Abstract
Smokers with airflow obstruction have an increased risk of atherosclerosis, but the relationship between the pathogenesis of these diseases is not well understood. To determine whether hypercholesterolemia alters lung inflammation and emphysema formation, we examined the lung phenotype of two hypercholesterolemic murine models of atherosclerosis at baseline and on a high-fat diet. Airspace enlargement developed in the lungs of apolipoprotein E-deficient (Apoe−/−) mice exposed to a Western-type diet for 10 wk. An elevated number of macrophages and lymphocytes accompanied by an increase in matrix metalloproteinase-9 (MMP-9) activity and MMP-12 expression was observed in the lungs of Apoe−/− mice on a Western-type diet. In contrast, low-density lipoprotein receptor-deficient (Ldlr−/−) mice did not exhibit lung destruction or inflammatory changes. Most importantly, we revealed augmented expression of the downstream targets of the Toll-like receptor (TLR) pathway, interleukin-1 receptor-associated kinase 1, and granulocyte colony-stimulating factor, in the lungs of Apoe−/− mice fed with a Western-type diet. In addition, we demonstrated overexpression of MMP-9 in Apoe−/− macrophages treated with TLR4 ligand, augmented with the addition of oxidized LDL, suggesting that emphysema in these mice results from the activation of the TLR pathway secondary to known abnormal cholesterol efflux. Our findings indicate that, in Apoe−/− mice fed with an atherogenic diet, abnormal cholesterol efflux leads to increased systemic inflammation with subsequent lung damage and emphysema formation.
Keywords: atherosclerosis, proteases, apolipoprotein E, Toll-like receptor
emphysema is a chronic obstructive pulmonary disease (COPD) characterized by the abnormal destruction of alveolar walls accompanied by the enlargement of airspaces (44). Today more than 4.9 million people in the United States are diagnosed with this condition (40). The incidence of emphysema in cigarette smokers is much higher than in nonsmokers, and the association between the progression of the disease and the degree of cigarette smoking has been documented by several studies (27, 59). Cigarette smoke exposure causes increased inflammation (22), protease/antiprotease imbalance (43), apoptosis (55), and oxidative stress (41). These processes are believed to contribute to the alveolar destruction that is characteristic of emphysema. It has also been shown that smokers face an increased risk for numerous diseases, including atherosclerosis. Cigarette smoke raises the levels of oxidized low-density lipoprotein (LDL) cholesterol and damages vessel endothelium, leading to the development of atherosclerosis (49). Of note, smokers with airflow limitation have more prominent atherosclerosis than smokers with normal lung function, suggesting a link between atherosclerosis and obstructive lung disease (32).
One of the major hallmarks of emphysema and atherosclerosis is inflammation originating from the infiltration of macrophages and lymphocytes into the airway and vessel wall, respectively. Atherosclerotic lesions have increased numbers of lipid-laden macrophages (23). Likewise, smokers with airflow limitations exhibit an increase in the number of macrophages and T-lymphocytes in the lung, the presence of which correlate with the development and progression of emphysema (34, 60). Animal models of atherosclerosis and emphysema demonstrate similar inflammatory profiles (50, 54). Therefore, we examined the lungs of two murine models of atherosclerosis to assess the potential consequences of hyperlipidemia on lung structure.
The most widely used murine models for atherosclerosis are apolipoprotein E (ApoE)-deficient mice and LDL receptor-deficient mice, which both develop hypercholesterolemia (33, 58). Under normal conditions, ApoE accepts cholesterol from cells and transports it back to the liver, where it can be excreted. The loss of ApoE and the LDL receptor result in hypercholesterolemic states as a result of impaired lipoprotein production and metabolism (33, 58). One of the critical roles of ApoE is to promote cholesterol efflux from macrophages (8, 37). Deficiency of endogenous ApoE expression leads to the deleterious effects of cholesterol-overloaded macrophages, which are also known as foam cells (42). ApoE promotes macrophage cholesterol efflux through the ABCA-1 and ABCG-1 cell surface transporters, which facilitate the efflux of phospholipids and cholesterol onto lipid-poor apolipoproteins, thereby initiating the formation of high-density lipoprotein (HDL) particles (53). Accumulation of oxidized LDL in the macrophages of the atherosclerotic plaque stimulates the secretion of various cytokines and proteases, which leads to degradation of the extracellular matrix (45). In contrast to ApoE, the LDL receptor mediates the binding and endocytosis of excess circulating LDL cholesterol to liver cells, where the cholesterol is catabolized and ultimately secreted in the feces via the biliary pathway (17). The goal of the present study was to examine the lung phenotype in Apoe−/− and Ldlr−/− to determine whether the lung inflammation or parenchymal structure is altered in these mouse models of atherosclerosis.
MATERIALS AND METHODS
Animal experiments.
Two murine models of atherosclerosis were used to study the effect of hypercholesterolemia on the lung structure. The control group consisted of female C57BL6/J mice obtained from Jackson Laboratories fed chow diet (n = 5) or Western-type diet (n = 5) for 10 wk. The first experimental group included 8-wk-old female Apoe−/− mice (n = 6) subjected to a Western diet for 10 wk, compared with Apoe−/− mice on a chow diet (n = 5). In the second experimental group of animals, the Ldlr−/− model of atherosclerosis was used to observe the effect of the diet on the lungs. Ldlr−/− mice were exposed to a Western diet (n = 6) or chow (n = 6) for 10 wk. Female mice were selected on the basis of their increased susceptibility to the atherosclerotic plaque formation (56). Mice were fed an atherogenic high-fat Western-type diet (20% protein, 50% carbohydrate, 21% fat, 0.21% cholesterol; D12079B; Research Diets, New Brunswick, NJ). The animal experiments were repeated at least two times for each subgroup. Animals were housed at Columbia University Medical Center according to animal welfare guidelines. Food and drinking water were provided ad libitum. The Columbia University Institutional Animal Care and Use Committee approved all animal studies.
Histology and immunohistochemistry.
After exposure to the Western or chow diet, mice were anesthetized with isoflurane and killed by carbon dioxide inhalation. The trachea was cannulated with a 16-gauge argon catheter secured with a silk suture. The lungs were lavaged first with PBS (1 ml) to collect bronchoalveolar lavage (BAL) fluid, and then pressure was perfused with 10% formalin to 25 cm H2O for 20 min. Tissues were stored in formalin for at least 24 h before paraffin embedding and sectioning (4 μm). Sections were stained with hematoxylin and eosin (H&E) for histological analysis including morphometry and quantification of inflammatory cells. Morphometric analysis of the H&E-stained lungs was performed as previously described (20, 46). Morphometric assessment was conducted to determine the average distance between alveolar walls (mean linear intercept), the fractional volume of parenchyma tissue per lung, the alveolar surface area per unit volume, and macrophage and lymphocyte counts. Forty histological fields (x40 magnification) were analyzed from at least four separate, randomly chosen sections from each mouse to calculate morphometric parameters. We adhered to a strict protocol for blinding and randomization (30), including a random number generator to select slides for analysis, as well as use of the OptiScan II motorized stage (Prior Scientific, Rockland, MA) controlled by Image-Pro Plus 7.0 software (Media Cybernetics, Bethesda, MD).
Serum total cholesterol measurements.
Utilizing the Wako Cholesterol E kit (Wako Pure Chemical Industries, Osaka, Japan), total serum cholesterol measurements were performed. Blood was obtained from mice at the time of death via cardiac puncture, and serum was separated and frozen for later analysis. Due to high cholesterol levels, serum was diluted five times with sterile PBS. With the use of a 96-well flat-bottomed plate, 200 μl of color reagent and 2 μl of standard or sample were added to each well. After a 5-min incubation at 37°C, wavelength at 595 nm was measured. Utilizing the standard curve, serum cholesterol values were calculated. Serum cholesterol levels were repeated on three subsequent days to ensure accuracy.
Cell culture.
Peritoneal macrophages were obtained from Apoe−/− and Apoe+/+ mice following intraperitoneal thioglycolate injection. The macrophages were cultured in DMEM containing 5% FBS for 24 h and subsequently treated with ligands for Toll-like receptor 2 (TLR2) (peptidoglycan, 2 μg/ml), TLR3 (polyinosine-polycytidylic acid, 2.5 μg/ml), and TLR4 (lipid A, the active component of lipopolysaccharide, 100 ng/ml) (InvivoGen, San Diego, CA). Macrophages were also treated with both TLR4 ligand and oxidized LDL (100 μg/ml). All cell culture experiments were repeated at least three times on subsequent days to ensure accuracy.
Western blotting.
Freshly dissected lungs of Apoe−/− and Ldlr−/− mice (10 mg) were homogenized in 1 ml of protein lysis buffer (PBS containing Triton X-100 0.1%), and centrifuged (14,000 g for 10 min). Fifty micrograms of the lung lysates of each group were subjected to Western Blot analysis. Rabbit polyclonal antibodies against phospho- (ph-) ERK, total ERK, ph-JNK (Cell Signaling, Beverly, MA), and interleukin-1 receptor activated kinase (IRAK)-1 (Santa Cruz Biotechnology, Santa Cruz, CA) were used, following the manufacturer's instructions. A goat polyclonal antibody against β-actin (Santa Cruz) was utilized to assess for equal protein loading. Western blots were performed twice for accuracy.
Zymography.
Gelatin zymography was performed using bronchoalveolar lavage fluid to detect proteases having gelatinolytic activity, including matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9), as previously reported (26). Zymography was performed twice to ensure accuracy of the results.
Quantitative RT-PCR.
Total RNA was extracted from specimens of lung tissue 0.3 cm3 in size with the use of the RNeasy kit (Qiagen, Germantown, MD). TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA) were performed to assess gene-transcript levels with the use of an ABI Prism 7900HT Sequence Detection System (Applera, Foster City, CA). Applied Biosystems primer and probe sets included the following: MMP-9 (Mm00442991_m1) and MMP-12 (Mm00500554_m1). GAPDH was used as the housekeeping gene. qRT-PCR analysis was performed on lung tissue from four animals in each group. To calculate the relative quantity of MMP-9 and MMP-12, we used the 2−ΔΔCT method implemented in the software. The data are shown as the fold change in gene expression normalized to the endogenous reference gene GAPDH and relative to the controls. All real-time PCR was performed in triplicate and repeated on subsequent days to ensure accuracy of results.
Statistical analysis.
Statistics were performed using Prism 5.0d software. Linear regression models were used to examine the relationship between total serum cholesterol and macrophage count in Apoe−/− and Ldlr−/− mice. Separate slopes and intercepts were fit for the two strains, and the two were compared using an F-statistic. A secondary analysis included strain-specific indicators of western diet as an additional predictor.
For isolated two-group comparisons, a Mann-Whitney U-test was performed, with P value <0.05 considered statistically significant. Of note, Mann-Whitney U-test on real-time PCR results was performed using ΔCT values; data are visually represented as mean relative expression ± SE.
RESULTS
Development of emphysema in Apoe−/− mice exposed to an atherogenic high-fat diet.
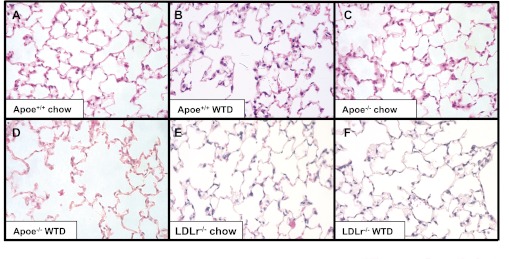
The effect of hypercholesterolemia on the development of emphysema in the Apoe−/− mice subjected to an atherogenic high-fat diet for 10 wk was estimated by measuring the mean linear intercept. At baseline, Apoe+/+ mice fed a Western-type diet for 10 wk demonstrated no difference in mean linear intercept compared with chow-fed Apoe+/+ controls (34.5 ± 2.6 μm vs. 34.5 ± 2.9 μm, respectively). Morphometric analysis demonstrated that Apoe−/− mice fed a Western-type diet for 10 wk developed statistically significant airspace enlargement compared with Apoe+/+ mice fed a Western-type diet (41.5 ± 2.8 μm vs. 34.5 ± 2.6 μm, respectively) (Table 1, Fig. 1). Ldlr−/− mice subjected to a Western-type diet for 10 wk did not exhibit emphysematous changes in their lungs (32.7 ± 2.4 μm for chow-fed mice vs. 36 ± 5.4 μm for Western-type diet) (Table 1, Fig. 1). Surface area to unit volume measurements confirmed these findings, with a decrease surface area to unit volume in the Apoe−/− mice fed Western-type diet compared with Apoe+/+ fed Western-type diet (48.4 ± 3.2 mm−1 vs. 58.3 ± 4.4 mm−1) (Table 1). Fractional volume measurements did not differ between groups (Table 1).
Table 1.
Lung morphometric parameters in the study population
| Animals | Diet, 10 wk | Mean Linear Intercept, μm | Surface Area/ Unit Volume, mm−1 | Fractional Volume, % |
|---|---|---|---|---|
| Apoe+/+ mice (n = 5) | Chow | 34.5 ± 2.9 | 58.3 ± 5 | 28.4 ± 2.9 |
| Apoe+/+ mice (n = 5) | Western-type | 34.5 ± 2.6 | 58.3 ± 4.4 | 28.2 ± 2.2 |
| Apoe−/− mice (n = 5) | Chow | 38.5 ± 3.2 | 52.2 ± 4.4 | 28.6 ± 2.5 |
| Apoe−/− mice (n = 6) | Western-type | 41.5 ± 2.8* | 48.4 ± 3.2* | 29.8 ± 3.7 |
| LDLr−/− mice (n = 6) | Chow | 32.7 ± 2.4 | 61.5 ± 4.8 | 29.2 ± 5 |
| LDLr−/− mice (n = 6) | Western-type | 36 ± 5.4 | 56.4 ± 7.1 | 27.5 ± 5.5 |
Values are means ± SE. Apolipoprotein E-deficient (Apoe−/−) mice fed with a Western-type diet exhibit increased mean linear intercept and decreased surface area to volume measurements as compared to Apoe+/+ mice fed with the same diet. The low-density lipoprotein receptor-deficient (Ldlr−/−) mice do not exhibit such changes, despite similar increases in total serum cholesterol. Fractional volume remains similar among all groups.
P < 0.05 versus Apoe+/+ Western-type diet.
Fig. 1.
Emphysematous changes in the lungs of apolipoprotein E-deficient (Apoe−/−) mice subjected to a Western-type diet for 10 wk. Hematoxylin and eosin-stained lung sections. A: Apoe+/+ mouse fed a chow diet for 10 wk. B: Apoe+/+ mouse fed a Western-type diet (WTD) for 10 wk. C: Apoe−/− mouse fed a chow diet. D: Apoe−/− mouse fed a WTD. E: low-density lipoprotein receptor-deficient (Ldlr−/−) mouse fed a chow diet. F: Ldlr−/− mouse fed a WTD.
Inflammatory response to hypercholesterolemia in the lungs of Apoe−/− mice.
As provided in Table 2, serum cholesterol is substantially increased with Western-type diet in the Apoe−/− and Ldlr−/− mice, but not in the Apoe+/+ mice. To evaluate the impact of hypercholesterolemia on the inflammation in the lungs of Apoe−/− and Ldlr−/− mice, macrophages were quantified in tissue sections from all experimental groups. Macrophage count is also increased with Western-type diet in the Apoe−/− mice, but not in the Ldlr−/− or Apoe+/+ mice. The linear regression analysis indicated that Apoe−/− mice macrophage levels increased with serum cholesterol and that the macrophage levels in the Ldlr−/− mice did not increase with serum cholesterol. The estimated intercept and slope parameters (standard errors) for the Apoe−/− mice were 0.15 (1.57) and 0.01 (0.003), whereas for the Ldlr−/− mice were 4.67 (1.07) and −0.001 (0.002). The F2,19 statistic for comparing strains was 13.97 (P < 0.01). These data indicate that the two strains have substantially different macrophage levels at the same cholesterol levels. When the model was refit with the indicator of diet included as an additional predictor, diet evidenced substantial independent explanatory effects on macrophage count, but serum cholesterol evidenced no independent effect.
Table 2.
Diet-dependent alterations in cholesterol and pulmonary macrophages are strain dependent
| Total Serum Cholesterol |
Macrophage per mm2 |
|||
|---|---|---|---|---|
| Genotype | Chow diet | Western-type diet | Chow diet | Western-type diet |
| Apoe+/+ mice | 63 ± 17.78 | 89 ± 11.9 | 3.11 ± 0.44 | 4.02 ± 0.34 |
| Apoe−/− mice | 335 ± 41.49 | 610.33 ± 58.19 | 3.56 ± 0.40 | 9.67 ± 0.42 |
| LDLr−/− mice | 225.33 ± 32.01 | 666.17 ± 60.24 | 4.61 ± 0.24 | 3.77 ± 0.67 |
Values are mean ± SE of total serum cholesterol and macrophage count (macrophage per mm2) separated by mouse strain and diet type.
Increased expression of MMP-9 and MMP-12 in the lungs of Apoe−/− mice subjected to a Western-type diet.
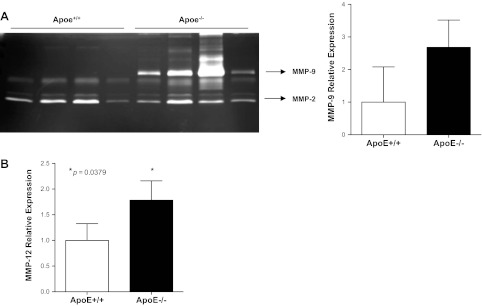
We performed gelatin zymography to determine the activity of MMP-9 in the lungs of Apoe−/− mice subjected to a Western-type diet for 10 wk. Increased activity of MMP-9 was observed in the BAL fluid from Apoe−/− mice (Fig. 2A). MMP-2 activity was detected in the BAL fluid of Apoe−/− and Apoe+/+ mice, but levels were not altered. Quantitative RT-PCR analysis demonstrated a trend toward elevation of MMP-9 on mRNA level in the lungs of Apoe−/− mice (Fig. 2A). RT-PCR analysis revealed augmented expression of MMP-12 at the mRNA level in the lungs of Apoe−/− mice (n = 8) compared with controls (n = 8) (Fig. 2B).
Fig. 2.
Augmented expression of matrix metalloproteinase-9 (MMP-9) and MMP-12 in the lungs of Apoe−/− mice subjected to a WTD for 10 wk. A: MMP-9 activity was detected by gelatin zymography in the bronchoalveolar lavage (BAL) fluid of Apoe−/− mice subjected to a WTD for 10 wk but not in the BAL of Apoe+/+ mice fed with chow diet. There was a trend to increased expression of MMP-9 in lung homogenates in Apoe−/− mice as detected by real-time PCR. MMP-2 activity was detected in the BAL fluid of Apoe−/− and Apoe+/+ mice, but activity was not altered between groups. B: MMP-12 expression is elevated in the lungs of Apoe−/− mice fed a WTD for 10 wk compared with Apoe+/+ controls.
Activation of TLR signaling pathway in response to hypercholesterolemia in the lungs of Apoe−/− mice.
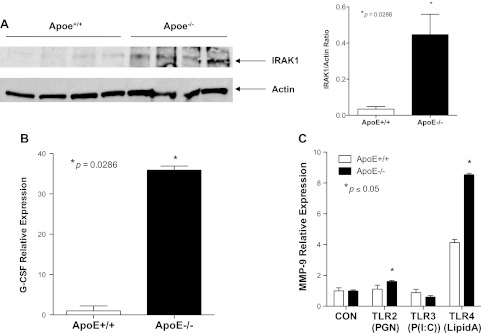
IRAK is a key regulator in the signaling pathway of TLRs. Once activated, IRAK initiates a cascade of signaling events, ultimately leading to the induction of inflammatory genes such as granulocyte colony-stimulating factor (G-CSF) (13, 52). To evaluate the involvement of the TLR pathway in the development of emphysema in the Apoe−/− mice, we analyzed the expression of its downstream targets IRAK-1 and G-CSF in the lungs of the Apoe−/− mice. The analysis of IRAK-1 and G-CSF expression revealed their upregulation in the lungs of Apoe−/− mice fed a Western-type diet for 10 wk but not in Apoe+/+ mice on the chow diet (Fig. 3, A and B), indicating that the TLR pathway is activated when mice are on the atherogenic diet and not at baseline.
Fig. 3.
Activation of Toll-like receptor (TLR) signaling in the lungs of Apoe−/− mice subjected to a WTD for 10 wk. A: expression of interleukin-1 receptor activated kinase (IRAK)-1, a downstream regulator of TLR signaling, is increased in the lungs of Apoe−/− mice fed with a WTD compared with wild-type control. Densitometry confirms this 15-fold increase in signaling (P = 0.0108 by 2-tailed t-test). B: increased granulocyte colony-stimulating factor (G-CSF) expression in the lungs of Apoe−/− mice fed a WTD compared with Apoe+/+ mice. C: treatment of macrophages isolated from Apoe−/− and Apoe+/+ mice with TLR2, TLR3, and TLR4 ligands for 24 h. The induction of MMP-9 expression by the TLR-4 ligand in the macrophages isolated from Apoe−/− and Apoe+/+ mice was detected by qRT-PCR analysis.
TLR signaling mediates MMP-9 expression induced in the macrophages of Apoe−/− mice.
To determine whether Apoe−/− macrophages were more responsive to TLR activation, peritoneal macrophages from Apoe−/− mice and their Apoe+/+ controls were treated with various TLR ligands. Activation of TLR4 increased mRNA expression of MMP-9 by eightfold in Apoe−/− macrophages and by fourfold in Apoe+/+ macrophages (Fig. 3C). Increases in MMP-9 mRNA expression in Apoe−/− macrophages were also seen after TLR2 activation although not to the extent seen with TLR4 ligand. Interestingly, the TLR3 ligand did not exhibit any effect on MMP-9 expression in either macrophage group, suggesting that the macrophage responsiveness was TLR4/2 specific (Fig. 3C).
Activation of ERK and JNK, downstream regulators of the TLR signaling pathway, in the lungs of Apoe−/− mice subjected to a Western-type diet.
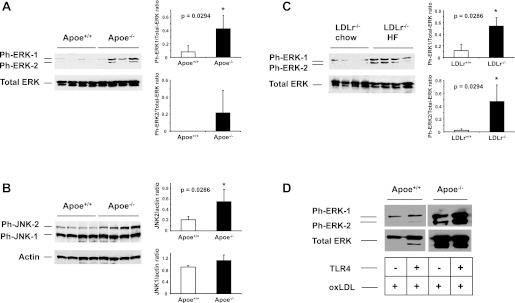
Mitogen-activated protein (MAP) kinases such as extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinases (JNK) are activated by the TLR signaling pathway (28). Phosphorylation of ERK and JNK results in the activation of transcription factors regulating inflammatory genes (29). To determine whether Apoe−/− and Ldlr−/− mice exposed to a Western-type diet have increased levels of phospho-ERK and phospho-JNK in the lung, we analyzed lung lysates by Western blotting. This analysis revealed elevated expression of phospho-ERK and phospho-JNK in the lungs of Apoe−/− mice (Fig. 4, A and B). Interestingly, we also observed augmented expression of phospho-ERK in the lungs of Ldlr−/− mice, suggesting that activation of ERK potentially occurs early under hypercholesterolemic conditions (Fig. 4C). In addition, we revealed that the activation of ERK in Apoe−/− macrophages by oxidized LDL was further increased by the addition of TLR4 ligand (Fig. 4D).
Fig. 4.
Western blot analysis of ERK and JNK phosphorylation in the lungs of mice. A–B: Western blot analysis of phospho-ERK (ph-ERK) and phospho-JNK (ph-JNK) in the lungs of Apoe−/− mice after 10 wk of WTD. Densitometric analysis of the signal was performed to determine ph-ERK/total ERK and JNK/actin ratios. C: Western blot analysis of ph-ERK in the lungs of Ldlr−/− mice fed a WTD for 10 wk. D: peritoneal macrophages from Apoe−/− and Apoe+/+ treated with TLR4 ligand (100 ng/ml) and oxidized LDL (100 μg/ml). Activation of ERK in Apoe−/− macrophages treated with oxidized LDL was augmented by the addition of TLR4 ligand.
DISCUSSION
In the present study, Apoe−/− mice subjected to an atherogenic Western-type diet for 10 wk developed emphysematous changes with increased inflammation, enlargement of airspaces, and destruction of alveolar walls. In contrast, Apoe+/+ mice and Ldlr−/− mice fed an atherogenic diet did not exhibit similar changes. The two strains have substantially different macrophage levels at the same cholesterol levels, with diet demonstrating substantial independent effects on macrophage count. In addition to observing TLR2/4 pathway activation in the lungs of Apoe−/− mice fed with Western-type diet, we also detected increases in two major elastinolytic proteases, including augmented activity of MMP-9 and expression of MMP-12. Macrophages from Apoe−/− mice were sensitive to TLR4 activation, the effects of which were augmented in the presence of oxidized LDL. The subsequent inflammatory cell recruitment, mediated by G-CSF production, and protease production likely contribute to the observed destruction of the lung extracellular matrix in the Apoe−/− mice.
Studies have suggested that COPD is a systemic disease rather than an independent disease state (18). Atherosclerosis is one of the leading causes of mortality in COPD (2). The major hallmark of both diseases is the presence of chronic inflammation with the recruitment of macrophages and lymphocytes (5). It has been demonstrated that murine models of smoke-induced emphysema exhibit a marked increase in the number of macrophages and neutrophils and have augmented myeloperoxidase activity (20). Arunachalam and colleagues (3) recently demonstrated that ApoE-deficient mice exposed to cigarette smoke manifest an increased inflammation response, characterized by infiltration of macrophages and neutrophils into the lung and increased oxidative stress. Our data demonstrate that, even in the absence of smoking, an atherogenic diet fed to Apoe−/− mice induces increased inflammation through TLR4 activation, with subsequent augmented protease production that results in emphysema. These results are unique to the Apoe−/− mice, as the Ldlr−/− mice, which achieve the same level of hyperlipidemia, do not develop pulmonary inflammation and lung destruction. Hypercholesterolemia alone, as is seen in the Ldlr−/− mice, is not sufficient to induce increased inflammation and protease production, despite the activation of MAP kinases in the lung. It is possible that diet serves as a better proxy for serum cholesterol than a single, possibly labile, measure of serum cholesterol. However, this may also indicate that the effect of diet in the Apoe−/− mice is not solely through serum cholesterol levels.
The increased susceptibility to emphysema formation in Apoe−/− mice compared with Ldlr−/− mice is likely attributed to additional alterations in cholesterol efflux present within the Apoe−/− macrophages (53, 65). ApoE promotes macrophage cholesterol efflux (53, 65) through the ABCA-1 and ABCG-1 cell surface transporters, ultimately initiating the formation of HDL particles (53). Interestingly, Abca1−/− and Abcg1−/− mice manifesting abnormal cholesterol efflux exhibit pulmonary inflammation (6, 9). Macrophages from these mice exhibit increased expression of inflammatory genes via TLR4 signaling, suggesting a link between alterations in cholesterol efflux and lung inflammation through TLR4 signaling (63).
In the present study, we provide evidence for the role of TLR4 signaling in the development of emphysema in an atherosclerosis animal model devoid of cigarette smoke exposure. The TLR4 signaling pathway plays a crucial role in the regulation of the immune response in atherosclerosis and in various lung-associated pathologies (7, 12) and is regulated by oxidized LDL (15, 39, 61). TLR4 activation reduces the expression of the genes involved in cholesterol transport and metabolism, ultimately causing a pathological lipid accumulation in macrophages (14). Additionally, free cholesterol accumulation in macrophage membranes activates TLRs, resulting in downstream MMP induction in atherosclerosis (51).
Numerous studies have shown that activation of TLR2 or TLR4 leads to the recruitment of IRAK-1, IRAK-4, and TNF receptor-associated factor 6 (24). This association results in the activation of ERK and JNK, MAP kinases involved in both TLR signaling and MMP production (21, 38). The present study provides evidence for activation of the TLR4 signaling pathway in the lungs of Apoe−/− mice fed with an atherogenic diet. Activation of this pathway is indicated in the analysis of downstream targets of TLR4 in the lung, including IRAK-1 and G-CSF. In vitro experiments on macrophages from Apoe+/+ and Apoe−/− mice further confirm this association.
An imbalance of proteases, including elastase, MMP-1, MMP-9, and MMP-12, is known to contribute to the development of emphysema and atherosclerosis (1, 16, 25, 31, 36, 64). In the present study, increased activity of MMP-9 and expression of MMP-12 were demonstrated in the lungs of Apoe−/− mice fed an atherogenic diet. It should be noted that, whereas BAL fluid from Apoe−/− mice fed with Western-type diet showed clear increase in MMP-9 activity, this result was not seen at the mRNA expression level in whole lung homogenates. The whole lung homogenates contain cell types that do not express MMP-9 and likely alter the amount of difference seen between the groups; the zymography results provide a more accurate assessment of the augmented MMP-9 activity at the alveolar level. Our laboratory has demonstrated that the transgenic overexpression of MMP-9 in macrophages causes spontaneous emphysema attributable to elastin degradation (19), which appears in contrast to studies demonstrating that an MMP-9-deficient mouse still develops smoke-induced emphysema (4). However, in the MMP-9-deficient mice, the presence of other proteases, including MMP-12, is potentially sufficient to destroy the lung. Therefore, it is likely that the combined upregulation of MMP-9 and MMP-12 in the lungs of Apoe−/− mice causes disruption of the lung extracellular matrix and ultimately contributes to the observed emphysematous changes seen in this model.
These findings have significant public health implications in the age of increasing incidence of obesity, atherosclerosis, and emphysema. It is already well established that patients with COPD have increased cardiovascular morbidity and mortality, especially in a younger cohort (47). However, the reverse is also true, with the development of COPD in patients with atherosclerosis substantially reducing cardiovascular disease survival rates (35). In patients with atherosclerosis, even a decline in forced expiratory volume in 1 s, independent of smoking history and other atherosclerosis risk factors, is associated with early mortality from cardiovascular causes (11, 48). Leptin, recently implicated in the exacerbation of inflammation in the obese, increases expression of IRAK-1 in macrophages (57) and is known to influence a broad array of TLR signaling, including TLR4, in adipocytes (10). A polymorphism within the leptin promoter region has been identified to be associated with increased circulating leptin levels and worsening severity of COPD (62). It is possible that the mechanism linking atherosclerosis and emphysema is attributed to a persistent systemic inflammation, from multiple different sources, all ultimately acting through the TLR4 signaling pathway with the resultant inflammatory and protease cascade contributing to the development and/or disease progression of both emphysema and atherosclerosis.
In summary, our study indicates that, in Apoe−/− mice fed a Western-type diet, severe systemic hypercholesterolemia accompanied by the abnormal cholesterol efflux induces pulmonary inflammation through a TLR4/inflammatory/MMP cascade, all of which ultimately result in emphysema development. The synergistic effect of dyslipidemia and defective cholesterol efflux observed in atherosclerosis may also contribute to emphysema, providing a mechanistic link between these two inflammatory disease states. Our findings also add to the increasing evidence for the role of TLR signaling in lung diseases and in mediating pulmonary inflammation. Therefore, we suggest that peripheral systemic changes in lipid transport and metabolism can ultimately lead to local lung destruction and the development of emphysema, a novel and clinically relevant mechanism in disease pathogenesis.
GRANTS
This work was supported by grants from the National Institutes of Health (HL086936, J. D'Armiento), American Heart Association (0840108, J. D'Armiento) and Flight Attendant Medical Research Institute (103236, M. Goldklang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.P.G., P.G., T.Z., J.T., V.L., and J.M.D. performed experiments; M.P.G., J.T., D.R., V.L., and J.M.D. analyzed data; M.P.G., D.R., V.L., and J.M.D. interpreted results of experiments; M.P.G., P.G., J.T., V.L., and J.M.D. prepared Figs.; M.P.G., P.G., V.L., and J.M.D. drafted manuscript; M.P.G., J.T., D.R., and J.M.D. edited and revised manuscript; M.P.G., D.R., and J.M.D. approved final version of manuscript; J.M.D. conception and design of research.
REFERENCES
- 1. Abboud RT, Vimalanathan S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int J Tuberc Lung Dis 12: 361–367, 2008 [PubMed] [Google Scholar]
- 2. Anthonisen NR, Connett JE, Enright PL, Manfreda J. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med 166: 333–339, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Arunachalam G, Sundar IK, Hwang JW, Yao H, Rahman I. Emphysema is associated with increased inflammation in lungs of atherosclerosis-prone mice by cigarette smoke: implications in comorbidities of COPD. J Inflamm 7: 34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atkinson JJ, Lutey BA, Suzuki Y, Toennies HM, Kelley DG, Kobayashi DK, Ijem WG, Deslee G, Moore CH, Jacobs ME, Conradi SH, Gierada DS, Pierce RA, Betsuyaku T, Senior RM. The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema. Am J Respir Crit Care Med 183: 876–884, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Back M. Atherosclerosis, COPD and chronic inflammation. Respir Med 4: 60–65, 2008 [Google Scholar]
- 6. Baldan A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol 180: 3560–3568, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol 286: L887–L892, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Basu SK, Brown MS, Ho YK, Havel RJ, Goldstein JL. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci USA 78: 7545–7549, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol 289: L980–L989, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Batra A, Pietsch J, Fedke I, Glauben R, Okur B, Stroh T, Zeitz M, Siegmund B. Leptin-dependent toll-like receptor expression and responsiveness in preadipocytes and adipocytes. Am J Pathol 170: 1931–1941, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beaty TH, Newill CA, Cohen BH, Tockman MS, Bryant SH, Spurgeon HA. Effects of pulmonary function on mortality. J Chronic Dis 38: 703–710, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med 10: 416–421, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Bozinovski S, Jones JE, Vlahos R, Hamilton JA, Anderson GP. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J Biol Chem 277: 42808–42814, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell 12: 805–816, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Choi SH, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res 104: 1355–1363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D'Armiento J, Dalal SS, Okada Y, Berg RA, Chada K. Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema. Cell 71: 955–961, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Defesche JC. Low-density lipoprotein receptor—its structure, function, and mutations. Semin Vasc Med 4: 5–11, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet 370: 797–799, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Foronjy R, Nkyimbeng T, Wallace A, Thankachen J, Okada Y, Lemaitre V, D'Armiento J. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol 294: L1149–L1157, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, Okada Y, D'Armiento JM. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med 173: 623–631, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geraghty P, Dabo AJ, D'Armiento J. TLR-4 contributes to cigarette smoke (CS) induced matrix metalloproteinase-1 (MMP-1) expression in chronic obstructive pulmonary disease. J Biol Chem 286: 30211–30218, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, Kheradmand F. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med 1: e8, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352: 1685–1695, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Harju K, Glumoff V, Hallman M. Ontogeny of Toll-like receptors Tlr2 and Tlr4 in mice. Pediatr Res 49: 81–83, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277: 2002–2004, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem 102: 196–202, 1980 [DOI] [PubMed] [Google Scholar]
- 27. Higgins M. Risk factors associated with chronic obstructive lung disease. Ann NY Acad Sci 624: 7–17, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Hirata N, Yanagawa Y, Ebihara T, Seya T, Uematsu S, Akira S, Hayashi F, Iwabuchi K, Onoe K. Selective synergy in anti-inflammatory cytokine production upon cooperated signaling via TLR4 and TLR2 in murine conventional dendritic cells. Mol Immunol 45: 2734–2742, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Hodgkinson CP, Patel K, Ye S. Functional Toll-like receptor 4 mutations modulate the response to fibrinogen. Thromb Haemost 100: 301–307, 2008 [PubMed] [Google Scholar]
- 30. Hsia CC, Hyde DM, Ochs M, Weibel ER. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181: 394–418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Imai K, Dalal SS, Chen ES, Downey R, Schulman LL, Ginsburg M, D'Armiento J. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med 163: 786–791, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Iwamoto H, Yokoyama A, Kitahara Y, Ishikawa N, Haruta Y, Yamane K, Hattori N, Hara H, Kohno N. Airflow limitation in smokers is associated with subclinical atherosclerosis. Am J Respir Crit Care Med 179: 35–40, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Knowles JW, Maeda N. Genetic modifiers of atherosclerosis in mice. Arterioscler Thromb Vasc Biol 20: 2336–2345, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larsson K. Aspects on pathophysiological mechanisms in COPD. J Intern Med 262: 311–340, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Leavitt BJ, Ross CS, Spence B, Surgenor SD, Olmstead EM, Clough RA, Charlesworth DC, Kramer RS, O'Connor GT. Long-term survival of patients with chronic obstructive pulmonary disease undergoing coronary artery bypass surgery. Circulation 114: I430–434, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Lemaitre V, D'Armiento J. Matrix metalloproteinases in development and disease. Birth Defects Res C Embryo Today 78: 1–10, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Mazzone T, Reardon C. Expression of heterologous human apolipoprotein E by J774 macrophages enhances cholesterol efflux to HDL3. J Lipid Res 35: 1345–1353, 1994 [PubMed] [Google Scholar]
- 38. Mercer BA, Kolesnikova N, Sonett J, D'Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J Biol Chem 279: 17690–17696, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Michelsen KS, Doherty TM, Shah PK, Arditi M. TLR signaling: an emerging bridge from innate immunity to atherogenesis. J Immunol 173: 5901–5907, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Pleis JR, Lucas JW, Ward BW. Summary health statistics for US. adults—National Health Interview Survey, 2008. Vital Health Stat 10 10: 1–157, 2009 [PubMed] [Google Scholar]
- 41. Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 154: 1055–1060, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler Thromb 14: 141–147, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Shapiro SD. The pathogenesis of emphysema: the elastase:antielastase hypothesis 30 years later. Proc Assoc Am Physicians 107: 346–352, 1995 [PubMed] [Google Scholar]
- 44. Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc 5: 475–477, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaw PX. Rethinking oxidized low-density lipoprotein, its role in atherogenesis and the immune responses associated with it. Arch Immunol Ther Exp (Warsz) 52: 225–239, 2004 [PubMed] [Google Scholar]
- 46. Shiomi T, Okada Y, Foronjy R, Schiltz J, Jaenish R, Krane S, D'Armiento J. Emphysematous changes are caused by degradation of type III collagen in transgenic mice expressing MMP-1. Exp Lung Res 29: 1–15, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Sidney S, Sorel M, Quesenberry CP, Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest 128: 2068–2075, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 127: 1952–1959, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Stokes J., 3rd Cardiovascular risk factors. Cardiovasc Clin 20: 3–20, 1990 [PubMed] [Google Scholar]
- 50. Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke 37: 1923–1932, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL, Tall AR. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ Res 104: 455–465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Szczepanski MJ, Czystowska M, Szajnik M, Harasymczuk M, Boyiadzis M, Kruk-Zagajewska A, Szyfter W, Zeromski J, Whiteside TL. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res 69: 3105–3113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tall AR, Costet P, Wang N. Regulation and mechanisms of macrophage cholesterol efflux. J Clin Invest 110: 899–904, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taraseviciene-Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. J Clin Invest 118: 394–402, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 29: 88–97, 2003 [DOI] [PubMed] [Google Scholar]
- 56. van Ree JH, van den Broek WJ, Dahlmans VE, Groot PH, Vidgeon-Hart M, Frants RR, Wieringa B, Havekes LM, Hofker MH. Diet-induced hypercholesterolemia and atherosclerosis in heterozygous apolipoprotein E-deficient mice. Atherosclerosis 111: 25–37, 1994 [DOI] [PubMed] [Google Scholar]
- 57. Vaughan T, Li L. Molecular mechanism underlying the inflammatory complication of leptin in macrophages. Mol Immunol 47: 2515–2518, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Veniant MM, Withycombe S, Young SG. Lipoprotein size and atherosclerosis susceptibility in Apoe(-/-) and Ldlr(-/-) mice. Arterioscler Thromb Vasc Biol 21: 1567–1570, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Vestbo J, Hogg JC. Convergence of the epidemiology and pathology of COPD. Thorax 61: 86–88, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wouters EF. Local and systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 26–33, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104: 3103–3108, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Ye XW, Xiao M, Ye J, Zhang XY, Xiao J, Feng YL, Wen FQ. The polymorphism -2548G/A in leptin and severity of chronic obstructive pulmonary disease. Int J Immunogenet 38: 45–50, 2011 [DOI] [PubMed] [Google Scholar]
- 63. Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 118: 1837–1847, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, Princen HM, Kooistra T. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol 27: 1706–1721, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Zhu Y, Bellosta S, Langer C, Bernini F, Pitas RE, Mahley RW, Assmann G, von Eckardstein A. Low-dose expression of a human apolipoprotein E transgene in macrophages restores cholesterol efflux capacity of apolipoprotein E-deficient mouse plasma. Proc Natl Acad Sci USA 95: 7585–7590, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]