Abstract
Cystic fibrosis (CF) is characterized by inflammatory lung disease that significantly contributes to morbidity and mortality. Airway epithelial cells play a role in the inflammatory signaling in CF and have been reported to exhibit a number of dysfunctions in signaling cascades that modulate inflammation. Previously, we reported that the activity of nuclear factor erythroid-derived-like 2 (Nrf2), a transcription factor that regulates antioxidant and cytoprotective protein expression, is diminished in CF epithelia (7). In this report, we examined the mechanism of Nrf2 dysregulation in vitro in human airway epithelial cell lines and primary cells and in vivo in nasal epithelia excised from ΔF508 CF mutant mice. We found that cAMP-mediated signaling markedly reduces Nrf2 activity in CF vs. non-CF cells. Rp-cAMPS, a cAMP competitor, significantly corrected Nrf2 activity in CF cells, predominantly by increasing the nuclear accumulation of the transcription factor. Furthermore, we found that Rp-cAMPS significantly decreased NF-κB activation following inflammatory stimulation of CF cells. Further investigation revealed that Nrf2 and NF-κB compete for the transcriptional coactivator cAMP responsive element-binding protein (CREB) binding protein (CBP) and that Rp-cAMPS shifts CBP association in favor of Nrf2. Thus our findings provide a link between feedback to CF transmembrane regulator dysfunction and dysregulation of an inflammatory signaling pathway that modulates the coordinated activities of Nrf2 and NF-κB. Furthermore, our studies suggest that strategies that shift CBP association away from NF-κB and toward Nrf2 could have potential therapeutic efficacy for reducing inflammation in patients with CF.
Keywords: H2O2-mediated inflammatory signaling, airway epithelia, cAMP responsive element-binding protein
in addition to dysfunction of the cystic fibrosis (CF) transmembrane regulator (CFTR) chloride channel, CF airway epithelia are characterized by a number of secondary defects including abnormal epithelial sodium channel function (15) and inflammatory signaling (5, 30). Excessive inflammatory responses have been reported in patients with CF (29) and CF mice (11). Furthermore, there is evidence that resolution of inflammation in CF is impaired (17, 27). Some studies have demonstrated that inflammation is not innate to CF (2). Nevertheless, it is widely accepted that inflammation diminishes lung function and leads to mortality and that controlling inflammation slows lung deterioration (16). Despite the significance of inflammation in CF disease, the molecular underpinnings linking CFTR dysfunction and inflammatory signaling remain to be established.
A potential mechanism for excessive inflammation in CF is the activation of NF-κB by oxidative stress, which results from an imbalance of oxidants (i.e., H2O2) and antioxidants (i.e., glutathione) in the cell (9, 32, 34). Redox signaling pathways have been shown to play a key role in the activation of inflammatory cascades in the lung (37, 44). For example, hydrogen peroxide (H2O2) has been reported to mediate IL-1β (18) and TNF-α (8, 20) receptor signaling cascades. Furthermore, a number of studies have demonstrated that H2O2 directly activates NF-κB (25, 36). Antioxidants, which reduce H2O2 levels, inhibit the production of cytokines following the stimulation of the IL-1β and TNF-α receptors (7, 8, 18), indicating an essential role of oxidants in the propagation of normal inflammatory signaling. We previously reported that intracellular steady-state levels of H2O2 were elevated in CF model primary and immortalized epithelia and that this elevation in H2O2 promoted hyperinflammatory responses (7).
A key modulator of redox balance and signaling is the transcription factor, nuclear-factor-E2-related factor 2 (Nrf2), which drives the expression of a battery of genes that protect against electrophilic and oxidative stress (reviewed in Ref. 45). It plays a central role in the cellular antioxidant defense system by inducing the expression of detoxifying and antioxidant factors that maintain and restore redox balance. During homeostasis, Nrf2 is sequestered in the cytoplasm by its interaction with Kelch-like erythroid cell-derived protein with cap'n'collar homology-associated protein 1 (Keap1) (28). Keap1 is a substrate adaptor that recruits Nrf2 to a cullin-3-based ubiquitin ligase for subsequent ubiquitin modification. Increased oxidant load in cells results in the oxidation of cysteine residues on Keap1 and dissociation of the Keap1:Nrf2 complex from the cullin-3 scaffold (46). Nrf2 is subsequently translocated to the nucleus where it binds to the antioxidant response elements (AREs) in the promoters of its target genes (4, 24). After the reestablishment of redox homeostasis, Nrf2 activity is terminated, but a complete mechanistic understanding of how this happens is lacking. Recent evidence suggests that Keap1 is chaperoned to the nucleus by prothymosin (PTM)-α, where interaction with PTM-α is exchanged for the binding of Nrf2 (33). The Keap1-Nrf2 complex is then exported from the nucleus and reassembled with the cytoplasmic cullin-3-based ligase, and Nrf2 is ubiquitinated and targeted for degradation. Removal from the nucleus serves as one mechanism to limit the transcriptional activity of Nrf2. Another level of control is modulation of the availability of transcription coactivators in the nucleus, such as cAMP responsive element-binding protein (CREB) binding protein (CBP), which has been shown to interact with and activate Nrf2 (13).
Previously, we demonstrated that steady-state intracellular H2O2 is significantly elevated in CF epithelial cells (7). However, despite this increase in H2O2, Nrf2 activity and the expression of a number of antioxidant proteins were significantly diminished in CF epithelia, both in culture and in vivo (7). This response is paradoxical because increased steady H2O2 is expected to promote Nrf2 activation and increase the expression of Nrf2 target genes. Therefore, we hypothesized that the suppression of Nrf2 activity in CF epithelia may be mediated by the modulation of one or more Nrf2 binding partners in the nucleus, following dissociation of the transcription factor from Keap1. This hypothesis is consistent with our earlier studies showing that the absence of CFTR activity results in the alteration of cAMP-mediated signaling and phosphorylation of CREB (26). Together, these findings indicate that cAMP-mediated regulation of CBP may be a nexus for modulating the relative activities of critical proinflammatory and cytoprotective transcription factors such as NF-κB and Nrf2.
Here, we report that the cAMP-binding competitor Rp-cAMPS significantly rescues Nrf2 activity in human CF epithelial cell lines and primary bronchial epithelia. Using an ARE-driven luciferase reporter assay, we found that Rp-cAMPS significantly increased Nrf2 activity in CF epithelia. This increase in Nrf2 activity was sufficient to rectify the decrease in Nrf2 target gene expression and results in a reduction of the elevated intracellular H2O2 and cytokine production by CF cells. We explored the mechanism of Nrf2 activation by Rp-cAMPS and found that treatment with the compound increased the nuclear fraction of Nrf2 in CF cells to non-CF control levels by increasing the interaction with CBP of Nrf2. Furthermore, the Rp-cAMPS-mediated increase in Nrf2-CBP association is concurrent with a decrease in CBP interaction with NF-κB p65. Taken together, these data demonstrate for the first time a mechanism for the inactivation of Nrf2 in CF epithelia, namely that altered cAMP-mediated signaling in response to the loss of CFTR function (26) decreases CBP levels available to interact with Nrf2, thereby significantly decreasing the accumulation of Nrf2 in the nucleus and suppressing its transcriptional activity.
MATERIALS AND METHODS
Materials.
Cell culture media were purchased from Invitrogen (Carlsbad, CA). Reagents for reverse-phase liquid chromatography and mass spectrometric analysis were supplied by Thermo Fisher Scientific (Waltham, MA). Lipofectamine was purchased from Invitrogen. Luciferase assay reagent was purchased from Promega (Madison, WI). Nrf1 and Nrf2 promoter-driven reporter plasmids were purchased from Panomics (Fremont, CA). NF-κB promoter-driven luciferase reporter plasmid was purchased from Clontech Laboratories (Mountain View, CA). Intracellular H2O2 was measured using Amplex Red purchased from Invitrogen. Western blotting for Nrf2 was conducted with an antibody generated in rabbits against a His6-S-tagged full-length Nrf2 protein expressed and purified from bacteria. Alternatively, we used the following antibodies purchased from Santa Cruz Biotechnology (Santa Cruz, CA): for Nrf2, the H300 antibody; for CBP, β-actin, and Lamin B, we used the sc-1211, AC-15, and C-20 antibodies, respectively; for NF-κB p65, we used the SC-7151 antibody; and for secondary antibodies, we used ones conjugated to horseradish peroxidase (HRP). Rp-cAMPS was purchased from BioLog (Hayward, CA).
Cell culture.
We tested three types of cultured epithelia, two immortalized cell line pairs and one primary cell model. For the immortalized cells, we studied the 16HBEo− sense (S) and antisense (AS) or the 9HTEo− pCEP and pCEP-R matched cell pairs. Cell pair identity was confirmed by Western blotting for CFTR for the 9HTEo− or the R-domain for the 16HBEo− cells. The 16HBEo− matched cell line pair was derived from human bronchial epithelia (HBE) and was stably transfected with S or AS of the CFTR nucleotides 1–131. The 9HTEo− cell line pair was derived from human tracheal epithelia (HTE) and was stably transfected with pCEP (empty vector) or pCEP-R (pCEP vector encoding the regulatory domain of CFTR). Both cell lines were cultured as previously described (17). For studies in primary cells, we used CF or non-CF HBE grown at an air-liquid interface on semipermeable filters (gift from Chantest, Cleveland, OH). Once plated, the cells were cultured in media containing Ultraser G (Biosepra, Paris, France). The cells were allowed to become differentiated and form tight junctions, as assessed by resistance measurements, and then studied.
Cell treatments.
Cell lines and primary cells in culture were treated with Rp-cAMPS, TNF-α, or IL-1β. For all treatments, media was replaced every 24 h with fresh media containing fresh reagents. For experiments with primary epithelia, reagents were added to both the apical and basolateral compartments and replaced daily. For experiments testing the effect of the inhibition of cAMP-mediated signaling, cells were treated with Rp-cAMPS as previously described (26). Briefly, Rp-cAMPS was added at a final concentration of 50 μM to media for 72 h. For experiments on the effect of inflammatory stimulation, 10 ng/ml each of TNF-α and IL-1β were added to the media overnight, and the cells were processed for assays the following day. No significant increase in cell death was observed in any treated vs. untreated cells at 72 h, as assessed by Trypan blue staining.
Analysis of Nrf2 activity.
We tested Nrf2 activity by two methods: reporter gene expression driven by an ARE promoter and proteomic analysis of a subset of antioxidant proteins whose expression is regulated by Nrf2. For reporter gene expression studies, cell line models were transfected cell line models with a firefly luciferase expression plasmid (pNrf2-fluc) under the control of the Nrf2 promoter for glutathione-S-transferase (GST) 1. To control for transfection efficiency between cell lines, we used a Renilla luciferase expression plasmid under the control of the cytomegalovirus (CMV) promoter (pCMV-rluc). Cells were grown to 80% confluence in 24-well plates and transfected with DNA complexed with lipofectamine, as previously described (10). A sample (1 μg) of total DNA (0.9 μg pNrf2-luc + 0.1 μg pCMV-rluc) was used for the transfection of each sample well. Three hours following transfection, cells were washed and media was replaced. One day following transfection, cells were homogenized and the homogenates centrifuged at 14,000 g. Supernatants were collected and examined for firefly and Renilla luciferase activity by dual luciferase assay. Firefly luciferase data (Nrf1 or Nrf2 promoter activity) were normalized to Renilla luciferase values.
We also measured Nrf2 activity by using 2D SDS-PAGE to examine the expression of seven proteins under its transcriptional control, as previously described (7). The densities of 2D gel bands peroxiredoxin (PRDX) 1 and 6, thioredoxin (TRX) 1, GST1, catalase (CAT), and NAD(P)H dehydrogenase quinone (NQO) 1 were measured by a BioRad GS-800 densitometer using the 2D gel analysis software PD Quest. PRDX proteins and catalase play a major role in regulating H2O2 levels in cells.
Measurement of intracellular H2O2.
Because the expression of Nrf2-regulated proteins markedly affects H2O2 levels (12), we measured H2O2 in a number of experiments. This outcome is also an important link to changes in inflammation, as H2O2 is a potent modulator of inflammatory signaling cascades (44). We used the Amplex Red assay to assess levels of intracellular H2O2, as previously described (7). The assay has a limit of detection of 50 nM H2O2. Briefly, following treatment with Rp-cAMPS, TNF-α, and/or IL-1β for specified times, cells were rinsed, rapidly lysed, and analyzed or frozen immediately. For analysis, an aliquot of 25 μl of cell lysate was mixed with 25 μl of working solution containing 100 μM Amplex Red reagent and 0.2 U/ml HRP and incubated for 30 min at room temperature. Standards and samples are then analyzed for fluorescence levels at an emission wavelength of 590 nm (excitation, 544 nm). Levels of intracellular H2O2 were normalized to protein concentration.
Subcellular protein fractionation.
We used fractionation to examine the levels of Nrf2 in different compartments of cell line and primary cell models. We purified cytoplasmic, membrane, nuclear, and cytoskeletal fractions from cultured cells (cell lines and primary cells) using a subcellular protein fractionation kit (Pierce kit no. 78840; Thermo Fisher Scientific). Cells were harvested using 0.25% trypsin-EDTA, centrifuged at 500 g for 5 min, resuspended, washed in ice-cold PBS, and then centrifuged at 500 g. Each fractionation was carried out on a 10-μl volume of packed cells. Cells were resuspended in 100 μl of ice-cold cytoplasmic extraction buffer and incubated at 4°C for 10 min with gentle mixing. Following centrifugation at 500 g for 5 min, the supernatant (cytoplasmic fraction) was collected. Membrane extraction buffer (100 μl) was then added to the pellet and the sample vortexed vigorously, incubated at 4°C for 10 min, and centrifuged at 3,000 g for 5 min. The supernatant (membrane fraction) was collected. Nuclear extraction buffer (100 μl) was then added to the pellet and the sample vortexed vigorously, incubated at 4°C for 30 min, centrifuged at 5,000 g, and the supernatant (nuclear fraction) collected. All extracts were stored at 4°C overnight and examined by Western blot the next day.
Animal experiments.
Studies conducted in animals were reviewed and approved by the Case Western Reserve University Institutional Animal Care and Use Committee (IACUC). For studies on the association of CBP with p65 and Nrf2, ΔF508 mutant CF mice (Cftrtm1Kth congenic on the C57BL/6 background) received 50 mg/kg per day Rp-cAMPS for 12 days or saline (control) administered by Alzet osmotic pumps. Animals were then killed by CO2 inhalation, and nasal epithelia were excised as previously described (42).
Coimmunoprecipitation and Western blot analyses.
We used coimmunoprecipitation to examine the association of CBP with NF-κB or Nrf2 in the human epithelial 9HTEo− cell line pairs and nasal epithelial tissues excised from ΔF508 mutant mice. Cells or tissues were harvested following treatment with or without Rp-cAMPS as described in Cell treatments and Animal experiments, respectively. Samples were lysed for 30 min in 25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM EDTA, and 2% glycerol on ice and homogenized for 60 s. Homogenates were centrifuged at 14,000 g for 5 min at 4°C to clear cellular debris, and protein concentration was determined by Bradford assay. Lysates (60 to 100 μg total protein; equal for all samples) were then incubated with antibody against CBP (1:200) for 3 h at 4°C with shaking and then incubated with protein A-agarose (100 μl) for 1 h at 4°C with shaking, and the Protein A-CBP complex was pulled down by brief centrifugation (10 s, 14,000 g, 4°C). The pellet was washed three times in PBS with 0.1% Tween-20, suspended in SDS-PAGE sample buffer, and run on an acrylamide gel.
For Western blot analysis of the levels of NF-κB p65, Nrf2, and Keap1 in cultured cell whole-cell or subcellular fractions and mouse nasal epithelia, we prepared samples to contain equal concentrations of protein to allow for equal gel loading (20 μg for whole cell and cytoplasmic fractions, and 10 μg for nuclear fractions). Whole cell or subcellular fraction proteins were separated by SDS-PAGE at 200 V for 1 h. Proteins were then transferred to a wetted PVDF membrane at 100 V for about 30 min. Membrane was blocked in 2.5–5.0% milk for 1 h at room temperature (or overnight at 4°C) on a rocker with gentle agitation. Membrane was then incubated with primary antibodies against Nrf2 (1:250), Keap1 (1:200), or NF-κB p65 (1:500) for 1 h at room temperature (or overnight at 4°C) on a rocker with gentle agitation. The blot was then washed four times in PBS + 0.5% Tween-20 (3–5 min each wash) while gently agitated. After the final wash, blots were incubated with respective HRP-conjugated secondary antibodies (1:10,000) for 1 h at room temperature (or overnight at 4°C) on a rocker with gentle agitation. Following incubation with secondary Ab, blots were washed four times in PBS + 0.5% Tween-20 (3–5 min each wash) while gently agitated, incubated with SuperSignal West Femto Chemiluminescent Substrate (Pierce kit no. 34094; Thermo Fisher Scientific) detection reagent for 5 min, and exposed to film. Films were scanned by a BioRad GS-800 densitometer using QuantityOne software. The observed bands for NF-κB p65, Nrf2, and Keap1 were 62 kDa, 105 kDa, and 68 kDa, respectively. Band densities were normalized to protein (with equally loaded wells) and β-actin (whole cell or cytoplasmic fraction comparisons) or Lamin B or nucleolin (nuclear fraction comparisons).
Analysis of cytokine production.
To test the impact of cAMP-mediated signaling on inflammatory cytokine production, we measured the levels of IL-6 and IL-8 secreted by cell line pairs either treated or untreated with Rp-cAMPS in response to stimulation with IL-1β (10 ng/ml) and TNF-α (10 ng/ml). 9HTEo− pCEP (non-CF) and pCEPR (CF) cells were either treated or untreated with Rp-cAMPS for 48 h. Culture media was then replaced with media containing IL-1β and TNF-α with or without Rp-cAMPS. Following stimulation for 1 h, stimulation media was removed, and incubation with or without Rp-cAMPS was continued for 24 h. Media was collected and stored for analysis by Luminex ELISA for cytokine levels, which were normalized to whole cell protein.
Analysis of NF-κB activity.
To measure NF-κB activity, we used a firefly luciferase expression plasmid driven by the NF-κB promoter elements of the IL-8 gene. Cells were transfected with the NF-κB reporter plasmid and then after 24 h were incubated with or without Rp-cAMPS for 72 h. Fresh media with or without Rp-cAMPS was replaced daily. Cells were stimulated for 1 h with IL-1β and TNF-α 24 h before harvest (at the 48 h of treatment time point), washed, and then replaced in fresh media with or without Rp-cAMPS. Cells were cotransfected with a Renilla luciferase plasmid to control for transfection efficiency. Homogenates were centrifuged, and the supernatants were assayed for firefly (NF-κB promoter activity) and Renilla luciferase activities by dual luciferase assay. Firefly luciferase data were normalized to Renilla luciferase levels in each sample to obtain NF-κB activity.
Analyses and statistics.
Analyses in cells and animal tissues were conducted with n = 3–18 for each condition depending on required effect size (based on our experience) and are expressed as averages ± SE. N value represents experiments conducted on separate days with different sets of cultured cells (cell lines) or donors (primary cells and animals). For Westerns, samples from different donors or conditions (at least 3) were run together or separately on at least three different gels run on different days. Representative images are shown in figures. Statistical analyses of two groups were conducted using paired t-test, whereas comparisons of three or more groups were conducted using ANOVA for parametric populations (1).
RESULTS
cAMP signaling modulates Nrf2 activity.
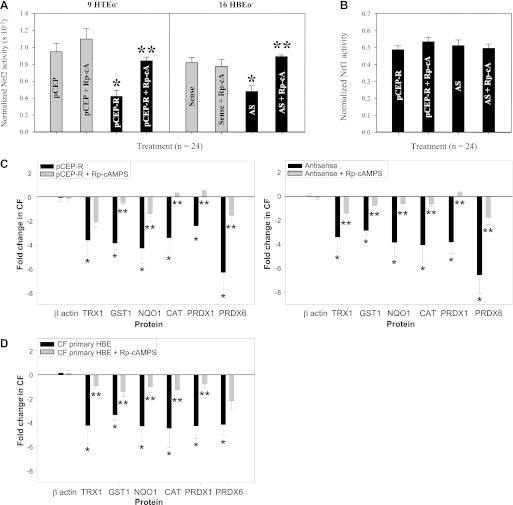
Previously, we demonstrated that cholesterol accumulation in CF cells was reversible upon treatment with the cAMP-binding competitor Rp-cAMPS, suggesting that an aspect(s) of cAMP-mediated signaling contributes to CF cellular phenotypes (26). We have also shown that CF cells exhibit a significant decrease in Nrf2 activity, and this has a profound impact on redox regulation in CF cells (7). This deficit in Nrf2-mediated cytoprotection in CF epithelia contributes to inflammation, insufficient expression of Nrf2 target genes, and a diminished antioxidant response (7). The mechanism of Nrf2 dysregulation in CF cells is not clear, but it is known that, under normal conditions, cAMP-mediated signaling can modulate Nrf2 activity (13, 22). Therefore, we investigated the influence of cAMP-mediated signaling on Nrf2 activation in CF model cells. When assessed by Nrf2 promoter-driven luciferase expression, 9HTEo− pCEPR (CF) and 16HBEo− AS (CF) cell pairs exhibit significantly lower levels of Nrf2 activity (by 52.4% and 34.8%, respectively) than non-CF matched controls, in the absence of treatment (Fig. 1A). Rp-cAMPS significantly reversed this defect in CF cell pairs, producing an increase in Nrf2 activity of 83.7–104% compared with untreated CF cells (Fig. 1A). Nevertheless, Rp-cAMPS failed to completely return Nrf2 activity to normal levels (Fig. 1A). The Rp-cAMPS effect was specific to Nrf2, as no change in Nrf1 activity was detected by luciferase reporter assay in CF or non-CF matched cell pairs (Fig. 1B).
Fig. 1.
Rp-cAMPS corrects nuclear-factor-E2-related factor 2 (Nrf2) activity in cystic fibrosis (CF) airway epithelia toward non-CF levels. To measure Nrf2 transcriptional activity, CF cell lines and non-CF matched controlled were transfected with a firefly luciferase expression cassette under the control of a Nrf2 promoter and a Renilla luciferase cassette under the control of the cytomegalovirus (CMV) promoter (transfection control). To examine the consequence of Nrf2 activity, the whole cell expression levels of proteins under the control of Nrf2 were analyzed by 2D gel electrophoresis. Both Nrf2 promoter activity and Nrf2-regulated protein expression were analyzed in the absence or presence of 50 μM Rp-cAMPS (Rp-cA) for 72 h. A: Nrf2 promoter activity normalized to Renilla control in cell line models. B: Nrf1 promoter activity normalized to Renilla control in CF cell lines. C: whole cell β-actin and Nrf2-regulated protein expression in cell line models (n = 12). D: whole cell β-actin and Nrf2-regulated protein expression in CF primary cells (n = 3). *Significant difference (P < 0.05) from non-CF control. **Significant difference (P < 0.05) from untreated CF matched cells. Error bars represent SE. AS, antisense; TRX1, thioredoxin; GST1, glutathione-S-transferase; NQO1, NAD(P)H dehydrogenase quinone; CAT, catalase; PRDX, peroxiredoxin; HBE, human bronchial epithelium; HTE, human tracheal epithelium.
To further examine Nrf2 function, we used 2D gel SDS-PAGE and proteomic analyses to determine the levels of proteins under the control of Nrf2 transcriptional activity. We first examined the 9HTEo− cell line pair. Consistent with our previous findings (7), we found a significant decrease in the expression of a number of Nrf2 target gene products, including TRX1, GST1, NQO1, and PRDX1, 3, and 6 (Fig. 1C). Decreases in these proteins at a whole cell level ranged from 2.46–6.32-fold in CF vs. non-CF cells (Fig. 1C). Treatment with Rp-cAMPS partially corrected the decrease in TRX1, GST1, NQO1, CAT, and PRDX 1 and 6 (Fig. 1C). Rp-cAMPS had no impact on β-actin levels (Fig. 1C), which is not regulated by Nrf2. We observed a similar effect of Rp-cAMPS in CF primary epithelial cells grown on filters at an air-liquid interface (Fig. 1D). These data are consistent with the activation of Nrf2 by Rp-cAMPS observed in the luciferase reporter studies and demonstrate an Nrf2-mediated functional impact of modulating cAMP-mediated signaling in CF cells.
Rp-cAMPS reduces peroxide production in CF cell lines and primary cells.
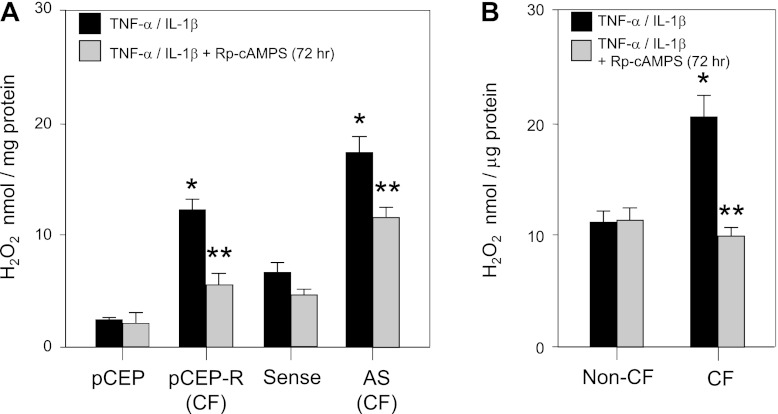
Our reporter gene and proteomic analyses indicated that Rp-cAMPS activates Nrf2 and influences the expression of a number of Nrf2 target gene products that can modulate the steady-state levels of H2O2 in the cell. Previously, we showed that H2O2 is a potent proinflammatory mediator (7). This proinflammatory effect is attributed to a number of functions of H2O2 in cells, including facilitating TNF-α (8, 20) and IL-1β (19) receptor signaling cascades, and the direct modification and activation of NF-κB (25, 36). Given the importance of inflammatory signaling in CF pathology, and its sensitivity to H2O2 levels, which are influenced by Nrf2 activity, we measured the impact of Rp-cAMPS on steady-state levels of H2O2 in our cell-culture models. We found that, in both CF cell lines and primary CF epithelia that have been stimulated with TNF-α and IL-1β, treatment with Rp-cAMPS significantly decreased steady-state H2O2 (Fig. 2). Following treatment with Rp-cAMPS, H2O2 levels in stimulated 9HTEo− pCEPR cells decreased by 53.6%, whereas levels in stimulated 16HBEo− AS cells were reduced by 32.9%, compared with untreated controls (Fig. 2A). Notably, Rp-cAMPS had little influence on H2O2 production in non-CF cell pairs. These observations extended to our primary CF cell model (Fig. 2B), in which Rp-cAMPS decreased steady-state intracellular H2O2 levels by 23–55% in three different donor vs. untreated same-donor controls. Taken together, our findings are consistent with partial and significant activation of Nrf2 by Rp-cAMPS. These studies also reveal that treatment with Rp-cAMPS elicits a CF-cell-specific inflammation-related functional outcome of modulating cAMP-mediated signaling with no apparent effect on normal control cells.
Fig. 2.
Rp-cAMPS modulates H2O2 levels in CF airway epithelia. H2O2 plays a key role the propagation of inflammatory signaling following stimulation with TNF-α and IL-1β. To examine the normalization of H2O2 levels following stimulation, Amplex red was used to measure whole cell H2O2 24 h poststimulation. Matched cell line pairs were grown to confluence and then maintained in the absence (solid bars) or presence (shaded bars) of 50 μM Rp-cAMPS for 72 h. Cells were then stimulated with TNF-α/IL-1β (10 ng/ml each) overnight and lysed, and intracellular levels of H2O2 were analyzed. A: intracellular H2O2 levels in CF cell line model pairs. B: intracellular H2O2 levels in non-CF and CF primary airway epithelia grown at an air-liquid interface. *Significant difference (P < 0.05) from non-CF control. **Significant difference (P < 0.05) from untreated CF cells. Error bars represent SE.
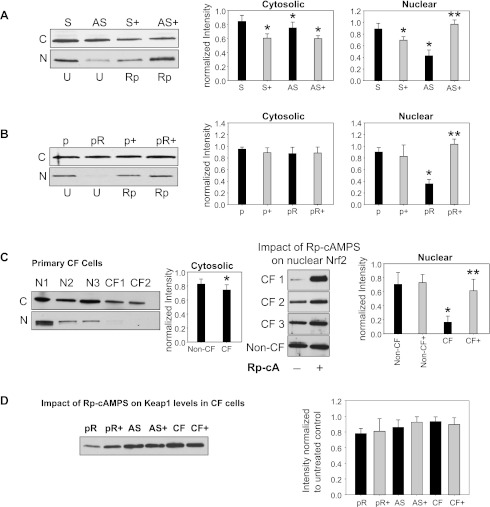
Rp-cAMPS increases nuclear levels of Nrf2 independently of Keap1 expression.
To further examine the mechanism by which Rp-cAMPS rescues Nrf2 function in CF cells, we examined Nrf2 localization in response to treatment. Levels of Nrf2 protein in the AS (CF) cell pair are slightly diminished in the cytoplasmic fraction compared with S (non-CF) controls (Fig. 3A). A significant decrease of Nrf2 accumulation in the nuclear fraction was observed in CF cells (Fig. 3A). Similar results were observed in the pCEPR (CF)/pCEP (non-CF) cell pair (Fig. 3B), as well as in primary CF epithelia compared with non-CF controls (Fig. 3C). Treatment with Rp-cAMPS only impacted cytoplasmic levels of Nrf2 in the S (non-CF)/AS (CF) cell line pair (Fig. 3A) but did not have a significant impact on cytoplasmic levels in the pCEPR (CF)/pCEP (non-CF) cell line pair or primary CF cells (Fig. 3, B and C, respectively). A robust impact of Rp-cAMPS on nuclear levels of Nrf2 in all CF cell models was observed (Fig. 3). These findings are consistent with the decrease in Nrf2 function in CF vs. non-CF cells observed in our Nrf2 promoter analyses and the examinations of Nrf2-regulated protein expression. Further studies revealed that the levels of Keap1 in CF cells were not influenced by Rp-cAMPS (Fig. 3D). This finding indicates that a decreased level of Keap1 is not the chief effect by which Rp-cAMPS activates Nrf2 in CF cells.
Fig. 3.
Rp-cAMPS modulates nuclear Nrf2, but not Kelch-like erythroid cell-derived protein with cap'n'collar homology-associated protein 1 (Keap1) levels in CF airway epithelia. The impact of Rp-cAMPS (Rp-cA, shaded bars) on Nrf2 and Keap1 levels were examined. Cells were maintained in the absence or presence of 50 μM Rp-cAMPS for 72 h. Cytoplasmic and nuclear fractions of cells were homogenized, subjected to SDS-PAGE, transferred to PVDF membrane, and probed for Nrf2 by Western blot. For Keap1 levels, whole cell homogenates were examined. Gel wells are loaded with equal amounts of protein (20 μg for cytoplasmic and whole cell fractions and 10 μg for nuclear fractions). Band intensities were normalized to β-actin levels and then to respective untreated non-CF controls (control value set as 1). Nrf2 levels in 16HBEo− (n = 12 under each condition) (A), 9HTEo− (n = 12 under each condition) (B), and human primary airway epithelia (n = 3 different donors under each condition) (C). D: whole cell Keap1 levels in CF cell models (n = 12 for cell lines, or n = 3 for primary cells, under each condition). All samples were analyzed at least 3 times on different gels. Representative gels shown. *Significant difference (P < 0.05) from non-CF control. **Significant difference (P < 0.05) from untreated (U) CF cells. Error bars represent SE.
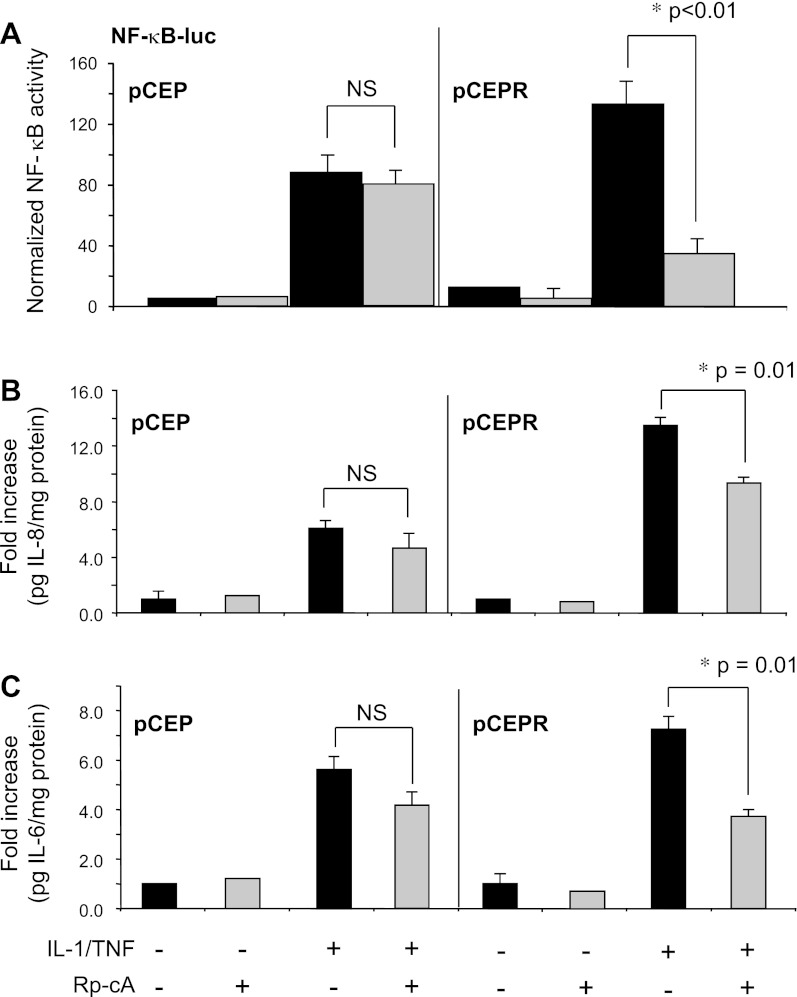
cAMP-mediated signaling influences NF-κB activation and cytokine production in CF-model cells.
The above data demonstrate that Rp-cAMPS treatment increases Nrf2 function in CF cells by increasing nuclear translocation and/or retention of the transcription factor independent of Keap1 levels. Another candidate cofactor related to cAMP-mediated signaling that has been associated with Nrf2 nuclear translocation is CBP (40). Liu et al. (23) reported that the p65 subunit of NF-κB competes with Nrf2 for CBP binding. If Rp-cAMPS influences Nrf2 regulation through CBP, this would predict that Rp-cAMPS should reduce NF-κB activation and subsequent cytokine production in a CF-specific manner. To assess whether the cAMP pathway impacts CF inflammatory signaling, we examined the effect of Rp-cAMPS on NF-κB activation, IL-6 production, and IL-8 production all in response to stimulation by IL-1β and TNF-α. NF-κB activity, as measured by luciferase reporter assay, was significantly diminished by Rp-cAMPS in 9HTEo- pCEPR (CF) cells vs. non-CF controls (Fig. 4A). These findings translated into significant differences at the level of cytokine production. Inflammatory stimulation of the 9HTEo- cell pair resulted in increases in secreted IL-8 and IL-6 levels. Rp-cAMPS had little influence on secreted IL-8 or IL-6 in non-CF control cells but significantly reduced levels in CF-model cells (Fig. 4, B and C). A similar result was observed for primary airway epithelial cells, where Rp-cAMPS treatment significantly decreased IL-8 production by 32.7% in primary CF bronchial epithelia vs. a non-significant decrease in non-CF cells (Fig. 4D). These data indicate that cAMP-related signaling plays a larger role in modulating NF-κB mediated inflammation in CF vs. non-CF epithelial cells.
Fig. 4.
NF-κB activity in CF epithelia is modulated by Rp-CAMPS. NF-κB activity was assessed by promoter analysis and cytokine measurements in 9HTEo-pCEP (non-CF) or pCEPR (CF), as well as primary epithelial cells, grown at an airway-liquid interface. Cells were maintained in the absence (solid bars) or presence (shaded bars) of 50 μM Rp-cAMPS (Rp-cA) for the duration of the experiments (72 h). NF-κB activity was measured 24 h following stimulation with 10 ng/ml TNF-α/IL-1β. Cytokine levels in media were measured in media, normalized to total cell protein, and expressed as fold change from untreated non-CF control. A: NF-κB-driven Firefly luciferase expression normalized to Renilla luciferase (n = 4 for each condition). B: IL-8 levels from the 9HTEo- cell line pair (n = 3 for each condition). C: IL-6 levels from the 9HTEo- cell line pair (n = 3 for each condition). D: IL-8 levels from primary airway epithelial cells (n = 3 for each condition). *Significant difference (P < 0.05) from stimulated CF in the absence of Rp-cAMPS. **Significant difference (P < 0.01) from stimulated CF in the absence of Rp-cAMPS. Error bars represent SE.
Rp-cAMPS inhibition of cAMP-mediated signaling modulates CBP interaction with NF-κB and Nrf2.
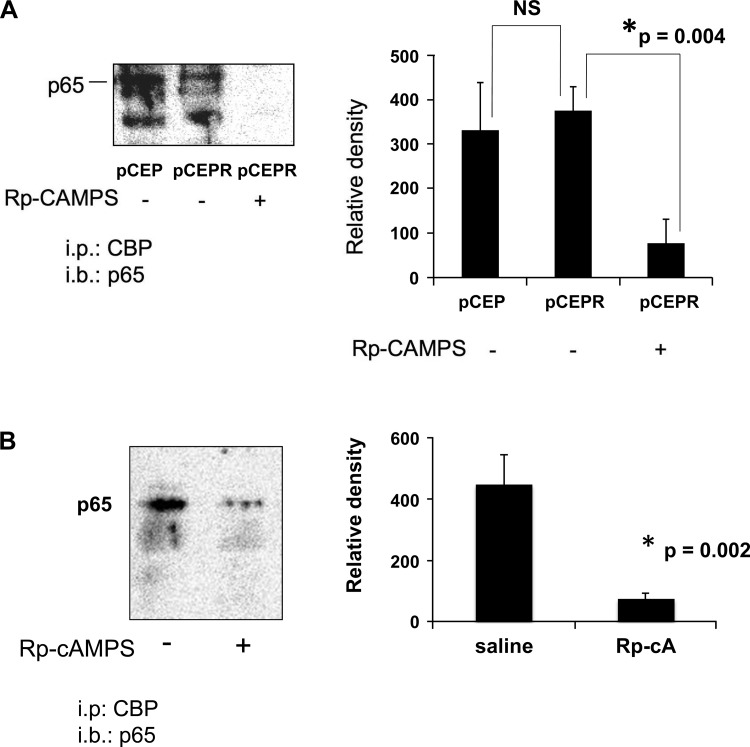
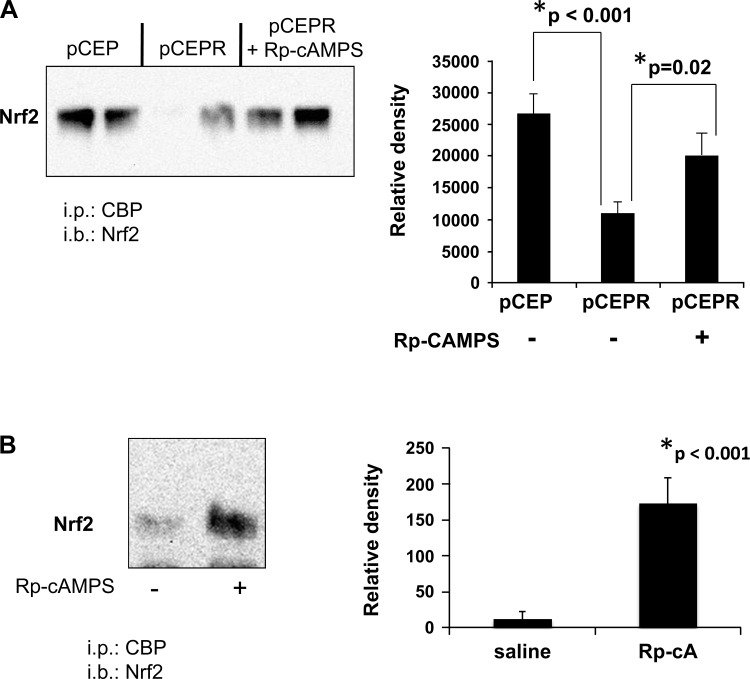
The above studies demonstrate that Rp-cAMPS treatment significantly reduces NF-κB activity and increases Nrf2 activity in CF cells, correcting both respective functions to levels approaching those in non-CF levels. These findings led us to hypothesize that Rp-cAMPS influences CF-related regulation of Nrf2 and NF-κB through a common factor, namely, CBP. We tested this hypothesis using coimmunoprecipitation assays and found that CBP binding to NF-κB p65 is not significantly different in CF cell models vs. non-CF controls (Fig. 5A) and is evident in excised nasal epithelia from ΔF508 mice (Fig. 5B). Conversely, association of CBP with Nrf2 was dramatically diminished in CF cell models (Fig. 6A) and essentially undetectable in excised nasal epithelia from ΔF508 mice (Fig. 6B). Because CBP acts to transactivate both NF-κB and Nrf2, our data are consistent with an increase in NF-κB activity and a concomitant decrease in Nrf2 activity. Treatment with Rp-cAMPS reversed CBP association patterns with NF-κB and Nrf2 in both CF cell models and nasal epithelial cells from CF mice (Figs. 5 and 6, respectively). These data are consistent with the activation of Nrf2 and the inhibition of NF-κB that we observe following treatment with Rp-cAMPS.
Fig. 5.
Rp-cAMPS decreases cAMP responsive element-binding protein (CREB) binding protein (CBP) association with NF-κB p65. CBP association with NF-κB p65 was assessed by immunoprecipitation. Whole cell homogenates of the 9HTEo- cell pair or excised nasal epithelia from DF508 mice were incubated with anti-CBP, immunoprecipitated, subjected to SDS-PAGE, transferred to PVDF membrane, and probed for p65. Cells were maintained in the absence or presence of 50 μM Rp-cAMPS for 72 h, whereas DF508 mice received saline or 50 mg/kg per day of Rp-cAMPS for 12 days by osmotic pump before assay. A: CBP interaction with p65 in the 9HTEo- pCEP (non-CF) and pCEPR (CF) matched cell pair (n = 5). B: CBP interaction with p65 in excised epithelia (untreated controls, n = 7); Rp-cAMPS treated, n = 8. All samples were analyzed at least 3 times on different gels. Representative gels shown. *Significant difference (P < 0.05) from CF in the absence of Rp-cAMPS. Error bars represent SE.
Fig. 6.
Rp-cAMPS increases CBP association with Nrf2. CBP association with Nrf2 was assessed by immunoprecipitation. Whole cell homogenates of the 9HTEo- cell pair or excised nasal epithelia from DF508 mice were incubated with anti-CBP, immunoprecipitated, subjected to SDS-PAGE, transferred to PVDF membrane, and probed for Nrf2. Cells were maintained in the absence or presence of 50 μM Rp-cAMPS for 72 h. DF508 mice received saline or 50 mg/kg per day of Rp-cAMPS for 12 days by osmotic pump before assay. A: CBP association in the 9HTEo- pCEP (non-CF) and pCEPR (CF) matched cell pair (n = 3). B: CBP interaction with Nrf2 in excised epithelia (n = 8). All samples were analyzed at least 3 times on different gels. Representative gels shown. *Significant difference (P < 0.05) from CF in the absence of Rp-cAMPS. Error bars represent SE.
DISCUSSION
Given the critical role of inflammation in CF disease progression, the current study was designed to identify the molecular pathways that modulate inflammatory signaling in CF epithelial cells. We hypothesized that dysfunction of CFTR induces a feedback elevation of cAMP-mediated signaling in CF and results in the aberrant regulation of NF-κB and Nrf2, two transcription factors that play central roles in inflammatory signaling. A number of studies have demonstrated that the activities of NF-κB and Nrf2 are inversely linked (23) and that the interplay between these activities controls the severity of the inflammatory response (21, 31). In addition, disruption of Nrf2 and a related increase in NF-κB activity have been associated with increased inflammation in a number of airway diseases with CF-like pathologies, including asthma (38) and chronic obstructive pulmonary disease (35).
Previously, we reported that Nrf2 activity is markedly reduced in CF cell lines and primary cells, as well as in lungs and excised nasal epithelia from CF mice (7). In that work, we reported a decrease in Nrf2 activity of 50–80% vs. non-CF controls. When examined the levels of Nrf2, we found that whole cell levels of Nrf2 were only slightly decreased, whereas levels of Nrf2 in the nucleus were markedly diminished, and this contributed to increased inflammatory responses (7). These observations are consistent with our present studies in primary CF cells grown at an air-liquid interface. Therefore, based on our studies, it is presently our hypothesis that the whole cell levels of Nrf2 in CF cells are not significantly relevant to Nrf2 activity in CF cells, but rather the levels of Nrf2 in the nucleus are the chief contributor to the alteration in activity. Furthermore, in this report, we present in vitro and in vivo evidence that altered interaction with CBP is a primary underlying mechanism for a pathological imbalance in the levels of NF-κB and Nrf2 in the nucleus and consequently significantly favors a pro- rather than anti-inflammatory phenotype. Treatment with a competitor of cAMP can correct abnormalities in NF-κB and Nrf2 activities and interaction with the shared transcriptional coactivator, CBP. This result is consistent with our previous findings that cAMP-mediated signaling is altered in CF cells (26).
Our findings in cultured cell lines and corroborating data from a CF mouse model and human primary CF epithelia indicate that cAMP-mediated signaling in CF cells specifically causes an increase of NF-κB activity coupled to a concomitant decrease in Nrf2 function, contributing to the proinflammatory state in CF. Although additional mechanisms likely contribute to Nrf2 dysregulation, diminished antioxidant capacity, and related excessive inflammatory signaling in CF epithelia, inhibition of cAMP-mediated signaling significantly corrects these abnormalities. Notably, this mechanism links a response to CFTR dysfunction (e.g., altered cAMP-mediated signaling) to the clinically relevant manifestations of excessive inflammation (3, 11, 29) and diminished antioxidant and anti-inflammatory signaling present in CF (6, 7, 39, 41).
Our data indicate that nuclear levels of Nrf2 are decreased in CF. One possible mechanism for this deficiency is that a decreased interaction with CBP decreases nuclear dwell time and DNA binding of the transcription factor (13, 40). Alternatively, nuclear Nrf2 accumulation may be suppressed by increased levels of Keap1. We do not currently favor this notion, as we did not detect a change in Keap1 levels in CF cells relative to non-CF cells. Further evidence against excessive Keap1-mediated Nrf2 suppression in CF epithelia derives from our previous work showing that CF cells have elevated intracellular levels of H2O2 (7), an environment expected to reduce Keap1-Nrf2 binding (45).
Interestingly, the results in the 9HTEo− cell line pair model, which recapitulates the phenotypic condition present in of epithelia of patients with CF, indicate that feedback cAMP-mediated signaling ultimately causes the dysregulation of NF-κB and Nrf2 in response to a lack of CFTR function. These data are consistent with our previous studies showing that inhibition of CFTR with CFTRinh-172 was sufficient to inhibit Nrf2 activity and thereby increase oxidant-mediated elevations in cytokine levels (7). Recently, Kelly and colleagues (14) concluded that increases in oxidants and inflammatory signaling in CF cell are independent of CFTR channel function loss, as CFTRinh-172 elicited these changes in cells that lack detectable levels of CFTR. However, these studies did show elevated levels of intracellular oxidants in the absence of CFTRinh-172 in CF vs. non-CF controls. Therefore, whereas CFTRinh-172 may have off-target effects, these studies do not preclude an effect from lack of CFTR function in cells that do express the channel.
In CF, the balance between CBP binding to NF-κB and Nrf2 is continually disrupted in a cAMP-dependent manner. Although persistent cAMP-mediated signaling may be present in CF cells as a response to inactive CFTR, the measurement of cAMP levels in CF cells has been very limited, mainly due to methodological difficulties. A single study has reported that levels of cAMP in CF nasal and tracheal epithelia, while trending higher, are not significantly different from non-CF controls (43). Levels of cAMP were not examined following inflammatory stimulation, the condition under which we observe the most significant inhibition of NF-κB by Rp-cAMPS. Noticeably, our findings on the effects of Rp-cAMPS in CF cells are consistent with a decrease in CBP-NF-κB p65 complex formation and a concomitant increase in CBP availability for binding to Nrf2. The consequence of this switch is a decrease in inflammatory signaling and an increase in antioxidant/anti-inflammatory activity. Thus the restoration of CBP distribution between Nrf2 and NF-κB represents a potential new avenue of therapeutic intervention.
Our test-compound Rp-cAMPS, although effective, is not a likely therapeutic agent because of the broad importance of the cAMP pathway in many physiological systems. We have noted in many of our treated animals slowed wound healing and some wound dehiscence, likely attributable to the Rp-cAMPS downregulation of inflammation and inhibition of granulation tissue formation. Although this is not entirely surprising, it also suggests that, as a therapeutic agent, Rp-cAMPS would likely have a narrow therapeutic window between the desired outcome of decreased airway inflammation and of potential adverse effects. These unwanted effects could potentially be limited by alternative modes of delivery (i.e., nebulization), but further study of Rp-cAMPS and its mechanism of action will be necessary. Nevertheless, the efficacy of Rp-cAMPS points to a potential impact of cAMP feedback-signaling in CF and may point to other sites of intervention that are more feasible, including strategies to mitigate the interaction between CBP with NF-κB p65. Alternatively, approaches that enhance the activity of Nrf2, especially by promoting its interaction with CBP may be desirable for CF therapy. The clear implication of this study is that therapeutically targeting CBP interactions should be considered.
GRANTS
This work was funded by the NHLBI [1R01HL109362-01 (A. Ziady), and R21HL104358 (T. Kelley)] and the Cystic Fibrosis Foundation (CFF ZIADY-Y03FGO, CFFT ZIADY08U0, and CFF KELLEY08G0). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.G.Z., S.P., and T.J.K. conception and design of research; A.G.Z., A.S., S.L.S., D.A.C., R.M., and T.J.K. performed experiments; A.G.Z., A.S., S.L.S., D.A.C., R.M., and T.J.K. analyzed data; A.G.Z., S.P., and T.J.K. interpreted results of experiments; A.G.Z., S.L.S., and T.J.K. prepared Figs.; A.G.Z. drafted manuscript; A.G.Z., A.S., and T.J.K. edited and revised manuscript; A.G.Z., A.S., S.P., and T.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
Dr. Ziady wrote the manuscript and along with Mr. Shank and Dr. Myers conducted experiments in the Ziady lab. Dr. Sokolow participated in writing the manuscript and along with Ms. Corey and Dr. Kelley conducted experiments in the Kelley lab. Dr. Plafker contributed to the design of the studies and provided the Nrf2 Ab. The authors thank the Cell Culture and Animal Cores of the Case Western Reserve University CF Center for facilitating studies in in vitro and in vivo models, respectively.
REFERENCES
- 1. Armitage P, Berry G, Matthews JNS. Analysis of non-normal data. In: Statistical Methods In Medical Research, edited by Blackwell Science Blackwell Publishing, 2002, p. 272– 311 [Google Scholar]
- 2. Becker MN, Sauer MS, Muhlebach MS, Hirsh AJ, Wu Q, Verghese MW, Randell SH. Cytokine secretion by cystic fibrosis airway epithelial cells. Am J Respir Crit Care Med 169: 645– 653, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Blackwell TS, Stecenko AA, Christman JW. Dysregulated NF-κB activation in cystic fibrosis: evidence for a primary inflammatory disorder. Am J Physiol Lung Cell Mol Physiol 281: L69– L70, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem 278: 44675– 44682, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med 152: 2111– 2118, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis 139: 370– 372, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Kinter M, Shank S, Cotton C, Kelley TJ, Ziady AG. Dysfunction of Nrf-2 in CF epithelia leads to excess intracellular H2O2 and inflammatory cytokine production. PLoS One 3: e3367, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen XL, Zhang Q, Zhao R, Medford RM. Superoxide, H2O2, and iron are required for TNF-α-induced MCP-1 gene expression in endothelial cells: role of Rac1 and NADPH oxidase. Am J Physiol Heart Circ Physiol 286: H1001– H1007, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Christman JW, Lancaster LH, Blackwell TS. Nuclear factor kappa B: a pivotal role in the systemic inflammatory response syndrome and new target for therapy. Intensive Care Med 24: 1131– 1138, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA 84: 7413– 7417, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 100: 2810– 2815, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2). Mol Cell Biol 24: 36– 45, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katoh Y, Itoh K, Yoshida E, Miyagishi M, Fukamizu A, Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 6: 857– 868, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Kelly M, Trudel S, Brouillard F, Bouillaud F, Colas J, Nguyen-Khoa T, Ollero M, Edelman A, Fritsch J. Cystic fibrosis transmembrane regulator inhibitors CFTR(inh)-172 and GlyH-101 target mitochondrial functions, independently of chloride channel inhibition. J Pharmacol Exp Ther 333: 60– 69, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Knowles MR, Stutts MJ, Spock A, Fischer N, Gatzy JT, Boucher RC. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science 221: 1067– 1070, 1983 [DOI] [PubMed] [Google Scholar]
- 16. Konstan MW. Ibuprofen therapy for cystic fibrosis lung disease: revisited. Curr Opin Pulm Med 14: 567– 573, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Kube D, Sontich U, Fletcher D, Davis PB. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am J Physiol Lung Cell Mol Physiol 280: L493– L502, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Li Q, Engelhardt JF. Interleukin-1beta induction of NFkappaB is partially regulated by H2O2-mediated activation of NFkappaB-inducing kinase. J Biol Chem 281: 1495– 1505, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol 26: 140– 154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid Redox Signal 11: 1249– 1263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 76: 1485– 1489, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin W, Shen G, Yuan X, Jain MR, Yu S, Zhang A, Chen JD, Kong AN. Regulation of Nrf2 transactivation domain activity by p160 RAC3/SRC3 and other nuclear co-regulators. J Biochem Mol Biol 39: 304– 310, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Liu GH, Qu J, Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta 1783: 713– 727, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Lo SC, Li X, Henzl MT, Beamer LJ, Hannink M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. EMBO J 25: 3605– 3617, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loukili N, Rosenblatt-Velin N, Rolli J, Levrand S, Feihl F, Waeber B, Pacher P, Liaudet L. Oxidants positively or negatively regulate nuclear factor kappaB in a context-dependent manner. J Biol Chem 285: 15746– 15752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manson ME, Corey DA, White NM, Kelley TJ. cAMP-mediated regulation of cholesterol accumulation in cystic fibrosis and Niemann-Pick type C cells. Am J Physiol Lung Cell Mol Physiol 295: L809– L819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKeon DJ, Condliffe AM, Cowburn AS, Cadwallader KC, Farahi N, Bilton D, Chilvers ER. Prolonged survival of neutrophils from patients with Delta F508 CFTR mutations. Thorax 63: 660– 661, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10: 549– 557, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Muhlebach MS, Reed W, Noah TL. Quantitative cytokine gene expression in CF airway. Pediatr Pulmonol 37: 393– 399, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med 160: 186– 191, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Nair S, Doh ST, Chan JY, Kong AN, Cai L. Regulatory potential for concerted modulation of Nrf2- and Nfkb1-mediated gene expression in inflammation and carcinogenesis. Br J Cancer 99: 2070– 2082, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol 20: 7311– 7318, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niture SK, Jaiswal AK. Prothymosin-alpha mediates nuclear import of the INrf2/Cul3 Rbx1 complex to degrade nuclear Nrf2. J Biol Chem 284: 13856– 13868, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18: 6853– 6866, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 28: 219– 242, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem 234: 239– 248, 2002 [PubMed] [Google Scholar]
- 37. Rahman I, Yang SR, Biswas SK. Current concepts of redox signaling in the lungs. Antioxid Redox Signal 8: 681– 689, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202: 47– 59, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol 75: 2419– 2424, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol 29: 2658– 2672, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Velsor LW, Kariya C, Kachadourian R, Day BJ. Mitochondrial oxidative stress in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Respir Cell Mol Biol 35: 579– 586, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. White NM, Jiang D, Burgess JD, Bederman IR, Previs SF, Kelley TJ. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 292: L476– L486, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Widdicombe JH. Cystic fibrosis and beta-adrenergic response of airway epithelial cell cultures. Am J Physiol Regul Integr Comp Physiol 251: R818– R822, 1986 [DOI] [PubMed] [Google Scholar]
- 44. Yao H, Yang SR, Kode A, Rajendrasozhan S, Caito S, Adenuga D, Henry R, Edirisinghe I, Rahman I. Redox regulation of lung inflammation: role of NADPH oxidase and NF-kappaB signalling. Biochem Soc Trans 35: 1151– 1155, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 38: 769– 789, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol 24: 10941– 10953, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]