Abstract
In the title compound, [Ru3(C6H12Cl3O3P)(C29H30P2)(CO)9], the bis(di-o-tolylphosphanyl)methane ligand bridges one Ru—Ru bond and the monodentate phosphite ligand bonds to the third Ru atom. Both ligands are equatorial with respect to the Ru3 triangle. Each Ru atom bears one equatorial and two axial terminal carbonyl ligands. The dihedral angles between the two benzene rings in the diphenylphosphanyl groups are 79.52 (10) and 69.88 (10)°. In the crystal, molecules are linked via C—H⋯O hydrogen bonds into chains along [100].
Related literature
For general background to triangulo-triruthenium compounds with general structure Ru3(CO)12-n
L
n (L = group 15 ligand) see: Bruce et al. (1985 ▶,1988a
▶,b
▶); Shawkataly et al. (1998 ▶, 2004 ▶, 2010 ▶, 2011 ▶). For the preparation of the di-o-tolylphosphanyl ligand, see: Filby et al. (2006 ▶). For the stability of the temperature controller used in the data collection, see: Cosier & Glazer (1986 ▶).
Experimental
Crystal data
[Ru3(C6H12Cl3O3P)(C29H30P2)(CO)9]
M r = 1265.25
Monoclinic,
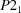
a = 10.1705 (6) Å
b = 20.7490 (12) Å
c = 12.3584 (7) Å
β = 109.241 (1)°
V = 2462.3 (2) Å3
Z = 2
Mo Kα radiation
μ = 1.23 mm−1
T = 100 K
0.63 × 0.30 × 0.09 mm
Data collection
Bruker SMART APEXII DUO CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.513, T max = 0.899
23611 measured reflections
11176 independent reflections
11085 reflections with I > 2σ(I)
R int = 0.016
Refinement
R[F 2 > 2σ(F 2)] = 0.016
wR(F 2) = 0.042
S = 1.05
11176 reflections
590 parameters
1 restraint
H-atom parameters constrained
Δρmax = 0.78 e Å−3
Δρmin = −0.50 e Å−3
Absolute structure: Flack (1983 ▶), 5368 Friedel pairs
Flack parameter: 0.016 (10)
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812023707/rz2762sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812023707/rz2762Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C11—H11A⋯O1i | 0.93 | 2.57 | 3.204 (3) | 126 |
Symmetry code: (i) 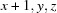 .
.
Acknowledgments
The authors would like to thank the Malaysian Government and Universiti Sains Malaysia (USM) for the Research Grant Nos. 1001/PJJAUH/811188 and 1001/PFIZIK/811160. IAK is grateful to USM for a Visiting Researcher position, SSS thanks USM for a fellowship and CKQ thanks USM for an Incentive Grant.
supplementary crystallographic information
Comment
A large number of substituted derivatives of the type Ru3(CO)12-nLn (L = group 15 ligand) have been reported (Bruce et al.,1985, 1988a,b). As part of our study on the substitution of transition metal-carbonyl clusters with mixed-ligand complexes, we have published several structures of triangulo-triruthenium-carbonyl clusters containing mixed P/As and P/Sb ligands (Shawkataly et al., 1998, 2004, 2010, 2011). Herein we report the synthesis and structure of the title compound.
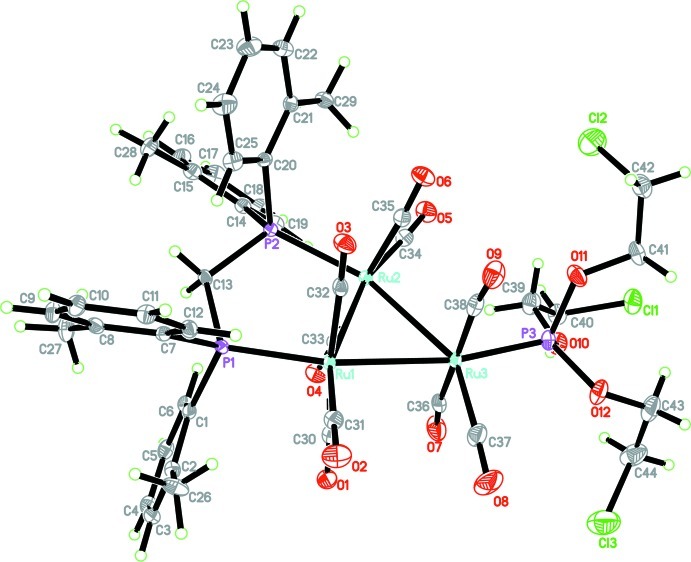
In the title triangulo-triruthenium compound, the bis(di-o-tolylphosphanyl)methane ligand bridges the Ru1–Ru2 bond and the monodentate phosphite ligand bonds to the Ru3 atom. Both phosphorous ligands are equatorial with respect to the Ru3 triangle. Moreover, each Ru atom carries one equatorial and two axial terminal carbonyl ligands (Fig. 1). The dihedral angles between the two benzene rings (C1–C6/C7–C12 and C14–C19/C20–C25) are 79.52 (10) and 69.88 (10)° for the two diphenylphosphanyl groups respectively.
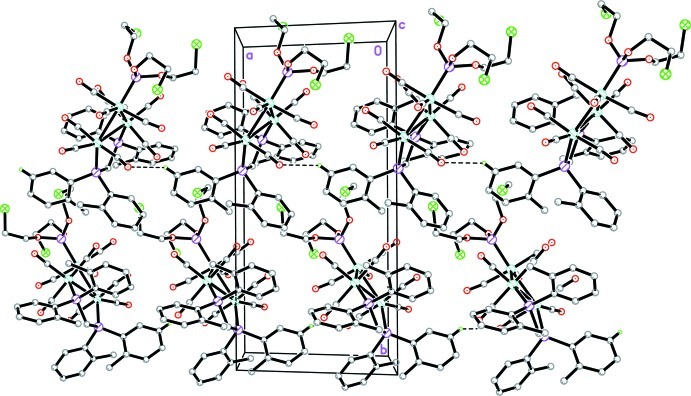
In the crystal structure, Fig. 2, molecules are linked via intermolecular C11–H11A···O1 hydrogen bonds (Table 1) into one-dimensional chains along [100].
Experimental
All manipulations were performed under a dry oxygen-free nitrogen atmosphere using standard Schlenk techniques. All solvents were dried over sodium and distilled from sodium benzophenone ketyl under dry oxygen free nitrogen. Tris(2-chloroethyl)phosphite (Aldrich) was used as received and bis(di-o-tolylphosphanyl)methane (Filby et al., 2006) was prepared by reported procedure. Ru3(CO)10(µ-(2-CH3C6H4)2PCH2P(2-CH3C6H4)2) was prepared by reacting Ru3(CO)12 with bis(di-o-tolylphosphanyl)methane in presence of sodium benzophenone ketyl radical in THF (Shawkataly et al.,2011). The title compound was obtained by refluxing equimolar quantities of Ru3(CO)10(µ-(2-(CH3C6H4)2PCH2P(2-CH3C6H4)2) and tris(2-chloroethyl)phosphite in hexane under nitrogen atmosphere. Crystals suitable for X-ray diffraction were grown by slow solvent/solvent diffusion of CH3OH into CH2Cl2.
Refinement
All H atoms were positioned geometrically and refined using a riding model with C–H = 0.93 or 0.97 Å and Uiso(H) = 1.2 or 1.5 Ueq(C). A rotating group model was applied to the methyl groups.
Figures
Fig. 1.
The molecular structure of the title compound showing 50% probability displacement ellipsoids for non-H atoms.
Fig. 2.
The crystal structure of the title compound, viewed down the c axis. H atoms not involved in hydrogen bonds (dashed lines) have been omitted for clarity.
Crystal data
| [Ru3(C6H12Cl3O3P)(C29H30P2)(CO)9] | F(000) = 1260 |
| Mr = 1265.25 | Dx = 1.707 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2yb | Cell parameters from 9989 reflections |
| a = 10.1705 (6) Å | θ = 2.9–30.1° |
| b = 20.7490 (12) Å | µ = 1.23 mm−1 |
| c = 12.3584 (7) Å | T = 100 K |
| β = 109.241 (1)° | Plate, brown |
| V = 2462.3 (2) Å3 | 0.63 × 0.30 × 0.09 mm |
| Z = 2 |
Data collection
| Bruker SMART APEXII DUO CCD area-detector diffractometer | 11176 independent reflections |
| Radiation source: fine-focus sealed tube | 11085 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.016 |
| φ and ω scans | θmax = 27.5°, θmin = 1.8° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −13→13 |
| Tmin = 0.513, Tmax = 0.899 | k = −26→26 |
| 23611 measured reflections | l = −15→16 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.016 | H-atom parameters constrained |
| wR(F2) = 0.042 | w = 1/[σ2(Fo2) + (0.0205P)2 + 0.6778P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max = 0.001 |
| 11176 reflections | Δρmax = 0.78 e Å−3 |
| 590 parameters | Δρmin = −0.50 e Å−3 |
| 1 restraint | Absolute structure: Flack (1983), 5368 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.016 (10) |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cryosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100 K. |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ru1 | 0.944298 (14) | 0.325120 (7) | 0.809961 (11) | 0.00983 (3) | |
| Ru2 | 0.765192 (14) | 0.257429 (7) | 0.616918 (12) | 0.01032 (3) | |
| Ru3 | 0.788240 (15) | 0.218119 (7) | 0.844371 (12) | 0.01256 (3) | |
| Cl1 | 0.27592 (6) | 0.02481 (3) | 0.67579 (5) | 0.02873 (12) | |
| Cl2 | 0.70147 (10) | −0.02232 (4) | 0.58668 (6) | 0.05110 (19) | |
| Cl3 | 0.49238 (9) | 0.17761 (4) | 1.09900 (7) | 0.0544 (2) | |
| P1 | 0.97101 (5) | 0.41265 (2) | 0.69855 (4) | 0.01024 (9) | |
| P2 | 0.84641 (5) | 0.31652 (2) | 0.48990 (4) | 0.01023 (9) | |
| P3 | 0.66767 (5) | 0.12666 (3) | 0.84199 (4) | 0.01523 (10) | |
| O1 | 0.72264 (15) | 0.40026 (8) | 0.87717 (13) | 0.0205 (3) | |
| O2 | 1.14022 (17) | 0.35167 (9) | 1.05085 (13) | 0.0280 (4) | |
| O3 | 1.17618 (15) | 0.24273 (7) | 0.77020 (13) | 0.0210 (3) | |
| O4 | 0.55800 (15) | 0.36598 (8) | 0.61476 (13) | 0.0214 (3) | |
| O5 | 0.55140 (16) | 0.17020 (8) | 0.45254 (14) | 0.0241 (3) | |
| O6 | 0.96944 (16) | 0.14721 (8) | 0.62476 (13) | 0.0222 (3) | |
| O7 | 0.50273 (18) | 0.28256 (8) | 0.79736 (17) | 0.0318 (4) | |
| O8 | 0.8773 (2) | 0.24850 (11) | 1.09856 (14) | 0.0409 (5) | |
| O9 | 1.05262 (17) | 0.13615 (9) | 0.88318 (15) | 0.0279 (4) | |
| O10 | 0.50288 (16) | 0.12107 (8) | 0.77775 (13) | 0.0226 (3) | |
| O11 | 0.73473 (16) | 0.06597 (7) | 0.80111 (15) | 0.0242 (3) | |
| O12 | 0.66222 (17) | 0.10944 (8) | 0.96670 (14) | 0.0262 (3) | |
| C1 | 0.86732 (19) | 0.48302 (9) | 0.71299 (16) | 0.0127 (4) | |
| C2 | 0.9109 (2) | 0.52284 (10) | 0.81082 (17) | 0.0162 (4) | |
| C3 | 0.8240 (2) | 0.57358 (10) | 0.81935 (18) | 0.0177 (4) | |
| H3A | 0.8521 | 0.5998 | 0.8840 | 0.021* | |
| C4 | 0.6980 (2) | 0.58619 (10) | 0.73529 (19) | 0.0194 (4) | |
| H4A | 0.6438 | 0.6209 | 0.7425 | 0.023* | |
| C5 | 0.6533 (2) | 0.54589 (10) | 0.63914 (18) | 0.0175 (4) | |
| H5A | 0.5681 | 0.5531 | 0.5824 | 0.021* | |
| C6 | 0.7371 (2) | 0.49519 (10) | 0.62942 (17) | 0.0156 (4) | |
| H6A | 0.7064 | 0.4683 | 0.5658 | 0.019* | |
| C7 | 1.14826 (18) | 0.44366 (9) | 0.72276 (16) | 0.0120 (3) | |
| C8 | 1.1781 (2) | 0.49736 (10) | 0.66462 (16) | 0.0143 (4) | |
| C9 | 1.3178 (2) | 0.51406 (10) | 0.68754 (17) | 0.0183 (4) | |
| H9A | 1.3393 | 0.5497 | 0.6508 | 0.022* | |
| C10 | 1.4259 (2) | 0.47910 (10) | 0.76359 (18) | 0.0176 (4) | |
| H10A | 1.5178 | 0.4909 | 0.7757 | 0.021* | |
| C11 | 1.3966 (2) | 0.42688 (10) | 0.82107 (17) | 0.0162 (4) | |
| H11A | 1.4683 | 0.4035 | 0.8725 | 0.019* | |
| C12 | 1.25792 (19) | 0.40972 (9) | 0.80080 (16) | 0.0138 (4) | |
| H12A | 1.2378 | 0.3749 | 0.8401 | 0.017* | |
| C13 | 0.9135 (2) | 0.39790 (9) | 0.54139 (15) | 0.0132 (4) | |
| H13A | 0.8412 | 0.4289 | 0.5047 | 0.016* | |
| H13B | 0.9916 | 0.4068 | 0.5151 | 0.016* | |
| C14 | 0.72006 (19) | 0.33745 (9) | 0.34803 (15) | 0.0133 (4) | |
| C15 | 0.7560 (2) | 0.36585 (10) | 0.25707 (16) | 0.0165 (4) | |
| C16 | 0.6482 (2) | 0.37698 (12) | 0.15341 (17) | 0.0233 (5) | |
| H16A | 0.6698 | 0.3957 | 0.0930 | 0.028* | |
| C17 | 0.5111 (2) | 0.36118 (12) | 0.13777 (18) | 0.0267 (5) | |
| H17A | 0.4427 | 0.3688 | 0.0675 | 0.032* | |
| C18 | 0.4757 (2) | 0.33404 (11) | 0.22648 (17) | 0.0213 (4) | |
| H18A | 0.3837 | 0.3234 | 0.2169 | 0.026* | |
| C19 | 0.58066 (19) | 0.32297 (11) | 0.33074 (16) | 0.0167 (4) | |
| H19A | 0.5568 | 0.3053 | 0.3909 | 0.020* | |
| C20 | 0.99155 (19) | 0.27865 (9) | 0.45717 (15) | 0.0124 (4) | |
| C21 | 0.9691 (2) | 0.22669 (10) | 0.37912 (16) | 0.0146 (4) | |
| C22 | 1.0841 (2) | 0.20189 (10) | 0.35464 (18) | 0.0198 (4) | |
| H22A | 1.0705 | 0.1683 | 0.3022 | 0.024* | |
| C23 | 1.2183 (2) | 0.22580 (12) | 0.40620 (19) | 0.0244 (5) | |
| H23A | 1.2925 | 0.2090 | 0.3871 | 0.029* | |
| C24 | 1.2399 (2) | 0.27481 (11) | 0.48596 (19) | 0.0235 (5) | |
| H24A | 1.3294 | 0.2904 | 0.5222 | 0.028* | |
| C25 | 1.1277 (2) | 0.30073 (10) | 0.51188 (17) | 0.0177 (4) | |
| H25A | 1.1432 | 0.3333 | 0.5664 | 0.021* | |
| C26 | 1.0458 (2) | 0.51396 (11) | 0.90788 (18) | 0.0226 (4) | |
| H26A | 1.0368 | 0.5314 | 0.9770 | 0.034* | |
| H26B | 1.1193 | 0.5360 | 0.8902 | 0.034* | |
| H26C | 1.0674 | 0.4689 | 0.9182 | 0.034* | |
| C27 | 1.0691 (2) | 0.53731 (11) | 0.57765 (19) | 0.0219 (4) | |
| H27A | 1.1129 | 0.5736 | 0.5555 | 0.033* | |
| H27B | 1.0015 | 0.5522 | 0.6108 | 0.033* | |
| H27C | 1.0237 | 0.5115 | 0.5114 | 0.033* | |
| C28 | 0.9023 (2) | 0.38464 (11) | 0.26288 (18) | 0.0202 (4) | |
| H28A | 0.8987 | 0.4074 | 0.1943 | 0.030* | |
| H28B | 0.9580 | 0.3465 | 0.2698 | 0.030* | |
| H28C | 0.9428 | 0.4119 | 0.3282 | 0.030* | |
| C29 | 0.8274 (2) | 0.19827 (10) | 0.31925 (17) | 0.0175 (4) | |
| H29A | 0.8374 | 0.1588 | 0.2823 | 0.026* | |
| H29B | 0.7729 | 0.2282 | 0.2630 | 0.026* | |
| H29C | 0.7815 | 0.1898 | 0.3744 | 0.026* | |
| C30 | 0.79802 (19) | 0.36992 (10) | 0.84677 (15) | 0.0142 (4) | |
| C31 | 1.0657 (2) | 0.34207 (10) | 0.95981 (17) | 0.0170 (4) | |
| C32 | 1.0840 (2) | 0.27162 (9) | 0.78005 (16) | 0.0141 (4) | |
| C33 | 0.63816 (19) | 0.32577 (11) | 0.62185 (15) | 0.0155 (4) | |
| C34 | 0.6298 (2) | 0.20390 (10) | 0.51678 (17) | 0.0168 (4) | |
| C35 | 0.8977 (2) | 0.18882 (10) | 0.62918 (16) | 0.0158 (4) | |
| C36 | 0.6115 (2) | 0.26232 (11) | 0.81124 (18) | 0.0206 (4) | |
| C37 | 0.8431 (2) | 0.23582 (11) | 1.00343 (19) | 0.0243 (5) | |
| C38 | 0.9560 (2) | 0.16876 (11) | 0.86278 (18) | 0.0198 (4) | |
| C39 | 0.4397 (2) | 0.12387 (12) | 0.65567 (19) | 0.0251 (5) | |
| H39A | 0.4861 | 0.0943 | 0.6193 | 0.030* | |
| H39B | 0.4474 | 0.1671 | 0.6284 | 0.030* | |
| C40 | 0.2891 (2) | 0.10560 (11) | 0.62675 (19) | 0.0222 (4) | |
| H40A | 0.2432 | 0.1080 | 0.5445 | 0.027* | |
| H40B | 0.2432 | 0.1354 | 0.6631 | 0.027* | |
| C41 | 0.6882 (2) | −0.00005 (10) | 0.7999 (2) | 0.0231 (4) | |
| H41A | 0.5883 | −0.0027 | 0.7622 | 0.028* | |
| H41B | 0.7099 | −0.0160 | 0.8777 | 0.028* | |
| C42 | 0.7620 (3) | −0.03933 (11) | 0.7364 (2) | 0.0281 (5) | |
| H42A | 0.7480 | −0.0847 | 0.7480 | 0.034* | |
| H42B | 0.8611 | −0.0306 | 0.7672 | 0.034* | |
| C43 | 0.5575 (3) | 0.07229 (13) | 0.9934 (2) | 0.0329 (6) | |
| H43A | 0.5991 | 0.0512 | 1.0668 | 0.040* | |
| H43B | 0.5230 | 0.0391 | 0.9357 | 0.040* | |
| C44 | 0.4365 (3) | 0.11351 (15) | 0.9983 (2) | 0.0378 (6) | |
| H44A | 0.3706 | 0.0867 | 1.0193 | 0.045* | |
| H44B | 0.3891 | 0.1313 | 0.9229 | 0.045* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ru1 | 0.00896 (6) | 0.01019 (7) | 0.00947 (6) | −0.00107 (5) | 0.00188 (5) | 0.00001 (5) |
| Ru2 | 0.00989 (6) | 0.01045 (7) | 0.00997 (6) | −0.00169 (5) | 0.00238 (5) | −0.00079 (5) |
| Ru3 | 0.01346 (7) | 0.01283 (7) | 0.01105 (7) | −0.00329 (6) | 0.00359 (5) | 0.00087 (5) |
| Cl1 | 0.0268 (3) | 0.0272 (3) | 0.0268 (3) | −0.0127 (2) | 0.0015 (2) | 0.0012 (2) |
| Cl2 | 0.0782 (6) | 0.0479 (4) | 0.0265 (3) | 0.0071 (4) | 0.0162 (3) | 0.0035 (3) |
| Cl3 | 0.0605 (5) | 0.0611 (5) | 0.0514 (4) | −0.0233 (4) | 0.0316 (4) | −0.0232 (4) |
| P1 | 0.0102 (2) | 0.0094 (2) | 0.0102 (2) | −0.00029 (17) | 0.00210 (17) | −0.00042 (16) |
| P2 | 0.00951 (19) | 0.0108 (2) | 0.00951 (19) | 0.00008 (17) | 0.00197 (16) | −0.00049 (17) |
| P3 | 0.0132 (2) | 0.0138 (2) | 0.0174 (2) | −0.00183 (19) | 0.00349 (19) | 0.00325 (18) |
| O1 | 0.0175 (7) | 0.0215 (8) | 0.0251 (8) | 0.0008 (6) | 0.0105 (6) | −0.0009 (6) |
| O2 | 0.0252 (8) | 0.0373 (9) | 0.0151 (7) | −0.0007 (7) | −0.0022 (6) | −0.0044 (7) |
| O3 | 0.0179 (7) | 0.0183 (8) | 0.0275 (8) | 0.0005 (6) | 0.0084 (6) | −0.0041 (6) |
| O4 | 0.0161 (7) | 0.0217 (8) | 0.0252 (7) | 0.0030 (6) | 0.0051 (6) | −0.0028 (6) |
| O5 | 0.0201 (7) | 0.0240 (8) | 0.0247 (8) | −0.0077 (6) | 0.0025 (6) | −0.0061 (6) |
| O6 | 0.0220 (7) | 0.0196 (8) | 0.0218 (7) | 0.0047 (6) | 0.0031 (6) | −0.0028 (6) |
| O7 | 0.0283 (8) | 0.0205 (8) | 0.0540 (11) | 0.0045 (7) | 0.0236 (8) | 0.0057 (8) |
| O8 | 0.0530 (11) | 0.0525 (13) | 0.0161 (8) | −0.0167 (10) | 0.0097 (8) | −0.0062 (8) |
| O9 | 0.0220 (8) | 0.0316 (9) | 0.0301 (9) | 0.0071 (7) | 0.0086 (7) | 0.0129 (7) |
| O10 | 0.0199 (7) | 0.0241 (8) | 0.0213 (7) | −0.0052 (6) | 0.0032 (6) | 0.0011 (6) |
| O11 | 0.0228 (8) | 0.0144 (8) | 0.0388 (9) | −0.0035 (6) | 0.0146 (7) | −0.0032 (7) |
| O12 | 0.0275 (8) | 0.0288 (9) | 0.0238 (8) | −0.0068 (7) | 0.0105 (7) | 0.0066 (7) |
| C1 | 0.0142 (8) | 0.0104 (9) | 0.0147 (8) | 0.0001 (7) | 0.0064 (7) | 0.0020 (7) |
| C2 | 0.0178 (9) | 0.0140 (10) | 0.0171 (9) | −0.0012 (7) | 0.0064 (8) | 0.0010 (7) |
| C3 | 0.0216 (10) | 0.0136 (10) | 0.0211 (10) | −0.0017 (8) | 0.0111 (8) | −0.0029 (8) |
| C4 | 0.0213 (10) | 0.0143 (10) | 0.0270 (11) | 0.0056 (8) | 0.0138 (9) | 0.0051 (8) |
| C5 | 0.0147 (9) | 0.0182 (10) | 0.0201 (9) | 0.0037 (8) | 0.0064 (8) | 0.0078 (8) |
| C6 | 0.0149 (9) | 0.0154 (10) | 0.0152 (9) | −0.0008 (7) | 0.0033 (7) | 0.0011 (7) |
| C7 | 0.0104 (8) | 0.0135 (9) | 0.0127 (8) | −0.0016 (7) | 0.0045 (7) | −0.0032 (7) |
| C8 | 0.0166 (9) | 0.0119 (9) | 0.0139 (8) | −0.0007 (7) | 0.0044 (7) | −0.0025 (7) |
| C9 | 0.0211 (10) | 0.0158 (10) | 0.0193 (9) | −0.0042 (8) | 0.0086 (8) | 0.0011 (8) |
| C10 | 0.0119 (8) | 0.0198 (10) | 0.0226 (10) | −0.0034 (7) | 0.0077 (8) | −0.0022 (8) |
| C11 | 0.0133 (9) | 0.0181 (10) | 0.0168 (9) | 0.0009 (7) | 0.0045 (7) | −0.0014 (7) |
| C12 | 0.0134 (8) | 0.0128 (9) | 0.0155 (9) | −0.0011 (7) | 0.0052 (7) | −0.0010 (7) |
| C13 | 0.0159 (9) | 0.0118 (9) | 0.0105 (8) | −0.0024 (7) | 0.0022 (7) | −0.0004 (7) |
| C14 | 0.0146 (8) | 0.0126 (10) | 0.0102 (8) | 0.0014 (7) | 0.0008 (7) | −0.0006 (7) |
| C15 | 0.0178 (9) | 0.0158 (10) | 0.0132 (8) | 0.0011 (8) | 0.0017 (7) | −0.0014 (7) |
| C16 | 0.0285 (11) | 0.0278 (12) | 0.0126 (9) | 0.0037 (9) | 0.0054 (8) | 0.0029 (8) |
| C17 | 0.0230 (11) | 0.0334 (13) | 0.0162 (9) | 0.0048 (10) | −0.0037 (8) | 0.0025 (9) |
| C18 | 0.0159 (9) | 0.0231 (12) | 0.0205 (9) | 0.0023 (8) | 0.0001 (8) | −0.0015 (8) |
| C19 | 0.0162 (8) | 0.0160 (9) | 0.0161 (8) | 0.0014 (8) | 0.0030 (7) | −0.0008 (8) |
| C20 | 0.0138 (8) | 0.0125 (9) | 0.0117 (8) | 0.0026 (7) | 0.0052 (7) | 0.0025 (7) |
| C21 | 0.0180 (9) | 0.0133 (10) | 0.0124 (8) | 0.0020 (7) | 0.0048 (7) | 0.0026 (7) |
| C22 | 0.0221 (10) | 0.0185 (11) | 0.0190 (9) | 0.0036 (8) | 0.0071 (8) | −0.0022 (8) |
| C23 | 0.0161 (9) | 0.0305 (12) | 0.0287 (11) | 0.0081 (9) | 0.0102 (8) | 0.0009 (9) |
| C24 | 0.0121 (9) | 0.0309 (13) | 0.0269 (11) | 0.0008 (8) | 0.0058 (8) | −0.0016 (9) |
| C25 | 0.0146 (9) | 0.0210 (10) | 0.0157 (9) | −0.0009 (8) | 0.0024 (7) | −0.0014 (8) |
| C26 | 0.0204 (10) | 0.0223 (11) | 0.0196 (10) | 0.0031 (9) | −0.0007 (8) | −0.0090 (8) |
| C27 | 0.0192 (10) | 0.0190 (10) | 0.0237 (11) | −0.0020 (8) | 0.0020 (8) | 0.0089 (8) |
| C28 | 0.0225 (10) | 0.0224 (11) | 0.0166 (9) | −0.0004 (8) | 0.0077 (8) | 0.0035 (8) |
| C29 | 0.0182 (9) | 0.0163 (10) | 0.0179 (9) | −0.0006 (7) | 0.0057 (8) | −0.0043 (7) |
| C30 | 0.0117 (8) | 0.0161 (10) | 0.0126 (8) | −0.0036 (7) | 0.0010 (7) | 0.0012 (7) |
| C31 | 0.0149 (9) | 0.0172 (10) | 0.0186 (9) | −0.0007 (7) | 0.0052 (8) | −0.0009 (7) |
| C32 | 0.0155 (9) | 0.0126 (10) | 0.0125 (8) | −0.0044 (7) | 0.0023 (7) | −0.0001 (7) |
| C33 | 0.0138 (8) | 0.0194 (10) | 0.0128 (8) | −0.0050 (8) | 0.0036 (7) | −0.0022 (8) |
| C34 | 0.0147 (9) | 0.0194 (11) | 0.0160 (9) | −0.0003 (7) | 0.0046 (7) | 0.0002 (7) |
| C35 | 0.0167 (9) | 0.0168 (10) | 0.0117 (8) | −0.0041 (8) | 0.0016 (7) | −0.0011 (7) |
| C36 | 0.0258 (10) | 0.0134 (10) | 0.0262 (10) | −0.0049 (9) | 0.0137 (8) | −0.0003 (8) |
| C37 | 0.0266 (11) | 0.0261 (12) | 0.0212 (11) | −0.0078 (9) | 0.0091 (9) | 0.0011 (8) |
| C38 | 0.0194 (10) | 0.0228 (11) | 0.0166 (9) | −0.0056 (8) | 0.0051 (8) | 0.0047 (8) |
| C39 | 0.0241 (11) | 0.0271 (12) | 0.0212 (11) | −0.0020 (9) | 0.0035 (9) | 0.0032 (9) |
| C40 | 0.0178 (10) | 0.0223 (11) | 0.0231 (11) | −0.0012 (8) | 0.0019 (8) | 0.0008 (8) |
| C41 | 0.0248 (11) | 0.0125 (10) | 0.0292 (11) | −0.0033 (8) | 0.0053 (9) | 0.0018 (8) |
| C42 | 0.0339 (12) | 0.0194 (11) | 0.0287 (12) | 0.0029 (10) | 0.0071 (10) | 0.0029 (9) |
| C43 | 0.0349 (13) | 0.0343 (14) | 0.0338 (13) | −0.0105 (11) | 0.0170 (11) | 0.0080 (11) |
| C44 | 0.0358 (14) | 0.0510 (18) | 0.0310 (13) | −0.0176 (12) | 0.0169 (11) | −0.0111 (12) |
Geometric parameters (Å, º)
| Ru1—C31 | 1.890 (2) | C10—C11 | 1.381 (3) |
| Ru1—C30 | 1.931 (2) | C10—H10A | 0.9300 |
| Ru1—C32 | 1.933 (2) | C11—C12 | 1.395 (3) |
| Ru1—P1 | 2.3483 (5) | C11—H11A | 0.9300 |
| Ru1—Ru3 | 2.8415 (2) | C12—H12A | 0.9300 |
| Ru1—Ru2 | 2.8492 (2) | C13—H13A | 0.9700 |
| Ru2—C34 | 1.881 (2) | C13—H13B | 0.9700 |
| Ru2—C35 | 1.931 (2) | C14—C19 | 1.395 (3) |
| Ru2—C33 | 1.933 (2) | C14—C15 | 1.419 (3) |
| Ru2—P2 | 2.3462 (5) | C15—C16 | 1.404 (3) |
| Ru2—Ru3 | 2.8614 (2) | C15—C28 | 1.517 (3) |
| Ru3—C37 | 1.894 (2) | C16—C17 | 1.382 (3) |
| Ru3—C38 | 1.938 (2) | C16—H16A | 0.9300 |
| Ru3—C36 | 1.938 (2) | C17—C18 | 1.382 (3) |
| Ru3—P3 | 2.2543 (5) | C17—H17A | 0.9300 |
| Cl1—C40 | 1.803 (2) | C18—C19 | 1.395 (3) |
| Cl2—C42 | 1.782 (3) | C18—H18A | 0.9300 |
| Cl3—C44 | 1.782 (3) | C19—H19A | 0.9300 |
| P1—C7 | 1.8434 (19) | C20—C25 | 1.402 (3) |
| P1—C1 | 1.844 (2) | C20—C21 | 1.414 (3) |
| P1—C13 | 1.8600 (19) | C21—C22 | 1.399 (3) |
| P2—C20 | 1.8313 (19) | C21—C29 | 1.507 (3) |
| P2—C14 | 1.8518 (19) | C22—C23 | 1.394 (3) |
| P2—C13 | 1.8534 (19) | C22—H22A | 0.9300 |
| P3—O11 | 1.5916 (16) | C23—C24 | 1.382 (3) |
| P3—O12 | 1.6007 (16) | C23—H23A | 0.9300 |
| P3—O10 | 1.6050 (16) | C24—C25 | 1.392 (3) |
| O1—C30 | 1.147 (2) | C24—H24A | 0.9300 |
| O2—C31 | 1.148 (3) | C25—H25A | 0.9300 |
| O3—C32 | 1.152 (2) | C26—H26A | 0.9600 |
| O4—C33 | 1.150 (3) | C26—H26B | 0.9600 |
| O5—C34 | 1.157 (3) | C26—H26C | 0.9600 |
| O6—C35 | 1.143 (3) | C27—H27A | 0.9600 |
| O7—C36 | 1.142 (3) | C27—H27B | 0.9600 |
| O8—C37 | 1.141 (3) | C27—H27C | 0.9600 |
| O9—C38 | 1.151 (3) | C28—H28A | 0.9600 |
| O10—C39 | 1.433 (3) | C28—H28B | 0.9600 |
| O11—C41 | 1.448 (3) | C28—H28C | 0.9600 |
| O12—C43 | 1.439 (3) | C29—H29A | 0.9600 |
| C1—C6 | 1.408 (3) | C29—H29B | 0.9600 |
| C1—C2 | 1.410 (3) | C29—H29C | 0.9600 |
| C2—C3 | 1.401 (3) | C39—C40 | 1.502 (3) |
| C2—C26 | 1.507 (3) | C39—H39A | 0.9700 |
| C3—C4 | 1.383 (3) | C39—H39B | 0.9700 |
| C3—H3A | 0.9300 | C40—H40A | 0.9700 |
| C4—C5 | 1.401 (3) | C40—H40B | 0.9700 |
| C4—H4A | 0.9300 | C41—C42 | 1.494 (3) |
| C5—C6 | 1.384 (3) | C41—H41A | 0.9700 |
| C5—H5A | 0.9300 | C41—H41B | 0.9700 |
| C6—H6A | 0.9300 | C42—H42A | 0.9700 |
| C7—C12 | 1.400 (3) | C42—H42B | 0.9700 |
| C7—C8 | 1.412 (3) | C43—C44 | 1.516 (4) |
| C8—C9 | 1.399 (3) | C43—H43A | 0.9700 |
| C8—C27 | 1.512 (3) | C43—H43B | 0.9700 |
| C9—C10 | 1.392 (3) | C44—H44A | 0.9700 |
| C9—H9A | 0.9300 | C44—H44B | 0.9700 |
| C31—Ru1—C30 | 89.14 (8) | H13A—C13—H13B | 107.2 |
| C31—Ru1—C32 | 90.58 (8) | C19—C14—C15 | 118.81 (17) |
| C30—Ru1—C32 | 173.49 (8) | C19—C14—P2 | 116.59 (14) |
| C31—Ru1—P1 | 105.43 (6) | C15—C14—P2 | 124.59 (14) |
| C30—Ru1—P1 | 90.73 (6) | C16—C15—C14 | 117.61 (19) |
| C32—Ru1—P1 | 95.61 (6) | C16—C15—C28 | 117.38 (19) |
| C31—Ru1—Ru3 | 102.45 (6) | C14—C15—C28 | 125.01 (17) |
| C30—Ru1—Ru3 | 80.17 (6) | C17—C16—C15 | 122.5 (2) |
| C32—Ru1—Ru3 | 93.55 (6) | C17—C16—H16A | 118.8 |
| P1—Ru1—Ru3 | 150.492 (13) | C15—C16—H16A | 118.8 |
| C31—Ru1—Ru2 | 160.62 (6) | C18—C17—C16 | 120.02 (19) |
| C30—Ru1—Ru2 | 96.02 (5) | C18—C17—H17A | 120.0 |
| C32—Ru1—Ru2 | 82.20 (5) | C16—C17—H17A | 120.0 |
| P1—Ru1—Ru2 | 93.203 (13) | C17—C18—C19 | 118.66 (19) |
| Ru3—Ru1—Ru2 | 60.372 (6) | C17—C18—H18A | 120.7 |
| C34—Ru2—C35 | 87.53 (8) | C19—C18—H18A | 120.7 |
| C34—Ru2—C33 | 95.85 (8) | C18—C19—C14 | 122.40 (19) |
| C35—Ru2—C33 | 174.03 (8) | C18—C19—H19A | 118.8 |
| C34—Ru2—P2 | 102.19 (6) | C14—C19—H19A | 118.8 |
| C35—Ru2—P2 | 92.71 (6) | C25—C20—C21 | 119.32 (17) |
| C33—Ru2—P2 | 91.38 (6) | C25—C20—P2 | 119.52 (15) |
| C34—Ru2—Ru1 | 165.79 (6) | C21—C20—P2 | 121.15 (14) |
| C35—Ru2—Ru1 | 93.48 (6) | C22—C21—C20 | 118.00 (18) |
| C33—Ru2—Ru1 | 82.04 (5) | C22—C21—C29 | 118.86 (18) |
| P2—Ru2—Ru1 | 91.930 (13) | C20—C21—C29 | 123.11 (17) |
| C34—Ru2—Ru3 | 106.46 (6) | C23—C22—C21 | 122.2 (2) |
| C35—Ru2—Ru3 | 83.32 (6) | C23—C22—H22A | 118.9 |
| C33—Ru2—Ru3 | 91.00 (5) | C21—C22—H22A | 118.9 |
| P2—Ru2—Ru3 | 150.848 (13) | C24—C23—C22 | 119.33 (19) |
| Ru1—Ru2—Ru3 | 59.680 (6) | C24—C23—H23A | 120.3 |
| C37—Ru3—C38 | 91.93 (10) | C22—C23—H23A | 120.3 |
| C37—Ru3—C36 | 93.62 (10) | C23—C24—C25 | 119.89 (19) |
| C38—Ru3—C36 | 173.94 (9) | C23—C24—H24A | 120.1 |
| C37—Ru3—P3 | 98.61 (7) | C25—C24—H24A | 120.1 |
| C38—Ru3—P3 | 90.61 (6) | C24—C25—C20 | 121.15 (19) |
| C36—Ru3—P3 | 86.15 (6) | C24—C25—H25A | 119.4 |
| C37—Ru3—Ru1 | 90.90 (7) | C20—C25—H25A | 119.4 |
| C38—Ru3—Ru1 | 85.24 (6) | C2—C26—H26A | 109.5 |
| C36—Ru3—Ru1 | 97.10 (6) | C2—C26—H26B | 109.5 |
| P3—Ru3—Ru1 | 169.752 (15) | H26A—C26—H26B | 109.5 |
| C37—Ru3—Ru2 | 149.88 (7) | C2—C26—H26C | 109.5 |
| C38—Ru3—Ru2 | 92.86 (6) | H26A—C26—H26C | 109.5 |
| C36—Ru3—Ru2 | 83.55 (6) | H26B—C26—H26C | 109.5 |
| P3—Ru3—Ru2 | 111.042 (14) | C8—C27—H27A | 109.5 |
| Ru1—Ru3—Ru2 | 59.948 (5) | C8—C27—H27B | 109.5 |
| C7—P1—C1 | 105.53 (9) | H27A—C27—H27B | 109.5 |
| C7—P1—C13 | 100.44 (8) | C8—C27—H27C | 109.5 |
| C1—P1—C13 | 103.79 (9) | H27A—C27—H27C | 109.5 |
| C7—P1—Ru1 | 118.08 (6) | H27B—C27—H27C | 109.5 |
| C1—P1—Ru1 | 112.05 (6) | C15—C28—H28A | 109.5 |
| C13—P1—Ru1 | 115.32 (6) | C15—C28—H28B | 109.5 |
| C20—P2—C14 | 104.47 (8) | H28A—C28—H28B | 109.5 |
| C20—P2—C13 | 103.56 (9) | C15—C28—H28C | 109.5 |
| C14—P2—C13 | 99.99 (8) | H28A—C28—H28C | 109.5 |
| C20—P2—Ru2 | 114.25 (6) | H28B—C28—H28C | 109.5 |
| C14—P2—Ru2 | 117.94 (6) | C21—C29—H29A | 109.5 |
| C13—P2—Ru2 | 114.67 (6) | C21—C29—H29B | 109.5 |
| O11—P3—O12 | 106.47 (9) | H29A—C29—H29B | 109.5 |
| O11—P3—O10 | 105.82 (9) | C21—C29—H29C | 109.5 |
| O12—P3—O10 | 95.64 (9) | H29A—C29—H29C | 109.5 |
| O11—P3—Ru3 | 112.45 (6) | H29B—C29—H29C | 109.5 |
| O12—P3—Ru3 | 111.51 (7) | O1—C30—Ru1 | 172.48 (16) |
| O10—P3—Ru3 | 122.83 (6) | O2—C31—Ru1 | 179.19 (19) |
| C39—O10—P3 | 123.43 (14) | O3—C32—Ru1 | 173.73 (17) |
| C41—O11—P3 | 125.37 (14) | O4—C33—Ru2 | 174.15 (16) |
| C43—O12—P3 | 127.12 (16) | O5—C34—Ru2 | 176.56 (18) |
| C6—C1—C2 | 118.54 (18) | O6—C35—Ru2 | 173.01 (17) |
| C6—C1—P1 | 120.05 (15) | O7—C36—Ru3 | 172.27 (19) |
| C2—C1—P1 | 121.25 (15) | O8—C37—Ru3 | 177.7 (2) |
| C3—C2—C1 | 118.49 (18) | O9—C38—Ru3 | 172.67 (18) |
| C3—C2—C26 | 117.62 (18) | O10—C39—C40 | 107.98 (18) |
| C1—C2—C26 | 123.88 (18) | O10—C39—H39A | 110.1 |
| C4—C3—C2 | 122.49 (19) | C40—C39—H39A | 110.1 |
| C4—C3—H3A | 118.8 | O10—C39—H39B | 110.1 |
| C2—C3—H3A | 118.8 | C40—C39—H39B | 110.1 |
| C3—C4—C5 | 119.07 (19) | H39A—C39—H39B | 108.4 |
| C3—C4—H4A | 120.5 | C39—C40—Cl1 | 109.75 (16) |
| C5—C4—H4A | 120.5 | C39—C40—H40A | 109.7 |
| C6—C5—C4 | 119.36 (19) | Cl1—C40—H40A | 109.7 |
| C6—C5—H5A | 120.3 | C39—C40—H40B | 109.7 |
| C4—C5—H5A | 120.3 | Cl1—C40—H40B | 109.7 |
| C5—C6—C1 | 122.00 (19) | H40A—C40—H40B | 108.2 |
| C5—C6—H6A | 119.0 | O11—C41—C42 | 107.60 (18) |
| C1—C6—H6A | 119.0 | O11—C41—H41A | 110.2 |
| C12—C7—C8 | 119.44 (17) | C42—C41—H41A | 110.2 |
| C12—C7—P1 | 116.78 (14) | O11—C41—H41B | 110.2 |
| C8—C7—P1 | 123.74 (14) | C42—C41—H41B | 110.2 |
| C9—C8—C7 | 117.83 (18) | H41A—C41—H41B | 108.5 |
| C9—C8—C27 | 117.79 (18) | C41—C42—Cl2 | 112.09 (17) |
| C7—C8—C27 | 124.38 (18) | C41—C42—H42A | 109.2 |
| C10—C9—C8 | 122.10 (19) | Cl2—C42—H42A | 109.2 |
| C10—C9—H9A | 119.0 | C41—C42—H42B | 109.2 |
| C8—C9—H9A | 119.0 | Cl2—C42—H42B | 109.2 |
| C11—C10—C9 | 120.03 (18) | H42A—C42—H42B | 107.9 |
| C11—C10—H10A | 120.0 | O12—C43—C44 | 112.3 (2) |
| C9—C10—H10A | 120.0 | O12—C43—H43A | 109.2 |
| C10—C11—C12 | 118.98 (18) | C44—C43—H43A | 109.2 |
| C10—C11—H11A | 120.5 | O12—C43—H43B | 109.2 |
| C12—C11—H11A | 120.5 | C44—C43—H43B | 109.2 |
| C11—C12—C7 | 121.60 (18) | H43A—C43—H43B | 107.9 |
| C11—C12—H12A | 119.2 | C43—C44—Cl3 | 111.89 (19) |
| C7—C12—H12A | 119.2 | C43—C44—H44A | 109.2 |
| P2—C13—P1 | 117.51 (10) | Cl3—C44—H44A | 109.2 |
| P2—C13—H13A | 107.9 | C43—C44—H44B | 109.2 |
| P1—C13—H13A | 107.9 | Cl3—C44—H44B | 109.2 |
| P2—C13—H13B | 107.9 | H44A—C44—H44B | 107.9 |
| P1—C13—H13B | 107.9 | ||
| C31—Ru1—Ru2—C34 | 43.1 (3) | C7—P1—C1—C6 | 131.77 (16) |
| C30—Ru1—Ru2—C34 | −61.6 (3) | C13—P1—C1—C6 | 26.60 (18) |
| C32—Ru1—Ru2—C34 | 112.1 (3) | Ru1—P1—C1—C6 | −98.46 (15) |
| P1—Ru1—Ru2—C34 | −152.7 (3) | C7—P1—C1—C2 | −52.89 (18) |
| Ru3—Ru1—Ru2—C34 | 13.5 (3) | C13—P1—C1—C2 | −158.06 (16) |
| C31—Ru1—Ru2—C35 | −50.52 (19) | Ru1—P1—C1—C2 | 76.88 (16) |
| C30—Ru1—Ru2—C35 | −155.25 (8) | C6—C1—C2—C3 | −1.3 (3) |
| C32—Ru1—Ru2—C35 | 18.44 (8) | P1—C1—C2—C3 | −176.74 (15) |
| P1—Ru1—Ru2—C35 | 113.67 (6) | C6—C1—C2—C26 | 178.31 (19) |
| Ru3—Ru1—Ru2—C35 | −80.18 (6) | P1—C1—C2—C26 | 2.9 (3) |
| C31—Ru1—Ru2—C33 | 125.52 (19) | C1—C2—C3—C4 | −0.4 (3) |
| C30—Ru1—Ru2—C33 | 20.79 (8) | C26—C2—C3—C4 | 179.9 (2) |
| C32—Ru1—Ru2—C33 | −165.52 (8) | C2—C3—C4—C5 | 1.7 (3) |
| P1—Ru1—Ru2—C33 | −70.29 (6) | C3—C4—C5—C6 | −1.2 (3) |
| Ru3—Ru1—Ru2—C33 | 95.86 (6) | C4—C5—C6—C1 | −0.6 (3) |
| C31—Ru1—Ru2—P2 | −143.36 (18) | C2—C1—C6—C5 | 1.9 (3) |
| C30—Ru1—Ru2—P2 | 111.91 (6) | P1—C1—C6—C5 | 177.35 (15) |
| C32—Ru1—Ru2—P2 | −74.39 (6) | C1—P1—C7—C12 | 130.80 (15) |
| P1—Ru1—Ru2—P2 | 20.834 (17) | C13—P1—C7—C12 | −121.58 (15) |
| Ru3—Ru1—Ru2—P2 | −173.017 (13) | Ru1—P1—C7—C12 | 4.65 (17) |
| C31—Ru1—Ru2—Ru3 | 29.66 (18) | C1—P1—C7—C8 | −51.49 (18) |
| C30—Ru1—Ru2—Ru3 | −75.07 (6) | C13—P1—C7—C8 | 56.13 (17) |
| C32—Ru1—Ru2—Ru3 | 98.62 (6) | Ru1—P1—C7—C8 | −177.64 (13) |
| P1—Ru1—Ru2—Ru3 | −166.149 (13) | C12—C7—C8—C9 | 0.4 (3) |
| C31—Ru1—Ru3—C37 | 17.63 (10) | P1—C7—C8—C9 | −177.22 (15) |
| C30—Ru1—Ru3—C37 | −69.28 (9) | C12—C7—C8—C27 | 179.29 (19) |
| C32—Ru1—Ru3—C37 | 109.01 (9) | P1—C7—C8—C27 | 1.6 (3) |
| P1—Ru1—Ru3—C37 | −143.02 (8) | C7—C8—C9—C10 | 0.9 (3) |
| Ru2—Ru1—Ru3—C37 | −172.05 (7) | C27—C8—C9—C10 | −178.0 (2) |
| C31—Ru1—Ru3—C38 | −74.23 (9) | C8—C9—C10—C11 | −1.4 (3) |
| C30—Ru1—Ru3—C38 | −161.14 (8) | C9—C10—C11—C12 | 0.5 (3) |
| C32—Ru1—Ru3—C38 | 17.15 (8) | C10—C11—C12—C7 | 0.8 (3) |
| P1—Ru1—Ru3—C38 | 125.12 (7) | C8—C7—C12—C11 | −1.3 (3) |
| Ru2—Ru1—Ru3—C38 | 96.09 (6) | P1—C7—C12—C11 | 176.55 (15) |
| C31—Ru1—Ru3—C36 | 111.39 (9) | C20—P2—C13—P1 | −103.00 (12) |
| C30—Ru1—Ru3—C36 | 24.48 (8) | C14—P2—C13—P1 | 149.33 (11) |
| C32—Ru1—Ru3—C36 | −157.23 (8) | Ru2—P2—C13—P1 | 22.14 (13) |
| P1—Ru1—Ru3—C36 | −49.26 (7) | C7—P1—C13—P2 | 126.57 (11) |
| Ru2—Ru1—Ru3—C36 | −78.29 (6) | C1—P1—C13—P2 | −124.43 (11) |
| C31—Ru1—Ru3—P3 | −140.61 (10) | Ru1—P1—C13—P2 | −1.50 (13) |
| C30—Ru1—Ru3—P3 | 132.49 (10) | C20—P2—C14—C19 | 135.20 (16) |
| C32—Ru1—Ru3—P3 | −49.23 (10) | C13—P2—C14—C19 | −117.87 (16) |
| P1—Ru1—Ru3—P3 | 58.74 (9) | Ru2—P2—C14—C19 | 7.10 (18) |
| Ru2—Ru1—Ru3—P3 | 29.71 (8) | C20—P2—C14—C15 | −43.81 (19) |
| C31—Ru1—Ru3—Ru2 | −170.32 (6) | C13—P2—C14—C15 | 63.13 (18) |
| C30—Ru1—Ru3—Ru2 | 102.77 (5) | Ru2—P2—C14—C15 | −171.90 (14) |
| C32—Ru1—Ru3—Ru2 | −78.94 (5) | C19—C14—C15—C16 | −0.9 (3) |
| P1—Ru1—Ru3—Ru2 | 29.03 (3) | P2—C14—C15—C16 | 178.10 (16) |
| C34—Ru2—Ru3—C37 | −160.58 (15) | C19—C14—C15—C28 | 179.6 (2) |
| C35—Ru2—Ru3—C37 | 114.00 (15) | P2—C14—C15—C28 | −1.4 (3) |
| C33—Ru2—Ru3—C37 | −64.18 (15) | C14—C15—C16—C17 | −0.2 (3) |
| P2—Ru2—Ru3—C37 | 30.44 (15) | C28—C15—C16—C17 | 179.3 (2) |
| Ru1—Ru2—Ru3—C37 | 16.00 (14) | C15—C16—C17—C18 | 0.8 (4) |
| C34—Ru2—Ru3—C38 | 100.60 (9) | C16—C17—C18—C19 | −0.2 (3) |
| C35—Ru2—Ru3—C38 | 15.18 (9) | C17—C18—C19—C14 | −0.9 (3) |
| C33—Ru2—Ru3—C38 | −163.00 (8) | C15—C14—C19—C18 | 1.5 (3) |
| P2—Ru2—Ru3—C38 | −68.38 (7) | P2—C14—C19—C18 | −177.58 (17) |
| Ru1—Ru2—Ru3—C38 | −82.82 (7) | C14—P2—C20—C25 | 128.42 (16) |
| C34—Ru2—Ru3—C36 | −74.50 (9) | C13—P2—C20—C25 | 24.15 (17) |
| C35—Ru2—Ru3—C36 | −159.92 (8) | Ru2—P2—C20—C25 | −101.27 (15) |
| C33—Ru2—Ru3—C36 | 21.90 (9) | C14—P2—C20—C21 | −52.72 (17) |
| P2—Ru2—Ru3—C36 | 116.52 (7) | C13—P2—C20—C21 | −157.00 (15) |
| Ru1—Ru2—Ru3—C36 | 102.08 (6) | Ru2—P2—C20—C21 | 77.59 (16) |
| C34—Ru2—Ru3—P3 | 8.85 (7) | C25—C20—C21—C22 | −3.8 (3) |
| C35—Ru2—Ru3—P3 | −76.57 (6) | P2—C20—C21—C22 | 177.39 (15) |
| C33—Ru2—Ru3—P3 | 105.24 (6) | C25—C20—C21—C29 | 177.93 (18) |
| P2—Ru2—Ru3—P3 | −160.13 (3) | P2—C20—C21—C29 | −0.9 (3) |
| Ru1—Ru2—Ru3—P3 | −174.578 (16) | C20—C21—C22—C23 | 1.4 (3) |
| C34—Ru2—Ru3—Ru1 | −176.58 (6) | C29—C21—C22—C23 | 179.8 (2) |
| C35—Ru2—Ru3—Ru1 | 98.00 (6) | C21—C22—C23—C24 | 1.3 (3) |
| C33—Ru2—Ru3—Ru1 | −80.18 (6) | C22—C23—C24—C25 | −1.5 (3) |
| P2—Ru2—Ru3—Ru1 | 14.44 (3) | C23—C24—C25—C20 | −0.9 (3) |
| C31—Ru1—P1—C7 | 41.43 (9) | C21—C20—C25—C24 | 3.6 (3) |
| C30—Ru1—P1—C7 | 130.74 (9) | P2—C20—C25—C24 | −177.51 (16) |
| C32—Ru1—P1—C7 | −50.72 (9) | C31—Ru1—C30—O1 | 26.9 (13) |
| Ru3—Ru1—P1—C7 | −158.18 (7) | C32—Ru1—C30—O1 | 114.4 (14) |
| Ru2—Ru1—P1—C7 | −133.19 (7) | P1—Ru1—C30—O1 | −78.5 (13) |
| C31—Ru1—P1—C1 | −81.49 (9) | Ru3—Ru1—C30—O1 | 129.7 (13) |
| C30—Ru1—P1—C1 | 7.82 (9) | Ru2—Ru1—C30—O1 | −171.8 (13) |
| C32—Ru1—P1—C1 | −173.65 (9) | C30—Ru1—C31—O2 | 144 (15) |
| Ru3—Ru1—P1—C1 | 78.90 (7) | C32—Ru1—C31—O2 | −29 (15) |
| Ru2—Ru1—P1—C1 | 103.89 (7) | P1—Ru1—C31—O2 | −125 (15) |
| C31—Ru1—P1—C13 | 160.09 (9) | Ru3—Ru1—C31—O2 | 65 (15) |
| C30—Ru1—P1—C13 | −110.61 (9) | Ru2—Ru1—C31—O2 | 39 (15) |
| C32—Ru1—P1—C13 | 67.93 (9) | C31—Ru1—C32—O3 | −22.0 (16) |
| Ru3—Ru1—P1—C13 | −39.52 (8) | C30—Ru1—C32—O3 | −109.4 (16) |
| Ru2—Ru1—P1—C13 | −14.53 (7) | P1—Ru1—C32—O3 | 83.6 (16) |
| C34—Ru2—P2—C20 | −88.92 (9) | Ru3—Ru1—C32—O3 | −124.5 (16) |
| C35—Ru2—P2—C20 | −0.86 (9) | Ru2—Ru1—C32—O3 | 176.1 (16) |
| C33—Ru2—P2—C20 | 174.80 (9) | C34—Ru2—C33—O4 | −46.4 (17) |
| Ru1—Ru2—P2—C20 | 92.72 (7) | C35—Ru2—C33—O4 | −170.7 (13) |
| Ru3—Ru2—P2—C20 | 80.27 (7) | P2—Ru2—C33—O4 | 56.0 (17) |
| C34—Ru2—P2—C14 | 34.39 (9) | Ru1—Ru2—C33—O4 | 147.8 (17) |
| C35—Ru2—P2—C14 | 122.45 (9) | Ru3—Ru2—C33—O4 | −153.1 (17) |
| C33—Ru2—P2—C14 | −61.90 (9) | C35—Ru2—C34—O5 | −44 (3) |
| Ru1—Ru2—P2—C14 | −143.98 (7) | C33—Ru2—C34—O5 | 141 (3) |
| Ru3—Ru2—P2—C14 | −156.42 (7) | P2—Ru2—C34—O5 | 48 (3) |
| C34—Ru2—P2—C13 | 151.76 (9) | Ru1—Ru2—C34—O5 | −138 (3) |
| C35—Ru2—P2—C13 | −120.18 (9) | Ru3—Ru2—C34—O5 | −126 (3) |
| C33—Ru2—P2—C13 | 55.47 (9) | C34—Ru2—C35—O6 | 39.2 (15) |
| Ru1—Ru2—P2—C13 | −26.61 (7) | C33—Ru2—C35—O6 | 163.9 (12) |
| Ru3—Ru2—P2—C13 | −39.05 (8) | P2—Ru2—C35—O6 | −62.9 (15) |
| C37—Ru3—P3—O11 | −117.02 (10) | Ru1—Ru2—C35—O6 | −155.0 (15) |
| C38—Ru3—P3—O11 | −24.98 (10) | Ru3—Ru2—C35—O6 | 146.1 (15) |
| C36—Ru3—P3—O11 | 149.90 (10) | C37—Ru3—C36—O7 | −73.4 (15) |
| Ru1—Ru3—P3—O11 | 40.96 (12) | C38—Ru3—C36—O7 | 82.8 (18) |
| Ru2—Ru3—P3—O11 | 68.33 (7) | P3—Ru3—C36—O7 | 25.0 (15) |
| C37—Ru3—P3—O12 | 2.49 (10) | Ru1—Ru3—C36—O7 | −164.8 (15) |
| C38—Ru3—P3—O12 | 94.54 (9) | Ru2—Ru3—C36—O7 | 136.7 (15) |
| C36—Ru3—P3—O12 | −90.59 (9) | C38—Ru3—C37—O8 | 104 (6) |
| Ru1—Ru3—P3—O12 | 160.47 (9) | C36—Ru3—C37—O8 | −78 (6) |
| Ru2—Ru3—P3—O12 | −172.16 (7) | P3—Ru3—C37—O8 | −165 (6) |
| C37—Ru3—P3—O10 | 114.83 (10) | Ru1—Ru3—C37—O8 | 19 (6) |
| C38—Ru3—P3—O10 | −153.13 (10) | Ru2—Ru3—C37—O8 | 5 (6) |
| C36—Ru3—P3—O10 | 21.74 (10) | C37—Ru3—C38—O9 | 48.1 (16) |
| Ru1—Ru3—P3—O10 | −87.20 (11) | C36—Ru3—C38—O9 | −108.2 (17) |
| Ru2—Ru3—P3—O10 | −59.83 (8) | P3—Ru3—C38—O9 | −50.5 (16) |
| O11—P3—O10—C39 | −63.21 (19) | Ru1—Ru3—C38—O9 | 138.8 (16) |
| O12—P3—O10—C39 | −172.12 (18) | Ru2—Ru3—C38—O9 | −161.6 (16) |
| Ru3—P3—O10—C39 | 67.74 (18) | P3—O10—C39—C40 | 170.33 (15) |
| O12—P3—O11—C41 | 52.09 (19) | O10—C39—C40—Cl1 | −60.7 (2) |
| O10—P3—O11—C41 | −48.89 (19) | P3—O11—C41—C42 | 169.99 (16) |
| Ru3—P3—O11—C41 | 174.49 (15) | O11—C41—C42—Cl2 | −70.8 (2) |
| O11—P3—O12—C43 | −82.9 (2) | P3—O12—C43—C44 | −87.7 (3) |
| O10—P3—O12—C43 | 25.5 (2) | O12—C43—C44—Cl3 | −56.7 (3) |
| Ru3—P3—O12—C43 | 154.15 (18) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C11—H11A···O1i | 0.93 | 2.57 | 3.204 (3) | 126 |
Symmetry code: (i) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RZ2762).
References
- Bruce, M. I., Liddell, M. J., Hughes, C. A., Patrick, J. M., Skelton, B. W. & White, A. H. (1988a). J. Organomet. Chem. 347, 181–205.
- Bruce, M. I., Liddell, M. J., Shawkataly, O. bin, Hughes, C. A., Skelton, B. W. & White, A. H. (1988b). J. Organomet. Chem. 347, 207–235.
- Bruce, M. I., Shawkataly, O. bin & Williams, M. L. (1985). J. Organomet. Chem. 287, 127–131.
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst. 19, 105–107.
- Filby, M., Deeming, A. J., Hogarth, G. & Lee, M.-Y. (2006). Can. J. Chem. 84, 319–329.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Shawkataly, O. bin, Khan, I. A., Hafiz Malik, H. A., Yeap, C. S. & Fun, H.-K. (2011). Acta Cryst. E67, m197–m198. [DOI] [PMC free article] [PubMed]
- Shawkataly, O. bin, Khan, I. A., Yeap, C. S. & Fun, H.-K. (2010). Acta Cryst. E66, m94–m95. [DOI] [PMC free article] [PubMed]
- Shawkataly, O. bin, Ramalingam, K., Fun, H.-K., Abdul Rahman, A., & Razak, I. A. (2004). J. Cluster Sci. 15, 387–394.
- Shawkataly, O. bin, Ramalingam, K., Lee, S. T., Parameswary, M., Fun, H.-K. & Sivakumar, K. (1998). Polyhedron, 17, 1211–1216.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812023707/rz2762sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812023707/rz2762Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report