Abstract
Peptidase S (PEPS) is a metallopeptidase that cleaves N-terminal residues from proteins and peptides. PEPS is used as a cell maintenance enzyme with critical roles in peptide turnover. The promoter region located upstream of the initiation site plays an important role in regulating gene expression. Polymorphism in the promoter region can alter gene expression and lead to biological changes. In the current study, polymorphisms in the promoter region of the PEPS gene were investigated. Polymerase chain reaction (PCR)-restriction fragment length polymorphism and DNA sequencing methods were used to screen sequence variations in the promoter region of DNA samples from 743 Chinese Holstein cattle. Two polymorphisms (g. −534 T>C and g. −2545 G>A) were identified and eight haplotypes were classified by haplotype analysis. The two genetic polymorphisms and haplotypes were associated with fat percentage and somatic cell score in Chinese Holstein cattle. The results of real-time PCR showed that cow kidneys exhibit the highest PEPS expression level. Moreover, bioinformatics analysis predicted that the single-nucleotide polymorphism g. −534 T>C is located in the core promoter region and in the transcription factor binding sites. The promoter activities of the polymorphism of −543 T>C were measured by luciferase assay in the human kidney epithelial cell line 293T. Transcriptional activity is significantly lower in cell lines transfected with the reporter construct containing 2.5 kb upstream fragments with −543 C than in those with wild-type −543 T. The results indicated that genetic variation at locus −543 influences PEPS promoter activity. The genetic variation in the promoter region of PEPS gene may regulate PEPS gene transcription and might have consequences at a regulatory level.
Introduction
Milk yield and composition traits, which are under the control of multiple genes, are economically important traits in dairy cattle. Some progresses in breed improvement have been achieved for milk production trait selection, but they entailed exorbitant costs and time. On the other hand, marker-assisted selection can improve selection accuracy, making possible the attainment of genetic progress faster and at a lower cost. Thus, studying the genetic variations of candidate genes and their association to milk production and mastitis-related traits is of utmost importance (Liefers et al., 2002; Kuss et al., 2003; Yahyaoui et al., 2003; Taylor et al., 2006; Khatib et al., 2007).
The bovine peptidase S (PEPS) gene for milk performance traits is located on chromosome 6 close to quantitative trait loci (QTL) (Sheely et al., 2009). PEPS is also known as leucine aminopeptidase 3 (LAP3), which catalyses the hydrolysis of N-terminal amino acid residues from a polypeptide chain. PEPS is involved in the processing and regular turnover of intracellular proteins and in the catalysis of the removal of unsubstituted N-terminal amino acids from various peptides. The PEPS gene is conserved in humans, chimpanzees, dogs, mice, rats, bovines, chickens, zebrafish, and mosquitoes (Wallner et al., 1993). In mammals, PEPS contributes to the processing of bioactive peptides (oxytocin, vasopressin, and enkephalins) and vesicle trafficking to the plasma membrane; it also plays a role in major histocompatibility complex class I (MHCI) antigen presentation (Matsui et al., 2006; Kloetzel and Ossendorp, 2004).
The promoter region located upstream of the initiation site plays an important role in regulating gene expression. A polymorphism in the promoter region may modify the transcription factor binding sites, thus affecting gene expression. These functional polymorphisms represent an important but relatively unexplored class of genetic variation (Hudson, 2003). In some cases, a natural binding site created or abolished by a regulatory single-nucleotide polymorphism (SNP) can account for observed differences in gene expression (Chorley et al., 2008).
However, data on bovine PEPS promoter SNPs are limited. In the present work, the promoter polymorphisms in the PEPS gene of Chinese Holstein cattle were investigated. Moreover, the correlations of polymorphisms in the promoter region with milk production traits in Chinese Holstein cattle were evaluated. PEPS promoter polymorphism was functionally characterized using a reporter gene assay.
Materials and Methods
Animals and DNA extraction
The dataset in our study included 743 Chinese Holstein cattle from eight farms, which are mainly the daughters of 23 sires in Jinan, Tianjin, and Qingdao Agriculture Development Area, China. The milk traits included 305-day milk yield, fat percentage, protein percentage, and somatic cell score (SCS), which were provided by the Dairy Cattle Research Center of Shandong Province, Academy of Agricultural Sciences Dairy Herd Improvement Laboratory using the milk composition analyzer (Foss MilkScan FT 6000). Genomic DNA was extracted from cattle blood using a phenol/chloroform solution according to the method used by Ju et al. (2011). DNA concentration was spectrophotometrically estimated and diluted to 50 ng/μL. All DNA samples were stored at −20°C for subsequent analysis.
Prediction function elements of the promoter region
Polymorphisms affecting gene transcription and expression are attracting increasing attention because they might be responsible for a significant proportion of heritable phenotypic variations (Xie et al., 2005). The ∼2.5 kb 5′ flanking region upstream of PEPS gene putative transcription start site was screened to identify the Cytosine phosphate Guanine (CpG) islands. The CpG islands were used to reveal (http://zeus2.itb.cnr.it/cgi-bin/wwwcpg.pl?page=ex) the promoter region using Web Promoter Scan (www-bimas.cit.nih.gov/molbio/signal/) and transcription factors using TFSEARCH (www.cbrc.jp/research/db/TFSEARCH.html). Findings were used to investigate the potential effects of some of the polymorphisms on transcription.
5′ Flanking region of PEPS gene polymorphism analysis
Based on the sequence of the bovine PEPS gene (GenBank accession No. NC_007304.3), the primers P1 (Table 1) were designed to amplify the 924-bp gene fragment, which encompasses a part of the bovine PEPS gene 5′ flanking region and a part of the preceding intron 1. Polymerase chain reactions (PCRs) were performed in a 25 μL reaction, which included 50 ng of genomic DNA, 1.0 μL of each primer with concentrations of 10 μM, 2.5 μL of 10×buffer, 0.9 μL of 50 mM Mg2+, 0.8 μL of 10 μM dNTP, and 0.5 μL of 5 U/μL Taq DNA polymerase (TaKaRa). The thermal profile consisted of denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, and a final extension at 72°C for 7 min. The PCR products were evaluated by staining them with ethidium bromide using 1% agarose gel electrophoresis. The pooled DNA samples of PCR products from 30 randomly selected Chinese Holstein cattle were purified with a DNA fragment recovery kit and sent to TaKaRa Biotechnology Co., Ltd., for sequencing. DNAMAN software was used to observe the nucleotide changes of the sequence based on the deposited bovine reference sequence (NCBI gene no.: NC_007304.3) and to screen the polymorphisms of the PEPS gene 5′ flanking region.
Table 1.
List of Primer Sets Used in This Work
| Primers | Primer sequences (5'–3') | Annealing temperature (°C) | Fragment size (bp) |
|---|---|---|---|
| P 1 | F: GAGGATGCGGAGACTGAGAC | 55 | 924 |
| R: ACGGCACAAGAACGGAATAC | |||
| P 2 | F: GGCTAACTGCCTACAATGTTGGAC | 55 | 282 |
| R: GGCTTAGAGTGTAGGGATC | |||
| P 3 | F: GCTCCAGCTTCTACAAAG | 58 | 433 |
| R: TGTAACACAGCCAGAAGAGAC | |||
| P4 | F: ATGCTCAACCTCAAAACTCC | 60 | 143 |
| R: ATCACTCAGACCAGCGAAAC | |||
| β-actin | F: GCACAATGAAGATCAAGATCATC | 60 | 150 |
| R: CTAACAGTCCGCCTAGAAGCA |
For the restriction fragment length polymorphism (RFLP) of the PEPS gene, the shorter 282- and 433-bp-long fragments of the PEPS gene were PCR amplified using primers P2 and P3, respectively (Table 1). The PCR products were digested with HinfI and BglI endonucleases. The restriction of DNA fragments was electrophoretically analyzed in 10% polyacrylamide gel, and then genotyped after stained with 0.1% silver nitrate. To confirm the identity of the analyzed fragment of the PEPS gene, the PCR products representing TT, TC, CC and GG, GA, and AA genotypes were sequenced.
Statistical analysis
Polymorphism information content, heterozygosity, and effective number of alleles were calculated using POPGENE32 (ver. 1.31). SHEsis software (http://analysis.bio-x.cn) was used to analyze the pairwise linkage disequilibrium (LD) and haplotype frequencies (Shi and He, 2005). The general linear model procedure from the Statistical Analysis Software (2000; SAS Institute, Inc.) was used to determine the relationship between the polymorphisms of the PEPS gene and milk production traits. The linear model is expressed as
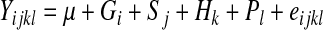 |
Where Yijkl is the milk yield or the observed number of milk, μ is the mean, Gi is the fixed effect of the ith genotype or ith haplotype (i=1–3), Sj is the fixed effect of the jth season (j=1–2), Hk is the fixed effect of the kth farm (k=1–20), Pl is the fixed effect of the lth parity (l=1–4), and eijkl is the random residual effect. The additive and dominant genetic effects were also estimated by SAS according to Hill et al. (2004).
Fluorescence quantitative real-time PCR
Heart, liver, spleen, lung, kidney, muscle, intestine, and mammary tissue samples were collected from 15 Chinese Holstein cattle in the slaughterhouse. Tissue samples were obtained immediately after slaughter, then snapped and frozen in liquid nitrogen until RNA isolation. Each tissue was dissolved in TRIzol reagent (Bioteke) for total RNA extraction according to the manufacturer's instructions. RNA was then treated with RNase-free DNase (Promega) to remove all genomic DNA contaminants. RNA quality was assessed by measuring the relative absorbance at 260 and 280 nm. Electrophoresis on agarose gels under denaturing conditions was performed to confirm the integrity of the ribosomal RNA bands. cDNA was synthesized from 1 μg of total RNA using the transcriptor first-strand cDNA synthesis kit (TaKaRa).
Real-time PCR was performed in a 20 μL mixture containing 50 ng cDNA, 0.4 μM each of the sense and antisense primers, 6.8 μL ddH2O, 10.0 μL SYBR® Premix Ex TaqTM (2×), and 0.4 μL ROX Reference Dye (50×) (TaKaRa). The β-actin gene was used as an endogenous control to normalize the differences in the amount of total cDNA added to each reaction. The reaction mixture was denatured for 30 s at 95°C and was followed by 40 cycles of 5 s at 95°C and 31 s at 60°C. PCR was monitored by the ABI PRISM 7000HT Fast Real-Time PCR system (Applied Biosystems) using primers P4 and β-actin (Table 1). The relative gene expression among different tissues was analyzed by the standard curve-based method (Larionov et al., 2005) and was calculated using Student's t-test. The level of significance was set at p<0.05.
Constructions of PEPS luciferase reporter gene promoter vector
Based on the results of bioinformatics prediction and association analysis, g. −543 T>C SNP was chosen for further function studies. PCR was used to amplify the PEPS gene −543 T and −543 C promoter fragments from bovine genomic DNA samples and then subcloned into the pEASY-T3 vector (Invitrogen) to verify whether the g. −543 T>C SNP affects the PEPS gene promoter activity. The constructs were named pEASY-T3/−543 T and pEASY-T3/−543 C, respectively. The fragments were digested in KpnI and MluI, and then ligated into the corresponding site of the pGL3 vector (Promega) to create the PEPS luciferase constructs. The constructs were sequenced to facilitate correct insertion and proper orientation for the assessment of functional promoter activity and to ensure that no errors were introduced by PCR.
Human kidney epithelial cell line 293T cell culture and transfection
The 293T cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) with 10% fetal bovine serum (HyClone) and 100 units/mL penicillin with 100 μg/mL streptomycin at 37°C with 10% CO2. For transfection studies, cells were plated in 96-well plates, cultured for 24 h, and transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. In the experiments, 10 ng of pRL-TK vector (Promega) was cotransfected to adjust transfection efficiency, and the empty pGL3-Basic vector was used as a control. The cells were harvested 24 h after transfection using a passive lysis buffer (Promega). Reporter activity was measured in the cell extract using Luminescencer-MCA (ATTO). Firefly and Renilla luciferase activities were sequentially determined in the same samples using the dual luciferase assay kit (Promega).
Results
Prediction of the promoter region of bovine PEPS
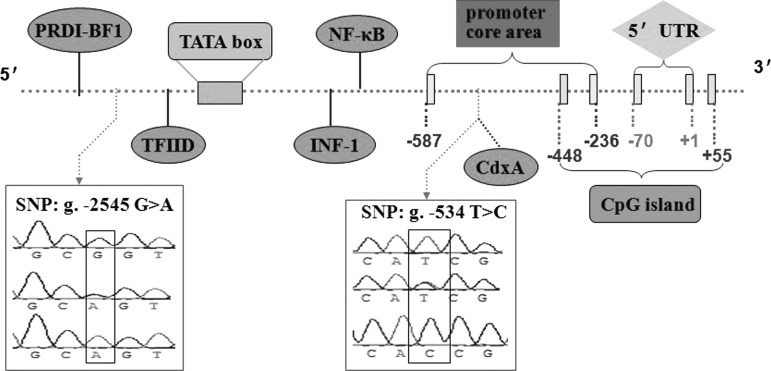
The sequence fragment of the 5′-proximal region of the bovine PEPS gene was successfully amplified using primer P1. In the 5′ flanking region of the bovine PEPS gene, there is a CpG island in the −448 bp to +55 bp from the translation start codon. All of the bases are numbered and the “A” of the translation start codon is considered as +1. The predicted results showed that the −587 bp to −236 bp region is the promoter core area, and typical TATA box, INF-1, NF-κB, CdxA, PRDI-BF1, and TFIID transcription factor binding site also exist in 5′ flanking region (Fig. 1).
FIG. 1.
Bioinformatics analysis and single-nucleotide polymorphisms (SNPs) of a 2500-bp 5′ flanking sequence in the bovine PEPS gene. The translation start site (ATG) is marked A with +1. The transcription factor binding sites are shown with ellipse.
SNP analysis of PEPS gene in Chinese Holstein
In the current study, primer P1 was used to amplify the promoter region of the bovine PEPS gene. PCR products were detected by 1% agarose gel and genotyped by PCR-RFLP. The polymorphic DNA fragments were sequenced. Comparison of the bovine PEPS gene sequence (NC_007304.3) and our sequences revealed two novel SNPs, namely, g. −534 T>C and g. −2545 G>A. Digestions of the PCR products of the PEPS gene g. −534 T>C locus with HinfI produced 22-, 85-, and 175-bp fragments for genotype TT; 22-, 85-, 175-, and 197-bp fragments for genotype TC; and 85- and 197-bp fragments for genotype CC. Digestions of the PCR products of the PEPS gene g. −2545 G>A locus with BglI produced 202-, 143-, and 88-bp fragments for genotype GG; 345-, 202-, 143-, and 88-bp fragments for genotype GA; and 345- and 88-bp fragments for genotype AA (Fig. 2).
FIG. 2.
Polymerase chain reaction-restriction fragment length polymorphism patterns of the bovine PEPS gene.
Genetic parameter analysis
The distribution of genotypic and allelic frequencies of g. −534 T>C and g. −2545 G>A, as well as their genetic diversity, are shown in Table 2. The C and G alleles are the dominant alleles at loci g. −534 T>C and g. −2545 G>A, respectively. The χ2 test results showed that both loci deviate from the Hardy–Weinberg disequilibrium, which implies significant differences in genotypic and allelic distributions within the two loci in the population analyzed. Genetic parameter analysis indicated that the locus g. −534 T>C has moderate polymorphism within the population. However, the locus g. −2545 G>A has low polymorphism.
Table 2.
Genotypic and Allelic Frequencies and Hardy–Weinberg Equilibrium χ2 Test of PEPS Gene
| Loci/samples | Genotype frequencies/samples | Allelic frequencies | Polymorphic information content | He | Ne | Hardy–Weinberg equilibrium χ2test |
|---|---|---|---|---|---|---|
| TT(46) 0.0614 | T 0.4129 | |||||
| TC(522) 0.7029 | C 0.5871 | |||||
| g. −534 T>C (743) | CC(175) 0.2357 | 0.3673 | 0.4848 | 1.9410 | Disequilibrium (p<0.05) | |
| GG(627) 0.8442 | G 0.9157 | |||||
| GA(106) 0.1429 | ||||||
| g. −2545 G>A (743) | AA(10) 0.0129 | A 0.0843 | 0.1424 | 0.1544 | 1.1825 | Disequilibrium (p<0.05) |
LD analysis and haplotype construction
Pairwise LD showed the two mutations with weak LD (D′=0.242, r2=0.004). Four haplotypes were constructed, namely, H1 (CA), H2 (CG), H3 (TA), and H4 (TG). Haplotype frequencies are 5.8%, 52.9%, 2.6%, and 38.6%, respectively. Eight haplotype combinations were found, namely, H1H1(CCAA), H2H1(CCGA), H2H2(CCGG), H3H1(CTAA), H4H1(CTGA), H4H2(CTGG), H4H3(TTGA), and H4H4(TTGG). The haplotype combination analysis showed that H2H4 and H2H2 haplotype combinations have the most frequency.
Associations between single SNP, haplotype combinations, and milk production traits
As shown in Table 3, at locus g. −534 T>C, the cows with genotype CC have higher SCS than the ones with genotype TC (p<0.05). Cows with genotype CC have higher fat percentage than genotype TT (p<0.05). The additive effect of fat percentage is significant (p<0.05). At locus g. −2545 G>A, the cows with genotype GA have higher SCS and fat percentage than the ones with genotype GG (p<0.05). However, no significant association of these SNPs with 305-day milk yield and protein percentage was detected in the analyzed populations (p>0.05). A number of significant associations was observed between the PEPS promoter haplotypes and milk performance traits (Table 4). The number of subjects with the haplotype combination H3H4 and H1H2 is significantly higher than the numbers of subjects with the haplotype combinations H4H4 with fat percentage (p<0.05). For SCS, the number of subjects with the haplotype combinations H2H4 is significantly lower than those with the haplotype combinations H1H2 and H2H2 (p<0.05).
Table 3.
Least Square Means (±Standard Error) of Milk Performance Traits of Different Genotypes in Bovine PEPS Gene of Chinese Holstein
| Loci | Genotype | Fat percentage (%) | Protein percentage (%) | 305-day milk yield (kg) | SCS |
|---|---|---|---|---|---|
| TT | 3.15±0.39b | 3.09±0.22 | 6497.7±726.8 | 4.65±0.68a,b | |
| g. −534 T>C | TC | 3.45±0.36a,b | 3.03±0.20 | 6622.1±670.7 | 4.41±0.62b |
| CC | 3.49±0.37a | 3.01±0.20 | 6490.0±685.6 | 4.78±0.64a | |
| Additive effect | 0.17±0.09 | −0.04±0.04 | 3.81±155.93 | 0.07±0.15 | |
| p-Value | 0.0482 | 0.3891 | 0.9805 | 0.6480 | |
| Dominance effect | 0.12±0.10 | −0.02±0.05 | 128.26±179.31 | −0.31±0.17 | |
| p-Value | 0.2110 | 0.7188 | 0.4747 | 0.0699 | |
| GG | 3.41±0.36b | 3.04±0.20 | 6611.8±671.4 | 4.40±0.62b | |
| g.−2545 G>A | GA | 3.63±0.37a | 3.01±0.20 | 6566.5±689.9 | 4.86±0.64a |
| AA | 3.61±0.48a,b | 3.05±0.27 | 6111.0±1117.0 | 4.93±0.84a,b | |
| Additive effect | 0.10±0.16 | 0.01±0.09 | −240.37±449.31 | 0.26±0.28 | |
| p-Value | 0.5299 | 0.9249 | 0.5929 | 0.3454 | |
| Dominance effect | 0.12±0.19 | −0.03±0.11 | 195.07±482.61 | 0.20±0.33 | |
| p-Value | 0.5186 | 0.7746 | 0.6862 | 0.5524 |
Values with different superscripts (a, b) within the same row in the same locus denote significant difference, p<0.05.
SCS, somatic cell score.
Table 4.
Least Square Mean and Standard Error for Milk Production Traits of Different PEPS Haplotype Combinations in 743 Chinese Holstein Cows
| Haplotype combination | Number of combination | Fat percentage (%) | Protein percentage (%) | 305-day milk yield (kg) | SCS |
|---|---|---|---|---|---|
| H1H1 (CCAA) | 3 | 3.04±0.78 | 3.03±0.43 | 6218.6±1912.4 | 5.35±1.35 |
| H1H2 (CCGA) | 28 | 3.77±0.40a | 3.03±0.22 | 6385.1±754.3 | 5.13±0.71a |
| H2H2 (CCGG) | 141 | 3.45±0.37 | 3.00±0.20 | 6511.7±692.1 | 4.70±0.63a |
| H1H3 (CTAA) | 8 | 3.76±0.51 | 3.05±0.28 | 6092.2±1229.1 | 4.86±0.89 |
| H1H4 (CTGA) | 75 | 3.58±0.38 | 2.96±0.21 | 6584.3±703.5 | 4.76±0.65 |
| H2H4 (CTGG) | 442 | 3.43±0.36 | 3.04±0.20 | 6636.5±673.9 | 4.35±0.62b |
| H3H4 (TTGA) | 4 | 3.78±0.37a | 3.08±0.26 | 6368.7±1229.4 | 4.63±1.14 |
| H4H4 (TTGG) | 42 | 3.12±0.39b | 3.02±0.22 | 6442.6±734.2 | 4.53±0.68 |
H1=CA; H2=CG; H3=TA; H4=TG. Values with different superscripts (a, b) within the same row in the same locus denote significant difference, p<0.05.
Expression of the bovine PEPS mRNA
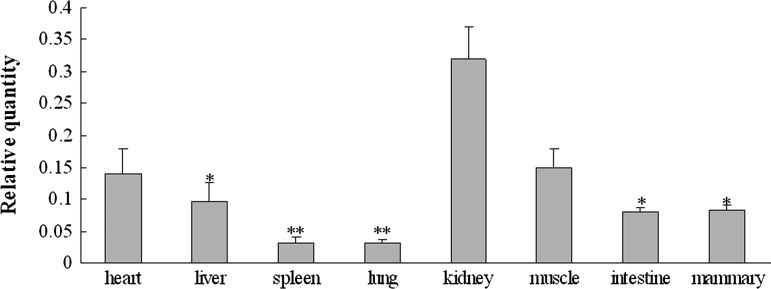
The differences in the PEPS gene expression levels in different tissues of Chinese Holstein cattle were also investigated (Fig. 3). Quantitative data indicated that the PEPS gene is expressed in the heart, liver, spleen, lung, kidney, muscle, intestine, and mammary tissues at considerably varied expression levels. The PEPS gene mRNA expression levels in the kidney tissue are the highest, and are significantly higher than those of the spleen, lung, liver, intestine, and mammary glands (p<0.05). The mRNA expression in the kidney is 3.3 times greater than that of the liver, 10 times greater than that of the spleen and lung, and 4 times greater than that of the mammary gland and intestine.
FIG. 3.
Expression differences of PEPS gene in different tissues of Chinese Holstein cattle; *p<0.05, **p<0.01.
Polymorphisms affecting PEPS promoter activity
The role of the single nucleotide at −543 for the promoter function was examined because the polymorphism g. −543 T>C was found at the promoter core region and transcription factor binding sites. Two reporter plasmids pEASY-T3/−543 T and pEASY-T3/−543 C were then constructed, and their transcription activity was analyzed by transient reporter assay in the 293T cells. As shown in Figure 4, the luciferase activity derived from the −543 T promoter is higher than that from the −543 C promoter (p<0.5). The 293T cells have luciferase values that were 1.6 times higher, which were associated to the −543 T promoter compared with the −543 C promoter.
FIG. 4.
Comparative activity analysis of bovine PEPS promoter when T changes to C at locus −543.
Discussion
The PEPS gene plays a critical role in the metabolism of several peptides, such as regulation of hormone levels, protein maturation, and inactivation of the protein and protein digestion in the terminal stage (Taylor, 1993; Noboru et al., 2000). The PEPS gene also catalyzes the hydrolysis of leucine residues from the amino-termini of protein or peptide substrates and participates in the conversion of peptides released by endoproteases or proteasome to their amino acid constituents (Rawlings and Barrett, 2004). Reduced enzymatic activity of PEPS in humans may cause hypertension. Sheely et al. (2009) showed that the PEPS gene for the BTA6 QTL positional candidates is differentially expressed in bovine mammary tissue across different stages of the lactation cycle (pregnancy, lactation, and involution). A 2.2-fold increase in expression in tissues from lactating mammary glands was observed for the PEPS gene compared with tissue from late pregnancy. Khatkar et al. (2004) reported that there are a number of QTL for milk production traits on BTA6. Olsen et al. (2005) mapped a QTL to a 420-kb region in bovine chromosome 6 containing six milk production candidate genes including the PEPS gene. Zheng et al. (2011) studied the five SNPs in the introns 12 and exon 13 of the PEPS gene, and SNP g.25415 T>C was found to be significantly associated with protein percentage. In the current study, two novel SNPs in the promoter region of the bovine PEPS gene were genotyped, and their combined haplotypes were associated with milk quality traits. There were notably significant associations with fat percentage and SCS traits.
Real-time PCR studies of eight tissues from Chinese Holstein cattle were carried out to understand the PEPS gene expression profiles. Results indicate a differential expression of the bovine PEPS gene in different tissues. The results are validated by the results of Cuypers et al. (1982), which reported that among the PEPS gene expressed in many tissues including lens, kidney, pancreas, muscle, liver, and mammary glands, the PEPS expression level in kidney tissue is the highest. This finding provided a basis of selection of transfected cell lines for further study.
Transcription of a gene can be regulated by SNPs within the regulatory regions (Aslan et al., 2011). SNPs in transcription factor binding sites can lead to allele-specific binding of transcription factors and can modulate gene expression (Hohjoh and Tokunaga, 2001). Numerous SNPs in gene regulatory regions have been associated with variations in enzyme levels and diseases. A promoter SNP (1323 T>C) in the G-substrate gene (GSBS) correlates with hypercholesterolemia (Ono et al., 2003). The UGT1A1 gene has a TATA box polymorphism that reduces the expression of UGT1A1, which leads to Gilbert's syndrome (Grant et al., 2004). The steroid metabolism gene CYP17 has a GC box polymorphism in its proximal promoter and has been associated with higher levels of circulating estradiol (Feigelson et al., 1998). Of the two promoter SNPs in the current study, g. −534 T>C was identified as potential regulatory SNPs.
SNPs in transcription factor binding sites may cause differences in gene expression with a significant potential in increasing phenotypic diversity (Wang et al., 2005). In the current research, g. −534 T>C is associated with fat percentage and SCS traits. The presence of the C allele at g. −534 T>C created a CdxA transcription factor binding motif, which was eliminated with the presence of the T allele. The CdxA transcription factor family is highly expressed in digestive organs and plays a critical role in the development of the intestines (Margalit et al., 1993). This finding suggests a possible role of g. −534 T>C in bovine fat content. Mutants −543 T and −543 C promoter fragments were constructed and transiently transfected into 293T cells to study the SNP g. −543 T>C impact on PEPS promoter activity. A human kidney epithelial cell line 293T was used as the transfected cell because of a lack in bovine kidney epithelial cell line in the market. Luciferase reporter assays demonstrated that the promoter with −543 T has a stronger activity than that with the −543 C in the statistical analysis. The C allele with g. −534 T>C decreased by 33% in the reporter gene transcription in 293T cells, which might have important physiological effects (Yan et al., 2002).
Conclusion
In the current study, two novel SNPs in the promoter region of the bovine PEPS gene were genotyped and their combined haplotypes were associated with milk quality traits. There were notable significant associations between fat percentage and SCS traits. In addition, the PEPS gene mRNA expression profiles in different tissues were studied by real-time PCR. The promoter activities of the polymorphism of g. −534 T>C were measured by luciferase assay in the human kidney epithelial cell line 293T. The results indicate that genetic variation at locus −543 influences the activity of the PEPS promoter. SNPs in the PEPS gene promoter could potentially contribute to genome-assisted selection of SNP panels to improve milk production traits on a breed basis.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (No. 31000543); the Program of the Ministry of Science and Technology, China (Nos. 2011BAD19B02 and 2011BAD19B04); the Major Project of National Transgene in China (Nos. 2011ZX08007-001 and 2011ZX08007-004); the Modern Agro-Industry Technology Research System (No. CARS-37); the Well-Bred Program of Shandong Province (No. 2010LZ10-06); and the Key Scientific and Technological Project of Shandong Province (No. 2009GG20002033).
Disclosure Statement
No competing financial interests exist in the present research.
References
- Aslan O. Hamill R.M. Mullen A.M. Davey G.C. Gil M. Gladney C.D. Sweeney T. Association between promoter polymorphisms in a key cytoskeletal gene (Ankyrin 1) and intramuscular fat and water-holding capacity in porcine muscle. Mol Biol Rep. 2011 doi: 10.1007/s11033-011-1169-4. [DOI] [PubMed] [Google Scholar]
- Chorley B.N. Wang X. Campbell M.R. Pittman G.S. Noureddine M.A. Bell D.A. Discovery and verification of functional single nucleotide polymorphisms in regulatory genomic regions: current and developing technologies. Mutat Res. 2008;659:147–157. doi: 10.1016/j.mrrev.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers H.T. van Loon-Klaassen L.A. Egberts W.T. de Jong W.W. Bloemendal H. The primary structure of leucine aminopeptidase from bovine eye lens. J Biol Chem. 1982;7:7077–7085. [PubMed] [Google Scholar]
- Feigelson H.S. Shames L.S. Pike M.C. Coetzee G.A. Stanczyk F.Z. Henderson B.E. Cytochrome P450c17alpha gene (CYP17) polymorphism is associated with serum estrogen and progesterone concentrations. Cancer Res. 1998;58:585–587. [PubMed] [Google Scholar]
- Grant D.J. Hall I.J. Eastmond D.A. Jones I.M. Bell D.A. Bilirubin UDP-glucuronosyltransferase 1A1 (UGT1A1) gene promoter polymorphisms and HPRT, glycophorin A, and micronuclei mutant frequencies in human blood. Mutat Res. 2004;560:1–10. doi: 10.1016/j.mrgentox.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Hill W.G. Mackay T.F. Falconer D.S. Introduction to quantitative genetics. Genetics. 2004;167:1529–1536. doi: 10.1093/genetics/167.4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohjoh H. Tokunaga K. Allele-specific binding of the ubiquitous transcription factor OCT-1 to the functional single nucleotide polymorphism (SNP) sites in the tumor necrosis factor-alpha gene (TNFA) promoter. Genes Immun. 2001;2:105–109. doi: 10.1038/sj.gene.6363721. [DOI] [PubMed] [Google Scholar]
- Hudson T.J. Wanted: regulatory SNPs. Nat Genet. 2003;33:439–440. doi: 10.1038/ng0403-439. [DOI] [PubMed] [Google Scholar]
- Ju Z.H. Li Q.L. Huang J.M. Hou M.H. Li R.L. Li J.B. Zhong J.F. Wang C.F. Three novel SNPs of the bovine Tf gene in Chinese native cattle and their associations with milk production traits. Genet Mol Res. 2011;1:340–352. doi: 10.4238/vol10-1gmr1038. [DOI] [PubMed] [Google Scholar]
- Khatib H. Zaitoun I. Chang Y.M. Maltecca C. Boettcher P. Evaluation of association between polymorphism within the thyroglobulin gene and milk production traits in dairy cattle. J Anim Breed Genet. 2007;124:26–28. doi: 10.1111/j.1439-0388.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- Khatkar M.S. Thomson P.C. Tammen I. Raadsma H.W. Quantitative trait loci mapping in dairy cattle: review and metaanalysis. Genet Sel Evol. 2004;36:163–190. doi: 10.1186/1297-9686-36-2-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel P.M. Ossendorp F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr Opin Immunol. 2004;1:76–81. doi: 10.1016/j.coi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Kuss A.W. Gogol J. Geldermann H. Association of a polymorphic AP-2 binding site in the 5’ flanking region of the bovine β-lactoglobulingene with milk proteins. J Dairy Sci. 2003;86:2213–2218. doi: 10.3168/jds.s0022-0302(03)73811-9. [DOI] [PubMed] [Google Scholar]
- Larionov A. Krause A. Miller W. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics. 2005;6:62. doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liefers S.C. te Pas M.F. Veerkamp R.F. Van der Lende T. Associations between leptin gene polymorphisms and production, live weight, energy balance, feed intake, and fertility in Holstein heifers. J Dairy Sci. 2002;85:1633–1638. doi: 10.3168/jds.S0022-0302(02)74235-5. [DOI] [PubMed] [Google Scholar]
- Margalit Y. Yarus S. Shapira E. Gruenbaum Y. Fainsod A. Isolation and characterization of target sequences of the chicken CdxA homeobox gene. Nucleic Acids Res. 1993;21:4915–4922. doi: 10.1093/nar/21.21.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M. Fowler J.H. Walling L.L. Leucine aminopeptidases: diversity in structure and function. Biol Chem. 2006;12:1535–1544. doi: 10.1515/BC.2006.191. [DOI] [PubMed] [Google Scholar]
- Noboru Y. Seiji N. Takanobu S. Atsuo I. Mitsuaki I. Tomomitsu O. Masafumi T. Hiroshi N. Shigehiko M. Placenta leucine aminopeptidase/oxytocinase in maternal serum and placenta during normal pregnancy. Life Sci. 2000;15:1401–1410. doi: 10.1016/s0024-3205(00)00451-3. [DOI] [PubMed] [Google Scholar]
- Olsen H.G. Lien S. Gautier M. Nilsen H. Roseth A. Berg P.R. Sundsaasen K.K. Svendsen M. Meuwissen T.H.E. Mapping of a milk production quantitative trait locus to a 420-kb region on bovine chromosome 6. Genetics. 2005;169:275–283. doi: 10.1534/genetics.104.031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. Ezura Y. Emi M. Fujita Y. Takada D. Sato K. Ishigami T. Umemura S. Takahashi K. Kamimura K. Bujo H. Saito Y. A promoter SNP (_1323 T>C) in G-substrate gene (GSBS) correlates with hypercholesterolemia. J Hum Genet. 2003;48:447–450. doi: 10.1007/s10038-003-0055-x. [DOI] [PubMed] [Google Scholar]
- Rawlings N.D. Barrett A.J. Introduction: metallopeptidases and their clans. In: Barrett Alan J., editor; Rawlings Neil D., editor; Fred Woessner J., editor. Handbook of Proteolytic Enzymes. 2nd. Elsevier/Academic Press; San Diego, CA: 2004. [Google Scholar]
- Sheely P.A. Riley L.G. Raadsma H.W. Williamson P. Wynn P.C. A function genomics approach to evaluate genes located in a QTL interval for milk production traits on BTA6. Anim Genet. 2009;40:492–499. doi: 10.1111/j.1365-2052.2009.01862.x. [DOI] [PubMed] [Google Scholar]
- Shi Y.Y. He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15:97–98. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- Taylor A. Aminopeptidases: structure and function. FASEB J. 1993;7:290–298. doi: 10.1096/fasebj.7.2.8440407. [DOI] [PubMed] [Google Scholar]
- Taylor V.J. Beever D.E. Bryant M.J. Wathes D.C. Pre-pubertal measurements of the somatotrophic axis as predictors of milk production in Holstein-Friesian dairy cows. Domest Anim Endocrinol. 2006;31:1–18. doi: 10.1016/j.domaniend.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Wallner B.P. Hession C. Tizard R. Frey A.Z. Zuliani A. Mura C. Jahngen-Hodge J. Taylor A. Isolation of bovine kidney leucine aminopeptidase cDNA: comparison with the lens enzyme and tissue-specific expression of two mRNAs. Biochemistry. 1993;36:9296–9301. doi: 10.1021/bi00087a006. [DOI] [PubMed] [Google Scholar]
- Wang X.T. Tomso D.J. Liu X.M. Bell Douglas A. Single nucleotide polymorphism in transcriptional regulatory regions and expression of environmentally responsive genes. Toxicol Appl Pharmacol. 2005;207:S84–S90. doi: 10.1016/j.taap.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Xie X.H. Lu J. Kulbokas E.J. Golub T.R. Mootha V. Lindblad-Toh K. Lander E.S. Kellis M. Systematic discovery of regulatory motifs in human romoters and 3′UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahyaoui M.H. Angiolillo A. Pilla F. Sanchez A. Folch J.M. Characterization and genotyping of the Caprine κ-casein variants. J Dairy Sci. 2003;86:2715–2720. doi: 10.3168/jds.S0022-0302(03)73867-3. [DOI] [PubMed] [Google Scholar]
- Yan H. Weishi Y. Velculescu V.E. Vogelstein B. Kinzler K.W. Allelic variation in human gene expression. Science. 2002;16:1143. doi: 10.1126/science.1072545. [DOI] [PubMed] [Google Scholar]
- Zheng X. Ju Z.H. Wang J. Li Q.L. Huang J.M. Zhang A.W. Zhong J.F. Wang C.F. Single nucleotide polymorphisms, haplotypes and combined genotypes of LAP3 gene in bovine and their association with milk production traits. Mol Biol Rep. 2011;38:4053–4061. doi: 10.1007/s11033-010-0524-1. [DOI] [PubMed] [Google Scholar]