Executive Summary
Objective
This review was conducted to assess the effectiveness of negative pressure wound therapy.
Clinical Need: Target Population and Condition
Many wounds are difficult to heal, despite medical and nursing care. They may result from complications of an underlying disease, like diabetes; or from surgery, constant pressure, trauma, or burns. Chronic wounds are more often found in elderly people and in those with immunologic or chronic diseases. Chronic wounds may lead to impaired quality of life and functioning, to amputation, or even to death.
The prevalence of chronic ulcers is difficult to ascertain. It varies by condition and complications due to the condition that caused the ulcer. There are, however, some data on condition-specific prevalence rates; for example, of patients with diabetes, 15% are thought to have foot ulcers at some time during their lives. The approximate community care cost of treating leg ulcers in Canada, without reference to cause, has been estimated at upward of $100 million per year.
Surgically created wounds can also become chronic, especially if they become infected. For example, the reported incidence of sternal wound infections after median sternotomy is 1% to 5%. Abdominal surgery also creates large open wounds. Because it is sometimes necessary to leave these wounds open and allow them to heal on their own (secondary intention), some may become infected and be difficult to heal.
Yet, little is known about the wound healing process, and this makes treating wounds challenging. Many types of interventions are used to treat wounds.
Current best practice for the treatment of ulcers and other chronic wounds includes debridement (the removal of dead or contaminated tissue), which can be surgical, mechanical, or chemical; bacterial balance; and moisture balance. Treating the cause, ensuring good nutrition, and preventing primary infection also help wounds to heal. Saline or wet-to-moist dressings are reported as traditional or conventional therapy in the literature, although they typically are not the first line of treatment in Ontario. Modern moist interactive dressings are foams, calcium alginates, hydrogels, hydrocolloids, and films. Topical antibacterial agents—antiseptics, topical antibiotics, and newer antimicrobial dressings—are used to treat infection.
The Technology Being Reviewed
Negative pressure wound therapy is not a new concept in wound therapy. It is also called subatmospheric pressure therapy, vacuum sealing, vacuum pack therapy, and sealing aspirative therapy.
The aim of the procedure is to use negative pressure to create suction, which drains the wound of exudate (i.e., fluid, cells, and cellular waste that has escaped from blood vessels and seeped into tissue) and influences the shape and growth of the surface tissues in a way that helps healing. During the procedure, a piece of foam is placed over the wound, and a drain tube is placed over the foam. A large piece of transparent tape is placed over the whole area, including the healthy tissue, to secure the foam and drain the wound. The tube is connected to a vacuum source, and fluid is drawn from the wound through the foam into a disposable canister. Thus, the entire wound area is subjected to negative pressure. The device can be programmed to provide varying degrees of pressure either continuously or intermittently. It has an alarm to alert the provider or patient if the pressure seal breaks or the canister is full.
Negative pressure wound therapy may be used for patients with chronic and acute wounds; subacute wounds (dehisced incisions); chronic, diabetic wounds or pressure ulcers; meshed grafts (before and after); or flaps. It should not be used for patients with fistulae to organs/body cavities, necrotic tissue that has not been debrided, untreated osteomyelitis, wound malignancy, wounds that require hemostasis, or for patients who are taking anticoagulants.
Review Strategy
The inclusion criteria were as follows:
Randomized controlled trial (RCT) with a sample size of 20 or more
Human study
Published in English
Summary of Findings
Seven international health technology assessments on NPWT were identified. Included in this list of health technology assessments is the original health technology review on NPWT by the Medical Advisory Secretariat from 2004. The Medical Advisory Secretariat found that the health technology assessments consistently reported that NPWT may be useful for healing various types of wounds, but that its effectiveness could not be empirically quantified because the studies were poorly done, the patient populations and outcome measures could not be compared, and the sample sizes were small.
Six RCTs were identified that compared NPWT to standard care. Five of the 6 studies were of low or very low quality according to Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria. The low and very low quality RCTs were flawed owing to small sample sizes, inconsistent reporting of results, and patients lost to follow-up. The highest quality study, which forms the basis of this health technology policy assessment, found that:
There was not a statistically significant difference (≥ 20%) between NPWT and standard care in the rate of complete wound closure in patients who had complete wound closure but did not undergo surgical wound closure (P = .15).
The authors of this study did not report the length of time to complete wound closure between NPWT and standard care in patients who had complete wound closure but who did not undergo surgical wound closure
There was no statistically significant difference (≥ 20%) in the rate of secondary amputations between the patients that received NPWT and those that had standard care (P = .06)
There may be an increased risk of wound infection in patients that receive NPWT compared with those that receive standard care.
Conclusion
Based on the evidence to date, the clinical effectiveness of NPWT to heal wounds is unclear. Furthermore, saline dressings are not standard practice in Ontario, thereby rendering the literature base irrelevant in an Ontario context. Nonetheless, despite the lack of methodologically sound studies, NPWT has diffused across Ontario.
Discussions with Ontario clinical experts have highlighted some deficiencies in the current approach to wound management, especially in the community. Because NPWT is readily available, easy to administer, and may save costs, compared with multiple daily conventional dressing changes, it may be used inappropriately. The discussion group highlighted the need to put in place a coordinated, multidisciplinary strategy for wound care in Ontario to ensure the best, continuous care of patients.
Objective
This assessment, which includes an Ontario systems analysis, assessed the effectiveness of negative pressure wound therapy (NPWT) across all reported indications and settings.
Negative pressure wound therapy is used by hospitals, home care agencies, and community care facilities. Home care agencies use the most NPWT systems in Ontario (40% of total NPWT systems used), followed by long-term care facilities, (29%), and hospitals (27%) (Personal communication, November 2004).
Background
Clinical Need: Target Population and Condition
Many wounds are difficult to heal, despite active medical and nursing care. They may result from complications of an underlying disease, like diabetes, or from surgery, trauma, or burns. Chronic wounds are more often found in elderly people and in people with immunological or chronic disease. They may lead to deficits in the ability to function and quality of life, to amputation, or even to death.
The prevalence of chronic ulcers is difficult to ascertain. It varies by condition and complications due to the condition that caused the ulcer. In a systematic review, Graham et al. (1) reported that the prevalence of lower limb ulcers ranged from 0.12% to 0.32% in the general population. (This translates to between 15,600 and 41,600 people in Ontario in 2004.) However, the authors stated that variations owing to heterogeneous populations, study designs, clinical definitions, and inclusion and exclusion criteria, made it difficult to pool the studies on prevalence.
Despite the paucity of good general prevalence data, condition-specific prevalence information has been reported. Of patients with diabetes, 15% are thought to have foot ulcers at some time during their lives, typically due to peripheral neuropathy and vascular disease, deformity, or infection (2) (about 105,000 people in Ontario). The approximate community care cost of treating leg ulcers in Canada, without reference to cause, has been estimated at upward of $100 million per year. (3)
In a 2004 Canadian study, (4) the prevalence of pressure ulcers was reported as follows:
25% in acute care settings (95% confidence interval [CI], 23.8%–26.3%)
30% in non-acute care settings (95% CI, 28.3%–31.4%)
15% in community care setting (95% CI, 13.4%–16.8%)
22% in mixed health care settings (95% CI, 20.9%–23.4%)
26% in all health care settings combined (95% CI, 25.2%–26.8%) w
Surgically created wounds can also become chronic, especially if they become infected. For example, the reported incidence of sternal wound infections after median sternotomy is 1% to 5%. (5) These patients are more likely to have longer hospital stays and higher rates of morbidity and mortality. Treatments for sternal infection are mediastinal antibiotic irrigation, pectoralis muscle flaps, aggressive surgical debridement, delayed closure, or plastic reconstruction with muscle flaps. (5) Abdominal surgery also creates large open wounds. Because it is sometimes necessary to leave these wounds open to allow them to heal on their own (secondary intention), some may become infected and resist healing. (5)
Existing Treatments Other Than Technology Being Reviewed
Little is known about the wound healing process, making the treatment of chronic wounds challenging. (6) Many types of interventions are used to treat wounds. The following are examples of some of the most common treatments.
Conventional Treatment
Current best practice for the treatment of ulcers and other chronic wounds includes debridement, bacterial balance, and moisture balance. (7) Treating the cause, ensuring good nutrition, and preventing primary infection helps wounds to heal. Saline or wet-to-moist dressings are reported as traditional or conventional therapy in the literature, although this is generally not recommended as the first line of treatment in Ontario (Personal communication, November 2004). Modern moist interactive dressings are foams, calcium alginates, hydrogels, hydrocolloids, and films. (8)
Debridement
Debridement is the removal of necrotic (dead) or contaminated tissue from inside or beside a wound. This typically is done in conjunction with other local treatments to control excessive bacterial growth and improve moisture balance. Debridement can be achieved surgically (cutting dead tissue away from wound), mechanically (using saline to dry dead tissue, which can be then removed), or chemically by using autolytic agents (e.g., alginates, films, foams, hydrocolloids, and hydrogels). The advantages of debridement are as follows:
It allows a provider to see the complete wound.
Draining the exudate (i.e., fluid, cells, and cellular debris that has escaped from blood vessels) and removing dead tissue may lower the chance of infection.
A deep swab can be taken from viable tissue.
It encourages healing by restoring a chronic wound to an acute wound. (8)
Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy has been in use for about 40 years. It is thought to improve wound healing by supplying oxygen to the wounds. (9;10) During the procedure, a patient is placed in a compression chamber with increased pressure between 2.0 and 2.5 atmospheres absolute for 60 to 120 minutes, once or twice daily. In the chamber, the patient inhales 100% oxygen. Treatment usually runs for 15 to 20 sessions. Adverse effects include ear, sinus, and lung damage; short-sightedness; claustrophobia; and oxygen poisoning. (9;10) In September 2005, the Medical Advisory Secretariat conducted a health technology literature review on the role of hyperbaric oxygen therapy to treat the foot ulcers of people with diabetes.
Topical Warming
The usual skin temperature of ulcers is about 33°C. This temperature limits the movement and growth of tissue. An increase to 38°C may help to induce healing in wounds by increasing the flow of oxygen. A protocol of this intervention was recently submitted for a Cochrane review. (11)
Other Therapies
Many other therapies are used to treat chronic wounds:
Electrotherapy: This includes ultrasound, laser therapy, electrical stimulation, and electromagnetic waves. During electrotherapy, the cells in the wound are stimulated electrically through electrodes or pulsed magnetic fields to induce healing. (12;13) The Centers for Medicare & Medicaid Services in the United States fund electromagnetic or electrical stimulation for 1 course of treatment for severe pressure ulcers, or arterial, diabetic or venous stasis ulcers when 30 days of traditional treatments have failed to reduce the wound’s size. (14) However, the cost of the devices is not covered. Some Ontario physicians use electrical stimulation as a secondary treatment when traditional, local, wound care therapies fail (Personal communication, November 2004). The Alberta Heritage Foundation (15) recently reported a lack of evidence to support the use of low-level laser therapy for wound healing.
Systemic therapy: This comprises nutrition, pentoxifylline, flavonoids, thromboxane alpha-2 agonists, and sulodexide. A well-balanced diet, including vitamin supplementation, is thought to be associated with wound healing, although empiric evidence is insufficient to date. (16)
Antimicrobial therapy: This therapy is used for infection control and includes topical ketanserin, dimethylsulphoxide, ciprofloxacin, and levamisole. (17)
Topical engineered skin substitutes/autologous blood-derived/growth factor products: There are many types of these dressings. They are thought to activate skin growth to promote healing. In December 2003, the Centers for Medicaid & Medicare Services decided that these are not “reasonable and necessary” for the treatment of chronic wounds; therefore, they do not cover them. (18)
Environmental therapies: These are foam beds, mattresses, cushions, and compression hosiery. (13)
New Technology Being Reviewed
Negative pressure wound therapy is also called negative topical pressure, subatmospheric pressure therapy, vacuum sealing, vacuum pack therapy, and sealing aspirative therapy. There are 2 manufacturers of NPWT licensed by Health Canada: KCI USA Inc. distributes the V.A.C. therapy system and BlueSky Medical Group Inc. distributes the Versatile 1 Wound Vacuum System. The Versatile 1 system has been licensed in Canada since April 2005. The V.A.C. therapy system has been licensed by Health Canada since 2003.
The aim of NPWT is to use negative pressure to create suction, which drains the wound and influences the shape and growth of the surface tissues in a way that promotes healing. During the procedure, a piece of foam is placed over the wound and a drain tube is placed over the foam. A large piece of transparent tape is placed over the whole area, including the surrounding healthy tissue, to secure the foam and drain the wound. The tube is connected to a vacuum source, and fluid is drawn from the wound through the foam into a disposable canister. Thus, the entire wound area is subjected to negative pressure. The device can be programmed to provide varying degrees of pressure either continuously or intermittently.
Indications for NPWT are as follows: (19)
Chronic and acute wounds
Subacute wounds (dehisced incisions)
Chronic, diabetic wounds or pressure ulcers
Meshed grafts (before and after)
Flaps
Contraindications for NPWT are as follows: (19)
Fistulae to organs/body cavities
Necrotic tissue that has not been debrided or eschar (sloughed-off dead tissue, or a scab)
Untreated osteomyelitis
Wound malignancy
Taking anticoagulants
Wounds that require hemostasis (i.e., that the flow of blood be stopped) for local bleeding
Documented precautions include these: (19)
Placing dressing on exposed blood vessels or organs
Active bleeding
Although the exact mechanism is unknown, NPWT may promote wound healing by doing the following: (19)
Stimulating blood cells in the wound bed
Promoting granulation tissue growth
Promoting a closed moist environment
Extracting interstitial fluid that can retard cell growth and proliferation
Negative pressure wound therapy is designed to be used in a variety of settings. It may be used by plastic surgeons to “prepare the wound bed for skin grafting and to improve the ‘take’ of skin grafts,” (20) and in cardiac and general surgery. It may also be used in hospitals, long-term care facilities, and patients’ homes to treat chronic ulcers caused by an underlying disease (like diabetes) or constant pressure.
Few evidence-based practice guidelines describe the characteristics of patients likely to benefit most from NPWT, when it should be used during wound treatment, and how long treatment should be. Nonetheless, NPWT has been accepted in many jurisdictions in Ontario, in other Canadian provinces (Personal communication, September 2004), and internationally. (20-22;22)
Regulatory Status
As noted, there are 2 manufacturers of NPWT systems licensed by Health Canada. KCI USA Inc. (San Antonio, Texas, United States) distributes the V.A.C. therapy system and BlueSky Medical Group Inc. (Carlsbad, California, United States) distributes the Versatile 1 Wound Vacuum System (licence 68201; Class 2 device). The V.A.C. therapy system is the system that is most commonly reported in the literature.
Insurance Coverage
No Canadian province provides direct funding for NPWT to providers. The Edmonton Capital Health Authority, however, has a managed-care system in place for NPWT (Personal communication, November 2004). One province is considering physician reimbursement for the use of NPWT in plastic surgery (Personal communication, December 2004).
Negative pressure wound therapy has diffused into Ontario hospitals, long-term care homes, and community care access centres (CCACs), as discussed in more detail further in this review (section on diffusion). Currently, the global budgets of hospitals and CCAC agencies pay for NPWT. In long-term care homes, NPWT is paid for through a high-intensity, needs-based funding scheme. This is described in detail in the diffusion section further in this assessment. There is no fee-for-service reimbursement for physicians under the Ontario Health Insurance Plan.
The Food and Drug Administration (FDA) in the United States approved V.A.C. therapy in December 2002 as a Class 2 device (510(k) number K021500) that must be sold on the order of a physician. The Centers for Medicaid & Medicare Services fund V.A.C. therapy for the treatment of wounds for up to 30 days. If the wound has not healed sufficiently by that time, the funding is discontinued. This decision is based on the assumption that the pump is used to initiate wound healing, not to complete the wound healing process. Most of the funding by the Centers for Medicaid & Medicare Services (80%) goes to the medical equipment supplier, (21) who may stipulate the conditions for providing this therapy based on specific patient indications and the consistent reporting and documenting of the progress of healing during therapy (www.umd.nycpic.com/rev15_1431NegativePressureWound.html; accessed October 2004). The equipment supplier charges the patient an additional co-payment of 20%. Secondary insurance may cover this cost (www.cms.hhs.gov/mcd; accessed September 2004).
In general, it appears that many health insurance companies in the United States provide coverage for NPWT under strict circumstances. (23-25) These companies have concluded that NPWT is medically necessary when either of the following conditions is met:
Patients with stage III or IV ulcers or wounds in a home setting for at least 30 days. Patients must meet certain criteria prior to receiving NPWT including documented wound measurements, treatment with moist dressings, adequate nutrition, debridement of necrotic tissue, appropriate positioning (elevation) to relieve pressure on wound, and appropriate diabetes care (if necessary).
Patients with stage III or IV wounds in inpatient setting. NPWT is an option if other more conservative means of wound healing are ineffective. Or if a patient has a wound where there is medical necessity for accelerated formation of granulation tissue which cannot be achieved by topical wound treatments.
The provision of coverage is stipulated rigidly. Written documentation on the patient’s nutritional status, history of wound treatment, treatment of underlying medical conditions, and treatment course every 2 weeks is mandatory. If the wound hasn’t healed within 3 months, coverage may be discontinued. The insurance companies have also indicated that NPWT will only be covered for up to 4 months in most cases. After 4 months, coverage will only continue if there is evidence of improvement with NPWT.
Adverse Events
According to the United States Food and Drug Administration’s Manufacturer and User Facility Device Experience database, there were more than 100 adverse events associated with NPWT reported between January 1, 2000 and June 29, 2006. (26) In the first 6 months of 2006, there were 24 separate reports of adverse events associated with negative pressure wound therapy. Eight of the 24 reported events were due to having accidentally left foam dressing in the wound after it was healed. Four adverse events were due to using NPWT over exposed organs, which is a contraindication to using NPWT cited by both manufacturers. Two adverse events involved wound progression despite using NPWT, and another 2 were unknown. There were 6 adverse events that may or may not have been associated with NPWT use, including 1 case of cellulitis, 1 amputation, 1 wound infection, 2 cases of skin irritation around the wound where the adhesive tape was applied and 1 case of toxic shock syndrome. One patient had developed tissue formation in the foam dressing. Finally, 1 patient tripped over the tubing attached to the NPWT unit and suffered a hip fracture.
Literature Review on Effectiveness
Research Questions
Is negative pressure wound therapy effective for healing wounds (including pressure or diabetic ulcers, sternal wounds, and skin grafts) compared with standard care?
If NPWT is effective, is it cost-effective compared with standard care?
Methods
The published health technology assessments on negative pressure wound therapy were extracted. All literature on NPWT was searched until March 2006.
The following databases were searched: MEDLINE, EMBASE, MEDLINE In-Process and Non-Indexed Citations, INAHTA, Cochrane Database of Systematic Reviews, and a vacuum therapy Web site (http://www.vacuumtherapy.co.uk/index.htm).
The keywords for the search were as follows:
Wound healing
Foot ulcer or skin ulcer or varicose ulcer or leg ulcer
Wounds, non penetrating
Chronic and ulcer or wound
Leg or foot arterial or diabetic and ulcer or wound
Suction
Pressure
Vacuum
Vacuum assisted closure or V.A.C. therapy
Negative pressure
Topical negative pressure
Subatmospheric pressure therapy
The criteria for inclusion were as follows:
Peer-reviewed, published randomized controlled trials (RCT) sample size of 20 or more
Human study
Negative pressure wound therapy
Note: In the 2004 health technology literature review on V.A.C. therapy, nonrandomized trials were also included in the analysis. However, due to the availability of RCTs now, nonrandomized trials were excluded from the update.
Results of Literature Review
Summary of Existing Health Technology Assessments
Seven international health technology assessments (27-33) on NPWT were identified (Table 1). Included in this list of health technology assessments is the original health technology review on NPWT by the Ontario Health Technology Advisory Committee from 2004. The health technology assessments consistently reported that NPWT may be useful for healing various types of wounds, but that its effectiveness could not be empirically quantified because the studies were poorly done, the patient populations and outcome measures could not be compared, and the sample sizes were small (Table 1).
Table 1: Conclusions and Limitations From Published Health Technology Assessments on Negative Pressure Wound Therapy*.
| Health Technology Assessment | Conclusions | Comments on Available Evidence |
|---|---|---|
| AHRQ/Blue Cross Blue Shield, 2005 (31) | Insufficient evidence to make conclusions regarding effectiveness of NPWT. |
|
| McGill University Health Centre, Technology Assessment Unit, 2005 (32) | No additional [NPWT] pumps should be purchased or rented until clear evidence of efficacy becomes available. |
|
| Cochrane, United Kingdom, 2004 (27) | Evidence of effectiveness is weak. Interpret results with “extreme caution.” |
|
| Ontario Health Technology Advisory Committee, 2004 (33) | Do not provide additional funding for [NPWT], based on the dearth of existing evidence of effectiveness. | The results of 4 health technology assessments, 1 small RCT, and 10 case series were reviewed. The results of the assessments consistently criticized the lack of evidence. The small RCT and case series provided insufficient evidence to support the use of NPWT. |
| ASERNIP-S, Australia, 2003 (28) | NPWT may promote better and faster healing with few complications, but there are limitations to the available evidence. |
|
| CCOHTA, Canada, 2003 (29) | Effectiveness is not established. |
|
| Centre for Clinical Excellence, Australia, 2003 (30) | Effectiveness is not confirmed. There were serious methodological flaws in the studies evaluated. |
|
AHRQ indicates Agency for Healthcare Research and Quality; ASERNIP-S, Australian Safety and Efficacy Register of New Interventional Procedures – Surgical; CCOHTA, Canadian Coordinating Office for Health Technology Assessment; NPWT, negative pressure wound therapy; RCT, randomized controlled trial.
Summary of Results of Medical Advisory Secretariat Review
Six RCTs (34-39) comparing NPWT to standard wound therapy met the inclusion criteria for this review. Two additional RCTs (40;41) were identified but excluded, because they included fewer than 20 patients (N = 10 for each RCT).
Each of the 6 RCTs was evaluated based on the quality of study. Five of the 6 were considered of low or very low quality. The most recent RCT, by Armstrong and Lavery, (34) was the highest quality RCT identified. This RCT had an adequate sample size and an intent-to-treat analysis. The other RCTs were flawed with various problems including small sample sizes, inconsistent reporting of results, and patients lost to follow-up. Table 2 describes the quality of the RCTs assess using Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (42) included in the review.
Table 2: Quality of RCTs Comparing NPWT to Standard Care for Wounds Using GRADE Criteria*.
| Study, Year | Adequate Sample Size and Method of Randomization | Blinding | Equal Groups at Baseline |
Outcome Measure | Intent-To-Treat Analysis | Other Limitations | Overall Quality |
|---|---|---|---|---|---|---|---|
| Armstrong, 2005 (34) | Yes: to detect a 20% difference between groups; N= 162 Sealed envelopes, 1:1 ratio |
No: “Given the effect of NWPT on the wound bed, an experienced observer would recognize an NPWT-treated wound” | Statistical comparison not reported in study; authors reported groups were equal in response to The Lancet regarding comments from other researchers. | Complete wound closure | Yes. | Few results reported for patients who had complete wound closure but did not undergo surgical wound closure. | Moderate |
| Moisidis, 2004 (35) | Unclear; N = 22 Randomization method not reported |
Yes. | Yes: all patients received both forms of wound healing. | Percentage of epithelialization: difficult to measure | No. - 2 patients lost with no explanation; given that is was a 2-week study of patients with chronic wounds, it is difficult to understand how a patient could be lost. |
Length of follow-up not reported; 2-week study, but authors reported that “all wounds healed without the need for further debridement or regrafting.” | Low |
| Moues, 2004 (36) | Unclear; authors reported that power calculation was performed N = 54 Sealed envelopes |
No due to the “obvious visible suction marks present in wounds treated with V.A.C.” | Yes. | Wound surface area (wound traced then measured): no volume measurement | No. Depending on the outcome—the results of the entire sample aren’t reported. |
No clear definition for “ready for surgery,” which was the endpoint of treatment. | Low |
| Wanner, 2003 (37) | Unclear; N = 34; 12 patients dropped out, leaving N = 22. Randomization process unknown |
Unknown if the assessment of wounds was blinded. | Yes; however, few baseline characteristics reported. | Reduction in wound volume (wound sealed and filled with saline, volume of saline measured): authors said this method is reproducible. | No; 12 patients dropped out. | Very specific population; therefore, questionable generalizability. Duration of wound is not reported. |
Low |
| Ford, 2002 (38) | Unclear; N = 28 (41 wounds) Random table of letters |
Yes. Blinded clinicians measured wound dimensions and obtained plaster wound impressions. |
Unclear if groups were equivalent at baseline. Patients in the NPWT group were younger than patients in control group (42 yrs vs. 54 yrs). | Reported using photographs, plaster impressions, and wound dimensions; these results are not reported. Only the number of patients with complete wound closure was reported. | No; 6 patients were lost to follow-up and excluded from the results. | Concluded that NPWT “promotes an increased rate of wound healing,” but did not report rate of wound healing or measurement of the rate of wound healing. | Very low |
| Joseph, 2000 (39) | Unclear; N = 24 (36 wounds) Randomized by label colour |
Yes. | No: patients in the NPWT group had larger wounds than did patients in the control group. | Wound size measurements | No patients were lost. | Interpretation of Kaplan-Meier curve unclear. Calculation of effectiveness unclear. |
Very low |
GRADE indicates Grading of Recommendations Assessment, Development and Evaluation working group; (42) NPWT, negative pressure wound therapy; RCT, randomized controlled trial.
Blinding
According to 2 of the RCTs, (34;36) blinding is not possible in studies comparing NPWT to standard care because of the effect of NPWT on the wound bed. Both studies indicated that an experienced observer would be able to recognize a NPWT-treated wound. Nevertheless, 3 of the other RCTs (35;38;39) reported using blinding in their assessments of the wounds. The “blinded” RCT by Moisidis et al. (35) did not report a significant difference between quantitative wound healing in the NPWT group compared with the standard care group, but they did report a significant difference in qualitative wound healing. Thus, based on the knowledge that it is possible to know which wounds have received which treatment, the qualitative assessment of wound healing is potentially biased.
Baseline Characteristics
In terms of baseline characteristics, most RCTs indicated that the groups were similar at baseline. In the RCT by Moisidis et al. (35) all patients received both wound treatments. Each wound was split in half; one-half of the wound was treated with NPWT, and the other half was treated with standard care. Each one-half of the wound was randomly assigned to one treatment or the other.
The RCT by Joseph et al. (39) reported that the groups were equal at baseline; however, there appears to be an error in the reporting of the results. In the table outlining the baseline characteristics of the groups, Joseph et al. reported the mean initial wound volume was 38 cc for the NPWT group and 24 cc for the standard treatment group. They reported that this difference was not significant (P = .08). Joseph et al. also included a table of characteristics of each of the 36 wounds (in 24 patients) included in the study. When the Medical Advisory Secretariat calculated the mean initial wound volume from the individual data, they found that, for the NPWT group, it was 53 cc (median 50 cc, range 3–150 cc) and 25 cc for the standard care group (median 18 cc, range 3–80 cc). Thus, the mean initial wound volume was more than twice as much for the NPWT group compared with the standard care group. Thus, the groups were not equal at baseline and the results of the RCT are unreliable.
Quality of Outcome Measures
Establishing objective outcome measures for wound healing is difficult owing to the nature of wound healing; therefore, judgment as to whether a wound has healed or not may be subjective. The FDA has published a guidance paper (43) for industry regarding designing studies for wounds treatments. The FDA has recommended that “[s]tudies should be designed to measure the incidence of complete wound closure…” The FDA also indicated that it does not consider partial healing to be an appropriate outcome for wound healing because the clinical benefit of a statistically significant reduction in wound size has not been established.
Only the RCT by Armstrong and Lavery indicated that their primary outcome was complete wound closure. The other studies measured wound volume, depth, length, and width (i.e., partial healing) as primary outcomes. Some studies reported making plaster impressions of the wound to observe changes in size over time. Others sealed the wound, then filled the wound with saline, and then drained and measured the saline to determine wound volume. The RCT by Moisidis et al. (35) reported measuring percentage of epithelialization, which was evaluated by “gross inspection” (i.e. visual inspection with no quantitative measure of wound healing). Measurement by inspection is unlikely accurate or reproducible.
The RCTs by Moues et al. (36) and Wanner et al. (37) were using the negative pressure wound therapy systems to initiate wound healing prior patients for surgical wound closure, unlike the other RCTs which were using NPWT and standard care for the purpose of complete wound healing. One of the hypotheses of Moues et al. is grounded in an a priori assumption that NPWT heals wounds better than standard care does.
Characteristics of Patients in Randomized Controlled Trials
Most of the RCTs had relatively small sample sizes, with the exception of the RCT by Armstrong and Lavery, (34) which included 162 patients. The mean age ranged between 42 and 64 years across the 6 RCTs. The mean wound duration also varied considerably across the studies. In the RCT by Moisidis et al. the mean wound duration was 18 days (range, 0–90 days), compared with 1.5 months (SD, 5.0) in the RCT by Armstrong and Lavery. The mean wound size was also variable across studies. It was 128 cm2 in the RCT by Moisdis et al., (35) which was much larger than the mean size of the wounds in the other RCTs (Table 3). Moreover, some studies reported wound area, whereas others reported wound volume.
Table 3: Characteristics of Recent Randomized Controlled Trials on Wound Healing*.
| Study, Year | N | % Male | Mean Age, Years | Mean Duration of Wound | Mean Size of Wound | Mean Length of Follow-Up |
|---|---|---|---|---|---|---|
| Armstrong, 2005 (34) | 162 | 81 | 59 (SD, 12.8) | 1.5 ± 5.0 months | 20.7 ± 20.6 cm2 | Until complete healing or 16 weeks (whichever was first) |
| Moisidis, 2004 (35) | 22 (20 after 2 pts were lost to follow-up) | 60 | Median, 64 (range, 27–88) | 18 days (range, 0–90 days) | 128 cm2 (range, 35–450 cm2) | Not reported. Study only lasted 2 weeks; however, authors reported “all wounds healed without the need for further debridement or regrafting” |
| Moues, 2004 (36) | 54 | 65 | 47 | Stratified early(< 4 weeks) vs. late (> 4 weeks) | Not reported | 30 days |
| Wanner, 2003 (37) | 22 | 68 | NPWT: 49 (range, 25–73) Control: 53 (range, 34–77) |
Not reported | NPWT: 50 ml; range, 3–132 ml Control: 42 ml; range 5–68 ml |
Not reported |
| Ford, 2002 (38) | 28 (41 full decubitus ulcers) 22 (35 ulcers) completed study |
Not reported | NPWT: 41.7 Control: 54.4 |
Minimum 4 weeks | Not reported | 6-week trial (Patients followed for 3–10 months.) |
| Joseph, 2000 (39) | 24 (36 wounds) | Overall:50 (NPWT:66) |
NPWT: 56 Control: 49 |
Minimum 4 weeks | NPWT: 53 cm3 Control: 24 cm3 Overall: 38 cm3 |
6-week trial |
NPWT indicates negative pressure wound therapy.
In terms of inclusion and exclusion criteria, the 6 RCTs included a variety of patients who required treatment for various wound types. For instance, in the RCT by Armstrong and Lavery, (34) they were treating surgical wounds after amputation. Moues et al., (36) on the other hand, were treating nonhealing wounds due to infection or contamination.
Table 4 describes the inclusion and exclusion criteria of each of the studies. In addition, it lists the treatment regimen for patients receiving NPWT compared with the regimen for those receiving standard care. Of note, 2 of the studies reported changing the NPWT dressings less frequently than is recommended by the manufacturer of V.A.C. therapy. Standard protocol indicates that the NPWT dressings should be changed every 48 hours. In the RCT by Moues et al. the dressings were changed every 5 days. In their study, Wanner et al. indicated that dressings were changed every 2 to 7 days depending on the fluid produced from the wound.
Table 4: Inclusion and Exclusion Criteria and Treatment Regimen in the RCTs Comparing NPWT to Standard Care*.
| Study, Year | Inclusion Criteria | Exclusion Criteria | NPWT Regimen | Standard Care Regimen |
|---|---|---|---|---|
| Armstrong, 2005 (34) |
|
|
|
|
| Moisidis, 2004 (35) |
|
|
|
|
| Moues, 2004 (36) |
|
|
|
|
| Wanner, 2003 (37) |
|
|
|
|
| Ford, 2002 (38) |
|
|
|
|
| Joseph, 2000 (39) |
|
|
|
|
NPWT indicates negative pressure wound therapy.
Outcomes of Randomized Controlled Trials
The 6 RCTs comparing NPWT to standard care reported a wide variety of outcomes, none of which were consistently reported. Table 5 outlines the outcomes reported by each of the studies. Based on the low quality of the methods and reporting of 5 of the RCTs, the results of these studies will not be described in detail. The grave limitations in these studies does not allow for reliable conclusions to be drawn, thus, only the study of moderate quality by Armstrong and Lavery will be described in detail.
Table 5: Outcomes of the RCTs Comparing Negative Pressure Wound Therapy to Standard Care.
| Study, Year | Treatment | Complete Closure Without Surgery, No. (%) | Second Outcome Measured |
Third Outcome Measured | Fourth Outcome Measured | Adverse Events, No. (%) |
|---|---|---|---|---|---|---|
| Armstrong, 2005 (34) | NPWT | 31 (40) | Total patients with complete closure 43 (56%) | Median time to wound closure 56 days | Rate of amputation 2 (3%) (second amputation) | - 40 (52) any adverse event -13 (17) wound infection -9 (12) treatment-related |
| Standard care | 25 (29) P = .15 |
33 (39%) P = .04 |
77 days P ≤.05 |
9 (11%)(second amputation) P = .06RR, 0.225 (95% Cl, 0.05-1.1) |
-46 (54) any adverse event -5 (6) wound infection -11 (13)treatment-related |
|
| Moisidis, 2004 (35) | NPWT | 6 (30) | Quantitative graft take (measured by a “blinded” clinician) 86% | Qualitative graft take 50% NPWT better than control 35% equivalent 15% worse P < .05 |
NR | NR |
| Standard care | 7 (35) P = NS |
86.8% P = .55 (MAS calculation) | NR | NR | ||
| Moues, 2004 (36) | NPWT | NR | Median time to ready for surgical closure: 6 days (0.52 SEM) | NR | NR | NR |
| Standard care | NR | 7 days (0.81 SEM) P = .19 | NR | NR | NR | |
| Wanner, 2003 (37) | NPWT | NR | Mean time to 50% initial wound volume: 27 days | NR | NR | NR |
| Standard care | NR | 28 days | NR | NR | NR | |
| Ford, 2002 (38) | NPWT | 2 (10) | NR | NR | NR | 1 pt (5) with diabetes suffered from sepsis, requiring amputation 6 pts (30) underwent flap surgery |
| Standard care | 2 (13) | NR | NR | NR | 6 pts (40) underwent flap surgery | |
| Joseph, 2000 (39) | NPWT | NR | At 6 weeks, there was a mean wound volume reduction of 78%. | NR | NR | - 3 (17) osteomyelitis with calcaneal fractures |
| Standard care | NR | 30% (P = .038) Significant difference in depth and width but not length. |
NR | NR | - 8 (44) wound infections and fistulae |
CI indicates confidence interval; MAS, Medical Advisory Secretariat; NPWT, negative pressure wound therapy; NR, not reported; pt, patient; RCT, randomized controlled trial; RR, relative risk; SEM, standard error of the mean.
Three studies reported on the number of patients with complete wound closure without undergoing surgery. All 3 studies reported no significant difference between NPWT and standard care for complete wound closure without surgery. Complete wound closure was not the outcome of interest in the studies by Moues et al. and Wanner et al., because the patients in those studies were preparing to undergo surgical wound closure.
The RCT by Moisidis et al. reported no significant difference between the groups in quantitative graft take. However, there was a significant improvement in the NPWT group for the qualitative graft take outcome. It is important to note that the clinicians in the Moisidis et al. study were blinded; but, as reported in 2 other RCTs, blinding is not possible in this population because of the effects of NPWT on the wound bed. So, the qualitative results reported by Moisidis et al. are likely biased.
Joseph et al. (39) reported that the mean wound volume had decreased by 78% in the NPWT group and by 30% in the standard care group (P = .038). They also found significantly greater reduction in wound depth and width for patients in the NPWT group compared with those in the standard therapy group, but no significant difference in wound length between groups. Of note, the treatment groups were not equal at baseline on wound volume. The mean initial wound volume for patients in the NPWT group was twice as large as that for patients in the standard care group (53 cc in NPWT group vs. 25 cc in standard care group). Wanner et al. (37) have suggested that vacuum application causes wound edges to be pulled together, thus reducing the volume of the wound, and they noted that this phenomenon would also exist initially after the vacuum was removed. Thus, it is not possible to ascertain whether the reduced wound volume in the NPWT group in Joseph and colleagues’ study was due to the formation of granulation tissue, or the mechanical situation created by the vacuum. Nonetheless, the treatment groups were not evenly matched at baseline on arguably the most important variable in this study – wound volume; therefore, the results of this study do not offer any evidence to support or refute the use of NPWT.
Armstrong and Lavery Randomized Controlled Trial
The study by Armstrong and Lavery (34) was a multicentre RCT. One hundred and sixty-two patients who had had partial foot amputations were randomized to receive either NPWT (n = 77) or standard moist wound care (n = 85; control group). Randomization was based on a 1:1 ratio using sealed envelopes. Patients were followed-up for 16 weeks.
The sample size was based on the hypothesis that there would be at least a 20% difference in the rate of complete wound closure (primary outcome) and the rate of secondary amputations (secondary outcome) at 16 weeks between the groups.
There was no blinding in the study because, due to the “effect of the NPWT on the wound bed, an experienced observer would recognize an NPWT-treated wound.” (34)
The decision for patients to undergo surgical wound closure was not randomized; instead, it was decided by the physician investigator based on clinical impression. Thus, for the patients who underwent surgical wound closure and had complete wound closure, it is difficult to assess the effect of NPWT compared with standard care because it cannot be separated from the effect of surgical wound closure on complete wound closure. More patients in the NPWT group (n = 12 [15.6%]) underwent surgical wound closure than did patients in the control group (n = 8 [9.4%])
The authors stated that the “primary objective of the study was to determine whether NPWT delivered by the V.A.C. therapy system was clinically efficacious in treating amputation wounds of the diabetic foot to improve the proportion of wounds with complete closure. Secondary objectives included assessment of the rates of wound healing or facilitation of surgical wound closure, foot salvage, and treatment-related complications.”
In a response to comments regarding their study in The Lancet, (44) Armstrong and Lavery indicated that “the primary objective was to assess complete wound closure with or without surgical intervention.” As mentioned previously, the addition of a second, nonrandomized intervention (i.e., surgical wound closure) adds a confounding variable to the study that makes it impossible to analyze the effect of NPWT alone. Thus, for the purpose of this review, the results for patients with complete wound healing without surgical wound closure will be reported where possible, because this reflects the true efficacy of NPWT.
Primary Outcome: Complete Wound Closure
Armstrong and Lavery reported that there was a significant improvement for patients using NPWT when patients who underwent surgical closure and had complete closure were combined with the patients who had complete closure without surgery (55.8% for NPWT versus 38.8% for control, P = .04).
According to a MAS calculation, the proportion of patients with complete wound closure without surgical wound closure in the NPWT compared to those in the control group was 40.3% versus 29.4%, respectively (P = .15). It is important to note that this is a post hoc analysis with low statistical power to detect a significant difference between groups (Type II error).
Time to Complete Wound Closure
The authors of the study did not report time to complete closure for patients who did not undergo surgical wound closure; thus, it is not possible to comment on whether or not NPWT decreases the time to complete wound closure. Armstrong and Lavery did report that the time to wound closure was shorter for patients who received NPWT with or without surgical wound closure; however, it is not possible to ascertain whether this difference was due to the effect of NPWT or whether it was due to the surgical wound closure. It is also not stated in the article the time at which patients underwent surgical wound closure.
Secondary Outcome: Rate of Secondary Amputations
Armstrong and Lavery did not find a 20% difference between the NPWT group and the control group for secondary amputations (3% for NPWT versus 11% for control, P = .06).
Adverse Effects
Forty (52%) patients in the NPWT group and 46 (54%) patients in the control group reported having one or more adverse events throughout the course of the study, a difference that was not significant (P = .875).
The most commonly reported adverse event was wound infection. Thirteen patients (17%) in the NPWT group had wound infections (4 were severe), and 5 patients (6%) in the control group had wound infections (2 were severe). The authors reported that this 11% difference in the rate of wound infection was not significant. The authors reported that none of the wound infections reported in the NPWT group was treatment-related; however, they noted that 2 of 6 of the wound infections in the control group were treatment-related. In a response to comments regarding the study, (44) the authors stated that adverse events that were determined to be treatment-related were made based on “the investigator’s impression.”
Because the randomization process was successful and the groups were comparable at baseline, it is assumed that the intervention caused the difference in the rate of wound infections. Therefore, the Medical Advisory Secretariat performed a post hoc calculation to determine if the difference in the rate of wound infections was statistically significant between groups, and calculated a statistically significant higher rate of wound infection in the NPWT group compared to the control group (P = .04, based on a 2-tailed Fisher’s exact test). It is important to note that Armstrong and Lavery (34) ranked the severity of the wound infections, which was not taken into consideration in the Medical Advisory Secretariat calculation.
Summary
There were 6 RCTs that met the inclusion criteria for this review. However, due to the poor quality of the studies, the review focused mainly on the highest quality RCT available, by Armstrong and Lavery. (34) Therefore, based on the results of the Armstrong and Lavery RCT, the following conclusions can be made if the confounding effect of surgical wound closure is excluded:
It is unclear if there is a statistically significant difference (≥ 20%) between NPWT and standard care in the rate of complete wound closure in patients who had complete wound closure but did not undergo surgical wound closure.
It is not reported in the Armstrong and Lavery study if there is a significant difference in the time to complete closure between NPWT and standard care in patients who had complete wound closure but did not undergo surgical wound closure.
Armstrong and Lavery did not find a statistically significant difference (≥ 20%) in the rate of secondary amputations between the patients receiving NPWT and those receiving standard care.
There may be an increased risk of wound infection in patients receiving NPWT compared with those receiving standard care.
Economic Analysis
Ontario-Based Economic Analysis
Note: The Medical Advisory Secretariat did not do a formal cost-effectiveness analysis, because the effectiveness of NPWT has not been established. The Medical Advisory Secretariat describes the usage of NPWT in Ontario below.
Negative pressure wound therapy has already diffused into Ontario and is provided in several health care settings. NPWT is not a provincially funded service under the physician fee-for-service reimbursement plan (Ontario Health Insurance Plan); however, NPWT therapy and nursing care are publicly paid for through the global budgets of hospitals, home care agencies, and long-term care homes.
Negative pressure wound therapy is not coded as a procedure in hospital discharge abstract data or in data from long-term care homes or home care agencies. Because of the many wound indications and many settings where wound care is delivered, this means that the existing administrative data is not specific enough to estimate how many patients in Ontario are receiving NPWT or treatment for wounds generally.
According to the manufacturer of the V.A.C. therapy systems, about 380 V.A.C. therapy systems were leased to patients daily in Ontario in 2004 (Personal communication, November 2004). This makes up about one-half of the total rental units in Canada. Public funds pay for 365 of these units. Another 55 V.A.C. therapy systems in Ontario are owned. Most often, NPWT is given to inpatients, to patients admitted to long-term care facilities, or to patients well enough to be treated in their homes by nurses, as coordinated by CCACs. Each of these settings must address unique issues pertaining to NPWT. Each is discussed in turn below.
Acute Care
Patients may receive NPWT while inpatients for non-healing surgical, infectious, or chronic wounds. Because NPWT is not covered under any of the provincial administrative data, its use cannot be tracked or monitored for hospital inpatients. Currently, 27% of all NPWT rentals are for patients in Ontario hospitals. This translates into about 103 rentals per day (Personal communication, October 2004).
Long-Term Care Homes
Patients may be discharged from a hospital to a long-term care home for rehabilitation after a surgical procedure, or they may be admitted because they cannot cope at home. There are about 600 long-term care or chronic care homes in Ontario. In 2004, about one-third (at least 202 facilities) had patients receiving NPWT. These patients make up about 29% of the total NPWT use in Ontario, or about 110 people per day (Personal communication, October 2004).
Negative pressure wound therapy may be a regimen that is covered under the “high-intensity needs” funding schedule, which reimburses long-term care homes for patients who require wound treatment under an enterostomal therapist’s (wound specialist) orders. The home is accountable for ensuring the ongoing assessment of the wound, which can be done by the therapist. The home is also responsible for monitoring the patient while he or she is receiving NPWT. In 2003, the cost of wound treatments in Ontario long-term care homes was about $16.8 million, but it is not known how many patients this comprises (Personal communication, December 2004).
Home Care
Patients may be discharged from a hospital to their home with a physician’s order to continue NPWT, or they may initiate NPWT at home if it is requested by a health care professional. In Ontario, CCACs are responsible for coordinating home care. Clinical staff (e.g., nurses and physiotherapists) and home-making staff typically are contracted by CCACs through private agencies. About 33 of the 42 CCACs in Ontario had patients who were receiving NPWT in late 2004. About 152 (40%) patients who receive NPWT in Ontario are in home care (Personal communication, November 2004).
Certain conditions apply to the use of NPWT at home. For example, the patient must be willing to use NPWT according to the provided clinical protocol and be able to change the NPWT canister when necessary. If the patient is not cognitively and physically able, a relative or other caregiver must be available to aid in these tasks when the home care nurses are not there.
Costs in Ontario
For uninfected wounds, it is recommended that NPWT dressings be changed 3 times per week, compared with daily or twice-daily dressing changes (7 or 14 per week) with conventional therapy.
Because NPWT has already diffused into the province, an estimate of the NPWT and conventional therapy costs for the same number of patients was attempted. The Medical Advisory Secretariat did not do a cost-effectiveness analysis, because the effectiveness of NPWT has not been determined.
At this time, the V.A.C. therapy system (currently the most commonly used NPWT system in Ontario) must be rented. All cost estimates are in Canadian dollars.
Table 7: Cost of Renting the Vacuum-assisted Closure Therapy System in Ontario.
| Component of System | Cost (Cdn) |
|---|---|
| Rental of V.A.C. therapy system | - $92/day (effective May 1, 2006) |
| Canisters (collect liquid removed from wound) changed on a weekly basis, unless there is a lot of liquid being removed the wound, in which case the canister would be changed more frequently | - $315–$465 for 5–10 canisters (depending on size) |
| Foam and drape (the foam is used to fill the wound) changed 3 times per week | - $300–$400 for 5–10 foam pieces* |
More expensive foam is available for exceptionally large wounds.
Existing Guidelines for Use of Technology
According to the literature, the effectiveness of NPWT is unclear within the broader context of wound care. There are many guidelines for the use of NPWT, but little consensus as to which patients will benefit most and what duration of therapy is most beneficial. Following are some examples of NPWT guidelines. Each is written from a particular perspective.
Physician Perspective
A Canadian consensus panel, comprising providers who do and do not typically prescribe NPWT, was assembled to define the role of NPWT in wound care. (7) A Delphi panel technique with representatives from the fields of dermatology, plastic surgery, physiotherapy, family medicine, orthopedic surgery, and nursing was used to gain consensus on a series of statements developed from a set of regional guidelines. The group defined appropriate patients for NPWT as those for whom the first line of treatment was unsuccessful.
The task force concluded that a patient-centred approach should be used when treating patients with chronic, nonhealing wounds (Figure 1). Knowing the underlying cause of the wound and subsequent treatment of this cause by a clinician is critical to helping a wound to heal, and to the long-term effectiveness of any surface wound treatment.
Figure 1: Preparing the Wound Bed*.
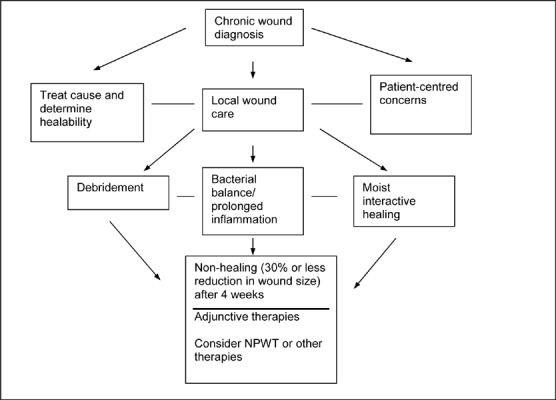
Reprinted from Ostomy Wound Management, Vol. 49 (11); Sibbald RG, Mahoney J; Therapy Canadian Consensus Group VAC. A consensus report on the use of vacuum assisted closure in chronic, difficult-to-heal wounds; p. 52-66, used with permission from Ostomy/Wound Management.
The group also noted that adjunctive treatments for wound care are expensive; therefore, they may not be efficient or appropriate as a first-line therapy to treat chronic wounds. Adjunctive therapies are usually initiated if a wound has not shrunk by at least 30% after 4 weeks of active moist treatment; however, other factors, such as a patient’s general health, the initial size of the wound, and the location of the wound, must be considered. Negative pressure wound therapy is considered an adjunctive therapy.
The group also developed guidelines for how to use the NPWT system, but it did not discuss the appropriate duration of therapy (Tables 8 and 9). It also did not look at NPWT in the context of other adjunctive therapies for wound care.
Table 8: Recommendations for Negative Pressure Wound Therapy by Type of Ulcer*.
| Chronic Wound Type | Target Pressure, mm Hg |
Type of pressure | Appropriate Dressing Change Interval, Hours | Comments |
|---|---|---|---|---|
| Pressure ulcer | 125 | Continuous over 48 hours and then intermittent (5 min. on/2 min. off | 48 | Continuous therapy could be extended if maintaining the seal is problematic where there are high exudate levels. |
| Diabetic neurotrophic foot ulcer | 50–75 | Continuous | 48 | Debridement on a regular basis is essential. |
| Heel ulcer | 50–75 | Continuous | 48 | Responds well to post-grafting |
| Venous leg ulcer | 50 and increase to 75 in no pain | Continuous | 48 |
|
Sibbald et al. (7)
Table 9: Guidelines for Negative Pressure Wound Therapy From the Vacuum-assisted Closure Therapy Consensus Group*.
| Negative pressure wound therapy should be considered for patients who are willing and able to comply with treatment and who meet the following criteria: |
|
| Negative pressure wound therapy may be considered when any of the following apply: |
|
Sibbald et al.; (7) Reprinted from Ostomy Wound Management, Vol. 49 (11); Sibbald RG, Mahoney J; Therapy Canadian Consensus Group VAC. A consensus report on the use of vacuum-assisted closure in chronic, difficult-to-heal wounds; p. 52-66, used with permission from Ostomy/Wound Management.
American guidelines on the use of NPWT to treat people with foot ulcers due to diabetes were published in April 2004 by the Tucson expert consensus conference on NPWT. (45) This document aimed to address specific issues, including the duration of NPWT, a definition of adherence for patients who may be eligible for the therapy, and the relationship between debridement and NPWT. Much of it has information on the prevalence of diabetes and diabetes care for peripheral neuropathic conditions resulting from diabetes. The guidelines may help practitioners who use NPWT to treat people with foot ulcers owing to diabetes, although they appear to rely heavily on expert opinion, rather than on empirical research.
Home Care Perspective
Guidelines have also been developed by many of the CCACs in Ontario. Table 10 shows an example of a protocol developed by the North York CCAC and Toronto-area CCACs. Overall in Toronto, the CCACs rely heavily on the educational and clinical expertise of the manufacturer. As Table 8 suggests, the CCAC protocol does not specify precisely and quantitatively which patients would benefit most from NPWT or how long therapy should be to get the best results.
Table 10: Toronto-Area Guidelines for Negative Pressure Wound Therapy for Wounds*†.
| Category | Product | Indications | Comments | Change Schedule |
|---|---|---|---|---|
| NPWT | Wound NPWT | Consider NPWT with patients who:
|
Follow individual CCAC protocols for NPWT Commonly used with:
|
Wound NPWT dressings must be changed 3 times per At least 2 nurses must be trained to do the dressing changes for NPWT per client. |
| The doctor has ordered NPWT (data information sheet must be completed.) |
Reprinted with permission from Toronto Area Community Care Access Centres.
CCAC indicates community care access centre; NPWT, negative pressure wound therapy.
Other Jurisdictions
The Capital Health Authority (46) in Edmonton, Alberta has had wound care guidelines in place since August 2001. These guidelines suggest NPWT should be used with standard therapy as needed (e.g., debridement, systemic antibiotic therapy, adequate nutrition, pressure relief, revascularization, etc.) in these cases:
Pressure ulcers and other chronic wounds
Acute and traumatic wounds with soft tissue loss
Dehisced wounds (abdominal, sternal orthopedic)
Infected wounds, postoperative meshed grafts
Perhaps in arterial ulcers following revascularization
When other wound therapies fail
Contraindications are as follows:
Necrotic tissue in wound
Untreated infection
Fistulae
Malignant wounds
Osteomyelitis (inflammation of the bone) with sequestrum (tissue that has become sequestered)
The Capital Health Authority suggests that NPWT should not be used or should be discontinued in these situations:
A patient has an allergic reaction, bleeding, bruising, or unmanaged pain in response to NPWT.
Other treatments are more economical or feasible.
NPWT does not reduce wound size or granulation growth is not witnessed after 2 to 3 weeks.
The wound gets worse.
An occlusive seal cannot be attained.
A patient cannot comply with therapy, or it is not feasible to use NPWT in a given setting.
In Edmonton, each hospital has a wound care manager. Only certain physicians may order NPWT before it can be initiated.
There are currently 6 V.A.C. therapy systems for about 10,000 home care recipients in the Edmonton Capital Health Authority. (46) Home care nurses must assess the wound and the feasibility of supplying the therapy in the home before NPWT can be initiated. The Edmonton Capital Health Authority criteria stipulate that the following:
The request for NPWT is made 48 hours before patient is discharged from hospital.
NPWT will be used for at least 1 week.
Concerns about client adherence are satisfied. (The patient is medically stable.)
If the patient has a pressure ulcer, supports are in place in the home to help wound healing.
A physician is available and willing to discuss the patient’s progress with the home care nurse.
Patients with acute wounds are given priority for NPWT over those with pressure wounds. This is because, according to Capital Health Authority experience, acute wounds heal better with NPWT. The mean length of NPWT is about 30 days, but it varies according to indication. (47) According to 1 home health care provider, about 7% of the home care population in the Capital Health Authority has wounds, and a small proportion of these will be eligible for NPWT. This is based on the Capital Health Authority’s in-house prevalence and tracking studies (Personal communication January, 2005).
In continuing care, NPWT is available only in certain facilities. A wound care coordinator is required to start therapy.
In Edmonton, the NPWT vendor has provided much of the education and clinical support for the therapy. Finally, NPWT should not impede hospital transfer or discharge as “other methods of wound care can be used in place of NPWT.” (46)
Discussion with Wound Care Experts
In late 2004, a multidisciplinary group of clinical experts met with government health officials to discuss the use of NPWT in particular, and wound care in general, in Ontario. This discussion elicited the following information:
The use of NPWT varies across the province. There are providers that typically use NPWT for their patients and those that never use it to treat wounds.
Wound care expertise varies widely among community nurses across Ontario.
The types of treatment for wounds vary widely across the province.
Wound care education for nurses is not standardized across the province.
Best-practice protocols for wound care in general, and NPWT in particular, have been developed. Provincial health care provider groups are using them, but not systematically.
Community nurses typically do not have access to medical support in their wound care decisions.
Primary care physicians may rely on the expertise of nurses to manage chronic wounds for their patients in the community. This point was empirically shown in a recent Canadian survey, (3) which suggested 61% of primary care physicians that responded to the survey did not know enough about wound care, while 58% reported that they rely on community nurses for up-to-date treatments.
Current funding schemes and processes for wound care may not be conducive to providing the most optimal care for patients.
There is a need for a seamless management and continuous patient wound care strategy in Ontario.
Appraisal
Considerations
Diffusion – International, National, Provincial
NPWT is used internationally.
380 V.A.C. therapy systems per day were rented in Ontario in 2004.
In Ontario, 40% (152 units) of V.A.C. rentals are in a home care setting, 29% (110 units) are in the hospital, and 27% (103 units) are in long-term care centres.
In hospitals, NPWT is paid for through global budgets. Ward nurses or enterostomal therapists usually administer it. In long-term care settings, NPWT is paid for through a high-intensity needs-based funding plan. External enterostomal therapists do most of the patient care.
In home care settings, NPWT is funded through the CCAC base budget. External agency nurses administer and monitor wound care for patients.
The manufacturer provides NPWT training and clinical assistance to providers, if necessary.
NPWT requires dressing changes 3 times a week for uninfected wounds. Traditional dressings may need to be changed once or twice a day or more.
Target Population
The prevalence of leg ulcers in Ontario may range from 0.12% to 0.32%, or from 15,600 to 41,600 people (based on an Ontario population of 13 million).
The prevalence of pressure ulcers in Ontario acute and/or long-term care settings may be as high as 6,000 cases.
The number of people in Ontario who have chronic wounds is not known.
Patient Outcomes – Medical, Clinical
Based on the available evidence, the effectiveness of NPWT remains unproven.
No studies have compared NPWT with conventional treatment using the same NPWT foam (i.e., without the negative pressure).
-
According to Ontario clinical expertise:
Patients should not be offered NPWT as a first line of wound treatment.
Saline dressings are not considered the standard treatment of choice in Ontario; therefore, the current literature base is not relevant to the experience of Ontario providers.
Some current guidelines suggest that if NPWT does not elicit at least a 30% reduction in wound size in 4 weeks of treatment, it should be discontinued. No protocols identify the patients who might benefit the most from NPWT.
In general, there is little focus in the literature on the provision of whole patient care (e.g., treatment of the cause and nutritional status).
Best-practice guidelines suggest that NPWT may be used as one of a series of adjunctive, secondary therapies, after whole patient care and wound care dressings have failed.
System Pressures
CCACs: These agencies assume the burden of community wound care management.
Long-term care homes: These also assume the burden of community wound care management.
External nursing agencies: These are hired by the CCACs and long-term care homes to manage wound care in the community. Education and clinical support remain the responsibility of these agencies. Standardized approaches to wound care management are not in place in the province.
Family physicians: It is not clear what their role is in wound care in Ontario.
The approach to wound treatment in Ontario does not appear to be integrated within the broader context of care of other chronic conditions. There is no multidisciplinary approach to wound care management or continuity of patient care in Ontario.
Stakeholder Analysis
Patients: NPWT must be activated for at least 22 hours per day to achieve optimal outcomes (Personal communication November, 2004). This could lead to quality of life issues for patients.
Nurses: There is little medical support for community nurses. Standardized wound care training is not systematically available for nurses across the province.
Family physicians: They rely heavily on the expertise of nurses to treat chronic wounds.
Wound care specialists: They typically see patients after treatment in the community has failed.
Conclusion
Based on the evidence, the clinical effectiveness of NPWT to heal chronic wounds is unproven. Furthermore, saline dressings are not the standard of practice for first line treatment in Ontario, thereby rendering the literature base irrelevant in an Ontario context. Nonetheless, despite the lack of methodologically sound studies, NPWT use has diffused across Ontario.
Discussions with Ontario clinical experts have highlighted some deficiencies in the current approach to chronic wound management, especially in the community. Because NPWT is readily available and easy to administer compared with multiple daily conventional dressing changes, it may be used inappropriately. The discussion group highlighted the need to put in place a coordinated, multidisciplinary strategy for wound care in Ontario to ensure the best, continuous care of patients.
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Negative pressure wound therapy: an evidence-based analysis. Ontario Health Technology Assessment Series 2006;6(14).
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL:www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-4327-2 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practicing medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
Abbreviations
- AHRQ
Agency for Healthcare Research and Quality
- ASERNIP-S
Australian Safety and Efficacy Register of New Interventional Procedures
- CCAC
Community care access centre
- CCOHTA
Canadian Coordinating Office for Health Technology Assessment
- CI
Confidence interval
- FDA
United States Food and Drug Administration
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- NPWT
Negative pressure wound therapy
- RCT
Randomized controlled trial
- RR
Relative risk
- SEM
Standard error of the mean
- SD
Standard deviation
- V.A.C.
Vacuum-assisted closure
Glossary
- Enterostomal
Relating to or having undergone enterostomy, the creation of a permanent opening into the intestine through the abdominal wall, usually surgically.
- Epithelialization
The process of becoming covered with or converted to epithelium, as in wound healing.
- Exudate
Fluid, cells, and cellular debris that has escaped from blood vessels and has been deposited in tissues or on tissue surfaces, usually due to inflammation.
- Debridement
The removal, often surgical, of foreign material and dead or contaminated tissue from or next to a traumatic or infected lesion until surrounding healthy tissue is exposed.
- Hyperbaric oxygen therapy
A treatment that provides more oxygen (100%) to a person’s body tissues. This is done by placing the patient in an airtight chamber in which the atmospheric pressure is increased. It is used to help heal wounds and for other indications, such as decompression sickness.
- Necrosis
The death of a portion of living tissue or bone that is surrounded by healthy tissue or bone.
- Necrotizing fasciitis
A rare bacterial infection also called flesh-eating disease.
- Osteomyelitis
Inflammation of the bone caused by infection.
- Osteoradionecrosis
The death of bone tissue as an adverse effect of radiation treatment.
- Sternal infection
An infection in the sternum, also called the breastbone.
References
- 1.Graham ID, Harrison MB, Nelson A, Lorimer K, Fisher A. Prevalence of lower-limb ulceration: a systematic review of prevalence studies. Adv Skin Wound Care. 2003;16(6):305–316. doi: 10.1097/00129334-200311000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–387. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 3.Graham ID, Harrison MB, Shafey M, Keast D. Knowledge and attitudes regarding care of leg ulcers. Can Fam Physician. 2003;49:897–902. Survey of family physicians. [PMC free article] [PubMed] [Google Scholar]
- 4.Woodbury MG, Houghton PE. Prevalence of pressure ulcers in Canadian healthcare settings. Ostomy Wound Manage. 2004;50(10):22–38. [PubMed] [Google Scholar]
- 5.Fleck TM, Fleck M, Moidl R, Czerny M, Koller R, Giovanoli P, et al. The vacuum-assisted closure system for the treatment of deep sternal wound infections after cardiac surgery. Ann Thorac Surg. 2002;74(5):1596–1600. doi: 10.1016/s0003-4975(02)03948-6. [DOI] [PubMed] [Google Scholar]
- 6.Clark M, Price PE. Is wound healing a true science or a clinical art? Lancet. 2004;364(9443):1388–1389. doi: 10.1016/S0140-6736(04)17240-1. [DOI] [PubMed] [Google Scholar]
- 7.Sibbald RG, Mahoney J VAC Therapy Canadian Consensus Group. A consensus report on the use of vacuum-assisted closure in chronic, difficult to heal wounds. Ostomy Wound Manage. 2003;49:52–66. [PubMed] [Google Scholar]
- 8.Shilt JS, Yoder JS, Manuck TA, Jacks L, Rushing J, Smith BP. Role of vacuum-assisted closure in the treatment of pediatric lawnmower injuries. J Pediatr Orthop. 2004;24(5):482–487. doi: 10.1097/00004694-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Kranke P, Bennett M, Roeckl-Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds (Cochrane Review) Cochrane Database Syst Rev. 2004. Issue 1. Art. No.: CD004123. DOI: 10.1002/14651858.CD004123. [DOI] [PubMed]
- 10.Medical Services Advisory Committee (MSAC) Hyperbaric oxygen therapy (HBOT). Assessment report [report on the Internet] 2000. Jun 15, 2006.
- 11.Forder R, Clark M. Topical warming for treating chronic wounds (Protocol) Cochrane Database Syst Rev. 2002. Issue 3. Art. No.: CD003947. DOI: 10.1002/14651858.CD003947.
- 12.Fernandez-Chimeno M, Houghton P, Holey L. Electrical stimulation for chronic wounds (Protocol) Cochrane Database Syst Rev. 2004. Issue 1. Art. No.: CD004550. DOI: 10.1002/14651858.CD004550.
- 13.Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: beds, compression, laser therapy, therapeutic ultrasound, electrotherapy, electromagnetic therapy. Health Technol Assess. 2001;5(9) doi: 10.3310/hta5090. [DOI] [PubMed] [Google Scholar]
- 14.Schaum KD. Decision on national coverage of electromagnetic therapy for wounds. Adv Skin Wound Care. 2002;17(6):316–317. doi: 10.1097/00129334-200407000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Simon A. Low level laser therapy for wound healing: an update [report on the Internet] IP22 Information Paper. 2004. May 10, 2006. Edmonton, The Alberta Heritage Foundation for Medical Research (AHFMR)
- 16.Lansdown AB. Nutrition 2: a vital consideration in the management of skin wounds. Br J Nurs. 2004;13(20):1199–210. doi: 10.12968/bjon.2004.13.20.17011. [DOI] [PubMed] [Google Scholar]
- 17.Oapos MS, Cullum N, Majid M, Sheldon T. Systematic review of wound care management: animicrobial agents, diabetic foot ulceration. Health Technol Assess. 2000;4(21) [PubMed] [Google Scholar]
- 18.Schaum KD. 2004 Medicare legislation and regulations impact wound management. Adv Skin Wound Care. 2004;17(3):113–114. doi: 10.1097/00129334-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 19.KCI. The V.A.C. therapy clinical guidelines: a reference source for clinicians [report on the Internet] Kinetic Concepts, Inc. 2005. Jul 1, 2006.
- 20.Birchall L, Street L, Clift H. Developing a trust-wide centralised approach to the use of TNP. J Wound Care. 2002;11:311–314. doi: 10.12968/jowc.2002.11.8.26425. [DOI] [PubMed] [Google Scholar]
- 21.Schaum KD. Medicare Part B negative pressure wound therapy pump policy. [A partner for Medicare Part A PPS]. Home Healthc Nurse. 2002;20(1):57–58. doi: 10.1097/00004045-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Isago T, Nozaki M, Kikuchi Y, Honda T, Nakazawa H. Negative-pressure dressings in the treatment of pressure ulcers. J Dermatol. 2003;30(4):299–305. doi: 10.1111/j.1346-8138.2003.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 23.AETNA. Negative pressure wound therapy [report on the Internet] Aetna. 2006. Mar 23, 2006. Number 0334. Clinical Policy Bulletins.
- 24.Harvard Pilgrim Health Care. TA 6.29 Negative pressure wound healing therapy for wound healing [report on the Internet] Technology Assessment Policy. 2005. Mar 23, 2006. Report No. TA 6.29.
- 25.Cigna. Negative pressure wound therapy/vacuum assisted closure (VAC) for non-healing wounds [report on the Internet] Cigna Healthcare Coverage Position. 2005. Mar 23, 2006.
- 26.Manufacturer and User Facility Device Experience (MAUDE) [database on the Internet] Center for Devices and Radiological Health; U.S. Food and Drugs Administration. Jan 30, 2006. Jun 30, 2006.
- 27.Evans D, Land L. Topical negative pressure for treating chronic wounds (Cochrane Review) Cochrane Database Syst Rev. 2001. Issue 1. Art. No.: CD001898. DOI: 10.1002/14651858.CD001898. [DOI] [PubMed]
- 28.Pham C, Middleton P, Maddern G. Vacuum-assisted closure for the management of wounds: an accelerated systematic review [report on the Internet] ASERNIP-S Report No. 37. 2003. Apr 23, 2006. Australian Safety and Efficacy Register of New Interventional Procedures - Surgical (ASERNIPS)
- 29.Fisher A, Brady B. Vacuum assisted wound closure therapy [report on the Internet] Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA) 2003. May 15, 2006. Issues in Emerging Health Technologies.
- 30.Higgins S. The effectiveness of vacuum assisted closure (VAC) in wound healing [report on the Internet] Clayton, Victoria: Centre for Clinical Effectiveness (CCE) 2003. Apr 15, 2006.
- 31.Samson DJ, Lefevre F, Aronson N. Wound-healing technologies: low-level laser and vacuum-assisted closure [report on the Internet] Evidence report/Technology Assessment No. 111. 2005. Mar 21, 2006. Agency for Healthcare Research and Quality. [PMC free article] [PubMed]
- 32.Costa V, Brophy J, McGregor M. Montreal: McGill University Health Centre (MUHC); Apr 20, 2006. Vacuum-assisted wound closure therapy (V.A.C.®) [report on the Internet] Report No. 19. 2005. [Google Scholar]
- 33.Ontario Health Technology Advisory Committee. Vacuum Assisted Closure Therapy for Wound Care. Medical Advisory Secretariat, editor. Toronto, Ontario: Ministry of Health and Long-Term Care; 2004. Apr 27, 2006. [Google Scholar]
- 34.Armstrong DG, Lavery LA Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366(9498):1704–1710. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]
- 35.Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg. 2004;114(4):917–922. doi: 10.1097/01.prs.0000133168.57199.e1. [DOI] [PubMed] [Google Scholar]
- 36.Moues CM, Vos MC, Van Den Bemd GJCM, Stijnen T, Hovius SER. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 37.Wanner MB, Schwarzl F, Strub B, Zaech GA, Pierer G. Vacuum-assisted wound closure for cheaper and more comfortable healing of pressure sores: a prospective study. Scand J Plast Reconstr Surg Hand Surg. 2003;37(1):28–33. doi: 10.1080/713796078. [DOI] [PubMed] [Google Scholar]
- 38.Ford CN, Reinhard ER, Yeh D, Syrek D, De las MA, Bergman SB, et al. Interim analysis of a prospective, randomized trial of vacuum-assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg. 2002;49(1):55–61. doi: 10.1097/00000637-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Joseph E, Hamori C, Bergman S, Roaf E, et al. A prospective randomized trial of vacuum-assisted closure versus standard therapy of chronic non-healing wounds. Wounds. 2000;12(3):60–67. [Google Scholar]
- 40.McCallon SK, Knight C, Valiulus JP, et al. Vacuum-assisted closure versus saline-moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy Wound Manage. 2000;46(8):28–32. [PubMed] [Google Scholar]
- 41.Genecov DG, Schneider AM, Morywas MJ, et al. A controlled subatmospheric dressing increases the rate of skin graft donor site reepithelialization. Ann Plast Surg. 1998;40(3):219–225. doi: 10.1097/00000637-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 42.GRADE Working Group. Grading quality of evidence and strength of recommendations. Br Med J. 2004;328:1–8. [Google Scholar]
- 43.Food and Drug Administration (FDA) Guidance for Industry: chronic cutaneous ulcer and burn wounds developing products for treatment [report on the Internet] U.S. Department of Health and Human Services. 2006. May 17, 2006.
- 44.Armstrong DG, Lavery LA. Negative pressure therapy in diabetic foot wounds. Lancet. 2006;367:725–727. doi: 10.1016/S0140-6736(06)68294-9. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong DG, Attinger CE, Boulton AJ, Frykberg RG, Kirsner RS, Lavery LA, et al. Guidelines regarding negative wound therapy (NPWT) in the diabetic foot. Ostomy Wound Manage. 2004;50(4B Suppl):3S–27S. [PubMed] [Google Scholar]
- 46.Capital Health Authority. Adjunctive therapies. VAC - vacuum assisted closure. Regional Wound Care Guidelines. Edmonton, Alberta: Capital Health; 2001. [Google Scholar]
- 47.Phillips DE, Rao SJ. Negative pressure therapy in the community: Analysis of outcomes. Wound Care Canada. 2003;2(1):42–45. [Google Scholar]


