Executive Summary
Objective
To assess the effectiveness, and cost effectiveness of EECP in patients with severe anginal symptoms, secondary to chronic coronary disease, who are unresponsive to exhaustive pharmacotherapy and not candidates for surgical/percutaneous revascularization procedures (e.g., angioplasty, coronary bypass surgery).
To assess the effectiveness, and cost effectiveness of EECP in patients with heart failure.
Clinical Need
Angina
Angina is a clinical syndrome characterized by discomfort in the chest, jaw, shoulder, back or arm. Angina usually occurs in patients with coronary artery disease (CAD) involving ≥1 large epicardial artery. However it can also occur in people with valvular heart disease, hypertrophic cardiomyopathy, and uncontrolled hypertension.
Conventional approaches to restoring the balance between oxygen supply and demand focus on the disruption of the underlying disease through: drug therapy (β blockers, calcium channel blockers, nitrates, antiplatelet agents, ACE inhibitors, statins); life-style modifications (smoking cessation, weight loss); or revascularization techniques such as coronary artery bypass graft surgery (CABG) or percutaneous coronary interventions (PCI). (1) Limitations of each of these approaches include: adverse drug effects, procedure-related mortality and morbidity, restenosis after PCI, and time dependent graft attrition after CABG. Furthermore, an increasing number of patients are not appropriate candidates for standard revascularization options, due to co-morbid conditions (HF, peripheral vascular disease), poor distal coronary artery targets, and patient preference. The morbidity and mortality associated with repeat surgical revascularization procedures are significantly higher, and often excludes these patients from consideration for further revascularizations. (2)
Patients with CAD who have chronic ischemic symptoms that are unresponsive to both conventional medical therapy and revascularization techniques have refractory angina pectoris. It has been estimated that greater than 100,000 patients each year in the US may be diagnosed as having this condition. (3) Patients with refractory angina have marked limitation of ordinary physical activity or are unable to perform any ordinary physical activity without discomfort (CCS functional class III/IV). Also, there must be some objective evidence of ischemia as demonstrated by exercise treadmill testing, stress imaging studies or coronary physiologic studies. (1)
Dejongste et al. (4)estimated that the prevalence of chronic refractory angina is about 100,000 patients in the United States. This would correspond to approximately 3,800 (100,000 x 3.8% [Ontario is approximately 3.8% of the population of the United States]) patients in Ontario having chronic refractory angina.
Heart Failure
Heart failure results from any structural or functional cardiac disorder that impairs the ability of the heart to act as a pump.
A recent study (5) revealed 28,702 patients were hospitalized for first-time HF in Ontario between April 1994 and March 1997. Women comprised 51% of the cohort. Eighty-five percent were aged 65 years or older, and 58% were aged 75 years or older.
Patients with chronic HF experience shortness of breath, a limited capacity for exercise, high rates of hospitalization and rehospitalization, and die prematurely. (6) The New York Heart Association (NYHA) has provided a commonly used functional classification for the severity of HF (7):
Class I: No limitation of physical activity. No symptoms with ordinary exertion.
Class II: Slight limitations of physical activity. Ordinary activity causes symptoms.
Class III: Marked limitation of physical activity. Less than ordinary activity causes symptoms. Asymptomatic at rest.
Class IV: Inability to carry out any physical activity without discomfort. Symptoms at rest.
The National Heart, Lung, and Blood Institute (7) estimates that 35% of patients with HF are in functional NYHA class I; 35% are in class II; 25%, class III; and 5%, class IV. Surveys (8) suggest that from 5% to 15% of patients with HF have persistent severe symptoms, and that the remainder of patients with HF is evenly divided between those with mild and moderately severe symptoms.
To date, the diagnosis and management of chronic HF has concentrated on patients with the clinical syndrome of HF accompanied by severe left ventricular systolic dysfunction. Major changes in treatment have resulted from a better understanding of the pathophysiology of HF and the results of large clinical trials. Treatment for chronic HF includes lifestyle management, drugs, cardiac surgery, or implantable pacemakers and defibrillators. Despite pharmacologic advances, which include diuretics, angiotensin-converting enzyme inhibitors, beta-blockers, spironolactone, and digoxin, many patients remain symptomatic on maximally tolerated doses. (6)
The Technology
Patients are typically treated by a trained technician in a medically supervised environment for 1 hour daily for a total of 35 hours over 7 weeks. The procedure involves sequential inflation and deflation of compressible cuffs wrapped around the patient’s calves, lower thighs and upper thighs. In addition to 3 sets of cuffs, the patient has finger plethysmogram and electrocardiogram (ECG) attachments that are connected to a control and display console.
External counterpulsation was used in the United States to treat cardiogenic shock after acute myocardial infarction. (9;10) More recently, an enhanced version namely “enhanced external counterpulsation” (EECP) was introduced as a noninvasive procedure for outpatient treatment of patients with severe, uncontrollable cardiac ischemia. EECP is said to increase coronary perfusion pressure and reduce the myocardial oxygen demand. Currently, EECP is not applicable for all patients with refractory angina pectoris. For example, many patients are considered ineligible for therapy due to co-morbidities, including those with severe pulmonary vascular disease, deep vein thrombosis, phlebitis and irregular heart rhythms, and heart failure. (1)
Very recently, investigation began into EECP as an adjunctive treatment for patients with HF. Anecdotal reports suggested that EECP may benefit patients with coronary disease and left ventricular dysfunction. The safety and effectiveness of EECP in patients with symptomatic heart failure and coronary disease and its role in patients with nonischemic heart failure secondary to LV dysfunction is unclear. Furthermore, the safety and effectiveness of EECP in the different stages of HF and whether it is only for patients who are refractive to pharmacotherapy is unknown.
2003 Health Technology Assessment by the Medical Advisory Secretariat
The Medical Advisory Secretariat health technology assessment (originally published in February 2003) reported on the effectiveness of EECP for patients with angina and HF. The report concluded that there was insufficient evidence to support the use of EECP in patients with refractory stable CCS III/IV angina as well as insufficient evidence to support the use of EECP in patients with HF.
Review Strategy
The aim of this literature review was to assess the effectiveness, safety, and cost effectiveness of EECP for the treatment of refractory stable CCS III/IV angina or HF.
The standard search strategy used by the Medical Advisory Secretariat was used. This included a search of all international health technology assessments as well as a search of the medical literature from December 2002 to March 2006.
A modification of the GRADE approach (11) was used to make judgments about the quality of evidence and strength of recommendations systematically and explicitly. GRADE provides a framework for structured reflection and can help to ensure that appropriate judgments are made. GRADE takes into account a study’s design, quality, consistency, and directness in judging the quality of evidence for each outcome. The balance between benefits and harms, quality of evidence, applicability, and the certainty of the baseline risks are considered in judgments about the strength of recommendations.
Summary of Findings
The Cochrane and INAHTA databases yielded 3 HTAs or systematic reviews on EECP treatment (Blue Cross Blue Shield Technology Evaluation Center [BCBS TEC], ECRI, and the Centers for Medicare and Medicaid Services [CMS]). A search of Medline and Embase December 2005 – March 2006 (after the literature search cutoff from the most recent HTA) was conducted using key words enhanced external counterpulsation, EECP, angina, myocardial ischemia, congestive heart failure. This search produced 1 study which met the inclusion criteria. This level 4a study was inferior in quality to the RCT which formed the basis of the 2003 Medical Advisory Secretariat recommendation.
BCBS reviewed the evidence through November 2005 to determine if EECP improves health outcomes for refractory chronic stable angina pectoris or chronic stable HF. (12) BCBS concluded that the available evidence is not sufficient to permit conclusions of the effect of EECP on health outcomes. Both controlled trials had methodologic flaws (MUST EECP and MUST EECP quality of life studies). The case series and observational studies for both indications while suggestive of a treatment benefit from EECP have shortcomings as well.
On March 20 2006, CMS posted their proposed coverage decision memorandum for external counterpulsation therapy. (13) Overall, CMS stated that the evidence is not adequate to conclude that external counterpulsation therapy is reasonable and necessary for:
Canadian Cardiovascular Society Classification (CCSC) II angina
-
Heart failure
NYHA class II/III stable HF symptoms with an EF≤35%
NYHA class II/III stable HF symptoms with an EF≤40%
NYHA class IV HF
Acute HF
Cardiogenic shock
Acute MI
In January 2005, ECRI (14) stated that there was insufficient evidence available to draw conclusions about the long-term effectiveness of EECP, with respect to morbidity, survival, or quality of life, for any coronary indication (refractory angina, congestive heart failure, cardiogenic shock and acute MI).
GRADE Quality of the Studies
According to the GRADE Working Group criteria, the quality of the trials was examined (Table 1). (11)
Quality refers to the criteria such as the adequacy of allocation concealment, blinding and followup.
Consistency refers to the similarity of estimates of effect across studies. If there is important unexplained inconsistency in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the size of the differences in effect and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the people interventions and outcome measures are similar to those of interest. For example, there may be uncertainty about the directness of the evidence if the people of interest are older, sicker or have more comorbidity than those in the studies.
As stated by the GRADE Working Group, the following definitions were used in grading the quality of the evidence. (11)
| High | Further research is very unlikely to change our confidence n the estimate of effect. |
| Moderate | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Table 1: GRADE Quality of Studies.
| Outcome | Design | Quality | Consistency | Directness | Overall Quality |
|---|---|---|---|---|---|
| Angina | 1 RCT |
Poor Unclear dropouts Subanalyses Patients/providers not blinded Greater adverse effects in EECP group. |
Exercise duration not significantly different between arms. Authors’ results and conclusions not match. | Not all patients have severe, refractory
stable angina. Large exclusion criteria. |
Very Low
|
| Case series Registry studies |
Uncontrolled. | Concomitant therapy in
patients. Dropouts not accounted for. |
Rationale for treatment unclear in some studies – e.g., patient “tune ups”. | ||
| Heart Failure | 1 unpublished RCT |
Poor Unclear dropouts Subanalyses Patients/providers not blinded |
Conflicting co-primary endpoint
results. Exacerbation of HF in some patients. |
Large exclusion criteria. | Very Low
|
| Case series Registry studies |
Uncontrolled. | Concomitant therapy in
patients. Dropouts not accounted for. |
Registry studies have patients enrolled for angina, not HF. |
Economic Analysis - Literature Review
No economic analysis of EECP was identified in the published literature.
Estimated Prevalence of Angina in Ontario
3,800 patients with chronic refractory angina:
The number of patients with chronic refractory angina in the US is estimated to be approximately 100,000 (4), this corresponds to about 3,800 patients in Ontario (3.8% × 100,000) with refractory angina.
3,800 patients × $7,000 Cdn (approximate cost for a full course of therapy) ~ $26.6M Cdn.
Estimated Prevalence of Heart Failure in Ontario
23,700 patients EF ≤ 0.35:
This estimate is from an expert (personal communication) at the Institute for Clinical Evaluative Sciences (ICES), where they examined a sample of echocardiography studies drawn from a diagnostic lab in 2001. They found that the prevalence of EF ≤ 0.35 was 8.3%, and if generalized to all patients undergoing echocardiography, there would be 23,700 patients.
23,700 patients with EF ≤35% × $7,000 Cdn ~ $166 M Cdn.
Conclusions
There is insufficient evidence to support the effectiveness and safety of EECP treatment for patients with refractory stable CCS III-IV angina or HF.
As per the GRADE Working Group, overall recommendations consider 4 main factors. (11)
The tradeoffs, taking into account the estimated size of the effect for the main outcome, the confidence limits around those estimates and the relative value placed on the outcome.
The quality of the evidence.
Translation of the evidence into practice in a specific setting, taking into consideration important factors that could be expected to modify the size of the expected effects such as proximity to a hospital or availability of necessary expertise.
Uncertainty about the baseline risk for the population of interest.
The GRADE Working Group also recommends that incremental costs of healthcare alternatives should be considered explicitly alongside the expected health benefits and harms. (11) Recommendations rely on judgments about the value of the incremental health benefits in relation to the incremental costs. The last column in Table 2 is the overall trade-off between benefits and harms and incorporates any risk/uncertainty.
For angina and heart failure, the overall GRADE and strength of the recommendations is “weak” – the quality of the evidence is “low” (uncertainties due to methodological limitations in the study design in terms of study quality and directness), and the corresponding risk/uncertainty is increased due to a budget impact of approximately $26.6 M Cdn or $166 M Cdn respectively while the cost-effectiveness of EECP is unknown and difficult to estimate considering that there are no high quality studies of effectiveness.
Table 2: Overall GRADE and Strength of Recommendation (Including Uncertainty).
| Quality | Estimated Prevalence in Ontario | Cost-Effectiveness | Cost in Ontario | Overall Grade and Strength of
Recommendation (Including Uncertainty) |
|
|---|---|---|---|---|---|
| Severe (CCS III/IV) refractory angina | Low | ~ 3,800 | ? Unknown | ~ $26.6M Cdn | Weak |
| Heart failure | Low | ~ 23,700 | ? Unknown | ~ $166 M Cdn | Weak |
Objective
To assess the effectiveness, and cost effectiveness of EECP in patients with severe anginal symptoms, secondary to chronic coronary disease, who are unresponsive to exhaustive pharmacotherapy and not candidates for surgical/percutaneous revascularization procedures (e.g., angioplasty, coronary bypass surgery).
To assess the effectiveness, and cost effectiveness of EECP in patients with heart failure.
Background
Clinical Indications
Angina
Angina is a clinical syndrome characterized by discomfort in the chest, jaw, shoulder, back or arm. Typically, it is aggravated by exertion or emotional stress and relieved by nitroglycerin. Angina usually occurs in patients with coronary artery disease (CAD) involving ≥1 large epicardial artery. However it can also occur in people with valvular heart disease, hypertrophic cardiomyopathy, and uncontrolled hypertension.
Angina represents an imbalance between myocardial oxygen supply and demand and is the symptom that most often brings patients with ischemic heart disease to medical attention. (1) The Canadian Cardiovascular Society (CCS) has established a classification of the grading of angina effort (Table 1).
Table 1: Canadian Cardiovascular Society Grading of Angina.
| Functional Class | Effort associated with angina |
|---|---|
| I | Ordinary physical activity does not cause angina, such as walking or climbing stairs. Angina with strenuous or prolonged exertion at work or recreation. |
| II | Slight limitations of ordinary physical activity. Walking or climbing stairs rapidly, walking uphill, walking or climbing stairs after meals, or in a cold or in wind, or under emotional stress or only during the few hours after wakening. Walking more than 2 blocks on the level and climbing more than 1 flight or ordinary stairs at a normal pace and in normal conditions. |
| III | Marked limitation of ordinary physical activity. Walking 1-2 blocks on the level and climbing more than 1 flight in normal conditions. |
| IV | Inability to carry on any physical activity without discomfort – anginal syndrome may be present at rest. |
From: Campeau L. Grading of angina pectoris [letter]. Circulation, 54:522-523, 1976. Copyright 1976, American Heart Association, Inc.
Chronic stable angina is the initial manifestation of ischemic heart disease in approximately one-half of patients. (15) The reported annual incidence of angina is 213/100,000 population greater than 30 years old. (16) The prevalence of angina was estimated by extrapolating from the number of myocardial infarctions (MI) in the United States. (15) Approximately one-half of patients presenting at the hospital with MI have preceding angina. (17) A current estimate is that there are 1,100,000 patients with MI each year in the US (18), and about one-half of these survive until hospitalization. Similar annual rates of MI in patients with angina symptoms have been reported (3%-3.5%). (19;20) On this basis, it was estimated that there are 30 patients with stable angina for every patient with infarction who is hospitalized. Therefore, the number of patients with stable angina was estimated to be 30 x 550,000 or 16,500,000. This estimate does not include patients who do not seek medical attention for their chest pain.
For the 1996/97 period, the hospitalization rate for angina, including the general category of ischemic heart disease and angina pectoris specifically, averaged 277 admissions per 100,000 population aged ≥20 years in Ontario. (21) These rates were higher for the elderly, averaging 1,363 and 1,113 per 100,000 population, for men and women respectively, >75 years of age. (21)
The most important determinants of the prognosis of chronic stable angina are underlying left ventricular systolic function at rest, co-morbid conditions, and severity and extent of CAD. (22) The goals of treatment in chronic stable angina are to (22;23):
1) Prolong life and reduce the incidence of acute coronary syndromes (unstable angina, myocardial infarction).
2) Decrease the frequency and severity of angina symptoms and increase angina-free exercise duration (functional capacity).
Conventional approaches to restoring the balance between oxygen supply and demand focus on the disruption of the underlying disease through: drug therapy (β blockers, calcium channel blockers, nitrates, antiplatelet agents, ACE inhibitors, statins); life-style modifications (smoking cessation, weight loss); or revascularization techniques such as coronary artery bypass graft surgery (CABG) or percutaneous coronary interventions (PCI). (1) Limitations of each of these approaches include: adverse drug effects, procedure-related mortality and morbidity, restenosis after PCI, and time dependent graft attrition after CARB. Furthermore, an increasing number of patients are not appropriate candidates for standard revascularization options, due to co-morbid conditions (CHF, peripheral vascular disease), poor distal coronary artery targets, and patient preference. The morbidity and mortality associated with repeat surgical revascularization procedures are significantly higher, and often excludes these patients from consideration for further revascularizations. (2)
Patients with CAD who have chronic ischemic symptoms that are unresponsive to both conventional medical therapy and revascularization techniques have refractory angina pectoris. It has been estimated that greater than 100,000 patients each year in the US may be diagnosed as having this condition. (3) Patients with refractory angina have marked limitation of ordinary physical activity or are unable to perform any ordinary physical activity without discomfort (CCS functional class III/IV). Also, there must be some objective evidence of ischemia as demonstrated by exercise treadmill testing, stress imaging studies or coronary physiologic studies. (1)
Recent therapeutic options emerging for patients with refractory angina include: transmyocardial revascularization; percutaneous myocardial revascularization by laser; minimally invasive coronary bypass surgery; transcutaneous electrical nerve stimulation (TENS); percutaneous CABG; and growth factor technology. (1;2) Many of these options are invasive and carry risk of complications.
Dejongste et al. (4)estimated that the prevalence of chronic refractory angina is about 100,000 patients in the United States. This would correspond to approximately 3,800 (100,000 x 3.8% [Ontario is approximately 3.8% of the population of the United States]) patients in Ontario having chronic refractory angina.
External counterpulsation was used in the United States to treat cardiogenic shock after acute myocardial infarction. (9;10) More recently, an enhanced version namely “enhanced external counterpulsation” (EECP) was introduced as a noninvasive procedure for outpatient treatment of patients with severe, uncontrollable cardiac ischemia (technology is described on page 10). EECP is said to increase coronary perfusion pressure and reduce the myocardial oxygen demand. This combination of effects would be expected to benefit patients with symptoms due to myocardial ischemia. A number of studies have reported sustained improvements in angina control for up to a year or more beyond the treatment phase. Currently, EECP is not applicable for all patients with refractory angina pectoris. For example, many patients are considered ineligible for therapy due to co-morbidities, including those with severe pulmonary vascular disease, deep vein thrombosis, phlebitis and irregular heart rhythms, and heart failure. (1)
Heart Failure
Heart failure results from any structural or functional cardiac disorder that impairs the ability of the heart to act as a pump. One to 5 percent of the general population in Europe have heart failure. (8;24) About half of the patients with heart failure are women and in affluent societies, approximately 40% of men and 60% of women with this condition will be > 75 years of age.
A recent study (5) revealed 28,702 patients were hospitalized for first-time HF in Ontario between April 1994 and March 1997. Women comprised 51% of the cohort. Eighty-five percent were aged 65 years or older, and 58% were aged 75 years or older.
Patients with chronic HF experience shortness of breath, a limited capacity for exercise, high rates of hospitalization and rehospitalization, and die prematurely. (6) The New York Heart Association (NYHA) has provided a commonly used functional classification for the severity of HF (7):
Class I: No limitation of physical activity. No symptoms with ordinary exertion.
Class II: Slight limitations of physical activity. Ordinary activity causes symptoms.
Class III: Marked limitation of physical activity. Less than ordinary activity causes symptoms. Asymptomatic at rest.
Class IV: Inability to carry out any physical activity without discomfort. Symptoms at rest.
The National Heart, Lung, and Blood Institute (7) estimates that 35% of patients with HF are in functional NYHA class I; 35% are in class II; 25%, class III; and 5%, class IV. Surveys (8) suggest that from 5% to 15% of patients with HF have persistent severe symptoms, and that the remainder of patients with HF is evenly divided between those with mild and moderately severe symptoms.
To date, the diagnosis and management of chronic HF has concentrated on patients with the clinical syndrome of HF accompanied by severe left ventricular systolic dysfunction. Major changes in treatment have resulted from a better understanding of the pathophysiology of HF and the results of large clinical trials. Treatment for chronic HF includes lifestyle management, drugs, cardiac surgery, or implantable pacemakers and defibrillators. Despite pharmacologic advances, which include diuretics, angiotensin-converting enzyme inhibitors, beta-blockers, spironolactone, and digoxin, many patients remain symptomatic on maximally tolerated doses. (6)
Patients with heart failure are at increased risk of pulmonary emboli due to a diminished cardiac output, high venous pressures promoting venous stasis, associated chronic venous insufficiency and decreased activity. (25) Counterpulsation intermittently compresses the venous beds in the lower extremities, increasing venous return and potentially mobilizing deep venous thrombi. The sudden increase in preload could also potentially cause pulmonary congestion, acute right HF, or exacerbate ischemia by increasing wall stress and causing hypoxia. (25)
Very recently, investigation began into EECP as an adjunctive treatment for patients with CHF. Anecdotal reports suggested that EECP may benefit patients with coronary disease and left ventricular dysfunction. The safety and effectiveness of EECP in patients with symptomatic heart failure and coronary disease and its role in patients with nonischemic heart failure secondary to LV dysfunction is unclear. Furthermore, the safety and effectiveness of EECP in the different stages of HF and whether it is only for patients who are refractive to pharmacotherapy is unknown.
Acute Myocardial Infarction
External counterpulsation appears to no longer be a routine treatment for patients with acute myocardial infarction (MI). In 1980, Amsterdam et al. (10) reported on patients receiving external pressure circulatory assistance in acute MI. However, the results of this study are not relevant considering the current standard of care for acute MI. (26) Zheng et al. (27) reported on an uncontrolled study that showed symptomatic benefit in 52 patients with acute MI, the device, which incorporated pressure on 4 limbs and buttocks, was different from the modern EECP. No trials have been published since Zheng et al. that evaluate patient outcomes after treatment with the modern enhanced external counterpulsation in patients with acute MI.
Cardiogenic Shock
The body of evidence to support the use of external counterpulsation for cardiogenic shock consists of a small case series from 1974 by Soroff et al. (9) and 2 more current small case series in hemodynamically stable patients, not in patients who have cardiogenic shock. (28;29)
Treatment Protocol
Patients are typically treated by a trained technician in a medically supervised environment for 1 hour daily for a total of 35 hours over 7 weeks. The procedure involves sequential inflation and deflation of compressible cuffs wrapped around the patient’s calves, lower thighs and upper thighs (Appendix 2). In addition to 3 sets of cuffs, the patient has finger plethysmogram and electrocardiogram (ECG) attachments that are connected to a control and display console.
Alternative Technologies
Alternative emerging technologies for refractory angina pectoris have been proposed. These include techniques that may reduce anginal pain by neural stimulation or blockade, and procedures that may enhance coronary myocardial perfusion.
Treatment for heart failure includes: lifestyle management, drugs (angiotensin converting enzyme inhibitors, β blockers, diruetics, spironolactone, digoxin), cardiac surgery or implantable pacemakers and defibrillators.
Mechanism of Action
EECP uses a mechanism similar to the intra-aortic balloon pump. Inflation and deflation of the cuffs are activated by events in the cardiac cycle via ECG. During diastole (cardiac relaxation and filling), the cuffs inflate sequentially from the calves upward to raise diastolic aortic pressure, and subsequently, coronary artery perfusion pressure (Appendix 3). At the onset of systole (cardiac contraction), the cuffs are rapidly deflated. The sudden drop in intra-aortic pressure unloads the left ventricle during systole, thus reducing the work of the ventricle in ejecting blood and reducing oxygen requirements of the cardiac muscle.
The exact mechanism by which EECP may improve anginal symptoms is undefined and controversial. Basic science studies suggested that an increase in sheer stress in the coronary circulation activates multiple pathways, leading to possible angiogenesis or opening of previously dormant vessels, or both. (30) Animal models have suggested an increase in the collateral circulation with counterpulsation. (1)
Possible mechanisms of benefit from EECP in HF include an increase in nitric oxide levels with subsequent coronary and systemic vasodilation (31) and decreased levels of endothelin-1 which is a potent endothelium-derived vasoconstrictor and is believed to contribute to the pathogenesis of HF. (32)
Regulatory Status
Table 2 summarizes the EECP systems licensed by Health Canada as of October 2005.
Table 2: EECP Systems Licensed by Health Canada.
| Product | Manufacturer | License Number | License Date | Indication |
|---|---|---|---|---|
| Enhanced external counterpulsation (EECP) MC2 | Vasomedical Inc. | 21738 | June 26, 2000 | External counterpulsation device using compressive air cuffs applied to legs for the treatment of patients suffering from stable or unstable angina pectoris, acute myocardial infarction and cardiogenic shock |
| EECP therapy model TS3 system | Vasomedical inc. | 60902 | Nov. 14, 2002 | Treatment of patients suffering from stable or unstable angina pectoris, acute myocardial infarction congestive heart failure or cardiogenic shock. |
| EECP therapy system model TS4 | Vasomedical inc. | 64215 | April 22, 2004 | Treatment of patients with stable or unstable angina, congestive heart failure, acute myocardial infarction or cardiogenic shock |
| Lumenair EECP therapy system | Vasomedical inc. | 66866 | December 17, 2004 | Treatment of patients with stable or unstable angina, congestive heart failure, acute myocardial infarction and cardiogenic shock. |
Review of the 2003 Health Assessment of EECP by the Medical Advisory Secretariat Technology
The Medical Advisory Secretariat health technology assessment (originally published in February 2003) reported on the effectiveness of EECP for patients with angina and HF. The report concluded that there was insufficient evidence to support the use of EECP in patients with refractory stable CCS III/IV angina as well as insufficient evidence to support the use of EECP in patients with HF.
A brief summary of the 2003 Medical Advisory Secretariat analysis follows, with emphasis on the controlled studies that were identified. Detailed analyses of these trials are found in Appendices 4 and 5.
Angina
Patients with refractory stable angina have marked limitation of ordinary physical activity or are unable to perform any ordinary physical activity without discomfort (Canadian Cardiovascular Society Functional Class III-IV), despite conventional therapy and revascularization techniques. There must also be some objective evidence of ischemia as demonstrated by exercise treadmill testing, stress imaging studies or coronary physiologic studies. No controlled study has limited assessment of EECP therapy strictly to patients with refractory CCS III-IV angina.
Patients who receive EECP are typically treated by a trained technician in a medically supervised environment for 1 hour daily for a total of 35 hours over 7 weeks. The procedure involves sequential inflation and deflation of compressible cuffs wrapped around the patient’s calves, lower thighs and upper thighs.
One randomized controlled trial (RCT) (Level 2 evidence) evaluated EECP treatment for patients with angina (Multicenter Study of Enhanced External Counterpulsation [MUST-EECP]). (33) Methodological limitations included:
No significant difference was noted between the treatment (n=57) and control (n=58) group for exercise duration, angina count and nitroglycerin usage (using an intent to treat analysis in the latter two endpoints). The study was specifically designed to have 80% power to detect a 45 second difference in “exercise duration” between the two study groups. Therefore, the specific endpoint (exercise duration) that the RCT was designed to examine did not reveal a statistically significant difference between EECP treated and control patients.
Arora et al. (33) stated that “the primary efficacy analyses for exercise treadmill test parameters were performed on an observed case basis using the intent to treat population”. However, an intent to treat analysis was not calculated for “exercise duration” or an electrophysiologic endpoint (≥1 mm ST segment depression). Intent to treat analysis addresses a situation when treatment is not received in full or part by a patient who is randomized to receive treatment. In such a circumstance, there is concern that those patients who do not receive the allocated treatment may differ in some way from those who do receive it.
Subanalyses (angina count and nitroglycerin usage) were calculated using: 1) an intent to treat population and 2) only patients who completed ≥34 sessions. Arora et al. did not specify why two different analyses were used to assess these endpoints.
Angina counts and nitroglycerin usage were further sub-analyzed according to percentage change in angina counts and drug usage. The difference between the two treatment groups with respect to the percent change in each parameter was tested using a chi square test. However, such a test is not robust given the large number of categories with low observed and expected values (<5 per category).
More patients in the active EECP group reported adverse events than in the inactive EECP group: 39 (55%) vs. 17(26%), p<0.001).
Patients with the most severe angina (Canadian Cardiovascular Society Class IV) were excluded from the trial. Only patients with Class I-III were enrolled into the study.
There was no significant difference between the treatment groups in terms of cardiovascular medications at baseline. However, of patients in the inactive EECP group, only 82% were taking nitrates, 91% ASA, 55% calcium channel blockers, 77% beta blockers, and 50% lipid lowering drugs. At baseline, of patients randomized to active EECP, 79% were taking nitrates, 87% ASA, 62% calcium channel blockers, 70% beta blockers, and 62% lipid lowering drugs. Not all patients were receiving exhaustive pharmacotherapy.
Not all the CCS I-III angina patients included in the trial had chronic ischemic symptoms that were unresponsive to exhaustive conventional medical therapy and revascularization techniques, or were not amenable to a revascularization procedure.
The RCT had methodological limitations including: unclear detail of patient dropouts; subanalyses that the trial was not designed to conduct; large exclusion criteria decreasing generalizability of results; and patient self-reporting of angina counts and drug usage.
Despite all of the above methodological limitations, there was one statistically significant improvement in an electrophysiologic endpoint (≥1 mm ST-segment depression) for EECP treated patients compared to controls. The clinical significance of this one endpoint (that was not calculated using an intent to treat analysis) in relation to the lack of significance to other endpoints including exercise duration, angina counts and nitroglycerin usage requires clarification.
Arora et al. only examined the immediate effect of EECP treatment, within one week of completion of treatment for patients who had CCS I-III angina. Arora et al. stated that “MUST-EECP examines only the immediate effect of treatment. Its long-term effects on symptoms and clinical events are not known”. (33) The safety and long-term effects of EECP treatment for angina have not been assessed using a RCT design.
An extension of MUST-EECP examined quality of life (QoL) between a subset of the treatment and control groups up to 1 year after treatment (34):
The substudy had methodological issues including: the study was not originally designed to examine QoL; lack of baseline data for QoL scores between treatment and control groups; the recording of any changes or events in the patients’ statuses was inconsistent and unable to be analyzed; the sample size was determined by power requirements for a previous trial, not the QoL study; and there was a low patient response available for analysis. Similar to the methodological limitation in the original study, there is decreased generalizability of the results due to a large initial exclusion criteria.
Only 54% (n=71) of patients from the original study completed questionnaires for the QoL parameters at baseline, end of treatment and the 1 year follow-up.
Similar to the study by Arora et al. (33) patients with severe angina (CCS IV) were excluded from the trial.
Of patients allocated to inactive EECP (n=35), 31% were CCS III angina. However, only 6% of patients allocated to active EECP (n=36) were CCS III angina.
Exercise treadmill test results, angina episodes and drug usage were not recorded at the 1-year follow-up.
Patient interpretation and scoring of the quality of life parameters may have varied.
There may have been post-treatment behaviour differences between the study groups.
Despite the above limitations, from baseline to the end of treatment the only statistically significant difference between the active EECP (n=35) and inactive EECP (n=36) groups was for “social functioning”, p<0.05. This parameter was from the Study 36 Item Short Form Health Survey (SF-36) which measures general health. From baseline to one year after treatment, there was a statistically significant difference between the active EECP (n=35) and inactive EECP (n=36) groups for “bodily pain”, “social functioning” (both from SF-36), and “cardiac specific health functioning” (from a different scale, the cardiac version of the Quality of Life Index) p<0.05.
The original MUST-EECP study revealed that there was no statistically significant difference between active and inactive EECP treated patients for: 1) exercise duration, 2) angina counts, and 3) nitroglycerin usage. (33) As well, the active EECP treated patients experienced significantly more adverse effects. (33) It is therefore unclear in the study by Arora et al. (34) why the actively EECP treated patients experienced significant improvement in social functioning at the end of treatment. Furthermore, it is unclear in the study by Arora et al. (34) why the actively treated EECP group demonstrated significantly greater improvement at 12 months for “bodily pain”, “social functioning” and “cardiac specific health functioning”. This may be accounted for by the fact that there were more CCS III angina patients in the control group (31%) than the active treatment group (6%). Specific details of the nature of “bodily pain”, “social functioning” and “cardiac specific health functioning” and their improvement in the EECP treated (n=35) and control (n=36) patients are required.
Arora et al. stated “studies in an appropriately sized study population are warranted and desirable” to confirm the findings. (34)
“Soft” semi-objective endpoints were used in many of the studies (e.g., patient recollection of angina episodes and drug usage, exercise performance); paucity of “harder” more objective endpoints (e.g., time to 1 mm ST segment depression).
The major limitation to the registry and case series studies is the lack of a comparison group. Another limitation is a lack of accurate and restrictive selection of patients receiving EECP treatment. One case series study reported that patients received supplementary EECP “tune ups” with no objective or subjective evidence of the patient’s deterioration.
A prospective design of varying treatment lengths is required to clarify any degree to which treatment should be individualized beyond the conventionally accepted 35-hour treatment.
Heart Failure
To date there is scanty, insufficient evidence published on the effectiveness and safety of EECP treatment for HF. No RCT has examined EECP in HF patients.
It is unclear which HF patients, if any, should receive EECP (moderate or severe or both, chronic or acute, left ventricular ejection fraction).
It is unknown whether HF patients should receive EECP treatments as refractory stable angina patients, and if they do, the optimal number of treatments is not determined.
There is a lack of consistent, definitive selection criteria for angina or HF patients receiving EECP treatment.
For studies assessing EECP treatment in HF patients there were major methodological weaknesses:
No information on sample size calculation was provided.
Some conclusions were based on subjective assessment, in particular side effects attributed to EECP despite the fact that the study design did not permit this analysis.
A large number of patients did not complete the case series study. A detailed account of the analysis should have been provided.
Large exclusion criteria in the case series study decreases the generalizability of the results.
Economic Analysis
No cost-effectiveness analyses or general economic analyses were identified that evaluated EECP treatment in patients with refractory stable angina or HF.
Updated Literature Review on Effectiveness
Objective
To assess the effectiveness, and cost effectiveness of EECP in patients with severe anginal symptoms, secondary to chronic coronary disease, who are unresponsive to exhaustive pharmacotherapy and not candidates for surgical/percutaneous revascularization procedures (e.g., angioplasty, coronary bypass surgery).
To assess the effectiveness, and cost effectiveness of EECP in patients with heart failure.
Methodology
Inclusion criteria
English language articles (December 2002 – March 2006).
Journal articles that report primary data on the effectiveness or cost effectiveness of EECP treatment obtained in a clinical setting, or analysis of primary data maintained in registries or databases.
Study design and methods must be clearly described.
Systematic reviews, randomized controlled trials (RCTs), non-randomized controlled trials and/or cohort studies that have ≥20 patients, cost effectiveness studies.
Exclusion criteria
Studies that are duplicate publications (superceded by another publication by the same investigator group, with the same objective and data).
Non-English articles.
Non-systematic reviews, letters and editorials.
Animal and in-vitro studies.
Case reports.
Studies that do not examine the outcomes of interest.
Patients
Human subjects with:
severe refractory angina pectoris who are refractive to optimal medical therapy and are not candidates for surgical revascularization procedures, or congestive heart failure
Intervention
EECP treatment.
Controls do not undergo EECP treatment but receive optimal medical management.
Literature Search
The Medical Advisory Secretariat performed a computer-aided search of the following databases. The search strategies can be viewed in Appendix 1.
OVID Medline
Medline In-Process and Other Non-Indexed Citations
Embase
Cochrane CENTRAL
Cochrane DSR
INAHTA
The bibliographies of relevant papers were searched to identify studies that may have been missed through the database search. Relevant Web sites were also identified and searched.
Outcomes of Interest
Change in CCS anginal class
Adverse effects
Change in exercise duration
Hospitalizations
Quality of life
Change in drug usage
Economics analysis data
Strength of Recommendation
The GRADE approach was used to systematically and explicitly make judgments about the quality of evidence and strength of recommendations. (11) GRADE provides a framework for structured reflection and can help to ensure that appropriate judgments are made. GRADE takes into account study design, study quality, consistence and directness in judging the quality of evidence for each outcome. The balance between benefits and harms, quality of evidence, applicability and the certainty of the baseline risks are all considered in judgments about the strength of recommendations.
Results of Literature Search
The Cochrane and INAHTA databases yielded 3 HTAs or systematic reviews on EECP treatment. A search of Medline and Embase December 2005 – March 2006 (after the literature search cutoff from the most recent HTA) was conducted using key words enhanced external counterpulsation, EECP, angina, myocardial ischemia, congestive heart failure. This search produced 1 study which met the inclusion criteria. (35) The quality of the article is presented in Table 3. Only 1 observational study was identified so that the quality of evidence was inferior to the RCT assessed in the 2003 Medical Advisory Secretariat review.
Table 3: Quality of Evidence.
| Study Design | Level of Evidence | Number of Studies |
|---|---|---|
| Large randomized controlled trial, systematic reviews of RCTs | 1 | |
| Large randomized controlled trial unpublished but reported to an international scientific meeting | 1(g) | |
| Small randomized controlled trial | 2 | |
| Small randomized controlled trial unpublished but reported to an international scientific meeting | 2(g) | |
| Nonrandomized study with contemporaneous controls | 3a | |
| Nonrandomized study with historical controls | 3b | |
| Nonrandomized study presented at international conference | 3(g) | |
| Surveillance (database or register) | 4a | 1 |
| Case series (multi-site) | 4b | |
| Case series (single site) | 4c | |
| Retrospective review, modeling | 4d | |
| Case series presented at international conference | 4(g) |
g=grey literature
International Health Technology Assessments
An updated search for health technology assessments published late 2002 onwards yielded two English-language assessments: a January 2005 assessment published by ECRI (14), and a December 2005 assessment published by the Blue Cross/Blue Shield Technology Evaluation Centre (TEC). (12) Two French-language assessments, Agence D’Evaluation des Technologies et des Modes D’Intervention en Sante (AETMIS), 2004 (36) and Comite d’Evaluation et de Diffusion des Innovations Technologique (CEDIT), 2005 (37) have not been reviewed in this assessment.
Blue Cross Blue Shield Technology Evaluation Center (BCBS TEC) December 2005
BCBS reviewed the evidence through November 2005 to determine if EECP improves health outcomes for refractory chronic stable angina pectoris or chronic stable HF. (12)
Studies were selected for assessment if they:
Included patients with either documented stable angina or stable HF (NYHA class II/III).
Included at least 20 patients
Used a standard 35 hour treatment protocol
Reported clinical outcomes of treatment in terms of improved symptoms and/or quantitative physiologic measures of cardiovascular status.
Additionally an unpublished study of EECP in HF was included. The Prospective Evaluation of Enhanced External Counterpulsation in Congestive Heart failure (PEECH) study was presented at the 2005 American College of Cardiology Scientific Sessions. The PEECH study was also presented to the Center for Medicaid and Medicare Services (CMS) for consideration of coverage determination.
BCBS concluded that the available evidence is not sufficient to permit conclusions of the effect of EECP on health outcomes. Both controlled trials had methodologic flaws (MUST EECP and MUST EECP quality of life studies). The case series and observational studies for both indications while suggestive of a treatment benefit from EECP have shortcomings as well.
A commentary of the new studies that BCBS analyzed for their assessment is described below. A summary of the studies included in the BCBS review is found in Appendices 6 and 7.
Review of Evidence – Angina
The only RCT for review was the MUST-EECP study (previously reviewed by Medical Advisory Secretariat [see Appendix 4]). Other evidence included 1 small comparative trial (nonrandomized, nonblinded), several single centre uncontrolled small studies with minimal to no followup and several multicentre registry studies comparing various patient subgroups. Details of these studies are provided in Appendices 6 and 7.
Shechter et al. (38) reported a small nonrandomized, nonblinded study of 40 patients with CCS class III/IV angina. Twenty patients who received EECP were compared to 20 age and gender matched patients who refused EECP. Nitroglycerin use was significantly reduced and the treatment group had a significant average CCS class reduction from 3.5 (0.5) to 1.9 (0.3) compared to controls (3.3[0.6] to 3.5 [0.5]).
BCBS stated that a limitation to the study by Schecter et al. was a placebo effect since the control group consisted of patients who refused EECP and therefore could have had less enthusiasm for the efficacy of the procedure.
Further limitations to the study by Shechter et al. include:
Nonblinded
Nonrandomized
Lack of a sample size calculation
Small sample size.
BCBS reported that, overall, the single centre case series are (12):
Characterized by small sample sizes (range 8-60 patients) and a variety of outcome measures including symptom assessment and testing of physiologic parameters and imaging techniques.
Many of the small physiologic-based studies were conducted either as pilot studies or in efforts to understand the mechanism of action of EECP with little information about the clinical effectiveness of EECP.
Most studies provide minimal to no followup beyond the 7 week treatment period.
Approximately 15-25% of patients are not evaluable, either due to dropout or loss to followup.
Lack of comparison groups make it impossible to rule out either placebo effect or spontaneous recovery among patients with milder disease.
Five registry studies from the EECP Clinical Consortium (initiated in 1995) and the International EECP Patient Registry (IEPR, initiated in 1997) reported outcomes of patients treated in a number of different institutions. It is unclear how many institutions contribute patients to both registries since the enrollment times of patients in the studies overlap to a great extent; it is unknown how many patients have been reported multiple times among the different studies.
Several IEPR registry studies from 2001-2005 compare various subsets within the registry, including patients aged 80 years and older to less than 80 years (39) and those with and without HF (25), prior coronary revascularization procedures (40), self reported diabetes mellitus (41), and significant treated and untreated left main disease. (42) The sicker subgroup in each study (those aged 80 or more, with HF, with prior revascularization, with diabetes, with significant left main disease) had more events than the less sick group. However, since none of the studies includes a nontreated control group, the event rates for the patient subsets are not informative.
Limitations to the registry studies include:
No comparison group
Very few studies provide data in which it can be determined what proportion of patients initially starting EECP are actually analyzed.
Review of Evidence – Heart Failure
There were no published controlled trials of EECP compared to usual treatment in HF, although the results of the recently completed PEECH trial were presented at the 2005 American College of Cardiology Scientific Sessions. A single (n=26) multicentrre feasibility study and several registry studies make up the rest of the body of evidence for this indication. (12) Of note, the registry studies were established to track acute and long term outcome data for consecutive patients treated for chronic angina.
Multicentre Registry Studies
Three studies from the IEPR of patients with angina and concomitant HF showed the feasibility of using EECP for HF. (43-45) Angina and HF are often comorbid conditions. Even though HF was initially a relative contraindication to EECP treatment as of 2002, 36% of patients in the IEPR registry had concomitant angina and HF. With the exception of MACE, the IEPR registry outcomes are pertinent to angina rather than typical HF outcomes, so the results do not inform the efficacy of EECP for HF.
The studies also define HF differently (e.g., one with ejection fraction, one with an undefined history of clinical HF, and one that compared high and low ejection fraction among those with a history of clinical HF).
A fourth registry study from the Cardiomedics External Counterpulsation Patient registry (46), used HF outcomes to study a patented graduated pressure treatment regimen (35 hours). The 127 patients included had angina and concomitant HF (NYHA class III/IV).
The CardiAssist device was used with a gradually increasing pressure (values not stated) applied over the 7 week treatment period. The use of lower pressure was thought to prevent HF exacerbations and increase the comfort and tolerability of the procedure. Vijayaraghavan et al. noted that there were not withdrawals during therapy and o losses to followup. (46) Patients were divided into 3 groups based on their low/mid/high diastolic augmentation (DA) ratio. For angina patients the most effective DA ratio (also called effectiveness ratio) is thought to be 1.5-2.0 which may be too high for HF patients.
Because there was no indication of how patients ended up in their low/mid/high subgroup (whether pressures were applied a priori, whether this was their response to therapy, or whether this was their tolerance of therapy), the outcomes reported by BCBS were aggregated for the entire cohort. (12) Outcomes included 1 year mortality, hospitalizations (compared to 1 year prior to EECP), change in NYHA functional class at 1 year (group mean), and LVEF measured before EECP and at 1 ear.
Patients in the mid and high DA groups experienced greater 1 year mortality than the low DA group (8.8% versus 7.7% versus 1.9% for high, mid and low groups respectively), which may be explained by worse baseline NYHA functional class distributions in these groups. LVEF improved by 20.4% in the surviving patients from a baseline of 32.3% to 38.9%. The group mean NYHA functional class improved from an average of 2.8 to 1.9. The cohort underwent hospitalization for any cause at an average rate of 2.4 per person in the year prior to treatment to 0.5 per person in the year following EECP.
All patients received medical therapy “consistent with conventional clinical practice”, however there is no documentation of other therapeutic changes that may have occurred to contribute to the observed outcomes over the course of 1 year. (12)
Limitations to the study by Vijavaraghavan et al. include:
Low level of evidence; registry study.
Sample size calculation. What was the primary question that the study was designed to address?
NYHA class is categorical. It is unclear why the average NYHA class was reported as 2.8 and 1.9.
It is unclear how patients were allotted to low/medium/high. Any subgroup analysis would be at best hypothesis generating.
To date, the CardiAssist device is not licensed for use by Health Canada.
The design and methods of the PEECH trial are published and the results were presented to the American college of Cardiology Meeting in 2005 and were submitted to CMS for coverage consideration but are not yet published in a peer reviewed journal. (47)
PEECH was a randomized multicenter study of EECP compared to usual care in 187 optimal medically managed patients with NYHA functional class II/III HF with EF<35% of ischemic or idiopathic etiology. EECP was administered as 35 one hour sessions over the course of 7 weeks. Patients were followed for 6 months. Included patients were able to exercise for at least 3 min, were clinically stable, had minimal to no edema, and were on beta blockers and angiotensin converting enzyme inhibitors/angiotensin receptor blockers.
Similar to MUST-EECP, a large set of exclusion criteria were applied including:
acute coronary syndrome in the 6 weeks prior,
nonbypassed left main coronary with >50% stenosis,
coronary artery bypass graft surgery in the past 3 months,
percutaneous coronary intervention the past 6 months,
cardiac catheterization in the past 2 weeks,
arrhythmias that interfere with machine triggering,
chronic obstructive pulmonary disease with 1 second forced expiratory volume of <1.5L,
clinically significant valvular heart disease,
acute myocarditis,
implantable cardiac defibrillator (if it triggered n the past 3 months),
uncontrolled hypertension,
pregnancy,
women of childbearing potential,
participation in other clinical studies in the past 30 days,
history of any of the following: deep vein thrombosis, phlebitis, stasis ulcer, pulmonary embolism or aortic aneurysm.
The co-primary endpoints were the percentage of patients with at least a 60 second increase in exercise duration from baseline to 6 months or the percentage of patients with at least a 1.25ml/min/kg increase in Peak VO2 from baseline to 6 months. Secondary outcomes of interest were quality of life measures, change in NYHA functional class, change in peak VO2 and adverse effects. Outcomes were assessed at baseline, at the end of treatment and 6 months after the end of treatment.
Patients were not blinded to treatment allocation. Blinded investigators performed patient evaluations nonblinded investigators supervised or performed patient treatment visits and daily interactions.
According to the PEECH investigators, there were no differences between the patients at baseline in terms of ischemic etiology, NYHA class, mean EF and optimal medical therapy.
Using intent to treat analysis, 35% of the EECP therapy and 25% of the control group increased exercise time by at least 60 seconds (p=0.016) at 6 months. There was no significant difference observed in the proportion of patients achieving an increase in peak VO2>1.25 ml/kg at 6 months. There was a higher proportion of patients in the control group (24%) who achieved an increase in VO2 compared to the active treatment group (23%).
For the secondary endpoints:
Exercise time increased by 24.7 seconds in the active treatment group and decreased by 9.9 seconds in the control group (p=0.013) at 6 months.
Functional class (p<0.01) significantly improved in the active treatment group compared to the control group at 6 months.
There was no significant difference between the groups in terms of quality of life at 6 months.
There was no significant difference between the groups in terms of change in peak VO2.
For adverse events:
11 patients (11.8%) dropped out of the treatment arm due to adverse events compared to 3 patients (3.2%) in the control group (no p value reported). According to the PEECH investigators, there were no statistical differences between groups for adverse events or serious adverse events.
During the treatment period, 7 patients in the EECP group had serious adverse events including 1 with worsening HF and 1 with pulmonary embolism. In the control group, 8 patients were reported to have serious adverse events.
Limitations to the PEECH trial include:
Study not published in the peer reviewed literature.
Patients were not blinded.
Large patient dropout: 23.7% in the EECP group and 13.8% in the control group.
Why did EECP patients (compared to control patients) have a significantly increased exercise time by at least 60 seconds, but not have a significant increase in peak VO2>1.25 ml/kg at 6 months?
Placebo effect: a quarter of the control patients had in increase in exercise time by at least 60 seconds.
All patients had NYHA class II/III HF, yet overall only around 85% of patients were taking beta blockers and 76% of patients were taking ACE inhibitors.
Short term followup (6 months)
Large drop out rate
The clinical significance of a >1.25 ml/min per kg increase in VO2 is unclear.
Centers for Medicare and Medicaid Services (CMS) March 2006
On March 20 2006, CMS posted their proposed coverage decision memorandum for external counterpulsation therapy. (13) Overall, CMS stated that the evidence is not adequate to conclude that external counterpulsation therapy is reasonable and necessary for:
Canadian Cardiovascular Society Classification (CCSC) II angina
-
Heart failure
NYHA class II/III stable HF symptoms with an EF<35%
NYHA class II/III stable HF symptoms with an EF<40%
NYHA class IV HF
Acute HF
Cardiogenic shock
Acute MI
In their analysis, CMS noted that:
EECP has been reported to have effects similar to physical training. It is not clear how the effects of EECP are different from exercise in patients who can participate in physical conditioning. Or “in other words, will an exercise program of walking achieve similar results?” (13)
One manufacturer suggests that standard therapy pressures (e.g., up to 300 mm Hg) are appropriate treatment for congestive HF, while another manufacturer claims that higher pressures are harmful in HF, suggesting that graduated lower pressures are the appropriate therapy. A comparison trial would address this question.
To date, the CardiAssist device is not licensed for use by Health Canada.
CMS reiterated that the accumulated data do support the current use of EECP in patients who are not amenable to surgical intervention. In 1999, coverage for EECP was provided for patients who have been diagnosed with disabling angina (CCS class III/IV) and who are not readily amenable to surgical intervention. However, CMS did not provide a critical assessment of the evidence to support the 1999 coverage decision. (26)
ECRI
January 2005
In an assessment of EECP updated in January 2005, ECRI (14) stated that there was insufficient evidence available to draw conclusions about the long-term effectiveness of EECP, with respect to morbidity, survival, or quality of life, for any coronary indication (refractory angina, congestive heart failure, cardiogenic shock and acute MI). No published data were available on EECP for acute MI or cardiogenic shock, and studies with limited heart failure data did not meet the ECRI study selection criteria. Hence, ECRI concluded that EECP should only be utilized in patients that are unable to tolerate invasive procedures and have no other treatment options.
ECRI (14) noted that the one RCT which was identified by the literature search, Arora et al. (33) showed that EECP may provide no significant short-term benefit or improvement in angina episodes or nitroglycerin use over sham treatment. In addition, ECRI noted the following methodological issues with the MUST-EECP study:
Short-follow-up (one week after treatment)
Patient selection criteria (exclusion of Class IV angina patients, inclusion of Class I patients who may have been responsive to other forms of therapy
Differences in baseline characteristics in the controlled study, i.e., mean time since angina diagnosis
Vague details about high patient dropout rate
Incomplete data i.e., only 52% (71/139) of patients completed the quality of life questionnaire at baseline, end of treatment and at 1 year.
No cost-effectiveness analysis comparing this treatment to other available options.
Because there were no published controlled trials with ≥2 years of follow-up, the most current data from the multicenter IEPR registry was also considered in the ECRI evaluation. Michaels et al. (42) described two-year outcome data from 1,097 IEPR patients at trial sites with ≥ 85% follow-up retention.
ECRI (14) noted the following limitations of the IEPR registry data:
Lack of a control group
Potential selection bias: Voluntary data reporting only provides coverage for about 20% to 25% of patients receiving EECP, also the registry includes patients with varying patient characteristics, which makes it difficult to assess outcomes
Inconsistent application of technology making accurate interpretation of data difficult.
Potential bias from self-reported data based on mail or telephone interviews
Incomplete data: Only 1,097 patients from 24 sites contributing >85% of follow-up results provided two-year follow-up data, so statistics reported are based on incomplete data.
Registry reports also do not provide specific detail as to why patients discontinued treatment.
Studies Published Since the Blue Cross Blue Shield Technology Evaluation Center Report
One study was published in January 2006. This level 4a study was inferior in quality to the RCT which formed the basis of the 2003 Medical Advisory Secretariat recommendation. The study will nevertheless be assessed for the purposes of this update.
Soran et al. (35) reviewed the 2 year outcomes of patients who had severe LV dysfunction (EF ≤35%) that was treated with EECP for angina. The IEPR included 363 patients who had angina with LV dysfunction. Angina was severe (CCS III/IV) in 93% of patients. More than 50% reported quality of life as 4 or 5 (i.e., poor on the 5 point scale where 5 is worst).
Eighty-one percent of patients completed a course of EECP; 12% discontinued due to a clinical event and 7% stopped due to patient preference. Women and those who had a history of HF were less likely to complete the treatment course (75% of women versus 82% of men, p=0.15; therapy completed by 78% of those who had HF versus 85% of those who did not, p=0.08).
The authors stated “There was a significant difference in the rate of exacerbation of HF between those who did not complete treatment and had previous HF and those who had no HF (16% of those who stopped treatment versus 0%, p=0.05).” It is unclear why the authors compared exacerbation of HF in patients with HF versus patients with no HF. It is also unclear how the authors define HF.
Overall, 16.1% of patients with LV dysfunction had an adverse event during EECP treatment. The adverse effects were:
| Death | 0.8% |
| MI | 0.3% |
| Coronary bypass | 0.3% |
| PCI | 0.8% |
| Death/MI/coronary bypass/PCI | 1.9% |
| Unstable angina pectoris | 4.1% |
| HF | 3.3% |
| Skin breakdown | 2.5% |
| Musculoskeletal | 2.2% |
The use of beta blockers, calcium channel blockers angiotensin converting enzyme inhibitors, angiotensin receptor blockers, antiplatelets and hypolipidemic medication was similar at baseline, immediately after EECP and at 2 years. The authors stated that 52% of patients discontinued nitroglycerine use. It is unknown if this was determined immediately after treatment or at 2 years.
All patients had angina and LV dysfunction, yet before EECP was started, only 71% were on beta blockers, 30% were on calcium channel blockers, 60% were on ACE inhibitors, 76% were on antiplatelets and 76% were on lipid lowering drugs.
After completion of treatment, there was a significant decrease in severity of angina (p<0.001). The authors state that “patients were interviewed by telephone or at a clinic visit and data concerning interim clinical events, hospitalizations and current symptomatology were recorded.” Patient self-reporting of angina severity creates uncertainty regarding the accuracy of the results.
The registry initially had 363 patients with LV≤35%. The authors reported that for the “post EECP” followup (either the 6 month or 1 year followup), there were angina data for 358 patients and for the 2 year followup, there were angina data for 265 patients. Ninety-eight out of 363 patients (27%) did not have 2 year followup data; it is unknown what happened to the 98 patients.
At 2 years, 83% survived and the event free survival rate as 70%. Forty-three percent of patients had cardiac hospitalizations, and 81% had no HF events.
Repeat EECP was performed in 20% of patients. Failure to complete the original treatment course was the only significant independent predictor of repeat EECP (hazard ratio 2.9, 95% confidence interval 1.7 to 4.9).
Limitations to the study by Soran et al. include:
Registry study; no control group.
The authors did not report LVEF after treatment with EECP.
The analysis was on a subgroup of patients enrolled in a registry who received treatment for an indication that the registry was not initially established to treat.
The IEPR is a registry that enrolls consecutive patients who underwent EECP “for chronic angina.” Later in the article the authors stated that the database was queried to select the cohort of patients who underwent EECP “for LV dysfunction.”
Ninety-eight out of 363 patients (27%) did not have 2 year followup data.
It is unclear why the authors are comparing exacerbation of HF in patients with HF versus patients with no HF.
The authors reported that 4.1% of patients developed unstable angina and 3.3% developed HF during EECP treatment.
Not all patients in the IEPR have severe (CCS III/IV) angina. Seven percent have mild/moderate angina.
It is unclear if all the patients were who received EECP for the treatment of angina and/or LV dysfunction were: 1) optimally treated with drugs; and 2) still refractory to drug treatment.
Limitations to Registry Studies in the EECP Literature
Registry studies are Level 4 evidence.
-
Registry studies are not controlled (outcomes of patients in a registry are not compared to patients who receive no treatment).
Registry studies do not control for extraneous factors that might otherwise account for observed differences. For example, in EECP registries patient outcomes are not controlled for other interventions likely to affect angina such as drug therapy and surgical interventions. Since many of the patients had not been optimally treated with standard anti angina therapy prior to EECP, some of the subsequent improvement could have been attributed to the introduction of other/newer effective interventions following EECP treatment.
Since there is no comparison group, it is difficult to determine whether improvement is in fact specifically due to the treatment of interest.
Some of EECP registry studies poorly accounted for patient dropouts. One trial reported safety and tolerability findings for 2,991 patients, but reported outcomes for only 2,289 patients.
Reporting of consistent and restrictive selection criteria for patients in the EECP registry studies is lacking. For example, one registry study reported that over 30% of patients were suitable for conventional revascularization but chose EECP to avoid or postpone surgery. (48) Another registry study reported that EECP was chosen as treatment for diverse reasons; one reason being patient or physician preference. (49) A case series study reported that patients underwent additional treatment with EECP for reasons such as “tune ups” (“with no objective or subjective evidence of worsening of the patient’s condition”). (50)
-
There are 2 EECP registries:
The EECP Clinical Consortium was organized in September 1995 “to evaluate across a broad range of providers and patients the practice, effectiveness and safety of EECP by consecutively tracking the results and side effects of EECP therapy at participating centers. Participation includes over 100 centers, treating patients with varied demographics, operating in diverse practice settings (hospital based, physician’s office or rehabilitation facility) and with substantial differences in treatment experience with EECP”. (49)
The International EECP Patient Registry (IEPR) housed at the University of Pittsburgh Graduate School of Public Health was initiated in January 1998 to determine the patterns of use, safety and efficacy of EECP. “The IEPR sequentially tracks across a broad spectrum of participating providers (over 102 participating centres) the demographics, entry characteristics and outcomes of all angina patients treated with EECP”. (25)
There is uncertainty as to how many institutions may have contributed patients to both registries, as well as how many patients may have been reported multiple times among different studies.
GRADE Quality of the Evidence
According to the GRADE Working Group criteria, the quality of the trials was examined (Table 4). (11)
Table 4: GRADE Quality of Studies.
| Outcome | Design | Quality | Consistency | Directness | Overall Quality |
|---|---|---|---|---|---|
| Angina | 1 RCT |
Poor Unclear dropouts Subanalyses Patients/providers not blinded Greater adverse effects in EECP group. |
Exercise duration not significantly different between arms. Authors’ results and conclusions not match. | Not all patients have severe, refractory stable
angina. Large exclusion criteria. |
Very Low
|
| Case series Registry studies |
Uncontrolled. | Concomitant therapy in patients. Dropouts not accounted for. |
Rationale for treatment unclear in some studies – e.g., patient “tune ups”. | ||
| Heart Failure | 1 unpublished RCT (PEECH) |
Poor Unclear dropouts Subanalyses Patients/providers not blinded |
Conflicting co-primary endpoint
results. Exacerbation of HF in some patients. |
Large exclusion criteria. | Very Low
|
| Case series Registry studies |
Uncontrolled. | Concomitant therapy in patients. Dropouts not accounted for. |
Registry studies have patients enrolled for angina, not HF. |
Quality refers to the criteria such as the adequacy of allocation concealment, blinding and followup.
Consistency refers to the similarity of estimates of effect across studies. If there is important unexplained inconsistency in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the size of the differences in effect and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the people interventions and outcome measures are similar to those of interest. For example, there may be uncertainty about the directness of the evidence if the people of interest are older, sicker or have more comorbidity than those in the studies.
As stated by the GRADE Working Group, the following definitions were used in grading the quality of the evidence. (11)
| High | Further research is very unlikely to change our confidence n the estimate of effect. |
| Moderate | Frther research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Economic Analysis
Literature Review
No economic analysis of EECP was identified in the published literature.
Ontario-Based Budget Impact Analysis
Notes and Disclaimer
The Medical Advisory Secretariat uses a standardized costing methodology for all of its economic analyses. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital costs: Ontario Case Costing Initiative (OCCI) cost data is used for all program costs when there are 10 or more hospital separations, or one-third or more of hospital separations in the Ontario Ministry of Health and Long-Term Care’s data warehouse are for the designated International Classification of Diseases-10 diagnosis codes and Canadian Classification of Health Interventions procedure codes. Where appropriate, costs are adjusted for hospital-specific or peer-specific effects. In cases where the technology under review falls outside the hospitals that report to the OCCI, PAC-10 weights converted into monetary units are used. Adjustments may need to be made to ensure the relevant case mix group is reflective of the diagnosis and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the Medical Advisory Secretariat normally defaults to considering direct treatment costs only. Historical costs have been adjusted upward by 3% per annum, representing a 5% inflation rate assumption less a 2% implicit expectation of efficiency gains by hospitals.
Non-hospital costs: These include physician services costs obtained from the Provider Services Branch of the ministry, device costs from the perspective of local health care institutions, and drug costs from the Ontario Drug Benefit formulary list price.
Discounting: For all cost-effective analyses, discount rates of 5% and 3% are used as per the Canadian Coordinating Office for Health Technology Assessment and the Washington Panel of Cost-Effectiveness, respectively.
Downstream cost savings: All cost avoidance and cost savings are based on assumptions of utilization, care patterns, funding, and other factors. These may or may not be realized by the system or individual institutions.
In cases where a deviation from this standard is used, an explanation has been given as to the reasons, the assumptions, and the revised approach.
The economic analysis represents an estimate only, based on assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied for the purpose of developing implementation plans for the technology.
Estimated Prevalence of Angina in Ontario
3,800 patients with chronic refractory angina:
The number of patients with chronic refractory angina in the US is estimated to be approximately 100,000 (4), this corresponds to about 3,800 patients in Ontario (3.8% × 100,000) with refractory angina.
3,800 patients × $7,000 Cdn (approximate cost for a full course of therapy) ~ $26.6M Cdn.
Estimated Prevalence of Heart Failure in Ontario
23,700 patients EF ≤ 0.35:
This estimate is from an expert in the field (personal communication) at the Institute for Clinical Evaluative Sciences (ICES), who examined a sample of echocardiography studies drawn from a diagnostic lab in 2001. They found that the prevalence of EF ≤ 0.35 was 8.3%, and if generalized to all patients undergoing echocardiography, there would be 23,700 patients in Ontario.
23,700 patients with EF ≤35% × $7,000 Cdn ~ $166 M Cdn.
Existing Guidelines Regarding Use of EECP
2005 American College of Cardiology/American Heart Association (ACC/AHA) Taskforce on Practice Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult
The Taskforce noted that the results of early trials of EECP for heart failure have been encouraging; however, until more data are available, routine use of EECP is not recommended for the management of patients with symptomatic reduced LVEF. (51)
2003 American College of Cardiology/American Heart Association (ACC/AHA) Guideline Update for the Management of Patients with Chronic Stable Angina
The guideline update stated that additional clinical trial data are necessary before EECP can be recommended definitively. (15) The ACC/AHA uses the classifications listed in Table 5.
Table 5: American College of Cardiology/American Heart Association (ACC/AHA) Classification of Evidence.
| Class I | Conditions for which there is evidence or general agreement that a given procedure or treatment is useful and effective. |
| Class II | Conditions for which there is conflicting evidence or a divergence of opinion about the usefulness/efficacy of a procedure or treatment. |
| Class IIa | Weight of evidence/opinion is in favour of usefulness/efficacy. |
| Class IIb | Usefulness/efficacy is less well established by evidence/opinion. |
| Class III | Conditions for which there is evidence and/or general agreement that the procedure/treatment is not useful/effective and in some cases may be harmful. |
| Level of Evidence A | Data derived from multiple randomized clinical trials. |
| Level of Evidence B | Data derived from a single randomized trial or nonrandomized studies. |
| Level of Evidence C | Consensus opinion of experts. |
According to ACC/AHA, the use of EECP treatment for chronic stable angina is considered to be Class IIb (Level of Evidence: B). Therefore, the usefulness/efficacy of EECP, as derived from a single randomized trial or nonrandomized studies, is less well established by evidence/opinion.
2004 American College of Physicians
Clinical guidelines on the management of chronic stable angina and suspected or known coronary artery disease concluded that evidence in support of EECP is lacking, and hence they do not support its use except in patients who cannot be managed adequately by medical therapy, and are not eligible for interventional or surgical revascularization. (52)
2006 Centers for Medicare and Medicaid Services (CMS)
Effective July 1999, CMS coverage of EECP therapy was provided for patients who have been diagnosed with disabling angina (Class III or Class IV), and who in the opinion of a cardiologist or cardiothoracic surgeon, are not readily amenable to surgical intervention. (26)
In June 2005, CMS accepted a request from Vasomedical Inc. to reconsider expanding coverage to include treatment of patients with Class II angina and for use in patients with NYHA Class II/III stable heart failure symptoms with an ejection fraction ≤ 35%. (26)
On March 20 2006, CMS posted their proposed coverage decision memorandum for external counterpulsation therapy. (13) Overall, CMS stated that the evidence is not adequate to conclude that external counterpulsation therapy is reasonable and necessary for:
Canadian Cardiovascular Society Classification (CCSC) II angina
-
Heart failure
NYHA class II/III stable HF symptoms with an EF≤35%
NYHA class II/III stable HF symptoms with an EF≤40%
NYHA class IV HF
Acute HF
Cardiogenic shock
Acute MI
Aetna
In a clinical policy bulletin updated in June 2005,(53) Aetna considers EECP medically necessary when both of the following criteria are met:
Disabling angina (New York Heart Association Class III or Class IV angina).
Disabling angina Class III or Class IV angina which is refractory to maximum medical therapy and not readily amenable to surgical intervention such as percutaneous transluminal coronary angioplasty (PTCA) or cardiac bypass.
The use of EECP for all other indications (e.g., heart failure) is considered experimental and investigational, and therefore is not covered by Aetna. Further, hydraulic versions of EECP devices are not considered medically necessary.
Blue Cross/Blue Shield of North Carolina
Corporate Medical Policy published by Blue Cross/Blue Shield and last updated in June 2004 does not provide coverage of EECP for any indication, as it is considered investigational. (54)
CIGNA
A CIGNA HealthCare Coverage Position (55) dated May 2005 provides EECP coverage when both of the following conditions are met:
Individuals with symptoms of severe chronic stable angina (NYHA Functional Class III or IV or equivalent).
Individuals who are not considered candidates for PTCA or revascularization.
All other indications are considered experimental and investigational, and are not covered by CIGNA (i.e., unstable angina, CHF, etc.)
Regence Group
Regence Group Medical Policy (56) updated October 2004 provides coverage for EECP treatment when all of the following criteria are met:
The patient has disabling Class III or Class IV (CCSC classification) or equivalent
The patient is refractive to maximum medical therapy
The patient is not readily amenable to surgical intervention such as PTCA or cardiac bypass
All other indications are considered investigational.
Conclusion
There is insufficient evidence to support the effectiveness and safety of EECP treatment for patients with refractory stable CCS III-IV angina or HF.
As per the GRADE Working Group, overall recommendations consider 4 main factors. (11)
The tradeoffs, taking into account the estimated size of the effect for the main outcome, the confidence limits around those estimates and the relative value placed on the outcome.
The quality of the evidence.
Translation of the evidence into practice in a specific setting, taking into consideration important factors that could be expected to modify the size of the expected effects such as proximity to a hospital or availability of necessary expertise.
Uncertainty about the baseline risk for the population of interest.
The GRADE Working Group also recommends that incremental costs of healthcare alternatives should be considered explicitly alongside the expected health benefits and harms. (11) Recommendations rely on judgments about the value of the incremental health benefits in relation to the incremental costs. The last column in Table 6 is the overall trade-off between benefits and harms and incorporates any risk/uncertainty.
Table 6: Overall GRADE and Strength of Recommendation (Including Uncertainty).
| Quality | Estimated Prevalence in Ontario |
Cost- Effectiveness |
Cost in Ontario | Overall Grade and Strength of
Recommendation (Including Uncertainty) |
|
|---|---|---|---|---|---|
| Severe (CCS III/IV) refractory angina | Low | ~ 3,800 | ? Unknown | ~ $26.6M Cdn | Weak |
| Heart failure | Low | ~ 23,700 | ? Unknown | ~ $166 M Cdn | Weak |
For angina and heart failure, the overall GRADE and strength of the recommendations is “weak” – the quality of the evidence is “low” (uncertainties due to methodological limitations in the study design in terms of study quality and directness), and the corresponding risk/uncertainty is increased due to a budget impact of approximately $26.6 M Cdn or $166 M Cdn respectively while the cost-effectiveness of EECP is unknown and difficult to estimate considering that there are no high quality studies of effectiveness.
Appendices
Appendix 1 – Enhanced External Counterpulsation Search Strategy
Search date: September 28, 2005
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, Embase, Cochrane DSR and CENTRAL, and INAHTA/CRD
Database: Ovid MEDLINE(R) <1966 to September Week 3 2005>
Search Strategy:
--------------------------------------------------------------------------------
exp COUNTERPULSATION/ (2648)
external.mp. (129587)
1 and 2 (168)
(external counterpulsation or eecp).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (171)
3 or 4 (216)
limit 5 to (humans and english language and yr=“2002 - 2005”) (71)
from 6 keep 1-10 (10)
from 6 keep 1-71 (71)
Database: EMBASE <1996 to 2005 Week 40>
Search Strategy:
--------------------------------------------------------------------------------
exp COUNTERPULSATION/ (406)
external.mp. (50382)
1 and 2 (107)
(external counterpulsation or eecp).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (122)
3 or 4 (127)
limit 5 to (humans and english language and yr=“2002 - 2005”) (67)
Appendix 2: Example of Patient Undergoing Enhanced External Counterpulsation Treatment.
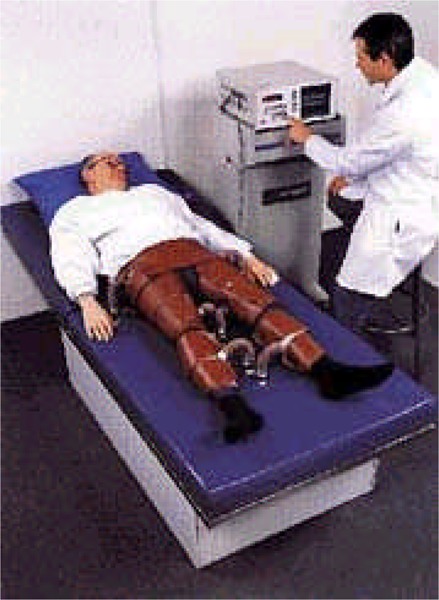
In addition to the 3 sets of cuffs, the patient has finger plethysmogram and electrocardiogram (ECG) attachments which are connected to the control and display console.
From: http://cardiology.ucsf.edu/clinical/eecp/ (accessed January 2003)
Appendix 3: Mechanism of Diastolic Augmentation and Systolic Afterload Reduction Using Enhanced External Counterpulsation
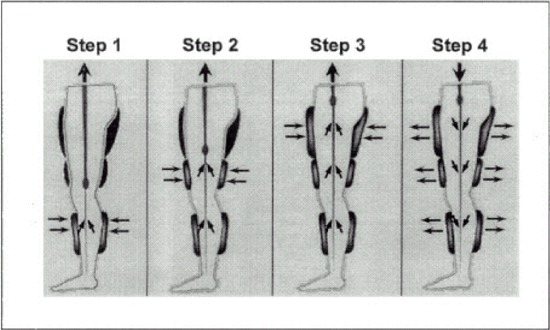
Note the electrocardiographically-gated sequential diastolic activation to “milk” lower extremity venous and arterial blood from the periphery to the central vasculature [steps 1-3]. All cuffs deflate simultaneously immediately prior to systole [step 4].
Reprinted with permission from Mayo Clinic Proceedings; From Singh M, Holmes DR, Tajik AJ, Barsness GW. Noninvasive revascularization by enhanced external counterpulsation: a case study and literature review. Mayo Clinic Proceedings. 2000;75(9):961-96.
Appendix 4: Detailed Analysis of the Studies Included in the 2003 Medical Advisory Secretariat Review of EECP: Angina
Multicenter Study of Enhanced External Counterpulsation (MUST-EECP)
• Arora et al. (33) conducted a multicentre, prospective, randomized, single-blinded, sham-controlled trial to assess the safety and efficacy of EECP in 139 outpatients with angina and documented CAD. Treatment effect was determined by comparing changes in exercise treadmill test (ETT) parameters (exercise duration, ≥1 mm ST segment depression), symptoms (frequency of anginal episodes), and nitroglycerin (NTG) usage between groups. Symptoms and NTG usage were assessed using the patients’ recollection. Once randomized, patients underwent 35 hours of either active counterpulsation (CP) or inactive CP. Treatment sessions, each lasting 1 hour could be given once or twice per day. Within one week after completion of 35 treatment sessions, a post-treatment ETT was performed.
-
Inclusion criteria consisted of:
21-81 years old
symptoms consistent with CCS angina class I, II or III
documented evidence of CAD
ETT positive for ischemia
-
Exclusion criteria consisted of:
Myocardial infarction (MI) or CABG in the preceding 3 months
Cardiac catheterization in the preceding 2 weeks
Unstable angina
Overt CHF
Left ventricular ejection fraction <30%
Significant valvular heart disease
Blood pressure >180/100 mm Hg
Permanent pacemaker or implantable defibrillator
Nonbypassed left main stenosis >50%
Severe symptomatic peripheral vascular disease
History of varicosities
Deep vein thrombosis
Phlebitis or stasis ulcer
Bleeding diathesis
Warfarin use with International Normalized Ratio >2.0
Atrial fibrillation or frequent ventricular premature beats that would interfere with EECP
triggering or baseline ECG abnormalities that would interfere with interpretation of exercise ECG
Pregnant women
Women of childbearing potential
Subjects unable to undergo treadmill testing
Subjects enrolled in a cardiac rehabilitation program/other research program
All medications (except on demand NTG) remained unchanged from baseline to end of treatment. Patients were questioned daily by research nurses about any adverse reaction experienced since the previous session. There was 80% power to detect a 45 second difference in exercise duration between the 2 study groups (using a 2 sided test, and 0.05 level of significance).
The pressures that can be applied to the cuffs range from 0 to 350 mm Hg. The pressure applied to the cuffs was 300 mm Hg in the active-CP group and 75 mm Hg in the inactive-CP group. This was considered sufficient to preserve the appearance and feel of an EECP application but insufficient to alter measurably the patient’s blood pressure. The means of patients’ diastolic to systolic pressure and area under the curve ratios achieved were 1.41±0.51 (mean±SD) and 1.59±0.6 respectively in active-CP. Changes in these parameters were reported to be undetectable in the inactive-CP group
One hundred and thirty-nine patients were randomized. In both the inactive counterpulsation (CP) group (n=67) and the active-CP group (n=72), 1 patient withdrew prior to first treatment. Exercise duration data were available for 57 patients in the active-CP group. Fourteen subjects in the active-CP could not be evaluated for exercise duration due to:
4 protocol violations
7 adverse experiences
3 dropped out for personal reasons
Exercise duration data were available for 58 patients in the inactive-CP group. Eight subjects in the inactive-CP could not be evaluated for exercise duration due to:
7 protocol violations
1 adverse experience
There was no significant difference between groups in change in exercise duration from baseline to post-treatment, p>0.3. Data for the time to ≥1 mm ST segment depression were available for 56 patients in each treatment group. Digoxin use invalidated analysis in 1 patient from the active-CP group and 2 in the inactive-CP group. There was a significant difference between groups in the change in time to exercise induced ischemia (time to ≥1 mm ST segment depression), p=0.01.
Using intent-to-treat (ITT) analysis there was no significant difference between groups in the change in angina counts (p<0.09).
NTG usage was not significantly different between groups when analyzed using ITT (p>0.1).
Both treatment groups reported a high incidence of adverse events at each treatment session (Table 7). More patients in the active-CP group reported adverse events than in the inactive-CP group: 39 (55%) vs. 17(26%), p<0.001). Thirty-seven of the 70 events reported by the 39 patients in the active-CP group were considered device-related. Ten of the 25 events reported by the 17 patients in the inactive-CP group were considered device-related.
Table 7: Adverse Events in MUST-EECP.(33).
| Adverse Events | Inactive-CP (n=66) |
Active-CP (n=71) |
P value |
|---|---|---|---|
| Number of patients with adverse events | 17(25.8%) | 39(54.9%) | <0.001 |
| Non-device related | |||
| Viral syndrome | 0 | 1 | >0.5 |
| Anxiety | 0 | 2 | =0.5 |
| Dizziness | 1 | 3 | >0.5 |
| Tinnitus | 0 | 1 | >0.5 |
| GI disturbance | 1 | 1 | >0.5 |
| Headache | 0 | 1 | >0.5 |
| Blood pressure change | 1 | 1 | >0.5 |
| Epistaxis | 0 | 2 | =0.5 |
| Angina | 1 | 1 | >0.5 |
| “Other” chest pain | 3 | 9 | =0.3 |
| A/V arrhythmia | 3 | 9 | >0.2 |
| Heart rate change (sinusal) | 3 | 0 | =0.1 |
| Respiratory | 2 | 4 | >0.5 |
| Total non-device related | 15 | 33 | <0.005 |
| Device related | |||
| Paresthesia | 1 | 2 | >0.5 |
| Edema, swelling | 0 | 2 | =0.5 |
| Skin abrasion, bruise, blister | 2 | 13 | =0.005 |
| Pain (legs, back) | 7 | 20 | =0.01 |
| Total device related | 10 | 37 | <0.001 |
Reprinted from the Journal of the American College of Cardiology, v. 33, Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, Nesto RW. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise induced myocardial ischemia and anginal episodes, 1833-1840, Copyright 1999, with permission from the American College of Cardiology Foundation.
Five patients withdrew from the study due to leg complaints (pain, abrasion).
The study by Arora et al. (33) had several methodological limitations:
No significant difference was noted between the treatment and control group for exercise duration, angina count and nitroglycerin usage (using an intent to treat analysis in the latter two endpoints). The study was specifically designed to have 80% power to detect a 45 second difference in “exercise duration” between the two study groups. Therefore, the specific endpoint (exercise duration) that the RCT was statistically designed to examine did not reveal a statistically significant difference between EECP treated and control patients.
Arora et al. (33) stated that “the primary efficacy analyses for exercise treadmill test parameters were performed on an observed case basis using the intent to treat population”. However, an intent to treat analysis was not calculated for “exercise duration” or an electrophysiologic endpoint (≥1 mm ST segment depression). Intent to treat analysis addresses a situation when treatment is not received in full or part by a patient who is randomized to receive treatment. In such a circumstance, there is concern that those patients who do not receive the allocated treatment may differ in some way from those who do receive it.
Subanalyses that the trial was not designed to conduct (angina count and nitroglycerin usage) were calculated using: 1) an intent to treat population and 2) only patients who completed ≥34 sessions. Arora et al. did not specify why two different analyses were used to assess these endpoints.
Angina counts and nitroglycerin usage were further sub-analyzed according to percentage change in angina counts and drug usage. The difference between the two treatment groups with respect to the percent change in each parameter was tested using a chi square test. However, such a test is not robust given the large number of categories with low observed and expected values (<5 per category).
More patients in the active EECP group reported adverse events than in the inactive EECP group: 39 (55%) vs. 17(26%), p<0.001).
Patients with the most severe angina (Canadian Cardiovascular Society Class IV) were excluded from the trial. Only patients with Class I-III were enrolled into the study.
There was no significant difference between the treatment groups in terms of cardiovascular medications at baseline. However, of patients in the inactive EECP group, only 82% were taking nitrates, 91% ASA, 55% calcium channel blockers, 77% beta blockers, and 50% lipid lowering drugs. At baseline, of patients randomized to active EECP, 79% were taking nitrates, 87% ASA, 62% calcium channel blockers, 70% beta blockers, and 62% lipid lowering drugs. Not all patients were receiving exhaustive pharmacotherapy.
Not all the CCS I-III angina patients included in the trial had chronic ischemic symptoms that were unresponsive to exhaustive conventional medical therapy and revascularization techniques, or were not amenable to a revascularization procedure.
Unclear detail of patient dropouts. For example, there were numerical inconsistencies between the text, figure and tables.
Large exclusion criteria decreasing generalizability of results. Many patients were excluded from this study before randomization (approximately 500 patients were screened).
Patient self-reporting of angina counts and drug usage, hence there is uncertainty regarding the accuracy of the results.
Despite all of the above methodological limitations, there was one statistically significant improvement in an electrophysiologic endpoint (≥1 mm ST-segment depression) for EECP treated patients compared to controls. The clinical significance of this one endpoint (that was not calculated using an intent to treat analysis) in relation to the lack of significance to other endpoints including exercise duration, angina counts and nitroglycerin usage requires clarification.
The ability of Arora et al. to enforce blinding is difficult since personnel applying the EECP treatment could have inadvertently suggested the form of treatment, and patients may have independently guessed their correct study group.
Arora et al. only examined the immediate effect of EECP treatment, within one week of completion of treatment for patients who had CCS I-III angina. Arora et al. stated that “MUST-EECP examines only the immediate effect of treatment. Its long-term effects on symptoms and clinical events are not known”. (33)
The safety and long-term effects of EECP treatment for angina have not been assessed using a RCT design.
Multicenter Study of Enhanced External Counterpulsation (MUST-EECP) Quality of Life
• In an extension of the MUST-EECP trial, Arora et al. (34) examined the effects of EECP compared to the sham controlled group in terms of patients’ functioning, their senses of well-being and other Health Related Quality of Life (HQOL) parameters from baseline to the end of treatment, and from baseline to 12 months after treatment. Arora et al. (34) stated that based on the relevance to angina pectoris and the characteristics of the study population, a subset of four of the nine HQOL scales were pre specified to serve as the primary parameters. The HQOL parameters assessed were: physical functioning (PF), bodily pain (BP), social functioning (SF), and cardiac-specific health and functioning (CSHF). Patients who had complete data sets for each of the four scales at each of the three time points comprised the study cohort. (34)
Arora et al. (34) selected PF, BP, and SF from the Medical Outcomes Study 36 Item Short Form Health Survey (SF-36). The SF-36 is a measure of general health and comprises 36 items that yield 8 multi item scales that measure: physical functioning, work role disability due to physical health problems, bodily pain, general health perceptions, vitality, social functioning, work role disability due to emotional problems, mental health and a single item evaluation of change in health. (34)
Cardiac specific health functioning was measured on the cardiac version of the Quality of Life Index (QLI) as a condition-specific measure. (34) The QLI focuses on a respondent’s satisfaction with the areas of life important to him/her. (34) The questionnaire is self-administered in two parts: The first part measures satisfaction with various aspects of life as they are impacted by the respondent’s cardiac health. The second part measures the importance of these same aspects of life to the respondent personally. Scores are calculated by weighting the satisfaction responses according to the importance of those responses, so that they reflect the level of satisfaction with those aspects of life that matter most to the individual. (34)
Within group and between group statistical tests were conducted.
One hundred and thirty-nine patients were enrolled in MUST-EECP, but 2 patients dropped out prior to receiving treatment. A total of 137 patients received at least one EECP treatment. In 5 cases, the unique patient identifiers that would allow their data to be used in the HQOL substudy were illegible, therefore 132 of the original 137 could have been analyzed for the substudy. Seventy-one patients (54%) from the original MUST-EECP trial provided completed questionnaires at baseline, end of treatment and 12 months post-treatment. Patients completed questionnaires independently, and study coordinators did not discuss the responses with the patients.
At baseline, patients in the active-CP group (n=36) had a longer history of angina (p=0.002) and previous MI (p<0.05) than the inactive-CP group (n=35). Arora et al. (34) state that the HQOL scores at baseline were similar between the two groups, however, no further data or p value was reported.
Baseline to End of Treatment
From baseline to end of EECP treatment (EOT), both active-CP and inactive-CP reported significant improvements in physical functioning, bodily pain, and cardiac-specific health and functioning, p<0.05 (within group analyses).
The only statistically significant difference between the active-CP and inactive-CP groups was for social functioning, p<0.05 (between group analysis).
Baseline to One Year after End of Treatment
-
At 1-year follow-up, the active-CP group maintained significant improvements across the 4 primary HQOL parameters (p<0.05), however, the inactive-CP group only maintained significant improvement in the physical functioning parameter, p<0.05 (within group analyses).
There was a statistically significant difference between the active-CP and inactive-CP groups for BP, SF, and CSHF, p<0.05 (between group analysis).
Methodological issues related to this substudy (34) include:
Of patients allocated to inactive EECP (n=35), 31% were CCS III angina. However, only 6% of patients allocated to active EECP (n=36) were CCS III angina.
The original MUST-EECP study revealed that there was no statistically significant difference between active and inactive CP treated patients for: 1) exercise duration, 2) angina counts, and 3) nitroglycerin usage. (33) As well, the active CP treated patients experienced significantly more adverse effects. (33) It is therefore unclear in the study by Arora et al. (34) why the actively CP treated patients experienced significant improvement in “social functioning” at the end of treatment. Furthermore, it is unclear in the study by Arora et al. (34) why the actively treated CP group (n=35) demonstrated significantly greater improvement than the inactive CP group (n=36) at 12 months in BP, SF and CSHF. This may be accounted for by the fact that there were more CCS III angina patients in the control group (31%) than the active treatment group (6%). Specific details of the nature of “bodily pain”, “social functioning”, and “cardiac specific health and functioning” and their improvement in the subset of EECP treated and control patients are required.
Explanation is required as to why the sham treated group reported significant (p<0.05) improvements between baseline and end of treatment for “physical functioning”, “bodily pain”, and “cardiac specific health and functioning”. The sham treated group also reported significant (p<0.05), maintained improvement from baseline to 12 months post treatment for “physical functioning”.
Baseline data for HQOL scores between active and inactive-CP were not reported.
Patient interpretation and scoring of the quality of life parameters may have varied. For example, a patient with arthritis or depression may have scored differently than a patient without similar comorbidities.
There may have been post-treatment behaviour differences between the 2 active-CP and inactive-CP groups.
During the 1 year post-treatment period, the recording of any changes or events in the patients’ statuses was inconsistent and unable to be analyzed by the authors.
The sample size was determined by statistical requirements for the original MUST-EECP study, not the HQOL substudy.
The substudy also had a low patient response that was available for analysis (54% or 71 out of the original 139 patients enrolled in MUST-EECP).
Similar to the methodological limitations in the original RCT (33), there is decreased generalizability of the results due to a large initial exclusion criteria.
It is unknown whether the HQOL results correlate with the MUST-EECP exercise outcomes and symptoms at EOT.
Exercise treadmill test results, angina episodes and drug usage were not recorded at the 1-year follow-up.
Arora et al. (34) stated “studies in an appropriately sized study population are warranted and desirable” to confirm the findings.
Registry Studies - Angina
• Barsness et al. (48), using the International EECP Patient Registry (IEPR), evaluated the safety and acute effectiveness of EECP treatment in patients with angina. The report of Barsness et al., used results from the first 43 centres joining the registry at the end of the fist 18 months of operation. From January 1998-April 1999, 978 patients were entered into the registry for whom data at baseline and completion were available.
Approximately 69% of patients had CCS III/IV angina, with a mean of 8.6 episodes of angina per week. Over 80% of the patients had previous revascularization with either PCI or CABG and 7% had received a previous course of treatment with EECP before the registry had started. Other medical history included:
68% MI
28% CHF
32% Noncardiac vascular disease
The total number of patients who completed treatment was 825 (84.4%). Patients failed to complete treatment due to a medical event or due to patient choice.
Angina class was improved post EECP:
Decrease in at least one CCS class: 81.0%
Mean decrease in anginal episodes per week: 6.4±12.6
Decrease in use of nitroglycerin: 61.7%
Overall for the patient assessment of quality of life post treatment, there was an improvement in health status for 67.4% of patients, in quality of life for 63.3% and in satisfaction with life for 66.7% compared to pre EECP treatment.
The overall adverse event rate was 1.1% (11 patients) reporting withdrawal due to a serious cardiac event (death, MI, CABG, or PCI). These serious events occurred after a mean treatment time of 19 hours, and none occurred within 48 hours of a treatment session. Clinical events were the reason for discontinuing treatment in 43.8% of patients withdrawing, other withdrawals were reported as being due to the patient’s decision.
Fifteen percent of patients starting EECP therapy did not complete the prescribed course of treatment.
The major limitations of this study are:
Lack of a control group to assess the extent of reported improvement.
Lack of information as to why patients did not complete treatment.
EECP treatment was given to patients with all classes of angina.
Over 30% of patients were suitable for conventional revascularization but chose EECP to avoid or postpone surgery.
• In a subsequent study, Holubkov et al. (57) compared the baseline characteristics and 1 year outcome in 2 cohorts of PCI candidates presenting with stable angina. One group of 323 patients was from IEPR who were candidates for PCI but received EECP while another group of 448 patients from the National Heart, Lung and Blood Institute (NHLBI) Dynamic Registry of Coronary Interventions underwent elective PCI. Outcome measures included mortality from all causes and patients’ self-reported level of exertional angina.
There were more patients with CCS I and IV angina in the IEPR. Baseline angina was not equivalent in the 2 cohorts, p<0.001. At study entry, more IEPR patients had prior surgical intervention (CABG and PCI, p<0.001), and history of MI (p<0.001), CHF (p<0.01), and diabetes mellitus (p<0.001).
Initial Results
Among 323 patients treated with EECP, 85.8% completed a full course of treatment. Reasons for failure to complete EECP treatment included disruption of treatment due to medical event or voluntary discontinuation. Among PCI treated patients, 92.1% had a successful initial procedure.
One Year Results
At 1 year, survival and rates of CABG during follow-up was comparable in the 2 cohorts (p=NS). A higher proportion of NHLBI patients underwent PCI during follow-up (IEPR 6.3%, 95%CI 4.0%-9.9%; NHLBI 17.2%, 95% CI 14.0%-21.1%).
For patients alive and contacted at one year, IEPR patients reported using more calcium channel blockers (p<0.001), angiotensin receptor blockers (p<0.01) and long acting nitrates (p<0.001). However, NHLBI patients reported using significantly more short acting nitroglycerin for angina than IEPR patients, p<0.001.
Class III, IV or unstable symptoms were reported in 15.5% of EECP patients and in 9.5% of PCI registry patients, p=0.02. More PCI registry patients (73.4%) reported no angina after 1 year than IEPR patients (43.7%), p<0.001.
This study comparing patients from 2 registries had methodological problems. There were different baseline characteristics and data were not directly comparable. The classification of an IEPR patient as a PCI candidate was not performed by a PCI “operator” who would be qualified to make this determination based on detailed angiographic and clinical evidence. Lastly, angina was self-reported by patients via mail or telephone, thereby introducing variability in the definition and assessment of follow-up symptoms.
• Lawson et al. (49) used the EECP Clinical Consortium to evaluate the practice, effectiveness, and safety of EECP by consecutively tracking the results and side effects of EECP therapy at participating centres. A cohort of 2,289 consecutive patients from 84 centres had completed follow-up as of September 1999. All patients had angina on entry (CCS I-IV). EECP was chosen as treatment for reasons including:
Angina refractory to medical or surgical therapy
Patient or physician preference
Poor candidate for surgery due to lack of graft material or targets or operative risk
The entire patient registry (n=2,991) was reviewed for safety and tolerability and 91 adverse patient experiences were observed, though not necessarily directly related to EECP:
23 musculoskeletal and skin trauma (joint and muscle pain, leg swelling, bruising, abrasion)
8 MI
4 angina, chest pain or silent ischemia
1 ECG change
2 arrhythmia
1 pulmonary edema
8 deaths
unreported number of medical problems (GI bleeding and pneumonia) and undefined events
The majority of CCS II-IV patients either improved their anginal class (n=1,531 or 73.4%) or remained unchanged after EECP (n=554 or 26.5%). Overall, the average CCS anginal class before treatment (2.78) improved to an average CCS class of 1.81 post EECP (p<0.001). Anginal class improved 2 or more CCS classes in 48.9% of patients in pretreatment CCS IV and 34.9% of patients in CCS III. The average anginal class of patients treated in CCS II-IV decreased from 2.99 before treatment to 1.84 post-treatment (no p value provided).
Improvement after EECP treatment was analyzed using mulitvariate analysis using logistic regression. Patients in lower CCS classes who received fewer treatments were 20% less likely to improve in CCS class than those undergoing 35 hours of treatment (no further data provided). Compared to patients with CCS I and II, pre EECP CCS IV patients significantly improved [OR 3.30 (95% CI 2.49-4.39), p<0.0001]. Similarly, pre EECP CCS III patients improved compared to patients with CCS I and II, OR 4.38 (3.46-5.56), p<0.0001. Patients who received treatment for >35 hours did not demonstrate benefit over the standard 35 hour course, OR 1.02 (95% CI 0.77-1.36). On reason may be due to the fact that patients in lower CCS classes are less impaired and therefore have less room for improvement. For patients who received <35 hours of treatment, the OR for improvement was 0.08(0.08-0.10), p<0.0001. Lack of improvement in <35 hours of therapy could be due to patient dropout due to failure to improve and/or failure to improve due to dropout.
Response to EECP treatment was significantly related to age with younger patients showing a significantly greater likelihood of benefit (age 68-74 years: OR 1.19 (0.90-1.58), p=NS; 58-67 years: OR 1.31(0.99-1.75), p=0.06; <57 years: OR 1.64(1.21-2.22), p<0.001). The baseline CCS class was similar across all quartiles. Possible explanations include: more extensive disease in the elderly and less lower extremity muscle mass and calcified noncompliant arterial walls or ‘lead pipe effect’.
Since this is a registry study the inherent major limitation is a lack of a control group. The entire patient registry contained safety and tolerability data for 2,991 patients. Follow-up was completed for 2,289 patients. Lawson et al. (49) did not explicitly account for the difference of 702 patients. The appropriate treatment length of EECP remains speculative. A prospective design of varying treatment lengths is required to clarify the dose response and any degree to which treatment should be individualized beyond the conventionally accepted 35 hour treatment.
Case Series - Angina
• Lawson et al. (50) examined sustained clinical benefit from EECP treatment by assessing major adverse cardiovascular events (MACE) over a 5 year post treatment period. Significant MACE chosen as endpoints were death, MI, CABG, PTC, and cardiac related hospitalization. A single centre cohort of consecutive angina patients treated with EECP from 1989-1991 was followed for a mean of 5 years (range 4-7 years). Patients had stable but limiting angina despite medical and/or CABG/PTC. However, the CCS functional class of these patients was not reported in order to verify angina severity. A radionuclide stress test was also required prior to EECP treatment. Patients received an initial 35-36 hour course of EECP administered at 1-2 hour daily sessions for 5 days/week.
Exclusion from treatment consisted of:
Decompensated heart failure
Aortic valve insufficiency
MI within the prior 3 months or unstable angina
Severe peripheral vascular disease (occlusive arterial disease or thrombophlebitis)
Arrhythmias interfering with timing (atrial fibrillation, frequent ectopy, pacemakers)
Uncontrolled hypertension (blood pressure > 180/110 mm Hg)
Significant bleeding diathesis
Thirty-three patients were enrolled in this study. In the early post-treatment results, anginal symptoms (frequency, severity, ease of precipitation, and duration of episodes) decreased in all patients (no data reported). Radionuclide stress tests demonstrated a significant (p<0.01) improvement in perfusion defects in 26/33 (79%) patients who were termed “responders”. Stress tests in the remaining 7 patients (“nonresponders”) were unchanged. A decrease in antianginal medication use was seen in 31% of the “responders” and in 43% of the “nonresponders” (p=NS).
Over the mean 5 year follow-up, 13/33 (39%) patients underwent additional treatment with EECP. The cumulative mean hours of EECP administered for the entire group over the 5 years was 55.7 hours (49.75 hours in responders and 77.8 hours in nonresponders). The reasons for additional therapy included:
Worsening or recurrent angina
Persistent ischemic defects on radionuclide stress imaging
“Tune ups” (with no objective or subjective evidence of the patient’s condition worsening)
Four patients died:
1 died of ventricular tachycardia/ventricular fibrillation 1 year post-treatment
1 died of complications of angioplasty 3 years post-treatment
1 died of CHF 3 years post-treatment
1 died during sleep 6 years post-treatment
Interim events occurred in 9 patients:
4 MI
6 revascularization procedures
1 aortic valve replacement
1 unstable angina
Overall, 21/33 (64%) of EECP treated patients remained alive 5 years post therapy without cardiovascular morbidity or repeat revascularization. Of the 26 “responders”, there was one death and 5 MACE. Of the 7 “nonresponders” there were 3 deaths and 3 MACE.
The authors arguably stated from this single-centre case series, which consisted of a very small patient group (n=33), that the marked difference in mortality and MACE noted between the sub-analyzed “responders” (6/26) and “nonresponders” (6/7) (p<0.01) supports a true treatment effect of EECP. The data are insufficient to make that conclusion.
Similarly, Lawson et al. (50) stated the 5 year survival of EECP treated patients (29/33 or 88%) is similar to mortality rates previously reported in large medical/revascularization trials. (58-60) However, this is a historical comparison of invasive and noninvasive treatments and most of the patients treated within the small EECP case series also had prior PCI and/or CABG.
Of note is the lack of accurate and restrictive selection of patients receiving EECP treatment since some patients received supplementary EECP “tune ups” with no objective or subjective evidence of the patient’s deterioration. The reporting of patient adverse events was unclear throughout the paper.
Appendix 5 - Detailed Analysis of the Studies Included in the 2003 Medical Advisory Secretariat Review of EECP: Heart Failure
• Lawson et al. (25) evaluated angina patients with a history of HF who were treated with EECP. Early and 6-month changes in CCS angina class and adverse events were compared with a cohort of patients without a history of HF.
Of 1,957 patients registered in the International EECP Patient Registry (IEPR), 548 had a history of HF at baseline. Significantly fewer HF patients completed the course of EECP (p<0.001), and exacerbation of HF was more frequent during treatment (p<0.001).
Exacerbation of HF during treatment was not attributed to EECP by the investigators. However, the study design did not allow this conclusion to be tested or reached since there was no direct comparison between HF with EECP and HF without EECP.
At 6 months, patients with HF maintained their reduction in angina but were significantly more likely to have experienced death (p<0.001) or a major adverse cardiac event (MACE) (p<0.001).
At 6 months follow-up, 82% of patients in the CHF cohort without interim MACE reported that their angina was the same or less than immediately after treatment. The odds ratio (OR) and 95% confidence interval (95% CI) for patients with HF compared to non-HF treated patients during EECP treatment and during 6 month follow-up was 27.08 (82.3-89.1) and 2.01 (1.16-3.49) for exacerbation of CHF and 1.58 (0.99-2.54) and 1.77 (1.34-2.39) for MACE respectively.
Based on the low incidence of pulmonary embolism and clinically important arrhythmias during or following EECP treatment or in the follow-up in the HF patients (no data provided) the investigators concluded that the theoretical concerns of EECP precipitating pulmonary embolism and atrial/ventricular arrhythmias were not confirmed. However, the frequency of exacerbation of HF during treatment was high, and despite the fact that Lawson et al. (2001) concluded that given the severity of the disease status of the patients the treatment and 6-month follow-up MACE were within expectations, the study design did not necessarily allow this conclusion to be reached. Although patients within the registry were described as having a “history” of heart failure information about the HF patients was lacking including whether the HF was acute or chronic, stable or unstable, or medically refractory. There was also no information on the severity or duration of HF.
• Soran et al. (45) performed a prospective, uncontrolled, mulitcentre feasibility study of EECP treatment in CHF patients. Thirty-two patients were enrolled, 11 of whom had idiopathic dilated cardiomyopathy and 21, ischemic cardiomyopathy.
Inclusion criteria consisted of:
Age 21-81
HF (NYHA II or III) despite conventional therapy
LVEF ≤35%
Ability to exercise on treadmill with exertion limited by shortness of breath or fatigue
Absence of medication changes for 2 weeks prior to 1st study visit
There were 19 exclusion criteria:
Exercise limited by chest pain
Electrocardiographic changes consistent with myocardial ischemia or claudication
Peripheral edema above the ankle
Unstable angina
Serum digoxin > 1.5 mg/mL
Serum creatinine level > 2 mg/dL
MI or CABG within past 3 months
Cardiac catherterization within past 2 weeks
Acute myocarditis
History of sudden death
Presence of ICD or pacemaker
History of clinically significant aortic stenosis, mitral stenosis, or aortic regurgitation
Chronic obstructive pulmonary disease with forced expiratory volume at 1 sec of ≤1.5 L
Coagulation abnormalities resulting in an international normalized ration > 2.0
Severe hypertension
Arrhythmias that would interfere significantly with triggering of EECP device
History of deep vein thrombosis, phlebitis, stasis ulcers, and/or pulmonary emboli
Any condition that precluded diastolic augmentation during initiation of EECP
Calcium channel blocker or beta blocker withdrawn < 3 months prior to the study
HF treatment was optimized and stable prior to enrollment. Each patient received 35 one-hour EECP treatment sessions administered once a day, 5 days a week for 7 weeks. During the study, all drugs were kept as constant as possible; injected inotropic agents were not permitted. Patients were followed-up for 6 months. The primary outcome was safety; other outcomes of interest were changes in exercise capacity and quality of life. The analysis was calculated using observed cases only; a secondary analysis was conducted using the intent-to-treat principle, and reportedly produced similar results.
Twenty-six patients received EECP, and of these 3 discontinued during the treatment period due to adverse events (no details provided).
Of 23 patients who completed treatment, 19 completed the whole study per protocol, protocol violations being reported in the remaining 4 patients.
Therefore, a total of 19 patients were evaluated for efficacy at the 6-month follow-up.
There were 46 adverse events reported in 23 patients. These included arrhythmia, atrial fibrillation, angina pectoris and worsening HF both during the treatment and follow-up period.
Of the 46 adverse events, 14 were classified as serious and led to hospitalization in 8 patients. Twenty-two of the adverse events occurred during the treatment period, 10 of which occurred during a treatment session.
According to the investigators, none of the adverse events, including worsening of HF, reported during the 6-month follow-up period were related to direct or indirect effects of EECP therapy. However, the study design did not allow this conclusion to be drawn.
Peak oxygen uptake increased significantly from baseline at 1-week post treatment (p=0.05), and at the 6-month follow-up (p<0.001). At the 1-week and 6-month follow-up, overall mean exercise duration increased significantly (p<0.001 and p<0.028 respectively).
Twenty-four patients had a post-treatment QOL assessment (including 1 patient who was discontinued from the study but had the QOL assessment 1 month after treatment). The overall changes between the test results at baseline and 1-week post-treatment were significant for total score, and physical and emotional dimension (p<0.01).
For the 22 patients who completed the 6-month follow-up, the QOL total score indicated improvement over baseline values, however, only the change in emotional dimension remained significant, p<0.01.
This study had a number of methodological weaknesses. The sample size was small, a large number of patients were withdrawn from the study (32 enrolled, 19 completed follow-up), and some endpoints were soft or subjective. There was no information on sample size calculation. Some conclusions were based on subjective assessment, in particular side effects attributed to EECP despite the fact that the study design did not permit this analysis. Furthermore, in a study with a small sample size and large numbers of patients who did not complete the study, a detailed account of the ITT analysis should have been provided. Finally, the large number of exclusion criteria reduces the generalizability of this study.
Appendix 6: Studies Evaluating EECP in Patients With Refractory Stable Angina
| Study | Type | Size | Endpoints | Results |
|---|---|---|---|---|
| MUST-EECP Arora et al., (33) 1999 | Randomized Prospective placebo controlled multicentre | 139 patients randomized CCS class I, II, or III | Exercise duration Daily average anginal
attacks Nitroglycerin usage Time to 1 mm ST segment depression |
Increase in time to 1 mm ST segment depression for
active CP group, p=0.01 (adjusted mean: active CP 37±11 s
vs. inactive CP –4+12s) Decrease in anginal episodes, p=NS (intent to treat) Decrease nitroglycerin use, p=NS (intent to treat) Increase in exercise duration, p=NS Active CP group reported more adverse events, p<0.001. |
| MUST-EECP (34) Arora et al., 2002 | Randomized Prospective placebo controlled multicentre | 71/139 randomized patients completed questionaires
at baseline, end of therapy, and 12 months. Same study patients as Arora et al., (1999). |
Health Related Quality of Life (HQOL) at end of treatment and 12 months | At end of treatment and 12-month follow-up, patients who had active EECP reported greater improvement than those having inactive EECP in all 4 primary HQOL quality of life parameters (p<0.05). |
| Schecter et al., (38) 2003 | Comparative | 40 patients (20 ECP and 20 age/gender matched controls who refused EECP) | Nitroglycerin tablets in the previous 24 hours. Change in CCS angina class. | EECP patients and nitroglycerine tablet
usage: Pre EECP 4.2 (2.7); Post EECP 0.4 (0.5); p<0.001. Control patients and nitroglycerine usage: Pre EECP 4.5 (2.3); Post EECP 4.4 (2.6); p=0.87. EECP patients and change in CCS angina class: Pre EECP 3.5 (0.5); Post EECP 1.9 (0.3); p<0.0001. Control patients and change in CCS angina class: Pre EECP 3.3 (0.6); Post EECP 3.5 (0.5); p=0.89. |
| Barsness et al., (48) 2001 | IEPR Registry | 978 patients 69% of patients had CCS class III/IV [Candidates = patients suitable for revascularization. Noncandidates = patients not suitable for revascularization] | Anginal class Quality of life Adverse effects | Angina class improved post EECP Pre EECP: CCS I 5.5%; II 24.8%; III 48.1%; IV 21.6%. Post EECP: 36.0%; 32.4%; 9.8%; 2.8% Decrease in at least 1 anginal class: 81% overall Mean decrease in anginal episodes per week: 6.4±12.6 overall Decrease in use of nitroglycerin: 61.7% overall Overall, 11 patients (1.1%) withdrew due to a serious cardiac event. Clinical events were cited as the reason for discontinuing treatment in 43.8% of patients withdrawing. 15% of patients starting EECP treatment did not complete full course of treatment. |
| Holubkov et al., (57) 2002 | Registry | 2 cohorts: 1. IEPR, n=323 (PCI candidates at time of index EECP) 2. NHLBI, n=448 (patients who underwent PCI) |
Mortality Patients’ self-reported level of exertional angina. | 85.8% of EECP patients completed full course of
treatment. 92.1% of PCI treated patients had a successful initial procedure. At 1 year, survival and rates of CABG were comparable, p=NS. IEPR patients reported more usage of Ca channel blockers [50.6% vs. 33.7%](p<0.001); angiotensin receptor blockers [5.6% vs. 1.9%](p<0.01); and long acting nitrates [53.0% vs. 30.3%](p<0.001). PCI registry patients reported using more short acting nitroglycerin [43.3% vs. 82.2%](p<0.001). Class III/IV or unstable symptoms were reported in 15.5% of EECP patients and 9.5% of PCI patients, p=0.02. 73.4% of PCI patients reported no angina after 1 year compared to IEPR patients (43.7%), p<0.001. |
| Lawson et al., (49) | Registry EECP Consortium | Cohort of 2,289 consecutive patients from 84 centres had completed follow-up. CCS I-IV. | Adverse effects Anginal class | 91 adverse patient experiences were observed out of
the entire patient registry (n=2,991). Overall, the average CCS class improved after EECP [average CCS class 2.78 vs. 1.81 p<0.001]. Pre EECP CCS IV (compared to CCS I and II) improvement: OR 3.30 (2.49-4.39), p<0.0001. PreEECP CCS III (compared to CCS I and II) improvement: OR 4.38 (3.46-5.56), p<0.0001. Patients receiving >35 hrs of treatment did not benefit more over the standard 35 hour course OR 1.02 (0.77-1.36). |
| Lawson et al., (50) | Case series | Single centre cohort of 33 consecutive angina patients followed for mean of 5 years. | Major adverse cardiovascular events (MACE) | In early post treatment: Radionuclide stress tests demonstrated a significant (p<0.01) improvement in perfusion defects in 26/33 (79%) of patients = responders. In the remaining 7 patients, stress tests were unchanged = nonresopnders. Over 5 years: 13/33 patients underwent additional EECP treatment.. 4 patients died, 9 patients experienced interim adverse events. 21/33 patients remained alive post therapy without cardiovascular morbidity or repeat revascularization. |
| Bonetti et al., (61) 2003 | Case series | Single centre cohort of 23 patients. | Change in CCS functional class. DASI |
17/23 (74%) improved 1+ CCS class and improved DASI score (maintained at 1 month followup). |
| Tartaglia et al., (62) 2003 | Caser series | Single centre cohort of 25 patients. | Exercise duration (seconds) Significant change on ECG during ETT Change in CCS functional class. |
Exercise duration: PreEECP 357 seconds; Post EECP 449 seconds; P<0.001. Of 16 patients with ST-segment depression preEECP, 10 (63%) had significant delay and 3 (19%) had no ST-segment depression post EECP. Reduction of 1+ angina class 24/25 (96%). |
| Werner et al., (63) 2003 | Case series | Single centre cohort of 48 patients. | Symptom limited bicycle test (Watts) Symptom limited bicycle test (minutes) Angina count/week Nitroglycerin use/week |
Symptom limited bicycle test: PreEECP 117.3 watts; PostEECP 137.8 watts; p<0.005. Symptom limited bicycle test: PreEECP 7.4 min; PostEECP 9.0 min; p<0.05. Angina count/week: PreEECP 6.4; PostEECP 3.3; p<0.05. Nitroglycerin use/week: PreEECP 5.5; PostEECP 2.7; p=Not significant. |
| Bagger et al., (64) 2004 | Case series | Single centre cohort of 26 patients | CCS angina class (group mean) Exercise duration |
CCS angina class: PreEECP 3.1; PostEECP 2.2; p<0.05. Exercise duration: Improved by 21% in the 78% who were able to perform the exercise test. |
| Michaels et al.,(42) 2004 | Case series | Registry study. 1,097 patients from sites with >85% followup. |
2 year followup Multiple clinical
endpoints. Change in CCS class. Quality of life improvement (Likert scale). |
Death 8.5% MI 8.9% Unstable angina 21.8% HF exacerbation 11.7% Cardiac hospitalization 39.3% Noncardiac hospitalization 40.9% Revascularization procedure 15% Repeat EECP 16.1% Event free 40.8% Change in CCS class: Among those event free, sustained reduction in CCS class at 2 years. Quality of Life: Improvement in 47% at 2 years. |
| Lawson et al. (65) 2005 | Registry | N=2007 EECP patients completing at least 30 h of EECP, with 1 year follow-up and info on acute angina reduction | Determine predictors of 1-year angina status in patients who demonstrated initial clinical improvement (responders [R] vs. those who did not show benefit non-responders [NR] after EECP; anginal class, weekly anginal episodes, frequency of nitroglycerin use; quality of life | Angina reduced by at least 1 CCS class in 83%
immediately post-EECP (1665), 17% no initial reduction
(342). In R, weekly angina decreased from 10.4 to 1.7 (p<.001). NR sig. decrease from 11.5 to 5.8 (p<.05). Nitroglycerin use decreased in R from 9.3 – 1.6 (p<.001), but not significantly in the NR (10.5 – 8.0). QoL reported in 63% or R vs. 37% of NR. The only significant independent predictor of lack of initial response to EECP was baseline anginal class (I, II, III, versus IV with odds ratios 5.0, 4.8, 1.4 and CI 2.4 – 10.4, 3.2 – 7.0 and 1.0 – 2.0, respectively. At 1 year, 15% of NR vs. 8% of R had PCI or CABG (p<.0001). |
| Michaels et al. (66) 2005 | Registry | N=1192 patients who completed a 1st course of EECP, and were enrolled in IEPR sites that provided >/=85% follow-up at 2 years; 90% had CCS class III or IV at baseline. | CCS class, angina episodes, nitroglycerin use | After initial course, 86% reported a decrease by at
least 1 CCS class and nitroglycerin use discontinued by 57% of
patients. Within 2 years post-EECP, 194 patients (18%) underwent a repeat EECP course of which 78% (152) had available data on 2nd course. Among repeaters, 70% demonstrated sig. decrease in angina, although distribution of CCS class not sig. different. At 2-year follow-up anginal symptoms remained sig. worse in patients who had repeat EECP (6 episodes per week in repeaters vs. 3 per week in non (p<.01), more nitroglycerin use in repeaters (63% vs. 45%, p<.001). |
| Lawson et al. (44) 2004 | Registry | Patients divided into 3 groups: those without left main coronary artery disease (LMD; n=2,377), those with LMD and prior CABG (LMD-CABG) (n=431); and those with LMD and no prior CABG (LMD-NCABG (n=53) | CCS class Weekly angina episodes Weekly nitroglycerin use MACE | Post EECP improvements in CCS by at least one class
in all three groups (NS between-group differences), No LMD
(74%), LMD-CABG (75%), LMD-NCABG (65%). No between-group differences in mean decrease in weekly angina episodes (7.1 vs. 8.0 vs. 7.6) or frequency of weekly nitroglycerin use (6.6 vs. 8.1 vs. 8.9). At 6 month follow-up, CCS class further improved, and there was a further reduction in mean weekly angina episodes (4.7 vs. 4.6 vs. 5.3) and nitroglycerin use (6.5 vs. 6.8 vs. 8.2). MACE 8 months after EECP treatment was 11.2% in non-LMD, 15.6% in LMD with CABG, and 24.3% in LMD without CABG. Late mortality in LMD non-CABG was 13.2% (CI 3.3-23.1) versus 4.8% (CI 2.7 – 7.1) in LMD with CABG, and 2.8% (CI 2.1 – 3.5) without LMD. |
| Fitzgerald et al., (40) 2003 | Registry | IEPR; N=4454 patients with prior CABG or PCI (CCS Class III/IV for 83.9%) compared with a group of patients (Pumpers, 77.2% Class II/III) who were candidates for either/both procedures but chose EECP as their initial revascularization treatment | Angina class; nitroglycerin use, angina episodes | Post-EECP a reduction by at least 1 CCS class was
seen in 74.8% of Pumper vs. 72.7% non-pumper,
p=NS. Decrease in angina episodes per week: 7.6% NP vs. 5.2 in pumpers, p<.001. Nitroglycerin use: 17.4% non-pumpers vs. 15.2% (5.9 times per week) pumpers, as compared to baseline of 71.4% (9.8 times per week) non-pumpers, 46.9 % (7.1 times per week) pumpers. |
| Lawson et al., (67) 2003 | Registry | N=4592 patients with no prev. EECP treatment on enrollment in the IEPR registry; 82.3% in CCS class III or IV at baseline. | CCS functional class; angina frequency, nitroglycerin use, changes in medications, quality of life, interim MACE; Determination of patient characteristics which influence EECP success | 83.1% completed prescribed EECP course. MACE over the course of therapy included death (.3%), MI (.9%), CABG (.2%), PCI (.8%), exacerbation of HF in 1.9%, unstable angina in 2.8%. CCS improvement of >/= 1 class in 73% of patients, >/=2 classes in 38.2%, >/=3 classes in 17.3% did not change in 26.0% and worsened in 1.1%. Mean angina episodes per week at baseline were 10.1 and decreased to 2.5 per week post EECP. Baseline nitroglycerin use was 9.5 times per week pre EECP, 2.5 times post-EECP. Overall success rate in patients with diabetes, prior CABG, and HF was 70% (success defined as 1 CCS class decrease). |
| Linnemeier et al.; (39) 2003 | Registry | N=249 IEPR patients >/=80 years (elderly); Elderly more likely to be female, have a history of CHF (41% vs. 29%, p<.001), and less likely to have had prior revascularization (74% vs. 86%, p<.001); about 87% of elderly CCS Class III/IV pre-EECP, somewhat more severe than in younger (Class not reported). | MACE; cardiac hospitalization; angina frequency, nitroglycerin use; CCS class, Quality of Life, | Sig. fewer >/=80 years (elderly) completed treatment (76% versus 84%, p=.05); Treatment non-completion not stopped due to clinical event any more than in younger (11% versus 9%), rather, patient discontinued in 11% of elderly vs. 7% of non-elderly). At 6-month follow-up low rates of MACE in both groups NS except for death (8 in younger versus 6 in elderly (p=.05) and cardiac hospitalization, 12 in younger vs. 6 in elderly (p=.05). Very little change in medication use recorded, and NS between group differences. Sig, improvement post-EECP in patient-assessed QoL, health, and satisfaction (all p<.001). |
| Linnemeier et al., (41) 2003 | Case series | N=1532 IEPR patients of which 665 (43%) reported a diagnosis of diabetes; patients with DM sig. more likely to have a history of heart failure | CCS class, MACE, angina episodes per week, nitroglycerin use, quality of life | In post-EECP period, MI (1.7% vs. .2%,
p<.01) and HF (3.3% vs. 1.3%, p<.01) occurred sig.
more often in the DM group. Post-EECP angina decreased by at least 1 CCS class in DM (69%) and ND (72%) of patients, diff.=NS. At 1 year, follow-up available for 86% of DM and 89.9% of ND; At 1-year, sig, increase in death (3.9% ND vs 7.5 % DM, p<.01), MACE (16.3% ND vs. 22.6% DM, p<.01, and CHF (6.1% ND vs. 12.8% DM, p<.001). |
Appendix 7: Studies Evaluating EECP in Patients With Heart Failure
| Study | Type | Size | Endpoints | Results |
|---|---|---|---|---|
| Lawson et al., (25) 2001. | Registry (IEPR) | Angina patients: 548 patients = HF history 1409 patients = nonHF history |
Adverse effects Angina symptoms | Significantly fewer HF patients completed course of
EECP (p<0.001); exacerbation of HF was more frequent
during treatment (p<0.001); angina class in HF patients
decreased less (p<0.01); and HF patients reported more
musculoskeletal problems (p<0.05). At 6 months, HF patients maintained reduction in angina but were more likely to have a MACE, p<0.001. |
| Soran et al., (45) 2002 | Prospective, uncontrolled case series | 32 HF patients NYHA class II and III LVEF <35% | Safety Exercise capacity Quality of life Patients followed up for 6 months. | 6 patients withdrew before treatment. 26 patients received EECP, including follow-up at 1 week.
46 adverse events in 23 patients: 14 events led to hospitalization in 8 patients 22 adverse events occurred during treatment period 12 adverse events occurred outside treatment session 24 adverse events occurred during 6 month follow-up At 1 week follow-up, overall mean exercise duration increased, p<0.001. AT 6 month follow-up, overall mean exercise duration increased, p<0.028. 24 patients had a posttreatment QOL assessment. Overall changes were significant for total score, and physical and emotional dimension, p<0.01. |
| PEECH [Prospective Evaluation of Enhanced External Counterpulsation in Heart Failure] (68-70) |
Prospective Controlled RCT | 187 pats. with Class 11/111 NYHA stable CHF, and an ejection fraction </=35%, with ACE inhibitor or ARD for at least 1 month prior to enrollment, and and a beta-blocker for at least 3 months prior | Co-primary endpoints were % of pts. with at least a 60 second increase in exercise duration from baseline to 6-months, or the % of pts. with at least a 1.25 ml/min/kg increase in Peak VO2 from baseline; Exercise duration measured using a standardized ETT on a treadmill (modified Naughton protocol), or, peak VO2 at 6-months; Secondary endpoints inc. changes in exercise time and Peak VO2, NYHA class, quality of life, adverse events, and predefined clinical outcomes | ITT analysis indicates that 35% of the EECP group
versus 25% of the control group increased exercise time by at
least 60 seconds (p=0.016) at 6 months. No difference in Peak
VO2 at 6 months. Secondary endpoints: Exercise time sig.
Improved at 1 week, 3 and 6 months (24.7 second increase in EECP
group, versus decrease of 9.9 seconds in the controls, p=0.01).
NYHA class sig. improved in EECP group with 31.3% of patients
reporting improvement in NYHA class versus 14.3% in the controls
at 6-months (p<.01), Improvement in QoL NS at 6 months,
but sig. at 3 months and 1 week. Adverse events: 27 (30%) of EECP and 36 (26%) of control group required hospitalization during treatment (p value?). During follow-up, 21 subjects in EECP and 23 in control reported serious adverse events, 1 report in EECP group of DVT, and 1 case of worsening heart failure in the control group. |
| Lawson et al., (43) 2005 | Registry | N=746 IEPR angina patients with a history of heart failure; divided into two groups, LVEF</=35%, [S] (n=355) of which 90.9% had CCS class III/IV angina, and LVEF>35%[D] (n=391), 92% with CCS class III/IV angina; some sig. differences between groups at baseline, incl. prior MI, gender, and some medications | Changes in angina functional class; changes in quality of life; changes in angina pattern and nitroglycerin use; medication changes; interim events (incl. cardiac hospitalizations and MACE | In both groups similar decreases in angina class,
(72.2% S versus 71.9% D) anginal episodes and nitroglycerin use.
Overall MACE rate over the course of therapy in S group was 3.1%
versus 2.3% in D group. 87% of patients were available for 1-year follow-up: Reduction of at least 1 CCS class seen in 76% of S and 78% of D, and 71% of each group reported either no angina or CCS class I/II. Adverse events: Death sig. more frequent in S group (14.1%) versus D group (9.2%), p=0.39, PCI more frequent in D group (7.3% versus 5.8%). |
| Soran et al., (35) 2006 | Registry | N=363 (7% of 5000 IEPR patients) EF </= 35% | Angina class, QoL | 81% completed full course of therapy (no reasons
provided for drop-out rate although during therapy heart failure
exacerbation and events reported 5.4%). Immed post-EECP 77% improved more than 1 anginal class, 15.6% - 18% had no angina post-treatment (p<.001). At 2-years 265 patients completed follow-up and 55% had sustained improvement of anginal class. QoL indicated 58% improved immed. post-EECP and at 2-years, 63% improved (p<.001). At 2 years, 70% of patients had event-free survival; adverse event rates during, post-EECP, and at 2–years not provided though the authors state that observed events were within expectations. |
| Vijayaraghavan et al.,(46) 2005 |
Cardiometrics ECP registry | 127 NYHA class III-V CHR patients with a comorbidity of CCS class III-IV angina; sig. differences in baseline characteristics | Comparative changes in mortality, LVEF, NYHA and CCS class, incidence of all-cause hospitalizations | 1 year follow-up data, no drop-outs. 127 patients divided into 3 groups based on EECP pressure applied which was based on LVEF and response to EECP; n=54 in Low D/S ratio (av. 7:1); 39 patients in Mid (av. 1.08:1); n=34 in High D/S (av. 1.32:1) which is the usual EECP treatment pressure. Mortality: 1 death in Low (1.5%), 3 in Mid (7.69%), 3 deaths in High (8.82%), the 1.85% annual mortality in the Low group was a 75.3% reduction from the 7.69% in the Mid (p<.001) and a 77.8% reduction from the 8.82% in the High DS ratio group (p<.0001). LVEF improved by 23%, 20.1%, 17.5%, and 20.4% in the Low (p=.05), Mid (p=NS), High (p=NS) and Total (p=NA) survivors. NYHA class: Low D/S improved by an av. of 36.6% (p=.0001); improved by 29.6% av. in Mid group (p<.005); high D/S improved by 29.6% (p<.01). Hospitalizations reduced by 87.5% in Low D/S (p<.0001); 83.2% in Mid (p<.0001); 46.2% in High (p<.01). |
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Enhanced external counterpulsation (EECP): an evidence-based analysis. Ontario Health Technology Assessment Series 2006; 6(5)
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo@moh.gov.on.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo@moh.gov.on.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo@moh.gov.on.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 1-4249-0801-9 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and information from practicing medical experts and industry, adds important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to maximize patient outcomes.
If you are aware of any current additional evidence to inform an existing Evidence-Based Analysis, please contact the Medical Advisory Secretariat: MASInfo@moh.gov.on.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to do so, this document may not fully reflect all scientific research available. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superceded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas
Abbreviations/Acronyms
- CABG
Coronary arterial bypass graft
- CAD
Coronary artery disease
- CCS
Canadian Cardiovascular Society
- CHF
Congestive heart failure
- ECG
Electrocardiogram
- EF
Ejection fraction
- EECP
Enhanced external counterpulsation
- HF
Heart failure
- IEPR
International EECP Patient Registry
- ITT
Intent to treat
- MI
Myocardial infarction
- MUST-EECP
Multicenter Study of Enhanced External Counterpulsation
- NYHA
New York Heart Association
- PCI
Percutaneous coronary interventions
- PEECH
Prospective Evaluation of Enhanced External Counterpulsation in Congestive Heart Failure Study
- RCT
Randomized controlled trial
- TENS
Transcutaneous electrical nerve stimulation
References
- 1.Kim MC, Kini A, Sharma SK. Refractory angina pectoris: mechanism and therapeutic options. J Am Coll Cardiol. 2002;39(6):923–34. doi: 10.1016/s0735-1097(02)01716-3. [DOI] [PubMed] [Google Scholar]
- 2.Singh M, Holmes DR Jr, Tajik AJ, Barsness GW. Noninvasive revascularization by enhanced external counterpulsation: a case study and literature review. Mayo Clin Proc. 2000;75(9):961–5. doi: 10.4065/75.9.961. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee D, Bhatt DL, Roe MT, Patel V, Ellis SG. Direct myocardial revascularization and angiogenesis--how many patients might be eligible? Am J Cardiol. 1999;84(5):598–600. doi: 10.1016/s0002-9149(99)00387-2. [DOI] [PubMed] [Google Scholar]
- 4.DeJongste MJL, Tio RA, Foreman RD. Chronic therapeutically refractory angina pectoris. Heart. 2004;90(2):225–30. doi: 10.1136/hrt.2003.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jong P, Vowinckel E, Liu P, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population based study. Arch Intern Med. 2002;162(15):1689–94. doi: 10.1001/archinte.162.15.1689. [DOI] [PubMed] [Google Scholar]
- 6.Hare JM. Cardiac resynchronization therapy for heart failure. N Engl J Med. 2005;346(24):1902–5. doi: 10.1056/NEJMed020028. [DOI] [PubMed] [Google Scholar]
- 7.Farwell D, Patel NR, Hall A, Ralph S, Sulke AN. How many people with heart failure are appropriate for biventricular resynchronization? Eur Heart J. 2000;21(15):1246–50. doi: 10.1053/euhj.1999.1985. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JG, Thackray S, Goodge L, Kaye G, Cooklin M. Outcome studies with device therapy in patients with heart failure. J Cardiovasc Electrophysiol. 2002;13(1 Suppl):S73–S91. doi: 10.1111/j.1540-8167.2002.tb01958.x. [DOI] [PubMed] [Google Scholar]
- 9.Soroff HS, Cloutier CT, Birtwell WC, Begley LA, Messer JV. External counterpulsation. Management of cardiogenic shock after myocardial infarction. JAMA. 1974;229(11):1441–50. doi: 10.1001/jama.229.11.1441. [DOI] [PubMed] [Google Scholar]
- 10.Amsterdam EA, Banas J, Criley JM, Loeb HS, Mueller H, Willerson JT, et al. Clinical assessment of external pressure circulatory assistance in acute myocardial infarction. Report of a cooperative clinical trial. Am J Cardiol. 1980;45(2):349–56. doi: 10.1016/0002-9149(80)90658-x. [DOI] [PubMed] [Google Scholar]
- 11.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Technology Evaluation Center (TEC) External counterpulsation for treatment of chronic stable angina pectoris and chronic heart failure [report on the Internet] 12. Vol. 20. Blue Cross Blue Shield Association (BCBSA); 2005. [[cited 2006 Mar. 6]]. Available at: http://www.bcbs.com/tec/vol20/20_12.html . [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. Decision memo for external counterpulsation (ECP) therapy (CAG-00002R2) [report on the Internet] Centers for Medicare and Medicaid Services (CMS); Mar, 2006. [[cited 2006 Mar. 25]]. Available at: http://www.cms.hhs.gov/mcd/viewdecisionmemo.asp?id=162 . [Google Scholar]
- 14.ECRI. External Counterpulsation (ECP) for Coronary Indications. Target Report #233. Jan, 2005. ECRI.
- 15.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41(1):159–68. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 16.Elveback LR, Connolly DC, Melton LJ. III. Coronary heart disease in residents of Rochester, Minnesota. [VII. Incidence, 1950 through 1982]. Mayo Clin Proc. 1986;61(11):896–900. doi: 10.1016/s0025-6196(12)62612-3. [DOI] [PubMed] [Google Scholar]
- 17.Rouleau JL, Talajic M, Sussex B, Potvin L, Warnica W, Davies RF, et al. Myocardial infarction patients in the 1990s--their risk factors, stratification and survival in Canada: the Canadian Assessment of Myocardial Infarction (CAMI) Study. J Am Coll Cardiol. 1996;27(5):1119–27. doi: 10.1016/0735-1097(95)00599-4. [DOI] [PubMed] [Google Scholar]
- 18.American Heart Association. Dallas, TX: American Heart Association; 1999. 1999 Heart and Stroke Statistical Update. [Google Scholar]
- 19.Kannel WB, Feinleib M. Natural history of angina pectoris in the Framingham study. [Prognosis and survival]. Am J Cardiol. 1972;29(2):154–63. doi: 10.1016/0002-9149(72)90624-8. [DOI] [PubMed] [Google Scholar]
- 20.Elveback LR, Connolly DC. Coronary heart disease in residents of Rochester, Minnesota. V. [Prognosis of patients with coronary heart disease based on initial manifestation]. Mayo Clin Proc. 1985;60(5):305–11. doi: 10.1016/s0025-6196(12)60537-0. [DOI] [PubMed] [Google Scholar]
- 21.Babinski ASH. Naylor C, Slaughter P, editors. Hospitalization for cardiovascular medical diagnoses. Toronto: Institute for Clinical Evaluative Sciences. 1999. [[cited 2006 Mar. 6]]. pp. 15–50. Available from: http://www.ices.on.ca/file/4_CVA_Chapter2.pdf .
- 22.Thadani U. Treatment of stable angina. Curr Opin Cardiol. 1999;14(4):349–58. doi: 10.1097/00001573-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Fihn SD, Williams SV, Daley J, Gibbons RJ. American College of Cardiology, American Heart Association et al. Guidelines for the management of patients with chronic stable angina: treatment. Ann Intern Med. 2001;135(8 Pt 1):616–32. doi: 10.7326/0003-4819-135-8_part_1-200110160-00014. [DOI] [PubMed] [Google Scholar]
- 24.Cowie MR, Zaphiriou A. Management of chronic heart failure. BMJ. 2002;325(7361):422–5. doi: 10.1136/bmj.325.7361.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson WE, Kennard ED, Holubkov R, Kelsey SF, Strobeck JE, Soran O, et al. Benefit and safety of enhanced external counterpulsation in treating coronary artery disease patients with a history of congestive heart failure. Cardiology. 2001;96(2):78–84. doi: 10.1159/000049088. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Medicare and Medicaid Services. NCA tracking sheet for external counterpulsation (ECP) therapy (CAG-00002R2) [report on the Internet] Centers for Medicare and Medicaid Services (CMS) Jun, 2005. [[cited 2005 Oct. 5]]. Available at: https://www.cms.hhs.gov/mcd/viewtrackingsheet.asp?id=162 .
- 27.Zheng ZS, Li TM, Kambic H, Chen GH, Yu LQ, Cai SR, et al. Sequential external counterpulsation (SECP) in China. Trans Am Soc Artif Intern Organs. 1983;29:599–603. [PubMed] [Google Scholar]
- 28.Taguchi I, Ogawa K, Oida A, Abe S, Kaneko N, Sakio H. Comparison of hemodynamic effects of enhanced external counterpulsation and intra-aortic balloon pumping in patients with acute myocardial infarction. Am J Cardiol. 2000;86(10):1139–41, A9. doi: 10.1016/s0002-9149(00)01175-9. [DOI] [PubMed] [Google Scholar]
- 29.Michaels AD, Accad M, Ports TA, Grossman W. Left ventricular systolic unloading and augmentation of intracoronary pressure and Doppler flow during enhanced external counterpulsation. Circulation. 2002;106(10):1237–42. doi: 10.1161/01.cir.0000028336.95629.b0. [DOI] [PubMed] [Google Scholar]
- 30.Soran O, Crawford LE, Schneider VM, Feldman AM. Enhanced external counterpulsation in the management of patients with cardiovascular disease. Clin Cardiol. 1999;22(3):173–8. doi: 10.1002/clc.4960220304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu GF, Qiang SZ, Zheng ZS, Zhang MQ, Lawson WE, Hui JCK. A neurohormonal mechanism for the effectiveness of EECP [abstract] Circulation. 1999;100(18) 1832 Abstract 4390. [Google Scholar]
- 32.Garlichs C, Zhang H, Werner D, John A, Trägner P, Daniel WG. Reduction in serum endothelin-1 levels by pneumatic external compression. Can J Cardiol. 1998;14(Suppl F):87F. [Google Scholar]
- 33.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, et al. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33(7):1833–40. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 34.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, et al. Effects of enhanced external counterpulsation on Health-Related Quality of Life continue 12 months after treatment: a substudy of the Multicenter Study of Enhanced External Counterpulsation. J Investig Med. 2002;50(1):25–32. doi: 10.2310/6650.2002.33514. [DOI] [PubMed] [Google Scholar]
- 35.Soran O, Kennard ED, Kfoury AG, Kelsey SF. IEPR Investigators. Two year clinical outcomes after enhanced external counterpulsation (EECP) therapy in patients with refractory angina pectoris and left ventricular dysfunction (report from the International EECP Patient Registry) Am J Cardiol. 2006;97(1):17–20. doi: 10.1016/j.amjcard.2005.07.122. [DOI] [PubMed] [Google Scholar]
- 36.Dery V. Efficacy of external counterpulsation in the treatment of chronic angina [report on the Internet] Montreal, Quebec: Agence d’Evaluation des Technologies et des Modes d’Intervention en Sante (AETMIS); 2004. [[cited 2006 Feb. 1]]. AETMIS 03-08. Available at: http://www.aetmis.gouv.qc.ca/ [Google Scholar]
- 37.Comite d’Evaluation et de Diffusion des Innovations Technologiques. External counterpulsation - preliminary report. Paris: Comite d’Evaluation et de Diffusion des Innovations Technologiques (CEDIT) 2005. [[cited 2005 Jan. 3]]. Available at: http://cedit.aphp.fr. /
- 38.Shechter M, Matetzky S, Feinberg MS, Chouraqui P, Rotstein Z, Hod H. External counterpulsation therapy improves endothelial function in patients with refractory angina pectoris. J Am Coll Cardiol. 2003;42(12):2090–5. doi: 10.1016/j.jacc.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Linnemeier G, Michaels AD, Soran O, Kennard ED IEPR Investigators. Enhanced external counterpulsation in the management of angina in the elderly. Am J Geriatr Cardiol. 2003;12(2):90–4. doi: 10.1111/j.1076-7460.2003.01749.x. [DOI] [PubMed] [Google Scholar]
- 40.Fitzgerald CP, Lawson WE, Hui JC, Kennard ED. IEPR Investigators. Enhanced external counterpulsation as initial revascularization treatment for angina refractory to medical therapy. Cardiology. 2003;100(3):129–35. doi: 10.1159/000073930. [DOI] [PubMed] [Google Scholar]
- 41.Linnemeier G, Rutter MK, Barsness G, Kennard ED, Nesto RW. IEPR Investigators. Enhanced External Counterpulsation for the relief of angina in patients with diabetes: safety, efficacy and 1-year clinical outcomes. Am Heart J. 2003;146(3):453–8. doi: 10.1016/S0002-8703(03)00251-5. [DOI] [PubMed] [Google Scholar]
- 42.Michaels AD, Linnemeier G, Soran O, Kelsey SF, Kennard ED. Two-year outcomes after enhanced external counterpulsation for stable angina pectoris (from the International EECP Patient Registry [IEPR]) Am J Cardiol. 2004;93(4):461–4. doi: 10.1016/j.amjcard.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 43.Lawson WE, Silver MA, Hui JC, Kennard ED, Kelsey SF. Angina patients with diastolic versus systolic heart failure demonstrate comparable immediate and one-year benefit from enhanced external counterpulsation. J Card Fail. 2005;11(1):61–6. doi: 10.1016/j.cardfail.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Lawson WE, Hui JC, Barsness GW, Kennard ED, Kelsey SF. IEPR Investigators. Clin Cardiol. 2004;27(8):459–63. doi: 10.1002/clc.4960270808. Effectiveness of enhanced external counterpulsation in patients with left main disease and angina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soran O, Kennard ED, Kelsey SF, Holubkov R, Strobeck J, Feldman AM. Enhanced external counterpulsation as treatment for chronic angina in patients with left ventricular dysfunction: a report from the International EECP Patient Registry (IEPR) Congest Heart Fail. 2002;8(6):297–302. doi: 10.1111/j.1527-5299.2002.00286.x. [DOI] [PubMed] [Google Scholar]
- 46.Vijayaraghavan K, Santora L, Kahn J, Abbott N, Torelli J, Vardi G. New graduated pressure regimen for external counterpulsation reduces mortality and improves outcomes in congestive heart failure: a report from the Cardiomedics External Counterpulsation Patient Registry. Congest Heart Fail. 2005;11(3):147–52. doi: 10.1111/j.1527-5299.2005.04068.x. [DOI] [PubMed] [Google Scholar]
- 47.Vasomedical Inc. Enhanced external counterpulsation (EECP) therapy formal application for revision to National Coverage Language Coverage Issues Manual 20.20. [[updated 2005; cited 2005 Oct. 4]]. Available at: http://www.cms.hhs.gov/DeterminationProcess/downloads/id162.pdf .
- 48.Barsness G, Feldman AM, Holmes DR, et al. The International EECP Patient Registry (IEPR): design, methods, baseline characteristics and acute results. Clin Cardiol. 2001;24:435–42. doi: 10.1002/clc.4960240604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawson WE, Hui JCK, Lang G. Treatment benefit in the enhanced external counterpulsation consortium. Cardiology. 2000;94(1):31–5. doi: 10.1159/000007043. [DOI] [PubMed] [Google Scholar]
- 50.Lawson WE, Hui JCK, Cohn PF. Long-term prognosis of patients with angina treated with enhanced external counterpulsation: five year followup study. Clin Cardiol. 2000;23:254–8. doi: 10.1002/clc.4960230406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure) J Am Coll Cardiol. 2005;46(6):e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 52.Snow V, Barry P, Fihn SD, Gibbons RJ, Owens DK, Williams SV, et al. Primary care management of chronic stable angina and asymptomatic suspected or known coronary artery disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2004 2005 Jan 4;141142(7)(1):562–7. 79. doi: 10.7326/0003-4819-141-7-200410050-00014. Erratum in: Ann Intern Med. [DOI] [PubMed] [Google Scholar]
- 53.Aetna. External counterpulsation (ECP) [report on the Internet]. Clinical Policy Bulletins Number: 0262 (updated) Aetna Inc; June July. 2005. [[cited 2005 Oct. 5]]. Available at: http://www.aetna.com/cpb/data/CPBA0262.html . [Google Scholar]
- 54.Blue Cross Blue Shield of North Carolina. Enhanced external counterpulsation [report on the Internet] Jun, 2004. [[cited 2005 Oct. 4]]. Available at: http://www.bcbsnc.com/services/medical-policy/pdf/enhanced_external_counterpulsation_eecp.pdf .
- 55.CIGNA. External counterpulsation [report on the Internet] May 15, 2005. [[cited 2005 Oct. 15]]. CIGNA. Available at: http://www.cigna.com/health/provider/medical/procedural/coverage_positions/medical/mm_0058_coveragepositioncriteria_eecp.pdf .
- 56.The Regence Group. Medicine section - Enhanced external counterpulsation (EECP) October May. 2004. [[cited 2005 Oct. 5]]. Policy No. #66. Available at: http://www.regence.com/trgmedpol/medicine/med66.html .
- 57.Holubkov R, Kennard ED, Foris JM, Kelsey SF, Soran O, Williams DO, et al. Comparison of patients undergoing enhanced external counterpulsation and percutaneous coronary intervention for stable angina pectoris. Am J Cardiol. 2002;89(10):1182–6. doi: 10.1016/s0002-9149(02)02301-9. [DOI] [PubMed] [Google Scholar]
- 58.Emond M, Mock MB, Davis KB, Fisher LD, Holmes DR, Chaitman BR, et al. Long term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) registry. Circulation. 1994;90(6):2645–57. doi: 10.1161/01.cir.90.6.2645. [DOI] [PubMed] [Google Scholar]
- 59.Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10 year results from randomized trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344(8922):563–70. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 60.Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med. 1996;335(4):217–25. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 61.Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41(10):1761–8. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 62.Tartaglia J, Stenerson J Jr, Charney R, Ramasamy S, Fleishman BL, Gerardi P, et al. Exercise capability and myocardial perfusion in chronic angina patients treated with enhanced external counterpulsation. Clin Cardiol. 2003;26(6):287–90. doi: 10.1002/clc.4950260610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Werner D, Kropp J, Schellong S, Friedel C, Voigt JU, Ludwig J, et al. Practicability and limitations of enhanced external counterpulsation as an additional treatment for angina. Clin Cardiol. 2003;26(11):525–9. doi: 10.1002/clc.4960261110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bagger JP, Hall RJ, Koutroulis G, Nihoyannopoulos P. Effect of enhanced external counterpulsation on dobutamine-induced left ventricular wall motion abnormalities in severe chronic angina pectoris. Am J Cardiol. 2004;93(4):465–7. doi: 10.1016/j.amjcard.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 65.Lawson WE, Hui JC, Kennard ED, Barsness G, Kelsey SF. IEPR Investigators. Predictors of benefit in angina patients one year after completing enhanced external counterpulsation: initial responders to treatment versus nonresponders. Cardiology. 2005;103(4):201–6. doi: 10.1159/000085170. [DOI] [PubMed] [Google Scholar]
- 66.Michaels AD, Barsness GW, Soran O, Kelsey SF, Kennard ED, Hui JC, et al. Frequency and efficacy of repeat enhanced external counterpulsation for stable angina pectoris (from the International EECP Patient Registry) Am J Cardiol. 2005;95(3):394–7. doi: 10.1016/j.amjcard.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 67.Lawson WE, Kennard ED, Hui JC, Holubkov R, Kelsey SF IEPR Investigators. Analysis of baseline factors associated with reduction in chest pain in patients with angina pectoris treated by enhanced external counterpulsation. Am J Cardiol. 2003;92(4):439–43. doi: 10.1016/s0002-9149(03)00662-3. [DOI] [PubMed] [Google Scholar]
- 68.Feldman AM. Prospective evaluation of EECP in congestive heart failure (PEECH) trial [abstract] American College of Cardiology 54th Annual Scientific Session. 2005. Mar 6-9, 2005. Orlando, Florida.
- 69.Cleland JG, Coletta AP, Freemantle N, Velavan P, Tin L, Clark AL. Clinical trials update from the American College of Cardiology meeting: CARE-HF and the remission of heart failure, Women’s Health Study, TNT, COMPASS-HF, VERITAS, CANPAP, PEECH and PREMIER. Eur J Heart Fail. 2005;7(5):931–6. doi: 10.1016/j.ejheart.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Feldman AM, Silver MA, Francis GS, De Lame PA, Parmley WW. Treating heart failure with enhanced external counterpulsation (EECP): design of the Prospective Evaluation of EECP in Heart Failure (PEECH) trial. J Card Fail. 2005;11(3):240–5. doi: 10.1016/j.cardfail.2004.10.001. [DOI] [PubMed] [Google Scholar]


