Abstract
In hyperbaric oxygen (HBO2) at or above 3 atmospheres absolute (ATA), autonomic pathways link central nervous system (CNS) oxygen toxicity to pulmonary damage, possibly through a paradoxical and poorly characterized relationship between central nitric oxide production and sympathetic outflow. To investigate this possibility, we assessed sympathetic discharges, catecholamine release, cardiopulmonary hemodynamics, and lung damage in rats exposed to oxygen at 5 or 6 ATA. Before HBO2 exposure, either a selective inhibitor of neuronal nitric oxide synthase (NOS) or a nonselective NOS inhibitor was injected directly into the cerebral ventricles to minimize effects on the lung, heart, and peripheral circulation. Experiments were performed on both anesthetized and conscious rats to differentiate responses to HBO2 from the effects of anesthesia. EEG spikes, markers of CNS toxicity in anesthetized animals, were approximately four times as likely to develop in control rats than in animals with central NOS inhibition. In inhibitor-treated animals, autonomic discharges, cardiovascular pressures, catecholamine release, and cerebral blood flow all remained below baseline throughout exposure to HBO2. In control animals, however, initial declines in these parameters were followed by significant increases above their baselines. In awake animals, central NOS inhibition significantly decreased the incidence of clonic-tonic convulsions or delayed their onset, compared with controls. The novel findings of this study are that NO produced by nNOS in the periventricular regions of the brain plays a critical role in the events leading to both CNS toxicity in HBO2 and to the associated sympathetic hyperactivation involved in pulmonary injury.
Keywords: neuronal nitric oxide synthase, central nervous system oxygen toxicity, pulmonary oxygen toxicity
epileptiform seizures and acute lung injury are major pathophysiological effects of hyperbaric oxygen (HBO2), primarily above oxygen partial pressures of 3 atmospheres absolute (ATA; Refs. 3, 4, 8). These insults are associated with a massive central nervous system (CNS)-mediated sympathetic hyperactivation that abruptly increases pulmonary vascular pressure (16, 17). Systemic, nonspecific inhibition of nitric oxide synthase (NOS) prevents seizures and forestalls or mitigates lung injury, implicating nitric oxide (NO) in CNS and pulmonary oxygen toxicity (5, 12, 14, 30). Furthermore, neuronal NOS-null (nNOS−/−) mice are relatively protected compared with endothelial NOS-null (eNOS−/−), inducible NOS-null (iNOS−/−), and wild-type animals (11), suggesting a role nNOS in HBO2-induced seizures and the associated acute lung injury.
It is not known how or where these interactions between NO and autonomic discharges are initiated. In normoxia, central NO release is associated with sympathetic restraint (32), but in HBO2 increased NO production is correlated with sympathetic activation. The connection between increased central NO production and sympathetic outflow in HBO2 has not been investigated until now nor have anatomical sources of this increased NO production been identified.
Our goal was to test the hypothesis that elevated NO production in the periventricular regions of the brain is a factor in both CNS toxicity and the sympathetic outflow that leads to acute pulmonary injury in HBO2. We therefore assessed CNS and autonomic discharges, cardiopulmonary hemodynamic responses, and lung damage in rats treated with NOS inhibitors before exposure to HBO2 at 5 or 6 ATA. We chose the intracerebral route of administration to limit the action of NOS inhibitors to the periventricular regions of the brain and to minimize direct effects of loss of NO vasodilator control on the lung, heart, and peripheral circulation.
METHODS
Experiments were performed on both anesthetized and conscious rats, to distinguish effects of HBO2 from those of anesthesia, using published methods (17) briefly described here. Male Sprague-Dawley rats weighing 346 ± 47 g were used according to protocols approved by the Duke University Institutional Animal Use and Care Committee (anesthetized rats) and by the Russian Academy of Sciences Animal Ethics Committee, following animal use and care guidelines of the Sechenov Institute of Evolutionary Physiology and Biochemistry (St. Petersburg, Russia; conscious rats).
Acute Experiments on Anesthetized Rats at 6 ATA O2
Surgical preparation.
Anesthesia was induced with urethane (750 mg/kg ip) together with α-chloralose (75 mg/kg ip) and maintained by intravenous administration of one-fourth of the initial doses, as needed. Separate polyethylene catheters (PE 50) were inserted in the left femoral artery and vein, and either into the right atrium or the right ventricle through the right jugular vein or into the left ventricle through the right carotid artery. For determining cardiac output, a thermistor (model 511; Yellow Springs Instruments) was advanced into the ascending aorta via the right carotid artery through a PE 50 catheter. The positions of the cardiac catheters and the thermistor were verified at autopsy. Because simultaneous insertion of a thermistor in the aorta and catheterization of both cardiac ventricles could adversely affect cardiovascular hemodynamics, cardiac output and ventricular pressures were measured in separate groups.
The trachea was intubated, and anesthetized animals were ventilated with 30% O2 in N2 (termed “air” in this report). To prevent voluntary respiratory movements, pancuronium bromide (0.5 mg/kg iv) was administered to deeply anesthetized rats (verified by observing cardiovascular responses to toe pinch) to permit control of arterial Pco2 by adjusting the ventilator. Previous control studies indicated that this regimen of supplemental anesthesia is adequate for HBO2 exposures.
A burr hole was drilled in the skull 1.5 mm posterior to bregma and 1.5 mm lateral to the midline, and a guide cannula, for intracerebroventricular injection, was inserted 3.2 mm below the brain surface into the right lateral ventricle. A second hole was drilled over the confluence of the superior sagittal and transverse dural venous sinuses, using lambda as a landmark, and a platinum disk electrode was fixed gently on the intact dura mater and covered with mineral oil. Two stainless steel cranial screws were positioned over the left and right parietal cortices for EEG recording.
Hemodynamic measurements.
Arterial and venous blood pressures were measured continuously through catheters inserted into a femoral artery and the right atrium, using pressure transducers (Viggo-Spectramed, Oxnard, CA). Heart rate (HR) was determined from arterial pulse waves. Pulses were averaged electronically to obtain mean arterial blood pressure (MABP) and mean venous blood pressure (MVBP).
Cardiac output (CO) was calculated from transpulmonary thermodilution curves generated from the output of the thermistor in the ascending aorta after injection of 0.075 ml of room-temperature glucose solution (2.5%) into the right atrium by an infusion pump (Harvard Apparatus, Holliston, MA) operated from outside the chamber. CO was normalized for body weight (ml·min−1·100 g body wt−1). Right and left ventricular pressures (RVP and LVP) were measured using the ventricular catheters. Right ventricular systolic pressure (RVSP) and left ventricular end-diastolic pressure (LVEDP), averaged over 10 s, were used as indicators of pulmonary arterial pressure and pulmonary venous pressure, respectively, and pulmonary vascular resistance (PVR) was calculated from the means: PVR = RVSP − LVEDP/CO.
Total cerebral blood flow (tCBF) was measured by hydrogen clearance using the platinum disk electrode on the dura mater. To initiate a measurement, 2.5% H2 in air was introduced through the ventilator for 40 s, H2 washout curves were captured using a polarographic amplifier, and absolute tCBF (ml·g−1·min−1) was calculated using Mathematica 3.0 software (Wolfram Research).
EEG and renal sympathetic nerve activity.
The EEG was monitored continuously, with the first appearance of a train of spikes marking the onset of CNS oxygen toxicity. To record renal sympathetic nerve activity (RSNA), the left kidney was exposed through the retroperitoneal space, and a pair of platinum wire electrodes were placed under a branch of the renal nerve. The nerve-electrode junctions were electrically isolated from surrounding tissue with a silicone gel (Wacker SilGel 604 A/B). The RSNA signal above background was averaged over 10 s and expressed as percent change from that observed in animals breathing air at 1 ATA. All hemodynamic and neuronal parameters were recorded digitally with a data acquisition system (WinDaq, D-1200AC; DATAQ Instruments, Springfield, OH).
Arterial Po2, Pco2, and pH were determined before compression while animals breathed air at 1 ATA and then immediately after decompression while the animals still breathed 100% O2 (Synthesis 15 blood gas analyzer; Instrumentation Laboratories). Rectal temperature was monitored continuously with a thermistor and maintained at 37 ± 0.5°C, using a heating pad.
HBO2 exposures.
Anesthetized rats equipped with catheters, electrodes, and probes were individually exposed to 6 ATA O2 in a hyperbaric chamber (Duke Center for Hyperbaric Medicine and Environmental Physiology). After a 60-min stabilization period, during which the animal breathed air, baseline physiological parameters were recorded three times at 10-min intervals. The ventilator was then supplied with 100% O2, and the chamber was pressurized with air to 6 ATA at 0.6 ATA/min. Chamber temperature and relative humidity were maintained at 23 ± 0.5°C and 60 ± 2%, respectively. Exposure to HBO2 was limited to 60 min. Decompression to 1 ATA was accomplished at 0.6 ATA/min, while rats breathed 100% O2. Immediately after decompression, animals were euthanized with sodium pentobarbital (250 mg/kg), and bilateral bronchoalveolar lavage (BAL) was performed. Total protein content in BAL fluid (BALF), as a marker of lung injury, was measured with the bicinchoninic acid assay, using bovine serum albumin as a standard (40).
Experiments on Awake, Chronically Instrumented Rats at 5 ATA O2
In two series of experiments, awake Sprague-Dawley rats were exposed to O2 at 5 ATA for 60 min in a hyperbaric chamber at the Institute of Evolutionary Physiology and Biochemistry, Russian Academy Sciences. This pressure was chosen because oxygen convulsion latencies in awake rats in 5 ATA HBO2 were similar to those in anesthetized rats at 6 ATA (17).
Animals from the first series were anesthetized with sodium pentobarbital (50 mg/kg), and a cannula was inserted into the right lateral cerebral ventricle, as described above, and fixed to the skull with dental cement. After 7–10 days of recovery from surgery, the freely moving rats were exposed to HBO2. Animals from the second series were also equipped with an intracerebroventricular injection cannula, as well as cranial screws for recording EEG and an arterial catheter for recording MABP and taking blood samples. The right carotid artery was exposed while the animals were under sodium pentobarbital anesthesia (50 mg/kg), and PE 50 tubing filled with 0.9% NaCl solution containing glucose (2.5%) and heparin (300 IU/ml) was inserted, secured with a ligature, and then tunneled subcutaneously to exit at the back of the neck. Each animal was allowed to recover from surgery for 5–7 days, with the catheter refilled daily with fresh solution.
Freely moving rats from series 1 were placed two or three in a partitioned cage and exposed to 100% O2 at 5 ATA for 60 min. Temperature and humidity were controlled (22–24°C and 60–65%) and CO2 was kept <1%. The animals were observed continuously, and tonic-clonic convulsion latencies were noted. After decompression, the animals were euthanized, and transpulmonary protein leakage was assessed with BAL protein assay, as above.
Chronically catheterized rats from series 2 were placed individually in the hyperbaric chamber and lightly restrained by a hammock. During HBO2 exposure, arterial BP and HR were monitored by means of the arterial catheter, and the EEG was recorded. Immediately after decompression, arterial blood samples were drawn and assayed for norepinephrine using HPLC (Coulochem II analyzer; ESA, Chelmsford, MA), as described previously (45).
Experimental Groups
Twelve groups of anesthetized rats were exposed to 6 ATA O2; and MABP, MVBP, EEG, and RSNA were measured continuously; and tCBF was determined every 15 min. CO, RVP, and LVP were measured separately (Table 1). The first three groups of animals were intracerebroventricularly injected with 250 μg of the nonselective NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) in 10 μl of artificial cerebral spinal fluid (aCSF); the second three groups were similarly injected with 250 μg of the selective nNOS inhibitor 3-bromo 7-nitroindazole (BrNI) in 10 μl of solution containing 90% aCSF and 10% DMSO. Control animals were injected with 10 μl of either aCSF alone or aCSF + DMSO alone. All intracerebroventricular injections were performed immediately before compression.
Table 1.
Experiments with anesthetized rats at 6 ATA
| Group (n) | MABP/MVBP | EEG | RSNA | tCBF | CO | RVP | LVP | ICVI |
|---|---|---|---|---|---|---|---|---|
| 1 (10) | X | X | X | X | X | l-NAME | ||
| 2 (9) | X | X | X | l-NAME | ||||
| 3 (9) | X/X | X | X | X | X | l-NAME | ||
| 4 (10) | X | X | X | X | BrNI | |||
| 5 (8) | X | X | X | X | BrNI | |||
| 6 (9) | X/X | X | X | X | BrNI | |||
| 7 (8) | X | X | X | X | aCSF | |||
| 8 (8) | X | X | X | aCSF | ||||
| 9 (8) | X/X | X | X | X | aCSF | |||
| 10 (7) | X | X | X | X | X | aCSF + DSMO | ||
| 11 (7) | X | X | X | aCSF + DSMO | ||||
| 12 (7) | X/X | X | X | X | aCSF + DSMO |
n, Number of animals/group; ATA, atmospheres absolute; MABP, mean arterial blood pressure; MVBP, mean venous blood pressure; EEG, electroencephalogram; RSNA, renal sympathetic nerve activity; tCBF, total cerebral blood flow; CO, cardiac output; RVP, right ventricular pressure; LVP, left ventricular pressure; ICVI, intracerebroventricular injection; l-NAME, NG-nitro-l-arginine methyl ester; BrNI, 3-bromo 7-nitroindazole; aCSF, artificial cerebral spinal fluid.
Experiments on conscious rats were performed on either freely moving or those loosely supported in a hammock (Table 2). Freely moving rats were divided into two groups: those injected with l-NAME in aCSF or control animals injected with aCSF alone. Lightly restrained rats were divided into three groups: those pretreated with l-NAME in aCSF or aCSF alone and exposed to 5 ATA O2 for 60 min and a third group pretreated with aCSF alone and exposed to 5 ATA for 30 min. The different durations of HBO2 exposure were used to investigate the temporal profile of sympathetic activation based on plasma catecholamines measured in blood samples withdrawn after decompression.
Table 2.
Experiments with conscious rats at 5 ATA
| Group (n) | HBO2, min | Freely Moving | Hammock | Seizure Latency | BP/HR | EEG | NE | ICVI |
|---|---|---|---|---|---|---|---|---|
| 1 (14) | 60 | X | X | l-NAME | ||||
| 2 (14) | 60 | X | X | aCSF | ||||
| 3 (10) | 60 | X | X | X | l-NAME | |||
| 4 (10) | 60 | X | X | X | X | aCSF | ||
| 5 (8) | 30 | X | X | X | aCSF |
HBO2, hyperbaric oxygen; BP/HR, blood pressure and heart rate (BP/HR); NE, norepinephrine.
Statistical Analysis
Data were analyzed using StatView software (SAS Institute, Cary, NC). Physiological variables in HBO2 are expressed as percentages of baseline values measured in air at 1 ATA, except for CBF, which is given as milliliters per grams per minute. Changes within an experimental group were analyzed using paired t-tests, with P < 0.05 as the threshold for significance. Comparisons between groups were made by repeated ANOVA, with significance determined using the protected Fisher's least significant difference test. All values are expressed as means ± SD. Rats that did not exhibit seizures during the 60-min HBO2 exposure were considered to have had seizure latencies of 60 min.
RESULTS
Effects of Central NOS Inhibition on HBO2 Toxicity in Anesthetized Rats
EEG seizures and lung injury.
During exposures to O2 at 6 ATA for 60 min, ∼80% of the control animals exhibited trains of EEG spikes indicating generalized seizures, compared with ∼20% for those treated with a NOS inhibitor. Seizures occurred in 19 (79%) of the aCSF groups (n = 24), with a mean latency of 48 ± 9.7 min, and in 17 (81%) of the aCSF + DMSO groups (n = 21), with a mean latency of 46 ± 9.2 min. Of rats pretreated with NOS inhibitors, trains of spikes occurred in 6 (21%) of the l-NAME groups (n = 28), with a mean latency of 57.5 ± 2.1 min, and in 5 (19%) of the BrNI groups (n = 27),with a mean latency of 58.3 ± 1.9 min. Mean total BALF protein values were 1.74 ± 0.24 and 1.61 ± 0.21 mg/ml for aCSF and aCSF + DSMO groups, respectively. BALF protein values were 0.67 ± 0.18 and 0.73 ± 0.19 for l-NAME and BrNI groups.
Autonomic responses.
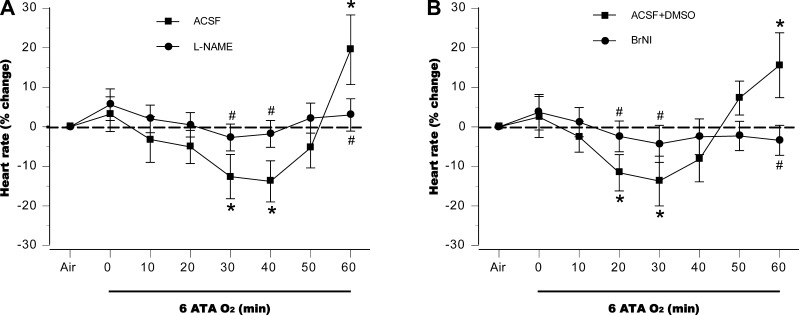
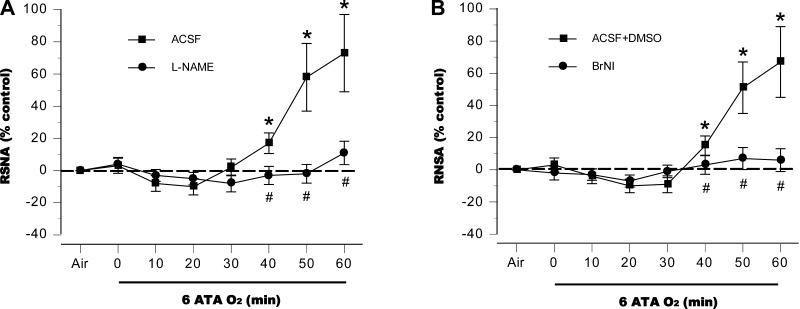
In control rats, autonomic responses to HBO2, measured by HR and RSNA, were biphasic. Starting from baselines established after compression to 6 ATA was complete, HR decreased for 30 to 40 min in both control groups but then rose, peaking 15–20% above preexposure levels by the end of the exposures (Fig. 1, A and B). l-NAME aggravated the initial bradycardia compared with control animals and significantly attenuated the subsequent tachycardia (Fig. 1A), and a similar pattern was observed in the BrNI group (Fig. 1B). RSNA decreased slightly for the first 20 min in both control groups; a subsequent rise reached 70% above baseline at the end of the exposure (Fig. 2, A and B). By contrast, RSNA did not change significantly from baseline in the two NOS-inhibited groups (Fig. 2, A and B).
Fig. 1.
Heart rate (HR). Responses in anesthetized control animals exposed to hyperbaric oxygen (HBO2) at 6 atmospheres absolute (ATA) were biphasic for control groups, artificial cerebral spinal fluid (aCSF; A) or aCSF + DSMO (B), decreasing at first, followed by increases that peaked ∼15–20% above baseline by the end of hyperbaric exposure. In animals with central NOS inhibition, decreases in HR were sustained throughout the 60-min hyperbaric exposure. *P < 0.05 vs. air; #P < 0.05 vs. control (aCSF or aCSF + DSMO) compared at same time points.
Fig. 2.
Renal sympathetic nerve activity (RSNA). In anesthetized control animals exposed to HBO2 at 6 ATA RSNA decreased slightly at first; however, after 30 min, sympathetic activity progressively increased to well above baseline. No significant increases in RSNA were observed in the two nitric oxide synthase (NOS)-inhibited groups, NG-nitro-l-arginine methyl ester (l-NAME; A) and 3-bromo 7-nitroindazole (BrNI; B). *P < 0.05 vs. air; #P < 0.05 vs. control (aCSF or aCSF + DSMO) compared at same time points.
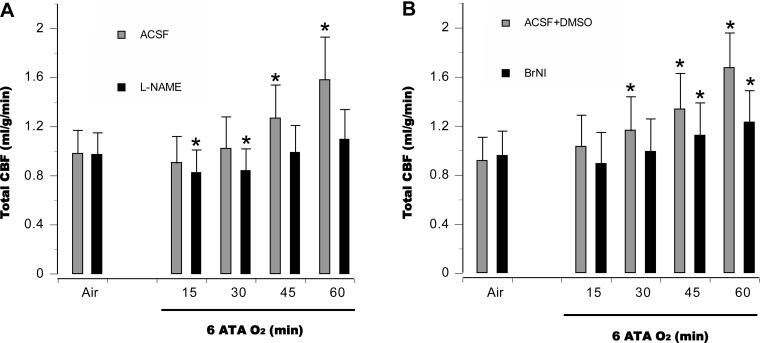
CBF and central hemodynamic changes.
In aCSF control animals, tCBF was relatively unchanged for the first 30 min at 6 ATA but was significantly elevated at 45 min, when EEG spikes appeared, and continued to rise throughout the 60-min exposure (Fig. 3A). In the aCSF + DMSO control groups, tCBF increased as early as 30 min and continued to rise (Fig. 3B). In l-NAME groups, cerebral vasoconstriction was observed for the first 30 min, after which tCBF rose to near control levels by the end of the HBO2 exposure (Fig. 3A). For the BrNI group, tCBF remained close to baseline for the first 30 min and was significantly above baseline after 45 min (Fig. 3B).
Fig. 3.
Cerebral hemodynamics. In anesthetized control animals, total cerebral blood flow (tCBF) was relatively stable for the first 30 min at 6 ATA and then increased, peaking as EEG spikes appeared. In anesthetized animals pretreated with l-NAME (A), tCBF was less than in control animals for the first 30 min, and minor increases were observed over the rest of hyperoxic exposure. In the BrNI group (B), tCBF within 30 min of HBO2 did not differ from baseline; however, subsequent increases were significant, but less pronounced than in control animals. *P < 0.05 vs. air in A and B.
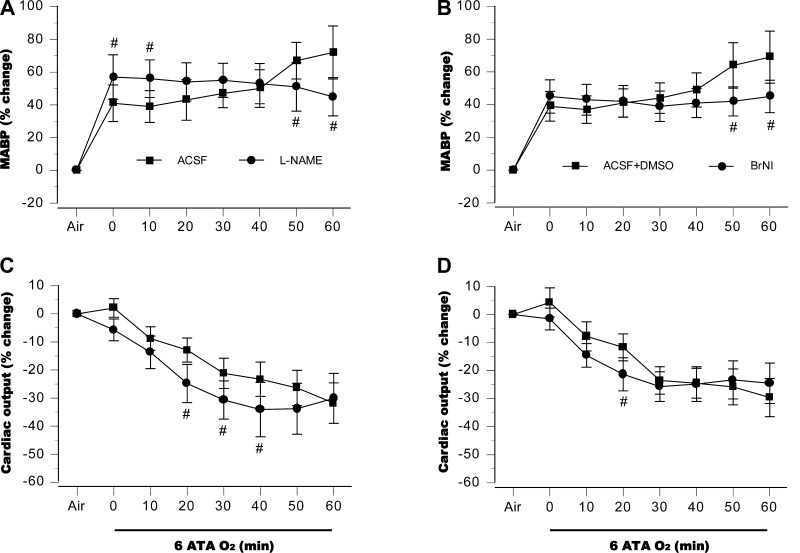
Central hemodynamic responses to HBO2 are illustrated in Fig. 4. In control animals, MABP rose in two steps, first during compression, then before the first EEG spikes (Fig. 4, A and B). In the l-NAME group, MABP was higher at the onset of 6 ATA, but no second-phase increase was observed (Fig. 4A). In rats treated with BrNI, initial changes in MABP were similar to those seen in control animals (and lower than seen in the l-NAME group), but again, no second increase was observed (Fig. 4B). CO fell gradually in both control groups and in the BrNi group, whereas in the l-NAME group a more pronounced decrease appeared midway in the hyperbaric exposure (Fig. 4, C and D).
Fig. 4.
Systemic hemodynamics. In control animals, mean arterial blood pressure (MABP) rose in 2 steps, initially during compression and a then immediately before the first EEG spikes, whereas in the l-NAME group, MABP elevation was higher at the onset of 6 ATA, but no second rise was observed (A). In rats treated with BrNI, initial changes in MABP were similar to those seen in control animals, but as with l-NAME treated animals, the second increase in blood pressure was not observed (B). Cardiac output fell gradually in both controls groups, but the NOS-inhibited groups showed a more pronounced decrease (C and D). #P < 0.05 vs. aCSF in A and C, or aCSF + DSMO in B and D.
Cardiopulmonary hemodynamic responses.
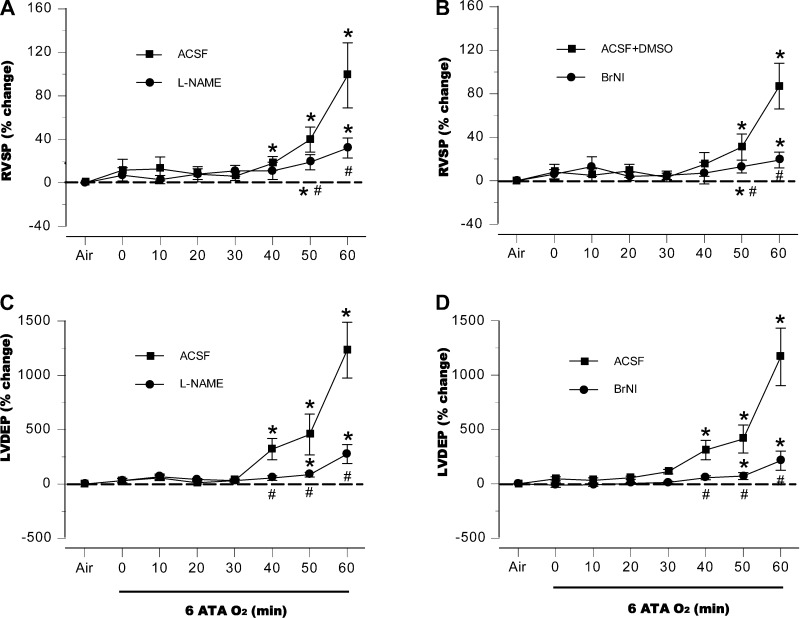
In both control groups, systolic RVP (RVSP) remained relatively stable for the first 30–40 min at 6 ATA but rose rapidly as EEG spikes appeared. In rats pretreated with l-NAME or BrNI, RVSP was initially the same as in the control groups, and only slight increases were observed later (Fig. 5, A and B). End-diastolic LVP (LVEDP) did not change in any of these four groups of animals for the first 30–40 min but increased abruptly in the control animals before the appearance of the first EEG spikes. Only attenuated increases appeared in the NOS-inhibited groups in the second half of the HBO2 exposure (Fig. 5, C and D).
Fig. 5.
Cardiopulmonary hemodynamics. In both control groups, systolic right ventricular pressure (RVSP) remained relatively stable for the first 30–40 min at 6 ATA but rose dramatically as EEG spikes appeared. In rats pretreated with l-NAME or BrNI, no significant changes occurred until the end of the hyperbaric exposure, where peak values were significantly above values in air, but well below those in control animals at the same time points (A and B). End-diastolic LVP (LVEDP) in these animals did not change for the first 30–40 min, but increased abruptly, slightly before the appearance of the first EEG spikes (C and D). In the l-NAME and BrNI groups, LVEDP did not change significantly above values seen in air until the end of the 60-min exposure; but as with RVP, maximum increases remained well below those for control animals at the same time points. *P < 0.05 vs. air; #P < 0.05 vs. control (aCSF or aCSF + DSMO) compared at same time points.
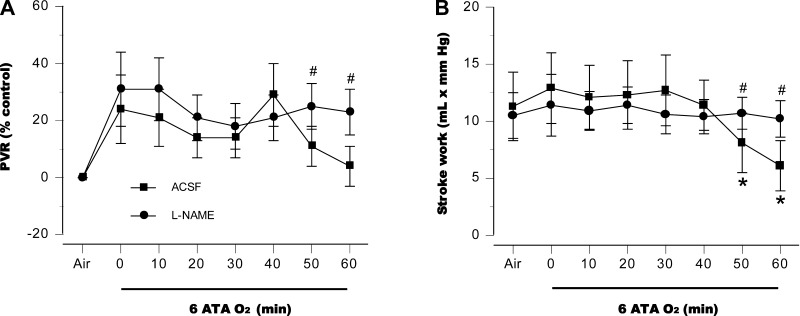
Intracerebroventricular inhibition of NOS also prevented significant changes in pulmonary vascular resistance (Fig. 6A). LV function became impaired in control rats, as manifested by a decrease in stroke work, whereas myocardial function was maintained throughout the HBO2 exposure in the l-NAME group (Fig. 6B). Similar changes in PVR and stroke work were observed in the BrNI group (data not shown).
Fig. 6.
Pulmonary vascular resistance (PVR) and stroke work. Central NOS inhibition prevented changes in pulmonary vascular resistance, compared with aCSF control animals (A). Control rats had significant impairment of LV function, as demonstrated by a decrease in stroke work; in the l-NAME group myocardial function persisted throughout the HBO2 exposure (B). Similar changes in PVR and stroke work were observed in the BrNI group (data not shown). *P < 0.05 vs. air; #P < 0.05 vs. aCSF control animals, compared at same time points.
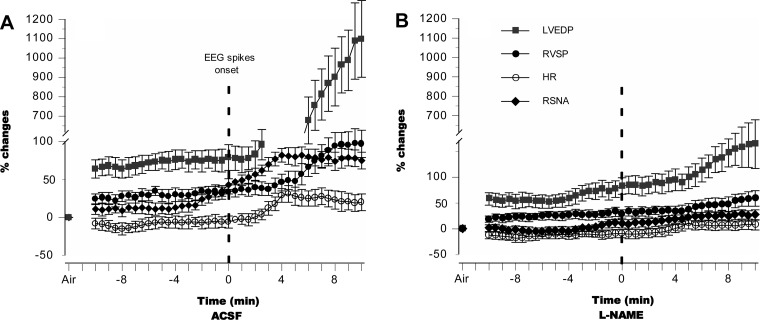
To examine the relationships among CNS, autonomic, and hemodynamic responses to HBO2, we compared temporal profiles of LVEDP, RVSP, HR, and RSNA with respect to EEG discharges, setting the zero point at the onset of spikes for each animal. In the control groups, RSNA increased before EEG spikes appeared, followed first by increases in LVEDP and then in RVSP (Fig. 7A). By comparison, l-NAME limited EEG spiking as well as autonomic activation and mitigated changes in both cardiopulmonary hemodynamics and myocardial function, conferring protection on the lung (Fig. 7B).
Fig. 7.
Temporal profiles of autonomic and cardiac responses to HBO2. In control animals that exhibited seizures, RSNA increased immediately before EEG spikes appeared (normalized at time zero), followed first by increases in LVEDP and then in RVSP (A). In l-NAME-pretreated animals that did not exhibit seizures, autonomic activation and changes in cardiopulmonary hemodynamics and myocardial function were attenuated, thus protecting the lung (B). In the absence of seizures, time zero in B is set at 48 min of HBO2, the mean value of seizure latency observed in control animals.
Effects of Central NOS Inhibition on HBO2 Toxicity in Awake Rats
Oxygen seizures, sympathetic activation, and lung injury.
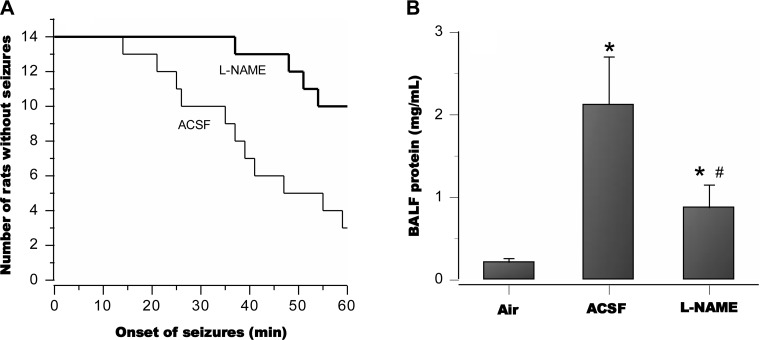
In awake and freely moving animals pretreated with intracerebroventricular aCSF alone and then exposed to HBO2 at 5 ATA for 60 min, 11 of 14 (79%) exhibited tonic-clonic convulsions, with a mean latency of 37 ± 8.2 min. By contrast, only 4 of 14 (28%) animals pretreated with l-NAME, exhibited convulsions during the 60 min exposure (Fig. 8A). Compared with controls, mean seizure latency in the affected animals increased to 56 ± 2.1 min. BALF protein was profoundly elevated in the aCSF control group but greatly attenuated in the l-NAME group (Fig. 8B). The lung wet-to-dry weight ratio was 5.6 ± 0.47 for aCSF rats and 5.0 ± 0.29 for those pretreated with l-NAME (P < 0.01).
Fig. 8.
Seizures and lung injury in awake animals in HBO2 at 5 ATA; 79% of freely moving control animals pretreated with aCSF alone exhibited tonic-clonic convulsions with a mean latency of 37 ± 8.2 min. By contrast, 28% of animals pretreated with l-NAME exhibited convulsions, with a mean latency of 56 ± 3.1 min (A). Postmortem assay of bronchoalveolar lavage fluid (BALF) in the aCSF control animals revealed pronounced pulmonary protein leakage, whereas the l-NAME group showed much less injury (B). *P < 0.05 vs. air; #P < 0.05 vs. aCSF controls.
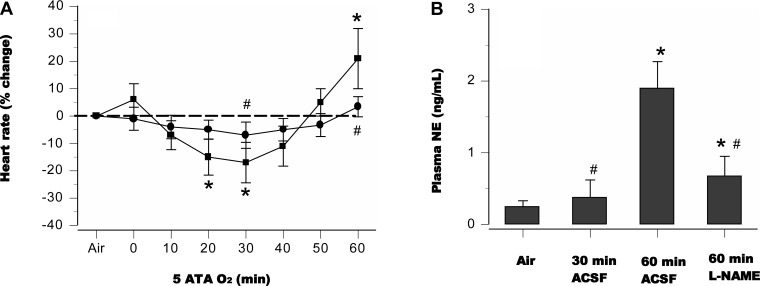
In lightly restrained awake rats pretreated with aCSF alone and exposed to 5 ATA O2 for 60 min, CNS O2 toxicity, signaled by generalized EEG spikes, occurred in 8 of 10 animals (80%), with a latency of 35 ± 5 min, whereas in the l-NAME group only 3 of 10 rats (30%) exhibited EEG spikes. Untreated and l-NAME groups demonstrated differences in autonomic discharges, as indicated by the temporal profile of HR and plasma norepinephrine content (Fig. 9). Among lightly restrained rats pretreated with aCSF and exposed to HBO2 for 30 min, only one of eight animals (12.5%) exhibited EEG spikes, and plasma norepinephrine levels were lower than in controls exposed to HBO2 for 60 min (Fig. 9B).
Fig. 9.
HR and norepinephrine release in awake animals. Autonomic discharges in rats treated with l-NAME (●) differed from those in controls (■), as shown by changes in HR and plasma norepinephrine (A and B). *P < 0.05 vs. air; #P < 0.05 vs. 60-min aCSF controls.
DISCUSSION
In the context of our previous work, we investigated here one of the three NO-mediated pathophysiological mechanisms that together lead to CNS and pulmonary toxicity in HBO2. These include direct cytotoxic effects of reactive oxygen and nitrogen species on susceptible brain regions; vascular responses that deliver excessive amounts of O2 due to hyperoxic vasodilation; and neuronal responses (described here) in which increased sympathetic outflow induces pulmonary hypertension and damage.
Cerebral NO, Seizures, and Pulmonary Injury
Seizures and severe lung damage associated with increased sympathetic activation occurred in both anesthetized and awake control groups. By contrast, central NOS inhibition prevented or delayed seizures, attenuated increases in sympathetic outflow, and mitigated lung damage in HBO2. Thus NO in the brain is implicated in both CNS and pulmonary HBO2 toxicity, which are linked by sympathetic activity. Cerebroventricular cannulation neither altered nor masked CNS and autonomic responses to HBO2. All control rats equipped with cannulas exhibited signs of CNS and pulmonary O2 toxicity similar to those seen in animals without cannulas, when exposed to the same levels of HBO2 (17). Moreover, the presence of DMSO in aCSF did not significantly alter the temporal profiles of CNS, autonomic, or cardiovascular responses to HBO2.
Sympathetic hyperactivation was signaled by large increases in RSNA that usually preceded generalized EEG spikes. These events were accompanied by positive chronotropic and negative inotropic effects on the heart, with marked increases in systemic arterial blood pressure, pulmonary blood volume, RVSP, and LVEDP. This indicates a hemodynamic mechanism for pulmonary damage that mirrors the neurogenic pulmonary edema that occurs, for example, after traumatic brain injury (41). The hallmarks of neurogenic pulmonary edema in HBO2 include a rapid increase in sympathetic outflow that elicits a catecholaminergic response with transient but dramatic systemic and pulmonary vasoconstriction and impairment of left ventricular contractility. This leads to acute pulmonary hypertension with extravasation of red blood cells and proteins into the alveoli (16, 17).
In past studies (5, 12, 14, 23, 30, 38), systemic administration of NOS inhibitors mitigated or prevented CNS toxicity in HBO2. In the present study, injection of NOS inhibitors directly into the cerebral ventricles was similarly effective, and selective inhibition of nNOS was nearly equivalent to nonselective NOS inhibition. These novel findings clearly implicate central NO production by nNOS in the sympathetic outflow that occurs in prolonged exposure to extreme HBO2 and that precedes EEG spikes and pulmonary injury.
Cerebral Targets of NO-Mediated Sympathetic Activation
Specific brain structures in which nNOS was inhibited in this study cannot be precisely ascertained, but areas contiguous to the ventricular system are probably important. Thus portions of the cerebral cortex and basal telencephalon enclose the lateral ventricles; the thalamus and hypothalamus border the third ventricle; and the cerebellum, pons, and the medulla adjoin the fourth ventricle (31). In the rat, intracerebroventricular infusion of l-NAME inhibits NOS activity in cortex, striatum, hippocampus, and cerebellum but particularly in the thalamus (36).
The effect of an agent injected in the cerebral ventricles depends on its concentration, diffusivity and interactions with the luminal wall, as well as on the flow of CSF. In the adult rat, CSF secretion is slow, 12.2 ± 0.3 μl/min (44), and the total volume is 300–500 μl (27); this makes it is unlikely that a 10-μl injection would significantly disturb normal pressure and flow. The CSF should turn over in ∼30 min, but radiolabeled agents can appear throughout the ventricular chambers within 5 min (44). Because so many factors influence the circulation CSF, we cannot precisely identify the anatomical targets of NOS inhibition.
NO-Mediated Alterations of CBF and Sympathetic Activity in HBO2
In HBO2, NO availability in the brain varies with Po2 and time (1, 18, 26, 42). At 5 ATA, NO falls at first, then gradually rises above preexposure levels (14). The initial fall reflects the reaction of NO with the superoxide anion (O2−; Refs. 13, 30); however, after prolonged exposure to HBO2, NO can predominate (14). At 6 ATA, initial NO production is comparable to that seen after 60 min at 5 ATA (11). NO levels always exceed preexposure values immediately before the appearance of EEG spikes in HBO2 (14, 38). In HBO2, changes in CBF correlate closely with brain NO. The initial response to HBO2 at 5 ATA is cerebral vasoconstriction (13), but later, CBF increases as NO production increases and then EEG spikes appear (7, 10, 12). At 6 ATA, instead of initial cerebral vasoconstriction, control groups exhibited a pronounced increase in tCBF. Of the two centrally administered NOS inhibitors, only l-NAME lowered tCBF (Fig. 3), consistent with our observation that cerebral vasoconstriction involves inactivation of endothelially derived NO by superoxide anion, since vasodilation and hyperemia occurring later in HBO2 depend on NO from both endothelial NOS and nNOS (2).
We do not yet know how NO elicits sympathetic hyperactivation in HBO2, but there are several putative mechanisms to consider. In normobaric normoxia, central NO production inhibits overall sympathetic outflow (24, 32, 34, 35, 37, 43, 46). At first glance our results contrast with findings in normobaric normoxia, but whether NO promotes or suppresses certain processes depends on its rates of production and degradation and sites of generation (6, 29). In HBO2 increased production of NO and O2− react to form the peroxynitrite anion (ONOO−), which damages proteins by nitration. S-nitrosylation of thiols on proteins such as GAD or GABA-T can alter their activity. Indeed, even in normoxia, abnormally high NO concentrations modify GAD and GABA-T, which are responsible for GABA synthesis and uptake (25). Thus in HBO2 NO can shift the Glu/GABA equilibrium.
Normally, NO modulates glutamate excitatory neurotransmission mainly through NMDA receptors and GABA inhibitory neurotransmission through GABAA receptors (21). This is consistent with the observation that the predominant nNOS splice variant in brain (nNOSa) contains a binding domain selective for NMDA receptors (29). Glu-mediated excitability in subcortical structures is associated with inhibition of GABAergic transmission (20), and HBO2 does decrease GABA in the brain (15, 19, 28, 47). However, since NOS inhibitors also decrease GABA release and the NO donor S-nitroso-N-acetyl penicillamine increases it, NO may mediate both excitatory and inhibitory functions in vivo, depending on concentration (22, 39). Inhibition of GABA-T by high NO concentrations is also documented (33). The effects of NO on the synthesis or catabolism of GABA have not been studied in HBO2.
In normorbaric normoxia or mild hyperoxia, therefore, NO can inhibit sympathetic outflow by upregulating GABA release, but high NO production in HBO2, together with ONOO− formation, reduces the inhibitory effect of GABA on sympathetic outflow. Preliminary data indicate that S-nitrosylation of glutamic acid decarboxylase in HBO2 at 5 ATA shifts the inhibitory/excitatiory equilibrium and predisposes to CNS seizures (9). This type of neurochemical event may comprise a novel mechanism through which NO can trigger sympathetic excitation.
Conclusions
This study implicates central NO in a mechanism that links CNS with pulmonary HBO2 toxicity through acute neurogenic pulmonary hypertension. Cardiovascular alterations and acute pulmonary hypertension in HBO2 result from a massive sympathetic outflow that overwhelms protective baroreflex-mediated cardiac vagal activity. Overall, these findings support a novel physiological mechanism by which NO modulates the sympathetic outflow to the cardiovascular system from specific CNS sites by explicit pathways that are under investigation.
GRANTS
This work was supported by Office of Naval Research Grant N00014-04-1-0171 (to C. A. Piantadosi, B. W. Allen, and I. T. Demchenko), National Institute of Allergy and Infectious Diseases Grant RO1-AI064789 (to C. A. Piantadosi), and Russian Foundation for Basic Research Grant 12-04-01446a (to I. T. Demchenko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.T.D., A.I.K., and C.A.P. conception and design of research; I.T.D., A.N.M., and A.I.K. performed experiments; I.T.D., A.N.M., A.I.K., and C.A.P. analyzed data; I.T.D., A.N.M., A.I.K., and B.W.A. interpreted results of experiments; I.T.D. prepared figures; I.T.D. and B.W.A. drafted manuscript; I.T.D., C.A.P., and B.W.A. edited and revised manuscript; I.T.D., C.A.P., and B.W.A. approved final version of manuscript.
REFERENCES
- 1. Allen BW, Demchenko IT, Piantadosi CA. Two faces of nitric oxide: implications for cellular mechanisms of oxygen toxicity. J Appl Physiol 106: 662–667, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Atochin DN, Demchenko IT, Astern J, Boso AE, Piantadosi CA, Huang PL. Contributions of endothelial and neuronal nitric oxide synthases to cerebrovascular responses to hyperoxia. J Cereb Blood Flow Metab 23: 1219–1226, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Balentine JD. Pathology of Oxygen Toxicity. New York: Academic, 1982 [Google Scholar]
- 4. Bean J, Zee D, Thom B. Pulmonary changes with convulsions induced by drugs and oxygen at high pressure. J Appl Physiol 21: 865–872, 1966 [DOI] [PubMed] [Google Scholar]
- 5. Bitterman N, Bitterman H. l-Arginine-NO pathway and CNS oxygen toxicity. J Appl Physiol 84: 1633–1638, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Giuffrida Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8: 766–775, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Chavko M, Braisted JC, Outsa NJ, Harabin AL. Role of cerebral blood flow in seizures from hyperbaric oxygen exposure. Brain Res 791: 75–82, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Clark JM, Lambertsen CJ. Pulmonary Oxygen Toxicity: A Review. Pharmacol Rev 23: 37–133, 1971 [PubMed] [Google Scholar]
- 9. Demchenko I, Atochin D, Suliman H, Tatro L, Allen B, Huang P, Piantadosi C. Neuronal NOS and glutamate decarboxylase S-nitrosylation before oxygen seizures. Undersea Hyperbaric Medical Society Annual Meeting Maui, HI: UHMS, 2007 [Google Scholar]
- 10. Demchenko IT, Atochin DN, Boso AE, Astern J, Huang PL, Piantadosi CA. Oxygen seizure latency and peroxynitrite formation in mice lacking neuronal or endothelial nitric oxide synthases. Neurosci Lett 344: 53–56, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Demchenko IT, Atochin DN, Gutsaeva DR, Godfrey RR, Huang PL, Piantadosi CA, Allen BW. Contributions of nitric oxide synthase isoforms to pulmonary oxygen toxicity, local vs. mediated effects. Am J Physiol Lung Cell Mol Physiol 294: L984–L990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demchenko IT, Bennett PB, Piantadosi CA. The role of nitric oxide in hyperbaric neurotoxicity. In: High Pressure Biology and Medicine, edited by Bennett PB, Demchenko IT, Marquis RE. Rochester, NY: Univ. of Rochester Press, 1998, p. 331–338 [Google Scholar]
- 13. Demchenko IT, Boso AE, Bennett PB, Whorton AR, Piantadosi CA. Hyperbaric oxygen reduces cerebral blood flow by inactivating nitric oxide. Nitric Oxide 4: 597–608, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Demchenko IT, Boso AE, Whorton AR, Piantadosi CA. Nitric oxide production is enhanced in rat brain before oxygen-induced convulsions. Brain Res 917: 253–261, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Demchenko IT, Piantadosi CA. Nitric oxide amplifies the excitatory to inhibitory neurotransmitter imbalance accelerating oxygen seizures. Undersea Hyperb Med 33: 169–174, 2006 [PubMed] [Google Scholar]
- 16. Demchenko IT, Welty-Wolf KE, Allen BW, Piantadosi CA. Similar but not the same: normobaric and hyperbaric pulmonary oxygen toxicity, the role of nitric oxide. Am J Physiol Lung Cell Mol Physiol 293: L229–L238, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Demchenko IT, Zhilyaev SY, Moskvin AN, Piantadosi CA, Allen BW. Autonomic activation links CNS oxygen toxicity to acute cardiogenic pulmonary injury. Am J Physiol Lung Cell Mol Physiol 300: L102–L111, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elayan IM, Axley MJ, Prasad PV, Ahlers ST, Auker CR. Effect of hyperbaric oxygen treatment on nitric oxide and oxygen free radicals in rat brain. J Neurophysiol 83: 2022–2029, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Faiman MD, Nolan RJ, Baxter CF, Dodd DE. Brain γ-aminobutyric acid, glutamic acid decarboxylase, glutamate, and ammonia in mice during hyperbaric oxygenation. J Neurochem 28: 861–865, 1977 [DOI] [PubMed] [Google Scholar]
- 20. Ferraro G, Sardo P. Nitric oxide and brain hyperexcitability. In Vivo 18: 357–366, 2004 [PubMed] [Google Scholar]
- 21. Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci 27: 2783–2802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Getting SJ, Segieth J, Ahmad S, Biggs CS, Whitton PS. Biphasic modulation of GABA release by nitric oxide in the hippocampus of freely moving rats in vivo. Brain Res 717: 196–199, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Hagioka S, Takeda Y, Zhang S, Sato T, Morita K. Effects of 7-nitroindazole and N-nitro-l-arginine methyl ester on changes in cerebral blood flow and nitric oxide production preceding development of hyperbaric oxygen-induced seizures in rats. Neurosci Lett 382: 206–210, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Hirai T, Musch TI, Morgan DA, Kregel KC, Claassen DE, Pickar JG, Lewis SJ, Kenney MJ. Differential sympathetic nerve responses to nitric oxide synthase inhibition in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 269: R807–R813, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Jayakumar AR, Sujatha R, Paul V, Asokan C, Govindasamy S, Jayakumar R. Role of nitric oxide on GABA, glutamic acid, activities of GABA-T and GAD in rat brain cerebral cortex. Brain Res 837: 229–235, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Korkmaz A, Oter S, Sadir S, Topal T, Uysal B. Exposure timerelated oxidative action of hyperbaric oxygen in rat brain. Neurochem Res 33: 160–166, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Levinger IM. The cerebral ventricles of the rat. J Anat 108: 447–451, 1971 [PMC free article] [PubMed] [Google Scholar]
- 28. Mialon P, Joanny P, Gibey R, Cann-Moisan C, Caroff J, Steinberg J, Barthélémy L. Amino acids and ammonia in the cerebral cortex, the corpus striatum and the brain stem of the mouse prior to the onset and after a seizure induced by hyperbaric oxygen. Brain Res 676: 352–357, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Mungrue IN, Bredt DS. nNOS at a glance: implications for brain and brawn. J Cell Sci 117: 2627–2629, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Oury TD, Ho Y, Piantadosi CA, Crapo JD. Extracellular superoxide dismutase, nitric oxide, and central nervous system O2 toxicity. Proc Nat Acad Sci USA 89: 9715–9719, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ozdemir MB, Akdogani E, Yonguc N. Three dimensional (3D) reconstruction of the rat ventricles. Neuroanatomy 4: 49–51, 2005 [Google Scholar]
- 32. Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med (Maywood) 226: 814–824, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Paul V, Jayakumar AR. A role of nitric oxide as an inhibitor of γ-aminobutyric acid transaminase in rat brain. Brain Res Bull 51: 43–46, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Nat Acad Sci USA 86: 3375–3378, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sakuma I, Togashi H, Yoshioka M, Saito H, Yanagida M, Tamura M, Kobayashi T, Yasuda H, Gross S, Levi R. NG-methyl-l-arginine, an inhibitor of l-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ Res 70: 607–611, 1992 [DOI] [PubMed] [Google Scholar]
- 36. Salter M, Duffy C, Garthwaite J, Strijbos PJLM. Substantial regional and hemispheric differences in brain nitric oxide synthase (NOS) inhibition following intracerebroventricular administration of Nω-nitro–arginine (l-NA) and its methyl ester (l-NAME). Neuropharmacology 34: 639–649, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Sander M, Hansen J, Victor RG. The sympathetic nervous system is involved in the maintenance but not initiation of the hypertension induced by nω-nitro-l-arginine methyl ester. Hypertension 30: 64–70, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Sato T, Takeda Y, Hagioka S, Zhang S, Hirakawa M. Changes in nitric oxide production and cerebral blood flow before development of hyperbaric oxygen-induced seizures in rats. Brain Res 918: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Segovia G, Mora F. Role of nitric oxide in modulating the release of dopamine, glutamate, and GABA in striatum of the freely moving rat. Brain Res Bull 45: 275–279, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85, 1985 [DOI] [PubMed] [Google Scholar]
- 41. Theodore J, Robin ED. Pathogenesis of neurogenic pulmonary edema. Lancet 2: 746–753, 1975 [DOI] [PubMed] [Google Scholar]
- 42. Thom SR, Fisher D, Zhang J, Bhopale VM, Ohnishi ST, Kotake Y, Ohnishi T, Buerk DG. Stimulation of perivascular nitric oxide synthesis by oxygen. Am J Physiol Heart Circ Physiol 284: H1230–H1239, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Togashi H, Sakuma I, Yoshioka M, Kobayashi T, Yasuda H, Kitabatake A, Saito H, Gross SS, Levi R. A central nervous system action of nitric oxide in blood pressure regulation. J Pharmacol Exp Therap 262: 343–347, 1992 [PubMed] [Google Scholar]
- 44. Wan X, Fu TC, Funk A, London RE. Differential clearance of nitroxide MRI contrast agents from rat cerebral ventricles. Brain Res Bull 36: 91–96, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Fice DS, Yeung PKF. A simple high-performance liquid chromatography assay for simultaneous determination of plasma norepinephrine, epinephrine, dopamine and 3,4-dihydroxyphenyl acetic acid. J Pharm Biomed Anal 21: 519–525, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Yang Z, Coote JH. Role of GABA and NO in the paraventricular nucleus-mediated reflex inhibition of renal sympathetic nerve activity following stimulation of right atrial receptors in the rat. Exp Physiol 88: 335–342, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, Su Y, Oury TD, Piantadosi CA. Cerebral amino acid, norepinephrine and nitric oxide metabolism in CNS oxygen toxicity. Brain Res 606: 56–62, 1993 [DOI] [PubMed] [Google Scholar]