Abstract
The paucity of cell culture models for childhood brain tumors prompted us to establish pediatric cell lines for use in biological experiments and preclinical developmental therapeutic studies. Three cell lines were established, CHLA-200 (GBM), CHLA-259 (anaplastic medulloblastoma) and CHLA-266 (atypical teratoid rhabdoid tumor, AT/RT). Consistent with an AT/RT origin, CHLA-266 lacked INI1 expression and had monosomy 22. All lines had unique DNA short tandem repeat “fingerprints” matching that of the patient’s tumor tissue and were adherent on tissue culture plastic, but differed in morphology and doubling times. CHLA-200 had a silent mutation in TP53. CHLA-259 and CHLA-266 had wild-type TP53. All three lines were relatively resistant to multiple drugs when compared to the DAOY medulloblastoma cell line, using the DIMSCAN fluorescence digital image microscopy cytotoxicity assay. RNA expression of MYC and MYCN were quantified using RT-PCR (Taqman). CHLA-200 expressed MYC, DAOY and CHLA-259 expressed MYCN, and CHLA-266 expressed both MYCN and MYC. CHLA-200 was only tumorigenic subcutaneously, but CHLA-259 and CHLA-266 were tumorigenic both subcutaneously and in brains of NOD/SCID mice. Immunohistochemistry of the xenografts revealed GFAP staining in CHLA-200 and PGP 9.5 staining in CHLA-259 and CHLA-266 tumors. As expected, INI1 expression was lacking in CHLA-266 (AT/RT). These three new cell lines will provide useful models for research of pediatric brain tumors.
Keywords: Pediatric anaplastic astrocytoma, AT/RT, Medulloblastoma, Cell lines, Multi-drug resistance, Xenograft
Introduction
Tumors of the central nervous system (CNS) comprise 22% of all malignancies occurring in children up to 14 years of age and 10% of tumors occurring among 15–19-year-olds, making them the most common solid tumor in children, and second in incidence only to leukemias [1]. With the improved treatment of leukemias, brain tumors are now the leading cause of death from childhood cancer. Among pediatric brain tumors, astrocytomas and medulloblastomas/embryonal tumors are the most common, accounting for 52 and 21%, respectively [2, 3]. Atypical teratoid/rhabdoid tumor (AT/RT), first described in 1996, is a highly malignant and increasingly-recognized CNS tumor that primarily occurs in very young children and typically has biallelic deletion and/or mutations in the INI1/hSNF5 gene [4, 5].
Advances in neurosurgery, radiation therapy, and chemotherapy are responsible for a considerable improvement in long-term survival of children with brain tumors, but a significant proportion of patients continue to die of their disease [6]. One reason may be an intrinsic or acquired resistance to radiation and chemotherapy, i.e. that many brain tumors could intrinsically manifest a multidrug resistance (MDR) phenotype [7]. Tumor cell lines derived from CNS tumors are essential tools for studying the biology of the disease, for understanding mechanisms of resistance to therapy, and for carrying out preclinical therapeutic testing. However, to date only few continuous pediatric brain tumor cell lines have been established. Here we report the characterization of three new pediatric brain tumor lines: CHLA-200 (GBM), CHLA-259 (medulloblastoma) and CHLA-266 (atypical teratoid/rhabdoid tumor).
Materials and methods
Brain tumor tissue and cell line establishment
Between year 1996 and 2004, 161 clinical samples were received. Five cell lines were successfully established, and of them three grew well, and are described here. Tumors were obtained at surgery except one that was obtained at autopsy approximately 2 h after death (Table 1). Informed consent was obtained according to institutionally-approved protocols. Tissues were minced (cross scalpels) in a petri dish and cultured in 12.5 cm2 filter-top cell culture flasks in lscoves DMEM medium supplemented with 20% fetal bovine serum, 4 mM l-glutamine and 0.1% ITS stock solution (ITS = insulin, selenium and transferrin, Mediatech, VA). Gentamicin (50 µg/ml) was used for the initial few weeks of culture and then withdrawn to facilitate detection of mycoplasma. Cells were detached from the flask by using Puck’s Saline with 1 mM EDTA and routinely cultured in a humidified incubator at 37°C in 5% carbon dioxide + 95% air. The three cell lines generated have been cryopreserved at low passages and propagated for at least 50 serial passages. Although the cell lines were established and grown routinely in serum containing medium as described above, the cell lines could also be grown as spheroids in serum-free Neurobasal®-A Medium + B-27 and N-2 supplements (Invitrogen, Carlsbad, CA), supplemented also with epidermal and fibroblast growth factors (10 ng/ml final concentration), (Promega, Madison, WI), and 2 mM l-glutamine (Mediatech, Inc, Manassas, VA).
Table 1.
Clinical features of the three malignant brain tumors used to establish the tumor cell lines
| Cell line | Diagnosis | Age (years) | Phase of therapy | Location |
|---|---|---|---|---|
| CHLA-200 | GBM | 12 | Autopsy (s/p multiple recurrences treated with chemotherapy and radiation) | Parietal lobe |
| CHLA-259 | Anaplastic medulloblastoma | 14 | At diagnosis (before chemotherapy) | Posterior fossa (4th ventricle), no metastases |
| CHLA-266 | Atypical teratoid/rhabdoid tumor | 1.5 | At diagnosis (before chemotherapy) | Posterior fossa with supratentorial metastases |
Cell growth
Cells (105 cells) were plated in triplicate in 25 cm2 plastic tissue culture flasks. Cells were counted every 24 or 48 h after the initiation of cell culture. Cell population doubling time was determined using the following equation: doubling time (hours) = 0.693 (t−t0)/In (Nt−N0), where t0 is the time when exponential growth began, t is time in hours, Nt is cell number at time t, and N0 is cell number at t0.
Drugs and chemicals
Cisplatin (CDDP), fenretinide (4-HPR), melphalan (L-PAM), topotecan (TPT), and vincristine (VINC) were obtained from the Developmental Therapeutics Program, National Cancer Institute (NCI) (Bethesda, MD). 4-hydroperoxycyclophosphamide (4-HC) was kindly provided by Susan Ludeman at Duke University, North Carolina. Etoposide (ETOP) was obtained from Bristol–Myers Squibb Co. (Princeton, NJ). Fluorescein diacetate (FDA) was purchased from Eastman Kodak Company (Rochester, NY) and eosin Y from Sigma Chemical Co. (St. Louis, MO).
Genotyping of cell lines
We confirmed cell line identity by comparing each cell line to the original tumor tissue using short tandem repeat (STR) genotyping [8]. We used the AmpFlSTR Identifiler PCR Amplification kit (Applied Biosystems, Foster City, CA) following the manufacturer’s recommendations to amplify genomic DNA. The product was separated on a 3100-Avant Genetic Analyzer (ABI) and the results were analyzed using GeneMapper ID v. 3.2. software (ABI). Loci examined were 15 STR loci: D8S1179, D21S11, D7S820, CSF1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TPOX, D18S51, D5S818, and FGA, and the gender-specific locus amelogenin (http://www.cogcell.org/clid.shtml).
TP53 sequencing
Exons 5–10 of TP53 in the three brain tumor cell lines were sequenced at University of Southern California Norris Comprehensive Cancer Center using the dye-based BigDye V3.1TM method, where 3′-fluorescent-labeled dideoxynucleotides (dye terminators) are incorporated into DNA extension products (cycle sequencing) [9]. DNA sequencing was performed on ABI 3730 DNA Analyzer (Applied Biosystems, Forster City, CA). Primers were synthesized at Integrated DNA Technologies (Coralville, IA). For primer sequences see data archive at www.COGcell.org.
Western blot analysis
After incubation with or without L-PAM for 16 h, cells were detached, collected, lysed in RIPA lysis buffer (Upstate, Lake Placid, NY) containing 4 µl/ml aprotinin, 15 µl/ml PMSF and 40 µl Protease Inhibitor Cocktail (Sigma, St. Louis, MO), sonicated briefly, and centrifuged 10 min at 12,000×g. Cell lysates (20 µg/lane for p53 and MDM2 blots and 40 µg/lane for p21 blots) were resolved on a 4–20% continuous gradient Tris–glycine gel, transferred to nitrocellulose membrane (Whatman, Brentford, UK), and incubated with primary antibodies: Mouse-anti-human-p53 (BD Biosciences, CA), dilution 1:1,000; rabbit-anti-human-MDM2 (Santa Cruz, CA), dilution 1:500; goat-anti-human-p21 (Santa Cruz, CA) dilution 1:200) and HRP-labeled secondary antibodies (Santa Cruz, CA). Immunoblots were visualized by ECL chemiluminescence (Pierce Biotechnology, Rockford, IL). Densitometry analysis of western blots was performed using ImageJ version 1.63 (NIH, MD) and calculated using β-actin as a reference.
Cytotoxicity assay
Cytotoxicity was determined using the DIMSCAN assay system, which has a dynamic range of four logs of cell kill [10, 11]. The range of drug concentrations we used approximated clinically-achievable plasma levels, but may over-model the limited levels achievable in the CNS [9, 12, 13]. CHLA-200 and DAOY (fast growing) were plated at 3,000 cells/well into 96-well plates; CHLA-266 cells (intermediate) were plated at 6,000 cells/well, and CHLA-259 cells (slow growing) were plated 8,000 cells/well. All cell lines were seeded in 150 µl of complete medium per well. Cells were cultured in atmospheric oxygen (21% O2) + 5% CO2 and were allowed to attach 1 day before addition of cytotoxic drugs in complete medium in replicates of 12 wells/condition. To measure cytotoxicity, FDA and eosin-Y (the latter to quench background fluorescence) were added to the 96-well plate (final concentration, 10 µg/ml) and incubated for 30 min. Total fluorescence per well (after digital thresholding to further eliminate background fluorescence) was then measured using a DIMSCAN system and results were expressed as the survival fraction of treated cells compared with control cells. The concentration of drug that was cytotoxic and/or growth inhibitory for 90% of cells (IC90) was calculated using the software “Calcusyn” (Biosoft, Cambridge, UK).
Quantitative real-time reverse transcriptase PCR
Primers and probes were designed using Primer Express (version 1.5, PE Biosystems) software. For primer sequences see data archive at www.COGcell.org. Total RNA was extracted from the cells using TRIzol (Invitrogen, Carlsbad, CA). All quantitative real time RT-PCR reactions were performed by Abl 7700 Sequence Detection System (Perkin-Elmer Applied Biosystems, Forster City, CA). Every reaction included Master mix reagents (Taq-Man one step RT-PCR Master Mix Reagents Kit, Applied Biosystems, Forster City, CA), 200 nM sense primer, 200 nM antisense primer, 100 nM TaqMan probe and 5 µl RNA sample (50 ng). TaqMan PCR data were analyzed using Sequence Detector (V1.7, Applied Biosystems, Forster City, CA). RNA isolated from a fibroblast cell line (CRL-2076, ATCC) and from the SMS-KCNR and CHLA-20 neuroblastoma cell lines were used as controls [9]. The mRNA level of each sample was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Tumor implantation
Work was performed according to a protocol approved by the Children’s Hospital Los Angeles Institutional Animal Care and Use Committee. Mice, 4–6-week-old female NOD/SCID (NOD/LtSz-scid/scid) and nu/nu were maintained under filter air barrier conditions and sterilized food and water. For intracranial injection, NOD/SCID mice were anesthetized and the head was fixed in a stereotaxic frame (Kopf Instruments, Tujunga, CA) using ear bars [14, 15]. A small hole was made into the skull 2.0 mm lateral and 0.5 mm anterior to the bregma using a 27G needle. Tumor cells (2 × 105 cells) in 2 µl IMDM without supplements were injected into the right caudate/putatmen to a depth of 3.3 mm through a 30G needle at a rate of 0.1 µl/min using a micro-injection system (Harvard Apparatus, MA). The needle was retained in place for additional 5 min and then was withdrawn slowly over 5 min. Mice were monitored daily for signs of distress and intracranial tumor size was followed by magnetic resonance imaging (MRI) at 1–3 week intervals, depending on the size of the tumor at prior MRI and the condition of the mice. Mice were sacrificed when the tumor reached a diameter of 4–5 mm or when the mice displayed signs of distress, whichever occurred earlier. For subcutaneous injection, 15 × 106 tumor cell and matrigel mix were injected s.c. into 4–6-week-old female athymic (nu/nu) mice between the shoulder blades.
Brain tumor imaging
MRI was performed using a 7 Tesla Bruker Pharmascan Instrument (Bruker, Ettlingen, Germany) using a 19 mm radiofrequency coil as previously described [16]. Fifteen to 20 min prior to scanning, mice were injected intraperitoneally with 100 µl gadopentate dimeglumine (Magnevist, Bayer HealthCare Pharmaceuticals, Wayne, NJ) for tumor enhancement. Before scanning, mice were sedated with 5% isoflurane and held in an anesthetic plane with 1.5–2% for the entirety of the scans. Scans were performed using the following parameters: TR = 3076.5 ms, TE = 36.2 ms, number of excitations = 4, field of view = 2.6 cm, and final voxel sizes of 0.100 × 0.100 × 0.670 mm slice thickness, with contiguous slices. Resultant images were visualized using the Bruker Pharmascan software and largest tumor axial dimensions were measured.
Histological and immunohistological examination
Mouse brains were removed under deep anesthesia following vascular perfusion with PBS followed by buffered 4% paraformaldehyde. Half of the brain was snap frozen in OCT and the other half fixed in 4% buffered paraformaldehyde for paraffin embedding. Sections (6 µm) were cut and stained with haematoxylin and eosin (H&E). Immunohistochemistry was performed using an automatic stainer BenchMark® (Ventana Medical Systems, AZ) according to manufacturer’s instructions. The antibodies used were as follows: synaptophysin (DAKO, Glostrup, Denmark, 1:50), PGP 9.5 (Accurate Chemical, NY, 1:400), GFAP (Ventana Medical Systems, AZ, prediluted), vimentin (Ventana Medical Systems, AZ, prediluted), INI1 (BD Biosciences, CA, 1:200).
Statistical analysis
Each experiment was done using at least three replicates or performed three times unless indicated otherwise. In vitro cytotoxicity data were analyzed and graphed using the DIMSCAN Data Analyzer Program, and Sigmaplot 2000. For qRT-PCR gene expression data, the error bars represent SD and were calculated using Sigmaplot 2000. Results with a P value <0.05 were considered significant.
Results
Cell line morphology and growth characteristics
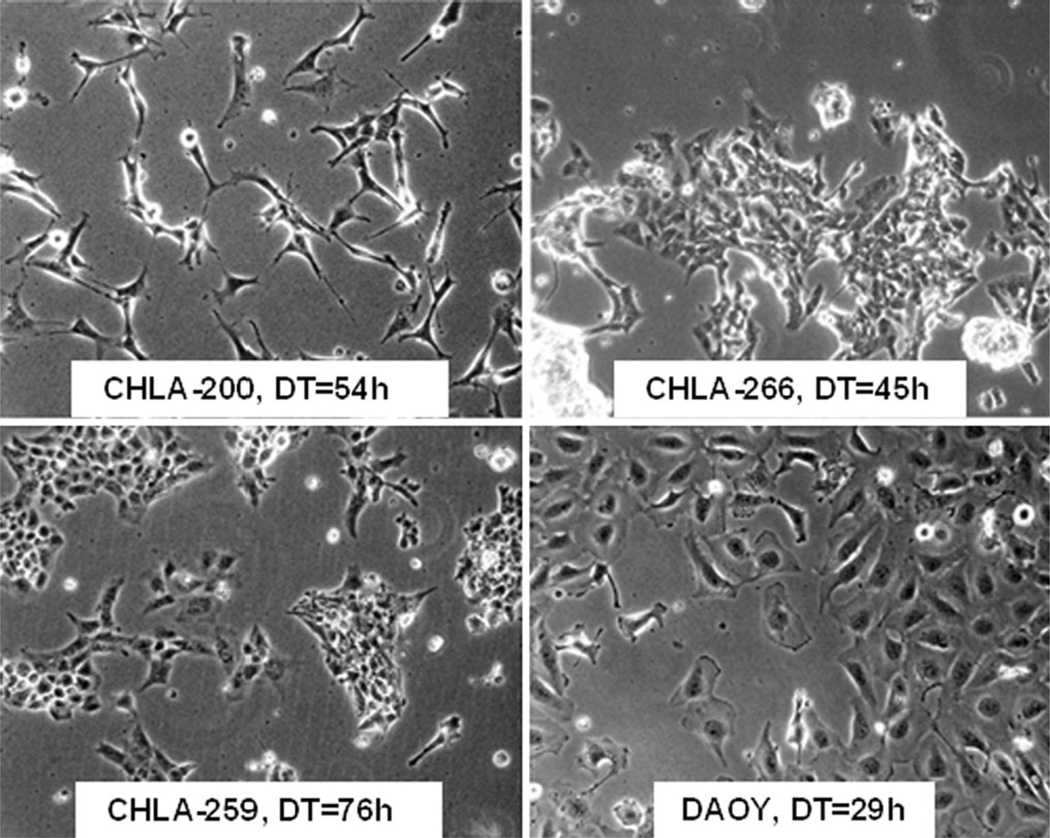
The paucity of cell culture models for childhood brain tumors prompted us to establish pediatric cell lines for use in biological and preclinical therapeutic studies. We established three pediatric brain cell lines summarized in Table 1. CHLA-200 was generated at autopsy from a recurrent glioma that at diagnosis was a frontoparietal anaplastic astrocytoma and at autopsy (2 years 2 months later) was gliomatosis cerebri consisting of GBM in the parietal lobe (where sample for the cell line was obtained), diffuse astrocytoma in the hemispheres, brainstem and cerebellum, and anaplastic ependymoma in the thalamus. CHLA-259 was an anaplastic medulloblastoma and CHLA-266 was an atypical teratoid rhabdoid tumor (AT/RT), both obtained from surgical specimens at diagnosis. Generated cell lines were examined by STR assay and matched their corresponding tumor specimens (Table 2). Figure 1 shows the morphology of the new cultured tumor cell lines. DAOY, a medulloblastoma cell line (ATCC) [17], was used for comparison. These new cell lines have been continuously cultured for more than 3 years and 50 passages and can thus be classified as permanent cell lines. In tissue culture all three lines were adherent. CHLA-200 (Fig. 1a) showed an elongated multipolar cell shape with long fibrillary processes. CHLA-259 and CHLA-266 (Fig. 1b, c) grew as a monolayer and were similar in morphology, with CHLA-259 morphology being less homogenous than CHLA-266. Doubling times of CHLA-200 (GBM), CHLA-259 (medulloblastoma), and CHLA-266 (AT/RT) were 54, 76, and 45 h, respectively (Fig. 1). As expected, in neuralbasal medium all three cell lines proliferated slower and grew as spheroids (data not shown).
Table 2.
Short tandem repeats of the new cell lines show distinct genotypes
| Locus | CHLA-200 | CHLA-259 | CHLA-266 |
|---|---|---|---|
| D8S1179 | 10, 15 | 13, 14 | 10, 14 |
| D21S11 | 29, 32.2 | 29, 30 | 29 |
| D7S820 | 8, 12 | 10, 12 | 10, 12 |
| CSF1PO | 9, 11 | 10, 11 | 10, 11 |
| D3S1358 | 14, 16 | 14, 15 | 15, 18 |
| TH01 | 6 | 6, 8 | 6, 7 |
| D13S317 | 11 | 9, 13 | 9, 10 |
| D16S539 | 12 | 11 | 10, 12 |
| D2S1338 | 18, 22 | 17, 18 | 20 |
| D19S433 | 15 | 16 | 13, 14 |
| vWA | 17 | 17, 18 | 15, 17 |
| TPOX | 8 | 8, 10 | 8, 11 |
| D18S51 | 15, 17 | 13 | 17, 19 |
| AMEL | X, Y | X, Y | X |
| D5S818 | 9, 12 | 12 | 10, 12 |
| FGA | 21, 26 | 20, 26 | 23, 24 |
Fig. 1.
Morphology of pediatric brain tumor cell lines in log phase growth. Doubling time (DT) of each cell line is shown in the panel
Drug response profile
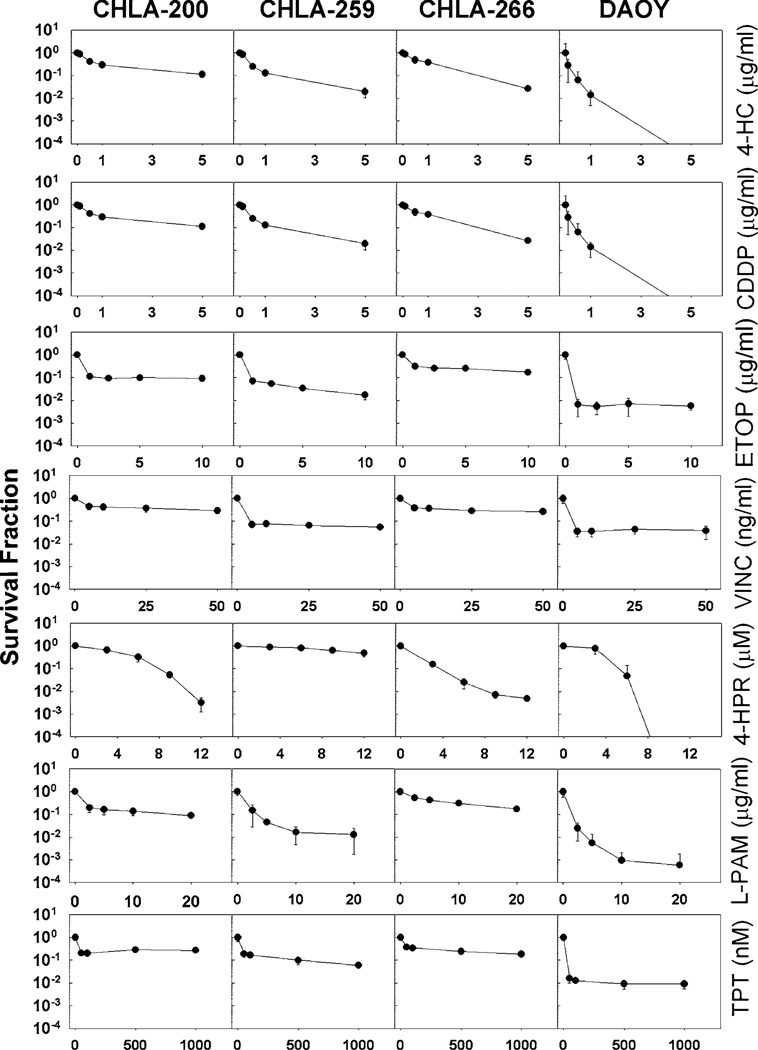
We next examined the cytotoxicity profiles of the new cell lines using several anti-neoplastic drugs that are used to treat pediatric brain tumors: CDDP, cyclophosphamide (in its active form 4-hydroperoxycyclophosphamide = 4-HC), ETOP, and VINC. For comparison we also tested TPT and L-PAM, that are often employed against neuroblastoma, a neural crest non-CNS tumor [9]. 4-HPR, an investigational synthetic retinoid, was also tested since it was reported to have preclinical activity in gliomas [18], medulloblastomas [19] as well as in rhabdoid tumor [20], a non-CNS tumor that is similar to AT/RT. Cytotoxicity assays were performed using a fluorescence microplate cytotoxicity digital image microscopy assay (DIMSCAN) [10]. CHLA-200 (GBM), which was obtained at autopsy from a heavily treated (VINC, carmustin, procarbazine, irradiation, and temozolomide), multiply-recurrent tumor was resistant to six of the seven tested drugs (Fig. 2; Table 3). CHLA-259, grown from an anaplastic medulloblastoma, which typically carries a relatively poor prognosis, was similar to DAOY in sensitivity to 4-HC, VINC and L-PAM, but more resistant to CDDP, ETOP, 4-HPR, and TPT (Fig. 2; Table 3). CHLA-266, that as an AT/RT is anticipated to be resistant to multiple cytotoxic agents, was indeed, resistant to six of the seven drugs we tested. Thus, this new panel of pediatric brain tumor cell lines may serve as useful models for studying these relatively drug-resistant childhood brain tumors.
Fig. 2.
CHLA-200, CHLA-259 and CHLA-266 show higher resistance to most chemotherapy drugs tested as compared to DAOY cells. Cytotoxicity was analyzed after 4 days exposure using the DIM-SCAN assay to cisplatin (CDDP), cyclophosphamide (as 4-hydroperoxycyclophosphamide, 4-HC), etoposide (ETOP), melphalan (L-PAM), topotecan (TPT), vincristin (VINC), and ferentinide (4-HPR). For each drug concentration n = 12
Table 3.
CHLA-200, CHLA-259, and CHLA-266 show significant drug resistance compared to DAOY
| IC90 | 4-HC (µg/ml) | CDDP (µg/ml) | ETOP (µg/ml) | VNC (ng/ml) | 4-HPR (µM) | L-PAM (µg/ml) | TPT (nM) |
|---|---|---|---|---|---|---|---|
| CHLA-200 | 2.6 ± 2.0* | 4.6 ± 0.2*** | 2.1 ± 1.0* | >50*** | 6.5 ± 0.2 | 12.2 ± 5.5* | >1,000*** |
| CHLA-259 | 1.7 ± 0.3 | 1.8 ± 0.8* | 0.6 ± 0.2* | 1.6 ± 1.4 | >12* | 1.3 ± 1 | 72.2 ± 34.2* |
| CHLA-266 | 6.9 ± 3.7* | 2.2 ± 0.5** | >10*** | >50*** | 3.5 ± 0.2 | >20** | >1,000*** |
| DAOY | 1.6 ± 0.3 | 0.3 ± 0.02 | 0.15 ± 0.03 | 2.2 ± 1.0 | 4.2 ± 1.2 | 1.7 ± 1.2 | 3.5 ± 2.0 |
Shown are IC90 values calculated from experiments performed in Fig. 2. Experiments were performed at least three times and P values were assessed compared to DAOY
4-HC Activated cyclophosphamide, CPPD cisplatin, ETOP etoposide, VINC vincristine, 4-HPR fenretinide, L-PAM melphalan, TPT topotecan P values (compared to DAOY): NS: >0.05;
P < 0.05,
P < 0.01,
P < 0.001
p53 signaling
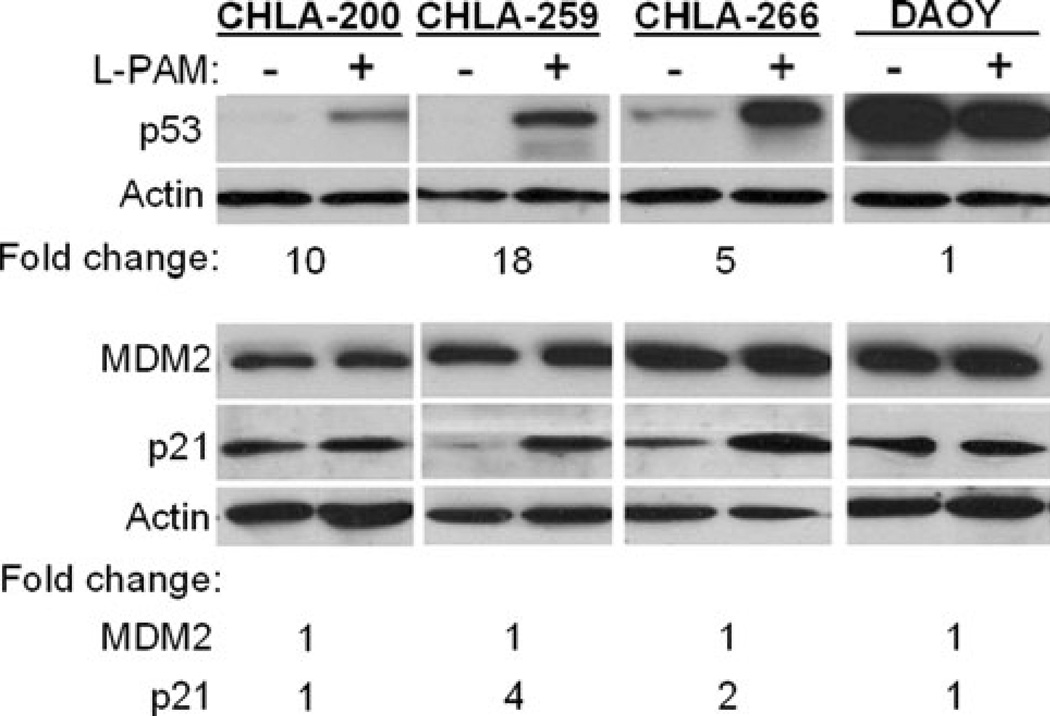
Since pathogenesis of many tumors is linked to mutations of TP53, we analyzed TP53 exons 5–10 of the new cell lines for mutations using automated fluorescence dideoxynucleotide sequencing. CHLA-200 had a common polymorphism at codon 213, which does not affect coding of the p53 protein (CGA to CGG, both translate to arginine) [21]. CHLA-259 and CHLA-266 have wild-type TP53. Our laboratory previously studied p53 functionality in neuroblastoma cells using a luciferase reporter assay and also examination of expression of p53 targets by western blot following genotoxic stress and found that results were similar in the two approaches [9]. Therefore, here we assessed the functionality of the p53 pathway by examination of changes in protein expression of p53 and its downstream targets p21 and MDM2 following 16 h exposure to the DNA damaging agent, L-PAM (10 µg/ml). For comparison, we included in our analysis the TP53-mutated cell line, DAOY (single point mutation of TP53 at base 725, resulting in substitution of phenylalanine for a cysteine at amino acid 242) [22]. As shown in Fig. 3, under baseline growth conditions CHLA-200, CHLA-259, and CHLA-266 expressed undetectable or low levels of p53, which increased 10-, 18-, and 5-fold, respectively, following exposure to L-PAM, suggesting functional signaling leading to p53. Following exposure to L-PAM, levels of p21, that normally increases in response to p53 increase and activation, did not increase in CHLA-200 (GBM), suggesting altered signaling downstream from p53 despite increased p53 protein expression. In CHLA-259 (medulloblastoma) and CHLA-266 (AT/RT) p21 did increase, suggesting functional p53-p21 axis. However, MDM2 expression (also a downstream target of p53) was not affected by L-PAM in any of the four cell lines tested, indicating an altered p53-MDM2 axis reponse. In the p53-mutated DAOY cells, levels of p53, p21, and MDM2 were unaffected by exposure to L-PAM, consistent with the report that DAOY cells lack functional p53. Thus, all three new cell lines had altered p53 signaling response despite CHLA-259 and CHLA-266 having wild-type TP53 and CHLA-200 having a polymorphism that is thought to not affect the amino acid sequence of p53. The functional significance of these findings is not known at this time.
Fig. 3.
Melphalan (L-PAM) induced variable p53 expression and signaling in CHLA-200, CHLA-259, CHLA-266, and DAOY cell lines. Cells (1 × 106) plated in six well plates were incubated with L-PAM (6 µg/ml) or vehicle in 2 ml medium. After 16 h cells were collected, cell lysates (20 µg for p53 and MDM2 and 10 µg for p21) were resolved by SDS-PAGE and detected by western blotting. Fold increase in expression in presence of drug compared to control was calculated by densitometry using actin as loading control, and is denoted below the blots. This is a representative experiment of three with similar results
Oncogene mRNA expression
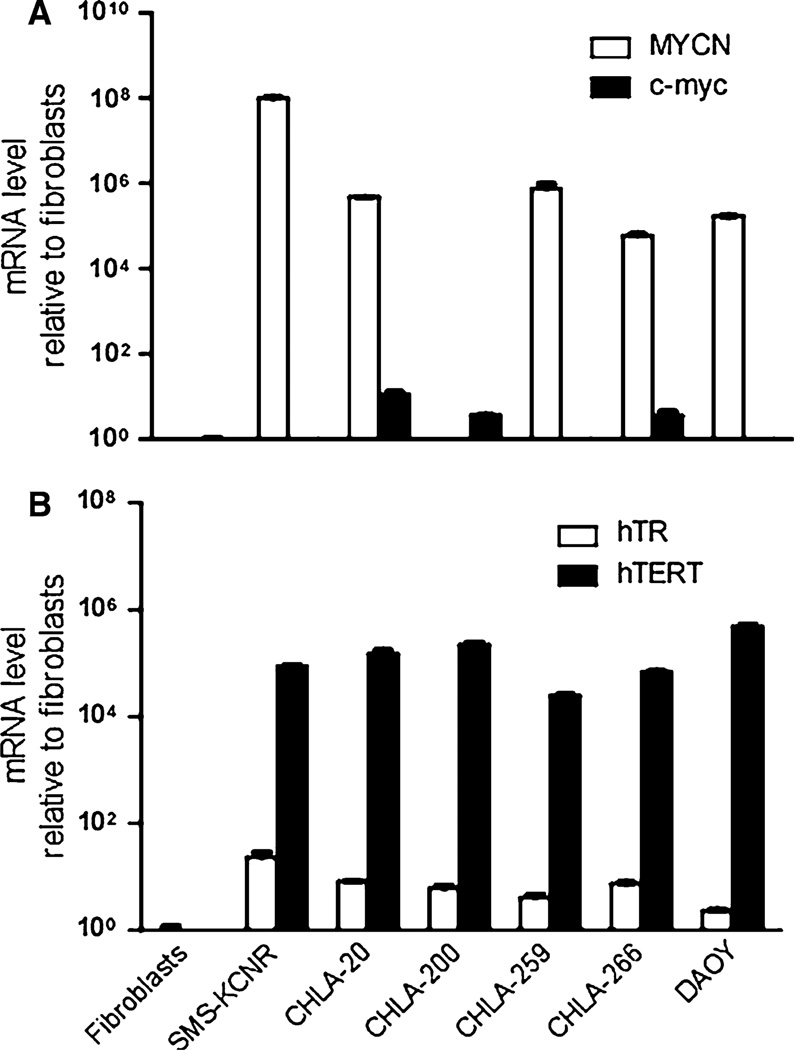
To characterize the three new cell lines we measured expression of MYC family genes by RT-PCR. Two well-characterized neuroblastoma cell lines, CHLA-20 and SMS-KCNR (both overexpress MYCN) were included for comparison, and fibroblasts were used as a reference against which expression was compared. MYC family genes MYC and MYCN have been widely studied due to their involvement in proliferation, differentiation, and potential role in tumorigenesis [23]. In neuroblastoma MYCN amplification is associated with poor prognosis [24]. In medulloblastoma MYCN and/or MYC can be amplified and highly expressed and are emerging as markers of poor prognosis [25–27]. Indeed, both medulloblastoma lines (CHLA-259 and DAOY) showed high MYCN but no increase in MYC expression. CHLA-266 (AT/RT) also had high expression of MYCN, but also had 3.6-fold ± 0.8 (SD; P = 0.0064) increase in MYC expression. CHLA-200 (GBM) on the other hand, had 3.6-fold ± 0.1 (SD; P <0.0001) increase of MYC expression compared to fibroblasts, but no increase in MYCN (Fig. 4a).
Fig. 4.
The new pediatric brain tumor cell lines express telomerase and MYC genes. Quantitative RT-PCR of the three cell lines and DAOY: a mRNA expression of MYC and MYCN oncogenes, b mRNA of telomerase: hTR and hTERT. Bars represent means ± SD of triplicate samples in one representative experiment out of three experiments with similar results
Since many malignant cell lines express telomerase and maintain telomere length through an indefinite number of cell divisions [28] to avoid senescence, we also examined telomerase expression. The core telomerase enzyme consists of an RNA component (human telomerase RNA: hTR) and a catalytic protein component (human telomerase reverse transcriptase: hTERT) [29, 30]. As expected of continuous cell lines, the three new brain tumor cell lines and DAOY, as well as the two neuroblastoma cell lines, expressed high levels of both hTR and hTERT compared to the normal fibroblasts (Fig. 4b).
Intracranial growth
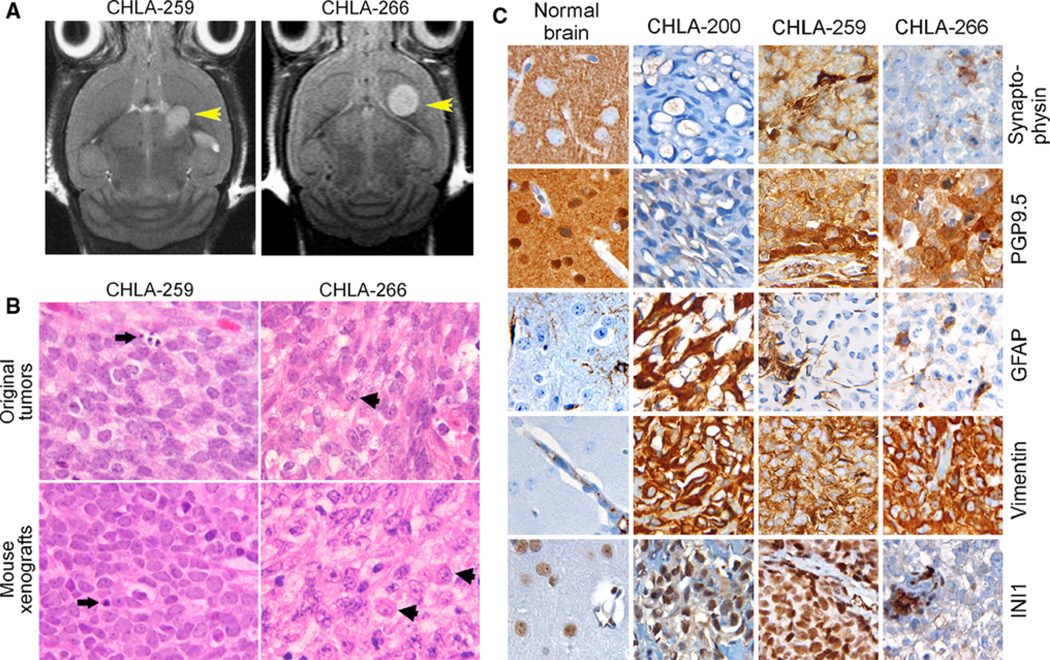
Last, the utility of tumor cell lines as pre-clinical models is greater if they can serve as in vivo tumor models in mice. To test this, we injected the three new cell lines both intracranially into the caudate/putamen nuclei of NOD/SCID mice, and subcutaneously in nu/nu mice [14, 15]. All three cell lines gave rise to subcutaneous tumors (data not shown). However, the time to detection of subcutaneous tumors of CHLA-200 was more than 4 months compared with CHLA-259 (43 days) and CHLA-266 (56 days). Importantly, CHLA-259 and CHLA-266, but not CHLA-200, also developed into tumors when injected intracranially, and were first detected by MRI on days 27 and 26, respectively (Fig. 5a). Mean time to symptoms of mice with orthotopic tumors was 58 days ± 19 for CHLA-259 (n = 8) and 45 days ± 5 for CHLA-266 (n = 6).
Fig. 5.
CHLA-259 and CHLA-266 form intracranial tumors in mice. a MRI images of tumors in mouse brains: Left panel CHLA-266, day 82, 2.1 × 2.0 mm tumor, Right panel CHLA-259, day 120, 1.9 × 2.4 mm tumor. b H&E stains of the original patient tumors (top panels) and the mouse brain tumors from orthotopic implantation of their respective cell lines (bottom). Original magnification is 4009. Arrows Apoptotic cells in CHLA-259, Arrowheads typical rhabdoid cells in CHLA-266. c Immunohistochemical staining for synaptophysin, PGP 9.5, GFAP, vimentin and INI1 of the mouse xenograft of CHLA-200 (subcutaneous), CHLA-266 and CHLA-259 (both intracranial) compared to normal brain
Morphology of the mouse intracranial CHLA-259 and CHLA-266 tumors recapitulated the histology of the original patient tumor (Fig. 5b). For CHLA-259 (medulloblastoma), both the original tumor and mouse intracranial tumors were composed of densely packed primitive cells with round-to-oval hyperchromatic nuclei surrounded by scanty cytoplasm, as typically seen in medulloblastomas, and apoptosis was frequent (arrows). For CHLA-266 (AT/RT), both the original tumor and mouse intracranial tumors contained a population of pleiomorphic neoplastic cells. Typical rhadboid cells (arrowheads) were seen which had round to lobulated eccentric nuclei, often with a prominent nucleolus, and cytoplasm that was eosinophilic or vacuolated. Cell borders were typically distinct and mitosis and apoptosis were present. Thus, in both tumors, the morphology of the mouse orthotopic tumors was similar to the morphology of the tumors resected from the patients.
Xenografts of the three new cell lines also represented the immunohistochemical characteristics of the original tumor lineage (Fig. 5c). The immunohistochemical stains of the intracranial (CHLA-259, CHLA-266) and subcutaneous (CHLA-200) tumors in the mice were as anticipated for these types of tumors: synaptophysin, an integral membrane glycoprotein in synaptic vesicles that is expressed in all neurons in the brain, was expressed diffusely in CHLA-259, but not in CHLA-266 and CHLA-200. In the normal brain synaptophysin only stained neuropil and neural outer menbranes. PGP 9.5, that is expressed in neurons and in cells of the neuroendocrine system, was diffusely expressed in both embryonal tumors, CHLA-259 and CHLA-266, but not in CHLA-200. In the normal brain PGP 9.5 stained neuropil, nucleus, and the cytoplasm of normal neurons. GFAP (glial fibrillary acidic protein), that comprises glial filaments and is a glioma marker, was extensively expressed by CHLA-200 cells, but was only found in resident astrocytic filaments in the other tumors and the normal brain. Vimentin, an intermediary filament present in mesenchymal cells, endothelial cells and glia, was abundant in all three brain tumors. In normal brain vimentin was only expressed in the vascular wall structures. The tumor suppressor gene, INI1, a member of the chromatin remodeling complex SWI/SWF, is expressed in nuclei of all cells and is typically absent from rhabdoid and AT/RT tumors. Indeed, INI1 was expressed all nuclei in the normal brain as well as in CHLA-259 and CHLA-200 tumors. However, CHLA-266 AT/RT showed no INI1 staining in the tumor cell nuclei, with cells of a capillary structure in the tumor continuing to express it and serving as an internal control (Fig. 5). The karyotype of CHLA-266 demonstrated monosomy 22, suggesting that lack of INI1 expression is due to loss of one allele of INI1 and a mutation in the other allele (data not shown).
Discussion
Low passage primary cell cultures are frequently considered superior to continuous cell lines in brain tumor research. However, their availability, especially for cancers with low incidence, can be limiting to scientific progress. Moreover, when one desires permanent long term and stable manipulation of cells, this is hard to achieve with primary cultures, and primary cultures can be stressed from adaptation to the culture environment. Therefore, to allow timely research progress it is also necessary to utilize continuously growing cell lines. This is especially true for pediatric brain tumors, which are far less frequent than adult gliomas. Here we characterized three new continuous pediatric brain tumor cell lines that we now make available to the research community.
Although medulloblastoma is the most common malignant brain tumor of childhood, a relatively small number of human medulloblastoma cell lines have been generated thus far [17, 31–34]. Existing pediatric GBM cell lines are even fewer [31, 35]. This is in marked contrast to other related primitive neuronal tumors such as neuroblastoma and Ewing’s family tumors, where many cell lines and transplantable xenograft models have been successfully established. We have established three new cell lines from primary pediatric brain tumors: CHLA-200 (GBM, from gliomatosis cerebri), CHLA-259 (anaplastic medulloblastoma), and CHLA-266 (atypical teratoid/rhabdoid tumor, AT/RT), all of which grow continuously in cell culture and are tumorigenic in vivo (CHLA-259 and CHLA-266 were tumorigenic orthotopically in the brain and all three were tumorigenic subcutaneously). The establishment of CHLA-200 from a post-mortem sample suggests that such an approach can increase the number of post-chemotherapy brain tumor cell lines available for research, although these lines may behave differently compared to those obtained at diagnosis.
Successful therapy of malignant brain tumors requires multi-modality therapy. While single drugs are only transiently and partially effective, testing cell lines against the single agents can provide the initial information about their sensitivity to such drugs. Despite this, one needs to take into account that under tissue culture conditions the cells are out of their natural microenvironment and may thus show an altered response. The drugs we compared comprise three groups: (1) drugs used in established protocols against medulloblastomas and AT/RT (CDDP, cyclophosphamide in its activated form 4-HC, ETOP, and VINC) [12, 36–39], (2) drugs frequently used in our laboratory that are in protocols to treat neuroblastoma, a neural crest non-CNS tumor (topotecan, TPT and melphalan, L-PAM) [9], and (3) an investigational synthetic retinoic, 4-HPR, that has been reported to have preclinical activity in gliomas [18], medulloblastoma [19] and rhabdoid tumors [20], but had not been reported in AT/RT. DAOY medulloblastoma cells are frequently used as a model for testing drugs and we therefore used DAOY to compared responses of the new cell lines to the drugs.
CHLA-259 (anaplastic medulloblastoma) sensitivity to 4-HC and VINC was similar to DAOY, but CHLA-259 were less sensitive to CDDP and ETOP. CHLA-200 (GBM) and CHLA-266 (AT/RT) were all less sensitive to the four drugs (4-HC, CDDP, ETOP and VINC) compared to DAOY cells (Table 3). Both CHLA-266 (AT/RT) and CHLA-259 (anaplastic medulloblastoma) were generated from tumors at diagnosis that had not been exposed to chemotherapy before, indicating that they were inherently resistant to these drugs. CHLA-200 (GBM) that was isolated from a recurrent tumor that had previously been treated with VINC, not surprisingly showed marked resistance to VINC (Table 3). However, despite never being treated with cyclophosphamide, CDDP or ETOP, CHLA-200 was also resistant to them, consistent with GBMs being resistant to multiple chemotherapies. The last drug, an investigational synthetic retinoid, 4-HPR, was tested since it had been reported to have preclinical activity in gliomas [18], medulloblastoma [19] and rhabdoid tumors [20]. In rhabdoid tumors 4-HPR decreased cyclin D1, thus inhibiting rhabdoid tumor cell line proliferation in vitro [20]. Since AT/RT is similar to rhabdoid tumors in its molecular pathogenesis (lack of INI1) and high therapy resistance, this prompted us to investigate sensitivity of the AT/RT cell line, CHLA-266, to 4-HPR. In our experiments, 4-HPR had activity at clinically-relevant concentrations against CHLA-266 (AT/RT), CHLA-200 (GBM) and DAOY (TP53-mutated medulloblastoma), but not against CHLA-259 (anaplastic medulloblastoma). This is the first indication that AT/RT (CHLA-266) and DAOY cell lines are responsive to 4-HPR.
TP53 mutations are infrequent in pediatric CNS tumors (~10%) [40, 41]. TP53 sequence was wildtype in CHLA-259 (anaplastic medulloblastoma) and CHLA-266 (AT/RT). CHLA-200 (GBM) only had a silent TP53 polymorphism at exon 6 codon 213 (CGA to CGG) that is similar to a constitutional polymorphism reported in up to 10.8% of Italians [21] and is not thought to cause deficient p53 pathway signaling. Despite the normal amino acid sequence of p53 in the three lines, each of them showed at least some degree of abnormal signaling. The expression of p53 in cancer cells with wt TP53 is usually low and increases briefly upon exposure to DNA-damaging agents [42–44]. CHLA-259 and CHLA-266 were able to increase the level of p53 and its downstream target, p21 following L-PAM challenge, which suggests that signaling upstream of p53 and from p53 to p21 was functional. However, despite this increase in p53 in response to L-PAM, we did not observe concomitant changes in expression of MDM2, suggesting altered p53 response and possible defects in the downstream pathway. Interestingly, p53 pathway functionality in CHLA-200 cells was only manifest as increase in p53 expression in response to L-PAM, but failed to increase expression of p21 or MDM2, suggesting that p53 signaling was also defective in the CHLA-200 cell line. Interestingly, despite their lack of p53, DAOY cells were relatively more sensitive to most of the drugs we tested compared to the new cell lines.
In order to achieve immortality tumors need to maintain telomere length and telomere integrity [28]. In most cases, this is achieved through reactivation of a reverse transcriptase mechanism, by which telomerase adds TTAGGG units to telomeres. Cargioli et al. [45] have established permanent cell line models via transduction of hTERT and SV40 into early passages of temporary cell lines, which demonstrated the need for telomerase activity. Similar to our cell lines other glioma and medulloblastoma tumors express high level of hTERT and hTR [32, 46]. However, to date, this is the first report of hTERT expression in AT/RT tissues or cell lines.
Several reports have described MYC mRNA expression in medulloblastoma: 18 of 59 cases (31%) and 30 of 72 cases (42%) [47, 48]. Moreover, Stearns et al. [49] reported that MYC overexpression caused anaplasia in DAOY and UW228 medulloblastoma cell lines and tumors derived from them. Neither our medulloblastoma cell line, CHLA-259, nor DAOY expressed MYC. Hayashi et al. [50] described correlation between MYC expression and grade of astrocytoma. Accordingly, CHLA-200, originating from a high-grade glioma (GBM, WHO grade IV), did express MYC. Additionally, MYC expression was mildly increased in CHLA-266 (AT/RT), similar to another reported AT/RT cell line [51].
Genomic amplification and high expression of MYCN is often observed in high-risk poor-prognosis neuroblastoma [52–54]. Amplification and high expression of MYCN also occurs in CNS embryonal tumors (medulloblastoma, PNET, and AT/RT), and is associated with worse prognosis [25–27, 55]. CHLA-259, an anaplastic medulloblastoma, showed high MYCN expression, as did the AT/RT line, CHLA-266. We found no expression of MYCN in CHLA-200 (GBM), as expected.
In conclusion, we have established three continuous pediatric brain tumor cell lines, CHLA-200 (GBM), CHLA-259 (anaplastic medulloblastoma), and CHLA-266 (AT/RT). These cell lines showed multi-drug resistance and two of them were tumorigenic intracranially in immunocompromised mice. These new cell lines will provide useful models for studies of pediatric brain tumor biology and for preclinical testing of novel drugs for possible activity against multidrug resistant pediatric brain tumors. The three cell lines are available from the Children’s Oncology Group Cell Line and Xenograft Repository (www.COGcell.org).
Acknowledgments
This work was supported by the Devin Hock Memorial Fund of the Michael Hoefflin Foundation. This work was also partially supported by a Pre-Institute Award from the Pediatric Brain Tumor Foundation of the US, by National Institutes of Health grants CA98568 and a gift from the Grayson’s Fund to AEE, and CA46274 to JAB, and by RP 110763 from the Cancer Prevention and Research Institute of Texas to CPR.
Abbreviations
- AT/RT
Atypical teratoid/rhabdoid tumor
- VINC
Vincristine
- 4-HC
4-Hydroperoxycyclophosphamide
- L-PAM
Melphalan
- CDDP
Cisplatin
- 4-HPR
Fenretinide
- TPT
Topotecan
- ETOP
Etoposide
- FBS
Fetal bovine serum
- ITS
Insulin, selenium, and transferrin
- GFAP
Glial fibrillary acidic protein
- CNS
Central Nervous System
Contributor Information
Jingying Xu, Developmental Therapeutics Program, Division of Hematology-Oncology, USC-CHLA Institute for Pediatric Clinical Research, Children’s Hospital Los Angeles, Los Angeles, CA 90027, USA; Department of Pediatrics and Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90027, USA.
Anat Erdreich-Epstein, Department of Pediatrics and Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90027, USA; Division of Hematology-Oncology, Children’s Hospital Los Angeles, Los Angeles, CA 90027, USA.
Ignacio Gonzalez-Gomez, Children’s Hospital Los Angeles, Los Angeles, CA 90027, USA; Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90027, USA.
Elizabeth Y. Melendez, Children’s Hospital Los Angeles, Los Angeles, CA 90027, USA
Goar Smbatyan, Children’s Hospital Los Angeles, Los Angeles, CA 90027, USA.
Rex A. Moats, Children’s Hospital Los Angeles, Los Angeles, CA 90027, USA Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90027, USA.
Michael Rosol, Children’s Hospital Los Angeles, Los Angeles, CA 90027, USA; Department of Radiology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90027, USA.
Jaclyn A. Biegel, Division of Human Genetics, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA
C. Patrick Reynolds, Email: patrick.reynolds@ttuhsc.edu, Developmental Therapeutics Program, Division of Hematology-Oncology, USC-CHLA Institute for Pediatric Clinical Research, Children’s Hospital Los Angeles, Los Angeles, CA 90027, USA; Department of Pediatrics and Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90027, USA; Cancer Center, School of Medicine, Texas Tech University Health Sciences Center, 3601 4th Street, Stop 9445, Lubbock, TX 79430, USA.
References
- 1.CBTRUS. Central Brain Tumor Registry of the US. 2005–2006 Statistical report: primary brain tumors in the United States, 1998–2002. 2005
- 2.Gurney JG, Smith MA, Bunin GR. CNS and miscellaneous intracranial and intraspinal neoplasms. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. Cancer incidence and survival among children and adolescents: United States SEER program, 1975–1995. Bethesda: National Cancer Institute. SEER Program, NIH; 1999. pp. 51–63. [Google Scholar]
- 3.Bredel M. Anticancer drug resistance in primary human brain tumors. Brain Res Brain Res Rev. 2001;35:161–204. doi: 10.1016/s0165-0173(01)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg. 1996;85:56–65. doi: 10.3171/jns.1996.85.1.0056. [DOI] [PubMed] [Google Scholar]
- 5.Reddy AT. Atypical teratoid/rhabdoid tumors of the central nervous system. J Neurooncol. 2005;75:309–313. doi: 10.1007/s11060-005-6762-8. [DOI] [PubMed] [Google Scholar]
- 6.Robertson PL. Advances in treatment of pediatric brain tumors. NeuroRx. 2006;3:276–291. doi: 10.1016/j.nurx.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeAngelis LM. Chemotherapy for brain tumors–a new beginning. N Engl J Med. 2005;352:1036–1038. doi: 10.1056/NEJMe058010. [DOI] [PubMed] [Google Scholar]
- 8.Collins PJ, Hennessy LK, Leibelt CS, Roby RK, Reeder DJ, Foxall PA. Developmental validation of a single-tube amplification of the 13 CODIS STR loci, D2S1338, D19S433, and amelogenin: the AmpFlSTR Identifiler PCR Amplification Kit. J Forensic Sci. 2004;49:1265–1277. [PubMed] [Google Scholar]
- 9.Keshelava N, Zuo JJ, Chen P, Waidyaratne SN, Luna MC, Gomer CJ, Triche TJ, Reynolds CP. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 2001;61:6185–6193. [PubMed] [Google Scholar]
- 10.Keshelava N, Frgala T, Krejsa J, Kalous O, Reynolds CP. DIMSCAN: a microcomputer fluorescence-based cytotoxicity assay for preclinical testing of combination chemotherapy. Methods Mol Med. 2005;110:139–153. doi: 10.1385/1-59259-869-2:139. [DOI] [PubMed] [Google Scholar]
- 11.Frgala T, Kalous O, Proffitt RT, Reynolds CP. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther. 2007;6:886–897. doi: 10.1158/1535-7163.MCT-04-0331. [DOI] [PubMed] [Google Scholar]
- 12.Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, Graf N, Emser A, Pietsch T, Wolff JE, Kortmann RD, Kuehl J. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 13.Villablanca JG, Krailo MD, Ames MM, Reid JM, Reaman GH, Reynolds CP. Phase I trial of oral fenretinide in children with high-risk solid tumors: a report from the children’s oncology group (CCG 09709) J Clin Oncol. 2006;24:3423–3430. doi: 10.1200/JCO.2005.03.9271. [DOI] [PubMed] [Google Scholar]
- 14.Yamada S, Khankaldyyan V, Bu X, Suzuki A, Gonzalez-Gomez I, Takahashi K, McComb JG, Laug WE. A method to accurately inject tumor cells into the caudate/putamen nuclei of the mouse brain. Tokai J Exp Clin Med. 2004;29:167–173. [PubMed] [Google Scholar]
- 15.Burgos JS, Rosol M, Moats RA, Khankaldyyan V, Kohn DB, Nelson MD, Jr, Laug WE. Time course of bioluminescent signal in orthotopic and heterotopic brain tumors in nude mice. Biotechniques. 2003;34:1184–1188. doi: 10.2144/03346st01. [DOI] [PubMed] [Google Scholar]
- 16.Otto-Duessel M, Khankaldyyan V, Gonzalez-Gomez I, Jensen MC, Laug WE, Rosol M. In vivo testing of Renilla luciferase substrate analogs in an orthotopic murine model of human glioblastoma. Mol Imaging. 2006;5:57–64. [PubMed] [Google Scholar]
- 17.Jacobsen PF, Jenkyn DJ, Papadimitriou JM. Establishment of a human medulloblastoma cell line and its heterotransplantation into nude mice. J Neuropathol Exp Neurol. 1985;44:472–485. doi: 10.1097/00005072-198509000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Puduvalli VK, Saito Y, Xu R, Kouraklis GP, Levin VA, Kyritsis AP. Fenretinide activates caspases and induces apoptosis in gliomas. Clin Cancer Res. 1999;5:2230–2235. [PMC free article] [PubMed] [Google Scholar]
- 19.Damodar Reddy C, Guttapalli A, Adamson PC, Vemuri MC, O’Rourke D, Sutton LN, Phillips PC. Anticancer effects of fenretinide in human medulloblastoma. Cancer Lett. 2006;231:262–269. doi: 10.1016/j.canlet.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Alarcon-Vargas D, Zhang Z, Agarwal B, Challagulla K, Mani S, Kalpana GV. Targeting cyclin D1, a downstream effector of INI1/hSNF5, in rhabdoid tumors. Oncogene. 2006;25:722–734. doi: 10.1038/sj.onc.1209112. [DOI] [PubMed] [Google Scholar]
- 21.Serra A, Gaidano GL, Revello D, Guerrasio A, Ballerini P, Dalla Favera R, Saglio G. A new TaqI polymorphism in the p53 gene. Nucleic Acids Res. 1992;20:928. doi: 10.1093/nar/20.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raffel C, Thomas GA, Tishler DM, Lassoff S, Allen JC. Absence of p53 mutations in childhood central nervous system primitive neuroectodermal tumors. Neurosurgery. 1993;33:301–305. doi: 10.1227/00006123-199308000-00018. Discussion 305–306. [DOI] [PubMed] [Google Scholar]
- 23.Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- 24.Vasudevan SA, Nuchtern JG, Shohet JM. Gene profiling of high risk neuroblastoma. World J Surg. 2005;29:317–324. doi: 10.1007/s00268-004-7820-7. [DOI] [PubMed] [Google Scholar]
- 25.Pfister S, Remke M, Benner A, Mendrzyk F, Toedt G, Felsberg J, Wittmann A, Devens F, Gerber NU, Joos S, Kulozik A, Reifenberger G, Rutkowski S, Wiestler OD, Radlwimmer B, Scheurlen W, Lichter P, Korshunov A. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol. 2009;27:1627–1636. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 26.Swartling FJ, Grimmer MR, Hackett CS, Northcott PA, Fan QW, Goldenberg DD, Lau J, Masic S, Nguyen K, Yakovenko S, Zhe XN, Gilmer HC, Collins R, Nagaoka M, Phillips JJ, Jenkins RB, Tihan T, Vandenberg SR, James CD, Tanaka K, Taylor MD, Weiss WA, Chesler L. Pleiotropic role for MYCN in medulloblastoma. Genes Dev. 2010;24:1059–1072. doi: 10.1101/gad.1907510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL, Zhao W, Nicholson SL, Taylor RE, Bailey S, Clifford SC. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 29.Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 31.Rooprai HK, Merzak A, Bullock P, Pilkington GJ. Establishment and characterization of two paediatric brain tumour cell lines in vitro. Anticancer Res. 1997;17:4127–4134. [PubMed] [Google Scholar]
- 32.Di Tomaso E, Pang JC, Lam HK, Tian XX, Suen KW, Hui AB, Hjelm NM. Establishment and characterization of a human cell line from paediatric cerebellar glioblastoma multiforme. Neuropathol Appl Neurobiol. 2000;26:22–30. doi: 10.1046/j.1365-2990.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 33.Friedman HS, Burger PC, Bigner SH, Trojanowski JQ, Wikstrand CJ, Halperin EC, Bigner DD. Establishment and characterization of the human medulloblastoma cell line and transplantable xenograft D283 Med. J Neuropathol Exp Neurol. 1985;44:592–605. doi: 10.1097/00005072-198511000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Keles GE, Berger MS, Srinivasan J, Kolstoe DD, Bobola MS, Silber JR. Establishment and characterization of four human medulloblastoma-derived cell lines. Oncol Res. 1995;7:493–503. [PubMed] [Google Scholar]
- 35.Yachnis AT, Neubauer D, Muir D. Characterization of a primary central nervous system atypical teratoid/rhabdoid tumor and derivative cell line: immunophenotype and neoplastic properties. J Neuropathol Exp Neurol. 1998;57:961–971. doi: 10.1097/00005072-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, Muraszko K, Langston J, Sposto R. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 37.Chi SN, Zimmerman MA, Yao X, Cohen KJ, Burger P, Biegel JA, Rorke-Adams LB, Fisher MJ, Janss A, Mazewski C, Goldman S, Manley PE, Bowers DC, Bendel A, Rubin J, Turner CD, Marcus KJ, Goumnerova L, Ullrich NJ, Kieran MW. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27:385–389. doi: 10.1200/JCO.2008.18.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner SL, Asgharzadeh S, Green A, Horn B, McCowage G, Finlay J. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008;51:235–240. doi: 10.1002/pbc.21578. [DOI] [PubMed] [Google Scholar]
- 39.Dhall G, Grodman H, Ji L, Sands S, Gardner S, Dunkel IJ, McCowage GB, Diez B, Allen JC, Gopalan A, Cornelius AS, Termuhlen A, Abromowitch M, Sposto R, Finlay JL. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50:1169–1175. doi: 10.1002/pbc.21525. [DOI] [PubMed] [Google Scholar]
- 40.Orellana C, Hernandez-Marti M, Martinez F, Castel V, Millan JM, Alvarez-Garijo JA, Prieto F, Badia L. Pediatric brain tumors: loss of heterozygosity at 17p and TP53 gene mutations. Cancer Genet Cytogenet. 1998;102:93–99. doi: 10.1016/s0165-4608(97)00343-9. [DOI] [PubMed] [Google Scholar]
- 41.Pollack IF, Finkelstein SD, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Finlay JL, Sposto R. Age and TP53 mutation frequency in childhood malignant gliomas: results in a multiinstitutional cohort. Cancer Res. 2001;61:7404–7407. [PubMed] [Google Scholar]
- 42.Maltzman W, Czyzyk L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol. 1984;4:1689–1694. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fritsche M, Haessler C, Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene. 1993;8:307–318. [PubMed] [Google Scholar]
- 44.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 45.Cargioli TG, Ugur HC, Ramakrishna N, Chan J, Black PM, Carroll RS. Establishment of an in vivo meningioma model with human telomerase reverse transcriptase. Neurosurgery. 2007;60:750–759. doi: 10.1227/01.NEU.0000255397.00410.8F. Discussion 759–760. [DOI] [PubMed] [Google Scholar]
- 46.Fan X, Wang Y, Kratz J, Brat DJ, Robitaille Y, Moghrabi A, Perlman EJ, Dang CV, Burger PC, Eberhart CG. hTERT gene amplification and increased mRNA expression in central nervous system embryonal tumors. Am J Pathol. 2003;162:1763–1769. doi: 10.1016/S0002-9440(10)64311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herms J, Neidt I, Luscher B, Sommer A, Schurmann P, Schroder T, Bergmann M, Wilken B, Probst-Cousin S, Hernaiz-Driever P, Behnke J, Hanefeld F, Pietsch T, Kretzschmar HA. C-MYC expression in medulloblastoma and its prognostic value. Int J Cancer. 2000;89:395–402. [PubMed] [Google Scholar]
- 48.Eberhart CG, Kratz J, Wang Y, Summers K, Stearns D, Cohen K, Dang CV, Burger PC. Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol. 2004;63:441–449. doi: 10.1093/jnen/63.5.441. [DOI] [PubMed] [Google Scholar]
- 49.Stearns D, Chaudhry A, Abel TW, Burger PC, Dang CV, Eberhart CG. c-myc overexpression causes anaplasia in medulloblastoma. Cancer Res. 2006;66:673–681. doi: 10.1158/0008-5472.CAN-05-1580. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi S, Yamamoto M, Ueno Y, Ikeda K, Ohshima K, Soma G, Fukushima T. Expression of nuclear factor-kappa B, tumor necrosis factor receptor type 1, and c-Myc in human astrocytomas. Neurol Med Chir (Tokyo) 2001;41:187–195. doi: 10.2176/nmc.41.187. [DOI] [PubMed] [Google Scholar]
- 51.Fujisawa H, Takabatake Y, Fukusato T, Tachibana O, Tsuchiya Y, Yamashita J. Molecular analysis of the rhabdoid predisposition syndrome in a child: a novel germline hSNF5/INI1 mutation and absence of c-myc amplification. J Neurooncol. 2003;63:257–262. doi: 10.1023/a:1024345221792. [DOI] [PubMed] [Google Scholar]
- 52.Brodeur GM, Hayes FA, Green AA, Casper JT, Wasson J, Wallach S, Seeger RC. Consistent N-myc copy number in simultaneous or consecutive neuroblastoma samples from sixty individual patients. Cancer Res. 1987;47:4248–4253. [PubMed] [Google Scholar]
- 53.Slavc I, Ellenbogen R, Jung WH, Vawter GF, Kretschmar C, Grier H, Korf BR. myc gene amplification and expression in primary human neuroblastoma. Cancer Res. 1990;50:1459–1463. [PubMed] [Google Scholar]
- 54.Matthay KK. MYCN expression in neuroblastoma: a mixed message? J Clin Oncol. 2000;18:3591–3594. doi: 10.1200/JCO.2000.18.21.3591. [DOI] [PubMed] [Google Scholar]
- 55.Grimmer MR, Weiss WA. Childhood tumors of the nervous system as disorders of normal development. Curr Opin Pediatr. 2006;18:634–638. doi: 10.1097/MOP.0b013e32801080fe. [DOI] [PubMed] [Google Scholar]