Abstract
Atrial natriuretic peptide (ANP, also known as NPPA) and brain natriuretic peptide (BNP, also known as NPPB) have been determined as genetic factors for several diseases, including stroke and myocardial infarction, in human and rat models. To investigate the potential association between polymorphisms of the NPPA gene and stroke in a Korean population, nine single-nucleotide polymorphisms (SNPs) of NPPA and NPPB genes were genotyped in a total of 941 Korean subjects, including 674 stroke patients (109 hemorrhagic and 565 ischemic) and 267 unaffected controls. Genotype comparisons of the targeted alleles revealed that there were no significant associations between stroke patients and control subjects, or among hemorrhagic, ischemic, and control groups. However, in logistic analysis for Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification of ischemic stroke, nonsynonymous rs5065 (STOP152Arg) and rs5067 in 3′UTR of NPPA, which were in complete linkage disequilibrium, showed significant associations with cardioembolic stroke. These two SNPs showed higher frequencies in cardioembolic stroke patients than those in controls and ischemic patients with small-vessel occlusion (p=0.002, adjusted p=0.02). It was also found that NPPA rs5065C allele in all of the Korean subjects existed as heterozygous compared with Caucasian and African populations. Although further replications in larger cardioembolic stroke subjects are required, our preliminary findings suggest that the nonsynonymous rs5065C of the NPPA gene, which could produce a new or dysfunctional transcript, is possibly associated with cardioembolism.
Introduction
Strokes occur when blood supply to a part of brain is interrupted or severely reduced with developing loss of brain functions. Strokes can be classified into two major categories: ischemic and hemorrhagic. Ischemia is caused by interruption of the blood supply, while hemorrhage is caused by rupture of a blood vessel or an abnormal vascular structure. About 80% of strokes are due to ischemia, whereas the remainders are due to hemorrhage. Furthermore, stroke is a complex neurological disorder that most likely results from complicated interactions between genetics, lifestyle, and environment. Family, twin, and population studies have shown significant genetic contributions to disease occurrence (Liao et al., 1997; Rastenyte et al., 1998; Hademenos et al., 2001).
A genome-wide screen using F2 cohort from mating between stroke-prone spontaneously hypertensive rat and stroke-resistant spontaneously hypertensive rat revealed NPPA and NPPB genes, which are located at chromosome 1p36.21 and 1p36.2, respectively, as blood pressure-independent genetic factors predisposing to a complex form of stroke (Rubattu et al., 1996; Jeffs et al., 1997). Natriuretic peptides, as polypeptide hormones primarily secreted from the heart, play important roles in the balance of electrolytes, water, and fat, and also in the regulation of cardiac hemodynamics through their cardiovascular, renal, and neural effects (Rubattu et al., 2008; Potter et al., 2009). In addition, the expression, regulation, and therapeutic applications of natriuretic peptides during development and diseases have been demonstrated (Houben et al., 2005; Houweling et al., 2005; Potter et al., 2009). Moreover, polymorphisms of NPPA and NPPB genes, which encode atrial natriuretic peptide (ANP, also known as NPPA) and brain natriuretic peptide (BNP, also known as NPPB), respectively, have been shown to be associated with vascular diseases, including stroke, blood pressure, and hypertension (Rubattu et al., 2004; Conen et al., 2009; Newton-Cheh et al., 2009).
Based on disease etiology and clinical features, the Trial of Org 10172 in Acute Stroke Treatment (TOAST) subclassified ischemic stroke into five subtypes as follows: large-artery atherosclerosis (LAA), small-vessel occlusion (SVO), cardioembolism (CE), stroke of other determined etiology (SOE), and stroke of undetermined etiology (SUE) (Adams et al., 1993). The interrater reliability of TOAST classification was also assessed recently (Meschia et al., 2006), providing its efficiency in strategies for treatment and management of stroke recurrence. LAA, which approximately accounts for 10%–25% of ischemic strokes, is characterized by significant stenoses of cerebral cortical artery or brain artery. SVO (15%–25%) is one of the traditional clinical lacunar syndromes that are due to occlusion of a single penetrating artery, without evidence of cerebral cortical dysfunction. CE (15%–35%) patients have arterial occlusions derived from an embolus in the heart, and the condition accounts for about 20% of ischemic strokes. Since SUE includes patients with no or more than two causes of stroke, disease occurrence is occasionally high depending on the evaluation (Yip et al., 1997).
Materials and Methods
Study subjects
This study included 267 unaffected controls and 674 stroke subjects recruited for about 3 years (between 2006 and 2009) and diagnosed from three oriental medical hospitals of multicenter. Patient subjects consisted of 109 hemorrhagic cases and 565 ischemic patients. Computed tomography (CT) and magnetic resonance imaging (MRI) evaluations of all participants were included for diagnosis. Written informed consent was obtained from all participants, and the study protocol was approved by the Institutional Review Board of each hospital. Patients with subdural hemorrhage, epidural hemorrhage, and stroke from injuries (e.g., traffic accident and fall) were excluded from this study. Ischemic patients were additionally determined according to the TOAST classification based on clinical features and CT/MRI evaluation. Genomic DNAs and clinical data of healthy controls were obtained from the center for genome science of Korea National Institute of Health.
Genotyping
Genomic DNA was extracted from peripheral blood lymphocytes using Exgene blood SV kit (GeneAll), according to the manufacturer's protocol. We mainly targeted the nonsynonymous single-nucleotide polymorphism (SNPs) of the NPPB and NPPA genes with heterozygosity above 0.01. SNPs in promoter region of the NPPA gene were also included due to the significant effect on NPPA expression. The rs198389 of the NPPB gene was added because this SNP was recently reported to be associated with elevating plasma BNP levels (Takeishi et al., 2007). Genotyping was performed with 20 ng of genomic DNA by TaqMan assay in the ABI prism 7900HT sequence detection system (Applied Biosystems). Genotyping data were obtained using the ABI-PRISM sequence detection system software version 2.3. The genotype quality score for keeping data was set at 0.25. As a result of this process, 674 stroke patients and 267 normal controls were successfully genotyped.
Data analysis and statistics
Blood test and clinical data of patients in each group and controls were analyzed using STATA/SE 10.1 for windows (StataCorp LP). Hardy–Weinberg equilibrium for individual polymorphism was evaluated to check the data quality and genotype error using chi-square test, which compared the observed numbers of each genotype with those expected for the population with one degree of freedom. Patients with SOE and SUE were excluded from association analysis due to the small number of subjects. Monomorphic rs5227 and rs35640285 of the NPPB gene were also excluded from association analysis. Associations of the genetic variants were assessed by comparing allele frequencies in each case and control, or between each group using the multivariable logistic analysis by STATA/SE 10.1 for windows. Dominant, additive, and recessive models adjusted for age, sex, and histories of hypertension and diabetes mellitus as covariables were tested. p-Values <0.05 were taken as statistically significant. Haploview v4.1 software was used to determine linkage disequilibrium (LD) of SNPs in the NPPA and NPPB genes. Lewontin's D′ (|D′|) and the LD coefficient r2 between all pairs of biallelic loci were examined (Barrett et al., 2005). Statistical power of single associations was calculated using the Power for Genetic Association Analyses (PGA) software (Menashe et al., 2008).
Results
Study subjects
Clinical and demographic characteristics of 674 Korean patients with stroke and 267 unaffected controls who participated in this study are presented in Table 1. The average high-density lipoprotein cholesterol, Na+, and K+ in total stroke patients were significantly decreased compared with those of normal controls, whereas histories of hypertension and diabetes mellitus were higher in cases compared with those of controls as expected (p<0.0001, Table 1). In subtypes of ischemic stroke, the proportion of males was higher than females in SVO, while lower in CE, about a 1.5-fold, respectively. Also, the level of triglyceride in CE group was decreased compared with that of other groups (Table 1).
Table 1.
Clinical Profiles of Study Subjects
| |
Strokes |
TOAST classification of ischemic stroke |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical profiles | Total | Ischemic | Hemorrhagic | LAA | SVO | CE | SOE | SUE | Normal controls | pa |
| Number of subjects (n) | 674 | 565 | 109 | 123 | 395 | 33 | 7 | 7 | 267 | |
| Sex (male/female) | 379/295 | 317/248 | 62/47 | 63/60 | 235/160 | 13/20 | 3/4 | 3/4 | 115/152 | |
| Age (year) | 65.3±12.2 | 59.3±13.1 | 66.5±11.7 | 65.3±12.9 | 66.9±10.9 | 69.5±10.1 | 50.1±25.9 | 63.6±7.3 | 49.8±7.9 | <0.001 |
| Total cholesterol (mg/dL) | 180.9±41.4 | 182.7±41.7 | 171.8±38.6 | 177.6±51.0 | 184.5±38.7 | 180.8±40.5 | 178.3±46.0 | 182.3±23.5 | 184.3±33.6 | 0.242 |
| Triglyceride (mg/dL) | 149.8±90.2 | 150.1±91.9 | 147.7±79.1 | 149.6±93.4 | 153.2±92.1 | 119.3±73.3 | 140.6±145.7 | 130.0±36.6 | 144.7±91.8 | 0.444 |
| High-density lipoprotein cholesterol (mg/dL) | 39.3±12.1 | 39.4±11.9 | 38.4±13.4 | 37.3±10.9 | 39.9±12.1 | 38.8±11.0 | 48.1±15.3 | 47.3±14.5 | 45.2±10.3 | <0.001 |
| Na (mM) | 140.1±4.9 | 140.2±5.1 | 139.5±3.6 | 139.0±8.6 | 140.5±3.5 | 140.5±2.7 | 141.0±5.7 | 142.4±3.0 | 142.5±2.2 | <0.001 |
| K (mM) | 4.0±0.5 | 4.0±0.5 | 4.0±0.5 | 3.9±0.5 | 4.0±0.5 | 4.0±0.6 | 4.0±0.0 | 4.1±0.4 | 4.5±0.5 | <0.001 |
| Cl (mM) | 104.2±6.8 | 104.5±7.2 | 102.9±4.1 | 103.1±13.6 | 105.0±3.8 | 104.1±4.3 | 104.1±4.0 | 105.0±2.2 | 103.2±2.4 | 0.001 |
| History of hypertension (%) | 62 | 62.4 | 60 | 62.4 | 60.9 | 80.7 | 28.6 | 71.4 | 10.9 | <0.001 |
| History of diabetes mellitus (%) | 28 | 31.4 | 9.6 | 30.1 | 32.1 | 30.3 | 14.3 | 42.9 | 1.5 | <0.001 |
p-Value was compared between total strokes and normal controls.
LAA, large-artery atherosclerosis; SVO, small-vessel occlusion; CE, cardioembolism; SOE, stroke of other determined etiology; SUE, stroke of undetermined etiology; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Association studies in strokes and normal controls
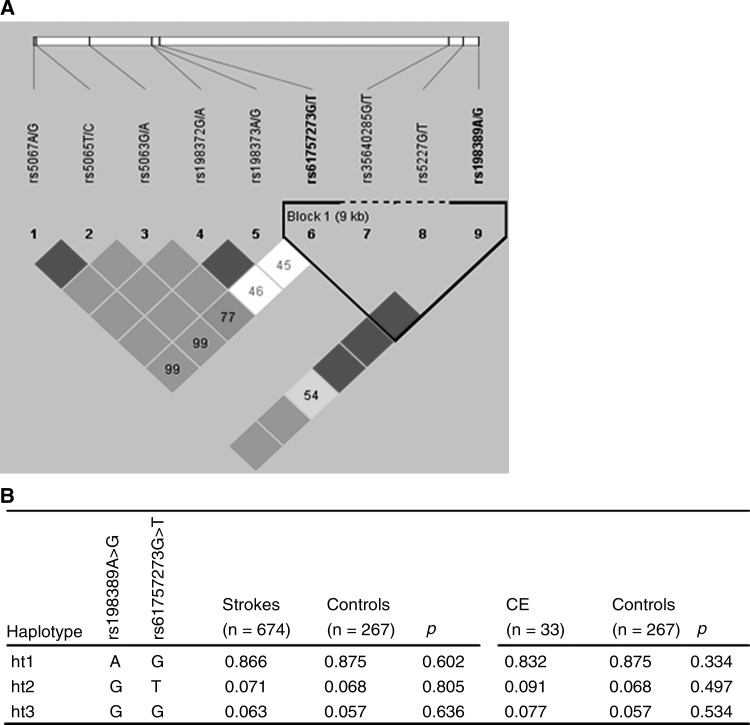
Monomorphic NPPB rs5227 and rs35640285, which were identified in Korean population, were excluded from analysis. All alleles genotyped in this study were in Hardy–Weinberg equilibrium (p>0.05). Among seven polymorphic variants of NPPB and NPPA, rs198373 and rs198372 as well as rs5065 and rs5067 showed complete LD (r2=1), and rs198389 and rs61757273 were observed to have tight LD upon construction of haplotype block (Fig. 1A). To investigate the associations between SNPs and stroke, logistic analysis adjusted for age, sex, and histories of hypertension and diabetes mellitus as covariables was performed. The minor allele frequency (MAF) of rs198373 and rs198372 in the promoter region of the NPPA gene was about 3.5-fold higher in patients with stroke than in controls, but no significant association was found in logistic analysis (p>0.05, Table 2). However, in additional comparison among hemorrhagic and ischemic strokes and controls, nominal significances between these two SNPs (rs198373 and rs198372) and ischemic stroke when compared with unaffected controls (p=0.032, Supplementary Table S1; Supplementary Data are available online at www.liebertonline.com/dna) have been detected. In the case of association analysis with haplotypes, no significant signals were observed (Fig. 1B). These results indicate that genomic variants of NPPB and NPPA have no critical effects on the development of stroke itself.
FIG. 1.
Haplotypes and LD of the NPPA gene. (A) Linkage disequilibrium (LD) and haplotype block of nine single-nucleotide polymorphisms in the NPPB and NPPA genes. LD plot was prepared using Haploview. Colors are used to display pairwise LD as follows: red, LOD ≥2 and D′=1; shades of pink/red, LOD ≥2 and D′<1; white, LOD<2 and D′<1. The numbers indicate pairwise r2 values shown as a percentage. (B) Haplotypes composed of rs198389 in NPPB and rs61757273 in NPPA, and its associations with stroke and cardioembolic stroke (CE).
Table 2.
Logistic Analyses for the NPPB and NPPA Polymorphisms in Total Strokes and Normal Controls
| |
|
|
|
|
|
MAF |
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SNP ID | Variation | Position | Amino acid change | Heterozygositya | Strokes (n=674) | Control (n=267) | OR (95% CI) | p-Value | Statistical power (%) |
| NPPB | rs198389 | A/G | Promoter | 0.523 | 0.132 | 0.125 | 1.2 (0.8–1.8) | 0.367 | 100 | |
| rs5227 | G/T | Exon 1 | Arg25Leu | 0.363 | 0.000 | 0.000 | — | — | 100 | |
| rs35640285 | G/T | Exon 2 | Val94Phe | 0.025 | 0.000 | 0.000 | — | — | 100 | |
| NPPA | rs61757273 | G/T | Promoter | 0.066 | 0.071 | 0.068 | 1.3 (0.8–2.2) | 0.313 | 100 | |
| rs198373 | A/G | Promoter | 0.069 | 0.007 | 0.002 | 8.1 (0.8–78.2) | 0.072 | 31.68 | ||
| rs198372 | G/A | Promoter | 0.133 | 0.007 | 0.002 | 8.1 (0.8–78.6) | 0.071 | 31.68 | ||
| rs5063 | G/A | Exon 1 | Val32Met | 0.133 | 0.094 | 0.099 | 1.1 (0.7–1.7) | 0.625 | 100 | |
| rs5065 | T/C | Exon 3 | STOP152Arg | 0.253 | 0.007 | 0.006 | 1.3 (0.2–7.4) | 0.779 | 73.02 | |
| rs5067 | A/G | 3′UTR | 0.223 | 0.007 | 0.006 | 1.3 (0.2–7.4) | 0.785 | 73.02 | ||
p-Values of additive model were obtained by logistic analysis adjusted for age, sex, and histories of hypertension and diabetes mellitus as covariables.
Heterozygosity is indicated from Build130 of National Center for Biotechnology Information (NCBI).
MAF, minor allele frequency; UTR, untranslated region; OR, odds ratio; CI, confidence interval; SNP, single-nucleotide polymorphism.
STOP152Arg variant of the NPPA gene was associated with cardioembolic stroke
Since TOAST classification is also important for etiology and treatment of stroke, further associations of subtypes of ischemic stroke were analyzed. Patients with SOE and SUE were excluded from association analysis due to the small number of subjects. Interestingly, the minor alleles of rs5065 nonsynonymous variant and rs5067 in 3′-untranslated region (3′UTR) of the NPPA gene were significantly frequent in cardioembolic stroke patients as compared with normal controls (adjusted p=0.002, Table 3) and SVO patients (adjusted p=0.020, Table 4). In addition, rs198373 and rs198372 of NPPA generated nominal evidence of association with SVO compared with those of normal controls (adjusted p<0.05, Table 3). Except for the relationship between CE and SVO, no additional associations were observed among other comparisons of subtypes of TOAST classification (Supplementary Table S2). Therefore, these results provide a possibility that two NPPA SNPs, the nonsynonymous rs5065 and 3′UTR rs5067, which are in complete LD, could be susceptibility factors for cardioembolic stroke, rather than stroke itself, at least in a Korean population. However, further association analysis for haplotypes showed no significant association with stroke and CE (p>0.05, Fig. 1B).
Table 3.
Associations Among Cardioembolism, Large-Artery Atherosclerosis, Small-Vessel Occlusion and Normal Controls
| |
|
CE (n=33) vs. Control (n=267) |
LAA (n=123) vs. Control (n=267) |
SVO (n=395) vs. Control (n=267) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
MAF |
|
|
MAF |
|
MAF |
|
|
|||
| Gene | SNP | CE | Controls | p-Value | Statistical power (%) | LAA | Controls | p-Value | SVO | Controls | p-Value | Statistical power (%) |
| NPPB | rs198389A/G | 0.156 | 0.125 | 0.822 | 45.46 | 0.135 | 0.125 | 0.564 | 0.133 | 0.125 | 0.373 | 100 |
| rs5227G/T | 0.000 | 0.000 | — | 0.000 | 0.000 | — | 0.000 | 0.000 | — | |||
| rs35640285G/T | 0.000 | 0.000 | — | 0.000 | 0.000 | — | 0.000 | 0.000 | — | |||
| NPPA | rs61757273G/T | 0.091 | 0.068 | 0.415 | 30.06 | 0.095 | 0.068 | 0.147 | 0.065 | 0.068 | 0.169 | 99.99 |
| rs198373A/G | 0.000 | 0.002 | — | 0.000 | 0.002 | — | 0.010 | 0.002 | 0.041 | 18.56 | ||
| rs198372G/A | 0.000 | 0.002 | — | 0.000 | 0.002 | — | 0.010 | 0.002 | 0.040 | 18.56 | ||
| rs5063G/A | 0.076 | 0.099 | 0.200 | 30.12 | 0.111 | 0.099 | 0.438 | 0.090 | 0.099 | 0.647 | 99.99 | |
| rs5065T/C | 0.030 | 0.006 | 0.002 | 7.29 | 0.008 | 0.006 | 0.669 | 0.004 | 0.006 | 0.952 | 25.55 | |
| rs5067A/G | 0.030 | 0.006 | 0.002 | 7.29 | 0.008 | 0.006 | 0.674 | 0.004 | 0.006 | 0.945 | 25.55 | |
Bold value indicates the statistical significance of P<0.05.
p-Values of additive model were obtained by logistic analysis adjusted for age, sex, and histories of hypertension and diabetes mellitus as covariables.
Table 4.
Associations Between Polymorphisms of NPPA and Ischemic Stroke with Cardioembolism
| |
|
|
MAF |
p-Value |
|
MAF |
p-Value |
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SNP | Functional position | CE (n=33) | Control (n=267) | Unadjusted | Adjusteda | Statistical power (%) | CE (n=33) | SVO (n=395) | Unadjusted | Adjusteda | Statistical power (%) |
| NPPB | rs198389A>G | Promoter | 0.156 | 0.125 | 0.488 | 0.822 | 45.46 | 0.156 | 0.133 | 0.596 | 0.444 | 66.11 |
| rs5227G>T | Arg25Leu | 0.000 | 0.000 | — | — | 0.000 | 0.000 | — | — | |||
| rs35640285G>T | Val94Phe | 0.000 | 0.000 | — | — | 0.000 | 0.000 | — | — | |||
| NPPA | rs61757273G>T | Promoter | 0.091 | 0.068 | 0.497 | 0.415 | 30.06 | 0.091 | 0.065 | 0.414 | 0.296 | 40.97 |
| rs198373A>G | Promoter | 0.000 | 0.002 | — | — | 0.000 | 0.010 | — | — | |||
| rs198372G>A | Promoter | 0.000 | 0.002 | — | — | 0.000 | 0.010 | — | — | |||
| rs5063G>A | Val32Met | 0.076 | 0.099 | 0.549 | 0.200 | 30.12 | 0.076 | 0.090 | 0.690 | 0.802 | 45.15 | |
| rs5065T>C | STOP152Arg | 0.030 | 0.006 | 0.039 | 0.002 | 7.29 | 0.030 | 0.004 | 0.023 | 0.020 | 7.32 | |
| rs5067A>G | 3′UTR | 0.030 | 0.006 | 0.039 | 0.002 | 7.29 | 0.030 | 0.004 | 0.023 | 0.020 | 7.32 | |
Bold value indicates the statistical significance of P<0.05.
p-Values of additive model were obtained by logistic analysis.
Adjusted for age, sex, and histories of hypertension and diabetes mellitus as covariates.
Discussion
Blood pressure-lowering properties of natriuretic hormones have been implicated in response to increased wall stresses. Moreover, mice lacking NPPA demonstrated an elevated blood pressure, whereas transgenic mice overexpressing NPPA or NPPB showed substantially decreased levels of blood pressure (Steinhelper et al., 1990; Ogawa et al., 1994; John et al., 1995; Potter et al., 2009). Genetic polymorphisms of the NPPA gene, such as rs5068 in 3′UTR and rs5065 in exon, have been implicated in the risk of diseases, including hypertension and stroke (Rubattu et al., 2004; Newton-Cheh et al., 2009). Recently, natriuretic peptides have received attention as a cardiovascular risk factor and its potential therapeutic application has been a subject of investigation (Schmitt et al., 2003; Rubattu et al., 2008). In addition, a pharmacogenetic association between the structural variant inducing STOP152Arg in the NPPA gene and modification of antihypertensive medication effects has also been described in patients with hypertension (Lynch et al., 2008). From the results, rs5065 T or C allele carriers showed different cardiovascular disease outcomes depending on the classes of antihypertensive drugs.
Compared with the occurrence of even homozygous rare alleles at rs5065T/C of the NPPA gene in Caucasian and African populations with 2%–18% frequency, only heterozygous genotype was observed from all 941 Koreans in this study, thereby resulting in a low frequency of the allele. This low frequency was also observed in a Chinese ethnic group (Wang et al., 2009). Moreover, the frequency of this C allele approaches about 40% in Africans as estimated in dbSNP database. When comparing incidences of stroke, African population also showed a higher incidence rate than whites (Gross et al., 1984), suggesting that STOP152Arg or at least NPPA gene polymorphisms might be important factors in the etiology of vascular diseases. In addition, although in rat NPPA, the C-terminal region of the molecule was demonstrated to be essential for high-affinity binding to substrates, such as insulin and insulin-degrading enzyme (Muller et al., 1991). Considering that rs5065T/C induces amino acid change from stop codon to arginine and is associated with stroke, blood pressure/hypertension, and even asthma (Rubattu et al., 2004; Conen et al., 2007; Lima et al., 2008), population difference and/or phenotype of NPPA rs5065 might also have important roles in several diseases or functions of the protein. On the other hand, there were confusing representations of the MAF of NPPB rs5227 in dbSNP for populations with two different sets of HapMap results. Based on our results from 943 Korean subjects, however, this SNP is monomorphic in most populations, except for a low MAF in Africans (about 0.067). In addition, rs35640285 has been discovered as a nonpolymorphic site in a Korean population, whereas this SNP is about 1% prevalent in Caucasians. Although there are minor differences in MAF among populations, these two SNPs are not likely to have effects on the expression of the gene, or to be associated with diseases due to its low MAF.
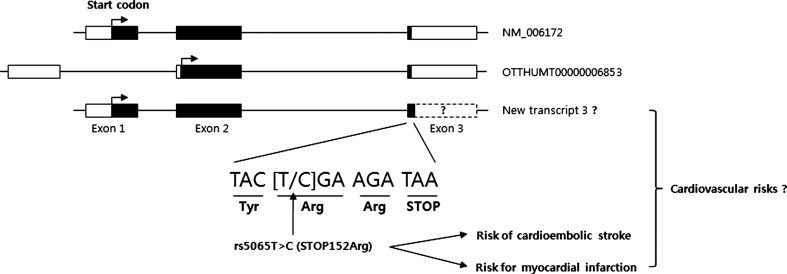
Indeed, the full-length cDNA (BC005893.1) with the rs5065C variant was cloned from the prostate (Strausberg et al., 2002). Although the pathophysiological role of the variant has not been uncovered, its association with high plasma ANP and BNP levels in heart failure patients has been reported (Vassalle et al., 2007). In addition, another transcript (OTTHUMT00000006853 from Ensembl database) for NPPA peptide has been identified. Considering that the rs5065C variant plays an important role on the endothelial dysfunction and vasorelaxation to develop stroke and that ANP and BNP have cardiovascular responses (Rubattu et al., 2004; Potter et al., 2009), it is also possible that newly translated natriuretic peptide from the rs5065C variant could be associated with cardioembolic stroke (Fig. 2). In addition, this putative peptide, which probably has unknown function or dysfunction, could play a role in the development of the cardiovascular diseases (Rubattu et al., 2008).
FIG. 2.
Schematic representation of human NPPA transcripts and the proposed effects of a new transcript potentially derived from STOP152Arg. The coding exons are represented by black blocks, and 5′UTR and 3′UTR by white blocks. The nonsynonymous variant rs5065 (STOP152Arg) has been suggested as a potential risk of cardioembolic stroke in this study as well as a risk of myocardial infarction in another study (Gruchala et al., 2003), whereas further studies are required to elucidate the effects of new transcript potentially derived from STOP152Arg on cardiovascular diseases (Rubattu et al., 2008).
A potential strength of this study is the recruitment of patients from multicenter. Furthermore, all of the patients were evaluated with CT and MRI for more accurate diagnosis. However, possible study limitations include the small number of CE patients due to the specificity of recruitment from oriental medical hospitals rather than from emergency of Western-oriented hospitals and the self-reported history of hypertension and diabetes mellitus, which was included for multivariable analysis. Considering the periodic medical examination and the recent concern in health, however, the self-reported histories from subjects are credible references compared with those from direct diagnosis. On the other hand, despite a few CE cases with rs5065 and rs5067 variants, recent genome-wide association studies using common SNPs with MAF over 5% have showed that only a limited amount of the heritable component has been identified in diseases pathogenesis, suggesting that rare variants may confer a substantial risk for complex diseases (Cirulli and Goldstein, 2010). Finally, there were no matches of age between cases and controls. Therefore, further replication studies including larger number of CE patients matched with similar age group will provide a more confident association with increased statistical powers. In addition, cardio-embolism-related clinical information, such as atrial fibrillation and cardiac valvular diseases, is suggested to be included in future replication studies.
This study found nominal associations of rs198373 and rs198372 in promoter region of the NPPA gene with ischemic stroke when compared with unaffected controls (p=0.032, Supplementary Table S1). However, even though these two SNPs of SVO subgroup showed higher MAFs than CE and LAA subgroups, no statistically significant associations were found (Supplementary Table S2), with no literature evidence for the higher prevalence of NPPA variations in LAA patients. Nevertheless, we do not rule out the potential associations of rs198373 and rs198372 with ischemic stroke; therefore, we additionally performed in silico analysis using the Signal Scan program (www-bimas.cit.nih.gov/molbio/signal/) to investigate whether these polymorphisms could be putative binding sites for regulators. Intriguingly, the AACCAAT and CCAAT sequences including the “C” allele of rs198372G/A (or C/T) were predicted as elements for the binding of several regulatory factors (ACF, alpha-CBF, etc.), whereas the ATCAATA sequence including the “T” allele of the SNP was observed to be an element only for the binding of Pit-1 factor (Supplementary Table S3). This suggests that the rs198372 variation could affect the expression of NPPA, leading to a possible role in the risk of ischemic stroke.
In this study, lower blood Na+ and K+ levels in total stroke patients were found compared with those of normal controls. NPPA has been known to be involved in the homeostatic control of Na+ and K+ in the body (Pedersen et al., 1999). More recently, higher potassium intake has been found to be associated with lower risks of stroke, coronary heart disease, and total cardiovascular disease (D'Elia et al., 2011), suggesting that lower concentration of potassium might be related to vascular diseases. In the case of sodium, considering that heart failure is a common cause to hyponatremia, which is characterized by decrease in serum Na+ concentration (Kazory, 2010), further studies of the association between abnormality on natriuretic peptides and the blood Na+ level in patients with vascular diseases are needed. Another intriguing finding in this study is that the percentage of hypertension history is higher in patients with CE than in those with LAA and SVO (Table 1). Despite inclusion of several populations and no further stratification into LAA and SVO, another report also showed a small increase of the hypertension history in cardioembolic stroke subjects compared with noncardioembolic stroke subgroup (Viehman et al., 2007). Although further studies are required, one potential explanation is that the higher percentage of hypertension history in patients with cardioembolic stroke could be related to atrial fibrillation (Krahn et al., 1995; Giacalone et al., 2010), with the potential involvement of NPPA variations.
Taken together, NPPA has been known to modulate arterial and cardiopulmonary baroreceptor-mediated blood outflow, leading to the baroreflex control of the circulation (Luchner and Schunkert, 2004). Synthetic analogs of NPPA have also been investigated as a potential therapy for the treatment of heart failure (Potter et al., 2009). Although further functional and replication studies in a large cohort with cardioembolic stroke are required, our preliminary findings may provide supporting information to the disease etiology, suggesting that genetic variations of the NPPA gene might be susceptibility factors for cardioembolic stroke through potential alternative transcript and/or transcriptional stability by rs5065 (STOP152Arg) and rs5067 (3′UTR), respectively.
Supplementary Material
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea Government (MEST; no. 2009-0063466). The Center for Genome Science of Korea National Institute of Health kindly provided us control genomic DNA and its clinical data.
Disclosure Statement
No competing financial interests exist.
References
- Adams H.P., Jr. Bendixen B.H. Kappelle L.J. Biller J. Love B.B. Gordon D.L. Marsh E.E., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Barrett J.C. Fry B. Maller J. Daly M.J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Cirulli E.T. Goldstein D.B. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet. 2010;11:415–425. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]
- Conen D. Cheng S. Steiner L.L. Buring J.E. Ridker P.M. Zee R.Y. Association of 77 polymorphisms in 52 candidate genes with blood pressure progression and incident hypertension: the Women's Genome Health Study. J Hypertens. 2009;27:476–483. doi: 10.1097/hjh.0b013e32832104c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conen D. Glynn R.J. Buring J.E. Ridker P.M. Zee R.Y. Natriuretic peptide precursor a gene polymorphisms and risk of blood pressure progression and incident hypertension. Hypertension. 2007;50:1114–1119. doi: 10.1161/HYPERTENSIONAHA.107.097634. [DOI] [PubMed] [Google Scholar]
- D'Elia L. Barba G. Cappuccio F.P. Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol. 2011;57:1210–1219. doi: 10.1016/j.jacc.2010.09.070. [DOI] [PubMed] [Google Scholar]
- Giacalone G. Abbas M.A. Corea F. Prevention strategies for cardioembolic stroke: present and future perspectives. Open Neurol J. 2010;4:56–63. doi: 10.2174/1874205X01004020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C.R. Kase C.S. Mohr J.P. Cunningham S.C. Baker W.E. Stroke in south Alabama: incidence and diagnostic features—a population based study. Stroke. 1984;15:249–255. doi: 10.1161/01.str.15.2.249. [DOI] [PubMed] [Google Scholar]
- Gruchala M. Ciecwierz D. Wasag B. Targonski R. Dubaniewicz W. Nowak A. Sobiczewski W. Ochman K. Romanowski P. Limon J. Rynkiewicz A. Association of the ScaI atrial natriuretic peptide gene polymorphism with nonfatal myocardial infarction and extent of coronary artery disease. Am Heart J. 2003;145:125–131. doi: 10.1067/mhj.2003.52. [DOI] [PubMed] [Google Scholar]
- Hademenos G.J. Alberts M.J. Awad I. Mayberg M. Shepard T. Jagoda A. Latchaw R.E. Todd H.W. Viste K. Starke R. Girgus M.S. Marler J. Emr M. Hart N. Advances in the genetics of cerebrovascular disease and stroke. Neurology. 2001;56:997–1008. doi: 10.1212/wnl.56.8.997. [DOI] [PubMed] [Google Scholar]
- Houben A.J. van der Zander K. de Leeuw P.W. Vascular and renal actions of brain natriuretic peptide in man: physiology and pharmacology. Fundam Clin Pharmacol. 2005;19:411–419. doi: 10.1111/j.1472-8206.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- Houweling A.C. van Borren M.M. Moorman A.F. Christoffels V.M. Expression and regulation of the atrial natriuretic factor encoding gene Nppa during development and disease. Cardiovasc Res. 2005;67:583–593. doi: 10.1016/j.cardiores.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Jeffs B. Clark J.S. Anderson N.H. Gratton J. Brosnan M.J. Gauguier D. Reid J.L. Macrae I.M. Dominiczak A.F. Sensitivity to cerebral ischaemic insult in a rat model of stroke is determined by a single genetic locus. Nat Genet. 1997;16:364–367. doi: 10.1038/ng0897-364. [DOI] [PubMed] [Google Scholar]
- John S.W. Krege J.H. Oliver P.M. Hagaman J.R. Hodgin J.B. Pang S.C. Flynn T.G. Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- Kazory A. Hyponatremia in heart failure: revisiting pathophysiology and therapeutic strategies. Clin Cardiol. 2010;33:322–329. doi: 10.1002/clc.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn A.D. Manfreda J. Tate R.B. Mathewson F.A. Cuddy T.E. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- Liao D. Myers R. Hunt S. Shahar E. Paton C. Burke G. Province M. Heiss G. Familial history of stroke and stroke risk. The Family Heart Study. Stroke. 1997;28:1908–1912. doi: 10.1161/01.str.28.10.1908. [DOI] [PubMed] [Google Scholar]
- Lima J.J. Mohapatra S. Feng H. Lockey R. Jena P.K. Castro M. Irvin C. Johnson J.A. Wang J. Sylvester J.E. A polymorphism in the NPPA gene associates with asthma. Clin Exp Allergy. 2008;38:1117–1123. doi: 10.1111/j.1365-2222.2008.02955.x. [DOI] [PubMed] [Google Scholar]
- Luchner A. Schunkert H. Interactions between the sympathetic nervous system and the cardiac natriuretic peptide system. Cardiovasc Res. 2004;63:443–449. doi: 10.1016/j.cardiores.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Lynch A.I. Boerwinkle E. Davis B.R. Ford C.E. Eckfeldt J.H. Leiendecker-Foster C. Arnett D.K. Pharmacogenetic association of the NPPA T2238C genetic variant with cardiovascular disease outcomes in patients with hypertension. JAMA. 2008;299:296–307. doi: 10.1001/jama.299.3.296. [DOI] [PubMed] [Google Scholar]
- Menashe I. Rosenberg P.S. Chen B.E. PGA: power calculator for case-control genetic association analyses. BMC Genet. 2008;9:36. doi: 10.1186/1471-2156-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschia J.F. Barrett K.M. Chukwudelunzu F. Brown W.M. Case L.D. Kissela B.M. Brown R.D., Jr. Brott T.G. Olson T.S. Rich S.S. Silliman S. Worrall B.B. Interobserver agreement in the trial of org 10172 in acute stroke treatment classification of stroke based on retrospective medical record review. J Stroke Cerebrovasc Dis. 2006;15:266–272. doi: 10.1016/j.jstrokecerebrovasdis.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D. Baumeister H. Buck F. Richter D. Atrial natriuretic peptide (ANP) is a high-affinity substrate for rat insulin-degrading enzyme. Eur J Biochem. 1991;202:285–292. doi: 10.1111/j.1432-1033.1991.tb16374.x. [DOI] [PubMed] [Google Scholar]
- Newton-Cheh C. Larson M.G. Vasan R.S. Levy D. Bloch K.D. Surti A. Guiducci C. Kathiresan S. Benjamin E.J. Struck J. Morgenthaler N.G. Bergmann A. Blankenberg S. Kee F. Nilsson P. Yin X. Peltonen L. Vartiainen E. Salomaa V. Hirschhorn J.N. Melander O. Wang T.J. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y. Itoh H. Tamura N. Suga S. Yoshimasa T. Uehira M. Matsuda S. Shiono S. Nishimoto H. Nakao K. Molecular cloning of the complementary DNA and gene that encode mouse brain natriuretic peptide and generation of transgenic mice that overexpress the brain natriuretic peptide gene. J Clin Invest. 1994;93:1911–1921. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen E.B. Pedersen H.B. Jensen K.T. Pulsatile secretion of atrial natriuretic peptide and brain natriuretic peptide in healthy humans. Clin Sci (Lond) 1999;97:201–206. [PubMed] [Google Scholar]
- Potter L.R. Yoder A.R. Flora D.R. Antos L.K. Dickey D.M. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;191:341–366. doi: 10.1007/978-3-540-68964-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastenyte D. Tuomilehto J. Sarti C. Genetics of stroke—a review. J Neurol Sci. 1998;153:132–145. doi: 10.1016/s0022-510x(97)00286-4. [DOI] [PubMed] [Google Scholar]
- Rubattu S. Sciarretta S. Valenti V. Stanzione R. Volpe M. Natriuretic peptides: an update on bioactivity, potential therapeutic use, and implication in cardiovascular diseases. Am J Hypertens. 2008;21:733–741. doi: 10.1038/ajh.2008.174. [DOI] [PubMed] [Google Scholar]
- Rubattu S. Stanzione R. Di Angelantonio E. Zanda B. Evangelista A. Tarasi D. Gigante B. Pirisi A. Brunetti E. Volpe M. Atrial natriuretic peptide gene polymorphisms and risk of ischemic stroke in humans. Stroke. 2004;35:814–818. doi: 10.1161/01.STR.0000119381.52589.AB. [DOI] [PubMed] [Google Scholar]
- Rubattu S. Volpe M. Kreutz R. Ganten U. Ganten D. Lindpaintner K. Chromosomal mapping of quantitative trait loci contributing to stroke in a rat model of complex human disease. Nat Genet. 1996;13:429–434. doi: 10.1038/ng0896-429. [DOI] [PubMed] [Google Scholar]
- Schmitt M. Cockcroft J.R. Frenneaux M.P. Modulation of the natriuretic peptide system in heart failure: from bench to bedside? Clin Sci (Lond) 2003;105:141–160. doi: 10.1042/CS20030044. [DOI] [PubMed] [Google Scholar]
- Steinhelper M.E. Cochrane K.L. Field L.J. Hypotension in transgenic mice expressing atrial natriuretic factor fusion genes. Hypertension. 1990;16:301–307. doi: 10.1161/01.hyp.16.3.301. [DOI] [PubMed] [Google Scholar]
- Strausberg R.L. Feingold E.A. Grouse L.H. Derge J.G. Klausner R.D. Collins F.S. Wagner L. Shenmen C.M. Schuler G.D. Altschul S.F. Zeeberg B. Buetow K.H. Schaefer C.F. Bhat N.K. Hopkins R.F. Jordan H. Moore T. Max S.I. Wang J. Hsieh F. Diatchenko L. Marusina K. Farmer A.A. Rubin G.M. Hong L. Stapleton M. Soares M.B. Bonaldo M.F. Casavant T.L. Scheetz T.E. Brownstein M.J. Usdin T.B. Toshiyuki S. Carninci P. Prange C. Raha S.S. Loquellano N.A. Peters G.J. Abramson R.D. Mullahy S.J. Bosak S.A. McEwan P.J. McKernan K.J. Malek J.A. Gunaratne P.H. Richards S. Worley K.C. Hale S. Garcia A.M. Gay L.J. Hulyk S.W. Villalon D.K. Muzny D.M. Sodergren E.J. Lu X. Gibbs R.A. Fahey J. Helton E. Ketteman M. Madan A. Rodrigues S. Sanchez A. Whiting M. Madan A. Young A.C. Shevchenko Y. Bouffard G.G. Blakesley R.W. Touchman J.W. Green E.D. Dickson M.C. Rodriguez A.C. Grimwood J. Schmutz J. Myers R.M. Butterfield Y.S. Krzywinski M.I. Skalska U. Smailus D.E. Schnerch A. Schein J.E. Jones S.J. Marra M.A. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi Y. Toriyama S. Takabatake N. Shibata Y. Konta T. Emi M. Kato T. Kawata S. Kubota I. Linkage disequilibrium analyses of natriuretic peptide precursor B locus reveal risk haplotype conferring high plasma BNP levels. Biochem Biophys Res Commun. 2007;362:480–484. doi: 10.1016/j.bbrc.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Vassalle C. Andreassi M.G. Prontera C. Fontana M. Zyw L. Passino C. Emdin M. Influence of ScaI and natriuretic peptide (NP) clearance receptor polymorphisms of the NP System on NP concentration in chronic heart failure. Clin Chem. 2007;53:1886–1890. doi: 10.1373/clinchem.2007.088302. [DOI] [PubMed] [Google Scholar]
- Viehman J.A. Saver J.L. Liebeskind D.S. Starkman S. Ali L.K. Buck B. Razinia T. Ovbiagele B. Utility of urinalysis in discriminating cardioembolic stroke mechanism. Arch Neurol. 2007;64:667–670. doi: 10.1001/archneur.64.5.667. [DOI] [PubMed] [Google Scholar]
- Wang X. Cheng S. Brophy V.H. Erlich H.A. Mannhalter C. Berger K. Lalouschek W. Browner W.S. Shi Y. Ringelstein E.B. Kessler C. Luedemann J. Lindpaintner K. Liu L. Ridker P.M. Zee R.Y. Cook N.R. A meta-analysis of candidate gene polymorphisms and ischemic stroke in 6 study populations: association of lymphotoxin-alpha in nonhypertensive patients. Stroke. 2009;40:683–695. doi: 10.1161/STROKEAHA.108.524587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip P.K. Jeng J.S. Lee T.K. Chang Y.C. Huang Z.S. Ng S.K. Chen R.C. Subtypes of ischemic stroke. A hospital-based stroke registry in Taiwan (SCAN-IV) Stroke. 1997;28:2507–2512. doi: 10.1161/01.str.28.12.2507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.