Abstract
In the dinuclear title compound, [Na2(C13H15ClN5O5S)2(H2O)6]n, two Na+ cations, disposed about a centre of inversion, are linked by two bridging water molecules. The coordination geometry is based on an O5 donor set defined by four water molecules and a 4-aminobenzenesulfonate O atom in a distorted trigonal–bipyramidal geometry. In the crystal, significant O—H⋯O, O—H⋯N and N—H⋯O hydrogen bonds lead to the formation of a three-dimensional architecture.
Related literature
For commercial and synthetic applications of related compounds, see: Candiani & Frigerio (2007 ▶); Hollink et al. (2005 ▶); Konstantion & Petrova (2002 ▶).
Experimental
Crystal data
[Na2(C13H15ClN5O5S)2(H2O)6]
M r = 931.72
Triclinic,
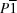
a = 7.5628 (7) Å
b = 8.6274 (8) Å
c = 15.532 (2) Å
α = 97.348 (2)°
β = 93.363 (4)°
γ = 102.410 (7)°
V = 977.75 (18) Å3
Z = 1
Mo Kα radiation
μ = 0.38 mm−1
T = 113 K
0.50 × 0.04 × 0.04 mm
Data collection
Rigaku Saturn724 CCD diffractometer
Absorption correction: multi-scan (CrystalClear; Molecular Structure Corporation & Rigaku, 2005 ▶) T min = 0.834, T max = 0.985
8262 measured reflections
3418 independent reflections
1459 reflections with I > 2σ(I)
R int = 0.106
Refinement
R[F 2 > 2σ(F 2)] = 0.062
wR(F 2) = 0.131
S = 0.87
3418 reflections
283 parameters
20 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.52 e Å−3
Δρmin = −0.52 e Å−3
Data collection: CrystalClear (Molecular Structure Corporation & Rigaku, 2005 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: CrystalClear.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812023732/tk5096sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812023732/tk5096Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯N4i | 0.87 (2) | 1.96 (3) | 2.823 (5) | 172 (5) |
| O2—H2⋯O3ii | 0.86 (3) | 1.94 (3) | 2.772 (4) | 165 (4) |
| O6—H6A⋯O5iii | 0.99 | 1.83 | 2.817 (4) | 174 |
| O6—H6B⋯N3iv | 0.99 | 2.23 | 3.009 (5) | 135 |
| O7—H7A⋯O8v | 0.87 (2) | 2.02 (3) | 2.861 (5) | 164 (4) |
| O7—H7B⋯O2ii | 0.82 (2) | 1.95 (2) | 2.767 (4) | 169 (5) |
| O8—H8A⋯O5vi | 0.80 (2) | 2.03 (3) | 2.797 (4) | 162 (4) |
| O8—H8B⋯O3iii | 0.82 (2) | 2.15 (3) | 2.907 (5) | 154 (4) |
| N5—H5⋯O1iv | 0.90 (4) | 2.01 (4) | 2.828 (5) | 151 (4) |
Symmetry codes: (i)  ; (ii)
; (ii) 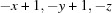 ; (iii)
; (iii) 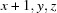 ; (iv)
; (iv) 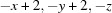 ; (v)
; (v) 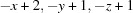 ; (vi)
; (vi) 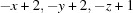 .
.
Acknowledgments
This work was supported financially by the Research Center for Engineering Technology of Polymeric Composites of Shanxi Province, College of Materials Science and Engineering, North University of China,
supplementary crystallographic information
Comment
Cyanuric chloride derivatives are widely used in commercial chemicals, especially in pesticides, reactive dyes, fluorescent brighteners, liposome and polymer photostabilizers (Hollink et al., 2005; Candiani & Frigerio, 2007). The widespread use of these compounds is due to their higher reactive activity (Konstantion & Petrova, 2002). The title compound belongs to the cyanuric chloride derivatives and its structure is reported herein.
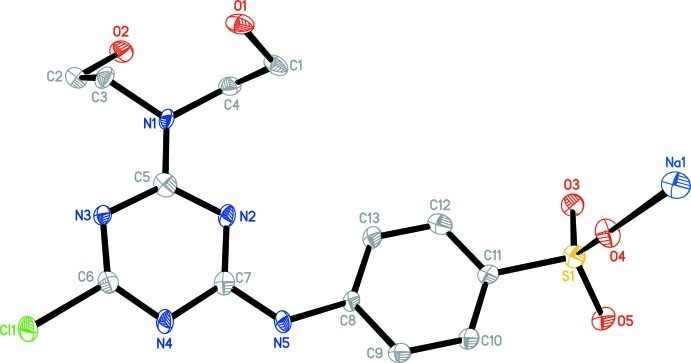
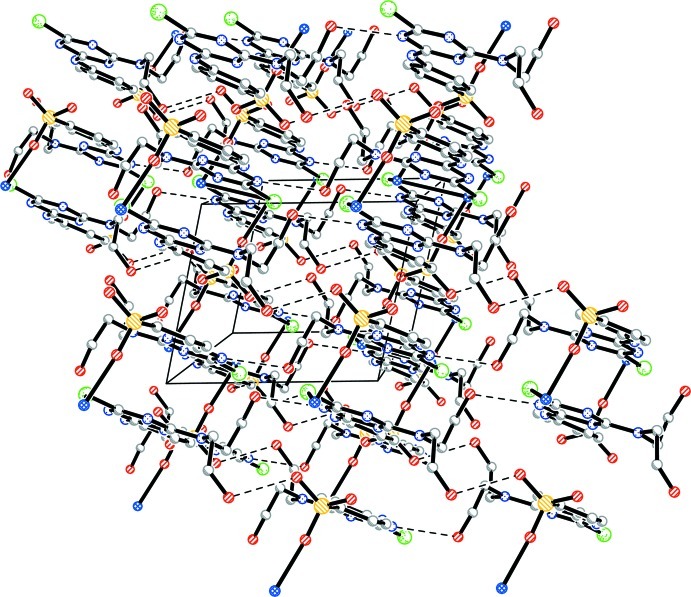
The asymmetric unit is shown in Fig. 1. The dihedral angle between the benzene ring and the triazine ring is 8.6 (2)°. As shown in Fig. 2, the crystal packing displays O—H···O, O—H···N and N—H···O hydrogen bonds, Table 1.
Experimental
Cyanuric chloride (0.1 mol) was dissolved in acetone (120 ml). This solution was poured into distilled water (150 ml) with crushed ice (150 g). The reaction system was stirred maintaining the temperature at 0–5 °C in the ice-bath. An aqueous solution of sodium sulfate (0.1 mol) was slowly dropped into the above reaction vessel within 0.5 h, and then a 20% aqueous solution of sodium carbonate was added drop-wise to the reaction mixture to keep the pH at 7–8. Then the mixture was kept stirring for 5 h at 0–5 °C. After the reaction was completed, the white precipitate was filtered, washed with acetone and water twice, respectively, and dried at room temperatuer under vacuum to constant weight. This white powder is intermediate I. The intermediate I (0.05 mol) and a mixed solution of water and acetone (160 ml) were added into 250 ml four-neck flask. After stirring for 0.5 h at 25 °C, an aqueous solution of diethanol amine (0.06 mol) was slowly dropped into the reaction vessel within 0.5 h, and then a 20% aqueous solution of sodium carbonate was added drop-wise to the reaction mixture to keep the pH at 8–9. Then the mixture was kept stirring for 6 h at 45 °C. After the reaction was completed, the solution was rotary evaporated, washed with anhydrous alcohol twice, and dried at room temperature under vacuum to constant weight. The target product was obtained. Crystals of the title compound were obtained by slow evaporation of its methanol/n-hexane solution held at room temperature.
Refinement
Carbon-bound H-atoms were placed in calculated positions [C—H = 0.95 to 0.99 Å, Uiso(H) = 1.2Ueq(C)] and were included in the refinement in the riding model approximation. The positions of the O- and N- bound H-atoms were refined with light distance restraints, and with Uiso(H) = 1.2Ueq(water-O,N) and Uiso(H) = 1.5Ueq(hydroxyl-O)].
Figures
Fig. 1.
The molecular structure of (I). Displacement ellipsoids are drawn at the 30% probability level. H atoms have been omitted.
Fig. 2.
A view of the crystal packing for (I). Hydrogen bonds are shown as dashed lines.
Crystal data
| [Na2(C13H15ClN5O5S)2(H2O)6] | Z = 1 |
| Mr = 931.72 | F(000) = 484 |
| Triclinic, P1 | Dx = 1.582 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.5628 (7) Å | Cell parameters from 3065 reflections |
| b = 8.6274 (8) Å | θ = 2.4–26.1° |
| c = 15.532 (2) Å | µ = 0.38 mm−1 |
| α = 97.348 (2)° | T = 113 K |
| β = 93.363 (4)° | Prism, colourless |
| γ = 102.410 (7)° | 0.50 × 0.04 × 0.04 mm |
| V = 977.75 (18) Å3 |
Data collection
| Rigaku Saturn724 CCD diffractometer | 3418 independent reflections |
| Radiation source: rotating anode | 1459 reflections with I > 2σ(I) |
| Multilayer monochromator | Rint = 0.106 |
| Detector resolution: 14.22 pixels mm-1 | θmax = 25.0°, θmin = 2.4° |
| ω and φ scans | h = −8→8 |
| Absorption correction: multi-scan (CrystalClear; Molecular Structure Corporation & Rigaku, 2005) | k = −10→10 |
| Tmin = 0.834, Tmax = 0.985 | l = −18→18 |
| 8262 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.062 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.131 | H atoms treated by a mixture of independent and constrained refinement |
| S = 0.87 | w = 1/[σ2(Fo2) + (0.0293P)2] where P = (Fo2 + 2Fc2)/3 |
| 3418 reflections | (Δ/σ)max = 0.001 |
| 283 parameters | Δρmax = 0.52 e Å−3 |
| 20 restraints | Δρmin = −0.52 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Na1 | 0.9529 (2) | 0.8060 (2) | 0.46061 (12) | 0.0216 (5) | |
| S1 | 0.55329 (16) | 0.91677 (15) | 0.34804 (8) | 0.0191 (3) | |
| Cl1 | 1.02875 (15) | 1.27203 (14) | −0.25219 (8) | 0.0221 (4) | |
| O1 | 0.9492 (4) | 0.5459 (4) | −0.0875 (2) | 0.0213 (9) | |
| H1 | 0.934 (6) | 0.443 (3) | −0.099 (3) | 0.032* | |
| O2 | 0.4874 (4) | 0.5163 (4) | −0.3219 (2) | 0.0234 (9) | |
| H2 | 0.504 (6) | 0.421 (3) | −0.330 (3) | 0.035* | |
| O3 | 0.4056 (4) | 0.7718 (3) | 0.32366 (19) | 0.0210 (8) | |
| O4 | 0.7162 (4) | 0.8814 (4) | 0.3889 (2) | 0.0216 (9) | |
| O5 | 0.4936 (4) | 1.0469 (4) | 0.40039 (19) | 0.0194 (8) | |
| N1 | 0.7584 (5) | 0.7287 (4) | −0.1875 (2) | 0.0161 (10) | |
| N2 | 0.7670 (5) | 0.9391 (4) | −0.0807 (2) | 0.0162 (10) | |
| N3 | 0.8817 (5) | 0.9841 (4) | −0.2196 (2) | 0.0150 (9) | |
| N4 | 0.8923 (5) | 1.2084 (4) | −0.1092 (2) | 0.0175 (10) | |
| N5 | 0.7900 (5) | 1.1689 (5) | 0.0233 (3) | 0.0170 (10) | |
| H5 | 0.851 (5) | 1.272 (5) | 0.029 (3) | 0.020* | |
| C1 | 0.7906 (5) | 0.5903 (5) | −0.0571 (3) | 0.0195 (12) | |
| H1A | 0.8270 | 0.6892 | −0.0142 | 0.023* | |
| H1B | 0.7234 | 0.5042 | −0.0271 | 0.023* | |
| C2 | 0.6672 (6) | 0.6188 (5) | −0.1302 (3) | 0.0207 (12) | |
| H2A | 0.6108 | 0.5145 | −0.1660 | 0.025* | |
| H2B | 0.5682 | 0.6632 | −0.1048 | 0.025* | |
| C3 | 0.8018 (6) | 0.6556 (5) | −0.2719 (3) | 0.0192 (12) | |
| H3A | 0.8331 | 0.5519 | −0.2650 | 0.023* | |
| H3B | 0.9100 | 0.7264 | −0.2901 | 0.023* | |
| C4 | 0.6466 (6) | 0.6270 (5) | −0.3431 (3) | 0.0227 (13) | |
| H4A | 0.6151 | 0.7303 | −0.3507 | 0.027* | |
| H4B | 0.6862 | 0.5828 | −0.3989 | 0.027* | |
| C5 | 0.8033 (6) | 0.8880 (6) | −0.1625 (3) | 0.0189 (12) | |
| C6 | 0.9213 (6) | 1.1378 (5) | −0.1863 (3) | 0.0169 (12) | |
| C7 | 0.8149 (6) | 1.0990 (6) | −0.0576 (3) | 0.0158 (12) | |
| C8 | 0.7265 (6) | 1.0979 (5) | 0.0957 (3) | 0.0130 (11) | |
| C9 | 0.7404 (6) | 1.2041 (5) | 0.1720 (3) | 0.0180 (12) | |
| H9 | 0.7876 | 1.3154 | 0.1720 | 0.022* | |
| C10 | 0.6857 (6) | 1.1488 (5) | 0.2486 (3) | 0.0180 (12) | |
| H10 | 0.6997 | 1.2221 | 0.3010 | 0.022* | |
| C11 | 0.6109 (6) | 0.9878 (5) | 0.2493 (3) | 0.0149 (11) | |
| C12 | 0.5925 (6) | 0.8800 (6) | 0.1722 (3) | 0.0203 (12) | |
| H12 | 0.5421 | 0.7691 | 0.1720 | 0.024* | |
| C13 | 0.6486 (5) | 0.9361 (5) | 0.0955 (3) | 0.0166 (11) | |
| H13 | 0.6335 | 0.8634 | 0.0428 | 0.020* | |
| O6 | 1.1240 (4) | 1.0471 (3) | 0.41494 (19) | 0.0191 (8) | |
| H6A | 1.2558 | 1.0514 | 0.4142 | 0.023* | |
| H6B | 1.0732 | 1.0718 | 0.3594 | 0.023* | |
| O7 | 0.8355 (4) | 0.5359 (4) | 0.4260 (2) | 0.0349 (11) | |
| H7A | 0.825 (6) | 0.466 (5) | 0.462 (2) | 0.042* | |
| H7B | 0.747 (5) | 0.518 (5) | 0.390 (2) | 0.042* | |
| O8 | 1.2365 (4) | 0.7394 (4) | 0.4857 (2) | 0.0226 (9) | |
| H8A | 1.297 (5) | 0.813 (4) | 0.518 (2) | 0.027* | |
| H8B | 1.267 (5) | 0.717 (5) | 0.4365 (17) | 0.027* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Na1 | 0.0141 (10) | 0.0229 (12) | 0.0293 (12) | 0.0041 (9) | 0.0094 (9) | 0.0061 (9) |
| S1 | 0.0148 (7) | 0.0226 (8) | 0.0217 (8) | 0.0051 (6) | 0.0093 (6) | 0.0052 (6) |
| Cl1 | 0.0211 (7) | 0.0231 (8) | 0.0241 (8) | 0.0047 (6) | 0.0125 (6) | 0.0067 (6) |
| O1 | 0.0155 (18) | 0.0163 (19) | 0.035 (2) | 0.0071 (17) | 0.0074 (16) | 0.0067 (18) |
| O2 | 0.0204 (19) | 0.019 (2) | 0.028 (2) | −0.0016 (18) | 0.0032 (16) | 0.0022 (18) |
| O3 | 0.0175 (18) | 0.020 (2) | 0.024 (2) | −0.0023 (16) | 0.0067 (16) | 0.0032 (16) |
| O4 | 0.0116 (17) | 0.033 (2) | 0.026 (2) | 0.0147 (16) | 0.0025 (15) | 0.0102 (16) |
| O5 | 0.0145 (18) | 0.024 (2) | 0.022 (2) | 0.0094 (15) | 0.0118 (15) | 0.0004 (15) |
| N1 | 0.018 (2) | 0.016 (2) | 0.014 (2) | 0.0025 (19) | 0.0096 (18) | 0.0017 (18) |
| N2 | 0.013 (2) | 0.021 (2) | 0.015 (2) | 0.0022 (19) | 0.0068 (18) | 0.0026 (19) |
| N3 | 0.012 (2) | 0.015 (2) | 0.018 (2) | 0.0017 (18) | 0.0083 (18) | 0.0018 (18) |
| N4 | 0.015 (2) | 0.022 (3) | 0.016 (2) | 0.002 (2) | 0.0080 (19) | 0.0045 (19) |
| N5 | 0.016 (2) | 0.018 (2) | 0.016 (2) | −0.0009 (19) | 0.0084 (19) | 0.004 (2) |
| C1 | 0.015 (3) | 0.016 (3) | 0.030 (3) | 0.004 (2) | 0.008 (2) | 0.010 (2) |
| C2 | 0.023 (3) | 0.016 (3) | 0.026 (3) | 0.008 (2) | 0.009 (2) | 0.004 (2) |
| C3 | 0.019 (3) | 0.019 (3) | 0.019 (3) | 0.004 (2) | 0.009 (2) | 0.000 (2) |
| C4 | 0.028 (3) | 0.018 (3) | 0.023 (3) | 0.005 (3) | 0.005 (2) | 0.006 (2) |
| C5 | 0.009 (3) | 0.023 (3) | 0.025 (3) | 0.002 (2) | 0.002 (2) | 0.005 (2) |
| C6 | 0.015 (3) | 0.020 (3) | 0.020 (3) | 0.009 (2) | 0.004 (2) | 0.007 (2) |
| C7 | 0.008 (3) | 0.023 (3) | 0.018 (3) | 0.007 (2) | 0.002 (2) | 0.004 (2) |
| C8 | 0.009 (3) | 0.018 (3) | 0.014 (3) | 0.007 (2) | 0.007 (2) | 0.003 (2) |
| C9 | 0.016 (3) | 0.015 (3) | 0.024 (3) | 0.003 (2) | 0.010 (2) | 0.001 (2) |
| C10 | 0.017 (3) | 0.018 (3) | 0.020 (3) | 0.004 (2) | 0.013 (2) | 0.002 (2) |
| C11 | 0.011 (2) | 0.017 (3) | 0.020 (3) | 0.009 (2) | 0.010 (2) | 0.002 (2) |
| C12 | 0.016 (3) | 0.018 (3) | 0.028 (3) | 0.004 (2) | 0.005 (2) | 0.005 (2) |
| C13 | 0.013 (3) | 0.021 (3) | 0.017 (3) | 0.004 (2) | 0.007 (2) | 0.003 (2) |
| O6 | 0.0091 (17) | 0.030 (2) | 0.021 (2) | 0.0056 (15) | 0.0023 (15) | 0.0114 (16) |
| O7 | 0.021 (2) | 0.034 (3) | 0.048 (3) | −0.0018 (19) | −0.0035 (19) | 0.017 (2) |
| O8 | 0.018 (2) | 0.026 (2) | 0.022 (2) | 0.0009 (17) | 0.0065 (17) | 0.0023 (18) |
Geometric parameters (Å, º)
| Na1—O7 | 2.290 (4) | N5—H5 | 0.90 (4) |
| Na1—O4 | 2.306 (3) | C1—C2 | 1.505 (5) |
| Na1—O6i | 2.346 (4) | C1—H1A | 0.9900 |
| Na1—O8 | 2.361 (4) | C1—H1B | 0.9900 |
| Na1—O6 | 2.415 (3) | C2—H2A | 0.9900 |
| Na1—Cl1ii | 3.239 (2) | C2—H2B | 0.9900 |
| Na1—Na1i | 3.322 (4) | C3—C4 | 1.522 (5) |
| Na1—H8B | 2.68 (4) | C3—H3A | 0.9900 |
| S1—O4 | 1.461 (3) | C3—H3B | 0.9900 |
| S1—O5 | 1.467 (3) | C4—H4A | 0.9900 |
| S1—O3 | 1.478 (3) | C4—H4B | 0.9900 |
| S1—C11 | 1.766 (5) | C8—C9 | 1.386 (6) |
| Cl1—C6 | 1.746 (5) | C8—C13 | 1.393 (6) |
| Cl1—Na1ii | 3.238 (2) | C9—C10 | 1.391 (6) |
| O1—C1 | 1.423 (5) | C9—H9 | 0.9500 |
| O1—H1 | 0.87 (2) | C10—C11 | 1.384 (6) |
| O2—C4 | 1.451 (5) | C10—H10 | 0.9500 |
| O2—H2 | 0.86 (3) | C11—C12 | 1.400 (6) |
| N1—C5 | 1.343 (5) | C12—C13 | 1.397 (6) |
| N1—C3 | 1.467 (5) | C12—H12 | 0.9500 |
| N1—C2 | 1.474 (5) | C13—H13 | 0.9500 |
| N2—C7 | 1.344 (5) | O6—Na1i | 2.346 (4) |
| N2—C5 | 1.354 (6) | O6—H6A | 0.9900 |
| N3—C6 | 1.323 (5) | O6—H6B | 0.9900 |
| N3—C5 | 1.365 (6) | O7—H7A | 0.87 (2) |
| N4—C6 | 1.324 (5) | O7—H7B | 0.82 (2) |
| N4—C7 | 1.374 (5) | O8—H8A | 0.80 (2) |
| N5—C7 | 1.364 (6) | O8—H8B | 0.82 (2) |
| N5—C8 | 1.408 (5) | ||
| O7—Na1—O4 | 95.45 (13) | C1—C2—H2A | 108.7 |
| O7—Na1—O6i | 121.20 (14) | N1—C2—H2B | 108.7 |
| O4—Na1—O6i | 85.08 (12) | C1—C2—H2B | 108.7 |
| O7—Na1—O8 | 86.24 (13) | H2A—C2—H2B | 107.6 |
| O4—Na1—O8 | 159.53 (15) | N1—C3—C4 | 113.2 (4) |
| O6i—Na1—O8 | 111.51 (12) | N1—C3—H3A | 108.9 |
| O7—Na1—O6 | 146.92 (15) | C4—C3—H3A | 108.9 |
| O4—Na1—O6 | 81.63 (11) | N1—C3—H3B | 108.9 |
| O6i—Na1—O6 | 91.52 (12) | C4—C3—H3B | 108.9 |
| O8—Na1—O6 | 85.87 (12) | H3A—C3—H3B | 107.7 |
| O7—Na1—Cl1ii | 74.72 (11) | O2—C4—C3 | 111.2 (4) |
| O4—Na1—Cl1ii | 70.57 (9) | O2—C4—H4A | 109.4 |
| O6i—Na1—Cl1ii | 152.67 (10) | C3—C4—H4A | 109.4 |
| O8—Na1—Cl1ii | 90.36 (10) | O2—C4—H4B | 109.4 |
| O6—Na1—Cl1ii | 73.26 (9) | C3—C4—H4B | 109.4 |
| O7—Na1—Na1i | 167.15 (14) | H4A—C4—H4B | 108.0 |
| O4—Na1—Na1i | 80.42 (10) | N1—C5—N2 | 115.8 (5) |
| O6i—Na1—Na1i | 46.62 (8) | N1—C5—N3 | 118.7 (5) |
| O8—Na1—Na1i | 101.92 (11) | N2—C5—N3 | 125.5 (4) |
| O6—Na1—Na1i | 44.91 (8) | N3—C6—N4 | 130.0 (5) |
| Cl1ii—Na1—Na1i | 114.70 (8) | N3—C6—Cl1 | 116.6 (4) |
| O7—Na1—H8B | 82.4 (8) | N4—C6—Cl1 | 113.4 (3) |
| O4—Na1—H8B | 142.8 (7) | N2—C7—N5 | 121.3 (5) |
| O6i—Na1—H8B | 127.8 (6) | N2—C7—N4 | 125.9 (5) |
| O8—Na1—H8B | 17.3 (6) | N5—C7—N4 | 112.8 (4) |
| O6—Na1—H8B | 80.6 (9) | C9—C8—C13 | 119.3 (4) |
| Cl1ii—Na1—H8B | 73.1 (6) | C9—C8—N5 | 114.7 (4) |
| Na1i—Na1—H8B | 108.3 (8) | C13—C8—N5 | 126.0 (4) |
| O4—S1—O5 | 112.25 (18) | C8—C9—C10 | 120.4 (4) |
| O4—S1—O3 | 112.36 (19) | C8—C9—H9 | 119.8 |
| O5—S1—O3 | 112.56 (18) | C10—C9—H9 | 119.8 |
| O4—S1—C11 | 107.4 (2) | C11—C10—C9 | 120.6 (4) |
| O5—S1—C11 | 105.8 (2) | C11—C10—H10 | 119.7 |
| O3—S1—C11 | 105.9 (2) | C9—C10—H10 | 119.7 |
| C6—Cl1—Na1ii | 125.53 (17) | C10—C11—C12 | 119.4 (4) |
| C1—O1—H1 | 112 (3) | C10—C11—S1 | 120.3 (4) |
| C4—O2—H2 | 110 (3) | C12—C11—S1 | 120.2 (4) |
| S1—O4—Na1 | 173.9 (2) | C13—C12—C11 | 119.7 (4) |
| C5—N1—C3 | 121.7 (4) | C13—C12—H12 | 120.1 |
| C5—N1—C2 | 121.4 (4) | C11—C12—H12 | 120.1 |
| C3—N1—C2 | 116.9 (4) | C8—C13—C12 | 120.5 (4) |
| C7—N2—C5 | 114.2 (4) | C8—C13—H13 | 119.8 |
| C6—N3—C5 | 112.4 (4) | C12—C13—H13 | 119.8 |
| C6—N4—C7 | 111.9 (4) | Na1i—O6—Na1 | 88.48 (12) |
| C7—N5—C8 | 129.8 (4) | Na1i—O6—H6A | 113.9 |
| C7—N5—H5 | 106 (3) | Na1—O6—H6A | 113.9 |
| C8—N5—H5 | 122 (3) | Na1i—O6—H6B | 113.9 |
| O1—C1—C2 | 112.1 (4) | Na1—O6—H6B | 113.9 |
| O1—C1—H1A | 109.2 | H6A—O6—H6B | 111.1 |
| C2—C1—H1A | 109.2 | Na1—O7—H7A | 126 (3) |
| O1—C1—H1B | 109.2 | Na1—O7—H7B | 111 (3) |
| C2—C1—H1B | 109.2 | H7A—O7—H7B | 112 (3) |
| H1A—C1—H1B | 107.9 | Na1—O8—H8A | 106 (3) |
| N1—C2—C1 | 114.4 (4) | Na1—O8—H8B | 104 (3) |
| N1—C2—H2A | 108.7 | H8A—O8—H8B | 120 (3) |
| O5—S1—O4—Na1 | 90 (2) | C5—N2—C7—N5 | −178.9 (4) |
| O3—S1—O4—Na1 | −38 (2) | C5—N2—C7—N4 | 0.8 (6) |
| C11—S1—O4—Na1 | −154 (2) | C8—N5—C7—N2 | 4.3 (7) |
| O7—Na1—O4—S1 | 48 (2) | C8—N5—C7—N4 | −175.4 (4) |
| O6i—Na1—O4—S1 | −73 (2) | C6—N4—C7—N2 | −1.9 (6) |
| O8—Na1—O4—S1 | 141.4 (19) | C6—N4—C7—N5 | 177.8 (4) |
| O6—Na1—O4—S1 | −166 (2) | C7—N5—C8—C9 | 171.8 (4) |
| Cl1ii—Na1—O4—S1 | 119 (2) | C7—N5—C8—C13 | −10.3 (7) |
| Na1i—Na1—O4—S1 | −120 (2) | C13—C8—C9—C10 | 3.2 (7) |
| C5—N1—C2—C1 | −76.6 (5) | N5—C8—C9—C10 | −178.8 (4) |
| C3—N1—C2—C1 | 101.9 (4) | C8—C9—C10—C11 | −2.2 (7) |
| O1—C1—C2—N1 | −50.9 (5) | C9—C10—C11—C12 | 0.7 (6) |
| C5—N1—C3—C4 | −94.4 (5) | C9—C10—C11—S1 | 176.5 (3) |
| C2—N1—C3—C4 | 87.0 (5) | O4—S1—C11—C10 | −86.3 (4) |
| N1—C3—C4—O2 | −61.9 (5) | O5—S1—C11—C10 | 33.7 (4) |
| C3—N1—C5—N2 | −175.5 (4) | O3—S1—C11—C10 | 153.4 (3) |
| C2—N1—C5—N2 | 2.9 (6) | O4—S1—C11—C12 | 89.4 (4) |
| C3—N1—C5—N3 | 3.9 (6) | O5—S1—C11—C12 | −150.5 (4) |
| C2—N1—C5—N3 | −177.7 (3) | O3—S1—C11—C12 | −30.8 (4) |
| C7—N2—C5—N1 | 179.5 (4) | C10—C11—C12—C13 | −0.4 (7) |
| C7—N2—C5—N3 | 0.1 (7) | S1—C11—C12—C13 | −176.2 (3) |
| C6—N3—C5—N1 | −179.1 (4) | C9—C8—C13—C12 | −2.9 (6) |
| C6—N3—C5—N2 | 0.3 (7) | N5—C8—C13—C12 | 179.3 (4) |
| C5—N3—C6—N4 | −1.8 (7) | C11—C12—C13—C8 | 1.5 (7) |
| C5—N3—C6—Cl1 | 178.1 (3) | O7—Na1—O6—Na1i | 171.9 (2) |
| C7—N4—C6—N3 | 2.6 (7) | O4—Na1—O6—Na1i | 84.80 (11) |
| C7—N4—C6—Cl1 | −177.4 (3) | O6i—Na1—O6—Na1i | 0.0 |
| Na1ii—Cl1—C6—N3 | 16.5 (4) | O8—Na1—O6—Na1i | −111.45 (12) |
| Na1ii—Cl1—C6—N4 | −163.6 (2) | Cl1ii—Na1—O6—Na1i | 156.93 (10) |
Symmetry codes: (i) −x+2, −y+2, −z+1; (ii) −x+2, −y+2, −z.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···N4iii | 0.87 (2) | 1.96 (3) | 2.823 (5) | 172 (5) |
| O2—H2···O3iv | 0.86 (3) | 1.94 (3) | 2.772 (4) | 165 (4) |
| O6—H6A···O5v | 0.99 | 1.83 | 2.817 (4) | 174 |
| O6—H6B···N3ii | 0.99 | 2.23 | 3.009 (5) | 135 |
| O7—H7A···O8vi | 0.87 (2) | 2.02 (3) | 2.861 (5) | 164 (4) |
| O7—H7B···O2iv | 0.82 (2) | 1.95 (2) | 2.767 (4) | 169 (5) |
| O8—H8A···O5i | 0.80 (2) | 2.03 (3) | 2.797 (4) | 162 (4) |
| O8—H8B···O3v | 0.82 (2) | 2.15 (3) | 2.907 (5) | 154 (4) |
| N5—H5···O1ii | 0.90 (4) | 2.01 (4) | 2.828 (5) | 151 (4) |
Symmetry codes: (i) −x+2, −y+2, −z+1; (ii) −x+2, −y+2, −z; (iii) x, y−1, z; (iv) −x+1, −y+1, −z; (v) x+1, y, z; (vi) −x+2, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: TK5096).
References
- Candiani, G. & Frigerio, M. (2007). Chem. Med. Chem. 2, 292–296.
- Hollink, E., Simanek, E. E. & Bergbreiter, D. E. (2005). Tetrahedron Lett. 46, 2005–2008.
- Konstantion, T. N. & Petrova, P. (2002). Dyes Pigm. 52, 115–120.
- Molecular Structure Corporation & Rigaku (2005). CrystalClear MSC, The Woodlands, Texas, USA, and Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812023732/tk5096sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812023732/tk5096Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report