Abstract
Changes in the local mechanical environment and tissue mechanical properties affect the biological activity of cells and play a key role in a variety of diseases, such as cancer, arthritis, nephropathy, and cardiovascular disease. Constitutive relations have long been used to predict the local mechanical environment within biological tissues and to investigate the relationship between biological responses and mechanical stimuli. Recent constitutive relations for soft tissues consider both material and structural properties by incorporating parameters that describe microstructural organization, such as fiber distributions, fiber angles, fiber crimping, and constituent volume fractions. The recently developed technique of imaging the microstructure of a single artery as it undergoes multiple deformations provides quantitative structural data that can reduce the number of estimated parameters by using parameters that are truly experimentally intractable. Here, we employed nonlinear multiphoton microscopy to quantify collagen fiber organization in mouse carotid arteries and incorporated measured fiber distribution data into structurally motivated constitutive relations. Microscopy results demonstrate that collagen fibers deform in an affine manner over physiologically relevant deformations. The incorporation of measured fiber angle distributions into constitutive relations improves the model's predictive accuracy and does not significantly reduce the goodness of fit. The use of measured structural parameters rather than estimated structural parameters promises to improve the predictive capabilities of the local mechanical environment, and to extend the utility of intravital imaging methods for estimating the mechanical behavior of tissues using in situ structural information.
Introduction
The mechanical constitutive response of biological tissues depends on the structural constituents that make up the tissue. Subtle changes in the content and organization of key structural constituents can have a significant effect on the local mechanical behavior of tissues and play a role in numerous diseases, such as cardiovascular disease (1), cancer (2,3), arthritis (4,5), and nephropathy (6). In the vasculature, changes in tissue mechanical properties through cell-mediated remodeling at multiple length scales play a key role in many physiological and pathophysiological processes (e.g., arterial stiffening with age and the development of aneurysms, hypertension, and atherosclerosis), as well as in the outcomes of many clinical interventions (e.g., restenosis of vascular grafts).
Constitutive relations predict the local mechanical environment within biological tissues and serve as a fundamental tool for elucidating the relationship between biological responses and mechanical stimuli. Most biological tissues are materially nonlinear, viscoelastic (or pseudoelastic), and often heterogeneous and anisotropic. They experience finite deformations and contain residual strains in traction-free states. Accurate prediction of the local stresses within tissues under physiologically relevant loading remains challenging; however, incorporating local tissue microstructure into constitutive relations promises to provide a more physically relevant basis for the quantification of material heterogeneities and improve prediction of the local mechanical environment (7).
Recent constitutive relations for arteries provide a framework for describing both material and structural properties (7–10). These models incorporate parameters that describe microstructural organization, such as fiber distributions (8), fiber orientation (7), fiber crimping (9), and constituent volume fractions (10). Although these constitutive relations often require the estimation of structural parameters along with material parameters, the recently developed technique of imaging the microstructure of a single artery as it undergoes multiple deformations (11–13) has the potential to provide quantitative structural data that would reduce the number of requisite parameters to be estimated.
Our purpose in this study was to quantify collagen fiber angles of mouse carotid arteries under various physiologically relevant loading conditions and to incorporate the resulting fiber distribution data into structurally motivated constitutive relations. We quantified collagen fiber angle distributions in mouse carotid arteries under various biaxial loading conditions. In parallel with cylindrical biaxial biomechanical testing, we used experimentally derived structural data to perform parameter estimation studies. In contrast to previous studies, we incorporated directly measured collagen fiber distributions from mechanically loaded arteries into the constitutive models. We imaged collagen fibers across the adventitia of the artery by detecting the second harmonic generation (SHG) signal (14), and used a fast Fourier transform (FFT) technique to calculate fiber angles through the depth of the vessel wall under each loading condition (15). We found that incorporating experimentally measured fiber angles in a four-fiber model (16) and using an experimentally measured fiber distribution in a distributed fiber model (8) yielded similar goodness-of-fit values. In addition, we also found that representing the measured fiber distribution as a sum of three distributions, each with different material properties, resulted in a better goodness-of fit-than did using the collagen histogram with uniform material properties. When we used mean estimated parameters to predict the mechanical response of arteries, we found that dividing the collagen histogram into three distribution families, with distinct material parameters, described by Gaussian terms yielded the greatest predictive accuracy with the lowest variances between samples compared with other models. We also found that we could reasonably approximate deformations of engaged collagen fibers using affine motion transformations. Establishing the predictive capabilities of constitutive relations based on measured microstructural data may provide a foundation for predicting the mechanical responses of a variety of tissues a priori, given in vivo quantification of structural metrics.
Materials and Methods
Surgical preparation and vessel isolation
Adult male wild-type mice (7.8 ± 0.2 weeks old, n = 6) on the C57-BL6 X 129/SvEv background were euthanized with an overdose of CO2. Under sterile conditions, both common carotid arteries were excised, placed in phosphate-buffered saline solution (PBS), dissected free of perivascular tissue, and mounted on the glass cannulae of our biomechanical testing device via sterile suture (17). All animal procedures were approved by the Institutional Animal Care and Use Committee of the Georgia Institute of Technology.
Biomechanical testing
A custom experimental apparatus was used to perform cylindrical biaxial biomechanical testing and multiphoton microscopy (17). Axial force-length data were collected over cyclic axial extensions at constant pressures (P = 60, 100, and 140 mmHg), and pressure-diameter data were collected from 0 to 160 mmHg at constant axial extensions. The in vivo axial stretch ratio was defined according to the intersection of axial force-length curves collected at different pressures (18).
Multiphoton microscopy
Freshly isolated arteries were mounted on our testing device under controlled transmural pressure and axial stretch, and were imaged on an LSM 510 META inverted confocal microscope (Carl Zeiss, Oberkochen, Germany) fitted with a tunable multiphoton laser (Coherent, Santa Clara, CA). The laser was set to 800 nm and a filter was configured to detect 350–450 nm backward scattering SHG signals from collagen (19). Vessels were imaged under physiological loading conditions of λz = 1.54 and P = 110 mmHg as well as three distinct geometries for affine motion analysis. Physiological loading conditions were determined through biaxial tests and from previously published data (20). The three loading configurations for affine motion calculations included higher axial stretch (λz = 1.55) with reduced pressure (P = 40 mmHg), reduced axial stretch (λz = 1.25) with higher physiological pressure (P = 110 mmHg), and an intermediate condition with moderate axial stretch (λz = 1.45) and moderate pressure (P = 80 mmHg). These configurations produced geometries of high axial stretch with low circumferential stretch, high circumferential stretch with low axial stretch, and a nearly equi-biaxial stretch. The configurations represent the limits of axial and circumferential deformations for the vessel, as well as the limits of deformation and rotation of engaged collagen fibers experienced during a typical biaxial mechanical test. An LSM Image Browser (Carl Zeiss) was used to export image stacks to .tiff files for analysis.
Estimation of collagen fiber angle distribution
We estimated the angular distribution of collagen fibers for each optical slice in a z-stack using MATLAB (The MathWorks, Natick, MA) code that was modified from a previously reported fast Fourier series algorithm (15). We determined the inner and outer bounds of the collagen fiber layer within the image stack by examining the orthogonal views of the reconstructed vessel. Each optical slice was low-pass filtered, converted to a binary image using Otsu's method to threshold (21,22), and windowed with a 2D Tukey window (23). A fast Fourier transform was performed and a power spectrum was generated (Fig. 1). This power spectrum was filtered and used to generate a histogram of frequency intensities between −90° and 90° in 4° increments. The fiber angle distribution at each wall location was averaged across the entire image stack to obtain a mean fiber distribution for each vessel. For each loading configuration, fiber angle distributions at corresponding normalized wall locations were averaged across all samples to obtain a representative surface of fiber distributions as a function of wall location.
Figure 1.
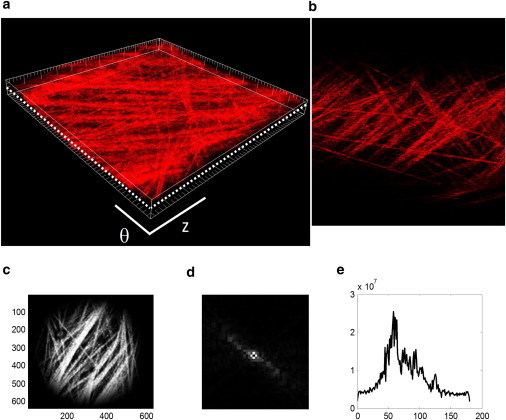
(a) Image stack. (b) Each optical slice within the image stack was analyzed to measure fiber orientation distributions (dotted line indicates a single optical slice). Image slices were taken at 0.5 μm intervals across the entire thickness of the adventitial layer. Image slices were divided into square segments and windowed (c), and a fast Fourier transform algorithm (d) was used to determine the distribution of fiber angles (e). Fiber distributions at normalized wall locations were averaged to determine a mean distribution for each experimental group. Panels a and b show a 225 μm × 225 μm square segment of the artery.
Prediction of fiber angles under different loading conditions
Fiber angles measured under physiological loading conditions were transformed to angles in the stress-free state according to relations found in Lanir (24):
| (1) |
where λ1 and λ2 are tissue stretches, γ is the magnitude of simple shear of the tissue, and λ is the stretch of a fiber originally oriented at angle α and rotated to angle β. In this study, the mapping of points was taken from a stress-free open sector to an inflated and extended cylindrical artery; thus, shear strains were not imposed (8). In this case, Eq. 1 yields the following relation:
| (2) |
where αR is the predominant fiber angle at the stress-free state; and are in vivo axial and circumferential stretch ratios, respectively; and αphys is the predominant fiber angle measured under physiological loading conditions. This relation was also used to transform fiber distributions between different states of axial and circumferential stretch.
Parameter estimation
We estimated the parameters using previously described methods (8,25). Instead of describing the kinematics in detail, we list the strain energy functions used to incorporate structural data. The ultimate goal of structural models is to use measured fiber orientation data as a direct input for the structural parameters of the model. We incorporated the measured fiber orientations in three ways, as described below. Conceptual differences between the constitutive relations used are illustrated in Fig. 2.
Figure 2.
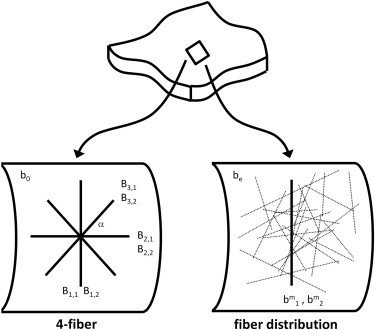
Simple four-fiber model and more descriptive fiber distribution models were used to estimate material parameters and predict mechanical response. Both types of models depict the artery as a network of fibers embedded in an isotropic matrix (bo, be). The four-fiber model consists of families of orthogonal fibers (b1,1, b1,2, b2,1, b2,2) and symmetric diagonal fibers (b3,1, b3,2) oriented at ±α0, and each fiber family can have different material properties. The fiber distribution model consists of a circumferential family of fibers (bm1, bm2) representing SMC and a distribution of fibers representing collagen fibers (dotted lines).
Four-fiber family model
A commonly used four-fiber constitutive model was used to estimate material and structural parameters (16,25,26). The fiber-family model has the strain energy function
| (3) |
where b0, , and are material parameters (with k denoting a fiber family), I1 = tr(C) = Crr + Cθθ + Czz is the first invariant of the right Cauchy-Green stain tensor C, is the stretch of the kth fiber family, Mk = sin(αk)eθ + cos(αk)ez is the unit vector along the kth fiber direction in the reference configuration, and αk is the associated angle between the axial and diagonal fiber directions. This model assumes that the tissue is comprised of an isotropic amorphous solid with embedded structural fibers that are oriented in four distinct directions: one axial (α2 = 0°), one circumferential (α1 = 90°), and two diagonal (α3 = −α4 = α; Fig. 2). To achieve material symmetry, the fibers oriented at +α0 are constrained to have the same material properties as the fibers at −α0; i.e., Under compression, the fiber families do not contribute to the mechanical response in an exponential fashion, as they do in tension.
We chose a four-fiber family for several reasons. First, structural data from arteries generally show that muscle is oriented nearly circumferentially and collagen fiber distributions have peaks along preferred diagonal directions (7). In addition, collagen (and elastin) has been suggested to have fibers oriented in the axial direction. Thus, general histological observations support the use of a four-fiber family model. Second, Zeinali-Davarani et al. (28) showed that when the number of fiber families increases from 2 to 3, and 3 to 4, there is significant improvement in the goodness-of-fit between model predictions and experimental results; however, when the number of fiber families increases above 4, there is little improvement in the goodness-of-fit.
Few studies have experimentally verified the ability to predict microstructural configuration of tissues (12); instead, most studies solved for the direction of the diagonal fiber family (i.e., α was solved for along with the material parameters). Here, we performed two regressions: one in which the diagonal fiber angle (denoted α) is solved for (along with the material parameters, via regression techniques) and one in which the fiber angle is prescribed based on multiphoton microscopy results.
Direct incorporation of collagen distribution histograms
One major limitation of using the peak angles in a four-fiber model is that information on the shape of the distribution functions is lost. Such a model does not distinguish between tissues with highly aligned collagen fibers and those with widely distributed collagen fibers. Thus, to directly incorporate the measured distribution of fibers, we binned the angular intensities for collagen fibers at 4° increments between −90° and 90°, and treated each bin as a collagen fiber family. All 45 collagen fiber families were assumed to have the same material parameters. The strain energy function for this approach was adapted from a previously reported model (8,29) and is as follows:
| (4) |
where ϕk is the intensity of the histogram bar at the kth fiber angle, and be, are material parameters. The parameters be, are analogous to the parameters b0, , respectively, from Eq. 3.
Fiber distribution model
Another way to incorporate the measured distributions of fibers is to fit the fiber distribution to a continuous distribution function. To that end, we used a previously described fiber distribution model (8,29), incorporating material parameters and distributed fiber orientations (Fig. 2). In the previous version of the fiber distribution model, the fiber distribution was defined using a sum of normal distributions; however, in this study we used either a sum of three Gaussian distributions or a single Gaussian distribution. A single Gaussian distribution represents the shape of the measured fiber distribution in less detail but uses fewer material parameters. The strain energy function is given as
| (5) |
where be, are analogous to the material parameters b0, , respectively, from Eq. 3; are material parameters for the collagen fibers; and ϕ(α) represents a Gaussian distribution:
| (6) |
such that ap, bp, and cp are constants determined by fitting Eq. 6 to the collagen orientation histogram using the MATLAB curve-fitting toolbox. A sum of three Gaussian distributions describes the measured distribution more precisely and can separate the aggregate distribution into distinct axial and diagonal distributions, which can then be assigned different material properties. The strain energy function in this case is given as
| (7) |
where be, are analogous to the material parameters b0, , respectively, from Eq. 3; are material parameters for the collagen fibers; and ϕa(α), ϕb(α), and ϕc(α) follow the same form as Eq. 6.
We first estimated the parameters while allowing all structural information to be determined through nonlinear regression, and then estimated them a second time using fiber angles and distributions measured from multiphoton images.
Predictive power of models using measured microstructural data
The mean best-fit parameters for each model were used to predict the mechanical response of arteries used in biaxial tests. The prediction error was defined as the difference between predicted and experimentally measured mechanical responses.
Statistical analysis
We compared the mean values using unpaired, two-tailed t-tests, with significance taken at p < 0.05. For comparisons of more than two means, a one-way analysis of variance was used with Tukey's post hoc test. Bartlett's test was used determine differences in variances, with significance taken at p < 0.05.
Results
Multiphoton microscopy
Optical image slices can be reconstructed to reveal the structure of collagen fibers within the blood vessel wall (Fig. 1, a and b). Collagen fibers exist in a variety of orientations that can be quantified and imaged through the entire thickness of the wall.
Image processing
At physiological pressures and axial stretches, the fiber angle distributions appeared to vary in shape throughout the vessel wall (Fig. 3 a). The mean fiber angle distribution surface suggests that fibers near the lumen (normalized wall location of 0) may be predominantly distributed in a diagonal direction, whereas fibers at the outermost surface (normalized wall location of 1) of the adventitia may have a preference for axial alignment. When we average across the entire thickness of the adventitia, the resulting physiological fiber distribution suggests a mean bimodal distribution with a peak in the axial direction at 0° and a second peak at −32° (Fig. 3 b). The mean histogram of all vessels was fitted to one or a sum of three Gaussian distributions for use in parameter estimation studies (Fig. 3 c).
Figure 3.
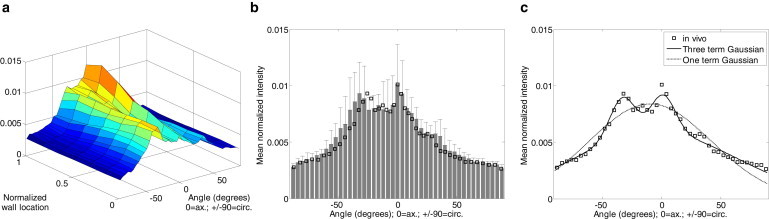
Distribution of fiber alignment varies through the depth of the vessel wall. (a) Fiber distributions for samples imaged under physiological loading were averaged across normalized wall locations to obtain a mean local fiber distributions. (b) The fiber distribution through the wall was also averaged to generate a mean distribution, which was transformed into a stress-free configuration (square markers). (c) The in vivo configuration was fitted to three- and one-term Gaussian curves, which were then used for parameter estimation.
Affine deformation assumption
Fiber angle distributions were measured for arteries imaged under three geometric configurations: high axial with low circumferential stretch, low axial with high circumferential stretch, and an intermediate configuration (Fig. 4). In the configuration with high axial and low circumferential stretch (Fig. 4, a and b), fibers were highly aligned in the axial direction. As pressure was increased and axial stretch was reduced, fibers rotated in the circumferential direction and became less aligned axially (Fig. 4, c–f). The mean fiber distributions were also predicted (square markers in Fig. 4, b and f) using Eq. 2 and the fiber distribution of the intermediate configuration as the reference configuration. Although the overall shape of the distributions suggests that fibers rotate in directions consistent with stretching in the axial and circumferential directions, the correlation coefficients, R2, between the predicted and measured fiber distributions varied with the type of deformation (Fig. 4, b and f).
Figure 4.
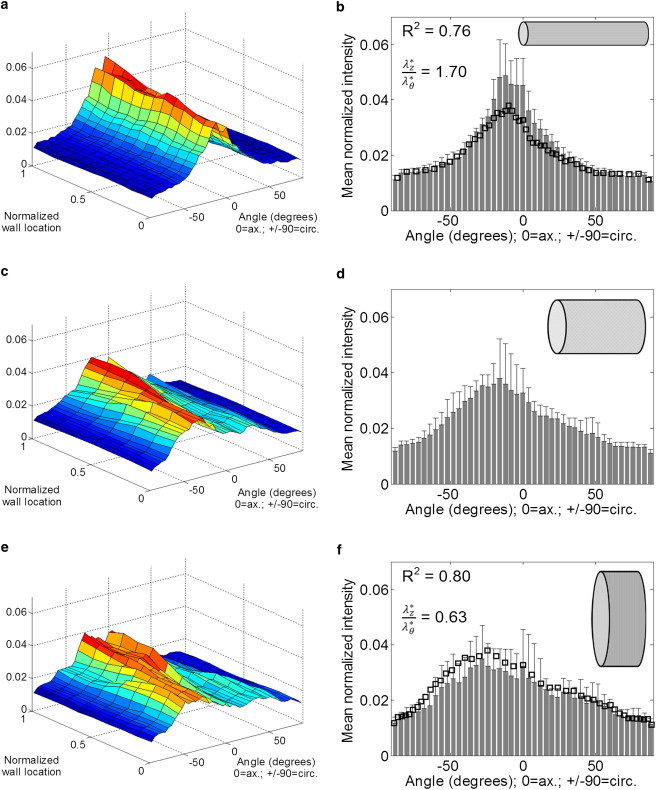
(a–d) Fibers through the depth of the vessel wall become more aligned axially when the vessel is stretched axially. The rotations of the fibers agree with affine motion assumptions, with R2 ≥ 0.76. Local fiber distributions were averaged at normalized wall locations to generate a fiber distribution surface (a, c, and e), and fiber distributions surfaces were averaged through the depth of the wall to obtain the mean fiber distributions (b, d, and f). The inset figures correspond to approximate loading geometries (not to scale), and open squares indicate the predicted distribution based on the fiber distribution measured in the intermediate state and assuming affine deformations. R2 values represent correlation coefficients between measured and predicted fiber orientations.
Parameter estimation
We estimated material and structural parameters using 1), a four-fiber model (Table S1 in the Supporting Material); 2), direct incorporation of collagen distribution histograms (Table S2); and 3), one-term and three-term Gaussian fiber distribution models (Table S3). For the four-fiber model, the in vivo distribution was transformed to the stress-free state and the nonaxially aligned peak (−25.47°) was used as the diagonal fiber angle (Fig. 3 b). The collagen distribution histogram was fitted to a single Gaussian distribution and to a sum of three Gaussian distributions (Fig. 3 c). A one-term Gaussian distribution was used because it requires a similar number of parameters as the four-fiber model, and a sum of three Gaussian distributions was used because it accurately captures the shape of the measured in vivo fiber distribution (Fig. 3 c).
The largest fitting errors resulted from directly using the collagen distribution histogram and from using one Gaussian distribution in the strain energy function (Fig. 5). These two models assume that all collagen fibers within the adventitia have the same material parameters while approximating the shape of the fiber angle distribution to different degrees of accuracy. Separating the collagen distribution histogram into three fiber distribution families with three sets of material parameters resulted in a significantly reduced fitting error (Fig. 5). These results suggest that although estimating both structural and material parameters produces the best goodness-of-fit, the use of measured structural information does not impose a significant penalty on the fitting error.
Figure 5.
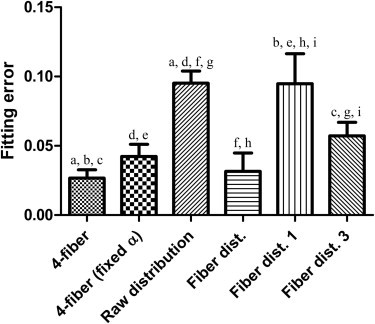
Mean fitting errors increased when experimentally measured fiber distributions were used (columns 3, 5, and 6). The first and fourth columns indicate error when estimating all material and structural parameters. The second column indicates error in the four-fiber model when using a mean fiber angle, and the remaining columns reflect error when using either the collagen histogram (column 3) or the collagen histogram fitted to a single Gaussian distribution (column 5) or a sum of three Gaussian distributions (column 6). (Symbols a–i indicate p < 0.05; for all other pairwise comparisons, p > 0.05.)
Prediction of mechanical response using mean parameters
The confidence intervals (CIs) for all models overlap; however, using a sum of three Gaussian distributions to describe collagen fiber orientations resulted in a smaller 95% CI range (Fig. 6). The large variations in errors of many models resulted in statistically insignificant differences in mean errors; however, the relatively close clustering of data points near the mean suggests a trend in which using three Gaussian distributions to describe fiber angle distributions results in more accurate and more consistent model predictions (Fig. 6 b). It appears that in addition to replicating the measured fiber distribution, it is also necessary to separate the distribution into distinct fiber distribution families to obtain the most consistent predictions. Directly using the collagen fiber histogram resulted in the largest 95% CI for prediction error, whereas approximating the collagen distribution using three Gaussian terms resulted in the most consistent prediction errors.
Figure 6.
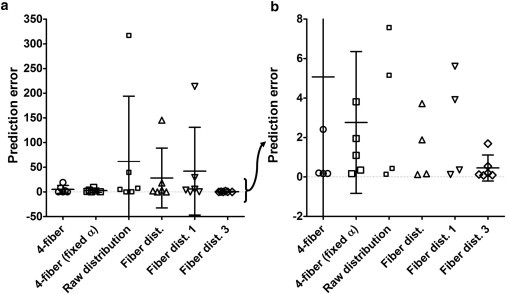
(a) Fiber distribution model using three Gaussian terms is able to approximate experimental mechanical response data with the smallest 95% CI. An examination of the data over a smaller range reveals that errors for the fiber distribution model using a sum of three Gaussian distributions are clustered near 0.5, whereas errors for all other models are distributed over a wider range of errors. This suggests that closely matching the in vivo fiber distribution in a constitutive model predicts the mechanical response more consistently and with smaller errors (b). Horizontal bars indicate means, and symbols designate errors of individual samples. The means were not statistically different (p > 0.05); however, variances differed significantly (p < 0.0001).
Discussion
Constitutive relations have the power to both predict the mechanical response of tissues under load and to elucidate the interactions between various constituents of biological tissues. Prediction of mechanical behavior is desired when excising, removing, and testing biological tissues is not feasible, such as in a live animal or patient. Recent formulations of constitutive relations incorporated parameters for the physical structure of arteries in predicting their mechanical response (7,8,30,31). Structurally motivated constitutive relations provide a more physically meaningful description of arteries and can offer improved predictive capabilities; however, they often increase the number of parameters that must be estimated, thus increasing the possibility of nonunique solutions. Advances in imaging techniques can aid in quantifying many structural parameters that previously had to be estimated, and reduce the number of requisite free parameters by using parameters that are truly experimentally intractable. The results presented here suggest that the approach of using measured structural parameters and separating fiber distributions into fiber families produces fewer prediction errors compared with using estimated structural parameters. Thus, it may be possible to accurately predict the mechanical response of an artery by measuring its structural parameters and using previously determined material parameters. One possible way to obtain in situ fiber orientation data would be to employ intravital microscopy, which has previously been used to image tissues such as tumors, blood vessels, and extracellular matrix of the interstitium (32–35).
With the increasing use of structurally motivated constitutive relations, the level of structural detail required for a constitutive model remains to be determined. In many cases, this depends on the particular constitutive relation, its purpose in a study, and whether the structural parameters can be quantified. When studying diseased or genetic knockout animals, the use of measurable structural data, even a single diagonal fiber angle (12), may increase the level of confidence in the resulting material parameters. However, when calculating local wall stresses from mechanical test data, a Fung-type phenomenological model may be sufficient. Hollander et al. (31) included radially oriented interlamellar elastic struts in a structural constitutive model and found that eliminating certain structural parameters did not significantly affect predictive power. A key difference in our study is that we experimentally measured structural information and found that including measured structural detail improved the model predictions at a small cost to goodness-of-fit. When estimating all model parameters, it is likely that a high degree of detail is unnecessary to recapitulate mechanical test data. However, when comparing diseased tissues or genetically modified animals, including measured structural information may increase the level of confidence in the resulting material parameters. Further studies incorporating measured structural data into constitutive relations will help determine an optimal balance between the structural detail required and the number of parameters in a constitutive model.
We used multiphoton imaging to measure the collagen fiber angle distributions of intact, pressurized, and stretched mouse carotid arteries. This technique allowed us to image the entire wall thickness of a single artery under various loading conditions. When we used experimentally measured structural information, a fiber distribution model using a sum of three Gaussian distributions yielded fitting errors similar to those obtained with a four-fiber model using a single mean fiber angle (Fig. 5). In contrast, directly using the measured collagen histogram resulted in a significantly larger fitting error. This suggests that all collagen fibers within the arteries may not have uniform material properties, and reinforces the need for more experimental studies of material properties. When we predicted the mechanical response of arteries using mean material parameters, we found that separating the fiber distribution into separate distribution families produced lower mean errors (p > 0.05) with lower variances (p < 0.0001). The large variance obtained with direct use of the measured collagen angle histogram suggests that obtaining only the shape of the distribution is insufficient for an accurate prediction of mechanical response, and the distribution may need to be further separated into different distribution families (Fig. 6 b). Although the mean prediction error for the fiber distribution model was not statistically lower, the clustering of data points near the mean suggests better predictive accuracy. In a previous study, Wicker et al. (12) also showed that using a fixed fiber angle in the four-fiber model altered the fitting error; however, they did not report the level of statistical significance. In structurally motivated constitutive relations, the four-fiber model depicts tissues as two orthogonal families of fibers and two families of symmetric diagonal fibers; however, images collected in this study reveal axial fibers and only one family of diagonal fibers (Fig. 3 b). This study also shows that fiber angle distributions appear to vary through the wall of the artery (Fig. 3 a), which is consistent with previous studies showing variations in fiber angle distributions throughout the wall of arteries from other species (12,36). Variations in fiber angles may be reconciled with the use of averaging (as in this study), weighted averaging (as performed in previous studies (12)), or a multilayered model (7).
Incorporating measured structural information also produced changes in certain material parameters. Using a fixed fiber angle in the four-fiber model resulted in changes in parameters and . The results obtained from summing three Gaussian distributions included a modulus-like parameter, bca1, which reached the lower limits (0.0001) for the range of possible parameters (Table S3). The parameters bca1 and bca2 correspond to a family of collagen fibers with a mean angle of −1.22°; thus, the mechanical response may partially overlap that of parameters bcc1 and bcc2. This suggests that using three terms to describe the fiber distribution may not be necessary to capture the salient features of the mechanical response of the artery; however, three Gaussian terms were necessary to adequately capture the shape of the fiber angle distribution, especially the nonzero values at ±90°. The changes in material parameters obtained with measured versus estimated structural information highlights the need for further studies of microstructural data of biological tissues, as well as studies to identify material parameters.
It is well known that increasing the number of parameters in constitutive relations often leads to decreases in fitting errors (28). Here, the use of three Gaussian terms to describe the mean fiber distribution resulted in a 40% decrease in fitting error compared with the model using one Gaussian term. An 11-parameter, six-fiber model (data not shown) was also evaluated and resulted in only a 7.5% decrease in fitting error compared with the four-fiber model. This finding suggests that obtaining a detailed description of the fiber orientation distribution, as opposed to merely adding fibers to the commonly used four-fiber model, significantly improves the fitting error.
Constitutive models that predict the mechanical response of tissues often employ the assumption that the tissue undergoes affine deformations at all stretches; however, previous studies suggested that this is not universally true (37). In this study we examined the deformation of fibers while the vessel was pressurized and stretched to three different aspect ratios, and the results suggest that, qualitatively, fibers undergo expected rotations with measured fiber orientations matching predicted orientations to varying extents throughout the range of fiber angles. Our results suggest that agreement between measured and predicted fiber distributions may vary according to the type of deformation (Fig. 4, b and f). With R2 values of 0.76 and 0.80, the results suggest a reasonable agreement between measured and predicted fiber distributions. In contrast to the physiological fiber distribution (Fig. 3 a), the geometries examined for affine motion analysis yielded fiber distributions that were predominantly nonbimodal. This may result from differences in fiber distribution through the wall of the vessel, and from the combination of axial stretches and pressures used to create the three distinct geometries. The degree of affine motion is of interest for a variety of biological tissues as well as engineered tissues (37,38). Constitutive relations typically assume affine motions (39,40), and biological tissues do not always undergo affine motions in all cases (37,41,42). For example, bovine pericardium appears to undergo nonaffine biaxial deformations (37), whereas human supraspinatus tendons undergo both affine and nonaffine deformations depending on the loading conditions (41). In addition, Hepworth et al. (42) found that collagen fibers in porcine skin undergo affine deformations when examined at the millimeter scale. Although engineered and native tissues may contain similar constituents, the microstructure of engineered tissues may be different and not necessarily follow affine deformations (38). When using mathematical models to analyze biological or engineered tissues, one must determine whether the affine motion assumption applies to the deformations of interest. When microstructural deformations deviate from tissue level deformations to an unacceptable degree, these errors must be considered.
In this study we examined a range of deformations that included only straightened fibers. Although fibers may be undulated in unloaded and stress-free states, they are likely to be straightened under physiological and pathophysiological states, such as hypertension. Although inferences cannot be made as to whether motions of undulated fibers are affine or nonaffine, the results suggest that the imaging and analysis techniques demonstrated here can be readily applied to study fiber motions under normal and disease conditions. Future attempts to image and quantify fiber motions in other tissues will help test assumptions underlying constitutive relations.
There are several limitations to this study that permit room for future improvement. In contrast to other studies (36), the collagen fibers in the medial layer of our mouse carotid arteries were not visible through SHG detection. Neglecting medial collagen fibers may introduce errors in stress analysis with certain constitutive models (7). Also, it is possible that fibers that are too thin to observe play a small role in the tissue's mechanical response with respect to more visible structures. Images collected in this study, as well as in previous studies, suggest that the fiber angle distribution changes between the adventitial and luminal surfaces of the arterial wall (12,36). Incorporation of fiber angle distributions that vary through the wall may improve the results of parameter estimation studies (43). Here, we used mean material parameters to evaluate the predictive capabilities of constitutive relations; however, the use of a larger sample size and a more comprehensive method, such as bootstrapping, may enable a more robust analysis of the predictive power of constitutive models (44). In this study we also used a mean fiber distribution rather than the local fiber distributions of biaxially tested arteries. Although it is preferable to image and test the same artery whenever possible, the results obtained from studying the predictive capabilities of models suggest that using mean structural parameter values may be sufficient. An in-depth analysis of fiber crimping and imaging under a wider variety of loading conditions may allow a better estimation of the limits of affine motions. In addition to enhancing our understanding of material behavior, constitutive relations may also be used to predict mechanical behavior a priori.
In this study, we incorporated microstructural information and collagen fiber angle distributions into structurally based constitutive relations, and showed that the addition of experimentally measured structural parameters does not significantly increase fitting errors for structurally descriptive constitutive relations. When evaluating the predictive capabilities of structurally motivated constitutive relations, one can obtain lower and more consistent prediction errors by using a measured fiber angle distribution and separating it into three Gaussian distribution. This finding suggests that when material parameters are known, the measurement of the in vivo fiber angle distribution may be sufficient to predict the mechanical response of an artery. We also show that the deformation of collagen fibers can be qualitatively predicted using affine motion assumptions. In the absence of detailed fiber distribution information, the four-fiber model remains useful for capturing the mechanical response of arteries. In combination, the techniques used in this study for measuring fiber distributions under different loading conditions and for incorporating measured fiber distributions in constitutive models provide what to our knowledge is a novel approach for reducing the number of parameters to be estimated and using measurable structural parameters to predict the mechanical behavior of arteries. In a broader context, the image analysis and modeling techniques described here can be applied to commonly collected experimental data and used to obtain additional insights into the structure-function relationship of healthy and diseased tissues.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (HL-070531).
Supporting Material
References
- 1.Arnett D.K., Evans G.W., Riley W.A. Arterial stiffness: a new cardiovascular risk factor? Am. J. Epidemiol. 1994;140:669–682. doi: 10.1093/oxfordjournals.aje.a117315. [DOI] [PubMed] [Google Scholar]
- 2.Huang S., Ingber D.E. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Ingber D.E. Extracellular matrix as a solid-state regulator in angiogenesis: identification of new targets for anti-cancer therapy. Semin. Cancer Biol. 1992;3:57–63. [PubMed] [Google Scholar]
- 4.Alexopoulos L.G., Haider M.A., Guilak F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J. Biomech. Eng. 2003;125:323–333. doi: 10.1115/1.1579047. [DOI] [PubMed] [Google Scholar]
- 5.Kleemann R.U., Krocker D., Duda G.N. Altered cartilage mechanics and histology in knee osteoarthritis: relation to clinical assessment (ICRS Grade) Osteoarthritis Cartilage. 2005;13:958–963. doi: 10.1016/j.joca.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Wyss H.M., Henderson J.M., Miller R.T. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 2011;300:C397–C405. doi: 10.1152/ajpcell.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holzapfel G., Gasser T.C., Ogden R.W. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. 2000;61:1–48. [Google Scholar]
- 8.Hansen L., Wan W., Gleason R.L. Microstructurally-motivated constitutive modeling of mouse arteries cultured under altered axial stretch. J. Biomech. Eng. 2009;131:11. doi: 10.1115/1.3207013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watton P.N., Hill N.A., Heil M. A mathematical model for the growth of the abdominal aortic aneurysm. Biomech. Model. Mechanobiol. 2004;3:98–113. doi: 10.1007/s10237-004-0052-9. [DOI] [PubMed] [Google Scholar]
- 10.Humphrey J., Rajagopal K. A constrained mixture model for growth and remodeling of soft tissues. Math. Models Methods Appl. Sci. 2002;12:407–430. [Google Scholar]
- 11.Wan W., Yanagisawa H., Gleason R.L., Jr. Biomechanical and microstructural properties of common carotid arteries from fibulin-5 null mice. Ann. Biomed. Eng. 2010;38:3605–3617. doi: 10.1007/s10439-010-0114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wicker B.K., Hutchens H.P., Humphrey J.D. Normal basilar artery structure and biaxial mechanical behaviour. Comput. Methods Biomech. Biomed. Engin. 2008;11:539–551. doi: 10.1080/10255840801949793. [DOI] [PubMed] [Google Scholar]
- 13.Chen H., Liu Y., Kassab G.S. The layered structure of coronary adventitia under mechanical load. Biophys. J. 2011;101:2555–2562. doi: 10.1016/j.bpj.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoumi A., Lu X., Tromberg B.J. Imaging coronary artery microstructure using second-harmonic and two-photon fluorescence microscopy. Biophys. J. 2004;87:2778–2786. doi: 10.1529/biophysj.104.042887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng C.P., Hinz B., Swartz M.A. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J. Cell Sci. 2005;118:4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 16.Baek S., Gleason R., Humphrey J. Theory of small on large: potential utility in computations of fluid–solid interactions in arteries. Comput. Methods Appl. Mech. Eng. 2007;196:3070–3078. [Google Scholar]
- 17.Gleason R.L., Gray S.P., Humphrey J.D. A multiaxial computer-controlled organ culture and biomechanical device for mouse carotid arteries. J. Biomech. Eng. 2004;126:787–795. doi: 10.1115/1.1824130. [DOI] [PubMed] [Google Scholar]
- 18.Van Loon P. Length-force and volume-pressure relationships of arteries. Biorheology. 1977;14:181–201. [PubMed] [Google Scholar]
- 19.Zoumi A., Yeh A., Tromberg B.J. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc. Natl. Acad. Sci. USA. 2002;99:11014–11019. doi: 10.1073/pnas.172368799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanagisawa H., Davis E.C., Olson E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhuri S., Nguyen H., Frank C.B. A Fourier domain directional filtering method for analysis of collagen alignment in ligaments. IEEE Trans. Biomed. Eng. 1987;34:509–518. doi: 10.1109/tbme.1987.325980. [DOI] [PubMed] [Google Scholar]
- 22.Otsu N. Threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cyber. 1979;9:62–66. [Google Scholar]
- 23.Harris F.J. On the use of windows for harmonic analysis with the discrete Fourier transform. Proc. IEEE. 1978;66:51–83. [Google Scholar]
- 24.Lanir Y. A structural theory for the homogeneous biaxial stress-strain relationships in flat collagenous tissues. J. Biomech. 1979;12:423–436. doi: 10.1016/0021-9290(79)90027-7. [DOI] [PubMed] [Google Scholar]
- 25.Gleason R.L., Dye W.W., Humphrey J.D. Quantification of the mechanical behavior of carotid arteries from wild-type, dystrophin-deficient, and sarcoglycan-delta knockout mice. J. Biomech. 2008;41:3213–3218. doi: 10.1016/j.jbiomech.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberth J.F., Taucer A.I., Humphrey J.D. Mechanics of carotid arteries in a mouse model of Marfan syndrome. Ann. Biomed. Eng. 2009;37:1093–1104. doi: 10.1007/s10439-009-9686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reference deleted in proof.
- 28.Zeinali-Davarani S., Choi J., Baek S. On parameter estimation for biaxial mechanical behavior of arteries. J. Biomech. 2009;42:524–530. doi: 10.1016/j.jbiomech.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Wan W., Hansen L., Gleason R.L., Jr. A 3-D constrained mixture model for mechanically mediated vascular growth and remodeling. Biomech. Model. Mechanobiol. 2010;9:403–419. doi: 10.1007/s10237-009-0184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zulliger M.A., Rachev A., Stergiopulos N. A constitutive formulation of arterial mechanics including vascular smooth muscle tone. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1335–H1343. doi: 10.1152/ajpheart.00094.2004. [DOI] [PubMed] [Google Scholar]
- 31.Hollander Y., Durban D., Lanir Y. Experimentally validated microstructural 3D constitutive model of coronary arterial media. J. Biomech. Eng. 2011;133:031007. doi: 10.1115/1.4003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidani M., Wyckoff J., Condeelis J. Probing the microenvironment of mammary tumors using multiphoton microscopy. J. Mammary Gland Biol. Neoplasia. 2006;11:151–163. doi: 10.1007/s10911-006-9021-5. [DOI] [PubMed] [Google Scholar]
- 33.Friedl P., Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- 34.Brendza R.P., Bacskai B.J., Holtzman D.M. Anti-Aβ antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J. Clin. Invest. 2005;115:428–433. doi: 10.1172/JCI23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herz J., Siffrin V., Niesner R.A. Expanding two-photon intravital microscopy to the infrared by means of optical parametric oscillator. Biophys. J. 2010;98:715–723. doi: 10.1016/j.bpj.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timmins L.H., Wu Q., Greenwald S.E. Structural inhomogeneity and fiber orientation in the inner arterial media. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1537–H1545. doi: 10.1152/ajpheart.00891.2009. [DOI] [PubMed] [Google Scholar]
- 37.Billiar K.L., Sacks M.S. A method to quantify the fiber kinematics of planar tissues under biaxial stretch. J. Biomech. 1997;30:753–756. doi: 10.1016/s0021-9290(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 38.Chandran P.L., Barocas V.H. Affine versus non-affine fibril kinematics in collagen networks: theoretical studies of network behavior. J. Biomech. Eng. 2006;128:259–270. doi: 10.1115/1.2165699. [DOI] [PubMed] [Google Scholar]
- 39.Lanir Y. Constitutive equations for fibrous connective tissues. J. Biomech. 1983;16:1–12. doi: 10.1016/0021-9290(83)90041-6. [DOI] [PubMed] [Google Scholar]
- 40.Sacks M.S. Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J. Biomech. Eng. 2003;125:280–287. doi: 10.1115/1.1544508. [DOI] [PubMed] [Google Scholar]
- 41.Lake S.P., Cortes D.H., Elliott D.M. Evaluation of affine fiber kinematics in human supraspinatus tendon using quantitative projection plot analysis. Biomech. Model. Mechanobiol. 2012;11:197–205. doi: 10.1007/s10237-011-0303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hepworth D.G., Steven-fountain A., Vincent J.F. Affine versus non-affine deformation in soft biological tissues, measured by the reorientation and stretching of collagen fibres through the thickness of compressed porcine skin. J. Biomech. 2001;34:341–346. doi: 10.1016/s0021-9290(00)00183-4. [DOI] [PubMed] [Google Scholar]
- 43.Gasser T.C., Ogden R.W., Holzapfel G.A. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface. 2006;3:15–35. doi: 10.1098/rsif.2005.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferruzzi J., Vorp D.A., Humphrey J.D. On constitutive descriptors of the biaxial mechanical behaviour of human abdominal aorta and aneurysms. J. R. Soc. Interface. 2011;8:435–450. doi: 10.1098/rsif.2010.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


