Executive Summary
Objective
The aim of this review was to assess the effectiveness of ultrasound screening for asymptomatic abdominal aortic aneurysm (AAA).
Clinical Need
Abdominal aortic aneurysm is a localized abnormal dilatation of the aorta greater than 3 cm. In community surveys, the prevalence of AAA is reported to be between 2% and 5.4%. Abdominal aortic aneurysms are found in 4% to 8% of older men and in 0.5% to 1.5% of women aged 65 years and older. Abdominal aortic aneurysms are largely asymptomatic. If left untreated, the continuing extension and thinning of the vessel wall may eventually result in rupture of the AAA. Often rupture may occur without warning, causing acute pain. Rupture is always life threatening and requires emergency surgical repair of the ruptured aorta. The risk of death from ruptured AAA is 80% to 90%. Over one-half of all deaths attributed to a ruptured aneurysm take place before the patient reaches hospital. In comparison, the rate of death in people undergoing elective surgery is 5% to 7%; however, symptoms of AAA rarely occur before rupture. Given that ultrasound can reliably visualize the aorta in 99% of the population, and its sensitivity and specificity for diagnosing AAA approaches 100%, screening for aneurysms is worth considering as it may reduce the incidence of ruptured aneurysms and hence reduce unnecessary deaths caused by AAA-attributable mortality.
Review Strategy
The Medical Advisory Secretariat used its standard search strategy to retrieve international health technology assessments and English-language journal articles from selected databases to determine the effectiveness of ultrasound screening for abdominal aortic aneurysms. Case reports, letters, editorials, nonsystematic reviews, non-human studies, and comments were excluded.
Questions asked:
Is population-based AAA screening effective in improving health outcomes in asymptomatic populations?
Is AAA screening acceptable to the population? Does this affect the effectiveness the screening program?
How often should population-based screening occur?
What are appropriate treatment options after screening based on the size of aneurysms?
Are there differences between universal and targeted screening strategies?
What are the harms of screening?
Summary of Findings
Population-based ultrasound screening is effective in men aged 65 to 74 years, particularly in those with a history of smoking. Screening reduces the incidence of AAA ruptures, and decreases rates of emergency surgical repair for AAA and AAA-attributable mortality.
Acceptance rates decline with increasing age and are lower for women. Low acceptance rates may affect the effectiveness of a screening program.
A one-time screen is sufficient for a population-based screening program with regard to initial negative scans and development of large AAAs.
There is no difference between early elective surgical repair and surveillance for small aneurysms (4.0–5.4 cm). Repeated surveillance of small aneurysms is recommended.
Targeted screening based on history of smoking has been found to detect 89% of prevalent AAAs and increase the efficiency of screening programs from statistical modeling data.
Women have not been studied for AAA screening programs. There is evidence suggesting that screening women for AAA should be considered with respect to mortality and case fatality rates in Ontario. It is important that further evaluation of AAAs in women occur.
There is a small risk of physical harm from screening. Less than 1% of aneurysms will not be visualized on initial screen and a re-screen may be necessary; elective surgical repair is associated with a 6% operative morality rate and about 3% of small aneurysms may rupture during surveillance. These risks should be communicated through informed consent prior to screening.
There is little evidence of severe psychological harms associated with screening.
Conclusions
Based on this review, the Medical Advisory Secretariat concluded that there is sufficient evidence to determine that AAA screening using ultrasound is effective and reduces negative health outcomes associated with the condition.
Moreover, screening for AAA is cost-effective, comparing favorably for the cost of per life year gained for screening programs for cervical cancer, hypertension, and breast cancer that are in practice in Ontario, with a high degree of compliance, and can be undertaken with a minimal effort at fewer than 10 minutes to screen each patient.
Overall, the clinical utility of an invitation to use ultrasound screening to identify AAA in men aged 65 to 74 is effective at reducing AAA-attributable mortality. The benefit of screening women is not yet established. However, Ontario data indicate several areas of concern including population prevalence, detection of AAA in women, and case management of AAA in women in terms of age cutoffs for screening and natural history of disease associated with age of rupture.
Objective
The aim of this review was to assess the effectiveness of ultrasound screening for asymptomatic abdominal aortic aneurysm (AAA).
Background
Clinical Need: Target Population and Condition
An AAA is a localized, abnormal dilatation of the aorta greater than 3 cm or 50% of the aortic diameter at the diaphragm. (1) A true AAA involves all 3 layers of the vessel wall. If left untreated, the continuing extension and thinning of the vessel wall may eventually result in rupture of the AAA. The risk of death from ruptured AAA is 80% to 90%. (2) One study analyzing national hospital database information in the United States found no significant change in the AAA incidence, rates of elective AAA repair, or ruptured AAA presented in hospitals. (3) The investigators concluded that technological and treatment advances over the past 19 years have not affected the outcomes of patients with AAA, and the ability to identify and to treat patients with AAA has not improved.
Classification of Abdominal Aortic Aneurysms
An AAA may be symptomatic or asymptomatic. It may be classified according to its size: (4)
Small aneurysms are smaller than 5 cm in diameter.
Medium aneurysms are 5 to 7 cm in diameter.
Large aneurysms are greater than 7 cm in diameter.
Symptoms of an Abdominal Aortic Aneurysm
Abdominal aortic aneurysms usually do not produce symptoms. However, as they expand, they may become painful. Compression or erosion of adjacent tissue by aneurysms also may cause symptoms. The formation of mural thrombi, a type of blood clot, within the aneurysm may predispose people to peripheral embolization, where blood vessels become blocked. Occasionally, an aneurysm may leak into the vessel wall and the periadventitial area, causing pain and local tenderness. More often, acute rupture occurs without any warning, causing acute pain and hypotension. This complication is always life threatening and requires an emergency operation.
Incidence and Prevalence
In community surveys, the prevalence of AAA is reported to be between 1% and 5.4%. (2) The prevalence is related to age and vascular risk factors. It is more common in men and in those with a positive family history. Abdominal aortic aneurysms are found in 4% to 8% of older men aged over 65 years and 0.5% to 1.5% in women aged over 65 years. (5) The incidence of AAAs (greater than 3.0 cm) in the general population is about 1.0% to 1.5%. (6)
Naylor et al (7) reported that in Canada, AAAs are the tenth leading cause of death in men aged 65 years or older. (7) The rate of AAA repair in Ontario has increased from 38 per 100,000 population in 1981/1982 to 54 per 100,000 population in 1991/1992. From 1989/1990 to 1991/1992, the rate of AAA repair in Ontarians aged 45 years and over was 53 per 100,000. (7)
In the United States, about 200,000 new cases are diagnosed each year, and 50,000 to 60,000 surgical AAA repairs are performed. (8) Ruptured AAAs are responsible for about 15,000 deaths in the United States annually. One in 10 men aged over 80 years has some aneurysmal change in his aorta. (8) Moreover, due to the ageing population, the absolute number of AAAs is set to increase.
Risk Factors for an Abdominal Aortic Aneurysm
Traditional risk factors for AAA include these:
Male sex
Older age
Family history of aneurysm
Smoking (ever, current)
Presence of atherosclerosis (coronary artery disease, cerebral vascular disease, claudication)
Presence of hypertension
Presence of vascular risk factors
Height
Obesity
Presence of chronic obstructive pulmonary disorder
Presence of diabetes
Numerous studies support a lower risk of developing AAA for women compared with men. A paper reporting on two large cohort studies (9) has shown lower prevalence of AAA in black people and people with diabetes. Additionally, obese people are less likely to be diagnosed due to a lower specificity with diagnosis using manual examination of palpable mass and increased difficulty visualizing the aorta through ultrasound. Therefore, obese individuals may be more at risk for ruptured undiagnosed AAAs.
Prognosis of an Abdominal Aortic Aneurysm
The risk of rupture of an untreated AAA is a continuous function of aneurysm size as represented by the maximal diameter of the AAA. The annual rupture rate is near 0 for aneurysms less than 4 cm in diameter. The risk is about 1% per year for aneurysms 4 to 4.9 cm, 11% per year for aneurysms 5 to 5.9 cm, and 25% per year or more for aneurysms greater than 6 cm. (4)
The 1-year mortality rate of patients with AAAs who do not undergo surgical treatment is about 25% if the aneurysms are 4 to 6 cm in diameter. This increases to 50% for aneurysms exceeding 6 cm. Other major causes of mortality for people with AAAs include coronary heart disease and stroke.
Treatment of Abdominal Aortic Aneurysms
Treatment of an aneurysm is indicated under any one of the following conditions:
The AAA is greater than 5.5 cm in diameter.
The patient is symptomatic.
The AAA is rapidly expanding irrespective of the absolute diameter.
Open surgical (OSR) repair of AAA is still the gold standard. It is a major operation involving the excision of the dilated area and placement of a sutured woven graft. The surgery may be performed under emergency situations following the rupture of an AAA, or it may be performed electively. Elective OSR is generally considered appropriate for healthy patients with aneurysms starting at 5 to 6 cm in diameter. (4) Treatment of smaller aneurysms through surgical repair is generally not considered appropriate because of the lower risk of rupture and the potential harms associated with surgical repair. The surgical treatment cutoff at 5.5 cm is generally considered appropriate as the increased exponential risk associated with an aneurysm greater than 6 cm and a potential 0.5 cm error associated with estimation of aortic dilation during diagnosis of an AAA. (Personal Communication, September 2005) Coronary artery disease is the major underlying illness contributing to morbidity and mortality in OSR. Other medical comorbidities, such as chronic renal failure, chronic lung disease, and liver cirrhosis with portal hypertension, may double or triple the usual risk of OSR.
Serial noninvasive follow-up of small aneurysms (less than 5.5 cm) is an alternative to immediate surgery.
Endovascular repair of AAA is the third treatment option and is the topic of another health technology policy assessment review conducted by the Medical Advisory Secretariat (10) and a field evaluation study conducted by the Program for Assessment of Technology in Health. (11)
Rationale for Screening
Ruptured aneurysms often occur without warning as AAAs are largely asymptomatic. Ruptured aneurysms are always life threatening and require emergency surgical repair of the abdominal aorta. The risk of death from a ruptured AAA is 80% to 90%. (2) Over one-half of all deaths from ruptured aneurysms take place before the patient reaches a hospital. (12) In comparison, mortality for people undergoing elective surgery is 5% to 7%. (13)
However, symptoms for AAA rarely occur prior to rupture. Possible detection of aneurysms at a size when rupture is unlikely to occur is viable through screening. Ultrasound as a screening test for AAA can visualize the aorta in 99% of patients and has a sensitivity and specificity approaching 100% in screening settings for AAAs. (14) In addition, ultrasound is noninvasive, fast, relatively inexpensive, and does not expose patients to radiation. The feasibility of population-based ultrasound screening for AAA has been established through large randomized screening trials. (13;15-17)
Existing Methods Other Than Technology Being Reviewed
Two diagnostic methods, palpation of the abdomen during physical examination and abdominal ultrasound, have been advocated as screening modalities for AAA.
Abdominal palpation has been found to be highly sensitive for diagnosis of an AAA large enough in patients who do not have a large girth. (18) In a study by Fink et al., (18) the sensitivity of abdominal palpation increased with AAA diameter. For AAAs measuring 3.0 to 3.9 cm, 4.0 to 4.9 cm, and 5.0 cm and greater, sensitivity was 61%, 69%, and 82% respectively. Previously, physical examination for AAA has been recommended for the periodic health examination of older men. (2;19) However, more recently, physical examination has not been considered a suitable alternative to ultrasound due to high false positive and false negative rates. (5)
New Technology Being Reviewed
Ultrasound for Abdominal Aortic Aneurysms
Abdominal ultrasound is considered the gold standard for AAA screening. It is noninvasive, fast, accurate, and relatively inexpensive.
Ultrasound is an extremely sensitive and specific screening test for AAA of all sizes, at least in cases where the diagnosis and size of the aneurysm can be confirmed at surgery. Reported sensitivities range from 82% to 99%, with sensitivity approaching 100% in some studies and in series of screening patients with a pulsatile mass. (14) In one evaluation (20) of a British screening program, ultrasound measurement had a sensitivity of 100% for AAAs of 4.5 cm or more and a specificity of 100% for AAAs up to 3.0 cm. The positive predictive value of ultrasound for AAA screening was 100% (95% confidence interval [CI], 97%–100%). However, in a small proportion of patients, visualization of the aorta will be inadequate due to obesity, bowel gas, or periaortic disease.
Benefits and Adverse Events
Ultrasound screening can reliably visualize the aorta in 99% of people, has high levels of sensitivity and specificity, and provides the opportunity to detect an AAA at a stage when rupture is unlikely to occur. Early intervention at the presymptomatic stage may reduce the frequency of rupture and subsequently decrease mortality and the requirement for emergency hospital treatment. Elective surgery for an AAA is associated with a 5% to 7% mortality rate compared to a fatality rate of 80% to 90% for emergency repair of a ruptured AAA. (13)
There are opposing views on the risks and benefits of establishing ultrasound screening programs for AAA because of the operative mortality rates associated with surgical repair, particularly for an AAA that would never have ruptured if it had not been detected through screening or left untreated. However, ultrasound screening is reasonably cheap and noninvasive, and AAAs may cause a substantial number of mortalities.
Insurance Coverage
The Ontario Health Insurance Plan (OHIP) Schedule of Benefits for Physician Services includes fee codes “J135/J1435” for complete abdominal scans and codes “J128/J428” for limited abdominal scans (aorta only, follow-up scans) as insured services. Both complete abdominal scans and partial abdominal scans are appropriate for screening AAAs. Ultrasound technologists and sonographers, also have the scope of practice to undertake ultrasound screening of the abdomen. However, sonography is not a regulated health profession under the Health Professions Act, and there are no formal uniform requirements for the operation of ultrasound equipment.
Regulatory Status
There are more than 500 different types of ultrasound devices approved and licensed under Health Canada’s medical devices listing. Ultrasound devices are well-developed technologies that are common tests accounting for the bulk of operating expenditures on diagnostic imaging.
Literature Review on Effectiveness
Objective
The aim of this review was to assess the effectiveness of ultrasound screening for asymptomatic AAAs.
Questions Asked
Is population-based AAA screening effective at improving health outcomes in asymptomatic populations?
Is AAA screening acceptable to the population? Does this affect the effectiveness of the screening program?
How often should population-based screening occur?
What are appropriate treatment options after screening based on the size of aneurysm?
Are there differences between universal and targeted screening strategies?
What are the harms of screening?
Methods
Search Strategy
The Medical Advisory Secretariat completed a computer-aided search limited to human studies. Case reports, letters, editorials, nonsystematic reviews, and comments were excluded. An in-depth quality assessment of each study included in this health technology policy assessment was performed. The USPSTF review was of exceptional quality; hence, the current literature search was an update to the USPSTF review published in 2004. (5)
Inclusion Criteria
English-language articles (September 2004 to August 2005)
Journal articles that reported primary data on the effectiveness or cost-effectiveness of data obtained in a clinical setting, or analysis of primary data maintained in registries or databases
Study design and methods that were clearly described
Systematic reviews, randomized controlled trials (RCTs), non-RCTS, or cohort studies that had at least 20 patients, and cost-effectiveness studies
Exclusion Criteria
Duplicate publications (superseded by another publication by the same investigator group, with the same objective and data)
Non-English-language articles
Non-systematic reviews, letters, and editorials
Animal and in-vitro studies
Case reports
Studies that did not examine the outcomes of interest
The intervention of interest was invitation to AAA screening.
Databases Searched
Cochrane database of systematic reviews
ACP Journal Club
DARE
INAHTA
EMBASE
MEDLINE
Reference sections from reviews and extracted articles
Outcomes of Interest
Effect of screening on AAA rupture incidence
Effect of screening on rates of emergency and elective AAA repair
Effect of screening on AAA-related mortality
Effect of screening on all-cause mortality
Frequency of screening
Case management post-screening related to size of AAA
Risk factors for AAAs and impact on screening
Harms of screening
Quality of life
Economic analysis of screening programs
Quality of Evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (21) will be used to summarize the overall quality of the body of evidence (defined as 1 or more studies) supporting the research question explored in this systematic review. This system has 4 levels of quality: very low, low, moderate, and high. The criteria for assigning the GRADE level are outlined below.
Type of evidence
RCT: given a high GRADE level to start
Observational study: given a low GRADE level to start
Any other evidence: given a very low GRADE level to start
Decrease grade if:
Serious limitation to study quality (-1, reduce GRADE level by 1 so a high GRADE level will become a moderate grade) or very serious limitation to study quality (-2, reduce GRADE level by 2 so a high GRADE level will become low grade)
Important inconsistency (-1, reduce GRADE level by 1)
Some (-1) or major (-2) uncertainty about directness
Imprecise or sparse data (-1)
High probability of reporting bias (-1)
Increase GRADE level if:
Strong evidence of association-significant relative risk of >2 (< 0.5) based on consistent evidence from 2 or more observation studies, with no plausible confounders (+1, increase GRADE level by 1, so a moderate grade will become high. However a high grade will remain high)
Very strong evidence of association-significant relative risk of > 5 (< 0.2) based on direct evidence with no major threats to validity (+2, increase GRADE level by 2, so a low grade will become a high grade)
Evidence of a dose response gradient (+1)
All plausible confounders would have reduced the effect (+1).
Overall GRADE Level definitions
High: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: Any estimate of effect is very uncertain.
Grading of Recommendations
Incorporating the quality evidence as the evidence base, recommendations either for or against the use of the technology are then weighed against risks and benefits of implementation in order to be given a strength of recommendation using the GRADE criteria as outlined below.
| Grade of recommendation | Clarity of risk/benefit | Strength of Supporting Evidence | Implications |
|---|---|---|---|
| 1A | Benefits clearly outweigh risk and burdens, or vice versa | Consistent evidence from well performed randomized, controlled trials or overwhelming evidence of some other form. Further research is unlikely to change our confidence in the estimate of benefit and risk. | Strong recommendation, can apply to most patients in most circumstances without reservation |
| 1B | Benefits clearly outweigh risk and burdens, or vice versa | Evidence from randomized, controlled trials with important limitations (inconsistent results, methodological flaws, indirect or imprecise), or very strong evidence of some other form. Further research (if performed) is likely to have an impact on our confidence in the estimate of benefit and risk and may change the estimate. | Strong recommendation, likely to apply to most patients |
| 1C | Benefits appear to outweigh risk and burdens, or vice versa | Evidence from observational studies, unsystematic clinical experience, or from randomized, controlled trials with serious flaws. Any estimate of effect is uncertain. | Relatively strong recommendation; might change when higher quality evidence becomes available. |
| 2A | Benefits closely balanced with risks and burdens | Consistent evidence from well performed randomized, controlled trials or overwhelming evidence of some other form. Further research is unlikely to change our confidence in the estimate of benefit and risk. | Weak recommendation, best action may differ depending on circumstances or patients’ or societal values |
| 2B | Benefits closely balanced with risks and burdens; some uncertainty in the estimates of benefits, risks, and burdens | Evidence from randomized, controlled trials with important limitations (inconsistent results, methodological flaws, indirect or imprecise), or very strong evidence of some other form. Further research (if performed) is likely to have an impact on our confidence in the estimate of benefit and risk and may change the estimate. | Weak recommendation, alternative approaches likely to be better for some patients under some circumstances. |
| 2C | Uncertainty in the estimates of benefits, risks, and burdens; benefits may be closely balanced with risks and burdens | Evidence from observation studies, unsystematic clinical experience, or from randomized, controlled trials with serious flaws. Any estimate of effect is uncertain. | Very weak recommendation; other alternatives may be equally reasonable. |
Results of Literature Review
Table 1: Quality of Evidence of Included Studies*.
| Study Design | Level of Evidence |
Number of Eligible Studies |
|---|---|---|
| Large RCT, systematic reviews of RCT | 1 | 6, 3 |
| Large RCT unpublished but reported to an international scientific meeting | 1(g) | 0 |
| Small RCT | 2 | 0 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | 0 |
| Non-RCT with contemporaneous controls | 3a | 7 |
| Non-RCT with historical controls | 3b | 0 |
| Non-RCT presented at international conference | 3(g) | 0 |
| Surveillance (database or register) | 4a | 1 |
| Case series (multisite) | 4b | 0 |
| Case series (single site) | 4c | 0 |
| Retrospective review, modeling | 4d | 2 |
| Case series presented at international conference | 4(g) | 0 |
RCT refers to randomized controlled trial; g, non-peer-reviewed grey literature.
Summary of Existing Health Technology Assessments
Recommendation of the Canadian Task Force on Preventive Health Care
The recommendation statement of the Canadian Task Force on Preventive Health Care (CTFPHC) was published in 1994. (22) The CTFPHC indicated that abdominal ultrasound is a sensitive and specific test for diagnosis of AAAs of all sizes. The CTFPHC concluded that while “there is insufficient evidence to recommend for or against screening with physical examination or ultrasound, the prudent physician may choose to include a targeted physical examination for AAA in males over age 60 in the periodic health examination.” The quality of the CTFPHC review was high.
Recommendation of the United States Preventive Services Task Force
The recommendation statement of the United States Preventive Services Task Force (USPSTF) (23) was recently published in June 2005. In an update to a review published in the 1990s, The USPSTF recommended one-time screening for AAA by ultrasonography in men aged 65 to 75 who have ever smoked. The USPSTF found that abdominal ultrasonography, performed in a setting with adequate quality assurance, is an accurate screening test for AAA. The quality of the USPSTF review was high and served as the basis for the current health technology policy assessment on ultrasound screening for AAA.
The USPSTF made no recommendations for or against screening for AAA in men aged 65 to 75 who have never smoked. The prevalence of large AAAs in men who have never smoked is much lower compared with the AAA prevalence in men who have ever smoked. Because screening and early treatment may lead to harm, including an increased number of surgeries with associated morbidity and mortality, and psychological harm, the USPSTF concluded that the balance between the benefits and harm of screening for AAA is too close to make a general recommendation in this population.
The USPSTF concluded that the harm of screening women for AAA outweighs the benefits because of the low prevalence of AAAs in women would only prevent a small number of deaths from screening programs, and the corresponding risks associated with screening and early treatment such as increased numbers elective surgeries associated with morbidity and mortality, and psychological harm.
Randomized Controlled Trials on Screening for Abdominal Aortic Aneurysm
The results of 4 AAA screening trials ranging from fair to poor in quality show an association between an invitation to attend screening and a reduction in AAA-related mortality. (Appendices 2 and 3)
The Western Australia trial (15) reported AAA-related mortality for different age groups. In this study, 41,000 men aged 65 to 83 years were randomized to invitation to screening and control groups. Overall, there was no significant difference in AAA-related mortality between those invited to screening and controls (odds ratio [OR], 0.87; 95% CI, 0.55–1.38). However, in a post hoc analysis, an invitation to screening was associated with a significant reduction in AAA-related mortality for men aged 65 to 75 years from the time of scheduled screening (OR, 0.19; 95% CI, 0.04–0.89).
In the Multicentre Aneurysm Screening Study (MASS), (17) 67,800 men aged 65 to 74 years were randomized either to receive an invitation for an abdominal ultrasound scan or not. There were 65 (0.19%) aneurysm-related deaths in the invited group, and 113 (0.33%) in the control group (risk reduction 42%; 95% CI, 22%–58%, P = .0002). Thirty-day mortality was 6% after elective surgery for an aneurysm, and 37% after emergency surgery. The results show that screening combined with early intervention can significantly reduce mortality rate associated with AAA.
Hospital costs and benefits of screening for AAA were studied in a randomized population screening trial in Viborg, Denmark. (24) This study showed that screening reduced hospital mortality from AAA by about 68% (95% CI, 41%–89%). Six people died from AAA-attributable mortality in the screening group in hospital compared with 19 in the control group (P < .001). In addition, the frequency of emergency operations was reduced by 74% (95% CI, 54%–89%) in the screened group.
The incidence of ruptured AAA was studied in a randomized trial in Chichester, United Kingdom. (16) In this study, 15,775 men and women aged 65 to 80 were randomized into 2 groups. One group was invited for ultrasound screening for AAA, and the other acted as age- and sex-matched controls. Abdominal aortic aneurysms were detected in 7.6% of men (4% overall). Aortic surgery was offered to the screened group if certain criteria (such as age of the patient or aneurysm size) were met. The incidence of rupture was reduced by 55% in men in the group invited for screening, compared with controls (OR, 0.40; 95% CI, 0.18–0.91). The incidence of rupture in women was low in both the invited (0.6%) and control (0.04%) groups (OR, 1.49; 95% CI, 0.25–8.94).
Randomized Controlled Trials on Treatment of Small Aneurysms
Two high quality RCTs investigated whether elective surgical repair of small AAAs (4.0–5.4 cm) improved survival compared with ultrasound surveillance. Both studies found no difference in survival rates between the groups randomized to early elective surgical repair of AAA and the groups randomized to surveillance.
In the Aneurysm Detection and Management Veterans Affairs Cooperative Study Group (ADAM), 1, 136 men and women aged 50 to 79 years were randomized either to early surgical repair of aneurysm or were assigned to the surveillance group to undergo ultrasonography or computed tomography until aneurysm growth was sufficient to proceed to regularly assigned elective surgical repair (5.5 cm). (9) There were no differences in survival between the 2 groups (OR, 1.21; 95% CI, 0.95–1.54).
The United Kingdom Small Aneurysm Trial (UKSAT) (25) randomly assigned 1090 patients aged 60 to 76 years to receive either early elective surgery or surveillance by ultrasound. After 8 years of follow-up, results indicated no long-term differences in survival between the early elective surgery and the surveillance groups (OR, 0.83; 95% CI, 0.35–0.69).
Studies Examining Risk Factors for AAA
Risk factors for AAA were derived from: one meta-analysis of AAA risk factors, one multivariate analysis of the Perth screening trials, 2 multivariate analyses from the ADAM small aneurysm trials, other data specific to Ontario and Canada obtained from Statistics Canada for smoking prevalence from the National Population Health Survey, and data obtained from the Ontario Provincial Health Planning Database and analyzed by the Medical Advisory Secretariat.
In a meta-analysis (26) of risk factors associated with AAA prevalence in asymptomatic populations, study investigators completed a systematic review using the MEDLINE and EMBASE databases to identify groups at high-risk for AAA. Population-based studies investigating risk factors associated with screening-detected AAAs included in English-, German-, French-, and Italian-language studies. Results from 14 studies considered sex, smoking, hypertension, diabetes, history of myocardial infarction, and peripheral vascular disease as risk factors for AAA. The investigators concluded that history of smoking, and peripheral or coronary artery disease should be further examined.
The Perth screening trial, (27) previously described in this report, included a questionnaire addressing demographic, behavioural, and medical factors relevant to AAA risk factors. Statistically significant results from a multivariate analysis of risk factors associated with AAA prevalence included decreased risk for Mediterranean-born men versus Australian-born men and lower risk for those who did regular vigorous exercise. Increased risk was associated with smoking in the past, current smoking, coronary and peripheral artery disease, and a waist-to hip ratio greater than 0.9 cm.
The ADAM small aneurysm trial (28) included a prescreening questionnaire prior to ultrasound screening for AAA in 73,451 veterans aged 50 to 59 years in 15 veterans affairs medical centres across the United States. The prescreening questionnaire was tested and validated before the study started, and results were compared to a subset of patients later entered into the small aneurysm trial as cases and controls. Questions asked about demographics, possible risk factors for AAA, and medical conditions diagnosed by a physician. Two multivariate logistic regression models were reported that characterized risk factors for AAA: one model that compared patients with small borderline aneurysms of 3.0 to 3.9 cm to patients with infrarenal aortic diameters of less than 3.0 cm, and another model that compared patients with AAAs larger than 4.0 cm to patients with infrarenal aortic diameters of less than 3.0 cm. Smoking was the risk factor most associated with AAA. Excess prevalence of AAAs associated with smoking accounted for 78% of all aneurysms larger than 4.0 cm in the study. Female sex, black race, and diabetes were not risk factors associated with AAA. Age, height, coronary artery disease, atherosclerosis, high cholesterol levels, and hypertension were positive risk factors for AAA.
Summary of Findings
Each study that met the inclusion criteria for the literature review was included in the analysis that assessed the effectiveness of ultrasound screening for AAA. Each study was critically appraised for quality. Results from the screening trials were stratified by sex because of the delayed age of onset in women and the differences in available research literature. Screening program outcomes included in the analysis of the effectiveness of ultrasound screening for AAA were the incidence of AAA rupture, surgical repair rates, AAA-attributable mortality, and all-cause mortality. Additional material deemed relevant to enabling the Ontario Health Technology Advisory Committee to make recommendations about this technology was also included. This additional material includes factors affecting program uptake; the case for repeated AAA screening; age at AAA rupture; case management of small aneurysms; targeted screening approaches based on AAA risk factors; a special report on women; and harms associated with ultrasound screening for AAA. An additional cost-effectiveness analysis is included in the report following the analysis of the effectiveness of AAA screening.
Similar to the USPSTF review, when appropriate, a meta-analysis was conducted by the Medical Advisory Secretariat across AAA screening trial and small aneurysm trial outcomes. All meta-analyses for AAA screening trial outcomes included men ages 65 years and older; women were excluded as they were only included in one trial and their risk was heterogeneous to the risk of AAA outcomes in men. No meta-analysis specific to women was conducted, as only one screening trial included women. The meta-analysis for the small aneurysm trials included men and women because small aneurysm trials outcomes were not reported by sex. However, due to the small proportion of women enrolled in the small aneurysm trials, results may not be representative of case management for small aneurysms in women.
Effectiveness of Screening
Does AAA Screening Reduce the Incidence of Aneurysm Ruptures?
All 4 screening trials had data on incidence of AAA rupture (Table 2). The odds ratios for males for rupture incidence ranged from 0.20 to 0.87. Statistically significant reductions in the incidence of AAA rupture with invitation to screening were found for males in the Viborg (OR, 0.20; 95% CI, 0.07–0.58), MASS (OR, 0.50; 95% CI 0.37–0.68) and Chichester (OR, 0.40; 95% CI 0.18–0.91) studies. In the meta-analysis, the pooled odds ratio among men showed a statistically significant reduction in the incidence of AAA rupture (OR, 0.50; 95% CI 0.31–0.80) with invitation to screening (Appendix 4).
Table 2: Incidence of Abdominal Aortic Aneurysm Rupture in Population-Based Abdominal Aortic Aneurysm Screening Trials*.
| Viborg (N = 12,639) |
MASS (N = 67,800) |
Chichester Men (N = 6,433) |
Chichester Women (N = 9,342) |
Perth, Australia (N = 41,000) |
|
|---|---|---|---|---|---|
| Invited, % | 0.1 | 0.2 | 0.3 | 0.06 | 0.2 |
| Prevalence of AAA, no. (%) | 191(4.0) | 1,333 (4.9) | 178 (7.6) | 40 (1.3) | 875 (7.2) |
| Control, % | 0.4 | 0.3 | 0.6 | 0.04 | 0.2 |
| Odds ratio | 0.20 | 0.50 | 0.40 | 1.49 | 0.87 |
| (95% CI) | (0.07–0.58) | (0.37–0.68) | (0.18–0.91) | (0.25–8.94) | (0.55–1.38) |
AAA indicates abdominal aortic aneurysm; CI, confidence interval.
Does AAA Screening Reduce Rates of Emergency Operations? Increase Rates of Elective Repair?
Rates of elective surgical repair and emergency surgical repair of AAAs were reported in all of the screening trials. Odds ratios for emergency surgical repair associated with an invitation to screening ranged from 0.20 to 1.13 (Table 3). Lower rates of emergency surgical repair were significant in the Viborg (OR, 0.20; 95% CI, 0.08–0.48) and MASS (OR, 0.50; 95% CI, 0.32–0.80) trials. Invitation to screening was associated with increased rates of elective surgical repair; odds ratios ranged from 1.99 to 5.62. All 4 trials reported statistically significantly results for men of increased elective repair associated with invitation to screening.
Table 3: Rates of Surgical Repair for AAA in Population-Based AAA Screening Trials*.
| Viborg (N = 12,639) |
MASS (N = 67,800) |
Chichester Men (N = 6,433) |
Chichester Women (N = 6,433) |
Perth, Australia (N = 41,000) |
||
|---|---|---|---|---|---|---|
| Emergency Repair | Invited, no. | 6 | 27 | 3 | 1 | 9 |
| Controls, no. | 30 | 54 | 8 | 1 | 8 | |
| Odds ratio | 0.20 | 0.50 | 0.38 | 1.00 | 1.13 | |
| (95% CI) | (0.08–0.48) | (0.32–0.80) | (0.10–1.42) | (0.06–15.93) | (0.43–2.92) | |
| Elective Repair | Invited, no. | 50 | 332 | 28 | 4 | 107 |
| Controls, no. | 14 | 92 | 5 | 2 | 54 | |
| Odds ratio | 3.58 | 3.65 | 5.62 | 1.99 | 2.02 | |
| (95% CI) | (1.29–6.49) | (2.89–4.65) | (2.14–14.37) | (0.36–10.88) | (1.46–2.80) |
AAA indicates abdominal aortic aneurysm; CI, confidence interval.
Overall, the results of the meta-analysis indicate that an invitation to screening was associated with higher rates of elective surgical repair (OR, 3.18; 95% CI 2.11–4.79) of AAAs and lower rates of emergency surgical repair (OR, 0.46; 95% CI 0.24–0.88) of AAAs (Appendix 5 and Appendix 6).
Does Population-Based AAA Screening Reduce AAA-Related Mortality?
Each of the screening trials found a reduction in AAA-attributable mortality, which is defined as a death certificate, hospital record, or vitality statistic indicating death that contains an ICD code including AAA as a cause of death. The point estimates of the odds ratios ranged from 0.31 to 1.00 (Table 4). The Viborg trial (OR, 0.31; 95% CI 0.13–0.79) and the MASS trial (OR, 0.58; 95% CI 0.42–0.78) showed statistically significant reductions in AAA-attributable mortality. In meta-analysis, the pooled odds ratio of population-based AAA screening showed a statistically significant reduction in AAA-attributable mortality (OR, 0.57; 95% CI 0.45–0.74) (Appendix 7). However, the USPSTF (5) found that the MASS study, the largest study with the narrowest confidence intervals, contributed most of the weight to the pooled estimates of AAA-attributable mortality.
Table 4: Mortality Attributed to Abdominal Aortic Aneurysms in Population-Based Screening Trials on Abdominal Aortic Aneurysm*.
| Viborg (N = 12,639) |
MASS (N = 67,800) |
Chichester Men (N = 6,433) |
Chichester Women (N = 9,342) |
Perth, Australia (N = 41,000) |
|
|---|---|---|---|---|---|
| Invited, % | 0.1 | 0.2 | 0.7 | < 0.01 | < 0.01 |
| Controls, % | 0.4 | 0.3 | 1.0 | < 0.01 | < 0.01 |
| Odds ratio | 0.31 | 0.58 | 0.59 | 1.00 | 0.72 |
| (95% CI) | (0.13–0.79) | (0.42–0.78) | (0.27–1.29) | (0.14–7.07) | (0.39–1.32) |
CI indicates confidence interval.
Does Population-Based AAA Screening Reduce All-Cause Mortality?
All-cause mortality was calculated in each of the screening trials; odds ratio ranged from 0.90 to 1.07 (Table 5). With the exception of the Viborg study (OR, 0.90; 95% CI 0.82–0.99) all confidence intervals crossed 1.00 indicating no significant differences in reduction of all-cause mortality through an invitation to screen. Results of the trials were pooled using a random-effects model, and an invitation to screen was associated with a nonsignificant reduction in all-cause mortality (OR, 0.97; 95% CI 0.93–1.01). (See Appendix 8.)
Table 5: All-Cause Mortality in Population-Based Screening Trials*.
| Viborg (N = 12,639) |
MASS (N = 67,800) |
Chichester Men (N = 6,433) |
Chichester Women (N = 9,342) |
Perth, Australia (N = 41,000) |
|
|---|---|---|---|---|---|
| Invited, % | 14.8 | 11.1 | 16.6 | 10.7 | 11.5 |
| Controls, % | 16.1 | 11.4 | 15.7 | 10.2 | 13.3 |
| Odds ratio | 0.90 | 0.97 | 1.07 | 1.05 | 0.98 |
| (95% CI) | (0.82–0.99) | (0.93–1.02) | (0.93–1.22) | (0.92–1.19) | (0.91–1.04) |
CI indicates confidence interval.
Factors Affecting Program Uptake and Impact on Population Screening Program Outcomes
Screening acceptance rates ranged from 63% to 80% across trials, with a mean screening acceptance rate of 72% (Appendix 3). The Chichester, Viborg, and MASS trials examined factors associated with screening acceptance. Additionally, the groups invited to screening in the MASS, Perth, and Chichester trials were examined to determine the screening outcomes for those refusing the invitation, or screening “refusers,” to those that did not comply with subsequent follow-up screening, or “non-compliers.”
In the Chichester study, older people of both sexes and women were less likely to accept screening (Table 6). For males in the Chichester study, 75% (18 of 24) of all the deaths in the invited group were attributable to refusers and non-compliers of screening. Fifteen of 17 (88%) deaths of people with no follow-up were attributable to people who refused the initial invitation to screening. Of the remaining deaths in the invited group during surveillance, 60% (3 of 5) of the deaths were of people who did not comply with requests for follow-up screening.
Table 6: Acceptance Rates for Abdominal Aortic Aneurysm Screening: Men and Women by Age Group in the Chichester Trial*.
| Age, years | 65 | 66–70 | 71–75 | 76–80 |
|---|---|---|---|---|
| Men Accepted Screening, % | 80.5 | 76.3 | 73.6 | 66.2 |
| Women Accepted Screening, % | 72.7 | 68.7 | 66.3 | 58.3 |
Scott (16)
The Viborg trial assessed the acceptability of screening in the screening population. (29) They found that screening acceptance rates decreased with age from 81.1% in men aged 65 years to 65.1% in men aged 73 years. Men with existing cardiovascular conditions (cardiac, pulmonary, or peripheral vascular disease) had higher screening program attendance (85%) compared with the 69% overall screening acceptance rate in the trial.
In the MASS trial, (30) older age was associated with lower screening acceptance rates. Comparing age at randomization, those aged 70 to 74 years were less likely than those aged 65 to 69 years to accept screening (79% versus 81% OR, 0.92; 95% CI, 0.87–0.97), and were also less likely to comply with follow-up (79% versus 84% OR, 0.70; 95% CI, 0.52–0.94). Older people (70–74 years) also had a higher prevalence of AAA (6% versus 4% OR; 1.50, 95% CI, 1.34–1.68), suggesting that those at greater risk for AAA rupture were also less likely to attend and comply with the screening program.
Study investigators estimated socioeconomic status using a census-derived social deprivation score created from postal codes from the 1991 census, ranked within the 8,414 wards in England, and treating the score as a quartile variable based on the hypothesis that people at lower socioeconomic levels are less likely to attend screening. They found that lower social deprivation scores were associated with lower rates of screening acceptance in comparison to the highest social deprivation quartile (Q4, 75% vs. Q1, 85%); less compliance for follow-up (Q4, 80% versus Q1, 83%), and higher prevalence rates of AAA (Q4, 6 % vs. Q1, 4%). In addition, health outcomes related to AAA screening were of little or no benefit for refusers compared with those who accepted screening. Those who were invited to and refused screening had no benefit in terms of AAA outcomes. People who refused the invitation to screening did not have any better outcomes in comparison to the control arm of the trial, and the refusers group did not exhibit the same benefits from screening as the screened group. In comparison to the invited group who accepted screening, there was no improvement in ruptured aneurysms (OR, 1.00; 95% CI, 0.67–1.47 vs. OR, 0.53; 95% CI, 0.41–0.69), for AAA-attributable deaths (OR, 0.88; 95% CI, 0.58–1.34 vs. OR, 0.40; 95% CI, 0.30–0.54) and all-cause mortality rates (46.4/1000 person-years vs. 24.1/1000 person-years).
Although the Perth trial did not include detailed information regarding characteristics associated with acceptance rates, study investigators included a breakdown of results stratified by age groups and acceptance of invitation to screening. (15) In men aged 65 to 74, all 11 (100%) screening group deaths were from those who refused screening. In men aged 75 to 83, 13 of the 20 (65%) screening group deaths were attributed to refusers.
Does Repeated Population-based Screening for AAA of Those Found To Have No AAA in an Initial Screen Decrease Health Outcomes?
The Chichester screening trial and a British screening program conducted analyses to determine if repeated screening is needed for people in which no AAA is found on initial ultrasound scan. Evidence from these studies suggests that a single ultrasound screening is sufficient to exclude future risks of AAA ruptures and AAA-attributable death.
Results from the population-based screening program (31) in Gloucestershire, England monitored a cohort of 223 65-year-old men with initial negative ultrasound scans for AAA for follow-up with repeat ultrasound scans. At 12 years follow-up, 86 had men died from causes not related to AAA, 8 men were lost to follow-up, and none of the 129 men remaining were found to have a clinically significant increase in aneurismal diameter over the 12-year follow-up.
In the Chichester trial, (32) 1,011 men aged 65 to 80 with an aortic diameter of less than 3.0 cm on initial scan were followed-up for 10 years. After the 10-year follow up, the incidence for new aneurysms was 4%, and none of the aneurysms was larger than 4.0 cm.
Similarly, results from the ADAM study (9) and an United Kingdom study (33) indicate that a single scan for ultrasound screening of AAA is sufficient.
Small Aneurysms
Case Management of Small Aneurysms Detected Through Screening
Immediate repair of aneurysms that measure 3.0 to 3.9 cm in diameter is generally not considered an option due to the rare risk of rupture. Continued surveillance of AAAs 3.0 to 3.9 cm is recommended in general practice. (34)
Treatment consensus for aneurysms between 4.0 and 5.4 cm in diameter has not yet been reached. Thus, there is no agreement on whether they should be managed with early surgical repair or if surveillance would be more appropriate to avoid unnecessary risk of operative morbidity and mortality. Early surgical repair may be advantageous to avoid ruptures at small diameters, and based on the assumptions that the patient will be younger, have fewer contraindications to surgical repair, have lower mortality rates, and fewer surgical complications than if surgery were delayed to an older age. Given that rates of operative mortality for elective repair are 1% to 5% in referral centers and 4% to 8% in community settings, (8;35) it may also be argued that early surgical repair may pose greater risks to patients than repeated surveillance of the aneurysm until the aneurysm reaches a diameter of 5.5 cm.
Two clinical trials (9;25;36) randomized patients with small aneurysms (4.0–5.4 cm) to receive either early surgical repair or repeated surveillance found no differences in survival between the groups (Appendix 9). Both studies used measures of AAA-attributable mortality and all-cause mortality to determine survival. Specifically, the ADAM trial (9) found no difference between groups in survival for either all-cause mortality (OR, 1.21; 95% CI, 0.95–1.54) or AAA-attributable mortality (OR, 1.15; 95% CI, 0.56–1.77). Similarly, the UK Small Aneurysm Trial (25) found no difference between the early surgery and surveillance groups in AAA-attributable morality (OR, 0.69; 95% CI, 0.44–1.07) and little difference for all-cause mortality (OR, 0.83; 95% CI, 0.69–1.00). In the meta-analysis, there was no significant difference between groups in survival for either AAA-attributable mortality (OR, 0.77; 95% CI 0.54–1.12) or all-cause mortality (OR, 0.99; 95% CI, 0.66–1.48). (See Appendices 10 and 11.)
Targeted Screening for High-Risk Groups
Traditional risk factors for AAAs include age, male sex, cardiovascular risk factors, smoking, and diabetes (Appendix 12). (26) Two studies derived multivariate analysis on risk factors for AAAs (Appendix 13). (9;15) Risk factors based on multivariate odds ratios for aneurysms greater than 4.0 cm from a study of 126,696 American veterans included age for each 7-year interval (OR, 1.65; 95% CI, 1.53–1.78); male sex (OR, 5.00; 95% CI, 1.47–14.3); family history of AAA (OR, 1.95; 95% CI, 1.56–2.43); history of smoking (OR, 5.57; 95% CI, 4.1–7.31); coronary artery disease (OR, 1.62; 95% CI, 1.41–1.84); high cholesterol levels (OR, 1.54; 95% CI, 1.31–1.80). Significant inverse risks for AAA were deep venous thrombosis (OR, 0.67; 95% CI, 0.50–0.88); diabetes mellitus (OR, 0.67; 95% CI, 0.50–0.88), and black race (OR, 0.49; 95% CI 0.35–0.69). (9)
Age, sex, and history of smoking are the most significant risk factors in identifying populations at higher risk for AAA.
Screening Based on Smoking History
Smoking is the most significant risk factor for AAA.(9) The prevalence of AAA by age and smoking history in one study found that the prevalence of AAAs was higher for people who were older and those had a history of smoking (Table 7). History of smoking was defined as 100 cigarettes or more smoked in a lifetime. The prevalence of AAAs greater than 3.0 cm in diameter in people who had smoked was 5.1%; in people who had never smoked, it was 1.5% (OR, 3.6; 95% CI, 3.3–4.0).
Table 7: Prevalence of Abdominal Aortic Aneurysms 4.0 cm or Larger by Age Group and Smoking History*.
| Age, Years |
Patients Who Never Smoked, No. |
Prevalence of AAA, % |
Patients Who Ever Smoked, No. † |
Prevalence of AAA, % |
|---|---|---|---|---|
| 50–54 | 1,152 | 0.0 | 4,359 | 0.3 |
| 55–59 | 1,481 | 0.0 | 5,819 | 0.9 |
| 60–64 | 2,985 | 0.2 | 11,119 | 1.5 |
| 65–69 | 4,198 | 0.5 | 14,129 | 1.9 |
| 70–74 | 4,679 | 0.5 | 13,008 | 2.5 |
| 75–79 | 2,544 | 0.8 | 5,669 | 2.7 |
From the ADAM trial; (9) AAA indicates abdominal aortic aneurysm.
Ever smoking defined as 100+ cigarettes per lifetime
The USPSTF modeled the impact of an invitation to screening based on smoking status and the data provided from the 4 population-based screening trials (Appendix 14). (5) AAA prevalence was estimated at 5.1% in the overall population, the prevalence for smokers was 6.4%, and the prevalence for never-smokers was 1.8%. The USPSTF used the pooled odds ratio from its meta-analysis to derive the reduction in AAA-related mortality assuming that both smokers and non-smokers would benefit equally from the invitation to screening. Predicting outcomes through using an invitation to screen based on a history of smoking would detect about 89% of prevalent AAAs. Results indicated the number needed to screen (NNS) to prevent one aneurysm-attributable death was 500 for men who have ever smoked, 1,783 for never-smokers, and 645 for the entire cohort. Using United States census data, they found, as predicted, an estimated reduction of 89% in aneurysm deaths attributable to smoking.
Using similar methods to the USPSTF, the Medical Advisory Secretariat modeled the impact of screening based on smoking status using assumptions based on a meta-analysis of the population-based screening trials combined with Ontario-specific population estimates from the Ministry of Finance (37) and Canadian estimates from the National Population Health Survey for sex-adjusted ever-smoking prevalence. (38) (See Table 8.) Results showed that the NNS for ever-smokers was 288; for never-smokers, it was 1,024. These NNS are comparable to the NNS in Ontario mammography screening programs for breast cancer in women (NNS60-69 = 695 and NNS50-59 = 1,532) and the NNS for colon cancer (NNS = 808). (39)
Table 8: Results of Modeling a Hypothetical Cohort in Ontario of Males Aged 65 to 75 (N = 413,500)*.
| Results (Assumptions) (38) |
Ever-Smokers (80.1%) |
Non-Smokers (19.9%) |
|---|---|---|
| Population of males aged 65–74 in Ontario (history of smoking prevalence 80.1%, 19.9%) | 331,214 | 82,286 |
| Total number of AAAs | ||
| (AAA prevalence 6.4%, 1.8%) | 21,198 | 1,481 |
| Number of AAA-attributable deaths in no-screen group | ||
| (0.72/1000 person-years) | 1,526 | 107 |
| Number of AAA-attributable deaths in screening group | ||
| (Odds ratio, 0.57) | 2,678 | 187 |
| Number of deaths prevented | 1,151 | 80 |
| Number needed to screen to prevent 1 AAA-attributable death | 288 | 1,024 |
Medical Advisory Secretariat; AAA indicates abdominal aortic aneurysm.
Establishing a targeted screening program based on history of smoking has been recommended in the Canadian literature and by the USPSTF. (5;40)
Screening Women
The Chichester trial from the United Kingdom was the only study to include women in an AAA screening trial. (16) The Chichester trial randomized women aged 65 to 80 years (N = 9342) to either an invited-screening group or a control group. Of the women invited to screening, 65% accepted, compared with 73% of men (P < .0001). The prevalence of AAAs in screened women was 1.3%; in men, it was 7.6%, with increased rates at older ages (Table 9).
Table 9: Prevalence of Abdominal Aortic Aneurysms in Chichester Trial by Sex*.
| Percentage with AAA | ||
|---|---|---|
| Age (years) | Men | Women |
| 65 | 5.9 | 0.0 |
| 66–70 | 5.9 | 1.0 |
| 71–75 | 9.0 | 1.8 |
| 76–80 | 9.2 | 1.6 |
| Total | 7.6 | 1.3 |
| AAA, abdominal aortic aneurysm | ||
(41)
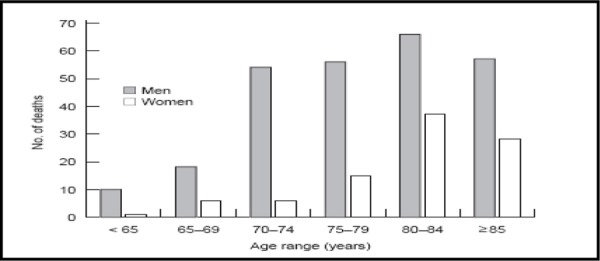
At 5-year follow-up, there were no statistically significant differences between women invited to screening and the control group for AAA-related mortality (OR, 1.0; 95% CI 0.14–7.07) or all-cause mortality (OR, 1.05; 95% CI, 0.9–1.19) in an intention–to-treat analysis. Analysis at 10-year follow-up failed to detect a statistically significant benefit of screening in women. The incidence of AAA ruptures was equal in the screening and control groups (Table 9). (41) Compared with men in the Chichester trial, women had much lower rates of AAA-attributable mortality (Figure 1).
Figure 1: Number of Deaths in the Chichester Trial From Abdominal Aortic Aneurysm Ruptures by Age and Sex in the Control Population*.
(41)
Of note, the Chichester trial had insufficient power to detect a statistically significant effect between screening groups. Due to the low prevalence and event rates of AAAs in women aged 65 to 80 years, a sample size of 350,000 is needed to achieve power; this is the total number of all women age 65 in the United Kingdom (Personal communication, December 2005). However bias may have occurred with regards to an older age-adjusted prevalence of AAA in women, hence skewing the results of the Chichester trial. For example, in men, most deaths from ruptured AAAs occur in those younger than 80 years of age, whereas in women, over 70% of deaths from ruptured AAAs occur after 80 years of age, which may in turn affect the age cutoffs for screening. (5) Therefore, by choosing to screen women based on age-adjusted prevalence in males, this may underestimate the effectiveness if a screening program in women, by missing AAAs prior to their development.
Level 4a Evidence: Surveillance Data in Ontario
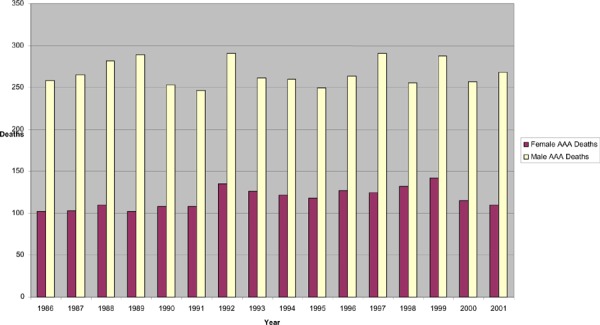
Although the incidence of AAA is much lower in women than in men, (41) in Ontario, women account for one-third of all AAA-related deaths (Figure 2). Discharge data from the Ontario Population Health Planning Database indicate that the case-fatality rates of women admitted to hospital are significantly higher than AAA case-fatality rates in men. (Table 10) Recent case-fatality rates from discharge data indicate that women’s case-fatality rates from ruptured aneurysms are about 65%, whereas men’s case-fatality rates for ruptured aneurysms are about 50% (OR 2002-2004, 2.05; 95% CI, 1.56–2.69). Mortality and case-fatality estimates in Ontario differ from the expected case-fatality rates based on prevalence data in the literature. It is expected that women would have one-quarter to one-sixth of all aneurysms; hence, AAA-attributable deaths based on estimates of AAA prevalence would be expected to mirror the prevalence at 0.5% to 1.5%, versus the prevalence in men of 4% to 8%. Factors for an unexpectedly higher estimate of women’s AAA-related mortality may include the following: that increased mortality rates are a surrogate for increasing prevalence rates; that there is a physiological difference in women that accounts for higher AAA-attributable mortality rates; or that some women’s aneurysms are not detected or treated as promptly as they are in men.
Figure 2: Deaths Attributable to Abdominal Aortic Aneurysms by Sex in Ontario, 1996–2001*.
Medical Advisory Secretariat; AAA indicates abdominal aortic aneurysm.
Table 10: Case Fatality Rates for Ruptured Abdominal Aortic Aneurysms by Sex in Ontario 2002-2004*.
| Fiscal Year | Female Case Fatality Rate, % (n/N) |
Male Case Fatality Rate, % (n/N) |
Odds Ratio (95% Confidence Interval) |
|---|---|---|---|
| 2002 | 65.5 (76/116) | 50.4 (169/335) | 1.87 (1.20–2.89) |
| 2003 | 64.2 (88/137) | 50.4 (176/349) | 1.77 (1.17–2.65) |
| 2004 | 72.1 (75/104) | 48.0 (141/294) | 2.81 (1.73–4.56) |
Medical Advisory Secretariat
No study to date has primarily examined the natural history of disease in women. However, there is a general consensus that there is delayed onset of disease for AAA in women (Personal communication, September 2005). In the Chichester trial, most of the deaths in men from ruptured AAAs occurred in those younger than 80 years of age, whereas in women, over 70% of deaths from ruptured AAAs occurred after 80 years of age, which may in turn affect the age cutoffs for screening. (5) Coupled with the findings in the small aneurysm trials that AAAs in women rupture earlier and at smaller diameters than in men, this may also have an impact on the age intervals for screening. (9;25)
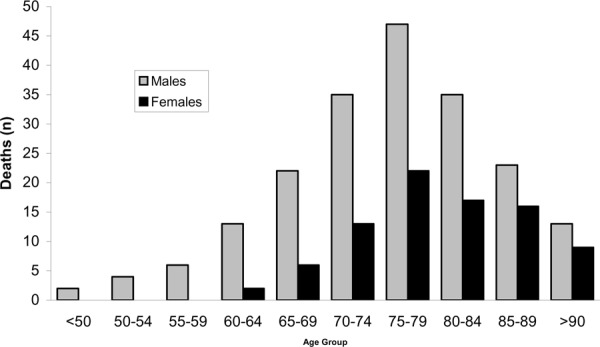
Vital statistics data from the Ontario Population Health Planning Database also indicate that there is a delayed onset of AAA rupture in women (Figure 3). Although deaths from AAA rupture peaked at 75 to 79 years of age for men and women, the incidence of AAA-attributable deaths indicate that the age of onset is later in women. Thus, the age-adjusted mortality and incidence rates of AAA rupture in women may require setting the age of screening for an AAA screening program later in this population to screen for aneurysms at an age and size when the aneurysms are detectable.
Figure 3: Age of Death from Ruptured AAA in Ontario by Age and Sex 2001*.
AAA indicates abdominal aortic aneurysm; data from PHPDB Vital Statistics
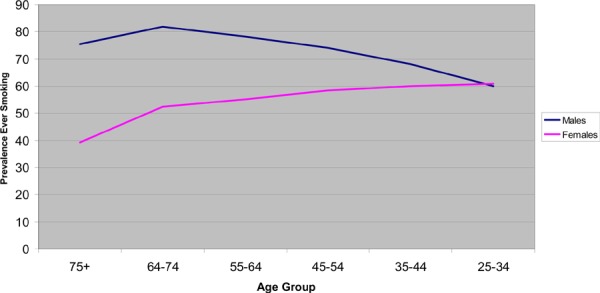
As noted, the relationship between smoking and incidence of AAAs has been established. The increased risk for AAAs associated with smoking is 6-fold. Prevalence rates for history of smoking are lower for women aged 65 to 74 years than for men aged 65 to 74 years (52.4% versus 81.9%) (Figure 4). (38) Given that future prevalence rates of smoking are projected to increase for women and decrease in men, due to the “smoking cohort” in women a hypothesis suggesting a potential increase in AAA prevalence may occur in the future. If indeed higher smoking rates among younger generations of women increase the prevalence of AAA, then this may require monitoring for screening policy and programming implications.
Figure 4: Smoking Rates Among Females in Canada*.
Statistics Canada (38)
Harm of Screening
Physical Harm of Screening
An ethical dilemma presents itself with treatment of AAA. Regardless of which cut-off points are chosen either to proceed under continued surveillance or to opt for elective surgery, some AAAs under surveillance will rupture, and some patients undergoing elective surgery will die from operative mortality for an AAA that never would have ruptured if left untreated. (24) This is in addition to the potential harms of morbidity associated with surgical repair of AAAs.
The rates of physical harm associated with the repair of large aneurysms vary between and within hospitals, surgical specialty, surgeon volume, and hospital volume. The lowest rates of surgical mortality are associated with experienced surgeons who have performed a high volume of AAA repairs in a high-volume hospital. (42) Data from the United States hospital administrative database examined surgical outcomes for intact AAA repair in 16,450 patients from 1994 to 1996. (5) Higher mortality rates were associated with older people (OR,70-79 1.8; 95% CI, 1.4–2.3; OR,>79 3.8; 95% CI, 2.9–4.9). (43) Additionally, in-hospital mortality was associated with being female (OR, 1.6; 95% CI, 1.3–1.9), preoperative renal failure (OR, 9.5; 95% CI, 7.7–11.7), and having more than 3 preoperative medical conditions (OR, 11.2; 95% CI, 3.6–35.4).
In the 4 screening trials, operative mortality for elective surgery ranged from 0% to 6%, with a weighted mean of 6%, indicating a relatively low risk of death (Table 11).
Table 11: Mortality Rate Owing to Elective Surgical Repair of Abdominal Aortic Aneurysms*.
| MASS | Viborg | Chichester Men | Chichester Women | Perth, Australia |
|
|---|---|---|---|---|---|
| Elective Surgery Mortality Rate, % | 6 | 4.3 | 6 | 0 | 0 |
Pooled estimate: 6%
Data from the small aneurysm screening trials indicated that mortality from ruptured aneurysms was relatively rare. It ranged from 1.9% in the ADAM trial to 4% in the United Kingdom Small Aneurysm Trial (Table 12).
Table 12: Mortality Rate Owing to Ruptured Abdominal Aortic Aneurysms During Surveillance in Small Aneurysm Screening Trials (4.0– 5.4 cm)*.
| United Kingdom Small Aneurysm Trial |
ADAM | |
|---|---|---|
| Rate of rupture during surveillance, % | 4.0 | 1.9 |
Pooled estimate: 3%
In the ADAM trial, (9) types of harm associated with elective surgical repair or surveillance included reoperation, myocardial infarction, amputation, paraplegia, stroke, pulmonary embolism, dialysis, late graft failure, and rehospitalization. The surveillance group had a higher risk of myocardial infarction but had lower rates of hospitalization (Table 13).
Table 13: Types of Harm Associated With Surveillance or Immediate Repair of Abdominal Aortic Aneurysms Measuring 4.0– 5.4 cm in the ADAM Trial*.
| Type of Harm | Immediate Repair | Surveillance |
|---|---|---|
| Reoperation required, no. | 9 | 4 |
| Myocardial infarction, no. | 5 | 13 |
| Amputation, no. | 2 | 2 |
| Paraplegia, no. | 0 | 2 |
| Stroke, no. | 3 | 2 |
| Pulmonary embolism, no. | 4 | 1 |
| Dialysis, no. | 1 | 2 |
| Late graft failure, no. | 2 | 1 |
| Rehospitalization, no. | 108 | 56 |
| Any complication, no. | 275 | 193 |
From the ADAM trial. (36)
Psychological Harm of Screening
Screening program evaluations traditionally evaluate program effectiveness in terms of morbidity, mortality, and burden of the disease avoided. However, screening programs should also evaluate the psychological impact of screening in terms of quality of life (QoL). The negative psychological consequences of AAA screening can include identifying the possibility of having serious disease without symptoms, considering harms of treatment, mortality associated with surveillance and elective repair, and reacting to undergoing changes to lifestyle in accordance to general health screening for cardiovascular risk. (44)
Seven case-control studies based on samples of other study populations examined QoL with respect to AAA screening and surveillance: 2 screening trials, 3 small aneurysm trials, and 2 screening programs (Appendix 16). Results suggested there is no significant long-term psychological harm associated with population-based screening for AAA.
The MASS trial (17) examined QoL using the SF-36 scale in samples of the study population: 599 who had AAAs greater than 3.0 cm, 631 who had a negative screen, and 727 control subjects. Those screened and found to be positive for an AAA had slightly higher anxiety scores (P = .02), no difference in depression scores (P = .09), and lower scores on the SF-36 mental (P = .003) and physical (P = .0003) scales at 6 weeks post-screening compared with those who were screened and found to be negative for AAA. Results for all study participants invited to screening were within group population norms. Results in the control population were not reported. Those undergoing surgery had lower SF-36 mental health scores at 3-month follow-up (P = .004), but not at 12 month follow-up compared with their baseline scores. Surgery was associated with better self-rated health at 3-month (P = .0003) and 12-month (P = .007) follow-up.
The Viborg trial (44) measured QoL using the Screen QL scale in 231 control subjects and 271 people in the invited-to-screening group at screening and then at 1 month after screening. People that screened positive for AAAs scored significantly lower on the health and sum QoL measures. Undergoing surgery was associated with higher psychosomatic distress scores in this group compared with those under surveillance for AAA expansion, but there were no differences between groups after surgery. Scores were significantly lower for those invited to screening before they had the scan, compared with after the scan. This could have reflected anxiety about attending AAA screening or relief when no AAA was identified.
A screening program (45) in Gloucestershire, United Kingdom, studied 161 participants before screening and at 12 months after screening using the General Health Questionnaire, which measures anxiety and depression, and the linear analogue anxiety scale. No differences between the invited and control groups were found at baseline or at follow-up on the anxiety scores from the General Health Questionnaire. However, both groups showed significant reductions in anxiety scores based on the General Health Questionnaire after screening.
In the ADAM trial, (46) 1,136 patients randomized either to early surgical repair or to surveillance were followed-up for about 5 years. Quality of Life was measured using the SF-36 scale. The early repair group had higher scores for general health (P < .001), but more people in the early repair group became impotent after treatment than did people in the surveillance group (P < .03). Additionally, maximum physical activity level was not statistically significantly different between groups at baseline, but it decreased significantly over time in the repair group (P < .02).
The UK Small Aneurysm Trial (47) administered the SF-36 scale to all study participants (N = 1090). At baseline there were no significant differences between the early repair and surveillance groups. At 12-month follow-up, patients in the early repair group reported significant improvement in self-rated health and lower body pain scores compared with the surveillance group.
A cross-sectional case-control comparison (48) was undertaken of men aged 65 to 83 years from the Perth screening trials using the Medical Outcomes Study Short Form-36, EuroQoL EQ-5D and Hospital Anxiety and Depression Scale. They were also asked about quality of life. The 2,009 men who attended AAA screening completed a short prescreening questionnaire about their perception of their general health. Twelve months after screening, 498 men (157 with an AAA and 341 with a normal aorta) were sent 2 questionnaires to complete, one for them, and one for their partners. Each addressed QoL life of the respondent. Men with an AAA were more limited in performing physical activities than those with a normal aorta (t-test of means, P = .04). After screening, men with an AAA were significantly less likely to have pain or discomfort than were those with a normal aorta (multivariate odds ratio, 0.5; 95% CI, 0.3–0.9), and they reported fewer visits to their doctor. The mean level of self-perceived general health increased for all men from before to after screening (from 63.4 to 65.4). Apart from physical functioning, screening was not associated with decreases in health and well-being. On average, a high proportion of men rated their health over the year after screening as being either the same or improved, as evidenced by the increase in mean level of self-perceived general health for all men from before to after screening (from 63.4 to 65.4) regardless of whether or not they were found to have an AAA.
Twenty-four patients with screening-detected AAA and 45 controls with aortas of a normal diameter were studied in a prospective, controlled, population-based study from a sample of a screening program in Sweden.(49) Prior to and 12 months after AAA screening, all participants completed the Short-Form 36. At 12 months, 10 AAA-specific questions were added. Findings suggested that screening for AAA results in impairment of QoL among those who have the disease and had a low QoL before screening. Lower levels of physical functioning (P < .03), social functioning (P < .05) and mental health scores (P < .02) and in the mental health cluster (P = .003) were reported for people in the AAA group. However, the decrease in the mental health cluster scores within the AAA group was all owing to 6 patients with a low baseline score (a scale score within the 25th percentile in at least 4 scales). Among those who had an age-adjusted normal QoL prior to screening and who were found to have the disease, and among those who were found to have normal aortas, no negative effect on QoL was observed. Thus, low QoL before screening may be a risk factor for negative mental effects of diagnosing an AAA by screening.
Summary of Medical Advisory Secretariat Review
Population-based ultrasound screening is effective in men aged 65 to 74 years at reducing the incidence of AAA ruptures and the rates of emergency surgical repair for AAA, and AAA-attributable mortality (Grade 1B).
Screening acceptance rates decline with increasing age and are lower for women. Low acceptance rates may affect the effectiveness of a screening program (Grade 1B).
A one-time screen is sufficient for a population-based screening program with regard to initial negative scans and development of large AAAs (Grade 1B).
There is no improvement in mortality outcomes for people who have early elective surgical repair compared with those who undergo surveillance for small aneurysms (4.0–5.4 cm). Therefore, conservative treatment of repeated surveillance of small aneurysms is recommended (Grade 1B).
Targeted screening based on smoking history has been found to detect 89% of prevalent AAAs and increase the efficiency of screening programs from statistical modeling data. Smoking is the biggest risk factor for developing AAAs, in particular, large AAAs (Grade 1A).
Few studies have examined the effectiveness of AAA screening programs for women. There is evidence suggesting that screening women for AAA should be considered with respect to mortality and case-fatality rates in Ontario. However, questions are unanswered with respect to a delayed age of onset for AAAs and the potential harms of screening and treatment at a later age. It is important that further evaluation of AAAs in women occur to determine if screening for AAA is appropriate and, if so, what the optimal age is to screen women (Grade 2B).
There is a small risk of physical harm from screening. Elective surgical repair is associated with a 6% operative morality rate, and about 3% of small aneurysms may rupture during surveillance. Additionally, less than 1% of aneurysms will not be visualized on initial screening and a may require another screen, potentially causing harm to the patient. These risks should be communicated through an informed consent process prior to screening (Grade 1B).
There is little evidence of psychological harm associated with screening (Grade 2C).
Economic Analysis
Notes & Disclaimer
The Medical Advisory Secretariat uses a standardized costing methodology for all of its economic analyses of technologies. The main cost categories and the associated methodology from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative (OCCI) cost data is used for all program costs when there are 10 or more hospital separations, or one-third or more of hospital separations in the ministry’s data warehouse are for the designated International Classification of Diseases-10 diagnosis codes and Canadian Classification of Health Interventions procedure codes. Where appropriate, costs are adjusted for hospital-specific or peer-specific effects. In cases where the technology under review falls outside the hospitals that report to the OCCI, PAC-10 weights converted into monetary units are used. Adjustments may need to be made to ensure the relevant case mix group is reflective of the diagnosis and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the Medical Advisory Secretariat normally defaults to considering direct treatment costs only. Historical costs have been adjusted upward by 3% per annum, representing a 5% inflation rate assumption less a 2% implicit expectation of efficiency gains by hospitals.
Non-Hospital: These include physician services costs obtained from the Provider Services Branch of the Ontario Ministry of Health and Long-Term Care, device costs from the perspective of local health care institutions, and drug costs from the Ontario Drug Benefit formulary list price.
Discounting: For all cost-effective analyses, discount rates of 5% and 3% are used as per the Canadian Coordinating Office for Health Technology Assessment and the Washington Panel of Cost-Effectiveness, respectively.
Downstream cost savings: All cost avoidance and cost savings are based on assumptions of utilization, care patterns, funding, and other factors. These may or may not be realized by the system or individual institutions.
In cases where a deviation from this standard is used, an explanation has been given as to the reasons, the assumptions and the revised approach.
The economic analysis represents an estimate only, based on assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied for the purpose of developing implementation plans for the technology.
Literature Review: Objectives and Methods
The Medical Advisory Secretariat did a cost analysis of ultrasound screening for AAA. Previous health technology assessments and the peer-reviewed literature were searched using the keywords listed in the methods for the literature review.
Results of Literature Review on Economics
The Medical Advisory Secretariat found 5 studies that did an economic analysis of screening for AAA. The studies measured cost-effectiveness and economic impact of AAA screening for different approaches to screening and using different economic analysis methods (Appendix 17). Scenarios for screening in women were discussed in only one study. The other studies focused on older male populations.
Wanhainen et al. (50) developed a simulation model to evaluate cost-effectiveness strategies for AAA screening in men. Using a Markov model, they analyzed screening strategies for screening at ages 60, 65, and 70 years, with screening or rescreening after 5 or 10 years after negative results from initial screening to determine the number of life-years gained. In addition, screening for higher-risk groups including smokers, siblings of AAA patients, patients with angina or claudication, and those with popliteal aneurysm were analyzed. Findings indicated that screening 65-year-old men once ($8,309 US/life year gained [LYG]) and screening 60-year-old men with another screen after 5 years if they had a negative scan the first time ($11,648 US/LYG) were each found to be cost-effective. These were comparable to cost per LYG in other population-based screening programs such as breast cancer, cervical cancer, and hypertension. However, the study’s authors noted that screening at 60 years old with another screen 5 years later would have the advantage in terms of LYG with low additional costs per LYG.
The authors of one economic analysis from the Netherlands (51) based their analysis on assumptions from another screening study from the Netherlands where all eligible men were screened for AAA by their family physician. A Markov model was designed to compare the effects of one-time screening for a cohort of men aged 60 to 65 years with the current no-screen strategy in the Netherlands. Life expectancy for the no-screen group was 16.99 years beyond their current age. The screening groups calculated life expectancy was 17.27 years from their current age. The net increased life expectancy for the men in the screening cohort was 3.5 months or 104 days. The cost of detection and prevention by chance and treatment attempts of ruptured AAA was 196 euros for males in the no-screen group. The cost of mass screening for AAA was 530 euros for each individual. The cost per life year saved (LYS) by screening was 1,177 euros per LYG.
Another economic analysis (52) evaluated a “quick-screen” program for AAAs in comparison to a full conventional scan of the abdomen; it then modeled results for a hypothetical cohort of 70-year-old men undergoing screening and those not undergoing screening. Using the quick-screen method, 25 patients were screened to determine how long and how accurate the quick screen was. The mean time for the quick screen was 4 minutes; the conventional scan took 24 minutes. The accuracy of the quick screen was 100%. Investigators then used a Markov model to determine the cost-effectiveness ratios (CERs) of the traditional full scan and quick-screen methods. The traditional full scan had a CER of $11,215 (US). The quick screen program had a CER of $6,859 (US).
Using data from the Viborg screening trials, another group pf investigators (24) analyzed hospital costs and benefits to determine the number of LYS of an AAA screening program. Direct costs in the study were based on the costs of the patients enrolled in the screening trial. The cost per prevented hospital death was 67,855 (DKK; 5,655 GBP), which investigators found to be equivalent to 7,540 (DKK; 628 GBP) per LYS.
Economic analysis based on data from the MASS trial (53) examined the cost of screening per patient, the cost of follow-up visits, and the costs associated with surgery. The investigators determined cost-effectiveness at 4 years of screening follow-up in addition to projecting longer-term cost-effectiveness at 10 years follow-up. The mean cost per LYG was £28,389 (United Kingdom pounds) at 4 years of follow-up and the 10 year projection for AAA screening cost-effectiveness was £8,000 per LYS. However, given that the trial had only a 4-year follow-up, estimations of accumulating costs and increasing benefits were speculative; therefore, the 10-year cost-effectiveness projections were likely substantially underestimated.
One Canadian study (54) used a multi-state life model and took the direct costs of surveillance from the UK Small Aneurysm Trial and converted into Canadian currency to determine the most cost-effective way to manage AAA. The investigators evaluated cost-effectiveness taking into account the following variables: the diameter of the aorta at the time of elective surgery, detecting the AAA early, selecting a screening population based on risk factors such as sex, age, and smoking status. The most cost-effective diameters for repair of AAAs were 5.5 cm and 7.5 cm. The most-cost effective early detection rate was 0.2/year for latent AAAs, corresponding to a screening interval of 5 years. Lastly, there was no improvement in cost-effectiveness for selective screening by sex or smoking status (Table 14). However, downstream costs of surgeries and repeated follow-up medical visits may not have been fully considered and included in the model, thereby leading to an underestimate of the cost and cost-effectiveness of AAA screening.
Table 14: Cost-Effectiveness: Selective Screening for Abdominal Aortic Aneurysm*.
| Target Population | Age Group, Years | Cost/Quality Adjusted Life Year (Cdn) |
|---|---|---|
| No screening | 50+ | 1,093 |
| Universal screening | 50+ | 741 |
| 65–79 | 864 | |
| Men only | 50+ | 859 |
| 65–79 | 947 | |
| Smokers only | 50+ | 900 |
| 65–79 | 991 |
From Connelly et al. (54)
Budget Impact Analysis
Three options were analyzed for up-front budget impact. A 3-year period was used with repeat screenings for 2 subsequent years for the prevalent cases. It was based on the assumption that the 6.4% prevalence rate for men was also applicable for women. Quick-screen ultrasound screening was chosen because of the shorter time needed to screen each patient and its lower cost. The quick screen was shown to be cost-effective in one economic analysis. (52) Moreover, it is acceptable to health care providers and has high levels of diagnostic accuracy in a screening setting (Personal communication, October 2005). Each ultrasound screening of the aorta includes a technical fee of $32.90 (Cdn) and a professional fee of $20.90 (Cdn). The 3 options are:
Option 1: All males aged 65 to 74 years that have ever smoked (80.1% smoking rate)
Option 2: Males and females aged 65 to 74 years that have ever smoked (80.1% and 46.25% smoking rates, respectively)
Option 3: Universal screening of males and women aged 65 to 74 years
The total budget impact of screening is shown in Figure 5.
Figure 5: Budget Impact of Screening Options (2006–2011)*.
Option 1: All males aged 65 to 74 years that have ever smoked (80.1% smoking rate); Option 2: Males and females aged 65 to 74 years that have ever smoked (80.1% and 46.25% smoking rates, respectively); Option 3: Universal screening of males and women aged 65 to 74 years.
The downstream impact of screening is an immediate increase in the number of AAA surgeries owing to the prevalence in the population. Depending on the timeline for the screening period (3 or 5 years), this eventually decreases to take into account only the incident rate. The downstream costs account for only hospital-based direct costs (OCCI data). Ontario Health Insurance Plan costs have not been factored into these costs. It also assumes that, based on current evidence, 2% of those screened have AAAs greater than 4 cm, and of those with an AAA greater than 5.5 cm, surgery is needed in 100% of cases. For sensitivity analysis, between 50% and 60% of this subset are candidates for surgery, defined as having an AAA greater than 5.5 cm. With screening, the number of urgent cases can be reduced (with ruptured AAAs accounting for 15% or urgent repair and unruptured cases for an additional 15% of urgent surgical repairs), as these would move to elective surgeries. This represents cost savings of between $0.8 million (option 1 low) and $3.0 million (option 2 high). Figure 6 shows the potential increase in the number of surgeries using 2003/2004 as the base year.
Figure 6: Impact of Abdominal Aortic Aneurysm Screening Program in Ontario on Surgical Repair of Abdominal Aortic Aneurysm, 5-Year Estimates: 2006–2011.
Option 1: All males aged 65 to 74 years that have ever smoked (80.1% smoking rate); Option 2: Males and females aged 65 to 74 years that have ever smoked (80.1% and 46.25% smoking rates, respectively)
The budget impact of these additional surgeries has a wide range depending on the option and the period for the screening of the entire cohort under review. (See Table 15 and Figure 7.)
Table 15: Differences in Costs of Elective and Urgent Repair of Abdominal Aortic Aneurysms, Ruptured and Unruptured.
| Surgery Costs (Hospital costs only) |
Elective | Urgent | Difference |
|---|---|---|---|
| Ruptured | $23,311 | $30,157 | $6,846 |
| Unruptured | $17,996 | $23,879 | $5,883 |
Figure 7: Incremental Costs of Surgical Repair of Abdominal Aortic Aneurysm Following Implementation of a Screening Program Based on Repair of Prevalent Cases Across a 5-year Estimate (2006–2011)*.
Option 1: All males aged 65 to 74 years that have ever smoked (80.1% smoking rate); Option 2: Males and females aged 65 to 74 years that have ever smoked (80.1% and 46.25% smoking rates, respectively)
Existing Guidelines for Use of Technology
The technology itself, ultrasound, is in widespread use throughout Canada and internationally. Although there are several screening trials and screening programs for AAA, there are less formal guidelines regarding ultrasound screening for AAA in asymptomatic populations.
Canada
In 1994, the Canadian Task Force in Preventive Health care completed a review of population-based AAA screening. They found ‘insufficient evidence to recommend for or against screening with physical examination or ultrasound, however the prudent physician may choose to include a targeted physical examination for AAA in males over age 60 in the periodic health examination.”
United Kingdom
The United Kingdom National Screening Committee is conducting an analysis of ultrasound screening for AAA. It has set up a working group to appraise the policy implications, and this working group will report by the end of 2005.
United States
The USPSTF found good evidence that screening for AAA and surgical repair of large AAAs (≥5.5 cm) in men aged 65 to 75 years who have ever smoked (current and former smokers) leads to decreased AAA-specific mortality. They also found good evidence that abdominal ultrasonography, performed in a setting with adequate quality assurance (that is, in an accredited facility with credentialed technologists), is an accurate screening test for AAA. Finally, they found good evidence that there are important types of harm associated with screening and early treatment, including more surgery, with associated clinically significant morbidity and mortality, and short-term psychological harm. On the basis of the moderate magnitude of net benefit, the USPSTF concluded that the benefits of screening for AAA in men aged 65 to 75 years who have ever smoked outweigh the harms.
The USPSTF found good evidence that screening for AAA in men age 65 to 75 years who have never smoked leads to decreased AAA-specific mortality. There is, however, a lower prevalence of large AAAs in men who have never smoked compared with men who have ever smoked; thus, the potential benefit of screening men who have never smoked is small. Moreover, there is good evidence that screening and early treatment lead to important types of harm, including more surgery, with associated clinically significant morbidity and mortality, and short-term psychological harm. The USPSTF concluded that the balance between the benefits and harms of screening for AAA is too close to make a general recommendation in this population.
Because of the low prevalence of large AAAs in women, the number of AAA-related deaths that can be prevented by screening this population is small. There is good evidence that screening and early treatment result in important types of harm, including more surgery, with associated morbidity and mortality, and psychological harm. The USPSTF concluded that the harms of screening women for AAA outweigh the benefits.
Society for Vascular Surgery and the Society for Vascular Medicine and Biology
The Society for Vascular Surgery and the Society for Vascular Medicine and Biology recommend screening all men aged 60 to 85 for AAA; women aged 60 to 85 with cardiovascular risk factors; and men and women aged 50 and older with a family history of AAA. (34) These groups further recommend the following courses of action after screening: no further testing if aortic diameter is less than 3.0 cm; yearly ultrasonographic screening if aortic diameter is between 3.0 and 4.0 cm; ultrasonography every 6 months if aortic diameter is between 4.0 and 4.5 cm; and referral to a vascular specialist if aortic diameter is greater than 4.5 cm.
Appraisal/Policy Development
Policy Considerations
Patient Outcomes – Medical, Clinical
Ultrasound screening for AAA is a fast noninvasive procedure that can be performed in a hospital or an outpatient clinic. This health technology policy assessment has shown AAA screening via ultrasound to be effective in decreasing negative health outcomes associated with AAA rupture.
Ethics
Benefits of AAA screening include early identification of a treatable condition that can greatly reduce AAA-attributable mortality of a fatal condition. However, regardless of which cut-offs of aneurysmal diameter are chosen to proceed under continued surveillance or to opt for elective surgery, some AAAs under surveillance will rupture, and others undergoing elective surgery will die from elective surgical mortality for an AAA which never would have ruptured if left untreated. (24)
Ultrasound Screening for AAA in Women
Smaller aneurysms in women may be of more clinical significance than they are in men, as woman have a normal aortic diameter of 14 to 18 mm, compared with men whose normal aortic diameter may be up to 24 mm. (55) A 5 cm aneurysm in a woman stretches the aortic wall to a greater extent, and aneurysms in women rupture more frequently and at smaller diameters. (25) Two Canadian studies (56;57) suggest there is evidence of a gender bias in the literature regarding the diagnosis of AAA and in patient selection for surgical treatment of AAA. For this reason, leaving women out of screening programs may be inappropriate. (55;58) Although there is an inadequate scientific evidence base to support screening women for AAA, ultrasound screening is relatively inexpensive and should be considered for this population taking into account the smaller aortic diameter in women and the later ages at which aortas rupture.
Demographics
Abdominal aortic aneurysm affects more men than women. It becomes more prevalent with age, increasing at age 65 years through to the 80s. Abdominal aortic aneurysm is most prevalent in people with a history of smoking. In addition, family history of AAA is a major risk factor. There are about 331,214 men aged 65 to 74 years in Ontario who have a history of smoking and about 211,825 women aged 65 to 74 years in Ontario who have a history of smoking. Non-smoking males are estimated to number 82,286; non-smoking females, 246,175.
Diffusion – International, National, Provincial
Ultrasound is already a well-diffused procedure. It is common practice throughout the province, Canada, and the world to screen for AAAs using ultrasound. Screening programs are underway in the United States, United Kingdom, Denmark, Sweden, and Australia.
Cost
The present estimates for AAA screening programs are generally considered cost-effective. (50;52) Screening has been found to be cost-effective and increase LYS. In an Ontario-based analysis, using a quick-screen approach (a partial abdominal scan that views only the abdominal aorta,) the impact of screening is high. Despite the initial costs of establishing screening in Ontario, screening results in cost-avoidance of emergency repairs, less morbidity from operative complications, and fewer unnecessary deaths due to ruptured aneurysms, all of which result in long-term cost savings. The cost of each ultrasound for AAA screening in Ontario is $32.90 for the technical fee and $20.90 for the professional fee. The savings from AAA screening result from the cost difference between urgent emergency repair and the lower cost (and associated lower complication and operative mortality rates) of elective surgical repair of AAA. Cost savings translate into $6,826 for each emergency ruptured repair avoided and $5,883 for each unruptured repair avoided when performing surgical repair electively. Lastly, AAA screening compares favourably with cited estimates of $26,00 to $44,000 (US; 2003 values) in terms of LYG for screening programs for cervical cancer, hypertension, and breast cancer, all of which have screening programs in practice in Ontario. (50)
Stakeholder Analysis
The adoption of an AAA screening program in Ontario would require the buy-in of family physicians, ultrasonographers, medical imaging specialists and radiologists, and vascular surgeons. Family physicians would offer AAA screening in practice that could be another procedure ordered during the same time as other routine health checks in the population being screened. An influx of screening would affect the system’s capacity to perform the screening, stressing the need for ultrasonographers to perform the screening, the number of ultrasound machines, and the location in which screening would take place. Using the quick-scan approach, the ultrasound would take less than 10 minutes to do, which would save cost and time compared with the traditional full abdominal scan as a screening test. Radiologists and medical imaging specialists would be asked to assess the results of the ultrasound and send the results to the family physician. However, expert opinion in medical imaging suggests that the impact on workload would be low to interpret the results of the screening ultrasound for AAA (Personal communication, October 2005). However, it is expected that there would be an increase in radiologists’ workload and associated costs with the implementation of an ultrasound-screening program for AAA. Lastly, vascular surgeons would be affected, as they would receive more patient referrals for AAA and would have to do more elective surgical repairs. However, despite the increase in cases for surveillance of small aneurysms and elective repair, urgent and emergency repairs would be avoided, and these are more difficult procedures that result in more operative complications and higher mortality rates (Personal communication, September 2005).
System Pressures
There are substantial system pressures related to AAA screening, including those that pertain to ultrasound screening, patient waiting rooms, ultrasonographers to conduct an ultrasound screen, radiologists and medical imaging experts to interpret the screen, operating room time, availability of hospital beds, and the number of vascular surgeons in the province. There are also pressures associated with follow-up care of patients, including repeated surveillance of small aneurysms. These system pressures relate to the capacity of the current health care system to recruit patients into screening, to have ultrasound machines and diagnostic facilities to accommodate screening of such a large screening population, to have adequately trained ultrasonographers, who are not a licensed body under the Regulated Health Professions Act, to provide high quality images of the abdominal aorta. Introducing screening into a large population would yield a significant number of prevalent and incident aneurysms. This in turn would require monitoring by health care professionals and, if appropriate, early surgical repair, which would increase the workloads of radiologists and vascular surgeons in terms of operating times and costs.
Appendices
Appendix 1: World Health Organization Screening Principles Applied to the Case of Population-Based Abdominal Aortic Aneurysm Screening
| WHO Criteria | Notes |
|---|---|
| Important Health problem | The prevalence of abdominal aortic aneurysms (AAAs) is between 1% and 5.4% in community surveys. (2) However, AAAs are found in 4% to 8% of older men and 0.5% to 1.5% of older women. (5) The incidence of AAAs greater than 3 cm in the general population is about 1.0% to 1.5%. (6) The risk of death from ruptured AAA is 80% to 90%. (2) |
| Accepted Treatment | The evidence base for treatment by size of aneurysm is clear. (25) Early treatment affects prognosis, as AAAs rarely cause symptoms prior to rupture. Mortality in people undergoing elective surgery is only 5% to 7%, compared with the 80% to 90% mortality rate of people with ruptured aneurysms. (13) Open surgical repair (OSR) of AAA is still the gold standard. It is a major operation involving the excision of dilated area and placement of a sutured woven graft for healthy patients with large aneurysms (5–6 cm). (4) Other medical comorbid conditions such as chronic renal failure, chronic lung disease, and liver cirrhosis with portal hypertension, may double or triple the usual risk of OSR, and other modes of treatment are considered. Serial noninvasive follow-up of small aneurysms is an alternative to immediate surgery. Endovascular repair of AAA is the third treatment option and is the topic of another health technology policy assessment and is under field evaluation. |
| Latent or early recognizable stage is recognizable | Ultrasound can reliably visualize the aorta in 99% of people, which enables the identification of an AAA at a size when rupture is unlikely to occur. (13) Through ultrasound, both presymptomatic borderline conditions (3–5 cm) and early clinical conditions (> 5.5 cm) can be identified. |
| Suitable test | Evidence indicates that diagnostic ultrasound is safe for the unborn child, unlike other tests, which are invasive or use ionizing radiation. Ultrasound is an extremely sensitive and specific test for AAAs of all sizes, at least in cases where the diagnosis and size of the aneurysm can be confirmed at surgery. Reported sensitivities range from 82% to 99%, with sensitivity approaching 100% in some series of patients with a pulsatile mass. (14;18) In a small proportion of patients, visualization of the aorta will be inadequate due to obesity, bowel gas, or periaortic disease. |
| Test is acceptable | Four large-population-based randomized controlled trials reported acceptance rates of ultrasonography screening acceptance for AAAs of between 65% and 80%. |
| Natural history of disease understood | An AAA is a localized, abnormal dilatation of the aorta greater than 3 cm or 50% of the aortic diameter at the diaphragm. (1) A true AAA involves all 3 layers of the vessel wall. If left untreated, the continuing extension and thinning of the vessel wall may eventually result in rupture of the AAA. The risk of death from ruptured AAA is 80% to 90%. (2) |
| Agreed policy on whom to treat as patients | No further testing is required for persons with an aortic diameter less than 3.0 cm. Patients with an aortic diameter between 3 and 4.5 cm are invited for surveillance, and patients with a diameter greater than 4.5 cm are referred to a vascular specialist. People with aneurysms greater than 5.5 cm or that have a growth rate of 1 cm/year or greater are referred to elective OSR. (4) |
| Cost of case finding is effective | Cases are found through family physicians’ recommendations for AAA screening. Cost-effectiveness of case finding is maximized for older male populations, including individuals with high risk factors for AAA. Randomized controlled trials and economic evaluations show that AAA screening significantly decreases morbidity and mortality for men aged 65 to 74 years. (13; 15-17) However, analyses for males younger and older than 65–74 and for females do not show significant gains in morbidity and mortality are achieved with AAA screening; therefore, cost-effectiveness is decreased. (15;16) |
| Case finding is a continuous process | Continuing case finding occurs through 2 mechanisms: through age-related recommendations several cohorts will be offered ultrasound screening for AAA; and continuing surveillance of preclinical abdominal aortic aneurysms. |
Appendix 2: Summary of Randomized Screening Trials on AAA Screening*
| Viborg | MASS | Chichester | Perth | |
|---|---|---|---|---|
| Study, year | Lindholt, 1995 (13) | Ashton, 2002 (17) | Scott, 1995 (16) | Norman, 2004 (15) |
| Recruitment | ||||
| Study design | RCT | RCT | RCT | RCT |
| Location | Denmark | United Kingdom | United Kingdom | Australia |
| Sex | Men | Men | Men and Women | Men |
| Age, years | 65–73 | 65–74 | 65–80 | 65–83 |
| Sample size | 12,639 | 67,800 | 15,775 | 41,000 |
| Randomized | ||||
| Randomization | Individual | Individual | Individual | Individual |
| Invited to screening | 6333 | 33839 | 7887 | 19352 |
| Accepted screening, % | 76 | 80 | 68 | 63 |
| Uninvited controls | 6,319 | 33,961 | 7,888 | 19,352 |
| Groups similar at baseline | Yes | Yes | Yes | Yes |
| Outcome Ascertainment | ||||
| Blinded assessors | Yes | No | No | Yes |
| Death registry | Yes | Yes | Yes | Yes |
| Hospital records | Yes | Yes | Yes | Yes |
| Outcome ascertainment, % | 100 | 99 | NR | NR |
| Analysis | ||||
| Sufficient sample size | Yes | Yes | No | Yes |
| Intention to treat analysis | Yes | Yes | Yes | |
| Follow-up (years) | 5 | 4 | 5,10 | 5 |
| Appropriate statistical analysis | Yes | Yes | Yes | Yes |
| Quality | Good | Good | Fair | Fair–Good |
AAA indicates abdominal aortic aneurysm; RCT, randomized controlled trial; NR, not reported
Appendix 3: Results of Trials on Population-Based Screening for Abdominal Aortic Aneurysms
| Viborg | MASS | Chichester Women | Chichester Men | Perth | Meta-analysis Fixed-Effects Model Men 65+ |
|||
|---|---|---|---|---|---|---|---|---|
| Study, year | Lindholt et al., 2005 (13) | Ashton et al., 2002 (17) | Scott et al., 1995 (16) | Scott et al., 1995 (16) | Norman et al., 2004 (15) | Medical Advisory Secretariat, 2005 | ||
| Randomized | 12,639 | 67,800 | 9,342 | 6,433 | 41,000 | |||
| Invited to screening, no. | 6,333 | 33,839 | 4,682 | 3,205 | 19,352 | |||
| Accepted screening, no. | 4,852 | 27,147 | 3,052 | 2,342 | 63% | |||
| Screened, % | 76.6 | 80 | 65 | 73 | 63 | 72 | ||
| AAAs in screened, no. (%) | 191 (4.0) | 1333 (4.9) | 40 (1.3) | 178 (7.6) | 875 (7.2) | 5.5 | ||
| Uninvited controls, no. | 6,319 | 33,961 | 4,660 | 3,228 | 19,352 | |||
| Duration of follow-up, yrs | 5.75 | 2.9–5.2 | 5 | 5 | 2.5 | |||
| AAA-specific mortality | ||||||||
| Invited, no. (%) | 6 (0.09) | 65 (0.19) | 2 (0.04) | 10 (0.31) | 18 (0.09) | |||
| Controls, no. (%) | 19 (0.30) | 113 (0.33) | 2 (0.04) | 17 (0.36) | 25 (0.13) | |||
| OR (95% CI) | 0.31 (0.13–0.79) | 0.58 (0.42–0.78) | 1.00 (0.14–7.07) | 0.59 (0.27–1.29) | 0.72 (0.39–1.32) | 0.57 (0.45–0.74) | ||
| All-cause mortality | ||||||||
| Invited, no. (%) | 939 (0.15) | 3750 (11.1) | 503 (10.7) | 532 (16.6) | 1976 (10.2) | |||
| Controls, no. (%) | 1019 (0.16) | 3855 (11.4) | 476 (10.2) | 508 (15.7) | 2020 (10.4) | |||
| OR (95% CI) | 0.90 (0.82–0.99) | 0.97 (0.93–1.02) | 1.05 (0.92–1.19) | 1.07 (0.93–1.22) | 0.98 (0.91–1.04) | 0.97 (0.93–1.01) | ||
| Emergency Repair | ||||||||
| Invited, no. (%) | 6 (0.09) | 27 (0.05) | 1 (0.02) | 3 (0.09) | 9 (0.05) | |||
| Controls, no. (%) | 30 (0.47) | 54 (0.04) | 1 (0.01) | 8 (0.24) | 8 (0.04) | |||
| OR (95% CI) | 0.20 (0.08–0.48) | 0.50 (0.32–0.80) | 1.00 (0.06–15.93) | 0.38 (0.10–1.42) | 1.13 (0.43–2.92) | 0.46 (0.24–0.88) | ||
| Elective Repair | ||||||||
| Invited, no. (%) | 50 (0.79) | 332 (0.55) | 4 (0.08) | 28 (0.87) | 107 (0.55) | |||
| Controls, no. (%) | 14 (0.22) | 92 (0.28) | 2 (0.04) | 5 (0.15) | 54 (0.28) | |||
| OR (95% CI) | 3.58 (1.98–6.49) | 3.65 (2.89–4.65) | 1.99 (0.36–10.88) | 5.62 2.14–14.73) | 2.02 (1.46–2.80) | 3.18 (2.11–4.79) | ||
| AAA Rupture | ||||||||
| Invited, no. (%) | 4 (0.10) | 65 (0.17) | 3 (0.06) | 8 (0.25) | 33 (0.17) | |||
| Controls, no. (%) | 20 (0.30) | 134 (0.20) | 2 (0.04) | 20 (0.62) | 38 (0.20) | |||
| OR (95% CI) | 0.20 (0.07–0.58) | 0.50 (0.37–0.68) | 1.49 (0.25–8.94) | 0.40 (0.18–0.91) | 0.87 (0.54–1.38) | 0.50 (0.31–0.80) | ||
| Operative Mortality | ||||||||
| Elective repair, % | 6 | 6 | 0 | 0 | 4.3 | 6 | ||
| Emergency repair, % | 39 | 37 | 33 | 25 | 50 | 37 | ||
AAA indicates abdominal aortic aneurysm; CI, confidence interval; OR, odds ratio.
Appendix 4: Meta-Analysis and Pooled Odds Ratio for the Association Between Invitation to Screening and Rupture Incidence in Men Aged 65 Years and Older*
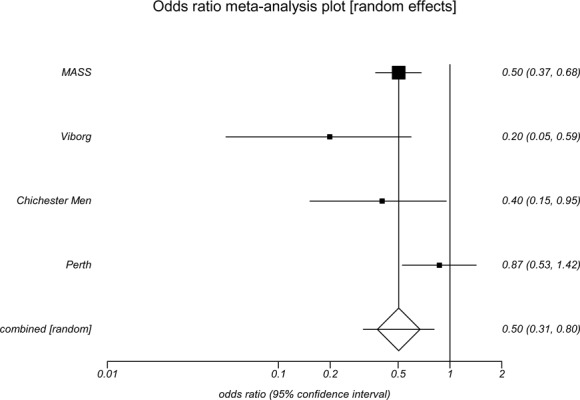
AAA indicates abdominal aortic aneurysm; Medical Advisory Secretariat
Appendix 5: Meta-Analysis and Pooled Odds Ratio for the Association Between Invitation to Screening and Emergency Surgical Repair of AAA in Men Aged 65 Years and Older*
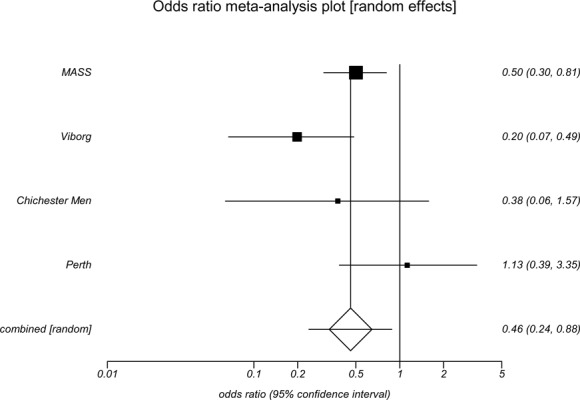
AAA indicates abdominal aortic aneurysm; Medical Advisory Secretariat
Appendix 6: Meta-Analysis and Pooled Odds Ratio for the Association Between Invitation to Screening and Elective Surgical Repair of AAA in Men Aged 65 Years and Older*
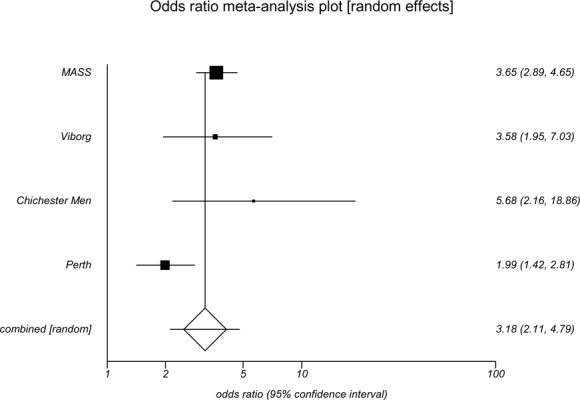
AAA indicates abdominal aortic aneurysm; Medical Advisory Secretariat
Appendix 7: Meta-Analysis and Pooled Odds Ratio for the Association Between Invitation to Screening and AAA-Attributable Mortality for Men Aged 65 Years and Older*
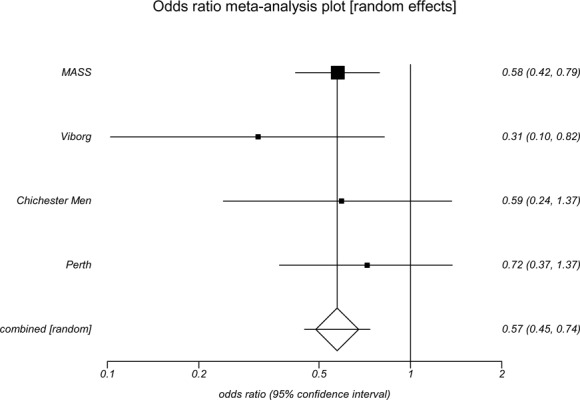
AAA indicates abdominal aortic aneurysm; Medical Advisory Secretariat
Appendix 8: Meta-Analysis and Pooled Odds Ratio for the Association Between Invitation to Screening and All Cause Mortality for Men Aged 65 Years and Older*
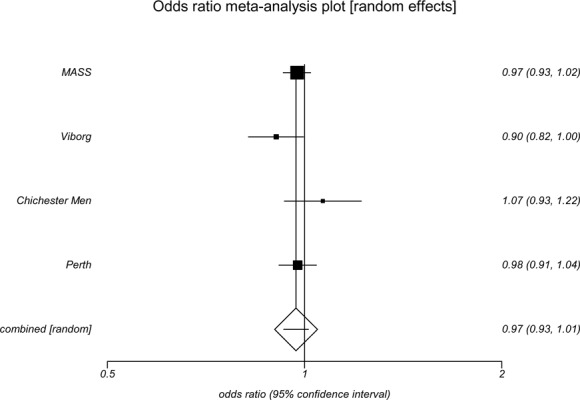
AAA indicates abdominal aortic aneurysm; Medical Advisory Secretariat
Appendix 9: Characteristics of Trials on Small Aneurysms
| ADAM | UKSTAT | |
|---|---|---|
| Study, year | Lederle et al., 2002 (9;36) | UKSTAT, 2002 (25) |
| Recruitment | ||
| Study design | RCT | RCT |
| Location | United States, multi-site | United Kingdom |
| Males, % | 99.2 | 82.8 |
| Age | 50–70 | 60–76 |
| Mean age, years | 69 | 68 |
| Randomized | ||
| Follow-up, years | 4.9 | 8.0 |
| Immediate repair, no. | 569 | 563 |
| Surveillance, no. | 567 | 527 |
| Interventions | ||
| Immediate Repair | 1.5 weeks | 3 months |
| Surveillance intervals | 6 months | 6 months |
| Repair criteria for surveillance | 5.5 cm | 5.5 cm |
| Outcome ascertainment | ||
| Surveillance compliance, % | 87.0 | 93.0 |
| Immediate repair, % | 85.3 | 100 |
| Surveillance, % | 100 | 100 |
| Results | ||
| AAA deaths OR (95% CI) | 1.00 (0.52–1.90) | 0.69 (0.44–1.07) |
| All deaths OR (95% CI) | 1.22 (0.93–1.61) | 0.81 (0.64–1.03) |
| Quality | Good | Good |
AAA indicates abdominal aortic aneurysm; CI, confidence interval; OR, odds ratio.
Appendix 10: Meta-Analysis and Pooled Odds Ratio for the Association Between Immediate Surgical Repair and AAA-Attributable Mortality in Small AAAs*
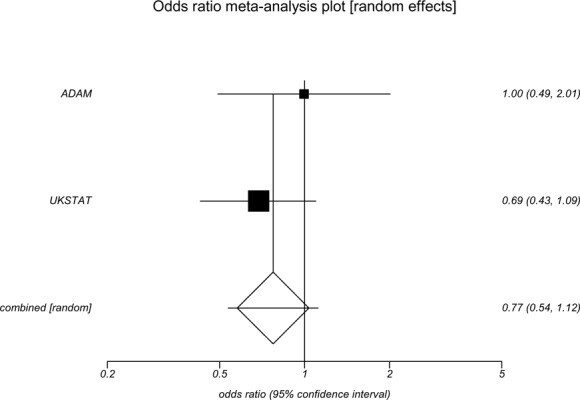
Medical Advisory Secretariat
Appendix 11: Meta-Analysis and Pooled Odds Ratio for the Association Between Immediate Surgical Repair and All-Cause Mortality in Small AAAs*
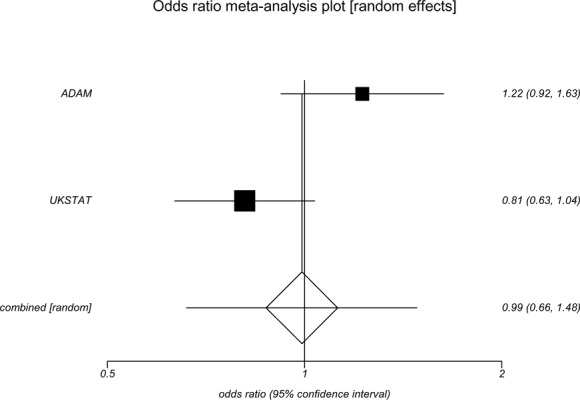
Medical Advisory Secretariat
Appendix 12: Results of Meta-Analysis Risk Factors for Asymptomatic AAA*
| Risk Factor | Number of Studies | Pooled Odds Ratio (95% Confidence Interval) Random-Effects Model |
|---|---|---|
| Sex (male versus female) | 6 | 5.69 (3.36–9.64) |
| History of Myocardial Infarction (yes versus no) | 6 | 2.28 (1.90–2.74) |
| Peripheral vascular disease (yes versus no) | 8 | 2.50 (2.12–2.95) |
| Smoking (yes versus no) | 11 | 2.41 (1.94–3.01) |
| Hypertension (yes versus no) | 9 | 1.33 (1.14–1.55) |
| Diabetes (yes versus no) | 6 | 1.02 (0.81–1.29) |
AAA indicates abdominal aortic aneurysm; Cornuz et al. (26)
Appendix 13: Comparison of Risk Factors for AAA in People With AAA Versus Those Without AAA in Multivariate Models*
| Risk Factor | Perth Trial (27) | ADAM Trial (28) | ADAM Trial (28) |
|---|---|---|---|
| Referent Groups | AAA > 3.0 cm | AAA 3.0–3.9 cm | AAA > 4.0 cm |
| MVOR (95% CI) | MVOR (95% CI) | MVOR (95% CI) | |
| Age | |||
| 65–69 | 1.00 | NR | NR |
| 70–74 | 1.6 (1.3–1.9) | NR | NR |
| 75–79 | 2.1 (1.7–2.6) | NR | NR |
| 80–84 | 2.6 (1.9–3.4) | NR | NR |
| Age (65+) at 7 year intervals | NR | 1.52 (1.45–1.60) | 1.65 (1.53–1.78) |
| Sex (male) | NA | 1.62 (1.06–2.40) | 5.00 (1.47–14.3) |
| Family history of smoking | 1.7 (1.4–5.3) | 1.96 (1.68–3.11) | 1.95 (1.56–2.43) |
| Ever smoked | NA | 2.72 (2.37–3.11) | 5.57 (4.10–7.31) |
| Ex-smoker | 2.3 (1.9–2.6) | NA | NA |
| Current (1–24/day) | 4.7 (3.6–6.1) | NA | NA |
| Current (25+/day) | 6.0 (3.9–9.1) | NA | NA |
| Claudication | 1.4 (1.1–1.8) | 1.39 (1.20–1.62) | 0.96 (0.74–1.25) |
| Deep venous thrombosis | NA | 0.90 (0.76–1.06) | 0.67 (0.50–0.88) |
| History of myocardial infarction | 1.7 (1.4–2.1) | NA | NA |
| Coronary artery disease | NA | 1.42 (1.30–1.55) | 1.62 (1.41–1.84) |
| History of coronary artery bypass | 1.6 (1.3–2.0) | NA | NA |
| Hypertension | NA | 1.25 (1.14–1.37) | 1.16 (1.01–1.32) |
| Hypertension treatment (current) | 1.5 (1.3–1.7) | NA | |
| High cholesterol levels | NA | 1.33 (1.20–1.62) | 1.54 (1.31–1.80) |
| Diet to control high cholesterol | 1.4 (1.1–1.7) | NA | NA |
| Height (per cm/per 7cm/per 7cm) | 1.03 (1.02–1.05) | 1.20 (1.14–1.37) | 1.21 (1.10–1.30) |
| Black race (versus white) | NA | 0.72 (0.59–0.87) | 0.49 (0.35–0.69) |
| Diabetes mellitus | NA | 0.68 (0.60–0.77) | 0.67 (0.50–0.88) |
AAA indicates abdominal aortic aneurysm; MVOR, multivariate-adjusted odds ratio; NA, not applicable; NR, not reported. Waist circumference, waist-to-hip-ratio, and weight not statistically significant in models.
Appendix 14: Prevalence of AAAs 4.0 cm or Larger Detected by Screening in Men*
| Age, Years | Patients Who Never Smoked, No. |
Prevalence of AAA, % |
Patient Who Ever Smoked, No.† |
Prevalence of AAA, % |
|---|---|---|---|---|
| 50–54 | 1,152 | 0 | 4,359 | 0.3 |
| 55–59 | 1,481 | 0 | 5,819 | 0.9 |
| 60–64 | 2,985 | 0.2 | 11,119 | 1.5 |
| 65–69 | 4,198 | 0.5 | 14,129 | 1.9 |
| 70–74 | 4,679 | 0.5 | 13,008 | 2.5 |
| 75–79 | 2,544 | 0.8 | 5,669 | 2.7 |
AAA indicates abdominal aortic aneurysm; from the ADAM trial. (9)
More than 100 cigarettes per lifetime.
Appendix 15: Model of a Hypothetical Cohort for Abdominal Aortic Aneurysm Screening for Men Aged 65 to 74 Years From USPSTF (2005)
| Assumptions* | Baseline | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|
| AAA Prevalence | 5.5% | |||
| Screening Attendance | 72.0% | |||
| AAA-Related Deaths, 1000 person-years | 0.72 | |||
| OR AAA-related death with screening | 0.57 | 0.45 | 0.74 | |
| All-Cause Deaths, 1000 person-years | 28.70 | |||
| OR deaths from all causes | 0.98 | 0.95 | 1.02 | |
| AAA-ruptures | ||||
| No Screening Program | 0.40% | 0.35% | 0.46% | |
| With Screening Program | 0.18% | 0.15% | 0.23% | |
| Elective Surgery | ||||
| No Screening Program | 0.28% | 0.24% | 0.34% | |
| With Screening Program | 0.96% | 0.88% | 1.06% | |
| Emergency Surgery | ||||
| No Screening Program | 0.23% | 0.19% | 0.28% | |
| With Screening Program | 0.11% | 0.08% | 0.14% | |
| Operative Mortality | ||||
| Elective AAA repair | 6% | 6% | 6% | |
| Emergency AAA repair | 37% | 37% | 37% | |
| Results | Baseline | Lower bound | Upper bound | |
| Total AAAs in Cohort | 5,500 | 5,500 | 5,500 | |
| AAA-Related Deaths, n | ||||
| No Screening Program | 359 | 359 | 359 | |
| With Screening Program | 205 | 162 | 266 | |
| AAA Deaths Prevented | 154 | 197 | 93 | |
| NNS to Prevent 1 Death from AAA | 648 | 506 | 1,072 | |
| Deaths from All Causes, n | ||||
| No Screening Program | 13,368 | 13,368 | 13,368 | |
| With Screening Program | 13.119 | 12.744 | 13.616 | |
| All-Cause Deaths Prevented (Caused) | 249 | 624 | (248) | |
| Elective Surgical Procedures, n | ||||
| Invited for Screening | 960 | 880 | 1060 | |
| Not Screened | 280 | 240 | 340 | |
| Emergency Surgical Procedures, n | ||||
| Not Screened | 230 | 190 | 280 | |
| Invited for Screening | 110 | 80 | 140 | |
| AAA Ruotures. n | ||||
| No Screening Program | 400 | 350 | 460 | |
| With Screening Program | 180 | 150 | 230 | |
| AAA Ruptures Prevented | 220 | 200 | 230 | |
| NNS to Prevent 1 AAA Rupture | 455 | 435 | 500 | |
| Deaths. Elective Surgery. n | ||||
| No Screening Program | 17 | 14 | 20 | |
| With Screening Program | 58 | 53 | 64 | |
| Deaths. Emergency Surgery. n | ||||
| No Screening Program | 85 | 70 | 104 | |
| With Screening Program | 41 | 30 | 52 | |
| Total Surgical Deaths, n | ||||
| No Screening Program | 102 | 85 | 124 | |
| With Screening Program | 98 | 82 | 115 | |
CL confidence interval, NNS, number needed to screen.
OR = Odds ratio with screening program compared to no screening program. Estimates of event rates and relative risks incorporate variability across screening trials in prevalence, acceptance of screening, accuracy of screening, and adherence to clinical management protocols for surgery and surveillance.
Appendix 16: Summary of Studies on Abdominal Aortic Aneurysm That Measured Quality of Life
| Reference | Design (N) | Participants | Measure | Results |
|---|---|---|---|---|
| MASS Ashton et al, 2002 | Case-control (males) post screen (6 weeks) | AAA = 599 No AAA = 631 |
SF-36, EuroQOL, SF-Spielberger state anxiety scale | Those with a positive AAA scan were found to have lower scores for SF-36 mental (P = .003) and physical health scores (P = .003), self-rated health (P = .003), no difference in depression scores (P = .09) and had higher anxiety levels (P = .02) in comparison to those who did not have an AAA. All results were within population norms. |
| West Australia Spencer et al, 2004 | Prescreen (males) post screen only (12 months) | Prescreen 2009 AAA = 50, No AAA = 341 |
Hospital anxiety Depression Scale, EuroQoL EQ-5D | Cross-sectional post-screen results found men with AAA had statistically significant (P < .05) lower dimension levels for physical functioning compared with men with no AAA. The men with AAA had less pain and discomfort at follow-up (multivariate odds ratio, 0.5; 95% CI, 0.3–0.9) and fewer hospital visits. All men’s post-screen general health improved regardless of AAA status. |
| Norsjo, Sweden Wanhainen et al, 2004 | Case-control from screening program(males and females) pre and post screen (12 months) | AAA = 27 No AAA = 59 |
SF-36 | There were no significant differences between the AAA and no AAA groups before or after screening. There were significant decreases post-screening for the AAA group in physical functioning (P < .03), social functioning (P < .05) and mental health (P < .02). Results indicate lower levels of QoL prior to screening resulted in lower QoL outcomes after screening. |
| Gloucestershire Trial Lucarotti et al, 1997 | Case-control pre and post screen (1 months) | AAA = 61 No AAA = 100 |
General Health Questionnaire | No significant differences between groups pre and post screen Significant fall (P < .04) in anxiety levels for both groups 1 month after screening |
| UK Small Aneurysm Trial Forbes at el, 1998 | RCT pre-randomization and post screen(12 months) | Early surgery = 391 AAA surveillance = 399 |
SF-36 | Early surgery group had significant self-perceived health improvement Surveillance group had significant decreases in physical functioning, role functioning, social functioning, and bodily pain |
| ADAM Lederle et al, 2003 | Nested case control from RCT prescreen and postscreen at repeated intervals (5 years) | Early surgery = 569 AAA surveillance = 567 |
SF-36, impotence, max activity level | General health improved for the elective group post-screen, mental health was significantly (P < .05) higher immediately post screen but then showed no difference at 12 months and beyond with the surveillance group Physical health(functioning and role) had slight significant(P < .05) improvements in repeated measures for the surveillance group |
| Viborg Lindholt et al, 2000 | Nested Case-control from RCT | Non-attender = 231 At screening = 271 Post screen (1mo) = 286 AAA = 127 AAA surg post surveillance = 29 |
ScreenQL | 1 month after screening, those with AAA had decreased health perception and QOL versus non-invited controls Invited attendees has initial lower scored for emotional health, psychosomatic stress, social, family and marriage roles in comparison to invited non-attendees One month post-screen invited attendees no longer had lower scores than invited non-attainders and surpassed invited non-attendees in psychosomatic distress, self-reported QOL, investigators suggest that there was increased anxiety about attending screening and relief when no AAA was found. |
Appendix 17: Results of Economic Literature Review on Cost-Effectiveness of Screening for Abdominal Aortic Aneurysm
| Study, Year | Subset/Methods | Results |
|---|---|---|
| Boll et al., 2003 (Netherlands) | Males aged 60–65; one-time screen, Markov Model | Cost per life-year gained: $2, 764 (Cdn) |
| Lee at al., 2002 US | Males, high-risk patients, Markov Model | Cost-effectiveness ratio: $11, 215 (US) |
| Lindholt et al., 2002 (Denmark) | Males aged 65–73 | Cost per life-year saved: $1,400 (Cdn) |
| Wanhainenen et al., 2005 (Sweden) | Males, different ages and risks, Markov Model | Cost per life-year gained: $8,309–$14,084 (US) |
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Ultrasound screening for abdominal aortic aneurysm: an evidence-based analysis. Ontario Health Technology Assessment Series 2006; 6(2)
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo@moh.gov.on.ca
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas
Print copies can be obtained by contacting MASinfo@moh.gov.on.ca
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo@moh.gov.on.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 1-4249-0330-0 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and information from practicing medical experts and industry, adds important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to maximize patient outcomes.
If you are aware of any current additional evidence to inform an existing Evidence-Based Analysis, please contact the Medical Advisory Secretariat: MASInfo@moh.gov.on.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to do so, this document may not fully reflect all scientific research available. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superceded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas
Abbreviations
- AAA
Abdominal aortic aneurysm
- ADAM
Aneurysm Detection and Management Trial
- CER
Cost-effectiveness ratio
- CI
Confidence interval
- CTFPHC
Canadian Task Force on Preventive Health Care
- EVAR
Endovascular aneurysm repair
- LYR
Life year gained
- LYS
Life year saved
- MASS
Multicentre Aneurysm Screening Study
- OR
Odds ratio
- OSR
Open surgical repair
- QALY
Quality adjusted life year
- QoL
Quality of life
- USPSTF
United States Preventive Services Task Force
- WHO
World Health Organization
References
- 1.Braunwald E, Fauci AS, Kasper DL, Hauser S, Longo DL, Jameson JL. 15th ed. New York: McGraw-Hill; 2001. Harrison’s principles of internal medicine. [Google Scholar]
- 2.Patterson C Canadian Task Force on Preventative Health Care. Screening for abdominal aortic aneurysms [monograph on the Internet] Ottawa: Health Canada. 1994. [[cited 2004 Oct. 10]]. pp. 672–678. Available from: http://www.ctfphc.org/Full_Text/Ch55full.htm .
- 3.Heller JA, Weinberg A, Arons R, Krishnanastry KV, Lyon RT, Deitch JS, et al. Two decades of aneurysmal repair: have we made any progress? J Vasc Surg. 2005;32(6):1091–1100. doi: 10.1067/mva.2000.111691. [DOI] [PubMed] [Google Scholar]
- 4.Hallett JW Jr. Management of abdominal aortic aneurysms. Mayo Clin Proc. 2000;75(4):395–399. doi: 10.4065/75.4.395. [DOI] [PubMed] [Google Scholar]
- 5.Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142(3):203–211. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 6.Salo JA, Soisalon-Soininen S, Bondestam S, Mattila PS. Familial occurrence of abdominal aortic aneurysm. Ann Intern Med. 1999;130:637–642. doi: 10.7326/0003-4819-130-8-199904200-00003. [DOI] [PubMed] [Google Scholar]
- 7.Naylor CD, Anderson GM, Goel VM, editors. Patterns of health care in Ontario. Ottawa: Canadian Medical Association [for] the Institute for Clinical Evaluative Sciences. ICES Practice Atlas 1994 Jun;1 [Google Scholar]
- 8.Brewster DC. Presidential Address: what would you do if it was your father? [Reflections on endovascular abdominal aortic repair]. J Vasc Surg. 2001;33(6):1139–1147. doi: 10.1067/mva.2001.115374. [DOI] [PubMed] [Google Scholar]
- 9.Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, et al. The aneurysm detection and management study screening program: validation cohort and final results. [Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators]. Arch Intern Med. 2000;160(10):1425–30. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 10.Medical Advisory Secretariat. Endovascular repair of abdominal aortic aneurysm [report on the Internet] Ministry of Health and Long-Term Care. Health Technology Literature Review. Mar, 2002. [[cited 2004 Oct. 1]]. Available at: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/rev_evar_030102.pdf .
- 11.Bowen J, De Rose G, Hopkins R, Novick T, Blackhouse G, Tarride J-E, et al. Systematic review and cost-effectiveness analysis of elective endovascular repair compared to open surgical repair of abdominal aortic aneurysms [report on the Internet] Program for Assessment of Technology in Health (PATH) Jul, 2005. [[cited 2005 July 1]]. Available at: http://www.path-hta.ca/EVAR%20Interim%20Report%20Jul%202005.pdf .
- 12.Wilmink TB, Quick CR, Hubbard CS, Day NE. The influence of screening on the incidence of ruptured abdominal aortic aneurysms. J Vasc Surg. 1999;30(2):203–208. doi: 10.1016/s0741-5214(99)70129-1. [DOI] [PubMed] [Google Scholar]
- 13.Lindholt JS, Juul S, fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ. 2005;330(7494):750. doi: 10.1136/bmj.38369.620162.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quill DS, Colgan MP, Sumner DS. Ultrasonic screening for the detection of abdominal aortic aneurysms. Surg Clin North Am. 1989;69(4):713–720. doi: 10.1016/s0039-6109(16)44878-4. [DOI] [PubMed] [Google Scholar]
- 15.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm [erratum appears in BMJ. 2005 Mar 12;330(7491):596] BMJ. 2004;329(7477):1259. doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott RA, Wilson SE, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82:1066–70. doi: 10.1002/bjs.1800820821. [DOI] [PubMed] [Google Scholar]
- 17.Ashton HA, Buxton MJ, Day NE, Kim LG, Scott RA, Thompson SG, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360(9345):1531–9. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 18.Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med. 2000;160(6):833–6. doi: 10.1001/archinte.160.6.833. [DOI] [PubMed] [Google Scholar]
- 19.Oboler SK, LaForce FM. The periodic physical examination in asymptomatic adults. Ann Intern Med. 1989;110(3):214–26. doi: 10.7326/0003-4819-110-3-214. [DOI] [PubMed] [Google Scholar]
- 20.Wilmink A, Forshaw M, Quick CRG, Hubbard CS, Day NE. Accuracy of serial screening for abdominal aortic aneurysms by ultrasound. J Med Screen. 2002;9(3):125–7. doi: 10.1136/jms.9.3.125. [DOI] [PubMed] [Google Scholar]
- 21.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olabarriaga SD, Rouet JM, Fradkin M, Breeuwer M, Niessen WJ. Segmentation of thrombus in abdominal aortic aneurysms from CTA with nonparametric statistical grey level appearance modeling. IEEE Trans Med Imaging. 2005;24(4):477–485. doi: 10.1109/tmi.2004.843260. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Preventive Services Task Force. Ann Intern Med. 2005;142(3):198–202. doi: 10.7326/0003-4819-142-3-200502010-00004. Screening for abdominal aortic aneurysm: recommendation statement. [DOI] [PubMed] [Google Scholar]
- 24.Lindholt JS, Juul S, fasting H, Henneberg EW. Hospital costs and benefits of screening for abdominal aortic aneurysms. [Results from a randomized population screening trial]. Eur J Vasc Endovasc Surg. 2002;23(1):55–60. doi: 10.1053/ejvs.2001.1534. [DOI] [PubMed] [Google Scholar]
- 25.UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352:1649–55. [PubMed] [Google Scholar]
- 26.Cornuz J, Sidoti PC, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health. 2004;14(4):343–349. doi: 10.1093/eurpub/14.4.343. [DOI] [PubMed] [Google Scholar]
- 27.Jamrozik K, Norman PE, Spencer CA, Parsons RW, Tuohy R, Lawrence-Brown M, et al. Screening for abdominal aortic aneurysm: lessons form a population-based study. Med J Aust. 2000;173(7):345–350. doi: 10.5694/j.1326-5377.2000.tb125684.x. [DOI] [PubMed] [Google Scholar]
- 28.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Ann Intern Med. 1997;126(6):441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 29.Lindholt JS, Juul S, Henneberg EW, fasting H. Is screening for abdominal aortic aneurysm acceptable to the population? [Selection and recruitment to hospital-based mass screening for abdominal aortic aneurysm]. J Public Health Med. 1998;20:211–7. doi: 10.1093/oxfordjournals.pubmed.a024745. [DOI] [PubMed] [Google Scholar]
- 30.Kim LG, Thompson SG, Marteau TM, Scott RA Multicentre Aneurysm Screening Study Group. Screening for abdominal aortic aneurysms: the effects of age and social deprivation on screening uptake, prevalence and attendance at follow-up in the MASS trial. J Med Screen. 2004;11(1):50–53. doi: 10.1177/096914130301100112. [DOI] [PubMed] [Google Scholar]
- 31.Crow P, Shaw E, Earnshaw JJ, Poskitt KR, Whyman MR, Heather BP. A single normal ultrasound scan at age 65 years rules out significant aneurysm disease for life in men. Br J Surg. 2001;88(7) doi: 10.1046/j.0007-1323.2001.01822.x. [DOI] [PubMed] [Google Scholar]
- 32.Scott RA, Vardulaki KA, Walker NM, Day NE, Duffy SW, Ashton HA. The long-term benefits of a single scan for abdominal aortic aneurysm at age 65. Eur J Vasc Endovasc Surg. 2001;21(6):535–40. doi: 10.1053/ejvs.2001.1368. [DOI] [PubMed] [Google Scholar]
- 33.Emerton ME, Shaw E, Poskitt KR, Heather BP. Screening for abdominal aortic aneurysm: a single scan is enough. Br J Surg. 1994;81(8):1112–3. doi: 10.1002/bjs.1800810810. [DOI] [PubMed] [Google Scholar]
- 34.Kent KC, Zwolak RM, Jaff MR, Hollenbeck ST, Thompson RW, Schermerhorn ML, et al. Screening for abdominal aortic aneurysms - A consensus statement. Vasc Med. 2004;9(1):87–89. doi: 10.1016/j.jvs.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Brewster DC, Cribinenwett JL, Hellett JW, Johnston KW, Krupski WC, Matsumura JS, et al. Guidelines for the treatment of AAA. [Report of subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery]. J Vasc Surg. 2003;37(5):1106–17. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 36.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1437–44. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 37.Ontario Ministry of Finance. Ontario Population Projections 2004 to 2031. Queen’s Printer for Ontario. Feb, 2005. [[cited 2005 Feb. 10]]. Available at: http://www.fin.gov.on.ca/english/demographics/demog05e.pdf .
- 38.Statistics Canada; Health Indicators. Table 2.1.1.1 Smoking, by age group and sex, household population aged 12 and over, Canada excluding territories, 1994/95-1998/99. [report on the Internet] Statistics Canada. Jan 4, 2001. [[cited 2004 Oct. 15]]. Available at: http://www.statcan.ca/english/freepub/82-221-XIE/00601/tables/htmltables/P2111.htm .
- 39.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317(307):12. doi: 10.1136/bmj.317.7154.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole CW, Millar WJ, Laupacis A, Johnston KW. Selective screening for abdominal aortic aneurysm. Chronic Dis Can. 1997;17(2):51–55. [PubMed] [Google Scholar]
- 41.Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg. 2002;89:283–5. doi: 10.1046/j.0007-1323.2001.02014.x. [DOI] [PubMed] [Google Scholar]
- 42.Dimick JB, Cowan JA, Stanley JC, Henke PK, Pronovost PJ, Upchurch GR. Surgeon specialty and provider volumes are related to outcome of intact abdominal aortic aneurysm repair in the United States. J Vasc Surg. 2003;38(4):739–44. doi: 10.1016/s0741-5214(03)00470-1. [DOI] [PubMed] [Google Scholar]
- 43.Huber TS, Wang JG, Derrow AE, Dame DA, Ozaki CK, Zelenock GB, et al. Experience in the United States with intact abdominal aortic aneurysms repair. J Vasc Surg. 2003;38(4):739–52. doi: 10.1067/mva.2001.112703. [DOI] [PubMed] [Google Scholar]
- 44.Lindholt JS, Vammen S, Fasting H, Henneberg EW. Psychological consequences of screening for abdominal aortic aneurysm and conservative treatment of small abdominal aortic aneurysms. Eur J Endovasc Surg. 2000;20:79–83. doi: 10.1053/ejvs.1999.1087. [DOI] [PubMed] [Google Scholar]
- 45.Lucarotti ME, Heather BP, Shaw E, Poskitt KR. Psychological morbidity associated with abdominal aortic aneurysm screening. Eur J Vasc Endovasc Surg. 1997;14:499–501. doi: 10.1016/s1078-5884(97)80131-1. [DOI] [PubMed] [Google Scholar]
- 46.Lederle FA, Johnson GR, Wilson SE, Acher CW, Ballard DJ, Littooy FN, et al. Quality of life, impotence, and activity level in a randomized trial of immediate repair versus surveillance of small abdominal aortic aneurysm. [Aneurysm Detection and Management Veterans Affairs Cooperative Study]. J Vasc Surg. 2003;38:745–52. doi: 10.1016/s0741-5214(03)00423-3. [DOI] [PubMed] [Google Scholar]
- 47.UK Small Aneurysm Trial Participants. Health service costs and quality of life for early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352(9141):1656–60. [PubMed] [Google Scholar]
- 48.Spencer CA, Norman PE, Jamrozik K, Tuohy R, Lawrence-Brown M. Is screening for abdominal aortic aneurysm bad for your health and well-being? ANZ J Surg. 2004;74(12):1069–1075. doi: 10.1111/j.1445-1433.2004.03270.x. [DOI] [PubMed] [Google Scholar]
- 49.Wanhainen A, Rosen C, Rutegard J, Bergqvist D, Bjorck M. Low quality of life prior to screening for abdominal aortic aneurysm: a possible risk factor for negative mental effects. Ann Vasc Surg. 2004;18(3):287–293. doi: 10.1007/s10016-004-0021-x. [DOI] [PubMed] [Google Scholar]
- 50.Wanhainen A, Lundkvist J, Bergqvist D, Bjorck M. Cost-effectiveness of different screening strategies for abdominal aortic aneurysm. J Vasc Surg. 2005;41(5):741–751. doi: 10.1016/j.jvs.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 51.Boll APM, Severens JL, Verbeek ALM, van der Vliet JA. Mass screening on abdominal aortic aneurysm in men aged 60 to 65 years in the Netherlands. [Impact on life expectancy and cost-effectiveness using a Markov model]. Eur J Vasc Endovasc Surg. 2003;26:75–80. doi: 10.1053/ejvs.2002.1773. [DOI] [PubMed] [Google Scholar]
- 52.Lee TY. The cost-effectiveness of a “quick-screen” program for abdominal aortic aneurysms. 2002;132(2):399–407. doi: 10.1067/msy.2002.126510. Surgery. [DOI] [PubMed] [Google Scholar]
- 53.Multicentre Aneurysm Screening Study Group. Multicentre aneurysm screening study (MASS): cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trial. BMJ. 2002;325:1135–41. doi: 10.1136/bmj.325.7373.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Connelly JB, Hill GB, Millar WJ. The detection and management of abdominal aortic aneurysm: a cost-effectiveness analysis. Clin Invest Med. 2002;25(4):127–33. [PubMed] [Google Scholar]
- 55.Daly KJ, Torella F, Ashleigh R, McCollum CN. Screening, diagnosis and advances in aortic aneurysm surgery. Gerontology. 2004;50(6):349–359. doi: 10.1159/000080172. [DOI] [PubMed] [Google Scholar]
- 56.Johnston KW. Influence of sex on the results of abdominal aortic aneurysm repair. J Vasc Surg. 1994;20(6):914–26. doi: 10.1016/0741-5214(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 57.Parsons GF, Gentleman JF, Johnston KW. Gender differences in abdominal aortic aneurysm surgery. Health Rep. 1997;9(1) [Google Scholar]
- 58.Greenhalgh RM, Powell JT. Screening men for aortic aneurysm. Br Med J. 2002;325:1223–4. doi: 10.1136/bmj.325.7373.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]


