Abstract
Trafficking and recruitment of eosinophils during allergic airway inflammation is mediated by the phosphatidylinositol 3-kinase (PI3K) family of signaling molecules. The role played by the p110δ subunit of PI3K (PI3K p110δ) in regulating eosinophil trafficking and recruitment was investigated using a selective pharmacological inhibitor (IC87114). Treatment with the PI3K p110δ inhibitor significantly reduced murine bone marrow-derived eosinophil (BM-Eos) adhesion to VCAM-1 as well as ICAM-1 and inhibited activation-induced changes in cell morphology associated with reduced Mac-1 expression and aberrant cell surface localization/distribution of Mac-1 and α4. Infused BM-Eos demonstrated significantly decreased rolling and adhesion in inflamed cremaster muscle microvessels of mice treated with IC87114 compared with vehicle-treated mice. Furthermore, inhibition of PI3K p110δ significantly attenuated eotaxin-1-induced BM-Eos migration and prevented eotaxin-1-induced changes in the cytoskeleton and cell morphology. Knockdown of PI3K p110δ with siRNA in BM-Eos resulted in reduced rolling, adhesion, and migration, as well as inhibition of activation-induced changes in cell morphology, validating its role in regulating trafficking and migration. Finally, in a mouse model of cockroach antigen-induced allergic airway inflammation, oral administration of the PI3K p110δ inhibitor significantly inhibited airway eosinophil recruitment, resulting in attenuation of airway hyperresponsiveness in response to methacholine, reduced mucus secretion, and expression of proinflammatory molecules (found in inflammatory zone-1 and intelectin-1). Overall, these findings indicate the important role played by PI3K p110δ in mediating BM-Eos trafficking and migration by regulating adhesion molecule expression and localization/distribution as well as promoting changes in cell morphology that favor recruitment during inflammation.
Keywords: phosphatidylinositol 3-kinase, adhesion molecule expression, migration, cell morphology, allergy, intravital microscopy
allergic airway inflammation is associated with increased pulmonary recruitment of inflammatory cells, especially eosinophils, elevated levels of Th2 cytokines, proinflammatory chemokines, and growth factors, as well as increased expression of adhesion molecules that together contribute to the overall pathogenesis of disease including the development of bronchoconstriction and airway hyperresponsiveness (AHR) associated with asthma (21). Recruitment of eosinophils during allergic inflammation is achieved by a multistep cascade involving consecutive steps of adhesive interactions between circulating eosinophils and the activated endothelium that is mediated by selectins, integrins, and their ligands (10). A complex interaction between inflammatory cell receptor signaling and downstream lipid and protein kinases is essential to this inflammatory cascade (41). Among the many signaling molecules, phosphatidylinositol 3-kinase (PI3K) plays an important role in leukocyte trafficking (20, 23). The PI3K family (I, II and III) of signaling molecules is central to a diverse array of cellular functions, including growth, proliferation, migration, and survival (4, 22). The PI3K pathway has been implicated in many pathologies including cancer (26, 31), diabetes (32), thrombosis (30), rheumatoid arthritis (47), and asthma (39, 58).
Class I PI3Ks are activated upon stimulation of receptors such as receptor tyrosine kinases or G protein-coupled receptors and are subdivided into two groups, class IA and IB (25, 47). Class IA PI3Ks phosphorylate phosphatidylinositol-(4,5)-phosphate to produce phosphatidylinositol-(3,4,5)-phosphate, which acts as a second messenger by recruiting pleckstrin homology domain-containing proteins to the plasma membrane to activate signaling pathways that promote proliferation, survival, differentiation, and chemotaxis (4, 25, 62). They are heterodimers consisting of a regulatory subunit (p85α, p85β, p50α, p55α, or p55γ) and a catalytic subunit (p110α, p110β, or p110δ) (4). Deregulation of signaling via the p110δ subunit of PI3K (PI3K p110δ) is known to result in severe defects of innate and adaptive immune responses (47). Therefore, targeting of PI3K p110δ has considerable therapeutic potential (3, 47). For instance, CAL-101, a close analog of IC87114 that is a highly selective inhibitor of p110δ (50), is currently in clinical testing for the treatment of a variety of hematological malignancies (9) as well as allergic rhinitis (http://clinicaltrials.gov/ct2/show/NCT00836914). The molecular mechanisms defining isoform selectivity of PI3K p110δ inhibitors based on the crystal structure of the p110δ catalytic core in the free form as well as in complexes was recently elucidated using a broad panel of p110δ-selective PI3K inhibitors (9).
The importance of PI3K p110δ in various functions of leukocytes such as B cell, T cells, NK cells, myeloid cell activities, macrophages, and mast cells is well illustrated (17). In vivo, studies have demonstrated an important role for PI3K p110δ in ovalbumin (OVA)-induced allergic asthma in mice that were deficient in p110δ activity (37) or by using the p110δ-selective inhibitor, IC87114 (35). In both studies, blockade of p110δ activity resulted in significant inhibition of airway eosinophilia. However, the mechanism by which PI3K p110δ regulates eosinophil trafficking, thus affecting recruitment to sites of inflammation such as the lung, is not known. Using IC87114, we have examined in detail the regulation of eosinophil trafficking and migration by PI3K p110δ in vitro as well as in vivo under conditions of flow by intravital microscopy (IVM) and in a model of allergic airway inflammation induced by cockroach antigen (CRA), which is a clinically significant allergen because it is an important contributor to the development of asthma in children and adults (6).
MATERIALS AND METHODS
Murine eosinophils.
Eosinophils were cultured from bone marrow (BM) obtained from 6–8-wk-old male or female BALB/c mice (Charles River, Wilmington, MA) as detailed previously (14). Differentiation of BM-derived eosinophils (BM-Eos) was assessed as described in our previous studies (8). Briefly, starting from day 8 of culture, cytocentrifuged preparations of BM cultures were stained with Hema 3 System (Fisher Scientific, Rockford, IL) and evaluated for expression of eosinophil-associated major basic protein (MBP) by confocal microscopy using a FLUOVIEW FV1000/BX61 Confocal Laser Scanning Biological Microscope equipped with an UPlanSApo lens (20×/0.85 oil) and a PlanApo N lens (60×/1.42 oil). FV10-ASW 2.0 software was used for image acquisition (Olympus, Melville, NY). Expression of Siglec-F was evaluated by flow cytometry using polyethylene-conjugated rat anti mouse Siglec-F (5 μg/ml; BD Biosciences, San Diego, CA) with a FACScan flow cytometer (BD Biosciences) and analyzed with FlowJo software (version 7.1; Tree Star, Ashland, OR). Cells between days 11 and 13 of culture that were 99% Hema 3 positive and expressed both MBP (by confocal microscopy) and Siglec-F (by flow cytometry) were used in studies.
Eosinophil expression of PI3K isoforms.
Total RNA from BM-Eos was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations and reverse transcribed using random primers and SuperScript III Reverse Transcriptase (Invitrogen) as recommended by the manufacturer. cDNA obtained after reverse transcription of total RNA was amplified using Go Taq Green master mix (Promega, Madison, WI) and forward and reverse primers for PI3K p110α, p110β, p110γ, selected from qPrimerDepot-A quantitative real-time PCR primer database (http://mouseprimerdepot.nci.nih.gov/), and PI3K-p110δ (Thermo Scientific, Dharmacon, Solaris Mouse qPCR Gene expression assay, Mouse PIK3CD). The primer sequence for β-actin was derived from a previous study (57). PCR amplification was performed in a DNA Engine PTC-0200 cycler (Bio-Rad Laboratories, Hercules, CA) under the following conditions: initial denaturation at 95°C for 2 min followed by 38 cycles of amplification [95°C for 20 s (denature), 62°C for 1 min (anneal), and 72°C for 30 s (extension)], and a final extension at 72°C for 5 min. PCR products were separated on 2% agarose gels, stained with ethidium bromide, and visualized using the FluorChem HD2 imaging system (Alpha Innotech, San Leandro, CA). Scanned images of the gels were analyzed using ImageJ image analysis system (1) to quantitate the integrated density of the bands.
Adhesion assay.
To evaluate the effect of IC87114, a selective inhibitor of PI3K p110δ (50) on BM-Eos adhesion, static adhesion assays were performed in 96-well flat-bottom cell culture plates coated with 10 μg/ml of recombinant murine (rm) vascular cell adhesion molecule (VCAM)-1 or rm intercellular adhesion molecule (ICAM)-1 as described previously (7). Briefly, BM-Eos were fluorescently labeled with Calcein-AM (1 μM, Invitrogen), treated with 10 μM IC87114 (Gilead Sciences, Seattle, WA), synthesized as described previously (51), or DMSO (vehicle control) for 20 min and then allowed to interact with rm VCAM-1- or ICAM-1-coated wells at 37°C. Nonadherent cells were removed by gentle washing (4–5 times) with PBS. The number of adherent cells in each case was quantitated against a standard curve generated with fluorescently labeled eosinophils using a FLUOStar Optima microplate reader (BMG Labtech, Durham, NC). Viability of BM-Eos after treatment with 10 μM IC87114 assessed by Trypan Blue exclusion was 99 ± 0.75%.
Assessment of changes in cell morphology.
To evaluate the effect of IC87114 on cytoskeletal/morphological changes, BM-Eos (5 × 104 cell) were allowed to attach to rm VCAM-1- or ICAM-1-coated (10 μg/ml) coverslips in the presence of 10 μM IC87114 or DMSO alone (control) for 20 min at 37°C. In some experiments, attached cells were exposed to 100 nM eotaxin-1 for an additional 5 min after treatment with IC87114 or DMSO at 37°C. Cells were gently washed two times with PBS and fixed with paraformaldehyde containing saponin and FITC-conjugated phalloidin (Invitrogen) at 2.5 U/ml as described previously (33) and evaluated by confocal microscopy. Differences in cell morphology between vehicle- and IC87114-treated cells with and without exposure to eotaxin-1 were assessed by manually counting the total number of adherent cells in five randomly selected fields of each coverslip and identifying the number of cells exhibiting morphological changes (cell spreading with several membrane protrusions, i.e., leading edges, lamellipodia, or filopodia from a round cell body), which were expressed as a percentage of the total number of adhered cells in the field (7).
Expression of cell surface receptors.
BM-Eos were treated with either IC87114 or vehicle alone as described in previous sections. After washing with PBS, expression of α4 (CD49d), lymphocyte function associated antigen (LFA-1) (CD11a), Mac-1 (CD11b), and L-selectin (CD62L) was assessed by flow cytometry using rat mAbs against murine α4 (PS/2), CD11a (eBiosciences, San Diego, CA), CD11b (eBiosciences), and CD62L (BD Biosciences), respectively, with FITC-conjugated goat anti-rat IgG as the secondary antibody. Expression of CCR3 was evaluated using FITC-conjugated rat anti-mouse CCR3 (R & D Systems, Minneapolis, MN). Depending on the mAb, rat IgG2a or 2b was used as the isotype-matched control. All antibodies were used at a final concentration of 5 μg/ml. In addition, BM-Eos were adhered to rm VCAM-1, treated with IC87114 (or DMSO) as described in earlier sections, fixed, and then evaluated for α4 expression as described previously (7). Briefly, cells were first incubated with donkey IgG in PBS as blocking buffer for 30 min at room temperature followed by overnight incubation with goat anti-mouse α4 (5 μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA) or goat IgG as isotype control. To evaluate Mac-1 expression, cells adhered to rm ICAM-1 were fixed and incubated overnight with rat anti-mouse Mac-1 (10 μg/ml, eBiosciences) or rat IgG2b as isotype control in 0.1% BSA in PBS. Rhodamine-conjugated donkey anti-goat IgG (for α4) and goat anti-rat IgG (for Mac-1) at 5 μg/ml (Jackson ImmunoResearch Laboratories, West Grove, PA) were used as the secondary antibody. Cells were evaluated by confocal microscopy.
Assessment of eosinophil trafficking in vivo by IVM.
Effect of IC87114 on BM-Eos trafficking in vivo by IVM was assessed as described in our recent studies (7). Male BALB/c mice, 25–30 g, were injected intrascrotally with 250 ng of rm TNF-α (R & D Systems) in 250 μl of isotonic saline. Approximately 3 h later, IC87114 [100 μl of 7.5 mg/ml in polyethylene glycol (PEG)-400 (Sigma-Aldrich, St. Louis, MO), 30 mg/kg] or vehicle alone was administered intraperitoneally. Mice were anesthetized, and surgery was initiated to exteriorize cremaster muscle microcirculation as described previously (63). BM-Eos (1 × 107) were fluorescently labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE, Invitrogen) in PBS for 15 min at 37°C. Labeled cells were washed, resuspended in PBS, and then administered via the carotid artery to the prepared animal placed on the stage of an intravital microscope (Leica Microsystems, Bannockburn, IL) ∼4–5 h post-TNF-α. Interaction of fluorescently labeled BM-Eos with the vascular endothelium of the cremaster muscle microvessels was made visible by stroboscopic epi-illumination using a Xenon lamp, and all images were recorded using StreamPix digital video recording software (NorPix, Montreal, Quebec, Canada). This was followed by offline analysis of recorded digital images to determine the number of rolling and adherent cells as well as the velocity of rolling eosinophils as described in our previous studies (53, 64). On average, 4–12 microvessels were analyzed per mouse.
Knockdown of PI3K p110δ in eosinophils.
BM-Eos cultured in 24-well plates in eosinophil culture medium (8) at 37°C were transfected with ON-TARGETplus SMARTpool PIK3CD siRNA (PI3K p110δ siRNA) or ON-TARGETplus Nontargeting Pool siRNA (scrambled siRNA) as a negative control (both from Thermo Scientific, Dharmacon Products) using INTERFERin siRNA transfection reagent (Polyplus-Transfection, New York, NY) according to the manufacturer's recommendations. Cells were harvested 2 days posttransfection and assessed for silencing of PI3K p110δ by Western blot analysis (10% polyacrylamide gels) with polyclonal antibodies against PI3K p110δ (1:250, Santa Cruz Biotechnology). Anti-β-actin antibodies (1:5,000; BD Biosciences) were used to monitor levels of actin expression as an internal control. Scanned images of the blots were analyzed using ImageJ, and density of the PI3K p110δ bands was normalized against β-actin after background subtraction in each case. Results are expressed as percent change in PI3K p110δ expression.
PI3K p110δ expression after silencing was also assessed by quantitative real-time RT-PCR using PerfeCTa SYBR Green FastMix for iQ (Quanta Biosciences, Gaithersburg, MD). cDNA obtained by reverse transcription of total RNA isolated from BM-Eos treated with scrambled siRNA or PI3K p110δ siRNA was amplified using primers specific for PI3K p110δ and β-actin (described in an earlier section) with SYBR Green PCR Master Mix (Quanta Biosciences). The reaction was carried out in the iQ5 multicolor real-time PCR detection System (Bio-Rad) under the following conditions: initial denaturation at 95°C for 10 min followed by 50 cycles of 95°C for 10 s and 60°C for 1 min. After PCR amplification, a melting curve was generated for every PCR product to check the specificity of the PCR reaction. All samples were run in duplicate. The relative amount of mRNA for each sample was calculated based on its threshold cycle suggested by the software (iQ5 Optical System software) compared with the threshold cycle of the housekeeping gene β-actin. The results were expressed as fold change (2−ΔΔCT) in expression after subtraction of internal β-actin control.
In addition, the effect of PI3K p110δ silencing on BM-Eos rolling in vitro (described below), adhesion, and activation-induced changes in cell morphology (as described earlier) and migration (described below) was evaluated.
Chemotaxis assay.
Migration of BM-Eos in response to eotaxin-1 was evaluated as described previously for mast cells (33) but with minor modifications. Cells were labeled with calcein-AM, treated with 10 μM IC87114 or DMSO for 20 min, and then added to the upper wells (5 × 104 cells in 50 μl/well) of HTS Transwell 96-well plates (Corning, Acton, MA) containing gelatin-coated 5.0-μm-pore polycarbonate membranes. Murine eotaxin-1 (100 nM; Peprotech, Rocky Hill, NJ) or media alone was added to the lower wells of the plates. Migration of labeled cells in response to eotaxin-1 was assessed after 2 h at 37°C and quantitated against a generated standard curve using a microplate reader as described above. Results are expressed as the number of cells after subtracting background migration in wells without eotaxin-1.
The effect of PI3K p110δ silencing on BM-Eos migration was assessed using 96-well Transwell chambers as described previously (7). Migration of BM-Eos treated with PI3K p110δ-specific or scrambled siRNA toward eotaxin-1 or media alone (background) added to the lower wells of the chamber was evaluated using an Olympus CK2 inverted microscope with a ×40 objective. Cells in 8–20 different fields of each well were counted. The assay was performed three times in duplicate. The average number of cells per field per well was determined, and results were expressed as percentage migration.
Assessment of eosinophil rolling in vitro.
Rolling of BM-Eos after PI3K p110δ knockdown was assessed on VCAM-1 under conditions of flow in an in vitro parallel plate flow chamber as described in our previous studies (45, 64). Briefly, BM-Eos transfected with PI3K p110δ-specific or scrambled siRNA were infused (2 × 105/ml) into a flow chamber containing glass coverslips coated with rm VCAM-1 (10 μg/ml in PBS, 200 μl/cover slip) at a flow rate of 1 ml/min (wall shear stress, 1.0–2.0 dynes/cm2) for 5 min with the aid of a constant infusion syringe pump (Harvard Apparatus, Holliston, MA). BM-Eos treated with DMSO (vehicle for IC87114) served as a control. Coverslips treated with PBS alone were used to determine background rolling. Interaction of the infused cells with VCAM-1 was observed using a Leitz Wetzlar inverted microscope. Images were recorded for subsequent offline analysis to manually determine the number of interacting cells. Results were expressed as the number of rolling cells per minute.
Induction of airway inflammation.
BALB/c mice (8 wk) maintained under standard pathogen-free conditions were used. All studies involving mice were performed following standards and procedures approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Test mice were sensitized intraperitoneally with 10 μg of CRA (Hollister Stier, Spokane, WA) in 100 μl saline mixed with 100 μl of Freund's adjuvant incomplete (IFA; Sigma-Aldrich). On day 14 after sensitization, mice were challenged intranasally (i.n.) with 1 μg of CRA in saline to localize the response to the lung followed by additional i.n challenges with 4 μg of CRA on days 21 and 23. To test the effect of the PI3K p110δ inhibitor IC87114, CRA-exposed mice were administered orally with IC87114 dissolved in PEG 400 (50 mg/kg) two times each day on days 21, 22 and 23 or with the vehicle PEG 400 alone. The first dose of IC87114 (or PEG 400 alone) was administered 1 h before challenge with CRA, and the second dose was administered 12 h after the first dose. Control mice received saline with IFA for sensitization and saline with PEG 400 for challenge.
Measurement of airway responsiveness.
Twenty-four hours after the last allergen challenge, airway responsiveness was measured in control and CRA-exposed mice that were treated with IC87114 or the vehicle alone by whole body plethysmography (Buxco, Troy, NY) as described (64). Briefly, mice were first exposed to saline followed by increasing concentrations (3–50 mg/ml in saline) of methacholine (MCh; Sigma-Aldrich) using an Aerosonic ultrasonic nebulizer (DeVilbiss, Somerset, PA). Each exposure was for 3 min, and the enhanced pause (PenH) in breathing after each nebulization was recorded every 10 s over a 5-min period and averaged for each MCh concentration. Results were expressed as PenH values.
Bronchoalveolar lavage fluid and lung tissue collection.
After measurement of airway responsiveness, mice were euthanized, and bronchoalveolar lavage fluid (BALF) was collected (single wash with 1.0 ml saline) for determination of total cell counts in a hemocytometer as well as differential cell counts from cytocentrifuged slides stained with the Hema 3 staining system (Fisher Diagnostics, Middletown, VA) based on morphological and histological criteria. BALF supernatant was centrifuged at 1,000 g to remove cellular debris and stored at −70°C for further evaluation. Left lungs were first perfused with 4% paraformaldehyde to preserve pulmonary structure, fixed in 4% paraformaldehyde for 48 h at 4°C, and then paraffin embedded. Right lungs were snap-frozen and stored at −80°C for subsequent analysis.
Measurement of BALF cytokines and chemokines.
The relative production of Th1 (IL-2 and IFN-γ) and Th2 (IL-4, IL-5, IL-13) type cytokines in the BALF was measured by BD Cytometric Bead Array technique with Flex Set kits (BD Biosciences) according to the manufacturer with a flow cytometer and FlowJo software for analysis. BALF eotaxin-1, monocyte chemoattractant protein (MCP)-1, and regulated on activation, T cells expressed and secreted (RANTES) levels were measured by ELISA using kits (R & D Systems) according to the manufacturers' recommendations. Optical density of the color developed was measured at 450/570 nm with a microplate reader (FLUOstar OPTIMA; BMG LABTECH, Durham, NC), and the concentration of each chemokine in the BALF was determined against a standard curve generated.
Immunohistology.
Paraffin-embedded tissue sections (4 μm thick) were deparaffinized and hydrated through a sequential alcohol series before staining with Harris Modified Hematoxylin and Shandon instant eosin (H&E) (Thermo Fisher Scientific) to determine cellular infiltration. For all immunohistological analysis, tissue sections were subjected to antigen retrieval followed by quenching of endogenous peroxidase activity before staining with specific antibodies. Sections were briefly counterstained (5 s) with hematoxylin. Appropriate VECTASTAIN ABC kits using biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA) and the Peroxidase AEC (3-amino-9-ethylcarbazole) substrate kit (Vector Laboratories) were used for detection. Stained slides were analyzed using a Nikon Microphot EPI-FL microscope, and images were captured with an Olympus DP71 camera. Semiquantitative scoring of lung tissue inflammation was carried out as described previously (12). Quantitation of lung tissue eosinophils was performed using rat mAb against murine MBP with rat IgG as a control (64). Analysis for expression of found in inflammatory zone 1 (FIZZ) and intelectin-1 was performed as described in our previous studies (8, 18) and quantitated using ImageJ as described earlier (64). Goat IgG (for FIZZ1) and sheep IgG (for intelectin-1) were used as control antibodies. Airway mucus production was assessed by staining lung sections with periodic acid-Schiff's (PAS) reagent using a kit (Sigma-Aldrich) according to the manufacturer's recommendation. PAS-positive areas in horizontally sectioned airways were quantitated using ImageJ. Results are expressed as PAS, FIZZ1, or intelectin-1 positive area (μm2)/100 μm basement membrane length of airways.
IC87114 levels in plasma.
Concentration of the IC87114 in plasma of mice was determined after liquid-liquid extraction by liquid chromatography/mass spectroscopy. The lower quantification limit was 5 ng/ml. Plasma samples from vehicle-treated animals were used as the baseline control.
Statistical analysis.
Results are expressed as the means ± SE. Statistical significance was determined using the unpaired Student's t-test. A two-tailed test was used to establish a statistically significant effect of IC87114 and of PI3K p110δ silencing in all the cellular studies and in recruitment of total inflammatory cells in the allergen-challenged animal model. A one-tailed test was used for all other analyses (Figs. 6, C–G, and 7). A P value <0.05 was considered as significant.
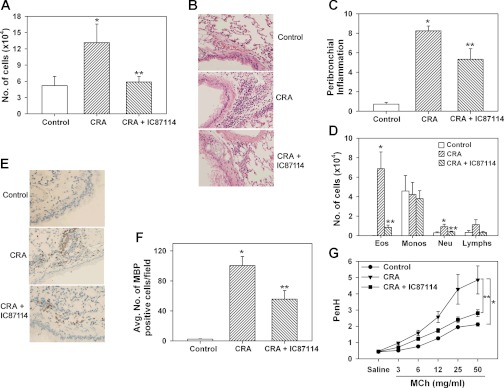
Fig. 6.
Attenuation of cockroach antigen (CRA)-induced airway inflammation in a mouse model by inhibiting PI3K p110δ activity. A: recruitment of total inflammatory cells to the airways determined by microscopic evaluation of Hema 3-stained cytocentrifuged slides of bronchoalveolar lavage fluid (BALF) in control and CRA-exposed mice treated with IC87114 (50 mg/kg) or vehicle alone (PEG-400). B and C: cellular infiltration of lung tissue after hematoxylin and eosin staining of paraformaldehyde-fixed lung sections (representative images are shown at magnification ×200) and scoring of peribronchial inflammation. Combined data from 2 experiments are shown in C. n = 7–9 mice/group. D: differential cell counts in BALF of control and CRA-exposed mice treated with IC87114 or vehicle alone. Eos, eosinophils; Monos, monocytes and macrophages; Neu, neutrophils; Lymphs, lymphocytes. Combined data from experiments repeated at least 3 times are shown in A and D. n = 6 mice for control group and 13–17 mice for CRA-exposed groups. E and F: infiltration of lung tissue by eosinophils after staining with rat mAb against murine major basic protein (MBP). Representative images are shown in E. Magnification ×200. MBP-positive cells in 5–6 randomly selected nonoverlapping microscopic fields were counted at magnification of ×400. Combined data from 2 experiments are shown in F. n = 4 mice for control group and 7 for CRA-exposed groups. G: airway hyperresponsiveness (AHR) assessed by whole body plethysmography in control and CRA-exposed mice treated with IC87114 or vehicle alone. The enhanced pause (PenH) in breathing when exposed to increasing amounts of nebulized methacholine (MCh) after initial exposure to saline was monitored. Combined data from 2 experiments are shown. n = 5 mice for control group and 6–10 mice for CRA-exposed groups. Data represent means ± SE in A, C, D, F, and G. *P < 0.03 in A, <0.01 in C, <0.01 for eosinophils and < 0.02 for neutrophils in D, <0.01 in F and <0.02 in G for comparison of control vs. CRA-exposed vehicle-treated mice; **P < 0.05 in A, <0.02 in C, <0.01 for eosinophils and <0.05 for neutrophils in D, <0.02 in F, and <0.05 in G for comparison of CRA-exposed vehicle-treated vs. CRA-exposed IC87114-treated groups.
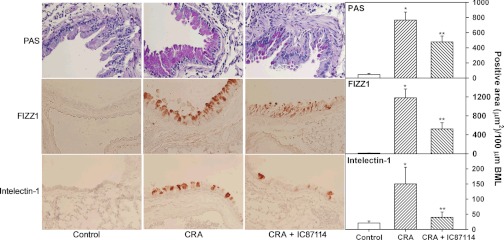
Fig. 7.
CRA-induced mucus secretion and expression of proinflammatory molecules found in inflammatory zone 1 (FIZZ1) and intelectin-1 are reduced by inhibition of PI3K p110δ activity. Lung sections from control and CRA-exposed mice treated with IC87114 or vehicle alone were evaluated for mucus secretion based on periodic acid-Schiff's (PAS) staining as well as expression of FIZZ1 and intelectin-1 by immunohistology. Representative microscopic images (left, magnification ×200) and combined quantitative data (right) of 3–6 airways from control group (n = 5) and 8–13 airways from CRA-exposed groups (n = 6–7) are shown. Data represent means ± SE. *P < 0.02 for PAS-positive staining and expression of FIZZ1 and intelectin-1 for control vs. CRA-exposed vehicle-treated mice; **P < 0.05 for PAS-positive staining and intelectin-1 expression and <0.01 for FIZZ1 expression comparing CRA-exposed IC87114-treated mice with vehicle-treated group.
RESULTS
Inactivation of PI3K p110δ with IC87114 inhibits eosinophil adhesion and trafficking.
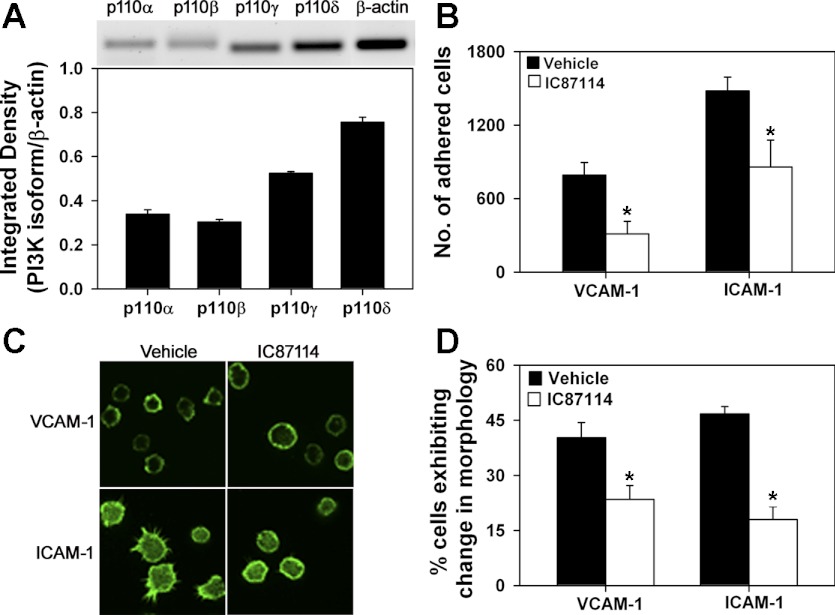
To understand the role played by PI3K p110δ in regulating eosinophil trafficking and migration, key steps involved in recruitment during airway inflammation, we first evaluated the level of expression of PI3K p110δ relative to the catalytic subunit of the other class I PI3Ks (α, β, and γ) in BM-Eos by RT-PCR and found that PI3K p110δ is the predominant catalytic subunit expressed followed by p110γ, with expression of α- and β-isoforms being much lower (Fig. 1A). Next, using IC87114, a p110δ-selective inhibitor, the role of PI3K p110δ in mediating eosinophil adhesion to endothelial adhesion molecules was evaluated (Fig. 1B). Adhesion of IC87114-treated BM-Eos to VCAM-1 and ICAM-1 was significantly inhibited compared with cells treated with the vehicle alone (DMSO).
Fig. 1.
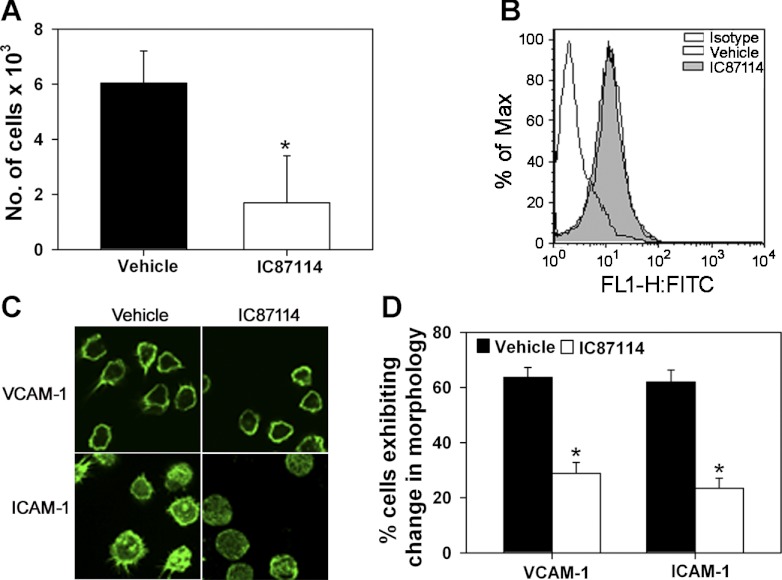
Phosphatidylinositol 3-kinase (PI3K) p110δ promotes eosinophil adhesion and changes in cell morphology. A: expression of PI3K p110α, p110β, p110γ, and p110δ in bone marrow-derived eosinophil (BM-Eos) by RT-PCR with isoform-specific primers. Expression of β-actin served as the internal control. Representative image is shown. Combined data from 3 experiments with BM-Eos from 3 different mice are shown for densitometry. B: adhesion of fluorescently labeled BM-Eos treated with IC87114 or vehicle to immobilized recombinant murine (rm) vascular cell adhesion molecule (VCAM)-1 or rm intercellular adhesion molecule (ICAM)-1 in 96-well plates under static conditions. The number of cells adhered was quantitated against a standard curve generated with fluorescently labeled eosinophils using a microplate reader. Combined data from 3 experiments in triplicate are shown. C: confocal microscopy of FITC-phalloidin-stained BM-Eos adhering to VCAM-1 or ICAM-1 on coverslips in the presence of vehicle or 10 μM IC87114 for 20 min. Magnification ×600. D: quantitation of adherent BM-Eos exhibiting changes in morphology after IC87114 treatment. Cells in 5 different fields of each coverslip exhibiting cell spreading with leading edges, lamellipodia, or filopodia after treatment with vehicle or IC87114 were counted and expressed as a percentage of the total number of cells in the field. Combined data of 3 experiments for adhesion to VCAM-1 and 2 experiments for adhesion to ICAM-1 are shown. Data represent means ± SE in A, B, and D. *P < 0.03 in B and < 0.01 in D for comparison of IC87114- vs. vehicle-treated group. Data in C are representative of 2–4 independent experiments.
Because cytoskeletal reorganization and changes in cell shape/morphology in response to activation are critical for cell attachment and subsequently for directional movement and migration, the importance of PI3K p110δ in activation-induced changes in cell morphology was evaluated by confocal microscopy. Interestingly, associated with the decreased adhesion, eosinophils treated with IC87114 exhibited distinct differences in cell morphology compared with cells treated with the vehicle alone following phalloidin staining. Vehicle-treated cells adhering to VCAM-1 largely exhibited cell spreading with formation of lamellipodia and membrane ruffles with phalloidin binding mostly localized to the cell periphery/margin (Fig. 1C, top, left). Similarly, a larger fraction of the cells adhering to ICAM-1 exhibited cell spreading with lamellipodia and distinct filopodia with phalloidin binding dispersed all over the cell (Fig. 1C, bottom, left). In both cases, a fraction of cells that were round without any extensions from the main cell body were also present. In contrast, with IC87114-treated cells, a larger fraction of the cells adhering to VCAM-1 or ICAM-1 appeared round with fewer cells demonstrating lamellipodia and filopodia (Fig. 1C, top, right and bottom, right). Quantitation of cells exhibiting changes in morphology (cell spreading with leading edges, lamellipodia or filopodia) indicated that, after treatment with IC87114, significantly fewer eosinophils adherent on VCAM-1 and ICAM-1 exhibited formation of leading edges, lamellipodia, or filopodia compared with vehicle-treated cells (Fig. 1D).
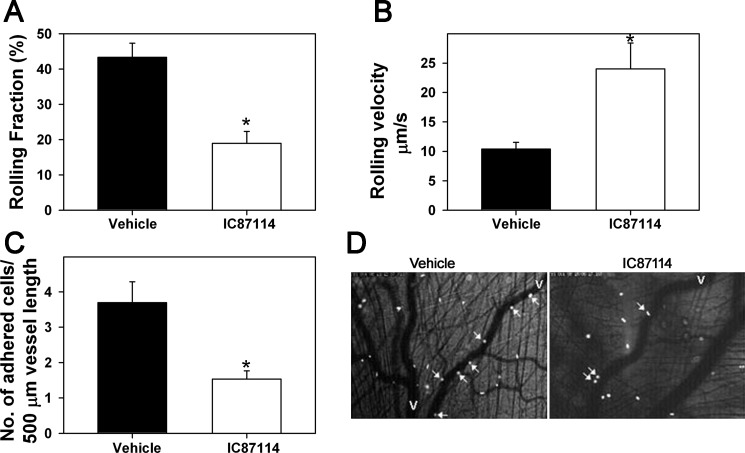
The requirement of PI3K p110δ for BM-Eos trafficking (rolling and adhesion) under conditions of physiological blood flow in inflamed microvessels of the cremaster muscle of mice administered with IC87114 in PEG 400 or vehicle alone was investigated by IVM. Infused CFSE labeled-murine eosinophils exhibited significantly decreased rolling in inflamed postcapillary venules of mice treated with IC87114 compared with microvessels of vehicle-treated mice (Fig. 2, A and D, P < 0.01). This was associated with a significant increase in the velocities of rolling eosinophils in postcapillary venules of IC87114-treated mice compared with the velocities of eosinophils rolling within inflamed venules of vehicle-treated mice (Fig. 2B, P < 0.01). Furthermore, a significantly decreased number of labeled eosinophils were found adhered to the vessel wall in IC87114-treated mice compared with mice treated with vehicle (Fig. 2, C and D, P < 0.01).
Fig. 2.
Effect of PI3K p110δ inhibition on eosinophil trafficking in vivo. A: rolling of infused murine eosinophils in inflamed cremaster muscle microvessels of mice treated with IC87114 [100 μl of 7.5 mg/ml (30 mg/kg)] or vehicle, polyethylene glycol (PEG-400), evaluated by intravital microscopy (IVM). The number of rolling cells is expressed as rolling fraction, which is a percentage of the total number of cells passing through the same reference point. B: rolling velocity of interacting eosinophils determined by offline analysis of recorded video images by choosing 4–6 rolling eosinophils per venule and measuring the time taken for the cells to travel between 2 reference points (50–200 μm). Results represent mean rolling velocity of 144 cells in the control group and 54 cells in the IC87114-treated group. C: adhesion of infused eosinophils in cremaster muscle microvessels of mice treated with IC87114 or vehicle. D: representative photomicrographs showing examples of rolling and adherent eosinophils in cremaster muscle microvessels of vehicle- and IC87114-treated mice (magnification ×100). White arrows indicate rolling and/or adherent cells. V; veins. Combined data from 4 mice for the control group and 5 mice for IC87114-treated group are shown in A–C. Rolling and adhesion were evaluated in 4–12 vessels/mouse for the control group and 4–8 vessels/mouse for IC87114-treated group. Data represent means ± SE for A–C. *P < 0.01 for comparison of IC87114- vs. vehicle-treated group.
IC87114 treatment alters expression of adhesion molecules on eosinophils.
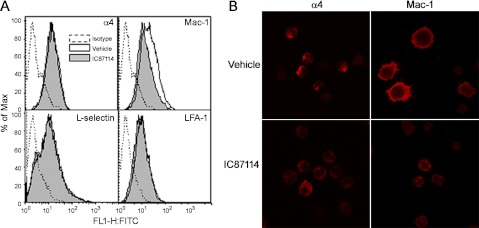
Because BM-Eos treated with IC87114 exhibit decreased adhesion to endothelial adhesion molecules in vitro as well as decreased adhesive interactions with the vascular endothelium in vivo, the effect of IC87114 on expression of α4 and β2 integrins, which together drive eosinophil rolling, tethering and arrest, as well as that of L-selectin, was investigated by flow cytometry. Whereas treatment of eosinophils with IC87114 did not alter the level of expression of α4, LFA-1, or L-selectin compared with vehicle-treated cells, cell surface expression of Mac-1 (CD11b) was reduced (Fig. 3A). Confocal microscopy studies confirmed the decreased expression of Mac-1 by eosinophils after IC87114 treatment and also demonstrated distinct alterations in Mac-1 distribution in these cells (Fig. 3B, top, right and bottom, right). Whereas vehicle-treated eosinophils adhering to ICAM-1 exhibited intense Mac-1 expression at the cell periphery/margin including filopodia with less expression in the main body of the cell, IC87114-treated eosinophils demonstrated decreased Mac-1 expression, which was dispersed all over the cell. Although no major difference was observed in the level of expression of α4, modest differences were observed in the distribution of α4 by confocal microscopy. Vehicle-treated eosinophils adhering to VCAM-1 exhibited α4 expression mostly localized at one end of the cell, whereas IC87114 treatment resulted in more dispersed expression of α4 (Fig. 3B, top, left and bottom, left).
Fig. 3.
Treatment with PI3K p110δ inhibitor alters adhesion molecule expression and distribution by eosinophils. A: expression of adhesion molecules by vehicle- and IC87114-treated BM-Eos by flow cytometry using rat monoclonal antibodies (mAbs) against α4 (CD49), lymphocyte function associated antigen (LFA)-1 (CD11a), Mac-1 (CD11b), and CD62L. Depending on the mAb, rat IgG2a or 2b was used as the isotype-matched control. All antibodies were used at a final concentration of 5 μg/ml. B: immunofluorescence staining to evaluate distribution of Mac-1 and α4 on the cell surface of vehicle- or IC87114-treated eosinophils adhered to ICAM-1 and VCAM-1, respectively. Adhered cells were stained with goat anti-mouse α4 (5 μg/ml) or rat anti-mouse Mac-1 (10 μg/ml) followed by rhodamine-conjugated secondary antibodies. Goat IgG (for anti-α4) or rat IgG2b (for anti-Mac-1) was used as the isotype control. Magnification ×600. Data shown in A and B are representative of 3 independent experiments with eosinophils from 3 different mice.
Diminished chemokine-induced migration by eosinophils after blockade of PI3K p110δ activity.
In vitro chemotaxis assays were performed to determine whether PI3K p110δ regulates BM-Eos migration. Preincubation of eosinophils with IC87114 significantly inhibited eotaxin-1-induced migration compared with cells treated with vehicle (Fig. 4A, P < 0.05) although IC87114 treatment did not alter expression of the eotaxin-1 receptor CCR3 (Fig. 4B). Furthermore, as expected, eotaxin-1 induced distinct changes in cell morphology of vehicle-treated eosinophils adherent on VCAM-1 including formation of leading edges and filopodia (Fig. 4C, top, left). A smaller fraction of cells that had an irregular shape but did not exhibit any leading edges and/or filopodia was also present. In contrast, a larger fraction of the IC87114-treated eosinophils adherent on VCAM-1 appeared irregular without any leading edges and/or filopodia after eotaxin-1 treatment with fewer polarized cells (Fig. 4C, top, right). Similar results were obtained with eosinophils adherent on ICAM-1 (Fig. 4C, bottom, left and bottom, right). Quantitation of the number of cells that exhibited changes in morphology indicated that, first, eotaxin-1 induced changes in morphology of VCAM-1- and ICAM-1-adhered eosinophils over and above that observed with adhesion to the these endothelial adhesion molecules in the absence of eotaxin-1 (Fig. 4D, ∼62% vs. 40–45% in Fig. 1D) and that, second, significantly fewer eosinophils adherent on VCAM-1 or ICAM-1 exhibited cell polarization with formation of leading edges and filopodia in response to eotaxin-1 when pretreated with IC87114 compared with cells that were pretreated with DMSO (Fig. 4D).
Fig. 4.
Inactivation of PI3K p110δ with IC87114 inhibits eosinophil migration and prevents eotaxin-1-induced changes in cell morphology. A: migration of fluorescently labeled BM-Eos treated with IC87114 or vehicle toward 100 nM murine eotaxin-1 in Transwell plates after 2 h at 37°C. Combined data from 5 independent experiments in duplicate are shown. B: expression of CCR3, the receptor for eotaxin-1, by vehicle- and IC87114-treated eosinophils by flow cytometry using FITC-conjugated anti-mouse CCR3 (5 μg/ml). Rat IgG2a was used as the isotype control. Histogram shown is representative of 3 experiments with eosinophils from 3 different mice. C: confocal microscopy of FITC-phalloidin-stained BM-Eos adhering to VCAM-1 or ICAM-1 in the presence of vehicle or 10 μM IC87114 for 20 min followed by eotaxin-1 (100 nM, 5 min). Magnification ×600. D: quantitation of adherent vehicle- and IC87114-treated BM-Eos exhibiting changes in morphology after eotaxin-1 treatment. Adhered cells in 5 different fields of each coverslip exhibiting cell spreading with distinct leading edges, lamellipodia, or filopodia after eotaxin-1 treatment were counted and expressed as a percentage of the total number of cells in the field. Combined data of 3 experiments for adhesion on VCAM-1 and 2 experiments for adhesion on ICAM-1 are shown. Data represent means ± SE in A and D. *P < 0.05 in A and < 0.01 in D for comparison of IC87114- vs. vehicle-treated eosinophils.
Inactivation of PI3K p110δ in eosinophils with siRNA results in decreased rolling, adhesion, and migration.
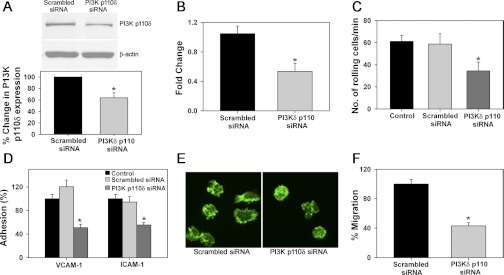
Western blot analysis for PI3K p110δ expression in BM-Eos treated with PI3K p110δ-specific siRNA revealed decreased PI3K p110δ expression compared with cells treated with nonspecific scrambled siRNA (Fig. 5A, top and bottom). This correlated with a decrease in PI3K p110δ mRNA expression by qPCR (Fig. 5B). Evaluation of eosinophil trafficking on VCAM-1 in vitro under flow conditions demonstrated significantly decreased rolling of PI3K p110δ-silenced cells on VCAM-1 (Fig. 5C), as well as decreased adhesion to VCAM-1 and ICAM-1 (Fig. 5D) with fewer of the adhered cells exhibiting morphological changes (Fig. 5E, shown for VCAM-1) compared with cells treated with scrambled siRNA. In addition, migration of PI3K p110δ-silenced BM-Eos in response to eotaxin-1 was significantly lower compared with control siRNA-treated cells (Fig. 5F), confirming the requirement of PI3K p110δ for these cellular events during eosinophil trafficking and recruitment.
Fig. 5.
Knockdown of PI3K p110δ with siRNA attenuates eosinophil rolling, adhesion, activation-induced shape change, and migration. A: expression of PI3K p110δ in BM-Eos after transfection with scrambled siRNA or PI3K p110δ siRNA by Western blot analysis using polyclonal antibodies against PI3K p110δ (1:250, top) along with densitometry (bottom). B: expression level of PI3K p110δ mRNA in BM-Eos after transfection with scrambled siRNA or PI3K p110δ siRNA detected by qPCR. Expression levels of β-actin served as the internal control. C: rolling of BM-Eos transfected with scrambled siRNA or PI3K p110δ siRNA on rm VCAM-1-coated coverslips under conditions of flow in vitro. Rolling of BM-Eos treated with DMSO (vehicle for IC87114) is shown for comparison. D: adhesion of fluorescently labeled BM-Eos after transfection with scrambled siRNA or PI3K p110δ siRNA to immobilized rm VCAM-1 or rm ICAM-1 as described in Fig. 1. Adhesion of untreated BM-Eos is shown as a control. E: confocal microscopy of FITC-phalloidin-stained BM-Eos transfected with scrambled siRNA or PI3K p110δ siRNA adhered to rm VCAM-1 (magnification ×600). F: migration of BM-Eos transfected with scrambled siRNA or PI3K p110δ siRNA toward 100 nM murine eotaxin-1 in Transwell plates after 2 h at 37°C. Data in A (top) and E are representative of 2–4 independent experiments. Combined data (mean ± SE) of 3–4 independent experiments are shown for A (bottom), B, C, D, and F. *P < 0.01 in B, D, and F and < 0.03 in A (bottom) and C for comparison of scrambled siRNA vs. PI3K p110δ siRNA-transfected eosinophils.
Treatment with the IC87114 inhibits allergen-induced airway inflammation in a mouse model.
CRA is a clinically relevant allergen contributing to the development of asthma in children and adults (6). The ability of IC87114 to inhibit CRA-induced eosinophilia and airway inflammation was evaluated in a mouse model. CRA-exposed mice treated with vehicle alone (PEG 400) demonstrated a marked increase (P < 0.05) in recruitment of inflammatory cells to the airways compared with control mice (saline exposed and treated with vehicle) as indicated by the total number of cells recovered from the BALF (Fig. 6A) and by cellular infiltration in lung tissue sections visualized by H&E staining observed predominantly around proximal airways (Fig. 6B). IC87114 treatment significantly attenuated recruitment of inflammatory cells to the airways in CRA-challenged mice (Fig. 6, A–C, P < 0.05 in 6A and 6C). Analysis of BALF differential cell counts demonstrated a marked reduction in recruitment of eosinophils in IC87114-treated CRA-exposed mice compared with vehicle-treated CRA-exposed mice (Fig. 6D, P < 0.01). In addition, a significant reduction in the number of neutrophils (P < 0.05) and a modest, but not statistically significant, reduction in the number of lymphocytes recruited to the airways was noted (Fig. 6D). CRA by itself did not induce an influx of monocyte/macrophages to the airways, which remained unaffected by IC87114 treatment. Quantitation of lung tissue eosinophils by immunohistology with mAb against eosinophil-specific MBP demonstrated several MBP-positive cells in lung sections of vehicle-treated CRA-exposed mice, whereas only a negligible number were detected in control lung sections (P < 0.05). Importantly, there was a marked reduction in the number of MBP-positive cells detected in the lungs of IC87114-treated CRA-exposed mice compared with CRA-exposed mice treated with vehicle alone (Fig. 6, E and F, P < 0.01). Along with the decreased airway eosinophilia, CRA-exposed mice treated with IC87114 exhibited markedly decreased PenH with MCh challenge compared with vehicle-treated mice (Fig. 6G, P < 0.05) that is suggestive of reduced AHR. The average peak plasma concentration of IC87114 was about 10.8 μM at 2 h after administration at a dosage of 50 mg/kg. These plasma concentrations are in the range found to be effective in inhibiting PI3K p110δ-mediated eosinophil adhesion and migration (based on in vitro studies), supporting a major role for this kinase in allergen-induced airway inflammation.
Evaluation of Th2 cytokines in the airways after allergen exposure indicated that, compared with control mice, CRA-exposed mice treated with vehicle alone had only a marginal increase in IL-4 and IL-13 levels (not statistically significant) although IL-5 levels in these mice was significantly higher (P < 0.05). Treatment of CRA-exposed mice with IC87114 lowered IL-5 levels although statistical significance was not achieved (Table 1). Levels of the Th1 cytokines IL-2 and IFN-γ remained unaltered by CRA exposure (data not shown). Levels of the eosinophil-active chemokines, eotaxin-1 and MCP-1, in the BALF was found to increase only modestly after CRA exposure and was unaltered after IC87114 treatment, remaining similar to levels observed in CRA-exposed mice treated with vehicle (Table 1). However, BALF RANTES levels were significantly higher in CRA-exposed mice compared with control mice but were only marginally reduced after IC87114 treatment (P > 0.05). In contrast, IC87114 treatment significantly inhibited mucus secretion in CRA-exposed mice as observed by the decreased number of PAS-positive cells in the bronchial epithelium of proximal airways compared with mice treated with vehicle alone (Fig. 7, top). In addition, expression of the proinflammatory molecules FIZZ1 and intelectin-1, both known to be associated with airway inflammation after allergen exposure (13, 19, 40, 56), was markedly reduced in the epithelium of proximal airways in CRA-exposed mice treated with IC87114 compared with those treated with vehicle alone (Fig. 7, middle and bottom). Overall, blockade of PI3K p110δ with IC87114 leads to a significant inhibition of airway eosinophilia induced by CRA as well as attenuation of the associated inflammatory responses, such as AHR, mucus secretion, and expression of proinflammatory molecules, although CRA-induced inflammatory cytokine (IL-5) and chemokine (RANTES) levels remained relatively unaffected.
Table 1.
BALF Th2 cytokines and chemokines in CRA-challenged mice with and without treatment with IC87114
| Cytokine | Control (n = 6 mice) | CRA (n = 12 mice) | CRA + IC87114 (n = 14 mice) |
|---|---|---|---|
| IL-4 | 1.97 ± 0.06 | 7.09 ± 2.97 | 2.8 ± 0.48 |
| IL-5 | 3.83 ± 0.16 | 21.73 ± 8.55* | 6.68 ± 0.74 |
| IL-13 | 0.31 ± 0.04 | 2.49 ± 0.94 | 0.89 ± 0.15 |
| Eotaxin-1 | 31.23 ± 5.96 | 44.64 ± 5.23 | 45.13 ± 5.41 |
| MCP-1 | 13.87 ± 2.97 | 18.91 ± 2.54 | 16.46 ± 2.0 |
| RANTES | 35.62 ± 5.18 | 61.62 ± 13.18* | 50.83 ± 5.55 |
Values are means ± SE, pg/ml.
P < 0.05 for comparison of control vs. cockroach antigen (CRA)-challenged group. BALF, bronchoalveolar lavage fluid; MCP, monocyte chemoattractant protein; RANTES, regulated on activation, T cells expressed and secreted.
DISCUSSION
Eosinophil accumulation and subsequent activation in bronchial tissues plays a critical role in the pathogenesis of allergic airway inflammation including asthma (29, 34). PI3Ks are implicated in the pathogenesis of asthma, and the contribution of the individual PI3K isoforms in airway inflammation and AHR has been reported using specific inhibitors (35) or genetically engineered mice (37, 58). A role for PI3K p110δ in promoting allergic airway inflammation has been previously demonstrated in an OVA-challenged mouse model of allergic airway inflammation using IC87114, a PI3K p110δ-selective inhibitor (35). Although these studies demonstrate that various aspects of OVA-induced airway inflammation are attenuated in IC87114-treated mice, it is not known whether the PI3K p110δ inhibitor has a direct effect on eosinophils, thereby inhibiting their trafficking and recruitment, or whether it is more indirect via inhibition of other players or mediators critical for eosinophil recruitment.
Eosinophil trafficking to sites of inflammation is mediated by a cascade of adhesive interactions between the leukocyte and the activated endothelium such as rolling followed by firm adhesion and chemokine-induced transmigration (10, 48). In the present study, we found that PI3K p110δ is the predominant isoform expressed in murine BM-Eos and that inhibition of PI3K p110δ with the p110δ-selective inhibitor IC87114 reduced cell adhesion to endothelial adhesion molecules VCAM-1 and ICAM-1 in vitro. Interestingly, changes in the cytoskeletal/cell morphology exhibited by eosinophils upon adhesion to these molecules were also significantly inhibited after IC87114 treatment. It is known that, upon activation, there is reorganization of the cytoskeleton and leukocytes undergo distinct shape change from a round free-flowing form to a polarized morphology associated with cell spreading, formation of leading edges, and filopodia to enable attachment (2). Decreased eosinophil adhesion in our studies can be attributed to inhibition of these events by PI3K p110δ inactivation as observed with IC87114 or siRNA treatment. The requirement of PI3K p110δ for eosinophil-endothelial interactions mediating cellular trafficking (rolling and adhesion) was also investigated in vivo under conditions of shear stress within inflamed blood vessels. There was, not only a reduction in the fraction of eosinophils that rolled along the vessel wall, but also an inhibition in the number of adhered cells in mice treated with IC87114 1 h before assessment. The role of PI3K p110δ in regulating eosinophil trafficking is also supported by the diminished rolling of PI3K p110δ-silenced eosinophils under conditions of flow in vitro. Furthermore, the reduced rolling and adhesion observed in microvessels of IC87114-treated mice is unlikely to be attributable to effects of the inhibitor on hemodynamic blood flow within these vessels because studies have demonstrated comparable wall shear rates in vessels of IC87114-treated and vehicle-treated mice (42). In contrast to eosinophils, PI3K p110δ does not appear to be necessary for neutrophil attachment to endothelial cells (42). In fact, eosinophils appear to behave somewhat similar to monocytes, which exhibit decreased adhesion to VCAM-1 (but not ICAM-1) associated with inhibition of cell spreading and formation of extensions when pretreated with IC87114 (15).
Interactions with the vascular endothelium during eosinophil trafficking are largely driven by α4β1-VCAM-1 and β2-integrin-ICAM-1 interactions (10). Indeed, decreased adhesion of IC87114-treated eosinophils to VCAM-1 and ICAM-1 in our studies was associated with distinct alterations in surface expression and distribution of Mac-1 with modest changes in α4 distribution, but not level of expression, that may account for the decreased attachment to ICAM-1 and VCAM-1. Although we did not evaluate β1 expression per se in the present study, studies with monocytes have demonstrated that PI3K p110δ can regulate basal very late activation antigen-4 (α4β1) activation without effecting total surface expression (as in our study) by altering β1 conformation (15). In contrast to eosinophils, PI3K p110δ inactivation did not appear to interfere with the ability of β2-integrins to bind to ICAM in neutrophils (42). However, leukotriene B4-induced neutrophil emigration was inhibited by IC87114 (42, 44). Likewise, eosinophil migration in response to eotaxin-1 was also significantly inhibited by IC87114, which was corroborated by PI3K p110δ silencing. Interestingly, IC87114 treatment had no affect on CCR3 expression, suggesting that inhibition of migration may be attributable to alternate effects at the level of cell motility that may be caused by changes in the cytoskeleton and cell morphology. It is well known that, upon stimulation, there is reorganization of the cytoskeleton of leukocytes to enable attachment, directional movement, and migration across endothelial cells. Studies with neutrophils have demonstrated that PI3K p110δ activity is required for changes in cell morphology such as spreading and for polarization in response to a chemoattractant such as formyl-Met-Leu-Phe (51). PI3K p110δ signaling has been shown to be critical for β2-integrin-mediated migration of neutrophils, and polarized cells demonstrate enrichment of PI3K p110δ and generation of PI3K products at the leading edge (52). Similarly, MCP-1-induced cell spreading of monocytes upon ICAM-1 and formation of filopodia are known to be PI3K p110δ dependent (15). Indeed, treatment of BM-Eos adhered to VCAM-1 or ICAM-1 with IC87114 before exposure to eotaxin-1 prevented eotaxin-1-induced changes in the cytoskeletal/cell morphology with more cells remaining spherical without polarizing even when exposed to eotaxin-1. Overall, inhibition of activation-induced cytoskeletal reorganization and changes in cell morphology can not only inhibit eosinophil rolling and attachment but also migration, which could lead to reduced recruitment to inflammatory sites in vivo. Recent studies using a PI3K p110γ-selective inhibitor demonstrate a role for PI3K p110γ in PAF-induced, but not eotaxin-1-induced, migration of human peripheral blood eosinophils; however, the specific role of PI3K p110δ was not investigated in these cells (24).
As a final confirmation that inhibition of PI3K p110δ can attenuate eosinophil recruitment to sites of inflammation in vivo, we used a model of allergic airway inflammation mediated by CRA. Although IC87114 has previously been shown to suppress OVA-induced allergic inflammation (35), we opted to use CRA as the allergen in the present study because it is prevalent in many settings at levels that can contribute to allergic sensitization, asthma morbidity (6, 11), and increased hospitalization in children with asthma (43). In our studies, CRA-challenged mice that were administered IC87114 demonstrated a significant reduction in total cellular recruitment to the airways (BALF and lung tissue), largely attributable to the significant decrease in eosinophil recruitment compared with CRA-challenged mice that received only the vehicle. Furthermore, these mice had markedly reduced PenH in response to MCh challenge compared with CRA-challenged mice that received only vehicle, suggesting that allergen-induced AHR may be attenuated. Once recruited, activated eosinophils, through the release of mediators (leukotrienes) and cationic proteins (MBP), are known to promote the development of AHR (34, 36, 38). Studies with human eosinophils have shown that cockroach extracts induce, not only Mac-1 expression, but also degranulation (54, 61), a process that requires Mac-1-dependent cellular adhesion (16, 28). Thus decreased expression of Mac-1 by IC87114-treated BM-Eos associated with reduced adhesion may contribute to reduced recruitment and eosinophil effector functions, AHR, and overall attenuation of airway inflammation in IC87114-treated mice.
The Th2 cytokine and chemokine responses to CRA in the present study were not observed to be as robust as that reported in a comparable study with OVA as the allergen (35), which may be attributable to the relatively small increase in the number of lymphocytes recruited to the airways after CRA exposure. However, these levels appear to be somewhat consistent with those reported in other studies involving CRA-induced allergic inflammation (5, 59). Protein levels of IL-5 and RANTES in the BALF were significantly higher in CRA-challenged mice compared with control unchallenged mice and may contribute in part to the airway inflammation. Whereas IL-5 supports recruitment of eosinophils by regulating proliferation, differentiation, and release from the BM (49), RANTES is known to function as a chemoattractant for eosinophils (46). CRA induced only a marginal increase in levels of IL-4, IL-13, eotaxin-1, and MCP-1. Furthermore, IC87114 treatment resulted in a tendency to lower the levels of the Th2 cytokines and RANTES in CRA-challenged mice; however, statistical significance was not achieved, and levels of eotaxin-1 and MCP-1 remained unaltered. These observations are in contrast to studies in the OVA-induced model of allergic inflammation, where IC87114 treatment resulted in a significant reduction in IL-5, IL-4, IL-13, eotaxin-1, and RANTES (35). Monocytes/macrophages are an important source of MCP-1, and, unlike eosinophils, the recruitment of these cells in response to CRA was not inhibited by IC87114 treatment in this study or in the OVA model (35) and may explain the persistent MCP-1 levels. These studies, further supported by our in vitro observations, suggest that the reduced eosinophil recruitment in IC87114-treated mice may be caused by a more direct effect of the inhibitor on eosinophil trafficking (rolling, adhesion, and migration) and may not entirely be driven by Th2 cytokines and eosinophil-active chemokines. Endothelial-expressed PI3K p110δ may also contribute to the modulation of airway inflammation in IC87114-treated mice in the present study because expression of endothelial adhesion molecules VCAM-1 and ICAM-1 that promote eosinophil rolling and adhesion has been shown to be reduced in the lungs of OVA-challenged mice treated with IC87114 (35) and could thus result in decreased eosinophil recruitment. Nonetheless, it is important to note that, in the in vivo trafficking studies within inflamed blood vessels described herein, mice were administered with IC87114 ∼1 h before evaluation of BM-Eos trafficking, which is unlikely to have a significant impact on the expression of VCAM-1. This, together with the inhibition of eosinophil rolling on VCAM-1 in in vitro studies after PI3K p110δ silencing, indicates the direct role of PI3K p110δ in regulating eosinophil trafficking under conditions of flow.
Finally, other indices of allergic inflammation such as mucus secretion and expression of proinflammatory molecules such as FIZZ1 (18, 27), which is associated with remodeling activity (13, 56) and intelectin-1, that is thought to have a potential role in airway mucus secretion (40), were significantly reduced by IC87114 treatment. Eosinophils, through the release of leukotrienes, are considered important mediators of mucus secretion (38), and reduced eosinophil recruitment in IC87114-treated mice may contribute to the decreased mucus secretion. In IC87114-treated mice, in the absence of a significant modulation of IL-4, which is one of the regulators of both intelectin-1 (40) and FIZZ1 (55) expression, the mechanism responsible for decreased expression of these two molecules by airway cells is not entirely clear. In addition to eosinophils, cockroach extract exerts its effects on various inflammatory cells such as epithelial cells and fibroblasts during allergic responses (60). It is possible that PI3K p110δ expressed by other airway cells such as goblet cells and epithelial cells may play a role directly or indirectly in promoting expression of these inflammatory molecules, thus accounting for their decreased expression in IC87114-treated CRA-exposed mice.
Overall, our findings indicate an essential and direct role for eosinophil-expressed PI3K p110δ in directed cell movement enabling rolling, adhesion, and chemokine-induced migration in vitro and trafficking within inflamed blood vessels in vivo by promoting activation-induced cell morphological changes and regulating adhesion molecule expression or localization/distribution, which is critical for recruitment during conditions of inflammation. In a murine model of CRA-induced airway inflammation, inhibition of PI3K p110δ with IC87114 significantly attenuated eosinophilia and airway inflammation (AHR, mucus secretion, expression of proinflammatory molecules FIZZ1 and intelectin-1) while causing only a marginal reduction in allergen-induced Th2 cytokine (IL-5) and chemokine (RANTES) levels.
GRANTS
This work was supported by National Institutes of Health grants AI35796 and HL0793041 to P. Sriramarao.
DISCLOSURES
Kamal D. Puri is an employee of Gilead Sciences, Seattle, WA, and has financial interest in this company (stock options and salary). The remaining authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
Author contributions: B.N.K., S.G.H., X.N.G., M.R.H., N.S.B., and Y.G. performed experiments; B.N.K., S.G.H., X.N.G., M.R.H., N.S.B., Y.G., S.P.R., and P.S. analyzed data; B.N.K., S.G.H., X.N.G., N.S.B., M.N.B., K.D.P., S.P.R., and P.S. interpreted results of experiments; B.N.K., X.N.G., S.P.R., and P.S. prepared figures; B.N.K. and P.S. drafted manuscript; B.N.K., S.G.H., X.N.G., M.R.H., N.S.B., Y.G., M.N.B., K.D.P., S.P.R., and P.S. approved final version of manuscript; K.D.P., S.P.R., and P.S. edited and revised manuscript; S.P.R. and P.S. conception and design of research.
REFERENCES
- 1. Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophoton Int 11: 36–42, 2004 [Google Scholar]
- 2. Alon R, Grabovsky V, Feigelson S. Chemokine induction of integrin adhesiveness on rolling and arrested leukocytes local signaling events or global stepwise activation? Microcirculation 10: 297–311, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Ameriks MK, Venable JD. Small molecule inhibitors of phosphoinositide 3-kinase (PI3K) delta and gamma. Curr Top Med Chem 9: 738–753, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Anderson KE, Jackson SP. Class I phosphoinositide 3-kinases. Int J Biochem Cell Biol 35: 1028–1033, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Arizmendi NG, Abel M, Puttagunta L, Asaduzzaman M, Davidson C, Karimi K, Forsythe P, Vliagoftis H. Mucosal exposure to cockroach extract induces allergic sensitization and allergic airway inflammation. Allergy Asthma Clin Immunol 7: 22–31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arruda LK. Cockroach allergens. Curr Allergy Asthma Rep 5: 411–416, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Bahaie NS, Hosseinkhani RM, Ge XN, Kang BN, Ha SG, Blumenthal MN, Jessberger R, Rao SP, Sriramarao P. Regulation of eosinophil trafficking by SWAP-70 and its role in allergic airway inflammation. J Immunol 188: 1479–1490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bahaie NS, Kang B, Frenzel EM, Hosseinkhani MR, Ge X, Greenberg Y, Ha S, Demetriou M, Rao SP, Sriramarao P. N-glycans differentially regulate eosinophil and neutrophil recruitment during allergic airway inflammation. J Biol Chem 286: 38231–38241, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berndt A, Miller S, Williams O, Le DD, Houseman BT, Pacold JI, Gorrec F, Hon WC, Ren P, Liu Y, Rommel C, Gaillard P, Ruckle T, Schwarz MK, Shokat KM, Shaw JP, Williams RL. The p110δ structure: mechanisms for selectivity and potency of new PI(3)K inhibitors. Nat Chem Biol 6: 117–124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Broide D, Sriramarao P. Cellular adhesion in inflammation. In: Middleton's Allergy Principles and Practice, edited by Adkinson NF. New York: Elsevier, 2008, p. 149–165 [Google Scholar]
- 11. Cohn RD, Arbes SJ, Jr, Jaramillo R, Reid LH, Zeldin DC. National prevalence and exposure risk for cockroach allergen in US households. Environ Health Perspect 114: 522–526, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doherty TA, Soroosh P, Broide DH, Croft M. CD4+ cells are required for chronic eosinophilic lung inflammation but not airway remodeling. Am J Physiol Lung Cell Mol Physiol 296: L229–L235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dong L, Wang SJ, Camoretti-Mercado B, Li HJ, Chen M, Bi WX. FIZZ1 plays a crucial role in early stage airway remodeling of OVA-induced asthma. J Asthma 45: 648–653, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally Competent Eosinophils Differentiated Ex Vivo in High Purity from Normal Mouse Bone Marrow. J Immunol 181: 4004–4009, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira AM, Isaacs H, Hayflick JS, Rogers KA, Sandig M. The p110delta isoform of PI3K differentially regulates beta1 and beta2 integrin-mediated monocyte adhesion and spreading and modulates diapedesis. Microcirculation 13: 439–456, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Fujiu T, Kato M, Kimura H, Tachibana A, Suzuki M, Nako Y, Morikawa A. Cellular adhesion is required for effector functions of human eosinophils via G-protein coupled receptors. Ann Allergy Asthma Immunol 89: 90–98, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Fung-Leung WP. Phosphoinositide 3-kinase delta (PI3KÎ′) in leukocyte signaling and function. Cell Signal 23: 603–608, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Ge XN, Bahaie NS, Kang BN, Hosseinkhani RM, Ha SG, Frenzel EM, Liu FT, Rao SP, Sriramarao P. Allergen-induced airway remodeling is impaired in galectin-3 deficient mice. J Immunol 185: 1205–1214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gu N, Kang G, Jin CE, Xu Y, Zhang Z, Erle DJ, Zhen G. Intelectin is required for IL-13-induced monocyte chemotactic protein-1 and -3 expression in lung epithelial cells and promotes allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol 298: L290–L296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Günzl P, Schabbauer G. Recent advances in the genetic analysis of PTEN and PI3K innate immune properties. Immunobiology 213: 759–765, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol 71: 489–507, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Harris SJ, Foster JG, Ward SG. PI3K isoforms as drug targets in inflammatory diseases: lessons from pharmacological and genetic strategies. Curr Opin Investig Drugs 10: 1151–1162, 2009 [PubMed] [Google Scholar]
- 23. Harris SJ, Parry RV, Westwick J, Ward SG. Phosphoinositide lipid phosphatases: natural regulators of phosphoinositide 3-kinase signaling in T lymphocytes. J Biol Chem 283: 2465–2469, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Hasan AM, Mirna M, Hameed MS, Williams GT, Dent G. Phosphoinositide 3-kinase gamma mediates chemotactic responses of human eosinophils to platelet-activating factor. Int Immunopharmacol 10: 1017–1021, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans 34: 647–662, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Hickey FB, Cotter TG. BCR-ABL regulates phosphatidylinositol 3-kinase-p110γ transcription and activation and is required for proliferation and drug resistance. J Biol Chem 281: 2441–2450, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV, Jr, Shelton DL, Hébert CC. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J 19: 4046–4055, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human granulocyte-macrophage colony stimulating factor and platelet activating factor. J Immunol 152: 5457–5467, 1994 [PubMed] [Google Scholar]
- 29. Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science 305: 1776–1779, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, Kenche V, Anderson KE, Dopheide SM, Yuan Y, Sturgeon SA, Prabaharan H, Thompson PE, Smith GD, Shepherd PR, Daniele N, Kulkarni S, Abbott B, Saylik D, Jones C, Lu L, Giuliano S, Hughan SC, Angus JA, Robertson AD, Salem HH. PI3-kinase p110[beta]: a new target for antithrombotic therapy. Nat Med 11: 507–514, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA 103: 1289–1294, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell 125: 733–747, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, Metcalfe DD. 5-Hydroxytryptamine induces mast cell adhesion and migration. J Immunol 177: 6422–6432, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305: 1773–1776, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Lee KS, Lee HK, Hayflick JS, Lee YC, Puri KD. Inhibition of phosphoinositide 3-kinase δ attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J 20: 455–465, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Lefort J, Nahori MA, Ruffie C, Vargaftig BB, Pretolani M. In vivo neutralization of eosinophil-derived major basic protein inhibits antigen-induced bronchial hyperreactivity in sensitized guinea pigs. J Clin Invest 97: 1117–1121, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nashed BF, Zhang T, Al-Alwan M, Srinivasan G, Halayko AJ, Okkenhaug K, Vanhaesebroeck B, Hayglass KT, Marshall AJ. Role of the phosphoinositide 3-kinase p110delta in generation of type 2 cytokine responses and allergic airway inflammation. Eur J Immunol 37: 416–424, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Ogawa Y, Calhoun WJ. The role of leukotrienes in airway inflammation. J Allergy Clin Immunol 118: 789–798, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Park SJ, Min KH, Lee YC. Phosphoinositide 3-kinase delta inhibitor as a novel therapeutic agent in asthma. Respirology 13: 764–771, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Pemberton AD, Rose-Zerilli MJ, Holloway JW, Gray RD, Holgate ST. A single-nucleotide polymorphism in intelectin 1 is associated with increased asthma risk. J Allergy Clin Immunol 122: 1033–1034, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Pribila JT, Shimizu Y. Signal transduction events regulating integrin function and T cell migration: new functions and complexity. Immunol Res 27: 107–128, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Puri KD, Doggett TA, Douangpanya J, Hou Y, Tino WT, Wilson T, Graf T, Clayton E, Turner M, Hayflick JS, Diacovo TG. Mechanisms and implications of phosphoinositide 3-kinase [delta] in promoting neutrophil trafficking into inflamed tissue. Blood 103: 3448–3456, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Rabito FA, Carlson J, Holt EW, Iqbal S, James MA. Cockroach exposure independent of sensitization status and association with hospitalizations for asthma in inner-city children. Ann Allergy Asthma Immunol 106: 103–109, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Randis TM, Puri KD, Zhou H, Diacovo TG. Role of PI3Kdelta and PI3Kgamma in inflammatory arthritis and tissue localization of neutrophils. Eur J Immunol 38: 1215–1224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rao SP, Wang Z, Zuberi RI, Sikora L, Bahaie NS, Zuraw BL, Liu FT, Sriramarao P. Galectin-3 functions as an adhesion molecule to support eosinophil rolling and adhesion under conditions of flow. J Immunol 179: 7800–7807, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Resnick MB, Weller PF. Mechanisms of eosinophil recruitment. Am J Respir Cell Mol Biol 8: 349–355, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Rommel C, Camps M, Ji H. PI3K[delta] and PI3K[gamma]: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol 7: 191–201, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol 119: 1303–1310, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Rothenberg ME. Eosinophilia. N Engl J Med 338: 1592–1600, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Sadhu C, Dick K, Tino WT, Staunton DE. Selective role of PI3K delta in neutrophil inflammatory responses. Biochem Biophys Res Commun 308: 764–769, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol 170: 2647–2654, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Schymeinsky J, Then C, Sindrilaru A, Gerstl R, Jakus Z, Tybulewicz VL, Scharffetter-Kochanek K, Walzog B. Syk-mediated translocation of PI3Kdelta to the leading edge controls lamellipodium formation and migration of leukocytes. PLoS One 2: e1132, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sikora L, Rao SP, Sriramarao P. Selectin-dependent rolling and adhesion of leukocytes in nicotine-exposed microvessels of lung allografts. Am J Physiol Lung Cell Mol Physiol 285: L654–L663, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Sohn MH, Lee YA, Jeong KY, Sim S, Kim KE, Yong TS, Shin MH. German cockroach extract induces activation of human eosinophils to release cytotoxic inflammatory mediators. Int Arch Allergy Immunol 134: 141–149, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Stutz AM, Pickart LA, Trifilieff A, Baumruker T, Prieschl-Strassmayr E, Woisetschlager M. The Th2 cell cytokines IL-4 and IL-13 regulate found in inflammatory zone 1/resistin-like molecule [alpha] gene expression by a STAT6 and CCAAT/enhancer-binding protein-dependent mechanism. J Immunol 170: 1789–1796, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Sun Y, Wang J, Li H, Han X. Found in inflammatory zone 1 induces angiogenesis in murine models of asthma. Lung 186: 375–380, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, Aronica M, Li X. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol 182: 1631–1640, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Takeda M, Ito W, Tanabe M, Ueki S, Kato H, Kihara J, Tanigai T, Chiba T, Yamaguchi K, Kayaba H, Imai Y, Okuyama K, Ohno I, Sasaki T, Chihara J. Allergic airway hyperresponsiveness, inflammation, and remodeling do not develop in phosphoinositide 3-kinase gamma-deficient mice. J Allergy Clin Immunol 123: 805–812, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Thomas MS, Kunkel SL, Lukacs NW. Regulation of cockroach antigen-induced allergic airway hyperreactivity by the CXCR3 ligand CXCL9. J Immunol 173: 615–623, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Wada K, Matsuwaki Y, Moriyama H, Kita H. Cockroach induces inflammatory responses through protease-dependent pathways. Int Arch Allergy Immunol 155: 135–141, 2011 [DOI] [PubMed] [Google Scholar]
- 61. Wada K, Matsuwaki Y, Yoon J, Benson LM, Checkel JL, Bingemann TA, Kita H. Inflammatory responses of human eosinophils to cockroach are mediated through protease-dependent pathways. J Allergy Clin Immunol 126: 169–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Williams R, Berndt A, Miller S, Hon WC, Zhang X. Form and flexibility in phosphoinositide 3-kinases. Biochem Soc Trans 37: 615–626, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Zanardo RCO, Bonder CS, Hwang JM, Andonegui G, Liu L, Vestweber D, Zbytnuik L, Kubes P. A down-regulatable E-selectin ligand is functionally important for PSGL-1-independent leukocyte-endothelial cell interactions. Blood 104: 3766–3773, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Zuberi RI, Ge XN, Jiang S, Bahaie NS, Kang BN, Hosseinkhani RM, Frenzel EM, Fuster MM, Esko JD, Rao SP, Sriramarao P. Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation. J Immunol 183: 3971–3979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]