Abstract
Background
We investigated whether luciferase immunoprecipitation systems (LIPS) can be the basis for a more rapid, specific, and standardized assay for the diagnosis of Strongyloides stercoralis infection.
Methods
A LIPS assay was developed based on immunoglobulin (Ig) G or IgG4 antibody to a recombinant Strongyloides antigen (NIE) and was compared with an NIE enzyme-linked immunosorbent assay (ELISA). A second antigen, S. stercoralis immunoreactive antigen (SsIR), was tested alone and in combination with NIE. The assays were tested using serum samples from patients with parasitologically proven S. stercoralis or filarial infections and from healthy, uninfected control subjects.
Results
The NIE LIPS assay based on IgG antibody easily differentiated between S. stercoralis–infected and uninfected patients (P < .0001) and demonstrated improved specificity compared with the NIE ELISA (100% vs. 95%). Serum from filaria-infected patients did not cross-react when tested with the NIE LIPS assay. When SsIR was used in combination with NIE in the LIPS format, sensitivity and specificity improved to 100%, with a 7-fold difference between positive and negative values. No advantage was found in using a LIPS assay based on IgG4. At post-treatment follow-up, a significant decline in antibody titers was detected using the NIE ELISA (P < .0017) and the NIE LIPS assay (P < .0001).
Conclusions
LIPS addresses several limitations of current ELISAs and represents a major advance in the diagnosis of S. stercoralis infection.
Although Strongyloides stercoralis often causes chronic and clinically asymptomatic infection, the number of Strongyloides parasites can increase substantially in immunocompromised hosts, leading to hyperinfection, dissemination, and death if unrecognized [1]. Early recognition of S. stercoralis infection is challenging because of scanty and intermittent excretion of larvae in chronically infected immunocompetent hosts [2]. Despite this, the mainstay of diagnostic testing for S. stercoralis infection has been stool examination, although more recently ELISAs have been used to measure antibodies to crude larval antigen. Serologic approaches to the diagnosis of S. stercoralis infection, however, have been hampered by poor specificity, reliance on crude parasite extracts, and the time needed to perform the assays [3–5].
A major drawback to ELISA-based diagnosis of S. stercoralis infection has been a reliance on crude antigen that must be prepared by isolating worms from the feces of heavily infected patients or experimental animals. Thus, investigators have turned to recombinant antigens, which can be purified easily and produced in large amounts [6]. Indeed, a 31-kDa recombinant antigen (termed NIE) derived from an S. stercoralis L3 cDNA library provided the basis for an ELISA that approaches the sensitivity and specificity of the crude antigen–based ELISA [5]. An attractive alternative to ELISA-based methods, luciferase immunoprecipitation systems (LIPS), has been successfully applied to the characterization of antibody responses to Pneumocystis jirovecii, HIV, and hepatitis viruses [7]. LIPS is a relatively straightforward technology for identifying serum containing antigen-specific antibodies and for generating quantitative antibody response profiles. Briefly, this approach involves fusion of a protein antigen to the enzyme reporter Renilla luciferase (Ruc), expression of the Ruc-antigen fusion in mammalian COS cells, immobilization of the Ruc-antigen fusion on protein A/G beads, and quantitation of antigen-specific antibody by the addition of a coelenterazine substrate and the measurement of light production [8]. This assay represents a major improvement over ELISA technology in that it produces a low background often with a 7-log dynamic range, thereby generating values with substantial separation between negative and positive antibody responses. The low background and high signal seen in the LIPS method can be credited, in part, to the use of a solution-phase immunoprecipitation assay that allows detection of a large number of conformational epitopes. The use of mammalian cells produces antigens free of contaminating bacterial proteins. An additional advantage of LIPS is that, once the Ruc-antigen constructs are made, relatively little time is needed to perform the assay.
Recently, we reported the use of Ruc-antigen fusion proteins, produced in COS1 monkey kidney cells, in an immunoprecipitation assay to measure human antibody responses to tumor-associated proteins [8] and to a variety of infectious agents [7]. In this study, we have broadened the application of LIPS to the diagnosis and monitoring of S. stercoralis infection. To develop a more rapid, specific, and standardized assay, we first developed a LIPS assay based on IgG (or IgG4) antibody to NIE and compared it with a standard NIE ELISA. Our data, generated using serum samples from S. stercoralis–infected patients, filaria-infected patients, and uninfected control subjects, demonstrated the clear benefit of the LIPS method. Moreover, when a second antigen, S. stercoralis immunoreactive antigen (SsIR), was used in combination with NIE in the LIPS format, we found an even greater degree of sensitivity and specificity. Finally, we assessed the ability of LIPS to evaluate the success of treatment in the follow-up of S. stercoralis–infected patients.
METHODS
Patient populations
Informed consent was obtained from all patients. Protocols involving adult human subjects were approved by the Institutional Review Board at the National Institutes of Health. Serum samples were obtained from patients (n = 31) within 1 month after S. stercoralis larvae were found in their stools. Healthy, uninfected control subjects (n = 36) had no history of travel to an area of endemicity. Filaria-infected patients (n = 39) had proven loiasis or onchocerciasis with at least 1 stool sample negative for S. stercoralis larvae. Six of these patients were coinfected with other intestinal helminths; 4 patients had Trichuris trichiura and 2 had hookworm, as determined by stool examination. Serum samples from a separate group of patients with parasitologically proven S. stercoralis infection (n = 36) were obtained before and after definitive treatment (single- or 2-dose ivermectin or 3 days of thiabendazole), as described elsewhere [9]. The mean duration of follow-up for these patients was 17.47 months (range, 6 –32 months).
Antigens and plasmids
Purified recombinant NIE and NIE glycerol stocks were prepared as described elsewhere [5]. Full-length NIE was amplified and cloned into pCR 2.1 TOPO (Invitrogen). A plasmid containing SsIR was constructed by GenScript Corporation, with codon bias optimized for mammalian cell expression. The nucleotide sequence for the humanized SsIR protein fragment has been deposited in GenBank with the accession number EU285565, based on the protein sequence with accession number AAB97359. Strongyloides cDNA plasmid clones were amplified by polymerase chain reaction (PCR) using specific linker-primer adapters. The primer adapter sequences used to clone each protein or protein fragment were as follows: for NIE, 5′-GAGGGATCCAATTCGGCACGAGATGAAAAT-3′ and 5′-GAGCTCGAGTTATTGTTTACGTTGTAAAAC-3′; for SsIR, 5′-GAGGGATCCAACTCCGCCCGCGTGGA-3′ and 5′-GAGCTCGAGTCAATCCCGCTCGTCCTC-3′.
Generation of Ruc-antigen fusion constructs
pREN2, a mammalian Ruc expression vector, was used to generate all plasmids. PCR products for NIE and SsIR were restricted with BamHI and XhoI and were ligated into BamHI-XhoI– cut pREN2. The resulting pREN2 expression vector was prepared using a Qiagen Midi Kit. Automated DNA sequencing was used to confirm the integrity of the DNA constructs. The cloned DNA fragment for SsIR polypeptide encoded aa 1–156 (AAB97359), whereas the NIE polypeptide encoded aa 1– 88 (AAD46493) followed by a short (11-aa) peptide sequence (PNCLMNKINIE) before the stop codon. With our standard transfection conditions, the Ruc-NIE fusion protein was highly expressed, yielding >1 × 108 luminometer units (LU) per 100-mm2 plate of COS1 cells (enough for ~100 –200 serologic tests). The Ruc-SsIR fusion protein was expressed to a higher level, yielding >5 × 1010 LU per 100-mm2 plate of COS1 cells (enough for ~5000 serologic tests).
LIPS analysis
Ruc-antigen fusion extracts were prepared from transfected COS1 cells as described elsewhere [8]. For the present study, the immunoprecipitation assay was adapted to a 96-well plate format to handle large numbers of serum samples. A master plate of patient serum samples was first generated by diluting patient serum 1:10 in assay buffer A (20 mmol/L Tris [pH 7.5], 150 mmol/L NaCl, 5 mmol/L MgCl2, and 1% Triton X-100) in a 96-well polypropylene microtiter plate (Nunc). For evaluating antibody titers by LIPS assay, the following were added to each well of a second polypropylene plate in which the assay was conducted: 40 μL of buffer A, 10 μL of diluted human serum (1 μL equivalent), and 50 μL of 1 × 107 LU of Ruc-antigen from the COS1 cell extract diluted in buffer A. This plate, containing 100 μL of the antigen-antibody reaction mixture, was then incubated on a rotary shaker for 1 h at room temperature. Next, 7 μL of a 30% suspension of protein A/G beads in PBS (Pierce Biotechnology) was added to the bottom of a 96-well filter HTS plate (Millipore). The 100-μL antigen-antibody reaction mixture from each microtiter well was then transferred to the well of the filter plate, and this plate was further incubated for 1 h at room temperature on a rotary shaker. The filter plate containing the mixture was then applied to a vacuum manifold. The retained protein A/G beads were washed under suction twice with 0.2 mL of buffer A, 8 times with 0.1 mL of buffer A, and twice with 0.1 mL of PBS. After the final washing, the plate was blotted and LU determined in a Berthold LB 960 Centro microplate luminometer using a coelenterazine substrate mix (Promega). For experiments using the cocktail of NIE and SsIR, 1 × 107 LU inputs of each antigen were added together and processed in the manner described above. All data presented in LU were obtained from the average of at least 2 independent experiments and corrected for background by subtracting the values for beads incubated with COS1 cell extract but no serum.
For anti-IgG4 determinations, the same protocol was adapted so that anti-IgG4 antibody beads were used instead of protein A/G beads. The anti-IgG4 antibody beads were generated by combining 10 mg of an anti-IgG4 monoclonal antibody with UltraLink preactivated beads (Pierce Biotechnology), as described by the manufacturer. Coupling efficiency was >90%.
ELISA
For measurement of anti-NIE antibodies by ELISA, 96-well flat-bottom Immulon 4 microtiter plates (Thermo Lab-Systems) were coated with recombinant NIE in coating buffer (1.0 mol/L NaHCO3 and 1.0 mol/L Na2CO3 [pH 9.6]) at a concentration of 0.125 μg−1 and kept at 4°C overnight. The wells were blocked with 5% bovine serum albumin after being washed 4 times in 0.5% PBS-Tween. Plates were incubated for 1 h at 37°C. A standard positive IgG curve based on serial 2-fold dilutions of serum from one patient was used. Patient serum samples (at a dilution of 1:100) and standards were tested in triplicate. After a 1-h incubation at 37°C and washing, goat anti– human IgG conjugated with alkaline phosphatase (Jackson Immunoresearch) was added at a concentration of 0.6 μg/mL. Plates were measured at 410 nm in an ELISA reader (SpectraMax Plus; Molecular Devices) after being washed and developed with p-nitrophenol phosphate substrate. For IgG4 measurement by ELISA, the protocol described above was used, except that mouse anti– human IgG4 (clone HP6023) was added at a 1:5000 dilution, and goat anti–mouse alkaline phosphatase (Jackson Immunoresearch) was used at a concentration of 1.2 μg/mL. A standard positive IgG4 curve was developed using a pool of 7 patient serum samples with high IgG4 values, from which the other values were interpolated.
Statistical analysis
Groups were compared using the non-parametric Mann-Whitney U test (2-tailed) for continuous variables and Fisher’s exact test for categorical variables (2-tailed). Spearman rank correlation coefficients were used to determine the relationship between ELISA and LIPS values.
RESULTS
Comparisons between LIPS assay and ELISA for the detection of an anti-NIE response
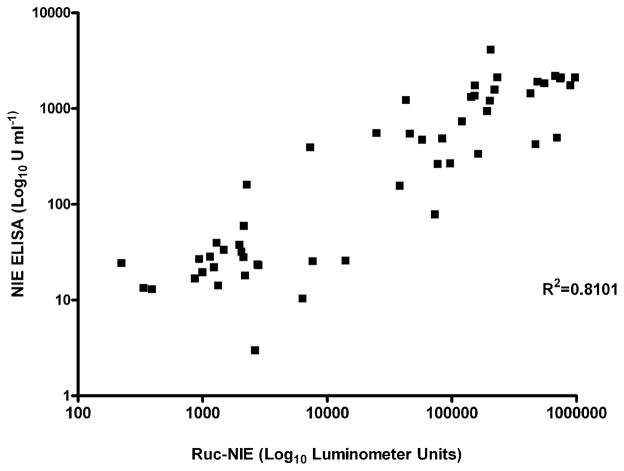
Both the NIE LIPS assay and the NIE ELISA accurately differentiated between S. stercoralis–infected patients and uninfected control subjects. As shown in figure 1, a significant difference was found between values for S. stercoralis–infected patients and control subjects by both LIPS assay (P < .0001) and ELISA (P < .0001). The relative values correlated well between the 2 assays (R2 = 0.8101; P < .0001). These results are shown in figure 2. LIPS assay produced a dynamic range of values (for S. stercoralis–infected patients, 7292.5–1,320,302 LU; for control subjects, 0 –14,013.5 LU), in contrast to the ELISA (for S. stercoralis–infected patients, 78.80 –4117.59 U/mL−1; for control subjects, 2.99 –160.9 U/mL−1).
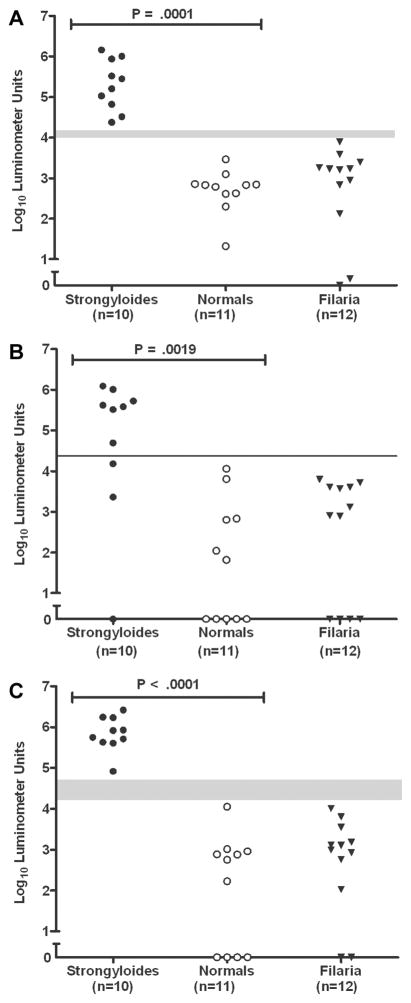
Figure 1.
Distribution of levels of antibody to NIE, as determined by ELISA (A) and luciferase immunoprecipitation systems (LIPS) assay (B), in patients infected with Strongyloides stercoralis, filaria, or helminths and in healthy, uninfected control subjects (normals). The 6 helminth-infected patients were a subset of the filaria-infected group. C, Levels of antibody to S. stercoralis immunoreactive antigen, as determined by LIPS assay. A significant difference between S. stercoralis–infected and uninfected control subjects was found for all 3 assays (P < .0001; Mann-Whitney U test). No significant difference was found between the control subjects and the filaria-infected patients for the NIE LIPS assay (P = .6909; Mann-Whitney U test).
Figure 2.
Anti-NIE titers determined by ELISA vs. luciferase immunoprecipitation systems assay for Strongyloides stercoralis–infected patients and healthy, uninfected control subjects (R 2 = 0.8101; P < .0001). Ruc, Renilla luciferase.
For the NIE LIPS assay, only 1 S. stercoralis–infected patient had a titer that fell below the arbitrary cutoff value of 24,772 LU (figure 1B). By comparison, for the NIE ELISA, the titer for 1 S. stercoralis–infected patient fell below and the titer for 1 control subject fell above the arbitrary cutoff of 156 U/mL−1 (figure 1A). This finding is reflected in the calculated specificity of 100% for the NIE LIPS assay, compared with 94.5% for the NIE ELISA (table 1).
Table 1.
Comparative performance of ELISA and the luciferase immunoprecipitation systems (LIPS) method for the diagnosis of Strongyloides stercoralis infection.
| Test | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| ELISA | ||||
| IgG NIE | 97 | 95 | 88 | 99 |
| IgG4 NIE | 45 | 100 | 100 | 64 |
| LIPS assay | ||||
| Ruc-NIE | ||||
| IgG | 97 | 100 | 100 | 99 |
| IgG4 | 87 | 100 | 100 | 90 |
| Ruc-SsIR | 97 | 97 | 94 | 99 |
| Ruc-NIE/Ruc-SsIR | 100 | 100 | 100 | 100 |
NOTE. Data are percentages. NPV, negative predictive value; PPV, positive predictive value; Ruc, Renilla luciferase; SsIR, S. stercoralis immunoreactive antigen.
Ability of LIPS assay to distinguish between S. stercoralis infection and other infections that typically cross-react in ELISAs
Because systemic helminth infections (particularly filarial infections) have been shown to cross-react in the current S. stercoralis ELISA [3, 4], we included serum samples from 39 filaria-infected individuals in our comparison. For the NIE LIPS assay, no filaria-infected patients had values above the cutoff of 24,772 LU (figure 1B). By contrast, 2 filaria-infected patients had values above the cutoff of 156 U/mL−1 for the NIE ELISA (figure 1A). Although a statistically significant difference between the titers for control subjects and those for the filaria-infected patients was found for the NIE ELISA (P = .0080), no such difference was found for the NIE LIPS assay (P = .6909). In addition, in all 6 patients with non-Strongyloides intestinal helminth infections, titers fell below the positive cutoff for both the NIE ELISA and the NIE LIPS assay.
Detection of anti-NIE IgG4 by ELISA and LIPS assay
Both the NIE ELISA and the NIE LIPS assay were modified to detect IgG4 antibody. The IgG4 NIE ELISA demonstrated slightly improved specificity compared with the IgG NIE ELISA (for IgG4, 100%; for IgG, 97%). For the NIE LIPS assay, no advantage was found in using an IgG4-based test rather than an IgG-based test, because the IgG4 assay demonstrated lower sensitivity (for IgG4, 87%; for IgG, 97%) with no change in specificity (tables 1 and 2).
Table 2.
Positive IgG and IgG4 antibody responses to NIE among Strongyloides stercoralis–infected, filaria-infected, or uninfected individuals.
| Patient group | IgG
|
IgG4
|
||
|---|---|---|---|---|
| LIPS assay | ELISA | LIPS assay | ELISA | |
| S. stercoralis–infected patients | 30/31 (97) | 30/31 (97) | 27/31 (87) | 13/29 (45) |
|
| ||||
| Filaria-infected patients | 0/44 (0) | 4/44 (9) | 0/43 (0) | 0/37 (0) |
|
| ||||
| Healthy, uninfected control subjects | 0/36 (0) | 1/35 (3) | 0/36 (0) | 0/29 (0) |
NOTE. Data are proportion (%) of subjects with a positive response. LIPS, luciferase immunoprecipitation systems.
Anti-SsIR antibodies for the diagnosis and monitoring of S. stercoralis infection
Although the NIE LIPS assay performed extremely well, 1 S. stercoralis–infected patient had a titer below the cutoff value of 24,772 LU (figure 1B). To maximize the difference between positive and negative antibody responses, additional testing was performed using a Ruc construct fused to another antigen, SsIR, chosen because of its presumed highly abundant expression in the expressed sequence tag Strongyloides databases. A significant difference was found between anti-SsIR levels for patients with S. stercoralis infection and control subjects (P < .0001) (figure 1C). It is interesting to note that, for uninfected or filaria-infected individuals, the SsIR LIPS assay had more completely nonreactive antibody titers (32/74) than did the NIE LIPS assay (0/74) (P < .0001). Although the calculated sensitivity for the SsIR LIPS assay using an arbitrary cutoff of 23,565 LU was the same as that for the NIE LIPS assay (97%), the specificity of the SsIR LIPS assay was slightly lower (97%) than that of the NIE LIPS assay (100%). These values are shown in table 1.
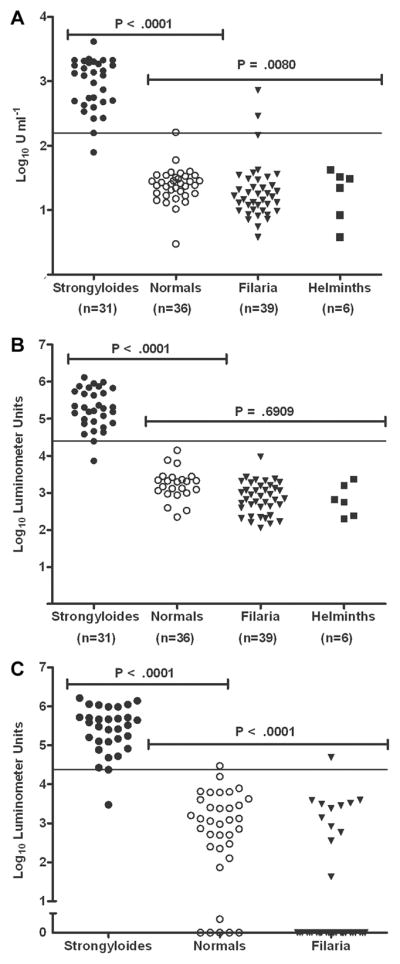
We next tested both fusion proteins together using a Ruc-SsIR and Ruc-NIE multiplex system on a select group of 33 patient serum samples with either inconsistent values by the individual assays or values close to our designated cutoffs for either assay (figure 3). The combination Ruc-NIE/Ruc-SsIR test unequivocally differentiated infected from uninfected individuals (P < .0001), with a 7-fold difference between the lowest titer in a S. stercoralis–infected patient (82,431 LU) and the highest titer in a control subject (11,202 LU) (figure 3C). Indeed, no significant difference between filaria-infected and uninfected individuals was found in this multiplexed system (P = .0994). For Ruc-NIE performed in the same group of patients in parallel (figure 3A), a 4-fold difference was found between infected (23,738 LU) and uninfected (6309 LU) individuals. Additionally, no significant cross-reactivity was observed with serum samples from filaria-infected patients.
Figure 3.
Distribution of levels of antibodies to Ruc-NIE (A), Ruc–SsIR (B), and Ruc-SsIR/Ruc-NIE (C) in 33 patients with inconsistent results between the Ruc-NIE– and Ruc-SsIR– based tests or with borderline results close to the designated cutoff (Ruc, Renilla luciferase; SsIR, Strongyloides stercoralis immunoreactive antigen). The solid line in panel B indicates the cutoff for the SsIR luciferase immunoprecipitation systems assay (23,565 luminometer units [LU]); the gray bars in panels A and C highlight the separation between positive and negative values. The difference between the lowest antibody level for an S. stercoralis–infected patient and the highest for a healthy, uninfected control subject (normals) was 71,229 LU for the Ruc-NIE/Ruc-SsIR combination and 15,919 LU for Ruc-NIE alone. P values were calculated using the Mann-Whitney U test.
Characterization of the serologic response after treatment by LIPS assay and ELISA
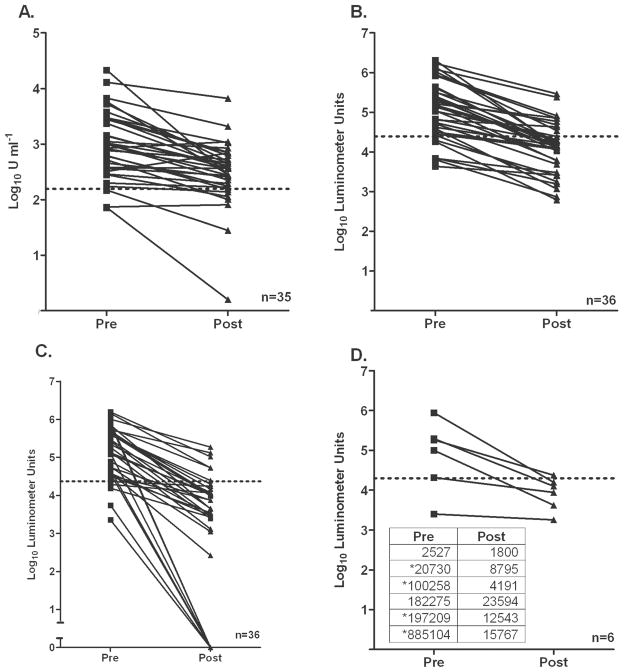
Pre- and posttreatment antibody levels were evaluated using both the LIPS method and ELISA. Although all 3 assays could detect a significant decline in antibody levels with treatment (for ELISA, P < .0017; for the NIE LIPS assay, P < .0001; for the SsIR LIPS assay, P < .0001), it is interesting to note that reversion from positive to negative serologic status after treatment was found more frequently with the NIE LIPS assay than with the NIE ELISA (figure 4). Antibody titers detected by the NIE LIPS assay became negative after treatment for 58% (21/36) of patients, compared with 17% (6/35) of patients with the NIE ELISA (P = .0213). Use of Ruc-SsIR appeared to improve the performance of LIPS slightly, because 69% (25/36) of patients had antibody titers that fell below the cutoff of 23,565 LU; however, no significant difference was found between the NIE LIPS assay and the SsIR LIPS assay in this regard (P = .7074). We tested the Ruc-SsIR/Ruc-NIE combination assay in 6 patients who did not demonstrate seroreversion with either the NIE LIPS or SsIR LIPS assay and found that positive titers became negative after therapy in 4 of 6 patients, using a cutoff of 20,000 LU (figure 4D). One patient had a low pretreatment titer (2527 LU). The other patient’s posttreatment titer declined from 182,275 to 23,594 LU but did not fall below the cutoff of 20,000 LU.
Figure 4.
Antibody titers before and after treatment for Strongyloides stercoralis infection, as determined by NIE ELISA (A), the NIE luciferase immunoprecipitation systems (LIPS) assay (B), the S. stercoralis immunoreactive antigen (SsIR) LIPS assay (C), and the SsIR/NIE LIPS assay (D). A significant difference was found between antibody levels at baseline and at the last follow-up time point (for the NIE ELISA, P < .0017; for the NIE LIPS assay, P < .0001; for the SsIR LIPS assay, P < .0001; for the SsIR/NIE LIPS assay, P < .0001; Mann-Whitney U test). Dotted lines indicate arbitrary cutoff values determined for each assay. Seroreversion was seen for 58% (21/36) of patients by the NIE LIPS assay, on the basis of a cutoff of 24,772 LU; 17% (6/35) of patients by the NIE ELISA, on the basis of a cutoff of 156 U mL−1; and 69% (25/36) of patients by the Ruc-SsIR assay, on the basis of a cutoff of 23,565 LU. The mean duration of follow-up for 36 patients was 17.47 months (range, 6 –32 months). A combination assay, Ruc-SsIR/Ruc-NIE, was tested in 6 patients who did not serorevert by either the NIE LIPS or SsIR LIPS assay (D). Four of 6 selected patients tested by the Ruc-SsIR/Ruc-NIE assay demonstrated seroreversion on the basis of a cutoff value of 20,000 LU. Pre- and posttreatment titers for each patient are shown in the inset in panel D. Asterisks indicate samples demonstrating seroreversion.
DISCUSSION
The LIPS format represents a novel approach to the diagnosis of S. stercoralis infection. This study establishes that the NIE LIPS assay, similar to an optimized NIE ELISA, can reliably distinguish patients with parasitologically proven S. stercoralis infection from healthy, uninfected control subjects (P < .0001). Both the NIE ELISA and the NIE LIPS assay have equivalent sensitivity (97%), although the NIE LIPS assay has slightly better specificity (100% vs. 95%). When a second antigen, SsIR, is used in combination with NIE in the LIPS format, the sensitivity and specificity of the assay improves to 100%, with a substantial 7-fold difference between positive and negative titers (figure 2C). This observation was based on serum samples from 33 patients who had borderline or inconsistent antibody titers when either antigen was used alone. When the SsIR/NIE multiplexed assay was tested using a separate group of 6 S. stercoralis–infected patients, however, 1 patient had a low pretreatment titer (figure 4D). It is interesting to note that this patient also had low titers across both individual assays (the NIE LIPS assay and the SsIR LIPS assay). Although the immune status of the patient is unknown, it could be postulated that this patient had not mounted any antibody response to infection. The observation that the LIPS method performs better when 2 antigens are used supports the notion that different epitopes may be immunogenic to varying degrees among individuals [10]. It also raises the possibility that a single test could profile antibody responses to antigens from a variety of infectious agents. Such a test would be of great value in identifying individuals with polyparasitism or with multiple infections that cross phyla [11, 12].
The NIE LIPS assay demonstrates improved specificity compared with the NIE ELISA (100% vs. 95%), with no cross-reaction in serum samples from filaria-infected patients. Cross-reactivity has been a major problem with serologic tests for S. stercoralis infection in patients from areas in which filarial infection is endemic [3, 4]. One reason for the improved performance of the LIPS method may be the choice of antigen, because NIE has been shown elsewhere to perform well in this regard [5]. The significant dynamic range of the LIPS technique further accentuates differences between antibody titers in filaria-infected and S. stercoralis–infected patients.
The development of an accurate and rapid test that relies on recombinant antigens represents a major improvement over the currently available diagnostic tests for S. stercoralis infection. The preparation of crude antigen for serologic tests is often time consuming and depends on the excretion of larvae from heavily infected humans or experimental animals. Recombinant antigens, unlike the crude antigen in use, can be purified easily and produced in large amounts.
Previous studies have indicated that the IgG antibody response detected by the standard ELISA declines (but remains positive) within 6 months of treatment [13]. We sought to characterize whether the LIPS method can be used to monitor changes in antibody response over time (mean follow-up, 17 months). A significant decline in antibody titers was found with all 3 assays (for ELISA, P < .0017; for the NIE LIPS assay, P < .0001; for the SsIR LIPS assay, P < .0001) as well as with the SsIR/NIE multiplexed assay (P < .0001). Titers determined by the SsIR LIPS assay became seronegative after treatment in up to 70% of patients, suggesting that the greater sensitivity afforded by the LIPS method allows us to observe changes in antibody titers that were undetectable with the conventional ELISA. This finding can be attributed to the low background and high signal seen with LIPS, which result from the use of a solution-phase immunoprecipitation assay and the use of more-pure antigen preparations. Whether the ability of the LIPS method to detect changes in antibody titers after treatment lends itself to being used as a test of cure, however, remains to be determined.
Although the IgG4 response has been characterized as a specific marker of S. stercoralis infection [14], we did not find any advantage to using the IgG4-based NIE LIPS assay. The IgG4-based NIE LIPS assay compromised sensitivity (87% for IgG4 vs. 97% for IgG) while maintaining the 100% specificity found with the IgG-based NIE LIPS assay.
A LIPS assay can be performed more rapidly than a conventional ELISA (in <2.5 h). A faster version of this assay can be performed in <2 min by eliminating the incubation step (P.D.B., unpublished data). This version has been applied to the diagnosis of other infections with accurate results (P.D.B., unpublished data), although it was not tested in this study. Rapid diagnostic testing in a hospital setting can be helpful in critically ill patients with suspected hyper-infection syndrome. Further studies may be useful in exploring the usefulness and accuracy of this format.
Because some investigators have suggested that the accuracy of some serologic assays may be limited in immunocompromised patients [15, 16], it is important to note that our study did not focus specifically on this population. Further studies may be useful in clarifying the performance of the LIPS method in immunocompromised hosts.
In conclusion, LIPS represents a major advance in the diagnosis of S. stercoralis infection because it addresses several outstanding limitations associated with the ELISA format. LIPS can identify S. stercoralis–infected patients more rapidly and accurately than an ELISA. Because of the extraordinary dynamic range of LIPS, there is a substantial difference between positive and negative values, which enables 100% sensitivity and specificity. Unlike most serologic assays to date, LIPS based on NIE antigen does not cross-react with serum from filaria-infected patients. Finally, the recombinant antigens on which this assay is based can be purified easily and produced in large amounts, unlike the crude antigen currently in use.
Acknowledgments
We thank Joseph Kubofcik and National Institute of Allergy and Infectious Diseases intramural editor Brenda Rae Marshall for their assistance.
Financial support: Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH); National Institute of Dental and Craniofacial Research, NIH.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: American Society of Tropical Medicine and Hygiene conference, Philadelphia, 12 November 2007 (abstract 135).
References
- 1.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–17. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato Y, Kobayashi J, Toma H, Shiroma Y. Efficacy of stool examination for detection of Strongyloides infection. Am J Trop Med Hyg. 1995;53:248–50. doi: 10.4269/ajtmh.1995.53.248. [DOI] [PubMed] [Google Scholar]
- 3.Conway DJ, Atkins NS, Lillywhite JE, et al. Immunodiagnosis of Strongyloides stercoralis infection: a method for increasing the specificity of the indirect ELISA. Trans R Soc Trop Med Hyg. 1993;87:173– 6. doi: 10.1016/0035-9203(93)90477-8. [DOI] [PubMed] [Google Scholar]
- 4.Muck AE, Pires ML, Lammie PJ. Influence of infection with non-filarial helminths on the specificity of serological assays for antifilarial immunoglobulin G4. Trans R Soc Trop Med Hyg. 2003;97:88–90. doi: 10.1016/s0035-9203(03)90033-2. [DOI] [PubMed] [Google Scholar]
- 5.Ravi V, Ramachandran S, Thompson RW, Andersen JF, Neva FA. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Mol Biochem Parasitol. 2002;125:73– 81. doi: 10.1016/s0166-6851(02)00214-1. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran S, Thompson RW, Gam AA, Neva FA. Recombinant cDNA clones for immunodiagnosis of strongyloidiasis. J Infect Dis. 1998;177:196–203. doi: 10.1086/513817. [DOI] [PubMed] [Google Scholar]
- 7.Burbelo PD, Ching KH, Mattson TL, Light JS, Bishop LR, Kovacs JA. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems) Biochem Biophys Res Commun. 2007;352:889–95. doi: 10.1016/j.bbrc.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 8.Burbelo PD, Goldman R, Mattson TL. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 2005;5:22. doi: 10.1186/1472-6750-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gann PH, Neva FA, Gam AA. A randomized trial of single and two-dose ivermectin versus thiabendazole for treatment of strongyloidiasis. J Infect Dis. 1994;169:1076–9. doi: 10.1093/infdis/169.5.1076. [DOI] [PubMed] [Google Scholar]
- 10.Thomas V, Ogunba EO, Fabiyi A. The application and limitations of immunodiagnostic techniques in parasitic infections. Afr J Med Sci. 1978;7:107–12. [PubMed] [Google Scholar]
- 11.van Regenmortel MH. The concept and operational definition of protein epitopes. Philos Trans R Soc Lond B Biol Sci. 1989;323:451– 66. doi: 10.1098/rstb.1989.0023. [DOI] [PubMed] [Google Scholar]
- 12.Buck AA, Anderson RI, MacRae AA. Epidemiology of poly-parasitism. III. Effects on the diagnostic capacity of immunological tests. Tropenmed Parasitol. 1978;29:145–55. [PubMed] [Google Scholar]
- 13.Karunajeewa H, Kelly H, Leslie D, et al. Parasite-specific IgG response and peripheral blood eosinophil count following albendazole treatment for presumed chronic strongyloidiasis. J Travel Med. 2006;13:84–91. doi: 10.1111/j.1708-8305.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- 14.Genta RM, Lillibridge JP. Prominence of IgG4 antibodies in the human responses to Strongyloides stercoralis infection. J Infect Dis. 1989;160:692–9. doi: 10.1093/infdis/160.4.692. [DOI] [PubMed] [Google Scholar]
- 15.Schaffel R, Nucci M, Carvalho E, et al. The value of an immunoenzymatic test (enzyme-linked immunosorbent assay) for the diagnosis of strongyloidiasis in patients immunosuppressed by hematologic malignancies. Am J Trop Med Hyg. 2001;65:346–50. doi: 10.4269/ajtmh.2001.65.346. [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Fattah MM, Nasr ME, Yousef SM, et al. Efficacy of ELISA in diagnosis of strongyloidiasis among the immune-compromised patients. J Egypt Soc Parasitol. 1995;25:491–7. [PubMed] [Google Scholar]