Abstract
Patients with severe acute lung injury are frequently administered high concentrations of oxygen (>50%) during mechanical ventilation. Long-term exposure to high levels of oxygen can cause lung injury in the absence of mechanical ventilation, but the combination of the two accelerates and increases injury. Hyperoxia causes injury to cells through the generation of excessive reactive oxygen species. However, the precise mechanisms that lead to epithelial injury and the reasons for increased injury caused by mechanical ventilation are not well understood. We hypothesized that alveolar epithelial cells (AECs) may be more susceptible to injury caused by mechanical ventilation if hyperoxia alters the mechanical properties of the cells causing them to resist deformation. To test this hypothesis, we used atomic force microscopy in the indentation mode to measure the mechanical properties of cultured AECs. Exposure of AECs to hyperoxia for 24 to 48 h caused a significant increase in the elastic modulus (a measure of resistance to deformation) of both primary rat type II AECs and a cell line of mouse AECs (MLE-12). Hyperoxia also caused remodeling of both actin and microtubules. The increase in elastic modulus was blocked by treatment with cytochalasin D. Using finite element analysis, we showed that the increase in elastic modulus can lead to increased stress near the cell perimeter in the presence of stretch. We then demonstrated that cyclic stretch of hyperoxia-treated cells caused significant cell detachment. Our results suggest that exposure to hyperoxia causes structural remodeling of AECs that leads to decreased cell deformability.
Keywords: acute respiratory distress syndrome, atomic force microscopy, force maps, elastic modulus
the mortality rate of 40–60% for patients with acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) remains remarkably high in the United States (36). Patients with ALI and ARDS exhibit compromised respiratory function that is partly related to edema formation attributable to loss of endothelial and epithelial barrier integrity (26, 40, 42). Fluid accumulation in the alveolar space ultimately diminishes the efficiency of gas exchange, causing hypoxemia. Although patients are often initially treated with oxygen by mask, the extent of injury frequently requires mechanical ventilation with supplemental oxygen. Although mechanical ventilation is necessary for the survival of patients with the most severe physiological impairment, additional injury can occur, and this is referred to as ventilator-induced lung injury (12, 23, 33). Both mechanical ventilation and long-term exposure to hyperoxia can independently cause lung injury that is similar histologically to that seen in ARDS (1, 10, 24). Furthermore, animal studies have shown that the use of high levels of inspired oxygen (FiO2 >50%) can accelerate and increase lung injury when used in conjunction with high-stretch mechanical ventilation (2, 10, 17, 19, 20, 31). However, the mechanisms responsible for this enhanced injury are not well understood.
In animal studies, hyperoxia exposure for 3 days caused injury to alveolar type I (ATI) cells, hyperproliferation of alveolar type II (ATII) cells, diffuse hyaline membrane deposition, edema, interstitial fibrosis, and pulmonary vascular remodeling (3, 4, 8, 15, 31). It has been proposed that hyperoxia-induced lung injury results from increased production of reactive oxygen species, causing either direct tissue and protein damage or activation of signaling pathways that promote cell injury (6, 14, 16, 37). This injury is linked to the activation of molecular pathways, leading to both apoptosis and necrosis, but the precise pathways have not been fully elucidated (7, 38, 44).
Previous studies have shown that oxidative stress changed cell morphology by disrupting microfilaments and the spatial organization of actin (9, 25, 30). Exposure of lung macrophages to hyperoxia caused excessive polymerization of actin, which adversely affected the antibacterial function of the cells (25). Endothelial cells exposed to hyperoxia exhibited an increase in actin stress fibers (30). On the basis of these earlier studies, we hypothesized that changes in cytoskeletal structure caused by hyperoxia might decrease the mechanical deformability of the cells and thereby enhance the development of injury when cells are stretched during mechanical ventilation.
To test this hypothesis, we measured the elastic modulus of cultured alveolar epithelial cells (AECs) using atomic force microscopy (AFM) in the indentation mode. The elastic modulus, derived from direct contact of the probe with the live cell, provides a measure of the local deformability of the cell. Cells exposed to hyperoxia exhibited a significant increase in elastic modulus, and this was consistent with cytoskeletal changes observed by fluorescence microscopy. Moreover, we used a computational approach to predict that the increase in elastic modulus leads to increased internal cell stresses when they are exposed to stretch, which could lead to mechanical injury of epithelial cells. We then demonstrated that cyclic stretch of hyperoxia-treated cells caused increased detachment of the cells, which did not occur in control cells.
MATERIALS AND METHODS
Cell culture.
Two cell types were utilized in this study: primary rat ATII cells and a mouse alveolar epithelial cell line (MLE-12) [ American Type Culture Collection (ATCC), Manassas, VA ]. Primary rat ATII cells were isolated according to the methods described previously (11, 43). Briefly, ATII cells were isolated from male Sprague-Dawley rats by elastase digestion and differential adherence on IgG-coated dishes. ATII cells were cultured on Corning (Corning, Corning, NY) cell culture dishes coated with a rat lung fibroblast matrix deposited by RLF-6 cells (ATCC). Freshly isolated cells were seeded at a density of 3.5 × 106 cells/ml in 3 ml of ATII culture medium (DMEM; GIBCO, Carlsbad, CA) containing 10% heat-inactivated FBS (Cell-gro, Herndon, VA, or GIBCO), 1 mM glutamine, 1% penicillin/streptomycin, and 0.25 μM amphotericin B on 60-mm cell culture dishes. Mouse alveolar epithelial MLE-12 cells were cultured in MLE-12 culture medium (DMEM with 10% heat-inactivated FBS, 1 mM glutamine, 1% penicillin/streptomycin). The plating density was 1.0 × 106 cells/ml.
ATII and MLE-12 cells were divided into two groups once confluent, hyperoxia and normoxia. In a 37°C incubator, confluent cells were exposed to either room air (with 5% CO2) or to hyperoxia (∼80–90% O2, 5% CO2, balance N2) in a contained chamber for 24 h (ATII cells) or 48 h (MLE-12 cells). ATII cells were placed in the hyperoxia chamber 3 days after cell isolation. Because ATII cells undergo differentiation into a type I-like phenotype between 2 and 5 days in culture, and because of the potential increased sensitivity of the primary cells, we limited the exposure to hyperoxia to 24 h. To determine the effects of longer exposure, we examined the response of MLE-12 cells after 48 h. To determine the effect of cytoskeletal disruption, MLE-12 cells were treated with cytochalasin D (1 μg/ml; Sigma Aldrich, St. Louis, MO) for 30 min. Cells were then rinsed three times and transferred immediately to the AFM or fixed for microscopy.
Fluorescence staining and confocal microscopy.
Following treatment, monolayers were fixed in 10% formalin for 10 min and stained simultaneously for F-actin using rhodamine-phalloidin (Cytoskeleton, Denver, CO) and for microtubules using an antibody against α-tubulin conjugated with Alexa488 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. For both fluorophores, a 1:250 dilution was applied for one 1 h. Images were acquired on a Zeiss 710 (Zeiss, Jena, Germany) laser confocal inverted microscope with six laser lines on a ×40 objective, using the Zen 2010 software. Further image analysis was performed with ImageJ 1.42 image processing software (W.S. Rasband; NIH, Bethesda, MD; http://rsbweb.nih.gov/ij/).
Indentation with AFM.
To measure the elastic modulus of cells, we utilized the AFM as a nano-indenter with a flexible cantilever beam. The AFM indentation is achieved when the cantilever is lowered (z-displacement) enough to come in contact with the cell and cause indentation. The force required to compress the cell structures is related to the elastic modulus of the cell, the nominal stiffness of the cantilever beam (its spring constant), and the geometry of the tip (see below) (18, 32, 34). Because the cytoskeletal structure of the cell below the indented location is heterogeneous, the resulting force-indentation curve is an average resistance of the nearby affected proteins, such as microtubules, actin, cortical filaments, etc.
Live cells were indented using an AFM (MFP3D; Asylum Research, Santa Barbara, CA) with triangular silicon nitride cantilevers (SiNi; Budget Sensors, Sofia, Bulgaria). Although the nominal stiffness of the cantilever beams was 0.27 N/m, because of the large variation in the actual values, the cantilever stiffness was measured at the beginning of each experiment by generating a force-deflection (F-d) curve on a relatively stiff substrate (the culture dish). Next, at randomly selected spots on the confluent monolayers of cells, force-indentation curves were recorded over a rectangle that is 3 μm × 50 μm. A MATLAB (MathWorks, Natick, MA) code that was developed in our group was used to batch process the force-indentation curves and to determine the elastic modulus from these curves. Using the Hertz model for a pyramidal indenter, the elastic modulus at each location was calculated by the following relationship E = F [ 2(1 − ν2) ] / [ 1.4906 δ2 tan(θ) ] (1), where E is elastic modulus, F is force, ν is Poisson's ratio, θ is the tip half-opening angle, and δ is the sample indentation (18, 32, 34). In the analyses, the Poisson's ratio is assumed to be 0.49, which is consistent with prior literature suggesting cells to be nearly incompressible. The indentation depth was chosen to be 0.3 μm to avoid the substrate stiffness concerns, but to allow a substantial indentation to record an average local mechanical response.
Initially we recorded complete maps (50 μm by 50 μm) with 5,184 individual data force-indentation curves. However, these required ∼90 min to acquire, and we decided to record smaller maps from more locations. To compare treatment groups, we performed measurements at 15 to 20 different locations within a culture dish with 2 × 72 force-indentation curves at each location. We limited the indentation depth on the cell to 0.3 μm corresponding to the analyzed portion of the curve. We computed the median modulus at each location and averaged these over the dish to obtain a measurement for that condition (over 2,000 measurements for each data point).
Cell detachment.
Confluent monolayers of MLE-12 cells were exposed to either normoxia or hyperoxia for 48 h as described above and then exposed to cyclic stretch using the Flexercell FX-4000T tension unit (Flexcell International, Hillsborough, NC). Cells were exposed to 20% linear strain for 60 min at a frequency of 15 cycles/min. Cells were then fixed in 10% formalin for 5 min, and phase-contrast images were collected at ×10 magnification using an EVOS microscope (Advanced Microscopy Group, Bothell, WA) with three to five fields for each well. The images were then analyzed using MATLAB software to outline areas of cell detachment and determine the percentage of total area with denuded cells. Unstretched cells were used as controls, and measurements of each condition were made from three different experiments.
Statistical analysis.
We utilized MATLAB and SigmaStat for the statistical analyses. We averaged the medians of each small E-map taken from different locations within a dish. Because of potential variations in cells, we performed measurements of elastic modulus on control and treated cells on the same day and performed paired comparisons. We conducted a paired t-test by isolations or cell-seeding events to determine whether significant differences exist between normoxia- and hyperoxia-treated cells on a given isolation or cell seeding events. A P value <0.05 was considered significant.
RESULTS
Hyperoxia caused remodeling of actin and microtubule structures.
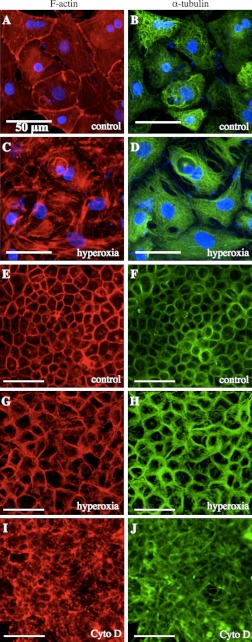
To examine whether hyperoxia caused remodeling of the cytoskeleton through reorganization of actin and microtubules, we exposed ATII and MLE-12 cells to either normoxia or 80–90% O2 and then stained for F-actin and α-tubulin. Representative images of ATII and MLE-12 cells treated with normoxia or hyperoxia are shown in Fig. 1. In control ATII cells, we observed typical F-actin staining with thin filaments both in the cortical regions and in the central area of the cell (Fig. 1A). Following exposure to hyperoxia, larger and thicker F-actin bands were observed both in the cell interior and in cortical regions (Fig. 1C). Microtubules appeared to be more densely distributed in hyperoxia-treated ATII cells and somewhat restricted from the cortical regions (Fig. 1, B and D). In control MLE-12 cells, there were fewer F-actin filaments spanning the cell compared with ATII cells, in part due to the prominent nuclei (not shown in these images), but there was strong cortical staining (Fig. 1E). Hyperoxia caused an increase in both cortical actin and F-actin filaments in MLE-12 cells (Fig. 1G). There was also stronger cortical staining of microtubules in hyperoxia-treated MLE-12 cells (Fig. 1, F and H). In both cell types, there was some nuclear damage following hyperoxia treatment.
Fig. 1.
Hyperoxia treatment caused changes in the distribution of F-actin (red, A, C, E, G) and microtubules (green, B, D, F, H) in alveolar type II (ATII) cells (A–D) and MLE-12 cells (E–H). Treatment with cytochalasin D (cytoD) caused disruption of actin and microtubules in MLE-12 cells (I–J). Each pair of images was from the same field. Scale bars are 50 μm (n = 3).
Hyperoxia increased the elastic modulus of alveolar epithelial cells.
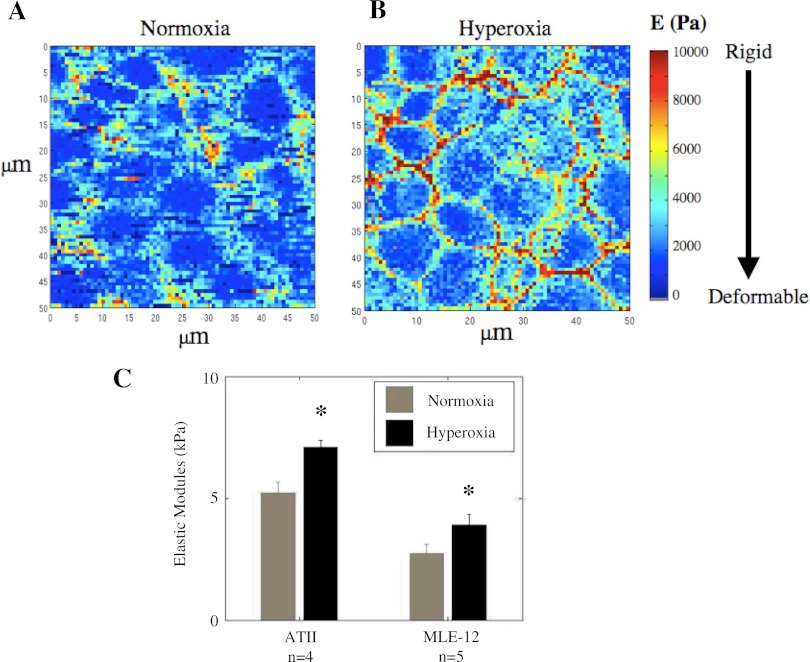
Because hyperoxia caused significant changes in actin and microtubule distribution, we hypothesized that this would also lead to changes in the elastic moduli. To test this, we exposed ATII and MLE-12 cells to either normoxia or 80–90% O2 and then measured the elastic modulus. Examples of detailed E-maps for normoxia- and hyperoxia-treated MLE-12 cells are shown in Fig. 2. These maps illustrate the variability of elastic modulus within a given field of cells and show that hyperoxia treatment caused more sights of increased stiffness. As shown in Fig. 2C, hyperoxia caused a significant increase in modulus for both ATII and MLE-12 cells, indicating a decrease in the deformability of the cells.
Fig. 2.
Detailed E-maps of a confluent monolayer of MLE-12 cells treated with normoxia (A) and hyperoxia (B). In these detailed maps, each pixel corresponds to a single force-indentation curve. C: elastic moduli (E) of ATII and MLE-12 cells incubated with normoxia or hyperoxia. ATII cells were exposed to either normoxia or hyperoxia for 24 h on the third day after isolation. MLE-12 cells were exposed for 48 h. Hyperoxia caused a significant increase in the elastic modulus in each type of cell (*significantly different from normoxia, P < 0.05; paired t-test; error bars indicate SE). Each bar corresponds to 4 separate isolations in the case of ATII cells (n = 4) and 5 different cell-seeding events in the case of MLE-12 cells (n = 5). Each data point represents the mean value from 15 to 20 locations in a given dish in which the median value was determined from 144 measurements near that location.
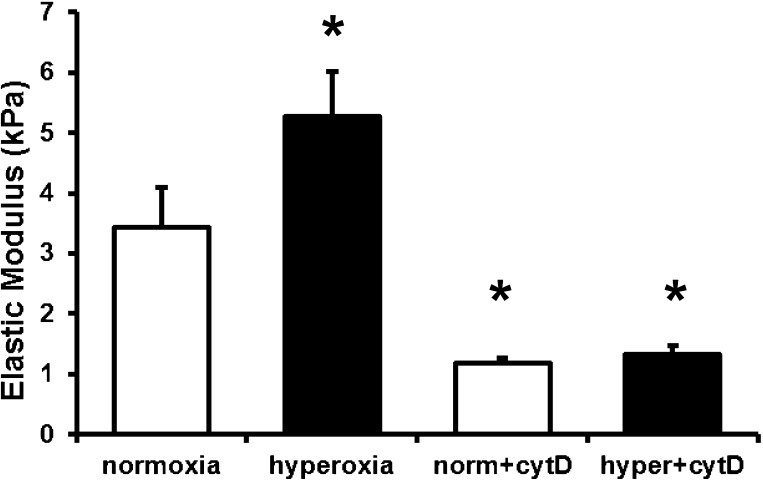
Cytochalasin D reduced the elastic modulus.
To determine the extent to which the mechanical response that we measured (elastic modulus) was dependent on the actin cytoskeleton, we measured the elastic modulus of MLE-12 cells following treatment with either normoxia or hyperoxia followed by treatment with cytochalasin D (cytoD) to disrupt F-actin. As shown in Fig. 1, I–J, cytoD treatment caused disruption of F-actin as well as microtubules in normoxia-treated cells. CytoD treatment caused similar disruption in hyperoxia-treated cells (not shown). Importantly, cytoD caused a significant reduction in elastic modulus in both normoxia- and hyperoxia-treated MLE-12 cells (Fig. 3).
Fig. 3.
Elastic moduli of MLE-12 cells incubated with normoxia or hyperoxia (as described in Fig. 2) followed by treatment with cytochalasin D (cytD) for 30 min. Each bar corresponds to 3 different cell-seeding events (n = 3) with each data point corresponding to the mean value of the median from ∼144 measurements from 15–20 different locations (*significantly different from normoxia, P < 0.05; error bars represent SE).
Finite element analysis predicts higher internal stress near the cell edge in hyperoxia-treated cells.
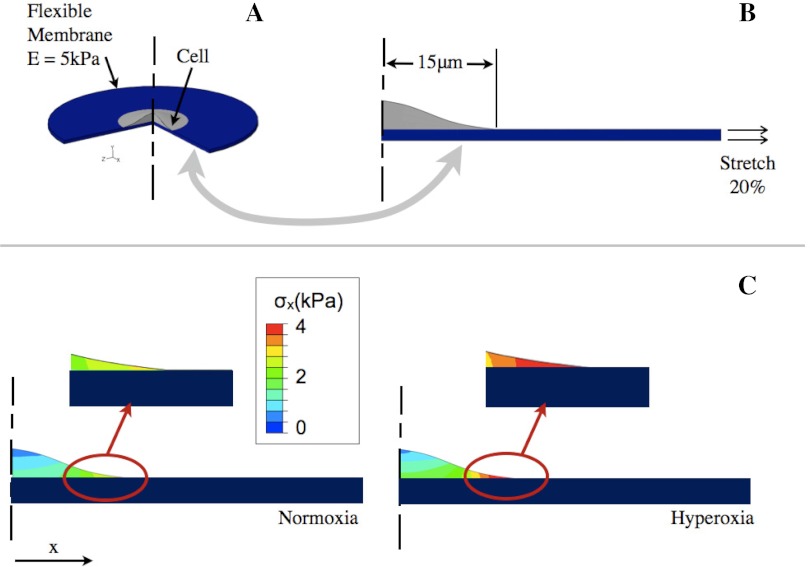
The increase in elastic modulus of the cells could lead to a modification of the stress-strain profiles experienced by cells exposed to injurious levels of distention. To investigate whether the decrease in cell deformability caused by hyperoxia would alter the response of the cell to a large deforming stress, we developed a finite element model of a cell residing on a flexible substrate. We utilized finite element analysis (ABAQUS; Simulia, Providence, RI) to show how internal stresses would be altered when a cell was exposed to quasistatic injurious stretch applied in the plane of a flexible substrate. As shown in Fig. 4A, we generated a two-dimensional (2D) axis-symmetric model (right) of a cell (gray) residing on a flexible membrane (blue), which is mechanically equivalent to the three-dimensional system shown on the left. We conducted two different analyses in which the membrane had an elastic modulus of E = 5.0 kPa (5) in both cases, and the modulus of the cell, Ecell, was modified according to our experimental findings of normoxia- and hyperoxia-treated primary ATII cells. A significant increase in the cell elastic modulus could lead to elevated stress levels because of increased dissimilarity between the cell elastic modulus and that of the alveolar wall on which the cells reside.
Fig. 4.
A: 3D depiction of the cell on the flexible membrane. B: 2D finite element model of the cell-membrane system where a lateral stretch is applied on the membrane. C: contour plots of the stress in the direction parallel to the 20% stretch load showing an increased tensile stress (σx) in the hyperoxia-treated cells near the cell boundary.
The results of this analysis (Fig. 4C) show that a 20% deforming stretch of the membrane would cause higher internal stresses in the hyperoxic cells compared with the control cells (indicated by the warmer colors in the map). For example, the stress in the thinner regions of the cell near the perimeter is higher in the hyperoxia cell compared with the normoxia cell. The cell is likely experiencing a more complex stress field near the perimeter, which is not shown for simplicity.
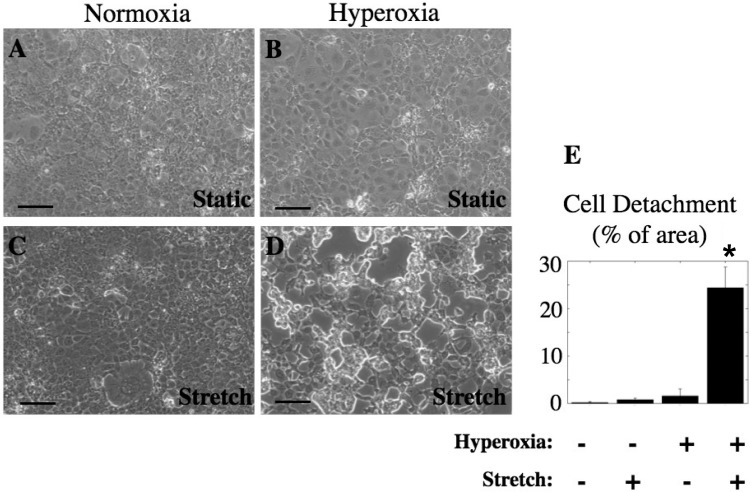
Cyclic stretch caused detachment of hyperoxia-treated cells.
To test the hypothesis that hyperoxia-treated cells are more susceptible to stretch-induced injury, we exposed MLE-12 cells to either normoxia or hyperoxia for 48 h, followed by cyclic stretch (20%, 15 cycles/min) for 60 min and then measured cell detachment. As shown in Fig. 5, there was little or no cell detachment when normoxia-treated cells were exposed to stretch, but there was significant cell detachment following stretch of hyperoxia-treated cells (significantly different from control, P < 0.05; n = 3).
Fig. 5.
Hyperoxia treatment followed by cyclic stretch caused detachment of MLE-12 cells. Cells were treated for 48 h with normoxia (A and C) or hyperoxia (B and D) and then kept static (A and B) or exposed to 20% cyclic stretch, 15 cycles/min (C and D) for 60 min. E: percentage of area of detached cells was determined for at least 5 fields from at least 3 different wells (n = 3; *significantly different from unstretched normoxia, P < 0.05).
DISCUSSION
In this study, we provide novel data demonstrating a significant increase in the elastic modulus of cells following exposure to hyperoxia that correlated with cytoskeletal remodeling. Qualitative analyses of fluorescence images suggest that, although ATII and MLE-12 cells have different structural appearance under control conditions, hyperoxia caused cytoskeletal remodeling in both. ATII cells exposed to hyperoxia contain F-actin that is in short but thick bundles, indicating a stressed condition of the whole cell. This finding is consistent with other studies that showed stress fiber formation or excessive polymerization of actin attributable to extended treatment with high levels of oxygen (9, 25, 30). In MLE-12 cells the changes in F-actin and microtubules were more apparent in the cortical regions, perhaps attributable to the presence of a relatively large nucleus in these cells (not shown). We are not aware of previous studies demonstrating changes in microtubules in response to hyperoxia. Whereas previous studies have indicated the role of F-actin and microtubules in contributing to the stiffness of cells by AFM (28, 35), we have shown for the first time that hyperoxia-induced changes in actin and microtubules are consistent with increased cell stiffness in two types of cells.
As the elastic modulus of a cell increases, it becomes more resistant to deformation. The consequences of such an increase in modulus in lung cells may be significant given that these cells undergo cyclic deformation during the breathing cycle (27, 29, 39). It has been estimated that the epithelial cells that reside on the lung basement membrane undergo a tensile strain that is ∼4% during normal tidal breathing (13, 27). During mechanical ventilation of patients with ARDS, there may be substantial heterogeneity of strain levels in the alveoli attributable to the presence of edema fluid, overinflation of air-filled regions, and collapse of airspaces (22, 26, 41). If mechanical ventilation with high levels of inspired oxygen causes increased resistance to deformation of AECs in overdistended alveoli, this could result in increased injury.
To reduce cell injury, the basement membrane and cells should deform, with nearly identical intrinsic mechanical responses. This similarity of mechanical responses limits the magnitude of cytoskeletal stresses from reaching excessive levels and causing adverse effects such as damage to focal adhesions or cell-to-cell junctions. Our measurements in isolated cells predict that hyperoxia would cause an imbalance between the elastic modulus of the cell and the alveolar walls. In such a case the cellular stresses resulting from an expansion of the basement membrane should increase as a function of the extent of the dissimilarity of the materials. Our finite element analysis supports this and shows that the dissimilarity induces higher levels of stress near the perimeter of cells. Under such conditions, the cell cytoskeleton may remodel to adjust to the deforming stress, but we have not yet investigated this possibility.
To test whether the increase in interfacial stress between the cell and the substrate could lead to injury, we mechanically stretched cells that were exposed to hyperoxia. Although we found little detachment of control, normoxia-treated cells, stretch of hyperoxia-treated cells caused significant cell detachment (Fig. 5). According to the finite element analysis, the most likely explanation for the formation of gaps in the monolayer would be the disruption of cell-substrate connections. This is due to the high in-plane stress at the cell periphery, which would apply a combined shear and tensile stress on the cell-substrate junctions. However, other potential forms of injury such as plasma membrane wounding are also possible.
There are other limitations to our study. The amount of time required to perform AFM indentation measurements limits our ability to measure maps with high spatial resolution while sampling a sufficient number of cells. Because of this limitation, we chose to conduct more measurements of smaller fields (15–20 locations) rather than obtain high spatial resolution maps (as shown in Fig. 2). This allowed us to contact a greater number of cells with a given monolayer. Considering that we recorded a minimum of 15 E-maps that spanned 50 μm, it is likely that we contacted 50 or more nonadjacent cells.
Another potential limitation is the possibility that measurements may be affected by the underlying substrate. Because AFM indentation causes compression of the cells that are on a relatively hard substrate, there is a concern that, if the cells are too thin, then the measurements may be affected by the presence of the substrate. To address this concern, we confirmed that the cells were tall enough to be indented an optimal 0.3 μm. Because of the chosen indentation depth, our measurements are less likely to be affected by the nucleus. In our E-maps we did not observe a high elastic modulus because of the presence of the nucleus, which is known to be higher than the cell cytoplasm (21).
In this study, we assumed that the cell was nearly incompressible, i.e., a Poisson's ratio of 0.49. Although cells are mostly composed of incompressible fluid, there may exist local fluctuations in Poisson's ratio. However, it is unlikely that such a spatial variation in Poisson's ratio would account for the significant changes in elastic modulus that we observed in hyperoxia-treated cells. The Poisson's ratio for an object composed of mostly fluid would be unlikely to fall below 0.45, and such a change would not account for the differences that we observed.
The elastic modulus of control ATII cells was significantly higher than that of control MLE-12 cells. MLE-12 cells were relatively taller than the ATII cells (data not shown), and F-actin fibers located centrally in the primary cells were not as evident in the MLE-12 cells. As indicated by comparing Figs. 1 and 2, there appear to be more prominent increases in elastic modulus in MLE-12 cells in what appears to be the cell-cell junctions, but we cannot confirm this without simultaneous imaging of the cells with force map measurements. In ATII cells, the changes appear to occur throughout the cells. Nevertheless, the fold change in the elastic modulus of hyperoxic over the normoxic cells was nearly the same for primary and MLE-12 cells, 1.42 and 1.63, respectively. Treatment of MLE-12 cells with cytoD caused a significant decrease in the elastic modulus, suggesting that F-actin contributes to the mechanical response of these cells to indentation. In addition, cytoD caused a reduction in elastic modulus in hyperoxia-treated cells to the level of normoxia-treated cells, suggesting that the changes in elastic modulus with hyperoxia were attributable to changes in actin. We should note here that we exposed the MLE-12 cells to hyperoxia for 48 h, whereas ATII cells were exposed for 24 h. As mentioned in materials and methods, we wanted to limit the time of exposure of ATII cells to minimize the effects of differentiation of ATII cells into ATI cells during the experiment. In future studies, we plan to investigate the development of the changes in mechanical properties with time of exposure to hyperoxia.
Our finite element analysis is limited as a result of simplifications, but it is useful in illustrating the principles of how stresses may be altered in cells when there are differences in material properties between the cells and the substrate. Although this model oversimplifies the in vivo conditions, which are likely to include stresses in multiple directions and transmission of forces attributable to cell-cell contacts, our analysis suggests that stretch induces stresses near the cell borders. Our analysis also ignores the potential for changes in the mechanical properties of the extracellular matrix on which the cells are attached. It is possible that such changes also occur with hyperoxia, but there are likely differences in the time course of such events with cytoskeletal changes likely to occur sooner. Our simplistic analysis indicating increased stress within cells during stretch is supported by our experimental results demonstrating detachment of hyperoxia-treated cells following stretch (Fig. 5).
In summary, we utilized AFM indentation to show that the elastic modulus of live alveolar epithelial cells was increased following exposure to hyperoxia. We provided additional qualitative evidence showing the remodeling of ATII and MLE-12 cells showing significant alterations in F-actin and microtubules. Hyperoxia-treated cells showed increased detachment when subsequently exposed to mechanical stretch. Our findings regarding the changes in elastic modulus in conjunction with finite element modeling suggest a potential mechanism for enhanced epithelial injury during mechanical ventilation with hyperoxia.
GRANTS
This work was supported by NIH grant HL094366.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.R., K.W., A.B., P.S.M., V.K.G., S.E.S., and C.M.W. conception and design of research; E.R., K.W., A.B., P.S.M., V.K.G., and C.M.W. performed experiments; E.R., K.W., A.B., P.S.M., and C.M.W. analyzed data; E.R., K.W., P.S.M., V.K.G., S.E.S., and C.M.W. interpreted results of experiments; E.R., K.W., and C.M.W. prepared Figs.; E.R., K.W., and C.M.W. drafted manuscript; E.R., K.W., P.S.M., S.E.S., and C.M.W. edited and revised manuscript; E.R., K.W., and C.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kathy Troughten at the University of Tennessee Health Science Center for help in confocal microscopy. We are grateful to Dr. Manik Ghosh, Charlean L. Luellen, and Dr. Lynn M. Crosby at the University of Tennessee Health Science Center for help, advice, and technical assistance. We thank Dr. Lindner and Dr. Pinkhasshik at the University of Memphis for the unlimited access to the AFM.
REFERENCES
- 1. Altemeier WA, Sinclair SE. Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care 13: 73–78, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bailey TC, Martin EL, Zhao L, Veldhuizen RA. High oxygen concentrations predispose mouse lungs to the deleterious effects of high stretch ventilation. J Appl Physiol 94: 975–982, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Barazzone C, Belin D, Piguet PF, Vassalli JD, Sappino AP. Plasminogen activator inhibitor-1 in acute hyperoxic mouse lung injury. J Clin Invest 98: 2666–2673, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet PF. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol 19: 573–581, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Cavalcante F, Ito S, Brewer K, Sakai H, Alencar A, Almeida M, Andrade JJ, Majumdar A, Ingenito E, Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol 98: 672–679, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chow CW, Herrera Abreu MT, Suzuki T, Downey GP. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol 29: 427–431, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol 122: 456–468; quiz 469–470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis 122: 123–143, 1980 [DOI] [PubMed] [Google Scholar]
- 9. Dalle-Donne I, Rossi R, Milzani A, Di Simplicio P, Colombo R. The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic Biol Med 31: 1624–1632, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Davis JM, Penney DP, Notter RH, Metlay L, Dickerson B, Shapiro DL. Lung injury in the neonatal piglet caused by hyperoxia and mechanical ventilation. J Appl Physiol 67: 1007–1012, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Desai L, Sinclair S, Chapman K, Hassid A, Waters C. High tidal volume mechanical ventilation with hyperoxia alters alveolar type II cell adhesion. Am J Physiol Lung Cell Mol Physiol 293: L769–L778, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Frank JA, Matthay MA. Science review: mechanisms of ventilator-induced injury. Crit Care 7: 233–241, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fredberg J, Kamm R. Stress transmission in the lung: pathways from organ to molecule. Annu Rev Physiol 68: 507–541, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Horowitz S. Pathways to cell death in hyperoxia. Chest 116: 64S–67S, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Jones R, Zapol WM, Reid L. Pulmonary artery remodeling and pulmonary hypertension after exposure to hyperoxia for 7 days. A morphometric and hemodynamic study. Am J Pathol 117: 273–285, 1984 [PMC free article] [PubMed] [Google Scholar]
- 16. Lee PJ, Choi AM. Pathways of cell signaling in hyperoxia. Free Radic Biol Med 35: 341–350, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Li LF, Liao SK, Ko YS, Lee CH, Quinn DA. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: a prospective, controlled animal experiment. Crit Care 11: R25, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin DC, Dimitriadis EK, Horkay F. Robust strategies for automated AFM force curve analysis–I. Non-adhesive indentation of soft, inhomogeneous materials. J Biomech Eng 129: 430–440, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Liu YY, Liao SK, Huang CC, Tsai YH, Quinn DA, Li LF. Role for nuclear factor-kappaB in augmented lung injury because of interaction between hyperoxia and high stretch ventilation. Transl Res 154: 228–240, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Makena PS, Luellen CL, Balazs L, Ghosh MC, Parthasarathi K, Waters CM, Sinclair SE. Preexposure to hyperoxia causes increased lung injury and epithelial apoptosis in mice ventilated with high tidal volumes. Am J Physiol Lung Cell Mol Physiol 299: L711–L719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maniotis A, Chen C, Ingber D. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA 94: 849–854, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matthay MA, Bhattacharya S, Gaver D, Ware LB, Lim LH, Syrkina O, Eyal F, Hubmayr R. Ventilator-induced lung injury: in vivo and in vitro mechanisms. Am J Physiol Lung Cell Mol Physiol 283: L678–L682, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Mendez JL, Hubmayr RD. New insights into the pathology of acute respiratory failure. Curr Opin Crit Care 11: 29–36, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Nash G, Blennerhassett JB, Pontoppidan H. Pulmonary lesions associated with oxygen therapy and artificial ventilation. N Engl J Med 276: 368–374, 1967 [DOI] [PubMed] [Google Scholar]
- 25. O'Reilly PJ, Hickman-Davis JM, Davis IC, Matalon S. Hyperoxia impairs antibacterial function of macrophages through effects on actin. Am J Respir Cell Mol Biol 28: 443–450, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Oeckler R, Hubmayr R. Alveolar microstrain and the dark side of the lung. Crit Care 11: 177, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oldmixon E, Hoppin FJ. Alveolar septal folding and lung inflation history. J Appl Physiol 71: 2369–2379, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Pelling AE, Dawson DW, Carreon DM, Christiansen JJ, Shen RR, Teitell MA, Gimzewski JK. Distinct contributions of microtubule subtypes to cell membrane shape and stability. Nanomedicine 3: 43–52, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Perlman C, Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol 103: 1037–1044, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Phillips PG, Higgins PJ, Malik AB, Tsan MF. Effect of hyperoxia on the cytoarchitecture of cultured endothelial cells. Am J Pathol 132: 59–72, 1988 [PMC free article] [PubMed] [Google Scholar]
- 31. Quinn DA, Moufarrej RK, Volokhov A, Hales CA. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol 93: 517–525, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Radmacher M. Measuring the elastic properties of biological samples with the AFM. IEEE Eng Med Biol Mag 16: 47–57, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J Suppl 42: 2s–9s, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Rico F, Roca-Cusachs P, Gavara N, Farré R, Rotger M, Navajas D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys Rev E Stat Nonlin Soft Matter Phys 72: 021914, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Rotsch C, Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys J 78: 520–535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Sinclair SE, Chi E, Lin HI, Altemeier WA. Positive end-expiratory pressure alters the severity and spatial heterogeneity of ventilator-induced lung injury: an argument for cyclical airway collapse. J Crit Care 24: 206–211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 294: L632–L641, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Tschumperlin D, Margulies S. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 275: L1173–L1183, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Ware L, Matthay M. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Waters C, Roan E, Navajas D. Mechanobiology in Lung Epithelial Cells: Measurements, Perturbations, and Responses. Bethesda, MD: American Physiological Society, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilson TA, Anafi RC, Hubmayr RD. Mechanics of edematous lungs. J Appl Physiol 90: 2088–2093, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Wirtz H, Dobbs L. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250: 1266–1269, 1990 [DOI] [PubMed] [Google Scholar]
- 44. Zaher TE, Miller EJ, Morrow DM, Javdan M, Mantell LL. Hyperoxia-induced signal transduction pathways in pulmonary epithelial cells. Free Radic Biol Med 42: 897–908, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]