in the hippocampus, effects linked to sex differences in the modulation of learning by stress (del Abril et al. 1987; Han and De Vries 2003; Bangasser and Shors 2008; Leuner and Shors 2004; Dalla et al. 2009; Bangasser et al. 2005). In addition to the perinatal surge, a second protracted hormonal surge occurs during puberty that establishes sex differences in the brain and some behaviors (e.g., ingestive and sexual behaviors) (Swithers et al. 2008; Schulz et al. 2004; Schulz et al. 2009). Evuarherhe (2009) demonstrated that the pubertal androgen surge is required for programming HPA axis sensitivity to testosterone in adulthood, underscoring the importance of this second surge to the masculinazation of stress responses (Evuarherhe et al. 2009). As sex differences in depression appear after puberty, it is likely that the pubertal increase in gonadal hormones determines the sex bias in this disease.
In some cases sex differences occur independent of gonadal hormones and instead arise from differential representation of genes on the X and Y chromosomes in non-gonadal cells (for review see, Arnold 2009; Arnold and Chen 2009). For example, sex differences in nociceptive responses are not related to surges in gonadal hormones or circulating hormone levels, but rather are established by genes on the X or Y chromosomes (Gioiosa et al. 2008). Similar direct genetic effects could also contribute to the sex differences in CRF1 receptor function or LC dendritic morphology.
Sex differences in CRF function: Overview and implications
During a stressful event, the coordinated release of CRF in distinct brain regions affects multiple systems resulting in the integration of endocrine, behavioral and autonomic responses. Among the targets of CRF, the HPA axis and the LC-norepinephrine system mediate certain endocrine and arousal responses, respectively. Although short-term activation of these systems is adaptive in dealing with an immediate life-threatening challenge, their persistent activation is pathological and thought to underlie the dysregulated HPA axis and hyperarousal that are core components of several mood and anxiety disorders. Because these disorders occur twice as frequently in women as in men, here we detailed how sex differences CRF function at the level of the hypothalamus and LC contribute to the sex bias in these diseases. First, we described how estrogen can increase hypothalamic CRF expression resulting in greater HPA axis activation in females. Second, we focused on how sex differences at three levels of the LC-norepinephrine system could predispose females to hyperarousal, a core feature of several psychiatric diseases (Fig. 2). Specifically, the longer and more complex LC dendritic tree of females would allow for enhanced limbic input, forming a structural basis for heightened arousal in response to emotional events in females. At the level of the CRF1 receptor, increased CRF1 receptor coupling to Gs in females would result in female LC neurons that more sensitive to low levels of CRF. The lack of CRF1 receptor association with -arrestin2 and compromised internalization following stress in females would render female LC neurons less adaptable to conditions of CRF hypersecretion, as are thought to occur during chronic stress, depression, and PTSD. Together, these sex differences would enhance CRF function in the hypothalamic and LC systems of females vs. males. Thus, when confronted with extreme or prolonged stressor exposure, CRF-mediated endocrine and arousal systems would be more likely to shift into a dysregulated state in females.
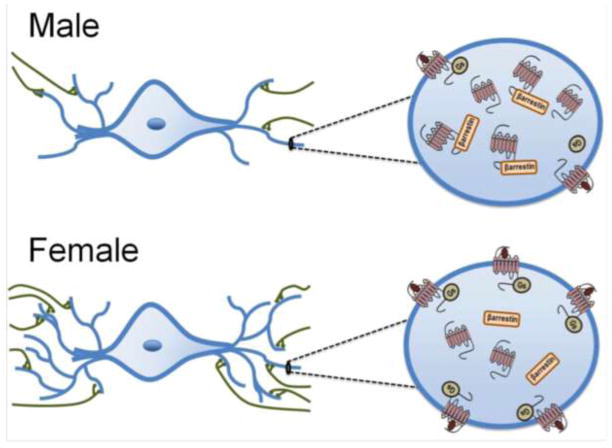
Figure 2.
This schematic illustrates sex differences in the LC-arousal system. The images on the left depict LC neurons with dendrites (blue) receiving afferent inputs (in green). Because the LC dendritic tree is longer and more complex in females, they are more likely to receive projections from afferent structures that terminate in the peri-LC. The images on the right depict magnified views of LC dendrites in order to illustrate sex differences in the CRF1 receptor coupling and trafficking. In males, CRF1 receptors (red) associate with β-arrestin2 following ligand binding and become localized in the cytoplasm. CRF1 receptors of females are highly coupled to Gs and following ligand binding they are predominantly found on the plasma membrane.
The focus of this review was on sex differences in the HPA and LC stress response systems. However, CRF has actions in other brain regions that are implicated in behavioral and autonomic aspects of the stress response and in symptoms of stress-related psychiatric disorders. It is possible that the sex differences described here for the LC extend, at least in part, to these components of the CRF system to further contribute to sex differences in other aspects of the stress response.
Sex differences in CRF function have important implications for drug development. Currently CRF antagonists are being designed to treat mood and anxiety disorders. Because the data reviewed here suggest that CRF hypersecretion is more likely in females, CRF antagonists may be more effective in women than men. However, sex differences in the CRF1 receptor could translate to a sex difference in drug efficacy. The fact that CRF1 receptors in females readily bind Gs, but fail to bind β-arrestin2, suggests a conformational change in the CRF1 receptors of females vs. males. This difference in receptor conformation may alter the ability of CRF antagonists to bind, potentially affecting how these compounds would work in women. If this idea is supported, it would suggest that gender-based pharmacotherapies that consider sex differences in pharmacodynamics would be required to effectively treat stress-related psychiatric disorders in both men and women. Finally, these data would underscore the importance of including female as well as male subjects in preclinical pharmaceutical research.
Acknowledgments
The authors acknowledge the support of PHS grants MH40008 to RJV and MH092438 to DAB.
References
- Abercrombie ED, Keller RW, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279(34):35518–35525. doi: 10.1074/jbc. M405878200 [pii] [DOI] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, Moorjani P, Haddad S, Basu A, Fagerness J, Saxe G, Smoller JW, Koenen KC. Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers. 2011;30 (2–3):89–99. doi: 10.3233/DMA-2011-0761. D7566715733N1LM2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21 (4):377–386. doi: 10.1111/j.1365-2826.2009.01831.x. JNE1831 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30 (1):1–9. doi: 10.1016/j.yfrne.2008.11.001. S0091-3022(08)00050-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981a;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981b;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res. 1996;107:380–402. doi: 10.1016/s0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Brain. Academic Press; 1995. pp. 183–213. [Google Scholar]
- Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8 (3):324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD., Jr Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156 (4):585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15 (9):877, 896–904. doi: 10.1038/mp.2010.66. mp201066 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Santollo J, Shors TJ. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav Neurosci. 2005;119 (6):1459–1466. doi: 10.1037/0735-7044.119.6.1459. 2006-00645-004 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. The bed nucleus of the stria terminalis modulates learning after stress in masculinized but not cycling females. J Neurosci. 2008;28 (25):6383–6387. doi: 10.1523/JNEUROSCI.0831-08.2008. 28/25/6383 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav. 2011;103 (3–4):342–351. doi: 10.1016/j.physbeh.2011.02.037. S0031-9384(11)00101-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banki CM, Karmacsi L, Bissette G, Nemeroff CB. Cerebrospinal fluid neuropeptides in mood disorder and dementia. J Affect Disord. 1992;25 (1):39–45. doi: 10.1016/0165-0327(92)90091-j. [DOI] [PubMed] [Google Scholar]
- Bao AM, Fischer DF, Wu YH, Hol EM, Balesar R, Unmehopa UA, Zhou JN, Swaab DF. A direct androgenic involvement in the expression of human corticotropin-releasing hormone. Mol Psychiatry. 2006;11 (6):567–576. doi: 10.1038/sj.mp.4001800. 4001800 [pii] [DOI] [PubMed] [Google Scholar]
- Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128 (Pt 6):1301–1313. doi: 10.1093/brain/awh448. awh448 [pii] [DOI] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. Gender difference in age-related number of corticotropin-releasing hormone-expressing neurons in the human hypothalamic paraventricular nucleus and the role of sex hormones. Neuroendocrinology. 2007;85 (1):27–36. doi: 10.1159/000099832. 000099832 [pii] [DOI] [PubMed] [Google Scholar]
- Battaglia G, Webster EL, De Souza EB. Characterization of corticotropin-releasing factor receptor-mediated adenylate cyclase activity in the rat central nervous system. Synapse. 1987;1 (6):572–581. doi: 10.1002/syn.890010610. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in the neocortex and hippocampus. J Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–383. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology. 1994;59 (3):228–234. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- Bissette G, Klimek V, Pan J, Stockmeier C, Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28(7):1328–1335. doi: 10.1038/sj.npp.1300191. [pii] [DOI] [PubMed] [Google Scholar]
- Bohler HC, Jr, Zoeller RT, King JC, Rubin BS, Weber R, Merriam GR. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res Mol Brain Res. 1990;8 (3):259–262. doi: 10.1016/0169-328x(90)90025-9. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154 (5):624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. Gender differences in trauma and posttraumatic stress disorder. J Gend Specif Med. 2002;5 (1):34–40. [PubMed] [Google Scholar]
- Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10 (3):198–210. doi: 10.1177/1524838009334448. 1524838009334448 [pii] [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Peterson EL, Lucia VC. Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. Psychol Med. 1999;29 (4):813–821. doi: 10.1017/s0033291799008612. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55 (7):626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lepine JP, Levinson D, Matschinger H, Mora ME, Browne MO, Posada-Villa J, Viana MC, Williams DR, Kessler RC. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. 1741-7015-9-90 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FM, Bilezikjian LM, Perrin MH, Rivier J, Vale W. Corticotropin releasing factor receptor-mediated stimulation of adenylate cyclase activity in the rat brain. Brain Res. 1986;381 (1):49–57. doi: 10.1016/0006-8993(86)90688-8. [DOI] [PubMed] [Google Scholar]
- Cibelli G, Corsi P, Diana G, Vitiello F, Thiel G. Corticotropin-releasing factor triggers neurite outgrowth of a catecholaminergic immortalized neuron via cAMP and MAP kinase signalling pathways. Eur J Neurosci. 2001;13(7):1339–1348. doi: 10.1046/j.0953-816x.2001.01510.x. ejn1510 [pii] [DOI] [PubMed] [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J Neurosci. 2004;24 (44):9914–9920. doi: 10.1523/JNEUROSCI.2446-04.2004. 24/44/9914 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A, Frankenhaeuser M. Stress responses in male and female engineering students. J Human Stress. 1978;4 (2):43–48. doi: 10.1080/0097840X.1978.9934986. [DOI] [PubMed] [Google Scholar]
- Conti LH, Foote SL. Effects of pretreatment with corticotropin-releasing factor (CRF) on the electrophysiological responsivity of the locus coeruleus to subsequent CRF challenge. Neuroscience. 1995;69:209–219. doi: 10.1016/0306-4522(95)00222-5. [DOI] [PubMed] [Google Scholar]
- Conti LH, Foote SL. Reciprocal cross-desensitization of locus coeruleus electrophysiological responsivity to corticotropin-releasing factor and stress. Brain Res. 1996;722:19–29. doi: 10.1016/0006-8993(96)00175-8. [DOI] [PubMed] [Google Scholar]
- Conti LH, Youngblood KL, Printz MP, Foote SL. Locus coeruleus electrophysiological activity and responsivity to corticotropin-releasing factor in inbred hypertensive and normotensive rats. Brain Res. 1997;774 (1–2):27–34. doi: 10.1016/s0006-8993(97)81683-6. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205 (5):807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31 (3):544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Grigoriadis DE, Page ME, Rivier J, Valentino RJ. Pharmacological comparison of two corticotropin-releasing factor antagonists: in vivo and in vitro studies. J Pharmacol Exp Ther. 1994;268 (1):359–365. [PubMed] [Google Scholar]
- Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281 (1):163–172. [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65 (2):541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Valentino RJ. Long-term regulation of locus ceruleus sensitivity to corticotropin-releasing factor by swim stress. J Pharmacol Exp Ther. 1999;289 (3):1211–1219. [PubMed] [Google Scholar]
- Dalla C, Whetstone AS, Hodes GE, Shors TJ. Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci Lett. 2009;449 (1):52–56. doi: 10.1016/j.neulet.2008.10.051. S0304-3940(08)01427-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJ, Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 1999;413(1):113–128. doi: 10.1002/(SICI)1096-9861(19991011)413:1<113::AID-CNE8>3.0.CO;2-B. [pii] [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Gold PW, Geracioti TD, Jr, Listwak SJ, Kling MA. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry. 1993;150 (4):656–657. doi: 10.1176/ajp.150.4.656. [DOI] [PubMed] [Google Scholar]
- De Souza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology. 1995;20 (8):789–819. doi: 10.1016/0306-4530(95)00011-9. 0306453095000119 [pii] del. [DOI] [PubMed] [Google Scholar]
- Abril A, Segovia S, Guillamon A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res. 1987;429 (2):295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG. Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int J Dev Neurosci. 2008;26 (3–4):259–268. doi: 10.1016/j.ijdevneu.2008.02.004. S0736-5748(08)00038-5 [pii] [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.ph.69.013107.100021. [DOI] [PubMed] [Google Scholar]
- Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. beta-arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283 (9):5669–5676. doi: 10.1074/jbc.M708118200. M708118200 [pii] [DOI] [PubMed] [Google Scholar]
- Duncko R, Kiss A, Skultetyova I, Rusnak M, Jezova D. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology. 2001;26(1):77–89. doi: 10.1016/s0306-4530(00)00040-8. S0306-4530(00)00040-8 [pii] [DOI] [PubMed] [Google Scholar]
- Evuarherhe O, Leggett JD, Waite EJ, Kershaw YM, Atkinson HC, Lightman SL. Organizational role for pubertal androgens on adult hypothalamic-pituitary-adrenal sensitivity to testosterone in the male rat. J Physiol. 2009;587 (Pt 12):2977–2985. doi: 10.1113/jphysiol.2008.168393. jphysiol.2008.168393 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab. 2007;292 (4):E1173–1182. doi: 10.1152/ajpendo.00102.2006. 00102.2006 [pii] [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann B, Kindermann W. Energy metabolism and regulatory hormones in women and men during endurance exercise. Eur J Appl Physiol Occup Physiol. 1989;59 (1–2):1–9. doi: 10.1007/BF02396572. [DOI] [PubMed] [Google Scholar]
- Gallucci WT, Baum A, Laue L, Rabin DS, Chrousos GP, Gold PW, Kling MA. Sex differences in sensitivity of the hypothalamic-pituitary-adrenal axis. Health Psychol. 1993;12 (5):420–425. doi: 10.1037//0278-6133.12.5.420. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Pinos H, Collado P, Pasaro E, Fernandez R, Segovia S, Guillamon A. The expression of brain sexual dimorphism in artificial selection of rat strains. Brain Res. 2005;1052 (2):130–138. doi: 10.1016/j.brainres.2005.05.066. S0006-8993(05)00817-6 [pii] [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Pinos H, Fernandez R, Collado P, Pasaro E, Segovia S, Guillamon A. Sexual dimorphism in hybrids rats. Brain Res. 2006;1123 (1):42–50. doi: 10.1016/j.brainres.2006.09.053. S0006-8993(06)02797-1 [pii] [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav. 2008;53 (1):124–130. doi: 10.1016/j.yhbeh.2007.09.003. S0018-506X(07)00212-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111 (1):22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7 (3):254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gold PW, Wong ML, Chrousos GP, Licinio J. Stress system abnormalities in melancholic and atypical depression: molecular, pathophysiological, and therapeutic implications. Mol Psychiatry. 1996;1 (4):257–264. [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J Neurochem. 2001;76 (2):509–519. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Guillamon A, de Blas MR, Segovia S. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res. 1988;468 (2):306–310. doi: 10.1016/0165-3806(88)90143-5. [DOI] [PubMed] [Google Scholar]
- Haas DA, George SR. Gonadal regulation of corticotropin-releasing factor immunoreactivity in hypothalamus. Brain Res Bull. 1988;20(3):361–367. doi: 10.1016/0361-9230(88)90065-2. 0361-9230(88)90065-2 [pii] [DOI] [PubMed] [Google Scholar]
- Hajos-Korcsok E, Robinson DD, Yu JH, Fitch CS, Walker E, Merchant KM. Rapid habituation of hippocampal serotonin and norepinephrine release and anxiety-related behaviors, but not plasma corticosterone levels, to repeated footshock stress in rats. Pharmacol Biochem Behav. 2003;74(3):609–616. doi: 10.1016/s0091-3057(02)01047-x. S009130570201047X [pii] [DOI] [PubMed] [Google Scholar]
- Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol. 2003;54 (3):502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28 (4):464–476. doi: 10.1006/hbeh.1994.1044. S0018-506X(84)71044-0 [pii] [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. NYAS5011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek RP, Van Haaren F, Feenstra MG, Endert E, Van de Poll NE. Sex- and time-dependent changes in neurochemical and hormonal variables induced by predictable and unpredictable footshock. Physiol Behav. 1991;49 (6):1251–1256. doi: 10.1016/0031-9384(91)90359-v. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Yates FE, Glenister DW, Urquhart J. Variations in hepatic inactivation of corticosterone with changes in food intake: an explanation of impaired corticosteroid metabolism following noxious stimuli. Endocrinology. 1960;67:222–238. doi: 10.1210/endo-67-2-222. [DOI] [PubMed] [Google Scholar]
- Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Nemeroff CB, Holsboer F. Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety. 1998;8 (2):71–79. [PubMed] [Google Scholar]
- Heuser IJ, Gotthardt U, Schweiger U, Schmider J, Lammers CH, Dettling M, Holsboer F. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol Aging. 1994;15 (2):227–231. doi: 10.1016/0197-4580(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96 (4):934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- Iteke O, Bakare MO, Agomoh AO, Uwakwe R, Onwukwe JU. Road traffic accidents and posttraumatic stress disorder in an orthopedic setting in South-Eastern Nigeria: a controlled study. Scand J Trauma Resusc Emerg Med. 2011;19:39. doi: 10.1186/1757-7241-19-39. 1757-7241-19-39 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34 (2):226–237. doi: 10.1016/j.psyneuen.2008.09.003. S0306-4530(08)00232-1 [pii] [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24 (43):9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH. The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of norepinephrine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur J Pharmacol. 2000;387:279–286. doi: 10.1016/s0014-2999(99)00793-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. S0165032702004263 [pii] [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52 (12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med. 1995;57 (1):23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61 (2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54 (6):648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46(9):1167–1180. doi: 10.1016/s0006-3223(99)00164-x. S0006-3223(99)00164-X [pii] [DOI] [PubMed] [Google Scholar]
- Kosoyan HP, Grigoriadis DE, Tache Y. The CRF(1) receptor antagonist, NBI-35965, abolished the activation of locus coeruleus neurons induced by colorectal distension and intracisternal CRF in rats. Brain Res. 2005;1056 (1):85–96. doi: 10.1016/j.brainres.2005.07.010. S0006-8993(05)01049-8 [pii] [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69 (1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. S0301-0511(04)00170-X [pii] [DOI] [PubMed] [Google Scholar]
- Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41 (3):1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. S1053-8119(08)00225-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SM, Curtis AL, Brons R, Valentino RJ. Locus coeruleus activation by colon distention: role of corticotropin-releasing factor and excitatory amino acids. Brain Res. 1997;756 (1–2):114–124. doi: 10.1016/s0006-8993(97)00116-9. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273 (30):18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308 (5721):512–517. doi: 10.1126/science.1109237. 308/5721/512 [pii] [DOI] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. New spines, new memories. Mol Neurobiol. 2004;29 (2):117–130. doi: 10.1385/MN:29:2:117. MN:29:2:117 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Wang H, Tang J, Wang X, Qiu D, Liu W, Cao Z, Li W. Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett. 2006;404 (3):358–362. doi: 10.1016/j.neulet.2006.06.016. S0304-3940(06)00587-8 [pii] [DOI] [PubMed] [Google Scholar]
- Livezey GT, Miller JM, Vogel WH. Plasma norepinephrine, epinephrine and corticosterone stress responses to restraint in individual male and female rats, and their correlations. Neurosci Lett. 1985;62 (1):51–56. doi: 10.1016/0304-3940(85)90283-6. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J Pharmacol Exp Ther. 2005;314 (1):201–206. doi: 10.1124/jpet.105.084913. jpet.105.084913 [pii] [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004;16(3):272–278. doi: 10.1111/j.0953-8194.2004.01167.xJNE1167. [pii] [DOI] [PubMed] [Google Scholar]
- Mark TL, Shern DL, Bagalman JE, Cao Z. copyright Mental Health America. Mental Health America; Alexandria: 2007. Ranking America’s Mental Health: An Analysis of Depression Across the States. [Google Scholar]
- McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have ‘organizational’ effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res. 1998;105(2):295–307. doi: 10.1016/s0165-3806(97)00155-7. S0165380697001557 [pii] [DOI] [PubMed] [Google Scholar]
- Melia KR, Duman RS. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci. 1991;88:8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci. 2004;24 (6):1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1 (4):336–342. [PubMed] [Google Scholar]
- Nemeroff CB, Bissette G, Akil H, Fink M. Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy. Corticotrophin-releasing factor, beta-endorphin and somatostatin. Br J Psychiatry. 1991;158:59–63. doi: 10.1192/bjp.158.1.59. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226 (4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- O’Donnell T, Hegadoren KM, Coupland NC. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology. 2004;50 (4):273–283. doi: 10.1159/000080952. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Comp Physiol. 2007;293 (1):R209–222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133 (2):183–204. doi: 10.1037/0033-2909.133.2.183. 2007-02367-001 [pii] [DOI] [PubMed] [Google Scholar]
- Owens MJ, Bissette G, Nemeroff CB. Acute effects of alprazolam and adinazolam on the concentrations of corticotropin-releasing factor in the rat brain. Synapse. 1989;4 (3):196–202. doi: 10.1002/syn.890040304. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43 (4):425–473. [PubMed] [Google Scholar]
- Owens MJ, Vargas MA, Knight DL, Nemeroff CB. The effects of alprazolam on corticotropin-releasing factor neurons in the rat brain: acute time course, chronic treatment and abrupt withdrawal. J Pharmacol Exp Ther. 1991;258 (1):349–356. [PubMed] [Google Scholar]
- Page ME, Abercrombie ED. Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse. 1999;33:304–313. doi: 10.1002/(SICI)1098-2396(19990915)33:4<304::AID-SYN7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Page ME, Berridge CW, Foote SL, Valentino RJ. Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci Lett. 1993;164:81–84. doi: 10.1016/0304-3940(93)90862-f. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Franco C, Sodano R, Freidenberg B, Gordis E, Anderson DA, Forsyth JP, Wulfert E, Frye CA. Sex differences in salivary cortisol in response to acute stressors among healthy participants, in recreational or pathological gamblers, and in those with posttraumatic stress disorder. Horm Behav. 2010;57 (1):35–45. doi: 10.1016/j.yhbeh.2009.06.003. S0018-506X(09)00130-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkonigg A, Kessler RC, Storz S, Wittchen HU. Traumatic events and post-traumatic stress disorder in the community: prevalence, risk factors and comorbidity. Acta Psychiatr Scand. 2000;101 (1):46–59. doi: 10.1034/j.1600-0447.2000.101001046.x. [DOI] [PubMed] [Google Scholar]
- Premont RT. Once and future signaling: G protein-coupled receptor kinase control of neuronal sensitivity. Neuromolecular Med. 2005;7 (1–2):129–147. doi: 10.1385/NMM:7:1-2:129. NMM:7:1-2:129 [pii] [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60 (4):436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Hoffman GE, Rabin BS, Sved AF. Injection of corticotropin-releasing hormone into the locus coeruleus or foot shock increases neuronal Fos expression. Neuroscience. 1998;85:259–268. doi: 10.1016/s0306-4522(97)00574-5. [DOI] [PubMed] [Google Scholar]
- Redei E, Li L, Halasz I, McGivern RF, Aird F. Fast glucocorticoid feedback inhibition of ACTH secretion in the ovariectomized rat: effect of chronic estrogen and progesterone. Neuroendocrinology. 1994;60 (2):113–123. doi: 10.1159/000126741. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Vines CM, Cimino DF, Prossnitz ER. Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem. 2004;279(23):24578–24584. doi: 10.1074/jbc.M402121200M402121200. [pii] [DOI] [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23 (11):2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149 (1):122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci. 2005;22 (1):93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacol Biochem Behav. 1999;64 (4):739–751. doi: 10.1016/s0091-3057(99)00148-3. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150 (1):8–13. doi: 10.1016/j.neuroscience.2007.09.043. S0306-4522(07)01098-6 [pii] [DOI] [PubMed] [Google Scholar]
- Sandanger I, Nygard JF, Sorensen T, Moum T. Is women’s mental health more susceptible than men’s to the influence of surrounding stress? Soc Psychiatry Psychiatr Epidemiol. 2004;39 (3):177–184. doi: 10.1007/s00127-004-0728-6. [DOI] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003;54 (12):1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Schulz C, Lehnert H. Activation of noradrenergic neurons in the locus coeruleus by corticotropin-releasing factor, a microdialysis study. Neuroendocrinology. 1996;63:454–458. doi: 10.1159/000127071. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55 (5):597–604. doi: 10.1016/j.yhbeh.2009.03.010. S0018-506X(09)00066-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45(4):242–249. doi: 10.1016/j.yhbeh.2003.12.007S0018506X04000121. [pii] [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol. 2004;16 (12):989–998. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Postnatal masculinization alters the HPA axis phenotype in the adult female rat. J Physiol. 2005a;563 (Pt 1):265–274. doi: 10.1113/jphysiol.2004.078212. jphysiol.2004.078212 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS. Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat. Endocrinology. 2005b;146 (4):1973–1982. doi: 10.1210/en.2004-1201. en.2004-1201 [pii] [DOI] [PubMed] [Google Scholar]
- Seeman TE, Singer B, Wilkinson CW, McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26 (3):225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Fu L, Ennis M, Liu W, Aston-Jones G. Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. J Comp Neurol. 1996;365:56–68. doi: 10.1002/(SICI)1096-9861(19960129)365:1<56::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of beta-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36 (9):457–469. doi: 10.1016/j.tibs.2011.06.003. S0968-0004(11)00086-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagin GN, Zhou J, Harris RB, Ryan DH. CRF receptor antagonist attenuates immobilization stress-induced norepinephrine release in the prefrontal cortex in rats. Brain Res Bull. 1997;42(6):431–434. doi: 10.1016/s0361-9230(96)00368-1. S0361923096003681 [pii] [DOI] [PubMed] [Google Scholar]
- Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, Laird NM, Kagan J, Snidman N, Hirshfeld-Becker D, Tsuang MT, Sklar PB, Slaugenhaupt SA. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry. 2003;54 (12):1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. Corticotropin-Releasing Factor in the Norepinephrine Nucleus, Locus Coeruleus, Facilitates Behavioral Flexibility. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.218. npp2011218 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46(9):1192–1204. doi: 10.1016/s0006-3223(99)00219-x. S0006-3223(99)00219-X [pii] [DOI] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One. 2011;6(11):e28128. doi: 10.1371/journal.pone.0028128PONE-D-11-14868. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Roubos EW, Peeters BW, Kozicz T. Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. J Neurosci Res. 2012;90 (1):179–192. doi: 10.1002/jnr.22737. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163 (4):467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36 (3):165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C, Hines M, Gorski R. Effect of androgens on the brain and other organs during development and aging. Psychoneuroendocrinology. 1992;17 (4):375–383. doi: 10.1016/0306-4530(92)90042-6. [DOI] [PubMed] [Google Scholar]
- Swinny JD, Valentino RJ. Corticotropin-releasing factor promotes growth of brain norepinephrine neuronal processes through Rho GTPase regulators of the actin cytoskeleton in rat. Eur J Neurosci. 2006;24 (9):2481–2490. doi: 10.1111/j.1460-9568.2006.05129.x. [DOI] [PubMed] [Google Scholar]
- Swithers SE, McCurley M, Hamilton E, Doerflinger A. Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm Behav. 2008;54 (3):471–477. doi: 10.1016/j.yhbeh.2008.05.009. S0018-506X(08)00166-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19 (2):474–490. doi: 10.1210/me.2004-0275. [DOI] [PubMed] [Google Scholar]
- Tjoumakaris SI, Rudoy C, Peoples J, Valentino RJ, Van Bockstaele EJ. Cellular interactions between axon terminals containing endogenous opioid peptides or corticotropin-releasing factor in the rat locus coeruleus and surrounding dorsal pontine tegmentum. J Comp Neurol. 2003;466 (4):445–456. doi: 10.1002/cne.10893. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132 (6):959–992. doi: 10.1037/0033-2909.132.6.959. 2006-20202-007 [pii] [DOI] [PubMed] [Google Scholar]
- Troop RC. Influence of gonadal hormones on the metabolism of cortisone. Endocrinology. 1959;64 (5):671–675. doi: 10.1210/endo-64-5-671. [DOI] [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31 (5):642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Chen S, Zhu Y, Aston-Jones G. Evidence for divergent projections of corticotropin-releasing hormone neurons of Barrington’s nucleus to the locus coeruleus and spinal cord. Brain Res. 1996;732:1–15. doi: 10.1016/0006-8993(96)00482-9. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39 (1):1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci. 1988;8 (3):1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555 (1):25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Van Bockstaele E, Aston-Jones G. Corticotropin-releasing factor innervation of the locus coeruleus region: distribution of fibers and sources of input. Neuroscience. 1992;48:689–705. doi: 10.1016/0306-4522(92)90412-u. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology (Berl) 2001;158 (4):331–342. doi: 10.1007/s002130000673. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Functional interactions between stress neuromediator and the locus coeruleur-noradrenaline system. In: Steckler TKN, editor. Handbook of Stress and the Brain. Elsevier; The Netherlands: 2005. pp. 465–486. [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583 (2–3):194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG. Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus ceruleus during hemodynamic stress. Neuroendocrinology. 1988;48 (6):674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;92 (4):1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364 (3):523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q [pii]10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10 (10):743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Valentino RJ. A.E. Bennett Research Award. Anatomic basis for differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biol Psychiatry. 1999;46(10):1352–1363. doi: 10.1016/s0006-3223(99)00213-9. S0006322399002139 [pii] [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146(1):137–146. doi: 10.1210/en.2004-0846en.2004-0846. [pii] [DOI] [PubMed] [Google Scholar]
- Violin JD, Dewire SM, Barnes WG, Lefkowitz RJ. G protein-coupled receptor kinase and beta-arrestin-mediated desensitization of the angiotensin II type 1A receptor elucidated by diacylglycerol dynamics. J Biol Chem. 2006;281 (47):36411–36419. doi: 10.1074/jbc.M607956200. M607956200 [pii] [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28 (8):416–422. doi: 10.1016/j.tips.2007.06.006. S0165-6147(07)00151-4 [pii] [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463(1–3):199–216. doi: 10.1016/s0014-2999(03)01282-2. S0014299903012822 [pii] [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586 (8):2157–2170. doi: 10.1113/jphysiol.2007.150078. jphysiol.2007.150078 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry. 2008;13 (8):786–799. 741. doi: 10.1038/mp.2008.38. mp200838 [pii] [DOI] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci U S A. 2003;100(19):10782–10787. doi: 10.1073/pnas.1834556100. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R. Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci. 1998;16(3–4):289–295. doi: 10.1016/s0736-5748(98)00021-5. S0736-5748(98)00021-5 [pii] [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159 (2):883–895. doi: 10.1016/j.neuroscience.2008.12.058. S0306-4522(08)01848-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17 (3):126–139. doi: 10.1016/j.molmed.2010.11.004. S1471-4914(10)00182-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34(3):171–181. doi: 10.1016/s0022-3956(00)00016-9. S0022-3956(00)00016-9 [pii] [DOI] [PubMed] [Google Scholar]