Abstract
Combined anti-retroviral therapeutic drugs effectively increase the lifespan of HIV-1-infected individuals who then have a higher prevalence of HAND (HIV-1 associated neurocognitive disorder). Soluble factors including HIV-1 proteins released from HIV-1-infected cells have been implicated in the pathogenesis of HAND, and particular attention has been paid to the HIV-1 Tat (transactivator of transcription) protein because of its ability to directly excite neurons and cause neuronal cell death. Since HIV-1 Tat enters cells by receptor-mediated endocytosis and since endolysosomes play an important role in neuronal cell life and death, we tested here the hypothesis that HIV-1 Tat neurotoxicity is associated with changes in the endolysosome structure and function and also autophagy. Following the treatment of primary cultured rat hippocampal neurons with HIV-1 Tat or as controls mutant-Tat or PBS, neuronal viability was determined using a triple staining method. Preceding observations of HIV-1 Tat-induced neuronal cell death, we observed statistically significant changes in the structure and membrane integrity of endolysosomes, endolysosome pH and autophagy. As early as 24 h after HIV-1 Tat was applied to neurons, HIV-1 Tat accumulated in endolysosomes, endolysosome morphology was affected and their size increased, endolysosome membrane integrity was disrupted, endolysosome pH increased, specific activities of endolysosome enzymes decreased and autophagy was inhibited, as indicated by the significant changes in three markers for autophagy. In contrast, statistically significant levels of HIV-1 Tat-induced neuronal cell death were observed only after 48 h of HIV-1 Tat treatment. Our findings suggest that endolysosomes are involved in HIV-1 Tat-induced neurotoxicity and may represent a target for therapeutic intervention against HAND.
Keywords: autophagy, endosome, HIV-1 Tat, lysosome, neuronal cell death, pH
Abbreviations: AM, acetoxymethyl ester, Atg5, autophagy-related gene-5; CXCR4, CXC chemokine receptor type 4; EEA1, early endosome antigen 1; HAND, HIV-1 associated neurocognitive disorder; LAMP1, lysosome-associated membrane protein 1; LC3, light chain 3; LDL, low-density lipoprotein; MAP, microtubule-associated protein; Tat, transactivator of transcription
INTRODUCTION
More than 40 million people worldwide are infected with HIV-1, and combined anti-retroviral therapeutic drugs have effectively increased the lifespan of people living with HIV. Increased as well is the prevalence of HAND (HIV-1-associated neurocognitive disorder) with recent epidemiological studies indicating that the prevalence of HAND in the U.S.A. is greater than 50% of HIV-1-infected people (Ellis et al., 2010; Heaton et al., 2010). Clinically, HAND represents a set of conditions ranging from subtle neuropsychological impairments to profoundly disabling HIV-associated dementia. Although the underlying mechanisms for HAND pathogenesis are not fully understood, soluble factors including HIV-1 viral products and pro-inflammatory mediators released from the infected glia and monocytes have been implicated (Ghafouri et al., 2006; King et al., 2006; Wallace 2006; Ances and Ellis, 2007). Among the viral products, HIV-1 Tat (transactivator of transcription) protein has been shown to be neuroexcitatory and neurotoxic, and it continues to be implicated as a causative agent in HAND (Sabatier et al., 1991; Weeks et al., 1995; Haughey et al., 1999; Nath et al., 2000; Perez et al., 2001; King et al., 2006; Buscemi et al., 2007b; Agrawal et al., 2012).
HIV-1 Tat is a non-structural transcriptional regulator essential for the replication of HIV-1. The first exon of HIV-1 Tat encodes the first 72 amino acids and the second exon encodes another 14–32 amino acids. Tat1–72 is sufficient for transactivation, which requires the arginine-rich domain of Tat between amino acid residues 49 and 57. Nanomolar concentrations of HIV-1 Tat have been reported in sera of HIV-1-infected patients, but these levels are almost certainly underestimated, given how avidly HIV-1 Tat binds to proteins and cells (Westendorp et al., 1995; Xiao et al., 2000). HIV-1 Tat can be transported across the blood–brain barrier from the systemic circulation (Kim et al., 2003; Banks et al., 2005), can be secreted by infected macrophages and microglia, and has been detected in brain of patients with HIV-1-associated dementia (Westendorp et al., 1995; Ellis et al., 2000; Nath, 2002).
HIV-1 Tat enters neurons via receptor-mediated endocytosis involving CD26 (Gutheil et al., 1994), CXCR4 (CXC chemokine receptor type 4) (Xiao et al., 2000), heparin sulphate proteoglycans (Tyagi et al., 2001) and LDL (low-density lipoprotein) receptor-related proteins (Liu et al., 2000; Vendeville et al., 2004; King et al., 2006; Deshmane et al., 2011). Endocytosis is a very rapid and early event, which results in the accumulation of HIV-1 Tat in endolysosomes from which it can be released into the cytoplasm and uptaken into the nucleus (Vives et al., 1997; Liu et al., 2000; Caron et al., 2004), most probably through the mechanisms involving the high H+ gradient maintained by vacuolar H+-ATPase (Vendeville et al., 2004). The endolysosome system is very dynamic, and lysosomes and other acidic sub-cellular compartments are involved in endocytosis and autophagy (Jeyakumar et al., 2005; Nixon and Cataldo, 2006). Endosomes and lysosomes process proteins and other materials that are endocytosed, whereas autophagy predominantly process cytosolic proteins.
Neurons are highly polarized long-lived post-mitotic cells, which possess an elaborate endolysosome system critical for the maintenance of neuronal function (Nixon and Cataldo, 1995, 2006). Increasingly, endolysosome dysfunction has been implicated in neuronal damage and in the pathogenesis of a variety of neurological disorders including AD (Alzheimer's disease), Parkinson's disease and HAND (Gelman et al., 2005; Spector and Zhou, 2008; Zhou and Spector, 2008). Here, we tested the hypothesis that HIV-1 Tat induces neuronal damage by affecting the structure and function of endolysosomes. We report that HIV-1 Tat enlarged endolysosomes, disrupted endolysosome membrane integrity, elevated endolysosome pH, decreased specific activities of endolysosome enzymes and inhibited autophagy. Our findings suggest that the disturbed structure and function of endolysosomes play an early and important role in HIV-1 Tat-induced neuronal damage.
MATERIALS AND METHODS
Hippocampal neuron primary cultures
Primary cultures of hippocampal neurons were prepared from embryonic day 18 Sprague–Dawley rats as described previously (Buscemi et al., 2007) using a protocol approved by the University of North Dakota Animal Care and Use Committee adherent with the Guide for the Care and Use of Laboratory Animals (NIH publication number 80–23). Pregnant dams (embryonic day 18) were killed by asphyxiation with CO2. The fetuses were removed, decapitated, and meninges-free hippocampi were isolated, trypsinized and plated on to 35-mm poly-d-lysine-coated glass-bottom tissue culture dishes. Neurons were grown in the Neurobasal™ medium with l-glutamine, antibiotic/antimycotic and B27 supplement, and were maintained at 37°C and 5% CO2 for 10–14 days at which time they were used for the experimentation. Typically, the purity of the neuronal cultures was greater than 95% as determined by neuronal staining with mouse anti-NeuN or goat anti-MAP2 (microtubule-associated protein 2) antibodies (Millipore); astrocytes were identified using a mouse anti-GFAP (glial fibrillary acidic protein) antibody (Sigma). Neurons were treated either with HIV-1 Tat1–72 (100 nM, a gift from Dr Avindra Nath, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A.), mutant Tat (TatΔ31–61, 100 nM) or PBS as vehicle.
Neuronal cell viability assay
Neuronal cell viability was determined using a triple staining method as described previously (Buscemi et al., 2007). Neurons were stained with Hoechst 33342 (10 μg/ml), ethidium homodimer-1 (4 μM) and calcein (1 μg/ml). Hoechst 33342, which labels DNA, was used as a marker for identifying condensed nuclei characteristic of apoptotic cell death. Cells dead or dying as a result of loss of membrane integrity were unable to exclude ethidium homodimer-1 dye. Cells were considered viable when cytoplasm was stained with green fluorescence after the cleavage of the non-fluorescent calcein AM (acetoxymethyl ester) to calcein. Fields were chosen randomly and at least five images from five separate fields of culture dishes for every experimental condition were taken with our Axiovert 200 M fluorescence microscope (Zeiss) and filter-based imaging system. The number of dead or dying neurons (ethidium-labelled red nuclei and blue-condensed nuclei without green cytoplasmic staining) and total neuron numbers were counted manually. More than 900 neurons were counted per experimental condition. Neuronal viability was reported as a percentage of total neurons.
Measurement of endolysosome pH
Endolysosome pH was measured using a ratio-metric lysosome pH indicator dye (LysoSensor Yellow/Blue DND-160 from Invitrogen); a dual excitation dye that permits pH measurements in acidic organelles independently of dye concentration. Neurons were loaded with 2 μM LysoSensor for 5 min at 37°C. Light emitted at 520 nm in response to λex at 340 and 380 nm was measured for 20 ms every 30 s using a filter-based imaging system (Zeiss). The ratios of light excited at 340/380 nm and emitted at 520 nm were converted to pH using a calibration curve established using 10 μM of the H+/Na+ ionophore monensin, and 20 μM of the H+/K+ ionophore nigericin dissolved in 20 mM Mes, 110 mM KCl, and 20 mM NaCl adjusted to pH 3.0–7.0 with HCl/NaOH.
Living cell imaging
The morphology of endolysosomes in living neurons was determined using a LysoTracker dye. After treatments, neurons were loaded with LysoTracker Red DND-99 (50 nM, Invitrogen) and calcein AM (1 μg/ml, Invitrogen) for 30 min at 37°C. Fields were chosen at random and at least five images from every experimental condition were acquired by confocal microscopy (Olympus). The sizes of endolysosomes (LysoTracker) were analysed with the particle-analysing program in Image J software. For measurement of Tat endocytosis, neurons were incubated with FITC–Tat (100 nM, AnaSpec) for 1 day at 37°C, followed by loading of LysoTracker Red DND-99 (50 nM) and Hoechst 33342 (10 μg/ml) for an additional 30 min. Images were taken with an Axiovert 200 M fluorescence microscope (Zeiss).
Immunocytochemistry
Neurons were fixed with cold methanol (−20°C) for 10 min, washed with PBS, blocked with 5% goat serum, and incubated overnight at 4°C with primary antibodies targeting EEA1 (early endosome antigen 1; 1:500, rabbit polyclonal, Santa Cruz) or LAMP1 (lysosome-associated membrane protein-1; 1:500, rabbit polyclonal, Sigma). After washing with PBS, neurons were incubated with Alexa Fluor® 546-conjugated goat anti-rabbit antibodies (Invitrogen). Neurons were examined by confocal microscopy (Olympus). Controls for specificity included staining neurons with primary antibodies without fluorescence-conjugated secondary antibodies (background controls) and staining neurons with only secondary antibodies.
Endolysosome membrane permeability
Endolysosome membrane permeability was determined by measuring the leakage of endolysosome fluorescent dye Lucifer Yellow CH (Invitrogen). Neurons were incubated with Lucifer Yellow (100 μg/ml) for 16 h, followed by incubation with Tat at 37°C for 1 and 2 days. Levels of dye inside of neurons were detected by confocal microscopy (Olympus).
Immunoblotting
Neurons were lysed with RIPA buffer (Pierce) containing protease inhibitor cocktail (Sigma). After centrifugation (14000 g for 10 min at 4°C), supernatants were collected, and protein concentrations were determined with a DC protein assay (Bio-Rad). Equal amounts of proteins (10 μg) were separated by SDS/12% PAGE, and, following transfer, PVDF membranes (Millipore) were incubated overnight at 4°C with anti-EEA1 (1:1000, Santa Cruz Biotechnology), anti-LAMP1 (1:1000, Sigma), anti-acid phosphatase (1:1000, mouse monoclonal, Abcam), anti-cathepsin B (1:500, mouse monoclonal, Sigma), anti-cathepsin D (1:1000, mouse monoclonal, Sigma), anti-LC3 (light chain 3) b (1:1000, rabbit polyclonal, Abcam), anti-Atg5 (autophagy-related gene-5; 1:2000, mouse monoclonal, Millipore) or anti-p62 (1:1000, rabbit polyclonal, Sigma) antibodies; anti-β-actin (1:10000, mouse monoclonal, Abcam) antibody was used as a gel-loading control. The blots were developed with enhanced chemiluminescence, and bands were visualized and analysed by LabWorks 4.5 software on a BioSpectrum® imaging System (UVP). Quantification was performed by densitometry and the results were analysed as total integrated densitometric volume values (arbitrary units).
Measurement of activities of endolysosome enzymes
Enzyme activities of acid phosphatase were determined using an acid phosphatase assay kit (Sigma); a luminescence-based assay that uses 4-nitrophenyl phosphate as substrate (Chen et al., 2010). Enzyme activities of cathepsins D and B were determined using assay kits (BioVision); fluorescence-based assays that used cathepsin D or cathepsin B preferred substrates labelled with MCA (Chen et al., 2010). Enzyme activities were expressed as absorbance per 10 μg of protein. Specific activities of each enzyme were expressed as a ratio of enzyme activity to protein levels as determined by immunoblotting.
Statistical analysis
All data were expressed as means±S.E.M. Statistical significance for multiple comparisons was determined by one-way ANOVA plus a Tukey post hoc test. P<0.05 was considered to be statistically significant.
RESULTS
In order to compare the effects of HIV-1 Tat on neuronal damage and the structure and function of endolysosomes, we needed to first determine the time course and extent to which HIV-1 Tat decreased neuronal viability. HIV-1 Tat1–72 induced significant amounts of neuronal cell death starting from 48 h of treatment (n = 5, P<0.05) with a maximum of 50% neuronal cell death (n = 5, P<0.001) after the treatment for 96 h (Figure 1). These data were consistent with previous studies that have shown similar neurotoxic effects of HIV-1 Tat (Kruman et al., 1998; Haughey et al., 1999; Bonavia et al., 2001; Aksenov et al., 2003; Buscemi et al., 2007; Eugenin et al., 2007). No statistically significant increases in neuronal cell death were observed with either mutant TatΔ31–61 or PBS, consistent with previous reports that this deletion mutant of Tat is not directly toxic to neurons (Buscemi et al., 2007).
Figure 1. HIV-1 Tat-induced neuronal cell death in a time-dependent manner.
Significant amounts of neuronal cell death were observed after 2-day incubation with HIV-1 Tat (100 nM) and reached a maximal level of 50% cell death by the fourth day. No significant neuronal cell death was observed in neurons treated with either mutant Tat or PBS (n = 5, *P<0.05 and ***P<0.001).
In neurons and other cells, HIV-1 Tat uses receptor-mediated endocytotic mechanisms (Liu et al., 2000; Vendeville et al., 2004; King et al., 2006) to enter cells where HIV-1 Tat accumulates first in endolysosomes. Since the basic region of amino acids 49–57 of HIV-1 Tat is required for binding to membrane receptor proteins (Sabatier et al., 1991) and exerting its neurotoxic effects (Weeks et al., 1995), we used a FITC-labelled Tat47–57 to determine first the extent to which HIV-1 Tat accumulated in endolysosomes in neurons. After incubating neurons with the FITC-labelled HIV-1 Tat47–57 (FITC-Tat), we observed significant intracellular accumulation of FITC-Tat, which was mainly compartmentalized in endolysosomes as identified with LysoTracker dye (red, Figure 2A).
Figure 2. HIV-1 Tat-altered the structure of neuronal endolysosomes.
(A) HIV-1 Tat is accumulated in endolysosomes of primary cultured neurons. FITC-labelled Tat47-57 peptide (100 nM, green) was co-localized with endolysosomes (red, LysoTracker) and DAPI (4′,6-diamidino-2-phenylindole; blue) was used for staining nuclei. Bar = 50 μm. (B) Live cell imaging showed that HIV-1 Tat (100 nM) treatment increased the sizes of neuronal endolysosomes. LysoTracker (red) was used to identify endolysosomes, and calcein AM (green) was used to stain living cells (top panel), and the sizes of endolysosomes were quantified with Image J software. HIV-1 Tat-enlarged endosomes as identified with EEA1 staining (middle panel). HIV-1 Tat-enlarged lysosomes as identified with LAMP1 staining (bottom panel). (C) Quantification of the top panel of (B) showed that HIV-1 Tat (100 nM) treatment for 1 and 2 days increased significantly the size of neuronal endolysosomes (n = 15).
Since alterations in the structure and function of endolysosomes have been implicated in the neuropathogenesis of a number of neurological disorders, we next determined the extent to which HIV-1 Tat-affected endolysosome morphology. In living neurons, we identified endolysosomes with LysoTracker, and we found that treatment with HIV-1 Tat for 1 and 2 days increased significantly (n = 15, P<0.05) the size of endolysosomes (Figures 2B and 2C). Treatment with mutant Tat did not affect endolysosome morphology (L. Hiu, X. Chen and J.D. Geiger, unpublished data). Using immunocytochemistry methods, we found that endosomes labelled with EEA1 antibody and lysosomes labelled with LAMP1 antibody were relatively small and evenly distributed in neurons treated with PBS or mutant Tat, but were markedly enlarged and clumped together in HIV-1 Tat-treated neurons (Figure 2B, middle and bottom panels).
The observation that HIV-1 Tat alters endolysosome morphology led us to determine next the extent to which HIV-1 Tat-affected endolysosome function. Since pH is critical for endolysosome function, we determined the extent to which HIV-1 Tat-affected endolysosome pH using LysoSensor dye, which permits ratio-metric assessment of pH changes in acidic organelles. We found that HIV-1 Tat, but not mutant Tat treatment for 1 or 2 days elevated significantly (n = 20, P<0.001) endolysosome pH in cultured hippocampal neurons (Figure 3). As endolysosome pH affects endolysosome enzyme activity, we next determined the protein levels and activity of endolysosome enzymes as evaluations of endolysosome function. Treatment of neurons with HIV-1 Tat for 1 or 2 days increased significantly protein levels of the endolysosome enzymes acid phosphatase (Figure 4A, n = 4, P<0.01 at 1 day and n = 4, P<0.05 at 2 days), cathepsin B (Figure 4C, n = 4, P<0.05) and cathepsin D (Figure 4E, n = 4, P<0.01 at 1 day and P<0.05 at 2 days). However, the specific activity levels of acid phosphatase (Figure 4B), cathepsin B (Figure 4D) and cathepsin D (Figure 4F) were decreased significantly (n = 4, P<0.001) in HIV-1 Tat-treated cultures.
Figure 3. HIV-1 Tat-elevated endolysosome pH in primary cultured neurons.
Endolysosome pH was measured ratio-metrically using LysoSensor dye. HIV-1 Tat (100 nM) treatment for 1 and 2 days elevated significantly endolysosome pH (n = 20).
Figure 4. HIV-1 Tat-altered the expression and activity of endolysosome enzymes.
(A, C, E) HIV-1 Tat (100 nM) increased protein levels of acid phosphatase (ACP), cathepsin B (Cat B), and cathepsin D (Cat D). Representative Western blots and quantitative data from each of the enzymes are shown (n = 4). Actin was used as a loading control. (B, D, F) HIV-1 Tat (100 nM) decreased significantly specific enzyme activity of ACP, Cat B and Cat D (n = 4).
Endolysosome dysfunction has been implicated in initiating stress pathways that lead to cellular dysfunction and death (Roberg and Ollinger, 1998; Turk et al., 2002; Guicciardi et al., 2004; Kroemer and Jaattela, 2005; Kurz et al., 2008). Here, we determined the extent to which HIV-1 Tat-affected endolysosome membrane integrity using Lucifer Yellow dye (Yang et al., 1998). We found that while control neurons displayed a discrete punctate pattern of perinuclear fluorescent staining (Figure 5), HIV-1 Tat-treated neurons displayed increased endolysosome membrane leakage as evidenced by diffuse fluorescent staining (Figure 5) in the cytoplasm.
Figure 5. HIV-1 Tat-disrupted endolysosome membrane integrity.
Endolysosome membrane integrity was evaluated by measuring the leakage of Lucifer Yellow dye. Control neurons displayed a discrete punctate fluorescent staining pattern in perinuclear regions with no fluorescence in cytoplasm (left panel), whereas neurons treated with HIV-1 Tat for 1 day displayed endolysosome membrane leakage as indicated by diffusion of fluorescence into cytoplasm (right panel, bar = 10 μm).
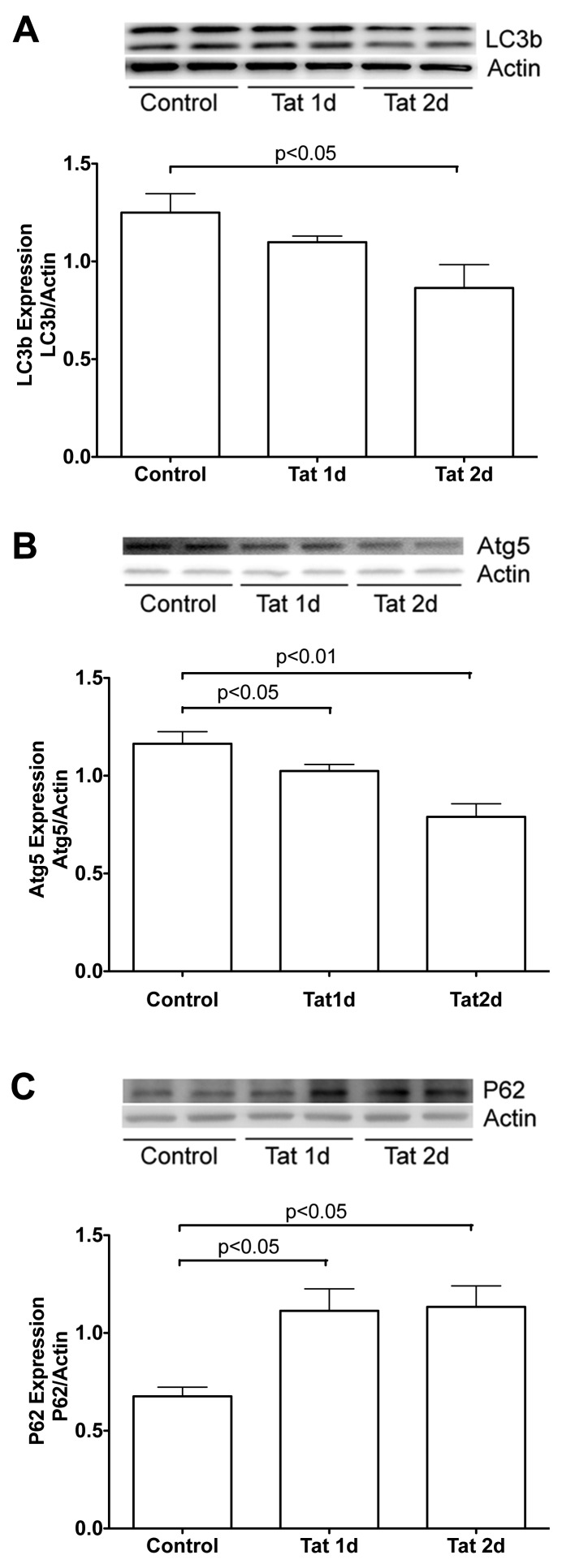
Endolysosomes also function to control autophagy, a process important for normal physiological functions of neurons. Dysfunctions in autophagy have been implicated in the pathogenesis of a variety of neurodegenerative disorders (Wong and Cuervo, 2010) including HAND (Alirezaei et al., 2008b; Alirezaei et al., 2008a; Spector and Zhou, 2008; Zhou and Spector, 2008; Zhu et al., 2009; Zhou et al., 2011). Based on the findings that HIV-1 Tat disrupts autophagy in immune cells (Van Grol et al., 2010), we determined the extent to which HIV-1 Tat-affected autophagy in primary cultured hippocampal neurons. Three markers were used to evaluate the status of autophagy; MAP1 LC3 which regulates the initiation process of autophagy, Atg5 which regulates the elongation process of autophagy, and p62 which inhibits autophagy. We found that HIV-1 Tat treatment decreased significantly protein levels of LC3 (Figure 6A, n = 4, P<0.05 at 2 day treatment) and Atg5 (Figure 6B, n = 4, P<0.05 at 1 day and P<0.01 at 2 days treatment), but increased significantly protein levels of p62 (Figure 6C, n = 4, P<0.05 at 1 and 2 days treatment).
Figure 6. HIV-1 Tat-inhibited autophagy.
Autophagy was estimated by measuring protein levels of LC3, Atg5 and p62. (A) HIV-1 Tat (100 nM) decreased significantly protein levels of LC3. (B) HIV-1 Tat (100 nM) reduced significantly protein levels of Atg5. (C) HIV-1 Tat (100 nM) increased significantly protein levels of p62. Representative Western blots and quantitative data from each of proteins are shown, and actin was used as a loading control (n = 4).
DISCUSSION
Combined highly active anti-retroviral therapeutic drugs have increased dramatically the lifespan of people now living with AIDS. However, this increased lifespan is accompanied by increased prevalence of HAND, which affects up to 50% of people with HIV-1 infection (Ellis et al., 2010; Heaton et al., 2010). The underlying mechanisms for HAND pathogenesis are not fully understood, but one mechanism that appears to be important yet relatively understudied is the involvement of endolysosomes. Disturbed endolysosomes have been noted in brain of HIV-1-infected individuals (Gelman et al., 2005; Spector and Zhou, 2008; Zhou and Spector, 2008), but the mechanisms for these pathological observations are not known. Since neurons are long-lived post-mitotic cells with extreme polarity, they possess an elaborate endolysosome system that contains hydrolases that degrade macromolecules, high concentrations of readily releasable calcium (Christensen et al., 2002; Moreno and Docampo, 2009; Patel and Docampo, 2010), and high concentrations of potentially redox-active iron (Brun and Brunk, 1970; Kidane et al., 2006). When dysfunctional, endolysosomes can contribute to altered calcium homoeostasis (Korkotian et al., 1999; Pelled et al., 2005; Lloyd-Evans et al., 2008) and increased oxidative stress (Pivtoraiko et al., 2009). Disruptions in endolysosome functions perturb numerous cellular functions and can ultimately result in the initiation of cell death pathways (Kroemer and Jaattela, 2005; Kurz et al., 2008). Therefore it is of potential significance to further understand the pathogenesis of HAND that we found that HIV-1 Tat-enlarged endolysosomes, elevated endolysosome pH, decreased specific activities of endolysosome enzymes, disrupted endolysosome membrane integrity and inhibited autophagy, all of which occurred prior to significant increases in HIV-1 Tat-induced neuronal cell death. Thus, the altered structure and function of endolysosomes could underlie, at least in part, the pathogenesis of HAND.
HIV-1 Tat protein continues to be implicated in the pathogenesis of HAND, in part, because there is significant neuronal dysfunction even though neurons are not infected by HIV-1 virus (Nuovo et al., 1994; Merino et al., 2011). HIV-Tat has been shown to, for example, activate NMDA (N-methyl-D-aspartate) receptors (Nath et al., 2000; Haughey et al., 2001; Eugenin et al., 2003), alter calcium homoeostasis (Kruman et al., 1998; Haughey et al., 1999; Bonavia et al., 2001), and increase oxidative stress (Kruman et al., 1998; Aksenov et al., 2001; Perry et al., 2005). HIV-1 Tat is actively secreted by infected glial cells and, following binding to neuronal cell surface receptors, it enters the endolysosome system following receptor-mediated endocytosis (Mann and Frankel, 1991; Liu et al., 2000). Although we did not determine the extent to which previously identified receptors mediate the endocytosis of HIV-1 Tat in neurons, including CD26 (Gutheil et al., 1994), CXCR4 (Xiao et al., 2000), heparin sulphate proteoglycans (Tyagi et al., 2001), and the LDL receptor-related protein (Liu et al., 2000; Deshmane et al., 2011), we did observe the presence of HIV-1 Tat in neuronal endolysosomes.
Neurons are long-lived post-mitotic cells that possess an elaborate endolysosome system to exercise quality control. Substrates for degradation are delivered to lysosomes by two general routes: endocytosis and autophagy. Endocytosis is not only responsible for taking up extracellular nutrients, but also is responsible for maintaining the integrity of axons and synapses because neurons are extremely polar cells with especially large volumes of cytoplasm. In contrast, autophagy is responsible for removing unwanted cytosolic proteins and ‘worn out’ organelles (Nixon and Cataldo, 1995, 2006). Alterations in the structure and function of neuronal endolysosomes have been noted in HAND (Gelman et al., 2005; Spector and Zhou, 2008; Zhou and Spector, 2008) and because HIV-1 Tat can accumulate in neuronal endolysosomes, we determined the extent to which HIV-1 Tat disturbed neuronal endolysosome structure and function. We demonstrated that HIV-1 Tat disturbed endolysosomes and inhibited autophagy as indicated by changes to marker proteins. There exist three types of autophagy in cells: macroautophagy, microautophagy and chaperone-mediated autophagy. As the best-studied type of autophagy, macroautophagy includes three stages such as autophagosome membrane origination, autophagosome formation and autolysosome formation. Accordingly, we focused our studies of the effects of HIV-1 Tat on macroautophagy using three markers: LC3 which mediates the initiation process of (Winslow and Rubinsztein, 2008), Atg5 which drives the elongation process of (Mizushima, 2007), and p62 which inhibits autophagy (Ichimura and Komatsu, 2010; Bjorkoy et al., 2005; Pankiv et al., 2007). Our findings suggest that HIV-1 Tat directly affects the morphology and function of endolysosomes and autophagy.
Low pH is critical for the degradation of internalized materials, the trafficking and fusion of endolysosomes and the formation of autophagy (Marshansky and Futai, 2008; Ravikumar et al., 2010; Williamson et al., 2010). Thus, central to the observed changes might be the ability of HIV-1 Tat to elevate endolysosome pH. Our observations of elevated endolysosome pH could help to explain alterations in the digestive capability of endolysosomes as evidenced by decreased specific activities of three different endolysosome enzymes, which results in increased accumulation of internalized material thus altering the structure and size of endolysosomes. In addition, elevation of endolysosome pH could result in alterations in the trafficking and fusion of endolysosomes (Hart and Young, 1991; Saftig and Klumperman, 2009). Furthermore, elevation of endolysosome pH could result in alterations in the fusion of autophagosomes with lysosomes (Kawai et al., 2007) thus inhibiting autophagy. Together, elevation of endolysosome pH could exaggerate neuronal injury and degeneration (Wong and Cuervo, 2010), and contribute directly to HIV-1 Tat-induced neurotoxicity.
Although the underlying mechanisms whereby HIV-1 Tat elevates endolysosome pH are unknown, the arginine-rich domain of HIV-1 Tat between amino acid residues 49 and 57 could be responsible for HIV-1 Tat-induced elevation of endolysosome pH because a series of other arginine-rich peptides including penetratin, an amino acid domain from the Antennapedia protein (sequences 43–58) of Drosophila, a flock house virus coat peptide (sequences 35–49) and oligoarginines (R9) all have the ability to elevate endolysosome pH (L. Hui, X. Chen and J. D. Geiger, unpublished data). It has been shown that most of these arginine-rich peptides have the ability to escape endolysosomes using the high proton gradient (Drin et al., 2003; Potocky et al., 2003; Fischer et al., 2004; Magzoub et al., 2005; Henriques et al., 2006), and here we postulate that a proton-dependent peptide transporter might be present on endolysosome membranes. Such a peptide transporter could transport HIV-1 Tat out of endolysosomes, and during the transporting process protons leak out and endolysosome pH is elevated. Another way that HIV-1 Tat could increase pH is by directly disrupting the membrane integrity of endolysosomes and this is consistent with our observations of increased leakage of Lucifer Yellow dye into cytosol. Consistent with these findings are previous reports that low pH induces the exposure of a highly conserved tryptophan residue and allows the insertion of HIV-1 Tat into endolysosome membranes (Yezid et al., 2009). Furthermore, disrupted endolysosome membrane integrity per se could lead to neuronal dysfunction and ultimately cell death because the increased endolysosome membrane permeability occurs in several models of apoptosis (Roberg and Ollinger, 1998; Turk et al., 2002; Guicciardi et al., 2004; Kroemer and Jaattela, 2005; Kurz et al., 2008) and is an early event in the apoptotic cascade that precedes destabilization of mitochondria and caspase activation (Kroemer and Jaattela, 2005; Kurz et al., 2008). These potential mechanisms warrant further investigation.
Our finding that HIV-1 Tat disturbed the structure and function of endolysosomes in primary cultured neurons prior to any significant increase in HIV-1 Tat-induced neurotoxicity suggests that the effects of HIV-1 Tat on endolysosomes may cause considerable neuronal dysfunction. Further elucidation of the involvement of endolysosomes in HAND might lead to the design of novel therapeutic strategies, including blocking the entry of HIV-1 Tat into endolysosomes and blocking HIV-1 Tat-induced elevation of endolysosome pH.
Footnotes
This work was supported by the National Center for Research Resources [grant no. 2P20RR17699], a component of the National Institutes of Health.
REFERENCES
- Agrawal L, Louboutin JP, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 Tat neurotoxicity: a model of acute and chronic exposure, and neuroprotection by gene delivery of antioxidant enzymes. Neurobiol Dis. 2012;45:657–670. doi: 10.1016/j.nbd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Fox HS. Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy. 2008a;4:963–966. doi: 10.4161/auto.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS One. 2008b;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Banks WA, Robinson SM, Nath A. Permeability of the blood–brain barrier to HIV-1 Tat. Exp Neurol. 2005;193:218–227. doi: 10.1016/j.expneurol.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on Huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- Brun A, Brunk U. Histochemical indications for lysosomal localization of heavy metals in normal rat brain and liver. J Histochem Cytochem. 1970;18:820–827. doi: 10.1177/18.11.820. [DOI] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis. 2007;26:661–670. doi: 10.1016/j.nbd.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron NJ, Quenneville SP, Tremblay JP. Endosome disruption enhances the functional nuclear delivery of Tat-fusion proteins. Biochem Biophys Res Commun. 2004;319:12–20. doi: 10.1016/j.bbrc.2004.04.180. [DOI] [PubMed] [Google Scholar]
- Chen X, Wagener JF, Morgan DH, Hui L, Ghribi O, Geiger JD. Endolysosome mechanisms associated with Alzheimer's disease-like pathology in rabbits ingesting cholesterol-enriched diet. J Alzheimers Dis. 2010;22:1289–1303. doi: 10.3233/JAD-2010-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- Deshmane SL, Mukerjee R, Fan S, Sawaya BE. High-performance capillary electrophoresis for determining HIV-1 Tat protein in neurons. PLoS One. 2011;6:e16148. doi: 10.1371/journal.pone.0016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278:31192–31201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Gamst AC, Capparelli E, Spector SA, Hsia K, Wolfson T, Abramson I, Grant I, McCutchan JA. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54:927–936. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, D'Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-Tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-Tat induces formation of an LRP–PSD–95-NMDAR–nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci USA. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R, Kohler K, Fotin-Mleczek M, Brock R. A stepwise dissection of the intracellular fate of cationic cell-penetrating peptides. J Biol Chem. 2004;279:12625–12635. doi: 10.1074/jbc.M311461200. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Soukup VM, Holzer CE III, Fabian RH, Schuenke KW, Keherly MJ, Richey FJ, Lahart CJ. Potential role for white matter lysosome expansion in HIV-associated dementia. J Acquir Immune Defic Syndr. 2005;39:422–425. doi: 10.1097/01.qai.0000164250.41475.f2. [DOI] [PubMed] [Google Scholar]
- Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23:2881–2890. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- Gutheil WG, Subramanyam M, Flentke GR, Sanford DG, Munoz E, Huber BT, Bachovchin WW. Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): a possible mechanism for Tat's immunosuppressive activity. Proc Natl Acad Sci USA. 1994;91:6594–6598. doi: 10.1073/pnas.91.14.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PD, Young MR. Ammonium chloride, an inhibitor of phagosome-lysosome fusion in macrophages, concurrently induces phagosome–endosome fusion, and opens a novel pathway: studies of a pathogenic mycobacterium and a nonpathogenic yeast. J Exp Med. 1991;174:881–889. doi: 10.1084/jem.174.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 protein Tat. J Neurochem. 1999;73:1363–1374. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin Jr DR, Woods SP, Ake C, Vaida F. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques ST, Melo MN, Castanho MA. Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochem J. 2006;399:1–7. doi: 10.1042/BJ20061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Komatsu M. Selective degradation of p62 by autophagy. Semin Immunopathol. 2010;32:431–436. doi: 10.1007/s00281-010-0220-1. [DOI] [PubMed] [Google Scholar]
- Jeyakumar M, Dwek RA, Butters TD, Platt FM. Storage solutions: treating lysosomal disorders of the brain. Nat Rev Neurosci. 2005;6:713–725. doi: 10.1038/nrn1725. [DOI] [PubMed] [Google Scholar]
- Kawai A, Uchiyama H, Takano S, Nakamura N, Ohkuma S. Autophagosome–lysosome fusion depends on the pH in acidic compartments in CHO cells. Autophagy. 2007;3:154–157. doi: 10.4161/auto.3634. [DOI] [PubMed] [Google Scholar]
- Kidane TZ, Sauble E, Linder MC. Release of iron from ferritin requires lysosomal activity. Am J Physiol Cell Physiol. 2006;291:C445–455. doi: 10.1152/ajpcell.00505.2005. [DOI] [PubMed] [Google Scholar]
- Kim TA, Avraham HK, Koh YH, Jiang S, Park IW, Avraham S. HIV-1 Tat-mediated apoptosis in human brain microvascular endothelial cells. J Immunol. 2003;170:2629–2637. doi: 10.4049/jimmunol.170.5.2629. [DOI] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV Tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Korkotian E, Schwarz A, Pelled D, Schwarzmann G, Segal M, Futerman AH. Elevation of intracellular glucosylceramide levels results in an increase in endoplasmic reticulum density and in functional calcium stores in cultured neurons. J Biol Chem. 1999;274:21673–21678. doi: 10.1074/jbc.274.31.21673. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Kurz T, Terman A, Gustafsson B, Brunk UT. Lysosomes and oxidative stress in aging and apoptosis. Biochim Biophys Acta. 2008;1780:1291–1303. doi: 10.1016/j.bbagen.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 Tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, Churchill GC, Schuchman EH, Galione A, Platt FM. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14:1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- Magzoub M, Pramanik A, Graslund A. Modeling the endosomal escape of cell-penetrating peptides: transmembrane pH gradient driven translocation across phospholipid bilayers. Biochemistry. 2005;44:14890–14897. doi: 10.1021/bi051356w. [DOI] [PubMed] [Google Scholar]
- Mann DA, Frankel AD. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991;10:1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol. 2008;20:415–426. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino JJ, Montes ML, Blanco A, Bustos MJ, Oreja-Guevara C, Bayon C. HIV-1 neuropathogenesis: therapeutic strategies against neuronal loss induced by gp120/Tat glycoprotein in the central nervous system. Rev Neurol. 2011;52:101–111. [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Moreno SN, Docampo R. The role of acidocalcisomes in parasitic protists. J Eukaryot Microbiol. 2009;56:208–213. doi: 10.1111/j.1550-7408.2009.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47:186–194. [PubMed] [Google Scholar]
- Nixon RA, Cataldo AM. The endosomal–lysosomal system of neurons: new roles. Trends Neurosci. 1995;18:489–496. doi: 10.1016/0166-2236(95)92772-i. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Cataldo AM. Lysosomal system pathways: genes to neurodegeneration in Alzheimer's disease. J Alzheimer's Dis. 2006;9:277–289. doi: 10.3233/jad-2006-9s331. [DOI] [PubMed] [Google Scholar]
- Nuovo GJ, Becker J, Burk MW, Margiotta M, Fuhrer J, Steigbigel RT. In situ detection of PCR-amplified HIV-1 nucleic acids in lymph nodes and peripheral blood in patients with asymptomatic HIV-1 infection and advanced-stage AIDS. J Acquir Immune Defic Syndr. 1994;7:916–923. [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Patel S, Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelled D, Trajkovic-Bodennec S, Lloyd-Evans E, Sidransky E, Schiffmann R, Futerman AH. Enhanced calcium release in the acute neuronopathic form of Gaucher disease. Neurobiol Dis. 2005;18:83–88. doi: 10.1016/j.nbd.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Perez A, Probert AW, Wang KK, Sharmeen L. Evaluation of HIV-1 Tat induced neurotoxicity in rat cortical cell culture. J Neurovirol. 2001;7:1–10. doi: 10.1080/135502801300069575. [DOI] [PubMed] [Google Scholar]
- Perry SW, Norman JP, Litzburg A, Zhang D, Dewhurst S, Gelbard HA. HIV-1 transactivator of transcription protein induces mitochondrial hyperpolarization and synaptic stress leading to apoptosis. J Immunol. 2005;174:4333–4344. doi: 10.4049/jimmunol.174.7.4333. [DOI] [PubMed] [Google Scholar]
- Pivtoraiko VN, Stone SL, Roth KA, Shacka JJ. Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death. Antioxid Redox Signal. 2009;11:481–496. doi: 10.1089/ars.2008.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocky TB, Menon AK, Gellman SH. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J Biol Chem. 2003;278:50188–50194. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- Roberg K, Ollinger K. Oxidative stress causes relocation of the lysosomal enzyme cathepsin D with ensuing apoptosis in neonatal rat cardiomyocytes. Am J Pathol. 1998;152:1151–1156. [PMC free article] [PubMed] [Google Scholar]
- Sabatier JM, Vives E, Mabrouk K, Benjouad A, Rochat H, Duval A, Hue B, Bahraoui E. Evidence for neurotoxic activity of Tat from human immunodeficiency virus type 1. J Virol. 1991;65:961–967. doi: 10.1128/jvi.65.2.961-967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- Spector SA, Zhou D. Autophagy: an overlooked mechanism of HIV-1 pathogenesis and neuroAIDS? Autophagy. 2008;4:704–706. doi: 10.4161/auto.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk B, Stoka V, Rozman-Pungercar J, Cirman T, Droga-Mazovec G, Oresic K, Turk V. Apoptotic pathways: involvement of lysosomal proteases. Biol Chem. 2002;383:1035–1044. doi: 10.1515/BC.2002.112. [DOI] [PubMed] [Google Scholar]
- Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 Tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- Van Grol J, Subauste C, Andrade RM, Fujinaga K, Nelson J, Subauste CS. HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3. PLoS One. 2010;5:e11733. doi: 10.1371/journal.pone.0011733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendeville A, Rayne F, Bonhoure A, Bettache N, Montcourrier P, Beaumelle B. HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Mol Biol Cell. 2004;15:2347–2360. doi: 10.1091/mbc.E03-12-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Wallace DR. HIV neurotoxicity: potential therapeutic interventions. J Biomed Biotechnol. 2006;2006:65741. doi: 10.1155/JBB/2006/65741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks BS, Lieberman DM, Johnson B, Roque E, Green M, Loewenstein P, Oldfield EH, Kleinman HK. Neurotoxicity of the human immunodeficiency virus type 1 Tat transactivator to PC12 cells requires the Tat amino acid 49–58 basic domain. J Neurosci Res. 1995;42:34–40. doi: 10.1002/jnr.490420105. [DOI] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Williamson WR, Wang D, Haberman AS, Hiesinger PR. A dual function of V0-ATPase a1 provides an endolysosomal degradation mechanism in Drosophila melanogaster photoreceptors. J Cell Biol. 2010;189:885–899. doi: 10.1083/jcb.201003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow AR, Rubinsztein DC. Autophagy in neurodegeneration and development. Biochim Biophys Acta. 2008;1782:723–729. doi: 10.1016/j.bbadis.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E, Cuervo AM. Autophagy gone away in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci USA. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AJ, Chandswangbhuvana D, Margol L, Glabe CG. Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Aβ1–42 pathogenesis. J Neurosci Res. 1998;52:691–698. doi: 10.1002/(SICI)1097-4547(19980615)52:6<691::AID-JNR8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Yezid H, Konate K, Debaisieux S, Bonhoure A, Beaumelle B. Mechanism for HIV-1 Tat insertion into the endosome membrane. J Biol Chem. 2009;284:22736–22746. doi: 10.1074/jbc.M109.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. AIDS. 2008;22:695–699. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Masliah E, Spector SA. Autophagy is increased in post-mortem brains of persons with HIV-1-associated encephalitis. J Infect Dis. 2011;203:1647–1657. doi: 10.1093/infdis/jir163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Vergote D, Pardo C, Noorbakhsh F, McArthur JC, Hollenberg MD, Overall CM, Power C. CXCR3 activation by lentivirus infection suppresses neuronal autophagy: neuroprotective effects of antiretroviral therapy. FASEB J. 2009;23:2928–2941. doi: 10.1096/fj.08-128819. [DOI] [PMC free article] [PubMed] [Google Scholar]