Abstract
BACKGROUND
After surgery, cytokines and chemokines are released at the surgical wound site, which can contribute to postoperative pain, local inflammation, and tissue repair. Multiple cell types are present that can release cytokines/chemokines at the wound site and, thus, the exact cellular source of these molecules is unclear. We sought to better understand the contribution of neutrophils to cytokine/chemokine gene expression at the surgical wound site during the initial postsurgery phase of total hip arthroplasty (THA).
METHODS
Hip drain fluid was collected at 24 h postsurgery from six patients undergoing standardized THA. In addition, venous blood was collected presurgery and 24 h postsurgery. Neutrophils were isolated, total RNA extracted, and a biotinylated cRNA probe generated. The probes were hybridized with a cDNA microarray containing approximately 100 oligonucleotide sequences representing various human cytokines/chemokines or receptor genes. Changes in gene expression seen in the microarray were verified by reverse transcription polymerase chain reaction.
RESULTS
In the microarray analysis of hip drain neutrophils, interleukin-1 receptor antagonist (IL1RN), interleukin-18 receptor 1 (IL18R1), macrophage migration inhibitory factor (MIF), and macrophage inflammatory protein 3α (CCL20) were upregulated, whereas interleukin-8 receptor β (IL8RB/CXCR2) was consistently downregulated, compared with presurgery blood neutrophils. All of these changes were confirmed by reverse transcription polymerase chain reaction.
CONCLUSION
There is a distinct cytokine gene expression profile in neutrophils at the THA surgical wound site at 24 h postsurgery when compared with that found in presurgery circulating neutrophils. Understanding these changes may allow us to knowledgeably manipulate neutrophil activity to reduce postoperative pain and inflammation without impairing wound healing.
Despite advances in both surgical and anesthetic treatments, several studies demonstrate that approximately 80% of postoperative patients experience moderate or severe pain postsurgery.1 Surgery induces a state of inflammatory response and understanding the pathophysiology of this response can potentially yield new targets for therapy. After hip replacement surgery, factors contributing to postoperative pain include prostaglandin E2 and cytokines, both of which are increased in wound site exudates.2 The cellular origin of such immuno- or neuroactive molecules in wound exudates has not yet been determined because they may arise from a variety of cell types and from plasma extravasates.
In the classic model, delineating the phases of healing in cutaneous wounds, there is an initial stage in which blood platelets release clotting factors as well as growth factors and cytokines, such as platelet-derived growth factor and TGF-β.3 This is followed by an inflammatory stage in which polymorphonuclear neutrophils cells and soon afterward macrophages migrate into the wound site.4–6 Typically, at 24 h after a cutaneous injury, the neutrophil population is at its maximum at the wound site and the activity of these neutrophils may play a critical role in recovery.5 At the same time, several studies have suggested that various leukocyte populations may secrete a variety of proalgesic compounds (e.g., interleukin [IL]-6) and analgesic compounds (e.g., IL-10) that can either sensitize or reduce the excitability of nociceptive primary afferent nerve endings, respectively, and modulate nociceptive input into the central nervous system.7,8 Matching the cell type(s) to the released molecules may provide insight into our understanding of inflammatory and nociceptive processes and wound healing in the periphery.
At present, the local cellular response to deep surgical trauma, such as hip replacement surgery, has not been well characterized. Exudates from hip drains (HDs) obtained after surgery contain leukocytes that have undergone emigration from the bloodstream to the wound site. In this study, we showed that at 24 h almost all of these leukocytes in the HD are neutrophils. Thus, the HD fluid at 24 h allows for clarifying the contribution of one population of leukocytes to tissue damage and wound healing without contamination from other cell types. This study analyzed multiple cytokine messenger RNAs from 24 h HD and circulating blood neutrophils to identify which cytokines and cytokine receptors are altered after major surgery.
METHODS
Patient Selection
After IRB approval (April 18, 2005) from Rush University Medical Center, patients scheduled for total hip arthroplasty (THA) signed informed consent forms and were enrolled in the study. Patients were ≤80-yr-old and were without recent trauma or systemic infection within 3 mo of surgery date. Patients who had used corticosteroid medications within 3 mo of the surgery date were also excluded.
Study Protocol
All nonsteroidal antiinflammatory therapy was discontinued 14 days before surgery (routine clinical practice to avoid perioperative bleeding). At the preoperative visit, demographic data were collected and all preoperative medications, including the dose, route, and duration, were recorded.
A standardized surgical technique of noncemented THA was performed through an anterolateral approach in all patients. All patients underwent standardized surgical management with combined spinal/epidural anesthesia.2
Sample Collection
Venous blood (10 mL) was collected in tubes with lithium heparin before surgical incision and at 24 h after the start of surgery. When the hip replacement was completed, a standard drain was placed in the deepest portion of the wound, in proximity to the newly replaced joint. Drain exudates were collected over a 60-min period, 23–24 h from start of surgery, in a 400 mL capacity Hemovac reservoir. The approximate volume of fluid obtained over this 60-min period was 10 mL, and no patient had excessive bleeding. Preliminary analysis with cell slide smears demonstrated that, at this time point, 95%–98% of HD leukocytes are neutrophils. Cells from either blood or HD fluid were fractionated by placing the sample over a separation gel (1-Step Polymorphs, Accurate Chemicals, Westbury, NY) and centrifuging at 500g for 30 min. A lower band of polymorphonuclear neutrophil cells appears in the gel, and this fraction was aspirated off and washed twice in Hanks balanced salt solution. A cell count and analysis of the percentage of each type of white blood cell was performed, and the cell pellet frozen at −80°C. The frozen pellet was then sent to the National Institutes of Health for analysis.
Microarray Analysis of Gene Expression
Total RNA was isolated from the frozen neutrophil pellets using TRIzol reagent (Invitrogen, San Diego, CA) and was further purified by the RNeasy Mini kit (Qiagen, Valencia, CA) with an additional step of DNase treatment. Neutrophil pellets from all samples were processed in an identical manner. RNA was quantified fluorometrically by the RiboGreen reagent (Molecular Probes, Eugene, OR). Biotinylated cRNA was generated using total RNA as a template with a microarray cRNA synthesis kit (SuperArray, Frederick, MD). The labeled cRNA probe was hybridized to an inflammatory cytokine-targeted microarray containing genes encoding cytokines and ILs associated with the inflammatory responses and their receptors, representing various human cytokine and cytokine receptor genes and control genes (Oligo GEArray, OHS-011, 113 oligonucleotide sequences; SuperArray). Chemiluminescence images were captured by a charge-coupled device camera (AlphaImager, Alpha Innotech, San Leandro, CA) and analyzed using ImageQuant 5 software (Molecular Dynamics, Piscataway, NJ). Eighteen membranes were used to obtain gene expression for presurgical circulating neutrophils (B0), postsurgical circulating neutrophils (B24), and HD neutrophils for the six patients. Per membrane, genes were normalized to β-actin expression (also located on the membrane) and the normalized values were put in three groups: B0, B24, and HD. Transcripts demonstrating a twofold change9 and a P < 0.05 (using Wilcoxon’s signed rank test) between the B0 and HD groups were chosen for further evaluation.
Reverse Transcription Polymerase Chain Reaction
To confirm the results of array screening, transcripts identified as altered in the gene microarray were further analyzed by reverse transcription polymerase chain reaction (RT-PCR) using the extracted RNA obtained from each patient (see above). Per gene, B0, B24, and HD samples from all patients were examined simultaneously. RT-PCR was performed using the Access RT-PCR system (Promega, Madison, WI). The PCR primer pairs and product sizes of genes that were successfully amplified are listed in Table 1. The RT-PCR analysis was performed according to the manufacturer’s instruction in 25 μL reaction mixture containing exactly 8 ng of RNA. RT-PCR steps were 1 cycle of 45 min at 45°C for reverse transcription, 1 cycle of 2 min at 94°C for inactivation of transcriptase, 28–35 cycles of 30 s at 94°C for denaturation, 1 min at 55°C for annealing, 2 min at 68°C for extension, and final extension at 68°C for 7 min. For each gene, all patient samples were amplified the same time for the same number of cycles: 28 cycles for IL1RN, 30 cycles for IL18R1, MIF, IL8RB, and CCL20, and 23 cycles for β-actin. The RT-PCR products were separated by electrophoresis on 2% agarose/ethidium bromide gels and images were acquired by an AlphaImager charge-coupled device camera. The relative intensities of the RT-PCR products, as visualized on the gel, were analyzed quantitatively using ImageQuant 5 software. The results were normalized to β-actin. Comparisons of gene expression from B0, B24, and HD samples were made by repeated measures mixed model with Tukey-Kramer post hoc test (P < 0.05 for significance).
Table 1.
Reverse Transcription Polymerase Chain Reaction (RT-PCR) Primer Pairs and Length of PCR Products
| Gene | Primer pairs | Product (bp) |
|---|---|---|
| β-Actin | CTCCTGAGCGCAAGTACTCCGTCACCTTCACCGTTCCAGT | 299 |
| IL1RN | CTCCTGGGGGTTCTTTCTTCTAGGGAACTTTGCACCCAAC | 251 |
| IL8RB | ATTCTGGGCATCCTTCACAGTGAGGCTTGGAATGTGACTG | 250 |
| IL18R1 | GAAGAACGCCGAGTTTGAAGATTTTCTTCCCCGAACATCC | 250 |
| MIF | AGAACCGCTCCTACAGCAAGATTTCTCCCCACCAGAAGGT | 234 |
| CCL20 | CTGGCCAATGAAGGCTGTGACAAGTCCAGTGAGGCACA | 266 |
RESULTS
Samples from six patients undergoing THA were analyzed. Patient ages ranged from 46 to 80 yr and were equally distributed between genders. For both blood and HD samples, 96% ± 3% of the leukocytes in the cell pellet were neutrophils. Lymphocytes, eosinophils, and monocytes were always <10%. In the microarray analysis, genes significantly upregulated in the HD neutrophils when compared with the presurgery blood sample neutrophils were IL-1 receptor antagonist (IL1RN), IL-18 receptor 1 (IL18R1), macrophage migration inhibitory factor (MIF), and macrophage inflammatory protein 3α (CCL20), whereas IL-8 receptor β (IL8RB/CXCR2), CCR3, CX3CR1, CCR5, and LTB were downregulated (Fig. 1, Table 2). All of the genes expressed in the microarray are tabulated in the online supplement table (see Supplemental Digital Content 1, available at: http://links.lww.com/A1335), which shows that most cytokines or chemokines did not show a twofold change or a statistically significant change in HD neutrophils when compared with the presurgery blood sample neutrophils.
Figure 1.
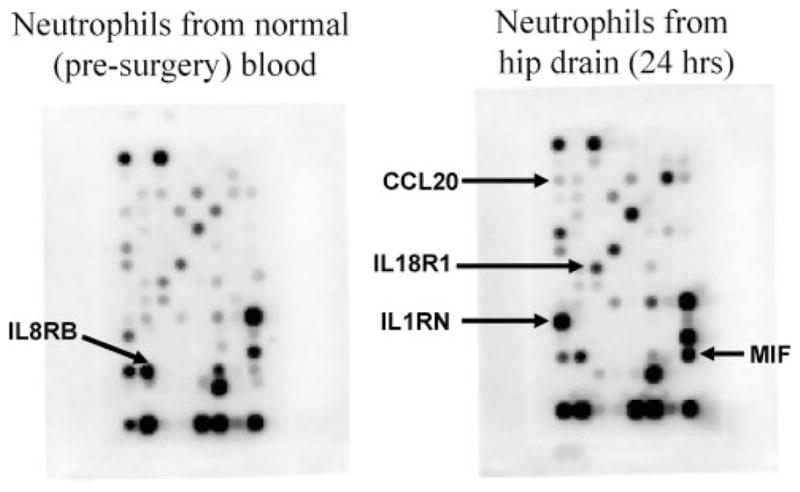
Microarray analysis for genes associated with cytokines, chemokines, and their receptors. The oligo GEArray membrane containing approximately 100 genes was hybridized with biotin-labeled cRNA generated with RNAs from neutrophils from presurgical blood (left) and from hip drain neutrophils of the same patient (right). Transcripts which were consistently altered in patients are indicated by an arrow.
Table 2.
Genes Upregulated or Downregulated in Hip Drain Neutrophils After Total Hip Arthroplasty Using Microarray Analysis
| Symbol | Gene name | Fold change |
|---|---|---|
| IL8RB | Interleukin-8 receptor β/CXCR2 | 0.23 |
| MIF | Macrophage migration inhibitory factor | 2.7 |
| IL18R1 | Interleukin-18 receptor 1 | 3.1 |
| IL1RN | Interleukin-1 receptor antagonist | 3.2 |
| CCL20 | LARC/MIP-3α | 5.3 |
| CCR3 | CC-CKR-3/CKR3 | 0.19 |
| CX3CR1 | CCRL1/CMKBRL1 | 0.35 |
| CCR5 | CC-CKR-5 | 0.43 |
| LTB | Cytokine P33 | 0.26 |
Genes that show more than a twofold upregulation or downregulation in 24 h hip drain neutrophils versus presurgery blood neutrophils with P < 0.05.
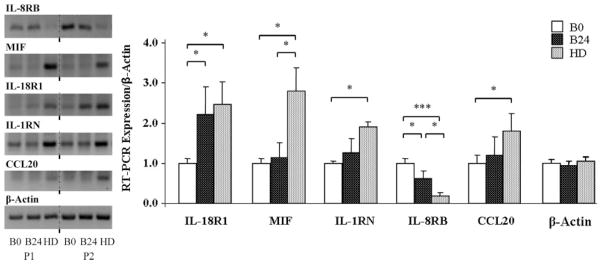
Changes seen in the gene microarray were confirmed by RT-PCR (Fig. 2) for ILIRN, IL18R1, MIF, and CCL20 for which expression in the HD neutrophil population was upregulated compared with those in circulating presurgery blood. In addition, IL18R1 expression was also increased in 24 h blood compared with presurgery blood. RT-PCR also confirmed that IL8RB/CRCX2 expression in HD was downregulated compared with presurgery blood. IL8RB/CXCR2 was also decreased in 24 h blood but not to the same extent as in the HD population. As a control, Figure 2 also demonstrates that β-actin expression did not differ between HD and blood neutrophils.
Figure 2.
Left, Gel electrophoresis of reverse transcription polymerase chain reaction (RT-PCR) expression of neutrophil mRNA showing genes that were significantly upregulated or downregulated in the gene microarray. Results from two representative patients are displayed for presurgery blood (B0), 24 h blood (B24), and 24 h postsurgery hip drain (HD). β-Actin was the internal control. Right, RT-PCR analysis of mRNA levels, relative to β-actin, showing four genes upregulated (MIF, IL18R1, IL1RN, CCL20) and one gene downregulated (IL8RB/CXCR2) in 24 h postsurgery HD neutrophils versus presurgery blood neutrophils. Data presented as mean ± SEM; *P < 0.05, ***P < 0.001. In the graph, β-actin expression of B0, B24, and HD neutrophils were normalized to the B0 average.
We also attempted to examine the expression of CCR3, CCR5, CXCR1, and LTB via RT-PCR. Genes CCR5 and CX3CR1 had very weak signals in the microarray and could not be amplified during RT-PCR. Although LTB had an adequate signal on the microarray, it could not be amplified appropriately with two different primer pairs in the RT-PCR. CCR3 also gave a low signal on the arrays and did not show downregulation in the RT-PCR gel. Based on the low baseline signals for these four genes and/or the difficulty in amplifying them (e.g., LTB), we consider them marginally or nonexpressed in this cell population.
DISCUSSION
A complex array of cytokines and chemokines are found in the extracellular milieu after surgery-induced tissue damage.2,10 In such a complex cellular milieu, identifying the sources of individual cytokines/chemokines is difficult because multiple cell types at the wound site express these molecules. By investigating HD exudates from patients who had surgical hip replacement, we were able to obtain an almost pure population of neutrophils enabling us to better assess the contribution of this cell type to cytokine/chemokine production in the extracellular space. This approach demonstrated that neutrophils collected at 24 h after THA express altered transcript levels in a distinct set of cytokines compared with presurgical circulating neutrophils.
One of the cytokines that is induced in HD neutrophils, but not in 24-h circulating neutrophils, after THA encodes the proinflammatory cytokine MIF. Functionally, MIF can induce the expression of proinflammatory cytokines and cyclooxygenase 2.11 MIF has also been suggested to amplify neurogenic inflammation induced by the pronociceptive neuropeptide substance P.12 The secretion of MIF protein in patients suffering from endometriosis has been shown to correlate with pain,13,14 suggesting the possibility that this molecule is involved with sensitization of nociceptive nerve terminals. MIF can also activate macrophages and inhibit apoptosis of these cells thereby sustaining macrophage activity during inflammation.15,16 Macrophages, which are generally recruited to the wound site after neutrophils, may in-turn release factors that can sensitize primary afferent fibers and contribute to inflammation. It may be interesting then to determine whether MIF produced by HD neutrophils is involved in the recruitment of macrophages and the reported increase in cytokines and prostaglandin.2
At the site of inflammation, it is common to observe the induction of both proinflammatory and antiinflammatory cytokines.10 The observed induction of the antiinflammatory cytokine IL1RN in the HD neutrophils is consistent with this duality. IL1RN functions to antagonize the binding of the proinflammatory cytokine IL-1 to its receptor.17 IL-1β protein was previously reported to be elevated in the HD after THA, has well-documented roles in inflammation, and like MIF, has been demonstrated to sensitize nerve terminals to pain.2,18 The increase in IL1RN after THA may thus be a natural process for attenuating the effects of IL-1β, suggesting that neutrophils at this point may also participate in postoperative repair. Concordant with this hypothesis, chronic treatment with IL1RN or anakinra, a recombinant analog of IL1RN, has been demonstrated to reduce basal nociceptive sensitivity in mice.19,20 Moreover, anakinra has been used clinically to reduce joint inflammation in rheumatoid arthritis patients.21 In this context, it would be interesting to determine whether further supplementation with exogenous IL1RN after THA could reduce pain and inflammation without having an adverse affect on wound healing.
We also detected significant alterations in message levels for two cytokine/chemokine receptors. The gene encoding IL18R1, which is the receptor for the proinflammatory cytokine IL-18, is significantly increased in HD neutrophils. Increased IL18R1 mRNA has also been reported in synovial fluid neutrophils of patients suffering from rheumatoid arthritis.22 It has been reported that IL-18 recruits neutrophils to the inflamed site via IL18R1,22 which is consistent with the massive, sustained infiltration of neutrophils after THA. IL-18 also activates neutrophils through this receptor, causing them to release various cytokines and chemokines.22 Regarding nociception, intraplantar injection of IL-18 causes mechanical hyperalgesia,23 although the exact role of the neutrophils in this process needs further examination. Finally, the fact that IL18R1 is also induced in 24-h circulating neutrophils suggests that this receptor may have been involved in the emigration from the blood to the wound site.
We also observed that IL8RB/CXCR2, a high affinity receptor for IL-8/CXCL8, is strongly downregulated in HD neutrophils. IL-8, which promotes chemoattraction and activation of neutrophils,24,25 has been reported to be increased in HD exudates2 after THA. Thus, it is possible that IL8RB-expressing neutrophils are recruited to the wound site via IL-8. IL-8 can also regulate the expression of IL8RB, because in vitro studies have shown that this molecule causes a rapid downregulation of its receptors.26,27 It is possible then that the downregulation of IL8RB in HD neutrophils may be caused by the increased levels of IL-8 in the HD exudate. Given that the HD neutrophils have reached their final destination site, a mechanism for decreasing receptors such as IL8RB seems likely in these cells. Understanding why receptors with similar functions (e.g., IL18R1) are not downregulated suggests a multiplicity of regulatory processes and provides different targets for manipulation.
The transcript encoding macrophage inflammatory protein 3α (MIP 3α/CCL20) was increased in HD neutrophils at 24 h after THA. The encoding molecule is a strong chemoattractant for lymphocytes, while weakly attracting neutrophils. Thus, a release of CCL20 from the neutrophils may be a signal for recruitment of cells other than neutrophils. Regarding pain, CCL20 has not been thoroughly investigated, although it is increased in conditions in which pain is elevated. For example, neutrophils in synovial fluid of some patients with rheumatoid arthritis show increased CCL20 mRNA, but CCL20 expression in the rheumatoid study was not detectable in circulating blood neutrophils.28 CCL20 has also been reported to be altered in oral wound healing models.29
Although this study focused on the most consistently altered genes within a 113-gene microarray, it cannot be excluded that additional genes are altered in HD neutrophils. Moreover, the 24-h time point may not be the optimal time for detecting alterations in other genes, although 24 h is the time point when neutrophils predominate at wound sites.4–6 Although it would be of interest to analyze cytokine and chemokine mRNA in neutrophils at later time points (e.g., Days 2–4), the risk of infection makes it unsafe to maintain a drain beyond the 24-h recovery period. It is also important to note that a lack of alteration in neutrophil mRNA does not mean that the corresponding protein is not secreted by another cell type. Thus, cytokines that were reported in HD fluid in our previous study,2 IL-1β, IL-6, and IL-8, may have originated, for example, from tissue macrophages surrounding the HD catheter. A limitation of this study is that we did not show that MIF and CCL20 are also produced and released into the HD exudates after THA as would be suggested by the upregulation of the encoding mRNAs. The expression of ILIRN, IL18R1, and IL8RB proteins in isolated HD neutrophils remains to be evaluated using methods such as Western blots or flow cytometry30 as well as the functional consequence in ex vivo experiments.
In contrast to the presurgery neutrophils, the neutrophils taken from the HD and circulating blood at 24 h were obtained while the patients were receiving epidural anesthesia. Thus, it is possible that anesthesia may have influenced the cytokine/chemokine profile in our study. However, a previous study failed to detect a difference in plasma IL-6 and tumor necrosis factor-α levels after THA with general versus regional spinal/epidural anesthesia, suggesting that the cell types involved in this study may not have been affected by the anesthetic.31
The data obtained here demonstrate that opponent processes (anti- and proinflammatory) occur in HD neutrophils after THA. Deciphering their roles in events such as pain processing, inflammation, and tissue repair could aid in our approach to treating patients pre- and postoperatively.
Acknowledgments
Supported by the Intramural Research Program, NIDCR, NIH, DHHS, and University Anesthesiologists S.C., Chicago, IL.
Footnotes
Reprints will not be available from the author.
References
- 1.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 2.Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, Moric M, Caicedo MS, Tuman KJ. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–10. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 4.Englelhardt E, Toksoy A, Goebeler M, Debus S, Brocker EB, Gillitzer R. Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–60. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–21. [PubMed] [Google Scholar]
- 6.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 7.Cunha FQ, Ferreira SH. Peripheral hyperalgesic cytokines. Adv Exp Med Biol. 2003;521:22–39. [PubMed] [Google Scholar]
- 8.Rittner HL, Machelska H, Stein C. Leukocytes in the regulation of pain and analgesia. J Leukoc Biol. 2005;78:1215–22. doi: 10.1189/jlb.0405223. [DOI] [PubMed] [Google Scholar]
- 9.Schena M, Shalon D, Heller R, Chai A, Brown PO, Davis RW. Parallel human genome analysis: microarray-based expression monitoring of 1000 genes. Proc Natl Acad Sci USA. 1996;93:10614–9. doi: 10.1073/pnas.93.20.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang HY, Mitchell K, Keller JM, Iadarola MJ. Peripheral inflammation increases Scya2 expression in sensory ganglia and cytokine and endothelial related gene expression in inflamed tissue. J Neurochem. 2007;103:1628–43. doi: 10.1111/j.1471-4159.2007.04874.x. [DOI] [PubMed] [Google Scholar]
- 11.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer-Siegler KL, Vera PL. Intraluminal antibodies to macrophage migration inhibitory factor decrease substance P induced inflammatory changes in the rat bladder and prostate. J Urol. 2004;172:1504–9. doi: 10.1097/01.ju.0000140213.54457.97. [DOI] [PubMed] [Google Scholar]
- 13.Akoum A, Metz CN, Al-Akoum M, Kats R. Macrophage migration inhibitory factor expression in the intrauterine endometrium of women with endometriosis varies with disease stage, infertility status, and pelvic pain. Fertil Steril. 2006;85:1379–85. doi: 10.1016/j.fertnstert.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 14.Morin M, Bellehumeur C, Therriault MJ, Metz C, Maheux R, Akoum A. Elevated levels of macrophage migration inhibitory factor in the peripheral blood of women with endometriosis. Fertil Steril. 2005;83:865–72. doi: 10.1016/j.fertnstert.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 15.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375–82. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA. 2002;99:345–50. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arend WP, Guthridge CJ. Biological role of interleukin 1 receptor antagonist isoforms. Ann Rheum Dis. 2000;59(suppl 1):i60–4. doi: 10.1136/ard.59.suppl_1.i60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 19.Wolf G, Yirmiya R, Goshen I, Iverfeldt K, Holmlund L, Takeda K, Shavit Y. Impairment of interleukin-1 (IL-1) signaling reduces basal pain sensitivity in mice: genetic, pharmacological and developmental aspects. Pain. 2003;104:471–80. doi: 10.1016/S0304-3959(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 20.Baamonde A, Curto-Reyes V, Juarez L, Meana A, Hidalgo A, Menendez L. Antihyperalgesic effects induced by the IL-1 receptor antagonist anakinra and increased IL-1beta levels in inflamed and osteosarcoma-bearing mice. Life Sci. 2007;81:673–82. doi: 10.1016/j.lfs.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Furst DE. Anakinra: review of recombinant human interleukin-I receptor antagonist in the treatment of rheumatoid arthritis. Clin Ther. 2004;26:1960–75. doi: 10.1016/j.clinthera.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Leung BP, Culshaw S, Gracie JA, Hunter D, Canetti CA, Campbell C, Cunha F, Liew FY, McInnes IB. A role for IL-18 in neutrophil activation. J Immunol. 2001;167:2879–86. doi: 10.4049/jimmunol.167.5.2879. [DOI] [PubMed] [Google Scholar]
- 23.Verri WA, Jr, Schivo IR, Cunha TM, Liew FY, Ferreira SH, Cunha FQ. Interleukin-18 induces mechanical hypernociception in rats via endothelin acting on ETB receptors in a morphine-sensitive manner. J Pharmacol Exp Ther. 2004;310:710–7. doi: 10.1124/jpet.103.063990. [DOI] [PubMed] [Google Scholar]
- 24.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–80. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- 25.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–3. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- 26.Samanta AK, Oppenheim JJ, Matsushima K. Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem. 1990;265:183–9. [PubMed] [Google Scholar]
- 27.Chuntharapai A, Kim KJ. Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol. 1995;155:2587–94. [PubMed] [Google Scholar]
- 28.Schlenk J, Lorenz HM, Haas JP, Herrmann M, Hohenberger G, Kalden JR, Röllinghoff M, Beuscher HU. Extravasation into synovial tissue induces CCL20 mRNA expression in polymorphonuclear neutrophils of patients with rheumatoid arthritis. J Rheumatol. 2005;32:2291–8. [PubMed] [Google Scholar]
- 29.McGrory K, Flaitz CM, Klein JR. Chemokine changes during oral wound healing. Biochem Biophys Res Commun. 2004;324:317–20. doi: 10.1016/j.bbrc.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 30.van Eeden SF, Klut ME, Walker BA, Hogg JC. The use of flow cytometry to measure neutrophil function. J Immunol Methods. 1999;232:23–43. doi: 10.1016/s0022-1759(99)00148-9. [DOI] [PubMed] [Google Scholar]
- 31.Høgevold HE, Lyberg T, Kähler H, Haug E, Reikerås O. Changes in plasma IL-1beta, TNF-alpha and IL-6 after total hip replacement surgery in general or regional anaesthesia. Cytokine. 2000;12:1156–9. doi: 10.1006/cyto.2000.0675. [DOI] [PubMed] [Google Scholar]