Executive Summary
Issue
Systematic reviews and analyses of administrative data were performed to determine the appropriate use of bone mineral density (BMD) assessments using dual energy x-ray absorptiometry (DXA), and the associated trends in wrist and hip fractures in Ontario.
Background
Dual Energy X-ray Absorptiometry Bone Mineral Density Assessment
Dual energy x-ray absorptiometry bone densitometers measure bone density based on differential absorption of 2 x-ray beams by bone and soft tissues. It is the gold standard for detecting and diagnosing osteoporosis, a systemic disease characterized by low bone density and altered bone structure, resulting in low bone strength and increased risk of fractures. The test is fast (approximately 10 minutes) and accurate (exceeds 90% at the hip), with low radiation (1/3 to 1/5 of that from a chest x-ray). DXA densitometers are licensed as Class 3 medical devices in Canada. The World Health Organization has established criteria for osteoporosis and osteopenia based on DXA BMD measurements: osteoporosis is defined as a BMD that is >2.5 standard deviations below the mean BMD for normal young adults (i.e. T-score <–2.5), while osteopenia is defined as BMD that is more than 1 standard deviation but less than 2.5 standard deviation below the mean for normal young adults (i.e. T-score< –1 & ≥–2.5). DXA densitometry is presently an insured health service in Ontario.
Clinical Need
Burden of Disease
The Canadian Multicenter Osteoporosis Study (CaMos) found that 16% of Canadian women and 6.6% of Canadian men have osteoporosis based on the WHO criteria, with prevalence increasing with age. Osteopenia was found in 49.6% of Canadian women and 39% of Canadian men. In Ontario, it is estimated that nearly 530,000 Ontarians have some degrees of osteoporosis. Osteoporosis-related fragility fractures occur most often in the wrist, femur and pelvis. These fractures, particularly those in the hip, are associated with increased mortality, and decreased functional capacity and quality of life. A Canadian study showed that at 1 year after a hip fracture, the mortality rate was 20%. Another 20% required institutional care, 40% were unable to walk independently, and there was lower health-related quality of life due to attributes such as pain, decreased mobility and decreased ability to self-care. The cost of osteoporosis and osteoporotic fractures in Canada was estimated to be $1.3 billion in 1993.
Guidelines for Bone Mineral Density Testing
With 2 exceptions, almost all guidelines address only women. None of the guidelines recommend blanket population-based BMD testing. Instead, all guidelines recommend BMD testing in people at risk of osteoporosis, predominantly women aged 65 years or older. For women under 65 years of age, BMD testing is recommended only if one major or two minor risk factors for osteoporosis exist. Osteoporosis Canada did not restrict its recommendations to women, and thus their guidelines apply to both sexes. Major risk factors are age greater than or equal to 65 years, a history of previous fractures, family history (especially parental history) of fracture, and medication or disease conditions that affect bone metabolism (such as long-term glucocorticoid therapy). Minor risk factors include low body mass index, low calcium intake, alcohol consumption, and smoking.
Current Funding for Bone Mineral Density Testing
The Ontario Health Insurance Program (OHIP) Schedule presently reimburses DXA BMD at the hip and spine. Measurements at both sites are required if feasible. Patients at low risk of accelerated bone loss are limited to one BMD test within any 24-month period, but there are no restrictions on people at high risk. The total fee including the professional and technical components for a test involving 2 or more sites is $106.00 (Cdn).
Method of Review
This review consisted of 2 parts. The first part was an analysis of Ontario administrative data relating to DXA BMD, wrist and hip fractures, and use of antiresorptive drugs in people aged 65 years and older. The Institute for Clinical Evaluative Sciences extracted data from the OHIP claims database, the Canadian Institute for Health Information hospital discharge abstract database, the National Ambulatory Care Reporting System, and the Ontario Drug Benefit database using OHIP and ICD-10 codes. The data was analyzed to examine the trends in DXA BMD use from 1992 to 2005, and to identify areas requiring improvement.
The second part included systematic reviews and analyses of evidence relating to issues identified in the analyses of utilization data. Altogether, 8 reviews and qualitative syntheses were performed, consisting of 28 published systematic reviews and/or meta-analyses, 34 randomized controlled trials, and 63 observational studies.
Findings of Utilization Analysis
Analysis of administrative data showed a 10-fold increase in the number of BMD tests in Ontario between 1993 and 2005.
OHIP claims for BMD tests are presently increasing at a rate of 6 to 7% per year. Approximately 500,000 tests were performed in 2005/06 with an age-adjusted rate of 8,600 tests per 100,000 population.
Women accounted for 90 % of all BMD tests performed in the province.
In 2005/06, there was a 2-fold variation in the rate of DXA BMD tests across local integrated health networks, but a 10-fold variation between the county with the highest rate (Toronto) and that with the lowest rate (Kenora). The analysis also showed that:
With the increased use of BMD, there was a concomitant increase in the use of antiresorptive drugs (as shown in people 65 years and older) and a decrease in the rate of hip fractures in people age 50 years and older.
Repeat BMD made up approximately 41% of all tests. Most of the people (>90%) who had annual BMD tests in a 2-year or 3-year period were coded as being at high risk for osteoporosis.
18% (20,865) of the people who had a repeat BMD within a 24-month period and 34% (98,058) of the people who had one BMD test in a 3-year period were under 65 years, had no fracture in the year, and coded as low-risk.
Only 19% of people age greater than 65 years underwent BMD testing and 41% received osteoporosis treatment during the year following a fracture.
Men accounted for 24% of all hip fractures and 21 % of all wrist fractures, but only 10% of BMD tests. The rates of BMD tests and treatment in men after a fracture were only half of those in women.
In both men and women, the rate of hip and wrist fractures mainly increased after age 65 with the sharpest increase occurring after age 80 years.
Findings of Systematic Review and Analysis
Serial Bone Mineral Density Testing for People Not Receiving Osteoporosis Treatment
A systematic review showed that the mean rate of bone loss in people not receiving osteoporosis treatment (including postmenopausal women) is generally less than 1% per year. Higher rates of bone loss were reported for people with disease conditions or on medications that affect bone metabolism. In order to be considered a genuine biological change, the change in BMD between serial measurements must exceed the least significant change (variability) of the testing, ranging from 2.77% to 8% for precisions ranging from 1% to 3% respectively. Progression in BMD was analyzed, using different rates of baseline BMD values, rates of bone loss, precision, and BMD value for initiating treatment. The analyses showed that serial BMD measurements every 24 months (as per OHIP policy for low-risk individuals) is not necessary for people with no major risk factors for osteoporosis, provided that the baseline BMD is normal (T-score ≥ –1), and the rate of bone loss is less than or equal to 1% per year. The analyses showed that for someone with a normal baseline BMD and a rate of bone loss of less than 1% per year, the change in BMD is not likely to exceed least significant change (even for a 1% precision) in less than 3 years after the baseline test, and is not likely to drop to a BMD level that requires initiation of treatment in less than 16 years after the baseline test.
Serial Bone Mineral Density Testing in People Receiving Osteoporosis Therapy
Seven published meta-analysis of randomized controlled trials (RCTs) and 2 recent RCTs on BMD monitoring during osteoporosis therapy showed that although higher increases in BMD were generally associated with reduced risk of fracture, the change in BMD only explained a small percentage of the fracture risk reduction.
Studies showed that some people with small or no increase in BMD during treatment experienced significant fracture risk reduction, indicating that other factors such as improved bone microarchitecture might have contributed to fracture risk reduction.
There is conflicting evidence relating to the role of BMD testing in improving patient compliance with osteoporosis therapy.
Even though BMD may not be a perfect surrogate for reduction in fracture risk when monitoring responses to osteoporosis therapy, experts advised that it is still the only reliable test available for this purpose.
A systematic review conducted by the Medical Advisory Secretariat showed that the magnitude of increases in BMD during osteoporosis drug therapy varied among medications. Although most of the studies yielded mean percentage increases in BMD from baseline that did not exceed the least significant change for a 2% precision after 1 year of treatment, there were some exceptions.
Bone Mineral Density Testing and Treatment After a Fragility Fracture
A review of 3 published pooled analyses of observational studies and 12 prospective population-based observational studies showed that the presence of any prevalent fracture increases the relative risk for future fractures by approximately 2-fold or more. A review of 10 systematic reviews of RCTs and 3 additional RCTs showed that therapy with antiresorptive drugs significantly reduced the risk of vertebral fractures by 40 to 50% in postmenopausal osteoporotic women and osteoporotic men, and 2 antiresorptive drugs also reduced the risk of nonvertebral fractures by 30 to 50%. Evidence from observational studies in Canada and other jurisdictions suggests that patients who had undergone BMD measurements, particularly if a diagnosis of osteoporosis is made, were more likely to be given pharmacologic bone-sparing therapy. Despite these findings, the rate of BMD investigation and osteoporosis treatment after a fracture remained low (<20%) in Ontario as well as in other jurisdictions.
Bone Mineral Density Testing in Men
There are presently no specific Canadian guidelines for BMD screening in men. A review of the literature suggests that risk factors for fracture and the rate of vertebral deformity are similar for men and women, but the mortality rate after a hip fracture is higher in men compared with women. Two bisphosphonates had been shown to reduce the risk of vertebral and hip fractures in men. However, BMD testing and osteoporosis treatment were proportionately low in Ontario men in general, and particularly after a fracture, even though men accounted for 25% of the hip and wrist fractures. The Ontario data also showed that the rates of wrist fracture and hip fracture in men rose sharply in the 75- to 80-year age group.
Ontario-Based Economic Analysis
The economic analysis focused on analyzing the economic impact of decreasing future hip fractures by increasing the rate of BMD testing in men and women age greater than or equal to 65 years following a hip or wrist fracture. A decision analysis showed the above strategy, especially when enhanced by improved reporting of BMD tests, to be cost-effective, resulting in a cost-effectiveness ratio ranging from $2,285 (Cdn) per fracture avoided (worst-case scenario) to $1,981 (Cdn) per fracture avoided (best-case scenario). A budget impact analysis estimated that shifting utilization of BMD testing from the low risk population to high risk populations within Ontario would result in a saving of $0.85 million to $1.5 million (Cdn) to the health system. The potential net saving was estimated at $1.2 million to $5 million (Cdn) when the downstream cost-avoidance due to prevention of future hip fractures was factored into the analysis.
Other Factors for Consideration
There is a lack of standardization for BMD testing in Ontario. Two different standards are presently being used and experts suggest that variability in results from different facilities may lead to unnecessary testing. There is also no requirement for standardized equipment, procedure or reporting format. The current reimbursement policy for BMD testing encourages serial testing in people at low risk of accelerated bone loss. This review showed that biannual testing is not necessary for all cases. The lack of a database to collect clinical data on BMD testing makes it difficult to evaluate the clinical profiles of patients tested and outcomes of the BMD tests. There are ministry initiatives in progress under the Osteoporosis Program to address the development of a mandatory standardized requisition form for BMD tests to facilitate data collection and clinical decision-making. Work is also underway for developing guidelines for BMD testing in men and in perimenopausal women.
Conclusion
Increased use of BMD in Ontario since 1996 appears to be associated with increased use of antiresorptive medication and a decrease in hip and wrist fractures.
Data suggest that as many as 20% (98,000) of the DXA BMD tests in Ontario in 2005/06 were performed in people aged less than 65 years, with no fracture in the current year, and coded as being at low risk for accelerated bone loss; this is not consistent with current guidelines. Even though some of these people might have been incorrectly coded as low-risk, the number of tests in people truly at low risk could still be substantial.
Approximately 4% (21,000) of the DXA BMD tests in 2005/06 were repeat BMDs in low-risk individuals within a 24-month period. Even though this is in compliance with current OHIP reimbursement policies, evidence showed that biannual serial BMD testing is not necessary in individuals without major risk factors for fractures, provided that the baseline BMD is normal (T-score < –1). In this population, BMD measurements may be repeated in 3 to 5 years after the baseline test to establish the rate of bone loss, and further serial BMD tests may not be necessary for another 7 to 10 years if the rate of bone loss is no more than 1% per year. Precision of the test needs to be considered when interpreting serial BMD results.
Although changes in BMD may not be the perfect surrogate for reduction in fracture risk as a measure of response to osteoporosis treatment, experts advised that it is presently the only reliable test for monitoring response to treatment and to help motivate patients to continue treatment. Patients should not discontinue treatment if there is no increase in BMD after the first year of treatment. Lack of response or bone loss during treatment should prompt the physician to examine whether the patient is taking the medication appropriately.
Men and women who have had a fragility fracture at the hip, spine, wrist or shoulder are at increased risk of having a future fracture, but this population is presently under investigated and under treated. Additional efforts have to be made to communicate to physicians (particularly orthopaedic surgeons and family physicians) and the public about the need for a BMD test after fracture, and for initiating treatment if low BMD is found.
Men had a disproportionately low rate of BMD tests and osteoporosis treatment, especially after a fracture. Evidence and fracture data showed that the risk of hip and wrist fractures in men rises sharply at age 70 years.
Some counties had BMD utilization rates that were only 10% of that of the county with the highest utilization. The reasons for low utilization need to be explored and addressed.
Initiatives such as aligning reimbursement policy with current guidelines, developing specific guidelines for BMD testing in men and perimenopausal women, improving BMD reports to assist in clinical decision making, developing a registry to track BMD tests, improving access to BMD tests in remote/rural counties, establishing mechanisms to alert family physicians of fractures, and educating physicians and the public, will improve the appropriate utilization of BMD tests, and further decrease the rate of fractures in Ontario. Some of these initiatives such as developing guidelines for perimenopausal women and men, and developing a standardized requisition form for BMD testing, are currently in progress under the Ontario Osteoporosis Strategy.
Issue
At the request of the Ontario Health Technology Advisory, the Medical Advisory Secretariat conducted a review of the utilization of and evidence on bone mineral density (BMD) testing for the identification and diagnosis of osteoporosis.
Background
Clinical Need
Bone mineral density testing has been used to detect and diagnose osteoporosis, a systemic skeletal disease characterized by low bone mass and micro-architectural deteriorations of bone tissue, with a consequent decrease in bone strength and increased susceptibility to fracture (1)
Burden of Illness
Extrapolating the age-specific rates to Canada, Goeree et al. (2) estimated that in 1993, approximately 1.8 million Canadian females had osteoporosis, particularly postmenopausal and elderly women. The Canadian Multicentre osteoporosis Study (CaMos) estimated that 16% of Canadian women and 6.6% of Canadian men have osteoporosis as defined by the World Health Organization. Osteopenia was found in 49.6% of Canadian women and 39% of Canadian men. (3)
Osteoporosis predisposes individuals to fragility (low trauma) fractures, defined as fractures relating to a fall from the standing position. (4) Fracture sites most likely to be associated with osteoporosis are the pelvis, spine, wrist, proximal femur, and proximal humerus. Vertebral fractures in the thoracic or lumbar spine are highly suggestive of osteoporosis, but are frequently undetected because they are asymptomatic. Colle’s fracture appears 10 years before hip fracture, and is a determining factor for hip fracture. (5;6)
Recent data suggest that approximately 3% of Canadians over the age of 25 sustain a fragility fracture each year, with the majority of serious fractures occurring in people over age 50 years. The risk of fragility fractures is particularly high in women. In many Western countries, the remaining life-time risk of a hip fracture in white women at the age of menopause was estimated to lie between 15 and 17%, with the remaining life-time risk for all fractures reaching 30 to 40%. (7) Fractures occurring at the spine and the forearm are associated with significant morbidity, while hip fractures are associated with significant increase in mortality. (7) Papadimitroupoulos et al. (8) reported in 1997 that the incidence of hip fracture and death rates during acute hospitalization in Canada increased exponentially with increasing age, and projected an increase in the number of age-adjusted hip fractures from 23,375 in 1993-1994, to 88,124 in 2041. A similar trend was observed for the province of Ontario. Jaglal et al. (9) reported in 1996 that between 1981 and 1992, the overall hip fracture rate in Ontario (based on hospital discharge data) was 3.3 per 1,000 persons (4.6 per 1,000 women vs 1.7 per 1,000 men). With the aging of the population, Jaglal et al. (9) projected that the number of hip fractures in Ontario would double by 2010, and that hospital bed-days due to hip fracture would increase by 84%, from 214,000 in 1990 to 393,000 in 2010.
Osteoporotic fractures in the elderly population are associated with higher mortality than in the general population (10;11) The mortality rate reaches 20% in the year after a hip fracture, and 20% of the survivors will eventually require long-term care in an institution. (2) A 2001 Ontario study reported that among community dwelling people who had a hip fracture, only 59.4% resided in the community 1 year following a hip fracture, and 5.6% of people who survived their first fracture experienced a subsequent hip fracture. (12)
Fragility fractures also have a significant impact on a person’s functional capacity and quality of life. A Canadian study reported that 40% of people were still unable to walk independently one year following a hip fracture. (13) Adachi et al. (14) reported a negative association between past osteoporotic fractures and health-related quality of life (HRQL) in both women and men that was dependent on fracture type and gender. For example, HRQL was significantly lower in both women and men who had experienced a hip fracture or a rib fracture compared with people without these fractures. The same study also found that women who had a past clinical vertebral deformity or a fracture in the lower limb had lower HRQL, largely because of pain, decreased mobility, and impaired ability for self-care, while a fracture in the lower limb was associated with decreased dexterity in men. Adachi et al. (14) reported that even a subclinical vertebral deformity in women was related to decreased cognition and increased pain, resulting in a lower HRQL.
Goeree et al. (2) estimated that osteoporosis and osteoporotic fractures in people 45 years and older cost the Canadian health system $1.3 billion (Cdn) in 1993, including $465 million in acute health care, $563 million in long-term care, and $279 million for chronic care hospitals. Based on the above estimation and population, Ontario’s proportion of the total osteoporosis cost would be approximately $400 million. The greatest portion of the cost was attributed to hip fractures. (2) In a 2001 Ontario study, the same investigators estimated the mean 1-year cost of a hip fracture in people aged 50 years and older to be $26,527 (Cdn) (95% confidence interval [CI], $24,564 – $28,490). The annual economic impact of hip fractures in Canada was expected to rise from $650 million (Cdn) at the time of the study to $2.4 billion (Cdn) by 2041. (12)
Because of the burden of illness from fractures, attempts are made to identify people at risk of osteoporosis and fractures, and intervene in order to reduce the risk of fractures.
Bone Mass
Low bone mass has been found to be a major risk factor for fragility fractures. Bone is composed of an organic phase of mainly collagen I, an inorganic phase consisting mainly of calcium phosphate crystals, and a cellular component of osteoblasts and osteoclasts. Every year, the human body replaces 10% of its bone mass. Bone resorption occurs at the osteoclasts. Formation of new bone in the osteoblasts involves synthesis of the organic matrix, followed by deposition of calcium crystals, and a gradual maturation process, resulting in an increase in the amount and size of calcium crystals. After reaching peak bone mass at age 25 to 29 years, bone density begins to decline until age 65, and the rate of decline slightly decreases thereafter. (15;16)
Diagnosis of Osteoporosis – World Health Organization
Bone mineral density measurement has been the most common test used to screen for and diagnose osteoporosis. It measures the amount of calcium per unit area (grams/square cm) or per unit volume (grams/cubic cm) in the bone. Results of BMD tests are expressed as T-score and Z-score.
T-score is the number of standard deviations (SD) from the mean BMD for young (25–45 year olds) adults. A T-score of – 2.5 represents a BMD value that is 2.5 SD below the mean BMD for young adults.
Z-score is the number of SDs from the mean BMD value for people of the same age and gender.
T-scores and Z-scores vary according to the technique and reference populations.
Bone mass is a major determinant of bone strength. Laboratory studies have shown a high correlation between bone mineral content and the force needed to break a bone. The World Health Organization (WHO) had established criteria for the diagnosis of osteoporosis in postmenopausal Caucasian women based on BMD and the associated risks of fractures. (17) According to these criteria, a T-score of at least –1 is considered normal; a T-score between –1 and of –2.5 indicates below normal bone density, and osteoporosis is considered to be present if the T-score is less than –2.5 (Table 1).
Table 1: World Health Organization Criteria for Osteoporosis*.
| T-Score: World Health Organization Criteria for Osteoporosis in Women | |
|---|---|
| Normal | BMD > -1.0 below the young adult reference range |
| Low Bone Mass (Osteopenia) | BMD is -1.0 to -2.5 SD below the young adult reference range |
| Osteoporosis | BMD < -2.5 SD below the young adult reference range |
| Severe Osteoporosis | BMD < -2.5 SD below the young adult reference range and the patient has one or more fractures |
BMD refers to bone mineral density
It should be noted that BMD is a continuous value. Despite the WHO definitions, there is no established threshold or cut-off value of BMD to distinguish low-and high-risk people. The WHO committee did not have enough data to create definitions for men or other ethnic groups.
The assessment of BMD has been used for the selection of patients for osteoporosis treatment.
Diagnosis of Osteoporosis for Other Groups
The WHO presently does not have diagnosis criteria for women less than 65 years of age or for men. Osteoporosis Canada is developing guidelines for BMD testing in men and in perimenopausal women (age 40 – 60 years).
In 2004, the International Society for Clinical Densitometry (ISCD) published its official position (18) that included diagnostic definitions for other populations in addition to the WHO classification (Table 2).
Table 2: International Society for Clinical Densitometry Official Position on Definition of Osteoporosis for Men and Premenopausal Women.
| Men | |||
|---|---|---|---|
| Age (years) | T-score | Risk factors for fracture | Diagnosis |
| 50–65 | ≤ 2.5 | Present | Osteoporosis may be diagnosed |
| ≥ 65 years Any age | ≤ 2.5 Low BMD |
Secondary causes or risk factors present | Osteoporosis diagnosed May be diagnosed |
| <50 | Diagnosis cannot be made on basis of densitometric criteria alone. | ||
| Premenopausal women | |||
| Age (years) | Z-score | Risk factors for fracture | Diagnosis |
| 20 - menopause | Low | Secondary causes or risk factors present | Diagnosis may be made |
Premenopausal Women (20 years to menopause)
The ISCD Official Position states that the WHO classification should not be applied to healthy premenopausal women, and Z-scores rather than T-scores should be used. Furthermore, the document further states that the diagnosis of osteoporosis in premenopausal women should not be made on the basis of densitometric criteria alone. (18)
Children
The Official position states that the WHO classification and T-scores should not be applied to children, and Z-scores should be used. The bone density may be described as low for chronological age if the Z-score is below –2.0. However, the ISCD cautions that the diagnosis of osteoporosis in children should not be made on the basis of densitometric criteria alone. (18)
Major Risk Factors for Osteoporotic Fractures in Postmenopausal Women
Most studies on risk factors for fragility fractures were conducted in postmenopausal women.
Canadian study
The Canadian Multicentre Osteoporosis Study (CaMos) followed 5,143 postmenopausal Canadian women for 3 years and analyzed the association of potential risk factors for incident fractures. Papaioannou et al.(19) reported the following findings of this study:
Low BMD was associated with increased fracture risk. The strength of the association was strongest for measurements at the femoral neck (Relative risk [RR] 2.729, 95% confidence interval [CI]: 1.742 to 4.275 for vertebral fracture and RR 1.389, 95% CI: 1.06 to 1.816 for main nonvertebral fractures).
A previous fracture was associated with increased future fracture risk. A prevalent vertebral fracture or a prevalent forearm fracture predicts future fragility fractures with an RR greater than 2. (This will be discussed in greater detail in the systematic review section.)
A 5-point lower quality of life as measured by the physical component of SF-36, was associated with increased risk of incident vertebral fractures, main nonvertebral fractures, and all nonvertebral fractures.
Height was associated with an increased risk of any nonvertebral fractures, whereas change in height was associated with vertebral fracture risks.
Weight loss was associated with increased risk of main nonvertebral fractures. Comorbid conditions also increased the risk of any nonvertebral fractures (RR 3.084, 95% CI 1.560 to 60.99 for kidney disease and RR 1.683, 95% CI: 1.084 to 2.613) for inflammatory bowel disease.
Other primary studies support the following as risk factors for osteoporotic fractures in postmenopausal women:
Bone Mineral Density
A low baseline BMD has been found to be a strong predictor of fragility fractures. A T-score of-2 in the spine in women is associated with a 4- to 6-fold increase in the risk of new vertebral fractures, (20) and every SD below the mean femoral neck BMD for young adults increases the age-adjusted risk of hip fracture by 2.6 (21) However, classifying postmenopausal women as osteoporotic based on BMD alone only accounted for 18% of the documented osteoporotic fractures in the National Osteoporosis Risk Assessment, (22) suggesting that other risk factors need to be used to identify women with BMD above the osteoporotic threshold but who may be at high risk of fractures.
Age
Age is another determinant of risk of fracture independent of BMD. Kanis et al. (23) showed that the same T-score is associated with a much higher risk of fracture at a more advanced age. For example a T-score of –2.5 at age 70 years is associated with a 10-year risk of osteoporotic fracture of 24% compared with 12% at age 50 years with the same T-score.
History of Previous Fractures
This risk factor will be discussed in greater detail in the literature review section.
Family history of fractures
Increased risk of fracture has been reported for people with a maternal history of fractures, particularly hip fractures. This effect appears to be most pronounced on future hip fractures. For example, Taylor et al. (24) reported in the Study of Osteoporotic Fractures that in elderly white women, a history of maternal hip fracture increased the risk for subsequent hip fracture independent of BMD (hazard ratio [HR] adjusted for BMD 1.35, 95% CI, 1.14–1.5]). Albrand et al. (25) reported in the Os des Femmes de Lyon (OFELY) cohort study that the odds ratio (OR) for 5-year risk of fragility fractures was 1.77 (95% CI, 1.01–3.09, P = .04) in healthy postmenopausal women who had a maternal history of fragility fractures. However, Bensen et al. (26) found that maternal history of fracture was a significant predictor of future fractures only at the rib (OR, 2.89; 95% CI, 1.035–8.081).
Propensity to falls
A tendency to fall has been identified as a predominant nonskeletal predictor of fragility fractures in the elderly. (27) It has been reported that about 90% of hip fractures involve falls. (28) Kaptoge et al. (29) found in the prospective multinational Europian Prospective Osteoporosis Study (EPOS) that BMD appeared to be less important in explaining variations in incidence of upper limb fractures in women across diverse populations in Europe, compared with the effect of location-specific risks of falling and factors that may be associated with the likelihood of falling. The nature of the fall likely determines the type of fracture, while bone density and factors that increase or attenuate the force of impact of the fall determine whether a fracture will occur when a faller lands on a particular bone. (28) The majority of falls in old age likely result from a combination of factors relating to aging and poor health, such as decreases in muscle strength and function, gait disorders, and loss of balance. (30) Epilepsy, use of seizure medication, Parkinson’s disease, and wearing corrective lenses are factors that tend to be associated with increased risk of pelvis fracture in men and women. (31)
Other risk factors
The reduction in estrogen associated with menopause was found to be the strongest risk factor for osteoporosis in women. Other risk factors for osteoporosis are low body weight (body mass index [BMI]<20 kg/cm2), lack of weight bearing activity, cigarette smoking, low dietary calcium/vitamin D, certain medications (e.g. corticosteroids, chronic anticonvulsant therapy), and some health conditions (e.g. malabsorption syndrome and primary hyperparathyroidism).
All the above risk factors should be used in conjunction with bone mineral measurements to assess an individual’s overall risk of fragility fractures and need for treatment.
Prevention and Treatment of Osteoporosis
Reducing the prevalence of osteoporosis requires preventative life style changes such as increasing dietary intake of vitamin D and calcium, increasing physical activity, smoking cessation, and moderating alcohol intake. Current available treatments for osteoporosis are mainly medications that reduce bone resorption (hormone replacement, bisphosphonates, estrogen receptor modulators, salmon calcitonin, and parathyroid hormone). The effectiveness of these treatments will be discussed in greater detail later.
Because of the silent nature of osteoporosis, early detection and treatment to prevent fractures in people with low BMD has been recommended. As clinical risk factors do not have adequate accuracy to identify patients with osteoporosis, BMD measurements are used in the setting of clinical risk factors for fractures to assess whether there is also low BMD that further increases the risk for fractures. (15)
Technology
There are many techniques for measuring bone mineral density. They fall into 2 main categories: those that use ionizing radiation and those that do not. Ionizing techniques include:
Dual Energy X-Ray Absorptiometry
BMD measurement of the entire skeleton or specific sites such as the spine or hip using x-ray absorptiometry is based on the absorption of x-rays by the calcium crystals in the bone. A dual energy x-ray absorptiometry (DXA) machine sends a thin beam of low-dose x-rays with 2 distinct energy peaks through the bones of patients. One peak is absorbed mainly by soft tissue and the other by bone. BMD is calculated from the difference in absorption between the bone and the soft tissue. Computer software calculates the numerical density of the bone from the image and compares it with the mean of healthy young adults, and to the age-matched control of the reference population. A radiologist interprets the data and creates a concise report on the patient’s bone density status. The BMD test with DXA takes approximately 10 to 30 minutes, and the dose of radiation received by the patient is equivalent to one-fifth to one-half of the dose from a chest x-ray. The accuracy of DXA at the hip exceeds 90%. New developments in DXA include the use of multi-element detector array with true fan-beam, single sweep scanning, and concomitant lateral vertebral assessment to screen for vertebral fractures. Small DXA devices are also available for measuring BMD in the heel or forearm in as little as 15 seconds. The distal radius is often used because it contains trabecular and cortical bone. Presence of osteomalacia or osteoarthritis may result in a high BMD value that does not reflect higher bone strength. (32)
Single X-ray absorptiometry (SXA) is similar to DXA but uses a single beam to measure BMD of the wrist or the heel.
Quantitative Computed Tomography
Quantitative computed tomography (QCT) can be performed on the spine using standard CT devices. QCT assesses 3-dimensional bone density and permits isolated measurement of trabecular bone density; however, QCT is not widely used because its reproducibility is poor and it exposes patients to far too high a radiation dose to be acceptable. The clinical utility of smaller peripheral QCT devices is also being investigated.
Other ionizing radiation techniques include radiograph of proximal phalanges, single photon absorptiometry (SPA) and double photon absorptiometry (DPA). These methods are no longer in use for BMD measurements. (personal communication, clinical expert, August 2006)
Techniques that do not use ionizing radiation are:
Quantitative Ultrasonography)
The transmission of sound through bone reflects its density and structure and can be assessed quantitatively using the speed of sound or broadband ultrasound attenuation. Quantitative ultrasonography (QUS) of the heel resembles other peripheral measurements in terms of ability to predict fractures. QUS is noninvasive, involves no exposure to ionizing radiation, and is less expensive and portable. However, there is a need for normative data, quality assurance programs, standardization, and attention to precision, sensitivity, and accuracy. (33) Experts advised that it has not been widely used because of low precision. (Personal communications October 2006)
Bone Markers
Measurement of biochemical bone markers in the blood may provide information on bone remodelling. Bone markers are specific for bone formation (e.g. bone alkaline phosphatase) or bone resorption (e.g. deoxypyridinoline), and may be influenced by age, gender, ethnicity, menopause status, diseases, recent fractures, immobility, treatment, and timing of sample collection.(34) Bone markers cannot be used to diagnose osteoporosis. However, studies suggest that bone markers used in conjunction with BMD may improve the prediction of fracture risk. (35;36) Since bone marker levels change quickly with the initiation of osteoporosis treatment, they may be used as a surrogate marker for treatment efficacy. The use of bone markers is limited by its variability.
Devices for Measurement of Microscopic Bone
Since microstructure is a determinant of bone strength, techniques are being investigated for measuring this parameter. In recent years, methods of high-resolution magnetic resonance imaging (MRI) are being investigated for the assessment of bone density and its microstructure. (37;38)
A new portable device called a mechanical response tissue analyzer is being investigated as a means of measuring the mechanical properties of the ulna and tibia to reflect both mineral content, and geometry/ structure of the bone. This device is not yet available for clinical use.
Gold Standards for Bone Mineral Density Measurement
Bone mineral density measurement yields different results depending on the technique used and the site of measurement. Correlation between results from different techniques has been poor. Presently, BMD measured with DXA at the hip and/or spine is considered the gold standard for the noninvasive diagnosis of osteoporosis, and has been used by the WHO to define osteoporosis. (17) Diagnosis is based on the lowest BMD obtained. The ISCD (18) recommends that BMD should be measured at both posterior-anterior spine (L1–L4) and hip (proximal femur, femoral neck, or trochanter) in all patients, and forearm (33% radius) BMD should be measured when the hip or spine cannot be measured or interpreted or in cases of hyperparathyroidism or very obese patients. The ISCD indicated that spine BMD should be interpreted with caution in the elderly because degenerative arthritis in the posterior elements of the spine may result in an artifactual increase in measured BMD. Furthermore, the ISCD also stated that the WHO classification for the diagnosis of osteoporosis and osteopenia should not be used with peripheral BMD measurements other than BMD at 33% radius. (18)
Reference Standards for Interpretation of Bone Mineral Density Tests
Based on the WHO definitions of osteoporosis, the T-scores and Z-scores will vary depending on the reference standards used. In Canada and the United States, the densitometers are programmed to use normative data from the United States National Health and Nutrition Examination Survey (NHANES III, a population-based study), for white, black, and Asian subgroups, and for men and women. (16;39;40) Peak bone mass for the Canadian population has been established in the Canadian Multicenter Osteoporosis Study (CaMos) using 10,061 women and men aged 25 years or more randomly selected from 9 regions across Canada. (3) However, this database has not been used routinely in the interpretation of BMD tests. There is controversy over whether thresholds derived from women can be applied to men. Studies have shown that for hip and vertebral fractures, the 10-year risks of fracture are similar in men and women for T-scores close to the diagnostic thresholds, lending support that T-scores derived from women are applicable to men, and that diagnostic thresholds should be the same in men and women. (41)
Reporting of Bone Mineral Density Measurements
Parameters usually included in current BMD reports are shown in Appendix 1. In the Recommendations for Bone Mineral Density Reporting in Canada, the Canadian Association of Radiologists recommended including fracture risk (low, moderate, or high) in BMD reports, stratified by gender and age group, and T-score, as well as a patient questionnaire that identifies the patient’s clinical risk factors. This recommendation aims to integrate BMD measurements with other clinical factors to quantify fracture risk assessment. (42)
Under the Ontario Osteoporosis Strategy, Osteoporosis Canada is submitting guidelines for BMD testing and reporting.
Licensing of Bone Mineral Density Devices in Canada
Health Canada has licensed numerous bone mineral densitometers as class 3 devices. These are summarized in Appendix 2.
Guidelines/Recommendations on Bone Mineral Density Measurements
Many health agencies and jurisdictional governments have developed recommendations for BMD testing. These recommendations are summarized in Appendix 3. None recommended using BMD to screen for osteoporosis in the general population.
Almost all of these recommendations target women over 65 years, and for women younger than 65 years of age, only those with risk factors for osteoporosis are targeted. The British Columbia Guidelines for Bone Density Measurement in Women emphasized that even in the presence of risk factors, BMD measurement should only performed when the results are likely to alter patient care. (43) The Canadian Task Force for Preventive Care recommended the use of risk assessment instruments for case finding, to further identify people at risk who should undergo BMD testing.(44)
Only Osteoporosis Canada (45) and the ISCD made recommendations for men (See section on BMD Testing in Men).
Health Insurance Coverage of Bone Mineral Density Tests in Ontario
Conditions for BMD Tests in the Ontario Health Insurance Plan (OHIP)
In the OHIP Schedule of Benefits (46), only bone mineral testing by axial technique using DXA at the hip/and or spine is an insured service. The conditions for the service to be insured include:
The service is rendered for the prevention and management of osteoporosis or osteopenia
When only one site is measured, because measurement at 2 sites is technically unfeasible due to prosthesis, the site must be either the hip or the spine.
When more than one site is measured, the site must include both the hip and spine
When the patient is a low-risk patient, BMD measurement has not been provided to the patient on an insured basis within the preceding 24 months (start counting from April 1, 1998).
For the purpose of this service, “high-risk patients” means a patient at risk of accelerated bone loss due to either states of high bone turnover such as primary hyperthyroidism and glucocorticoid induced osteopenia, or due to such other conditions as have been determined by the Scientific Advisory Board of the Osteoporotic Society of Canada OSC (presently Osteoporosis Canada) which prevail at the time the service is rendered. “Low-risk patient” means any patient who is not a high-risk patient (Table 3).
Table 3: Ontario Health Insurance Plan Fee Codes and Conditions for DXA Bone Mineral Densotometry.
| Codes for BMD Tests | H (Technical) Fee ($ Cdn) | P (Professional) Fee ($ Cdn) |
|---|---|---|
| Low risk Patients | ||
| X152 (one site) | 43.95 | 41.30 |
| X163 (2 or more sites) | 56.00 | 49.40 |
| For high-risk patients: | ||
| X149 (one site) | 43.95 | 41.30 |
| X155 (2 or more sites) | 56.60 | 49.40 |
| X157 BMD measurement using radiographic technique other than axial DXA | 0.00 | 0.00 |
Source: Ontario Health Insurance (OHIP) Schedule of Benefits and Fees: Schedule of Benefits for Physician Services Under the Health Insurance Act. 2006
The OSC guidelines (47) recommend BMD testing for everyone over the age of 65 years and also for people over age 50 if they have at least one major or two minor risk factors. According to the OSC guidelines:
Major risk factors for osteoporosis include:
Age greater than 65 years
Vertebral compression fractures
Fragility fracture after age 40
Family history of osteoporotic fractures
Medical therapy that affects bone metabolism (e.g. systemic glucocorticoid therapy lasting more than 3 months)
Conditions that affect bone metabolism (primary hyperparathyroidism, malabsorption syndrome, hypogonadism, early menopause)
Propensity to fall
Minor risk factors for osteoporosis include:
Weight less than 57 kg (125 lbs) or weight loss>10% of weight at age 25
Rheumatoid arthritis
Past history of clinical hyperthyroidism
Chronic convulsant therapy or chronic heparin therapy
Low dietary calcium intake
Smoker
Excessive alcohol intake
Excessive caffeine intake
Previously Published Information on the Use of Bone Mineral Density Tests in Ontario
The main purpose of BMD tests is to identify people with osteoporosis and treat them effectively in order to reduce their risk of fragility fractures. Several studies had been published on the use of BMD tests in Ontario. The most recent publication by Jaglal et al. (48) reported that BMD tests in the province increased 10-fold between 1992 and 2001. The same study also suggested that the increase in BMD use was accompanied by an increase in the use of bone sparing medication, and a decrease in the rate of hip fractures and wrist fractures in people aged 65 years or more.
Medical Advisory Secretariat Health Technology and Policy Assessment
There are 2 main parts in this assessment:
Part 1 - Objectives-
To analyze the trend in the utilization of BMD tests in Ontario, particularly since 2001, in order to determine whether BMD tests are being used appropriately in the province (i.e., any gaps and misuses)
To determine whether the concurrent increased use of osteoporotic treatment and decreased rates of fractures in people aged 65 years and over identified in 2001 have persisted.
Part 2 - Objectives
To conduct a literature review relating to gaps and potential misuses.
Analyze information from the literature review to develop recommendations and address the gaps and misuses identified from the analysis of utilization data.
PART 1 - Analyses of Utilization Data
Analyses of administrative data were conducted to update the trend of BMD test utilization in Ontario, and the corresponding trends in the incidence of fragility fractures in the province.
Questions to be addressed by the analyses:
What is the trend in BMD testing in Ontario based on gender, age, and geographic location since 2001?
Is the use of BMD tests consistent with the recommendations of the Canadian Task Force for Preventive Care?
What proportions of BMD tests are repeats and what is the interval between initial and repeat BMD tests?
Are there gaps in access to BMD tests in the province and, if so, what are these gaps?
Is there any inappropriate use of BMD?
What is the current trend in the use of antiresorptive drugs in Ontario since 2003?
Is there any relationship between the use of BMD tests and antiresorptive drugs, and changes in the prevalence of hip and wrist fractures in Ontario since 2001?
Method of data abstraction
The Institute of Clinical Evaluative Sciences (ICES), under the direction of Susan Jaglal, Ph.D., abstracted data from administrative databases. For all data, only Ontario residents were included.
BMD data: Physician claims for DXA BMD measurements for fiscal years 2002/03 to 2005/06 were obtained from the OHIP claims database. OHIP files were linked using a unique identifier to the Registered Persons Database to obtain the age and sex of the patient for each tests performed. This provided the number and rates of BMD tests by sex and 5-year age groups. Only BMD for people aged greater than 40 and less than or equal to 105 years were included in the analysis. Any record of BMD measurements performed in the previous 2 years were obtained for people who had a BMD test in either 2003/04, 2004/05 or 2005/06, in order to examine trends in serial BMD testing. For those with no BMD in the previous 2 years, OHIP records were searched for the last 5 years.
Fracture data: Information on hip fractures was obtained from the Canadian Institute for Health Information (CIHI) hospital discharge abstract database. The age and sex were identified for each person. Fracture rates were reported for 5-year age groups beginning with age 40. Overall fracture rates for each fiscal year were age-adjusted using population estimates from Statistics Canada. Any record of BMD tests (from the OHIP database) and antiresorptive treatment (from the Ontario Drug Benefit [ODB] database) in the first year following the fracture was also obtained for people 65 years of age or older.
All ODB claims for antiresorptive drugs (for people aged ≥ 65 years and ≤ 105 years) were obtained for fiscal years 2002/03 to 2005/06.
The codes and criteria for data abstraction are shown in Appendix 4. The data were analyzed to identify trends in BMD use from 1992 to 2005.
Findings – Use of DXA Bone Mineral Density Assessment in Ontario
Increase in Volume of Bone Mineral Density Tests
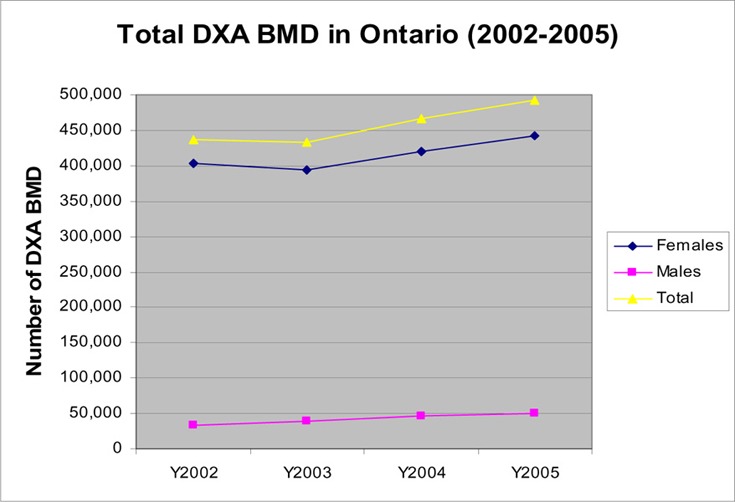
The number of BMD claims increased almost10-fold between 1993 and 2005, reaching 500,000 tests in 2005. Figure 1 shows that the largest increase in BMD testing occurred in 1996/97 (64%) and 1997/98 (56%). A change to OHIP coverage was implemented in October 1999, restricting reimbursement to once in any 24-month period for people at low risk. The use of BMD in 2000/01 increased by 12% compared with an increase of 20% in the previous year. The use of BMD actually decreased slightly in 2003/2004, probably due to restricted access to hospitals during the SARS epidemics in Toronto. In the last 2 fiscal years (2004 & 2005), the volume of BMD tests increased at a rate of 6 to 7% per year (an average increase of 30,000 tests per year). Approximately 90% of all BMD tests were performed in women (Figure 2).
Figure 1: Number of Dual-Energy Absorptiometry Bone Mineral Density Tests in Ontario (Fiscal years 1992/93 –2005/06).
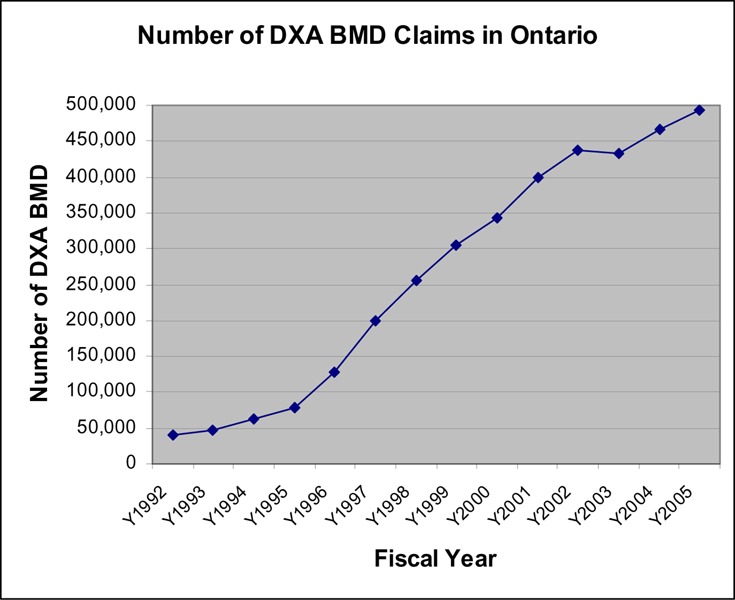
Figure 2: Number of Dual-Energy Absorptiometry Bone Mineral Density Tests in Women and Men (Fiscal years 2002/03 – 2005/06).
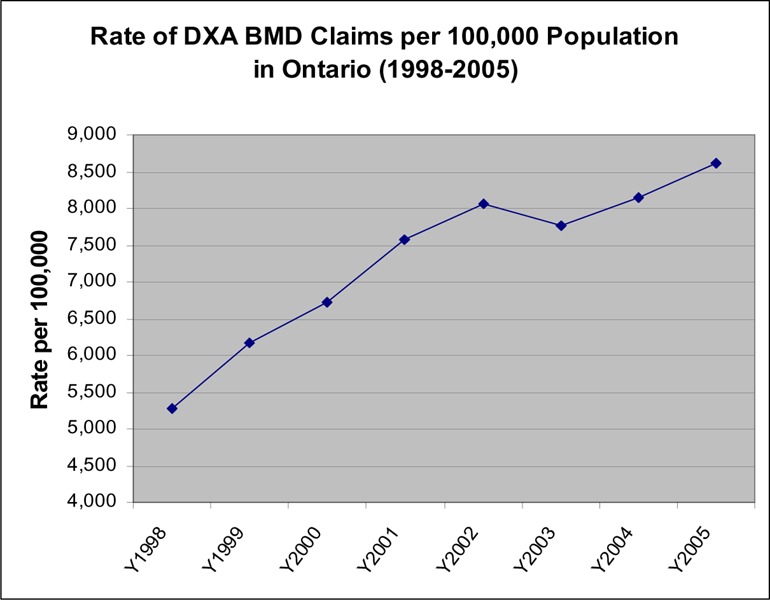
The rate of BMD use per 100,000 population was examined to determine the increase in BMD use independent of population growth. Figure 3 shows that with the exception of 2003/04, the rate of BMD tests has increased steadily and is still increasing in the last 2 fiscal years at a rate of 5% to 6% per year. It was estimated that more than 80% of BMD tests were ordered by family practice physicians.
Figure 3: Age-Adjusted Rate of DXA Bone Mineral Density Claims per 100,000 Population in Ontario (1998/09–2005/06).
Use of Bone Mineral Density Testing in Women
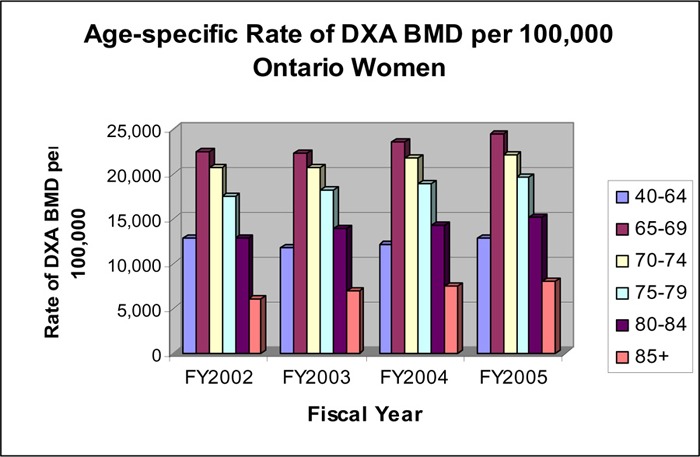
Since women accounted for 90% of all BMD tests, the data pertaining to women were further analyzed. Figure 4 shows that the rate of BMD use was highest in women aged 65 to 69 years, followed by the 70 to 74 and 75 to 79 age groups. The rate was about the same for women under age 40 to 64 years and women aged 80 to 84 years. Women older than age 85 years had the lowest rate of BMD tests. In the most recent 4 fiscal years, the increase in the rate of BMD use occurred in the greater than 65 year age groups, while the rate for the 45 to 65 year age group remained more or less constant.
Figure 4: Age-Specific Rate per 100,000 Ontario Women of Dual-Energy X-Ray Absorptiometry Bone Mineral Density.
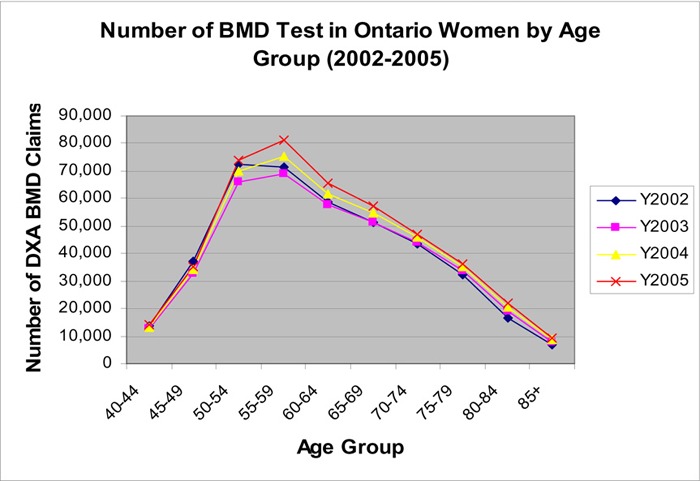
Even though women under age 65 years had the second lowest rate of BMD tests, due to the number of women in this age group, they accounted for 61% of all BMD tests. The highest number of BMD performed was in women in the 55 to 59 and 50 to 55 age groups (Figure 5).
Figure 5: Number of DXA BMD in Ontario Women By Age Groups.
Geographical Variation in Rates of DXA Bone Mineral Density Testing in Ontario
The rate of BMD tests varied across geographical areas. The rates were highest in the Central, Mississauga Halton, Toronto Central, Hamilton Niagara Haldimand Brant, and Central East local health integrated networks (LHINs). LHINs in remote areas had the lowest rates of BMD testing (Erie St. Clair, North East and North West). The greatest geographical variation in rates of BMD testing was between the Central LHIN and the North West LHIN. However, trend analysis shows that this gap is slowly decreasing over time. In 2002, the rate was almost 3-fold higher in the Central LHIN compared with the North West LHIN. In 2003, this gap has decreased to 2.2-fold (Table 4).
Table 4: Rates of Bone Mineral Density Testing per 100,000 Population by Local Health Integrated Network*.
| LHIN | Rate of BMD Tests per 100,000 | |||
|---|---|---|---|---|
| 2002 | 2003 | 2004 | 2005 | |
| Erie St. Clair | 5,175 | 5,244 | 5,334 | 5,496 |
| South West | 6,070 | 5,612 | 5,605 | 6,101 |
| Waterloo Wellington | 7,609 | 7,575 | 7645 | 7898 |
| Hamilton Niagara Haldimand Brant | 9568 | 9346 | 9,591 | 10,020 |
| Central West | 7,819 | 7,562 | 7,918 | 8,600 |
| Mississauga Halton | 9,875 | 9,554 | 9,944 | 10,446 |
| Toronto Central | 9,468 | 9,238 | 9,973 | 10,424 |
| Central | 9,888 | 9,400 | 10,282 | 10,890 |
| Central East | 8,709 | 8,192 | 8,662 | 9,061 |
| South East | 6,407 | 6,028 | 6,173 | 6,655 |
| Champlain | 7,719 | 7,465 | 7,542 | 7,965 |
| North Simcoe Muskoka | 7,032 | 7,008 | 7,501 | 7,574 |
| North East | 5,098 | 5,150 | 5,277 | 5,734 |
LHIN refers to local health integrated network
Jaglal et al. (49) previously reported a 17-fold variation in the BMD rate across counties in 2000. Although the gap in BMD rates across counties had narrowed by 2004, there was still an almost 10-fold variation between the highest rate (203.76 per 1,000 women for Toronto) and the lowest rate (21.04 per 1,000 women for Kenora) (Table 5). The overall rate was 142 per 1,000 women. Most of the more remote or rural counties had lower BMD rates.
Table 5: Age-Adjusted* Rate of Bone Mineral Density per 1,000 Women by County of Referring Physician’s Office for Fiscal 2004.
| County name | Standardized rate per 1,000 women |
|---|---|
| Toronto | 203.76 |
| Halton | 194.43 |
| Hamilton | 181.55 |
| Ottawa | 159.43 |
| Brant | 146.88 |
| York | 146.63 |
| Peel | 143.80 |
| Wellington | 140.77 |
| Frontenac | 137.58 |
| Dufferin | 135.95 |
| Muskoka | 127.39 |
| Haldimand-Norfolk | 126.43 |
| Niagara | 125.36 |
| Waterloo | 124.32 |
| Lanark | 123.68 |
| Durham | 117.58 |
| Parry Sound | 117.04 |
| Simcoe | 115.52 |
| Middlesex | 113.30 |
| Renfrew | 108.04 |
| Bruce | 108.01 |
| Northumberland | 101.85 |
| Peterborough | 101.23 |
| Oxford | 100.79 |
| Lambton | 100.60 |
| Nipissing | 97.58 |
| Timiskaming | 97.26 |
| Sudbury, Greater | 94.38 |
| Essex | 93.27 |
| Thunder Bay | 90.67 |
| Prescott-Russell | 88.12 |
| Hastings | 86.20 |
| Leeds-Grenville | 81.07 |
| Grey | 77.95 |
| Cochrane | 77.45 |
| Huron | 76.82 |
| Lennox-Addington | 75.31 |
| Kawartha Lakes | 74.89 |
| Kent | 73.10 |
| Algoma | 70.43 |
| Haliburton | 70.25 |
| Stormont-Dundas-Glengarry | 68.03 |
| Manitoulin | 61.26 |
| Prince Edward | 59.51 |
| Perth | 57.48 |
| Elgin | 57.27 |
| Sudbury District | 27.61 |
| Rainy River | 25.09 |
| Kenora | 21.04 |
| Overall rate | 141.99 |
Age included: 40 – 105 years
Pattern of Serial Bone Mineral Density Testing
Bone mineral density tests performed in 2003/04, 2004/05, and 2005/06 were analyzed according to the history of BMD tests in the previous 2 years. Four patterns of BMD testing were observed: category 1 includes patients who had a BMD test in the previous year (annually for 2 years); in category 2, a BMD test was performed 2 years prior to the current tests (a repeat test in a 24 month period); category 3 represents people with a BMD test annually 3 years in a row; and category 4 represents people with no BMD in the previous 2 years (Table 6). The distribution of BMD tests among the 4 categories was quite consistent for the 3 fiscal years studied. The data showed that approximately 59% of people tested did not have a BMD test in the previous 2 years, while 41% had a repeat test during a 3-year period. About 17 to 18% had a BMD annually within a 2-year or 3-year period, and 23% to 24% had a repeat BMD test in 2-year period.
Table 6: Stratification of Bone Mineral Density Tests by Pattern of Repeat Testing (2003-2005)*.
| Pattern of Repeat BMD | As % of all Bone Mineral Density Tests | |||||
|---|---|---|---|---|---|---|
| Category | Year 1 | Year 2 | Year 3 | 2003/04 | 2004/05 | 2005/06 |
| 1 | x | x | 10.9% | 10.0% | 10.7% | |
| 2 | x | x | 23.2% | 23.9% | 23.5% | |
| 3 | x | x | x | 7.4% | 7.1% | 6.8% |
| 4 | x | 58.5% | 58.9% | 59.1% | ||
X = BMD performed
The profiles of patients in each of the categories were analyzed according to age (<65 vs ≥65 years), presence or absence of fracture in the most recent year, and the risk for osteoporosis according to the fee code.
For people who had a BMD test in 2005/06 but no BMD in the previous 2 years (category 4), 34% (>98,000 BMD tests) were performed in people under age 65 years, who had no fracture in the year of study, and were also coded as low risk in the OHIP claims (Table 7). Although the risk level of these patients cannot be validated and some might have been miscoded as low risk, even if 50% were coded correctly, it would mean 49,000 BMD tests were performed in people less than 65 years of age and at low risk of osteoporosis and fractures, which is not consistent with current Canadian guidelines for BMD testing.
Table 7: Age and Risk Profile of People According to Pattern of Serial Bone Mineral Density Tests*,∓.
| As % of BMD in the category | |||||||
|---|---|---|---|---|---|---|---|
| Pattern of repeat BMD | Number of BMD (%) in <65 yrs, no fracture & coded as low risk | ||||||
| Category | Year 1 | Year 2 | Year 3 | 2003/04 | 2004/05 | 2005/06 | |
| 1 | x | x | 2,866 (6%) | 2,625 (6%) | 2,900 (6%) | ||
| 2 | x | x | 20,565 (21%) | 21,621 (20%) | 20,865 (18%) | ||
| 3 | x | x | x | 916 (3%) | 935 (3%) | 872 (3%) | |
| 4 | x | 89,186 (35%) | 93,894 (34%) | 98,058 (34%) | |||
| Total | |||||||
X = BMD performed
TBMD refers to bone mineral density.
More than 24,000 BMD tests performed in 2005/06 were repeat BMDs. Of the people who had annual BMD tests for 2 or 3 years (categories 1 or 3), more than 90% were considered high risk (Table 7) and were in compliance with OHIP since there are no restrictions on BMD testing in high-risk people. However, approximately 3,500 annual repeats were performed in people rated as low risk, in contravention to the OHIP conditions, since people at low risk are limited to one BMD in any 24 month period.
In each of the 3 years studied, approximately 21,000 repeat BMD tests within a 24 month period were performed in people under 65 years of age, were coded as low risk, and had no fracture during the year of the study (Table 7). Although these repeat tests were compliant with OHIP reimbursement policies for BMD, these policies were last revised in 1999. The evidence for serial BMD tests every 2 years in people at low risk of osteoporosis needs to be re-examined.
Bone Mineral Density Tests and Treatment After a Fragility Fracture
The percentages of people (age > 65 years) who underwent BMD testing and/or had a ODB claim for osteoporosis drugs during the first year after a fragility fracture are summarized in Table 8, and 1 year after a hip fracture in Table 9. For people who had no BMD during the first year after a fracture, the database was searched to verify that no BMD tests were performed in the 5 years prior to the fracture.
Table 8: Bone Mineral Density Test and Osteoporosis Treatment Within One Year After a Hip or Wrist Fracture (People ≥ age 65 years)*.
| After a Hip or Wrist Fracture | ||
|---|---|---|
| 2003 | 2004 | |
| % of people ≥ age 65 who had a DXA BMD within 1 yr after any fracture | 18.5 | 19.3 |
| % of osteoporosis treatment within 1 year in those who had DXA BMD after fracture | 65.8 | 66 |
| % of osteoporotic treatment within 1 year in those who did not have DXA BMD after fracture | 31.9 | 35 |
| % of people > age 65 who received treatment within 1 year after any fracture | 38.2 | 41 |
| Total number of people age >65 who did not have DXA BMD within 1 yr after any fracture | 8,601 | 9,069 |
BMD refers to bone mineral density; DXA, dual-energy x-ray absorptiometry.
Table 9: Osteoporosis Treatment Within One Year After a Hip Fracture (People ≥ age 65 years)*.
| After a Hip Fracture | ||
|---|---|---|
| 2003 | 2004 | |
| % of people ≥ age 65 who had a DXA BMD within 1 yr after a hip fracture | 12.5 | 12.6 |
| % of osteoporosis treatment within 1 year in those who had DXA BMD after a hip fracture | 72.9 | 75 |
| % of osteoporosis treatment within 1 year in those who did not have DXA BMD after hip fracture | 36.8 | 41 |
| % of people > age 65 who received osteoporosis treatment within 1 year after a hip fracture | 41.3 | 45 |
| Total number of people age >65 who did not have DXA BMD within 1 yr after any fracture | 4,432 | 4,503 |
BMD refers to bone mineral density; DXA, dual-energy x-ray absorptiometry.
The analysis showed that only approximately 19% of people who had a fragility fracture in 2003 or 2004 had a BMD test within the first year following the fracture or in the previous 5-year period, and approximately 40% received antiresorptive pharmacologic treatment during the 1-year period. This means that 81% of patients age 65 years and older did not have bone density assessment following a fragility fracture, representing approximately 9,100 people in 2004/2005. Patients were more likely to receive antiresorptive treatment if they had a BMD assessment after fracture compared with those who did not (64% vs 35%) (Table 8). Approximately 3,200 people had ODB prescription for antiresorptive drugs in the year after a hip or wrist fracture without any baseline BMD measurements (after fracture or in the previous 5 years).
The percentage of people who had a BMD test during the first year following a hip fracture was even lower (approximately 13%) while the percentage that received antiresorptive treatment in the same period was 45%. Approximately 4,500 patients who had a hip fracture did not undergo a BMD assessment 1 year after the fracture. The rate of treatment was higher in patients who had a BMD test compared with patients with no BMD test after the hip fracture (75% vs 41%) (Table 9).
Gender Disparity in Bone Mineral Density Testing and Treatment
Due to greater bone mass, osteoporotic fractures tend to occur later in men compared with women. Osteoporosis is being recognized as a growing concern in men because of the increasing average life expectancy in men. Although the prevalence of osteoporosis (as defined by WHO) in Canadian men 50 years of age and over is 5% compared with 16% in women of the same age, osteoporotic fracture in men results in higher mortality rates than in women (Appendix 5). Standardized mortality ratios for people who have had major fractures compared with the general population ranges from 2.3 to 3.2 for men, and from 1.7 to 2.2 for women. (50) Osteoporotic fractures in men also results in increased morbidity and reduced quality of life due to decreases in mobility, independent living, and dexterity.(48) Moreover, radiographic studies show that the prevalence of vertebral deformity (fracture) is about 25% in both genders.
Current utilization data suggests that BMD tests have been underutilized as a screening tool in men. Even though men accounted for about 24% of hip fractures and 21% of wrist fractures in people aged 50 years and older (2004 & 2005), only 10% of all BMDs were performed in men. Men were also less likely than women to undergo BMD measurements (13 % vs 21%) or receive antiresorptive treatment (20% vs 46%) following a fracture (Table 10).
Table 10: Percentage of Men and Women ≥ Age 65 Who Had a Bone Mineral Density Test or Received Antiresorptive Treatment One Year After a Fracture.
| 2003 | 2004 | |||
|---|---|---|---|---|
| Men (%) | Women (%) | Men (%) | Women (%) | |
| % of all hip fractures | 24 | 76 | 24 | 76 |
| % of all wrist fractures | 21 | 79 | 21 | 79 |
| % of all BMD claims | 9.8 | 90.2 | 10.3 | 89.7 |
| Within 1 year after Any Fracture | ||||
| % who received BMD tests | 12 | 20 | 13 | 20 |
| % who had antiresorptive Treatment | 19 | 43 | 20 | 46 |
Use of Antiresorptive Medication in Ontario
Figure 6 shows that the number of Ontarians aged 65 years and older who filled prescriptions for antiresorptive medications has increased steadily since 1996 The rate of increase in the number of people was 12.2% and 9.2% for 2004/05 and 2005/06 respectively, reaching a peak of more than 280,000 people, and 1.626 million prescriptions in 2005/06. The type and percentage of the different antiresorptive drugs prescribed for people receiving ODB coverage are summarized in Table 11.
Figure 6: Number of Men & Women Age ≥ 65 Filling Prescriptions for Antiresorptive Medications.
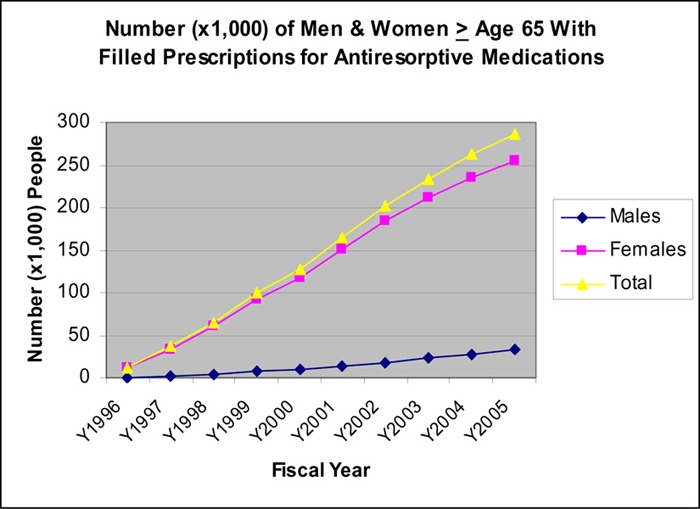
Table 11: Number and Percent of Antiresorptive Prescriptions Filled for People ≥ 65 Years in 2005/06.
| Alendronate daily |
Alendronate once weekly |
Didrocal | Miacalcin | Raloxifene | Risedronate daily |
Risedronate once weekly |
Total | |
|---|---|---|---|---|---|---|---|---|
| 2002 | 109,360 | 9,986 | 475,772 | 50,93 | 35,354 | 97,868 | 3,449 | 736,882 |
| 2003 | 80,431 | 202,259 | 467,062 | 4,542 | 42,067 | 88,663 | 133,355 | 1,018,379 |
| 2004 | 50,231 | 403,494 | 428,698 | 31,12 | 45,941 | 54,709 | 375,251 | 1,361,436 |
| 2005 | 36,355 | 547,895 | 396,422 | 2,284 | 51,765 | 38,661 | 552,824 | 1,626,206 |
| 2005 % of | 2.24 | 33.69 | 24.38 | 0.14 | 3.18 | 2.38 | 33.99 | 100 |
| Total |
Hip and Wrist Fracture in Ontario
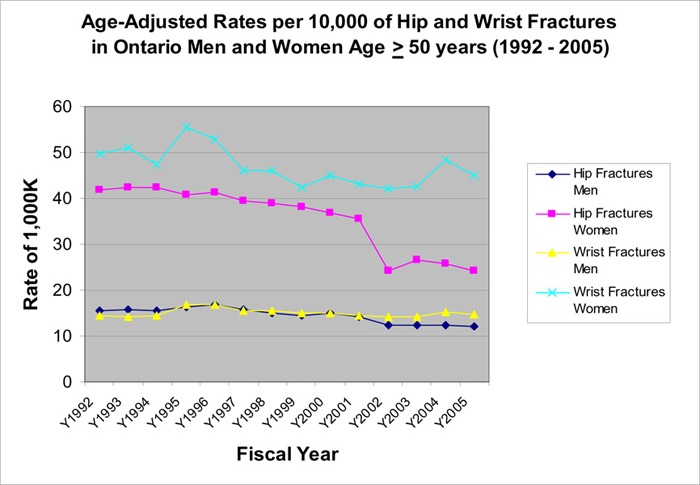
The rate of both wrist fractures and hip fractures in women started to decline in 1996 (Figure 9). This decline continued for hip fractures, reaching a low of 24.2 per 10,000 women (41% reduction since 1992). The rate of wrist fractures in women plateaued in 2003 and started to rise again in 2004, and reached a rate of 44.9 per 10,000. The rates of wrist fractures and hip fractures had also declined in men with a larger decrease in the rate of hip fractures (Figure 7).
Figure 9: Rate per 100,000 Hip Fractures in Ontario Women (2005/06).
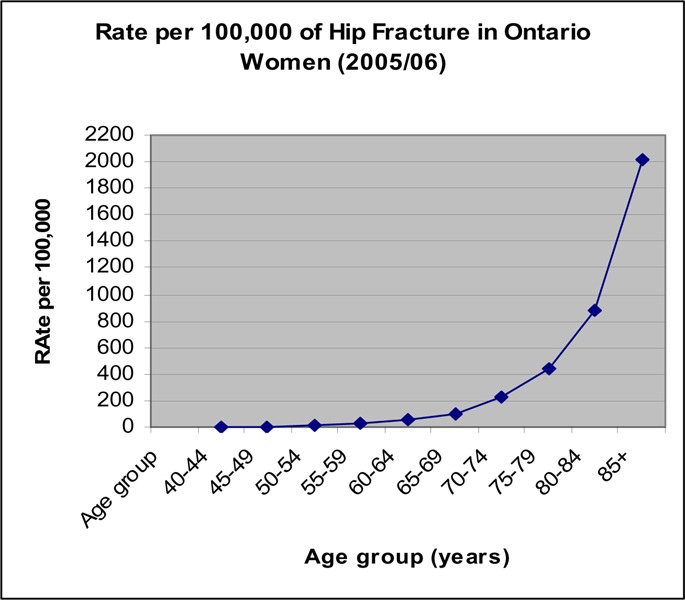
Figure 7: Age-Adjusted Rates Per 10,000 of Hip and Wrist Fractures in Ontario Men and Women.
The total number of hip fractures has remained relatively stable in the last 4 years but the number of wrist fractures started to rise again in 2003 (Figure 8).
Figure 8: Number of Hip and Wrist Fractures in Ontario Men and Women Age ≥ 50 Years (1992 – 2005).
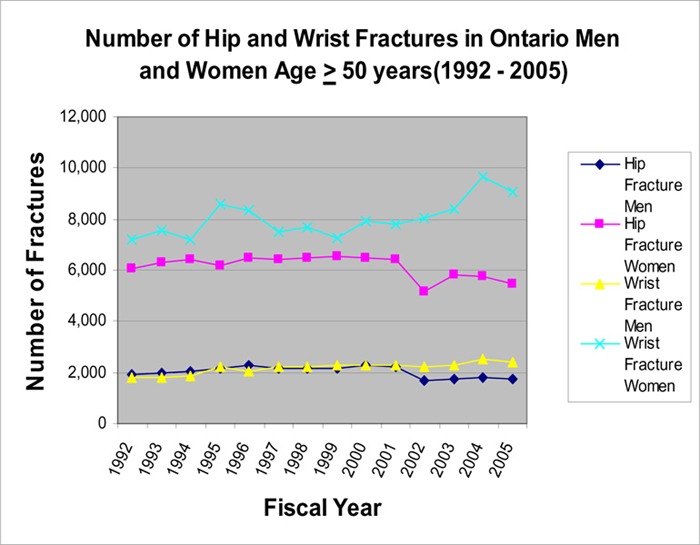
Based on the above analysis, a steady increase in the use of antiresorptive drugs and a decrease in the rate of hip and wrist fracture (particularly hip fracture) occurred in the same period (1997 - present), during which BMD testing escalated.
Age-Specific Rate of Fractures in Men And Women
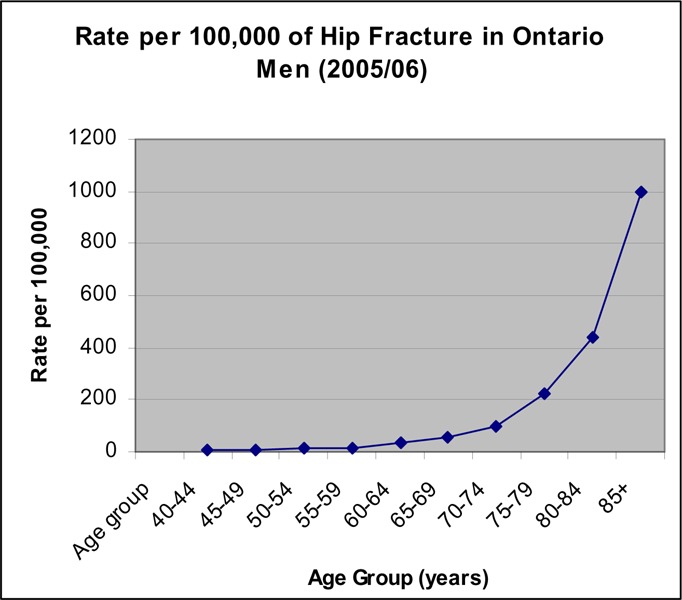
The rate of hip fractures was low for people under 65 years of age (Figures 9 and 10). For women, the rate began to increase in the age group of 60 to 65 years, and continued to increase exponentially with the sharpest increase occurring after age 80 years (Figure 9). The same pattern occurred in men but the increase in rate appeared to occur about 5 years later than in women (Figure 10).
Figure 10: Rate per 100,000 of Hip Fractures in Men (2005/06).
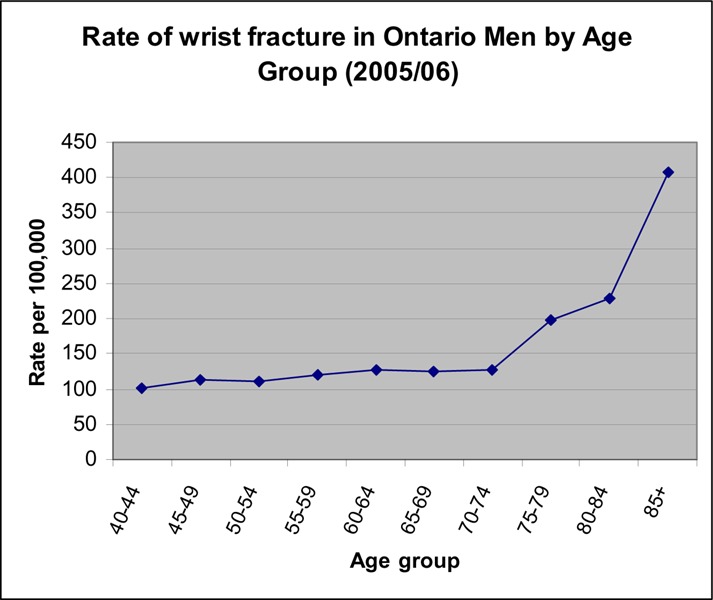
There seems to be a small increase in the rate of wrist fractures around age 55 to 60 years in women, with the highest rise in rate occurring at age 70 years (Figure 11). For men, the rate of wrist fractures remains low until age 70 years (Figure 10).
Figure 11: Rate per 100,000 Wrist Fractures in Ontario Women (2005/06).
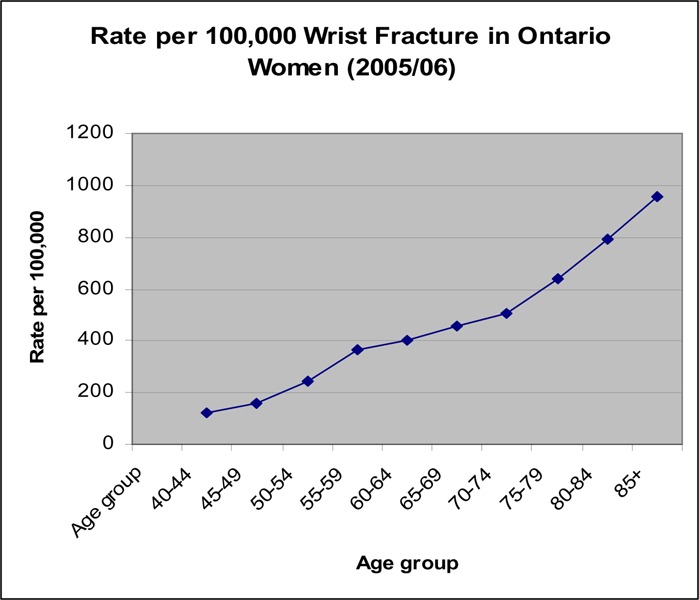
Figure 12: Rate per 100,000 of Wrist Fractures in Men (2005/06).
Summary of Findings on Bone Mineral Densitometry Utilization
Dual-energy z-ray absorptiometry BMD tests increased 10-fold between 1993 and 2005, reaching approximately 500,000 tests per year. There was a concomitant increase in the use of antiresorptive drugs and a decrease in the rate of hip and wrist fractures with the increase in BMD tests in Ontario.
The growth in the rate of BMD testing appeared to be slowing down to 6 to 7% per year. The increase in rate occurred mainly in people age 65 years or older.
Most of the hip and wrist factures occurred after age 65 years in men and women. The highest rate of fractures occurred in people aged 80 years and older.
Likely overuse: The current OHIP reimbursement policies do not have any risk-based restrictions on initial testing and provides payment for BMD testing every 24 months for low-risk patients. Up to 20% of the BMD tests might have been conducted unnecessarily in people under age 65 years and at low risk of osteoporosis.
-
Gaps in BMD testing:
– Less than 20% of the people who had a fragility fracture underwent BMD testing. The percentage of men that underwent BMD assessment after a fracture was even lower (about 10%).
– In 2005/06, there was a 10-fold variation in rates of BMD testing across counties, an improvement over the 17-fold variation reported in 2000. This gap in access to BMD testing needs to be examined and addressed.
Even though there appears to be room for improvement in the utilization of BMD testing, the increase in BMD use in Ontario appears to be having a positive impact on the rates of hip and wrist fractures, Future strategies should not focus on a blanket reduction in the use of BMD assessment. Instead, they need to ensure that the test is being performed in people at high risk of osteoporosis and fractures. A literature review was conducted on issues relating to the overuse and gaps in BMD testing to inform future polices relating to BMD service in the province.
PART 2 - Literature Review
Objectives:
To determine the utility and frequency of serial BMD tests in (1) people who are not receiving osteoporotic therapy and (2) people who are receiving drug therapy to monitor response.
To determine the role of BMD testing after fragility fractures.
To identify factors affecting the use of BMD after fractures
To identify men at risk of developing fragility fractures.
To determine the role of BMD testing in men.
To determine cost-effectiveness of BMD tests in Ontario.
Research questions
How does precision of BMD tests affect frequency of tests?
What are the rates of BMD loss in men and women not receiving osteoporosis treatment?
What are the rates of BMD change in men and women receiving pharmacological therapy for osteoporosis?
Do changes in BMD accurately reflect reductions in fracture risk during osteoporosis treatment?
Can BMD monitoring improve patient adherence to osteoporosis therapy?
What is the impact of a prevalent fragility fracture on the risk of future fractures?
Are current treatments for osteoporosis effective in reducing risk of fractures in osteoporotic women and men?
Does the use of BMD influence the likelihood of osteoporosis treatment?
What are the predictors of fragility fractures in men?
How should policy be changed to address issues identified in the utilization analyses?
What is the budget impact and cost-effectiveness ratio of the above policy changes relating to BMD tests in Ontario?
Search Strategy
Separate search strategies were developed to address the main questions analyzed in the systematic review. The detailed search strategies are shown in Appendix 6. All searches were run between May 5 and August 30th, 2006 in the following databases: OVID MEDLINE, OVID MEDLINE In-Process & Other Non-indexed Citations, OVID EMBASE, Cochrane Library, and the INAHTA/CRD database. All searches were limited to human subjects and English-language articles. Additional searches of websites and references of publications were also performed to ensure comprehensiveness.
The first search strategy (detailed in Appendix 6a) was developed to locate published articles that evaluated the relationship between changes in BMD (as a result of pharmacologic therapies for osteoporosis), and fracture and fracture risk. The pharmacological therapies of interest included etidronate, alendronate, risedronate, raloxifene, estrogen replacement, parathyroid hormone, and calcitonin. This search was limited to articles published between January 2000 and August 29, 2006, and yielded 297 citations.
The second search strategy (detailed in Appendix 6b) was developed to locate published articles dealing with BMD testing and treatment rates for osteoporosis after a fragility fracture. This search was limited to articles published between January 2005 and August 30, 2006, as a very comprehensive systematic review (51) was published in 2005 which included studies published through 2004. This search yielded 333 citations.
The third search strategy (detailed in Appendix 6c) was developed to identify randomized controlled trials (RCTs) of specific pharmacological treatments for osteoporosis. This search was limited to meta-analyses, systematic reviews, and randomized controlled trials published from January 2001 to June 19, 2006, and yielded 458 citations. As an addendum to this search, major RCTS published between 1997 and 2001 were included to ensure comprehensiveness
The fourth search strategy (detailed in Appendix 6d) examined the predictive value of BMD in men. The search was limited to articles published between January 1, 2001 and May 4, 2006, and yielded 381 citations.
General Inclusion Criteria
This review included published English-language journal articles that reported primary epidemiological or clinical data if:
The design and method were clearly described.
The report was not superseded by a publication with the same purpose, by the same group or a later publication that included the data from the same study (unless the article addressed different outcomes).
Unless otherwise stated, the reports were published between January 1, 2000 and August 30, 2006.
Specific Inclusion Criteria
For rate of change of bone loss for women and men not receiving osteoporosis treatment
Systematic reviews or population-based studies that provided rate of change in BMD for the general population not receiving osteoporosis treatment; RCTs on medical treatment of primary osteoporosis (etidronate, alendronate, risedronate, or raloxifene) that provided rate of change in BMD from baseline for the placebo arm
-
Description:
Patients: general population in population-based studies relating to BMD changes; postmenopausal women or men in placebo arm of RCTs on medical treatment for primary osteoporosis.
Intervention: Receiving a placebo or calcium/vitamin supplementation
Outcome of interest: Rate of change in BMD (change from baseline within a known period of time)
For effectiveness of BMD tests in monitoring response to osteoporosis treatment
RCTs and systematic reviews and meta-analyses of RCTs
-
Description:
Patients: Women and/or men with primary osteopenia or osteoporosis
Intervention: Bisphosphonates (etidronate, alendronate, risedronate, or raloxifene)
Comparison: Placebo or calcium/vitamin D supplement only
Outcomes of interest: Change in BMD, rate of incident fractures, correlation between BMD change and fracture risk reduction; percentage of treatment effect explained by changes in BMD
For rate of change in BMD during treatment for osteoporosis
RCTs on medical treatment of primary osteoporosis (etidronate, alendronate, risedronate, or raloxifene) that provided rate of change in BMD from baseline for the treatment arm.
-
Description:
Patients: Women and/or men with primary osteoporosis or osteopenia
Intervention: Pharmacologic treatment for osteoporosis: bisphosphonates and estrogen receptor modulator covered by the Ontario Drug Benefits Program (etidronate, alendronate, risedronate, or raloxifene), and parathyroid hormone
Comparison: Placebo or calcium /vitamin D supplement only
Outcome of interest: Rate of change in BMD from baseline during treatment
For patient compliance with osteoporosis therapy
Systematic reviews of RCTs, RCTs, prospective or retrospective observational studies
-
Description:
Patients: Women and/or men with primary osteopenia or osteoporosis
Intervention: Bisphosphonates (etidronate, alendronate, risedronate, or raloxifene) and BMD tests
Comparison: Same drug treatment and no BMD monitoring, placebo or calcium /vitamin D supplement only
Outcomes of interest: Percentage patient compliance, and patient outcome (increase in BMD and/or decrease in incidence of fractures)
For prevalence of BMD tests after a fragility fracture
Systematic reviews, RCTs or observational studies conducted in Canada
-
Description:
Patients: Women and/or men who had a fragility fracture in Canadian jurisdictions
Intervention: Follow-up survey, interview, or chart audit on follow-up care (BMD test and/or osteoporosis treatment)
Outcome of interest: Percentage of patients that underwent BMD tests and/or osteoporosis treatment after a fragility fracture; factors that increases BMD tests after fracture.
For impact of a previous fracture on the risk of future fractures in men and women
Systematic reviews (2000-2006), prospective primary population-based studies (2003-2006)
-
Description:
Patients: Perimenopausal or postmenopausal women or men who had suffered a fragility fracture (vertebral/spine, nonvertebral, hip, Colle’s, or any fragility fracture)
Intervention: Did not receive osteoporosis therapy
Outcomes of interest: Increase in risk of incident fractures (vertebral, nonvertebral, hip, Colle’s, or any fractures) after a prevalent fracture compared with people without the fracture; expressed as relative risk, odds ratio, or hazard ratio.
For effectiveness of osteoporosis treatment in reducing risk of fractures
Systematic reviews of RCTs on osteoporosis treatments
-
Description:
Patients: Women and/or men with primary osteoporosis or osteopenia
Intervention: Treatment for osteoporosis including bisphosphonates and estrogen receptor modulator covered by the Ontario Drug Benefits Program (etidronate, alendronate, risedronate, or raloxifene), calcitonin, HRT, parathyroid hormone therapy, and vitamin D therapy
Comparison: placebo or calcium /vitamin D supplement
Outcomes of interest: Reduction in risk of fractures (vertebral, nonvertebral, Coles’, hip) compared with the controlled group (not receiving treatment), expressed as relative risk, odds ratio, or hazard ratio.
For impact of BMD test on treatment
Systematic reviews, RCTs, and prospective and retrospective observational studies
-
Description:
Patients: Women and/or those who had undergone a BMD test
Intervention: Prevalence of BMD tests and osteoporosis treatment
Outcome of interest: Percentage of treatment after BMD test compared with people with no BMD test; predictors of treatment for osteoporosis
For predictors of fractures in men
Systematic reviews, and observational studies
-
Description:
Patients: Men who had a fragility fracture
Intervention: Interview, clinical examination, surveys
Outcomes of interest: Ability of different risk factors to predict fragility fractures, including BMD. Accuracy of BMD tests in predicting risk of fractures (e.g. risk gradient [relative risk of fracture per standard deviation in T-score] or odds ratio1 (OR), sensitivity, specificity, predictive values, and/or area under the curve) Other risk factors for fractures
Exclusion Criteria (All questions)
Studies were excluded if they:
Focused on nonprimary osteoporotic disease associated with concomitant diseases such as hypoparathyroidism or drug treatment (e.g., corticosteroid therapy after organ transplantation)
Opinion article, or letter to the editor that provided no primary data
Nonsystematic reviews or case reports (except where indicated)
Provided a previously published report, or had a more current update on the same study
Were full text articles in a language other than English
Results of Literature Search
One researcher selected reports based on inclusion and exclusion criteria. In total, 28 systematic reviews/meta-analyses and 97 primary studies (34 randomized controlled trials and 63 observational studies) were included.
Quality Assessment and Data Abstraction
One researcher reviewed the full-text reports and extracted data using data extraction forms. For RCTs, the quality of studies was assessed using criteria adapted from Jadad et al. (52) The quality of observational studies was evaluated based on method of patient selection, sample size, statistical analysis and completeness of follow-up. Levels of evidence were assigned to studies included in each section (Tables 12A to 12I).
Table 12I: Level of Evidence of Included Studies*.
| Type of Study (Design) | Level of Evidence |
No. of Eligible Studies |
|---|---|---|
| Large RCT, Systematic reviews of RCTs | 1 | |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3a | |
| Non-RCT with historical control | 3b | |
| Non-RCT unpublished but reported to an international scientific meeting | 3(g) | |
| Systematic review of observational studies | 4a | 8 |
| Surveillance (database or register) | 4a | 4 |
| Case series, multisite | 4b | 12 |
| Case series, single-site | 4c | 8 |
| Case series unpublished but presented to an international scientific meeting | 4(g) | |
| Total | 32† | |
g refers to grey literature; RCT, randomized controlled trial.
1 systematic review and 1 observational study had been referenced in an earlier section.
For rate of change in BMD without osteoporosis treatment
Table 12A: Level of Evidence of Included Studies on Rate of Change in Bone Mineral Density Without Osteoporosis Treatment*.
| Type of Study (Design) | Level of Evidence |
No. of Eligible Studies |
|---|---|---|
| Large RCT, Systematic reviews of RCTs | 1 | 7 † |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | 5 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3 a | |
| Non-RCT with historical control | 3b | |
| Non-RCT unpublished but reported to an international scientific meeting | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series, multisite | 4b | 5 |
| Case series, single-site | 4c | 7 |
| Case series unpublished but presented to an international scientific meeting | 4(g) | |
| Total | 24 | |
g refers to grey literature; RCT, randomized controlled trial.
Large RCTs
Effectiveness of Bone Mineral Densitometry for Monitoring Response to Osteoporosis Treatment
Table 12B: Level of Evidence of Included Studies on the Use of Bone Mineral Densitometry for Monitoring Response to Osteoporosis Treatment*.
| Type of Study (Design) | Level of Evidence |
No. of Eligible Studies |
|---|---|---|
| Large RCT, Systematic reviews of RCTs | 1 | 9† |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3 a | |
| Non-RCT with historical control | 3b | |
| Non-RCT unpublished but reported to an international scientific meeting | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series, multisite | 4b | |
| Case series, single-site | 4c | |
| Case series unpublished but presented to an international scientific meeting | 4(g) | |
| Total | 9 | |
g refers to grey literature; RCT, randomized controlled trial.
Included 7 meta-analysis of RCTs and 2 large RCTs
For rate of change in BMD during treatment for antiresorptive drugs
Table 12C: Level of Evidence of Included Studies on Rate of Bone Mineral Density Change During Treatment for Osteoporosis*.
| Type of Study (Design) | Level of Evidence |
No. of Eligible Studies |
|---|---|---|
| Large RCT, Systematic reviews of RCTs | 1 | 12 large RCTs |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | 19 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3 a | |
| Non-RCT with historical control | 3b | |
| Non-RCT unpublished but reported to an international scientific meeting | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series, multisite | 4b | |
| Case series, single-site | 4c | |
| Case series unpublished but presented to an international scientific meeting | 4(g) | |
| Total | 31† | |
g refers to grey literature; RCT, randomized controlled trial.
12 of these studies were also referenced in a previous section.
For BMD monitoring and Patient Compliance With Osteoporosis Therapy
Table 12D: Level of Evidence of Studies on Patient Compliance With Osteoporosis Therapy*.
| Type of Study (Design) | Level of Evidence |
No. of Eligible Studies |
|---|---|---|
| Large RCT, Systematic reviews of RCTs | 1 | |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3 a | |
| Non-RCT with historical control | 3b | |
| Non-RCT unpublished but reported to an international scientific meeting | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series, multisite | 4b | |
| Case series, single-site | 4c | 4 |
| Case series unpublished but presented to an international scientific meeting | 4(g) | |
| Total | 4 | |
g refers to grey literature; RCT, randomized controlled trial.
Table 12E: Level of Evidence on Prevalence of Bone Mineral Densitometry Use After a Fragility Fracture*.
| Type of Study (Design) | Level of Evidence |
No. of Eligible Studies |
|---|---|---|
| Large RCT, Systematic reviews of RCTs | 1 | |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3 a | |
| Non-RCT with historical control | 3b | |
| Non-RCT unpublished but reported to an international scientific meeting | 3(g) | |
| Surveillance (database or register) | 4a | |
| Systematic review of observational studies | 4a | |
| Case series, multisite | 4b | 2 |
| Case series, single-site | 4c | 4 |
| Case series unpublished but presented to an international scientific meeting | 4(g) | |
| Total | 6 | |
g refers to grey literature; RCT, randomized controlled trial.
For association between a previous fracture and the risk of future fractures
Table 12F: Level of Evidence of Included Studies on Impact of Previous Fracture on Risk of Future Fractures *.
| Type of Study (Design) | Level of Evidence |
No. of Eligible Studies |
|---|---|---|
| Large RCT, Systematic reviews of RCTs | 1 | |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3 a | |
| Non-RCT with historical control | 3b | |
| Non-RCT unpublished but reported to an international scientific meeting | 3(g) | |
| Surveillance (database or register) | 4a | |
| Pooled analysis of observational studies | 4 | 3 |
| Case series, multisite (Population-based observational) | 4b | 13 |
| Case series, single-site | 4c | |
| Case series unpublished but presented to an international scientific meeting | 4(g) | |
| Total | 16 | |
g refers to grey literature; RCT, randomized controlled trial.
For effectiveness of osteoporosis treatment in reducing risk of fractures
Table 12G: Level of Evidence of Studies on Effectiveness of Osteoporosis Treatment in Reducing Risk of Fractures *.
| Type of Study (Design) | Level of Evidence |
No. of Eligible Studies |
|---|---|---|
| Large RCT, Systematic reviews of RCTs | 1 | 11† |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | 2‡ |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3 a | |
| Non-RCT with historical control | 3b | |
| Non-RCT unpublished but reported to an international scientific meeting | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series, multisite | 4b | |
| Case series, single-site | 4c | |
| Case series unpublished but presented to an international scientific meeting | 4(g) | |
| Total | 13 | |
g refers to grey literature; RCT, randomized controlled trial.
Systematic reviews of RCTs.
1 of these RCTs was referenced in an earlier section.
For impact of BMD Test on Osteoporosis Treatment
Table 12H: Level of Evidence of Included Studies on the Impact of Bone Mineral Density on Treatment *.
| Type of Study (Design) | Level of Evidence |
No. of Eligible Studies |
|---|---|---|
| Large RCT, Systematic reviews of RCTs | 1 | |
| Large RCT, unpublished but reported to an international scientific meeting | 1(g) | |
| Small RCT | 2 | |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3 a | |
| Non-RCT with historical control | 3b | |
| Non-RCT unpublished but reported to an international scientific meeting | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series, multisite | 4b | |
| Case series, single-site | 4c | 5 |
| Case series unpublished but presented to an international scientific meeting | 4(g) | |
| Total | 5 | |
g refers to grey literature; RCT, randomized controlled trial.
For predictive accuracy of Bone Mineral Density in men
Eight meta-analyses of cohort studies and 28 primary studies met the selection criteria. The studies are classified in Table 7A.
Literature Review - Findings
Potential Overuse: Serial Bone Mineral Density Tests
Analysis of the administrative data showed that more than 18% (21,000) of all BMD tests in 2005/06 were repeat tests performed within 24 months in people at low risk of developing osteoporosis, in compliance with current OHIP reimbursement policies. The importance of a baseline BMD in assessing the risk of fractures has been well established; however, the utility and frequency of serial BMD measurements are less well defined. For this reason, evidence relating to the utility and frequency of serial BMD screening and serial BMD monitoring during osteoporosis treatment were examined.
How Frequently Should Bone Mineral Densitometry be Repeated in Patients not Receiving Osteoporotic Treatment?
For people who are not receiving osteoporosis treatment, the purpose of repeating BMD measurements is to monitor the progression of bone loss in order to initiate treatment when necessary. The time interval from baseline test for the BMD to drop to a treatment level depends on 4 parameters:
Precision of the BMD test
The baseline BMD
The rate of BMD loss; and
The BMD level at which treatment should be initiated.
The last parameter would depend on the presence or absence of other risk factors for fractures.
What is the Precision of Bone Mineral Density Testing?
The reliability of a follow-up BMD test depends on the precision (reproducibility) of the specific test, i.e., the ability of the test to produce the same results in repeated measurements of the same individual. Factors that can affect the precision of a BMD test are equipment, operator, patient population, site of measurement, and positioning of the patient. (53)
Precision can be stated either as the SD, or as the percent coefficient of variation (%CV), defined as %SD/mean. Percent CV of DXA tests have been reported to range from 1.8% to 2.3% at the lumbar spine, 2.3% to 3.6% at the femoral neck, and 1.7% to 2.5% for the total hip. (54) Precision may vary widely among DXA facilities. In a 7-centre pharmaceutical trial, 6 of the sites showed BMD test precisions at the posteroanterior spine ranging from 0.969 to 2.101%, and at the femoral neck ranging from 1.475 to 3.362%. However, the 7th site yielded an average PA spine precision of 3.565%, and a femoral neck precision of 4.349%. A change in BMD between the baseline and a repeat test may reflect the precision (reproducibility) of the test rather than a real biological change in bone density. Hence, interpretation of serial measurements can only be accomplished with the knowledge of the precision of the specific DXA facility where the test was performed. (53)
Changes in 2 BMD measurements at the same skeletal site in an individual may be related to measurement errors unless they are greater than the least significant change (LSC) (LSC at 95% CI = 2.77 × % CV) or the smallest detectable difference (SDD = 2 × SD in g/cm2). (55) Maghraoui et al. (56) recently reported at their center an LSC of 3.56% (total hip) and 5.60% (spine) and an SDD of 0.02g/cm2 (total hip) and 0.04 g/cm2(spine). There are indications that absolute precision errors derived from the SD are preferred because they are independent of the level of BMD. Precision errors and LSCs of a BMD facility are usually measured by performing 2 or more scans on a group of patients and then calculating the root-mean squared standard deviation of the replicate measurements. The ISCD recommends either measuring 30 subjects twice, or 15 subjects 3 times.(53)
Table 13 illustrates the impact of precision on the interval of serial BMD measurements. For a BMD facility with a 1% precision, the LSC is close to 3%. At a rate of BMD change of 1% per year, the shortest time interval to repeat the BMD measurement to obtain a result that exceeds the least significant change is 3 years. Similarly, at the same rate of BMD change (1%), it would take 6 years for the BMD change to exceed the LSC (6%) for a BMD test that has a 2% precision. Conversely, in situations where a rapid rate of bone change is expected (e.g., in certain disease conditions or during treatment), the LSC may be exceeded within a shorter period of time (e.g., 1 year for a precision of 1% and a rate of change of 3%/year).
Table 13: Precision of Bone Mineral Density Test, Rate of Bone Mineral Density Change, and Frequency of Test.
| Precision Error of Densitometry test (%) |
Least significant BMD Change (statistically meaningful difference) (%) |
Rate of change in BMD (% per year) |
Follow-up Period required for change in BMD to exceed the least significant change (Years) |
|---|---|---|---|
| 1 | +/- 2.77 | 1 | 3 |
| 1 | +/- 2.77 | 2 | 1.5 |
| 1 | +/–2.77 | ≥3 | 1 |
| 1 | +/–2.77 | ≥6 | 0.5 |
| 2 | +/– 5.54 | 1 | 6 |
| 2 | +/– 5.54 | 2 | 3 |
| 2 | +/– 5.54 | 3–5.5 | 2 |
| 2 | +/–5.54 | ≥6 | 1 |
| 3 | +/–8.31 | 1 | 8.3 |
| 3 | +/–8.31 | 2 | 4.1 |
| 3 | +/–8.31 | 3 | 3 |
| 3 | +/–8.31 | 4–8 | 2 |
| 3 | +/–8.31 | >8.31 | 1 |
In order to reduce the random error, repeat BMD measurements should be made at the same facility using the same instrument and same scanning procedure. The Canadian standards and guidelines for DXA densitometry recommend a documented quality control program at each DXA facility to ensure minimal radiation control, proper calibration, and ongoing monitoring of precision. (54)
Baseline Bone Mineral Density Measurements
A baseline BMD has been shown to predict 10-year risks of fractures. Initiation of osteoporosis treatment is usually made on the basis of risk of fracture and BMD values. The closer the baseline BMD is closer to the osteoporotic range, the sooner treatment may be needed, and hence more frequent monitoring of BMD will be necessary.
Rate of Bone Mineral Density Loss (no treatment) – Systematic Review
The rate of bone loss influences how quickly the BMD may reach a level that requires treatment. This in turn, will determine the interval between the baseline BMD and repeat BMD measurement.
A systematic review was conducted to determine the rate of BMD changes in women and men who were not receiving osteoporotic treatment. The review included 1 prospective population-based study in men and women, 5 prospective population-based cohort studies in women (sample ranging from 50 to 1,035), and 6 cohort studies in men (sample ranging from 214 to 5,995. Rates of bone loss from baseline in the placebo arm of RCTs on osteoporosis treatment were also reviewed, including 10 RCTs for postmenopausal women, and 2 RCTs for men with primary osteoporosis. These studies are described in Appendix 7, and mean rates of BMD changes are summarized in Appendices 8 and 9. The data suggest that in postmenopausal women, the mean rate of BMD loss at the spine, femoral neck, or total hip is generally 1% per year or less. Chapurlat (57) showed that apart from a small but significant bone loss at the hip, trochanter and anteriorposterior spine in premenopausal women (< 0.1% to 0.3% per year), there was no significant bone loss at other skeletal sites. Even though perimenopausal women showed more rapid bone loss compared with premenopausal women, the mean rate of loss is still less than 1% per year at all skeletal sites. (57) Even in the placebo arm of osteoporotic treatment studies, postmenopausal women with osteopenia or osteoporosis had a mean rate of bone loss of 1% per year or less. It should be noted that even though the mean rate of bone loss is up to 1% per year, there were individual women who had lost more than 1% of BMD per year. In women who have other risk factors such as lactose intolerance or surgical menopause (without hormone replacement), the rate of bone loss can be as high as 3% per year. (58;59)
Appendices 9A and 9B show that the mean rate of bone loss in men who do not have other major risk factors is also generally less than 1% per year; however, there were exceptions. Ensrud et al. (60) reported that men who had lost at least 5% of their body weight had a significant increase in the rate of bone loss compared with people with stable weight or weight gain (mean –1.2% to –1.7%, range –0.9% to –2.%) (Appendix 9B). The increased rate of bone loss occurred regardless of baseline BMI or whether the weight loss was voluntary. A similar relationship between weight loss and increased bone loss was observed in women. (61)
The higher the rate of bone loss, the sooner BMD may progress to a level at which treatment becomes necessary, and hence more frequent monitoring may be required.
Bone Mineral Density Level for Initiation of Treatment
Apart from baseline BMD and the rate of bone loss, presence of risk factors for fractures will also influence the BMD threshold at which treatment needs to be initiated and hence the frequency of BMD monitoring.
As previously discussed, the most important predictors of fractures independent of BMD are:
Advanced age (>75 years)
History of fragility fractures including vertebral compression fractures (particularly at age>75 yrs)
Family history of osteoporotic fractures (especially maternal hip fractures)
Systemic glucocorticoid use (>7.5g/day for >3 months), chemotherapy
Conditions such as hyperparathyroidism, hypogonadism, early menopause
Propensity to fall
Experts advised that for people with any of the above risk factors, treatment may be considered at a higher BMD level (e.g. at a T-score of –1.5 to –2), whereas for people who did not have any of these risk factors, treatment may not be necessary until a T-score of –2 to –2.5).
A Model to Estimate Frequency of Repeat Bone Mineral Densitometry for People not Receiving Osteoporosis Treatment
The progression of BMD over time was computed for 3 scenarios using baseline T-scores of 0 (peak BMD), –1 (lower limit of normal) and –1.5 (osteopenic), and using a rate of change of BMD loss of 1% to 5% in each scenario (Tables 14A to 14 C). It was assumed that pharmacologic treatment would begin at a T-score of –2 (before the patient becomes osteoporotic). For each scenario, the time intervals for the BMD loss to exceed the LSC for test precisions of 1% to 3% were computed. The time intervals to reach the treatment threshold were also identified.
Table 14A: Scenario: T-score = 0 at baseline.
| BMD | in | g/cm2 | BMD | in | g/cm2 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate of bone loss %/yr | Baseline BMD gm/cm2 Year 0 | Year 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Year 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
| 1% | 0.849 | 0.841 | 0.832 | 0.824* | 0.816 | 0.807 | 0.799Ψ | 0.791 | 0.783 | 0.776** | 0.768 | 0.760 | 0.753 | 0.745 | 0.738 | 0.730 | 0.723 | 0.716 | 0.709 | 0.702 | 0.695 | 0.688 | 0.681 | 0.674 | 0.667 | 0.660 |
| 2% | 0.849 | 0.832 | 0.815* | 0.799Ψ | 0.783 | 0.767** | 0.752 | 0.737 | 0.722 | 0.708 | 0.694 | 0.680 | 0.666 | 0.653 | 0.640 | 0.627 | 0.615 | 0.603 | 0.591 | 0.579 | 0.567 | 0.556 | 0.545 | 0.534 | 0.523 | 0.513 |
| 3% | 0.849 | 0.824* | 0.799Ψ | 0.775** | 0.752 | 0.729 | 0.707 | 0.686 | 0.665 | 0.645 | 0.626 | 0.607 | 0.589 | 0.571 | 0.554 | 0.538 | 0.522 | 0.506 | 0.491 | 0.476 | 0.462 | 0.448 | 0.435 | 0.422 | 0.409 | 0.397 |
| 4% | 0.849 | 0.815* | 0.782Ψ | 0.751** | 0.721 | 0.692 | 0.665 | 0.638 | 0.612 | 0.588 | 0.564 | 0.542 | 0.520 | 0.499 | 0.479 | 0.460 | 0.442 | 0.424 | 0.407 | 0.391 | 0.375 | 0.360 | 0.346 | 0.332 | 0.319 | 0.306 |
| 5% | 0.849 | 0.807Ψ | 0.766** | 0.728 | 0.692 | 0.657 | 0.624 | 0.593 | 0.563 | 0.535 | 0.508 | 0.483 | 0.459 | 0.436 | 0.414 | 0.393 | 0.374 | 0.355 | 0.338 | 0.321 | 0.305 | 0.289 | 0.275 | 0.261 | 0.248 | 0.236 |
Table 14C: Scenario C: T-score = -1.5 at baseline.
| Rate of bone loss %/year | Baseline BMD gm/cm2 Year 0 | Year 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Year 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1% | 0.683 | 0.676 | 0.669 | 0.663* | 0.656 | 0.650 | 0.643† | 0.637 | 0.630 | 0.624‡ | 0.618 | 0.612 | 0.605 | 0.599 | 0.593 | 0.587 | 0.582 | 0.576 | 0.570 | 0.565 | 0.559 | 0.553 | 0.548 | 0.542 | 0.537 | 0.532 |
| 2% | 0.683 | 0.669 | 0.656* | 0.643† | 0.630 | 0.617‡ | 0.605 | 0.593 | 0.581 | 0.569 | 0.558 | 0.547 | 0.536 | 0.525 | 0.515 | 0.504 | 0.494 | 0.484 | 0.474 | 0.465 | 0.456 | 0.447 | 0.438 | 0.429 | 0.420 | 0.412 |
| 3% | 0.683 | 0.663* | 0.643† | 0.623‡ | 0.605 | 0.587 | 0.569 | 0.552 | 0.535 | 0.519 | 0.504 | 0.489 | 0.474 | 0.460 | 0.446 | 0.433 | 0.420 | 0.407 | 0.395 | 0.383 | 0.372 | 0.361 | 0.350 | 0.339 | 0.329 | 0.319 |
| 4% | 0.683 | 0.656* | 0.629† | 0.604‡ | 0.580 | 0.557 | 0.535 | 0.513 | 0.493 | 0.473 | 0.454 | 0.436 | 0.418 | 0.402 | 0.386 | 0.370 | 0.355 | 0.341 | 0.327 | 0.314 | 0.302 | 0.289 | 0.278 | 0.267 | 0.256 | 0.246 |
| 5% | 0.683 | 0.649† | 0.616‡ | 0.586 | 0.556 | 0.528 | 0.502 | 0.477 | 0.453 | 0.430 | 0.409 | 0.388 | 0.369 | 0.351 | 0.333 | 0.316 | 0.301 | 0.286 | 0.272 | 0.258 | 0.245 | 0.233 | 0.221 | 0.210 | 0.200 | 0.190 |
Change from baseline exceeds LSC for 1% precision
Change exceeds LSC for 2% precision
Change exceeds LSC for 3% precision
The model showed that regardless of the baseline BMD, for a normal rate of bone loss of 1% per year (as in the majority of people), it will take approximately 3 years for the BMD loss to exceed the LSC of a BMD test with a 1% precision (Tables 14A – 14C). This means that for the majority of people, even with the most precise BMD test, repeating the test less than 3 years after the baseline test is not likely to provide meaningful information. The less precise the test, the longer it would take to detect a BMD change that can be considered significant (e.g. 9 years for a test with a 3% precision error). It is, therefore, important that BMD facilities have high precision so that genuine biological bone loss in people who require frequent monitoring can be detected Conversely, as the rate of bone loss increases, it will take less time to exceed the LSC and for a genuine change in BMD to be detected. For example, at a rate of bone loss of 1% per year, the LSC for a 1% precision will be exceeded in 3 years whereas with a bone loss of 3% per year, the LSC for a test with the same precision will be exceeded in 1 year (Tables 14A–C).
Table 14A shows that for a person with a normal baseline T-score of 0 and a normal rate of bone loss up to 1% per year, the BMD probably will not drop to an osteoporotic level within 25 years. Even at an increased rate of bone loss of 3%, it would take 13 years to reach a T-score of –2 and 10 years to reach a T-score of –2.5, levels at which treatment is usually considered.
At a baseline T-score of –1 (lower limit of the normal BMD range) and at a normal rate of bone loss (up to 1% per year), it would take approximately 16 years for the BMD to drop to a T-score of –2, the level treatment may need to be initiate (Table 14B). Repeating the BMD test every 2 years after the baseline BMD test in this population will not serve any clinical purpose.
Table 14B: Scenario B: T-score = -1 at baseline.
| BMD | in | g/cm2 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate of bone loss %/year | Baseline BMD gm/cm2 Year 0 | Year 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Year 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 |
| 1% | 0.738 | 0.731 | 0.723 | 0.176 * | 0.709 | 0.702 | 0.695 Ψ | 0.688 | 0.681 | 0.674 ** | 0.667 | 0.661 | 0.654 | 0.648 | 0.641 | 0.635 | 0.628 | 0.622 | 0.616 | 0.609 | 0.603 | 0.597 | 0.591 | 0.585 | 0.579 | 0.574 |
| 2% | 0.738 | 0.723 | 0.709* | 0.695 Ψ | 0.681 | 0.667** | 0.654 | 0.641 | 0.628 | 0.615 | 0.603 | 0.591 | 0.579 | 0.568 | 0.556 | 0.545 | 0.534 | 0.523 | 0.513 | 0.503 | 0.493 | 0.483 | 0.473 | 0.464 | 0.454 | 0.445 |
| 0.674 | ||||||||||||||||||||||||||
| 3% | 0.738 | 0.716 * | 0.694 Ψ | ** | 0.653 | 0.634 | 0.615 | 0.596 | 0.578 | 0.561 | 0.544 | 0.528 | 0.512 | 0.497 | 0.482 | 0.467 | 0.453 | 0.439 | 0.426 | 0.413 | 0.401 | 0.389 | 0.377 | 0.366 | 0.355 | 0.344 |
| 0.653 | ||||||||||||||||||||||||||
| 4% | 0.738 | 0.708* | 0.680Ψ | ** | 0.627 | 0.602 | 0.578 | 0.555 | 0.532 | 0.511 | 0.491 | 0.471 | 0.452 | 0.434 | 0.417 | 0.400 | 0.384 | 0.369 | 0.354 | 0.340 | 0.326 | 0.313 | 0.301 | 0.289 | 0.277 | 0.266 |
| 5% | 0.738 | 0.701Ψ | 0.666** | 0.633 | 0.601 | 0.571 | 0.542 | 0.515 | 0.490 | 0.465 | 0.442 | 0.420 | 0.399 | 0.379 | 0.360 | 0.342 | 0.325 | 0.309 | 0.293 | 0.279 | 0.265 | 0.251 | 0.239 | 0.227 | 0.216 | 0.205 |
However, if a person with a normal baseline T-score of –1 has increased rate of bone loss of 3% per year, the time interval for the BMD to drop to the treatment level (T-score of –2) will be shorten to 5 years (Table 14B). If the person is osteopenic at baseline (e.g. with a T-score of –1.5), and is losing BMD at an accelerated rate of 3% per year, the BMD may drop to a T-score of -2 within 3 years (Table 14C). The lower the baseline BMD and the higher the rate of bone loss, the sooner treatment may need to be initiated, and the closer the BMD needs to be monitored.
Thus, for people with a normal baseline BMD and a normal rate of bone loss without major risk of fractures, BMD measurements do not need to be repeated every 2 years. A repeat BMD measurement after the baseline test will be required (in 3–5 years depending on the precision of the test facility) to establish the rate of bone loss. The frequency of further serial testing should be guided by the baseline BMD and the rate of bone loss (see Tables 14A to 14C). For people that have osteopenic baseline BMD, have accelerated rate of bone loss, or have major predisposing factors for osteoporosis, BMD needs to be monitored more frequently so that treatment can be initiated promptly (Table 15).
Table 15: Interval Between Baseline BMD and Treatment Threshold Based on Baseline BMD, Rate of Bone Loss, and Risks of Fractures.
| Age | Sex | Other risk factors for fractures * |
Baseline BMD (gm/cm2) † |
Rate of BMD loss (% per year) |
BMD Threshold to initiate treatment (gm/cm2) † |
Time interval to reach threshold to initiate treatment yrs. |
|---|---|---|---|---|---|---|
| >65 yrs | Women | None | T-score =0 BMD = 0.849 |
3 | T-score = -2.5 BMD = 0.572 |
12 |
| >65 | Women | None | T-score up to –1 BMD = 0.738) |
1 | T-score = –2.5 BMD = 0.572 |
24 |
| >65 | Women | None | T-score up to –1 (0.738) | 2 | T-score = –2.5 BMD = 0.572 |
11 |
| >65 | Women | None | T-score up to –1 (0.738) | 3 | T-score = –2.5 BMD = 0.572 |
8 |
| >65 | Women | None | T-score up to –1 BMD = 0.738) |
1 | T-score = –2 BMD = 0.627 |
16 |
| >65 | Women | None | T-score up to –1 (0.738) | 2 | T-score = –2 BMD = 0.627 |
7 |
| >65 | Women | None | T-score up to –1 (0.738) | 3 | T-score = –2 BMD = 0.627 |
5 |
| >65 yrs | Women | None | T-score –1.5 0.683 osteopenic |
3 | T-score = –2.5 BMD = 0.572 |
5 |
| >65 yrs | Women | None | T-score–1.5 0.683 osteopenic |
3 | T-score = –2 BMD = 0.627 |
2 |
| Women | Yes * | T-score = -1 BMD>0.738 |
1 | T-score =– 1.5 BMD = 0.683 |
6 | |
| Women | Yes * | T-score = -1 BMD>0.738 |
2 | T-score = –1.5 BMD = 0.683 |
3 | |
| Women | Yes * | T-score = -1 BMD>0.738 |
3 | T-score =– 1.5 BMD = 0.683 |
2 | |
| Any age | Women & men | Glucocortic oids therapy | Any BMD | Regardless of BMD | Guidelines Every 6 to 12 months |
History of fracture & advanced age, condition or medication associated with rapid bone loss, and/or history of fracture in first-degree relative
BMD in gm/cm2 based on femoral neck for Caucasian women (Hologic densitometers)
For men and women receiving glucocorticoid treatment, BMD loss can be as high as 8% in 20 weeks. (62) Hence, for patients receiving 7.5 mg corticosteroid per day or more for at least 3 months, BMD monitoring may be performed every 6 to 12 months depending whether the person is receiving osteoporosis treatment.
Table 14: Bone Loss Over Time Based on Baseline BMD and Rate of Bone Loss.
| T-score | BMD gm/cm2 | Baseline BMD | Least Significant change (g/cm2) | ||
|---|---|---|---|---|---|
| 0 | 0.849 | T-score | 1% precision | 2% precision | 3% precision |
| 0.02 | 0.07 | ||||
| -1 | 0.738 | 0 | 4 | 0.047 | 1 |
| 0.02 | 0.06 | ||||
| -1.5 | 0.683 | -1 | 0 | 0.041 | 1 |
| 0.01 | 0.05 | ||||
| -2 | 0.627 | -1.5 | 9 | 0.038 | 7 |
| -2.5 | 0.572 | Assume starting treatment at a T-score of –2 (0.627g/cm2) | |||
Summary
Current OHIP reimbursement policy provides coverage for serial BMD tests in any 24-month period for people at low risk of accelerated bone loss. OHIP claims for 2005/06 showed that approximately 21,000 BMD tests were repeat tests within a 24 month period in low-risk individuals
With a normal rate of bone loss (≤ 1% per year), as in the majority of people, even with a high precision BMD test, the change in BMD is not likely to exceed the random test variability in less than 3 years after the baseline test.
It is reasonable to conduct the first repeat BMD test 3 to 5 years (depending on the precision of the test) after the baseline test to establish the rate of bone loss in the individual person.
Analysis of evidence showed that for people with a normal baseline BMD (T-score ≥ –1), a normal rate of bone loss (≤ 1% per year), and no major risk for osteoporosis other than age greater than 65 years, the BMD is not likely to drop to a level requiring osteoporosis treatment in less than 16 years. For this population, a second repeat BMD test may not be necessary for 7 to 10 years.
For people with BMD in the osteopenic range (e.g. T-score < –1, but >–2.5), who have accelerated rate of bone loss (>1% per year), or who have one or more major risk factors* for fractures, more frequent monitoring will be necessary and the frequency needs to be determined on an individual basis.
| *Major Risk Factors | |
| - | History of a fragility fracture and age greater than 75 years |
| - | Fragility fracture in first degree relative (particularly maternal history of hip fracture) |
| - | A condition associated with rapid bone loss |
| - | Medication associated with rapid bone loss |
For men or women receiving 7.5mg/day or more of glucocorticoid therapy for >3 or more months, BMD loss can be as high as 8% in 20 weeks. It is, therefore, appropriate to repeat BMD measurements at 6 months if treatment has not been initiated and every year after initiation of treatment, regardless of baseline BMD.
Should Serial Bone Mineral Density Measurements be Used to Monitor Response to Treatment?
In order to determine whether BMD is useful for monitoring of response to treatment, the following questions must be answered:
Do BMD changes during osteoporotic treatment predict reduction in risk of fracture?
Can BMD monitoring improve patient adherence to osteoporotic treatment?
Do Bone Mineral Density Changes During Treatment Accurately Reflect Changes in Risk of Fracture?
Systematic Review: Relationship Between BMD Changes and Reduction in Fracture Risk During Treatment
To be considered an appropriate surrogate for fracture as a treatment endpoint, BMD should be related causally to a decreased propensity to fracture, and the change in BMD must largely capture the intervention’s effect on the propensity to fracture. (63) Seven meta-analyses and 2 RCTs on this subject were identified (Table 16).
Table 16: Summary of Meta-Analysis and Studies (Changes in Bone Mineral Density on Fracture Risk Reduction).
| Study | Type of Study |
Antiosteoporotic drug |
Type of BMD /Fracture |
Treatment effect - Reduction in fracture risk compared with placebo % |
% of treatment effect explained by change in BMD |
|---|---|---|---|---|---|
| Wasnich et al., 2000 (64) | Meta-analysis 13 RCTs | 3 bisphosphonates Raloxifene, HRT, calcitonin | Spine & hip BMD/vertebral fractures | 54 (vertebral) | 76 (41/54) |
| Cummings et al., 2002 (65) | Meta-analysis 12 RCTs | 4 bisphosphonates Raloxifene HRT Calcitonin | Spine BMD/Vertebral fractures | 16 - 78 FIT (47) | 44 (20/45) 161 (vertebral) |
| Hochberg et al., 2002 (66) | Meta-analysis 18 RCTs | 4 bisphosphonates Raloxifene HRT Calcitonin | Spine & hip BMD/nonvertebral fractures | (6% ⇓ spine BMD) 39% risk reduction (3% ⇓ hip BMD) 46% risk reduction |
Explained significant part of risk reduction |
| Guyatt et al., 2002 (63) | Summarized 8 meta-analysis | 3 bisphosphonates, raloxifene, HRT, Calcitonin, vitamin D & calcium | Vertebral fractures Nonvertebral fractures |
45 (vertebral) | 25 No association for nonvertebral fractures |
| Delmas et al., 2004 (67) | Meta-analysis 16 RCT | bisphosphonates Raloxifene, HRT, calcitonin | Spine & hip BMD/nonvertebral fractures | No association | |
| Watts et al., 2004 (68) | Meta-analysis 3 pivotal RCTs | Risedronate | Femoral neck BMD/vertebral fracture | BMD ⇓ ⇓ Risk 0-<5% 49% ≥5% 41% ≥0% 44% |
Spine BMD 18 Femoral neck BMD 11 |
| Watts et al., 2005 (69) | Meta-analysis 3 pivotal RCTs | Risedronate | Spine & femoral neck BMD/nonvertebral fractures | 32 | Spine BMD 12% Femoral neck BMD 7% |
| Sarkar et al., 2002 (70) (MORE) | Placebo controlled RCT | Raloxifene 3 years | Spine or femoral neck BMD/vertebral fracture | 36 (vertebral) | 4 |
| Chapurlat et al., 2005 (71) (FIT) | Multicenter, Placebo controlled RCT | Alendronate 3–4 years | Spine & hip BMD/Vertebral fractures | People who adhered to treatment showed reduction in vertebral fracture even with (≤4) decrease in BMD |
FIT refers to Fracture Intervention Trial; MORE, Multiple Outcome Raloxifene Evaluation; RCT, randomized controlled trial.
The quality assessment and description of these meta-analyses is summarized in Appendices 10, 11 and 12.
Two meta-analyses reported that an increase in BMD is responsible for most of the reduction in fracture risk.
Wasnich et al. (64) conducted a meta-analysis of 13 randomized, placebo-controlled trials of antiresorptive drugs including etidronate, alendronate, tiludronate, raloxifene, hormone replacement therapy (HRT), and calcitonin with a total follow-up of 63,822 person years. Overall, trials reported that patients with a larger increase in BMD tended to have greater reductions in vertebral fracture risk. Poisson regression showed that treatments that increase spine BMD by 8% reduced vertebral fracture risk by 54%, and changes in BMD explained most of the total treatment effect on fracture risk (41% risk reduction). A risk reduction of 20 to 22% was not associated with any measurable change in spine BMD.
Hochberg et al. (66) also found a significant association between the amount of increase in BMD at the spine and hip and nonvertebral fracture risk reduction. Hochberg et al. (66) conducted a meta-analysis using trial level summary data from 18 randomized, double-blind, and placebo-controlled trials on antiresorptive drugs including etidronate, alendronate, tiludronate, risedronate, raloxifene, estradiol, and calcitonin, with a total sample size of 26,494 women with incident nonvertebral fractures and 69,369 women-years. The analyses showed that larger increases in BMD at both the lumbar spine and hip during treatment were significantly associated with a greater reduction in the risk of nonvertebral fracture (P= .02 and .06 respectively). Each 1% increase in spine BMD at 1 year was associated with an 8% reduction in nonvertebral fracture risk (P= .02) An antiresorptive drug that increased spine BMD by 6% at 1 year reduced nonvertebral fracture risk by about 39%, and one that increased hip BMD by 3% at 1 year reduced nonvertebral fracture risk by about 46%. Hochberg et al. concluded that changes in BMD appeared to explain a significant part of the fracture risk reduction.
There is growing evidence that an increase in BMD alone does not fully account for the reduction in fracture risks.
Guyatt et al. (63) summarized the results of 8 meta-analyses that examined the magnitude of effects of osteoporosis therapies on fracture and bone density and conducted an analysis of the relationship between changes in bone density and magnitude of fracture reduction. Based on a regression analysis using data from systematic reviews and results from a large RCT of parathyroid hormone, Guyatt et al. (63) found a 20% reduction in the relative risk for vertebral fracture which was not associated with changes in BMD, and an additional 25% reduction in relative risk that was associated with changes in BMD. Based on the analysis, Guyatt et al. concluded that BMD is not helpful for predicting the impact of antiresorptive treatment on nonvertebral fractures.
Dalmas et al. (67) repeated the meta-analysis by Hochberg et al., (66) using individual patient data from all but 3 of the same studies. The results showed that there was no association between the extent of a reduction in nonvertebral fracture risk and an increase in BMD at the spine or hip at 1 year or at study end point. Larger increases in the lumbar spine BMD at 1 year were not associated with a greater reduction in nonvertebral fracture risk (P= .12), nor was there any association with increases in hip BMD (PP= .11).
Even when there was a significant increase in BMD, it has not been shown that BMD gain is a determinant of treatment effectiveness. In a meta-analysis by Cummings et al., (65) using individual patient data of 12 blinded, randomized, placebo-controlled trials of antiresorptive drugs, a 1% increase in spine BMD in the treatment group (compared with placebo group) was associated with a 0.03 decrease in relative risk of spine fractures (95% CI, 0.02–0.05, P= .002). The total treatment effect was a 45% reduction in vertebral fracture risk, but the reduction in BMD was only expected to reduce fracture risks by 20% (relative risk [RR] = 0.8). A separate analysis of the Fracture Intervention Trial (FIT) of alendronate treatment showed that the 3.9% increase in BMD after 1 year of alendronate therapy explained only 16% of the total decrease in the risk of vertebral fracture.
Similar findings were reported for trials on risedronate. Watts et al. conducted 2 meta-analyses (68;69) using individual patient data from 3 pivotal double-blind, placebo controlled trials on risedronate (2.5 mg or 5 mg) with a follow-up period of up to 3 years. The 3 trials are the Vertebral Efficacy with Risedronate Therapy North America (VERT-NA), the VERT Multinational (VERT-MN), and the Hip Intervention Trial (HIP). The first meta-analysis examined the relationship between changes in BMD and vertebral fracture risk reduction. The analysis showed that patients who had an increase in BMD had lower vertebral fracture risk than patients showing a decrease in BMD. However, the reduction in vertebral fracture risk was similar for patients who had a less than 5% increase in BMD and those who had an increase in BMD of 5% or greater. Watts et al. (68) reported that changes in lumbar spine BMD only explained 18% and changes in hip BMD explained 11% of the raloxifene treatment effect. The second meta-analysis explored the relationship between BMD changes and reduction in nonvertebral fracture risk. The analysis showed a similar incidence of nonvertebral fractures for people who had an increase in spine BMD (6.4%) and people who had a decrease in spine BMD (7.8%). Incidence of nonvertebral fractures was also similar regardless of an increase or decrease in femoral neck BMD (7.6% vs 7.5%). Watts et al. (69) determined that changes in spine BMD explained 12% and changes in femoral neck BMD explained 7% of the raloxifene treatment effect on nonvertebral fracture risk.
Two reports published after the above meta-analyses also suggest that changes in BMD do not predict changes in fracture risks (Appendix 13). The Multiple Outcome Raloxifene Evaluation (MORE Trial) (70) enrolled 7,705 postmenopausal women with osteoporosis randomized either to raloxifene treatment (60mg or 120 mg/day) or to a placebo, for 3 years. DXA BMD of the lumbar spine and femoral neck was measured at baseline and annually during follow-up. Sarkar et al. (70) reported that at 3 years, the incidence of fractures was the same regardless of the change in follow-up BMD (0.3%, 3.13% or 6.06%). Even patients with no change in BMD showed a reduction in fracture risk. The percentage change in BMD only accounted for 4% of the observed vertebral risk reduction, while the other 96% of risk reduction remained unexplained. (70)
Chapurlat et al. (71) conducted a post hoc analysis of the relationship between BMD changes and risk of vertebral fracture in women who adhered to alendronate therapy or a placebo. Changes in vertebral fractures were compared among patients who had gained BMD (0% – 4%, or >4%) and those who had lost BMD (lost 0%–4%, or lost >4%) at 1 year. The analyses showed that after one year of alendronate therapy, women who had lost 0 to 4% of BMD at the hip or the lumbar spine compared with the controls had substantial reduction in the risk of vertebral fractures (OR 0.47 & 0.40 respectively), similar to women who had gained 0 to 4% BMD (OR 0.49 & 0.49 respectively) during alendronate therapy. Women who had lost BMD at both the hip and lumbar spine (OR, 0.71; 95% CI, 0.26–1.93) and those who had lost more than 4% BMD during alendronate therapy did not appear to have statistically significant reduction in the risk of vertebral fracture. (71) These findings were not consistent with an earlier analysis of the same trial by Hochberg et al. (72) showing that patients who had gained at least 3% in spine BMD over 12 months of alendronate therapy had the lowest incidence of new vertebral fractures at 36 to 48 months, while those whose BMD remained stable or declined had the greatest fracture risk. (72)
Among clinical trials, the percentage of patients with no significant increase in BMD during drug therapy ranged from 4% to 14%. It was reported that about 10% of elderly patients treated with bisphosphonates lost BMD more than the LSC (Table 17). (73)
Table 17: Spine Bone Mineral Density Response to Therapy in Clinical Trials.
| Drug | Study | Months | BMD increased |
BMD not increased |
|---|---|---|---|---|
| Alendronate | FIT (1) | 24 | 96% | 49% |
| Risedronate | Vert-NAa | 36 | 86% | 14% |
| Risedronate | Vert-MNa | 36 | 90% | 10% |
| Teriparatide | Neer (2) | 19 | 96% | 4% |
Data on file at Proctor & Gamble Pharmaceuticals.
Note: Comparison among trials cannot be made because of the study of different populations over varying lengths of time.
Used with permission; This article was published in the Journal of Clinical Densitometry, 6(4), Lewiecki EM, Nonresponders to Osteoporosis Therap, 307–314. Copyright Elsevier 2003.
Can Bone Mineral Density Monitoring Improve Patient Adherence to Treatment?
Consistent compliance with osteoporosis medication is required to reduce the risk of fractures, but compliance with such medications is low. (74;75) Four observational studies examined the role of BMD testing in influencing patient compliance with osteoporotic drug therapy. These are summarized in Table 18.
Table 18: Factors Affecting Adherence to Osteoporosis Treatment.
| Study | Design/Patient | Method | Finding |
|---|---|---|---|
| Solomon et al., 2005 (76) | Retrospective cohort N = 40,002 pts > 65 yrs Medicare drug benefit recipients with claims for osteoporosis medications Mean age = 80 yrs Average comorbidity 2 | Determine factors influencing compliance with treatment defined as <66% of days with medication during a 60 day period | Compliance: 45.2% after 1 year & 52.1% after 5 years Multivariate analysis: independent factor that increased compliance: female gender, younger age, less comorbidity, fewer nonosteoporosis med. BMD test or fracture before & after initiation, residence in nursing home before initiation of med. Models explained only 6% of variation in compliance. |
| Pickney et al., 2005 (77) | Retrospective cohort N = 1,014 people who had DXA BMD at a rural US hospital (96% females) | Mail questionnaire @ mean interval of @follow-up = 18 months. Response rate = 71% of 1,492 | Of the patients who were prescribed medication after BMD tests, 50% of osteoporotic patients and 26% of those with osteopenia remained on treatment. Patients with osteopenia and osteoporosis who correctly recalled the results of their BMD tests were more likely to have been prescribed a medication (69% vs 35%) and to have remained on the initial medication (59% vs 28%) compared with those who incorrectly recalled their BMD results. |
| Rossini et al., 2006 (78) | Prospective cohort study N = 9,851 postmenopausal women prescribed an osteoporosis drug for at least 1 year |
Questionnaire survey Mean follow-up 14 months |
Persistence: 19.1% discontinued medication, >50% within the first 6 months. 9.5% had poor compliance with recommended dosing. Persistence was associated with previous vertebral fractures and BMD T-score< –2.5 or corticosteroid treatment. (RR for discontinuation when no BMD = 1.28) Better compliance was associated with early menopause, low BMD, & previous vertebral fractures. |
Solomon et al. (76) found that a BMD test before or after initiation of drug therapy, along with younger age, less comorbidity, and a fracture, were factors that increased compliance with osteoporosis drug therapy. However, other unknown factors appear to influence compliance. Pickney et al. (77) reported that patients who can recall their BMD results accurately were more likely to have been prescribed an osteoporosis medication and to have remained on the initial medication. Rossini et al. (78) reported that more than 50% of patients who discontinued their osteoporosis medication did so in the first 6-months. They also reported that a BMD T-score of –2.5, a previous vertebral fracture, and corticosteroid treatment were associated with persistence with osteoporosis drug treatment.
Clowes et al. (79) compared compliance to raloxifene in women randomized to no monitoring (n=25), nurse monitoring (n=25) or marker monitoring (n=25). Compliance and persistence with drug therapy was monitored electronically without the patients’ knowledge using a device on the container of the drug. The results showed that patients who were followed up by the nurse had significantly better adherence at one year compared with those without monitoring (increased cumulative adherence to therapy by 27%, P=0.04). Monitoring by marker did not result in an additional improvement in adherence or persistence to therapy compared with nurse-monitoring alone. Patients who were given information of a good response to treatment (measured using change in hip and spine BMD and bone turnover marker) showed significantly better compliance with treatment. The highest frequency of nonadherence occurred in the first 3 months of therapy.
Summary of Findings – Bone mineral density as a surrogate for fracture risk reduction
Differences in results of meta-analyses relating to the relationship between BMD changes and fracture risk reduction during osteoporosis treatment were likely due to differences in methodology, i.e. use of trial level means vs individual patient data.
-
There is growing evidence from meta-analyses using individual patient data to suggest that:
Although improvement in BMD is generally associated with a reduction in risk of fractures, the risk reduction is not proportional to the increase in BMD. Greater BMD changes during treatment were not necessarily associated with greater decreases in fracture risk.
Patients may also have significant reduction in fracture risk despite no change or losses in BMD.
Observed BMD changes only accounted for a relatively small proportion of the antifracture treatment effect.
Improvement in spine BMD measured by DXA substantially underestimates the degree to which antiresorptive drugs reduce the risk of vertebral fractures.
It appears that an increase in BMD plays a role in reducing the risk of vertebral fractures with antiresorptive drugs, but there may be other important determinants of bone strength, such as geometry, microarchitecture, activation frequency, and material properties that are not captured by standard densitometry.
Evidence showed that patient compliance with osteoporosis medical treatment is generally low (≤50% at 1 year) and most of the discontinuation of treatment occurred in the first 3 to 6 months after initiation of treatment, suggesting that BMD monitoring even at one year may be too late to prevent patients from discontinuing treatment.
Many factors appear to influence patient compliance with osteoporosis treatment. Evidence suggest that monitoring (e.g. by a nurse) and feedback on response to treatment (e.g. changes in BMD) may improve patient compliance.
Although there is conflicting evidence regarding the influence of BMD monitoring on patient compliance, experts advised that in practice, BMD monitoring plays an important role to motivate patient adherence to osteoporosis therapy because bone-sparing drugs such as bisphosphonates are difficult to take and BMD is the only sensitive and reliable tool available to provide some feedback to patients.
How Often Should Bone Mineral Densitometry Be Repeated During Treatment for Osteoporosis?
Systematic Review: Changes in Bone Mineral Density from Baseline During Osteoporosis Treatment
The frequency of BMD monitoring during treatment would depend on the expected rate of increase in BMD in response to treatment and the precision of the test. If the change in BMD is not expected to exceed the LSC in the first year, repeating the test after one year of treatment probably is not likely to provide meaningful results.
A systematic review was conducted on the rate of change in BMD during drug therapy for osteoporosis. Twenty-nine RCTs on etidronate, alendronate, risedronate, parathyroid hormone and HRT that provided data on BMD changes from baseline in the treatment arm were included (Appendices 14 & 15). Mean percent BMD changes from baseline are summarized in Appendix 16. Some of these results are also presented graphically in Figures 13 to 16. The largest increase in BMD with treatment occurred during the first year of treatment. However, the increase in BMD even during the first year rarely exceeded 5.54%, the LSC for a BMD test with a 2% precision.
Figure 13: Mean (+/-Standard Deviation of Mean) Percent Bone Mineral Density Changes From Baseline in (A) Lumbar Spine and (B) Femoral Neck in Patients Treated with 400 mg Etidronate and Calcium Compared With Calcium Alone.
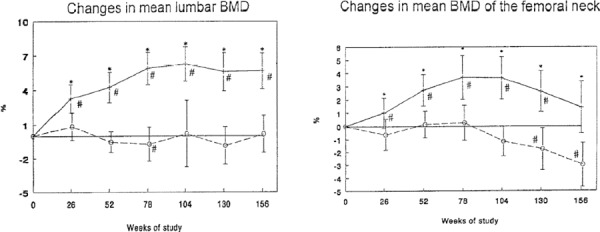
Used with permission. Montessori ML, Scheele WH, Netelenbos JC, Kerkhoff JF, Bakker K. The use of etidronate and calcium versus calcium alone in the treatment of postmenopausal osteopenia: results of three years of treatment. Osteoporosis International 1997; 7(1): 52-58. http://www.iofbonehealth.org/publications/osteoporosis-international.html
Figure 16: Mean Percent Change in Spine Bone Mineral Density in Four Placebo-Controlled Trials in Postmenopausal Women.
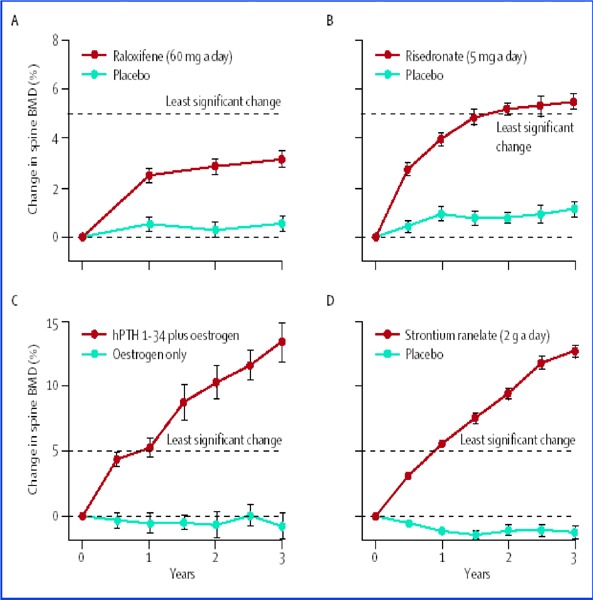
(A). Multiple Outcomes of Raloxifene Evaluation (MORE) Trial on 60 mg raloxifene vs a placebo (Ettinger 1999)(B) The VERT trial on 5 mg risedronate vs a placebo (Harris 1999, JAMA) (C) Parathyroid hormone and estrogen vs estrogen alone (Neer 2001) (D) Strontium ranelate (2g) vs placebo (Reginster 2005). Only the strontium ranelate achieved a mean BMD increase that was close to the 5.4% least significant change after the first year.
Used with permission; This article was published in Lancet, 366(9503), Fogelman I, Blake GM. Bone densitometry: an update. Lancet, 2068-2070, Copyright Elsevier 2005.
In the RCT comparing patients receiving 400 mg etidronate daily for 14 days followed by 76 days of 500 mg elemental calcium daily to patients receiving 500 mg elemental calcium alone, the etidronate group showed an increase in BMD from baseline of 5.67% in the spine and 1.44% in the femoral neck at 3-years follow-up. The BMD increase from baseline at 1 year was 3.7% at the spine and 1.02% at the femoral neck. Although these changes exceeded the LSC in the research (0.78% based on the reported CV of 0.28%), (80) it is doubtful whether this value will exceed the LSCs of BMD tests in the clinical setting.
Figures 14A to 14D show that with 2.5 mg and 5 mg of alendronate, the increase in BMD never exceeded the LSC of a 2% precision test regardless of the site of measurement. (81)
Figure 14: Mean Percent Change in Bone Mineral Density of (A) Lumber Spine (B) Total Hip (C) Total Body (D) Distal Third Forearm in Postmenopausal Women on 5mg Alendronate, 2.5 mg Alendronate, and Placebo.
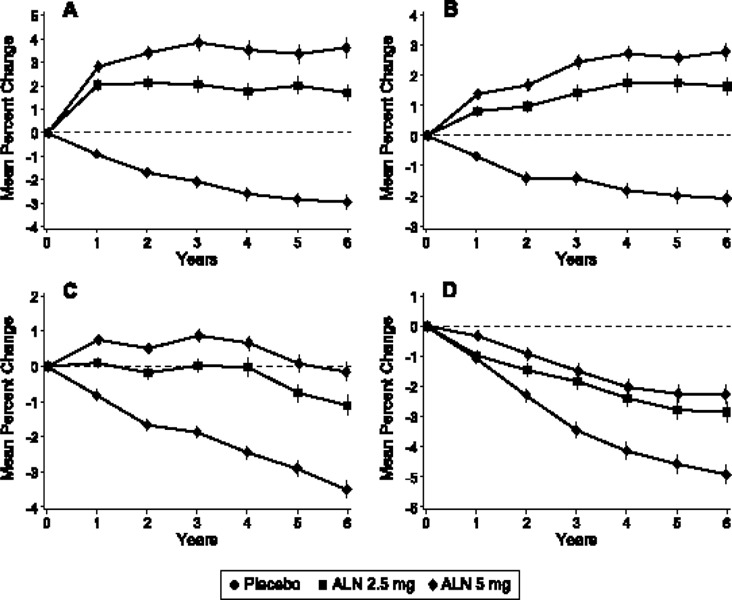
Copyright 2004, Endocrine Society. McClung MR, Wasnich RD, Hosking DJ, Christiansen C, Ravn P, Wu M et al. Prevention of postmenopausal bone loss: six-year results from the Early Postmenopausal Intervention Cohort Study. The Journal of Clinical Endocrinology and Metabolism, 2004; 89(10): 4879–4885.
Figures 15A and 15D illustrate changes in lumbar spine and hip BMD during 10 years of treatment with alendronate. Only the mean increase in BMD for patients on 20 mg of alendronate exceeded the LSC after the first year. The mean BMD in patients treated with 10 mg of alendronate exceeded the LSC only after 2 years of treatment, and patients receiving 5 mg alendronate after 3 years of treatment. (82)
Figure 15: Mean Percent Change in Spine (A) and Total Hip (B) Bone Mineral Density Over 10 Years in Postmenopausal Women on 10 mg, 5 mg, Alendronate & Discontinued After 2 Years on 5 mg Alendronate.
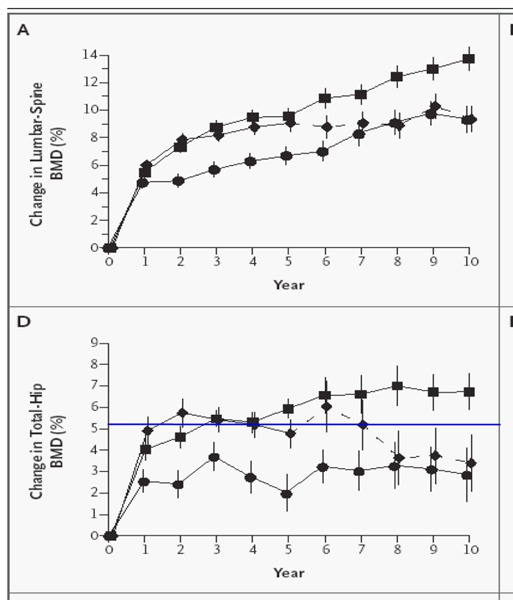
Source: Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP et al. Ten years’experience with alendronate for osteoporosis in postmenopausal women. New England Journal of Medicine 350(12):1189-99, 2004. Copyright ©2004 Massachusettes Medical Society. All rights reserved.
Legend
• 5 mg alendronate group
♦ discontinued after 2 yrs on 5 mg alendronate
▪ 10-mg alendronate group
Mean +/- standard deviation percent changes in BMD over 10 years at the lumbar spine (A) and total hip (D)
Horizontal line indicates least significant change for a BMD test with precision of 2%
Among raloxifene, risedronate, parathyroid hormone or strontium ranelate, only strontium ranelate resulted in an increase in BMD close to LSC of a 2% precision test after the first year of treatment (Figure 16). (83)
Although most of the drug studies showed that it took more than 1 year to achieve an increase in BMD greater than a LSC of 5.4%, there were a few exceptions. Evio et al. (84) reported that treatment of postmenopausal women with 10 mg alendronate resulted in a 6.8% increase in spine BMD from baseline after 1 year of therapy, and a combination of alendronate and HRT resulted in an increase of 8.4% from baseline BMD. Black et al. (85) reported a 6.3% increase in the lumbar spine BMD in postmenopausal osteoporotic women after 1 year of treatment with parathyroid hormone, and a 6.1% increase after 1 year of treatment with combined parathyroid and alendronate. Ringe et al. (86) reported an increase of 8% from baseline lumbar spine BMD in osteoporotic men after 1 year of treatment with 10 mg of alendronate.
Cummings et al. (55) reported on the findings of BMD monitoring in 2 RCTs, the MORE Trial and the FIT trial. The FIT trial is a multicenter RCT that compared postmenopausal women with femoral neck BMD of 0.68 g/cm2 or less assigned to treatment of alendronate sodium, and a similar cohort being treated with a placebo. The MORE Trial included postmenopausal women aged 80 years or younger with femoral neck BMD T-score of-2.5 or less or who had at least 1 moderate or 2 mild vertebral fractures detected by spine radiographs. Study subjects were randomly assigned to treatment with 60 or 120 mg/day of raloxifene hydrochloride or an identical looking placebo. BMD was monitored annually for 2 years in the lumbar spine and hip in the FIT study and in the femoral neck in the MORE study. Cummings et al. (55) analyzed changes in BMD in both studies with a total of 3,954 patients. The results showed that in both studies, women with the greatest loss of BMD during the first year of treatment were the most likely to gain BMD during the second year of treatment. Women taking alendronate whose hip BMD decreased by more than 4% during the first year, 83% (95% CI, 82% – 84%) had increases in hip BMD during the second year, with an overall mean increase of 4.7%. In contrast, those women who seemed to gain at least 8% during the first year lost an average of 1% (85% CI, 0.1%–1.9%) during the second year. Similar results were observed among women taking raloxifene for 2 years. Cummings et al. (55) attributed this phenomenon to the principle of regression to the mean, a natural correction of random error in the earlier estimation of change in BMD. In regression to the mean, individuals who have measurements that differ from the mean of a population tend to have repeat measurements that are closer to the mean, and this tendency is greatest for measurements that are farther from the mean.
Expert Opinion
Experts consulted by the Medical Advisory Secretariat indicated that even though BMD may not be a perfect surrogate for reduction in fracture risk and monitoring response to osteoporosis therapy, BMD testing is presently the most reliable test available for this purpose. The test serves a few purposes. It enables practitioners to identify people who are losing BMD during treatment and to assess whether the patient is taking the medication appropriately. The test also enables physicians to make adjustments to the treatment regimen as required. Bone-sparing drugs such as bisphosphonates are difficult to take and feedback on BMD changes plays an important role in motivating patients to continue with osteoporosis treatment.
Summary Statements - Frequency of Bone Mineral DEnsity Monitoring During Treatment
Although most of the studies showed that mean increases in BMD during treatment did not exceed the LSC after the first year of treatment, there were exceptions to individual patients (due to variability) and exceptions with medications (e.g. parathyroid hormone). Hence it is appropriate to repeat BMD testing 1 to 2 years after initiation of therapy depending on the type of medications used.
After one year of treatment, BMD results may not reflect responsiveness to osteoporosis therapy. People who had no BMD increase during the first year of treatment tend to have a greater increase in the second year. For these reasons, people who have little or no increase in BMD during treatment should not discontinue their therapy.
A lack of response or decrease in BMD after the first year of treatment should alert the physician that the patient may not be taking the treatment appropriately.
Gap: Bone Mineral Density Testing and Treatment After a Fragility Fracture
Analysis of BMD utilization data in Ontario showed that less than 20% of people 65 years of age and older who had a fragility fracture underwent BMD testing, and only 40% received osteoporosis treatment. About half of the people who received treatment did not have a BMD test. The percentage of men undergoing BMD assessment after a fracture was even lower (about 10%). These findings are consistent with results of studies performed in Canada as well as in other jurisdictions. (Appendix 17)
A systematic review by Papaioannou et al. (87) reported that the prevalence of BMD testing after a fragility fracture ranged from 22% in an Ontario community fracture clinic at 1 year after fracture, to 26% after rehabilitation in an Edmonton study. A retrospective population-based study conducted in the province of Quebec showed that the rate of BMD testing after a fragility fracture was approximately 5% in men and 13% in women, while the rate of treatment was 10% in men and 30% in women. (88)
Low rates of BMD testing and/or treatment after fractures were reported for many countries including the United States, (89) the United Kingdom, (90) and France. (91) A systematic review conducted by Elliot-Gibson et al. (51) that included 37 observational studies, reported that the rate of investigation after a fragility fracture, primarily using BMD measurements, ranged from 0.5 to 32% (median 11%). Only 25% of studies reported treatment rates greater than 10%. Giangregorio et al. (92) conducted a systematic review of 35 studies and reported that BMD was measured in 1 to 32% of patients, and laboratory tests were performed in 1 to 49% of patients for investigation of osteoporosis following a fracture. A diagnosi of osteoporosis diagnosis made in 1 to 45% of patients. A subsequent refracture occurred in 1 to 22% of patients during 6-month to 5-year follow-up. (92)
In order to determine the importance of BMD testing and osteoporosis treatment after a fragility fracture, the Medical Advisory Secretariat conducted 3 systematic reviews to examine (1) the impact of a fragility fracture on the risk of future fractures, (2) the impact of a BMD on the likelihood of osteoporosis treatment and (3) the effectiveness of treatment in reducing the risk of fractures.
Medical Advisory Secretariat Systematic Review: Impact of a Previous Fracture on Risk of Subsequent Fractures
Accelerated bone mineral loss of 5.4% from the contralateral femoral neck and 2.4% from the lumbar spine in the year following a fracture has been reported. (93) Since fractures are the most important consequence of low BMD, a systematic review was conducted to examine the relationship between a previous fracture and risk of subsequent fractures. The review included 3 meta-analysis, (94-96) and 13 observational studies published after the meta-analyses. The meta-analyses and current review included large international multicenter population studies with one study exceeding 200,000 people. The studies are mainly prospective longitudinal studies with follow-up periods ranging from 5 to 10 years. The description of these studies is summarized in Appendix 18, and the findings of the studies regarding the impact of a previous fracture on risks of subsequent fracture risks is summarized separately for women and for men in Tables 19 and 20.
Table 19: Impact of Previous Fractures on the Risk of Subsequent Fractures in Women*,†.
| Study | Prevalent fracture | Incident Vertebral fracture RR (95% CI)) | Incident Hip Fractures RR (95% CI) | Incident fracture RR (95% CI) |
|---|---|---|---|---|
| Klotzbuecher et al., 2000 (94) Meta-analysis (peri/post menopausalwomen) (33 studies 1996–1999) |
Wrist Vertebral Hip Pooled |
1.7 (1.4, 2.1) 4.4 (3.6, 5.4) 2.5 (1.8, 3.5) 2.0 (1.6,2.4) |
1.9 (1.6–2.2) 2.3 (2.0,2.87) 2.3 (1.5, 3.7) 1.8 (1.6,2.2) |
3.3 (2.0, 5.3) (Wrist) 1.4 (1.2–1.7) (Wrist) 2.4 (1.9, 3.2) pooled 1.9 (1.3, 2.8) Wrist 2.0 (1.8, 2.1) Pooled |
| Kanis et al., 2004 (95) Meta-analysis (11 Studies 1994–2003) |
Prior fracture | Without BMD 1.77 (1.49–2.11) With BMD 1.56 (1.23–1.98) |
Without BMD – any fracture 1.84 (1.72–1.96) Osteoporotic fracture 1.85 (1.70–2.01) With BMD Any fracture 1.73 (1.59–1.88) Osteoporotic fracture 1.74 (1.57–1.92) |
|
| Haentjens et al., 2003 (96) Meta-analysis For postmenopausal women (9 Studies 1982–2001) |
Colles Spine |
RR 1.53 (1.34–1.74) RR 2.20 (1.92–2.51) |
||
| Johnell et al., 2004 (98) Immediately following fracture for women |
@ age 60 years Shoulder Spine Hip @ age 80 years Shoulder Spine Hip |
10.2 9.1 5.9 1.8 3.5 1.4 |
Hip 18 Hip 7.1 Hip 16.9 Hip 1.5 Hip 3.2 Hip 1.5 |
Forearm 5.2 Forearm 2.3 ‡ Forearm 1.4 ‡ Forearm 2.5 Forearm 1.3 ‡ Forearm 0.7 |
| Papaioannou et al., 2005 (19) (Post-menopausal women) |
Forearm Other nonvertebral |
Main Nonvertebral RR 3.626 (1.876 –7.008) Any nonvertebral RR 2.521 (1.442–4.409) Main nonvertebral 1.975 (1.08–3.540) Any nonvertebral 1.624 (1.030–2.559) |
||
| Bensen et al., 2005 (Canada) (26) | Previous fracture after age 50 years | OR 1.37 (0.931–2.012) | OR 1.08 (0.477– 23.078) | Wrist Fractures OR 1.96 (1.19 –3.22) Rib fracturesOR 2.16 (1.20–3.87) |
| Schousboe et al., 2005 (102) | Prior wrist fracture since age 50 years | OR adjusted for age 1.72 (1.31–2.25) Adjusted for age & BMD 1.39 (1.05–1.83) |
HR adjusted or age 1.43 (1.17–1.74) HR adjusted for age & BMD 1.12 (0.92–1.38) |
|
| Van der Klift et al., 2004 (103) (Mulivariate analysis) | Vertebral Nonvertebral | 4.1 (2.5–6.7) 1.1 (0.7–1.8) |
||
| Porthouse et al., 2004 (104) (UK) (Multivariate analysis) |
Previous fractures | Hip fractures) OR 2.31 (1.31–4.08, P =.004) Non vertebral fractures OR 2.67 (2.10–3.40, P =.000) |
Wrist fractures OR 2.29 (1.56–3.34) | |
| Taylor et al., 2004 (24) SOF (Multivariate analysis) |
Any previous fracture since age 50 yrs | 1.57 (1.34–1.85) without BMD 1.35 (1.14–1.58) with BMD |
||
| Colon-Emeric et al., 2003(105) | Hip | For men & women Unadjusted HR for nonhip fractures skeletal fracture (2.04–3.12) P< .0001 Adjusted HR 1.62 (1.30–2.02) P< .0001 |
||
| Naves et al., 2003 (106) (Longitudinal) |
Vertebral (men & women) | RR 4.7 (1.8–11.9) | Hip RR 6.7 (2.0–22.7) | Colles RR 3 (1.1–7.8) |
| Albrand et al., 2003 (OFELY) (25) Healthy postmenopausal women |
All prevalent fracture (univariate): All prevalent fracture after age 45 yrs (univariate) (multivariate |
Fragility fractures OR 2.72 (1.67–4.40) P<.0001 OR 2.72 (1.67–4.44) OR 3.33 (1.42–7.79) P =.006 |
||
| Pongchaiyakul et al., 2005 (99) | Vertebral deformity | Adjusted HR 11.1 (3.8– 32.3)*,* | HR 2.8 (0.6–11.7)*,* | Colles’ fracture HR 1.7 (0.5–5.2)*,* |
| Hasseius et al., 2003 EVOS (101) Longitudinal |
Vertebral deformity –3 SD vertebral height –5 SD |
Any incident fracture HR 1.8 (1.1–2.9) Any fragility fracture HR 2.0 (1.1 – 3.5) Any incident fracture HR 2.7 (1.4 – 5.1) Any fragility fracture HR 3.8 (1.9–7.5) |
CI refers to confidence interval; HR, hazard ratio; OR, odds ratio; RR Relative risk; SD, standard deviation.
Increased risk still observed after stratification by calcaneal BMD, estrogen use, maternal fracture history, & number of falls
Increase in risk of forearm fracture following a spine or hip fracture was no statistically significant
Table 20: Impact of Previous Fractures on the Risk of Subsequent Fractures in Men*.
| Study | Prevalent fracture | RR for Incident Vertebral fracture (95% CI) | RR for Incident Hip Fractures (95% CI) | RR for All incident fracture (95% CI) |
|---|---|---|---|---|
| Klotzbuecher et al., 2000 (94) Meta-analysis |
Wrist Vertebral Hip Pooled |
3.3–10.7 | No data | 1.8–2.5 |
| Kanis et al., 2004 (95) Meta-analysis |
Prevalent fracture | No data | Without BMD 2.30 (1.56–3.41) With BMD 1.97 (1.12–3.48) |
Without BMD Any fracture 2.02 (1.73–2.38) Osteoporotic fracture 1.93 (1.61–2.33) With BMD Any fracture 2.04 (1.67–2.48) Osteoporotic fracture 1.91 (1.50–2.43) |
| Haentjens et al., 2003 (96) For men ≥ 50 years old Meta-analysis |
Colle’s (men) Spine (men) |
No data | RR 3.26 (2.08–5.11) RR 3.54 (2.01–6.23) |
No data |
| Johnell et al., 2004 (98) Immediately following fracture |
Age 60 Shoulder Spine Hip Age 80 Shoulder Spine Hip |
6.4 6.6 3.7 1.4 3.1 1.1 |
Hip 125 Hip 40.4 Hip 97.5 Hip 2.2 Hip 1.9 Hip 1.9 |
Forearm 43.1 Forearm 9.9 Forearm 6.0 Forearm 15.9 Forearm 4.2 Forearm RR 2.4 |
| Szulc et al., 2005 (97) MINOS (Longitudinal) |
Any prevalent fracture | No data | No data | Any incident fracture 2.07 (1.15–3.76) adjusted |
| Van der Klift et al., 2004 (103) (Longitudinal) |
Vertebral Nonvertebral | 2.2 (0.9–5.0) 2.4 (1.2–4.8) |
No data | No data |
| Colon-Emeric et al., 2003 (105) | Hip (men) | No data | No data | For men & women Unadjusted HR for nonhip fractures skeletal fracture (2.04–3.12) P< .0001 Adjusted HR 1.62 (1.30–2.02) P< .0001 |
| Pongchaiyakul et al., 2005 (99) | Vertebral deformity | Adjusted HR 5.5 (1.3–22.4) |
No data | Colles fracture Adjusted HR 1.9 (0.6–5.9) |
| Naves et al., 2003 (106) (Longitudinal) | Vertebral (men & women) | 4.7 (1.8–11.9) | Hip 6.7 (2.0–22.7) | Colles 3 (1.1–7.8) |
CI refers to confidence interval; HR, hazard ratio; RR Relative risk; SIR Standardized incidence rate (=observed/expected).
Findings
Klotzbuecher et al. (94), conducted a literature review and pooled statistical analysis of the risk of future fracture in people who had a history of prior fracture. The analysis included 33 studies, including prospective cohort studies, case-controlled studies, and cross-sectional surveys published between January 1996 and September 1999. The analysis showed that in women, a prevalent wrist fracture increased the risk of future wrist fractures 3-fold (RR, 3.3; 95% CI, 2.0–5.3), risk of hip fracture 2-fold (RR 1.9, 95% CI 1.6–2.2]), and vertebral fracture by 70% (RR, 1.7; 95% CI, 1.4–2.1). A prevalent vertebral fracture was the strongest predictor for future vertebral fractures, increasing the risk by more than 4-fold (RR, 4.4;, 95% CI, 3.6–5.4). A prevalent vertebral fracture also increased the risk for future hip fracture (RR, 2.3; 95% CI, 2.0–2.87) and wrist fracture (RR, 1.4; [95% CI, 1.2–1.7]). A prevalent hip fracture increased the risk of all fractures by 2-fold or more (RR 2.5 for vertebral fracture, 2.3 for hip fracture and 2.4 for pooled fractures). Klozbuecher reported that in men, a wrist fracture increased the risk of vertebral fractures (RR, 3.3–10) and all incident fractures (RR, 1.8–2.5).
Similar results were reported by Kanis et al., (95) who conducted a pooled analysis of 11 large international prospective cohort studies including 877 men and 4,686 women with 250,000 person years. A previous fracture was associated with a significant increased risk of any subsequent fracture, osteoporotic fracture, and hip fracture at all ages compared with people without a prior fracture. Men and women had similar risk, with relative ratios ranging from 1.93 to 2.30 for men, and from 1.77 to 1.85 for women. The risk ratio was stable with age except in the case of hip fracture where the RR decreased significantly with age.
Haentjens et al. (96) conducted a pooled analysis of 9 cohort studies (1982–2001) to determine the relative risks of subsequent hip fracture in elderly men (>/=50 years) and postmenopausal women who had suffered a Colle’s fracture or spine fracture. The analysis included studies with sample sizes between 36 to 1,905 and follow-up periods ranging from 241 to 40,832 person years. The results were consistent with those of both Klozbuecher et al. and Kanis et al. The impact of a spine fracture on future hip fractures did not differ significantly between genders (RR 3.54 for men vs. 2.20 for women, P= .11). Fractures of the distal part of the radius increased the relative risk of hip fractures significantly more in men than in women (RR 3.26 in men vs. 1.53 in women, PP = .002).
Results of primary studies published since the above systematic reviews lent support to the above findings.
Papaioannou et al. (19) analyzed data for 5,143 postmenopausal women from the Canadian Multicentre Osteoporosis study and reported that a personal history of prior fracture was one of seven independent predictors for incident vertebral and nonvertebral fractures. Prior vertebral fracture appeared to increase the likelihood of developing a clinical vertebral fracture, but was not associated with nonvertebral fractures. A prior forearm fracture appeared to have a greater association with developing an incident main nonvertebral fracture (RR, 3.626; 95% CI, 1.876 –7.008) as compare with any other incident nonvertebral fracture, and is a superior predictor of all incident nonvertebral fractures (RR, 2.521; 95% CI, 1.442– 4.409).
Similar results were reported by Szulc et al. (97) for the Osteoporosis Study in Men sponsored by the French National Institute of Health and Research (MINOS) which included 759 men 50 years of age or older, followed for a mean of 7.5 years. In this cohort, prevalent fractures were associated with a 2-fold increase in the risk of incident fracture (OR 1.28–1.89) when adjusted for age, weight, and BMD, regardless of the site of measurement. For example, for total hip, the OR was 2.07 (95% CI, 1.15–3.76, P = 2.07).
Johnell et al. (98) reported that fracture risk was significantly higher than the general population immediately after a spine, hip or shoulder fracture, especially in younger men (60 years) in whom the RR for new hip fracture reached 125 and RR for or new forearm fracture was 43 immediately after a shoulder fracture. Johnell et al. found that the risk decreased with time.
In addition to fractures, vertebral deformity (usually defined as a reduction of at least 3 SDs in vertebral height from the same-sex normal) has also been found to increase the risk of fractures. Vertebral deformity is a hallmark of osteoporosis and affects at least 20% of the elderly population. (99)
The multicenter European Vertebral Osteoporosis Study (EVOS) (100), a study included in the meta-analysis by Kanis et al., followed 6,344 men and 6,788 women aged 50 years and older from 31 European centers for a median follow-up of 3 years. In this study, Ismail et al. (100) reported that baseline prevalent vertebral deformity was associated with a significant increase in the risk of subsequent hip fracture in women (RR, 4.5; 95% CI, 2.1–9.4), but not in men. The study also found that increasing number of vertebral deformities was associated with an increased risk of all types of limb fractures except distal forearm fracture in both men and women. (Ismail 2001)
A smaller Swedish cohort (101) from the EVOS study (described above), consisting of 298 men and 300 women followed for 10 years, reported that a prevalent vertebral deformity significantly predicted future fractures of any type in both men (age-adjusted HR, 2.7; 95% CI, 1.4–5.3), and in women (age-adjusted HR, 1.8; 95% CI, 1.1–2.9) compared with no vertebral deformity. The predictive value of a prevalent vertebral deformity remained significant after adjusting for age, weight, alcohol consumption, smoking, general health, and previous hip fracture. (101)
Pongchaiyakul et al. (99) conducted a prospective longitudinal study of 114 men and 186 women from the Dubbo Osteoporosis Epidemiology Study (DOES). During 10-year follow-up, people with baseline vertebral deformity had a significantly higher incidence of subsequent fractures (44%) than those without baseline vertebral deformity (27%) with an adjusted relative hazard for any fracture of 2.5 (95% CI, 1.5– 3.9). However, after adjusting for age, sex, and body weight, the effect is only statistically significant for any fractures in women (HR 3.1, 95% CI 1.8–5.4) and in vertebral fractures in both sexes, with an adjusted HR of 5.5 (95% CI, 1.3–22.4) in men and an adjusted HR of 11.1 (95% CI, 3.8–32.3) in women.
Summary:
A fragility fracture at the hip, spine, or wrist is an independent predictor of subsequent fractures not only at the site of the prevalent fracture, but also at other skeletal sites. The presence of a prevalent fracture increases the risk for any incident fractures by approximately 2 fold or more.
Medical Advisory Secretariat Systematic Review: Effectiveness of Osteoporosis Treatment for Reducing Risk of Fractures
Some guidelines have indicated that BMD measurements should only be made if the results will be used to make treatment decisions. Hence the availability of effective treatment for osteoporosis is an important factor for BMD assessments. The ultimate goal of treating osteoporosis is to reduce the risk of future fractures. The Medical Advisory Secretariat reviewed the evidence relating to the effectiveness of osteoporosis drugs listed in the ODB program. These are HRT, antiresorptive bisphosphonates (e.g. etidronate, alendronate, and risedronate), estrogen receptor modifier (e.g. raloxifene), calcitonin, calcium supplements, vitamin D supplements, and parathyroid hormone therapy.
The Osteoporosis Methodology Group and the Osteoporosis Research Advisory Group conducted 9 systematic reviews and meta-analyses of RCTs (107-116) on osteoporosis therapies for postmenopausal women, comparing osteoporotic drugs with placebo or calcium/vitamin D supplements. There were no direct comparisons between treatments. Quality assessment of the RCTs is summarized in Table 21.
Table 21: Methodology of Studies by Therapy.
| Medication | No. of studies | Blinding | Concealed allocation |
Intention to treat | Loss to follow-up |
|---|---|---|---|---|---|
| Etidronate (107) | 13 | 6 | 9 | 12 | 1 trial <1%, 5 trials 5–20%, 7 trials >20% |
| Alendronate(108) | 11 | 11 | 11 | 10 | 2 trials <5%, 6 trials 5–20%, 3 trials >20% |
| Risedronate(109) | 8 | 8 | 6 | 8 | 5 trials >20%, 1 trial>35% |
| Raloxifene(110) | 7 | 7 | 7 | 7 | 1 trial<10%, 4 trials >10%, 1 trial>35% |
| HRT(111) | 57 | 31 | 5 | N/a* | N/a |
| Calcitonin (112) | 30 | 16 | 15 | 4 | 4 trials<1%, 2 trials 2–4%, 13 trials 5–20%, 9 trials >20% |
| Calcium (113) | 15 | 13 | 13 | 1 | 13 trials 5–20%, 2 trials >20% |
| Vitamin D (114) | 25 | 18 | 10 | 9 | 8 trials 10–20%, 13 trials >20% |
| Parathyroid Hormone (115) | 12 | 6 level 1 (adequate sample size, blinding of subjects & assessors), 6 level 2 | 2 trials < 5%, 8 trials 5% – 20% 2 trials >20% |
||
Data incomplete
Used with permission. Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C et al. Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocrine Reviews 23(4):570-8, 2002. Copyright 2002, The Endocrine Society.
Results of the meta-analyses are summarized in Table 22. Tests for heterogeneity were not statistically significant for studies in alendronate, risedronate, and etidronate, indicating consistent results from study to study. The vitamin D results are relatively consistent. Due to potential publication bias for studies in calcitonin with the pooled estimate driven by 3 small trials with large RR reductions, the RR reduction from a large RCT was presented instead of the pooled estimate. (116)
Table 22: Meta-Analysis of Effect of Osteoporotic Drug Treatment on Risk of Fracture and Bone Mineral Density Compared With Placebo in Women with Postmenopausal Osteoporosis*.
| Drug | Vertebral Fracture RR (95% CI) | Nonvertebral Fracture RR (95% CI) | Weighted mean difference in BMD in Lumbar Spine (95% CI) |
Follow-up period for BMD |
|---|---|---|---|---|
| Etidronate (107) | 0.63(0.44–0.92) P =.02 |
0.99(0.69–1.42) P =97 |
4.06(3.12–5.00) † Combined hip 2.35 (3.94, 7.44) † |
1–3 yrs |
| Alendronate vs Placebo (108) | (≥ 5mg) 0.52 (CI, 0.43, 0.65) P =<.01 |
(≥ 10 mg) 0.51 (95% CI, 0.38, 0.69) P<.01 |
Spine 7.48 (6.12–8.85) † Combined hip 5.6 (4.8–6.39) † |
(2–3 yrs) (3–4 yrs) |
| Risedronate(109) | 0.64(0.54, 0.77) P =.01 |
0.73 (0.61, 0.87) P< .01 |
Lumbar spine 4.54 (4.12,.97) † Combined hip 2.73(2.32, 3.15) † |
1.5–3 yrs |
| Raloxifene(110) | 0.60(0.50,0.70) P =.01 |
0.91 (0.79,1.06) P =.24 |
Lumbar spine 2.51 (2.21,2.82) † Combined hip 2.11(1.68, 2.53) † |
2–3 yrs |
| HRT (111) | 0.66(0.41, 1.07 P =.12 |
0.87(0.71,1.08) P =.10 |
Lumbar spine 6.76 (5.63, 7.89) † Combined hip 4.12 (3.45, 4.8) † |
2 yrs |
| Calcitonin (112) | 0.79(0.62, 1.00) P =.05 |
0.80(0.59, 1.09) P =.16 |
Lumbar spine 3.74 (2.04, 5.43) ‡ Combined hip 3.8 (–0.32, 7.91) |
1–5 yrs |
| Calcium (113) | 0.77(0.54, 1.09) P =.14 |
0.86(0.43,1.72) P =.66 |
Lumbar spine 1.66 (0.92, 2.38) † Combined hip 1.64 (0.70, 2.57) † |
2 yrs |
| Vitamin D (114) | 0.63 (0.45, 0.88) P< .01 |
0.77(0.57, 1.04) P =.09 |
Lumbar spine 0.41(–1.40, 2.22) § Combined hip 1.00 (0.22, 1.78) † |
2–5 yrs |
| Parathyroid hormone (115) | 0.31 – 0.35 in postmenopausal women with priorfracture. | 0.3, P = .042 Effect on hip fracture has not been assessed. |
20 or 40 ug/day ⇑ BMD @ lumbar spine & proximal femur in postmenopausal women with prior vertebral fractures. ⇑ in lumbar spine BMD & ⇓ fracture risk greater than alendronate | 11 months – 2 yrs |
CI refers to confidence interval; mg, milligram; RR, relative risk; Yrs, years.
P<.01
P = .07
P = .66
Source: Adapted from Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C et al. Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocrine Reviews 23(4):570-8, 2002
There was statistically significant reduction in the pooled RR for vertebral fractures with Vitamin D, alendronate, etidronate, risedronate, raloxifene and calcitonin compared with placebo, with RRs ranging from 0.6 to 0.79 (Table 24). (107-110;112;114) The CIs around the pooled estimates suggest that the relative risk reduction is unlikely to be less than one-third for alendronate and unlikely to be less than a quarter for risedronate and raloxifene. Calcium supplements and HRT show trends toward reduction in vertebral fracture, but did not reach statistical significance. (117) (116) The number needed to treat (NNT) to prevent one vertebral fracture in the high-risk population was 72 for alendronate and 94–99 for vitamin D, etidronate, risedronate, and raloxifene. (116)
Table 24: Factors Influencing Ordering of Bone Mineral Density Tests in Ontario*.
| Factors that affect ordering of BMD |
Jaglal, 2003 (survey) (130) % of respondents |
Ridout & Hawker, 2000 (129) (Survey) % of respondents |
|---|---|---|
| Risk factors for osteoporosis | 72.4 | 75.8 (M); 84.9(F) |
| Height loss | 61.3 | 19.7 (M); 31.2(F) |
| Recent fracture | 57.7 | 42.4 (M); 48.4(F) |
| Osteopenia on X-ray | 53 | NR |
| Family history of osteoporosis or fracture | 45.8 | NR |
| Decision-making for HRT | 39.1 | 70.8 (M); 86.5(F) |
| Patient is menopausal | 27.0 | NR |
| Patient request | NR | 57.3 (M & F) |
| Follow-up | NR | 48.7 (M); 65.6 (F) |
| Patient had back pain | NR | 21.6 (M); 17.7(F) |
F refers to females; M, Males; NR, Not reported; HTR, hormone replacement therapy.
Alendronate and risedronate were the only 2 drugs that had a significant pooled treatment effect on nonvertebral fracture reduction with RRs of 0.51 and 0.73 respectively (P< .01). The treatment effects were very similar with alendronate across all fracture types. To prevent one nonvertebral fracture in the high-risk population, the NNT was 24 for alendronate, and 43 for risedronate. (116)
With the exceptions of vitamin D and calcitonin, all drugs showed a significant increase in lumbar and spine BMD compared with placebo. The largest treatment effects on the lumbar spine BMD were seen with alendronate and HRT, with intermediate effects seen with risedronate and etidronate. Alendronate, raloxifene, calcium, risedronate, and HRT showed convincing, relatively large effects on BMD in all sites compared with placebo. Greater increases in BMD were observed with higher doses of risedronate, alendronate, and HRT. A dose effect was not observed for calcium or calcitonin. (116)
There were fewer studies on the efficacy of osteoporosis treatment in men that used fracture risk reduction as an outcome. One meta-analysis on alendronate and 2 RCTs on risedronate are summarized in Table 23.
Table 23: Meta-Analysis and RCTs of Effect of Osteoporotic Drug Treatment on Risk of Fracture and BMD Compared with Placebo or Calcium & Vitamin D Supplements in Men with Low BMD*.
| Medication | Vertebral Fracture RR or OR (95% CI) | Nonvertebral Fracture RR or OR (95% CI) |
Increase in BMD % | Follow-up Months |
|---|---|---|---|---|
| Sawka et al., 2005 Alendronate (118) Meta-analysis (2 RCTs) |
OR 0.44 (0.23–0.83) | OR 0.6 (0.29–1.44) | L-spine 7.15 FN 2.5 (Statistically significant) |
2–3 years |
| Sato et al., 2005 (121) Risedronate Double blind RCT |
No data | Hip fracture RR 0.19 (0.04–0.89) NNT for hip fracture = 16 (95% CI: 9–32) |
8 compared with control | 18 |
| Ringe et al., 2006 (122) Risedronate Open label RCT |
RR 0.40 (P = .028) | No significant difference | L-spine 3.7 FN 2.3 compared with control (P< .0001) |
12 |
OR refers to odds ratio; RCT, Randomized controlled trial; RR, Relative Risk.
Sawka et al. (118) conducted a meta-analysis on 2 RCTs that compared the effect of alendronate to alfacalciferol or calcium supplements as a treatment of primary osteoporosis in men. One of the studies was a double- blind study (sample size = 77) (119) and the other was an open-label RCT with a sample size of 118. (120) The analysis showed that alendronate significantly reduced the incidence of vertebral fracture in men with primary osteoporosis, with an OR of 0.44 (95% CI, 0.23–0.83). The OR for nonvertebral fracture was 0.6, but this did not reach statistical significance (95% CI, 0.29–1.44). (Table 23)
In an RCT on risedronate, Sato et al. (121) reported a significant risk reduction of hip fracture (RR 0.19, [95% CI,0.04–0.89) with a mean NNT of 16 (95% CI, 9–32). Ringe et al. (86) reported in a small RCT that risedronate significantly reduced the incidence of vertebral fracture from 12.7 to 5.1% (P = 0.28) while there was no significant reduction in the risk of nonvertebral fractures. (Table 23)
No RCTs were found regarding the effectiveness of etidronate or raloxifene in osteoporotic men.
Adverse events
Upper gastrointestinal (GI) events:
The most common adverse event is upper GI side effects. The pooled RR for GI effects in clinical trials of bisphosphonates compared with controls was not statistically significant (e.g. for alendronate, RR = 1.03, 95% CI, 0.98–1.07, P = 0.23). The pooled RR of discontinuing medication as a result of adverse effects from alendronate in 9 clinical trials was also not statistically significant.(108) An endoscopy study (123) on patients receiving risedronate or alendronate reported that overall, gastric ulcers 3 mm and over were observed in 6% of 300 patients on risedronate and 12% of 297 patients during treatment with alendronate. Helitobacter pylori infection did not increase the incidence of bisphosphonate-related gastric ulcers. In the same study, upper GI events were reported by 5.7% of subjects in the risedronate group, and 8.8% in the alendronate group. The symptoms did not predict the presence of mucosal damage.
Osteonecrosis of the jaws
Since 2004, osteonecrosis of the jaws relating to bisphosphonates therapy has been reported. Woo et al. (124) conducted a systematic review of 10 case series on this complication in 2006. A total of 368 cases were reported, of which 4.1% were patients receiving bisphosphonates for treatment of osteoporosis, while 95% of the cases had multiple myeloma and metastatic carcinoma. These cases manifested as exposure of portions of the bone of the mandible alone (65%), maxilla only (26%), or both. Most lesions were on the posterior lingual mandible near the mylophoid ridge and 60% of the cases occurred after a tooth extraction or other dentoalveolar surgery. Most of the published cases (94%) involved new generation intravenous bisphosphonates such as zoledronic acid and pamidronate, which are not listed on the ODB formulary. For bisphosphonates currently covered by the ODB, oral alendronate accounted for 4.2% and risedronate for 0.3% of the cases. Conservative debridement of necrotic bone, pain control, infection management, use of antimicrobial oral rinses, and withdrawal of bisphosphonates were recommended. (124)
Summary
There is high-quality evidence that in postmenopausal osteoporotic women, treatment with alendronate, etidronate, risedronate, raloxifene, calcitonin, and vitamin D significantly reduces the risk of vertebral fractures, and only alendronate and risedronate also reduce the risk of nonvertebral fractures. There is some evidence that alendronate and risedronate are also effective in reducing the risk of vertebral fractures in osteoporotic men. The most common adverse event is upper GI side effects including ulcers. Osteonecrosis of the jaw has been reported with the majority of cases being patients receiving high dose intravenous bisphosphonate therapy in association with cancer therapy. The degree of risk for osteonecrosis in patients taking oral bisphosphonates such as alendronate, for osteoporosis is uncertain and warrants careful monitoring (124)
Medical Advisory Secretariat Systematic Review: Impact of BMD Test on Likelihood of Osteoporosis Treatment
Since evidence shows that there are treatments that effectively increase BMD and reduce risk of fracture, the next question is whether BMD testing is likely to increase the likelihood of treating people with low BMD and at risk of fractures.
There is evidence from 4 prospective observational studies and 1 retrospective study that people with osteopenia or osteoporosis diagnosed with BMD were more likely to receive counselling for osteoporosis prevention, and treatment for low BMD.
Hamel et al.(125) conducted a Canadian prospective cohort study to determine the impact of low BMD and history of stroke on treatment patterns in 1,300 women (aged >20 years) undergoing their first BMD testing. A questionnaire was administered at baseline and again 3 months after BMD testing. Logistic regression showed that treatment decisions were influenced by BMD testing, but not by a history of fracture. There was a substantial care gap in the treatment of patients with osteoporosis either with bisphosphonate or estrogen. (125)
Fitt et al. (126) conducted a prospective study of 385 women age 50 years or older who were referred to a tertiary care hospital to undergo bone density measurement. The proportion of women with osteoporosis receiving HRT or bisphosphonate therapy increased from 15.2% to 63.3% after diagnosis with densitometry. Independent factors associated with the initiation of either therapy were actual BMD results showing osteoporosis (OR, 7.2; 95% CI, 1.7–30.3), subjects’ perception that their scan showed osteopenia or osteoporosis (OR, 13.5; 95% CI, 4.0–45.5), or they were unclear about the results (OR, 3.4; 95% CI, 1.6-18.8), compared with the perception that the results were normal.
Gallagher et al. (127) conducted a questionnaire survey of 1,004 women (age 40–69 years) regarding receipt of osteoporosis prevention counselling, BMD testing, and information on treatment options. Most of the women with osteopenia or osteoporosis reported receiving information about various treatment options (estrogen therapy, calcium, weight bearing exercise), but only 33% reported communication about pharmaceutical alternatives to estrogen replacement therapy, and 20% about vitamin D supplementation. Multivariate analyses showed that women with multiple risk factors for osteoporosis were not being identified for preventive counselling interventions or BMD testing, and the main trigger to physician counselling of women about osteoporosis and its prevention was an osteopenia or osteoporosis diagnosis (OR range 2.15–5.04). (127)
Pressman et al. (128) analyzed information from the Kaiser Foundation Health Plan Persons database to determine the impact of BMD results on the use of osteoporotic drugs in 8,020 women aged 45 years or older who had BMD testing. Logistic regression was used to explore the association between BMD diagnosis and initiation of drug therapy for osteoporosis including HRT, alendronate, etidronate, raloxifene, and calcitonin, within 6 months after BMD testing. The regression analyses showed that diagnosis of osteopenia or osteoporosis by BMD testing increased the likelihood of initiating osteoporotic treatment by 4-fold and 15-fold respectively. Other factors such as age, high exposure to corticosteroid, and history of osteoporotic fracture also increased the likelihood of treatment initiation, but the association was much weaker. (128)
In a United Kingdom survey (90) of 218 people approximately 3 months after a minimal or medium trauma fracture, factors associated with osteoporosis treatment were explored. Multivariate analysis showed that prior bone density scan was 1 of 2 independent predictive factors for receipt of osteoporosis therapy (OR, 8.9; 95% CI, 3.4–23.3). The other predictive factor was age greater than 50 years (OR, 15.2; 95% CI, 1.9–118).
Summary
Evidence from observational studies in Canada and other jurisdictions suggests that patients who have undergone BMD measurements and particularly if a diagnosis of osteoporosis is made, are more likely to be given pharmacologic bone-sparing therapy.
Factors Influencing the Use of Bone Mineral Density Tests
Based on a survey of practitioners, Elliot-Gibson et al. (51) reported that barriers to post fracture osteoporosis investigation and treatment cited by physicians in Canada and Ireland were cost of therapy, patient reluctance, time and cost of diagnosing osteoporosis, side effects of medication, lack of access to BMD testing, and lack of time to address secondary prevention. Moreover, until recently, many orthopaedic surgeons did not feel that osteoporosis was their responsibility and, therefore, did not investigate or treat this disease.
Ridout and Hawker (129) conducted a survey of 457 family physicians in province of Ontario (Canada) using a self-administered questionnaire, to examine the use of bone densitometry in the primary care setting. The results showed that few Ontario physicians were significantly limited in their use of BMD. The most often cited reasons for ordering the test were presence of risk factors for osteoporosis (79.4%) and decision-making for HRT (Table 24). The only significant limitations to use identified by more than 10% of respondents were travel distance to a densitometer, and concerns regarding the cost of the test. Results also suggest a positive correlation between the frequency of BMD and the physicians’ reported confidence in the use of the test (correlation coefficient r = 0.25, P = .001). Components of a BMD report perceived to be most useful by physicians were the statement of fracture risk, comparison with age-matched controls and suggestion for investigation and management, supporting the inclusion of clinical data in BMD reports.
Jaglal et al. (130) conducted a mailed survey of a stratified random sample of 1,000 Ontario family physicians from the College of Family Physicians’ database. Three hundred and sixty-four practicing respondents (182 male, 182 females) completed the full questionnaire. There were no statistically significant differences in responses by gender or region of practice. More than 80% of family physicians wanted to be more informed about BMD testing and the pharmacological and nonpharmacological management of osteoporosis. The presence of risk factors was one of the most influential reasons (72%) for ordering BMD testing. (Table 24) Information in peer-reviewed journals was thought to be the most credible. More than 80% were interested in a decision aid that incorporates information on risk factors, fracture risk and a treatment algorithm.
Solomon et al. (131) conducted a cross-sectional survey of 494 physicians in 6 New England states to identify factors associated with ordering few BMD scans. The cohort included physicians in general/family practice, internal medicine, and obstetrics/gynaecology. The mean number of self-reported BMD referrals was 10 (SD11) (median 7) per month. In adjusted logistic models, several factors were found to be significantly associated with referring fewer than 4 patients per month for BMD scans. Internists and family practice physicians, physicians who practised in an urban or rural/small town setting, physicians who spent less than 50% of their time in patient care, and physicians who saw a low proportion of postmenopausal women, were more likely to report ordering fewer BMD tests. Physicians who believed that calcium and vitamin D alone are adequate treatment for osteoporosis and that osteoporosis treatment should not be based on BMD measurements also reported ordering fewer BMD tests. Solomon et al. suggests that the above factors should help provide a rational basis for designing educational strategies aimed at physicians.
Other studies suggest that the beliefs of the orthopaedic surgeon are important determinants of BMD testing and osteoporosis treatment after fractures.
Khandwala et al. (132) surveyed 5 orthopaedic surgeons in Saskatchewan who indicated that osteoporosis treatment was not initiated mainly because they would not be involved in the postoperative follow-up of these patients, and that they believed medical treatment of osteoporosis was the sole responsibility of the primary care physicians.
According to a survey (133) of 3,422 orthopaedic surgeons in France, Germany, Italy, Spain, the United Kingdom, and New Zealand, less than one-fifth of the orthopaedic surgeons arranged for a surgically treated patient with a fragility fracture to have a BMD test. Twenty per cent said that they never refer a patient after a fragility fracture for BMD tests. Only half of the orthopaedic surgeons in Southern Europe know about the importance of some external risk factors for hip fractures. (133)
In a questionnaire survey (4) of 117 orthopaedic surgeons and 113 family physicians in the United Kingdom, 81% of the orthopaedic surgeons and 96% of family physicians agreed that low trauma fractures in patients over 50 years old required investigation for osteoporosis. However, only a small percentage of orthopaedic surgeons would routinely assess and start treatment for osteoporosis or refer to an osteoporosis clinic in patients over 50 years old following a Colle’s fracture or a femoral neck fracture (17%). Similarly, without prompting from the orthopaedic surgeon, only 33% of general practitioners would routinely investigate for osteoporosis after a Colle’s fracture and 38% after a femoral neck fracture. Prompting from the orthopaedic surgeons would increase osteoporosis investigation by 22% for Colle’s fracture and 21% for femoral neck fracture. In comparison to wrist fracture and femoral neck fracture, patients with vertebral wedge fractures are relatively well investigated and treated by orthopaedic surgeons (71%) and general practitioners (64%). (4)
Summary
Surveys of physicians and patients suggest that the following are factors that may increase the appropriate use of BMD in high-risk patients:
Improve Communication between orthopaedic surgeons and primary care physicians.
Alert primary care physicians of fragility fractures and prompt osteoporotic investigation and treatment
Improve physician confidence in the use of BMD
Improve how BMD results are reported to be more meaningful to physicians
Algorithms that assist the integration of clinical risks factors and BMD results.
Improve knowledge on decision-making based on BMD
Improve knowledge on pharmacologic therapy for osteoporosis
Easy access to a BMD facility
Patients with fragility fracture represent a target population in whom the use of BMD testing and subsequent treatment can be optimized. Since orthopaedic surgeons are usually the first medical practitioner to see osteoporotic fractures, they play a crucial role in initiating osteoporosis investigation and treatment in patients with a fragility fracture. The World Orthopaedic Osteoporosis Organization strongly advocates a leading role for orthopaedic surgeons in the management of osteoporosis in their fragility fracture patients. (51;134)
Interventions That May Increase the Use of Bone Mineral Densitometry After a Fracture
Many initiatives have been developed in Canada and other jurisdictions to improve the investigation and treatment of osteoporosis in patients after a fragility fracture. A few examples are provided below.
The most recent development is an integrated-care delivery model for post-fracture care in Ontario developed by Jaglal et al. (135). Based on a questionnaire survey of 178 hospitals, 4 patient focus groups, 4 physician focus groups and 34 key informant interviews with community-based organizations, Jaglal et al. concluded that there is a lack of communication between hospitals and primary care physicians regarding occurrence of a fracture, and that there is a lack of continuity and integration of care provided by different health care professionals. The integrated model developed by Jaglal et al. (135) focuses on improving emergency department/fracture clinic communications, increasing follow-up investigation for osteoporosis by family physicians, incorporating other health care professionals and community programs and telemedicine multidisciplinary osteoporosis clinic for areas without access to a family physician.
Hawker et al. (136) conducted an intervention study in 298 fragility fracture patients (139 in intervention and 139 controls) in 5 large fracture clinics in Toronto, Ontario. Intervention consisted of informing patients during their fracture clinic visit about the risk of having osteoporosis in association with their fracture, informing the patients’ primary physicians, and doing an interview with patients. Despite a longer follow-up period for controls, patients in the intervention group were more likely to have follow-up up with a physician regarding diagnosis of osteoporosis (OR, 1.81; 95% CI, 1.12-2.93) and more likely to have received a BMD testing (adjusted OR, 5.22; 95% CI, 2.43-11.19). The intervention was also associated with an increased likelihood of having been recommended treatment for osteoporosis (adjusted OR, 2.07; 95% CI, 0.93-4.56) but the result did not reach statistical significance. (136).
Bogoch et al. (137) in Toronto, Ontario implemented an Osteoporosis Exemplary Care Program to identify, educate, evaluate, refer, and treat patients considered to be at risk for osteoporosis because of a typical fragility fracture. The program required system modification including a dedicated coordinator and coordination among the orthopaedic unit, Metabolic Bone Disease Clinic, and nuclear medicine unit to provide a continuum of care for the patients. Also included in the program is ongoing education of physicians, staff, and patients to increase knowledge and awareness of osteoporosis. Of the 430 patients who went through the program, more than 95% were appropriately, diagnosed, treated, or referred for osteoporosis care.
Majumdar et al. (138) conducted a controlled trial of a multi-faceted intervention in emergency clinics in Edmonton, Alberta involving 102 patients (55 interventions, 47 controls) years of age and older who were treated for a wrist fracture and their physicians (n= 101). For the intervention group, physicians were faxed reminders that contained osteoporosis treatment guidelines endorsed by local opinion leaders and patient education. Control patients received usual care and information about falls and home safety. Within 6 months of fracture, the intervention increased the rates of BMD testing to 62% vs. 17% in controls (relative increase 3.6, P <.001) and the rates of osteoporosis treatment to 40% compared with 10% in controls (relative increase 3.8, P =.002). Intervention patients were more likely to report a diagnosis of osteoporosis but other patient-reported outcomes did not differ significantly between the 2 groups.
Charalambous et al. (139) introduced a protocol that included either a referral to the osteoporosis clinic, referral to BMD test, prophylaxis treatment, or a note to the patient’s family physician to arrange further osteoporosis investigation. Two months after implementation of the protocol, the number of appropriately managed fracture patients increased from 22% to 75% for hip fracture patients and 0% to 81% in outpatients.
Chevalley et al. (140) referred low-trauma fracture patients under their care to a multidisciplinary osteoporosis team for osteoporosis management. The management proposed was not necessarily the management finally prescribed. Even fewer patients were following the treatment protocol at 6-month follow-up.
Johnson et al. (141) increased the likelihood of a BMD tests by 11.5 times in people attending a fracture clinic by providing education about osteoporosis and offering an opportunity for BMD tests. Full evaluation was provided to patients diagnosed with osteoporosis or osteopenia, or had other risk factors. Vitamin D and calcium supplementation was recommended for people with osteopenia. BMD information was placed in the electronic medical record and low BMD results were also sent to the primary care provider for implementation of specific recommendations. The percentage of people identified for osteoporosis or osteopenia treatment increased 5-fold compared with preintervention (OR, 5.3; 95% CI, 2.8-10.1). The intervention resulted in more patients being treated for low bone mass (23.5% vs. 9.5%, P = .002), OR 2.9 (95% CI, 1.4-5.9).
Gap: Underutilization of Bone Mineral Densitometry in Men
Which Men Are at Risk of Fragility Fractures?
Factors that predispose men to fragility fractures are less well established than in women. The follow sections reviewed 7 systematic reviews and 24 observational studies that pertain to predictors of fragility fractures in men. These studies are summarized in Appendices 19 to 20.
Bone Mineral Density – Does Bone Mineral Density Predict Fractures in Men?
The clinical use of bone densitometry is in the relation between BMD and fracture risk. BMD has been found to be one of the most important determinants of fractures in women. The effectiveness of a BMD test depends on its ability to predict fracture risk, often expressed as the gradient of risk, which is the RR of fracture for every standard deviation decrease in age-adjusted mean BMD (RR/SD). The larger the gradient of risk, the higher is the predictive value. One meta-analysis and 7 studies addressed the predictive value of BMD in men (Table 25).
Table 25: Studies on Bone Mineral Density as a Predictor for Fragility Fractures in Men.
| Study | Design | Patient Sample size (N) & Age |
Relative risk RR or Odds Ratio OR or Hazard Ratio HR (95% CI) for fractures |
Other findings |
|---|---|---|---|---|
| Johnell 2005 (142) | Meta-analysis | N = 38,973 25% men |
RR/SD @ hip for men 2.42 (1.90–3.09) | Gradient of risk not significantly different between men & women |
| Gonelli 2005 (143) | Cross-sectional study | N= 401 men | Hip BMD predicts hip fractures OR 3.42 | |
| Szulc 2005 (97) | Prospective Longitudinal study (7.5 years) | N = 759 men Age >50 years |
OR 1.28 (L spine) to 1.89 (whole body) per SD decrease in BMD Predictive accuracy: AUC 0.643–0.712 | 13.7% of incident fractures occurred in men with low BMD at trochanter and 44% in men with low BMD at ultradistal radius |
| Van der Klift 2002 (144) | Longitudinal (6.3 years) | N =1,377 men 1,624 women |
Low spine BMD RR 2.3(1.6–3.3) | |
| Schuit 2004 (145) | Prospective population-based longitudinal cohort study (Rotterdam) Mean follow-up 6.8 yrs | N = 3,075 men & 4,731 women ≥55 years | HR/SD decrease in BMD Men: all nonvertebral 1.4 (1.2–1.6); hip 2.3 (1.6–3.3) Women: all nonvertebral 1.5 (1.4-1.60; hip 2.1 (1.7–2.5) | T-score < –2.5 identified 21% of nonvertebral fractures in elderly men & 44% in elderly women |
| Pande 2000 (146) | Case-controlled study | N = 62 of 100 consecutive Caucasian men >50 years of age with low trauma fracture & had BMD test vs 100 controls | OR for fracture per SD reduction in BMD = 1.8 for L-spine, 3.1 for femoral neck, 3.9 for trochanter, 4.0 for intertrochanter area, 3.7 for ward’s triangle. | |
| Cauley 2004 SOF(147) | Cross sectional study | N = 317 men & 2,067 Caucasian women Age>50 years |
0.1g/cm2 decrease in BMD associated with 30–40% increase in risk of vertebral fracture in men | |
| Kudlacek 2000 (148) | Cross-sectional study | N = 136 men & 337 women Mean age 60.7 yrs |
Men fractured at a higher BMD level than women OR for gender 3.1 |
Meta-Analysis
The meta-analysis by Johnell et al. (142) was based on individual data from 12 cohort studies conducted in Europe, North America, Australia, and Asia, consisting of 38,973 patients (75% female) with a follow-up of 168,366 person-years. The characteristics of these studies are summarized in Table 26.
Table 26: Summary of Studies Included in the Meta-Analysis of Predictive Value of Bone Mineral Density.
| Study | Publication Year | Country | Sample Size | Age (years) Range/Mean | Female % | Person years | Any fracture | Osteo-porotic fracture | Hip Fracture |
|---|---|---|---|---|---|---|---|---|---|
| CaMos | 1999 | Canada | 8,317 | ≥25 60.9 |
69 | 23,707 | 508 | 262 | 27 |
| EVOS/EPOS | 2002 | Europe | 4,967 | 50-79 63.4 |
55 | 14,702 | 270 | 270 | 15 |
| DOES | 1994 | Australia | 2,071 | ≥60 70.4 |
61 | 15,884 | 516 | 406 | 104 |
| EPIDOS | 1996 | France | 1,180 | ≥75 82.4 |
100 | 3,941 | No data | No data | 289 |
| Gothenburg I | 1997 1998 |
Gothenburg | 1,643 | ≥70 77.6 |
58 | 13, 008 | 283 | 283 | 217 |
| Gothenburg II | 2000 | Gothenburg | 7,090 | 21–89 58.9 |
100 | 29,712 | 441 | 312 | 29 |
| Hiroshima | 1997 2003 |
Japan | 2,596 | 65.1 | 69 | 9,803 | 186 | 89 | 31 |
| Kuopio | 1998 | Finland | 1,755 | 47-56 52.5 |
100 | 8,385 | 177 | No data | No data |
| OFELY | 1997 2000 |
France | 431 | 31-89 64.1 |
100 | 2,140 | 54 | No data | No data |
| Rochester | 1998 2003 |
United States | 993 | 56.7 | 65 | 6,185 | 286 | 241 | 42 |
| Rotterdam | 1998 | Netherlands | 5,776 | ≥55 67.9 |
58 | 34,055 | 682 | 501 | 154 |
| Sheffield | 2004 | United kingdom | 2,152 | 80.0 | 100 | 6,844 | 291 | 242 | 63 |
| Total | 38,973 | 64.6 | 75 | 168,366 | 3,694 | 2,606 | 971 |
Source: Adapted from J Bone Miner Res 2005; 20; 1185-1194 with permission of the American Society for Bone and Mineral Research.
BMD was assessed at the femoral neck by DXA. The Z-score for each cohort was computed from the regression of BMD by age. The results showed no difference in the gradient of risk (predictive power) afforded by BMD at the femoral neck between men and women. The gradients of risk were highest for the prediction of hip fracture, lowest for any fracture, and intermediate for osteoporotic fracture. For hip fracture risk, the gradient of risk per SD was marginally higher in men than in women, but this was not apparent when gradients of risk were examined by unit of BMD or by age. (Table 27)
Table 27: Gradient of Risk Per Standard Deviation Decrease in Z-score and in T-score of Bone Mineral Density in Men and Women*.
| Fracture | RR/SD Decrease in Z-score | RR/SD Decrease in T-score | |||
|---|---|---|---|---|---|
| Gradient of risk RR/SD |
95% CI | Gradient of Risk | 95% CI | ||
| Any fracture | |||||
| Men | 1.47 | 1.34–1.60 | 1.44 | 1.32–1.58 | |
| Women | 1.45 | 1.39–1.51 | 1.46 | 1.39–1.58 | |
| Combined | 1.45 | 1.39–1.51 | 1.46 | 1.40–1.52 | |
| Osteoporotic | |||||
| Men | 1.60 | 1.43–1.79 | 1.55 | 1.40–1.73 | |
| Women | 1.53 | 1.46–1.62 | 1.56 | 1.47–1.64 | |
| Combined | 1.55 | 1.47–1.62 | 1.56 | 1.49–1.64 | |
| Hip | |||||
| Men | 2.42 | 1.90–3.09 | 2.28 | 1.81–2.87 | |
| Women | 2.03 | 1.87–2.21 | 2.18 | 1.99–2.38 | |
| Combined | 2.07 | 1.91–2.24 | 2.21 | 2.03–2.41 | |
CI refers to confidence interval; RR, relative risk; SD, standard deviation.
The study showed that for any fracture and for osteoporotic fractures, the gradient of risk increased significantly with age in both men and women, reaching a plateau at about age 80 (Figure 17). For the prediction of hip fracture, the gradient of risk decreased with age, with no differences between men and women (Figure 18). However, the absolute risk still rose markedly with age. The age-specific risk of hip fracture at a given hip BMD in men was the same in women with the same BMD and age. (142)
Figure 17: Gradient of Risk of DXA Bone Mineral Density for Osteoporotic Fractures in Men and Women.
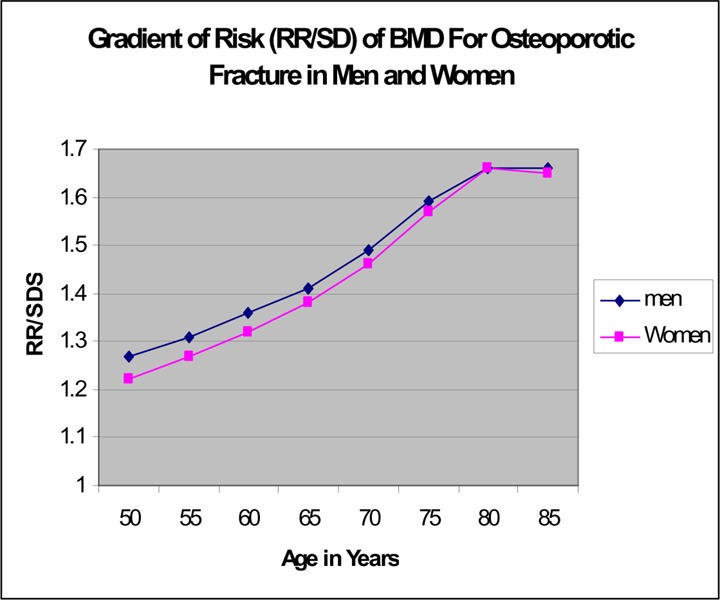
Source: Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res 2005; 20(7): 1185-1194
Figure 18: Gradient of Risk (RR/SD change in Z-score) of Hip Fracture in Men and Women Combined.
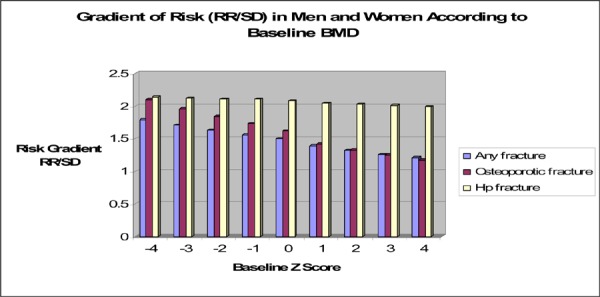
For predicting any fractures or osteoporotic fractures, there was a higher gradient of risk the lower the baseline BMD. For example at a baseline Z-score of –4, the relative risk for osteoporotic fracture was 2.1 per SD (95% CI, 1.63 – 2.71) and at a Z-score of –1, the relative risk was 1.73 per SD (95% CI, 1.59– 1.89). The baseline Z-score did not have a significant impact on the gradient of risk for predicting hip fractures (Figure 18).
Gradients of risk did not change as time elapsed after BMD measurement for all fractures, but had a nonsignificant attenuation for predicting osteoporotic fracture or hip fracture. The decrease in predictive ability was small and did not markedly affect the computation of 10-year fracture probability. (142)
Primary Studies on Bone Mineral Density and Risks of Fractures in Men
The 7 studies not included in the meta-analysis by Johnell et al. (142) included 3 population-based longitudinal studies, 3 cross-sectional studies, and 1 case-controlled study (Table 25). Three of the studies included only men and the other 5 included both men and women. The number of men included in the studies ranged from 62 (146) to 22,444. (145) Follow-up periods of the longitudinal studies ranged from 6.3 years to 16 years. Most of the studies expressed the relationship between BMD and fracture risk in RR, OR or HR for fracture per SD decrease in T-score or Z-score.
Van der Klift et al.,(144) Gonnelli et al., (143) Schuit et al., (145) and Pande et al., (146) all reported that low BMD increased the risk of fragility fractures (Table 25). The reported mean predictive values of BMD in men, expressed as RR/SD, OR/SD, or HR/SD decrease in BMD ranged from 2.42 to 3.42 for the hip and 1.28 to 2.3 for the spine (Table 25). Cauley et al. (147) reported that a 0.10 g/cm2 decrease in real BMD was associated with a 30 to 40% increased odds of a vertebral fracture in men compared with a 60 to 90% increased odds in women.
In the MINOS study, Szulc et al. (97) provided accuracy data based on BMD cut-offs. This study followed 759 French men aged 50 years or more for 90 months to compare the predictive value of BMD T-scores of –2.0 and –2.5 at different sites for osteoporotic fracture. The study found that BMD was predictive of osteoporotic fractures at all sites with ORs varying from 1.28 to 1.89 per 1 SD decrease in BMD (P< .05– .0001). The sensitivity and specificity of the 2 BMD thresholds are summarized in Table 28.
Table 28: Comparison of the Sensitivity and Specificity of Thresholds of T-scores of –2 and –2.5 for the Identification of Men at Risk of Incident Fracture.
| Site of BMD measurement | Threshold BMD* | Sensitivity % | Specificity % | |||
|---|---|---|---|---|---|---|
| T-score < –2.5 |
T-score< –2.0 |
T-score < –2.5 | T-score < –2.0 |
T-score< –2.5 | T-score< –2.0 | |
| Lumbar spine | 0.780 | 0.840 | 9.9 | 21.1 | 95.2 | 89.2 |
| Femoral neck | 0.627 | 0.696 | 5.5 | 17.8 | 98.7 | 90.4 |
| Trochanter | 0.524 | 0.584 | 5.5 | 13.7 | 98.8 | 95.4 |
| Total hip | 0 749 | 0.818 | 9.6 | 21.9 | 97.9 | 89.0 |
| Whole body | 1.010 | 1.058 | 6.9 | 13.7 | 98.2 | 93.2 |
| Distal Forearm | 0.448 | 0.526 | 29.7 | 40.5 | 88.5 | 79.5 |
| Ultradistal radius | 0.364 | 0.388 | 33.8 | 44.6 | 86.0 | 77.0 |
Calculated based on the reference data obtained in young men from the MINOS cohort.
Used with permission. Szulc P, Munoz F, Duboeuf F, Marchand F, Delmas PD. Bone mineral density predicts osteoporotic fractures in elderly men: the MINOS study. Osteoporosis International 2005 16(10):1184-92. http://www.iofbonehealth.org/publications/osteoporosis-international.html
The above data shows that despite a strong association between BMD and fracture risk, the sensitivity of BMD to detect men at high risk of fracture is low. Only 14 to 45% of fractures were observed in men with a T-score of less than –2, and 27 to 45% of fractures occurred in men with a T-score between –1 and –2. The low sensitivity was explained by the limited number of fractures that occurred in men with low BMD regardless of the measured site. Area under the Receiver Operator Characteristics curve ranged from 0.643 (95% CI, 0.592–0.693) for femoral neck to 0.697 (95% CI, 0.627–0.765) for the distal forearm, indicating that BMD itself has a limited value for detecting individual men who will actually have a fracture in the future. Similarly, Schuit et al. (145) reported that a T-score less than –2.5 identified only 21% of nonvertebral fractures in elderly men, even lower than the 44% in women. (97)
The meta-analysis by Kanis et al. (95) found that both the risk of fracture at a specific age and BMD are similar for both genders, but Cauley et al. (147), based on an analysis of the data from 2 longitudinal studies, found that the areal BMD of men with a vertebral fracture was 20 to 38% greater (P <.05) than the areal BMD of a woman with a fracture. For areal BMD, the curves for men and women had different slopes, suggesting a different probability of fracture at absolute levels of areal BMD. Kudlacek et al. (148) also reported that men fractured at a higher BMD value than women (OR for gender 3.1).
Factors that influence BMD in men
A systematic review is on factors that influences BMD in men is being conducted under a guideline initiative of the Osteoporosis Strategy at the ministry. Hence these factors will not be addressed in this report.
Summary Statements
DXA BMD is predictive of hip fractures and osteoporotic fractures at all skeletal sites in men.
DX BMD is equally predictive of fracture in both men and women.
The risk of fracture in men increases 1.5- to 3-fold for each standard deviation reduction in BMD.
DXA BMD at the hip predicts hip fractures with higher gradients of risk than other fractures.
BMD’s ability to predict hip fractures is at least as good as that of blood pressure in predicting stroke, and considerably better than the use of serum cholesterol to predict coronary artery disease.(Kanis 2002)
The predictive value of BMD in men increases with age and with deterioration in baseline BMD.
In men as well as in women, the predictive value of BMD is not significantly attenuated with time after assessment over a 10-year interval, suggesting that it can be used to compute long-term fracture probabilities.
There is conflicting evidence regarding whether men and women have similar fracture risk at the same BMD measurement and same age.
Although BMD is a strong predictor of osteoporotic fractures, it has low accuracy for predicting which individual will actually have a fracture.
Other Risk Factors for Fractures in Men
Knowing risk factors other than BMD that predisposes men to fragility fractures will assist in case finding for BMD testing. Hence meta-analysis and studies on predictors (other than BMD) for fractures in men will be reviewed.
Meta-Analysis:
The CaMos study (19) (discussed in the Background section) identified low BMD, previous history of fragility fracture, comorbid conditions (kidney disease and inflammatory bowel disease) as significant predictors of fragility fractures in women.
Espallargues et al. (149) conducted a large systematic review to identify factors associated with the development of low bone mass and classify these risk factors according to the strength of their association with fracture incidence. This review included 94 cohort studies, 72 case-controlled studies, and 1 RCT. The quality of the studies was assessed to be moderate. Only 57% of the studies included men compared with 94% for women. Based on both qualitative and quantitative analysis, Espallargues et al. classified risk factors for fractures according to level of risks. Table 29 summarizes the high and moderate-risk factors for fracture-related bone mass loss that have point estimates for RR from the meta-analysis.
Table 29: Bone-Mass Related High-Risk Factors for Fractures*.
| High-risk factors | High-risk factors | ||
|---|---|---|---|
| Meta-analysis RR (95% CI) | Range of reported RR | ||
| Aging (>70-80 yrs) | 1.27 (1.22–1.77) for 10 yr increase in age | Primary hyperparathyroidism | 2.56–3.49 |
| Low body weight | 2.35 (1.7–3.14) low vs. high body weight | Diabetes Mellitus Type I | 2.91–3.79 |
| Weight loss | 1.3 (1.09–1.55) for 10 kg weight loss | Anorexia Nervosa | No data |
| Reduced physical inactivity | Risk interval 1.18–7.1 | Gastrectomy | 1.83 (risk interval) |
| Corticosteroids | 1.78(1.37–2.32) 2.15 (1.59–2.91) |
Pernicious anemia | 2.9–3.8 |
| Anticonvulsants | 2.64 (1.82–3.82) † | Prior Osteoporotic fractures | 1.35–21.5 |
CI refers to confidence interval; RR, relative risk;
for femoral neck fractures
Source: Espallargues M, Sampietro-Colom L, Estrada MD, Sola M, Del Rio L, Setoain J et al. Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature. Osteoporos Int 2001; 12(10): 811-822
Moderate-risk factors for fractures included female sex, active smoking, low sunlight exposure, family history of osteoporotic fracture, and surgical menopause. (149)
This systematic review was not specific to men. Moreover, it could not determine whether these factors were independent predictors of fracture when all risk factors were combined or whether they provide additional information beyond other factors since the results were not based on individual patient data.
Studies relating to risk factors for fragility fractures in men are reviewed in the following sections.
Old Age
Kanis et al. (23) explored the relationship between 10-year probabilities of fractures, BMD, and age in Swedish men and women. This study showed that age provided an independent element of risk not captured by BMD. In men, forearm risk is stable with age. For other fractures in men, as for all fractures in women, fracture risk increases with age up to 80 years. Thereafter, the 10-year probability plateaued or decreased since mortality exceeded the fracture risk. (23)
The 10-year probability for hip fracture in men with a T-scoreless than or equal to –2.5 at the femoral neck is 5.1% at age 50 and increases to 24.3% at age 80 with the same T-score. At a T score of –2.5, any difference in 10-year fracture probabilities for hip and vertebral fracture between men and women is not marked, since the same BMD measured at the same site at the same age carries a similar fracture risk in both sexes. For example, a femoral neck T-score of –2.5 at age 85 carries a risk of hip fracture of 10.5 for men and 10.0 for women. At the same age, the 10-year probabilities are higher in women than men.. (23)
Szulc et al. (97) reported that in the MINOS study, the incidence of fractures increased with age (OR = 1.29 per 10-year increase, 95% CI, 1.00–1.64, P = .05). In men aged more than 75 years, the fracture incidence was almost 3 times higher than in men aged less than 55 years. The age-related increment of the fracture incidence was mild but significant and independent of BMD. The results also suggest that age itself is not an independent risk factor for fracture, but rather a surrogate for age-related risk factors such as the risk of falls, lower limb disability, or impaired balance.
Cauley e al. (147) also found that the risk of vertebral fracture increased with age in both men and women. The prevalence was 14%, 20%, and 28% among women and 11%, 13%, and 29% among men aged less than 69 yrs, 70-79 years and 80+ years respectively.
History of Previous Fractures
Evidence presented in the section on previous fractures showed that a history of previous fractures increases the rate of subsequent fractures in men as well as in women.
Body Weight/Body Mass Index and Weight Loss
Previous studies have shown that low BMI and weight loss are associated with increased bone loss in older men (150) (60) (151). Table 30 summarizes 1 meta-analysis (152) and 7 primary studies that explored the impact of body weight, BMI, and weight loss on the risk of fractures in men.
Table 30: Impact of Body Weight, Body Mass Index, or Weight Loss on Risk of Fractures in Men.
| Study | Design (Follow-up) |
Patient | Type of fracture |
Predictive value of Body weight or BMI |
Other findings |
|---|---|---|---|---|---|
| De Laet 2005 (152) | Meta-analysis | >6,000 men & women | Overall: RR of 0.98 per unit increase in BMI Risk gradient highest in BMI<20 kg (RR>1.95) | Independent of age & sex Dependent on BMD | |
| Szulc 2005 MINOS (97) | Longitudinal study (7.5 years) | N = 759 males Age >50 years | Incident osteoporotic fracture | (Unadjusted) OR 1.15 (95% CI, 1.03–1.28) per 5 kg ⇓ in body weight (P< .02) | Fracture incidence 2x higher in men in lowest quartile of body weight compared with men in highest quartile |
| Roy 2003 (153) | Longitudinal (3.8 years) | N = 3,173 men & 3,402 women | Vertebral | Men in lowest quintile BMI compared with others RR = 1.99 (1.01–3.93) | ⇑ in BMI& body weight associated with significant ⇓ in risk of vertebral fractures in men |
| Kelsey 2005 (31) | Case control | N = 192 men & women, 2,402 controls | Pelvis fracture | OR = 0.65 (95% CI, 0.52–0.81) per 5 unit ⇓ in BMI | High BMI protects against pelvis fracture. |
| Holmberg 2005 (154) | Longitudinal (16 yrs for men) | N = 22,444 men 10,902 women | Hip fracture | Age adjusted for BMI RR = 0.63 (0.53–0.76, P = .0001)/SD increase | A high BMI protects against hip fracture |
| Kanis 1999 (155) MEDOS | Case control | N = 730 men >/=50 yrs with a hip fracture Control = 1,132 | Hip fracture | 6.8% increase in fracture risk with each unit decrease in BMI. RR = 0.68 (P< .009) | Effect of BMI on risk of hip fracture was and linear. No significant impact from height. |
| Meyer 1998 (156) | Longitudinal (12 years) | N = 19,151 men & 19,938 women | Hip fracture | Weight variability associated with ⇑ risk of hip fracture (most vs least) RR = 2.70 (95% CI, 1.25–5.86) in men | Effect of weight variability was independent of BMI. Also an association between weight loss and hip fracture RR 2.01 (95% CI, 1.19–3.41) |
| Langlois 1998 (157) | Longitudinal | N = 2,413 white men >age 50 years | Hip fracture | ≥10% weight loss increased hip fracture risk RR = 1.85 (95% CI,:1.04– 3.31) | 5–10% weight loss-no significant ⇑ in fracture risk. Extreme weight gain – only borderline ⇓ in fracture risk. |
BMI refers to body mass index; CI, confidence interval; kg, kilogram; OR, odds ratio; RR, relative risk; SD, standard deviation.
Meta-analysis
De Laet et al. (152) conducted a meta-analysis of 12 prospective studies (>6,000 men and women, >250,000 person years) to explore the relationship of BMI with fracture risk in men and women. The effect of BMI, BMD, age and gender on the risk of any fracture, any osteoporotic fracture, and hip fracture were analyzed using a Poisson regression model in each cohort, separately and for merged results. Without information of BMD, low BMI significantly increases the age-adjusted risk of any fractures, osteoporotic fractures, and hip fractures. The effect of BMI on fracture risk is independent of age or gender, but dependent on BMD. The overall risk gradient for men and women was an RR of 0.98 per unit increase in BMI. The RR for fracture risk with BMI was nonlinear, the RR was markedly higher at the lower values of BMI, particularly with a BMI less than or equal to 20 kg/ m2. For example, the risk for hip fractures decreased by 17% (RR 0.83, 95% CI 0.69–0.99) when the BMI increased from 25 kg/m2 to 30 kg/m2. However, the risk for hip fractures doubled (RR 1.95, 95% CI 1.71–2.22) when the BMI decreased from 25 kg/m2 to 20 kg/ m2. De Laet et al. (152) commented that obesity should not be regarded as an important protective factor for hip fracture risk. Rather leanness should be regarded as a significant risk factor for fractures. The authors also suggested that low BMI could be used to enhance the predictive value of BMI in case finding. After adjustment for BMD, BMI was only predictive of hip fracture risk for men and women at a BMI of 20 kg/m2 or less.
Primary studies
The 7 primary studies included 5 longitudinal studies and 2 case-controlled studies. The number of men included in the studies ranged from 192 to 22,444. The follow-up period for the longitudinal studies ranged from 3.8 to 16 years.
A high BMI has been found to protect against hip and pelvis fractures in both men and women. Kelsey et al. (31) reported an OR of 0.65 (95% CI, 0.52–0.81) for pelvis fracture with a 5 unit increase in BMI, while Holmberg et al. (31) reported an age-adjusted relative risk of 0.63 (95% CI, 0.53–0.76, P = .0001) for hip fracture with every SD increase in BMI.
The Mediterranean Osteoporosis Study (MEDOS) (155) compared 730 men aged 50 years and older who had a hip fracture with 1,132 matched controls who did not have a hip fracture. The results showed that a low BMI was associated with a significantly increased risk of hip fracture in a linear dose dependent manner. In univariate analysis, the risk of hip fracture decreased by a mean of 6.8% (95% CI, 4–9) for every unit increase in BMI. The risk decreased significantly with increasing weight but the increase in risk with increasing height was small and not statistically significant. Other protective factors were consumption of cheese, and exposure to sunlight. A high consumption of alcohol and a long duration of smoking increased the risk for fractures. In multivariate analysis, BMI, leisure exercise, exposure to sunlight, and consumption of tea, alcohol, and tobacco remained independent risk factors, accounting for 54% of hip fractures. The use of risk factors to predict hip fractures had relatively low sensitivity (59.6%) and specificity (61.0%). According to the MEDOS study, these potentially modifiable risk factors are similar to those reported in women from the same study. (155)
The European Prospective Osteoporosis Study (EPOS) (153) which followed more than 3,000 men for a mean of 3.8 years, found that only in men was an increase in BMI significantly associated with a reduced risk of incident vertebral fracture as defined qualitatively (RR 0.76 per 1 SD change in BMI adjusted for age and centre, 95% CI, 0.60–0.97). Neither the height nor weight had significantly influenced the risk of vertebral fracture in either gender. Men in the lowest BMI quintile had an increased risk of incident vertebral fracture (RR, 1.99; 95% CI, 1.01–3.93). (153)
Szulc et al. (97) reported in the MINOS study that in men, the unadjusted fracture risk increased with decreasing body weight (OR = 1.15 per 5 kg decrease in body weight, 95% CI, 1.03–1.28, P< .02). The study also found that men in the lowest quartile of body weight (<71 kg), the fracture incidence was twice as high as in men in the highest quartile. Similar to EPOS, body height was not associated with risk of fracture.
Numerous studies have reported an association between weight loss and increased risk of fracture. Two studies that yielded data on men were found. Langlois et al. (157) analyzed data from the 3 sites of the Established Populations for Epidemiologic Studies of the Elderly (EPESE) with 2,413 community-dwelling white men 67 years of age or older, followed for a mean of 8 years. The overall incident rate of hip fractures was 5.3 per 1,000 person-years. Extreme weight loss (>/=10%) beginning at age 50 years in older men was associated with a significant increase in risk of hip fracture. The unadjusted RR of hip fracture among older men with extreme weight loss was 3 times that of both men with lesser weight loss and those with little change in weight. After adjustment for other risk factors for hip fracture including BMI, older men with a weight loss of at least 10% still had a 2-fold increase in risk of fractures (RR, 1.85, 95% CI, 1.04–3.31). Men with a lesser decrease in weight (5% to <10%) did not have a significant increase in risk of fracture. Conversely, men with weight gain of 10% or less had a borderline significant decrease in the risk of hip fracture (RR, 0.38; 95% CI, 0.14–1.00). Men with extreme weight loss were associated with several indicators of poor health, suggesting that weight loss is a marker of frailty that may increase the risk of hip fracture. (157)
Meyer et al. (156) conducted a prospective longitudinal study of 19,151 men and 19,938 women (mean age 49 years) in Norway over a mean follow-up period of 12 years. Weight variability was calculated from 3 consecutive weight measurements during follow-up. The results showed that in both men and women, those people with the most weight variability had an increased risk of hip fracture (RR, 1.24; 95% CI, 1.25–5.86 in men, and RR, 2.07; 95% CI, 1.24–3.46 in women). Overall, the effect of weight variability was not affected by adjustment for BMI and linear trend in weight change. In men, those losing weight also had significantly higher risk of fracture compared wit men gaining weight (RR, 2.01; 95% CI, 1.19 –3.41). (156)
Parental History of Fractures
Meta-analysis
Kanis et al. (158) conducted a meta-analysis of 7 prospective population studies (12,567 men, 22,361 women, and 134,374 person-years) to explore the relationship between family history of fracture and fracture risk. The risk of fracture was estimated by applying Poisson regression to each cohort and each sex separately. Covariates used in the model included time since start of follow-up, age at baseline, family history of fracture, BMD, and the interaction term, current age × family history and BMD. The results of each cohort and the 2 sexes were weighted according to the variance and merged to determine the weighted mean and standard deviation. The meta-analysis showed that in men, a history of any fracture in a parent was associated with a significant increase in risk ratio for hip fracture (RR, 2.02; 95% CI, 1.18– 3.46) and a sibling history was associated with a significant increase in the risk of any fracture (RR, 1.66; 95% CI, 1.23–2.24) and osteoporotic fracture (RR, 1.58; 95% CI, 1.07–2.32). In women, parental history of any fracture was associated with an increased risk of any fractures, osteoporotic fractures and hip fractures, but there was no significant impact from sibling history of fracture. The risk ratios were generally higher in men compared with women, but the difference was not statistically significant. In men, a family history of hip fracture was not associated with a significant increase in risk of fractures whereas in women, parental history of hip fracture was associated with increased risk of any fracture, osteoporotic fracture, and hip fracture. A sibling history of hip fracture was not associated with significant changes in risk of fractures in men or women.
Long-Term Glucocorticoid Therapy
Glucocorticoids decrease intestinal absorption of calcium and phosphate and increase urinary excretion of calcium. In addition, long-term exposure to glucocorticoids inhibits osteoblast proliferation and reduces sex hormone production. The combined result is a loss of BMD reported as high as 8% in the trabecular bone and 2% in the cortical bone of the lumbar spine over a 20-week period at a mean dose of 7.5 mg/day prednisone. (62) No studies that included only men were found. One meta-analysis (159) was found that included 42,542 men and women with 176,000 person-years from 7 prospective population studies (2 including women only). Three case controlled studies, and 5 cohort studies that were not included in the meta-analysis were also reviewed. (Table 31) All included both men and women.
Table 31: Relative Risks for Fractures in People Receiving Corticosteroid Therapy Glucocorticoid*.
| Study | Type of Glucocorticoid Therapy |
RR vertebral fracture | RR non vertebral fracture | RR hip fracture |
|---|---|---|---|---|
| Kanis et al., 2004 (159) (Meta-analysis) | Ever use vs no use: RR for fractures: no significant difference between men and women | Any fracture (point estimate) Age 50: 1.98 Age 85: 1.66 All ages: 1.57 |
Osteoporotic fracture Age 50: 2.63 Age 85: 1.71 All ages: 1.66 |
Hip fracture Age 50: 4.42 Age 85: 2.48 All ages: 2.25 |
| Van Staa et al., 2000 (161) (Retrospective cohort) |
Oral 7.5mg vs 2.5mg | 2.83 (2.35–2.40) | 1.44(1.34–1.54) | 2.2(1.85–2.64) |
| Vestergaard et al., 2003 (160) (Prospective case controlled) |
Oral vs control 30mg for 4 days Short course of 450 mg7.5 mg/day × 6 months>1,500 mg |
Adjusted OR 0.96 (0.89–1.04) 1.17 (1.01–1.35) 1.36 (1.19–1.56) 1.65 (1.43–1.92) |
||
| Steinbuch et al., 2004 (162) (Retrospective cohort) |
Oral Overall Low dose<10 mg High dose>10 mg <90 days >90 days Test for trend significant P<0.01 – <.001 |
Adjusted 2.92 (2.0–4.3) 2.73 (1.80–4.15) 3.15 (2.07 –4.79) 2.88 (1.96–4.23) 3.27 (1.82–5.87) |
1.68 (1.5–1.9) 1.81 (1.61–2.04) 1.53 (1.35–1.74) 1.68 (1.51 –1.87) 1.69 (1.38–2.07) |
Adjusted 1.87 (1.2–2.9) 1.73 (1.04–2.90) 2.04 (1.22–3.41) 1.69 (1.06–2.70) 3.41 (1.72–6.75) |
| Vestergaard et al., 2005 163) (Prospective case controlled) |
Oral >2.5 mg Inhaled >7.5 mg |
Increased risk of any fracture, hip, spine, & forearm Limited increase in the risk of any fractures but no increased risk in hip, spine, or forearm fracture |
||
| Hubbard et al., 2006 164) (Retrospective cohort) |
Inhaled vs control | Any fracture Adjusted HR 2.53 (1.65–3.89) Trend P <.0001 Adjusted for oral corticosteroid HR 4.21 (2.19–8.13) |
||
| Donnan et al., 2005 (165) (Retrospective cohort) |
Inhaled vs general population | Women compared with men 5.19 (2.95–9.16) | All fractures adjusted 1.90 (1.68–2.16) | |
| Van Staa et al., 2001 (166) (Retrospective cohort) |
Inhaled vs control Inhaled vs bronchodilator |
1.51 (1.22–1.85) 1.00(0.94–1.06) |
1.15(1.10–1.20) | 1.22(1.04–1.43) |
| Hubbard et al., 2002 (167) (Case controlled) |
Inhaled vs control Inhaled vs control (adjusted) |
OR 1.26 (1.17–1.36) OR 1.19 (1.10–1.28) |
HR refers to hazard ratio; OR, odds ratio; RR, relative risk.
Adapted from J Bone Miner Res 2004; 19; 893-9 with permission of the American Society for Bone and Mineral Research
The meta-analysis and primary studies suggest that oral corticosteroid therapy significantly increases the risk of vertebral fractures, nonvertebral fractures, hip fractures, osteoporotic fractures, and all fractures, even after adjustment for BMD. The impact becomes significant at a dose of 7.5 mg for 6 months. (160) The meta-analysis by Kanis et al. suggests greatest impact for hip fractures (RR 2.48-4.42), and in younger men (50 years vs 85 years). A dose response (161;162) and a duration effect (RR 3.27 for vertebral fracture for >90-day therapy vs. RR 2.88 for <90-day therapy) were observed. (162)
Increased risk of any fractures associated with the use of inhaled glucocorticoid therapy was reported (163-165). A slight but significant increase in the risk of hip fractures was also found (RR 1.19–1.26) in people using inhaled glucocorticoid therapy. (166;167) Van Staa et al. reported an increased risk for both vertebral (RR 1.51) and nonvertebral fractures (RR 1.15) in a retrospective cohort study. (166)
Level of Sex Steroids
Sex hormones are important for the growth and maintenance of the skeleton. In women, reduced serum levels of estradiol are associated with an increased risk of incident fractures. (168;169) However, there was conflicting evidence regarding the relationship between levels of sex hormones and risk of fracture in men.
Barrett-Connor et al. (170) studied 352 men (mean age 66 years) and 288 postmenopausal women (mean age 72 years) in the Rancho Bernardo Study. The results showed that men with at least one vertebral fracture had significantly lower levels of total and bioavailable estradiol with no significant differences for other hormones. There was a graded association between increasing concentrations of total and bioavailable estradiol and decreasing fracture prevalence. Men in the lowest quintile of total or bioavailable estradiol had significantly higher odds for vertebral fracture than those in the highest quintile. The OR for total estradiol was 4.16 (95% CI, 1.22–14.19) and for bioavailable estradiol 5.08 (95% CI, 1.20–21.51). Testosterone levels were not associated with vertebral fractures in men in quintile analysis. In women, vertebral fractures were not associated with any of the hormones or with other covariates including BMI, weight loss, alcohol consumption, current smoking, exercise, current use of thiazide diuretics, thyroid hormones, or calcium supplementation.
Mellstrom et al. (171) explored the relationship of sex hormone levels and self–reported prevalent fractures (after age 50 years) in 2,908 elderly men (mean age 75.4 years) in the cross-sectional Swedish MrOS Study. Mellstrome et al. reported that the free level of testosterone was an independent positive predictor of BMD in total hip, total body, femur trochanter and arm, but not in the lumbar spine. Free estradiol was an independent positive predictor of BMD at all sites, especially the lumbar spine. Free estradiol and free testosterone were stronger predictors of BMD than the respective total sex hormone levels. Free testosterone levels below the median were positive predictors of prevalent fractures after 50 years of age (OR, 1.26; 95% CI, 1.04–1.53, P< .05), osteoporotic fractures (OR, 1.47; 95% CI, 1.09– 1.98, P< .05), and prevalent x-ray confirmed vertebral fractures (OR, 1.85; 95% CI, 1.29–2.66, P< .001). The predictive value of free testosterone was not affected by adjustment for BMD, age, height, weight, smoking status, physical activity, and calcium intake. Free estradiol below the median did not significantly predict any of the fracture-related parameters. However, free estradiol in the lowest 10 percentile was a strong positive predictor of X-ray-verified vertebral fractures (adjusted OR, 2.31; 95% CI, 1.39–3.86, P< .001) and height loss of greater than 5mm (OR, 1.63; 95% CI, 1.14–2.34), suggesting that a threshold level exists for free estradiol to affect bone health. (171)
Alcoholism
Kanis et al. (172) conducted a meta-analysis of 3 prospective cohort studies from Canada, Australia, and the Netherlands to quantify, in an international setting, the risk associated with alcohol consumption. The meta-analysis included 16,971 people (5,939 males and 11,032 females with a total follow-up of 75,433 person years). BMD was measured at the femoral neck by DXA at all centres. The risk of fracture was estimated by applying Poisson regression to each cohort and each sex separately. Covariates included current time, current age, alcohol intake, and alcohol intake times current age. There was no significant heterogeneity in risk between cohorts. Intake of alcohol was higher in men than in women (19% of men vs. 4% of women had more than 2 units per day, 8% of men vs. 1% of women took >/= 5 units per day). When assessed as a continuous variable, high intakes of alcohol were associated with an increased risk of osteoporotic fracture or of hip fracture which was not statistically significant (RR for hip fracture 1.07 (95% CI 1–1.3) for men and 1.11 (95% CI 0.98–1.26) for women. When the risk ratio was assessed according to units of alcohol consumed, the risk ratio increased with more than 2 units per day in both men and women. (RR hip fracture = 1.38; 95% CI, 1.21–3.03 for men and RR hip fracture 1.33; 95% CI, 1.01–1.75 for women). The risk ratio increased with higher categories of intake. There was no effect on risk ratio when BMD was added to the model. When intake was dichotomized at more than 2 units daily, there was no confounding effect of smoking or BMI on the association. (A high intake of alcohol confers a significant risk of future fracture, which is over and above that which can be explained by variations in BMD). There was a threshold effect with no increased risk of osteoporosis or hip fracture in individuals who consumed 2 units or less per day of alcohol.
Smoking
Kanis et al. (173) conducted a meta-analysis of 10 prospective cohort studies to explore the risk associated with smoking on future fractures. The meta-analysis included 59,232 people (43,832 women and 15,400 men). Mean age ranged from 52.3 to 80 years and, based on self-reporting, 18% had a history of current smoking. Current smoking was associated with a significantly increased risk of any kind of fracture including osteoporotic or hip fracture in both men and women. For hip fractures alone, there was no difference in risk ratio between men and women. For men and women combined, risk with current smoking was highest for hip fracture (RR = 1.84), lowest for overall fractures (RR = 1.25) and intermediate for osteoporotic fracture (RR = 1.29). Risk ratio was adjusted downward when taking BMD into account and was no longer significant for osteoporotic fracture in women. In men and women combined, low BMD accounted for 45% of the risk for overall fractures associated with smoking, 40% for osteoporotic fractures, and 23% for hip fractures. In multivariate analysis including BMI, and BMD, the risk ratio for smoking remained significant for overall fractures and for hip fractures. A history of smoking was also associated with a significant risk increase for any fracture (RR, 1.19; 95% CI, 1.07–1.51) and specifically for an osteoporotic (RR, 1.18; 95% CI, 1.09–1.27), or hip fracture (RR, 1.38; 95% CI, 1.15–1.65). In summary, smoking carries a modest but significant risk for future fractures. The effect of smoking is over and above that which can be explained by variations in BMD. The risk was greater for hip fracture than for all fractures and osteoporotic fractures.
Summary Statement on BMD in men
DXA BMD at the hip and spine predicts vertebral, non-vertebral, hip fractures, and osteoporotic fractures.
The risk factors for fragility fractures in men are similar to those found in women. The risk factors most predictive of fractures in men (relative risk >/=2) are:
Bone mineral density: a low base line baseline density is predictive of fractures
Age: the risk of fractures increased with age (for same BMD, risk of fracture at 75 years is 3 times the risk at 55 years of age)
A history of previous fractures increases subsequent fractures by 1.5 - to 19-fold (particularly hip fracture)
Family history of fractures, specifically parental history of fractures (RR, 2.02; 95% CI, 1.18–3.46)
Long-term glucocorticoid use
Low levels of free estradiol increases the risk of fractures.
Evidence supports the use of BMD in conjunction with assessment of other risks for fractures (age, history of fractures, family history, and body weight, etc.) to determine risk of fractures in men as in women. Efforts are being focused on developing 5 to 10 year probabilities of fractures based on BMD, age and other risk factors.
Guidelines for Bone Mineral Density Testing in Men
There are presently no Canadian guidelines for BMD testing that are specific to men. Most guidelines address only women. Osteoporosis Canada’s current guidelines recommend screening for both men and women age 65 years and older as well as for younger individuals (between age 50 and age 64) who have risk factors for fractures. Osteoporosis Canada is preparing for submission to Ontario’s Osteoporosis Strategy new guidelines for BMD testing in men. These have not yet been released. (Personal communication, November 17, 2006)
The 2004 official position of the International Society for Clinical Densitometry (ISCD) (18) recommends BMD testing in all men 70 years of age and older and in younger men who have experienced a fragility fracture or had a condition or are taking medication associated with low BMD. ISCD also provided diagnostic definitions for osteoporosis in men (Table 32).
Table 32: International Society for Clinical Densitometry Official Position on Definition of Osteoporosis for Men and Premenopausal Women.
| Men Age (years) | T-score | Risk factors for fracture | Diagnosis |
|---|---|---|---|
| 50–65 | ≤2.5 | Present | Osteoporosis may be diagnosed |
| ≥ 65 years | ≤2.5 | Osteoporosis diagnosed | |
| Any age | Low BMD | Secondary causes or risk factors present | May be diagnosed |
| <50 | Diagnosis cannot be made on basis of densitometric criteria alone. |
Are There Effective Treatments for Osteoporosis in Men?
The section on treatment of osteoporosis showed that, based on evidence from small RCTs, alendronate and risedronate significantly reduce the risk of vertebral fractures in men with osteoporosis, and there is evidence to suggest that risedronate reduces the risk of hip fracture in this population.
Conclusions Regarding Bone Mineral Densitometry in Men
Rates of wrist fractures and hip fractures in Ontario men increased rapidly in the 70 to 74 age group and the 75 to 80 age group.
DXA BMD predicts hip fractures and osteoporotic fractures at all skeletal sites in men as well as in women
The risk of fracture in men increases 1.5- to 3-fold for each standard deviation reduction in BMD.
DXA BMD at the hip predicts hip fractures with higher gradients of risk than other fractures.
The predictive power of DXA BMD for hip fracture in men decreases with age increases with age and with deterioration in baseline BMD. Whereas the predictive power of BMD for osteoporotic fracture in men increases with age.
In men as well as in women, the predictive value of BMD is not significantly attenuated with time after assessment over a 10-year interval, suggesting that it can be used to compute long-term fracture probabilities.
There is conflicting evidence regarding whether men and women have similar fracture risk at the same BMD measurement and same age.
Although BMD is a strong predictor of osteoporotic fractures, it has low accuracy for predicting which individual will actually have a fracture.
-
Since only a small portion of fractures (<50%) occurred in people with low BMD (T-score<-2), other non-BMD risk factors need to be considered when determining the overall risk of fractures and when selecting people for BMD testing. Major non-BMD risk factors for fractures in men are:
Age
History of fragility fractures or loss of vertebral height greater than 3 SD
BMI or weight loss of 10% or more
Family history of fractures (particularly maternal history of hip fracture)
History of corticosteroid use
Alcohol intake in excess of 2 units (18 gram alcohol) per day
History of smoking
There are available treatments that increase BMD and reduce risk of vertebral and hip fractures in osteoporotic men.
Although no formal guidelines have yet been issued for men, epidemiological information and evidence supports the use of BMD testing in:
Economic Analysis
Literature Review
In a Canadian burden of illness study on Osteoporosis, Goeree et al. (2) reported that hip fractures caused greater morbidity, higher mortality and more expenditures than any other fractures combined. In addition, approximately one-third of hip fracture patients may become totally dependent on support and in turn become largely dependent on long-term institutionalization for care. They also reported that between 12 to 40% of all hip fracture patients die within 6 months, and the excess mortality rate has been reported to be between 12 to 25% during the first year following a hip fracture.
According to Wiktorowicz et al. (12), the mean one year cost of hip fractures in Canada was estimated at $26,527 (Cdn). However, this cost varied by the patient’s place of residence, age and survival to one year. The authors estimated the average cost (Cdn dollars) of hospitalization for those admitted for a hip fracture to be approximately $21,385 for community residents returned to the community, $44,156 for community residents transferred to long-term care, $33,729 for long-term care residents and $15,498 for those who died within 1 year of hospitalization. They estimated that the annual economic implication of hip fracture in Canada was $650 million in 2001 and was expected to increase to $2.4 billion by 2041. They also reported that among patients who experienced a fracture, the risk of a future fractures increased 20-fold.
In addition to reducing the burdens associated with osteoporosis, one of the objectives of increasing BMD testing is to improve patient management associated with low BMD. A study by Stock et al. (174) suggests that only 30% of physicians who receive a short version of a BMD test report understand it compared with86% of those who receive a more comprehensive BMD test report. The longer reports led to greater modifications in pharmacologic treatment of osteoporosis and less confusion about reports by physicians.
Ontario-Based Economic Analysis/Budget Impact Analysis
Notes & Disclaimer
The Medical Advisory Secretariat uses a standardized costing methodology for all of its economic analysis of technologies. The main cost categories and the associated methodology from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative (OCCI) cost data is used for all program costs when there are ten or more hospital separations or one-third or more of hospital separations in the ministry’s data warehouse are for the designated ICD-10 diagnosis and CCI procedure codes. Where appropriate, costs are adjusted for both hospital specific or peer-specific effects. In cases where the technology under review falls outside the hospitals that report to the OCCI, PAC-10 weights converted into monetary units are utilized. Adjustments may need to be made to ensure that the relevant Case Mix Group is reflective of the diagnosis and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis/procedure, the MAS normally defaults to considering direct treatment costs only. Historical costs have been adjusted upward by 3% per annum representing a 5% inflation rate assumption less a 2% implicit expectation of efficiency gains by hospitals. Non-Hospital: These include physician services costs obtained from the Provider Services Branch of the Ontario Ministry of Health and Long Term Care, device costs from the perspective of local health care institutions and pharmaceutical costs from the Ontario Drug Benefit formulary list price. Discounting: For all cost-effective analysis, discount rates of 5% and 3% are used as per the Canadian Coordinating Office for Health Technology Assessment (CCOHTA) and the Washington Panel of Cost-Effectiveness, respectively. Downstream cost savings: All cost avoidance and cost savings are based on assumptions of utilization, care patterns, funding and other factors. These may or may not be realized by the system or individual institutions.
In cases where a deviation from this standard is used, an explanation has been given as to the reasons, the assumptions and the revised approach.
The economic analysis represents an estimate only, based on assumptions and costing methodologies that have been explicitly stated above. These estimates will change if different assumptions and costing methodologies are applied for the purpose of developing implementation plans for the technology.
Cost-Effectiveness Analysis of Increasing BMD Testing and Improving BMD Reporting Following a Fragility Fracture
Analyses of Ontario data suggest that only about 19% of people underwent BMD testing and about 40% received treatment after a fragility fracture. The analysis also suggests that people were more likely to be treated with antiresorptive drugs following a fracture if they had undergone BMD testing during the first year after the fracture. There is evidence from RCTs that antiresorptive drugs significantly reduce the risk of fragility fractures. These findings support increasing the use of BMD tests following a fragility fracture. A cost-effectiveness analysis was conducted on increasing the use of BMD testing in and improving reporting for postmenopausal women aged 65 years and older during the first year following a wrist or hip fracture. The following data were used in the analysis.
Incidence
Based on administrative data, 2,737 males and 10,706 females aged 65 years and older in Ontario experienced either a hip or wrist fracture in 2005. These figures represent approximately 1% of the total population of males and females aged 65 and over in Ontario.
Costs
All costs are in Canadian dollars unless specified otherwise.
Physician Costs
Total cost of a BMD = $106 (46)
Hospital Costs (Average Costs)
Due to a lack of data from the Ontario Case Costing Initiative, all hospital costs were obtained from a Canadian study on the economics of hip fractures by Wiktorowicz et al. (12) and adjusted to present value using a discounting rate of 5%. Annual hospital costs associated with hip fractures were estimated for 4 separate categories of patients as follows:
Community residents returned to the community = $27,159
Community residents transferred to long-term care (LTC) = $56,078
LTC residents = $42,836
Patients who die within one year of hospitalization = $19,817
Other Costs
The average annual cost of treatment for low BMD with biphosphonates = $391. This estimate was based on weekly doses of 70mgs of alendronate (fosamax) and 35mgs of risedronate sodium (actonel). The cost of a 70mg tablet of alendronate is estimated at $6.195, and the cost of a 35mg tablet of risedronate sodium is estimated at $8.85. (175)
Decision Analysis
A decision analysis was conducted using TreeAge Pro 2006 software to compare 3 different options for bone mineral densitometry testing among women aged 65 years and older with a previous history of wrist or hip fracture over a one-year time frame. These 6 different options were as follows:
1) Status Quo testing (22% of women 65+with previous hip/wrist fracture)
2) Status Quo testing (22% of women 65+with previous hip/wrist fracture) with improved reporting of BMD tests
3) Increased BMD testing (50% of women 65+with previous hip/wrist fracture)
4) Increased BMD testing (50% of women 65+with previous hip/wrist fracture) with improved reporting of BMD tests
5) Increased BMD testing (80% of women 65+with previous hip/wrist fracture) with improved reporting of BMD tests
6) Increased BMD testing (80% of women 65+with previous hip/wrist fracture)
The rationale behind introducing the “improved reporting” arms of the decision analysis is that, according to expert opinion, physicians may lack the ability to interpret the tests and are not able to fully understand them. Improved reporting has the potential to improve management and lead to higher treatment rates in women with low BMD. This would then lead to a reduction in future hip fractures that are the most resource intensive kinds of fractures. According to Cadarette et al., (176) approximately 80% of women in Ontario aged 65 years of age and older with a prior fracture were found to have low BMD upon testing; ideally these women should be on treatment. This objective of improved reporting to capture the targeted 80% of women aged 65 and older with a previous hip or wrist fracture who have low BMD was used to model the “improved reporting” arms of the decision analysis.
Assumptions
The assumptions used in the decision analysis were as follows:
Of the patients who experienced hip fracture, 8% sustained a repeat hip fracture within the first year. (177)
Risk of an incident hip fracture following a prevalent wrist fracture is similar to that following a prevalent hip fracture.
Approximately 80% of women with a prior wrist fracture had low BMD; these women should be on treatment.
Treatment with biphosphonates reduces the risk of a future hip fracture by approximately 50%. (116)
Figure 19 illustrates the different strategies that were considered to determine the most cost-effective strategy for increased BMD testing and reporting.
Figure 19: Decision Tree to Determine the Cost-effectiveness of Various Strategies for Bone Mineral Density Testing Among Women Aged 65 Years and Older Following A Wrist or Hip Fracture in Ontario.
Table 33 illustrates the probabilities that were used in each of the chance nodes (indicated by green circles) in the decision analysis.
Table 33: Probabilities Used in the Decision Analysis*.
| Decision Node | BMD testing after wrist/hip fracture | Women received treatment after BMD test | Woman received treatment without BMD test | Future hip fracture without treatment | Future hip fracture with treatment | Alive after future (second) hip fracture | Second hip fracture survivors who were “community residents returning to the community” | Second hip fracture survivors who were “community residents transferred to LTC” | Second hip fracture survivors who were “LTC residents” |
|---|---|---|---|---|---|---|---|---|---|
| Status Quo (22%) | 0.22 | 0.66 | 0.32 | 0.12 | 0.06 | 0.78 | 0.67 | 0.12 | 0.21 |
| Status Quo (22%) with improved reporting | 0.22 | 0.80 | 0.32 | 0.12 | 0.06 | 0.78 | 0.67 | 0.12 | 0.21 |
| Increased BMD testing (50%) | 0.50 | 0.66 | 0.32 | 0.12 | 0.06 | 0.78 | 0.67 | 0.12 | 0.21 |
| Increased BMD testing (50%) with improved reporting | 0.50 | 0.80 | 0.32 | 0.12 | 0.06 | 0.78 | 0.67 | 0.12 | 0.21 |
| Increased BMD testing (80%) | 0.80 | 0.66 | 0.32 | 0.12 | 0.06 | 0.78 | 0.67 | 0.12 | 0.21 |
| Increased BMD testing (80%) with improved reporting | 0.80 | 0.80 | 0.32 | 0.12 | 0.06 | 0.78 | 0.67 | 0.12 | 0.21 |
BMD refers to BMD testing; LTC, long-term care.
Table 34 summarized the results from the decision analysis for all 6 different strategies under consideration.
Table 34: Results of the Decision Analysis for Ontario Women Aged 65+ With a Previous Hip or Wrist Fracture.
| Strategy | Cost (Cdn $) | Number hip fractures | Cost/fracture ($ Cdn) | Total number of fractures | Total fractures avoided | Cost Avoided ($ Cdn) | |
|---|---|---|---|---|---|---|---|
| 1 | 2,138 | 0.06 | 33,302 | 687 | |||
| 2 | 2,112 | 0.06 | 33,524 | 674 | 13 | 278,356 | |
| 3 | 2,059 | 0.06 | 34,089 | 647 | 41 | 845,774 | |
| 4 | 1,999 | 0.06 | 34,705 | 617 | 71 | 1,488,134 | |
| 5 | 1,974 | 0.06 | 35,000 | 604 | 84 | 1,755,784 | |
| 6 | 1,878 | 0.05 | 36,255 | 555 | 133 | 2,783,560 |
A decision analysis showed that increasing the rate of BMD testing following a fragility fracture in people age 65 years and older and improving BMD reports to physicians would be cost-effective, resulting in a cost-effectiveness ratio ranging from $2,285 (Cdn) per fracture avoided (worst-case scenario) to $1,981 (Cdn) per fracture avoided (best-case scenario).
The analysis also showed that approximately $0.3 million to $2.8 million (Cdn) could potentially be saved in annual downstream costs due to a reduction in hip re-fracture rates among women 65 and over with a previous history of hip or wrist fractures, if they were tested for low BMD within one year after a wrist or hip fracture. If the total number of males aged 65 and over were included in the analysis under the same assumptions that were made for females, the total savings would increase to a range of $0.3 million to $3.5 million.
Budget Impact Analysis (Improving Use of Bone Mineral Densitometry)
Analysis of Ontario utilization data suggests that baseline and serial BMD tests may have been overused in people at low risk of accelerated bone loss, and underused in high-risk men and in people following a fragility fracture. In order to obtain the full benefit of BMD tests, use of BMD in the province should be shifted from low-risk individuals to those at high risk of osteoporosis and fractures. An analysis was conducted on the budget impact of increasing the rates of BMD testing in high-risk men and in people aged 50 years and over following a hip or wrist fracture, while reducing unnecessary testing and lengthening the interval of serial testing in low-risk individuals. Tables 35 and 36 summarize these data according to current utilization within each category as well as expected utilization within each category.
Table 35: Potential Reductions in Inappropriate DXA Bone Mineral Density Testing Within Ontario*.
| Targets for decreasing inappropriate DXA BMD tests | Current number of DXA BMD tests/year | Expected number of DXA BMD tests/year |
|---|---|---|
| Interval between serial testing in low risk individuals (from every 2 years to every 3–5 years) | 35,800 | 26,200–30,800 |
| Decreasing BMD testing in people <65 years and at low risk for accelerated bone loss | 98,000 | 19,600–49,000 |
| Decrease annual testing in < 65 years low-risk individuals | 3,800 | 1,900–3,000 |
BMD refers to BMD testing; DXA, dual-energy x-ray absorptiometry.
Table 36: Potential Increases in DXA Bone Mineral DensityTesting in High-Risk Ontarians*.
| Target areas for increasing use of BMD | Number of DXA BMD claims 2005/06 |
Expected Number of DXA BMD tests if increase utilization in target areas |
|---|---|---|
| BMD after fragility fracture (≥50 years of age) | 4,800 | 15,400–22,400 |
| BMD testing in men | 50,700 | 87,300–109,100 |
BMD refers to BMD testing; DXA, dual-energy x-ray absorptiometry.
At present, there are approximately 35,800 repeat BMDs (within 24-month of the previous test) were performed annually in low risk individuals under the age of 65 years. Analysis in a previous section on repeat BMD in low-risk people not receiving osteoporosis treatment showed that the appropriate interval between two consecutive BMD tests in this population can be between 3 to 5 years, depending on the precision of the test. Increasing the interval of serial testing for low risk individuals from every 2 years (to every 3 to 5 years) could potentially reduce repeat testing by 5,000 to 9,700 per year. For initial and annual testing in low-risk people, the projected utilization was calculated assuming that 50 to 80% of the 2005/06 claims coded as low risk were coded correctly.
The projected increase in BMD tests after a fragility fracture (in people ≥ age 50 years) was estimated based on the assumption of a 50% and 80% increase in BMD testing used in the decision model. The increase in BMD tests among men was estimated assuming that they should account for approximately 20% of all BMD tests (Table 36).
Using these estimated increases and decreases in BMD tests, the net budget impact was determined. The total cost of a BMD test is $106. (46) Table 37 summarizes the annual budget impact of shifting the utilization of BMD tests from areas of overuse to areas of underuse as estimated above. The annual cost avoidance due to a decrease in annual hip refracture rates was also included to estimate overall costs.
Table 37: Budget Impact Summary.
| Low Estimate | High Estimate | |
|---|---|---|
| Total Reduction in DXA BMD Tests | 55,900 | 91,000 |
| Total Increase in DXA BMD Tests | 47,800 | 76,500 |
| Net Reduction in DXA BMD Tests | 8,100 | 14,500 |
| Net Savings ($ million Cdn) | 0.85 | 1.5 |
| Net Savings (including down-stream cost avoidance from decrease in future hip fractures) ($ million Cdn) | 1.2 | 5 |
Unmeasured Costs
This economic analysis did not include the cost avoidance associated with a decrease in refracture rates in fractures other than hip fractures. Hip refractures are the most resource intensive types of fractures and therefore were the focus of this analysis.
Factors for Consideration
Reimbursement Policy
The Ontario Schedule of Benefits (46) presently funds BMD testing every 24 months for low-risk persons. This review showed that for low-risk persons who have a normal baseline BMD (T score <10) and a normal rate of bone loss (≤ 1%/year) established through a repeat BMD at a 3-5 year interval, further testing is probably not necessary for another 7 years.
Factors Influencing the Use of Bone Mineral Densitometry
Access to a BMD Facility may be a factor in northern or rural communities. Current OHIP data show a 10-fold variation in the use of DXA BMD.
Physician knowledge regarding who should be referred for BMD testing may result in inappropriate testing of individuals not at risk.
Practitioner confidence in interpreting BMD reports.
Format of BMD reports - Need to facilitate practitioner interpretation and decision-making by integrating BMD results with clinical risks to predict risk of fractures.
Lack of support mechanisms to alert family physicians of fractures and the need to investigate for osteoporosis (e.g. letter from fracture clinics, other systems for communities with no/limited number of fracture clinics)
Current Initiatives in the Ministry of Health and Long-Term Care
The Ontario Osteoporosis Strategy was announced by the Minister on February 2nd, 2005. The implementation of the strategy began soon thereafter. The Osteoporosis Strategy has five components aimed at health promotion and disease management.
The Strategy (Osteoporosis Action Plan) prepared by the Osteoporosis Action Plan Committee for the Ministry of Health and Long-Term Care and Osteoporosis Canada, identified potential gaps and inappropriate use of BMD and made the following recommendations:
The Ministry of Health and Long-Term Care should develop a mandatory Recommended Use Requisition for BMD testing that would support both appropriate clinical practice and data gathering.
Take steps to ensure that Ontarians have appropriate, equitable and timely access to BMD testing including developing an algorithm for BMD testing for subgroups, a standard of care policy for BMD testing with performance indicators, ensure DXA technologists and reports are adhering to new ISCD standards, and harmonize policies on BMD testing.
Establish a BMD database and assess appropriate use of BMD testing and rate of change in practice.
In response to the above recommendations, the Ministry of Health and Lon-Term Care, through the Osteoporosis Strategy, has funded the following agencies to develop guidelines and requisition relating to BMD testing:
Osteoporosis Canada is currently determining the guidelines for BMD testing for males and for perimenopausal women.
Women’s College Hospital is developing guidelines for BMD testing in women aged 40-60 years.
Women’s College will be funded for developing a Recommended Use Requisition form to be used across Ontario for BMD testing.
Lack of Standardization of Bone Mineral Density Testing in Ontario
BMD results are influenced by the precision of the test that is, in turn, dependent on the equipment used, the standards adopted, and the skill of the technician conducting the measurements. In Ontario, at least 2 different standards for BMD testing are being used: standards of the International Society of Clinical Densitometry used mainly in hospitals, and standards of the Canadian Association of Radiologists, used by independent health facilities. Experts suggest that variability in results obtained from different test facilities may lead to unnecessary retesting.
Lack of Standardized Reporting
There is presently no requirement for standardized equipment, procedure, or reporting format.
Lack of Information on Patient Outcomes
Presently, information relating to the use of BMD in Ontario can only be obtained from administrative databases that do not provide information on patient risks, test results, or patient outcomes. A registry for BMD tests would provide data for further assessment of BMD tests and form the basis for future standards and policy.
Conclusions
The increased use of BMD in Ontario since 1996 appears to be associated with an increased use of antiresorptive medication, and a decrease in hip and wrist fractures. Trends showed that BMD use has been moving in the right direction, since the growth in BMD use was mainly in people age 65 years and older. However, some areas for improvement were identified.
Potential Overuse
BMD screening in low-risk individuals under 65 years of age: Approximately 20% (98,000) of the DXA BMD tests in Ontario in 2005/06 were performed in people aged less then 65 years, with no fracture in the current year, and coded as at low risk of accelerated bone loss. This is not consistent with current guidelines. Even making allowance for some incorrect coding for risk level, the data suggest that the number of tests in people truly at low risk undergoing BMD could still be substantial.
Approximately 4% (21,000) of the DXA BMD tests in 2005/06 were repeat BMDs in low-risk individuals within a 24 month period. Even though this is in compliance with current OHIP reimbursement policies, evidence shows that biannual serial BMD testing is not necessary in individuals without major risk factors for fractures, provided that the baseline BMD is normal (T score < –1). In this population, BMD measurements may be repeated in 3 to 5 years after the baseline test to establish the rate of bone loss, and further serial BMD tests may not be necessary for another 7 to 10 years if the rate of bone loss is no more than 1% per year. Precision of the test needs to be considered when interpreting serial BMD results.
Potential Gaps in BMD Exam
After a fragility fracture: Less than 20% of men and women greater than 65 years of age received a BMD test after a fragility fracture despite evidence that a fragility fracture in the hip, spine, wrist, or shoulder increases the risk of future fractures by 2-fold or more, and that current available treatment using bisphosphonates can reduce the risk of fractures in osteoporotic individuals by approximately 40 to 50%.
BMD in men: BMD has been shown to be an effective predictor of fracture risk in men. Even though Ontario men accounted for 25% of wrist and hip fractures, they received a disproportionately low rate of BMD investigation in general, and especially after a fracture. Ontario data showed that the risk of fractures started to rise sharply in the 70 to75 age group, and 2 bisphosphonates have been shown to reduce risk of fractures in osteoporotic men
Disparity in BMD use across counties: Some remote counties had BMD utilization rates that were only 10% of those of other counties. The reason for the low utilization needs to be explored and addressed.
Appropriate Use
Baseline BMD testing in people with risk factors for accelerated bone loss and/or fractures (including age>65 years)
-
More frequent serial monitoring (generally every 1-2 years with the exception of glucocorticoid therapy) at the discretion of the physician for people who have one or more of the following factors:
– Low baseline BMD (T score <–1)
– Rate of bone loss greater than 1% per year
– Medication or conditions that affect bone metabolism
Although BMD may not be the perfect surrogate for reduction in fracture risk as a measure of response to osteoporosis treatment, experts advised that it is presently the only reliable test for monitoring response to treatment and helps motivate patients to continue treatment. Patients should not discontinue treatment if there is no increase after the first year of treatment. Evidence showed that it may take 2 years to establish a response to drug therapy, hence serial monitoring every 1 to 2 years depending on the type of osteoporosis drug therapy appears to be appropriate. Lack of response during treatment should prompt the physician to examine whether the patient is taking the medication appropriately.
Other Issues
Although BMD may not be the perfect surrogate for reduction in fracture risk during osteoporosis treatment, experts advise that it is presently the only reliable test for monitoring response to treatment. No response or bone loss during treatment should alert physicians to ensure that the patient is adhering to the treatment appropriately.
Lack of data for monitoring the use and outcomes of BMD tests.
Lack of awareness of guidelines for BMD tests.
BMD reports difficult to interpret.
Focus for future actions
Future efforts to improve the appropriate use of BMD tests in Ontario need to focus on:
Aligning reimbursement policy for BMD tests with current guidelines
Developing specific guidelines for BMD testing in men and perimenopausal women
Requiring BMD facilities to comply with standards for precision and reporting
Improving BMD reports to assist clinical decision-making
Developing a registry to track BMD tests and outcomes
Improving access to BMD tests in remote/rural counties
Establishing mechanisms to alert family physicians of fragility fractures for follow-up investigation and treatment of osteoporosis
Educating physicians and the public of the appropriate use of BMD tests
Some initiatives such as developing guidelines for men and perimenopausal women, and developing a standardized requisition form for BMD testing, are currently in progress under the Ontario Osteoporosis Strategy.
Appendices
Appendix 1: Examples of A Bone Densitometry Report
Reproduced with permission from Sociological Research Online: Green, E., Griffiths, F., Thompson, Di. ‘Are My Bones Normal Doctor?’ The Role of Technology in Understanding and Communicating Health Risks for Midlife Women. Sociological Research Online; Volume 11, Issue 4, published 31/12/2006
Reproduced with permission from Sociological Research Online: Green, E., Griffiths, F., Thompson, Di. ‘Are My Bones Normal Doctor?’ The Role of Technology in Understanding and Communicating Health Risks for Midlife Women. Sociological Research Online; Volume 11, Issue 4, published 31/12/2006
Reproduced with permission from Sociological Research Online: Green, E., Griffiths, F., Thompson, Di. ‘Are My Bones Normal Doctor?’ The Role of Technology in Understanding and Communicating Health Risks for Midlife Women. Sociological Research Online; Volume 11, Issue 4, published 31/12/2006
Appendix 2: Bone Densitometers Licensed by Health Canada
| Manufacturer | Device Name | License no. | Device Category |
|---|---|---|---|
| Norland, A Coopersurgical Company, Fort Atkinson, USA. | XR Series X-ray Bone Densitometer Excell Bone Densitometer ExcellPlus Bone Densitometer Apollo Bone Densitometers |
11901 34427 34438 |
3 3 3 |
| Hologic Inc., Bedford, MA, USA. | Sahara Clinical Bone sonometer Delphi Bone Densitometer Discovery QDR series Bone Densitometer System Explorer QDR Series X-ray Bone Densitometer QDR 4000 Bone Densitometer QDR 4500 Bone Densitometer |
28433 36534 62783 64782 36088 36571 |
3 3 3 3 3 3 |
| GE Medical Systems Ultrasound and Primary Care Diagnostics, LLC. Madison, WI, USA. |
DPX Bone Densitometer DPX Bravo Bone Densitometer DPX Duo Densitometer Lunar IDXA Bone Densitometer |
12214 70581 |
3 3 |
| Osteometer Meditech, Inc. Hawthorne, CA, USA. | X-ray Bone Densitometer Dexacare G4 X-ray Bone Densitometer |
64222 69957 |
3 3 |
| Strategic Medizintechnik GMBH, Pforzheim, DE | XCT 2000 X-ray Bone Densitometer XCT 3000 X-ray Bone Densitometer 960 X-ray Bone Densitometer |
11662 | 3 |
Source: E-mail communication, May 9, 2006
Appendix 3: Guidelines on Bone Mineral Density Testing
| Recommendation by | Year | BMD measurement recommended for | Recommend method | Frequency |
|---|---|---|---|---|
| Ontario Osteoporosis Strategy (178) | 2003 | Ontario Guidelines for the Prevention and Treatment of Osteoporosis does not address BMD testing. Ontario Health Insurance Plan |
DXA at the hip and/or spine | Annual for people at high-risk of osteoporosis and once every 2 years for people at low risk |
| Canadian Task Force on Preventive Health Care (44) | 2004 | Postmenopausal women (Grade B) who (a) are ≥ 65 yrs old (b) <60 kg (c) have history of previous fracture, (d) have an ORAL score ≥9, or (e) have a score ≥6 on the SCORE questionnaire (grade B) Insufficient evidence to recommend using bone turnover markers to predict fracture | Use SCORE questionnaire or ORAL instrument to predict low BMD (grade A) BMD screening using DEXA to prevent fractures in post menopausal women with a risk factor (Grade I) |
- |
| Osteoporosis Canada (Former Osteoporosis Society of Canada) (45) | 2002 | BMD measurement for men or women age >65 (Grade A) -Targeted case-finding for those with increased risk (1 major or 2 minor)* adults age 50 – 65 yrs – |
Hip or spine DXA the most accurate tool. Access to BMD measurement should not be limited by decision tools based on clinical risk factors (Grade A) Quantitative ultrasound may be considered for diagnosis of osteoporosis but not for follow-up at this time. Bone turnover markers should not yet be used for routine clinical management. |
-Monitor using central DXA in 1–2 years after initiating therapy -monitor height loss with thoracolumbar spine X-ray |
| Manitoba (179) | 2000 | Manitoba Bone Density Program Targeted testing for: -Vertebral or nonvertebral fragility fractures proven by x-ray. -Osteopenia or osteoporosis proven by x-ray -Systemic corticosteroid therapy>3 months/year -Prolonged amenorrhea prior to age 45 years if results needed to decide on hormonal or drug therapy -Women>age 65 years if results needed to decide on hormonal or drug therapy |
Follow-up of previous BMD (initial recommended interval 3 years for most patients, 1 year for patients on systemic corticosteroid therapy | |
| BC Health Services (43) | 2005 |
|
DXA Risk factors same as those identified by Osteoporosis Canada. Did not recommend screening for women <65 or as part of routine evaluation around the time of menopause. |
Follow-up BMD measurements not considered necessary prior to 2 yrs after previous measurement except in people on high dose of prednisone for >/= 3 months or with existing fractures with very low bone density |
| US Preventive Service Task Force (180) | 2002 | -Women ≥ 65 years -Women ≥ 60 years & at increased risk for osteoporosis -Postmenopausal women <60 or between 60–64 yrs not at increased risk: no recommendation for or against screening |
Number needed to screen to prevent 1 hip fracture in 5 years = approximately 1,000 or less | |
| International Society for Clinical Densitometry 2004 (18) | 2004 | -Women≥ 65 years- Postmenopausal women <65 yrs with risk factors -Men ≥ 70 yrs -Adults with fragility fracture or disease associated with low bone mass or bone loss. -Adults taking medication associated with low bone mass or bone loss -People receiving treatment or in whom evidence of bone loss would lead to treatment |
DXA @ posterior-anterior spine & hip & @ forearm if spine or hip not feasible. |
Appendix 4: Codes and Criteria for Extraction of Bone Mineral Densitometry Utilization and Fracture Data
CODES
OHIP Codes for BMD testing:
Low-risk patient X152, × 153
High-risk patient X149, X155
Codes for determining fractures:
Data Sources
The data sources for this analysis will include the National Ambulatory Care Reporting System (NACRS) for visits to the emergency department to identify fractures and Canadian Institute for Health Information (CIHI) hospital claims databases to exclude fractures related to malignancies and epilepsy.
Inclusion Criteria
In NACRS data
only keep records with codes beginning with “71310…” to identify ED visits
valid Ontario health card number
Ontario resident
Exclusion Criteria
Age > 105 years
Age < 40 years at time of ED visit
Death in ED or upon arrival (visit disposition)
-
External causes of Injury not including falls
Falls not from a standing height (W11-17)
Striking against or struck by other objects (W22)
Exposure to inanimate mechanical forces (W20, W21, W23 -W49)
Exposure to animate mechanical forces (W50-W64)
Accidental drowning and submersion (W65-W74)
Other accidental threats to breathing (W75-W84)
Exposure to electric current, radiation and extreme ambient air temperature and pressure (W85-W99)
Transport accidents (V01-V99)
Exposure to smoke, fire and flames (X00-X09)
Contact with heat and hot substances (X10-X19)
Contact with venomous animals and plants (X20-X29)
Exposure to forces of nature (X30-X39)
Accidental poisoning by and exposure to noxious substances (X40-X49)
Overexertion, travel and privation (X50-X57)
Accidental exposure to other and unspecified factors (X58-X59)
Intentional self-harm (X60-X84)
Assault (X85-Y09)
Event of undetermined intent (Y10-Y34)
Legal intervention and operations of war (Y35-Y36)
Complications of medical and surgical care (Y40-Y84)
Sequelae of external causes of morbidity and mortality (Y85-Y89)
Fracture Codes
In NACRS data look in diagnosis code fields 1 to 3 to identify fractures and in DAD most responsible diagnosis. Identify fracture patients in NACRS and DAD and if in both then use DAD for assigning fracture since required a hospitalization and expect coding to be better than in ED.
-
Hip fracture (Data source – CIHI; ICD-10 code S72)
S72 Fracture of femur S72.0 Fracture of neck of femur
Fracture of hip NOSS72.1 Pertrochanteric fracture
Intertrochanteric fracture
Trochanteric fractureS72.2 Subtrochanteric fracture S72.3 Fracture of shaft of femur S72.4 Fracture of lower end of femur S72.7 Multiple fractures of femur S72.8 Fractures of other parts of femur S72.9 Fracture of femur, part unspecified -
Wrist Fracture (Data source – NACRS and CIHI; ICD-10 codes S52 and S62.0-S62.4, and S62.8 which are fracture of other and unspecified parts of wrist and hand)
Fracture of forearm S52.0 Fracture of upper end of ulna
Coronoid process
Elbow NOS
Monteggia’s fracture-dislocation
Olecranon process
Proximal endS52.1 Fracture of upper end of radius
Head
Neck
Proximal endS52.2 Fracture of shaft of ulna S52.3 Fracture of shaft of radius S52.4 Fracture of shafts of both ulna and radius S52.5 Fracture of lower end of radius
Colles’ fracture
Smith’s fractureS52.6 Fracture of lower end of both ulna and radius S52.7 Multiple fractures of forearm S62 Fracture at wrist and hand level S62.0 Fracture of navicular [scaphoid] bone of hand S62.1 Fracture of other carpal bone(s) S62.2 Fracture of first metacarpal bone Bennett’s fracture S62.3 Fracture of other metacarpal bone S62.4 Multiple fractures of metacarpal bones S62.8 Fracture of other and unspecified parts of wrist and hand Subjects were excluded from analysis if hospitalization data within 2 years prior to inclusion revealed a history of epilepsy (ICD-10 G40), pathological fracture (ICD-10 M80, M84.4), or malignant neoplasms of the breast (ICD-10 C50), bone (ICD-10 C40, C41), colon (ICD-10 C18), rectum (ICD-10 C20), or lung (ICD-10 C34), and multiple myeloma (ICD-10 C90).
Exclusions for previous fracture records (look-back)
History of epilepsy (ICD-9 345 or 780.3), trauma (ICD-9 E800 to E848), pathological fracture (ICD-9 198.5, 733.1), or malignancies of the breast (ICD-9 170), bone (ICD-9 174), colon (ICD-9 153), rectum (ICD-9 154), or lung (ICD-9 162), and multiple myeloma (ICD-9 203.0) or metastatic cancer (ICD-9 199)
Appendix 5: Studies on Mortality after Fractures in Men
| Study | Design | Patients | Method | Finding |
|---|---|---|---|---|
| Johnell et al., 2004 (181) Sweden |
Longitudinal study, Malmo, Sweden 5 year follow-up |
N = 2,847 with low energy fractures @ spine, hip, & forearm | Poisson model to calculate age & sex-specific mortality rate & compare with that of general population | Higher mortality rate for men than women after hip, spine & shoulder fracture but rate relative to general population similar for both sexes. Rate decreased over 5 years (hip - from RR of 13 to RR of 4.3 for age 60). Mortality risk highest for spine and did not increase over general population risk for forearm fracture. |
| Pande et al., 2006 (11) UK |
Prospective case control 2-year follow-up |
N = 100 Consecutive men hospitalized with low trauma hip fracture. Mean age = 79.9 years Control n=100 matched without fracture, mean age 75 yrs | BMD measurement. Mortality from registers. Kaplan Meier survival curve analysis and a Cox proportional hazard model to determine factors for increased mortality. |
Significantly more patients with hip fracture had comorbid conditions and T-score<–2.5 (83% vs 39%) compared with control. Mortality @ 1 yr, 47% pts vs 1% for control. Mortality @ 2 year 63% for treatment group vs 12% in control. (log rank test62.6, P=.0001) Most common causes of death: bronchopneumonia, heart failure, & ischemic heart disease Factors associated with mortality after hip fracture: older age, residence in nursing/residential home before fracture, comorbid disease & poor functional activity before fracture. Often disabled with poor quality of life. – 7% could not walk, 12% required residential accommodation lower QOL |
| Barrett et al., 2003 (182) Male & female |
Case-control study | N = 81,181 Medicare recipient vs matched control with no fracture | Compared 90 day & 1-year mortality rate and pulmonary embolism rate | 90 day mortality rate for entire US Medicare population after hip fracture was 13%, and after pelvic fracture was 9%. At 1-year after hip fracture, risk of death was 1.6 times that of matched control. Fractures of pelvis, nonhip, femur, & proximal humerus also associated with substantial mortality even a year after fracture. Death rates increased for age for both fracture cases & controls. For both men & women, relative risks of death (compared with controls) decreased with age for hip and pelvis fractures. |
| Jalava et al., 2003 (183) | N=677 women (84 men) and men with primary or secondary osteoporosis 352 had morphometric vertebral fracture | 3.2 years follow-up | Mortality = 5.5% People with prevalent vertebral fracture had a 4.4 fold higher mortality rate compared with people with no prevalent fracture. After adjustment for medication, number of disease, use of oral corticosteroid, alcohol intake, serum albumin and erythrocyte sedimentation rate, renal function, height, weight, gender and age, the point estimate remained elevated but no longer statistically significant (HR 2.4 95% CI, 0.93–6.23). |
|
| Holmberg et al., 2005 (154) | Longitudinal | 22,444 men & 10,902 women identify incident hip fracture 137 women and 181 men had low energy hip fracture | Men 16 yrs Women 11 yrs Follow-up |
Nonfracture population: mortality rate during follow-up 6.4% in women & 15% in men. Mean age of death 61 years. In hip fracture population: mortality rate was 16.8% in women @ average of 2.5 years. & 40.5% in men at average of 3.25 yrs follow-up. Mean age of death 64 yrs for women & 66.4 yrs for men. |
Appendix 6: Search Strategies
a) Bone Density/Pharmacological Therapy/Fracture Risk - Final Search Strategy
Database: Ovid MEDLINE(R) <1996 to August Week 3 2006>
Search Strategy:
------------------------------------------------------------------
((BMD or bone mineral density or bone density) adj3 (monitor$ or correlat$ or measure$ or change$ or increase$ or decrease$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (7468)
(fracture adj1 risk).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (1931)
exp Fractures, Bone/pc, dt [Prevention & Control, Drug Therapy] (2938)
exp Risk Assessment/ (61879)
exp Fractures, Bone/ (31539)
4 and 5 (887)
1 and (2 or 3 or 6) (1046)
exp Osteoporosis/ (15566)
7 and 8 (751)
exp Drug Monitoring/ (5675)
exp dose-response relationship, drug/ (120614)
exp Monitoring, Physiologic/ (39676)
exp Raloxifene/ (1258)
exp Alendronate/ (1271)
exp Etidronic Acid/ or risedronate.mp. (1015)
risedronic acid.mp. (379)
exp Parathyroid Hormone/ (5897)
exp Calcitonin/ (2734)
exp Estrogen Replacement Therapy/ (7868)
or/10-19 (177082)
9 and 20 (266)
limit 21 to (humans and english language and yr=”2000 - 2006”) (187)
(systematic$ review$ or meta-analysis or metaanalysis or random$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (295397)
22 and 23 (103)
22 (187)
limit 25 to (case reports or comment or editorial or letter or “review”) (78)
25 not 26 (109)
24 or 27 (136)
Database: EMBASE <1980 to 2006 Week 34>
Search Strategy:
------------------------------------------------------------------
((BMD or bone mineral density or bone density) adj3 (monitor$ or correlat$ or measure$ or change$ or increase$ or decrease$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (10272)
(fracture$ adj1 risk).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (2554)
exp Fracture/ (66670)
exp Risk Assessment/ (129908)
3 and 4 (2981)
2 or 5 (4801)
exp Fracture/pc, dt [Prevention, Drug Therapy] (4248)
6 or 7 (8143)
*osteoporosis/ or exp idiopathic osteoporosis/ or exp postmenopause osteoporosis/ or exp primary osteoporosis/ or exp senile osteoporosis/ (19622)
1 and 8 and 9 (779)
exp drug efficacy/ or exp drug effect/ or exp treatment response/ or exp therapy effect/ or exp drug monitoring/ (546504)
exp alendronic acid/ or exp risedronic acid/ (4793)
exp Etidronic Acid/ (3953)
exp Parathyroid Hormone/ (16795)
exp Raloxifene/ (4068)
exp CALCITONIN/ (11434)
exp Estrogen Replacement Therapy/ (7463)
or/11-17 (580318)
10 and 18 (391)
limit 19 to (humans and english language and yr=”2000 - 2006”) (256)
(systematic$ review$ or meta-analysis or metaanalysis or random$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (372370)
20 and 21 (127)
20 (256)
limit 23 to (editorial or letter or note or “review”) (111)
Case Report/ (900658)
23 not (24 or 25) (145)
22 or 26 (181)
b) Treatment Rates/Fragility Fractures – Final Search Strategy
Search date: August 30, 2006
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations,
EMBASE, INAHTA, Cochrane Library
Database: Ovid MEDLINE(R) <1996 to August Week 3 2006>
Search Strategy:
---------------------------------------------------------------------
exp Fractures, Bone/pc, dt, rh, th, et [Prevention & Control, Drug Therapy, Etiology] (12404)
exp Osteoporosis/co, di, dt [Complications, Diagnosis, Drug Therapy] (7515)
1 and 2 (2855)
(secondary adj2 prevent$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (5477)
exp Guideline Adherence/ (7330)
exp Recurrence/ or exp Aftercare/ (51972)
undertreatment.mp. (601)
exp “Continuity of Patient Care”/ (4771)
(postfracture or post-fracture).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (195)
(fracture$ adj2 (second$ or after or new or subsequent or future or prior or previous or recent or recur$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (3922)
((assessment or investigation or diagnosis or detection or treatment) adj1 rate$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (4984)
exp “Outcome and Process Assessment (Health Care)”/ (265994)
exp Physician’s Practice Patterns/ (15759)
or/4-13 (341043)
3 and 14 (842)
limit 15 to (humans and english language and yr=”2005 - 2006”) (190)
(systematic$ review$ or meta-analysis or metaanalysis or random$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (295397)
16 and 17 (48)
16 (190)
limit 19 to (case reports or comment or editorial or letter or “review”) (50)
19 not 20 (140)
18 or 21 (149)
Database: EMBASE <1980 to 2006 Week 34>
Search Strategy:
---------------------------------------------------------------------
exp Secondary Prevention/ (5553)
exp Recurrent Disease/ (42031)
exp Clinical Pathway/ (1332)
exp Clinical Protocol/ (32436)
exp Treatment Planning/ (57500)
exp Follow Up/ (197697)
(postfracture or post-fracture).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (283)
(fracture$ adj3 (second$ or after or new or subsequent or future or prior or previous or recent or recur$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (8086)
((assessment or investigation or diagnosis or detection or treatment) adj2 rate$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (11632)
exp Health Care Quality/ (562894)
(second$ adj1 prevent$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (9953)
undertreatment.mp. (811)
((guideline$ or reommend$) adj1 adhere$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (141)
or/1-13 (778838)
exp Fracture/ (66670)
exp OSTEOPOROSIS/co, dm, dt, th [Complication, Disease Management, Drug Therapy, Therapy] (12503)
15 and 16 (4044)
14 and 17 (1562)
limit 18 to (human and english language and yr=”2005 - 2006”) (363)
(systematic$ review$ or meta-analysis or metaanalysis or random$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (372370)
19 and 20 (111)
19 (363)
limit 22 to (editorial or letter or note or “review”) (177)
Case Report/ (900658)
22 not (23 or 24) (174)
21 or 25 (216)
c) RCTs/Pharmacological Treatments for Osteoporosis – Final Search Strategy
Database: Ovid MEDLINE(R) <1996 to June Week 1 2006>
Search Strategy:
--------------------------------------------
exp Osteoporosis/ (15175)
exp Alendronate/ or fosamax.mp. or alendronate.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (1559)
exp Etidronic Acid/ or etidronate.mp. or didronel.mp. or actonel.mp. or risedronate.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (1098)
exp Raloxifene/ or keoxifene.mp. or raloxifene.mp. or evista.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (1589)
or/2-4 (3686)
1 and 5 (1665)
limit 6 to (humans and english language and yr=”2001 - 2006”) (811)
(systematic$ review$ or meta-analysis or metaanalysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (24066)
7 and 8 (36)
7 (811)
limit 10 to (case reports or comment or editorial or letter or “review”) (349)
10 not 11 (462)
limit 12 to (controlled clinical trial or metaanalysis or randomized controlled trial) (235)
random$.mp. (274191)
exp Double-Blind Method/ (41825)
12 and (13 or 14 or 15) (275)
9 or 16 (300)
Database: EMBASE <1980 to 2006 Week 24>
Search Strategy:
------------------------------------------------
exp osteoporosis/ (33497)
exp Alendronic Acid/ or alendronate.mp. or fosamax.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (4218)
exp Etidronic Acid/ or etidronate.mp. or didronel.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (3980)
exp Risedronic Acid/ or risedronate.mp. or actonel.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1870)
exp RALOXIFENE/ or raloxifene.mp. or evista.mp. or Keoxifene.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (3985)
or/2-5 (10302)
1 and 6 (4774)
limit 7 to (human and english language and yr=”2001 - 2006”) (2246)
(meta-analysis or metaanalysis).ti, ab. (11725)
systematic$ review$.ti, ab. (8588)
8 and (9 or 10) (35)
8 (2246)
limit 12 to (editorial or letter or note or “review”) (1221)
Case Report/ (889259)
12 not (13 or 14) (933)
11 or 15 (956)
Randomized Controlled Trial/ (106396)
(meta-analysis or metaanalysis).ti,ab. (11725)
Double Blind Procedure/ (60047)
triple-blind$.mp. (112)
random$.mp. (335676)
systematic$ review$.ti, ab. (8588)
or/17-22 (360329)
16 and 23 (359)
d) BMD/Predictive Value/Men – Final Search Strategy
Database: Ovid MEDLINE(R) <1996 to April Week 4 2006>
Search Strategy:
------------------------------------
*bone density/ or low bone mass.mp. or low bone density.mp. or low bone mineral density.mp. or low BMD.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (8908)
*Bone Resorption/ (2214)
exp Bone Demineralization, Pathologic/ (154)
or/1-3 (11008)
exp osteoporosis/ (14982)
exp Bone fractures/ (30310)
5 or 6 (40879)
exp Risk Assessment/ (57739)
predict$.mp. (270122)
8 or 9 (319644)
4 and 7 and 10 (927)
limit 11 to (humans and english language and yr=”2001 - 2006” and male) (203)
(systematic$ review$ or meta-analysis or metaanalysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (23584)
12 and 13 (8)
12 (203)
limit 15 to (case reports or comment or editorial or letter or “review”) (36)
15 not 16 (167)
14 or 17 (172)
Database: EMBASE <1980 to 2006 Week 18>
Search Strategy:
-----------------------------------------
*bone density/ or low bone mass.mp. or low bone density.mp. or low bone mineral density.mp. or low BMD.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (6932)
*Osteolysis/ (4835)
*Bone Demineralization/ (304)
or/1-3 (11959)
*OSTEOPOROSIS/ (15218)
*Fragility Fracture/ (452)
*Bone Fragility/ (64)
or/5-7 (15612)
exp prediction/ (92003)
(predict$ adj3 (fracture$ or osteoporosis)).mp. (1968)
exp Risk Assessment/ (122668)
or/9-11 (206780)
8 and 12 (1499)
limit 13 to (human and english language and yr=”2001 - 2006”) (764)
limit 14 to male (246)
(systematic$ review$ or meta-analysis or metaanalysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (38680)
15 and 16 (7)
15 (246)
limit 18 to (editorial or letter or note or “review”) (43)
18 not 19 (203)
17 or 20 (205)
Appendix 7: Description of Observational Studies on Rate of Change of Bone Mineral Density in Women – No Osteoporosis Treatmen
| Design | Inclusion/Exclusion | Method | Results | |
|---|---|---|---|---|
| Melton et al., 2000 (Longitudinal study) (184) US |
Prospective population-based cohort study (348 men & 351 women) N= 351 women 4-yr follow-up |
Age 21-93 yrs | DXA BMC & areal BMD for total body, L-spine (2-4), proximal femur, & forearm @ baseline, 1, 2, & 4 yrs. Rate of bone loss cross-sectional vs longitudinal, for ≥70 yrs vs <70 yrs | Cross-sectional data overestimated Rate of bone loss Rate of bone loss greater for ≥70 yrs than for <70 yrs group; may be due to younger women on HRT |
| Chapurlat et al., 2000 (57) OFELY (Longitudinal study - 3 years) France |
Prospective population-based cohort study N = 272 3-year follow-up |
Premenopausal Perimenopausal Age 31-59 yrs healthy Caucasian randomly selected Excluded diseases or medications affecting bone, pregnancy | Annual DXA BMD total body, L-spine & hip, serum estrogen, osteocalcin, procollagen peptide, bone alkaline phosphatase, urine biomarkers for bone resorption or formation | Perimenopausal – no significant bone loss, small but significant increase @ FN, total hip & L-spine. Perimenopausal: rapid & diffuse bone loss related to decreased estrogen secretion (–0.1%/yr to –0.6%/yr) |
| Bainbridge et al., 2004 US (185) (part of Michigan Bone Health Study) |
Prospective population-based cohort N = 614 Caucasian women 6 years follow-up |
Pre, peri & post menopausal community-dwelling (age 24-50yrs) from sampling lists. | Annual DXA BMD @ FN & L-spine (2-4), info on BMI, reproductive history, menstrual status, diet, lifestyle & medical history Linear regression analysis for association between risk factors & rate of bone loss | |
| Goulding et al., 1999 (59) Prospective cohort 1 year New Zealand Single center |
Prospective cohort study N = 80 1 year follow-up |
Healthy women 40–79 years No previous test for lactose intolerance Excluded: therapy or conditions affecting bone e.g., GI surgery, radiotherapy, hyperthyroidism, hyperthyroidism etc. |
Effect of aging on lactose malabsorption & determine Ca intake & BMD loss Test for lactose malabsorption Baseline & @ 1 year: DXA BMD @ radius hip, spine & total body. Urinary biomarkers for bone formation & bone resorption | Ca intake only reduced in malabsorber 70 – 79 yrs BMD change in 1 year: Lactose malabsorber: FN –2.2% Trochanter –2.7% L-spine +0.8% Lactose absorber FN= –1.5% Trochanter –1.6% L-spine +0.8% Difference not statistically significant |
| Sellmeyer et al., 2001 (186) Prospective cohort (7 yrs) Multicenter |
Prospective cohort study N = 1,035 |
Caucasian women>60 yrs Mean age 73–74 years from population listings (no exclusion criteriastated) |
Animal protein intake from food frequency questionnaire, DXA BMD @ total hip & subregions @ baseline, @ year 2, & mean of 3.6 years later. Hip fractures assessed for 7+/-1.5 years. Linear regression analysis | High Animal/vegetable protein ratio bone loss @ femoral neck =0.78%/yr vs 0.21%/yr with a low ratio Hip fracture higher with a high ratio (RR = 2.7, P=0.04) |
| Chittacharoen et al., 1997 (58) 50 surgical menopausal women without hormone replacement vs perimenopausal |
RCT N = 50 surgical (S) menopausal women & 50 controls (perimenopausalwomen) N = 50 S menopause < 9 yrs S. menopause > 9 yrs |
Study patients: women who had surgical & menopause and did not receive hormone replacement | Both study & control groups had DSA @ lumbar spine, femoral neck, total body, distal radius & midradius. Body, height, weight & BMD also assessed. Rate & pattern of bone loss were compared. For the surgical menopausal group was stratified according to postmenopausal period less than9 ears vs longer than 9 years. | BMD were significantly lower for the surgical menopausal women @ all skeletal sites measured. With postmenopausal period less than 9 years, the rate of bone loss was higher @ the lumbar spine & distal radius while for postmenopausal period longer than 9 years, the rate of bone loss was higher at the femoral neck compared to bone loss @ other sites. |
Appendix 8: Summary - Rate of Change in Bone Mineral Density in Women Not Receiving Osteoporosis Treatment
| N/Mean age | Rate of BMD change in Spine (% per year) | Rate of BMD change in Femoral Neck (% per year) | Rate of BMD change in Total hip (% per year) | |
|---|---|---|---|---|
| Observational studies | ||||
| Melton et al., 2000 (184) (Longitudinal study) US |
N= 351 women <70 years old ≥70 yrs old |
Longitudinal 0.32 (lateral spine) 0.87 |
0.31 –0.24 |
0.15 –0.64 |
| Chapurlat et al., 2000 (57) OFELY (Longitudinal study - 3 years) France |
N = 272 Premenopausal Perimenopausal |
0.3 –0.4 |
0.03 –0.6 |
0.3 –0.1 |
| Bainbridge et al., 2004 (185) US (Prospective cohort 6 years) |
N = 614 Premenopausal Perimenopausal Postmenopausal |
(%/yr of T-score) –0.09 –0.082 –0.13 |
–0.08 –0.085 –0.094 |
NR |
| Goulding et al., 1999 (59) Prospective cohort 1 year New Zealand |
N = 80 40–79 years Lactose absorber Lactose Malabsorber |
L2–L4 0.8 0.8 |
–1.5 –2.2 (NS) |
NR |
| Sellmeyer et al., 2001 (186) Prospective cohort (7 years) |
N = 1,035 Caucasian 73–74 years |
High animal protein –0.78 Low animal protein –0.21 |
||
| Chittacharoen et al., 1997 (58) 50 surgical menopausal women without hormone replacement vs perimenopausal |
N = 50 S menopause < 9 years S.menopause > 9 years |
3.05 |
2.70 |
2.70 (distal radius) |
| Rate of BMD change in Women in the Placebo Arm of RCTs on Antiresorptive Therapy | ||||
| Etidronate studies | ||||
| Montessori et al., 1997*(80) | Postmenopausal osteopenic women Mean age 62.9 yrs Mean lumber BMD 0.675 (Z-score< –1)on calcium alone | 0.17 (95% CI –1.56; 1.90) in 3 years Average (+0.06 per year) |
–2.97 (95% CI-4.75; –1.19) in 3 years Average –0.96 per year, maximum – 1.6 per year |
|
| Alendronate | ||||
| Wasnich et al., 1999(187) | Early post-menopausal Caucasian women on Placebo, T-score< –2 | – 1.9 (in 2 years) Average –0.95 per year | NR | –2 (in 2 years) |
| % BMD Changes From Baseline in Women in the Placebo Arm of RCTs on Antiresorptive Therapy | ||||
| Alendronate studies | ||||
| Bone et al., 1997 (188) women age 60–85 yrs, T-score< –2 (5mg) † |
Osteoporotic elderly women on placebo Mean age 71.1 yrs Mean spine BMD 0.71 (0.09)g/cm2 | 0.56 (SE 0.44) in 2 years (Average +0.28/year) |
-1.51 (SE 0.58) in 2 years (average –0.75 per year) |
|
| McClung et al., 2004 (81) † Early postmenopausal women (5mg) Mean age 51–55 years) |
Placebo Mean age 53.7 year Mean L-spine BMD 0.95 g/cm2 |
–3.2(SE 0.34) in 6 years (average – 0.53% per year) |
-3.5 (SE 0.32) in 6 year (average –0.6% per year) |
-2.3 (SE 0.26) in 6 years (Average –0.4% per year) |
| Cummings et al. 1998 (189) | Placebo Mean age= 67.7 (6.1) years Mean BMD (spine) 0.842 (0.13). FN 0.593 (0.06) L-spine |
+1.5 in 4 years (Average +0.37% per year) | -0.8 in 4 years (Average –0.2% per year) | –1.6 in 4 years Average –0.4% per year) |
| Bell et al., 2002 (190) T-score≤ –1.75, |
African American Women, mean age 65.9 yrs, mean L-spine BMD 0.80 (0.01)g/cm2 on placebo | +0.9 (SE 0.6) in 2 years Average +0.45 per year |
+0.5 (SE 1.1) in 2 years Average +0.25 per year |
–1.1 (SE 0.7) in 2 years Average –0.55 per year |
| Pols et al., 1999 (191) | Women postmenopausal>3 yrs, <85years old Placebo Mean spine BMD =0.72 (0.08) mg/cm2 |
0.1 (SD 3.4) in 1 year | –0.2 (SD 4.5) in 1 year | 0.1 (SD 3.0) in 1 year |
| Risedronate studies | ||||
| Hooper et al., 2005 (192) | Postmenopausal women, mean age 52.6 yrs Mean spine T-score – 0.432 on placebo |
–2.5 in 2 years Average –1.25 per year | –2.5 in(2 years Average –1.25 per year | –1.8 in 2 years Average –0.9 per year |
| Clemmesen et al., 1997 (193) |
Postmenopausal women mean age = 70 years, mean spine BMD 0.747 g/cm2 on placebo | 1.7 in 3 years Average 0.6 per year | –2.6 in 3 years Average –0.86 | Trochanter –0.04 in 3 year Average –0.013 per year |
| Harris et al., 1999 (194) postmenopausal women 1<85 yrs >/=1 vertebral fracture |
Postmenopausal women <85 years with ≥ 1 vertebral fracture | 1.1 In 3 years Average 0.33 per year | –1.2 in 3 years Average –0.4 per year | Trochanter –0.7 in 3 years Average –0.23 per year |
CI refers to confidence interval; L, lumbar; NR, not reported
BMD changes estimated from graph
L-spine refers to lumbar-spine; SD refers to standard deviation; SE, standard error.
Appendix 9A: Rate of Change in Bone Mineral Density in Men Not Receiving Osteoporosis Treatment
| Study | Sample size Age |
Rate of BMD change Spine % per year (SD) | Rate of BMD change Femoral Neck % per year(SD) | Rate of BMD change Total hip % per year |
|---|---|---|---|---|
| Melton et al., 2000 (195) (Longitudinal) US | N=348 men <70 years >70 years |
–0.18 –0.36 |
0.52 –0.19 |
0.30 –0.29 |
| Van Pottelbergh et al., 2003 (196) Belgium | N = 214 Age 71–86 yrs |
NA | –0.04 (NS) | –0.39 |
| Knoke et al., 2003 (150) (Rancho Bernardo Study) US |
N = 1,214 Age>50yrs Mean = 70.6 yrs |
–0.5 (men & women) 29% of men lost at least 1%/yr (especially in people with >1%/yr weight loss |
||
| Bakhireva et al., 2004 (151) US |
N=507 Caucasian men Age 45–92 yrs Mean 70.8yrs |
0.22 | –0.34 | –0.47 |
| Cauley et al., 2005 (197) (MrOS) US |
N = 5,995 Age≥ 65yrs |
+7% for every 5 yrs increase in age (Average +1.4%/yr) | –2.6% every 5 yrs increase in age (Average –0.52%/yr) | |
| Ensrud et al., 2005 (60) (MrOs) US |
N = 1,342 Age >65 yrs |
Weight gain 0.1 Stable weight –0.3 Weight loss>5% –1.4 r |
||
| Naves et al., 2005 (198) Spain | N = 308 Age ≥50 yrs |
–0.0021 (0.11) g/cm2/yr |
–0.0011 (.009) g/cm2/yr | 0.0008 (.011) g/cm2/yr |
| % BMD Change From Baseline in Men in the Placebo arms of Randomized Controlled Trials* | ||||
| Gonelli et al., 2003 (119) † N=77 RCT |
Men with primary osteoporosis treated with placebo & calcium alone Mean age = 56.6 years, mean FN BMD 0.622 g/cm2 | –1.2 (3 years) Average –0.4 per year |
–1.2 (3 years) Average –0.4 per year |
–0.3 (3 years) Average –0.1 per year |
| Ringe et al., 2004 (120) † N=134 Open label RCT |
Men with primary osteoporosis on alfacalcidol, mean age = 53.3 Mean FN T-score = –2.56 |
3.5 in 3 years Average 1.16 per year |
2.3 in 3 years Average 0.76 per year |
|
FN refers to femoral neck; MrOS, Osteoporosis Study in Men; NS, not significant; RCT, randomized controlled trial; SD, standard deviation; yr, year.
BMD changes estimated from graph
Appendix 9B*: Annualized Percent Change in Hip Bone Density in Men Aged 65 years and Over- Stratified by Body Mass Index and Weight Changes*
| % Change/year in Total Hip Bone Mineral Density (95% Confidence Interval) | ||||
|---|---|---|---|---|
| Gained >5% weight | Weight Stable | Lost >5% weight | P-value test for trend | |
| Entire cohort † | 0.1 (–0.1, 0.4) | –0.3 (–0.4, –0.3) | –1.4 (–1.6, –1.2) | <.001 |
| BMI<25 kg/m2 | 0.2 (–0.3, 0.7) | –0.4 (–0.5, –0.2) | –1.4 (–1.9, –0.9) | <.001 |
| BMI 25.0–29.9 kg/m2 | 0.2 (-0.2, 0.5) | –0.4 (–0.5, –0.3) | –1.2 (–1.5, –0.9) | <.001 |
| BMI >30 kg/m2 | –0.2 (–0.8, 0.5) | –0.3 (–0.5, 0.0) | –1.7 (–2.2, –1.2) | <.001 |
| BMI>30 kg/m2, trying to lose weight | 0.5 (–0.3, 1.3) | –0.1 (–0.4, 0.1) | –1.7 (–2.4, –1.1) | <.001 |
kg refers to kilogram; m, meter.
Adjusted for age, health status, physical activity level, smoking status, alcohol use, total calcium intake, history of one or more select medical conditions (including stroke, diabetes, hyperthyroidism, Parkinson’s disease, coronary heart disease, congestive heart failure, chronic obstructive lung disease, and non skin cancer), body mass index, lean mass, leg power, and total hip bone density.
Used with permission. Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. Journal of Clinical Endocrinology & Metabolism 2005; 90(4):1998-2004. Copright 2005, The Endocrine Society.
Appendix 10: Assessment of Systematic Reviews - Change in Bone Mineral Density and Reduction in Fracture Risk During Antiresorptive Treatment (Postmenopausal Women With Osteoporosis*
| Include only RCTs | Clearly defined question | Description of search strategy | Inclusion/exclusion criteria | Study selection & data abstraction by > 1 person | Assessed Quality of Studies | Method of meta-analysis described | Use Individual patient data in Meta-analysis | |
|---|---|---|---|---|---|---|---|---|
| Wasnich et al., 2000 (64) | √ | √ | Based on review articles & abstracts | √ | NR | NR | √ | NR |
| Cummings et al., 2002 (65) | √ | √ | √ | √ | √ | NR | √ | No |
| Hochberg et al., 2002 (66) | √ | √ | NR | √ | NR | NR | √ | No |
| Guyatt et al., 2002 (63) | √ | √ | Not applicable | From meta-analysis | NR | √ | √ | Summary of meta-analysis |
| Delmas et al., 2004 (67) | √ | √ | No, based on studies from another meta-analysis | √ | NR | NR | √ | √ |
| Watts et al., 2004 (68) | √ | √ | NR | √ † | NR | NR | √ | √ |
| Watts et al., 2005 (69) | √ | √ | NR | √ † | NR | NR | √ | √ |
CI refers to confidence interval; NR, not reported.
Provided bases for selecting the 3 trials
Appendix 11: Studies Included in Meta-Analyses on Changes in Bone Mineral Density Versus Fracture Risk Reduction During Treatment
| Wasnich et al., 2000 (64) | Hochberg et al., 2002 (66) | Delmas et al., 2004 (67) | Cummings et al., 2002 (65) | Watts et al., 2004 (68) & 2005 (69) |
|---|---|---|---|---|
|
Raloxifene Ensrud 1998 Lufkin 1998 |
Raloxifene Ettinger 1999 Lufkin 1998 |
Raloxifene Etinger 1999 Lufkin 1998 |
Raloxifene Ettinger 1999 |
|
|
Alendronate Liberman 1995 Black 1993 |
Alendronate Liberman 1995 Black 2000 |
Alendronate Liberman 1995 Black 2000 |
Alendronate Liberman 1995 Black 1996 |
|
| Cummings 1998 Bone 1997 |
Pols 1999 Bone 1997 Adami 1995 Bone 2000 Chestnut 1995 |
Pols 1999 Bone 1997 Adami 1995 Bone 2000 Chestnut 1995 |
Cummings 1998 | |
|
Etidronate Storm 1990 |
Etidronate Harris 1993 Storm 1990 |
Etidronate Harris 1993 |
Etidronate Harris 1993 Storm 1990 |
|
| Risedronate |
Risedronate Harris 1999 McClung 2001 Reginster 2000 Clemmesen 1997 |
Risedronate Harris 1999 McClung 2001 Reginster 2000 Clemmesen 1997 |
Risedronate Harris 1999 Reginster 2000 |
Risedronate Harris 1999 McClung 2001 Reginster 2000 |
|
Estrogen Lufkin 1992 |
Estrogen |
Estrogen Lufkin 1992 |
||
|
Calcitonin Silverman 1998 Overgaard 1992 |
Calcitonin Chestnut 2000 Overgaard 1992 |
Calcitonin Chestnut 2000 |
Calcitonin Chestnut 2000 Overgaard 1992 |
|
|
Tiludronate Genant 1998 |
Tiludronate Reginster 2001 |
Tiludronate Reginster 2001 |
Tiludronate Gennat 1998 |
Appendix 12: Summary - Meta-Analysis on the Relationship Between Bone Mineral Density Changes and Reduction in Fracture Risk During Antiresorptive Treatment (Postmenopausal Women With Osteoporosis)
| Meta-analysis/Study | Year of studies | Antiresorptive Drug (number of studies) | Type of BMD & Type of Fracture Studied | % change in BMD vs % change in fracture risk | Other Findings |
|---|---|---|---|---|---|
| Meta-Analysis | |||||
| Wasnich et al., 2000 (64) | 13 Randomized placebo controlled trials † | Alendronate (4) Etidronate (2) HRT (1) Raloxifene (2) Calcitonin (2) Tiludronate (2) |
% Changes in spine BMD & hip BMD vs RR of vertebral fracture | The model predicts: -treatments that increase spine BMD by 8% would reduce vertebral fracture risk by 54% and change in BMD explained mot of the effect (41% risk reduction) Treatments that increased hip BMD by 5% would reduce vertebral fracture risk by 50% with 38% attributable to BMD |
Poisson regression:CI large for individual trials. Substantial variability inantifracture efficacy at anygiven level of change in BMD. Overall trials reportinglarger increase in BMDtended to have greaterreductions in vertebralfracture risk. -A small but significant riskreduction of 20-22% withno measurable change inspine BMD. |
| Hochberg et al., 2002 (66) | 18 double-blind placebo controlled RCTs † (n = 26,494) 1–3.5 yrs follow-up | Etidronate (2)* Alendronate (7) Tilodronate (2) Estrogen (2) Risedronate (4) Calcitonin (3) Raloxifene (2) | Spine & Hip BMD, bone markers (BCM), & nonvertebral fractures | At 1 year: 6% ⇑ in spine BMD (treatment vs placebo) was associated with a 39% reduction in nonvertebral fracture risk. 3% ⇑ in hip BMD (over placebo) was associated with 46% risk reduction 70% decrease in bone resorption marker associated with 40% risk reduction | There was a significant association between the amount of increase in BMD @ the spine &hip at 1 year and ⇓ in risk of incident nonvertebral fractures (P= .02 & .006) without an independent effect of treatment. Multiple regression line not perfectly linear but BMD or BCM appear to explain a significant part of the risk reduction. The association remained significant in sensitivity analysis. Changes in BMD were correlated with changes in bone markers (P</= .002) |
| Guyatt et al., 2002 (63) | Summary of 8 meta-analysis of placebo controlled RCTs *,† | Calcium (15) Vitamin D (25) Alendronate (11) Etidronate (11) Risedronate (8) Calcitonin (30) Raloxifene (7) HRT (56) |
Relative risk of vertebral & nonvertebral fractures, absolute difference in event rates, changes in BMD, relationship between BMD changes & fracture risk reduction | Reduction in vertebral fracture risk: Alendronate, calcitonin about 50% Vitamin D, etidronate, risedronate, raloxifene about 33.3% Nonvertebral fracture: significant reduction only for Alendronate (50%) (At least 31%) and risedronate (33.3%) (At least 13%) & may be less in low-risk population. |
Based on Poisson regression analysis of individual trial data: Vertebral fracture relative risk reduction 20% with no effect on BMD and an additional 25% associated with BMD. (BMD explained 25/45% of risk reduction)Nonvertebral fracture risk reduction: No significant relationship between BMD and risk reduction. BMD is not helpful for predicting the impact of antiosteoporosis treatment on nonvertebral fractures. |
| Dalmas et al., 2004 (67) | 16 placebo-controlled RCTs† | Same as Hochberg with omission of 3 studies to correct for discrepancies in reported BMD & person years | Spine & Hip BMD, & nonvertebral fractures | No association between the extent of reduction in nonvertebral fracture risk and increases in BMD at the spine or hip at one year or at study end point. Larger increase in BMD @ 1 year were not associated with greater reduction in nonvertebral fracture risk (P = .12) for L spine & P=.11 for hip). Larger increases in BMD from baseline to study endpoint were not associated with greater reduction in nonvertebral fracture risk (P=.47 for L spine and .60 for hip). | |
| Meta-Analysis | |||||
| Cummings et al., 2002 (65) | 12 blinded placebo-controlled RCTs † 1990-2000 (n = 21,404) | Etidronate (2) Alendronate (3) Tiludronate (1) Estradiol (1) Risedronate (2) Raloxifene (1) Calcitonin (2) (Vit D & Calcium for both groups) | DXA Spine BMD & Vertebral fractures | 1% ⇑ spine BMD (Treatment vs placebo) was associated with 0.03 ⇓ in RR of spine fractures (95% CI, 0.02-0.05, P=.002) | Based on ⇑ in BMD, expected to reduce fracture risk by 20% (RR= 0.8), but treatment reduced risk of vertebral fracture by 45% Alendronate: 3.9%⇑ in BMD @ 1 year explained 16%of the ⇓ (47%) in risk of vertebral fracture |
| Watts et al., 2004 (68) | 3 pivotal double-blind parallel placebo controlled RCTs (VERT & HIP) (n=3,224) Meta-analysis using individual patient data | Risedronate 2.5 mg or 5mg (n = 2,047) or placebo (n=1,177)047) For up to 3 years + 1,000 mg Calcium & Vit D supplement (if serum 25 OH vitamin D<40 mol/L) | DXA spine & femoral neck BMD @ baseline and 6-month intervals. Lateral thoracolumbar (T4–L4) radiographs were @ baseline & annually for 3 years to identify vertebral fractures | Incidence of vertebral fracture: 15% for spine BMD⇑<0%; 9.5% for BMD ⇑ = 0 - <5%; 10.2% for BMD ⇑ >5% Changes in spine BMD explained 18% of treatment effect & femoral neck BMD explained 11% of treatment effect |
Risk reduction similar for BMD ⇑ <5% & BMD ⇑ >5% (49% vs 41%, P=.77) Patients showing an increase in BMD had lower fracture risk than patients showing a decrease in BMD, greater increases in BMD did not necessarily predict greater reduction in fracture risk. |
| Watts et al., 2005 (69) | 3 pivotal double-blind parallel place bo-controlled RCTs (VERT) (n=3,979) Meta-analysis using individual patient data |
Risedronate 2.5 mg or 5mg or place bo For up to 3 years + 1,000 mg Calcium & Vit D supplement (if serum 25 OH vitamin D<40 mol/L) | 3DXA L-Spine & femoral neck BMD @ baseline & 6-month intervals and Radiologically confirmed nonvertebral fractures | Risedronate ⇓ risk fracture by 32% (HR 0.68, 95% CI,, 0.54-0.85, P< .001). Changes in L-spine BMD explained 12% and change in FN BMD explained 7% of effect of risedronate on nonvertebral fracture incidence. | Similar incidence of nonvertebral fracture whether there is an increase or decrease in BMD with treatment (7.8% for pts whose spine BMD decreased vs 6.4% in patients with increased spine BMD. Similar for FN BMD (Incidence 7.6% for pts with increased FN BMD vs 7.5 for people with increased FN BMD) Changes in BMD as measured by DXA did not predict the degree of reduction in nonvertebral fracture. |
RCT refers to randomized controlled trials;
These included studies on more than one antiresorptive drug
VERT refers to Vertebral Efficacy with Risedronate Therapy study; HR, hazard ratio; RR, relative risk; CI, confidence interval; FN femoral neck, DXA, dual energy x-ray absorptiometry; T, thoracic; L, lumbar
These included studies on more than one antiresorptive drug
Appendix 13: Clinical Trials on Relationship Between Bone Mineral Density Changes and Reduction in Fracture Risk During Antiresorptive Treatment*,†
| Meta-analysis/Study | Year of studies | Anti-resorptive Drug | Type of BMD & Type of Fracture Studied | % change in BMD vs % change in fracture risk | Other Findings |
|---|---|---|---|---|---|
| Clinical Trials | |||||
| Sarkar et al., 2002 (70) MORE Trial |
Placebo controlled RCT N = 7,705 postmenopausal women with osteoporosis |
Randomized to Raloxifene (60 or 120 mg/day) or placebo Raloxifene patients pooled for logistic regression analysis |
DXA lumbar spine or femoral neck BMD @ baseline & annually ANND vertebral fractures | @ 3 years, raloxifene group had a 36% lower risk of vertebral fracture compared with placebo group. | Women with the lowest baseline lumber spine or femoral neck BMD had the greatest risk for femoral fractures. % change in BMD accounted for 4% of the observed vertebral fracture risk reduction, and the other 96% of the risk reduction remains unexplained. |
| Chapurlat et al., 2005 (71) Fracture Intervention Trial (FIT) |
Multicenter placebo controlled RCT. N = 5,383 women With low BMD Randomly assigned to either alendronate or placebo (post hocanalysis) |
Alendronate: 5mg/D 1st 2 yrs then increased to 10 mg If calcium intake<1,000 mg, also receive calcium & 250 IU vitamin D supplement Alendronate & Placebo patients each divided into 4 categories BMD loss >4%, 0-4%, gain 0-4%, >4% |
Baseline & annual L-spine & hip BMD with QDR & Vertebral fractures Lateral spine radiographs obtained at baseline and @ end of 3 year follow-up for FIT & 4 years for FIT-II. | Lost: -spine BMD Alendronate 10% Placebo 40% Lost Hip BMD Alendronate 19% Placebo 47% Among patients who adhere to treatment with alendronate, even those who lose BMD benefit from a substantial reduction in risk of vertebral fracture. |
@ 1 year: Reduction in risk of vertebral fracture, alendronate vs placebo: Lost 0%-4% L-spine BMD OR 0.4 (95% CI, 0.16–0.99) Lost >4% L-spine BMD OR 0.15(95% CI, 0.02–1.29) Gained 0%–4% spineBMD OR 0.49 (95% CI, 0.3–0.78) Gained>4% spine BMD OR 0.46 (0.32–0.66) Lost 0-4% hip BMD OR 0.47 (0.27–0.81) Lost >4% hip BMD OR 0.61 (0.11–3.45) Gained 0%–4% hipBMD OR 0.49 (0.34_0.71) Gained >4% OR 0.34 (0.18–0.62) No benefit fromalendronate for people who lost BMD@ bothhip & spine |
| Cummings et al., 2005 FIT Trial MORE Trial |
2 double-blind Multicenter placebo controlled RCTs FIT. N = 2,634 MORE N=3,954 post menopausal women with osteoporosis |
FIT: women completed 2 yrs of treatment with alendronate (5 mg/day) MORE: women completed 2 yrs of treatment with raloxifene (60 or 120 mg/day) |
changes in L-spine and total hip BMD in year 1 compared with year 2 | FIT: 92% of pts who had the >4% loss in hip BMD in year 1 gained BMD in year 2 (92%), & gained the most: average 4.8%. Patients who had the greatest gain in hip BMD (>8%) in year one were least likely to gain BMD in year 2 (36%). Average, lost 1% BMD. Similar pattern for spine BMD and in the placebo group |
MORE: Women who lost >4% FN BMD during year 1 of raloxifene had a 79% chance of gaining BMD in year 2, with an average gain of 4.0%. Patients who gained>8% in yr 1 had a 22% of gaining BMD in year 2 and on average lost 2.8% in yr 2. Concluded that most women who lost BMD during the first year of treatment with alendronate or raloxifene will gain BMD if the same treatment is continued for a second year, illustrating the principle of regression to the mean & that effective treatment for osteoporosis should not be changed because of loss of BMD during the first year of use. |
FIT refers to Fracture Intervention Trial; MORE, Multiple Outcomes of Raloxifene Evaluation Trial; OR, odds ratio; CI, confidence interval
These included studies on more than one antiresorptive drug
FIT refers to Fracture Intervention Trial; MORE, Multiple Outcomes of Raloxifene Evaluation Trial.
Appendix 14: Quality Assessment of Randomized Controlled Trials Providing Bone Mineral Density Change From Baseline During Osteoporosis Drug Therapy
| Study | Sample size | Clear Inclusion/exclusion criteria | Method of randomization | Concealment | Blinding | Power calculation | % complete follow-up | Intention to treat analysis |
|---|---|---|---|---|---|---|---|---|
| Lyritis et al., 1997 | 100 | √ | Not stated | No | No | Not stated | 73% | Not stated |
| Montessori et al., 1997* (80) | 80 | √ | √ by computer in blocks of 4 | Not stated | No | Not stated | 80% | √ |
| Ishida et al., 2004 | 396 | √ | √ | Not stated | √ end point evaluation | Not stated | 9% withdrawn | √ |
| Alendronate | ||||||||
| Bone et al., 1997 (188) (multicenter) | 994 | √ | Not stated | Not stated | √ | Not stated | 81% @ 3 yrs 51%@ 10 yrs | √ Primary evaluation |
| Pols et al., 1999 (191) (Multicenter) | 1,908 | √ | Not stated | Not stated | √ | √ | 88% study group 90% Placebo | √ Primary evaluation |
| Cummings et al., 1998 (189) (Multicenter) | 4,432 | √ | √ in blocks of 10 by in blocks of 10 by computer generated codes | √ | √ All blinded to treatment & BMD results | √ | 96% | √ |
| Wasnich et al., 1999 (187) (EPIC) | 262 | √ | Not stated | Not stated | Unclear | Not stated | 79% | √ for treatment group |
| Bell et al., 2002 (190) (multicenter) | 65 | √ | Not stated | Not stated | √ | Not stated | 72% study group 67% placebo | √ Primary analysis |
| McClung et al., 2004 (81) (multicenter) Prevention | 529 | √ | Not stated | Not stated | Single blind | Not stated | 71.3% @ 6 years | √ modified |
| Evio et al., 2004 (84) (Single center) | 60 | √ | Not stated | Not stated | Single | Not stated | 77% | Not stated |
| Sambrook et al., 2004 (199) (multicenter) | 487 | √ | √ Computer generated random allocation | √ | √ | Not stated | Alendronate 88% Raloxifene 86% | √ modified |
| Alendronate | ||||||||
| Black et al., 2003 (85) (Multicenter) | 238 | √ | Not stated | Not stated | √ | √ | 95% @ 1 year | √ |
| Gonelli et al., 2003 (119) (Single center) | 77 | √ | Not stated | Not stated | Open label | Not stated | 86% | No |
| Ringe et al., 2004 (200) (Single center) | 167 | √ | Not stated | No | Open label Radiologist blinded to allocation | Not stated | 88% | √ |
| Risedronate | ||||||||
| Hooper et al., 2005 (192) | 383 | √ | √ computer generated | Not stated | √ | √ 90% power for 3% difference | 77% | √ |
| Clemmesen et al., 1997 (193) | 132 | √ | Not stated | Not stated | √ | Not stated | 73% @ 2 years | √ |
| Fogelman et al., 2000 (201) (Multicenter) | 543 | √ | Not stated | Not stated | √ | √ | 70% @ 3years 65.4% | √ |
| Harris et al., 1999 (194) | 2,458 | √ | √ computer generated | √ | √ | Not stated | 57% | √ |
| Reginster et al., 2000 (202) | 1,226 | √ | Not stated | Not stated | √ | √ 90% power to detect 40% fracture reduction | 81% @ 1 year 44%@3 years |
√ |
| Ringe et al., 2006 (86) | 316 | √ | Not stated | Not stated | Open label | Not stated | 100% @ 1 year | √ |
| Raloxifene | ||||||||
| Ettinger et al., 1999(203) | 7,705 | √ | Not stated | √ | √ | √ | 77% | √ |
| Sambrook et al., 2004 (199) (multicenter) Morii et al., 2003 (204) | See study under alendronate 284 | √ | Not stated | √ | √ | Not stated | 87% | √ |
| Parathyroid hormone | ||||||||
| Black et al., 2003 (85) Postmenopaus al | See previous table | |||||||
| Hodsman et al., 1997 (205) | 217 (Placebo 53) | √ | Not stated | Not stated | Double blind √ |
√ | 95% | √ |
| Neer et al., 2001 (206) | 1,637 | √ | Not stated | Not stated | √ Double blind |
Not stated | 81% for radiographs | Not stated |
| Body et al., 2002 (207) | PTH (20 or 40 ug) vs placebo 146 (PTH vs alendronate) | √ | Not stated | Not stated | Double blind √ |
Not stated | 74% | √ |
| McClung et al., 2005 (208) (multicenter) | 203 (PTH vs alendronate) |
√ | Not stated | Not stated | Double blind √ |
Not stated | 85% | √ modified |
| Lane et al., 1998 (209) | 51 (PTH vs PTH+estrogen) |
√ | √ Computer generated table |
Not stated | Not stated | Not stated | 94% | Not stated |
| Cosman et al., 2005 (210) | 126 Alendronate vs Alendronate + PTH |
√ | √ Bycomputer in blocks of 18 |
Not stated | No blinding (patient & physician); blinded outcome assessment | Not stated | 78% | √ |
| Finkelstein et al., 2003 (211) | 83 (men) Alendronate vs PTH vs both |
√ | √ By computer |
No blinding to treatment; blinded assessment of BMD | Not stated | 76% | √ Include those with at least 1 BMD test |
|
| Kurland et al., 2000(212) | 23 (men) PTH vs placebo |
√ Inclusion Exclusion not specific | √ By computer in blocks of 4 |
√ | √ Double-blind |
Not stated | Not clear | √ |
Appendix 15: Description of Meta-analysis and Randomized Controlled Trials on Osteoporosis Drug Therapy in Men*
| Study | Patient/Drug | Effect on BMD | Effect on fracture | Authors’ conclusion |
|---|---|---|---|---|
| Alendronate Sawka et al., 2005 (118) | Meta-analysis Of Orwell & Ringe 2004 1996–2004 Alendronate vs placebo or vitamin D or calcium |
Using Bayesian random effects model OR vertebral fracture in alendronate treated men 0.44 (95% CI, 0.23–0.83) OR nonvertebral fracture 0.6 (95% CI, 0.29–1.44) |
Alendronate decreases risk of vertebral fractures in men with low bone mineral or fractures. Insufficient data for effect on non-vertebral fractures |
|
| Orwoll et al., 2000 (213) Double blind RCT |
Men mean age 63 yrs with femoral neck T-score>/= –2 or L-spine T-score< –1 (Alendronate 10 mg + Ca+vit D) vs (placebo+Ca+vit D) (N= 146/95) Follow-up = 2 years |
BMD increase significantly ↑ 7.15 in lumbar spine & 2.5% in femoral neck in Alendronate vs ↑ of 1.8% in L-spine & 0.6% @ hip of placebo. BMD significantly higher in Alendronate group @ each site. | 3% alendronate vs 13% control had >/=10 mm height loss Vertebral fractures 0.8% alendronate vs7.1% control (P = 0.02) Effect independentof age. |
Significantly lower bone marker level in alendronate group |
| Ringe et al., 2004 (200) Open label RCT |
Men with primary osteoporosis Alendronate 10 mg vs alfacalciferol (N = 68/66) Mean age 52.1/53.3 @ 3 years (58/60 completed treatment) |
BMD over baseline L-spine 11.5% alendronate vs 3.5 control (P = .0001) Femoral neck 5.8% vs 2.3% (P = .0015) 87% of alendronate vs 46% of control group had increase in spine BMD>/=3%, 63% vs 33% had increase in hip BMD>/=3% |
Vertebral fractures occurred in 10.3% alendronate vs 24.2% control (P = .04) 57% reduction in vertebral fracture risk Change in height: –7.1mm in alendronate vs 13.1mm in control (P = .03) |
No significant difference in nonvertebral fractures Both treatments well tolerated. Hypercalciuria reported in 15.1% control vs 4.4% alendronate (P = .04) |
| Gonnelli et al., 2003 (119) RCT |
Primary osteoporosis Alendronate 10 mg+ Ca vs Ca alone (n=39/38) 3 years |
Alendronate group significant increase in spine BMD in each of 3 yrs of follow-up 4.2-8.8% Increased total hip BMD only significant in year 3 (3.9%) | BMD at lumbar spine appear to be the best method for monitoring effect of alendronate on bone mass in osteoporotic men (> least significant change al each year). | |
| Risedronate | ||||
| Sato et al., 2005 (121) Double blind RCT |
Ambulatory men after stroke >/=65 yrs Risedronate 2.5 mg oral vs placebo (n=140/140) 18 months |
BMD Alendronate group +2.5% vs control –3.5% (P<.001) Serum Ca+ decreased & PTH & 1,25(0H)2 increased in risedronate group but stayed low in control | Number of fall similar Hip fracture 2/140 inrisedronate vs 10/140 control RR 0.19 (95% CI, 0.04–0.89) NNT for hip fracture16 (9–32) |
|
| Ringe et al., 2006 (122) Single centre open label RCT |
Men with primary & secondary osteoporosis (Risedronate 5 mg+1g Ca & vitamin D) vs (placebo) N= 158/158 Mean age 55.8 yrs vs 58 yrs(NS) 12 months |
Increase in spine BMD Risedronate 4.7% vs 1% control (P<.0001) Total hip BMD +2.7 %vs +0.4% Femoral neck 1.8% vs 0.2% (P <.0001) |
New vertebral fractures risedronate 5.1% vs 12.7% control (P = .028) No significant difference in non-vertebral fracture risk. Height change 1.1mm risedronate vs –4.6mm control |
Improvement in back pain greater in risedronate than control (P < .0001) |
Ca refers to calcium; L-spine, lumbar spine; OR, odds ratio.
Appendix 16: Rate of Bone Mineral Density Change From Baseline During Osteoporosis Treatment*
| Study | Site | Mean % Change in BMD From Baseline (95% Confidence Interval During Treatment | |||
|---|---|---|---|---|---|
| After 1 year | After 2 years (SE when reported) | After 3 years | |||
| Etidronate | |||||
| Lyritis et al., 1997 (214) Postmenopausal osteoporotic women 90 day cyclical (400 mg × 20 days) |
Spine FN |
Approximately 8† 1† |
12† 1.7† |
||
| Montessori et al., 1997 * (80) | Spine FN |
4.25 (95% CI, 2.90; 5.59) 2.73 (95% CI, 1.51; 3.95) |
5.67 (4.04; 7.29) 1.44 (–0.60; 3.47) |
||
| Ishida et al., 2004 (215) Postmenopausal osteoporotic women, age 50–75 years 200mg x2 weeks then none for 10 weeks |
Distal radius | – 0.5% | |||
| Alendronate | |||||
| Bone et al., 1997 (188) women age 60–85 yrs, T-score< –2 (5mg) * |
L spine FN Trochanter |
4.5 (0.38) 1.01 (0.45) 3.54 (0.52) |
6.23 (0.43) 1.89 (0.49) 4.13(0.59) |
||
| Wasnich et al., 1999 (187) Early Caucasian postmenopausal (5mg) |
L-spine Total hip |
4.2 3 |
|||
| McClung et al., 2004 † (81) Early postmenopausal women (5mg) Mean age 51–55 years) |
L-spine Total hip |
† 3 1.4 (hip) |
3.5 | 4 | |
| Iwamoto et al., 2005 (216) Women 55–86 yrs old BMD<70% of young adult mean (5mg) |
L spine | 9.3 | |||
| Gonelli et al., 2003 (119) Men with primary Osteoporosis (5 mg) |
Femoral FN L-spine |
2.1† 4.2† |
3.2 6.3 |
4.2 8.8 |
|
| Evio et al., 2004 (84) Postmenopausal women age 65–80 yrs (mean 71), T-score≤ –2.5 (10 mg+/- HRT) |
L-spine Alendronate Alend+HRT FN Alindronate HRT Alend+HRT |
6.8 8.4 3.3 4.9 |
9.1 11.2 1* 5.8 2.7 |
||
| Sambrook et al., 2004 (217) Postmenopausal women Mean age 62 yrs, T-score ≤–2 Alendronate 70mgx1/wk |
L-spine Total hip |
4.8 2. |
|||
| Cummings et al. 1998 (189) Women age 55–80 yrs BMD @ femoral neck ≤ 0.68 g/cm2 (T-score ≤ –2) (5mg increased to 10 mg/d) |
L-spine FN Total hip |
† 4.1 2 2 |
Year 4 8.3 3.8 3.4 |
||
| Bell et al., 2002 (190) African-American women (45–88 yrs), T-score≤ –1.75, Alendronate(10 mg) | L-spine FN Trochanter Total hip |
About 6† About 4 About 4 About 3 |
6.5 (0.7) 4.5 (1.0) 6.4 (0.6) 4.1 (0.7) |
||
| Pols et al., 1999 (191) Women postmenopausal>3 yrs,<85yrsold (10mg) |
L-spine FN Trochanter Total hip |
5 2.3 4.1 3.1 |
|||
| Black et al., 2003 (85) Postmenopausal women T-score < –2.5 or ≥ –2 + risk factors Alendronate 10 mg/day or PTH 100 ug or both |
L-spine Alendronate PTH Both Total Hip Alendronate PTH Both Distal radius Alendronate PTH Both |
4.6 6.3 6.1 unchanged 0.3 1.9 –3.4 –1.1 |
|||
| Ringe et al., 2004 * (200) Men with primary osteoporosis (10 mg) |
Femoral neck L-spine |
3.2 8 |
5.2 10.2 |
5.8 (0.4) 11.5 (0.7) |
|
| Risedronate 5mg | |||||
| Hooper et al., 2005 (192) Women, early postmenopausal |
F. N Total hip |
About +1.4 About +2 |
0.8 2% |
||
| Clemmesen et al., 1997 (193) postmenopausal >1yr, 1–4 vertebral fractures (2.5mg) |
L spine FN |
1.2 1.7 (cyclic) 2.9 1.3 (cyclic) |
0.8 2.3 (cyclic) 0.9 2.4 (cyclic) |
||
| Harris et al., 1999 * (194) postmenopausal women l<85 yrs >/=1 vertebral fracture (5mg) |
L spine FN Trochanter |
4 2 2.6 |
5 1.8 >3 |
5.4 1.6 3.3 |
|
| Reginster et al., 2000 (202) Women>5 yrs postmenopausal <85 yrs >/=2 vertebral fractures (5mg) |
L spine† FN† |
4.4 1.6 |
6 1.1 |
7 2.1 |
9.3 2.2 |
| Ringe et al., 2006 (122) Men (primary & secondary) |
L. spine FN Total hip |
4.7 1.8 2.7 |
|||
| Raloxifene | |||||
| Sarkar et al., 2002 (70) | L spine Hip |
2.7 2.2 |
|||
| Ettinger et al., 1999 (203) MORE >/=2 yrs postmenopausal women & 31–80 yrs old (120mg) |
L spine FN (Estimated fromgraph)* |
2.8 1.5 |
3 1.9 |
3.2 1.3 |
|
| Sambrook et al., 2004, (199) Mean age 62 yrs, postmenopausal Raloxifene 60mg/d |
L-spine Total hip |
2.2 0.8 |
|||
| Morii, 2003 (204) (60mg) Japanese women osteoporotic, PM |
L-spine | 3.5 | |||
| Parathyroid hormone | |||||
| Black et al., 2003 (85) Postmenopausal T-score< –2.5 or –2+ a major risk factor (100 ug) |
L-spine FN |
6.3 0.93 |
|||
| Hodsman et al., 1997 (205) Postmenopausal T-score<-2.5 |
L-spine FN |
3.0 (50ug) 5.1 (75 ug) 7.8 (100 ug) 0.5 (100 ug) |
|||
| Neer et al., 2001 (206) Postmenopausal & history of fracture | L-spine FN |
(1.5 yrs) 9.7 (20ug) 13.7 (40 ug) 2.8 (20ug) 5.1 (40 ug) |
|||
| Body et al., 2002 (207)(Teriparatide) Postmenopausal women |
L-spine | (14 months) 14.2 |
|||
| McClung et al., 2005 (208) (Teripartide) Postmenopausal Osteoporosis (20 ug) |
L-spine FN |
(18 months) 10.3 3.9 |
|||
| Lane et al., 1998 (209) Postmenopausal + cortisone induced- osteoporosis (daily or cyclic PTH 25ug+estrogen) |
L-spine FN |
11.1 2.9 |
|||
| Cosman et al., 2005 (210) Women T-score <–2.5 or –2+fracture (25 ug) PTH + alendronate |
L-spine | (15 month) 6.1 (daily) 5.4 (cyclic) |
|||
| Finkelstein et al., 2003 (211) Men T-score< –2 (40 ug) |
L-spine FN |
8* 1* |
30 months 25.8 6.4 |
||
| Kurland et al., 2000 (212) Men T-score <–2.5 or Z-score< –2 (400IU 1-34) |
L-spine FN |
9.6 | 18 months 13.5 2.9 |
||
FN refers to femoral neck; L spine, lumbar spine; PTH, parathyroid hormone; SE, standard error.
BMD changes estimated from graph
FN refers to femoral neck; L spine, lumbar spine; PTH, parathyroid hormone; SE, standard error.
BMD changes estimated from graph
Appendix 17: Canadian Studies on Prevalence of Osteoporosis Investigation, Diagnosis and Treatment After a Fragility Fracture*
| Canadian Studies | ||||||
|---|---|---|---|---|---|---|
| Hajcsar et al., 2000 (134) | Papaioannou et al., 2004 (87) | Khan et al., 2001 (218) | Juby et al., 2002 (219) | Khandwala et al., 2005 (132) | Vanasse et al., 2005 (88) | |
| Study design | Retrospective analysis | Retrospective analysis | Retrospective analysis | Prospective cohort study | Retrospective review of medicalrecords | Retrospective population-based cohort study |
| Setting | 3 Ontario Community hospital fracture clinic | 4 tertiary care hospitals Hamilton, Ontario | 1 tertiary care hospital, Edmonton, Alberta | Tertiary hospital, Edmonton, Alberta- pts from seniors’ clinic & a day program | 1 hospital in Saskatoon, Saskatchewan | Province of Quebec – data from Quebec Health Insurance Board |
| Sample Complete follow-up Age, years |
N=108 (89% women) Representing 56.1% of patients with fragility fractures 108 >18 |
All patients with hip fracture ≥ 50 |
N = 156 (83% women) 72% of patients with fragility fracture of distal radius/ulna N = 112 (72%) Mean 64 (range 41–91 years) |
N = 145 (73% women) All patients witha new hipfracture 145 complete questionnaire >65, Mean 72 & 77.7 |
N = 174 Admitted with fragility hip fracture 174 Mean age 82.5 (9.8) yrs |
N = 25,852 (77% women) with a fragility fracture (vertebral, hip, wrist, or humerus) in 1999 & 2000 25,852 ≥ age 65 |
| History of prior fracture % | 39.8 | 39.2 | 16 | 22 | ||
| Time after fracture, years | 1 | 1 | 0.5–3 | Acute & after rehabilitation | @ discharge | |
| BMD testing, % Clinical diagnosis % Calcium % Vitamin D % HRT % Bisphosphonate % Any osteoporosis specific therapy % Other findings |
35 (total) 22.2 (after index fracture) 18.5 32.4 13 16 7.4 - |
- 1.7 4.7 0 0 - |
- BMD or clinical 50 61.6 (Ca or Vitamin D) - - 37.5 (HRT or bisphosphonate) |
Overall = 26% 35% women 2.6% men 14.5 40 17.9 29% of women - 18.3 (3% in men) |
0% Evaluation recommended in 1% 3% 1% Recommended in 4% implemented in 3% No significant difference in intervention rates by sex & history of fractures. |
Men 4.6 Women 13.1 Men 9.9 Women 29.7 Regional BMD range 0–16% Use of BMD ⇓ with ⇑ distance from BMD facility |
Ca refers to calcium; N, sample size; HRT, hormone replacement therapy.
Appendix 18: Description of Studies: Impact of Prevalent Fractures on Risk of Incident Studies
| Study | Design | Population | Prevalent fractures | Incident fractures |
|---|---|---|---|---|
| Klotzbuecher et al., 2000 (94) | Meta-analysis 33 studies 1996–1999 | peri/postmenopausalwomen | Wrist, vertebral, hip, pooled | Vertebral, hip, wrist, pooled |
| Kanis et al., 2004 (95) | Meta-analysis(11 large population–based studies 1994–2003 | 15,259 men and 44,902 women | Prior fractures | Hip, any fracture, osteoporotic fracture (with and without effect of BMD) |
| Haentjens et al., 2003 (96) | Meta-analysis of 9 cohort studies 1982–2001 | Colle’s fractures & spine fractures | Hip fractures | |
| Johnell et al., 2004 (98) Immediately following fracture |
Prospective longitudinal cohort study in Sweden 5-year follow-up | 1,918 men and women identified by radiology to have a fracture at the spine, hip or shoulder | Spine Hip Shoulder |
Relative risk of Hip, spine, and forearm over time stratified by age 60 & age 80 years |
| Papaioannou et al., 2005 (19) (Post-menopausal women) |
Prospective multi-site population based Canadian cohort study 3-year follow-up | 5,143 postmenopausal women who participated in the Canadian multicenter Osteoporosis Study mean age of group 66.4 (SD 9.6) –74.4 (SD 10.0) years | Vertebral Forearm Nonvertebral | Vertebral Main nonvertebral (wrist, hip, humerus, pelvis or rib) Any nonvertebral fractures |
| Bensen et al., 2005 (26) (Canada) | Analysis of prospective multisite Canadian CANDOO database | 3,426 postmenopausal women registered in the CANDOO | Previous fractures after age 50 years | OR for vertebral fractures, hip fractures, wrist fracture, & rib fracture |
| Schousboe et al., 2005 (102) | Study of Osteoporotic Fractures (SOF) - Prospective cohort study in the US Mean follow-up 3.7 years | 9,704 elderly community dwelling women, mean age 73.2 years (with wrist fracture) & 71.5 (no wrist fractures) | Previous wrist fractures since age 50 years | Hip fractures Radiographic vertebral fracture |
| Van der Klift et al., 2004 (103) | Prospective population-based cohort study – Part of the Rotterdam Study Mean follow-up 6.3 years | 4,216 men & women (2467 women) age>55 years mean age for subgroups 65.2– 68.6 years | Vertebral fracture | Vertebral fracture |
| Porthouse et al., 2004 (104) | UK comprehensive cohort study with anested randomized controlled trial on hipprotectors 2 year follow-up | 4,292 women ≥ 70 years Mean age 76.9 years |
Previous fracture | Hip, nonvertebral, wrist fractures |
| Taylor et al., 2004 (24) | Study of Osteoporotic Fractures (SOF) - Prospective cohort study in the US | 6,787 community-dwelling, ambulatory Caucasian women ≥age 65 (mean age 73.3 (SD 4.9) years from SOF with complete data | Any previous fractures since age 50 years | Hip fractures |
| Colon-Emeric et al., 2003 (105) | Analysis of data from the Baltimore Hip Studies and the Established Populations for Epidemiologic Studies of the Elderly (EPESE) Mean follow-up 6.0 and 1.6 years respectively |
Baltimore study: 549 men & women >/= 65 years of age with acute hip fracture (Mean age 80.9 (SD 7.4) years EPESE: 10,680 community-dwelling men & women age >/= 65 years. Mean age 73.8 (SD 6.7) years | Hip fracture | Hazard ratio for subsequent nonhip skeletal fracture |
| Naves et al., 2003 (106) (Longitudinal) |
Prospective cohort study – Spanish cohort of the EVOS study Follow-up 8 years |
316 women and 308 men age> 50 years randomly selected from the EVOS cohort. Mean age 65 (SD 9) for men and women |
Prevalent vertebral fracture Prevalent and Incident vertebral fracture Intraobserver agreement = 92%, interobserver agreement of 90% |
Hip, Colles’, vertebral Hip, Colles’ |
| Albrand et al., 2003 (OFELY) (25) Healthypostmenopausalwomen |
Longitudinal cohort study of healthy ambulatory Caucasian volunteers in Rhone district of France, followed for a mean of 5.3+1.1 years | 672 postmenopausal healthy ambulatory Caucasian women (mean age 59.1 years (SD 9.8 years) | All prevalent fractures after age 45 years | Fragility fractures |
| Pongchaiyakul et al., 2005 (99) | Part of ongoing Dubbo Osteoporosis Epidemiology study (DOES) – longitudinal, population-based study of risk factors for fracture & mortality in Australia (5 year follow-up) | 114 men and 186 women (age> 60 years & free of illnesses that affect bone metabolism) randomly selected from the DOES database Mean age 69.8 years with vertebral deformity) & 69.4 years with no vertebral deformity | Asymptomatic vertebral deformity (at least –3SD in vertebral height) confirmed on radiograph | Any fracture Hip fracture Vertebral fracture Colles’ fracture Major fractures (major upper or lower limb and/or rib fractures) |
| Hasseius et al., 2003 EVOS (101) Longitudinal | European Vertebral Osteoporosis Study – multicenter study to evaluate vertebral deformity – men and women followed for 10 years | Men & women age 50–80 years 213 men (mean age 63 years) and 257 women (mean age 64 years) |
Vertebral deformity (–3 SD or –5 SD in vertebral height | Any incident fracture Any fragility fracture |
| Szulc et al., 2005 (97) | A prospective study of osteoporosis and of its determinants in men (MINOS) in France Follow-up 7.5 years | 791 men aged 51-85 years were followed prospectively for BMD and fractures | Prevalent fractures | Fractures Total hip fractures |
Appendix 19: Description of Studies on Risk Factors of Fragility Fractures in Men*
| Study | Sample size/Follow-up | Inclusion Criteria | Exclusion Criteria | Independent assessment of risk factors & fractures | Statistical Method | Complete follow-up |
|---|---|---|---|---|---|---|
| Johnell et al., 2004 (98) Malmo |
Population based longitudinal N = 1,918 men & women Follow-up = 5 years (osteoporotic fracture on future fractures & mortality) |
Patients in Malmo with an osteoporotic fracture @ the spine, shoulder, or hip identified from radiograph (1990–1994) |
Incomplete radiographic follow-up | Not stated for evaluation of incident fractures | Poisson model to calculate rate of new fractures after a fracture taking mortality into account. The rate was calculated as a function of age, sex, & time after fracture. | Lost to follow-up due to moving out of Malmo: 2.5% of men & 2.7% of women |
| Van der Klift et al., 2004 (103) Rotterdam Study | Population based longitudinal N = 1,377 men & 1,624 women Mean follow-up = 6.3 years | Age ≥ 55 years living in Ommoord, Rotterdam & Had 2nd follow-up visit in the Rotterdam study | No baseline visit; data on one or more risk factors were missing | Not stated for morphometric evaluation of baseline & follow-up radiograph of thoracolumbar spine Interviews re medical history, drug use, diet, falls & non-vertebral fractures after age 50 years | Test for significance of risk factors on incident vertebral fracture, using unadjusted & adjusted (age, BMD, prevalent nonvertebral fractures) models of logistic regression | 71% of original selected subjects had complete data. Accounted for exclusions |
| Szulc et al., 2005 (97) MINOS | Population-based longitudinal N = 759 men Mean follow-up = 7.5 years | Men age ≥ 50 years & had DXA absorptiometry @ baseline in MINOS study |
Had high trauma fractures; fractures of figures, toes or skull; fractures before 40, | Not stated for evaluation of incident fractures or DXA absorptiometry | Logistic regression to determine increase in fracture risk/SD decrease in BMD adjusted for risk factors. Did not use Cox proportional hazard model. | Analysis included 96% of original cohort who had reliable data about fracture incidence |
| Colon-Emeric et al., 2003 (105) US | Two cohort studies† EPSE n = 10,680 BHS = 549 Mean follow-up EPSE 6 years BHN 1.6 years Outcome: self-reported nonhip skeletal fractures during follow-up |
EPSE: community dwelling adults≥65 yrs BHS included community dwelling men & women >65 yrs admitted to hospital with acute hip fracture |
Excluded: patients with no follow-up visits or who reported a history of nonhip fractures. | Not applicable | Survival analysis; Cox proportional Hazard model stratified by site. Model adjusted for race, sex, age, BMI, stroke, cancer, difficulty walking acrossa room etc. |
All included patients |
| Roy et al., 2003 (153) EPOS |
Prospective Multicenter longitudinal N=3,173 men & 3,402 women Mean follow-up = 3.8 years | Age 50–79 years randomly sampled from population registers in study centres & had repeat lateral thoraco-lumbar spine radiographs – completed questionnaire | <50 years | Yes, baseline& follow-up radiographs evaluated morphometrically & qualitatively at central radiological facility | Poisson regression used to explore relationship between patient risk factor variables & incident vertebral fracture, adjusted for age & centre | |
| Cauley et al., 2004 (147) SOF STORM |
N = 317 of 523 men from STORM study 2,067 women from SOF study who had info on prevalent vertebral fractures | Caucasian men & women in 2 separate longitudinal cohort studies (population-based list) | Inclusion: living in community of Monongahela Valley (Pittsburgh) - walk without assistance of another person Exclusion: people with bilateral hip replacement | Morphometric definition of vertebral fractures given. No masking of assessment stated. |
Chi-square test for categorical variables. Logistic regression analysis to calculate OR of having a vertebral fracture per 0.01g/cm2 decrease in BMD. Analyzed effect of gender & age. |
Not clear. |
| Schuit et al., 2004 (145) Rotterdam Study |
Population-based cohort study N = 3,075 men & 4,731 women Mean follow-up = 6.8 years (BMD & nonvertebral fractures) |
Inhabitants of Ommoord ≥55 years who consented to participate Underwent Clinical exam & DXA BMD of FN | No informed consent for follow-up registration | Reported events verified & coded independently by research physicians & confirmed by a medical expert. | RR for first fracture associated with 1 SD decrease in femoral neck BMD using a Cox proportional hazard model | Results reported entire cohort. FN BMD available in 74.2% of participants |
| Van Potelbergh et al., 2003 (196) | Longitudinal population study N = 214 men | Healthy; age > 70 years recruited from population register of a semi rural community in Belgium | Past or current disorders or treatments that may affect androgen status &/or bone metabolism; incomplete data | |||
| Pande et al., 2000 (146) | Case-control study N=100 men - study subjects & 100 age-matched controls | Study subjects Consecutive men>/=50 years old admitted to a UK hospital with a low trauma hip fracture | Excluded trauma fractures & residence outside Cornwall, & active malignancy | Not stated for DXA BMD measurements @ L-spine & proximal femur; hip axis length (HAL) recorded by automated software | Association between BMD, HAL and risk of hip fracture determined using logistic regression, adjusted for age, & subsequent height & weight | 62 study patients & 100 controls had data concerning BMD. Accounted for lack of data |
| Naves et al., 2003 (106) EVOS (Spain) | Cross sectional. N = 316 women and 308 men Follow-up 8 years (Effect of vertebral on risk of further osteoporotic fractures) | Random selection from register of Oviedo in Spain; Age ≥ 50 years. | Not stated | Semi quantitative & morphometric evaluation of lateral radiographs of dorsal & lumbar spine taken @ baseline & 4th year of follow-up Blinded for incidence of new fractures | RR of different osteoporotic fractures (with vs no vertebral fractures). K-M survival curves. Cox multiple regression to compare survival (with vs no prevalent vertebral fractures. | Analysis included all patients. |
| Van Staa et al., 2002 (220) UK |
Population-based –register N= 119,317 women & 103,052 men from General Practice Research Database GPRD | All patients age ≥ 20 years registered in GPRD& had a fracture recorded in 1988–1998 | Not stated | Not stated | Standardized incidence ratios of observed to expected number of fracture cases during follow-up Adjusted for age & incidence | Analysis included all patients |
| Pongchaiyakul et al., 2005 (99) DOES Australia |
300 Men 114 Women 186 Follow-up median 10.2 (SD 4) years |
Randomly selected from database of a large population (Dubbo) study – residence in an isolated Australia city | Inclusion: -60 years of ageas of June 1989 -Free of anyillness likely toaffect bonemetabolism | Presence of vertebral deformity read in a masked fashion to BMD. Precision of BMD: 1.3–1.5% | Cox proportional hazards model, adjusted for age, sex, BMD & body weight | 100% |
| Ismail et al., 2001 (100) EVOS (Europe) | Multicenter longitudinal N = 6,344 men & 6,788 women Median follow-up = 3 years | Age ≥ 50 years recruited from population registers in 36 European centres through stratified sampling | Not stated | Not stated for Morphological identification of spine deformity in radiographs & confirmation of reported fractures | Cox proportional hazard regression analysis to assess the predictive risk of vertebral deformity on future limb fractures, | Reported results for all patients |
| Hasserius et al., 2003 (101) EVOS (Sweden) |
Longitudinal N = 298 men & 300 women Randomly selected from population of city of Malmo Follow-up = 10 years (spine deformity on risk of future fractures & mortality) |
Age 50–80 years | Not stated | Not stated for morphological identification of spine deformity in radiographs Mortality & fracture (all types, fragility, hip) incidence |
Cox proportional hazard regression model Multivariate analysis adjusted for age, alcohol intake, smoking, general health & previous hip fracture | Reported results for all patients. |
| Leifke et al., 2005 (221) | Case-control N = 27, men and 12 controls (after non-immobilizing stroke, no fracture) | Study group: 1–3.5 months after a minimal trauma hip fracture; ≥ 65 years |
Men with secondary osteoporosis | Laboratory assay of sex hormones (T, non-SHGB-bound T, E2, iPTH | Yes | 100% |
| Mellstrom et al., 2006 (171) MrOS (Multinational) | Multicenter Cross-sectional N = 2,908 men | Men age 69–80 years randomly selected from population registers in Sweden, Hong Kong, & US; able to walk without aids, | Bilateral hip prosthesis | Not stated for: BMD (total hip, femoral trochanter & L-spine. Assay of serum total T, E2, & SHBG Self-reported fractures. | Pearson correlation for univariate associations. Linear regression for independent predictors. Odds ratios to determine predictive values. |
All 2,908 patients included in analysis, * CV of DXA BMD tests ranged from 0.5% to 3% |
| Barret-Connor et al., 2000 (170) Rancho Bernardo Study | Longitudinal study N = 352 men & 288 postmenopausal women Mean follow-up = 8.4 years |
Community – dwelling, ambulatory residents of Rancho Bernardo; Caucasian; age ≥ 50 years. | Women on estrogen; | Masked assessment not stated for lateral radiograph of thoracic & L-spine. Blood assay of free & bioavailable sex hormones | Pearson’s correlation used to assess association between hormone levels & age; Mann-Whitney test for difference by fracture status using median age. | 14 of original cohort excluded because of suspected hormone use, endocrine disease, or uninterpretable radiograph |
| Kanis et al., 1999(155) MEDOS |
Case control study N = 730 men Identified by surveillance of hospitals, clinics, & nursing homes + 1,132 age-matched controls |
Caucasian, age≥ 50 years, in catchment area in Southern Europe & had a hip fracture | Poor mental sautés, refusal, or concurrent illness | Questionnaire : assessed height, body weight, physical activity, mental score, intake of alcohol, tobacco, exposure to sun light | RR estimated from OR & adjusted using logistic regression models. The multivariate analysis used unconditional logistic regression. | All study subjects and controls included in the analysis |
| Kanis et al., 2002(222) NHANES III Sweden | Population-based study | Fracture risks obtained from Malmo and applied to the population of Sweden | Calculated 10-yr probability in 5-year age groups using hazard of first fracture @ each of the hip, spine, shoulder & distal forearm & the death hazard | |||
| Meyer et al., 1998 (156) (Norway) Hip fracture | Longitudinal study N = 19,151 men & 19,938 women Mean follow-up =11.6 years |
Men & women born in 1925–1940, living in 3 Norwegian counties & attended 3 consecutive health examinations by the National Health Screening Service | Not stated | Hip fractures (cervical & trochanteric) identified from registers& verified through medical records | Age adjusted incidence rates using Cox proportional hazards regression; RR from multivariate analysis; association between weight variability & trend in weight change by Pearson’s correlation. | All patients included in analysis. |
| Langlois et al., 1998 (157) EPESE US |
Longitudinal study N = 2,413 men Follow-up = 8 years |
Caucasian community-dwelling men in 3 US counties; age ≥ 67 years; & participated in the baseline interview. | Non-white men; no match to Medicare hospitalization file, had previous hip fractures, or missing body weight or height data. | Identified men hospitalized for hip fracture during follow-up. BMI & weight change determined. |
Adjusted RR for hip fracture by category of weight change from age 50 years - from a Cox proportional hazard model stratified by community | Results reported for all 2,413 |
| Gonnelli et al., 2005 (143) | Cross sectional study N = 401 mens consecutively referred |
Referred for assessment of bone status Jan 2000–Dec2002 | Excluded : extensive exclusion criteria including >15% below or 30% above ideal body weight, cannot have spine DXA BMD test, secondary causes of osteoporosis, etc | Not stated for (DXA BMD @ spine, FN, total hip, trochanter & intertrochanter & ultrasound). |
Pearson correlation coefficient (BMD & fracture risk), Receiver Operator characteristic analysis & AUC | Results reported for all 401 patients |
| Kudlacek et al., 2000 (148) Austria |
Cross sectional N = 136 men & 337 women | Patients referred to a single center for screening or diagnosis of suspected osteoporosis | Secondary osteoporosis or receiving medical treatment for osteoporosis, history of severe trauma | Not stated for fracture assessment or BMD measurements Lumbar spine BMD by QCT |
Logistic regression model to estimate probability of a fracture depending on BMD, matched & compared for sex groups. | Reported for all patients |
| Kelsey et al., 2005 (31) US |
Case control N = 192 consecutive men and women Controls : 2,402 randomly selected from KP medical centre from same period (not matched) | Pelvis fracture identified from radiology or medical reports, confirmed by radiograph, bone scan or MRI. Controls- | Prior pelvis fracture after age 45 ; Fractures from disease such as Paget’s or cancer. | Not stated Risk factors by standardized questionnaire by trained interviews. Measurement of physical functioning |
Odds rations adjusted using unconditional logistic regression for sampling variables of age group, sex, & race/ethnicity | Reported for all patients |
| Holmberg et al., 2005 (154) Malmo, Sweden |
Longitudinal N = 22,444 men (Mean age 44 years) & 10,902 women (mean age 50 years) Follow-up = 16 years for men & 11 years for women | Population of Malmo, Sweden between 1974–1984 who consented to the study | Identification of hip fracture from radiology register (excluded high energy fractures) Questionnaire on risk factors (fracture history, family history, lifestyle factors) |
Age adjusted Cox proportional hazard model to analyze each variable and in categories. Analysis in a final multivariate Cox regression model | ||
| Van Staa et al., 2000 (161) UK |
Retrospective cohort study N = 244,235 (101,599 men) study patients & 244,235 matched controls from the GPRD | Study patients: Received ≥ 1 Prescription for oral corticosteroid Grouped according to dosage of glucocorticoid |
Not stated | Not stated | Cox proportional hazards models to calculate adjusted RRs in comparison between oral corticosteroid dose groups. Poisson regression was used in the analysis of the cumulative vs daily doses | All patients included in analysis (outcomes: risk of vertebral, non-vertebral, & hip fractures) |
| Van Staa et al., 2001 (166) UK |
Retrospective cohort study N = 170,818 (77,763 men) inhaled corticosteroid users, 108,786 bronchodilator users, 170,818 controls from the GPRD Follow-up: from 91 days after last inhaled prescription until a fracture or censored | Age≥18 years Inhaled corticosteroid or bronchodilator group: received ≥ 1 prescription for the respective drug during the study period | Inhaled corticosteroid user who received a prescription for oral corticosteroid 6 months prior or 91 days after the last inhaled corticosteroid prescription. | Not stated | Adjusted RRs for fractures estimated using Cox proportional hazards models that included age, gender, & selected confounding variables. | All patients included in analysis (Outcome: risk of vertebral, nonvertebral, hip, & forearm fracture) |
| Hubbard et al., 2002 (167) | Case-control study N = 16,341 (3,432 men) study patients& 29,889 matched controls from GPRD | Study group: All patients in GPRD with recorded diagnosis of hip fracture. Controls: 2:1 ratio matched for age, sex, general practice, & start date for collection of prescribing data. |
Not stated | Not stated | Relationship (ORs) between inhaled corticosteroid & hip fracture quantified by conditional logistic regression. Bivariate & multivariate models used to determine impact of confounders. | Not stated Outcome: relationship between exposure to inhaledcorticosteroid & risk of hip fracture) |
| Hubbard et al., 2006 (164) UK |
Prospective cohort analysis N = 1,671 patients (868 men) from computerized general practice records of 31 practices (January 1995 – February 1999) | Age≥ 75 years; Diagnosis of asthma, COPD, or both | Not stated | Not stated | Cox regression modeling for fracture risk of people with vs those without exposure; controlled for age & gender. Multivariate model to explore potential confounders | Included all patients in analysis. (Outcomes: risk of all fractures (exposed vs non-exposed) (oral, inhaled, injected) |
| Vestergaard et al., 2003 (160) Denmark |
Case-control study N = 6,660 study patients (1,974 men) + 33,272 age-matched population controls (no fracture) |
Study patients: In County Hospital Discharge Register ; a 1st diagnosis of hip fracture (1994–2001) | Had hip fracture before 1993; had an address outside the county; residence in county <5 years. | Not stated | OR for hip fractures based on cumulative dose by conditional logistic regression. Univariate & multivariate analysis for potential confounders | Included all patients in analysis |
| Vestergaard et al., 2005 (163) Denmark |
Case-control study N = 124, 655 study patients (60,084 men) +373,962 controls matched on age & gender (random selection) |
Study patients: All people in Denmark who had a fracture in year 2000 (National Hospital Discharge Register | None stated | Not stated | Conditional logistic regression for crude & adjusted ORs of any fractures (adjusted for exposure variables, comorbid conditions & other drug use. | Included all patients in analysis (oral, inhaled, injected, or topical corticosteroid) |
| Steinbuch et al., 2004 (162) US |
Retrospective cohort study N = 17,957 (7,509 men) + 17,957 controls matched for age, sex, & date of claim from same database | Study patients: in an admin claims database, age 18–64 years, had ≥ 1 claim for oral glucocorticoid 1995–1996; continued enrolment in the drug & medical plan during follow-up | Study patients: excluded patients with a pharmacy claim for an injectable steroid during the study period | Not stated | RR for of fracture (study group vs controls) estimated using a Cox proportional hazards model, adjusted for age group, sex, prior fracture, & prior exposure; & for amount, duration & pattern | All patients included in analysis (Outcomes: risk of hip, non-vertebral, vertebral, & forearm fracture during 1 year after first exposure) |
| Donnan et al., 2005 (165) UK |
Retrospective cohort study N = 20,226 (men 40.5% of total person years) + 248,723 controls January 1993–January 1997 | Age> 18 years from an administrative claims database; ≥ 1 dispensed prescription for oral corticosteroids; Controls: unexposed to glucocorticoids | None stated | Not stated | Regression analysis for RR of fractures (vertebral, non-vertebral, hip & wrist) per 1,000 person years for exposed vs non-exposed to glucocorticoids. Population attributable risk estimated from adjusted RR | All patients included in analysis (Outcomes: risk of non-traumatic vertebral & non-vertebral fracture during follow-up |
BHS refers to Baltimore Hip Studies; EPESE, Epidemiologic Studies of the Elderly.
AUC refers to area under the curve; FN, femoral neck.
K-M refers to Kaplan-Meier.
CV refers to coefficient of variation; E2, estradiol; iPTH, intact parathormone; L-spine, lumbar spine; SHBG, sex hormone binding globulin; T refers to testosterone.
EPESE refers to Established Populations for Epidemiologic Studies of the Elderly; FN, femoral neck; RR, relative risk.
GPRD refers to General Practice Research Database; OR, odds ratio; RR, relative risk.
Appendix 20: Summary of Risk Factors Predicting Fractures in Men*
| Study | Design | Age | BMD | 2 BMI Kg/m2 or Weight loss | Previous fractures/V. deformities | Sex Hormones | Other Risk Factors | |
|---|---|---|---|---|---|---|---|---|
| Klotzbuecher et al., 2000 (94) | Meta-analysis 33 studies | Prior fracture @ any site predicts future fracture RR 1.9–2.6 (men & women) | ||||||
| Kanis et al., 2004 US (95) | Meta-analysis (RR similar for men & women) | Low BMD explained a minority of risk for any fracture (8%), 22% hip fracture. | RR 1.86, 95% CI, 1.75–1.98 for hip & osteoporotic fractures independent of BMD | |||||
| Haentjens et al., 2003 (96) | Meta-analysis 9 cohort studies (Colles or spine) | Colles RR 3.26 Spine RR 3.54 |
||||||
| De Laet et al., 2005 (152) | Meta-analysis | |||||||
| Kanis et al., 2004 (158) | Meta-analysis | Parental history of fracture increased risk of fracture | ||||||
| Johnell et al., 2004 (98) | Longitudinal N=268 men & women | Osteop. Fracture RR 3.7–125 | ||||||
| Van der Klift et al., 2004 (103) Rotterdam Study (Incident vertebral fractures) | Prospective population-based single-cohort N =1,377 men & 1,624 women Follow-up 6.3 years |
Women: age RR (1.8-2.6) depending on age | Men Low L-spine BMD RR 2.3 (1.6–3.3) Women low L-spine BMD RR 2.1 (95% CI, 1.6–2.6) |
Men-prevalent vertebral (RR 2.2)& non-vertebral fracture (RR 2.4) | Women: menopause before at or before age 45 years | Women use of walking aid (RR 2.5) Current smoking (RR 2.1) |
||
| Szulc et al., 2005 (97) MINOS | Prospective cohortn=759 >50 years Follow-up, 7.5yrs | OR 1.29 (1–1.64, P =.05) per 10 year increase in age Risk @ age 75 yrs 3x risk @<55 yrs | Adjusted OR 1.28–1.89 per SD decrease in BMD AUC 0.643–0.712 | Unadjusted fracture risk ⇑ with ⇓ in body weight OR 1.15 per 5 kg ⇓ weight (1.03–1.28) P<.02) | 2-fold ⇑ in riskregardless of site of previous fracture | |||
| Colon-Emericet al., 2003 (105) US BHS 1.6 yrs, EPESE 6 yrs | EPSE † n = 10,680 BHS n = 549 Mean age 73.8 yrs & 80.9 years respectively | Hip fracture linked to 2.5-X increase in nonhip fractures in men & women | ||||||
| Naves et al., 2003 (106) EVOS (Spain) |
Longitudinal N = 308 men &316 women≥ 50 years old Men & women. Follow-up of 8years |
Prevalent V fracture predict vertebral (RR 4.7), hip (RR 6.7) & Colles fracture (RR 3) | ||||||
| Van Staa et al., 2002 (220) UK |
Longitudinal N = 103,052 & men, 119,317 women | Any prevalent fracture SIR 4.2–13.4 | ||||||
| Pongchaiyakul et al., 2005 (99) Australia |
Longitudinal study N=114 men and 186 women Follow-up men 10.5 yrs, women 10 yrs |
Asymptomatic deformity associated with higher incidence of subsequent fractures & mortality in both sexes | ||||||
| Ismail et al., 2001 (100) EVOS (Europe) |
Multicenter Longitudinal N = 6,344 men & 6,788 women age ≥ 50 yrs Median follow up = 3 years | Vertebral deformity is strong predictor of hip & limb fracture in women but not in men | ||||||
| Hasserius et al., 2003 (101) EVOS (Sweden) |
Longitudinal N = 298 men & 300 women age ≥ 50 yrs Follow-up = 10 years | V. deformity predicted mortality & risk of any fracture in women & men | ||||||
| Roy et al., 2003 (153) EPOS (Lifestylefactors) |
Longitudinal N=3,173 men Mean follow-up 3.8 years (risk of vertebral fracture) | Risk increased with age | Not studied | Men in lowest quintile wt compared with others, RR for V. fracture 1.99 (95% FI 1.01–3.93) | Not studied | Smoking & alcohol or milk intake not associated with risk ofvertebral fractures | ||
| Cauley et al., 2004 (147) SOF STORM |
(n=317 Caucasian men Age & 2,067 Caucasian women >50 yrs old | Prevalence of vertebral fractures increased with age in both men & women | 0.1g/cm2 ⇓ in BMD associated with 30–40% ⇑ in men & 60–70% ⇑ in women of risk of V. fracture | |||||
| Kanis et al., 1999 (155) MEDOS |
Case control N=730 men ≥50 yrs w a hip fracture & 1,132 controls Interview: re Risk factors for hip fracture in men. | 6.8% ⇑ in fracture risk with each unit ⇓ in BMI. RR = 0.68 (P< .009) |
Gender at each threshold probabilities higher in women than in men, esp. hip & spine fractures | Recreational Physical activity RR = 0.64 (P<.002) | ||||
| Kanis et al., 2002 (222) NHANES III Sweden |
10-yr probabilities of fractures by age & T-score Threshold for T-score 0.577g/cm2 osteoporosis 0.740g/cm2 low bone mass | 10-year probabilities for fractures (except forearm) increased with age, effect independent of BMD. | 10-year probabilities of fractures decreased with T-score | |||||
| Meyer et al., 1998 (156) (Norway) Hip fracture |
Longitudinal study N = 19,151 men & 19,938 women Mean age 49 yrs Follow-up: 12 year |
Men & women who had the most weight variability had increased risk of hip fractures independent of BMI. | ||||||
| Langlois et al., 1998 (157) EPESE US |
Longitudinal study N = 2,413 white men in community Age ≥ 67 years | >10% wt loss since age 50 associated with 2-fold increase in risk of hip fracture | ||||||
| Gonnelli et al., 2005 (143) | X-section n=401 men | Hip BMD predicts hip fracture OR 3.42 | ||||||
| Schuit et al., 2004 (145) The Netherlands |
T-score < –2.5 identified only 21% of nonvertebral fractures in elderly men & 44% in elderly women. | |||||||
| Van Potelbergh et al., 2003 (196) | (n=540 men, 2,264 women) 65-89 yrs for men | x | ||||||
| Pande et al., 2000(146) | Cross sectional study N = 62 men with low trauma hip fracture > age 50 years 100 controls |
Adjusted for age, height & weight, OR for fracture per 1SD reduction in BMD =1.8 L-spine, 3.1 for femoral neck, 3.9 for trochanter, 4.0 for intertrochanter area 3.7 for wards triangle | No association between hip axis length & risk of fracture. | |||||
| Kanis et al., 2005(173) | Meta-analysis 10 studies N =15,400 men & 43,832 women 250,000 person-years | Current or history of smoking ⇑ risk of fracture >explain edby | ||||||
| Kanis et al., 2005 (172) | Meta-analysis 3 studies N = 5,939 men & 11,032 women | >2 units alcohol/d ay ⇑ risk of any, osteop, & hip fractures (men & women) | ||||||
| Leifke et al., 2005(221) | Systematic review + cross sectional study N= 27 after hip fracture Control = 138 healthy youth & 110 (60–80 yr old males) | Review = conflicting finding X-sectional study: hip fracture associated with >2 SD below control | ||||||
| Mellstrom et al., 2006 (171) MrOS (Sweden) | Cross-sectional N=2,908 men Mean age 75.4 yrs DXA BMD, fractures rates & serum sex steroids | Free testosterone predicts BMD & osteoporotic fractures & vertebral fractures | ||||||
| Barret-Connor et al., 2000 (170) Rancho Bernardo Study | Longitudinal N = 352 men, 288 women, ≥age 50 yrs. Predictors of vertebral fractures |
Not studied | Low total &bio available estradiol associated with 4–5 fold ⇑ odds ofvertebral | No | ||||
| Kanis et al., 2005 (223) 151,957 person years |
Meta-analysis of 6 prospective cohort studies including CaMos N = 39,563 men & women (69% women) | Low intake of milk was it was associated with increased risk of osteoporotic fracture only after age 80 yrs. | ||||||
| Kudlacek et al., 2000(148) | Cross sectional study N = 136 men & 337 women mean age 60.7 & 59.7 yrs respectively 52 & 96 had a spine fracture |
Men fracture @ a higher BMD level than women OR for gender 3.1 | ||||||
| Kelsey et al., 2005 (31) US |
Case control N = 192 consecutive men & women with pelvic fracture, age =/>45 years 2,402 controls @ 5 Kaiser Permanente centres 21% of cases & 50% controls from racial/ethnic minority groups |
Propensity to fall & indications of frailty associated with increased risk. Need help to perform physical function. Inactivity during leisure time in past year. Self reported diseases: |
High BMI OR = 0.65 (0.52–0.81) per 5 units increase - protects loss of bone mass |
Number of fractures since age 45 Adjusted OR = 1.42 (1.03–1.96) Maternal history of hip fracture OR = 1.72 (1.02–2.90) |
Recent use of menopausal hormone OR = 0.55 (0.33–0.91) Protectsloss of bonemass Hysterectomy OR = 1.75 (1.15–2.66) | Current smoker OR = 2.17 (1.34–3.52) | ||
| Holmberg et al., 2005 (154) Malmo, Sweden |
Prospective population-based observational study N=22,444 men & 10,902 women mean age 44 (27–61) Followed-up: 16 yrs for men & 11 yrs for women Cox regression model | Similar risk factors for cervical hip fractures in men except admission for mental disorder is not a risk factor for cervical fracture | For hip fracture in men: High BMI protective in RR 0.63 (0.53–0.76, P=.0001) Sleep disturbance RR = 1.84 (1.25–2.70, P = .002) Multivariate analysis: All 7 variables were associated with ⇑ risk of hip fractures & cervical hip fractures Diabetes strongest association to cervical hip fracture. |
Previous fracture increased risk in women RR 4.76 (2.74–8.26) for hip fracture | Self-reported diabetes strongest predictor RR 4.07 RR 7.75 (4.37–13.7, P = .001) High BP & elevated resting pulse. Elevated serum glutamy transferace RR = 1.45 (1.28–1.65) |
Hip fracture in men Current smoking or self rated poor health RR 2.72 (1.94–3.80, P=.001) Hospital admission for mental disorder RR = 2.64(1.46-4.76, P=.01) |
||
| Vestergaard et al., 2003 (160) | Prospective case-controlled N = 6,660 people with hip fractures & 33,272 matched controls 5 year study |
Use of oral glucocorticoid – 7.5mg/day × 6 months OR 1.36 (95% CI 1.19–1.56) >1500mg: OR 1.65 (95% CI 1.43–1.92) |
||||||
| Vestergaard et al., 2005 (163) Denmark |
Prospective community-based case-controlled N=124,655 & 373,962 matched controls | Oral corticosteroid>2.5 mg/day increased risk of hip, spine & forearm, but no increase for inhaled corticosteroid | ||||||
| Steinbuch et al., 2004 (162) US |
Retrospe ctivecohort study N = 17,957 (7,509 men) + 17,957 controls Patients had a claim or oral corticosteroid | Oral corticosteroid increased risk of vertebral, nonvertebral & hip fractures RR ranged from 1.53 to 3.41 depending on dose & duration | ||||||
| Donnan et al., 2005 (165) UK |
Retrospective cohort study population ≥18 | Inhaled corticosteroid risk of all fractures RR 1.9 (1.68–2.16) | ||||||
| Van Staa et al., 2000 (161) UK |
Retrospe ctivecohort study N = 244,235 oral corticosteroid users & 24,235 controls | Oral corticosteroid – effect on risk of fractures was dose dependent; risk doubled as dose ⇑ from 2.5 mg to 7.5 mg | ||||||
| Van Staa et al., 2001 (166) UK |
Retrospective cohort study N = 17,818 inhaled corticosteroid users (55% men), 108,786 bronchodilator users, and 170,818 controls | Inhaled corticosteroid increased risk for vertebral, non vertebral & hip fractures RR ranged from 1.15 to 1.51 | ||||||
| Hubbard et al., 2002 (167) | Case control study N = 16,341 people with hip fractures (21% men) (meanage 79 years) & 29,889 matched controls | Inhaled corticosteroid increased risk forhip fractures OR 1.26 (95% CI1.17–1.36) | ||||||
| Hubbard et al., 2006(164) | Case control study N = 1,671 people with asthma or COPD (mean age 80.6 yrs) | Inhaled corticosteroid increased risk for any fractures HR 2.53 (95% CI 1.65–3.89) |
AUC refers to area under the curve; CI, confidence interval; OR, odds ratio; RR, relative risk; SD, standard deviation.
Established Population for Epidemiologic Studies of Elderly (PETESE) and 1990–1991 cohort of Baltimore Hip Studies (BHS)
BMI refers to body mass index; OR, odds ratio.
BMI refers to body mass index; RR, relative risk; OR, odds ratio.
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Utilization of DXA bone mineral densitometry in Ontario: an evidence-based analysis. Ontario Heath Technology Assessment Series 2006; 620)
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo@moh.gov.on.ca
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas
Print copies can be obtained by contacting MASinfo@moh.gov.on.ca
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo@moh.gov.on.ca
Telephone: 416-314-1092
ISSN 1915-7398
ISBN 978-1-4249-4319-7
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and information from practicing medical experts and industry, adds important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to maximize patient outcomes.
If you are aware of any current additional evidence to inform an existing Evidence-Based Analysis, please contact the Medical Advisory Secretariat: MASInfo@moh.gov.on.ca.. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to do so, this document may not fully reflect all scientific research available. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superceded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website foralist of all evidence-based analyses: http://www.health.gov.on.ca/ohtas
Abbreviations
- AHS
Adult Health Study (Hiroshima)
- BMD
Bone mineral density
- BMI
Body mass index
- CaMos
The Canadian Multicenter Osteoporosis Study
- CI
Confidence interval
- CV
The Dubbo Osteoporosis Epidemiology Study (Australia)
- DOES
Coefficient of variation
- DPA
Dual photon absorptiometry
- DXA
Dual energy x-ray absorptiometry
- EPESE
Established Populations for Epidemiologic Studies of the Elderly
- EPIDOS
Epidémiologie des Ostéoporoses Study
- EVOS
The European Vertebral Osteoporosis Study
- HR
Hazard ratio
- HRQL
Health related quality of life
- ICES
Institute for Clinical Evaluative Sciences
- LHIN
Local health integrated network
- MINOS
A study in men sponsored by IN SERM (French National Institute of Health and Medical Research) and it concerns osteoporosis
- MORE
Multiple Outcomes Raloxifene Evaluation
- MEDOS
The Mediterranean Osteoporosis Study (men & women)
- MrOS
Multicenter Study in Elderly Men in Sweden, Hong Kong and the US.
- ODB
Ontario Drug Benefits program
- OFELY
Os des Femmes de Lyon (cohort study)
- OHIP
Ontario Health Insurance Plan
- OR
Odds ratio
- OSTPRE
The Kuopiao Osteoporosis Risk Factor and Prevention Study (Finland)
- QCT
Quantitative computed tomography
- QUS
Quantitative ultrasound
- RCT
Randomized controlled trial
- RR
Relative risk
- SD
Standard deviation
- SOF
Study of Osteoporotic Fractures
- SPA
Single photon absorptiometry
- STORM
Study of Osteoporotic Risk in Men
- VERT
Vertebral Efficacy with Risedronate Therapy Study
- WHO
World Health Organization
Glossary
- Bisphosphonates
A class of nonhormonal medications that inhibits the resorption of bone
- Body mass index
(Body weight in kilograms)/ (height in meters)2
- Clinical vertebral fracture
Symptomatic vertebral fracture
- Colle’s fracture
A break across the end of the main bone of the forearm (the radius)
- Dual energy x-ray absorptiometry
Measurement of BMD using 2 X-ray beams with differing energy levels aimed at the patient’s bones; when soft tissue absorption is subtracted out, the BMD can be determined from the absorption of each beam by bone
- Dual photon absorptiometry
Measurement of the bone mineral content in the axial skeleton, particularly the lumbar spine, by comparing the transmission of the 2 separate photoelectric energy peaks emitted by gadolinium 153 through both soft and bone tissues
- Incident fracture
A new fracture that occurred during the study
- Morphometric vertebral fracture
Vertebral fracture diagnosed on the basis of reduced vertebral body height
- Morphometric
Relative to measurements of the shape of an individual; body proportions
- Odds ratio
A measure of association in which a value of “1.0” means that there is no relationship between variables, whereas an odds ratio less than 1.0 indicates an inverse or negative association and an odds ratio greater than 1.0 indicates a positive relation
- Osteoporosis
A condition that affects especially older women and is characterized by decrease in bone mass with decreased density and enlargement of bone spaces producing porosity and brittleness
- Prevalent fracture
A fracture that occurred before the study
- Relative risk
The ratio of the probability of developing an outcome among those receiving the treatment of interest or exposed to a risk factor, compared with the probability of developing the outcome if the risk factor or intervention is not present
- Single photon absorptiometry
The passage of a highly collimated monoenergetic beam of photons across peripheral sites such as the radius or heel, and monitoring the transmitted radiation with a sodium iodide scintillation detector; Differential photon absorption between bone and soft tissue allows calculation of the total bone mineral content in the path of the beam, measuredasgrams per centimetre
- T-score
The number of standard deviations from the mean bone mineral density for young adults
- Z-score
The number of standard deviations from the mean bone mineral density value for people of the same age and sex
Footnotes
Odds ratios are reliable estimates of the relative risk when they include unbiased samples of cases and controls (i.e., population-based studies) and when they are used in studies of rare events (i.e., the end point occurs in <15% of the subjects) (Haentjens 2003)
References
- 1.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–50. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Goeree R, O’Brien B, Pettit D, Cuddy L, Ferraz M, Adachi J. An assessment of the burden of illness due to osteoporosis in Canada. J SOGC. 1996 July Suppl;18:15–24. [Google Scholar]
- 3.Tenenhouse A, Joseph L, Kreiger N, Poliquin S, Murray TM, Blondeau L, et al. Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: the Canadian Multicentre Osteoporosis Study (CaMos) Osteoporos Int. 2000;11(10):897–904. doi: 10.1007/s001980070050. [DOI] [PubMed] [Google Scholar]
- 4.Chami G, Jeys L, Freudmann M, Connor L, Siddiqi M. Are osteoporotic fractures being adequately investigated? A questionnaire of GP & orthopaedic surgeons. BMC Fam Pract. 2006;7(7) doi: 10.1186/1471-2296-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuddihy MT, Gabriel SE, Crowson CS, O’Fallon WM, Melton LJ, III. Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int. 1999;9(6):469–75. doi: 10.1007/s001980050172. [DOI] [PubMed] [Google Scholar]
- 6.Eastell R. Forearm fracture. 1996;18(3 Suppl):203S–7S. doi: 10.1016/8756-3282(95)00503-x. Bone. [DOI] [PubMed] [Google Scholar]
- 7.Kanis JA, Delmas P, Burckhardt P, Cooper C, Torgerson D. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos Int. 1997;7(4):390–406. doi: 10.1007/BF01623782. [DOI] [PubMed] [Google Scholar]
- 8.Papadimitropoulos EA, Coyte PC, Josse RG, Greenwood CE. Current and projected rates of hip fracture in Canada. CMAJ. 1997;157(10):1357–63. [PMC free article] [PubMed] [Google Scholar]
- 9.Jaglal SB, Sherry PG, Schatzker J. The impact and consequences of hip fracture in Ontario. Can J Surg. 1996;39(2):105–11. [PMC free article] [PubMed] [Google Scholar]
- 10.Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11(7):556–61. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 11.Pande I, Scott DL, O’Neill TW, Pritchard C, Woolf AD, Davis MJ. Quality of life, morbidity, and mortality after low trauma hip fracture in men. Ann Rheum Dis. 2006;65(1):87–92. doi: 10.1136/ard.2004.034611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiktorowicz ME, Goeree R, Papaioannou A, Adachi JD, Papadimitropoulos E. Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteoporos Int. 2001;12(4):271–8. doi: 10.1007/s001980170116. [DOI] [PubMed] [Google Scholar]
- 13.Adachi JD, Loannidis G, Berger C, Joseph L, Papaioannou A, Pickard L, et al. The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int. 2001;12(11):903–8. doi: 10.1007/s001980170017. [DOI] [PubMed] [Google Scholar]
- 14.Adachi JD, Ioannidis G, Pickard L, Berger C, Prior JC, Joseph L, et al. The association between osteoporotic fractures and health-related quality of life as measured by the Health Utilities Index in the Canadian Multicentre Osteoporosis Study (CaMos) Osteoporos Int. 2003;14(11):895–904. doi: 10.1007/s00198-003-1483-3. [DOI] [PubMed] [Google Scholar]
- 15.Lorrain J, Paiement G, Chevrier N, Lalumiere G, Laflamme GH, Caron P, et al. Population demographics and socioeconomic impact of osteoporotic fractures in Canada. Menopause. 2003;10(3):228–34. doi: 10.1097/00042192-200310030-00010. [DOI] [PubMed] [Google Scholar]
- 16.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–89. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 17.Genant HK, Cooper C, Poor G, Reid I, Ehrlich G, Kanis J, et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos Int. 1999;10(4):259–64. doi: 10.1007/s001980050224. [DOI] [PubMed] [Google Scholar]
- 18.Lewiecki EM, Watts NB, McClung MR, Petak SM, Bachrach LK, Shepherd JA, et al. Official positions of the international society for clinical densitometry. J Clin Endocrinol Metab. 2004;89(8):3651–5. doi: 10.1210/jc.2004-0124. [DOI] [PubMed] [Google Scholar]
- 19.Papaioannou A, Joseph L, Ioannidis G, Berger C, Anastassiades T, Brown JP, et al. Risk factors associated with incident clinical vertebral and nonvertebral fractures in postmenopausal women: the Canadian Multicentre Osteoporosis Study (CaMos) Osteoporos Int. 2005;16(5):568–78. doi: 10.1007/s00198-004-1735-x. [DOI] [PubMed] [Google Scholar]
- 20.Ross PD, Davis JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med. 1991;114(11):919–23. doi: 10.7326/0003-4819-114-11-919. [DOI] [PubMed] [Google Scholar]
- 21.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341(8837):72–5. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 22.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286(22):2815–22. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 23.Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12(12):989–95. doi: 10.1007/s001980170006. [DOI] [PubMed] [Google Scholar]
- 24.Taylor BC, Schreiner PJ, Stone KL, Fink HA, Cummings SR, Nevitt MC, et al. Long-term prediction of incident hip fracture risk in elderly white women: study of osteoporotic fractures. J Am Geriatr Soc. 2004;52(9):1479–86. doi: 10.1111/j.1532-5415.2004.52410.x. [DOI] [PubMed] [Google Scholar]
- 25.Albrand G, Munoz F, Sornay-Rendu E, Duboeuf F, Delmas PD. Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the OFELY study. Bone. 2003;32(1):78–85. doi: 10.1016/s8756-3282(02)00919-5. [DOI] [PubMed] [Google Scholar]
- 26.Bensen R, Adachi JD, Papaioannou A, Ioannidis G, Olszynski WP, Sebaldt RJ, et al. Evaluation of easily measured risk factors in the prediction of osteoporotic fractures. BMC Musculoskelet Disord. 2005;6(47) doi: 10.1186/1471-2474-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardsell P, Johnell O, Nilsson BE, Nilsson JA. The predictive value of fracture, disease, and falling tendency for fragility fractures in women. Calcif Tissue Int. 1989;45(6):327–30. doi: 10.1007/BF02556001. [DOI] [PubMed] [Google Scholar]
- 28.Nevitt MC, Cummings SR. Type of fall and risk of hip and wrist fractures: the study of osteoporotic fractures. The Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 1993;41(11):1226–34. doi: 10.1111/j.1532-5415.1993.tb07307.x. [DOI] [PubMed] [Google Scholar]
- 29.Kaptoge S, Benevolenskaya LI, Bhalla AK, Cannata JB, Boonen S, Falch JA, et al. Low BMD is less predictive than reported falls for future limb fractures in women across Europe: results from the European Prospective Osteoporosis Study. Bone. 2005;36(3):387–98. doi: 10.1016/j.bone.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Hanssens L, Reginster JY. Relevance of bone mineral density, bone quality and falls in reduction of vertebral and non-vertebral fractures. J Musculoskelet Neuronal Interact. 2003;3(3):189–93. [PubMed] [Google Scholar]
- 31.Kelsey JL, Prill MM, Keegan TH, Quesenberry CP Jr, Sidney S. Risk factors for pelvis fracture in older persons. Am J Epidemiol. 2005;162(9):879–86. doi: 10.1093/aje/kwi295. [DOI] [PubMed] [Google Scholar]
- 32.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359(9321):1929–36. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 33.ICSI Technology Assessment Committee. Densitometry as a diagnostic tool for the identification and treatment of osteoporosis in women [report on the Internet] Jan, 2000. [[cited 2006 Oct. 10]]. TA #31 (revised) Institute For Clinical Systems Improvement. Available at: http://www.icsi.org/knowledge/detail.asp?catID=107&itemID=277 .
- 34.Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C. Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone. 2002;31(1):57–61. doi: 10.1016/s8756-3282(02)00791-3. [DOI] [PubMed] [Google Scholar]
- 35.Garnero P, Dargent-Molina P, Hans D, Schott AM, Breart G, Meunier PJ, et al. Do markers of bone resorption add to bone mineral density and ultrasonographic heel measurement for the prediction of hip fracture in elderly women? The EPIDOS prospective study. Osteoporos Int. 1998;8(6):563–9. doi: 10.1007/s001980050100. [DOI] [PubMed] [Google Scholar]
- 36.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15(8):1526–36. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 37.Boehm HF, Link TM. Bone imaging: traditional techniques and their interpretation. Curr Osteoporos Rep. 2004;2(2):41–6. doi: 10.1007/s11914-004-0002-6. [DOI] [PubMed] [Google Scholar]
- 38.Ludescher B, Martirosian P, Lenk S, Machann J, Dammann F, Schick F, et al. High-resolution magnetic resonance imaging of trabecular bone in the wrist at 3 tesla: initial results. Acta Radiol. 2005;46(3):306–9. doi: 10.1080/02841850510021076. [DOI] [PubMed] [Google Scholar]
- 39.Kanis JA, Gluer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11(3):192–202. doi: 10.1007/s001980050281. [DOI] [PubMed] [Google Scholar]
- 40.Looker AC, Orwoll ES, Johnston CC Jr, Lindsay RL, Wahner HW, Dunn WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12(11):1761–8. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 41.Kanis JA, Johnell O, Oden A, De Laet C, Mellstrom D. Diagnosis of osteoporosis and fracture threshold in men. Calcif Tissue Int. 2001;69(4):218–21. doi: 10.1007/s00223-001-1046-6. [DOI] [PubMed] [Google Scholar]
- 42.Siminoski K, Leslie WD, Frame H, Hodsman A, Josse RG, Khan A, et al. Recommendations for bone mineral density reporting in Canada. Can Assoc Radiol J. 2005;56(3):178–88. [PubMed] [Google Scholar]
- 43.Guidelines and Protocols Advisory Committee. British Columbia Health Services. Bone density measurement in women [report on the Internet].www.healthservices.gov.bc.ca/msp/protoguides . 2005. [[cited 2006 Sept. 15].]. Revised. Available at: http://www.health.gov.bc.ca/msp/protoguides/gps/bone_density.pdf .
- 44.Cheung AM, Feig DS, Kapral M, Diaz-Granados N, Dodin S. Prevention of osteoporosis and osteoporotic fractures in postmenopausal women: recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ. 2004;170(11):1665–7. doi: 10.1503/cmaj.1030757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown JP, Josse RG. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002;167(10 Suppl):S1–34. [PMC free article] [PubMed] [Google Scholar]
- 46.Schedule of benefits for physician services under the Health Insurance Act. Oct 1, 2005. [[cited 2006 Oct. 10]]. Ministry of Health and Long-Term Care. Available at: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/d_radiol.pdf .
- 47.Scientific Advisory Council of the Osteoporosis Society of Canada. [2002 Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada]. CMAJ. 2002;167(10 Suppl):S1–S34. [PMC free article] [PubMed] [Google Scholar]
- 48.Jaglal SB, Weller I, Mamdani M, Hawker G, Kreder H, Jaakkimainen L, et al. J Bone Miner Res. 2005;20(6):898–905. doi: 10.1359/JBMR.041231. Population trends in BMD testing, treatment, and hip and wrist fracture rates: are the hip fracture projections wrong? [DOI] [PubMed] [Google Scholar]
- 49.Jaglal SB. Stewart D, Cheung A, Ferris L, Hyman I, Cohen M, William J, editors. Bone density testing [monograph on the Internet] Ontario Womens’ Health Status Report. Ontario Women’s Health Council. 2002. [[cited 2006 Sept. 15]]. pp. 113–120. Available from: http://www.womenshealthcouncil.on.ca/userfiles/page_attachments/chaptersPDF/Chapter9.pdf .
- 50.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–82. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 51.Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int. 2004;15(10):767–78. doi: 10.1007/s00198-004-1675-5. [DOI] [PubMed] [Google Scholar]
- 52.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 53.Baim S, Wilson CR, Lewiecki EM, Luckey MM, Downs RW Jr, Lentle BC. Precision assessment and radiation safety for dual-energy X-ray absorptiometry: position paper of the International Society for Clinical Densitometry. J Clin Densitom. 2005;8(4):371–8. doi: 10.1385/jcd:8:4:371. [DOI] [PubMed] [Google Scholar]
- 54.Khan AA, Brown J, Faulkner K, Kendler D, Lentle B, Leslie W, et al. Standards and guidelines for performing central dual X-ray densitometry from the Canadian Panel of International Society for Clinical Densitometry. J Clin Densitom. 2002;5(4):435–45. doi: 10.1385/jcd:5:4:435. [DOI] [PubMed] [Google Scholar]
- 55.Cummings SR, Palermo L, Browner W, Marcus R, Wallace R, Pearson J, et al. Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. [Fracture Intervention Trial Research Group]. JAMA. 2000;283(10):1318–21. doi: 10.1001/jama.283.10.1318. [DOI] [PubMed] [Google Scholar]
- 56.El Maghraoui A, Achemlal L, Bezza A. Monitoring of dual-energy X-ray absorptiometry measurement in clinical practice. J Clin Densitom. 2006;9(3):281–6. doi: 10.1016/j.jocd.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Chapurlat RD, Gamero P, Sornay-Rendu E, Arlot ME, Claustrat B, Delmas PD. Longitudinal study of bone loss in pre- and perimenopausal women: evidence for bone loss in perimenopausal women. Osteoporos Int. 2000;11(6):493–8. doi: 10.1007/s001980070091. [DOI] [PubMed] [Google Scholar]
- 58.Chittacharoen A, Theppisai U, Sirisriro R, Thanantaseth C. Pattern of bone loss in surgical menopause: a preliminary report. J Med Assoc Thai. 1997;80(11):731–7. [PubMed] [Google Scholar]
- 59.Goulding A, Taylor RW, Keil D, Gold E, Lewis-Barned NJ, Williams SM. Lactose malabsorption and rate of bone loss in older women. Age Ageing. 1999;28(2):175–80. doi: 10.1093/ageing/28.2.175. [DOI] [PubMed] [Google Scholar]
- 60.Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90(4):1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- 61.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51(12):1740–7. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 62.Laan RF, van Riel PL, van de Putte LB, van Erning LJ, van’t Hof MA, Lemmens JA. Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis. [A randomized, controlled study]. Ann Intern Med. 1993;119(10):963–8. doi: 10.7326/0003-4819-119-10-199311150-00001. [DOI] [PubMed] [Google Scholar]
- 63.Guyatt GH, Cranney A, Griffith L, Walter S, Krolicki N, Favus M, et al. Summary of meta-analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures. Endocrinol Metab Clin North Am. 2002;31(3):659–79. doi: 10.1016/s0889-8529(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 64.Wasnich RD, Miller PD. Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab. 2000;85(1):231–6. doi: 10.1210/jcem.85.1.6267. [DOI] [PubMed] [Google Scholar]
- 65.Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, Lacroix AZ, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drug. Am J Med. 2002;112(4):281–9. doi: 10.1016/s0002-9343(01)01124-x. [DOI] [PubMed] [Google Scholar]
- 66.Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD. Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab. 2002;87(4):1586–92. doi: 10.1210/jcem.87.4.8415. [DOI] [PubMed] [Google Scholar]
- 67.Delmas PD, Li Z, Cooper C. Relationship between changes in bone mineral density and fracture risk reduction with antiresorptive drugs: some issues with meta-analyses. J Bone Miner Res. 2004;19(2):330–7. doi: 10.1359/JBMR.0301228. [DOI] [PubMed] [Google Scholar]
- 68.Watts NB, Cooper C, Lindsay R, Eastell R, Manhart MD, Barton IP, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7(3):255–61. doi: 10.1385/jcd:7:3:255. [DOI] [PubMed] [Google Scholar]
- 69.Watts NB, Geusens P, Barton IP, Felsenberg D. Relationship between changes in BMD and nonvertebral fracture incidence associated with risedronate: reduction in risk of nonvertebral fracture is not related to change in BMD. J Bone Miner Res. 2005;20(12):2097–104. doi: 10.1359/JBMR.050814. [DOI] [PubMed] [Google Scholar]
- 70.Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res. 2002;17(1):1–10. doi: 10.1359/jbmr.2002.17.1.1. [DOI] [PubMed] [Google Scholar]
- 71.Chapurlat RD, Palermo L, Ramsay P, Cummings SR. Risk of fracture among women who lose bone density during treatment with alendronate. [The Fracture Intervention Trial]. Osteoporos Int. 2005;16(7):842–8. doi: 10.1007/s00198-004-1770-7. [DOI] [PubMed] [Google Scholar]
- 72.Hochberg MC, Ross PD, Black D, Cummings SR, Genant HK, Nevitt MC, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. [Fracture Intervention Trial Research Group]. Arthritis Rheum. 1999;42(6):1246–54. doi: 10.1002/1529-0131(199906)42:6<1246::AID-ANR22>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 73.Lewiecki EM. Nonresponders to osteoporosis therapy. J Clin Densitom. 2003;6(4):307–14. doi: 10.1385/jcd:6:4:307. [DOI] [PubMed] [Google Scholar]
- 74.Caro JJ, Ishak KJ, Huybrechts KF, Raggio G, Naujoks C. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004;15(12):1003–8. doi: 10.1007/s00198-004-1652-z. [DOI] [PubMed] [Google Scholar]
- 75.McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004;48(3):271–87. doi: 10.1016/j.maturitas.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 76.Solomon DH, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, et al. Compliance with osteoporosis medications. Arch Intern Med. 2005;165(20):2414–9. doi: 10.1001/archinte.165.20.2414. [DOI] [PubMed] [Google Scholar]
- 77.Pickney CS, Arnason JA. Correlation between patient recall of bone densitometry results and subsequent treatment adherence. Osteoporos Int. 2005;16(9):1156–60. doi: 10.1007/s00198-004-1818-8. [DOI] [PubMed] [Google Scholar]
- 78.Rossini M, Bianchi G, Di Munno O, Giannini S, Minisola S, Sinigaglia L, et al. Determinants of adherence to osteoporosis treatment in clinical practice. Osteoporos Int. 2006;17(6):914–21. doi: 10.1007/s00198-006-0073-6. [DOI] [PubMed] [Google Scholar]
- 79.Clowes JA, Peel NF, Eastell R. The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(3):1117–23. doi: 10.1210/jc.2003-030501. [DOI] [PubMed] [Google Scholar]
- 80.Montessori ML, Scheele WH, Netelenbos JC, Kerkhoff JF, Bakker K. The use of etidronate and calcium versus calcium alone in the treatment of postmenopausal osteopenia: results of three years of treatment. Osteoporos Int. 1997;7(1):52–8. doi: 10.1007/BF01623461. [DOI] [PubMed] [Google Scholar]
- 81.McClung MR, Wasnich RD, Hosking DJ, Christiansen C, Ravn P, Wu M, et al. Prevention of postmenopausal bone loss: six-year results from the Early Postmenopausal Intervention Cohort Study. J Clin Endocrinol Metab. 2004;89(10):4879–85. doi: 10.1210/jc.2003-031672. [DOI] [PubMed] [Google Scholar]
- 82.Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–99. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 83.Fogelman I, Blake GM. Bone densitometry: an update. Lancet. 2005;366(9503):2068–70. doi: 10.1016/S0140-6736(05)67867-1. [DOI] [PubMed] [Google Scholar]
- 84.Evio S, Tiitinen A, Laitinen K, Ylikorkala O, Valimaki MJ. Effects of alendronate and hormone replacement therapy, alone and in combination, on bone mass and markers of bone turnover in elderly women with osteoporosis. J Clin Endocrinol Metab. 2004;89(2):626–31. doi: 10.1210/jc.2003-030198. [DOI] [PubMed] [Google Scholar]
- 85.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349(13):1207–15. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 86.Ringe JD, Faber H, Farahmand P, Dorst A. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26(5):427–31. doi: 10.1007/s00296-005-0004-4. [DOI] [PubMed] [Google Scholar]
- 87.Papaioannou A, Giangregorio L, Kvern B, Boulos P, Ioannidis G, Adachi JD. The osteoporosis care gap in Canada. BMC Musculoskelet Disord. 2004. pp. 5–11. [DOI] [PMC free article] [PubMed]
- 88.Vanasse A, Dagenais P, Niyonsenga T, Gregoire JP, Courteau J, Hemiari A. Bone mineral density measurement and osteoporosis treatment after a fragility fracture in older adults: regional variation and determinants of use in Quebec. BMC Musculoskeletal Disord. 2005. pp. 6–33. [DOI] [PMC free article] [PubMed]
- 89.Hooven F, Gehlbach SH, Pekow P, Bertone E, Benjamin E. Follow-up treatment for osteoporosis after fracture. Osteoporos Int. 2005;16(3):296–301. doi: 10.1007/s00198-004-1676-4. [DOI] [PubMed] [Google Scholar]
- 90.Bliuc D, Ong CR, Eisman JA, Center JR. Barriers to effective management of osteoporosis in moderate and minimal trauma fractures: a prospective study. Osteoporos Int. 2005;16(8):977–82. doi: 10.1007/s00198-004-1788-x. [DOI] [PubMed] [Google Scholar]
- 91.Malochet-Guinamand S, Chalard N, Billault C, Breuil N, Ristori JM, Schmidt J. Osteoporosis treatment in postmenopausal women after peripheral fractures: impact of information to general practitioners. Joint Bone Spine. 2005;72(6):562–6. doi: 10.1016/j.jbspin.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 92.Giangregorio L, Papaioannou A, Cranney A, Zytaruk N, Adachi JD. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum. 2006;35(5):293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Dirschl DR, Henderson RC, Oakley WC. Accelerated bone mineral loss following a hip fracture: a prospective longitudinal study. Bone. 1997;21(1):79–82. doi: 10.1016/s8756-3282(97)00082-3. [DOI] [PubMed] [Google Scholar]
- 94.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA III, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721–39. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 95.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35(2):375–82. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 96.Haentjens P, Autier P, Collins J, Velkeniers B, Vanderschueren D, Boonen S. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women. J Bone Joint Surg Am. 2003;85(A10):1936–43. doi: 10.2106/00004623-200310000-00011. A meta-analysis. [DOI] [PubMed] [Google Scholar]
- 97.Szulc P, Munoz F, Duboeuf F, Marchand F, Delmas PD. Bone mineral density predicts osteoporotic fractures in elderly men: the MINOS study. Osteoporos Int. 2005;16(10):1184–92. doi: 10.1007/s00198-005-1970-9. [DOI] [PubMed] [Google Scholar]
- 98.Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15(3):175–9. doi: 10.1007/s00198-003-1514-0. [DOI] [PubMed] [Google Scholar]
- 99.Pongchaiyakul C, Nguyen ND, Jones G, Center JR, Eisman JA, Nguyen TV. Asymptomatic vertebral deformity as a major risk factor for subsequent fractures and mortality: a long-term prospective study. J Bone Miner Res. 2005;20(8):1349–55. doi: 10.1359/JBMR.050317. [DOI] [PubMed] [Google Scholar]
- 100.Ismail AA, Cockerill W, Cooper C, Finn JD, Abendroth K, Parisi G, et al. Prevalent vertebral deformity predicts incident hip though not distal forearm fracture: results from the European Prospective Osteoporosis Study. Osteoporos Int. 2001;12(2):85–90. doi: 10.1007/s001980170138. [DOI] [PubMed] [Google Scholar]
- 101.Hasserius R, Karlsson MK, Nilsson BE, Redlund-Johnell I, Johnell O. Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int. 2003;14(1):61–8. doi: 10.1007/s00198-002-1316-9. [DOI] [PubMed] [Google Scholar]
- 102.Schousboe JT, Fink HA, Taylor BC, Stone KL, Hillier TA, Nevitt MC, et al. Association between self-reported prior wrist fractures and risk of subsequent hip and radiographic vertebral fractures in older women: a prospective study. J Bone Miner Res. 2005;20(1):100–6. doi: 10.1359/JBMR.041025. [DOI] [PubMed] [Google Scholar]
- 103.van der KM, de Laet CE, McCloskey EV, Johnell O, Kanis JA, Hofman A, et al. Risk factors for incident vertebral fractures in men and women: the Rotterdam Study. J Bone Miner Res. 2004;19(7):1172–80. doi: 10.1359/JBMR.040215. [DOI] [PubMed] [Google Scholar]
- 104.Porthouse J, Birks YF, Torgerson DJ, Cockayne S, Puffer S, Watt I. Risk factors for fracture in a UK population: a prospective cohort study. QJM. 2004;97(9):569–74. doi: 10.1093/qjmed/hch097. [DOI] [PubMed] [Google Scholar]
- 105.Colon-Emeric CS, Biggs DP, Schenck AP, Lyles KW. Risk factors for hip fracture in skilled nursing facilities: who should be evaluated? Osteoporos Int. 2003;14(6):484–9. doi: 10.1007/s00198-003-1384-5. [DOI] [PubMed] [Google Scholar]
- 106.Naves M, Diaz-Lopez JB, Gomez C, Rodriguez-Rebollar A, Rodriguez-Garcia M, Cannata-Andia JB. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos Int. 2003;14(6):520–4. doi: 10.1007/s00198-003-1405-4. [DOI] [PubMed] [Google Scholar]
- 107.Cranney A, Guyatt G, Krolicki N, Welch V, Griffith L, Adachi JD, et al. A meta-analysis of etidronate for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2001;12(2):140–51. doi: 10.1007/s001980170147. [DOI] [PubMed] [Google Scholar]
- 108.Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, Robinson V II, et al. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev. 2002;23(4):508–16. doi: 10.1210/er.2001-2002. [DOI] [PubMed] [Google Scholar]
- 109.Cranney A, Tugwell P, Adachi J, Weaver B, Zytaruk N, Papaioannou A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23(4):517–23. doi: 10.1210/er.2001-3002. [DOI] [PubMed] [Google Scholar]
- 110.Cranney A, Tugwell P, Zytaruk N, Robinson V, Weaver B, Adachi J, et al. Meta-analyses of therapies for postmenopausal osteoporosis. IV. [Meta-analysis of raloxifene for the prevention and treatment of postmenopausal osteoporosis]. Endocr Rev. 2002;23(4):524–8. doi: 10.1210/er.2001-4002. [DOI] [PubMed] [Google Scholar]
- 111.Wells G, Tugwell P, Shea B, Guyatt G, Peterson J, Zytaruk N, et al. Meta-analyses of therapies for postmenopausal osteoporosis. V. [Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women]. Endocr Rev. 2002;23(4):529–39. doi: 10.1210/er.2001-5002. [DOI] [PubMed] [Google Scholar]
- 112.Cranney A, Tugwell P, Zytaruk N, Robinson V, Weaver B, Shea B, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VI. [Meta-analysis of calcitonin for the treatment of postmenopausal osteoporosis]. Endocr Revyear. 2002;23(4):540–51. doi: 10.1210/er.2001-6002. [DOI] [PubMed] [Google Scholar]
- 113.Shea B, Wells G, Cranney A, Zytaruk N, Robinson V, Griffith L, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VII. [Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis]. Endocr Rev. 2002;23(4):552–9. doi: 10.1210/er.2001-7002. [DOI] [PubMed] [Google Scholar]
- 114.Papadimitropoulos E, Wells G, Shea B, Gillespie W, Weaver B, Zytaruk N, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VIII. [Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women]. Endocr Rev. 2002;23(4):560–9. doi: 10.1210/er.2001-8002. [DOI] [PubMed] [Google Scholar]
- 115.Cranney A, Papaioannou A, Zytaruk N, Hanley D, Adachi J, Goltzman D, et al. Parathyroid hormone for the treatment of osteoporosis: a systematic review. CMAJ. 2006;175(1):52–9. doi: 10.1503/cmaj.050929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C, et al. Meta-analyses of therapies for postmenopausal osteoporosis. IX. [Summary of meta-analyses of therapies for postmenopausal osteoporosis]. Endocr Rev. 2002;23(4):570–8. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- 117.Cranney A, Wells GA. Hormone replacement therapy for postmenopausal osteoporosis. Clin Geriatr Med. 2003;19(2):361–70. doi: 10.1016/s0749-0690(02)00078-2. [DOI] [PubMed] [Google Scholar]
- 118.Sawka AM, Papaioannou A, Adachi JD, Gafni A, Hanley DA, Thabane L. Does alendronate reduce the risk of fracture in men? [A meta-analysis incorporating prior knowledge of anti-fracture efficacy in women]. BMC Musculoskelet Disord. 2005;6(39) doi: 10.1186/1471-2474-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gonnelli S, Cepollaro C, Montagnani A, Bruni D, Caffarelli C, Breschi M, et al. Alendronate treatment in men with primary osteoporosis: a three-year longitudinal study. Calcif Tissue Int. 2003;73(2):133–9. doi: 10.1007/s00223-002-1085-7. [DOI] [PubMed] [Google Scholar]
- 120.Ringe JD, Dorst A, Faber H, Ibach K. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24(2):110–3. doi: 10.1007/s00296-003-0388-y. [DOI] [PubMed] [Google Scholar]
- 121.Sato Y, Iwamoto J, Kanoko T, Satoh K. Risedronate sodium therapy for prevention of hip fracture in men 65 years or older after stroke. Arch Intern Med. 2005;165(15):1743–8. doi: 10.1001/archinte.165.15.1743. [DOI] [PubMed] [Google Scholar]
- 122.Ringe JD, Faber H, Farahmand P, Dorst A. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26(5):427–31. doi: 10.1007/s00296-005-0004-4. [DOI] [PubMed] [Google Scholar]
- 123.Thomson AB, Marshall JK, Hunt RH, Provenza JM, Lanza FL, Royer MG, et al. 14 day endoscopy study comparing risedronate and alendronate in postmenopausal women stratified by Helicobacter pylori status. J Rheumatol. 2002;29(9):1965–74. [PubMed] [Google Scholar]
- 124.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144(10):753–61. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 125.Hamel ME, Sebaldt RJ, Siminoski K, Adachi JD, Papadimitropoulos E, Petrie A, et al. Influence of fracture history and bone mineral density testing on the treatment of osteoporosis in two non-academic community centers. Osteoporos Int. 2005;16(2):208–15. doi: 10.1007/s00198-004-1776-1. [DOI] [PubMed] [Google Scholar]
- 126.Fitt NS, Mitchell SL, Cranney A, Gulenchyn K, Huang M, Tugwell P. Influence of bone densitometry results on the treatment of osteoporosis. CMAJ. 2001;164(6):777–81. [PMC free article] [PubMed] [Google Scholar]
- 127.Gallagher TC, Geling O, Comite F. Missed opportunities for prevention of osteoporotic fracture. Arch Intern Med. 2002;162(4):450–6. doi: 10.1001/archinte.162.4.450. [DOI] [PubMed] [Google Scholar]
- 128.Pressman A, Forsyth B, Ettinger B, Tosteson AN. Initiation of osteoporosis treatment after bone mineral density testing. Osteoporos Int. 2001;12(5):337–42. doi: 10.1007/s001980170099. [DOI] [PubMed] [Google Scholar]
- 129.Ridout R, Hawker GA. Use of bone densitometry by Ontario family physician. Osteoporos Int. 2000;11(5):393–9. doi: 10.1007/s001980070105. [DOI] [PubMed] [Google Scholar]
- 130.Jaglal SB, McIsaac WJ, Hawker G, Carroll J, Jaakkimainen L, Cadarette SM, et al. Information needs in the management of osteoporosis in family practice: an illustration of the failure of the current guideline implementation process. Osteoporos Int. 2003;14(8):672–6. doi: 10.1007/s00198-003-1421-4. [DOI] [PubMed] [Google Scholar]
- 131.Solomon DH, Connelly MT, Rosen CJ, Dawson-Hughes B, Kiel DP, Greenspan SL, et al. Factors related to the use of bone densitometry: survey responses of 494 primary care physicians in New England. Osteoporos Int. 2003;14(2):123–9. doi: 10.1007/s00198-002-1326-7. [DOI] [PubMed] [Google Scholar]
- 132.Khandwala HM, Kolla N, Grover VK. Evaluation and treatment of osteoporosis in patients with a fragility hip fracture. Endocr Pract. 2005;11(6):370–5. doi: 10.4158/EP.11.6.370. [DOI] [PubMed] [Google Scholar]
- 133.Dreinhofer KE, Anderson M, Feron JM, Herrera A, Hube R, Johnell O, et al. Multinational survey of osteoporotic fracture management. Osteoporos Int. 2005;16(Suppl 2):S44–S53. doi: 10.1007/s00198-004-1700-8. [DOI] [PubMed] [Google Scholar]
- 134.Hajcsar EE, Hawker G, Bogoch ER. Investigation and treatment of osteoporosis in patients with fragility fractures. CMAJ. 2000;163(7):819–22. [PMC free article] [PubMed] [Google Scholar]
- 135.Jaglal SB, Cameron C, Hawker GA, Carroll J, Jaakkimainen L, Cadarette SM, et al. Development of an integrated-care delivery model for post-fracture care in Ontario, Canada. Osteoporos Int. 2006;17(9):1337–45. doi: 10.1007/s00198-006-0076-3. [DOI] [PubMed] [Google Scholar]
- 136.Hawker G, Ridout R, Ricupero M, Jaglal S, Bogoch E. The impact of a simple fracture clinic intervention in improving the diagnosis and treatment of osteoporosis in fragility fracture patients. Osteoporos Int. 2003;14(2):171–8. doi: 10.1007/s00198-003-1377-4. [DOI] [PubMed] [Google Scholar]
- 137.Bogoch ER, Elliot-Gibson V, Beaton DE, Jamal SA, Josse RG, Murray TM. Effective initiation of osteoporosis diagnosis and treatment for patients with a fragility fracture in an orthopaedic environment. J Bone Joint Surg Am. 2006;88(1):25–34. doi: 10.2106/JBJS.E.00198. [DOI] [PubMed] [Google Scholar]
- 138.Majumdar SR, Rowe BH, Folk D, Johnson JA, Holroyd BH, Morrish DW, et al. A controlled trial to increase detection and treatment of osteoporosis in older patients with a wrist fracture. Ann Intern Med. 2004;141(5):366–73. doi: 10.7326/0003-4819-141-5-200409070-00011. [DOI] [PubMed] [Google Scholar]
- 139.Charalambous CP, Kumar S, Tryfonides M, Rajkumar P, Hirst P. Management of osteoporosis in an orthopaedic department: audit improves practice. Int J Clin Pract. 2002;56(8):620–1. [PubMed] [Google Scholar]
- 140.Chevalley T, Hoffmeyer P, Bonjour JP, Rizzoli R. An osteoporosis clinical pathway for the medical management of patients with low-trauma fracture. Osteoporos Int. 2002;13(6):450–5. doi: 10.1007/s001980200053. [DOI] [PubMed] [Google Scholar]
- 141.Johnson SL, Petkov VI, Williams MI, Via PS, Adler RA. Improving osteoporosis management in patients with fractures. Osteoporos Int. 2005;16(9):1079–85. doi: 10.1007/s00198-004-1814-z. [DOI] [PubMed] [Google Scholar]
- 142.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–94. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 143.Gonnelli S, Cepollaro C, Gennari L, Montagnani A, Caffarelli C, Merlotti D, et al. Quantitative ultrasound and dual-energy X-ray absorptiometry in the prediction of fragility fracture in men. Osteoporos Int. 2005;16(8):963–8. doi: 10.1007/s00198-004-1771-6. [DOI] [PubMed] [Google Scholar]
- 144.van der Klift M, de Laet CE, McCloskey EV, Hofman A, Pols HA. The incidence of vertebral fractures in men and women: the Rotterdam Study. J Bone Miner Res. 2002;17(6):1051–6. doi: 10.1359/jbmr.2002.17.6.1051. [DOI] [PubMed] [Google Scholar]
- 145.Schuit SC, van der KM, Weel AE, de Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34(1):195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 146.Pande I, O’Neill TW, Pritchard C, Scott DL, Woolf AD. Bone mineral density, hip axis length and risk of hip fracture in men: results from the Cornwall Hip Fracture Study. Osteoporos Int. 2000;11(10):866–70. doi: 10.1007/s001980070046. [DOI] [PubMed] [Google Scholar]
- 147.Cauley JA, Zmuda JM, Wisniewski SR, Krishnaswami S, Palermo L, Stone KL, et al. Bone mineral density and prevalent vertebral fractures in men and women. Osteoporos Int. 2004;15(1):32–7. doi: 10.1007/s00198-003-1462-8. [DOI] [PubMed] [Google Scholar]
- 148.Kudlacek S, Schneider B, Resch H, Freudenthaler O, Willvonseder R. Gender differences in fracture risk and bone mineral density. Maturitas. 2000;36(3):173–80. doi: 10.1016/s0378-5122(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 149.Espallargues M, Sampietro-Colom L, Estrada MD, Sola M, Del Rio L, Setoain J, et al. Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature. Osteoporos Int. 2001;12(10):811–22. doi: 10.1007/s001980170031. [DOI] [PubMed] [Google Scholar]
- 150.Knoke JD, Barrett-Connor E. Weight loss: a determinant of hip bone loss in older men and women. Am J Epidemiol. 2003;158(12):1132–8. doi: 10.1093/aje/kwg265. The Rancho Bernardo Study. [DOI] [PubMed] [Google Scholar]
- 151.Bakhireva LN, Barrett-Connor E, Kritz-Silverstein D, Morton DJ. Modifiable predictors of bone loss in older men: a prospective study. Am J Prev Med. 2004;26(5):436–42. doi: 10.1016/j.amepre.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 152.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 153.Roy DK, O’Neill TW, Finn JD, Lunt M, Silman AJ, Felsenberg D, et al. Determinants of incident vertebral fracture in men and women: results from the European Prospective Osteoporosis Study (EPOS) Osteoporos Int. 2003;14(1):19–26. doi: 10.1007/s00198-002-1317-8. [DOI] [PubMed] [Google Scholar]
- 154.Holmberg AH, Johnell O, Nilsson PM, Nilsson JA, Berglund G, Akesson K. Risk factors for hip fractures in a middle-aged population: a study of 33,000 men and women. Osteoporos Int. 2005;16(12):2185–94. doi: 10.1007/s00198-005-2006-1. [DOI] [PubMed] [Google Scholar]
- 155.Kanis J, Johnell O, Gullberg B, Allander E, Elffors L, Ranstam J, et al. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporos Int. 1999;9(1):45–54. doi: 10.1007/s001980050115. [DOI] [PubMed] [Google Scholar]
- 156.Meyer HE, Tverdal A, Selmer R. Weight variability, weight change and the incidence of hip fracture: a prospective study of 39,000 middle-aged Norwegians. Osteoporos Int. 1998;8(4):373–8. doi: 10.1007/s001980050077. [DOI] [PubMed] [Google Scholar]
- 157.Langlois JA, Visser M, Davidovic LS, Maggi S, Li G, Harris TB. Hip fracture risk in older white men is associated with change in body weight from age 50 years to old age. Arch Intern Med. 1998;158(9):990–6. doi: 10.1001/archinte.158.9.990. [DOI] [PubMed] [Google Scholar]
- 158.Kanis JA, Johansson H, Oden A, Johnell O, De Laet C, Eisman JA, et al. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35(5):1029–37. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 159.Kanis JA, Johansson H, Oden A, Johnell O, De Laet C, Melton III LJ, et al. A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res. 2004;19(6):893–9. doi: 10.1359/JBMR.040134. [DOI] [PubMed] [Google Scholar]
- 160.Vestergaard P, Olsen ML, Paaske JS, Rejnmark L, Sorensen HT, Mosekilde L. Corticosteroid use and risk of hip fracture: a population-based case-control study in Denmark. J Intern Med. 2003;254(5):486–93. doi: 10.1046/j.1365-2796.2003.01219.x. [DOI] [PubMed] [Google Scholar]
- 161.van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fracture. J Bone Miner Res. 2000;15(6):993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 162.Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15(4):323–8. doi: 10.1007/s00198-003-1548-3. [DOI] [PubMed] [Google Scholar]
- 163.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with systemic and topical corticosteroids. J Intern Med. 2005;257(4):374–84. doi: 10.1111/j.1365-2796.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- 164.Hubbard R, Tattersfield A, Smith C, West J, Smeeth L, Fletcher A. Use of inhaled corticosteroids and the risk of fracture. Chest. 2006;130(4):1082–8. doi: 10.1378/chest.130.4.1082. [DOI] [PubMed] [Google Scholar]
- 165.Donnan PT, Libby G, Boyter AC, Thompson P. The population risk of fractures attributable to oral corticosteroids. Pharmacoepidemiol Drug Saf. 2005;14(3):177–86. doi: 10.1002/pds.1075. [DOI] [PubMed] [Google Scholar]
- 166.van Staa TP, Leufkens HG, Cooper C. Use of inhaled corticosteroids and risk of fractures. J Bone Miner Res. 2001;16(3):581–8. doi: 10.1359/jbmr.2001.16.3.581. [DOI] [PubMed] [Google Scholar]
- 167.Hubbard RB, Smith CJ, Smeeth L, Harrison TW, Tattersfield AE. Inhaled corticosteroids and hip fracture: a population-based case-control study. Am J Respir Crit Care Med. 2002;166(12 Pt 1):1563–6. doi: 10.1164/rccm.200206-606OC. [DOI] [PubMed] [Google Scholar]
- 168.Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. [Study of Osteoporotic Fractures Research Group]. N Engl J Med. 1998;339(11):733–8. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 169.Goderie-Plomp HW, van der KM, de Ronde W, Hofman A, de Jong FH, Pols HA. Endogenous sex hormones, sex hormone-binding globulin, and the risk of incident vertebral fractures in elderly men and women: the Rotterdam Study. J Clin Endocrinol Metab. 2004;89(7):3261–9. doi: 10.1210/jc.2002-022041. [DOI] [PubMed] [Google Scholar]
- 170.Barrett-Connor E, Mueller JE, von Muhlen DG, Laughlin GA, Schneider DL, Sartoris DJ. Low levels of estradiol are associated with vertebral fractures in older men, but not women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85(1):219–23. doi: 10.1210/jcem.85.1.6327. [DOI] [PubMed] [Google Scholar]
- 171.Mellstrom D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H, et al. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res. 2006;21(4):529–35. doi: 10.1359/jbmr.060110. [DOI] [PubMed] [Google Scholar]
- 172.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16(7):737–42. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 173.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, et al. Smoking and fracture risk: a meta-analysis. 2005;16(2):155–62. doi: 10.1007/s00198-004-1640-3. Osteoporosis International. [DOI] [PubMed] [Google Scholar]
- 174.Stock JL, Waud CE, Coderre JA, Overdorf JH, Janikas JS, Heiniluoma KM, et al. Clinical reporting to primary care physicians leads to increased use and understanding of bone densitometry and affects the management of osteoporosis. [A randomized trial]. Ann Intern Med. 1998;128(12 Pt 1):996–9. doi: 10.7326/0003-4819-128-12_part_1-199806150-00006. [DOI] [PubMed] [Google Scholar]
- 175.Ontario drug benefit formulary. E-formulary [database on the Internet]. Ontario Ministry of Health and Long-Term Care. [[updated 2006; cited 2006 Oct. 27].]. Available at: http://www.health.gov.on.ca/english/providers/program/drugs/odbf_eformulary.html .
- 176.Cadarette SM, Beaton DE, Gignac MAM, Jaglal JB, Hawker GA. Validity of self reported DXA testing and DXA results. J Bone Miner Res. 2006;21(Suppl 1) [Google Scholar]
- 177.Bischoff HA, Solomon DH, Dawson-Hughes B, et al. Repeat hip fractures in a population-based sample of Medicare recipients in the US: rates, timing and gender differences. J Bone Miner Res. 2001;16(Suppl 1) [Google Scholar]
- 178.Osteoporosis Action Plan Committee. Osteoporosis action plan: an osteoporosis strategy for Ontario. Report of the Osteoporosis Action Plan Committee to the Ministry of Health and Long-Term Care [report on the Internet] Feb, 2003. [[cited 2006 Oct. 15].]. Ministry of Health and Long-Term Care; Osteoporosis Society of Canada. Available at: http://health.gov.on.ca/english/public/pub/ministry_reports/osteo/osteo_0205.pdf .
- 179.Manitoba Health. Manitoba bone density program [Web page] [[updated 2006; cited 2006 June 14].]. Available at: http:/www.gov.mb.ca/health/programs/mbd/index.html .
- 180.US Preventive Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137(6):526–8. doi: 10.7326/0003-4819-137-6-200209170-00014. [DOI] [PubMed] [Google Scholar]
- 181.Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int. 2004;15(11):897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- 182.Barrett JA, Baron JA, Beach ML. Mortality and pulmonary embolism after fracture in the elderly. Osteoporos Int. 2003;14(11):889–94. doi: 10.1007/s00198-003-1494-0. [DOI] [PubMed] [Google Scholar]
- 183.Jalava T, Sarna S, Pylkkanen L, Mawer B, Kanis JA, Selby P, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res. 2003;18(7):1254–60. doi: 10.1359/jbmr.2003.18.7.1254. [DOI] [PubMed] [Google Scholar]
- 184.Melton LJ, III, Khosla S, Achenbach SJ, O’Connor MK, O’Fallon WM, Riggs BL. Effects of body size and skeletal site on the estimated prevalence of osteoporosis in women and men. Osteoporos Int. 2000;11(11):977–83. doi: 10.1007/s001980070037. [DOI] [PubMed] [Google Scholar]
- 185.Bainbridge KE, Sowers M, Lin X, Harlow SD. Risk factors for low bone mineral density and the 6-year rate of bone loss among premenopausal and perimenopausal women. Osteoporos Int. 2004;15(6):439–46. doi: 10.1007/s00198-003-1562-5. [DOI] [PubMed] [Google Scholar]
- 186.Sellmeyer DE, Stone KL, Sebastian A, Cummings SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Am J Clin Nutr. 2001;73(1):118–22. doi: 10.1093/ajcn/73.1.118. Study of Osteoporotic Fractures Research Group. [DOI] [PubMed] [Google Scholar]
- 187.Wasnich RD, Ross PD, Thompson DE, Cizza G, Yates AJ. Skeletal benefits of two years of alendronate treatment are similar for early postmenopausal Asian and Caucasian women. Osteoporos Int. 1999;9(5):455–60. doi: 10.1007/s001980050171. [DOI] [PubMed] [Google Scholar]
- 188.Bone HG, Downs RW Jr, Tucci JR, Harris ST, Weinstein RS, Licata AA, et al. Dose-response relationships for alendronate treatment in osteoporotic elderly women. [Alendronate Elderly Osteoporosis Study Centers]. J Clin Endocrinol Metab. 1997;82(1):265–74. doi: 10.1210/jcem.82.1.3682. [DOI] [PubMed] [Google Scholar]
- 189.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 190.Bell NH, Bilezikian JP, Bone HG III, Kaur A, Maragoto A, Santora AC, et al. Alendronate increases bone mass and reduces bone markers in postmenopausal African-American women. J Clin Endocrinol Metab. 2002;87(6):2792–7. doi: 10.1210/jcem.87.6.8575. [DOI] [PubMed] [Google Scholar]
- 191.Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9(5):461–8. doi: 10.1007/pl00004171. [DOI] [PubMed] [Google Scholar]
- 192.Hooper MJ, Ebeling PR, Roberts AP, Graham JJ, Nicholson GC, D’Emden M, et al. Risedronate prevents bone loss in early postmenopausal women: a prospective randomized, placebo-controlled trial. Climacteric. 2005;8(3):251–62. doi: 10.1080/13697130500118126. [DOI] [PubMed] [Google Scholar]
- 193.Clemmesen B, Ravn P, Zegels B, Taquet AN, Christiansen C, Reginster JY. A 2-year phase II study with 1-year of follow-up of risedronate (NE-58095) in postmenopausal osteoporosis. Osteoporos Int. 1997;7(5):488–95. doi: 10.1007/pl00004152. [DOI] [PubMed] [Google Scholar]
- 194.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. [Vertebral Efficacy With Risedronate Therapy (VERT) Study Group]. JAMA. 1999;282(14):1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 195.Melton LJ III, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL. Determinants of bone loss from the femoral neck in women of different ages. J Bone Miner Res. 2000;15(1):24–31. doi: 10.1359/jbmr.2000.15.1.24. [DOI] [PubMed] [Google Scholar]
- 196.Van Pottelbergh I, Goemaere S, Kaufman JM. Bioavailable estradiol and an aromatase gene polymorphism are determinants of bone mineral density changes in men over 70 years of age. J Clin Endocrinol Metab. 2003;88(7):3075–81. doi: 10.1210/jc.2002-021691. [DOI] [PubMed] [Google Scholar]
- 197.Cauley JA, Fullman RL, Stone KL, Zmuda JM, Bauer DC, Barrett-Connor E, et al. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int. 2005;16(12):1525–37. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 198.Naves M, Diaz-Lopez JB, Gomez C, Rodriguez-Rebollar A, Serrano-Arias M, Cannata-Andia JB. Prevalence of osteoporosis in men and determinants of changes in bone mass in a non-selected Spanish population. Osteoporos Int. 2005;16(6):603–9. doi: 10.1007/s00198-004-1727-x. [DOI] [PubMed] [Google Scholar]
- 199.Sambrook PN, Geusens P, Ribot C, Solimano JA, Ferrer-Barriendos J, Gaines K, et al. Alendronate produces greater effects than raloxifene on bone density and bone turnover in postmenopausal women with low bone density: results of EFFECT (Efficacy of FOSAMAX versus EVISTA Comparison Trial) International. J Intern Med. 2004;255(4):503–11. doi: 10.1111/j.1365-2796.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- 200.Ringe JD, Dorst A, Faber H, Ibach K. Alendronate treatment of established primary osteoporosis in men: 3-year results of a prospective, comparative, two-arm study. Rheumatol Int. 2004;24(2):110–3. doi: 10.1007/s00296-003-0388-y. [DOI] [PubMed] [Google Scholar]
- 201.Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab. 2000;85(5):1895–900. doi: 10.1210/jcem.85.5.6603. [DOI] [PubMed] [Google Scholar]
- 202.Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. [Vertebral Efficacy with Risedronate Therapy (VERT) Study Group]. Osteoporos Int. 2000;11(1):83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 203.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. [Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators]. JAMA. 1999;282(7):637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 204.Morii H, Ohashi Y, Taketani Y, Fukunaga M, Nakamura T, Itabashi A, et al. Effect of raloxifene on bone mineral density and biochemical markers of bone turnover in Japanese postmenopausal women with osteoporosis: results from a randomized placebo-controlled trial. Osteoporos Int. 2003;14(10):793–800. doi: 10.1007/s00198-003-1424-1. [DOI] [PubMed] [Google Scholar]
- 205.Hodsman AB, Fraher LJ, Watson PH, Ostbye T, Stitt LW, Adachi JD, et al. A randomized controlled trial to compare the efficacy of cyclical parathyroid hormone versus cyclical parathyroid hormone and sequential calcitonin to improve bone mass in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 1997;82(2):620–8. doi: 10.1210/jcem.82.2.3762. [DOI] [PubMed] [Google Scholar]
- 206.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 207.Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, et al. A randomized double-blind trial to compare the efficacy of teriparatide. [[recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis]. J Clin Endocrinol Metab. 2002;87(10):4528–35. doi: 10.1210/jc.2002-020334. [DOI] [PubMed] [Google Scholar]
- 208.McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165(15):1762–8. doi: 10.1001/archinte.165.15.1762. [DOI] [PubMed] [Google Scholar]
- 209.Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest. 1998;102(8):1627–33. doi: 10.1172/JCI3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med. 2005;353(6):566–75. doi: 10.1056/NEJMoa050157. [DOI] [PubMed] [Google Scholar]
- 211.Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349(13):1216–26. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 212.Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85(9):3069–76. doi: 10.1210/jcem.85.9.6818. [DOI] [PubMed] [Google Scholar]
- 213.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343(9):604–10. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 214.Lyritis GP, Tsakalakos N, Paspati I, Skarantavos G, Galanos A, Androulakis C. The effect of a modified etidronate cyclical regimen on postmenopausal osteoporosis: a four-year study. Clin Rheumatol. 1997;16(4):354–60. doi: 10.1007/BF02242451. [DOI] [PubMed] [Google Scholar]
- 215.Ishida Y, Kawai S. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: The Yamaguchi Osteoporosis Prevention Study. Am J Med. 2004;117(8):549–55. doi: 10.1016/j.amjmed.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 216.Iwamoto J, Takeda T, Sato Y, Uzawa M. Comparison of effect of treatment with etidronate and alendronate on lumbar bone mineral density in elderly women with osteoporosis. Yonsei Med J. 2005;46(6):750–8. doi: 10.3349/ymj.2005.46.6.750. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 217.Sambrook PN, Rodriguez JP, Wasnich RD, Luckey MM, Kaur A, Meng L, et al. Alendronate in the prevention of osteoporosis: 7-year follow-up. Osteoporos Int. 2004;15(6):483–8. doi: 10.1007/s00198-003-1571-4. [DOI] [PubMed] [Google Scholar]
- 218.Khan SA, de Geus C, Holroyd B, Russell AS. Osteoporosis follow-up after wrist fractures following minor trauma. Arch Intern Med. 2001;161(10):1309–12. doi: 10.1001/archinte.161.10.1309. [DOI] [PubMed] [Google Scholar]
- 219.Juby AG, Geus-Wenceslau CM. Evaluation of osteoporosis treatment in seniors after hip fracture. Osteoporos Int. 2002;13(3):205–10. doi: 10.1007/s001980200015. [DOI] [PubMed] [Google Scholar]
- 220.van Staa TP, Leufkens HG, Cooper C. Does a fracture at one site predict later fractures at other sites? [A British cohort study]. Osteoporos Int. 2002;13(8):624–9. doi: 10.1007/s001980200084. [DOI] [PubMed] [Google Scholar]
- 221.Leifke E, Wichers C, Gorenoi V, Lucke P, von zur Muhlen A, Brabant G. Low serum levels of testosterone in men with minimal traumatic hip fractures. Exp Clin Endocrinol Diabetes. 2005;113(4):208–13. doi: 10.1055/s-2005-837652. [DOI] [PubMed] [Google Scholar]
- 222.Kanis JA, Johnell O, Oden A, De Laet C, Jonsson B, Dawson A. Ten-year risk of osteoporotic fracture and the effect of risk factors on screening strategies. Bone. 2002;30(1):251–8. doi: 10.1016/s8756-3282(01)00653-6. [DOI] [PubMed] [Google Scholar]
- 223.Kanis JA, Johansson H, Oden A, De Laet C, Johnell O, Eisman JA, et al. A meta-analysis of milk intake and fracture risk: low utility for case finding. Osteoporos Int. 2005;16(7):799–804. doi: 10.1007/s00198-004-1755-6. [DOI] [PubMed] [Google Scholar]


