Abstract
Thermal lesions were produced in 12 male Wistar rats, positioning a massive aluminum bar 10 mm in diameter (51 g), preheated to 99°C ± 2°C/10 min. on the back of each animal for 15 sec. After 7, 14, 21, and 28 days, animals were euthanized. The edema intensity was mild, with no bubble and formation of a thick and dry crust from the 3rd day. The percentage of tissue shrinkage at 28 days was 66.67 ± 1.66%. There was no sign of infection, bleeding, or secretion. Within 28 days reepithelialization was incomplete, with fibroblastic proliferation and moderate fibrosis and presence of modeled dense collagen fibers. It is concluded that the model established is applicable in obtaining deep second-degree thermal burns in order to evaluate the healing action of therapeutic agents of topical use.
1. Introduction
Burns are tissue lesions from thermal origin for exposure to flames, hot surfaces and liquids, extreme cold, chemicals, radiation, or friction [1]. Even with improved prognosis [2] and progress in the use of biological skin substitutes [3], burns are an important cause of mortality [4].
Burns are classified depending on the lesion severity into superficial or first degree, when lesion is restricted to the epidermis or skin causing redness; partial thickness or second degree that can be superficial when reaching the epidermis and superficial dermis, showing hypersensitivity and pain, or deep when it extends to the deepest layer of the dermis and may have reduced sensitivity with red and/or white coloration of the tissue; full-thickness or third degree when lesion involves the subcutaneous layer, with no sensitivity and white coloring [5].
The use of animals as experimental models in different areas of biological research was encouraged by Claude Bernard [6], who around 1865, described in his paper entitled “Introduction to the Study of Experimental Medicine” the use of animals as a model for study and transposition into human physiology. Experimental models are essential in mammals when studying on burns. There are literature reports on the use of rabbits [7], pigs [8], dogs [9], rats [10], and mice [11] as models in the study of burns.
The healing of skin lesions induces the burn-injured tissue inflammation, edema, and hypertrophic and unsightly scars [12]. Thus, the choice of a topical agent or the type of coverage to be used in treating burns should be conducted based on the assessment of lesion characteristics and evidence reported in the specific literature. These products must have features such as antimicrobial or bacteriostatic activity, absence of toxicity and hypersensitivity, compliance, reduced healing time, and cost/benefit [13]. However, many of the methods used in healing injuries caused by burns are controversial [14].
In this context, the objective of this study was to establish an experimental protocol for induction of deep second-degree thermal lesions in Wistar male rats to obtain clinical and histopathologic data that will facilitate understanding of results concerning the evolution of the healing action of topical therapeutic agents.
2. Materials and Methods
2.1. Animals
The experiment was conducted at the Department of Experimental Surgery, Federal University of Pernambuco, using albino Wistar male rats (Rattus norvegicus) weighing 250 ± 50 g, kept in individual cages of polypropylene measuring 30 × 20 × 19 cm and controlled lighting conditions (12 h light/dark photoperiod), temperature (24 ± 2°C), receiving water, and food (Labina) ad libitum. The experimental procedure was approved by the ethics committee on animal experimentation of the Federal University of Pernambuco (Case no. 23076.015015/2009-31).
2.2. Thermal Burn Experimental Model
Initially, 12 animals were weighed and intramuscularly preanesthetized with atropine sulfate (0.04 mg/kg) and 10 minutes after subjected to anesthetic combination of 10% ketamine (90 mg/kg) and 2% xylazine (10 mg/kg) intramuscularly [15, 16]. With the animal properly anesthetized trichotomy of back was performed and antisepsis with 1% polyvinylpyrrolidone iodine. Thermal injuries were made with a solid aluminum bar 10 mm in diameter (Figure 1(a)), previously heated in boiling water and so that the temperature reached 100°C measured with a thermometer. The bar is maintained in contact with the animal skin on the dorsal proximal region for 15 sec (Figure 1(b)). The pressure exerted on the animal skin corresponded to the mass of 51 g of aluminum bar used in the burn induction. Immediately after the procedure, analgesia with dipyrone sodium (40 mg/kg) was performed intramuscularly, being maintained for three consecutive days sodium dipyrone at 200 mg/kg orally administered in the drinking water supplied to animals.
Figure 1.
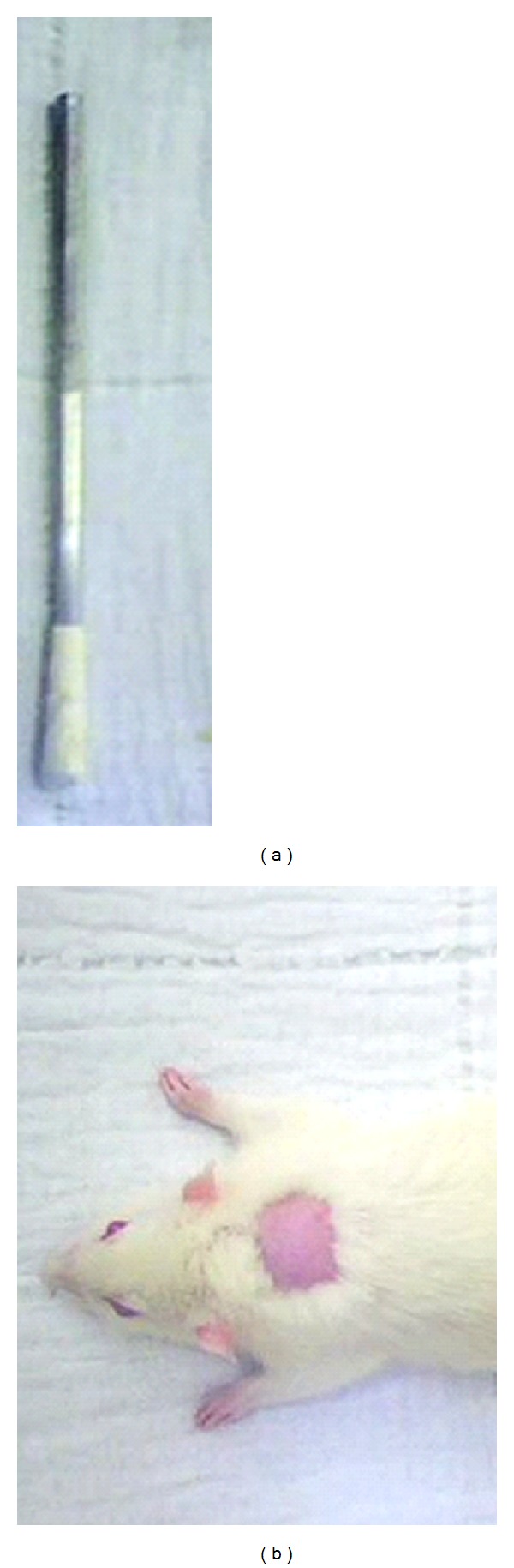
Experimental model of second-degree thermal burn in male Wistar rats. (a) Solid aluminum bar of 10 mm in diameter and 51 g used in the induction of thermal burns by direct heat transference. (b) Proximal dorsal region chosen for burn induction.
2.3. Clinical Evaluation
The clinical course of skin lesions by burns was evaluated for 28 consecutive days based on the following aspects: blistering, swelling, redness, crust, bleeding, secretion, granulation tissue, and scar tissue.
The wound retraction was evaluated using a caliper in 7, 14, 21, and 28 days after burn induction. Wound contraction was expressed as reduction in percentage of original wound size. % wound contraction on day X = [(area on day 0 − open area on day X)/area on day 0] × 100 [17].
2.4. Microbiological Evaluation
Microbiological evaluation was carried out using “swabs” in the injury area at the moment of surgery and respective days of biopsies. This sample was transferred to a Petri dish of 20 × 150 mm containing nutrient agar medium in a laminar flow chamber. After 24 h incubation, plates inoculated in triplicate for each sample were evaluated. This routine evaluation was performed to evaluate the degree of contamination of injuries.
2.5. Histological Analysis
At the preestablished times for biopsy (7, 14, 21, and 28 days after burn induction), three animals randomly selected underwent anesthesia combination of 10% ketamine (90 mg/kg) and 2% xylazine (10 mg/kg), intramuscularly [15, 16] for tissue samples collection. Euthanasia was performed by excessive doses of sodium pentobarbital intraperitoneally (100 mg/kg) [18].
Tissue samples were immediately fixed by immersion in 4% formaldehyde (v/v) prepared in PBS (0.01 M, pH 7.2), followed by routine histological processing paraffin embedding, microtomy with 4 μm cuts, and Masson's trichrome staining. Histological study was performed by comparative descriptive analysis of the experimental groups in binocular optical microscope (Zeiss-Axiostar model) where were evaluated the evolution of skin healing after thermal trauma.
The histological analysis was performed by independent pathologist who was experienced in the examination of burn wound specimens, in the following ways: (1) inflammatory response, characterized by the presence of polymorphonuclear leukocytes (PNM), (2) granular tissue, characterized by the presence of fibroblasts, myofibroblasts, and neovascularization, (3) fibrosis, characterized by the density of collagen fibers identified by the intensity of blue color observed under optical microscopy due to staining by Masson's trichrome. A score was made for all parameters evaluated : − = absent, + = mild presence, ++ = moderate presence, and +++ = strong presence.
2.6. Statistical Analysis
Data were analyzed using nonparametric tests. To detect differences between groups, the Kruskal-Wallis was used. All results were expressed as mean values for group ± standard deviation and analyzed considering P < 0.05 as statistically significant.
3. Results and Discussion
3.1. Study Design
This experimental model was established to standardize thermal burn injuries in order to obtain injuries with the same size and depth degree. The choice of Wistar rats due to these animals shows a great ease of handling, accommodation and resistance to surgical aggressions, and infectious processes, with low mortality [19, 20]. However, the choice of male rats is due to variations in hormonal cycles in females that could intervene in the process of tissue repair [21]. The result of clinical evaluation showed no signs of infection, secretion, bleeding, or death in both groups. If wounds are not well treated, they can be infected. Infected wounds heal more slowly, reepithelisation is more prolonged, and there is also the risk of systemic infection [22].
Shaving the back of the animals was performed by manual traction of hair (Figure 2(a)) thus preventing secondary skin lesions that often occurs by the use of laminated devices [23]. The option to induce only one burning in the dorsal-proximal aimed at preventing the animal itself could reach the burn so that altering the outcome of the clinical evaluation of lesions. The use of individual aluminum bar for each animal in the experimental group is important in reducing the interval between the induction of a burn and another within the same group, thus avoiding large variations in the assessment of healing time. The size of lesions showed uniform average distribution of 10 ± 1 mm in diameter (Figure 2(b)). Similar studies by Heredero and colleagues [20] and Meyer and Silva [24] revealed that it is not possible to perform a perfectly uniform burn in all experimental rats.
Figure 2.
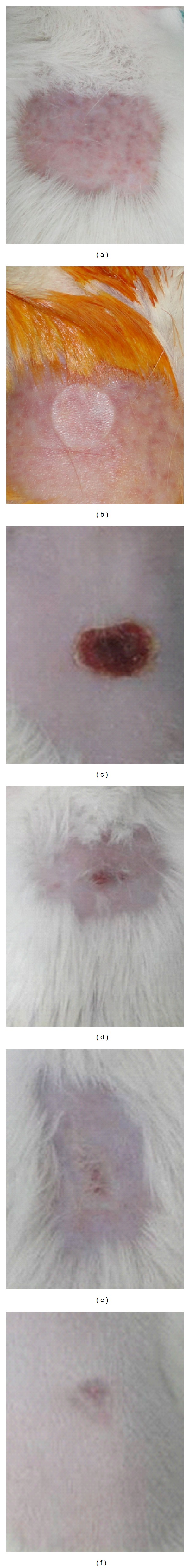
Clinical evolution observed in the experimental model of deep second-degree thermal burns in male Wistar rats. (a) Animal's skin after shaving. (b) Thermal lesion obtained with 10 mm diameter bar, with presence of mild edema. (c) Injured tissue on day 7 after burn induction, presence of thin and dry crust with homogeneous staining and discreet detachment on the edges. (d) Damaged tissue on day 14 after burn induction, presence of granulation tissue in the center of the lesion with a second discreet crust and formation of scar tissue at the edge. (e) Injured tissue on day 21 after the burn induction, discreet presence of granulation tissue with the presence of scar tissue. (f) Injured tissue at day 28 after burn induction, tissue with incomplete healing.
According to Vale [25], the burn depth depends on the intensity of the thermal agent, generator or heat transmitter and time of contact with the tissue, which is the determinant of the aesthetic and functional result of the burn. Medeiros et al. [26] caused thermal burns by using 5 cm2 aluminum plate heated to 130°C, which were pressed into the skin of the back for 5 seconds. However, this method can generate lesions with different depths depending on the pressure during the procedure. In our study, the pressure was equivalent to the mass of the aluminum bar (51 g) there being no interference by researcher, thus ensuring the reproducibility of thermal injuries.
The standardization of procedures, systematization, and organization of knowledge about the interrelationships of models is necessary to provide more reliable knowledge advance [27]. The most common method for obtaining second-degree thermal burns uses hot water as heat transfer agent. Khorasani et al. [28] induced second-degree thermal burns on the back of rats using submersion in hot water (90°C) for 6 seconds. In this experiment, 10% body surface of the animal was injured producing lesions of variable size. According to Orgaes et al. [23], burns when reaching 26% to 30% of total body surface area of these mice cause mortality rates of 40% after three days, 52.5% after 7 days, 57.5% after 15 days, and 62.5% after 25 days.
3.2. Macroscopic Evaluation
Results of this study revealed thermal burns white in color, painful, with no bubbles, mild edema until the 3rd day after injury (Figure 2(b)). Similar definition is reported by [29, 30] that describes the deep second-degree burns and injuries that have pale color with pain in lower intensity compared to superficial second-degree burn. In our evaluation variation of the degree of hyperemia in the first three days of experiment that changed from slight to absent was observed (Table 1). The formation of a thick and dry crust was observed from day 3 after burn induction. Signs of the scar tissue formation at the edge of the lesion were observed from day 14 (Figure 2(d)).
Table 1.
Clinical parameters evaluated in the experimental model of deep second degree thermal burns in male Wistar rats.
| Time | Animal | Clinical signs of the experimental model | |||
|---|---|---|---|---|---|
| Edema | Hyperemia | Crust | Scar tissue | ||
| 7th day | 1 | + | + | * | − |
| 2 | + | − | * | − | |
| 3 | + | − | * | − | |
|
| |||||
| 14th day | 1 | − | + | − | + |
| 2 | + | − | − | + | |
| 3 | − | − | − | + | |
|
| |||||
| 21st day | 1 | − | − | − | +++ |
| 2 | − | − | − | +++ | |
| 3 | − | − | − | +++ | |
|
| |||||
| 28th day | 1 | − | − | − | ++ |
| 2 | − | − | − | + | |
| 3 | − | − | − | + | |
The intensity of clinical signs was scored as −: absent; *: present, +: mild, ++: moderate, +++: strong.
The burn healing occurs by second intention, which is a slow process with high risk of infection, producing scar retraction, which depending on the area of injury can cause extensive scarring and consequently high cost in treatment [31]. The contraction of skin lesions occurs centripetally fromthe injury edges being caused by the action of myofibroblasts present at the site. In turn, myofibroblasts may promote lesion retraction from 50 to 70% of original size [32]. The percentage of lesion contraction at the end of the experiment was 66.67 ± 1.66%. Values obtained in this study are similar to those published by Zohdi and colleagues [33], who observed 72.75 ± 1.8% of reduction in control rats treated with hydrogel without drug (placebo) at 28 days of study (Figure 3).
Figure 3.
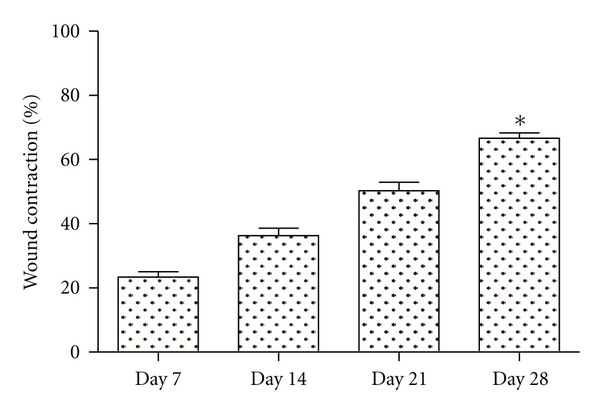
Contraction area percentage of deep second-degree thermal burns in the experimental model in male Wistar rats. n = 3. Values are mean ± SEM. *P < 0.05.
According to Mandelbaum and colleagues [34], the mechanism of tissue repair is the integration of dynamic cellular and molecular processes involving biochemical and physiological phenomena aiming at ensuring tissue restoration. For this reason, only the clinical evaluation of a burn injury does not provide information on the evolution degree of tissue healing, being of fundamental importance of the histopathologic evaluation of these lesions.
3.3. Microscopic Evaluation
The histopathological findings confirmed the acquisition of deep second-degree burns based on the observation of total autolysis of both the dermis and epidermis, without reaching the hypodermis. These data are consistent with reports of several authors who characterize it as deep second-degree burn injuries that cause partial or total destruction of nerve endings, hair follicles, and sweat glands [25, 35, 36].
Thermal injury was observed on the 7th day and extensive inflammatory exudate featuring an intense inflammatory reaction. Inngjerdinger et al. [22] describe in their study the occurrence in the control group, treated with saline solution, an acute inflammatory process on the 6th day of evaluation. By day 14 the inflammatory response was classified as moderate with presence of macrophages, progressing to discreet at day 21. By day 28 signs of inflammatory response in the animals evaluated was not observed (Table 2).
Table 2.
Histopathological analysis on the degree of inflammatory intensity, presence of granulation tissue, and fibrosis in the skin after deep second-degree thermal burn. Samples were obtained on days 7, 14, 21, and 28 after burn wound induction.
| Time | Animal | Inflammatory response | Granulation tissue | Fibrosis |
|---|---|---|---|---|
| 7th day | 1 | +++ | + | − |
| 2 | +++ | + | − | |
| 3 | +++ | + | − | |
|
| ||||
| 14th day | 1 | + | ++ | + |
| 2 | ++ | +++ | + | |
| 3 | ++ | +++ | + | |
|
| ||||
| 21st day | 1 | + | + | + |
| 2 | + | + | ++ | |
| 3 | + | ++ | ++ | |
|
| ||||
| 28th day | 1 | − | − | ++ |
| 2 | − | − | ++ | |
| 3 | − | − | ++ | |
Intensity of the evaluated parameters was scored as −: absent, +: mild presence, ++: moderate presence; +++: strong presence.
Tissue still presented a complete destruction of the dermis and epidermis and maintenance of the hypodermis (Figure 4(a)) on the 7th day after lesion induction. Histopathology section of the burned skin of control animals on 5th day showed denuded epidermis, diffuse infiltration of plasma cells, lymphocytes, and polymorphs [37]. After 14 days the histopathological evaluation revealed moderate autolysis of the tissue, with discrete neovascularization and fibroblast proliferation, with loose collagen fibers, not modeled with mild fibrosis and crust absence (Figure 4(b)). Yaman and colleagues [38] confirm the presence of crust formed by remnants of necrotic tissue and infiltration of mononuclear cells on the 4th day of experimentation in the control group. The crust detachment was only observed by these authors on the 14th day of study.
Figure 4.
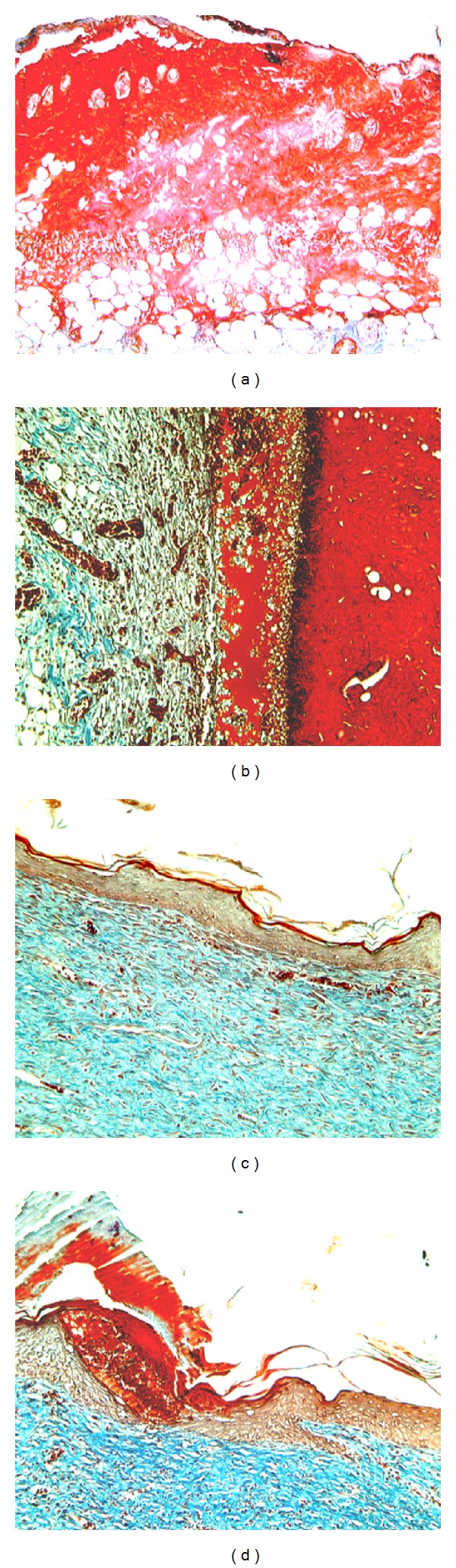
Histopathological aspects of deep second-degree thermal burns. Masson's trichrome staining. 100x Magnification. (a) Animal showing thin crust and epithelial tissue with complete destruction of dermis and epidermis and hypodermis maintenance at the 7th day after the thermal lesion induction. (b) Animal at day 14, with crust and tissue reepithelialization, showing collagen, not modeled and slight fibrosis. (c) Animal at day 21, tissue repithelialization showing intense fibroblastic proliferation with the presence of dense collagen, not modeled and moderate fibrosis. (d) Animal at day 28, with incomplete tissue repithelialization, moderate fibroblastic proliferation, presence of modeled dense collagen mesh, and moderate fibrosis.
By day 21 we observed the absence of autolysis, discrete neovascularization and intense fibroblastic proliferation, with dense collagen, not modeled and moderate fibrosis (Figure 4(c)). By the end of the experiment at 28 days, histological observations showed incomplete reepithelialization of the injured tissue with autolysis and absent neovascularization, showing moderate fibroblastic proliferation and fibrosis with the presence of modeled dense collagen fibers (Figure 4(d)).
Wound healing includes number of stages like clotting, inflammation, granulation, fibrosis, arrangement of collagen with spasm of wound, and epithelization. The time required for complete healing of deep second-degree burns, without the application of specific therapeutic agents, can be three to six weeks or more, and these burns will leave a scar tissue that may hypertrophy and contract itself [29, 30].
4. Conclusion
In this new model of second-degree thermal burns, injuries are easy to create and easily reproducible. There are similarities with the human second-degree burns in clinical and pathologic aspects. Thus, the animal model presented in this study is applicable in evaluating the use of therapeutic agents in the healing evolution of deep second-degree burns.
References
- 1.Jorge SA, Dantas SRPE. Abordagem Multiprofissional do Tratamento de Feridas. São Paulo, Brazil: Atheneu; 2003. [Google Scholar]
- 2.Barret JP, Herndon DN. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Archives of Surgery. 2003;138(2):127–132. doi: 10.1001/archsurg.138.2.127. [DOI] [PubMed] [Google Scholar]
- 3.Ramos-E-Silva M, Ribeiro de Castro MC. New dressings, including tissue-engineered living skin. Clinics in Dermatology. 2002;20(6):715–723. doi: 10.1016/s0738-081x(02)00298-5. [DOI] [PubMed] [Google Scholar]
- 4.Sheridan RL, Hinson MI, Liang MH, et al. Long-term outcome of children surviving massive burns. Journal of the American Medical Association. 2000;283(1):69–73. doi: 10.1001/jama.283.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Rangel MF, Pereira APJT. Atendimento inicial e definitivo do grande queimado. Jornal Brasileiro de Medicina. 2007;92(2):20–22. [Google Scholar]
- 6.Bernard C. An introduction to the study of experimental medicine (1865) In: Images from the history of medicine division, National Library of Medicine, http://www.ihm.nlm.nih.gov.
- 7.Bashkaran K, Zunaina E, Bakiah S, Sulaiman SA, Sirajudeen K, Naik V. Anti-inflammatory and antioxidant effects of Tualang honey in alkali injury on the eyes of rabbits: experimental animal study. BMC Complementary and Alternative Medicine. 2011;11, article 90 doi: 10.1186/1472-6882-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer AJ, Hirth D, McClain SA, Clark RA. Lack of agreement between gross visual and histological assessment of burn reepithelialization in a porcine burn model. Journal of Burn Care and Research. 2012;33(2):286–290. doi: 10.1097/BCR.0b013e3182331de2. [DOI] [PubMed] [Google Scholar]
- 9.Hu S, Che JW, Tian YJ. Effect of oral fluid resuscitation on pulmonary vascular permeability and lung water content in burn dogs in shock stage. Zhonghua Shao Shang Za Zhi. 2009;25(3):184–187. [PubMed] [Google Scholar]
- 10.Campelo P, Campelo MW, Britto GA, Ayala AP, Guimarães SB, Vasconcelos PR. An optimized animal model for partial and total skin thickness burns studies. Acta Cirúrgica Brasileira. 2011;26(1):38–42. doi: 10.1590/s0102-86502011000700008. [DOI] [PubMed] [Google Scholar]
- 11.Asai A, Tsuda Y, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Pathogenic role of macrophages in intradermal infection of methicillin-resistant Staphylococcus aureus in thermally injured mice. Infection and Immunity. 2010;78(10):4311–4319. doi: 10.1128/IAI.00642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar V, Abbas AK, Fausto N, Aster J. Robbins & Cotran Pathologic Basis of Disease. 8th edition. Philadelphia, Pa, USA: WB Saunders; 2010. [Google Scholar]
- 13.Ferreira E, Lucas R, Rossi LA, Andrade D. Treatment of the burned patient: a review of the literature. Revista da Escola de Enfermagem da USP. 2003;37(1):44–51. doi: 10.1590/s0080-62342003000100006. [DOI] [PubMed] [Google Scholar]
- 14.Atiyeh BS, Hayek SN, Gunn SW. New technologies for burn wound closure and healing—review of the literature. Burns. 2005;31(8):944–956. doi: 10.1016/j.burns.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Massone F. Anestesiologia Veterinária: Farmacologia e Técnicas. 2nd edition. Rio de Janeiro, Brazil: Guanabara Koogan; 1994. [Google Scholar]
- 16.Hillyer EV, Quesenberry KE. Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery. New York, NY, USA: WB Sounders; 1997. [Google Scholar]
- 17.Kumar MS, Sripriya R, Raghavan HV, Sehgal PK. Wound healing potential of Cassia fistula on infected albino rat model. Journal of Surgical Research. 2006;131(2):283–289. doi: 10.1016/j.jss.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 18.de Lucca RR, Alexandre SR, Marques T, Souza NL, Merusse JL, Neves SP. Manual Para Técnicos em Bioterismo. São Paulo, Brazil: Winner Graph; 1996. [Google Scholar]
- 19.Marchini FB, Martins DMFS, Teves DC, Simões MJ. Efeito do óleo de rosa mosqueta na cicatrização de feridas abertas. Revista Paulista de Medicina. 1988;106(6, article 356) [PubMed] [Google Scholar]
- 20.Heredero FXS, Hamann C, Martin JMO, Arias CR, Menchero SC. Experimental burn models. Annals of Burns and Fire Disasters. 1996;9(2):96–97. [Google Scholar]
- 21.Teves DC, Cabral ACV, Simões MJ, Kulay JRL. Biologia das reparações teciduais. Jornal Brasileiro de Medicina. 1986;50:39–44. [Google Scholar]
- 22.Inngjerdinger K, Nergard CS, Diallo D, Monoro PP, Paulsin BS. An ethnopharmacological survey of plants used for wound healing in Dongoland, Mali, West Africa. Journal of Ethnopharmacology. 2004;92:233–244. doi: 10.1016/j.jep.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Orgaes FAFS, Lyra MC, Rodrigues OF, Jr., Gonella HA. Estudo histopatológico do uso de heparina tópica em queimaduras por escaldo em ratos. Revista Brasileira de Cirurgia Plástica. 2007;22(1):39–44. [Google Scholar]
- 24.Meyer TN, Silva AL. A standard burn model using rats. Acta Cirúrgica Brasileira. 1999;14(4) [Google Scholar]
- 25.Vale ECS. Primeiro atendimento em queimaduras: a abordagem do dermatologista. Anais Brasileiros de Dermatologia. 2005;80(1):9–19. [Google Scholar]
- 26.Medeiros AC, Ramos AMO, Dantas Filho AM, Azevedo RCF, Araújo FLFB. Tratamento tópico de queimaduras do dorso de ratos com ácido hialurônico. Acta Cirúrgica Brasileira. 1999;14(4) [Google Scholar]
- 27.Fagundes DJ, Taha MO. Modelo animal de doença: critérios de escolha e espécies de animais de uso corrente. Acta Cirúrgica Brasileira. 2004;19(1):59–65. [Google Scholar]
- 28.Khorasani G, Hosseinimehr SJ, Zamani P, Ghasemi M, Ahmadi A. The effect of saffron (Crocus sativus) extract for healing of second-degree burn wounds in rats. The Keio Journal of Medicine. 2008;57(4):190–195. doi: 10.2302/kjm.57.190. [DOI] [PubMed] [Google Scholar]
- 29.Tecklin JS. Fisioterapia Pediátrica. 3rd edition. Porto Alegre—Rio Grande do Sul, Brazil: Artmed; 2002. [Google Scholar]
- 30.Johnson RM, Richard R. Partial-thickness burns: identification and management. Advances in Skin & Wound Care. 2003;16(4):178–187. doi: 10.1097/00129334-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Coelho COM, Rezende CMF, Tenório APM. Contração de feridas após cobertura com substitutos temporários de pele. Ciência Rural. 1999;29(2):297–303. [Google Scholar]
- 32.Swaim SF, Hinkle SH, Bradley DM. Wound contraction: basic and clinical factors. Compendium on Continuing Education for the Practicing Veterinarian. 2001;23(1):20–33. [Google Scholar]
- 33.Zohdi RM, Zakaria ZAB, Yusof N, Mustapha NM, Abdullah MN. Gelam (Melaleuca spp.) honey-based hydrogel as burn wound dressing. Evidence-Based Complementary and Alternative Medicine. 2012;2012:7 pages. doi: 10.1155/2012/843025. Article ID 843025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandelbaum SR, di Santis EP, Mandelbaum MH. Cicatrização: conceitos atuais e recursos auxiliares—parte I. Anais Brasileiros de Dermatologia. 2003;78(4):393–410. [Google Scholar]
- 35.Lima OS, Lima Verde FS, Lima Filho OS. Medicina Perioperatória. Vol. 91. Rio de Janeiro, Brazil: Sociedade de Anestesiologia do Estado do Rio de Janeiro, 91. Capítulo; 2006. Queimados: alterações metabólicas, fisiopatologia, classificação e interseções com o tempo de jejum; pp. 803–815. [Google Scholar]
- 36.Mélega JM. Cirurgia Plástica—Fundamentos e Arte: Princípios Gerais. 1st edition. Rio de Janeiro, Brazil: Guanabara Koogan; 2002. [Google Scholar]
- 37.Chandran PK, Kuttan R. Effect of Calendula officinalis flower extract on acute phase proteins, antioxidant defense mechanism and granuloma formation during thermal burns. Journal of Clinical Biochemistry and Nutrition. 2008;43(2):58–64. doi: 10.3164/jcbn.2008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaman I, Durmus AS, Ceribasi S, Yaman M. Efects of Nigella sativa and silver sulfadiazine on burn wound healing in rats. Veterinarni Medicina. 2010;55(12):619–624. [Google Scholar]


