Abstract
β-Adrenergic receptors (β-AR) increase epithelial sodium channel (ENaC) activity to promote lung fluid clearance. However, the effect of selective β-AR agonist on highly selective cation (HSC) channels or nonselective cation (NSC) channels in alveolar type 1 (T1) and type 2 (T2) cells is unknown. We hypothesized that stimulation with β1-AR agonist (denopamine) or β2-AR agonist (terbutaline) would increase HSC and/or NSC channel activity in alveolar epithelial cells. We performed single-channel measurements from T1 and T2 cells accessed from rat lung slices. Terbutaline (20 μM) increased HSC ENaC activity (open probability, NPo) in T1 (from 0.96 ± 0.61 to 1.25 ± 0.71, n = 5, P <0.05) and T2 cells (from 0.28 ± 0.14 to 1.0 ± 0.30, n = 8, P = 0.02). Denopamine (20 μM) increased NSC NPo in T1 cells (from 0.34 ± 0.09 to 0.63 ± 0.14, n = 7, P = 0.02) and in T2 cells (from 0.47 ± 0.09 to 0.68 ± 0.10, P = 0.004). In vivo X-ray imaging of lung fluid clearance and ICI 118,551 selective inhibition of β2-ARs confirmed patch-clamp findings. cAMP concentrations increased following treatment with denopamine or terbutaline (n = 3, P < 0.002). The effects of systemic (intraperitoneal, IP) and local (intratracheal, IT) modes of delivery on lung fluid clearance were assessed. IT delivery of denopamine promoted alveolar flooding, whereas IP delivery promoted delayed fluid clearance. In summary, β-AR agonists differentially regulate HSC and NSC in T1 and T2 cells to promote lung fluid clearance in vivo, and the mode of drug delivery is critical for maximizing β-AR agonist efficacy.
Keywords: terbutaline, denopamine, β1-adrenergic receptor, β2-adrenergic receptor
alveolar fluid homeostasis is vital for maintaining effective gas exchange. Excess alveolar fluid, or pulmonary edema, is removed from the alveolar space by active salt and water transport across the alveolar and distal airway epithelium (22). Epithelial sodium channels (ENaC) are the rate-limiting factor in alveolar fluid clearance because these channels actively transport Na+ across the apical cell membrane to create an osmotic gradient, resulting in the net clearance of fluid from the airspace. ENaC channels can be further divided into highly selective cation (HSC) channels and nonselective cation (NSC) channels based on specific measurements of conductance and amiloride sensitivity. HSCs, as the name implies, are ion channels that are highly selective for Na+ (Na+:K+ of >40:1), whereas NSC are less selective and can transport multiple cations (Na+:K+ of 1:1). On the basis, in part, of the differences in the biophysical properties of HSC and NSC channels, the efficacy of these channels in promoting lung fluid clearance may differ.
β-Adrenergic receptor (β-AR) agonists have been shown to attenuate inflammation and decrease alveolar flooding via β-AR stimulation of alveolar Na+ transport (24). β2-ARs are known to stimulate apical sodium channels and basolateral Na+-K+ ATPase on type 2 (T2) alveolar epithelial cells (8, 21, 24). Studies from knockout mice strongly suggest that functional β2-ARs are needed to promote fluid clearance via Na+ channels under physiological conditions (26). However, the utility of β-AR agonists in the treatment of acute lung injury remains unclear. Data from randomized, placebo-controlled clinical trials suggest that aerosolized β2-AR agonists are not effective in improving clinical outcomes in individuals with acute respiratory distress syndrome (ARDS) (23). However, a previous study described improved alveolar fluid clearance in humans receiving an intravenous administration of salbutamol, a β2-AR agonist (27).
Denopamine, a β1-AR agonist, has been shown to increase lung fluid clearance in hyperoxic exposed rats via Cl− transport through cystic fibrosis transmembrane conductance regulator (CFTR) (29). The effects of selective β1-AR agonist or β2-AR agonist on HSC channel or NSC channel activity in the alveoli have not been thoroughly investigated. Therefore, we tested the hypothesis that stimulation with a β1-AR agonist (denopamine) or a β2-AR agonist (terbutaline) would increase HSC and/or NSC channel activity in alveolar epithelial cells. Accordingly, we performed single-channel measurements from alveolar T1 cells and T2 cells accessed from lung slices, and we performed radiographic assays of lung fluid volume that were recently developed in our laboratory (13) to unambiguously determine the effect of denopamine and terbutaline on HSC and NSC channels and lung fluid balance.
MATERIALS AND METHODS
Animals.
Twelve-week-old female C57Bl/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) for in vivo lung fluid clearance studies. Male Sprague Dawley rats were purchased from Charles River (Wilmington, MA) and used as a source for lung tissue slices and primary cell isolation. All procedures conformed to National Institutes of Health animal care and use guidelines and were approved by the Institutional Animal Care and Use Committee of Emory University.
Reagents.
Unless stated otherwise, all reagents were purchased from Sigma-Aldrich (St. Louis, MO). Elastase was purchased from Worthington Biochemical (Lowell, MA).
Lung slice preparation.
Lung slice preparation was performed as previously described (14). Lung slices were maintained in 50:50 ice-cold DMEM/F12 (containing 10% FBS, 2 mM l-glutamine, 1 μM dexamethasone, 84 μM gentamicin, and 20 U/ml penicillin-streptomycin). Lung slices were treated and alveolar T1 cells were patched within 6 h of the initial tissue preparation.
Isolation of rat primary T2 cells.
Rat primary T2 cells were isolated using a previously published protocol (7). Briefly, the lungs were perfused via the pulmonary artery and then extracted en bloc. The trachea was cannulated and the lungs instilled with elastase solution. Then the lung lobes were excised, minced into ∼1–2-mm pieces, and sequentially filtered through 100-μm and 40-μm filters to produce a cell suspension. The cell suspension was added to mouse IgG-coated plates and panned for 1 h at 37°C. The cell suspension was collected and centrifuged, and the number of cells was counted. Approximately 5 × 104 cells were seeded to patch-clamp rings coated with crude rat-tail collagen and fed DMEM/F12. Air-interface was established, and T2 cells were used within 24 h for the patch-clamp experiments.
Tracheal instillation of saline and radiographic assessment of lung fluid clearance.
Tracheal instillations were performed as previously described (13). Briefly, mice were anesthetized with intraperitoneal (IP) injection of 15 mg/kg xylazine (Anased 100 mg/m; Lloyd Laboratories, Shenandoah, IA) and 125 mg/kg ketamine (ketamine hydrochloride injection USP 50 mg/ml; Bioniche Pharma USA, Lake Forest, IL) prepared in PBS (pH 7.4; Invitrogen, Grand Island, NY) for a final IP injection volume of 250 μl/20 g mouse. The instillate vehicle for all experiments consisted of 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, and 10 mM HEPES with pH = 7.4, hereafter referred to as control saline. Tracheal instillation volumes of 5 μl/g body wt (100 μl; final volume in 20 g C57Bl/6J mice) were delivered using a 26-gauge, 5/8-inch needle, and 1-ml syringe. IP injections of 20 μM β-agonists occurred immediately before tracheal instillation of saline, and all animals were immediately assayed for lung fluid clearance using X-ray imaging.
X-ray imaging.
Immediately after we received a tracheal instillation, lung fluid clearance was assessed using radiographic imaging as previously described (13). Briefly, animals were placed in a Kodak In-Vivo Multispectral Imaging System (Carestream Health, Rochester, NY) connected to a rodent nose cone with continuous anesthesia (1.5 l/min isoflurane) and supplemental oxygen (100%). Animals were X-ray imaged at 5-min intervals up to 240 min with an acquisition period of 120 s. X-ray settings were as follows: 2X2 binding, 180-mm field of view, 149-μA X-ray current, 35 kVp, and 0.4-mm aluminum filter. X-ray density was quantified using Carestream Health MI software with a 5-mm2 region of interest (ROI). In all whole animal studies, the ROI was the left upper lobe of the lung as described in (13).
Single-channel patch-clamp analysis of Na+ channels in alveolar cells.
Single-channel patch-clamp analysis was performed as previously described (14). Voltages are given as the negative of the patch-pipette potential (−Vp), which is the displacement of the patch potential from the resting potential. Positive potentials represent depolarization, and negative potentials represent hyperpolarization of the cell membrane away from the resting potential. We used the product of the number of channels (N) and the single-channel open probability (Po) as a measure of ENaC activity within a patch as previously described (31). NPo was calculated using FETCHAN and Clampfit 10.1 software (Molecular Devices, Sunnyvale, CA). Chord conductances were calculated between hyperpolarizing potentials for HSC channels and between hyperpolarizing and depolarizing potentials for NSC channels. To determine the effect of β-AR agonists on amiloride-sensitive channels in alveolar cells, patch-clamp electrodes were back-filled with 25 nM (to inhibit HSC activity) or 10 μM amiloride (to inhibit NSC activity) as previously reported (14).
Immunohistochemistry and confocal microscopy.
Mouse lung slices were fixed using an 8% paraformaldehyde solution and then incubated with goat poly-clonal anti-α-ENaC antibody (1:50; Santa Cruz Biotechnology, Santa Cruz, CA); goat poly-clonal anti-β1-AR antibody (1:1,000; Sigma); or rabbit poly-clonal anti-β2-AR antibody (1:1,000; Sigma). Erythrina crista galli lectin (1:1,000; Vector Laboratories, Burlingame, CA) was used to detect alveolar T1 cells. Samples were then incubated with Alexa_546 donkey anti-goat secondary antibody (1:10,000; Invitrogen,), Alexa_532 goat anti-rabbit secondary antibody (1:10,000; Sigma), or Alexa_633 donkey anti-goat antibody (1:10,000; Sigma) for 1 h and mounted on slides using Vectashield with DAPI. Thin optical sections (1-μm z-stack images) were obtained using an Olympus BX61WI microscope designed for confocal fluorescence observations alongside FluoView FV10-ASW 1.7 software.
Western blot.
Whole lung homogenates were used to quantify α-ENaC. Mouse lung was perfused via the pulmonary artery with PBS, and then the heart and lungs were removed en bloc and rinsed with PBS with 1× protease inhibitors. The lungs were homogenized and then lysed in 2 ml of RIPA buffer (150 mM NaCl, 10 mM NaPO4, 0.15 SDS, 1% Nonidet P-40, 0.25% Na+ deoxycholate). All proteins were electrophoresed on 10% acrylamide gels under denaturing conditions. Protein lysates were then transferred to Protan nitrocellulose membrane (Schleicher Schuell) for immunolabeling. The membrane was blocked in TBS with Tween buffer (10 mM Tris, pH 7.5, 70 mM NaCl, and 0.1% Tween) with 5% dry milk and then incubated with α-ENaC (1:200; Santa Cruz Biotechnology) or α-ENaC C-20 peptide (1:200; Santa Cruz) antibody overnight at 4°C. The next day, the immunoblot was incubated with IgG alkaline phosphatase-labeled secondary antibody (1:10,000; KPL, Gaithersburg, MD) diluted in TBS with Tween for 1 h at room temperature. After 5 washes, alkaline phosphatase signal was detected using Nitro-Block chemiluminescence enhancer and CDP-Star substrate (Tropix, Bedford, MA) in combination with Kodak Image Station 2000MM and Carestream MI software.
Cyclic AMP.
cAMP levels were assessed from whole lung homogenates of mice using the cAMP Direct Immunoassay Kit (Abcam, Cambridge, MA) per the manufacturer's instructions. Briefly, the lungs of mice instilled with saline and treated with an IP injection of either saline (control), 20 μM terbutaline, or 20 μM denopamine, then extracted, and immediately flash frozen in liquid nitrogen and stored in 500 μl of 0.1 N HCl at −80°C. Tissue samples were homogenized on ice and the homogenates centrifuged. The cell supernatant was acetylated, and cAMP levels were determined spectrophotometrically (optical density 450 nm).
Statistical analysis.
ENaC NPo values were examined before and after treatment with either terbutaline or denopamine. Therefore, the same patch-clamp recording before drug treatment could be used as its own control, and statistical analysis was determined by paired t-test, P values ≤0.05 were considered significant. Data are presented as means ± SE.
RESULTS
The selective β2-AR agonist, terbutaline, activates HSC channels in T1 and T2 cells.
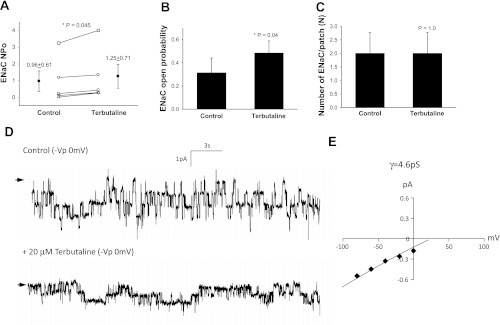
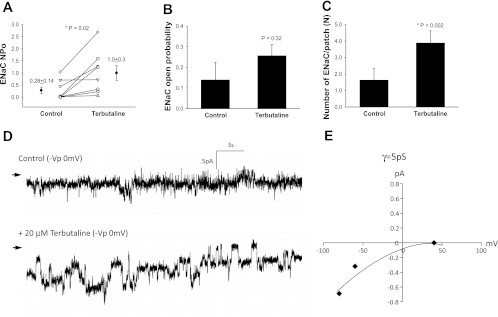
Terbutaline increased NPo from 0.96 ± 0.61 to 1.25 ± 0.71 (n = 5, P = 0.045, Fig. 1A) with a clear increase in open probability (Fig. 1, B and D) and no changes in the density of functional ENaC (Fig. 1C). The mean conductance of channels measured in T1 cells was 4.6 pS, suggesting that terbutaline activated HSC channels (Fig. 1E). In comparison, terbutaline increased NPo in T2 cells from 0.28 ± 0.14 to 1.0 ± 0.30 (n = 8, P = 0.02, Fig. 2A) with no change in ENaC open probability (P = 0.32, Fig. 2B), but an increase in the number of active channels was observed (Fig. 2, C and D). The mean conductance of T2 cells treated with terbutaline was 5 pS, which suggests that terbutaline activated HSC channels (Fig. 2E). Collectively, these data show that selective β2-AR agonist increase net sodium transport across the alveolar epithelium by increasing HSC open probability in T1 cells and by increasing the number of functional HSC ENaC in the plasma membrane of T2 cells.
Fig. 1.
Terbutaline increases highly selective cation (HSC)-epithelial sodium channel (ENaC) activity in type 1 (T1) cells. A: results from 5 independent observations shown on dot-plot graphs with y-axis = ENaC activity (NPo), where N = number of channels and Po = open probability. NPo increased from 0.96 ± 0.61 to 1.25 ± 0.71 with stimulation by 20 μM terbutaline; P = 0.045. B: results from 5 independent observations shown on bar graph with y-axis = ENaC open channel probability (Po); P = 0.04. C: 5 independent observations shown on bar graph with y-axis = number of ENaC per patch (N); P = 1.0. Asterisk denotes significant difference. D: representative recordings taken from a continuous cell-attached patch-clamp analysis of a T1 cell before (control) and after 20 μM terbutaline treatment. Arrow indicates closed state, and downward deflections depict inward entry of Na ions. E: I/V curve of ENaC channels in T1 cells following terbutaline treatment. Conductance measurements (γ = 4.6 pS) indicate the channels are HSC channels.
Fig. 2.
Terbutaline increases HSC-ENaC in T2 cells. A: results from 8 independent observations shown on dot-plot graphs with y-axis = ENaC activity (NPo). NPo increased from 0.28 ± 0.14 to 1.0 ± 0.30 with stimulation by 20 μM terbutaline; P = 0.02. B: results from 8 independent observations shown on bar graph with y-axis = ENaC open channel probability (Po); P = 0.32. C: results from 8 independent observations shown on bar graph with y-axis = number of ENaC per patch. The number of ENaC increased significantly in T2 cells following terbutaline challenge; P = 0.002. Asterisk denotes significant difference. D: representative recordings taken from a continuous cell-attached patch-clamp analysis of a T2 cell before (control) and after 20 μM terbutaline treatment. Arrow indicates closed state, and downward deflections from closed state depict inward entry of Na ions. E: I/V curve of ENaC channels in T2 cells following terbutaline treatment. Conductance measurements (γ = 5 pS) indicate the channels are HSC channels.
Denopamine, a selective β1-AR agonist, activates NSC channels in the alveoli.
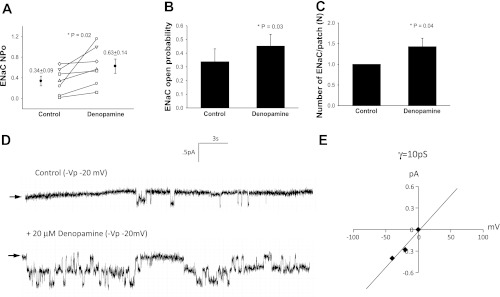
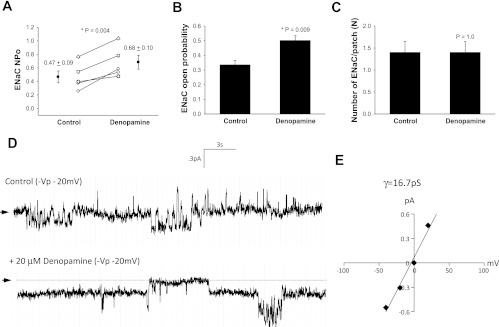
Denopamine increased ENaC (NPo) from 0.34 ± 0.09 to 0.63 ± 0.14 (n = 7, P = 0.02, Fig. 3A) while significantly altering ENaC open probability (n = 7, P = 0.03, Fig. 3, B and D) and the number of functional ENaC measured at the apical surface (n = 7, P = 0.04, Fig. 3, C and D) in alveolar T1 cells. The mean conductance was 10 pS, suggesting that predominantly NSC were activated (Fig. 3E). T2 cells treated with 20 μM of denopamine increased ENaC NPo (0.47 ± 0.09 to 0.68 ± 0.10, n = 5, P = 0.004, Fig. 4A). In these cells, the open probability of ENaC increased (n = 5, P = 0.009, Fig. 4, B and D) and denopamine had no effect on the number of ENaC (n = 5, P = 0.10, Fig. 4C). The mean conductance was 16.7 pS, suggesting that denopamine activated NSC channels (Fig. 4E).
Fig. 3.
Denopamine increases NSC-ENaC in T1 cells. A: results for 7 independent observations shown on dot-plot graphs with y-axis = ENaC activity. NPo increased from 0.34 ± 0.09 to 0.63 ± 0.14 with stimulation by 20 μM denopamine; P = 0.02. B: results from 7 independent observations shown on bar graph with y-axis = ENaC open channel probability (Po). Treatment with 20 μM denopamine significantly increased Po of channels in T1 cells; P = 0.03. C: results from 7 independent observations shown on bar graph with y-axis = number of ENaC per patch. The number of ENaC increased significantly in T1 cells following denopamine challenge; P = 0.04. Asterisk denotes significant difference. D: representative recordings taken from a continuous cell-attached patch-clamp analysis of a T1 cell before (control) and after 20 μM denopamine treatment. Arrow indicates closed state, and downward deflections from closed state depict inward entry of Na ions. E: I/V curve of ENaC channels in T1 cells following denopamine treatment. Conductance measurements (γ = 10 pS) indicate the channels are mostly nonselective cation (NSC) channels.
Fig. 4.
Denopamine increases NSC open probability in T2 cells. A: results for 5 independent observations shown on dot-plot graphs with y-axis = ENaC number of open channels (NPo). NPo increased from 0.47 ± 0.09 to 0.68 ± 0.10 with stimulation by 20 μM denopamine; P = 0.004. B: results from 8 independent observations shown on bar graph with y-axis = ENaC open channel probability (Po); P = 0.09. C: results from 8 independent observations shown on bar graph with y-axis = number of ENaC per patch. The number of ENaC did not increase in T2 cells following denopamine challenge; P = 0.35. Asterisk denotes significant difference. D: representative recordings taken from a continuous cell-attached patch-clamp analysis of a T2 cell before (control) and after 20 μM denopamine treatment. Arrow indicates closed state, and downward deflections from closed state depict inward entry of Na ions. E: I/V curve of ENaC channels in T2 cells following denopamine treatment. Conductance measurements (γ = 16.7 pS) indicate the channels are mostly NSC channels.
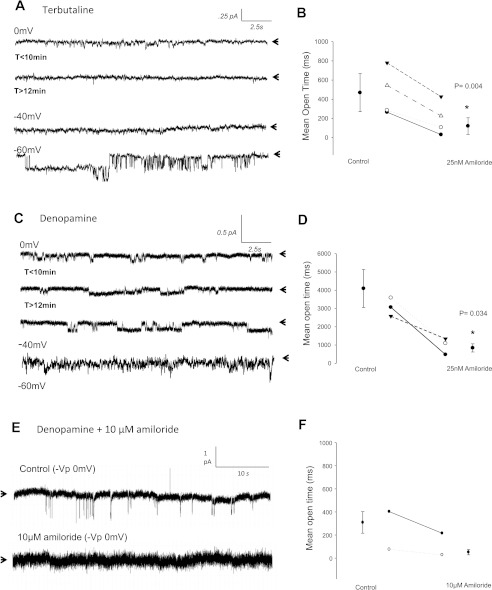
To confirm that terbutaline and denopamine differentially regulate HSC and NSC activity (respectively) in alveolar cells, we performed patch-clamp studies in which amiloride was back-filled in the patch electrode. In this way, we were able to obtain high GΩ seals between the glass electrode and cell membrane and verify the presence of active channels before amiloride blocked the gating property of ENaC from the apical side. Figure 5 shows that Na+ channels were present in alveolar cells pretreated with terbutaline before amiloride diffusion and inhibition of sodium channels at the cell membrane (after ∼10 min). Because the mean open time of amiloride-sensitive channels was effectively decreased from 714 ± 361 ms to 448 ± 328 ms (n = 4; P = 0.004, Fig. 5B) using submicromolar amounts of amiloride, and because we could restore sodium current at a strongly polarized potentials of −60 mV (−Vp), we conclude that terbutaline primarily effects HSC activity in alveolar T1 cells. The amiloride sensitivity of HSC channels in T2 cells activated by terbutaline has recently been reported (8). Similarly, we verified that denopamine stimulated larger conducting channels (between 10–16.7 pS) by performing additional patch-clamp experiments with either 25 nM or 10 μM amiloride back-filled in the patch electrode. As expected, 25 nM of amiloride decreased the mean open time of channels from 4,096 ± 1,037 ms to 448 ± 328 ms following denopamine treatment in alveolar T1 cells (n = 4; P = 0.03, Fig. 5D). Complete blockage of denopamine-activated NSC current, however, required 10 μM amiloride and is shown in Fig. 5, E–F.
Fig. 5.
Amiloride sensitivity confirms HSC and NSC channel activity following terbutaline and denopamine treatment in T1 cells. A–B: recordings taken from a continuous cell-attached patch-clamp analysis of terbutaline-treated T1 cells before (t < 10 min) and after 25 nM amiloride diffusion to apical membrane (t > 12 min). Strongly depolarizing potentials of −40 and −60 mV (−Vp) restored HSC channel activity. B: 4 independent observations with y-axis = mean open time showing 25 nM amiloride sensitivity of HSCs. 25 nM amiloride decreased mean open time from 714 ± 361 ms to 448 ± 328 ms; P = 0.004. C–D: recordings taken from a continuous cell-attached patch-clamp analysis of denopamine-treated T1 cells before (control) and after treatment with 25 nM amiloride. Arrows in representative trace indicate closed state of channel, with downward deflections from the closed state depicting entry of Na ions. Submicromolar concentrations of amiloride decreases NSC channel activity but fails to completely block gating properties at 0 mV, −40 mV, −60 mV (−Vp) potentials. D: results from 3 independent observations with y-axis = mean open time. 25 nM amiloride decreased mean open time from 4,096 ± 1,037 ms to 842 ± 223 ms; P = 0.034. E–F: recordings obtained from a continuous cell-attached patch-clamp analysis of denopamine-treated T1 cells before (control) and after treatment with 10 μM amiloride. Again, arrows in representative trace indicate closed state of channel and depict complete blockade of NSC activity with high μM amounts of amiloride.
IP injection of β-AR agonists promotes lung fluid clearance.
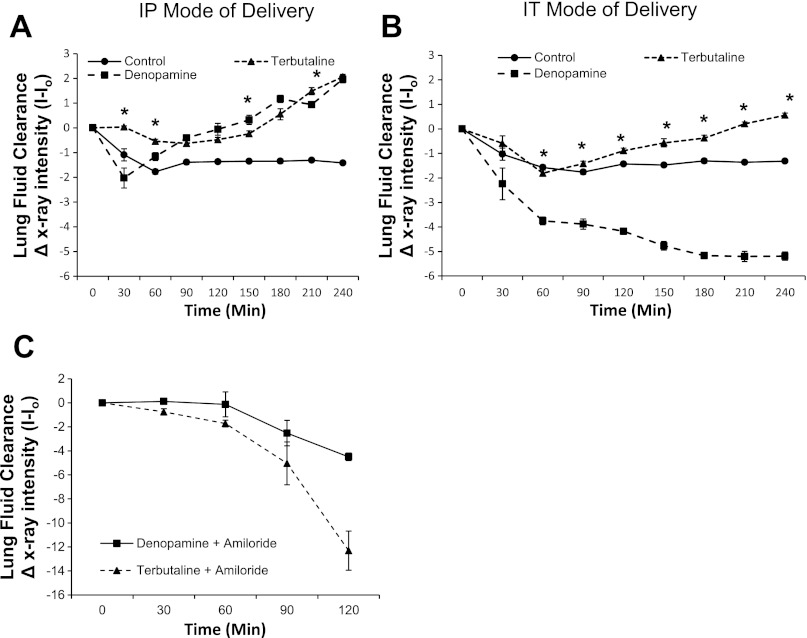
We assessed the effect of stimulation with β-AR agonists on lung fluid clearance using live X-ray fluoroscopy (13), which enabled quantification of airspace fluid content in C57Bl/6J mouse lung following an tracheal instillation of saline. First, in Fig. 6A, we IP injected 20 μM denopamine or terbutaline immediately before tracheally instilling 5 μl/g body wt (∼100 μl) instillate. Freely breathing, anesthetized animals were X-ray imaged every 5 min up to 4 h (2-min exposure times) to compare the rate of alveolar fluid clearance against uninjected (control) groups of mice that also received a saline challenge. Figure 6A shows that an IP injection of terbutaline or denopamine increased the rate of alveolar fluid clearance compared with control mice receiving a 100-μl tracheal instillation of saline. Then, in Fig. 6B, we compared the effect of administering either 20 μM of terbutaline or denopamine intratracheally (IT). We found that IT instillation of terbutaline also resulted in lung fluid clearance, albeit the response was delayed and was not as robust as IP injection of terbutaline. Additionally, IT instillation of denopamine resulted in alveolar flooding. On the basis of these data, we conclude that the parenteral route (IP injection of denopamine) was more successful than a local delivery (IT instillation of denopamine) in promoting lung fluid clearance in mice following a saline challenge. Furthermore, we instilled 1 mM amiloride (reconstituted in saline) immediately following IP injection of β-AR agonists. Figure 6C shows that lung fluid clearance was indeed abrogated by amiloride, verifying that ENaC plays a very important role in maintaining lung fluid volumes in vivo, as suggested by single-channel data presented in Figs. 1–6.
Fig. 6.
The mode of delivery of selective β-adrenergic receptor (AR) agonists affect lung fluid clearance in nonventilated mice following saline challenge of 5 μl/g body wt. A: graph depicting lung fluid clearance following saline challenge in mice receiving an intraperitoneal (IP) injection of 20 μM terbutaline or 20 μM denopamine. Control group represents mice receiving only a tracheal instillation of saline; asterisk denotes significant difference, i.e., increase in lung fluid clearance (P < 0.05) between terbutaline and denopamine treatment groups. B: graph depicting lung fluid clearance following intratracheal (IT) administration of 20 μM terbutaline or 20 μM denopamine (total instilled volume = 5 μl/g body wt). Control group represents mice receiving only a tracheal instillation of saline; asterisk denotes significant difference (P < 0.001) between terbutaline and denopamine treatment groups. C: graph depicting alveolar flooding following a tracheal instillation of 1 mM amiloride (final volume = 5 μl/g body wt) in mice receiving IP injection of either 20 μM terbutaline or 20 μM denopamine. Control group represents mice receiving only an IT instillation of saline; n = 10 per group.
Stimulation with β-AR agonists increases α-ENaC protein expression in the lung.
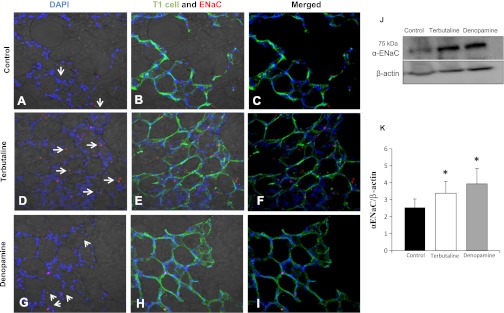
Immunohistochemical detection data of α-ENaC protein expression under control conditions (Fig. 7, A–C) and following terbutaline (Fig. 7, D–F) or denopamine (Fig. 7, G–I) treatment are provided. T2 cells (cuboidal and located at the junction of alveoli) showed robust increases in the signal intensity of anti-α-C20 ENaC and anti-goat Alexa Red-conjugated antibody labeling following β1- and β2 AR agonist treatment, whereas T1 cells responded with increases in ENaC expression only after terbutaline treatment. Western blot analysis (Fig. 7, J and K) similarly shows an increase in α-ENaC expression in the lungs of terbutaline- and denopamine-treated mice compared with untreated controls.
Fig. 7.
β-Adrenergic agonists increase α-ENaC protein expression in the lung. Tissue slices were prepared from the lungs of mice treated with either 20 μM terbutaline (IP) or 20 μM denopamine (IP) and assessed for α-ENaC expression using immunohistochemistry and confocal microscopy. A–C: images from control animals. D–F: animals treated with 20 μM terbutaline (IP). G–I: 20 μM denopamine (IP); from left to right the panels show nuclei (DAPI, blue) and α-ENaC (red) labeling overlayed on bright-field illumination of lung slices; nuclei (DAPI, blue), α-ENaC (red), and Erythrina crista galli lectin-labeled T1 cells (green) overlayed on bright-field illumination of lung slices; or finally, DAPI, α-ENaC, and T1 cell signal without bright-field illumination. Arrows point to labeled ENaC, which may not be clearly visible with bright-field illumination. Western blot analysis of enriched T2 cells (J) and quantification normalized to β-actin levels (K) verified immunohistochemical observation of α-ENaC increases following terbutaline and denopamine treatment. Asterisk denotes statistically significant difference (P < 0.05) compared with control.
β1 Receptor expression in the alveoli.
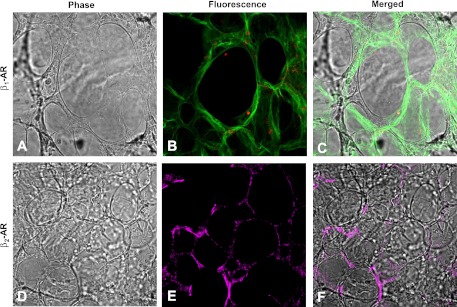
Although β1 receptor expression is commonly reported in the pulmonary vasculature and β2 receptors typically described in airway cells (6), our immunohistochemical labeling reveal that both β1 and β2 receptors can also be found in the alveoli. Figure 8A shows β1-AR expression is primarily localized to cuboidal T2 cells, whereas β2-ARs (Fig. 8B) are present in both T1 and T2 cells. This observation lead us to investigate the possibility that denopamine transactivates β2-ARs in T1 cells to stimulate ENaC activity.
Fig. 8.
β-AR distribution in the alveoli. A: rat lung slices under bright-field illumination. B: β1-AR expression (red) in lung slices counterstained with Erythrina crista galli lectin for T1 cells (green). A and B are merged in C. D–F: anti-β2-AR labeling was detected using secondary antibodies conjugated to Alexa 532 fluorophore and pseudo-colored magenta.
Denopamine transactivates β2-ARs to promote lung fluid clearance.
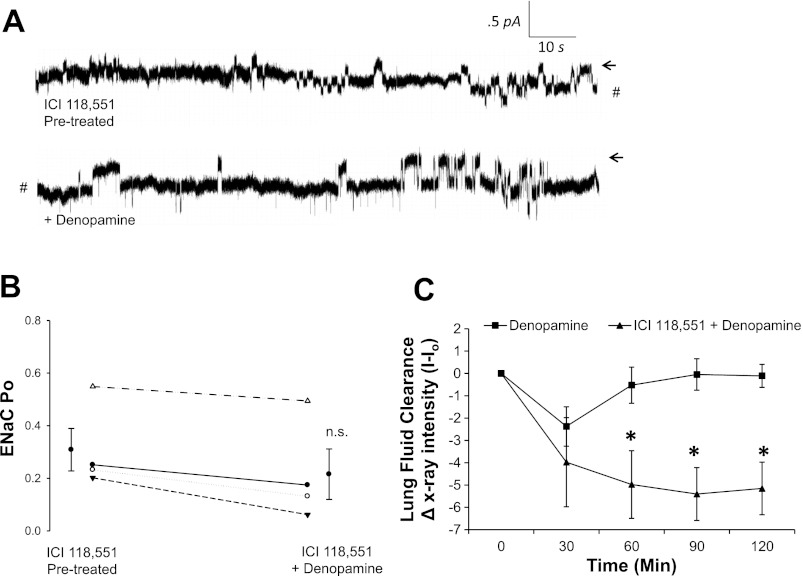
In Fig. 9A, we pretreated T1 cells with a highly selective β2-AR antagonist (ICI 118,551) to test the hypothesis that denopamine increases NSC activity in T1 cells via transactivation of β2-ARs. In support of this hypothesis, we observed no change in ENaC NPo following denopamine treatment (as previously reported in Fig. 3) in cells that had been pretreated with ICI 118, 551. Furthermore, Fig. 9C shows that ICI 118,551 abrogated the effect of denopamine on lung fluid clearance previously reported in Fig. 6.
Fig. 9.
Denopamine transactivates β2-ARs to promote lung fluid clearance. A: T1 cells were pretreated with 50 nM of ICI 118,551, a highly selective β2-AR antagonist, and with subsequent application of denopamine to the saline bath after a control recording period. Arrows indicate closed state, and downward deflections from the closed state depict entry of Na ions. #Break in continuous cell-attached patch-clamp recording. B: results for 4 independent observations shown on dot-plot graphs with y-axis = ENaC activity (Po) in T1 cells pretreated with 50 nM ICI 118,551 and then stimulated with 20 μM denopamine. C: lung fluid clearance in mice receiving an IP injection of 20 μM denopamine (■) and pulmonary edema in 50 nM ICI 118,551 and 20 μM denopamine (▴)-treated animals; asterisk denotes statistical significance (P < 0.05).
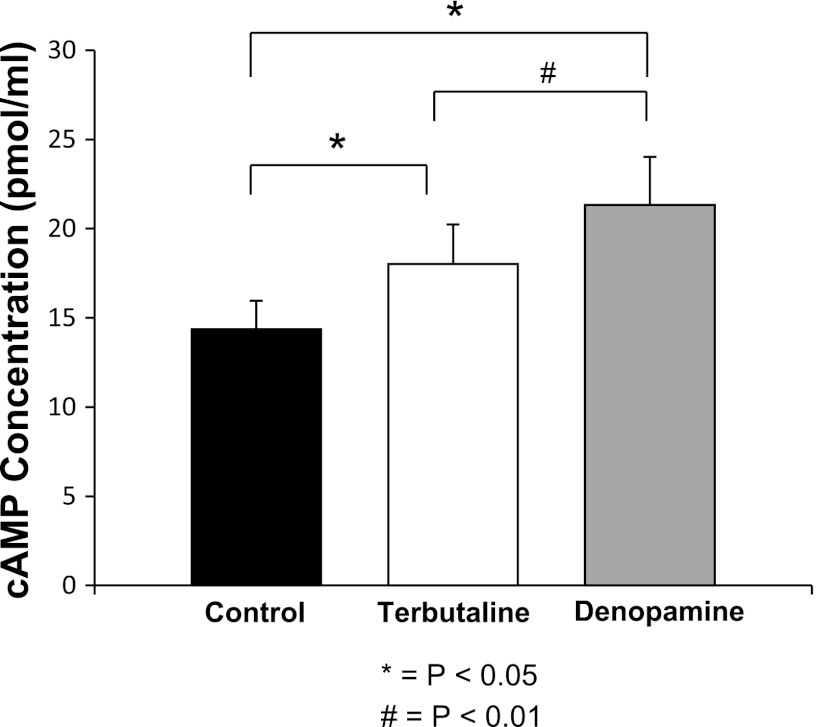
Figure 10 shows that both β-AR agonists significantly increased cAMP levels compared with controls (n = 3), with denopamine yielding a greater increase in cAMP levels compared with animals treated with terbutaline (P < 0.05). This observation further suggests that transactivation of alveolar T1 cells by β2-ARs occurs and provides a plausible signaling mechanism by which it could happen.
Fig. 10.
Cyclic AMP levels from whole lung homogenates of mice given an IT instillation of saline. Results from 3 independent observations shown on bar graph with y-axis = cAMP concentration. IP treatment with 20 μM of terbutaline or 20 μM denopamine increased cAMP levels in whole lung homogenates (P < 0.05). In addition, we observed a significant increase in cAMP in denopamine-treated mice compared with terbutaline (P < 0.002), likely secondary to the increased concentration of β1-ARs in the highly vascularized lung.
DISCUSSION
Major findings of this study are that β-AR agonists selectively increase either HSC or NSC channels to promote fluid clearance in the lung and that IT delivery of denopamine significantly worsened alveolar flooding. These results suggest that β-AR agonists differentially affect ENaC channels by promoting the upregulation of HSC or NSC channels and that the mode of drug delivery influences the effectiveness of β-AR agonists. These observations have implications for our understanding of the role of β-AR agonists in the treatment of acute lung injury.
β-AR expression in the alveolar epithelium.
β-Adrenergic agonists have been shown to regulate both ion and liquid transport in the lungs (3, 30). In the present study, however, we examined β-AR expression and the electrophysiological properties of alveolar T1 cells and T2 cells to clarify the role β-AR agonists (terbutaline and denopamine) play in the regulation of amiloride-sensitive epithelial sodium channels in the alveoli. Although it is clear that the dominant subtype of β-ARs in the lung are β2-ARs (6, 20), the expression profile of other β-ARs in the lung is not clear. Using immunohistochemical labeling of lung tissue, we show in Fig. 8 that β2-ARs are expressed in all cells that make up the alveolar surface area (i.e., T1 and T2 cells), whereas β1-ARs are only expressed in T2 cells. This observation provides insight into the respiratory effects of β-blocker therapy, as well as mechanistic insight into crossactivation of G protein-coupled signaling. For example, in a recent study, carvedilol (a pharmacological blocker of β1-AR and β2-AR) significantly decreased lung function (i.e., diffusion capacity of CO in the alveoli) (1), whereas selective β1-AR inhibition did not significantly impact lung function. On the basis of our new results, the β-blockers used in the aforementioned studies may have differentially attenuated sodium channel activity and as a result limited gas exchange. Also, it is possible that the impact on lung function would have been more pronounced had carvedilol been given as the β-blocker because this antagonist targets both β1-AR and β2-AR, which are expressed in both T1 cells and T2 cells. Pharmacological use of β1-AR antagonists in patients with heart failure did not alter gas exchange in the lung, possibly because β1-ARs are only expressed in T2 cells, and normal sodium channel activity remains in T1 cells that make up the majority of the alveolar surface area (6, 9, 17, 24).
Although our immunohistochemical labeling of lung tissue identified β1-AR expression only in T2 cells, we report that 20 μM denopamine stimulated ENaC activity in both T1 (where β1-labeling was not detected) and T2 cells. Because denopamine increased sodium transport in T1 cells lacking β1-AR expression, we evaluated whether β2-adrenoceptors were transactivated by the high concentration of denopamine applied to the cells. Transactivation of various receptors has been described in human airway (28) and intestinal epithelial cells (5) in response to overstimulation. In Fig. 9, we show that ENaC activity does not change following stimulation with denopamine in T1 cells pretreated with ICI 118,551. In contrast, T1 cells stimulated with denopamine demonstrated an increase in ENaC activity in the absence of ICI 118,551 (Fig. 3). Furthermore, the lack of β1-AR in T1 cells suggests that transactivation of β2-AR occurs in T1 cells to increase ENaC activity. This observation was validated with in vivo lung fluid clearance studies (Fig. 9C). In addition, the robust increase in cAMP levels measured following 20 μM denopamine stimulation in Fig. 10 supports the notion that denopamine transactivates β2-AR by cAMP.
β-AR regulation of salt and water balance in the lung.
The systematic characterization of the biophysical properties of the alveolar epithelium following β-AR stimulation is needed to gain a comprehensive understanding of the direction of net salt and water movement in the lung. Multiple studies describe the effect of β-AR agonists on promoting lung fluid clearance, but these studies lack specificity for cell type and the type of ENaC channel activated (8, 15, 18, 21, 22, 25, 29). Furthermore, our knowledge of the role of alveolar T1 cells in ion transport is limited because it has only recently been discovered that T1 cells possess ion channels (17). More specifically, the role of alveolar T1 cells in ion and water transport in vivo has not been conclusively demonstrated. In the present study, we show that systemic stimulation with either a β1- or β2-AR agonist promoted lung fluid clearance in nonventilated mice. Moreover, the transactivation of β2-AR from high doses of a β1-AR agonist resulted in lung fluid clearance and enhanced Na+ transport only in mice not receiving a selective β2-AR antagonist. Alveolar T1 cells cover 95% of the alveolar surface area, possess β2-AR (Fig. 8), and express amiloride-sensitive current, which collectively support a role for alveolar T1 cells in promoting lung fluid clearance in vivo.
Denopamine, a β1-AR agonist, increased NSC channel activity in alveolar T1 cells and T2 cells, albeit in different manners; denopamine increased the Po of NSC in T2 cells and increased NPo in T1 cells (with half the T1 patch-clamp recordings exhibiting increases in N, and all recordings exhibited increases in Po). The variability in T1 cell activation on N or Po following denopamine treatment arises from receptor transactivation and therefore varied from cell to cell. Terbutaline stimulates β2-ARs with specificity and increased HSC channel activity in both T1 (with increases in Po) and T2 cells (increased N).
In vivo alveolar clearance measurements confirm single-channel analysis.
In the present study, we observed that the basal rate of fluid clearance in saline-instilled mice remained relatively unchanged. Interestingly, however, alveolar fluid clearance rates have been shown to increase within 1 h in studies that utilize mechanical ventilation and positive end-expiratory pressure as measures of lung function (12, 16). Perhaps (and in a manner that is not completely understood) the positive pressure employed with mechanical ventilation of the lung leads to stretch activation of epithelial sodium channels, thereby facilitating net fluid clearance out of the lungs (2, 19). In our studies, we have minimized any putative effects of mechanical ventilation on alveolar fluid clearance by studying spontaneously breathing mice under anesthesia.
We are then able to determine the effect of β-AR agonists on lung fluid clearance. In addition, we are able to make conclusions about the efficacy of HSC and NSC stimulation of T1 and T2 cells by examining the results obtained from patch-clamp analysis presented in Figs. 1–4 with in vivo lung clearance data obtained using X-ray imaging (in Fig. 6). As expected, activation of HSC channels in alveolar T1 cells and T2 cells resulted in a robust increase in the rate of alveolar fluid clearance following IP injections of terbutaline. Activation of NSC channels in alveolar T1 cells produced a slower rate of alveolar fluid as evidenced by an initial decrease in lung fluid clearance that significantly improved over time such that by 240 min the rate of lung fluid clearance was comparable to terbutaline. These data are further supported by the amiloride inhibition studies (Fig. 5) that demonstrate significant reduction in HSC and NSC dwell time in the presence of amiloride.
The physiological importance of NSC channels in the lung, with Na:K permeability near 1.4, is not clear. Our single-channel recordings of denopamine in alveolar T1 cells and T2 cells, coupled with real-time fluid clearance measurements (13), reveal that increases in NSC channels are responsible for latent increases in the rate of alveolar fluid clearance. Figure 7A shows that animals injected IP with denopamine had a slower rate of saline clearance compared with animals injected IP with terbutaline (at t = 60 min). However, within 180 min of treatment animals injected IP with denopamine had greater lung fluid clearance compared with animals receiving terbutaline. The time course of lung fluid clearance indicates that HSC channels, activated by terbutaline in T1 and T2 cells (shown in Figs. 1–2), are primarily responsible for the immediate clearance of the saline challenge (within 1 h). Denopamine activation of NSC (Figs. 3–4) promotes lung fluid clearance, but the rate of clearance is slower than terbutaline.
Considerations for the use of β-AR agonists as potential therapy for acute lung injury.
The potential use of β-AR agonists in the treatment and prevention of alveolar flooding makes terbutaline, or denopamine, appealing as potential therapeutics. The utility of aerosolized β2-adrenergic agonists in the treatment of acute lung injury is unclear because data from the ARDS Clinical Network Trial, a randomized, placebo-controlled trial, clearly describe no change in clinical outcomes of individuals treated with albuterol (23). However, other studies describe improvement in lung fluid clearance associated with intravenous infusion of a β2-adrenergic agonist (27), a finding supported by data presented in the present study. The lack of a response to β-adrenergic agonists in the ARDS Clinical Network Trials is perplexing, but previously published studies suggest that β-ARs may become desensitized from infection, a common cause of ARDS (4, 10).
The effective use of these pharmacological compounds requires a better understanding of the physiological consequences (as well as benefits) that may arise. In the present study, we used common modes of drug delivery (systemic/parenteral and local) for β-AR agonists to determine their effect on lung fluid clearance. We observed differences in the rate of lung fluid clearance based on systemic (IP) and local (IT) delivery of β-adrenergics (Fig. 7). Mice receiving an IP injection of denopamine demonstrated significant increases in the rate of lung fluid clearance at all time points studied. However, mice receiving an IT instillation of the same compound exhibited alveolar flooding, possibly by altering CFTR channel activity (11, 19, 26). The marked difference in effect on lung fluid volume indicates that local (IT) delivery of denopamine disrupted normal fluid removal mechanisms in the lung and could have stimulated secretion into the airways. In contrast, we observed significant increases in the rate of lung fluid clearance in terbutaline (IP and IT)-treated mice. Collectively, these observations suggest that the mode of drug delivery is a critical component to maximizing the efficacy of β-AR agonists to treat acute lung injury.
Conclusions.
In summary, β-AR agonists increase net Na+ reabsorption in the lung by increasing open probability (Po) or the number of functional ENaC channels. We have extended on initial observations on the effect of β-AR agonists on alveolar epithelial sodium channels (8) to include characterization of HSC or NSC channel activation following terbutaline and denopamine in all the cells that make up the alveolar epithelium. We show that denopamine increased cellular levels of cAMP to a greater extent than terbutaline and provide evidence that denopamine transactivates NSC transport of sodium in T1 cells. Parenteral delivery of denopamine or terbutaline similarly increased alveolar fluid clearance 240 min following a saline challenge. However, intratracheal instillation of denopamine promoted alveolar flooding. Collectively, these data suggest a potential role for targeted β-AR agonist in promoting lung fluid clearance. Additional work is needed to maximize the beneficial effects of β-AR stimulation.
GRANTS
This work was supported by NIH grants 5R00HL092226-04 awarded to M. Helms, 5R37DK037963-25 awarded to D. Eaton, and 5R01HL063306-09 awarded to L. Jain.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.A.D., D.C.E., L.J., and M.N.H. conception and design of research; C.A.D., L.H.K., and L.Y. performed experiments; C.A.D., L.H.K., and L.Y. analyzed data; C.A.D., L.H.K., and M.N.H. interpreted results of experiments; C.A.D. and L.H.K. prepared figures; C.A.D. and M.N.H. drafted manuscript; C.A.D., D.C.E., L.J., and M.N.H. edited and revised manuscript; C.A.D., L.H.K., L.Y., D.C.E., L.J., and M.N.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the technical assistance obtained from Preston C. Goodson and the 2011 APS Professional Skills Training: Writing and Reviewing for Scientific Publication workshop.
REFERENCES
- 1. Agostoni P, Palermo P, Contini M. Respiratory effects of beta-blocker therapy in heart failure. Cardiovasc Drugs Ther 23: 377–384, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Awayda MS, Ismailov, Berdiev BK, Benos DJ. A cloned renal epithelial Na+ channel protein displays stretch activation in planar lipid bilayers. Am J Physiol Cell Physiol 268: C1450–C1459, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Berthiaume Y, Matthay MA. Alveolar edema fluid clearance and acute lung injury. Respir Physiol Neurobiol 159: 350–359, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buckner CK, Clayton DE, Ain-Shoka AA, Busse WW, Dick EC, Shult P. Parainfluenza 3 infection blocks the ability of a beta adrenergic receptor agonists to inhibit antigen-induced contraction of guinea pig isolated airway smooth muscle. J Clin Invest 67: 376–384, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buffin-Meyer B, Crassous PA, Delage C, Denis C, Schaak S, Paris H. EGF receptor transactivation and PI3-kinase mediate stimulation of ERK by alpha(2A)-adrenoreceptor in intestinal epithelial cells: a role in wound healing. Eur J Pharmacol 574: 85–93, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Carstairs JR, Nimmo AJ, Barnes PJ. Autoradiographic visualization of beta-adrenoceptor subtypes in human lung. Am Rev Respir Dis 132: 541–547, 1985 [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Chen Z, Narasaraju T, Jin N, Liu L. Isolation of highly pure alveolar epithelial type I and type II cells from rat lungs. Lab Invest 84: 727–735, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Chen XJ, Eaton DC, Jain L. Beta-adrenergic regulation of amiloride-sensitive lung sodium channels. Am J Physiol Lung Cell Mol Physiol 282: L609–L620, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 126: 332–337, 1982 [DOI] [PubMed] [Google Scholar]
- 10. Davis IC, Xu A, Gao Z, Hickman-Davis JM, Factor P, Sullender WM, Matalon S. Respiratory syncytial virus induces insensitivity to β-adrenergic agonists in mouse lung epithelium in vivo. Am J Physiol Lung Cell Mol Physiol 293: L281–L289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces. J Gen Physiol 119: 199–207, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garica-Delgado M, Touma-Fernandez A, Chamoro-marin V, Ruiz-Aguilar A, Aguilar-Alonso E, Fernandez-Mondejar E. Alveolar fluid clearance in healthy pigs and influence of positive end-expiratory pressure. Crit Care 14: R36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodson P, Kumar A, Jain L, Kundu K, Murthy N, Koval M, Helms MN. NADPH oxidase regulates alveolar epithelial sodium channel (ENaC) activity and lung fluid balance in vivo via O2- signaling. Am J Physiol Lung Cell Mol Physiol 302: L410–L419, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helms MN, Jain L, Self JL, Eaton DC. Redox regulation of epithelial sodium channels examined in alveolar type 1 and 2 cells patch-clamped in lung slice tissue. J Biol Chem 283: 22875–22883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jain L, Chen XJ, Ramosevac S, Brown LA, Eaton DC. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. Am J Physiol Lung Cell Mol Physiol 280: L646–L658, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Jayr C, Garat C, Meignan M, Pittet JF, Zelter M, Matthay MA. Alveolar liquid and protein clearance in anesthetized ventilated rats. J Appl Physiol 76: 2636–2642, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 103: 4964–4969, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loeh B, Baloglu E, Ke A, Bartsch P, Mairbaurl H. Beta2-adrenergic stimulation blunts inhibition of epithelial ion transport by hypoxia of rat alveolar epithelial cells. Cell Physiol Biochem 25: 123–134, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Ma HP, Li L, Zhou ZH, Eaton DC, Warnock DG. ATP masks stretch activation of epithelial sodium channels in A6 distal nephron cells. Am J Physiol Renal Physiol 282: F501–F505, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Mak JC, Nishikawa M, Haddad EB, Kwon OJ, Hirst SJ, Twort CH, Barnes PJ. Localisation and expression of beta-adrenoceptor subtype mRNAs in human lung. Eur J Pharmacol 302: 215–221, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Matalon S, Benos DJ, Jackson RM. Biophysical and molecular properties of amiloride-inhibitable Na+ channels in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 271: L1–L22, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 61: 627–661, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT. Randomized, placebo-controlled clinical trial of an aerosolized beta-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 184: 561–568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 82: 569–600, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Minakata Y, Suzuki S, Grygorczyk C, Dagenais A, Berthiaume Y. Impact of β-adrenergic agonist on Na+ channel and Na+-K+-ATPase expression in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 275: L414–L422, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, Matalon S, Hollenberg S, Factor P. Upregulation of alveolar epithelial active Na+ transport is dependent on beta2-adrenergic receptor signaling. Circ Res 94: 1091–1100, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 173: 281–287, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Rider CF, King EM, Holden NS, Giembycz MA, Newton R. Inflammatory stimuli inhibit glucocorticoid-dependent transactivation in human pulmonary epithelial cells: rescue by long-acting beta2-adrenoceptor agonists. J Pharmacol Exp Ther 338: 860–869, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Sakuma T, Hida M, Nambu Y, Osanai K, Toga H, Takahashi K, Ohya N, Inoue M, Watanabe Y. Beta1-adrenergic agonist is a potent stimulator of alveolar fluid clearance in hyperoxic rat lungs. Jpn J Pharmacol 85: 161–166, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Xu JG, Li TP, Wang P, Shen HY. [Effect of terbutaline on sodium transport in alveolar type I and type II cells]. Nan Fang Yi Ke Da Xue Xue Bao 30: 966–968, 2010 [PubMed] [Google Scholar]
- 31. Yu L, Bao HF, Self JL, Eaton DC, Helms MN. Aldosterone-induced increases in superoxide production counters nitric oxide inhibition of epithelial Na channel activity in A6 distal nephron cells. Am J Physiol Renal Physiol 293: F1666–F1677, 2007 [DOI] [PubMed] [Google Scholar]