Abstract
Serotonin (5-HT) and fibronectin (FN) have been associated with pulmonary hypertension (PH). We previously reported that FN is posttranslationally modified by tissue transglutaminase (TGase) to form serotonylated FN (s-FN) in pulmonary artery smooth muscle cells and that serotonylation stimulates their proliferation and migration, hallmarks of PH. We hypothesized that s-FN and its binding to TGase are elevated in human and experimental PH. To assess this hypothesis, FN isolation and electrophoretic, immunoblotting, and densitometric techniques were used. Mean ratio of serum s-FN to total FN level (s-FN/FN) was elevated in 19 consecutive pulmonary arterial hypertension (PAH) patients compared with 25 controls (0.3 ± 0.18 vs. 0.05 ± 0.07, P < 0.001). s-FN/FN also was increased in lungs of mice and rats with hypoxia-induced PH and in rats with monocrotaline-induced PH. In mice, the increase was detected at 1 wk of hypoxia, preceding the development of PH. Hypoxic rats had elevated serum s-FN/FN. Enhanced binding of TGase to its substrate FN occurred in serum from patients with PAH (mean 0.50 ± 0.51 vs. 0.063 ± 0.11, P = 0.002) and s-FN/FN and TGase-bound FN were highly correlated (R2 = 0.77). TGase-bound FN also was increased in experimental PH. We conclude that increased serotonylation of FN occurs in human and experimental PH and may provide a biomarker for the disease.
Keywords: serotonin, transglutaminase, transglutamidation, hypoxia- and monocrotaline-induced pulmonary hypertension
strong lines of evidence from human and experimental animal models support a role for serotonin (5-HT) and the serotonin transporter (SERT) in the pathogenesis of pulmonary hypertension (PH). Herve et al. (11, 12) first identified elevation of plasma 5-HT and reduced platelet 5-HT in association with pulmonary arterial hypertension (PAH) in patients with familial platelet storage disease. About this time it also became recognized that PH is associated with appetite-suppressing derivatives of fenfluramine (e.g., Ref. 1), which along with 5-HT is a substrate for the 5-HT transporter (SERT) (19, 30). In the mid-1980s our laboratory reported that exposure of endothelial and smooth muscle cells (SMCs) to hypoxia enhanced cellular transcription of SERT and that this was related to increased cellular glycolysis (17, 18). This observation fitted well with known hypoxia-induced PH, later reports of elevated plasma 5-HT in mice developing PH after hypoxic exposure (3) and elevated plasma levels of 5-HT in humans with the idiopathic form of PAH (15). Eddahibi and associates (6) carried these observations further with studies on transgenic mice in which SERT knockout animals failed to develop hypoxia-induced PH and animals selectively overexpressing vascular SERT with a SM22-targeted promoter spontaneously developed PH (9). Furthermore, these investigators reported elevated expression of SERT in cultured pulmonary artery SMCs from patients with PH. A promoter polymorphism in SERT has been described in humans, and the LL genotype has been associated with higher in vitro SERT activity and an increased risk of PH in patients with chronic obstructive lung disease (COPD) (5). Our laboratory described a mitogenic effect of 5-HT on SMCs (8, 22); 5-HT from platelets was later associated with liver regeneration (23). The mitogenic effect of 5-HT on SMCs is related to generation of reactive oxygen species associated with cellular internalization of 5-HT (20).
SERT, a looped 12-transmembrane protein, belongs to a monoamine transporter family that is known to actively transport 5-HT intracellularly concurrent with sodium movement. Although initially identified and cloned by Blakely et al. (2) from neural tissue, SERT is now known to be present in many different types of cells throughout the body, and its presence on the endothelial surface is important in the clearance of 5-HT from the blood as it passes through the pulmonary circulation. Although our early studies demonstrated that 5-HT is actively transported intracellularly in pulmonary vascular cells (18, 21) and that this transport is enhanced by exposure of cells to hypoxia or agents that produce cellular anaerobiosis (17, 22), the mechanism by which intracellular 5-HT participates in SMC growth has remained unknown.
New insight regarding the possible mechanism by which intracellular 5-HT might exert an intracellular effect comes from our in vitro studies with SMCs showing that 5-HT serotonylates (transaminates) intracellular proteins through the enzyme transglutaminase (TGase) (25). In this study one protein substrate that is highly transaminated by 5-HT is fibronectin (FN). This is of interest because FN is known to participate in clinical and experimental PH (4, 14, 28).
As a result of our in vitro studies we hypothesized that serotonylated FN (s-FN) is increased in PAH. We measured s-FN in the lungs and blood of patients with PAH and extended our studies to animal models of PH, including rodents exposed to hypoxia (16, 26) and rats administered monocrotaline (MCT). In addition, we determined the degree of TGase binding to its substrate FN in both animals and humans with PH.
MATERIALS AND METHODS
Acquisition of human lungs and blood.
Human lung tissue was obtained from 19 idiopathic PAH patients undergoing lung transplantation and from 9 normal controls (normal lung adjacent to lung tumors and COPD patients who had had partial lung resections) at the Cleveland Clinic. Demographics of the PAH patients are contained in Table 1. Thirteen of the patients had idiopathic PAH, 3 familial PAH, and 3 associated PAH. No hemodynamic or therapeutic data were available. Lung tissue was snap frozen directly after explantation for protein extraction. Serum samples were from patients with PAH from the Tufts Medical Center Pulmonary Hypertension Center and were obtained at the time of diagnostic right heart catheterization. Demographics for these 19 patients are noted in Table 2. Eighteen of the 19 patients had idiopathic PAH and one had PAH secondary to lupus erythematosis. Fourteen of the 19 patients had not received therapy for their PH; the others were on various forms of conventional therapy. Sera from 25 controls were from both healthy individuals and randomized samples from patients without known PAH that were stored in the Tufts Medical Center Clinical Laboratories.
Table 1.
Demographic data for patients with explanted lungs and control group
| Variable | Value |
|---|---|
| Patients | |
| Age, yr | 44.9 ± 10.2 |
| Female | 12 (63.2%) |
| Male | 7 (36.8%) |
| PAH | 19 (100%) |
| Idiopathic | 13 (68.4%) |
| Familial | 3 (15.8%) |
| Associated | 3 (15.8%) |
| Caucasian | 19 (100%) |
| Controls | |
| Age, yr | 57.3 ± 7.6 |
| Female | 6/9 (66.7%) |
| Male | 3/9 (33.3%) |
| Caucasian | 9/9 (100%) |
Values are means ± SD. PAH, pulmonary arterial hypertension.
Table 2.
Demographic data for PAH serum samples and control group
| Variable | Value |
|---|---|
| Patients | |
| Age, yr | 58.4 ± 14.8 |
| Female | 13 (68.4%) |
| Caucasian | 18 (94.7%) |
| Idiopathic PAH | 18 (94.7%) |
| BMI, kg/m2 | 30.9 ± 7.86 |
| RAP, mmHg | 9.7 ± 7.3 |
| mPA, mmHg | 51.5 ± 10.9 |
| PW, mmHg | 10 ± 4.3 |
| PVR, dyn·s·cm−5 | 945 ± 339 |
| CO, l/min | 3.90 ± 1.30 |
| CI, l·min·m−2 | 2.09 ± 0.61 |
| NYHA FC | |
| 1 | 0 |
| 2 | 5 (26.3%) |
| 3 | 9 (47.4%) |
| 4 | 2 (10.5%) |
| Controls | |
| Age, yr | 50.8 ± 18.4 |
| Female | 15/25 (60%) |
| Male | 10/25 (40%) |
Clinical and demographic data for 19 patients with Pulmonary Arterial Hypertension. Eighteen patients were idiopathic; one was systemic lupus erythematosus. Values are means ± SD; categorical data are presented as n (%). BMI, body mass index; RAP, right atrium pressure; mPA, mean pulmonary artery pressure; PW, wedge pressure; PVR, pulmonary vascular resistance; CO, cardiac output; CI, cardiac index; NYHA FC, New York Heart Association functional classification.
Animals.
Adult male C57Bl/6 mice (∼20 g body wt) and Sprague-Dawley rats (∼250 g body wt) were obtained from Taconic Laboratories. Animals were housed under controlled temperature (∼22°C) and lighting (12:12-h light-dark cycle), with free access to food and water. Mice and rats were exposed to chronic hypoxia. In other experiments, rats were injected with 60 mg/kg MCT (Sigma, St. Louis, MO) subcutaneously, and measurements were obtained after 21 days.
Exposure to chronic hypoxia.
Animals were randomized to exposures to normoxia or chronic hypoxia (11% O2, 3 wk for rat experiments and 5 wk for mouse experiments). There were three to four animals in each group and each experiment was repeated three times. Hypoxic chambers were opened three times weekly for food, water, and cleaning. Twelve-hour light-exposure cycles, standard rodent chow, and water ad libitum were provided to all animals. The chamber temperature was maintained at 22–24°C. Animals exposed to normoxia were kept in the same room adjacent to the hypoxic chamber. For a time course, a separate group of mice was exposed to 0, 1, 3, 7, and 35 days of hypoxia vs. room air and lungs were collected for s-FN measurements.
Hemodynamic measurements and heart weights.
At the end of the exposure period (hypoxia or MCT), animals were anesthetized with pentobarbital (20 mg/kg ip) and ketamine (60 mg/kg im) and hemodynamics were measured. The trachea was cannulated and the lungs were ventilated with room air. For rats, a catheter was advanced into the right ventricle (RV) via the right jugular vein for continuous RV pressure recording. For mice, a 26-gauge needle attached to a Statham P23-G pressure transducer by a short segment of P-50 tubing was inserted directly into the RV using a transthoracic approach. After hemodynamic measurements were performed rat blood was collected via the RV catheter; serum was immediately separated by centrifugation and frozen at −80°C. Animals were euthanized with a pentobarbital injection (120 mg/kg ip). The thorax was opened immediately; the lungs were removed, quickly frozen in liquid nitrogen, and stored at −80°C. Heart chambers were weighed and the ratio of RV/LV+S was determined as an index of RV hypertrophy.
Extraction of protein from lung tissues.
Tissue extractions were done identically for human and rodent lungs. For preparation of proteins from lungs, ∼300 mg (for human) or 150 mg (for mice or rats) of frozen lung tissue was homogenized in 1 ml ice-cold tissue homogenization buffer consisting of 50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100, 10% glycerol containing protease (Sigma) and phosphatase (EMD, Gibbstown, NJ) inhibitors. The homogenate was centrifuged at 14,000 rpm for 10 min at 4°C. Protein extracts were stored at −80°C.
Immunoprecipitation of protein from tissue extracts and sera.
Lung tissue protein extracts were normalized for protein concentrations by using the Bradford reagent (Bio-Rad, Hercules, CA). One-half milliliter of serum was diluted with an equal volume of homogenization buffer (same as extraction buffer) containing protease and phosphatase inhibitors. Sera and tissue extracts were precleared with protein A-agarose and protein G-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C for 1 h, then incubated with antibody to FN (Santa Cruz Biotechnology) at 4°C overnight and immunoprecipitated with protein G-agarose and protein A-agarose beads (Santa Cruz Biotechnology) at 4°C for another 4 h with constant rotation.
Western blotting.
Immunoprecipitates were resolved by SDS-PAGE (4–20%) and transferred onto a PVDF membrane. The membrane was blocked with 5% fat-free milk in TBST [10 mM Tris·HCl, pH 8.0, 150 mM NaCl, and 0.1% (vol/vol) Tween-20] for 1 h at room temperature and then incubated with primary antibody (1:1,000 for antibody to TGase and 1:2,000 for antibody to 5-HT) at 4°C overnight. The membrane was washed three times in PBST and incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibody (Bio-Rad, Hercules, CA) in a dilution of 1:3,000 for TGase and 1:5,000 for 5-HT, followed by ECL detection (Thermo Fisher Scientific, Pittsburgh, PA). Densitometry measurements were performed with the software UN-SCAN-IT from Silk Scientific (Orem, UT). Fold changes were expressed in relation to the total protein of FN.
Statistics.
Values are shown as means ± SD. Statistical significance was determined by one-way ANOVA followed by Bonferroni multiple-comparison test. When only two groups were compared, statistical differences were assessed with unpaired two-tailed t-test, or Wilcoxon Mann-Whitney U-test when the normality passed, or failed, respectively. The relationship between serum levels of s-FN/FN and FN-bound TGase was determined by a linear regression analysis. Calculations were performed using SigmaStat software (Systat Software). A P value less than 0.05 was considered statistically significant. The number of samples or animals in each group is indicated in the figure legends and in the tables.
Study approval.
The human studies were approved by the appropriate Institutional Review Boards on human subjects at the Cleveland Clinic and Tufts Medical Center, and all subjects gave written, informed consent. All animal studies were approved by the Institutional Animal Care and Use Committee at Tufts Medical Center and were conducted in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
RESULTS
s-FN/FN in excised lungs of patients with PAH receiving transplanted lungs.
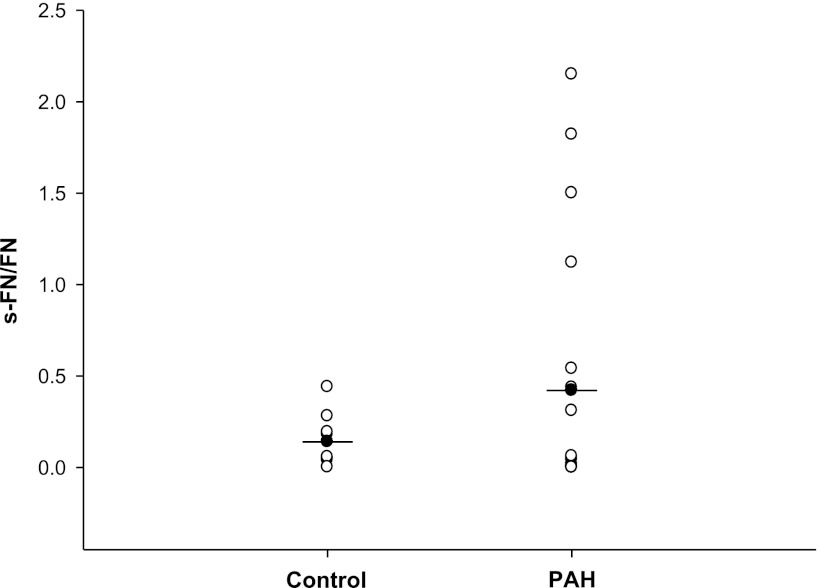
The mean value of s-FN/FN for lungs of patients with PAH was 0.423 ± 0.69 (n = 19) compared with 0.143 ± 0.14 (n = 9) for control lung samples obtained from noncancerous lung tissue of lung cancer patients or patients with COPD (Fig. 1). Considerably higher levels of s-FN/FN out of the range of control values (from 1.12 to 2.15) occurred in four of the lung samples from patients with PAH. There were no recognizable factors noted for these four patients (two of subtype idiopathic PAH, one familial, and one associated PAH) that might account for the difference in values. A statistical analysis failed to show a significant difference between s-FN/FN of the control and PAH samples (P = 0.2).
Fig. 1.
Serotonylation of fibronectin (FN) in lungs of patients with pulmonary arterial hypertension (PAH). Serotonylated FN (s-FN)/FN was determined in lungs of patients with PAH as noted in materials and methods and compared with that of lungs of control subjects (lung tissue adjacent to resected tumor or resected tissue from chronic obstructive pulmonary disease patients). Mean values are shown; n = 19 for PAH patients and 9 for controls; P = 0.24.
s-FN/FN in sera of patients with PAH.
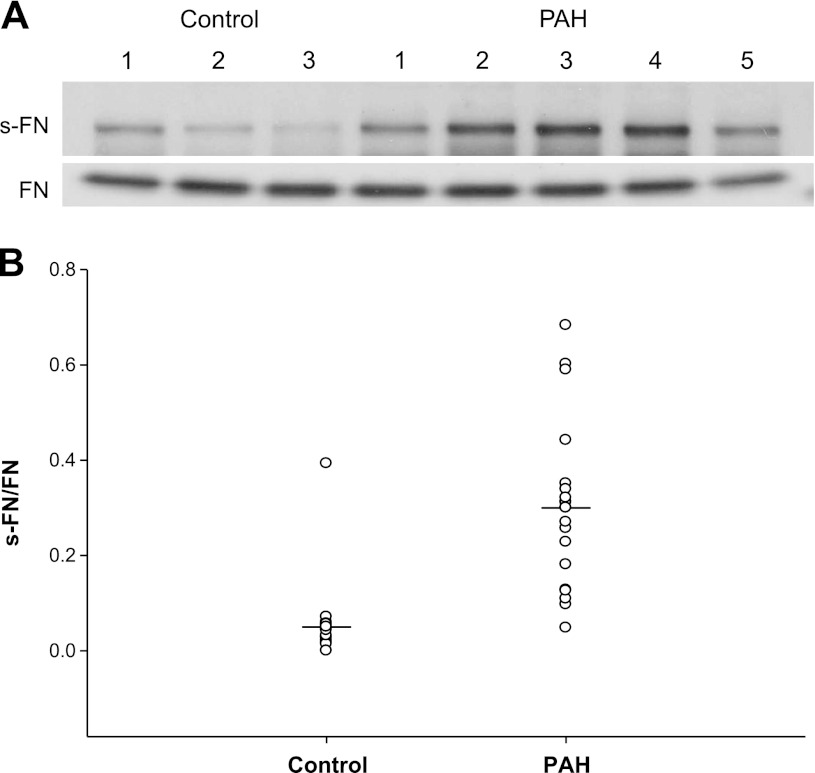
Because of the limited number of lung samples from patients with PAH, we turned our attention to measurements in sera collected from patients with PAH and compared these with normal controls or controls obtained from otherwise to be discarded sera of patients with various diseases other than PAH from our clinical laboratory. No sera had been collected from PAH patients from whom lung samples had been obtained. Thus the sera we used were from another group of patients followed in our PH clinic. These sera samples from patients with PAH showed significantly increased s-FN (Fig. 2A), and the mean value of s-FN/FN was 0.3 compared with a mean of 0.05 for controls (P < 0.001) (Fig. 2B). One control sample from the clinical laboratory was abnormally high (0.393), but an assessment of clinical data from this patient failed to reveal obvious PH or an explanation for the elevated value.
Fig. 2.
Serotonylation of FN in serum of patients with PAH. s-FN/FN was determined in sera from patients with PAH and compared with that from controls. A: representative electrophoretic blots of s-FN and FN in serum from 5 PAH patients and 3 controls. B: comparisons for all patients studied; we show mean s-FN/FN of patients with PAH (n = 19) vs. controls (n = 25). Mean value for PAH = 0.3 ± 0.18 vs. 0.05 ± 0.07 for controls; P < 0.001.
Elevation of s-FN/FN in lungs and blood of rodents exposed to hypoxia.
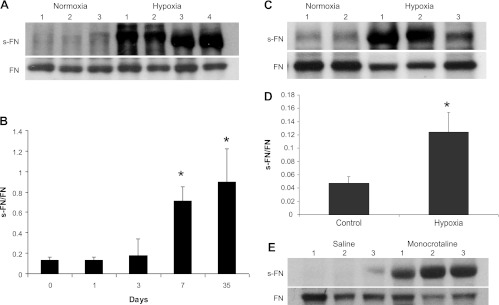
We next assessed whether similar enhancement of s-FN could be detected in animal models of PH. A well-known model of rodent exposure to hypoxia was used. With this exposure PH is produced within 5 wk of hypoxia in mice (26) and within 3 wk of the hypoxic environment in rats (16). All animals exposed to hypoxia used for Western blotting in this study developed PH as determined by measurements of RV (RV) peak pressures and RV/left ventricular (LV) + septal (S) weights (Table 3). As seen in Fig. 3A, Western blots of s-FN showed significant elevation of s-FN in lungs of mice exposed to hypoxia for 5 wk. Statistical elevation of s-FN was also observed in these animals within 7 days of exposure to hypoxia (Fig. 3B), a time that precedes the development of PH (*P < 0.05). Similar elevation of s-FN was noted in lungs of rats exposed to hypoxia for 3 wk (Fig. 3C). s-FN/FN in rat serum also was elevated at 3 wk (Fig. 3D) (*P < 0.05). Another method to induce experimental PH is with use of MCT (29). In additional experiments, rats were injected subcutaneously with 60 mg/kg MCT. PA pressures and RV/LV + S weights were measured on all animals (Table 3) and lungs were extracted for protein and subjected to gel electrophoresis as noted in materials and methods. Similar to results obtained with exposure to hypoxia, there was elevation in s-FN/FN in lungs at 3 wk, a time at which the animals had developed PH (Fig. 3E and Table 3).
Table 3.
Representative hemodynamic data of mice and rats under hypoxia or given MCT
| Mice 35 Days |
Rats 21 Days |
Rats 21 Days |
||||
|---|---|---|---|---|---|---|
| Normoxia N = 3 | Hypoxia N = 4 | Normoxia N = 3 | Hypoxia N = 3 | Saline N = 3 | MCT N = 3 | |
| RV pressure, mmHg | 19 ± 4 | 35 ± 6* | 28 ± 5 | 45 ± 6* | 23 ± 1 | 55 ± 15* |
| RV/LV+S | 0.22 ± 0.04 | 0.31 ± 0.02* | 0.28 ± 0.01 | 0.46 ± 0.02* | 0.23 ± 0.04 | 0.41 ± 0.07* |
Summary of hemodynamic parameters for animal experiments.
P ≤ 0.02 between control and treated animals. RV, right ventricle; LV, left ventricle; S, septum; MCT, monocrotaline.
Fig. 3.
FN serotonylation in lungs of mice and rats exposed to hypoxia or monocrotaline. Mice or rats were exposed to hypoxia for 5 or 3 wk, respectively. Three to 4 animals were used for each group and each experiment was repeated 3 times. Lungs were excised, tissues were extracted, and FN and s-FN/FN were measured as noted in materials and methods. A: representative blots of s-FN and FN in mouse lungs. B: s-FN/FN was calculated from lungs of mice and a time course is illustrated (n = 4 at each time point); *P < 0.05. C: representative blotted electrophoresis sample from lungs of rats exposed to hypoxia for 3 wk. D: sera were collected from rats exposed to hypoxia for 3 wk and FN was immunoprecipitated. The precipitate was run on electrophoresis. S-FN and FN were immunoblotted and measured as noted in materials and methods. The s-FN-to-FN ratio was determined and mean values ± SD are noted (*P < 0.05, n = 4 for each group). E: treatment with monocrotaline (60 mg/kg) was carried out as noted in materials and methods. Lung tissue was harvested at 21 days and FN was immunoprecipitated. Electrophoresis with immunoblotting was carried out as noted in materials and methods.
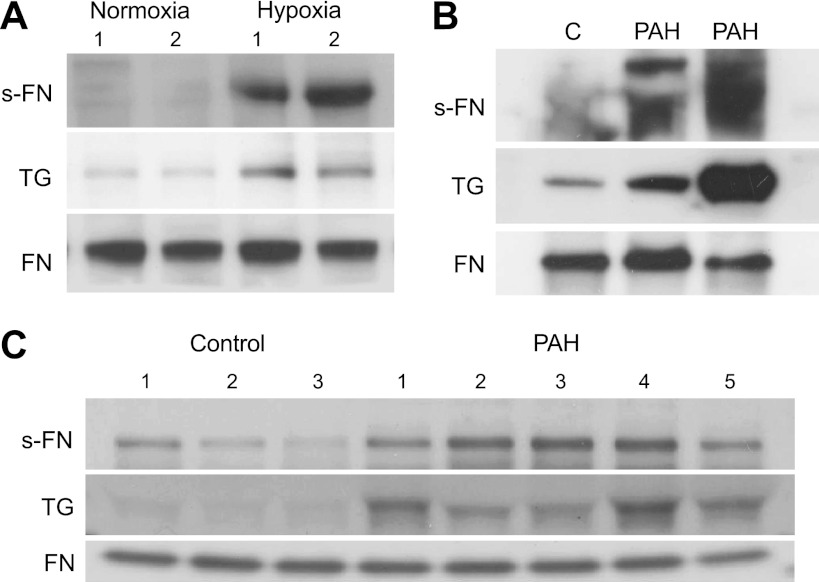
Binding of transglutaminase to FN in human and animal tissue studies.
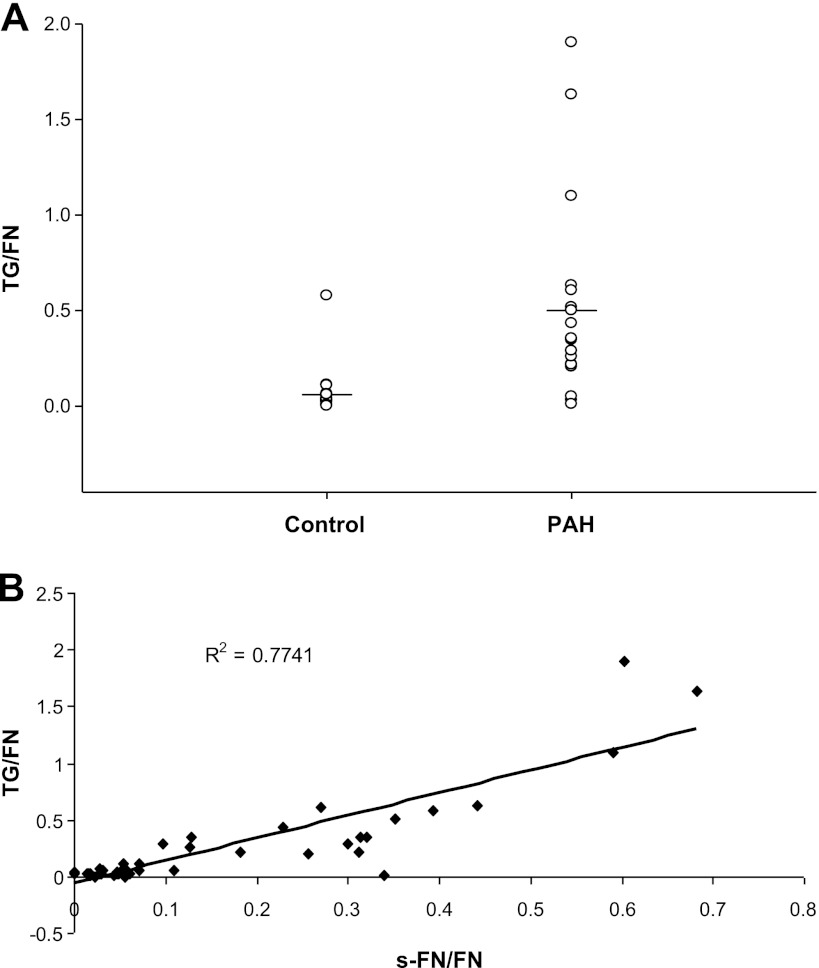
Efforts were made to determine whether enhanced binding of TGase to its substrate FN could be detected in the animal and human studies. The enhanced binding was found in both lungs of rats developing PH and patients with PAH as seen in Fig. 4, A and B. Elevated TGase binding to FN also occurred for serum from patients with PAH as illustrated in Fig. 4C. The TGase-to-FN ratio was increased in sera of patients with PAH compared with controls (Fig. 5A) and the TGase-to-FN ratios for these patients correlated with those of s-FN/FN (correlation coefficient 0.77) (Fig. 5B).
Fig. 4.
Transglutaminase (TGase) binding to FN. A: rats were exposed to hypoxia for 3 wk. FN was immunoprecipitated from lungs and run on gel electrophoresis as noted in materials and methods. S-FN, TGase, and FN were blotted. A representative sample is shown. B: FN was immunoprecipitated from human lungs and run on gel electrophoresis as noted in materials and methods. TGase, s-FN, and FN were probed. Selected PAH lung samples had known high s-FN/FN values. A representative blot is shown. C: after precipitation and gel electrophoresis of FN in human serum, TGase, s-FN and FN were blotted. A representative blot is shown.
Fig. 5.
TGase/FN in serum of patients with PAH. A: FN was immunoprecipitated and run on gel electrophoresis as noted in materials and methods. Mean control TGase/FN = 0.063 ± 0.11, n = 25, vs. mean PAH TGase/FN = 0.50 ± 0.51, n = 19, P = 0.002. B: correlation of bound TGase with s-FN/FN in human serum. P < 0.001.
DISCUSSION
There is increasing awareness of the participation of protein transamidation in organ cellular function. When serotonin is the substrate for this action, the process is referred to as protein serotonylation and the enzyme that catalyzes the reaction is a tissue transglutaminase. Serotonylation of small GTPases was first identified as a signal transduction pathway that triggers platelet α-granule release occurring in hemostasis (31). Later studies showed that protein serotonylation participates in insulin secretion from pancreatic cells (27) and that fibronectin is serotonylated in C6 glioma cells (13), an indication of a possible role in neurotransmission. Guilluy et al. (10) reported an elevation of serotonylated Rho A and Rho kinase activation in circulating platelets of humans with PH and lungs of 5-HT transporter overexpressing mice. Our own studies showed a role for protein transamidation in serotonin-induced proliferation and migration of pulmonary artery SMCs and suggested that FN is a major cellular protein that participates in serotonylation (25). In cell culture the s-FN is released into extracellular medium. Studies with systemic vascular rings have shown that serotonylation of proteins is not important only in smooth muscle growth and migration but also in vascular contraction (32).
Our present findings demonstrate that enhanced serotonylation of FN occurs in sera of patients with PAH. Furthermore, as shown with immunoprecipitation and immunoblotting techniques, there is enhanced binding of FN with transglutaminase, the enzyme participating in serotonylation. Serotonylated FN also was elevated in lung tissue and sera of experimental rodent models of PH. These observations, coupled with our previous studies (25) and those of other investigators demonstrating enhanced cellular uptake of 5-HT associated with PH, are consistent with a process of intracellular FN transamidation by the 5-HT and subsequent release of the s-FN into the intercellular space and serum. However, it is also possible that FN in serum may be directly transamidated by 5-HT.
Studies by Li et al. (24) utilizing cystamine as an inhibitor of TGase suggest that tissue TGase is a regulator of RV hypertrophy produced by exposure of animals to hypoxia. However, their data showed only moderate decreases in indices of RV weights of hypoxia-exposed animals given cystamine compared with controls and although hemodynamic data were not included the authors stated that cystamine failed to influence elevation of pulmonary artery pressure. Of concern in this article is a failure to directly measure inhibition of TGase activity by cystamine in these experiments. Hence, no firm conclusions can be drawn from the data about a causal role of TGase activity in PH. Our results showing that elevation in serotonylation of FN precedes elevation in pulmonary artery pressure in mice exposed to hypoxia suggest that the protein serotonylation may not be secondary to the pressure response alone. Mechanistically, our findings are consistent with increased intracellular transport of 5-HT via SERT previously reported in PAH (7). Furthermore, the results are consistent with a hypothesis that exposure to hypoxia stimulates this transport process (17). Serotonylation of FN and other proteins is dependent on 5-HT transport and results in SMC proliferation and migration (25) and enhanced vascular contractility (32) that accompany PH. Whether or not TGase activity is elevated in the process is unknown. It also remains to be determined whether altered protein transamidation is causally related to the development of PH or RV cardiac responses.
Although we believe we have established proof of concept that enhanced protein serotonylation occurs in human and experimental PH and these data offer a compelling and novel insight into serotonin's role in its pathogenesis, there are several limitations and unanswered questions that deserve consideration. We cannot presently explain why we see enhanced serotonylation of fibronectin in serum whereas that in lung tissue was elevated in only a small number of patients with PAH. Because of solubility issues for fibronectin from tissue, technical factors may be a consideration. It must be recognized that the human lung and sera studies were undertaken in two different populations of patients. Human lung was obtained from patients undergoing lung transplantation for end-stage PAH and therefore represent more advanced and prolonged cases of PAH. Unfortunately, sera were not available from these patients and thus no direct comparisons could be made. We were limited in not being able to obtain larger numbers of samples of human lung from patients with PAH. However, we have found enhanced serotonylation of FN in lungs and sera of animal models of PH. The kinetics of the relationship between serotonylated FN in lungs and blood remains to be determined. It is possible that any enhanced serotonylation of FN in lung tissue decays earlier than that in serum so that elevated tissue levels are not detected at later times. It would be difficult to replicate prolonged (for many years) PH that occurs in humans in animal experimental models.
Our observation of elevated levels of s-FN/FN in serum of patients with PAH along with other data in the literature suggests a possible role in the pathogenesis of PAH. Furthermore, determinations of s-FN/FN or TGase/FN may provide novel biomarkers for this disease. The histological localization and pattern of development of serotonylation, its possible relationship to idiopathic or associated PAH and susceptibility to therapeutic agents need further investigation with larger cohorts of patients. Finally, it should be noted that we elected to examine FN as the serotonylated protein based on high serotonylation of this protein in studies of pulmonary artery cells in culture treated with 5-HT (25). Other proteins also may be serotonylated in PAH and may reflect its pathogenesis or serve as better biomarkers than FN.
GRANTS
The research was supported by RO1HL085260 (B. L. Fanburg).
AUTHOR CONTRIBUTIONS
L. Wei did all of the protein isolation, electrophoresis, immunoblotting and densitometry work and assisted with composition of the manuscript. R. R. Warburton did all of the animal studies, including instrumentation, blood collecting and organ procurement. I. R. Preston and K. E. Roberts assisted with procurement of human blood samples, review of data and composition of the manuscript. S. A. A. Comhair and S. Erzurum participated in acquisition and identification of tissue from human explanted lungs. N. Hill contributed to discussions of assessment of data and design of experimentation. B. L. Fanburg composed the experimental plan of the study, assessed all data and participated in composition of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Atanassoff PG, Weiss BM, Schmid ER, Tornic M. Pulmonary hypertension and dexfenfluramine. Lancet 339: 436, 1992 [PubMed] [Google Scholar]
- 2. Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature 354: 66–70, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Callebert J, Esteve JM, Herve P, Peoc'h K, Tournois C, Drouet L, Launay JM, Maroteaux L. Evidence for a control of plasma serotonin levels by 5-hydroxytryptamine(2B) receptors in mice. J Pharmacol Exp Ther 317: 724–731, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol 189: 1–13, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Eddahibi S, Chaouat A, Morrell N, Fadel E, Fuhrman C, Bugnet AS, Dartevelle P, Housset B, Hamon M, Weitzenblum E, Adnot S. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation 108: 1839–1844, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Eddahibi S, Hanoun N, Lanfumey L, Lesch KP, Raffestin B, Hamon M, Adnot S. Attenuated hypoxic pulmonary hypertension in mice lacking the 5-hydroxytryptamine transporter gene. J Clin Invest 105: 1555–1562, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest 108: 1141–1150, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fanburg BL, Lee SL. A new role for an old molecule: serotonin as a mitogen. Am J Physiol Lung Cell Mol Physiol 272: L795–L806, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res 98: 1323–1330, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, Savale L, Humbert M, Fadel E, Adnot S, Loirand G, Pacaud P. RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am J Respir Crit Care Med 179: 1151–1158, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Herve P, Drouet L, Dosquet C, Launay JM, Rain B, Simonneau G, Caen J, Duroux P. Primary pulmonary hypertension in a patient with a familial platelet storage pool disease: role of serotonin. Am J Med 89: 117–120, 1990 [DOI] [PubMed] [Google Scholar]
- 12. Herve P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, Poubeau P, Cerrina J, Duroux P, Drouet L. Increased plasma serotonin in primary pulmonary hypertension. Am J Med 99: 249–254, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Hummerich R, Schloss P. Serotonin—more than a neurotransmitter: transglutaminase-mediated serotonylation of C6 glioma cells and fibronectin. Neurochem Int 57: 67–75, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Jones PL, Cowan KN, Rabinovitch M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol 150: 1349–1360, 1997 [PMC free article] [PubMed] [Google Scholar]
- 15. Kereveur A, Callebert J, Humbert M, Herve P, Simonneau G, Launay JM, Drouet L. High plasma serotonin levels in primary pulmonary hypertension. Effect of long-term epoprostenol (prostacyclin) therapy. Arterioscler Thromb Vasc Biol 20: 2233–2239, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Klinger JR, Siddiq FM, Swift RA, Jackson C, Pietras L, Warburton RR, Alia C, Hill NS. C-type natriuretic peptide expression and pulmonary vasodilation in hypoxia-adapted rats. Am J Physiol Lung Cell Mol Physiol 275: L645–L652, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Lee SL, Fanburg BL. Glycolytic activity and enhancement of serotonin uptake by endothelial cells exposed to hypoxia/anoxia. Circ Res 60: 653–658, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Lee SL, Fanburg BL. Serotonin uptake by bovine pulmonary artery endothelial cells in culture. II. Stimulation by hypoxia. Am J Physiol Cell Physiol 250: C766–C770, 1986 [DOI] [PubMed] [Google Scholar]
- 19. Lee SL, Wang WW, Fanburg BL. Dexfenfluramine as a mitogen signal via the formation of superoxide anion. FASEB J 15: 1324–1325, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Lee SL, Wang WW, Fanburg BL. Superoxide as an intermediate signal for serotonin-induced mitogenesis. Free Radic Biol Med 24: 855–858, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Lee SL, Wang WW, Lanzillo JJ, Fanburg BL. Regulation of serotonin-induced DNA synthesis of bovine pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 266: L53–L60, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Lee SL, Wang WW, Moore BJ, Fanburg BL. Dual effect of serotonin on growth of bovine pulmonary artery smooth muscle cells in culture. Circ Res 68: 1362–1368, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science 312: 104–107, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Li X, Wei XL, Meng LL, Chi MG, Yan JQ, Ma XY, Jia YS, Liang L, Yan HT, Zheng JQ. Involvement of tissue transglutaminase in endothelin 1-induced hypertrophy in cultured neonatal rat cardiomyocytes. Hypertension 54: 839–844, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Wei L, Laskin DL, Fanburg BL. Role of protein transamidation in serotonin-induced proliferation and migration of pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 44: 548–555, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, Walther DJ. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol 7: e1000229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rabinovitch M. Pathobiology of pulmonary hypertension. Extracellular matrix. Clin Chest Med 22: 433–449, viii, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Rosenberg HC, Rabinovitch M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am J Physiol Heart Circ Physiol 255: H1484–H1491, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation 100: 869–875, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 115: 851–862, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Watts SW, Priestley JR, Thompson JM. Serotonylation of vascular proteins important to contraction. PLoS One 4: e5682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]