Executive Summary
Objective
The objective of this health technology policy assessment was to determine the effectiveness and cost-effectiveness of using intravascular ultrasound (IVUS) as an adjunctive imaging tool to coronary angiography for guiding percutaneous coronary interventions.
Background
Intravascular Ultrasound
Intravascular ultrasound is a procedure that uses high frequency sound waves to acquire 3-dimensional images from the lumen of a blood vessel. The equipment for performing IVUS consists of a percutaneous transducer catheter and a console for reconstructing images. IVUS has been used to study the structure of the arterial wall and nature of atherosclerotic plaques, and obtain measurements of the vessel lumen. Its role in guiding stent placement is also being investigated. IVUS is presently not an insured health service in Ontario.
Clinical Need
Coronary artery disease accounts for approximately 55% of cardiovascular deaths, the leading cause of death in Canada. In Ontario, the annual mortality rate due to ischemic heart disease was 141.8 per 100,000 population between 1995 and 1997. Percutaneous coronary intervention (PCI), a less invasive approach to treating coronary artery disease, is used more frequently than coronary bypass surgery in Ontario. The number of percutaneous coronary intervention procedures funded by the Ontario Ministry of Health and Long-term Care is expected to increase from approximately 17, 780 in 2004/2005 to 22,355 in 2006/2007 (an increase of 26%), with about 95% requiring the placement of one or more stents. Restenosis following percutaneous coronary interventions involving bare metal stents occurs in 15% to 30% of the cases, mainly because of smooth muscle proliferation and migration, and production of extracellular matrix. In-stent restenosis has been linked to suboptimal stent expansion and inadequate lesion coverage, while stent thrombosis has been attributed to incomplete stent-to-vessel wall apposition. Since coronary angiography (the imaging tool used to guide stent placement) has been shown to be inaccurate in assessing optimal stent placement, and IVUS can provide better views of the vessel lumen, the clinical utility of IVUS as an imaging tool adjunctive to coronary angiography in coronary intervention procedures has been explored in clinical studies.
Method
A systematic review was conducted to answer the following questions:
What are the procedure-related complications associated with IVUS?
Does IVUS used in conjunction with angiography to guide percutaneous interventions improve patient outcomes compared to angiographic guidance without IVUS?
Who would benefit most in terms of clinical outcomes from the use of IVUS adjunctive to coronary angiography in guiding PCIs?
What is the effectiveness of IVUS guidance in the context of drug-eluting stents?
What is the cost-effectiveness ratio and budget impact of adjunctive IVUS in PCIs in Ontario?
A systematic search of databases OVID MEDLINE, EMBASE, MEDLINE In-Process & Other Non-Indexed Citations, The Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) database for the period beginning in May 2001 until the day of the search, November 4, 2005 yielded 2 systematic reviews, 1 meta-analysis, 6 randomized controlled trials, and 2 non-randomized studies on left main coronary arteries. The quality of the studies ranged from moderate to high. These reports were combined with reports from a previous systematic review for analysis. In addition to qualitative synthesis, pooled analyses of data from randomized controlled studies using a random effect model in the Cochrane Review Manager 4.2 software were conducted when possible.
Findings of Literature Review & Analysis
Safety
Intravascular ultrasound appears to be a safe tool when used in coronary interventions. Periprocedural complications associated with the use of IVUS in coronary interventions ranged from 0.5% in the largest study to 4%. Coronary rupture was reported in 1 study (1/54). Other complications included prolonged spasms of the artery after stenting, dissection, and femoral aneurysm.
Effectiveness
Based on pooled analyses of data from randomized controlled studies, the use of intravascular ultrasound adjunctive to coronary intervention in percutaneous coronary interventions using bare metal stents yielded the following findings:
For lesions predominantly at low risk of restenosis:
There were no significant differences in preintervention angiographic minimal lumen diameter between the IVUS-guided and angiography-guided groups.
IVUS guidance resulted in a significantly larger mean postintervention angiographic minimal lumen diameter (weighted mean difference of 0.11 mm, P = .0003) compared to angiographic guidance alone.
The benefit in angiographic minimal lumen diameter from IVUS guidance was not maintained at 6-month follow-up, when no significant difference in angiographic minimal lumen diameter could be detected between the two arms (weighted mean difference 0.08, P = .13).
There were no statistically significant differences in angiographic binary restenosis rates between IVUS-guidance and no IVUS guidance (Odds ratio [OR] 0.87 in favour of IVUS, 95% Confidence Interval [CI] [0.64–1.18], P = 0.37).
IVUS guidance resulted in a reduction in the odds of target lesion revascularization (repeat percutaneous coronary intervention or coronary bypass graft) compared to angiographic guidance alone. The reduction was statistically significant at a follow-up period of 6 months to 1 year, and at a follow-up period of 18 month to 2 years (OR 0.52 in favour of IVUS, 95% CI [0.33–0.81], P = .004).
Total revascularization rate (either target lesion or target vessel revascularization) was significantly lower for IVUS-guided patients at 18 months to 2.5 years after intervention (OR 0.43 in favour of IVUS, 95% CI [0.29–0.63], p < .0001).
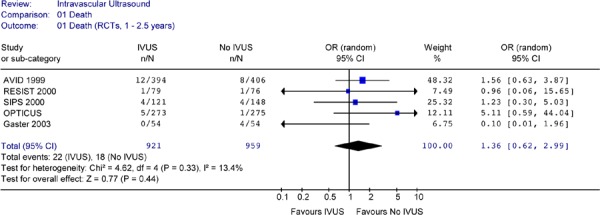
There were no statistically significant differences in the odds of death (OR 1.36 in favour of no IVUS, P =0.65) or myocardial infarction (OR 0.95 in favour of IVUS, P = 0.93) between IVUS-guidance and angiographic guidance alone at up to 2.5 years of follow-up
The odds of having a major cardiac event (defined as death, myocardial infarction, and target lesion or target vessel revascularization) were significantly lower for patients with IVUS guidance compared to angiographic guidance alone during follow-up periods of up to 2.5 years (OR 0.53, 95% CI [0.36–0.78], P = 0.001). Since there were no significant reductions in the odds of death or myocardial infarction, the reduction in the odds of combined events reflected mainly the reduction in revascularization rates.
For lesions at High Risk of Restenosis:
There is evidence from one small, randomized controlled trial (n=150) that IVUS-guided percutaneous coronary intervention in long de novo lesions (>20 mm) of native coronary arteries resulted in statistically significant larger minimal lumen Diameter, and statistically significant lower 6-month angiographic binary restenosis rate. Target vessel revascularization rate and the rate of combined events were also significantly reduced at 12 months.
A small subgroup analysis of a randomized controlled trial reported no benefit in clinical or angiographic outcomes for IVUS-guided percutaneous coronary interventions in patients with diabetes compared to those guided by angiography. However, due to the nature and size of the analysis, no firm conclusions could be reached.
Based on 2 small, prospective, non-randomized controlled studies, IVUS guidance in percutaneous coronary interventions of left main coronary lesions using bare metal stents or drug-eluting stents did not result in any benefits in angiographic or clinical outcomes. These findings need to be confirmed.
Interventions Using Drug-Eluting Stents
There is presently no evidence on whether the addition of IVUS guidance during the implantation of drug-eluting stents would reduce incomplete stent apposition, or improve the angiographic or clinical outcomes of patients.
Ontario-Based Economic Analysis
Cost-effectiveness analysis showed that PCIs using IVUS guidance would likely be less costly and more effective than PCIs without IVUS guidance. The upfront cost of adjunctive use of IVUS in PCIs ranged from $1.56 million at 6% uptake to $13.04 million at 50% uptake. Taking into consideration cost avoidance from reduction in revascularization associated with the use of IVUS, a net saving of $0.63 million to $5.2 million is expected. However, since it is uncertain whether the reduction in revascularization rate resulting from the use of IVUS can be generalized to clinical settings in Ontario, further analysis on the budget impact and cost-effectiveness need to be conducted once Ontario-specific revascularization rates are verified.
Factors to be Considered in the Ontario Context
Applicability of Findings to Ontario
The interim analysis of an Ontario field evaluation that compared drug-eluting stents to bare metal stents showed that the revascularization rates in low-risk patients with bare metal stents were much lower in Ontario compared to rates reported in randomized controlled trials (7.2% vs >17 %). Even though IVUS is presently not routinely used in the stenting of low-risk patients in Ontario, the revascularization rates in these patients in Ontario were shown to be lower than those reported for the IVUS groups reported in published studies. Based on this information and previous findings from the Ontario field evaluation on stenting, it is uncertain whether the reduction in revascularization rates from IVUS guidance can be generalized to Ontario. In light of the above findings, it is advisable to validate the reported benefits of IVUS guidance in percutaneous coronary interventions involving bare metal stents in the Ontario context.
Licensing Status
As of January 16, 2006, Health Canada has licensed 10 intravascular ultrasound imaging systems/catheters for transluminal intervention procedures, most as class 4 medical devices.
Current Funding
IVUS is presently not an insured procedure under the Ontario Health Insurance Plan and there are no professional fees for this procedure. All costs related to the use of IVUS are covered within hospitals’ global budgets. A single use IVUS catheter costs approximately $900CDN and the procedure adds approximately 20 minutes to 30 minutes to a percutaneous coronary intervention procedure.
Diffusion
According to an expert consultant, current use of IVUS in coronary interventions in Ontario is probably limited to high-risk cases such as interventions in long lesions, small vessels, and bifurcated lesions for which images from coronary angiography are indeterminate. It was estimated that IVUS is being used in about 6% of all percutaneous coronary interventions at a large Ontario cardiac centre.
Expert Opinion
IVUS greatly enhances the cardiac interventionists’ ability to visualize and assess high-risk lesions such as long lesions, narrow lesions, and bifurcated lesions that may have indeterminate angiographic images. Information from IVUS in these cases facilitates the choice of the most appropriate approach for the intervention.
Conclusion
The use of adjunctive IVUS in PCIs using bare metal stents in lesions predominantly at low risk for restenosis had no significant impact on survival, myocardial infarction, or angiographic restenosis rates up to 2.5 years after intervention.
The use of IVUS adjunctive to coronary angiography in percutaneous coronary interventions using bare metal stents in lesions predominantly at low risk for restenosis significantly reduced the target lesion and target vessel revascularization at a follow-up period of 18 months to 2.5 years.
One small study suggests that adjunctive IVUS in PCIs using bare metal stents in long lesions (>20 mm) significantly improved the 6-month angiographic restenosis rate and one-year target lesion revascularization rate. These results need to be confirmed with large randomized controlled trials.
Based on information from the Ontario field evaluation on stenting, it is uncertain whether the reduction in revascularization rate resulting from the use of IVUS in the placement of bare metal stents can be generalized to clinical settings in Ontario.
There is presently insufficient evidence available to determine the impact of adjunctive IVUS in percutaneous interventions in high-risk lesions (other than long lesions) or in PCIs using drug-eluting stents.
Objective
The objective of this health technology policy assessment was to determine the effectiveness and cost-effectiveness of intravascular ultrasound (IVUS) as an adjunct to coronary angiography to guide percutaneous coronary interventions (PCIs).
Clinical Need
Coronary Artery Disease
Cardiovascular disease is the leading cause of death in Canada, accounting for 36% of all deaths in 1999. (1) More than 55% of cardiovascular deaths were due to ischemia resulting from coronary artery disease (CAD). In Ontario, between 1995 and 1997, the average annual mortality rate due to cardiovascular disease was 245.7 per 100,000 population, and the mortality rate due to ischemic heart disease was 141.8 per 100,000 population. (2)
The coronary artery and its branches supply the heart with oxygenated blood. CAD results from narrowing (stenosis) of the lumen of one or more coronary arteries due to fatty deposits (plaques) on the interior vessel wall. A greater than 50% narrowing of the artery could impede the flow of blood, and decrease the supply of oxygen to the heart muscle, causing angina. Total blockage of a coronary artery results in myocardial infarction (cell death), and if left untreated, may lead to heart failure and death.
Medical therapy for CAD aims to increase blood supply to the heart muscle, and reduce the heart muscle’s demand for oxygen. Medication usually includes nitroglycerine, beta blockers, and calcium channel blockers. Antiplatelet drugs such as aspirin are also recommended for patients with CAD. Other medications may be required for to treat risk factors of CAD such as hypercholesteremia and hypertension.
When medical therapy fails to control the angina, the remaining treatment options are coronary bypass graft (CABG) and percutaneous coronary interventions. CABG is a procedure which uses a piece of a vein or artery from the leg or the chest to bypass the blocked segment.
PCIs are percutaneous catheter procedures that do not require open surgery. PCIs consist of the following procedures performed alone or in combination:
Balloon dilation: This involves the insertion of a transluminal catheter into an artery in the groin area, navigating it to the area of the narrowed coronary artery, and inflating a balloon at the tip of the catheter to dilate the artery. It may be performed in isolation or with stenting and/or atherectomy.
Coronary stenting: This procedure is the transluminal deployment, at the site of the stenosis, of one or more tube-like or mesh-like devices (stents) mounted on a balloon catheter. The stent may be self-expanding or expanded by inflation of the balloon. The stents remain inside the vessel after deflation of the balloon and withdrawal of the catheter. The metal stent acts as a scaffold to prevent recoil and closure of the vessel. Randomized controlled trials have demonstrated that stents significantly reduce the incidence of angiographic stenosis and repeat angioplasty in patients with discrete, new lesions in large target vessels. (3;4)
Atherectomy: This is a procedure that removes calcified plaques from within a coronary artery using a transluminal cutting balloon, an atherectomy device, or laser. It may be performed in isolation or prior to balloon dilatation and/or stenting
Due to their less invasive nature, PCIs have become the treatments of choice for many CAD patients. In Ontario, PCIs are used twice as often as CABGs. The predominant PCI procedure performed in Ontario is stenting with or without balloon pre-dilatation, guided by coronary angiography alone in the majority of cases. The number of PCI procedures funded by the Ontario Ministry of Health and Long-Term Care is expected to increase from approximately 17,780 in 2004/2005 to 22,355 in 2006/2007 (an increase of 26%), with about 95% requiring the placement of one or more stents (MOHLTC data). However, despite improved stent design and the use of antiplatelet drugs, the effectiveness of stenting using bare metal stents is still hampered by the recurrence of luminal narrowing due to in-stent restenosis .
In-Stent Restenosis (ISR)
Stenting causes injury to the luminal wall of the coronary artery, resulting in neointimal hyperplasia (proliferation and migration of vascular smooth muscle cells and production of extracellular matrix) inside the stent, the main cause of ISR. ISR has been shown to occur in 15% to 30% of people implanted with bare metal stents. A 2005 interim report on a large observational study (5) in Ontario (n = 9,103) showed that the incidence of restenosis had been reduced with the use of new generation bare metal stents (7.2%) in low risk populations characterized by short and wide lesions in non-diabetic patients. However, the rate of ISR still ranged from 9% to 11% with the use of bare metal stents in patients with long or narrow lesions, and from 8% to 21% if these lesions occurred in people with diabetes. (5) Studies suggest that suboptimal stent deployment such as incomplete stent apposition (ISA), inadequate lesion coverage, and inadequate stent expansion may be contributing factors to the development of ISR. (6;7) Post-intervention lumen diameter has been identified as an important independent predictor of restenosis rate. (8) Moreover, incomplete stent apposition has been associated with subsequent stent thrombosis. (9) In stenting procedures, attempts are made to achieve a large post-procedural lumen, in order to compensate for subsequent late lumen loss due to neointimal growth. (10) It is believed that an imaging technology that can accurately assess stent lumen size and residual stenosis during stent implantation is important in achieving optimal stent deployment.
Coronary Angiography
Coronary angiography is a technique for imaging coronary arteries using x-ray fluoroscopy following the injection of a radiographic contrast medium into the coronary arteries through a percutaneous catheter. Assessments can be conducted visually; however, computerized quantitative coronary angiography (QCA) reduces inter-reader variability. Coronary angiography has been the gold standard for diagnosing CAD, revealing the location, extent, and severity of coronary arterial blockages. Coronary angiography is the imaging tool usually used to guide stent placement. In this application, the extent of the stenosis before and after stenting is based on measurement of the minimal lumen diameter (MLD) within the lesion, and comparing it to the mean luminal diameter of normal segments proximal and distal to the lesion.
Although angiography has been the predominant method used to define coronary anatomy in stenting procedures, intravascular ultrasound (IVUS) had revealed insufficiently dilated stents after final balloon dilation in 60% to 80% of cases despite a satisfactory result according to angiography. (6;11;12) Studies suggest that quantitative coronary angiography overestimates lumen dimensions after stenting, and the adequacy of stent placement in stenotic lesions. (13;14) These findings may be explained by limitations of coronary angiography: (15)
Angiography provides a 2-dimensional planer silhouette of the contrast filled lumen of the vessel which can misrepresent the true extent of luminal narrowing
CAD and mechanical interventions during PCIs may increase lumen irregularity, affecting the accuracy of angiography.
In angiographic images, outward remodelling of the vessel wall may conceal early atherosclerosis
In vessels with diffuse CAD, angiography may not detect disease in the “normal segments” chosen as a reference, resulting in an underestimation of the extent of atherosclerosis in the target lesion.
Angiography does not allow an assessment of the plaque burden
Because of limitations of coronary angiography, IVUS has been investigated as an adjunct to coronary angiography to guide balloon dilatation and stenting procedures. It is believed that the addition of IVUS can accurately assess stent lumen, resulting in the use of larger balloons and higher pressure to achieve optimal stent lumen.
The Technology – Intravenous Ultrasound
Intravascular Ultrasound (IVUS)
Intravascular ultrasound (IVUS) is a procedure that uses ultrasound to provide images from inside the lumen of a blood vessel. It is presently not an insured health service in Ontario.
An IVUS system consists of a catheter mounted with a miniature transducer at the tip (Figure 1) and a console (Figure 2) for processing the data and displaying the images. The transducer may be mechanical, consisting of a single rotating transducer driven by a flexible drive cable, or it may be electronic, consisting of a set of transducing crystals arranged circularly. Combined IVUS and stent delivery catheters have been developed, but were not in use in Ontario at the time of this report (Personal communication, March 2006)
Figure 1: Example of an IVUS Imaging Catheter.
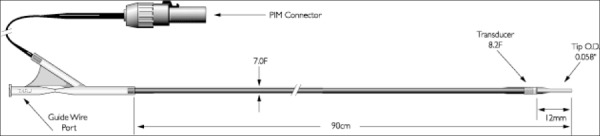
Visions® PV 8.2F technical drawing from Volcano Therapeutics: http://www.volcanotherapeutics.com/products/ivus-imaging.lvisions-pv82f.asp
Figure 2: Example of an IVUS Processing & Display Console.
Galaxy2™ Imaging System Image provided courtesy of Boston Scientific. ©2006 Boston Scientific Corporation or it’s affiliates. All rights reserved.
IVUS of a coronary artery is performed in a catheterization laboratory. The IVUS catheter is inserted into an artery in the groin area, and navigated to a coronary artery. The catheter is usually positioned distal to the lesion or stent, and withdrawn through the lesion/stent at a constant speed manually or with an automatic mechanical pullback device. (16) The miniature transducer produces high frequency sound waves. Structures such as blood, tissues, and plaques in the artery reflect sound waves differently because of differences in density. (Figure 3) The reflected ultrasound waves are processed electronically to reconstruct black and white images displayed on a monitor and recorded on videotape (Figure 4). Cardiologists may interpret these images on-line or off-line to obtain information about lumen dimensions, stent expansion, and plaque structure.
Figure 3: How IVUS Works.
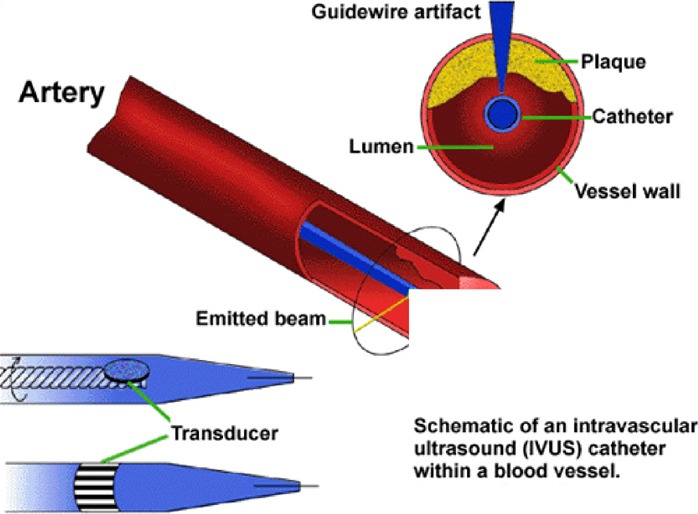
From American Heart Journal, Vol. 130, Kimura BJ, Bhargava V, DeMaria AN. Value and limitations of intravascular ultrasound imaging in characterizing coronary atherosclerotic plaque, pp: 386-396, Copyright 1995 with permission from Elsevier; Adaptation used with permission from the Oak Ridge Institute for Science and Education (ORAU):http://www.orau.pov/ehsd/lvus.GIF
Figure 4: An IVUS Image of a Coronary Artery.
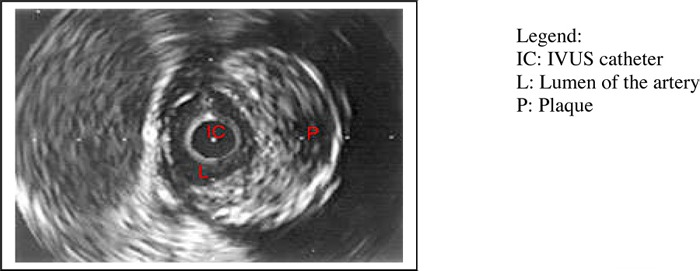
Image copyright Texas Heart Institute, www.texasheart.org; Used with permission
An advantage of IVUS is its ability to provide 3-dimensional images of a cross section (Figure 4) or longitudinal section of the blood vessel. It can be used in the diagnosis of coronary artery disease by assessing the degree of narrowing in the blood vessel and the extent and composition of the plaque, and by detecting the presence of dissection, plaque rupture, and thrombus. IVUS findings have also been used to predict the likely functional severity of lesions.
This review focuses on the therapeutic role of IVUS in the provision of serial monitoring during PCI procedures, and in the assessment of adequacy of balloon dilatation and stent placement.
Regulatory Status of Intravascular Ultrasound Systems and Catheters
As of January 16, 2006, Health Canada has licensed the IVUS systems and catheters listed in Table 1. Most of the IVUS devices are licensed as Class 4 medical devices. The only exception is license 61746 (the Galaxy system), which is Class 3, and license 67817 (Pioneer catheter), which is class 2 (Health Canada, March 2006).
Table 1: Health Canada Licensed Intravascular Ultrasound Devices.
| Intravascular Ultrasound Systems | January 16, 2006 | |||
|---|---|---|---|---|
| Company Name | Licence | Licence Name | Trade Name | Purpose/Intended Use |
| Boston Scientific Corporation | 14428 | Intravascular Ultrasound Imaging System and | Clearview Ultra Imaging System Intravascular Ultrasound ND | Intended for the ultrasound examination of intravascular/intraluminal pathology. Indicated in patients who are candidates for transluminal interventional procedures such as angioplasty, atherectomy, the placement of stents or other interventional procedures. |
| 14431 | Intravascular Ultrasound Imaging System and Accessories | Clearview Ultra Imaging System Intravascular Ultrasound | Intended for the ultrasound examination of intravascular/intraluminal pathology. Indicated in patients who are candidates for transluminal interventional procedure such as angioplasty atherectomy, the placement of stents or other interventional procedures. | |
| 14435 | Intravascular Ultrasound Imaging System and accessories | Clearview Ultra Imaging System Intravascular Ultrasound | Intended for the ultrasound examination of intravascular/intraluminal pathology. Indicated in patients who are candidates for transluminal interventional procedures such as angioplasty, atherectomy, the placement of stents or other interventional procedures. | |
| 61746 | Galaxy Intravascular Ultrasound Imaging System | Galaxy Intravascular Ultrasound System | The Galaxy IVUS is used in conjunction with a variety of Imaging catheters. Is intended for ultrasound examination of intravascular and intracardiac pathology. Indicated for transluminal coronary interventional procedures. | |
| 61747 | Atlantis Coronary Imaging Catheters | Atlantis SR Intravascular Catheters | Intended for ultrasound examination of coronary intravascular pathology only. Intravascular ultrasound imaging is indicated in patients who are candidates for transluminal coronary interventional procedures | |
| MEDTRONIC INC. | 67817 | Pioneer Catheter | Pioneer Catheter | The Pioneer Catheter is a short term, intravascular catheter. It uses an extendable, hollow needle and intravascular ultrasound to facilitate redirection and placement of a guide wire into peripheral vessels. |
| SIEMENS MEDICAL SOLUTIONS USA, INC | 21099 | ACUNAV Diagnostic Ultrasound Cardiac Catheter | ACUNAV Diagnostic Ultrasound Cardiac Catheter | This is an ultrasound-tipped catheter device, which is used directly within the vasculature and/or right heart for intravascular or intracardiac ultrasound, imaging. For use in visualization of vascular anatomy, cardiac and great vessel anatomy and physiology, or other devices in the heart and measurements of blood flow. |
| VOLCANO CORPORATION | 61230 | Visions Five - 64 Intravascular Ultrasound Imaging catheter | Visions Five/64 OTW Visons Five/64 F/X |
The Visions Five-64 Intravascular Ultrasound catheter is designed for use in the evaluation of vascular morphology in blood vessels of the coronary and peripheral vasculature by providing a cross-sectional image of such vessel. |
| 61982 | JOVUS AVANAR F/X Ultrasound Imaging Catheter | AVANAR F/X Ultrasound Imaging Catheter | For use in the evaluation of vascular morphology in blood vessels of the coronary and peripheral vasculature by providing a cross-sectional image of such vessel. Not currently indicated for use in cerebral vessels. For use as an adjunct to conventional angiographic procedures to provide an image of the vessel lumen and wall structure | |
| 65543 | Eagle Eye Gold Intravascular Ultrasound Imaging Catheter | Eagle Eye Gold Intravascular Ultrasound Imaging Catheter | Designed for use in the evaluation of vascular morphology blood vessels of the coronary and peripheral vasculaure by providing a cross-sectional image of such vessels, is not currently for use in cerebral vessels. | |
Literature Review
Objective
To determine the incremental value in terms of patient outcomes and the cost-effectiveness of using intravascular ultrasound adjunctive to coronary angiography to guide percutaneous coronary interventions.
Research Questions
What are the procedure-related complications associated with IVUS?
Does IVUS used in conjunction with angiography to guide percutaneous interventions improve patient outcomes compared to angiographic guidance without IVUS?
Who would benefit most in terms of clinical outcomes from the use of IVUS adjunctive to coronary angiography in guiding PCIs?
What is the effectiveness of IVUS guidance in the context of drug-eluting stents?
What is the cost-effectiveness ratio and budget impact of adjunctive IVUS in PCIs in Ontario?
Methods
Search Strategy
The preliminary search yielded two systematic reviews and one meta-analysis. The most recent systematic review was a Medical Services Advisory Committee (MSAC) review published in 2001. (17) This review included a comparison of IVUS-guided and angiography-guided PCI and included literature published up to May 2001. Therefore, the literature search for the current Medical Advisory Secretariat review was conducted for the period beginning in May 2001 until the day of the search, November 4, 2005.
Databases searched included: OVID MEDLINE, EMBASE, MEDLINE In-Process & Other Non-Indexed Citations, The Cochrane Library, and the International Agency for Health Technology Assessment (INAHTA) database. The database search was supplemented with a review of relevant Web sites, along with the bibliographies of relevant articles and reports.
The detailed search strategy is shown in Appendix 1. Only English-language studies in humans were included. Case reports, letters, comments, editorials and nonsystematic reviews were excluded. The following criteria were used to select studies for the review.
Inclusion Criteria
Systematic reviews or randomized controlled trials (RCTs) including unpublished reports presented at international conferences. Non-randomized comparative studies were included only when RCTs were not available to answer a specific question. Studies will meet the following description:
Patients: Patients with coronary stenosis undergoing balloon dilatation, stent implantation (bare metal or drug-eluting stents) with a sample size ≥ 20.
Intervention: IVUS guidance in conjunction with angiographic guidance
Comparator: Angiographic guidance alone
Outcomes of interest: short term and long-term major adverse cardiac events (MACE, consisting of death, myocardial infarction (MI), target lesion revascularization (TLR), target vessel revascularization (TVR)), angiographic stenosis, acute gain, net gain, costs, and/or cost-effectiveness ratio
Follow-up: At least 6 months
Exclusion Criteria
Nonsystematic reviews, non-randomized studies when RCTs are available, editorials, letters, comments, case series, and case reports, animal studies
Non-English language reports
Technical reports
Review and Selection
Two systematic reviews and one meta-analysis were found. Excluding these reports, the search yielded 318 citations. A medical information specialist and one researcher reviewed all abstracts and full text if necessary, to identify citations that did not meet the selection criteria. When uncertain, another researcher was consulted, and decision was based on consensus. Of the 318 citations, 310 reports were excluded (Table 2) and 8 reports met the inclusion criteria (Table 3A)
Table 2: Excluded Studies and Reasons.
| Reason for Exclusion | Number of Reports |
|---|---|
| Did not evaluate the effectiveness of IVUS | 236 |
| Drug study | 25 |
| Non-comparative studies | 18 |
| Non-systematic review | 15 |
| Case reports | 5 |
| Different comparator or disease state or no outcomes of interest | 5 |
| Therapeutic IVUS | 6 |
| Total Excluded | 310 |
Table 3A: Summary of Studies and Reports Included in the Medical Advisory Secretariat Review.
| Current search | Total Reports Included | |||
|---|---|---|---|---|
| Systematic Reviews | 2000 NHS systematic review 2001 MSAC systematic review 2003 Meta-analysis (Casella) |
2 systematic reviews and 1 meta-analysis | ||
| Primary Studies | ||||
| RCTs | Total Patients | Previous MSAC review | Current search (Selected) | Reports on Primary Studies |
| Gaster et al | 108 | 2 (2001, 2003) | 2 | |
| SIPS | 269 | 1 (Frey, 2000) | 2 (Mueller 2002, 2003) | 3 |
| OPTICUS | 550 | 1 (2001) | 1 | |
| RESIST | 158 | 2 (Schiele 1998, 2000) | 2 | |
| TULIP | 150 | 1 (Oemrawsingh 2003) | 1 | |
| AVID (Abstracts) | 800 | 2 (Russo 1999, 2000) | 2 Abstracts | |
| Total Non-randomized | 2,035 | |||
| Agostoni 2005 | 58 | 1 (on DES) | 1 | |
| Park 2001 | 127 | 1 (Left main coronary) | 1 | |
| Guedes 2005 | 387 | 1 (Safety of IVUS) | ||
| Total | 572 | 4 reports & 2 abstracts | 8 reports | 12 reports & 2 abstracts on 6 RCTs & 3 prospective non-randomized controlled studies |
Data Extraction and Quality Assessment
Eight reports on primary studies were selected, including: 6 reports on 3 RCTs, and 2 reports on 2 prospective non-randomized studies. Some of the reports provided follow-up to previously published studies. Studies from the MSAC systematic review that met the inclusion criteria were included in this review, bringing the total to 12 reports and 2 abstracts on 6 RCTs and 2 prospective non-randomized (i.e. 8 primary studies) (Table 3A). The non-randomized, controlled studies were on PCIs in left main coronary arteries. One researcher abstracted data from the studies using a standard form.
NHS National Health Service; MSAC Medical Services Advisory Committee (Australia); DES drug-eluting stent
A researcher reviewed the full text of all included reports and extracted data using a standard data extraction guide. The quality of the reports was assessed using MAS criteria (Appendices 3 and 4) and the level of evidence was graded (Table 3B).
Table 3B: Level of Evidence of Studies Included in the Review.
| Study Design | Level of Evidence | Number of Eligible Studies |
|---|---|---|
| Large RCTs | 1 | 1 |
| Large RCT unpublished but reported to an international scientific meeting | 1(g) | 1 |
| Small RCT | 2 | 3 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls (prospective) | 3a | 3 |
| Non-RCT with historical controls | 3b | |
| Non-RCT presented at international conference | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series (multisite) | 4b | |
| Case series (single site) | 4c | |
| Retrospective review, modelling | 4d | |
| Case series presented at international conference | 4(g) | |
| Total number of primary studies | 8 |
g= literature
RCT represents randomized controlled trial; HTA, health technology assessment
Data Synthesis
Revman 4.2 (The Cochrane meta-analysis software) was used to test for heterogeneity of the odds ratios of death, MI, target lesion revascularization, target vessel revascularization, binary restenosis rates, and MACE. Mean weighted differences were computed for angiographic MLDs, acute gain, net gain, and percent diameter stenosis. A point estimate with the 95% confidence interval was generated when appropriate. A descriptive synthesis was provided when statistical analysis was not appropriate.
Assessment of Overall Quality of Evidence
The quality of the overall evidence was assessed using GRADE. (18) The GRADE system was used to summarize the overall quality of evidence supporting the findings relating to each key outcome measure. This system rates the overall quality based on the assessment of four key elements:
Study design - (type of evidence), broadly categorized as randomized trials and observational studies.
Study quality - refers to whether there were limitations relating to the methods and execution that may result in biases. The assessment is based on appropriate criteria such as adequacy of allocation concealment, blinding and follow-up.
Consistency - refers to the similarity of estimates of effect across studies. Important unexplained inconsistency in the results decreases the confidence in the estimate of effects for the outcome.
Directness - refers to the extent to which the people, interventions, and outcome measures are similar to those of interest.
Quality grades were assigned as follows:
Type of evidence
Randomized trial = high
Observational study = low
Any other evidence = very low
Decrease grade if:
Serious (-1, reduce GRADE level by 1 so a high grading will become moderate) or very serious (-2, reduce GRADE level by 2 so a high grading will become low) limitation to study quality
Important inconsistency (-1)
Some (-1) or major (-2) uncertainty about directness
Imprecise or sparse data (-1)
High probability of reporting bias (-1)
Increase grade if:
Strong evidence of association-significant relative risk of >2 (<0.5) based on consistent evidence from two or more observation studies, with no plausible confounders (+1, increase GRADE level by 1, so a moderate grade will become high. However a high grade will remain high)
Very strong evidence of association-significant relative risk of > 5 (<0.2) based on direct evidence with no major threats to validity (+2)
Evidence of a dose response gradient (+1)
All plausible confounders would have reduced the effect (+1).
| High: | ⊕⊕⊕⊕ Further research is very unlikely to change our confidence in the estimate of effect. | |
| Moderate: | ⊕⊕⊕Ο Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | |
| Low: | ⊕⊕ΟΟ Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | |
| Very low: | ⊕ΟΟΟ Any estimate of effect is very uncertain. |
Findings of Literature Review
Summary of Systematic Reviews and Meta-analysis
The two systematic reviews and one meta-analysis are summarized in Appendix 2.
The 2000 review by Berry et al (19) for the National Health Service (NHS) in the United Kingdom mainly focuses on economic modeling. Almost all studies included in the Berry et al review were non-randomized, and in most cases, only had 6-month outcomes. Berry et al concluded that the evidence available was too weak to have any reliable implications for clinical practice, and recommended adequately powered and well-designed RCTs.
The Medical Services Advisory Committee in Australia published a systematic review on IVUS in 2001. (17) The review examined the accuracy of IVUS in the diagnosis of CAD, prediction of outcome, impact on patient management, and as an adjunct to angiography in stent placement. The review found that IVUS was relatively safe, provided additional information that complements information from coronary angiography, and had good sensitivity and specificity for detection of plaque dissections and media rupture, but low sensitivity for the detection of plaque rupture and thrombus formation. Meta-analysis of data from 5 RCTs with up to 12 months follow-up showed that IVUS-guided stenting resulted in a statistically significant reduction in the odds of target lesion revascularization 9 to 12 months after the procedure, but the upper limit of the 95% confidence interval approached the point of no effect (odds ratio = 1). MSAC also found the long-term outcome and impact of IVUS on survival and Q-wave MI unclear. Based on these findings, MSAC concluded that there was insufficient evidence regarding the effectiveness and cost-effectiveness of IVUS as a diagnostic or therapeutic tool, and did not recommend public funding for this procedure at the time.
The 2003 meta-analysis performed by Casella et al (20) included 5 RCTs and 3 registries. The five RCTs were the same studies included in the MSAC review. This meta-analysis found no statistically significant difference in major adverse cardiac events (MACE) between IVUS-guided and angiography-guided stenting. Angiographic binary restenosis and target vessel revascularization rates were lower in IVUS-guided compared to angiography-guided stenting, and these differences were found to be statistically significant. Casella et al stated that this effect was driven mostly by results of the registry studies. When only results from RCTs were included, the upper limit of the 95% CI of odds ratio for binary restenosis rate was close to 1 (1.06).
Summary of Randomized Controlled Studies Included in Current Medical Advisory Secretariat Review
Ten reports and two abstracts on the following 6 randomized controlled trials were found. These trials will be discussed briefly.
OPTICUS: Optimization with ICUS to reduce stent restenosis (21)
RESIST: Restenosis after Intravascular ultrasound Stenting Study (22;23)
SIPS: Strategy for Intracoronary Ultrasound-Guided PTCA and Stenting Trial (24;25)
TULIP: Thrombocyte activity evaluation and effects of Ultrasound guidance in Long Intracoronary Stent Placement (26)
AVID: Angiographic Versus Ultrasound-Directed Stent Placement (12;27)
Gaster 2001 & 2003: Prospective randomized study on clinical outcome and cost-effectiveness following intravascular ultrasound guided-PCI (28;29)
The characteristics of these RCTs are summarized in Table 4 and the quality assessment is summarized in Appendices 3 and 4.
Table 4: Included Reports on Randomized Controlled Trials.
| Study | Enrolment period | Design & Sample | Sample size | Type of lesion | Intervention Strategy | Follow-up (Months) | Primary End Point | Secondary End points |
|---|---|---|---|---|---|---|---|---|
| Gaster 2001 (28) | May 1996–Dec 1998 | RCT Single center (Denmark) | IVUS 54 Angio 54 | De novo in native coronary in males | Provisional stenting IVUS 87% Angio 85% | 6 | Incidence of angiographic diameter stenosis | Death, MI, CABG & repeat PCI |
| Gaster 2003 (29) | May 1996–Dec 1998 | RCT Single center | IVUS 54 Angio 54 | De novo in native coronary in males | Provisional stenting | 2.5 year | Death, Ml, CABG & repeat PCI | |
| SIPS (Frey 2000) (25) (Mueller 2003) (24) | Feb–May 1996 | RCT Single center | IVUS 121 Angio 148 | De novo & stenotic in native coronary | Provisional stenting IVUS 49.7% Angio 49.5% | 2 years | 6-month angiographic MLD | 6-month & 2 year Death, MI, clinical TLR |
| SIPS diabetes (Mueller 2002) (30) | Feb–May 1996 | (Switzerland) RCT Single center Subgroup analysis | IVUS 19 Angio 24 People with diabetes | De novo & stenotic in native coronary | Provisional stenting | 28 | MACE @ 28 months | 6 month angiographic restenosis rate |
| Opticus (Mudra 2001 (21) | Oct 1996–Feb 1998 | RCT Multicenter(Europe) | IVUC 273 Angio 277 | </=25 mm Diameter >/=2.5mm | Stenting in all patients | 12 | Angiographic 6-month restenosis, MLD, %diameter stenosis | Death, MI &TVR& meeting angiographic & IVUS criteria |
| RESIST (Schiele 1998) (22) (Schiele 2000) (23) | Jan 1995–Feb 1997 | RCT* Multicenter (France) | IVUS 79 Angio 79 | De novo in native coronary >70% stenosis | randomized after satisfactory QCA stent placement in all patients | 18 | 6 month restenosis rate | 6 month QCA MLD & IVUS CSA |
| TULIP (Oemrawsingh 2003) (Longlesions)(26) | June 1998–Jan 2001 | RCT Single centre (The Netherlands) |
IVUS 74 Angio 76 | Long de novolesions ≥20 mm, native coronary, diameter ≤3 mm stent | Stenting in all patients | 12 | Angiographic MLD Death, Ml & TLR @ 6 months | |
| AVID (Russo, 1997 (31) &2000 (27)(Abstracts) | ? | RCT Multicenter (USA) |
IVUS 394 Angio 406 | De novo & restenotic in coronary vessels ≥2.5mm in diameter | Stenting in all patients | 12 | TLR @ 12 months |
MLD=minimal lumen diameter
RCT=randomized controlled trial
MACE=major adverse cardiac events
PTCA=percutaneous transluminal coronary angiography
CSA=Cross sectional area
ICUS: Intracoronary ultrasound
QCA=Quantitative coronary angiography
CAD=Coronary artery disease
TLR=Target lesion revascularization
TVR=Target vessel revascularization
MI=myocardial infarction
40% power
Description of Randomized Controlled Trials
The 6 prospective randomized controlled studies had sample sizes ranging from 108 to 800 (median n=269). However, only abstracts were available for the AVID study. (27) OPITCUS, (21) RESIST, (22) and AVID (27) were multicenter studies while the remainder were single-center studies. All studies had 6-month angiographic follow-up, and clinical follow-up ranged from 12 months to 2.5 years. There were multiple reports for the SIPS study, the RESIST study, (22) and the study by Gaster et al. (28) Since each of the reports provided information on different parameters, all were included in the review.
All studies included patients with CAD undergoing PTCA and/or stenting. CAD was defined as >50% diameter stenosis in a coronary artery in most studies, except in RESIST where CAD was defined as >70% diameter stenosis. The mean age of the patients ranged from 54.7 years to 61 years. Both males and females were included in all studies, except the study by Gaster et al that included only males. The RESIST study had a prospectively designed sub-study for people with diabetes. The inclusion and exclusion criteria are summarized in Appendix 5, and baseline profiles of the subjects are summarized in Appendices 6 and 7.
Type of Lesions
Gaster, (28) RESIST, (22) OPTICUS, (21) and TULIP (26) included only de novo lesions in native coronary arteries, whereas SIPS (25) and AVID (27) included both de novo and restenotic lesions. AVID is the only study that did not limit vessels to native coronary arteries. Gaster, RESIST, and SIPS did not have any angiographic limitations for the lesions and coronary arteries. OPTICUS limited lesion length to no longer than 25 mm, and OPTICUS as well as AVID required vessel diameter to be no smaller than 2.5mm. The TULIP study is the only RCT that exclusively studied long lesions (≥20 mm) (Table 4). Mean lesion length was 29 mm for IVUS group and 27 mm for the angiography group in the TULIP study, compared to mean lesion lengths of 7.7 mm to 13.4 mm in other studies.
Strategy for Stenting
Different stenting strategies were employed (Table 4). Two of the 6 studies (SIPS and Gaster et al) (25;28) employed a provisional stenting strategy that used stents only when optimal lumen dimensions could not be obtained with balloon dilation alone, or when there was significant dissection. The percentage of people who received a stent in each of these studies was not significantly different between the two arms (about 50% in each arm in SIPS, and 89% for IVUS and 85% for angiography alone in Gaster et al). In the other studies, all patients underwent stent placement. In almost all studies, patients were randomized to IVUS guidance or angiography-guidance before balloon dilatation and/or stent placement. The only exception was the RESIST study that randomized patients after quantitative coronary angiography (QCA) showed satisfactory stent placement. (22)
The study protocols generally required QCA to be performed before and immediately after stent placement, and at 6-month follow-up. In the IVUS-guided group, IVUS was performed preintervention in some studies, and after QCA showed satisfactory stent deployment in all studies. The postintervention IVUS results were used to guide further stent expansion with larger balloons, higher pressure, and/or additional stents. IVUS was repeated after each expansion until the criteria for optimal stent placement were met, or when no further expansion was feasible. In some studies, a “documentary IVUS” was also performed on patients in the angiography-guided (control) group, but operators were blinded to the IVUS results, and no further stent expansions were performed. Follow-up care generally included antiplatelet therapy consisting of aspirin and clopidogrel (Appendix 11).
Endpoints
The primary and secondary end points varied among studies (Table 4). Most studies used angiographic results as primary end points. These included one or more of binary restenosis rates, minimal lumen diameter (MLD), minimal lumen area (MLA), and percentage diameter stenosis. Clinical end points often included target lesion or target vessel revascularization rates, death, MI, and the combined end point MACE. However, the definition of MACE varied among the studies. The end points and definitions for MACE are summarized in Appendix 8.
Criteria of Optimal PTCA and Stent Placement
The RCTs used different IVUS and angiographic criteria for optimal stent placement and balloon dilatation. These criteria are summarized in Appendices 9 and 10.
Quality of Randomized Studies
The quality assessment of the individual studies is summarized in Appendix 3. The quality ranged from moderate to good.
Non-Randomized Comparative Studies
Based on the current literature search and a previous meta-analysis (20), non-randomized controlled studies were identified. These studies are briefly summarized in Table 5a. Because of potential biases resulting from non-random patient allocation and lack of standards for optimal PCI procedures, these studies were not included in this review.
Table 5a: Characteristics of Non-Randomized Studies Excluded From the Review.
| Study | Enrolment period | Design | Sample size | Type of lesion | Intervention Strategy | Follow-up-Months | Primary End Point | Secondary End points |
|---|---|---|---|---|---|---|---|---|
| Albiero et al | 1993–95 | Multicenter Case series (Retrospective) | IVUS 158 Angio 154 | De novo, native | Stenting in all patients | 6 | 6-month angiographic restenosis rate | MACE, repeat PTCA & CABG |
| Blasini et al | 1994-95 | Single centre case series (Retrospective) | IVUS 105 Angio 107 | De novo, native, SVG, restenotic | Stenting in all patients | 6 | 6-month angiographic restenosis rate, MLD, % diameter stenosis | |
| Choi | 1997 | Single centre case series (Retrospective) | IVUS 178 Angio 100 | De novo, native | Stenting in all patients | 6 | Resource utilization | Death, MI, re PTCA, CABG, TVR, MACE |
| CRUISE Fitzgerald 2000 | April 1996–May 1997 | Prospective multicenter, observational; substudy of a RCT | IVUS 270 Angio 229 | De novo & restenotic, native coronary, up to 2 stents | Stenting in all patients | 9 | Angiographic MSD & IVUS MSD & MSA @ 6 months | MACE (death, MI, TVR) @ 9 months |
| PRESTO Orford 2004 | ? | Prospective multicenter, observational; substudy of a RCT | IVUS 796 Angio 8,274 | De novo &r restenotic, native coronary artery or vein graft lesions | Stenting in all patients | 9 | Incidence of death, MI or ischemia driven TVR @ 9 months | - |
Angio angiography; IVUS intravascular ultrasound; MACE major adverse cardiac events; Ml myocardial infarction; PTCA percutaneous coronary interventions; CABG coronary artery bypass; TVR target vessel revascularization; MSD minimal stent diameter
Since there were no randomized studies on the role of IVUS in PCIs in left main coronary arteries or using drug-eluting stents, two non-randomized studies on these topics were included. A case-controlled study on safety was also included. These are summarized in Table 5b and will be discussed in the section on high-risk lesions.
Table 5b: Summary of Non-Randomized Studies Included in Review.
| Study | Enrolment period | Design | Sample size | Type of lesion | Intervention Strategy | Follow-up Months | Primary End Point | Secondary End points |
|---|---|---|---|---|---|---|---|---|
| Park 2001 (32) | November 1995–April 2000 | Case-controlled | IVUS 77 Angio 50 | Unprotected left main coronary arteries | Stenting in all patients | 6 | Postintervention & follow-up Angiographic MLD | - |
| Agostoni 2005 (33) | April 2002–December 2003 | Case-controlled | IVUS 24 Angio 34 | Unprotected left main coronary arteries | Stenting with drug-eluting stents in all patients | 14 | MACE (death, non-fatal MI, & TVR) | - |
| Guedes 2005 (34) | ? | Multicenter | In same patients : IVUS 387 No IVUS 387 | De novo in native coronary | (No PCI) IVUS vs No IVUS in 2 segments of same coronary artery | 19 | Mean change in angiographic MLD, lesion progression (≥0.4 mm decrease in MLD), new lesion, & complications | |
Angio angiography-guided; IVUS intravascular ultrasound; MLD minimal lumen diameter; MACE major adverse cardiac events; MI myocardial infarction; TVR target vessel revascularization
Synthesis of Outcomes
This synthesis only included RCTs. Since the mean lesion lengths in the TULIP study were much longer compared to the other RCTs, and lesion length has been shown to be a predictor of restenosis rate, results of the TULIP study were excluded from the meta-analysis. This study is discussed separately under the section “IVUS Guidance for High Risk PCIs”. The AVID study results were reported only in published abstracts, and, therefore, details regarding baseline patient characteristics and angiographic outcomes were not available. To determine the impact of this study on the overall results, pooled analyses were conducted with and without the AVID study.
Angiographic Outcomes
Preintervention Minimal Luminal Diameter
Preintervention MLD were found to be similar between the IVUS and no IVUS groups both in individual studies and in pooled analysis (Figure 5)
Figure 5: Forest Plot of Preintervention Minimal Luminal Diameter of Randomized Controlled Trials.
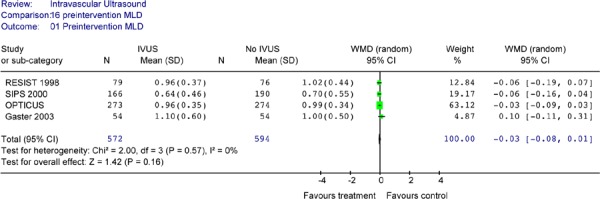
MLD minimal lumen diameter; IVUS intravascular ultrasound; WMD weighted mean difference; SD standard deviation; CI confidence interval
Postprocedure Minimal Luminal Diameter
All studies except the AVID study reported some angiographic findings (Table 6). All studies showed a trend towards larger postintervention MLD for the IVUS group compared to the No IVUS group, but only OPTICUS reached statistical significance.
Table 6: Angiographic Minimal Luminal Diameter After Procedure and at Follow-up.
| Post Procedure Mean MLD (SD) (mm) | Follow-up MLD (SD) (mm) | |||||
|---|---|---|---|---|---|---|
| IVUS | Angiography | P value | IVUS | Angiography | P value | |
| OPTICUS | 3.02 (0.49) | 2.91(0.41) | .006 | 1.95 (0.72) | 1.91 (0.68) | .52 |
| SIPS | 2.49 (0.66) | 2.38 (0.67) | .12 | 1.71 (0.9) | 1.56 (0.9) | .19 |
| RESIST | 2.57 (0.41) | 2.46 (0.46) | .11 | 1.70 (0.64) | 1.60 (0.65) | .20 |
| Gaster | 2.3 | 2.2 | NS | |||
MLD minimal lumen diameter; SD standard deviation; mm millimeter
The Forest plot of postintervention angiographic MLD yielded a weighted mean difference of 0.11mm in favour of IVUS (95% CI [0.05 – 0.17]) that is statistically significant (P = .0003) (Figure 6). A test of heterogeneity was not significant (P = 1.00).
Figure 6: Forest Plot of Postintervention Angiographic Minimal Lumen Diameter (Randomized Controlled Trials).
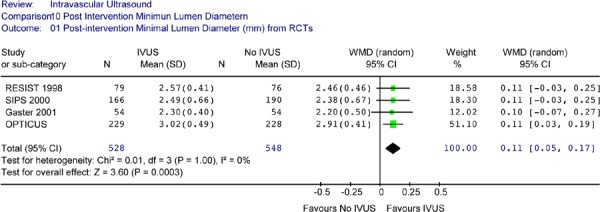
RCT randomized controlled trial; IVUS intravascular ultrasound; WMD weighted mean difference; SD standard deviation; CI confidence interval
Angiographic 6-Month Minimal Luminal Diameter
Six-month follow-up angiographic MLD was only available for 3 RCTs (Table 6) all of which were not significantly different between the IVUS and no IVUS group. A Forest plot of the MLD reported by the three RCTs showed no statistically significant differences in angiographic mean minimal lumen diameter between the IVUS-guided and the no IVUS-guidance group at 6 months (Figure 7). The weighted mean difference was 0.08mm (95% CI [-0.02, 0.17], P = .13). There was no significant heterogeneity detected (P = .66).
Figure 7: Forest Plot of 6-Month Angiographic Minimal Lumen Diameter (Randomized Controlled Trials).
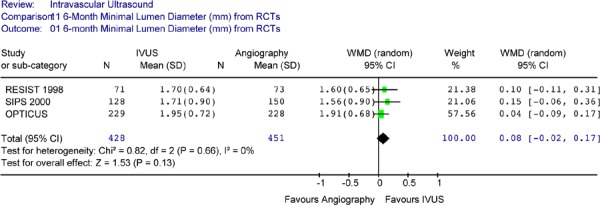
RCT randomized controlled trials; IVUS intravascular ultrasound; WMD weighted mean difference; SD standard deviation; CI confidence interval
Acute and Net Gain in Minimal Luminal Diameter
Acute gain is the increase in MLD over baseline immediately following the intervention. Late loss is the decrease in MLD that occurred in the period between the procedure and follow-up. Net gain is the net increase in MLD at follow-up compared to baseline. These can be expressed as:
Acute gain = Postintervention MLD – Preintervention MLD
Net gain = Follow-up MLD – Preintervention MLD = Acute gain – late loss
The ideal scenario is to achieve a large acute gain in MLD (large postprocedure MLD) and a small late loss in order to sustain a large net gain and hence a large MLD (minimal restenosis) at follow-up. Angiographic acute lumen gain and late lumen gain (at 6 months) are summarized in Table 8. IVUS guidance resulted in significantly higher postintervention acute lumen gain compared to angiography guidance in OPTICUS, SIPS, and RESIST studies.
Table 8: Angiographic Gain in Lumen Diameter.
| Acute Lumen Gain (SD) (mm) | Net Gain @ follow-up (SD) (mm) | |||||
|---|---|---|---|---|---|---|
| IVUS | Angiography | P value | IVUS | Angiography | P value | |
| OPTICUS(21) | 2.07 (0.50) | 1.91 (0.66) | < .0001 | 1.0 (0.74) | 0.91 (0.66) | 0.19 |
| SIPS (25) | 1.85 (0.72) | 1.67 (0.76) | .02 | 1.06 (0.91) | 0.87 (1.01) | 0.12 |
| RESIST (22) | 1.62 (0.43) | 1.45 (0.53) | .04 | 0.74 (0.65) | 0.60 (0.70) | .85 |
| Gaster 2003 (29) | 1.2 (0.6) | 1.2 (0.5) | NS | - | - | - |
SD standard deviation; mm milimeter; IVUS intravascular ultrasound
The larger acute lumen gain for IVUS-guidance was confirmed in the pooled analysis (Weighted mean difference of 0.17 mm in favour of IVUS, 95% CI [0.08, 0.22], p< .0001) (Figure 8).
Figure 8: Forest Plot of Acute Lumen Gain.
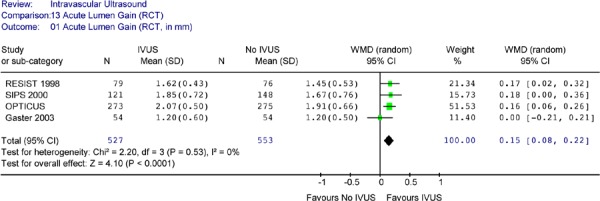
IVUS intravascular ultrasound; WMD weighted mean difference; SD standard deviation; CI confidence interval
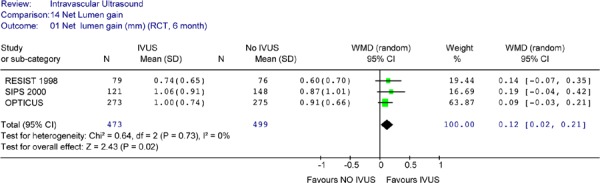
Only three RCTs provided data on net lumen gain at follow-up. Despite the presence of late lumen loss, all three RCTs showed a trend towards a larger net lumen gain at 6-month follow-up for IVUS guidance compared to angiographic guidance alone, although none reached statistical significance. However, pooled analysis showed that the IVUS group still had significantly larger net lumen gain at 6 months compared to the no IVUS group (Figure 9). The test for heterogeneity was negative (P = .73). A Forest plot yielded a weighted mean difference of 0.12 mm in favour of IVUS (95% CI of [0.02, 0.24], P = .02), which is statistically significant; however the lower limit was close to 0 (no difference).
Figure 9: Forest Plot of Net Lumen Gain at 6-Month Angiographic Follow-Up.
RCT randomized controlled trials; IVUS intravascular ultrasound; WMD weighted mean difference; SD standard deviation; CI confidence interval
Postprocedure Diameter Stenosis
Percentage angiographic diameter stenosis after the PCI procedure and at 6-month follow-up are summarized in Table 9. Residual diameter stenosis immediately after PCI procedures ranged from 2.8% to 27% (median 12%) for IVUS guidance and 6% to 26% (median 13%) for angiography guidance. One of the four RCTs (OPTICUS) (21) showed statistically significant lower angiographic residual diameter stenosis immediately after the procedure.
Table 9: Angiographic Diameter Stenosis After Procedure and at Follow-Up.
| Diameter Stenosis (after Procedure) (%) | Diameter Stenosis (6-month follow-up) (%) | |||||
|---|---|---|---|---|---|---|
| IVUS | Angiography | P value | IVUS | Angiography | P value | |
| OPTICUS (21) | 2.8 (7.8) | 6.0(8.0) | < .0001 | 34.8(20.6) | 36.8(19.6) | .29 |
| SIPS (25) | 18.8 (17.3) | 22.5 (19.7) | .07 | 44.5 (26.8) | 46.2 (28.2) | .61 |
| RESIST (22) | 16(10) | 19(9) | 0.35 | 38 (20) | 42(18) | .13 |
| Gaster 2003 (29) | 27(10) | 26(14) | NS | |||
IVUS intravascular ultrasound
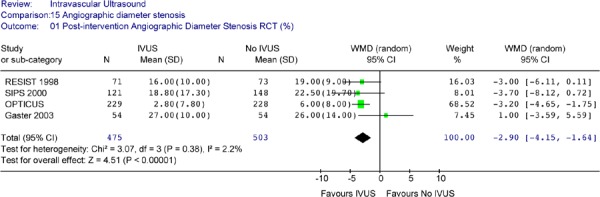
The Forest plot (Figure 10) suggests that that immediately after the intervention, IVUS guidance resulted in a statistically significant reduction in angiographic diameter stenosis in the target lesion (weighted mean difference of – 2.90%, 95% CI [-4.15, –1.64], P<. 00001]. No heterogeneity was detected (P= .38).
Figure 10: Forest Plot of Postintervention Angiographic Diameter Stenosis.
RCT randomized controlled trials; IVUS intravascular ultrasound; WMD weighted mean difference; SD standard deviation; CI confidence interval
Six-Month Diameter Stenosis
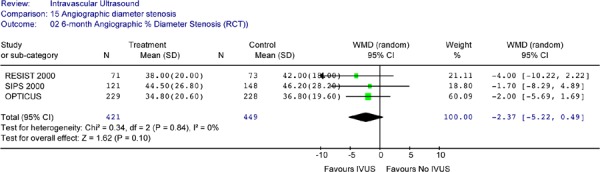
Three RCTs reported angiographic diameter stenosis at 6-month follow-up. None of these studies showed a statistically significant difference in diameter stenosis between the two groups (Table 9). This finding was not changed by pooled analysis (Figure 11).
Figure 11: Forest Plot of 6-Month Angiographic Percent Diameter Stenosis.
RCT randomized controlled trials; IVUS intravascular ultrasound; WMD weighted mean difference; SD standard deviation; CI confidence interval
Angiographic Binary Restenosis Rate
Only 4 randomized controlled trials reported the angiographic binary restenosis rate. The reported 6-month binary restenosis rates are summarized in Table 10. Restenosis rates were provided based on number of patients for all studies except the SIPS trial, which reported 29% restenosis rate for the IVUS group and 35% for the No IVUS group based on number of lesions (166 for IVUS, 190 for No IVUS). This analysis adopted the approach from a 2001 MSAC systematic review that converted the number of restenotic lesions to patients using the average number of lesions per patient (1.37 for IVUS, 1.28 for no IVUS) reported for SIPS. (17)
Table 10: Comparison of 6 Month Angiographic Binary Restenosis Rate Between IVUS-Guided and Angiography-Guided Percutaneous Coronary Intervention.
| 6-Month Angiographic Binary Restenosis Rate (%) | |||
|---|---|---|---|
| IVUS Group | Angiography Alone | P value | |
| OPTICUS(21) | 56/229 (24.5) | 52/228 (22.8) | .68 |
| SIPS (25) | 27/93 (29) | 41/117(35) | |
| RESIST (22) | 16/71(22.5) | 21/73(28.8) | .25 |
| Gaster 2003 (29) | 8/54(16) | 13/54(25) | NS |
NS not significant
The Forest Plot of angiographic binary restenosis rates from 4 RCTs yielded an odds ratio of 0.87 in favour of IVUS (95% CI [0.65 to 1.18]) that is not statistically significant (P = .37) (Figure 12).
Figure 12: Forest Plot of 6-Month Angiographic Binary Restenosis (Randomized Controlled Trials).
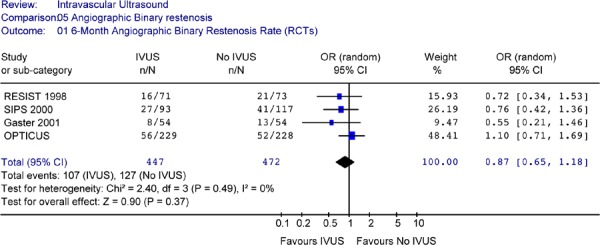
RCT randomized controlled trials; IVUS intravascular ultrasound; n number of patients with binary restenosis; N total sample size; OR odds ratio; CI confidence interval
Summary Statements on Angiographic Outcomes
Based on pooled analysis:
Preintervention MLDs were not significantly different between the IVUS-guided patients and angiographically-guided patients.
IVUS-guidance resulted in significantly larger postintervention angiographic MLD compared to angiographic guidance alone. However, there was no statistically significant difference in angiographic MLD between the two arms at 6 months follow-up.
IVUS also resulted in a statistically larger acute gain measured immediately postintervention using quantitative angiography. At 6 months, the net lumen gain was only marginally larger in the IVUS group compared to the no IVUS group.
Immediately after the PCI procedure, IVUS-guidance resulted in a significantly greater reduction in percent diameter stenosis (measured by quantitative angiography) compared to angiography guidance. However, there was no statistically significant difference in percent diameter stenosis between the two groups at 6 months follow-up.
PCI guided by IVUS did not result in a significant improvement in 6-month binary restenosis rate based on quantitative coronary angiography compared to PCI guided by angiography alone.
The key question is whether the improvement in lumen size after intervention and at 6-month follow-up led to improved clinical outcomes such as survival, frequency of MI, and revascularization in the target lesion and target vessel.
Clinical Outcomes
Survival
Six RCTs provided survival data. However, follow-up periods varied from 6 months to 2.5 years. Some studies provided data for more than one follow-up period. Analysis was conducted for 12-month follow-up, 18-month to 2.5-year follow-up, and 1-year to 2.5-year follow-up (Figures 13–15).
Figure 13: Forest Plot of Mortality Rates in Randomized Controlled Trials (Reported at 12 Months.
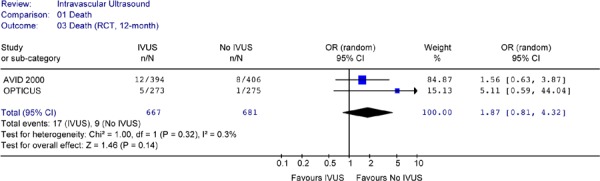
Figure 15: Forest Plot of Mortality Rates of all RCTs (at 1 to 2.5 years).
RCT randomized controlled trials; IVUS intravascular ultrasound; n number of deaths; N total sample size; OR odds ratio; CI confidence interval
Table 11: Mortality Rates (Number and %) in IVUS Versus no IVUS.
| Studies (RCTs) | Follow-up (Month) | Death - IVUS | Death – No IVUS | ||||
|---|---|---|---|---|---|---|---|
| Deaths | N | % | Deaths | N | % | ||
| OPTICUS (21) | 6 | 3 | 273 | 1 | 275 | ||
| OPTICUS (21) | 12 | 5 | 273 | 1.8 | 1 | 275 | 0.4 |
| AVID (Russo 1997) (31) | 12 | 12 | 394 | 3 | 8 | 406 | 2 |
| RESIST (22) | 18 | 1 | 79 | 1.3 | 1 | 76 | 1.3 |
| SIPS (25) | 2 year | 4 | 121 | 3.3 | 4 | 148 | 2.7 |
| Gaster, 2003 (29) | 2.5 years | 0 | 54 | 0 | 4 | 54 | 3.7 |
IVUS intravascular ultrasound; N sample size
Figure 14: Forest Plot of Mortality Rates: IVUS Versus no IVUS Guidance in Randomized Controlled Trials (Reported at 18 Months to 2.5 Years).
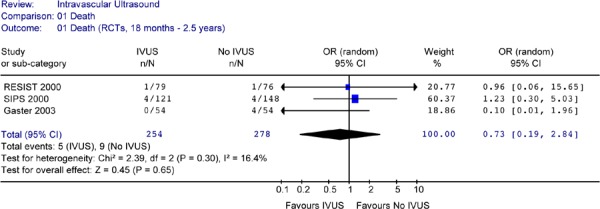
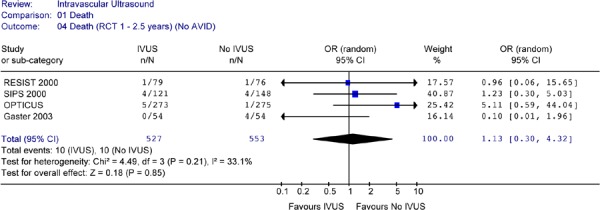
None of the studies showed a statistically significant difference in mortality rates between the two arms. Pooled analysis of mortality rates by period or combined showed no heterogeneity. The Forest plots of data from RCTs (Figures 13 to 15) showed no statistically significant difference in the odds of cardiac death between the IVUS and the no IVUS groups regardless of the length of follow-up. Repeating the analysis without the AVID study did not change this result and the odds ratio was still statistically insignificant (P = 0.85) (Figure 16).
Figure 16: Forest Plot of Mortality Rates of All RCTs (at 1 to 2.5 years) (No AVID).
AVID Angiographic Versus Ultrasound-Directed Stent Placement study; IVUS intravascular ultrasound; n number of patients with binary restenosis; N total sample size; OR odds ratio; CI confidence interval
Myocardial Infarction
Data on myocardial infarction was provided by 4 RCTs (Table 12). Myocardial infarction rates were measured at different time points ranging from 12 months to 2.5 years post intervention for these studies
Table 12: Rates of Myocardial Infarction in Intravascular Ultrasound-Guided Versus no Ultrasound-Guided Coronary Stent Implantation.
| Studies (RCTs) | Follow-up (Months) | IVUS | No IVUS | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| MI | N | % | Ml | N | % | |||
| OPTICUS, (Mudra 2001) (21) | 12 | 1 | 273 | 0.4 | 2 | 275 | 0.7 | 1 |
| AVID (Russo 1997) (31) | 12 | 26 | 394 | 6.6 | 20 | 406 | 4.9 | - |
| SIPS (Frey) 2000 (25) | 2 years | 1 | 121 | 0.8 | 6 | 148 | 4.1 | .16 |
| Gaster 2003 (29) | 2.5 years | 2 | 54 | 3.7 | 0 | 54 | 0 | NS |
RCTs randomized controlled trials; IVUS intravascular ultrasound; MI myocardial infarction
None of the studies reported a statistically significant difference in the rates of MI between the IVUS-guided and the no IVUS guidance group. Pooled analyses were performed for reported rates of MIs at 12 months and at 2 years to 2.5 years. The Forest plots showed did not show any statistically significant difference in myocardial infarction rates between the IVUS-guided group and the no IVUS group regardless of the length of follow-up (Figures 17 to 20).
Figure 18: Forest Plot of Myocardial Infarction in Randomized Studies (At 2 to 2.5 Years of Follow-Up).
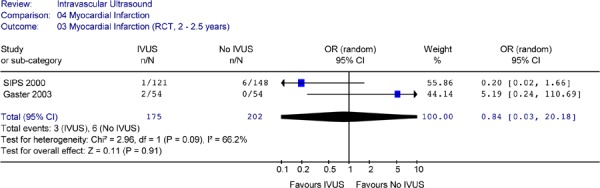
IVUS intravascular ultrasound; n number of patients with myocardial infarction; N total sample size; OR odds ration; CI confidence interval
Figure 19: Forest Plot of Myocardial Infarction in Randomized Studies (12 Months to 2.5 Years Follow-up).
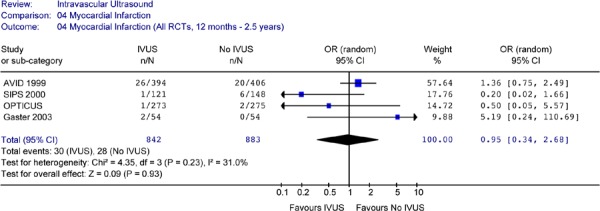
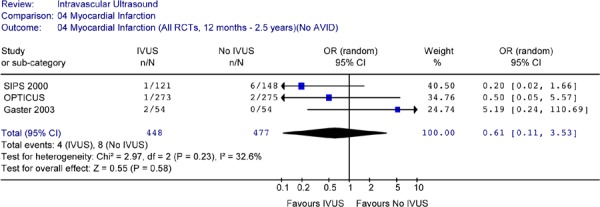
Repeating the pooled analysis without the AVID study did not change the above results. The odds ratio for myocardial infarction (OR 0.61, 95% CI [0.11–3.53]) between the two groups was still statistically insignificant (P = 0.58) (Figure 17b).
Figure 17: Forest Plot of Myocardial Infarction in Randomized Studies (At 12 Months Follow-Up).
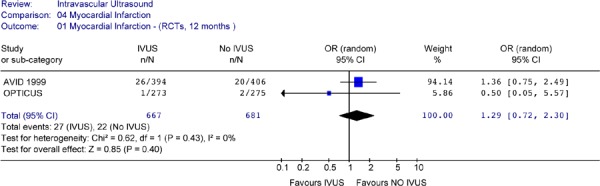
Figure 20: Forest Plot of Myocardial Infarction in Randomized Studies (12 Months to 2.5 Years Follow-up) (No AVID).
IVUS intravascular ultrasound; n number of patients with binary restenosis; N total sample size; OR odds ratio; CI confidence interval
Summary:
Results from individual studies and pooled analysis showed no statistically significant difference in the odds of cardiac death or myocardial infarction between IVUS-guided PCI and angiographically-guided PCI for up to 2.5 years of follow-up.
Target Lesion Revascularization
Target lesion revascularization was defined as CABG or repeat PCIs involving the target lesion. Five RCTs provided target lesion revascularization rate (Table 13).
Table 13: Target Lesion Revascularization of Randomized Controlled Trials.
| Studies | Follow-up | IVUS | No IVUS | P | ||||
|---|---|---|---|---|---|---|---|---|
| (Months) | TLR | N | % | TLR | N | % | ||
| RESIST (Schiele 2000) (23) † | 6 | 19 | 79 | 24 | 27 | 76 | 36 | |
| SIPS (Frey 2000) (25) † | 9* | 19 | 121 | 16 | 37 | 148 | 25 | |
| AVID (Russo 2000) (27) | 12 | 33 | 394 | 8.4 | 50 | 406 | 12.3 | .08 |
| RESIST (Schiele, 2000) (23) † | 18 | 21 | 79 | 27 | 31 | 76 | 41 | ** |
| SIPS (Frey 2000) (25) † | 2 year | 21 | 121 | 17.4 | 43 | 148 | 29 | .02 |
Extrapolated from KM survival curve.
Clinically driven
Odds ratio of 1.9 in favour of IVUS (95% CI 0.97; 2.4)
Aside from the TULIP study, which will be reviewed separately, three RCTs (RESIST, SIPS, and AVID) reported TLR rates and two RCTs (OPTICUS and Gaster et al) reported TVR rates. In the pooled analysis, it is assumed that all TLRs were clinically or ischemia driven, although this was specified explicitly only in the SIPS, RESIST, and TULIP studies. Clinically driven TLR is defined having angiographic stenosis (>50% diameter stenosis) in the target segment as well as having demonstrable ischemia (e.g. angina or positive stress test). Whether the TLRs were clinically or angiographically driven, the same protocol should have been applied to both arms of each study.
Since the TLR rates were reported at different periods of follow-up, pooled analysis was conducted for two periods: 6 to 12 months (RESIST, SIPS, and AVID) and 18 months to 2 years (RESIST and SIPS). In a 2001 systematic review, MSAC estimated the 9-month TLR for the SIPS trial based on Kaplan Meier curves for freedom from TLR. These 9-month TLR rates were incorporated in the current analysis.
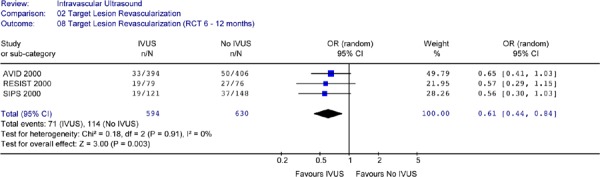
For the studies that reported TLR at 6 to 12 months of follow-up, none reported a statistically significant difference in TLR rates between IVUS guidance and angiography guidance alone. The Forest plot (Figure 21) yielded an odds ratio of 0.61 (95% CI [0.0.44, 0.84]) in favour of IVUS and this is statistically significant (P = .003). The caveat that should be noted is the different time points of TLR in this analysis.
Figure 21: Forest Plot: Target Lesion Revascularization for IVUS Guidance Versus no IVUS Guidance (RCTs, at 6 to 12 Months Follow-Up).
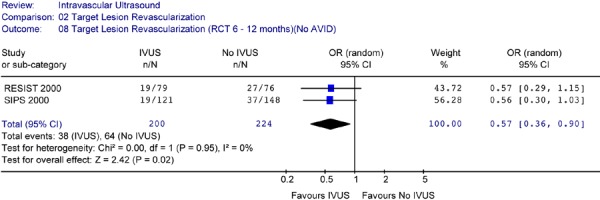
When the analysis was repeated without the AVID study, the reduction in the odds of having target lesion revascularization for IVUS is still statistically significant (OR 0.57 in favour of IVUS, 95% CI[0.36–0.90], P = 0.02) (Figure 22).
Figure 22: Forest Plot of Target Lesion Revascularization (6 Months to 1 Year)(Without AVID).
IVUS intravascular ultrasound; n number of patients with myocardial infarction; N total sample size; OR odds ration; CI confidence interval
Target Lesion Revascularization at Follow-Up Longer Than One Year
Only the RESIST trial and the SIPS trial reported TLRs beyond 12 months of follow-up. Both the 18-month TLR rate in the RESIST study and the 2-year TLR rate in the SIPS study appeared to be lower for the IVUS-guidance; however, only the 2-year rate in SIPS reached statistical significance (P = .02). The primary end point of the RESIST trial was 6-month restenosis rate, and it might not have been adequately powered to detect a statistically significant difference in TLR.
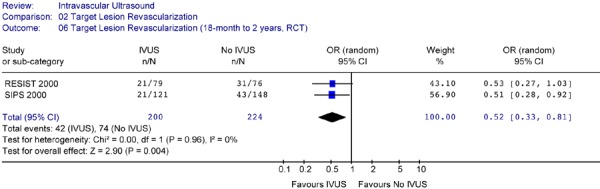
The Forest plot (Figure 23) for the two trials yielded an OR of 0.52 (CI [0.33, 0.81]) in favour of IVUS guidance (P =0.004), indicating that at follow-up periods ranging from 18 months to 2 years, IVUS-guidance resulted in a significantly lower rate of TLR compared to angiography-guidance alone. There was no statistical heterogeneity (P = .96).
Figure 23: Forest Plot of Target Lesion Revascularization – Intravascular Ultrasound Guidance Versus no IVUS Guidance (At 18 Months to 2 Years Follow-Up).
RCT randomized controlled trial; IVUS intravascular ultrasound; n number of patients with target lesion revascularization; N total sample size; OR odds ratio; CI confidence interval
Summary:
The foregoing meta-analysis indicates that:
Based on data from three RCTs, at 6 months to 12 months follow-up, the odds of having target lesion revascularization was significantly lower for the IVUS-guided PCI compared to angiography-guided PCI. This reduction was statistically significant (odds ratio of 0.61 (95% CI [0.0.44, 0.84]) (P = .003).
Based on two studies (RESIST and SIPS) that reported longer-term results, IVUS-guidance resulted in a statistically significant 48% reduction in the odds of target lesion revascularization at follow-up ranging from 18 months to 2 years after the intervention (OR 0.52 in favour of IVUS, 95% CI [0.33–0.81], P = .004).
Target Vessel Revascularization
Two RCTs (OPTICUS and Gaster) (21;28;29) provided data on target vessel revascularization defined as repeat PCI or CABG (Table 14). TVR usually refers to revascularization of a vessel where the target lesion was located. Neither of these studies showed a statistically significant difference in TVR at 6 months between the IVUS and No IVUS groups. This finding did not change for the OPTICUS study at 12 months. However, in the study by Gaster et al, a statistically significant reduction in TVR rate was observed in the IVUS-guided group compared to the group without IVUS guidance at a median follow-up of 2.5 years (42% vs 78%, P = .004).
Table 14: Target Vessel Revascularization.
| Studies (RCTs) | Follow-up (Months) | IVUS | Angio | P | ||||
|---|---|---|---|---|---|---|---|---|
| TVR | N | % | TVR | N | % | P | ||
| Gaster 2001 (28) | 6 | 10 | 54 | 19 | 18 | 54 | 33 | |
| OPTICUS (Mudra 2001) (21) | 6 | 30 | 273 | 11 | 27 | 275 | 10 | |
| OPTICUS (Mudra 2001) (21) | 12 | 41 | 273 | 15 | 38 | 275 | 13.8 | NS |
| Gaster 2003 (29) | 2.5 years | 23 | 54 | 43 | 42 | 54 | 78 | .004 |
IVUS intravascular ultrasound; Angio angiography; TVR target vessel revascularization; N sample size
The Forest plot of TVR rates at 6 months or at 1 to 2.5 years showed significant heterogeneity and no statistically significant difference in TVR rates between the IVUS-guided and the angiography-guided groups (Figures 24 & 25).
Figure 24: Forest Plot of Odds Ratio for Target Vessel Revascularization from RCTs at 6 Months Follow-up.
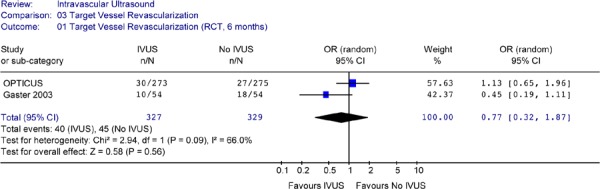
Figure 25: Forest Plot of Target Vessel Revascularization of Randomized Controlled Trials (12 Months to 2.5 Years).
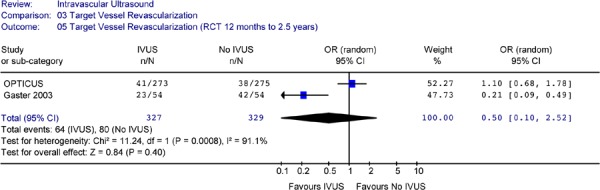
Summary:
Only one RCT showed significant reduction in TVR at 2.5 years after intervention. However meta-analysis of 2 RCTs or 2 non-randomized studies showed significant heterogeneity, and no statistically significant difference in the odds of having a target vessel revascularization between IVUS guidance and angiography guidance in PCI procedures.
Combined Target Lesion or Target Vessel Revascularization
Table 15: Combined Target Lesion or Target Vessel Revascularization in Randomized Studies.
| Studies | Follow-up (Months) | IVUS | No IVUS | P | ||||
|---|---|---|---|---|---|---|---|---|
| TLR | N | % | TLR | N | % | |||
| OPTICUS (Mudra 2001) (21) | 6 | 30 | 273 | 11 | 27 | 275 | 10 | |
| Gaster 2001 (RCT) (28) | 6 | 10 | 54 | 19 | 18 | 54 | 33 | |
| RESIST (Schiele 2000) (23) † | 6 | 19 | 79 | 24 | 27 | 76 | 36 | |
| SIPS (Frey 2000) (25)† | 9* | 19 | 121 | 16 | 37 | 148 | 25 | |
| OPTICUS (Mudra 2001) (21) | 12 | 41 | 273 | 15 | 38 | 275 | 13.8 | NS |
| AVID (Russo 2000) (27) | 12 | 33 | 394 | 8.4 | 50 | 406 | 12.3 | .08 |
| RESIST (Schiele, 2000) (23)† | 18 | 21 | 79 | 27 | 31 | 76 | 41 | ** |
| SIPS (Frey 2000) (25)† | 2 year | 21 | 121 | 17.4 | 43 | 148 | 29 | .02 |
| Gaster 2003 (29) (PCI+CABG) | 2.5 years | 23 | 54 | 43 | 42 | 54 | 78 | .004 |
IVUS intravascular ultrasound; TLR target lesion revascularization; N sample size: NS not significant
Extrapolated from KM survival curve
Clinically driven
Pooled analysis was conducted to include revascularization data as reported by RCTs (either TLR or TVR) for the follow-up period of 6 to 12 months and the period of 18 months to 2.5 years. The Forest plot of the 5 RCTs did not detect any statistical heterogeneity among the studies. The plot showed that IVUS significantly reduced revascularization rates at a follow-up period ranging from 6 to 12 months (OR 0.69 in favour of IVUS guidance, 95% CI [0.51–0.94], P = .02) (Figure 26).
Figure 26: Forest Plot of Combined TLR and TVR from Randomized Studies (at 6 to 12 Months Follow-Up).
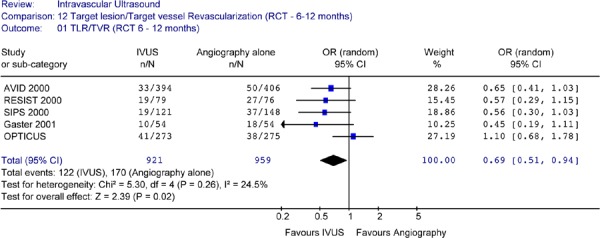
RCT randomized controlled trial; IVUS intravascular ultrasound; n number of patients with target lesion or target vessel revascularization; N total sample size; OR odds ratio; CI confidence interval
There was some uncertainty about the results in Figure 27. When the above pooled analysis was repeated without the results of the AVID study, the reduction in the odds of having a repeat revascularization for IVUS guidance compared to angiographic guidance alone was no longer statistically significant (OR 0.69, 95% CI[0.45–1.05], P = 0.08) (Figure 27).
Figure 27: Forest Plot of Combined Target Lesion and Target Vessel Revascularization at 6 to 12 Months (Without AVID).
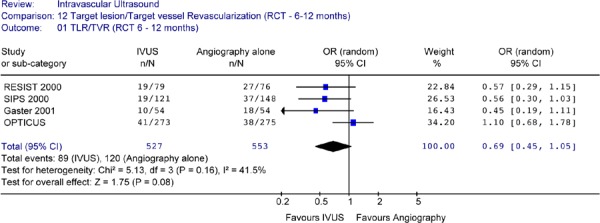
RCT randomized controlled trial; IVUS intravascular ultrasound; n number of patients with target lesion or target vessel revascularization; N total sample size; OR odds ratio; CI confidence interval
Three studies reported revascularization rates at follow-up periods ranging from 18 months to 2.5 years. The Forest plot of these data detected no heterogeneity and showed a significant reduction in the odds of revascularization in the IVUS-guided group vs the no IVUS group (OR 0.41 in favour of IVUS, 95% CI [0.24–0.29], P = .0008) (Figure 28)
Figure 28: Forest Plot of Target Lesion and Target Vessel Revascularization from RCTs (at 18 Months to 2.5 Years Follow-Up).
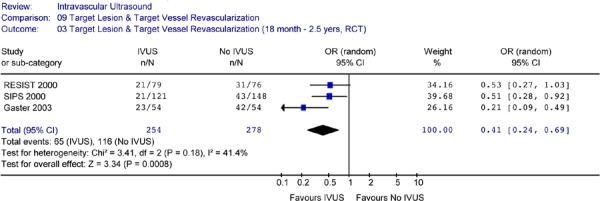
RCT randomized controlled trial; IVUS intravascular ultrasound; n number of patients with target lesion or target vessel revascularization; N total sample size; OR odds ratio; CI confidence interval
Summary
Pooled analysis showed that the odds of having a target lesion or target vessel revascularization were significantly reduced with IVUS guidance compared to angiographic guidance alone at a follow-up ranging from 18 months to 2.5 years.
Major Adverse Cardiac Events (MACE)
Of the 5 RCTs, AVID did not provide any data on combined event rates. OPTICUS reported combined event rates at 12 months, while the other three studies reported rates ranging from 18 months to 2.5 years (Table 16).(21) For SIPS, two different sets of MACE were reported for the SIPS trial. The combined event rates reported by Mueller for SIPS (which included clinically driven TVR) were used in the analysis. Two RCTs (RESIST and OPTICUS) reported no statistically significant difference in the rate of MACE between IVUS guidance and angiographic guidance alone. According to Mueller et al, (24) IVUS guidance resulted in significantly lower incidence of MACE at two years (19.8% for IVUS vs 31.1% for angiography, P = .04). The study by Gaster et al (29) reported that 78% of IVUS patients remained event-free compared to 59% of the no IVUS group at a median of 2.5 years follow-up (OR 2.5, P =0.04).
Table 16: Rates of Major Adverse Cardiac Events for RCTs and a Non-Randomized Study.
| Studies (RCTs) | Follow-up (Months) | IVUS | Angio | ||||
|---|---|---|---|---|---|---|---|
| MACE | N | % | MACE | N | % | ||
| Opticus, Mudra 2001 (21) | 12 | 51 | 273 | 18.6 | 44 | 275 | 16 |
| RESIST, Schiele 2000 (23) | 18 | 20 | 79 | 25 | 28 | 76 | 37 |
| SIPS, Frey 2000 (25) | 2 years | 24 | 121 | 20 | 46 | 148 | 31 |
| Gaster 2003 (29) | 2.5 years | 12 | 54 | 22 | 22 | 54 | 40 |
IVUS intravascular ultrasound; MACE major adverse cardiac event
A Forest plot of combined rates for all 4 studies showed significant heterogeneity (P = .05) (Figure 29). Since OPTICUS had shorter follow-up, and it is the only study that showed a trend of favouring no IVUS, the analysis was repeated without the OPTICUS study. The Forest plot of the combined event rates from RESIST, SIPS, and Gaster et al did not detect heterogeneity (P = 0.82) (Figure 30). The plot showed that IVUS guidance resulted in a significant reduction of combined event rates at a follow-up ranging from 18 months to 2.5 years (OR 0.53 in favour of IVUS-guidance, 95% CI [0.36–0.78], P = .001) (Figure 30).
Figure 29: Forest Plot of Odds Ratios of Major Adverse Cardiac Events (MACE) at 1 to 2.5 Years (RCTs).
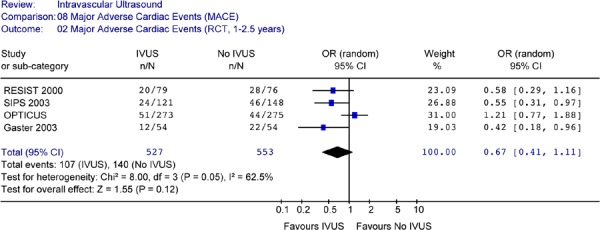
IVUS intravascular ultrasound; MACE major adverse cardiac event
Figure 30: Forest Plot of Combined Event Rates from RCTs (at 18 Months to 2.5 Years Follow-Up).
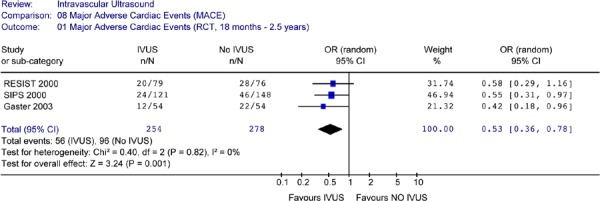
IVUS intravascular ultrasound; MACE major adverse cardiac event
Summary Statements
The only combined event rate reported at one year did not show a statistically significant difference in combined event rates between the two groups.
Based on a pooled analysis of 3 RCTs, IVUS guidance showed a statistically significant reduction in the odds of a major adverse cardiac event at 18 months to 2.5 years after the intervention compared to angiographic guidance.
Since there is no difference in the odds of death and MI between the two groups, the statistically significant reduction in the odds of combined adverse event in the IVUS-guided group at 18 months to 2.5 years after intervention reflected the reduction in revascularization rate in the same period.
Safety of IVUS
Guedes et al (34) conducted a multicenter case controlled study on the safety of IVUS itself (without PCI) on atherosclerotic, native coronary arteries. The study compared a segment of an atherosclerotic coronary artery that had IVUS with another segment of the same artery that did not undergo IVUS. Quantitative coronary angiography was performed at baseline and again at 18 months to 24 months follow-up. Guedes et al reported that IVUS by itself did not significantly accelerate the progression of atherosclerosis in native coronary artery disease. Among 387 patients in whom both IVUS-related and non-IVUS arteries were assessed, mean coronary change score was –0.060 (SD 0.21) mm for IVUS coronary arteries compared to –0.04 (SD 0.21) mm for the non-IVUS coronary arteries (P = .50). Lesion progression was found in 11.6% of IVUS related coronary arteries and 9% of non-IVUS arteries (P = .27). New coronary lesions were found in 3.6% of IVUS related coronary arteries compared to 3.9% of non-IVUS coronary arteries. The most frequent side-effect was coronary spasm (1.9% of a total of 475 IVUS examinations). Coronary spasm was relieved by the intracoronary injection of nitroglycerine. One major complication (occlusion) of a coronary artery was reported and it was successfully treated with balloon dilatation.
Complications relating to the adjunctive use of IVUS in PCIs reported by studies included in this review are summarized in Table 17. No serious complications were reported. Complication rates ranged from 0.5% to 4%. Complications reported included dissection or spasms of the coronary artery, femoral aneurysm, and rupture of the coronary artery, requiring emergency CABG in a small number of cases.
Table 17: Complications Relating to IVUS Guidance in PCI Procedures.
| Studies | Complications | Emergency CABG |
|---|---|---|
| OPTICUS (21) | No serious complication associated with IVU Prolonged spasms after stenting possibly related to IVUS (2.1%) | |
| AVID (27) | Procedural complications related to addition of IVUS (0.5%) | |
| Gaster et al, 2003 (29) | No statistically significant difference between the 2 groups. No death, Q-wave MI, CVA, or bleeding @ Puncturesite associated with IVUS Femoral aneurysm in 2% of each group |
2% of IVUS patients required emergency CABG |
| RESIST (23) | No major complication induced by IVUS-guided over dilation Dissection (Type C) (4%) in IVUS |
|
| SIPS (25) | Dissection NHLBI Type C or worse 3% in IVUS & 3.2% I Angiography group |
|
| TULIP (26) | Coronary rupture (1/54) Side branch occlusion (1/54 IVUS, 2/54 angio) |
1/54 in IVUS group due to coronary rupture |
| Park 2001 (32) | Not addressed | |
| Agostoni 2005 (33) | 2 periprocedural non-Q-wave MI, 3 deaths in patient with severe CAD & comorbidity |
IVUS intravascular ultrasound; CABG coronary artery bypass graft; NHLBI National Heart, Lung and Blood Institute
Schiele et al (22) stated that the complication rate of IVUS guidance was around 5% and the coronary rupture rate of 1.2% in early introduction at their centre. With experience and use of appropriate balloon size, complication rate has been reduced to an average of 1.05% with no coronary artery rupture.
IVUS Guidance in High-Risk Percutaneous Coronary Interventions
Long Lesions
The TULIP study (26) examined the impact of IVUS-guided elective stenting in long, de novo, non-ostial lesions (≥ 20 mm in length). The mean lesion length was 29 mm for the IVUS group and 27 mm for the no IVUS group, compared to mean lesion lengths of the cohorts ranging from 7.72 mm to 14.5 mm in the other studies (Appendix 7). TULIP compared 73 patients randomized to stenting with IVUS guidance to 71 patients randomized to stenting with angiographic guidance alone. There were no statistically significant differences in baseline clinical and angiographic characteristics between the two groups. The primary end points were 6-month angiographic MLD and the combined event rate of cardiac death, MI, and ischemia-driven TLR. The study had 80% power to detect a ≥0.25mm difference in 6-month MLD at a significance level of 0.05. Clinical follow-up was available for 100% of the patients at 6 months and 96% at 12 months. Six-month follow-up angiography was available in 88% of IVUS patients and 86% of No IVUS patients. Analysis was based on intention-to-treat.
Results of the study are summarized in Table 18. The mean MLD was similar for both groups at baseline, but was significantly larger for the IVUS group postintervention as well as at 6 months. The 6-month restenosis rate (>/=50% diameter restenosis) was significantly lower for the IVUS group (23% vs 46%, P = .008). The ischemia driven TLR rate was also significantly lower in the IVUS group compared to the no IVUS group both at 6 months (4% vs 14%, P = .37) and at 12 months (10% vs 23%, P = .018). The combined event rate (cardiac death, MI, and ischemia-driven TLR) was significantly lower for the IVUS group both at 6 months (6% vs 20%, P = .01) and at 12 months (12% vs 27%, P = .026). Since there were no significant differences in the incidence of death or MI between the two groups, the differences in the 6-month and 12-month combined event rates between the two groups were driven mainly by a lower TLR rate in the IVUS group compared to the no IVUS group (Table 18).
Table 18: Outcomes of the TULIP Study.
| Variable | IVUS (n = 73) | No IVUS (n= 71) | P Value |
|---|---|---|---|
| Angiographic MLD - Pre- intervention (SD), mm | 1.02 (0.42) | 0.99 (0.41) | NS |
| - Post - intervention (SD), mm | 3.01 (0.40) | 2.80 (0.31) | .008 |
| - 6 month follow-up (SD), mm | 1.82 (0.53) | 1.51 (0.71) | .042 |
| Angiographic Diameter Stenosis - Pre - intervention (SD), % | 65 (13) | 65 (10) | NS |
| - Post intervention (SD), % | 12 (7) | 13 (9) | NS |
| - 6-month follow-up (SD), % | 38 (15) | 45 (20) | NS |
| 6-month angiographic restenosis, % | 23 | 46 | .008 |
| 6-month death, % | 0 | 1 | NS |
| 12-month death, % | 2.7 | 1.4 | NS |
| 6-month MI, % | 1 | 7 | NS |
| 6-month ischemia-driven TLR, % | 4 | 14 | .037 |
| 6-month MACE (Death +MI +TLR), % | 6 | 20 | .01 |
| 12-month ischemia-driven TLR, % | 10 | 23 | .018 |
| 12-month MACE (Death +MI+TLR) | 12 | 27 | .026 |
MLD minimal lumen diameter; IVUS intravascular ultrasound; SD standard deviation; TLR target lesion revascularization; MI myocardial infarction
Left Main Coronary Artery
The initial experiences of patients undergoing unprotected left main coronary artery (LMCA) interventions were discouraging because of high procedural complications and early mortality. (35;36) Some groups considered IVUS guidance mandatory for percutaneous treatment of left main coronary artery disease.
In a prospective non-randomized study, Park et al (32) studied the effect of IVUS guidance in elective stenting of unprotected left main coronary artery stenosis. At the discretion of the operator, IVUS was used to guide PCI in 77 patients while 50 patients had PCI with angiographic guidance alone. MLD was significantly larger for the IVUS group both before and after intervention, but the mean MLD was the same for both the IVUS and the no IVUS group at follow-up (2.7+/-1.0, P= .976). There were no statistically significant differences in the angiographic restenosis rate for the two groups at 6-month follow-up (18.6% for IVUS vs 19.5% for no IVUS, P = .556). However, Park et al indicated that IVUS before stenting helped evaluate the actual size of the LMCA especially in the case of ostial lesions with a certain degree of negative modeling. In these cases, IVUS provided useful information for changing the treatment strategy from debulking with stenting to stenting alone.
In another prospective non-randomized study, Agostoni et al (33) studied the early outcomes of PCI for the unprotected left main coronary artery using a sirolimus or paclitaxel drug-eluting stent with IVUS guidance (n=24) or without IVUS guidance (n = 34). Use of IVUS guidance was left at the discretion of the operator.
There were no statistically significant differences between the two arms regarding age, cardiac risk factors, previous PCI, previous MI, unstable angina, serum creatinine level, and baseline angiographic measurements. However, the ejection fraction was significantly lower (44+/-14 vs 52+/-10%, P = .02) and there was a higher proportion of 3-vessel coronary artery disease (73% vs 46%, P = .03) in the no IVUS group compared to the IVUS group. The only procedural difference was the use of bigger balloons in the IVUS group (4 mm vs 3.7mm diameter) compared to the no IVUS group. Angiographic success was defined as residual stenosis <30% by visual estimate in the presence of Thrombolysis In MI (TIMI) grade 3 (37) flow (full perfusion with normal flow). IVUS criteria for stent optimization were complete stent-to-vessel wall apposition, stent CSA>80% of average reference CSA, and full lesion coverage. The primary outcome was the occurrence of MACE defined as death (cardiac or non-cardiac), non-fatal MI, and TVR. TVR was defined as a repeat intervention to treat a lesion within the stent or within 5 mm distal or proximal to the stent, including the ostium of the left anterior artery or circumflex artery, or both. Outcomes are summarized in Table 19.
Table 19: Outcomes of IVUS Guidance Versus Angiographic Guidance in Stenting in the Left Main Coronary Artery.
| Variable | IVUS (n = 24) | No IVUS (n= 34) | P Value |
|---|---|---|---|
| Quantitative Coronary Angiography | |||
| Reference vessel diameter (SD) (mm) | 3.37 (0.40) | 3.21 (0.56) | .24 |
| Lesion length (SD), mm | 7.47 (3.05) | 7.33 (3.11) | .89 |
| Minimal Lumen Diameter | |||
| - Pre- intervention (SD), mm | 1.19 (0.40) | 1.13 (0.39) | .53 |
| - Post - intervention (SD), mm | 2.93 (0.45) | 2.83 (0.50) | .45 |
| Diameter Stenosis | |||
| - Pre - intervention (SD), % | 62.0 (11.3) | 62.4 (13.8) | .91 |
| - Post intervention (SD), % | 14.5 (10.1) | 12.1 (11.1) | .24 |
| At median follow-up of 433 days | |||
| MACE (Death +MI +TVR), % | 8 | 20 | .18 |
| MACE, Distal left main lesion, % (N) | 20 (2/10) | 27 (6/22) | .69 |
| MACE, Non-distal left main lesions | 0/14 | 1/12 | NS |
IVUS intravascular ultrasound; SD standard deviation; MACE major adverse cardiac event; MI myocardial infarction
TVR target vessel revascularization
IVUS was performed in 41%, but only 14/24 had IVUS both before and after the intervention. IVUS guidance permitted optimization of stent deployment in 29% of cases. IVUS identified 4 cases (17%) of incomplete stent apposition, 1 case (4%) of stent under expansion, and 2 cases (8%) of incomplete lesion coverage, prompting additional post-dilatation in the first two situations, and a second stent deployment in the last situation. At a mean follow-up of 433 days, the incidence of MACE was 8% (2/24) in the IVUS group and 30% (7/34) of the no IVUS group (P = .18). IVUS was performed in 54% of non-distal left mains and the rate of MACE was low (1 non cardiac death in no IVUS group). In the distal LM group, IVUS was performed in 31% (less often than non-distal patients, P = .08), MACE occurred more frequently than the non-distal group, but were not significantly different between IVUS guidance (20%) and the no-IVUS guidance (27%, P = .69) groups. At multivariate analysis, distal left main disease was the significant predictor of adverse events with a hazard ratio of 7.7 (95% CI 1–62.6, P = .05). (33)
Patients with Diabetes
A prospectively designed substudy of the randomized controlled SIPS trial explored whether routine use of IVUS guidance during PCI improves long-term outcomes in people with diabetes. (30) Forty-three patients with diabetes were randomized to either IVUS guidance (n=19) or angiography guidance alone (n=24). According to the SIPS protocol, the study used a provisional stenting strategy that discouraged stenting unless the angiographic results were unsatisfactory or there was significant dissection. The stent rates for this subgroup analysis were not provided. However, for the entire SIPS cohort, the overall stent rate was approximately 50% for each arm. Baseline patient and lesion characteristics were well matched between the two groups. More than 50% of the lesions were complex ACC/AHA lesion type B2 or C. At 2-years, there were no statistically significant differences in the combined primary end point of death, non-fatal MI, and TVR, or in the individual outcomes (Table 20). Kaplan Meier survival analysis showed that IVUS guidance yielded slightly better long-term event-free survival, but this improvement was not statistically significant (Figure 31), and the restenosis rates were equally high for both groups (53% for IVUS vs 52% for angiography). Follow-up with quantitative coronary angiography at 6 months showed that the MLD, the per cent diameter stenosis, and the incidence of restenosis were all very similar for both groups. Differences in angiographic late loss, net gain, or late loss index between the two groups were not statistically significant (Table 21). Mueller et al concluded that IVUS guidance during provisional stenting seems to slightly attenuate the negative effect to diabetes on clinical long-term outcome. However, the angiographic restenosis rate remains high for both groups.
Table 20: Two-Year Clinical Outcomes of IVUS Versus Angiography Guidance in Patients with Diabetes.
Figure 31: Event-free Survival in People With and Without Diabetes Guided by IVUS or Angiography Alone.
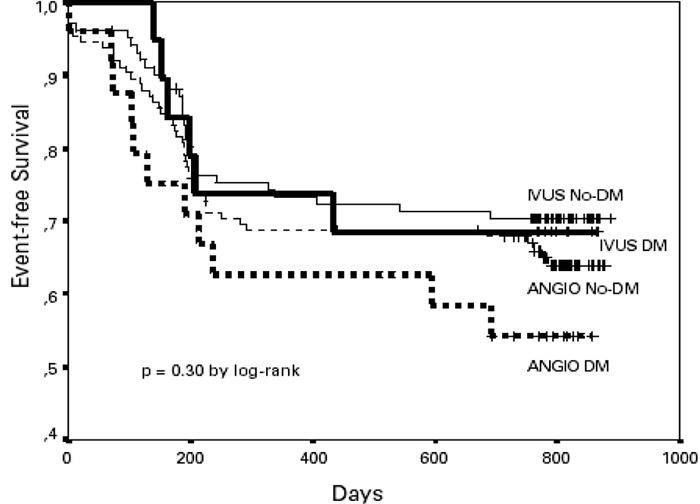
Reproduced with permission from EMH Swiss Medical Publishers Ltd., and the author; From Mueller C, Mc Hodgson JB, Brutsche M, Perruchoud AP, Marsch S, Hunziker P et al. Impact of intracoronary ultrasound guidance on long-term outcome of percutaneous coronary interventions in diabetics-insights from the randomized SIPS trial. Swiss Med Wkly 2002; 132(21-22): 279-284
IVUS DM = Diabetics guided by IVUS
ANGIO DM = Diabetics guided by angiography
IVUS no-DM = non-diabetics guided by IVUS
ANGIO No-DM = Non-diabetics guided by angiography
Table 21: Six Month Angiographic Outcomes of IVUS Guidance Versus Angiography Guidance in Patients with Diabetes.
| IVUS | Angiography alone | P value | |
|---|---|---|---|
| Baseline | |||
| MLD (SD), (mm) | 0.6 (0.36) | 0.66(0.57) | 0.62 |
| Stenosis (SD), (%) | 81 (11) | 77 (18) | 0.38 |
| Immediately after Intervention | |||
| MLD (SD), (mm) | 2.44 (0.64) | 2.36 (0.58) | 0.25 |
| Acute gain (SD), (mm) | 1.85 (0.67) | 1.60 (0.64) | 0.16 |
| Stenosis (SD), % | 22 (18) | 21 (18) | 0.86 |
| At 6-month follow-up | |||
| MLD (SD), (mm) | 1.27 (1.02) | 1.11 (0.89) | 0.61 |
| Late loss (SD), (mm) | 1.26 (1.07) | 1.10 (0.85) | 0.61 |
| Net gain (SD), (mm) | 0.71 (0.98) | 0.55 (1.03) | 0.63 |
| Re-stenosis, n (%) | 8 (53) | 13 (52) | 0.94 |
IVUS intravascular ultrasound; MLD minimal lumen diameter; SD standard deviation; mm millimeter
Other High-Risk Percutaneous Coronary Interventions
Although the AVID study (27) did not show a statistically significant difference in TLR rates at one-year based on intention to treat analysis, Russo et al reported that IVUS guidance resulted in significant reduction in TLR rates in the following subgroups:
Patients treated for saphenous vein grafts (TLR was 5.1% for IVUS vs 20.8% for angiography guidance, P = .03)
Patients with reference diameter ≥ 2.5mm (TLR was 4.9% for IVUS vs 10.8% for angiography guidance, P = .02)
Patients with pre-procedural stenosis >50% (When lesion >70%, TLR was 3.4% for IVUS vs 14.4% for angiography guidance, P = .003)
The above information was reported in a published abstract. Details regarding patient characteristics and the subgroup analysis were not available.
Summary
There is evidence from one small, randomized controlled trial that IVUS guided PCI in long de novo non-ostial lesions (>20 mm) of native coronary arteries resulted in statistically significant larger MLD, and statistically significant lower 6-month angiographic binary restenosis rate. Target vessel revascularization rate and rates of combined event were also significantly reduced at 6 months and 12 months follow-up.
A small subgroup analysis of an RCT reported no benefit in clinical or angiographic outcome for IVUS-guided PCI in patients with diabetes compared to angiography-guided PCI. However, due to the nature and size of the study, no firm conclusion can be reached.
Based on 2 small, prospective, non-randomized controlled studies, IVUS guidance in PCI of left main coronary lesions using bare-metal stents or drug-eluting stents did not result in any benefit in angiographic or clinical outcomes. Because of the size and study designs, no firm conclusion can be drawn.
There is presently insufficient information to reach any conclusion on the angiographic or clinical impact of IVUS guidance on high-risk lesions.
Intravascular Ultrasound in Deployment of Drug-Eluting Stents
Randomized studies have demonstrated dramatically reduced restenosis rates and revascularization rates with the use of sirolimus- or paclitaxel-eluting stents compared to bare metal stents (Appendix 13). IVUS studies have confirmed that the reduction in in-stent restenosis was attributed mainly to a near elimination of neointimal hyperplasia development inside the drug-eluting stents. (38)
Questions have been raised whether IVUS guidance is necessary in the placement of drug-eluting stents because of the low restenosis and revascularization rates. Since IVUS guidance was used not only to achieve a larger postintervention lumen in PCIs, but also to ensure complete stent apposition in order to reduce the risk of stent thrombosis, the incidence of incomplete stent apposition, and the risk of stent thrombosis associated with the use of drug-eluting stents need to be examined.
Incidence of Incomplete Stent Apposition (Drug-Eluting Stents)
Incomplete stent apposition (ISA) is often defined as more than 1 stent strut not apposed to the vessel wall at the time of post-procedural IVUS. Neointimal hyperplasia may close the gap between the stent struts and the vessel wall. If the gap remains unchanged at follow-up, persistent ISA exists. Late acquired ISA occurs when the stent is well opposed to the vessel wall at the post-procedural IVUS but is noted to have incomplete apposition at the time of follow-up IVUS.
ISA had been reported in randomized controlled trials on the safety and efficacy of drug-eluting stents. Serruy et al (39) reported that in the double-blind “Randomized Study with the sirolimus-eluting Velocity Balloon Expandable Stent” (RAVEL) study that compared 48 patients with sirolimus-eluting stent implantation with 47 patients with bare metal stent implantation, ISA was significantly higher in the sirolimus-eluting stent group compared to the bare metal stent group (21% vs 4%, P = .001) at 6 months. The study was not able to determine which cases resulted from suboptimal stent implantation or which cases were late acquired. However, the study reported that patients with persistent ISA were asymptomatic and event-free at one-year follow-up.
Ako et al (40) reported, at the 52nd Annual Scientific Session of the American College of Cardiology, an analysis of 141 patients in the randomized SIRIUS study for whom serial quantitative IVUS results were available. This analysis compared the IVUS findings of 80 patients implanted with a sirolimus-eluting stent to 61 patients with bare metal stent. At 6-month follow-up, the total incidence of ISA was higher in the sirolimus group compared to the bare metal stent group (16.3% vs 9.8%). The rates of persistent ISA were similar in both groups (7.6% for sirolimus-eluting stent vs 9.8% for bare metal stent). However, late acquired ISA was observed only in the group with sirolimus-eluting stent (8.7%). It was noted that in the sirolimus group, all persistent ISA occurred at the edges of the stent, whereas for late acquired ISA, only 22% occurred at the edges while 78% occurred in the mid-portion of the stent. There were no differences reported between the two groups with respect to stent lumen or follow-up external elastic membrane.
Stent thrombosis occurred in 0.4% of the entire sirolimus-eluting stent cohort (n = 533) versus 0.8% of the entire bare metal stent cohort (n=525) of the SIRIUS trial, (41) and there were no negative clinical events reported for any ISA cases at 12-month clinical follow-up and no increase in the rates of late stent thrombosis in patients with late ISA. (40)
Tanabe et al (42)also reported ISA in TAXUS II, a substudy of the randomized controlled TAXUS I trial, that compared the IVUS findings of 229 patients implanted with moderate-release or slow-release paclitaxel-eluting stents to 240 patients implanted with bare metal stents. There was less frequent ISA in the moderate-release Taxus stents (2.6%) compared to bare metal stents (7.9%, P = .028), and slow-release Taxus stents (11.5%). The majority of ISA resolved spontaneously so that at 6 months, no patient with a moderate-release paclitaxel stent showed persistent ISA, while persistent ISA was observed in 4.4% of the slow-release paclitaxel group and in 3.3% of patients with bare metal stents. Incidence of late acquired ISA was similar in all three groups (5.4% to 9.5%) (Table 22)
Table 22: Incomplete Stent Apposition in TAXUS II Trial.
| Paclitaxel stent -Moderate Release |
Paclitaxel stent - Slow Release |
Bare Metal Stent | P value | |
|---|---|---|---|---|
| Post procedure ISA % | 2.6* | 11.5 | 7.9* | *.028 |
| Persistent ISA % | 0** | 4.4 | 3.3** | ** .056 |
| Late acquired ISA % | 9.5 | 8.0 | 5.4 |
Tanabe et al reported that ISA had no clinical repercussions such as stent thrombosis or TLR (Table 23)
Table 23: Late Acquired Incomplete Stent Apposition and Adverse Events in TAXUS II.
| Paclitaxel-eluting Stent | Bare Metal Stent | P value | |||
|---|---|---|---|---|---|
| With late ISA | No late ISA | With Late ISA | No late ISA | ||
| Stent thrombosis % | 0 | 0.9 | 0 | 0 | |
| Target lesion revascularization % | 0 | 3.3 | 5.3 | 12.7 | |
The TAXUS III trial (43) studied 28 patients with in-stent restenosis (lesion length<30mm) in vessels with a diameter ranging from 3.0 to 3.5mm. At 6-months follow-up, binary angiographic restenosis was documented in 4 (16%) of the 25 patients with follow-up angiography, mostly (75%) in a gap between 2 paclitaxel-eluting stents. IVUS showed one case each of incomplete apposition and insufficient stent expansion without angiographic restenosis. No late acquired ISA at 6-month or late sub-acute stent thrombosis (up to 12-month follow-up) was found.
Risk of Stent Thrombosis with Drug-Eluting Stents
A meta-analysis conducted by Moreno et al (44) that included 10 RCTs comparing drug-eluting stents with bare metal stents in 5,030 patients found that the use of drug-eluting stents did not increase the incidence of stent thrombosis in patients (OR 1.05; 95% CI: 0.51 to 2.15, P =1.0). The meta-analysis showed that the mean stented length was longer in patients suffering from stent thrombosis. Bavry et al (45) conducted a meta-analysis on 8 trials (3,817 patients) that compared the risk of stent thrombosis associated with the use of paclitaxel-eluting stents compared to bare metal stents. The results suggest that standard dose paclitaxel-eluting stents do not increase the risk for thrombosis for up to 12 months (risk ratio = 1.06, 95% CI 0.55 to 2.04, P = .86)
Evidence on the Role of IVUS in Drug-Eluting Stent Implantation
The ability of IVUS guidance to prevent ISA and improve angiographic and clinical outcomes needs to be compared with that of angiographic guidance in the placement of drug-eluting stents.
There were no randomized studies that compared IVUS guidance with angiographic guidance in the placement of drug-eluting stents. One non-randomized study, by Agostoni et al (33) (previously described under the section Left Main Coronary Artery), compared the impact of IVUS-guidance with angiographic-guidance alone in the placement of drug-eluting stents in lesions of the left main coronary artery. The study showed no statistically significant difference in MLD and percent diameter stenosis postintervention, or in MACE (cardiac or non-cardiac death, non-fatal MI, and TVR) at a median follow-up of approximately 1.2 years. However, this was a small study (total sample = 58), likely with selection bias (non-randomized, allocation at the discretion of the operator), and there were no data on angiographic follow-up or revascularization rates. Hence, no firm conclusion can be drawn based on this study alone.
In summary:
Sirolimus- and paclitaxel-eluting stents have been shown to reduce both restenosis rates and target lesion revascularization rates compared to bare metal stents.
A large field evaluation in Ontario showed that the benefit of reduced target vessel revascularization associated with the use of drug-eluting stents appears to lie mainly in high-risk patients (with diabetes, long-lesions, and/or narrow vessels).
High-quality studies have reported persistent and late acquired incomplete stent apposition associated with the use of sirolimus- and paclitaxel-eluting stents. With sirolimus-eluting stents, late acquired ISA occurred in drug-eluting stents but not in bare metal stents.
Incomplete stent apposition, associated with the use of sirolimus- and paclitaxel-eluting stents, was not associated with increased risks of stent thrombosis or other adverse clinical events. However, it should be noted these findings were based on short-term studies. The long-term implications of persistent and late acquired incomplete stent apposition are presently unknown.
The risk of stent thrombosis did not increase compared to bare metal stents.
There is presently no evidence that the use of IVUS in the implantation of drug-eluting stents would reduce incomplete stent apposition, or improve the angiographic or clinical outcomes of the patients.
Limitations in Evidence
Results of the meta-analysis need to be interpreted with caution because of some limitations in the data and the analysis. Although the pooled analysis did not show statistical heterogeneity in the data, there was clinical heterogeneity among the studies. These included heterogeneity in:
Study population
The five studies included in the analysis used different inclusion and exclusion criteria resulting in differences in patient profiles and lesion characteristics (Appendices 6 and 7). For example, 2/5 of the studies included recent acute MI and 3/5 included unstable angina, 3/5 included restenotic lesions and 1/5 included saphaneous vein grafts. Two studies had limits on maximum lesion lengths varying from 15 mm to 25mm. With the exception of the TULIP study, the lesions were not considered long lesions; mean lesion length varied from 7.7 mm to 13.4 mm among the studies. Mean size of the reference diameter before intervention ranged from 2.8 mm to 3.13 mm.
Aside from TULIP, the study by Gaster et al (28;29)had the longest mean lesion length (13.3 mm & 13.4 mm vs 7.7 mm–11.6 mm in the other studies); the SIPS study had the highest mean preintervention diameter stenosis, the smallest preintervention MLD, and the highest percent of prior MIs and diabetes (19% & 24%). The OPTICUS study had the highest percentage of ACC/AHA Type B2 to Type C lesions (76% & 78% vs 43% – 51% in other studies).
The average risk profile of the patients in the included studies cannot be considered high-risk. However, there are some high-risk patients within each study and hence the study population is rather mixed in terms of risk for restenosis. This might have accounted for the failure to detect significant differences in outcomes between the IVUS groups and the no IVUS groups. Aside from the AVID study, the other studies did not identify subgroups that would benefit from IVUS guidance. Hence future study needs to focus on this area.
Criteria for optimal stent placement
Studies used different criteria for optimal stent placement. Although all studies set out the desired lumen size relative to the size of the reference segment, different measures were used to define lumen size. OPTICUS (21)and SIPS (25)used the MUSIC criteria and therefore used minimal lumen area (MLA), Gaster et al (28)and RESIST (22) used cross-sectional area (CSA), and SIPS used MLD. AVID defined the final lumen size in terms of residual stenosis. The target lumen size as a percentage of the reference segment lumen varied from 80% to 90%. Although all studies aimed for complete stent-to-vessel wall apposition, only AVID specified lack of dissection as one of the criteria for optimal stent placement. Only the criteria in the two non-randomized trials required full lesion coverage.
Definition of outcome measures also varied among the studies.
Different definitions were used for combined events since some included cardiac death while others included all–cause mortality. Similarly, some studies included target lesion revascularization while others included target vessel revascularization in the combined events. Some studies included clinically/ischemic driven target lesion revascularizations while others did not clearly state whether the revascularization was angiographically- or clinically-driven. Angiographically-driven revascularization rate is expected to be higher than clinically-driven revascularization rate since not all angiographic diameter stenoses exceeding 50% require intervention.
Different protocols
The studies also used different protocols relating to stenting (provisional versus primary stenting), hence the percentage of patients who underwent stenting ranged from 50% to 100%. RESIST and AVID randomized patients only after the optimal post-stent angiographic results were obtained, thus ensuring comparability of the two arms, whereas the other studies randomized before stenting. There were inter-study and intra-study variations in the type of stents used (Appendix 10). This might have an impact on outcomes since coil stents had been shown to be more prone to recoil than tubular slotted stents. Although all studies used an antiplatelet therapy including aspirin and ticlopidine after intervention, the dose and duration varied among studies (Appendix 11).
Including data from published abstracts
The largest RCT included in this review only had published abstracts. There is uncertainty about the results since there is a lack of detail concerning patient profiles, lesion characteristics, protocols, postintervention antiplatelet therapy, and method of analysis.
Other limitations included small sample sizes (108 in Gaster et al (28)), inability to blind the operators, and lack of blinding in the assessment of IVUS results. Moreover, almost all studies were conducted in the mid- to late 1990s and they might not reflect the most current technology in stenting, coronary angiography, or IVUS.
Overall Quality of Evidence by GRADE System
The overall quality of the main findings from the analysis were assessed using the GRADE system (18) and are summarized in Tables 24 and 25.
Table 24: Quality of Evidence on Predominantly Low-Risk Lesions.
| Outcome | Design | Quality | Consistency | Directness | Overall Quality |
|---|---|---|---|---|---|
| Post PCI MLD | 4 RCTs | 2 studies small sample (N = 108 & 155) Blinded angio assessment Some limitations* | Larger in IVUS | All measured with QCA | Good |
| 6-month MLD | 3 RCTs | Small sample in 1 study Some limitations | Larger in IVUS, not significant Some inconsistency** | Same as above | Good |
| MACE (18m - 2.5 yrs) | 3 RCTs | Small sample in 2 studies Some limitations* | Lower in IVUS 47% relative ⇓ in odds Some inconsistency** | Patient record | Moderate |
| TLR or TVR (18m – 2.5yrs) | 3 RCTs | Small sample in 2 studies Some limitations* | Lower in IVUS 59% relative ⇓ in odds Some inconsistency** | Patient record | Moderate |
| Death (Up to 2.5 yrs) | 5 RCTs | Small sample in 3 studies Some limitations* | No significant difference No inconsistency | Patient record & registry | Good |
| MI | 4 RCTs | 1 study small sample Some limitations* | No significant difference No inconsistency | Patient record | Good |
MLD minimal lumen diameter; RCT randomized controlled trial; MACE major adverse cardiac event; TLR target lesion revascularization; TVR target vessel revascularization; MI myocardial infarction
Limitations in quality: Some heterogeneity in definitions, criteria for optimal stent placement, basis for revascularization, and stenting protocols.
Inconsistency among studies: some of the studies showed significant difference while others did not.
Table 25: Overall Quality of Evidence on High-Risk Lesions.
| Outcome | Design | Quality | Consistency | Directness | Overall Quality |
|---|---|---|---|---|---|
|
Long Lesions Significant ⇓6m MLD Significant ⇓ 6-m restenosis, 1-yr MACE & TLR |
1 small RCT | N=144 Narrow spectrum Blinded Angiographic analysis Power calculation ITT Some limitation* |
NA | Criteria for optimal stent implantation Definition for outcomes |
Moderate Needs to be confirmed with larger RCTs |
| Diabetes No significant difference in MACE, death, MI or TVR |
1 small subgroup analysis of RCT | Prospectively designed subgroup analysis of RCT Small sample (N = 54) Significant limitations** |
NA | Same as above | Weak evidence – hypothesis generating |
|
Unprotected left main C artery No significant difference in angio outcome sor MACE |
2 small non-randomized Case-controlled Studies |
Small samples (N = 58&127) Use of lVUS @ discretion of operator Selection bias No power calculation Possible type 2 error |
Measured different parameters | Criteria for optimal stent implantation | Poor Cannot draw conclusion |
m month; MLD minimal lumen diameter; MACE major adverse cardiac event; TLR target lesion revascularization; TVR target vessel revascularization; MI myocardial infarction
Limitations: single center, small sample, narrow spectrum (de novo, native coronary, non-ostial, only one type of stent)
Limitations: subgroup analysis, small sample, inadequate power to detect a difference
Applicability of Findings to Ontario
A large Ontario observational study (5) compared drug-eluting stents to bare metal stents in 20,431 PCI procedures. Preliminary reports on 9,103 cases that had at least 9 months of follow-up indicate that in non-post MI non-diabetic patients, the TVR rate for bare metal stents was lower than previously reported in RCTs from, and was not statistically different from that of drug-eluting stents (7.2% vs 5.4% in DES). (5) In non-post MI non-diabetic patients, TVR was significantly lower for DES compared to bare metal stents in long lesions (4.7% vs 9.0%, p<0.05) and in narrow lesions (6.4% vs 10.7%, p<0.05). A similar pattern was observed in non-post MI patients with diabetes (6% vs 20.6%) for long and narrow lesions (Table 26). (5)
Table 26: Adjusted Target Vessel Revascularization Rates of Drug-Eluting Stent Versus Bare Metal Stents in Ontario Field Evaluation of Drug-Eluting Stents.
| Drug-eluting stents (%) | Bare metal stents (%) | P value | |||
|---|---|---|---|---|---|
| Non-post MI, non- diabetic | |||||
| All lesions | 5.4 | 7.2 | NS | ||
| Long lesions | 4.7 | 9.0 | < .05 | ||
| Narrow lesions | 6.4 | 10.7 | < .05 | ||
| Long or narrow lesions | 5.4 | 9.5 | < .05 | ||
| Non-post MI, diabetic | |||||
| Long lesions | 7.9 | 18.6 | < .05 | ||
| Narrow lesions | 5.7 | 11.9 | < .05 | ||
| Long & narrow lesions | 6.0 | 20.6 | < .05 | ||
| Long or narrow lesions | 6.9 | 14.3 | < .05 | ||
Bowen et al., 2005 (5)
MI myocardial infarction
Table 27 compares the results of the field evaluation to the findings from the current review.
Table 27: Revascularization Rates for Bare Metal Stents.
| Revascularization Rate % Ontario Field Evaluation |
Revascularization Rate % Current IVUS review |
||
|---|---|---|---|
| IVUS | No IVUS | ||
| Low risk lesions | 7.2 | 13.2 | 17.7 |
| Long lesions | 9.0 | 10 | 23 |
| Narrow lesions | 10.7 | ? | ? |
| Diabetes, long & narrow lesions | 20.6 | ? | ? |
IVUS intravascular ultrasound
Comparison of data from this review with data from the Ontario field evaluation showed that for low-risk patients, the revascularization rate for PCIs with bare metal stents in Ontario was lower that the revascularization rate in studies where IVUS was used to guide stenting in all patients (IVUS arm). It is understood that IVUS is presently not routinely used in low-risk stenting in Ontario. This raises the question as to whether a reduction in revascularization rates with the use of IVUS can be generalized to clinical settings in Ontario. This finding suggests that results from this systematic review need to be validated in the Ontario context.
Economic Analysis
Ontario-Based Economic Analysis
Notes & Disclaimer
The Medical Advisory Secretariat uses a standardized costing methodology for all of its economic analyses of technologies. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative (OCCI) cost data is used for all program costs when there are 10 or more hospital separations, or one-third or more of hospital separations in the ministry’s data warehouse are for the designated International Classification of Diseases-10 diagnosis codes and Canadian Classification of Health Interventions procedure codes. Where appropriate, costs are adjusted for hospital-specific or peer-specific effects. In cases where the technology under review falls outside the hospitals that report to the OCCI, PAC-10 weights converted into monetary units are used. Adjustments may need to be made to ensure the relevant case mix group is reflective of the diagnosis and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the Medical Advisory Secretariat normally defaults to considering direct treatment costs only. Historical costs have been adjusted upward by 3% per annum, representing a 5% inflation rate assumption less a 2% implicit expectation of efficiency gains by hospitals.
Non-Hospital: These include physician services costs obtained from the Provider Services Branch of the Ontario Ministry of Health and Long-Term Care, device costs from the perspective of local health care institutions, and drug costs from the Ontario Drug Benefit formulary list price.
Discounting: For all cost-effective analyses, discount rates of 5% and 3% are used as per the Canadian Coordinating Office for Health Technology Assessment and the Washington Panel of Cost-Effectiveness, respectively.
Downstream cost savings: All cost avoidance and cost savings are based on assumptions of utilization, care patterns, funding, and other factors. These may or may not be realized by the system or individual institutions.
In cases where a deviation from this standard is used, an explanation has been given as to the reasons, the assumptions and the revised approach.
The economic analysis represents an estimate only, based on assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied for the purpose of developing implementation plans for the technology.
In Ontario, the current rate of using IVUS for PCI procedures is 6%. In 2005/06, that approximated to 1,000 cases out of the 18,830 cases of PCI using stents. In comparison, the number of CABG cases in Ontario is 9,488.
There are at the moment no specific physician fee codes associated with the use of IVUS, and IVUS is used for complex cases at the discretion of the surgeon.
The Ontario Ministry of Health provides specific funding for all PCI procedures. The rate is $3,959 for PCI using BMS and $6,159 for DES. The ministry’s funding amount includes the cost of procedure in the hospital, stents (device) and the use of drugs G IIb/III.
The number of PCI stent procedures in Ontario is shown in Table 28, which indicates a 12% increase in the last two years.
Table 28: Number of PCI Stent Cases.
| Year | Cases |
|---|---|
| 2004 | 14,582 |
| 2005 | 16,687 |
| 2006 (Funded) | 18,830 |
| 2007 (Projected) | 21,239 |
The costs of surgery were obtained from the Program for Assessment of Technology in Health (PATH) as part of a comprehensive economic analysis conducted in 2005 (see Table 29).(5) The device cost for this analysis was based on the mean cost of BMSs.
Table 29: Surgical Costs.
| PCI (stents) | PCI (no stents) | CABG | |
|---|---|---|---|
| Hospital | $ 6,048 | $ 6,048 | $ 15,838 |
| Physician Fees | $ 1,069 | $ 967 | $ 2,965 |
| Device | $ 2,486 | ||
| Total | $ 9,603 | $ 7,015 | $ 18,803 |
The surgical costs were based on the use of BMSs, which accounted for 74% of all PCI procedures.
The cost of the IVUS catheter is approximately $900 and there are additional devices required with the use of IVUS (stents, balloons, guide catheters and sheaths). These additional device costs are estimated at $328. The total incremental cost for IVUS is therefore $1,228. These costs do not take into account capital costs, staff training costs or the costs associated with increased use of surgical time of between 20 to 30 minutes, with a PCI stent procedure averaging an hour of surgical time. The estimated cost associated with increased use of operating room time is $1,255 using data from the Ontario Case Costing Initiatiative (OCCI). (46)
Decision Analytic Model
A decision analytic model was developed for cost-effectiveness analysis. The model compared two strategies for stenting – with IVUS guidance and without IVUS guidance (Figure 32).
Figure 32: Decision Tree for IVUS Guidance Versus no IVUS Guidance in PCIs.
For each strategy, the combined rate for total vessel revascularization and total lesion revascularization was used for the analysis. From meta-analysis of the results from the trials (RESIST 2000 (23), SIPS 2000 (25) and Gaster 2003 (29)), this rate was determined to be 25.6% using IVUS and 41.7% if IVUS is not used, giving a differential of 16.1% in combined TVR and TLR rate.
The number needed to treat (NNT) is 6. That is, in order to prevent one revascularization, 6 patients need to have their PCI procedures guided by IVUS.
In the case of revascularization, the patient undergoes one of the three surgical procedures (PCI with stent, PCI without stent or CABG). These rates are 73%, 12% and 15% respectively based on current utilization pattern. The model assumes that these rates remain the same in both strategies, as there are no data available that indicates a difference in the type of procedures used for revascularization based on whether IVUS is used for PCI.
For sensitivity analysis, distribution functions were used for costs and effect (quality adjusted life years or QALY). Costs were based on a provincial perspective, which included hospital, physician and devices costs. The total number of QALYs over a one-year period came from the results of the 2005 PATH study (5). The averages were 0.86 for no revascularization, 0.82 for PCI with or without stent, and 0.8 for CABG.
Probabilistic Sensitivity Analysis
A probabilistic sensitivity analysis was conducted with the results shown in Figure 34. The results show that the IVUS usage does show improvement in QALYs though the increment is very small. The mean incremental QALY is 0.0067 with a 95% probability interval of between 0.0059 and 0.0076. In terms of cost, there is a mean cost savings of $491 with a 95% probability interval that lies between a cost savings of $1,108 and incremental cost of $138 (-$1,108, $138).
Figure 34: Probabilistic Sensitivity Graph.
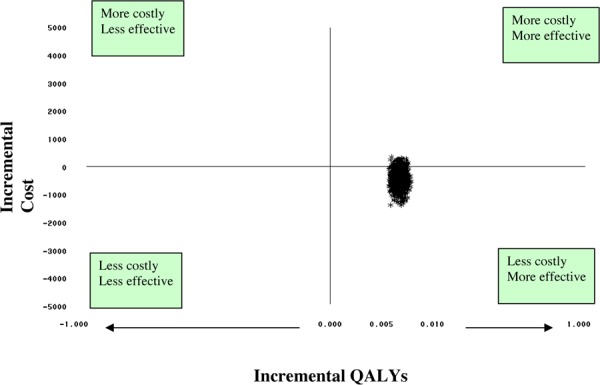
Budget Impact
The incremental upfront budget impact based on the estimate projected PCI stent cases of 21,239 in Ontario (for 2006/07) ranges from $1.56 million to $13.04 million depending on the percentage of IVUS usage in PCI procedures (see Table 30).
Table 30: Net Budget Impact.
| Percentage of PCI using IVUS | 6% | 10% | 20% | 50% |
|---|---|---|---|---|
| Incremental upfront Budget Impact ($, millions) | $1.56 | $2.61 | $5.21 | $13.04 |
| Downstream Costs Avoided ($, millions) | $2.19 | $3.65 | $7.30 | $18.25 |
| Net Budget Impact | -$0.63 | -$1.04 | -$2.09 | -$5.21 |
The downstream costs avoided are based on the number of repeat revascularization avoided due to the use of IVUS. These numbers are based on figures derived over a follow-up period of between 18 months to 2.5 years. The number of revascularization avoided ranged from 205 to 1,710 annually, dependent on the rate of IVUS usage.
The next budget impact is therefore negative with annual savings starting at $0.6M. These figures are based on using conservative cost figures.
Though the results from the economic analysis show that IVUS is cost-effective with potential savings to the Ontario health system, it is uncertain whether the reduction in revascularization rate resulting from the use of IVUS can be generalized to clinical settings in Ontario. As such, further analysis on the budget impact and cost-effectiveness needs to be conducted once Ontario-specific revascularization rates are verified.
Appraisal/Policy Development
Target Population
The projected number of PCI procedures in Ontario for 2006/2007 is 22,355. The projected number of PCI procedures requiring stenting is 21,239. If IVUS were to be used in all stenting procedures, the potential volume would be 21, 239.
Patient Outcomes
Pooled analysis of randomized controlled trials showed that even though IUVS guidance during PCI procedures did not have any impact on survival or myocardial infarction rates, it significantly reduced revascularization rates at more than 2 years after the initial PCI procedures involving bare metal stents.
Applicability of Findings to the Ontario Context
Even though IVUS is presently not routinely used in stenting of low-risk patients in Ontario, the revascularization rates in these patients in Ontario were shown to be lower than those reported for the IVUS groups in predominantly non-high risk stenting studies. In light of this information and previous findings from the Ontario field evaluation on stenting, it is uncertain whether the reduction in revascularization rates from IVUS guidance can be generalized to Ontario.
Financial Impact
The incremental cost of the IVUS catheter and additional devices is approximately $1,228 CDN per PCI procedure. IVUS also adds approximately 20 minutes to 30 minutes to the PCI procedure, thus incurring additional human resources and catheterization laboratory costs. The estimated upfront incremental cost ranged from $1.56 million at 6% uptake to $13 million at 50% uptake. The downstream cost avoidance was estimated to range from $2.19 million (6% uptake) to $18.25 million (50% uptake). There is an estimated net saving of $0.63 million (6% uptake) to $5.21 million (50% uptake). However, there is a high degree of variability on these estimates because they are dependent on the reduction in revascularization rates relating to the use of IVUS, and these rates have not been validated in the Ontario context.
Current Funding Status of Intravascular Ultrasound in Ontario
According to the July 1, 2006 Schedule of Benefit under the Health Insurance Act (47), intravascular ultrasound is unlisted, and is, therefore, not an insured health service in Ontario. If IVUS is performed at a hospital, the hospital will be responsible for covering the cost within its global budget, and the physician who performs the procedure will not be able to bill the Ontario Health Insurance Plan (OHIP) for professional fees for the service.
Diffusion
The MOHLTC was informed that almost all cardiac intervention centres in Ontario have access to IVUS. The most commonly used system in Ontario is the Galaxy IVUS Imaging System by Boston Scientific Corporation.
One academic health science centre recorded using IVUS in 182 of 2,879 PCI procedures in a 1.5-year period (November 2003 – March 2005), an uptake of 6.3%. It was believed that the uptake in other cardiac centres would be similar or lower (Personal communication, March 2006).
The additional device cost, the extra procedure time, and the lack of a physician fee code in the Schedule of Benefits are probably the main factors limiting the diffusion of this technology.
Conclusion
IVUS appears to be a safe imaging tool.
The use of adjunctive IVUS in PCIs using bare metal stents in lesions predominantly at low risk of restenosis had no significant impact on survival or myocardial infarction up to 2.5 years after intervention.
The use of intravascular ultrasound adjunctive to coronary angiography in percutaneous coronary interventions using bare metal stents in lesions predominantly at low risk of restenosis significantly reduced the (target lesion or target vessel) revascularization rate at 18 months to 2.5 years follow-up.
Based on one small study, adjunctive IVUS in PCIs using bare metal stents in long lesions (>20 mm) significantly improved the 6-month angiographic restenosis rate and one-year target lesion revascularization rate.
Based on information from an Ontario field evaluation on stenting, it is uncertain whether the reduction in revascularization rate with the use of IVUS can be generalized to Ontario.
There is presently insufficient evidence available to determine the impact of adjunctive IVUS in PCIs in high-risk lesions (other than long lesions) or in PCIs using drug-eluting stents.
Using IVUS to guide PCIs may be cost-effective and may result in net cost-saving to the health system if the reduction in revascularization rates associated with the use of IVUS can be validated in Ontario clinical settings.
Glossary
- Acute lumen gain
The difference between post intervention minimal lumen diameter and preintervention minimal lumen diameter
- Atherectomy
Removal of atherosclerotic plaque from an artery using a rotary cutter inside a special catheter guided radiographically, it does not extend to the tunica intima as endarterectomy does.
- Balloon dilatation
Also known as balloon angioplasty: Elimination of areas of narrowing in blood vessels using a balloon-tip catheter that is inflated inside an artery, stretching the intima and leaving a ragged interior surface after deflation, which triggers a healing response and breaking up of plaque.
- Binary restenosis
Recurrence of stenosis greater than 50% measured with coronary angiography during follow-up after coronary interventions
- Coronary angiography
The radiographic visualization of coronary arteries following introduction of contrast material; used as a diagnostic aid in such conditions as myocardial infarction.
- Coronary artery bypass graft
A section of vein or other conduit grafted between the aorta and a coronary artery distal to an obstructive lesion in the latter.
- Coronary artery disease
Formation of deposits of yellowish plaques containing cholesterol, lipoid material, and lipophages within the coronary arteries which may cause angina pectoris, myocardial infarction, and sudden death.
- Drug-eluting stent
A stent coated with a pharmacological compound, which may be designed to inhibit cell proliferation that causes re-narrowing at the site of the stented artery.
- Elastic recoil
The immediate reduction in vessel lumen that occurs after balloon deflation and accounts for a 50% loss in acute lumen gain during standard balloon angioplasty. Difference between the minimal measured balloon size and IVUS –derived minimal lumen area within the stent (Bermejo Circulation 1998;98:112)
- In-stent restenosis
Restenosis within a stent that has been placed in the artery.
- Intravascular ultrasound
Visualization of the interior of blood vessels by ultrasound; the transducer is mounted on the end of a catheter that is introduced percutaneously.
- Late lumen loss
The difference in minimum lumen diameter (MLD) when a follow-up angiogram is compared to a postprocedure angiogram.
- Major adverse cardiac events
A combination of clinical events that usually includes cardiac death, myocardial infarction, and target lesion revascularization or target vessel revascularization
- Acute myocardial infarction
Occurring during the period when circulation to a region of the heart is obstructed and necrosis is occurring; it is usually characterized by severe pain, frequently associated with pallor, perspiration, nausea, dyspnea, and dizziness; electrographic abnormalities may include Q-wave, ST segment, and T-wave alterations.
- Neointimal hyperplasia
Proliferation of smooth muscle cells inside a blood vessel
- Net lumen gain
The difference between follow-up minimal lumen diameter and preintervention minimal lumen diameter
- Restenosis
A re-narrowing or blockage of an artery at the same site where treatment, such as an angioplasty or stent procedure, has already taken place.
- Stent
A metal wire or tube introduced into a stenotic blood vessel to create and maintain luminal patency; it may be self expanding or balloon-expandable.
- Target lesion revascularization
Repeat percutaneous coronary intervention of the previously treated lesion
- Target Vessel Revascularization
Repeat PCI of a previously treated target vessel or bypass surgery of the target vessel.
- Thrombolysis in Myocardial Infarction grade (TIMI)
-
A grading system of coronary perfusion widely adopted for angiographic trials of thrombolysis
TIMI grade 0 – Complete occlusion
TIMI grade 1 – some penetration of the obstruction by contrast material
TIMI grade 2 – perfusion of entire coronary artery
TIMI grade 3 – full perfusion with normal flow.
- Thrombosis
Formation, development, or presence of a stationary blood clot along the wall of a blood vessel, frequently causing vascular obstruction.
Appendices
Appendix 1: Search Strategy - Intravascular Ultrasound
Search date: November 4, 2005
Databases searched: OVID Medline, In-Process and Other Non-Indexed Citations, Embase, INAHTA,
Cochrane DSR and CENTRAL
Database: Ovid MEDLINE(R) <1966 to October Week 3 2005> Search Strategy:
--------------------------------------------------------------------------------
exp Ultrasonography, Interventional/ (5280)
((intracoronary or intravascular) adj2 (ultrasound or ultrasonography or ultrasonic)).mp. [mP =title, original title, abstract, name of substance word, subject heading word] (2843)
ivus.mp. (1101)
or/1-3 (6477)
exp ANGIOPLASTY/ (30528)
exp ATHERECTOMY/ (1469)
exp Stents/ (21327)
exp Coronary Artery Bypass/ (30026)
or/5-8 (70137)
4 and 9(1800)
exp CORONARY ANGIOGRAPHY/ (27133)
10 and 11 (715)
limit 12 to (humans and english language and yr=“2001 - 2005”) (302)
(systematic review$ or systematic overview$ or metaanalysis or meta-analysis).mp. [mP =title, original title, abstract, name of substance word, subject heading word] (21420)
13 and 14(1)
13 (302)
limit 16 to (case reports or comment or editorial or letter or “review” or “review literature” or review, multicase or “review of reported cases”) (85)
16 not 17 (217)
15 or 18 (217)
Database: EMBASE <1980 to 2005 Week 44> Search Strategy:
--------------------------------------------------------------------------------
exp INTRAVASCULAR ULTRASOUND/ (2402)
((intracoronary or intravascular) adj2 (ultrasound or ultrasonography or ultrasonic)).mp. [mp =title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (3659)
ivus.mp. (1159)
exp ANGIOPLASTY/ (28075)
exp ATHERECTOMY/(1802)
exp Stent/ (23498)
exp Coronary Artery Bypass Graft/ (19527)
or/1-3 (3722)
or/4-7 (60834)
8 and 9 (1729)
exp angiocardiography/ (24160)
10 and 11 (609)
limit 12 to (human and english language and yr=“2001 - 2006”) (271)
(systematic review$ or systematic overview$ or metaanalysis or meta-analysis).mp. [mp =title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (34386)
13 and 14 (0)
13 (271)
limit 16 to (editorial or letter or note or “review”) (49)
16 not 17 (222)
Appendix 2: Summary of Systematic Reviews and Meta-Analysis
| Report | Scope of comparison between IVUS & coronary angiography | Studies included | Meta-analysis performed? | Conclusions |
| Berry et al, 2000 NHS HTA Program, UK (19) |
Effectiveness & cost-effectiveness in: Primary stenting Optimization of PTCA Other coronary interventions Therapy of in-stent restenosis Economic modelling |
Published 1990-end of 1998: 1 study on IVUS-guided PTCA 15 studies on IVUS-guided stenting (1RCT) 5 on IVUS only -6 months outcome available |
Pooled analysis for overall event rates Decision analytical model |
The evidence available is too weak for there to be any reliable implications for clinical practice. Further study with an adequately powered, well-designed RCTs was recommended |
| Medical Services Advisory Committee, 2001 Australia (17) |
Diagnostic accuracy for CAD Prediction of outcome Change inmanagement As an adjunct to coronary interventions Economic modeling |
Published 1999-August 2001 Adjunct in PCIs: 5 RCTs |
As an adjunct in PCIs: Death rates MACE Ml TLR/TVR Restenosis MLD |
Insufficient evidence pertaining to the effectiveness and cost-effectiveness of IVUS as a diagnostic or therapeutic tool. Recommended that public funding should not be supported at the time for this procedure. |
| Casella 2003 (20) | Meta-analysis of Long-term clinical outcomes of IVUS-guided vsangiography-guided stenting | 5 RCTs 3 Registries |
6-month MACE Binary restenosis MLD Acute gain Late loss Net gain |
IVUS-guidance stent implantation; -has a neutral effect on long-term death & non-fatal MI compared to angiography-guided optimization. -lowers 6-month angiographic restenosis & target vessel revascularization. |
IVUS intravascular ultrasound; PTCA percutaneous transluminal coronary interventions; RCT randomized controlled trial; PCI percutaneous coronary intervention; MACE major adverse cardiac events; MI myocardial infarction; TLR target lesion revascularization; MLD minimal lumen diameter
Appendix 3: Quality Assessment of Randomized Controlled Trials
| Study | Method of Randomization/ Concealment |
Spectrum | Blinding – patient & operator | Interpretation of CAG, IVUS & clinical outcomes | Documentary IVUS in Angio group | Statistical Power | ITT |
|---|---|---|---|---|---|---|---|
| Gaster 2001, 2003 (28) | Drawing lots from sealed opaque envelops Concealed |
Males with stable angina scheduled for PCI | No | CAG – blinded IVUS- unblinded Clinical assessment-blinded |
Yes | No stated | Yes |
| SIPs 2000 (25) | Day-to-day block schedule each morning Concealment not stated |
All patients undergoing PTCA or primary stenting in vessel 2.2–4.6 mm in diameter |
No | Not stated | Not stated | Powered to detect 0.104mm chronic difference in MLD | Yes |
| OPTICUS 2001 (21) | By fax from central office before start of procedure Concealed |
Patients with angina or documented ischemia, no long lesions or small vessel | No | Angiographic & IVUS measured blind | Not stated | Powered to detect 10% absolute ↓ in binary restenosis rate | Yes |
| RESIST 1998, (22) 2000 (23) | After satisfactory QCA stent deployment Method of randomization unknown Concealment unknown |
Symptomatic CAD>70% stenosis in 1 or more coronary undergoing PTCA+ stenting | No | Angio analysis blinded to IVUS results | Yes | 40% power to detect a 15% absolute ↓ in restenosis rate | Yes |
| TULIP 2003 (26) | Just before the procedure. Method not stated. Concealment unknown |
Consecutive patients for elective PCI, Only long lesions ≥ 20 mm, no narrow vessels. | No | Angio analysis blinded to IVUS assignment | Not stated | Powered to detect >0.25mm difference in MLD in 6 months | Yes |
| AVID 1997 (31) 2000 (27)(Abstract) | Method not stated Concealment unknown |
Undergoing elective stenting in vessel >2.5mm, & @ low risk for complications | No | Not stated | Yes | Not reported | Not reported |
Angio or QCA quantitative coronary angiography; IVUS intravascular ultrasound; CAD coronary artery disease; MLD minimal lumen diameter; ITT intention-to-treat analysis
Appendix 4: Quality Assessment of Non-Randomized Observational Studies
| Study | Enrolment & assignment | Spectrum | Blinding – patient & operator | Interpretation of CAG, IVUS & clinical outcomes | Document ary IVUS in Angio group | Statistical Power | ITT |
|---|---|---|---|---|---|---|---|
| Park 2001 (32) | Prospective non-randomized controlled study with consecutive patients IVUS @ discretion of operator Some had atherectomy before stenting |
Only patients with symptomatic unprotected left main coronary artery stenosis | No | IVUS & QCA by Computer software QCA by two independent angiographers No blinding mentioned Angiographic follow-up done: IVUS 59/77 Angio 41/50 |
No | Not stated | No |
| Agostoni 2005 (33) | Prospective non-randomized observational studies | Only patients with symptomatic unprotected left main coronary artery stenosis | No | All analysis performed on line using computer software No off-line analysis mentioned |
No | Not stated | No clear |
Angio or QCA quantitative coronary angiography; IVUS intravascular ultrasound
Appendix 5: Inclusion and Exclusion Criteria of Primary Studies
| Study | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Gaster 2001 (28) | Male patients referred for PCI of a de novo lesion on a native coronary artery | Restenotic lesion, SVG Patients in whom follow-up was deemed unlikely Acute MI<3 months before PCI Unstable angina <1month of PCI Left bundle branch block Atrial fibrillation Elevated level of serum creatinine>200 umol/l Thyrotoxicosis Polycythemia. Total occlusion or unobtainable preprocedure IVUS pullback. |
| SIPs 2000 (25) | Patients undergoing elective or urgent PTCA or primary stenting in vessels of diameter between 2.2mm to 4.6mm |
Chronic total occlusion Lesions in saphenous vein grafts>4.6mm |
| OPTICUS 2001 (21) | Angina or documented ischemia Lesion length </=25mm |
Contraindication to antiplatelet therapy Acute angina at rest Complete akinesia in myocardium supplied by target artery Significant left main lesion, bifurcation lesion, & involvement of a side branch>/=2 mm in diameter with ostial stenosis |
| RESIST 1998(22) 2000 (23) | Single<20mm long stent deployment Balloon/artery ratio for stent placement between 1.0 &1.2 Balloon inflation pressure >12 atmospheres Optimal angiographic results after stent implantation without dissection or residual stenosis >20% on QCA |
Vessel diameter <3.0 mm by visual QCA Coronary lesion >12mm in length Previous CABG Contraindication to antiplatelet therapy Treatment of acute or chronic total occlusion Saphenous vein graft stenosis Acute coronary syndrome <7 days. |
| TULIP 2003 (26) | Consecutive patients referred for elective PCI De novo, nonostial stenosis >/=20mm long in a native coronary artery Reference diameter allowed implantation of >/=3mm stent No involvement of significant side branches (diameter>/=2.0mm) |
MI<2weeks Total occlusion Contraindication for combined antiplatelet therapy with ASA &ticlopidine |
| AVID 1997(31) 2000 (27) (Abstract) |
Low risk for complications who were undergoing elective stent placement in vessels>2.5mm in diameter Successful stent deployment & underwent IVUS |
Vessel<2.5mm |
| Park 2001 (32) | Consecutive patients with symptomatic left main coronary artery diseaseor documented myocardial infarction & angiographic evidence of ≥50% diameter stenosis of the LMCA. Excluded: contraindications | Contraindication to antiplatelet or anticoagulation therapy Left ventricular dysfunction (ejection fraction >40%) |
| Agostoni 2005 (33) | Patients undergoing elective PCI using drug-eluting stents for symptomatic CAD (stenosis >50%) by visual estimation in unprotected left main coronary artery | Acute MI Cardiogenic shock undergoing emergency PCI Protected left main (>/=1 patent bypass graft on the left coronary artery |
Angio or QCA quantitative coronary angiography; PCI percutaneous coronary intervention; PTCA percutaneous transluminal coronary interventions; LMCA left main coronary artery; SVG saphenous vein graft; umol micromole; I litre
Appendix 6: Comparison of Study Population Based on Inclusion/Exclusion Criteria
| Study | Urgent/ Emergent PCI |
Recent acute MI | Unstable angina | Multi-vessel Disease | Restenotic lesion | SVG | Lesion length mm | Vessel size(diameter mm) | Others |
|---|---|---|---|---|---|---|---|---|---|
| Gaster 2001 (28) | No | No | No | Yes | No | No | No limit | No limit | Males only |
| SIPS 2000 (25) | No | Yes | Yes | Yes | Yes | Yes | No limit | 2.2–4.6 mm | Exclude artery with total obstruction/atherectomy |
| OPTICUS 2001 (21) | Yes | Yes | Yes | Yes | Yes | No | ≤25 ≤2 stents No bifurcated lesions |
>2.5 No L main |
Exclude: complete akinetic myocardium, |
| RESIST 1998(22) | Yes | No | Yes | Yes | Yes | No | ≤ 15 | ≥3.0 | Stenosis >70% |
| AVID 1997(31) 2000 (27) | No | ? | ? | Yes | No | Yes | No limit | ≥2.5 | Successful stenting |
| TULIP 2003 (26) | No | No | Yes | Yes | No | No | ≥20 Nonostial | Allow stent≥3 | No significant side branch involved |
| Park 2001 (32) | Yes | Yes | Yes | No | No limit | No limit | Unprotected left main only | ||
| Agostoni 2005 (33) | No | No | Yes | Yes | Yes | No | No limit | No limit | Unprotected left main only |
Yes=included
No=excluded
Appendix 7: Summary of Baseline Patient Profiles
| Study | Group | Mean age(Years) | Restenotic Lesion % | ACC/AHA lesion type B2 - C | Mean lesion length (mm) | Mean reference diameter (mm) | Minimal lumen diameter (mm) | Diameter stenosis % | Prior MI % | Prior CABG or PCI % | Diabetes % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gaster | IVUS | 57 | 0 | 47 | 13.4 | 2.8 (.5) | 1.1 | 60 | 54 | 13 | 4 | |
| 2001 (28) | Angio | 57 | 0 | 46 | 13.3 | 2.8 (.5) | 1.0 | 64 | 44 | 19 | 11 | |
| SIPS | IVUS | 60 | 38 | 51 | 9.74 | 3.01 (.59) | 0.64 | 79.1 | 69 | 13 | 19 | |
| 2000 (25) | Angio | 61 | 51 | 42 | 9.71 | 3.0 (.7) | 0.70 | 76.8 | 77 | 15 | 24 | |
| OPTICUS | IVUS | 60.1 | - | 76 | 11.2 | 2.97 (.63) | 0.96 | 67.6 | 32 | 23 | 17 | |
| 2001(21) | Angio | 61.5 | 78 | 11.6 | 3.13 (.52) | 0.99 | 66.7 | 32 | 24 | 17 | ||
| RESIST | IVUS | 57 | - | 43 | 7.72 | 2.94(.57) | 0.96 | 65 | 68 | - | 11 | |
| 1998(22) | Angio | 56 | 48 | 8.05 | 3.06(.59) | 1.02 | 64 | 63 | 11 | |||
| AVID | IVUS | ? | ? | ? | ? | ? | ? | ? | ? | |||
| 1997 (31) | Angio | |||||||||||
| 2000 (27) | ||||||||||||
| TULIP | IVUS | 61 | - | ? | 29 | 2.95 (.57) | 1.02 | 65 | - | - | 16 | |
| 2003 (26) | Angio | 63 | 27 | 2.96(.53) | 0.99 | 65 | 21 | |||||
| Park 2001 | IVUS | 54.7 | 52 | ** | 1.1 | |||||||
| (32) (LMCA) | Angio | 56.7 | 60 | 1.0 | ||||||||
| Agostoni | IVUS | 54.7 | 52 | 7.47 | 1.2 | 62 | 37 | 50 | ||||
| 2005 (33) (LMCA) | Angtio | 56.7 | 60 | 7.33 | 1.0 | 62.4 | 50 | 21 |
P < .01
Ostial lesion = 4.2 +/-1.5mm, lesion in body & distal =12.4 +/- 3.6mm
LMCA = Left main coronary artery IVUS= intravascular ultrasound Angio = angiography
Appendix 8: Endpoints and Definition of Major Adverse Cardiac Events in Included Studies
| Study | Primary End point | Secondary End point | Definition of MACE | ||
|---|---|---|---|---|---|
| Gaster 2001 (28) |
6-month Recurrence of stenosis defined as: QCA diameter stenosis ≥50% or Recurrence = QCA diameter stenosis≥ 50% + CFR<2.5+ angina or Recurrence = QCA diameter stenosis≥ 50% + FFR<0.75 + angina or TVR |
Death | Q-wave MI | Clinically driven TVR = CABG + repeat target vessel PCI | |
| Repeat angio & IVUS @ 6 months | |||||
| Gaster 2003 (29) | Rehospitalization rate MACE Length of stay | Death | Q-wave MI | Clinically driven TVR = CABG + repeat target vessel PCI | |
| OPTICUS (Mudra 2001) (21) |
6-month Binary angiographic restenosis rate (>50% ↓ lumen diameter) MLD % Diameter stenosis |
1, 6 & 12 month MACE Fulfillment of ultrasound & angiographic target criteria |
Death | Q-wave MI | Clinically driven TLR= CABG + repeat PTCA |
| SIPS (Frey 2000) (25) | 6-month angiographic MLD | Acute MLD Acute & chronic cost QOL, 2-year MACE & clinically driven TLR |
Death | MI | (Total) TVR = CABG +re PTCA |
| SIPS (Mueller 2003) (24) | MACE at 28 months | 6-month angiographic restenosis rate | Death | MI | Clinically driven TLR |
| RESIST (Schiele 1998) (22) | 6-month angiographic restenosis rate (>50% stenosis) stent+5mm proximal or distal by QCA | 6 month angiographic MLD & IVUS CSA | |||
| RESIST (Schiele 2000) (23) | Six-month angiographic end points & 18 month MACE | Death | MI | TVR =CABG + re PTCA (18 month) | |
| TULIP(Oemrawsingh, 2003) (26) (long lesion) | Angiographic MLD MACE @ 6 month | Angiographic & procedural success, Angiographic restenosis & %diameter stenosis @ 6 months MACE @ 12 months |
C. Death | MI | Ischemia driven TLR |
| AVID Abstract 1997 (31) 2000 (27) | TLR @ 12 months | TLR (driven by?) | |||
| Studies on Unprotected Left Main Coronary Artery | |||||
| Park 2001 (32) | MACE | C. Death | MI* | TLR | |
| Agostoni 2005 (33) | Occurrence of MACE @ median follow-up of 433 days | - | All Death | MI* | TVR = CABG + re PTCA in stent+5mm distal or proximal |
CFR = coronary flow reserve
FFR = fractional flow reserve
CABG = coronary artery bypass graft
TLR = Target lesion revascularization
TVR = Target vessel revascularization
C. death = Cardiac death
rePTCA = repeat percutaneous transluminal coronary angioplasty
Non-fatal
Appendix 9a: Intravenous Ultrasound Criteria for Optimal Stent Placement in Randomized Controlled Studies
| Study | Coronary vessel treated | IVUS Criteria for Optimal Stent Placement | |||
|---|---|---|---|---|---|
| Minimal lumen size as a % of reference segment lumen size | Absence of Dissection | Complete apposition of stent against vessel wall | Others | ||
| RCTs | |||||
| Gaster 2001 & 2003 (28;29) | Native | ≥90% of average of proximal & distal reference lumen CSA =100% of smaller reference vessel lumen CSA | ν | Lumen CSA @ proximal stent entrance>90% of the proximal reference lumen CSA | |
| Opticus (21) | Native | MUSIC Criteria:In-stent MLA≥ 90% of average reference LA or 100% LA of reference segment with the lowest LA. In-stent LA of proximal stent entrance > 90% of proximal reference LA. If in-stent LA> 9 mm2, in-stent MLA>80% of average reference LA or >90% of LA of the reference segment with the lowest LA |
ν | Symmetric stent expansion defined by LDmin/LDmax≥ 0.7 | |
| SIPS (24;25) | Native | No stents: MLA>65% mean reference area Stenting: MUSIC criteria (see OPITCUS) |
ν | ||
| RESIST (22;23) | Native | Intrastent CSA ≥ 80% of mean reference lumen CSA | |||
| TULIP (26) | MLD>80% of mean reference MLD In-stent MLA≥distal reference LA |
ν | |||
| AVID (12) | Native & SVG | <10% residual stenosis | ν | ν | |
| Non-randomized | |||||
| Agostoni 2005 (33) | LMCA | Stent Lumen CSA>80% of average reference CSA by visual estimation | ν | Full lesion coverage. | |
| Park 2001 (32) | LMCA | Lumen CSA of target lesions >90% of distal reference lumen CSA | ν | Full lesion coverage | |
IVUS = Intravascular ultrasound
MLD = Minimal lumen diameter
CSA = Cross sectional area LA = luminal
area MLA = Minimal lumen area
SVG = Saphenous vein graft
LMCA = Left main coronary artery
Appendix 9b: Interventions that Met Optimal Stent Placement Criteria
| Study | Interventions that met Optimal Stent Placement Criteria (%) | ||
|---|---|---|---|
| IVUS (%) | Angio (%) | P Value | |
| Gaster 2001 (28) | 64 | 16 | < .01 |
| AVID 1997 (31), 2000 (27) | 42 | No data | |
| OPTICUS 2001 (21) | 82.2 | 70.7 | < .0001 |
| RESIST 1998 (22) | 80 | 59 | < .01 |
| SIPS 2000 (25) | 69 | No data | |
| TULIP 2003 (26) | 89 | No data | |
| Agostoni 2005 (33) | Additional 29% | ||
Appendix 10: Stents used in Randomized Controlled Trials
| Study | Type of Stents Used |
|---|---|
| Gaster 2001 (28) | No specific type of stent required (QCA off-line in core lab, blinded) |
| AVID 1997(31), 2000(27) | No data |
| OPTICUS 2001 (21) | Customized stents from 2 companies used: JJIS Double spiral bridge Power Grip, Crown stent (Cordis J & J,) or NIR (Medinol, Boston Scientific Corp. |
| RESIST 1998 (22) | Palmaz-Schatz stent (Johnson & Johnson), MicroStent (Applied Vascular Engineering), NIR stent (Boston Scientific Corporation), or Freedom stent (Global Therapeutic) |
| SIPS 2000 (25) | Majority (83.4%) had Palmaz-Schatz stent (Johnson & Johnson) |
| TULIP 2003 (26) | AVE GFX-XL (Medtronic /AVE) stents |
| Agostoni 2005 (33) | Sirolimus eluting stents – Cypher (Johnson & Johnson-Cordis unit, Roden, The Netherlands) Paclitaxel-eluting stents–Taxus (Boston Scientific Corp. Natick, Massachusetts) |
| Park 2001 (32) | Different types of stents (slotted-tube or coiled stents) |
Appendix 11: Drug Therapy after Percutaneous Coronary Intervention
| Study | Drug Therapy after Intervention |
|---|---|
| Gaster 2001 (28) | No information |
| AVID 1997 (31), 2000 (27) | Aspirin and ticlopidine (dose not stated) |
| OPTICUS 2001 (21) | >/=100 mg aspirin per day for an indefinite duration and ticlopidine 250 mg BID started immediately after the procedure and maintained for 4 weeks. |
| RESIST 1998 (22) | 250 mg aspirin daily and 500 mg ticlopidine for 1 month |
| SIPS 2000 (25) | Oral aspirin (100 mg daily) Preprocedure. Stented patients treated with 250-500 mg aspirin IV & 250 mg ticlopidine bid. Glycoprotein lib/lia receptor inhibitors only under emergency situations. |
| TULIP 2003 (26) | 240 ASA plus 500 mg ticlopidine for 4 weeks and >/=80 mg of ASA indefinitely. |
| Agostoni 2005 (33) | Aspirin therapy lifelong. Clopidogrel (300 mg loading dose before procedure and 75 mg/day) prescribed for 6 months. |
Appendix 12: Definition of Clinically-Driven or Ischemia-Driven Target Lesion Revascularization
RESIST
Clinically driven TLR defined as angiographic restenosis >50% and angina and/or demonstrable myocardial ischemia.
SIPS
Clinically driven TLR defined as having angina pain at the time of admission to hospital and angiographic restenosis in the target segment treated either by PTCA or CABG
TULIP
Ischemia driven TLR defined as angiographic restenosis>50% and angina or positive stress test.
Appendix 13: Outcomes of Studies on Drug-Eluting Stents
| RAVEL Morice 2002(48) | Sousa 2003 (49) | SIRIUS Moses 2003 (41) | TAXUS I Grube 2003 (50) | TAXUS II Colombo 2003 (51) | TAXUS III Tanabe 2003 (43) | |
|---|---|---|---|---|---|---|
| Drug-eluting stent used | Sirolimus Bx Velocity | Sirolimus Bx Velocity | Sirolimus | Paclitaxel SR NIRx | Paclitaxel SR &MR | Paclitaxel SR NIRx |
| Sample size DES BMS |
120 118 |
25 |
533 525 |
31 30 |
229 240 |
28 |
| Angiographic follow-up data @ | 6 months | 12 months | 240 days | 6 months | 6 months | |
| Postintervention MLD, mm DES BMS |
In-stent 2.43 (0.41) 2.41 (0.40) |
In-stent 2.71 (0.3) |
In-stent 2.67 (0.4) 2.68 (0.42) |
2.95 (0.34) 2.87 (0.43) |
SR 2.53 (0.29) 2.58 (0.37) MR 2.53(0.36) 2.52(0.34) |
Instent 2.4(0.44) |
| Follow-up MLD, mm DES BMS DES BMS |
2.42 (0.49) 1.64 (0.59)* |
2.35 (0.6) |
2.50 (0.58) 1.69 (0.79)* |
2.60 (0.49) 2.19 (0.65) P =.007 |
SR 2.23 (0.47) 1.79 (0.54)** MR 2.24 (0.47) 1.76 (0.57)** |
1.84 (0.63) |
| Late lumen loss, mm DES BMS DES BMS |
–0.01 (0.33) 0.80 (0.53)* |
0.36 (0.46) | 0.17 (0.45) 1.00 (0.70)* |
0.36 (0.48) 0.71 (0.47) P =.008 |
SR 0.31 (0.38) 0.79 (0.45)** MR 0.30 (0.39) 0.77 (0.50)** |
0.54 (0.51) |
| Neointimal hyperplasia volume, mm3 DES BMS |
2.55 (4.9) | 14.8 (10.8) 21.6 (10.7) P =.028 |
||||
| Angiographic restenosis % DES BMS DES BMS |
0 26.6* |
3.2 35.4* |
0 10 P =.112 |
SR 2.3 17.9** MR 4.7 20.2** |
||
| Target lesion revascularization % DES BMS |
1 year TVR 0.8 24 |
@270 days 4.1 16.6* |
0 10 P =.237 |
SR 4.7 12.9 (P =.03) MR 3.8 (P =.002) 16.0 |
21.4 |
P< .001
< .0001
DES = Drug-eluting stent
BMS = Bare metal stent
SR= Slow release
MR = Moderate release
Appendix 14: Detailed Summary of Randomized Controlled Trials
| Study | Objective | Endpoints | Design | Follow-up | Patients & Lesion | Method | Results | Conclusion & Limitation | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gaster 2001 (28) | Cost-effectiveness of ICUS guided PCI vs CAG guided PCI Enrolment period May 1996 – December 1998 |
Angiographic diameter restenosis≥5 0%, CFR<2.5 or FFR<0.75 & angina OR TVR |
Single center RCT ICUS n=54 CAG n=54 |
6 month angiographic & clinical | Patients Males scheduled for PCI Stable angina No angiographic criteria Lesion De novo lesions in native coronary artery Mean length: 13.4+/-11.7 mm |
- Strategy of provisional stenting -IVUS, CAG & IC-Doppler for all patients @ 6 months -TLR @ operator’s discretion Blinding Operator of CAG group blinded to IVUS results; clinical assessor blinded to test results. |
Lower cost for ICUS group mainly due to fewer re-interventions & fewer extra days of hospitalization in the ICUS guided group. |
-Small sample -Only some patients received stents -cost estimates based on a limited number of procedures-only cost of single use equipment included in costing |
||||||||||||||||||||||||||||||||||||||||
| Gaster 2003 (29) | Assess MACE rates & cost-effectiveness of ICUS guided PCI vs CAG guided PCI 5 years after enrollment of 1st patient Enrolment period May 1996 – December 1998 |
Freedom from occurrence of MACE (death, Q-wave MI & TVR) | Single center RCT ICUS n = 54 CAG n = 54 |
Median 2.5 years – patient record review | Patients Males scheduled for PCI Stable angina No angiographic criteria Lesion De novo lesions in native coronary artery Mean length: 13.4+/-11.7 mm |
- Strategy of provisional stenting -IVUS, CAG & IC- Doppler for all patients @ 6 months -TLR @ operator’s discretion Blinding Operator of CAG group blinded to IVUS results; clinical assessor blinded to test results. |
OR for MACE 2.5 in favour of ICUS P =0.04
Lower cost for ICUS guided group due to less CAG, PCI or CABG and lower rate of hospitalization due to angina and fewer outpatient visits. |
Authors’ conclusion IVUS guidance resulted in continued improvement of long-term clinical outcome & cost-effectiveness. Study supports a more liberal use of IVUS guidance of coronary interventions, particularly in procedures performed on patients with stable angina. | ||||||||||||||||||||||||||||||||||||||||
| Mudra 2001 (21) (OPTICUS) | To compare stent implantation guided by ICUS with that guided by CAG in experienced ICUS centres Enrolment period October 1996–February 1998 |
Primary: incidence of angiographic restenosis (>50% lumen diameter reduction), MLD, & % diameter stenosis after 6 months 2nd: MACE (death, MI, TVR), & fulfillment of angiographic & ultrasound target criteria | Multicenter RCT (26) Concealment ICUS = 273 CAG = 277 |
1& 6 month angiographic 12 month clinical |
Patients Patients with angina or documented ischemia Lesion Length ≤ 25 mm Needed 1–2 stents Vessel diameter ≥ 2.5 mm |
-Stent implantation guided by ICUS or CAG Pre intervention ICUS recommended in the ICUS group. All ultrasound with motorized pullback. Criteria for optimal stent deployment ICUS group: MUSIC study criteria CAG group: <10%residual diameter Blinding For angiographic & ultrasound measurements ITT analysis |
-Significantly higher early lumen gain (2.07 mm vs 1.91 mm, p<0.0001) in IVUS group after intervention -No significant difference in MLD, diameter stenosis, net MLD gain and restenosis rate between the two groups at 6 month follow-up (restenosis 24.8% IVUS vs 22.8% Angio, P =0.68). -No significant difference in death, TVR, MI or MACE at 12-month follow-up. |
|||||||||||||||||||||||||||||||||||||||||
| Frey, 2000 (25) SIPS |
To test the hypothesis that routine ICUS guidance of PCI improves outcome Enrolment period: February 1996 – May 1996 |
Primary: 6 month angiographic MLD | Single center RCT of consecutive patients ICUS 121 CAG 148 |
6-month angiographic 6-month & 2 year clinical by review of clinical record |
Patients undergoing elective or urgent PTCA or primary stenting Vessel diameter 2.2 –4.6 mm Lesion: de novo & restenotic in native coronary artery Blinding Not reported |
Strategy of provisional stenting – stenting discouraged unless significant dissection after PTCA or angiographic results unsatisfactory. Criteria for optimal results: ICUS: no stents- MLA in lesion >65% of mean reference area. Stent implantation - MUSIC criteria. CAG: No stents - <35% residual angiographic diameter stenosis; stenting: <10% diameter stenosis with no evidence of uncovered dissection |
-No significant difference inangiographic findings except significantly higher acute gain in MLD for ICUS group (1.85 vs 1.67 mm, P =0.02), however, no significant difference in net gain.-No significant difference in death, MI or MACE rate at 2 years -ICUS guided group had significantly lower clinically driven TLR @ 2 years (17% vs 29% in CAG, P =0.02) |
A strategy of IVUS guided intervention can be applied to a wide range of patients in routine clinical practice. | ||||||||||||||||||||||||||||||||||||||||
| Mueller 2002 (30) SIPS diabetes subgroup analysis |
To investigate whether routine use of ICUS guidance during PCI improves long-term outcomes in people with diabetes. Enrolment period February 1996 – May 1996 |
Primary: MACE (death, non-fatal MI, & TVR) @ 28 months Secondary: 6-month angiographic restenosis rate |
Prospectively designed subgroup analysis of RCT ICUS 19 CAG 24 |
Angiographic – 6 month Clinical: 18 & 28 months |
Consecutive diabetic patients undergoing elective or urgent PTCA or primary stenting Vessel diameter 2.2 –4.6 mm Lesion: de novo & restenotic in native coronary artery Blinding Analysis of QCA blinded to clinical data |
Strategy of provisional stenting – stenting discouraged unless significant dissection after PTCA or angiographic results unsatisfactory. Criteria for optimal results: ICUS: no stents- MLA inlesion >65% of mean reference area. Stent implantation - MUSIC criteria. CAG: No stents - <35% residual angiographic diameter stenosis; stenting: <10% diameter stenosis with no evidence of uncovered dissection |
-Baseline patient & lesion characteristics well matched between the two groups ->50% of lesions were complex-B2 or C, 1/3 were re-stenotic. No statistically significant difference in MLD, net gain, or restenosis rate at 6 month QAC. No statistically significant difference in death (1in each groups), MI (0 for both groups), TVR (26% vs 42%, P =0.3), MACE or days in hospitals. Total costs* were similar for the two groups ($16,725 for ICUS vs $16,230 for CAG) *Costs = initial hospitalization, cardiac related hospitalization during follow-up – including costs of cath lab resources, personnel, inpatient care, TVR, cardiac medication & indirect costs. |
Authors’ Conclusion Routine ICUS guidance during provisional stenting seems to slightly attenuate the negative effect of diabetes on clinical long-term outcome. However, the angiographic restenosis rate remains very high. Limitations | ||||||||||||||||||||||||||||||||||||||||
| Mueller 2003 (24) SIPS Cost-effectiveness analysis |
To determine whether routine ICUS guidance intervention is cost effective. Enrolment period February 1996 – May 1996 |
Primary: incremental cost-effectiveness | Prospectively designed economic analysis included in an RCT (SIPS) | 6-month angiographic 6-month & 2 year clinical by review of clinical record 2-year cost analysis |
See Frey 2000 | Collected cost data: Direct costs: initial hospitalization, outpatient visits & cardiac related hospitalization during 2 year follow-up (cath lab resources, personnel, cardiac medication) Indirect costs Expenses for hospital care calculated from intensity of care & length of stay. Incremental cost effectiveness = (cost ICUS – cost CAG)/(MACE-free ICUS – MACE free CAG) |
2-year MACE-free survival was significantly higher for the ICUS group (80.% vs 69% for CAG, p<0.04) Total costs similar: ICUS $15,947+/-8,545 CAG $16,103+/-9,954 P =0.89 Cost-effectiveness ICUS -$1,417/MACE free survival gained. In 55.3% of boostrapping replications, IVUS was less expensive and more effective, and in 43.2% of replications, ICUS was more expensive and more effective. Sensitivity analysis revealed that the cost-effectiveness of ICUS was very successful. |
Authors’ conclusion When used in a provisional stenting strategy, routine IVUS imaging is cost-saving half the time. Limitations Some of the clinical benefits observed in the IUVS group might be due to the unique variable diameter focal design of the combination balloon catheter. Compared to angiography, experience in IVUS was limited. The rate of stenting was low compared to current practice. | ||||||||||||||||||||||||||||||||||||||||
| Schiele 1998 (22) RESIST |
To investigate impact of IVUS guided stent implantation on 6 month restenosis rate Enrolment period Jan 1995–Feb 1997 |
Primary: 6-month restenosis rate >50% QAC-2nd: 6 month QAC MLD & IVUS CSA | Multicenter, single blinded RCT IVUS 79 CAG 76 |
6-month clinical & angiographic MACE: death, MI, TVR |
Symptomatic CAD & demonstrated ischemia Lesion: de novo in native vessel >70% stenosis All had PTCA+stenting |
Randomized after satisfactory stent deployment No further dilation in angio group. IVUS group: additional overdilation until IVUS criteria met. Both on-line & offline IVUS assessment. Blinding Angiograms analyzed off line by operator blinded to IVUS data. |
Overdilation in 31 (39%) of IVUS pts. 80% IVUS group reached IVUS criterion vs 59% of angio group Immediately after PCI -No significant difference in MLD or residual stenosis IVUS group had higher CSA (7.16+/-2.48 vs 7.95+/-2.21 mm2, P =0.04), acute gain (1.45mm vs 162, P =0.04) & stent lumen CSA 6 month follow-up: -No significant difference in restenosis rate (22.5% IVUS vs 28.8% angio, P =0.25). 19.9% increase in lumen CSA in IVUS group (5.36 vs 4.47 mm2, P =0.03) -The only independent predictor of restenosis in multivariate analysis was post procedure lumen CSA @ stent level (OR 0.70 per additional mm2 in stent lumen CSA, 95% CI 0.47 to 0.93). -No major complication. 3 cases of coronary non occlusive dissection. |
Cannot rule out beneficial effect of IVUS (possible type 2 error.) Limitations -Sample size calculation based on unsubstantiated restenosis rates. -Lack of statistical power resulting in type 2 error Different types of stents were used requiring different implantation pressure. (40% power) |
||||||||||||||||||||||||||||||||||||||||
| Schiele 2000 (23) RESIST Cost analysis (France) |
To compare acute & long-term medical costs of IVUS-guided & angiography-guided stenting Enrolment period Jan 1995–Feb 1997 |
18-month MACE Cumulated medical costs @ 18 months |
Multicenter, single blinded RCT IVUS 79 CAG 76 |
6 month angiographic & 18 month clinical | Symptomatic CAD & demonstrated ischemia Lesion: de novo in native coronary artery>70% stenosis All had PTCA+stenting |
Calculate accumulated hospital & procedure costs using a cost accounting system for all initial & repeat lesion revascularization. | Event free survival @ 18 months: IVUS 75%, Angio 63% (P =0.12) 6 month TLR: IVUS 19/79, Angio 27/76 Revascularization rate (18 month) – angio driven: IVUS 27% (21/79), Angio 41%(31/76)? Initial stent implantation cost – 18% higher in IVUS Total procedure cost @ 18 month – 8.7% higher in IVUS Total medical cost @ 18 month – 3.2% higher in IVUS Sensitivity analysis showed total medical costs +1% and 7.6% higherin IVUS |
- Lower revascularization rate in IVUS did not totally offset higher initial stent implanation cost IVUS guidance in stent implantation did not considerably increase the medical costs. Limitation: Angiographicall driven revascularization probably increased the costs of both groups |
||||||||||||||||||||||||||||||||||||||||
| Oemrawsingh (26) 2003 TULIP (Long lesions) |
To compare 6-month outcome of stent implantation for long lesions in patients randomized to IVUS or angiography guidance Enrolment period June 1998–Jan 2001 |
Primary: Angiographic MLD & death +MI +ischemia driven TLR @ 6 months Secondary: Angiographic & procedural success & angiographic stenosis @ 6 month, death +MI+TLR @months |
Single centre RCT of consecutive patients IVUS 74 Angio 76 Blinded analysis of angiograms |
6 month angiographic & clinical 12 month clinical MACE = death +MI+ischemia driven TLR |
Patients: Referred for elective PCI Lesion: de novo in native coronary Lesion length >20 mm in length Vessel allow implantation of >3 mm stents |
3 angiograms for every patient. IVUS pullback & dilatation until criteria met. Angiographic criteria for successful stent placement: Complete coverage of stenotic segment, angiographic residual diameter stenosis<30%, & absence of angiographic dissection IVUS criteria for successful stent placement: Complete stent apposition, in-stent MLD>80% of mean reference MLD & in-stent LML>distal reference lumen area |
-Comparable baseline characteristics longer stents & larger # ofstents in IVUS group At 6 months: Angiographic MLD significantly larger (1.82 vs1.51 mm, P =0.042) &restenosis rate significantly lower (23% vs 46%, P =0.008) in IVUS group 6 & 12 months TLR (4% vs 14%, 10% vs 23%, P =0.018) & combined events (6% vs 20%, 12% vs27%, P =0.026) significantly lower in IVUS group both @ 6months & 12 months respectively. Other parameters not significantly different |
Angiographic and clinical outcome up to 12 month after long stent placement by IVUS is superior to guidance by angiography. | ||||||||||||||||||||||||||||||||||||||||
| Russo et al 1997 (31) (Abstract) AVID study 2000 (27) Angiography Versus Ultrasound-Directed Stent Placement (AVID) |
To assessthe effect of IVUS on patient outcome after elective coronary stent placement | Primary: TLR @ 12 months | Multicenter RCT (parallel group) IVUS 394 Angio 406 Total 800 |
30 day angiographic 6month and 12 month clinical | Patients at low risk for complications who were undergoing elective stent placement Lesions Any lesion in vessels >2.5mm |
Patients randomized after optimal stent placement (<10% residual stenosis on angiography) Blinded IVUS performed IVUS Criteria for optimal stent placement: (<10% stenosis, full apposition, nodissection) Additional therapy in 1.5% of angio patients & 41.6% of IVUS guided patients. Mean post procedural MLD significantly larger in IVUS group (2.97 vs 2.88 mm, P =0.02) Relatively low incidence (~0.5%) of procedural complications related to additional therapy in IVUS group |
Based on all vessels entered into the study: 12-month TLR not significantly lower in IVUS (8.4% vs 12.4%, P =0.08, 95% CI –8.4% to 0.8%) 12-month TLR significantly lower in the IVUS group (4.9% vs 10.8%, P =0.02) when protocol violators (preprocedure reference diameter <2.5mm) were excluded. Significantly lower TLR in IVUS patients treated for saphenous vein grafts (5.1% vs 20.8%, P =0.03). Also if lesion >50%, particularly when >70%. (3.4% for IVUS vs 14.4% for angiography) & vessels >2.5mm. |
Although in the overall population, only a nonsignificant trend favoured IVUS guidance, the positive results when protocol violators were excluded suggest that IVUS may be of significant benefit in patients with vein graft lesions, more severe stenosis, and vessels <3.5 mm and >2.5mm. |
ICUS=intracoronary ultrasound
CAG = coronary angiography
CFR = coronary flow reserve FFR=fractional flow reserve
TVR=Target vessel revascularization
MLA = minimal lumen diameter
MLD = minimal lumen diameter
PCI = percutaneous coronary intervention
CABG = coronary artery bypass graft
QCA = quantitative coronary angiography
Appendix 15: Detailed Summary of Prospective Non-Randomized Controlled Trials
| Study | Objective | Endpoints | Design | Follow-up | Patients & Lesion | Method | Results | Conclusion & Limitation | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Park 2001 (32) | Effect of debulking & IVUS guidance on elective stenting of unprotected left main coronary artery (LMCA) stenosis Nov 1995 – April 2000 |
Procedural success: <30% residual diameter stenosis by QCA & no procedural or in hospital complications Angiographic MLD, restenosis rates MACE = cardiac death, non-fatal MI & TLR |
Non-randomized observation al study Patients IVUS 77 No IVUS 50 Use of IVUS @ discretion of the operator |
Angiographic follow-up @ 6 months Clinical following -up up to 2 years |
Patients: Consecutive patients Inclusion criteria -Symptomatic LMCA disease or documented MI -Angiographic evidence of ≥50% diameter stenosis of LMCA Exclusion criteria -Contraindication to antiplatelet or anticoagulation therapy -LVEF<40% |
IVUS Group Preintervention (56) and postintervention (77) IVUS IVUS criteria for optimal stenting Complete stent tovessel wall apposition; lumen CSA≥90% of distal reference lumen CSA; full lesion coverage QCA: analyzed by 2 independent angiographers using on-line QCA system. Angiographic stenosis defined as diameter stenosis>50% @follow-up Directional atherectomy performed before stenting in 40 lesions. All pts received aspirin + coumadinor aspirin + ticlopidine At least 48 hours before stenting |
For entire cohort: MACE free survival 86.9% @ 1year & 2 years. Survival rate 98.1% @ 1 year &97% @ 2 year |
Stenting of unprotected LMCA stenosis might be associated with favourable long-term outcome in selected patients. Guidance with IVUS may optimize the immediate results & debulking before stenting seems to be effective in reducing the restenosis rate. Large-scale RCT needed. |
|||||||||||||||||||||||||||||||||||||||||
| Agostoni 2005 (33) | Assess short & midterm clinical impact of IVUS guidance in elective percutaneo ustreatment of unprotected left main coronary artery disease with drug-eluting stents |
Major adverse cardiac events defined as cardiac or non-cardiac death, non-fatal MI, & target vessel revascularization | Non-randomized cohort | Clinical Median 433 days(range 178–780 days) | Elective patients with symptomatic coronary artery disease & >50% occlusion of left main coronary artery. IVUS n = 24 No IVUS n = 34 |
Vessels measured Q baseline & after procedure with quantitative coronary angiography Unprotected left main coronary artery stented with drug-eluting stent (s) under guidance of coronary angiography or additional IVUS at the discretion of the operator. External elastic membrane areas & lumen cross-sectional area measured with computerized planimetry. Criteria for optimal stent placement: Complete stent-to-stent wall apposition, adequate stent expansion (>80% reference cross-sectional area), full lesion coverage. |
Incidence of MACE IVUS 8% No IVUS 20% (P = .18) Univariate analysis: Distal left main involvement & reference vessel diameter were the only significant predictors of MACE. Multivariate analysis: Distal left main disease was theonly significant predictor of MACE (Hazard ratio 7.7, 95% CI 1–62.6, P =.05). |
IVUS was not associated with additional clinical benefit with respect to angiographic-assisted stent deployment Major Limitation: Small sample Non-randomized No angiographic follow-up |
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Intravascular ultrasound to guide percutaneous coronary interventions: an evidence-based analysis. Ontario Health Technology Assessment Series 2006;6(12).
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.qov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit www.health.qov.on.ca/enqlish/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-4324-1 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practicing medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.qov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
Abbreviations
- BMS
Bare metal stent
- CABG
Coronary artery bypass graft
- CAD
Coronary artery disease
- CFR
Coronary flow reserve
- CSA
Cross sectional area
- DES
Drug-eluting stent
- ICUS
Intracoronary ultrasound
- IVUS
Intravascular ultrasound
- MACE
Major adverse cardiac events
- MI
Myocardial infarction
- MLA
Minimal lumen area
- MLD
Minimal lumen diameter
- PCI
Percutaneous coronary intervention
- PTCA
Percutaneous transluminal coronary angioplasty
- QALY
Quality adjusted life year
- QCA
Quantitative coronary angiography
- QOL
Quality of life
- TLR
Target lesion revascularization
- TVR
Target vessel revascularization
References
- 1.Manuel DG, Leung M, Nguyen K, Tanuseputro P, Johansen H. Burden of cardiovascular disease in Canada. Can J Cardiol. 2003;19(9):997–1004. [PubMed] [Google Scholar]
- 2.Filate WA, Johansen HL, Kennedy CC, Tu JV. Regional variations in cardiovascular mortality in Canada. Can J Cardiol. 2003;19(11):1241–1248. [PubMed] [Google Scholar]
- 3.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. [Benestent Study Group]. N Engl J Med. 1994;331(8):489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 4.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med. 1994;331(8):496–501. doi: 10.1056/NEJM199408253310802. Stent Restenosis Study Investigators. [DOI] [PubMed] [Google Scholar]
- 5.Bowen J, Hopkins R, He Y, Blackhouse G, Lazzam C, Tu JV, et al. Systematic review and cost-effectiveness analysis of drug eluting stents compared to bare metal stents for percutaneous coronary interventions in Ontario. Interim Report for the Ontario Ministry of Health and Long-Term Care [report on the Internet] [Report No.: HTA002-0512. Contract No.: 06129. December 2005]. Hamilton, Ontario: Program for Assessment of Technology in Health; [[cited 2006 Mar. 15]]. Available at: http://www.path-hta.ca/DESreport.pdf . [Google Scholar]
- 6.Nakamura S, Colombo A, Gaglione A, Almagor Y, Goldberg SL, Maiello L, et al. Intracoronary ultrasound observations during stent implantation. Circulation. 1994;89(5):2026–2034. doi: 10.1161/01.cir.89.5.2026. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald PJ, Oshima A, Hayase M, Metz JA, Bailey SR, Baim DS, et al. Final results of the Can Routine Ultrasound Influence Stent Expansion (CRUISE) study. Circulation. 2000;102(5):523–530. doi: 10.1161/01.cir.102.5.523. [DOI] [PubMed] [Google Scholar]
- 8.Kuntz RE, Safian RD, Carrozza JP, Fishman RF, Mansour M, Baim DS. The importance of acute luminal diameter in determining restenosis after coronary atherectomy or stenting. Circulation. 1992;86(6):1827–1835. doi: 10.1161/01.cir.86.6.1827. [DOI] [PubMed] [Google Scholar]
- 9.Uren NG, Schwarzacher SP, Metz JA, Lee DP, Honda Y, Yeung AC, et al. Predictors and outcomes of stent thrombosis: an intravascular ultrasound registry. Eur Heart J. 2002;23(2):124–132. doi: 10.1053/euhj.2001.2707. [DOI] [PubMed] [Google Scholar]
- 10.Cheneau E, Pichard AD, Satler LF, Suddath WO, Weissman NJ, Waksman R. Intravascular ultrasound stent area of sirolimus-eluting stents and its impact on late outcome. Am J Cardiol. 2005;95(10):1240–1242. doi: 10.1016/j.amjcard.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg SL, Colombo A, Nakamura S, Almagor Y, Maiello L, Tobis JM. Benefit of intracoronary ultrasound in the deployment of Palmaz-Schatz stents. J Am Coll Cardiol. 1994;24(4):996–1003. doi: 10.1016/0735-1097(94)90861-3. [DOI] [PubMed] [Google Scholar]
- 12.Russo RJ. Ultrasound-guided stent placement. Cardiol Clin. 1997;15(1):49–61. doi: 10.1016/s0733-8651(05)70318-0. [DOI] [PubMed] [Google Scholar]
- 13.Laskey W, Boyle J, Johnson LW. Multivariable model for prediction of risk of significant complication during diagnostic cardiac catheterization. [The Registry Committee of the Society for Cardiac Angiography & Interventions]. Cathet Cardiovasc Diagn. 1993;30(3):185–190. doi: 10.1002/ccd.1810300302. [DOI] [PubMed] [Google Scholar]
- 14.Mudra H, Blasini R, Regar E, Klauss V, Rieber J, Theisen K. Intravascular ultrasound assessment of the balloon-expandable Palmaz-Schatz coronary stent. Coron Artery Dis. 1993;4(9):791–799. doi: 10.1097/00019501-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Nissen SE, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation. 2001;103(4):604–616. doi: 10.1161/01.cir.103.4.604. [DOI] [PubMed] [Google Scholar]
- 16.Choi JW, Goodreau LM, Davidson CJ. Resource utilization and clinical outcomes of coronary stenting: a comparison of intravascular ultrasound and angiographical guided stent implantation. Am Heart J. 2001;142(1):112–118. doi: 10.1067/mhj.2001.115793. [DOI] [PubMed] [Google Scholar]
- 17.Medical Services Advisory Committee. Intravascular ultrasound [report on the Internet]. MS AC Application 1032. Copyright: University of York. Canberra, Australia: Commonwealth of Australia; Dec, 2001. [[cited 2006 Mar. 3]]. Database of Abstracts of Reviews of Effectiveness. Produced by the NHS Centre for Reviews and Dissemination, University of York. Available at: http://www.health.gov.au/internet/msac/publishing.nsf/Content/1032-l/$FILE/msacl032.pdf . [Google Scholar]
- 18.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry E, Kelley S, Hutton J, Lindsay HSJ, Blaxhill JM, Evans JA, et al. Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review with decision analytical modelling, of outcomes and cost-effectiveness [report on the Internet] Health Technology Assessment 4[35] Nov, 2000. [[cited 2006 Apr. 15]]. Available at: http://www.hta.nhsweb.nhs.uk/fullmono/mon435.pdf . [PubMed]
- 20.Casella G, Klauss V, Ottani F, Siebert U, Sangiorgio P, Bracchetti D. Impact of intravascular ultrasound-guided stenting on long-term clinical outcome: a meta-analysis of available studies comparing intravascular ultrasound-guided and angiographically guided stenting. Catheter Cardiovasc Interv. 2003;59(3):314–321. doi: 10.1002/ccd.10537. [DOI] [PubMed] [Google Scholar]
- 21.Mudra H, Di Mario C, de Jaegere P, Figulla HR, Macaya C, Zahn R, et al. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS Study) Circulation. 2001;104(12):1343–1349. doi: 10.1161/hc3701.096064. [DOI] [PubMed] [Google Scholar]
- 22.Schiele F, Meneveau N, Vuillemenot A, Zhang DD, Gupta S, Mercier M, et al. Impact of intravascular ultrasound guidance in stent deployment on 6-month restenosis rate: a multicenter, randomized study comparing two strategies—with and without intravascular ultrasound guidance. [RESIST Study Group. REStenosis after Ivus guided STenting]. J Am Coll Cardiol. 1998;32(2):320–328. doi: 10.1016/s0735-1097(98)00249-6. [DOI] [PubMed] [Google Scholar]
- 23.Schiele F, Meneveau N, Seronde MF, Caulfield F, Pisa B, Arveux P, et al. Medical costs of intravascular ultrasound optimization of stent deployment. [Results of the multicenter randomized ’REStenosis after Intravascular ultrasound STenting’ (RESIST) study]. Int J Cardiovasc Intervent. 2000;3(4):207–213. doi: 10.1080/14628840050515957. [DOI] [PubMed] [Google Scholar]
- 24.Mueller C, Hodgson JM, Schindler C, Perruchoud AP, Roskamm H, Buettner HJ. Cost-effectiveness of intracoronary ultrasound for percutaneous coronary interventions. Am J Cardiol. 2003;91(2):143–147. doi: 10.1016/s0002-9149(02)03099-0. [DOI] [PubMed] [Google Scholar]
- 25.Frey AW, Hodgson JM, Muller C, Bestehorn HP, Roskamm H. Ultrasound-guided strategy for provisional stenting with focal balloon combination catheter: results from the randomized Strategy for Intracoronary Ultrasound-guided PTCA and Stenting (SIPS) trial. Circulation. 2000;102(20):2497–2502. doi: 10.1161/01.cir.102.20.2497. [DOI] [PubMed] [Google Scholar]
- 26.Oemrawsingh PV, Mintz GS, Schalij MJ, Zwinderman AH, Jukema JW, van der Wall EE, et al. Intravascular ultrasound guidance improves angiographic and clinical outcome of stent implantation for long coronary artery stenoses: final results of a randomized comparison with angiographic guidance (TULIP Study) Circulation. 2003;107(1):62–67. doi: 10.1161/01.cir.0000043240.87526.3f. [DOI] [PubMed] [Google Scholar]
- 27.Russo RJ. Angiography versus intravascular ultrasound-directed stent placement (AVID) [abstract] Circulation. 2000;101:e9017. doi: 10.1161/CIRCINTERVENTIONS.108.778647. [DOI] [PubMed] [Google Scholar]
- 28.Gaster AL, Slothuus U, Larsen J, Thayssen P, Haghfelt T. Cost-effectiveness analysis of intravascular ultrasound guided percutaneous coronary intervention versus conventional percutaneous coronary intervention. Scand Cardiovasc J. 2001;35(2):80–85. doi: 10.1080/140174301750164673. [DOI] [PubMed] [Google Scholar]
- 29.Gaster AL, Slothuus SU, Larsen J, Korsholm L, von Birgelen C, Jensen S, et al. Continued improvement of clinical outcome and cost effectiveness following intravascular ultrasound guided PCI: insights from a prospective, randomised study. Heart. 2003;89(9):1043–1049. doi: 10.1136/heart.89.9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller C, Mc Hodgson JB, Brutsche M, Perruchoud AP, Marsch S, Hunziker P, et al. Impact of intracoronary ultrasound guidance on long-term outcome of percutaneous coronary interventions in diabetics-insights from the randomized SIPS trial. Swiss Med Wkly. 2002;132(21-22):279–284. doi: 10.4414/smw.2002.09940. [DOI] [PubMed] [Google Scholar]
- 31.Russo RJ, Nicosia A, Teirstein PS. Angiography versus intravascular ultrasound-directed stent placement (AVID)(Abstract) Journal of the American College of Cardiology. 1997;29(Supplement I):60A. [Google Scholar]
- 32.Park SJ, Hong MK, Lee CW, Kim JJ, Song JK, Kang DH, et al. Elective stenting of unprotected left main coronary artery stenosis: effect of debulking before stenting and intravascular ultrasound guidance. J Am Coll Cardiol. 2001;38(4):1054–1060. doi: 10.1016/s0735-1097(01)01491-7. [DOI] [PubMed] [Google Scholar]
- 33.Agostoni P, Valgimigli M, Van Mieghem CA, Rodriguez-Granillo GA, Aoki J, Ong AT, et al. Comparison of early outcome of percutaneous coronary intervention for unprotected left main coronary artery disease in the drug-eluting stent era with versus without intravascular ultrasonic guidance. Am J Cardiol. 2005;95(5):644–647. doi: 10.1016/j.amjcard.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 34.Guedes A, Keller PF, LAllier PL, Lesperance J, Gregoire J, Tardif JC. Long-term safety of intravascular ultrasound in nontransplant, nonintervened, atherosclerotic coronary arteries. J Am Coll Cardiol. 2005;45(4):559–564. doi: 10.1016/j.jacc.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 35.O’Keefe JH Jr, Hartzler GO, Rutherford BD, McConahay DR, Johnson WL, Giorgi LV, et al. Left main coronary angioplasty: early and late results of 127 acute and elective procedures. Am J Cardiol. 1989;64(3):144–147. doi: 10.1016/0002-9149(89)90447-5. [DOI] [PubMed] [Google Scholar]
- 36.Ellis SG, Tamai H, Nobuyoshi M, Kosuga K, Colombo A, Holmes DR, et al. Contemporary percutaneous treatment of unprotected left main coronary stenoses: initial results from a multicenter registry analysis 1994-1996. Circulation. 1997;96(11):3867–3872. doi: 10.1161/01.cir.96.11.3867. [DOI] [PubMed] [Google Scholar]
- 37.The Thrombolysis in Myocardial Infarction (TIMI) trial. [Phase I findings TIMI Study Group]. N Engl J Med. 1985;312(14):932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 38.Sousa JE, Costa MA, Abizaid A, Abizaid AS, Feres F, Pinto IM, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103(2):192–195. doi: 10.1161/01.cir.103.2.192. [DOI] [PubMed] [Google Scholar]
- 39.Serruys PW, Degertekin M, Tanabe K, Abizaid A, Sousa JE, Colombo A, et al. Intravascular ultrasound findings in the multicenter, randomized, double-blind RAVEL (RAndomized study with the sirolimus-eluting VElocity balloon-expandable stent in the treatment of patients with de novo native coronary artery Lesions) trial. Circulation. 2002;106(7):798–803. doi: 10.1161/01.cir.0000025585.63486.59. [DOI] [PubMed] [Google Scholar]
- 40.Ako J, Moreno R, Honda Y. Late incomplete stent apposition following sirolimus-eluting stent: serial quantitative intravascular ultrasound analysis from the SIRIUS trial [abstract] J Am Coll Cardiol. 2006;41(33A Abstract):805–4. [Google Scholar]
- 41.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 42.Tanabe K, Serruys PW, Degertekin M, Grube E, Guagliumi G, Urbaszek W, et al. Incomplete stent apposition after implantation of paclitaxel-eluting stents or bare metal stents: insights from the randomized TAXUS II trial. Circulation. 2005;111(7):900–905. doi: 10.1161/01.CIR.0000155607.54922.16. [DOI] [PubMed] [Google Scholar]
- 43.Tanabe K, Serruys PW, Grube E, Smits PC, Selbach G, van der Giessen WJ, et al. TAXUS III Trial: in-stent restenosis treated with stent-based delivery of paclitaxel incorporated in a slow-release polymer formulation. Circulation. 2003;107(4):559–564. doi: 10.1161/01.cir.0000048184.96491.8a. [DOI] [PubMed] [Google Scholar]
- 44.Moreno R, Fernandez C, Hernandez R, Alfonso F, Angiolillo DJ, Sabate M, et al. Drug-eluting stent thrombosis: results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. 2005;45(6):954–959. doi: 10.1016/j.jacc.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 45.Bavry AA, Kumbhani DJ, Helton TJ, Bhatt DL. What is the risk of stent thrombosis associated with the use of paclitaxel-eluting stents for percutaneous coronary intervention?: a meta-analysis. J Am Coll Cardiol. 2005;45(6):941–946. doi: 10.1016/j.jacc.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 46.Ministry of Health and Long-Term Care. Ontario Case Costing Initiative (OCCI) [Web page]. 2006. [[cited 2006 July 1]]. Available at: http://www.occp.com/
- 47.Ministry of Health and Long-Term Care. Schedule of benefits for physician services under the Health Insurance Act [Web page]. 2006. [[cited 2006 July 1]]. Available at: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/physservmn.html .
- 48.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban HE, Perin M, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 49.Sousa JE, Costa MA, Abizaid A, Sousa AG, Feres F, Mattos LA, et al. Sirolimus-eluting stent for the treatment of in-stent restenosis: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2003;107(1):24–27. doi: 10.1161/01.cir.0000047063.22006.41. [DOI] [PubMed] [Google Scholar]
- 50.Grube E, Silber S, Hauptmann KE, Mueller R, Buellesfeld L, Gerckens U, et al. TAXUS I: six-and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107(1):38–42. doi: 10.1161/01.cir.0000047700.58683.a1. [DOI] [PubMed] [Google Scholar]
- 51.Colombo A, Drzewiecki J, Banning A, Grube E, Hauptmann K, Silber S, et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108(7):788–794. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]


