Executive Summary
Objective
Due to continuing advances in the development of structures, devices, and systems with a length of about 1 to 100 nanometres (nm) (1 nm is one billionth of a metre), the Medical Advisory Secretariat conducted a horizon scanning appraisal of nanotechnologies as new and emerging technologies, including an assessment of the possibly disruptive impact of future nanotechnologies.
The National Cancer Institute (NCI) in the United States proclaimed a 2015 challenge goal of eliminating suffering and death from cancer. To help meet this goal, the NCI is engaged in a concerted effort to introduce nanotechnology “to radically change the way we diagnose, treat and prevent cancer.” It is the NCI’s position that “melding nanotechnology and cancer research and development efforts will have a profound, disruptive effect on how we diagnose, treat, and prevent cancer.”
Thus, this appraisal sought to determine the systemic effects of nanotechnologies that target, image and deliver drugs, for example, with respect to health human resources, training, and new specialties; and to assess the current status of these nanotechnologies and their projected timeline to clinical utilization.
Clinical Need: Target Population and Condition
Cancer is a heterogeneous set of many malignant diseases. In each sex, 3 sites account for over one-half of all cancers. In women, these are the breast (28%), colorectum (13%) and lungs (12%). In men, these are the prostate (28%), lungs (15%), and the colorectum (13%).
It is estimated that 246,000 people in Ontario (2% of the population) have been diagnosed with cancer within the past 10 years and are still alive. Most were diagnosed with cancer of the breast (21%), prostate (20%), or colon or rectum (13%).
The number of new cancer cases diagnosed each year in Ontario is expected to increase from about 53,000 in 2001 to 80,000 in 2015. This represents more than a 50% increase in new cases over this period. An aging population, population growth, and rising cancer risk are thought to be the main factors that will contribute to the projected increase in the number of new cases.
The Technology Being Reviewed - Medical Advisory Secretariat Definition of Nanotechnology
First-Generation Nanotechnologies
Early application of nanotechnology-enabled products involved drug reformulation to deliver some otherwise toxic drugs (e.g., antifungal and anticancer agents) in a safer and more effective manner.
Examples of first-generation nanodevices include the following:
liposomes;
albumin bound nanoparticles;
gadolinium chelate for magnetic resonance imaging (MRI);
iron oxide particles for MRI;
silver nanoparticles (antibacterial wound dressing); and
nanoparticulate dental restoratives.
First-generation nanodevices have been in use for several years; therefore, they are not the focus of this report.
Second-Generation Nanotechnologies
Second-generation nanotechnologies are more sophisticated than first- generation nanotechnologies, due to novel molecular engineering that enables the devices to target, image, deliver a therapeutic agent, and monitor therapeutic efficacy in real time. Details and examples of second-generation nanodevices are discussed in the following sections of this report.
Review Strategy
The questions asked were as follows:
What is the status of these multifunctional nanotechnologies? That is, what is the projected timeline to clinical utilization?
What are the systemic effects of multifunctional nanodevices with integrated applications that target, image, and deliver drugs? That is, what are the implications of the emergence of nanotechnology on health human resources training, new specialties, etc.?
The Medical Advisory Secretariat used its usual search techniques to conduct the literature review by searching relevant databases. Outcomes of interest were improved imaging, improved sensitivity or specificity, improved response rates to therapeutic agents, and decreased toxicity.
Results
The search yielded 1 health technology assessment on nanotechnology by The Centre for Technology Assessment TA-Swiss and, in the grey literature, a technology review by RAND. These, in addition to data from the National Cancer Institute (United States) formed the basis for the conclusions of the review.
With respect to the question as to how soon until nanotechnology is used in patient care, overall, the use of second-generation nanodevices, (e.g., quantum dots [QDs]), nanoshells, dendrimers) that can potentially target, image, and deliver drugs; and image cell response to therapy in real time are still in the preclinical benchwork stage.
Table 1 summarizes the projected timelines to clinical utilization.
Medical Advisory Secretariat Estimated Timeline for Ontario
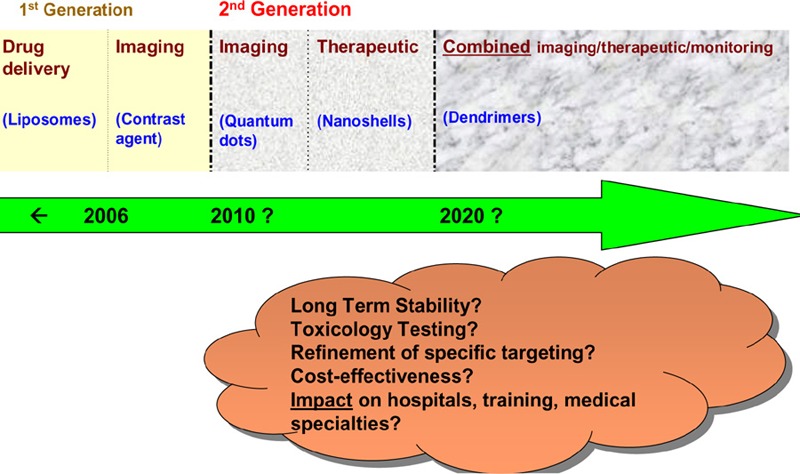
Upon synthesizing the estimated timelines from the NCI, the Swiss technology assessment and the RAND reports (Figure 1), it appears that:
the clinical use of separate imaging and therapeutic nanodevices is estimated to start occurring around 2010;
the clinical use of combined imaging and therapeutic nanodevices is estimated to start occurring around 2020;
changes in the way disease is diagnosed, treated and monitored are anticipated; and
the full (and realistic) extent of these changes within the next 10 to 20 years is uncertain.
Figure 1: Medical Advisory Secretariat Estimated Timeline for the Clinical Use of Second-Generation Nanodevices in Ontario.
With respect to the question on potential systemic effects of second-generation nanodevices (i.e., the implications of the emergence of these nanodevices on health human resources training, new specialties etc.), Table 2 summarizes the findings from the review.
Table 2: Potential Systemic Effects Caused by Second Generation Nanodevices*.
| Imaging | Therapeutic | Combined (Detect, Image, Treat, Monitor) |
|---|---|---|
| Increased sensitivity and specificity of QDs or other nanodevices could lead to the replacement of existing technologies (e.g., PSA testing, mammogram). | Sudden demand in use of MRI due to use of nanodevices that are activated in the presence of a magnetic field. | Universal demand to detect cancer– how will patients be prioritized for this? Sudden demand in use of MRI due to use of nanodevices that are activated in the presence of a magnetic field. |
| Cost: possibly more expensive than current screening modalities. | Possibly more expensive than existing therapies (gold nanoshells) | Many functions can be performed on one device → possibly faster, more cost-effective than individual devices. |
| Report of results: possibly faster than existing technologies. | Possibly faster determination of therapeutic efficacy (vs. existing technologies) | Increase in life expectancy of population? Free-up beds in hospitals? |
| Nanodevices may be able to pinpoint with more accuracy when cancer starts. Ethical question: when does disease start? Increased demand for imaging by people who are asymptomatic and concerned they may get cancer. |
Nano-radiologist or medical nano-oncologist provides treatment rather than conventional radiologists or medical oncologists. Creation of nano-nursing compared to conventional nursing. |
Nano-radiologist or medical nano-oncologist provides treatment, rather than conventional radiologists or medical oncologists. Creation of nano-nursing compared to conventional nursing. Uncertainty regarding how many “traditional” cancer radiologists/oncologists should be retained and trained. |
| New branch of (nano) radiology compared to conventional radiology New/longer training in biochemistry and targeting ligands will be required by nanoradiologists. | More training required for new nano-treatments Patient education – people may be concerned regarding the use of nanodevices inside their bodies. | Longer time to specialize in medicine in order to use nanotechnology clinically? Insufficient number of dendrimer specialists to treat everyone with personalized dendrimers |
| Restricted to specialized centres Possible in-house nanodevice production required to keep up with the demand for use. | Will the same specialized centres that offer imaging also offer treatment? How many specialized centres will be required? |
Only specialized centres can perform this combined imaging/treatment Possible nano-monitoring from patient home via wireless technology. This may free hospital beds for other patients. |
| After imaging with nanodevices, specifically targeted therapeutic nanodevices may also be required for immediate treatment of the patient. Will both of these nanodevices be commercially available in sufficient quantities? | Possible waiting time for preparation of appropriately targeted nanodevice after imaging (will a therapeutic nanodevice be immediately available?) Will a patient receive conventional treatment if there is a waiting period required to prepare the therapeutic nanodevice? |
Will there be a patient waiting time required for preparation of personalized dendrimers (hours, days, weeks after a patient sees a physician)? |
MRI indicates magnetic resonance imaging; PSA, prostate-specific antigen; QD, quantum dot.
Uncertainties Not Addressed in the Literature
The United States National Nanotechnology Initiative (NNI) funds a variety of research in the economic, ethical, legal, and cultural implications of the use of nanotechnology, as well as the implications for science, education and quality of life.
There are many uncertainties that are sparsely or not addressed at all in the literature regarding second generation nanodevices. These include the following:
long-term stability and toxicology of nanodevices;
cost-effectiveness of nanodevices;
refinement of specific targeting;
effects on hospitals, physician/nurse training, creation/removal of specialties; and
that pertaining to the question, where does disease begin if therapy is applied before the symptoms have appeared?
Objective
Due to continuing advances in the development of structures, devices, and systems with a length of about 1 to 100 nanometres (nm) (1 nm is one billionth of a metre), the Medical Advisory Secretariat conducted a horizon scanning appraisal of nanotechnologies as new and emerging technologies, including an assessment of the possibly disruptive impact of future nanotechnologies.
The National Cancer Institute (NCI) (1) in the United States proclaimed a 2015 challenge goal of eliminating suffering and death from cancer. To help meet this goal, the NCI is engaged in a concerted effort to introduce nanotechnology “to radically change the way we diagnose, treat and prevent cancer.” (1) It is the NCI’s position that “melding nanotechnology and cancer research and development efforts will have a profound, disruptive effect on how we diagnose, treat, and prevent cancer.” (1)
Thus, this appraisal sought to determine the systemic effects of nanotechnologies that target, image and deliver drugs, for example, with respect to health human resources, training, and new specialties; and to assess the current status of these nanotechnologies and their projected timeline to clinical utilization.
Background
Clinical Need: Target Population and Condition
Cancer is a heterogeneous set of many malignant diseases. In both sexes, 3 sites account for over half of all cancers. (2) In women, these include breast (28%), colorectum (13%) and lung (12%) and in men, these include prostate (28%), lung (15%) and colorectum (13%).
(2)
It is estimated that 246,000 people in Ontario (2% of the population) have been diagnosed with cancer sometime in the past 10 years and are still alive. (3) Most were diagnosed with cancer of the breast, (21%), prostate (20%) or colon or rectum (13%). (3)
The number of new cancer cases diagnosed each year in Ontario is expected to increase from approximately 53,000 in 2001 to 80,000 in 2015. (4) This represents more than a 50% increase in new cases over this time period. An aging population, population growth, and rising cancer risk are thought to be the main factors that will contribute to the projected increase in the number of new cases. (4)
New Technology Being Reviewed
In 2001, the United States National Nanotechnology Initiative (NNI) was formed. (5) The NNI is a multi-agency governmental program aimed at accelerating the discovery, development, and deployment of nanoscale science, engineering, and technology. As of March 2005, 22 agencies are participating in the NNI (including the National Institutes of Health). Total funding for the NNI to date exceeds $5 billion (US). (5)
Nanotechnology is defined by the NNI as (6):
research and technology development at the atomic, molecular, or macromolecular level that leads to the controlled creation and use of structures, devices, or systems with a length scale of 1 to 100 nm;
creating and using structures, devices, and systems that have novel properties and functions because of their small and/or intermediate size; and
having the ability to control or manipulate on the atomic scale.
Most animal cells are 10,000 to 20,000 nm in diameter. Nanoscale devices can interact with biomolecules on both the cell surface and within the cell (e.g., deoxyribonucleic acid [DNA] and proteins). Nanoscale devices smaller than 50 nm can easily enter most cells, while those smaller than 20 nm can move out of blood vessels as they circulate throughout the body. (7) Nanoscale devices may, therefore, be able to penetrate biological barriers such as the blood-brain barrier or the stomach epithelium, barriers that that normally make it difficult for therapeutic and imaging agents to reach certain tumours.
Nanomedicine is an offshoot of nanotechnology. It refers to a medical intervention at the molecular scale. (8) Research in nanotechnology began with applications outside of medicine and is based on discoveries in physics and chemistry.
Nanotechnology is made out of a wide range of materials that are designed with chemically modifiable surfaces in order to attach a variety of ligands (molecules that bind to other molecules) that can turn these nanomaterials into biosensors, molecular-scale fluorescent tags, imaging agents, targeted molecular delivery vehicles, and other biological tools. (9)
In theory, nanomaterials can be engineered from nearly any chemical substance; for example, semiconductor nanocrystals, organic dendrimers, carbon fullerenes (“buckyballs,” which are spherical molecules of carbon atoms linked together like a soccer ball), and carbon nanotubes. An important feature of nanomaterials is that they can exhibit very different physical, chemical, and biological properties from their corresponding macroscale state, because quantum mechanics phenomena become prevalent only at nanometre scales. Such differences include altered magnetic properties, electrical conductivity, optical sensitivity, and increased surface area. (6;9-12) Because of these special properties, nanodevices may pose different safety issues than their macroscale counterparts. (13)
Recently, nanodevices are being used to detect cancer at its earliest stages; that is, pinpointing its location within the body, as well as to deliver anticancer drugs specifically to malignant cells, and then determine if these drugs are killing malignant cells. (7) As research continues, it is hoped that nanodevices will result in advances in early detection; molecular imaging, assessment and therapeutic efficacy; targeted and multifunctional therapeutics; and, ultimately, in the prevention and control of cancer. (7)
Medical Advisory Secretariat’s Definition of Nanotechnology
The Medical Advisory Secretariat defines nanotechnology as either first-generation or second-generation nanotechnologies.
First-Generation Nanotechnologies
Early application of nanotechnology-enabled products involved drug reformulation to deliver some otherwise toxic drugs (e.g., antifungal and anticancer agents) more safely and effectively. (9) Examples of first-generation nanodevices include:
liposomes;
albumin bound nanoparticles;
gadolinium chelate for magnetic resonance imaging (MRI);
iron oxide particles for MRI;
silver nanoparticles (antibacterial wound dressing); and
nanoparticulate dental restoratives.
Because first-generation nanodevices have been in use for several years, they are not the focus of this report.
Second-Generation Nanotechnologies
Second-generation nanotechnologies are more sophisticated than first-generation nanotechnologies, owing to novel molecular engineering that enables the devices to target, image, and deliver a therapeutic agent; and monitor therapeutic efficacy in real-time. Details and examples of second-generation nanodevices are discussed in the following sections of this report.
Use of the Term “Nano”
The term “nano” is increasingly becoming a selling point for consumers, as well as funding agencies. (14) Thus, the apparent surge in the use of nanotechnology may be the result of companies relabelling their goods to meet consumer preferences. For example, most cosmetic creams already contain nanoscale particles to penetrate the skin, so companies are able to use this in their marketing strategies. (14)
An example of how the term “nano” is increasingly becoming a selling point for funding agencies comes from a chemist in Sweden and former president of the European Tissue Engineering Society (14) who recently stated that a project he heads recently won $1.7 million (€) from the European Union’s framework program following a call for nanobiotechnolgy projects. According to the chemist, “I could have very well written the proposal without nano in there. I didn’t lie to get the money; I just used the word they like to hear.”
United States Food and Drug Administration’s Definition of Nanotechnology
The United States Food and Drug Administration (FDA) has not established its own formal definition, although the agency participated in the development of the NNI definition of nanotechnology. Using that definition, nanotechnology relevant to the FDA might include research and technology development that both satisfies the NNI definition and is related to a product regulated by the FDA.
At present, the FDA considers that existing regulatory requirements may be adequate for most nanotechnology products that they regulate. These products are in the same size range as the cells and molecules with which the FDA reviewers and scientists associate every day. Every degradable medical device (e.g., iron oxide MRI contrast agent) or injectable drug generates particulates that pass through this size range during the processes of their absorption and elimination by the body. To date, the FDA has no knowledge of reports of adverse reactions related to the nano size of resorbable drug or medical device products. However, if new risks are identified beyond-first generation nanodevices (e.g., from new materials or manufacturing techniques), new tests or other requirements may be required.
In August 2006, the FDA Nanotechnology Task Force was formed. The goal of the task force is to determine regulatory approaches that encourage development of innovative, safe, and effective FDA-regulated products that use nanomaterials, and to identify and recommend ways to address knowledge or policy gaps.
Nanotechnology in Europe
In June 2005, the European Commission adopted an action plan for 2005 to 2009 defining actions for the “immediate implementation of a safe integrated and responsible strategy for nanosciences and nanotechnologies.” (15)
The European Commission raised the following points with regard to implementing nanotechnology (16):
Nanotechnology is raising questions as to what should and should not be patentable (e.g., on the level of individual molecules).
Existing regulations rely on macroscale parameters that may turn out to be inappropriate for nanoscale applications (e.g., occupational exposure to loose nanoparticles/quantum dots). For example, thresholds are often defined in terms of production volumes or mass, below which a substance may be exempt from regulation. The relevance of such thresholds for nanoparticles may need to be examined.
Nanotechnology in Canada
The National Institute for Nanotechnology is an integrated multidisciplinary institution involving researchers in physics, chemistry, engineering, biology, informatics, pharmacy, and medicine. (17) It was established in 2001 and is a partnership between the National Research Council and the University of Alberta.
The National Institute of Bioimaging and Bioengineering in the United States awarded a 5-year $8.3 million (Cdn) grant to support a research partnership between the National Research Council of Canada’s National Institute for Nanotechnology, the University of Alberta, Los Alamos National Laboratory, and the La Jolla Bioengineering Institute. (17) It is a multidisciplinary initiative involving engineers, biologists, and chemists from academia, government, and industry in the United States and Canada for the development of new technology platforms and their applications in drug discovery and biomedical diagnostics. (17)
Nanotechnology in the United States
In October 2005, the National Science Foundation (18) in the United States announced a series of initiatives that will greatly expand efforts to inform the general public about nanotechnology and to explore the implications of that field for society as a whole (e.g., freedom, privacy, and security; and human identity, enhancement, and biology). According to the National Science Foundation’s senior advisor for nanotechnology, “The nanotechnology field has been evolving rapidly since 2000, with technological, economic, social, environmental and ethical implications that could change our world.” (18)
National Cancer Institute: The 2015 Challenge Goal
The NCI in the United States proclaimed a 2015 challenge goal of eliminating suffering and death from cancer. (1) To help meet this goal, the NCI is engaged in a concerted effort to introduce nanotechnology “to radically change the way we diagnose, treat and prevent cancer.” (1) It is the NCI’s position that “melding nanotechnology and cancer research and development efforts will have a profound, disruptive effect on how we diagnose, treat, and prevent cancer.” (1)
The NCI undertook fact-finding discussions with cancer research and clinical oncology communities during 2003 and 2004. The discussions increased the NCI’s awareness that there are a number of barriers that could slow the translation of cancer nanotechnology research into clinically useful advances in diagnosing, treating and preventing cancer. These include the following:
Cross-disciplinary collaborations:
Barriers to multidisciplinary and multiple partner collaborations must fall. In response, the NCI undertook alternative funding mechanisms to encourage and facilitate more collaboration among the public, private, and nonprofit sectors.
Gap between late discovery and early development of diagnostics and therapeutics:
Many products that reach clinical development fail because of a lack of solid science to support regulatory filings.
To conduct clinical trials, there is often insufficient financial and intellectual support for smaller companies to move products through the testing and regulatory approval processes.
Regulatory uncertainty:
There is no clear pathway for approval of nanoscale devices. In particular, there is concern that each new use of a given nanoscale device will require full scale preclinical and clinical testing—a requirement that would prolong development and increase costs.
Difficulty of gaining regulatory approval for combination nanodevices (diagnostic and therapeutic products or multiple therapeutic agents in the same construct).
Standardization and characterization:
Few standards and sparse reference physical and biological characterization data that researchers can use to choose which nanodevices might be most suitable for a given clinical or research application.
Lack of standard assay and characterization methods makes it difficult to compare results from different laboratories.
In vivo behaviour:
It is reasonable to expect that pharmacokinetics, pharmacodynamics, toxicokinetics, and biodistribution of nanodevices will differ markedly from current imaging and therapeutic agents.
There is very little in vivo data on these characteristics. There is also little ongoing research that will generate these data.
Technology transfer and knowledge exchange:
There is a need for new mechanisms for sharing and cross-licensing intellectual property.
National Cancer Institute: Alliance for Nanotechnology in Cancer
In September 2004, the NCI initiated a $144.3 million (US) 5-year research effort, the Alliance for Nanotechnology in Cancer, to develop and translate cancer-related nanotechnology research into clinical practice. (19) The NCI alliance is a comprehensive initiative, encompassing the public and private sectors, to accelerate the application of nanotechnology to cancer.
A cancer nanotechnology plan has been developed to guide the implementation of the NCI alliance. (20) Under this plan, the NCI has established 4 major programs:
-
Centres of Cancer Nanotechnology Excellence
The aims of this program are to design and test nanomaterials and nanodevices and translate their use into clinical research.
-
Multidisciplinary Research Training and Team Development
The aim of this program is to create incentives to integrate nanotechnology into the mainstream of basic and applied cancer research.
-
Nanotechnology Platforms for Cancer Research
These aim to focus on translational research in the following 6 major challenge areas where nanotechnology can have the greatest impact:
-
Molecular imaging and early detection
Nanocantilevers, nanowires, and nanochannels offer the potential for detecting molecular signals associated with malignancy. Nanoscale harvesters could collect those signals for analysis. This has been shown to be feasible with nanoparticulate albumin, which can collect proteins that signal the presence of malignant ovarian tissue.
Another area in which nanotechnology shows potential is in the detection of gene mutations. For example, nanotubes show promise in detecting mutations.
-
In vivo nanotechnology imaging systems
First-generation nanoscale imaging contrast agents are providing new methods for spotting tumours much earlier in their development.
-
Reporters of efficacy
The greatest possibility for immediate results in this area focuses on detecting cell death following cancer therapy. Such systems could be constructed using nanoparticles containing an imaging contrast agent and a targeting molecule that recognizes a biochemical signal only seen when cells die.
Another approach may be to use targeted nanoparticles that would bind avidly or irreversibly to a tumour and then be released back into the blood as cells in the tumour die following therapy. If labelled with a fluorescent probe, the particles could be detected in the urine. If also labelled with an imaging contrast agent, the construct could double as a diagnostic imaging probe.
-
Multifunctional therapeutics
Because of multifunctional capabilities, nanoscale devices can contain targeting, imaging, and therapeutic agents at levels that produce high local drug concentrations.
Many nanoparticles respond to an externally applied field (e.g., magnetic focused heat or light). For example, nanoparticulate hydrogels can be targeted to sites of tumour angiogenesis (where new blood vessels are sprouting). Once they have bound to vessels undergoing angiogenesis, localized heat can be applied to “melt” the hydrogel and release an antiangiogenic drug (a drug that works to block angiogenesis). Similarly, iron oxide nanoparticles can be heated to temperatures lethal to cancer cells by increasing the magnetic field at the location where these nanoparticles are bound to tumour cells.
-
Prevention and control
Nanotechnology can be used to develop devices capable of signalling when cancer markers appear in the body and delivering agents that can reverse premalignant changes or kill cells that have the potential to become malignant.
-
Research enablers
Nanotechnology offers a wide range of tools that can track the movements of cells and individual molecules. Using such tools can allow researchers to study, monitor, and alter systems that go wrong in the cancer process, and identify areas were future therapies may be directed.
-
-
Nanotechnology Characterization Laboratory
The aim of this program is to perform and standardize the preclinical characterization (pharmacology, toxicology, and efficacy testing) of nanodevices (in conjunction with the FDA) to allow accelerated regulatory review and translation of basic research into the clinical domain.
Nanotechnologies With Potential To Improve Cancer Detection, Diagnosis, and Treatment
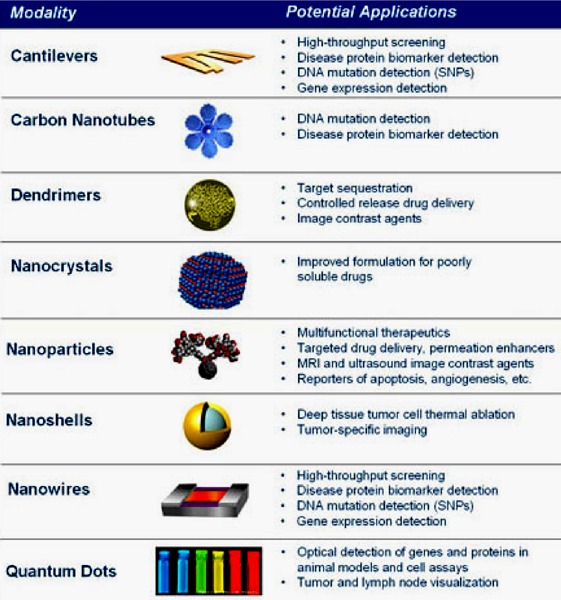
Several narrative reviews (21-23) have been published that describe the broad scope of technologies and applications that the integration of nanotechnology and drug delivery research has generated; these technologies have many diverse and distinct features. Table 1 lists examples of nanotechnologies that the NCI considers to have potential to improve cancer detection, diagnosis, and treatment.
Table 1: Summary of Timelines to Clinical Use*.
| Organization/Year | Second-Generation Nanodevice | Estimate of When Nanodevice Will be in Clinical Use |
|---|---|---|
| NCI 2001 | Imaging/detection (e.g., QDs) Therapeutic (e.g., nanoshell) Combined (e.g., dendrimer) |
~2006–2016 ~2006–2016 ~2016–2020 |
| NCI 2004 | Imaging/detection Therapeutic Combined |
~2009–2019 ~2009–2019 ~2019–2024 |
| RAND 2006 | Combined | unlikely before 2021 |
| Swiss 2003 | Imaging Therapeutic Combined |
~2005–2010 ~2008–2013 ~ 2010–2013 |
NCI refers to National Cancer Institute; QD, quantum dot.
Table 1: Examples of Nanotechnologies That the National Cancer Institute Considers to Have Potential to Improve Cancer Detection, Diagnosis and Treatment.
Source: Barker A. Nanotechnology and cancer prevention. St. Gallen Oncology Conference [report on the Internet]. February 16, 2006. National Cancer Institute; National Institutes of Health; NCI Alliance for Nanotechnology in Cancer. [cited 2006 Sept. 15]. Available at: http://nano.cancer.gov/meetings_events/ADB_0_16_2006.pdf
The Use of Nanotechnology in Diagnosis
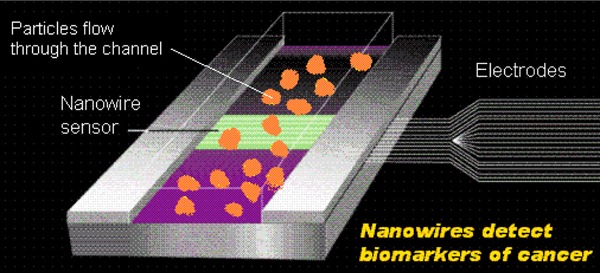
Nanowire Sensors
Nanowires are made of carbon, silicon, and other materials that have unique properties. (7) When used as sensors, nanowires lay across a small fluid channel (Figure 1). As particles flow through the channel (e.g., from the blood), the nanowire sensors pick up the molecular signatures of the particles and relay this information through a connection of electrodes outside the body.
Figure 1: Nanowire Sensors Pick Up the Molecular Signatures of Particles and Relay This Information Through Electrodes.
Source: National Cancer Institute. Nanowire sensor {Web page]. [updated 2006; cited 2006 Sept. 26]. Available at: http://nano.cancer.gov/resource_center/nanotech_nanowires.asp#
Nanowires have the potential to be used to detect the presence of altered genes associated with cancer. (7)
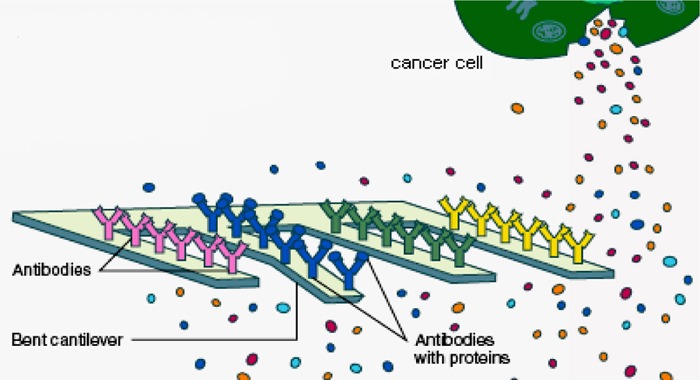
Cantilevers
Nanoscale cantilevers are flexible beams anchored at one end resembling a row of diving boards, and are engineered to bind to molecules associated with cancer. Antibodies on the cantilever fingers selectively bind to proteins secreted by the cancer cell; the change in surface tension then causes the cantilevers to bend (Figure 2). This change can be measured in real time and the concentration of different molecular secretions determined.
Figure 2: Cantilever Detection of Cancer Molecules.
Source: Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Cancer nanotechnology. Going small for big advances: using nanotechnology to advance cancer diagnosis, prevention and treatment [report on the Internet]. January, 2004. U.S. Department of Health and Human Services. [cited 2006 Nov. 2]. Available at: http://nano.cancer.gov/resource_center/cancer_nanotechnology_brochure.pdf
Nanotubes
Nanotubes are carbon rods that can detect the presence of altered genes. {National Cancer Institute, 2005 801 /id} They may help pinpoint the exact location of such changes. To prepare DNA for nanotube analysis, a bulky molecule (pink triangle in Figure 3) must be attached to regions of DNA that are associated with cancer. Designer tags can be used to target specific mutations in the DNA. A nanotube tip is then used to trace the physical shape of the DNA and pinpoint the mutated regions. Because the location of mutations can influence the effects they have on a cell, nanotubes may be important in predicting disease. (24;25)
Figure 3: A Nanotube Traces the Shape of Deoxyribonucleic Acid and Pinpoints a Mutation.
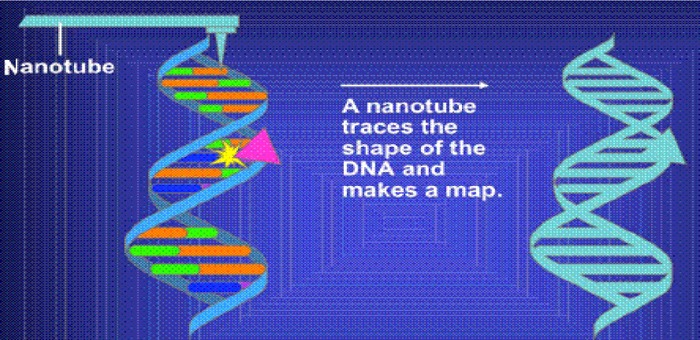
Source: National Cancer Institute. Slide 14. Nanotubes: mapping mutations [Web page]. U.S. National Institutes of Health. [updated 2005 Jan. 28; cited 2006 Sept. 15]. Available at: http://www.cancer.gov/cancertopics/understandingcancer/nanodevices/Slidel4
Nanopores
A nanopore has a hole that allows DNA to pass through one strand at a time to identify the shape and electrical properties of each base on the strand. (26) The nanopore can be used to decipher the encoded information, including errors in the code known to be associated with cancer (Figure 4).
Figure 4: Nanopores Can Read the Encoded Information in Deoxyribonucleic Acid Strands as They Pass Through the Hole.
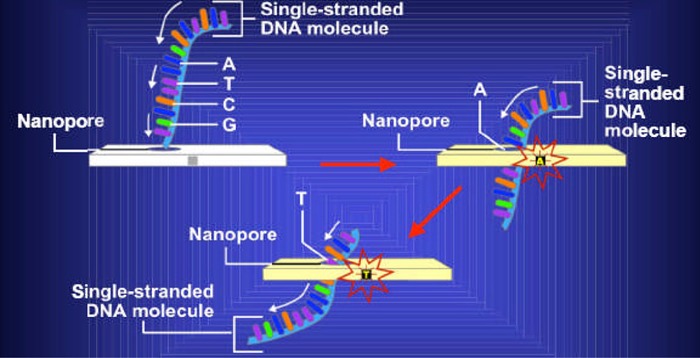
Source: National Cancer Institute. Slide 12. Nanopores [Web page]. U.S. National Institutes of Health. [updated 2005 Jan. 28; cited 2006 Oct. 23]. Available at: http://www.cancer.gov/cancertopics/understandingcancer/nanodevices/Slide12
The Use of Nanotechnology in Imaging
Nanoparticles can be engineered to target cancer cells for use in the molecular imaging of a malignant lesion. A nanoparticle can be injected to preferentially bind to the cancer cell, thereby identifying the lesion and making it visible.
Nanocrystals or Quantum Dots
Quantum dots (QDs) are nanometre-sized semiconductor crystals that glow when they are stimulated by ultraviolet light. The wavelength or colour of the light emitted from a QD depends on the size of the crystal. (27)
Structurally, QDs consist of a metalloid crystalline core and a “cap” or “shell” that shields the core and renders the QD available biologically. Quantum dots consist of a variety of metal complexes such as semiconductors, noble metals, and magnetic transition metals (e.g., indium arsenate, gallium arsenate, zinc selenium, cadmium selenium, cadmium tellurium, and lead selenium). (28) Biocompatible coatings or functional groups are added to the QD core-shell to improve water solubility, QD core durability and suspension characteristics, and assign a desired bioactivity (e.g., drug delivery or molecular imaging). Compromise of the coating may reveal the metalloid core, which may be toxic either as a composite core (e.g., cadmium telluride), or upon dissolution of the QD core, to constituent metals (e.g., cadmium). (28)
Compared with organic dyes and fluorescent proteins, QDs have unique optical and electronic properties such as size and composition-tunable fluorescence emission from visible to infrared wavelengths, large absorption coefficients across a wide spectral range, and very high levels of brightness and photostability. (29) Due to the broad excitation profiles, QDs are well suited to optical multiplexing in which multiple colours and intensities are combined to encode genes, proteins, and small molecule libraries. (29)
Gao et al. (29) created QD conjugates containing a special polymer coating (including a QD capping ligand) for in vivo protection, targeting ligands for tumour recognition, and several molecules (poly ethylene glycol) for improved biocompatibility and circulation (Figure 5). By attaching a targeting ligand, high-affinity binding of QD-antibody conjugates to tumour antigens occurs (Figure 6).
Figure 5: Structure of a Multifunctional Quantum Dot Probe*.

MW refers to molecular weight; PEG, polyethylene glycol; QD, quantum dot; TOPO, tri-n-octylphosphine oxide.
Reprinted by permission from Macmillan Publishers Ltd: [Nature Biotechnology]: Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots; 22(8): 969-76, Copyright 2004
Figure 6: High Affinity Binding of Quantum Dot Conjugates to a Tumour*.
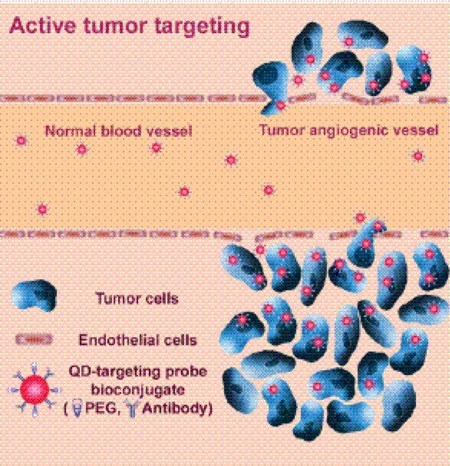
PEG refers to polyethylene glycol; QD, quantum dot.
Reprinted by permission from Macmillan Publishers Ltd: [Nature Biotechnology]: Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots; 22(8): 96976, Copyright2004
Figure 7 shows imaging results obtained from a QD with prostate-specific membrane antigen antibody conjugates injected into the tail vein of a tumour-bearing mouse and a control mouse (no tumour). (29) The fluorescence signals indicate a prostate tumour growing in a live mouse (mouse on right side). Control studies using a healthy mouse (no tumour) had the same amount of QD injected and showed no localized fluorescence signals (mouse on left side). After in vivo imaging, histological tests confirmed that the QD signals came from an underlying tumour. The QDs in deep organs such as the liver and spleen were not detected because of the limited penetration depth of visible light. (29)
Figure 7: Imaging of Quantum Dot Prostate-Specific Membrane Antigen Antibody Conjugates in Live Animals With Tumour Xenografts.
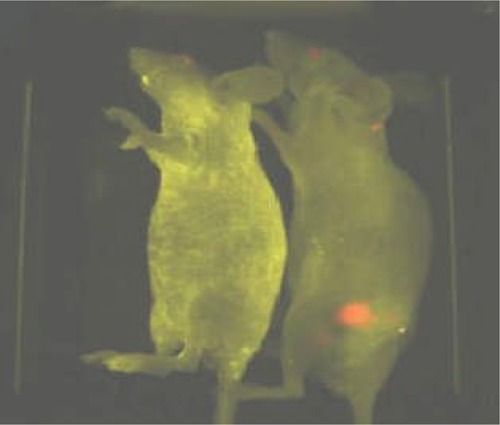
Reprinted by permission from Macmillan Publishers Ltd: [Nature Biotechnology]: Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots; 22(8): 969-76, Copyright 2004
The Use of Nanotechnology in Drug Delivery
Nanospheres/Nanocapsules/Nanoparticles
Depending on the method of preparation, nanoparticles, nanocapsules, and nanospheres can be obtained with different properties and release characteristics for the attached drug. (30)
Nanoparticles are solid colloidal particles consisting of macromolecular substances. The drug of interest is dissolved, entrapped, adsorbed, attached, or encapsulated into the nanoparticle matrix.
Nanocapsules are vesicular systems in which the drug is confined to a cavity surrounded by a unique polymer membrane.
Nanospheres are matrix systems in which the drug is physically and uniformly dispersed.
Inorganic (or Ceramic) Nanoparticles
Inorganic nanoparticles (e.g., silica, alumina, and titania) can protect entrapped enzymes or drugs against denaturation induced by external pH and temperature, and are reportedly compatible with biological systems. Moreover, their surfaces can be easily modified with different functional groups to allow targeting to a desired site. (30) For example, Roy et al. (31) reported on a nanoparticle-based drug carrier for photodynamic therapy. In vitro studies showed that irradiation of a photosensitizing drug entrapped in silica-based nanoparticles results in the generation of singlet oxygen which can cause damage to tumour cells.
The Use of Nanotechnology in Combination/Diagnostic Therapy
Dendrimers
Dendrimers are man-made macromolecular compounds that comprise a series of branches around an inner core. (30) The interaction of dendrimer macromolecules with the molecular environment is mainly controlled by their terminal groups. Due to their globular shape and internal cavities, dendrimers can encapsulate therapeutic agents within the macromolecule interior as well as attach to surface groups.
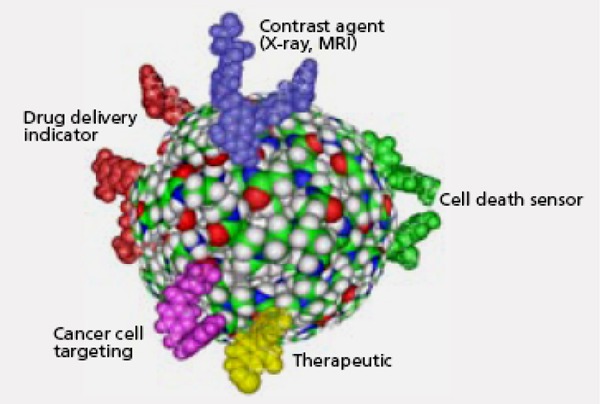
One of the advantages of using nanoparticles as delivery devices for therapeutic and imaging agents is their ability to attach tumour-targeting molecules to the nanoparticle surface (Figure 8).
Figure 8: Example of a Multifunctional Dendrimer Capable of Detecting Cancer and Delivering Drugs.
Source: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Cancer nanotechnology. Going small for big advances: using nanotechnology to advance cancer diagnosis, prevention and treatment [report on the Internet]. January 2004. U.S. Department of Health and Human Services. [cited 2006 Nov. 2]. Available at: http://nano.cancer.gov/resource_center/cancer_nanotechnology_brochure.pdf.
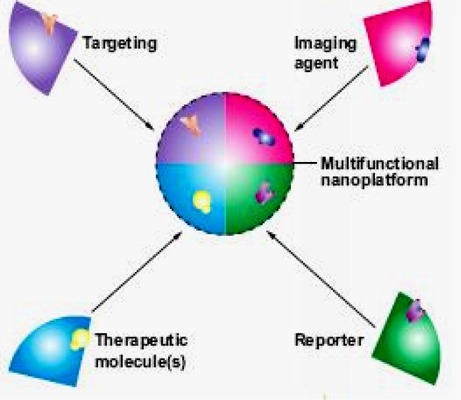
The ultimate goal is to create customizable nanodevices that would be able to circulate through the body, detect cancer-associated molecular changes, image a cancer cell, release a therapeutic agent in a controlled time-release manner, and monitor the effectiveness of the intervention in real time (Figure 9).
Figure 9: General Platforms Can Be Used to Create a Diverse Set of Multifunctional Diagnostic and Therapeutic Devices.
Source: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Cancer nanotechnology. Going small for big advances: using nanotechnology to advance cancer diagnosis, prevention and treatment [report on the Internet]. January 2004. U.S. Department of Health and Human Services. [cited 2006 Nov. 2]. Available at: http://nano.cancer.gov/resource_center/cancer_nanotechnology_brochure.pdf.
Nanoshells
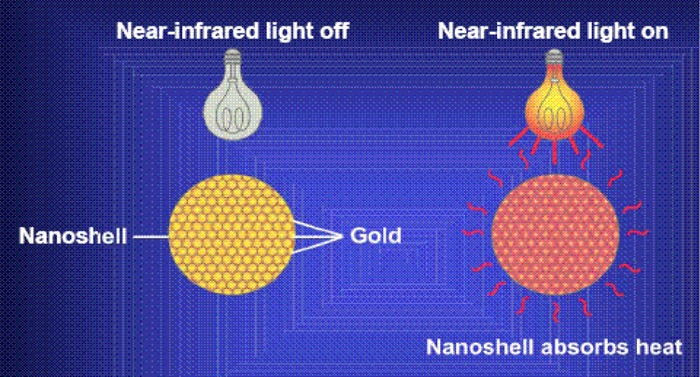
Nanoshells, which have a silica core and metallic outer layer (e.g., gold), are layered nanoparticles with optical resonances that can be “tuned” by the control of the relative size of their layers. Nanoshells have been applied in a number of biological applications, such as the detection of immunoglobulin in whole blood and for thermal ablation of cancerous cells both in vitro and in vivo. (32) Nanoshells can be conjugated to antibodies that recognize cancer cells. Some nanoshells can absorb near-infrared light (Figure 10), which may penetrate several centimetres of human tissue; cells in the vicinity of these nanoshells can be overheated locally and subsequently damaged. (33)
Figure 10: Nanoshells Absorb Heat Upon Exposure to Near Infrared Light.
Source: National Cancer Institute. Slide 18. Nanoshells [Web page]. U.S. National Institutes of Health. [updated 2005 Jan. 28; cited 2006 Sept. 15]. Available at: http://www.cancer.gov/cancertopics/understandingcancer/nanodevices/Slide18
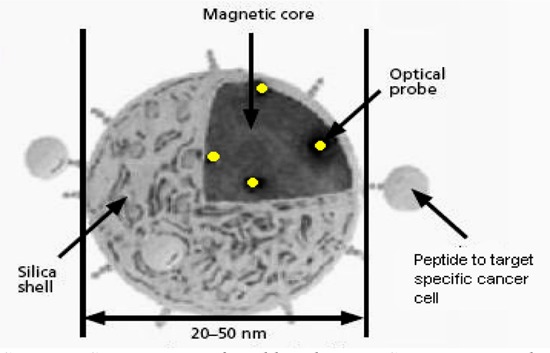
In vitro studies have examined the use of magnetic fields to create magnetocytolysis (lysis of cells in a magnetic field) of cancer cells that were targeted by specific peptides. Bergey et al. (34) demonstrated that a multifunctional nanoparticle (composed of an iron oxide core, a fluorescent probe to aid in optical tracking and a peptide to target specific cancer cells) can destroy in vitro cancer cells upon exposure to a magnetic field similar to that used for MRI in diagnostic settings (Figure 11).
Figure 11: An Optical Probe Coated Iron Oxide Core Is Encapsulated in a Silica Shell With a Targeting Agent on the Surface.
Source: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Cancer nanotechnology. Going small for big advances: using nanotechnology to advance cancer diagnosis, prevention and treatment [report on the Internet]. January 2004. U.S. Department of Health and Human Services. [cited 2006 Nov. 2]. Available at: http://nano.cancer.gov/resource_center/cancer_nanotechnology_brochure.pdf.
National Toxicology Program
The National Toxicology Program (NTP) in the United States has declared that published studies on the inhalation of ultrafine particles suggest that particle size can have an impact on toxicity that is equal to, if not more so than, the impact of chemical composition. (35) (25) Surface properties can be changed by coating nanoscale particles with different materials, but surface chemistry is also influenced by the size of the particle. The interaction of surface area and particle composition in eliciting biological responses adds an extra dimension of complexity in evaluating potential adverse events that may result from exposure to nanomaterials. There are some indications in the literature (36) that manufactured nanoscale materials may distribute in the body in unpredictable ways. (35) An example is quantum dots.
Quantum Dots
In general, there are discrepancies in the literature (36) regarding the toxicity of QDs that can be attributed to several factors: the lack of toxicology-based studies, the variety of QD dosage/exposure concentrations reported in the literature, and the widely varying physicochemical properties of individual QDs.
Absorption, distribution, metabolism, and excretion characteristics are variable for QDs because of the variation in QD physicochemical properties; and QD size, charge, concentration, stability, and outer coating bioactivity. Quantum dot dosage/exposure concentrations reported in the literature (animal and cell culture studies) (36) vary widely in units of measurement (e.g., micrograms/millilitre, molarity, milligrams per kilogram body weight, QDs per cell). (28)
The NTP (35) is engaged in a broad-based research program to address potential human health hazards associated with the manufacture and use of nanoscale materials. This includes testing hypotheses focused on the relationship of key physicochemical parameters of selected manufactured nanomaterials to their potential toxicity. Initial parameters of greatest concern to the NTP are size, shape, surface chemistry, and composition.
Ongoing research activities are focusing initially on 4 classes of nanoscale materials:
metal oxides;
fluorescent crystalline semiconductors (quantum dots);
fullerenes; and
carbon nanotubes.
Depending on the type of study, results from NTP studies are anticipated to be available in the next 1 to 5 years. Results from longer-term studies on rodents will likely take several years beyond that.
Literature Review
Research Questions
What is the status of these multifunctional nanotechnologies? That is, what is the projected timeline to clinical utilization?
What are the systemic effects of multifunctional nanodevices with integrated applications that target, image, and deliver drugs? That is, what are the implications of the emergence of nanotechnology on health human resources training, new specialties, etc.?
Methods
Inclusion criteria
English-language articles (January 2000–June 2006)
Journal articles that report primary data on the effectiveness or cost-effectiveness of nanotechnology treatment obtained in a clinical setting, or analysis of primary data maintained in registries or databases
Clearly described study design and methods
Systematic reviews, randomized controlled trials (RCTs), non-RCTs, and/or cohort studies that have at least 20 patients; and cost-effectiveness studies
Exclusion criteria
Studies that are duplicate publications (superseded by another publication by the same investigator group, with the same objective and data)
Studies with less than 10 patients
Non-English-language articles
Nonsystematic reviews, letters, and editorials
Animal and in vitro studies
Case reports
Studies that do not examine the outcomes of interest
Literature Search
Cochrane Library
INAHTA
EMBASE
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations
Reference section from reviews and extracted articles
Grey literature (conference proceedings, ongoing clinical trials)
Outcomes of Interest
Improved imaging; improved sensitivity/specificity
Improved response rates to therapeutic agents
Decreased toxicity
Results of Literature Review
The Cochrane and INAHTA databases yielded 1 health technology assessment on nanotechnology from Switzerland. (37) A search of MEDLINE and EMBASE starting from the Swiss health technology assessment cut-off date produced 647 citations; of these, none met the inclusion criteria.
A search of the grey literature identified a technology review by RAND. (38)
Projected Timeline of Nanotechnology to Clinical Use
With respect to the question of the translation of nanotechnologies into the clinical arena for patient care, overall, the literature shows that the use of second-generation nanodevices, (e.g., quantum dots, nanoshells, dendrimers) that might be able to target, image, and deliver drugs; and image cell response to therapy in real time are still in the preclinical bench-work stage.
According to the NCI’s Nanotechnology and Cancer Fact Sheet that was posted on their Web site in 2001, nanodevices are in various stages of discovery and development. “Experts believe that quantum dots, nanopores and other devices for detection and diagnosis may be available for clinical use in 5 to 15 years. Therapeutic agents are expected to be available within a similar time frame. Devices that integrate detection and therapy could be used clinically in about 15 or 20 years.” (39)
The Centre for Technology Assessment TA-Swiss, 2003
The TA-Swiss authors (37) convened a Delphi panel of over 70 nanotechnology specialists from different scientific disciplines to discuss the opportunities and risks that could be linked to the use of nanotechnology.
Three rounds of surveys were conducted. The first 2 were directed at researchers (more than one-half from the United States) who were actively involved in nanoscience. The aim was to work out how nanotechnology would be likely to develop. They looked at the following questions:
Where are the earliest breakthroughs likely to be?
How long before these breakthroughs arrive?
What are the technical hurdles that will have to be overcome?
The third survey round focused on the legal, social, and ethical consequences of the new technology. Three-quarters of the specialists were from Germany.
The specialists estimated that, in cancer therapy, nanoparticles (e.g., QDs, encapsulated drugs, and magnetic particles) will be usable in 5 to 10 years. From 2005 to 2008, QDs for diagnostic purposes could be available for wide application. Targeted release of drugs by nanoparticles could be possible by about 2010.
In addition, the specialists suggested that the treatment of cancer would offer the greatest opportunities for nanotechnology breakthroughs (Figure 12).
Figure 12: Therapy Potential and Probability of Realization for Nanotechnology by 2020.
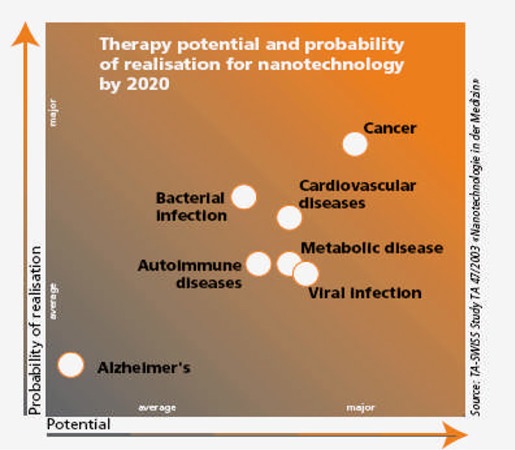
Source: TA-Swiss. Cutting nature’s building blocks down to size: abridged version of the TA-SWISS study “Nanotechnology in Medicine” [report on the Internet]. TA 47A/2003. 2003. [cited 2006 Sept. 15]. Available at: http://www.ta-swiss.ch/www-remain/reports_archive/publications/2003/TA_47A_KF_Nano_e.pdf.
Comments Regarding Estimated Timeframes
The experts failed to agree about whether the development of nanotechnology over the next 20 years is certain. Some were prepared for nanoscience to encounter some unexpected obstacles and surprising solutions; others regarded research in nanotechnology as relatively clear and predictable.
The time span between the first successes in the laboratory and general everyday application is underestimated. Potential problems include the lack of long-term stability of nanostructures and the manufacture of sufficiently large commercially viable quantities of nanotechnology products (in particular, 3-dimensional nanostructures).
All specialists stated that there are unrealistic expectations placed on nanotechnology. For instance, the time and costs between initial laboratory studies at the research stage and application in clinical practice is underestimated.
Nanotechnology-supported diagnoses will be more targeted and faster than conventionally supported medical investigations. There was uncertainty regarding cost-effectiveness (details not reported).
Nanotechnology-based treatment of diseases is advancing more slowly than is diagnosis of diseases.
Potential beneficial effects are not expected on a relatively large scale until after 2020. More than 50% of the specialists estimated that nano-based therapies will lead to major changes in medicine over the next 20 years.
The panel stated that nanotechnology is unlikely to make therapy cheaper than conventional treatment.
Toxicity
The panel commented that the effect of nanoparticles in nontargeted regions of the body (e.g., crossing the blood-brain barrier) is unknown.
Ethical Considerations
The panel stated that there is an ethical consideration regarding where does disease begin if therapy is applied before the symptoms have appeared?
RAND Report: The Global Technology Revolution 2020, In-Depth Analyses, 2006
Silberglitt et al. (38) suggested 3 areas in which nano-enabled drug delivery systems will have significant effects: improving existing pharmaceuticals, rescuing drug candidates, and creating new therapeutic opportunities.
Improving Existing Pharmaceuticals
Drug delivery systems have been used to make old drugs new. Reformulation strategies may extend product lines and life cycles for pharmaceutical developers, and may improve efficacy, dosing schedules, routes, and convenience for patients. As manufacturers feel the pressure of patent expiration on blockbuster drugs, reformulations with nano-enabled drug delivery systems could see acceleration in the next 15 years.
Fast growth is predicted for anticancer agents, and protein and peptide therapeutics, due to shortcomings in existing formulations. Nano-enabled delivery systems may offer improvements in toxicity, stability, specific targeting, and administration routes for these drugs.
Rescuing Drug Candidates
About one-half of new drug candidates fail to reach the market because of problems with absorption, distribution, metabolism, and toxicity; development of these candidates would require improved formulations. Nano-enabled delivery systems may be used in the next 15 years to help pharmaceutical developers bring failed drug candidates to market.
Creating New Therapeutic Opportunities (Delivery of RNA/DNA and Multifunctional Delivery Systems)
These new areas of research could significantly affect drug delivery but are less advanced than the other 2 areas discussed above. While it is unlikely that they will appear on the market in the next 15 years, technological breakthroughs could occur that advance these systems faster than expected.
Summary of Literature Addressing Timelines to Clinical Use
Table 2 summarizes the projected timelines to clinical utilization as reported by NCI, (40) the RAND report, (38) and the Swiss technology assessment. (37)
Table 2: Summary of Timelines to Clinical Use*.
| Organization/Year | Second-Generation Nanodevice | Estimate of When Nanodevice Will Be in Clinical Use |
|---|---|---|
| NCI 2001 (39) | Imaging/detection (e.g., QDs) | ~2006–2016 |
| Therapeutic (e.g., nanoshell) | ~2006–2016 | |
| Combined (e.g., dendrimer) | ~2016–2020 | |
| NCI 2004 (40) | Imaging/detection | ~2009–2019 |
| Therapeutic | ~2009–2019 | |
| Combined | ~2019–2024 | |
| RAND 2006 (38) | Combined | unlikely before 2021 |
| Centre for Technology Assessment TA Swiss 2003 (37) | Imaging | ~ 2005–2010 |
| Therapeutic | ~ 2008–2013 | |
| Combined | ~ 2010–2013 |
NCI indicates National Cancer Institute; QD, quantum dot.
Medical Advisory Secretariat Estimated Timeline for Ontario
Upon synthesizing the estimated timelines from the NCI, the TA Swiss health technology assessment, and the RAND report (Figure 13), it appears that:
Figure 13: Medical Advisory Secretariat Estimated Timeline for the Clinical Use of Second-Generation Nanodevices in Ontario*.
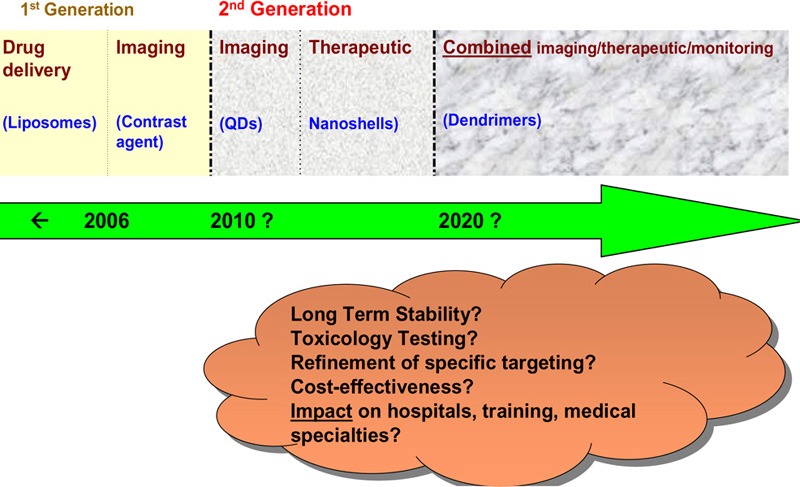
QD refers to quantum dot.
the clinical use of separate imaging and therapeutic nanodevices is estimated to start occurring around 2010;
the clinical use of combined imaging and therapeutic nanodevices is estimated to start occurring around 2020;
changes in the way disease is diagnosed, treated and monitored are anticipated; and
the full (and realistic) extent of these changes within the next 10–20 years is uncertain.
Systemic Effects of Implementing Second-Generation Nanodevices
Table 3 shows the potential systemic effects of second-generation nanodevices (i.e., the implications of the emergence of these nanodevices on health human resources training, new specialties, etc.).
Table 3: Potential Disruptions Caused by Second-Generation Nanodevices*.
| Imaging | Therapeutic | Combined (Detect, Image, Treat, Monitor) |
|---|---|---|
| Increased sensitivity and specificity of QDs or other nanodevices could lead to the replacement of existing technologies (e.g., PSA testing, mammogram). | Sudden demand in use of MRI due to use of nanodevices that are activated in the presence of a magnetic field. | Universal demand to detect cancer–how will patients be prioritized for this? Sudden demand in use of MRI due to use of nanodevices that are activated in the presence of a magnetic field. |
| Cost: possibly more expensive than current screening modalities. | Possibly more expensive than existing therapies (gold nanoshells). | Many functions can be performed on one device → possibly faster, more cost-effective than individual devices. |
| Report of results: possibly faster than existing technologies. | Possibly faster determination of therapeutic efficacy (vs. existing technologies). | Increase in life expectancy of population? Free-up beds in hospitals? |
| Nanodevices may be able to pinpoint with more accuracy when cancer starts. Ethical question – when does diseasestart? Increased demand for imaging by people who are asymptomatic and concerned they may get cancer. |
Nano-radiologist or medical nano-oncologist provides treatment compared to conventional. radiologists or medical oncologists. Creation of nano-nursing compared with conventional nursing. |
Nano-radiologist or medical nano-oncologist provides treatment compared to conventional radiologists. or medical oncologists. Creation of nano-nursing compared to conventional nursing. Uncertainty regarding how many “traditional” cancer radiologists/oncologists should be retained and trained. |
| New branch of (nano) radiology compared to conventional radiology. New/longer training in biochemistry and targeting ligands will be required by nanoradiologists. |
More training required for new nano-treatments. Patient education: people may be concerned regarding the use of nanodevices inside their bodies. |
Longer time to specialize in medicine in order to use nanotechnology clinically? Insufficient number of dendrimer specialists to treat everyone with personalized dendrimers |
| Restricted to specialized centres Possible in-house nanodevice production required to keep up with the demand for use. | Will the same specialized centres that offer imaging also offer treatment? How many specialized centres will be required? | Only specialized centres can perform this combined imaging/treatment. Possible nano-monitoring from patient home via wireless technology. This may free hospital beds for other patients. |
| After imaging with nanodevices, specifically targeted therapeutic nanodevices may also be required for immediate treatment of the patient. Will both of these nanodevices be commercially available in sufficient quantities? | Possible waiting time for preparation of appropriately targeted nanodevice after imaging. (Will a therapeutic nanodevice be immediately available?) Will a patient receive conventional treatment if there is a waiting period required to prepare the therapeutic nanodevice? |
Will there be a patient waiting time required for preparation of personalized dendrimers (hours, days, weeks after a patient sees a physician)? |
MRI indicates magnetic resonance imaging; PSA, prostate-specific antigen testing; QD, quantum dot.
Uncertainties Not Addressed in the Literature
The United States’ NNI funds a variety of research in the economic, ethical, legal, and cultural implications of the use of nanotechnology, as well as the implications for science and education and quality of life.
There are many uncertainties that are sparsely or not addressed at all in the literature regarding second-generation nanodevices. These include:
long term stability and toxicology of nanodevices;
cost-effectiveness of nanodevices;
refinement of specific targeting;
effects on hospitals, physician/nurse training, creation/removal of specialties; and
that pertaining to the question, where does disease begin if therapy is applied before the symptoms have appeared?
Appendices
Appendix 1: Literature Search Strategy
Search date: July 14, 2006
Databases searched: OVID MEDLINE, In-Process & Other Non-Indexed Citations, EMBASE, Cochrane Library, INAHTA
Database: Ovid MEDLINE(R) <1996 to July Week 1 2006>
Search Strategy:
--------------------------------------------------------------------------------
exp Nanostructures/ (3903)
exp Nanotechnology/ (5928)
(Nanotub$ or nanosize$ or nanosphere$ or nanoparticle$ or nanoprobe$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (5309)
ferumoxtran-10.mp. (33)
dendritic compounds.mp. or exp Dendrimers/ (89)
(nanoscale or nanotechnology).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (5766)
or/1-6 (10818)
exp neoplasms/ (642994)
7 and 8 (569)
limit 9 to (humans and english language and yr=“2003 - 2006”) (322)
exp Health Manpower/ (1417)
exp Sociology/ (298595)
exp education/ (162559)
exp Specialties, Medical/ (112591)
exp bioethical issues/ or exp bioethics/ (5297)
exp public policy/ (34734)
or/11-16 (506184)
*Nanotechnology/ (2936)
17 and 18 (56)
exp Nanotechnology/ma, ed, lj [Manpower, Education, Legislation & Jurisprudence] (30)
19 or 20 (71)
limit 21 to (humans and english language and yr=“2003 - 2006”) (27)
10 (322)
(systematic$ review$ or metaanalysis or meta-analysis or random$).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (290686)
23 and 24 (6)
23 (322)
limit 26 to (case reports or comment or editorial or letter or “review”) (75)
26 not 27 (247)
22 or 25 or 28 (272)
Database: EMBASE <1980 to 2006 Week 27>
Search Strategy:
--------------------------------------------------------------------------------
exp nanotechnology/ (2236)
exp nanoparticle/ or exp nanotube/ (9830)
(Nanotub$ or nanosize$ or nanosphere$ or nanoparticle$ or nanoprobe$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (11610)
exp Ultrasmall Superparamagnetic Iron Oxide/ or ferumoxtran-10.mp. (374)
(nanoscale or nanotechnology).mp. [mp=title, abstract, subjectheadings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (3224)
dendritic compounds.mp. or exp Dendrimer/ [mp=title, abstract, subjectheadings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (1529)
or/1-6 (15217)
exp Neoplasm/ (1232761)
exp CANCER/ (48461)
exp Tumor/ (8092)
7 and (8 or 9 or 10) (1138)
limit 11 to (human and english language and yr=“2003 - 2006”) (584)
(systematic$ review$ or metaanalysis or meta-analysis or random$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (366367)
12 and 13 (13)
12 (584)
limit 15 to (editorial or letter or note or“review”) (212)
Case Report/ (892512)
15 not (16 or 17) (366)
exp SOCIETY/ (1782940)
exp Health Care Manpower/ (1023)
exp EDUCATION/ (219373)
exp ethics/ (50464)
exp Policy/ (16550)
exp Medical Specialist/ (23488)
or/19-24 (1788439)
*nanotechnology/ (934)
25 and 26 (194)
limit 27 to (human and english language and yr=“2003 - 2006”) (80)
limit 28 to (editorial or letter or note) (25)
Case Report/ (892512)
28 not (29 or 30) (55)
18 or 31(416)
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Nanotechnology: an evidence-based analysis. Ontario Health Technology Assessment Series 2006; 6(19)
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo@moh.gov.on.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo@moh.gov.on.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo@moh.gov.on.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-4326-5 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and information from practicing medical experts and industry, adds important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to maximize patient outcomes.
If you are aware of any current additional evidence to inform an existing Evidence-Based Analysis, please contact the Medical Advisory Secretariat: MASInfo@moh.gov.on.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to do so, this document may not fully reflect all scientific research available. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superceded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas
Abbreviations
- DNA
Deoxyribonucleic acid
- FDA
Food and Drug Administration
- MRI
Magnetic resonance imaging
- MW
Molecular weight
- NCI
National Cancer Institute
- Nm
Nanometre
- NNI
National Nanotechnology Initiative
- NTP
National Toxicology Program
- PEG
Polyethylene glycol
- PSA
Prostate specific antigen
- QD
Quantum dot
- TOPO
Tri-n-octylphosphine oxide
Glossary
- Carbon nanotube
A nanotube consisting of cylindrically arranged graphite carbon.
- Nano
One one-billionth (10-9).
- Nanomaterial
A material that has engineered properties because of nanometre scale structuring.
- Nanometre (nm)
One one-billionth of a metre.
- Nanoparticle
A particle that is 1 to 100 nm in diameter.
- Nanotechnology
Applications developed using materials that have at least one critical dimension on the nanometre length scale.
- Photobleaching
The progressive loss of fluorescence signal intensity due to exposure to light. This can result in a decreased signal to noise ratio.
- Quantum dots
Nanometre-sized semiconductor crystals that when surface modified to be water soluble and biocompatible, can be attached to targeting molecules and used as fluorescent probes. The wavelength of light from quantum dots is largely controlled by their size and material composition and therefore an entire family of distinct colours can be generated by the same material.
References
- 1.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Cancer nanotechnology plan. A strategic initiative to transform clinical oncology and basic research through the directed application of nanotechnology [report on the Internet] Jul, 2004. [[cited 2006 June 5]]. U.S. Department of Health and Human Services. Available at: http://nano.cancer.gov/about_alliance/cancer_nanotechnology_plan.pdf .
- 2.Cancer Care Ontario. Cancer incidence and mortality in Ontario, 1964-2002 [Web page] [[updated 2006; cited 2007 Mar. 9]]. Available at: http://www.cancercare.on.ca/index_statisticsAllSites.htm .
- 3.Cancer Care Ontario. Cancer fact: what cancers are Ontarians living with? [Web page] [[updated 2004; cited 2007 Mar. 9]]. Available at: http://www.cancercare.on.ca/index_ontarioCancerFacts.htm .
- 4.Cancer Care Ontario. Cancer Fact: population aging and growth are the main contributors to the future burden of cancer [Web page] [[updated 2005; cited 2007 Mar. 9]]. Available at: http://www.cancercare.on.ca/index_ontarioCancerFacts.htm .
- 5.National Science and Technology Council. The national nanotechnology initiative. Research and development leading to a revolution in technology and industry. Supplement to the President’s FY 2006 budget [report on the Internet] Mar, 2005. [[cited 2006 June 5]]. Available at: http://www.nano.gov/NNI_06Budget.pdf .
- 6.National Science and Technology Council. Nanotechnology: shaping the world atom by atom [report on the Internet] Sep, 1999. [[cited 2006 Oct. 15]]. Available at: http://www.wtec.org/loyola/nano/IWGN.Public.Brochure/IWGN.Nanotechnology.Brochure.pdf"> .
- 7.NCI Alliance for Nanotechnology in Cancer. Nanotechnology in cancer. Tools to relieve human cancer. Technology backgrounder [Web page] [[updated 2006; cited 2006 Oct. 16]]. Available at: http://nano.cancer.gov/resource_center/tech_backgrounder.asp .
- 8.Office of Portfolio Analysis and Strategic Initiatives (OPASI). NIH roadmap for medical research. Nanomedicine: overview [Web page] [[updated 2006 Oct. 16; cited 2006 June 6]]. Available at: http://nihroadmap.nih.gov/nanomedicine/index.asp .
- 9.Ferrari M, Downing G. Medical nanotechnology: shortening clinical trials and regulatory pathways? Biodrugs. 2005;19(4):203–10. doi: 10.2165/00063030-200519040-00001. [DOI] [PubMed] [Google Scholar]
- 10.National Nanotechnology Initiative. What is nanotechnology? [Web page] [[updated 2006; cited 2006 June 6]]. Available at: http://www.nano.gov/html/facts/whatIsNano.html .
- 11.U.S. Food and Drug Administration. FDA and nanotechnology products. Frequently asked questions [Web page] [[updated 2006; cited 2006 June 5]]. Available at: www.fda.gov/nanotechnology/faqs.html .
- 12.LaVan DA, Lynn DM, Langer R. Moving smaller in drug discovery and delivery. Nat Rev Drug Discov. 2002;1(1):77–84. doi: 10.1038/nrd707. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. FDA forms internal nanotechnology task force [Web page] [[updated 2006 Aug. 9; cited 2006 Aug. 10]]. Available at: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01426.html .
- 14.Brumfiel G, Hogan J. Consumer products leap aboard the nano bandwagon. Nature. 2006;440(262) doi: 10.1038/440262b. [DOI] [PubMed] [Google Scholar]
- 15.Euractiv. Commission defines action plan for nanotechnologies [Web page] EurActiv.com . [[updated 2005 June 14; cited 2006 June 5]]. Available at: www.euractiv.com/en/science/commission-defines-action-plan-nanotechnolgies/article-140902?
- 16.European Commission. Towards a European strategy for nanotechnology. Communication from the Commission [report on the Internet] 2004. [[cited 2006 June 5]]. Available at: http://ec.europa.eu/research/industrial_technologies/pdf/nanotechnology_communication_en.pdf .
- 17.National Institute for Nanotechnology. Bioengineering research partnership [Web page] [[updated 2002 Sept. 2; cited 2006 Oct. 24]]. Available at: http://nint-innt.nrc-cnrc.gc.ca/research/supra_projects_e.html .
- 18.National Science Foundation. New grants are awarded to inform the public and explore the implications of nanotechnology [Web page] [[updated 2005 Oct. 6; cited 2006 Sept. 19]]. Available at: http://nsf.gov/news/news_summ.jsp?cntn_id=104505&org=olpa&from=news .
- 19.Brower V. Is nanotechnology ready for primetime? J Natl Cancer Inst. 2006;98(1):9–11. doi: 10.1093/jnci/djj028. [DOI] [PubMed] [Google Scholar]
- 20.NCI Alliance for Nanotechnology in Cancer. Mission and goals [Web page] [[updated 2006; cited 2006 May 23]]. Available at: http://nano.cancer.gov/about_alliance/mission.asp .
- 21.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 22.Orive G, Hernandez RM, Gascon AR, Dominguez-Gilo A, Pedraz JL. Drug delivery in biotechnology: present and future. Curr Opin Biotechnol. 2003;14:659–64. doi: 10.1016/j.copbio.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan DC, Ferrari M. Nanotechnology and tumor imaging: seizing an opportunity. Mol Imaging. 2004;3(4):364–9. doi: 10.1162/15353500200404139. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Slide 14. Nanotubes: mapping mutations [Web page] [[updated 2005 Jan. 28; cited 2006 Sept. 15]]. Available at: http://www.cancer.gov/cancertopics/understandingcancer/nanodevices/Slide14 .
- 25.National Cancer Institute. Slide 13. Nanotubes: marking mutations. [[updated 2005 Jan. 28; cited 2006 June 6]]. [Web page] Available at: http://cancer.gov/cancertopics/understandingcancer .
- 26.National Cancer Institute. Slide 12. Nanopores [Web page]. U.S. National Institutes of Health. [[updated 2005 Jan. 28; cited 2006 Oct. 23]]. Available at: http://www.cancer.gov/cancertopics/understandingcancer/nanodevices/Slide12 .
- 27.National Cancer Institute. Slide 15. Quantum dots [Web page]. U.S. National Institutes of Health. [[updated 2006 Jan. 28; cited 2006 Sept. 15]]. Available at: http://www.cancer.gov/cancertopics/understandingcancer/nanodevices/Slide15 .
- 28.Hardman R. A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 2006;114:165–72. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22(8):969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 30.Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today. 2003;8:1112–20. doi: 10.1016/s1359-6446(03)02903-9. [DOI] [PubMed] [Google Scholar]
- 31.Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, et al. Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: A novel drug-carrier system for photodynamic therapy. J Am Chem Soc. 2003;125(26):7860–5. doi: 10.1021/ja0343095. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B. Price RE et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100(23):13549–54. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Cancer Institute. Slide 18. Nanoshells [Web page]. U.S. National Institutes of Health. [[updated 2005 Jan. 28; cited 2006 Sept. 15]]. Available at: http://www.cancer.gov/cancertopics/understandingcancer/nanodevices/Slide18 .
- 34.Bergey EJ, Levy L, Wang X, Krebs LJ, Lal M, Kim KS, et al. DC Magnetic field induced magnetocytolysis of cancer cells targeted by LHRH magnetic nanoparticles in vitro. Biomed Microdevices. 2002;4:293–9. [Google Scholar]
- 35.National Toxicology Program. NTP nanotechnology safety initiative. National Toxicology Program fact sheet [Web page] [[updated 2006 Feb. 28; cited 2006 July 15]]. Available at: http://ntp.niehs.nih.gov/index.cfm?objectid=7E6B19D0-BDB5-82F8-FAE73011304F542A .
- 36.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.TA-Swiss. Cutting nature’s building blocks down to size: abridged version of the TA-SWISS study “Nanotechnology in Medicine” [report on the Internet] 2003. [[cited 2006 Sept. 15]]. TA 47A/2003. Available at: http://www.ta-swiss.ch/www-remain/reports archive/publications/2003/TA_47A_KF_Nano_e.pdf .
- 38.Silberglitt R, Anton PS, Howell DR, Wong A. Santa Monica, CA: RAND Corporation. RAND Technical Report; 2006. The global technology revolution 2020, in-depth analyses. Bio/nano/materials/information trends, drivers, barriers, and social implications [report on the Internet] Available at: http://www.rand.org/pubs/technical_reports/2006/RAND_TR303.pdf . [Google Scholar]
- 39.National Cancer Institute. Nanotechnology and cancer: fact sheet [Web page] [[updated 2001 Aug. 11; cited 2006 Sept. 20]]. Available at: http://www.cancer.gov/newscenter/nanotech .
- 40.Michalowski J, Kerrigan D, Kelly J, Hollen B. Understanding cancer and related topics. Understanding nanodevices [PowerPoint presentation] [[updated 2004; cited 2006 Oct. 10]]. Available at: http://www.cancer.gov/cancertopics/understandingcancer/nanodevices .


