Abstract
Collagen fibrils are nanostructured biological cables essential to the structural integrity of many of our tissues. Consequently, understanding the structural basis of their robust mechanical properties is of great interest. Here we present what to our knowledge is a novel mode of collagen fibril disruption that provides new insights into both the structure and mechanics of native collagen fibrils. Using enzyme probes for denatured collagen and scanning electron microscopy, we show that mechanically overloading collagen fibrils from bovine tail tendons causes them to undergo a sequential, two-stage, selective molecular failure process. Denatured collagen molecules—meaning molecules with a reduced degree of time-averaged helicity compared to those packed in undamaged fibrils—were first created within kinks that developed at discrete, repeating locations along the length of fibrils. There, collagen denaturation within the kinks was concentrated within certain subfibrils. Additional denatured molecules were then created along the surface of some disrupted fibrils. The heterogeneity of the disruption within fibrils suggests that either mechanical load is not carried equally by a fibril's subcomponents or that the subcomponents do not possess homogenous mechanical properties. Meanwhile, the creation of denatured collagen molecules, which necessarily involves the energy intensive breaking of intramolecular hydrogen bonds, provides a physical basis for the toughness of collagen fibrils.
Introduction
Collagen fibrils are hierarchically structured biological cables (Fig. 1) that serve as the principal tensile elements of our tendons, ligaments, cartilage, skin, bones, arteries, heart valves, intestines, intervertebral disks, and more. Their component collagen molecules are slender triple-helical proteins stabilized by extensive interchain hydrogen bonding (1–3). To form fibrils, collagen molecules first pack in a quasicrystalline or liquid-crystal lattice (4,5), and are then covalently bound to each other by cross-links (6,7). The intermediate structures produced called subfibrils, which are akin to the individual strands of a wire, are then bundled in a straight or helical arrangement to form a fibril (8–12). The fibrils are precision-constructed with respect to both the crystalline packing of molecules and lateral registration of subfibrils (8,11). This produces 67.0-nm-long (13,14) striations in both the fibrils and subfibrils: a distance known as the D-period (15,16).
Figure 1.
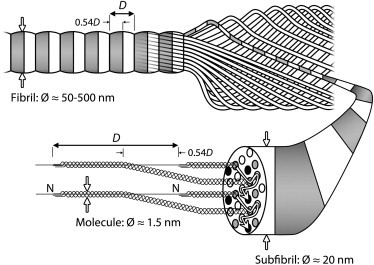
Structural hierarchy of the collagen fibril. (Counterclockwise from bottom left) Beginning with their N-termini in position 1, collagen molecules transition through four subsequent lattice positions as they pass through the gap region (length 0.54D) of successive D-periods (14) (as indicated by the arrows on the cross-sectional surface of the enlarged subfibril). Cross-links are formed between adjacent molecules in positions 1 (shaded circles) and 5 (solid circles). Subfibrils, which appear striated due to the axial and lateral consistency of this molecular packing, wind together at an angle of ≤10° to their long axis to form a tendon fibril (10), while remaining in-register laterally and thus conferring their striated appearance to their parent fibril (11). Note that the striations have been removed from all subfibrils except for the enlarged one for clarity. The symbol Ø indicates diameter.
Collagen fibrils are not unique to humans; rather, they have been supporting life for >600,000,000 years, and are now an integral component of nearly all members of the animal kingdom (17,18). Conservation of the collagen fibrils' architecture during the course of evolution is readily appreciated by the consistency of the axial packing of collagen molecules across different animal species. For example, the mean D-periods measured for native human, calf, lamb, and rat tendon fibrils are 67.0, 67.0, 66.9, and 67.0 nm, respectively (19,20). For skin fibrils, where the apparent D-period is reduced due to a more helical arrangement of the subfibrils (10,19), a similar consistency is also seen across species (19,21,22).
The ubiquitous and long-standing nature of collagen fibrils is a testament to their mechanical performance. Collagen fibrils provide tissues with extensibility at low stiffness by virtue of their crimp (23,24), allowing our joints the range of motion we enjoy and our intestines the ability to expand markedly when required. Cross-linking between molecules gives fibrils high tensile strength at higher extensions (25–27), protecting our muscles by preventing joint hyperextension, and our arteries from aneurysm. Finally, fibrils are capable of absorbing large amounts of strain energy—∼10 times more than steel wire (28,29)—preventing brittle fracture of overloaded tissues and allowing, instead, subrupture events such as sprains and strains.
Owing to their great importance, the mechanisms underlying the mechanics of collagen fibrils have received much attention. During the initial phase of stretching within the “toe” region of the stress-strain curve, collagen fibrils straighten, removing the tissue's crimp, and align with the applied load (23,24,30). As stretching progresses into the “heel” region of the stress-strain curve, the kinks along individual collagen molecules are straightened (31–33). Finally, during the linear region of the stress-strain curve, where the applied deformation and resulting load are directly proportional, the cross-linked telopeptide regions of collagen molecules are stretched as the molecules shift longitudinally relative to their neighbors (20,31).
Unlike the molecular level events within collagen fibrils that take place before plastic deformation (preyield), those that occur from the yield point to tissue failure (postyield) have received relatively little study and remain poorly understood. Some authors have reported that fibrils dissociate into fine substructures when extended beyond their yield point (34–36); however, this response may only be relevant to poorly cross-linked tissues (37). Other authors have reported finding an increase in denatured collagen molecules within overloaded or ruptured tendons (38,39); however, the mechanism by which such denaturation occurs remains unclear and the location of its occurrence with respect to fibril structure remains undefined. As a result of these knowledge gaps, we know very little about the roots of one of collagen's fundamental material properties: toughness.
In this study, we have used enzyme probes combined with scanning electron microscopy (SEM) to explore how mechanical overload alters both the structure of collagen fibrils and the conformation of their constituent molecules. We show that when overloaded, collagen fibrils undergo a mode of distributed partial failure involving molecular denaturation at discrete, repeating locations along a fibril's length. This process, which necessarily involves the energy-intensive rupture of hydrogen bonds, provides what to our knowledge is the first physically derived description of how collagen fibrils absorb strain energy and continue to bear load after yielding. In addition to its mechanical significance, this novel (to our knowledge) mode of fibril failure may play a critical role in our bodies' ability to locate, remove, and replace damaged tissue.
Materials and Methods
Tendons were dissected from the dorsal, proximal region of the tails of steers age 24–36 months, fresh from slaughter for food at a local abattoir. Dissected tendons were immediately placed between layers of gauze moistened with phosphate-buffered saline (PBS), double-bagged, and then stored at −86°C.
Ten tendons, dissected from three tails, were used for this study. The 10 tendons were removed from the freezer and allowed to thaw at room temperature in their sealed bags. Each tendon was then vertically suspended by one of its ends and digitally photographed four times. Between each of the four photographs, tendons were rotated 90° about their longitudinal axis. The photographs of each tendon were later used to calculate its mean, elliptical cross-sectional area.
Before the mechanical damage procedure detailed below, a 10-mm length was removed from one end of five randomly selected tendons. These lengths were then divided in half longitudinally. One longitudinal half from each of the five tendons was retained as a control sample.
After the control samples had been prepared, the ends of each tendon sample to be damaged were clamped in the grips of a servo-hydraulic materials testing system. The distance between the grips was increased until the load cell, positioned in series with one of the grips, first measured an increase in load. The distance between the grips was then measured; this was recorded as the initial gauge length of the tendon sample. Gauge lengths ranged from 31 to 49 mm. Each tendon was then stretched at a strain rate of 0.25%/s to an elongation equivalent to a final strain of 45%. During this procedure, tendons were misted with PBS to prevent dehydration. All tendons stretched in this manner experienced a frank rupture: in some cases extending through the entire thickness of the tendon, and in other cases only extending part way.
Using a scalpel, a 10-mm length was removed from each of the ruptured tendons. Each such length began at one of the ruptured edges and extended longitudinally into macroscopically undamaged tissue (Fig. 2). These lengths were then divided in half longitudinally, yielding paired samples. Each sample was placed in an individual specimen tube filled with PBS and left overnight at 4°C.
Figure 2.
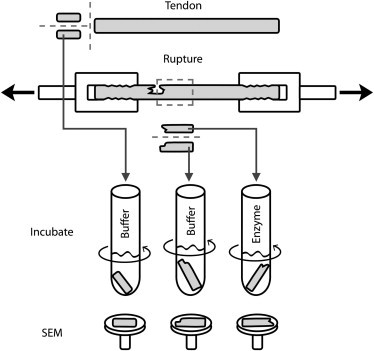
Three types of samples were prepared from bovine tail tendons: 1), control samples, taken before mechanical damage and subsequently exposed only to buffer solution; 2), undigested samples, each containing part of the mechanically induced rupture and subsequently exposed to buffer; and 3), digested samples, each also containing part of the mechanically induced rupture, but exposed instead to trypsin or α-chymotrypsin in buffer to remove any denatured collagen. Inspection of the samples was carried out using SEM. (Dashed lines) Planes along which each tendon was cut.
To assess whether the damaged tendons contained denatured collagen, one of the longitudinal halves from each pair was incubated the following day with either 1000 U acetylated trypsin (n = 5) or 9U α-chymotrypsin TLCK (n = 5) per mg dry collagen (enzymes from Sigma-Aldrich, St. Louis, MO) following the methods of Willett et al. (39). These two serine proteases, each with distinct cleavage specificities (40), are able to digest only nonhelical (denatured) collagen (41–43). These samples formed the digested sample group (n = 10). The mass of dry collagen in each sample was calculated from the sample's wet mass based on previously published biochemical data for bovine tail tendons (39). The incubation was conducted under constant agitation for 8 h at 30°C in 0.1 M Tris-HCl buffer with 20 mM CaCl2. The concentration of enzyme in the digestion solutions was 2580 U/mL for trypsin, and 23.2 U/mL for chymotrypsin. The second longitudinal half from each pair was incubated under the same conditions, but with buffer only. These samples formed the undigested sample group (n = 10). The control samples, taken before mechanical damage, were also incubated with buffer only (n = 5).
After the incubation was complete, the samples in each of the three experimental groups (control; damaged and undigested; damaged and digested) were immediately transferred to new tubes containing 2.5% SEM grade glutaraldehyde (Sigma-Aldrich) in PBS. In preparation for SEM, samples were rinsed in distilled water, dehydrated in graded ethanol, and then critical-point-dried. Samples were mounted on SEM stubs using carbon paste, and then coated with gold-palladium. The longitudinal cut surface of each sample was then inspected using a model No. S-4700 SEM (Hitachi, Chula Vista, CA) operating at 3 kV, 15 μA, using magnifications up to 70,000×. Measurements on the resulting digital images were made using the software ImageJ (Ver. 1.44; National Institutes of Health, Bethesda, MD).
Results
SEM examination of our specimens revealed that the tendon samples that were mechanically ruptured but undigested contained regions of regularly kinked fibrils, a feature not found in the control tendons (Fig. 3, A versus B). The paired samples, which were mechanically ruptured and then digested to remove any denatured collagen, also contained regions of regularly kinked fibrils; however, these fibrils were missing material within the kinks. That is, whereas the kinks appeared as solid masses in the undigested samples, in the digested samples they were composed of subfibrils separated by voids indicating the existence and subsequent removal of denatured collagen (arrow in Fig. 3, C versus D). No morphological differences between the tendon samples digested with trypsin and those digested α-chymotrypsin were found. Occasionally groups of damaged fibrils were found wound in a tight ball, suggesting elastic recoil after breaking (Fig. 4).
Figure 3.
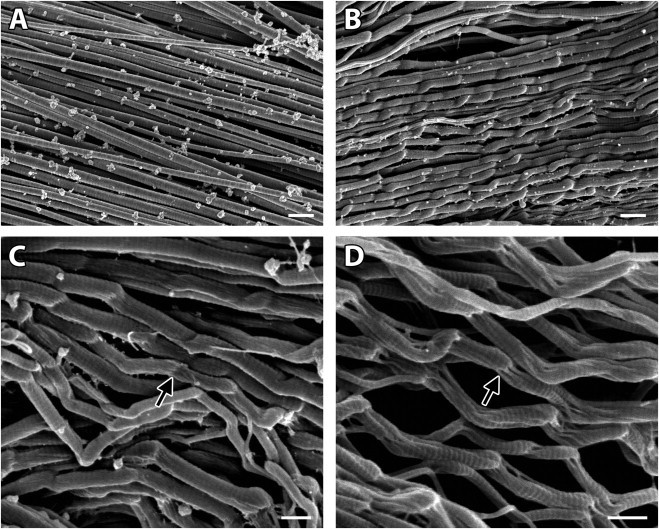
Mechanical overload caused molecular denaturation at discrete, repeating sites along the length of collagen fibrils. Whereas the fibrils in the control tendon samples were straight (A), fibrils with regularly repeating kinks were found in the tendons that were mechanically ruptured (B). Whereas the kinked regions in the undigested damaged fibrils appeared to be solid masses (arrow in C), the kinked regions in enzymatically digested samples contained voids (arrow in D), indicating that denatured collagen was created in these regions and had subsequently been digested. With the exception of the mechanical rupture procedure, both the control tendon samples (A) and the overloaded, undigested tendon samples (B and C) were subjected to the same treatments. Similarly, with the exception of the presence of enzymes during digestion procedure, both the overloaded, undigested tendon samples (B and C) and the overloaded, digested tendon samples (D) were subjected to the same treatments. Bars 500 nm in panel A (20,000×) and panel B (20,000×); bars 300 nm in panel C (30,100×) and panel D (40,100×). The tendon sample shown in panel D was incubated with α-chymotrypsin.
Figure 4.
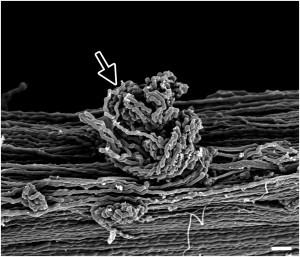
Elastic recoil of damaged collagen fibrils. On occasion, tightly wound balls of damaged collagen fibrils were found in the mechanically ruptured tendons, as shown here in an undigested sample (arrow), indicative of elastic recoil upon rupture of the fibrils. Bar, 1 μm.
To provide a quantitative description of the damaged fibrils, the distance between successive kinks on individual fibrils (here termed the “repeat distance”) was measured using images of the undigested samples (Fig. 5). While the resulting histogram revealed a wide distribution of repeat distances, the distances reached a limiting minimum value of ∼300 nm. Further, although a wide range of repeat distances was found in the population of all measured fibrils, the repeat distances were considerably less variable within a given fibril. For instance, the coefficient of variation for all repeat distances measured (n = 368) was 0.41; however, the mean value of the coefficients of variation for repeat distances within individual fibrils (n = 62) was 0.12.
Figure 5.
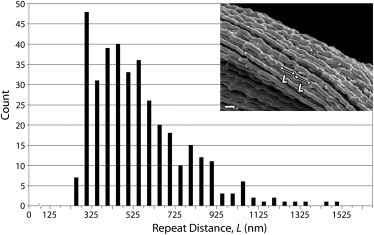
Repeat distance between successive kinks (L) on damaged collagen fibrils reached a limiting minimum value of ∼300 nm. Three-hundred-sixty-eight measurements of L were made along 62 fibrils using SEM images of the ruptured, undigested samples. (Inset) Micrograph, bar 400 nm, illustrates two successive measurements on a single fibril.
Although it was obvious that our damaged and digested collagen fibrils were missing material within their kinked regions, closer inspection showed that in some cases material from the fibril's surface between successive kinks had also been digested. Although the D-period striations, a sign of undisrupted molecular packing, were visible between the kinks along many damaged—but undigested—fibrils (arrow 1 in Fig. 6 A), others had a fuzzy surface texture, obscuring or completely hiding the fibrils' D-banding (arrow 2 and arrow 3 in Fig. 6 A). By contrast, the surface of the digested, damaged fibrils always displayed D-period striations. The fact that the fuzzy surface texture was never seen on the digested fibrils demonstrates its composition: a layer of denatured collagen produced via mechanical damage. Furthermore, in some cases, subfibrils displaying D-banding and running at angles ranging from 0.0 to 20.1° (mean 10.7 ± 6.3°; 12 measured fibrils) relative to their fibrils' long axis were visible on the fibril's surface between kinks following digestion. These subfibrils were apparently left in relief when the surrounding denatured collagen was digested (arrows in Fig. 6 B).
Figure 6.
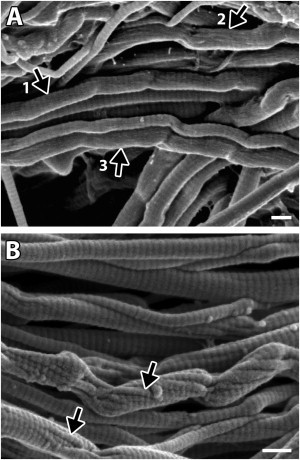
Disruption to some fibrils also involved a secondary mechanism causing denaturation of their surface molecules. (A) On the surface of fibrils between successive kinks in the ruptured, undigested tendon samples, the D-period bands were either visible (arrow 1), partly obscured (arrow 2), or completely obscured and replaced by a fuzzy surface texture (arrow 3). (B) In contrast, the D-period was always visible along surface of fibrils in the digested samples, indicating that the fuzzy surface texture in the undigested samples was created by a layer of denatured collagen that had subsequently been digested in these enzymatically treated samples. Additionally, subfibrils could be seen along some of these fibrils (arrows in B), having been left in relief when adjacent, denatured subfibrils were digested. Bars, 200 nm. The tendon sample shown in B was incubated with trypsin.
In addition to the two main features of progressive fibril disruption mentioned thus far, kink-based denaturation and surface denaturation, some fibrils also displayed marked distortion (fibril marked by arrows in Fig. 7, A and B). The surface subfibrils of these fibrils appeared to be oriented obliquely to the subfibrils beneath them, i.e., to those making up the fibril's interior between kinks (arrow 1 in Fig. 7, A and B). The differing orientations of their subcomponents gave these fibrils the appearance of having two distinct structural elements: a core and surrounding sheath. Remarkably, the heterogeneity of damage to these fibrils was so great that, despite having many denatured subfibrils (arrow 2 in Fig. 7, A versus B), D-banding could be seen along some of the subfibrils that remained after digestion (arrow 3 in Fig. 7 B).
Figure 7.
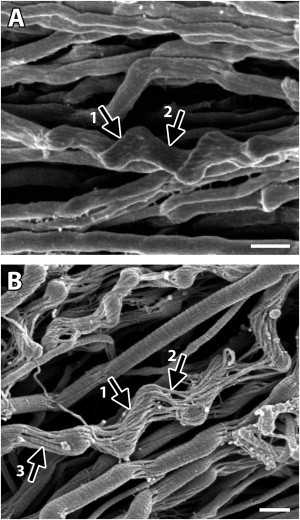
Mechanical overload did not affect all subfibrils of a fibril equally. In addition to possessing kinks and surface denaturation, some fibrils were also highly distorted, having surface subfibrils that were oriented obliquely to those beneath them (arrow 1 in A and B). Comparison between a damaged, undigested fibril (arrow 2 in panel A) and a similarly damaged but digested fibril (arrow 2 in B) shows that even in severely disrupted fibrils a significant number of subfibrils remained undigested, indicating that their molecules had retained their native triple-helical conformation. Further, in some cases D-banding could be seen along these subfibrils (arrow 3 in panel B), indicating that the molecules had retained their quasicrystalline packing arrangement. Bars 300 nm. The tendon sample shown in panel B was incubated with trypsin.
Zones of damaged (kinked) fibrils were found throughout the mechanically ruptured tendon samples. The continuity of these zones was more limited laterally than longitudinally: that is, whereas damaged fibrils often neighbored normal fibrils, a zone of damaged fibrils could be followed longitudinally for distances on the order of millimeters. Individual damaged fibrils could be followed for several hundred microns, a distance limited only by our inability to track them after their departure from the sample's surface. Although the zones of damage often incorporated a large number of neighboring fibrils and extended over relatively long lengths, it should be pointed out that, qualitatively, the total surface area of these zones was small compared to the total surface area of the samples, consistent with earlier findings from our laboratory (39).
Discussion
The mechanically induced morphologic change to collagen fibrils documented in this study, characterized by repeating sites of kink-based molecular denaturation occurring along a fibril's length, represents what we consider an entirely novel mode of disruption that has neither been previously observed nor predicted. These findings provide what to our knowledge are new insights into both the native structure of collagen fibrils, as well as the structural mechanisms underlying a tendon's mechanics after yielding, particularly in regard to their toughness.
It is important to clarify what we mean by “molecular denaturation” in this article. When used in the context of collagen, molecular denaturation is used to describe the uncoiling of the collagen molecule's triple-helix. This uncoiling may be permanent as in the heat-activated transition of collagen to gelatin, or temporary as in the uncoiling and recoiling of local regions of the helix that occurs in solubilized collagen (often referred to as “micro-unfolding”). When present in a solution as monomers, micro-unfolding events allow collagen molecules to be readily cleaved by trypsin and α-chymotrypsin (43,44). In stark contrast to this situation, collagen molecules that are natively packed in a fibril undergo few micro-unfolding events, making them highly resistant to cleavage via trypsin and α-chymotrypsin. For example, Willett et al. (39) (on whose work we based the enzyme concentrations used for digestion in this study) found that only ∼1.5% of a tendon's collagen was solubilized after exposure to trypsin for 48 h at 37°C (an 8 h digestion at 30°C was used in this study). The value for α-chymotrypsin under these same conditions was even smaller, ∼0.5%. In this study, molecules from the mechanically damaged regions of fibrils were easily removed by trypsin and α-chymotrypsin. This indicates that, over the course of the enzymatic digestion, the removed molecules had spent significantly more time in an uncoiled state than did adjacent molecules from undamaged regions of the fibrils. Thus, the results presented here do not indicate that collagen molecules were permanently uncoiled, but this possibility is not excluded. Consequently, our use of the term “denaturation” in this study means a time-averaged decrease in helicity relative to the molecule's native state.
Few authors have previously studied the morphologic response of fiber-forming collagen structures to mechanical overload at the fibril level. Of those that have, most report the dissociation of fibrils into their subfibrillar components (34–36), similar to that occurring as the result of chemical- or enzyme-induced fibril dissociation (11,12). An exception to these studies are the early observations of Nemetschek et al. (37), who reported a change in the mode of fibril disruption from dissociation to kinking with increasing tendon age. Although the results of Nemetschek et al. (37) do not indicate the presence of regularly repeating kinks or of denatured molecules along the length of fibrils, it is likely that the kink-based mode of fibril disruption shown here also results from our use of a more-mature tissue model.
Using atomistic modeling, the unwinding of a collagen molecule's triple-helix due to an imposed strain has been predicted to occur (45). The results of the work presented here do not necessarily validate that research group's model. First, it is not clear that their proposed mechanism—hydrogen-bond rupture due to helix straightening—is physically possible within a functional, cross-linked tissue. Indeed, the model in question was conducted using an 8-nm-long segment of a single 300-nm-long collagen molecule. Second, if the mechanism proposed by those authors is indeed possible, there is no evidence that that mechanism, helix straightening, is responsible for the collagen denaturation documented here.
Mechanically induced collagen denaturation has also been previously explored using physical experimentation, although to a very limited extent. In 1975, Steven et al. (38) reported observing denatured collagen at the ruptured ends of tendons. Their observations were conducted at a much larger scale, that of the tendon fiber or fascicle, and are not directly comparable to ours. Using chemical assays, Willett et al. (39) subsequently confirmed that overloading tendons can alter the native helicity of collagen molecules, and suggested that the underlying mechanism involved the molecule's thermally labile domain and a polymer-in-a-box concept. Again, there is no evidence from our study that the collagen denaturation we document here was achieved via the mechanism advocated by Willett et al. (39).
Although we can neither confirm a previously proposed denaturation mechanism, nor inform of an alternative mechanism, our results illustrate an important and basic point: we do not yet fully understand the native structure of collagen fibrils. For mechanical overload to produce repeating kinks along the length of a fibril, it would seem that the fibril must contain repeating regions of structural irregularity. Here, it is important to emphasize that we are speaking of repetition with a much larger period than that occurring within a fibril's 67-nm D-banding (46,47). To the authors' knowledge, no such irregularity has previously been reported. Similarly, because enzymatic digestion produced discrete voids within these kinks, the overload damage must be restricted to a subset of subfibrils within each fibril. This fact is best appreciated by viewing Fig. 7, which shows severely damaged fibrils. Again, to the authors' knowledge no previous study has indicated that the subfibrils making up a fibril either undergo uneven load-sharing or possess heterogeneous strengths. Both of these realizations make the (currently) accepted model of uniformly structured collagen fibrils less convincing.
During the elastic stretching of tendons, strain energy may be dissipated as heat via viscous mechanisms (48) including the movement of water molecules (49,50), and the shearing of collagen molecules both between (51,52) and within fibrils (20,31). The shearing of proteoglycans, particularly decorin that links neighboring fibrils (53,54), probably also dissipates some energy, but this appears to be negligible compared to the other mechanisms involved (55,56). In contrast to these preyield events, the physical mechanisms contributing to a soft tissue's toughness postyield appear to have received little research attention, and remain undefined. This is quite unlike the situation for bone, where numerous toughness studies have been conducted (57–61). To the authors' knowledge, only one research group has studied the molecular level processes that occur during the failure of unmineralized collagen fibrils; however, the results, garnered by modeling fibrils devoid of cross-links, do not apply to functional tissue (62).
Here, based on our ultrastructural observations of overloaded tendons, we offer what to our knowledge are the first insights into the physical mechanisms underlying the postyield toughness of unmineralized collagen fibrils. Taken together, our finding that mechanical overload produces repeating kinks in collagen fibrils, combined with the observation that trypsin and chymotrypsin selectively digest material within these kinks, provides direct evidence of a toughness mechanism at play in collagen fibrils: selective molecular failure. Treatment of the damaged collagen fibrils using trypsin or chymotrypsin could only produce voids by removing collagen molecules that had undergone a loss of helicity to some extent. This reduction in helicity may result from forced-induced uncoiling and/or the splitting of subfibrils, thereby providing the lateral space necessary for microunfolding to occur. In either case, the damaged collagen fibrils had absorbed strain energy during the overload procedure resulting in molecular denaturation at discrete, repeating locations along their length. Further, these kink-based sites of molecular denaturation could be followed along a fibril's length for considerable distances, highlighting, qualitatively, the amount of deformational energy the individual fibrils had absorbed. It is important to note that the kink structures presented here were seen after elastic recoil had occurred: when the sample ruptured and/or when the sample was unloaded. Therefore, the number of observed kinks on a given fibril is a record of the denaturation events that occurred during the sample's loading history.
In addition to denatured collagen molecules within the kinked regions of fibrils, we have also found denatured molecules along the surface of fibrils. Our finding that denatured collagen is only created along the surface of some of the kinked fibrils, and never along the surface of unkinked fibrils, strongly suggests that the surface denaturation process occurs secondary to kink formation. Additionally, our observation that the D-banding along some fibrils was visible beneath a surface layer of denatured collagen, and that subfibrils appeared in relief after digestion, suggests that the denaturation process between kinks progresses from a fibril's surface inward. These results suggest that collagen fibrils possess not just one, but two, distinct and likely sequential mechanisms to absorb strain energy by generating denatured collagen via selective molecular failure: the first associated with the formation of kinks, and the second associated with the fibril's surface molecules.
We have deliberately referred to the mechanism for collagen toughness described above as occurring via absorption rather than dissipation of strain energy. Indeed, the physical mechanisms underlying a material's toughness are traditionally said to dissipate strain energy (63). For example, when stretching a ductile alloy it would be common to say that strain energy is dissipated via the creation of dislocations or new surfaces. Here, the term “dissipation” is used because the strain energy expended is not elastically recoverable despite being stored within the now-restructured alloy; it is not meant to indicate that energy has been transferred from the alloy to the surrounding environment. Conversely, in biomechanics we often use the term dissipation when describing the conversion of strain energy into heat due to a tissue's viscous components. To avoid any confusion with this phenomenon, we have chosen to use the term “energy absorption” here: collagen molecules may absorb strain energy via the breakage of hydrogen bonds, which results in a loss of helical structure and increases the internal energy of individual α-chains.
It is worthwhile outlining the potential biologic significance of our findings. The healing or remodeling process in a damaged collagenous structure requires: 1), identification of the damaged structure, 2), removal of the damaged components at least, and 3), replacement of that structure. The periodic regions of kink-based molecular denaturation observed in our study extend along individual fibrils for hundreds of microns. Rather than failing in a localized all-or-nothing manner by ductile or brittle fracture, this longitudinally distributed smart mode of fibril failure may aid in, or even be critical to, healing processes postfailure. It is possible that cell-collagen binding sites required for the detection of damaged collagen are hidden within the collagen molecule's native triple-helix, becoming available for binding only after molecular denaturation has occurred. Native collagen fibrils under low tensile loads are understood to be highly resistant to proteolytic cleavage by collagenase (64–66). In contrast, collagen that had been denatured as part of the damage process might be more efficiently removed from the extracellular matrix. Lastly, it is intriguing to consider that, within each damaged fibril, the portion of remaining, undamaged subfibrils may serve as a scaffold upon which to conduct the rebuilding process for the replacement structure.
The mechanical properties of collagen fibrils in vivo are not static: cross-linking of collagen molecules is known to vary according to both the functional mechanical demands of a tissue (67), and the animal/patient's age (68). Although the results of this study, conducted using young adult bovine tail tendons, should be translatable to any mammalian tendon owing to the evolutionary preservation of the collagen fibril's architecture, it remains to be determined how our results may vary with the type of cross-links present, collagen type, and developmental or healing status. Although such experiments are necessary from a basic science standpoint, they will also be pertinent to the understanding and/or implications of various pathologies. For instance, increased cross-linking of collagen fibrils by the advanced glycation end product pentosidine has recently been linked to a decrease in the toughness of cortical bone (58). An accumulation of pentosidine causing a reduction in toughness, as may occur in elderly persons or those with diabetes (69), may have its origins in alterations to the native failure mode of collagen molecules, possibly impacting the biologic repair processes mentioned above.
Finally, this study may be relevant to tissue engineers. If collagen denaturation in response to mechanical overload proves to be a biologically significant event, developing a functional tissue will not only involve matching the native tissue's strength, but also its molecular level failure characteristics. This same consideration would also apply to the exogenous cross-linking of native tissues. In this respect, the results presented here, when combined with similar future studies, may provide a valuable benchmark.
Conclusions
Mechanically overloading bovine tail tendons produced collagen fibrils with regularly repeating regions of denatured collagen molecules. This form of damage, involving molecular failure at select repeating locations along a fibril's length, has not been described previously in the collagen literature and, to the authors' knowledge, is quite unlike any other known material failure mechanism. Although further experimentation is required to determine the universality of this fibril damage mechanism with respect to both species and age, it is fascinating to consider that this unique mode of failure may have evolved to serve both mechanical and biologic functions. Creating the observed pattern of disruption, which necessitates unwinding the triple-helix of collagen molecules at repeating locations along a fibril's length, would require a net energy input, providing a physical basis for the mechanical toughness of fibrils. Meanwhile, rather than simply failing locally at the location of maximum stress, a longitudinally distributed mode of failure, presumably caused by some currently undocumented structural heterogeneity, might provide a great increase in the number of potential sites for damage detection by cells. After removal of the denatured collagen, which is produced heterogeneously across a fibril's width, the undamaged subfibrils may provide a scaffold on which to conduct the rebuilding process.
Acknowledgments
The authors thank Dalhousie's Institute for Research in Materials, particularly Pat Scallion for assistance with the scanning electron microscopy.
S.P.V. is grateful for the postdoctoral funding provided by the Killam Trust. This work was supported by a grant to J.M.L. from the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Bella J., Berman H.M. Crystallographic evidence for C-α-H···O=C hydrogen bonds in a collagen triple helix. J. Mol. Biol. 1996;264:734–742. doi: 10.1006/jmbi.1996.0673. [DOI] [PubMed] [Google Scholar]
- 2.Bella J., Eaton M., Berman H.M. Crystal-structure and molecular-structure of a collagen-like peptide at 1.9 Å resolution. Science. 1994;266:75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 3.Shoulders M.D., Raines R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hukins D.W.L., Woodhead-Galloway J. Collagen fibrils as examples of smectic a biological fibers. Mol. Crystals Liq. Crystals. 1977;41:33–39. [Google Scholar]
- 5.Woodhead-Galloway J., Machin P.A. Modern theories of liquids and diffuse equatorial x-ray-scattering from collagen. Acta Crystallogr. A. 1976;32:368–372. [Google Scholar]
- 6.Eyre D.R., Wu J.J. Collagen cross-links. In: Brinckmann J., Notbohm H., Muller P.K., editors. Collagen. Springer; New York: 2005. pp. 207–229. [Google Scholar]
- 7.Rich A., Crick F.H.C. The molecular structure of collagen. J. Mol. Biol. 1961;3:483–506. doi: 10.1016/s0022-2836(61)80016-8. [DOI] [PubMed] [Google Scholar]
- 8.Hashizume H., Hitomi J., Ushiki T. Growth of collagen fibrils produced by human osteosarcoma cells: high-resolution scanning electron microscopy. Arch. Histol. Cytol. 1999;62:327–335. doi: 10.1679/aohc.62.327. [DOI] [PubMed] [Google Scholar]
- 9.Lillie J.H., MacCallum D.K., Occhino J.C. Collagen structure: evidence for a helical organization of the collagen fibril. J. Ultrastruct. Res. 1977;2:134–143. doi: 10.1016/s0022-5320(77)90025-9. [DOI] [PubMed] [Google Scholar]
- 10.Reale E., Benazzo F., Ruggeri A. Differences in the microfibrillar arrangement of collagen fibrils. Distribution and possible significance. J. Submicrosc. Cytol. 1981;13:135–143. [PubMed] [Google Scholar]
- 11.Scott J.E. Proteoglycan:collagen interactions and subfibrillar structure in collagen fibrils. Implications in the development and ageing of connective tissues. J. Anat. 1990;169:23–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao T., Weinhold P.S., Dahners L.E. Some observations on the subfibrillar structure of collagen fibrils as noted during treatment with NKISK and cathepsin G with mechanical agitation. J. Electron Microsc. (Tokyo) 2011;60:177–182. doi: 10.1093/jmicro/dfr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle B.B., Hukins D.W., Woodhead-Galloway J. Origins and implications of the D stagger in collagen. Biochem. Biophys. Res. Commun. 1974;60:858–864. doi: 10.1016/0006-291x(74)90320-9. [DOI] [PubMed] [Google Scholar]
- 14.Orgel J.P., Irving T.C., Wess T.J. Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. USA. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle B.B., Hulmes D.J., Woodhead-Galloway J. Axially projected collagen structures. Proc. R. Soc. Lond. B Biol. Sci. 1974;187:37–46. doi: 10.1098/rspb.1974.0059. [DOI] [PubMed] [Google Scholar]
- 16.Hodge A.J., Schmitt F.O. The charge profile of the tropocollagen macromolecule and the packing arrangement in native-type collagen fibrils. Proc. Natl. Acad. Sci. USA. 1960;46:186–197. doi: 10.1073/pnas.46.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boot-Handford R.P., Tuckwell D.S. Fibrillar collagen: the key to vertebrate evolution? A tale of molecular incest. Bioessays. 2003;25:142–151. doi: 10.1002/bies.10230. [DOI] [PubMed] [Google Scholar]
- 18.Exposito J.Y., Cluzel C., Lethias C. Evolution of collagens. Anat. Rec. 2002;268:302–316. doi: 10.1002/ar.10162. [DOI] [PubMed] [Google Scholar]
- 19.Brodsky B., Eikenberry E.F., Cassidy K. An unusual collagen periodicity in skin. Biochim. Biophys. Acta. 1980;621:162–166. doi: 10.1016/0005-2795(80)90072-0. [DOI] [PubMed] [Google Scholar]
- 20.Mosler E., Folkhard W., Koch M.H. Stress-induced molecular rearrangement in tendon collagen. J. Mol. Biol. 1985;182:589–596. doi: 10.1016/0022-2836(85)90244-x. [DOI] [PubMed] [Google Scholar]
- 21.Gathercole L.J., Shah J.S., Nave C. Skin tendon differences in collagen D-period are not geometric or stretch-related artifacts. Int. J. Biol. Macromol. 1987;9:181–183. [Google Scholar]
- 22.Stinson R.H., Sweeny P.R. Skin collagen has an unusual D-spacing. Biochim. Biophys. Acta. 1980;621:158–161. doi: 10.1016/0005-2795(80)90071-9. [DOI] [PubMed] [Google Scholar]
- 23.Broom N.D. Simultaneous morphological and stress-strain studies of the fibrous components in wet heart valve leaflet tissue. Connect. Tissue Res. 1978;6:37–50. doi: 10.3109/03008207809152285. [DOI] [PubMed] [Google Scholar]
- 24.Viidik A. Simultaneous mechanical and light microscopic studies of collagen fibers. Z. Anat. Entwicklungsgesch. 1972;136:204–212. doi: 10.1007/BF00519178. [DOI] [PubMed] [Google Scholar]
- 25.Opsahl W., Zeronian H., Riggins R.S. Role of copper in collagen cross-linking and its influence on selected mechanical properties of chick bone and tendon. J. Nutr. 1982;112:708–716. doi: 10.1093/jn/112.4.708. [DOI] [PubMed] [Google Scholar]
- 26.Bailey A.J. Intermediate labile intermolecular crosslinks in collagen fibers. Biochim. Biophys. Acta. 1968;160:447–453. doi: 10.1016/0005-2795(68)90216-x. [DOI] [PubMed] [Google Scholar]
- 27.Haut R.C. The effect of a lathyritic diet on the sensitivity of tendon to strain rate. J. Biomech. Eng. 1985;107:166–174. doi: 10.1115/1.3138537. [DOI] [PubMed] [Google Scholar]
- 28.Bhonsle S., Van Karsen C. Mechanical and fatigue properties of stress relieved type 302 stainless steel wire. J. Mater. Eng. Perform. 1992;1:363–369. [Google Scholar]
- 29.Shen Z.L., Dodge M.R., Eppell S.J. In vitro fracture testing of submicron diameter collagen fibril specimens. Biophys. J. 2010;99:1986–1995. doi: 10.1016/j.bpj.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruehlmann S.B., Matyas J.R., Duncan N.A. ISSLS prize winner. Collagen fibril sliding governs cell mechanics in the annulus fibrosus: an in situ confocal microscopy study of bovine discs. Spine. 2004;29:2612–2620. doi: 10.1097/01.brs.0000146465.05972.56. [DOI] [PubMed] [Google Scholar]
- 31.Folkhard W., Mosler E., Koch M.H.J. Quantitative-analysis of the molecular sliding mechanism in native tendon collagen-time-resolved dynamic studies using synchrotron radiation. Int. J. Biol. Macromol. 1987;9:169–175. [Google Scholar]
- 32.Misof K., Rapp G., Fratzl P. A new molecular model for collagen elasticity based on synchrotron x-ray scattering evidence. Biophys. J. 1997;72:1376–1381. doi: 10.1016/S0006-3495(97)78783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraser R.D.B., MacRae T.P., Suzuki E. Molecular conformation and packing in collagen fibrils. J. Mol. Biol. 1983;167:497–521. doi: 10.1016/s0022-2836(83)80347-7. [DOI] [PubMed] [Google Scholar]
- 34.Kastelic J., Baer E. Deformation in tendon collagen. Symp. Soc. Exp. Biol. 1980;34:397–435. [PubMed] [Google Scholar]
- 35.Knörzer E., Folkhard W., Nemetschek T. New aspects of the etiology of tendon rupture. An analysis of time-resolved dynamic-mechanical measurements using synchrotron radiation. Arch. Orthop. Trauma. Surg. 1986;105:113–120. doi: 10.1007/BF00455845. [DOI] [PubMed] [Google Scholar]
- 36.Torp S., Baer E., Friedman B. Effects of age and of mechanical deformation on the ultrastructure of tendon. Colston Papers. 1975;26:223–250. [Google Scholar]
- 37.Nemetschek T., Jonak R., Riedl H. Kinking deformities in collagen [Knickdeformationen an kollagen] Arch. Orthop. Unfallchir. 1977;89:249–257. doi: 10.1007/BF00416953. [DOI] [PubMed] [Google Scholar]
- 38.Steven F.S., Minns R.J., Finlay J.B. Evidence for the local denaturation of collagen fibrils during the mechanical rupture of human tendons. Injury. 1975;6:317–319. doi: 10.1016/0020-1383(75)90181-3. [DOI] [PubMed] [Google Scholar]
- 39.Willett T.L., Labow R.S., Lee J.M. Increased proteolysis of collagen in an in vitro tensile overload tendon model. Ann. Biomed. Eng. 2007;35:1961–1972. doi: 10.1007/s10439-007-9375-x. [DOI] [PubMed] [Google Scholar]
- 40.Hedstrom L. Serine protease mechanism and specificity. Chem. Rev. 2002;102:4501–4524. doi: 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- 41.Bank R.A., Krikken M., te Koppele J.M. A simplified measurement of degraded collagen in tissues: application in healthy, fibrillated and osteoarthritic cartilage. Matrix Biol. 1997;16:233–243. doi: 10.1016/s0945-053x(97)90012-3. [DOI] [PubMed] [Google Scholar]
- 42.Bruckner P., Prockop D.J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal. Biochem. 1981;110:360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- 43.Ryhänen L., Zaragoza E.J., Uitto J. Conformational stability of type I collagen triple helix: evidence for temporary and local relaxation of the protein conformation using a proteolytic probe. Arch. Biochem. Biophys. 1983;223:562–571. doi: 10.1016/0003-9861(83)90621-5. [DOI] [PubMed] [Google Scholar]
- 44.Kuznetsova N.V., McBride D.J., Leikin S. Changes in thermal stability and microunfolding pattern of collagen helix resulting from the loss of α2(I) chain in osteogenesis imperfecta murine. J. Mol. Biol. 2003;331:191–200. doi: 10.1016/s0022-2836(03)00715-0. [DOI] [PubMed] [Google Scholar]
- 45.Gautieri A., Buehler M.J., Redaelli A. Deformation rate controls elasticity and unfolding pathway of single tropocollagen molecules. J. Mech. Behav. Biomed. Mater. 2009;2:130–137. doi: 10.1016/j.jmbbm.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Barenberg S.A., Filisko F.E., Geil P.H. Ultrastructural deformation of collagen. Connect. Tissue Res. 1978;6:25–35. doi: 10.3109/03008207809152284. [DOI] [PubMed] [Google Scholar]
- 47.Minary-Jolandan M., Yu M.F. Nanomechanical heterogeneity in the gap and overlap regions of type I collagen fibrils with implications for bone heterogeneity. Biomacromolecules. 2009;10:2565–2570. doi: 10.1021/bm900519v. [DOI] [PubMed] [Google Scholar]
- 48.Ker R.F. Dynamic tensile properties of the plantaris tendon of sheep (Ovis aries) J. Exp. Biol. 1981;93:283–302. doi: 10.1242/jeb.93.1.283. [DOI] [PubMed] [Google Scholar]
- 49.Helmer K.G., Nair G., Grigg P. Water movement in tendon in response to a repeated static tensile load using one-dimensional magnetic resonance imaging. J. Biomech. Eng. 2006;128:733–741. doi: 10.1115/1.2244573. [DOI] [PubMed] [Google Scholar]
- 50.Hannafin J.A., Arnoczky S.P. Effect of cyclic and static tensile loading on water content and solute diffusion in canine flexor tendons: an in vitro study. J. Orthop. Res. 1994;12:350–356. doi: 10.1002/jor.1100120307. [DOI] [PubMed] [Google Scholar]
- 51.Cheng V.W.T., Screen H.R.C. The micro-structural strain response of tendon. J. Mater. Sci. 2007;42:8957–8965. [Google Scholar]
- 52.Puxkandl R., Zizak I., Fratzl P. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:191–197. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raspanti M., Viola M., Tira M.E. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J. Struct. Biol. 2008;164:134–139. doi: 10.1016/j.jsb.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Scott J.E., Thomlinson A.M. The structure of interfibrillar proteoglycan bridges (shape modules) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J. Anat. 1998;192:391–405. doi: 10.1046/j.1469-7580.1998.19230391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fessel G., Snedeker J.G. Evidence against proteoglycan mediated collagen fibril load transmission and dynamic viscoelasticity in tendon. Matrix Biol. 2009;28:503–510. doi: 10.1016/j.matbio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Svensson R.B., Hassenkam T., Magnusson S.P. Tensile force transmission in human patellar tendon fascicles is not mediated by glycosaminoglycans. Connect. Tissue Res. 2011;52:415–421. doi: 10.3109/03008207.2010.551569. [DOI] [PubMed] [Google Scholar]
- 57.Fantner G.E., Hassenkam T., Hansma P.K. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 2005;4:612–616. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 58.Nyman J.S., Roy A., Wang X. Age-related factors affecting the postyield energy dissipation of human cortical bone. J. Orthop. Res. 2007;25:646–655. doi: 10.1002/jor.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterlik H., Roschger P., Fratzl P. From brittle to ductile fracture of bone. Nat. Mater. 2006;5:52–55. doi: 10.1038/nmat1545. [DOI] [PubMed] [Google Scholar]
- 60.Tai K., Dao M., Ortiz C. Nanoscale heterogeneity promotes energy dissipation in bone. Nat. Mater. 2007;6:454–462. doi: 10.1038/nmat1911. [DOI] [PubMed] [Google Scholar]
- 61.Zimmermann E.A., Schaible E., Ritchie R.O. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc. Natl. Acad. Sci. USA. 2011;108:14416–14421. doi: 10.1073/pnas.1107966108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buehler M.J. Nature designs tough collagen: explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA. 2006;103:12285–12290. doi: 10.1073/pnas.0603216103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kysar J.W. Energy dissipation mechanisms in ductile fracture. J. Mech. Phys. Solids. 2003;51:795–824. [Google Scholar]
- 64.Flynn B.P., Bhole A.P., Ruberti J.W. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8) PLoS ONE. 2010;5:e12337. doi: 10.1371/journal.pone.0012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nabeshima Y., Grood E.S., Herman J.H. Uniaxial tension inhibits tendon collagen degradation by collagenase in vitro. J. Orthop. Res. 1996;14:123–130. doi: 10.1002/jor.1100140120. [DOI] [PubMed] [Google Scholar]
- 66.Ruberti J.W., Hallab N.J. Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem. Biophys. Res. Commun. 2005;336:483–489. doi: 10.1016/j.bbrc.2005.08.128. [DOI] [PubMed] [Google Scholar]
- 67.Aldous I.G., Veres S.P., Lee J.M. Differences in collagen cross-linking between the four valves of the bovine heart: a possible role in adaptation to mechanical fatigue. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H1898–H1906. doi: 10.1152/ajpheart.01173.2008. [DOI] [PubMed] [Google Scholar]
- 68.Couppé C., Hansen P., Magnusson S.P. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J. Appl. Physiol. 2009;107:880–886. doi: 10.1152/japplphysiol.00291.2009. [DOI] [PubMed] [Google Scholar]
- 69.Sell D.R., Nagaraj R.H., Monnier V.M. Pentosidine: a molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes Metab. Rev. 1991;7:239–251. doi: 10.1002/dmr.5610070404. [DOI] [PubMed] [Google Scholar]


