Abstract
Purpose
Live/killed mycobacteria and culture supernatants can suppress asthmatic reactions. This study investigated whether mycobacterial secretory proteins have therapeutic effects on asthma.
Methods
Mycobacterium bovis bacille Calmette-Guérin (BCG; 2×105 CFUs) and mycobacterial secretory proteins (Ag85 complex, 38-kDa protein or MPB70; 4 or 20 µg) were administered intraperitoneally to female BALB/c mice with established airway hyperresponsiveness. One week after treatment, the mice underwent a methacholine challenge test, and then inflammatory cell numbers in bronchoalveolar lavage fluid (BAL) and around bronchi (<500 µm), and cytokine levels in splenocyte supernatants, were assessed.
Results
BCG and all of the tested secretory proteins significantly improved airway sensitivity compared to baseline values (P<0.05). The secretory protein Ag85 complex significantly suppressed airway reactivity also (P<0.05), while 38-kDa protein significantly suppressed reactivity and maximal narrowing (P<0.05). The number of eosinophils in BAL and around bronchi, and the goblet cell proportion, were also significantly reduced in mice in both the BCG and secretory protein groups compared to the asthma control group. IFN-γ/IL-5 ratios were significantly higher in mice treated with BCG, 4 µg MPB70 or 4 µg 38-kDa protein than in asthma control mice (P<0.05), and were negatively associated with airway hyperresponsiveness, peribronchial eosinophil numbers and goblet cell proportion (all P<0.05). IL-17A was positively correlated with IL-5 (r=0.379, P<0.001), maximal airway narrowing, peribronchial eosinophil numbers and goblet cell proportion (all P<0.05).
Conclusions
Secretory proteins from BCG and M. tuberculosis and live BCG were effective against established asthma, their effects being accompanied by increased IFN-γ/IL-5 ratios. Thus, allergic asthma could be effectively treated with mycobacterial secretory proteins.
Keywords: Ag85 complex, asthma, MPB70, mycobacteria, 38-kDa protein
INTRODUCTION
The prevalence of asthma and allergic disease has increased substantially during the past three or four decades.1,2 Although it has been stabilized or has decreased in most high-prevalence countries (such as English-speaking countries) since 2000, its prevalence is still increasing in population-dense countries in Africa, Latin America and parts of Asia.3,4 Overall, the global burden of asthma is continuing to rise.
The hygiene/old friends hypothesis was proposed to explain the rise in the prevalence of asthma/allergy.5 This hypothesis states that an increase in the prevalence of asthma/allergy is related to diminished exposure to infections and certain relatively harmless environmental organisms or components that the immune system has learned to tolerate.6 Many investigators, including our group,7-12 have shown that Mycobacterium bovis bacille Calmette-Guérin (BCG) and other mycobacterial infections suppress airway hyperresponsiveness (AHR) and eosinophilic inflammation, likely through a T-helper 1 (Th1) or regulatory T cell (Treg) response. Furthermore, we found that BCG and M. tuberculosis culture supernatants also suppressed asthmatic reactions.13
The antigen 85 complex (Ag85) is a major constituent of the secreted proteins of M. tuberculosis and BCG that can induce interferon (IFN)-γ secretion from Th1 cells.14 38-kDa protein is also actively secreted from M. tuberculosis15 and stimulates IFN-γ secretion.16 MPB70, another protein that induces IFN-γ production, is a major secreted protein of BCG but is only secreted in small quantities from M. tuberculosis.17 This study aimed to determine which of the enriched secretory proteins of mycobacteria have therapeutic effects on asthma.
MATERIALS AND METHODS
Experimental animals
Specific pathogen-free, 6-week-old female BALB/c mice (Daehan BioLink, Eumsung, Korea) were fed standard mouse chow and given water ad libitum in an animal care room at Chonnam National University Medical School, Korea. Animal care and treatment followed the guidelines of the Chonnam National University Research Institute of Medical Sciences and the study was approved by the Chonnam National University Institutional Animal Care and Use Committees (CNU IACUC-H-2009-9). Mice were divided into nine groups: one normal control group, one asthma control group, one live BCG-treated group (n=10 per group), and six mycobacterial secretory protein-treated groups (n=8 per group).
Study design
Mice were sensitized and provoked with ovalbumin (Grade V; Sigma-Aldrich, St. Louis, MO, USA), and those that developed AHR to methacholine (Sigma) were selected as the experimental animals, as described previously (Fig. 1).18 AHR was defined as airway sensitivity >95% of the confidence interval of the non-sensitized normal control group, and the asthma threshold was 16.3 mg/mL. Mice with established AHR were treated intraperitoneally with live BCG or mycobacterial secretory proteins. One week after treatment, they underwent a second provocation test with ovalbumin, followed by a methacholine challenge test, and then were sacrificed so that inflammatory cell numbers in bronchoalveolar lavage (BAL) fluid and around bronchi (<500 µm), and cytokine levels in the splenocyte culture supernatants, could be quantified.
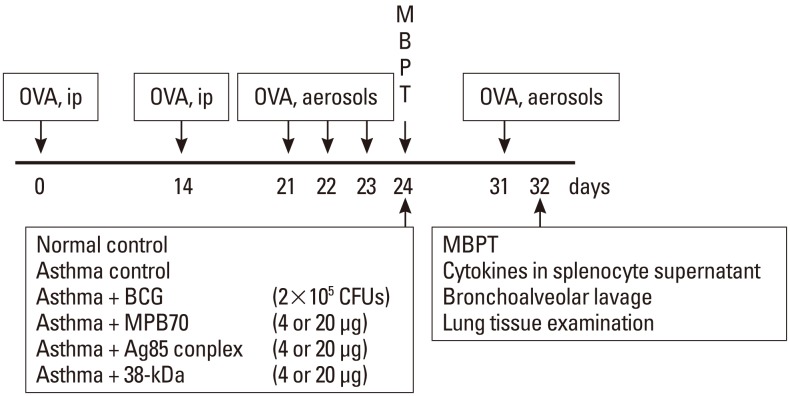
Fig. 1.
The time course of the experiment. OVA, ovalbumin; ip, intraperitoneal; BCG, bacille Calmette-Guérin; MBPT, methacholine bronchoprovocation test.
Ovalbumin sensitization and provocation, and administration of mycobacterial proteins
Mice were sensitized through two intraperitoneal injections of 200 µL phosphate-buffered saline (PBS; BioWhittaker™, MD, USA) containing 20 µg ovalbumin and 2.25 mg aluminum hydroxide (Imject Alum; Pierce Biotechnology, Rockford, IL, USA) 2 weeks apart. One week after the second sensitization, the mice were provoked with 1% ovalbumin aerosol using an Ultra-Neb ultrasonic nebulizer (DeVilbiss, Somerset, PA, USA) in an OCP3000 animal body plethysmograph (All Medicus, Anyang, Korea) for 30 minutes per day for three successive days. The normal control group was sensitized and provoked with PBS alone.
Pathogenic M. tuberculosis H37Rv (ATCC 27294) and M. bovis BCG (Tokyo 172 strain; Korean National Tuberculosis Association, Seoul, Korea) were cultured at 37℃as surface pellicles on Sauton's medium. After 6 weeks, the bacilli were removed by filtration through filter paper. The culture supernatants were sequentially sterilized using membrane filters (pore size 1.2 and 0.2 µm) and concentrated by ultrafiltration (Amicon Centriprep-10, Millipore, Billerica, MA, USA).
Ag85 (0.632 mg/mL) and 38-kDa protein (0.95 mg/mL) were purified from the culture supernatants of M. tuberculosis, as described previously.15,19,20 MPB70 protein (1.8 mg/mL) from BCG culture supernatants was purified by a two-step process: hydroxylapatite chromatography followed by anion-exchange chromatography. Briefly, a 50%-80% ammonium sulfate precipitate of BCG culture filtrate was dissolved and dialyzed against 1 mM potassium phosphate buffer (PB, pH 7.0), loaded to a column of hydroxylapatite equilibrated with 1 mM PB, and eluted with 1 mM PB (because MPB70 was not retained on the column). Further purification was performed by anion-exchange chromatography using DEAE-Sepharose CL-6B. MPB70 protein was eluted with 0 to 50 mM NaCl in 20 mM Tris-HCl (pH 8.0). The purified proteins were dialyzed against PBS, filter sterilized, and frozen at -80℃. Protein concentrations were determined using a protein assay kit (Pierce) with bovine serum albumin as the standard.
To prepare a live bacterial stock, BCG was cultured at 36℃ for 3 weeks in a sealed 1:1 semisolid mixture of Middlebrook 7H10 agar (Becton Dickinson, Cockeysville, MD, USA) and Middlebrook 7H9 broth (Becton Dickinson) supplemented with 10% oleic acid-albumin-dextrose-catalase (Becton Dickinson). Colonies were counted before preparation of the inoculum. Live BCG (2×105 CFUs) or purified mycobacterial secretory proteins (4 or 20 µg) were administered intraperitoneally to mice with established AHR.
Lung function tests
Airway responsiveness to methacholine was measured using the OCP3000 body plethysmograph, as described previously.21 Briefly, the animals inhaled PBS aerosol and then progressively doubled concentrations of methacholine (starting from 3.125 mg/mL and increasing to 100 mg/mL at 24-hour after the final ovalbumin inhalation challenge). Aerosols were generated using an Ultra-Neb nebulizer, and 3 mL solution was aerosolized for 3 minutes. Enhanced pause (Penh) was used to represent airway resistance. The methacholine concentration required for a 200% increase in Penh from the post-saline value (PC200), the maximal response to methacholine (Maximal Penh), and the slope of the methacholine dose-response curve (by linear regression) (DRS) were calculated as indices of airway sensitivity, excessive airway narrowing, and airway reactivity, respectively. As a three-fold improvement in PC20 is considered significant in occupational asthma,22 the fold increase in PC200 (i.e., ratio of second PC200 value to first PC200 value) was calculated to evaluate the degree of improvement after treatment.
Analysis of cells in BAL fluid and lung tissue
Forty-eight hours after the last ovalbumin provocation, the mice were sacrificed by intraperitoneal injection of 5 mg/kg thiopental sodium. BAL samples were taken, and total and differential cell counts in the BAL fluid were determined, as described previously.21 Briefly, the lungs were lavaged with 3.6 mL cold 0.9% saline via the trachea. After counting total cells, cytospin preparations (CF-120; Sakura Fine-Technical, Tokyo, Japan) were stained with Diff-Quick (International Reagents, Kobe, Japan). The percentage of each cell type was calculated from the average of 300 consecutive cells counted on each of two slides (magnification, ×400).
The left lung was fixed in 4% formalin and embedded in paraffin for histopathological analysis. The embedded tissue was sectioned to a thickness of 4 µm and stained with hematoxylin-eosin and periodic acid-Schiff. Small airways (circumference <500 µm) were selected23 and numbers of eosinophils, lymphocytes, and neutrophils within the peribronchial area from the basement membrane to a depth of 100 µm were determined using a Nikon microscope using AnalySIS® Pro image analysis software (Soft Imaging System GmbH, Münster, Germany) and expressed as number of cells/mm2.24 Goblet cell hyperplasia was evaluated according to a 5-point scoring system (0-4) as follows: 0, no goblet cells; 1, <25%; 2, 25%-50%; 3, 50%-75%; and 4, ≥75%.25
Cytokine assays
Splenocyte culture and cytokine measurements were performed as described previously.21 Briefly, splenocytes were cultured in RPMI 1640 medium (BioWhittaker, Walkersville, MD, USA) supplemented with 10% fetal bovine serum (Gibco BRL, Grand Island, NY, USA) and a 1% penicillin-streptomycin-amphotericin B mixture (BioWhittaker), and were stimulated with 2.5 µg/mL concanavalin A (Sigma) for 48 hours. IFN-γ, IL-5, and IL-17A concentrations in the supernatants of stimulated splenocytes were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (BioLegend, Inc., San Diego, CA, USA). The sensitivities of the assays for IFN-γ, IL-5, and IL-17A were 4, 8, and 7.8 pg/mL, respectively.
Statistical analysis
PC200 values were log10-transformed before analysis, and all data are expressed as the mean±SEM. The Kruskal-Wallis test and Mann-Whitney U test were used to determine the significance of the intergroup differences, and the Wilcoxon signed-rank test was used to determine the significance of the intragroup differences. Associations between variables were examined using Spearman's rank correlation coefficient. A P value of <0.05 was considered statistically significant.
RESULTS
Methacholine-AHR
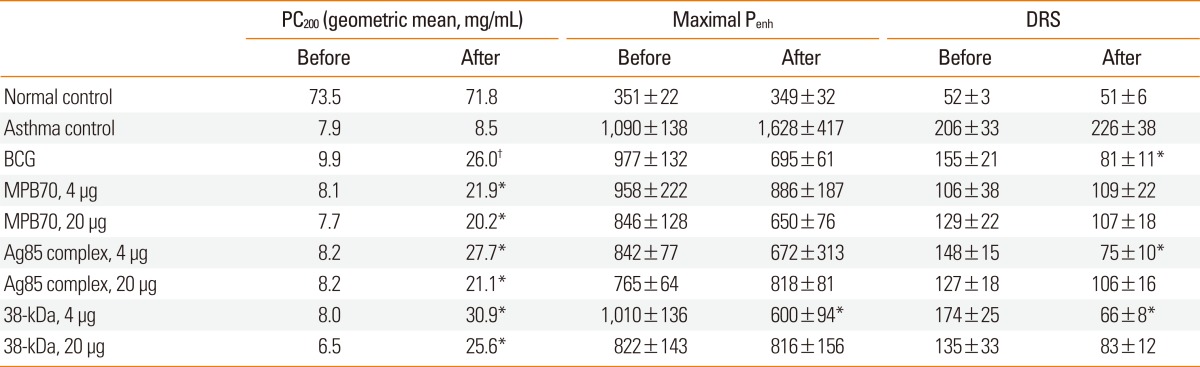
The Penh values after PBS inhalation in the treatment groups were not significantly different than those in the asthma control group either before or after the treatment with live BCG or mycobacterial secretory proteins. In addition, the pre-treatment PC200, Maximal Penh, and DRS values in the treatment groups were not significantly different than those in the asthma control group (Table 1). However, treatment with BCG or mycobacterial secretory proteins, but not PBS, significantly improved airway sensitivity (PC200). While the maximal Penh value was only significantly reduced in the 38-kDa protein-treated group, the DRS was significantly reduced in animals treated with BCG, 4 µg Ag85, or 4 µg 38-kDa protein. The fold increase in PC200 value after treatment (second PC200 value/first PC200 value) was significantly higher in the BCG-treated group (1.44±0.09) than in the asthma control group (1.06±0.06) (P<0.01), and was also significantly higher in all groups treated with mycobacterial secretory proteins. The fold increases in PC200 value in the mycobacterial secretory protein-treated groups were not significantly different than those in the BCG group.
Table 1.
Airway sensitivity (PC200), excessive airway narrowing (maximal Penh), and airway reactivity (DRS) in mice before and after treatment with BCG or mycobacterial secretory proteins
Data are expressed as the mean±standard error.
PC200, concentration of methacholine required for a 200% increase in enhanced pause (Penh) index from baseline; DRS, dose-response slope; BCG, bacille Calmette-Guérin. *P<0.05, †P<0.01 vs. pretreatment values.
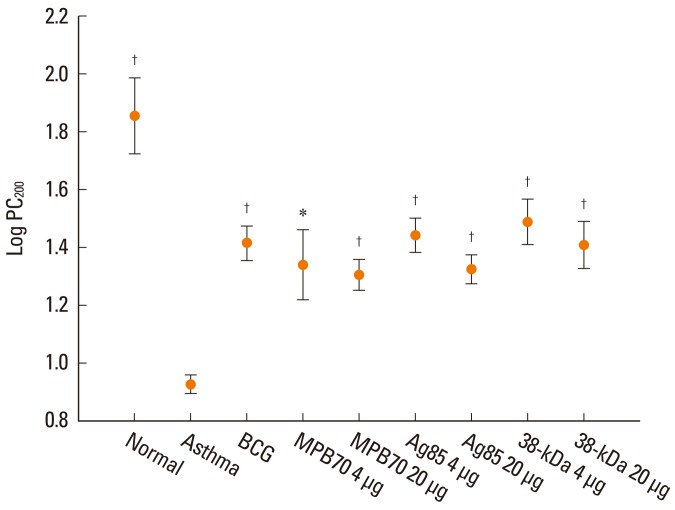
The PC200 value after treatment was significantly lower in the asthma control group than in the normal control group (Fig. 2). The PC200 values in all treatment groups were significantly higher than in the asthma control group. Similarly, the maximal Penh and DRS values were significantly higher in the asthma control group than in the normal control group. In addition, the values in the treatment groups, except for maximal Penh values in the 4 µg MPB70 and 20 µg 38-kDa protein groups and for DRS values in the 20 µg MPB70 and 20 µg Ag85 groups, were significantly lower than those in the asthma control group. Compared to the normal control group, all treatment groups showed significant differences in PC200 and maximal Penh values. However, the DRS values for the BCG, 4 µg Ag85, and 4 µg 38-kDa protein groups were not significantly different than those for the normal control group.
Fig. 2.
PC200 was significantly higher in all treatment groups than in the asthma control group. PC200: concentration of methacholine required for a 200% increase in enhanced pause index from baseline. *P<0.05, †P<0.001 vs. the asthma control group.
Cells in BAL fluid and lung tissue
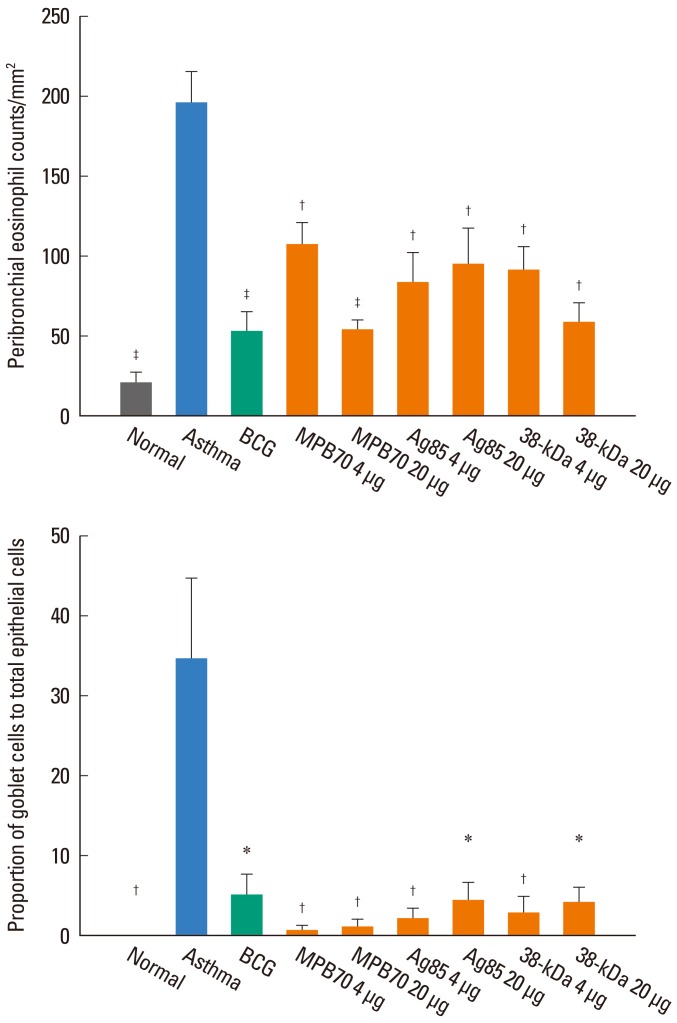
The proportion of eosinophils in the BAL fluid was significantly higher in the asthma control group than in the normal control group, and significantly lower in all treatment groups than in the asthma control group. However, the eosinophil proportions in all of the treatment groups were significantly higher than that in the normal control group (data not shown). Similarly, the number of eosinophils in the peribronchial tissue was significantly higher in the asthma control group than in the normal control group, and significantly lower in all treatment groups than in the asthma control group (Fig. 3). Numbers of neutrophils/lymphocytes in all treatment groups were not significantly different than those in the asthma control group. In addition, the proportion of goblet cells in the bronchial epithelium was significantly higher in the asthma control group than in the normal control group, and all treatment groups showed a significantly lower proportion of goblet cells compared to the asthma control group (Fig. 3). However, peribronchial eosinophil numbers and goblet cell proportions were significantly higher in all treatment groups than in the normal control group.
Fig. 3.
Peribronchial eosinophil numbers and goblet cell proportion in the bronchial epithelium were significantly reduced in all treatment groups compared to the asthma control group. *P<0.05, †P<0.01, ‡P<0.001 vs. the asthma control group.
Concentrations of cytokines in splenocyte culture supernatants
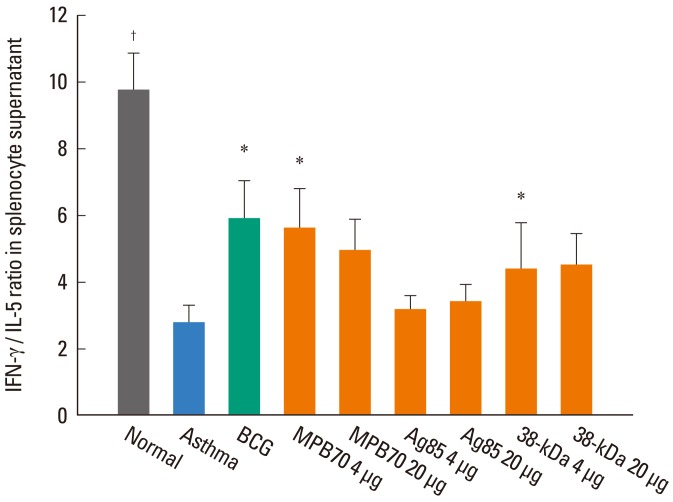
In the supernatants of concanavalin A-stimulated splenocytes, the IFN-γ level was significantly lower (499±58 vs. 860±97 pg/mL, P<0.05) and the IL-5 level significantly higher (211±81 vs. 90±10, P<0.001) in the asthma control group than in the normal control group, resulting in a significantly lower IFN-γ/IL-5 in the asthma control group (2.8±0.5 vs. 9.8±1.1, P<0.001) (Fig. 4). The IFN-γ/IL-5 ratio was significantly higher in the groups treated with BCG (5.9±1.1, P<0.05), 4 µg MPB70 (5.6±1.2, P<0.05), and 4 µg 38-kDa protein (5.0±0.8, P<0.05) compared to the asthma control group. The IFN-γ/IL-5 ratio was significantly lower in the group treated with 4 µg Ag85 (3.2±0.4, P<0.05) than in the 4 µg MPB70 group. The IFN-γ/IL-5 ratios for all treatment groups were significantly lower than that for the normal control group. The IL-17A level was significantly higher in the asthma control group than in the normal control group (171.0±22.7 vs. 73.8±7.1 pg/mL, P<0.01). However, IL-17A levels in all treatment groups, while significantly higher than that in the normal control group, were not significantly different than that in the asthma control group.
Fig. 4.
IFN-γ/IL-5 ratios in splenocyte culture supernatants were higher in the BCG, 4 µg MPB70, and 4 µg 38-kDa protein groups than in the asthma control group. *P<0.05, †P<0.001 vs. the asthma control group.
For all subjects, the IFN-γ/IL-5 ratios in the splenocyte culture supernatants were significantly associated with AHR, peribronchial eosinophil, and neutrophil numbers, and goblet cell hyperplasia (Table 2). IL-17A level showed a significant relationship with maximal airway narrowing, peribronchial eosinophil numbers, and goblet cell hyperplasia, and was also significantly correlated with IL-5 level (r=0.379, P<0.001).
Table 2.
Correlation coefficients for cytokine levels in splenocyte culture supernatants and markers of asthmatic airway reactions in mice treated with BCG or mycobacterial secretory proteins
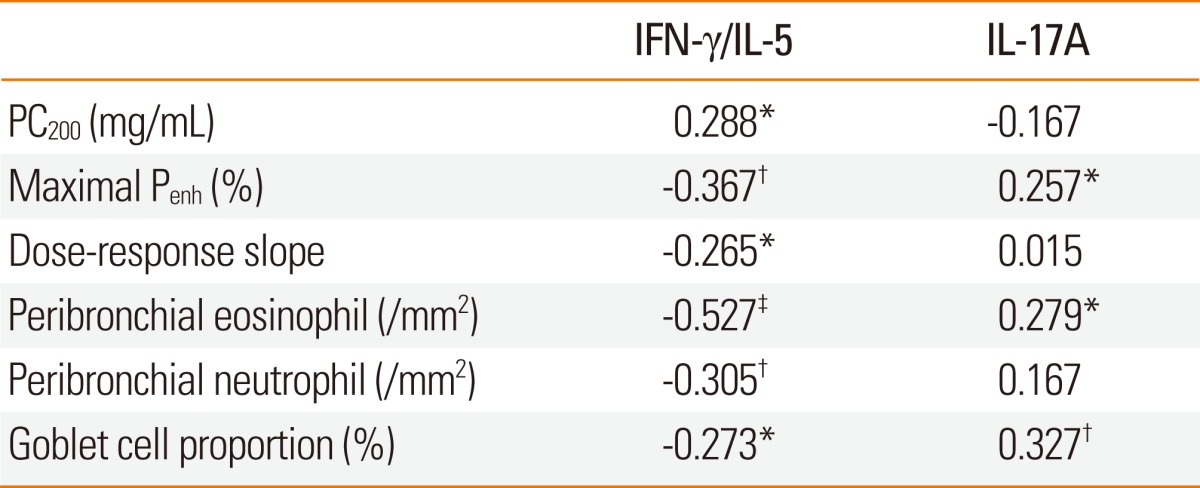
BCG, bacille Calmette-Guérin.
*P<0.05, †P<0.01, ‡P<0.001.
DISCUSSION
Treatment with mycobacterial secretory proteins had therapeutic effects on AHR and eosinophilic airway inflammation in mice. These results are consistent with those of previous studies showing that mycobacteria such as BCG have suppressive effects on asthma.7-9,11,13,21,26 However, whole BCG bacteria, even if heat-killed, could not be repeatedly administered to patients with asthma due to side effects.27 The results of this study suggest that mycobacterial secretory proteins such as Ag85, 38-kDa protein, and MPB70, which may have fewer side effects than whole bacteria, may be effective for the treatment of asthma as a result of suppression of AHR and eosinophilic airway inflammation. In our previous study,13 heat-killed preparations of BCG and M. tuberculosis did not effectively suppress airway sensitivity, while Shirtcliffe et al.27 reported that heat-killed BCG had no therapeutic effect on patients with asthma. However, because both BCG and M. tuberculosis culture supernatants effectively suppressed asthmatic reactions in our previous study,13 and because secretory components in culture supernatants effectively suppressed asthmatic reactions in the present study, it is anticipated that mycobacterial secretory proteins would be effective for treating human asthma as well.
The mycobacterial cell wall glycolipids lipoarabinomannan (LAM) and phosphatidylinositol mannan (PIM) can suppress airway eosinophilia through IL-10 production by T cells.28 In addition, M. tuberculosis chaperonin 60.1 can also suppress airway eosinophilia and methacholine-AHR.29 Mannose-capped LAM from M. bovis BCG Tokyo-172 enhances the differentiation of human peripheral blood naïve CD4 T cells into Th1 cells.30 Mannose-capped LAM31 and chaperonin 60.129 from M. tuberculosis can suppress asthmatic reactions through Treg cell induction. Thus, many mycobacterial cell wall components seem to have therapeutic effects on asthma through the above mechanisms. However, because heat-killed mycobacteria were ineffective against AHR in our previous study,13 and because Major et al.32 also showed that heat-killed BCG was less effective in suppressing airway eosinophilia than live BCG, these mycobacterial cell wall components seem to be heat-labile. Means et al.33 showed that a cell-associated mycobacterial factor that was distinct from LAM and that mediated Toll-like receptor (TLR)4-dependent activation was heat-sensitive, while a factor in the culture supernatant that activated cells in a TLR2-dependent manner was heat-stable. The mycobacterial secretory proteins in this study might be such heat-stable mycobacterial factors.
The Ag85 complex, which consists of secreted proteins from M. tuberculosis, is composed of Ag85A, Ag85B, and Ag85C, of which Ag85A and Ag85B induce a strong Th1-like response.34 Wu et al.35 showed in 2009 that an Ag85B DNA vaccine suppressed eosinophilic airway inflammation in a murine model of asthma, and Mori et al.36 reported that Ag85B suppressed Th2 cytokine-mediated acute-phase atopic inflammatory reactions by increasing Foxp3+ Treg cell numbers in an atopic dermatitis mouse model. Because 38-kDa protein16 and MPB7017 induce IFN-γ production, they may also be effective for treating asthma. To the best of our knowledge this study is the first to prove it.
In a large epidemiological study, an increase in tuberculosis notification rates of 25 per 100,000 was associated with a 4.7% decrease in the prevalence of wheezing,37 while BCG vaccination had only a weak protective effect against asthma.38 Therefore, secretory proteins from M. tuberculosis may have a strong therapeutic effect against asthma compared to BCG. In this study, 38-kDa protein and Ag85 of M. tuberculosis suppressed all three components of AHR (airway sensitivity, excessive airway narrowing, and airway reactivity), while MPB70 of BCG suppressed only airway sensitivity. Because M. tuberculosis induces the production of four times more IFN-γ than does BCG,39 we speculated that secretory proteins of M. tuberculosis would induce the production of greater amounts of IFN-γ than do BCG proteins, but this was not the case. The IFN-γ/IL-5 ratio was higher in the MPB70-treated group than in the Ag85-treated group in this study. Hence, the superior effect of M. tuberculosis on AHRmay not primarily depend on IFN-γ but Treg cells. However, further studies are required to confirm this. In this study, the lower dose of 38-kDa protein and Ag85 from M. tuberculosis tested (4 µg) was more effective at suppressing AHR than the higher dose (20 µg), similar to the observation of Lin et al.,18 who found that addition of high-dose dehydroepiandrosterone to BCG reduced the efficacy of BCG. Although the reason for this paradoxical response is not clear, a plausible explanation can be found in a report by Yoshida et al.,40 who showed that ongoing AHR following allergen challenge in IFN-γ-deficient mice was resolved by treatment with IFN-γ for 1 week, but aggravated by treatment for 4 weeks. Thus, the superfluous IFN-γ in mice treated with higher doses might not have a beneficial effect on asthma, but may instead be detrimental, even if it is transient, as brief Th1-related exacerbation of asthma may follow respiratory viral infection.41 Further investigation is necessary to determine the proper doses of mycobacterial secretory proteins for effective treatment of asthma.
Although the changes in the IFN-γ/IL-5 ratio after treatment did not coincide well with the effects of mycobacterial component treatment on AHR or airway cells, the ratios tended to be higher in the treatment groups than the asthma control group, and the IFN-γ/IL-5 ratio was significantly correlated with AHR, airway eosinophilia, and goblet cell proportion. These results are consistent with previous reports showing that mycobacteria7-11,21,26,42 and their components29 produced suppressive effects by shifting the Th1/Th2 balance to Th1. Because Treg cells can suppress airway eosinophilia through suppression of both Th1 and Th2 cells by secreting IL-10 and transforming growth factor-β,12 it is necessary to check these cytokine levels. However, we were unable to do this because they were undetectable in a preliminary study. Meanwhile, IL-17A was significantly associated with maximal airway narrowing and goblet cell proportion in this study. IL-17 produced by Th17 cells was recently reported to mediate neutrophilic airway inflammation and AHR.43 In contrast to this previous report,43 IL-17A levels were associated with peribronchial infiltration of eosinophils, but not neutrophils, in the present study. IL-17A levels showed a significant relationship with IL-5 levels, corroborating a report by Bullens et al.,44 who showed that IL-17 mRNA level was correlated with IL-5 mRNA level in sputum from patients with asthma. Thus, while its role in asthma is unclear, IL-17A seems unlikely to promote the therapeutic effects of mycobacterial secretory proteins.
Various mycobacterial lipoproteins are recognized by TLR2 in association with TLR1/TLR6 or by TLR4 on the surface of antigen-presenting cells (APCs) and induce naïve T cells to differentiate, mainly into Th1/Treg cells (immune polarization), with the help of IL-12 and IL-10 secreted from APCs.45 Lipomannan, the precursor of LAM, is recognized by TLR2, ManLAMs by the mannose receptor or DC-SIGN, PIM by TLR2,46,47 chaperonin 60.1 by TLR4,29 and 38-kDa glycolipoprotein by both TLR2 and TLR4. However, for Ag85, there is only one report showing that it does not stimulate TLR2 or TLR4,48 and for MPB70, no pattern recognition receptor has yet been identified. Therefore, further investigation in this area is necessary.
MPB70, a BCG secretory protein, was as effective as live BCG for the treatment of established asthma in this study. In our previous study,21 among the various strains of BCG, the Tokyo 172 strain, but not the Pasteur F1173P2, Tice, or Connaught strains, significantly suppressed AHR. Because MPB70 is present at high concentrations in Tokyo strain culture supernatants, but only in very small amounts in Pasteur, Tice, and Copenhagen strain culture supernatants,49 the strain-dependency of the effects of BCG on asthma may be affected by differences in MPB70 levels between the various strains.
Because cytokine levels in the splenocyte culture supernatants may not exactly reflect the local airway status, it would be better to measure cytokine levels directly in the airways. We tried for the first time to detect cytokine mRNAs in the lungs of mice by semi-quantitative RT-PCR, but the results were disappointingly not interpretable and we therefore did not present them in this study. We did not obtain any data on innate immunity because we have no experience with TRL studies. Further experiments on this are desirable in the future. Although Hamelmann et al.50 reported that an increase in Penh in response to inhaled methacholine is a valid indicator of AHR, AHR measurements must be validated using a more rigorous method.
In conclusion, mycobacterial secretory proteins were, like live BCG, effective for the treatment of established asthma, and increased the IFN-γ/IL-5 ratio. Thus, allergic asthma can be effectively treated with mycobacterial secretory proteins.
ACKNOWLEDGMENTS
We are indebted to Ms. Young-Ah Koh for her technical support. This study was supported by a grant (CRI09066-1) from the Chonnam National University Hospital Research Institute of Clinical Medicine. The authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Ninan TK, Russell G. Respiratory symptoms and atopy in Aberdeen schoolchildren: evidence from two surveys 25 years apart. BMJ. 1992;304:873–875. doi: 10.1136/bmj.304.6831.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malik G, Tagiyeva N, Aucott L, McNeill G, Turner SW. Changing trends in asthma in 9-12 year olds between 1964 and 2009. Arch Dis Child. 2011;96:227–231. doi: 10.1136/adc.2010.189175. [DOI] [PubMed] [Google Scholar]
- 3.Annesi-Maesano I, Mourad C, Daures JP, Kalaboka S, Godard P. Time trends in prevalence and severity of childhood asthma and allergies from 1995 to 2002 in France. Allergy. 2009;64:798–800. doi: 10.1111/j.1398-9995.2008.01886.x. [DOI] [PubMed] [Google Scholar]
- 4.Asher MI. Recent perspectives on global epidemiology of asthma in childhood. Allergol Immunopathol (Madr) 2010;38:83–87. doi: 10.1016/j.aller.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Cookson WO, Moffatt MF. Asthma: an epidemic in the absence of infection? Science. 1997;275:41–42. doi: 10.1126/science.275.5296.41. [DOI] [PubMed] [Google Scholar]
- 6.Rook GA, Hamelmann E, Brunet LR. Mycobacteria and allergies. Immunobiology. 2007;212:461–473. doi: 10.1016/j.imbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Koh YI, Choi IS, Kim WY. BCG infection in allergen-presensitized rats suppresses Th2 immune response and prevents the development of allergic asthmatic reaction. J Clin Immunol. 2001;21:51–59. doi: 10.1023/a:1006745116360. [DOI] [PubMed] [Google Scholar]
- 8.Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guérin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998;187:561–569. doi: 10.1084/jem.187.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herz U, Gerhold K, Grüber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998;102:867–874. doi: 10.1016/s0091-6749(98)70030-2. [DOI] [PubMed] [Google Scholar]
- 10.Wang CC, Rook GA. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998;93:307–313. doi: 10.1046/j.1365-2567.1998.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopfenspirger MT, Agrawal DK. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J Immunol. 2002;168:2516–2522. doi: 10.4049/jimmunol.168.5.2516. [DOI] [PubMed] [Google Scholar]
- 12.Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8:625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 13.Han ER, Choi IS, Eom SH, Kim HJ. Preventive effects of mycobacteria and their culture supernatants against asthma development in BALB/c mice. Allergy Asthma Immunol Res. 2010;2:34–40. doi: 10.4168/aair.2010.2.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiker HG, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung SB, Yang CS, Lee JS, Shin AR, Jung SS, Son JW, Harding CV, Kim HJ, Park JK, Paik TH, Song CH, Jo EK. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006;74:2686–2696. doi: 10.1128/IAI.74.5.2686-2696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonseca DP, Benaissa-Trouw B, van Engelen M, Kraaijeveld CA, Snippe H, Verheul AF. Induction of cell-mediated immunity against Mycobacterium tuberculosis using DNA vaccines encoding cytotoxic and helper T-cell epitopes of the 38-kilodalton protein. Infect Immun. 2001;69:4839–4845. doi: 10.1128/IAI.69.8.4839-4845.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Attiyah R, Shaban FA, Wiker HG, Oftung F, Mustafa AS. Synthetic peptides identify promiscuous human Th1 cell epitopes of the secreted mycobacterial antigen MPB70. Infect Immun. 2003;71:1953–1960. doi: 10.1128/IAI.71.4.1953-1960.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin XH, Choi IS, Koh YA, Cui Y. Effects of combined bacille Calmette-Guérin and dehydroepiandrosterone treatment on established asthma in mice. Exp Lung Res. 2009;35:250–261. doi: 10.1080/01902140802626656. [DOI] [PubMed] [Google Scholar]
- 19.Lim JH, Park JK, Jo EK, Song CH, Min D, Song YJ, Kim HJ. Purification and immunoreactivity of three components from the 30/32-kilodalton antigen 85 complex in Mycobacterium tuberculosis. Infect Immun. 1999;67:6187–6190. doi: 10.1128/iai.67.11.6187-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JS, Paik TH, Yoo YC, Lee J, Shin A, Song CH, Jo EK, Kim HJ, Park JK. Purification of native Ag85 complex, 38-kDa and MTB12 protein antigens from the culture filtrate of Mycobacterium tuberculosis. J Bacteriol Virol. 2006;36:211–220. [Google Scholar]
- 21.Choi IS, Lin XH, Koh YA, Koh YI, Lee HC. Strain-dependent suppressive effects of BCG vaccination on asthmatic reactions in BALB/c mice. Ann Allergy Asthma Immunol. 2005;95:571–578. doi: 10.1016/S1081-1206(10)61021-6. [DOI] [PubMed] [Google Scholar]
- 22.Malo JL, Chan-Yeung M. Occupational asthma. In: Adkinson NF Jr, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Middleton's Allergy Principles & Practice. 6th ed. Philadelphia: Mosby Inc.; 2003. pp. 1333–1352. [Google Scholar]
- 23.Ozdemir C, Akkoc T, Bahceciler NN, Kucukercan D, Barlan IB, Basaran MM. Impact of Mycobacterium vaccae immunization on lung histopathology in a murine model of chronic asthma. Clin Exp Allergy. 2003;33:266–270. doi: 10.1046/j.1365-2222.2003.01595.x. [DOI] [PubMed] [Google Scholar]
- 24.Maestrelli P, Saetta M, Di Stefano A, Calcagni PG, Turato G, Ruggieri MP, Roggeri A, Mapp CE, Fabbri LM. Comparison of leukocyte counts in sputum, bronchial biopsies, and bronchoalveolar lavage. Am J Respir Crit Care Med. 1995;152:1926–1931. doi: 10.1164/ajrccm.152.6.8520757. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka H, Masuda T, Tokuoka S, Komai M, Nagao K, Takahashi Y, Nagai H. The effect of allergen-induced airway inflammation on airway remodeling in a murine model of allergic asthma. Inflamm Res. 2001;50:616–624. doi: 10.1007/PL00000243. [DOI] [PubMed] [Google Scholar]
- 26.Choi IS, Lin XH, Koh YA, Cui Y. Inoculation route-dependent and allergen-specific suppressive effects of bacille Calmette-Guérin vaccination on asthmatic reactions in BALB/c mice. Lung. 2007;185:179–186. doi: 10.1007/s00408-007-9003-4. [DOI] [PubMed] [Google Scholar]
- 27.Shirtcliffe PM, Easthope SE, Weatherall M, Beasley R. Effect of repeated intradermal injections of heat-inactivated Mycobacterium bovis bacillus Calmette-Guérin in adult asthma. Clin Exp Allergy. 2004;34:207–212. doi: 10.1111/j.1365-2222.2004.01861.x. [DOI] [PubMed] [Google Scholar]
- 28.Sayers I, Severn W, Scanga CB, Hudson J, Le Gros G, Harper JL. Suppression of allergic airway disease using mycobacterial lipoglycans. J Allergy Clin Immunol. 2004;114:302–309. doi: 10.1016/j.jaci.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 29.Riffo-Vasquez Y, Spina D, Page C, Tormay P, Singh M, Henderson B, Coates A. Effect of Mycobacterium tuberculosis chaperonins on bronchial eosinophilia and hyper-responsiveness in a murine model of allergic inflammation. Clin Exp Allergy. 2004;34:712–719. doi: 10.1111/j.1365-2222.2004.1931.x. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Hasegawa A, Hosokawa H, Yamashita M, Motohashi S, Naka T, Okamoto Y, Fujita Y, Ishii Y, Taniguchi M, Yano I, Nakayama T. Human Th1 differentiation induced by lipoarabinomannan/lipomannan from Mycobacterium bovis BCG Tokyo-172. Int Immunol. 2008;20:849–860. doi: 10.1093/intimm/dxn043. [DOI] [PubMed] [Google Scholar]
- 31.Garg A, Barnes PF, Roy S, Quiroga MF, Wu S, García VE, Krutzik SR, Weis SE, Vankayalapati R. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur J Immunol. 2008;38:459–469. doi: 10.1002/eji.200737268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Major T, Wohlleben G, Reibetanz B, Erb KJ. Application of heat killed Mycobacterium bovis-BCG into the lung inhibits the development of allergen-induced Th2 responses. Vaccine. 2002;20:1532–1540. doi: 10.1016/s0264-410x(01)00496-0. [DOI] [PubMed] [Google Scholar]
- 33.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 34.Lozes E, Huygen K, Content J, Denis O, Montgomery DL, Yawman AM, Vandenbussche P, Van Vooren JP, Drowart A, Ulmer JB, Liu MA. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine. 1997;15:830–833. doi: 10.1016/s0264-410x(96)00274-5. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Xu J, Cai C, Gao X, Li L, Zhong N. Ag85B DNA vaccine suppresses airway inflammation in a murine model of asthma. Respir Res. 2009;10:51. doi: 10.1186/1465-9921-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori H, Yamanaka K, Matsuo K, Kurokawa I, Yasutomi Y, Mizutani H. Administration of Ag85B showed therapeutic effects to Th2-type cytokine-mediated acute phase atopic dermatitis by inducing regulatory T cells. Arch Dermatol Res. 2009;301:151–157. doi: 10.1007/s00403-008-0873-y. [DOI] [PubMed] [Google Scholar]
- 37.von Mutius E, Pearce N, Beasley R, Cheng S, von Ehrenstein O, Björkstén B, Weiland S. International patterns of tuberculosis and the prevalence of symptoms of asthma, rhinitis, and eczema. Thorax. 2000;55:449–453. doi: 10.1136/thorax.55.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grüber C, Meinlschmidt G, Bergmann R, Wahn U, Stark K. Is early BCG vaccination associated with less atopic disease? An epidemiological study in German preschool children with different ethnic backgrounds. Pediatr Allergy Immunol. 2002;13:177–181. doi: 10.1034/j.1399-3038.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- 39.Chernousova LN, Smirnova TG, Afanasieva EG, Karpov VL, Timofeev AV. Ex vivo production of interferon-gamma, tumor necrosis factor-alpha, and interleukin-6 by mouse macrophages during infection with M. bovis and M. tuberculosis H37Rv. Bull Exp Biol Med. 2007;144:709–712. doi: 10.1007/s10517-007-0412-4. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida M, Leigh R, Matsumoto K, Wattie J, Ellis R, O'Byrne PM, Inman MD. Effect of interferon-γ on allergic airway responses in interferon-γ-deficient mice. Am J Respir Crit Care Med. 2002;166:451–456. doi: 10.1164/rccm.200202-095OC. [DOI] [PubMed] [Google Scholar]
- 41.van Schaik SM, Obot N, Enhorning G, Hintz K, Gross K, Hancock GE, Stack AM, Welliver RC. Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice. J Med Virol. 2000;62:257–266. doi: 10.1002/1096-9071(200010)62:2<257::aid-jmv19>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 42.Choi IS, Koh YI. Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial. Ann Allergy Asthma Immunol. 2002;88:584–591. doi: 10.1016/S1081-1206(10)61890-X. [DOI] [PubMed] [Google Scholar]
- 43.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barlan I, Bahceciler NN, Akdis M, Akdis CA. Bacillus Calmette-Guerin, Mycobacterium bovis, as an immunomodulator in atopic diseases. Immunol Allergy Clin North Am. 2006;26:365–377. doi: 10.1016/j.iac.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Quesniaux V, Fremond C, Jacobs M, Parida S, Nicolle D, Yeremeev V, Bihl F, Erard F, Botha T, Drennan M, Soler MN, Le Bert M, Schnyder B, Ryffel B. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect. 2004;6:946–959. doi: 10.1016/j.micinf.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 47.Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007;9:1087–1098. doi: 10.1111/j.1462-5822.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 48.Simons MP, Moore JM, Kemp TJ, Griffith TS. Identification of the mycobacterial subcomponents involved in the release of tumor necrosis factor-related apoptosis-inducing ligand from human neutrophils. Infect Immun. 2007;75:1265–1271. doi: 10.1128/IAI.00938-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harboe M, Nagai S, Patarroyo ME, Torres ML, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]