Abstract
Glycosylation is one of the most abundant protein modifications in nature, having roles in protein stability, secretion and function. Alterations in mucin-type O-glycosylation are responsible for a number of human diseases and developmental defects, as well as associated with certain types of cancer. However, the mechanistic role of this form of glycosylation in many of these instances is unclear. Here we describe how one glycosyltransferase responsible for initiating mucin-type O-glycosylation (pgant3), specifically modulates integrin-mediated cell adhesion by influencing the secretion and localization of an integrin ligand. The integrin ligand Tiggrin, is normally O-glycosylated and localized to the basal matrix, where adhesion of two opposing cell layers takes place. In pgant3 mutants, Tiggrin is no longer O-glycosylated and fails to be properly secreted to the basal cell layer interface, resulting in disruption of proper cell adhesion. pgant3-mediated effects are dependent upon the enzymatic activity of PGANT3 and cannot be rescued by another pgant family member, indicating a unique role for this glycosyltransferase. These results provide in vivo evidence for the role of O-glycosylation in the secretion of specific extracellular matrix proteins, that thereby influences the composition of the cellular “microenvironment” and modulates cell adhesion events. The studies described in this review provide insight into the long-standing association between aberrant O-glycosylation and tumorigenesis, as changes in tumor environment and cell adhesion are hallmarks of cancer progression.
Introduction
Mucin-type O-glycosylation is an evolutionarily conserved and widespread post-translational modification of proteins initiated by a family of enzymes known as the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases (ppGalNAcTs in mammals and PGANTs in Drosophila) [1-3]. O-glycosylated proteins are either membrane-bound or secreted, forming components of the cell membrane and cellular environment, leading to the hypothesis that they play important roles in cell interactions, signaling, polarity and protection. Indeed, aberrant mucin-type O-glycosylation is known to be responsible for certain diseases and developmental defects. For example, defects in the extension of mucin-type O-glycans are responsible for Tn Syndrome, where aberrantly formed O-glycans on red blood cells are recognized by the immune system, leading to hemolytic anemia [4]. Also, the loss of function of the human GALNT3 causes familial tumoral calcinosis by influencing the stability of the growth factor, FGF23 [5]. Loss of function of another glycosyltransferase in Drosophila (PGANT35A) results in lethality and defects in the proper formation of the embryonic respiratory system [6,7]. Additionally, mice deficient for ppGalNAcT-1 display reduced lymphocyte homing and bleeding disorders [8]. Other studies have identified associations between aberrant O-glycosylation and certain types of cancer [9-13]. For example, mutations in human GALNT12 are associated with colon cancer [9]. In mice, disruption of O-glycan extension results in increased susceptibility to colitis and colorectal tumors [10]. Other ppGalNAcT family members have been identified as biomarkers for the detection and diagnosis of certain types of cancer [11-13]. However, the precise mechanistic role of O-glycosylation in these complex diseases remains unknown.
Here, we describe the role of mucin-type O-glycosylation in cell interactions during development. Using Drosophila as a model system, we found that the loss of O-glycosylation disrupts cell adhesion in the developing wing. The Drosophila wing is comprised of two epithelial cell layers that adhere to one another by virtue of a secreted basal matrix that mediates integrin binding. Loss of integrins or the components with which they interact, disrupts cell adhesion between the two layers, creating localized blisters on the wings [14-17]. Mutations in one glycosyltransferase (pgant3) result in abnormal cell adhesion during Drosophila wing development by disrupting the secretion of an integrin-binding extracellular matrix (ECM) protein. Our studies demonstrate that O-glycosylation can influence the composition of the ECM or cellular “microenvironment”, and thus alter cell interactions. This provides insight into the roles of O-glycans in the cellular interactions occurring in both development and disease.
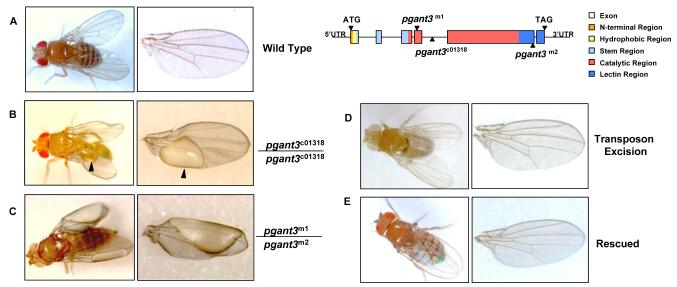
pgant3 plays a unique role in wing formation during development
To investigate the biological roles of pgant3, we examined a transposon insertion mutation of pgant3 (pgant3c01318) from the Exelixis Drosophila Stock Collection [18], which contains a piggyBac transposable element in the fourth intron of pgant3 (Fig. 1). This transposon insertion results in a significant reduction/loss of pgant3 expression. Homozygous transposon insertion flies (pgant3c01318/pgant3c01318) are viable yet display wing blistering (Fig. 1) [20]. Additionally, flies carrying the transposon insertion over a deficiency of the pgant3 region ((pgant3c01318/ Df(2R)Exel6283) also display wing blistering. Furthermore, when the transposon was precisely excised from the pgant3 gene, pgant3 gene expression levels and wing integrity were restored (Fig. 1) [19]. These data support a role for pgant3 in proper wing formation.
Fig.1.
RNA interference (RNAi) to pgant3 in vivo was also employed to confirm a role for pgant3 in wing integrity [19]. After crossing two independent UAS-pgant3IR transgenic lines (that contain inducible pgant3 dsRNA) to a tubulin-Gal4 driver line (which induces expression of pgant3 dsRNA ubiquitously), we found that 95-97% of the progeny expressing pgant3 dsRNA (i.e. RNAi to pgant3) displayed wing blistering. Induction of pgant3 RNAi exclusively in the developing wing also resulted in wing blistering, indicating a specific requirement for pgant3 expression in the wing [19].
To determine whether the catalytic activity of pgant3 is required for proper wing formation, we generated point mutations in the coding region of pgant3 using ethylmethane sulfonate (EMS) mutagenesis. One mutation, pgant3m1, changes a conserved arginine to a cysteine at amino acid position 130 and the other, pgant3m2, creates a stop codon at amino acid 609, deleting the C-terminal 59 amino acids of the enzyme (Fig. 1). The pgant3m1 point mutant, like wild type pgant3, encodes a protein that is stably expressed and localized properly to the Golgi apparatus. However, this mutation results in loss of pgant3 enzymatic activity. All pgant3 mutant flies containing this catalytically inactive version of pgant3 (pgant3m1/pgant3m2, pgant3m1/pgant3c01318, pgant3m1/Df(2R)Exel6283,) showed a high frequency of wing blistering, indicating that the catalytic activity of pgant3 is required for proper wing formation [20].
Finally, we demonstrated that expression of wild type pgant3 in the pgant3 mutant backgrounds can rescue wing integrity (Fig. 1). Interestingly, expression of another pgant family member (pgant35A) in the pgant3 mutant background was unable to rescue the wing phenotype, indicating a specific requirement for PGANT3 activity [19]. Taken together, our observations demonstrate a unique role for PGANT3 catalytic activity in proper wing blade formation.
pgant3 is involved in integrin-mediated cell adhesion
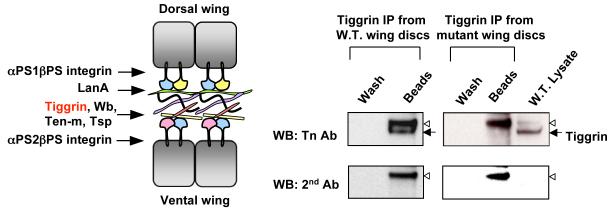
Drosophila wing has been used as a model to study integrin-mediated cell adhesion [14-17]. Integrins interact with components of the basal extracellular matrix, as well as intracellular proteins, to regulate the cell adhesion between the two wing layers (Fig. 2A). Loss of integrins or their interacting partners results in loss of cell adhesion between the dorsal and ventral cell layers, resulting in a fluid filled blister present on the wing surface. Thus, the blistered wing phenotype of pgant3 mutants suggested that O-glycosylation is involved in integrin-mediated adhesion. Indeed, genetic interaction studies revealed that pgant3 is genetically interacting with integrins to modulate cell adhesion in the wing [20].
Fig.2.
To address the role of pgant3 in integrin-mediated cell adhesion in another system, we performed RNAi to pgant3 in two Drosophila cell lines. S2R+ is an embryonic cell line that adheres to surfaces via an integrin-dependent mechanism. The S2 cell line is a non-adherent, embryonic line that can be induced to spread and adhere on concanavalin-A (ConA) coated surfaces in an integrin-independent manner. Both cell lines have been used to study cell adhesion [21-23]. We treated each cell line with non-specific dsRNA (YFP) as a negative control, dsRNA to the βPS integrin (mys) (as a positive control for integrin-mediated cell adhesion) or dsRNA to pgant3. As expected, mys dsRNA caused disruption of adhesion of S2R+ cells but had no effect on the adherence of S2 cells to ConA. Interestingly, S2R+ cells treated with pgant3 dsRNA displayed a similar non-adherent phenotype, while S2 cells treated with pgant3 dsRNA were unaffected [20]. These data further support a specific role for pgant3 in integrin-mediated cell adhesion.
PGANT3 glycosylates an extracellular matrix integrin ligand
To visualize the location of O-glycosylated proteins during Drosophila wing development, we stained larval and pupal wings with an antibody (Tn Ab) that specifically detects the O-linked GalNAc on serines or threonines. Optical cross-sections through the larval wing discs showed strong staining for O-linked glycoproteins along the basal surface, the area that will eventually mediate contacts between the two epithelial cell layers of the wing. Wing discs from homozygous pgant3 mutants showed a dramatic decrease in staining of O-glycoproteins along this basal surface that was restored in mutant discs expressing wild type pgant3 [19]. In developing pupal wings, O-linked glycoproteins were found along the basal adhesive surface between the dorsal and ventral cell layers of the wing, where integrin adhesive contacts are formed. In pgant3 mutant pupal wings, the staining of O-glycans was decreased along this surface and the localization of integrins normally present there was more diffuse [20]. Together, these results showed a specific localization of O-linked glycoproteins to the adhesive basal matrix of the wing.
To identify the substrates of PGANT3, we compared the O-linked glycoprotein profiles of wild type and pgant3 mutants by Western blotting using the Tn antibody. Western blots examining O-glycoproteins demonstrated that one band (~200 kDa) was present in wild type but absent or severely reduced in pgant3 mutants. Furthermore, the glycosylation of this protein was restored in wing discs from pgant3 mutants expressing wild type pgant3 [19]. Bioinformatic searches for ~200 kDa proteins expressed in developing wings identified the extracellular matrix protein Tiggrin as a candidate. Tiggrin is a known integrin binding protein that is involved in muscle-tendon cell adhesion [24-26]. Immunoprecipatiation of Tiggrin from wing discs revealed that Tiggrin is normally O-glycosylated in wild type discs but is not glycosylated in mutant discs (Fig. 2B) [19]. To determine if O-glycosylation influences Tiggrin function, we examined whether or not pgant3 and tiggrin interact genetically to influence wing blade adhesion. Combining pgant3 and tiggrin mutations resulted in a significant increase in wing blistering frequencies (beyond additive effects), indicating that these genes act together in establishing wing blade adhesion [19]. These results conclusively demonstrate that the extracellular matrix component, Tiggrin, is the substrate of PGANT3 in vivo and O-glycosylation of Tiggrin plays a role in cell adhesion between the two wing cell layers.
PGANT3 influences secretion of Tiggrin
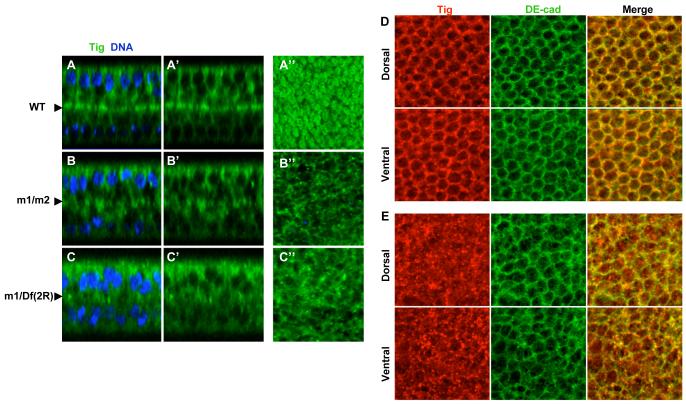
Confocal imaging revealed that Tiggrin normally co-localizes with β-integrin along the basal adhesive surfaces of the dorsal and ventral cell layers of the wing (Fig. 3A). However, Tiggrin is no longer properly localized to this surface in pgant3 mutants (Fig. 3A) [20]. pgant3 mutants displayed a reduction in O-glycosylation and a loss of Tiggrin along the cell layer interface [20]. O-glycosylation and Tiggrin localization were restored upon restoration of pgant3 activity. These results suggest that loss of O-glycosylation specifically disrupts Tiggrin localization.
Fig.3.
The loss of Tiggrin at the basal adhesive surface could be caused by altered Tiggrin secretion or Tiggrin degradation. However, no significant difference in the amount of Tiggrin was seen between mutant and wild type samples on western blots, suggesting that loss of Tiggrin at the interface between two cell layers is not due to degradation. To examine the effects of O-glycosylation on secretion of Tiggrin, we stained pupal wings from wild type and pgant3 mutants for Tiggrin and also for DE-cadherin, to outline the cells. Confocal imaging revealed that Tiggrin is localized along the periphery of cells in wild type wings (co-localizing with DE-cadherin) while it is largely absent from this region in pgant3 mutant wing cells (Fig. 3B) [20]. Additionally, in mutant wing cells, Tiggrin accumulates in punctate structures inside the cell borders. These results indicate that O-glycosylation influences Tiggrin secretion, thereby influencing matrix composition and cell adhesion.
Conclusions
In this review, we summarize how O-glycosylation influences the secretion and localization of a known integrin binding ECM protein, altering the microenvironment of the wing and disrupting cell adhesion during development. In our studies, we found that Tiggrin is O-glycosylated by PGANT3 and co-localizes with integrin at the adhesive interface between the two cell layers of the developing wing. In pgant3 mutants, Tiggrin is no longer O-glycosylated and fails to be secreted to the basal adhesive matrix, resulting in cell adhesion defects between the two wing surfaces. Expression of wild type PGANT3 enzymatic activity restores O-glycosylation and localization of Tiggrin as well as wing integrity. Our studies provide the first evidence that mucin-type O-glycosylation can influence the secretion of an ECM protein, and thus alter the composition of the cellular microenvironment. We are currently investigating the role of other pgant family members in secretion and establishment of the microenviroment during development. The ability of O-glycosylation to modulate the environment of a cell has implications for the role of this protein modification in other aspects of development and provides insight into the role of O-glycosylation in disease states where matrix composition and cell adhesion are affected.
Supplementary Material
Acknowledgement
This research was originally published in the Journal of Biological Chemistry.
Liping Zhang, Ying Zhang and Kelly G. Ten Hagen. A mucin-type O-glycosyltransferase modulates cell adhesion during Drosophila development. J. Biol. Chem. 2008; 283: 34076-34086. © the American Society for Biochemistry and Molecular Biology.
Liping Zhang, Duy T. Tran and Kelly G. Ten Hagen. An O-glycosyltransferase promotes cell adhesion during development by influencing secretion of an extracellular matrix integrin ligand. J. Biol. Chem. 2010; 285:19491-19501. © the American Society for Biochemistry and Molecular Biology.
Funding This research was supported by the Intramural Research Program of the NIDCR at the National Institutes of Health.
References
- 1.Ten Hagen KG, Fritz TA, Tabak LA. All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 2003;13:1R–16R. doi: 10.1093/glycob/cwg007. [DOI] [PubMed] [Google Scholar]
- 2.Ten Hagen KG, Zhang L, Tian E, Zhang Y. Glycobiology on the fly: developmental and mechanistic insights from Drosophila. Glycobiology. 2009;19:102–111. doi: 10.1093/glycob/cwn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian E, Ten Hagen KG. Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconjugate J. 2009;26:325–334. doi: 10.1007/s10719-008-9162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ju T, Cummings RD. Protein glycosylation: chaperone mutation in Tn syndrome. Nature. 2005;437:1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 5.Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 6.Ten Hagen KG, Tran DT. A UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase is essential for viability in Drosophila melanogaster. J Biol Chem. 2002;277:22616–22622. doi: 10.1074/jbc.M201807200. [DOI] [PubMed] [Google Scholar]
- 7.Tian E, Ten Hagen KG. A UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase is required for epithelial tube formation. J Biol Chem. 2007;282:606–614. doi: 10.1074/jbc.M606268200. [DOI] [PubMed] [Google Scholar]
- 8.Tenno M, Ohtsubo K, Hagen FK, Ditto D, Zarbock A, Schaerli P, von Andrian UH, Ley K, Le D, Tabak LA, Marth JD. Initiation of protein O glycosylation by the polypeptide GalNAcT-1 in vascular biology and humoral immunity. Mol Cell Biol. 2007;27:8783–8796. doi: 10.1128/MCB.01204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guda K, Moinova H, He J, Jamison OL, Natale L, Lutterbaugh J, Lawrence ES, Willson JK, Lowe JB, Wiesner GL, Parmigiani G, Barnholtz-Sloan J, Dawson DW, Velculescu VE, Kinzler KW, Papadopoulos N, Vogelstein B, Willis J, Gerken TA, Markowitz SD. Inactivating germ-line and somatic mutations in polypeptide N-acetylgalactosaminyltransferase 12 in human colon cancers. Proc Natl Acad Sci U S A. 2009;106:12921–12925. doi: 10.1073/pnas.0901454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berois N, Blanc E, Ripoche H, Mergui X, Trajtenberg F, Cantais S, Barrois M, Dessen P, Kagedal B, Benard J, Osinaga E, Raguenez G. ppGalNAc-T13: a new molecular marker of bone marrow involvement in neuroblastoma. Clin Chem. 2006;52:1701–1712. doi: 10.1373/clinchem.2006.067975. [DOI] [PubMed] [Google Scholar]
- 12.Freire T, Berois N, Sonora C, Varangot M, Barrios E, Osinaga E. UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a potential new marker for detection of bone marrow-disseminated breast cancer cells. Int J Cancer. 2006;119:1383–1388. doi: 10.1002/ijc.21959. [DOI] [PubMed] [Google Scholar]
- 13.Wu C, Guo X, Wang W, Wang Y, Shan Y, Zhang B, Song W, Ma S, Ge J, Deng H, Zhu M. N-Acetylgalactosaminyltransferase-14 as a potential biomarker for breast cancer by immunohistochemistry. BMC Cancer. 2010;10:123–130. doi: 10.1186/1471-2407-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brower DL. Platelets with wings: the maturation of Drosophila integrin biologyl. Curr Opin Cell Biol. 2003;15:607–613. doi: 10.1016/s0955-0674(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 15.Brower DL, Bunch TA, Mukai L, Adamson TE, Wehrli M, Lam S, Friedlander E, Roote CE, Zusman S. Nonequivalent requirements for PS1 and PS2 integrin at cell attachments in Drosophila: genetic analysis of the alpha PS1 integrin subunit. Development. 1995;121:1311–1320. doi: 10.1242/dev.121.5.1311. [DOI] [PubMed] [Google Scholar]
- 16.Brabant MC, Brower DL. PS2 integrin requirements in Drosophila embryo and wing morphogenesis. Dev Biol. 1993;157:49–59. doi: 10.1006/dbio.1993.1111. [DOI] [PubMed] [Google Scholar]
- 17.Walsh EP, Brown NH. A screen to identify Drosophila genes required for integrin-mediated adhesion. Genetics. 1998;150:791–805. doi: 10.1093/genetics/150.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Zhang Y, Hagen KG. A mucin-type O-glycosyltransferase modulates cell adhesion during Drosophila development. J Biol Chem. 2008;283:34076–34086. doi: 10.1074/jbc.M804267200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Tran DT, Ten Hagen KG. An O-glycosyltransferase promotes cell adhesion during development by influencing secretion of an extracellular matrix integrin ligand. J Biol Chem. 2010;285:19491–19501. doi: 10.1074/jbc.M109.098145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boutros M, Kiger AA, Armknecht S, Kerr K, Hild M, Koch B, Haas SA, Paro R, Perrimon N. Genome-wide RNAi analysis of growth and viability in Drosophila cells. Science. 2004;303:832–835. doi: 10.1126/science.1091266. [DOI] [PubMed] [Google Scholar]
- 22.Jani K, Schock F. Zasp is required for the assembly of functional integrin adhesion sites. J Cell Biol. 2007;179:1583–1597. doi: 10.1083/jcb.200707045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiger AA, Baum B, Jones S, Jones MR, Coulson A, Echeverri C, Perrimon N. A functional genomic analysis of cell morphology using RNA interference. J Biol. 2003;2:27.1–27.15. doi: 10.1186/1475-4924-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogerty FJ, Fessler LI, Bunch TA, Yaron Y, Parker CG, Nelson RE, Brower DL, Gullberg D, Fessler JH. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development. 1994;120:1747–1758. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- 25.Graner MW, Bunch TA, Baumgartner S, Kerschen A, Brower DL. Splice variants of the Drosophila PS2 integrins differentially interact with RGD-containing fragments of the extracellular proteins tiggrin, ten-m, and D-laminin 2. J Biol Chem. 1998:18235–18241. doi: 10.1074/jbc.273.29.18235. [DOI] [PubMed] [Google Scholar]
- 26.Bunch TA, Graner MW, Fessler LI, Fessler JH, Schneider KD, Kerschen A, Choy LP, Burgess BW, Brower DL. The PS2 integrin ligand tiggrin is required for proper muscle function in Drosophila. Development. 1998;125:1679–1689. doi: 10.1242/dev.125.9.1679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.