Executive Summary
Objective
The objective of this analysis was to determine the strength of association between age, gender, ethnicity, family history of disease and refractive error and the risk of developing glaucoma or ARM?
Clinical Need
A routine eye exam serves a primary, secondary, and tertiary care role. In a primary care role, it allows contact with a doctor who can provide advice about eye care, which may reduce the incidence of eye disease and injury. In a secondary care role, it can via a case finding approach, diagnose persons with degenerative eye diseases such as glaucoma and or AMD, and lead to earlier treatment to slow the progression of the disease. Finally in a tertiary care role, it provides ongoing monitoring and treatment to those with diseases associated with vision loss.
Glaucoma is a progressive degenerative disease of the optic nerve, which causes gradual loss of peripheral (side) vision, and in advanced disease states loss of central vision. Blindness may results if glaucoma is not diagnosed and managed. The prevalence of primary open angle glaucoma (POAG) ranges from 1.1% to 3.0% in Western populations, and from 4.2% to 8.8% in populations of African descent. It is estimated up to 50% of people with glaucoma are aware that they have the disease. In Canada, glaucoma disease is the second leading cause of blindness in people aged 50 years and older. Tonometry, inspection of the optic disc and perimetry are used concurrently by physicians and optometrists to make the diagnosis of glaucoma. In general, the evidence shows that treating people with increased IOP only, increased IOP and clinical signs of early glaucoma or with normal-tension glaucoma can reduce the progression of disease.
Age-related maculopathy (ARM) is a degenerative disease of the macula, which is a part of the retina. Damage to the macula causes loss of central vision affecting the ability to read, recognize faces and to move about freely. ARM can be divided into an early- stage (early ARM) and a late-stage (AMD). AMD is the leading cause of blindness in developed countries. The prevalence of AMD increases with increasing age. It is estimated that 1% of people 55 years of age, 5% aged 75 to 84 years and 15% 80 years of age and older have AMD. ARM can be diagnosed during fundoscopy (ophthalmoscopy) which is a visual inspection of the retina by a physician or optometrist, or from a photograph of the retina. There is no cure or prevention for ARM. Likewise, there is currently no treatment to restore vision lost due to AMD. However, there are treatments to delay the progression of the disease and further loss of vision.
The Technology
A periodic oculo-visual assessment is defined “as an examination of the eye and vision system rendered primarily to determine if a patient has a simple refractive error (visual acuity assessment) including myopia, hypermetropia, presbyopia, anisometropia or astigmatism.” This service includes a history of the presenting complaint, past medical history, visual acuity examination, ocular mobility examination, slit lamp examination of the anterior segment, ophthalmoscopy, and tonometry (measurement of IOP) and is completed by either a physician or an optometrist.
Review Strategy
The Medical Advisory Secretariat conducted a computerized search of the literature in the following databases: OVID MEDLINE, MEDLINE, In-Process & Other Non-Indexed Citations, EMBASE, INAHTA and the Cochrane Library. The search was limited to English-language articles with human subjects, published from January 2000 to March 2006. In addition, a search was conducted for published guidelines, health technology assessments, and policy decisions. Bibliographies of references of relevant papers were searched for additional references that may have been missed in the computerized database search. Studies including participants 20 years and older, population-based prospective cohort studies, population-based cross-sectional studies when prospective cohort studies were unavailable or insufficient and studies determining and reporting the strength of association or risk- specific prevalence or incidence rates of either age, gender, ethnicity, refractive error or family history of disease and the risk of developing glaucoma or AMD were included in the review. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was used to summarize the overall quality of the body of evidence.
Summary of Findings
A total of 498 citations for the period January 2000 through February 2006 were retrieved and an additional 313 were identified when the search was expanded to include articles published between 1990 and 1999. An additional 6 articles were obtained from bibliographies of relevant articles. Of these, 36 articles were retrieved for further evaluation. Upon review, 1 meta-analysis and 15 population-based epidemiological studies were accepted for this review
Primary Open Angle Glaucoma
Age
Six cross-sectional studies and 1 prospective cohort study contributed data on the association between age and PAOG. From the data it can be concluded that the prevalence and 4-year incidence of POAG increases with increasing age. The odds of having POAG are statistically significantly greater for people 50 years of age and older relative to those 40 to 49 years of age. There is an estimated 7% per year incremental odds of having POAG in persons 40 years of age and older, and 10% per year in persons 49 years of age and older. POAG is undiagnosed in up to 50% of the population. The quality of the evidence is moderate.
Gender
Five cross-sectional studies evaluated the association between gender and POAG. Consistency in estimates is lacking among studies and because of this the association between gender and prevalent POAG is inconclusive. The quality of the evidence is very low.
Ethnicity
Only 1 cross-sectional study compared the prevalence rates of POAG between black and white participants. These data suggest that prevalent glaucoma is statistically significantly greater in a black population 50 years of age and older compared with a white population of similar age. There is an overall 4-fold increase in prevalent POAG in a black population compared with a white population. This increase may be due to a confounding variable not accounted for in the analysis. The quality of the evidence is low.
Refractive Error
Four cross-sectional studies assessed the association of myopia and POAG. These data suggest an association between myopia defined as a spherical equivalent of -1.00D or worse and prevalent POAG. However, there is inconsistency in results regarding the statistical significance of the association between myopia when defined as a spherical equivalent of -0.5D. The quality of the evidence is very low.
Family History of POAG
Three cross-sectional studies investigated the association between family history of glaucoma and prevalent POAG. These data suggest a 2.5 to 3.0 fold increase in the odds having POAG in persons with a family history (any first-degree relative) of POAG. The quality of the evidence is moderate.
Age-Related Maculopathy
Age
Four cohort studies evaluated the association between age and early ARM and AMD. After 55 years of age, the incidence of both early ARM and AMD increases with increasing age. Progression to AMD occurs in up to 12% of persons with early ARM. The quality of the evidence is low
Gender
Four cohort studies evaluated the association between gender and early ARM and AMD. Gender differences in incident early ARM and incident AMD are not supported from these data. The quality of the evidence is lows.
Ethnicity
One meta-analysis and 2 cross-sectional studies reported the ethnic-specific prevalence rates of ARM. The data suggests that the prevalence of early ARM is higher in a white population compared with a black population. The data suggest that the ethnic-specific differences in the prevalence of AMD remain inconclusive.
Refractive Error
Two cohort studies investigated the association between refractive error and the development of incident early ARM and AMD. The quality of the evidence is very low.
Family History
Two cross-sectional studies evaluated the association of family history and early ARM and AMD. Data from one study supports an association between a positive family history of AMD and having AMD. The results of the study indicate an almost 4-fold increase in the odds of any AMD in a person with a family history of AMD. The quality of the evidence, as based on the GRADE criteria is moderate.
Economic Analysis
The prevalence of glaucoma is estimated at 1 to 3% for a Caucasian population and 4.2 to 8.8% for a black population. The incidence of glaucoma is estimated at 0.5 to 2.5% per year in the literature. The percentage of people who go blind per year as a result of glaucoma is approximately 0.55%.
The total population of Ontarians aged 50 to 64 years is estimated at 2.6 million based on the April 2006 Ontario Ministry of Finance population estimates. The range of utilization for a major eye examination in 2006/07 for this age group is estimated at 567,690 to 669,125, were coverage for major eye exams extended to this age group. This would represent a net increase in utilization of approximately 440,116 to 541,551.
The percentage of Ontario population categorized as black and/or those with a family history of glaucoma was approximately 20%. Therefore, the estimated range of utilization for a major eye examination in 2006/07 for this sub-population is estimated at 113,538 - 138,727 (20% of the estimated range of utilization in total population of 50-64 year olds in Ontario), were coverage for major eye exams extended to this sub-group. This would represent a net increase in utilization of approximately 88,023 to 108,310 within this sub-group.
Costs
The total cost of a major eye examination by a physician is $42.15, as per the 2006 Schedule of Benefits for Physician Services.(1) The total difference between the treatments of early-stage versus late-stage glaucoma was estimated at $167. The total cost per recipient was estimated at $891/person.
Current Ontario Policy
As of November 1, 2004 persons between 20 years and 64 years of age are eligible for an insured eye examination once every year if they have any of the following medical conditions: diabetes mellitus type 1 or 2, glaucoma, cataract(s), retinal disease, amblyopia, visual field defects, corneal disease, or strabismus. Persons between 20 to 64 years of age who do not have diabetes mellitus, glaucoma, cataract(s), retinal disease, amblyopia, visual field defects, corneal disease, or strabismus may be eligible for an annual eye examination if they have a valid “request for major eye examination” form completed by a physician (other than that who completed the eye exam) or a nurse practitioner working in a collaborative practice. Persons 20-64 years of age who are in receipt of social assistance and who do not have one of the 8 medical conditions listed above are eligible to receive an eye exam once every 2 years as a non-OHIP government funded service. Persons 19 years of age or younger and 65 years of age or older may receive an insured eye exam once every year.
Considerations for Policy Development
As of July 17, 2006 there were 1,402 practicing optometrists in Ontario. As of December 31, 2005 there were 404 practicing ophthalmologists in Ontario. It is unknown how many third party payers now cover routine eye exams for person between the ages of 20 and 64 years of age in Ontario.
Objective
The objective of this analysis was to determine the strength of association between age, gender, ethnicity, family history of disease and refractive error and the risk of developing glaucoma or ARM?
Background
Clinical Need: Target Population and Condition
A routine eye exam serves a primary, secondary, and tertiary care role. In a primary care role, it allows contact with a doctor who can provide advice about eye care, which may reduce the incidence of eye disease and injury. In a secondary care role, it can via a case finding approach, diagnose persons with degenerative eye diseases such as glaucoma and or AMD, and lead to earlier treatment to slow the progression of the disease. Finally in a tertiary care role, it provides ongoing monitoring and treatment to those with diseases associated with vision loss.
Glaucoma
Glaucoma is a progressive degenerative disease of the optic nerve which causes gradual loss of peripheral (side) vision, and in advanced disease states loss of central vision.(6) There are two main types of glaucoma, primary open angle (POAG) and angle-closure glaucoma (ACG) of which POAG is the most common type. The earliest symptom of POAG is loss of peripheral vision, which can often go unnoticed. (7;8). Blindness may results if glaucoma is not diagnosed and managed (7). POAG is diagnosed by assessing characteristic degenerative changes in the optic disc and damage to visual fields (9)
Epidemiology
The prevalence of POAG ranges from 1.1% to 3.0% in Western populations, and from 4.2% to 8.8% in populations of African descent (10). The disease process can begin as early as 40 years of age and it is estimated that in developed countries, only 50% of people with glaucoma are aware they have the disease. (11) In Canada, glaucoma disease is the second leading cause of blindness in people aged 50 years and older, with POAG accounting for 90% of all cases.(12). It is estimated that 10% of people with POAG go blind in 1 eye and 4% go blind in both eyes. (Personal communication, clinical expert, July 6, 2006). The rate of progression to blindness has been difficult to determine with some studies reporting visual field loss rates of 2%-3% per year.(13). However, quantifying the lifetime risk of blindness for patients is difficult (14)
Some but not all people with POAG have increased intraocular pressure (IOP), which is the pressure of the fluid inside the eye. However, an estimated 25% to 50% of people with POAG have normal IOP. Those people with POAG whose IOP falls within the normal range (10-21mmHG) are said to have normal-tension or low-tension glaucoma (15). Risk factors for glaucoma include an increase in IOP, a family history of glaucoma, older age and being of African descent (9). Because the natural history of PAOG is not well-defined, some people with POAG who experience no disease progression will not experience a huge change in their vision, while others with more rapid progression may experience some loss of vision within 10 years of diagnosis. It is difficult to predict how fast the disease will progress. However, it is thought that people with higher IOP levels, poorer baseline visual field integrity and those who are older are at greater risk for rapid disease progression. Population studies indicate that approximately 50% of people with glaucoma have been diagnosed and treated (16). Prevalence and incidence are measures of burden of disease as is the patient perspectives on the impact of vision loss on functionality. (17) The degree of functional loss associated with glaucoma has not been adequately described. (6)
Diagnostic Tests for POAG
There are three methods of detecting POAG: tonometry, inspection of the optic disc and perimetry. All 3 tests are used concurrently to make the diagnosis of glaucoma.
Tonometry
Tonometry is a test that measures IOP. Because normal IOP has a diurnal fluctuation of as much as 5 mmHG if used alone to diagnose glaucoma, tonometry may not accurately detect the presence or absence of disease (7). Furthermore, as previously mentioned not everyone with glaucoma has an increased IOP. It is estimated that up to half of people with glaucoma have increases in IOP above normal in a random measurement. The positive predictive value for diagnosing glaucoma with tonometry has been reported to be 2% to 5% (7). IOP measurements above the upper limit of normal (21mmHG) have an estimated sensitivity of 47% and specificity of 92% for diagnosing POAG. The sensitivity of the classical cut-off for IOP of greater than 21mmHG is less than 50%. Furthermore, there is no IOP level where a reasonable balance of sensitivity and specificity is obtained.(6)
Inspection of the optic disc
Inspection or visualization of the optic disc called a fundoscopy (ophthalmoscopy) can be done by a physician or optometrist to determine if there is damage to the optic nerve. A dilated eye exam with direct ophthalmoscopy by an ophthalmologist has an estimated sensitivity of 59% and specificity of 73% for detecting glaucomatous-associated optic disc changes (15). Characteristic changes in the structure of the optic nerve have been used as diagnostic indicators of glaucoma. However, the ability of these parameters to accurately classify people into disease and non-disease states is relatively poor. Similar to tonometry, there is no cut-point that achieves an adequate sensitivity and specificity balance.
Perimetry
Perimetry is a test that evaluates the visual fields. The integrity of the visual fields determines where a person perceives visual stimuli. It is estimated that up to 30% to 50% of the optic nerve fibres must be lost before a classic glaucomatous visual field defect occurs with any consistency. Perimetry has been used as a screening test for glaucoma however the equipment is costly and not generally available to family physicians. The specificity and sensitivity of perimetry will vary depending on the method used as well as the cut-off point for defining visual field defects and the reference standard employed (15). To establish the presence of visual field defects, several visual field measurements are needed (9). Perimetry can be done by an automated instrument (Humphrey’s automated perimitry) or using a Goldmann perimetry device and a perimetrist (7). Measurement of visual fields can be difficult and the reliability of a single measurement may be low. Currently there is no defined standard of progression of visual field defects (9).
Treatments for POAG
Treatment for POAG is aimed at reducing IOP with topical agents often used as first-line therapy including pilocarpine, beta-adrenergic blockers such as timolol and betaxolol and systemic agents such as acetazolamide.(18). Surgery (laser trabeculoplasty or trabeculectomy) has also been used, usually when medical treatment has failed (18). In general, the evidence shows that treating people with increased IOP (16), increased IOP and clinical signs of early glaucoma (19) and normal-tension glaucoma (20) can reduce the progression of disease.
The Early Manifest Glaucoma Trial (19) evaluated the effectiveness of reducing IOP in patients 50 to 80 years of age with newly detected and previously untreated open-angle glaucoma (POAG, normal-tension glaucoma or exfoliation glaucoma) Patients were randomized to receive either a full 360 degree trabeculoplasty plus betaxolol hydrochloride eye drops at a dose of 5mg/ml twice daily (treatment group) or no treatment (control group). After a median follow-up time of 6 years, 58/129 (45%) of the treated patients compared with 78/126 (62%) of the controls showed disease progression (risk difference [RD], -17%, 95% confidence interval [CI], -.29, -.05, number-needed-to- treat [NNT] = 5.9) which was defined by progression of either glaucomatous visual field defects or optic disc cupping. The median time to progression was 48 months in the control group and 66 months in the treatment group (statistical significance not reported).
The Ocular Hypertension Treatment Study (16) determined the safety and efficacy of topical ocular hypotension medication (bextaxolol hydrochloride eye drops) in delaying or preventing the onset of POAG (evidenced by visual field defects or optic disc deterioration) in people 40 to -80 years of age with increased IOP (≥21mmHg) and no evidence of glaucomatous damage. At 60 months follow- up, the cumulative probability of developing POAG was 4.4% in the treatment group compared with 9.5% in the control (untreated) group (hazard ratio, 0.40; 95% CI, 0.27-0.59). The authors concluded that treatment with topical ocular hypotensive medication effectively delays the onset of POAG in people with increased IOP and that treatment should be considered for persons with ocular hypertension who are at moderate or high risk for developing POAG (16).
The Collaborative Normal-Tension Glaucoma Study (20) group determined the effect of treatment on disease progression in persons with normal-tension glaucoma (IOP ≤20mmHg). Disease progression was determined by glaucomatous optic disc progression or visual field loss. One eye of each participant was randomized to either no treatment (control) or treatment (topical medication or surgical treatment) in order to lower IOP by 30% from baseline values. Using the protocol definition of progression (deepening of an existing scotoma, the expansion of an existing scotoma, a new or expanded threat to fixation, or a fresh scotoma in a previously normal area of the visual field) and baseline values in the treatment group that were obtained after a 30% reduction in IOP had been achieved, 28/79 (35%) eyes in the control group showed disease progression compared with 7/61 (11.5%) of the treatment group eyes (P<.0001 survival curve analysis). Cataracts developed in 11 (14%) control group eyes and 23 (38%) treatment group (P=.0011) eyes. Of the 23 cataracts that developed in the treatment group 16 (26%) had been treated surgically and 7 (11%) received medical treatment. In a subsequent intention-to-treat analysis, where the outcomes of treatment managements were compared to the baseline values obtained at the time of randomization (before 30% reduction in IOP was achieved), 31/79 (39%) eyes in the control group progressed compared with 22/66 (33%) eyes in the treatment group (P=.21). However, the power to detect a difference in these observed rates with a 5% (two-sided) level of statistical significance was 11%. (9;20). In a subsequent analysis which censored the data from eyes that developed cataracts, the benefit of IOP reduction on the progression of visual field defects was statistically significant (P=.0018). This suggests that if IOP is reduced with treatments that do not cause adverse visual effects such as cataracts, a reduction in the progression of disease can be achieved in patients with normal-tension glaucoma. (20)
The major harms to glaucoma treatment include an increased risk of cataract formation associated with surgical intervention (15). The magnitude of any treatment in reducing impairment in vision-related function and quality of life is uncertain (9;15).
Age-Related Maculopathy
Age-related maculopathy (ARM) is a degenerative disease of a specific part of the retina called the macula (21). The macula is the central 25mm2 of the retina that is responsible for visual acuity (sharpness of vision) and central vision (seeing objects straight ahead) (21). Damage to the macula causes loss of central vision affecting the ability to read, recognize faces and to move about freely (21). Degeneration of the macula is thought to occur because of a breakdown of the retinal pigment epithelium (RPE), which is the lining underneath the retina responsible for supplying the retina with oxygen and nutrients. ARM can be divided into an early- stage (early ARM) and a late-stage ARM (referred to as AMD) (21).
In early ARM, yellow spots under the retina called drusen are seen by a doctor during opthalmosocopy (22). Drusen are thought to be abnormal extracelluar deposits that range in size, shape and consistency from small well-defined hard drusen, to large ill-defined soft drusen. (23) Drusen may also be crystalline or calcific in nature. The size and shape of drusen determine in part how far the disease has progressed (24). In general, vision loss is not usually associated with early ARM. However, it is estimated that 0.5% to 50% of people with early ARM will develop AMD within 5 years (24).
There are 2 main types of AMD: dry and wet (7;22). Dry AMD, also called geographic atrophy, atrophic or non-neovascular AMD is characterized by well-defined areas of RPE atrophy (thinning out) (21;22). The most common symptom of dry AMD is blurred central vision which gradually worsens (25). If only one eye is affected, symptoms may not be noticeable (25). In wet AMD, also called exudative, neovascular (meaning forming new blood vessels) or disciform macular degeneration, abnormal blood vessels begin to grow and leak underneath the retina causing scarring and distortion of the retina. This type of AMD can lead to rapid and severe vision loss. A common symptom of wet AMD is that straight lines will appear wavy. Central vision can deteriorate rapidly in wet AMD (25).
Dry AMD comprises approximately 90% of all AMD cases. However, while less frequent, the wet form of AMD can be more devastating because of the risk of severe and sudden vision loss due to retinal detachment (7). Because of this, wet AMD accounts for the majority of cases of blindness caused by AMD (26).
Epidemiology
AMD is the leading cause of blindness in developed countries (21). The prevalence of AMD is estimated at 1% in people who are 55 years of age, increasing with increasing age to 5% in person aged 75 to 84 years, (21) and 15% in those 80 years of age and older (7). The prevalence of AMD is estimated to be higher in Caucasians than among people of African descent. Some of the other reported risk factors include age, family history of AMD, smoking, hypertension, atherosclerosis (hardening of the arteries), obesity, and chronic infection (24).
Diagnostic Test for ARM
ARM can be diagnosed during fundoscopy (ophthalmoscopy) which is a visual inspection of the retina by a physician or optometrist, or from a photograph of the retina (fundus photography) (21). During this examination, pale yellow spots called drusen can be seen many years before a person’s vision is affected. Detecting the presence of drusen during an eye exam can help identify people with this eye disease. The size, number and consistency (hard, soft, and crystalline) of the drusen as well as changes in the retina (loss and/or hypertrophy of RPE, retinal thickening and/or bleeding) indicate the advancement of the disease. Advanced disease is associated with severe vision loss. Fluorscein angiography can evaluate the vascularity (blood supply) of the retina to determine if the blood vessels are leaking (wet AMD). The Amsler chart (Figures 1 and 2) has also been used for patient self-monitoring. It is a 10x10 cm grid with twenty 5-mm squares drawn with white lines on a black background (opposite to figures 1 and 2). The grid is viewed periodically to check for metamorphopsia, or distortion of straight lines, which is one of the earliest symptoms associated with wet AMD.
Figure 1: Normal Vision.
Reproduced from the Macular Degeneration Network: http://www.macular-degeneration.org/WetDry/WetamslerMain.html
Figure 2: Distortion of Straight Lines.
Amsler Grid
Treatments
There is no cure or prevention for ARM. Likewise, there is currently no treatment to restore vision lost due to AMD. However, there are treatments to delay the progression of the disease and further loss of vision.
Antioxidants
The Age-Related Eye Disease Study (AREDS), a randomized controlled clinical trial (RCT), evaluated the efficacy of antioxidants compared with placebo in 3640 people aged 55 to 80 years of age who had either no AMD, mild, moderate or advanced AMD. Study participants were randomized to 1 of 4 treatment groups: antioxidant group (500 mg vitamin C, 400IU vitamin E, 15mg of beta-carotene); zinc group (80mgs of zinc oxide and 2 mg cupric (copper) oxide; antioxidants plus zinc group; or placebo group and followed for an average of 6.3 years. The outcome measure was the proportion of eyes developing advanced AMD in 5 years. Results showed that those people without AMD or with mild AMD did not benefit from antioxidant and/or zinc treatment. Those with moderate and advanced AMD however did benefit from treatment, showing a lower risk of progression to advanced AMD and preservation of visual acuity compared with people treated with a placebo at 7 years follow-up. People with the highest risk of AMD progression who were treated with antioxidants plus zinc had an odds ratio (OR) of 0.66 [99% CI, 0.47-0.91] and an absolute risk reduction (ARR) of 0.06 for the proportion of eyes developing advanced AMD in 5 years, equal to a NNT of 17. Those in the zinc treatment group had an OR of 0.71 [99% CI, 0.52-0.99] and an ARR of 0.36, equal to an NNT of 27. However, those treated with antioxidants only had an OR of 0.76 [99% OR CI, 0.55-1.05] which was not statistically significant (24). The authors concluded that all individuals older than 55 years of age should have a dilated fundoscopy examination of both eyes to determine if they have moderate AMD and if found should consider treatment with vitamin E, C, and beta-carotene plus zinc. (24). Adverse effects of treatment included a skin color change associated with beta-carotene and an increased risk of hospitalization due to genitourinary disease in men (26). Beta-carotene supplementation has also been connected to an increased risk of lung cancer in patients who smoke. (Numbers needed to harm [NNH] to produce 1 case of lung cancer in 3 years, 1190, and 294 in 6 years) (24). In other studies, high-dose vitamin E (≥ 400 IU/d) has been associated with a significant increase in the rate of heart failure in people with heart disease or diabetes (RR 1.13 [95% CI, 1.01-1.26] (24).
Laser Photocoagulation
Thermal laser photocoagulation has been used to coagulate the leaking capillaries of wet AMD. Results of RCTs indicate that in approximately 15% of the population with wet AMD (those with well-defined choroidal neovascularization [CNV]), treatment with thermal laser photocoagulation significantly reduced the relative risk (RR) of severe vision loss over 5 years (24). CNV can recur within 2 years of treatment in approximately 50% of patients treated (24). In 1995 the Canadian Task Force on the Periodic Health Examination stated that “the benefits of photocoagulation offer a rational for early detection and observation of AMD” (7). Photocoagualation will not restore vision already lost (24). Likewise, if retinal detachment has occurred photocoagulation will not be effective (7). Photocoagulation treatment is most beneficial for patients with a visual acuity of 20/60 or better and re-intervention may be required as some vessels may reopen(7). The earlier a person seeks treatment after the onset of symptoms, the greater chance of treating AMD (7). It is estimated that up to 50% of patients with wet AMD may benefit from treatment if the condition is identified early enough (7). Laser photocoagulation therapy is not indicated if the lesion is located directly under the fovea (centre of the macula) (personal communication with clinical expert Sept. 20, 2006).
Photodynamic Therapy
Photodynamic therapy has been investigated as a treatment for wet AMD. It involves the intravenous injection (into a vein) of photosensitive dye (verteporfin [Visudyne]) followed by the use of a laser to activate the photosensitive dye. The activated dye clots the leaking blood vessels underneath the retina (24). Unlike laser photocoagulation, photodynamic therapy targets the deleterious new growth of blood vessels while avoiding damage to the retina (26).
Pharmacological Treatment
Vascular endothelial growth factor (VEGF) inhibitors block the neovascularization process of AMD (24). Pegaptanib (Macugen) is the first VEGF inhibitor drug for the treatment of wet AMD to be approved by the United States Food and Drug Administration (FDA). It is also approved by Health Canada. (27). The long-term complications associated with chronic treatment with VEGF inhibitors are unknown (24).
Biological Treatment
On June 30, 2006, the FDA announced that it had approved Lucentis (ranibizumab injection), a new biologic treatment for wet AMD. Lucentis is injected into the eye once a month. It is the first treatment which can maintain the vision of more than 90% of patients with wet AMD (28;29). Lucentis contains an active substance which until now has never been approved for marketing in any form in the United States. (30) Intravitreal injections of Avastin (Bevacizumab), an anticancer drug, have been shown anecdotally to reverse vision loss in some patients with wet AMD. It is presently being used off label for this indication (31).
Visual Acuity
Visually Acuity is the term used to describe how clearly or sharply a person sees. Many people are familiar with the standard visual acuity measurement of 20/20 (meaning 20feet/20feet) or in metric 6/6 (meaning 6 meters /6meters), which is a measure of a person’s visual acuity; the larger the denominator the worse a person’s vision. Therefore, someone with 20/30 (6/9) vision cannot see as well as someone with 20/20 (6/6) vision. Visual acuity can be improved by wearing corrective lenses (glasses or contact lenses). Visual impairment is defined as a visual acuity of less than 20/60 (6/18) in the better eye with the best correction. Legal blindness is defined by visual acuity as well and is different in different jurisdictions. In Canada, legal blindness is defined as a visual acuity of less than 20/200 (6/60). This means that if a person with 20/20 vision can read a sign 30 feet away, a person with 20/200 vision will need to be 3 feet away from the sign to read it. Put another way, a person with 20/200 vision will see at 20 feet what a person with 20/20 vision will see at 200 feet. Table 1 reports the classification of severity of visual impairment recommended by the World Health Organization Study Group on the Prevention of Blindness, Geneva, 1972. Low vision comprises categories 1 and 2. Blindness comprises categories 3, 4 and 5 as well as category 9, unqualified visual loss.
Table 1: *Categories of Visual Impairment defined by Visual Acuity.
| Category of Visual Impairment |
Visual Acuity with Best Possible Correction | |
|---|---|---|
| Maximum less than | Minimum equal to or better than | |
| 1 | 20/70 | 20/200 |
| 2 | 20/200 | 20/400 |
| 3 | 20/400 | 20/1200 |
| 4 | 20/1200 | Light perception |
| 9 | undetermined | |
Source: International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Canada (ICD-10-CA)
Vision Standards for Driving in Ontario
On May 29, 2005, Ontario introduced new vision standards for all license classes. The new standards are set out in Ontario Regulation 340/94 of the Highway Traffic Act (32). For class G and M drivers’ licenses which includes passenger-carrying vehicles and motorcycles, visual acuity not worse than 20/50 with both eyes open and examined together, and a horizontal visual field of 120 continuous degrees along the horizontal meridian and 15 continuous degrees above and below fixation, with both eyes open and examined together, is required. For commercial-class drivers licenses (classes A, B, C, D, E, F) which may include but is not limited to a tractor trailer, school bus, dump truck, ambulance, or fire truck, a visual acuity not poorer than 20/30 with both eyes open and examined together, with the worse eye no poorer than 20/100 and a horizontal visual field of 150 continuous degrees along the horizontal meridian and 20 continuous degrees above and below fixation, is required.
Technology Being Reviewed
As described in the Schedule of Benefits for Physician Services (1), a periodic oculo-visual assessment is defined “as an examination of the eye and vision system rendered primarily to determine if a patient has a simple refractive error (visual acuity assessment) including myopia, hypermetropia, presbyopia, anisometropia or astigmatism.” (3) This service includes a history of the presenting complaint, past medical history, visual acuity examination, ocular mobility examination, slit lamp examination of the anterior segment, ophthalmoscopy, and tonometry (measurement of IOP).
Literature Review
Research Question
What is the strength of association between age, gender, ethnicity, family history of disease and refractive error and the risk of developing glaucoma or ARM?
Methods
The Medical Advisory Secretariat conducted a computerized search of the literature in the following databases:
OVID MEDLINE
MEDLINE In-Process & Other Non-Indexed Citations
EMBASE
INAHTA
Cochrane Library
The search was limited to English-language articles with human subjects, published from January 2000 to March 2006. Letters, editorial, comments, case reports, and nonsystematic reviews were excluded. The literature search strategy is available in Appendix 1.
In addition, a search was conducted for published guidelines, health technology assessments, and policy decisions. Bibliographies of references of relevant papers were searched for additional references that may have been missed in the computerized database search.
The criteria for selecting studies for this review were as follows:
Inclusion Criteria
Studies including participants 20 years and older
Population-based prospective cohort studies
Population-based cross-sectional studies if data from prospective cohort studies were not available or insufficient
Studies determining and reporting the strength of association (OR, RR) or risk- specific prevalence or incidence rates of at least one of the following: age, gender, ethnicity, refractive error and family history of disease and the risk of developing glaucoma or AMD
Exclusion criteria
Non-English language studies
Duplicate publications
Studies that did not examine the outcome(s) of interest
Studies with a participation rate less than 70%
Clinical Outcomes
Strength of association (OR, RR) between glaucoma and:
Age,
Gender
Ethnicity
Family history of glaucoma
Refraction error
Strength of association (OR, RR) between early ARM and AMD and:
Age
Gender
Ethnicity
Family History of AMD
Refraction error
Study Eligibility
One reviewer not blinded to author, institution, and journal of publication evaluated the eligibility of the citations retrieved from the literature search. Articles were excluded based on information reported in the title and abstract, and the full document of potentially relevant articles was retrieved for further assessment. Where the relevance of the article was inconclusive from the abstract or title, the full publication was retrieved for further assessment.
Data Extraction
One reviewer extracted data from the included studies. Information extracted included response rate, sampling frame, sampling method, sample size, population demographic characteristics, study measurements, outcome measures and reliability assessments.
Assessment of Study Methodological Quality
One reviewer evaluated the internal validity of the primary studies.
Summarizing the Quality of the Body of Evidence
Quality of Evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (33;34) was used to summarize the overall quality of the body of evidence (defined as 1 or more studies). This system has 4 levels of quality: very low, low, moderate, and high. The criteria for assigning the GRADE level are available in Appendix 2.
Summary of Medical Advisory Secretariat Review
A total of 498 citations for the period January 2000 through February 2006 were retrieved using the literature search strategy outlined in Appendix 1, and an additional 313 were identified when the search was expanded to include articles published between 1990-1999. An additional 6 articles were obtained from bibliographies of relevant articles. Of these, 36 articles were retrieved for further evaluation. Upon review, 1 meta-analysis and 15 population-based epidemiological studies (cross-sectional and prospective cohort studies) were accepted for this review.
Table 2: Quality of Evidence of Included Studies.
| Study Design | Level of Evidence |
Number of Eligible Studies |
|---|---|---|
| Large RCT,* systematic reviews of RCT | 1 | |
| Large RCT unpublished but reported to an international scientific meeting | 1(g)† | |
| Small RCT | 2 | |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3a | |
| Non-RCT with historical controls | 3b | |
| Non-RCT presented at international conference | 3(g) | |
| Surveillance (database or register) | 4a | |
Case series (multisite)
|
4b | 1 |
Case series (single site)
|
4c | 5 10 |
| Retrospective review, modeling | 4d | |
| Case series presented at international conference | 4(g) |
RCT refers to randomized controlled trial.
g indicates grey literature.
Glaucoma
Six population-based cross-sectional studies determining the prevalence of and risk variables for POAG met the inclusion and exclusion criteria set out for this review. One of these, the Barbados Eye Study (35) conducted a 4-year prospective cohort study on a sub-set of glaucoma-free participants to determine the incidence of and risk factors for POAG. All studies evaluated risk factors for POAG using a multivariate analysis. The participation rate was greater than 80% of eligible participants in all studies except for the Baltimore Eye Study, which was 79%. The characteristics of each study are reported in Table 3.
Table 3: Study Characteristics.
| Study Name | Design | Years Study Conducted |
Sample Size * (Participation rate %) |
Population | Mean Age (SD), years [range] |
|---|---|---|---|---|---|
| Chennai Glaucoma Study Southern India (36) |
Cross-sectional | 2001-2003 | 3934/4800 (82%) |
≥ 40 years East Indian |
53.8 (10.7) |
| Aravind Comprehensive Eye survey Southern India (5) |
Cross-sectional | 1995-1997 | 5150/5539 (93%) |
≥40 years East Indian |
Median 51.0 [40-90] |
| Blue Mountains Study Australia(37-39) |
Cross-sectional | 1992-1994 | 3654/4433 (82.4%) |
≥49 years Predominately Caucasian |
Males: 75.9 [49-97] Females: 65.9 [52-96] |
| Barbados Eye Study Barbados, West Indies(35;40;41) |
Prospective Cohort |
1992-1997 (4- year followup of cross sectional sample) |
†3427/4040 (85%) |
40-84 years 93.1% Black 4.0% mixed race 2.9% Caucasian |
57.5 (11.5) |
| Cross-sectional | 1988-1992 |
‡4123/4314 (95.5%) |
No POAG: 57.8 (11.8) POAG: 69.2 (10.4) |
||
| Baltimore Eye Study USA(42;43) |
Cross-sectional | 1985-1988 | 5308/6702 (79.2%) |
≥40 years 45% Black 55% Caucasian |
|
| Beaver Dam Study USA (44;45) |
Cross sectional | 1987-1990 | 4926/5924 (83.1%) |
43-84 years 99% Caucasian |
60.6 (11.3) |
Participation rate = number participated/ number eligible
4040= surviving cohort
Black population in study sample as a fraction of total black population in study sample
Internal Validity of Studies
Three studies including the Chennai, Aravind, and Baltimore studies reported using a probability sampling strategy (Table 4). Specifically, the Baltimore study used a stratified cluster randomization to obtain equal numbers of black and white participants. The Blue Mountains and Beaver Dam studies each used a total sample of all people living in the area of interest identified by a census. Sample size calculations were reported for the Chennai Glaucoma study only and were based on a prevalence estimate of glaucoma. Protocol standardized measurements of glaucoma were used in all studies. Inter- and intra-rater reliability assessments were determined and reported for the Chennai, Blue Mountains and Baltimore studies. The Beaver Dam study reported a high consensus rating on visual field tests. The Aravind study reported methods to reduce measurement error through ongoing standardization throughout the course of the study however, actual measures of reliability were not reported.
Table 4: Internal Validity of Studies.
| Study | Sampling Method | Sample Size Calculation a priori | Standardized measurements | Reliability Assessments |
|---|---|---|---|---|
| Chennai Glaucoma Study Southern India (36) |
Multistage sampling | Yes | Yes | Inter-rater reliability |
| Aravind Comprehensive Eye survey Southern India (5) |
3 stage cluster sampling |
Not reported | Yes | Study ophthalmologists were standardized to each other and to a senior ophthalmologist considered the reference standard. Standardization was repeated during the study. |
| Blue Mountains Study Australia(37-39) |
census | Not reported | Yes | Inter- and Intra-rater reliability |
| Barbados Eye Study Barbados, West Indies(35;40;41) |
Simple Random Sampling |
Not reported | Yes | Inter- and intra-rater reliability |
| Baltimore Eye Study USA(42;43) |
Stratified Multistage sampling |
Not reported | Yes | Intra-rater reliability |
| Beaver Dam Study USA (44;45) |
census | Not reported | Yes | Consensus of ¾ raters on 94.8% of visual field tests. |
Diagnostic Methods
The diagnostic methods and definition of POAG for each study are reported in Table 5. All studies used structural damage to the optic disc and functional damage measured by visual field loss to diagnose definite glaucoma. Only the Beaver Dam study used IOP as an additional diagnostic criterion for definite glaucoma. While category 3 of the International Society of Geographical and Epidemiologic Ophthalmology classification used in the Chennai Glaucoma incorporated IOP as a diagnostic criterion, only 1 case of glaucoma was diagnosed based on this criterion. A cup-to-disc ratio (CDR) of at least 0.7 was specified as structural damage in all studies.
Table 5: Diagnostic Criteria for POAG used in Studies.
| Study Name, | Diagnostic criteria for POAG | Definition of POAG |
|---|---|---|
| Chennai Glaucoma Study Southern India (36) |
Diagnosed using the 3 categories of the International Society of Geographical and Epidemiologic Ophthalmology classification. Category 1: structural and functional evidence. *CDR or CDR asymmetry ≥97.5th percentile of the normal population or a neuroretinal rim width reduced to ≤ 0.1 CDR (between 11-10 or 5-7 o’clock) with a definite visual field defect on automated perimetry consistent with glaucoma Category 2: advanced structural damage + unproven visual field loss for cases where visual fields could not be assessed or had a CDR and CDR asymmetry ≥ 99.5th percentile for the normal population. Category 3: Optic disc not seen; visual field testing impossible. Visual acuity <3/60 and IOP ≥ 99.5th percentile of normal population or visual acuity <3/60 and the eye shows evidence of glaucoma filtering surgery, or medical record were available confirming glaucomatous visual morbidity. |
Definite POAG: 1 of the 3 categories + an open and normal appearing angle on gonioscopy. (Only 1 case was diagnosed based on IOP criterion in this study) |
| Aravind Comprehensive Eye survey Southern India (5) |
Optic nerve damage: †VCDR>0.8 or a narrowest neuro-retinal rim width <0.2 (including classic notching) or asymmetry >0.2 between eyes coupled with a visual field defect on automated perimetry in the matching location In cases where visual fields were not available: Presence of significant optic disc excavation compatible with glaucoma, or end-stage glaucoma with severe central vision loss, or total optic disc cupping |
Evidence of glaucomatous optic nerve damage which included either glaucomatous change in the appearance of the optic nerve head and/or nerve fiber bundle pattern perimetric defects typical of glaucomatous damage. Definition did not rely on IOP |
| Blue Mountains Study Australia (37-39) |
Matching optic disc cupping with rim thinning (CDR ≥0.7 or cup-disc asymmetry ≥0.3) and characteristic visual field loss on automated perimetry. | Optic disc defects and visual field loss on automated perimetry. Definition did not rely on IOP. |
| Barbados Eye Study Barbados, West Indies (35;40;41) |
Optic disc criteria: At least 2 signs of optic disc damage including either a HCDR or VCDR of ≥ 0.7, narrowest remaining neuroretinal rim of 0.1 disc diameters or less, notching asymmetry in CDR between eyes of > 0.2 and disc hemorrhages. | Criteria for definite PAOG were based on the presence of both visual field defects (Humphrey automated perimetery) and optic disc damage in at least 1 eye. Definition did not rely on IOP |
| Baltimore Eye Study USA (42;43) |
Glaucomatous optic nerve damage and visual field defects on automated perimetry or Goldmann perimetry if automated was not possible in the presence of normal angles and in the absence of other likely causes. 97% of all cases were diagnosed using the following criteria: |
A final classification of definite, probable and uncertain-unknown was made after considering glaucomatous optic nerve damage based on appearance of visual fields, optic disc, and nerve fiber layer, angle grade based on slit-lamp examination and gonioscopy. |
| 1. ≥ 2 abnormal visual fields with excellent congruence between fields | Definition did not rely on IOP | |
| 2. End-stage disease with visual acuity ≤ 20/200 and 100% cupping | ||
| 3. ≥ 1 abnormal visual field with some but not perfect confluence between fields and a CDR ≥ 0.8 or a difference between the 2 eyes of ≥ 0.3/ | ||
| 4. ≥ 1 abnormal visual field with some but not perfect congruence between fields | ||
| 5. 1 visual field performed with typical field defects. | ||
| 6. 1 visual field either typically abnormal or compatible with glaucoma and cupping or nerve-fiber layer loss | ||
| 7. asymmetric cupping with a difference between the 2 eyes of ≥ 0.4 | ||
| Beaver Dam Study USA (44;45) |
1. Visual field defects with suprathreshold static perimetry using multiple stimulus patterns. | Definite glaucoma: having at least 2 of the first 3 criteria. |
| 2. CDR ≥ 0.8 or difference in CDR of 0.2 or more in involved eye. | Probable glaucoma: criterion 4 and fewer than 2 of the other 3 criteria in the same eye. | |
| 3. IOP ≥ 22 mmHg | ||
| 4. History of taking drops or having surgery for glaucoma |
CDR=cup to disc ratio,
VCDR=vertical cup to disc ratio
Prevalence and Incidence
Crude prevalence rates for POAG ranged between 1.1% and 7.0% in the 6 cross-sectional studies. (Table 6) Higher prevalence rates were seen in populations of African descent compared with Caucasians. The Barbados Eye Study, a study in a predominately black population, reported a prevalence rate of 7.0% while the Baltimore Eye Study reported a prevalence of 4.2% in the sub-set of African- Americans participating in the study which was greater than that for the sub-set of Caucasians (1.1%) in the same study. Prevalence was similar amongst studies with Caucasian populations and ranged from 1.1%-2.4%. Of note, at least 50% and up to 98% of prevalent cases did not know they had PAOG before participating in the study (rate of undiagnosed prevalent POAG). The 4-year incidence of POAG in the Barbados Eye Study was 2.2%.
Table 6: Crude Prevalence and Incidence of POAG (definite POAG where otherwise indicated) in Studies.
| Study | Diagnostic Methods |
Number of Prevalent cases in study sample |
Crude Prevalence of POAG % (95% CI) |
Rate of undiagnosed prevalent POAG (%) |
Number of Incidence Cases in study sample |
Crude Incidence of POAG % (95% CI) |
|---|---|---|---|---|---|---|
| Chennai Glaucoma Study Southern India (36) |
*I, VFD, T | †64/3924 | 1.6 (1.4-1.8) | 98.5 | not applicable (N/A) | N/A |
| Aravind Comprehensive Eye survey Southern India (5) |
I, VFD | 64/5150 | 1.2 (0.9-1.5) | 93 | N/A | N/A |
| Blue Mountains Study Australia(37-39) |
I, VFD | 108/3654 | 2.4 (not reported) ‡3.0 (2.5-3.6) |
51 | N/A | N/A |
| Barbados Eye Study Barbados, West Indies(35;40;41) |
I, VFD | 302/4314 | 7.0 (not reported) | 51 | 67/2989 | 2.2 (1.7-2.8) |
| Baltimore Eye Study USA (42;43) |
I, VFD | 100/2395 32/2913 |
Black population: 4.2 (3.4-5.0) Caucasian population: 1.1 (0.7-1.6) |
50 | ||
| Beaver Dam Study USA (44;45) |
I, VFD, T | 104/4926 | 2.1 (not reported) | Not reported |
I= inspection of optic disc; VFD = visual field defects by perimetry; T = Tonometry
1 case diagnosed with IOP
Includes definite and probable POAG
Multivariate Statistics
A limitation of this review is that both cross-sectional and prospective cohort studies that completed multivariate statistical analysis adjusted their models for different risk factors. This could account for variability in the magnitude of the strength of association point estimate (OR or RR) amongst studies for similar risk factors. Because of this, consistency in the direction, statistical significance, prevalence and incidence of POAG amongst studies was important in determining significant associations. A description of the variables used in each multivariate model from each study is examined below.
Cross-Sectional Design
The Chennai Glaucoma Study(36) completed a multivariable logistic regression analysis adjusting for age, sex, IOP (mmHg), central corneal thickness (µm), Myopia (yes/no) and hypertension (yes/no).
The Aravind Comprehensive Eye Survey (5) completed a multivariate analysis adjusting for age, sex, diabetes (yes/no), hypertension (yes/no), pseudoexfoliation (yes/no), and myopia (none, mild, moderate or severe).
The Blue Mountains Eye Study (37-39) completed a multivariate logistic regression adjusting for age (per year), IOP (maximum of both eyes mmHg), family history (reported history of glaucoma in parents, siblings and children), myopia (≤-1.0D), pseudoexfoliation, diabetes (yes/no) and hypertension (yes/no). The R2 for the model was reported to be 0.22.
The Barbados Eye Study (35;40;41) completed a multivariate logistic regression adjusting for age, sex, body mass index, cataract history, IOP (>21mmHg), family history and interaction of family history with gender (Leske 1995).
The Baltimore Eye Study (42;43) completed a multivariate logistic regression adjusting for age and race on the association of a positive family history (any first-degree relative including parents, full siblings and children with POAG) with POAG. (Tielsch 1994)
The Beaver Dam Study (44;45) completed a multivariate logistic regression analysis adjusting the model for education, hypertension and diabetes. (Wong 2003)
Prospective Cohort Design: Statistics
The Barbados Eye Study (35) did not complete a multivariate analysis of risk factors but presented age-specific incidence rates of glaucoma.
Results
Age and POAG
The prevalence rates of POAG were used to determine the association between age and prevalent POAG in 6 cross-sectional studies, and age and incident POAG in 1 prospective cohort study. In general, there was an increasing risk of prevalent and incident glaucoma with increasing age. Participants age 40 to 49 were used as a reference group for multivariate analyses.
Cross-Sectional Studies
The main objective of the cross-sectional studies was to determine the prevalence of POAG. Both the Chennai Glaucoma Study and the Aravind Eye Study reported that the odds of prevalent POAG increased with increasing age in a population of Southern India (Table 7). Likewise, in both studies the odds of prevalent POAG was statistically significantly greater for people 50 years of age and older relative to those 40 to 49 (Table 7). In the Blue Mountains Study, (39) reported a statistically significant 10% per year incremental odds of prevalent POAG in a predominately white population, and in the predominately black population of the Barbados Eye Study, Leske et al. (41) reported a 7% per year incremental odds. The small increase per incremental year of age reported in the Blue Mountains Eye Study compared to the Barbados Eye Study might be explained by the slightly older population (people ≥ 49 yrs.) in the former study compared with the latter (people age 40-84). In the Beaver Dam Eye Study Klein et al. reported that the odds of having prevalent POAG increased 74% for every 10 year incremental age increase, which seems consistent with the per-year incremental age increase reported in the Barbados Eye Study (7%/year) but slightly lower than that reported in the Blue Mountains Eye Study (10%/year). Again, this discrepancy might be explained by the slightly younger population of the Beaver Dam Eye Study, 43 to 84 years of age, compared to that of the Blue Mountains Eye Study (≥ 49 years).
Table 7: Association of Age and Prevalent POAG in Cross-Sectional Studies.
| Age (Years) | Odds Ratio (95% CI) | ||||
|---|---|---|---|---|---|
|
*Chennai Glaucoma Study (36) ≥ 40 years |
†Aravind Eye Study (5) ≥ 40 years |
‡Blue Mountains Study (39) ≥49 years |
§Barbados Eye Study (41) 40-84 years |
||Beaver Dam Eye Study (44) 43-84 years |
|
| 40-49 | 1.0 | 1.0 | |||
| 50-59 | 2.59 (1.17-5.73) | 4.5 (1.6-12.2) | |||
| 60-69 | 4.15 (1.97-8.76) | 4.0 (1.4-11.3) | |||
| (70-79) | 5.26 (2.34-11.80) | 7.2 (2.3-22.4) (≥ 70 years) |
|||
| ≥ 80 | Not reported | Not reported | |||
| Age (per year) |
1.10 (1.08-1.13) | 1.07 (1.05-1.08) | |||
| Age (per 10 year increment) |
1.74 (1.45-2.09) | ||||
Adjusted for age, gender, IOP, CCT, Myopia and hypertension
Adjusted for age, gender, diabetes, hypertension, pseudoexfoliation, and myopia (mild, moderate, severe)
Adjusted for age, Maximum IOP of 2 eyes, glaucoma family history, myopia, pseudoexfoliation, diabetes, and hypertension
Adjusted for age, gender, BMI, cataract surgery, IOP, family history of OAG, interaction of gender and family history of POAG
Adjusted for gender
Prospective Cohort Studies
The main objective of the Barbados Eye Study (35) was to determine the incidence of POAG. Leske et al.(35) reported an age-specific increase in the incidence of POAG (Figure 3 and Table 8).
Figure 3: Age Specific Incidences of POAG in Barbados Eye Study.
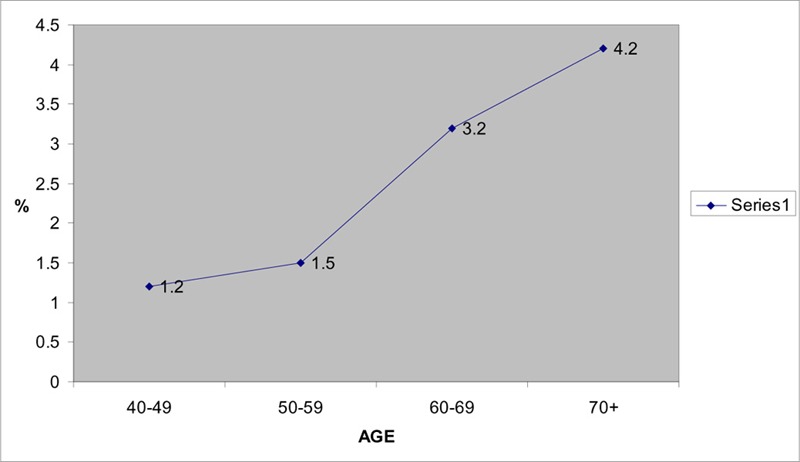
Table 8: Age Specific Incidence of Definite POAG.
| Study | Number of Incident Cases (%, 95% CI) |
||||
|---|---|---|---|---|---|
| 40-49 years | 50-59 years | 60-69 years | ≥ 70 years | Crude Incidence | |
| Barbados Eye Study (35) |
12/980 (1.2; 0.6-2.1) |
12/821 (1.5; 0.8-2.5) |
22/682 (3.2; 2.0-4.8) |
21/506 (4.2; 2.6-6.3) |
67/2989 (2.2;1.7-2.8) |
Source: Leske MC, Connell AM, Wu SY, Nemesure B, Li X, Schachat A et al. Incidence of open-angle glaucoma: the Barbados Eye Studies. Arch Ophthalmol 2001; 119(1):89-95
Conclusion
The odds of prevalent POAG is statistically significantly greater for people 50 years of age and older relative to those 40 to 49 years of age in 2 populations of Southern India. There is an estimated 7% per year incremental odds of prevalent POAG in persons 40 years of age and older, and 10% per year in persons 49 years of age and older. Prevalent POAG is undiagnosed in up to 50% of the population. From the data it can be concluded that the prevalence and 4-year incidence of POAG increases with increasing age. The quality of the evidence, as based on the GRADE criteria, is moderate (Table 9) (34).
Table 9: GRADE Profile Question: Does prevalent POAG increase with age?
| Quality Assessment | Summary of Findings | ||||||
|---|---|---|---|---|---|---|---|
| Studies | Design | Quality | Consistency | Directness | Other modifying factors |
Number of Subjects POAG/Total Study Sample Total |
Quality |
| Chennai Aravind Blue Mtns. Barbados Beaver Dam |
Cross sectional | No Issues |
Yes, consistency in direction of association amongst studies |
None | Strong evidence of association with increasing age s(OR > 2.0) | 64/3924 64/5150 108/3654 302/4314 104/4926 642 / 21968 |
Moderate |
| GRADE | Low | Low | Low | Low | Moderate | Moderate | |
Gender and POAG
Five cross-sectional studies evaluated the association between gender and POAG using a multivariate analysis of which 4 reported actual point estimates (Table 10). Both the Aravind Comprehensive Eye Survey and the Barbados Eye Study reported a statistically significant association between being male and prevalent POAG. In the Barbados Eye study, Leske et al.(41) reported a statistically significant association of prevalent PAOG for black males compared with black females, and this association was compounded with a family history of glaucoma (interaction family history and being male in the Barbados Eye Study, 3.15, 95% CI, 1.38-7.18). Results of the Chennai Glaucoma Study reported a nonstatistically significant association between gender and POAG, and that for the Blue Mountains Eye study was marginally significant. The Beaver Dam study reported a nonstatistically significant association between gender and POAG, however the actual point estimate obtained from the multivariate analysis was not reported.
Table 10: Association of POAG and Gender with by Multivariate Analysis.
| *OR 95% CI | ||
|---|---|---|
| Study | Males | Females |
| Chennai Glaucoma Study (36) |
§0.98 (0.58-1.62) | |
| Aravind Comprehensive Eye Survey (5) |
‡2.60 (1.5-4.6) | |
| Blue Mountains Eye Study (37) |
|| 1.55 (1.03-2.32) | |
| Barbados Eye Study (41) |
†1.66 (1.24-2.24) | |
| Beaver Dam (44) | Not statistically significant Point estimate not Reported |
Odds ratio and 95% Confidence Intervals
After controlling for age, gender, body mass index, cataract history, IOP, family history and interaction between gender and family history.
After controlling for age, gender, diabetes, hypertension, pseudoexfoliation, and myopia
After controlling for age, gender, IOP, CCT, myopia, and hypertension
Age-adjusted
Conclusion
Consistency in estimates is lacking among studies and because of this the association between gender and prevalent POAG is inconclusive. The quality of the evidence, as based on the GRADE criteria, is very low (Table 11). (34).
Table 11: GRADE Profile Question: What is the association between gender and prevalent POAG?
| Quality Assessment | Summary of Findings | ||||||
|---|---|---|---|---|---|---|---|
| Studies | Design | Quality | Consistency | Directness | Other modifying factors | Number of Subjects POAG/Total Study Sample Total | Quality |
| Chennai Aravind Blue Mtns. Barbados Beaver Dam |
Cross sectional | No Issues |
Some Inconsistency across studies in direction of association | No issues | None | 64/3924 64/5150 108/3654 302/4314 104/4926 642/21968 |
Very low |
| GRADE | Low | Low | Very low | Very low | Very low | Very low | |
Ethnicity and POAG
Only 1 cross-sectional study, The Baltimore Eye Study (42), directly compared the prevalence rates of POAG between black and white participants. The sample size included 5308 of 7104 eligible participants, for a response rate of 74.7%. The study reported a higher age-specific prevalence rate of definite POAG (Table 10) for black participants compared with white participants. Statistically significant age-specific odds ratios are reported for black participants 50 years of age and older compared with white participants of similar age (Table 12). There was no significant difference in age and ethnicity adjusted prevalence rates by gender. Higher rates of prevalent POAG were reported in black participants at an earlier age relative to white participants. Limitations of this research include the possibility of a confounding variable inflating the association between ethnicity and POAG. However, higher rates of prevalent POAG were reported in the Barbados Eye study (7%), whose population was predominately black participants compared to studies of predominately white participants Table 6). The authors of the Baltimore Eye Study stated that the proportion of persons in this study who reported seeing an eye care professional within the last year were similar among both black and white participants. In a companion report, the authors also reported no association in the age-race adjusted OR (1.03, 95% CI 0.85-1.25) with self-reported diabetes (both insulin dependant and noninsulin-dependant) and POAG in this study population (46).
Table 12: Prevalence of Definite POAG by Age and Race.
| Age (years) | Number of POAG Cases |
Number of cases/1000 |
Observed Prevalence Rate |
†Adjusted Prevalence Rate % (95% CI) |
‡OR (95% C.I.) | |
|---|---|---|---|---|---|---|
| 40-49 | ||||||
| *W | 1/543 | 1.8 | 0.18 (0.02-1.03) | 0.92 (0-2.72) | 1.0 | |
| *B | 6/632 | 9.5 | 0.95 (0.35-2.07) | 1.23 (0.23-2.24) | 5.2 (0.6-43.4) | |
| 50-59 | ||||||
| W | 2/618 | 3.2 | 0.32 (0.03-1.17) | 0.41 (0-098) | 1.0 | |
| B | 25/699 | 35.8 | 3.58 (2.32-5.26) | 4.05 (2.47-5.63) | 11.4 (2.7-48.4) | |
| 60-69 | ||||||
| W | 7/915 | 7.7 | 0.77 (0.31-1.57) | 0.88 (0.14-1.62) | 1.0 | |
| B | 31/614 | 5.05 | 5.05 (3.4-7.2) | 5.51 (3.57-7.46) | 6.9 (3.0-15.8) | |
| 70-79 | ||||||
| W | 18/631 | 28.5 | 2.85 (1.70-4.50) | 2.89 (1.44-4.34) | 1.0 | |
| B | 27/349 | 77 | 7.74 (4.9-10.5) | 9.15 (5.83-12.48) | 2.85 (1.5-5.3) | |
| ≥80 | ||||||
| W | 4/206 | 19.4 | 1.94(0.49-4.95) | 1.29(0.80-1.78) | 1.0 | |
| B | 11/101 | 109 | 10.89 (4.8-16.9) | 11.26 (4.52-17.00) | 6.21 (1.9-19.9) | |
| Overall | ||||||
| W | 32/2913 | 11 | 1.10 (0.75-1.55) | 1.29 (0.80-1.78) | 1.0 | |
| B | 100/2395 | 42 | 4.18 (3.38-4.98) | 4.74 (3.81-5.67) | 3.9 (2.6-5.9) |
W=Caucasian; B=Black
Adjusted for non-response rate (patient did not have the exam) to definitive ophthalmologic examination. Adjustment was done by applying the rate of disease among the cohort that presented for the definitive examination to those that did not have it with age, race and “reason for referral” strata. Therefore the rate of disease was the assumed to be the same for those who received and those that did not receive the definitive examination in each age, race, and reason for referral stratum.
Odds Ratio, Calculated by MAS using number of POAG cases in column 2 of Table. Black participants relevant to Caucasian participants in same age group (e.g. OR for black participants 40-49 years relevant to Caucasian participants 40-49 years)
Modified from Tielsch JM, Sommer A, Katz J, Royall R, Quigley HA, Javitt J. Racial Variations in the prevalence of primary open-angle glaucoma. JAMA 1991; 266(3):369-374
Conclusion
These data suggest that prevalent glaucoma is statistically significantly greater in a black population 50 years of age and older compared with a white population of similar age. There is an overall 4-fold increase in prevalent POAG in a black population compared with a white population. The increase may be due to a confounding variable not accounted for in the analysis. Results of the Baltimore study are consistent with higher prevalence rates reported in other studies of black populations (13). The quality of the evidence, as based on the GRADE criteria, is low (Table 13)(34).
Table 13: GRADE Profile Question: What is the association between ethnicity and prevalent POAG?
| Quality Assessment | Summary of Findings | ||||||
|---|---|---|---|---|---|---|---|
| Studies | Design | Quality | Consistency | Directness | Other modifying factors | Number of Subjects POAG/Total Study Sample Total |
Quality |
| Baltimore Eye Study |
Cross sectional | No Issues |
1 study | No Issues | Strong evidence of association however all possible confounders not considered in study | 132/5308 | Low |
| GRADE | Low | Low | Low | Low | Low | Low | |
Refractive Error and POAG
Four cross-sectional studies assessed the association of myopia and POAG (Table 14). Two studies (5;36) defined myopia as a spherical equivalent of -0.5 Diopters (D). Of these, the Chennai Glaucoma Study reported that myopia was not statistically significantly associated with POAG, whereas the Aravind Comprehensive Eye Survey did, however only for mild and severe myopia which were both undefined in terms of diopters in the report. Both the Beaver Dam Study and The Blue Mountains Eye Study defined myopia as a spherical equivalent of -1.00D or worse and reported a statistically significant association between myopia and prevalent POAG. The Beaver Dam study reported a 60% increase in the odds of having prevalent POAG with myopia whereas The Blue Mountains Study reported more than twice the odds of having prevalent POAG with myopia. The Blue Mountains study also reported a statistically significant association with low and moderate to high myopia and prevalent POAG in persons 60 years of age or older. No conclusions could be made for persons younger than 60 as there was insufficient data (insufficient number of cases of prevalent POAG) for this age group. Of note, a dose response effect was found between increasing prevalent POAG and increasing myopia in person ≥ 60 years of age.
Table 14: Myopia and Risk of Prevalent POAG by Multivariate Analysis.
| Study | Age (years) Mean (SD)[Range] | OR (95% CI) | Definition of Myopia (Spherical Equivalent, S.E.) |
Dose Response Effect Found? |
|---|---|---|---|---|
| Chennai Glaucoma Study (36) | 53.8(10.7) | *0.68 (0.40-1.17) | S.E. worse than -0.5 D in phakic eye | Not assessed |
| Aravind Comprehensive Eye Survey (5) | Median:51.0 [40-90] |
†Mild:2.9 (1.3-6.9) †Moderate:2.1 (1.0-4.6) †Severe3.9 (1.6-9.5) |
S.E. worse than -0.5D in either phakic eye No definitions of mild, moderate or severe reported in study. | No |
| Beaver Dam Eye Study (45) |
60.6(11.3) |
‡1.6 (1.1, 2.4) (2 eyes) ‡1.6 (0.9-2.6) (right eyes only) |
S.E. of -1.00D or worse | No |
| Blue Mountains Eye Study(38) |
Males: 75.9 [49-97] Females: 65.9 [52-96] |
§2.1 (1.2-3.8) ||2.3 (1.3-4.1) |
S.E. of -1.00D or worse | Yes as assessed in people ≥ 60 years of age |
| ||¶2.3(1.3-4.1) | Low: S.E ≥-1.00 to < -3.0 D | |||
| ||¶3.3(1.7-6.4) | Moderate to High: S.E. ≥ -3.00 D |
Adjusted for age, gender, IOP, central corneal thickness and hypertension
Adjusted for age, gender, diabetes, hypertension, pseudoexfoliation, myopia
Adjusted for age, gender, education, hypertension, diabetes
Age and gender adjusted
Adjusted for gender, age, glaucoma family history, diabetes, hypertension, history of typical migraine, steroid use, and presence of pseudoexfoliation.
Low, and moderate to severe Myopia for people 60+ years of age. Insufficient data for persons less than 60 years of age to include in analyses.
Conclusion
These data suggest an association with myopia defined as a spherical equivalent of -1.00D or worse and prevalent POAG. However, there is inconsistency in results regarding the statistical significance of the association between myopia when defined as a spherical equivalent of -0.5D or worse and prevalent POAG. The quality of the evidence, as based on the GRADE criteria, is very low (Table 15) (34).
Table 15: GRADE Profile Question: What is the association between myopia and prevalent POAG?
| Quality Assessment | Summary of Findings | ||||||
|---|---|---|---|---|---|---|---|
| Studies | Design | Quality | Consistency | Directness | Other modifying factors |
Number of Subjects POAG/Total Study Sample Total | Quality |
| Chennai Aravind Beaver Dam Blue Mtns. |
Cross-sectional | *Serious | †Some inconsistency | None | none | 64/3924 64/5150 104/4926 108/3654 340/17,654 |
Low |
| GRADE | Low | Very Low |
Very Low | Very Low | Very Low | Very Low | |
Aravind study did not define mild, moderate or severe myopia therefore difficult to interpret results
Some inconsistency at -0.5D. The magnitude of odds ratio association at 1.0D is not consistently > 2.0 (defined as strong association in Grade)
Family History and POAG
Currently, taking a family history is the only practical method of assessing familial influence of glaucoma (39). Three cross-sectional studies investigated the association between family history of glaucoma and prevalent POAG (Table 16). The Blue Mountain Eye Study reported a statistically significant association between a positive family history of glaucoma in any first-degree relative and prevalent POAG. The Barbados Eye Study which evaluated the association of family history and prevalent POAG in a predominately black population reported a statistically significant association between family history and prevalent POAG, and a statistically significant interaction between being male with a family history of POAG and prevalent POAG. Males with a family history of POAG had an odds of 7.9 (95% CI 4.1-15.23) of having prevalent POAG compared with women who had a 2.5-fold increase (OR 2.5, 95% CI 1.4-4.2). Similarly the Baltimore Eye Study found an overall statistically significant association between a family history of POAG and prevalent POAG and reported a higher OR for participants who were black and had a family history of POAG compared with a white cohort.
Table 16: Association Between Any First Degree Relative History of POAG and Prevalent POAG by Multivariate Analysis.
| Study | OR (95% CI) |
|---|---|
| Blue | *3.2 (1.8-5.6) |
| Mountains | |
| Eye Study (39) |
|
| Barbados | †2.43 (1.43-4.15) |
| Eye Study (41) |
‡3.15 (1.38-7.18) |
| Baltimore | §2.85 (1.8-4.5) |
| Eye Study | ¶B: 3.11 (1.86-5.19) |
| (43) | ||W: 2.18 (.84-5.67) |
Adjusted for age, max. IOP of 2 eyes, family history, myopia, pseudoexfoliation (PXF), diabetes and hypertension
Adjusted for age, gender, BMI, cataract history, POAG x gender interaction and family history
interaction of family history and being male (black population) (males vs. females)
adjusted for age and race; B=black population, W=White population
age adjusted
Both the Blue Mountains Eye Study and the Baltimore Eye Study reported that the family history risk factor was subject to recall and survival bias. In the Blue Mountains Eye Study, prevalent POAG cases diagnosed before study participation were twice as likely to report a family history of POAG compared with those prevalent POAG cases newly diagnosed in the study (39). Similarly, The Baltimore Eye study reported a 4-fold increase in the association between family history of POAG and prevalent POAG in participants diagnosed with POAG (OR 4.3, 95% CI 2.5-7.4) before study participation compared with those diagnosed during study participation. (O.R 1.6, 95% CI 0.77-3.44).
Conclusion
These data suggest a 2.5 to 3.0 increased odds in prevalent POAG in persons with a family history (any first-degree relative) with POAG. The quality of the evidence, as based on the GRADE criteria, is moderate 9 Table 17) (34).
Table 17: GRADE Profile Question: What is the association between family history of POAG and prevalent POAG?
| Quality Assessment | Summary of Findings | ||||||
|---|---|---|---|---|---|---|---|
| Studies | Design | Quality | Consistency | Directness | Other modifying factors |
Number of Subjects POAG/Total Study Sample Total |
Quality |
| Blue Mtns. Barbados Baltimore |
Cross-sectional | No Issues |
Yes | No Issues | Strong evidence of association (OR >2.O) | 108/3654 302/4314 132/4926 542/12,894 |
Moderate |
| GRADE | Low | Low | Low | Low | Moderate | Moderate | |
Summary of Glaucoma Research
Age
Quality of Evidence is Moderate
The incidence and prevalence of POAG increases with age
Prevalent POAG is statistically significantly greater in people 50 years of age and older compared with those 40 to 49 years of age in some populations
Gender
Quality of Evidence is Very Low
The association between gender and prevalent POAG is inconclusive.
Ethnicity
Quality of Evidence is Low
Prevalent POAG is statistically significantly greater in black populations 50 years of age and older compared with a white population 50 years of age and older.
Black populations have a statistically significant 4-fold increase in prevalent POAG.
Burden of disease (measured by crude prevalence rate) is higher in black populations compared with white populations.
Refractive Error
Quality of Evidence is Very Low
Myopia defined as a spherical equivalent of -1.00D or worse is associated with a 1.6 to 2.3 fold increase in prevalent POAG.
Family History
Quality of Evidence is Moderate
A positive family history (any first-degree relative) of POAG is associated with a statistically significant 3-fold increase in prevalent POAG.
Other
An estimated 50% of prevalent POAG cases are unaware they have POAG.
Assessment of age, gender, family history, refractive error and ethnicity as risk factors for POAG within a well-designed prospective cohort study is lacking.
Age-Related Maculopathy
Four prospective cohort studies were evaluated which assessed the relationship between age, gender and refractive error and early ARM and AMD. The characteristics of these studies can be found in Table 18.
Table 18: Characteristics of Cohort Studies.
| Study Name | Design | Follow up (years) | Years Study Conducted | Sample Size (*Response rate %) |
Population | Mean Age (SD), years [range] |
|---|---|---|---|---|---|---|
| Barbados Incidence Study of Eye Disease II (BISED II)(47) |
Population based cohort | 9 | 1997-2003 | 2612/3448 (81) |
93% black 40-84 years of age 60% female |
55 (10.3) |
| The Rotterdam Study(48) |
Population based cohort |
6.5 | 1997-1999 | 3636/5109 (71.2) |
The Netherlands ≥55 years of age | 51.0 (not reported) [not reported] |
| The Beaver Dam Eye Study(49) |
Population based cohort |
5 | 1993-1995 | 3583/4541 (79) |
United States 99% Caucasian 43-86 years of age |
†60.1 [43-86] |
| The Blue Mountains Study(50) |
Population Based cohort |
5 | 1997-1999 | 2311/3111 (74) |
Australia ≥49 years of age |
64.5 (not reported) |
response rate = number of persons with gradable fundus photographs at follow-up / total surviving cohort at follow-up.
Source: Tomany et al: 2004 (51)
Internal Validity of Studies
Internal validity characteristics of the 4 prospective studies are reported in Table 19. Probability sampling was used in the Barbados Incidence Study of Eye Diseases (47) and the Rotterdam study (48) and a total sample (census) of a defined population was used for the Beaver Dam (49) and Blue Mountains Studies (50). Sample size calculations were not reported for any study. Protocol standardized measurements for outcome measures were used in each study. Reliability was assessed through processes to determine agreement between photographic graders in each study. The Blue Mountains (50) eye study also determined intra-rater reliability for various components of age-related maculopathy (drusen type, number and maximum size, increased pigment area)
Table 19: Internal Validity Characteristics.
| Study | Sampling Method |
Sample Size Calculation a Prior | Standardized measurements | Reliability Assessments |
|---|---|---|---|---|
| Barbados Incidence Study of Eye Diseases Study Barbados, West Indies (47) |
Simple Random Sampling |
Not reported | Yes | Photographs were graded independently by 2 graders and discrepancies were resolved by consensus. When agreement could not be reached a third party (study retinal specialist) determined the final grading. |
| Rotterdam Study (48) | Cluster sampling | Not reported | Yes | Photographs were graded by 3 graders. Graders were trained according to the Wisconsin ARM grading system. Consensus sessions and inter-rater comparisons were completed at regular intervals throughout the course of the study. |
| Beaver Dam Study USA (49) |
Census, the total population of Beaver Dam Wisconsin, USA was canvassed | Not reported | Yes | Two gradings were performed for each eye. A preliminary masked grading was done by 1 of 2 senior graders. Right and left eyes of the same subject were done consecutively. A second grading was then completed by 1 of 3 experienced graders. Results of the first and second gradings were compared for inconsistencies. Disagreements between gradings were resolved by a third grader. |
| Blue Mountains (50) | Census, the total population from 2 postcode areas west of Sydney, Australia was canvassed | Not reported | Yes | Photographs were assessed by 2 graders. Inter and intra-rater reliability of photographic grading was assessed on a random sample of eyes. |
Diagnostic Methods
Diagnostic methods and definitions of early ARM and AMD for the 4 cohort studies are reported in Table 20. Three (Barbados, Beaver Dam and Blue Mountains) studies used 30-degree stereoscopic fundus photography (47;49;50) and 1 (Rotterdam Study) used 35-degree stereoscopic fundus photography for diagnosis (48). The Blue Mountains study (50) did not specify if it was color photography. Among the 4 studies the retinal photographs were graded using a variety of protocols; 2 studies including the Blue Mountains (50) and Beaver Dam (49) studies used the Wisconsin ARM grading system, the Rotterdam Study (48) used the International Classification and Grading System for ARM and AMD and the Barbados Study (47) used a protocol specified grading system. Early ARM was defined in all studies as the presence of drusen and or pigmentation changes in the retinal pigment epithelium (hypo/hyper pigmentation). However, there were variations in size and consistency of drusen used to define early ARM among the studies. AMD was defined in all studies as either geographic atrophy or exudative AMD.
Table 20: Diagnostic Criteria used in Prospective Cohort Studies.
| Study Name, | Diagnostic Methods | Definitions |
|---|---|---|
| Barbados Incidence Study of Eye Disease II (47) |
30 degree color stereoscopic fundus photographs of the disc and macula and/or clinical evaluations of macula-related features. | Early ARM: any medium or large drusen or >20 small drusen with retinal pigment epithelium atrophy and/or pigment in at least one eye. |
| Grading: Graded at the Fundus Photography Reading Center in Baltimore using a standardized grading protocol. | AMD: presence of 1 or more of the following in at least one eye: geographic atrophy; exudative features such as fluid, lipid, or hemorrhage; disciform scar. | |
| The Rotterdam Study (48) | 35 degree stereoscopic color photographs centered on the fovea and a standard eye exam and stereoscopic Grading: Photographs were graded according to the International Classification and Grading System for ARM and AMD |
Early ARM as the presence of either soft distinct drusen (≥ 63μm) with hyperpigmentation and/or hypopigmentation of the RPE or soft indistinct or reticular drusen with or without pigmentary irregularities. |
| AMD: Atrophic AMD defined as any sharply demarcated round or oval area of apparent absence of the retinal pigment epithelium, larger than 175 μm, with visible choroidal vessels and no neovascular AMD. | ||
| Neovascular AMD was defined as the presence of a serous or a hemorrhagic retinal pigment epithelium detachment and/or a sub-retinal neovascular membrane and/or a sub-retinal hemorrhage and/or a peri-retinal fibrous scar. | ||
| Progression: Progression of ARM was studied by stratifying the ARM fundus signs into 5 mutually exclusive stages | ||
| Beaver Dam (49) | 30 degree stereoscopic color fundus photographs (96.9% of participants) centered on the disc, macula and a non-stereoscopic color fundus photograph including the fovea. | Early ARM: the presence of any type of drusen associated with retinal pigment epithelial depigmentation or increased retinal pigment |
| 2.3% of participants had 45 degree non-stereoscopic fundus photographs. | AMD: the appearance of either exudative macular degeneration or pure geographic atrophy | |
| Grading: Photographs were graded using the Wisconsin ARM Grading System | ||
| Blue Mountains Study (50) | 30 degree stereoscopic retinal photographs (96.9% of participants) of the macula and other retinal fields of both eyes were taken. Grading: Photographs were graded using the Wisconsin ARM grading system. |
Early ARM was defined as the presence of either: (1) large (>125µm diameter) indistinct soft or reticular drusen, or (2) both large distinct soft drusen and retinal pigmentary abnormalities (hyperpigmentation or hypopigmentation) and the absence of late stage ARM lesions (geographic atrophy and neovascular ARM. |
| AMD: included geographic atrophy involving the fovea and neovascular AMD |
Incidence
All studies reported cumulative incidences (Table 21). The 5-year crude incidence of early ARM determined in both the Beaver Dam (49) and the Blue Mountains study (50) ranged from 8.2% to 8.7%. The 6.5-year crude incidence of early ARM determined from data from the Rotterdam Study (48) was 7.1%, and the 9-year crude incidence estimated in the Barbados Incidence Eye Study was 12.6%. (47)
Table 21: Cumulative Incidence.
| Study | Diagnostic Methods | Follow-up years |
Number of Incidence Cases in study sample/number at risk |
Overall Crude Incidence Rates % (95% CI) |
|---|---|---|---|---|
| Barbados Incidence Eye Study II (47) |
Fundus Photography and/or clinical evaluation |
9 | Early ARM: 260/2070 AMD: 21/3045 |
Early AMD: 12.6 (11.0-14.1) AMD: 0.7 (0.4-1.1) |
| Rotterdam Study (48) |
Fundus Photography | 6.5 | Early ARM:413/5836 413/25,113 person-years |
Early ARM:7.1 *16.4 (14.9-18.1) 5-year risk: 7.9 |
| AMD: 47/6312 47/26,592 person-years |
AMD: 0.74 *1.8 (1.3-2.4) 5-year risk:0.9 |
|||
| The Beaver Dam Eye Study (49) |
Fundus Photography | 5 | Early ARM: 232/2834 AMD: 32/3502 |
Early ARM: 8.2 AMD: 0.9 |
| Blue Mountains Study (50) |
Fundus Photography | 5 | Early ARM: 191/2198 AMD: 25/2313 |
Early ARM: 8.7 (†NR) AMD: 1.1 (†NR) |
per 1000 Person-years
NR=not reported
The 5-year crude incidence of AMD ranged from 0.9% to 1.1% in both the Beaver Dam and Blue Mountains studies(49;50), while the Rotterdam study (48) reported a 6.5-year crude incidence rate of 0.74%, and the 9-year crude incidence rate of AMD in the Barbados Eye Study was 0.7% (47).
Age and Early ARM
In general, all 4 cohort studies (47-50) reported that the incidence of early ARM increased with increasing age (Figure 4). Direct comparison amongst the 4 cohort studies with respect to age-specific incidence rates of early ARM was somewhat difficult because of the different age-specific categories used in each study, as well as the different method of expressing incidence (percent vs. person-years), and the years of follow-up completed in each study (Tables 22-25). However, some trends are observed (Figure 4). In the Blue Mountains (50) and Beaver Dam (49) cohorts, the 5-year incidence of early ARM in persons 55 years of age at baseline was under 5%. In the Rotterdam study, the 6.5-year incidence in persons 55 years of age at baseline was 5 per 1000 person-years (48). The Barbados eye study (47) reported a 9-year incidence of early ARM of 12.7% in persons 55 years of age at baseline, which was sustained in persons 65 years of age at baseline but increased in persons older than 65 years at baseline. In the Blue Mountains (50), Beaver Dam (49) and Rotterdam (48) studies, the incidences of early ARM begins to increase markedly in persons older than 55 years at baseline. In the Barbados Incidence Study of Eye Disease (47) which was completed in a predominately black population, the 9-year incidence in early ARM began to increase in persons 65 years of age at baseline.
Figure 4: Age-Specific Incidence of Early ARM.
Figure Legend: Barbados (9-year) (47), Blue Mountains (50) and Beaver Dam (49) (5-year) Incidences expressed as percentages Rotterdam Study (48) 6.5 year incidences expressed as 1000 person-years
Table 22: Age specific 9-Year Incidence (%) of Early ARM in the Barbados Incidence Study of Eye Disease.
| 40-49 years | 50-59 years | 60-69 years | ≥70 years | 80+ years | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number at risk | % | Number at risk | % | Number at risk | % | Number at risk | % | Number at risk | % |
| 803 | 10.7 | 571 | 12.7 | 407 | 12.4 | 289 | 16.8 | Not assessed | Not assessed |
Source: Leske MC, Wu SY, Hennis A, Nemesure B, Yang L, Hyman L et al. Nine-year incidence of age-related macular degeneration in the Barbados Eye Studies. Ophthalmology 2006; 113(1): 29-35
Table 25: Age-Specific 5-Year Incidence Rates (%) of Early ARM in the Beaver Dam Eye Study.
| 43-54 years | 55-64 years | 65-74 years | ≥75 years | ||||
|---|---|---|---|---|---|---|---|
| Number at risk | % | Number at risk | % | Number at risk | % | Number at risk | % |
| 1135 | 3.9 | 855 | 4.7 | 660 | 16.1 | 184 | 22.8 |
Source: Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997; 104(1): 7-21
Table 23: Age-Specific 6.5-Year Incidence (per 1000 Person-Years) of Early ARM in the Rotterdam Study.
| 55-59 years | 60-64 years | 65-69 years | 70-74 years | 75-79 years | ≥80 years |
|---|---|---|---|---|---|
| 1.4 | 5.3 | 10.8 | 19.0 | 31.8 | 51.0 |
Source: van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol 2003; 121(4): 519-526
Table 24: Age-Specific 5-Year Incidence Rates (%) of Early ARM in the Blue Mountains Eye Study.
| <60 years | 60-69 years | 70-79 years | ≥80 years | ||||
|---|---|---|---|---|---|---|---|
| Number at risk | % | Number at risk | % | Number at risk | % | Number at risk | % |
| 717 | 3.2 | 907 | 7.4 | 486 | 18.3 | 88 | 14.8 |
Source: Mitchell P, Wang JJ, Foran S, Smith W. Five year incidence of age-related maculopathy lesions. Ophthalmology 2002; 109: 1092-1097
Age and AMD
In general, all 4 cohort studies (47-50) reported that the incidence of AMD increased with increasing age (Figure 5). Direct comparisons amongst the 4 cohort studies with respect to age-specific incidence rates of AMD is also difficult for reasons previously expressed for early ARM (Tables 26-29). However, an increase in the 5-year (Blue Mountains (50), Beaver Dam (49) studies) and 6.5-year (Rotterdam Study (48)) incidence rates of AMD is seen in persons 55 years of age at baseline [Figure 5]. In the Barbados study, (47) an increase in the 9-year incidence rate of AMD begins in persons 65 years of age at baseline (Figure 5).
Figure 5: Age-Specific Incidence of AMD.
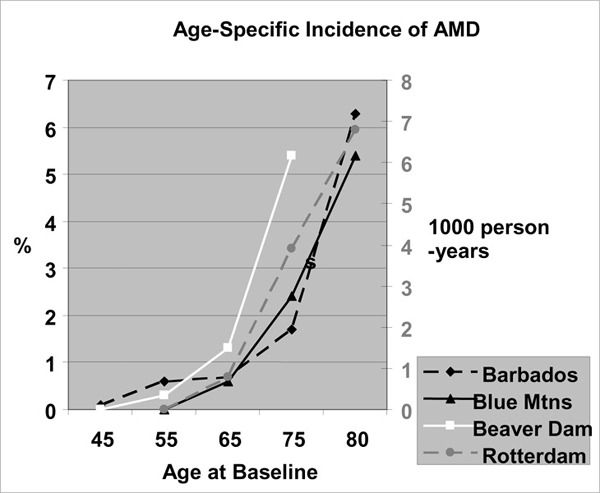
Figure Legend: Barbados (9-year) (47), Blue Mountains (5 year) (50) and Beaver Dam (5-year) (49) incidences expressed as percentages. Rotterdam 6.5 year incidences expressed as 1000 person-years (48)
Table 26: Age-Specific 9-Year Incidence Rates (%) of AMD in The Barbados Incidence Study of Eye Disease.
| 40-49 years | 50-59 years | 60-69 years | ≥70 years | 80+ years | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number at risk | % | Number at risk | % | Number at risk | % | Number at risk | % | Number at risk | % |
| *NR | 0.1 | NR | 0.6 | NR | 1.1 | NR | 2.3 | *NR | NR |
Not reported
Source: Leske MC, Wu SY, Hennis A, Nemesure B, Yang L, Hyman L et al. Nine-year incidence of age-related macular degeneration in the Barbados Eye Studies. Ophthalmology 2006; 113(1): 29-35
Table 29: Age-Specific 5-Year Incidence Rates (%) of AMD in the Beaver Dam Eye Study.
| 43-54 years | 55-64 years | 65-74 years | ≥75 years | ||||
|---|---|---|---|---|---|---|---|
| Number at risk | % | Number at risk | % | Number at risk | % | Number at risk | % |
| 1254 | 0.0 | 1033 | 0.3 | 901 | 1.3 | 314 | 5.4 |
Source: Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997; 104(1): 7-21
Table 27: Age-Specific 6.5 Year Incidence (Per 1000 Person-Years) of AMD in the Rotterdam Study.
| 55-59 years | 60-64 years | 65-69 years | 70-74 years | 75-79 years | ≥80 years |
|---|---|---|---|---|---|
| 0 | 0.2 | 0.8 | 1.8 | 3.9 | 6.8 |
Source: van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study.[erratum appears in Arch Ophthalmol. 2003 Jul;121(7):955]. Arch Ophthalmol 2003; 121(4): 519-526
Table 28: Age-Specific 5-Year Incidence Rates (%) of AMD in the Blue Mountains Eye Study.
| <60 years | 60-69 years | 70-79 years | ≥80 years | ||||
| Number at risk | % | Number at risk | % | Number at risk | % | Number at risk | % |
| 721 | 0 | 940 | 0.6 | 541 | 2.4 | 111 | 5.4 |
Source: Mitchell P, Wang JJ, Foran S, Smith W. Five year incidence of age-related maculopathy lesions. Ophthalmology 2002; 109: 1092-1097
Conclusion
The incidence of early ARM and AMD increases with increasing age. The 5-year incidence of early ARM is less than 5% in a Caucasian population 55 years of age and increases thereafter. The 9-year incidence of early ARM in a black population with a median age of 45 years is greater than 10% and increasing to 18% in persons 70 years of age and older.
The 5- and 9-year incidence of AMD is under 1% in persons 55 years of age and increases thereafter. The quality of the evidence, as based on the GRADE criteria, is low (34) (Table 30).
Table 30: GRADE Profile Question: What is the association between age and incident early ARM and AMD?
| Quality Assessment | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|
| Studies | Design | Quality | Consistency | Directness | Other modifying factors | Number of Subjects Early ARM/number at risk Total |
Number of Subjects AMD/number at risk Total |
Quality |
| Barbados Blue Mtns. Beaver Dam Rotterdam |
Prospective Cohort |
No Issues | Yes | No Issues | none | 260/2070 191/2198 232/2834 413/5836 1086/12,938 |
21/3045 25/2313 32/3502 47/6312 125/15,172 |
Low |
| GRADE | Low | Low | Low | Low | low | Low | ||
Progression of Early ARM
All 4 cohort studies assessed progression of disease for which 3 provided data.(47-49) In general all studies reported a positive relationship between the presence of early ARM and the development of AMD. Specifically, in the Barbados Incidence Study of Eye Disease, Leske et al. (47) reported that 1.7% of people with unilateral early ARM, and 2.2% with bilateral early ARM at baseline, progressed to AMD (Table 31). In the Beaver Dam study, Klein et al. Klein, 1997 664 /id observed that of the 197 people with bilateral early ARM at baseline who were examined at the 5-year follow-up, 23 (11.7%) progressed to AMD; 9 (4.6%) developed dry AMD while 14 (7.1%) developed wet AMD. In the Rotterdam Study, van Leeuwen et al. (48) reported that the 5-year risk of AMD increased with increasing stages of early ARM (Table 32). However, risk varied with age with younger people having a lower risk of progression to AMD than older persons at the same stage of early ARM (Figure 6).
Table 31: Progression from early ARM to AMD.
| % Progression | ||
|---|---|---|
| Study | Unilateral ARM (%, 95% CI) |
Bilateral Early ARM (%, 95% CI) |
| Barbados Study of Incidence Eye Disease (47) | ||
| Any AMD | 1.7 (0.7-2.8) | 2.2 (.04-4.5) |
| Dry AMD | 0.7 (.01-1.4) | 1.2 (0.0-2.9) |
| Wet AMD | 1.0 (0.2-1.8) | 1.0 (0.0-2.4) |
| The Beaver Dam Eye Study(49) | ||
| Any AMD | Not Reported | 11.7 (NR) |
| Dry AMD | 4.6 (NR) | |
| Wet AMD | 7.1 (NR) | |
Table 32: Rotterdam Study Progression from early ARM to AMD.
| 5-year Absolute Risk by Stage | |
|---|---|
| Rotterdam study | Stage 0: 0.0 |
| Stage 1: 0.9 | |
| Stage 2: 7.8 | |
| Stage 3: 28.0 | |
| Stage 4: 0.9 |
Source: van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol 2003; 121(4): 519-526
Figure 6: 5-Year Risk of AMD as a Function of early ARM in the Rotterdam Study (48).
Conclusion
Progression to AMD occurs in 1.7% to 11.7% of persons with early ARM. Rate of progression may be a function of age and stage of disease.
Gender and ARM
All 4 cohort studies assessed the association between gender and incident early ARM and AMD. In general, crude incidence rates of early ARM and AMD in women were slightly higher than men, however when adjusted for age, there was no difference between males and females.
In the Barbados Incidence Study of Eye Diseases, Leske et al.(47) reported that the 5-year incidence of early ARM or AMD was similar among men and women. In the Rotterdam Study van Leeuwen et al.(48) reported that the 6.5-year age-adjusted incidence rates of early ARM and AMD among men and women were not statistically significant different (Table 33). Both the Beaver Dam study (49) and the Blue Mountains study (50) reported slightly higher gender-specific incidence rates of early ARM and AMD in women compared with men. However, the Blue Mountains study (50) stated that these gender differences were small and insignificant. The Beaver Dam study (49) reported a statistically significant relative risk of early ARM in women 75 years of age compared with men (2.2; 95% CI 1.6-3.2).
Table 33: Gender Specific Crude Incidence of Early ARM and AMD.
| Incidence % (95 % C.I) | ||||
|---|---|---|---|---|
| Study | Early ARM | AMD | ||
| Men | Women | Men | Women | |
| Barbados Incidence Study of Eye Disease II (47) (9-year incidence) |
12.5 (10.1-14.9) | 12.6 (10.7-14.6) | 0.70 (0.3-1.2) | 0.70 (0.3-1.2) |
| Rotterdam Study (48) (6.5 year incidence) |
*17.1/1000 (†NR) | *16.0/1000 (NR) | *2/1000 (NR) | *1.6/1000 (NR) |
| The Beaver Dam Eye Study(49) (5-year incidence) |
7.1(NR) | 9.0 (NR) | 0.5(NR) | 1.3 (NR) |
| Blue Mountains Study(50) (5-year incidence) |
7.9 (NR) | 9.4 (NR) | 0.7 (NR) | 1.4 (NR) |
person-years
NR=not reported
Conclusion
Gender differences in either incident early ARM or AMD are not supported from these data. The quality of the evidence, as based on the GRADE criteria, is low (Table 34) (34).
Table 34: GRADE Profile Question: What is the association between gender and Incident Early ARM and AMD?
| Quality Assessment | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|
| Studies | Design | Quality | Consistency | Directness | Other modifying factors | Number of Subjects Early ARM/Number at risk Total |
Number of Subjects AMD/Number at risk Total |
Quality |
| Barbados Blue Mtns. Beaver Dam Rotterdam |
Pro-spective Cohort | No Issues | Yes | No Issues | none | 260/2070 191/2198 232/2834 413/5836 1086/12,938 |
21/3045 25/2313 32/3502 47/6312 125/15,172 |
Low |
| GRADE | Low | Low | Low | Low | Low | Low | ||
Refractive Error and ARM
Two cohort studies, the Rotterdam Study (23) and the Beaver Dam Study (52;53), investigated the association between refractive error and the development of incident early ARM and AMD. As part of the Rotterdam Study, Ikram et al.(23) examined the association between baseline refraction error and incident ARM (which included because of the small number of incident AMD cases, both incident early ARM and incident AMD cases) at a mean follow-up time of 5.2 years. Five refraction error categories were defined by diopters (D): advanced myopia (-3.0D or worse), myopia (better than -3.0D but worse than -0.5D), emmetropia (better than -0.5D and less than +0.5D), hyperopia (+0.5D or greater but less than +3.0D) and advanced hyperopia (+3.0D or greater). Logistic regression modeling was done to determine the relationship between baseline refraction and incident ARM correcting for age, gender, follow-up time, smoking, atherosclerosis and blood pressure at baseline. Results indicated a 4% increase in the odds of incident ARM for every 1 diopter of progress toward hyperopia (OR: 1.04; 95% CI, 1.00-1.09); however, this was not statistically significant (the authors however concluded that this association was statistically significant).
The Beaver Dam study assessed the relationship between refraction error and cumulative incident early ARM and AMD at 5- and 10-year follow-up intervals.(52;53) Myopia was defined as a refractive error of -0.50D or worse and hyperopia as +0.50 or greater. After controlling for age, hyperopia at baseline was associated with a statistically significantly reduced odds of incident early ARM at 5 years (OR 0.69; CI 0.50-0.97). However, the relationship between hyperopia and incident 5-year AMD was not statistically significant (OR 1.58, CI 0.28-9.06). At the 10 year-follow-up, there was no association between either myopia or hyperopia and incident early ARM or AMD, after controlling for age (Table 35).
Table 35: Age-Adjusted Relative Risk of 10-year cumulative incidence of Early ARM and AMD by Refractive Status in the Beaver Dam Study (52;53).
| Incident Early ARM (RR and 95% CI) | Incident AMD (RR and 95% CI) | |
|---|---|---|
| Myopia | 1.0 (0.7-1.3) | 0.5 (0.2-1.5) |
| Hyperopia | 0.9 (0.7-1.1) | 1.2 (0.6-2.3) |
Conclusion
These data do not support an association between either hyperopia or myopia and incident early ARM or AMD. The quality of the evidence, as based on the GRADE criteria, is low (Table 36) (34).
Table 36: GRADE Profile Question: What is the association between refractive error and Incident Early ARM and AMD?
| Quality Assessment | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|
| Studies | Design | Quality | Consistency | Directness | Other modifying factors | Number of Subjects Early ARM/Number at risk Total |
Number of Subjects AMD/Number at risk Total |
Quality |
| Rotterdam Beaver Dam. |
Prospective Cohort |
No Issues | Yes | No Issues | none | 413/5836 232/2834 645/8670 |
47/6312 32/3502 79/9814 |
Low |
| GRADE | Low | Low | Low | Low | low | Low | ||
Cross-sectional Studies
There were no cohort studies available that assessed family history of AMD and/or ethnicity as risk factors for age-related maculopathy, and therefore associations for these potential risk factors were determined from cross-sectional data. After a review of the studies, 1 meta-analysis (54) and 4 cross-sectional studies, 2 which evaluated family history (55;56), and 2 which evaluated ethnicity (57;58) were accepted for review. The characteristics of these studies are reported in Table 37.
Table 37: Characteristics of Cross-Sectional Studies.
| Study Name | Risk Factor Assessed |
Design | Years study Conducted | Sample Size (*Response rate %) |
Population | Mean Age (SD), years [range] |
|---|---|---|---|---|---|---|
| Blue Mtns. Eye Study (56) |
Family History | Population based cross-sectional | 1992-1994 | 3582/4433 (81) | Australia ≥ 49 years of age |
Not reported |
| Los Angeles Latino Eye Study Fraser-Bell, 2005 (55) |
Family History | Population based cross-sectional | 2000-2003 | 5875/7789 (75) | Latino population ≥40 years 42% male |
54.9 (NR) |
|
†The Eye Diseases Prevalence Research Group (54) |
Ethnicity | Meta-analysis of 7 population based cross-sectional studies | 2004 | 29658 (>80 each study) |
≥40 years black 19.7% white 79.3% |
Not reported |
| Atherosclerosis Risk in Communities (ARIC) Study (57) | Ethnicity | Population based cross-sectional | 1993-1995 | 11532/12849(89.7) | United States 48 years-72 years 43.3% male 36.9% black |
59.7 (10.7) |
| Multi-ethnic Study of Atherosclerosis (MESA) Klein, 2006(58) |
Ethnicity | Population based cross-sectional | 2002-2004 | 5887/6176 (95%) | United States Caucasian 39% Black 27% Hispanic 22% Chinese 12% |
Caucasian 63 (10.2) Black 62.4 (9.9) Hispanic 61.6 (10.2) Chinese 62.4 (10.2 |
Response rate: number of participants with gradable retinal photographs of at least 1 eye/number of eligible participants
Included The Baltimore Eye Survey, the Barbados Eye Study, the Beaver Dam Eye Study, the Blue Mountains Eye Study, the Rotterdam Study, the Melbourne Vision Impairment Project, Salisbury Eye Evaluation Project.
Internal Validity of Studies
Two studies including the ARIC (57) and the MESA (58) studies stated they used probability sampling, and 2 including the Blue Mountains (56) and the Los Angeles Latino Study (55) used a total sample of a population identified from either census tracts or a designated postal code area (Table 38). Sample size calculations were reported for the Los Angeles Latino study (55) and the MESA study (58). The Los Angeles Latino study (55) predicated the sample size on an adequate relative standard error for estimating the overall and age-specific prevalence of cataract, age-related maculopathy, diabetic retinopathy, and visual impairment. However, the MESA study’s sample size was calculated based on a 95% power to identify associations between risk factors and the presence of coronary calcium.(58) Protocol standardized measurements of early ARM and AMD were used in all studies. Intra- and inter-rater reliability was assessed in all studies.
Table 38: Internal Validity Characteristics.
| Study | Sampling Method |
Sample Size Calculation a Prior | Standardized measurements | Reliability Assessments |
|---|---|---|---|---|
| Blue Mountains Study Australia (56) | A total sample of a population from 2 postcode areas west of Sydney. | Not reported | Yes | Inter and Intra-rater reliability |
| Los Angeles Latino Eye Study (55) | A total sample of a population in 6 census tracts in and around the city of La Puente, Los Angeles County | Yes | Yes | Inter and intra rater reliability |
| Atherosclerosis Risk in Communities (ARIC) Study (57) | Probability Sample | Yes | Inter rater and intra rater reliability | |
| Multi-ethnic Study of Atherosclerosis (MESA) (58) | Probability Sampling | Yes | Yes | Inter and intra-rater reliability was assessed among the 9 graders on a random subset of images of 25 eyes |
The meta-analysis (54) determined the pooled prevalence of AMD from data of 7 population-based cross-sectional studies, each of which used a standard photographic grading system to determine AMD. The number of individuals with gradable photographs in at least 1 eye and the number found to have any AMD (wet or dry) in at least 1 eye were obtained from the investigators of each study. Pooled prevalence proportions were derived for each ethnic-gender-and age-specific stratum using minimum variation linear estimation. Logistic regression models were fit to the pooled prevalence proportions using the midpoint of each age interval as the independent variable.
Diagnostic Methods
The diagnostic methods and definitions of early ARM and AMD used in the 4 cross sectional studies are reported in Table 39. The 2 cross-sectional studies that contributed data on the association of family history (Blue Mountains Study (56) and Los Angles Latino Study (55)) of early ARM and AMD each used 30-degree stereoscopic retinal photography in the diagnosis of ARM, whereas the 2 studies contributing data on the association of ethnicity and early ARM and AMD (ARIC (57) and MESA (58) studies) used 45-degree stereoscopic retinal photographs. The definitions for early ARM and AMD were similar among studies. All studies used the Wisconsin Age-Related Maculopathy Grading System to grade the retinal photographs.
Table 39: Diagnostic Methods and Definitions.
| Study Name, | Diagnostic Methods | Definitions |
|---|---|---|
| Blue Mtns. Eye Study (56) | 30 degree stereoscopic retinal photographs of the macula and other retinal fields of both eyes Grading: The Wisconsin age-related grading system was used to grade individual ARM lesions developed by Klein et al. (58) |
Early ARM: was defined as the presence of either indistinct soft or reticular drusen or both distinct soft drusen and retinal pigmentary abnormalities in the absence of late AMD in either eye. AMD: includes neovascular AMD and geographical atrophy. Neovascular AMD was defined with serous or hemorrhagic detachment of the retinal pigment epithelium or sensory retina, the presence of subretinal or sub-retinal pigment epithelium hemorrhages or subretinal fibrous scars. Geographical atrophy was defined as discrete retinal depigmentation at least 175 μm in diameter with a sharp border and visible choroidal vessels. |
| Los Angeles Latino Eye Study(55) | 30-degree stereoscopic color retinal photographs Grading: grading was completed at the Wisconsin Ocular Epidemiology Reading Center using the Wisconsin Age-related Maculopathy Grading System developed by Klein et al. (58) |
Early ARM: absence of signs of advanced disease and the presence of soft indistinct or reticular drusen or hard or soft distinct drusen with pigmentary abnormalites (depigmentation or increased retinal pigment). AMD: presence of geographic atrophy or exudative AMD. |
| Atherosclerosis Risk in Communities (ARIC) Study (57) | 45 degree retinal photograph was taken of 1 eye, centered on the region of the optic disc and the macula. Pupillary dilatation was achieved after the participant spent 5 minutes in a dark room. No pharmacological mydriatic drops were used. Grading: grading was completed using the Wisconsin Age-Related Maculopathy grading system developed by Klein et al. (58) Photographs were evaluated at the Fundus Photographic Reading Center in Madison Wisconsin. |
Early ARM was defined as the presence of soft drusen alone, retinal pigment epithelium depigmentation alone, or a combination of soft drusen with increased retinal pigment and/or depigmentation in the absence of AMD. AMD was defined as the presence of signs of exudative ADM degeneration or pure geographic atrophy. |
| Multi-ethnic Study of Atherosclerosis (MESA)(58) | 45 degree 6.3 megapixel digital nonmydriatic camera. Two photographic fields were taken of each eye, the first entered on the optic disc and the second on the fovea. Grading: grading was completed the Wisconsin Age-Related Maculopathy Grading scheme developed by Klein et al. (58) |
Early ARM was defined by either the presence of any soft distinct or indistinct drusen and pigmentary abnormalities or the presence of a large soft drusen ≥ 125 μm in diameter, soft instinct drusen in the absence of signs of AMD. AMD was defined by the presence of any of the following: geographic atrophy or pigment epithelial detachment, sub-retinal hemorrhage or visible sub-retinal new vessel, or sub-retinal fibrous scar or laser treatment scar for AMD |
Prevalence
Crude Prevalence rates amongst the 4 cross–sectional studies ranged from 3.7% to 6.7% for early ARM and 0.13% to 2.0% for AMD (Table 40)
Table 40: Crude Prevalence of Early ARM and AMD in Cross-sectional Studies.
| Study | Diagnostic Methods | Number of Prevalent Cases in study sample/study sample | Overall Crude Prevalence Rates % (95% CI) |
|---|---|---|---|
| Blue Mtns. Eye Study (56) | Retinal Photography | Early AMD: 240/3582 AMD: 72/3582 |
Early AMD: 6.7 AMD: 2.0 |
| Los Angeles Latino Eye Study (55) |
Retinal Photography | Early ARM: 551/5875 AMD: 25/5875 |
Early ARM:9.4 AMD: 0.42 |
| Atherosclerosis Risk in Communities (ARIC) Study (57) | Retinal Photography | Early ARM:581/11532 AMD:15/11532 |
Early ARM:5.0 AMD:0.13 |
| Multi-ethnic Study of Atherosclerosis (MESA) (58) | Retinal Photography | Early ARM:221/5884 AMD:27/5884: |
Early ARM:3.7 AMD:0.46: |
Family History and ARM
Of the 4 cross-sectional studies included in this review, only 2 evaluated the association of family history and early ARM and AMD (55;56).
In the Blue Mountains Eye Study, (56) family history was statistically significantly associated with prevalent early ARM and AMD on multivariate analyses after adjusting for age, sex and current smoking status (Table 41). A statistically significant association between family history of AMD and prevalent wet AMD was also reported, however because of the small number of cases an association could not be determined for prevalent dry AMD. In the Los Angeles Latino Eye Study, (55) a statistically significant association between a positive family history of AMD and dry AMD was reported after adjusting for sex, smoking and alcohol use. However, the confidence interval is very wide indicating little precision in the point estimate (Table 41).
Table 41: Association Between Family History of AMD and Prevalent Early ARM and/or AMD.
| Family History of AMD |
Odds ratio (95% CI) |
||
|---|---|---|---|
|
*Blue Mountains Eye Study |
†Los Angeles Latino Eye Study |
||
| None | 1.00 | 1.00 | |
| Early ARM | 2.17 (1.04-4.55) | Not reported | |
| Any AMD | 3.9 (1.3-11.5) | Not reported | |
| Wet AMD only | 4.3 (1.4-13.5) | Not reported | |
| Dry AMD only | Sample too small | 18.83 (2.02-175.7) | |
adjusted for age, sex and current smoking
Adjusted for sex, smoking and alcohol use
Conclusion
Data from one study (56) supports an association between a positive family history of AMD and having prevalent AMD. The results of the study indicate an almost 4-fold increase in the odds of any AMD in a person with a family history of AMD. Data from the same study indicates a 4-fold increase in wet AMD in persons with a family history of AMD. However, the magnitude of the association between family history of disease and dry AMD is inconclusive. The quality of the evidence, as based on the GRADE criteria, is moderate (Table 42) (34).
Table 42: GRADE Profile Question: What is the association between family history of AMD and prevalent Early ARM and AMD?
| Quality Assessment | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|
| Studies | Design | Quality | Consistency | Directness | Other modifying factors | Number of Subjects Early ARM/Total Sample Total |
Number of Subjects AMD/Total Sample Total |
Quality |
| Blue Mtns. Los Angeles |
Cross-sectional | No Issues | *Unknown (not down graded) | None | Odds Ratios >2 for early ARM and Any AMD in Blue Mtns. Study | 240/3582 551/5875 791/9457 |
72/3582 25/5875 97/9457 |
Moderate |
| GRADE | Low | Low | Low | Low | Moderate | Moderate | ||
Because the Los Angeles study does not report OR for any AMD, inconsistency cannot be determined.
Ethnicity and ARM
One meta-analysis (54) and 2 cross-sectional studies (57;58) reported on the prevalence of age related maculopathy by ethnicity.
Meta-Analysis
The Eye Diseases Prevalence Research Group (54) completed a meta-analysis of prevalence rates of AMD from 7 population-based cross-sectional studies each of which used a standard photographic grading system to determine AMD. The average participation rate in each study was 80%. Characteristics of the studies included in the meta-analysis are provided in Table 43. Figure 7 reports the pooled age-specific prevalence rates of AMD in black and white populations from the meta-analysis. Pooled data from 6 of the studies contributed prevalence data for a white population and from 3 studies for a black population. Results of the meta-analysis indicated that the pooled prevalence of AMD was similar in both ethnic groups until age 75 years at which point the prevalence rate of AMD increased in a white population but not in a black population (Figure 7). The limitations of this meta-analysis included: the proportion of black participants included in the meta-analysis was 19.7%, there was limited data available for black participants in the younger age groups, and the number of ungradable photographs was higher in the 3 studies of a black population.
Table 43: Characteristics of Population-Based Cross-Sectional Studies Included in Meta-Analysis.
| Variable | Baltimore Eye Survey |
Barbados Eye Study |
Beaver Dam Eye Study |
Blue Mountains Eye Study |
Rotterdam Study |
Salisbury Eye Evaluation |
Melbourne Vision Impairment Project |
|---|---|---|---|---|---|---|---|
| Sample size | n=4361 | n=3413 | n=4752 | n=3632 | n=6774 | n=2387 | n=4339 |
| Years study conducted | 1985-1988 | 1988-1992 | 1988-1990 | 1992-1994 | 1990-1993 | 1993-1995 | 1991-1998 |
| Ages of Participants (years) | ≥40 | ≥40 | ≥40 | ≥50 | ≥55 | ≥65 | ≥40 |
| Ethnicity Distribution (%) | Black 42.3 White 57.7 |
Black 100 | White 100 | White 100 | White 100 | Black 23.7 White 74.3 |
White 100 |
Source: The Eye Disease Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004; 564-572
Figure 7: Data from The Eye Diseases Prevalence Research Group Meta-Analysis.
Figure Legend: W=White; B= Black White males vs. White females; OR men: 1.10; 95% CI 0.81-1.25 Black males vs. Black females; OR men: 0.64; 95% CI 0.31-1.32
Cross-Sectional Studies
Two cross-sectional studies, The Atherosclerosis Risk in Communities (ARIC)(57) and the Multi-Ethnic Study of Atherosclerosis (MESA) (58), met the inclusion criteria for this review and contributed data on the association between ethnicity and prevalent early ARM and ADM.
The Atherosclerosis Risk in Communities (ARIC) study (57) reported on the prevalence of Early ARM and AMD in white and black populations. Figures 8 and 9 summarize data from each study and the data used to derive these figures are reported in Tables 44 and 45. The age-specific prevalence of early ARM was similar amongst both ethnicities until 65 years of age, at which point the prevalence increases in a white population (Figure 8). The prevalence of AMD was slightly higher in a black population 55 to 64 years of age compared to a white population, decreasing thereafter in the black population and increasing in the white population (Figure 9). The authors report that the estimated age- and sex-adjusted prevalence of any ARM (early ARM and AMD) was statistically significantly lower in black participants compared with white participants (OR, 0.73; 95% CI, 0.58-0.91). There was no statistically significant interaction between ethnicity and age or sex.
Figure 8.
Figure 9.
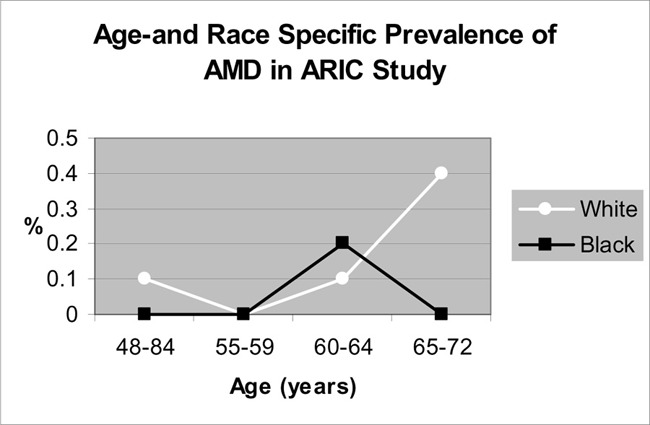
Table 44: Prevalence of Early ARM by Age and Race in the ARIC Study.
| Age (years) | Number of Early ARM Cases |
Per 1000 persons | Prevalence Rates % |
|---|---|---|---|
| 48-54 | |||
| White (W) | 54/1874 | 28 | 2.9 |
| Black (B) | 19/763 | 25 | 2.5 |
| 55-59 | |||
| W | 100/2460 | 41 | 4.1 |
| B | 25/761 | 33 | 3.3 |
| 60-64 | |||
| W | 136/2307 | 59 | 5.9 |
| B | 26/552 | 33 | 4.7 |
| 65-72 | |||
| W | 196/2343 | 84 | 8.4 |
| B | 23/472 | 49 | 4.9 |
| Overall | |||
| W | 486/8984 | 54 | 5.4 |
| B | 93/2548 | 36 | 3.6 |
Source: Klein R, Clegg L, Cooper LS, Hubbard LD, Klein BE, King WN et al. Prevalence of age-related maculopathy in the Atherosclerosis Risk in Communities Study. Arch Ophthalmol 1999; 117(9): 1203-1210
Table 45: Prevalence of AMD by Age and Race in the ARIC study.
| Age (years) | Number of Early ARM Cases |
Per 1000 persons | Prevalence Rates % |
|---|---|---|---|
| 48-54 | |||
| White (W) | 2/1874 | 1 | 0.1 |
| Black (B) | 0/763 | 0 | 0 |
| 55-59 | |||
| W | 0/2460 | 0 | 0 |
| B | 0/761 | 0 | 0 |
| 60-64 | |||
| W | 2/2307 | 1 | 0.1 |
| B | 1/552 | 2 | 0.2 |
| 65-72 | |||
| W | 9/2343 | 4 | 0.4 |
| B | 0/472 | 0 | 0 |
| Overall | |||
| W | 13/8984 | 1.4 | 0.14 |
| B | 1/2548 | .4 | 0.04 |
Source: Klein R, Clegg L, Cooper LS, Hubbard LD, Klein BE, King WN et al. Prevalence of age-related maculopathy in the Atherosclerosis Risk in Communities Study. Arch Ophthalmol 1999; 117(9): 1203-1210
The MESA study (58) reported the prevalence rates of early ARM and AMD in white, black, Hispanic and Chinese ethnic groups. The study included 39% whites, 27% blacks, 22% Hispanics and 12% Chinese participants. The estimated crude prevalence rates for each ethnic group are reported in Table 46. Figures 10 and 11 report the age- and ethnic-specific prevalence rates for early ARM and AMD. Black participants had a statistically significantly lower prevalence of early ARM compared with white participants, whereas the prevalence rates of Hispanic and Chinese cohorts did not differ statistically from the white cohort. Prevalence rates amongst the 4 ethnic groups did not differ statistically for AMD. Adjustments for age, gender, body mass index, smoking, and hypertension status did not change these relationships. Because of the low prevalence rate of AMD, the statistically insignificant difference in AMD between ethnic groups may represent an inadequacy in statistical power to detect differences between these group stratifications.
Table 46: MESA study.
| Early ARM | AMD | ||||
|---|---|---|---|---|---|
| Ethnic Group | Crude Prevalence (%) |
Age-gender adjusted Odds Ratio (95% CI) |
Crude Prevalence (%) |
Age-gender adjusted Odds Ratio (95% CI) |
|
| White | 4.8 | 1.0 | 0.6 | 1.0 | |
| Black | 2.1 | 0.45 (0.30-0.67) | 0.3 | 0.52 (0.17-1.60) | |
| Hispanic | 4.0 | 0.88 (0.63-1.25) | 0.2 | 0.48 (0.14-1.70) | |
| Chinese | 3.6 | 0.76 (0.49-1.19) | 1.0 | 1.91 (0.75-4.87) | |
Source: Klein R, Klein BE, Knudtson M, Wong TY, Cotch MF, Liu K et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology 2006; 113(373):380.
Figure 10.
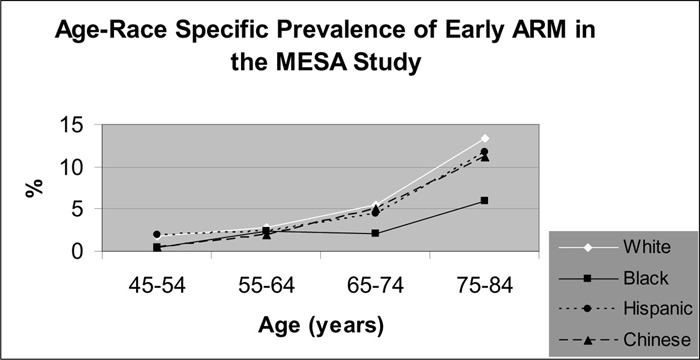
Figure 11.
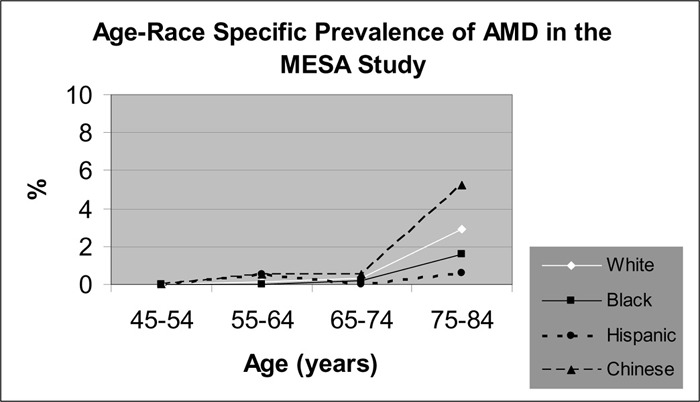
Conclusion
These data suggest that ethnic differences may exist in the prevalence rates of early ARM but not AMD. Regarding any ARM, data from the ARIC study suggest that prevalence rates of early ARM are higher in a white population 55 years of age and older compared with a black population. Data from the MESA study suggests a statistically significantly lower prevalence of early ARM in a black population compared with a white population. Data on the prevalence rates of early ARM in other ethnic groups are lacking.
Regarding AMD, the data is inconsistent. Data from the meta-analysis suggest that prevalence of AMD is greater in a white population compared with a black population after the age of 75 years. Data from the ARIC study suggests that the prevalence of AMD is greater in a white population compared with a black population after the age of 65 years. However, data from the MESA study suggested no statistically significant difference in the prevalence of AMD between white, black, Hispanic or Chinese ethnic populations. Inadequacies in the design of the MESA study may have contributed to it being underpowered to detect differences between ethnic strata.
The quality of the evidence, as based on the GRADE criteria, is very low (Table 47) (34).
Table 47: GRADE Profile Question: What is the association between ethnicity and prevalent Early ARM and AMD?
| Quality Assessment | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|
| Studies | Design | Quality | Consistency | Directness | Other modifying factors | Number of Subjects Early ARM/Total Sample Total |
Number of Subjects AMD/Total Sample Total |
Quality |
| Eye Disease Prevalence Group |
Cross-sectional | *Serious | †Some inconsistency | No Issues | none | Not assessed |
418/29,658 |
Very low |
| ARIC | ||||||||
| 581/11,532 | 15/11,532 | |||||||
| MESA | ||||||||
| 221/5884 | 27/5884 | |||||||
| 802/17,416 | 660/47,074 | |||||||
| GRADE | Low | Very low | Very Low | Very Low | Very low | Very low | ||
Type II statistical error possible in MESA study. MESA study primarily designed to determine association between risk factors and coronary calcium levels.
Inconsistency with association of ethnicity and AMD amongst ARIC and MESA studies.
Summary of Age-Related Maculopathy Research
Age
The incidence of early ARM and AMD increases with age.
Quality of evidence is low
The incidence of early ARM increases in persons 55 years of age and older.
The incidence of AMD increases in persons 55 years of age and older.
Progression to AMD occurs in up to 12% of persons with early ARM.
Family History
Quality of evidence is moderate
One study suggests that a family history of AMD is associated with approximately a 4-fold increase in the odds of prevalent AMD
Gender
Quality of evidence is low
The data suggest that age-adjusted gender differences in the incidence of early ARM and AMD are not apparent.
Refractive Error
Quality of evidence is low
The data suggest no association between refractive error (hyperopia or myopia) and the incidence of early ARM or AMD.
Ethnicity
Quality of evidence is very low.
The data suggests that the prevalence of early ARM is higher in a white population compared with a black population.
The data suggest that the ethnic-specific differences in the prevalence of AMD remain inconclusive.
Economic Analysis
Notes & Disclaimer
The Medical Advisory Secretariat uses a standardized costing methodology for all of its economic analyses of technologies. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative (OCCI) cost data is used for all program costs when there are 10 or more hospital separations, or one-third or more of hospital separations in the ministry’s data warehouse are for the designated International Classification of Diseases-10 diagnosis codes and Canadian Classification of Health Interventions procedure codes. Where appropriate, costs are adjusted for hospital-specific or peer-specific effects. In cases where the technology under review falls outside the hospitals that report to the OCCI, PAC-10 weights converted into monetary units are used. Adjustments may need to be made to ensure the relevant case mix group is reflective of the diagnosis and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the Medical Advisory Secretariat normally defaults to considering direct treatment costs only. Historical costs have been adjusted upward by 3% per annum, representing a 5% inflation rate assumption less a 2% implicit expectation of efficiency gains by hospitals.
Non-Hospital: These include physician services costs obtained from the Provider Services Branch of the Ontario Ministry of Health and Long-Term Care, device costs from the perspective of local health care institutions, and drug costs from the Ontario Drug Benefit formulary list price.
Discounting: For all cost-effective analyses, discount rates of 5% and 3% are used as per the Canadian Coordinating Office for Health Technology Assessment and the Washington Panel of Cost-Effectiveness, respectively.
Downstream cost savings: All cost avoidance and cost savings are based on assumptions of utilization, care patterns, funding, and other factors. These may or may not be realized by the system or individual institutions.
In cases where a deviation from this standard is used, an explanation has been given as to the reasons, the assumptions and the revised approach.
The economic analysis represents an estimate only, based on assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied for the purpose of developing implementation plans for the technology.
Ontario-Based Economic Analysis/Budget Impact Analysis
Diffusion
Figure 12 illustrates the utilization of publicly covered major eye exams by Ontarians aged 50 to 64, before and after the delisting of publicly covered major eye examinations in Nov 2004.
Figure 12: Utilization of OHIP (Physician and Optometrist) Insured Eye Exams for Persons 50-64 Years.
Demographics
The prevalence of glaucoma is estimated at 1 to 3% for a Caucasian population and 4.2 to 8.8% for a black population. The incidence of glaucoma is estimated at 0.5 to 2.5% per year in the literature. The percentage of people who go blind during their lifetime as a result of glaucoma is estimated at approximately 10%, while the average length of time between the diagnosis of glaucoma and diagnosis of blindness is estimated at 18 years (59). Therefore, the percentage of people who go blind per year as a result of glaucoma is approximately 0.55% (10%/18 years).
The total population of Ontarians aged 50 to 64 years is estimated at 2.6 million based on the April 2006 Ontario Ministry of Finance population estimates (60). On average, 567,690 Ontarians aged 50 to 64 received a major eye examination before November 2004 (based on The Ontario Provincial Health Planning Database (61) data). The total number of Ontarians aged 50 to 64 years that received a publicly insured eye examination in the fiscal year 2003 was 595,598. Assuming an increase of 24,509 per year from before November 2004, as illustrated in the figure 12, the estimated number of Ontarians aged 50 to 64 years who will utilize an eye examination in 2006/07 if the eligible age for publicly insured exams was decreased from 65 to 50 years is 669,125 (595,598+3*(24,509)). Therefore the range of utilization for a major eye examination in 2006/07 for this age group is estimated at 567,690 to 669,125. The total number of Ontarians aged 50 to 64 years that received a publicly insured eye examination after November 2004 was 127,5741. Given this, the net increase in utilization if the eligible age for a publicly insured eye exam was changed to 50 years is 440,116 to 541,551 ([567, 690-127, 574] to [669,125-127,574]).
The percentage of Ontario population categorized as black was approximately 19% while the percentage of Ontario population with a family history of glaucoma was approximately 1%. Therefore, the estimated range of utilization for a major eye examination in 2006/07 for this sub-population is estimated at 113,538 - 133,825 (20% of the estimated range of utilization in total population of 50-64 year olds in Ontario). The total number of 50-64 year olds in Ontario that received a publicly insured eye examination after November 2004 and who were of African descent or had a family history of glaucoma was 25,515 (0.2*127,5741). Therefore, the net increase in utilization of major eye exams within this sub-population (Option to decrease age from 65 to 50 years for people with African descent and/or family history of glaucoma only) = 88,023 to 108,310 (113,538-25,525 to 133,825-25,515).
Costs
All costs are in Canadian currency unless otherwise noted.
Professional Costs:
The total cost of a major eye examination by a physician is $42.15, as per the 2006 Schedule of Benefits for Physician Services.(1) The total cost of a major eye examination by an optometrist is $41.30, as per the 2004 Schedule of Benefits for Optometry Services (62). The total cost for completing a “requisition for a major eye examination” is $10.25, as per the 2006 Schedule of Benefits for Physician Services.
Health Care System Costs:
Iskedjian et al.(63), conducted an Ontario-based costing analysis on glaucoma in Canada and estimated the total cost of procedures associated with the treatment of glaucoma to be $344 for mild (< 5dB), $420 for moderate (5 to <12 dB) and $511 for severe (≥ 12 dB) forms of glaucoma. These estimates included the cost of the procedure itself, physician’s fee, assistant’s fee, and the anaesthetist’s fee. Costs associated with hospital resources and medications were not included. The total difference between the treatments of early-stage versus late-stage glaucoma was estimated at $167.
Other Provincial Costs:
The annual caseload of Ontario Disability Support Program recipients aged 50-64, with a disorder of the eye and adnexa, was estimated at 13,464 in 2005/06. The total financial assistance paid in 2005/06 was $12 million. Therefore, the total cost per recipient was estimated at $891/person. It has been assumed, for the purposes of this analysis, that this is approximately equal to the cost of glaucoma.
Costs
Reduce threshold from 65 years to 50 years
The estimated range of utilization for a major eye examination in 2006/07 is 567,690 to 669,125
The average utilization for a major eye examination in 2006/07 = (567,690+669,125)/2 = 618,408
The total number of 50 to 64 year olds in Ontario that received a publicly insured eye examination after November 2004 was 127,574
Net increase in utilization (option to decrease age from 65 to 50 years) = 440,116 to 541,551 ([567, 690-127, 574] to [669,125-127,574])
Incremental Budget Impact = $9.3 to $11.4 million/year ($42.15 * (440,116 to 541,551) * 0.5 (due to the fact that screening will be offered biannually))
Average increase in utilization (option to decrease age from 65 to 50 years) = (440,116 + 541,551)/2 = 490,834
The incidence of glaucoma = 1.5%
Number of glaucoma cases detected due to the increase in utilization of major eye exams among 50-64 year olds = 7,362/year (=0.015*490,834)
Cost avoidance due to early detection of glaucoma = $1.3 million/year ($167 * 7,362 glaucoma cases detected per year)
Number of potential blindness cases due to glaucoma avoided = 0.55% * 490,834 = 2,700/year
Total cost per person (2006/07) = $891/person (Ministry of Community and Social Services)
Cost avoidance due to prevention of blindness = ($891 * 2,700 potential blindness cases due to glaucoma avoided per year) = $2.4 million/year
Therefore:
The net budget impact to the MOHLTC was calculated by subtracting the cost avoidance due to early detection of glaucoma from the estimate budget impact
The Net Budget Impact to MOHLTC = $8 to 10.1 million/year
The average net budget impact to MOHLTC = $9 million/year ($1,222/case detected)
The net budget impact to Ontario was calculated by further subtracting the cost avoidance due to prevention of blindness from the above
Net Budget Impact to Ontario = $5.6 to $7.7 million/year
The average net budget impact to Ontario = $6.6 million/year ($2,444/case detected)
Existing Guidelines for Use of Technology
Existing Guidelines from Canada, United States and Europe for routine eye examinations are presented in Table 38.
Table 38 – Guidelines for Routine Eye Examinations.
| Organization | Year | Policy | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canadian Task Force on the Periodic Health Examination (7) | 1995 |
|
||||||||||||||||||||
| Canadian Ophthalmological Society | 2005 | Canadians are recommended to have an eye examination every 3 to 5 years until the age of 40 and then every 2 to 4 years until the age of 65. Those with risk factors for eye diseases should get an eye examination annually. People over 65 should have an examination every 2 years - or annually if they have any risk factors | ||||||||||||||||||||
| Canadian Association of Optometrists | No date | The minimum recommended frequency of examination for those at low risk is as follows:
The frequency of examination for those at high risk will be determined by the examining optometrist on the basis of one’s health and visual status at the preliminary examination. Some of the factors which may indicate high risk are as follows:
|
||||||||||||||||||||
| Centers for Medicare and Medicaid | 2005 | Glaucoma screening can lead to early detection and treatment, which can prevent, slow, or stop vision loss from the disease. Medicare covers annual glaucoma screening for people at high risk for the disease; this section describes this benefit and provides information and resources for health care professionals and organizations to support the delivery and promotion of this benefit for appropriate Medicare beneficiaries. What Medicare covers: Medicare covers annual glaucoma screening for the following persons considered to be at high risk for this disease:
Medicare will pay for glaucoma screening examinations when they are furnished by or under the direct supervision in the office setting of an ophthalmologist or optometrist, legally authorized to perform these services under State law. The beneficiary will pay 20% as the co-payment or coinsurance after meeting the yearly Part B deductible. A glaucoma screening examination includes the following:
Other helpful information:
|
||||||||||||||||||||
| American Academy of Ophthalmology | 2005 | Adults without risk factors:
Adults with risk factors for glaucoma (e.g. increased IOP, family history of glaucoma, African or Hispanic/Latino descent):
|
||||||||||||||||||||
| American Optometric Association | 2005 | Persons without risk factors 18-60: every 2 years 61 and older: annually Persons at risk (diabetes, hypertension, family history of ocular disease, or whose clinical findings increase their potential risk such as those working in occupations that are highly demanding visually or are eye hazardous, those taking prescription or non prescription drugs with ocular side effects, those wearing contact lenses those who have had eye surgery and those with other health concerns or conditions) 18-60: 1-2 years or as recommended 61 and older: annually or as recommended |
||||||||||||||||||||
| United Kingdom, College of Optometrists | 2005 | 16-69 years: every 2 years 70 and older: annually Persons 40 and older with a family history of glaucoma or with ocular hypertension who are not part of a monitory scheme: annually Diabetics who are not part of a diabetic retinopathy monitoring scheme: annually |
||||||||||||||||||||
| National Eye Institute | 2004 | People at high risk for glaucoma who do not have the disease should be examined every 2 years. People with seemingly normal vision should also be referred to an eye care professional, if they fit into any of the following categories:
|
||||||||||||||||||||
| Prevent Blindness America | In general, the recommended frequency of comprehensive eye examinations for people without symptoms or special risk factors is:
People with special risks, such as diabetes, a previous eye injury, surgery or a family history of glaucoma, may need an eye exam more frequently. |
|||||||||||||||||||||
| U.S. Preventative Services Task Force | 1996 | Routine vision screening with Snellen acuity testing is recommended for elderly persons (“B” recommendation). The optimal frequency for screening is not known and is left to clinical discretion. Selected questions about vision may also be helpful in detecting vision problems in elderly persons, but they do not appear as sensitive or specific as direct assessment of acuity. There is insufficient evidence to recommend for or against routine screening with ophthalmoscopy by the primary care physician in asymptomatic elderly patients (“C” recommendation). |
Canada
Existing provincial policies on eye examinations are presented in Table 39. In general, the majority of provinces do not insure routine eye examinations for persons between 20 and 64 years of age.
Table: 39 – Provincial Policies in Canada on Eye Examinations.
| Province | Policy |
|---|---|
| British Columbia | Routine eye examinations are a benefit only for those 18 years of age and under and 65 years of age and over. Medically required eye examinations are a benefit for all Medical Service Plan beneficiaries when there is a medical necessity (such as eye diseases, trauma or injury, or health conditions associated with significant risk to the eyes such as diabetes.) |
| Alberta | Basic Medical Services include:
|
| Saskatchewan | Routine Eye exams are limited to:
|
| Manitoba | If you are under 19 years of age or 65 and over you may receive one complete routine eye exam every two years. Exams for all ages will be covered if deemed medically necessary by your physician or optometrist. Routine eye exams for people 19-64 are not insured. |
| Quebec | Persons under 18 and 65 years of age and older are covered. People of any age on welfare are covered. |
| Nova Scotia | Provincial coverage for persons 10 years and younger and 65 years and older once every 2 years. |
| New Brunswick | Refractions for prescription eye glasses and optometrist services are not insured by either the New Brunswick Medicare or the province's hospital services. Persons of any age may have a provincially insured eye exam by an ophthalmologist for conditions other than refraction. |
| PEI | Optometrists' services are not covered for any persons of any age. With a referral from a family physician, services rendered by an ophthalmologist are covered by the province's Medicare plan. |
| Newfoundland | Routine eye examinations are not insured |
International
International Policies on eye examinations (other than the United States) are reported in Table 40.
Table 40 – International Policies on Routine Eye Examinations.
| Country | Policy |
|---|---|
| United Kingdom | You are entitled to a free NHS sight test if you:
|
Appraisal
Considerations
Diffusion – International, National, Provincial
Saskatchewan is the only Canadian province to offer publicly insured routine eye exams to persons between the ages of 18 and 64 years of age.
In the United States, Medicare will cover annual glaucoma screening tests for persons with diabetes, a family history of glaucoma, African Americans over the age of 50 years and Hispanics 65 years and older.
The Canadian Ophthalmological Society and the Canadian Optometrist society both advocate routine eye exams for persons between the ages of 20-64 years of age however the frequency of these assessments differs among these organizations. The Canadian Ophthalmological Society advocates examinations every 3 to 5 years until age 40 and then every 2 to 4 years until age 65 years of age whereas the Canadian Optometrist Society advocates every 1 to 2 years for persons between the ages of 20 to 64 years of age. Internationally, the frequency of eye exams varies similarly. The increased risk of glaucoma associated with African descent and family history of disease is recognized among national and international organizations.
It is unknown how many Ontarians have had an eye exam paid for by a third party insurer since November, 2004. Personal communication with authorities at the Canadian Life and Health Insurance Association (CLHIA) suggested that group insurance packages for major eye exams offered by employers vary significantly, as was demonstrated through a survey. Therefore, no conclusive statements could be derived regarding the extent of third payer participation in major eye examination insurance coverage.
Current Ontario Policy
Persons between 20 to 64 years of age are eligible for an OHIP-insured eye examination once every year if they have any of the following medical conditions: diabetes mellitus type 1 or 2, glaucoma, cataract, retinal disease, amblyopia, visual field defects, corneal disease, or strabismus. Persons 20 to 64 years of age who are in receipt of social assistance are eligible to receive an eye examination once every 2 years as a non-OHIP government funded service.
Persons 20 – 64 years of age diagnosed with glaucoma, cataract, retinal disease, amblyopia, visual field defects, corneal disease or strabismus during the course of an uninsured eye examination will from that point forward be eligible for an annual insured eye examination.
Persons between the ages of 20 and 64 who do not have diabetes mellitus, glaucoma, cataract, retinal disease, amblyopia, visual field defects, corneal disease, or strabismus can access an insured eye exam if they have a valid “request for major eye examination” form completed by a physician (other than that who completed the eye exam) or a nurse practitioner working in a collaborative practice. (3) If approved, the request is valid for a 5-year period.
Persons 20-64 years of age who are in receipt of social assistance and who do not have one of the 8 medical conditions listed in the previous paragraph are eligible to receive an eye exam once every 2 years as a non-OHIP government funded service.
Persons 19 years of age or younger and 65 years of age or older may receive an insured eye exam once every year.
Target Population
For glaucoma the target population is persons 50 years of age and older, those with a family history of glaucoma and persons of African descent. For macular degeneration the target population is persons 55 years of age and older and those with a family history of macular degeneration.
Patient Outcomes – Medical, Clinical
There is evidence that treatment for glaucoma and age related maculopathy can slow the progression of these diseases. The degree of functional loss associated with glaucoma has not been adequately described.
Financial Impact
Based on the economic analysis, if the policy were altered to cover the entire 50-64 year old population of Ontario for major eye exams, the net budget impact to the Ministry of Health and Long-Term Care would be approximately $8 million to $10.1 million. However, the net budget impact to the government of Ontario would be approximately $5.6 million to $7.7 million.
If the policy were changed to cover major eye exams for the 50-64 year old population who are of African descent and/or have a family history of glaucoma only, the net budget impact to the Ministry of Health and Long-Term Care would be approximately $1.6 million to $2 million. If considered from the government of Ontario perspective, the net budget impact would be lower at approximately $1.1 million to $1.6 million.
Stakeholder Analysis
As of July 17, 2006 there were 1,402 practicing optometrists in Ontario. As of December 31, 2005 there were 404 practicing ophthalmologists in Ontario (Personal contact The Ontario College of Physician and Surgeons)
It is unknown how many third party payers now cover routine eye exams for person between the ages of 20 and 64 years of age in Ontario.
Glossary
- Aphakic
The absence of the lens of the eye most commonly caused by extraction of a cataract. It may also occur congenitally or due to trauma.
- Cross-Sectional Study
A type of observational study where there is a simultaneous acquisition of disease and exposure information.
- Cohort
A subset of a population with a common feature such as age, sex or occupation etc
- Cornea
Is the outer, clear, round structure covering the iris and pupil. The cornea helps to focus the light rays entering the eye on the retina at the back of the eye. Vision problems such as myopia (nearsightedness) or astigmatism can be caused by changes in the shape of the cornea.
- Cup to Disc ratio
The ratio of the horizontal or vertical diameter of the cup to the horizontal or vertical diameter of the optic disc.
- Emmetropia
No refractive error or vision
- Fundoscopy
See ophthalmoscopy
- Gonioscopy
Is an eye test that checks to seed if the angle where the iris meets the cornea is open or closed. The angle is open for POAG and closed for Angle closure glaucoma.
- Hyperopia
Also called farsightedness, it is that error of refraction in which rays of light entering the eye parallel to the optic axis are brought to a focus behind the retina, usually as a result of the eyeball being too short from front to back.
- Incidence
The rate at which new cases of a disease occur during a specific time period
- Macula
The most sensitive portion of the retina. Area of retina with greatest concentration of photoreceptors and therefore provides high-resolution visual acuity. The fovea centralis is at the center of the macula.
- Myopia
Also called nearsightedness, it is that error of refraction in which rays of light entering the eye parallel to the optic axis are brought to a focus in front of the retina, usually as a result of the eyeball being too long from front to back.
- Neuroretinal rim
The area from the margin of the cup to the margin of the optic disc.
- Ophthalmoscopy
Also called fundoscopy; it is the examination of the interior of the eye with an ophthalmoscope
- Optic Nerve Cupping (optic disc cupping)
The optic nerve exit to the brain through the optic discs located at the back of the eye. The centre portion of the optic disc is called the “cup” which is usually small compared with the entire optic disc. In people with glaucoma, the optic nerve begins to die causing the cup portion of the optic disc to become larger compared with the optic discs. Optic nerve cupping is the increasing in size of the cup compared to the optic disc as disease progresses.
- Perimetry
A test which determines the extent and sensitivity of the peripheral visual field by use of a perimeter.
- Phakic
The state of having the native lens of the eye.
- Prevalence
The proportion of a population with the disease at a given time point.
- Pseudophakic
Having an artificial lens.
- Pseudophakos
An implantable artificial lens used in the treatment of cataracts.
- Retina Pigment Epithelium
Found underneath the retina and supplies blood to the outer two thirds of the retina and keeps the retina attached via osmotic forces.
- Scotoma
An area of lost or depressed vision within the visual field.
- Vitreous gel
Also called vitreous humour, it is a gel-like fluid filling the space in the eye behind the lens. It helps the globe maintain its shape and provides a clear media for vision.
Appendices
Appendix 1 Literature Search Strategy
Search date: February 23, 2006
Database: Ovid MEDLINE(R) <1996 to February Week 3 2006>
Search Strategy:
--------------------------------------------------------------------------------
exp Risk Factors/ (186400)
*Macular Degeneration/ (2538)
*Glaucoma/ (4024)
1 and (2 or 3) (541)
(risk factor$ adj3 (macular degeneration or maculopath$ or glaucoma)).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (125)
4 or 5 (629)
limit 6 to (humans and english language and yr=“2000 - 2006”) (331)
(systematic review$ or meta-analysis or metaanalysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word] (22113)
7 and 8 (5)
7 (331)
limit 10 to (case reports or comment or editorial or letter or “review”) (89)
10 not 11 (242)
9 or 12 (245)
Database: EMBASE <1980 to 2006 Week 07>
Search Strategy:
--------------------------------------------------------------------------------
exp Risk Factor/ (166223)
*Retina Macula Degeneration/ (1124)
*GLAUCOMA/ (11067)
1 and (2 or 3) (443)
(risk factor$ adj3 (macular degeneration or maculopath$ or glaucoma)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (852)
limit 5 to (human and english language and yr=“2000 - 2006”) (431)
(systematic review$ or metaanalysis or meta-analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (36634)
6 and 7 (6)
6 (431)
limit 9 to (editorial or letter or note or “review”) (174)
Case Report/ (873920)
9 not (10 or 11) (248)
8 or 12 (253)
Appendix 2 - GRADE
Type of evidence
RCT: given a high GRADE level to start
Observational study: given a low GRADE level to start
Any other evidence: given a very low GRADE level to start
Decrease GRADE if:
Serious limitation to study quality (-1, reduce GRADE level by 1 so a high GRADE level will become a moderate GRADE) or very serious limitation to study quality (-2, reduce GRADE level by 2 so a high GRADE level will become low GRADE)
Important inconsistency (-1, reduce GRADE level by 1)
Some (-1) or major (-2) uncertainty about directness
Imprecise or sparse data (-1)
High probability of reporting bias (-1)
Increase GRADE level if:
Strong evidence of association-significant relative risk of greater than 2 (< 0.5) based on consistent evidence from 2 or more observation studies, with no plausible confounders (+1, increase GRADE level by 1, so a moderate GRADE will become high. However a high GRADE will remain high)
Very strong evidence of association-significant relative risk of greater than 5 (< 0.2) based on direct evidence with no major threats to validity (+2, increase GRADE level by 2, so a low GRADE will become a high GRADE)
Evidence of a dose response gradient (+1)
All plausible confounders would have reduced the effect (+1).
Overall GRADE Level definitions
| High: | Further research is very unlikely to change our confidence in the estimate of effect. |
| Moderate: | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. |
| Low: | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. |
| Very low: | Any estimate of effect is very uncertain. |
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Routine eye examinations for persons 20-64 years of age: an evidence-based analysis. Ontario Health Technology Assessment Series 2006; 6(15)
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo@moh.gov.on.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo@moh.gov.on.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo@moh.gov.on.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-4317-3 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and information from practicing medical experts and industry, adds important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to maximize patient outcomes.
If you are aware of any current additional evidence to inform an existing Evidence-Based Analysis, please contact the Medical Advisory Secretariat: MASInfo@moh.gov.on.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to do so, this document may not fully reflect all scientific research available. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superceded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas
Abbreviations
- ARM
Age related maculopathy
- AMD
Age-related macular degeneration
- CI
Confidence interval
- FDA
Food and Drug Administration
- IOP
Intraocular pressure
- NNH
Numbers needed to harm
- NNT
Numbers needed to treat
- OHIP
Ontario Health Insurance Plan
- POAG
Primary open angle glaucoma
- RCT
Randomized controlled trial
- RD
Risk difference
- RPE
Retinal pigment epithelium
- RR
Relative Risk
- VEGF
Vascular endothelial growth factor
Footnotes
This number was estimated based on the 2005/06 estimate, since this was the only year in which a stable estimate of utilization after delisting was available
References
- 1.Ministry of Health and Long-Term Care. Schedule of benefits for physician services under the Health Insurance Act [Web page] 2006. [[cited 2006 July 7]]. Available at: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/physserv_mn.html .
- 2.Ministry of Health and Long-Term Care. Changes to OHIP coverage for eyecare services. Fact sheet [Web page] May, 2005. [[cited 2006 July 15]]. Available at: http://www.health.gov.on.ca/english/public/pub/ohip/pdf/eyecare.pdf .
- 3.Ontario Ministry of Health and Long-Term Care. Request for major eye examination [Web page] 2004. [[cited 2006 Aug. 8]]. Available at: http://www.forms.ssb.gov.on.ca/mbs/ssb/forms/ssbforms.nsf/AttachDocsPublish/014-4347-84~1/$File/4347-84E_.pdf .
- 4.Girard M. The Canadian National Institute for the Blind submission to the Commission on the Future of Health Care in Canada [report on the Internet] Dec 21, 2001. [[cited 2006 June 5]]. Available at: http://www.cnib.ca/eng/publications/health_care.htm .
- 5.Ramakrishnan R, Nirmalan PK, Krishnadas R, Thulasiraj RD, Tielsch JM, Katz J, et al. Glaucoma in a rural population of southern India: the Aravind comprehensive eye survey. [[erratum appears in Ophthalmology. 2004 Feb;111(2):331]]. Ophthalmology. 2003;110(8):1484–1490. doi: 10.1016/S0161-6420(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 6.Tielsch JM. The epidemiology and control of open angle glaucoma: a population-based perspective. Annu Rev Public Health. 1996;17:121–136. doi: 10.1146/annurev.pu.17.050196.001005. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Task Force on the Periodic Health Examination. [Periodic health examination, 1996 update: screening for visual problems among elderly patients]. Can Med Assoc J. 1995;152(8):1211–1222. [PMC free article] [PubMed] [Google Scholar]
- 8.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82(11):887–888. [PMC free article] [PubMed] [Google Scholar]
- 9.United States Preventative Task Force. Screening for glaucoma: recommendation statement. Ann Fam Med. 2005;3(2):171–172. doi: 10.1370/afm.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukesh BN, McCarty CA, Rait JL, Taylor HR. Five year incidence of open-angle glaucoma: the visual impairment project. Ophthalmology. 2002;109(6):1047–1051. doi: 10.1016/s0161-6420(02)01040-0. [DOI] [PubMed] [Google Scholar]
- 11.Tuulonen A, Airaksinen PJ, Erola E, Forsman E, Friberg K, Kaila M, et al. The Finnish evidence-based guideline for open-angle glaucoma. Acta Ophthalmol Scand. 2003;81(1):3–18. doi: 10.1034/j.1600-0420.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 12.Elolia R, Stokes J. Monograph series on aging-related diseases: XI Glaucoma. Chronic Dis Can. 2000;19(4):1–24. [PubMed] [Google Scholar]
- 13.Wilson RM. Progression of visual field loss in untreated glaucoma patients and suspects in St. lucia, West Indies. Trans Am Ophthalmol Soc. 2002;100:365–410. [PMC free article] [PubMed] [Google Scholar]
- 14.Allingham RR, Damji K, Freedman S, Moroi S, Shafranov G. Shields MB. Clinical epidemiology of glaucoma. Philadelphia: Lippincott Williams & Wilkins. 5. 2005. pp. 170–198. Shields' textbook of glaucoma.
- 15.Fleming D, Whitlock EP, Beil T, Smit B, Harris RP. Screening for primary open-angle glaucoma in the primary care setting: an update for the U.S. preventative services task force [report on the Internet] [[cited 2006 July 15]]. Available at: http://www.ahrq.gov/clinic/uspstf05/glaucoma/glaucup.htm .
- 16.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The ocular hypertension treatment study. Arch Ophthalmol. 2002;120(2002):701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 17.Ferris FL, Tielsch JM. Blindness and Visual Impairment. Arch Ophthalmol. 2004;122:451–452. doi: 10.1001/archopht.122.4.451. [DOI] [PubMed] [Google Scholar]
- 18.Feiner L, Piltz-Seymour JR. Collaborative Initial Galucoma Treatment Study: a summary of results to date. Curr Opin Ophthalmol. 2003;14:106–111. doi: 10.1097/00055735-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Heijl A, Leske C, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(1268):1278. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 20.Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 21.van Leeuwen R, Klaver C, Vingerling JR, Hofman A, de Jong PT. Epidemiology of age-related maculopathy: a review. Eur J Epidemiol. 2003;18:845–854. doi: 10.1023/a:1025643303914. [DOI] [PubMed] [Google Scholar]
- 22.Wormald R, Evans JR, Smeeth L, Henshaw K. Photodynamic therapy for neovascular age-related macular degeneration (Cochrane Review) Cochrane Database of Systematic Reviews. 2005. Issue 4. Art. No.: CD002030. DOI:10.1002/14651858.CD002030. [DOI] [PubMed]
- 23.Ikram MK, van Leeuwen R, Vingerling JR, Hofman A, de Jong PT. Relationship between refraction and prevalent as well as incident age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2003;44(9):3778–3782. doi: 10.1167/iovs.03-0120. [DOI] [PubMed] [Google Scholar]
- 24.Arroyo Jorge G. A 76-year-old man with macular degeneration. JAMA. 2006;295(20):2394–2406. doi: 10.1001/jama.295.20.2394. [DOI] [PubMed] [Google Scholar]
- 25.Parmet S, Lynm C, Glass RM. Age-related macular degeneration. JAMA. 2006;295(20):2438. doi: 10.1001/jama.295.20.2438. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb JL. Age-related macular degeneration. JAMA. 2002;288(18):2233–2236. doi: 10.1001/jama.288.18.2233. [DOI] [PubMed] [Google Scholar]
- 27.Health Canada. Macugen [Web page]. Health Product Database. 2006. [[cited 2006 June 6]]. Available at: http://www.hc-sc.gc.ca/drug2/product/p75262.html .
- 28.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus Verteporfin for Neovascular age-related macular dgeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 30.United States Food and Drug Administration (FDA) FDA approves new biologic treatment for wet age-related macular degeneration [Web page] 2006. [[cited 2006 July 4]]. Available at: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01405.html .
- 31.Steinbrook R. The price of sight-Ranibizumab, Bevacizumab, and the treatment of macular degeneration. N Engl J Med. 2006;355(14):1409–1412. doi: 10.1056/NEJMp068185. [DOI] [PubMed] [Google Scholar]
- 32.Ministry of Government Services. Highway Traffic Act. Ontario Regulation 340/94. 2005. [[cited 2006 July 4]]. Available at: http://www.e-laws.gov.on.ca/DBLaws/Regs/English/940340_e.htm .
- 33.GRADE Working Group. Grade [Web page] 2006. [[cited 2005 Aug. 8]]. Available at: www.gradeworkinggroup.org .
- 34.GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1–8. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leske MC, Connell AM, Wu SY, Nemesure B, Li X, Schachat A, et al. Incidence of open-angle glaucoma: the Barbados Eye Studies. Arch Ophthalmol. 2001;119(1):89–95. [PubMed] [Google Scholar]
- 36.Vijaya L, George R, Paul PG, Baskaran M, Arvind H, Raju P, et al. Prevalence of open angle- glaucoma in a rural South Indian Population. Invest Ophthalmol Vis Sci. 2006;46(12):4461–4467. doi: 10.1167/iovs.04-1529. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell P, Smith W, Attebo K, Healey P. Prevalence of open-angle glaucoma in Australia: the blue mountains eye study. Ophthalmology. 1996;103(1661):1669. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106(10):2010–2015. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell P, Rochtchina E, Lee AJ, Wang JJ. Bias in self-reported family history and relationship to glaucoma: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2002;9(5):333–345. doi: 10.1076/opep.9.5.333.10335. [DOI] [PubMed] [Google Scholar]
- 40.Leske MC, Connell AMS, Schachat A, Hyman L. The Barbados eye study: prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–829. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 41.Leske MC, Connell AM, Wu SY, Hyman LG, Schachat AP. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol. 1995;113(7):918–924. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- 42.Tielsch JM, Sommer A, Katz J, Royall R, Quigley HA, Javitt J. Racial Variations in the prevalence of primary open-angle glaucoma. JAMA. 1991;266(3):369–374. [PubMed] [Google Scholar]
- 43.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt J. Family history and risk of primary open angle glaucoma. Arch Ophthalmol. 1994;112:69–73. doi: 10.1001/archopht.1994.01090130079022. [DOI] [PubMed] [Google Scholar]
- 44.Klein BEK, Klein R, Sponsel WE, Franke T, Cantor LB, Martone J, et al. Prevalence of glaucoma: the Beaver Dam Study. Ophthalmology. 1992;99:1499–1504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- 45.Wong TY, Klein BEK, Klein R, Knudtson M, Lee KE. Refractive errors, intraocular pressure, and glaucoma in a white population. Ophthalmology. 2003;110(1):211–217. doi: 10.1016/s0161-6420(02)01260-5. [DOI] [PubMed] [Google Scholar]
- 46.Tielsch JM, Katz J, Quigley HA, Javitt J, Sommer A. Diabetes, intraocular pressure, and primary open-angle glaucoma in the Baltimore Eye Survey. Ophthalmology. 1995;102(48):53. doi: 10.1016/s0161-6420(95)31055-x. [DOI] [PubMed] [Google Scholar]
- 47.Leske MC, Wu SY, Hennis A, Nemesure B, Yang L, Hyman L, et al. Nine-year incidence of age-related macular degeneration in the Barbados Eye Studies. Ophthalmology. 2006;113(1):29–35. doi: 10.1016/j.ophtha.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 48.van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. [[erratum appears in Arch Ophthalmol. 2003 Jul;121(7):955]]. Arch Ophthalmol. 2003;121(4):519–526. doi: 10.1001/archopht.121.4.519. [DOI] [PubMed] [Google Scholar]
- 49.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104(1):7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell P, Wang JJ, Foran S, Smith W. Five year incidence of age-related maculopathy lesions. Ophthalmology. 2002;109:1092–1097. doi: 10.1016/s0161-6420(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 51.Tomany SC, Wang JJ, van Leeuwen R, Klein R, Mitchell P, Vingerling JR, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111(7):1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Klein R, Klein BE, Jensen SC, Cruickshanks KJ. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmol. 1998;116(4):506–513. doi: 10.1001/archopht.116.4.506. [DOI] [PubMed] [Google Scholar]
- 53.Wong TY, Klein R, Klein BE, Tomany SC. Refractive errors and 10-year incidence of age-related maculopathy. Invest Ophthalmol Vis Sci. 2002;43(9):2869–2873. [PubMed] [Google Scholar]
- 54.The Eye Disease Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004. pp. 564–572. [DOI] [PubMed]
- 55.Fraser-Bell S, Donofrio J, Wu J, Klein R, Azen SP, Varma R, et al. Sociodemographic factors and age-related macular degeneration in Latinos: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2005;139(1):30–38. doi: 10.1016/j.ajo.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 56.Smith W, Mitchell P. Family history and age-related maculopathy: the Blue Mountains Eye Study. Aust N Z J Ophthalmol. 1998;26(3):203–206. doi: 10.1111/j.1442-9071.1998.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 57.Klein R, Clegg L, Cooper LS, Hubbard LD, Klein BE, King WN, et al. Prevalence of age-related maculopathy in the Atherosclerosis Risk in Communities Study. Arch Ophthalmol. 1999;117(9):1203–1210. doi: 10.1001/archopht.117.9.1203. [DOI] [PubMed] [Google Scholar]
- 58.Klein R, Klein BE, Knudtson M, Wong TY, Cotch MF, Liu K, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113(373):380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Boivin JF, McGregor M, Archer C. Cost effectiveness of screening for primary open angle glaucoma. J Med Screen. 1996;3:154–163. doi: 10.1177/096914139600300309. [DOI] [PubMed] [Google Scholar]
- 60.Ministry of Finance. Ontario population projections update [Web page] 2006. [[cited 2006 June 15]]. Available at: http://www.fin.gov.on.ca/english/demographics/demog06.html .
- 61.Ministry of Health and Long-Term Care. Ontario Provincial Health Planning Database. 2006. [database]. [ver. 2]
- 62.Ministry of Health and Long-Term Care. Schedule of benefits for optometry services [report on the Internet]. 2006. [[cited 2006 July 20]]. Available at: http://www.health.gov.on.ca/english/providers/program/ohip/sob/optometry/optometry.pdf .
- 63.Iskedjian M, Walker J, Vincente C, Trope G, Buys Y, Einarson TR, et al. Cost of Glaucoma in Canada: analyses based on visual field and physician's assessment. J Glaucoma. 2003;12(6):456–462. doi: 10.1097/00061198-200312000-00002. [DOI] [PubMed] [Google Scholar]


