Abstract
Airway remodeling and exacerbated airway narrowing in asthma have been attributed to the regulation of intracellular Ca2+ by sarcoplasmic reticulum (SR) of the airway smooth muscle cells. The protein encoded by obscurin, cytoskeletal calmodulin and titin-interacting RhoGEF (OBSCN) is a crucial factor in determining the SR architecture in Obscn−/− mice. This study genotyped a total of 55 common single-nucleotide polymorphisms (SNPs) in 592 Korean asthmatics including 163 aspirin exacerbated respiratory disease (AERD) cases and 429 aspirin-tolerant asthma (ATA) controls. Eight SNPs, including two nonsynonymous polymorphisms rs1188722C>T (Leu2116Phe) and rs1188729G>C (Cys4642Ser), and one haplotype BL2_ht1 showed statistically significant associations with AERD development (p=0.003–0.03). Two variants, rs1188722C>T (Leu2116Phe) and rs369252C>A, also revealed nominal association with FEV1 decline by aspirin provocation in asthmatics (p=0.03–0.04). Intriguingly, rs1188722C>T (Leu2116Phe) is a highly conserved amino acid residue among species, suggesting its functional relevance to AERD. In addition, the A allele of rs369252C>A, which was more prevalent in AERD than in ATA, was predicted as a potential branch point (BP) site for alternative splicing (BP score=4.29). Although further functional evaluation is required, our findings suggest that OBSCN polymorphisms, in particular, highly conserved nonsynonymous Leu2116Phe variant, might contribute to aspirin hypersensitivity in asthmatics.
Introduction
Aspirin exacerbated respiratory disease (AERD) is characterized by development of severe bronchoconstriction after ingestion of aspirin or other nonsteroidal antiinflammatory drugs (Szczeklik and Stevenson, 2003). Frequently, AERD patients experience bronchial asthma and chronic rhinosinusitis accompanied with nasal polyposis. Aspirin hypersensitivity is prevalent in 0.6%–2.5% of the general population, and it increases up to 5%–10% in asthmatic patients (Hedman et al., 1999). Although the pathogenesis of AERD has not yet been clearly elucidated, overexpressions of proinflammatory cysteinyl leukotrienes (CysLTs) and CysLT receptors have been suggested as the major risk factors for AERD development (Babu and Salvi, 2000). Recently, aspirin hypersensitivity has been shown to be related with increased asthma severity and potential remodeling of upper and lower airways (Mascia et al., 2005; Warner and Knight, 2008).
Polymorphisms in many genes, such as cysteinyl leukotriene receptor 2 (Park et al., 2005) and thromboxane A2 receptor (Kim et al., 2005) in the arachidonate pathway and tumor necrosis factor (Kim et al., 2006) and angiotensin I converting enzyme (Kim et al., 2008) in the immune-related pathway, are associated with AERD in the Korean population. In addition, a genome-wide association study recently suggested centrosomal protein 68 kDa as a candidate gene for AERD development (Kim et al., 2010). However, considering that confounders with small effects may contribute to the complexity of asthma, genetic variants of other genes may also be related to the development of aspirin hypersensitivity in asthmatics.
Intracellular Ca2+ concentration regulated by sarcoplasmic reticulum (SR) has an important role in bronchial construction and bronchial wall thickening in asthma (Sweeney et al., 2002). In addition, the elevated concentration of cytoplasmic Ca2+ is a crucial event leading to the contraction of airway smooth muscle cells that causes extensive airway narrowing (Cox et al., 2007; Kellner et al., 2008). The SR is a complex internal membrane system that releases and reuptakes Ca2+ during muscle contraction and relaxation. Observations of SR architecture and organization from obscurin, cytoskeletal calmodulin and titin-interacting RhoGEF (Obscn) knockout mice have revealed that a lack of obscurin leads to changes in longitudinal SR architecture, with subsequent alterations in expressions of SR-associated proteins (Lange et al., 2009). Furthermore, the relation between aspirin and intracellular calcium homeostasis that is regulated by SR suggests that defects in the SR could lead to dysfunctions of aspirin (Dragomir et al., 2004).
The human OBSCN gene on chromosome 1q42.13 region is comprised of over 80 exons and encodes a ∼720 kDa protein. Although few associations between OBSCN genetic variations and human diseases have been reported, mutations (e.g., Arg4558His and Glu4574Lys) in the gene have been suggested to function in the predisposition to cancers, including glioblastoma and melanoma, and to provide new therapeutic opportunities (Balakrishnan et al., 2007; Bleeker and Bardelli, 2007). In addition, Arg4344Gln, another nonsynonymous variant in OBSCN, affects its binding to titin/connectin in hypertrophic cardiomyopathy (Arimura et al., 2007).
To investigate the potential association of OBSCN variations with AERD development and pathogenesis of bronchial constriction, this study analyzed a total of 55 common single-nucleotide polymorphisms (SNPs) in 163 AERD patients and 429 aspirin-tolerant asthma (ATA) controls in the Korean population.
Materials and Methods
Study subjects
Study subjects were recruited from hospitals of Soonchunhyang, Chungbuk National, Chonnam National, Seoul National, and Chung-Ang Universities. The Institutional Review Board of each hospital approved the study protocol. All subjects were Korean, and provided written informed consent. All patients showed clinical symptoms in accordance with the definition of asthma set forth in the Global Initiative for Asthma guidelines. Evaluation of asthma included histories of dyspnea and wheezing during the previous 12 months, along with one of the following: (1) a >15% increase in forced expiratory volume in 1 s (FEV1) or a >12% increase in FEV1 plus 200 mL follow-up inhalation of a short-acting bronchodilator, (2) <10 mg/mL PC20 methacholine, or (3) >20% increase in FEV1 following 2 weeks of treatment with inhaled steroids and long-acting bronchodilators. The oral aspirin provocation test was performed with slight modifications in increasing doses (Cormican et al., 2005), following the guidelines from EAACI/GA2LEN (Nizankowska-Mogilnicka et al., 2007). Based on the results of oral aspirin provocation tests, subjects were classified into two groups as follows: asthmatics who showed 20% or greater decrease in FEV1 or 15%–19% decrease in FEV1 with nasoocular or cutaneous reactions were stratified as AERD cases, whereas asthmatics who showed less than 15% decreases in FEV1 without nasoocular or cutaneous reactions were grouped as ATA controls.
SNP selection and genotyping
Based on the Asian population from the International HapMap Project database (http://hapmap.ncbi.nlm.nih.gov/index.html.en), a total of 55 common SNPs with a minor allele frequency (MAF) over 0.05 were selected for genotyping. Approximately 5 ng of genomic DNA, which was isolated from blood of patients using Wizard® Genomic DNA Purification Kit (Promega), was used to genotype each sample. Genotyping was performed in a total of 592 asthmatics, including 163 AERD patients and 429 ATA controls, using TaqMan assay on the ABI prism 7900HT sequence detection system (Applied Biosystems). Data quality was assessed by duplicate DNA (n=10 per reaction). SNPs that did not satisfy the following criteria were excluded from the study: (1) a minimum call rate of 95%, (2) no duplication error, (3) Hardy–Weinberg equilibrium of p>0.05.
Statistics
Analysis of linkage disequilibrium (LD) was performed using the Haploview v4.2 software downloaded from the Broad Institute (www.broadinstitute.org/mpg/haploview). LD coefficients (|D′| and r2) between all pairs of biallelic loci were used to determine LD among the SNPs. Haplotypes were estimated using the PHASE algorithm (Stephens et al., 2001). With adjustments for age, gender, smoking status, atopy, and body mass index as covariates, logistic analysis was used to assess the association of OBSCN genotype and haplotype with AERD and FEV1 decline by aspirin provocation using the Statistical Analysis System.
Results
Characteristics of study subjects
Despite need of demographic characteristics from a larger number of AERD patients, data from the subjects in this study showed that the mean age of the first medical examination of AERD patients (43.1 years) was 4 years earlier compared with that of ATA controls (47.3 years). In addition, smoking status, body mass index, and PC20 methacholine level of AERD patients' subgroup were lower than those of ATA controls (p<0.05). Most significantly, the mean FEV1 decline after aspirin provocation of AERD patients was about sevenfold higher than that of ATA controls (p<0.0001; Table 1).
Table 1.
Clinical Profiles of Study Subjects
| Clinical profile | All asthmatics (n=592) | AERD (n=163) | ATA (n=429) |
|---|---|---|---|
| Age [year, mean (range)] | 46.15 (15.40–77.88) | 43.13 (17.22–72.73)a | 47.30 (15.40–77.88) |
| Sex (n, male/female) | 206/386 | 59/104 | 147/282 |
| Total smoker (current smoker; ex-smoker) (%) | 27.70 (12.50; 15.20) | 21.47 (12.88; 8.59)a | 30.07 (12.35; 17.72) |
| Body mass index (kg/m2) | 24.24±3.39 | 23.39±3.25a | 24.58±3.39 |
| Basal FVC (%) | 84.28±17.87 | 85.16±18.67 | 83.97±17.58 |
| Basal FEV1 (%) | 84.66±20.60 | 83.65±20.18 | 85.03±20.76 |
| FEV1 decline by aspirin provocation (%) | 9.27±13.24 | 24.63±16.11b | 3.54±4.85 |
| Blood eosinophil (%) | 6.01±5.73 | 5.96±5.21 | 6.03±5.92 |
| PC20 methacholine (mg/mL) | 6.43±8.67 | 5.02±7.83a | 6.91±8.90 |
| Positive rate of skin test (%) | 56.42 | 52.76 | 57.81 |
| Presence of rhinosinusitis (%) | 41.39 | 62.58a | 33.33 |
| Presence of nasal polyp (%) | 29.56 | 50.92a | 21.45 |
| Total IgE (IU/mL) | 357.65±604.09 | 348.60±596.44 | 361.00±607.56 |
Age indicates a first medical examination.
Statistical significance of each clinical profile is compared between AERD cases and ATA controls.
p<0.05.
p<0.0001.
AERD, aspirin exacerbated respiratory disease; ATA, aspirin-tolerant asthma; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s.
Polymorphisms, LD, and haplotypes of OBSCN
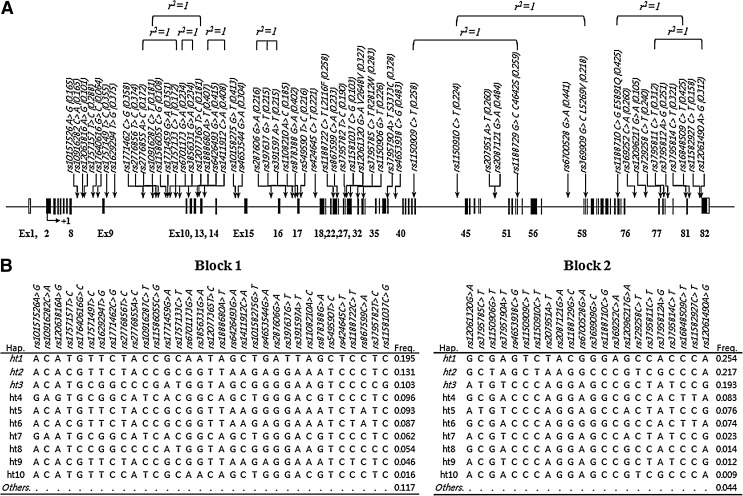
A total of 55 common polymorphisms, with MAF >0.05, were selected and successfully genotyped in 163 AERD and 429 ATA subjects (Fig. 1A and Table 2). Most SNPs are located at the intronic regions, except for one synonymous and six nonsynonymous variants in the coding regions. As a result of genotyping in Korean asthmatics, most SNPs showed high MAFs over 0.05 like other populations, with several complete LDs (r2= 1) as shown in Figure 1. Pair-wise comparisons revealed two LD blocks (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/dna), and the haplotypes were inferred using PHASE software (Fig. 1B). Only common haplotypes with frequency over 0.1 were used for the haplotype association analysis.
FIG. 1.
Physical map of single-nucleotide polymorphisms (SNPs) and haplotypes in OBSCN. (A) Complete linkage disequilibrium (LD) is denoted as r2=1. The coding exons are represented by black blocks, and 5′-untranslated region (UTR) and 3′UTR by white blocks. (B) Haplotypes of OBSCN. Haplotypes are estimated using PHASE algorithm (ver. 2.0). Haplotypes with frequency>0.1 (denoted as italics) are used for association analyses. Block indicates the LD block derived from LD coefficients (|D'| and r2) between all pairs of biallelic loci as shown in Supplementary Figure S1.
Table 2.
Association of OBSCN Variants with Aspirin Exacerbated Respiratory Disease
| |
|
|
MAF |
Co-dominant |
Dominant |
Recessive |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP (AA change) | Position | HWE | AERD (n=163) | ATA (n=429) | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| rs10157526A>G | Intron 8 | 0.541 | 0.150 | 0.167 | 0.87 (0.61–1.26) | 0.47 | 0.82 (0.54–1.23) | 0.33 | 1.29 (0.40–4.12) | 0.67 |
| rs10916282C>A | Intron 8 | 0.571 | 0.147 | 0.167 | 0.85 (0.59–1.23) | 0.39 | 0.79 (0.52–1.19) | 0.26 | 1.29 (0.40–4.12) | 0.67 |
| rs12063816A>G | Intron 8 | 0.551 | 0.095 | 0.098 | 0.98 (0.62–1.53) | 0.91 | 0.99 (0.61–1.59) | 0.95 | 0.75 (0.08–7.42) | 0.80 |
| rs1757157T>C | Intron 8 | 0.170 | 0.279 | 0.291 | 0.92 (0.68–1.23) | 0.57 | 0.93 (0.64–1.35) | 0.70 | 0.79 (0.38–1.65) | 0.53 |
| rs17640616G>C | Intron 8 | 0.299 | 0.052 | 0.066 | 0.71 (0.40–1.28) | 0.26 | 0.72 (0.40–1.30) | 0.28 | . | . |
| rs1757149T>C | Intron 9 | 0.591 | 0.334 | 0.364 | 0.86 (0.65–1.13) | 0.27 | 0.76 (0.52–1.10) | 0.14 | 0.98 (0.56–1.72) | 0.95 |
| rs1629294T>G | Intron 9 | 0.548 | 0.365 | 0.380 | 0.93 (0.71–1.22) | 0.58 | 0.79 (0.54–1.15) | 0.21 | 1.20 (0.71–2.02) | 0.50 |
| rs1771462C>G | Intron 9 | 0.583 | 0.337 | 0.366 | 0.88 (0.67–1.15) | 0.35 | 0.71 (0.49–1.03) | 0.07 | 1.24 (0.72–2.13) | 0.44 |
| rs2776856T>C | Intron 9 | 0.673 | 0.350 | 0.383 | 0.87 (0.66–1.14) | 0.30 | 0.66 (0.46–0.96) | 0.03 | 1.30 (0.78–2.18) | 0.32 |
| rs2776855A>C | Intron 9 | 0.331 | 0.160 | 0.181 | 0.84 (0.59–1.20) | 0.34 | 0.80 (0.53–1.20) | 0.28 | 0.98 (0.33–2.96) | 0.98 |
| rs10916287C>T | Intron 9 | 0.706 | 0.160 | 0.184 | 0.84 (0.59–1.19) | 0.33 | 0.81 (0.54–1.21) | 0.30 | 0.88 (0.30–2.61) | 0.82 |
| rs11586055C>G | Intron 9 | 0.474 | 0.107 | 0.114 | 0.94 (0.62–1.42) | 0.78 | 0.91 (0.58–1.45) | 0.70 | 1.18 (0.28–4.92) | 0.82 |
| rs1771459G>A | Intron 9 | 0.730 | 0.334 | 0.357 | 0.90 (0.68–1.18) | 0.44 | 0.73 (0.50–1.05) | 0.09 | 1.30 (0.76–2.25) | 0.34 |
| rs1757133C>T | Intron 9 | 0.331 | 0.160 | 0.181 | 0.84 (0.59–1.20) | 0.34 | 0.80 (0.53–1.20) | 0.28 | 0.98 (0.33–2.96) | 0.98 |
| rs6701173G>A | Intron 9 | 0.038a | 0.224 | 0.237 | 0.98 (0.71–1.35) | 0.89 | 0.92 (0.63–1.33) | 0.64 | 1.42 (0.59–3.45) | 0.44 |
| rs3856331G>A | Intron 9 | 0.038a | 0.224 | 0.237 | 0.98 (0.71–1.35) | 0.89 | 0.92 (0.63–1.33) | 0.64 | 1.42 (0.59–3.45) | 0.44 |
| rs12077365T>C | Intron 11 | 0.706 | 0.160 | 0.184 | 0.84 (0.59–1.19) | 0.33 | 0.81 (0.54–1.21) | 0.30 | 0.88 (0.30–2.61) | 0.82 |
| rs1888680A>T | Intron 12 | 0.420 | 0.439 | 0.396 | 1.16 (0.88–1.51) | 0.29 | 1.06 (0.72–1.57) | 0.77 | 1.48 (0.92–2.38) | 0.11 |
| rs6426493G>A | Intron 12 | 0.512 | 0.442 | 0.407 | 1.13 (0.86–1.47) | 0.39 | 1.01 (0.68–1.49) | 0.97 | 1.45 (0.90–2.32) | 0.12 |
| rs1411912C>A | Intron 13 | 0.382 | 0.439 | 0.397 | 1.15 (0.88–1.51) | 0.30 | 1.05 (0.71–1.56) | 0.80 | 1.48 (0.92–2.38) | 0.11 |
| rs10158275G>T | Intron 14 | 0.004a | 0.399 | 0.412 | 0.98 (0.74–1.30) | 0.89 | 1.13 (0.76–1.68) | 0.56 | 0.74 (0.43–1.30) | 0.30 |
| rs4653544G>A | Intron 14 | 0.753 | 0.304 | 0.304 | 0.98 (0.74–1.30) | 0.87 | 0.92 (0.64–1.34) | 0.68 | 1.12 (0.60–2.09) | 0.72 |
| rs287606G>A | Intron 15 | 0.055 | 0.212 | 0.217 | 1.02 (0.73–1.42) | 0.91 | 0.95 (0.65–1.39) | 0.81 | 1.65 (0.63–4.29) | 0.31 |
| rs397637G>T | Intron 16 | 0.035a | 0.212 | 0.216 | 1.03 (0.74–1.44) | 0.86 | 0.95 (0.65–1.39) | 0.81 | 1.85 (0.70–4.90) | 0.21 |
| rs391597A>T | Intron 16 | 0.035a | 0.212 | 0.216 | 1.03 (0.74–1.44) | 0.86 | 0.95 (0.65–1.39) | 0.81 | 1.85 (0.70–4.90) | 0.21 |
| rs1108210A>C | Intron 16 | 0.728 | 0.160 | 0.188 | 0.82 (0.58–1.16) | 0.25 | 0.79 (0.53–1.18) | 0.24 | 0.81 (0.28–2.35) | 0.69 |
| rs878388G>A | Intron 16 | 0.242 | 0.442 | 0.389 | 1.22 (0.93–1.60) | 0.15 | 1.16 (0.78–1.72) | 0.46 | 1.54 (0.95–2.51) | 0.08 |
| rs549590T>C | Intron 17 | 0.028a | 0.212 | 0.218 | 1.01 (0.73–1.41) | 0.94 | 0.93 (0.64–1.36) | 0.72 | 1.85 (0.70–4.90) | 0.21 |
| rs424645C>T | Intron 17 | 0.014a | 0.215 | 0.224 | 1.00 (0.72–1.39) | 0.99 | 0.92 (0.63–1.34) | 0.65 | 1.85 (0.70–4.90) | 0.21 |
| rs1188722C>T (L2116F) | Exon 23 | 0.597 | 0.291 | 0.242 | 1.26 (0.94–1.68) | 0.12 | 1.10 (0.76–1.60) | 0.62 | 2.52 (1.30–4.87) | 0.006 |
| rs867599C>A | Intron 25 | 0.991 | 0.258 | 0.225 | 1.15 (0.85–1.56) | 0.36 | 1.07 (0.74–1.56) | 0.71 | 1.78 (0.86–3.70) | 0.12 |
| rs3795782T>C | Intron 27 | 0.496 | 0.178 | 0.198 | 0.88 (0.62–1.24) | 0.46 | 0.86 (0.58–1.27) | 0.45 | 0.86 (0.30–2.50) | 0.79 |
| rs11581037C>G | Intron 28 | 0.309 | 0.098 | 0.108 | 0.89 (0.58–1.37) | 0.59 | 0.89 (0.55–1.42) | 0.62 | 0.78 (0.15–4.00) | 0.76 |
| rs12061320G>A (V2648V) | Exon 31 | 0.828 | 0.316 | 0.330 | 0.91 (0.69–1.21) | 0.52 | 0.75 (0.51–1.08) | 0.12 | 1.38 (0.78–2.44) | 0.27 |
| rs3795785C>T (R2812W) | Exon 32 | 0.687 | 0.288 | 0.282 | 1.01 (0.76–1.36) | 0.93 | 0.89 (0.61–1.28) | 0.52 | 1.60 (0.83–3.08) | 0.16 |
| rs1150906G>T | Intron 33 | 0.068 | 0.215 | 0.230 | 0.97 (0.70–1.34) | 0.84 | 0.91 (0.62–1.33) | 0.62 | 1.39 (0.55–3.51) | 0.49 |
| rs3795790A>T (S3373C) | Exon 39 | 0.811 | 0.317 | 0.331 | 0.91 (0.69–1.21) | 0.53 | 0.74 (0.51–1.08) | 0.12 | 1.39 (0.79–2.46) | 0.25 |
| rs4653938C>G | Intron 39 | 0.541 | 0.503 | 0.472 | 1.15 (0.89–1.49) | 0.29 | 0.88 (0.58–1.32) | 0.53 | 1.68 (1.11–2.54) | 0.02 |
| rs1150909C>T | Intron 43 | 0.636 | 0.288 | 0.244 | 1.22 (0.91–1.63) | 0.18 | 1.04 (0.72–1.51) | 0.83 | 2.52 (1.30–4.88) | 0.006 |
| rs1150910C>T | Intron 44 | 0.032a | 0.215 | 0.226 | 0.99 (0.71–1.38) | 0.95 | 0.92 (0.63–1.34) | 0.66 | 1.62 (0.63–4.18) | 0.32 |
| rs207951A>T | Intron 50 | 0.596 | 0.291 | 0.246 | 1.22 (0.91–1.63) | 0.18 | 1.06 (0.73–1.54) | 0.77 | 2.41 (1.25–4.63) | 0.008 |
| rs2087121G>A | Intron 50 | 0.393 | 0.506 | 0.472 | 1.16 (0.90–1.50) | 0.26 | 0.89 (0.59–1.33) | 0.56 | 1.70 (1.12–2.57) | 0.01 |
| rs1188729G>C (C4642S) | Exon 54 | 0.665 | 0.288 | 0.245 | 1.21 (0.91–1.62) | 0.19 | 1.04 (0.71–1.50) | 0.86 | 2.53 (1.31–4.89) | 0.006 |
| rs6700528G>A | Intron 57 | 0.831 | 0.433 | 0.446 | 0.92 (0.71–1.20) | 0.54 | 0.78 (0.53–1.15) | 0.21 | 1.09 (0.69–1.73) | 0.70 |
| rs369909G>C (L5269V) | Exon 59 | 0.089 | 0.206 | 0.221 | 0.97 (0.69–1.36) | 0.87 | 0.90 (0.61–1.32) | 0.57 | 1.64 (0.64–4.23) | 0.31 |
| rs1188710C>G (E5891Q) | Exon 73 | 0.077 | 0.442 | 0.418 | 1.07 (0.82–1.40) | 0.63 | 1.01 (0.68–1.50) | 0.96 | 1.23 (0.76–1.99) | 0.41 |
| rs369252C>A | Intron 76 | 0.596 | 0.291 | 0.245 | 1.24 (0.93–1.65) | 0.15 | 1.04 (0.72–1.51) | 0.84 | 2.71 (1.41–5.21) | 0.003 |
| rs12096217G>A | Intron 76 | 0.625 | 0.095 | 0.104 | 0.89 (0.58–1.37) | 0.59 | 0.88 (0.55–1.42) | 0.61 | 0.77 (0.15–3.94) | 0.75 |
| rs729258C>T | Intron 77 | 0.073 | 0.227 | 0.244 | 0.97 (0.70–1.34) | 0.86 | 0.93 (0.64–1.35) | 0.70 | 1.25 (0.50–3.10) | 0.64 |
| rs3795811C>T | Intron 78 | 0.745 | 0.304 | 0.316 | 0.91 (0.68–1.20) | 0.49 | 0.77 (0.53–1.11) | 0.16 | 1.31 (0.72–2.39) | 0.38 |
| rs3795812A>G | Intron 79 | 0.073 | 0.233 | 0.257 | 0.94 (0.68–1.30) | 0.71 | 0.89 (0.61–1.29) | 0.52 | 1.24 (0.53–2.93) | 0.62 |
| rs3795814C>T | Intron 80 | 0.778 | 0.307 | 0.329 | 0.87 (0.66–1.16) | 0.35 | 0.75 (0.52–1.08) | 0.12 | 1.17 (0.64–2.11) | 0.61 |
| rs16848509C>T | Intron 81 | 0.071 | 0.442 | 0.418 | 1.07 (0.81–1.40) | 0.63 | 1.01 (0.68–1.50) | 0.97 | 1.23 (0.76–2.00) | 0.40 |
| rs11582927C>T | Intron 81 | 0.098 | 0.144 | 0.167 | 0.81 (0.56–1.18) | 0.28 | 0.79 (0.52–1.19) | 0.26 | 0.85 (0.21–3.48) | 0.82 |
| rs12061490A>G | Intron 81 | 0.844 | 0.307 | 0.316 | 0.92 (0.69–1.23) | 0.57 | 0.77 (0.53–1.11) | 0.16 | 1.41 (0.78–2.56) | 0.25 |
p-Values are adjusted with initial diagnosed age, sex, smoking, atopy, and body mass index.
Bold values indicate the statistical significance (p<0.05).
p-Values of HWE are not significant after correction by number of markers.
SNP, single-nucleotide polymorphism; AA, amino acid; HWE, p-values of deviation from Hardy–Weinberg equilibrium; MAF, minor allele frequency; OR, odds ratio; CI, confidence interval.
Association analysis of OBSCN with the risk of AERD and FEV1 decline
Results of logistic analysis adjusted for age, gender, smoking status, atopy, and body mass index as covariates showed significant associations between eight SNPs in OBSCN and AERD development (p=0.003–0.03; Table 2). Intriguingly, the significant SNPs included two nonsynonymous variants, rs1188722C>T (Leu2116Phe) and rs1188729G>C (Cys4642Ser), in the coding regions (odds ratio [OR]=2.52–2.53, p=0.006; Table 2). In addition, haplotype BL2_ht1, unique to rs1150909C>T, rs207951A>T, rs1188729G>C, and rs369252C>A (Fig. 1B), was associated with AERD (OR=2.53, p=0.006; Table 3).
Table 3.
Association of OBSCN Haplotypes with Aspirin Exacerbated Respiratory Disease
| |
|
Frequency |
Co-dominant |
Dominant |
Recessive |
||||
|---|---|---|---|---|---|---|---|---|---|
| LD block | Haplotype | AERD (n=163) | ATA (n=429) | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Block 1 | BL1_ht1 | 0.193 | 0.196 | 1.05 (0.74–1.48) | 0.79 | 0.98 (0.67–1.45) | 0.93 | 1.86 (0.65–5.34) | 0.25 |
| BL1_ht2 | 0.135 | 0.133 | 0.97 (0.66–1.42) | 0.87 | 0.93 (0.61–1.43) | 0.74 | 1.35 (0.36–5.03) | 0.66 | |
| BL1_ht3 | 0.098 | 0.108 | 0.89 (0.58–1.37) | 0.59 | 0.89 (0.55–1.42) | 0.62 | 0.78 (0.15–4.00) | 0.76 | |
| Block 2 | BL2_ht1 | 0.282 | 0.239 | 1.21 (0.91–1.63) | 0.19 | 1.04 (0.71–1.51) | 0.85 | 2.53 (1.31–4.89) | 0.006 |
| BL2_ht2 | 0.215 | 0.219 | 1.03 (0.74–1.44) | 0.85 | 0.97 (0.67–1.42) | 0.88 | 1.62 (0.63–4.18) | 0.32 | |
| BL2_ht3 | 0.196 | 0.198 | 0.96 (0.69–1.34) | 0.82 | 0.83 (0.57–1.23) | 0.36 | 2.00 (0.84–4.79) | 0.12 | |
p-Values are adjusted with initial diagnosed age, sex, smoking, atopy, and body mass index.
Bold values indicate the statistical significance (p<0.05).
LD, linkage disequilibrium.
Since FEV1 decline by aspirin provocation is the important criteria for total pulmonary function, regression analysis for the association with the phenotype of FEV1 decline by aspirin provocation in asthmatics was performed. As a result, only two SNPs of OBSCN, rs1188722C>T (Leu2116Phe) in exon 23 and rs369252C>A in the intron 76, revealed nominal signals (p=0.03–0.04; Supplementary Table S1). However, no dramatic differences in FEV1 decline by aspirin provocation were observed in further comparison between AERD and ATA subgroups (Supplementary Tables S2 and S3). On the other hand, haplotypes of OBSCN showed no significant relation to the FEV1 decline (p>0.05; Supplementary Table 4), except a nominal link to BL2_ht1 in ATA subjects (p=0.04).
In silico analyses for potential functions of the significant variants
In the alignment of amino acid residues of the nonsynonymous Leu2116Phe and Cys4642Ser SNPs showing significant associations with AERD, the Leu2116Phe site was highly conserved among human and other species. Also, Cys4642Ser site was found to be highly conserved among mammals (Fig. 2).
FIG. 2.
Amino acid sequence alignments of OBSCN. (A) Multiple alignments of nonsynonymous rs1188722C>T (Leu2116Phe) using the ClustalW2 program (www.ebi.ac.uk/Tools/msa/clustalw2/) show a highly conserved residue among human and other species. (B) The rs1188729G>C (Cys4642Ser) site also shows a high conservation among mammals. Asterisk (*)indicates the identical residues, whereas colon (:) and period (.) are used to show the positions where the conserved position contains amino acids of the strong (:) and weak (.) similarity groups, respectively. Human (NP_443075.2), Pig (XP_003123677.1), Mouse (NP_954603.2), Rat (XP_340808.4), Chicken (XP_418501.2), and Zebrafish (XP_001341205.4).
In silico analysis using EMBL-EBI splice site prediction (www.ebi.ac.uk/asd-srv/wb.cgi?method=2) revealed that the ATG[C/A]C sequence containing the minor A allele of rs369252C>A in intron 76 was estimated to be a potential branch point (BP) site for alternative splicing, with BP score=4.29 (Supplementary Fig. S2). Interestingly, the rs369252C>A variant showed the strongest association with the risk of AERD (OR=2.71, p=0.003; Table 2) and was found to be related with FEV1 decline by aspirin provocation in an allelic dose-dependent manner (p=0.04 under co-dominant model; p=0.03 under recessive model; Supplementary Table S1).
Discussion
This study is the first to investigate association of OBSCN genetic variants and their haplotypes with the risk of AERD and the phenotypic FEV1 decline after aspirin provocation in asthmatics. This study found that most of the associated variants and one haplotype (BL2_ht1) in OBSCN were more frequent in AERD cases than in ATA controls. More interestingly, two nonsynonymous variants, rs1188722C>T (Leu2116Phe) and rs1188729G>C (Cys4642Ser), were identified as conserved residues among human and other species, suggesting that genetic variations of OBSCN might contribute to the development of AERD and could be molecular markers for the disease. However, there are several limitations in this study: (1) lack of normal controls; (2) no functional evaluation for the associated SNPs; (3) need for further replication study in larger cohorts.
Airway obstruction derived from bronchial hyperresponsiveness and airway hyperreactivity may threaten asthmatics' lives. Recently, many studies have suggested the important role of intracellular Ca2+, indicating that an increase of cytosolic Ca2+ concentration in bronchial smooth muscle cells might cause bronchial obstruction and bronchospam (Parekh and Penner, 1997; Sweeney et al., 2002). In the airway smooth muscle cells, the altered Ca2+ homeostasis of the SR has also been shown to affect airway remodeling, including changes in expressions of immune-related molecules (Kellner et al., 2008). Furthermore, the correlation between SR Ca2+ regulation and proinflammatory cytokines has been reported in human airway smooth muscle (Sathish et al., 2009). Therefore, considering the regulation of SR integrity and function in Obscn−/− mice, aspirin hypersensitivity in asthma could be mediated by bronchial smooth muscle contraction with the involvement of Ca2+ sensitization through OBSCN-mediated SR regulation.
Although little is known about mutations and functions of OBSCN and its association with human diseases since the generation of Obscn knockout mice has only been reported recently, missense mutations of OBSCN have been implicated in several human diseases, such as glioblastoma and melanoma as well as hereditary myopathies (Balakrishnan et al., 2007; Bleeker and Bardelli, 2007; Fukuzawa et al., 2008). Recently, the nonsynonymous Arg4344Gln variant of OBSCN has been suggested to be involved in the pathogenesis of hypertrophic cardiomyopathy, a condition that is characterized by dyspneas and reported to be vulnerable to the outflow tract obstruction (Brilakis and Nishimura, 2004), by affecting interaction with titin/connectin (Arimura et al., 2007). More intriguingly, increased expression of titin, which interacts with obscurin, has been determined in chronic obstructive pulmonary disease with the involvement of alternative splicing of the titin gene (Ottenheijm et al., 2006). These observations suggest that obscurin could also be related with obstructive diseases, possibly through expressional and/or functional changes.
To ascertain the potential functions of the significantly associated OBSCN variants, further in silico analysis was applied. In the prediction of functional site using eukaryotic linear motif (ELM) program (http://elm.eu.org/index.html), both the nonsynonymous variants rs1188722C>T (Leu2116Phe) and rs1188729G>C (Cys4642Ser) were not detected as the motif sites. However, additional multiple alignment comparing the conserved residues of human and other species has revealed that the Leu2116Phe site is a highly conserved residue, even in the Zebrafish (Fig. 2A). In addition, the Cys4642Ser variant showed a considerably high conservation among mammals (Fig. 2B). Nonsynonymous polymorphisms of many genes have been functionally evaluated in human diseases (Hindorff et al., 2009), including asthma and immune-mediated diseases, in which the nonsynonymous SNPs of chitotriosidase (CHIT1) affects its protein activity and subsequently leads to associations with the related phenotypes (e.g., positive tuberculosis and atopic diseases) (Ober and Chupp, 2009). Findings from this study suggest that two OBSCN nonsynonymous variants, rs1188722C>T (Leu2116Phe) and rs1188729G>C (Cys4642Ser), may be functionally relevance in AERD occurrence.
In silico analyses for splice sites provides relatively consistent results with microarray and polymerase chain reaction data (Coulombe-Huntington et al., 2009), suggesting that the extent of individual cis-regulation that affects alternative splicing might be greater than previously expected. In silico analysis from this study also predicted the ATG[C/A]C sequence containing rs369252C>A as a potential BP site for alternative splicing. The ATGAC sequence containing the minor A allele of rs369252C>A was predicted as a BP site, but not in the case of the ATGCC sequence containing the major C allele of the variant. Therefore, considering that many alternative transcripts of OBSCN have been discovered (Supplementary Fig. S2), intronic rs369252C>A in OBSCN may also have a role in the protein expression and subsequent association with AERD.
Although we were not able to compare with the frequencies of Korean normal controls, minor allele frequencies of 55 OBSCN SNPs among Korean asthmatics (AERD plus ATA in this study) and other Asian control populations (Chinese and Japanese) from the International HapMap Project database are shown in Supplementary Table S5. On the other hand, in further analysis of the association between OBSCN and FANCC polymorphisms, which were genotyped in the same study subjects (Kim et al., 2011), no relationships were found (data not shown). Recently, genetic variants of many genes, including those on leukotriene synthetic pathway or the 5-lipoxygenase pathway (e.g., LTC4S and ALOX5) (Sanak et al., 1997; Choi et al., 2004), have been determined as risk alleles, with modest OR, for the aspirin hypersensitivity in asthma. However, it has been suggested that common susceptibility loci with low penetrance may confer modest risk effects for diseases, under multiple and independently associated condition among the risk alleles (Hindorff et al., 2009). Considering the small fraction of each confounder for the association with diseases and the complexity of genetic and environmental contribution to asthma, genetic variations in OBSCN may add a functional responsibility for the occurrence of AERD and its related phenotypes, together with risk factors on other genes.
In conclusion, this study found associations of OBSCN variants with AERD and the FEV1 decline by aspirin provocation in asthmatics. One possible explanation is that the variation in the highly conserved Leu2116Phe locus may alter its protein function, leading to changes in the SR architecture that regulates Ca2+ homeostasis in the airway smooth muscle cells. The other possible explanation would be that the variation in the rs369252C>A locus could lead to an alternative splicing that produces a new dysfunctional product. In addition, further studies to investigate associations between OBSCN variants and AERD in larger cases from different ethnic origins are required.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Korea Health 21 R&D Project (A010249) and by grant number M1-0302-00-0073 from the Korea Science and Engineering Foundation (KOSEF) funded by the Korean government (MEST) (No. 2009-0080157) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the MEST (No. 2010-0007857), and also supported by a grant from the Korea Health 21 R&D Project (A010249).
Disclosure Statement
No competing interests exist.
References
- Arimura T. Matsumoto Y. Okazaki O. Hayashi T. Takahashi M. Inagaki N. Hinohara K. Ashizawa N. Yano K. Kimura A. Structural analysis of obscurin gene in hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2007;362:281–287. doi: 10.1016/j.bbrc.2007.07.183. [DOI] [PubMed] [Google Scholar]
- Babu K.S. Salvi S.S. Aspirin and asthma. Chest. 2000;118:1470–1476. doi: 10.1378/chest.118.5.1470. [DOI] [PubMed] [Google Scholar]
- Balakrishnan A. Bleeker F.E. Lamba S. Rodolfo M. Daniotti M. Scarpa A. van Tilborg A.A. Leenstra S. Zanon C. Bardelli A. Novel somatic and germline mutations in cancer candidate genes in glioblastoma, melanoma, and pancreatic carcinoma. Cancer Res. 2007;67:3545–3550. doi: 10.1158/0008-5472.CAN-07-0065. [DOI] [PubMed] [Google Scholar]
- Bleeker F.E. Bardelli A. Genomic landscapes of cancers: prospects for targeted therapies. Pharmacogenomics. 2007;8:1629–1633. doi: 10.2217/14622416.8.12.1629. [DOI] [PubMed] [Google Scholar]
- Brilakis E.S. Nishimura R.A. Dynamic respiratory changes in hypertrophic cardiomyopathy. Heart. 2004;90:296. doi: 10.1136/hrt.2003.024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.H. Park H.S. Oh H.B. Lee J.H. Suh Y.J. Park C.S. Shin H.D. Leukotriene-related gene polymorphisms in ASA-intolerant asthma: an association with a haplotype of 5-lipoxygenase. Hum Genet. 2004;114:337–344. doi: 10.1007/s00439-004-1082-1. [DOI] [PubMed] [Google Scholar]
- Cormican L.J. Farooque S. Altmann D.R. Lee T.H. Improvements in an oral aspirin challenge protocol for the diagnosis of aspirin hypersensitivity. Clin Exp Allergy. 2005;35:717–722. doi: 10.1111/j.1365-2222.2005.02261.x. [DOI] [PubMed] [Google Scholar]
- Coulombe-Huntington J. Lam K.C. Dias C. Majewski J. Fine-scale variation and genetic determinants of alternative splicing across individuals. PLoS Genet. 2009;5:e1000766. doi: 10.1371/journal.pgen.1000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. Thomson N.C. Rubin A.S. Niven R.M. Corris P.A. Siersted H.C. Olivenstein R. Pavord I.D. McCormack D. Chaudhuri R. Miller J.D. Laviolette M. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327–1337. doi: 10.1056/NEJMoa064707. [DOI] [PubMed] [Google Scholar]
- Dragomir E. Manduteanu I. Voinea M. Costache G. Manea A. Simionescu M. Aspirin rectifies calcium homeostasis, decreases reactive oxygen species, and increases NO production in high glucose-exposed human endothelial cells. J Diabet Complications. 2004;18:289–299. doi: 10.1016/j.jdiacomp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Fukuzawa A. Lange S. Holt M. Vihola A. Carmignac V. Ferreiro A. Udd B. Gautel M. Interactions with titin and myomesin target obscurin and obscurin-like 1 to the M-band: implications for hereditary myopathies. J Cell Sci. 2008;121:1841–1851. doi: 10.1242/jcs.028019. [DOI] [PubMed] [Google Scholar]
- Hedman J. Kaprio J. Poussa T. Nieminen M.M. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28:717–722. doi: 10.1093/ije/28.4.717. [DOI] [PubMed] [Google Scholar]
- Hindorff L.A. Sethupathy P. Junkins H.A. Ramos E.M. Mehta J.P. Collins F.S. Manolio T.A. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner J. Tantzscher J. Oelmez H. Edelmann M. Fischer R. Huber R.M. Bergner A. Mechanisms altering airway smooth muscle cell Ca+ homeostasis in two asthma models. Respiration. 2008;76:205–215. doi: 10.1159/000135606. [DOI] [PubMed] [Google Scholar]
- Kim J.H. Park B.L. Cheong H.S. Bae J.S. Park J.S. Jang A.S. Uh S.T. Choi J.S. Kim Y.H. Kim M.K. Choi I.S. Cho S.H. Choi B.W. Park C.S. Shin H.D. Genome-wide and follow-up studies identify CEP68 gene variants associated with risk of aspirin-intolerant asthma. PLoS One. 2010;5:e13818. doi: 10.1371/journal.pone.0013818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H. Park B.L. Pasaje C.F. Bae J.S. Park J.S. Park S.W. Uh S.T. Choi J.S. Kim Y.H. Kim M.K. Choi I.S. Cho S.H. Choi B.W. Park C.S. Shin H.D. Association of FANCC polymorphisms with FEV1 decline in aspirin exacerbated respiratory disease. Mol Biol Rep. 2011 doi: 10.1007/s11033-011–0989-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kim S.H. Choi J.H. Park H.S. Holloway J.W. Lee S.K. Park C.S. Shin H.D. Association of thromboxane A2 receptor gene polymorphism with the phenotype of acetyl salicylic acid-intolerant asthma. Clin Exp Allergy. 2005;35:585–590. doi: 10.1111/j.1365-2222.2005.02220.x. [DOI] [PubMed] [Google Scholar]
- Kim S.H. Ye Y.M. Lee S.K. Choi J.H. Holloway J.W. Park C.S. Park H.S. Association of TNF-alpha genetic polymorphism with HLA DPB1*0301. Clin Exp Allergy. 2006;36:1247–1253. doi: 10.1111/j.1365-2222.2006.02567.x. [DOI] [PubMed] [Google Scholar]
- Kim T.H. Chang H.S. Park S.M. Nam B.Y. Park J.S. Rhim T. Park H.S. Kim M.K. Choi I.S. Cho S.H. Chung I.Y. Park B.L. Park C.S. Shin H.D. Association of angiotensin I-converting enzyme gene polymorphisms with aspirin intolerance in asthmatics. Clin Exp Allergy. 2008;38:1727–1737. doi: 10.1111/j.1365-2222.2008.03082.x. [DOI] [PubMed] [Google Scholar]
- Lange S. Ouyang K. Meyer G. Cui L. Cheng H. Lieber R.L. Chen J. Obscurin determines the architecture of the longitudinal sarcoplasmic reticulum. J Cell Sci. 2009;122:2640–2650. doi: 10.1242/jcs.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia K. Haselkorn T. Deniz Y.M. Miller D.P. Bleecker E.R. Borish L. Aspirin sensitivity and severity of asthma: evidence for irreversible airway obstruction in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2005;116:970–975. doi: 10.1016/j.jaci.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Nizankowska-Mogilnicka E. Bochenek G. Mastalerz L. Swierczynska M. Picado C. Scadding G. Kowalski M.L. Setkowicz M. Ring J. Brockow K. Bachert C. Wohrl S. Dahlen B. Szczeklik A. EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy. 2007;62:1111–1118. doi: 10.1111/j.1398-9995.2007.01409.x. [DOI] [PubMed] [Google Scholar]
- Ober C. Chupp G.L. The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune-mediated diseases. Curr Opin Allergy Clin Immunol. 2009;9:401–408. doi: 10.1097/ACI.0b013e3283306533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheijm C.A. Heunks L.M. Hafmans T. van der Ven P.F. Benoist C. Zhou H. Labeit S. Granzier H.L. Dekhuijzen P.N. Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:527–534. doi: 10.1164/rccm.200507-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A.B. Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Park J.S. Chang H.S. Park C.S. Lee J.H. Lee Y.M. Choi J.H. Park H.S. Kim L.H. Park B.L. Choi Y.H. Shin H.D. Association analysis of cysteinyl-leukotriene receptor 2 (CYSLTR2) polymorphisms with aspirin intolerance in asthmatics. Pharmacogenet Genomics. 2005;15:483–492. doi: 10.1097/01.fpc.0000166456.84905.a0. [DOI] [PubMed] [Google Scholar]
- Sanak M. Simon H.U. Szczeklik A. Leukotriene C4 synthase promoter polymorphism and risk of aspirin-induced asthma. Lancet. 1997;350:1599–1600. doi: 10.1016/s0140-6736(05)64015-9. [DOI] [PubMed] [Google Scholar]
- Sathish V. Thompson M.A. Bailey J.P. Pabelick C.M. Prakash Y.S. Sieck G.C. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297:L26–L34. doi: 10.1152/ajplung.00026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M. Smith N.J. Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M. McDaniel S.S. Platoshyn O. Zhang S. Yu Y. Lapp B.R. Zhao Y. Thistlethwaite P.A. Yuan J.X. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol. 2002;92:1594–1602. doi: 10.1152/japplphysiol.00722.2001. [DOI] [PubMed] [Google Scholar]
- Szczeklik A. Stevenson D.D. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003;111:913–921. doi: 10.1067/mai.2003.1487. [DOI] [PubMed] [Google Scholar]
- Warner S.M. Knight D.A. Airway modeling and remodeling in the pathogenesis of asthma. Curr Opin Allergy Clin Immunol. 2008;8:44–48. doi: 10.1097/ACI.0b013e3282f3b5cb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.