Abstract
Objectives
To describe secular trends in Staphylococcus aureus bacteraemia (SAB) and to assess the impacts of infection control practices, including universal methicillin-resistant Staphylococcus aureus (MRSA) admission screening on associated clinical burdens.
Design
Retrospective cohort study and multivariate time-series analysis linking microbiology, patient management and health intelligence databases.
Setting
Teaching hospital in North East Scotland.
Participants
All patients admitted to Aberdeen Royal Infirmary between 1 January 2006 and 31 December 2010: n=420 452 admissions and 1 430 052 acute occupied bed days (AOBDs).
Intervention
Universal admission screening programme for MRSA (August 2008) incorporating isolation and decolonisation.
Primary and secondary measures
Hospital-wide prevalence density, hospital-associated incidence density and death within 30 days of MRSA or methicillin-sensitive Staphylococcus aureus (MSSA) bacteraemia.
Results
Between 2006 and 2010, prevalence density of all SAB declined by 41%, from 0.73 to 0.50 cases/1000 AOBDs (p=0.002 for trend), and 30-day mortality from 26% to 14% (p=0.013). Significant reductions were observed in MRSA bacteraemia only. Overnight admissions screened for MRSA rose from 43% during selective screening to >90% within 4 months of universal screening. In multivariate time-series analysis (R2 0.45 to 0.68), universal screening was associated with a 19% reduction in prevalence density of MRSA bacteraemia (−0.035, 95% CI −0.049 to −0.021/1000 AOBDs; p<0.001), a 29% fall in hospital-associated incidence density (−0.029, 95% CI −0.035 to −0.023/1000 AOBDs; p<0.001) and a 46% reduction in 30-day mortality (−15.6, 95% CI −24.1% to −7.1%; p<0.001). Positive associations with fluoroquinolone and cephalosporin use suggested that antibiotic stewardship reduced prevalence density of MRSA bacteraemia by 0.027 (95% CI 0.015 to 0.039)/1000 AOBDs. Rates of MSSA bacteraemia were not significantly affected by screening or antibiotic use.
Conclusions
Declining clinical burdens from SAB were attributable to reductions in MRSA infections. Universal admission screening and antibiotic stewardship were associated with decreases in MRSA bacteraemia and associated early mortality. Control of MSSA bacteraemia remains a priority.
Article summary
Article focus
This study describes the changing epidemiology of MRSA and MSSA bacteraemia in a large inpatient population from Scotland over a 5-year period.
Second, it evaluates the impact of universal MRSA admission screening, and other infection control practices, on hospital-wide rates of MRSA bacteraemia.
Key messages
Recent declines in clinical burdens from SAB in North East Scotland were attributable to a reduction in invasive MRSA infections.
Compared with a strategy of targeted screening in high-risk environments, universal admission screening may significantly reduce rates of MRSA bacteraemia and associated early mortality alongside improvements in antibiotic stewardship and infection control.
Strategies to reduce clinical burdens from MSSA bacteraemia are required if progress towards national targets for all SAB is to be sustained.
Strengths and limitations of this study
Without a contemporary control, this study did not prove causality but a temporal association between universal admission screening and rates of MRSA bacteraemia.
ARIMA modelling accounted for the non-independence of data and stochastic elements in time series of infections, and the dynamic effects of changes in other aspects of care.
Findings may be limited to large public hospitals with intensive care units and endemic MRSA but low rates of MRSA infection.
Introduction
Staphylococcus aureus is an important cause of serious, invasive and healthcare-associated infections worldwide.1 In high-income countries, it remains a leading cause of community and nosocomial bacteraemia,2 associated with mortality rates of 20%–50%3 4 and large economic burdens.5 In the UK, dramatic increases in Staphylococcus aureus bacteraemia (SAB) during the 1990s were attributed to methicillin-resistant Staphylococcus aureus (MRSA)3 6 and healthcare exposures,7 engendering aggressive public health responses.8 A decade of national mandatory surveillance of both methicillin-sensitive Staphylococcus aureus (MSSA) and MRSA bacteraemia has suggested impacts from infection control measures,9 10 but there remain over 12 000 cases annually.11 12
Despite a steep reduction in MRSA bacteraemia from a peak in 2003/2004, rates of MSSA bacteraemia have remained relatively stable.11 12 Reasons for this MRSA-specific decline are not fully understood.9 10 Meanwhile, studies assessing the importance of methicillin resistance to outcomes after SAB have yielded conflicting results.3 4 13–18 These uncertainties are reflected in different public health approaches: England and Wales implemented performance targets for reducing MRSA bacteraemia only,9 while NHS Scotland's strategy aimed to reduce all SAB to 70% of 2005/2006 levels by 2010.19 Some authors have warned that policy focusing on MRSA alone may have unintended adverse effects on control of MSSA.20 It is therefore important to understand the evolving epidemiology of both MRSA and MSSA bacteraemia.21
UK policy on reducing burdens from MRSA has advocated admission screening, with subsequent decolonisation and isolation, despite weaknesses in evidence.22–25 Studies on MRSA screening have generally assessed impacts on bacteraemia by surveillance in high-risk groups,26 27 while studies of universal surveillance have taken all MRSA infections as the primary outcome.25 28 In 2008, a universal screening strategy was piloted in three NHS Scotland trusts,29 30 providing an opportunity to assess effects on rates of MRSA bacteraemia, compared with a previous strategy of selective screening in high-risk environments.
This study aimed to describe the changing clinical epidemiology of SAB in a large inpatient population over a 5-year period and to evaluate the impact of infection control measures, including universal MRSA admission screening. Our prespecified null hypothesis was that universal screening would not significantly reduce rates of MRSA bacteraemia, after accounting for prior trends and changes in other aspects of care in time-series intervention analysis.
Methods
Study design
This retrospective cohort study described secular trends in SAB in all admissions to Aberdeen Royal Infirmary (ARI) between 2006 and 2010. A quasi-experimental before-and-after design used time-series data from the same period to assess the impact of introducing universal admission surveillance on MRSA bacteraemia alongside other infection control practices (figure 1). Controls were historic trends in MRSA bacteraemia and concurrent trends in MSSA bacteraemia.
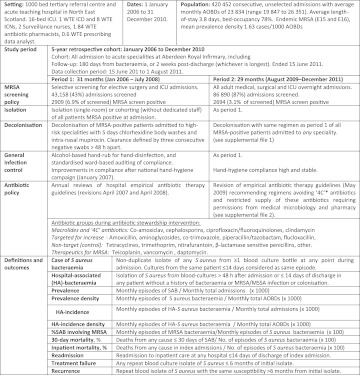
Figure 1.
Study overview in accordance with the ORION (Outbreak Reports and Intervention Studies Of Nosocomial infection) statement.31 *‘4C’ antibiotics are clindamycin, ciprofloxacin (all fluoroquinolones), cephalosporins (all generations), co-amoxiclav. AOBDs, acute occupied bed days; ICD, infection control doctor; ICN, infection control nurse; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; SAB, Staphylococcus aureus bacteraemia; WTE, whole-time equivalents calculated as 37.5 h/week×52 weeks =1950 h/year.
Setting
ARI is a tertiary referral centre and acute teaching hospital (1000 beds, 85 000 annual admissions), serving a population of 500 000 in North East Scotland (NHS Grampian). It provides a full range of acute medical and surgical services, with a 16-bedded intensive care unit (ICU) (800 admissions/year) and a cardiac ICU (six beds, 600 admissions/year). Microbiology services also serve the on-site 185-bedded maternity and 85-bedded children's hospitals.
Admission screening intervention
Universal admission screening for MRSA was introduced in NHS Grampian in August 2008 as part of an NHS Scotland Pathfinder Project detailed elsewhere.29 30 This 32-month pilot study (ending March 2011) tested a strategy suggested as most clinically and cost-effective by an NHS Scotland Health Technology Assessment (supplemental file 1).29 This involved screening of all overnight admissions to acute specialities (excluding obstetrics, paediatrics and psychiatry) by nasal (and wound or device as necessary) swabs, isolation or cohorting of all patients with known or new colonisation or infection with MRSA and decolonising of all MRSA-positive patients admitted to any specialty. Decolonisation therapy included 5 days of daily body wash with 4% chlorhexidine gluconate and thrice-daily mupirocin nasal ointment. Patients were re-swabbed a minimum of 2 days after decolonisation and could be removed from isolation on receipt of three successive negative swabs, taken ≥48 h apart. Elective patients were screened at preadmission assessment or on admission. Compliance with screening and infection control protocols was monitored. Prior to the intervention, MRSA screening was performed on selected high-risk patients only, including intensive care and elective surgical admissions, with an identical strategy of isolation and decolonisation.
Outcomes and potential confounders
SAB was defined as the isolation of any S aureus from ≥1 blood culture bottle. Cultures from the same patient within 14 days of the original isolate were considered to represent the same episode. Patients could be included more than once in analysis for different episodes. Hospital-associated (HA) bacteraemia was defined as isolation of S aureus from blood cultures >48 h after admission or within 14 days of discharge, without history of bacteraemia or MRSA colonisation or infection.
The primary outcome measure was prevalence density of MRSA and MSSA bacteraemia. Secondary outcomes are detailed in figure 1. Secular trends in longer term outcomes were also investigated with recurrence expressed as episodes per 1000 patient-months to avoid follow-up bias.
In examining secular trends and the impacts of universal MRSA admission screening, we considered changes in other aspects of care and case-mix including: MRSA importation pressure (No. of patients who are MRSA positive or with a history of MRSA at admission/1000 acute occupied bed days (AOBDs)) length of stay,3 13 bed occupancy,7 patient age,3 4 13 admitting department,3 hand hygiene9 32–35 and antibiotic usage.34–37 We considered the effects of other hospital-wide infection control measures with potential to affect MRSA including: a national hand-hygiene campaign (January 2007); and a mixed persuasive and restrictive antibiotic stewardship intervention (May 2009), limiting use of antibiotics associated with Clostridium difficile and resistant gram-positive or gram-negative infections (figure 1 and supplemental file 2).
Study population
All patients admitted to medical, surgical, paediatric and maternity services at ARI between 1 January 2006 and 31 December 2010 were eligible for inclusion in the study. This period was chosen as it included the time frame stated in national targets for reducing rates of SAB. A time series of 60 months with equivalent baseline and intervention periods (31 and 29 months) also facilitated a robust time-series analysis.38 Outpatients in all specialities were excluded. Admissions resulting in death or discharge within 24 h were retained in the main analysis so as to capture burdens from community-associated bacteraemia. Patients at risk of incident HA bacteraemia were those hospitalised for at least 48 h without previous documented SAB. Follow-up was until inhospital death, 180 days from bacteraemia or a minimum of 2 weeks after discharge (whichever was longest) and ended on 15 June 2011.
Data collection
Electronic laboratory records were screened to identify admission screening swabs, previous or current MRSA colonisation or infection, episodes of SAB and location of sampling. Patient identifiers were used to identify multiple samples from the same patient.
Health intelligence databases provided data on demographics, admission details and mortality for all admissions between 2006 and 2010. Aggregated data on bed occupancy were also provided by month and department. For episodes of bacteraemia, data were triangulated using the hospital's Patient Management System. Numbers of admissions within the last 12 months and age were taken as a proxy of patients' baseline health.
Details on use of ‘4C’ (ciprofloxacin, cephalosporins, clindamycin, co-amoxiclav) and macrolide antibiotics (defined daily doses/1000 AOBDs) and hand hygiene (litres of alcohol gel used/1000 AOBDs; monthly average hand-hygiene compliance assessed by nationally standardised audit of opportunity and technique) were ascertained from pharmacy and infection control departments.
Use of routinely collected data meant an almost complete data set. Data on outcomes after discharge were missing for six patients (0.7%) with SAB and for obstetric or neonatal inpatients without bacteraemia. Outcomes were explored using a complete-case analysis or departments with complete data.
Laboratory methods
Screening swabs were tested by latex slide test after plating on chromogenic agar (Brilliance–Oxoid, Basingstoke, UK), followed by confirmatory coagulase test. Antibiotic sensitivities were evaluated by disc-diffusion test. Processing of screening and clinical samples was carried out 24 h a day, 7 days a week. After confirmation by laboratory staff, results were made immediately available on an electronic laboratory reporting system. Between 09:00 and 17:00 daily, positive MRSA screens were verbally reported to nursing staff on relevant wards and infection control teams. Turnaround time was typically <24 h. All S aureus blood isolates were identified initially by agglutination, using the Prolex—Blue Staph Latex Kit (Pro-Lab, Richmond Hill, Canada), and subsequently by a Vitek instrument, using custom-made Staphylococcus sensitivity cards (Biomerieux, Marcy l'Etoile, France).
Statistical analysis
Clinical epidemiology and secular trends
Comparisons between characteristics of MRSA and MSSA and non-bacteraemic inpatient cohorts were made by χ2, Mann–Whitney U or independent samples t tests. Univariate linear or logistic regression was used to model associations between risk factors and rates of SAB. An indirect standardised mortality ratio was calculated to explore excess mortality in SAB, using all ARI inpatients between 2006 and 2010 as the reference population, and standardising by age, gender and specialty. Attributable mortality, defined as the excess mortality caused by bacteraemia, was calculated using matched controls from this inpatient reference group, as crude mortality rate in controls minus crude mortality rate after bacteraemia.
Restricting analysis to the SAB cohort, determinants of 30-day mortality were explored by multivariate logistic regression. A priori determinants of methicillin sensitivity, month and demographics were included in a multivariate model alongside significant variables from univariate analysis (p<0.10). Interaction terms were generated for terms significantly associated by Spearman rank correlation but retained only where contributing to model fit. Competing hazards of inpatient mortality and being discharged alive were further explored with multivariate Cox-regression, with censoring at date of discharge or death, respectively. Length of stay was included as a time-dependent determinant of mortality.16
Secular trends in demographics, clinical characteristics and outcomes in SAB cohorts were evaluated by logistic or linear regressions, with month of isolate as the sole explanatory variable. Trends in rates were examined using Poisson regression, with Poisson distribution, log-link function and the natural logarithm of AOBDs as the offset. Difference in trends by admitting department was assessed by an interaction term (department × month of study). Multivariate Poisson regression models assessed secular trends after adjusting for changes in case-mix.
Impacts of universal MRSA admission screening
We conducted intervention analyses to model the effects of universal screening on SAB while controlling for hand hygiene, antibiotic use and other dynamic explanatory factors, using the Linear Transfer Function (LTF) identification method suggested by Pankratz39 After ensuring stationary series, an initial transfer function model was created, with six lags for all explanatory variables and an autoregressive term of order 1. An iterative process of eliminating non-significant terms, and identifying further autoregressive (AR) or moving average (MA) terms for parts of the model remaining unexplained, determined the most parsimonious LTF model. Model parameters were estimated using unconditional least squares and goodness-of-fit evaluated by R2. Finally, diagnostic checks were used to determine whether models adequately represented times-series data. These included checking the statistical significance of parameters, AR parameter stationarity and MA parameter invertibility, and auto-correlation (ACF) and partial auto-correlation (PACF) functions of residuals to ensure remaining variability was random. Analysis of concurrent trends in MSSA bacteraemia controlled for unidentified aspects of care or infection control affecting the clinical epidemiology of SAB.
Intervention analysis was conducted using SCA software (Chicago, Illinois, USA, 1992) as described by Liu and Hudak.40 All other analyses were performed using SPSS V.19.0 for windows.
Results
Descriptive epidemiology
Cohort and rates of SAB
There were 430 452 admissions to ARI between 2006 and 2010, representing 1 430 052 AOBDs (8% ICU). The total number of days of follow-up was 7 578 805: median, 181 days (range 180–355 days) for episodes of SAB, and 16 days (14 to 129 days) for other admissions.
Eight hundred and sixty-seven episodes of SAB were identified in 795 patients, including 208 cases of MRSA bacteraemia (24%). Sixty-two per cent of MRSA and 44% of MSSA bacteraemia were HA (p<0.001). Overall prevalence density of SAB was 0.61/1000 AOBDs and HA incidence density was 0.29/1000 AOBDs. Prevalence and HA incidence were 2.1/1000 admissions and 3.0/1000 admissions, respectively. Patients with SAB were more likely to be male, older and admitted to medical or ICU settings than the remainder inpatient population (table 1).
Table 1.
Characteristics of inpatient cohorts with and without Staphylococcus aureus bacteraemia, 2006–2010
| Characteristic | Cohort |
p Value* |
||||
| None (n=419 585) | All SAB (n=867) | MSSA (n=659) | MRSA (n=208) | MRSA vs MSSA | All SAB vs none | |
| Overnight admissions | 262 412 (63) | 864 (99) | 656 (99) | 206 (99) | 0.801 | <0.001 |
| Hospital associated (onset >48 h) | 137 520 (33)† | 416 (48) | 287 (44) | 129 (62) | <0.001 | <0.001 |
| Demographics | ||||||
| Gender (female) | 242 668 (58) | 287 (33) | 227 (34) | 60 (29) | 0.14 | <0.001 |
| Mean age in years (SD) | 56 (5) | 58 (22) | 55 (22) | 65 (18) | <0.001 | <0.001 |
| Clinical background | ||||||
| Admitting department (all) | <0.001 | <0.001 | ||||
| Medical | 130 209 (31) | 600 (69) | 471 (72) | 129 (62) | 0.010 | <0.001 |
| Surgical | 173 851 (41) | 173 (20) | 118 (18) | 55 (26) | 0.007 | <0.001 |
| ICU | 6206 (1) | 60 (7) | 37 (6) | 23 (11) | 0.007 | <0.001 |
| Paediatrics/neonatal | 47 726 (10) | 27 (4) | 26 (4) | 1 (1) | 0.008 | <0.001 |
| Maternity | 61 593 (15) | 7 (1) | 7 (1) | 0 (0) | 0.136 | <0.001 |
| Previous S aureus bacteraemia | –‡ | 73 (9) | 49 (8) | 24 (12) | 0.068 | – |
| MRSA colonisation at admission (%)§ | 8134 (4.3) | 160 (19) | 72 (11) | 88 (43) | <0.001 | <0.001 |
| Admission within past 12 months | –‡ | 522 (60) | 366 (56) | 156 (75) | <0.001 | – |
| Median (IQR) time from admission to bacteraemia, days | – | 2 (0–9) | 1 (0–7) | 7 (0–19) | 0.002 | – |
| Outcomes | ||||||
| 30-day mortality | – | 173 (20) | 110 (17) | 63 (30) | <0.001 | – |
| Inhospital death | 7165 (2)¶ | 209 (25)¶ | 134 (21)¶ | 75 (36)¶ | <0.001 | <0.001 |
| Median (IQR) length of stay, days | 3.8 (3.4–3.9) | 20 (11–39) | 19 (10–37) | 27 (14–52) | <0.001 | <0.001 |
| Readmission (≤14 days)** | 26 534 (8)¶ | 119 (19)¶ | 86 (17)¶ | 33 (25)¶ | 0.036 | <0.001 |
| Treatment failure | – | 42 (4.8) | 31 (4.7) | 13 (6.3) | 0.732 | – |
| Recurrence rate (100 patient/years) | – | 0.78 | 0.81 | 1.26 | 0.627 | – |
Data are represented as n (%), mean (SD) or median (IQR).
χ2, Mann–Whitney U or independent samples t test.
Patients without bacteraemia admitted >48 h.
Data not available.
% Eligible admissions.
Excluding admissions to maternity and neonatal departments (data not available, n=65 849) and episodes of SAB with incomplete data (n=6) associated with survival at discharge.
In those alive at discharge.
ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; SAB, Staphylococcus aureus bacteraemia.
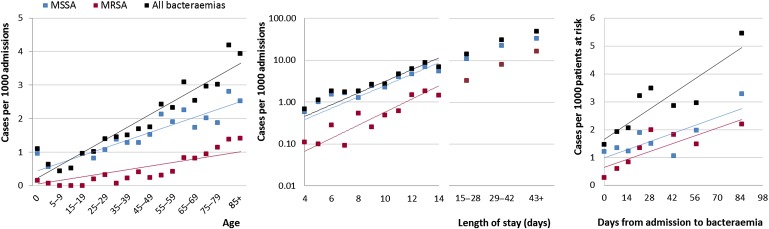
There were strong associations between rate of SAB and age, days since admission and length of stay (figure 2). Patients colonised with MRSA at admission were 17 times more likely to develop HA MRSA bacteraemia (0.78 cases/1000 AOBDs) than those not colonised (0.05 cases/1000 AOBDs) (crude OR (95% CI)=17.2 (15 to 20), p<0.001). Methicillin-resistant bacteraemia occurred more frequently in ICU or surgical settings, older patients, following MRSA colonisation and after prolonged or recent admission. Comparing community with HA bacteraemia, there were no significant differences in demographics or rates of previous admission in the past 12 months (41% vs 37%; p=0.10).
Figure 2.
Rates of Staphylococcus aureus bacteraemia by age group, length of stay and days from admission. p<0.01 for all linear regression lines. Note logarithmic scale for length of stay. Linear trend fitted after logarithmic transformation. MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Clinical outcomes
Inpatient and 30-day all-cause mortality rates after SAB were 25% and 20%, respectively, and outcomes were consistently worse than for patients without bacteraemia (table 1). Inpatient mortality was over six times higher than expected in the SAB cohort (standardised mortality ratio 6.4, 95% CI 5.7 to 7.0). Attributable inpatient mortality was 20% (MRSA 31%, MSSA 17%).
Methicillin resistance was associated with longer length of stay and increased readmission rates. The crude OR for mortality within 30 days of isolation of MRSA versus MSSA was 2.15 (95% CI 1.50 to 3.08; p<0.001). A final multivariate logistic regression model confirmed age, month of study (secular trend) and HA infection as independent risk factors for 30-day mortality; however, after adjustment for these covariates, methicillin resistance was not a significant determinant (table 2).
Table 2.
Multivariate logistic and Cox-regression models of risk factors for 30-day mortality, inpatient mortality and discharge alive
| 30 day mortality* |
Inpatient mortality† |
Discharge alive‡ |
||||
| OR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| MRSA | 1.38 (0.93 to 2.06) | 0.112 | 1.47 (1.09 to 1.98) | 0.012 | 1.09 (0.89 to 1.33) | 0.416 |
| Gender (female) | 1.41 (0.96 to 2.04) | 0.075 | 1.18 (0.89 to 1.58) | 0.244 | 1.06 (0.89 to 1.25) | 0.536 |
| Age (10/years) | 1.79 (1.58 to 1.97) | <0.001 | 1.42 (1.29 to 1.57) | <0.001 | 0.86 (0.83 to 0.90) | <0.001 |
| Hospital-associated SAB | 1.56 (1.08 to 2.26) | 0.018 | 2.27 (1.67 to 2.27) | <0.001 | 1.50 (1.26 to 1.80) | <0.001 |
| Secular trend per 3 months | 0.87 (0.77 to 0.99) | 0.028 | 0.92 (0.83 to 1.02) | 0.094 | – | – |
| Length of stay (7/days)§ | – | – | 1.02 (1.01 to 1.03) | <0.001 | 0.98 (0.97 to 0.98) | <0.001 |
| ICU admission | – | – | – | – | 0.70 (0.59 to 1.00) | 0.052 |
Logistic regression for 30-day mortality. This model had good calibration (Hosmer–Lemeshow goodness-of-fit p=0.93) and discrimination (area under receiver operator characteristic curve =0.77).
Cox (proportional hazards) regression. Model χ2 (df) =115 (6); p<0.001.
Cox (proportional hazards) regression. Model χ2 (df) =265 (6); p<0.001.
Entered as a time-dependent covariate.
ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; SAB, Staphylococcus aureus bacteraemia.
In a multivariate Cox-regression model, methicillin resistance was associated with a nearly 50% increased hazard of inpatient death (table 2), but there was no significant difference in discharge rate in survivors. Age, duration of hospitalisation and HA infection were independent predictors of hazard of inpatient death.
Secular trends
Trends in SAB and clinical outcomes
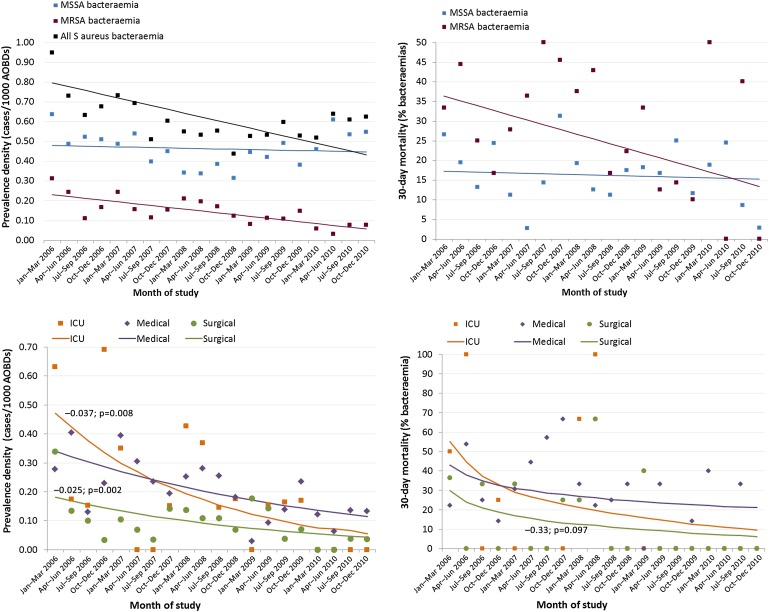
Prevalence density of all SAB declined from 0.73/1000 AOBDs to 0.50/1000 AOBDs (−41%; p=0.002 for trend) between January 2006 and December 2010. Prevalence density of MRSA bacteraemia fell 73% from 0.26 to 0.07/1000 AOBDs (p<0.001) and HA incidence density 82%, from 0.16 to 0.03/1000 AOBDs (p<0.001); however, rates of MSSA bacteraemia were unchanged (figure 3). An increasing proportion of MRSA bacteraemia was associated with previous colonisation or infection (table 3). Case-mix within the SAB cohort was otherwise stable.
Figure 3.
Secular trends in prevalence density and all-cause 30-day mortality after Staphylococcus aureus bacteraemia by methicillin resistance and admitting department (MRSA only). Data aggregated in 3-month blocks. Lines represent results of trend analysis, using Poisson regression with time (month) as sole explanatory variable. AOBDs, acute occupied bed days; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Table 3.
Characteristics of Staphylococcus aureus bacteraemia, associated outcomes and frequencies of all S aureus isolates by year of study: N (%), mean (SD) or median (IQR)
| Year |
p value* | |||||
| 2006 (n=218) | 2007 (n=188) | 2008 (n=151) | 2009 (n=152) | 2010 (n=158) | ||
| S aureus bacteraemia | ||||||
| N (%) involving | <0.001 | |||||
| MSSA | 156 (72) | 140 (74) | 100 (66) | 121 (80) | 142 (90) | |
| MRSA | 62 (28) | 48 (26) | 51 (34) | 31 (20) | 16 (10) | |
| Demographics | ||||||
| Gender (female) (%) | 66 (30) | 62 (33) | 55 (36) | 55 (36) | 49 (30) | 0.603 |
| Age (years) | 57 (22) | 56 (21) | 58 (22) | 56 (22) | 57 (20) | 0.744 |
| Clinical characteristics | ||||||
| Hospital associated (%) | 112 (51) | 108 (60) | 69 (46) | 75 (50) | 92 (58) | 0.750 |
| Previous S aureus bacteraemia, any (%) | 10 (5) | 18 (10) | 19 (13) | 14 (10) | 12 (8) | 0.787 |
| Previous MRSA colonisation or infection (%)† | 24 (39) | 24 (50) | 29 (57) | 16 (52) | 10 (63) | 0.056 |
| Admission within past 12 months (%) | 134 (62) | 101 (54) | 92 (61) | 96 (63) | 99 (62) | 0.503 |
| Outcomes | ||||||
| 30-day mortality (%) | 52 (24) | 39 (22) | 31 (21) | 27 (18) | 24 (15) | 0.013 |
| Inhospital death (%) | 63 (29) | 45 (25) | 34 (23) | 34 (23) | 33 (21) | 0.045 |
| Length of stay (days) | 19 (10–41) | 17 (7–36) | 27 (12–36) | 22 (12–44) | 19 (12–42) | 0.508 |
| Readmission (≤14 days) (%) | 25 (17) | 19 (14) | 25 (22) | 31 (27) | 19 (16) | 0.291 |
| All S aureus infection/colonisations | ||||||
| N (%) involving‡ | <0.001 | |||||
| MSSA | 1682 (72) | 1532 (75) | 1250 (74) | 1416 (83) | 1351 (90) | |
| MRSA | 638 (28) | 510 (25) | 448 (26) | 289 (17) | 151 (10) | |
Linear and logistic regressions with month of study as sole explanatory variable.
Data presented for MRSA bacteraemia only.
Data available for adult non-obstetric patients 2006–2010. Counts represent non-duplicate isolates (one per patient per year).
MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
30-Day mortality after MRSA bacteraemia declined from 37% to 13% (p=0.027), but no significant change was observed in mortality after MSSA bacteraemia (figure 3). By 2010, 90% of episodes of bacteraemia and 86% of associated inpatient deaths were attributable to MSSA. These MRSA-specific declines closely correlated with changes in rates of all MRSA or MSSA infection or colonisation. By admitting department, declines in MRSA prevalence density, HA incidence density and mortality were significantly steeper in ICU than medical or surgical departments (p<0.05 for interaction term) (figure 3).
Trends in inpatient case-mix
There were no significant trends in admitting specialty or gender among inpatients over the 5-year period. Mean age of adults and all patients increased between 2006 and 2010 (+1.7, 95% CI +1.3 to +2.2 years, for all patients; p<0.001), while mean length of stay (−1.3, 95% CI −1.6 to −1.11 days; p<0.001) and weighted average bed occupancy (−2.6%, 95% CI −4.8% to −0.4%; p=0.021) declined (supplemental file 3). Considering the associations noted earlier, these changes represented opposing upward (increasing age) and downward (reduced length of stay, bed occupancy) pressures on rates of bacteraemia. Secular trend in MRSA prevalence density (p=0.03 for trend) and HA incidence density (p=0.01) remained significant after adjusting for these changes in case-mix in a multivariate Poisson regression model.
Impacts of universal MRSA admission screening
Screening adherence and importation pressures
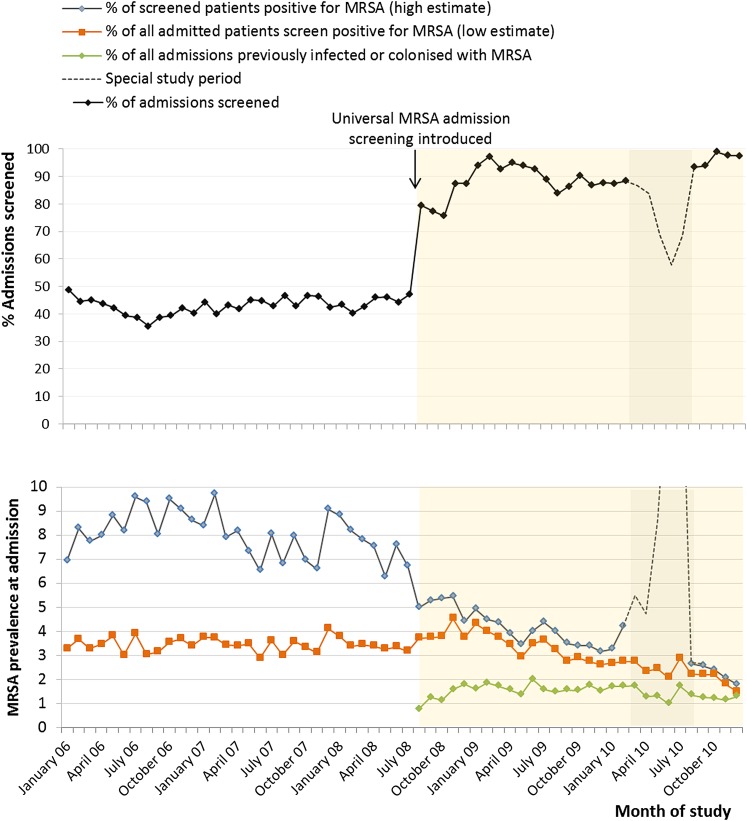
Forty-three per cent of all adult, non-obstetric overnight admissions and 84% of eligible patients in high-risk environments were screened prior to routine surveillance. During universal surveillance, 87% of eligible patients were screened (n=86 890). A target of 90% adherence was achieved within 4 months of initiation and sustained thereafter, excluding a special study period in which trial of additional throat, perineum and axillae swabs and discharge screening reduced patient participation (figure 4).
Figure 4.
Adherence to methicillin-resistant Staphylococcus aureus (MRSA) admission testing during universal surveillance (August 2008 to December 2010). Special study period (February 2010 to August 2010) involved a trial of axillae and groin swabs.
MRSA prevalence at admission (importation pressure) steadily declined during the period of universal surveillance, averaging 3.1%, with 1.7% known to be previously colonised or infected with MRSA. There was an increase in episodes of MRSA bacteraemia preceded by screening at admission (95% vs 81%; p=0.008) and identified as being colonised at admission (56% vs 38% of all bacteraemia; p=0.013; 30% vs 11% without history of MRSA; p=0.011) after introduction of universal surveillance. Data from all hospitals involved in the pathfinder study demonstrated that 78% of MRSA-positive patients were successfully isolated or cohorted and 41% received at least 1 day of decolonisation therapy.29 Given that <11% of admissions to ARI stayed for more than the minimum 10 days required to identify MRSA from screening, complete a full course of decolonisation and obtain confirmatory swabs, only 4.1% of MRSA-positive patient were identified as being successfully decolonised during the index admission (table 4).29
Table 4.
Characteristics preintervention and postintervention
| Characteristic | Selective screening only | Universal admission screening |
| Admission data | ||
| No. of all admissions | 210 745 | 209 707 |
| No. of AOBDs (at risk) | 748 569 | 681 483 |
| Mean (SD) length of stay in hospital, days | 3.96 (0.23) | 3.33 (0.51) |
| Bed occupancy (% all available beds occupied) | 79 | 77 |
| Case-mix | ||
| Mean (SD) age all patients, years | 45.9 (0.45) | 47.3 (0.57) |
| Mean (SD) age, ICU, medical and surgical adult services, years | 55.3 (0.41) | 56.4 (0.56) |
| Gender: n (%) of all admissions | ||
| Female | 122 538 (58) | 120 417 (57) |
| Male | 88 207 (42) | 89 290 (43) |
| Specialty, n (%) of all admissions | ||
| Surgical | 84 216 (40) | 89 808 (43) |
| Medical | 65 711 (31) | 65 098 (31) |
| ICU | 3312 (2) | 2954 (1) |
| Maternity | 31 862 (15) | 29 738 (14) |
| Paediatric/neonatal | 24 606 (12) | 23 147 (12) |
| MRSA screening, colonisation and all Staphylococcus aureus infections | ||
| N (%) of overnight admissions† screened for MRSA | 43 158 (43) | 86 890 (87) |
| Number of overnight admission screened per 1000 AOBDs | 58 | 128 |
| Number (%) of overnight admissions† positive for MRSA | 2909 (3.5) | 2694 (3.1) |
| Estimated n (%) of MRSA-positive patients isolated/cohorted | No data | 2101 (78) |
| Estimated n (%) of MRSA-positive patients receiving decolonisation therapy | No data | 1105 (41) |
| Estimated n (%) of MRSA-positive patients with confirmed eradication | No data | 110 (4.1) |
| Other infection control measures | ||
| Hand hygiene (dispensed alcohol gel in litres/1000 AOBDs)‡ | 38.1‡ | 37.2 |
| Mean (SD) monthly hand-hygiene compliance, % § | 60.5% (12.3) | 92.9% (3.7) |
| Mean (SD) monthly use of ‘4C’ and macrolide antibiotics (DDD/1000 AOBDs) | 698 (79.4) | 416 (107.7) |
| Monthly use of ‘4C’ and macrolide antibiotics as % of all antibiotic DDDs, % | 66 | 37 |
| Clinical burdens from S aureus bacteraemia | ||
| Prevalent MSSA bacteraemia (n) | 353 | 306 |
| Prevalent MRSA bacteraemia (n) | 144 | 64 |
| Hospital-associated incident MRSA bacteraemia (n) | 89 | 29 |
| Deaths within 30 days MSSA (n) | 62 | 48 |
| Deaths within 30 days MRSA (n) | 51 | 12 |
| Clinical burdens from other S aureus infections/colonisations | ||
| Prevalent MRSA infection (any) or colonisation (% admissions)¶ | 1457 (1.0) | 579 (0.4) |
| Prevalence density of any MRSA infection (cases/1000 AOBDs)¶ | 2.25 (0.53) | 0.96 (0.44) |
| Prevalent MSSA infection (any) or colonisation (% admissions)¶ | 3932 (2.6) | 3299 (2.1) |
| Prevalence density of any MSSA infection (cases/1000 AOBDs)¶ | 5.86 (1.15) | 5.49 (0.89) |
‘4C’ antibiotics are ciprofloxacin (all fluoroquinolones), cephalosporins, clindamycin, co-amoxiclav.
Adult non-obstetric patients only.
Data available from April 2008 only (35 months).
Average ward compliance weighted by admissions as assessed by standardised audit methods integrating opportunity and technique from January 2007.
Data available for adult non-obstetric patients 2006–2010. Counts represent non-duplicate isolates (one per patient per year).
AOBDs, acute occupied bed days; DDDs, defined daily doses; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus.
Patient characteristics by study period
Case-mix remained stable between periods of selective screening and universal admission screening (table 4). However, there were significant reductions in bed occupancy and length of stay. There was an abrupt and permanent decline in use of ‘4C’ and macrolide antibiotics within 3 months of the antibiotic stewardship intervention (month 11 of universal screening). Improvements in hand hygiene were suggested by audited compliance but not by consumption of alcohol-based hand-rub.
Time-series intervention analysis
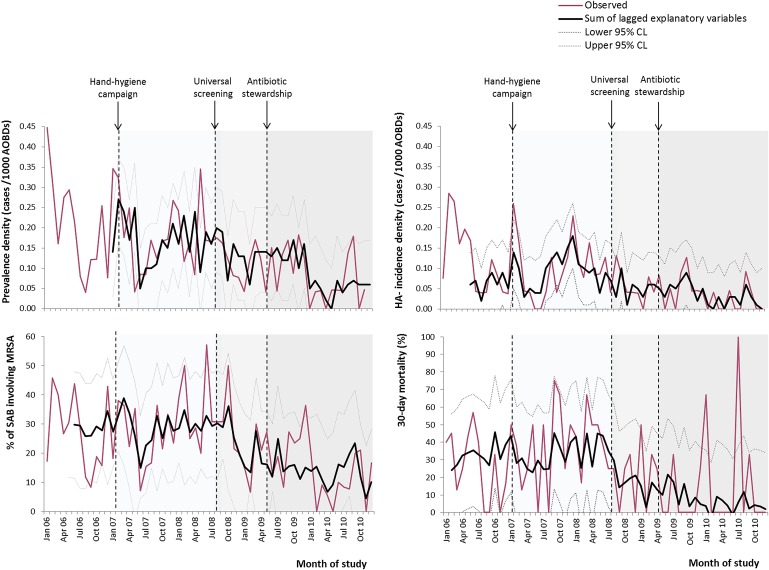
In multivariate transfer function models, adjusting for changes in other aspects of care and prior trends (table 5 and figure 5), universal screening was associated with a 19% reduction in prevalence density (absolute change, 0.189 to 0.154 (−0.035, 95% CI −0.049 to −0.021)/1000 AOBDs; p<0.001), a 29% reduction in HA incidence density (0.100 to 0.071 (−0.029, 95% CI −0.035 to −0.023)/1000 AOBDs; p<0.001) and a 46% fall in 30-day mortality (34% to 18.4% (−15.6%, 95% CI −24.1% to −7.1%); p<0.001). Using targeted screening as the comparison, during universal screening, the number needed to screen to avoid one additional episode of MRSA bacteraemia was 1978. Rates of bacteraemia and 30-day mortality were also positively associated with hospital-wide consumption of fluoroquinolone and cephalosporin antibiotics 1–6 months earlier. Assuming an average regimen of seven defined daily doses, the number needed to treat to cause one additional case of MRSA bacteraemia was 179 for cephalosporins and 204 for fluoroquinolones. Compared with forecasted consumption, reduction in the use of these antibiotics following the ‘4C’ antibiotic stewardship intervention was projected to have reduced prevalence density of MRSA bacteraemia by 0.027 (0.15 to 0.039)/1000 AOBDs. No significant relationships were identified with % hand-hygiene compliance, and effect sizes for screening were comparable across all departments. Final models explained 45%–68% of variance, and in all models, residuals were randomly distributed.
Table 5.
Multivariate transfer function models* for MRSA bacteraemia taking into account introduction of universal admission screening and changes in other aspects of care (January 2006 to December 2010)
| Term | Order† | Parameter‡ (SE) | T ratio | p Value |
| (a) Prevalence density of MRSA bacteraemia (cases per 1000 AOBDs), R2=0.678 | ||||
| Universal MRSA admission screening intervention | 3 | −0.0346 (0.0071) | −4.89 | <0.001 |
| Cephalosporin use (DDDs/1000 AOBDs) | 6 | +0.0008 (0.0004) | 2.03 | 0.046 |
| Fluoroquinolone use (DDDs/1000 AOBDs) | 5 | +0.0007 (0.0002) | +3.53 | <0.001 |
| MA§ | 4 | +0.7602 (0.0932) | +8.15 | <0.001 |
| AR¶ | 6 | −0.3100 (0.1309) | −2.37 | 0.019 |
| (b) Hospital-associated incidence density of MRSA bacteraemia (cases per 1000 AOBDs), R2=0.648 | ||||
| Universal MRSA admission screening intervention | 3 | −0.0290 (0.0032) | −8.92 | <0.001 |
| Fluoroquinolone use (DDDs/1000 AOBDs) | 5 | +0.0006 (0.0001) | 63.93 | <0.001 |
| MA1§ | 2 | +0.5801 (0.1273) | 4.56 | <0.001 |
| MA2§ | 3 | +0.2960 (0.1384) | 2.14 | 0.032 |
| MA3§ | 5 | +0.3028 (0.1298) | 2.33 | 0.014 |
| (c) % SABs involving MRSA (%), R2=0.504 | ||||
| Universal MRSA admission screening intervention | 3 | −13.490 (3.322) | −4.06 | <0.001 |
| Fluoroquinolone use (DDDs/1000 AOBDs) | 5 | +0.097 (0.047) | 2.06 | 0.042 |
| Bed occupancy, %** | 2 | +0.201 (0.094) | 2.14 | 0.032 |
| MA§ | 9 | −0.519 (0.115) | −4.51 | <0.001 |
| (d) 30-day mortality (%) after MRSA bacteraemia, R2=0.448 | ||||
| Universal MRSA admission screening intervention | 0 | −15.615 (4.349) | −3.59 | <0.001 |
| Fluoroquinolone use (DDDs/1000 AOBDs) | 1 | +0.222 (0.023) | 9.54 | <0.001 |
| MA§ | 8 | −0.306 (0.108) | −2.85 | 0.005 |
All series stationary before model identification.
Delay necessary to observe the effect (in months).
Size and direction of effect.
MA, moving average term representing abrupt changes in bacteraemia rates or mortality in immediate future.
AR, autoregressive term representing past values of bacteraemia rates or mortality.
% Bed occupancy, average bed occupancy weighted by admitting department, by month.
AOBDs, acute occupied bed days; DDDs, defined daily doses; MRSA, methicillin-resistant Staphylococcus aureus; SAB, Staphylococcus aureus bacteraemia.
Figure 5.
Observed trends and multivariate transfer model predictions (sum of lagged explanatory variables) for prevalence density, hospital-associated (HA) incidence density, 30-day mortality in methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia and % Staphylococcus aureus bacteraemia (SAB) involving MRSA. CL, confidence limit.
No significant associations were found between universal screening, hand hygiene or antibiotic use, and rates of MSSA bacteraemia. The %SAB involving MRSA fell by 52% (from 28.6% to 15.1% (−13.5%, 95% CI −20% to −7%); p=0.014).
Discussion
This retrospective cohort study identified a 41% decrease in prevalence density of all SAB in an inpatient population from Scotland between 2006 and 2010. Secular trends were attributable to steep reductions in MRSA bacteraemia. Introduction of a universal MRSA admission screening programme was associated with significant reductions in rates of MRSA bacteraemia and associated early mortality, while having no discernible impact on burdens from MSSA bacteraemia. Ecological and temporal associations between MRSA bacteraemia and use of fluoroquinolones and cephalosporins suggested that a subsequent antibiotic stewardship programme limiting use of these agents also contributed to control of MRSA bloodstream infections.
Strengths and limitations
Analyses of risk factors for SAB acquisition and outcomes were limited by a lack of information on comorbidities, severity of sepsis, source control and clinical management.4 13 41 However, age has been shown to be an appropriate proxy for comorbidity and risk of death,13 and our estimates of attributable mortality approximate those in more detailed analyses.4 The effect of MRSA on risk of 30-day mortality approximated estimates from a previous meta-analysis,17 and non-significance may be explained by a limited sample size.
Changes in strain distribution have been linked to secular trends in invasive S aureus infections,6 10 21 with declines in epidemic strains predating decreases in MRSA in the UK.10 Reflecting national data,10 regional studies of MRSA infections from the same period identified significant increase in EMRSA-15, with a reciprocal decline in EMRSA-16.42 43 Trends in strain may have confounded, or mediated, the associations between infection control measures and SAB epidemiology.10
Universal MRSA admission screening was introduced as part of an NHS Scotland Pathfinder Project, precluding the use of cross-over or controlled trial designs as elsewhere.44 45 Data on isolation-days captured, suggested as a measure of surveillance effectiveness,46 were also not available. We attempted to minimise threats to internal validity common to quasi-experimental studies of infection control measures.22 38 A definition of bacteraemia based on blood isolates rather than clinical suspicion made the study less vulnerable to detection bias while follow-up to a minimum of 2 weeks post-discharge prevented attrition bias arising for changes in length of stay. An attempt to identify and prevent selection and performance bias was made by identifying and controlling for, changes in case-mix, importation pressure,47 48 and other aspects of care,22 before and after the intervention. Investigation of concurrent trends in MSSA bacteraemia provided some control for impacts of general improvements in infection control or clinical management and supports an independent effect of screening on MRSA bacteraemia.34 38 ARIMA techniques account for non-independence of parameters and stochastic elements in time series. This is convergent with understanding of the spread of resistance and infectious disease within populations38 and minimises the potential for regression to the mean to account for trends.
Transfer function models including screening, antibiotic use and bed occupancy accounted for 45%–68% of variation in rates of bacteraemia, suggesting unmeasured factors affecting rates. Universal screening was one of several sequentially implemented control measures for MRSA in North East Scotland, including introduction of environmental swabbing and disinfection (2001), alcohol hand-gel (2002) and targeted admission screening (2003). The lack of accurate data on alcohol-based hand-gel consumption, and limited baseline data before the national hand-hygiene campaign, may explain a failure to identify significant effects of hand-improving hand hygiene33 as described in other time-series analyses.34 35 Introduction of screening was likely to be associated with improved awareness among healthcare workers and the public around MRSA, with potential improvements in adherence to general infection control policy. Performance in infection control may also have been influenced by internal audit of MRSA screening. However, non-declining trends in MSSA-suggested general infection control measures were an inadequate explanation for MRSA-specific declines.
Rates of MRSA colonisation, infection and bacteraemia, and effect sizes from intervention in the present study are comparable to those described in previous investigations of universal surveillance.28 44–46 Findings may be generalisable to other large public hospitals with ICUs in high-income countries, with endemic MRSA and relatively low rates of MRSA infection.
Comparison to literature
We identified a number of risk factors for developing SAB and associated early mortality consistent with previous findings, including older age,3 4 13 recent or prolonged hospitalisation,3 41 history of colonisation or infection,47 colonisation on admissions,47 48 and ICU admissions.9 Associations were significantly stronger for MRSA bacteraemia.48 Despite two meta-analyses suggesting an excess mortality in MRSA, compared with MSSA bacteraemia,15 17 there remains considerable debate about the importance of methicillin resistance to outcomes.4 13 18 Our findings suggest that much of the increase in mortality associated with methicillin resistance may be explained by infection of more vulnerable patients,13–18 often in the context of extended contact with healthcare.15 41
Reflecting the findings of an earlier study from Oxfordshire, which found that MRSA-related disease was responsible for increasing rates of SAB between 1997 and 2003,3 our findings suggest that subsequent declines have occurred, almost exclusively in MRSA-related disease. An equivalent upward pressure on MSSA rates has not been observed, consistent with observations that MRSA appears to add to, rather than displace MSSA infection.21 These findings match experience across the UK.8
Evidence on the role of universal screening in reducing all MRSA infections is conflicting,28 30 44–46 49–52 and benefits may depend on target population, screening technology and subsequent control interventions.49 A recent US study of routine surveillance for MRSA noted a significant downward trend in MRSA bacteraemia in ICU but not in other hospital settings.28 A second US study found a decrease in hospital-wide MRSA but not MSSA bacteraemia during universal screening.46 In agreement with these studies, we found that rates of MRSA bacteraemia declined in parallel with all MRSA infections,50 53 and there was no reciprocal rise in hospital-wide MSSA bacteraemia or infections.46 Hospital-wide reductions in bacteraemia, of similar magnitude to that seen in our study, were reported following introduction of screening in intensive care27 or high-risk patients only.26 However, we identified additional declines in both general and intensive care settings, despite a baseline scenario involving routine screening in high-risk patients. These findings also contrast with those from a 1-year review of the pathfinder study, which found that although declines in all MRSA infections/colonisations were greater in intervention than control hospitals during universal screening, the difference was non-significant.54 We note that this comparison was limited by low numbers in control hospitals, risks of contamination where control hospitals were in the same NHS board, a short baseline and follow-up period, and methods not accounting for non-independence of observations in time-series and lagged effects.
We are not aware of a previous time-series analysis describing significant impacts of universal screening on % mortality following SAB. A lack of improvement in mortality after MSSA bacteraemia did not suggest general improvements in care. The increased proportion of bacteraemia in those without history of MRSA identified as positive for MRSA at admission during universal screening may have facilitated prompt initiation of appropriate therapy. Other potential explanations include increased awareness of invasive MRSA infection in clinical staff with routine screening, greater marginal benefits of universal admission screening in ICU settings27 28 and changes in strain distribution.42 43
Our findings suggest additional considerations in assessing utility of universal surveillance. Patients colonised at admission were at high risk of developing HA MRSA bacteraemia, and early identification of colonised patients provides opportunities to reduce invasive infection by decolonisation.27 As elsewhere,27 declines in HA infection were steeper than those in rates including community-associated infection, coherent with reductions in transmission. Similarly, decline in importation pressure during universal surveillance suggested interruption of connections between prevalence of MRSA in hospital and community populations, focused in frequently admitted patients.55–57 However, approximately 50% of HA MRSA bacteraemia occurred in patients not colonised at admission highlighting the limitations in admission surveillance and the persistence of cross-transmission.58 Other lost opportunities to prevent transmission may arise in practice, given the respective 22% and 59% of MRSA-positive patients not isolated or receiving any decolonisation therapy during the pathfinder study. The latter is particularly concerning as effective decolonisation may be a prerequisite for cost-effectiveness of universal screening.59
As in previous studies from the region,35 36 we noted the importance of antibiotic use in hospital in determining rates of all MRSA infections in the region. Although both patient-level37 and ecological associations35 between fluoroquinolone and cephalosporin use and MRSA infection have been identified, we are not aware of an experimental or quasi-experimental study investigating impacts of limiting their use in the control of MRSA bacteraemia specifically. Independent effects of screening and antibiotic stewardship were of comparable magnitude suggesting complementary roles in the control of both invasive and other MRSA infections.36
Implications for practice, policy and research
Our study suggests that universal admission screening for MRSA may have an important effect on rates of MRSA bacteraemia and associated mortality, beyond selective screening of high-risk patients. However, there remains debate around the cost-effectiveness of universal surveillance in comparison to alternative control measures,51 59–63 risks of chlorhexidine resistance with widespread decolonisation9 and opportunity costs or unintended harms associated with isolation.64 Subsequent to the pathfinder study, NHS Scotland has suggested hospital-wide targeted surveillance based on clinical risk assessment as a minimum standard.65 This is convergent with an emerging consensus that admission screening based on clinical prediction rules may offer a more efficient and pragmatic approach outside the populations with high prevalence of MRSA.49 63 66 Our findings suggest the need to consider the greater marginal benefits in preventing bacteraemia and associated mortality, which impose disproportional healthcare and wider societal costs.67 Considered alongside subsequent experience of low adherence to clinical risk assessment-based screening in NHS Grampian, we suggest the need to re-evaluate the benefits of universal screening in Scotland. Irrespective of the chosen strategy, as the additional effects of antibiotic stewardship in this study and effects of bed occupancy suggest, benefits of admission screening will be optimised where integrated with a broader package of infection prevention and control measures.28 49 58
The concentration of both MRSA and MSSA bloodstream infections in susceptible patient groups with higher levels of healthcare contact suggests that some measures successfully limiting invasive MRSA infections may be generalisable to control of all SAB. A more rigorous approach to identify and limit iatrogenic sources of bacteraemia, including peripheral or central catheters,41 48 68 is required. Screening for MSSA with isolation and decolonisation has been suggested for selected high-risk patients.69
Equally, strategies are required that account for the distinct epidemiology of MSSA and MRSA bacteraemia. In contrast to MRSA, the majority of MSSA bacteraemia in this study were community associated and occurred in younger patients. Targeted measures are required to prevent invasive infection in at-risk groups, including IV drug users,41 70 surgical, diabetic and renal patients.69 71
Given the role of social and risk-networks in sustaining S aureus transmission,70 broadening control of SAB to the community is likely to require the commitment of multiple agencies and healthcare providers.
Changes in virulence of MSSA and MRSA may account for divergence in trends in outcomes.13 Genetic sequencing or typing could be used to quantify the contribution of clonal expansion to recent trends in SAB epidemiology. A recent multicentre study found large variation in management of SAB in the UK and called for high-level evidence to define optimal care.41 Future research and guidelines should consider both MSSA and MRSA bacteraemia.
In summary, this study described decreasing trends in SAB following a decade of infection control policies focusing on MRSA. Expansion from targeted to universal MRSA admission screening was associated with important reductions in MRSA bacteraemia when combined with isolation and decolonisation. However, findings also highlighted the need for strategies to reduce clinical burdens from invasive MSSA infection if progress towards national targets for SAB is to be sustained.21
Supplementary Material
Acknowledgments
The authors thank all the staff in the routine microbiology diagnostic laboratory and all the clinical staff at ARI, whose work made this possible. We are grateful to Richard Slessor and the health intelligence services, Aberdeen for providing data on admissions. We also thank Health Protection Scotland for help in the implementation of the pathfinder project.
Footnotes
To cite: Lawes T, Edwards B, López-Lozano J-M, et al. Trends in Staphylococcus aureus bacteraemia and impacts of infection control practices including universal MRSA admission screening in a hospital in Scotland, 2006–2010: retrospective cohort study and time-series intervention analysis. BMJ Open 2012;2:e000797. doi:10.1136/bmjopen-2011-000797
Contributors: BE and IG conceived this study. The statistical analysis was performed by TL and J-ML-L. All authors assisted in drafting the manuscript. IG is the guarantor for this study.
Funding: The NHS Pathfinder Study on universal admission screening was funded by the Scottish government Health Directorate. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: IG has received personal and grant financial support from companies manufacturing diagnostics and therapeutics for MRSA. BE has received grant financial support from Novarits. TL and J-ML-L have no conflicts to declare. TL, BE, J-ML-L and IG know of no other relationships or activities that could appear to have influenced the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Technical appendix and data available on request from the corresponding author.
References
- 1.Allegranzi B, Bagheri Nejad S, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 2011;377:228–41 [DOI] [PubMed] [Google Scholar]
- 2.Wilson J, Elgohari S, Livermore DM, et al. Trends among pathogens reported as causing bacteraemia in England, 2004-2008. Clin Microbiol Infect 2011;17:451–8 [DOI] [PubMed] [Google Scholar]
- 3.Wyllie DH, Crook DW, Peto TE. Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997-2003: cohort study. BMJ 2006;333:281–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blot SI, Vandewoude KH, Hoste EA, et al. Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002;162:2229–35 [DOI] [PubMed] [Google Scholar]
- 5.de Kraker ME, Davey PG, Grundmann H; On behalf of the BURDEN study group Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med 2011;8:e1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson AP, Pearson A, Duckworth G. Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother 2005;56:455–62 [DOI] [PubMed] [Google Scholar]
- 7.Clements A, Halton K, Graves N, et al. Overcrowding and understaffing in modern health-care systems: key determinants in meticillin-resistant Staphylococcus aureus transmission. Lancet Infect Dis 2008;8:427–34 [DOI] [PubMed] [Google Scholar]
- 8.Pearson A, Chronias A, Murray M. Voluntary and mandatory surveillance for methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) bacteraemia in England. J Antimicrob Chemother 2009;64(Suppl 1):i11–17 [DOI] [PubMed] [Google Scholar]
- 9.Edgeworth JD. Has decolonization played a central role in the decline in UK methicillin-resistant Staphylococcus aureus transmission? A focus on evidence from intensive care. J Antimicrob Chemother 2011;66(Suppl 2):ii41–7 [DOI] [PubMed] [Google Scholar]
- 10.Wyllie D, Paul J, Crook D. Waves of trouble: MRSA strain dynamics and assessment of the impact of infection control. J Antimicrob Chemother 2011;66:2685–8 [DOI] [PubMed] [Google Scholar]
- 11.Health Protection Agency Voluntary Reporting of Staphylococcus aureus Bacteraemia in England, Wales and Northern Ireland, 2010. London: Health Protection Agency, 2011 [Google Scholar]
- 12.Health Protection Scotland SNBTS Annual Infection Control Report: April 2010 to March 2011. Edinburgh: Health Protection Scotland, 2011 [Google Scholar]
- 13.Shurland S, Zhan M, Bradham DD, et al. Comparison of mortality risk associated with bacteremia due to Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol 2007;28:273–9 [DOI] [PubMed] [Google Scholar]
- 14.Wang JL, Chen SY, Wang JT, et al. Comparison of both clinical features and mortality risk associated with bacteremia due to community-acquired methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. Clin Infect Dis 2008;46:799–806 [DOI] [PubMed] [Google Scholar]
- 15.Whitby M, McLaws ML, Berry G. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: a meta-analysis. Med J Aust 2001;175:264–7 [DOI] [PubMed] [Google Scholar]
- 16.Wolkewitz M, Frank U, Philips G, et al. Mortality associated with in-hospital bacteraemia caused by Staphylococcus aureus:a multistate analysis with follow-up beyond hospital discharge. J Antimicrob Chemother 2011;66:381–6 [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteraemia: a meta-analysis. Clin Infect Dis 2003;36:53–9 [DOI] [PubMed] [Google Scholar]
- 18.Cosgrove SE, Qi Y, Kaye KS, et al. The impact of methicillin resistance in Staphylococcus aureus bacteraemia on patient outcomes: mortality, length of stay, and hospital Charges. Infect Control Hosp Epidemiol 2005;26:166–74 [DOI] [PubMed] [Google Scholar]
- 19.NHS Scotland HEAT Targets Due for Delivery in 2009/10: Summary of Performance. Edinburgh: NHS Scotland Performance and Business Management, 2011 [Google Scholar]
- 20.Paul J. Surveillance and management of all types of Staphylococcus aureus bacteraemia. BMJ 2006;333:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mostofsky E, Lipsitch M, Regev-Yochay G. Is methicillin-resistant Staphylococcus aureus replacing methicillin-susceptible S.aureus? J Antimicrob Chemother 2011;66:2199–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper BS, Stone SP, Kibbler CC, et al. Systematic review of isolation measures in the hospital management of methicillin resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling. Health Technol Assess 2003;7:191–4 [DOI] [PubMed] [Google Scholar]
- 23.Wilcox MH. Screening for MRSA. BMJ 2008;336:899–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeb M, Main C, Walker-Dilks C, et al. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev 2003;(4):CD003340. [DOI] [PubMed] [Google Scholar]
- 25.McGinigle KL, Gourlay ML, Buchanan IB. The use of active surveillance cultures in adult intensive care units to reduce methicillin-resistant Staphylococcus aureus-related morbidity, mortality, and costs: a systematic review. Clin Infect Dis 2008;46:1717–25 [DOI] [PubMed] [Google Scholar]
- 26.Clancy M, Graepler A, Wilson M, et al. Active screening in high-risk units is an effective and cost-avoidant method to reduce the rate of methicillin-resistant Staphylococcus aureus infection in the hospital. Infect Control Hosp Epidemiol 2006;27:1009–17 [DOI] [PubMed] [Google Scholar]
- 27.Huang SS, Yokoe DS, Hinrichsen VL, et al. Impact of routine intensive care unit surveillance cultures and resultant barrier precautions on hospital-wide methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2006;43:971–8 [DOI] [PubMed] [Google Scholar]
- 28.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011;364:1419–30 [DOI] [PubMed] [Google Scholar]
- 29.Reilly JS, Stewart S, Christie P, et al. Universal screening for methicillin resistant Staphyloccocus aureus: interim results from the NHS Scotland pathfinder project. J Hosp Infect 2010;74:35–41 [DOI] [PubMed] [Google Scholar]
- 30.Health Protection Scotland; National Services Scotland NHS Scotland MRSA Screening Pathfinder Programme Final Report Volume 1: An Investigation of the Clinical Effectiveness of MRSA Screening [Report]. Glasgow: Health Protection Scotland, 2011 [Google Scholar]
- 31.Stone SP, Cooper BS, Kibbler CC, et al. The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. Lancet Infect Dis 2007;7:282–8 [DOI] [PubMed] [Google Scholar]
- 32.Chen YC, Sheng WH, Wang JT, et al. Effectiveness and limitations of hand hygiene promotion on decreasing healthcare-associated infections. PLoS One 2011;6:e27163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sroka S, Gastmeier P, Meyer E. Impact of alcohol hand-rub use on meticillin-resistant Staphylococcus aureus: an analysis of the literature. J Hosp Infect 2010;74:204–11 [DOI] [PubMed] [Google Scholar]
- 34.Kaier K, Hagist C, Frank U, et al. Two time-series analyses of the impact of antibiotic consumption and alcohol-based hand disinfection on the incidences of nosocomial methicillin-resistant Staphylococcus aureus infection and Clostridium difficile infection. Infect Control Hosp Epidemiol 2009;30:346–53 [DOI] [PubMed] [Google Scholar]
- 35.Aldeyab MA, Monnet DL, López-Lozano JM, et al. Modelling the impact of antibiotic use and infection control practices on the incidence of hospital-acquired methicillin-resistant Staphylococcus aureus: a time-series analysis. J Antimicrob Chemother 2008;62:593–600 [DOI] [PubMed] [Google Scholar]
- 36.Monnet DL, Mackenzie FJ, Lopez-Lozano JM, et al. Antimicrobial drug use and methicillin-resistant Staphylococcus aureus, Aberdeen, 1996-2000. Emerg Infect Dis 2004;10:1432–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taconnelli E, De Angelis G, Cataldo MA, et al. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J Antimicrob Chemother 2008;61:26–38 [DOI] [PubMed] [Google Scholar]
- 38.Shardell M, Harris AD, El-Kamary SS, et al. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin Infect Dis 2007;45:901–7 [DOI] [PubMed] [Google Scholar]
- 39.Pankratz A. Forecasting with Dynamic Regression Models. New York (NY): Wiley, 1991 [Google Scholar]
- 40.Liu LM, Hudak GB. Forecasting and Time Series Analysis Using the SCA Statistical System. Chicago (IL): Scientific Computing Associates, 1994 [Google Scholar]
- 41.Thwaites GE; United Kingdom clinical infection research group (UKCIRG) The management of Staphylococcus aureus bacteraemia in the United Kingdom and Vietnam: a multi-centre evaluation. PLoS One 2010;5:e14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards B, Milne K, Lawes T, et al. Is Vancomycin MIC ‘creep’ method dependent? Analysis of methicillin-resistant S. aureus (MRSA) susceptibility trends in blood isolates from North East Scotland, 2006-10. J Clin Microbiol 2012;50:318–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt AC, Edwards B, Girvan EK, et al. Methicillin-resistant Staphylococcus aureus in northeastern Scotland in 2003 to 2007: evolving strain distribution and resistance patterns. J Clin Microbiol 2011;49:1975–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeyaratnam D, Whitty CJM, Phillips K, et al. Impact of rapid screening tests on acquisition of meticillin resistant Staphylococcus aureus: cluster randomised crossover trial. BMJ 2008;336:927–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harbarth S, Fankhauser C, Schrenzel J, et al. Universal screening for methicillin resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA 2008;299:1149–57 [DOI] [PubMed] [Google Scholar]
- 46.Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med 2008;148:409–18 [DOI] [PubMed] [Google Scholar]
- 47.Seybold U, Schubert S, Bogner JR, et al. Staphylococcus aureus infection following nasal colonisation: an approach to rapid risk stratification in a university healthcare system. J Hosp Infect 2011;79:297–301 [DOI] [PubMed] [Google Scholar]
- 48.Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol 2010;31:584–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harbarth S, Hawkey PM, Tenover F, et al. Update on screening and clinical diagnosis of meticillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 2011;37:110–17 [DOI] [PubMed] [Google Scholar]
- 50.Hacek DM, Paule SM, Thomson RB, et al. Implementation of a Universal Admission Surveillance and Decolonisation Program for Methicillin-Resistant Staphylococcus aureus (MRSA) reduces the number of MRSA and total number of S. aureus isolates reported by the clinical laboratory. J Clin Microbiol 2009;47:3749–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leonhardt KK, Yakusheva O, Phelan D, et al. Clinical effectiveness and cost benefit of universal versus targeted methicillin-resistant Staphylococcus aureus screening upon admission in hospitals. Infect Control Hosp Epidemiol 2011;32:797–803 [DOI] [PubMed] [Google Scholar]
- 52.Hardy K, Price C, Szczepura A, et al. Reduction in the rate of methicillin-resistant Staphylococcus aureus acquisition in surgical wards by rapid screening for colonization: a prospective, cross-over study. Clin Microbiol Infect 2009;16:333–9 [DOI] [PubMed] [Google Scholar]
- 53.Walker S, Peto TE, O'Conner L, et al. Are there better methods of monitoring MRSA control than bacteraemia surveillance? An observational database study. PLoS One 2008;3:e2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reilly JS, Stewart S, Christie P, et al. Universal screening for meticillin-resistant Staphylococcus aureus in acute care: risk factors and outcome from a multicentre study. J Hosp Infect 2012;80:31–5 [DOI] [PubMed] [Google Scholar]
- 55.MacKenzie FM, Lopez-Lozano JM, Monnet DL, et al. Temporal relationship between prevalence of meticillin-resistant Staphylococcus aureus (MRSA) in one hospital and prevalence of MRSA in the surrounding community: a time-series analysis. J Hosp Infect 2007;67:225–31 [DOI] [PubMed] [Google Scholar]
- 56.Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003;36:131–9 [DOI] [PubMed] [Google Scholar]
- 57.Wyllie DH, Peto TE, Crook D. MRSA bacteraemia in patients on arrival in hospital: a cohort study in Oxfordshire 1997-2003. BMJ 2005;331:992–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Velzen EV, Reilly JS, Kavanagh K, et al. A retrospective cohort study into acquisition of MRSA and associated risk factors after implementation of universal screening in Scottish hospitals. Infect Control Hosp Epidemiol 2011;32:889–96 [DOI] [PubMed] [Google Scholar]
- 59.Robotham JV, Graves N, Cookson BD, et al. Screening, isolation, and decolonisation strategies in the control of meticillin resistant Staphylococcus aureus in intensive care units: cost effectiveness evaluation. BMJ 2011;343:d5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murthy A, De Angelis G, Pittet D, et al. Cost-effectiveness of universal MRSA screening on admission to surgery. Clin Microbiol Infect 2010;16:1747–53 [DOI] [PubMed] [Google Scholar]
- 61.Hubben G, Bootsma M, Luteijn M, et al. Modelling the costs and effects of selective and universal hospital admission screening for Methicillin resistant Staphylococcus aureus. PloS One 2011;6:e14783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee BY, Bailey RR, Smith KJ, et al. Universal methicillin-resistant Staphylococcus aureus (MRSA) surveillance for adults at hospital admission: an economic model and analysis. Infect Control Hosp Epidemiol 2010;31:598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins J, Raza M, Ford M, et al. Review of a three-year meticillin-resistant Staphylococcus aureus screening programme. J Hosp Infect 2011;78:81–5 [DOI] [PubMed] [Google Scholar]
- 64.Kirkland KB. Taking off the gloves: towards a less dogmatic approach to the use of contact isolation. Clin Infect Dis 2009;48:766–71 [DOI] [PubMed] [Google Scholar]
- 65.Health Protection Scotland Protocol for CRA MRSA Screening National Rollout in Scotland. Glasgow: Health Protection Scotland, 2011 [Google Scholar]
- 66.Morgan DJ, Day HR, Furuno JP, et al. Improving efficiency in active surveillance for methicillin-resistant Staphylococcus aureus or vancomycin-resistant Enterococcus at hospital admission. Infect Control Hosp Epidemiol 2010;31:1230–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gould IM, Reilly J, Bunyan D, et al. Costs of healthcare-associated methicillin-resistant Staphylococcus aureus and its control. Clin Microbiol Infect 2010;16:1721–8 [DOI] [PubMed] [Google Scholar]
- 68.Hetem DJ, de Ruiter SC, Buiting AG, et al. Preventing Staphylococcus aureus bacteremia and sepsis in patients with Staphylococcus aureus colonization of intravascular catheters: a retrospective multicenter study and meta-analysis. Medicine 2011;90:284–8 [DOI] [PubMed] [Google Scholar]
- 69.Lucet JC, Regnier B. Screening and decolonization: does methicillin-susceptible Staphylococcus aureus hold lessons for methicillin-resistant S. aureus? Clin Infect Dis 2010;51:585–90 [DOI] [PubMed] [Google Scholar]
- 70.Gwizdala RA, Miller M, Bhat M, et al. Staphylococcus aureus colonization and infection among drug users: identification of hidden networks. Am J Public Health 2011;101:1268–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saxena AK, Panhotra BR, Venkateshappa CK, et al. The impact of nasal carriage of methicillin-resistant and methicillin-susceptible Staphylococcus aureus (MRSA & MSSA) on vascular access-related septiceamia among patients with type-II diabetes on dialysis. Ren Fail 2002;24:763–77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.